User login
Can this device take on enlarged prostates?
Inflating a drug-coated balloon in the prostate is the latest approach to treating a common cause of frequent or difficult urination in older men.
As the prostate naturally grows with age, the gland can obstruct the flow of urine – leading to frequent trips to the bathroom and disrupted nights. An estimated 50% of men aged 60 years and older have benign prostatic hyperplasia (BPH). That figure rises to more than 80% by age 70 and to 90% by age 80.
Transurethral resection of the prostate was the main surgical treatment for symptomatic BPH for much of the 20th century.
More recently, researchers have developed various minimally invasive surgical therapy (MIST) devices to treat the obstruction while limiting effects on sexual function. Some newer devices use lasers or water vapor to remove prostate tissue. Another approach uses implants to move and hold prostate tissue out of the way.
Now drug-coated balloons have entered the picture.
, paclitaxel – best known as a chemotherapy medication – to limit further growth and keep the lobes apart.
The Food and Drug Administration approved Optilume BPH in June. The results from a randomized controlled trial of the device were published in The Journal of Urology.
Uptake of MIST devices for BPH “has been variable due to a host of factors including mixed results, complexity of equipment, and costs,” the journal’s editor, D. Robert Siemens, MD, noted in the issue.
The developer of the device, Urotronic, said it expects that the newest treatment will be commercially available in the near future. Discussions about cost, insurance coverage, and how to train urologists to use it are ongoing, said Ian Schorn, the company’s vice president of clinical affairs.
Raevti Bole, MD, a urologic surgeon at Cleveland Clinic’s Glickman Urological and Kidney Institute, said BPH treatments ideally benefit patients for years, so she is eager to see how patients are doing 5 and 10 years after the Optilume BPH procedure. Studies should also examine its effects on fertility.
But given the safety and efficacy results reported 1 year after treatment, “I think this is something that a lot of people are going to be able to use in their practice and that their patients are going to benefit from,” Dr. Bole told this news organization.
She said she expects most urologists will be able to master the technology. The procedure’s minimal effect on sexual issues and the relatively short time needed to perform it are other advantages.
“All of those things are very positive in terms of whether patients are going to want to consider it and also whether surgeons are going to be able to realistically learn it and offer it at their centers,” Dr. Bole said.
In choosing a particular treatment, Dr. Bole discusses options with patients and takes into account factors such as trial data, the nature and severity of symptoms, treatment goals, comorbidities, and the size of the prostate.
Available MIST devices can vary by institution, and urologists can have different levels of experience with each device. If a patient is interested in an approach a surgeon does not offer, the surgeon can refer the patient to a colleague who does.
Active vs. sham treatment
Urologists may be familiar with another Optilume device, the Optilume urethral drug-coated balloon, that is used for urethral strictures.
The devices have similar names, and the underlying technology is similar, but there are major differences, Mr. Schorn said.
The BPH device expands between the lobes of the prostate, creating an anterior commissurotomy. A double-lobe balloon locks the device in place during inflation.
For the PINNACLE trial of the BPH device, which was conducted at 18 sites in the United States and Canada, Steven A. Kaplan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues enrolled 148 men with symptomatic BPH who were experiencing urinary flow obstruction.
The average age of the patients was 65 years; 100 of them were assigned to undergo active treatment with Optilume BPH. The rest received a sham procedure that mimicked active treatment.
At 3 months, men who received active treatment had an average improvement in the International Prostate Symptom Score of about 11 points. This improvement was maintained at 1 year. Those who received sham treatment experienced an 8-point improvement at 3 months that dissipated over time.
The rate of urine flow increased dramatically with Optilume BPH, the researchers reported.
Five serious adverse events were considered to be possibly related to the device. There were four cases of postprocedural hematuria that required cystoscopic management or extended observation, and one case of urethral false passage that required extended catheterization.
Nonserious adverse events in the men who underwent the Optilume procedure typically resolved in about a month and included hematuria (40%), urinary tract infection (14%), dysuria (9.2%), urge or mixed incontinence (8.2%), mild stress incontinence (7.1%), bladder spasms (6.1%), elevated prostate-specific antigen levels (6.1%), and urinary urgency (6.1%), according to the researchers.
In a subset of participants for whom pharmacokinetic data were available, systemic exposure to paclitaxel was minimal.
Four participants in the Optilume BPH arm (4.1%) reported ejaculatory dysfunction, compared with one man in the sham treatment arm (2.1%). There were no cases of treatment-related erectile dysfunction.
Most patients were treated under deep sedation or general anesthesia, and the average procedure time was 26 minutes.
After the procedure, patients received a Foley catheter, which remained in place for about 2 days, “which is not significantly different from water vapor thermal therapy, holmium laser enucleation of the prostate, or laser photovaporization in similar gland sizes,” Dr. Bole and Petar Bajic, MD, also with Cleveland Clinic, noted in a commentary accompanying the article in The Journal of Urology.
MIST devices can be ideal for patients who prioritize sexual function, but the need for a temporary catheter after the procedure can be a “major postoperative source of patient dissatisfaction,” they acknowledged.
“Consistent with other minimally invasive technologies, the Optilume BPH procedure is a straightforward procedure that can be conducted in an ambulatory or office outpatient setting with pain management at physician and patient discretion,” Dr. Kaplan and his coauthors wrote.
The study was featured on the cover of the journal, which the research team saw as an unusual but welcome spotlight for a treatment for BPH.
“We were thrilled that we got on the cover of The Journal of Urology, which is not a common thing for BPH technology,” Mr. Schorn said.
Urotronic funded the PINNACLE study. Dr. Bole has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Inflating a drug-coated balloon in the prostate is the latest approach to treating a common cause of frequent or difficult urination in older men.
As the prostate naturally grows with age, the gland can obstruct the flow of urine – leading to frequent trips to the bathroom and disrupted nights. An estimated 50% of men aged 60 years and older have benign prostatic hyperplasia (BPH). That figure rises to more than 80% by age 70 and to 90% by age 80.
Transurethral resection of the prostate was the main surgical treatment for symptomatic BPH for much of the 20th century.
More recently, researchers have developed various minimally invasive surgical therapy (MIST) devices to treat the obstruction while limiting effects on sexual function. Some newer devices use lasers or water vapor to remove prostate tissue. Another approach uses implants to move and hold prostate tissue out of the way.
Now drug-coated balloons have entered the picture.
, paclitaxel – best known as a chemotherapy medication – to limit further growth and keep the lobes apart.
The Food and Drug Administration approved Optilume BPH in June. The results from a randomized controlled trial of the device were published in The Journal of Urology.
Uptake of MIST devices for BPH “has been variable due to a host of factors including mixed results, complexity of equipment, and costs,” the journal’s editor, D. Robert Siemens, MD, noted in the issue.
The developer of the device, Urotronic, said it expects that the newest treatment will be commercially available in the near future. Discussions about cost, insurance coverage, and how to train urologists to use it are ongoing, said Ian Schorn, the company’s vice president of clinical affairs.
Raevti Bole, MD, a urologic surgeon at Cleveland Clinic’s Glickman Urological and Kidney Institute, said BPH treatments ideally benefit patients for years, so she is eager to see how patients are doing 5 and 10 years after the Optilume BPH procedure. Studies should also examine its effects on fertility.
But given the safety and efficacy results reported 1 year after treatment, “I think this is something that a lot of people are going to be able to use in their practice and that their patients are going to benefit from,” Dr. Bole told this news organization.
She said she expects most urologists will be able to master the technology. The procedure’s minimal effect on sexual issues and the relatively short time needed to perform it are other advantages.
“All of those things are very positive in terms of whether patients are going to want to consider it and also whether surgeons are going to be able to realistically learn it and offer it at their centers,” Dr. Bole said.
In choosing a particular treatment, Dr. Bole discusses options with patients and takes into account factors such as trial data, the nature and severity of symptoms, treatment goals, comorbidities, and the size of the prostate.
Available MIST devices can vary by institution, and urologists can have different levels of experience with each device. If a patient is interested in an approach a surgeon does not offer, the surgeon can refer the patient to a colleague who does.
Active vs. sham treatment
Urologists may be familiar with another Optilume device, the Optilume urethral drug-coated balloon, that is used for urethral strictures.
The devices have similar names, and the underlying technology is similar, but there are major differences, Mr. Schorn said.
The BPH device expands between the lobes of the prostate, creating an anterior commissurotomy. A double-lobe balloon locks the device in place during inflation.
For the PINNACLE trial of the BPH device, which was conducted at 18 sites in the United States and Canada, Steven A. Kaplan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues enrolled 148 men with symptomatic BPH who were experiencing urinary flow obstruction.
The average age of the patients was 65 years; 100 of them were assigned to undergo active treatment with Optilume BPH. The rest received a sham procedure that mimicked active treatment.
At 3 months, men who received active treatment had an average improvement in the International Prostate Symptom Score of about 11 points. This improvement was maintained at 1 year. Those who received sham treatment experienced an 8-point improvement at 3 months that dissipated over time.
The rate of urine flow increased dramatically with Optilume BPH, the researchers reported.
Five serious adverse events were considered to be possibly related to the device. There were four cases of postprocedural hematuria that required cystoscopic management or extended observation, and one case of urethral false passage that required extended catheterization.
Nonserious adverse events in the men who underwent the Optilume procedure typically resolved in about a month and included hematuria (40%), urinary tract infection (14%), dysuria (9.2%), urge or mixed incontinence (8.2%), mild stress incontinence (7.1%), bladder spasms (6.1%), elevated prostate-specific antigen levels (6.1%), and urinary urgency (6.1%), according to the researchers.
In a subset of participants for whom pharmacokinetic data were available, systemic exposure to paclitaxel was minimal.
Four participants in the Optilume BPH arm (4.1%) reported ejaculatory dysfunction, compared with one man in the sham treatment arm (2.1%). There were no cases of treatment-related erectile dysfunction.
Most patients were treated under deep sedation or general anesthesia, and the average procedure time was 26 minutes.
After the procedure, patients received a Foley catheter, which remained in place for about 2 days, “which is not significantly different from water vapor thermal therapy, holmium laser enucleation of the prostate, or laser photovaporization in similar gland sizes,” Dr. Bole and Petar Bajic, MD, also with Cleveland Clinic, noted in a commentary accompanying the article in The Journal of Urology.
MIST devices can be ideal for patients who prioritize sexual function, but the need for a temporary catheter after the procedure can be a “major postoperative source of patient dissatisfaction,” they acknowledged.
“Consistent with other minimally invasive technologies, the Optilume BPH procedure is a straightforward procedure that can be conducted in an ambulatory or office outpatient setting with pain management at physician and patient discretion,” Dr. Kaplan and his coauthors wrote.
The study was featured on the cover of the journal, which the research team saw as an unusual but welcome spotlight for a treatment for BPH.
“We were thrilled that we got on the cover of The Journal of Urology, which is not a common thing for BPH technology,” Mr. Schorn said.
Urotronic funded the PINNACLE study. Dr. Bole has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Inflating a drug-coated balloon in the prostate is the latest approach to treating a common cause of frequent or difficult urination in older men.
As the prostate naturally grows with age, the gland can obstruct the flow of urine – leading to frequent trips to the bathroom and disrupted nights. An estimated 50% of men aged 60 years and older have benign prostatic hyperplasia (BPH). That figure rises to more than 80% by age 70 and to 90% by age 80.
Transurethral resection of the prostate was the main surgical treatment for symptomatic BPH for much of the 20th century.
More recently, researchers have developed various minimally invasive surgical therapy (MIST) devices to treat the obstruction while limiting effects on sexual function. Some newer devices use lasers or water vapor to remove prostate tissue. Another approach uses implants to move and hold prostate tissue out of the way.
Now drug-coated balloons have entered the picture.
, paclitaxel – best known as a chemotherapy medication – to limit further growth and keep the lobes apart.
The Food and Drug Administration approved Optilume BPH in June. The results from a randomized controlled trial of the device were published in The Journal of Urology.
Uptake of MIST devices for BPH “has been variable due to a host of factors including mixed results, complexity of equipment, and costs,” the journal’s editor, D. Robert Siemens, MD, noted in the issue.
The developer of the device, Urotronic, said it expects that the newest treatment will be commercially available in the near future. Discussions about cost, insurance coverage, and how to train urologists to use it are ongoing, said Ian Schorn, the company’s vice president of clinical affairs.
Raevti Bole, MD, a urologic surgeon at Cleveland Clinic’s Glickman Urological and Kidney Institute, said BPH treatments ideally benefit patients for years, so she is eager to see how patients are doing 5 and 10 years after the Optilume BPH procedure. Studies should also examine its effects on fertility.
But given the safety and efficacy results reported 1 year after treatment, “I think this is something that a lot of people are going to be able to use in their practice and that their patients are going to benefit from,” Dr. Bole told this news organization.
She said she expects most urologists will be able to master the technology. The procedure’s minimal effect on sexual issues and the relatively short time needed to perform it are other advantages.
“All of those things are very positive in terms of whether patients are going to want to consider it and also whether surgeons are going to be able to realistically learn it and offer it at their centers,” Dr. Bole said.
In choosing a particular treatment, Dr. Bole discusses options with patients and takes into account factors such as trial data, the nature and severity of symptoms, treatment goals, comorbidities, and the size of the prostate.
Available MIST devices can vary by institution, and urologists can have different levels of experience with each device. If a patient is interested in an approach a surgeon does not offer, the surgeon can refer the patient to a colleague who does.
Active vs. sham treatment
Urologists may be familiar with another Optilume device, the Optilume urethral drug-coated balloon, that is used for urethral strictures.
The devices have similar names, and the underlying technology is similar, but there are major differences, Mr. Schorn said.
The BPH device expands between the lobes of the prostate, creating an anterior commissurotomy. A double-lobe balloon locks the device in place during inflation.
For the PINNACLE trial of the BPH device, which was conducted at 18 sites in the United States and Canada, Steven A. Kaplan, MD, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues enrolled 148 men with symptomatic BPH who were experiencing urinary flow obstruction.
The average age of the patients was 65 years; 100 of them were assigned to undergo active treatment with Optilume BPH. The rest received a sham procedure that mimicked active treatment.
At 3 months, men who received active treatment had an average improvement in the International Prostate Symptom Score of about 11 points. This improvement was maintained at 1 year. Those who received sham treatment experienced an 8-point improvement at 3 months that dissipated over time.
The rate of urine flow increased dramatically with Optilume BPH, the researchers reported.
Five serious adverse events were considered to be possibly related to the device. There were four cases of postprocedural hematuria that required cystoscopic management or extended observation, and one case of urethral false passage that required extended catheterization.
Nonserious adverse events in the men who underwent the Optilume procedure typically resolved in about a month and included hematuria (40%), urinary tract infection (14%), dysuria (9.2%), urge or mixed incontinence (8.2%), mild stress incontinence (7.1%), bladder spasms (6.1%), elevated prostate-specific antigen levels (6.1%), and urinary urgency (6.1%), according to the researchers.
In a subset of participants for whom pharmacokinetic data were available, systemic exposure to paclitaxel was minimal.
Four participants in the Optilume BPH arm (4.1%) reported ejaculatory dysfunction, compared with one man in the sham treatment arm (2.1%). There were no cases of treatment-related erectile dysfunction.
Most patients were treated under deep sedation or general anesthesia, and the average procedure time was 26 minutes.
After the procedure, patients received a Foley catheter, which remained in place for about 2 days, “which is not significantly different from water vapor thermal therapy, holmium laser enucleation of the prostate, or laser photovaporization in similar gland sizes,” Dr. Bole and Petar Bajic, MD, also with Cleveland Clinic, noted in a commentary accompanying the article in The Journal of Urology.
MIST devices can be ideal for patients who prioritize sexual function, but the need for a temporary catheter after the procedure can be a “major postoperative source of patient dissatisfaction,” they acknowledged.
“Consistent with other minimally invasive technologies, the Optilume BPH procedure is a straightforward procedure that can be conducted in an ambulatory or office outpatient setting with pain management at physician and patient discretion,” Dr. Kaplan and his coauthors wrote.
The study was featured on the cover of the journal, which the research team saw as an unusual but welcome spotlight for a treatment for BPH.
“We were thrilled that we got on the cover of The Journal of Urology, which is not a common thing for BPH technology,” Mr. Schorn said.
Urotronic funded the PINNACLE study. Dr. Bole has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Is this the best screening test for prostate cancer?
In the ReIMAGINE study, a group of researchers from the United Kingdom found that half of men with apparently “safe” levels of prostate-specific antigen (PSA) below 3 ng/mL had clinically significant prostate cancers when multiparametric MRI was added to screening. The researchers, whose paper appeared in BMJ Oncology, also found that one in six screened men had a prostate lesion on MRI.
Meanwhile, a large Swedish population-based study, published in JAMA Network Open, showed that pre-biopsy MRIs combined with PSA testing after adoption of guidelines recommending MRIs led to a decrease in the proportion of men with negative biopsies (28% to 7%) and the number of Gleason score 6 cancers (24% to 6%), while the proportion of Gleason score 7-10 cancers rose from 49% to 86%.
Researchers compared prostate MRI uptake rates in the Jönköping Region in southern Sweden over 9 years – 2011 through 2018 before prostate MRIs were recommended nationally, and 2018-2020 when MRIs became commonly used.
David Robinson, MD, PhD, associate professor at Linköping University and leader of the Swedish study, told this news organization: “MRI is now standard for men before biopsy” in that country. In Sweden, which has a high rate of mortality from prostate cancer – about 50 deaths per 100,000 men vs. 12 and 8 per 100,000 in the United Kingdom and United States, respectively – PSA testing is not routine. “Most men that are diagnosed with prostate cancer have no symptoms. They have asked for a PSA when they have visited their general practitioner,” Dr. Robinson said. “To take a PSA test is not encouraged but it is not discouraged either. It is up to each man to decide.”
PSA screening is not common in the United Kingdom. Caroline Moore, MD, chair of urology at University College London and principal investigator on ReIMAGINE, said only 20% of UK men older than age 50 undergo PSA tests because doctors in the United Kingdom are concerned about the sort of overdiagnosis and overtreatment of prostate cancer that has occurred in the United States since the mid-1990s, when PSA screening was adopted here.
The rate of PSA screening in the United States has declined with controversies over recommendations for screening, though they remain above European rates: 37% in 2019, down from 47% in 2005, according to a 2022 Veterans Administration study published in JAMA Oncology.
In the UK study, Dr. Moore’s hospital-based group asked general practitioners to send letters to 2,096 men aged 50-75 years who had not been diagnosed with prostate cancer, inviting them to undergo prostate health checks combining screening with PSA and 10-minute prostate MRIs.
Of the 457 men who responded to the letters, 303 completed both screening tests. Older White men were more likely to respond, and Black men responded 20% less often.
Of the men who completed screening, 29 (9.6%) were diagnosed with clinically significant cancer and 3 were diagnosed with clinically insignificant cancer, the researchers reported.
Dr. Moore said the PSA and MRI-first approach spared men from biopsies as well as the downsides of active surveillance, which include close monitoring with urology visits and occasional MRIs or biopsies over many years. Biopsies are considered undesirable because of pain and the risk for sepsis and other infections associated with transrectal biopsies.
But urologists in America were less convinced by the international data. William J. Catalona, MD, a urologist at Northwestern University in Chicago, who developed the PSA screening test in the 1990s, said he wasn’t surprised so many men in ReIMAGINE with low PSAs had advanced cancers. “Some of the most aggressive prostate cancers occur in men with a low PSA level – not new news,” he said.
Dr. Catalona also disagreed with the UK researchers’ emphasis on MRIs because the readings often are incorrect. A 2021 study in Prostate Cancer and Prostatic Diseases reported that multiparametric MRI had a false-negative rate of between 10% and 20%.
“MRI alone should not be considered more reliable than PSA. Rather, it should be considered complementary,” he said.
Michael S. Leapman, MD, MHS, associate professor of urology at the Yale Cancer Center, New Haven, Conn., said the UK findings point to a role for MRI as a “triage tool” to help identify men with elevated PSAs who should have a prostate biopsy.
But he said the research to date doesn’t support the use of MRI as a stand-alone test for prostate cancer. “In my opinion, it would have to demonstrate some tangible benefit to patients other than finding a greater number of cancers, such as improvement in cancer control, lower burden from the disease overall, or cancer-specific survival,” he said.
Major U.S. guidelines recommend including MRIs before biopsies. Dr. Leapman also pointed out that 2023 recommendations from the National Comprehensive Cancer Network state that MRI is “strongly recommended if available.” Yet fewer than half of U.S. urologists use MRIs as a screening tool, he said.
“My sense is that MRI is not available everywhere. We have also seen that wait times are too long in some centers, leading physicians and patients to opt for biopsy – particularly in cases with higher suspicion,” he said.
The studies from Sweden and the United Kingdom “demonstrate the strides being made in reducing overdetection of low-grade prostate cancer will increase detection of clinically significant Gleason 3+4 or higher” tumors, Dr. Leapman said. “It is unclear whether such patients in whom their otherwise low-risk disease is recast as ‘intermediate risk’ meaningfully stand to benefit in the long term from this detection.”
Dr. Robinson reported no relevant financial conflicts of interest. The Swedish Cancer Society, the Swedish Research Council, Region Jönköping, Futurum, and Clinical Cancer Research Foundation in Jönköping supported the Swedish study. Members of the ReIMAGINE study team disclosed research support from the United Kingdom’s National Institute of Health Research and various industry/other sources. The Medical Research Council and Cancer Research UK funded the ReIMAGINE study.
A version of this article appeared on Medscape.com.
In the ReIMAGINE study, a group of researchers from the United Kingdom found that half of men with apparently “safe” levels of prostate-specific antigen (PSA) below 3 ng/mL had clinically significant prostate cancers when multiparametric MRI was added to screening. The researchers, whose paper appeared in BMJ Oncology, also found that one in six screened men had a prostate lesion on MRI.
Meanwhile, a large Swedish population-based study, published in JAMA Network Open, showed that pre-biopsy MRIs combined with PSA testing after adoption of guidelines recommending MRIs led to a decrease in the proportion of men with negative biopsies (28% to 7%) and the number of Gleason score 6 cancers (24% to 6%), while the proportion of Gleason score 7-10 cancers rose from 49% to 86%.
Researchers compared prostate MRI uptake rates in the Jönköping Region in southern Sweden over 9 years – 2011 through 2018 before prostate MRIs were recommended nationally, and 2018-2020 when MRIs became commonly used.
David Robinson, MD, PhD, associate professor at Linköping University and leader of the Swedish study, told this news organization: “MRI is now standard for men before biopsy” in that country. In Sweden, which has a high rate of mortality from prostate cancer – about 50 deaths per 100,000 men vs. 12 and 8 per 100,000 in the United Kingdom and United States, respectively – PSA testing is not routine. “Most men that are diagnosed with prostate cancer have no symptoms. They have asked for a PSA when they have visited their general practitioner,” Dr. Robinson said. “To take a PSA test is not encouraged but it is not discouraged either. It is up to each man to decide.”
PSA screening is not common in the United Kingdom. Caroline Moore, MD, chair of urology at University College London and principal investigator on ReIMAGINE, said only 20% of UK men older than age 50 undergo PSA tests because doctors in the United Kingdom are concerned about the sort of overdiagnosis and overtreatment of prostate cancer that has occurred in the United States since the mid-1990s, when PSA screening was adopted here.
The rate of PSA screening in the United States has declined with controversies over recommendations for screening, though they remain above European rates: 37% in 2019, down from 47% in 2005, according to a 2022 Veterans Administration study published in JAMA Oncology.
In the UK study, Dr. Moore’s hospital-based group asked general practitioners to send letters to 2,096 men aged 50-75 years who had not been diagnosed with prostate cancer, inviting them to undergo prostate health checks combining screening with PSA and 10-minute prostate MRIs.
Of the 457 men who responded to the letters, 303 completed both screening tests. Older White men were more likely to respond, and Black men responded 20% less often.
Of the men who completed screening, 29 (9.6%) were diagnosed with clinically significant cancer and 3 were diagnosed with clinically insignificant cancer, the researchers reported.
Dr. Moore said the PSA and MRI-first approach spared men from biopsies as well as the downsides of active surveillance, which include close monitoring with urology visits and occasional MRIs or biopsies over many years. Biopsies are considered undesirable because of pain and the risk for sepsis and other infections associated with transrectal biopsies.
But urologists in America were less convinced by the international data. William J. Catalona, MD, a urologist at Northwestern University in Chicago, who developed the PSA screening test in the 1990s, said he wasn’t surprised so many men in ReIMAGINE with low PSAs had advanced cancers. “Some of the most aggressive prostate cancers occur in men with a low PSA level – not new news,” he said.
Dr. Catalona also disagreed with the UK researchers’ emphasis on MRIs because the readings often are incorrect. A 2021 study in Prostate Cancer and Prostatic Diseases reported that multiparametric MRI had a false-negative rate of between 10% and 20%.
“MRI alone should not be considered more reliable than PSA. Rather, it should be considered complementary,” he said.
Michael S. Leapman, MD, MHS, associate professor of urology at the Yale Cancer Center, New Haven, Conn., said the UK findings point to a role for MRI as a “triage tool” to help identify men with elevated PSAs who should have a prostate biopsy.
But he said the research to date doesn’t support the use of MRI as a stand-alone test for prostate cancer. “In my opinion, it would have to demonstrate some tangible benefit to patients other than finding a greater number of cancers, such as improvement in cancer control, lower burden from the disease overall, or cancer-specific survival,” he said.
Major U.S. guidelines recommend including MRIs before biopsies. Dr. Leapman also pointed out that 2023 recommendations from the National Comprehensive Cancer Network state that MRI is “strongly recommended if available.” Yet fewer than half of U.S. urologists use MRIs as a screening tool, he said.
“My sense is that MRI is not available everywhere. We have also seen that wait times are too long in some centers, leading physicians and patients to opt for biopsy – particularly in cases with higher suspicion,” he said.
The studies from Sweden and the United Kingdom “demonstrate the strides being made in reducing overdetection of low-grade prostate cancer will increase detection of clinically significant Gleason 3+4 or higher” tumors, Dr. Leapman said. “It is unclear whether such patients in whom their otherwise low-risk disease is recast as ‘intermediate risk’ meaningfully stand to benefit in the long term from this detection.”
Dr. Robinson reported no relevant financial conflicts of interest. The Swedish Cancer Society, the Swedish Research Council, Region Jönköping, Futurum, and Clinical Cancer Research Foundation in Jönköping supported the Swedish study. Members of the ReIMAGINE study team disclosed research support from the United Kingdom’s National Institute of Health Research and various industry/other sources. The Medical Research Council and Cancer Research UK funded the ReIMAGINE study.
A version of this article appeared on Medscape.com.
In the ReIMAGINE study, a group of researchers from the United Kingdom found that half of men with apparently “safe” levels of prostate-specific antigen (PSA) below 3 ng/mL had clinically significant prostate cancers when multiparametric MRI was added to screening. The researchers, whose paper appeared in BMJ Oncology, also found that one in six screened men had a prostate lesion on MRI.
Meanwhile, a large Swedish population-based study, published in JAMA Network Open, showed that pre-biopsy MRIs combined with PSA testing after adoption of guidelines recommending MRIs led to a decrease in the proportion of men with negative biopsies (28% to 7%) and the number of Gleason score 6 cancers (24% to 6%), while the proportion of Gleason score 7-10 cancers rose from 49% to 86%.
Researchers compared prostate MRI uptake rates in the Jönköping Region in southern Sweden over 9 years – 2011 through 2018 before prostate MRIs were recommended nationally, and 2018-2020 when MRIs became commonly used.
David Robinson, MD, PhD, associate professor at Linköping University and leader of the Swedish study, told this news organization: “MRI is now standard for men before biopsy” in that country. In Sweden, which has a high rate of mortality from prostate cancer – about 50 deaths per 100,000 men vs. 12 and 8 per 100,000 in the United Kingdom and United States, respectively – PSA testing is not routine. “Most men that are diagnosed with prostate cancer have no symptoms. They have asked for a PSA when they have visited their general practitioner,” Dr. Robinson said. “To take a PSA test is not encouraged but it is not discouraged either. It is up to each man to decide.”
PSA screening is not common in the United Kingdom. Caroline Moore, MD, chair of urology at University College London and principal investigator on ReIMAGINE, said only 20% of UK men older than age 50 undergo PSA tests because doctors in the United Kingdom are concerned about the sort of overdiagnosis and overtreatment of prostate cancer that has occurred in the United States since the mid-1990s, when PSA screening was adopted here.
The rate of PSA screening in the United States has declined with controversies over recommendations for screening, though they remain above European rates: 37% in 2019, down from 47% in 2005, according to a 2022 Veterans Administration study published in JAMA Oncology.
In the UK study, Dr. Moore’s hospital-based group asked general practitioners to send letters to 2,096 men aged 50-75 years who had not been diagnosed with prostate cancer, inviting them to undergo prostate health checks combining screening with PSA and 10-minute prostate MRIs.
Of the 457 men who responded to the letters, 303 completed both screening tests. Older White men were more likely to respond, and Black men responded 20% less often.
Of the men who completed screening, 29 (9.6%) were diagnosed with clinically significant cancer and 3 were diagnosed with clinically insignificant cancer, the researchers reported.
Dr. Moore said the PSA and MRI-first approach spared men from biopsies as well as the downsides of active surveillance, which include close monitoring with urology visits and occasional MRIs or biopsies over many years. Biopsies are considered undesirable because of pain and the risk for sepsis and other infections associated with transrectal biopsies.
But urologists in America were less convinced by the international data. William J. Catalona, MD, a urologist at Northwestern University in Chicago, who developed the PSA screening test in the 1990s, said he wasn’t surprised so many men in ReIMAGINE with low PSAs had advanced cancers. “Some of the most aggressive prostate cancers occur in men with a low PSA level – not new news,” he said.
Dr. Catalona also disagreed with the UK researchers’ emphasis on MRIs because the readings often are incorrect. A 2021 study in Prostate Cancer and Prostatic Diseases reported that multiparametric MRI had a false-negative rate of between 10% and 20%.
“MRI alone should not be considered more reliable than PSA. Rather, it should be considered complementary,” he said.
Michael S. Leapman, MD, MHS, associate professor of urology at the Yale Cancer Center, New Haven, Conn., said the UK findings point to a role for MRI as a “triage tool” to help identify men with elevated PSAs who should have a prostate biopsy.
But he said the research to date doesn’t support the use of MRI as a stand-alone test for prostate cancer. “In my opinion, it would have to demonstrate some tangible benefit to patients other than finding a greater number of cancers, such as improvement in cancer control, lower burden from the disease overall, or cancer-specific survival,” he said.
Major U.S. guidelines recommend including MRIs before biopsies. Dr. Leapman also pointed out that 2023 recommendations from the National Comprehensive Cancer Network state that MRI is “strongly recommended if available.” Yet fewer than half of U.S. urologists use MRIs as a screening tool, he said.
“My sense is that MRI is not available everywhere. We have also seen that wait times are too long in some centers, leading physicians and patients to opt for biopsy – particularly in cases with higher suspicion,” he said.
The studies from Sweden and the United Kingdom “demonstrate the strides being made in reducing overdetection of low-grade prostate cancer will increase detection of clinically significant Gleason 3+4 or higher” tumors, Dr. Leapman said. “It is unclear whether such patients in whom their otherwise low-risk disease is recast as ‘intermediate risk’ meaningfully stand to benefit in the long term from this detection.”
Dr. Robinson reported no relevant financial conflicts of interest. The Swedish Cancer Society, the Swedish Research Council, Region Jönköping, Futurum, and Clinical Cancer Research Foundation in Jönköping supported the Swedish study. Members of the ReIMAGINE study team disclosed research support from the United Kingdom’s National Institute of Health Research and various industry/other sources. The Medical Research Council and Cancer Research UK funded the ReIMAGINE study.
A version of this article appeared on Medscape.com.
Treating fractures in elderly patients: Beyond the broken bone
While half the fracture-prevention battle is getting people diagnosed with low bone density, nearly 80% of older Americans who suffer bone breaks are not tested or treated for osteoporosis. Fractures associated with aging and diminished bone mineral density exact an enormous toll on patients’ lives and cost the health care system billions of dollars annually according to Bone Health and Osteoporosis: A Report of the Surgeon General. But current gaps in patient education and bone density screening are huge.
“It’s concerning that older patients at risk for fracture are often not screened to determine their risk factors contributing to osteoporosis and patients are not educated about fracture prevention,” said Meryl S. LeBoff, MD, an endocrinologist at Brigham and Women’s Hospital, and chief of calcium and bone section, and professor of medicine, at Harvard Medical School, Boston. “Furthermore, the majority of highest-risk women and men who do have fractures are not screened and they do not receive effective, [Food and Drug Administration]–approved therapies.”
Recent guidelines
Screening with dual-energy x-ray absorptiometry (DEXA) is recommended for all women at age 65 and all men at age 70. But the occasion of a fracture in an older person who has not yet met these age thresholds should prompt a bone density assessment.
“Doctors need to stress that one in two women and one in four men over age 50 will have a fracture in their remaining lifetimes,” Dr. LeBoff said. ”Primary care doctors play a critical role in ordering timely bone densitometry for both sexes.
If an older patient has been treated for a fracture, the main goal going forward is to prevent another one, for which the risk is highest in the 2 years after the incident fracture.”
According to Kendall F. Moseley, MD, clinical director of the division of endocrinology, diabetes & metabolism at Johns Hopkins Medicine in Baltimore, “Elderly patients need to understand that a fracture at their age is like a heart attack of the bone,” she said, adding that just as cardiovascular risk factors such as high blood pressure and blood lipids are silent before a stroke or infarction, the bone thinning of old age is also silent.
Endocrinologist Jennifer J. Kelly, DO, director of the metabolic bone program and an associate professor at the University of Vermont Medical Center in Burlington, said a fracture in anyone over age 50 that appears not to have resulted from a traumatic blow, is a compelling reason to order a DEXA exam.
Nahid J. Rianon, MBBS/MD, DrPH, assistant professor of the division of geriatric medicine at the UTHealth McGovern Medical School, Houston, goes further: “Any fracture in someone age 50 and older warrants screening for osteoporosis. And if the fracture is nontraumatic, that is by definition a clinical diagnosis of osteoporosis regardless of normal results on bone density tests and they should be treated medically. There are aspects of bone that we still can’t measure in the clinical setting.”
If DEXA is not accessible, fracture risk over the next 10 years can be evaluated based on multiple patient characteristics and medical history using the online FRAX calculator.
Just a 3% risk of hip fracture on FRAX is considered an indication to begin medical osteoporosis treatment in the United States regardless of bone density test results, Dr. Rianon said.
Fracture management
Whether a senior suffers a traumatic fracture or an osteoporosis-related fragility fracture, older age can impede the healing process in some. Senescence may also increase systemic proinflammatory status, according to Clark and colleagues, writing in Current Osteoporosis Reports.
They called for research to develop more directed treatment options for the elderly population.
Dr. Rianon noted that healing may also be affected by a decrease in muscle mass, which plays a role in holding the bone in place. “But it is still controversial how changing metabolic factors affect bone healing in the elderly.”
However, countered Dr. Kelly, fractures in elderly patients are not necessarily less likely to mend – if osteoporosis is not present. “Many heal very well – it really depends more upon their overall health and medical history. Whether or not a person requires surgery depends more upon the extent of the fracture and if the bone is able to align and heal appropriately without surgery.”
Fracture sites
Spine. According to the American Academy of Orthopedic Surgeons the earliest and most frequent site of fragility fractures in the elderly is the spine. Most vertebral fracture pain improves within 3 months without specific treatment. A short period of rest, limited analgesic use, and possible back bracing may help as the fractures heal on their own. But if pain is severe and persistent, vertebral augmentation with percutaneous kyphoplasty or vertebroplasty may be an option. These procedures, however, can destabilize surrounding discs because of the greater thickness of the injected cement.
Hip. The most dangerous fractures occur in the hip. These carry at least a 20% risk of death in the first postoperative year and must be treated surgically. Those in the proximal femur, the head, or the femoral neck will usually need hip replacement, but if the break is farther down, it may be repaired with cement, screws, plates, and rods.
Distal radius. Outcomes of wrist fractures may be positive without surgical intervention, according to a recent retrospective analysis from Turkey by Yalin and colleagues. In a comparison of clinical outcomes in seniors aged 70-89 and assigned to cast immobilization or various surgical treatments for distal radius fractures, no statistically significant difference was found in patient-reported disability scores and range of motion values between casting and surgery in the first postoperative year.
Other sites. Fractures in the elderly are not uncommon in the shoulder, distal radius, cubitus, proximal humerus, and humerus. These fractures are often treated without surgery, but nevertheless signal a high risk for additional fractures.
Bone-enhancing medications
Even in the absence of diagnosed low bone density or osteoporosis, anabolic agents such as the synthetic human parathyroid hormones abaloparatide (Tymlos) and teriparatide (Forteo) may be used to help in some cases with a bad healing prognosis and may also be used for people undergoing surgeries such as a spinal fusion, but there are not clinical guidelines. “We receive referrals regularly for this treatment from our orthopedics colleagues, but it is considered an off-label use,” Dr. Kelly said.
The anabolics teriparatide and romosozumab (Evenity) have proved effective in lowering fractures in high-risk older women.
Post fracture
After recovering from a fracture, elderly people are strongly advised to make lifestyle changes to boost bone health and reduce risk of further fractures, said Willy M. Valencia, MD, a geriatrician-endocrinologist at the Cleveland Clinic. Apart from active daily living, he recommends several types of formal exercise to promote bone formation; increase muscle mass, strength, and flexibility; and improve endurance, balance, and gait. The National Institute on Aging outlines suitable exercise programs for seniors.
“These exercises will help reduce the risk of falling and to avoid more fractures,” he said. “Whether a patient has been exercising before the fracture or not, they may feel some reticence or reluctance to take up exercise afterwards because they’re afraid of having another fracture, but they should understand that their fracture risk increases if they remain sedentary. They should start slowly but they can’t be sitting all day.”
Even before it’s possible to exercise at the healing fracture site, added Dr. Rianon, its advisable to work other areas of the body. “Overall mobility is important, and exercising other parts of the body can stimulate strength and help prevent falling.”
In other postsurgical measures, a bone-friendly diet rich in calcium and vitamin D, as well as supplementation with these vital nutrients, is essential to lower the risk of falling.
Fall prevention is paramount, said Dr. Valencia. While exercise can improve, gait, balance, and endurance, logistical measures may also be necessary. Seniors may have to move to a one-floor domicile with no stairs to negotiate. At the very least, they need to fall-proof their daily lives by upgrading their eyeglasses and home lighting, eliminating obstacles and loose carpets, fixing bannisters, and installing bathroom handrails. Some may need assistive devices for walking, especially outdoors in slippery conditions.
At the end of the day, the role of the primary physician in screening for bone problems before fracture and postsurgical care is key. “Risk factors for osteoporosis and fracture risk must be added to the patient’s chart,” said Dr. Rianon. Added Dr. Moseley. “No matter how busy they are, my hope is that primary care physicians will not put patients’ bone health at the bottom of the clinical agenda.”
While half the fracture-prevention battle is getting people diagnosed with low bone density, nearly 80% of older Americans who suffer bone breaks are not tested or treated for osteoporosis. Fractures associated with aging and diminished bone mineral density exact an enormous toll on patients’ lives and cost the health care system billions of dollars annually according to Bone Health and Osteoporosis: A Report of the Surgeon General. But current gaps in patient education and bone density screening are huge.
“It’s concerning that older patients at risk for fracture are often not screened to determine their risk factors contributing to osteoporosis and patients are not educated about fracture prevention,” said Meryl S. LeBoff, MD, an endocrinologist at Brigham and Women’s Hospital, and chief of calcium and bone section, and professor of medicine, at Harvard Medical School, Boston. “Furthermore, the majority of highest-risk women and men who do have fractures are not screened and they do not receive effective, [Food and Drug Administration]–approved therapies.”
Recent guidelines
Screening with dual-energy x-ray absorptiometry (DEXA) is recommended for all women at age 65 and all men at age 70. But the occasion of a fracture in an older person who has not yet met these age thresholds should prompt a bone density assessment.
“Doctors need to stress that one in two women and one in four men over age 50 will have a fracture in their remaining lifetimes,” Dr. LeBoff said. ”Primary care doctors play a critical role in ordering timely bone densitometry for both sexes.
If an older patient has been treated for a fracture, the main goal going forward is to prevent another one, for which the risk is highest in the 2 years after the incident fracture.”
According to Kendall F. Moseley, MD, clinical director of the division of endocrinology, diabetes & metabolism at Johns Hopkins Medicine in Baltimore, “Elderly patients need to understand that a fracture at their age is like a heart attack of the bone,” she said, adding that just as cardiovascular risk factors such as high blood pressure and blood lipids are silent before a stroke or infarction, the bone thinning of old age is also silent.
Endocrinologist Jennifer J. Kelly, DO, director of the metabolic bone program and an associate professor at the University of Vermont Medical Center in Burlington, said a fracture in anyone over age 50 that appears not to have resulted from a traumatic blow, is a compelling reason to order a DEXA exam.
Nahid J. Rianon, MBBS/MD, DrPH, assistant professor of the division of geriatric medicine at the UTHealth McGovern Medical School, Houston, goes further: “Any fracture in someone age 50 and older warrants screening for osteoporosis. And if the fracture is nontraumatic, that is by definition a clinical diagnosis of osteoporosis regardless of normal results on bone density tests and they should be treated medically. There are aspects of bone that we still can’t measure in the clinical setting.”
If DEXA is not accessible, fracture risk over the next 10 years can be evaluated based on multiple patient characteristics and medical history using the online FRAX calculator.
Just a 3% risk of hip fracture on FRAX is considered an indication to begin medical osteoporosis treatment in the United States regardless of bone density test results, Dr. Rianon said.
Fracture management
Whether a senior suffers a traumatic fracture or an osteoporosis-related fragility fracture, older age can impede the healing process in some. Senescence may also increase systemic proinflammatory status, according to Clark and colleagues, writing in Current Osteoporosis Reports.
They called for research to develop more directed treatment options for the elderly population.
Dr. Rianon noted that healing may also be affected by a decrease in muscle mass, which plays a role in holding the bone in place. “But it is still controversial how changing metabolic factors affect bone healing in the elderly.”
However, countered Dr. Kelly, fractures in elderly patients are not necessarily less likely to mend – if osteoporosis is not present. “Many heal very well – it really depends more upon their overall health and medical history. Whether or not a person requires surgery depends more upon the extent of the fracture and if the bone is able to align and heal appropriately without surgery.”
Fracture sites
Spine. According to the American Academy of Orthopedic Surgeons the earliest and most frequent site of fragility fractures in the elderly is the spine. Most vertebral fracture pain improves within 3 months without specific treatment. A short period of rest, limited analgesic use, and possible back bracing may help as the fractures heal on their own. But if pain is severe and persistent, vertebral augmentation with percutaneous kyphoplasty or vertebroplasty may be an option. These procedures, however, can destabilize surrounding discs because of the greater thickness of the injected cement.
Hip. The most dangerous fractures occur in the hip. These carry at least a 20% risk of death in the first postoperative year and must be treated surgically. Those in the proximal femur, the head, or the femoral neck will usually need hip replacement, but if the break is farther down, it may be repaired with cement, screws, plates, and rods.
Distal radius. Outcomes of wrist fractures may be positive without surgical intervention, according to a recent retrospective analysis from Turkey by Yalin and colleagues. In a comparison of clinical outcomes in seniors aged 70-89 and assigned to cast immobilization or various surgical treatments for distal radius fractures, no statistically significant difference was found in patient-reported disability scores and range of motion values between casting and surgery in the first postoperative year.
Other sites. Fractures in the elderly are not uncommon in the shoulder, distal radius, cubitus, proximal humerus, and humerus. These fractures are often treated without surgery, but nevertheless signal a high risk for additional fractures.
Bone-enhancing medications
Even in the absence of diagnosed low bone density or osteoporosis, anabolic agents such as the synthetic human parathyroid hormones abaloparatide (Tymlos) and teriparatide (Forteo) may be used to help in some cases with a bad healing prognosis and may also be used for people undergoing surgeries such as a spinal fusion, but there are not clinical guidelines. “We receive referrals regularly for this treatment from our orthopedics colleagues, but it is considered an off-label use,” Dr. Kelly said.
The anabolics teriparatide and romosozumab (Evenity) have proved effective in lowering fractures in high-risk older women.
Post fracture
After recovering from a fracture, elderly people are strongly advised to make lifestyle changes to boost bone health and reduce risk of further fractures, said Willy M. Valencia, MD, a geriatrician-endocrinologist at the Cleveland Clinic. Apart from active daily living, he recommends several types of formal exercise to promote bone formation; increase muscle mass, strength, and flexibility; and improve endurance, balance, and gait. The National Institute on Aging outlines suitable exercise programs for seniors.
“These exercises will help reduce the risk of falling and to avoid more fractures,” he said. “Whether a patient has been exercising before the fracture or not, they may feel some reticence or reluctance to take up exercise afterwards because they’re afraid of having another fracture, but they should understand that their fracture risk increases if they remain sedentary. They should start slowly but they can’t be sitting all day.”
Even before it’s possible to exercise at the healing fracture site, added Dr. Rianon, its advisable to work other areas of the body. “Overall mobility is important, and exercising other parts of the body can stimulate strength and help prevent falling.”
In other postsurgical measures, a bone-friendly diet rich in calcium and vitamin D, as well as supplementation with these vital nutrients, is essential to lower the risk of falling.
Fall prevention is paramount, said Dr. Valencia. While exercise can improve, gait, balance, and endurance, logistical measures may also be necessary. Seniors may have to move to a one-floor domicile with no stairs to negotiate. At the very least, they need to fall-proof their daily lives by upgrading their eyeglasses and home lighting, eliminating obstacles and loose carpets, fixing bannisters, and installing bathroom handrails. Some may need assistive devices for walking, especially outdoors in slippery conditions.
At the end of the day, the role of the primary physician in screening for bone problems before fracture and postsurgical care is key. “Risk factors for osteoporosis and fracture risk must be added to the patient’s chart,” said Dr. Rianon. Added Dr. Moseley. “No matter how busy they are, my hope is that primary care physicians will not put patients’ bone health at the bottom of the clinical agenda.”
While half the fracture-prevention battle is getting people diagnosed with low bone density, nearly 80% of older Americans who suffer bone breaks are not tested or treated for osteoporosis. Fractures associated with aging and diminished bone mineral density exact an enormous toll on patients’ lives and cost the health care system billions of dollars annually according to Bone Health and Osteoporosis: A Report of the Surgeon General. But current gaps in patient education and bone density screening are huge.
“It’s concerning that older patients at risk for fracture are often not screened to determine their risk factors contributing to osteoporosis and patients are not educated about fracture prevention,” said Meryl S. LeBoff, MD, an endocrinologist at Brigham and Women’s Hospital, and chief of calcium and bone section, and professor of medicine, at Harvard Medical School, Boston. “Furthermore, the majority of highest-risk women and men who do have fractures are not screened and they do not receive effective, [Food and Drug Administration]–approved therapies.”
Recent guidelines
Screening with dual-energy x-ray absorptiometry (DEXA) is recommended for all women at age 65 and all men at age 70. But the occasion of a fracture in an older person who has not yet met these age thresholds should prompt a bone density assessment.
“Doctors need to stress that one in two women and one in four men over age 50 will have a fracture in their remaining lifetimes,” Dr. LeBoff said. ”Primary care doctors play a critical role in ordering timely bone densitometry for both sexes.
If an older patient has been treated for a fracture, the main goal going forward is to prevent another one, for which the risk is highest in the 2 years after the incident fracture.”
According to Kendall F. Moseley, MD, clinical director of the division of endocrinology, diabetes & metabolism at Johns Hopkins Medicine in Baltimore, “Elderly patients need to understand that a fracture at their age is like a heart attack of the bone,” she said, adding that just as cardiovascular risk factors such as high blood pressure and blood lipids are silent before a stroke or infarction, the bone thinning of old age is also silent.
Endocrinologist Jennifer J. Kelly, DO, director of the metabolic bone program and an associate professor at the University of Vermont Medical Center in Burlington, said a fracture in anyone over age 50 that appears not to have resulted from a traumatic blow, is a compelling reason to order a DEXA exam.
Nahid J. Rianon, MBBS/MD, DrPH, assistant professor of the division of geriatric medicine at the UTHealth McGovern Medical School, Houston, goes further: “Any fracture in someone age 50 and older warrants screening for osteoporosis. And if the fracture is nontraumatic, that is by definition a clinical diagnosis of osteoporosis regardless of normal results on bone density tests and they should be treated medically. There are aspects of bone that we still can’t measure in the clinical setting.”
If DEXA is not accessible, fracture risk over the next 10 years can be evaluated based on multiple patient characteristics and medical history using the online FRAX calculator.
Just a 3% risk of hip fracture on FRAX is considered an indication to begin medical osteoporosis treatment in the United States regardless of bone density test results, Dr. Rianon said.
Fracture management
Whether a senior suffers a traumatic fracture or an osteoporosis-related fragility fracture, older age can impede the healing process in some. Senescence may also increase systemic proinflammatory status, according to Clark and colleagues, writing in Current Osteoporosis Reports.
They called for research to develop more directed treatment options for the elderly population.
Dr. Rianon noted that healing may also be affected by a decrease in muscle mass, which plays a role in holding the bone in place. “But it is still controversial how changing metabolic factors affect bone healing in the elderly.”
However, countered Dr. Kelly, fractures in elderly patients are not necessarily less likely to mend – if osteoporosis is not present. “Many heal very well – it really depends more upon their overall health and medical history. Whether or not a person requires surgery depends more upon the extent of the fracture and if the bone is able to align and heal appropriately without surgery.”
Fracture sites
Spine. According to the American Academy of Orthopedic Surgeons the earliest and most frequent site of fragility fractures in the elderly is the spine. Most vertebral fracture pain improves within 3 months without specific treatment. A short period of rest, limited analgesic use, and possible back bracing may help as the fractures heal on their own. But if pain is severe and persistent, vertebral augmentation with percutaneous kyphoplasty or vertebroplasty may be an option. These procedures, however, can destabilize surrounding discs because of the greater thickness of the injected cement.
Hip. The most dangerous fractures occur in the hip. These carry at least a 20% risk of death in the first postoperative year and must be treated surgically. Those in the proximal femur, the head, or the femoral neck will usually need hip replacement, but if the break is farther down, it may be repaired with cement, screws, plates, and rods.
Distal radius. Outcomes of wrist fractures may be positive without surgical intervention, according to a recent retrospective analysis from Turkey by Yalin and colleagues. In a comparison of clinical outcomes in seniors aged 70-89 and assigned to cast immobilization or various surgical treatments for distal radius fractures, no statistically significant difference was found in patient-reported disability scores and range of motion values between casting and surgery in the first postoperative year.
Other sites. Fractures in the elderly are not uncommon in the shoulder, distal radius, cubitus, proximal humerus, and humerus. These fractures are often treated without surgery, but nevertheless signal a high risk for additional fractures.
Bone-enhancing medications
Even in the absence of diagnosed low bone density or osteoporosis, anabolic agents such as the synthetic human parathyroid hormones abaloparatide (Tymlos) and teriparatide (Forteo) may be used to help in some cases with a bad healing prognosis and may also be used for people undergoing surgeries such as a spinal fusion, but there are not clinical guidelines. “We receive referrals regularly for this treatment from our orthopedics colleagues, but it is considered an off-label use,” Dr. Kelly said.
The anabolics teriparatide and romosozumab (Evenity) have proved effective in lowering fractures in high-risk older women.
Post fracture
After recovering from a fracture, elderly people are strongly advised to make lifestyle changes to boost bone health and reduce risk of further fractures, said Willy M. Valencia, MD, a geriatrician-endocrinologist at the Cleveland Clinic. Apart from active daily living, he recommends several types of formal exercise to promote bone formation; increase muscle mass, strength, and flexibility; and improve endurance, balance, and gait. The National Institute on Aging outlines suitable exercise programs for seniors.
“These exercises will help reduce the risk of falling and to avoid more fractures,” he said. “Whether a patient has been exercising before the fracture or not, they may feel some reticence or reluctance to take up exercise afterwards because they’re afraid of having another fracture, but they should understand that their fracture risk increases if they remain sedentary. They should start slowly but they can’t be sitting all day.”
Even before it’s possible to exercise at the healing fracture site, added Dr. Rianon, its advisable to work other areas of the body. “Overall mobility is important, and exercising other parts of the body can stimulate strength and help prevent falling.”
In other postsurgical measures, a bone-friendly diet rich in calcium and vitamin D, as well as supplementation with these vital nutrients, is essential to lower the risk of falling.
Fall prevention is paramount, said Dr. Valencia. While exercise can improve, gait, balance, and endurance, logistical measures may also be necessary. Seniors may have to move to a one-floor domicile with no stairs to negotiate. At the very least, they need to fall-proof their daily lives by upgrading their eyeglasses and home lighting, eliminating obstacles and loose carpets, fixing bannisters, and installing bathroom handrails. Some may need assistive devices for walking, especially outdoors in slippery conditions.
At the end of the day, the role of the primary physician in screening for bone problems before fracture and postsurgical care is key. “Risk factors for osteoporosis and fracture risk must be added to the patient’s chart,” said Dr. Rianon. Added Dr. Moseley. “No matter how busy they are, my hope is that primary care physicians will not put patients’ bone health at the bottom of the clinical agenda.”
Osteoarthritis cases projected to balloon over next 30 years
TOPLINE:
METHODOLOGY:
- Researchers estimated the prevalence of osteoarthritis in 204 countries and territories from 1990 to 2020 and projected prevalence levels for the year 2050.
- Population-based surveys offered data from 26 countries for knee osteoarthritis, 23 countries for hip osteoarthritis, and 42 countries for hand osteoarthritis. Researchers used U.S. insurance claims to estimate prevalence for other osteoarthritis types.
- Similar analyses were conducted in 2010 and 2017.
TAKEAWAY:
- Osteoarthritis cases worldwide have grown by an estimated 132.2% since 1990. Population growth and aging were identified as major contributing factors.
- In 2020, an estimated 595 million people had osteoarthritis. From 2020 to 2050, cases of osteoarthritis in the knee are expected to grow by 74.9%, in the hand by 48.6%, in the hip by 78.6%, and in other locations by 95.1%.
- Years lived with disability (age-standardized rate) grew from an estimated 233 per 100,000 in 1990 to 255 per 100,000 in 2020, an increase of 9.5%.
- High body mass index contributed to 20.4% of cases.
IN PRACTICE:
In “a major challenge to health systems,” osteoarthritis may affect nearly 1 billion people in 2050.
SOURCE:
The Global Burden of Disease 2021 Osteoarthritis Collaborators, led by Jaimie D. Steinmetz, PhD, MSc, of the Institute for Health Metrics and Evaluation, Seattle, conducted the study, which was published in The Lancet Rheumatology.
LIMITATIONS:
Limited data, heavy reliance on U.S. insurance data, and other factors may have skewed the results.
DISCLOSURES:
The study was supported by the Bill & Melinda Gates Foundation, the Institute of Bone and Joint Research, and the Global Alliance for Musculoskeletal Health. Multiple authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers estimated the prevalence of osteoarthritis in 204 countries and territories from 1990 to 2020 and projected prevalence levels for the year 2050.
- Population-based surveys offered data from 26 countries for knee osteoarthritis, 23 countries for hip osteoarthritis, and 42 countries for hand osteoarthritis. Researchers used U.S. insurance claims to estimate prevalence for other osteoarthritis types.
- Similar analyses were conducted in 2010 and 2017.
TAKEAWAY:
- Osteoarthritis cases worldwide have grown by an estimated 132.2% since 1990. Population growth and aging were identified as major contributing factors.
- In 2020, an estimated 595 million people had osteoarthritis. From 2020 to 2050, cases of osteoarthritis in the knee are expected to grow by 74.9%, in the hand by 48.6%, in the hip by 78.6%, and in other locations by 95.1%.
- Years lived with disability (age-standardized rate) grew from an estimated 233 per 100,000 in 1990 to 255 per 100,000 in 2020, an increase of 9.5%.
- High body mass index contributed to 20.4% of cases.
IN PRACTICE:
In “a major challenge to health systems,” osteoarthritis may affect nearly 1 billion people in 2050.
SOURCE:
The Global Burden of Disease 2021 Osteoarthritis Collaborators, led by Jaimie D. Steinmetz, PhD, MSc, of the Institute for Health Metrics and Evaluation, Seattle, conducted the study, which was published in The Lancet Rheumatology.
LIMITATIONS:
Limited data, heavy reliance on U.S. insurance data, and other factors may have skewed the results.
DISCLOSURES:
The study was supported by the Bill & Melinda Gates Foundation, the Institute of Bone and Joint Research, and the Global Alliance for Musculoskeletal Health. Multiple authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers estimated the prevalence of osteoarthritis in 204 countries and territories from 1990 to 2020 and projected prevalence levels for the year 2050.
- Population-based surveys offered data from 26 countries for knee osteoarthritis, 23 countries for hip osteoarthritis, and 42 countries for hand osteoarthritis. Researchers used U.S. insurance claims to estimate prevalence for other osteoarthritis types.
- Similar analyses were conducted in 2010 and 2017.
TAKEAWAY:
- Osteoarthritis cases worldwide have grown by an estimated 132.2% since 1990. Population growth and aging were identified as major contributing factors.
- In 2020, an estimated 595 million people had osteoarthritis. From 2020 to 2050, cases of osteoarthritis in the knee are expected to grow by 74.9%, in the hand by 48.6%, in the hip by 78.6%, and in other locations by 95.1%.
- Years lived with disability (age-standardized rate) grew from an estimated 233 per 100,000 in 1990 to 255 per 100,000 in 2020, an increase of 9.5%.
- High body mass index contributed to 20.4% of cases.
IN PRACTICE:
In “a major challenge to health systems,” osteoarthritis may affect nearly 1 billion people in 2050.
SOURCE:
The Global Burden of Disease 2021 Osteoarthritis Collaborators, led by Jaimie D. Steinmetz, PhD, MSc, of the Institute for Health Metrics and Evaluation, Seattle, conducted the study, which was published in The Lancet Rheumatology.
LIMITATIONS:
Limited data, heavy reliance on U.S. insurance data, and other factors may have skewed the results.
DISCLOSURES:
The study was supported by the Bill & Melinda Gates Foundation, the Institute of Bone and Joint Research, and the Global Alliance for Musculoskeletal Health. Multiple authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
The most important study from ESC: FRAIL-AF
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
One of the hardest tasks of a clinician is applying evidence from trials to the person in your office. At the annual congress of the European Society of Cardiology, the surprising and unexpected results of the FRAIL-AF trial confirm the massive challenge of evidence translation.
FRAIL-AF investigators set out to study the question of whether frail, elderly patients with atrial fibrillation who were doing well with vitamin K antagonists (VKA) should be switched to direct-acting oral anticoagulants (DOAC).
Senior author Geert-Jan Geersing, MD, PhD, from the University Medical Center Utrecht (the Netherlands), told me that frustration led him to design this study. He was frustrated that colleagues assumed that evidence in nonfrail patients can always be translated to frail patients.
Dr. Geersing offered two reasons why common wisdom may be wrong. First was that the large DOAC versus warfarin trials included few elderly patients with frailty. Second, first author Linda Joosten, MD, made it clear in her presentation that frailty is a lot more than aging. It is a clinical syndrome, which entails a “high burden of comorbidities, dependency on others, and a reduced ability to resist stressors.”
The FRAIL-AF trial
The investigators recruited elderly, frail patients with fibrillation who were treated with VKAs and had stable international normalized ratios from outpatient clinics throughout the Netherlands. They screened about 2,600 patients and enrolled nearly 1,400. Most were excluded for not being frail.
Half the group was randomized to switching to a DOAC – drug choice was left to the treating clinician – and the other half remained on VKAs. Patients were 83 years of age on average with a mean CHA2DS2-VASc score of 4. All four classes of DOAC were used in the switching arm.
The primary endpoint was major or clinically relevant nonmajor bleeding, whichever came first, accounting for death as a competing risk. Follow-up was 1 year.
The results for switching to DOAC vs. VKA
Dr. Joosten started her presentation with this: “The results turned out to be different than we expected.” The authors designed the trial with the idea that switching to DOACs would be superior in safety to remaining on VKAs.
But the trial was halted after an interim analysis found a rate of major bleeding in the switching arm of 15.3% versus 9.4% in the arm staying on VKA (hazard ratio, 1.69; 95% confidence interval, 1.23-2.32; P = .0012).
The Kaplan-Meier event curves reveal that the excess risk of bleeding occurred after 100 days and increased with time. This argued against an early effect from transitioning the drugs.
An analysis looking at specific DOAC drugs revealed similar hazards for the two most common ones used – apixaban and rivaroxaban.
Thrombotic events were a secondary endpoint and were low in absolute numbers, 2.4% versus 2.0%, for remaining on VKA and switching to DOAC, respectively (HR, 1.26; 95% CI, 0.60-2.61).
The time in therapeutic range in FRAIL-AF was similar to that in the seminal DOAC trials.
Comments
Three reasons lead me to choose FRAIL-AF as the most important study from the 2023 ESC congress.
First is the specific lesson about switching drugs. Note that FRAIL-AF did not address the question of starting anticoagulation. The trial results show that if you have a frail older patient who is doing well on VKA, don’t change to a DOAC. That is important to know, but it is not what gives this study its heft.
The second reason centers on the investigators choice to do this trial. Dr. Geersing had a feeling that common wisdom was wrong. He did not try to persuade colleagues with anecdote or plausibility or meta-analyses of observational studies. He set out to answer a question in the correct way – with a randomized trial.
This is the path forward in medicine. I’ve often heard proponents of observational research declare that many topics in medicine cannot be studied with trials. I could hear people arguing that it’s not feasible to study mostly home-bound, elderly frail patients. And the fact that there exist so few trials in this space would support that argument.
But the FRAIL-AF authors showed that it is possible. This is the kind of science that medicine should celebrate. There were no soft endpoints, financial conflicts, or spin. If medical science had science as its incentive, rather than attention, FRAIL-AF easily wins top honors.
The third reason FRAIL-AF is so important is that it teaches us the humility required in translating evidence in our clinics. I like to say evidence is what separates doctors from palm readers. But using this evidence requires thinking hard about how average effects in trial environments apply to our patient.
Yes, of course, there is clear evidence from tens of thousands of patients in the DOAC versus warfarin trials, that, for those patients, on average, DOACs compare favorably with VKA. The average age of patients in these trials was 70-73 years; the average age in FRAIL-AF was 83 years. And that is just age. A substudy of the ENGAGE AF-TIMI 48 trial found that only 360 of more than 20,000 patients in the trial had severe frailty.
That lesson extends to nearly every common therapy in medicine today. It also casts great doubt on the soft-thinking idea of using evidence from trials to derive quality metrics. As if the nuance of evidence translation can be captured in an electronic health record.
The skillful use of evidence will be one of the main challenges of the next generation of clinicians. Thanks to advances in medical science, more patients will live long enough to become frail. And the so-called “guideline-directed” therapies may not apply to them.
Dr. Joosten, Dr. Geersing, and the FRAIL-AF team have taught us specific lessons about anticoagulation, but their greatest contribution has been to demonstrate the value of humility in science and the practice of evidence-based medicine.
If you treat patients, no trial at this meeting is more important.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
ADHD in older adults: A closer look
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).
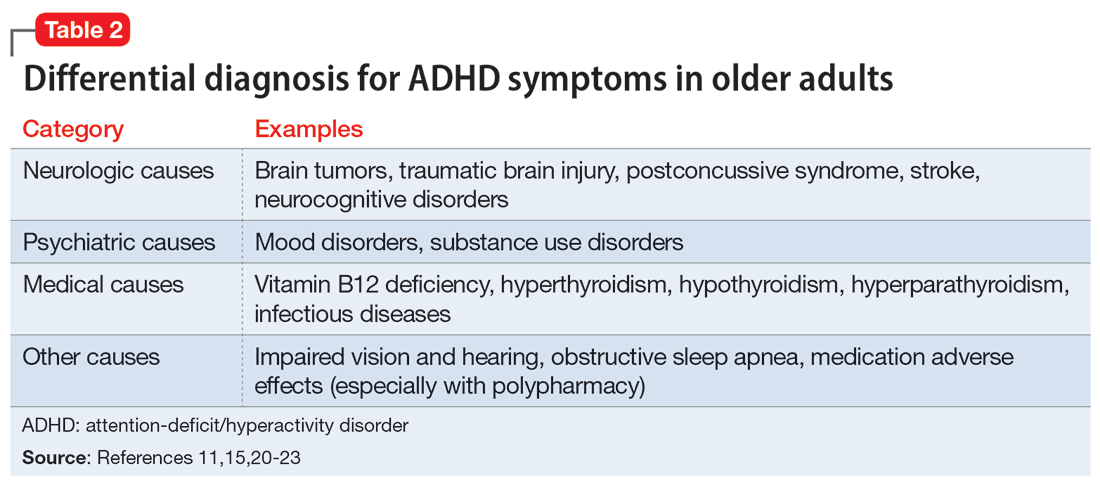
In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21
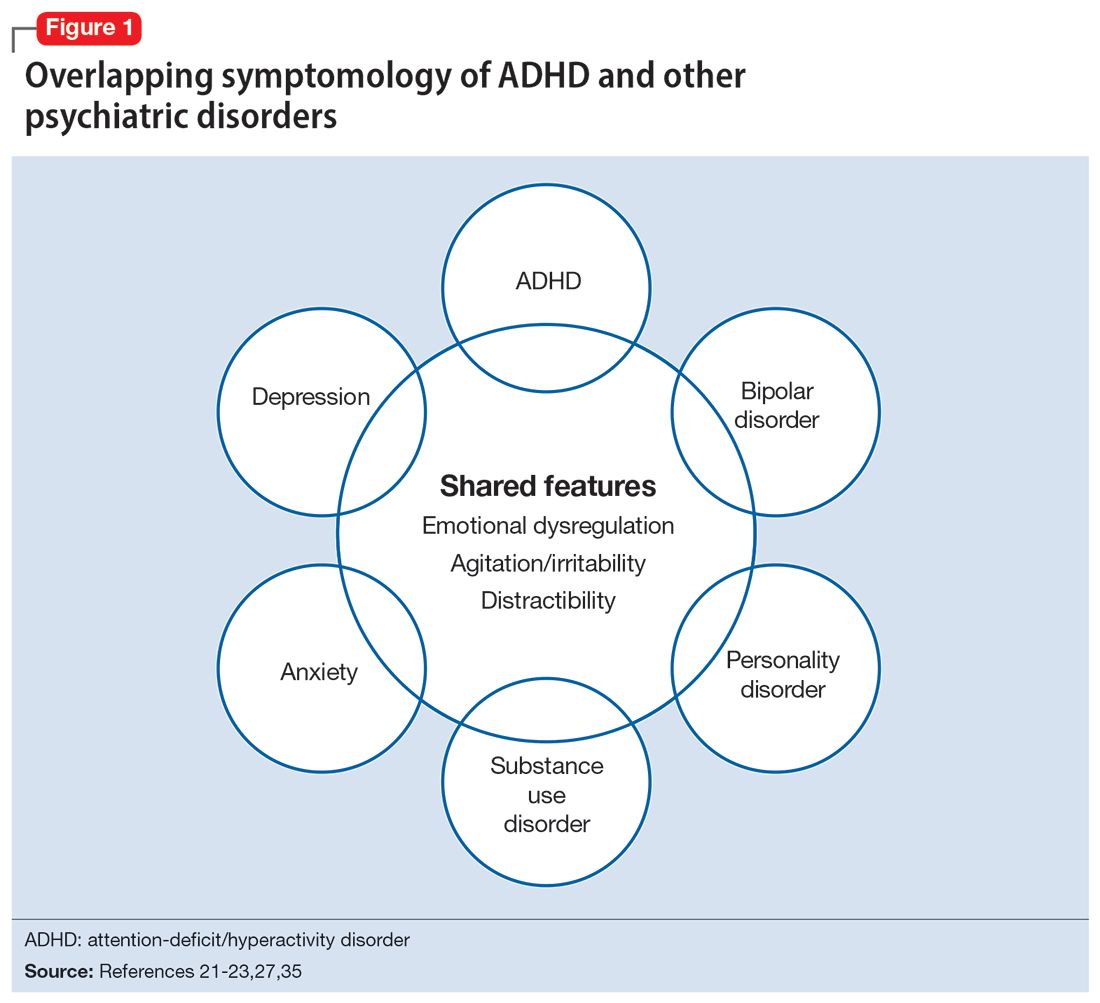
Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.
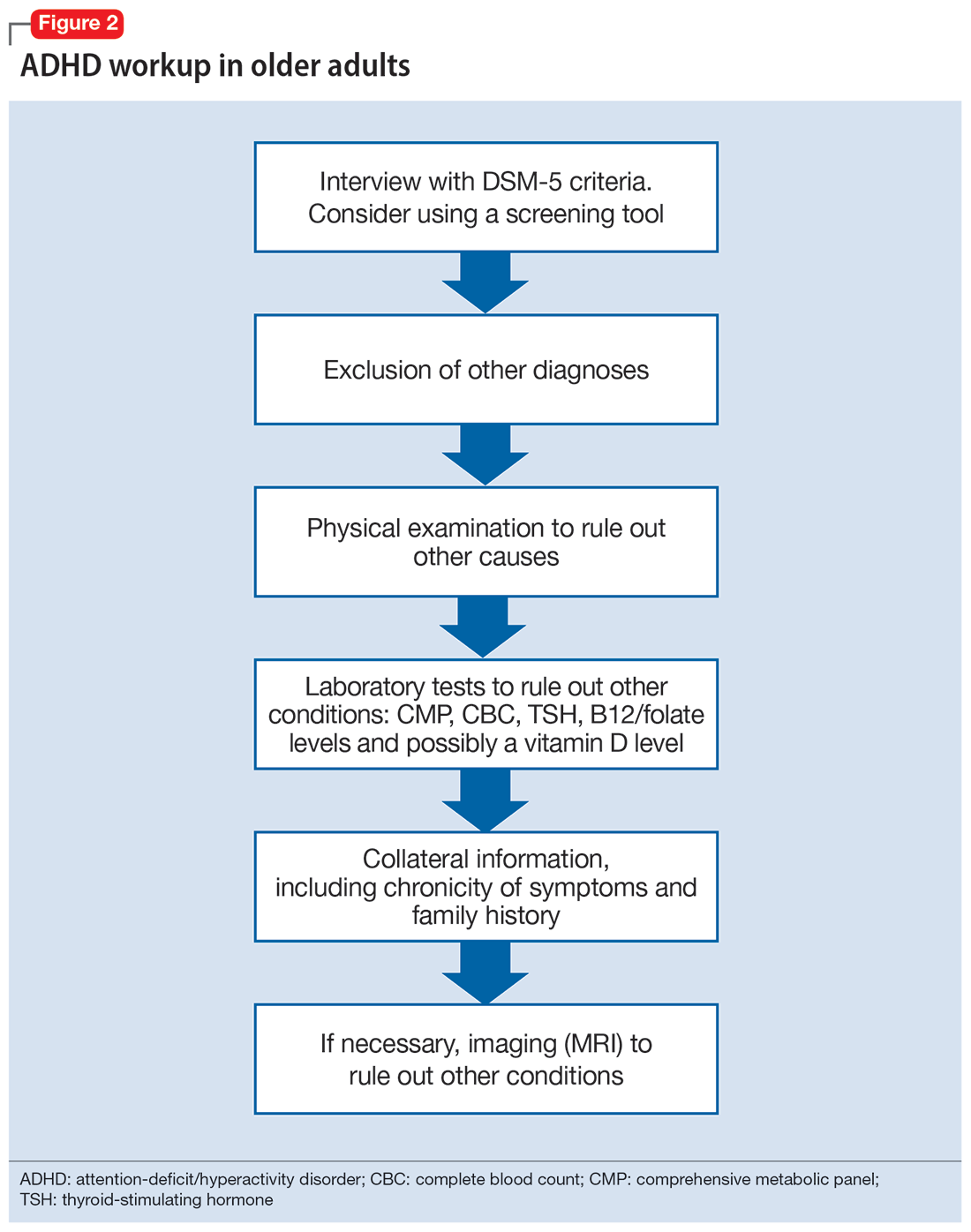
Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
For many years, attention-deficit/hyperactivity disorder (ADHD) was thought of as a disorder of childhood; however, it is now increasingly being recognized as a chronic, lifelong disorder that persists into adulthood in approximately two-thirds of patients.1 While our knowledge about ADHD in adults has increased, most research in this population focused on young or middle-aged adults; less is known about ADHD in older adults. Older adults with ADHD may be newly diagnosed at any point in their lives, or not at all.2 Because ADHD may present differently in older adults than in children or young adults, and because it may impair domains of life in different ways, a closer look at late-life ADHD is needed. This article summarizes the literature on the prevalence, impairment, diagnosis, and treatment of ADHD in adults age >60.
Challenges in determining the prevalence
Few studies have examined the age-specific prevalence of ADHD among older adults.3 Compared with childhood ADHD, adult ADHD is relatively neglected in epidemiological studies, largely due to the absence of well-established, validated diagnostic criteria.1,4 Some experts have noted that DSM-5’s ADHD criteria were designed for diagnosing children, and the children-focused symptom threshold may not be useful for adults because ADHD symptoms decline substantially with age.2 One study evaluating DSM-5 ADHD criteria in young adults (N = 4,000, age 18 to 19) found ADHD was better diagnosed when the required number of clinically relevant inattention and hyperactivity symptoms was reduced from 6 to 5 for each category.5 They also found the DSM-5 age-at-onset criterion of symptoms present before age 12 had a significant effect on ADHD prevalence, reducing the rate from 23.7% (95% CI, 22.38 to 25.02) to 5.4% (95% CI, 13.99 to 16.21).5 This suggests that strict usage of DSM-5 criteria may underestimate the prevalence of ADHD in adults, because ADHD symptoms may not be detected in childhood, or self-reporting of childhood ADHD symptoms in older adults may be unreliable due to aging processes that compromise memory and recall. These findings also indicate that fewer ADHD symptoms are needed to impair functioning in older age.
Determining the prevalence of ADHD among older adults is further complicated by individuals who report symptoms consistent with an ADHD diagnosis despite having never received this diagnosis during childhood.6-8 This may be due to the considerable number of children who meet ADHD criteria but do not get a diagnosis due to limited access to health care.9 Thus, many studies separately analyze the syndromatic (with a childhood onset) and symptomatic (regardless of childhood onset) persistence of ADHD. One epidemiological meta-analysis found the 2020 prevalence of syndromatic ADHD in adults age >60 was 0.77% and the prevalence of symptomatic ADHD was 4.51%, which translates to 7.91 million and 46.36 million affected older adults, respectively.8 Other research has reported higher rates among older adults.6,7,10 The variations among this research may be attributed to the use of different diagnostic tools/criteria, study populations, sampling methods, or DSM versions. Heterogeneity among this research also further supports the idea that the prevalence of ADHD is heavily dependent on how one defines and diagnoses the disorder.
Reasons for late-life ADHD diagnosis
There are many reasons a patient may not be diagnosed with ADHD until they are an older adult.11 In addition to socioeconomic barriers to health care access, members of different ethnic groups exhibit differences in help-seeking behaviors; children may belong to a culture that does not traditionally seek health care even when symptoms are evident.6,9 Therefore, individuals may not receive a diagnosis until adulthood. Some experts have discussed the similarity of ADHD to other neurodevelopmental disorders, such as autism spectrum disorder or social communication disorder, where ADHD symptoms may not manifest until stressors at critical points in life exceed an individual’s capacity to compensate.2
The life transition model contextualizes ADHD as being associated with demand/resource imbalances that come and go throughout life, resulting in variability in the degree of functional impairment ADHD symptoms cause in older adults.2,12 Hypothetically, events in late life—such as the death of a spouse or retirement—can remove essential support structures in the lives of high-functioning individuals with ADHD. As a result, such events surpass these individuals’ ability to cope, resulting in a late-life manifestation of ADHD.
The plausibility of late-onset ADHD
In recent years, many studies identifying ADHD in adults have been published,2,10,12-15 including some that discuss adult ADHD that spontaneously appears without childhood symptoms (ie, late-onset ADHD).2,4,12 Research of late-onset ADHD attracts attention because the data it presents challenge the current rationale that ADHD symptoms should be present before age 12, as defined by DSM-5 criteria. While most reports of late-onset ADHD pertain to younger adults, little evidence exists to reinforce the concept; to date just 1 study has reported cases of late-onset ADHD in older adults (n = 7, age 51 to 59).11 In this study, Sasaki et al11 acknowledged the strong possibility their cases may be late manifestations of long-standing ADHD. Late-onset ADHD is further challenged by findings that 95% of individuals initially diagnosed with late-onset ADHD can be excluded from the diagnosis with further detailed assessment that accounts for co-occurring mental disorders and substance use.16 This suggests false positive cases of late-onset ADHD may be a symptom of narrow clinical assessment that fails to encompass other aspects of a patient’s psychiatric profile, rather than an atypical ADHD presentation.
Comorbidity and psychosocial functioning
ADHD symptoms and diagnosis in older adults are associated with clinically relevant levels of depression and anxiety. The Dutch Longitudinal Aging Study Amsterdam (LASA) examined 1,494 older adults (age 55 to 85) using the Diagnostic Interview for ADHD in Adults version 2.0.10 The 231 individuals identified as having symptoms of ADHD reported clinically relevant levels of depressive and anxiety symptoms. ADHD was significantly associated with these comorbid symptoms.
Continue to: Little is known regarding...
Little is known regarding the manifestation of symptoms of ADHD in older age and the difficulties these older adults face. Older adults with ADHD are more often divorced and report more loneliness than older adults without this disorder, which suggests loneliness in older age may be more pressing for the older ADHD population.17 ADHD in older adults has also been associated with poor quality-of-life measures, including moderate to severe problems in mobility, self-care, usual activity, pain/discomfort, and anxiety/depression (Table 114,17).
Qualitative research has described a domino effect of a lifetime of living with ADHD. In one American study, older adults with ADHD (N = 24, age 60 to 74) reported experiencing a tangible, accumulated impact from ADHD on their finances and long-term relationships with family, friends, and coworkers.13 Another study utilizing the Dutch LASA data examined how ADHD may impact patient’s lives among participants who were unaware of their diagnosis.18 One-half of patients reported low self-esteem, overstepping boundaries, and feeling different from others. When compared to younger adults with ADHD, older adults report significantly greater impairments in productivity and a worse life outlook.19
Differential diagnosis
When assessing whether an older adult has ADHD, it is important to consider other potential causes of their symptoms (Table 211,15,20-23). The differential diagnosis includes impaired vision and hearing as well as medical illness (vitamin B12 deficiency, hyperthyroidism, hypothyroidism, hyperparathyroidism, and infectious diseases such as herpes simplex virus or syphilis).

In older adults, ADHD symptoms include frontal-executive impairments, inattentiveness, difficulty with organization or multitasking, forgetfulness, and challenges involving activities of daily living or socialization that can appear to be a mild or major neurocognitive disorder (Table 311,24,25). This includes major neurocognitive disorder due to Alzheimer’s disease, Lewy body disease, and vascular disease.2,26 However, frontotemporal lobar degeneration is reported to have more symptom overlap with ADHD.21,22,26,27 A way to differentiate between neurocognitive disorders and ADHD in older adults is to consider that patients with neurocognitive disorders often progress to visual hallucinations and more extreme personality changes than would be expected in ADHD.11 Each disease also has its own identifiable characteristics. Extreme changes in memory are often Alzheimer’s disease, personality changes suggest frontotemporal lobar degeneration, stepwise decline is classic for vascular disease, and parkinsonian features may indicate dementia with Lewy bodies.21 In addition, the onset of ADHD usually occurs in childhood and can be traced throughout the lifespan,2 whereas neurocognitive diseases usually appear for the first time in later life.2,28 There are nuances in the nature of forgetfulness that can distinguish ADHD from neurocognitive disorders. For instance, the forgetfulness in early-onset Alzheimer’s disease involves “the lack of episodic memories,” while in contrast ADHD is thought to be “forgetfulness due to inadvertence.”11 Furthermore, patients with neurocognitive disorders are reported to have more severe symptoms and an inability to explain why, whereas those with ADHD have a steady level of symptoms and can provide a more comprehensive story.24 Two recent studies have shown that weak performance on language tests is more indicative of a neurodegenerative process than of ADHD.29,30 Research has suggested that if an older adult shows a sudden, acute onset of ADHD-like symptoms, this is most likely reflective of cognitive decline or a mood disorder such as depression.2,15,24
Several other psychiatric conditions share many symptoms with ADHD. Overlapping symptomology between ADHD and mood and anxiety disorders presents challenges.27 Emotional dysregulation is a feature of adult ADHD, and this often causes a mood disorder to be diagnosed without considering other possible explanations.21,22,27,31-34 Features of mania can overlap with ADHD symptoms, including psychomotor agitation, talkativeness, and distractibility.27 Several other disorders also include distractibility, such as depression, anxiety, and substance use disorders.35 Depression and anxiety can be an outcome of untreated ADHD, or can co-occur with ADHD.21-23,27 ADHD can also co-occur with bipolar disorder (BD), substance use disorders, and personality disorders (borderline and antisocial personality disorder) (Figure 121-23,27,35). One suggested method of establishing an appropriate diagnosis is to study the efficacy of the treatment retrospectively. For example, if a patient is presumed to have depression and they do not respond to several selective serotonin reuptake inhibitors, this may be undetected ADHD.27 In addition, the argument about the chronicity of the symptoms should also be considered. ADHD symptoms are pervasive whereas BD symptoms are episodic.35 Depression can be chronic; however, there are often discrete major depressive episodes. It is important to have a clear timeline of the patient’s symptoms. Ask about age of onset, because in theory, ADHD is supposed to start in childhood.22 It is sometimes difficult to ascertain this information because many older adults grew up during a time where ADHD was not a recognized diagnosis.21

Continue to: Diagnosis and workup
Diagnosis and workup
The key aspects of diagnosing ADHD are the interview based on DSM-5 criteria, exclusion of other diagnoses, and collateral information. Research has shown that clinical interviews and longitudinal family histories provide critical information that can differentiate ADHD from other psychiatric conditions.35 DSM-5 criteria are adjusted for adults: 5 out of 9 criteria for inattention and/or hyperactivity-impulsivity must be fulfilled, as opposed to 6 out of 9 in children age <17.21,31,36 However, no criteria are specific for older adults.37 Since the differential diagnosis involves multiple entities, it is important to follow DSM-5 criteria for ADHD, which include eliminating other conditions that can explain these symptoms.15 Additionally, in DSM-5, the age-of-onset threshold for ADHD diagnosis was increased from 7 and younger to 12 and younger, addressing criticism that the previous cutoff was too restrictive.24,31 The age of onset of childhood symptoms can be challenging to verify in older adults. Older patients can have unreliable memories and their childhood records are not always available.2,20 In this population, childhood symptoms are mainly underreported but sometimes overreported.10,38 However, to establish a diagnosis, the patient should have experienced some symptoms of the disorder within their first 50 years of life, including having impaired functionality in multiple settings.15,26 The goal is to establish the chronicity of this condition to distinguish it from other psychiatric conditions.22 Overall, using DSM-5 criteria without any modifications may lead to underdiagnosis of ADHD in adults.23 At this time, however, DSM-5 remains the main criteria used to make a diagnosis.
While tools to assist in screening and diagnosing ADHD have been validated in adults, none have been validated specifically for older adults.22 Structured diagnostic interviews to diagnose ADHD include39:
- Adult ADHD Clinical Diagnostic Scale version 1.2
- ADHD Lifespan Functioning interview
- Conners’ Adult ADHD Diagnostic interview for DSM-IV
- Diagnostic Interview for ADHD in Adults version 2.0
- Structured Clinical Interview for DSM-5.
ADHD symptom measures that can be used for screening and to look at treatment response include39:
- ADHD Rating Scale 5
- Adult ADHD Self-Report Scale Symptom Checklist
- Barkley Adult ADHD Rating Scale IV
- Barkley Quick-Check for Adult ADHD Diagnosis
- Young ADHD Questionnaire
- RATE Scales.
Adult ADHD inventories consider problems that adults with ADHD face. These include39:
- Brown Attention Deficit Disorders Scales—Adult version
- Conners’ Adult ADHD Rating Scales
- Wender-Reimherr Adult Attention Deficit Disorder Scale.
Since these scales were not designed for older adults, they may miss nuances in this population.40
Continue to: It can be particularly...
It can be particularly perplexing to diagnose ADHD in older adults because the other possible causes of the symptoms are vast. During the interview, it is important to ask questions that may rule out other psychiatric, neurologic, and medical conditions.21 Screen for other diagnoses, and include questions about a patient’s sleep history to rule out obstructive sleep apnea.21 To screen for other psychiatric conditions, the Mini International Neuropsychiatric Interview 5.0.0 may be used.22 Other tools include the Saint Louis University AMSAD screen for depression, the Geriatric Depression Scale, and the Beck Anxiety Inventory.28,41 To screen for cognitive functioning, the Saint Louis University Mental Status Exam, Montreal Cognitive Assessment, or Mini-Mental State Examination can be used.22,28,42,43 Once screening is performed, a physical and neurologic examination is the best next step.26 Additionally, laboratory data and imaging can rule out other conditions; however, these are not routinely performed to diagnose ADHD.
Laboratory tests should include a comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone level, B12/folate level, and possibly a vitamin D level.11,36 These tests cover several conditions that may mimic ADHD. Brain MRI is not routinely recommended for diagnosing ADHD, though it may be useful because some research has found brain structural differences in individuals with ADHD.28,44,45 Neurocognitive disorders have notable MRI findings that distinguish them from ADHD and each other.24 If there is significant concern for neurocognitive disorders, more specific tests can be employed, such as CSF studies, to look for phosphorylated tau and beta amyloid markers.11
Ask about family history (first-degree relative with ADHD) and obtain collateral information to make sure no other diagnoses are overlooked. Family history can help diagnose this disorder in older adults because there is evidence that ADHD runs in families.2,25 This evidence would ideally come from someone who has known the patient their entire life, such as a sibling or parent.24 The collateral information will be especially helpful to discern the chronicity of the patient’s symptoms, which would point toward a diagnosis of ADHD. To summarize (Figure 2):
- obtain a thorough interview that may be supported by a screening tool
- rule out other conditions
- conduct a physical examination
- obtain laboratory results
- collect collateral information
- obtain neuroimaging if necessary.

Treatment
ADHD symptoms can be treated with medications and psychotherapy. Research has shown the efficacy of ADHD medications in older adults, demonstrating that treatment leads to better functioning in multiple settings and decreases the risk for developing comorbid psychiatric conditions (mood disorder, substance use disorders).25,27 Symptoms that improve with medication include attention, concentration, self-efficacy, functioning, self-esteem, psychomotor agitation, mood, energy, and procrastination.21,31,46 If a patient with ADHD also has other psychiatric diagnoses, treat the most impairing disorder first.22 This often means mood disorders and substance use disorders must be remedied before ADHD is treated.21
Medication options include stimulants and nonstimulants. First-line treatments are stimulant medications, including methylphenidate, amphetamines, and mixed amphetamine salts.12,22,27,31,35 Stimulants have shown significant efficacy in older adults, although the American Geriatrics Society’s Beers Criteria list stimulants as potentially inappropriate for older adults.33 Adults show significant improvement with methylphenidate.21,23,47 In an observational study, Michielsen et al46 found stimulants were safe and efficacious in older adults if patients are carefully monitored for adverse effects, especially cardiovascular changes. Second-line treatments include the nonstimulant atomoxetine.12,22,27,31 Clonidine and guanfacine are FDA-approved for treating ADHD in children, but not approved for adults.26 There is little evidence for other treatments, such as bupropion.12,22,27 All of these medications have adverse effects, which are especially important to consider in older adults, who experience age-related physiological changes.
Continue to: Medications for ADHD symptoms...
Medications for ADHD symptoms are thought to act via catecholaminergic mechanisms.21 As a result, adverse effects of stimulants can include headache, appetite suppression, nausea, difficulty sleeping, tremor, blurred vision, agitation, psychosis, increased heart rate, arrhythmia, and hypertension.22,27,32-34 Especially in older adults, adverse effects such as reduced appetite, disrupted sleep, or increased blood pressure or heart rate may be harmful.21,23 Using caffeine or pseudoephedrine can exacerbate these adverse effects.21 Atomoxetine’s adverse effects include appetite suppression, insomnia, dizziness, anxiety, agitation, fatigue, dry mouth, constipation, nausea, vomiting, dyspepsia, and increased heart rate or blood pressure.27,32,35 Genitourinary adverse effects have also been reported, including priapism (rare), decreased libido, and urinary hesitancy and retention.26,32 Before any medication is initiated, it is important to conduct a physical and neurologic examination and a detailed clinical interview.
Before starting medication, as with any medical treatment, conduct a risk vs benefit analysis. Record baseline values for the patient’s heart rate, blood pressure, and weight.23,26,27,31 During the interview, screen for family and personal cardiovascular conditions,27,33 and obtain an electrocardiogram for any patient with cardiovascular risks.23,26,27,31 Once the patient is deemed to be an appropriate candidate for pharmacologic treatment, begin with low doses and titrate the medication slowly until reaching a therapeutic level.23,48
Medications should be combined with psychotherapy (eg, cognitive-behavioral therapy or dialectical behavioral therapy) and other lifestyle changes (exercise, mindfulness, support groups).18,22,23,27,31,49 Psychotherapy can help patients come to terms with receiving an ADHD diagnosis later in life and help with organization and socialization.12,50 Pharmacologic treatments are thought to be helpful with attention challenges and emotional instability.50 Taken together, medications and behavioral interventions can help individuals experience an improved quality of life.
Future directions
Given the relatively recent interest in ADHD in older adults, there are several areas that need further research. For future editions of DSM, it may be prudent to consider establishing ADHD criteria specific to older adults. Research has also shown the need for clear diagnostic and validated tools for older adults.8 Few analyses have been undertaken regarding pharmacotherapy for this population. Randomized controlled clinical trials are needed.23,37,48 More research about the relative utility of psychotherapy and behavioral interventions would also be useful, given their potential to improve the quality of life for older adults with ADHD.
Bottom Line
Although generally thought of as a disorder of childhood, attention-deficit/ hyperactivity disorder (ADHD) has substantial effects in older adults. When the condition is appropriately diagnosed, pharmacologic treatment and psychotherapy are associated with improved quality of life for older patients with ADHD.
Related Resources
- Children and Adults with Attention-Deficit/Hyperactivity Disorder. Living with ADHD: A lifespan disorder. https://chadd.org/for-adults/living-with-adhd-a-lifespan-disorder/
- Attention Deficit Disorder Association. Support groups for adults. https://add.org/adhd-support-groups/
Drug Brand Names
Amphetamine/dextroamphetamine • Adderall
Atomoxetine • Straterra
Bupropion • Wellbutrin
Clonidine • Catapres
Guanfacine • Intuniv
Methylphenidate • Ritalin
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. doi:10.1016/S2215-0366(16)30190-0
2. Sharma MJ, Lavoie S, Callahan BL. A call for research on the validity of the age-of-onset criterion application in older adults being evaluated for ADHD: a review of the literature in clinical and cognitive psychology. Am J Geriatr Psychiatry. 2021;29(7):669-678. doi:10.1016/j.jagp.2020.10.016
3. Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299-304. doi:10.1016/j.psychres.2009.12.010
4. McGough JJ, Barkley RA. Diagnostic controversies in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161(11):1948-1956. doi:10.1176/appi.ajp.161.11.1948
5. Matte B, Anselmi L, Salum GA, et al. ADHD in DSM-5: a field trial in a large, representative sample of 18- to 19-year-old adults. Psychol Med. 2015;45(2):361-373. doi:10.1017/S0033291714001470
6. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
7. Guldberg-Kjär T, Johansson B. Old people reporting childhood AD/HD symptoms: retrospectively self-rated AD/HD symptoms in a population-based Swedish sample aged 65-80. Nord J Psychiatry. 2009;63(5):375-382. doi:10.1080/08039480902818238
8. Song P, Zha M, Yang Q, et al. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. 2021;11:04009. doi:10.7189/jogh.11.04009
9. Russell AE, Ford T, Williams R, et al. The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry Hum Dev. 2016;47(3):440-458. doi:10.1007/s10578/-015-0578-3
10. Michielsen M, Semeijn E, Comijs HC, et al. Prevalence of attention-deficit hyperactivity disorder in older adults in The Netherlands. Br J Psychiatry. 2012;201(4):298-305. doi:10.1192/bjp.bp.111.101196
11. Sasaki H, Jono T, Fukuhara R, et al. Late-manifestation of attention-deficit/hyperactivity disorder in older adults: an observational study. BMC Psychiatry. 2022;22(1):354. doi:10.1186/s12888-022-03978-0
12. Turgay A, Goodman DW, Asherson P, et al. Lifespan persistence of ADHD: the life transition model and its application. J Clin Psychiatry. 2012;73(2):192-201. doi:10.4088/JCP.10m06628
13. Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795-799. doi:10.1007/s1136-011-9981-9
14. Thorell LB, Holst Y, Sjöwall D. Quality of life in older adults with ADHD: links to ADHD symptom levels and executive functioning deficits. Nord J Psychiatry. 2019;73(7):409-416. doi:10.1080/08039488.2019.1646804
15. Sibley MH. Diagnosing ADHD in older adults: critical next steps for research. Am J Geriatr Psychiatry. 2021;29(7):679-681. doi:10.1016/j.jagp.2020.11.012
16. Sibley MH, Rohde LA, Swanson JM, et al. Late-onset ADHD reconsidered with comprehensive repeated assessments between ages 10 and 25. Am J Psychiatry. 2018;175(2):140-149. doi:10.1176/appi.ajp.2017.17030298
17. Michielsen M, Comijs HC, Aartsen MJ, et al. The relationships between ADHD and social functioning and participation in older adults in a population-based study. J Atten Disord. 2015;19(5):368-379. doi:10.1177/1087054713515748
18. Michielsen M, de Kruif JTCM, Comijs HC, et al. The burden of ADHD in older adults: a qualitative study. J Atten Disord. 2018;22(6):591-600. doi:10.1177/1087054715610001
19. Lensing MB, Zeiner P, Sandvik L, et al. Quality of life in adults aged 50+ with ADHD. J Atten Disord. 2015;19(5):405-413. doi:10.1177/1087054713480035
20. Fischer BL, Gunter-Hunt G, Steinhafel CH, et al. The identification and assessment of late-life ADHD in memory clinics. J Atten Disord. 2012;16(4):333-338. doi:10.1177/1087054711398886
21. Goodman DW, Mitchell S, Rhodewalt L, et al. Clinical presentation, diagnosis and treatment of attention-deficit hyperactivity disorder (ADHD) in older adults: a review of the evidence and its implications for clinical care. Drugs Aging. 2016;33(1):27-36. doi:10.1007/s40266-015-0327-0
22. Kooij JJ, Michielsen M, Kruithof H, et al. ADHD in old age: a review of the literature and proposal for assessment and treatment. Expert Rev Neurother. 2016;16(12):1371-1381. doi:10.1080/14737175.2016.1204914
23. Torgersen T, Gjervan B, Lensing MB, et al. Optimal management of ADHD in older adults. Neuropsychiatr Dis Treat. 2016;12:79-87. doi:10.2147/NDT.S59271
24. Callahan BL, Bierstone D, Stuss DT, et al. Adult ADHD: risk factor for dementia or phenotypic mimic? Front Aging Neurosci. 2017;9:260. doi:10.3389/fnagi.2017.00260
25. Mendonca F, Sudo FK, Santiago-Bravo G, et al. Mild cognitive impairment or attention-deficit/hyperactivity disorder in older adults? A cross sectional study. Front Psychiatry. 2021;12:737357. doi:10.3389/fpsyt.2021.737357
26. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017;20(1):4-11. doi:10.1136/eb-2016-102415
27. Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. doi:10.1186/s12888-017-1463-3
28. Klein M, Silva MA, Belizario GO, et al. Longitudinal neuropsychological assessment in two elderly adults with attention-deficit/hyperactivity disorder: case report. Front Psychol. 2019;10:1119. doi:10.3389/fpsyg.2019.01119
29. Prentice JL, Schaeffer MJ, Wall AK, et al. A systematic review and comparison of neurocognitive features of late-life attention-deficit/hyperactivity disorder and dementia with Lewy bodies. J Geriatr Psychiatry Neurol. 2021;34(5):466-481. doi:10.1177/0891988720944251
30. Callahan BL, Ramakrishnan N, Shammi P, et al. Cognitive and neuroimaging profiles of older adults with attention deficit/hyperactivity disorder presenting to a memory clinic. J Atten Disord. 2022;26(8):1118-1129. doi:10.1177/10870547211060546
31. Ramos-Quiroga, JA, Nasillo V, Fernández-Aranda, et al. Addressing the lack of studies in attention-deficit/hyperactivity disorder in adults. Expert Rev Neurother. 2014;14(5):553-567. doi:10.1586/14737175.2014.908708
32. Stahl SM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. 6th ed. Cambridge University Press; 2017.
33. Latronica JR, Clegg TJ, Tuan WJ, et al. Are amphetamines associated with adverse cardiovascular events among elderly individuals? J Am Board Fam Med. 2021;34(6):1074-1081. doi:10.3122/jabfm.2021.06.210228
34. Garcia-Argibay M, du Rietz E, Lu Y, et al. The role of ADHD genetic risk in mid-to-late life somatic health conditions. Transl Psychiatry. 2022;12(1):152. doi:10.1038/s41398-022-01919-9
35. Jain R, Jain S, Montano CB, Addressing diagnosis and treatment gaps in adults with attention-deficit/hyperactivity disorder. Prim Care Companion CNS Disord. 2017;19(5):17nr02153. doi:10.4088/PCC.17nr02153
36. Sasaki H, Jono T, Fukuhara R, et al. Late-onset attention-deficit/hyperactivity disorder as a differential diagnosis of dementia: a case report. BMC Psychiatry. 2020;20(1):550. doi:10.1186/s12888-020-02949-7
37. Surman CBH, Goodman DW. Is ADHD a valid diagnosis in older adults? Atten Defic Hyperact Disord. 2017;9(3):161-168. doi:10.1007/s12402-017-0217-x
38. Semeijn EJ, Michielsen M, Comijs HC, et al. Criterion validity of an attention deficit hyperactivity disorder (ADHD) screening list for screening ADHD in older adults aged 60-94 years. Am J Geriatr Psychiatry. 2013;21(7):631-635. doi:10.1016/j.jagp.2012.08.003
39. Ramsay JR. Assessment and monitoring of treatment response in adult ADHD patients: current perspectives. Neuropsychiatr Dis Treat. 2017;13:221-232. doi:10.2147/NDT.S104706
40. Das D, Cherbuin N, Easteal S, et al. Attention deficit/hyperactivity disorder symptoms and cognitive abilities in the late-life cohort of the PATH through life study. PLoS One. 2014;9(1):e86552. doi:10.1371/journal.pone.0086552
41. Kaya D, Isik AT, Usarel C, et al. The Saint Louis University Mental Status Examination is better than the Mini-Mental State Examination to determine the cognitive impairment in Turkish elderly people. J Am Med Dir Assoc. 2016;17(4):370.e11-370.e3.7E15. doi:10.1016/j.jamda.2015.12.093
42. Michielsen M, Comijs HC, Semeijn EJ, et al. Attention deficit hyperactivity disorder and personality characteristics in older adults in the general Dutch population. Am J Geriatr Psychiatry. 2014;22(12):1623-1632. doi:10.1016/j.jagp.2014.02.005
43. Khoury R, Chakkamparambil B, Chibnall J, et al. Diagnostic accuracy of the SLU AMSAD scale for depression in older adults without dementia. J Am Med Dir Assoc. 2020;21(5):665-668. doi:10.1016/j.jamda.2019.09.011
44. Çavuşoğlu Ç, Demirkol ME, Tamam L. Attention deficit hyperactivity disorder in the elderly. Current Approaches in Psychiatry. 2020;12(2):182-194. doi:10.18863/pgy.548052
45. Klein M, Souza-Duran FL, Menezes AKPM, et al. Gray matter volume in elderly adults with ADHD: associations of symptoms and comorbidities with brain structures. J Atten Disord. 2021;25(6):829-838. doi:10.1177/1087054719855683
46. Michielsen M, Kleef D, Bijlenga D, et al. Response and side effects using stimulant medication in older adults with ADHD: an observational archive study. J Atten Disord. 2021;25(12):1712-1719. doi:10.1177/1087054720925884
47. Manor I, Rozen S, Zemishlani Z, et al. When does it end? Attention-deficit/hyperactivity disorder in the middle aged and older populations. Clin Neuropharmacol, 2011;34(4):148-154. doi:10.1097/WNF.0b013e3182206dc1
48. Deshmukh P, Patel D. Attention deficit hyperactivity disorder and its treatment in geriatrics. Curr Dev Disord Rep. 2020;7(3):79-84.
49. Barkley RA. The important role of executive functioning and self-regulation in ADHD. 2010. Accessed August 10, 2023. https://www.russellbarkley.org/factsheets/ADHD_EF_and_SR.pdf
50. Corbisiero S, Bitto H, Newark P, et al. A comparison of cognitive-behavioral therapy and pharmacotherapy vs. pharmacotherapy alone in adults with attention-deficit/hyperactivity disorder (ADHD)-a randomized controlled trial. Front Psychiatry. 2018;9:571. doi:10.3389/fpsyt.2018.00571
Weight loss linked to mortality risk in older women
Weight loss of at least 5% over a 3-year period was associated with significantly increased mortality in women at age 90, 95, and 100 years compared with those whose weight remained stable, based on data from more than 50,000 individuals.
Previous studies of later-life weight changes and mortality have yielded inconsistent results driven by considerations of weight loss intentionality, and data on older adults in particular are limited, wrote Aladdin H. Shadyab, PhD, of the University of California, San Diego, and colleagues.
In a study published in the Journals of Gerontology: Medical Sciences, the researchers reviewed data from the Women’s Health Initiative, a prospective study of factors affecting chronic disease development in postmenopausal women. The study population included 54,437 women who entered the WHI between 1993 and 1998 at ages 50-79 years. The mean baseline age was 69.8 years; 89.5% of the participants were White, 5.7% were Black, 2.7% were Asian, 2.5% were Hispanic/Latino, and the remaining 1.0% were multiracial, American Indian/Alaskan Native, Native Hawaiian/Other Pacific Islander, or unknown.
The primary outcomes were the associations of short-term (3-year) and long-term (10-year) weight changes with survival to ages 90, 95, and 100 years.
A total of 30,647 women survived to at least 90 years (56.3%).
Overall, women with a short-term weight loss of 5% or more of body weight were 33% less likely to survive to age 90 years, 35% less likely to survive to age 95 years, and 38% less likely to survive to age 100 years than were those whose weight remained stable (odds ratios, 0.67, 0.65, and 0.62, respectively).
The associations were stronger in cases of unintentional short-term weight loss. Intentional weight loss from baseline to year 3 was associated with 17% lower odds of survival to age 90 compared to stable weight (OR, 0.83), but unintentional weight loss was associated with 51% lower odds of survival to age 90 (OR, 0.49).
Similarly, women with 10-year weight loss of at least 5% were 40% less likely to survive to 90 years and 49% less likely to survive to 95 years (OR, 0.60 and OR, 0.51, respectively). The sample size was too small to assess the relation of 10-year weight loss with survival to 100 years, and intentionality was not assessed for 10-year weight changes.
By contrast, weight gain of at least 5% had no significant effect on survival to ages 90, 95, or 100 years, but stable weight over time increased the odds of living to ages 90 to 100 years by 1.2-fold to 2-fold compared to either intentional or unintentional weight loss of at least 5%.
The trends in results were similar across body weight categories (normal weight, overweight, and obese as defined by body mass index). Baseline age and smoking status had no significant effect on the results.
Some of the proportion of self-reported intentional weight loss in the study population may have been unintentional, the researchers wrote in their discussion.
“It is important to note that perceived intentionality of weight loss may be influenced by the many societal pressures to lose weight, especially among women, and therefore overestimate the behavioral changes underlying experienced weight loss in older adults,” they said.
The findings were limited by several factors including the potential for inaccurate self-reported weight loss intention, and the likelihood that the mean older age of the population at baseline (older than 60 years) meant that they were more likely to live longer regardless of weight changes, the researchers noted. Other limitations included the primarily White study population, and other residual confounding factors such as ill health that might drive weight loss, the researchers noted.
However, the results were strengthened by the large sample size and long follow-up period, and suggest that “blanket recommendations for weight loss in older women are unlikely to lead to better survival at advanced ages,” they concluded.
Data support weight monitoring
The investigators acknowledged that their data do not affect clinical recommendations for moderate weight loss in older women to improve health outcomes, especially in those with overweight or obesity, but instead “support close monitoring of the amount and speed of weight loss, particularly when unintentional, as an indicator of underlying poor health and predictor of decreased lifespan in older women.”
Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, agreed with this conclusion. The current study suggests that when older women lose a significant amount of weight unintentionally, it could be a sign of failing health, he said.
Weight gain or loss in old age is very different from weight issues in younger people, where clinicians may be encouraging weight loss to improve health outcomes, Dr. Skolnik said in an interview.
A key take-home message for clinicians, in addition to monitoring weight in older patients, is to emphasize nutrition for individuals in their 80s, 90s, and beyond, he said.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shadyab had no financial conflicts to disclose. Dr. Skolnik had no financial conflicts to disclose and serves on the editorial advisory board of Family Practice News.
Weight loss of at least 5% over a 3-year period was associated with significantly increased mortality in women at age 90, 95, and 100 years compared with those whose weight remained stable, based on data from more than 50,000 individuals.
Previous studies of later-life weight changes and mortality have yielded inconsistent results driven by considerations of weight loss intentionality, and data on older adults in particular are limited, wrote Aladdin H. Shadyab, PhD, of the University of California, San Diego, and colleagues.
In a study published in the Journals of Gerontology: Medical Sciences, the researchers reviewed data from the Women’s Health Initiative, a prospective study of factors affecting chronic disease development in postmenopausal women. The study population included 54,437 women who entered the WHI between 1993 and 1998 at ages 50-79 years. The mean baseline age was 69.8 years; 89.5% of the participants were White, 5.7% were Black, 2.7% were Asian, 2.5% were Hispanic/Latino, and the remaining 1.0% were multiracial, American Indian/Alaskan Native, Native Hawaiian/Other Pacific Islander, or unknown.
The primary outcomes were the associations of short-term (3-year) and long-term (10-year) weight changes with survival to ages 90, 95, and 100 years.
A total of 30,647 women survived to at least 90 years (56.3%).
Overall, women with a short-term weight loss of 5% or more of body weight were 33% less likely to survive to age 90 years, 35% less likely to survive to age 95 years, and 38% less likely to survive to age 100 years than were those whose weight remained stable (odds ratios, 0.67, 0.65, and 0.62, respectively).
The associations were stronger in cases of unintentional short-term weight loss. Intentional weight loss from baseline to year 3 was associated with 17% lower odds of survival to age 90 compared to stable weight (OR, 0.83), but unintentional weight loss was associated with 51% lower odds of survival to age 90 (OR, 0.49).
Similarly, women with 10-year weight loss of at least 5% were 40% less likely to survive to 90 years and 49% less likely to survive to 95 years (OR, 0.60 and OR, 0.51, respectively). The sample size was too small to assess the relation of 10-year weight loss with survival to 100 years, and intentionality was not assessed for 10-year weight changes.
By contrast, weight gain of at least 5% had no significant effect on survival to ages 90, 95, or 100 years, but stable weight over time increased the odds of living to ages 90 to 100 years by 1.2-fold to 2-fold compared to either intentional or unintentional weight loss of at least 5%.
The trends in results were similar across body weight categories (normal weight, overweight, and obese as defined by body mass index). Baseline age and smoking status had no significant effect on the results.
Some of the proportion of self-reported intentional weight loss in the study population may have been unintentional, the researchers wrote in their discussion.
“It is important to note that perceived intentionality of weight loss may be influenced by the many societal pressures to lose weight, especially among women, and therefore overestimate the behavioral changes underlying experienced weight loss in older adults,” they said.
The findings were limited by several factors including the potential for inaccurate self-reported weight loss intention, and the likelihood that the mean older age of the population at baseline (older than 60 years) meant that they were more likely to live longer regardless of weight changes, the researchers noted. Other limitations included the primarily White study population, and other residual confounding factors such as ill health that might drive weight loss, the researchers noted.
However, the results were strengthened by the large sample size and long follow-up period, and suggest that “blanket recommendations for weight loss in older women are unlikely to lead to better survival at advanced ages,” they concluded.
Data support weight monitoring
The investigators acknowledged that their data do not affect clinical recommendations for moderate weight loss in older women to improve health outcomes, especially in those with overweight or obesity, but instead “support close monitoring of the amount and speed of weight loss, particularly when unintentional, as an indicator of underlying poor health and predictor of decreased lifespan in older women.”
Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, agreed with this conclusion. The current study suggests that when older women lose a significant amount of weight unintentionally, it could be a sign of failing health, he said.
Weight gain or loss in old age is very different from weight issues in younger people, where clinicians may be encouraging weight loss to improve health outcomes, Dr. Skolnik said in an interview.
A key take-home message for clinicians, in addition to monitoring weight in older patients, is to emphasize nutrition for individuals in their 80s, 90s, and beyond, he said.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shadyab had no financial conflicts to disclose. Dr. Skolnik had no financial conflicts to disclose and serves on the editorial advisory board of Family Practice News.
Weight loss of at least 5% over a 3-year period was associated with significantly increased mortality in women at age 90, 95, and 100 years compared with those whose weight remained stable, based on data from more than 50,000 individuals.
Previous studies of later-life weight changes and mortality have yielded inconsistent results driven by considerations of weight loss intentionality, and data on older adults in particular are limited, wrote Aladdin H. Shadyab, PhD, of the University of California, San Diego, and colleagues.
In a study published in the Journals of Gerontology: Medical Sciences, the researchers reviewed data from the Women’s Health Initiative, a prospective study of factors affecting chronic disease development in postmenopausal women. The study population included 54,437 women who entered the WHI between 1993 and 1998 at ages 50-79 years. The mean baseline age was 69.8 years; 89.5% of the participants were White, 5.7% were Black, 2.7% were Asian, 2.5% were Hispanic/Latino, and the remaining 1.0% were multiracial, American Indian/Alaskan Native, Native Hawaiian/Other Pacific Islander, or unknown.
The primary outcomes were the associations of short-term (3-year) and long-term (10-year) weight changes with survival to ages 90, 95, and 100 years.
A total of 30,647 women survived to at least 90 years (56.3%).
Overall, women with a short-term weight loss of 5% or more of body weight were 33% less likely to survive to age 90 years, 35% less likely to survive to age 95 years, and 38% less likely to survive to age 100 years than were those whose weight remained stable (odds ratios, 0.67, 0.65, and 0.62, respectively).
The associations were stronger in cases of unintentional short-term weight loss. Intentional weight loss from baseline to year 3 was associated with 17% lower odds of survival to age 90 compared to stable weight (OR, 0.83), but unintentional weight loss was associated with 51% lower odds of survival to age 90 (OR, 0.49).
Similarly, women with 10-year weight loss of at least 5% were 40% less likely to survive to 90 years and 49% less likely to survive to 95 years (OR, 0.60 and OR, 0.51, respectively). The sample size was too small to assess the relation of 10-year weight loss with survival to 100 years, and intentionality was not assessed for 10-year weight changes.
By contrast, weight gain of at least 5% had no significant effect on survival to ages 90, 95, or 100 years, but stable weight over time increased the odds of living to ages 90 to 100 years by 1.2-fold to 2-fold compared to either intentional or unintentional weight loss of at least 5%.
The trends in results were similar across body weight categories (normal weight, overweight, and obese as defined by body mass index). Baseline age and smoking status had no significant effect on the results.
Some of the proportion of self-reported intentional weight loss in the study population may have been unintentional, the researchers wrote in their discussion.
“It is important to note that perceived intentionality of weight loss may be influenced by the many societal pressures to lose weight, especially among women, and therefore overestimate the behavioral changes underlying experienced weight loss in older adults,” they said.
The findings were limited by several factors including the potential for inaccurate self-reported weight loss intention, and the likelihood that the mean older age of the population at baseline (older than 60 years) meant that they were more likely to live longer regardless of weight changes, the researchers noted. Other limitations included the primarily White study population, and other residual confounding factors such as ill health that might drive weight loss, the researchers noted.
However, the results were strengthened by the large sample size and long follow-up period, and suggest that “blanket recommendations for weight loss in older women are unlikely to lead to better survival at advanced ages,” they concluded.
Data support weight monitoring
The investigators acknowledged that their data do not affect clinical recommendations for moderate weight loss in older women to improve health outcomes, especially in those with overweight or obesity, but instead “support close monitoring of the amount and speed of weight loss, particularly when unintentional, as an indicator of underlying poor health and predictor of decreased lifespan in older women.”
Neil Skolnik, MD, professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, agreed with this conclusion. The current study suggests that when older women lose a significant amount of weight unintentionally, it could be a sign of failing health, he said.
Weight gain or loss in old age is very different from weight issues in younger people, where clinicians may be encouraging weight loss to improve health outcomes, Dr. Skolnik said in an interview.
A key take-home message for clinicians, in addition to monitoring weight in older patients, is to emphasize nutrition for individuals in their 80s, 90s, and beyond, he said.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Shadyab had no financial conflicts to disclose. Dr. Skolnik had no financial conflicts to disclose and serves on the editorial advisory board of Family Practice News.
FROM THE JOURNALS OF GERONTOLOGY: MEDICAL SCIENCES
Could retinal changes be a harbinger of Parkinson’s?
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Changes in retinal tissues known to be associated with Parkinson’s disease (PD) may occur up to 7 years before clinical symptoms of the disease appear, a new study suggests.
Researchers used artificial intelligence (AI) to analyze data from two population-level data sets and the world’s largest database of retinal images and associated clinical data to detect the retinal changes in patients with PD and in healthy individuals who developed the disease years later.
Prior research had shown that PD is associated with a thinning of the ganglion cell-inner plexiform layer (GCIPL) in the retina, something that investigators confirmed in this new study. But they also identified changes in the inner nuclear layer (INL), which is a new finding.
The study is the largest to date on retinal markers in PD and the first to show these changes in living patients.
“I think we are still several years away from converting these findings into individual level prediction for patients,” lead author, Siegfried Wagner, MD, MsC, Honorary Clinical Senior Research Fellow at Moorfields Eye Hospital and University College of London Institute of Ophthalmology in London, told this news organization. “The most important takeaway is that there are observable differences in the retina of individuals who go on to develop Parkinson’s disease.”
The findings were published online in Neurology.
Another look at OCT
Researchers used data from retinal eye scans taken by optical coherence tomography (OCT), a noninvasive three-dimensional imaging technology that is widely used by opticians.
Other studies have used OCT to detect retinal changes in multiple sclerosis and cognitive decline.
For this research, investigators identified markers in people with PD using ophthalmic imaging data from 700 patients and 105,770 controls who participated in the retrospective AlzEye study.
After adjustment for age, sex, ethnicity, hypertension, and diabetes, individuals with PD had significantly thinner GCIPL and reduced thickness of the INL.
To evaluate retinal changes in patients before a PD diagnosis, researchers then turned to 50,405 participants in the UK Biobank with no history of PD who received a retinal scan as part of their baseline visit. Of that group, 53 were diagnosed with PD during the study period.
Researchers found an association between new diagnoses of PD and reduced thickness of the GCIPL (hazard ratio [HR], 0.62; P = .002) and thinner INL, especially at the inferior subfield (HR, 0.66; P = .002). That association persisted even in people whose clinical symptoms developed within 2 years of the retinal scan.
“We wonder if the reduced INL thickness is indicating a direct dopaminergic impairment occurring within the inner retina,” Dr. Wagner said. “Dopaminergic amacrine cells only account for a small proportion of the cells in this layer but previous work in the laboratory shows observable abnormalities in Parkinson’s disease.”
Too early for diagnostics?
Commenting on the findings, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association, noted that the changes in the retinal thickness identified in the study were too small to be useful in the clinic as a screening tool for early PD.
“In order for that to happen, the specificity and sensitivity needs to be established,” she said. “Both specificity and sensitivity need to be high enough so that the test can be used to give clinically meaningful results – and reliably tell an individual with PD that he or she does have the disease and individual without PD that he or she doesn’t have the disease.”
Authors of an accompanying editorial agreed. Valeria Koska, MD, and Philipp Albrecht, MD, both of Heinrich Heine University Düsseldorf in Germany, noted that though the effect sizes of retinal changes were small, the study “sets new standards for the role of retinal morphology as potential biomarker in neurodegenerative disease.”
The study was funded by Fight for Sight UK, Medical Research Council, UK Research & Innovation, Basque Health Department, and the Wellcome Trust Study. Dr. Wagner reported funding from the Medical Research Council and the Rank Prize. Dr. Gilbert is employed by the American Parkinson Disease Association. Dr. Albrecht has received grant and personal fees and nonfinancial support from Allergan, Biogen, Celgene, Ipsen, Janssen Cilag, Merck, Merz Pharmaceuticals, Novartis, Roche, and Teva, outside the submitted work. Dr. Koska reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Dementia diagnosis a good time to reduce polypharmacy
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
Physicians may be missing opportunities to reduce harmful polypharmacy in elderly patients with newly diagnosed dementia, investigators for a large study of Medicare beneficiaries reported.
They found that those with an incident dementia diagnosis were somewhat more likely to initiate central nervous system–active medications and slightly more likely to discontinue cardiometabolic and anticholinergic medications, compared with controls.
According to the authors, time of diagnosis can be a potential inflexion point for deprescribing long-term medications with high safety risks, limited likelihood of benefit, or possible association with impaired cognition.
“Understanding the chronology of medication changes following a first dementia diagnosis may identify targets for deprescribing interventions to reduce preventable medication-related harms, said Timothy S. Anderson, MD, MAS, of the division of general medicine at Beth Israel Deaconess Medical Center, Boston, and colleagues in JAMA Internal Medicine.
“Our results provide a baseline to inform efforts to rethink the clinical approach to medication use at the time of a new dementia diagnosis.”
Hundreds of thousands of Americans are diagnosed annually with Alzheimer’s and related dementias, the authors pointed out, and the majority have multiple other chronic conditions. Worsening cognitive impairment may alter the risk-benefit balance of medications taken for these conditions.
Matched cohort study
The sample consisted of adults 67 years or older enrolled in traditional Medicare and Medicare Part D. Patients with an initial incident dementia diagnosis between January 2012 and December 2018 were matched with controls (as of last doctor’s office visit) based on demographics, geographic location, and baseline medication count. Data were analyzed from 2021 to June 2023.
The study included 266,675 adults with incident dementia and 266,675 controls. In both groups, 65.1% were 80 years or older (mean age, 82.2) and 67.8% were female. At baseline, patients with incident dementia were more likely than controls to use CNS-active medications (54.32% vs. 48.39%) and anticholinergic medications (17.79% vs. 15.96%) and less likely to use most cardiometabolic medications (for example, antidiabetics, 31.19% vs. 36.45%).
Immediately following the index diagnosis, the dementia cohort had greater increases in the mean number of medications used: 0.41 vs. –0.06 (95% confidence interval, 0.27-0.66) and in the proportion using CNS-active medications (absolute change, 3.44% vs. 0.79%; 95% CI, 0.85%-4.45%). The rise was because of an increased use of antipsychotics, antidepressants, and antiepileptics.
The affected cohort showed a modestly greater decline in anticholinergic medications: quarterly change in use: −0.53% vs. −0.21% (95% CI, −0.55% to −0.08%); and in most cardiometabolic medications: for example, quarterly change in antihypertensive use: –0.84% vs. –0.40% (95% CI, –0.64% to –0.25%). Still, a year post diagnosis, 75.2% of dementia patients were using five or more medications, for a 2.8% increase.
The drug classes with the steepest rate of discontinuation – such as lipid-lowering and antihypertensive medications – had low risks for adverse drug events, while higher-risk classes – such as insulins and antiplatelet and anticoagulant agents – had smaller or no reductions in use.
While the findings point to opportunities to reduce polypharmacy by deprescribing long-term medications of dubious benefit, interventions to reduce polypharmacy and inappropriate medications have been modestly successful for patients without dementia, the authors said. But the recent OPTIMIZE trial, an educational effort aimed at primary care clinicians and patients with cognitive impairment, reduced neither polypharmacy nor potentially inappropriate medications.
Luke D. Kim, MD, a geriatrician at the Cleveland Clinic in Ohio, agreed that seniors with dementia can benefit from reassessment of their pharmacologic therapies. “Older adults in general are more prone to have side effects from medications as their renal and hepatic clearance and metabolism are different and lower than those of younger individuals. But they tend to take multiple medications owing to more comorbidities,” said Dr. Kim, who was not involved in the study. “While all older adults need to be more careful about medication management, those with dementia need an even more careful approach as they have diminished cognitive reserve and risk more potential harm from medications.”
The authors noted that since decision-making models aligned with patient priorities for older adults without dementia led to reductions in overall medication use, that may be a path forward in populations with dementia.
The study was supported by grants from the National Institute on Aging, National Institutes of Health. The authors had no competing interests to disclose. Dr. Kim disclosed no competing interests relevant to his comments.
FROM JAMA INTERNAL MEDICINE
Most with early AD not eligible for new antiamyloid drugs
Only a small fraction of older adults in the early stages of Alzheimer’s disease (AD) meet eligibility criteria to receive treatment with newly approved antiamyloid drugs, largely because of the presence of medical conditions or neuroimaging findings, new research shows.
Applying the clinical trial criteria, only about 8%-17% of amyloid-positive individuals with early AD would be eligible for lecanemab (Leqembi), and even fewer, 5%-9%, would be eligible for aducanumab (Aduhelm), the researchers found.
This study highlights the “limited suitability” of most adults with mild cognitive impairment (MCI) or mild dementia with elevated brain amyloid for treatment with these anti–beta amyloid monoclonal antibodies, write Maria Vassilaki, MD, PhD, and colleagues with Mayo Clinic, Rochester, Minn.
The study was published online in Neurology
The authors of an accompanying editorial write that this study “provides an important estimate of treatment eligibility for amyloid-lowering monoclonal antibodies for early AD to help health systems make realistic plans for providing these treatments.”
More real-world data needed
Dr. Vassilaki and colleagues applied eligibility criteria for lecanemab and aducanumab to 237 older adults with MCI or mild dementia and increased brain amyloid burden from the Mayo Clinic Study of Aging (MCSA). Their mean age was 80.9 years, 55% were men, and most were White.
After applying lecanemab’s inclusion criteria, less than half of the study population was eligible to receive treatment (112 of 237, or 47%).
A total of 21 people were excluded because of a body mass index less than 17 or greater than or equal to 35; 48 due to a Clinical Dementia Rating (CDR) global score other than 0.5 or 1.0; 46 because they did not meet WMS-R Logical Memory II scores for age; 8 because of a Mini Mental State Examination (MMSE) score outside the bounds of 22-30; and two because of a CDR memory score less than 0.5.
Applying lecanemab’s exclusion criteria further narrowed the number of eligible participants from 112 to 19 (8% of 237).
Notable exclusions included cardiopulmonary contraindications, central nervous system–related exclusions such as brain cancer, Parkinson’s disease, epilepsy or brain injury, imaging findings, and history of cancer.
The results were similar for aducanumab, with 104 of the 237 participants (44%) meeting the trial’s inclusion criteria. Applying aducanumab’s exclusion criteria further reduced the number of eligible participants to 12 (5% of 237).
A sensitivity analysis including participants with MCI, without CDR global, MMSE, or WMS-R Logical Memory II score restrictions, resulted in a somewhat higher percentage of eligible participants (17.4% for lecanemab and 8.9% for aducanumab).
Shared decision-making
“Clinicians and health systems should be aware that by applying the clinical trial criteria, a smaller percentage might be eligible for these treatments than originally anticipated,” Dr. Vassilaki told this news organization. To help clinicians, there are published recommendations for the appropriate use of these treatments, she noted.
Given that clinical trial participants are typically healthier than the general population, Dr. Vassilaki said that research is needed to examine the safety and efficacy of antiamyloid therapies in larger, more diverse populations as well as in less healthy populations, before these therapies may be more widely available to people with AD.
“We can take advantage of the postmarketing surveillance of side effects, and also enrollment of patients receiving these treatments to registries could provide us with data useful for any necessary adjustment to drug use,” Dr. Vassilaki told this news organization.
‘Sharp focus’
This study “brings the issue of eligibility for amyloid-lowering antibody treatment into sharp focus,” Matthew Howe, MD, PhD, with Butler Hospital Memory & Aging Program, Providence, R.I., and colleagues note in their editorial.
“The results underscore the importance of careful patient selection to help identify patients most likely to benefit from treatment and exclude those at risk for serious outcomes,” they write.
They also write that appropriate use recommendations for lecanemab and aducanumab “will be revisited as more real-world data emerge, especially about safety.”
For now, clinicians “must exercise clinical judgment in selecting patients for treatment with shared decision-making with patients and families,” they add.
The study was supported by the National Institutes of Health, the National Institute on Aging, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Mayo Foundation for Medical Education and Research, the Liston Award, the GHR Foundation and the Schuler Foundation. Dr. Vassilaki has consulted for F. Hoffmann-La Roche and has equity ownership in Abbott Laboratories, Johnson & Johnson, Medtronic, Merck, and Amgen. Dr. Howe has no conflicts of interest.
A version of this article first appeared on Medscape.com.
Only a small fraction of older adults in the early stages of Alzheimer’s disease (AD) meet eligibility criteria to receive treatment with newly approved antiamyloid drugs, largely because of the presence of medical conditions or neuroimaging findings, new research shows.
Applying the clinical trial criteria, only about 8%-17% of amyloid-positive individuals with early AD would be eligible for lecanemab (Leqembi), and even fewer, 5%-9%, would be eligible for aducanumab (Aduhelm), the researchers found.
This study highlights the “limited suitability” of most adults with mild cognitive impairment (MCI) or mild dementia with elevated brain amyloid for treatment with these anti–beta amyloid monoclonal antibodies, write Maria Vassilaki, MD, PhD, and colleagues with Mayo Clinic, Rochester, Minn.
The study was published online in Neurology
The authors of an accompanying editorial write that this study “provides an important estimate of treatment eligibility for amyloid-lowering monoclonal antibodies for early AD to help health systems make realistic plans for providing these treatments.”
More real-world data needed
Dr. Vassilaki and colleagues applied eligibility criteria for lecanemab and aducanumab to 237 older adults with MCI or mild dementia and increased brain amyloid burden from the Mayo Clinic Study of Aging (MCSA). Their mean age was 80.9 years, 55% were men, and most were White.
After applying lecanemab’s inclusion criteria, less than half of the study population was eligible to receive treatment (112 of 237, or 47%).
A total of 21 people were excluded because of a body mass index less than 17 or greater than or equal to 35; 48 due to a Clinical Dementia Rating (CDR) global score other than 0.5 or 1.0; 46 because they did not meet WMS-R Logical Memory II scores for age; 8 because of a Mini Mental State Examination (MMSE) score outside the bounds of 22-30; and two because of a CDR memory score less than 0.5.
Applying lecanemab’s exclusion criteria further narrowed the number of eligible participants from 112 to 19 (8% of 237).
Notable exclusions included cardiopulmonary contraindications, central nervous system–related exclusions such as brain cancer, Parkinson’s disease, epilepsy or brain injury, imaging findings, and history of cancer.
The results were similar for aducanumab, with 104 of the 237 participants (44%) meeting the trial’s inclusion criteria. Applying aducanumab’s exclusion criteria further reduced the number of eligible participants to 12 (5% of 237).
A sensitivity analysis including participants with MCI, without CDR global, MMSE, or WMS-R Logical Memory II score restrictions, resulted in a somewhat higher percentage of eligible participants (17.4% for lecanemab and 8.9% for aducanumab).
Shared decision-making
“Clinicians and health systems should be aware that by applying the clinical trial criteria, a smaller percentage might be eligible for these treatments than originally anticipated,” Dr. Vassilaki told this news organization. To help clinicians, there are published recommendations for the appropriate use of these treatments, she noted.
Given that clinical trial participants are typically healthier than the general population, Dr. Vassilaki said that research is needed to examine the safety and efficacy of antiamyloid therapies in larger, more diverse populations as well as in less healthy populations, before these therapies may be more widely available to people with AD.
“We can take advantage of the postmarketing surveillance of side effects, and also enrollment of patients receiving these treatments to registries could provide us with data useful for any necessary adjustment to drug use,” Dr. Vassilaki told this news organization.
‘Sharp focus’
This study “brings the issue of eligibility for amyloid-lowering antibody treatment into sharp focus,” Matthew Howe, MD, PhD, with Butler Hospital Memory & Aging Program, Providence, R.I., and colleagues note in their editorial.
“The results underscore the importance of careful patient selection to help identify patients most likely to benefit from treatment and exclude those at risk for serious outcomes,” they write.
They also write that appropriate use recommendations for lecanemab and aducanumab “will be revisited as more real-world data emerge, especially about safety.”
For now, clinicians “must exercise clinical judgment in selecting patients for treatment with shared decision-making with patients and families,” they add.
The study was supported by the National Institutes of Health, the National Institute on Aging, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Mayo Foundation for Medical Education and Research, the Liston Award, the GHR Foundation and the Schuler Foundation. Dr. Vassilaki has consulted for F. Hoffmann-La Roche and has equity ownership in Abbott Laboratories, Johnson & Johnson, Medtronic, Merck, and Amgen. Dr. Howe has no conflicts of interest.
A version of this article first appeared on Medscape.com.
Only a small fraction of older adults in the early stages of Alzheimer’s disease (AD) meet eligibility criteria to receive treatment with newly approved antiamyloid drugs, largely because of the presence of medical conditions or neuroimaging findings, new research shows.
Applying the clinical trial criteria, only about 8%-17% of amyloid-positive individuals with early AD would be eligible for lecanemab (Leqembi), and even fewer, 5%-9%, would be eligible for aducanumab (Aduhelm), the researchers found.
This study highlights the “limited suitability” of most adults with mild cognitive impairment (MCI) or mild dementia with elevated brain amyloid for treatment with these anti–beta amyloid monoclonal antibodies, write Maria Vassilaki, MD, PhD, and colleagues with Mayo Clinic, Rochester, Minn.
The study was published online in Neurology
The authors of an accompanying editorial write that this study “provides an important estimate of treatment eligibility for amyloid-lowering monoclonal antibodies for early AD to help health systems make realistic plans for providing these treatments.”
More real-world data needed
Dr. Vassilaki and colleagues applied eligibility criteria for lecanemab and aducanumab to 237 older adults with MCI or mild dementia and increased brain amyloid burden from the Mayo Clinic Study of Aging (MCSA). Their mean age was 80.9 years, 55% were men, and most were White.
After applying lecanemab’s inclusion criteria, less than half of the study population was eligible to receive treatment (112 of 237, or 47%).
A total of 21 people were excluded because of a body mass index less than 17 or greater than or equal to 35; 48 due to a Clinical Dementia Rating (CDR) global score other than 0.5 or 1.0; 46 because they did not meet WMS-R Logical Memory II scores for age; 8 because of a Mini Mental State Examination (MMSE) score outside the bounds of 22-30; and two because of a CDR memory score less than 0.5.
Applying lecanemab’s exclusion criteria further narrowed the number of eligible participants from 112 to 19 (8% of 237).
Notable exclusions included cardiopulmonary contraindications, central nervous system–related exclusions such as brain cancer, Parkinson’s disease, epilepsy or brain injury, imaging findings, and history of cancer.
The results were similar for aducanumab, with 104 of the 237 participants (44%) meeting the trial’s inclusion criteria. Applying aducanumab’s exclusion criteria further reduced the number of eligible participants to 12 (5% of 237).
A sensitivity analysis including participants with MCI, without CDR global, MMSE, or WMS-R Logical Memory II score restrictions, resulted in a somewhat higher percentage of eligible participants (17.4% for lecanemab and 8.9% for aducanumab).
Shared decision-making
“Clinicians and health systems should be aware that by applying the clinical trial criteria, a smaller percentage might be eligible for these treatments than originally anticipated,” Dr. Vassilaki told this news organization. To help clinicians, there are published recommendations for the appropriate use of these treatments, she noted.
Given that clinical trial participants are typically healthier than the general population, Dr. Vassilaki said that research is needed to examine the safety and efficacy of antiamyloid therapies in larger, more diverse populations as well as in less healthy populations, before these therapies may be more widely available to people with AD.
“We can take advantage of the postmarketing surveillance of side effects, and also enrollment of patients receiving these treatments to registries could provide us with data useful for any necessary adjustment to drug use,” Dr. Vassilaki told this news organization.
‘Sharp focus’
This study “brings the issue of eligibility for amyloid-lowering antibody treatment into sharp focus,” Matthew Howe, MD, PhD, with Butler Hospital Memory & Aging Program, Providence, R.I., and colleagues note in their editorial.
“The results underscore the importance of careful patient selection to help identify patients most likely to benefit from treatment and exclude those at risk for serious outcomes,” they write.
They also write that appropriate use recommendations for lecanemab and aducanumab “will be revisited as more real-world data emerge, especially about safety.”
For now, clinicians “must exercise clinical judgment in selecting patients for treatment with shared decision-making with patients and families,” they add.
The study was supported by the National Institutes of Health, the National Institute on Aging, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Mayo Foundation for Medical Education and Research, the Liston Award, the GHR Foundation and the Schuler Foundation. Dr. Vassilaki has consulted for F. Hoffmann-La Roche and has equity ownership in Abbott Laboratories, Johnson & Johnson, Medtronic, Merck, and Amgen. Dr. Howe has no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY