User login
Liraglutide fixes learning limit tied to insulin resistance
A single injection of the GLP-1 receptor agonist liraglutide led to short-term normalization of associative learning in people with obesity and insulin resistance, a finding that suggests say the authors of a recent report in Nature Metabolism.
“We demonstrated that dopamine-driven associative learning about external sensory cues crucially depends on metabolic signaling,” said Marc Tittgemeyer, PhD, professor at the Max Planck Institute for Metabolism Research in Cologne, Germany, and senior author of the study. Study participants with impaired insulin sensitivity “exhibited a reduced amplitude of behavioral updating that was normalized” by a single subcutaneous injection of 0.6 mg of liraglutide (the starting daily dose for liraglutide for weight loss, available as Saxenda, Novo Nordisk) given the evening before testing.
The findings, from 30 adults with normal insulin sensitivity and normal weight and 24 adults with impaired insulin sensitivity and obesity, suggest that metabolic signals, particularly ones that promote energy restoration in a setting of energy deprivation caused by insulin or a glucagon-like peptide-1 (GLP-1) receptor agonist, “profoundly influence neuronal processing,” said Dr. Tittgemeyer. The findings suggest that impaired metabolic signaling such as occurs with insulin resistance in people with obesity can cause deficiencies in associative learning.
‘Liraglutide can normalize learning of associations’
“We show that in people with obesity, disrupted circuit mechanisms lead to impaired learning about sensory associations,” Dr. Tittgemeyer said in an interview. “The information provided by sensory systems that the brain must interpret to select a behavioral response are ‘off tune’ ” in these individuals.
“This is rather consequential for understanding food-intake behaviors. Modern obesity treatments, such as liraglutide, can normalize learning of associations and thereby render people susceptible again for sensory signals and make them more prone to react to subliminal interactions, such as weight-normalizing diets and conscious eating,” he added.
The normalization in associative learning that one dose of liraglutide produced in people with obesity “fits with studies showing that these drugs restore a normal feeling of satiety, causing people to eat less and therefore lose weight,” he explained.
Dr. Tittgemeyer noted that this effect is likely shared by other agents in the GLP-1 receptor agonist class, such as semaglutide (Ozempic, Wegovy, Novo Nordisk) but is likely not an effect when agents agonize receptors to other nutrient-stimulated hormones such as glucagon and the glucose-dependent insulinotropic polypeptide.
The findings “show that liraglutide restores associative learning in participants with greater insulin resistance,” a “highly relevant” discovery, commented Nils B. Kroemer, PhD, head of the section of medical psychology at the University of Bonn, Germany, who was not involved with this research, in a written statement.
The study run by Dr. Tittgemeyer and his associates included 54 healthy adult volunteers whom they assessed for insulin sensitivity with their homeostasis model assessment of insulin resistance. The researchers divided the cohort into groups; one group included 24 people with impaired insulin sensitivity, and one included 30 with normal insulin sensitivity. The average body mass index (BMI) of the normal sensitivity group was about 24 kg/m2; in the insulin-resistant subgroup, BMI averaged about 33 kg/m2.
The associative learning task tested the ability of participants to learn associations between auditory cues (a high or low tone) and a subsequent visual outcome (a picture of a face or a house). During each associative learning session, participants also underwent functional MRI of the brain.
Liraglutide treatment leveled learning
The results showed that the learning rate was significantly lower in the subgroup with impaired insulin sensitivity, compared with those with normal insulin sensitivity following treatment with a placebo injection. This indicates a decreased adaptation of learning to predictability variations in individuals with impaired insulin sensitivity.
In contrast, treatment with a single dose of liraglutide significantly enhanced the learning rate in the group with impaired insulin sensitivity but significantly reduced the learning rate in the group with normal insulin sensitivity. Liraglutide’s effect was twice as large in the group with impaired insulin sensitivity than in the group with normal insulin sensitivity, and these opposing effects of liraglutide resulted in a convergence of the two groups’ adaptive learning rates so that there wasn’t any significant between-group difference following liraglutide treatment.
After analyzing the functional MRI data along with the learning results, the researchers concluded that liraglutide normalized learning in individuals with impaired insulin sensitivity by enhancing adaptive prediction error encoding in the brain’s ventral striatum and mesocortical projection sites.
This apparent ability of GLP-1 analogues to correct this learning deficit in people with impaired insulin sensitivity and obesity has implications regarding potential benefit for people with other pathologies characterized by impaired dopaminergic function and associated with metabolic impairments, such as psychosis, Parkinson’s disease, and depression, the researchers say.
“The fascinating thing about GLP-1 receptor agonists is that they have an additional mechanism that relates to anti-inflammatory effects, especially for alleviating cell stress,” said Dr. Tittgemeyer. “Many ongoing clinical trials are assessing their effects in neuropsychiatric diseases,” he noted.
The study received no commercial funding. Dr. Tittgemyer and most of his coauthors had no disclosures. One coauthor had several disclosures, which are detailed in the report. Dr. Kroemer had no disclosures.
A version of this article first appeared on Medscape.com.
A single injection of the GLP-1 receptor agonist liraglutide led to short-term normalization of associative learning in people with obesity and insulin resistance, a finding that suggests say the authors of a recent report in Nature Metabolism.
“We demonstrated that dopamine-driven associative learning about external sensory cues crucially depends on metabolic signaling,” said Marc Tittgemeyer, PhD, professor at the Max Planck Institute for Metabolism Research in Cologne, Germany, and senior author of the study. Study participants with impaired insulin sensitivity “exhibited a reduced amplitude of behavioral updating that was normalized” by a single subcutaneous injection of 0.6 mg of liraglutide (the starting daily dose for liraglutide for weight loss, available as Saxenda, Novo Nordisk) given the evening before testing.
The findings, from 30 adults with normal insulin sensitivity and normal weight and 24 adults with impaired insulin sensitivity and obesity, suggest that metabolic signals, particularly ones that promote energy restoration in a setting of energy deprivation caused by insulin or a glucagon-like peptide-1 (GLP-1) receptor agonist, “profoundly influence neuronal processing,” said Dr. Tittgemeyer. The findings suggest that impaired metabolic signaling such as occurs with insulin resistance in people with obesity can cause deficiencies in associative learning.
‘Liraglutide can normalize learning of associations’
“We show that in people with obesity, disrupted circuit mechanisms lead to impaired learning about sensory associations,” Dr. Tittgemeyer said in an interview. “The information provided by sensory systems that the brain must interpret to select a behavioral response are ‘off tune’ ” in these individuals.
“This is rather consequential for understanding food-intake behaviors. Modern obesity treatments, such as liraglutide, can normalize learning of associations and thereby render people susceptible again for sensory signals and make them more prone to react to subliminal interactions, such as weight-normalizing diets and conscious eating,” he added.
The normalization in associative learning that one dose of liraglutide produced in people with obesity “fits with studies showing that these drugs restore a normal feeling of satiety, causing people to eat less and therefore lose weight,” he explained.
Dr. Tittgemeyer noted that this effect is likely shared by other agents in the GLP-1 receptor agonist class, such as semaglutide (Ozempic, Wegovy, Novo Nordisk) but is likely not an effect when agents agonize receptors to other nutrient-stimulated hormones such as glucagon and the glucose-dependent insulinotropic polypeptide.
The findings “show that liraglutide restores associative learning in participants with greater insulin resistance,” a “highly relevant” discovery, commented Nils B. Kroemer, PhD, head of the section of medical psychology at the University of Bonn, Germany, who was not involved with this research, in a written statement.
The study run by Dr. Tittgemeyer and his associates included 54 healthy adult volunteers whom they assessed for insulin sensitivity with their homeostasis model assessment of insulin resistance. The researchers divided the cohort into groups; one group included 24 people with impaired insulin sensitivity, and one included 30 with normal insulin sensitivity. The average body mass index (BMI) of the normal sensitivity group was about 24 kg/m2; in the insulin-resistant subgroup, BMI averaged about 33 kg/m2.
The associative learning task tested the ability of participants to learn associations between auditory cues (a high or low tone) and a subsequent visual outcome (a picture of a face or a house). During each associative learning session, participants also underwent functional MRI of the brain.
Liraglutide treatment leveled learning
The results showed that the learning rate was significantly lower in the subgroup with impaired insulin sensitivity, compared with those with normal insulin sensitivity following treatment with a placebo injection. This indicates a decreased adaptation of learning to predictability variations in individuals with impaired insulin sensitivity.
In contrast, treatment with a single dose of liraglutide significantly enhanced the learning rate in the group with impaired insulin sensitivity but significantly reduced the learning rate in the group with normal insulin sensitivity. Liraglutide’s effect was twice as large in the group with impaired insulin sensitivity than in the group with normal insulin sensitivity, and these opposing effects of liraglutide resulted in a convergence of the two groups’ adaptive learning rates so that there wasn’t any significant between-group difference following liraglutide treatment.
After analyzing the functional MRI data along with the learning results, the researchers concluded that liraglutide normalized learning in individuals with impaired insulin sensitivity by enhancing adaptive prediction error encoding in the brain’s ventral striatum and mesocortical projection sites.
This apparent ability of GLP-1 analogues to correct this learning deficit in people with impaired insulin sensitivity and obesity has implications regarding potential benefit for people with other pathologies characterized by impaired dopaminergic function and associated with metabolic impairments, such as psychosis, Parkinson’s disease, and depression, the researchers say.
“The fascinating thing about GLP-1 receptor agonists is that they have an additional mechanism that relates to anti-inflammatory effects, especially for alleviating cell stress,” said Dr. Tittgemeyer. “Many ongoing clinical trials are assessing their effects in neuropsychiatric diseases,” he noted.
The study received no commercial funding. Dr. Tittgemyer and most of his coauthors had no disclosures. One coauthor had several disclosures, which are detailed in the report. Dr. Kroemer had no disclosures.
A version of this article first appeared on Medscape.com.
A single injection of the GLP-1 receptor agonist liraglutide led to short-term normalization of associative learning in people with obesity and insulin resistance, a finding that suggests say the authors of a recent report in Nature Metabolism.
“We demonstrated that dopamine-driven associative learning about external sensory cues crucially depends on metabolic signaling,” said Marc Tittgemeyer, PhD, professor at the Max Planck Institute for Metabolism Research in Cologne, Germany, and senior author of the study. Study participants with impaired insulin sensitivity “exhibited a reduced amplitude of behavioral updating that was normalized” by a single subcutaneous injection of 0.6 mg of liraglutide (the starting daily dose for liraglutide for weight loss, available as Saxenda, Novo Nordisk) given the evening before testing.
The findings, from 30 adults with normal insulin sensitivity and normal weight and 24 adults with impaired insulin sensitivity and obesity, suggest that metabolic signals, particularly ones that promote energy restoration in a setting of energy deprivation caused by insulin or a glucagon-like peptide-1 (GLP-1) receptor agonist, “profoundly influence neuronal processing,” said Dr. Tittgemeyer. The findings suggest that impaired metabolic signaling such as occurs with insulin resistance in people with obesity can cause deficiencies in associative learning.
‘Liraglutide can normalize learning of associations’
“We show that in people with obesity, disrupted circuit mechanisms lead to impaired learning about sensory associations,” Dr. Tittgemeyer said in an interview. “The information provided by sensory systems that the brain must interpret to select a behavioral response are ‘off tune’ ” in these individuals.
“This is rather consequential for understanding food-intake behaviors. Modern obesity treatments, such as liraglutide, can normalize learning of associations and thereby render people susceptible again for sensory signals and make them more prone to react to subliminal interactions, such as weight-normalizing diets and conscious eating,” he added.
The normalization in associative learning that one dose of liraglutide produced in people with obesity “fits with studies showing that these drugs restore a normal feeling of satiety, causing people to eat less and therefore lose weight,” he explained.
Dr. Tittgemeyer noted that this effect is likely shared by other agents in the GLP-1 receptor agonist class, such as semaglutide (Ozempic, Wegovy, Novo Nordisk) but is likely not an effect when agents agonize receptors to other nutrient-stimulated hormones such as glucagon and the glucose-dependent insulinotropic polypeptide.
The findings “show that liraglutide restores associative learning in participants with greater insulin resistance,” a “highly relevant” discovery, commented Nils B. Kroemer, PhD, head of the section of medical psychology at the University of Bonn, Germany, who was not involved with this research, in a written statement.
The study run by Dr. Tittgemeyer and his associates included 54 healthy adult volunteers whom they assessed for insulin sensitivity with their homeostasis model assessment of insulin resistance. The researchers divided the cohort into groups; one group included 24 people with impaired insulin sensitivity, and one included 30 with normal insulin sensitivity. The average body mass index (BMI) of the normal sensitivity group was about 24 kg/m2; in the insulin-resistant subgroup, BMI averaged about 33 kg/m2.
The associative learning task tested the ability of participants to learn associations between auditory cues (a high or low tone) and a subsequent visual outcome (a picture of a face or a house). During each associative learning session, participants also underwent functional MRI of the brain.
Liraglutide treatment leveled learning
The results showed that the learning rate was significantly lower in the subgroup with impaired insulin sensitivity, compared with those with normal insulin sensitivity following treatment with a placebo injection. This indicates a decreased adaptation of learning to predictability variations in individuals with impaired insulin sensitivity.
In contrast, treatment with a single dose of liraglutide significantly enhanced the learning rate in the group with impaired insulin sensitivity but significantly reduced the learning rate in the group with normal insulin sensitivity. Liraglutide’s effect was twice as large in the group with impaired insulin sensitivity than in the group with normal insulin sensitivity, and these opposing effects of liraglutide resulted in a convergence of the two groups’ adaptive learning rates so that there wasn’t any significant between-group difference following liraglutide treatment.
After analyzing the functional MRI data along with the learning results, the researchers concluded that liraglutide normalized learning in individuals with impaired insulin sensitivity by enhancing adaptive prediction error encoding in the brain’s ventral striatum and mesocortical projection sites.
This apparent ability of GLP-1 analogues to correct this learning deficit in people with impaired insulin sensitivity and obesity has implications regarding potential benefit for people with other pathologies characterized by impaired dopaminergic function and associated with metabolic impairments, such as psychosis, Parkinson’s disease, and depression, the researchers say.
“The fascinating thing about GLP-1 receptor agonists is that they have an additional mechanism that relates to anti-inflammatory effects, especially for alleviating cell stress,” said Dr. Tittgemeyer. “Many ongoing clinical trials are assessing their effects in neuropsychiatric diseases,” he noted.
The study received no commercial funding. Dr. Tittgemyer and most of his coauthors had no disclosures. One coauthor had several disclosures, which are detailed in the report. Dr. Kroemer had no disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE METABOLISM
Apple cider vinegar, fenugreek best herbal remedies for T2D: Review
The review included 44 randomized clinical trials with more than 3,000 participants using six herbal remedies: apple cider vinegar, cinnamon, curcumin, fenugreek seeds, ginger, and saffron.
Apple cider vinegar, fenugreek seeds, curcumin (turmeric), and cinnamon resulted in statistically significant reductions in fasting blood glucose, compared with the control groups in the clinical trials. Out of all the remedies, the authors found apple cider vinegar to be the most effective for lowering fasting blood glucose levels.
The review also found that apple cider vinegar and fenugreek seeds had a statistically significant effect on reducing A1c, compared with the control groups. The authors found the herbal remedies made no difference to insulin level or homeostatic model assessment for insulin resistance (HOMA-IR).
The results are published online in Diabetes & Metabolic Syndrome: Clinical Research & Reviews. The authors said they hoped the review would help medical professionals and people with type 2 diabetes understand the effectiveness of different herbal remedies and consider incorporating these remedies into standard care.
“Some people use curcumin, some use ginger, some use apple cider [vinegar], but it’s not clear which is better,” said Shiv Mudgal, PhD, corresponding author of the paper and an associate professor in nursing at the All India Institute of Medical Sciences in Deoghar, India.
“We thought it would be nice to get some idea about how they work and how they compete with each other,” said Subodh Kumar, MD, the first author and an associate professor in pharmacology at the All India Institute of Medical Sciences in Deoghar, India.
They wanted to understand how the herbal remedies worked by including insulin level and HOMA-IR as measurable outcomes but found nothing conclusive. Instead, they speculated that the effect of apple cider vinegar and fenugreek seeds on blood glucose and A1c could be related to delayed gastric emptying, among other mechanisms.
However, the results should be interpreted with caution, said Dr. Kumar.
Apple cider vinegar had three clinical trials to back the finding, and fenugreek seeds had four studies supporting the results – fewer than the other included remedies. The authors also identified risks of bias from the randomization process and the allocation concealment process in several of the included trials.
Most of the studies included only short follow-up periods, meaning that the long-term effects of using these herbal remedies to help manage type 2 diabetes remain unclear.
The six herbal remedies included in the study were chosen out of dozens of popular complementary medicines for the strength and number of clinical trials backing their use.
The limited number included in the review is a drawback, according to Merlin Willcox, DPhil, a clinical lecturer in general practice at the University of Southampton, England, who was not involved in the research.
“It means they’ve left out stuff that’s potentially effective,” Dr. Willcox said in an interview.
Dr. Willcox, who has coauthored a review of herbal remedies for glycemic control in type 2 diabetes, said he was surprised that apple cider vinegar came out on top in this analysis.
His review concluded that aloe vera leaf gel, psyllium fiber, and fenugreek seeds appeared to be the most effective at reducing A1c, compared with the control groups of the included trials, out of 18 plant-based remedies.
There were no adverse effects associated with the herbal remedies, according to Dr. Mudgal. However, the evidence for the herbal remedies included in their review also lacked substantial follow-up periods assessing their long-term effects.
“You need to look at the evidence for each individual remedy; it’s not just about what plant it is, but it’s about what preparation, what dose. All of that comes into play,” Dr. Willcox said.
Up to 3.6 million people use herbal remedies to manage type 2 diabetes in the United States, according to a 2014 study cited by the review authors. The number is much higher elsewhere: As many as two-thirds of patients with diabetes in India and Saudi Arabia incorporate herbal remedies to help manage symptoms, whereas about half of patients with diabetes in the United Kingdom use herbal medicines.
Experts warn of the risks associated with using herbal medicines to complement traditional therapies.
“I caution my patients about dietary supplements and herbals because of the lack of high-quality data demonstrating efficacy and safety,” Katherine H. Saunders, MD, DABOM, cofounder of Intellihealth and clinical assistant professor of medicine at Weill Cornell Medicine, New York, said in an interview.
For Dr. Willcox, the risks relate to where patients get their information from. Many patients with type 2 diabetes are too scared to talk to their doctor about herbal medicines.
“They think their doctor is going to be negative or dismissive,” Dr. Willcox said. “So patients are getting their information from family and friends or from the Internet, which is not necessarily the most reliable, evidence-based source of information.”
The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
The review included 44 randomized clinical trials with more than 3,000 participants using six herbal remedies: apple cider vinegar, cinnamon, curcumin, fenugreek seeds, ginger, and saffron.
Apple cider vinegar, fenugreek seeds, curcumin (turmeric), and cinnamon resulted in statistically significant reductions in fasting blood glucose, compared with the control groups in the clinical trials. Out of all the remedies, the authors found apple cider vinegar to be the most effective for lowering fasting blood glucose levels.
The review also found that apple cider vinegar and fenugreek seeds had a statistically significant effect on reducing A1c, compared with the control groups. The authors found the herbal remedies made no difference to insulin level or homeostatic model assessment for insulin resistance (HOMA-IR).
The results are published online in Diabetes & Metabolic Syndrome: Clinical Research & Reviews. The authors said they hoped the review would help medical professionals and people with type 2 diabetes understand the effectiveness of different herbal remedies and consider incorporating these remedies into standard care.
“Some people use curcumin, some use ginger, some use apple cider [vinegar], but it’s not clear which is better,” said Shiv Mudgal, PhD, corresponding author of the paper and an associate professor in nursing at the All India Institute of Medical Sciences in Deoghar, India.
“We thought it would be nice to get some idea about how they work and how they compete with each other,” said Subodh Kumar, MD, the first author and an associate professor in pharmacology at the All India Institute of Medical Sciences in Deoghar, India.
They wanted to understand how the herbal remedies worked by including insulin level and HOMA-IR as measurable outcomes but found nothing conclusive. Instead, they speculated that the effect of apple cider vinegar and fenugreek seeds on blood glucose and A1c could be related to delayed gastric emptying, among other mechanisms.
However, the results should be interpreted with caution, said Dr. Kumar.
Apple cider vinegar had three clinical trials to back the finding, and fenugreek seeds had four studies supporting the results – fewer than the other included remedies. The authors also identified risks of bias from the randomization process and the allocation concealment process in several of the included trials.
Most of the studies included only short follow-up periods, meaning that the long-term effects of using these herbal remedies to help manage type 2 diabetes remain unclear.
The six herbal remedies included in the study were chosen out of dozens of popular complementary medicines for the strength and number of clinical trials backing their use.
The limited number included in the review is a drawback, according to Merlin Willcox, DPhil, a clinical lecturer in general practice at the University of Southampton, England, who was not involved in the research.
“It means they’ve left out stuff that’s potentially effective,” Dr. Willcox said in an interview.
Dr. Willcox, who has coauthored a review of herbal remedies for glycemic control in type 2 diabetes, said he was surprised that apple cider vinegar came out on top in this analysis.
His review concluded that aloe vera leaf gel, psyllium fiber, and fenugreek seeds appeared to be the most effective at reducing A1c, compared with the control groups of the included trials, out of 18 plant-based remedies.
There were no adverse effects associated with the herbal remedies, according to Dr. Mudgal. However, the evidence for the herbal remedies included in their review also lacked substantial follow-up periods assessing their long-term effects.
“You need to look at the evidence for each individual remedy; it’s not just about what plant it is, but it’s about what preparation, what dose. All of that comes into play,” Dr. Willcox said.
Up to 3.6 million people use herbal remedies to manage type 2 diabetes in the United States, according to a 2014 study cited by the review authors. The number is much higher elsewhere: As many as two-thirds of patients with diabetes in India and Saudi Arabia incorporate herbal remedies to help manage symptoms, whereas about half of patients with diabetes in the United Kingdom use herbal medicines.
Experts warn of the risks associated with using herbal medicines to complement traditional therapies.
“I caution my patients about dietary supplements and herbals because of the lack of high-quality data demonstrating efficacy and safety,” Katherine H. Saunders, MD, DABOM, cofounder of Intellihealth and clinical assistant professor of medicine at Weill Cornell Medicine, New York, said in an interview.
For Dr. Willcox, the risks relate to where patients get their information from. Many patients with type 2 diabetes are too scared to talk to their doctor about herbal medicines.
“They think their doctor is going to be negative or dismissive,” Dr. Willcox said. “So patients are getting their information from family and friends or from the Internet, which is not necessarily the most reliable, evidence-based source of information.”
The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
The review included 44 randomized clinical trials with more than 3,000 participants using six herbal remedies: apple cider vinegar, cinnamon, curcumin, fenugreek seeds, ginger, and saffron.
Apple cider vinegar, fenugreek seeds, curcumin (turmeric), and cinnamon resulted in statistically significant reductions in fasting blood glucose, compared with the control groups in the clinical trials. Out of all the remedies, the authors found apple cider vinegar to be the most effective for lowering fasting blood glucose levels.
The review also found that apple cider vinegar and fenugreek seeds had a statistically significant effect on reducing A1c, compared with the control groups. The authors found the herbal remedies made no difference to insulin level or homeostatic model assessment for insulin resistance (HOMA-IR).
The results are published online in Diabetes & Metabolic Syndrome: Clinical Research & Reviews. The authors said they hoped the review would help medical professionals and people with type 2 diabetes understand the effectiveness of different herbal remedies and consider incorporating these remedies into standard care.
“Some people use curcumin, some use ginger, some use apple cider [vinegar], but it’s not clear which is better,” said Shiv Mudgal, PhD, corresponding author of the paper and an associate professor in nursing at the All India Institute of Medical Sciences in Deoghar, India.
“We thought it would be nice to get some idea about how they work and how they compete with each other,” said Subodh Kumar, MD, the first author and an associate professor in pharmacology at the All India Institute of Medical Sciences in Deoghar, India.
They wanted to understand how the herbal remedies worked by including insulin level and HOMA-IR as measurable outcomes but found nothing conclusive. Instead, they speculated that the effect of apple cider vinegar and fenugreek seeds on blood glucose and A1c could be related to delayed gastric emptying, among other mechanisms.
However, the results should be interpreted with caution, said Dr. Kumar.
Apple cider vinegar had three clinical trials to back the finding, and fenugreek seeds had four studies supporting the results – fewer than the other included remedies. The authors also identified risks of bias from the randomization process and the allocation concealment process in several of the included trials.
Most of the studies included only short follow-up periods, meaning that the long-term effects of using these herbal remedies to help manage type 2 diabetes remain unclear.
The six herbal remedies included in the study were chosen out of dozens of popular complementary medicines for the strength and number of clinical trials backing their use.
The limited number included in the review is a drawback, according to Merlin Willcox, DPhil, a clinical lecturer in general practice at the University of Southampton, England, who was not involved in the research.
“It means they’ve left out stuff that’s potentially effective,” Dr. Willcox said in an interview.
Dr. Willcox, who has coauthored a review of herbal remedies for glycemic control in type 2 diabetes, said he was surprised that apple cider vinegar came out on top in this analysis.
His review concluded that aloe vera leaf gel, psyllium fiber, and fenugreek seeds appeared to be the most effective at reducing A1c, compared with the control groups of the included trials, out of 18 plant-based remedies.
There were no adverse effects associated with the herbal remedies, according to Dr. Mudgal. However, the evidence for the herbal remedies included in their review also lacked substantial follow-up periods assessing their long-term effects.
“You need to look at the evidence for each individual remedy; it’s not just about what plant it is, but it’s about what preparation, what dose. All of that comes into play,” Dr. Willcox said.
Up to 3.6 million people use herbal remedies to manage type 2 diabetes in the United States, according to a 2014 study cited by the review authors. The number is much higher elsewhere: As many as two-thirds of patients with diabetes in India and Saudi Arabia incorporate herbal remedies to help manage symptoms, whereas about half of patients with diabetes in the United Kingdom use herbal medicines.
Experts warn of the risks associated with using herbal medicines to complement traditional therapies.
“I caution my patients about dietary supplements and herbals because of the lack of high-quality data demonstrating efficacy and safety,” Katherine H. Saunders, MD, DABOM, cofounder of Intellihealth and clinical assistant professor of medicine at Weill Cornell Medicine, New York, said in an interview.
For Dr. Willcox, the risks relate to where patients get their information from. Many patients with type 2 diabetes are too scared to talk to their doctor about herbal medicines.
“They think their doctor is going to be negative or dismissive,” Dr. Willcox said. “So patients are getting their information from family and friends or from the Internet, which is not necessarily the most reliable, evidence-based source of information.”
The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM DIABETES & METABOLIC SYNDROME: CLINICAL RESEARCH & REVIEWS
Continuous glucose monitors for pregnant patients?
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone – having a blood glucose level below 140 mg/dL – than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Mark Ebell, MD, a professor of epidemiology at the University of Georgia, Athens, was more skeptical, pointing out that study participants might have used other methods in addition to the technology to lower their blood sugar levels.
The findings suggest that insulin pumps are more manageable than multiple, daily self-injections. About 1 in 9 women have diabetes in the United States, and 35% of people newly diagnosed with the condition are women of reproductive age.
Dr. Hamill said that in future research, use of a stricter criterion for baseline blood sugar levels (< 140 mg/dL) would be helpful, as would exploring how much time patients need to spend below that level for optimal outcomes.
“Those questions are really absent in the literature,” Dr. Hamill said. “Most of our obstetrical literature is comparing treatment types. All those things are secondary. It’s the blood sugar that confers the risk, and if we get the blood sugar better, risk is reduced.”
Dr. Hamill added that the benefits of these technologies for patients with gestational diabetes are unclear in consideration of the limited duration of the disease and the time required to implant or install a monitor and pump, as well as associated risks and the cost of the devices.
Dr. Sodhi said clinicians who see patients during family planning visits should review morbidities and medical problems related to diabetes.
“I think this is a study that’s maybe too early,” Dr. Sodhi said. “They did ‘guesstimates’ on what target blood glucose ranges to be looking at, but I think over time, we might, with more studies like this, be building a case to try to put these types of monitors in for patients who are young for the purpose of optimizing pregnancy outcomes.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone – having a blood glucose level below 140 mg/dL – than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Mark Ebell, MD, a professor of epidemiology at the University of Georgia, Athens, was more skeptical, pointing out that study participants might have used other methods in addition to the technology to lower their blood sugar levels.
The findings suggest that insulin pumps are more manageable than multiple, daily self-injections. About 1 in 9 women have diabetes in the United States, and 35% of people newly diagnosed with the condition are women of reproductive age.
Dr. Hamill said that in future research, use of a stricter criterion for baseline blood sugar levels (< 140 mg/dL) would be helpful, as would exploring how much time patients need to spend below that level for optimal outcomes.
“Those questions are really absent in the literature,” Dr. Hamill said. “Most of our obstetrical literature is comparing treatment types. All those things are secondary. It’s the blood sugar that confers the risk, and if we get the blood sugar better, risk is reduced.”
Dr. Hamill added that the benefits of these technologies for patients with gestational diabetes are unclear in consideration of the limited duration of the disease and the time required to implant or install a monitor and pump, as well as associated risks and the cost of the devices.
Dr. Sodhi said clinicians who see patients during family planning visits should review morbidities and medical problems related to diabetes.
“I think this is a study that’s maybe too early,” Dr. Sodhi said. “They did ‘guesstimates’ on what target blood glucose ranges to be looking at, but I think over time, we might, with more studies like this, be building a case to try to put these types of monitors in for patients who are young for the purpose of optimizing pregnancy outcomes.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone – having a blood glucose level below 140 mg/dL – than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Mark Ebell, MD, a professor of epidemiology at the University of Georgia, Athens, was more skeptical, pointing out that study participants might have used other methods in addition to the technology to lower their blood sugar levels.
The findings suggest that insulin pumps are more manageable than multiple, daily self-injections. About 1 in 9 women have diabetes in the United States, and 35% of people newly diagnosed with the condition are women of reproductive age.
Dr. Hamill said that in future research, use of a stricter criterion for baseline blood sugar levels (< 140 mg/dL) would be helpful, as would exploring how much time patients need to spend below that level for optimal outcomes.
“Those questions are really absent in the literature,” Dr. Hamill said. “Most of our obstetrical literature is comparing treatment types. All those things are secondary. It’s the blood sugar that confers the risk, and if we get the blood sugar better, risk is reduced.”
Dr. Hamill added that the benefits of these technologies for patients with gestational diabetes are unclear in consideration of the limited duration of the disease and the time required to implant or install a monitor and pump, as well as associated risks and the cost of the devices.
Dr. Sodhi said clinicians who see patients during family planning visits should review morbidities and medical problems related to diabetes.
“I think this is a study that’s maybe too early,” Dr. Sodhi said. “They did ‘guesstimates’ on what target blood glucose ranges to be looking at, but I think over time, we might, with more studies like this, be building a case to try to put these types of monitors in for patients who are young for the purpose of optimizing pregnancy outcomes.”
The authors have disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Human frailty is a cash cow
Doctor, if you are caring for patients with diabetes, I sure hope you know more about it than I do. The longer I live, it seems, the less I understand.
In a free society, people can do what they want, and that’s great except when it isn’t. That’s why societies develop ethics and even public laws if ethics are not strong enough to protect us from ourselves and others.
Sugar, sugar
When I was growing up in small-town Alabama during the Depression and World War II, we called it sugar diabetes. Eat too much sugar, you got fat; your blood sugar went up, and you spilled sugar into your urine. Diabetes was fairly rare, and so was obesity. Doctors treated it by limiting the intake of sugar (and various sweet foods), along with attempting weight loss. If that didn’t do the trick, insulin injections.
From then until now, note these trends.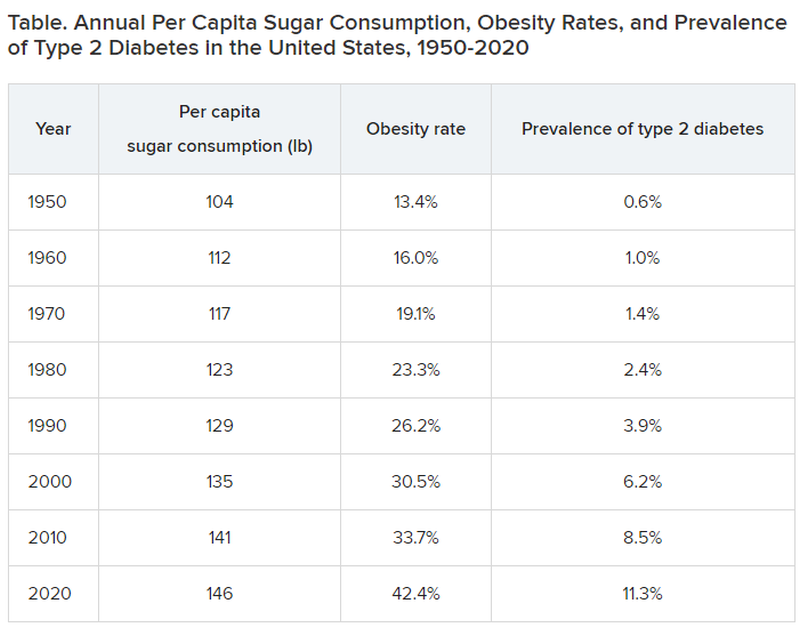
Type 2 diabetes was diagnosed even more infrequently before 1950:
- 1920: 0.2% of the population
- 1930: 0.3% of the population
- 1940: 0.4% of the population
In 2020, although 11.3% of the population was diagnosed with type 2 diabetes, the unknown undiagnosed proportion could be much higher.
Notice a correlation between sugar consumption and prevalence of diabetes? Of course, correlation is not causation, but at the same time, it sure as hell is not negation. Such concordance can be considered hypothesis generating. It may not be true causation, but it’s a good bet when 89% of people with diabetes have overweight or obesity.
What did the entire medical, public health, government, agriculture, nursing, food manufacturing, marketing, advertising, restaurant, and education constituencies do about this as it was happening? They observed, documented, gave lip service, and wrung their hands in public a bit. I do not believe that this is an organized active conspiracy; it would take too many players cooperating over too long a period of time. But it certainly may be a passive conspiracy, and primary care physicians and their patients are trapped.
The proper daily practice of medicine consists of one patient, one physician, one moment, and one decision. Let it be a shared decision, informed by the best evidence and taking cost into consideration. That encounter represents an opportunity, a responsibility, and a conundrum.
Individual health is subsumed under the collective health of the public. As such, a patient’s health is out of the control of both physician and patient; instead, patients are the beneficiaries or victims of the “marketplace.” Humans are frail and easily taken advantage of by the brilliant and highly motivated strategic planning and execution of Big Agriculture, Big Food, Big Pharma, Big Marketing, and Big Money-Driven Medicine and generally failed by Big Government, Big Public Health, Big Education, Big Psychology, and Big Religion.
Rethinking diabetes
Consider diabetes as one of many examples. What a terrific deal for capitalism. then it discovers (invents) long-term, very expensive, compelling treatments to slim us down, with no end in sight, and still without ever understanding the true nature of diabetes.
Gary Taubes’s great new book, “Rethinking Diabetes: What Science Reveals About Diet, Insulin, and Successful Treatments,” is being published by Alfred A. Knopf in early 2024.
It is 404 pages of (dense) text, with 401 numbered references and footnotes, a bibliography of 790 references, alphabetically arranged for easy cross-checking, and a 25-page index.
Remember Mr. Taubes’s earlier definitive historical treatises: “Good Calories, Bad Calories” (2007), “Why We Get Fat” (2010), “The Case Against Sugar” (2016), and “The Case for Keto” (2020)?
This new book is more like “Good Calories, Bad Calories”: long, dense, detailed, definitive, and of great historical reference value, including original research information from other countries in other languages. The author told me that the many early research reference sources were available only in German and that his use of generative artificial intelligence as an assistant researcher was of great value.
Nonphysician author Mr. Taubes uses his deep understanding of science and history to inform his long-honed talents of impartial investigative journalism as he attempts to understand and then explain why after all these years, the medical scientific community still does not have a sound consensus about the essence of diabetes, diet, insulin, and proper prevention and treatment at a level that is actually effective – amazing and so sad.
To signal these evolved and evolving conflicts, the book includes the following chapters:
- “Rise of the Carbohydrate-Rich and Very-Low-Carbohydrate Diets”
- “The Fear of Fat and High-Fat Diets”
- “Insulin and The End of Carbohydrate Restriction and Low Blood Sugar”
Yes, it is difficult. Imagine the bookend segments: “The Nature of Medical Knowledge” and “The Conflicts of Evidence-Based Medicine.” There is also a detailed discussion of good versus bad science spanning three long chapters.
If all that reads like a greatly confused mess to you then you’re beginning to understand. If you are a fan of an unbiased explication of the evolution of understanding the ins and outs of scientific history in richly documented detail, this is a book for you. It’s not a quick nor easy read. And don’t expect to discover whether the newest wonder drugs for weight loss and control of diabetes will be the long-term solution for people with obesity and diabetes worldwide.
Obesity and overweight are major risk factors for type 2 diabetes. About 90% of patients with diabetes have either overweight or obesity. Thus, the complications of these two conditions, which largely overlap, include atherosclerotic cardiovascular disease; myocardial infarction; stroke; hypertension; metabolic syndrome; lower-extremity gangrene; chronic kidney disease; retinopathy; glaucoma; cataracts; disabling osteoarthritis; breast, endometrial, colon, and other cancers; fatty liver; sleep apnea; and peripheral neuropathy. These diseases create a major lucrative business for a wide swathe of medical and surgical specialties, plus hospital, clinic, device, pharmaceutical, and food industries.
In summary, we’ve just been through 40 years of failure to recognize the sugar-elephant in the room and intervene with serious preventive efforts. Forty years of fleshing out both the populace and the American medical-industrial complex (AMIC). Talk about a sweet spot. The only successful long-term treatment of obesity (and with it, diabetes) is prevention. Don’t emphasize losing weight. Focus on preventing excessive weight gain, right now, for the population, beginning with yourselves. Otherwise, we continue openly to perpetuate a terrific deal for the AMIC, a travesty for everyone else. Time for some industrial grade penance and a course correction.
Meanwhile, here we are living out Big Pharma’s dream of a big populace, produced by the agriculture and food industries, enjoyed by capitalism after failures of education, medicine, and public health: a seemingly endless supply of people living with big complications who are ready for big (expensive, new) medications to fix the world’s big health problems.
Dr. Lundberg is editor in chief, Cancer Commons. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Doctor, if you are caring for patients with diabetes, I sure hope you know more about it than I do. The longer I live, it seems, the less I understand.
In a free society, people can do what they want, and that’s great except when it isn’t. That’s why societies develop ethics and even public laws if ethics are not strong enough to protect us from ourselves and others.
Sugar, sugar
When I was growing up in small-town Alabama during the Depression and World War II, we called it sugar diabetes. Eat too much sugar, you got fat; your blood sugar went up, and you spilled sugar into your urine. Diabetes was fairly rare, and so was obesity. Doctors treated it by limiting the intake of sugar (and various sweet foods), along with attempting weight loss. If that didn’t do the trick, insulin injections.
From then until now, note these trends.
Type 2 diabetes was diagnosed even more infrequently before 1950:
- 1920: 0.2% of the population
- 1930: 0.3% of the population
- 1940: 0.4% of the population
In 2020, although 11.3% of the population was diagnosed with type 2 diabetes, the unknown undiagnosed proportion could be much higher.
Notice a correlation between sugar consumption and prevalence of diabetes? Of course, correlation is not causation, but at the same time, it sure as hell is not negation. Such concordance can be considered hypothesis generating. It may not be true causation, but it’s a good bet when 89% of people with diabetes have overweight or obesity.
What did the entire medical, public health, government, agriculture, nursing, food manufacturing, marketing, advertising, restaurant, and education constituencies do about this as it was happening? They observed, documented, gave lip service, and wrung their hands in public a bit. I do not believe that this is an organized active conspiracy; it would take too many players cooperating over too long a period of time. But it certainly may be a passive conspiracy, and primary care physicians and their patients are trapped.
The proper daily practice of medicine consists of one patient, one physician, one moment, and one decision. Let it be a shared decision, informed by the best evidence and taking cost into consideration. That encounter represents an opportunity, a responsibility, and a conundrum.
Individual health is subsumed under the collective health of the public. As such, a patient’s health is out of the control of both physician and patient; instead, patients are the beneficiaries or victims of the “marketplace.” Humans are frail and easily taken advantage of by the brilliant and highly motivated strategic planning and execution of Big Agriculture, Big Food, Big Pharma, Big Marketing, and Big Money-Driven Medicine and generally failed by Big Government, Big Public Health, Big Education, Big Psychology, and Big Religion.
Rethinking diabetes
Consider diabetes as one of many examples. What a terrific deal for capitalism. then it discovers (invents) long-term, very expensive, compelling treatments to slim us down, with no end in sight, and still without ever understanding the true nature of diabetes.
Gary Taubes’s great new book, “Rethinking Diabetes: What Science Reveals About Diet, Insulin, and Successful Treatments,” is being published by Alfred A. Knopf in early 2024.
It is 404 pages of (dense) text, with 401 numbered references and footnotes, a bibliography of 790 references, alphabetically arranged for easy cross-checking, and a 25-page index.
Remember Mr. Taubes’s earlier definitive historical treatises: “Good Calories, Bad Calories” (2007), “Why We Get Fat” (2010), “The Case Against Sugar” (2016), and “The Case for Keto” (2020)?
This new book is more like “Good Calories, Bad Calories”: long, dense, detailed, definitive, and of great historical reference value, including original research information from other countries in other languages. The author told me that the many early research reference sources were available only in German and that his use of generative artificial intelligence as an assistant researcher was of great value.
Nonphysician author Mr. Taubes uses his deep understanding of science and history to inform his long-honed talents of impartial investigative journalism as he attempts to understand and then explain why after all these years, the medical scientific community still does not have a sound consensus about the essence of diabetes, diet, insulin, and proper prevention and treatment at a level that is actually effective – amazing and so sad.
To signal these evolved and evolving conflicts, the book includes the following chapters:
- “Rise of the Carbohydrate-Rich and Very-Low-Carbohydrate Diets”
- “The Fear of Fat and High-Fat Diets”
- “Insulin and The End of Carbohydrate Restriction and Low Blood Sugar”
Yes, it is difficult. Imagine the bookend segments: “The Nature of Medical Knowledge” and “The Conflicts of Evidence-Based Medicine.” There is also a detailed discussion of good versus bad science spanning three long chapters.
If all that reads like a greatly confused mess to you then you’re beginning to understand. If you are a fan of an unbiased explication of the evolution of understanding the ins and outs of scientific history in richly documented detail, this is a book for you. It’s not a quick nor easy read. And don’t expect to discover whether the newest wonder drugs for weight loss and control of diabetes will be the long-term solution for people with obesity and diabetes worldwide.
Obesity and overweight are major risk factors for type 2 diabetes. About 90% of patients with diabetes have either overweight or obesity. Thus, the complications of these two conditions, which largely overlap, include atherosclerotic cardiovascular disease; myocardial infarction; stroke; hypertension; metabolic syndrome; lower-extremity gangrene; chronic kidney disease; retinopathy; glaucoma; cataracts; disabling osteoarthritis; breast, endometrial, colon, and other cancers; fatty liver; sleep apnea; and peripheral neuropathy. These diseases create a major lucrative business for a wide swathe of medical and surgical specialties, plus hospital, clinic, device, pharmaceutical, and food industries.
In summary, we’ve just been through 40 years of failure to recognize the sugar-elephant in the room and intervene with serious preventive efforts. Forty years of fleshing out both the populace and the American medical-industrial complex (AMIC). Talk about a sweet spot. The only successful long-term treatment of obesity (and with it, diabetes) is prevention. Don’t emphasize losing weight. Focus on preventing excessive weight gain, right now, for the population, beginning with yourselves. Otherwise, we continue openly to perpetuate a terrific deal for the AMIC, a travesty for everyone else. Time for some industrial grade penance and a course correction.
Meanwhile, here we are living out Big Pharma’s dream of a big populace, produced by the agriculture and food industries, enjoyed by capitalism after failures of education, medicine, and public health: a seemingly endless supply of people living with big complications who are ready for big (expensive, new) medications to fix the world’s big health problems.
Dr. Lundberg is editor in chief, Cancer Commons. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Doctor, if you are caring for patients with diabetes, I sure hope you know more about it than I do. The longer I live, it seems, the less I understand.
In a free society, people can do what they want, and that’s great except when it isn’t. That’s why societies develop ethics and even public laws if ethics are not strong enough to protect us from ourselves and others.
Sugar, sugar
When I was growing up in small-town Alabama during the Depression and World War II, we called it sugar diabetes. Eat too much sugar, you got fat; your blood sugar went up, and you spilled sugar into your urine. Diabetes was fairly rare, and so was obesity. Doctors treated it by limiting the intake of sugar (and various sweet foods), along with attempting weight loss. If that didn’t do the trick, insulin injections.
From then until now, note these trends.
Type 2 diabetes was diagnosed even more infrequently before 1950:
- 1920: 0.2% of the population
- 1930: 0.3% of the population
- 1940: 0.4% of the population
In 2020, although 11.3% of the population was diagnosed with type 2 diabetes, the unknown undiagnosed proportion could be much higher.
Notice a correlation between sugar consumption and prevalence of diabetes? Of course, correlation is not causation, but at the same time, it sure as hell is not negation. Such concordance can be considered hypothesis generating. It may not be true causation, but it’s a good bet when 89% of people with diabetes have overweight or obesity.
What did the entire medical, public health, government, agriculture, nursing, food manufacturing, marketing, advertising, restaurant, and education constituencies do about this as it was happening? They observed, documented, gave lip service, and wrung their hands in public a bit. I do not believe that this is an organized active conspiracy; it would take too many players cooperating over too long a period of time. But it certainly may be a passive conspiracy, and primary care physicians and their patients are trapped.
The proper daily practice of medicine consists of one patient, one physician, one moment, and one decision. Let it be a shared decision, informed by the best evidence and taking cost into consideration. That encounter represents an opportunity, a responsibility, and a conundrum.
Individual health is subsumed under the collective health of the public. As such, a patient’s health is out of the control of both physician and patient; instead, patients are the beneficiaries or victims of the “marketplace.” Humans are frail and easily taken advantage of by the brilliant and highly motivated strategic planning and execution of Big Agriculture, Big Food, Big Pharma, Big Marketing, and Big Money-Driven Medicine and generally failed by Big Government, Big Public Health, Big Education, Big Psychology, and Big Religion.
Rethinking diabetes
Consider diabetes as one of many examples. What a terrific deal for capitalism. then it discovers (invents) long-term, very expensive, compelling treatments to slim us down, with no end in sight, and still without ever understanding the true nature of diabetes.
Gary Taubes’s great new book, “Rethinking Diabetes: What Science Reveals About Diet, Insulin, and Successful Treatments,” is being published by Alfred A. Knopf in early 2024.
It is 404 pages of (dense) text, with 401 numbered references and footnotes, a bibliography of 790 references, alphabetically arranged for easy cross-checking, and a 25-page index.
Remember Mr. Taubes’s earlier definitive historical treatises: “Good Calories, Bad Calories” (2007), “Why We Get Fat” (2010), “The Case Against Sugar” (2016), and “The Case for Keto” (2020)?
This new book is more like “Good Calories, Bad Calories”: long, dense, detailed, definitive, and of great historical reference value, including original research information from other countries in other languages. The author told me that the many early research reference sources were available only in German and that his use of generative artificial intelligence as an assistant researcher was of great value.
Nonphysician author Mr. Taubes uses his deep understanding of science and history to inform his long-honed talents of impartial investigative journalism as he attempts to understand and then explain why after all these years, the medical scientific community still does not have a sound consensus about the essence of diabetes, diet, insulin, and proper prevention and treatment at a level that is actually effective – amazing and so sad.
To signal these evolved and evolving conflicts, the book includes the following chapters:
- “Rise of the Carbohydrate-Rich and Very-Low-Carbohydrate Diets”
- “The Fear of Fat and High-Fat Diets”
- “Insulin and The End of Carbohydrate Restriction and Low Blood Sugar”
Yes, it is difficult. Imagine the bookend segments: “The Nature of Medical Knowledge” and “The Conflicts of Evidence-Based Medicine.” There is also a detailed discussion of good versus bad science spanning three long chapters.
If all that reads like a greatly confused mess to you then you’re beginning to understand. If you are a fan of an unbiased explication of the evolution of understanding the ins and outs of scientific history in richly documented detail, this is a book for you. It’s not a quick nor easy read. And don’t expect to discover whether the newest wonder drugs for weight loss and control of diabetes will be the long-term solution for people with obesity and diabetes worldwide.
Obesity and overweight are major risk factors for type 2 diabetes. About 90% of patients with diabetes have either overweight or obesity. Thus, the complications of these two conditions, which largely overlap, include atherosclerotic cardiovascular disease; myocardial infarction; stroke; hypertension; metabolic syndrome; lower-extremity gangrene; chronic kidney disease; retinopathy; glaucoma; cataracts; disabling osteoarthritis; breast, endometrial, colon, and other cancers; fatty liver; sleep apnea; and peripheral neuropathy. These diseases create a major lucrative business for a wide swathe of medical and surgical specialties, plus hospital, clinic, device, pharmaceutical, and food industries.
In summary, we’ve just been through 40 years of failure to recognize the sugar-elephant in the room and intervene with serious preventive efforts. Forty years of fleshing out both the populace and the American medical-industrial complex (AMIC). Talk about a sweet spot. The only successful long-term treatment of obesity (and with it, diabetes) is prevention. Don’t emphasize losing weight. Focus on preventing excessive weight gain, right now, for the population, beginning with yourselves. Otherwise, we continue openly to perpetuate a terrific deal for the AMIC, a travesty for everyone else. Time for some industrial grade penance and a course correction.
Meanwhile, here we are living out Big Pharma’s dream of a big populace, produced by the agriculture and food industries, enjoyed by capitalism after failures of education, medicine, and public health: a seemingly endless supply of people living with big complications who are ready for big (expensive, new) medications to fix the world’s big health problems.
Dr. Lundberg is editor in chief, Cancer Commons. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA approves new tubeless insulin pump
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
The product received CE Mark in Europe in 2018 and is now available in 19 markets worldwide. It offers users a choice of bolusing directly from the pump or from a handheld remote-control device. The pump can be detached and reattached without wasting insulin.
The remote-control device also incorporates blood glucose monitoring and bolus advice, although it currently does not integrate with continuous glucose monitoring (CGM) devices.
A Roche spokesperson said in an interview, “For future product generations, we are exploring possibilities to integrate CGM data into the system. Already today, the diabetes manager allows users to manually enter a glucose value that can be used to calculate a bolus. To do so, people with diabetes could use their CGM device of choice in conjunction with the Accu-Chek Solo micropump system.”
Roche will provide an update on next steps for further developments and time lines for launch “in due course,” according to a company statement.
A version of this article appeared on Medscape.com.
Type 1 diabetes management improves as technology advances
Significant reductions in hemoglobin A1c have occurred over time among adults with type 1 diabetes as their use of diabetes technology has increased, yet there is still room for improvement, new data suggest.
The new findings are from a study involving patients at the Barbara Davis Center for Diabetes Adult Clinic between Jan. 1, 2014, and Dec. 31, 2021. They show that as technology use has increased, A1c levels have dropped in parallel. Moreover, progression from use of stand-alone continuous glucose monitors (CGMs) to automated insulin delivery systems (AIDs), which comprise insulin pumps and connected CGMs, furthered that progress.
The findings “are in agreement with American Diabetes Association standards of care, and recent international consensus recommending CGM and AID for most people with type 1 diabetes, and early initiation of diabetes technology from the onset of type 1 diabetes,” write Kagan E. Karakus, MD, of the University of Colorado’s Barbara Davis Center, Aurora, and colleagues in the article, which was published online in Diabetes Care.
“It’s very rewarding to us. We can see clearly that the uptake is going up and the A1c is dropping,” lead author Viral N. Shah, MD, of the Barbara Davis Center, told this news organization.
On the flip side, A1c levels rose significantly over the study period among nonusers of technology. “We cannot rule out provider bias for not prescribing diabetes technology among those with higher A1c or from disadvantaged socioeconomic backgrounds,” Dr. Karakus and colleagues write.
Also of note, even with use of the most advanced AID systems available during the study period, just under half of patients were still not achieving A1c levels below 7%. “The technology helps, but it’s not perfect,” Dr. Shah observed.
This study is the first to examine the relationship of A1c with technology use over time, in contrast to prior cross-sectional studies. “The intention here was to look at the landscape over a decade,” Dr. Shah said.
As overall use of technology use rose, A1c levels fell
The analysis included data for 4,174 unique patients (mean number of patients, 1,988/yr); 15,903 clinic visits were included over the 8-year study period. Technology use was defined as CGM use without an AID system or with an AID system.
Over the study period, diabetes technology use increased from 26.9% to 82.7% of the clinic population (P < .001). At the same time, the overall proportion patients who achieved the A1c goal of less than 7% increased from 32.3% to 41.7%, while the mean A1c level dropped from 7.7% to 7.5% (P < .001).
But among the technology nonusers, A1c rose from 7.85% in 2014 to 8.4% in 2021 (P < .001).
Regardless of diabetes technology use, White patients (about 80% of the total study population) had significantly lower A1c than non-White patients (7.5% vs. 7.7% for technology users [P = .02]; 8.0% vs. 8.3% for nontechnology users [P < .001]).
The non-White group was too small to enable the researchers to break down the data by technology type. Nonetheless, Dr. Shah said, “As a clinician, I can say that the penetration of diabetes technology in non-White populations remains low. These are also the people more vulnerable for socioeconomic and psychosocial reasons.”
The A1c increase among technology nonusers may be a result of a statistical artifact, as the number of those individuals was much lower in 2021 than in 2014. It’s possible that those remaining individuals have exceedingly high A1c levels, bringing the average up. “It’s still not good, though,” Dr. Shah said.
The more technology, the lower the A1c
Over the study period, the proportion of stand-alone CGM users rose from 26.9% to 44.1%, while use of AIDs rose from 0% in 2014 and 2015 to 38.6% in 2021. The latter group included patients who used first-generation Medtronic 670G and 770G devices and second-generation Tandem t:slim X2 with Control-IQ devices.
Between 2017 and 2021, AIDs users had significantly lower A1c levels than nontechnology users: 7.4% vs. 8.1% in 2017, and 7.3% vs. 8.4% in 2021 (P < .001 for every year). CGM users also had significantly lower A1c levels than nonusers at all time points (P < .001 per year).
The proportions achieving an A1c less than 7% differed significantly across users of CGMs, AIDs, and no technology (P < .01 for all years). In 2021, the percentage of people who achieved an A1c less than 7% were 50.9% with AIDs and 44.1% for CGMs vs, just 15.2% with no technology.
Work to be done: Why aren’t more achieving < 7% with AIDs?
Asked why only slightly more than half of patients who used AIDs achieved A1c levels below 7%, Dr. Shah listed three possibilities:
First, the 7% goal doesn’t apply to everyone with type 1 diabetes, including those with multiple comorbidities or with short life expectancy, for whom the recommended goal is 7.5%-8.0% to prevent hypoglycemia. “We didn’t separate out patients by A1c goals. If we add that, the number might go up,” Dr. Shah said.
Second, AID technology is continually improving, but it’s not perfect. Users still must enter carbohydrate counts and signal the devices for exercise, which can lead to errors. “It’s a wonderful technology for overnight control, but still, during the daytime, there are so many factors with the user interface and how much a person is engaged with the technology,” Dr. Shah explained.
Third, he said, “Unfortunately, obesity is increasing in type 1 diabetes, and insulin doses are increasing. Higher BMI [body mass index] and more insulin resistance can mean higher A1c. I really think for many patients, we probably will need an adjunct therapy, such as an SGLT2 [sodium-glucose cotransporter-2] inhibitor or a GLP-1 [glucagonlike peptide-1] agonist, even though they’re not approved in type 1 diabetes, for both glycemic and metabolic control including weight. I think that’s another missing piece.”
He also pointed out, “If someone has an A1c of 7.5%, I don’t expect a huge change. But if they’re at 10%, a drop to 8% is a huge change.”
Overall, Dr. Shah said, the news from the study is good. “In the past, only 30% were achieving an A1c less than 7%. Now we’re 20% above that. ... It’s a glass half full.”
Dr. Karakus has disclosed no relevant financial relationships. Dr. Shah has received, through the University of Colorado, research support from Novo Nordisk, Insulet, Tandem Diabetes, and Dexcom, and honoraria from Medscape, Lifescan, Novo Nordisk, and DKSH Singapore for advisory board attendance and from Insulet and Dexcom for speaking engagements.
A version of this article first appeared on Medscape.com.
Significant reductions in hemoglobin A1c have occurred over time among adults with type 1 diabetes as their use of diabetes technology has increased, yet there is still room for improvement, new data suggest.
The new findings are from a study involving patients at the Barbara Davis Center for Diabetes Adult Clinic between Jan. 1, 2014, and Dec. 31, 2021. They show that as technology use has increased, A1c levels have dropped in parallel. Moreover, progression from use of stand-alone continuous glucose monitors (CGMs) to automated insulin delivery systems (AIDs), which comprise insulin pumps and connected CGMs, furthered that progress.
The findings “are in agreement with American Diabetes Association standards of care, and recent international consensus recommending CGM and AID for most people with type 1 diabetes, and early initiation of diabetes technology from the onset of type 1 diabetes,” write Kagan E. Karakus, MD, of the University of Colorado’s Barbara Davis Center, Aurora, and colleagues in the article, which was published online in Diabetes Care.
“It’s very rewarding to us. We can see clearly that the uptake is going up and the A1c is dropping,” lead author Viral N. Shah, MD, of the Barbara Davis Center, told this news organization.
On the flip side, A1c levels rose significantly over the study period among nonusers of technology. “We cannot rule out provider bias for not prescribing diabetes technology among those with higher A1c or from disadvantaged socioeconomic backgrounds,” Dr. Karakus and colleagues write.
Also of note, even with use of the most advanced AID systems available during the study period, just under half of patients were still not achieving A1c levels below 7%. “The technology helps, but it’s not perfect,” Dr. Shah observed.
This study is the first to examine the relationship of A1c with technology use over time, in contrast to prior cross-sectional studies. “The intention here was to look at the landscape over a decade,” Dr. Shah said.
As overall use of technology use rose, A1c levels fell
The analysis included data for 4,174 unique patients (mean number of patients, 1,988/yr); 15,903 clinic visits were included over the 8-year study period. Technology use was defined as CGM use without an AID system or with an AID system.
Over the study period, diabetes technology use increased from 26.9% to 82.7% of the clinic population (P < .001). At the same time, the overall proportion patients who achieved the A1c goal of less than 7% increased from 32.3% to 41.7%, while the mean A1c level dropped from 7.7% to 7.5% (P < .001).
But among the technology nonusers, A1c rose from 7.85% in 2014 to 8.4% in 2021 (P < .001).
Regardless of diabetes technology use, White patients (about 80% of the total study population) had significantly lower A1c than non-White patients (7.5% vs. 7.7% for technology users [P = .02]; 8.0% vs. 8.3% for nontechnology users [P < .001]).
The non-White group was too small to enable the researchers to break down the data by technology type. Nonetheless, Dr. Shah said, “As a clinician, I can say that the penetration of diabetes technology in non-White populations remains low. These are also the people more vulnerable for socioeconomic and psychosocial reasons.”
The A1c increase among technology nonusers may be a result of a statistical artifact, as the number of those individuals was much lower in 2021 than in 2014. It’s possible that those remaining individuals have exceedingly high A1c levels, bringing the average up. “It’s still not good, though,” Dr. Shah said.
The more technology, the lower the A1c
Over the study period, the proportion of stand-alone CGM users rose from 26.9% to 44.1%, while use of AIDs rose from 0% in 2014 and 2015 to 38.6% in 2021. The latter group included patients who used first-generation Medtronic 670G and 770G devices and second-generation Tandem t:slim X2 with Control-IQ devices.
Between 2017 and 2021, AIDs users had significantly lower A1c levels than nontechnology users: 7.4% vs. 8.1% in 2017, and 7.3% vs. 8.4% in 2021 (P < .001 for every year). CGM users also had significantly lower A1c levels than nonusers at all time points (P < .001 per year).
The proportions achieving an A1c less than 7% differed significantly across users of CGMs, AIDs, and no technology (P < .01 for all years). In 2021, the percentage of people who achieved an A1c less than 7% were 50.9% with AIDs and 44.1% for CGMs vs, just 15.2% with no technology.
Work to be done: Why aren’t more achieving < 7% with AIDs?
Asked why only slightly more than half of patients who used AIDs achieved A1c levels below 7%, Dr. Shah listed three possibilities:
First, the 7% goal doesn’t apply to everyone with type 1 diabetes, including those with multiple comorbidities or with short life expectancy, for whom the recommended goal is 7.5%-8.0% to prevent hypoglycemia. “We didn’t separate out patients by A1c goals. If we add that, the number might go up,” Dr. Shah said.
Second, AID technology is continually improving, but it’s not perfect. Users still must enter carbohydrate counts and signal the devices for exercise, which can lead to errors. “It’s a wonderful technology for overnight control, but still, during the daytime, there are so many factors with the user interface and how much a person is engaged with the technology,” Dr. Shah explained.
Third, he said, “Unfortunately, obesity is increasing in type 1 diabetes, and insulin doses are increasing. Higher BMI [body mass index] and more insulin resistance can mean higher A1c. I really think for many patients, we probably will need an adjunct therapy, such as an SGLT2 [sodium-glucose cotransporter-2] inhibitor or a GLP-1 [glucagonlike peptide-1] agonist, even though they’re not approved in type 1 diabetes, for both glycemic and metabolic control including weight. I think that’s another missing piece.”
He also pointed out, “If someone has an A1c of 7.5%, I don’t expect a huge change. But if they’re at 10%, a drop to 8% is a huge change.”
Overall, Dr. Shah said, the news from the study is good. “In the past, only 30% were achieving an A1c less than 7%. Now we’re 20% above that. ... It’s a glass half full.”
Dr. Karakus has disclosed no relevant financial relationships. Dr. Shah has received, through the University of Colorado, research support from Novo Nordisk, Insulet, Tandem Diabetes, and Dexcom, and honoraria from Medscape, Lifescan, Novo Nordisk, and DKSH Singapore for advisory board attendance and from Insulet and Dexcom for speaking engagements.
A version of this article first appeared on Medscape.com.
Significant reductions in hemoglobin A1c have occurred over time among adults with type 1 diabetes as their use of diabetes technology has increased, yet there is still room for improvement, new data suggest.
The new findings are from a study involving patients at the Barbara Davis Center for Diabetes Adult Clinic between Jan. 1, 2014, and Dec. 31, 2021. They show that as technology use has increased, A1c levels have dropped in parallel. Moreover, progression from use of stand-alone continuous glucose monitors (CGMs) to automated insulin delivery systems (AIDs), which comprise insulin pumps and connected CGMs, furthered that progress.
The findings “are in agreement with American Diabetes Association standards of care, and recent international consensus recommending CGM and AID for most people with type 1 diabetes, and early initiation of diabetes technology from the onset of type 1 diabetes,” write Kagan E. Karakus, MD, of the University of Colorado’s Barbara Davis Center, Aurora, and colleagues in the article, which was published online in Diabetes Care.
“It’s very rewarding to us. We can see clearly that the uptake is going up and the A1c is dropping,” lead author Viral N. Shah, MD, of the Barbara Davis Center, told this news organization.
On the flip side, A1c levels rose significantly over the study period among nonusers of technology. “We cannot rule out provider bias for not prescribing diabetes technology among those with higher A1c or from disadvantaged socioeconomic backgrounds,” Dr. Karakus and colleagues write.
Also of note, even with use of the most advanced AID systems available during the study period, just under half of patients were still not achieving A1c levels below 7%. “The technology helps, but it’s not perfect,” Dr. Shah observed.
This study is the first to examine the relationship of A1c with technology use over time, in contrast to prior cross-sectional studies. “The intention here was to look at the landscape over a decade,” Dr. Shah said.
As overall use of technology use rose, A1c levels fell
The analysis included data for 4,174 unique patients (mean number of patients, 1,988/yr); 15,903 clinic visits were included over the 8-year study period. Technology use was defined as CGM use without an AID system or with an AID system.
Over the study period, diabetes technology use increased from 26.9% to 82.7% of the clinic population (P < .001). At the same time, the overall proportion patients who achieved the A1c goal of less than 7% increased from 32.3% to 41.7%, while the mean A1c level dropped from 7.7% to 7.5% (P < .001).
But among the technology nonusers, A1c rose from 7.85% in 2014 to 8.4% in 2021 (P < .001).
Regardless of diabetes technology use, White patients (about 80% of the total study population) had significantly lower A1c than non-White patients (7.5% vs. 7.7% for technology users [P = .02]; 8.0% vs. 8.3% for nontechnology users [P < .001]).
The non-White group was too small to enable the researchers to break down the data by technology type. Nonetheless, Dr. Shah said, “As a clinician, I can say that the penetration of diabetes technology in non-White populations remains low. These are also the people more vulnerable for socioeconomic and psychosocial reasons.”
The A1c increase among technology nonusers may be a result of a statistical artifact, as the number of those individuals was much lower in 2021 than in 2014. It’s possible that those remaining individuals have exceedingly high A1c levels, bringing the average up. “It’s still not good, though,” Dr. Shah said.
The more technology, the lower the A1c
Over the study period, the proportion of stand-alone CGM users rose from 26.9% to 44.1%, while use of AIDs rose from 0% in 2014 and 2015 to 38.6% in 2021. The latter group included patients who used first-generation Medtronic 670G and 770G devices and second-generation Tandem t:slim X2 with Control-IQ devices.
Between 2017 and 2021, AIDs users had significantly lower A1c levels than nontechnology users: 7.4% vs. 8.1% in 2017, and 7.3% vs. 8.4% in 2021 (P < .001 for every year). CGM users also had significantly lower A1c levels than nonusers at all time points (P < .001 per year).
The proportions achieving an A1c less than 7% differed significantly across users of CGMs, AIDs, and no technology (P < .01 for all years). In 2021, the percentage of people who achieved an A1c less than 7% were 50.9% with AIDs and 44.1% for CGMs vs, just 15.2% with no technology.
Work to be done: Why aren’t more achieving < 7% with AIDs?
Asked why only slightly more than half of patients who used AIDs achieved A1c levels below 7%, Dr. Shah listed three possibilities:
First, the 7% goal doesn’t apply to everyone with type 1 diabetes, including those with multiple comorbidities or with short life expectancy, for whom the recommended goal is 7.5%-8.0% to prevent hypoglycemia. “We didn’t separate out patients by A1c goals. If we add that, the number might go up,” Dr. Shah said.
Second, AID technology is continually improving, but it’s not perfect. Users still must enter carbohydrate counts and signal the devices for exercise, which can lead to errors. “It’s a wonderful technology for overnight control, but still, during the daytime, there are so many factors with the user interface and how much a person is engaged with the technology,” Dr. Shah explained.
Third, he said, “Unfortunately, obesity is increasing in type 1 diabetes, and insulin doses are increasing. Higher BMI [body mass index] and more insulin resistance can mean higher A1c. I really think for many patients, we probably will need an adjunct therapy, such as an SGLT2 [sodium-glucose cotransporter-2] inhibitor or a GLP-1 [glucagonlike peptide-1] agonist, even though they’re not approved in type 1 diabetes, for both glycemic and metabolic control including weight. I think that’s another missing piece.”
He also pointed out, “If someone has an A1c of 7.5%, I don’t expect a huge change. But if they’re at 10%, a drop to 8% is a huge change.”
Overall, Dr. Shah said, the news from the study is good. “In the past, only 30% were achieving an A1c less than 7%. Now we’re 20% above that. ... It’s a glass half full.”
Dr. Karakus has disclosed no relevant financial relationships. Dr. Shah has received, through the University of Colorado, research support from Novo Nordisk, Insulet, Tandem Diabetes, and Dexcom, and honoraria from Medscape, Lifescan, Novo Nordisk, and DKSH Singapore for advisory board attendance and from Insulet and Dexcom for speaking engagements.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE
Artificial sweeteners no help for weight loss: Review
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
FROM CURRENT OPINION IN CARDIOLOGY
Evaluating Pharmacists’ Time Collecting Self-Monitoring Blood Glucose Data
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
The American Diabetes Association recommends that patients on intensive insulin regimens self-monitor blood glucose (SMBG) to assist in therapy optimization.1 To be useful, SMBG data must be captured by patients, shared with care teams, and used and interpreted by patients and practitioners.2,3 Communication of SMBG data from the patient to practitioner can be challenging. Although technology can help in this process, limitations exist, such as manual data entry into systems, patient and/or practitioner technological challenges (eg, accessing interface), and compatibility and integration between SMBG devices and electronic health record (EHR) systems.4
The Boise Veterans Affairs Medical Center (BVAMC) in Idaho serves more than 100,000 veterans. It includes a main site, community-based outpatient clinics, and a clinical resource hub that provides telehealth services to veterans residing in rural neighboring states. The BVAMC pharmacy department provides both inpatient and outpatient services. At the BVAMC, clinical pharmacist practitioners (CPPs) are independent practitioners who support their care teams in comprehensive medication management and have the ability to initiate, modify, and discontinue drug therapy for referred patients.5 A prominent role of CPPs in primary care teams is to manage patients with uncontrolled diabetes and intensive insulin regimens, in which SMBG data are vital to therapy optimization. As collecting SMBG data from patients is seen anecdotally as time intensive, we determined the mean time spent by CPPs collecting patient SMBG data and its potential implications.
Methods
Pharmacists at BVAMC were asked to estimate and record the following: SMBG data collection method, time spent collecting data, extra time spent documenting or formatting SMBG readings, total patient visit time, and visit type. Time was collected in minutes. Extra time spent documenting or formatting SMBG readings included any additional time formatting or entering data in the clinical note after talking to the patient; if this was done while multitasking and talking to the patient, it was not considered extra time. For total patient visit time, pharmacists were asked to estimate only time spent discussing diabetes care and collecting SMBG data. Visit types were categorized as in-person/face-to-face, telephone, and telehealth using clinical video telehealth (CVT)/VA Video Connect (VVC). Data were collected using a standardized spreadsheet. The spreadsheet was pilot tested by a CPP before distribution to all pharmacists.
CPPs were educated about the project in March 2021 and were asked to record data for a 1-week period between April 5, 2021, and April 30, 2021. One CPP also provided delayed data collected from May 17 to 21, 2021, and these data were included in our analysis.
Descriptive statistics were used to determine the mean time spent by CPPs collecting SMBG data. Unpaired t tests were used to compare time spent collecting SMBG data by different collection methods and patient visit types. A P value of ≤ .05 was considered statistically significant. Data were organized in Microsoft Excel, and statistics were completed with JMP Pro v15.
Results
Eight CPPs provided data from 120 patient encounters. For all pa
When compared by the SMBG collection method, the longest time spent collecting SMBG data was with patient report (3.7 minutes), and the longest time spent documenting/formatting time was with meter download/home telehealth (2 minutes). There was no statistically significant difference in the time to collect SMBG data between patient report and other methods (3.7 minutes vs 2.8 minutes; P = .07).
When compared by visit type, there was not a statistically significant difference between time spent collecting SMBG data (3.8 minutes vs 3.2 minutes; P = .39) (Table 2).
Discussion
We found that the mean amount of time spent collecting and documenting/formatting SMBG data was only 4.6 minutes; however, this still represented a substantial portion of visit time. For telephone and CVT/VVC appointments, this represented > 25% of total visit time. While CPPs make important contributions to interprofessional team management of patients with diabetes, their cost is not trivial.6-8 It is worth exploring the most effective and efficient ways to use CPPs. Our results indicate that streamlining SMBG data collection may be beneficial.
Pharmacy technicians, licensed practical nurses/clinical associates, registered nurses/nurse care managers, or other team members could help improve SMBG data collection. Using other team members is also an opportunity for comanagement, for team collaboration, and for more patients to be seen. For example, if a CPP currently has 12 patient encounters that last 20 minutes each, this results in about 240 minutes of direct patient care. If patient encounters were 16 minutes, CPPS could have 15 patient encounters in 240 minutes. Saved time could be used for other clinical tasks involved in disease management or clinical reminder reviews. While there are benefits to CPPs collecting SMBG data, such as further inquiry about patient-reported values, other team members could also be trained to ask appropriate follow-up questions for abnormal blood glucose readings. In addition, leveraging current team members and optimizing their roles could prevent the need to acquire additional full-time equivalent employees.
Another opportunity to increase efficiency in SMBG data collection is with SMBG devices and EHR integration.4,9 However, integration can be difficult with different types of SMBG devices and EHR platforms. Education for patients and practitioners could help to ensure accurate and reliable data uploads; patient internet availability; data protection, privacy, and sharing; workflow management; and clear patient-practitioner expectations.10 For example, if patient SMBG data are automatically uploaded to practitioners, patients’ expectations for practitioner review of data and follow-up need to be determined.
We found a subset of patient encounters (n = 23) where data collection and documenting/formatting represented more than half of the total visit time. In this subset, 13 SMBG reports were pulled from a log or meter, 8 were patient reported, and 3 were meter download or home telehealth.
Limitations
A potential reason for the lack of statistically significant differences in SMBG collection method or visit type in this study includes the small sample size. Participation in this work was voluntary, and all participating CPPs had ≥ 3 years of practice in their current setting, which includes a heavy workload of diabetes management. These pharmacists noted self-established procedures/systems for SMBG data collection, including the use of Excel spreadsheets with pregenerated formulas. For less experienced CPPs, SMBG data collection time may be even longer. Pharmacists also noted that they may limit time spent collecting SMBG data depending on the patient encounter and whether they have gathered sufficient data to guide clinical care. Other limitations of this work include data collection from a single institution and that the time documented represented estimates; there was no external monitor.
Conclusions
In this analysis, we found that CPPs spend about 3 minutes collecting SMBG data from patients, and about an additional 1 minute documenting and formatting data. While 4 to 5 minutes may not represent a substantial amount of time for one patient, it can be when multiplied by several patient encounters. The time spent collecting SMBG data did not significantly differ by collection method or visit type. Opportunities to increase efficiency in SMBG data collection, such as the use of nonpharmacist team members are worth exploring.
Acknowledgments
Thank you to the pharmacists at the Boise Veterans Affairs Medical Center for their time and support of this work: Danielle Ahlstrom, Paul Black, Robyn Cruz, Sarah Naidoo, Anthony Nelson, Laura Spoutz, Eileen Twomey, Donovan Victorine, and Michelle Wilkin.
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
1. American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S85-S99. doi:10.2337/dc21-S007
2. Austin MM. The two skill sets of self-monitoring of blood glucose education: the operational and the interpretive. Diabetes Spectr. 2013;26(2):83-90. doi:10.2337/diaspect.26.2.83
3. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997;314(7085):964-967. doi:10.1136/bmj.314.7085.964
4. Lewinski AA, Drake C, Shaw RJ, et al. Bridging the integration gap between patient-generated blood glucose data and electronic health records. J Am Med Inform Assoc. 2019;26(7):667-672. doi:10.1093/jamia/ocz039
5. McFarland MS, Groppi J, Jorgenson T, et al. Role of the US Veterans Health Administration clinical pharmacy specialist provider: shaping the future of comprehensive medication management. Can J Hosp Pharm. 2020;73(2):152-158. doi:10.4212/cjhp.v73i2.2982
6. Schmidt K, Caudill J. Hamilton T. Impact of clinical pharmacy specialists on glycemic control in veterans with type 2 diabetes. Am J Health Syst Pharm. 2019;76(suppl 1):S9-S14. doi:10.1093/ajhp/zxy015
7. Sullivan J, Jett BP, Cradick M, Zuber J. Effect of clinical pharmacist intervention on hemoglobin A1c reduction in veteran patients with type 2 diabetes in a rural setting. Ann Pharmacother. 2016;50(12):1023-1027. doi:10.1177/1060028016663564
8. Bloom CI, Ku M, Williams M. Clinical pharmacy specialists’ impact in patient aligned care teams for type 2 diabetes management. J Am Pharm Assoc (2003). 2019;59(5):717-721. doi:10.1016/j.japh.2019.05.002
9. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. doi:10.1093/jamia/ocv206
10. Reading MJ, Merrill JA. Converging and diverging needs between patients and providers who are collecting and using patient-generated health data: an integrative review. J Am Med Inform Assoc. 2018;25(6):759-771. doi:10.1093/jamia/ocy006
Dementia diagnosis a good time to reduce polypharmacy
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
FROM JAMA INTERNAL MEDICINE
Simple blood test may predict heart and kidney risk in T2D
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
, suggests an analysis of the CREDENCE trial.
The research, published online in the journal Circulation, also revealed that patients treated with the sodium-glucose cotransporter-2 inhibitor canagliflozin (Invokana, Invokamet) had lower levels of the biomarkers after 1 year compared with those given placebo.
Examination of biomarker levels in more than 2,600 patients from CREDENCE showed that high baseline concentrations of the individual biomarkers were able to predict the future risk for a composite endpoint of renal and heart outcomes.
The combination of all four biomarkers into a single panel revealed that patients with the highest levels were more than four times as likely to experience the composite endpoint than were those with the lowest levels.
As two of the biomarkers used in the study have yet to have established prognostic thresholds, the results remain exploratory.
Lead author James L. Januzzi, MD, director of the Heart Failure and Biomarker Trials at the Baim Institute for Clinical Research, Boston, said that further study will help refine the predictive value of the panel.
“Given that the American Heart Association/American College of Cardiology and the American Diabetes Association now all recommend measurement of biomarkers to enhance the ability to predict risk in persons with type 2 diabetes, these results may considerably extend the reach of biomarker-based testing, refining accuracy even further,” he said in a press release.
In an interview, Dr. Januzzi said that “three out of the four biomarkers are already clinically and commercially available,” while the fourth, for insulin-like growth factor binding protein 7 (IGFBP7), is “on the near horizon.”
He stressed that the “future for multiple biomarker testing, however, will be less about ordering each individual test, and ultimately will revolve around panels of blood work that are ordered as a single test.”
Dr. Januzzi added that “rather than using the rather primitive approach that we took” of looking at the individual biomarkers in adjusted models, the next stage “will be to utilize algorithms to combine the results into a single value.
“A clinician will not have to struggle with looking at individual results but will just receive one aggregated test result that informs them whether a patient is at low, medium or higher risk,” he explained.
However, this will require determining the relative importance of each biomarker and weighting them in the final model.
Consequently, the current results “set the foundation for identifying some very powerful individual tests that may ultimately, in aggregate, help us to help our patients with diabetes avoid a major complication,” Dr. Januzzi said.
By revealing that some individuals with both type 2 diabetes and kidney disease are at higher risk than others, he also hopes the findings can be leveraged to treat patients with “varying degrees of intensity with proven therapies, including weight loss, dietary adjustment, and pharmacologic intervention.”
Dr. Januzzi added: “Diabetes affects a dramatic, and growing, percentage of our population, and this type of personalized strategy to reduce the major complications of this rather common disease is an important step forward.”
The authors noted that there is a “bidirectional relationship” between cardiovascular disease and chronic kidney disease (CKD), such that either diagnosis may increase the risk of, or exacerbate, the other.
Individuals with type 2 diabetes and CKD albuminuria, they added, are at particularly high risk for major cardiovascular events, and studies have shown that several circulating cardiorenal stress biomarkers may predict the onset and progression of CKD in type 2 diabetes, as well as predict cardiovascular events.
Several biomarkers associated with myocardial stress and necrosis
The recent CANVAS trial revealed that, among individuals with type 2 diabetes with and without CKD, several biomarkers were associated with myocardial stress and necrosis, and renal tubular injury, predicting the progression of CKD with albuminuria, and the risk for heart failure events.
Taking inspiration from those findings, the current researchers studied a panel of similar cardiac and renal biomarkers among participants from the CREDENCE trial, for which 4,401 patients with type 2 diabetes and CKD at high risk of progression were randomly assigned to canagliflozin or placebo.
The current analysis involved 2,627 participants who had baseline plasma samples available for analysis of four circulating biomarkers: N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (hs-cTnT), growth differentiation factor-15 (GDF-15), and IGFBP7.
Among those, 2,385 participants also had year 1 plasma samples available for analysis, while year 3 plasma samples were available for 895 individuals.
The results showed that, in general, median baseline concentrations of each biomarker in both treatment groups were elevated compared with healthy reference populations.
Baseline log-transformed concentrations of each biomarker were also strongly predictive of cardiac and renal outcomes, including heart failure and progression of CKD.
For example, each unit increase in baseline NT-proBNP concentrations was associated with a hazard ratio of 1.35 for the primary composite endpoint of end-stage kidney disease, doubling of serum creatinine levels, renal death, or cardiovascular disease (P < .001).
For each unit increase in hs-cTnT levels, the hazard ratio for the primary composite was 1.73 (P < .001), for GDF-15 it was 1.84 (P < .0001), and for IGFBP7 the hazard ratio was 3.14 (P < .001).
Combining the four biomarkers into a single multimarker panel revealed that, compared with individuals with a low-risk score, those with a high-risk score had a hazard ratio for the primary outcome of 4.01, whereas those with a moderate risk score had a hazard ratio of 2.39 (P < .001 for both).
For the individual outcome of heart failure hospitalization, the effect was even greater. A high-risk score was associated with a hazard ratio vs. a low-risk score of 6.04 (P < .001), whereas patients with a moderate risk score had a hazard ratio of 2.45 (P = .04).
The researchers also reported that, between baseline and year 1, concentrations of all four biomarkers rose from 6% to 29% in the placebo group, but from 3% to just 10% in those treated with canagliflozin.
“It was reassuring to discover that canagliflozin helped reduce risks the most in people with the highest chances for complications,” said Dr. Januzzi.
The CREDENCE trial and the current analysis were funded by Janssen Research & Development LLC. NT-proBNP, hs-cTnT, GDF-15, and IGFBP7 reagents were provided by Roche Diagnostics. Dr. Januzzi is funded in part by the Hutter Family Professorship. Dr. Januzzi declared relationships with Imbria Pharmaceuticals, Jana Care, Abbott, Applied Therapeutics, HeartFlow, Innolife, Roche Diagnostics, Beckman, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Merck, Novartis, Pfizer, Siemens, Abbott, AbbVie, CVRx, Intercept, and Takeda.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION