User login
Specialist offers pain medication pro tips for rheumatologists
LAS VEGAS – Be prepared to prescribe pain medications off label. Understand that some of these drugs are double- or even triple-action, although the three-in-ones aren’t necessarily the best option. And beware of prescribing both opioid painkillers and benzodiazepines over the long term.
Xavier Jimenez, MD, a psychiatrist and chronic pain specialist at the Cleveland Clinic, offered these held by Global Academy for Medical Education.
Here’s a closer look at his tips:
• “Avoid chronic opioid therapy for the most part because of the preponderance of negative effects,” Dr. Jimenez said. “You should also try as best as possible to avoid chronic benzodiazepine therapy, which hasn’t gotten as much media attention but has been linked to all sorts of negative outcomes.”
As he described it, a 2010 study (Pain Med. 2010 Jun;11[6]:805-14) revealed that “benzodiazepines predict opioid use better than pain itself. One thing you can do [to help patients] is start to remove the benzodiazepines.”
Are benzodiazepines even useful for rheumatic conditions? A 2012 Cochrane review of the use of diazepam and triazolam found that they “do not appear to be beneficial in improving pain over 24 hours or 1 week” (Cochrane Database Syst Rev. 2012 Jan 18;1:CD008922. doi: 10.1002/14651858.CD008922.pub2).
• When it comes to choosing medications for pain, “there isn’t really a good textbook algorithm. You’ll have to be creative,” he said. “There are a lot of off-label uses going on here. It’s the norm, not the exception.”
• Some painkillers can have antidepressant, anxiolytic (anti-anxiety) or analgesic effects, and a few like duloxetine (Cymbalta) have “double” or “triple action,” Dr. Jimenez said. Some doctors may be tempted to immediately go for something like duloxetine, but he questioned whether “it’s really that simple.”
“That’s done and is effective for a large proportion of these patients, but not everyone. You will have a lot of patients who’ve tried these and not benefited.”
Is Cymbalta appropriate for rheumatic disease? It may be, at least for chronic knee pain due to osteoarthritis: A 2013 review found that three studies supported its pain-relieving properties relative to placebo at about 4 weeks (Ther Adv Musculoskelet Dis. 2013 Dec;5[6]:291-304).
• Consider treating unresolved anxiety, which Dr. Jimenez said he sees more often in moderate and refractory chronic pain. “No one’s asking you to become psychiatrists,” he said, “but I imagine you are asked to act as psychiatrists.”
Researchers continue to explore connections between anxiety and rheumatic disease. A 2016 study of 56 patients with rheumatoid arthritis (RA) over 1 year found that “inflated [28-joint Disease Activity Score] despite well controlled inflammatory disease markers may indicate significant psychological morbidity and related non-inflammatory pain, rather than true disease activity” (BMC Musculoskelet Disord. 2016;17:155).
• Among the many painkiller options are: Gabapentin (“widely used, relatively safe”), pregabalin (keep an eye on kidney values) and topiramate/Topamax (“a pretty decent medication if you’ve got someone who’s not too depressed,” but watch out for the brain-dulling effect that’s spawned a nickname for the drug: “Dopamax”).
• Cannabinoids aren’t ready for prime time as a pain treatment. “Generally, the message here is ‘not yet,’ ” Dr. Jimenez said. “We don’t have enough compelling evidence to suggest cannabinoids are effective, uniformly or across rheumatic diseases. We know much more about the deficits than the benefits.”
What about rheumatic disease and cannabinoids specifically? A 2018 review of research said “preliminary clinical trials have explored the effects of cannabis on rheumatoid arthritis, osteoarthritis, and fibromyalgia; preliminary evidence has also found an association between the cannabinoid system and other rheumatic conditions, including systemic sclerosis and juvenile idiopathic arthritis.”
However, the report says, evidence so far is insufficient to recommend cannabis in rheumatic disease (Nat Rev Rheumatol. 2018;14:488-98).
For now, Dr. Jimenez advised: “Sit tight and wait.”
Dr. Jimenez reported no relevant disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Be prepared to prescribe pain medications off label. Understand that some of these drugs are double- or even triple-action, although the three-in-ones aren’t necessarily the best option. And beware of prescribing both opioid painkillers and benzodiazepines over the long term.
Xavier Jimenez, MD, a psychiatrist and chronic pain specialist at the Cleveland Clinic, offered these held by Global Academy for Medical Education.
Here’s a closer look at his tips:
• “Avoid chronic opioid therapy for the most part because of the preponderance of negative effects,” Dr. Jimenez said. “You should also try as best as possible to avoid chronic benzodiazepine therapy, which hasn’t gotten as much media attention but has been linked to all sorts of negative outcomes.”
As he described it, a 2010 study (Pain Med. 2010 Jun;11[6]:805-14) revealed that “benzodiazepines predict opioid use better than pain itself. One thing you can do [to help patients] is start to remove the benzodiazepines.”
Are benzodiazepines even useful for rheumatic conditions? A 2012 Cochrane review of the use of diazepam and triazolam found that they “do not appear to be beneficial in improving pain over 24 hours or 1 week” (Cochrane Database Syst Rev. 2012 Jan 18;1:CD008922. doi: 10.1002/14651858.CD008922.pub2).
• When it comes to choosing medications for pain, “there isn’t really a good textbook algorithm. You’ll have to be creative,” he said. “There are a lot of off-label uses going on here. It’s the norm, not the exception.”
• Some painkillers can have antidepressant, anxiolytic (anti-anxiety) or analgesic effects, and a few like duloxetine (Cymbalta) have “double” or “triple action,” Dr. Jimenez said. Some doctors may be tempted to immediately go for something like duloxetine, but he questioned whether “it’s really that simple.”
“That’s done and is effective for a large proportion of these patients, but not everyone. You will have a lot of patients who’ve tried these and not benefited.”
Is Cymbalta appropriate for rheumatic disease? It may be, at least for chronic knee pain due to osteoarthritis: A 2013 review found that three studies supported its pain-relieving properties relative to placebo at about 4 weeks (Ther Adv Musculoskelet Dis. 2013 Dec;5[6]:291-304).
• Consider treating unresolved anxiety, which Dr. Jimenez said he sees more often in moderate and refractory chronic pain. “No one’s asking you to become psychiatrists,” he said, “but I imagine you are asked to act as psychiatrists.”
Researchers continue to explore connections between anxiety and rheumatic disease. A 2016 study of 56 patients with rheumatoid arthritis (RA) over 1 year found that “inflated [28-joint Disease Activity Score] despite well controlled inflammatory disease markers may indicate significant psychological morbidity and related non-inflammatory pain, rather than true disease activity” (BMC Musculoskelet Disord. 2016;17:155).
• Among the many painkiller options are: Gabapentin (“widely used, relatively safe”), pregabalin (keep an eye on kidney values) and topiramate/Topamax (“a pretty decent medication if you’ve got someone who’s not too depressed,” but watch out for the brain-dulling effect that’s spawned a nickname for the drug: “Dopamax”).
• Cannabinoids aren’t ready for prime time as a pain treatment. “Generally, the message here is ‘not yet,’ ” Dr. Jimenez said. “We don’t have enough compelling evidence to suggest cannabinoids are effective, uniformly or across rheumatic diseases. We know much more about the deficits than the benefits.”
What about rheumatic disease and cannabinoids specifically? A 2018 review of research said “preliminary clinical trials have explored the effects of cannabis on rheumatoid arthritis, osteoarthritis, and fibromyalgia; preliminary evidence has also found an association between the cannabinoid system and other rheumatic conditions, including systemic sclerosis and juvenile idiopathic arthritis.”
However, the report says, evidence so far is insufficient to recommend cannabis in rheumatic disease (Nat Rev Rheumatol. 2018;14:488-98).
For now, Dr. Jimenez advised: “Sit tight and wait.”
Dr. Jimenez reported no relevant disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Be prepared to prescribe pain medications off label. Understand that some of these drugs are double- or even triple-action, although the three-in-ones aren’t necessarily the best option. And beware of prescribing both opioid painkillers and benzodiazepines over the long term.
Xavier Jimenez, MD, a psychiatrist and chronic pain specialist at the Cleveland Clinic, offered these held by Global Academy for Medical Education.
Here’s a closer look at his tips:
• “Avoid chronic opioid therapy for the most part because of the preponderance of negative effects,” Dr. Jimenez said. “You should also try as best as possible to avoid chronic benzodiazepine therapy, which hasn’t gotten as much media attention but has been linked to all sorts of negative outcomes.”
As he described it, a 2010 study (Pain Med. 2010 Jun;11[6]:805-14) revealed that “benzodiazepines predict opioid use better than pain itself. One thing you can do [to help patients] is start to remove the benzodiazepines.”
Are benzodiazepines even useful for rheumatic conditions? A 2012 Cochrane review of the use of diazepam and triazolam found that they “do not appear to be beneficial in improving pain over 24 hours or 1 week” (Cochrane Database Syst Rev. 2012 Jan 18;1:CD008922. doi: 10.1002/14651858.CD008922.pub2).
• When it comes to choosing medications for pain, “there isn’t really a good textbook algorithm. You’ll have to be creative,” he said. “There are a lot of off-label uses going on here. It’s the norm, not the exception.”
• Some painkillers can have antidepressant, anxiolytic (anti-anxiety) or analgesic effects, and a few like duloxetine (Cymbalta) have “double” or “triple action,” Dr. Jimenez said. Some doctors may be tempted to immediately go for something like duloxetine, but he questioned whether “it’s really that simple.”
“That’s done and is effective for a large proportion of these patients, but not everyone. You will have a lot of patients who’ve tried these and not benefited.”
Is Cymbalta appropriate for rheumatic disease? It may be, at least for chronic knee pain due to osteoarthritis: A 2013 review found that three studies supported its pain-relieving properties relative to placebo at about 4 weeks (Ther Adv Musculoskelet Dis. 2013 Dec;5[6]:291-304).
• Consider treating unresolved anxiety, which Dr. Jimenez said he sees more often in moderate and refractory chronic pain. “No one’s asking you to become psychiatrists,” he said, “but I imagine you are asked to act as psychiatrists.”
Researchers continue to explore connections between anxiety and rheumatic disease. A 2016 study of 56 patients with rheumatoid arthritis (RA) over 1 year found that “inflated [28-joint Disease Activity Score] despite well controlled inflammatory disease markers may indicate significant psychological morbidity and related non-inflammatory pain, rather than true disease activity” (BMC Musculoskelet Disord. 2016;17:155).
• Among the many painkiller options are: Gabapentin (“widely used, relatively safe”), pregabalin (keep an eye on kidney values) and topiramate/Topamax (“a pretty decent medication if you’ve got someone who’s not too depressed,” but watch out for the brain-dulling effect that’s spawned a nickname for the drug: “Dopamax”).
• Cannabinoids aren’t ready for prime time as a pain treatment. “Generally, the message here is ‘not yet,’ ” Dr. Jimenez said. “We don’t have enough compelling evidence to suggest cannabinoids are effective, uniformly or across rheumatic diseases. We know much more about the deficits than the benefits.”
What about rheumatic disease and cannabinoids specifically? A 2018 review of research said “preliminary clinical trials have explored the effects of cannabis on rheumatoid arthritis, osteoarthritis, and fibromyalgia; preliminary evidence has also found an association between the cannabinoid system and other rheumatic conditions, including systemic sclerosis and juvenile idiopathic arthritis.”
However, the report says, evidence so far is insufficient to recommend cannabis in rheumatic disease (Nat Rev Rheumatol. 2018;14:488-98).
For now, Dr. Jimenez advised: “Sit tight and wait.”
Dr. Jimenez reported no relevant disclosures. Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Patient outcome questionnaires take a beating in talk
LAS VEGAS – The purpose of patient-reported outcome data is to help physicians understand how patients are faring so they can make better clinical decisions. But commonly used assessments in rheumatoid arthritis aren’t doing their job, rheumatologist Clifton O. Bingham III, MD, said at the annual Perspectives in Rheumatic Diseases.
“We’re really missing the boat in terms of all these things that are important to our patients with rheumatoid arthritis,” said Dr. Bingham of Johns Hopkins Arthritis Center, Baltimore. For example, multiple instruments used in RA ignore important topics such as pain assessment, fatigue, and physical function, he explained at the conference, held by Global Academy for Medical Education.
There’s good news, however. “Can we come up with something that can overcome these barriers?” he asked. “Yes we can.”
First, Dr. Bingham described the weaknesses of patient questionnaires. “Just because an instrument has been used before, like the SF-36, doesn’t necessarily mean that it is measuring what it’s supposed to be measuring or that the information it gathers is important,” he said.
Even instruments that ask about issues important to RA patients can fail to produce a full picture. As Dr. Bingham noted, the Multidimensional Health Assessment Questionnaire (MD-HAQ) and Rheumatoid Arthritis Disease Activity Index (RADAI) instruments do ask about joint stiffness, but they’re only interested in whether patients felt stiff in the morning and how long the stiffness lasted.
In fact, Dr. Bingham said, a 2013 study found that 23% of 288 patients with RA reported having stiffness only in the morning; most had it all day and night or in the morning and evening (Young KO, et al. ACR 2013, Abstract 2273).
Dr. Bingham listed other problems with patient-reported outcome instruments, such as a question on the MD-HAQ about “fatigue or tiredness.” He said patients actually distinguish between the two: “Patients say they’re tired when they don’t get a good night’s sleep, but that’s not the same as fatigue from RA.”
Is there a better way? Dr. Bingham has explored this issue while serving on the executive committee of Outcome Measures in Rheumatology, an international group supported by drugmakers and other entities. (The funding is arms-length, and he is not compensated.)
Dr. Bingham supports the use of the recently developed Patient-Reported Outcome Measurement Information System (PROMIS), which was established by the National Institutes of Health.
“It lets us see how our patients are doing in their real lives and gives us information about their disease in terms of their activity,” Dr. Bingham said.
He pointed to the upcoming results of a survey of patients with RA that found a wide majority of patients believed many questions on the PROMIS-29 and PROMIS short form questionnaires – regarding pain interference, fatigue and physical function – to be sufficient. However, they did want to see more questions about physical function.
A survey of doctors also revealed that PROMIS results often led them to identify new symptoms or comorbidities, and they frequently took action as a result. In some cases, the results spurred adjustments to RA treatment or medication.
“These measures have the right stuff,” Dr. Bingham said.
He suggested exploring the use of PROMIS-29 in conjunction with other assessments as needed.
“You can administer the questionnaires on tablet computers,” said Dr. Bingham, who added that styluses with built-up handles can be useful for patients with RA when they respond to the questions.
“The information comes straight into your medical record,” he said, “and it can be shown to you and your patient at the same time.”
For more about PROMIS, visit healthmeasures.net.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – The purpose of patient-reported outcome data is to help physicians understand how patients are faring so they can make better clinical decisions. But commonly used assessments in rheumatoid arthritis aren’t doing their job, rheumatologist Clifton O. Bingham III, MD, said at the annual Perspectives in Rheumatic Diseases.
“We’re really missing the boat in terms of all these things that are important to our patients with rheumatoid arthritis,” said Dr. Bingham of Johns Hopkins Arthritis Center, Baltimore. For example, multiple instruments used in RA ignore important topics such as pain assessment, fatigue, and physical function, he explained at the conference, held by Global Academy for Medical Education.
There’s good news, however. “Can we come up with something that can overcome these barriers?” he asked. “Yes we can.”
First, Dr. Bingham described the weaknesses of patient questionnaires. “Just because an instrument has been used before, like the SF-36, doesn’t necessarily mean that it is measuring what it’s supposed to be measuring or that the information it gathers is important,” he said.
Even instruments that ask about issues important to RA patients can fail to produce a full picture. As Dr. Bingham noted, the Multidimensional Health Assessment Questionnaire (MD-HAQ) and Rheumatoid Arthritis Disease Activity Index (RADAI) instruments do ask about joint stiffness, but they’re only interested in whether patients felt stiff in the morning and how long the stiffness lasted.
In fact, Dr. Bingham said, a 2013 study found that 23% of 288 patients with RA reported having stiffness only in the morning; most had it all day and night or in the morning and evening (Young KO, et al. ACR 2013, Abstract 2273).
Dr. Bingham listed other problems with patient-reported outcome instruments, such as a question on the MD-HAQ about “fatigue or tiredness.” He said patients actually distinguish between the two: “Patients say they’re tired when they don’t get a good night’s sleep, but that’s not the same as fatigue from RA.”
Is there a better way? Dr. Bingham has explored this issue while serving on the executive committee of Outcome Measures in Rheumatology, an international group supported by drugmakers and other entities. (The funding is arms-length, and he is not compensated.)
Dr. Bingham supports the use of the recently developed Patient-Reported Outcome Measurement Information System (PROMIS), which was established by the National Institutes of Health.
“It lets us see how our patients are doing in their real lives and gives us information about their disease in terms of their activity,” Dr. Bingham said.
He pointed to the upcoming results of a survey of patients with RA that found a wide majority of patients believed many questions on the PROMIS-29 and PROMIS short form questionnaires – regarding pain interference, fatigue and physical function – to be sufficient. However, they did want to see more questions about physical function.
A survey of doctors also revealed that PROMIS results often led them to identify new symptoms or comorbidities, and they frequently took action as a result. In some cases, the results spurred adjustments to RA treatment or medication.
“These measures have the right stuff,” Dr. Bingham said.
He suggested exploring the use of PROMIS-29 in conjunction with other assessments as needed.
“You can administer the questionnaires on tablet computers,” said Dr. Bingham, who added that styluses with built-up handles can be useful for patients with RA when they respond to the questions.
“The information comes straight into your medical record,” he said, “and it can be shown to you and your patient at the same time.”
For more about PROMIS, visit healthmeasures.net.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – The purpose of patient-reported outcome data is to help physicians understand how patients are faring so they can make better clinical decisions. But commonly used assessments in rheumatoid arthritis aren’t doing their job, rheumatologist Clifton O. Bingham III, MD, said at the annual Perspectives in Rheumatic Diseases.
“We’re really missing the boat in terms of all these things that are important to our patients with rheumatoid arthritis,” said Dr. Bingham of Johns Hopkins Arthritis Center, Baltimore. For example, multiple instruments used in RA ignore important topics such as pain assessment, fatigue, and physical function, he explained at the conference, held by Global Academy for Medical Education.
There’s good news, however. “Can we come up with something that can overcome these barriers?” he asked. “Yes we can.”
First, Dr. Bingham described the weaknesses of patient questionnaires. “Just because an instrument has been used before, like the SF-36, doesn’t necessarily mean that it is measuring what it’s supposed to be measuring or that the information it gathers is important,” he said.
Even instruments that ask about issues important to RA patients can fail to produce a full picture. As Dr. Bingham noted, the Multidimensional Health Assessment Questionnaire (MD-HAQ) and Rheumatoid Arthritis Disease Activity Index (RADAI) instruments do ask about joint stiffness, but they’re only interested in whether patients felt stiff in the morning and how long the stiffness lasted.
In fact, Dr. Bingham said, a 2013 study found that 23% of 288 patients with RA reported having stiffness only in the morning; most had it all day and night or in the morning and evening (Young KO, et al. ACR 2013, Abstract 2273).
Dr. Bingham listed other problems with patient-reported outcome instruments, such as a question on the MD-HAQ about “fatigue or tiredness.” He said patients actually distinguish between the two: “Patients say they’re tired when they don’t get a good night’s sleep, but that’s not the same as fatigue from RA.”
Is there a better way? Dr. Bingham has explored this issue while serving on the executive committee of Outcome Measures in Rheumatology, an international group supported by drugmakers and other entities. (The funding is arms-length, and he is not compensated.)
Dr. Bingham supports the use of the recently developed Patient-Reported Outcome Measurement Information System (PROMIS), which was established by the National Institutes of Health.
“It lets us see how our patients are doing in their real lives and gives us information about their disease in terms of their activity,” Dr. Bingham said.
He pointed to the upcoming results of a survey of patients with RA that found a wide majority of patients believed many questions on the PROMIS-29 and PROMIS short form questionnaires – regarding pain interference, fatigue and physical function – to be sufficient. However, they did want to see more questions about physical function.
A survey of doctors also revealed that PROMIS results often led them to identify new symptoms or comorbidities, and they frequently took action as a result. In some cases, the results spurred adjustments to RA treatment or medication.
“These measures have the right stuff,” Dr. Bingham said.
He suggested exploring the use of PROMIS-29 in conjunction with other assessments as needed.
“You can administer the questionnaires on tablet computers,” said Dr. Bingham, who added that styluses with built-up handles can be useful for patients with RA when they respond to the questions.
“The information comes straight into your medical record,” he said, “and it can be shown to you and your patient at the same time.”
For more about PROMIS, visit healthmeasures.net.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES ON RHEUMATIC DISEASES
New insight into celiac disease: What you should know
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Mayo Clinic gastroenterologist Joseph A. Murray, MD, has a message for rheumatologists: You might think you know celiac disease, which often mimics rheumatic disorders, but there’s a good chance you don’t.
For one, researchers have discovered only in the past few years that many people with celiac disease (CD) don’t spend their days on the toilet. “It’s really come into the fore in the past 5 years that it can present totally without any GI symptoms,” Dr. Murray said in an interview following his presentation at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
For another, he said, CD “is much more common than we thought,” affecting 1% of whites.
This is all relevant to rheumatologists, Dr. Murray said, because celiac disease can cause rheumatic symptoms and is more likely to affect patients with certain rheumatic conditions.
In the big picture, he said during his presentation, researchers now understand that most people with CD do not suffer from diarrhea, the classic symptom associated with the disorder. A 2014 Italian study of 770 patients with CD found that just one-third had diarrhea (BMC Gastroenterol. 2014;14:194).
Instead of GI symptoms – or in addition to them – CD can cause numerous symptoms that may land patients in a rheumatologist’s office. According to Dr. Murray, these include joint pain (often without joint destruction), peripheral neuropathy, general aches and pains, and chronic fatigue.
“You could have a patient presenting with nondestructive joint problems and general fatigue who has celiac disease as a cause,” he said.
Or patients could have both CD and a rheumatic condition. Patients with lupus and Sjögren’s syndrome, for example, are at higher risk of CD, Dr. Murray said.
In addition, he said, “I will often see overlap between celiac disease and rheumatic arthritis.”
What should you do if you suspect a patient has CD? Dr. Murray suggests referring the patient to a gastroenterologist, although he cautioned physicians to keep in mind that a patient with the condition may not be able to adequately absorb oral medications.
He said it’s crucial to not direct the patient to begin a gluten-free diet. The main way to test a patient for CD is through serology prior to a gluten-free diet, he said.
There’s another hitch regarding a gluten-free diet. As he explained in his presentation, it’s often difficult to convince someone who’s already gone on a gluten-free diet to go off it so they can be challenged with gluten for weeks. In these cases, he said, patients may be afraid of the return of symptoms.
As for treatment, Dr. Murray said “patients can be dramatically improved by a gluten-free diet,” although it’s often difficult for them to tolerate. “Often they’re unhappy,” he said in his presentation. “It’s not an easy diet.”
He noted that patients may go gluten free even if it’s not clear they have to. “If the diet is nutritionally adequate,” he said, “I don’t argue with success.”
Dr. Murray disclosed consulting for various drug makers, receiving royalties from Torax, and receiving grant/contracted research support from drug makers, the Broad Medical Research Program at Crohn’s & Colitis Foundation, and Oberkotter Foundation.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
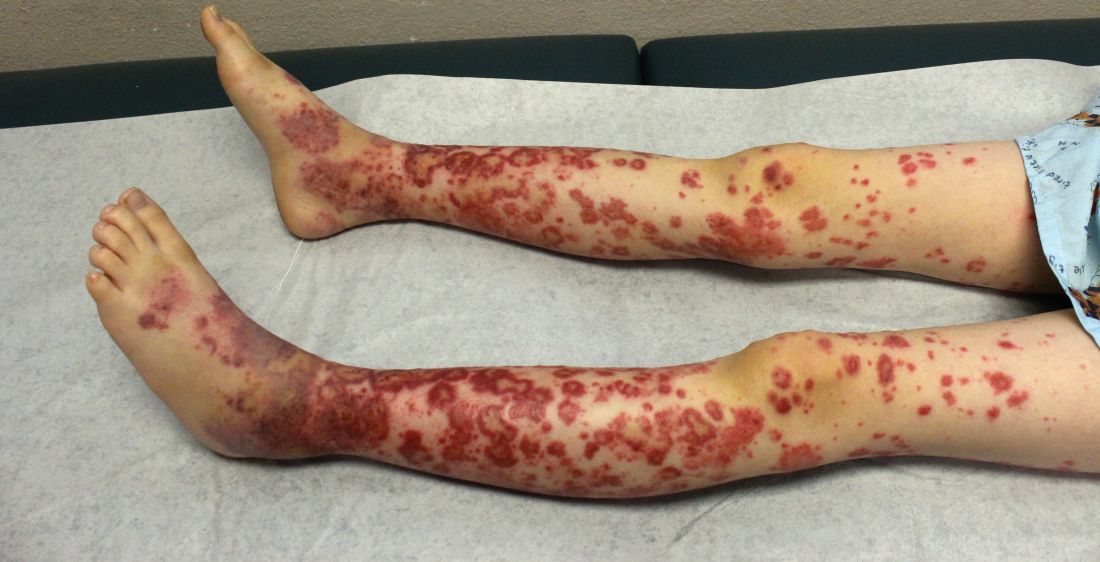
IgA vasculitis may be more common in adults than assumed
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – IgA vasculitis has a reputation as an illness of childhood, but rheumatologist Alexandra Villa-Forte, MD, MPH, cautioned colleagues that it can strike adults, too, often in a much more severe form. And, she warned, it’s likely not as rare as physicians assume.
“I believe it’s more common in adults than reported. There’s a huge problem with establishing the right way to make the diagnosis in adults, which is why it is missed,” said Dr. Villa-Forte of the Cleveland Clinic, who spoke at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education.
But even if IgA vasculitis (IV) is diagnosed correctly in adults, there are many questions about how to move forward, she said. “Treatment in adults remains a problem since we don’t have data.”
IV is also known as Henoch-Schönlein purpura, or HSP, and “spring fever” because it often appears in children in the spring following an upper respiratory infection.
The condition causes vasculitis, the swelling of small blood vessels in organs such as the skin, joints, kidneys, and intestines. Leaking blood vessels can cause skin rashes known as purpura.
The estimated ranges of disease are high, with the annual incidence in children estimated at 3-26 per 100,000 and in adults at 0.1-1.8 per 100,000. Dr. Villa-Forte noted that the male-to-female ratio is 1.5, and she said the condition is less common in African Americans.
“In children, this is a disease that is frequently self-limited. Most of them don’t need treatment, and most of them do not relapse,” Dr. Villa-Forte said. “In adults, it’s more resistant to treatment, frequently chronic, and frequently relapsing over the years.”
There are other differences in IV between children and adults. “In children, there is a clear seasonal pattern of disease that is not seen in adults,” she said, and it’s linked to preceding infections.
“In adults, there are multiple causes, and most of the time they’re not identified,” she said.
As for diagnosis, she suggests looking at clinical presentation and whether tissue biopsy shows cutaneous leukocytoclastic vasculitis with IgA deposits. She cautioned that increased serum IgA is seen in about 50% of adult patients, making it an unreliable indicator.
Prognosis is much better for children than adults. According to a 2014 study, 80% of children completely recover, compared with 40% of adults. Persistent hematuria or proteinuria occurs in 30% of children and 60% of adults, respectively, while chronic renal failure occurs in 2% of children and 10% of adults (J Korean Med Sci. 2014 Feb;29[2]:198-203).
It’s possible that the latter number may be higher, with as many as 30% of adults developing chronic kidney disease (CKD), Dr. Villa-Forte said.
An estimated 97% of nephritis develops within the first 6 months of disease onset in adults, she said, “and in adults, the active renal disease can persist for over 20 years.”
Guidelines suggest that patients be monitored for CKD for 6 months after disease onset, but Dr. Villa-Forte said it’s better to monitor them for 12 months.
What about treatment? “The major challenge in adults is the real absence between correlation between initial presentation and long-term renal outcome,” she said. “That makes for a difficult choice in terms of treatment selection.”
Fever and cutaneous lesions above the waist may predict renal involvement, she said, although that isn’t confirmed, and increasing proteinuria is a probable factor predicting progression/complications.
According to Dr. Villa-Forte, the value of early treatment hasn’t been proved. Due to the risk of kidney problems, she said, “we don’t feel like we can just watch patients – that we need to do something. But there’s not good data supporting that.”
Various protocols involving steroids, early plasmapheresis, rituximab (Rituxan), and other drug regimens lack evidence, she said, although use of steroids in early nephritis may be beneficial in adults.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Coping with a rheumatology clinic’s ‘worst nightmare’
LAS VEGAS – Cleveland Clinic rheumatologist Alexandra Villa-Forte, MD, MPH, has a vivid way of describing the challenge of maintaining remission in granulomatosis with polyangiitis (GPA): She called it “the worst nightmare in the clinic” in a presentation at the annual Perspectives in Rheumatic Diseases.
Dr. Villa-Forte should know. She’s treated more than 700 patients with GPA, also known as Wegener’s granulomatosis, a rare disorder that causes necrotizing vasculitis of small arteries and veins. She advises close monitoring of patients in remission, caution regarding toxicity, pneumonia prophylaxis, and an understanding of the limitations of existing medications.
An estimated 3 in 100,000 people have GPA, which can be fatal if it’s not treated. “All patients with active GPA should receive prednisone with a second agent,” she said at the conference, held by Global Academy for Medical Education. “We know that increasing the prednisone or using it alone will not result in sustained remission at all.”
According to her, new concepts in therapeutic guidance suggest four criteria should determine the specific agents used: Involvement of critical organ systems, severity of clinical manifestations, rate of change (such as rapidly progressing kidney failure), and individual patient factors such as comorbidities.
Patients facing immediate risk to life or organ function should get higher doses of corticosteroids, a cytotoxic agent, and perhaps plasmapheresis, she said.
Induction therapy should last 3-6 months, followed by maintenance therapy that keeps the condition at bay, Dr. Villa-Forte said. But things do not always go according to plan.
“The main challenge that we encounter is that relapses still occur during treatment. The patient could be on appropriate treatment and appropriate doses and they still relapse,” she said. “The second challenge is that patients who come off treatment have a very high rate of relapse.”
Research suggests that up to 70% of patients off medication relapse by 18 months, and 15%-35% of patients on medication relapse by 12-18 months, Dr. Villa-Forte said.
“Every time a patient relapses, it means induction therapy and high doses of prednisone are started all over again,” she said. “Multiple relapses are associated with a very high rate of complications. That’s what we’re trying to avoid through successful maintenance therapy.”
Which agent is best for maintenance? Research has suggested that there’s little difference in relapse rates between methotrexate and azathioprine, while mycophenolate mofetil was linked to more relapses than was azathioprine. Other research has found that rituximab (Rituxan) is superior to azathioprine at sustaining remission and overall survival (N Engl J Med. 2014 Nov 6;371[19]:1771-80).
Questions about rituximab remain, Dr. Villa-Forte said: Can remission be sustained with fewer infusions? Would higher doses produce long-term benefit after therapy is halted without boosting toxicity?
Regardless of the drug used, relapses from remission are still frequent, suggesting the need to focus on special treatment for patients at higher risk for relapse, she said.
For now, she gave these tips about how to treat patients in remission:
• Reduce treatment toxicity (it’s “significant over time and not better with rituximab”) and focus on disease activity and complications.
• Take regular labs and get a fresh urine sediment analysis with every visit. In some cases, the urine analysis in patients who seem healthy will reveal hematuria, a sign of nephritis. Then, “you have to make a decision about changing the treatment just by looking at the urine.”
• Pay attention to the risk of thrombosis.
• Put patients on Pneumocystis carinii pneumonia prophylaxis even if they’re only on a bit of prednisone.
• Be aware of risk factors for relapse, including a history of at least one relapse and stopping therapy before 48 months.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Cleveland Clinic rheumatologist Alexandra Villa-Forte, MD, MPH, has a vivid way of describing the challenge of maintaining remission in granulomatosis with polyangiitis (GPA): She called it “the worst nightmare in the clinic” in a presentation at the annual Perspectives in Rheumatic Diseases.
Dr. Villa-Forte should know. She’s treated more than 700 patients with GPA, also known as Wegener’s granulomatosis, a rare disorder that causes necrotizing vasculitis of small arteries and veins. She advises close monitoring of patients in remission, caution regarding toxicity, pneumonia prophylaxis, and an understanding of the limitations of existing medications.
An estimated 3 in 100,000 people have GPA, which can be fatal if it’s not treated. “All patients with active GPA should receive prednisone with a second agent,” she said at the conference, held by Global Academy for Medical Education. “We know that increasing the prednisone or using it alone will not result in sustained remission at all.”
According to her, new concepts in therapeutic guidance suggest four criteria should determine the specific agents used: Involvement of critical organ systems, severity of clinical manifestations, rate of change (such as rapidly progressing kidney failure), and individual patient factors such as comorbidities.
Patients facing immediate risk to life or organ function should get higher doses of corticosteroids, a cytotoxic agent, and perhaps plasmapheresis, she said.
Induction therapy should last 3-6 months, followed by maintenance therapy that keeps the condition at bay, Dr. Villa-Forte said. But things do not always go according to plan.
“The main challenge that we encounter is that relapses still occur during treatment. The patient could be on appropriate treatment and appropriate doses and they still relapse,” she said. “The second challenge is that patients who come off treatment have a very high rate of relapse.”
Research suggests that up to 70% of patients off medication relapse by 18 months, and 15%-35% of patients on medication relapse by 12-18 months, Dr. Villa-Forte said.
“Every time a patient relapses, it means induction therapy and high doses of prednisone are started all over again,” she said. “Multiple relapses are associated with a very high rate of complications. That’s what we’re trying to avoid through successful maintenance therapy.”
Which agent is best for maintenance? Research has suggested that there’s little difference in relapse rates between methotrexate and azathioprine, while mycophenolate mofetil was linked to more relapses than was azathioprine. Other research has found that rituximab (Rituxan) is superior to azathioprine at sustaining remission and overall survival (N Engl J Med. 2014 Nov 6;371[19]:1771-80).
Questions about rituximab remain, Dr. Villa-Forte said: Can remission be sustained with fewer infusions? Would higher doses produce long-term benefit after therapy is halted without boosting toxicity?
Regardless of the drug used, relapses from remission are still frequent, suggesting the need to focus on special treatment for patients at higher risk for relapse, she said.
For now, she gave these tips about how to treat patients in remission:
• Reduce treatment toxicity (it’s “significant over time and not better with rituximab”) and focus on disease activity and complications.
• Take regular labs and get a fresh urine sediment analysis with every visit. In some cases, the urine analysis in patients who seem healthy will reveal hematuria, a sign of nephritis. Then, “you have to make a decision about changing the treatment just by looking at the urine.”
• Pay attention to the risk of thrombosis.
• Put patients on Pneumocystis carinii pneumonia prophylaxis even if they’re only on a bit of prednisone.
• Be aware of risk factors for relapse, including a history of at least one relapse and stopping therapy before 48 months.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Cleveland Clinic rheumatologist Alexandra Villa-Forte, MD, MPH, has a vivid way of describing the challenge of maintaining remission in granulomatosis with polyangiitis (GPA): She called it “the worst nightmare in the clinic” in a presentation at the annual Perspectives in Rheumatic Diseases.
Dr. Villa-Forte should know. She’s treated more than 700 patients with GPA, also known as Wegener’s granulomatosis, a rare disorder that causes necrotizing vasculitis of small arteries and veins. She advises close monitoring of patients in remission, caution regarding toxicity, pneumonia prophylaxis, and an understanding of the limitations of existing medications.
An estimated 3 in 100,000 people have GPA, which can be fatal if it’s not treated. “All patients with active GPA should receive prednisone with a second agent,” she said at the conference, held by Global Academy for Medical Education. “We know that increasing the prednisone or using it alone will not result in sustained remission at all.”
According to her, new concepts in therapeutic guidance suggest four criteria should determine the specific agents used: Involvement of critical organ systems, severity of clinical manifestations, rate of change (such as rapidly progressing kidney failure), and individual patient factors such as comorbidities.
Patients facing immediate risk to life or organ function should get higher doses of corticosteroids, a cytotoxic agent, and perhaps plasmapheresis, she said.
Induction therapy should last 3-6 months, followed by maintenance therapy that keeps the condition at bay, Dr. Villa-Forte said. But things do not always go according to plan.
“The main challenge that we encounter is that relapses still occur during treatment. The patient could be on appropriate treatment and appropriate doses and they still relapse,” she said. “The second challenge is that patients who come off treatment have a very high rate of relapse.”
Research suggests that up to 70% of patients off medication relapse by 18 months, and 15%-35% of patients on medication relapse by 12-18 months, Dr. Villa-Forte said.
“Every time a patient relapses, it means induction therapy and high doses of prednisone are started all over again,” she said. “Multiple relapses are associated with a very high rate of complications. That’s what we’re trying to avoid through successful maintenance therapy.”
Which agent is best for maintenance? Research has suggested that there’s little difference in relapse rates between methotrexate and azathioprine, while mycophenolate mofetil was linked to more relapses than was azathioprine. Other research has found that rituximab (Rituxan) is superior to azathioprine at sustaining remission and overall survival (N Engl J Med. 2014 Nov 6;371[19]:1771-80).
Questions about rituximab remain, Dr. Villa-Forte said: Can remission be sustained with fewer infusions? Would higher doses produce long-term benefit after therapy is halted without boosting toxicity?
Regardless of the drug used, relapses from remission are still frequent, suggesting the need to focus on special treatment for patients at higher risk for relapse, she said.
For now, she gave these tips about how to treat patients in remission:
• Reduce treatment toxicity (it’s “significant over time and not better with rituximab”) and focus on disease activity and complications.
• Take regular labs and get a fresh urine sediment analysis with every visit. In some cases, the urine analysis in patients who seem healthy will reveal hematuria, a sign of nephritis. Then, “you have to make a decision about changing the treatment just by looking at the urine.”
• Pay attention to the risk of thrombosis.
• Put patients on Pneumocystis carinii pneumonia prophylaxis even if they’re only on a bit of prednisone.
• Be aware of risk factors for relapse, including a history of at least one relapse and stopping therapy before 48 months.
Dr. Villa-Forte had no relevant disclosures.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
Stop treating gout and start curing it, physician urges
LAS VEGAS – Brian F. Mandell, MD, PhD, of Cleveland Clinic, has a message about one of the most devastating conditions that rheumatologists see: Gout isn’t just a treatable disease. It’s a curable one.
Still, research shows time and time again that physicians manage gout “horrendously,” he told colleagues at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “The problem really lies with us,” he said. “We need to do a better job.”
At issue, he believes, is a failure to consider the basic workings of gout when making treatment decisions and advising patients. Lowering serum uric acid (SUA) via medication works, he said, but physicians too frequently don’t go far enough with this approach.
Gout appears to be on the rise in the United States, reflecting increases in related conditions such as obesity and diabetes. A study published this year found that the rate of new-onset gout more than doubled in Olmsted County, Minn., from 1989-1992 to 2009-2010, reaching an adjusted rate of 137/100,000 (J Rheumatol. 2018 Apr;45[4]:574-9).
According to Dr. Mandell, various mysteries regarding gout still need to be cleared up. For one, does resolution of gout also resolve conditions related to hyperuricemia, such as onset of hypertension, progressive chronic kidney disease, and nonalcoholic fatty liver along with higher all-cause mortality?
“We don’t know from interventional studies whether these are as reversible as the gouty arthritis,” he said.
It’s also unknown why so many hyperuricemic patients don’t get flares, with one study estimating that about 50% don’t get them over 15 years (Arthritis Rheumatol. 2017;69[Suppl 10]: Abstract 2843).
One fascinating theory, Dr. Mandell said, suggests “the microbiome is playing a huge [role] in the body’s response to deposits of crystals.”
Fortunately, he said, other mysteries about gout are being solved.
It’s now clear that lowering SUA below 6 mg/dL with medication will reduce flares, Dr. Mandell said. He pointed to a 2017 study of 314 patients with early gout that found 63% of patients who took febuxostat (Uloric) lowered their SUA below 6 mg/dL, compared with just 6% of the placebo group. The overall percentage of patients who had at least one gout flare over 2 years was 29% in the febuxostat group vs. 41% in the placebo group (Arthritis Rheumatol. 2017;69[12]:2386‐95).
It’s also clear that maintenance of lower SUA levels is crucial to prevent recurrence, Dr. Mandell said.
So why is management of hyperuricemia so poor? He ticked off various possible explanations: Maybe it’s the medications. Or perhaps patient compliance is low.
But the drugs are fine, he said, although he cautioned that too-rapid lowering of SUA levels can provoke attacks. He pointed to a 2014 study that suggests allopurinol can help nearly all patients get their SUA below 6 mg/dL, and in the study, the drug was “generally well tolerated” (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
As for compliance, Dr. Mandell said, it can be boosted by patient education. The problem, he said, is that physicians are failing patients by not up-titrating allopurinol despite evidence that this approach works.
He added that hyperuricemia can be managed even in patients on diuretic therapy (Arthritis Res Ther. 2018;20:53).
What about patients who are intolerant to allopurinol or don’t fully respond to it on the SUA front? Dr. Mandell said he likes to try febuxostat, although he noted that it’s tremendously more expensive than allopurinol in the United States with a price that could be 10 times higher.
The nonscored design of febuxostat pills makes dose adjustment difficult in patients, he said, and there are concerns about heart-related and all-cause deaths.
Lesinurad (Zurampic) may be helpful for patients with hyperuricemia that doesn’t response to high doses of xanthine oxidase inhibitors (XOI) or if they’re intolerant to lower inadequate doses, he said. Avoid the drug in patients with chronic kidney disease, he cautioned, and be aware that it’s not approved as a monotherapy. Instead, it’s approved by the Food and Drug Administration for use with an XOI.
As for other gout issues, Dr. Mandell said pegloticase (Krystexxa) via infusion can help patients who don’t respond to an XOI but infusion reactions can occur (mainly in nonresponders), and it’s extremely expensive (about $20,000 per month).
He added that anti–IL-1 therapy is effective in hospitalized patients with gout and doesn’t exacerbate other conditions.
Dr. Mandell disclosed various links to drug makers that produce treatments for gout. He has served as clinical investigator for Horizon, has been a consultant to AstraZeneca, Ironwood, and Horizon, and has received honoraria (unrestricted grants) for continuing medical education activities from Takeda and Horizon. He also reported soliciting advertisements for a journal and educational grants for CME activities.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Brian F. Mandell, MD, PhD, of Cleveland Clinic, has a message about one of the most devastating conditions that rheumatologists see: Gout isn’t just a treatable disease. It’s a curable one.
Still, research shows time and time again that physicians manage gout “horrendously,” he told colleagues at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “The problem really lies with us,” he said. “We need to do a better job.”
At issue, he believes, is a failure to consider the basic workings of gout when making treatment decisions and advising patients. Lowering serum uric acid (SUA) via medication works, he said, but physicians too frequently don’t go far enough with this approach.
Gout appears to be on the rise in the United States, reflecting increases in related conditions such as obesity and diabetes. A study published this year found that the rate of new-onset gout more than doubled in Olmsted County, Minn., from 1989-1992 to 2009-2010, reaching an adjusted rate of 137/100,000 (J Rheumatol. 2018 Apr;45[4]:574-9).
According to Dr. Mandell, various mysteries regarding gout still need to be cleared up. For one, does resolution of gout also resolve conditions related to hyperuricemia, such as onset of hypertension, progressive chronic kidney disease, and nonalcoholic fatty liver along with higher all-cause mortality?
“We don’t know from interventional studies whether these are as reversible as the gouty arthritis,” he said.
It’s also unknown why so many hyperuricemic patients don’t get flares, with one study estimating that about 50% don’t get them over 15 years (Arthritis Rheumatol. 2017;69[Suppl 10]: Abstract 2843).
One fascinating theory, Dr. Mandell said, suggests “the microbiome is playing a huge [role] in the body’s response to deposits of crystals.”
Fortunately, he said, other mysteries about gout are being solved.
It’s now clear that lowering SUA below 6 mg/dL with medication will reduce flares, Dr. Mandell said. He pointed to a 2017 study of 314 patients with early gout that found 63% of patients who took febuxostat (Uloric) lowered their SUA below 6 mg/dL, compared with just 6% of the placebo group. The overall percentage of patients who had at least one gout flare over 2 years was 29% in the febuxostat group vs. 41% in the placebo group (Arthritis Rheumatol. 2017;69[12]:2386‐95).
It’s also clear that maintenance of lower SUA levels is crucial to prevent recurrence, Dr. Mandell said.
So why is management of hyperuricemia so poor? He ticked off various possible explanations: Maybe it’s the medications. Or perhaps patient compliance is low.
But the drugs are fine, he said, although he cautioned that too-rapid lowering of SUA levels can provoke attacks. He pointed to a 2014 study that suggests allopurinol can help nearly all patients get their SUA below 6 mg/dL, and in the study, the drug was “generally well tolerated” (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
As for compliance, Dr. Mandell said, it can be boosted by patient education. The problem, he said, is that physicians are failing patients by not up-titrating allopurinol despite evidence that this approach works.
He added that hyperuricemia can be managed even in patients on diuretic therapy (Arthritis Res Ther. 2018;20:53).
What about patients who are intolerant to allopurinol or don’t fully respond to it on the SUA front? Dr. Mandell said he likes to try febuxostat, although he noted that it’s tremendously more expensive than allopurinol in the United States with a price that could be 10 times higher.
The nonscored design of febuxostat pills makes dose adjustment difficult in patients, he said, and there are concerns about heart-related and all-cause deaths.
Lesinurad (Zurampic) may be helpful for patients with hyperuricemia that doesn’t response to high doses of xanthine oxidase inhibitors (XOI) or if they’re intolerant to lower inadequate doses, he said. Avoid the drug in patients with chronic kidney disease, he cautioned, and be aware that it’s not approved as a monotherapy. Instead, it’s approved by the Food and Drug Administration for use with an XOI.
As for other gout issues, Dr. Mandell said pegloticase (Krystexxa) via infusion can help patients who don’t respond to an XOI but infusion reactions can occur (mainly in nonresponders), and it’s extremely expensive (about $20,000 per month).
He added that anti–IL-1 therapy is effective in hospitalized patients with gout and doesn’t exacerbate other conditions.
Dr. Mandell disclosed various links to drug makers that produce treatments for gout. He has served as clinical investigator for Horizon, has been a consultant to AstraZeneca, Ironwood, and Horizon, and has received honoraria (unrestricted grants) for continuing medical education activities from Takeda and Horizon. He also reported soliciting advertisements for a journal and educational grants for CME activities.
Global Academy for Medical Education and this news organization are owned by the same parent company.
LAS VEGAS – Brian F. Mandell, MD, PhD, of Cleveland Clinic, has a message about one of the most devastating conditions that rheumatologists see: Gout isn’t just a treatable disease. It’s a curable one.
Still, research shows time and time again that physicians manage gout “horrendously,” he told colleagues at the annual Perspectives in Rheumatic Diseases held by Global Academy for Medical Education. “The problem really lies with us,” he said. “We need to do a better job.”
At issue, he believes, is a failure to consider the basic workings of gout when making treatment decisions and advising patients. Lowering serum uric acid (SUA) via medication works, he said, but physicians too frequently don’t go far enough with this approach.
Gout appears to be on the rise in the United States, reflecting increases in related conditions such as obesity and diabetes. A study published this year found that the rate of new-onset gout more than doubled in Olmsted County, Minn., from 1989-1992 to 2009-2010, reaching an adjusted rate of 137/100,000 (J Rheumatol. 2018 Apr;45[4]:574-9).
According to Dr. Mandell, various mysteries regarding gout still need to be cleared up. For one, does resolution of gout also resolve conditions related to hyperuricemia, such as onset of hypertension, progressive chronic kidney disease, and nonalcoholic fatty liver along with higher all-cause mortality?
“We don’t know from interventional studies whether these are as reversible as the gouty arthritis,” he said.
It’s also unknown why so many hyperuricemic patients don’t get flares, with one study estimating that about 50% don’t get them over 15 years (Arthritis Rheumatol. 2017;69[Suppl 10]: Abstract 2843).
One fascinating theory, Dr. Mandell said, suggests “the microbiome is playing a huge [role] in the body’s response to deposits of crystals.”
Fortunately, he said, other mysteries about gout are being solved.
It’s now clear that lowering SUA below 6 mg/dL with medication will reduce flares, Dr. Mandell said. He pointed to a 2017 study of 314 patients with early gout that found 63% of patients who took febuxostat (Uloric) lowered their SUA below 6 mg/dL, compared with just 6% of the placebo group. The overall percentage of patients who had at least one gout flare over 2 years was 29% in the febuxostat group vs. 41% in the placebo group (Arthritis Rheumatol. 2017;69[12]:2386‐95).
It’s also clear that maintenance of lower SUA levels is crucial to prevent recurrence, Dr. Mandell said.
So why is management of hyperuricemia so poor? He ticked off various possible explanations: Maybe it’s the medications. Or perhaps patient compliance is low.
But the drugs are fine, he said, although he cautioned that too-rapid lowering of SUA levels can provoke attacks. He pointed to a 2014 study that suggests allopurinol can help nearly all patients get their SUA below 6 mg/dL, and in the study, the drug was “generally well tolerated” (Semin Arthritis Rheum. 2014 Aug;44[1]:25-30).
As for compliance, Dr. Mandell said, it can be boosted by patient education. The problem, he said, is that physicians are failing patients by not up-titrating allopurinol despite evidence that this approach works.
He added that hyperuricemia can be managed even in patients on diuretic therapy (Arthritis Res Ther. 2018;20:53).
What about patients who are intolerant to allopurinol or don’t fully respond to it on the SUA front? Dr. Mandell said he likes to try febuxostat, although he noted that it’s tremendously more expensive than allopurinol in the United States with a price that could be 10 times higher.
The nonscored design of febuxostat pills makes dose adjustment difficult in patients, he said, and there are concerns about heart-related and all-cause deaths.
Lesinurad (Zurampic) may be helpful for patients with hyperuricemia that doesn’t response to high doses of xanthine oxidase inhibitors (XOI) or if they’re intolerant to lower inadequate doses, he said. Avoid the drug in patients with chronic kidney disease, he cautioned, and be aware that it’s not approved as a monotherapy. Instead, it’s approved by the Food and Drug Administration for use with an XOI.
As for other gout issues, Dr. Mandell said pegloticase (Krystexxa) via infusion can help patients who don’t respond to an XOI but infusion reactions can occur (mainly in nonresponders), and it’s extremely expensive (about $20,000 per month).
He added that anti–IL-1 therapy is effective in hospitalized patients with gout and doesn’t exacerbate other conditions.
Dr. Mandell disclosed various links to drug makers that produce treatments for gout. He has served as clinical investigator for Horizon, has been a consultant to AstraZeneca, Ironwood, and Horizon, and has received honoraria (unrestricted grants) for continuing medical education activities from Takeda and Horizon. He also reported soliciting advertisements for a journal and educational grants for CME activities.
Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES