User login
Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism, is an important public health concern. Deep venous thrombosis is estimated to affect 10% to 20% of medical (nonsurgical) patients, 15% to 40% of stroke patients, and 10% to 80% of critical care patients who are not prophylaxed.1 Venous thromboembolism is associated with significant resource utilization, long-term sequelae, recurrent events, and sudden death.2
The current guidelines of the American College of Chest Physicians recommend use of pharmacologic thromboprophylaxis as the preferred strategy for nonsurgical (or medical) patients (IB, formerly IA, recommendation) and for critically ill patients (2C recommendation) at low risk for bleeding.1,3 Mechanical (or nonpharmacologic) thromboprophylaxis (eg, intermittent pneumatic compression) is an alternative for those at increased risk for bleeding (2C recommendation).3 Pharmacologic thromboprophylaxis in high-risk patients, similar to those studied in randomized controlled clinical trials, reduces the occurrence of symptomatic DVT by 34 events per 1,000 patients treated.3 However, data are conflicting regarding mortality benefit.4,5
Related: Trends in Venous Thromboembolism
The Joint Commission adopted any thromboprophylaxis (measure includes pharmacologic or nonpharmacologic strategies) as a core discretionary measure in the ORYX (National Quality Hospital Measures) program. The ORYX measurements are intended to support Joint Commission-accredited organizations in institutional quality improvement efforts. The thromboprophylaxis core measure became effective May 2009 and remains as an option for hospitals to meet the 4 core measure set accreditation requirement. A top-performing hospital should provide this measure to applicable patients ≥ 95% of the time, according to the Joint Commission.6 The Joint Commission does not encourage use of any risk assessment model (RAM), such as the Padua Prediction Score to preferentially select high-risk medical patients.3
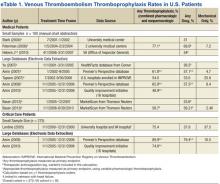
A disparity exists between thromboprophylaxis recommendations and practices in the nonsurgical patient, even when electronic prompts or alerts are available (eTables 1 and 2). In the U.S., pharmacologic thromboprophylaxis is administered to 23.6% to 81.1% of medical patients and 37.9% to 79.4% of critical care patients.7-21 In most cases, these rates are liberal estimates, because they include patients who are already on therapeutic anticoagulation or may have received only 1 prophylactic dose during hospitalization.8-11,13-20 When studies exclude patients receiving therapeutic (or treatment doses) anti-coagulation, pharmacologic thromboprophylaxis rates are substantially lower, typically 31% to 33% for medical patients and 37.9% for critical care patients.7,12,21 Furthermore, when studies examine appropriateness of thromboprophylaxis (eg, within the first 2 days of hospitalization or at the correct dose, correct time, or predefined duration), calculations are often less robust.10,11,13,14,22,23
The VHA uses thromboprophylaxis of surgical patients as an external peer review (EPR) performance measure (PM). With the great attention to this national measure, Altom and colleagues reported 89.9% of surgeries adhered.24 Before 2015, VTE thromboprophylaxis EPR PM did not exist. However, the VHA has initiated efforts to assure that providers are adherent to the new indications, which include VTE prophylaxis and treatment.
There is little published literature evaluating VHA performance.Quraishi and colleagues reported a pharmacologic prophylaxis rate of 63% in nonsurgical patients at a single VAMC, facilitated by the use of an admission VTE order set. Unfortunately, their estimate allowed inclusion of 5% of patients receiving treatment doses of anticoagulation and failed to provide any estimates on regimen appropriateness (eg, correct dose, correct time, or correct duration).18 Lentine and colleagues documented a pharmacologic thromboprophylaxis rate of 48% for a subset of veteran critical care patients who were not already receiving indicated therapeutic anticoagulants.21
Veterans have poorer health status, more medical conditions, and higher medical resource use than do nonveterans; therefore, it is postulated that veterans can derive clinical benefit from improved attention to thromboprophylaxis benchmarking, performance improvement, and potentially, implementation of electronic alerts or reminder tools.25 Nationally, VHA has no formal inpatient reminder tools to trigger use of thromboprophylaxis for high-risk medical patients, although individual health care systems may have created alerts or tools. Some studies demonstrated that order sets and electronic tools are helpful, whereas others demonstrated potential for harm.17-20,26,27
For any hospitalization at the VA Tennessee Valley Healthcare System (TVHS), the only electronic prompt to order VTE thromboprophylaxis occurs when the admission order set is completed. But the prompt can be readily bypassed if the quick admission orders are selected. Although no further electronic prompts in the Computerized Patient Record System (CPRS) are invoked following admission, the authors hypothesized that the rate of VTE thromboprophylaxis, specifically pharmacologic, in a subset of veterans with respiratory disease will be higher than the usual published rates.
Purpose and Relevance
This study’s primary aim was to assess the rate of pharmacologic VTE prophylaxis in veterans with pulmonary disease who were admitted for a nonsurgical stay. The 2 secondary aims were to determine whether thromboprophylaxis was appropriate and to characterize whether differences exist for pharmacologic prophylaxis according to level of care (medical critical care unit [CCU] vs acute care medical ward).
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: Rivaroxaban Update
This analysis emphasizes pharmacologic thromboprophylaxis instead of the combined endpoint of pharmacologic plus nonpharmacologic thromboprophylaxis traditionally used and will supplement the limited literature in 2 understudied cohorts: (1) nonsurgical veteran patients, specifically where advanced computerized thromboprophylaxis alerts are not in use; and (2) patients with the VTE risk factor of respiratory disease.1,7-9,12,13,15,16,18,21
Study Design
This observational study used retrospectively collected data. The data were extracted electronically from the VISN 9 data warehouse by a Decision Support Services analyst and manually validated by an investigator using the CPRS. Prior to initiation of research activities, the VHA Institutional Review Board and the Research and Development Committee at the facility level approved the study.
Sampling
Patients assigned to the treating specialties of medicine and medical critical care during fiscal years 2006 to 2008, admitted for ≥ 24 hours, and discharged with a diagnosis of chronic obstructive pulmonary disease (COPD), asthma, or acute, severe respiratory disease (eg, patients requiring mechanical ventilation) were eligible for inclusion. The authors also elected to include patients with asthma, because this diagnosis commonly overlaps with COPD and reflects real-world clinical practice and diagnostic challenges.28 Pneumonia and other infectious pulmonary conditions were not a qualifying diagnosis for study inclusion.
Patients were excluded if aged > 79 years, because it is difficult to maintain de-identification in a small sample of inpatients in this age category. Unfortunately, octogenarians have the highest rate of VTE per 100,000 population and would gain substantial benefit from prophylaxis.29 Similar to other VHA and non-VHA investigators, this study excluded patients who were prescribed therapeutic anticoagulation.7,12,21,30 The authors believe continuation of therapeutic (or treatment) anticoagulation does not measure a clinical decision to use pharmacologic thromboprophylaxis, and any interruption of therapeutic anticoagulation suggests that prophylactic anticoagulation is not warranted.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
Additionally, patients were excluded if length of stay (LOS) exceeded 14 days, if known or potential contraindications to thromboprophylaxis existed, or if laboratory data that were needed to assess for contraindications were missing from the electronic data set. Known or potential contraindications included active hemorrhage, hemorrhage within the past 3 months, recent administration of packed red blood cells, bacterial endocarditis, known coagulopathy, recent or current heparin-induced thrombocytopenia, or a potential coagulopathy (International Normalized Ratio > 1.5, platelets < 50,000, or an activated partial thromboplastin time > 41 sec).
Contraindications were conservative in construct and were similar to the exclusion-based VTE checklist for the nonsurgical patient.31 The authors did not examine the electronic data set for the contraindication of epidural or spinal anesthesia, because neither is commonly used in the medical ward or medical CCU. The authors also did not exclude patients with a creatinine clearance (CrCl) < 10 mL/min (a relative contraindication to VTE thromboprophylaxis), although these patients may be at an increased risk for bleeding complications.32
Endpoints and Measures
The primary endpoint of this study was the rate of any pharmacologic thromboprophylaxis (eg, ≥ 1 doses), similar to the endpoint selected by other investigators.7-9,12,13,15,16 Secondary endpoints included VTE protected time period on thromboprophylaxis, therapeutic appropriateness ratio for heparin and enoxaparin doses combined, and pharmacologic thromboprophylaxis rates according to level and location of care.
Sample Size
Although data have been forthcoming, at the time of study inception no studies documented the rate of pharmacologic thromboprophylaxis alone (defined as use of ≥ 1 dose of a pharmacologic agent) in patients with the VTE risk factor of respiratory disease.15,23 However, an average combined pharmacologic and nonpharmacologic thromboprophylaxis rate of 48.8% was determined from available studies.11,14 Although this percentage is an overestimate of pharmacologic thromboprophylaxis rates alone, this value was used to determine a sample size for the cohort.
About 122 subjects would be needed to provide 80% power and a significance level of < 0.05 to assess the hypothesis that pharmacologic prophylaxis rates at TVHS would exceed 60%. Additionally calculated was the sample size necessary to find a 20% expected difference in thromboprophylaxis rates according to location of care (eg, medical ward vs medical CCU), the secondary endpoint. This sample size was calculated to be 180 subjects, or 90 patients in each arm, to provide 80% power and a significance level (2-tailed alpha) of < 0.05. Subsequently, up to 130 patients from each location of care were randomly selected for study inclusion.
Data Analysis
A chi square test was used to compare groups on categorical variables. SPSS version 16.0 (SPSS Chicago, IL) was used for data analysis.
Results
A sample of 3,762 hospitalizations for veterans with COPD, asthma, or acute, severe respiratory disease who received inpatient care in the medical ward or medical CCU were extracted from the data warehouse.
Electronic Data Set
An investigator reviewed the electronic data set, and exclusion criteria that could be ascertained electronically were applied. The primary reasons for exclusion were age (18.4%), potential coagulopathy (14.5%), recent transfusion (14.6%), use of therapeutic anticoagulation (11%), or an extended LOS (7%). Less common reasons for exclusion were coagulation disorders (1.4%), heparin-induced thrombocytopenia (1.2%), recent hemorrhage (1.1%), or missing baseline laboratory values (3.2%). Subsequently, the potential sample of subjects declined to 1,018 (27%) hospitalizations. Of the remaining hospitalizations, 46 and 972 were medical CCU and nonsurgical (medical) inpatients, respectively.
In line with the sampling plan, 130 (13.4%) medical ward hospitalizations were selected using a random number generator. As the ICU sample was smaller than anticipated, the convenience sample of all 46 hospitalizations was used.
Manual Chart Abstraction
Manual chart abstraction (n = 176) clarified physician/provider decision making (eg, some patients were not appropriate for thromboprophylaxis due to upcoming invasive procedures), medical history that could not be extracted by ICD-9 coding (eg, recent non-VHA admissions for medical conditions that were contraindications to prophylaxis), and anticoagulation dosing. These exclusions led to an additional 52 (29.5%) excluded hospitalizations. Reasons for manual exclusion included recent bleeding or at high risk for bleeding (18, 34.6%), incorrect classification as nonsurgical or elective admission (5, 9.6%), no diagnosis of lung disease (21, 40.4%), invasive procedures planned (4, 7.7%), treatment anticoagulant doses selected (4, 7.7%), or patient transferred to a non-VA medical facility due to acuity level (1, 1.9%). One patient was excluded for multiple reasons.
Baseline Demographics
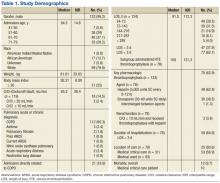
The sample was an elderly, male (98%), white (79.8%) cohort (Table 1). No patients were aged < 40 years. Racial information was missing for 5.6% of the patients. The chief pulmonary diagnosis was COPD, and few patients had new onset, acute, severe respiratory disease (3.2%) prompting admission, because pneumonia was not included as a qualifying diagnosis. Median body mass index (BMI) was 26.31. The median LOS was 3.8 days for the overall cohort and 4.1 days for those receiving pharmacologic thromboprophylaxis, although for the latter group a larger proportion of patients were hospitalized for < 3 days. Renal function, according to endpoint definitions, was for using enoxaparin as the appropriate strategy for thromboprophylaxis for the majority (97.5%) of hospitalizations.
Primary and Secondary Endpoints
Of those receiving pharmacologic thromboprophylaxis, heparin was prescribed most often (62.8%). One patient received both heparin and enoxaparin during a single hospitalization.
Pharmacologic thromboprophylaxis was more common in the medical CCU subgroup (80.6%) compared with the nonsurgical patient (56.9%). Pharmacologic thromboprophylaxis was used in 62.9% of patients (n = 124). However, the therapeutic appropriateness ratio was reduced to 58% of the entire sample (n = 124), because 6 patients of the cohort receiving thromboprophylaxis (n = 78) were prescribed suboptimal doses: Specifically, 1 patient was underdosed and 1 overdosed when prescribed enoxaparin (2, 2.6%). Four patients (5.1%) received underdoses of heparin, based on institutional guidance. For those prescribed pharmacologic thromboprophylaxis, the VTE protected time period ratio was 82.8% (Table 2). Overall inpatient mortality rate was low (12, 9.7%). Most deceased patients were managed in the medical CCU (10, 83.3%) and did receive pharmacologic thromboprophylaxis (10, 83.3%).
Discussion
This study demonstrated moderate rates of VTE pharmacologic thromboprophylaxis, because 62.9% of nonsurgical patients with respiratory disease who were hospitalized for various reasons were prophylaxed with either SC heparin or enoxaparin. This rate represents active clinical decision making, because there was no indication to prescribe anticoagulation at therapeutic doses. As expected, pharmacologic thromboprophylaxis was more common in the critical care subgroup (80.6%) compared with the nonsurgical patients (56.9%). Although the study did not meet the intended sample size for this subgroup analysis, results were statistically significant for location of care (P = .014) and may be beneficial for future study design by other investigators.
As early studies of nonsurgical and critical care patients document ≤ 40% of patients receive pharmacologic thromboprophylaxis, this study’s performance seems better.7,12,21 Recently, VHA investigators Quraishi and colleagues seemed to document similar findings. Although 63% of medical patients at the Dayton VAMC in Ohio received appropriate pharmacologic thromboprophylaxis, this value must be tempered by the proportion of subjects receiving therapeutic anticoagulation (5.4%).18
Similar to this study’s results, recent studies of nonveterans document pharmacologic thromboprophylaxis rates of 41% to 51.8%, 41% to 65.9%, and 74.6% to 89.9% in patients with respiratory disease, nonsurgical patients, and critical care patients, respectively. Although findings seem similar to this study’s results, adjustments in estimates again must be made, because these estimates included patients on therapeutic anticoagulation.12,14-16 This study’s results found that 58% of the patient cohort met the therapeutic appropriateness ratio, because they were administered pharmacologic thromboprophylaxis and received correct doses at indicated dosing intervals.
Because stringent exclusion criteria that minimized use of pharmacologic thromboprophylaxis in patients at risk for bleeding were applied, a higher rate of use was expected. This difference between expected and actual rates likely occurred because patient care is individualized and not all factors can be readily assessed in an observational study using retrospective data.
Additionally, for patients who remain ambulatory or have an invasive procedure, thromboprophylaxis may be appropriately delayed past the first 24-hour window of therapy or even temporarily interrupted. Subsequently, the measure of thromboprophylaxis initiation within the first 24 to 48 hours of admission was not elected. Instead, an alternative endpoint of VTE protected time period on thromboprophylaxis was selected. When thromboprophylaxis was used, the median period of protection was 83% of the time period hospitalized for this subgroup. Standardizing to a 7-day period, a VTE protected time period of 83% is coverage for 5.81 days. This would support the Joint Commission ORYX measure that allows for the receipt of thromboprophylaxis within 48 hours of admission to be counted as a success.6
Unfortunately, the authors did not assess whether mechanical thromboprophylaxis was provided to the remaining one-third of patients not receiving pharmacologic thromboprophylaxis. As a result, the complete data set is lacking, which would document whether the Joint Commission measure of ≥ 95% of the time was achieved. Therefore, the claim that TVHS is a top performing hospital for this ORYX measure cannot be made.
Although this study demonstrated a low mortality rate, this rate was not selected as a measure of interest, since one meta-analysis has demonstrated no mortality benefit from VTE thromboprophylaxis.4 Although in-hospital mortality may be an appropriate measure for critical care patients, most of the study patients did not meet this criterion.21 Last, mortality should be assessed no earlier than 30 days from admission.17 Subsequently, statistical assessment and conclusions from this measure are not relevant.
Limitations
A number of limitations hindered the generalizability of the results. This was an observational study using retrospectively collected data. The sample was narrowed to those with chronic respiratory disease, which has been less studied and typically examined in concert with acute processes, such as pneumonia. The demographic was primarily white males. The BMI of subjects enrolled in this study (26 kg/m2) was lower than the BMI of nonveteran subjects with COPD (28.6 kg/m2), nonveteran subjects with COPD and VTE (29 kg/m2), or veteran nonsurgical patients receiving thromboprophylaxis (29 kg/m2).18,34,35
The exclusion criteria resulted in a 73% reduction in the cohort and severely limited the number of medical critical care patients included. However, the problem of a small cohort was anticipated.
Other researchers conducting a prospective VHA thromboprophylaxis study found only 7.6% of veterans screened were eligible for enrollment, although 25% of subjects were anticipated by chart review. Two of the 3 primary reasons for trial exclusion were indication for therapeutic anticoagulation and contraindications to heparin (other than thrombocytopenia), and these were also primary reasons for exclusion in this study.30 Subsequently, the cohort appropriate for thromboprophylaxis in VHA seems relatively small.
Additionally, mobility is difficult to judge in a chart review. Day-to-day clinical assessments of mobility lead to individualization of care, including delayed initiation and timely termination of thromboprophylaxis. It is also possible that a significant portion of the patients had mechanical thromboprophylaxis, because they may have had an unrecognized risk factor for bleeding or patient preferences were considered. Last, some veterans may have classified as palliative care, and VTE prophylaxis may have been omitted for comfort care purposes.32
This study was not designed to evaluate the Padua Prediction Score, which categorizes risk and ration-alizes use of thromboprophylaxis for nonsurgical patients.3 This tool eliminates many of the established risk factors for VTE, including COPD, which was a qualifying diagnosis for inclusion in this study.1 It is not clear how the Padua Prediction Score would categorize the inpatient veteran population. Veterans clearly have poorer health status, more medical conditions, and higher medical resource use compared with the general patient population.25
Veterans with COPD have a higher comorbid illness burden than that of veterans without COPD.36 Chronic obstructive pulmonary disease is associated with VTE development, and when VTE develops in patients with COPD, mortality is greater than that of patients without COPD.37,38 VTE mortality may be related to an increased likelihood of fatal pulmonary embolism.39 Therefore, the authors recommend that VHA conduct studies to examine the Padua Prediction Score and potentially other RAMs that include COPD subjects, to determine what tool should be used in VHA.32
The authors also recommend that VHA evaluate how to improve thromboprophylaxis care with time-based studies. Since manual extraction to determine study inclusion was a time-consuming process, this time frame likely was a barrier to physician implementation of pharmacologic thromboprophylaxis. Therefore an electronic tool that serves as a daily reminder for subjects calculated as high risk for VTE but low risk for bleeding may improve clinical outcomes.
Conclusions
Overall, about one-third of patients did not receive potentially indicated pharmacologic thromboprophylaxis on the medical wards. Use of pharmacologic thromboprophylaxis in medical CCU patients was robust (80%). Doses and dosing intervals were appropriate for > 90% of patients, and therapy clearly was started early and continued for much of the at-risk period, as the VTE protected time period exceeded 80%. Although computerized tools were limited, the authors feel their modest pharmacologic thromboprophylaxis rate is related to the facility’s teaching hospital affiliation or the provider mix, because TVHS is one of the largest VA cardiology centers in the U.S.7,8,13
As it was challenging and time consuming to locate eligible subjects, it may also prove difficult for the admitting physician to have the same luxury of time to look for specific at-risk diagnoses in the medical record and evaluate for exclusions to therapy. If electronic alerts and reminder tools were included in clinical pharmacy inpatient templates, the authors believe the frequency of pharmacologic thromboprophylaxis would further improve in the facility. Also, the authors encourage VHA researchers to further evaluate VTE prophylaxis RAM, the role of daily electronic reminders, and tools to calculate VTE and bleeding risk.
Acknowledgements
The authors are grateful to James Minnis, PharmD, BCPS, and April Ungar, PharmD, BCPS, for their contributions to the study design. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Geerts WH, Bergquist D, Pineo G, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):381S-453S.
2. Go AS, Mozaffarian D, Roger VL, et al; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
3. Kahn SR, Lim W, Dunn AS, et al; American College of Chest Physicians. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
4. Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: A meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486.
5. Ruiz EM, Utrilla GB, Alvarez JL, Perrin RS. Effectiveness and safety of thromboprophylaxis with enoxaparin in medical inpatients. Thromb Res. 2011;128(5):440-445.
6. The Joint Commission. Specifications Manual for National Hospital Inpatient Quality Measures. http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality _measures.aspx. The Joint Commission Website. Accessed March 5, 2015.
7. Stark JE, Kilzer WJ. Venous thromboembolic prophylaxis in hospitalized medical patients. Ann Pharmacother. 2004;38(1):36-40.
8. Peterman CM, Kolansky DM, Spinler SA. Prophylaxis against venous thromboembolism in acutely ill medical patients: An observational study. Pharmacotherapy. 2006;26(8):1086-1090.
9. Herbers J, Zarter S. Prevention of venous thromboembolism in Department of Veterans Affairs hospitals. J Hosp Med. 2010;5(1):E21-E25.
10. Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health-Syst Pharm. 2007;64(1):69-76.
11. Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: Success or failure? J Thromb Haemost. 2007;5(8):1610-1616.
12. Tapson VF, Decousus H, Pini M, et al; IMPROVE Investigators. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: Findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945.
13. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: Adherence to the Seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21.
14. Amin A, Spyropoulos AC, Dobesh P, et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE Start). J Thromb Thrombolysis. 2010;29(3):326-339.
15. Baser O, Liu X, Phatak H, Wang L, et al. Venous thromboembolism prophylaxis and clinical consequences in medically ill patients. Am J Ther. 2013:20(2):132-142.
16. Baser O, Sengupta N, Dysinger A, Wang L. Thromboembolism prophylaxis in medical inpatients: Effect on outcomes and costs. Am J Manag Care. 2012;18(6):294-302.
17. Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969-977.
18. Quraishi MB, Mathew R, Lowes A, et al. Venous thromboembolism prophylaxis and the impact of standardized guidelines: Is a computer-based approach enough? J Clin Outcomes Manage. 2011;18(11):505-512.
19. Stinnett JM, Pendleton R, Skordos L, Wheeler M, Rodgers GM. Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates. Am J Hematol. 2005;78(3):167-172.
20. Cohn SL, Adekile A, Mahabir V. Improved use of thromboprophylaxis for deep vein thrombosis following an educational intervention. J Hosp Med. 2006;1(6):331-338.
21. Lentine KL, Flavin KE, Gould MK. Variability in the use of thromboprophylaxis and outcomes in critically ill medical patients. Am J Med. 2005;118(12):1373-1380.
22. Pendergraft T, Liu X, Edelsberg J, Phatak H, et al. Prophylaxis against venous thromboembolism in hospitalized medically ill patients. Circ Cardiovasc Qual Outcomes. 2013;6(1):75-82.
23. Rothberg MB, Lahti M, Pekow PS, Lindenauer PK. Venous thromboembolism prophylaxis among medical patients at US hospitals. J Gen Intern Med. 2010;25(6):489-494.
24. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597.
25. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21): 3252-3257.
26. Pham DQ, Pham AQ, Ullah E, McFarlane SI, Payne R. Short communication: Evaluating the appropriateness of thromboprophylaxis in an acute care setting using a computerised reminder, through order-entry system. Int J Clin Pract. 2008;62(1):134-137.
27. Khanna R, Vittinghoff E, Maselli J, Auerbach A. Unintended consequences of a standard admission order set on venous thromboembolism prophylaxis and patient outcomes. J Gen Intern Med. 2011;27(3):318-324.
28. Barr RG, Celli VR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348-355.
29. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations – United States, 2007-2009. (MMWR) Morbidity & Mortality Weekly Report. 2012;61(22):401-404.
30. Lederle FA, Sacks JN, Fiore L, et al. The prophylaxis of medical patients for thromboembolism pilot study. Am J Med. 2006;119(1):54-59.
31. Dobromirski M, Cohen AT. How I manage venous thromboembolism risk in hospitalized medical patients. Blood. 2012;120(8):1562-1569.
32. Polich AL, Etherton GM, Knezevich JT, Rousek JB, Masek CM, Hallbeck MS. Can eliminating risk stratification improve medical residents’ adherence to venous thromboembolism prophylaxis? Acad Med. 2011;86(12):1518-1524.
33. King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: A metaanalysis. Chest. 2007;131(2):507-516.
34. Piazza G, Goldhaber SZ, Kroll A, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with chronic obstructive pulmonary disease. Am J Med. 2012;125(10):1010-1018.
35. Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131(1):20-28.
36. Sharafkhaneh A. Peterson NJ, Yu HJ, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: A retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132.
37. Shetty R, Seddighzadeh A, Piazza G, Goldhaber SZ. Chronic obstructive pulmonary disease and deep venous thrombosis: a prevalent combination. J Thromb Thrombolysis. 2008;26(1):35-40.
38. Carson JL, Terrin ML, Duff A, Kelley MA. Pulmonary embolism and mortality in patients with COPD. Chest. 1996;110(5):1212-1219.
39. Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167(9):935-943.
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism, is an important public health concern. Deep venous thrombosis is estimated to affect 10% to 20% of medical (nonsurgical) patients, 15% to 40% of stroke patients, and 10% to 80% of critical care patients who are not prophylaxed.1 Venous thromboembolism is associated with significant resource utilization, long-term sequelae, recurrent events, and sudden death.2
The current guidelines of the American College of Chest Physicians recommend use of pharmacologic thromboprophylaxis as the preferred strategy for nonsurgical (or medical) patients (IB, formerly IA, recommendation) and for critically ill patients (2C recommendation) at low risk for bleeding.1,3 Mechanical (or nonpharmacologic) thromboprophylaxis (eg, intermittent pneumatic compression) is an alternative for those at increased risk for bleeding (2C recommendation).3 Pharmacologic thromboprophylaxis in high-risk patients, similar to those studied in randomized controlled clinical trials, reduces the occurrence of symptomatic DVT by 34 events per 1,000 patients treated.3 However, data are conflicting regarding mortality benefit.4,5
Related: Trends in Venous Thromboembolism
The Joint Commission adopted any thromboprophylaxis (measure includes pharmacologic or nonpharmacologic strategies) as a core discretionary measure in the ORYX (National Quality Hospital Measures) program. The ORYX measurements are intended to support Joint Commission-accredited organizations in institutional quality improvement efforts. The thromboprophylaxis core measure became effective May 2009 and remains as an option for hospitals to meet the 4 core measure set accreditation requirement. A top-performing hospital should provide this measure to applicable patients ≥ 95% of the time, according to the Joint Commission.6 The Joint Commission does not encourage use of any risk assessment model (RAM), such as the Padua Prediction Score to preferentially select high-risk medical patients.3
A disparity exists between thromboprophylaxis recommendations and practices in the nonsurgical patient, even when electronic prompts or alerts are available (eTables 1 and 2). In the U.S., pharmacologic thromboprophylaxis is administered to 23.6% to 81.1% of medical patients and 37.9% to 79.4% of critical care patients.7-21 In most cases, these rates are liberal estimates, because they include patients who are already on therapeutic anticoagulation or may have received only 1 prophylactic dose during hospitalization.8-11,13-20 When studies exclude patients receiving therapeutic (or treatment doses) anti-coagulation, pharmacologic thromboprophylaxis rates are substantially lower, typically 31% to 33% for medical patients and 37.9% for critical care patients.7,12,21 Furthermore, when studies examine appropriateness of thromboprophylaxis (eg, within the first 2 days of hospitalization or at the correct dose, correct time, or predefined duration), calculations are often less robust.10,11,13,14,22,23
The VHA uses thromboprophylaxis of surgical patients as an external peer review (EPR) performance measure (PM). With the great attention to this national measure, Altom and colleagues reported 89.9% of surgeries adhered.24 Before 2015, VTE thromboprophylaxis EPR PM did not exist. However, the VHA has initiated efforts to assure that providers are adherent to the new indications, which include VTE prophylaxis and treatment.
There is little published literature evaluating VHA performance.Quraishi and colleagues reported a pharmacologic prophylaxis rate of 63% in nonsurgical patients at a single VAMC, facilitated by the use of an admission VTE order set. Unfortunately, their estimate allowed inclusion of 5% of patients receiving treatment doses of anticoagulation and failed to provide any estimates on regimen appropriateness (eg, correct dose, correct time, or correct duration).18 Lentine and colleagues documented a pharmacologic thromboprophylaxis rate of 48% for a subset of veteran critical care patients who were not already receiving indicated therapeutic anticoagulants.21
Veterans have poorer health status, more medical conditions, and higher medical resource use than do nonveterans; therefore, it is postulated that veterans can derive clinical benefit from improved attention to thromboprophylaxis benchmarking, performance improvement, and potentially, implementation of electronic alerts or reminder tools.25 Nationally, VHA has no formal inpatient reminder tools to trigger use of thromboprophylaxis for high-risk medical patients, although individual health care systems may have created alerts or tools. Some studies demonstrated that order sets and electronic tools are helpful, whereas others demonstrated potential for harm.17-20,26,27
For any hospitalization at the VA Tennessee Valley Healthcare System (TVHS), the only electronic prompt to order VTE thromboprophylaxis occurs when the admission order set is completed. But the prompt can be readily bypassed if the quick admission orders are selected. Although no further electronic prompts in the Computerized Patient Record System (CPRS) are invoked following admission, the authors hypothesized that the rate of VTE thromboprophylaxis, specifically pharmacologic, in a subset of veterans with respiratory disease will be higher than the usual published rates.
Purpose and Relevance
This study’s primary aim was to assess the rate of pharmacologic VTE prophylaxis in veterans with pulmonary disease who were admitted for a nonsurgical stay. The 2 secondary aims were to determine whether thromboprophylaxis was appropriate and to characterize whether differences exist for pharmacologic prophylaxis according to level of care (medical critical care unit [CCU] vs acute care medical ward).
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: Rivaroxaban Update
This analysis emphasizes pharmacologic thromboprophylaxis instead of the combined endpoint of pharmacologic plus nonpharmacologic thromboprophylaxis traditionally used and will supplement the limited literature in 2 understudied cohorts: (1) nonsurgical veteran patients, specifically where advanced computerized thromboprophylaxis alerts are not in use; and (2) patients with the VTE risk factor of respiratory disease.1,7-9,12,13,15,16,18,21
Study Design
This observational study used retrospectively collected data. The data were extracted electronically from the VISN 9 data warehouse by a Decision Support Services analyst and manually validated by an investigator using the CPRS. Prior to initiation of research activities, the VHA Institutional Review Board and the Research and Development Committee at the facility level approved the study.
Sampling
Patients assigned to the treating specialties of medicine and medical critical care during fiscal years 2006 to 2008, admitted for ≥ 24 hours, and discharged with a diagnosis of chronic obstructive pulmonary disease (COPD), asthma, or acute, severe respiratory disease (eg, patients requiring mechanical ventilation) were eligible for inclusion. The authors also elected to include patients with asthma, because this diagnosis commonly overlaps with COPD and reflects real-world clinical practice and diagnostic challenges.28 Pneumonia and other infectious pulmonary conditions were not a qualifying diagnosis for study inclusion.
Patients were excluded if aged > 79 years, because it is difficult to maintain de-identification in a small sample of inpatients in this age category. Unfortunately, octogenarians have the highest rate of VTE per 100,000 population and would gain substantial benefit from prophylaxis.29 Similar to other VHA and non-VHA investigators, this study excluded patients who were prescribed therapeutic anticoagulation.7,12,21,30 The authors believe continuation of therapeutic (or treatment) anticoagulation does not measure a clinical decision to use pharmacologic thromboprophylaxis, and any interruption of therapeutic anticoagulation suggests that prophylactic anticoagulation is not warranted.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
Additionally, patients were excluded if length of stay (LOS) exceeded 14 days, if known or potential contraindications to thromboprophylaxis existed, or if laboratory data that were needed to assess for contraindications were missing from the electronic data set. Known or potential contraindications included active hemorrhage, hemorrhage within the past 3 months, recent administration of packed red blood cells, bacterial endocarditis, known coagulopathy, recent or current heparin-induced thrombocytopenia, or a potential coagulopathy (International Normalized Ratio > 1.5, platelets < 50,000, or an activated partial thromboplastin time > 41 sec).
Contraindications were conservative in construct and were similar to the exclusion-based VTE checklist for the nonsurgical patient.31 The authors did not examine the electronic data set for the contraindication of epidural or spinal anesthesia, because neither is commonly used in the medical ward or medical CCU. The authors also did not exclude patients with a creatinine clearance (CrCl) < 10 mL/min (a relative contraindication to VTE thromboprophylaxis), although these patients may be at an increased risk for bleeding complications.32
Endpoints and Measures
The primary endpoint of this study was the rate of any pharmacologic thromboprophylaxis (eg, ≥ 1 doses), similar to the endpoint selected by other investigators.7-9,12,13,15,16 Secondary endpoints included VTE protected time period on thromboprophylaxis, therapeutic appropriateness ratio for heparin and enoxaparin doses combined, and pharmacologic thromboprophylaxis rates according to level and location of care.
Sample Size
Although data have been forthcoming, at the time of study inception no studies documented the rate of pharmacologic thromboprophylaxis alone (defined as use of ≥ 1 dose of a pharmacologic agent) in patients with the VTE risk factor of respiratory disease.15,23 However, an average combined pharmacologic and nonpharmacologic thromboprophylaxis rate of 48.8% was determined from available studies.11,14 Although this percentage is an overestimate of pharmacologic thromboprophylaxis rates alone, this value was used to determine a sample size for the cohort.
About 122 subjects would be needed to provide 80% power and a significance level of < 0.05 to assess the hypothesis that pharmacologic prophylaxis rates at TVHS would exceed 60%. Additionally calculated was the sample size necessary to find a 20% expected difference in thromboprophylaxis rates according to location of care (eg, medical ward vs medical CCU), the secondary endpoint. This sample size was calculated to be 180 subjects, or 90 patients in each arm, to provide 80% power and a significance level (2-tailed alpha) of < 0.05. Subsequently, up to 130 patients from each location of care were randomly selected for study inclusion.
Data Analysis
A chi square test was used to compare groups on categorical variables. SPSS version 16.0 (SPSS Chicago, IL) was used for data analysis.
Results
A sample of 3,762 hospitalizations for veterans with COPD, asthma, or acute, severe respiratory disease who received inpatient care in the medical ward or medical CCU were extracted from the data warehouse.
Electronic Data Set
An investigator reviewed the electronic data set, and exclusion criteria that could be ascertained electronically were applied. The primary reasons for exclusion were age (18.4%), potential coagulopathy (14.5%), recent transfusion (14.6%), use of therapeutic anticoagulation (11%), or an extended LOS (7%). Less common reasons for exclusion were coagulation disorders (1.4%), heparin-induced thrombocytopenia (1.2%), recent hemorrhage (1.1%), or missing baseline laboratory values (3.2%). Subsequently, the potential sample of subjects declined to 1,018 (27%) hospitalizations. Of the remaining hospitalizations, 46 and 972 were medical CCU and nonsurgical (medical) inpatients, respectively.
In line with the sampling plan, 130 (13.4%) medical ward hospitalizations were selected using a random number generator. As the ICU sample was smaller than anticipated, the convenience sample of all 46 hospitalizations was used.
Manual Chart Abstraction
Manual chart abstraction (n = 176) clarified physician/provider decision making (eg, some patients were not appropriate for thromboprophylaxis due to upcoming invasive procedures), medical history that could not be extracted by ICD-9 coding (eg, recent non-VHA admissions for medical conditions that were contraindications to prophylaxis), and anticoagulation dosing. These exclusions led to an additional 52 (29.5%) excluded hospitalizations. Reasons for manual exclusion included recent bleeding or at high risk for bleeding (18, 34.6%), incorrect classification as nonsurgical or elective admission (5, 9.6%), no diagnosis of lung disease (21, 40.4%), invasive procedures planned (4, 7.7%), treatment anticoagulant doses selected (4, 7.7%), or patient transferred to a non-VA medical facility due to acuity level (1, 1.9%). One patient was excluded for multiple reasons.
Baseline Demographics
The sample was an elderly, male (98%), white (79.8%) cohort (Table 1). No patients were aged < 40 years. Racial information was missing for 5.6% of the patients. The chief pulmonary diagnosis was COPD, and few patients had new onset, acute, severe respiratory disease (3.2%) prompting admission, because pneumonia was not included as a qualifying diagnosis. Median body mass index (BMI) was 26.31. The median LOS was 3.8 days for the overall cohort and 4.1 days for those receiving pharmacologic thromboprophylaxis, although for the latter group a larger proportion of patients were hospitalized for < 3 days. Renal function, according to endpoint definitions, was for using enoxaparin as the appropriate strategy for thromboprophylaxis for the majority (97.5%) of hospitalizations.
Primary and Secondary Endpoints
Of those receiving pharmacologic thromboprophylaxis, heparin was prescribed most often (62.8%). One patient received both heparin and enoxaparin during a single hospitalization.
Pharmacologic thromboprophylaxis was more common in the medical CCU subgroup (80.6%) compared with the nonsurgical patient (56.9%). Pharmacologic thromboprophylaxis was used in 62.9% of patients (n = 124). However, the therapeutic appropriateness ratio was reduced to 58% of the entire sample (n = 124), because 6 patients of the cohort receiving thromboprophylaxis (n = 78) were prescribed suboptimal doses: Specifically, 1 patient was underdosed and 1 overdosed when prescribed enoxaparin (2, 2.6%). Four patients (5.1%) received underdoses of heparin, based on institutional guidance. For those prescribed pharmacologic thromboprophylaxis, the VTE protected time period ratio was 82.8% (Table 2). Overall inpatient mortality rate was low (12, 9.7%). Most deceased patients were managed in the medical CCU (10, 83.3%) and did receive pharmacologic thromboprophylaxis (10, 83.3%).
Discussion
This study demonstrated moderate rates of VTE pharmacologic thromboprophylaxis, because 62.9% of nonsurgical patients with respiratory disease who were hospitalized for various reasons were prophylaxed with either SC heparin or enoxaparin. This rate represents active clinical decision making, because there was no indication to prescribe anticoagulation at therapeutic doses. As expected, pharmacologic thromboprophylaxis was more common in the critical care subgroup (80.6%) compared with the nonsurgical patients (56.9%). Although the study did not meet the intended sample size for this subgroup analysis, results were statistically significant for location of care (P = .014) and may be beneficial for future study design by other investigators.
As early studies of nonsurgical and critical care patients document ≤ 40% of patients receive pharmacologic thromboprophylaxis, this study’s performance seems better.7,12,21 Recently, VHA investigators Quraishi and colleagues seemed to document similar findings. Although 63% of medical patients at the Dayton VAMC in Ohio received appropriate pharmacologic thromboprophylaxis, this value must be tempered by the proportion of subjects receiving therapeutic anticoagulation (5.4%).18
Similar to this study’s results, recent studies of nonveterans document pharmacologic thromboprophylaxis rates of 41% to 51.8%, 41% to 65.9%, and 74.6% to 89.9% in patients with respiratory disease, nonsurgical patients, and critical care patients, respectively. Although findings seem similar to this study’s results, adjustments in estimates again must be made, because these estimates included patients on therapeutic anticoagulation.12,14-16 This study’s results found that 58% of the patient cohort met the therapeutic appropriateness ratio, because they were administered pharmacologic thromboprophylaxis and received correct doses at indicated dosing intervals.
Because stringent exclusion criteria that minimized use of pharmacologic thromboprophylaxis in patients at risk for bleeding were applied, a higher rate of use was expected. This difference between expected and actual rates likely occurred because patient care is individualized and not all factors can be readily assessed in an observational study using retrospective data.
Additionally, for patients who remain ambulatory or have an invasive procedure, thromboprophylaxis may be appropriately delayed past the first 24-hour window of therapy or even temporarily interrupted. Subsequently, the measure of thromboprophylaxis initiation within the first 24 to 48 hours of admission was not elected. Instead, an alternative endpoint of VTE protected time period on thromboprophylaxis was selected. When thromboprophylaxis was used, the median period of protection was 83% of the time period hospitalized for this subgroup. Standardizing to a 7-day period, a VTE protected time period of 83% is coverage for 5.81 days. This would support the Joint Commission ORYX measure that allows for the receipt of thromboprophylaxis within 48 hours of admission to be counted as a success.6
Unfortunately, the authors did not assess whether mechanical thromboprophylaxis was provided to the remaining one-third of patients not receiving pharmacologic thromboprophylaxis. As a result, the complete data set is lacking, which would document whether the Joint Commission measure of ≥ 95% of the time was achieved. Therefore, the claim that TVHS is a top performing hospital for this ORYX measure cannot be made.
Although this study demonstrated a low mortality rate, this rate was not selected as a measure of interest, since one meta-analysis has demonstrated no mortality benefit from VTE thromboprophylaxis.4 Although in-hospital mortality may be an appropriate measure for critical care patients, most of the study patients did not meet this criterion.21 Last, mortality should be assessed no earlier than 30 days from admission.17 Subsequently, statistical assessment and conclusions from this measure are not relevant.
Limitations
A number of limitations hindered the generalizability of the results. This was an observational study using retrospectively collected data. The sample was narrowed to those with chronic respiratory disease, which has been less studied and typically examined in concert with acute processes, such as pneumonia. The demographic was primarily white males. The BMI of subjects enrolled in this study (26 kg/m2) was lower than the BMI of nonveteran subjects with COPD (28.6 kg/m2), nonveteran subjects with COPD and VTE (29 kg/m2), or veteran nonsurgical patients receiving thromboprophylaxis (29 kg/m2).18,34,35
The exclusion criteria resulted in a 73% reduction in the cohort and severely limited the number of medical critical care patients included. However, the problem of a small cohort was anticipated.
Other researchers conducting a prospective VHA thromboprophylaxis study found only 7.6% of veterans screened were eligible for enrollment, although 25% of subjects were anticipated by chart review. Two of the 3 primary reasons for trial exclusion were indication for therapeutic anticoagulation and contraindications to heparin (other than thrombocytopenia), and these were also primary reasons for exclusion in this study.30 Subsequently, the cohort appropriate for thromboprophylaxis in VHA seems relatively small.
Additionally, mobility is difficult to judge in a chart review. Day-to-day clinical assessments of mobility lead to individualization of care, including delayed initiation and timely termination of thromboprophylaxis. It is also possible that a significant portion of the patients had mechanical thromboprophylaxis, because they may have had an unrecognized risk factor for bleeding or patient preferences were considered. Last, some veterans may have classified as palliative care, and VTE prophylaxis may have been omitted for comfort care purposes.32
This study was not designed to evaluate the Padua Prediction Score, which categorizes risk and ration-alizes use of thromboprophylaxis for nonsurgical patients.3 This tool eliminates many of the established risk factors for VTE, including COPD, which was a qualifying diagnosis for inclusion in this study.1 It is not clear how the Padua Prediction Score would categorize the inpatient veteran population. Veterans clearly have poorer health status, more medical conditions, and higher medical resource use compared with the general patient population.25
Veterans with COPD have a higher comorbid illness burden than that of veterans without COPD.36 Chronic obstructive pulmonary disease is associated with VTE development, and when VTE develops in patients with COPD, mortality is greater than that of patients without COPD.37,38 VTE mortality may be related to an increased likelihood of fatal pulmonary embolism.39 Therefore, the authors recommend that VHA conduct studies to examine the Padua Prediction Score and potentially other RAMs that include COPD subjects, to determine what tool should be used in VHA.32
The authors also recommend that VHA evaluate how to improve thromboprophylaxis care with time-based studies. Since manual extraction to determine study inclusion was a time-consuming process, this time frame likely was a barrier to physician implementation of pharmacologic thromboprophylaxis. Therefore an electronic tool that serves as a daily reminder for subjects calculated as high risk for VTE but low risk for bleeding may improve clinical outcomes.
Conclusions
Overall, about one-third of patients did not receive potentially indicated pharmacologic thromboprophylaxis on the medical wards. Use of pharmacologic thromboprophylaxis in medical CCU patients was robust (80%). Doses and dosing intervals were appropriate for > 90% of patients, and therapy clearly was started early and continued for much of the at-risk period, as the VTE protected time period exceeded 80%. Although computerized tools were limited, the authors feel their modest pharmacologic thromboprophylaxis rate is related to the facility’s teaching hospital affiliation or the provider mix, because TVHS is one of the largest VA cardiology centers in the U.S.7,8,13
As it was challenging and time consuming to locate eligible subjects, it may also prove difficult for the admitting physician to have the same luxury of time to look for specific at-risk diagnoses in the medical record and evaluate for exclusions to therapy. If electronic alerts and reminder tools were included in clinical pharmacy inpatient templates, the authors believe the frequency of pharmacologic thromboprophylaxis would further improve in the facility. Also, the authors encourage VHA researchers to further evaluate VTE prophylaxis RAM, the role of daily electronic reminders, and tools to calculate VTE and bleeding risk.
Acknowledgements
The authors are grateful to James Minnis, PharmD, BCPS, and April Ungar, PharmD, BCPS, for their contributions to the study design. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism, is an important public health concern. Deep venous thrombosis is estimated to affect 10% to 20% of medical (nonsurgical) patients, 15% to 40% of stroke patients, and 10% to 80% of critical care patients who are not prophylaxed.1 Venous thromboembolism is associated with significant resource utilization, long-term sequelae, recurrent events, and sudden death.2
The current guidelines of the American College of Chest Physicians recommend use of pharmacologic thromboprophylaxis as the preferred strategy for nonsurgical (or medical) patients (IB, formerly IA, recommendation) and for critically ill patients (2C recommendation) at low risk for bleeding.1,3 Mechanical (or nonpharmacologic) thromboprophylaxis (eg, intermittent pneumatic compression) is an alternative for those at increased risk for bleeding (2C recommendation).3 Pharmacologic thromboprophylaxis in high-risk patients, similar to those studied in randomized controlled clinical trials, reduces the occurrence of symptomatic DVT by 34 events per 1,000 patients treated.3 However, data are conflicting regarding mortality benefit.4,5
Related: Trends in Venous Thromboembolism
The Joint Commission adopted any thromboprophylaxis (measure includes pharmacologic or nonpharmacologic strategies) as a core discretionary measure in the ORYX (National Quality Hospital Measures) program. The ORYX measurements are intended to support Joint Commission-accredited organizations in institutional quality improvement efforts. The thromboprophylaxis core measure became effective May 2009 and remains as an option for hospitals to meet the 4 core measure set accreditation requirement. A top-performing hospital should provide this measure to applicable patients ≥ 95% of the time, according to the Joint Commission.6 The Joint Commission does not encourage use of any risk assessment model (RAM), such as the Padua Prediction Score to preferentially select high-risk medical patients.3
A disparity exists between thromboprophylaxis recommendations and practices in the nonsurgical patient, even when electronic prompts or alerts are available (eTables 1 and 2). In the U.S., pharmacologic thromboprophylaxis is administered to 23.6% to 81.1% of medical patients and 37.9% to 79.4% of critical care patients.7-21 In most cases, these rates are liberal estimates, because they include patients who are already on therapeutic anticoagulation or may have received only 1 prophylactic dose during hospitalization.8-11,13-20 When studies exclude patients receiving therapeutic (or treatment doses) anti-coagulation, pharmacologic thromboprophylaxis rates are substantially lower, typically 31% to 33% for medical patients and 37.9% for critical care patients.7,12,21 Furthermore, when studies examine appropriateness of thromboprophylaxis (eg, within the first 2 days of hospitalization or at the correct dose, correct time, or predefined duration), calculations are often less robust.10,11,13,14,22,23
The VHA uses thromboprophylaxis of surgical patients as an external peer review (EPR) performance measure (PM). With the great attention to this national measure, Altom and colleagues reported 89.9% of surgeries adhered.24 Before 2015, VTE thromboprophylaxis EPR PM did not exist. However, the VHA has initiated efforts to assure that providers are adherent to the new indications, which include VTE prophylaxis and treatment.
There is little published literature evaluating VHA performance.Quraishi and colleagues reported a pharmacologic prophylaxis rate of 63% in nonsurgical patients at a single VAMC, facilitated by the use of an admission VTE order set. Unfortunately, their estimate allowed inclusion of 5% of patients receiving treatment doses of anticoagulation and failed to provide any estimates on regimen appropriateness (eg, correct dose, correct time, or correct duration).18 Lentine and colleagues documented a pharmacologic thromboprophylaxis rate of 48% for a subset of veteran critical care patients who were not already receiving indicated therapeutic anticoagulants.21
Veterans have poorer health status, more medical conditions, and higher medical resource use than do nonveterans; therefore, it is postulated that veterans can derive clinical benefit from improved attention to thromboprophylaxis benchmarking, performance improvement, and potentially, implementation of electronic alerts or reminder tools.25 Nationally, VHA has no formal inpatient reminder tools to trigger use of thromboprophylaxis for high-risk medical patients, although individual health care systems may have created alerts or tools. Some studies demonstrated that order sets and electronic tools are helpful, whereas others demonstrated potential for harm.17-20,26,27
For any hospitalization at the VA Tennessee Valley Healthcare System (TVHS), the only electronic prompt to order VTE thromboprophylaxis occurs when the admission order set is completed. But the prompt can be readily bypassed if the quick admission orders are selected. Although no further electronic prompts in the Computerized Patient Record System (CPRS) are invoked following admission, the authors hypothesized that the rate of VTE thromboprophylaxis, specifically pharmacologic, in a subset of veterans with respiratory disease will be higher than the usual published rates.
Purpose and Relevance
This study’s primary aim was to assess the rate of pharmacologic VTE prophylaxis in veterans with pulmonary disease who were admitted for a nonsurgical stay. The 2 secondary aims were to determine whether thromboprophylaxis was appropriate and to characterize whether differences exist for pharmacologic prophylaxis according to level of care (medical critical care unit [CCU] vs acute care medical ward).
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: Rivaroxaban Update
This analysis emphasizes pharmacologic thromboprophylaxis instead of the combined endpoint of pharmacologic plus nonpharmacologic thromboprophylaxis traditionally used and will supplement the limited literature in 2 understudied cohorts: (1) nonsurgical veteran patients, specifically where advanced computerized thromboprophylaxis alerts are not in use; and (2) patients with the VTE risk factor of respiratory disease.1,7-9,12,13,15,16,18,21
Study Design
This observational study used retrospectively collected data. The data were extracted electronically from the VISN 9 data warehouse by a Decision Support Services analyst and manually validated by an investigator using the CPRS. Prior to initiation of research activities, the VHA Institutional Review Board and the Research and Development Committee at the facility level approved the study.
Sampling
Patients assigned to the treating specialties of medicine and medical critical care during fiscal years 2006 to 2008, admitted for ≥ 24 hours, and discharged with a diagnosis of chronic obstructive pulmonary disease (COPD), asthma, or acute, severe respiratory disease (eg, patients requiring mechanical ventilation) were eligible for inclusion. The authors also elected to include patients with asthma, because this diagnosis commonly overlaps with COPD and reflects real-world clinical practice and diagnostic challenges.28 Pneumonia and other infectious pulmonary conditions were not a qualifying diagnosis for study inclusion.
Patients were excluded if aged > 79 years, because it is difficult to maintain de-identification in a small sample of inpatients in this age category. Unfortunately, octogenarians have the highest rate of VTE per 100,000 population and would gain substantial benefit from prophylaxis.29 Similar to other VHA and non-VHA investigators, this study excluded patients who were prescribed therapeutic anticoagulation.7,12,21,30 The authors believe continuation of therapeutic (or treatment) anticoagulation does not measure a clinical decision to use pharmacologic thromboprophylaxis, and any interruption of therapeutic anticoagulation suggests that prophylactic anticoagulation is not warranted.
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
Additionally, patients were excluded if length of stay (LOS) exceeded 14 days, if known or potential contraindications to thromboprophylaxis existed, or if laboratory data that were needed to assess for contraindications were missing from the electronic data set. Known or potential contraindications included active hemorrhage, hemorrhage within the past 3 months, recent administration of packed red blood cells, bacterial endocarditis, known coagulopathy, recent or current heparin-induced thrombocytopenia, or a potential coagulopathy (International Normalized Ratio > 1.5, platelets < 50,000, or an activated partial thromboplastin time > 41 sec).
Contraindications were conservative in construct and were similar to the exclusion-based VTE checklist for the nonsurgical patient.31 The authors did not examine the electronic data set for the contraindication of epidural or spinal anesthesia, because neither is commonly used in the medical ward or medical CCU. The authors also did not exclude patients with a creatinine clearance (CrCl) < 10 mL/min (a relative contraindication to VTE thromboprophylaxis), although these patients may be at an increased risk for bleeding complications.32
Endpoints and Measures
The primary endpoint of this study was the rate of any pharmacologic thromboprophylaxis (eg, ≥ 1 doses), similar to the endpoint selected by other investigators.7-9,12,13,15,16 Secondary endpoints included VTE protected time period on thromboprophylaxis, therapeutic appropriateness ratio for heparin and enoxaparin doses combined, and pharmacologic thromboprophylaxis rates according to level and location of care.
Sample Size
Although data have been forthcoming, at the time of study inception no studies documented the rate of pharmacologic thromboprophylaxis alone (defined as use of ≥ 1 dose of a pharmacologic agent) in patients with the VTE risk factor of respiratory disease.15,23 However, an average combined pharmacologic and nonpharmacologic thromboprophylaxis rate of 48.8% was determined from available studies.11,14 Although this percentage is an overestimate of pharmacologic thromboprophylaxis rates alone, this value was used to determine a sample size for the cohort.
About 122 subjects would be needed to provide 80% power and a significance level of < 0.05 to assess the hypothesis that pharmacologic prophylaxis rates at TVHS would exceed 60%. Additionally calculated was the sample size necessary to find a 20% expected difference in thromboprophylaxis rates according to location of care (eg, medical ward vs medical CCU), the secondary endpoint. This sample size was calculated to be 180 subjects, or 90 patients in each arm, to provide 80% power and a significance level (2-tailed alpha) of < 0.05. Subsequently, up to 130 patients from each location of care were randomly selected for study inclusion.
Data Analysis
A chi square test was used to compare groups on categorical variables. SPSS version 16.0 (SPSS Chicago, IL) was used for data analysis.
Results
A sample of 3,762 hospitalizations for veterans with COPD, asthma, or acute, severe respiratory disease who received inpatient care in the medical ward or medical CCU were extracted from the data warehouse.
Electronic Data Set
An investigator reviewed the electronic data set, and exclusion criteria that could be ascertained electronically were applied. The primary reasons for exclusion were age (18.4%), potential coagulopathy (14.5%), recent transfusion (14.6%), use of therapeutic anticoagulation (11%), or an extended LOS (7%). Less common reasons for exclusion were coagulation disorders (1.4%), heparin-induced thrombocytopenia (1.2%), recent hemorrhage (1.1%), or missing baseline laboratory values (3.2%). Subsequently, the potential sample of subjects declined to 1,018 (27%) hospitalizations. Of the remaining hospitalizations, 46 and 972 were medical CCU and nonsurgical (medical) inpatients, respectively.
In line with the sampling plan, 130 (13.4%) medical ward hospitalizations were selected using a random number generator. As the ICU sample was smaller than anticipated, the convenience sample of all 46 hospitalizations was used.
Manual Chart Abstraction
Manual chart abstraction (n = 176) clarified physician/provider decision making (eg, some patients were not appropriate for thromboprophylaxis due to upcoming invasive procedures), medical history that could not be extracted by ICD-9 coding (eg, recent non-VHA admissions for medical conditions that were contraindications to prophylaxis), and anticoagulation dosing. These exclusions led to an additional 52 (29.5%) excluded hospitalizations. Reasons for manual exclusion included recent bleeding or at high risk for bleeding (18, 34.6%), incorrect classification as nonsurgical or elective admission (5, 9.6%), no diagnosis of lung disease (21, 40.4%), invasive procedures planned (4, 7.7%), treatment anticoagulant doses selected (4, 7.7%), or patient transferred to a non-VA medical facility due to acuity level (1, 1.9%). One patient was excluded for multiple reasons.
Baseline Demographics
The sample was an elderly, male (98%), white (79.8%) cohort (Table 1). No patients were aged < 40 years. Racial information was missing for 5.6% of the patients. The chief pulmonary diagnosis was COPD, and few patients had new onset, acute, severe respiratory disease (3.2%) prompting admission, because pneumonia was not included as a qualifying diagnosis. Median body mass index (BMI) was 26.31. The median LOS was 3.8 days for the overall cohort and 4.1 days for those receiving pharmacologic thromboprophylaxis, although for the latter group a larger proportion of patients were hospitalized for < 3 days. Renal function, according to endpoint definitions, was for using enoxaparin as the appropriate strategy for thromboprophylaxis for the majority (97.5%) of hospitalizations.
Primary and Secondary Endpoints
Of those receiving pharmacologic thromboprophylaxis, heparin was prescribed most often (62.8%). One patient received both heparin and enoxaparin during a single hospitalization.
Pharmacologic thromboprophylaxis was more common in the medical CCU subgroup (80.6%) compared with the nonsurgical patient (56.9%). Pharmacologic thromboprophylaxis was used in 62.9% of patients (n = 124). However, the therapeutic appropriateness ratio was reduced to 58% of the entire sample (n = 124), because 6 patients of the cohort receiving thromboprophylaxis (n = 78) were prescribed suboptimal doses: Specifically, 1 patient was underdosed and 1 overdosed when prescribed enoxaparin (2, 2.6%). Four patients (5.1%) received underdoses of heparin, based on institutional guidance. For those prescribed pharmacologic thromboprophylaxis, the VTE protected time period ratio was 82.8% (Table 2). Overall inpatient mortality rate was low (12, 9.7%). Most deceased patients were managed in the medical CCU (10, 83.3%) and did receive pharmacologic thromboprophylaxis (10, 83.3%).
Discussion
This study demonstrated moderate rates of VTE pharmacologic thromboprophylaxis, because 62.9% of nonsurgical patients with respiratory disease who were hospitalized for various reasons were prophylaxed with either SC heparin or enoxaparin. This rate represents active clinical decision making, because there was no indication to prescribe anticoagulation at therapeutic doses. As expected, pharmacologic thromboprophylaxis was more common in the critical care subgroup (80.6%) compared with the nonsurgical patients (56.9%). Although the study did not meet the intended sample size for this subgroup analysis, results were statistically significant for location of care (P = .014) and may be beneficial for future study design by other investigators.
As early studies of nonsurgical and critical care patients document ≤ 40% of patients receive pharmacologic thromboprophylaxis, this study’s performance seems better.7,12,21 Recently, VHA investigators Quraishi and colleagues seemed to document similar findings. Although 63% of medical patients at the Dayton VAMC in Ohio received appropriate pharmacologic thromboprophylaxis, this value must be tempered by the proportion of subjects receiving therapeutic anticoagulation (5.4%).18
Similar to this study’s results, recent studies of nonveterans document pharmacologic thromboprophylaxis rates of 41% to 51.8%, 41% to 65.9%, and 74.6% to 89.9% in patients with respiratory disease, nonsurgical patients, and critical care patients, respectively. Although findings seem similar to this study’s results, adjustments in estimates again must be made, because these estimates included patients on therapeutic anticoagulation.12,14-16 This study’s results found that 58% of the patient cohort met the therapeutic appropriateness ratio, because they were administered pharmacologic thromboprophylaxis and received correct doses at indicated dosing intervals.
Because stringent exclusion criteria that minimized use of pharmacologic thromboprophylaxis in patients at risk for bleeding were applied, a higher rate of use was expected. This difference between expected and actual rates likely occurred because patient care is individualized and not all factors can be readily assessed in an observational study using retrospective data.
Additionally, for patients who remain ambulatory or have an invasive procedure, thromboprophylaxis may be appropriately delayed past the first 24-hour window of therapy or even temporarily interrupted. Subsequently, the measure of thromboprophylaxis initiation within the first 24 to 48 hours of admission was not elected. Instead, an alternative endpoint of VTE protected time period on thromboprophylaxis was selected. When thromboprophylaxis was used, the median period of protection was 83% of the time period hospitalized for this subgroup. Standardizing to a 7-day period, a VTE protected time period of 83% is coverage for 5.81 days. This would support the Joint Commission ORYX measure that allows for the receipt of thromboprophylaxis within 48 hours of admission to be counted as a success.6
Unfortunately, the authors did not assess whether mechanical thromboprophylaxis was provided to the remaining one-third of patients not receiving pharmacologic thromboprophylaxis. As a result, the complete data set is lacking, which would document whether the Joint Commission measure of ≥ 95% of the time was achieved. Therefore, the claim that TVHS is a top performing hospital for this ORYX measure cannot be made.
Although this study demonstrated a low mortality rate, this rate was not selected as a measure of interest, since one meta-analysis has demonstrated no mortality benefit from VTE thromboprophylaxis.4 Although in-hospital mortality may be an appropriate measure for critical care patients, most of the study patients did not meet this criterion.21 Last, mortality should be assessed no earlier than 30 days from admission.17 Subsequently, statistical assessment and conclusions from this measure are not relevant.
Limitations
A number of limitations hindered the generalizability of the results. This was an observational study using retrospectively collected data. The sample was narrowed to those with chronic respiratory disease, which has been less studied and typically examined in concert with acute processes, such as pneumonia. The demographic was primarily white males. The BMI of subjects enrolled in this study (26 kg/m2) was lower than the BMI of nonveteran subjects with COPD (28.6 kg/m2), nonveteran subjects with COPD and VTE (29 kg/m2), or veteran nonsurgical patients receiving thromboprophylaxis (29 kg/m2).18,34,35
The exclusion criteria resulted in a 73% reduction in the cohort and severely limited the number of medical critical care patients included. However, the problem of a small cohort was anticipated.
Other researchers conducting a prospective VHA thromboprophylaxis study found only 7.6% of veterans screened were eligible for enrollment, although 25% of subjects were anticipated by chart review. Two of the 3 primary reasons for trial exclusion were indication for therapeutic anticoagulation and contraindications to heparin (other than thrombocytopenia), and these were also primary reasons for exclusion in this study.30 Subsequently, the cohort appropriate for thromboprophylaxis in VHA seems relatively small.
Additionally, mobility is difficult to judge in a chart review. Day-to-day clinical assessments of mobility lead to individualization of care, including delayed initiation and timely termination of thromboprophylaxis. It is also possible that a significant portion of the patients had mechanical thromboprophylaxis, because they may have had an unrecognized risk factor for bleeding or patient preferences were considered. Last, some veterans may have classified as palliative care, and VTE prophylaxis may have been omitted for comfort care purposes.32
This study was not designed to evaluate the Padua Prediction Score, which categorizes risk and ration-alizes use of thromboprophylaxis for nonsurgical patients.3 This tool eliminates many of the established risk factors for VTE, including COPD, which was a qualifying diagnosis for inclusion in this study.1 It is not clear how the Padua Prediction Score would categorize the inpatient veteran population. Veterans clearly have poorer health status, more medical conditions, and higher medical resource use compared with the general patient population.25
Veterans with COPD have a higher comorbid illness burden than that of veterans without COPD.36 Chronic obstructive pulmonary disease is associated with VTE development, and when VTE develops in patients with COPD, mortality is greater than that of patients without COPD.37,38 VTE mortality may be related to an increased likelihood of fatal pulmonary embolism.39 Therefore, the authors recommend that VHA conduct studies to examine the Padua Prediction Score and potentially other RAMs that include COPD subjects, to determine what tool should be used in VHA.32
The authors also recommend that VHA evaluate how to improve thromboprophylaxis care with time-based studies. Since manual extraction to determine study inclusion was a time-consuming process, this time frame likely was a barrier to physician implementation of pharmacologic thromboprophylaxis. Therefore an electronic tool that serves as a daily reminder for subjects calculated as high risk for VTE but low risk for bleeding may improve clinical outcomes.
Conclusions
Overall, about one-third of patients did not receive potentially indicated pharmacologic thromboprophylaxis on the medical wards. Use of pharmacologic thromboprophylaxis in medical CCU patients was robust (80%). Doses and dosing intervals were appropriate for > 90% of patients, and therapy clearly was started early and continued for much of the at-risk period, as the VTE protected time period exceeded 80%. Although computerized tools were limited, the authors feel their modest pharmacologic thromboprophylaxis rate is related to the facility’s teaching hospital affiliation or the provider mix, because TVHS is one of the largest VA cardiology centers in the U.S.7,8,13
As it was challenging and time consuming to locate eligible subjects, it may also prove difficult for the admitting physician to have the same luxury of time to look for specific at-risk diagnoses in the medical record and evaluate for exclusions to therapy. If electronic alerts and reminder tools were included in clinical pharmacy inpatient templates, the authors believe the frequency of pharmacologic thromboprophylaxis would further improve in the facility. Also, the authors encourage VHA researchers to further evaluate VTE prophylaxis RAM, the role of daily electronic reminders, and tools to calculate VTE and bleeding risk.
Acknowledgements
The authors are grateful to James Minnis, PharmD, BCPS, and April Ungar, PharmD, BCPS, for their contributions to the study design. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Geerts WH, Bergquist D, Pineo G, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):381S-453S.
2. Go AS, Mozaffarian D, Roger VL, et al; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
3. Kahn SR, Lim W, Dunn AS, et al; American College of Chest Physicians. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
4. Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: A meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486.
5. Ruiz EM, Utrilla GB, Alvarez JL, Perrin RS. Effectiveness and safety of thromboprophylaxis with enoxaparin in medical inpatients. Thromb Res. 2011;128(5):440-445.
6. The Joint Commission. Specifications Manual for National Hospital Inpatient Quality Measures. http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality _measures.aspx. The Joint Commission Website. Accessed March 5, 2015.
7. Stark JE, Kilzer WJ. Venous thromboembolic prophylaxis in hospitalized medical patients. Ann Pharmacother. 2004;38(1):36-40.
8. Peterman CM, Kolansky DM, Spinler SA. Prophylaxis against venous thromboembolism in acutely ill medical patients: An observational study. Pharmacotherapy. 2006;26(8):1086-1090.
9. Herbers J, Zarter S. Prevention of venous thromboembolism in Department of Veterans Affairs hospitals. J Hosp Med. 2010;5(1):E21-E25.
10. Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health-Syst Pharm. 2007;64(1):69-76.
11. Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: Success or failure? J Thromb Haemost. 2007;5(8):1610-1616.
12. Tapson VF, Decousus H, Pini M, et al; IMPROVE Investigators. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: Findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945.
13. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: Adherence to the Seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21.
14. Amin A, Spyropoulos AC, Dobesh P, et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE Start). J Thromb Thrombolysis. 2010;29(3):326-339.
15. Baser O, Liu X, Phatak H, Wang L, et al. Venous thromboembolism prophylaxis and clinical consequences in medically ill patients. Am J Ther. 2013:20(2):132-142.
16. Baser O, Sengupta N, Dysinger A, Wang L. Thromboembolism prophylaxis in medical inpatients: Effect on outcomes and costs. Am J Manag Care. 2012;18(6):294-302.
17. Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969-977.
18. Quraishi MB, Mathew R, Lowes A, et al. Venous thromboembolism prophylaxis and the impact of standardized guidelines: Is a computer-based approach enough? J Clin Outcomes Manage. 2011;18(11):505-512.
19. Stinnett JM, Pendleton R, Skordos L, Wheeler M, Rodgers GM. Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates. Am J Hematol. 2005;78(3):167-172.
20. Cohn SL, Adekile A, Mahabir V. Improved use of thromboprophylaxis for deep vein thrombosis following an educational intervention. J Hosp Med. 2006;1(6):331-338.
21. Lentine KL, Flavin KE, Gould MK. Variability in the use of thromboprophylaxis and outcomes in critically ill medical patients. Am J Med. 2005;118(12):1373-1380.
22. Pendergraft T, Liu X, Edelsberg J, Phatak H, et al. Prophylaxis against venous thromboembolism in hospitalized medically ill patients. Circ Cardiovasc Qual Outcomes. 2013;6(1):75-82.
23. Rothberg MB, Lahti M, Pekow PS, Lindenauer PK. Venous thromboembolism prophylaxis among medical patients at US hospitals. J Gen Intern Med. 2010;25(6):489-494.
24. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597.
25. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21): 3252-3257.
26. Pham DQ, Pham AQ, Ullah E, McFarlane SI, Payne R. Short communication: Evaluating the appropriateness of thromboprophylaxis in an acute care setting using a computerised reminder, through order-entry system. Int J Clin Pract. 2008;62(1):134-137.
27. Khanna R, Vittinghoff E, Maselli J, Auerbach A. Unintended consequences of a standard admission order set on venous thromboembolism prophylaxis and patient outcomes. J Gen Intern Med. 2011;27(3):318-324.
28. Barr RG, Celli VR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348-355.
29. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations – United States, 2007-2009. (MMWR) Morbidity & Mortality Weekly Report. 2012;61(22):401-404.
30. Lederle FA, Sacks JN, Fiore L, et al. The prophylaxis of medical patients for thromboembolism pilot study. Am J Med. 2006;119(1):54-59.
31. Dobromirski M, Cohen AT. How I manage venous thromboembolism risk in hospitalized medical patients. Blood. 2012;120(8):1562-1569.
32. Polich AL, Etherton GM, Knezevich JT, Rousek JB, Masek CM, Hallbeck MS. Can eliminating risk stratification improve medical residents’ adherence to venous thromboembolism prophylaxis? Acad Med. 2011;86(12):1518-1524.
33. King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: A metaanalysis. Chest. 2007;131(2):507-516.
34. Piazza G, Goldhaber SZ, Kroll A, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with chronic obstructive pulmonary disease. Am J Med. 2012;125(10):1010-1018.
35. Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131(1):20-28.
36. Sharafkhaneh A. Peterson NJ, Yu HJ, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: A retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132.
37. Shetty R, Seddighzadeh A, Piazza G, Goldhaber SZ. Chronic obstructive pulmonary disease and deep venous thrombosis: a prevalent combination. J Thromb Thrombolysis. 2008;26(1):35-40.
38. Carson JL, Terrin ML, Duff A, Kelley MA. Pulmonary embolism and mortality in patients with COPD. Chest. 1996;110(5):1212-1219.
39. Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167(9):935-943.
1. Geerts WH, Bergquist D, Pineo G, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):381S-453S.
2. Go AS, Mozaffarian D, Roger VL, et al; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: A report from the American Heart Association. Circulation. 2013;127(1):e6-e245.
3. Kahn SR, Lim W, Dunn AS, et al; American College of Chest Physicians. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
4. Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: A meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486.
5. Ruiz EM, Utrilla GB, Alvarez JL, Perrin RS. Effectiveness and safety of thromboprophylaxis with enoxaparin in medical inpatients. Thromb Res. 2011;128(5):440-445.
6. The Joint Commission. Specifications Manual for National Hospital Inpatient Quality Measures. http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality _measures.aspx. The Joint Commission Website. Accessed March 5, 2015.
7. Stark JE, Kilzer WJ. Venous thromboembolic prophylaxis in hospitalized medical patients. Ann Pharmacother. 2004;38(1):36-40.
8. Peterman CM, Kolansky DM, Spinler SA. Prophylaxis against venous thromboembolism in acutely ill medical patients: An observational study. Pharmacotherapy. 2006;26(8):1086-1090.
9. Herbers J, Zarter S. Prevention of venous thromboembolism in Department of Veterans Affairs hospitals. J Hosp Med. 2010;5(1):E21-E25.
10. Yu HT, Dylan ML, Lin J, Dubois RW. Hospitals’ compliance with prophylaxis guidelines for venous thromboembolism. Am J Health-Syst Pharm. 2007;64(1):69-76.
11. Amin A, Stemkowski S, Lin J, Yang G. Thromboprophylaxis rates in US medical centers: Success or failure? J Thromb Haemost. 2007;5(8):1610-1616.
12. Tapson VF, Decousus H, Pini M, et al; IMPROVE Investigators. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: Findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945.
13. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: Adherence to the Seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21.
14. Amin A, Spyropoulos AC, Dobesh P, et al. Are hospitals delivering appropriate VTE prevention? The venous thromboembolism study to assess the rate of thromboprophylaxis (VTE Start). J Thromb Thrombolysis. 2010;29(3):326-339.
15. Baser O, Liu X, Phatak H, Wang L, et al. Venous thromboembolism prophylaxis and clinical consequences in medically ill patients. Am J Ther. 2013:20(2):132-142.
16. Baser O, Sengupta N, Dysinger A, Wang L. Thromboembolism prophylaxis in medical inpatients: Effect on outcomes and costs. Am J Manag Care. 2012;18(6):294-302.
17. Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969-977.
18. Quraishi MB, Mathew R, Lowes A, et al. Venous thromboembolism prophylaxis and the impact of standardized guidelines: Is a computer-based approach enough? J Clin Outcomes Manage. 2011;18(11):505-512.
19. Stinnett JM, Pendleton R, Skordos L, Wheeler M, Rodgers GM. Venous thromboembolism prophylaxis in medically ill patients and the development of strategies to improve prophylaxis rates. Am J Hematol. 2005;78(3):167-172.
20. Cohn SL, Adekile A, Mahabir V. Improved use of thromboprophylaxis for deep vein thrombosis following an educational intervention. J Hosp Med. 2006;1(6):331-338.
21. Lentine KL, Flavin KE, Gould MK. Variability in the use of thromboprophylaxis and outcomes in critically ill medical patients. Am J Med. 2005;118(12):1373-1380.
22. Pendergraft T, Liu X, Edelsberg J, Phatak H, et al. Prophylaxis against venous thromboembolism in hospitalized medically ill patients. Circ Cardiovasc Qual Outcomes. 2013;6(1):75-82.
23. Rothberg MB, Lahti M, Pekow PS, Lindenauer PK. Venous thromboembolism prophylaxis among medical patients at US hospitals. J Gen Intern Med. 2010;25(6):489-494.
24. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597.
25. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21): 3252-3257.
26. Pham DQ, Pham AQ, Ullah E, McFarlane SI, Payne R. Short communication: Evaluating the appropriateness of thromboprophylaxis in an acute care setting using a computerised reminder, through order-entry system. Int J Clin Pract. 2008;62(1):134-137.
27. Khanna R, Vittinghoff E, Maselli J, Auerbach A. Unintended consequences of a standard admission order set on venous thromboembolism prophylaxis and patient outcomes. J Gen Intern Med. 2011;27(3):318-324.
28. Barr RG, Celli VR, Mannino DM, et al. Comorbidities, patient knowledge, and disease management in a national sample of patients with COPD. Am J Med. 2009;122(4):348-355.
29. Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations – United States, 2007-2009. (MMWR) Morbidity & Mortality Weekly Report. 2012;61(22):401-404.
30. Lederle FA, Sacks JN, Fiore L, et al. The prophylaxis of medical patients for thromboembolism pilot study. Am J Med. 2006;119(1):54-59.
31. Dobromirski M, Cohen AT. How I manage venous thromboembolism risk in hospitalized medical patients. Blood. 2012;120(8):1562-1569.
32. Polich AL, Etherton GM, Knezevich JT, Rousek JB, Masek CM, Hallbeck MS. Can eliminating risk stratification improve medical residents’ adherence to venous thromboembolism prophylaxis? Acad Med. 2011;86(12):1518-1524.
33. King CS, Holley AB, Jackson JL, Shorr AF, Moores LK. Twice vs three times daily heparin dosing for thromboembolism prophylaxis in the general medical population: A metaanalysis. Chest. 2007;131(2):507-516.
34. Piazza G, Goldhaber SZ, Kroll A, Goldberg RJ, Emery C, Spencer FA. Venous thromboembolism in patients with chronic obstructive pulmonary disease. Am J Med. 2012;125(10):1010-1018.
35. Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131(1):20-28.
36. Sharafkhaneh A. Peterson NJ, Yu HJ, Dalal AA, Johnson ML, Hanania NA. Burden of COPD in a government health care system: A retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125-132.
37. Shetty R, Seddighzadeh A, Piazza G, Goldhaber SZ. Chronic obstructive pulmonary disease and deep venous thrombosis: a prevalent combination. J Thromb Thrombolysis. 2008;26(1):35-40.
38. Carson JL, Terrin ML, Duff A, Kelley MA. Pulmonary embolism and mortality in patients with COPD. Chest. 1996;110(5):1212-1219.
39. Huerta C, Johansson S, Wallander MA, García Rodríguez LA. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167(9):935-943.
Trends in Venous Thromboembolism
Methods of identifying and treating venous thromboembolism (VTE) have gotten better in the past 30 years, yet the annual event rate of VTE is still high. Indeed, it is on the rise, say researchers from University of Massachusetts in Worcester and McMaster University in Hamilton, Ontario, Canada. Is the increased rate of VTE events because diagnostic methods have improved, or are current prevention and treatment strategies falling short?
Maybe both, the researchers conclude. Clinicians are “clearly detecting more cases” than they were before the introduction of computed tomography pulmonary angiography. And between 1985 and 2009, the use of ≥ 1 noninvasive diagnostic methods for detecting VTE increased from about two-thirds of patients to nearly all patients (P < .001). But the researchers raise the question: How much of the increase in cases reflects small, clinically insignificant pulmonary embolisms?
Related: The Changing Landscape of VTE Treatment (Video)
They also point out that greater awareness of VTE as an important public health problem may have led clinicians to refer more patients for evaluation. Their study shows that the proportion of patients who received any form of objective diagnostic testing also has grown over time.
This study is the first population-based surveillance project of VTE to provide data about trends in annual event rates of first-time and recurrent VTE between 1985 and 2009. The study is based on data from 5,487 residents of Worcester, Massachusetts, who participated in the Worcester VTE study. That long-running study, the researchers say, afforded them a “unique opportunity” to examine trends in the magnitude, characteristics, and diagnostic workup for VTE “from the perspective of a well-characterized population.”
Between 1985 and 2009, 5,025 patients were diagnosed with acute pulmonary embolism or lower-extremity deep vein thrombosis. Of those, 3,887 had VTE for the first time, and 1,138 had recurrent VTE. The proportion of first-time VTE increased from about 66% in the initial cohort to about 80% in the 2009 cohort (P < .001). Interestingly, the rate of recurrent VTE dropped from 39 per 100,000 in 1985/86 to 19 per 100,000 in 2003 (95% confidence interval [CI], 15-23), then rose back to 35 per 100,000 in 2009 (95% CI, 29-40).
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
After adjustment for sex and age, the annual event rate was 142 per 100,000 for the entire study period, from 112 in 1985/86 to 168 in 2009. That’s higher than the incidence of the 2 leading cancers (prostate and breast) and > 15 times the rate of HIV in white Americans, the researchers point out. Also, the event rate of acute pulmonary embolism was nearly equivalent to the annual incidence of ischemic stroke. In other words, VTE is still a “major national health problem with a substantial disease burden”—one that may get even heavier. Given the aging of the U.S. population, the projected disease burden of VTE is expected to more than double by 2050.
Source
Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Am J Med. 2014;127(9):829-839.
doi: 10.1016/j.amjmed.2014.
Methods of identifying and treating venous thromboembolism (VTE) have gotten better in the past 30 years, yet the annual event rate of VTE is still high. Indeed, it is on the rise, say researchers from University of Massachusetts in Worcester and McMaster University in Hamilton, Ontario, Canada. Is the increased rate of VTE events because diagnostic methods have improved, or are current prevention and treatment strategies falling short?
Maybe both, the researchers conclude. Clinicians are “clearly detecting more cases” than they were before the introduction of computed tomography pulmonary angiography. And between 1985 and 2009, the use of ≥ 1 noninvasive diagnostic methods for detecting VTE increased from about two-thirds of patients to nearly all patients (P < .001). But the researchers raise the question: How much of the increase in cases reflects small, clinically insignificant pulmonary embolisms?
Related: The Changing Landscape of VTE Treatment (Video)
They also point out that greater awareness of VTE as an important public health problem may have led clinicians to refer more patients for evaluation. Their study shows that the proportion of patients who received any form of objective diagnostic testing also has grown over time.
This study is the first population-based surveillance project of VTE to provide data about trends in annual event rates of first-time and recurrent VTE between 1985 and 2009. The study is based on data from 5,487 residents of Worcester, Massachusetts, who participated in the Worcester VTE study. That long-running study, the researchers say, afforded them a “unique opportunity” to examine trends in the magnitude, characteristics, and diagnostic workup for VTE “from the perspective of a well-characterized population.”
Between 1985 and 2009, 5,025 patients were diagnosed with acute pulmonary embolism or lower-extremity deep vein thrombosis. Of those, 3,887 had VTE for the first time, and 1,138 had recurrent VTE. The proportion of first-time VTE increased from about 66% in the initial cohort to about 80% in the 2009 cohort (P < .001). Interestingly, the rate of recurrent VTE dropped from 39 per 100,000 in 1985/86 to 19 per 100,000 in 2003 (95% confidence interval [CI], 15-23), then rose back to 35 per 100,000 in 2009 (95% CI, 29-40).
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
After adjustment for sex and age, the annual event rate was 142 per 100,000 for the entire study period, from 112 in 1985/86 to 168 in 2009. That’s higher than the incidence of the 2 leading cancers (prostate and breast) and > 15 times the rate of HIV in white Americans, the researchers point out. Also, the event rate of acute pulmonary embolism was nearly equivalent to the annual incidence of ischemic stroke. In other words, VTE is still a “major national health problem with a substantial disease burden”—one that may get even heavier. Given the aging of the U.S. population, the projected disease burden of VTE is expected to more than double by 2050.
Source
Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Am J Med. 2014;127(9):829-839.
doi: 10.1016/j.amjmed.2014.
Methods of identifying and treating venous thromboembolism (VTE) have gotten better in the past 30 years, yet the annual event rate of VTE is still high. Indeed, it is on the rise, say researchers from University of Massachusetts in Worcester and McMaster University in Hamilton, Ontario, Canada. Is the increased rate of VTE events because diagnostic methods have improved, or are current prevention and treatment strategies falling short?
Maybe both, the researchers conclude. Clinicians are “clearly detecting more cases” than they were before the introduction of computed tomography pulmonary angiography. And between 1985 and 2009, the use of ≥ 1 noninvasive diagnostic methods for detecting VTE increased from about two-thirds of patients to nearly all patients (P < .001). But the researchers raise the question: How much of the increase in cases reflects small, clinically insignificant pulmonary embolisms?
Related: The Changing Landscape of VTE Treatment (Video)
They also point out that greater awareness of VTE as an important public health problem may have led clinicians to refer more patients for evaluation. Their study shows that the proportion of patients who received any form of objective diagnostic testing also has grown over time.
This study is the first population-based surveillance project of VTE to provide data about trends in annual event rates of first-time and recurrent VTE between 1985 and 2009. The study is based on data from 5,487 residents of Worcester, Massachusetts, who participated in the Worcester VTE study. That long-running study, the researchers say, afforded them a “unique opportunity” to examine trends in the magnitude, characteristics, and diagnostic workup for VTE “from the perspective of a well-characterized population.”
Between 1985 and 2009, 5,025 patients were diagnosed with acute pulmonary embolism or lower-extremity deep vein thrombosis. Of those, 3,887 had VTE for the first time, and 1,138 had recurrent VTE. The proportion of first-time VTE increased from about 66% in the initial cohort to about 80% in the 2009 cohort (P < .001). Interestingly, the rate of recurrent VTE dropped from 39 per 100,000 in 1985/86 to 19 per 100,000 in 2003 (95% confidence interval [CI], 15-23), then rose back to 35 per 100,000 in 2009 (95% CI, 29-40).
Related: Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
After adjustment for sex and age, the annual event rate was 142 per 100,000 for the entire study period, from 112 in 1985/86 to 168 in 2009. That’s higher than the incidence of the 2 leading cancers (prostate and breast) and > 15 times the rate of HIV in white Americans, the researchers point out. Also, the event rate of acute pulmonary embolism was nearly equivalent to the annual incidence of ischemic stroke. In other words, VTE is still a “major national health problem with a substantial disease burden”—one that may get even heavier. Given the aging of the U.S. population, the projected disease burden of VTE is expected to more than double by 2050.
Source
Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Am J Med. 2014;127(9):829-839.
doi: 10.1016/j.amjmed.2014.
Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.
The Changing Landscape of VTE Treatment
For more presentations from the 9th Annual Meeting of the Association of VA Hematology/Oncology (AVAHO), click here: AVAHO Meeting Presentations
For more presentations from the 9th Annual Meeting of the Association of VA Hematology/Oncology (AVAHO), click here: AVAHO Meeting Presentations
For more presentations from the 9th Annual Meeting of the Association of VA Hematology/Oncology (AVAHO), click here: AVAHO Meeting Presentations
Prevention of Venous Thromboembolism After Total Joint Replacement: Rivaroxaban Update
The number of total hip and knee replacement surgeries is increasing, and consequently, more patients are at risk of venous thromboembolism (VTE).1 Without thromboprophylaxis, the incidence of proximal deep vein thrombosis (DVT) is about 18% to 36% following total hip replacement (THR) and about 5% to 22% following total knee replacement (TKR). The incidence of total pulmonary embolism (PE) has been estimated at 0.9% to 28% in THR and 1.5% to 10% in TKR, and the incidence of fatal PE has been estimated to be as high as 2% following total joint replacement surgery.2 Despite the availability of effective anticoagulant agents for thromboprophylaxis, symptomatic VTE continues to occur in 1.3% to 10.0% of patients in the 3-month period following joint replacement surgery.2
VTE following THR or TKR represents a significant source of morbidity and mortality as well as a financial burden on the health care system. This burden is increased further by the complications of VTE—including a high rate of recurrence, postthrombotic syndrome (PTS), and pulmonary hypertension—which may be more debilitating than the primary event.3-5 Effective prophylaxis of VTE is paramount in reducing the incidence of these consequences.
Rivaroxaban, a Factor Xa inhibitor, is a novel anticoagulant that has recently been approved by the U.S. Food and Drug Administration (FDA) for prophylaxis of DVT in patients undergoing THR or TKR as well as for reduction of the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AFib).6 Clinical trials have shown that rivaroxaban is superior to enoxaparin, the standard of care, in preventing VTE after total joint replacement, and cost-effectiveness studies have demonstrated that rivaroxaban may potentially be a cost-saving agent.
This clinical review will address the economic and public health burden of VTE following THR and TKR and evaluate the novel anticoagulants that have been studied for this indication, including rivaroxaban. The safety and efficacy of rivaroxaban will be discussed, along with cost-effectiveness issues and practical management information.
The Economic Burden of VTE
VTE is the most common cause of hospital readmission following THR.2 The occurrence of VTE is reported to significantly increase the length of hospital stays and health care charges in patients undergoing TKR or THR. For example, one study reported health care charges for the index admission that were $9,297 higher for inpatients who experienced VTE compared with patients with TKR who did not have a VTE (P = .02).7 Similarly, for patients with THR, health care charges were $25,853 higher for inpatients who experienced VTE compared with patients who did not (P < .01).7
According to Spyropoulos and Lin, the total annual health care cost of a VTE (not limited to orthopedic patients) is $7,594 to $16,644, depending on the type of VTE (DVT or PE) and whether it was the primary or secondary diagnosis on discharge.8 These costs are significant, especially when multiplied by the number of THR and TKR surgeries performed in the U.S. Furthermore, VTE complications, such as PTS, represent an additional driver of health care expenditures.5,9,10
VTE Prophylaxis Guidelines
Both the American College of Chest Physicians (ACCP) and the American Academy of Orthopaedic Surgeons (AAOS) have published evidence-based guidelines for the prevention of VTE following total joint replacement.2,11 The 2012 version of the ACCP guidelines recommended the routine use of low-molecular-weight heparins (LMWHs), fondaparinux, low-dose unfractionated heparin, aspirin, adjusted-dose warfarin, rivaroxaban, apixaban, or dabigatran for thromboprophylaxis following THR or TKR. In contrast, the 2011 version of the AAOS guidelines makes no specific recommendations for pharmacologic agents.11 Despite the availability of evidence-based guidelines with specific recommendations, physician compliance with these guidelines is low.12
Before the approval of rivaroxaban, the vitamin K antagonist warfarin was the only available oral option for thromboprophylaxis following THR or TKR. The use of warfarin can be challenging, because it requires frequent monitoring and maintaining a patient within a specified international normalized ratio (INR) range. Several factors may influence time-in-therapeutic range, including drug-drug and drug-food interactions and genetic polymorphisms of vitamin K epoxide reductase complex subunit 1 (VKORC1) and cytochrome P450 (CYP)2C9.13 Warfarin does not provide short-term prophylaxis because of its delayed onset of action. It has been reported that 5 days after THR and TKR only about 30% of patients are within an INR of 2.0 to 3.0.14 Patients with an INR below 2.0 are at a 4- to 5-fold increased risk for VTE.14
Low-molecular-weight heparins, such as enoxaparin, do not require routine monitoring and have minimal drug interactions.15 The main drawback of LMWHs is their injectable mode of administration, which may influence prescribing habits and medication adherence. Patient education plays an essential role in ensuring adherence to LMWHs following orthopedic surgery.16
New Oral Anticoagulants
The new oral anticoagulants that have completed phase 3 trials for VTE prophylaxis following total joint replacement surgery include the selective Factor Xa inhibitors rivaroxaban and apixaban and the direct thrombin inhibitor dabigatran etexilate. Unlike warfarin, these agents do not require routine monitoring, a result of their predictable pharmacokinetic/pharmacodynamic (PK/PD) relationship, and they have few clinically significant drug interactions.17 Dabigatran and apixaban are not currently FDA approved for VTE prophylaxis following total joint replacement surgery; however, rivaroxaban was approved by the FDA in July 2011 for this indication.6
Apixaban
The Apixaban or Enoxaparin for Thromboprophylaxis After Knee Replacement (ADVANCE)-1 and ADVANCE-2 trials evaluated the use of Apixaban for thromboprophylaxis in patients undergoing TKR.18,19 In the ADVANCE-1 trial, patients undergoing TKR were randomized to receive either apixaban 2.5 mg orally twice daily or the North American preferred dosing of enoxaparin, 30 mg subcutaneously (SC) twice daily, with both agents started 12 to 24 hours after surgery and then taken for 10 to 14 days.18
Apixaban did not meet the prespecified statistical criteria for noninferiority compared with enoxaparin, although the rates of the primary efficacy endpoint (composite of VTE and all-cause mortality) were similar: 9.0% and 8.8% of patients, respectively. However, apixaban showed lower rates of clinically relevant bleeding and a similar adverse event (AE) profile compared with enoxaparin.
In the ADVANCE-2 trial, patients undergoing TKR received either apixaban 2.5 mg orally twice daily or enoxaparin 40 mg SC once daily for 10 to 14 days.19 The primary efficacy outcome (composite of total VTE and all-cause mortality) occurred at significantly lower rates in patients receiving apixaban (15% and 24%, respectively; relative risk [RR] 0.62; 95% confidence interval [CI] 0.51-0.74; P < .0001). Rates of major or clinically relevant nonmajor bleeding events occurred at similar rates between the 2 groups.
In the ADVANCE-3 trial, patients undergoing THR received either apixaban 2.5 orally twice daily or enoxaparin 40 mg SC once daily for 35 days.20 The primary efficacy outcome (composite of total VTE and all-cause mortality) occurred at significantly lower rates in patients receiving apixaban (1.4% and 3.9%, respectively; RR 0.36; 95% CI 0.22-0.54; P < .001). Rates of major or clinically relevant nonmajor bleeding events occurred at similar rates between both groups.
Dabigatran etexilate
Dabigatran etexilate has been evaluated in patients undergoing TKR in the Oral Dabigatran Etexilate vs Subcutaneous Enoxaparin for the Prevention of Venous Thromboembolism After Total Knee Replacement (RE-MODEL) and Oral Thrombin Inhibitor Dabigatran Etexilate vs North American Enoxaparin Regimen for Prevention of Venous Thromboembolism After Total Knee Replacement (RE-MOBILIZE) studies and in patients undergoing THR in the Oral Dabigatran vs Enoxaparin for Thromboprophylaxis After Primary Total Hip Arthroplasty (RE-NOVATE)-1 and RE-NOVATE‑2 trials.21-24
In RE-MODEL, patients undergoing TKR received either 150 mg or 220 mg of dabigatran etexilate once daily (starting with a half dose 1 to 4 hours after surgery) or enoxaparin 40 mg once daily (started the evening before surgery) for 6 to 10 days.23 Both doses of dabigatran had a similar incidence of the composite of total VTE and mortality and major bleeding. In RE-MOBILIZE, patients undergoing TKR received either 150 mg or 220 mg of dabigatran etexilate once daily or the North American regimen of enoxaparin, 30 mg SC twice daily for 12 to 15 days.24 Both dabigatran regimens had a significantly higher incidence of the composite of total VTE, failing to establish noninferiority to enoxaparin. The incidence of major bleeding was not significantly different among the 3 groups.
In RE-NOVATE I, patients undergoing THR received either dabigatran 150 mg or 220 mg twice daily, or enoxaparin 40 mg SC once daily for 28 to 35 days.21 Both doses of dabigatran had a similar incidence of the composite of total VTE and mortality and major bleeding. Because RE-NOVATE I did not include any study sites in North America, a second phase 3 trial, RE-NOVATE II, was conducted to include North American sites. RE-NOVATE II had an identical trial design to RE-NOVATE I and similar results (noninferior efficacy and comparable safety).22
Rivaroxaban
The phase 3 Rivaroxaban vs Enoxaparin for Thromboprophylaxis After Hip Arthroplasty (RECORD) program consisted of 4 double-blind randomized trials that compared the efficacy and safety of oral rivaroxaban to SC enoxaparin in THR (RECORD1 and 2) and TKR (RECORD3 and 4). The RECORD1 and 2 trials compared rivaroxaban 10 mg orally once daily for 31 to 39 days with enoxaparin 40 mg SC once daily (for 31-39 days in RECORD1 and for 10-14 days followed by placebo in RECORD2). The RECORD3 and 4 trials compared 10- to 14-day regimens of rivaroxaban 10 mg orally once daily with enoxaparin 40 mg SC once daily (RECORD3) and 30 mg twice daily (RECORD4). The 4 trials used the same efficacy and safety outcomes (Table).
In the RECORD1, 2, and 3 studies, rivaroxaban significantly reduced the composite of DVT, nonfatal PE, and all-cause mortality, as well as major VTE compared with enoxaparin. Major bleeding events as well as clinically relevant nonmajor bleeding and hemorrhagic wound complications were similar across both groups.25-27
In the RECORD4 study, rivaroxaban significantly reduced the composite of DVT, nonfatal PE, and all-cause mortality, but not major VTE, in patients undergoing TKR. Major bleeding events as well as clinically relevant nonmajor bleeding were numerically higher in the rivaroxaban group, but this was not statistically significant. Hemorrhagic wound complications were similar across both groups.28 This study was not required for approval and was not included in the final FDA-approved package insert.29
In a pooled analysis of the 4 RECORD trials presented at the FDA advisory committee, the incidence of major bleeding was significantly higher in the rivaroxaban group (24 events [0.39%]) compared with enoxaparin (13 events [0.21%]), with a nominal P value of .08 (significant at 10% nominal level) in the total treatment duration pool.30 In a pooled analysis by Turpie and colleagues who used the same data, this difference was not determined to be statistically different.31 In addition, this pooled analysis showed that the incidence of treatment-emergent hemorrhagic wound complications was similar in patients receiving rivaroxaban and enoxaparin and that fewer treatment-emergent serious AEs occurred in patients receiving rivaroxaban compared with patients receiving enoxaparin.31
In the FDA advisory committee, an “isolated signal” for a potentially increased risk of ischemic stroke was identified: In the safety population, ischemic stroke occurred in 5 patients who had received rivaroxaban and 1 patient who received enoxaparin.30 Furthermore, cardiovascular events in the safety population were concentrated around discontinuation of rivaroxaban, which was not the case for enoxaparin. The concern of stroke following rivaroxaban discontinuation was much more robust in Rivaroxaban vs Warfarin in Nonvalvular Atrial Fibrillation (ROCKET-AF), the phase 3 trial that compared rivaroxaban and warfarin for stroke prophylaxis in AFib.32 In the study ROCKET-AF, higher rates of stroke and systemic embolism were observed at the end of the trial among patients discontinuing rivaroxaban and switching to open-label warfarin compared with patients who had been taking warfarin and were transitioned to open-label warfarin. This observation led to a black box warning in the label of rivaroxaban regarding discontinuation of this agent.33
rivaroxaban management
The approved dose of rivaroxaban for the prophylaxis of VTE is 10 mg orally once daily with or without food.29 The first dose should be taken 6 to 10 hours after surgery, once hemostasis has been established. Rivaroxaban should be administered for 35 days to patients undergoing THR and for 12 days to patients undergoing TKR. Tablets may be crushed and administered in a gastric feeding tube, but they must not be administered via feeding tubes that deliver the contents into the proximal small intestine, because reduced drug absorption may result.29
The prescribing information for rivaroxaban includes a black box warning stating that epidural or spinal hematomas have occurred in patients treated with rivaroxaban who are receiving neuraxial (ie, spinal or epidural) anesthesia or undergoing spinal puncture. For such patients, the epidural catheter should not be removed earlier than 18 hours after the last administration of rivaroxaban.29 The next rivaroxaban dose should not be administered earlier than 6 hours after the catheter is removed.29 If a traumatic puncture occurs, rivaroxaban administration should be delayed for 24 hours.29 Factors that can increase the risk of developing epidural or spinal hematomas include the use of indwelling epidural catheters, concomitant use of drugs that affect hemostasis (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], platelet inhibitors, and other anticoagulants), a history of traumatic or repeated epidural or spinal punctures, and a history of spinal deformity or spinal surgery.
Rivaroxaban has not been studied in severe hepatic impairment (Child-Pugh C), and in subjects with moderate hepatic impairment (Child-Pugh B), rivaroxaban has led to increased drug exposure, with increased PD effects. Therefore, rivaroxaban should be avoided in patients with moderate to severe hepatic impairment or any hepatic disease associated with coagulopathy.29
Rivaroxaban should also be avoided in patients with severe renal impairment (a creatinine clearance [CrCl] of < 30 mL/min), because increased exposure with increased PD effects is expected. A combined analysis of the RECORD1, 2, and 3 trials did not show an increased bleeding risk for patients with moderate renal impairment (CrCl 30-50 mL/min) taking rivaroxaban. However, such patients should be observed for signs or symptoms of bleeding. Discontinuation should be considered in any patient who develops acute renal failure while taking rivaroxaban.
Drug interaction studies found that the concomitant use of rivaroxaban with drugs that are combined P-glycoprotein (P-gp) and CYP3A4 inhibitors led to increased rivaroxaban exposure and PD effects (ie, Factor Xa inhibition and prolonged prothrombin time). Rivaroxaban exposure was increased significantly when the drug was administered with combined P-gp and strong CYP3A4 inhibitors (eg, azole antifungal agents and protease inhibitors). Therefore, coadministration of rivaroxaban with these agents should be avoided, particularly in patients with any degree of renal impairment, because bleeding risk may increase. In cases where a change in exposure is considered unlikely to affect bleeding risk (ie, coadministration of weaker combined P-gp and CYP3A4 inhibitors, such as clarithromycin and erythromycin), no precautions are necessary.
If bleeding occurs during treatment with rivaroxaban, it may be appropriate to temporarily discontinue the drug and start supportive care. Rivaroxaban has a relatively short half-life (5-9 hours in healthy subjects aged 20-45 years and 11-13 hours in the elderly), meaning that drug effect decreases relatively quickly compared with warfarin. There is currently no direct antidote for rivaroxaban, but a study in healthy human subjects demonstrated that administration of prothrombin complex concentrates may be a potential option.34 Absolute contraindications to rivaroxaban treatment include patients with active pathological bleeding and those with severe hypersensitivity to the drug.
RIVAROXABAN ECONOMICS
Despite its high cost, economic analyses indicate that enoxaparin is a cost-effective agent for VTE prophylaxis compared with warfarin, which is well known to be inexpensive.35 An economic analysis that took into account prophylaxis failures and treatment complications as well as the direct costs associated with medical services, drugs, and laboratory tests showed a cost advantage for enoxaparin over warfarin that lasted for a substantial amount of time (19-31 days after hospital discharge).35
An economic model that followed patients for 1 year postsurgery specifically evaluated the costs associated with symptomatic VTE and major bleeding events in the RECORD trials, assuming the cost of rivaroxaban to be similar to that of enoxaparin 40 mg.36 Cost savings for rivaroxaban over enoxaparin were $82 to $291 per patient, depending on the indication (TKR or THR) and regimen, with cost savings increasing further if the costs of home nursing or training patients to self-administer enoxaparin are included.36 This economic model was also applied to THR and TKR figures from 2005 to show the global cost-effectiveness of rivaroxaban.37 This analysis showed that based on RECORD1, the use of rivaroxaban was associated with an average cost savings of $82 per patient and a reduction of 6 symptomatic events per 1,000 patients undergoing THR. Based on RECORD3, the use of rivaroxaban was associated with a cost savings of $284 per patient and a reduction of 18 symptomatic events per 1,000 patients undergoing TKR.
A later cost-effectiveness analysis by Duran and colleagues, published after FDA approval, included U.S. pricing information.38 In patients receiving extended-duration prophylaxis (35 days) following THR, rivaroxaban was associated with a cost savings of $695 per patient compared with enoxaparin. Compared with 14 days of enoxaparin, extended-duration rivaroxaban (35 days) prevented about 10 additional symptomatic VTE events per 1,000 patients and saved $244 per patient. In patients undergoing TKR, short-duration rivaroxaban
(10-14 days) prevented about 13 additional symptomatic VTE events per 1,000 patients while saving $411 per patient compared with short-duration enoxaparin (10-14 days). It should be noted that statistically significant differences were detected only in the base-case economic analysis, and differences in PE and bleeding events were not captured.
Conclusion
The prevalence of VTE after total joint replacement continues to pose a significant burden to our health care system in terms of morbidity, mortality, and health care costs. Novel anticoagulants such as rivaroxaban, which is now FDA-approved, represent promising alternatives to the traditional agents used for VTE prophylaxis. In addition to its superior efficacy and comparable safety profile to enoxaparin, rivaroxaban’s oral route of administration and straightforward management make it a promising alternative. In particular, the lack of a requirement for routine coagulation monitoring or dose adjustment should simplify treatment with the potential to improve compliance and adherence.
Some questions remain unanswered, such as lack of a direct antidote or widely accepted reversibility technique and how to monitor or assess anticoagulation status in emergency situations, such as overdose or pathologic bleeding. Importantly, early cost-effectiveness analyses indicate that rivaroxaban is cost-effective and potentially even cost-saving compared with enoxaparin and warfarin.Careful postmarketing surveillance will need to be conducted to establish its safety in real-world settings.
Acknowledgments
The authors would like to acknowledge Matthew Romo, PharmD, who provided editorial support with funding from Janssen Scientific Affairs, LLC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: Preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
2. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in Orthopedic Surgery Patients: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 9th ed. Chest. 2012(suppl 2);141:e278S-e325S.
3. Fanikos J, Piazza G, Zayaruzny M, Goldhaber SZ. Long-term complications of medical patients with hospital-acquired venous thromboembolism. Thromb Haemost. 2009;102(4):688-693.
4. Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257-2264.
5. Ruppert A, Lees M, Steinle T. Clinical burden of venous thromboembolism. Curr Med Res Opin. 2010;26(10):2465-2473.
6. FDA approves Xarelto® (rivaroxaban tablets) for the prophylaxis of deep vein thrombosis which may lead to a pulmonary embolism in patients undergoing knee or hip replacement surgery. BayNews.
July 1, 2011.
7. Oster G, Ollendorf DA, Vera-Llonch M, Hagiwara M, Berger A, Edelsberg J. Economic consequences of venous thromboembolism following major orthopedic surgery. Ann Pharmacother. 2004;38(3):377-382.
8. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13(6):475-486.
9. Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28(4):465-476.
10. MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome.
Am J Health Syst Pharm. 2006;63(suppl 20):S5-S15.
11. American Academy of Orthopaedic Surgeons. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty: evidence-based guidelines and evidence report. American Academy of Orthopaedic Surgeons Website. http://www.aaos.org/research/guidelines/VTE/VTE_full_guideline.pdf. Accessed January 29, 2014.
12. Friedman RJ, Gallus A, Gil-Garay E, Fitzgerald G, Cushner F. Practice patterns in the use of venous thromboembolism prophylaxis after total joint arthroplasty—insights from the Multinational Global Orthopaedic Registry (GLORY). Am J Orthop (Belle Mead NJ). 2010;39(suppl 9):14-21.
13. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):160S-198S.
14. Nordstrom BL, Kachroo S, Fraeman KH, et al. Warfarin prophylaxis in patients after total knee or hip arthroplasty—international normalized ratio patterns and venous thromboembolism. Curr Med Res Opin. 2011;27(10):1973-1985.
15. Lovenox [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2013.
16. Colwell CW Jr, Pulido P, Hardwick ME, Morris BA. Patient compliance with outpatient prophylaxis: An observational study. Orthopedics. 2005;28(2):143-147.
17. Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009;48(1):1-22.
18. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604.
19. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE-2 investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet. 2010;375(9717):807-815.
20. Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487-2498.
21. Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet. 2007;370(9591):949-956.
22. Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105(4):721-729.
23. Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: The RE-MODEL randomized trial. J Thromb Haemost. 2007;5(11):2178-2185.
24. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24(1):1-9.
25. Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty.
N Engl J Med. 2008;358(26):2765-2775.
26. Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: A double-blind, randomised controlled trial. Lancet. 2008;372(9632):31-39.
27. Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty.
N Engl J Med. 2008;358(26):2776-2786.
28. Turpie AG, Lassen MR, Davidson BL, et al; RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): A randomised trial. Lancet. 2009;373(9676):1673-1680.
29. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
30. Cardiovascular and Renal Drugs Advisory Committee. FDA Advisory Committee Briefing Document. 2009. http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4418b1-01-FDA.pdf. Accessed January 15, 2012.
31. Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105(3):444-453.
32. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
33. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
34. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
35. Friedman RJ, Dunsworth GA. Cost analyses of extended prophylaxis with enoxaparin after hip arthroplasty. Clin Orthop Rel Res. 2000;370:171-182.
36. Kwong LM. Cost-effectiveness of rivaroxaban after total hip or total knee arthroplasty. Am J Manag Care. 2011;17(suppl 1):S22-S26.
37. Friedman RJ, Sengupta N, Lees M. Economic impact of venous thromboembolism after hip and knee arthroplasty: Potential impact of rivaroxaban. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):299-306.
38. Duran A, Sengupta N, Diamantopoulos A, Forster F, Kwong L, Lees M. Cost and outcomes associated with rivaroxaban vs enoxaparin for the prevention of postsurgical venous thormboembolism from a US payer’s perspective. J Med Econ. 2011;14(6):824-834.
The number of total hip and knee replacement surgeries is increasing, and consequently, more patients are at risk of venous thromboembolism (VTE).1 Without thromboprophylaxis, the incidence of proximal deep vein thrombosis (DVT) is about 18% to 36% following total hip replacement (THR) and about 5% to 22% following total knee replacement (TKR). The incidence of total pulmonary embolism (PE) has been estimated at 0.9% to 28% in THR and 1.5% to 10% in TKR, and the incidence of fatal PE has been estimated to be as high as 2% following total joint replacement surgery.2 Despite the availability of effective anticoagulant agents for thromboprophylaxis, symptomatic VTE continues to occur in 1.3% to 10.0% of patients in the 3-month period following joint replacement surgery.2
VTE following THR or TKR represents a significant source of morbidity and mortality as well as a financial burden on the health care system. This burden is increased further by the complications of VTE—including a high rate of recurrence, postthrombotic syndrome (PTS), and pulmonary hypertension—which may be more debilitating than the primary event.3-5 Effective prophylaxis of VTE is paramount in reducing the incidence of these consequences.
Rivaroxaban, a Factor Xa inhibitor, is a novel anticoagulant that has recently been approved by the U.S. Food and Drug Administration (FDA) for prophylaxis of DVT in patients undergoing THR or TKR as well as for reduction of the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AFib).6 Clinical trials have shown that rivaroxaban is superior to enoxaparin, the standard of care, in preventing VTE after total joint replacement, and cost-effectiveness studies have demonstrated that rivaroxaban may potentially be a cost-saving agent.
This clinical review will address the economic and public health burden of VTE following THR and TKR and evaluate the novel anticoagulants that have been studied for this indication, including rivaroxaban. The safety and efficacy of rivaroxaban will be discussed, along with cost-effectiveness issues and practical management information.
The Economic Burden of VTE
VTE is the most common cause of hospital readmission following THR.2 The occurrence of VTE is reported to significantly increase the length of hospital stays and health care charges in patients undergoing TKR or THR. For example, one study reported health care charges for the index admission that were $9,297 higher for inpatients who experienced VTE compared with patients with TKR who did not have a VTE (P = .02).7 Similarly, for patients with THR, health care charges were $25,853 higher for inpatients who experienced VTE compared with patients who did not (P < .01).7
According to Spyropoulos and Lin, the total annual health care cost of a VTE (not limited to orthopedic patients) is $7,594 to $16,644, depending on the type of VTE (DVT or PE) and whether it was the primary or secondary diagnosis on discharge.8 These costs are significant, especially when multiplied by the number of THR and TKR surgeries performed in the U.S. Furthermore, VTE complications, such as PTS, represent an additional driver of health care expenditures.5,9,10
VTE Prophylaxis Guidelines
Both the American College of Chest Physicians (ACCP) and the American Academy of Orthopaedic Surgeons (AAOS) have published evidence-based guidelines for the prevention of VTE following total joint replacement.2,11 The 2012 version of the ACCP guidelines recommended the routine use of low-molecular-weight heparins (LMWHs), fondaparinux, low-dose unfractionated heparin, aspirin, adjusted-dose warfarin, rivaroxaban, apixaban, or dabigatran for thromboprophylaxis following THR or TKR. In contrast, the 2011 version of the AAOS guidelines makes no specific recommendations for pharmacologic agents.11 Despite the availability of evidence-based guidelines with specific recommendations, physician compliance with these guidelines is low.12
Before the approval of rivaroxaban, the vitamin K antagonist warfarin was the only available oral option for thromboprophylaxis following THR or TKR. The use of warfarin can be challenging, because it requires frequent monitoring and maintaining a patient within a specified international normalized ratio (INR) range. Several factors may influence time-in-therapeutic range, including drug-drug and drug-food interactions and genetic polymorphisms of vitamin K epoxide reductase complex subunit 1 (VKORC1) and cytochrome P450 (CYP)2C9.13 Warfarin does not provide short-term prophylaxis because of its delayed onset of action. It has been reported that 5 days after THR and TKR only about 30% of patients are within an INR of 2.0 to 3.0.14 Patients with an INR below 2.0 are at a 4- to 5-fold increased risk for VTE.14
Low-molecular-weight heparins, such as enoxaparin, do not require routine monitoring and have minimal drug interactions.15 The main drawback of LMWHs is their injectable mode of administration, which may influence prescribing habits and medication adherence. Patient education plays an essential role in ensuring adherence to LMWHs following orthopedic surgery.16
New Oral Anticoagulants
The new oral anticoagulants that have completed phase 3 trials for VTE prophylaxis following total joint replacement surgery include the selective Factor Xa inhibitors rivaroxaban and apixaban and the direct thrombin inhibitor dabigatran etexilate. Unlike warfarin, these agents do not require routine monitoring, a result of their predictable pharmacokinetic/pharmacodynamic (PK/PD) relationship, and they have few clinically significant drug interactions.17 Dabigatran and apixaban are not currently FDA approved for VTE prophylaxis following total joint replacement surgery; however, rivaroxaban was approved by the FDA in July 2011 for this indication.6
Apixaban
The Apixaban or Enoxaparin for Thromboprophylaxis After Knee Replacement (ADVANCE)-1 and ADVANCE-2 trials evaluated the use of Apixaban for thromboprophylaxis in patients undergoing TKR.18,19 In the ADVANCE-1 trial, patients undergoing TKR were randomized to receive either apixaban 2.5 mg orally twice daily or the North American preferred dosing of enoxaparin, 30 mg subcutaneously (SC) twice daily, with both agents started 12 to 24 hours after surgery and then taken for 10 to 14 days.18
Apixaban did not meet the prespecified statistical criteria for noninferiority compared with enoxaparin, although the rates of the primary efficacy endpoint (composite of VTE and all-cause mortality) were similar: 9.0% and 8.8% of patients, respectively. However, apixaban showed lower rates of clinically relevant bleeding and a similar adverse event (AE) profile compared with enoxaparin.
In the ADVANCE-2 trial, patients undergoing TKR received either apixaban 2.5 mg orally twice daily or enoxaparin 40 mg SC once daily for 10 to 14 days.19 The primary efficacy outcome (composite of total VTE and all-cause mortality) occurred at significantly lower rates in patients receiving apixaban (15% and 24%, respectively; relative risk [RR] 0.62; 95% confidence interval [CI] 0.51-0.74; P < .0001). Rates of major or clinically relevant nonmajor bleeding events occurred at similar rates between the 2 groups.
In the ADVANCE-3 trial, patients undergoing THR received either apixaban 2.5 orally twice daily or enoxaparin 40 mg SC once daily for 35 days.20 The primary efficacy outcome (composite of total VTE and all-cause mortality) occurred at significantly lower rates in patients receiving apixaban (1.4% and 3.9%, respectively; RR 0.36; 95% CI 0.22-0.54; P < .001). Rates of major or clinically relevant nonmajor bleeding events occurred at similar rates between both groups.
Dabigatran etexilate
Dabigatran etexilate has been evaluated in patients undergoing TKR in the Oral Dabigatran Etexilate vs Subcutaneous Enoxaparin for the Prevention of Venous Thromboembolism After Total Knee Replacement (RE-MODEL) and Oral Thrombin Inhibitor Dabigatran Etexilate vs North American Enoxaparin Regimen for Prevention of Venous Thromboembolism After Total Knee Replacement (RE-MOBILIZE) studies and in patients undergoing THR in the Oral Dabigatran vs Enoxaparin for Thromboprophylaxis After Primary Total Hip Arthroplasty (RE-NOVATE)-1 and RE-NOVATE‑2 trials.21-24
In RE-MODEL, patients undergoing TKR received either 150 mg or 220 mg of dabigatran etexilate once daily (starting with a half dose 1 to 4 hours after surgery) or enoxaparin 40 mg once daily (started the evening before surgery) for 6 to 10 days.23 Both doses of dabigatran had a similar incidence of the composite of total VTE and mortality and major bleeding. In RE-MOBILIZE, patients undergoing TKR received either 150 mg or 220 mg of dabigatran etexilate once daily or the North American regimen of enoxaparin, 30 mg SC twice daily for 12 to 15 days.24 Both dabigatran regimens had a significantly higher incidence of the composite of total VTE, failing to establish noninferiority to enoxaparin. The incidence of major bleeding was not significantly different among the 3 groups.
In RE-NOVATE I, patients undergoing THR received either dabigatran 150 mg or 220 mg twice daily, or enoxaparin 40 mg SC once daily for 28 to 35 days.21 Both doses of dabigatran had a similar incidence of the composite of total VTE and mortality and major bleeding. Because RE-NOVATE I did not include any study sites in North America, a second phase 3 trial, RE-NOVATE II, was conducted to include North American sites. RE-NOVATE II had an identical trial design to RE-NOVATE I and similar results (noninferior efficacy and comparable safety).22
Rivaroxaban
The phase 3 Rivaroxaban vs Enoxaparin for Thromboprophylaxis After Hip Arthroplasty (RECORD) program consisted of 4 double-blind randomized trials that compared the efficacy and safety of oral rivaroxaban to SC enoxaparin in THR (RECORD1 and 2) and TKR (RECORD3 and 4). The RECORD1 and 2 trials compared rivaroxaban 10 mg orally once daily for 31 to 39 days with enoxaparin 40 mg SC once daily (for 31-39 days in RECORD1 and for 10-14 days followed by placebo in RECORD2). The RECORD3 and 4 trials compared 10- to 14-day regimens of rivaroxaban 10 mg orally once daily with enoxaparin 40 mg SC once daily (RECORD3) and 30 mg twice daily (RECORD4). The 4 trials used the same efficacy and safety outcomes (Table).
In the RECORD1, 2, and 3 studies, rivaroxaban significantly reduced the composite of DVT, nonfatal PE, and all-cause mortality, as well as major VTE compared with enoxaparin. Major bleeding events as well as clinically relevant nonmajor bleeding and hemorrhagic wound complications were similar across both groups.25-27
In the RECORD4 study, rivaroxaban significantly reduced the composite of DVT, nonfatal PE, and all-cause mortality, but not major VTE, in patients undergoing TKR. Major bleeding events as well as clinically relevant nonmajor bleeding were numerically higher in the rivaroxaban group, but this was not statistically significant. Hemorrhagic wound complications were similar across both groups.28 This study was not required for approval and was not included in the final FDA-approved package insert.29
In a pooled analysis of the 4 RECORD trials presented at the FDA advisory committee, the incidence of major bleeding was significantly higher in the rivaroxaban group (24 events [0.39%]) compared with enoxaparin (13 events [0.21%]), with a nominal P value of .08 (significant at 10% nominal level) in the total treatment duration pool.30 In a pooled analysis by Turpie and colleagues who used the same data, this difference was not determined to be statistically different.31 In addition, this pooled analysis showed that the incidence of treatment-emergent hemorrhagic wound complications was similar in patients receiving rivaroxaban and enoxaparin and that fewer treatment-emergent serious AEs occurred in patients receiving rivaroxaban compared with patients receiving enoxaparin.31
In the FDA advisory committee, an “isolated signal” for a potentially increased risk of ischemic stroke was identified: In the safety population, ischemic stroke occurred in 5 patients who had received rivaroxaban and 1 patient who received enoxaparin.30 Furthermore, cardiovascular events in the safety population were concentrated around discontinuation of rivaroxaban, which was not the case for enoxaparin. The concern of stroke following rivaroxaban discontinuation was much more robust in Rivaroxaban vs Warfarin in Nonvalvular Atrial Fibrillation (ROCKET-AF), the phase 3 trial that compared rivaroxaban and warfarin for stroke prophylaxis in AFib.32 In the study ROCKET-AF, higher rates of stroke and systemic embolism were observed at the end of the trial among patients discontinuing rivaroxaban and switching to open-label warfarin compared with patients who had been taking warfarin and were transitioned to open-label warfarin. This observation led to a black box warning in the label of rivaroxaban regarding discontinuation of this agent.33
rivaroxaban management
The approved dose of rivaroxaban for the prophylaxis of VTE is 10 mg orally once daily with or without food.29 The first dose should be taken 6 to 10 hours after surgery, once hemostasis has been established. Rivaroxaban should be administered for 35 days to patients undergoing THR and for 12 days to patients undergoing TKR. Tablets may be crushed and administered in a gastric feeding tube, but they must not be administered via feeding tubes that deliver the contents into the proximal small intestine, because reduced drug absorption may result.29
The prescribing information for rivaroxaban includes a black box warning stating that epidural or spinal hematomas have occurred in patients treated with rivaroxaban who are receiving neuraxial (ie, spinal or epidural) anesthesia or undergoing spinal puncture. For such patients, the epidural catheter should not be removed earlier than 18 hours after the last administration of rivaroxaban.29 The next rivaroxaban dose should not be administered earlier than 6 hours after the catheter is removed.29 If a traumatic puncture occurs, rivaroxaban administration should be delayed for 24 hours.29 Factors that can increase the risk of developing epidural or spinal hematomas include the use of indwelling epidural catheters, concomitant use of drugs that affect hemostasis (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], platelet inhibitors, and other anticoagulants), a history of traumatic or repeated epidural or spinal punctures, and a history of spinal deformity or spinal surgery.
Rivaroxaban has not been studied in severe hepatic impairment (Child-Pugh C), and in subjects with moderate hepatic impairment (Child-Pugh B), rivaroxaban has led to increased drug exposure, with increased PD effects. Therefore, rivaroxaban should be avoided in patients with moderate to severe hepatic impairment or any hepatic disease associated with coagulopathy.29
Rivaroxaban should also be avoided in patients with severe renal impairment (a creatinine clearance [CrCl] of < 30 mL/min), because increased exposure with increased PD effects is expected. A combined analysis of the RECORD1, 2, and 3 trials did not show an increased bleeding risk for patients with moderate renal impairment (CrCl 30-50 mL/min) taking rivaroxaban. However, such patients should be observed for signs or symptoms of bleeding. Discontinuation should be considered in any patient who develops acute renal failure while taking rivaroxaban.
Drug interaction studies found that the concomitant use of rivaroxaban with drugs that are combined P-glycoprotein (P-gp) and CYP3A4 inhibitors led to increased rivaroxaban exposure and PD effects (ie, Factor Xa inhibition and prolonged prothrombin time). Rivaroxaban exposure was increased significantly when the drug was administered with combined P-gp and strong CYP3A4 inhibitors (eg, azole antifungal agents and protease inhibitors). Therefore, coadministration of rivaroxaban with these agents should be avoided, particularly in patients with any degree of renal impairment, because bleeding risk may increase. In cases where a change in exposure is considered unlikely to affect bleeding risk (ie, coadministration of weaker combined P-gp and CYP3A4 inhibitors, such as clarithromycin and erythromycin), no precautions are necessary.
If bleeding occurs during treatment with rivaroxaban, it may be appropriate to temporarily discontinue the drug and start supportive care. Rivaroxaban has a relatively short half-life (5-9 hours in healthy subjects aged 20-45 years and 11-13 hours in the elderly), meaning that drug effect decreases relatively quickly compared with warfarin. There is currently no direct antidote for rivaroxaban, but a study in healthy human subjects demonstrated that administration of prothrombin complex concentrates may be a potential option.34 Absolute contraindications to rivaroxaban treatment include patients with active pathological bleeding and those with severe hypersensitivity to the drug.
RIVAROXABAN ECONOMICS
Despite its high cost, economic analyses indicate that enoxaparin is a cost-effective agent for VTE prophylaxis compared with warfarin, which is well known to be inexpensive.35 An economic analysis that took into account prophylaxis failures and treatment complications as well as the direct costs associated with medical services, drugs, and laboratory tests showed a cost advantage for enoxaparin over warfarin that lasted for a substantial amount of time (19-31 days after hospital discharge).35
An economic model that followed patients for 1 year postsurgery specifically evaluated the costs associated with symptomatic VTE and major bleeding events in the RECORD trials, assuming the cost of rivaroxaban to be similar to that of enoxaparin 40 mg.36 Cost savings for rivaroxaban over enoxaparin were $82 to $291 per patient, depending on the indication (TKR or THR) and regimen, with cost savings increasing further if the costs of home nursing or training patients to self-administer enoxaparin are included.36 This economic model was also applied to THR and TKR figures from 2005 to show the global cost-effectiveness of rivaroxaban.37 This analysis showed that based on RECORD1, the use of rivaroxaban was associated with an average cost savings of $82 per patient and a reduction of 6 symptomatic events per 1,000 patients undergoing THR. Based on RECORD3, the use of rivaroxaban was associated with a cost savings of $284 per patient and a reduction of 18 symptomatic events per 1,000 patients undergoing TKR.
A later cost-effectiveness analysis by Duran and colleagues, published after FDA approval, included U.S. pricing information.38 In patients receiving extended-duration prophylaxis (35 days) following THR, rivaroxaban was associated with a cost savings of $695 per patient compared with enoxaparin. Compared with 14 days of enoxaparin, extended-duration rivaroxaban (35 days) prevented about 10 additional symptomatic VTE events per 1,000 patients and saved $244 per patient. In patients undergoing TKR, short-duration rivaroxaban
(10-14 days) prevented about 13 additional symptomatic VTE events per 1,000 patients while saving $411 per patient compared with short-duration enoxaparin (10-14 days). It should be noted that statistically significant differences were detected only in the base-case economic analysis, and differences in PE and bleeding events were not captured.
Conclusion
The prevalence of VTE after total joint replacement continues to pose a significant burden to our health care system in terms of morbidity, mortality, and health care costs. Novel anticoagulants such as rivaroxaban, which is now FDA-approved, represent promising alternatives to the traditional agents used for VTE prophylaxis. In addition to its superior efficacy and comparable safety profile to enoxaparin, rivaroxaban’s oral route of administration and straightforward management make it a promising alternative. In particular, the lack of a requirement for routine coagulation monitoring or dose adjustment should simplify treatment with the potential to improve compliance and adherence.
Some questions remain unanswered, such as lack of a direct antidote or widely accepted reversibility technique and how to monitor or assess anticoagulation status in emergency situations, such as overdose or pathologic bleeding. Importantly, early cost-effectiveness analyses indicate that rivaroxaban is cost-effective and potentially even cost-saving compared with enoxaparin and warfarin.Careful postmarketing surveillance will need to be conducted to establish its safety in real-world settings.
Acknowledgments
The authors would like to acknowledge Matthew Romo, PharmD, who provided editorial support with funding from Janssen Scientific Affairs, LLC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: Preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
2. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in Orthopedic Surgery Patients: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 9th ed. Chest. 2012(suppl 2);141:e278S-e325S.
3. Fanikos J, Piazza G, Zayaruzny M, Goldhaber SZ. Long-term complications of medical patients with hospital-acquired venous thromboembolism. Thromb Haemost. 2009;102(4):688-693.
4. Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257-2264.
5. Ruppert A, Lees M, Steinle T. Clinical burden of venous thromboembolism. Curr Med Res Opin. 2010;26(10):2465-2473.
6. FDA approves Xarelto® (rivaroxaban tablets) for the prophylaxis of deep vein thrombosis which may lead to a pulmonary embolism in patients undergoing knee or hip replacement surgery. BayNews.
July 1, 2011.
7. Oster G, Ollendorf DA, Vera-Llonch M, Hagiwara M, Berger A, Edelsberg J. Economic consequences of venous thromboembolism following major orthopedic surgery. Ann Pharmacother. 2004;38(3):377-382.
8. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13(6):475-486.
9. Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28(4):465-476.
10. MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome.
Am J Health Syst Pharm. 2006;63(suppl 20):S5-S15.
11. American Academy of Orthopaedic Surgeons. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty: evidence-based guidelines and evidence report. American Academy of Orthopaedic Surgeons Website. http://www.aaos.org/research/guidelines/VTE/VTE_full_guideline.pdf. Accessed January 29, 2014.
12. Friedman RJ, Gallus A, Gil-Garay E, Fitzgerald G, Cushner F. Practice patterns in the use of venous thromboembolism prophylaxis after total joint arthroplasty—insights from the Multinational Global Orthopaedic Registry (GLORY). Am J Orthop (Belle Mead NJ). 2010;39(suppl 9):14-21.
13. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):160S-198S.
14. Nordstrom BL, Kachroo S, Fraeman KH, et al. Warfarin prophylaxis in patients after total knee or hip arthroplasty—international normalized ratio patterns and venous thromboembolism. Curr Med Res Opin. 2011;27(10):1973-1985.
15. Lovenox [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2013.
16. Colwell CW Jr, Pulido P, Hardwick ME, Morris BA. Patient compliance with outpatient prophylaxis: An observational study. Orthopedics. 2005;28(2):143-147.
17. Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009;48(1):1-22.
18. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604.
19. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE-2 investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet. 2010;375(9717):807-815.
20. Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487-2498.
21. Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet. 2007;370(9591):949-956.
22. Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105(4):721-729.
23. Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: The RE-MODEL randomized trial. J Thromb Haemost. 2007;5(11):2178-2185.
24. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24(1):1-9.
25. Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty.
N Engl J Med. 2008;358(26):2765-2775.
26. Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: A double-blind, randomised controlled trial. Lancet. 2008;372(9632):31-39.
27. Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty.
N Engl J Med. 2008;358(26):2776-2786.
28. Turpie AG, Lassen MR, Davidson BL, et al; RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): A randomised trial. Lancet. 2009;373(9676):1673-1680.
29. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
30. Cardiovascular and Renal Drugs Advisory Committee. FDA Advisory Committee Briefing Document. 2009. http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4418b1-01-FDA.pdf. Accessed January 15, 2012.
31. Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105(3):444-453.
32. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
33. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
34. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
35. Friedman RJ, Dunsworth GA. Cost analyses of extended prophylaxis with enoxaparin after hip arthroplasty. Clin Orthop Rel Res. 2000;370:171-182.
36. Kwong LM. Cost-effectiveness of rivaroxaban after total hip or total knee arthroplasty. Am J Manag Care. 2011;17(suppl 1):S22-S26.
37. Friedman RJ, Sengupta N, Lees M. Economic impact of venous thromboembolism after hip and knee arthroplasty: Potential impact of rivaroxaban. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):299-306.
38. Duran A, Sengupta N, Diamantopoulos A, Forster F, Kwong L, Lees M. Cost and outcomes associated with rivaroxaban vs enoxaparin for the prevention of postsurgical venous thormboembolism from a US payer’s perspective. J Med Econ. 2011;14(6):824-834.
The number of total hip and knee replacement surgeries is increasing, and consequently, more patients are at risk of venous thromboembolism (VTE).1 Without thromboprophylaxis, the incidence of proximal deep vein thrombosis (DVT) is about 18% to 36% following total hip replacement (THR) and about 5% to 22% following total knee replacement (TKR). The incidence of total pulmonary embolism (PE) has been estimated at 0.9% to 28% in THR and 1.5% to 10% in TKR, and the incidence of fatal PE has been estimated to be as high as 2% following total joint replacement surgery.2 Despite the availability of effective anticoagulant agents for thromboprophylaxis, symptomatic VTE continues to occur in 1.3% to 10.0% of patients in the 3-month period following joint replacement surgery.2
VTE following THR or TKR represents a significant source of morbidity and mortality as well as a financial burden on the health care system. This burden is increased further by the complications of VTE—including a high rate of recurrence, postthrombotic syndrome (PTS), and pulmonary hypertension—which may be more debilitating than the primary event.3-5 Effective prophylaxis of VTE is paramount in reducing the incidence of these consequences.
Rivaroxaban, a Factor Xa inhibitor, is a novel anticoagulant that has recently been approved by the U.S. Food and Drug Administration (FDA) for prophylaxis of DVT in patients undergoing THR or TKR as well as for reduction of the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AFib).6 Clinical trials have shown that rivaroxaban is superior to enoxaparin, the standard of care, in preventing VTE after total joint replacement, and cost-effectiveness studies have demonstrated that rivaroxaban may potentially be a cost-saving agent.
This clinical review will address the economic and public health burden of VTE following THR and TKR and evaluate the novel anticoagulants that have been studied for this indication, including rivaroxaban. The safety and efficacy of rivaroxaban will be discussed, along with cost-effectiveness issues and practical management information.
The Economic Burden of VTE
VTE is the most common cause of hospital readmission following THR.2 The occurrence of VTE is reported to significantly increase the length of hospital stays and health care charges in patients undergoing TKR or THR. For example, one study reported health care charges for the index admission that were $9,297 higher for inpatients who experienced VTE compared with patients with TKR who did not have a VTE (P = .02).7 Similarly, for patients with THR, health care charges were $25,853 higher for inpatients who experienced VTE compared with patients who did not (P < .01).7
According to Spyropoulos and Lin, the total annual health care cost of a VTE (not limited to orthopedic patients) is $7,594 to $16,644, depending on the type of VTE (DVT or PE) and whether it was the primary or secondary diagnosis on discharge.8 These costs are significant, especially when multiplied by the number of THR and TKR surgeries performed in the U.S. Furthermore, VTE complications, such as PTS, represent an additional driver of health care expenditures.5,9,10
VTE Prophylaxis Guidelines
Both the American College of Chest Physicians (ACCP) and the American Academy of Orthopaedic Surgeons (AAOS) have published evidence-based guidelines for the prevention of VTE following total joint replacement.2,11 The 2012 version of the ACCP guidelines recommended the routine use of low-molecular-weight heparins (LMWHs), fondaparinux, low-dose unfractionated heparin, aspirin, adjusted-dose warfarin, rivaroxaban, apixaban, or dabigatran for thromboprophylaxis following THR or TKR. In contrast, the 2011 version of the AAOS guidelines makes no specific recommendations for pharmacologic agents.11 Despite the availability of evidence-based guidelines with specific recommendations, physician compliance with these guidelines is low.12
Before the approval of rivaroxaban, the vitamin K antagonist warfarin was the only available oral option for thromboprophylaxis following THR or TKR. The use of warfarin can be challenging, because it requires frequent monitoring and maintaining a patient within a specified international normalized ratio (INR) range. Several factors may influence time-in-therapeutic range, including drug-drug and drug-food interactions and genetic polymorphisms of vitamin K epoxide reductase complex subunit 1 (VKORC1) and cytochrome P450 (CYP)2C9.13 Warfarin does not provide short-term prophylaxis because of its delayed onset of action. It has been reported that 5 days after THR and TKR only about 30% of patients are within an INR of 2.0 to 3.0.14 Patients with an INR below 2.0 are at a 4- to 5-fold increased risk for VTE.14
Low-molecular-weight heparins, such as enoxaparin, do not require routine monitoring and have minimal drug interactions.15 The main drawback of LMWHs is their injectable mode of administration, which may influence prescribing habits and medication adherence. Patient education plays an essential role in ensuring adherence to LMWHs following orthopedic surgery.16
New Oral Anticoagulants
The new oral anticoagulants that have completed phase 3 trials for VTE prophylaxis following total joint replacement surgery include the selective Factor Xa inhibitors rivaroxaban and apixaban and the direct thrombin inhibitor dabigatran etexilate. Unlike warfarin, these agents do not require routine monitoring, a result of their predictable pharmacokinetic/pharmacodynamic (PK/PD) relationship, and they have few clinically significant drug interactions.17 Dabigatran and apixaban are not currently FDA approved for VTE prophylaxis following total joint replacement surgery; however, rivaroxaban was approved by the FDA in July 2011 for this indication.6
Apixaban
The Apixaban or Enoxaparin for Thromboprophylaxis After Knee Replacement (ADVANCE)-1 and ADVANCE-2 trials evaluated the use of Apixaban for thromboprophylaxis in patients undergoing TKR.18,19 In the ADVANCE-1 trial, patients undergoing TKR were randomized to receive either apixaban 2.5 mg orally twice daily or the North American preferred dosing of enoxaparin, 30 mg subcutaneously (SC) twice daily, with both agents started 12 to 24 hours after surgery and then taken for 10 to 14 days.18
Apixaban did not meet the prespecified statistical criteria for noninferiority compared with enoxaparin, although the rates of the primary efficacy endpoint (composite of VTE and all-cause mortality) were similar: 9.0% and 8.8% of patients, respectively. However, apixaban showed lower rates of clinically relevant bleeding and a similar adverse event (AE) profile compared with enoxaparin.
In the ADVANCE-2 trial, patients undergoing TKR received either apixaban 2.5 mg orally twice daily or enoxaparin 40 mg SC once daily for 10 to 14 days.19 The primary efficacy outcome (composite of total VTE and all-cause mortality) occurred at significantly lower rates in patients receiving apixaban (15% and 24%, respectively; relative risk [RR] 0.62; 95% confidence interval [CI] 0.51-0.74; P < .0001). Rates of major or clinically relevant nonmajor bleeding events occurred at similar rates between the 2 groups.
In the ADVANCE-3 trial, patients undergoing THR received either apixaban 2.5 orally twice daily or enoxaparin 40 mg SC once daily for 35 days.20 The primary efficacy outcome (composite of total VTE and all-cause mortality) occurred at significantly lower rates in patients receiving apixaban (1.4% and 3.9%, respectively; RR 0.36; 95% CI 0.22-0.54; P < .001). Rates of major or clinically relevant nonmajor bleeding events occurred at similar rates between both groups.
Dabigatran etexilate
Dabigatran etexilate has been evaluated in patients undergoing TKR in the Oral Dabigatran Etexilate vs Subcutaneous Enoxaparin for the Prevention of Venous Thromboembolism After Total Knee Replacement (RE-MODEL) and Oral Thrombin Inhibitor Dabigatran Etexilate vs North American Enoxaparin Regimen for Prevention of Venous Thromboembolism After Total Knee Replacement (RE-MOBILIZE) studies and in patients undergoing THR in the Oral Dabigatran vs Enoxaparin for Thromboprophylaxis After Primary Total Hip Arthroplasty (RE-NOVATE)-1 and RE-NOVATE‑2 trials.21-24
In RE-MODEL, patients undergoing TKR received either 150 mg or 220 mg of dabigatran etexilate once daily (starting with a half dose 1 to 4 hours after surgery) or enoxaparin 40 mg once daily (started the evening before surgery) for 6 to 10 days.23 Both doses of dabigatran had a similar incidence of the composite of total VTE and mortality and major bleeding. In RE-MOBILIZE, patients undergoing TKR received either 150 mg or 220 mg of dabigatran etexilate once daily or the North American regimen of enoxaparin, 30 mg SC twice daily for 12 to 15 days.24 Both dabigatran regimens had a significantly higher incidence of the composite of total VTE, failing to establish noninferiority to enoxaparin. The incidence of major bleeding was not significantly different among the 3 groups.
In RE-NOVATE I, patients undergoing THR received either dabigatran 150 mg or 220 mg twice daily, or enoxaparin 40 mg SC once daily for 28 to 35 days.21 Both doses of dabigatran had a similar incidence of the composite of total VTE and mortality and major bleeding. Because RE-NOVATE I did not include any study sites in North America, a second phase 3 trial, RE-NOVATE II, was conducted to include North American sites. RE-NOVATE II had an identical trial design to RE-NOVATE I and similar results (noninferior efficacy and comparable safety).22
Rivaroxaban
The phase 3 Rivaroxaban vs Enoxaparin for Thromboprophylaxis After Hip Arthroplasty (RECORD) program consisted of 4 double-blind randomized trials that compared the efficacy and safety of oral rivaroxaban to SC enoxaparin in THR (RECORD1 and 2) and TKR (RECORD3 and 4). The RECORD1 and 2 trials compared rivaroxaban 10 mg orally once daily for 31 to 39 days with enoxaparin 40 mg SC once daily (for 31-39 days in RECORD1 and for 10-14 days followed by placebo in RECORD2). The RECORD3 and 4 trials compared 10- to 14-day regimens of rivaroxaban 10 mg orally once daily with enoxaparin 40 mg SC once daily (RECORD3) and 30 mg twice daily (RECORD4). The 4 trials used the same efficacy and safety outcomes (Table).
In the RECORD1, 2, and 3 studies, rivaroxaban significantly reduced the composite of DVT, nonfatal PE, and all-cause mortality, as well as major VTE compared with enoxaparin. Major bleeding events as well as clinically relevant nonmajor bleeding and hemorrhagic wound complications were similar across both groups.25-27
In the RECORD4 study, rivaroxaban significantly reduced the composite of DVT, nonfatal PE, and all-cause mortality, but not major VTE, in patients undergoing TKR. Major bleeding events as well as clinically relevant nonmajor bleeding were numerically higher in the rivaroxaban group, but this was not statistically significant. Hemorrhagic wound complications were similar across both groups.28 This study was not required for approval and was not included in the final FDA-approved package insert.29
In a pooled analysis of the 4 RECORD trials presented at the FDA advisory committee, the incidence of major bleeding was significantly higher in the rivaroxaban group (24 events [0.39%]) compared with enoxaparin (13 events [0.21%]), with a nominal P value of .08 (significant at 10% nominal level) in the total treatment duration pool.30 In a pooled analysis by Turpie and colleagues who used the same data, this difference was not determined to be statistically different.31 In addition, this pooled analysis showed that the incidence of treatment-emergent hemorrhagic wound complications was similar in patients receiving rivaroxaban and enoxaparin and that fewer treatment-emergent serious AEs occurred in patients receiving rivaroxaban compared with patients receiving enoxaparin.31
In the FDA advisory committee, an “isolated signal” for a potentially increased risk of ischemic stroke was identified: In the safety population, ischemic stroke occurred in 5 patients who had received rivaroxaban and 1 patient who received enoxaparin.30 Furthermore, cardiovascular events in the safety population were concentrated around discontinuation of rivaroxaban, which was not the case for enoxaparin. The concern of stroke following rivaroxaban discontinuation was much more robust in Rivaroxaban vs Warfarin in Nonvalvular Atrial Fibrillation (ROCKET-AF), the phase 3 trial that compared rivaroxaban and warfarin for stroke prophylaxis in AFib.32 In the study ROCKET-AF, higher rates of stroke and systemic embolism were observed at the end of the trial among patients discontinuing rivaroxaban and switching to open-label warfarin compared with patients who had been taking warfarin and were transitioned to open-label warfarin. This observation led to a black box warning in the label of rivaroxaban regarding discontinuation of this agent.33
rivaroxaban management
The approved dose of rivaroxaban for the prophylaxis of VTE is 10 mg orally once daily with or without food.29 The first dose should be taken 6 to 10 hours after surgery, once hemostasis has been established. Rivaroxaban should be administered for 35 days to patients undergoing THR and for 12 days to patients undergoing TKR. Tablets may be crushed and administered in a gastric feeding tube, but they must not be administered via feeding tubes that deliver the contents into the proximal small intestine, because reduced drug absorption may result.29
The prescribing information for rivaroxaban includes a black box warning stating that epidural or spinal hematomas have occurred in patients treated with rivaroxaban who are receiving neuraxial (ie, spinal or epidural) anesthesia or undergoing spinal puncture. For such patients, the epidural catheter should not be removed earlier than 18 hours after the last administration of rivaroxaban.29 The next rivaroxaban dose should not be administered earlier than 6 hours after the catheter is removed.29 If a traumatic puncture occurs, rivaroxaban administration should be delayed for 24 hours.29 Factors that can increase the risk of developing epidural or spinal hematomas include the use of indwelling epidural catheters, concomitant use of drugs that affect hemostasis (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], platelet inhibitors, and other anticoagulants), a history of traumatic or repeated epidural or spinal punctures, and a history of spinal deformity or spinal surgery.
Rivaroxaban has not been studied in severe hepatic impairment (Child-Pugh C), and in subjects with moderate hepatic impairment (Child-Pugh B), rivaroxaban has led to increased drug exposure, with increased PD effects. Therefore, rivaroxaban should be avoided in patients with moderate to severe hepatic impairment or any hepatic disease associated with coagulopathy.29
Rivaroxaban should also be avoided in patients with severe renal impairment (a creatinine clearance [CrCl] of < 30 mL/min), because increased exposure with increased PD effects is expected. A combined analysis of the RECORD1, 2, and 3 trials did not show an increased bleeding risk for patients with moderate renal impairment (CrCl 30-50 mL/min) taking rivaroxaban. However, such patients should be observed for signs or symptoms of bleeding. Discontinuation should be considered in any patient who develops acute renal failure while taking rivaroxaban.
Drug interaction studies found that the concomitant use of rivaroxaban with drugs that are combined P-glycoprotein (P-gp) and CYP3A4 inhibitors led to increased rivaroxaban exposure and PD effects (ie, Factor Xa inhibition and prolonged prothrombin time). Rivaroxaban exposure was increased significantly when the drug was administered with combined P-gp and strong CYP3A4 inhibitors (eg, azole antifungal agents and protease inhibitors). Therefore, coadministration of rivaroxaban with these agents should be avoided, particularly in patients with any degree of renal impairment, because bleeding risk may increase. In cases where a change in exposure is considered unlikely to affect bleeding risk (ie, coadministration of weaker combined P-gp and CYP3A4 inhibitors, such as clarithromycin and erythromycin), no precautions are necessary.
If bleeding occurs during treatment with rivaroxaban, it may be appropriate to temporarily discontinue the drug and start supportive care. Rivaroxaban has a relatively short half-life (5-9 hours in healthy subjects aged 20-45 years and 11-13 hours in the elderly), meaning that drug effect decreases relatively quickly compared with warfarin. There is currently no direct antidote for rivaroxaban, but a study in healthy human subjects demonstrated that administration of prothrombin complex concentrates may be a potential option.34 Absolute contraindications to rivaroxaban treatment include patients with active pathological bleeding and those with severe hypersensitivity to the drug.
RIVAROXABAN ECONOMICS
Despite its high cost, economic analyses indicate that enoxaparin is a cost-effective agent for VTE prophylaxis compared with warfarin, which is well known to be inexpensive.35 An economic analysis that took into account prophylaxis failures and treatment complications as well as the direct costs associated with medical services, drugs, and laboratory tests showed a cost advantage for enoxaparin over warfarin that lasted for a substantial amount of time (19-31 days after hospital discharge).35
An economic model that followed patients for 1 year postsurgery specifically evaluated the costs associated with symptomatic VTE and major bleeding events in the RECORD trials, assuming the cost of rivaroxaban to be similar to that of enoxaparin 40 mg.36 Cost savings for rivaroxaban over enoxaparin were $82 to $291 per patient, depending on the indication (TKR or THR) and regimen, with cost savings increasing further if the costs of home nursing or training patients to self-administer enoxaparin are included.36 This economic model was also applied to THR and TKR figures from 2005 to show the global cost-effectiveness of rivaroxaban.37 This analysis showed that based on RECORD1, the use of rivaroxaban was associated with an average cost savings of $82 per patient and a reduction of 6 symptomatic events per 1,000 patients undergoing THR. Based on RECORD3, the use of rivaroxaban was associated with a cost savings of $284 per patient and a reduction of 18 symptomatic events per 1,000 patients undergoing TKR.
A later cost-effectiveness analysis by Duran and colleagues, published after FDA approval, included U.S. pricing information.38 In patients receiving extended-duration prophylaxis (35 days) following THR, rivaroxaban was associated with a cost savings of $695 per patient compared with enoxaparin. Compared with 14 days of enoxaparin, extended-duration rivaroxaban (35 days) prevented about 10 additional symptomatic VTE events per 1,000 patients and saved $244 per patient. In patients undergoing TKR, short-duration rivaroxaban
(10-14 days) prevented about 13 additional symptomatic VTE events per 1,000 patients while saving $411 per patient compared with short-duration enoxaparin (10-14 days). It should be noted that statistically significant differences were detected only in the base-case economic analysis, and differences in PE and bleeding events were not captured.
Conclusion
The prevalence of VTE after total joint replacement continues to pose a significant burden to our health care system in terms of morbidity, mortality, and health care costs. Novel anticoagulants such as rivaroxaban, which is now FDA-approved, represent promising alternatives to the traditional agents used for VTE prophylaxis. In addition to its superior efficacy and comparable safety profile to enoxaparin, rivaroxaban’s oral route of administration and straightforward management make it a promising alternative. In particular, the lack of a requirement for routine coagulation monitoring or dose adjustment should simplify treatment with the potential to improve compliance and adherence.
Some questions remain unanswered, such as lack of a direct antidote or widely accepted reversibility technique and how to monitor or assess anticoagulation status in emergency situations, such as overdose or pathologic bleeding. Importantly, early cost-effectiveness analyses indicate that rivaroxaban is cost-effective and potentially even cost-saving compared with enoxaparin and warfarin.Careful postmarketing surveillance will need to be conducted to establish its safety in real-world settings.
Acknowledgments
The authors would like to acknowledge Matthew Romo, PharmD, who provided editorial support with funding from Janssen Scientific Affairs, LLC.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
References
1. Iorio R, Robb WJ, Healy WL, et al. Orthopaedic surgeon workforce and volume assessment for total hip and knee replacement in the United States: Preparing for an epidemic. J Bone Joint Surg Am. 2008;90(7):1598-1605.
2. Falck-Ytter Y, Francis CW, Johanson NA, et al; American College of Chest Physicians. Prevention of VTE in Orthopedic Surgery Patients: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, 9th ed. Chest. 2012(suppl 2);141:e278S-e325S.
3. Fanikos J, Piazza G, Zayaruzny M, Goldhaber SZ. Long-term complications of medical patients with hospital-acquired venous thromboembolism. Thromb Haemost. 2009;102(4):688-693.
4. Pengo V, Lensing AW, Prins MH, et al; Thromboembolic Pulmonary Hypertension Study Group. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257-2264.
5. Ruppert A, Lees M, Steinle T. Clinical burden of venous thromboembolism. Curr Med Res Opin. 2010;26(10):2465-2473.
6. FDA approves Xarelto® (rivaroxaban tablets) for the prophylaxis of deep vein thrombosis which may lead to a pulmonary embolism in patients undergoing knee or hip replacement surgery. BayNews.
July 1, 2011.
7. Oster G, Ollendorf DA, Vera-Llonch M, Hagiwara M, Berger A, Edelsberg J. Economic consequences of venous thromboembolism following major orthopedic surgery. Ann Pharmacother. 2004;38(3):377-382.
8. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13(6):475-486.
9. Ashrani AA, Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28(4):465-476.
10. MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome.
Am J Health Syst Pharm. 2006;63(suppl 20):S5-S15.
11. American Academy of Orthopaedic Surgeons. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty: evidence-based guidelines and evidence report. American Academy of Orthopaedic Surgeons Website. http://www.aaos.org/research/guidelines/VTE/VTE_full_guideline.pdf. Accessed January 29, 2014.
12. Friedman RJ, Gallus A, Gil-Garay E, Fitzgerald G, Cushner F. Practice patterns in the use of venous thromboembolism prophylaxis after total joint arthroplasty—insights from the Multinational Global Orthopaedic Registry (GLORY). Am J Orthop (Belle Mead NJ). 2010;39(suppl 9):14-21.
13. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):160S-198S.
14. Nordstrom BL, Kachroo S, Fraeman KH, et al. Warfarin prophylaxis in patients after total knee or hip arthroplasty—international normalized ratio patterns and venous thromboembolism. Curr Med Res Opin. 2011;27(10):1973-1985.
15. Lovenox [package insert]. Bridgewater, NJ: sanofi-aventis U.S. LLC; 2013.
16. Colwell CW Jr, Pulido P, Hardwick ME, Morris BA. Patient compliance with outpatient prophylaxis: An observational study. Orthopedics. 2005;28(2):143-147.
17. Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet. 2009;48(1):1-22.
18. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604.
19. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE-2 investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): A randomised double-blind trial. Lancet. 2010;375(9717):807-815.
20. Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487-2498.
21. Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: A randomised, double-blind, non-inferiority trial. Lancet. 2007;370(9591):949-956.
22. Eriksson BI, Dahl OE, Huo MH, et al; RE-NOVATE II Study Group. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost. 2011;105(4):721-729.
23. Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: The RE-MODEL randomized trial. J Thromb Haemost. 2007;5(11):2178-2185.
24. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24(1):1-9.
25. Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty.
N Engl J Med. 2008;358(26):2765-2775.
26. Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: A double-blind, randomised controlled trial. Lancet. 2008;372(9632):31-39.
27. Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty.
N Engl J Med. 2008;358(26):2776-2786.
28. Turpie AG, Lassen MR, Davidson BL, et al; RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): A randomised trial. Lancet. 2009;373(9676):1673-1680.
29. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2009.
30. Cardiovascular and Renal Drugs Advisory Committee. FDA Advisory Committee Briefing Document. 2009. http://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4418b1-01-FDA.pdf. Accessed January 15, 2012.
31. Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost. 2011;105(3):444-453.
32. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
33. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc; 2011.
34. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: A randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573-1579.
35. Friedman RJ, Dunsworth GA. Cost analyses of extended prophylaxis with enoxaparin after hip arthroplasty. Clin Orthop Rel Res. 2000;370:171-182.
36. Kwong LM. Cost-effectiveness of rivaroxaban after total hip or total knee arthroplasty. Am J Manag Care. 2011;17(suppl 1):S22-S26.
37. Friedman RJ, Sengupta N, Lees M. Economic impact of venous thromboembolism after hip and knee arthroplasty: Potential impact of rivaroxaban. Expert Rev Pharmacoecon Outcomes Res. 2011;11(3):299-306.
38. Duran A, Sengupta N, Diamantopoulos A, Forster F, Kwong L, Lees M. Cost and outcomes associated with rivaroxaban vs enoxaparin for the prevention of postsurgical venous thormboembolism from a US payer’s perspective. J Med Econ. 2011;14(6):824-834.