User login
Prolonged dual-antiplatelet therapy after PCI challenged
WASHINGTON – Guidelines were recently modified to permit shorter duration of dual-antiplatelet therapy (DAPT) after percutaneous coronary intervention, but a series of ongoing trials are evaluating whether DAPT can be abandoned altogether in many if not most percutaneous coronary intervention (PCI) patients, according to a review of this major potential change in direction presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The 1-year duration of dual-antiplatelet therapy post PCI with a drug eluting stent is based on anecdotal historical data,” asserted Patrick W. Serruys, MD, PhD, professor of cardiology, Imperial College, London. Citing several sets of data consistent with the conclusion that single agents provide adequate protection against thrombus formation but reduced risk of bleeding relative to DAPT, he suggested that it is now critical to challenge the old standard.
It has long been understood that greater protection against thrombus formation with more aggressive antiplatelet therapy is purchased with a higher risk of bleeding, but there appears to be a fundamental change in orientation. Several new pieces of evidence, including data showing that shorter duration of DAPT is as good as longer duration, has placed this trade-off in doubt at least over the longer term.
To some degree, the current standard was based on the premise that thrombotic events are more important than bleeding events, according to Usman Baber, MD, assistant professor of cardiology, Icahn School of Medicine at Mount Sinai, New York. He said, “That thought process really dominated thinking for many years, but this is completely unsupported by the data.” Instead, he noted that hazard ratios after thrombotic and bleeding events are almost identical, but the risk of death after bleeding is more persistent, while risk of ischemic events typically diminishes after an initial peak.
There is no shortage of studies that have attempted to determine the ideal combination and duration of antiplatelet therapies after PCI, but the heterogeneity of study design has prohibited definitive conclusions. In particular, Dr. Serruys suggested that there is no level 1 evidence confirming the value of adding aspirin, which he emphasized has a relatively nonspecific effect, over that of P2Y12 inhibitor alone.
In the design phase of the GLOBAL LEADERS trial, Dr. Serruys recounted, he first argued for a design in which aspirin was eliminated altogether and then for a protocol with only a single week of aspirin, but was met with strong objections each time. In the end, the experimental protocol calls for 1 month of aspirin plus ticagrelor before patients are continued on ticagrelor alone. This is being compared with the current standard, which is aspirin plus ticagrelor or clopidogrel for 12 months followed by another 12 months of aspirin alone.
GLOBAL LEADERS is an all-comers trial in which patients are randomized before PCI. All patients at the 131 participating centers in 18 countries are receiving the same stent (BioMatrix Flex). The primary endpoint is all-cause mortality, and enrollment is completed. The results are expected in November of this year.
There are numerous other studies addressing the same question. Like GLOBAL LEADERS, the TWILIGHT trial is also investigator-initiated and is near the halfway mark for a 9,000-patient enrollment. In this study, patients are being randomized to aspirin plus ticagrelor or ticagrelor alone after they have achieved a successful placement of a drug-eluting stent. This trial, however, is restricted to those with diabetes, chronic kidney disease, or other high-risk features. The primary endpoint is major bleeding. Completion is expected in 2019.
The SMART-CHOICE trial is enrolling roughly 5,000 PCI patients receiving a drug-eluting stent. Patients are being randomized to a P2Y12 antagonist monotherapy plus aspirin or the P2Y12 antagonist alone. The primary endpoint is a composite of major adverse cardiovascular events as well as major bleeding events.
After the STOP DAPT trial showed that 3 months of DAPT after PCI was as safe as prolonged DAPT in patients receiving a everolimus-eluting chromium-cobalt stent (Cardiovasc Interv Ther. 2016;31:196-209), the same group of Japanese investigators conceived the STOP-DAPT2 trial. In this trial, 3,000 patients are being randomized a standard DAPT or clopidogrel monotherapy beginning 1 month after PCI. The primary outcome is similar to that of SMART-CHOICE.
In yet another trial cited by Dr. Serruys, patients will receive DAPT only if the PCI outcome is considered suboptimal. For those judged to have a good result, patients will receive ticagrelor alone. Outcomes at the end of 1 year will be monitored.
The movement toward antiplatelet monotherapy is driven by recognition that “the need to mitigate the risk of bleeding is an important as the need to mitigate thrombosis,” Dr. Baber explained. Like Dr. Serruys, he believes it is important to challenge the standard.
“By testing single, specific, and potent antiplatelet therapy and getting rid of the old and nonspecific platelet drug called acetylsalicylic acid, we may be able to simplify risk management after PCI,” agreed Dr. Serruys. If, as expected, the GLOBAL LEADERS and other monotherapy antiplatelet trials meet their endpoints, it will mean a major evolution in postprocedural risk management.
Dr. Serruys reported no financial relationships to disclose.
WASHINGTON – Guidelines were recently modified to permit shorter duration of dual-antiplatelet therapy (DAPT) after percutaneous coronary intervention, but a series of ongoing trials are evaluating whether DAPT can be abandoned altogether in many if not most percutaneous coronary intervention (PCI) patients, according to a review of this major potential change in direction presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The 1-year duration of dual-antiplatelet therapy post PCI with a drug eluting stent is based on anecdotal historical data,” asserted Patrick W. Serruys, MD, PhD, professor of cardiology, Imperial College, London. Citing several sets of data consistent with the conclusion that single agents provide adequate protection against thrombus formation but reduced risk of bleeding relative to DAPT, he suggested that it is now critical to challenge the old standard.
It has long been understood that greater protection against thrombus formation with more aggressive antiplatelet therapy is purchased with a higher risk of bleeding, but there appears to be a fundamental change in orientation. Several new pieces of evidence, including data showing that shorter duration of DAPT is as good as longer duration, has placed this trade-off in doubt at least over the longer term.
To some degree, the current standard was based on the premise that thrombotic events are more important than bleeding events, according to Usman Baber, MD, assistant professor of cardiology, Icahn School of Medicine at Mount Sinai, New York. He said, “That thought process really dominated thinking for many years, but this is completely unsupported by the data.” Instead, he noted that hazard ratios after thrombotic and bleeding events are almost identical, but the risk of death after bleeding is more persistent, while risk of ischemic events typically diminishes after an initial peak.
There is no shortage of studies that have attempted to determine the ideal combination and duration of antiplatelet therapies after PCI, but the heterogeneity of study design has prohibited definitive conclusions. In particular, Dr. Serruys suggested that there is no level 1 evidence confirming the value of adding aspirin, which he emphasized has a relatively nonspecific effect, over that of P2Y12 inhibitor alone.
In the design phase of the GLOBAL LEADERS trial, Dr. Serruys recounted, he first argued for a design in which aspirin was eliminated altogether and then for a protocol with only a single week of aspirin, but was met with strong objections each time. In the end, the experimental protocol calls for 1 month of aspirin plus ticagrelor before patients are continued on ticagrelor alone. This is being compared with the current standard, which is aspirin plus ticagrelor or clopidogrel for 12 months followed by another 12 months of aspirin alone.
GLOBAL LEADERS is an all-comers trial in which patients are randomized before PCI. All patients at the 131 participating centers in 18 countries are receiving the same stent (BioMatrix Flex). The primary endpoint is all-cause mortality, and enrollment is completed. The results are expected in November of this year.
There are numerous other studies addressing the same question. Like GLOBAL LEADERS, the TWILIGHT trial is also investigator-initiated and is near the halfway mark for a 9,000-patient enrollment. In this study, patients are being randomized to aspirin plus ticagrelor or ticagrelor alone after they have achieved a successful placement of a drug-eluting stent. This trial, however, is restricted to those with diabetes, chronic kidney disease, or other high-risk features. The primary endpoint is major bleeding. Completion is expected in 2019.
The SMART-CHOICE trial is enrolling roughly 5,000 PCI patients receiving a drug-eluting stent. Patients are being randomized to a P2Y12 antagonist monotherapy plus aspirin or the P2Y12 antagonist alone. The primary endpoint is a composite of major adverse cardiovascular events as well as major bleeding events.
After the STOP DAPT trial showed that 3 months of DAPT after PCI was as safe as prolonged DAPT in patients receiving a everolimus-eluting chromium-cobalt stent (Cardiovasc Interv Ther. 2016;31:196-209), the same group of Japanese investigators conceived the STOP-DAPT2 trial. In this trial, 3,000 patients are being randomized a standard DAPT or clopidogrel monotherapy beginning 1 month after PCI. The primary outcome is similar to that of SMART-CHOICE.
In yet another trial cited by Dr. Serruys, patients will receive DAPT only if the PCI outcome is considered suboptimal. For those judged to have a good result, patients will receive ticagrelor alone. Outcomes at the end of 1 year will be monitored.
The movement toward antiplatelet monotherapy is driven by recognition that “the need to mitigate the risk of bleeding is an important as the need to mitigate thrombosis,” Dr. Baber explained. Like Dr. Serruys, he believes it is important to challenge the standard.
“By testing single, specific, and potent antiplatelet therapy and getting rid of the old and nonspecific platelet drug called acetylsalicylic acid, we may be able to simplify risk management after PCI,” agreed Dr. Serruys. If, as expected, the GLOBAL LEADERS and other monotherapy antiplatelet trials meet their endpoints, it will mean a major evolution in postprocedural risk management.
Dr. Serruys reported no financial relationships to disclose.
WASHINGTON – Guidelines were recently modified to permit shorter duration of dual-antiplatelet therapy (DAPT) after percutaneous coronary intervention, but a series of ongoing trials are evaluating whether DAPT can be abandoned altogether in many if not most percutaneous coronary intervention (PCI) patients, according to a review of this major potential change in direction presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
“The 1-year duration of dual-antiplatelet therapy post PCI with a drug eluting stent is based on anecdotal historical data,” asserted Patrick W. Serruys, MD, PhD, professor of cardiology, Imperial College, London. Citing several sets of data consistent with the conclusion that single agents provide adequate protection against thrombus formation but reduced risk of bleeding relative to DAPT, he suggested that it is now critical to challenge the old standard.
It has long been understood that greater protection against thrombus formation with more aggressive antiplatelet therapy is purchased with a higher risk of bleeding, but there appears to be a fundamental change in orientation. Several new pieces of evidence, including data showing that shorter duration of DAPT is as good as longer duration, has placed this trade-off in doubt at least over the longer term.
To some degree, the current standard was based on the premise that thrombotic events are more important than bleeding events, according to Usman Baber, MD, assistant professor of cardiology, Icahn School of Medicine at Mount Sinai, New York. He said, “That thought process really dominated thinking for many years, but this is completely unsupported by the data.” Instead, he noted that hazard ratios after thrombotic and bleeding events are almost identical, but the risk of death after bleeding is more persistent, while risk of ischemic events typically diminishes after an initial peak.
There is no shortage of studies that have attempted to determine the ideal combination and duration of antiplatelet therapies after PCI, but the heterogeneity of study design has prohibited definitive conclusions. In particular, Dr. Serruys suggested that there is no level 1 evidence confirming the value of adding aspirin, which he emphasized has a relatively nonspecific effect, over that of P2Y12 inhibitor alone.
In the design phase of the GLOBAL LEADERS trial, Dr. Serruys recounted, he first argued for a design in which aspirin was eliminated altogether and then for a protocol with only a single week of aspirin, but was met with strong objections each time. In the end, the experimental protocol calls for 1 month of aspirin plus ticagrelor before patients are continued on ticagrelor alone. This is being compared with the current standard, which is aspirin plus ticagrelor or clopidogrel for 12 months followed by another 12 months of aspirin alone.
GLOBAL LEADERS is an all-comers trial in which patients are randomized before PCI. All patients at the 131 participating centers in 18 countries are receiving the same stent (BioMatrix Flex). The primary endpoint is all-cause mortality, and enrollment is completed. The results are expected in November of this year.
There are numerous other studies addressing the same question. Like GLOBAL LEADERS, the TWILIGHT trial is also investigator-initiated and is near the halfway mark for a 9,000-patient enrollment. In this study, patients are being randomized to aspirin plus ticagrelor or ticagrelor alone after they have achieved a successful placement of a drug-eluting stent. This trial, however, is restricted to those with diabetes, chronic kidney disease, or other high-risk features. The primary endpoint is major bleeding. Completion is expected in 2019.
The SMART-CHOICE trial is enrolling roughly 5,000 PCI patients receiving a drug-eluting stent. Patients are being randomized to a P2Y12 antagonist monotherapy plus aspirin or the P2Y12 antagonist alone. The primary endpoint is a composite of major adverse cardiovascular events as well as major bleeding events.
After the STOP DAPT trial showed that 3 months of DAPT after PCI was as safe as prolonged DAPT in patients receiving a everolimus-eluting chromium-cobalt stent (Cardiovasc Interv Ther. 2016;31:196-209), the same group of Japanese investigators conceived the STOP-DAPT2 trial. In this trial, 3,000 patients are being randomized a standard DAPT or clopidogrel monotherapy beginning 1 month after PCI. The primary outcome is similar to that of SMART-CHOICE.
In yet another trial cited by Dr. Serruys, patients will receive DAPT only if the PCI outcome is considered suboptimal. For those judged to have a good result, patients will receive ticagrelor alone. Outcomes at the end of 1 year will be monitored.
The movement toward antiplatelet monotherapy is driven by recognition that “the need to mitigate the risk of bleeding is an important as the need to mitigate thrombosis,” Dr. Baber explained. Like Dr. Serruys, he believes it is important to challenge the standard.
“By testing single, specific, and potent antiplatelet therapy and getting rid of the old and nonspecific platelet drug called acetylsalicylic acid, we may be able to simplify risk management after PCI,” agreed Dr. Serruys. If, as expected, the GLOBAL LEADERS and other monotherapy antiplatelet trials meet their endpoints, it will mean a major evolution in postprocedural risk management.
Dr. Serruys reported no financial relationships to disclose.
EXPERT ANALYSIS FROM CRT 2017
Aspirin use linked to increased ICH in trauma patients
WAIKOLOA, HAWAII – Among a group of anticoagulated trauma patients, those on aspirin had the highest rate and risk of intracranial hemorrhage (ICH), while those on novel oral anticoagulants were not at higher risk for ICH, ICH progression, or death, a multicenter study found.
“The number of patients on warfarin and antiplatelet agents has significantly increased over time,” Leslie Kobayashi, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “These oral antithrombotic agents have been associated with poor outcomes following traumatic injury, including increased rates of intracranial hemorrhage, increased progression of intracranial hemorrhage, and increased mortality.”
In a prospective, multicenter observational study conducted by the AAST’s Multi-institutional Trials Committee, Dr. Kobayashi and her associates set out identify injury patterns and outcomes in trauma patients taking the NOAs, and to test their hypothesis that patients taking NOAs would have higher rates of ICH, ICH progression, and death, compared with patients taking traditional oral anticoagulant therapies (OATs). Patients were included if they were admitted to the trauma service on warfarin, aspirin, clopidogrel, dabigatran, apixaban, or rivaroxaban. Pregnant patients, prisoners, and minors were excluded from the study. Data collected included demographics, mechanism of injury, vitals on admission, injuries/injury severity scores, labs, interventions, and reversal agents used such as vitamin K, prothrombin complexes, dialysis, and transfusion of fresh frozen plasma (FFP). Outcomes studied included ICH, ICH progression, and death.
In all, 16 Level 1 trauma centers enrolled 1,847 patients over a 2-year period. Their average age was 75 years, 46% were female, 77% were white, their median Injury Severity Score (ISS) was 9, and 99% sustained a blunt mechanism of trauma. The top two causes of injury were falls (71%) and motor vehicle crashes (15%). One-third of patients (33%) were on warfarin, while the remainder were on aspirin (26%), clopidogrel (24%), NOAs (10%), and 7% took multiple or other agents.
The mechanism of injury pattern was similar between patients taking NOAs and those taking OATs, with the exception of patients on aspirin being significantly less likely to have sustained a fall. Patients on aspirin also had a significantly higher median ISS. “Patients on NOAs presented more frequently in shock as defined by a systolic blood pressure of less than 90 mmHg, but this was not associated with increased need for packed red blood cell transfusion, bleeding requiring an intervention, need for surgical procedure, hospital LOS, complications, or death,” Dr. Kobayashi said.
About 30% of all patients studied underwent an attempt at reversal. The types of agents used to reverse the patients differed depending on drug agent, with antiplatelet patients more frequently getting platelets, and patients on warfarin more frequently receiving FFP, vitamin K, and prothrombin complex. “Interestingly, patients on the anti-Xa inhibitors more frequently received prothrombin complex as well,” she said. “This likely reflects some of the recent literature which suggests that there may be a therapeutic benefit to using prothrombin complex in patients taking the oral anti-Xa inhibitors but not in patients on dabigatran.”
Overall, bleeding, need for surgical procedure, need for neurosurgical procedure, complications, length of stay, and death were similar between those on NOAs and those on OATs. However, the rate of ICH was significantly higher in patients on aspirin. “What is even more surprising is that 89% of the patients in the aspirin-only group were on an 81-mg baby aspirin rather than the larger 325-mg dose,” Dr. Kobayashi said. This difference was significant on univariate analysis and was retained after multivariate logistic regression adjusted for differences between populations, with an OR for aspirin of 1.7 and a P value of .024. “This is not to suggest that patients on aspirin are doing markedly worse, compared to their counterparts, but I think most of us would have assumed that aspirin patients would have done better,” she commented. “I think we’ve definitively shown that is not the case.” Other independent predictors of ICH were advanced age (OR, 1.02), Asian race (OR, 3.1), ISS of 10 or greater (OR, 2.2), and a Glasgow coma score (GCS) of 8 or less (OR, 5.6).
Despite their increased risk for ICH, patients on aspirin were significantly less likely to undergo an attempt at reversal with any type of agent, at 16% with a P value of less than .001, on univariate analysis. “This was significantly lower than all other medications and was retained after multivariate logistic regression, with an OR of 0.3 and a P value of less than .001,” she said.
Progression of ICH did not differ by medication group. Other independent predictors included intraparenchymal location of hemorrhage (OR, 2.2), need for a neurosurgical procedure (OR, 5.1), an attempt at reversal (OR, 2.3) and a GCS of 8 or lower at admission (OR, 4.3). Similarly, multivariate analysis of death showed no significant differences between the different medication groups. Independent predictors included advanced age (OR, 1.06), GCS of 8 or less (OR, 13), progression of head injury (OR, 10), bleeding (OR, 2.3), and complications (OR, 2.1).
Dr. Kobayashi acknowledged that the study’s observational design is a limitation, as well as the fact that it lacked a control group of age-matched patients who were not taking anticoagulants. “Additionally, we had a relatively low number of patients on NOAs, at only 10% of the study population,” she said. “Lastly, there is potential for enrollment bias as all sites involved in this study were level one trauma centers.” She reported having no financial disclosures.
WAIKOLOA, HAWAII – Among a group of anticoagulated trauma patients, those on aspirin had the highest rate and risk of intracranial hemorrhage (ICH), while those on novel oral anticoagulants were not at higher risk for ICH, ICH progression, or death, a multicenter study found.
“The number of patients on warfarin and antiplatelet agents has significantly increased over time,” Leslie Kobayashi, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “These oral antithrombotic agents have been associated with poor outcomes following traumatic injury, including increased rates of intracranial hemorrhage, increased progression of intracranial hemorrhage, and increased mortality.”
In a prospective, multicenter observational study conducted by the AAST’s Multi-institutional Trials Committee, Dr. Kobayashi and her associates set out identify injury patterns and outcomes in trauma patients taking the NOAs, and to test their hypothesis that patients taking NOAs would have higher rates of ICH, ICH progression, and death, compared with patients taking traditional oral anticoagulant therapies (OATs). Patients were included if they were admitted to the trauma service on warfarin, aspirin, clopidogrel, dabigatran, apixaban, or rivaroxaban. Pregnant patients, prisoners, and minors were excluded from the study. Data collected included demographics, mechanism of injury, vitals on admission, injuries/injury severity scores, labs, interventions, and reversal agents used such as vitamin K, prothrombin complexes, dialysis, and transfusion of fresh frozen plasma (FFP). Outcomes studied included ICH, ICH progression, and death.
In all, 16 Level 1 trauma centers enrolled 1,847 patients over a 2-year period. Their average age was 75 years, 46% were female, 77% were white, their median Injury Severity Score (ISS) was 9, and 99% sustained a blunt mechanism of trauma. The top two causes of injury were falls (71%) and motor vehicle crashes (15%). One-third of patients (33%) were on warfarin, while the remainder were on aspirin (26%), clopidogrel (24%), NOAs (10%), and 7% took multiple or other agents.
The mechanism of injury pattern was similar between patients taking NOAs and those taking OATs, with the exception of patients on aspirin being significantly less likely to have sustained a fall. Patients on aspirin also had a significantly higher median ISS. “Patients on NOAs presented more frequently in shock as defined by a systolic blood pressure of less than 90 mmHg, but this was not associated with increased need for packed red blood cell transfusion, bleeding requiring an intervention, need for surgical procedure, hospital LOS, complications, or death,” Dr. Kobayashi said.
About 30% of all patients studied underwent an attempt at reversal. The types of agents used to reverse the patients differed depending on drug agent, with antiplatelet patients more frequently getting platelets, and patients on warfarin more frequently receiving FFP, vitamin K, and prothrombin complex. “Interestingly, patients on the anti-Xa inhibitors more frequently received prothrombin complex as well,” she said. “This likely reflects some of the recent literature which suggests that there may be a therapeutic benefit to using prothrombin complex in patients taking the oral anti-Xa inhibitors but not in patients on dabigatran.”
Overall, bleeding, need for surgical procedure, need for neurosurgical procedure, complications, length of stay, and death were similar between those on NOAs and those on OATs. However, the rate of ICH was significantly higher in patients on aspirin. “What is even more surprising is that 89% of the patients in the aspirin-only group were on an 81-mg baby aspirin rather than the larger 325-mg dose,” Dr. Kobayashi said. This difference was significant on univariate analysis and was retained after multivariate logistic regression adjusted for differences between populations, with an OR for aspirin of 1.7 and a P value of .024. “This is not to suggest that patients on aspirin are doing markedly worse, compared to their counterparts, but I think most of us would have assumed that aspirin patients would have done better,” she commented. “I think we’ve definitively shown that is not the case.” Other independent predictors of ICH were advanced age (OR, 1.02), Asian race (OR, 3.1), ISS of 10 or greater (OR, 2.2), and a Glasgow coma score (GCS) of 8 or less (OR, 5.6).
Despite their increased risk for ICH, patients on aspirin were significantly less likely to undergo an attempt at reversal with any type of agent, at 16% with a P value of less than .001, on univariate analysis. “This was significantly lower than all other medications and was retained after multivariate logistic regression, with an OR of 0.3 and a P value of less than .001,” she said.
Progression of ICH did not differ by medication group. Other independent predictors included intraparenchymal location of hemorrhage (OR, 2.2), need for a neurosurgical procedure (OR, 5.1), an attempt at reversal (OR, 2.3) and a GCS of 8 or lower at admission (OR, 4.3). Similarly, multivariate analysis of death showed no significant differences between the different medication groups. Independent predictors included advanced age (OR, 1.06), GCS of 8 or less (OR, 13), progression of head injury (OR, 10), bleeding (OR, 2.3), and complications (OR, 2.1).
Dr. Kobayashi acknowledged that the study’s observational design is a limitation, as well as the fact that it lacked a control group of age-matched patients who were not taking anticoagulants. “Additionally, we had a relatively low number of patients on NOAs, at only 10% of the study population,” she said. “Lastly, there is potential for enrollment bias as all sites involved in this study were level one trauma centers.” She reported having no financial disclosures.
WAIKOLOA, HAWAII – Among a group of anticoagulated trauma patients, those on aspirin had the highest rate and risk of intracranial hemorrhage (ICH), while those on novel oral anticoagulants were not at higher risk for ICH, ICH progression, or death, a multicenter study found.
“The number of patients on warfarin and antiplatelet agents has significantly increased over time,” Leslie Kobayashi, MD, said at the annual meeting of the American Association for the Surgery of Trauma. “These oral antithrombotic agents have been associated with poor outcomes following traumatic injury, including increased rates of intracranial hemorrhage, increased progression of intracranial hemorrhage, and increased mortality.”
In a prospective, multicenter observational study conducted by the AAST’s Multi-institutional Trials Committee, Dr. Kobayashi and her associates set out identify injury patterns and outcomes in trauma patients taking the NOAs, and to test their hypothesis that patients taking NOAs would have higher rates of ICH, ICH progression, and death, compared with patients taking traditional oral anticoagulant therapies (OATs). Patients were included if they were admitted to the trauma service on warfarin, aspirin, clopidogrel, dabigatran, apixaban, or rivaroxaban. Pregnant patients, prisoners, and minors were excluded from the study. Data collected included demographics, mechanism of injury, vitals on admission, injuries/injury severity scores, labs, interventions, and reversal agents used such as vitamin K, prothrombin complexes, dialysis, and transfusion of fresh frozen plasma (FFP). Outcomes studied included ICH, ICH progression, and death.
In all, 16 Level 1 trauma centers enrolled 1,847 patients over a 2-year period. Their average age was 75 years, 46% were female, 77% were white, their median Injury Severity Score (ISS) was 9, and 99% sustained a blunt mechanism of trauma. The top two causes of injury were falls (71%) and motor vehicle crashes (15%). One-third of patients (33%) were on warfarin, while the remainder were on aspirin (26%), clopidogrel (24%), NOAs (10%), and 7% took multiple or other agents.
The mechanism of injury pattern was similar between patients taking NOAs and those taking OATs, with the exception of patients on aspirin being significantly less likely to have sustained a fall. Patients on aspirin also had a significantly higher median ISS. “Patients on NOAs presented more frequently in shock as defined by a systolic blood pressure of less than 90 mmHg, but this was not associated with increased need for packed red blood cell transfusion, bleeding requiring an intervention, need for surgical procedure, hospital LOS, complications, or death,” Dr. Kobayashi said.
About 30% of all patients studied underwent an attempt at reversal. The types of agents used to reverse the patients differed depending on drug agent, with antiplatelet patients more frequently getting platelets, and patients on warfarin more frequently receiving FFP, vitamin K, and prothrombin complex. “Interestingly, patients on the anti-Xa inhibitors more frequently received prothrombin complex as well,” she said. “This likely reflects some of the recent literature which suggests that there may be a therapeutic benefit to using prothrombin complex in patients taking the oral anti-Xa inhibitors but not in patients on dabigatran.”
Overall, bleeding, need for surgical procedure, need for neurosurgical procedure, complications, length of stay, and death were similar between those on NOAs and those on OATs. However, the rate of ICH was significantly higher in patients on aspirin. “What is even more surprising is that 89% of the patients in the aspirin-only group were on an 81-mg baby aspirin rather than the larger 325-mg dose,” Dr. Kobayashi said. This difference was significant on univariate analysis and was retained after multivariate logistic regression adjusted for differences between populations, with an OR for aspirin of 1.7 and a P value of .024. “This is not to suggest that patients on aspirin are doing markedly worse, compared to their counterparts, but I think most of us would have assumed that aspirin patients would have done better,” she commented. “I think we’ve definitively shown that is not the case.” Other independent predictors of ICH were advanced age (OR, 1.02), Asian race (OR, 3.1), ISS of 10 or greater (OR, 2.2), and a Glasgow coma score (GCS) of 8 or less (OR, 5.6).
Despite their increased risk for ICH, patients on aspirin were significantly less likely to undergo an attempt at reversal with any type of agent, at 16% with a P value of less than .001, on univariate analysis. “This was significantly lower than all other medications and was retained after multivariate logistic regression, with an OR of 0.3 and a P value of less than .001,” she said.
Progression of ICH did not differ by medication group. Other independent predictors included intraparenchymal location of hemorrhage (OR, 2.2), need for a neurosurgical procedure (OR, 5.1), an attempt at reversal (OR, 2.3) and a GCS of 8 or lower at admission (OR, 4.3). Similarly, multivariate analysis of death showed no significant differences between the different medication groups. Independent predictors included advanced age (OR, 1.06), GCS of 8 or less (OR, 13), progression of head injury (OR, 10), bleeding (OR, 2.3), and complications (OR, 2.1).
Dr. Kobayashi acknowledged that the study’s observational design is a limitation, as well as the fact that it lacked a control group of age-matched patients who were not taking anticoagulants. “Additionally, we had a relatively low number of patients on NOAs, at only 10% of the study population,” she said. “Lastly, there is potential for enrollment bias as all sites involved in this study were level one trauma centers.” She reported having no financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point:
Major finding: The rate of ICH was significantly higher in patients on aspirin, compared with those on novel oral anticoagulant therapies (OR, 1.7; P = .024).
Data source: A prospective evaluation of 1,847 patients treated at 16 level one trauma centers over a 2-year period.
Disclosures: Dr. Kobayashi reported having no financial disclosures.
Recovery path complicated for trauma patients with VTE
CORONADO, CALIF. – Patients who develop a venous thromboembolism (VTE) following severe hemorrhage are more susceptible to complications, compared with their counterparts who do not; they also exhibit hypercoagulability and enhanced platelet function at admission, and have delayed recovery of coagulation and platelet function following injury.
Those are the key findings from a secondary analysis of data from the Pragmatic Randomized Optimal Platelet and Plasma Ratio (PROPPR) trial, which randomized 680 severely injured trauma patients from 12 level I trauma centers to receive 1:1:1 or 1:1:2 ratios of plasma to platelets to red blood cells (JAMA 2015;313[5]:471-82). “The prevention of VTE following traumatic injury is an ongoing challenge,” Belinda H. McCully, PhD, said at the annual meeting of the Western Surgical Association. “Despite prophylaxis, about 25% of patients present with VTE, which is associated with higher complications and an increased risk for mortality. Common risk factors for mortality include age, body mass index, extremity injury, and immobility, but the precise mechanisms that contribute to VTE development are not well understood. We do know that the three main factors contributing to thrombosis include static flow, endothelial injury, and hypercoagulability. Clinically, coagulation is the most feasible factor to assess, mainly through the use of conventional coagulation tests, thromboelastography, platelet levels, and platelet function assays.”
Dr. McCully of the division of trauma, critical care, and acute care surgery in the department of surgery at Oregon Health & Science University, Portland, and her associates hypothesized that enhanced, earlier recovery of coagulation function is associated with increased VTE risk in severely injured trauma patients. To test this hypothesis, they conducted a secondary analysis of the PROPPR database, excluding patients who received anticoagulants, to rule out any bias against VTE development, as well as patients who died within 24 hours, to reduce the survival bias. This left 558 patients: 475 who did not develop a VTE, and 83 who did (defined as those who developed deep vein thrombosis or pulmonary embolism). Patient characteristics of interest included age, sex, BMI, mechanism of injury, and injury severity, as well as the transfusion group, the type of blood products given, and the percentage of patients given procoagulants. The investigators also assessed length of stay and complication incidence previously defined by the trial. During the trial, blood samples were taken from admission up to 72 hours and were used to asses both whole blood coagulation using thromboelastography and platelet function using the Multiplate assay.
Dr. McCully reported that VTE patients and non-VTE patients demonstrated similar admission platelet function activity and inhibition of all platelet function parameters at 24 hours (P less than .05). The onset of platelet function recovery was delayed in VTE patients, specifically for arachidonic acid, adenosine-5’-diphosphate, and collagen. Changes in thromboelastography, clot time to initiation, formation, rate of formation, and strength and index of platelet function from admission to 2 hours indicated increasing hypocoagulability (P less than .05) but suppressed clot lysis in both groups. Compared with patients in the non-VTE group, the VTE group had lower mortality (4% vs. 13%) but increased total hospital days (a mean of 30 vs. 16; P less than .05).
Adverse outcomes were also more prevalent in the VTE group, compared with the non-VTE group, and included systemic inflammatory response syndrome (82% vs. 72%), acute kidney injury (36% vs. 26%), infection (61% vs. 31%), sepsis (60% vs. 28%), and pneumonia (34% vs. 19%; P less than 0.05 for all associations). Conversely, regression analysis showed that VTE was associated only with total hospital days (odds ratio, 1.12), while adverse events were similar between the two groups. “From this we can conclude that VTE development following trauma may be attributed to hypercoagulable thromboelastography parameters and enhanced platelet function at admission, and compensatory mechanisms in response to a delayed recovery of coagulation and platelet function,” Dr. McCully said.
She acknowledged certain limitations of the study, including the fact that it was a secondary analysis of prospectively collected data. “We also plan to assess plasma markers of clot strength and fibrinolysis, which is an ongoing process,” she said. “Despite excluding patients that died within 24 hours, there was still a survival bias in the VTE group.”
The PROPPR study was supported by the National Heart, Lung, and Blood Institute and by the Department of Defense. Dr. McCully reported having no relevant financial disclosures.
CORONADO, CALIF. – Patients who develop a venous thromboembolism (VTE) following severe hemorrhage are more susceptible to complications, compared with their counterparts who do not; they also exhibit hypercoagulability and enhanced platelet function at admission, and have delayed recovery of coagulation and platelet function following injury.
Those are the key findings from a secondary analysis of data from the Pragmatic Randomized Optimal Platelet and Plasma Ratio (PROPPR) trial, which randomized 680 severely injured trauma patients from 12 level I trauma centers to receive 1:1:1 or 1:1:2 ratios of plasma to platelets to red blood cells (JAMA 2015;313[5]:471-82). “The prevention of VTE following traumatic injury is an ongoing challenge,” Belinda H. McCully, PhD, said at the annual meeting of the Western Surgical Association. “Despite prophylaxis, about 25% of patients present with VTE, which is associated with higher complications and an increased risk for mortality. Common risk factors for mortality include age, body mass index, extremity injury, and immobility, but the precise mechanisms that contribute to VTE development are not well understood. We do know that the three main factors contributing to thrombosis include static flow, endothelial injury, and hypercoagulability. Clinically, coagulation is the most feasible factor to assess, mainly through the use of conventional coagulation tests, thromboelastography, platelet levels, and platelet function assays.”
Dr. McCully of the division of trauma, critical care, and acute care surgery in the department of surgery at Oregon Health & Science University, Portland, and her associates hypothesized that enhanced, earlier recovery of coagulation function is associated with increased VTE risk in severely injured trauma patients. To test this hypothesis, they conducted a secondary analysis of the PROPPR database, excluding patients who received anticoagulants, to rule out any bias against VTE development, as well as patients who died within 24 hours, to reduce the survival bias. This left 558 patients: 475 who did not develop a VTE, and 83 who did (defined as those who developed deep vein thrombosis or pulmonary embolism). Patient characteristics of interest included age, sex, BMI, mechanism of injury, and injury severity, as well as the transfusion group, the type of blood products given, and the percentage of patients given procoagulants. The investigators also assessed length of stay and complication incidence previously defined by the trial. During the trial, blood samples were taken from admission up to 72 hours and were used to asses both whole blood coagulation using thromboelastography and platelet function using the Multiplate assay.
Dr. McCully reported that VTE patients and non-VTE patients demonstrated similar admission platelet function activity and inhibition of all platelet function parameters at 24 hours (P less than .05). The onset of platelet function recovery was delayed in VTE patients, specifically for arachidonic acid, adenosine-5’-diphosphate, and collagen. Changes in thromboelastography, clot time to initiation, formation, rate of formation, and strength and index of platelet function from admission to 2 hours indicated increasing hypocoagulability (P less than .05) but suppressed clot lysis in both groups. Compared with patients in the non-VTE group, the VTE group had lower mortality (4% vs. 13%) but increased total hospital days (a mean of 30 vs. 16; P less than .05).
Adverse outcomes were also more prevalent in the VTE group, compared with the non-VTE group, and included systemic inflammatory response syndrome (82% vs. 72%), acute kidney injury (36% vs. 26%), infection (61% vs. 31%), sepsis (60% vs. 28%), and pneumonia (34% vs. 19%; P less than 0.05 for all associations). Conversely, regression analysis showed that VTE was associated only with total hospital days (odds ratio, 1.12), while adverse events were similar between the two groups. “From this we can conclude that VTE development following trauma may be attributed to hypercoagulable thromboelastography parameters and enhanced platelet function at admission, and compensatory mechanisms in response to a delayed recovery of coagulation and platelet function,” Dr. McCully said.
She acknowledged certain limitations of the study, including the fact that it was a secondary analysis of prospectively collected data. “We also plan to assess plasma markers of clot strength and fibrinolysis, which is an ongoing process,” she said. “Despite excluding patients that died within 24 hours, there was still a survival bias in the VTE group.”
The PROPPR study was supported by the National Heart, Lung, and Blood Institute and by the Department of Defense. Dr. McCully reported having no relevant financial disclosures.
CORONADO, CALIF. – Patients who develop a venous thromboembolism (VTE) following severe hemorrhage are more susceptible to complications, compared with their counterparts who do not; they also exhibit hypercoagulability and enhanced platelet function at admission, and have delayed recovery of coagulation and platelet function following injury.
Those are the key findings from a secondary analysis of data from the Pragmatic Randomized Optimal Platelet and Plasma Ratio (PROPPR) trial, which randomized 680 severely injured trauma patients from 12 level I trauma centers to receive 1:1:1 or 1:1:2 ratios of plasma to platelets to red blood cells (JAMA 2015;313[5]:471-82). “The prevention of VTE following traumatic injury is an ongoing challenge,” Belinda H. McCully, PhD, said at the annual meeting of the Western Surgical Association. “Despite prophylaxis, about 25% of patients present with VTE, which is associated with higher complications and an increased risk for mortality. Common risk factors for mortality include age, body mass index, extremity injury, and immobility, but the precise mechanisms that contribute to VTE development are not well understood. We do know that the three main factors contributing to thrombosis include static flow, endothelial injury, and hypercoagulability. Clinically, coagulation is the most feasible factor to assess, mainly through the use of conventional coagulation tests, thromboelastography, platelet levels, and platelet function assays.”
Dr. McCully of the division of trauma, critical care, and acute care surgery in the department of surgery at Oregon Health & Science University, Portland, and her associates hypothesized that enhanced, earlier recovery of coagulation function is associated with increased VTE risk in severely injured trauma patients. To test this hypothesis, they conducted a secondary analysis of the PROPPR database, excluding patients who received anticoagulants, to rule out any bias against VTE development, as well as patients who died within 24 hours, to reduce the survival bias. This left 558 patients: 475 who did not develop a VTE, and 83 who did (defined as those who developed deep vein thrombosis or pulmonary embolism). Patient characteristics of interest included age, sex, BMI, mechanism of injury, and injury severity, as well as the transfusion group, the type of blood products given, and the percentage of patients given procoagulants. The investigators also assessed length of stay and complication incidence previously defined by the trial. During the trial, blood samples were taken from admission up to 72 hours and were used to asses both whole blood coagulation using thromboelastography and platelet function using the Multiplate assay.
Dr. McCully reported that VTE patients and non-VTE patients demonstrated similar admission platelet function activity and inhibition of all platelet function parameters at 24 hours (P less than .05). The onset of platelet function recovery was delayed in VTE patients, specifically for arachidonic acid, adenosine-5’-diphosphate, and collagen. Changes in thromboelastography, clot time to initiation, formation, rate of formation, and strength and index of platelet function from admission to 2 hours indicated increasing hypocoagulability (P less than .05) but suppressed clot lysis in both groups. Compared with patients in the non-VTE group, the VTE group had lower mortality (4% vs. 13%) but increased total hospital days (a mean of 30 vs. 16; P less than .05).
Adverse outcomes were also more prevalent in the VTE group, compared with the non-VTE group, and included systemic inflammatory response syndrome (82% vs. 72%), acute kidney injury (36% vs. 26%), infection (61% vs. 31%), sepsis (60% vs. 28%), and pneumonia (34% vs. 19%; P less than 0.05 for all associations). Conversely, regression analysis showed that VTE was associated only with total hospital days (odds ratio, 1.12), while adverse events were similar between the two groups. “From this we can conclude that VTE development following trauma may be attributed to hypercoagulable thromboelastography parameters and enhanced platelet function at admission, and compensatory mechanisms in response to a delayed recovery of coagulation and platelet function,” Dr. McCully said.
She acknowledged certain limitations of the study, including the fact that it was a secondary analysis of prospectively collected data. “We also plan to assess plasma markers of clot strength and fibrinolysis, which is an ongoing process,” she said. “Despite excluding patients that died within 24 hours, there was still a survival bias in the VTE group.”
The PROPPR study was supported by the National Heart, Lung, and Blood Institute and by the Department of Defense. Dr. McCully reported having no relevant financial disclosures.
AT WSA 2016
Key clinical point:
Major finding: Compared with patients in the non-VTE group, the VTE group had lower mortality (4% vs. 13%) but increased total hospital days (a mean of 30 vs. 16; P less than .05).
Data source: A secondary analysis of 558 patients from the Pragmatic Randomized Optimal Platelet and Plasma Ratio (PROPPR) trial, which randomized severely injured trauma patients from 12 level I trauma centers to receive 1:1:1 or 1:1:2 ratios of plasma to platelets to red blood cells.
Disclosures: The PROPPR study was supported by the National Heart, Lung, and Blood Institute and by the Department of Defense. Dr. McCully reported having no relevant financial disclosures.
Initial outcomes of PERT at Cleveland Clinic
LOS ANGELES – Initial outcomes measures are beginning to emerge from Pulmonary Embolism Response Teams.
Members of the Cleveland Clinic’s PERT, which was established in 2014, presented some of their preliminary data during a presentation at the CHEST annual meeting.
The concept behind the PERT is to rapidly mobilize a team with varied expertise helpful for treating patients with pulmonary embolisms (PEs). While the PERT “can be activated by any (clinician) for any patient, even low-risk patients ... those with submassive and massive PEs [intermediate- and high-risk patients]” are the target patients, said Dr. Mahar of the Cleveland Clinic.
The first PERT was created at Massachusetts General Hospital in Boston in 2012, according to the National Consortium of Pulmonary Embolism Response Team’s website. As of May 2015, the PERT model has been adopted by physicians and health care professionals from more than 40 institutions.
Dr. Mahar reported that the Cleveland Clinic’s PERT is activated through a single pager that resides with a vascular medicine fellow during the day and a critical care fellow at night. When paged, the fellow promptly evaluates the patient and ensures a complete basic work-up, which includes an ECG, cardiac enzymes, N-terminal pro b-type natriuretic peptide, lower-extremity deep vein thrombosis scans, transthoracic echocardiogram, and confirmatory CT/PE protocol or ventilation/perfusion scan.
Based on the simplified Pulmonary Embolism Severity Index and Bova scores, the patient is risk stratified and the patient’s indications, and relative and absolute contraindications to advanced therapies are reviewed. The fellow next sends a group notification to the PERT via email and text message. The team then convenes online for a virtual meeting and case presentation that includes sharing of lab and test results and images.
The process sounds complex, but the surgeon, interventional radiologist, vascular medicine specialist, and cardiologist are on call and simultaneously get the message and respond, Dr. Mahar said. With a team approach, the decision to use advanced therapies – systemic lytics, surgery, catheter-directed lysis and extracorporeal membrane oxygenation – is expedited. “For example, over the last 2 years, four out of four patients who underwent surgical embolectomies had good outcomes without any deaths,” he said.
Based on a retrospective chart review from October 2014 through August 2016, Cleveland Clinic’s PERT had been activated for 134 patients, 112 of whom were found to have PEs, Dr. Mahar said during his presentation at the annual meeting of the American College of Chest Physicians (CHEST).
The number of low risk, submassive, and massive PEs were 14 (12%), 76 (68%), and 22 (20%), respectively. Just over half of the PE patients, 55% (60 patients), were treated with anticoagulation therapy alone. Inferior vena cava filters were placed in 32 patients (29%); 14 patients received catheter-directed thrombolysis, 3 received a suction thrombectomy, and 4 received a surgical embolectomy.
The 30-day all-cause mortality rate was 9%; the deaths occurred in six patients who had massive PEs, three patients with submassive PEs, and one patient with a low-risk PE. Six of the patients who died had been treated with anticoagulation, two had received catheter-directed thrombolysis, and one had received a full dose of systemic thrombolysis.
Bleeding complications occurred in 10 patients, 6 of whom were treated with anticoagulation alone and 4 of whom underwent catheter-directed thrombolysis.
Cleveland Clinic is a large entity with multiple resources, but the principles of PERT can be applied in smaller facilities, as well, according to Gustavo A. Heresi-Davila, MD, medical director of the Cleveland Clinic’s pulmonary thromboendarterectomy program and the lead researcher for the PERT project at the clinic. “I would emphasize the notion that a PERT has to be multidisciplinary, as people with different backgrounds and expertise bring complementary talent to the discussion of each case. I would not minimize the challenges of assembling such a team,” he said during an interview following the meeting.
The moderator of the meeting session, Robert Schilz, DO, PhD, noted, that the goal of PERT is to determine the best approach for an individual patient based on available resources. To establish a PERT, “you don’t have to be able to put a patient on ECMO [extracorporeal membrane oxygenation] in 15 minutes, and you don’t have to be able to do endarterectomies, embolectomies, and all the catheter-drive techniques emergently. But you do need to have the disposition to have efficient and standardized care, and the solutions may need to be very geographic. What hospital A may do may be very different from hospital B.”
Small hospitals can draw on their available resources, added Dr. Schilz, director of pulmonary vascular disease and lung transplantation at Case Western Reserve University, Cleveland. “Most hospitals have cardiologists on call 24/7, and many have some flavor of interventional radiology; others have clear referral and transfer schemes. Emergency department personnel at small rural hospitals can rapidly identify patients appropriate for transfer.”
Dr. Mahar added that PERTs are already being utilized in smaller hospitals and that he thinks that, in the next 5 years, having a PERT will be the standard protocol.
Dr. Mahar reported no disclosures.
Mary Jo Dales contributed to this report.
LOS ANGELES – Initial outcomes measures are beginning to emerge from Pulmonary Embolism Response Teams.
Members of the Cleveland Clinic’s PERT, which was established in 2014, presented some of their preliminary data during a presentation at the CHEST annual meeting.
The concept behind the PERT is to rapidly mobilize a team with varied expertise helpful for treating patients with pulmonary embolisms (PEs). While the PERT “can be activated by any (clinician) for any patient, even low-risk patients ... those with submassive and massive PEs [intermediate- and high-risk patients]” are the target patients, said Dr. Mahar of the Cleveland Clinic.
The first PERT was created at Massachusetts General Hospital in Boston in 2012, according to the National Consortium of Pulmonary Embolism Response Team’s website. As of May 2015, the PERT model has been adopted by physicians and health care professionals from more than 40 institutions.
Dr. Mahar reported that the Cleveland Clinic’s PERT is activated through a single pager that resides with a vascular medicine fellow during the day and a critical care fellow at night. When paged, the fellow promptly evaluates the patient and ensures a complete basic work-up, which includes an ECG, cardiac enzymes, N-terminal pro b-type natriuretic peptide, lower-extremity deep vein thrombosis scans, transthoracic echocardiogram, and confirmatory CT/PE protocol or ventilation/perfusion scan.
Based on the simplified Pulmonary Embolism Severity Index and Bova scores, the patient is risk stratified and the patient’s indications, and relative and absolute contraindications to advanced therapies are reviewed. The fellow next sends a group notification to the PERT via email and text message. The team then convenes online for a virtual meeting and case presentation that includes sharing of lab and test results and images.
The process sounds complex, but the surgeon, interventional radiologist, vascular medicine specialist, and cardiologist are on call and simultaneously get the message and respond, Dr. Mahar said. With a team approach, the decision to use advanced therapies – systemic lytics, surgery, catheter-directed lysis and extracorporeal membrane oxygenation – is expedited. “For example, over the last 2 years, four out of four patients who underwent surgical embolectomies had good outcomes without any deaths,” he said.
Based on a retrospective chart review from October 2014 through August 2016, Cleveland Clinic’s PERT had been activated for 134 patients, 112 of whom were found to have PEs, Dr. Mahar said during his presentation at the annual meeting of the American College of Chest Physicians (CHEST).
The number of low risk, submassive, and massive PEs were 14 (12%), 76 (68%), and 22 (20%), respectively. Just over half of the PE patients, 55% (60 patients), were treated with anticoagulation therapy alone. Inferior vena cava filters were placed in 32 patients (29%); 14 patients received catheter-directed thrombolysis, 3 received a suction thrombectomy, and 4 received a surgical embolectomy.
The 30-day all-cause mortality rate was 9%; the deaths occurred in six patients who had massive PEs, three patients with submassive PEs, and one patient with a low-risk PE. Six of the patients who died had been treated with anticoagulation, two had received catheter-directed thrombolysis, and one had received a full dose of systemic thrombolysis.
Bleeding complications occurred in 10 patients, 6 of whom were treated with anticoagulation alone and 4 of whom underwent catheter-directed thrombolysis.
Cleveland Clinic is a large entity with multiple resources, but the principles of PERT can be applied in smaller facilities, as well, according to Gustavo A. Heresi-Davila, MD, medical director of the Cleveland Clinic’s pulmonary thromboendarterectomy program and the lead researcher for the PERT project at the clinic. “I would emphasize the notion that a PERT has to be multidisciplinary, as people with different backgrounds and expertise bring complementary talent to the discussion of each case. I would not minimize the challenges of assembling such a team,” he said during an interview following the meeting.
The moderator of the meeting session, Robert Schilz, DO, PhD, noted, that the goal of PERT is to determine the best approach for an individual patient based on available resources. To establish a PERT, “you don’t have to be able to put a patient on ECMO [extracorporeal membrane oxygenation] in 15 minutes, and you don’t have to be able to do endarterectomies, embolectomies, and all the catheter-drive techniques emergently. But you do need to have the disposition to have efficient and standardized care, and the solutions may need to be very geographic. What hospital A may do may be very different from hospital B.”
Small hospitals can draw on their available resources, added Dr. Schilz, director of pulmonary vascular disease and lung transplantation at Case Western Reserve University, Cleveland. “Most hospitals have cardiologists on call 24/7, and many have some flavor of interventional radiology; others have clear referral and transfer schemes. Emergency department personnel at small rural hospitals can rapidly identify patients appropriate for transfer.”
Dr. Mahar added that PERTs are already being utilized in smaller hospitals and that he thinks that, in the next 5 years, having a PERT will be the standard protocol.
Dr. Mahar reported no disclosures.
Mary Jo Dales contributed to this report.
LOS ANGELES – Initial outcomes measures are beginning to emerge from Pulmonary Embolism Response Teams.
Members of the Cleveland Clinic’s PERT, which was established in 2014, presented some of their preliminary data during a presentation at the CHEST annual meeting.
The concept behind the PERT is to rapidly mobilize a team with varied expertise helpful for treating patients with pulmonary embolisms (PEs). While the PERT “can be activated by any (clinician) for any patient, even low-risk patients ... those with submassive and massive PEs [intermediate- and high-risk patients]” are the target patients, said Dr. Mahar of the Cleveland Clinic.
The first PERT was created at Massachusetts General Hospital in Boston in 2012, according to the National Consortium of Pulmonary Embolism Response Team’s website. As of May 2015, the PERT model has been adopted by physicians and health care professionals from more than 40 institutions.
Dr. Mahar reported that the Cleveland Clinic’s PERT is activated through a single pager that resides with a vascular medicine fellow during the day and a critical care fellow at night. When paged, the fellow promptly evaluates the patient and ensures a complete basic work-up, which includes an ECG, cardiac enzymes, N-terminal pro b-type natriuretic peptide, lower-extremity deep vein thrombosis scans, transthoracic echocardiogram, and confirmatory CT/PE protocol or ventilation/perfusion scan.
Based on the simplified Pulmonary Embolism Severity Index and Bova scores, the patient is risk stratified and the patient’s indications, and relative and absolute contraindications to advanced therapies are reviewed. The fellow next sends a group notification to the PERT via email and text message. The team then convenes online for a virtual meeting and case presentation that includes sharing of lab and test results and images.
The process sounds complex, but the surgeon, interventional radiologist, vascular medicine specialist, and cardiologist are on call and simultaneously get the message and respond, Dr. Mahar said. With a team approach, the decision to use advanced therapies – systemic lytics, surgery, catheter-directed lysis and extracorporeal membrane oxygenation – is expedited. “For example, over the last 2 years, four out of four patients who underwent surgical embolectomies had good outcomes without any deaths,” he said.
Based on a retrospective chart review from October 2014 through August 2016, Cleveland Clinic’s PERT had been activated for 134 patients, 112 of whom were found to have PEs, Dr. Mahar said during his presentation at the annual meeting of the American College of Chest Physicians (CHEST).
The number of low risk, submassive, and massive PEs were 14 (12%), 76 (68%), and 22 (20%), respectively. Just over half of the PE patients, 55% (60 patients), were treated with anticoagulation therapy alone. Inferior vena cava filters were placed in 32 patients (29%); 14 patients received catheter-directed thrombolysis, 3 received a suction thrombectomy, and 4 received a surgical embolectomy.
The 30-day all-cause mortality rate was 9%; the deaths occurred in six patients who had massive PEs, three patients with submassive PEs, and one patient with a low-risk PE. Six of the patients who died had been treated with anticoagulation, two had received catheter-directed thrombolysis, and one had received a full dose of systemic thrombolysis.
Bleeding complications occurred in 10 patients, 6 of whom were treated with anticoagulation alone and 4 of whom underwent catheter-directed thrombolysis.
Cleveland Clinic is a large entity with multiple resources, but the principles of PERT can be applied in smaller facilities, as well, according to Gustavo A. Heresi-Davila, MD, medical director of the Cleveland Clinic’s pulmonary thromboendarterectomy program and the lead researcher for the PERT project at the clinic. “I would emphasize the notion that a PERT has to be multidisciplinary, as people with different backgrounds and expertise bring complementary talent to the discussion of each case. I would not minimize the challenges of assembling such a team,” he said during an interview following the meeting.
The moderator of the meeting session, Robert Schilz, DO, PhD, noted, that the goal of PERT is to determine the best approach for an individual patient based on available resources. To establish a PERT, “you don’t have to be able to put a patient on ECMO [extracorporeal membrane oxygenation] in 15 minutes, and you don’t have to be able to do endarterectomies, embolectomies, and all the catheter-drive techniques emergently. But you do need to have the disposition to have efficient and standardized care, and the solutions may need to be very geographic. What hospital A may do may be very different from hospital B.”
Small hospitals can draw on their available resources, added Dr. Schilz, director of pulmonary vascular disease and lung transplantation at Case Western Reserve University, Cleveland. “Most hospitals have cardiologists on call 24/7, and many have some flavor of interventional radiology; others have clear referral and transfer schemes. Emergency department personnel at small rural hospitals can rapidly identify patients appropriate for transfer.”
Dr. Mahar added that PERTs are already being utilized in smaller hospitals and that he thinks that, in the next 5 years, having a PERT will be the standard protocol.
Dr. Mahar reported no disclosures.
Mary Jo Dales contributed to this report.
FROM CHEST 2016
Scoring formula consolidates stroke, bleeding risk in atrial fib patients
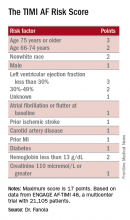
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
ROME – A new risk-stratification formula for atrial fibrillation patients starting oral anticoagulant therapy helps sort out their potential net benefit on edoxaban, compared with warfarin.
This risk score “could help guide selection of treatment” with a vitamin K antagonist such as warfarin or a new oral anticoagulant (NOAC) such as edoxaban, Christina L. Fanola, MD, said at the annual congress of the European Society of Cardiology.
“It’s a great time to think about this type of score, because so many more patients are being diagnosed with atrial fibrillation and there is a lot of clinical equipoise” over which anticoagulant to start patients on, said Dr. Fanola, a cardiologist at Brigham and Women’s Hospital in Boston. She said she and her associates hope to externally validate the score and test it in cohorts that received other NOACs, such as apixaban (Eliquis), dabigatran (Pradaxa), or rivaroxaban (Xarelto), but it is very possible that scoring might differ from one NOAC to the next. “Each NOAC may need its own scoring formula,” Dr. Fanola said in an interview.
A Cox proportional hazards model identified 10 demographic, clinical, and laboratory features that had significant, independent correlations to a primary outcome of disabling stroke, life-threatening bleeding, or death. After weighing the point allocation for each item by the strength of its association, the researchers developed a scoring formula in a model that could account for about 69% of the three combined adverse outcomes.
An analysis that applied the scoring formula back to the ENGAGE AF-TIMI 48 database showed that a low-risk score of 0-6 correlated with a 4% per year rate of disabling stroke, life-threatening bleed, or death; an intermediate-risk score of 7-9 correlated with a 10% per year incidence of this combined outcome, and a high-risk score of 10 or greater linked with a 21% annual event rate.
Dr. Fanola and her associates ran a further analysis that evaluated the efficacy of edoxaban, compared with warfarin, among the patients in each of these risk strata. The high-risk patients received a major benefit from edoxaban, with a 30% overall incidence of the combined endpoint during 3 years of follow-up, compared with a 51% rate among patients on warfarin, a 21-percentage-point reduction in adverse events. Intermediate-risk patients also received a significant benefit, with a 26% event rate on warfarin and an 18% rate on edoxaban. But low-risk patients had identical 10% event rates with either treatment.
These findings suggest that atrial fibrillation patients with a TIMI AF score that is high or intermediate would have a better chance for a good outcome on edoxaban, or perhaps a different NOAC, than on warfarin. Low-risk patients seem to have similar outcomes on edoxaban or warfarin, so other considerations can come into play for choosing between these drug options, such as the cost of treatment and the inconvenience of regular warfarin monitoring, Dr. Fanola said.
ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban. Dr. Fanola had no relevant financial disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE ESC CONGRESS 2016
Key clinical point:
Major finding: Among high-risk patients, edoxaban cut adverse events by 21 percentage points, compared with warfarin.
Data source: ENGAGE AF-TIMI 48, a multicenter trial with 21,105 patients.
Disclosures: ENGAGE AF-TIMI 48 was sponsored by Daiichi Sankyo, the company that markets edoxaban (Savaysa). Dr. Fanola had no relevant financial disclosures.
LMWH best for preventing PE in patients with major trauma
WAIKOLOA, HAWAII – Venous thromboembolism prophylaxis with low molecular weight heparin (LMWH), instead of unfractionated heparin (UH), is associated with lower risk of pulmonary embolism (PE) in patients with major trauma, results from a large study have shown.
The results of the study, based on data from the American College of Surgeons (ACS) Trauma Quality Improvement Program, suggest that LMWH-based strategies for thromboprophylaxis should be preferred after major trauma.
Dr. Byrne, a general surgery resident at Sunnybrook Health Science Center, Toronto, Ontario, Canada, went on to note that LMWH is often favored because of a randomized controlled trial which showed that LMWH was associated with fewer deep vein thromboses (N Engl. J. Med. 1996;335[10]:701-7). However, significant practice variability continues to exist.
“Practitioners might favor the shorter half-life of unfractionated heparin in patients where they perceive the risk for hemorrhagic complications is high,” he said. “There’s also recent evidence to suggest that dosing may be all important and that unfractionated heparin dosed three times daily may be equivalent to low molecular weight heparin. If this is true, it might suggest that the historically higher cost of low molecular weight heparin could favor the use of unfractionated heparin.”
Furthermore, there is a is a lack of evidence comparing either agent to prevent PE, he added. “This is an important gap in our knowledge, because PE frequently occurs in the absence of an identified DVT and carries a significant risk of death. At present, it is not known how practice patterns with respect to choice of prophylaxis type influence risk of PE at the patient or hospital levels.”
Due to a lack of evidence comparing agents to prevent PE, the researchers set out to compare the effectiveness of LMWH versus UH to prevent PE in patients with major trauma who were treated at trauma centers participating in the ACS Trauma Quality Improvement Program from 2012 to 2015. They included all adults with severe injury who received LMWH or UH and excluded those who died or were discharged within five days, and those with a bleeding disorder or chronic anticoagulation. The exposure was defined as thromboprophylaxis with LMWH versus UH, and the primary outcome was PE confirmed on radiologic imaging. Potential confounders were considered, including patient baseline characteristics, anatomic and global injury severity, presenting characteristics in the emergency department, acute intracranial injuries, orthopedic injuries, early surgical interventions, and timing of prophylaxis initiation.
Dr. Byrne and his associates then used three analytic approaches in the study: a propensity score matching methodology, a multivariable logistic regression model for PE, and a center-level analysis examining the influence of LMWH utilization on hospital rates of PE.
They identified 153,474 trauma patients from 217 trauma centers. Their median age was 50 years and 67% were male. Blunt trauma was most common (89%), with a mean Injury Severity Score score of 20. LMWH was the most common type of thromboprophylaxis used (74%), and PE was diagnosed in 2,722 patients (1.8%).
Compared with patients who received LMWH, those who received UH were older and were significantly more likely to have been injured by falling (42% vs. 28%), with higher rates of severe head injuries (43% vs. 24%) and intracranial hemorrhage (38% vs. 19%). Conversely, LMWH was most favored in patients with orthopedic injuries.
After propensity score matching, patients on LMWH suffered significantly fewer PEs (1.4% vs. 2.4%; odds ratio, 0.56). This result was consistent within propensity-matched subgroups, including for patients with blunt multisystem injuries (OR, 0.60), penetrating truncal injuries (OR, 0.65), shock in the ED (OR, 0.68), isolated severe traumatic brain injury (OR, 0.49), and isolated orthopedic injuries (OR, 0.28).
Results of a sensitivity analysis in which each propensity-matched pair was matched within the same trauma center yielded similar results. Specifically, patients who received LMWH were at significantly lower risk for developing PE (OR, 0.64). “Importantly, this analysis minimized residual confounding due to differences in hospital-level processes of care, such as prophylaxis dosing or frequency, mechanical prophylaxis use, and thromboembolism screening practices,” Dr. Byrne noted.
Multivariable logistic regression also showed that patients who received LMWH had lower odds of PE (OR, 0.59). Other significant predictors of PE included obesity (OR, 1.54), severe chest injury (OR, 1.31), femoral shaft fracture (OR, 1.60), and spinal cord injury (OR, 1.60). Delays in prophylaxis initiation beyond the first day in the hospital were associated with significantly higher rates of PE, with an 80% increased risk of PE for patients who had their prophylaxis initiated after the fourth day.
The researchers conducted a center-level analysis in an effort to answer the question whether practice patterns with respect to choice of prophylaxis type influence hospital rates of PE. Across all 217 trauma centers in the study, the median rate of LMWH use was 80%, while the mean rate of PE was 1.6%. When trauma centers were grouped into quartiles based on their unique rate of LMWH use, trauma centers in the highest quartile (median LMWH use: 95%) were 50 times more likely to use LMWH, compared to those in the lowest quartile (median LMWH use: 39%) after adjusting for patient case mix. Compared with the lowest quartile, trauma centers that used the greatest proportion of LMWH had significantly lower rates of PE (1.2% vs. 2.0%). After adjusting for patient baseline and injury characteristics, patients who were treated at trauma centers in the highest quartile had significantly lower odds of PE (OR, 0.59).
Dr. Byrne acknowledged certain limitations of the study, including the potential for residual confounding and the inability to account for the dosing and frequency of prophylaxis that was given. “We were only able to measure the type and timing of prophylaxis initiation. We don’t know what doses of prophylaxis were used, and it is possible that the trauma centers included in this study favored use of UH twice daily,” he said.
Therefore, it is possible that the results might have been different if they had been able to directly compare LMWH to UH administered three times a day. “We also couldn’t measure interruptions in dosing due to surgery or patient refusal,” he said. “However, if it the case that UH is more likely to be refused based on the need for more frequent dosing, perhaps that is another feather in the cap of low molecular weight heparin-based thromboprophylaxis strategies. Larger prospective studies are needed, that take into account prophylaxis type and dosing, and are powered to detect a difference with respect to PE.”
Dr. Byrne reported having no financial disclosures.
WAIKOLOA, HAWAII – Venous thromboembolism prophylaxis with low molecular weight heparin (LMWH), instead of unfractionated heparin (UH), is associated with lower risk of pulmonary embolism (PE) in patients with major trauma, results from a large study have shown.
The results of the study, based on data from the American College of Surgeons (ACS) Trauma Quality Improvement Program, suggest that LMWH-based strategies for thromboprophylaxis should be preferred after major trauma.
Dr. Byrne, a general surgery resident at Sunnybrook Health Science Center, Toronto, Ontario, Canada, went on to note that LMWH is often favored because of a randomized controlled trial which showed that LMWH was associated with fewer deep vein thromboses (N Engl. J. Med. 1996;335[10]:701-7). However, significant practice variability continues to exist.
“Practitioners might favor the shorter half-life of unfractionated heparin in patients where they perceive the risk for hemorrhagic complications is high,” he said. “There’s also recent evidence to suggest that dosing may be all important and that unfractionated heparin dosed three times daily may be equivalent to low molecular weight heparin. If this is true, it might suggest that the historically higher cost of low molecular weight heparin could favor the use of unfractionated heparin.”
Furthermore, there is a is a lack of evidence comparing either agent to prevent PE, he added. “This is an important gap in our knowledge, because PE frequently occurs in the absence of an identified DVT and carries a significant risk of death. At present, it is not known how practice patterns with respect to choice of prophylaxis type influence risk of PE at the patient or hospital levels.”
Due to a lack of evidence comparing agents to prevent PE, the researchers set out to compare the effectiveness of LMWH versus UH to prevent PE in patients with major trauma who were treated at trauma centers participating in the ACS Trauma Quality Improvement Program from 2012 to 2015. They included all adults with severe injury who received LMWH or UH and excluded those who died or were discharged within five days, and those with a bleeding disorder or chronic anticoagulation. The exposure was defined as thromboprophylaxis with LMWH versus UH, and the primary outcome was PE confirmed on radiologic imaging. Potential confounders were considered, including patient baseline characteristics, anatomic and global injury severity, presenting characteristics in the emergency department, acute intracranial injuries, orthopedic injuries, early surgical interventions, and timing of prophylaxis initiation.
Dr. Byrne and his associates then used three analytic approaches in the study: a propensity score matching methodology, a multivariable logistic regression model for PE, and a center-level analysis examining the influence of LMWH utilization on hospital rates of PE.
They identified 153,474 trauma patients from 217 trauma centers. Their median age was 50 years and 67% were male. Blunt trauma was most common (89%), with a mean Injury Severity Score score of 20. LMWH was the most common type of thromboprophylaxis used (74%), and PE was diagnosed in 2,722 patients (1.8%).
Compared with patients who received LMWH, those who received UH were older and were significantly more likely to have been injured by falling (42% vs. 28%), with higher rates of severe head injuries (43% vs. 24%) and intracranial hemorrhage (38% vs. 19%). Conversely, LMWH was most favored in patients with orthopedic injuries.
After propensity score matching, patients on LMWH suffered significantly fewer PEs (1.4% vs. 2.4%; odds ratio, 0.56). This result was consistent within propensity-matched subgroups, including for patients with blunt multisystem injuries (OR, 0.60), penetrating truncal injuries (OR, 0.65), shock in the ED (OR, 0.68), isolated severe traumatic brain injury (OR, 0.49), and isolated orthopedic injuries (OR, 0.28).
Results of a sensitivity analysis in which each propensity-matched pair was matched within the same trauma center yielded similar results. Specifically, patients who received LMWH were at significantly lower risk for developing PE (OR, 0.64). “Importantly, this analysis minimized residual confounding due to differences in hospital-level processes of care, such as prophylaxis dosing or frequency, mechanical prophylaxis use, and thromboembolism screening practices,” Dr. Byrne noted.
Multivariable logistic regression also showed that patients who received LMWH had lower odds of PE (OR, 0.59). Other significant predictors of PE included obesity (OR, 1.54), severe chest injury (OR, 1.31), femoral shaft fracture (OR, 1.60), and spinal cord injury (OR, 1.60). Delays in prophylaxis initiation beyond the first day in the hospital were associated with significantly higher rates of PE, with an 80% increased risk of PE for patients who had their prophylaxis initiated after the fourth day.
The researchers conducted a center-level analysis in an effort to answer the question whether practice patterns with respect to choice of prophylaxis type influence hospital rates of PE. Across all 217 trauma centers in the study, the median rate of LMWH use was 80%, while the mean rate of PE was 1.6%. When trauma centers were grouped into quartiles based on their unique rate of LMWH use, trauma centers in the highest quartile (median LMWH use: 95%) were 50 times more likely to use LMWH, compared to those in the lowest quartile (median LMWH use: 39%) after adjusting for patient case mix. Compared with the lowest quartile, trauma centers that used the greatest proportion of LMWH had significantly lower rates of PE (1.2% vs. 2.0%). After adjusting for patient baseline and injury characteristics, patients who were treated at trauma centers in the highest quartile had significantly lower odds of PE (OR, 0.59).
Dr. Byrne acknowledged certain limitations of the study, including the potential for residual confounding and the inability to account for the dosing and frequency of prophylaxis that was given. “We were only able to measure the type and timing of prophylaxis initiation. We don’t know what doses of prophylaxis were used, and it is possible that the trauma centers included in this study favored use of UH twice daily,” he said.
Therefore, it is possible that the results might have been different if they had been able to directly compare LMWH to UH administered three times a day. “We also couldn’t measure interruptions in dosing due to surgery or patient refusal,” he said. “However, if it the case that UH is more likely to be refused based on the need for more frequent dosing, perhaps that is another feather in the cap of low molecular weight heparin-based thromboprophylaxis strategies. Larger prospective studies are needed, that take into account prophylaxis type and dosing, and are powered to detect a difference with respect to PE.”
Dr. Byrne reported having no financial disclosures.
WAIKOLOA, HAWAII – Venous thromboembolism prophylaxis with low molecular weight heparin (LMWH), instead of unfractionated heparin (UH), is associated with lower risk of pulmonary embolism (PE) in patients with major trauma, results from a large study have shown.
The results of the study, based on data from the American College of Surgeons (ACS) Trauma Quality Improvement Program, suggest that LMWH-based strategies for thromboprophylaxis should be preferred after major trauma.
Dr. Byrne, a general surgery resident at Sunnybrook Health Science Center, Toronto, Ontario, Canada, went on to note that LMWH is often favored because of a randomized controlled trial which showed that LMWH was associated with fewer deep vein thromboses (N Engl. J. Med. 1996;335[10]:701-7). However, significant practice variability continues to exist.
“Practitioners might favor the shorter half-life of unfractionated heparin in patients where they perceive the risk for hemorrhagic complications is high,” he said. “There’s also recent evidence to suggest that dosing may be all important and that unfractionated heparin dosed three times daily may be equivalent to low molecular weight heparin. If this is true, it might suggest that the historically higher cost of low molecular weight heparin could favor the use of unfractionated heparin.”
Furthermore, there is a is a lack of evidence comparing either agent to prevent PE, he added. “This is an important gap in our knowledge, because PE frequently occurs in the absence of an identified DVT and carries a significant risk of death. At present, it is not known how practice patterns with respect to choice of prophylaxis type influence risk of PE at the patient or hospital levels.”
Due to a lack of evidence comparing agents to prevent PE, the researchers set out to compare the effectiveness of LMWH versus UH to prevent PE in patients with major trauma who were treated at trauma centers participating in the ACS Trauma Quality Improvement Program from 2012 to 2015. They included all adults with severe injury who received LMWH or UH and excluded those who died or were discharged within five days, and those with a bleeding disorder or chronic anticoagulation. The exposure was defined as thromboprophylaxis with LMWH versus UH, and the primary outcome was PE confirmed on radiologic imaging. Potential confounders were considered, including patient baseline characteristics, anatomic and global injury severity, presenting characteristics in the emergency department, acute intracranial injuries, orthopedic injuries, early surgical interventions, and timing of prophylaxis initiation.
Dr. Byrne and his associates then used three analytic approaches in the study: a propensity score matching methodology, a multivariable logistic regression model for PE, and a center-level analysis examining the influence of LMWH utilization on hospital rates of PE.
They identified 153,474 trauma patients from 217 trauma centers. Their median age was 50 years and 67% were male. Blunt trauma was most common (89%), with a mean Injury Severity Score score of 20. LMWH was the most common type of thromboprophylaxis used (74%), and PE was diagnosed in 2,722 patients (1.8%).
Compared with patients who received LMWH, those who received UH were older and were significantly more likely to have been injured by falling (42% vs. 28%), with higher rates of severe head injuries (43% vs. 24%) and intracranial hemorrhage (38% vs. 19%). Conversely, LMWH was most favored in patients with orthopedic injuries.
After propensity score matching, patients on LMWH suffered significantly fewer PEs (1.4% vs. 2.4%; odds ratio, 0.56). This result was consistent within propensity-matched subgroups, including for patients with blunt multisystem injuries (OR, 0.60), penetrating truncal injuries (OR, 0.65), shock in the ED (OR, 0.68), isolated severe traumatic brain injury (OR, 0.49), and isolated orthopedic injuries (OR, 0.28).
Results of a sensitivity analysis in which each propensity-matched pair was matched within the same trauma center yielded similar results. Specifically, patients who received LMWH were at significantly lower risk for developing PE (OR, 0.64). “Importantly, this analysis minimized residual confounding due to differences in hospital-level processes of care, such as prophylaxis dosing or frequency, mechanical prophylaxis use, and thromboembolism screening practices,” Dr. Byrne noted.
Multivariable logistic regression also showed that patients who received LMWH had lower odds of PE (OR, 0.59). Other significant predictors of PE included obesity (OR, 1.54), severe chest injury (OR, 1.31), femoral shaft fracture (OR, 1.60), and spinal cord injury (OR, 1.60). Delays in prophylaxis initiation beyond the first day in the hospital were associated with significantly higher rates of PE, with an 80% increased risk of PE for patients who had their prophylaxis initiated after the fourth day.
The researchers conducted a center-level analysis in an effort to answer the question whether practice patterns with respect to choice of prophylaxis type influence hospital rates of PE. Across all 217 trauma centers in the study, the median rate of LMWH use was 80%, while the mean rate of PE was 1.6%. When trauma centers were grouped into quartiles based on their unique rate of LMWH use, trauma centers in the highest quartile (median LMWH use: 95%) were 50 times more likely to use LMWH, compared to those in the lowest quartile (median LMWH use: 39%) after adjusting for patient case mix. Compared with the lowest quartile, trauma centers that used the greatest proportion of LMWH had significantly lower rates of PE (1.2% vs. 2.0%). After adjusting for patient baseline and injury characteristics, patients who were treated at trauma centers in the highest quartile had significantly lower odds of PE (OR, 0.59).
Dr. Byrne acknowledged certain limitations of the study, including the potential for residual confounding and the inability to account for the dosing and frequency of prophylaxis that was given. “We were only able to measure the type and timing of prophylaxis initiation. We don’t know what doses of prophylaxis were used, and it is possible that the trauma centers included in this study favored use of UH twice daily,” he said.
Therefore, it is possible that the results might have been different if they had been able to directly compare LMWH to UH administered three times a day. “We also couldn’t measure interruptions in dosing due to surgery or patient refusal,” he said. “However, if it the case that UH is more likely to be refused based on the need for more frequent dosing, perhaps that is another feather in the cap of low molecular weight heparin-based thromboprophylaxis strategies. Larger prospective studies are needed, that take into account prophylaxis type and dosing, and are powered to detect a difference with respect to PE.”
Dr. Byrne reported having no financial disclosures.
AT THE AAST ANNUAL MEETING
Key clinical point:
Major finding: After propensity score matching, patients on LMWH had significantly fewer PEs, compared with those on unfractionated heparin (1.4% vs. 2.4%; odds ratio, 0.56). Data source: A multicenter analysis of 2,722 trauma patients who were diagnosed with pulmonary embolism.
Disclosures: Dr. Byrne reported having no financial disclosures.
Pulmonary embolism common in patients hospitalized for syncope
When specifically looked for, pulmonary embolism was identified in approximately 17% of adults hospitalized for a first episode of syncope, according to a report published in the New England Journal of Medicine.
Most medical textbooks include pulmonary embolism (PE) in the differential diagnosis of syncope, but “current international guidelines, including those from the European Society of Cardiology and the American Heart Association, pay little attention to establishing a diagnostic workup for PE in these patients. Hence, when a patient is admitted to a hospital for an episode of syncope, PE – a potentially fatal disease that can be effectively treated – is rarely considered as a possible cause,” said Paolo Prandoni, MD, PhD, of the vascular medicine unit, University of Padua (Italy), and his associates in the PESY (Prevalence of Pulmonary Embolism in Patients With Syncope) trial.
The investigators used a systematic diagnostic work-up to determine the prevalence of PE in a cross-sectional study involving 560 adults hospitalized for syncope at 11 medical centers across Italy during a 2.5-year period. Most of these patients were elderly (mean age, 76 years), and most had clinical evidence indicating that a factor other than PE had caused their fainting. For this study, syncope was defined as a transient loss of consciousness with rapid onset, short duration (less than 1 minute), and spontaneous resolution, with obvious causes ruled out (such as epileptic seizure, stroke, or head trauma).
The “unexpectedly high” prevalence of PE was 17.3% overall, and it was consistent, ranging from 15% to 20%, across all 11 hospitals. The prevalence was even higher, at 25.4%, in the subgroup of 205 patients who had syncope of undetermined origin, as well as in 12.7% of the subgroup of 355 patients considered to have an alternative explanation for the disorder, Dr. Prandoni and his associates wrote (N Engl J Med. 2016 Oct 20. doi: 10.1056/NEJMoa1602172).
The researchers noted that this study likely underestimates the actual prevalence of PE among patients with syncope because it did not include patients who were not hospitalized, such as those who received only ambulatory care and those who presented to an emergency department but were not admitted.
The study was supported by the University of Padua. Dr. Prandoni and his associates reported having no relevant financial disclosures.
When specifically looked for, pulmonary embolism was identified in approximately 17% of adults hospitalized for a first episode of syncope, according to a report published in the New England Journal of Medicine.
Most medical textbooks include pulmonary embolism (PE) in the differential diagnosis of syncope, but “current international guidelines, including those from the European Society of Cardiology and the American Heart Association, pay little attention to establishing a diagnostic workup for PE in these patients. Hence, when a patient is admitted to a hospital for an episode of syncope, PE – a potentially fatal disease that can be effectively treated – is rarely considered as a possible cause,” said Paolo Prandoni, MD, PhD, of the vascular medicine unit, University of Padua (Italy), and his associates in the PESY (Prevalence of Pulmonary Embolism in Patients With Syncope) trial.
The investigators used a systematic diagnostic work-up to determine the prevalence of PE in a cross-sectional study involving 560 adults hospitalized for syncope at 11 medical centers across Italy during a 2.5-year period. Most of these patients were elderly (mean age, 76 years), and most had clinical evidence indicating that a factor other than PE had caused their fainting. For this study, syncope was defined as a transient loss of consciousness with rapid onset, short duration (less than 1 minute), and spontaneous resolution, with obvious causes ruled out (such as epileptic seizure, stroke, or head trauma).
The “unexpectedly high” prevalence of PE was 17.3% overall, and it was consistent, ranging from 15% to 20%, across all 11 hospitals. The prevalence was even higher, at 25.4%, in the subgroup of 205 patients who had syncope of undetermined origin, as well as in 12.7% of the subgroup of 355 patients considered to have an alternative explanation for the disorder, Dr. Prandoni and his associates wrote (N Engl J Med. 2016 Oct 20. doi: 10.1056/NEJMoa1602172).
The researchers noted that this study likely underestimates the actual prevalence of PE among patients with syncope because it did not include patients who were not hospitalized, such as those who received only ambulatory care and those who presented to an emergency department but were not admitted.
The study was supported by the University of Padua. Dr. Prandoni and his associates reported having no relevant financial disclosures.
When specifically looked for, pulmonary embolism was identified in approximately 17% of adults hospitalized for a first episode of syncope, according to a report published in the New England Journal of Medicine.
Most medical textbooks include pulmonary embolism (PE) in the differential diagnosis of syncope, but “current international guidelines, including those from the European Society of Cardiology and the American Heart Association, pay little attention to establishing a diagnostic workup for PE in these patients. Hence, when a patient is admitted to a hospital for an episode of syncope, PE – a potentially fatal disease that can be effectively treated – is rarely considered as a possible cause,” said Paolo Prandoni, MD, PhD, of the vascular medicine unit, University of Padua (Italy), and his associates in the PESY (Prevalence of Pulmonary Embolism in Patients With Syncope) trial.
The investigators used a systematic diagnostic work-up to determine the prevalence of PE in a cross-sectional study involving 560 adults hospitalized for syncope at 11 medical centers across Italy during a 2.5-year period. Most of these patients were elderly (mean age, 76 years), and most had clinical evidence indicating that a factor other than PE had caused their fainting. For this study, syncope was defined as a transient loss of consciousness with rapid onset, short duration (less than 1 minute), and spontaneous resolution, with obvious causes ruled out (such as epileptic seizure, stroke, or head trauma).
The “unexpectedly high” prevalence of PE was 17.3% overall, and it was consistent, ranging from 15% to 20%, across all 11 hospitals. The prevalence was even higher, at 25.4%, in the subgroup of 205 patients who had syncope of undetermined origin, as well as in 12.7% of the subgroup of 355 patients considered to have an alternative explanation for the disorder, Dr. Prandoni and his associates wrote (N Engl J Med. 2016 Oct 20. doi: 10.1056/NEJMoa1602172).
The researchers noted that this study likely underestimates the actual prevalence of PE among patients with syncope because it did not include patients who were not hospitalized, such as those who received only ambulatory care and those who presented to an emergency department but were not admitted.
The study was supported by the University of Padua. Dr. Prandoni and his associates reported having no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: When specifically looked for, pulmonary embolism was identified in approximately 17% of adults hospitalized for a first episode of syncope.
Major finding: The “unexpectedly high” prevalence of PE was 17.3% overall, and it was consistent, ranging from 15% to 20%, across all 11 hospitals in the study.
Data source: A cross-sectional study involving 560 adults hospitalized for syncope at 11 Italian medical centers during a 2.5 year period.
Disclosures: This study was supported by the University of Padua (Italy). Dr. Prandoni and his associates reported having no relevant financial disclosures.
Algorithm for suspected pulmonary embolism safely cut CT rate
ROME – A newly validated, simplified algorithm for the management of patients with suspected acute pulmonary embolism enables physicians to safely exclude the disorder in roughly half of patients without resorting to CT pulmonary angiography, Tom van der Hulle, MD, reported at the annual congress of the European Society of Cardiology.
“This is the largest study ever performed in the diagnostic management of suspected pulmonary embolism. Based on our results, I think the YEARS algorithm is ready to be used in daily clinical practice,” declared Dr. van der Hulle of the department of thrombosis and hemostasis at Leiden (the Netherlands) University Medical Center.
Using the YEARS algorithm, PE was reliably ruled out without need for CT pulmonary angiography – considered the standard in the diagnosis of PE – in 48% of patients. In contrast, adherence to the Wells rule would have meant that 62% of patients would have gotten a CT scan to rule out PE with a comparably high degree of accuracy.
But that 62% figure underestimates the actual CT rate in clinical practice. The reality is that although the guideline-recommended Wells rule and revised Geneva score have been shown to be safe and accurate, they are so complex, cumbersome, and out of sync with the flow of routine clinical practice that many physicians skip the algorithms and go straight to CT, Dr. van der Hulle said. This approach results in many unnecessary CTs, needlessly exposing patients to the risks of radiation and intravenous contrast material while driving up health care costs, he added.
Using the Wells rule or revised Geneva score, the patient evaluation begins with an assessment of the clinical probability of PE based upon a risk score involving seven or eight factors. Only patients with a low or intermediate clinical probability of PE get a D-dimer test; those with a high clinical probability go straight to CT.
The YEARS algorithm is much simpler than that, Dr. van der Hulle explained. Everyone who presents with suspected acute PE gets a D-dimer test while the physician simultaneously applies a brief, three-item clinical prediction rule. These three items were selected by the Dutch investigators because they were the three strongest predictors of PE out of the original seven in the Wells rule. They are hemoptysis, clinical signs of deep vein thrombosis such as leg swelling or hyperpigmentation, and the clinician’s global impression of PE as being the most likely diagnosis.
In the YEARS algorithm, the threshold for a positive D-dimer test warranting CT pulmonary angiography depends upon whether any of the three clinical predictors is present. If none is present, the threshold is 1,000 ng/mL or above; if one or more is present, the threshold for a positive D-dimer test drops to 500 ng/mL.
Using these criteria, PE was excluded without resort to CT in 1,306 patients with none of the three YEARS items and a D-dimer test result below 1,000 ng/mL, as well as in another 327 patients with one or more YEARS items present but a D-dimer below 500 ng/mL. Those two groups were left untreated and followed prospectively for 3 months.
The 964 patients with one or more YEARS predictors present and a D-dimer score of at least 500 ng/mL underwent CT imaging, as did the 352 with no YEARS items and a D-dimer of at least 1,000 ng/mL.
The prevalence of CT-confirmed PE in the study was 13.2%. Affected patients were treated with anticoagulants.
The primary study endpoint was the total rate of deep vein thrombosis during 3 months of follow-up after PE had been excluded. The rate was 0.61%, including a fatal PE rate of 0.20%. The rate in patients managed without CT was 0.43%, including a 0.12% rate of fatal PE. In patients managed with diagnostic CT, the deep vein thrombosis rate was 0.84%, with a fatal PE rate of 0.30%.
“I think these results are completely comparable to those in previous studies using the standard algorithms,” Dr. van der Hulle commented.
The study’s main limitation is that it wasn’t a randomized, controlled trial. But given the tiny event rates, detecting any small differences between management strategies would require an unrealistically huge sample size, he added.
Asked if he thinks physicians will actually use the new tool, Dr. van der Hulle replied that some physicians feel driven to be 100% sure that a patient doesn’t have PE, and they will probably keep overordering CT scans. But others will embrace the YEARS algorithm because it reduces wasted resources and minimizes radiation exposure, a particularly compelling consideration in young female patients.
Discussant Marion Delcroix, MD, had reservations. She said she appreciated the appeal of a simple algorithm, but she asked, “Couldn’t we do better with a bit more sophistication, perhaps by adjusting the D-dimer cutoff for age and also adding some other items, like oxygen saturation and estrogen use?
“My concern is about the applicability. The age of the study cohort is relatively young, at a mean of 53 years. The peak age of PE in a very large contemporary German database is 70-80 years. We don’t know if the YEARS score is any good in this older population,” asserted Dr. Delcroix, professor of medicine and respiratory physiology and head of the center for pulmonary vascular diseases at University Hospital in Leuven, Belgium.
“If the aim is to decrease the number of CT pulmonary angiograms for safety reasons, why not reintroduce compression ultrasound of the lower limbs in the diagnostic algorithm?” she continued. “It has been shown to effectively reduce the need for further imaging.”
Dr. Delcroix predicted that the YEARS algorithm study will prove “too optimistic” regarding the number of CT scans avoided, particularly in elderly patients.
The YEARS study was funded by the trial’s 12 participating Dutch hospitals. Dr. van der Hulle reported having no financial conflicts of interest.
ROME – A newly validated, simplified algorithm for the management of patients with suspected acute pulmonary embolism enables physicians to safely exclude the disorder in roughly half of patients without resorting to CT pulmonary angiography, Tom van der Hulle, MD, reported at the annual congress of the European Society of Cardiology.
“This is the largest study ever performed in the diagnostic management of suspected pulmonary embolism. Based on our results, I think the YEARS algorithm is ready to be used in daily clinical practice,” declared Dr. van der Hulle of the department of thrombosis and hemostasis at Leiden (the Netherlands) University Medical Center.
Using the YEARS algorithm, PE was reliably ruled out without need for CT pulmonary angiography – considered the standard in the diagnosis of PE – in 48% of patients. In contrast, adherence to the Wells rule would have meant that 62% of patients would have gotten a CT scan to rule out PE with a comparably high degree of accuracy.
But that 62% figure underestimates the actual CT rate in clinical practice. The reality is that although the guideline-recommended Wells rule and revised Geneva score have been shown to be safe and accurate, they are so complex, cumbersome, and out of sync with the flow of routine clinical practice that many physicians skip the algorithms and go straight to CT, Dr. van der Hulle said. This approach results in many unnecessary CTs, needlessly exposing patients to the risks of radiation and intravenous contrast material while driving up health care costs, he added.
Using the Wells rule or revised Geneva score, the patient evaluation begins with an assessment of the clinical probability of PE based upon a risk score involving seven or eight factors. Only patients with a low or intermediate clinical probability of PE get a D-dimer test; those with a high clinical probability go straight to CT.
The YEARS algorithm is much simpler than that, Dr. van der Hulle explained. Everyone who presents with suspected acute PE gets a D-dimer test while the physician simultaneously applies a brief, three-item clinical prediction rule. These three items were selected by the Dutch investigators because they were the three strongest predictors of PE out of the original seven in the Wells rule. They are hemoptysis, clinical signs of deep vein thrombosis such as leg swelling or hyperpigmentation, and the clinician’s global impression of PE as being the most likely diagnosis.
In the YEARS algorithm, the threshold for a positive D-dimer test warranting CT pulmonary angiography depends upon whether any of the three clinical predictors is present. If none is present, the threshold is 1,000 ng/mL or above; if one or more is present, the threshold for a positive D-dimer test drops to 500 ng/mL.
Using these criteria, PE was excluded without resort to CT in 1,306 patients with none of the three YEARS items and a D-dimer test result below 1,000 ng/mL, as well as in another 327 patients with one or more YEARS items present but a D-dimer below 500 ng/mL. Those two groups were left untreated and followed prospectively for 3 months.
The 964 patients with one or more YEARS predictors present and a D-dimer score of at least 500 ng/mL underwent CT imaging, as did the 352 with no YEARS items and a D-dimer of at least 1,000 ng/mL.
The prevalence of CT-confirmed PE in the study was 13.2%. Affected patients were treated with anticoagulants.
The primary study endpoint was the total rate of deep vein thrombosis during 3 months of follow-up after PE had been excluded. The rate was 0.61%, including a fatal PE rate of 0.20%. The rate in patients managed without CT was 0.43%, including a 0.12% rate of fatal PE. In patients managed with diagnostic CT, the deep vein thrombosis rate was 0.84%, with a fatal PE rate of 0.30%.
“I think these results are completely comparable to those in previous studies using the standard algorithms,” Dr. van der Hulle commented.
The study’s main limitation is that it wasn’t a randomized, controlled trial. But given the tiny event rates, detecting any small differences between management strategies would require an unrealistically huge sample size, he added.
Asked if he thinks physicians will actually use the new tool, Dr. van der Hulle replied that some physicians feel driven to be 100% sure that a patient doesn’t have PE, and they will probably keep overordering CT scans. But others will embrace the YEARS algorithm because it reduces wasted resources and minimizes radiation exposure, a particularly compelling consideration in young female patients.
Discussant Marion Delcroix, MD, had reservations. She said she appreciated the appeal of a simple algorithm, but she asked, “Couldn’t we do better with a bit more sophistication, perhaps by adjusting the D-dimer cutoff for age and also adding some other items, like oxygen saturation and estrogen use?
“My concern is about the applicability. The age of the study cohort is relatively young, at a mean of 53 years. The peak age of PE in a very large contemporary German database is 70-80 years. We don’t know if the YEARS score is any good in this older population,” asserted Dr. Delcroix, professor of medicine and respiratory physiology and head of the center for pulmonary vascular diseases at University Hospital in Leuven, Belgium.
“If the aim is to decrease the number of CT pulmonary angiograms for safety reasons, why not reintroduce compression ultrasound of the lower limbs in the diagnostic algorithm?” she continued. “It has been shown to effectively reduce the need for further imaging.”
Dr. Delcroix predicted that the YEARS algorithm study will prove “too optimistic” regarding the number of CT scans avoided, particularly in elderly patients.
The YEARS study was funded by the trial’s 12 participating Dutch hospitals. Dr. van der Hulle reported having no financial conflicts of interest.
ROME – A newly validated, simplified algorithm for the management of patients with suspected acute pulmonary embolism enables physicians to safely exclude the disorder in roughly half of patients without resorting to CT pulmonary angiography, Tom van der Hulle, MD, reported at the annual congress of the European Society of Cardiology.
“This is the largest study ever performed in the diagnostic management of suspected pulmonary embolism. Based on our results, I think the YEARS algorithm is ready to be used in daily clinical practice,” declared Dr. van der Hulle of the department of thrombosis and hemostasis at Leiden (the Netherlands) University Medical Center.
Using the YEARS algorithm, PE was reliably ruled out without need for CT pulmonary angiography – considered the standard in the diagnosis of PE – in 48% of patients. In contrast, adherence to the Wells rule would have meant that 62% of patients would have gotten a CT scan to rule out PE with a comparably high degree of accuracy.
But that 62% figure underestimates the actual CT rate in clinical practice. The reality is that although the guideline-recommended Wells rule and revised Geneva score have been shown to be safe and accurate, they are so complex, cumbersome, and out of sync with the flow of routine clinical practice that many physicians skip the algorithms and go straight to CT, Dr. van der Hulle said. This approach results in many unnecessary CTs, needlessly exposing patients to the risks of radiation and intravenous contrast material while driving up health care costs, he added.
Using the Wells rule or revised Geneva score, the patient evaluation begins with an assessment of the clinical probability of PE based upon a risk score involving seven or eight factors. Only patients with a low or intermediate clinical probability of PE get a D-dimer test; those with a high clinical probability go straight to CT.
The YEARS algorithm is much simpler than that, Dr. van der Hulle explained. Everyone who presents with suspected acute PE gets a D-dimer test while the physician simultaneously applies a brief, three-item clinical prediction rule. These three items were selected by the Dutch investigators because they were the three strongest predictors of PE out of the original seven in the Wells rule. They are hemoptysis, clinical signs of deep vein thrombosis such as leg swelling or hyperpigmentation, and the clinician’s global impression of PE as being the most likely diagnosis.
In the YEARS algorithm, the threshold for a positive D-dimer test warranting CT pulmonary angiography depends upon whether any of the three clinical predictors is present. If none is present, the threshold is 1,000 ng/mL or above; if one or more is present, the threshold for a positive D-dimer test drops to 500 ng/mL.
Using these criteria, PE was excluded without resort to CT in 1,306 patients with none of the three YEARS items and a D-dimer test result below 1,000 ng/mL, as well as in another 327 patients with one or more YEARS items present but a D-dimer below 500 ng/mL. Those two groups were left untreated and followed prospectively for 3 months.
The 964 patients with one or more YEARS predictors present and a D-dimer score of at least 500 ng/mL underwent CT imaging, as did the 352 with no YEARS items and a D-dimer of at least 1,000 ng/mL.
The prevalence of CT-confirmed PE in the study was 13.2%. Affected patients were treated with anticoagulants.
The primary study endpoint was the total rate of deep vein thrombosis during 3 months of follow-up after PE had been excluded. The rate was 0.61%, including a fatal PE rate of 0.20%. The rate in patients managed without CT was 0.43%, including a 0.12% rate of fatal PE. In patients managed with diagnostic CT, the deep vein thrombosis rate was 0.84%, with a fatal PE rate of 0.30%.
“I think these results are completely comparable to those in previous studies using the standard algorithms,” Dr. van der Hulle commented.
The study’s main limitation is that it wasn’t a randomized, controlled trial. But given the tiny event rates, detecting any small differences between management strategies would require an unrealistically huge sample size, he added.
Asked if he thinks physicians will actually use the new tool, Dr. van der Hulle replied that some physicians feel driven to be 100% sure that a patient doesn’t have PE, and they will probably keep overordering CT scans. But others will embrace the YEARS algorithm because it reduces wasted resources and minimizes radiation exposure, a particularly compelling consideration in young female patients.
Discussant Marion Delcroix, MD, had reservations. She said she appreciated the appeal of a simple algorithm, but she asked, “Couldn’t we do better with a bit more sophistication, perhaps by adjusting the D-dimer cutoff for age and also adding some other items, like oxygen saturation and estrogen use?
“My concern is about the applicability. The age of the study cohort is relatively young, at a mean of 53 years. The peak age of PE in a very large contemporary German database is 70-80 years. We don’t know if the YEARS score is any good in this older population,” asserted Dr. Delcroix, professor of medicine and respiratory physiology and head of the center for pulmonary vascular diseases at University Hospital in Leuven, Belgium.
“If the aim is to decrease the number of CT pulmonary angiograms for safety reasons, why not reintroduce compression ultrasound of the lower limbs in the diagnostic algorithm?” she continued. “It has been shown to effectively reduce the need for further imaging.”
Dr. Delcroix predicted that the YEARS algorithm study will prove “too optimistic” regarding the number of CT scans avoided, particularly in elderly patients.
The YEARS study was funded by the trial’s 12 participating Dutch hospitals. Dr. van der Hulle reported having no financial conflicts of interest.
Key clinical point:
Major finding: Applying the YEARS algorithm to a large population of patients with suspected PE, the 3-month incidence of deep vein thrombosis after PE had been excluded was 0.61%.
Data source: This was a prospective study of clinical outcomes in nearly 3,000 consecutive Dutch patients who presented with suspected acute PE and were managed in accord with the YEARS algorithm.
Disclosures: The YEARS algorithm validation study was funded by the trial’s 12 participating Dutch hospitals. The study presenter reported having no financial conflicts of interest.
Winning Ideas for Preventing and Reducing VTE
Inventive ways of identifying and treating patients with health care associated venous thromboembolism (HA-VTE) have garnered awards for 8 hospitals and health care systems in the HA-VTE Prevention Challenge, sponsored by the CDC.
The winners range from small community hospitals to large health care systems: Mayo Clinic, University of California Health, Center for Health Quality and Innovation; University of Wisconsin Health (Madison); Intermountain Healthcare (Murray, UT); Northwestern Memorial Hospital (Chicago); Johns Hopkins Hospital (Baltimore); Harborview Medical Center (Seattle); and Hutchinson (KS) Regional Medical Center.
All improved VTE prevention with innovative, effective, and sustainable initiatives and strategies.
Harborview Medical Center (HMC) developed an electronic tool for efficient, standardized review of HA-VTE. The tool uses natural language processing, allowing the HMC VTE Task Force to quickly gauge the accuracy of risk assessment and appropriateness of prophylaxis. It also developed tools to provide real-time, actionable information at the bedside, including lists that highlight patients who have not received chemical or mechanical prophylaxis in 24 hours. Those who have received vitamin K antagonists are identified to ensure patient/family education and appropriate follow-up. Treatment data “snapshots” are embedded in resident physician and nursing handoff tools to enhance multidisciplinary communication. All process and outcome measures are displayed on an internal web-based dashboard, with improvement opportunities highlighted.
As a result, HMC has had zero potentially preventable VTE events since the measure was implemented in January 2013—a national best practice. Improved VTE prophylaxis contributed to a 15% reduction in HA-VTE between 2011 and 2015. Among postoperative patients, the rate of VTE dropped 21%. Diagnosis, treatment, patient education, and outpatient follow-up have all improved. Moreover, HMC says, the lessons learned have formed the basis of ongoing improvement initiatives.
Four entrants (“Unique Populations and Interventions”) received honorable mentions: Michigan Hospital Medicine Safety Consortium, Ann Arbor; Sheppard Pratt Health System, Baltimore; Rotunda Hospital, Dublin, Ireland; and University of Cincinnati Medical Center.
Inventive ways of identifying and treating patients with health care associated venous thromboembolism (HA-VTE) have garnered awards for 8 hospitals and health care systems in the HA-VTE Prevention Challenge, sponsored by the CDC.
The winners range from small community hospitals to large health care systems: Mayo Clinic, University of California Health, Center for Health Quality and Innovation; University of Wisconsin Health (Madison); Intermountain Healthcare (Murray, UT); Northwestern Memorial Hospital (Chicago); Johns Hopkins Hospital (Baltimore); Harborview Medical Center (Seattle); and Hutchinson (KS) Regional Medical Center.
All improved VTE prevention with innovative, effective, and sustainable initiatives and strategies.
Harborview Medical Center (HMC) developed an electronic tool for efficient, standardized review of HA-VTE. The tool uses natural language processing, allowing the HMC VTE Task Force to quickly gauge the accuracy of risk assessment and appropriateness of prophylaxis. It also developed tools to provide real-time, actionable information at the bedside, including lists that highlight patients who have not received chemical or mechanical prophylaxis in 24 hours. Those who have received vitamin K antagonists are identified to ensure patient/family education and appropriate follow-up. Treatment data “snapshots” are embedded in resident physician and nursing handoff tools to enhance multidisciplinary communication. All process and outcome measures are displayed on an internal web-based dashboard, with improvement opportunities highlighted.
As a result, HMC has had zero potentially preventable VTE events since the measure was implemented in January 2013—a national best practice. Improved VTE prophylaxis contributed to a 15% reduction in HA-VTE between 2011 and 2015. Among postoperative patients, the rate of VTE dropped 21%. Diagnosis, treatment, patient education, and outpatient follow-up have all improved. Moreover, HMC says, the lessons learned have formed the basis of ongoing improvement initiatives.
Four entrants (“Unique Populations and Interventions”) received honorable mentions: Michigan Hospital Medicine Safety Consortium, Ann Arbor; Sheppard Pratt Health System, Baltimore; Rotunda Hospital, Dublin, Ireland; and University of Cincinnati Medical Center.
Inventive ways of identifying and treating patients with health care associated venous thromboembolism (HA-VTE) have garnered awards for 8 hospitals and health care systems in the HA-VTE Prevention Challenge, sponsored by the CDC.
The winners range from small community hospitals to large health care systems: Mayo Clinic, University of California Health, Center for Health Quality and Innovation; University of Wisconsin Health (Madison); Intermountain Healthcare (Murray, UT); Northwestern Memorial Hospital (Chicago); Johns Hopkins Hospital (Baltimore); Harborview Medical Center (Seattle); and Hutchinson (KS) Regional Medical Center.
All improved VTE prevention with innovative, effective, and sustainable initiatives and strategies.
Harborview Medical Center (HMC) developed an electronic tool for efficient, standardized review of HA-VTE. The tool uses natural language processing, allowing the HMC VTE Task Force to quickly gauge the accuracy of risk assessment and appropriateness of prophylaxis. It also developed tools to provide real-time, actionable information at the bedside, including lists that highlight patients who have not received chemical or mechanical prophylaxis in 24 hours. Those who have received vitamin K antagonists are identified to ensure patient/family education and appropriate follow-up. Treatment data “snapshots” are embedded in resident physician and nursing handoff tools to enhance multidisciplinary communication. All process and outcome measures are displayed on an internal web-based dashboard, with improvement opportunities highlighted.
As a result, HMC has had zero potentially preventable VTE events since the measure was implemented in January 2013—a national best practice. Improved VTE prophylaxis contributed to a 15% reduction in HA-VTE between 2011 and 2015. Among postoperative patients, the rate of VTE dropped 21%. Diagnosis, treatment, patient education, and outpatient follow-up have all improved. Moreover, HMC says, the lessons learned have formed the basis of ongoing improvement initiatives.
Four entrants (“Unique Populations and Interventions”) received honorable mentions: Michigan Hospital Medicine Safety Consortium, Ann Arbor; Sheppard Pratt Health System, Baltimore; Rotunda Hospital, Dublin, Ireland; and University of Cincinnati Medical Center.
Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.