User login
New AHA checklist: Only one in five adults has optimal heart health
About 80% of American adults have low to moderate cardiovascular (CV) health based on the American Heart Association checklist for optimal heart health, which now includes healthy sleep as an essential component for heart health.
With the addition of sleep, “Life’s Essential 8” replaces the AHA’s “Life’s Simple 7” checklist.
“The new metric of sleep duration reflects the latest research findings: Sleep impacts overall health, and people who have healthier sleep patterns manage health factors such as weight, blood pressure, or risk for type 2 diabetes more effectively,” AHA President Donald M. Lloyd-Jones, MD, said in a news release.
“In addition, advances in ways to measure sleep, such as with wearable devices, now offer people the ability to reliably and routinely monitor their sleep habits at home,” said Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University in Chicago.
The AHA Presidential Advisory – Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct on Cardiovascular Health – was published online in the journal Circulation.
A companion paper published simultaneously in Circulation reports the first study using Life’s Essential 8.
Overall, the results show that CV health of the U.S. population is “suboptimal, and we see important differences across age and sociodemographic groups,” Dr. Lloyd-Jones said.
Refining Life’s Simple 7
The AHA first defined the seven metrics for optimal CV health in 2010. After 12 years and more than 2,400 scientific papers on the topic, new discoveries in CV health and ways to measure it provided an opportunity to revisit each health component in more detail and provide updates as needed, the AHA explains.
“We felt it was the right time to conduct a comprehensive review of the latest research to refine the existing metrics and consider any new metrics that add value to assessing cardiovascular health for all people,” Dr. Lloyd-Jones said.
Four of the original metrics have been redefined for consistency with newer clinical guidelines or compatibility with new measurement tools, and the scoring system can now also be applied to anyone ages 2 and older. Here is a snapshot of Life’s Essential 8 metrics, including updates.
1. Diet (updated)
The tool includes a new guide to assess diet quality for adults and children at the individual and population level. At the population level, dietary assessment is based on daily intake of elements in the Dietary Approaches to Stop Hypertension (DASH) eating pattern. For individuals, the Mediterranean Eating Pattern for Americans (MEPA) is used to assess and monitor cardiovascular health.
2. Physical activity (no changes)
Physical activity continues to be measured by the total number of minutes of moderate or vigorous physical activity per week, as defined by the U.S. Physical Activity Guidelines for Americans (2nd edition). The optimal level is 150 minutes (2.5 hours) of moderate physical activity or more per week or 75 minutes per week of vigorous-intensity physical activity for adults; 420 minutes (7 hours) or more per week for children ages 6 and older; and age-specific modifications for younger children.
3. Nicotine exposure (updated)
Use of inhaled nicotine-delivery systems, which includes e-cigarettes or vaping devices, has been added since the previous metric monitored only traditional, combustible cigarettes. This reflects use by adults and youth and their implications on long-term health. Second-hand smoke exposure for children and adults has also been added.
4. Sleep duration (new)
Sleep duration is associated with CV health. Measured by average hours of sleep per night, the ideal level is 7-9 hours daily for adults. Ideal daily sleep ranges for children are 10-16 hours per 24 hours for ages 5 and younger; 9-12 hours for ages 6-12 years; and 8-10 hours for ages 13-18 years.
5. Body mass index (no changes)
The AHA acknowledges that body mass index (BMI) is an imperfect metric. Yet, because it’s easily calculated and widely available, BMI continues as a “reasonable” gauge to assess weight categories that may lead to health problems. BMI of 18.5-24.9 is associated with the highest levels of CV health. The AHA notes that BMI ranges and the subsequent health risks associated with them may differ among people from diverse racial or ethnic backgrounds or ancestry. This aligns with the World Health Organization recommendations to adjust BMI ranges for people of Asian or Pacific Islander ancestry because recent evidence indicates their risk of conditions such as CVD or type 2 diabetes is higher at a lower BMI.
6. Blood lipids (updated)
The metric for blood lipids (cholesterol and triglycerides) is updated to use non-HDL cholesterol as the preferred number to monitor, rather than total cholesterol. This shift is made because non-HDL cholesterol can be measured without fasting beforehand (thereby increasing its availability at any time of day and implementation at more appointments) and reliably calculated among all people.
7. Blood glucose (updated)
This metric is expanded to include the option of hemoglobin A1c readings or blood glucose levels for people with or without type 1 or 2 diabetes or prediabetes.
8. Blood pressure (no changes)
Blood pressure criteria remain unchanged from 2017 guidance that established levels less than 120/80 mm Hg as optimal, and defined hypertension as 130-139 mm Hg systolic pressure or 80-89 mm Hg diastolic pressure.
‘Concerning’ new data
Results of the first study using Life’s Essential 8 show that the overall CV health of the U.S. population is “well below ideal,” with 80% of adults scoring at a low or moderate level, the researchers report.
Data for the analysis came from 2013-2018 U.S. National Health and Nutrition Examination surveys (NHANES) of more than 13,500 adults aged 20-79 years and nearly 9,900 children aged 2-19 years. Among the key findings:
- The average CV health score based on Life’s Essential 8 was 64.7 for adults and 65.5 for children – in the moderate range on the 0-100 scale.
- Only 0.45% of adults had a perfect score of 100; 20% had high CV health (score of 80 or higher), 63% moderate (score of 50-79), and 18% had low CV health (score of less than 50).
- Adult women had higher average CV health scores (67) compared with men (62.5).
- In general, adults scored lowest in the areas of diet, physical activity, and BMI.
- CV health scores were generally lower at older ages.
- Non-Hispanic Asian Americans had a higher average CV health score than other racial/ethnic groups. Non-Hispanic Whites had the second highest average CV health score, followed, in order, by Hispanic (other than Mexican), Mexican, and non-Hispanic Blacks.
- Children’s diet scores were low, at an average of 40.6.
- Adult sociodemographic groups varied notably in CV health scores for diet, nicotine exposure, blood glucose, and blood pressure.
“These data represent the first look at the cardiovascular health of the U.S. population using the AHA’s new Life’s Essential 8 scoring algorithm,” Dr. Lloyd-Jones said.
“Life’s Essential 8 is a major step forward in our ability to identify when cardiovascular health can be preserved and when it is suboptimal. It should energize efforts to improve cardiovascular health for all people and at every life stage,” Dr. Lloyd-Jones added.
“Analyses like this can help policymakers, communities, clinicians, and the public to understand the opportunities to intervene to improve and maintain optimal cardiovascular health across the life course,” he said.
This research had no commercial funding. The authors have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About 80% of American adults have low to moderate cardiovascular (CV) health based on the American Heart Association checklist for optimal heart health, which now includes healthy sleep as an essential component for heart health.
With the addition of sleep, “Life’s Essential 8” replaces the AHA’s “Life’s Simple 7” checklist.
“The new metric of sleep duration reflects the latest research findings: Sleep impacts overall health, and people who have healthier sleep patterns manage health factors such as weight, blood pressure, or risk for type 2 diabetes more effectively,” AHA President Donald M. Lloyd-Jones, MD, said in a news release.
“In addition, advances in ways to measure sleep, such as with wearable devices, now offer people the ability to reliably and routinely monitor their sleep habits at home,” said Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University in Chicago.
The AHA Presidential Advisory – Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct on Cardiovascular Health – was published online in the journal Circulation.
A companion paper published simultaneously in Circulation reports the first study using Life’s Essential 8.
Overall, the results show that CV health of the U.S. population is “suboptimal, and we see important differences across age and sociodemographic groups,” Dr. Lloyd-Jones said.
Refining Life’s Simple 7
The AHA first defined the seven metrics for optimal CV health in 2010. After 12 years and more than 2,400 scientific papers on the topic, new discoveries in CV health and ways to measure it provided an opportunity to revisit each health component in more detail and provide updates as needed, the AHA explains.
“We felt it was the right time to conduct a comprehensive review of the latest research to refine the existing metrics and consider any new metrics that add value to assessing cardiovascular health for all people,” Dr. Lloyd-Jones said.
Four of the original metrics have been redefined for consistency with newer clinical guidelines or compatibility with new measurement tools, and the scoring system can now also be applied to anyone ages 2 and older. Here is a snapshot of Life’s Essential 8 metrics, including updates.
1. Diet (updated)
The tool includes a new guide to assess diet quality for adults and children at the individual and population level. At the population level, dietary assessment is based on daily intake of elements in the Dietary Approaches to Stop Hypertension (DASH) eating pattern. For individuals, the Mediterranean Eating Pattern for Americans (MEPA) is used to assess and monitor cardiovascular health.
2. Physical activity (no changes)
Physical activity continues to be measured by the total number of minutes of moderate or vigorous physical activity per week, as defined by the U.S. Physical Activity Guidelines for Americans (2nd edition). The optimal level is 150 minutes (2.5 hours) of moderate physical activity or more per week or 75 minutes per week of vigorous-intensity physical activity for adults; 420 minutes (7 hours) or more per week for children ages 6 and older; and age-specific modifications for younger children.
3. Nicotine exposure (updated)
Use of inhaled nicotine-delivery systems, which includes e-cigarettes or vaping devices, has been added since the previous metric monitored only traditional, combustible cigarettes. This reflects use by adults and youth and their implications on long-term health. Second-hand smoke exposure for children and adults has also been added.
4. Sleep duration (new)
Sleep duration is associated with CV health. Measured by average hours of sleep per night, the ideal level is 7-9 hours daily for adults. Ideal daily sleep ranges for children are 10-16 hours per 24 hours for ages 5 and younger; 9-12 hours for ages 6-12 years; and 8-10 hours for ages 13-18 years.
5. Body mass index (no changes)
The AHA acknowledges that body mass index (BMI) is an imperfect metric. Yet, because it’s easily calculated and widely available, BMI continues as a “reasonable” gauge to assess weight categories that may lead to health problems. BMI of 18.5-24.9 is associated with the highest levels of CV health. The AHA notes that BMI ranges and the subsequent health risks associated with them may differ among people from diverse racial or ethnic backgrounds or ancestry. This aligns with the World Health Organization recommendations to adjust BMI ranges for people of Asian or Pacific Islander ancestry because recent evidence indicates their risk of conditions such as CVD or type 2 diabetes is higher at a lower BMI.
6. Blood lipids (updated)
The metric for blood lipids (cholesterol and triglycerides) is updated to use non-HDL cholesterol as the preferred number to monitor, rather than total cholesterol. This shift is made because non-HDL cholesterol can be measured without fasting beforehand (thereby increasing its availability at any time of day and implementation at more appointments) and reliably calculated among all people.
7. Blood glucose (updated)
This metric is expanded to include the option of hemoglobin A1c readings or blood glucose levels for people with or without type 1 or 2 diabetes or prediabetes.
8. Blood pressure (no changes)
Blood pressure criteria remain unchanged from 2017 guidance that established levels less than 120/80 mm Hg as optimal, and defined hypertension as 130-139 mm Hg systolic pressure or 80-89 mm Hg diastolic pressure.
‘Concerning’ new data
Results of the first study using Life’s Essential 8 show that the overall CV health of the U.S. population is “well below ideal,” with 80% of adults scoring at a low or moderate level, the researchers report.
Data for the analysis came from 2013-2018 U.S. National Health and Nutrition Examination surveys (NHANES) of more than 13,500 adults aged 20-79 years and nearly 9,900 children aged 2-19 years. Among the key findings:
- The average CV health score based on Life’s Essential 8 was 64.7 for adults and 65.5 for children – in the moderate range on the 0-100 scale.
- Only 0.45% of adults had a perfect score of 100; 20% had high CV health (score of 80 or higher), 63% moderate (score of 50-79), and 18% had low CV health (score of less than 50).
- Adult women had higher average CV health scores (67) compared with men (62.5).
- In general, adults scored lowest in the areas of diet, physical activity, and BMI.
- CV health scores were generally lower at older ages.
- Non-Hispanic Asian Americans had a higher average CV health score than other racial/ethnic groups. Non-Hispanic Whites had the second highest average CV health score, followed, in order, by Hispanic (other than Mexican), Mexican, and non-Hispanic Blacks.
- Children’s diet scores were low, at an average of 40.6.
- Adult sociodemographic groups varied notably in CV health scores for diet, nicotine exposure, blood glucose, and blood pressure.
“These data represent the first look at the cardiovascular health of the U.S. population using the AHA’s new Life’s Essential 8 scoring algorithm,” Dr. Lloyd-Jones said.
“Life’s Essential 8 is a major step forward in our ability to identify when cardiovascular health can be preserved and when it is suboptimal. It should energize efforts to improve cardiovascular health for all people and at every life stage,” Dr. Lloyd-Jones added.
“Analyses like this can help policymakers, communities, clinicians, and the public to understand the opportunities to intervene to improve and maintain optimal cardiovascular health across the life course,” he said.
This research had no commercial funding. The authors have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
About 80% of American adults have low to moderate cardiovascular (CV) health based on the American Heart Association checklist for optimal heart health, which now includes healthy sleep as an essential component for heart health.
With the addition of sleep, “Life’s Essential 8” replaces the AHA’s “Life’s Simple 7” checklist.
“The new metric of sleep duration reflects the latest research findings: Sleep impacts overall health, and people who have healthier sleep patterns manage health factors such as weight, blood pressure, or risk for type 2 diabetes more effectively,” AHA President Donald M. Lloyd-Jones, MD, said in a news release.
“In addition, advances in ways to measure sleep, such as with wearable devices, now offer people the ability to reliably and routinely monitor their sleep habits at home,” said Dr. Lloyd-Jones, chair of the department of preventive medicine at Northwestern University in Chicago.
The AHA Presidential Advisory – Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct on Cardiovascular Health – was published online in the journal Circulation.
A companion paper published simultaneously in Circulation reports the first study using Life’s Essential 8.
Overall, the results show that CV health of the U.S. population is “suboptimal, and we see important differences across age and sociodemographic groups,” Dr. Lloyd-Jones said.
Refining Life’s Simple 7
The AHA first defined the seven metrics for optimal CV health in 2010. After 12 years and more than 2,400 scientific papers on the topic, new discoveries in CV health and ways to measure it provided an opportunity to revisit each health component in more detail and provide updates as needed, the AHA explains.
“We felt it was the right time to conduct a comprehensive review of the latest research to refine the existing metrics and consider any new metrics that add value to assessing cardiovascular health for all people,” Dr. Lloyd-Jones said.
Four of the original metrics have been redefined for consistency with newer clinical guidelines or compatibility with new measurement tools, and the scoring system can now also be applied to anyone ages 2 and older. Here is a snapshot of Life’s Essential 8 metrics, including updates.
1. Diet (updated)
The tool includes a new guide to assess diet quality for adults and children at the individual and population level. At the population level, dietary assessment is based on daily intake of elements in the Dietary Approaches to Stop Hypertension (DASH) eating pattern. For individuals, the Mediterranean Eating Pattern for Americans (MEPA) is used to assess and monitor cardiovascular health.
2. Physical activity (no changes)
Physical activity continues to be measured by the total number of minutes of moderate or vigorous physical activity per week, as defined by the U.S. Physical Activity Guidelines for Americans (2nd edition). The optimal level is 150 minutes (2.5 hours) of moderate physical activity or more per week or 75 minutes per week of vigorous-intensity physical activity for adults; 420 minutes (7 hours) or more per week for children ages 6 and older; and age-specific modifications for younger children.
3. Nicotine exposure (updated)
Use of inhaled nicotine-delivery systems, which includes e-cigarettes or vaping devices, has been added since the previous metric monitored only traditional, combustible cigarettes. This reflects use by adults and youth and their implications on long-term health. Second-hand smoke exposure for children and adults has also been added.
4. Sleep duration (new)
Sleep duration is associated with CV health. Measured by average hours of sleep per night, the ideal level is 7-9 hours daily for adults. Ideal daily sleep ranges for children are 10-16 hours per 24 hours for ages 5 and younger; 9-12 hours for ages 6-12 years; and 8-10 hours for ages 13-18 years.
5. Body mass index (no changes)
The AHA acknowledges that body mass index (BMI) is an imperfect metric. Yet, because it’s easily calculated and widely available, BMI continues as a “reasonable” gauge to assess weight categories that may lead to health problems. BMI of 18.5-24.9 is associated with the highest levels of CV health. The AHA notes that BMI ranges and the subsequent health risks associated with them may differ among people from diverse racial or ethnic backgrounds or ancestry. This aligns with the World Health Organization recommendations to adjust BMI ranges for people of Asian or Pacific Islander ancestry because recent evidence indicates their risk of conditions such as CVD or type 2 diabetes is higher at a lower BMI.
6. Blood lipids (updated)
The metric for blood lipids (cholesterol and triglycerides) is updated to use non-HDL cholesterol as the preferred number to monitor, rather than total cholesterol. This shift is made because non-HDL cholesterol can be measured without fasting beforehand (thereby increasing its availability at any time of day and implementation at more appointments) and reliably calculated among all people.
7. Blood glucose (updated)
This metric is expanded to include the option of hemoglobin A1c readings or blood glucose levels for people with or without type 1 or 2 diabetes or prediabetes.
8. Blood pressure (no changes)
Blood pressure criteria remain unchanged from 2017 guidance that established levels less than 120/80 mm Hg as optimal, and defined hypertension as 130-139 mm Hg systolic pressure or 80-89 mm Hg diastolic pressure.
‘Concerning’ new data
Results of the first study using Life’s Essential 8 show that the overall CV health of the U.S. population is “well below ideal,” with 80% of adults scoring at a low or moderate level, the researchers report.
Data for the analysis came from 2013-2018 U.S. National Health and Nutrition Examination surveys (NHANES) of more than 13,500 adults aged 20-79 years and nearly 9,900 children aged 2-19 years. Among the key findings:
- The average CV health score based on Life’s Essential 8 was 64.7 for adults and 65.5 for children – in the moderate range on the 0-100 scale.
- Only 0.45% of adults had a perfect score of 100; 20% had high CV health (score of 80 or higher), 63% moderate (score of 50-79), and 18% had low CV health (score of less than 50).
- Adult women had higher average CV health scores (67) compared with men (62.5).
- In general, adults scored lowest in the areas of diet, physical activity, and BMI.
- CV health scores were generally lower at older ages.
- Non-Hispanic Asian Americans had a higher average CV health score than other racial/ethnic groups. Non-Hispanic Whites had the second highest average CV health score, followed, in order, by Hispanic (other than Mexican), Mexican, and non-Hispanic Blacks.
- Children’s diet scores were low, at an average of 40.6.
- Adult sociodemographic groups varied notably in CV health scores for diet, nicotine exposure, blood glucose, and blood pressure.
“These data represent the first look at the cardiovascular health of the U.S. population using the AHA’s new Life’s Essential 8 scoring algorithm,” Dr. Lloyd-Jones said.
“Life’s Essential 8 is a major step forward in our ability to identify when cardiovascular health can be preserved and when it is suboptimal. It should energize efforts to improve cardiovascular health for all people and at every life stage,” Dr. Lloyd-Jones added.
“Analyses like this can help policymakers, communities, clinicians, and the public to understand the opportunities to intervene to improve and maintain optimal cardiovascular health across the life course,” he said.
This research had no commercial funding. The authors have no reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Thigh muscle fat predicts risk of developing heart failure
in a new study. The association was independent of other cardiometabolic risk factors and measures of adiposity such as body mass index.
The observation raises the possibility of new avenues of research aimed at modifying intramuscular fat levels as a strategy to reduce the risk of developing heart failure.
The study was published online in JACC: Heart Failure.
The authors, led by Kevin Huynh, MD, University of Texas Southwestern Medical Center, Dallas, explained that obesity is a known risk for heart failure, and has been incorporated into risk calculators for heart failure.
However, obesity is a complex and heterogeneous disease with substantial regional variability of adipose deposition in body tissues, they noted. For example, variability in visceral adipose tissue and subcutaneous adipose tissue has been shown to have a differential impact on both cardiovascular risk factors and clinical cardiovascular disease outcomes.
The fat deposition around and within nonadipose tissues (termed “ectopic fat”), such as skeletal muscle, is also a known risk factor for cardiovascular disease, independent of adiposity. However, the impact of peripheral skeletal muscle fat deposition on heart failure risk is not as well studied.
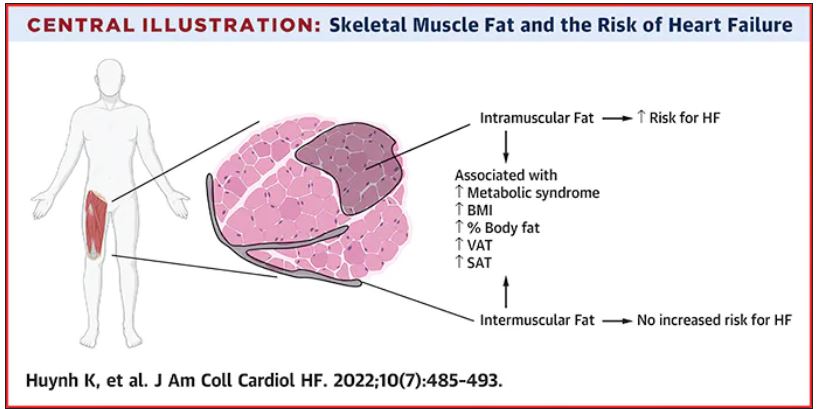
The researchers noted that ectopic fat in skeletal muscle can be measured through imaging and categorized as either intermuscular or intramuscular fat according to the location of muscle fat around or within skeletal muscle, respectively.
The researchers conducted the current study to characterize the association of both intermuscular and intramuscular fat deposition with heart failure risk in a large cohort of older adults.
They used data from 2,399 individuals aged 70-79 years without heart failure at baseline who participated in the Health ABC (Health, Aging and Body Composition) study. Measures of intramuscular and intermuscular fat in the thigh were determined by CT, and the participants were followed for an average of 12 years.
During the follow-up period, there were 485 incident heart failure events. Higher sex-specific tertiles of intramuscular and intermuscular fat were each associated with heart failure risk.
After multivariable adjustment for age, sex, race, education, blood pressure, fasting blood sugar, current smoking, prevalent coronary disease, and creatinine, higher intramuscular fat, but not intermuscular fat, was significantly associated with higher risk for heart failure.
Individuals in the highest tertile of intramuscular fat had a 34% increased risk of developing heart failure, compared with those in the lowest tertile. This finding was independent of other cardiometabolic risk factors, measures of adiposity including body mass index and percent fat, muscle strength, and muscle mass.
The association was slightly attenuated when adjusted for inflammatory markers, suggesting that inflammation may be a contributor.
The association between higher intramuscular fat and heart failure appeared specific to higher risk of incident heart failure with reduced ejection fraction, but not with heart failure with preserved ejection fraction.
The researchers noted that skeletal muscle is a pivotal endocrine organ in addition to the role it plays in the production of mechanical power.
They pointed out that there are differences in the biology of intermuscular and intramuscular fat deposition, and that excess intramuscular fat deposition is a result of dysregulated lipid metabolism and is associated with insulin resistance (a known risk factor for the development of heart failure), inflammation, and muscle wasting conditions.
They concluded that, in patients with heart failure, alterations in skeletal muscle function are most likely affected by multiple contributors, including inflammation, oxidative stress, and neurohormonal factors. “As these factors are also implicated in the pathogenesis of heart failure, intramuscular fat deposition may indicate a biological milieu that increases the risk of heart failure.”
New approaches to reduce heart failure risk?
In an accompanying editorial, Salvatore Carbone, PhD, Virginia Commonwealth University, Richmond, said the findings of the study are “exceptionally novel,” providing novel evidence that noncardiac body composition compartments, particularly intramuscular adipose tissue, can predict the risk for heart failure in a diverse population of older adults.
He called for further research to understand the mechanisms involved and to assess if this risk factor can be effectively modified to reduce the risk of developing heart failure.
Dr. Carbone reported that intramuscular adipose tissue can be influenced by dietary fat intake and can be worsened by accumulation of saturated fatty acids, which also contribute to insulin resistance.
He noted that saturated fatty acid–induced insulin resistance in the skeletal muscle appears to be mediated by proinflammatory pathways within the skeletal muscle itself, which can be reversed by monounsaturated fatty acids, like oleic acid, that can be found in the largest amount in food like olive oil, canola oil, and avocados, among others.
He added that sodium-glucose transporter 2 inhibitors, drugs used in the treatment of diabetes that have also been shown to prevent heart failure in individuals at risk, can also improve the composition of intramuscular adipose tissue by reducing its content of saturated fatty acids and increase the content of monosaturated fatty acids.
The study results suggest that the quality of intramuscular adipose tissue might also play an important role and could be targeted by therapeutic strategies, he commented.
Dr. Carbone concluded that “studies testing novel modalities of exercise training, intentional weight loss, diet quality improvements with and without weight loss (i.e., increase of dietary monounsaturated fatty acids, such as oleic acid), as well as pharmacological anti-inflammatory strategies should be encouraged in this population to test whether the reduction in intramuscular adipose tissue or improvements of its quality can ultimately reduce the risk for heart failure in this population.”
This research was supported by the National Institute on Aging and the National Institute of Nursing Research. Dr. Huynh and Dr. Carbone disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a new study. The association was independent of other cardiometabolic risk factors and measures of adiposity such as body mass index.
The observation raises the possibility of new avenues of research aimed at modifying intramuscular fat levels as a strategy to reduce the risk of developing heart failure.
The study was published online in JACC: Heart Failure.
The authors, led by Kevin Huynh, MD, University of Texas Southwestern Medical Center, Dallas, explained that obesity is a known risk for heart failure, and has been incorporated into risk calculators for heart failure.
However, obesity is a complex and heterogeneous disease with substantial regional variability of adipose deposition in body tissues, they noted. For example, variability in visceral adipose tissue and subcutaneous adipose tissue has been shown to have a differential impact on both cardiovascular risk factors and clinical cardiovascular disease outcomes.
The fat deposition around and within nonadipose tissues (termed “ectopic fat”), such as skeletal muscle, is also a known risk factor for cardiovascular disease, independent of adiposity. However, the impact of peripheral skeletal muscle fat deposition on heart failure risk is not as well studied.
The researchers noted that ectopic fat in skeletal muscle can be measured through imaging and categorized as either intermuscular or intramuscular fat according to the location of muscle fat around or within skeletal muscle, respectively.
The researchers conducted the current study to characterize the association of both intermuscular and intramuscular fat deposition with heart failure risk in a large cohort of older adults.
They used data from 2,399 individuals aged 70-79 years without heart failure at baseline who participated in the Health ABC (Health, Aging and Body Composition) study. Measures of intramuscular and intermuscular fat in the thigh were determined by CT, and the participants were followed for an average of 12 years.
During the follow-up period, there were 485 incident heart failure events. Higher sex-specific tertiles of intramuscular and intermuscular fat were each associated with heart failure risk.
After multivariable adjustment for age, sex, race, education, blood pressure, fasting blood sugar, current smoking, prevalent coronary disease, and creatinine, higher intramuscular fat, but not intermuscular fat, was significantly associated with higher risk for heart failure.
Individuals in the highest tertile of intramuscular fat had a 34% increased risk of developing heart failure, compared with those in the lowest tertile. This finding was independent of other cardiometabolic risk factors, measures of adiposity including body mass index and percent fat, muscle strength, and muscle mass.
The association was slightly attenuated when adjusted for inflammatory markers, suggesting that inflammation may be a contributor.
The association between higher intramuscular fat and heart failure appeared specific to higher risk of incident heart failure with reduced ejection fraction, but not with heart failure with preserved ejection fraction.
The researchers noted that skeletal muscle is a pivotal endocrine organ in addition to the role it plays in the production of mechanical power.
They pointed out that there are differences in the biology of intermuscular and intramuscular fat deposition, and that excess intramuscular fat deposition is a result of dysregulated lipid metabolism and is associated with insulin resistance (a known risk factor for the development of heart failure), inflammation, and muscle wasting conditions.
They concluded that, in patients with heart failure, alterations in skeletal muscle function are most likely affected by multiple contributors, including inflammation, oxidative stress, and neurohormonal factors. “As these factors are also implicated in the pathogenesis of heart failure, intramuscular fat deposition may indicate a biological milieu that increases the risk of heart failure.”
New approaches to reduce heart failure risk?
In an accompanying editorial, Salvatore Carbone, PhD, Virginia Commonwealth University, Richmond, said the findings of the study are “exceptionally novel,” providing novel evidence that noncardiac body composition compartments, particularly intramuscular adipose tissue, can predict the risk for heart failure in a diverse population of older adults.
He called for further research to understand the mechanisms involved and to assess if this risk factor can be effectively modified to reduce the risk of developing heart failure.
Dr. Carbone reported that intramuscular adipose tissue can be influenced by dietary fat intake and can be worsened by accumulation of saturated fatty acids, which also contribute to insulin resistance.
He noted that saturated fatty acid–induced insulin resistance in the skeletal muscle appears to be mediated by proinflammatory pathways within the skeletal muscle itself, which can be reversed by monounsaturated fatty acids, like oleic acid, that can be found in the largest amount in food like olive oil, canola oil, and avocados, among others.
He added that sodium-glucose transporter 2 inhibitors, drugs used in the treatment of diabetes that have also been shown to prevent heart failure in individuals at risk, can also improve the composition of intramuscular adipose tissue by reducing its content of saturated fatty acids and increase the content of monosaturated fatty acids.
The study results suggest that the quality of intramuscular adipose tissue might also play an important role and could be targeted by therapeutic strategies, he commented.
Dr. Carbone concluded that “studies testing novel modalities of exercise training, intentional weight loss, diet quality improvements with and without weight loss (i.e., increase of dietary monounsaturated fatty acids, such as oleic acid), as well as pharmacological anti-inflammatory strategies should be encouraged in this population to test whether the reduction in intramuscular adipose tissue or improvements of its quality can ultimately reduce the risk for heart failure in this population.”
This research was supported by the National Institute on Aging and the National Institute of Nursing Research. Dr. Huynh and Dr. Carbone disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in a new study. The association was independent of other cardiometabolic risk factors and measures of adiposity such as body mass index.
The observation raises the possibility of new avenues of research aimed at modifying intramuscular fat levels as a strategy to reduce the risk of developing heart failure.
The study was published online in JACC: Heart Failure.
The authors, led by Kevin Huynh, MD, University of Texas Southwestern Medical Center, Dallas, explained that obesity is a known risk for heart failure, and has been incorporated into risk calculators for heart failure.
However, obesity is a complex and heterogeneous disease with substantial regional variability of adipose deposition in body tissues, they noted. For example, variability in visceral adipose tissue and subcutaneous adipose tissue has been shown to have a differential impact on both cardiovascular risk factors and clinical cardiovascular disease outcomes.
The fat deposition around and within nonadipose tissues (termed “ectopic fat”), such as skeletal muscle, is also a known risk factor for cardiovascular disease, independent of adiposity. However, the impact of peripheral skeletal muscle fat deposition on heart failure risk is not as well studied.
The researchers noted that ectopic fat in skeletal muscle can be measured through imaging and categorized as either intermuscular or intramuscular fat according to the location of muscle fat around or within skeletal muscle, respectively.
The researchers conducted the current study to characterize the association of both intermuscular and intramuscular fat deposition with heart failure risk in a large cohort of older adults.
They used data from 2,399 individuals aged 70-79 years without heart failure at baseline who participated in the Health ABC (Health, Aging and Body Composition) study. Measures of intramuscular and intermuscular fat in the thigh were determined by CT, and the participants were followed for an average of 12 years.
During the follow-up period, there were 485 incident heart failure events. Higher sex-specific tertiles of intramuscular and intermuscular fat were each associated with heart failure risk.
After multivariable adjustment for age, sex, race, education, blood pressure, fasting blood sugar, current smoking, prevalent coronary disease, and creatinine, higher intramuscular fat, but not intermuscular fat, was significantly associated with higher risk for heart failure.
Individuals in the highest tertile of intramuscular fat had a 34% increased risk of developing heart failure, compared with those in the lowest tertile. This finding was independent of other cardiometabolic risk factors, measures of adiposity including body mass index and percent fat, muscle strength, and muscle mass.
The association was slightly attenuated when adjusted for inflammatory markers, suggesting that inflammation may be a contributor.
The association between higher intramuscular fat and heart failure appeared specific to higher risk of incident heart failure with reduced ejection fraction, but not with heart failure with preserved ejection fraction.
The researchers noted that skeletal muscle is a pivotal endocrine organ in addition to the role it plays in the production of mechanical power.
They pointed out that there are differences in the biology of intermuscular and intramuscular fat deposition, and that excess intramuscular fat deposition is a result of dysregulated lipid metabolism and is associated with insulin resistance (a known risk factor for the development of heart failure), inflammation, and muscle wasting conditions.
They concluded that, in patients with heart failure, alterations in skeletal muscle function are most likely affected by multiple contributors, including inflammation, oxidative stress, and neurohormonal factors. “As these factors are also implicated in the pathogenesis of heart failure, intramuscular fat deposition may indicate a biological milieu that increases the risk of heart failure.”
New approaches to reduce heart failure risk?
In an accompanying editorial, Salvatore Carbone, PhD, Virginia Commonwealth University, Richmond, said the findings of the study are “exceptionally novel,” providing novel evidence that noncardiac body composition compartments, particularly intramuscular adipose tissue, can predict the risk for heart failure in a diverse population of older adults.
He called for further research to understand the mechanisms involved and to assess if this risk factor can be effectively modified to reduce the risk of developing heart failure.
Dr. Carbone reported that intramuscular adipose tissue can be influenced by dietary fat intake and can be worsened by accumulation of saturated fatty acids, which also contribute to insulin resistance.
He noted that saturated fatty acid–induced insulin resistance in the skeletal muscle appears to be mediated by proinflammatory pathways within the skeletal muscle itself, which can be reversed by monounsaturated fatty acids, like oleic acid, that can be found in the largest amount in food like olive oil, canola oil, and avocados, among others.
He added that sodium-glucose transporter 2 inhibitors, drugs used in the treatment of diabetes that have also been shown to prevent heart failure in individuals at risk, can also improve the composition of intramuscular adipose tissue by reducing its content of saturated fatty acids and increase the content of monosaturated fatty acids.
The study results suggest that the quality of intramuscular adipose tissue might also play an important role and could be targeted by therapeutic strategies, he commented.
Dr. Carbone concluded that “studies testing novel modalities of exercise training, intentional weight loss, diet quality improvements with and without weight loss (i.e., increase of dietary monounsaturated fatty acids, such as oleic acid), as well as pharmacological anti-inflammatory strategies should be encouraged in this population to test whether the reduction in intramuscular adipose tissue or improvements of its quality can ultimately reduce the risk for heart failure in this population.”
This research was supported by the National Institute on Aging and the National Institute of Nursing Research. Dr. Huynh and Dr. Carbone disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Cardiologists concerned for patient safety after abortion ruling
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Hydroxychloroquine risk found in some older patients with RA
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Hydroxychloroquine should be initiated with caution in older patients with rheumatoid arthritis who also have heart failure or are at risk for it, say the authors of a study suggesting that the drug could increase their risk for major adverse cardiovascular events (MACE), compared with methotrexate.
A cohort study published online in the Journal of the American College of Cardiology looked at outcomes in 54,462 patients with RA aged 65 years or older and not previously treated with disease-modifying antirheumatic drugs. Half were initiated on methotrexate and half on hydroxychloroquine, making 27,231 propensity-matched pairs.
Across the entire cohort, hydroxychloroquine was not associated with a higher risk for sudden cardiac arrest, ventricular arrhythmia, or MACE, compared with methotrexate. When broken down into individual cardiovascular events, the data suggested a statistically significant 17% increase in the risk for cardiovascular mortality and 10% increase in all-cause mortality with hydroxychloroquine, although there were no differences in the risks for myocardial infarction or stroke.
However, a subgroup analysis revealed a significant 30% increase in the risk for MACE among patients starting hydroxychloroquine who also had a history of heart failure, compared with patients taking methotrexate. The researchers found no difference between the two drugs in patients without a history of heart failure. The study also suggested an overall 41% increase in the risk for hospitalization with heart failure with hydroxychloroquine, regardless of heart failure history.
Hydroxychloroquine was also associated with a 34% increase in the risk for cardiovascular mortality, a 22% increase in the risk for all-cause mortality, and a 74% increase in the risk for MI.
The lead author of the study, Elvira D’Andrea, MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School in Boston, said that hydroxychloroquine is used as a first-line treatment for RA, but there was limited evidence on its cardiovascular risks. The pandemic in particular shined a spotlight on these concerns and prompted the researchers to extend their original prepandemic study to encompass additional cardiovascular outcomes.
“The emerging concerns on its cardiovascular safety in early 2020 has led the rheumatological community, and patients regularly taking hydroxychloroquine for rheumatoid arthritis, to confusion,” Dr. D’Andrea said in an interview.
She advised that clinicians be cautious when initiating hydroxychloroquine in older patients with existing heart failure or who have risk factors for it. “Although heart failure is a known concern for hydroxychloroquine use, these findings helped to clarify the relationship between the use of hydroxychloroquine or methotrexate and heart failure. Clinicians should pay careful attention to clinical manifestations of cardiomyopathy or heart failure in older patients with rheumatoid arthritis treated with hydroxychloroquine.”
Hydroxychloroquine is associated with cardiotoxicity, particularly cardiomyopathy, which may help precipitate MACE or heart failure exacerbations in patients who already have deterioration of their cardiac tissue, the authors suggested.
Short follow-up period leaves risk attribution under question
In an accompanying editorial, Elizabeth Blair Solow, MD, and Bonnie L. Bermas, MD, of the University of Texas Southwestern Medical Center, Dallas, commented that the lack of an increased risk for arrhythmic events or MACE in the overall cohort taking hydroxychloroquine was reassuring. They also suggested the subgroup analysis findings among patients with preexisting heart failure were still “exploratory and hypothesis-generating” and should be interpreted with caution.
They noted that the follow-up time of the study was relatively short – a median of 209 days – given that hydroxychloroquine does not reach a steady-state level for 6 months.
“Evidence to date suggests cardiomyopathy from HCQ [hydroxychloroquine] takes years to develop, many months beyond the exposures described here, bringing into question as to whether HCQ itself increased HF hospitalizations,” the editorialists wrote.
The editorial also raised the question of whether the association observed in the study was related to a possible cardioprotective effect of methotrexate, given that previous studies have suggested this effect in older patients with RA.
The study authors did an exploratory analysis comparing hydroxychloroquine with sulfasalazine, which appeared to support their main findings of a possible cardiovascular effect of hydroxychloroquine. However, they qualified this by pointing out that the analysis involved small numbers of patients.
Senior investigator Seoyoung C. Kim, MD, ScD, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, also noted that the study only looked at outcomes in patients aged 65 years and older.
“It would be clinically important to further examine the cardiovascular safety of hydroxychloroquine versus methotrexate in a younger population with rheumatic conditions,” she said.
The study was supported by the National Institutes of Health, Brigham and Women’s Hospital, and Harvard Medical School. Four authors declared unrelated research grants from the pharmaceutical sector, with one also declaring stock options and consulting work with the pharmaceutical sector. No other conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Pig-heart transplant case published with new details, insights
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
It’s a given that the case of David Bennett, Sr, and his transplanted, genetically modified porcine heart will have a lot to teach, and the peer-reviewed publication this week lends welcome authority to some of its earliest lessons.
Mr. Bennett lived for 2 months after receiving the heart in the pioneering surgery, and the new case report compiles the available clinical, anatomic, and histologic evidence and other potential clues to the underlying cause or causes of death.
It also describes a mystery that came to light at autopsy: a grossly enlarged heart attributable to pervasive interstitial edema, and at the cellular level, a peculiar pattern of myocardial damage that included microvascular deterioration and, potentially as a result, cellular necrosis, according to the new report.
The myocardium itself was described as “thickened and stiff,” consistent with the “diastolic heart failure” that characterized Mr. Bennett’s final 10 days and the likely convergence of several underlying processes. Missing, however, was any conventional sign of graft rejection as it is understood clinically or in animal models, the report states.
If a form of tissue rejection was the cause of graft failure, any implicating cellular evidence may simply have been unrecognizable, given the unprecedented nature of the first pig-to-human heart transplantation, the donor animal’s multiple anti-inflammatory gene deletions, and partly investigational immunosuppression regimen, speculated Bartley P. Griffith, MD, University of Maryland, College Park.
“I’m betting against it being a fulminant rejection,” he told this news organization, “because we saw nothing like the [characteristic] platelet deposition or thrombosis of the capillaries.”
Dr. Griffith, who performed the xenotransplant surgery and led Mr. Bennett’s postoperative care, is lead author on the case report published in the New England Journal of Medicine. “Additional studies are underway to characterize the pathophysiologic mechanisms that resulted in this damage,” the report states.
The report builds on recent meeting presentations on the case, which, as previously reported, gave cursory details regarding the organ damage and other clinical developments during and after the surgery, including evidence that the transplanted heart contained porcine cytomegalovirus (PCMV).
Similar details also appeared in a third-person account based in part on personal communication with Dr. Griffith. The cardiac XTx review that focused on this University of Maryland experience was published June 15 in JACC: Basic to Translational Science, with lead author Jacinthe Boulet, MD, CM, Brigham and Women’s Hospital Heart, Boston.
“The question of how to move XTx forward remains uncertain, and appropriate selection of patients for experimental XTx will be one of the most important challenges to be addressed. The first issue we must contend with is whether we are ready to move to the next XTx in a human. We strongly believe this to be the case,” the review states. “Once early experience is gained, with successive iterations of XTx, the bar for success can be raised with maturation of the technology.”
Evidence has so far not implicated several other potential mechanisms underlying the graft failure that had been the focus of early speculations. For example, the transplanted pig heart was infected with PCMV, as previously reported. Mr. Bennett showed traces of PCMV DNA in his circulation, but no actual virus in his native cells. Still, PCMV remains a suspect.
Mr. Bennett also received intravenous immunoglobulin (IVIG) on several occasions to fight rejection, and also severe infections, including a nasty episode of sepsis. A reaction to the IVIG, derived from pooled donor antibodies, could potentially have caused the unusual myocardial damage seen by the University of Maryland team, Dr. Griffith observed. Alternatively, the damage might have been partly related to the patient’s overall severely diminished condition even before the transplant surgery or his rocky postoperative clinical course.
Indeed, Mr. Bennett’s condition worsened dramatically on postoperative day 50, and echocardiography showed a striking degree of myocardial wall thickening and heart enlargement, determined to be from edema. “The heart got amazingly stiff but maintained a systolic function that wasn›t too terrible, even to the very end. But his heart seemed as though it had swollen overnight,” Dr. Griffith said. “We had never seen that type of process, the suddenness of this swelling, in our nonhuman primate studies.”
The damage to the heart muscle appeared irreversible, based on myocardial biopsy results, so the decision was made to withdraw life support 60 days after the transplant surgery, the report notes.
Among the experience’s apparent lessons for future cardiac xenotransplantation, Dr. Griffith said, would be to select patients for the surgery who are in a bit more robust condition than Mr. Bennett was, who are perhaps ambulatory, not sarcopenic, and not recently on prolonged mechanical circulatory support. “We’re going to try to pick a patient who, on the front end, is less critically ill but who is just as likely not to benefit from continued medical therapy” and who isn’t a candidate for conventional heart transplantation, he said.
Because of universal efforts to manage conditions like diabetes, hypertension, and vascular disease in the population, and “because these conditions cause many of the cases of organ failure and fuel demand for transplantation, one might wonder whether the advances reported by Dr. Griffith and colleagues presage a decreasing demand for organ transplantation,” speculates an accompanying editorialfrom Jeffrey L. Platt, MD, and Marilia Cascalho, MD, PhD, University of Michigan, Ann Arbor.
“We think the answer is no. Since aging is associated with progressive decline in the function of the heart, kidneys, and other organs, advances that extend life expectancy will ultimately increase the prevalence of organ failure and potentially the demand for transplantation.”
The donor pig was developed and provided by Revivicor, and the investigational KPL-404 antibody drug used in the experience was provided by Kiniksa. Other disclosures for the case report and editorial from Dr. Platt and Dr. Cascalho are available at NEJM.com. Dr. Boulet reports no relevant relationships; disclosures for the other authors are in their report.
A version of this article first appeared on Medscape.com.
Class I recall for Medtronic’s HeartWare HVAD batteries
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to [email protected].
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to [email protected].
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Medtronic is recalling a single lot of HeartWare Ventricular Assist Device (HVAD) System batteries because of welding defects that may cause separation of the two cell battery packs used to power the system, according to an alert on the Food and Drug Administration website.
“The welding defect may cause the battery to malfunction and no longer provide power or prevent the battery from holding a full charge or properly recharging,” the FDA said.
The agency has identified this as a class I recall, the most serious type because of the potential for serious injury or death.
Medtronic reports one death associated with this recall and two complaints in the affected lot.
Back in April, as reported by this news organization, Medtronic alerted providers that patients implanted with the Medtronic HVAD System who develop pump thrombosis could have a welding defect in the internal pump that causes the pump to malfunction.
The batteries from the recalled lot have a model number of 1650DE, were manufactured from April 13 to 19, 2021 and distributed from April 20 to July 19, 2021. The recall affects a total of 429 devices.
On May 5, 2022, Medtronic sent an urgent medical device correction notice to customers asking them to identify and quarantine all affected batteries and notify affected patients. The notice includes a patient template to help communicate directly with patients.
It also includes a customer confirmation form to initiate an exchange. The completed form should be returned to [email protected].
Medtronic is replacing the affected batteries with new product and has implemented actions to improve control of the welding process.
The Medtronic HVAD System was approved as a bridge to heart transplantation in 2012. Since then, it’s been fraught with problems.
Earlier in June, the company announced it was stopping all sales of the device and advised physicians to stop implanting it, as reported by this news organization.
Problems related to the Medtronic HVAD System should be reported to the FDA’s MedWatch program.
A version of this article first appeared on Medscape.com.
Heart failure: Medicare cost sharing may put quadruple therapy out of reach
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
FROM THE JOURNAL Of the AMERICAN COLLEGE OF CARDIOLOGY
Experts elevate new drugs for diabetic kidney disease
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
ATLANTA – U.S. clinicians caring for people with diabetes should take a more aggressive approach to using combined medical treatments proven to slow the otherwise relentless progression of chronic kidney disease (CKD), according to a new joint statement by the American Diabetes Association and a major international nephrology organization presented during the annual scientific sessions of the American Diabetes Association (ADA).
The statement elevates treatment with an agent from the sodium-glucose cotransporter 2 (SGLT2) inhibitor class to first-line for people with diabetes and laboratory-based evidence of advancing CKD. It also re-emphasizes the key role of concurrent first-line treatment with a renin-angiotensin system inhibitor (an ACE inhibitor or angiotensin-receptor blocker), metformin, and a statin.
The new statement also urges clinicians to rapidly add treatment with the new nonsteroidal mineralocorticoid receptor antagonist finerenone (Kerendia) for further renal protection in the many patients suitable for treatment with this agent, and it recommends the second-line addition of a glucagon-like peptide-1 (GLP-1) receptor agonist as the best add-on for any patient who needs additional glycemic control on top of metformin and an SGLT2 inhibitor.
The consensus joint statement with these updates came from a nine-member writing group assembled by the ADA and the Kidney Disease: Improving Global Outcomes (KDIGO) organization.
“We’re going to try to make this feasible. We have to; I don’t think we have a choice,” commented Amy K. Mottl, MD, a nephrologist at the University of North Carolina, Chapel Hill. Dr. Mottl was not involved with writing the consensus statement but has been active in the Diabetic Kidney Disease Collaborative of the American Society of Nephrology, another group promoting a more aggressive multidrug-class approach to treating CKD in people with diabetes.
Wider use of costly drugs
Adoption of this evidence-based approach by U.S. clinicians will both increase the number of agents that many patients receive and drive a significant uptick in the cost and complexity of patient care, a consequence acknowledged by the authors of the joint statement as well as outside experts.
But they view this as unavoidable given what’s now known about the high incidence of worsening CKD in patients with diabetes and the types of interventions proven to blunt this.
Much of the financial implication stems from the price of agents from the new drug classes now emphasized in the consensus recommendations – SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists. All these drugs currently remain on-patent with relatively expensive retail prices in the range of about $600 to $1,000/month.
Commenting on the cost concerns, Dr. Mottl highlighted that she currently has several patients in her practice on agents from two or more of these newer classes, and she has generally found it possible for patients to get much of their expenses covered by insurers and through drug-company assistance programs.
“The major gap is patients on Medicare,” she noted in an interview, because the Federal health insurance program does not allow beneficiaries to receive rebates for their drug costs. “The Diabetic Kidney Disease Collaborative is currently lobbying members of Congress to lift that barrier,” she emphasized.
Improved alignment
Details of the KDIGO recommendations feature in a guideline from that organization that appeared as a draft document online in March 2022. The ADA’s version recently appeared as an update to its Standards of Medical Care in Diabetes – 2022, as reported by this news organization. A panel of five KDIGO representatives and four members appointed by the ADA produced the harmonization statement.
Recommendations from both organizations were largely in agreement at the outset, but following the panel’s review, the two groups are now “very well-aligned,” said Peter Rossing, MD, DMSc, a diabetologist and professor at the Steno Diabetes Center, Copenhagen, and a KDIGO representative to the writing committee, who presented the joint statement at the ADA meeting.
“These are very important drugs that are vastly underused,” commented Josef Coresh, MD, PhD, an epidemiologist and professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, who specializes in CKD and was not involved with the new statement.
“Coherence and simplicity are what we need so that there are no excuses about moving forward” with the recommended combination treatment, he stressed.
Moving too slow
“No one is resisting using these new medications, but they are just moving too slowly, and data now show that it’s moving more slowly in the United States than elsewhere. That may be partly because U.S. patients are charged much more for these drugs, and partly because U.S. health care is so much more fragmented,” Dr. Coresh said in an interview.
The new joint consensus statement may help, “but the fragmentation of the United States system and COVID-19 are big enemies” for any short-term increased use of the highlighted agents, he added.
Evidence for low U.S. use of SGLT2 inhibitors, finerenone, and GLP-1 receptor agonists is becoming well known.
Dr. Rossing cited a 2019 report from the CURE-CKD registry of more than 600,000 U.S. patients with CKD showing that less than 1% received an SGLT2 inhibitor and less than 1% a GLP-1 receptor agonist. Not all these patients had diabetes, but a subgroup analysis of those with diabetes, prediabetes, or hypertension showed that usage of each of these two classes remained at less than 1% even in this group.
A separate report at the ADA meeting documented that of more than 1.3 million people with type 2 diabetes in the U.S. Veterans Affairs Healthcare System during 2019 and 2020, just 10% received an SGLT2 inhibitor and 7% a GLP-1 receptor agonist. And this is in a setting where drug cost is not a limiting factor.
In addition to focusing on the updated scheme for drug intervention in the consensus statement, Dr. Rossing highlighted several other important points that the writing committee emphasized.
Lifestyle optimization is a core first-line element of managing patients with diabetes and CKD, including a healthy diet, exercise, smoking cessation, and weight control. Other key steps for management include optimization of blood pressure, glucose, and lipids. The statement also calls out a potentially helpful role for continuous glucose monitoring in patients with type 1 or type 2 diabetes and CKD.
The statement notes that patients who also have atherosclerotic cardiovascular disease usually qualify for and could potentially benefit from more intensified lipid management with ezetimibe or a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, as well as a potential role for treatment with antiplatelet agents.
‘If you don’t screen, you won’t find it’
Dr. Rossing also stressed the importance of regular screening for the onset of advanced CKD in patients. Patients whose estimated glomerular filtration rate (eGFR) drops below 60 mL/min/1.73m2, as well as those who develop microalbuminuria with a urinary albumin-to-creatinine ratio of at least 30 mg/g (30 mg/mmol), have a stage of CKD that warrants the drug interventions he outlined.
Guidelines from both the ADA and KDIGO were already in place, recommending annual screening of patients with diabetes for both these parameters starting at diagnosis of type 2 diabetes or 5 years following initial diagnosis of type 1 diabetes.
“If you don’t screen, you won’t find it, and you won’t be able to treat,” Dr. Rossing warned. He also highlighted the panel’s recommendation to treat these patients with an SGLT2 inhibitor as long as their eGFR is at least 20 mL/min/1.73m2. Treatment can then continue even when their eGFR drops lower.
Starting treatment with finerenone requires that patients have a normal level of serum potassium, he emphasized.
One reason for developing the new ADA and KDIGO statement is that “discrepancies in clinical practice guideline recommendations from various professional organizations add to confusion that impedes understanding of best practices,” write Katherine R. Tuttle, MD, and associates in a recent commentary.
The goal of the new statement is to harmonize and promote the shared recommendations of the two organizations, added Dr. Tuttle, who is executive director for research at Providence Healthcare, Spokane, Washington, and a KDIGO representative on the statement writing panel.
Dr. Mottl has reported being a consultant to Bayer. Dr. Rossing has reported being a consultant to or speaker on behalf of Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, MSD, Mundipharma, Novo Nordisk, Sanofi Aventis, and Vifor, as well as receiving research grants from AstraZeneca and Novo Nordisk. Dr. Coresh has reported no relevant financial relationships. Dr. Tuttle has reported being a consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Goldfinch Bio, Janssen, Novo Nordisk, and Travere; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Goldfinch Bio, Novo Nordisk, and Travere; and receiving research funding from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Goldfinch Bio, Novo Nordisk, and Travere.
A version of this article first appeared on Medscape.com.
AT ADA 2022
Air pollution tied to ventricular arrhythmias in those with ICDs
Ventricular arrhythmias more commonly occur on days when there are higher levels of air pollution, especially with fine particulate matter (PM), a new study suggests.
The investigators studied the relationship between air pollution and ventricular arrhythmias in Piacenza, Italy by examining 5-year data on patients who received an implantable cardioverter defibrillator (ICD).
They found a significant association between PM2.5 levels and ventricular arrhythmias, especially those treated with direct current shock. Moreover, higher levels of PM2.5 and PM10 were associated with increased risk of all ventricular arrhythmias.
“These data confirm that environmental pollution is not only a climate emergency but also a public health problem,” lead author Alessia Zanni, currently at Maggiore Hospital, Bologna, Italy, and previously at Piacenza Hospital, said in an interview.
“The study suggests that the survival of patients with heart disease is affected not only by pharmacological therapies and advances in cardiology, but also by the air that they breathe,” she said.
The results were presented at European Society of Cardiology Heart Failure 2022.
More ED visits
The World Health Organization estimates around 7 million people die every year from exposure to polluted air, “as 91% of the world’s population lives in areas where air contaminants exceed safety levels,” Dr. Zanni said. Furthermore, “air pollution has been defined as the fourth-highest ranking risk factor for mortality – more important than LDL cholesterol, obesity, physical activity, or alcohol use.”
She noted that Piacenza has “historically been very attentive to the issues of early defibrillation and cardiac arrest.” Her group had previously found a correlation between out-of-hospital cardiac arrests and air pollution in the general population.
Moreover, her group recently observed that ED visits for patients with ICDs “tended to cluster; on some special days, many patients with ICDs had cardiac arrhythmias, and during those days, air pollution levels were particularly high.”
Her group therefore decided to compare the concentration of air pollutants on days when patients suffered from an arrhythmia event versus pollution levels on days without an arrhythmia, she said.
Further piece in a complex puzzle
The researchers studied 146 patients with ICDs between January 2013 and December 2017, assigning exposures (short, mid, and long term) to these patients based on their residential addresses.
They extracted day-by-day urban PM10, PM2.5, CO, NO2, and O3 levels from the Environmental Protection Agency monitoring stations and then, using time-stratified case-crossover analysis methodology, they calculated the association of ventricular arrhythmia onset with 0- to 7-day moving averages of the various air pollutants prior to the event.
Patients had received their ICD to control cardiac dysfunction brought on by previous myocardial infarction (n = 93), genetic or inflammatory conditions (n = 53), secondary prevention after a lethal arrhythmia (n = 67), and primary prevention (n = 79).
Of the 440 ventricular arrhythmias recorded, 322 were treated with antitachycardia pacing, while the remaining 118 were treated with direct current shock.
The researchers found a significant association between PM2.5 levels and ventricular arrhythmia treated with shock, corresponding to a 15% increased risk or every additional 10mg/m3 (P < .019).
They also found that, when PM2.5 concentrations were elevated by 1 mg/m3 for an entire week, compared with average levels, there was a 2.4% higher likelihood of ventricular arrhythmias, regardless of the temperature, and when PM10 was 1 mg/m3 above average for a week, there was a 2.1% increased risk for arrhythmias (odds ratio, 1,024; 95% confidence interval, 1,009-1,040] and OR, 1,021; 95% CI, 1,009-1,033, respectively), Dr. Zanni reported.
“Since the majority of out-of-hospital cardiac arrest causes still remain unclear, our data add a further piece to the complex puzzle of cardiac arrest triggers,” Dr. Zanni commented. “We think that particulate matter can cause acute inflammation of the heart muscle and potentially act as a trigger for lethal cardiac arrhythmias.
“As these toxic particles are emitted from power plants, industries, and cars, we think that cardiovascular research should highlight these new findings to promote green projects among the general population, clarifying the risks to the health of the human being, and we think strategies to prevent air pollutant exposure in high-risk patients [with previous cardiac disease] should be developed,” she added.
Further, “we advise patients at risk, during days with high PM2.5 (> 35 mg/m3) and PM10 (> 50 mg/m3) to use a mask of the N95 type outdoors, to reduce time spent outdoors – particularly in traffic – and to improve home air filtration,” Dr. Zanni said.
Entering the mainstream
In a comment, Joel Kaufman, MD, MPH, professor of internal medicine and environmental health, University of Washington, Seattle, said the study “adds to a fairly substantial literature already on this topic of short-term exposure to air pollution.”
The evidence that air pollutants “can be a trigger of worsening of cardiovascular disease is fairly consistent at this time, and although the effect sizes are small, they are consistent,” said Dr. Kaufman, who was the chair of the writing group for the American Heart Association’s 2020 policy statement, “Guidance to Reduce Cardiovascular Burden of Ambient Air Pollutants.”
“The research into this issue has become clearer during the past 10 years but still is not in the mainstream of most cardiologists’ awareness. They tend to focus more on controlling cholesterol and performing procedures, etc., but there are modifiable risk factors like air pollution that are increasingly recognized as being part of the picture,” said Dr. Kaufman, who was not involved with the current study.
Dr. Zanni added: “It is important that politics work hand in hand with the scientific community in order to win the battle against global warming, which will reduce the number of cardiovascular deaths – the leading cause of death worldwide – as well as environmental integrity.”
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Dr. Zanni and coauthors and Dr. Kaufman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ventricular arrhythmias more commonly occur on days when there are higher levels of air pollution, especially with fine particulate matter (PM), a new study suggests.
The investigators studied the relationship between air pollution and ventricular arrhythmias in Piacenza, Italy by examining 5-year data on patients who received an implantable cardioverter defibrillator (ICD).
They found a significant association between PM2.5 levels and ventricular arrhythmias, especially those treated with direct current shock. Moreover, higher levels of PM2.5 and PM10 were associated with increased risk of all ventricular arrhythmias.
“These data confirm that environmental pollution is not only a climate emergency but also a public health problem,” lead author Alessia Zanni, currently at Maggiore Hospital, Bologna, Italy, and previously at Piacenza Hospital, said in an interview.
“The study suggests that the survival of patients with heart disease is affected not only by pharmacological therapies and advances in cardiology, but also by the air that they breathe,” she said.
The results were presented at European Society of Cardiology Heart Failure 2022.
More ED visits
The World Health Organization estimates around 7 million people die every year from exposure to polluted air, “as 91% of the world’s population lives in areas where air contaminants exceed safety levels,” Dr. Zanni said. Furthermore, “air pollution has been defined as the fourth-highest ranking risk factor for mortality – more important than LDL cholesterol, obesity, physical activity, or alcohol use.”
She noted that Piacenza has “historically been very attentive to the issues of early defibrillation and cardiac arrest.” Her group had previously found a correlation between out-of-hospital cardiac arrests and air pollution in the general population.
Moreover, her group recently observed that ED visits for patients with ICDs “tended to cluster; on some special days, many patients with ICDs had cardiac arrhythmias, and during those days, air pollution levels were particularly high.”
Her group therefore decided to compare the concentration of air pollutants on days when patients suffered from an arrhythmia event versus pollution levels on days without an arrhythmia, she said.
Further piece in a complex puzzle
The researchers studied 146 patients with ICDs between January 2013 and December 2017, assigning exposures (short, mid, and long term) to these patients based on their residential addresses.
They extracted day-by-day urban PM10, PM2.5, CO, NO2, and O3 levels from the Environmental Protection Agency monitoring stations and then, using time-stratified case-crossover analysis methodology, they calculated the association of ventricular arrhythmia onset with 0- to 7-day moving averages of the various air pollutants prior to the event.
Patients had received their ICD to control cardiac dysfunction brought on by previous myocardial infarction (n = 93), genetic or inflammatory conditions (n = 53), secondary prevention after a lethal arrhythmia (n = 67), and primary prevention (n = 79).
Of the 440 ventricular arrhythmias recorded, 322 were treated with antitachycardia pacing, while the remaining 118 were treated with direct current shock.
The researchers found a significant association between PM2.5 levels and ventricular arrhythmia treated with shock, corresponding to a 15% increased risk or every additional 10mg/m3 (P < .019).
They also found that, when PM2.5 concentrations were elevated by 1 mg/m3 for an entire week, compared with average levels, there was a 2.4% higher likelihood of ventricular arrhythmias, regardless of the temperature, and when PM10 was 1 mg/m3 above average for a week, there was a 2.1% increased risk for arrhythmias (odds ratio, 1,024; 95% confidence interval, 1,009-1,040] and OR, 1,021; 95% CI, 1,009-1,033, respectively), Dr. Zanni reported.
“Since the majority of out-of-hospital cardiac arrest causes still remain unclear, our data add a further piece to the complex puzzle of cardiac arrest triggers,” Dr. Zanni commented. “We think that particulate matter can cause acute inflammation of the heart muscle and potentially act as a trigger for lethal cardiac arrhythmias.
“As these toxic particles are emitted from power plants, industries, and cars, we think that cardiovascular research should highlight these new findings to promote green projects among the general population, clarifying the risks to the health of the human being, and we think strategies to prevent air pollutant exposure in high-risk patients [with previous cardiac disease] should be developed,” she added.
Further, “we advise patients at risk, during days with high PM2.5 (> 35 mg/m3) and PM10 (> 50 mg/m3) to use a mask of the N95 type outdoors, to reduce time spent outdoors – particularly in traffic – and to improve home air filtration,” Dr. Zanni said.
Entering the mainstream
In a comment, Joel Kaufman, MD, MPH, professor of internal medicine and environmental health, University of Washington, Seattle, said the study “adds to a fairly substantial literature already on this topic of short-term exposure to air pollution.”
The evidence that air pollutants “can be a trigger of worsening of cardiovascular disease is fairly consistent at this time, and although the effect sizes are small, they are consistent,” said Dr. Kaufman, who was the chair of the writing group for the American Heart Association’s 2020 policy statement, “Guidance to Reduce Cardiovascular Burden of Ambient Air Pollutants.”
“The research into this issue has become clearer during the past 10 years but still is not in the mainstream of most cardiologists’ awareness. They tend to focus more on controlling cholesterol and performing procedures, etc., but there are modifiable risk factors like air pollution that are increasingly recognized as being part of the picture,” said Dr. Kaufman, who was not involved with the current study.
Dr. Zanni added: “It is important that politics work hand in hand with the scientific community in order to win the battle against global warming, which will reduce the number of cardiovascular deaths – the leading cause of death worldwide – as well as environmental integrity.”
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Dr. Zanni and coauthors and Dr. Kaufman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ventricular arrhythmias more commonly occur on days when there are higher levels of air pollution, especially with fine particulate matter (PM), a new study suggests.
The investigators studied the relationship between air pollution and ventricular arrhythmias in Piacenza, Italy by examining 5-year data on patients who received an implantable cardioverter defibrillator (ICD).
They found a significant association between PM2.5 levels and ventricular arrhythmias, especially those treated with direct current shock. Moreover, higher levels of PM2.5 and PM10 were associated with increased risk of all ventricular arrhythmias.
“These data confirm that environmental pollution is not only a climate emergency but also a public health problem,” lead author Alessia Zanni, currently at Maggiore Hospital, Bologna, Italy, and previously at Piacenza Hospital, said in an interview.
“The study suggests that the survival of patients with heart disease is affected not only by pharmacological therapies and advances in cardiology, but also by the air that they breathe,” she said.
The results were presented at European Society of Cardiology Heart Failure 2022.
More ED visits
The World Health Organization estimates around 7 million people die every year from exposure to polluted air, “as 91% of the world’s population lives in areas where air contaminants exceed safety levels,” Dr. Zanni said. Furthermore, “air pollution has been defined as the fourth-highest ranking risk factor for mortality – more important than LDL cholesterol, obesity, physical activity, or alcohol use.”
She noted that Piacenza has “historically been very attentive to the issues of early defibrillation and cardiac arrest.” Her group had previously found a correlation between out-of-hospital cardiac arrests and air pollution in the general population.
Moreover, her group recently observed that ED visits for patients with ICDs “tended to cluster; on some special days, many patients with ICDs had cardiac arrhythmias, and during those days, air pollution levels were particularly high.”
Her group therefore decided to compare the concentration of air pollutants on days when patients suffered from an arrhythmia event versus pollution levels on days without an arrhythmia, she said.
Further piece in a complex puzzle
The researchers studied 146 patients with ICDs between January 2013 and December 2017, assigning exposures (short, mid, and long term) to these patients based on their residential addresses.
They extracted day-by-day urban PM10, PM2.5, CO, NO2, and O3 levels from the Environmental Protection Agency monitoring stations and then, using time-stratified case-crossover analysis methodology, they calculated the association of ventricular arrhythmia onset with 0- to 7-day moving averages of the various air pollutants prior to the event.
Patients had received their ICD to control cardiac dysfunction brought on by previous myocardial infarction (n = 93), genetic or inflammatory conditions (n = 53), secondary prevention after a lethal arrhythmia (n = 67), and primary prevention (n = 79).
Of the 440 ventricular arrhythmias recorded, 322 were treated with antitachycardia pacing, while the remaining 118 were treated with direct current shock.
The researchers found a significant association between PM2.5 levels and ventricular arrhythmia treated with shock, corresponding to a 15% increased risk or every additional 10mg/m3 (P < .019).
They also found that, when PM2.5 concentrations were elevated by 1 mg/m3 for an entire week, compared with average levels, there was a 2.4% higher likelihood of ventricular arrhythmias, regardless of the temperature, and when PM10 was 1 mg/m3 above average for a week, there was a 2.1% increased risk for arrhythmias (odds ratio, 1,024; 95% confidence interval, 1,009-1,040] and OR, 1,021; 95% CI, 1,009-1,033, respectively), Dr. Zanni reported.
“Since the majority of out-of-hospital cardiac arrest causes still remain unclear, our data add a further piece to the complex puzzle of cardiac arrest triggers,” Dr. Zanni commented. “We think that particulate matter can cause acute inflammation of the heart muscle and potentially act as a trigger for lethal cardiac arrhythmias.
“As these toxic particles are emitted from power plants, industries, and cars, we think that cardiovascular research should highlight these new findings to promote green projects among the general population, clarifying the risks to the health of the human being, and we think strategies to prevent air pollutant exposure in high-risk patients [with previous cardiac disease] should be developed,” she added.
Further, “we advise patients at risk, during days with high PM2.5 (> 35 mg/m3) and PM10 (> 50 mg/m3) to use a mask of the N95 type outdoors, to reduce time spent outdoors – particularly in traffic – and to improve home air filtration,” Dr. Zanni said.
Entering the mainstream
In a comment, Joel Kaufman, MD, MPH, professor of internal medicine and environmental health, University of Washington, Seattle, said the study “adds to a fairly substantial literature already on this topic of short-term exposure to air pollution.”
The evidence that air pollutants “can be a trigger of worsening of cardiovascular disease is fairly consistent at this time, and although the effect sizes are small, they are consistent,” said Dr. Kaufman, who was the chair of the writing group for the American Heart Association’s 2020 policy statement, “Guidance to Reduce Cardiovascular Burden of Ambient Air Pollutants.”
“The research into this issue has become clearer during the past 10 years but still is not in the mainstream of most cardiologists’ awareness. They tend to focus more on controlling cholesterol and performing procedures, etc., but there are modifiable risk factors like air pollution that are increasingly recognized as being part of the picture,” said Dr. Kaufman, who was not involved with the current study.
Dr. Zanni added: “It is important that politics work hand in hand with the scientific community in order to win the battle against global warming, which will reduce the number of cardiovascular deaths – the leading cause of death worldwide – as well as environmental integrity.”
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Dr. Zanni and coauthors and Dr. Kaufman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ESC HEART FAILURE 2022
Cause of death in pig heart recipient: New clues
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.
The underlying cause of David Bennett’s death on March 8, two months after he received the heart of a genetically altered pig, remains unknown and is only slightly less mysterious for what can likely be ruled out, suggests a progress report on the case from the director of the cardiac xenotransplantation program where the pioneering surgery took place.
Mr. Bennett died in “diastolic heart failure,” reported Muhammad M. Mohiuddin, MBBS, University of Maryland School of Medicine, Baltimore, “but the mechanism is still under investigation.”
Although the immediate cause could have been single or multiple, evidence so far does not point to immune rejection nor does it support a role for a recently proposed suspect, infection by porcine cytomegalovirus (PCMV), Dr. Mohiuddin observed in front of a standing-room-only audience June 6 at the American Transplant Congress (ATC) in Boston. The congress is a joint meeting of the American Society of Transplant Surgeons (ASTS) and the American Society of Transplantation (AST).
Rocky clinical course
Early characterizations of the patient’s death focused more on his diminished, end-stage clinical condition at the time of the surgery than on immune rejection or other direct effects of the xenograft or on the first-of-its-kind procedure itself.
The 57-year-old Mr. Bennett had presented to the University of Maryland team with nonischemic cardiomyopathy, on multiple inotropes, and requiring an intra-aortic balloon pump, Dr. Mohiuddin said in his ATC presentation. The patient had suffered multiple arrests and resuscitations, and by the time of surgery had been hospitalized for almost 2 months, including 40 days on veno-arterial extracorporeal membrane oxygenation (ECMO).
The transplant procedure itself went as planned until removal of the aortic cross clamp, which triggered a type-A aortic dissection. “We put a graft in the ascending aorta and a stent in the descending aorta. Even after 2 days, we found the dissection extending to the renal artery, so we had to go back and also put a stent in the renal artery,” Dr. Mohiuddin said.
Mr. Bennett also underwent two exploratory laparotomies in the first 10 days after transplantation, after CT imaging revealed signs of possible bowel inflammation and ischemia.
Further, he had to fight back a series of infections that led to major changes to his experimental drug regimen, which included immunosuppressants methylprednisolone and mycophenolate mofetil (MMF), the investigational anti-CD40 antibody KPL-404 (Kiniksa Pharmaceuticals), and the anti-inflammatories etanercept (Enbrel) and tocilizumab (Actemra).
One episode of sepsis, in particular, forced temporary withdrawal of MMF and a reduction in methylprednisolone dosage. It’s unknown whether the 30-day MMF suspension played a role in Mr. Bennett’s ultimate clinical deterioration and death, but it’s “highly possible,” Dr. Mohiuddin said in an interview.
Realistically, Mr. Bennett’s death was likely “multifactorial,” Dr. Mohiuddin said. He was in such poor clinical condition going into the procedure, and afterward confronted so many clinical challenges, that “it’s very difficult to say that one thing caused it.”
That hasn’t lessened speculation that the patient’s heart failed secondary to immunologic rejection or PCMV infection, either in Mr. Bennett or the donor pig.
A role for PCMV?
Weeks after Mr. Bennett’s death, as previously reported, his surgeon announced at a public forum that PCMV had been identified in the transplanted heart and in tissues of the donor pig. Mr. Bennett’s circulation showed traces of the viral DNA but not of the virus itself.
The presence of PCMV in transplanted porcine hearts is a well-recognized potential hazard in animal models but is considered avoidable with proper screening. In Mr. Bennett’s case, preoperative screening of the pig donor missed signs of the virus.
Still, PCMV could potentially have contributed to Mr. Bennett’s death, acknowledged Bartley P. Griffith, MD, University of Maryland School of Medicine, who had announced the PCMV finding in an AST-sponsored April 20 webcast.
Preclinical evidence does suggest that PCMV can harm a xenograft organ, observed David H. Sachs, MD, Columbia University Medical Center, New York, from the audience during the comment period after Dr. Mohiuddin’s presentation.
“Each species has a CMV, and they’re quite species-specific,” observed the renowned surgeon and xenotransplantation immunologist. “We showed almost 10 years ago that if PCMV was in a pig kidney, it led to a much shortened survival of the pig kidney in a baboon. There was never any evidence, however, that the CMV infected the baboon or any baboon cells.”
Dr. Sachs asked Dr. Mohiuddin for confirmation that Mr. Bennett displayed no more than DNAemia, circulating cell-free PCMV DNA presumably shed from the porcine heart, but no sign of the virus itself outside of the heart’s porcine cells.
Cell-free DNA had shown up in Mr. Bennett’s circulation about 20 days after the surgery, with concentrations rising until at least day 50. Post-hoc polymerase chain reaction (PCR) testing disclosed PCMV only in the pig’s spleen and porcine cells of the transplanted heart, Dr. Mohiuddin noted.
“We have not found any evidence that the patient was infected by PCMV,” nor was there evidence of any disease related to PCMV, Dr. Mohiuddin replied.
Nor of ongoing rejection
Mr. Bennett’s new heart passed a critical test in the first post-implantation hours by avoiding acute rejection, a potentially disastrous outcome that three of the pig’s 10 gene edits had been designed to prevent.
Although chronic immune rejection was always a concern despite Mr. Bennett’s novel immunosuppressant regimen, myocardial biopsy on postoperative days 34, 50, and 56 and necropsy showed “no signs of typical xenograft rejection,” Dr. Mohiuddin said at the ATC presentation. But “there’s a chance of atypical rejection which we were not accustomed to.”
By day 50, his diastolic function showed echocardiographic signs of deterioration, and “we started seeing interstitial edema with some extravasation of red blood cells, which we thought would resolve over a period of time,” he said. Eventually, however, “we saw that turn into fibroblasts and scar tissue.”
Mr. Bennett once again went on veno-arterial ECMO but died 10 days later. Once they had seen histologic evidence of fibrosis, Dr. Mohiuddin told this news organization, the team believed the myocardial injury was irreversible. “That was the reason we gave up on recovery.”
Mr. Bennett’s xenotransplantation journey has taught the field a lot, he said. “By no means was this a failure; we consider this a huge success. You can do all the experiments in animal models, but you won’t find out the true mechanism of rejection unless you do these kinds of human experiments.”
Looking ahead to clinical trials
Research involving humans is always subject to vagaries of human nature, including degree of adherence to prescribed therapy and – in xenotransplantation – precautions in place to mitigate any risks to public health. Such risks theoretically include transfer of porcine viruses or other pathogens to the patient and subsequent release into the general population.
Looking ahead to the possibility of clinical trials after this successful xenotransplantation experience, transplant nephrologist and epidemiologist Peter P. Reese, MD, PhD, University of Pennsylvania, Philadelphia, raised the potentially controversial issue in discussion following Dr. Mohiuddin’s presentation.
It’s known that Mr. Bennett had been repeatedly turned down for a conventional allograft transplant primarily because of his history of treatment noncompliance. Should such a record, Dr. Reese asked, be a relative contraindication to enrollment in any future xenotransplantation trials? Or does the field need a standardized gauge of a patient’s readiness, once discharged, to adhere not only to all medications – including those that fight infection – but also with rules established for public safety, such as routine contact reporting?
“It makes me wonder about choosing a noncompliant patient for these trials,” Dr. Reese said. “If we discharge a patient from the hospital who is at risk for a zoonotic infection that could spread if they basically refuse to cooperate with us or with public health authorities, it really could have negative consequences for the reputation of the field.”
Dr. Mohiuddin agreed such concerns are valid. Mr. Bennett “and all his immediate contacts” signed consent forms acknowledging their willingness to be followed should he be discharged. Mr. Bennett himself “signed a consent to inform us if he has any other intimate contact with someone,” he said in an interview.
“But those are only on paper.” Had Mr. Bennett survived to be discharged, Dr. Mohuiddin said, “no one knows how he would have behaved.”
Dr. Mohiuddin said the research staff had prepared to monitor Mr. Bennett at his home if that’s what it took. “We were ready to follow him as long as we could. There was a surveillance plan in place.”
A version of this article first appeared on Medscape.com.