User login
Almost one-third of ED patients with gout are prescribed opioids
Patients with gout who visit the emergency department are regularly prescribed opioids, based on a review of electronic medical records.
“In addition to regulatory changes, the burden of opioid prescription could be potentially reduced by creating prompts for providers in electronic record systems to avoid prescribing opioids in opioid-naive patients or using lower intensity and shorter duration of prescription,” wrote Deepan S. Dalal, MD, of Brown University, Providence, R.I., and coauthors. The study was published in Arthritis Care & Research.
To determine frequency, dose, and duration of opioid prescription at ED discharge, the researchers reviewed the records of 456 patients with acute gout who were discharged in Rhode Island between March 30, 2015, and Sept. 30, 2017. All data were gathered via electronic medical system records.
Of the 456 discharged patients, 129 (28.3%) were prescribed opioids; 102 (79%) were not on opioids at the time. A full prescription description was available for 119 of the 129 patients; 96 (81%) were prescribed oxycodone or oxycodone combinations. Hydrocodone was prescribed for 9 patients (8%) and tramadol was prescribed for 11 patients (9%).
The median duration of each prescription was 8 days (interquartile range, 5-14 days) and the average daily dose was 37.9 mg of morphine equivalent. Patients who were prescribed opioids tended to be younger and male. After multivariable analysis, diabetes, polyarticular gout attack, and prior opioid use were all associated with a more than 100% higher odds of receiving an opioid prescription.
The authors acknowledged their study’s limitations, including their inability to determine the physicians’ reasoning behind each prescription or the prescribing habits of each provider. In addition, they were only able to assess the prescriptions as being written and not the number of pills actually taken or not taken.
No conflicts of interest were reported.
SOURCE: Dalal DS et al. Arthritis Care Res. 2019 Jul 3. doi: 10.1002/acr.23928.
Patients with gout who visit the emergency department are regularly prescribed opioids, based on a review of electronic medical records.
“In addition to regulatory changes, the burden of opioid prescription could be potentially reduced by creating prompts for providers in electronic record systems to avoid prescribing opioids in opioid-naive patients or using lower intensity and shorter duration of prescription,” wrote Deepan S. Dalal, MD, of Brown University, Providence, R.I., and coauthors. The study was published in Arthritis Care & Research.
To determine frequency, dose, and duration of opioid prescription at ED discharge, the researchers reviewed the records of 456 patients with acute gout who were discharged in Rhode Island between March 30, 2015, and Sept. 30, 2017. All data were gathered via electronic medical system records.
Of the 456 discharged patients, 129 (28.3%) were prescribed opioids; 102 (79%) were not on opioids at the time. A full prescription description was available for 119 of the 129 patients; 96 (81%) were prescribed oxycodone or oxycodone combinations. Hydrocodone was prescribed for 9 patients (8%) and tramadol was prescribed for 11 patients (9%).
The median duration of each prescription was 8 days (interquartile range, 5-14 days) and the average daily dose was 37.9 mg of morphine equivalent. Patients who were prescribed opioids tended to be younger and male. After multivariable analysis, diabetes, polyarticular gout attack, and prior opioid use were all associated with a more than 100% higher odds of receiving an opioid prescription.
The authors acknowledged their study’s limitations, including their inability to determine the physicians’ reasoning behind each prescription or the prescribing habits of each provider. In addition, they were only able to assess the prescriptions as being written and not the number of pills actually taken or not taken.
No conflicts of interest were reported.
SOURCE: Dalal DS et al. Arthritis Care Res. 2019 Jul 3. doi: 10.1002/acr.23928.
Patients with gout who visit the emergency department are regularly prescribed opioids, based on a review of electronic medical records.
“In addition to regulatory changes, the burden of opioid prescription could be potentially reduced by creating prompts for providers in electronic record systems to avoid prescribing opioids in opioid-naive patients or using lower intensity and shorter duration of prescription,” wrote Deepan S. Dalal, MD, of Brown University, Providence, R.I., and coauthors. The study was published in Arthritis Care & Research.
To determine frequency, dose, and duration of opioid prescription at ED discharge, the researchers reviewed the records of 456 patients with acute gout who were discharged in Rhode Island between March 30, 2015, and Sept. 30, 2017. All data were gathered via electronic medical system records.
Of the 456 discharged patients, 129 (28.3%) were prescribed opioids; 102 (79%) were not on opioids at the time. A full prescription description was available for 119 of the 129 patients; 96 (81%) were prescribed oxycodone or oxycodone combinations. Hydrocodone was prescribed for 9 patients (8%) and tramadol was prescribed for 11 patients (9%).
The median duration of each prescription was 8 days (interquartile range, 5-14 days) and the average daily dose was 37.9 mg of morphine equivalent. Patients who were prescribed opioids tended to be younger and male. After multivariable analysis, diabetes, polyarticular gout attack, and prior opioid use were all associated with a more than 100% higher odds of receiving an opioid prescription.
The authors acknowledged their study’s limitations, including their inability to determine the physicians’ reasoning behind each prescription or the prescribing habits of each provider. In addition, they were only able to assess the prescriptions as being written and not the number of pills actually taken or not taken.
No conflicts of interest were reported.
SOURCE: Dalal DS et al. Arthritis Care Res. 2019 Jul 3. doi: 10.1002/acr.23928.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Though there are other effective conventional treatments, opioids are often prescribed for patients who present to the ED with gout.
Major finding: After multivariable analysis, diabetes, polyarticular gout attack, and prior opioid use were all associated with a more than 100% higher odds of opioid prescription.
Study details: A retrospective cohort study of 456 patients with acute gout discharged from EDs in Rhode Island.
Disclosures: The authors reported no conflicts of interest.
Source: Dalal DS et al. Arthritis Care Res. 2019 Jul 3. doi: 10.1002/acr.23928.
Lowering hyperuricemia improved endothelial function but failed as an antihypertensive
MADRID – Using allopurinol to reduce hyperuricemia in young adults with prehypertension or stage 1 hypertension failed to significantly lower blood pressure but succeeded in significantly improving endothelial function as measured by increased flow-mediated arterial dilation in a single-center crossover study with 82 participants.
The finding of improved endothelial function suggests that reducing hyperuricemia may be a new way to manage hypertension or prevent progression to stage 1 hypertension, improve cardiovascular health, and ultimately cut cardiovascular events, Angelo L. Gaffo, MD, said at the European Congress of Rheumatology. The results indicated that the BP-lowering effect of allopurinol treatment was strongest in people who entered the study with the highest serum urate levels, greater than 6.5 mg/dL, an indication that the next step in developing this approach should be targeting it to people with serum urate levels in this range, said Dr. Gaffo, a rheumatologist at the University of Alabama at Birmingham.
“It’s just a matter of finding the right population to see the blood pressure reduction effect,” Dr. Gaffo said in an interview.
He and his associates designed the SURPHER (Serum Urate Reduction to Prevent Hypertension) study to assess the impact of allopurinol treatment in people aged 18-40 years with prehypertension or stage 1 hypertension as defined by U.S. BP standards at the time they launched the study in 2016 (Contemp Clin Trials. 2016 Sep;50:238-44). Enrolled participants had to be nonsmokers; have an estimated glomerular filtration rate of greater than 60 mL/min per 1.73 m2; have a serum urate level of at least 5.0 mg/dL in men and at least 4.0 mg/dL in women; and be without diabetes, antihypertensive medications, prior urate-lowering treatment, or a history of gout. The 99 people who started the study averaged 28 years old, nearly two-thirds were men, 40% were African Americans, and 52% were white. The participants’ average body mass index was nearly 31 kg/m2, and their average BP was 127/81 mm Hg. Average serum urate levels were 6.4 mg/dL in men and 4.9 mg/dL in women. Participants received 300 mg/day allopurinol or placebo, and after 4 weeks crossed to the alternate regimen, with 82 people completing the full protocol. While on allopurinol, serum urate levels fell by an average of 1.3 mg/dL, a statistically significant drop; on placebo, the levels showed no significant change from baseline.
The primary endpoint was the change in BP on allopurinol treatment, which overall showed no statistically significant difference, compared with when participants received placebo. The results also showed no significant impact of allopurinol treatment, compared with placebo, in serum levels of high-sensitivity C-reactive protein, a measure of inflammation. However, for the secondary endpoint of change in endothelial function as measured by a change in flow-mediated dilation (FMD), the results showed a statistically significant effect of allopurinol treatment. While on allopurinol, average FMD increased from 10.3% at baseline to 14.5% on the drug, a 41% relative increase, while on placebo the average FMD rate showed a slight reduction. Allopurinol treatment was safe and well tolerated during the study.
The results also showed that among people with a baseline serum urate level of greater than 6.5 mg/dL (15 of the 82 study completers) systolic BP fell by an average of about 5 mm Hg.
The results suggested that the concept of reducing hyperuricemia in people with early-stage hypertension or prehypertension might be viable for people with higher serum urate levels than most of those enrolled in SURPHER, Dr. Gaffo said. He noted that prior study results in obese adolescents showed that treating hyperuricemia was able to produce a meaningful BP reduction (Hypertension. 2012 Nov;60[5]:1148-56).
SURPHER received no commercial funding. Dr. Gaffo has received research funding from Amgen and AstraZeneca.
MADRID – Using allopurinol to reduce hyperuricemia in young adults with prehypertension or stage 1 hypertension failed to significantly lower blood pressure but succeeded in significantly improving endothelial function as measured by increased flow-mediated arterial dilation in a single-center crossover study with 82 participants.
The finding of improved endothelial function suggests that reducing hyperuricemia may be a new way to manage hypertension or prevent progression to stage 1 hypertension, improve cardiovascular health, and ultimately cut cardiovascular events, Angelo L. Gaffo, MD, said at the European Congress of Rheumatology. The results indicated that the BP-lowering effect of allopurinol treatment was strongest in people who entered the study with the highest serum urate levels, greater than 6.5 mg/dL, an indication that the next step in developing this approach should be targeting it to people with serum urate levels in this range, said Dr. Gaffo, a rheumatologist at the University of Alabama at Birmingham.
“It’s just a matter of finding the right population to see the blood pressure reduction effect,” Dr. Gaffo said in an interview.
He and his associates designed the SURPHER (Serum Urate Reduction to Prevent Hypertension) study to assess the impact of allopurinol treatment in people aged 18-40 years with prehypertension or stage 1 hypertension as defined by U.S. BP standards at the time they launched the study in 2016 (Contemp Clin Trials. 2016 Sep;50:238-44). Enrolled participants had to be nonsmokers; have an estimated glomerular filtration rate of greater than 60 mL/min per 1.73 m2; have a serum urate level of at least 5.0 mg/dL in men and at least 4.0 mg/dL in women; and be without diabetes, antihypertensive medications, prior urate-lowering treatment, or a history of gout. The 99 people who started the study averaged 28 years old, nearly two-thirds were men, 40% were African Americans, and 52% were white. The participants’ average body mass index was nearly 31 kg/m2, and their average BP was 127/81 mm Hg. Average serum urate levels were 6.4 mg/dL in men and 4.9 mg/dL in women. Participants received 300 mg/day allopurinol or placebo, and after 4 weeks crossed to the alternate regimen, with 82 people completing the full protocol. While on allopurinol, serum urate levels fell by an average of 1.3 mg/dL, a statistically significant drop; on placebo, the levels showed no significant change from baseline.
The primary endpoint was the change in BP on allopurinol treatment, which overall showed no statistically significant difference, compared with when participants received placebo. The results also showed no significant impact of allopurinol treatment, compared with placebo, in serum levels of high-sensitivity C-reactive protein, a measure of inflammation. However, for the secondary endpoint of change in endothelial function as measured by a change in flow-mediated dilation (FMD), the results showed a statistically significant effect of allopurinol treatment. While on allopurinol, average FMD increased from 10.3% at baseline to 14.5% on the drug, a 41% relative increase, while on placebo the average FMD rate showed a slight reduction. Allopurinol treatment was safe and well tolerated during the study.
The results also showed that among people with a baseline serum urate level of greater than 6.5 mg/dL (15 of the 82 study completers) systolic BP fell by an average of about 5 mm Hg.
The results suggested that the concept of reducing hyperuricemia in people with early-stage hypertension or prehypertension might be viable for people with higher serum urate levels than most of those enrolled in SURPHER, Dr. Gaffo said. He noted that prior study results in obese adolescents showed that treating hyperuricemia was able to produce a meaningful BP reduction (Hypertension. 2012 Nov;60[5]:1148-56).
SURPHER received no commercial funding. Dr. Gaffo has received research funding from Amgen and AstraZeneca.
MADRID – Using allopurinol to reduce hyperuricemia in young adults with prehypertension or stage 1 hypertension failed to significantly lower blood pressure but succeeded in significantly improving endothelial function as measured by increased flow-mediated arterial dilation in a single-center crossover study with 82 participants.
The finding of improved endothelial function suggests that reducing hyperuricemia may be a new way to manage hypertension or prevent progression to stage 1 hypertension, improve cardiovascular health, and ultimately cut cardiovascular events, Angelo L. Gaffo, MD, said at the European Congress of Rheumatology. The results indicated that the BP-lowering effect of allopurinol treatment was strongest in people who entered the study with the highest serum urate levels, greater than 6.5 mg/dL, an indication that the next step in developing this approach should be targeting it to people with serum urate levels in this range, said Dr. Gaffo, a rheumatologist at the University of Alabama at Birmingham.
“It’s just a matter of finding the right population to see the blood pressure reduction effect,” Dr. Gaffo said in an interview.
He and his associates designed the SURPHER (Serum Urate Reduction to Prevent Hypertension) study to assess the impact of allopurinol treatment in people aged 18-40 years with prehypertension or stage 1 hypertension as defined by U.S. BP standards at the time they launched the study in 2016 (Contemp Clin Trials. 2016 Sep;50:238-44). Enrolled participants had to be nonsmokers; have an estimated glomerular filtration rate of greater than 60 mL/min per 1.73 m2; have a serum urate level of at least 5.0 mg/dL in men and at least 4.0 mg/dL in women; and be without diabetes, antihypertensive medications, prior urate-lowering treatment, or a history of gout. The 99 people who started the study averaged 28 years old, nearly two-thirds were men, 40% were African Americans, and 52% were white. The participants’ average body mass index was nearly 31 kg/m2, and their average BP was 127/81 mm Hg. Average serum urate levels were 6.4 mg/dL in men and 4.9 mg/dL in women. Participants received 300 mg/day allopurinol or placebo, and after 4 weeks crossed to the alternate regimen, with 82 people completing the full protocol. While on allopurinol, serum urate levels fell by an average of 1.3 mg/dL, a statistically significant drop; on placebo, the levels showed no significant change from baseline.
The primary endpoint was the change in BP on allopurinol treatment, which overall showed no statistically significant difference, compared with when participants received placebo. The results also showed no significant impact of allopurinol treatment, compared with placebo, in serum levels of high-sensitivity C-reactive protein, a measure of inflammation. However, for the secondary endpoint of change in endothelial function as measured by a change in flow-mediated dilation (FMD), the results showed a statistically significant effect of allopurinol treatment. While on allopurinol, average FMD increased from 10.3% at baseline to 14.5% on the drug, a 41% relative increase, while on placebo the average FMD rate showed a slight reduction. Allopurinol treatment was safe and well tolerated during the study.
The results also showed that among people with a baseline serum urate level of greater than 6.5 mg/dL (15 of the 82 study completers) systolic BP fell by an average of about 5 mm Hg.
The results suggested that the concept of reducing hyperuricemia in people with early-stage hypertension or prehypertension might be viable for people with higher serum urate levels than most of those enrolled in SURPHER, Dr. Gaffo said. He noted that prior study results in obese adolescents showed that treating hyperuricemia was able to produce a meaningful BP reduction (Hypertension. 2012 Nov;60[5]:1148-56).
SURPHER received no commercial funding. Dr. Gaffo has received research funding from Amgen and AstraZeneca.
REPORTING FROM EULAR 2019 CONGRESS
Arthritis joint pain, inactivity vary greatly across U.S.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
Almost 31% of the estimated 54 million adults in the United States with arthritis have severe joint pain, according to the Centers for Disease Control and Prevention.

Nationally, the prevalence of severe joint pain was 30.8% in adults with arthritis in 2017, but state-specific, age-standardized prevalences varied from a low of 20.8% in Colorado to 45.2% in Mississippi. Regionally, prevalences of both severe joint pain and physical inactivity in arthritis patients were highest in the Southeast, noted Dana Guglielmo, MPH, of the CDC’s National Center for Chronic Disease Prevention and Health Promotion, Atlanta, and associates (MMWR 2019 May 3;68(17):381-7).
The prevalence of arthritis itself was lowest in the District of Columbia at 15.7% and highest in West Virginia at 34.6%. Alabama, at 30.4%, was the only other state above 30%. Colorado had the lowest physical inactivity rate (23.2%), while Kentucky had the highest (44.4%), the investigators said.
The differences among arthritis patients were demographic as well as geographic in 2017. The prevalence of severe joint pain was 33.0% among those aged 18-44 years and 35.6% in those 45-64 but only 25.1% in those aged 65 and older. Whites had a 27.4% prevalence of severe joint pain, compared with 42.0% for Hispanics and 50.9% for blacks. For arthritis patients with a college degree, the age-standardized prevalence of severe joint pain was 15.1%, compared with 35.5% for high school graduates and 54.1% for those with less than a high school degree, based on data from the Behavioral Risk Factor Surveillance System.
“Although persons with arthritis report that pain, or fear of causing or worsening it, is a substantial barrier to exercising, physical activity is an inexpensive intervention that can reduce pain, prevent or delay disability and limitations, and improve mental health, physical functioning, and quality of life with few adverse effects,” wrote Ms. Guglielmo and associates. Adults with severe joint pain “should engage in regular physical activity according to their abilities and avoid physical inactivity [since] even small amounts of physical activity can improve physical functioning in adults with joint conditions.”
SOURCE: Guglielmo D et al. MMWR 2019 May 3;68(17):381-7.
FROM MMWR
Colchicine reduces inflammatory markers associated with metabolic syndrome
A small study offers a tantalizing hint that
The 3-month trial did not meet its primary endpoint – change in insulin sensitivity as measured by a glucose tolerance test – but it did hit several secondary goals, all of which were related to the inflammation that accompanies prediabetes, Jack A. Yanovski, MD, and colleagues wrote in Diabetes, Obesity, and Metabolism.
“Colchicine is well-known to have anti-inflammatory properties, although its effect on obesity-associated inflammation has not previously been investigated,” said Dr. Yanovski of the National institutes of Health and his coauthors. “Classically, it has been posited that colchicine blocks inflammation by impeding leukocyte locomotion, diapedesis, and, ultimately, recruitment to sites of inflammation. ... Recently, it has been shown that colchicine also inhibits the formation of the NLRP3 [NOD-like receptor family pyrin domain-containing 3] inflammasome, an important component of the obesity-associated inflammatory cascade.”
The NLRP3 inflammasome has been shown to play an important part in promoting the inflammatory state of obesity, the authors noted. When a cell senses danger, NLRP3 uses microtubules to create an inflammasome that then produces interleukin-1 beta gene and interleukin-18. One of colchicine’s known actions is to inhibit microtubule formation, suggesting that it could put the brakes on this process.
The study comprised 40 patients who had metabolic syndrome, significant insulin resistance, and elevated inflammatory markers. Among the exclusionary criteria were having a significant medical illness, a history of gout, and recent or current use of colchicine.
The patients were randomized to colchicine 0.6 mg or placebo twice daily for 3 months. No dietary advice was given during the study period. Of the 40 randomized patients, 37 completed the 3-month study, though none left because of adverse events.
Although there were no significant between-group differences in levels of fasting insulin, colchicine did significantly decrease inflammatory markers, compared with placebo. C-reactive protein dropped by 2.8 mg/L in the active group but increased slightly in the placebo group. The erythrocyte sedimentation rate also decreased in the colchicine group, compared with placebo (difference, –5.9 mm/hr; P = .07). The active group experienced an improvement in fasting insulin as measured by the homeostasis model assessment–estimated insulin resistance index and in glucose effectiveness, which suggests metabolic improvement.
“Larger trials are needed to investigate whether colchicine has efficacy in improving insulin resistance and/or preventing the onset of diabetes mellitus in at-risk individuals with obesity-associated inflammation,” the authors concluded.
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Institutes of Health. None of the authors reported any disclosures or conflicts of interest relating to this study.
SOURCE: Yanovski JA et al. Diabetes Obes Metab. 2019 Mar 14. doi: 10.1111/dom.13702.
A small study offers a tantalizing hint that
The 3-month trial did not meet its primary endpoint – change in insulin sensitivity as measured by a glucose tolerance test – but it did hit several secondary goals, all of which were related to the inflammation that accompanies prediabetes, Jack A. Yanovski, MD, and colleagues wrote in Diabetes, Obesity, and Metabolism.
“Colchicine is well-known to have anti-inflammatory properties, although its effect on obesity-associated inflammation has not previously been investigated,” said Dr. Yanovski of the National institutes of Health and his coauthors. “Classically, it has been posited that colchicine blocks inflammation by impeding leukocyte locomotion, diapedesis, and, ultimately, recruitment to sites of inflammation. ... Recently, it has been shown that colchicine also inhibits the formation of the NLRP3 [NOD-like receptor family pyrin domain-containing 3] inflammasome, an important component of the obesity-associated inflammatory cascade.”
The NLRP3 inflammasome has been shown to play an important part in promoting the inflammatory state of obesity, the authors noted. When a cell senses danger, NLRP3 uses microtubules to create an inflammasome that then produces interleukin-1 beta gene and interleukin-18. One of colchicine’s known actions is to inhibit microtubule formation, suggesting that it could put the brakes on this process.
The study comprised 40 patients who had metabolic syndrome, significant insulin resistance, and elevated inflammatory markers. Among the exclusionary criteria were having a significant medical illness, a history of gout, and recent or current use of colchicine.
The patients were randomized to colchicine 0.6 mg or placebo twice daily for 3 months. No dietary advice was given during the study period. Of the 40 randomized patients, 37 completed the 3-month study, though none left because of adverse events.
Although there were no significant between-group differences in levels of fasting insulin, colchicine did significantly decrease inflammatory markers, compared with placebo. C-reactive protein dropped by 2.8 mg/L in the active group but increased slightly in the placebo group. The erythrocyte sedimentation rate also decreased in the colchicine group, compared with placebo (difference, –5.9 mm/hr; P = .07). The active group experienced an improvement in fasting insulin as measured by the homeostasis model assessment–estimated insulin resistance index and in glucose effectiveness, which suggests metabolic improvement.
“Larger trials are needed to investigate whether colchicine has efficacy in improving insulin resistance and/or preventing the onset of diabetes mellitus in at-risk individuals with obesity-associated inflammation,” the authors concluded.
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Institutes of Health. None of the authors reported any disclosures or conflicts of interest relating to this study.
SOURCE: Yanovski JA et al. Diabetes Obes Metab. 2019 Mar 14. doi: 10.1111/dom.13702.
A small study offers a tantalizing hint that
The 3-month trial did not meet its primary endpoint – change in insulin sensitivity as measured by a glucose tolerance test – but it did hit several secondary goals, all of which were related to the inflammation that accompanies prediabetes, Jack A. Yanovski, MD, and colleagues wrote in Diabetes, Obesity, and Metabolism.
“Colchicine is well-known to have anti-inflammatory properties, although its effect on obesity-associated inflammation has not previously been investigated,” said Dr. Yanovski of the National institutes of Health and his coauthors. “Classically, it has been posited that colchicine blocks inflammation by impeding leukocyte locomotion, diapedesis, and, ultimately, recruitment to sites of inflammation. ... Recently, it has been shown that colchicine also inhibits the formation of the NLRP3 [NOD-like receptor family pyrin domain-containing 3] inflammasome, an important component of the obesity-associated inflammatory cascade.”
The NLRP3 inflammasome has been shown to play an important part in promoting the inflammatory state of obesity, the authors noted. When a cell senses danger, NLRP3 uses microtubules to create an inflammasome that then produces interleukin-1 beta gene and interleukin-18. One of colchicine’s known actions is to inhibit microtubule formation, suggesting that it could put the brakes on this process.
The study comprised 40 patients who had metabolic syndrome, significant insulin resistance, and elevated inflammatory markers. Among the exclusionary criteria were having a significant medical illness, a history of gout, and recent or current use of colchicine.
The patients were randomized to colchicine 0.6 mg or placebo twice daily for 3 months. No dietary advice was given during the study period. Of the 40 randomized patients, 37 completed the 3-month study, though none left because of adverse events.
Although there were no significant between-group differences in levels of fasting insulin, colchicine did significantly decrease inflammatory markers, compared with placebo. C-reactive protein dropped by 2.8 mg/L in the active group but increased slightly in the placebo group. The erythrocyte sedimentation rate also decreased in the colchicine group, compared with placebo (difference, –5.9 mm/hr; P = .07). The active group experienced an improvement in fasting insulin as measured by the homeostasis model assessment–estimated insulin resistance index and in glucose effectiveness, which suggests metabolic improvement.
“Larger trials are needed to investigate whether colchicine has efficacy in improving insulin resistance and/or preventing the onset of diabetes mellitus in at-risk individuals with obesity-associated inflammation,” the authors concluded.
The study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and by the National Institutes of Health. None of the authors reported any disclosures or conflicts of interest relating to this study.
SOURCE: Yanovski JA et al. Diabetes Obes Metab. 2019 Mar 14. doi: 10.1111/dom.13702.
FROM DIABETES, OBESITY, AND METABOLISM
Industry-funded rheumatology RCTs are higher quality
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
MAUI, HAWAII – Industry-funded randomized, controlled clinical trials published in the three top-rated rheumatology journals during the past 20 years are of significantly higher overall quality than the nonindustry-funded ones, Michael Putman, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
Dr. Putman, a second-year rheumatology fellow at Northwestern University, Chicago, analyzed all randomized, controlled trials (RCTs) of pharmacotherapy featuring a comparator – either placebo or an active agent – published in 1998, 2008, and 2018 in Annals of the Rheumatic Diseases, Rheumatology, and Arthritis & Rheumatology.
His main takeaway: “Rheumatologic interventions seem to work pretty well. The mean absolute risk reduction in the trials is 17.5%, so the average number of patients who need to be treated with a rheumatologic intervention is about five. This is why it’s such a great specialty to be a part of: A lot of our patients get better.”
He created an RCT quality rating scale that captured the strength of study design, methodology, and findings based upon whether a randomized trial used a double-blind design; identified a prespecified primary outcome; and featured patient-reported outcomes, power calculations, sensitivity analysis, adjustment for multiple hypotheses, and intention-to-treat analysis. He then applied the rating scale to the 85 published RCTs in the three study years.
Of note, 84% of the trials published in 2018 were industry funded, up from 74% in 2008 and 1998.
“Industry funds the vast majority of studies. Industry studies are significantly more likely to be appropriately double blinded, report patient-reported outcome measures, use intention to treat, and they have a higher overall quality,” according to Dr. Putman.
Indeed, the industry-funded studies averaged a 66% score on his quality grading scale, compared with 45% for nonindustry-funded studies.
Utilization of most of the quality metrics remained stable over time. The exceptions: Incorporation of intent-to-treat analysis increased from 58% in 1998 to 87% in 2018, and sensitivity analysis was employed in just 5% of the trials published in 1998, compared with 37% in 2008 and 26% in 2018.
The most important change over the past 2 decades, in his view, has been the shrinking proportion of RCTs featuring an active-drug, head-to-head comparator arm. In 1998, 42% of studies featured that design; for example, comparing methotrexate to sulfasalazine. By 2018, that figure had dropped to just 13%.
“Most of our trials today compare an active compound, such an interleukin-17 inhibitor, to a placebo. I think that’s a big change in how we do things,” Dr. Putman observed. “With 84% of our studies being funded by industry, the incentives in medicine right now don’t support active comparator research. It’s harder to show a difference between two things that work than it is to show a difference between something and nothing.”
However, he’d welcome a revival of head-to-head active comparator trials.
“I’d really love to have that happen,” he said. “We have basic questions we haven’t answered yet about a lot of our basic drugs: Like in myositis, should you start with Imuran [azathioprine], CellCept [mycophenolate mofetil], or methotrexate?”
Another striking change over time has been the dwindling proportion of published trials with a statistically significant finding for the primary outcome: 79% in 1998, 46% in 2008, and 36% last year. Dr. Putman suspects the explanation lies in the steady improvement in the effectiveness of standard background therapy for many conditions, which makes it tougher to show a striking difference between the add-on study drug and add-on placebo.
“We’re a victim of our own success,” he commented.
In any event, many key secondary outcomes in the RCTs were positive, even when the primary endpoint wasn’t, according to Dr. Putman, and there was a notable dearth of completely negative clinical RCTs published in the three top journals.
“The more cynical interpretation is there’s an incredible amount of publication bias, where we’re only publishing studies that show an effect and the journals or investigators are censoring the ones that don’t. The more charitable explanation, which is probably also true, is that by the time you get to putting on an RCT you kind of think, ‘This thing works.’ You’re not testing random stuff, so your pretest probability of a drug being effective when it enters into an RCT is probably shifted toward effectiveness,” Dr. Putman speculated.
He reported having no financial conflicts regarding his study.
REPORTING FROM RWCS 2019
Concomitant methotrexate boosts pegloticase efficacy in gout patients
MAUI, HAWAII – , Orrin M. Troum, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He cited what he considers to be a practice-changing, prospective, observational, proof-of-concept study presented by John Botson, MD, at the 2018 annual meeting of the American College of Rheumatology.
Dr. Botson, a rheumatologist at Orthopedic Physicians Alaska, in Anchorage, reported on nine patients with refractory tophaceous gout placed on an 8-mg infusion of pegloticase every 2 weeks as third-line therapy. But 1 month beforehand he put them on oral methotrexate at 15 mg once weekly along with folic acid at 1 mg/day in an effort to prevent the development of treatment-limiting anti-pegloticase antibodies. It’s the same strategy rheumatologists often use when patients with rheumatoid arthritis on a tumor necrosis factor inhibitor begin to develop anti-drug antibodies.
At the time of the ACR meeting, all nine patients had received at least nine infusions, and six had received at least 12 infusions over the course of 6 months. The response rate was 100%, defined as more than 80% of serum uric acid levels being below 6.0 mg/dL. All patients stayed on methotrexate with no dose adjustment. And there were no infusion reactions. In contrast, the response rate in the randomized trials of pegloticase was only 42%, and 26% of pegloticase recipients experienced infusion reactions within 6 months.
“Although this is not [Food and Drug Administration] approved, it makes a lot of sense. From my standpoint, this is something that I’m doing now for my patients starting on pegloticase if there’s no contraindication to using methotrexate,” said Dr. Troum, a rheumatologist at the University of Southern California in Los Angeles.
“I’ve been doing this, too. This really did change my practice,” added his fellow panelist Alvin F. Wells, MD, PhD, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
When they asked for a show of hands, only a handful of audience members indicated they are now using methotrexate in conjunction with pegloticase in their tophaceous gout patients.
Dr. Wells said his sole reservation about the practice involves using methotrexate in patients with an elevated creatinine level. What about using azathioprine or corticosteroids instead? he asked.
Dr. Troum replied that he monitors those patients carefully but sticks with the methotrexate because it’s only for a few months, which is the time frame in which patients are especially vulnerable to experiencing loss of response to pegloticase due to development of anti-drug antibodies.
Dr. Botson, who was in the Maui audience, rose to give a study update. With additional follow-up, he said, there has still been no signal of loss of response to pegloticase coadministered with methotrexate.
“A lot of us are starting to feel like immunosuppression, whether it’s with methotrexate or something else, is standard of care now,” according to the rheumatologist.
As to prescribing methotrexate in gout patients with renal insufficiency, he continued, he and his colleagues have given the matter quite a bit of thought.
“You’re talking about using methotrexate for 6 months in most of these cases. A lot of the patients who have really bad tophaceous gout already have renal insufficiency, and in the short term we haven’t really seen any problems with that. We work closely with a nephrologist on those cases. And a lot of nephrologists swear – although I don’t think the data are there – that they actually improve their renal function when we start to treat their tophaceous gout,” Dr. Botson said.
Dr. Troum and Dr. Wells reported serving as consultants to and on speakers bureaus for numerous pharmaceutical companies.
MAUI, HAWAII – , Orrin M. Troum, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He cited what he considers to be a practice-changing, prospective, observational, proof-of-concept study presented by John Botson, MD, at the 2018 annual meeting of the American College of Rheumatology.
Dr. Botson, a rheumatologist at Orthopedic Physicians Alaska, in Anchorage, reported on nine patients with refractory tophaceous gout placed on an 8-mg infusion of pegloticase every 2 weeks as third-line therapy. But 1 month beforehand he put them on oral methotrexate at 15 mg once weekly along with folic acid at 1 mg/day in an effort to prevent the development of treatment-limiting anti-pegloticase antibodies. It’s the same strategy rheumatologists often use when patients with rheumatoid arthritis on a tumor necrosis factor inhibitor begin to develop anti-drug antibodies.
At the time of the ACR meeting, all nine patients had received at least nine infusions, and six had received at least 12 infusions over the course of 6 months. The response rate was 100%, defined as more than 80% of serum uric acid levels being below 6.0 mg/dL. All patients stayed on methotrexate with no dose adjustment. And there were no infusion reactions. In contrast, the response rate in the randomized trials of pegloticase was only 42%, and 26% of pegloticase recipients experienced infusion reactions within 6 months.
“Although this is not [Food and Drug Administration] approved, it makes a lot of sense. From my standpoint, this is something that I’m doing now for my patients starting on pegloticase if there’s no contraindication to using methotrexate,” said Dr. Troum, a rheumatologist at the University of Southern California in Los Angeles.
“I’ve been doing this, too. This really did change my practice,” added his fellow panelist Alvin F. Wells, MD, PhD, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
When they asked for a show of hands, only a handful of audience members indicated they are now using methotrexate in conjunction with pegloticase in their tophaceous gout patients.
Dr. Wells said his sole reservation about the practice involves using methotrexate in patients with an elevated creatinine level. What about using azathioprine or corticosteroids instead? he asked.
Dr. Troum replied that he monitors those patients carefully but sticks with the methotrexate because it’s only for a few months, which is the time frame in which patients are especially vulnerable to experiencing loss of response to pegloticase due to development of anti-drug antibodies.
Dr. Botson, who was in the Maui audience, rose to give a study update. With additional follow-up, he said, there has still been no signal of loss of response to pegloticase coadministered with methotrexate.
“A lot of us are starting to feel like immunosuppression, whether it’s with methotrexate or something else, is standard of care now,” according to the rheumatologist.
As to prescribing methotrexate in gout patients with renal insufficiency, he continued, he and his colleagues have given the matter quite a bit of thought.
“You’re talking about using methotrexate for 6 months in most of these cases. A lot of the patients who have really bad tophaceous gout already have renal insufficiency, and in the short term we haven’t really seen any problems with that. We work closely with a nephrologist on those cases. And a lot of nephrologists swear – although I don’t think the data are there – that they actually improve their renal function when we start to treat their tophaceous gout,” Dr. Botson said.
Dr. Troum and Dr. Wells reported serving as consultants to and on speakers bureaus for numerous pharmaceutical companies.
MAUI, HAWAII – , Orrin M. Troum, MD, said at the 2019 Rheumatology Winter Clinical Symposium.
He cited what he considers to be a practice-changing, prospective, observational, proof-of-concept study presented by John Botson, MD, at the 2018 annual meeting of the American College of Rheumatology.
Dr. Botson, a rheumatologist at Orthopedic Physicians Alaska, in Anchorage, reported on nine patients with refractory tophaceous gout placed on an 8-mg infusion of pegloticase every 2 weeks as third-line therapy. But 1 month beforehand he put them on oral methotrexate at 15 mg once weekly along with folic acid at 1 mg/day in an effort to prevent the development of treatment-limiting anti-pegloticase antibodies. It’s the same strategy rheumatologists often use when patients with rheumatoid arthritis on a tumor necrosis factor inhibitor begin to develop anti-drug antibodies.
At the time of the ACR meeting, all nine patients had received at least nine infusions, and six had received at least 12 infusions over the course of 6 months. The response rate was 100%, defined as more than 80% of serum uric acid levels being below 6.0 mg/dL. All patients stayed on methotrexate with no dose adjustment. And there were no infusion reactions. In contrast, the response rate in the randomized trials of pegloticase was only 42%, and 26% of pegloticase recipients experienced infusion reactions within 6 months.
“Although this is not [Food and Drug Administration] approved, it makes a lot of sense. From my standpoint, this is something that I’m doing now for my patients starting on pegloticase if there’s no contraindication to using methotrexate,” said Dr. Troum, a rheumatologist at the University of Southern California in Los Angeles.
“I’ve been doing this, too. This really did change my practice,” added his fellow panelist Alvin F. Wells, MD, PhD, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
When they asked for a show of hands, only a handful of audience members indicated they are now using methotrexate in conjunction with pegloticase in their tophaceous gout patients.
Dr. Wells said his sole reservation about the practice involves using methotrexate in patients with an elevated creatinine level. What about using azathioprine or corticosteroids instead? he asked.
Dr. Troum replied that he monitors those patients carefully but sticks with the methotrexate because it’s only for a few months, which is the time frame in which patients are especially vulnerable to experiencing loss of response to pegloticase due to development of anti-drug antibodies.
Dr. Botson, who was in the Maui audience, rose to give a study update. With additional follow-up, he said, there has still been no signal of loss of response to pegloticase coadministered with methotrexate.
“A lot of us are starting to feel like immunosuppression, whether it’s with methotrexate or something else, is standard of care now,” according to the rheumatologist.
As to prescribing methotrexate in gout patients with renal insufficiency, he continued, he and his colleagues have given the matter quite a bit of thought.
“You’re talking about using methotrexate for 6 months in most of these cases. A lot of the patients who have really bad tophaceous gout already have renal insufficiency, and in the short term we haven’t really seen any problems with that. We work closely with a nephrologist on those cases. And a lot of nephrologists swear – although I don’t think the data are there – that they actually improve their renal function when we start to treat their tophaceous gout,” Dr. Botson said.
Dr. Troum and Dr. Wells reported serving as consultants to and on speakers bureaus for numerous pharmaceutical companies.
REPORTING FROM RWCS 2019
FDA approves liquid colchicine for gout
for prophylaxis of gout flares in adults, according to a statement from Romeg Therapeutics.
Colchicine has been used in capsule and tablet forms to treat this form of arthritis for decades. An advantage to the new formulation is that it allows physicians to “easily make dose adjustments,” according to the statement.
“Existing therapies do not adequately address the physician’s need to adjust dosages of colchicine to manage the toxicity profile for patients with renal and liver impairments, side effects, common drug-to-drug interactions, and age-related health disorders,” said Naomi Vishnupad, PhD, chief scientific officer of Romeg Therapeutics, in the statement.
According to the prescribing information for the drug on the FDA website, this formulation is indicated for prophylaxis rather than acute treatment of gout flares because the safety profile of acute treatment with it has not yet been studied. It is contraindicated in patients with hepatic and/or renal impairment. Gastrointestinal symptoms were the most commonly reported adverse reactions.
The drug is expected to be available this summer.
for prophylaxis of gout flares in adults, according to a statement from Romeg Therapeutics.
Colchicine has been used in capsule and tablet forms to treat this form of arthritis for decades. An advantage to the new formulation is that it allows physicians to “easily make dose adjustments,” according to the statement.
“Existing therapies do not adequately address the physician’s need to adjust dosages of colchicine to manage the toxicity profile for patients with renal and liver impairments, side effects, common drug-to-drug interactions, and age-related health disorders,” said Naomi Vishnupad, PhD, chief scientific officer of Romeg Therapeutics, in the statement.
According to the prescribing information for the drug on the FDA website, this formulation is indicated for prophylaxis rather than acute treatment of gout flares because the safety profile of acute treatment with it has not yet been studied. It is contraindicated in patients with hepatic and/or renal impairment. Gastrointestinal symptoms were the most commonly reported adverse reactions.
The drug is expected to be available this summer.
for prophylaxis of gout flares in adults, according to a statement from Romeg Therapeutics.
Colchicine has been used in capsule and tablet forms to treat this form of arthritis for decades. An advantage to the new formulation is that it allows physicians to “easily make dose adjustments,” according to the statement.
“Existing therapies do not adequately address the physician’s need to adjust dosages of colchicine to manage the toxicity profile for patients with renal and liver impairments, side effects, common drug-to-drug interactions, and age-related health disorders,” said Naomi Vishnupad, PhD, chief scientific officer of Romeg Therapeutics, in the statement.
According to the prescribing information for the drug on the FDA website, this formulation is indicated for prophylaxis rather than acute treatment of gout flares because the safety profile of acute treatment with it has not yet been studied. It is contraindicated in patients with hepatic and/or renal impairment. Gastrointestinal symptoms were the most commonly reported adverse reactions.
The drug is expected to be available this summer.
A couple of little known side effects of medications
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
A 46-year-old woman with diabetes and seizure disorder presents with nausea and fatigue. Her physical exam is unremarkable.
Meds: Glyburide 5 mg daily, metformin 850 mg b.i.d., phenytoin 300 mg daily, topiramate 400 mg daily, pantoprazole 40 mg daily.
Labs: Na 133, K 3.9, Cl 112, HCO3 13, Glu 158, Bun 18, Cr 1.0.
What is the most likely cause of this patient’s acidosis?
A. Phenytoin
B. Topiramate
C. Metformin
D. Pantoprazole
The correct answer to this question is topiramate.
Metformin has had warnings about risk of lactic acidosis occurring in patients with kidney disease, but there is no evidence that metformin is associated with lactic acidosis or raised serum lactate levels in patients with diabetes with normal renal function.1 and its use may decrease CV risk in patients with stage 3 CKD.2 This patient has a non–anion gap acidosis (anion gap is 8).
Topiramate acts as a carbonic anhydrase inhibitor, which causes impairment of both the normal reabsorption of filtered HCO3 by the proximal renal tubule and the excretion of hydrogen ion by the distal tubule.3 Acidosis occurs in most patients who are treated with topiramate. Dr. Ture and colleagues did a cross-sectional study to assess the frequency of metabolic acidosis in patients who were taking topiramate.4 Eighty patients who were on topiramate for seizure prevention prior to elective craniotomy were studied. Metabolic acidosis was present in 71% of the patients. Patients treated with topiramate also have a higher risk for kidney stones and uric acid elevation.
A 60-year-old patient presents with right great toe pain. On exam he has warmth and erythema of the 1st MTP joint. Aspiration of the joint shows uric acid crystals. He has had BP’s of 150-160 mm Hg systolic on his home BP monitoring over the past 6 months. In clinic today BP is 156/90 mm Hg. Labs: Bun 10, Cr 1.0, K 3.8, Uric acid 7.4.
Which blood pressure medication would you recommend?
A. Hydrochlorothiazide
B. Chlorthalidone
C. Lisinopril
D. Losartan
E. Irbesartan
In a patient with gout, diuretics should be avoided if possible, as they increase uric acid levels. Of the other three options, losartan offers the added benefit of lowering uric acid levels. Losartan has uricosuric effects and lowers uric acid levels, a property that is unique to losartan of the angiotensin receptor blockers (ARBs) that have been studied.5-6 The uric acid lowering appears to be a probenecid-like effect. Losartan has also been evaluated to see whether using it in combination with a thiazide diuretic can reduce the rise in uric acid that occurs with thiazides. Dr. Matsumura et al. looked at data from the COMFORT trial, focusing on the effect of combining losartan with hydrochlorothiazide on uric acid levels.7 They looked at a group of 118 patients on an ARB other than losartan plus a diuretic, who were then randomly assigned to losartan 50 mg/hydrochlorothiazide 12.5 mg or continuation of another ARB plus a diuretic. Blood pressure control was the same between groups, but the patients who received the losartan combination had lower uric acid levels (P = .01).
Pearls: Topiramate acts as a cerbonic anhydrase inhibitor and can cause a non–anion gap acidosis. Losartan has a modest uricosuric effect and can modestly lower uric acid levels. This is a unique property of losartan and is not shared by other ARBs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Salpeter SR et al. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2010;4:CD002967.
2. Charytan DM et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2019 Jan 22. doi: 10.1111/dom.13642.
3. Mirza N et al. Effect of topiramate on acid-base balance: extent, mechanism and effects. Br J Clin Pharmacol. 2009 Nov;68(5):655-61.
4. Ture H et al. The frequency and severity of metabolic acidosis related to topiramate. J Int Med Res. 2016;44(6):1376-80.
5. Würzner G et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001 Oct;19(10):1855-60.
6. Puig JG et al. Effect of eprosartan and losartan on uric acid metabolism in patients with essential hypertension. J Hypertens. 1999 Jul;17(7):1033-9.
7. Matsumura K et al. Effect of losartan on serum uric acid in hypertension treated with a diuretic: the COMFORT study. Clin Exp Hypertens. 2015;37(3):192-6.
Death data spur black-box warning for gout drug Uloric
, the Food and Drug Administration declared on Feb. 21. The agency is now mandating a black-box warning.
“Health care professionals should reserve Uloric for use only in patients who have failed or do not tolerate allopurinol,” the FDA announced. “Counsel patients about the cardiovascular risk with Uloric,” the agency suggested, and advise them to seek medical attention at once if they have cardiac symptoms such as chest pain, shortness of breath, rapid or irregular heartbeat, or dizziness.
The FDA’s move comes a decade after it approved febuxostat as a gout treatment. As the FDA noted in its announcement, “the number of medicines to treat gout is limited, and there is an unmet need for treatments for this disease.”
Research has suggested that both febuxostat and allopurinol have similar efficacy. Some experts have recommended febuxostat as an alternative for patients who shouldn’t take allopurinol (Semin Arthritis Rheum. 2013 Dec;43[3]:367-75).
However, research has raised concerns about febuxostat’s cardiac risk. In its Feb. 21 statement, the FDA pointed to the findings of a 2010-2017 postmarket clinical trial of 6,190 patients with gout who were treated with febuxostat or allopurinol (N Engl J Med. 2018;378:1200-10).
“In patients treated with Uloric, 15 deaths from heart-related causes were observed for every 1,000 patients treated for a year compared to 11 deaths from heart-related causes per 1,000 patients treated with allopurinol for a year,” the FDA said. “In addition, there were 26 deaths from any cause per 1,000 patients treated for a year with Uloric compared to 22 deaths per 1,000 patients treated for a year with allopurinol.”
, the Food and Drug Administration declared on Feb. 21. The agency is now mandating a black-box warning.
“Health care professionals should reserve Uloric for use only in patients who have failed or do not tolerate allopurinol,” the FDA announced. “Counsel patients about the cardiovascular risk with Uloric,” the agency suggested, and advise them to seek medical attention at once if they have cardiac symptoms such as chest pain, shortness of breath, rapid or irregular heartbeat, or dizziness.
The FDA’s move comes a decade after it approved febuxostat as a gout treatment. As the FDA noted in its announcement, “the number of medicines to treat gout is limited, and there is an unmet need for treatments for this disease.”
Research has suggested that both febuxostat and allopurinol have similar efficacy. Some experts have recommended febuxostat as an alternative for patients who shouldn’t take allopurinol (Semin Arthritis Rheum. 2013 Dec;43[3]:367-75).
However, research has raised concerns about febuxostat’s cardiac risk. In its Feb. 21 statement, the FDA pointed to the findings of a 2010-2017 postmarket clinical trial of 6,190 patients with gout who were treated with febuxostat or allopurinol (N Engl J Med. 2018;378:1200-10).
“In patients treated with Uloric, 15 deaths from heart-related causes were observed for every 1,000 patients treated for a year compared to 11 deaths from heart-related causes per 1,000 patients treated with allopurinol for a year,” the FDA said. “In addition, there were 26 deaths from any cause per 1,000 patients treated for a year with Uloric compared to 22 deaths per 1,000 patients treated for a year with allopurinol.”
, the Food and Drug Administration declared on Feb. 21. The agency is now mandating a black-box warning.
“Health care professionals should reserve Uloric for use only in patients who have failed or do not tolerate allopurinol,” the FDA announced. “Counsel patients about the cardiovascular risk with Uloric,” the agency suggested, and advise them to seek medical attention at once if they have cardiac symptoms such as chest pain, shortness of breath, rapid or irregular heartbeat, or dizziness.
The FDA’s move comes a decade after it approved febuxostat as a gout treatment. As the FDA noted in its announcement, “the number of medicines to treat gout is limited, and there is an unmet need for treatments for this disease.”
Research has suggested that both febuxostat and allopurinol have similar efficacy. Some experts have recommended febuxostat as an alternative for patients who shouldn’t take allopurinol (Semin Arthritis Rheum. 2013 Dec;43[3]:367-75).
However, research has raised concerns about febuxostat’s cardiac risk. In its Feb. 21 statement, the FDA pointed to the findings of a 2010-2017 postmarket clinical trial of 6,190 patients with gout who were treated with febuxostat or allopurinol (N Engl J Med. 2018;378:1200-10).
“In patients treated with Uloric, 15 deaths from heart-related causes were observed for every 1,000 patients treated for a year compared to 11 deaths from heart-related causes per 1,000 patients treated with allopurinol for a year,” the FDA said. “In addition, there were 26 deaths from any cause per 1,000 patients treated for a year with Uloric compared to 22 deaths per 1,000 patients treated for a year with allopurinol.”
No drop in gout prevalence, but no increase either
according to data from an ongoing, nationally representative survey.
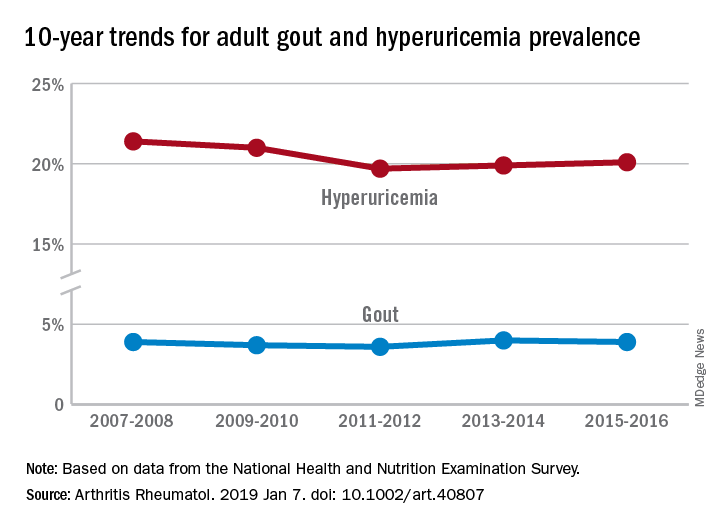
Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
according to data from an ongoing, nationally representative survey.

Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
according to data from an ongoing, nationally representative survey.

Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
FROM ARTHRITIS & RHEUMATOLOGY






