User login
The removal of the multiple-kilogram uterus using MIGSs
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
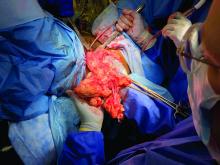
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
It has now been 30 years since the first total laparoscopic hysterectomy was performed. The benefits of minimally invasive gynecologic surgery (MIGS) – and of minimally invasive hysterectomy specifically – are now well documented. Since this milestone procedure, both instrumentation and technique have improved significantly.
This includes traditional laparoscopy, as well as the robotically assisted laparoscopic approach. However, certain patient characteristics also may influence the choice. A uterus that is undescended, combined with a narrow introitus, for instance, can be a contributory factor in choosing to perform laparoscopic hysterectomy. Additionally, so can an extremely large uterus and an extremely high body mass index (BMI).
These latter two factors – a very large uterus (which we define as more than 15-16 weeks’ gestational size) and a BMI over 60 kg/m2 – historically were considered to be contraindications to laparoscopic hysterectomy. But as the proficiency, comfort, and skill of a new generation of laparoscopic surgeons increases, the tide is shifting with respect to both morbid obesity and the very large uterus.
With growing experience and improved instrumentation, the majority of gynecologists who are fellowship-trained in MIGS are able to routinely and safely perform laparoscopic hysterectomy for uteri weighing 1-2 kg and in patients who have extreme morbid obesity. The literature, moreover, increasingly features case reports of laparoscopic removal of very large uteri and reviews/discussions of total laparoscopic hysterectomy being feasible.
In our own experience, total laparoscopic hysterectomy (TLH) of the very large uterus can be safely and advantageously performed using key instruments and refinements in technique, as well as thorough patient counseling regarding the risk of unexpected sarcomas. Recently, we safely performed total laparoscopic hysterectomy for a patient with a uterus that – somewhat unexpectedly – weighed 7.4 kg.
Surgical pearls
Performing safe and effective total laparoscopic hysterectomy for large uteri – and for morbidly obese patients – hinges largely on modifications in entry and port placement, patient positioning, and choice of instrumentation. With these modifications, we can achieve adequate visualization of critical anatomy and can minimize bleeding. Otherwise, the surgery itself is largely the same. Here are the principles we find most helpful.
Entry and port placement
Traditionally, for TLHs, a camera port is placed at the umbilicus to provide a full view of the pelvis. For the larger uterus – and in women who are extremely obese – we aim to introduce the laparoscope higher. A reliable landmark is the Palmer’s point in the left upper quadrant. From here, we can identify areas for the placement of additional trocars.
In general, we place ancillary 5-mm ports more cephalad and lateral to the uterus than we otherwise would. Such placement facilitates effective visualization while accommodating manipulation of the uterus and allows us to avoid bleeding around the vascular upper pedicles. Overall, we have much better control through all parts of the surgery when we operate lateral to the uterus.
Patient positioning
In addition to the Trendelenburg position, we have adopted an “airplaning” technique for patients with a very large uterus in which the bed is tilted from side to side so that the left and right sides of the body are rotated upward as needed. This allows for gravitational-assisted retraction when it otherwise is not possible.
Instrumentation
For morbidly obese patients, we use Kii Fios advanced fixation trocars. These come in 5- and 10-mm sizes and are equipped with an intraperitoneal balloon that can be inflated to prevent sliding of the trocar out of the abdominal wall.
By far the most valuable instrument for the morbidly obese and the very large uterus is a 30-degree laparoscope. With our higher port placement as described, the 30-degree scope provides visualization of critical structures that wouldn’t be possible with a 0-degree scope.
The Rumi uterine manipulator comes with cups that come in different sizes and can fit around the cervix and help delineate the cervicouterine junction. We use this manipulator for all laparoscopic hysterectomies, but it is a must for the very large uterus.
Extensive desiccation of the utero-ovarian pedicles and uterine arteries is critical, and for this we advise using the rotating bipolar RoBi instrument. Use of the conventional bipolar instrument allows us to use targeted and anatomically guided application of energy. This ensures certainty that vessels whose limits exceed the diameter for advanced bipolar devices (typically 7 mm) are completely sealed. In-depth knowledge of pelvic anatomy and advanced laparoscopic dissection is paramount during these steps to ensure that vital structures are not damaged by the wider thermal spread of the traditional bipolar device. For cutting, the use of ultrasonic energy is important to prevent energy from spreading laterally.
Lastly, we recommend a good suction irrigator because, if bleeding occurs, it tends to be heavy because of the enlarged nature of feeding vasculature. When placed through an umbilical or suprapubic port, the suction irrigator also may be used to help with the rotational vectors and traction for further uterine manipulation.
Technique
We usually operate from top to bottom, transecting the upper pedicles such as the infundibulopelvic (IP) ligaments or utero-ovarian ligaments first, rather than the round ligaments. This helps us achieve additional mobility of the uterus. Some surgeons believe that retroperitoneal dissection and ligation of the uterine arteries at their origin is essential, but we find that, with good uterine manipulation and the use of a 30-degree scope, we achieve adequate visualization for identifying the ureter and uterine artery on the sidewall and consequently do not need to dissect retroperitoneally.
When using the uterine manipulator with the colpotomy cup, the uterus is pushed upward, increasing the distance between the vaginal fornix and the ureters. Uterine arteries can easily be identified and desiccated using conventional bipolar energy. When the colpotomy cup is pushed cephalad, the application of the bipolar energy within the limits of the cup is safe. The thermal spread does not pose a threat to the ureters, which are displaced 1.5-2 cm laterally. Large fibroids often contribute to distorted anatomical planes, and a colpotomy cup provides a firm palpable surface between the cervix and vagina during dissection.
When dealing with large uteri, one must sometimes think outside the box and deviate from standard technique. For instance, in patients with distorted anatomy because of large fibroids, it helps to first control the pedicles that are most easily accessible. Sometimes it is acceptable to perform oophorectomy if the IP ligament is more accessible and the utero-ovarian pedicle is distorted by dilated veins and adherent to the uterus. After transection of each pedicle, we gain more mobility of the uterus and better visualization for the next step.
Inserting the camera through ancillary ports – a technique known as “port hopping” – helps to visualize and take down adhesions much better and more safely than using the camera from the umbilical port only. Port hopping with a 30-degree laparoscope helps to obtain a 360-degree view of adhesions and anatomy, which is exceedingly helpful in cases in which crucial anatomical structures are within close proximity of one another.
In general it is more challenging to perform TLH on a patient with a broad uterus or a patient with low posterior fibroids that are occupying the pelvis than on a patient with fibroids in the upper abdomen. The main challenge for the surgeon is to safely secure the uterine arteries and control the blood supply to the uterus.
Access to the pelvic sidewall is obtained with the combination of 30-degree scope, uterine manipulator, and the suction irrigator introduced through the midline port; the cervix and uterus are deviated upward. Instead of the suction irrigator or blunt dissector used for internal uterine manipulation, some surgeons use myoma screws or a 5-mm single-tooth tenaculum to manipulate a large uterus. Both of those instruments are valuable and work well, but often a large uterus requires extensive manipulation. Repositioning of any sharp instruments that pierce the serosa can often lead to additional blood loss. It is preferable to avoid this blood loss on a large uterus at all costs because it can be brisk and stains the surgical pedicles, making the remainder of the procedure unnecessarily difficult.
Once the uterine arteries are desiccated, if fibroids are obscuring the view, the corpus of the uterus can be detached from the cervix as in supracervical hysterectomy fashion. From there, the uterus can be placed in the upper abdomen while colpotomy can be performed.
In patients with multiple fibroids, we do not recommend performing myomectomy first, unless the fibroid is pedunculated and on a very small stalk. Improved uterine manipulation and retroperitoneal dissection are preferred over myomectomy to safely complete hysterectomy for the broad uterus. In our opinion, any attempt at myomectomy would lead to unnecessary blood loss and additional operative time with minimal benefit.
In patients with fibroids that grow into the broad ligament and pelvic sidewall, the natural course of the ureter becomes displaced laterally. This is contrary to the popular misconception that the ureter is more medially located in the setting of broad-ligament fibroids. To ensure safe access to the uterine arteries, the vesicouterine peritoneum can be incised and extended cephalad along the broad ligament and, then, using the above-mentioned technique, by pushing the uterus and the fibroid to the contralateral side via the suction irrigator, the uterine arteries can be easily accessed.
Another useful technique is to use diluted vasopressin injected into the lower pole of the uterus to cause vasoconstriction and minimize the bleeding. The concentration is 1 cc of 20 units of vasopressin in 100-400 cc of saline. This technique is very useful for myomectomies, and some surgeons find it also helpful for hysterectomy. The plasma half-life of vasopressin is 10-20 minutes, and a large quantity is needed to help with vasoconstriction in a big uterus. The safe upper limits of vasopressin dosing are not firmly established. A fibroid uterus with aberrant vasculature may require a greater-than-acceptable dose to control bleeding.
It is important to ensure that patients have an optimized hemoglobin level preoperatively. We use a hemoglobin level of 8 g/dL as a lowest cutoff value for performing TLH without preoperative transfusion. Regarding bowel preparation, neither the literature nor our own experience support its value, so we typically do not use it.
Morcellation and patient counseling
Uteri up to 12 weeks’ gestational size usually can be extracted transvaginally, and most uteri regardless of size can be morcellated and extracted through the vagina, providing that the vaginal fornix is accessible from below. In some cases, such as when the apex is too high, a minilaparotomic incision is needed to extract the uterus, or when available, power morcellation can be performed.
A major challenge, given our growing ability to laparoscopically remove very larger uteri, is that uteri heavier than about 2.5 kg in weight cannot be morcellated inside a morcellation bag. The risk of upstaging a known or suspected uterine malignancy, or of spreading an unknown malignant sarcoma (presumed benign myoma), should be incorporated in each patient’s decision making.
Thorough counseling about surgical options and on the risks of morcellating a very large uterus without containment in a bag is essential. Each patient must understand the risks and decide whether the benefits of minimally invasive surgery outweigh these risks. While MRI can sometimes provide increased suspicion of a leiomyosarcoma, malignancy can never be completed excluded preoperatively.
Removal of a 7.4-kg uterus
Our patient was a 44-year-old with a markedly enlarged fibroid uterus. Having been told by other providers that she was not a candidate for minimally invasive hysterectomy, she had delayed surgical management for a number of years, allowing for such a generous uterine size to develop.
The patient was knowledgeable about her condition and, given her comorbid obesity, she requested a minimally invasive approach. Preoperative imaging included an ultrasound, which had to be completed abdominally because of the size of her uterus, and an additional MRI was needed to further characterize the extent and nature of her uterus. A very detailed discussion regarding risk of leiomyosarcoma, operative complications, and conversion to laparotomy ensued.
Intraoperatively, we placed the first 5 mm port in the left upper quadrant initially to survey the anatomy for feasibility of laparoscopic hysterectomy. The left utero-ovarian pedicle was easily viewed by airplaning the bed alone. While the right utero-ovarian pedicle was much more skewed and enlarged, the right IP was easily accessible and the ureter well visualized.
The decision was made to place additional ports and proceed with laparoscopic hysterectomy. The 5-mm assistant ports were placed lateral and directly above the upper vascular pedicles. Operative time was 4 hours and 12 minutes, and blood loss was only 700 cc. Her preoperative hemoglobin was optimized at 13.3 g/dL and dropped to 11.3 g/dL postoperatively. The patient was discharged home the next morning and had a normal recovery with no complications.
Dr. Pasic is professor of obstetrics, gynecology & women’s health; director of the section of advanced gynecologic endoscopy; and codirector of the AAGL fellowship in minimally invasive gynecologic surgery at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Cesta is Dr. Pasic’s current fellow in minimally invasive gynecologic surgery as well as an instructor in obstetrics and gynecology at the University of Louisville. Dr. Pasic disclosed he is a consultant for Ethicon Endo, Medtronic, and Olympus and is a speaker for Cooper Surgical, which manufactures some of the instruments mentioned in this article. Dr. Cesta had no relevant financial disclosures.
Safely pushing the limits of MIGS surgery
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at [email protected].
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at [email protected].
In his excellent treatise on the history of hysterectomy, Chris Sutton, MBBch, noted that, while Themison of Athens in 50 bc and Soranus of Ephesus in 120 ad were reported to have performed vaginal hysterectomy, these cases essentially were emergency amputation of severely prolapsed uteri, which usually involved cutting both ureters and the bladder (J Minim Invas Gynecol. 2010 Jul;17[4]:421–35). It was not until 1801 that the first planned elective vaginal hysterectomy was performed, and it was not until the mid 19th century, in 1853, that Walter Burnham, MD, in Lowell, Mass., performed the first abdominal hysterectomy resulting in patient survival.
Seemingly incredible, it was only 125 years later, in autumn of 1988 at William Nesbitt Memorial Hospital in Kingston, Pa., that Harry Reich, MD, performed the first total laparoscopically assisted hysterectomy.
Since Dr. Reich’s groundbreaking procedure, the performance of laparoscopic hysterectomy has advanced at a feverish pace. In my own practice, I have not performed an abdominal hysterectomy since 1998. My two partners, who are both fellowship-trained in minimally invasive gynecologic surgery (MIGS), Aarathi Cholkeri-Singh, MD, who joined my practice in 2007, and Kristen Sasaki, MD, who joined my practice in 2014, have never performed an open hysterectomy since starting practice. Despite these advances, One of the most difficult situations is the truly large uterus – greater than 2,500 grams.
For this edition of the Master Class in Gynecologic Surgery, I have enlisted the assistance of Paya Pasic, MD, and Megan Cesta, MD, to discuss the next frontier: the removal of the multiple kilogram uterus.
Dr. Pasic is an internationally recognized leader in laparoscopic MIGS. He is professor of obstetrics, gynecology & women’s health; director, section of advanced gynecologic endoscopy, and codirector of the AAGL fellowship in MIGS at the University of Louisville (Ky.). Dr. Pasic is the current president of the International Society of Gynecologic Endoscopy. He is also a past president of the AAGL (2009). Dr. Pasic is published in the field of MIGS, having authored many publications, book chapters, monographs, and textbooks.
Dr. Cesta is Dr. Pasic’s current fellow in MIGS and an instructor in obstetrics and gynecology at the university.
It is truly a pleasure to welcome Dr. Pasic and Dr. Cesta to this edition of the Master Class in Gynecologic Surgery.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. He has no disclosures relevant to this Master Class. Email him at [email protected].
Performing gender-reaffirming surgery: Guidelines for the general ob.gyn.
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
According to the DSM-V, gender dysphoria in adolescents and adults “involves a difference between one’s experienced/expressed gender and assigned gender, and significant distress or problems functioning. It lasts at least 6 months,” and several other criteria must be met.1 Many patients with gender dysphoria also identify as transgender. A “transition” or “transitioning” is a process by which individuals come to inhabit their gender identity.2 A gender transition may take many forms, and only some people will choose to include medical assistance in their transition process. Although the scope of this article will not address these concerns, it should be noted that many people in the transgender and gender nonconforming community would object to the concepts of gender dysphoria and gender transition because they rely on a binary model of gender that may exclude individuals that see themselves as something other than “man or woman.”
There are both medical and surgical options for medical assistance in a gender transition. This article will focus on the surgical care of patients assigned female at birth who are seeking masculinizing surgical therapy. Many writers will discuss “gender-affirming” surgery, but we will use the term “gender-reaffirming” surgery because transgender patients have already affirmed their own genders and do not require surgery to inhabit this affirmation. Surgical options might include bilateral mastectomy, hysterectomy, bilateral salpingo-oophorectomy (BSO), metoidioplasty (surgical formation of a neophallus with existing genital tissue), or phalloplasty. There currently is no single surgical subspecialty that encompasses training in all forms of gender-reaffirming surgical therapies. In some areas of the country, centers of excellence have given rise to multidisciplinary teams that combine the skill sets of surgical subspecialists to provide a streamlined approach to gender-reaffirming surgery. Because of the scarcity of these integrated centers, most patients seeking gender-reaffirming surgeries will need to find individual subspecialists whose surgical training focuses on one area of the body. For example, patients seeking all possible surgical options may need a breast surgeon to perform their mastectomy, an ob.gyn. to perform their hysterectomy and BSO, a urologist to perform their metoidioplasty, and a plastic surgeon to perform their phalloplasty. In these scenarios,
There are many reasons why transgender men might desire hysterectomy/BSO as part of their transition. Removal of the uterus and cervix eliminates concerns surrounding menstruation, pregnancy, and cervical cancer screening, all of which may add to their experience of gender dysphoria. Furthermore, removal of the ovaries may simplify long-term hormonal therapy with testosterone by eliminating the need for estrogen suppression. Lastly, a hysterectomy/BSO is a lower-risk and more cost-effective masculinizing surgery, compared with metoidioplasty or phalloplasty.
While the technical aspect of performing a hysterectomy/BSO certainly is within the scope of training for a general ob.gyn., there are several nuances of which providers should be aware when planning gender-reaffirming surgery for a transgender man. During the preoperative planning phase, it is of utmost importance to provide an environment of safety so that the focus of the preop visit is not clouded by communication mishaps between office staff and the patient. These barriers can be avoided by implementing office intake forms that give patients the opportunity to inform the health care team of their chosen name and personal pronouns upon registration for the visit.
A pelvic exam is commonly performed by ob.gyns. to determine surgical approach for a hysterectomy/BSO. When approaching transgender male patients for preoperative pelvic exams, it is important to be mindful of the fact that this type of exam may trigger gender dysphoria. While pelvic exams should be handled in sensitive fashion regardless of a patient’s gender identity, a patient who is a transgender man may benefit from some added steps in discussing the pelvic exam. One approach is to acknowledge that these exams/discussions may be especially triggering of gender dysphoria, and ask if the patient would prefer certain words to be used or not used in reference to their anatomy. As with any patient, the provider should explain the purpose of the examination and offer opportunities for the patient to have some control in the exam such as by assisting with insertion of the speculum or designating a “safe word” that would signal the provider to stop or pause the exam. In some cases, patients may not be able to tolerate the pelvic exam while awake because of the degree of gender dysphoria that the exam would induce. Providers might consider noninvasive imaging studies to help with surgical planning if they find they need more information before scheduling the operation, or they may offer a staged procedure with exam under anesthesia prior to the definitive surgery.
In conclusion, performing a gender-reaffirming hysterectomy/BSO requires thoughtful preparation to ensure a safe surgical environment for this vulnerable population. Care should be taken to plan the operation with a culturally sensitive approach.
Dr. Joyner is an assistant professor at Emory University, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital, both in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no relevant financial disclosures.
References
1. American Psychiatric Association. What is Gender Dysphoria? https://www.psychiatry.org/patients-families/gender-dysphoria/what-is-gender-dysphoria
2. UCSF Transgender Care. Transition Roadmap. https://transcare.ucsf.edu/transition-roadmap
Molar pregnancy: The next steps after diagnosis
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Molar pregnancy is an uncommon but serious condition that affects young women of reproductive age. The diagnosis and management of molar pregnancy is familiar to most gynecologists. However, in the days and weeks following evacuation of molar pregnancy, clinicians face a critical time period in which they must be vigilant for the development of postmolar gestational trophoblastic neoplasia (GTN). If recognized early and treated appropriately, it almost always can be cured; however, errors or delays in the management of this condition can have catastrophic consequences for patients, including decreasing the likelihood of cure. Here we will review some of the steps and actions that can be taken immediately following the diagnosis of a molar pregnancy to expeditiously identify postmolar GTN and ensure patients are appropriately prepared for further consultation and intervention.
Postmolar GTN includes the diagnoses of invasive mole and choriocarcinoma that contain highly atypical trophoblasts with the capacity for local invasion and metastasis. Typically, the diagnosis is made clinically and not distinguished with histology. While molar pregnancies are a benign condition, invasive moles and choriocarcinoma are malignant conditions in which the molar tissue infiltrates the uterine myometrium, vasculature, and frequently is associated with hematogenous spread with distant metastases. It is a highly chemosensitive disease, and cure with chemotherapy typically is achieved with the ability to preserve fertility if desired even in advanced stage disease.1
After evacuation of a molar pregnancy, gynecologists should be on alert for the development of postmolar GTN if the following known risk factors are present: a history of a prior GTN diagnosis, complete mole on pathology (as opposed to partial mole), serum human chorionic gonadotropin (hCG) levels greater than 100,000 mIU/mL, age greater than 40 years, an enlarged uterus or large ovarian theca lutein cysts, and slow to normalize (more than 2 months) hCG. Symptoms for the development of postmolar GTN include persistent vaginal bleeding after evacuation, a persistently enlarged or enlarging uterine size, and adnexal masses. Ultimately, the diagnosis is made through plateaued or rising serum hCG assessments.2 (See graphic.)
Following the evacuation of a molar pregnancy, hCG levels should be drawn at the same laboratory every 1-2 weeks until normalization and then three consecutive normal values. Once this has been achieved, hCG levels should be tested once at 3 months and again at 6 months. During this 6 month period, patients should use reliable contraception, ideally, and through oral contraceptive pills that suppress the secretion of pituitary hCG if not contraindicated. Should a woman become pregnant during this 6-month surveillance, it becomes impossible to rule out occult postmolar GTN.
Typically after evacuation of a molar pregnancy, there is rapid fall in hCG levels, but this does not occur when the molar pregnancy has become invasive or is associated with choriocarcinoma. In these cases, after an initial drop in hCG levels, there is an observed rise or plateau in levels (as defined in the accompanying table), and this establishes the diagnosis of postmolar GTN. It is common for hCG to fall in fits and starts, rather than have a smooth, consistent diminution, and this can be worrying for gynecologists; however, provided there is a consistent reduction in values in accordance with the stated definitions, observation can continue.
Another source of confusion and concern is an HCG level that fails to completely normalize during observation, yet reaches a very low level. If this is observed, clinicians should consider the diagnosis of quiescent hCG, pituitary hCG, or phantom hCG.3 These can be difficult to distinguish from postmolar GTN, and consultation with a gynecologic oncologist with experience in the diagnosis and management of these rare tumors is helpful to determine if the persistent low levels in hCG require intervention.
Once a clinician has observed a plateau or rise in hCG levels, a gynecologic examination should be performed because the lower genital tract is a common site for metastatic postmolar GTN. If during this evaluation, a suspicious lesion is identified (typically a blue-black, slightly raised, hemorrhagic-appearing lesion), it should not be biopsied, but rather assumed to be a metastatic site. The vasculature of metastatic sites is extremely fragile, and biopsy or disruption can result in catastrophic hemorrhage, even from very small lesions.
In addition to physical examination, several diagnostic studies should be performed which may expedite the triage and management of the case. A pelvic ultrasound should evaluate the endometrial cavity for a new viable pregnancy, and residual molar tissue; sometimes, myometrial invasion consistent with an invasive mole can be appreciated. Chest x-ray or CT scan should be ordered to evaluate for pulmonary metastatic lesions. Additionally, CT scans of the abdomen and pelvis should be ordered, and if lung metastases are present, brain imaging with either MRI or CT scan also should be obtained. These imaging studies will provide the necessary information to stage the GTN (as metastatic or not).
Treatment for postmolar GTN is determined based on further prognostic categorization (“high risk” or “low risk”) in accordance with the WHO classification, which is derived using several prognostic clinical variables including age, antecedent pregnancy, interval from index pregnancy, pretreatment hCG, largest tumor size, sites and number of metastases, and response to previous chemotherapy.4 These assignments are necessary to determine whether single-agent or multiagent chemotherapy should be prescribed.
Laboratory studies are helpful to obtain at this time and include metabolic panels (which can ensure that renal and hepatic function are within normal limits in anticipation of future chemotherapy), and complete blood count ,which can establish viable bone marrow function prior to chemotherapy.
Once postmolar GTN has been diagnosed, it is most appropriate to refer the patient to a gynecologic oncologist with experience in the treatment of these relatively rare malignancies. At that point, the patient will be formally staged, and offered treatment based on these staging results.
Among women with low-risk, nonmetastatic GTN who desire future fertility it is appropriate to offer a repeat dilation and curettage (D&C) procedure rather than immediately proceeding with chemotherapy. Approximately two-thirds of women with low risk disease can avoid chemotherapy with repeat curettage.5 Risk factors for needing chemotherapy after repeat D&C include the presence of trophoblastic disease in the pathology specimen and urinary hCG levels greater than 1,500 mIU/mL at the time of curettage. In my experience, many women appreciate this option to potentially avoid toxic chemotherapy.
For women with low-risk, nonmetastatic postmolar GTN who do not desire future fertility, and hope to avoid chemotherapy, hysterectomy also is a reasonable first option. This can be performed via either minimally invasive, laparotomy, or vaginal route. If performing a minimally invasive procedure in the setting of GTN, there should be caution or avoidance of use of a uterine manipulator because the uterine wall typically is soft and prone to perforation, and bleeding can be significant secondary to disruption of the tumor.
If repeat D&C or hysterectomy are adopted instead of chemotherapy, it is important that patients are very closely monitored post operatively to ensure normalization of their hCG levels (as described above). If it fails to normalize, restaging scans and examinations should be performed, and referral for the appropriate chemotherapy regimen should be initiated without delay.
Postmolar GTN is a serious condition that usually can be cured with chemotherapy or, if appropriate, surgery. and refer to a gynecologic oncologist when criteria are met to ensure that overtreatment is avoided and essential therapy is ensured.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. Lancet Oncol. 2007 Aug;8(8):715-24.
2. J Natl Compr Canc Netw. 2019 Nov 1;17(11):1374-91.
3. Gynecol Oncol. 2009 Mar;112(3):663-72.
4. World Health Organ Tech Rep Ser. 1983;692:7-81.
5. Obstet Gynecol. 2016;128(3):535-42.
Consider sparing the uterus in prolapse procedures
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – A female pelvic medicine and reconstructive surgeon urged colleagues to consider uterus-sparing hysteropexies instead of hysterectomies in pelvic organ prolapse repairs.
said Beri M. Ridgeway, MD, of the Cleveland Clinic, at the Pelvic Anatomy and Gynecologic Surgery Symposium. Even so, “in the U.S., gynecologists rarely offer uterine preservation for women who desire repair of their uterovaginal prolapse.”
According to research compiled by Dr. Ridgeway, about 74,000 hysterectomies are performed each year in the United States to treat pelvic organ prolapse. The procedure became standard in the second half of the 20th century, in part to reduce cancer risk.
But attitudes evolved starting in the 1990s “as we have had better cancer screening and more focus on patient sexuality, patient autonomy, and quality of life,” Dr. Ridgeway said.
She offered these reasons to question hysterectomies to treat pelvic organ prolapse repairs:
- It’s not clear whether hysterectomies address the anatomic problems that produce prolapse in the first place. “Prolapse is caused by weakened or damaged tissue – connective tissue, muscles, etc.,” she said in an interview. “The problem is what is supporting the uterus, not the uterus itself.”
- Despite assumptions, women don’t necessarily prefer hysterectomy. Dr. Ridgeway pointed to a 2013 study in which researchers surveyed 213 women with prolapse symptoms about their preferred treatment, assuming that outcomes were the same. The results: 36% preferred uterine preservation, 20% preferred hysterectomy, and 44% reported no strong preference (Am J Obstet Gynecol. 2013 Nov;209[5]:470.e1-6.).
- Hysterectomies hasten menopause.
There has been a perception that uterus removal is appropriate in women who don’t wish to have any more children, Dr. Ridgeway said. “You had your babies, you’re done, you don’t need this anymore.” In fact, “that’s basically not true.”
As she explained, hysterectomy is linked to earlier menopause, and “even losing one ovary pushed patients into menopause significantly earlier.” She pointed to a 2016 Australian study, which found that “women who have a hysterectomy (with ovarian conservation) have a higher risk of hot flushes and night sweats that persist over an extended period” (Maturitas. 2016 Sep;91:1-7).
There’s no consensus on how hysterectomy affects sexual function. However, Dr. Ridgeway noted, it’s clear that pelvic floor disorders disrupt sexual function, and most women see improvement after surgical treatment.
The rate of uterine pathology is low in hysterectomy. Dr. Ridgeway highlighted a 2018 study of 24,076 women who underwent hysterectomy for benign indications. The study reported that “prevalence of occult corpus uteri, cervical, and ovarian malignancy was 1.44%, 0.60%, and 0.19%, respectively, among women undergoing hysterectomy and it varied by patient age and surgical route” (Obstet Gynecol. 2018 Apr;131[4]:642-51).
As an alternative, Dr. Ridgeway pointed to hysteropexy, which can be performed as a vaginal, laparoscopic, robot, or open procedure.
She highlighted a 2018 systematic review of pelvic organ prolapse surgeries that provided a meta-analysis and clinical practice guidelines. It found that “uterine-preserving prolapse surgeries improve operating time, blood loss, and risk of mesh exposure, compared with similar surgical routes with concomitant hysterectomy and do not significantly change short-term prolapse outcomes” (Am J Obstet Gynecol. 2018 Aug;219[2]:129-46.e2).
Dr. Ridgeway reported no relevant disclosures. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Beware nerve injuries in laparoscopic surgery
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – , and it’s not just nerves below the waist that are vulnerable.
But the risk can be lowered through careful positioning that avoids pressure and over-stretching, the two main causes of nerve injuries in these surgeries, Cleveland Clinic obstetrician-gynecologist and surgeon Tommaso Falcone, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Falcone offered tips about avoiding injuries to these nerves:
Brachial plexus. To avoid injury to this nerve, arms should be tucked and pronated or kept at less than a 90-degree angle from the body. Hyperextension injuries can occur when the arm is outstretched too much, said Dr. Falcone, who recommends putting a cushion under the elbow and wrist.
How can you know if an injury has occurred? The patient will typically wake up with a dropped wrist and numbness in the arm, he said, signs suggesting a stretch injury to the brachial plexus.
Ulnar nerve: Improper tucking of the arms can injure the ulnar nerve and cause numbness and weakness in the fourth and fifth fingers. Dr. Falcone recommends keeping the arms pronated if they’re tucked and supinated if they’re on arm boards. Again, he emphasized placing a cushion under the elbow and wrist.
Femoral nerve: Hyperflexion or hyperextension can compress the femoral nerve under the inguinal ligament and cause it to become ischemic, Dr. Falcone said.
Femoral nerve injury can lead to numbness (in the anterior and medial thigh), decreased patellar reflex, weakness of the quadriceps, and loss of knee extension flexibility. Patients may need to use a wheelchair for a time and undergo physical therapy, he said.
Obdurator nerve: Excessive lateral thigh abduction – outward movement – can stretch this nerve, Dr. Falcone said. Avoid excessive abduction by keeping the thigh-to-thigh angle under 90 degrees, he recommended. (This rule also helps to prevent femoral nerve injury.)
Signs of injury can include numbness on the medial aspect of the thigh and weakness in the adductor muscles. Physical therapy can be helpful, he said.
Sciatic nerve: Beware of hyperflexion of the hips followed by sudden straightening of the knees, he said. According to him, this can occur when candy-cane stirrups are used. Signs of injury can include foot drop and numbness (calf, dorsum, sole, and lateral side of the foot).
Peroneal nerve: Prolonged pressure against the knee can injure this nerve and cause foot drop and numbness (over the lower anterior leg and dorsum of the foot).
Dr. Falcone reports honoraria (Journal of Minimally Invasive Gynecology and Up-To-Date) and federal research funds. This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Uterine balloon tamponade found safe in postpartum hemorrhage
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
A new study summarizing and reanalyzing the .
Of 90 studies that reported efficacy data for uterine balloon tamponade (UBT), the procedure had overall success of 85.9% in treating postpartum hemorrhage (PPH). The pooled success rate was highest for women who were treated with a condom UBT, at 90.4%, compared with those treated with a Bakri balloon, at 83.2%, though the one randomized trial that compared the two devices head-to-head found no difference in success rates, wrote Sebastian Suarez, MD, and coauthors.
In all, the investigators looked at 91 studies involving 4,729 women who sustained PPH. The systematic review and meta-analysis included randomized controlled trials (RCTs), nonrandomized studies, and case series in which UBT was used to treat PPH.
Dr. Suarez, of Boston University Medical Center, and colleagues explained that postpartum hemorrhage (PPH) accounts for more maternal mortality and morbidity worldwide than any other complication of pregnancy, with the vast majority of PPH deaths occurring in low- and middle-income countries.
“While treatment of PPH varies depending on the cause, generally less invasive methods should be tried initially,” commented Angela Martin, MD, a maternal-fetal medicine specialist at the University of Kansas, Lawrence,in interview. Dr. Martin, who was not involved in the study, explained that “these options typically include administration of uterotonics or pharmacologic agents, and tamponade of the uterus with intrauterine balloons.” The hope in using less invasive options is that pelvic artery embolization, other surgical techniques, or even hysterectomy can be avoided in the face of the emergency of severe PPH.
One retrospective, nonrandomized study compared UBT plus standard of care with standard of care alone for uterine atony after vaginal delivery. The study found significantly less blood loss (759 mL vs. 1,582 mL) and a 0.22 relative risk of surgical interventions and 0.18 relative risk of blood transfusion for women receiving UBT. However, the authors assessed the evidence for UBT in this study to be of very low quality. Two other RCTs compared UBT and no UBT, and the authors’ meta-analysis of these two studies showed no significant differences between the two groups in risk of maternal death or surgical interventions. The evidence was considered very low quality in these studies as well.
UBT was also examined in uterine atony after Cesarean delivery; a subgroup analysis found overall less efficacy than that in cases of vaginal delivery. Other subgroup analyses found that UBT was more likely to be successful when uterine atony or placenta previa was the cause of PPH, compared with PPH from placenta accreta spectrum or retained products of conception. Also, the overall success of UBT was higher for PPH in vaginal delivery, compared with Cesarean delivery, at 87% versus 81%, regardless of the etiology of hemorrhage.
Looking at safety of UBT, Dr. Suarez and coinvestigators found 39 studies reporting various complications of UBT use for PPH, not all of which reported on all complications. The overall rate for fever or infection was 6.5% in studies reporting on this complication, and endometritis was recorded in 2.3% of participants in studies tracking that complication. Cervical tears, laceration of the lower segment of the vagina, uterine incision rupture or uterine perforation, and acute colonic pseudo-obstruction were all reported in 2% or less of the patients participating in studies that recorded these complications.
The authors excluded studies that included simultaneous use of surgical techniques and UBT and those that involved UBT for hemorrhage after pregnancy loss with a gestation less than 20 weeks’ duration. However, studies were included if UBT was used after surgical procedure failure.
To assess the primary outcome of UBT success rate, the authors used the raw ratio of cases of success divided by the total number of women treated with UBT. For the analysis, successful UBT use was considered to be arrest of PPH bleeding without maternal death or other surgical or radiological interventions after UBT placement, regardless of the definition of “UBT success” used in each study. Similarly, the authors considered “UBT failure” to have occurred in cases of maternal death or when additional surgical or radiological interventions happened after UBT placement.
The authors considered a composite primary outcome measure for the RCT and nonrandomized studies that was made up of maternal death and/or surgical or radiologic interventions.
Secondary outcome measures included UBT’s success rate for individual PPH causes, frequency of surgical and invasive procedures, and maternal outcomes such as death, blood loss, transfusion, ICU admission, and complication rates.
Overall, about half of the studies (n = 48; 53%) were conducted in low- and middle-income countries. Asian countries were the site of 46 studies, or 52% of the total. A quarter were conducted in Europe, and just five studies were conducted in the United States; the remainder were conducted in Africa or Latin America or were multinational studies.
Dr. Martin said that “[the review] findings provide reassurance that UBT can be implemented as a treatment option with a high success rate and low complication rate.” However, she noted, “There was a discrepancy between nonrandomized studies and RCTs on the efficacy and effectiveness of UBT.”
“Two randomized studies concluded there is no benefit to introduction of UBT in management of refractory PPH,” she continued. “The authors point out risk of bias and multiple methodological concerns that likely favored the control group in one effectiveness trial. Lack of benefit may have been due to suboptimal implementation strategies and lack of consistent UBT use.”
Dr. Martin concluded, “Overall, UBT success rates were consistently high across all study types. These findings are reassuring to the practicing clinician. There are many benefits to UBT including ease of use by a variety of health care providers, affordability, and its minimally invasive nature. Now there is evidence that UBT appears safe and has a high rate of success for management of PPH.”
The study’s senior author is a board member of the nonprofit organization Ujenzi Charitable Trust, which received Food and Drug Administration approval for the “Every Second Matters–Uterine Balloon Tamponade” device. Dr. Suarez reported that he had no financial conflicts of interest. The authors reported that there were no external sources of funding for the research. Dr. Martin serves on the editorial board of Ob.Gyn. News.
SOURCE: Suarez S et al. Am J Obstet Gynecol. 2020 Jan 6. doi: 10.1016/j.ajog.2019.11.1287.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Don’t neglect urinary tract in gynecologic procedures
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – – but when the time is appropriate, John B. Gebhart, MD, MS, urged.
You don’t need to stop a procedure to fix a bladder injury. Rather, mark the spot with a suture, finish what you are doing, then come back and fix the bladder injury, he advised.
“We need to be thinking [of the]urinary tract all the time in the procedures that we’re doing,” Dr. Gebhart, a urogynecologist and reconstructive pelvic surgeon from the Mayo Clinic, Rochester, Minn, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“Can you look at the bladder and see that it’s intact, that ureters are functioning like they should? You don’t need to have the skill set to place stents, but you should be able to look in and know you’re okay leaving the operating room,” he said.
According to Dr. Gebhart, urethral injuries can occur in these procedures: anterior repair, cystoscopy, midurethral sling, and treatment of diverticulitis or Skene’s duct abscess.
He offered these tips about urethral injuries:
- Use catheters, dyes, and urethroscopy to reveal injuries. “Putting in a catheter is great because it helps you identify injury because you can visually see it,” he said. “We can squirt some dye in the urethra and see if it’s leaking out. We can put in a zero-degree scope and do urethroscopy.”
- Consider linking multiple holes in the urethra. “Don’t make individual repairs,” he said. “Connect the holes, making them into one hole that you can fix in one setting.”
- Check your repairs for leakage. “I might take a little indigo carmine or methylene blue in a little [angiocatheter], squirt it down the urethra, and see if I’ve got anything leaking out from my repair site,” he said. “If I do, then I want to go back and repair that so that I’ve got a watertight closure.”
- Consider a catheter after repair. “If you do a repair, you want to place a catheter at the end of the splint in the urethra for 7 to 10 to 14 days to help prevent stricture afterwards.”
Dr. Gebhart also discussed bladder injuries, which he said can occur in anterior repair, cystoscopy, hysterectomy, midurethral slings, sacrocolpopexy, and other procedures.
He offered these tips:
- Use bladder backfilling to detect injury. “We can backfill the bladder with the little methylene blue stain–normal saline to help identify whether you’ve got a leak or an injury,” he said. “[Cystogram] can also be very helpful as well.”
- Don’t stop a hysterectomy to fix a bladder injury. “Mark the hole with a suture, finish the hysterectomy and get it out of the way, then come back and fix the hole in the bladder,” Dr. Gebhart said.
- After repair, drain the bladder with a catheter for 10-14 days. “You’re always better draining through a catheter a little longer than pulling the catheter too soon, putting a stretch on the bladder, and maybe compromising your repair,” he said.
He recommended performing a quick cystogram before pulling the catheter to make sure there’s no leak.
Dr. Gebhart disclosed consultant (Hologic) and advisory board (UroCure) relationships and royalties (UpToDate, Elsevier).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Beware the dangers of nerve injury in vaginal surgery
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – a pelvic surgeon urged colleagues.
“It’s a very high medical and legal risk. You have to think about the various nerves that can be influenced,” urogynecologist Mickey M. Karram, MD, said at the Pelvic Anatomy and Gynecologic Surgery Symposium.
Dr. Karram, director of urogynecology and reconstructive surgery at the Christ Hospital in Cincinnati and clinical professor of obstetrics and gynecology at the University of Cincinnati, offered these pearls:
- Understand the anatomy of nerves at risk. These include the ilioinguinal nerve, obturator neurovascular bundle, and pudendal nerve.
- Position the patient correctly. The buttocks should be at edge of table, Dr. Karram said, and there should be slight extension and lateral rotation of the thigh. Beware of compression of the lateral knee.
- Avoid compression from stirrups. If you still use candy-cane stirrups, he said, you can get compression along the lateral aspect of the knee. “You can [get] common perineal nerve injuries. You can also get femoral nerve injuries that are stretch injuries and over-extension injuries as well. Just be careful about this.” Dr. Karram said he prefers fin-type stirrups such as the Allen Yellofin brand. Also, he said, avoid compression injuries that result when there are too many people between the patient’s legs and someone leans on the thighs, he said.
- Free the retractor in abdominal procedures. “If you’re operating abdominally and use retractors, free the retractor at times,” he said. Otherwise, “you can get injuries to the genitofemoral nerve and the femoral nerve itself.”
- Beware buttock pain after sacrospinous fixation. “About 15%-20% of the time, you’ll get extreme buttock pain,” Dr. Karram said. “Assuming the buttock pain doesn’t radiate anywhere and doesn’t go down the leg, it’s definitely not a problem. If it goes down the leg, then you have to think about things like deligating pretty quickly.”
Dr. Karram disclosed consulting (Coloplast and Cynosure/Hologic) and speaker (Allergan, Astellas, Coloplast, and Cynosure/Hologic) relationships. He has royalties from Fidelis Medical and LumeNXT.
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019
Pelvic organ prolapse surgery isn’t as ‘simple’ as you think
LAS VEGAS – Surgical repair of pelvic organ prolapse often may seem like an uncomplicated procedure. But many factors play roles into decisions, and surgeons around the world vary widely in how they handle the operations, Mark D. Walters, MD, told colleagues at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“These prolapse repairs seem relatively simple at first, but they’re not simple at all,” he said.
Questions to ask prior to surgery
It’s important to first answer a number of questions, said Dr. Walters, professor and vice-chair of gynecology at the Cleveland Clinic. “When you see a patient like this, you may not realize how many decisions you’re making.”
These questions include:
- Is the patient sexually active or planning to be?
- Has she had a hysterectomy, and or is one necessary? If so, how should it be done? What does the patient think about a hysterectomy?
- Should the prolapse procedure be performed vaginally, open, laparoscopically, or robotically?
- Is adding a graft advisable? What kind?
- Should there be a sling to prevent stress urinary incontinence?”
Worldwide differences in surgical technique choice
Dr. Walters talked to colleagues from several nations and learned about these variations in surgical techniques.
Chinese surgeons use a variety of techniques with transvaginal mesh (TVM). Their use is more common in more populated cities because of the effect of medical education; native tissue procedures are more common in less-populated regions that are considered “backward.”
TVM with hysteropexy (“apical sling”) also is common in Latin America, while Middle Eastern surgeons have little training in female pelvic medicine and reconstructive surgery.
In Europe, France embraces mesh surgery and laparoscopy, while the United Kingdom has “completely abandoned” mesh surgery, and the Netherlands rarely uses it in favor of vaginal procedures.
In the United States, he said, TVM is “discouraged” while a variety of other procedures are used.
What procedures should surgeons embrace? There are many topics of debate, Dr. Walters said, including type of transvaginal repair (native tissue or mesh-augmented or sacrocolpopexy?), repair of “defects” in the vagina (even if they’re nonsymptomatic?) and the removal of the uterus (yes or no?).
Dr. Walters pointed to several explanations for this variation, including lack of high-quality research, confirmation bias, economic conflicts – surgeons are in the business of surgery, after all – and lack of insight into what women prefer.
Consider patient choice
In a survey, Dr. Walters polled women in their 50s with this question: “How much do you value your uterus?” Three women, he said, had widely varied opinions on a scale of 1-10, with one at 10 and another at 0.
“A doctor doesn’t know this and doesn’t have a way to ask, and the doctor has [his/her] own opinion about the value of the uterus,” he said. “Shouldn’t we know what patients think?”
How to measure success
He offered these tips about measuring success:
- Focus on symptomatic cure more than clinical cure.
- Remember that perfect anatomic support isn’t linked to health-related quality of life, and some loss of anatomic support is normal.
- Understand that commonly used definitions of anatomic success often aren’t clinically relevant.
Dr. Walters’ disclosures: royalties (Elsevier, UpToDate), website/lecturer (International Academy of Pelvic Surgery), and website editor (Foundation for Female Health Awareness).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – Surgical repair of pelvic organ prolapse often may seem like an uncomplicated procedure. But many factors play roles into decisions, and surgeons around the world vary widely in how they handle the operations, Mark D. Walters, MD, told colleagues at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“These prolapse repairs seem relatively simple at first, but they’re not simple at all,” he said.
Questions to ask prior to surgery
It’s important to first answer a number of questions, said Dr. Walters, professor and vice-chair of gynecology at the Cleveland Clinic. “When you see a patient like this, you may not realize how many decisions you’re making.”
These questions include:
- Is the patient sexually active or planning to be?
- Has she had a hysterectomy, and or is one necessary? If so, how should it be done? What does the patient think about a hysterectomy?
- Should the prolapse procedure be performed vaginally, open, laparoscopically, or robotically?
- Is adding a graft advisable? What kind?
- Should there be a sling to prevent stress urinary incontinence?”
Worldwide differences in surgical technique choice
Dr. Walters talked to colleagues from several nations and learned about these variations in surgical techniques.
Chinese surgeons use a variety of techniques with transvaginal mesh (TVM). Their use is more common in more populated cities because of the effect of medical education; native tissue procedures are more common in less-populated regions that are considered “backward.”
TVM with hysteropexy (“apical sling”) also is common in Latin America, while Middle Eastern surgeons have little training in female pelvic medicine and reconstructive surgery.
In Europe, France embraces mesh surgery and laparoscopy, while the United Kingdom has “completely abandoned” mesh surgery, and the Netherlands rarely uses it in favor of vaginal procedures.
In the United States, he said, TVM is “discouraged” while a variety of other procedures are used.
What procedures should surgeons embrace? There are many topics of debate, Dr. Walters said, including type of transvaginal repair (native tissue or mesh-augmented or sacrocolpopexy?), repair of “defects” in the vagina (even if they’re nonsymptomatic?) and the removal of the uterus (yes or no?).
Dr. Walters pointed to several explanations for this variation, including lack of high-quality research, confirmation bias, economic conflicts – surgeons are in the business of surgery, after all – and lack of insight into what women prefer.
Consider patient choice
In a survey, Dr. Walters polled women in their 50s with this question: “How much do you value your uterus?” Three women, he said, had widely varied opinions on a scale of 1-10, with one at 10 and another at 0.
“A doctor doesn’t know this and doesn’t have a way to ask, and the doctor has [his/her] own opinion about the value of the uterus,” he said. “Shouldn’t we know what patients think?”
How to measure success
He offered these tips about measuring success:
- Focus on symptomatic cure more than clinical cure.
- Remember that perfect anatomic support isn’t linked to health-related quality of life, and some loss of anatomic support is normal.
- Understand that commonly used definitions of anatomic success often aren’t clinically relevant.
Dr. Walters’ disclosures: royalties (Elsevier, UpToDate), website/lecturer (International Academy of Pelvic Surgery), and website editor (Foundation for Female Health Awareness).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
LAS VEGAS – Surgical repair of pelvic organ prolapse often may seem like an uncomplicated procedure. But many factors play roles into decisions, and surgeons around the world vary widely in how they handle the operations, Mark D. Walters, MD, told colleagues at the Pelvic Anatomy and Gynecologic Surgery Symposium.
“These prolapse repairs seem relatively simple at first, but they’re not simple at all,” he said.
Questions to ask prior to surgery
It’s important to first answer a number of questions, said Dr. Walters, professor and vice-chair of gynecology at the Cleveland Clinic. “When you see a patient like this, you may not realize how many decisions you’re making.”
These questions include:
- Is the patient sexually active or planning to be?
- Has she had a hysterectomy, and or is one necessary? If so, how should it be done? What does the patient think about a hysterectomy?
- Should the prolapse procedure be performed vaginally, open, laparoscopically, or robotically?
- Is adding a graft advisable? What kind?
- Should there be a sling to prevent stress urinary incontinence?”
Worldwide differences in surgical technique choice
Dr. Walters talked to colleagues from several nations and learned about these variations in surgical techniques.
Chinese surgeons use a variety of techniques with transvaginal mesh (TVM). Their use is more common in more populated cities because of the effect of medical education; native tissue procedures are more common in less-populated regions that are considered “backward.”
TVM with hysteropexy (“apical sling”) also is common in Latin America, while Middle Eastern surgeons have little training in female pelvic medicine and reconstructive surgery.
In Europe, France embraces mesh surgery and laparoscopy, while the United Kingdom has “completely abandoned” mesh surgery, and the Netherlands rarely uses it in favor of vaginal procedures.
In the United States, he said, TVM is “discouraged” while a variety of other procedures are used.
What procedures should surgeons embrace? There are many topics of debate, Dr. Walters said, including type of transvaginal repair (native tissue or mesh-augmented or sacrocolpopexy?), repair of “defects” in the vagina (even if they’re nonsymptomatic?) and the removal of the uterus (yes or no?).
Dr. Walters pointed to several explanations for this variation, including lack of high-quality research, confirmation bias, economic conflicts – surgeons are in the business of surgery, after all – and lack of insight into what women prefer.
Consider patient choice
In a survey, Dr. Walters polled women in their 50s with this question: “How much do you value your uterus?” Three women, he said, had widely varied opinions on a scale of 1-10, with one at 10 and another at 0.
“A doctor doesn’t know this and doesn’t have a way to ask, and the doctor has [his/her] own opinion about the value of the uterus,” he said. “Shouldn’t we know what patients think?”
How to measure success
He offered these tips about measuring success:
- Focus on symptomatic cure more than clinical cure.
- Remember that perfect anatomic support isn’t linked to health-related quality of life, and some loss of anatomic support is normal.
- Understand that commonly used definitions of anatomic success often aren’t clinically relevant.
Dr. Walters’ disclosures: royalties (Elsevier, UpToDate), website/lecturer (International Academy of Pelvic Surgery), and website editor (Foundation for Female Health Awareness).
This meeting was jointly provided by Global Academy for Medical Education and the University of Cincinnati. Global Academy and this news organization are owned by the same company.
EXPERT ANALYSIS FROM PAGS 2019