User login
Is genetic testing valuable in the clinical management of epilepsy?
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.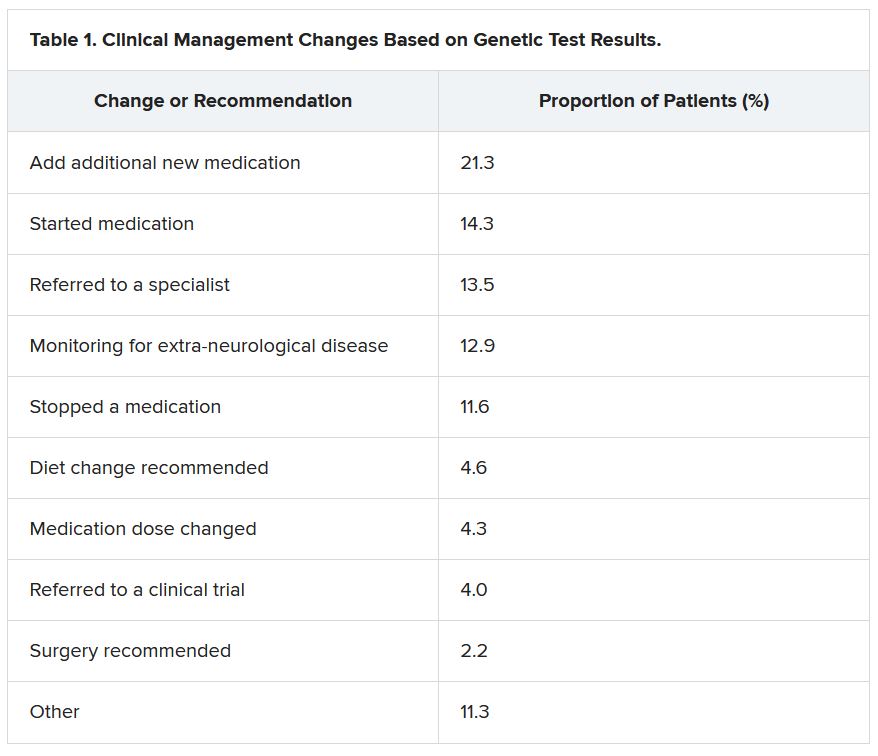
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”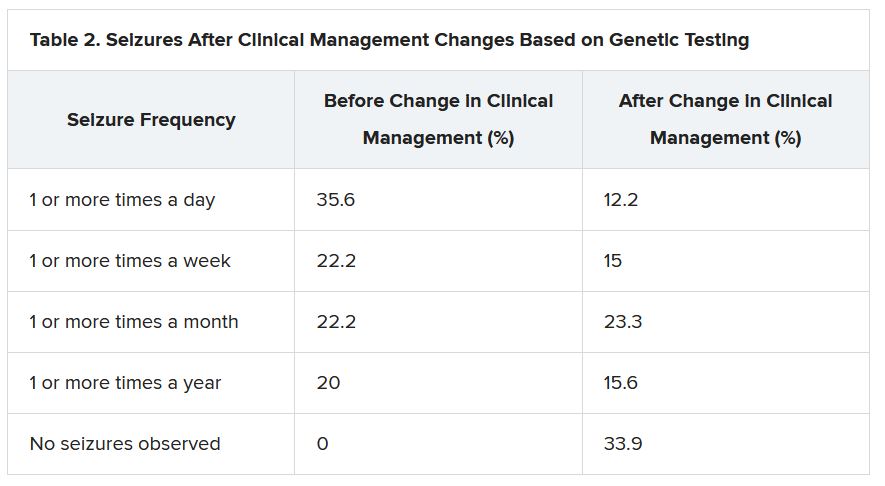
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows.
Results of a survey that included more than 400 patients showed that positive findings from genetic testing helped guide clinical management in 50% of cases and improved patient outcomes in 75%. In addition, the findings were applicable to both children and adults.
“Fifty percent of the time the physicians reported that, yes, receiving the genetic diagnosis did change how they managed the patients,” reported co-investigator Dianalee McKnight, PhD, director of medical affairs at Invitae, a medical genetic testing company headquartered in San Francisco. In 81.3% of cases, providers reported they changed clinical management within 3 months of receiving the genetic results, she added.
The findings were presented at the 2021 World Congress of Neurology (WCN).
Test results can be practice-changing
Nearly 50% of positive genetic test results in epilepsy patients can help guide clinical management, Dr. McKnight noted. However, information on how physicians use genetic information in decision-making has been limited, prompting her conduct the survey.
A total of 1,567 physicians with 3,572 patients who had a definitive diagnosis of epilepsy were contacted. A total of 170 (10.8%) clinicians provided completed and eligible surveys on 429 patients with epilepsy.
The patient cohort comprised mostly children, with nearly 50 adults, which Dr. McKnight said is typical of the population receiving genetic testing in clinical practice.
She reported that genetic testing results prompted clinicians to make medication changes about 50% of the time. Other changes included specialist referral or to a clinical trial, monitoring for other neurological disease, and recommendations for dietary change or for surgery.
“Of the physicians who changed treatment, 75% reported there were positive outcomes for the patients,” Dr. McKnight told meeting attendees. “Most common was a reduction or a complete elimination of seizures, and that was reported in 65% of the cases.”
In many cases, the changes resulted in clinical improvements.
“There were 64 individuals who were having daily seizures before the genetic testing,” Dr. McKnight reported via email. “After receiving the genetic diagnosis and modifying their treatment, their physicians reported that 26% of individuals had complete seizure control and 46% of individuals had reduced seizure frequency to either weekly (20%), monthly (20%) or annually (6%).”
The best seizure control after modifying disease management occurred among children. Although the changes were not as dramatic for adults, they trended toward lower seizure frequency.
“It is still pretty significant that adults can receive genetic testing later in life and still have benefit in controlling their seizures,” Dr. McKnight said.
Twenty-three percent of patients showed improvement in behavior, development, academics, or movement issues, while 6% experienced reduced medication side effects.
Dr. McKnight also explored reasons for physicians not making changes to clinical management of patients based on the genetic results. The most common reason was that management was already consistent with the results (47.3%), followed by the results not being informative (26.1%), the results possibly being useful for future treatments in development (19.0%), or other or unknown reasons (7.6%).
Besides direct health and quality of life benefits from better seizure control, Dr. McKnight cited previous economic studies showing lower health care costs.
“It looked like an individual who has good seizure control will incur about 14,000 U.S. dollars a year compared with an individual with pretty poor seizure control, where it can be closer to 23,000 U.S. dollars a year,” Dr. McKnight said. This is mainly attributed to reduced hospitalizations and emergency department visits.
Dr. McKnight noted that currently there is no cost of genetic testing to the patient, the hospital, or insurers. Pharmaceutical companies, she said, sponsor the testing to potentially gather patients for clinical drug trials in development. However, patients remain completely anonymous.
Physicians who wish to have patient samples tested agree that the companies may contact them to ask if any of their patients with positive genetic test results would like to participate in a trial.
Dr. McKnight noted that genetic testing can be considered actionable in the clinic, helping to guide clinical decision-making and potentially leading to better outcomes. Going forward, she suggested performing large case-controlled studies “of individuals with the same genetic etiology ... to really find a true causation or correlation.”
Growing influence of genetic testing
Commenting on the findings, Jaysingh Singh, MD, co-director of the Epilepsy Surgery Center at the Ohio State University Wexner Medical Center in Columbus, noted that the study highlights the value of gene testing in improving outcomes in patients with epilepsy, particularly the pediatric population.
He said the findings make him optimistic about the potential of genetic testing in adult patients – with at least one caveat.
“The limitation is that if we do find some mutation, we don’t know what to do with that. That’s definitely one challenge. And we see that more often in the adult patient population,” said Dr. Singh, who was not involved with the research.
He noted that there is a small group of genetic mutations when, found in adults, may dramatically alter treatment.
For example, he noted that if there is a gene mutation related to mTOR pathways, that could provide a future target because there are already medications that target this pathway.
Genetic testing may also be useful in cases where patients have normal brain imaging and poor response to standard treatment or in cases where patients have congenital abnormalities such as intellectual impairment or facial dysmorphic features and a co-morbid seizure disorder, he said.
Dr. Singh noted that he has often found genetic testing impractical because “if I order DNA testing right now, it will take 4 months for me to get the results. I cannot wait 4 months for the results to come back” to adjust treatment.
Dr. McKnight is an employee of and a shareholder in Invitae, which funded the study. Dr. Singh has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From WCN 2021
Customized brain stimulation: New hope for severe depression
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Personalized deep brain stimulation (DBS) appears to rapidly and effectively improve symptoms of treatment-resistant depression, new research suggests.
In a proof-of-concept study, investigators identified specific brain activity patterns responsible for a single patient’s severe depression and customized a DBS protocol to modulate the patterns. Results showed rapid and sustained improvement in depression scores.
“This study points the way to a new paradigm that is desperately needed in psychiatry,” Andrew Krystal, PhD, Weill Institute for Neurosciences, University of California, San Francisco, said in a news release.
“ by identifying and modulating the circuit in her brain that’s uniquely associated with her symptoms,” Dr. Krystal added.
The findings were published online Oct. 4 in Nature Medicine.
Closed-loop, on-demand stimulation
The patient was a 36-year-old woman with longstanding, severe, and treatment-resistant major depressive disorder. She was unresponsive to multiple antidepressant combinations and electroconvulsive therapy.
The researchers used intracranial electrophysiology and focal electrical stimulation to identify the specific pattern of electrical brain activity that correlated with her depressed mood.
They identified the right ventral striatum – which is involved in emotion, motivation, and reward – as the stimulation site that led to consistent, sustained, and dose-dependent improvement of symptoms and served as the neural biomarker.
In addition, the investigators identified a neural activity pattern in the amygdala that predicted both the mood symptoms, symptom severity, and stimulation efficacy.
The patient was implanted with the Food and Drug Administration–approved NeuroPace RNS System. The device was placed in the right hemisphere. A single sensing lead was positioned in the amygdala and the second stimulation lead was placed in the ventral striatum.
When the sensing lead detected the activity pattern associated with depression, the other lead delivered a tiny dose (1 milliampere/1 mA) of electricity for 6 seconds, which altered the neural activity and relieved mood symptoms.
Remission achieved
Once this personalized, closed-loop therapy was fully operational, the patient’s depression score on the Montgomery-Åsberg Depression Rating Scale (MADRS) dropped from 33 before turning treatment ON to 14 at the first ON-treatment assessment carried out after 12 days of stimulation. The score dropped below 10, representing remission, several months later.
The treatment also rapidly improved symptom severity, as measured daily with Hamilton Depression Rating Scale (HAMD-6) and visual analog scales.
“Success was predicated on a clinical mapping stage before chronic device placement, a strategy that has been utilized in epilepsy to map seizure foci in a personalized manner but has not previously been performed in other neuropsychiatric conditions,” the investigators wrote.
This patient represents “one of the first examples of precision psychiatry – a treatment tailored to an individual,” the study’s lead author, Katherine Scangos, MD, also with UCSF Weill Institute, said in an interview.
She added that the treatment “was personally tailored both spatially,” meaning at the brain location, and temporally – the time it was delivered.
“This is the first time a neural biomarker has been used to automatically trigger therapeutic stimulation in depression as a successful long-term treatment,” said Dr. Scangos. However, “we have a lot of work left to do,” she added.
“This study provides proof-of-principle that we can utilize a multimodal brain mapping approach to identify a personalized depression circuit and target that circuit with successful treatment. We will need to test the approach in more patients before we can determine its efficacy,” Dr. Scangos said.
First reliable biomarker in psychiatry
In a statement from the UK nonprofit Science Media Centre, Vladimir Litvak, PhD, with the Wellcome Centre for Human Neuroimaging, University College London, said that the study is interesting, noting that it is from “one of the leading groups in the field.”
The fact that depression symptoms can be treated in some patients by electrical stimulation of the ventral striatum is not new, Dr. Litvak said. However, what is “exciting” is that the authors identified a particular neural activity pattern in the amygdala as a reliable predictor of both symptom severity and stimulation effectiveness, he noted.
“Patterns of brain activity correlated with disease symptoms when testing over a large group of patients are commonly discovered. But there are just a handful of examples of patterns that are reliable enough to be predictive on a short time scale in a single patient,” said Dr. Litvak, who was not associated with the research.
“Furthermore, to my knowledge, this is the first example of such a reliable biomarker for psychiatric symptoms. The other examples were all for neurological disorders such as Parkinson’s disease, dystonia, and epilepsy,” he added.
He cautioned that this is a single case, but “if reproduced in additional patients, it will bring at least some psychiatric conditions into the domain of brain diseases that can be characterized and diagnosed objectively rather than based on symptoms alone.”
Dr. Litvak pointed out two other critical aspects of the study: the use of exploratory recordings and stimulation to determine the most effective treatment strategy, and the use of a closed-loop device that stimulates only when detecting the amygdala biomarker.
“It is hard to say based on this single case how important these will be in the future. There is no comparison to constant stimulation that might have worked as well because the implanted device used in the study is not suitable for that,” Dr. Litvak said.
It should also be noted that implanting multiple depth electrodes at different brain sites is a “traumatic invasive procedure only reserved to date for severe cases of drug-resistant epilepsy,” he said. “Furthermore, it only allows [researchers] to test a small number of candidate sites, so it relies heavily on prior knowledge.
“Once clinicians know better what to look for, it might be possible to avoid this procedure altogether by using noninvasive methods,” such as functional MRI or EEG, to match the right treatment option to a patient, Dr. Litvak concluded.
The research was funded by the National Institutes of Health, the Brain & Behavior Research Foundation, and the Ray and Dagmar Dolby Family Fund through the department of psychiatry at UCSF. Dr. Scangos has reported no relevant financial relationships. A complete list of author disclosures is available in the original article. Dr. Litvak is participating in a research funding application to search for electrophysiological biomarkers of depression symptoms using invasive recordings.
A version of this article first appeared on Medscape.com.
Data supporting cannabis for childhood epilepsy remain scarce
, according to two leading experts.
In a recent invited review article, Martin Kirkpatrick, MD, of the University of Dundee (Scotland), and Finbar O’Callaghan, MD, PhD, of University College London suggested that childhood epilepsy may be easy terrain for commercial interests to break ground, and from there, build their presence.
“Children with epilepsy are at risk of being used as the ‘Trojan horse’ for the cannabis industry,” Dr. Kirkpatrick and Dr. O’Callaghan wrote in Developmental Medicine & Child Neurology.
They noted that some of the first publicized success stories involving cannabis oil for epilepsy coincided with the rise of the medicinal and recreational cannabis markets, which will constitute an estimated 55-billion-dollar industry by 2027.
“Pediatric neurologists, imbued with the need to practice evidence-based medicine and wary of prescribing unlicensed medicines that had inadequate safety data, suddenly found themselves at odds with an array of vested interests and, most unfortunately, with the families of patients who were keen to try anything that would alleviate the effects of their child’s seizures,” the investigators wrote.
According to the review, fundamental questions about cannabis remain unanswered, including concerns about safety with long-term use, and the medicinal value of various plant components, such as myrcene, a terpene that gives cannabis its characteristic smell.
“A widely discussed issue is whether the terpenes add any therapeutic benefit, contributing to the so-called entourage effect of ‘whole-plant’ medicines,” the investigators wrote. “The concept is that all the constituents of the plant together create ‘the sum of all the parts that leads to the magic or power of cannabis.’ Although commonly referred to, there is little or no robust evidence to support the entourage effect as a credible clinical concept.”
Clinical evidence for treatment of pediatric epilepsy is also lacking, according to Dr. Kirkpatrick and Dr. O’Callaghan.
“Unfortunately, apart from the studies of pure cannabidiol (CBD) in Lennox–Gastaut and Dravet syndromes and tuberous sclerosis complex, level I evidence in the field of CBMPs and refractory epilepsy is lacking,” they wrote.
While other experts have pointed out that lower-level evidence – such as patient-reported outcomes and observational data – have previously been sufficient for drug licensing, Dr. Kirkpatrick and Dr. O’Callaghan noted that such exceptions “almost always” involve conditions without any effective treatments, or drugs that are undeniably effective.
“This is not the scenario with CBMPs,” they wrote, referring to current clinical data as “low-level” evidence “suggesting … possible efficacy.”
They highlighted concerns about placebo effect with open-label epilepsy studies, citing a randomized controlled trial for Dravet syndrome, in which 27% of patients given placebo had a 50% reduction in seizure frequency.
“We need carefully designed, good-quality CBMP studies that produce results on which we can rely,” Dr. Kirkpatrick and Dr. O’Callaghan concluded. “We can then work with families to choose the best treatments for children and young people with epilepsy. We owe this to them.”
A therapy of last resort
Jerzy P. Szaflarski, MD, PhD, of the University of Alabama at Birmingham, agreed that data are lacking for the use of CBMPs with patients who have epilepsy and other neurologic conditions; however, he also suggested that Dr. Kirkpatrick and Dr. O’Callaghan did not provide adequate real-world clinical context.
“Medical cannabis is not used as a first-, second-, or third-line therapy,” Dr. Szaflarski said. “It’s mostly used as a last resort in the sense that patients have already failed multiple other therapies.” In that respect, patients and parents are desperate to try anything that might work. “We have medical cannabis, and our patients want to try it, and at the point when multiple therapies have failed, it’s a reasonable option.”
While Dr. Szaflarski agreed that more high-quality clinical trials are needed, he also noted the practical challenges involved in such trials, largely because of variations in cannabis plants.
“The content of the cannabis plant changes depending on the day that it’s collected and the exposure to sun and how much water it has and what’s in the soil and many other things,” Dr. Szaflarski said. “It’s hard to get a very good, standardized product, and that’s why there needs to be a good-quality product delivered by the industry, which I have not seen thus far.”
For this reason, Dr. Szaflarski steers parents and patients away from over-the-counter CBMPs and toward Epidiolex, the only FDA-approved form of CBD.
“There is evidence that Epidiolex works,” he said. “I don’t know whether the products that are sold in a local cannabis store have the same high purity as Epidiolex. I tell [parents] that we should try Epidiolex first because it’s the one that is approved. But if it doesn’t work, we can go in that [other] direction.”
For those going the commercial route, Dr. Szaflarski advised close attention to product ingredients, to ensure that CBMPs are “devoid of any impurities, pesticides, fungicides, and other products that could be potentially dangerous.”
Parents considering CBMPs for their children also need to weigh concerns about long-term neurological safety, he added, noting that, on one hand, commercial products lack data, while on the other, epilepsy itself may cause harm.
“They need to consider the potential effects [of CBMPs] on their child’s brain and development versus … the effects of seizures on the brain,” Dr. Szaflarski said.
Dr. Kirkpatrick and Dr. O’Callaghan disclosed an application for a National Institute for Health Research–funded randomized controlled trial on CBMPs and joint authorship of British Paediatric Neurology Association Guidance on the use of CBMPs in children and young people with epilepsy. Dr. Szaflarski disclosed a relationship with Greenwich Biosciences and several other cannabis companies.
, according to two leading experts.
In a recent invited review article, Martin Kirkpatrick, MD, of the University of Dundee (Scotland), and Finbar O’Callaghan, MD, PhD, of University College London suggested that childhood epilepsy may be easy terrain for commercial interests to break ground, and from there, build their presence.
“Children with epilepsy are at risk of being used as the ‘Trojan horse’ for the cannabis industry,” Dr. Kirkpatrick and Dr. O’Callaghan wrote in Developmental Medicine & Child Neurology.
They noted that some of the first publicized success stories involving cannabis oil for epilepsy coincided with the rise of the medicinal and recreational cannabis markets, which will constitute an estimated 55-billion-dollar industry by 2027.
“Pediatric neurologists, imbued with the need to practice evidence-based medicine and wary of prescribing unlicensed medicines that had inadequate safety data, suddenly found themselves at odds with an array of vested interests and, most unfortunately, with the families of patients who were keen to try anything that would alleviate the effects of their child’s seizures,” the investigators wrote.
According to the review, fundamental questions about cannabis remain unanswered, including concerns about safety with long-term use, and the medicinal value of various plant components, such as myrcene, a terpene that gives cannabis its characteristic smell.
“A widely discussed issue is whether the terpenes add any therapeutic benefit, contributing to the so-called entourage effect of ‘whole-plant’ medicines,” the investigators wrote. “The concept is that all the constituents of the plant together create ‘the sum of all the parts that leads to the magic or power of cannabis.’ Although commonly referred to, there is little or no robust evidence to support the entourage effect as a credible clinical concept.”
Clinical evidence for treatment of pediatric epilepsy is also lacking, according to Dr. Kirkpatrick and Dr. O’Callaghan.
“Unfortunately, apart from the studies of pure cannabidiol (CBD) in Lennox–Gastaut and Dravet syndromes and tuberous sclerosis complex, level I evidence in the field of CBMPs and refractory epilepsy is lacking,” they wrote.
While other experts have pointed out that lower-level evidence – such as patient-reported outcomes and observational data – have previously been sufficient for drug licensing, Dr. Kirkpatrick and Dr. O’Callaghan noted that such exceptions “almost always” involve conditions without any effective treatments, or drugs that are undeniably effective.
“This is not the scenario with CBMPs,” they wrote, referring to current clinical data as “low-level” evidence “suggesting … possible efficacy.”
They highlighted concerns about placebo effect with open-label epilepsy studies, citing a randomized controlled trial for Dravet syndrome, in which 27% of patients given placebo had a 50% reduction in seizure frequency.
“We need carefully designed, good-quality CBMP studies that produce results on which we can rely,” Dr. Kirkpatrick and Dr. O’Callaghan concluded. “We can then work with families to choose the best treatments for children and young people with epilepsy. We owe this to them.”
A therapy of last resort
Jerzy P. Szaflarski, MD, PhD, of the University of Alabama at Birmingham, agreed that data are lacking for the use of CBMPs with patients who have epilepsy and other neurologic conditions; however, he also suggested that Dr. Kirkpatrick and Dr. O’Callaghan did not provide adequate real-world clinical context.
“Medical cannabis is not used as a first-, second-, or third-line therapy,” Dr. Szaflarski said. “It’s mostly used as a last resort in the sense that patients have already failed multiple other therapies.” In that respect, patients and parents are desperate to try anything that might work. “We have medical cannabis, and our patients want to try it, and at the point when multiple therapies have failed, it’s a reasonable option.”
While Dr. Szaflarski agreed that more high-quality clinical trials are needed, he also noted the practical challenges involved in such trials, largely because of variations in cannabis plants.
“The content of the cannabis plant changes depending on the day that it’s collected and the exposure to sun and how much water it has and what’s in the soil and many other things,” Dr. Szaflarski said. “It’s hard to get a very good, standardized product, and that’s why there needs to be a good-quality product delivered by the industry, which I have not seen thus far.”
For this reason, Dr. Szaflarski steers parents and patients away from over-the-counter CBMPs and toward Epidiolex, the only FDA-approved form of CBD.
“There is evidence that Epidiolex works,” he said. “I don’t know whether the products that are sold in a local cannabis store have the same high purity as Epidiolex. I tell [parents] that we should try Epidiolex first because it’s the one that is approved. But if it doesn’t work, we can go in that [other] direction.”
For those going the commercial route, Dr. Szaflarski advised close attention to product ingredients, to ensure that CBMPs are “devoid of any impurities, pesticides, fungicides, and other products that could be potentially dangerous.”
Parents considering CBMPs for their children also need to weigh concerns about long-term neurological safety, he added, noting that, on one hand, commercial products lack data, while on the other, epilepsy itself may cause harm.
“They need to consider the potential effects [of CBMPs] on their child’s brain and development versus … the effects of seizures on the brain,” Dr. Szaflarski said.
Dr. Kirkpatrick and Dr. O’Callaghan disclosed an application for a National Institute for Health Research–funded randomized controlled trial on CBMPs and joint authorship of British Paediatric Neurology Association Guidance on the use of CBMPs in children and young people with epilepsy. Dr. Szaflarski disclosed a relationship with Greenwich Biosciences and several other cannabis companies.
, according to two leading experts.
In a recent invited review article, Martin Kirkpatrick, MD, of the University of Dundee (Scotland), and Finbar O’Callaghan, MD, PhD, of University College London suggested that childhood epilepsy may be easy terrain for commercial interests to break ground, and from there, build their presence.
“Children with epilepsy are at risk of being used as the ‘Trojan horse’ for the cannabis industry,” Dr. Kirkpatrick and Dr. O’Callaghan wrote in Developmental Medicine & Child Neurology.
They noted that some of the first publicized success stories involving cannabis oil for epilepsy coincided with the rise of the medicinal and recreational cannabis markets, which will constitute an estimated 55-billion-dollar industry by 2027.
“Pediatric neurologists, imbued with the need to practice evidence-based medicine and wary of prescribing unlicensed medicines that had inadequate safety data, suddenly found themselves at odds with an array of vested interests and, most unfortunately, with the families of patients who were keen to try anything that would alleviate the effects of their child’s seizures,” the investigators wrote.
According to the review, fundamental questions about cannabis remain unanswered, including concerns about safety with long-term use, and the medicinal value of various plant components, such as myrcene, a terpene that gives cannabis its characteristic smell.
“A widely discussed issue is whether the terpenes add any therapeutic benefit, contributing to the so-called entourage effect of ‘whole-plant’ medicines,” the investigators wrote. “The concept is that all the constituents of the plant together create ‘the sum of all the parts that leads to the magic or power of cannabis.’ Although commonly referred to, there is little or no robust evidence to support the entourage effect as a credible clinical concept.”
Clinical evidence for treatment of pediatric epilepsy is also lacking, according to Dr. Kirkpatrick and Dr. O’Callaghan.
“Unfortunately, apart from the studies of pure cannabidiol (CBD) in Lennox–Gastaut and Dravet syndromes and tuberous sclerosis complex, level I evidence in the field of CBMPs and refractory epilepsy is lacking,” they wrote.
While other experts have pointed out that lower-level evidence – such as patient-reported outcomes and observational data – have previously been sufficient for drug licensing, Dr. Kirkpatrick and Dr. O’Callaghan noted that such exceptions “almost always” involve conditions without any effective treatments, or drugs that are undeniably effective.
“This is not the scenario with CBMPs,” they wrote, referring to current clinical data as “low-level” evidence “suggesting … possible efficacy.”
They highlighted concerns about placebo effect with open-label epilepsy studies, citing a randomized controlled trial for Dravet syndrome, in which 27% of patients given placebo had a 50% reduction in seizure frequency.
“We need carefully designed, good-quality CBMP studies that produce results on which we can rely,” Dr. Kirkpatrick and Dr. O’Callaghan concluded. “We can then work with families to choose the best treatments for children and young people with epilepsy. We owe this to them.”
A therapy of last resort
Jerzy P. Szaflarski, MD, PhD, of the University of Alabama at Birmingham, agreed that data are lacking for the use of CBMPs with patients who have epilepsy and other neurologic conditions; however, he also suggested that Dr. Kirkpatrick and Dr. O’Callaghan did not provide adequate real-world clinical context.
“Medical cannabis is not used as a first-, second-, or third-line therapy,” Dr. Szaflarski said. “It’s mostly used as a last resort in the sense that patients have already failed multiple other therapies.” In that respect, patients and parents are desperate to try anything that might work. “We have medical cannabis, and our patients want to try it, and at the point when multiple therapies have failed, it’s a reasonable option.”
While Dr. Szaflarski agreed that more high-quality clinical trials are needed, he also noted the practical challenges involved in such trials, largely because of variations in cannabis plants.
“The content of the cannabis plant changes depending on the day that it’s collected and the exposure to sun and how much water it has and what’s in the soil and many other things,” Dr. Szaflarski said. “It’s hard to get a very good, standardized product, and that’s why there needs to be a good-quality product delivered by the industry, which I have not seen thus far.”
For this reason, Dr. Szaflarski steers parents and patients away from over-the-counter CBMPs and toward Epidiolex, the only FDA-approved form of CBD.
“There is evidence that Epidiolex works,” he said. “I don’t know whether the products that are sold in a local cannabis store have the same high purity as Epidiolex. I tell [parents] that we should try Epidiolex first because it’s the one that is approved. But if it doesn’t work, we can go in that [other] direction.”
For those going the commercial route, Dr. Szaflarski advised close attention to product ingredients, to ensure that CBMPs are “devoid of any impurities, pesticides, fungicides, and other products that could be potentially dangerous.”
Parents considering CBMPs for their children also need to weigh concerns about long-term neurological safety, he added, noting that, on one hand, commercial products lack data, while on the other, epilepsy itself may cause harm.
“They need to consider the potential effects [of CBMPs] on their child’s brain and development versus … the effects of seizures on the brain,” Dr. Szaflarski said.
Dr. Kirkpatrick and Dr. O’Callaghan disclosed an application for a National Institute for Health Research–funded randomized controlled trial on CBMPs and joint authorship of British Paediatric Neurology Association Guidance on the use of CBMPs in children and young people with epilepsy. Dr. Szaflarski disclosed a relationship with Greenwich Biosciences and several other cannabis companies.
FROM DEVELOPMENTAL MEDICINE & CHILD NEUROLOGY
FDA OKs IV Briviact for seizures in kids as young as 1 month
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
All three brivaracetam formulations (tablets, oral solution, and IV) may now be used. The approval marks the first time that the IV formulation will be available for children, the company said in a news release.
The medication is already approved in the United States as monotherapy and adjunctive therapy in adults with epilepsy.
In an open-label follow-up pediatric study, an estimated 71.4% of patients aged 1 month to 17 years with partial-onset seizures remained on brivaracetam therapy at 1 year, and 64.3% did so at 2 years, the company reported.
“We often see children with seizures hospitalized, so it’s important to have a therapy like Briviact IV that can offer rapid administration in an effective dose when needed and does not require titration,” Raman Sankar, MD, PhD, distinguished professor and chief of pediatric neurology, University of California, Los Angeles, said in the release.
“The availability of the oral dose forms also allows continuity of treatment when these young patients are transitioning from hospital to home,” he added.
Safety profile
Dr. Sankar noted that with approval now of both the IV and oral formulations for partial-onset seizures in such young children, “we have a new option that helps meet a critical need in pediatric epilepsy.”
The most common adverse reactions with brivaracetam include somnolence and sedation, dizziness, fatigue, nausea, and vomiting. In the pediatric clinical trials, the safety profile for pediatric patients was similar to adults.
In the adult trials, psychiatric adverse reactions, including nonpsychotic and psychotic symptoms, were reported in approximately 13% of adults taking at least 50 mg/day of brivaracetam compared with 8% taking placebo.
Psychiatric adverse reactions were also observed in open-label pediatric trials and were generally similar to those observed in adults.
Patients should be advised to report these symptoms immediately to a health care professional, the company noted.
A version of this article first appeared on Medscape.com.
‘No justification’ for suicide warning on all antiseizure meds
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
, new research shows. “There appears to be no justification for the FDA to label every new antiseizure medication with a warning that it may increase risk of suicidality,” said study investigator Michael R. Sperling, MD, professor of neurology, Thomas Jefferson University, Philadelphia.
“How many patients are afraid of their medication and do not take it because of the warning – and are consequently at risk because of that? We do not know, but have anecdotal experience that this is certainly an issue,” Dr. Sperling, who is director of the Jefferson Comprehensive Epilepsy Center, added.
The study was published online August 2 in JAMA Neurology.
Blanket warning
In 2008, the FDA issued an alert stating that antiseizure medications increase suicidality. The alert was based on pooled data from placebo-controlled clinical trials that included 11 antiseizure medications – carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide.
The meta-analytic review showed that, compared with placebo, antiseizure medications nearly doubled suicide risk among patients treated for epilepsy, psychiatric disorders, and other diseases. As a result of the FDA study, all antiseizure medications that have been approved since 2008 carry a warning for suicidality.
However, subsequent analyses did not show the same results, Dr. Sperling and colleagues noted.
“Pivotal” antiseizure medication epilepsy trials since 2008 have evaluated suicidality prospectively. Since 2011, trials have included the validated Columbia Suicidality Severity Rating Scale, they noted.
Meta analysis showed no increased risk
Dr. Sperling and colleagues conducted a meta-analysis of 17 randomized placebo-controlled epilepsy trials of five antiseizure medications approved since 2008. These antiseizure medications were eslicarbazepine, perampanel, brivaracetam, cannabidiol, and cenobamate. The trials involved 5,996 patients, including 4,000 who were treated with antiseizure medications and 1,996 who were treated with placebo.
Confining the analysis to epilepsy trials avoids potential confounders, such as possible differences in suicidality risks between different diseases, the researchers noted.
They found no evidence of increased risk for suicidal ideation (overall risk ratio, antiseizure medications vs. placebo: 0.75; 95% confidence interval: 0.35-1.60) or suicide attempt (risk ratio, 0.75; 95% CI: 0.30-1.87) overall or for any individual antiseizure medication.
Suicidal ideation occurred in 12 of 4,000 patients treated with antiseizure medications (0.30%), versus 7 of 1,996 patients treated with placebo (0.35%) (P = .74). Three patients who were treated with antiseizure medications attempted suicide; no patients who were treated with placebo attempted suicide (P = .22). There were no completed suicides.
“There is no current evidence that the five antiseizure medications evaluated in this study increase suicidality in epilepsy and merit a suicidality class warning,” the investigators wrote. When prescribed for epilepsy, “evidence does not support the FDA’s labeling practice of a blanket assumption of increased suicidality,” said Dr. Sperling.
“Our findings indicate the nonspecific suicide warning for all epilepsy drugs is simply not justifiable,” he said. “The results are not surprising. Different drugs affect cells in different ways. So there’s no reason to expect that every drug would increase suicide risk for every patient,” Dr. Sperling said in a statement.
“It’s important to recognize that epilepsy has many causes – perinatal injury, stroke, tumor, head trauma, developmental malformations, genetic causes, and others – and these underlying etiologies may well contribute to the presence of depression and suicidality in this population,” he said in an interview. “Psychodynamic influences also may occur as a consequence of having seizures. This is a complicated area, and drugs are simply one piece of the puzzle,” he added.
Dr. Sperling said the FDA has accomplished “one useful thing with its warning – it highlighted that physicians and other health care providers must pay attention to their patients’ psychological state, ask questions, and treat accordingly.”
The study had no specific funding. Dr. Sperling has received grants from Eisai, Medtronic, Neurelis, SK Life Science, Sunovion, Takeda, Xenon, Cerevel Therapeutics, UCB Pharma, and Engage Pharma; personal fees from Neurelis, Medscape, Neurology Live, International Medical Press, UCB Pharma, Eisai, Oxford University Press, and Projects in Knowledge. He has also consulted for Medtronic outside the submitted work; payments went to Thomas Jefferson University. A complete list of authors’ disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Short-term approach is best for seizure prevention after intracerebral hemorrhage
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.
Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.

Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
(sICH), new research shows.
Investigators created a model that simulated common clinical scenarios to compare four antiseizure drug strategies – conservative, moderate, aggressive, and risk-guided. They used the 2HELPS2B score as a risk stratification tool to guide clinical decisions.
The investigators found that the short-term, early-seizure prophylaxis strategies “dominated” long-term therapy under most clinical scenarios, underscoring the importance of early discontinuation of antiseizure drug therapy.
“The main message here was that strategies that involved long-term antiseizure drug prescription (moderate and aggressive) fail to provide better outcomes in most clinical scenarios, when compared with strategies using short-term prophylaxis (conservative and risk-guided),” senior investigator Lidia M.V.R. Moura, MD, MPH, assistant professor of neurology, Harvard Medical School, Boston, said in an interview.
The study was published online July 26 in JAMA Neurology.
Common complication
“Acute asymptomatic seizures [early seizures ≤7 days after stroke] are a common complication of sICH,” the authors noted.
Potential safety concerns have prompted recommendations against the use of antiseizure medications for primary prophylaxis. However, approximately 40% of U.S. patients with sICH do receive prophylactic levetiracetam before seizure development. For these patients, the duration of prophylaxis varies widely.
“Because seizure risk is a key determinant of which patient groups might benefit most from different prophylaxis strategies, validated tools for predicting early ... and late ... seizure risks could aid physicians in treatment decisions. However, no clinical trials or prospective studies have evaluated the net benefit of various strategies after sICH,” the investigators noted.
“Our patients who were survivors of an intracerebral hemorrhage motivated us to conduct the study,” said Dr. Moura, who is also director of the MGH NeuroValue Laboratory. “Some would come to the clinic with a long list of medications; some of them were taking antiseizure drugs for many years, but they never had a documented seizure.” These patients did not know why they had been taking an antiseizure drug for so long.
“In these conversations, we noted so much variability in indications and variability in patient access to specialty care to make treatment decisions. We noted that the evidence behind our current guidelines on seizure management was limited,” she added.
Dr. Moura and colleagues were “committed to improve outcome for people with neurological conditions by leveraging research methods that can help guide providers and systems, especially when data from clinical trials is lacking,” so they “decided to compare different strategies head to head using available data and generate evidence that could be used in situations with many trade-offs in risks and benefits.”
To investigate, the researchers used a simulation model and decision analysis to compare four treatment strategies on the basis of type of therapy (primary vs. secondary prophylaxis), timing of event (early vs. late seizures), and duration of therapy (1-week [short-term] versus indefinite [long-term] therapy).
These four strategies were as follows:
- Conservative: short-term (7-day) secondary early-seizure prophylaxis with long-term therapy after late seizure
- Moderate: long-term secondary early-seizure prophylaxis or late-seizure therapy
- Aggressive: long-term primary prophylaxis
- Risk-guided: short-term secondary early-seizure prophylaxis among low-risk patients (2HELPS2b score, 0), short-term primary prophylaxis among patients at higher risk (2HELPS2B score ≥1), and long-term secondary therapy for late seizure
The decision tree’s outcome measure was the number of expected quality-adjusted life-years.
Primary prophylaxis was defined as “treatment initiated immediately on hospital admission.” Secondary prophylaxis was defined as “treatment started after a seizure” and was subdivided into secondary early-seizure prophylaxis, defined as treatment started after a seizure occurring in the first 7 days after the stroke, or secondary late-seizure therapy, defined as treatment started or restarted after a seizure occurring after the first poststroke week.
Incorporate early-risk stratification tool
The researchers created four common clinical scenarios and then applied the decision-making model to each. They found that the preferred strategies differed, depending on the particular scenario.

Sensitivity analyses revealed that short-term strategies, including the conservative and risk-guided approaches, were preferable in most cases, with the risk-guided strategy performing comparably or even better than alternative strategies in most cases.
“Our findings suggest that a strategy that incorporates an early-seizure risk stratification tool [2HELPS2B] is favored over alternative strategies in most settings,” Dr. Moura commented.
“Current services with rapidly available EEG may consider using a 1-hour screening with EEG upon admission for all patients presenting with sICH to risk-stratify those patients, using the 2HELPS2B tool,” she continued. “If EEG is unavailable for early-seizure risk stratification, the conservative strategy seems most reasonable.”
‘Potential fallacies’
Commenting on the study, José Biller, MD, professor and chairman, department of neurology, Loyola University Chicago, Maywood, Ill., called it a “well-written and intriguing contribution [to the field], with potential fallacies.”
The bottom line, he said, is that only a randomized, long-term, prospective, multicenter, high-quality study with larger cohorts can prove or disprove the investigators’ assumption.
The authors acknowledged that a limitation of the study was the use of published literature to obtain data to estimate model parameters and that they did not account for other possible factors that might modify some parameter estimates.
Nevertheless, Dr. Moura said the findings have important practical implications because they “highlight the importance of discontinuing antiseizure medications that were started during a hospitalization for sICH in patients that only had an early seizure.”
It is “of great importance for all providers to reassess the indication of antiseizure medications. Those drugs are not free of risks and can impact the patient’s health and quality of life,” she added.
The study was supported by grants from the National Institutes of Health. Dr. Moura reported receiving funding from the Centers for Disease Control and Prevention, the NIH, and the Epilepsy Foundation of America (Epilepsy Learning Healthcare System) as the director of the data coordinating center. Dr. Biller is the editor-in-chief of the Journal of Stroke and Cerebrovascular Diseases and a section editor of UpToDate.
A version of this article first appeared on Medscape.com.
Can a supplement that mimics the keto diet reduce seizures?
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
FROM BRAIN COMMUNICATION
Noted ability of Mozart’s music to reduce seizures explained?
– and now researchers believe they know why.
Investigators conducting new research found that the acoustic characteristics of Mozart’s Sonata for Two Pianos in D Major (K448) suppresses brain activity in patients with epilepsy, while a piece by the 18th century classical composer Franz Joseph Haydn did not have this effect.
Listening to this Mozart sonata and perhaps other musical pieces may eventually become a treatment for preventing epileptic seizures, said study investigator Ivan Rektor, MD, CSc, Epilepsy Centre at the Hospital St. Anne and professor at the Central European Institute of Technology, Masaryk University, Brno, Czech Republic.
“This research into the impact of listening to music could lead to the development of a music-related type of palliative neurostimulation therapy,” said Dr. Rektor.
The findings were presented at the 2021 Congress of the European Academy of Neurology and published online in the European Journal of Neurology.
Clinically controversial?
Epilepsy affects 6 million people in Europe. Furthermore, estimates show that about 15 million Europeans have had at least one seizure at some time in their lives. In addition, about 30% of patients with epilepsy are not adequately treated with antiseizure medications.
Researchers have been studying the impact of Mozart’s music on brain-wave activity since the 1990s. Various studies report a reduction in epileptiform discharges in patients with epileptic seizures, coma, and refractory nonconvulsive status.
A 2012 meta-analysis of 12 publications involving patients with epilepsy showed an overall reduction in the number of interictal epileptic discharges (IEDs) or abnormal electrical brain waves in 84% of participants who listened to Mozart’s music. A more recent meta-analysis also showed a significant reduction in epileptic seizures and IEDs.
American researchers also found Mozart’s music regulated abnormal interictal epileptiform activity (IEA), especially in those with a high baseline rate of interictal spikes.
However, the methodological quality of some of this research “has been limited,” Dr. Rektor noted. He added that use of music therapy in clinical practice is still considered “controversial.”
The new study included 18 treatment-resistant patients with epilepsy (50% men) who ranged in age from 19 to 55 years. Participants had intracerebral electrodes implanted in the brain before undergoing surgery.
Of the total study population, 15 had temporal lobe epilepsy and three had extratemporal epilepsy. Eleven were affected on the left side, six on the right side, and one bi-temporally. Duration of epilepsy ranged from 8 to 40 years.
Patients listened to the Mozart piece intermittently on one day and to Haydn’s “Surprise” Symphony No. 94 the next day. Researchers counted the number of ED discharges before, during, and after the patients listened to the music.
Surprising finding
Results showed that exposure to the Mozart piece was associated with a 32% reduction in IEDs, from 28 EDs pre-exposure to 19 during exposure. However, IEDs rose to 21 post-exposure.
Overall, the Haydn piece was associated with an increase in IEDs, from 23 pre-exposure to 26 during and post-exposure.
“We saw a clear decrease in epileptic spikes while listening and after listening to Mozart, while there was an increase in spikes while listening to Haydn,” Dr. Rektor said.
He added that all 18 patients responded “more or less” to the music and that the results were statistically significant.
Dr. Rektor noted that the investigators were not surprised by the Mozart effect but were somewhat taken aback by the opposite effect from listening to Haydn.
The impact differed between men and women. The Mozart piece had a larger effect on women. In addition, the Haydn piece led to a decrease in spikes in women but led to “a clear” increase in men, Dr. Rektor reported.
In an effort to explore why the two classical pieces had such different effects, the researchers examined the acoustic properties. They worked with acoustic engineers to examine three musical properties that might influence the number of spikes: rhythm (tempo or beats per minute), dynamics (energy), and timbre (how harsh or unpleasant, how noisy, and how many “high-frequency” parts the music has).
“We observed that K448 [Mozart’s piece] has a more harmonic spectrum and its spectral content doesn’t change quickly, which probably has a positive effect on epilepsy patients,” said Dr. Rektor.
Specific features of the music had a slightly different effect on men and women. Men were more sensitive to dissonance and high-frequency parts while women were more sensitive to energy.
A new theory
Researchers previously hypothesized that the Mozart effect in epilepsy was connected to the emotional impact of music. The neurotransmitter dopamine, which plays a role in the brain’s reward system, is released when listening to music. However, the new research seems to challenge that theory. The majority of the participants did not express a strong preference for classical music.
“We believe emotions didn’t play an important role in these patients,” Dr. Rektor said, adding that the impact was instead mostly related to acoustic signals.
The team also found that the reduction in IEDs was larger in the lateral temporal lobe, the part of the brain involved in translating acoustic signals, rather than in the mesiotemporal limbic region, which plays an important role in the emotional response to music.
Comparing men with women, there’s an “overlap” of brain activation in most brain areas. However, some areas are more activated in men and others in women, said Dr. Rektor.
While the Mozart Sonata for two pianos in D Major has become the “gold standard” in this type of research, Dr. Rektor said “it’s very probable” that other classical compositions with similar acoustic properties have the same effect in epilepsy.
The investigators are testing other musical pieces, both classical and nonclassical. The ultimate aim is to develop individualized musical patterns based on these acoustic features.
“If it works, we would like to use it as a noninvasive neurostimulation method,” Dr. Rektor said.
‘Inspiring research’
Commenting on the study, session chair Marte Bjørk, MD, PhD, associate professor, department of clinical medicine, University of Bergen, Norway, called it “inspiring.” She noted that she recently had a patient whose temporal lobe seizures were consistently triggered by music played on a children’s TV program. “So I have no doubt that music can be important for some patients,” Dr. Bjørk said.
She questioned whether factors other than gender may predict response to music.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and now researchers believe they know why.
Investigators conducting new research found that the acoustic characteristics of Mozart’s Sonata for Two Pianos in D Major (K448) suppresses brain activity in patients with epilepsy, while a piece by the 18th century classical composer Franz Joseph Haydn did not have this effect.
Listening to this Mozart sonata and perhaps other musical pieces may eventually become a treatment for preventing epileptic seizures, said study investigator Ivan Rektor, MD, CSc, Epilepsy Centre at the Hospital St. Anne and professor at the Central European Institute of Technology, Masaryk University, Brno, Czech Republic.
“This research into the impact of listening to music could lead to the development of a music-related type of palliative neurostimulation therapy,” said Dr. Rektor.
The findings were presented at the 2021 Congress of the European Academy of Neurology and published online in the European Journal of Neurology.
Clinically controversial?
Epilepsy affects 6 million people in Europe. Furthermore, estimates show that about 15 million Europeans have had at least one seizure at some time in their lives. In addition, about 30% of patients with epilepsy are not adequately treated with antiseizure medications.
Researchers have been studying the impact of Mozart’s music on brain-wave activity since the 1990s. Various studies report a reduction in epileptiform discharges in patients with epileptic seizures, coma, and refractory nonconvulsive status.
A 2012 meta-analysis of 12 publications involving patients with epilepsy showed an overall reduction in the number of interictal epileptic discharges (IEDs) or abnormal electrical brain waves in 84% of participants who listened to Mozart’s music. A more recent meta-analysis also showed a significant reduction in epileptic seizures and IEDs.
American researchers also found Mozart’s music regulated abnormal interictal epileptiform activity (IEA), especially in those with a high baseline rate of interictal spikes.
However, the methodological quality of some of this research “has been limited,” Dr. Rektor noted. He added that use of music therapy in clinical practice is still considered “controversial.”
The new study included 18 treatment-resistant patients with epilepsy (50% men) who ranged in age from 19 to 55 years. Participants had intracerebral electrodes implanted in the brain before undergoing surgery.
Of the total study population, 15 had temporal lobe epilepsy and three had extratemporal epilepsy. Eleven were affected on the left side, six on the right side, and one bi-temporally. Duration of epilepsy ranged from 8 to 40 years.
Patients listened to the Mozart piece intermittently on one day and to Haydn’s “Surprise” Symphony No. 94 the next day. Researchers counted the number of ED discharges before, during, and after the patients listened to the music.
Surprising finding
Results showed that exposure to the Mozart piece was associated with a 32% reduction in IEDs, from 28 EDs pre-exposure to 19 during exposure. However, IEDs rose to 21 post-exposure.
Overall, the Haydn piece was associated with an increase in IEDs, from 23 pre-exposure to 26 during and post-exposure.
“We saw a clear decrease in epileptic spikes while listening and after listening to Mozart, while there was an increase in spikes while listening to Haydn,” Dr. Rektor said.
He added that all 18 patients responded “more or less” to the music and that the results were statistically significant.
Dr. Rektor noted that the investigators were not surprised by the Mozart effect but were somewhat taken aback by the opposite effect from listening to Haydn.
The impact differed between men and women. The Mozart piece had a larger effect on women. In addition, the Haydn piece led to a decrease in spikes in women but led to “a clear” increase in men, Dr. Rektor reported.
In an effort to explore why the two classical pieces had such different effects, the researchers examined the acoustic properties. They worked with acoustic engineers to examine three musical properties that might influence the number of spikes: rhythm (tempo or beats per minute), dynamics (energy), and timbre (how harsh or unpleasant, how noisy, and how many “high-frequency” parts the music has).
“We observed that K448 [Mozart’s piece] has a more harmonic spectrum and its spectral content doesn’t change quickly, which probably has a positive effect on epilepsy patients,” said Dr. Rektor.
Specific features of the music had a slightly different effect on men and women. Men were more sensitive to dissonance and high-frequency parts while women were more sensitive to energy.
A new theory
Researchers previously hypothesized that the Mozart effect in epilepsy was connected to the emotional impact of music. The neurotransmitter dopamine, which plays a role in the brain’s reward system, is released when listening to music. However, the new research seems to challenge that theory. The majority of the participants did not express a strong preference for classical music.
“We believe emotions didn’t play an important role in these patients,” Dr. Rektor said, adding that the impact was instead mostly related to acoustic signals.
The team also found that the reduction in IEDs was larger in the lateral temporal lobe, the part of the brain involved in translating acoustic signals, rather than in the mesiotemporal limbic region, which plays an important role in the emotional response to music.
Comparing men with women, there’s an “overlap” of brain activation in most brain areas. However, some areas are more activated in men and others in women, said Dr. Rektor.
While the Mozart Sonata for two pianos in D Major has become the “gold standard” in this type of research, Dr. Rektor said “it’s very probable” that other classical compositions with similar acoustic properties have the same effect in epilepsy.
The investigators are testing other musical pieces, both classical and nonclassical. The ultimate aim is to develop individualized musical patterns based on these acoustic features.
“If it works, we would like to use it as a noninvasive neurostimulation method,” Dr. Rektor said.
‘Inspiring research’
Commenting on the study, session chair Marte Bjørk, MD, PhD, associate professor, department of clinical medicine, University of Bergen, Norway, called it “inspiring.” She noted that she recently had a patient whose temporal lobe seizures were consistently triggered by music played on a children’s TV program. “So I have no doubt that music can be important for some patients,” Dr. Bjørk said.
She questioned whether factors other than gender may predict response to music.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and now researchers believe they know why.
Investigators conducting new research found that the acoustic characteristics of Mozart’s Sonata for Two Pianos in D Major (K448) suppresses brain activity in patients with epilepsy, while a piece by the 18th century classical composer Franz Joseph Haydn did not have this effect.
Listening to this Mozart sonata and perhaps other musical pieces may eventually become a treatment for preventing epileptic seizures, said study investigator Ivan Rektor, MD, CSc, Epilepsy Centre at the Hospital St. Anne and professor at the Central European Institute of Technology, Masaryk University, Brno, Czech Republic.
“This research into the impact of listening to music could lead to the development of a music-related type of palliative neurostimulation therapy,” said Dr. Rektor.
The findings were presented at the 2021 Congress of the European Academy of Neurology and published online in the European Journal of Neurology.
Clinically controversial?
Epilepsy affects 6 million people in Europe. Furthermore, estimates show that about 15 million Europeans have had at least one seizure at some time in their lives. In addition, about 30% of patients with epilepsy are not adequately treated with antiseizure medications.
Researchers have been studying the impact of Mozart’s music on brain-wave activity since the 1990s. Various studies report a reduction in epileptiform discharges in patients with epileptic seizures, coma, and refractory nonconvulsive status.
A 2012 meta-analysis of 12 publications involving patients with epilepsy showed an overall reduction in the number of interictal epileptic discharges (IEDs) or abnormal electrical brain waves in 84% of participants who listened to Mozart’s music. A more recent meta-analysis also showed a significant reduction in epileptic seizures and IEDs.
American researchers also found Mozart’s music regulated abnormal interictal epileptiform activity (IEA), especially in those with a high baseline rate of interictal spikes.
However, the methodological quality of some of this research “has been limited,” Dr. Rektor noted. He added that use of music therapy in clinical practice is still considered “controversial.”
The new study included 18 treatment-resistant patients with epilepsy (50% men) who ranged in age from 19 to 55 years. Participants had intracerebral electrodes implanted in the brain before undergoing surgery.
Of the total study population, 15 had temporal lobe epilepsy and three had extratemporal epilepsy. Eleven were affected on the left side, six on the right side, and one bi-temporally. Duration of epilepsy ranged from 8 to 40 years.
Patients listened to the Mozart piece intermittently on one day and to Haydn’s “Surprise” Symphony No. 94 the next day. Researchers counted the number of ED discharges before, during, and after the patients listened to the music.
Surprising finding
Results showed that exposure to the Mozart piece was associated with a 32% reduction in IEDs, from 28 EDs pre-exposure to 19 during exposure. However, IEDs rose to 21 post-exposure.
Overall, the Haydn piece was associated with an increase in IEDs, from 23 pre-exposure to 26 during and post-exposure.
“We saw a clear decrease in epileptic spikes while listening and after listening to Mozart, while there was an increase in spikes while listening to Haydn,” Dr. Rektor said.
He added that all 18 patients responded “more or less” to the music and that the results were statistically significant.
Dr. Rektor noted that the investigators were not surprised by the Mozart effect but were somewhat taken aback by the opposite effect from listening to Haydn.
The impact differed between men and women. The Mozart piece had a larger effect on women. In addition, the Haydn piece led to a decrease in spikes in women but led to “a clear” increase in men, Dr. Rektor reported.
In an effort to explore why the two classical pieces had such different effects, the researchers examined the acoustic properties. They worked with acoustic engineers to examine three musical properties that might influence the number of spikes: rhythm (tempo or beats per minute), dynamics (energy), and timbre (how harsh or unpleasant, how noisy, and how many “high-frequency” parts the music has).
“We observed that K448 [Mozart’s piece] has a more harmonic spectrum and its spectral content doesn’t change quickly, which probably has a positive effect on epilepsy patients,” said Dr. Rektor.
Specific features of the music had a slightly different effect on men and women. Men were more sensitive to dissonance and high-frequency parts while women were more sensitive to energy.
A new theory
Researchers previously hypothesized that the Mozart effect in epilepsy was connected to the emotional impact of music. The neurotransmitter dopamine, which plays a role in the brain’s reward system, is released when listening to music. However, the new research seems to challenge that theory. The majority of the participants did not express a strong preference for classical music.
“We believe emotions didn’t play an important role in these patients,” Dr. Rektor said, adding that the impact was instead mostly related to acoustic signals.
The team also found that the reduction in IEDs was larger in the lateral temporal lobe, the part of the brain involved in translating acoustic signals, rather than in the mesiotemporal limbic region, which plays an important role in the emotional response to music.
Comparing men with women, there’s an “overlap” of brain activation in most brain areas. However, some areas are more activated in men and others in women, said Dr. Rektor.
While the Mozart Sonata for two pianos in D Major has become the “gold standard” in this type of research, Dr. Rektor said “it’s very probable” that other classical compositions with similar acoustic properties have the same effect in epilepsy.
The investigators are testing other musical pieces, both classical and nonclassical. The ultimate aim is to develop individualized musical patterns based on these acoustic features.
“If it works, we would like to use it as a noninvasive neurostimulation method,” Dr. Rektor said.
‘Inspiring research’
Commenting on the study, session chair Marte Bjørk, MD, PhD, associate professor, department of clinical medicine, University of Bergen, Norway, called it “inspiring.” She noted that she recently had a patient whose temporal lobe seizures were consistently triggered by music played on a children’s TV program. “So I have no doubt that music can be important for some patients,” Dr. Bjørk said.
She questioned whether factors other than gender may predict response to music.
The study authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EAN 2021
Young adults with epilepsy face higher mental illness risks
Young adults with epilepsy experience higher rates of anxiety, depression, and suicidality, compared with their counterparts in the general population, a new study shows.
The findings, based on a study of 144 young adults with epilepsy (YAWE), was published recently in Epilepsy & Behavior.
“People with epilepsy (PWE) are at a significantly higher risk of experiencing mental health difficulties, compared with healthy controls and individuals with other [long-term conditions] such as asthma and diabetes,” according to Rachel Batchelor, MSc, and Michelle D. Taylor, PhD, of the University of London (England) in Surrey.
Young adulthood, which encompasses people aged 18-25 years, has been identified as “a peak age of onset for anxiety and depression,” but mental health in young adults with epilepsy in particular has not been well studied, they wrote.
The survey measured current mental health symptoms, including anxiety, depression, and suicidality, as well as sociodemographic and epilepsy-related factors, coping strategies, and social support (Epilepsy Behav. 2021 May;118:107911. doi: 10.1016/j.yebeh.2021.107911).
The average age of the respondents was 21.6 years, 61% were female, and 88% were of White British ethnicity. A total of 88 participants were single, 48 were in a relationship, and 8 were married or engaged. About one-third (38%) worked full-time, and 28.5% were full-time university students, 18.8% worked part-time, and 8.3% were unemployed and not students. The average age of seizure onset was 12.4 years.
Overall, 116 (80.6%) of the survey respondents met the criteria for anxiety, 110 (76.4%) for depression, and 51 (35.4%) for suicidality.
Ratings of all three of these conditions were significantly higher in females, compared with males, the researchers noted. Anxiety, depression, and suicidality also were rated higher for individuals who waited more than 1 year vs. less than 1 year for an epilepsy diagnosis from the time of seizure onset, for those suffering from anti-seizure medication side effects vs. no side effects, and for those with comorbid conditions vs. no comorbid conditions.
Avoidant-focused coping strategies were positively correlated with anxiety, depression, and suicidality, while problem-focused coping and meaning-focused coping were negatively correlated, the researchers said. In addition, those who reported greater levels of support from friends had lower rates of anxiety and depression, and those who reported greater levels of support from family had lower rates of suicidality.
The study findings were limited by several factors, including the relatively homogenous population, and the absence of data on current anxiety and depression medications and additional professional support, the researchers noted.
However, the results extend the research on mental health in people with epilepsy, and the study is the first known to focus on the young adult population with epilepsy, they said.
“The high rates of anxiety, depression, and suicidality underscore the need for better integration of mental health provision into epilepsy care,” the researchers wrote. “While it would be premature to base recommendations for treating anxiety, depression, and suicidality in YAWE on the current study, investigating the efficacy of psychological interventions (for example, [acceptance and commitment therapy], [compassion-focused therapy], peer support, and family-based [therapy]) designed to address the psychosocial variables shown to independently predict mental health outcomes in YAWE would be worthy future research avenues,” they concluded.
The study received no outside funding, and the researchers disclosed no financial conflicts.
Young adults with epilepsy experience higher rates of anxiety, depression, and suicidality, compared with their counterparts in the general population, a new study shows.
The findings, based on a study of 144 young adults with epilepsy (YAWE), was published recently in Epilepsy & Behavior.
“People with epilepsy (PWE) are at a significantly higher risk of experiencing mental health difficulties, compared with healthy controls and individuals with other [long-term conditions] such as asthma and diabetes,” according to Rachel Batchelor, MSc, and Michelle D. Taylor, PhD, of the University of London (England) in Surrey.
Young adulthood, which encompasses people aged 18-25 years, has been identified as “a peak age of onset for anxiety and depression,” but mental health in young adults with epilepsy in particular has not been well studied, they wrote.
The survey measured current mental health symptoms, including anxiety, depression, and suicidality, as well as sociodemographic and epilepsy-related factors, coping strategies, and social support (Epilepsy Behav. 2021 May;118:107911. doi: 10.1016/j.yebeh.2021.107911).
The average age of the respondents was 21.6 years, 61% were female, and 88% were of White British ethnicity. A total of 88 participants were single, 48 were in a relationship, and 8 were married or engaged. About one-third (38%) worked full-time, and 28.5% were full-time university students, 18.8% worked part-time, and 8.3% were unemployed and not students. The average age of seizure onset was 12.4 years.
Overall, 116 (80.6%) of the survey respondents met the criteria for anxiety, 110 (76.4%) for depression, and 51 (35.4%) for suicidality.
Ratings of all three of these conditions were significantly higher in females, compared with males, the researchers noted. Anxiety, depression, and suicidality also were rated higher for individuals who waited more than 1 year vs. less than 1 year for an epilepsy diagnosis from the time of seizure onset, for those suffering from anti-seizure medication side effects vs. no side effects, and for those with comorbid conditions vs. no comorbid conditions.
Avoidant-focused coping strategies were positively correlated with anxiety, depression, and suicidality, while problem-focused coping and meaning-focused coping were negatively correlated, the researchers said. In addition, those who reported greater levels of support from friends had lower rates of anxiety and depression, and those who reported greater levels of support from family had lower rates of suicidality.
The study findings were limited by several factors, including the relatively homogenous population, and the absence of data on current anxiety and depression medications and additional professional support, the researchers noted.
However, the results extend the research on mental health in people with epilepsy, and the study is the first known to focus on the young adult population with epilepsy, they said.
“The high rates of anxiety, depression, and suicidality underscore the need for better integration of mental health provision into epilepsy care,” the researchers wrote. “While it would be premature to base recommendations for treating anxiety, depression, and suicidality in YAWE on the current study, investigating the efficacy of psychological interventions (for example, [acceptance and commitment therapy], [compassion-focused therapy], peer support, and family-based [therapy]) designed to address the psychosocial variables shown to independently predict mental health outcomes in YAWE would be worthy future research avenues,” they concluded.
The study received no outside funding, and the researchers disclosed no financial conflicts.
Young adults with epilepsy experience higher rates of anxiety, depression, and suicidality, compared with their counterparts in the general population, a new study shows.
The findings, based on a study of 144 young adults with epilepsy (YAWE), was published recently in Epilepsy & Behavior.
“People with epilepsy (PWE) are at a significantly higher risk of experiencing mental health difficulties, compared with healthy controls and individuals with other [long-term conditions] such as asthma and diabetes,” according to Rachel Batchelor, MSc, and Michelle D. Taylor, PhD, of the University of London (England) in Surrey.
Young adulthood, which encompasses people aged 18-25 years, has been identified as “a peak age of onset for anxiety and depression,” but mental health in young adults with epilepsy in particular has not been well studied, they wrote.
The survey measured current mental health symptoms, including anxiety, depression, and suicidality, as well as sociodemographic and epilepsy-related factors, coping strategies, and social support (Epilepsy Behav. 2021 May;118:107911. doi: 10.1016/j.yebeh.2021.107911).
The average age of the respondents was 21.6 years, 61% were female, and 88% were of White British ethnicity. A total of 88 participants were single, 48 were in a relationship, and 8 were married or engaged. About one-third (38%) worked full-time, and 28.5% were full-time university students, 18.8% worked part-time, and 8.3% were unemployed and not students. The average age of seizure onset was 12.4 years.
Overall, 116 (80.6%) of the survey respondents met the criteria for anxiety, 110 (76.4%) for depression, and 51 (35.4%) for suicidality.
Ratings of all three of these conditions were significantly higher in females, compared with males, the researchers noted. Anxiety, depression, and suicidality also were rated higher for individuals who waited more than 1 year vs. less than 1 year for an epilepsy diagnosis from the time of seizure onset, for those suffering from anti-seizure medication side effects vs. no side effects, and for those with comorbid conditions vs. no comorbid conditions.
Avoidant-focused coping strategies were positively correlated with anxiety, depression, and suicidality, while problem-focused coping and meaning-focused coping were negatively correlated, the researchers said. In addition, those who reported greater levels of support from friends had lower rates of anxiety and depression, and those who reported greater levels of support from family had lower rates of suicidality.
The study findings were limited by several factors, including the relatively homogenous population, and the absence of data on current anxiety and depression medications and additional professional support, the researchers noted.
However, the results extend the research on mental health in people with epilepsy, and the study is the first known to focus on the young adult population with epilepsy, they said.
“The high rates of anxiety, depression, and suicidality underscore the need for better integration of mental health provision into epilepsy care,” the researchers wrote. “While it would be premature to base recommendations for treating anxiety, depression, and suicidality in YAWE on the current study, investigating the efficacy of psychological interventions (for example, [acceptance and commitment therapy], [compassion-focused therapy], peer support, and family-based [therapy]) designed to address the psychosocial variables shown to independently predict mental health outcomes in YAWE would be worthy future research avenues,” they concluded.
The study received no outside funding, and the researchers disclosed no financial conflicts.
FROM EPILEPSY & BEHAVIOR
Risk factors identified for late seizure relapse after epilepsy surgery
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
according to a new study on the factors most associated with seizure recurrence in drug-resistant epilepsy.
“As our study analyzed late seizure relapse, our results are not applicable for short‐term seizure control. Vice versa, results for short‐term outcomes should not be transferred to long‐term outcomes,” Stephan Petrik of the Epilepsy Center at the University of Freiburg (Germany) and colleagues wrote. The study was published in the May 2021 issue of Epilepsia.
To assess the variables that increase risk of late seizure recurrence following surgery, the researchers retrospectively studied the medical records of patients who underwent resective epilepsy surgery at the University Hospital Freiburg (Germany) between 1999 and 2015. Of the 1,278 initial patients, a group of 99 participants (7.7%) with seizure relapses after at least 2 years of complete seizure freedom were matched with controls experiencing long-term seizure freedom. The two groups had similar mean durations of epilepsy from onset to surgery: 13.9 years in the relapse group and 13.0 years in the control group.
The mean follow-up was 9.7 years (standard deviation, 4.0; range, 2.9-18.5) in the relapse group and 8.2 years (SD, 3.5; range, 2.2-18.3) in the control group. The mean time to late seizure recurrence was 56.6 months, and two-thirds of patients relapsed in the 5 years after surgery. Twenty of the relapse patients only experienced a single seizure, and 41% of the patients who reported more than one seizure had a frequency of less than one per month.
The type of resection had no discernible impact on outcomes, although anterior temporal lobe resection did trend toward being associated with recurrence (odds ratio, 2.7; 95% confidence interval, 0.93-8.89; P = .06). Incomplete resection was significantly associated with late relapse but did not seem to affect timing: the mean duration of seizure freedom was 56.5 months with complete resection and 58.5 months with incomplete resection (P = .62). Additional preoperative PET scans were performed on 45% of patients in the relapse group, compared with 29% in the control group.
After multivariate analysis, predictors for late relapse included incomplete resection (OR, 3.81; 95% CI, 1.79-8.53; P < .001); the existence of additional, potentially epileptogenic lesions in the contralateral hemisphere on presurgical MRI (OR, 3.36; 95% CI, 1.18-10.62; P = .03); epilepsy onset during the first year of life (OR, 4.24; 95% CI, 1.4-15.89; P = .02); and preoperative PET scans being performed (OR, 2.47; 95% CI, 1.25-4.97; P = .01). Though use of preoperative and postoperative antiepileptic drugs (AEDs) was higher in the relapse group, along with complete withdrawal being more common in the control group (68%, compared with 51%), neither was deemed significant in multivariate analysis.
What to do about seizure relapse risk factors
“This is one of the best analyses of the factors that contribute to late seizure relapse,” Gregory K. Bergey, MD, director of the Johns Hopkins Epilepsy Center in Baltimore, said in an interview. “Am I surprised by their results? Not necessarily.”
What did jump out, he said, was AED use not being a predictor of recurrence, as well as all the patients with late relapse having lesional epilepsy. “As they point out, you can have relapse with nonlesional epilepsy, but very often it happens in the first year or 2. If someone is 2 years out and doesn’t have a lesion, they’re probably more likely to remain seizure free.”
Despite the researchers’ comprehensive review of risk factors, the question remains: What to do with this information?
“They’ve done a very good job of identifying that 7.7% of 1,200 who are at risk of a late relapse,” he said. “Now, take those patients with high-risk factors and launch a trial where you keep medicines the same or do something that would alter that outcome.”
“The problem is,” he added, “that’s a 10-year study. It’s easy for me to sit here and call for one of those. But still, as valuable as this was, it’s a retrospective study. Now you have to say, what are the implications of this? What can we do in the prospective fashion?”
The authors acknowledged their study’s other limitations, including a lack of information on the reasons for an incomplete resection, a notable decrease in follow-up visits more than 5 years after surgery, and potential selection bias. They added, however, that “matching by age at surgery, gender, and time to relapse/last follow‐up” should have helped reduce any significant bias.
No potential conflicts of interest were disclosed.
FROM EPILEPSIA

