User login
Boosting hypertension screening, treatment would cut global mortality 7%
If 80% of individuals with hypertension were screened, 80% received treatment, and 80% then reached guideline-specified targets, up to 200 million cases of cardiovascular disease (CVD) and 130 million deaths could be averted by 2050, a modeling study suggests.
Achievement of the 80-80-80 target “could be one of the single most important global public health accomplishments of the coming decades,” according to the authors.
“We need to reprioritize hypertension care in our practices,” principal investigator David A. Watkins, MD, MPH, University of Washington, Seattle, told this news organization. “Only about one in five persons with hypertension around the world has their blood pressure well controlled. Oftentimes, clinicians are focused on addressing patients’ other health needs, many of which can be pressing in the short term, and we forget to talk about blood pressure, which has more than earned its reputation as ‘the silent killer.’ ”
The modeling study was published online in Nature Medicine, with lead author Sarah J. Pickersgill, MPH, also from the University of Washington.
Two interventions, three scenarios
Dr. Watkins and colleagues based their analysis on two approaches to blood pressure (BP) control shown to be beneficial: drug treatment to a systolic BP of either 130 mm Hg or 140 mm Hg or less, depending on local guidelines, and dietary sodium reduction, as recommended by the World Health Organization.
The team modeled the impacts of these interventions in 182 countries according to three scenarios:
- Business as usual (control): allowing hypertension to increase at historic rates of change and mean sodium intake to remain at current levels
- Progress: matching historically high-performing countries (for example, accelerating hypertension control by about 3% per year at intermediate levels of intervention coverage) while lowering mean sodium intake by 15% by 2030
- Aspirational: hypertension control achieved faster than historically high-performing countries (about 4% per year) and mean sodium intake decreased by 30% by 2027
The analysis suggests that in the progressive scenario, all countries could achieve 80-80-80 targets by 2050 and most countries by 2040; the aspirational scenario would have all countries meeting them by 2040. That would result in reductions in all-cause mortality of 4%-7% (76 million to 130 million deaths averted) with progressive and aspirational interventions, respectively, compared with the control scenario.
There would also be a slower rise in expected CVD from population growth and aging (110 million to 200 million cases averted). That is, the probability of dying from any CVD cause between the ages of 30 and 80 years would be reduced by 16% in the progressive scenario and 26% in the aspirational scenario.
Of note, about 83%-85% of the potential mortality reductions would result from scaling up hypertension treatment in the progressive and aspirational scenarios, respectively, with the remaining 15%-17% coming from sodium reduction, the researchers state.
Further, they propose, scaling up BP interventions could reduce CVD inequalities across countries, with low-income and lower-middle-income countries likely experiencing the largest reductions in disease rates and mortality.
Implementation barriers
“Health systems in many low- and middle-income countries have not traditionally been set up to succeed in chronic disease management in primary care,” Dr. Watkins noted. For interventions to be successful, he said, “several barriers need to be addressed, including: low population awareness of chronic diseases like hypertension and diabetes, which leads to low rates of screening and treatment; high out-of-pocket cost and low availability of medicines for chronic diseases; and need for adherence support and provider incentives for improving quality of chronic disease care in primary care settings.”
“Based on the analysis, achieving the 80-80-80 seems feasible, though actually getting there may be much more complicated. I wonder whether countries have the resources to implement the needed policies,” Rodrigo M. Carrillo-Larco, MD, researcher, department of epidemiology and biostatistics, School of Public Health, Imperial College London, told this news organization.
“It may be challenging, particularly after COVID-19, which revealed deficiencies in many health care systems, and care for hypertension may have been disturbed,” said Dr. Carrillo-Larco, who is not connected with the analysis.
That said, simplified BP screening approaches could help maximize the number of people screened overall, potentially identifying those with hypertension and raising awareness, he proposed. His team’s recent study showed that such approaches vary from country to country but are generally reliable and can be used effectively for population screening.
In addition, Dr. Carrillo-Larco said, any efforts by clinicians to improve adherence and help patients achieve BP control “would also have positive effects at the population level.”
The study was supported by a grant from the Bill & Melinda Gates Foundation, with additional funding by a grant to Dr. Watkins from Resolve to Save Lives. No conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
If 80% of individuals with hypertension were screened, 80% received treatment, and 80% then reached guideline-specified targets, up to 200 million cases of cardiovascular disease (CVD) and 130 million deaths could be averted by 2050, a modeling study suggests.
Achievement of the 80-80-80 target “could be one of the single most important global public health accomplishments of the coming decades,” according to the authors.
“We need to reprioritize hypertension care in our practices,” principal investigator David A. Watkins, MD, MPH, University of Washington, Seattle, told this news organization. “Only about one in five persons with hypertension around the world has their blood pressure well controlled. Oftentimes, clinicians are focused on addressing patients’ other health needs, many of which can be pressing in the short term, and we forget to talk about blood pressure, which has more than earned its reputation as ‘the silent killer.’ ”
The modeling study was published online in Nature Medicine, with lead author Sarah J. Pickersgill, MPH, also from the University of Washington.
Two interventions, three scenarios
Dr. Watkins and colleagues based their analysis on two approaches to blood pressure (BP) control shown to be beneficial: drug treatment to a systolic BP of either 130 mm Hg or 140 mm Hg or less, depending on local guidelines, and dietary sodium reduction, as recommended by the World Health Organization.
The team modeled the impacts of these interventions in 182 countries according to three scenarios:
- Business as usual (control): allowing hypertension to increase at historic rates of change and mean sodium intake to remain at current levels
- Progress: matching historically high-performing countries (for example, accelerating hypertension control by about 3% per year at intermediate levels of intervention coverage) while lowering mean sodium intake by 15% by 2030
- Aspirational: hypertension control achieved faster than historically high-performing countries (about 4% per year) and mean sodium intake decreased by 30% by 2027
The analysis suggests that in the progressive scenario, all countries could achieve 80-80-80 targets by 2050 and most countries by 2040; the aspirational scenario would have all countries meeting them by 2040. That would result in reductions in all-cause mortality of 4%-7% (76 million to 130 million deaths averted) with progressive and aspirational interventions, respectively, compared with the control scenario.
There would also be a slower rise in expected CVD from population growth and aging (110 million to 200 million cases averted). That is, the probability of dying from any CVD cause between the ages of 30 and 80 years would be reduced by 16% in the progressive scenario and 26% in the aspirational scenario.
Of note, about 83%-85% of the potential mortality reductions would result from scaling up hypertension treatment in the progressive and aspirational scenarios, respectively, with the remaining 15%-17% coming from sodium reduction, the researchers state.
Further, they propose, scaling up BP interventions could reduce CVD inequalities across countries, with low-income and lower-middle-income countries likely experiencing the largest reductions in disease rates and mortality.
Implementation barriers
“Health systems in many low- and middle-income countries have not traditionally been set up to succeed in chronic disease management in primary care,” Dr. Watkins noted. For interventions to be successful, he said, “several barriers need to be addressed, including: low population awareness of chronic diseases like hypertension and diabetes, which leads to low rates of screening and treatment; high out-of-pocket cost and low availability of medicines for chronic diseases; and need for adherence support and provider incentives for improving quality of chronic disease care in primary care settings.”
“Based on the analysis, achieving the 80-80-80 seems feasible, though actually getting there may be much more complicated. I wonder whether countries have the resources to implement the needed policies,” Rodrigo M. Carrillo-Larco, MD, researcher, department of epidemiology and biostatistics, School of Public Health, Imperial College London, told this news organization.
“It may be challenging, particularly after COVID-19, which revealed deficiencies in many health care systems, and care for hypertension may have been disturbed,” said Dr. Carrillo-Larco, who is not connected with the analysis.
That said, simplified BP screening approaches could help maximize the number of people screened overall, potentially identifying those with hypertension and raising awareness, he proposed. His team’s recent study showed that such approaches vary from country to country but are generally reliable and can be used effectively for population screening.
In addition, Dr. Carrillo-Larco said, any efforts by clinicians to improve adherence and help patients achieve BP control “would also have positive effects at the population level.”
The study was supported by a grant from the Bill & Melinda Gates Foundation, with additional funding by a grant to Dr. Watkins from Resolve to Save Lives. No conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
If 80% of individuals with hypertension were screened, 80% received treatment, and 80% then reached guideline-specified targets, up to 200 million cases of cardiovascular disease (CVD) and 130 million deaths could be averted by 2050, a modeling study suggests.
Achievement of the 80-80-80 target “could be one of the single most important global public health accomplishments of the coming decades,” according to the authors.
“We need to reprioritize hypertension care in our practices,” principal investigator David A. Watkins, MD, MPH, University of Washington, Seattle, told this news organization. “Only about one in five persons with hypertension around the world has their blood pressure well controlled. Oftentimes, clinicians are focused on addressing patients’ other health needs, many of which can be pressing in the short term, and we forget to talk about blood pressure, which has more than earned its reputation as ‘the silent killer.’ ”
The modeling study was published online in Nature Medicine, with lead author Sarah J. Pickersgill, MPH, also from the University of Washington.
Two interventions, three scenarios
Dr. Watkins and colleagues based their analysis on two approaches to blood pressure (BP) control shown to be beneficial: drug treatment to a systolic BP of either 130 mm Hg or 140 mm Hg or less, depending on local guidelines, and dietary sodium reduction, as recommended by the World Health Organization.
The team modeled the impacts of these interventions in 182 countries according to three scenarios:
- Business as usual (control): allowing hypertension to increase at historic rates of change and mean sodium intake to remain at current levels
- Progress: matching historically high-performing countries (for example, accelerating hypertension control by about 3% per year at intermediate levels of intervention coverage) while lowering mean sodium intake by 15% by 2030
- Aspirational: hypertension control achieved faster than historically high-performing countries (about 4% per year) and mean sodium intake decreased by 30% by 2027
The analysis suggests that in the progressive scenario, all countries could achieve 80-80-80 targets by 2050 and most countries by 2040; the aspirational scenario would have all countries meeting them by 2040. That would result in reductions in all-cause mortality of 4%-7% (76 million to 130 million deaths averted) with progressive and aspirational interventions, respectively, compared with the control scenario.
There would also be a slower rise in expected CVD from population growth and aging (110 million to 200 million cases averted). That is, the probability of dying from any CVD cause between the ages of 30 and 80 years would be reduced by 16% in the progressive scenario and 26% in the aspirational scenario.
Of note, about 83%-85% of the potential mortality reductions would result from scaling up hypertension treatment in the progressive and aspirational scenarios, respectively, with the remaining 15%-17% coming from sodium reduction, the researchers state.
Further, they propose, scaling up BP interventions could reduce CVD inequalities across countries, with low-income and lower-middle-income countries likely experiencing the largest reductions in disease rates and mortality.
Implementation barriers
“Health systems in many low- and middle-income countries have not traditionally been set up to succeed in chronic disease management in primary care,” Dr. Watkins noted. For interventions to be successful, he said, “several barriers need to be addressed, including: low population awareness of chronic diseases like hypertension and diabetes, which leads to low rates of screening and treatment; high out-of-pocket cost and low availability of medicines for chronic diseases; and need for adherence support and provider incentives for improving quality of chronic disease care in primary care settings.”
“Based on the analysis, achieving the 80-80-80 seems feasible, though actually getting there may be much more complicated. I wonder whether countries have the resources to implement the needed policies,” Rodrigo M. Carrillo-Larco, MD, researcher, department of epidemiology and biostatistics, School of Public Health, Imperial College London, told this news organization.
“It may be challenging, particularly after COVID-19, which revealed deficiencies in many health care systems, and care for hypertension may have been disturbed,” said Dr. Carrillo-Larco, who is not connected with the analysis.
That said, simplified BP screening approaches could help maximize the number of people screened overall, potentially identifying those with hypertension and raising awareness, he proposed. His team’s recent study showed that such approaches vary from country to country but are generally reliable and can be used effectively for population screening.
In addition, Dr. Carrillo-Larco said, any efforts by clinicians to improve adherence and help patients achieve BP control “would also have positive effects at the population level.”
The study was supported by a grant from the Bill & Melinda Gates Foundation, with additional funding by a grant to Dr. Watkins from Resolve to Save Lives. No conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
Two distinct phenotypes of COVID-related myocarditis emerge
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
Researchers from France have identified two distinct phenotypes of fulminant COVID-19–related myocarditis in adults, with different clinical presentations, immunologic profiles, and outcomes.
Differentiation between the two bioclinical entities is important to understand for patient management and further pathophysiological studies, they said.
The first phenotype occurs early (within a few days) in acute SARS-CoV-2 infection, with active viral replication (polymerase chain reaction positive) in adults who meet criteria for multisystem inflammatory syndrome (MIS-A+).
In this early phenotype, there is “limited systemic inflammation without skin and mucosal involvement, but myocardial dysfunction is fulminant and frequently associated with large pericardial effusions. These cases more often require extracorporeal membrane oxygenation [ECMO],” Guy Gorochov, MD, PhD, Sorbonne University, Paris, said in an interview.
The second is a delayed, postinfectious, immune-driven phenotype that occurs in adults who fail to meet the criteria for MIS-A (MIS-A–).
This phenotype occurs weeks after SARS-CoV-2 infection, usually beyond detectable active viral replication (PCR–) in the context of specific immune response and severe systemic inflammation with skin and mucosal involvement. Myocardial dysfunction is more progressive and rarely associated with large pericardial effusions, Dr. Gorochov explained.
The study was published in the Journal of the American College of Cardiology.
Evolving understanding
The findings are based on a retrospective analysis of 38 patients without a history of COVID-19 vaccination who were admitted to the intensive care unit from March 2020 to June 2021 for suspected fulminant COVID-19 myocarditis.
Patients were confirmed to have SARS-CoV-2 infection by PCR and/or by serologic testing. As noted in other studies, the patients were predominantly young men (66%; median age, 27.5 years). Twenty-five (66%) patients were MIS-A+ and 13 (34%) were MIS-A–.
In general, the MIS-A– patients were sicker and had worse outcomes.
Specifically, compared with the MIS-A+ patients, MIS-A– patients had a shorter time between the onset of COVID-19 symptoms and the development of myocarditis, a shorter time to ICU admission, and more severe presentations assessed using lower left ventricular ejection fraction and sequential organ failure assessment scores.
MIS-A– patients also had higher lactate levels, were more likely to need venoarterial ECMO (92% vs 16%), had higher ICU mortality (31% vs. 4%), and a had lower probability of survival at 3 months (68% vs. 96%), compared with their MIS-A+ peers.
Immunologic differences
The immunologic profiles of these two distinct clinical phenotypes also differed.
In MIS-A– early-type COVID-19 myocarditis, RNA polymerase III autoantibodies are frequently positive and serum levels of antiviral interferon-alpha and granulocyte-attracting interleukin-8 are elevated.
In contrast, in MIS-A+ delayed-type COVID-19 myocarditis, RNA polymerase III autoantibodies are negative and serum levels of IL-17 and IL-22 are highly elevated.
“We suggest that IL-17 and IL-22 are novel criteria that should help to assess in adults the recently recognized MIS-A,” Dr. Gorochov told this news organization. “It should be tested whether IL-17 and IL-22 are also elevated in children with MIS-C.”
The researchers also observed “extremely” high serum IL-10 levels in both patient groups. This has been previously associated with severe myocardial injury and an increase in the risk for death in severe COVID-19 patients.
The researchers said the phenotypic clustering of patients with fulminant COVID-19–related myocarditis “seems relevant” for their management.
MIS-A– cases, owing to the high risk for evolution toward refractory cardiogenic shock, should be “urgently” referred to a center with venoarterial ECMO and closely monitored to prevent a “too-late” cannulation, especially under cardiopulmonary resuscitation, known to be associated with poor outcomes, they advised.
They noted that the five patients who died in their series had late venoarterial ECMO implantation, while undergoing multiple organ failures or resuscitation.
Conversely, the risk for evolution to refractory cardiogenic shock is lower in MIS-A+ cases. However, identifying MIS-A+ cases is “all the more important given that numerous data support the efficacy of corticosteroids and/or intravenous immunoglobulins in MIS-C,” Dr. Gorochov and colleagues wrote.
The authors of a linked editorial said the French team should be “commended on their work in furthering our understanding of fulminant myocarditis related to COVID-19 infection.”
Ajith Nair, MD, Baylor College of Medicine, and Anita Deswal, MD, MPH, University of Texas M.D. Anderson Cancer Center, both in Houston, noted that fulminant myocarditis is rare and can result from either of two mechanisms: viral tropism or an immune-mediated mechanism.
“It remains to be seen whether using antiviral therapy versus immunomodulatory therapy on the basis of clinical and cytokine profiles will yield benefits,” they wrote.
“Fulminant myocarditis invariably requires hemodynamic support and carries a high mortality risk if it is recognized late. However, the long-term prognosis in patients who survive the critical period is favorable, with recovery of myocardial function,” they added.
“This study highlights the ever-shifting understanding of the pathophysiology and therapeutic approaches to fulminant myocarditis,” Dr. Nair and Dr. Deswal concluded.
This research was supported in part by the Foundation of France, French National Research Agency, Sorbonne University, and Clinical Research Hospital. The researchers have filed a patent application based on these results. Dr. Nair and Dr. Deswal have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Hypertension heightens risk for severe COVID-19, even in the fully vaxxed
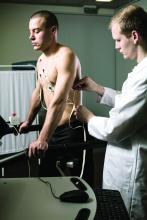
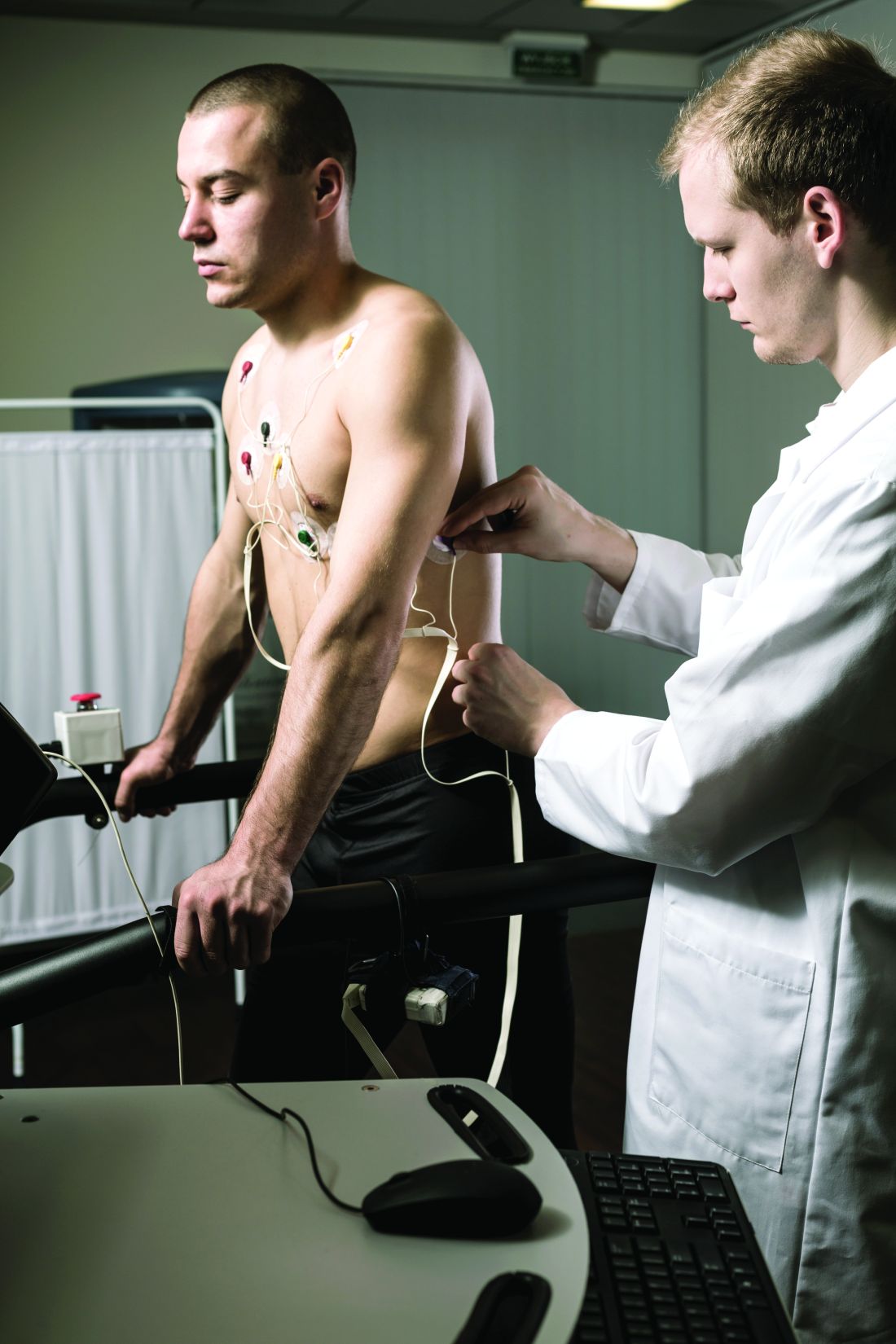
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM HYPERTENSION
‘Stunning variation’ in CV test, procedure costs revealed at top U.S. hospitals
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Wide variation in the cost of common cardiovascular (CV) tests and procedures, from stress tests to coronary interventions, was revealed in a cross-sectional analysis based on publicly available data from 20 top-ranked hospitals in the United States.
The analysis also suggested a low level of compliance with the 2021 Hospital Price Transparency Final Rule among the 20 centers.
“The variation we found in payer-negotiated prices for identical cardiovascular tests and procedures was stunning,” Rishi K. Wadhera, MD, MPP, MPhil, Beth Israel Deaconess Medical Center, Boston, told this news organization.
“For example, there was a 10-fold difference in the median price of an echocardiogram, and these differences were even larger for common procedures” such as percutaneous coronary intervention (PCI) and pacemaker implantation, he said. “It’s hard to argue that this variation reflects quality of care, given that we looked at a top group of highly ranked hospitals.”
“Even more striking was how the price of a cardiovascular test within the very same hospital could differ across commercial insurance companies,” he said. “For example, the price of a stress test varied 5-fold in one hospital, and in another hospital, more than 4-fold for a coronary angiogram.”
Dr. Wadhera is senior author on the study published online as a research letter in JAMA Internal Medicine, with lead author Andrew S. Oseran, MD, MBA, also from Beth Israel Deaconess Medical Center.
Difficulties with data, interpretation
The researchers looked at payer and self-pay cash prices for noninvasive and invasive CV tests and procedures at the U.S. News & World Report 2021 top 20–ranked U.S. hospitals, based in part on Current Procedural Terminology codes.
Price differences among the hospitals were derived from median negotiated prices for each test and procedure at the centers across all payers. The interquartile ratio (IQR) of prices for each test or procedure across payers was used to evaluate within-hospital price variation.
“Only 80% of the hospitals reported prices for some cardiovascular tests and procedures,” Dr. Wadhera said. “For the most part, even among the hospitals that did report this information, it was extremely challenging to navigate and interpret the data provided.”
Further, the team found that only 7 of the 20 hospitals reported prices for all CV tests and procedures. Centers that did not post prices for some tests or procedures are named in the report’s Figure 1 and Figure 2.
The number of insurance plans listed for each test or procedure ranged from 1 to 432 in the analysis. Median prices ranged from $204 to $2,588 for an echocardiogram, $463 to $3,230 for a stress test, $2,821 to $9,382 for right heart catheterization, $2,868 to $9,203 for a coronary angiogram, $657 to $25,521 for a PCI, and $506 to $20,002 for pacemaker implantation, the report states.
A similar pattern was seen for self-pay cash prices.
Within-hospital variation also ranged broadly. For example, the widest IQR ranges were $3,143-$12,926 for a right heart catheterization, $4,011-$14,486 for a coronary angiogram, $11,325-$23,392 for a PCI, and $8,474-$22,694 for pacemaker implantation.
The report cites a number of limitations to the analysis, among those, the need to rely on the hospitals themselves for data quality and accuracy.
‘More needed besides transparency’
“As a means to better understand health care costs, many opined that full price transparency would leverage market dynamics and result in lower costs,” observed Clyde W. Yancy, MD, MSc, professor of medicine and chief of cardiology at Northwestern Medicine, Chicago. The findings “by an expert group of outcomes scientists make clear that more is needed besides price transparency to lower cost,” he said in an interview.
That said, he added, “there are sufficient variations and allowances made for data collection that it is preferable to hold the current findings circumspect at best. Importantly, the voice of the hospitals does not appear.”
Although “price variation among the top 20 hospitals is substantial,” he observed, “without a better assessment of root cause, actual charge capture, prevailing market dynamics – especially nursing and ancillary staff costs – and the general influence of inflation, it is too difficult to emerge with a precise interpretation.”
Across the 20 hospitals, “there are likely to be 20 different business models,” he added, with negotiated prices reflecting “at least regional, if not institutional, variations.”
“These are complex issues. The several-fold price differences in standard procedures are a concern and an area worth further study with the intention of lowering health care costs,” Dr. Yancy said. “But clearly our next efforts should not address lowering prices per se but understanding how prices are set [and] the connection with reimbursement and actual payments.”
Dr. Wadhera discloses receiving personal fees from Abbott and CVS Health unrelated to the current study; disclosures for the other authors are in the report. Dr. Yancy is deputy editor of JAMA Cardiology.
A version of this article first appeared on Medscape.com.
Pig heart transplants and the ethical challenges that lie ahead
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”
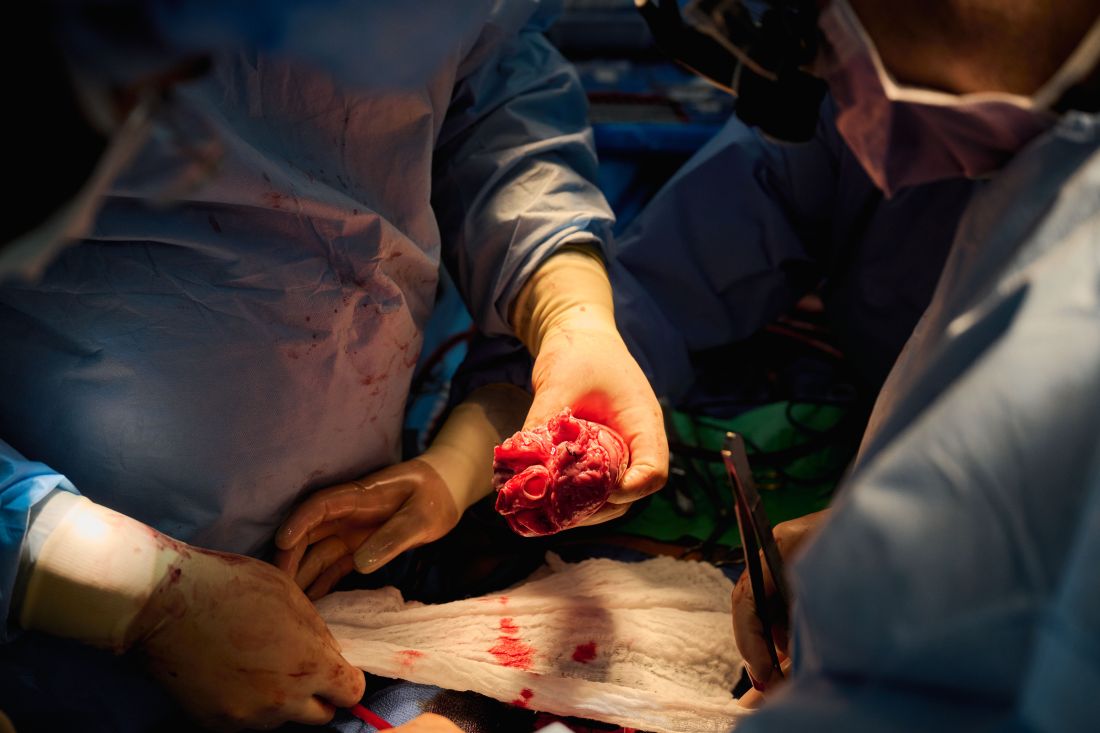
The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”

The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
The long-struggling field of cardiac xenotransplantation has had a very good year.
In January, the University of Maryland made history by keeping a 57-year-old man deemed too sick for a human heart transplant alive for 2 months with a genetically engineered pig heart. On July 12, New York University surgeons reported that heart function was “completely normal with excellent contractility” in two brain-dead patients with pig hearts beating in their chests for 72 hours.
The NYU team approached the project with a decedent model in mind and, after discussions with their IRB equivalent, settled on a 72-hour window because that’s the time they typically keep people ventilated when trying to place their organs, explained Robert A. Montgomery, MD, DPhil, director of the NYU Langone Transplant Institute.
“There’s no real ethical argument for that,” he said in an interview. The consideration is what the family is willing to do when trying to balance doing “something very altruistic and good versus having closure.”
Some families have religious beliefs that burial or interment has to occur very rapidly, whereas others, including one of the family donors, were willing to have the research go on much longer, Dr. Montgomery said. Indeed, the next protocol is being written to consider maintaining the bodies for 2-4 weeks.
“People do vary and you have to kind of accommodate that variation,” he said. “For some people, this isn’t going to be what they’re going to want and that’s why you have to go through the consent process.”
Informed authorization
Arthur L. Caplan, PhD, director of medical ethics at the NYU Langone Medical Center, said the Uniform Anatomical Gift Act recognizes an individual’s right to be an organ donor for transplant and research, but it “mentions nothing about maintaining you in a dead state artificially for research purposes.”
“It’s a major shift in what people are thinking about doing when they die or their relatives die,” he said.
Because organ donation is controlled at the state, not federal, level, the possibility of donating organs for xenotransplantation, like medical aid in dying, will vary between states, observed Dr. Caplan. The best way to ensure that patients whose organs are found to be unsuitable for transplantation have the option is to change state laws.
He noted that cases are already springing up where people are requesting postmortem sperm or egg donations without direct consents from the person who died. “So we have this new area opening up of handling the use of the dead body and we need to bring the law into sync with the possibilities that are out there.”
In terms of informed authorization (informed consent is reserved for the living), Dr. Caplan said there should be written evidence the person wanted to be a donor and, while not required by law, all survivors should give their permission and understand what’s going to be done in terms of the experiment, such as the use of animal parts, when the body will be returned, and the possibility of zoonotic viral infection.
“They have to fully accept that the person is dead and we’re just maintaining them artificially,” he said. “There’s no maintaining anyone who’s alive. That’s a source of a lot of confusion.”
Special committees also need to be appointed with voices from people in organ procurement, law, theology, and patient groups to monitor practice to ensure people who have given permission understood the process, that families have their questions answered independent of the research team, and that clear limits are set on how long experiments will last.
As to what those limits should be: “I think in terms of a week or 2,” Dr. Caplan said. “Obviously we could maintain bodies longer and people have. But I think, culturally in our society, going much past that starts to perhaps stress emotionally, psychologically, family and friends about getting closure.”
“I’m not as comfortable when people say things like, ‘How about 2 months?’ ” he said. “That’s a long time to sort of accept the fact that somebody has died but you can’t complete all the things that go along with the death.”
Dr. Caplan is also uncomfortable with the use of one-off emergency authorizations, as used for Maryland resident David Bennett Sr., who was rejected for standard heart transplantation and required mechanical circulatory support to stay alive.
“It’s too premature, I believe, even to try and rescue someone,” he said. “We need to learn more from the deceased models.”
A better model
Dr. Montgomery noted that primates are very imperfect models for predicting what’s going to happen in humans, and that in order to do xenotransplantation in living humans, there are only two pathways – the one-off emergency authorization or a clinical phase 1 trial.
The decedent model, he said, “will make human trials safer because it’s an intermediate step. You don’t have a living human’s life on the line when you’re trying to do iterative changes and improve the procedure.”

The team, for example, omitted a perfusion pump that was used in the Maryland case and would likely have made its way into phase 1 trials based on baboon data that suggested it was important to have the heart on the pump for hours before it was transplanted, he said. “We didn’t do any of that. We just did it like we would do a regular heart transplant and it started right up, immediately, and started to work.”
The researchers did not release details on the immunosuppression regimen, but noted that, unlike Maryland, they also did not use the experimental anti-CD40 antibody to tamp down the recipients’ immune system.
Although Mr. Bennett’s autopsy did not show any conventional sign of graft rejection, the transplanted pig heart was infected with porcine cytomegalovirus (PCMV) and Mr. Bennett showed traces of DNA from PCMV in his circulation.
Nailing down safety
Dr. Montgomery said he wouldn’t rule out xenotransplantation in a living human, but that the safety issues need to be nailed down. “I think that the tests used on the pig that was the donor for the Bennett case were not sensitive enough for latent virus, and that’s how it slipped through. So there was a bit of going back to the drawing board, really looking at each of the tests, and being sure we had the sensitivity to pick up a latent virus.”
He noted that United Therapeutics, which funded the research and provided the engineered pigs through its subsidiary Revivicor, has created and validated a more sensitive polymerase chain reaction test that covers some 35 different pathogens, microbes, and parasites. NYU has also developed its own platform to repeat the testing and for monitoring after the transplant. “The ones that we’re currently using would have picked up the virus.”
Stuart Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., said “the biggest thing from my perspective is those two amazing families that were willing let this happen. ... If 20 years from now, this is what we’re doing, it’s related to these families being this generous at a really tough time in their lives.”
Dr. Russell said he awaits publication of the data on what the pathology of the heart looks like, but that the experiments “help to give us a lot of reassurance that we don’t need to worry about hyperacute rejection,” which by definition is going to happen in the first 24-48 hours.
That said, longer-term data is essential to potential safety issues. Notably, among the 10 genetic modifications made to the pigs, four were porcine gene knockouts, including a growth hormone receptor knockout to prevent abnormal organ growth inside the recipient’s chest. As a result, the organs seem to be small for the age of the pig and just don’t grow that well, admitted Dr. Montgomery, who said they are currently analyzing this with echocardiography.
Dr. Russell said this may create a sizing issue, but also “if you have a heart that’s more stressed in the pig, from the point of being a donor, maybe it’s not as good a heart as if it was growing normally. But that kind of stuff, I think, is going to take more than two cases and longer-term data to sort out.”
Sharon Hunt, MD, professor emerita, Stanford (Calif.) University Medical Center, and past president of the International Society for Heart Lung Transplantation, said it’s not the technical aspects, but the biology of xenotransplantation that’s really daunting.
“It’s not the physical act of doing it, like they needed a bigger heart or a smaller heart. Those are technical problems but they’ll manage them,” she said. “The big problem is biological – and the bottom line is we don’t really know. We may have overcome hyperacute rejection, which is great, but the rest remains to be seen.”
Dr. Hunt, who worked with heart transplantation pioneer Norman Shumway, MD, and spent decades caring for patients after transplantation, said most families will consent to 24 or 48 hours or even a week of experimentation on a brain-dead loved one, but what the transplant community wants to know is whether this is workable for many months.
“So the fact that the xenotransplant works for 72 hours, yeah, that’s groovy. But, you know, the answer is kind of ‘so what,’ ” she said. “I’d like to see this go for months, like they were trying to do in the human in Maryland.”
For phase 1 trials, even longer-term survival with or without rejection or with rejection that’s treatable is needed, Dr. Hunt suggested.
“We haven’t seen that yet. The Maryland people were very valiant but they lost the cause,” she said. “There’s just so much more to do before we have a viable model to start anything like a phase 1 trial. I’d love it if that happens in my lifetime, but I’m not sure it’s going to.”
Dr. Russell and Dr. Hunt reported no relevant financial relationships. Dr. Caplan reported serving as a director, officer, partner, employee, advisor, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position) and is a contributing author and adviser for Medscape.
A version of this article first appeared on Medscape.com.
Overly tight sodium restriction may worsen HFpEF outcomes
Cutting out almost all salt when preparing meals was associated with a worse prognosis in patients with heart failure with preserved ejection fraction (HFpEF), according to the results of a new study.
Results from a post hoc analysis of the TOPCAT trial show that those with a cooking salt score of zero were at significantly higher risk of the primary outcome of cardiovascular (CV) death, HF hospitalization, and aborted cardiac arrest than those whose score was above zero. Survival was similar in both groups.
“Some patients restrict dietary salt intake as least as possible according to their physicians’ words or their own understanding. However, the present study found that, in patients with heart failure with preserved ejection fraction, overstrict salt restriction could lead to poor prognosis – mainly heart failure hospitalization,” explained professor Chen Liu, MD, and Weihao Liang, MD, Sun Yat-sen University First Affiliated Hospital, Guangzhou, Guangdong, China.
“Thus, when giving salt restriction advice to patients with heart failure with preserved ejection fraction, physicians should be careful instead of just saying “as least as possible,” they said in an email to this news organization.
The study was published in the journal Heart.
The authors note that HF guidelines recommend reduced salt intake, but there’s a lack of high-quality evidence to support those recommendations and no consensus on how low to go.
Previous studies have shown that reduced dietary sodium intake was associated with worse survival and higher readmission rate in patients with HF, whereas the SODIUM HF trial reported earlier this year that dietary sodium intake of less than 100 mmol (1,500 mg) per day did not improve 1-year clinical outcomes but moderately improved quality of life and New York Heart Association functional class.
“In daily clinical practice, we noticed that some physicians advised patients with heart failure to take salt as least as possible, but it could lead to hyponatremia and loss of appetite, which has been frequently reported to be associated with poor prognosis. Thus, we wanted to investigate the potential effect of overstrict salt restriction,” Dr. Liu and Dr. Liang explained.
The investigators examined data from 1,713 participants aged 50 and older with HFpEF (left ventricular ejection fraction 45% or greater) in the phase 3 TOPCAT trial, excluding those from Russia and Georgia. Patients self-reported how much salt they added to cooking staples, such as rice, pasta, potatoes, soup, meat, and vegetables, and were scored as 0 (none), 1 (⅛ teaspoon), 2 (¼ teaspoon), and 3 (½ teaspoon or more) points. Median follow-up was 2.9 years.
TOPCAT failed to show that spironolactone improved CV outcomes over placebo, but regional differences in data from Russia/Georgia and the Americas have raised concerns about its validity.
In the present analysis, almost half the participants (816) had a cooking salt score of 0, 56.4% were male, and 80.8% were White. They were more likely than participants with a salt score greater than zero to have a previous HF hospitalization, diabetes, poor renal function, and a lower ejection fraction (57% vs. 60%). Half were randomly assigned to spironolactone.
Compared with patients with a cooking salt score of 0, patients with a cooking salt score greater than 0 had significantly lower risks of the primary outcome (hazard ratio, 0.760; P = .002) and HF hospitalization (HR, 0.737; P = .003) but not all-cause (HR, 0.838) or CV (HR, 0.782) death.
The findings were consistent after full adjustment, with hazard ratios of 0.834 (P = .046), 0.791 (P = .024), 0.944, and 0.872, respectively.
Results of subgroup analyses suggested that patients aged 70 years or younger (HR, 0.644) and those of Black and other ethnicities (HR, 0.574) were at greater risk of the primary outcome from aggressive restriction of cooking salt.
“It was an interesting but unproved finding,” Dr. Liu and Dr. Liang observed. “One possible explanation is the difference in RAAS [renin-angiotensin-aldosterone system] physiology and its response to salt restriction among races, and the other is the difference in accustomed food, because the cooking salt score only accounted for sodium added during cooking but not sodium from ingredients.”
Spearman correlation analyses showed that the cooking salt score correlated significantly with systolic and diastolic blood pressure, serum sodium, and chloronium levels but not with plasma volume status, suggesting that low sodium intake did not have an intravascular volume contraction effect on patients with HFpEF.
The authors pointed out that the salt score was self-reported, hemodynamic parameters were seldom acquired in TOPCAT, and that reverse causation between low dietary sodium intake and worse HF might still exist, despite a propensity score-matching sensitivity analysis.
Reached for comment, Mary Norine Walsh, MD, the medical director of heart failure and cardiac transplantation, Ascension St. Vincent Heart Center, Indianapolis, said in an email that the authors appropriately excluded patients enrolled from Russia and Georgia because of concerns about the representativeness of patients with HFpEF in these two countries, which has been previously demonstrated.
“What limits the importance of the authors’ findings, which they acknowledge, is that the sodium intake for each patient was self-reported,” she said. “No confirmatory testing was done and recall bias could clearly have played a role.”
“Last, many patients with HFpEF have significant volume overload and dyspnea and appropriate sodium restriction is needed to help address symptoms and achieve a euvolemic state,” added Dr. Walsh, a past president of the American College of Cardiology.
Future trials are needed to determine an optimal salt restriction range for patients with heart failure, Dr. Liu and Dr. Liang suggested. “A randomized controlled trial may be hard to achieve because it is difficult to set a perfect control group. Therefore, an analysis using real-world data with a dose-response curve could be ideal.”
The study was funded by the National Natural Science Foundation of China, Guangdong Natural Science Foundation, and China Postdoctoral Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cutting out almost all salt when preparing meals was associated with a worse prognosis in patients with heart failure with preserved ejection fraction (HFpEF), according to the results of a new study.
Results from a post hoc analysis of the TOPCAT trial show that those with a cooking salt score of zero were at significantly higher risk of the primary outcome of cardiovascular (CV) death, HF hospitalization, and aborted cardiac arrest than those whose score was above zero. Survival was similar in both groups.
“Some patients restrict dietary salt intake as least as possible according to their physicians’ words or their own understanding. However, the present study found that, in patients with heart failure with preserved ejection fraction, overstrict salt restriction could lead to poor prognosis – mainly heart failure hospitalization,” explained professor Chen Liu, MD, and Weihao Liang, MD, Sun Yat-sen University First Affiliated Hospital, Guangzhou, Guangdong, China.
“Thus, when giving salt restriction advice to patients with heart failure with preserved ejection fraction, physicians should be careful instead of just saying “as least as possible,” they said in an email to this news organization.
The study was published in the journal Heart.
The authors note that HF guidelines recommend reduced salt intake, but there’s a lack of high-quality evidence to support those recommendations and no consensus on how low to go.
Previous studies have shown that reduced dietary sodium intake was associated with worse survival and higher readmission rate in patients with HF, whereas the SODIUM HF trial reported earlier this year that dietary sodium intake of less than 100 mmol (1,500 mg) per day did not improve 1-year clinical outcomes but moderately improved quality of life and New York Heart Association functional class.
“In daily clinical practice, we noticed that some physicians advised patients with heart failure to take salt as least as possible, but it could lead to hyponatremia and loss of appetite, which has been frequently reported to be associated with poor prognosis. Thus, we wanted to investigate the potential effect of overstrict salt restriction,” Dr. Liu and Dr. Liang explained.
The investigators examined data from 1,713 participants aged 50 and older with HFpEF (left ventricular ejection fraction 45% or greater) in the phase 3 TOPCAT trial, excluding those from Russia and Georgia. Patients self-reported how much salt they added to cooking staples, such as rice, pasta, potatoes, soup, meat, and vegetables, and were scored as 0 (none), 1 (⅛ teaspoon), 2 (¼ teaspoon), and 3 (½ teaspoon or more) points. Median follow-up was 2.9 years.
TOPCAT failed to show that spironolactone improved CV outcomes over placebo, but regional differences in data from Russia/Georgia and the Americas have raised concerns about its validity.
In the present analysis, almost half the participants (816) had a cooking salt score of 0, 56.4% were male, and 80.8% were White. They were more likely than participants with a salt score greater than zero to have a previous HF hospitalization, diabetes, poor renal function, and a lower ejection fraction (57% vs. 60%). Half were randomly assigned to spironolactone.
Compared with patients with a cooking salt score of 0, patients with a cooking salt score greater than 0 had significantly lower risks of the primary outcome (hazard ratio, 0.760; P = .002) and HF hospitalization (HR, 0.737; P = .003) but not all-cause (HR, 0.838) or CV (HR, 0.782) death.
The findings were consistent after full adjustment, with hazard ratios of 0.834 (P = .046), 0.791 (P = .024), 0.944, and 0.872, respectively.
Results of subgroup analyses suggested that patients aged 70 years or younger (HR, 0.644) and those of Black and other ethnicities (HR, 0.574) were at greater risk of the primary outcome from aggressive restriction of cooking salt.
“It was an interesting but unproved finding,” Dr. Liu and Dr. Liang observed. “One possible explanation is the difference in RAAS [renin-angiotensin-aldosterone system] physiology and its response to salt restriction among races, and the other is the difference in accustomed food, because the cooking salt score only accounted for sodium added during cooking but not sodium from ingredients.”
Spearman correlation analyses showed that the cooking salt score correlated significantly with systolic and diastolic blood pressure, serum sodium, and chloronium levels but not with plasma volume status, suggesting that low sodium intake did not have an intravascular volume contraction effect on patients with HFpEF.
The authors pointed out that the salt score was self-reported, hemodynamic parameters were seldom acquired in TOPCAT, and that reverse causation between low dietary sodium intake and worse HF might still exist, despite a propensity score-matching sensitivity analysis.
Reached for comment, Mary Norine Walsh, MD, the medical director of heart failure and cardiac transplantation, Ascension St. Vincent Heart Center, Indianapolis, said in an email that the authors appropriately excluded patients enrolled from Russia and Georgia because of concerns about the representativeness of patients with HFpEF in these two countries, which has been previously demonstrated.
“What limits the importance of the authors’ findings, which they acknowledge, is that the sodium intake for each patient was self-reported,” she said. “No confirmatory testing was done and recall bias could clearly have played a role.”
“Last, many patients with HFpEF have significant volume overload and dyspnea and appropriate sodium restriction is needed to help address symptoms and achieve a euvolemic state,” added Dr. Walsh, a past president of the American College of Cardiology.
Future trials are needed to determine an optimal salt restriction range for patients with heart failure, Dr. Liu and Dr. Liang suggested. “A randomized controlled trial may be hard to achieve because it is difficult to set a perfect control group. Therefore, an analysis using real-world data with a dose-response curve could be ideal.”
The study was funded by the National Natural Science Foundation of China, Guangdong Natural Science Foundation, and China Postdoctoral Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cutting out almost all salt when preparing meals was associated with a worse prognosis in patients with heart failure with preserved ejection fraction (HFpEF), according to the results of a new study.
Results from a post hoc analysis of the TOPCAT trial show that those with a cooking salt score of zero were at significantly higher risk of the primary outcome of cardiovascular (CV) death, HF hospitalization, and aborted cardiac arrest than those whose score was above zero. Survival was similar in both groups.
“Some patients restrict dietary salt intake as least as possible according to their physicians’ words or their own understanding. However, the present study found that, in patients with heart failure with preserved ejection fraction, overstrict salt restriction could lead to poor prognosis – mainly heart failure hospitalization,” explained professor Chen Liu, MD, and Weihao Liang, MD, Sun Yat-sen University First Affiliated Hospital, Guangzhou, Guangdong, China.
“Thus, when giving salt restriction advice to patients with heart failure with preserved ejection fraction, physicians should be careful instead of just saying “as least as possible,” they said in an email to this news organization.
The study was published in the journal Heart.
The authors note that HF guidelines recommend reduced salt intake, but there’s a lack of high-quality evidence to support those recommendations and no consensus on how low to go.
Previous studies have shown that reduced dietary sodium intake was associated with worse survival and higher readmission rate in patients with HF, whereas the SODIUM HF trial reported earlier this year that dietary sodium intake of less than 100 mmol (1,500 mg) per day did not improve 1-year clinical outcomes but moderately improved quality of life and New York Heart Association functional class.
“In daily clinical practice, we noticed that some physicians advised patients with heart failure to take salt as least as possible, but it could lead to hyponatremia and loss of appetite, which has been frequently reported to be associated with poor prognosis. Thus, we wanted to investigate the potential effect of overstrict salt restriction,” Dr. Liu and Dr. Liang explained.
The investigators examined data from 1,713 participants aged 50 and older with HFpEF (left ventricular ejection fraction 45% or greater) in the phase 3 TOPCAT trial, excluding those from Russia and Georgia. Patients self-reported how much salt they added to cooking staples, such as rice, pasta, potatoes, soup, meat, and vegetables, and were scored as 0 (none), 1 (⅛ teaspoon), 2 (¼ teaspoon), and 3 (½ teaspoon or more) points. Median follow-up was 2.9 years.
TOPCAT failed to show that spironolactone improved CV outcomes over placebo, but regional differences in data from Russia/Georgia and the Americas have raised concerns about its validity.
In the present analysis, almost half the participants (816) had a cooking salt score of 0, 56.4% were male, and 80.8% were White. They were more likely than participants with a salt score greater than zero to have a previous HF hospitalization, diabetes, poor renal function, and a lower ejection fraction (57% vs. 60%). Half were randomly assigned to spironolactone.
Compared with patients with a cooking salt score of 0, patients with a cooking salt score greater than 0 had significantly lower risks of the primary outcome (hazard ratio, 0.760; P = .002) and HF hospitalization (HR, 0.737; P = .003) but not all-cause (HR, 0.838) or CV (HR, 0.782) death.
The findings were consistent after full adjustment, with hazard ratios of 0.834 (P = .046), 0.791 (P = .024), 0.944, and 0.872, respectively.
Results of subgroup analyses suggested that patients aged 70 years or younger (HR, 0.644) and those of Black and other ethnicities (HR, 0.574) were at greater risk of the primary outcome from aggressive restriction of cooking salt.
“It was an interesting but unproved finding,” Dr. Liu and Dr. Liang observed. “One possible explanation is the difference in RAAS [renin-angiotensin-aldosterone system] physiology and its response to salt restriction among races, and the other is the difference in accustomed food, because the cooking salt score only accounted for sodium added during cooking but not sodium from ingredients.”
Spearman correlation analyses showed that the cooking salt score correlated significantly with systolic and diastolic blood pressure, serum sodium, and chloronium levels but not with plasma volume status, suggesting that low sodium intake did not have an intravascular volume contraction effect on patients with HFpEF.
The authors pointed out that the salt score was self-reported, hemodynamic parameters were seldom acquired in TOPCAT, and that reverse causation between low dietary sodium intake and worse HF might still exist, despite a propensity score-matching sensitivity analysis.
Reached for comment, Mary Norine Walsh, MD, the medical director of heart failure and cardiac transplantation, Ascension St. Vincent Heart Center, Indianapolis, said in an email that the authors appropriately excluded patients enrolled from Russia and Georgia because of concerns about the representativeness of patients with HFpEF in these two countries, which has been previously demonstrated.
“What limits the importance of the authors’ findings, which they acknowledge, is that the sodium intake for each patient was self-reported,” she said. “No confirmatory testing was done and recall bias could clearly have played a role.”
“Last, many patients with HFpEF have significant volume overload and dyspnea and appropriate sodium restriction is needed to help address symptoms and achieve a euvolemic state,” added Dr. Walsh, a past president of the American College of Cardiology.
Future trials are needed to determine an optimal salt restriction range for patients with heart failure, Dr. Liu and Dr. Liang suggested. “A randomized controlled trial may be hard to achieve because it is difficult to set a perfect control group. Therefore, an analysis using real-world data with a dose-response curve could be ideal.”
The study was funded by the National Natural Science Foundation of China, Guangdong Natural Science Foundation, and China Postdoctoral Science Foundation. The authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HEART
Heed cardiac risk of BTKis for CLL
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
The report discourages the use of the drugs in patients with heart failure, and it specifies that ibrutinib should be avoided in cases of ventricular fibrillation. The consensus statement appeared in the journal Blood Advances.
However, a physician who studies the intersection of cardiology and oncology questioned the report's methodology and said that it goes too far in its warnings about the use of BTKis. Also, the report is funded by AstraZeneca, which produces acalabrutinib, a rival BTKi product to ibrutinib.
“BTK inhibitors have revolutionized treatment outcomes and strategies in both the upfront and refractory CLL disease settings. Led by ibrutinib, the drugs are associated with dramatic improvements in long-term survival and disease outcomes for most CLL patients,” report co-author and cardiologist Daniel Addison, MD, co-director of the cardio-oncology program at the Ohio State University, said in an interview. “The main cardiac concerns are abnormal heart rhythms, high blood pressure, and heart weakness. It is not completely clear at this time why these things develop when patients are treated with these important drugs.”
For the new consensus statement, colleagues met virtually and examined peer-reviewed research. “Generally, this statement reflects available knowledge from cancer clinical trials,” Dr. Addison said. “Because of the design of these trials, cardiac analyses were secondary analyses. In terms of clinic use, this should be balanced against a large number of heart-focused retrospective examinations specifically describing the cardiac effects of these drugs. Most of the available heart-focused studies have not been prospective trials. Primary outcome heart-focused trials with BTK inhibitors are needed. This statement acknowledges this.”
The report recommends that all patients under consideration for BTKi therapy undergo electrocardiograms and blood pressure measurement, and it states that echocardiograms are appropriate for patients with heart disease or at high risk. Patients under 70 without risk factors may take ibrutinib, acalabrutinib, or zanubrutinib, while the latter two drugs are “generally preferred” in patients with established heart disease, well-controlled atrial fibrillation (AFib), hypertension, heart failure, or valvular heart disease.
The authors noted: “If the patient has difficult-to-manage AF[ib], recent acute coronary syndromes, or difficult to control heart failure, alternatives to BTKi treatment, including venetoclax, should be considered.”
As for patients with heart failure, the authors wrote that BTKis should be avoided, “but this is a relative contraindication, not an absolute one.” Ibrutinib should definitely be avoided because of the risk of AFib.
Finally, the authors stated that “the use of BTKis, especially ibrutinib, should be avoided in patients with a history of ventricular arrhythmias and cardiac arrest. Ibrutinib has been shown to increase the incidence of ventricular arrhythmias and sudden cardiac death. Although data are not yet available regarding whether second-generation BTKis [acalabrutinib or zanubrutinib] are also associated with these events, a Bcl-2 antagonist is preferred to any BTKi in these patients.”
Darryl P. Leong, MBBS, PhD, MPH, director of the cardio-oncology program at McMaster University, Hamilton, Ont., and Hamilton Health Sciences, said in an interview that the consensus statement has important limitations.
“The data extracted were not standardized. The authors of the original research were not contacted to provide data that might have been informative,” he said. “Finally and perhaps most importantly, I am uncertain that the quality of the data on which recommendations are made was well evaluated or described.”
Specifically, Dr. Leong said the report’s conclusions about heart failure and arrhythmias are not “necessarily well-supported by the evidence.”
He added: “While there is some evidence to suggest that BTKIs may increase heart failure risk, ibrutinib leads to substantial reductions in mortality. It is a large extrapolation to accept that a mostly theoretic risk of heart failure –with modest supporting empiric data – should outweigh proven reductions in death.”
As for the recommendation against the use of ibrutinib in patients with ventricular arrhythmias and cardiac arrest, he said the evidence cited by the report – an analysis of adverse event data prompted by a case report and a retrospective analysis – is limited. “The statement that ibrutinib increases the risk of ventricular arrhythmias and sudden death is more of a hypothesis at present, and the evidence to support this hypothesis is far from conclusive.”
As for the future, report co-author Dr. Addison said that “additional prospective and lab-based studies of these drugs are needed to guide how to best manage their cardiac effects in the future. This will be critical, as the use of these drugs continues to rapidly expand. Currently, we do not know a lot about why these heart issues really happen.”
The study was funded by AstraZeneca. Several authors reported multiple disclosures. Dr. Addison disclosed funding from AstraZeneca. Dr. Leong reported consulting and speaker fees from Janssen, maker of ibrutinib, as well as AstraZeneca.
FROM BLOOD ADVANCES
Adding social determinants of health to AI models boosts HF risk prediction in Black patients
The addition of social determinants of health (SDOH) to machine-learning risk-prediction models improved forecasts of in-hospital mortality in Black adults hospitalized for heart failure (HF) but didn’t show similar ability in non-Black patients, in a study based in part on the American Heart Association–sponsored Get with the Guidelines in Heart Failure (GWTG-HF) registry.
The novel risk-prediction tool bolstered by SDOH at the zip-code level – including household income, number of adults without a high-school degree, poverty and unemployment rates, and other factors – stratified risk more sharply in Black patients than more standard models, including some based on multivariable logistic regression.
“Traditional risk models that exist for heart failure assign lower risks to Black individuals if everything else is held constant,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, told this news organization.
“I think that is problematic, because if Black patients are considered lower risk, they may not get appropriate risk-based therapies that are being provided. We wanted to move away from this approach and use a more race-agnostic approach,” said Dr. Pandey, who is senior author on the study published in JAMA Cardiology, with lead author Matthew W. Segar, MD, Texas Heart Institute, Houston.
The training dataset for the prediction model consisted of 123,634 patients hospitalized with HF (mean age, 71 years), of whom 47% were women, enrolled in the GWTG-HF registry from 2010 through 2020.
The machine-learning models showed “excellent performance” when applied to an internal subset cohort of 82,420 patients, with a C statistic of 0.81 for Black patients and 0.82 for non-Black patients, the authors report, and in a real-world cohort of 553,506 patients, with C statistics of 0.74 and 0.75, respectively. The models performed similarly well, they write, in an external validation cohort derived from the ARIC registry, with C statistics of 0.79 and 0.80, respectively.
The machine-learning models’ performance surpassed that of the GWTG-HF risk-score model, C statistics 0.69 for both Black and non-Black patients, and other logistic regression models in which race was a covariate, the authors state.
“We also observed significant race-specific differences in the population-attributable risk of in-hospital mortality associated with the SDOH, with a significantly greater contribution of these parameters to the overall in-hospital mortality risk in Black patients versus non-Black patients,” they write.
For Black patients, five of the SDOH parameters were among the top 20 covariate predictors of in-hospital mortality: mean income level, vacancy and unemployment rates, proportion of the population without a high school degree, and proportion older than 65 years. Together they accounted for 11.6% of population-attributable risk for in-hospital death.
Only one SDOH parameter – percentage of population older than 65 years – made the top 20 for non-Black patients, with a population-attributable risk of 0.5%, the group reports.
“I hope our work spurs future investigations to better understand how social determinants contribute to risk and how they can be incorporated in management of these patients,” Dr. Pandey said.
“I commend the authors for attempting to address SDOH as a potential contributor to some of the differences in outcomes among patients with heart failure,” writes Eldrin F. Lewis, MD, MPH, Stanford University School of Medicine, Palo Alto, Calif., in an accompanying editorial.
“It is imperative that we use these newer techniques to go beyond simply predicting which groups are at heightened risk and leverage the data to create solutions that will reduce those risks for the individual patient,” Dr. Lewis states.
“We should use these tools to reduce racial and ethnic differences in the operations of health care systems, potential bias in management decisions, and inactivity due to the difficulty in getting guideline-directed medical therapy into the hands of people who may have limited resources with minimal out-of-pocket costs,” he writes.
The models assessed in the current report “set a new bar for risk prediction: Integration of a comprehensive set of demographics, comorbidities, and social determinants with machine learning obviates race and ethnicity in risk prediction,” contend JAMA Cardiology deputy editor Clyde W. Yancy, MD, and associate editor Sadiya S. Khan, MD, both from Northwestern University Feinberg School of Medicine, Chicago, in an accompanying editor’s note.
“This more careful incorporation of individual-level, neighborhood-level, and hospital-level social factors,” they conclude, “is now a candidate template for future risk models.”
Dr. Pandey discloses grant funding from Applied Therapeutics and Gilead Sciences; consulting for or serving as an advisor to Tricog Health, Eli Lilly, Rivus, and Roche Diagnostics; receiving nonfinancial support from Pfizer and Merck; and research support from the Texas Health Resources Clinical Scholarship, the Gilead Sciences Research Scholar Program, the National Institute on Aging GEMSSTAR Grant, and Applied Therapeutics. Dr. Segar discloses receiving nonfinancial support from Pfizer and Merck. Other disclosures are in the report. Dr. Lewis reported no disclosures. Dr. Yancy and Dr. Khan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The addition of social determinants of health (SDOH) to machine-learning risk-prediction models improved forecasts of in-hospital mortality in Black adults hospitalized for heart failure (HF) but didn’t show similar ability in non-Black patients, in a study based in part on the American Heart Association–sponsored Get with the Guidelines in Heart Failure (GWTG-HF) registry.
The novel risk-prediction tool bolstered by SDOH at the zip-code level – including household income, number of adults without a high-school degree, poverty and unemployment rates, and other factors – stratified risk more sharply in Black patients than more standard models, including some based on multivariable logistic regression.
“Traditional risk models that exist for heart failure assign lower risks to Black individuals if everything else is held constant,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, told this news organization.
“I think that is problematic, because if Black patients are considered lower risk, they may not get appropriate risk-based therapies that are being provided. We wanted to move away from this approach and use a more race-agnostic approach,” said Dr. Pandey, who is senior author on the study published in JAMA Cardiology, with lead author Matthew W. Segar, MD, Texas Heart Institute, Houston.
The training dataset for the prediction model consisted of 123,634 patients hospitalized with HF (mean age, 71 years), of whom 47% were women, enrolled in the GWTG-HF registry from 2010 through 2020.
The machine-learning models showed “excellent performance” when applied to an internal subset cohort of 82,420 patients, with a C statistic of 0.81 for Black patients and 0.82 for non-Black patients, the authors report, and in a real-world cohort of 553,506 patients, with C statistics of 0.74 and 0.75, respectively. The models performed similarly well, they write, in an external validation cohort derived from the ARIC registry, with C statistics of 0.79 and 0.80, respectively.
The machine-learning models’ performance surpassed that of the GWTG-HF risk-score model, C statistics 0.69 for both Black and non-Black patients, and other logistic regression models in which race was a covariate, the authors state.
“We also observed significant race-specific differences in the population-attributable risk of in-hospital mortality associated with the SDOH, with a significantly greater contribution of these parameters to the overall in-hospital mortality risk in Black patients versus non-Black patients,” they write.
For Black patients, five of the SDOH parameters were among the top 20 covariate predictors of in-hospital mortality: mean income level, vacancy and unemployment rates, proportion of the population without a high school degree, and proportion older than 65 years. Together they accounted for 11.6% of population-attributable risk for in-hospital death.
Only one SDOH parameter – percentage of population older than 65 years – made the top 20 for non-Black patients, with a population-attributable risk of 0.5%, the group reports.
“I hope our work spurs future investigations to better understand how social determinants contribute to risk and how they can be incorporated in management of these patients,” Dr. Pandey said.
“I commend the authors for attempting to address SDOH as a potential contributor to some of the differences in outcomes among patients with heart failure,” writes Eldrin F. Lewis, MD, MPH, Stanford University School of Medicine, Palo Alto, Calif., in an accompanying editorial.
“It is imperative that we use these newer techniques to go beyond simply predicting which groups are at heightened risk and leverage the data to create solutions that will reduce those risks for the individual patient,” Dr. Lewis states.
“We should use these tools to reduce racial and ethnic differences in the operations of health care systems, potential bias in management decisions, and inactivity due to the difficulty in getting guideline-directed medical therapy into the hands of people who may have limited resources with minimal out-of-pocket costs,” he writes.
The models assessed in the current report “set a new bar for risk prediction: Integration of a comprehensive set of demographics, comorbidities, and social determinants with machine learning obviates race and ethnicity in risk prediction,” contend JAMA Cardiology deputy editor Clyde W. Yancy, MD, and associate editor Sadiya S. Khan, MD, both from Northwestern University Feinberg School of Medicine, Chicago, in an accompanying editor’s note.
“This more careful incorporation of individual-level, neighborhood-level, and hospital-level social factors,” they conclude, “is now a candidate template for future risk models.”
Dr. Pandey discloses grant funding from Applied Therapeutics and Gilead Sciences; consulting for or serving as an advisor to Tricog Health, Eli Lilly, Rivus, and Roche Diagnostics; receiving nonfinancial support from Pfizer and Merck; and research support from the Texas Health Resources Clinical Scholarship, the Gilead Sciences Research Scholar Program, the National Institute on Aging GEMSSTAR Grant, and Applied Therapeutics. Dr. Segar discloses receiving nonfinancial support from Pfizer and Merck. Other disclosures are in the report. Dr. Lewis reported no disclosures. Dr. Yancy and Dr. Khan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The addition of social determinants of health (SDOH) to machine-learning risk-prediction models improved forecasts of in-hospital mortality in Black adults hospitalized for heart failure (HF) but didn’t show similar ability in non-Black patients, in a study based in part on the American Heart Association–sponsored Get with the Guidelines in Heart Failure (GWTG-HF) registry.
The novel risk-prediction tool bolstered by SDOH at the zip-code level – including household income, number of adults without a high-school degree, poverty and unemployment rates, and other factors – stratified risk more sharply in Black patients than more standard models, including some based on multivariable logistic regression.
“Traditional risk models that exist for heart failure assign lower risks to Black individuals if everything else is held constant,” Ambarish Pandey, MD, MSCS, University of Texas Southwestern Medical Center, Dallas, told this news organization.
“I think that is problematic, because if Black patients are considered lower risk, they may not get appropriate risk-based therapies that are being provided. We wanted to move away from this approach and use a more race-agnostic approach,” said Dr. Pandey, who is senior author on the study published in JAMA Cardiology, with lead author Matthew W. Segar, MD, Texas Heart Institute, Houston.
The training dataset for the prediction model consisted of 123,634 patients hospitalized with HF (mean age, 71 years), of whom 47% were women, enrolled in the GWTG-HF registry from 2010 through 2020.
The machine-learning models showed “excellent performance” when applied to an internal subset cohort of 82,420 patients, with a C statistic of 0.81 for Black patients and 0.82 for non-Black patients, the authors report, and in a real-world cohort of 553,506 patients, with C statistics of 0.74 and 0.75, respectively. The models performed similarly well, they write, in an external validation cohort derived from the ARIC registry, with C statistics of 0.79 and 0.80, respectively.
The machine-learning models’ performance surpassed that of the GWTG-HF risk-score model, C statistics 0.69 for both Black and non-Black patients, and other logistic regression models in which race was a covariate, the authors state.
“We also observed significant race-specific differences in the population-attributable risk of in-hospital mortality associated with the SDOH, with a significantly greater contribution of these parameters to the overall in-hospital mortality risk in Black patients versus non-Black patients,” they write.
For Black patients, five of the SDOH parameters were among the top 20 covariate predictors of in-hospital mortality: mean income level, vacancy and unemployment rates, proportion of the population without a high school degree, and proportion older than 65 years. Together they accounted for 11.6% of population-attributable risk for in-hospital death.
Only one SDOH parameter – percentage of population older than 65 years – made the top 20 for non-Black patients, with a population-attributable risk of 0.5%, the group reports.
“I hope our work spurs future investigations to better understand how social determinants contribute to risk and how they can be incorporated in management of these patients,” Dr. Pandey said.
“I commend the authors for attempting to address SDOH as a potential contributor to some of the differences in outcomes among patients with heart failure,” writes Eldrin F. Lewis, MD, MPH, Stanford University School of Medicine, Palo Alto, Calif., in an accompanying editorial.
“It is imperative that we use these newer techniques to go beyond simply predicting which groups are at heightened risk and leverage the data to create solutions that will reduce those risks for the individual patient,” Dr. Lewis states.
“We should use these tools to reduce racial and ethnic differences in the operations of health care systems, potential bias in management decisions, and inactivity due to the difficulty in getting guideline-directed medical therapy into the hands of people who may have limited resources with minimal out-of-pocket costs,” he writes.
The models assessed in the current report “set a new bar for risk prediction: Integration of a comprehensive set of demographics, comorbidities, and social determinants with machine learning obviates race and ethnicity in risk prediction,” contend JAMA Cardiology deputy editor Clyde W. Yancy, MD, and associate editor Sadiya S. Khan, MD, both from Northwestern University Feinberg School of Medicine, Chicago, in an accompanying editor’s note.
“This more careful incorporation of individual-level, neighborhood-level, and hospital-level social factors,” they conclude, “is now a candidate template for future risk models.”
Dr. Pandey discloses grant funding from Applied Therapeutics and Gilead Sciences; consulting for or serving as an advisor to Tricog Health, Eli Lilly, Rivus, and Roche Diagnostics; receiving nonfinancial support from Pfizer and Merck; and research support from the Texas Health Resources Clinical Scholarship, the Gilead Sciences Research Scholar Program, the National Institute on Aging GEMSSTAR Grant, and Applied Therapeutics. Dr. Segar discloses receiving nonfinancial support from Pfizer and Merck. Other disclosures are in the report. Dr. Lewis reported no disclosures. Dr. Yancy and Dr. Khan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The heartache of bereavement can be fatal in heart failure
that points to the need for greater integration of psychosocial risk factors in the treatment of HF.
The adjusted relative risk of dying was nearly 30% higher among bereaved patients with HF (1.29; 95% confidence interval, 1.27-1.30) and slightly higher for those grieving the loss of more than one family member (RR, 1.35).
The highest risk was in the first week after the loss (RR, 1.78) but persisted after 5 years of follow-up (RR, 1.30).
“Heart failure is a very difficult condition and has a very poor prognosis comparable to many, many cancers,” senior author Krisztina László, PhD, Karolinska Institutet, Stockholm, said in an interview. “So it’s important for us to be aware of these increased risks and to understand them better.”
The early risk for death could be related to stress-induced cardiomyopathy, or Takotsubo syndrome, as well as activation of the hypothalamic-pituitary-adrenal axis, the renin-angiotensin-aldosterone system, and sympathetic nervous system, she explained. Higher long-term risks may reflect chronic stress, leading to poorly managed disease and an unhealthy lifestyle.
“If we understand better the underlying mechanisms maybe we can give more specific advice,” Dr. László said. “At this stage, I think having an awareness of the risk and trying to follow patients or at least not let them fall out of usual care, asking questions, trying to understand what their needs are, maybe that is what we can do well.”
A recent position paper by the European Association of Preventive Cardiology pointed out that psychosocial risk factors, like depression and social isolation, can exacerbate heart failure and calls for better integration of psychosocial factors in the treatment of patients with chronic HF.
“We don’t do a very good job of it, but I think they are very important,” observed Stuart D. Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., and was not involved in the study.
“When we hear about a spouse dying, we might call and give condolences, but it’s probably a group of patients that for the next 6 months or so we need to watch more closely and see if there are things we can impact both medically as well as socially to perhaps prevent some of this increase in mortality,” he told this news organization.
Although several studies have linked bereavement with adverse health outcomes, this is just one of two studies to look specifically at its role in HF prognosis, Dr. László noted. A 2013 study of 66,000 male veterans reported that widowers had nearly a 38% higher all-cause mortality risk than did married veterans.
The present study extends those findings to 490,527 patients in the Swedish Heart Failure Registry between 2000 and 2018 and/or in the Swedish Patient Register with a primary diagnosis of HF between 1987 and 2018. During a mean follow-up of 3.7 years, 12% of participants had a family member die, and 383,674 participants died.
Results showed the HF mortality risk increased 10% after the death of a child, 20% with the death of a spouse/partner, 13% with a sibling’s death, and 5% with the death of a grandchild.
No increased risk was seen after the death of a parent, which is likely owed to a median patient age of about 75 years and “is in line with our expectations of the life cycle,” Dr. László said.
An association between bereavement and mortality risk was observed in cases of loss caused by cardiovascular disease (RR, 1.34) and other natural causes (RR, 1.27) but also in cases of unnatural deaths, such as suicide (RR, 1.13).
The overall findings were similar regardless of left ventricular ejection fraction and New York Heart Association functional class and were not affected by sex or country of birth.
Dr. Russell agreed that the death of a parent would be expected among these older patients with HF but said that “if the mechanism of this truly is kind of this increased stress hormones and Takotsubo-type mechanism, you’d think it would be worse if it was your kid that died. That shocked me a bit.”
The strong association between mortality and the loss of a spouse or partner was not surprising, given that they’re an important source of mutual social support, he added.
“If it’s a 75-year-old whose spouse dies, we need to make sure that we have the children’s phone number or other people that we can reach out to and say: ‘Can you check on them?’ ” he said. “And we need to make sure that somebody else is coming in with them because I would guess that probably at least half of what patients hear in a clinic visit goes in one ear and out the other and it’s going to make that much better. So we need to find who that new support person is for the patient.”
Asked whether there are efforts underway to incorporate psychosocial factors into current U.S. guidelines, Dr. Russell replied, “certainly within heart failure, I don’t think we’re really discussing it and, that may be the best part of this paper. It really makes us think about a different way of approaching these older patients.”
Dr. László said that future studies are needed to investigate whether less severe sources of stress may also contribute to poor HF prognosis.
“In our population, 12% of patients were affected, which is quite high, but there are patients with heart failure who experience on a daily basis other sources of stress, which are less severe but chronic and affect large numbers,” she said. “This may also have important public health implications and will be an important next step.”
The authors noted that they were unable to eliminate residual confounding by genetic factors or unmeasured socioeconomic-, lifestyle-, or health-related factors shared by family members. Other limitations are limited power to detect a modest effect in some of the subanalyses and that the findings may be generalizable only to countries with social and cultural contexts and health-related factors similar to those of Sweden.
The study was supported by grants from the Swedish Council for Working Life and Social Research, the Karolinska Institutet’s Research Foundation, and the China Scholarship Council. Dr. László is also supported by a grant from the Heart and Lung Foundation. All other authors and Dr. Russell reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that points to the need for greater integration of psychosocial risk factors in the treatment of HF.
The adjusted relative risk of dying was nearly 30% higher among bereaved patients with HF (1.29; 95% confidence interval, 1.27-1.30) and slightly higher for those grieving the loss of more than one family member (RR, 1.35).
The highest risk was in the first week after the loss (RR, 1.78) but persisted after 5 years of follow-up (RR, 1.30).
“Heart failure is a very difficult condition and has a very poor prognosis comparable to many, many cancers,” senior author Krisztina László, PhD, Karolinska Institutet, Stockholm, said in an interview. “So it’s important for us to be aware of these increased risks and to understand them better.”
The early risk for death could be related to stress-induced cardiomyopathy, or Takotsubo syndrome, as well as activation of the hypothalamic-pituitary-adrenal axis, the renin-angiotensin-aldosterone system, and sympathetic nervous system, she explained. Higher long-term risks may reflect chronic stress, leading to poorly managed disease and an unhealthy lifestyle.
“If we understand better the underlying mechanisms maybe we can give more specific advice,” Dr. László said. “At this stage, I think having an awareness of the risk and trying to follow patients or at least not let them fall out of usual care, asking questions, trying to understand what their needs are, maybe that is what we can do well.”
A recent position paper by the European Association of Preventive Cardiology pointed out that psychosocial risk factors, like depression and social isolation, can exacerbate heart failure and calls for better integration of psychosocial factors in the treatment of patients with chronic HF.
“We don’t do a very good job of it, but I think they are very important,” observed Stuart D. Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., and was not involved in the study.
“When we hear about a spouse dying, we might call and give condolences, but it’s probably a group of patients that for the next 6 months or so we need to watch more closely and see if there are things we can impact both medically as well as socially to perhaps prevent some of this increase in mortality,” he told this news organization.
Although several studies have linked bereavement with adverse health outcomes, this is just one of two studies to look specifically at its role in HF prognosis, Dr. László noted. A 2013 study of 66,000 male veterans reported that widowers had nearly a 38% higher all-cause mortality risk than did married veterans.
The present study extends those findings to 490,527 patients in the Swedish Heart Failure Registry between 2000 and 2018 and/or in the Swedish Patient Register with a primary diagnosis of HF between 1987 and 2018. During a mean follow-up of 3.7 years, 12% of participants had a family member die, and 383,674 participants died.
Results showed the HF mortality risk increased 10% after the death of a child, 20% with the death of a spouse/partner, 13% with a sibling’s death, and 5% with the death of a grandchild.
No increased risk was seen after the death of a parent, which is likely owed to a median patient age of about 75 years and “is in line with our expectations of the life cycle,” Dr. László said.
An association between bereavement and mortality risk was observed in cases of loss caused by cardiovascular disease (RR, 1.34) and other natural causes (RR, 1.27) but also in cases of unnatural deaths, such as suicide (RR, 1.13).
The overall findings were similar regardless of left ventricular ejection fraction and New York Heart Association functional class and were not affected by sex or country of birth.
Dr. Russell agreed that the death of a parent would be expected among these older patients with HF but said that “if the mechanism of this truly is kind of this increased stress hormones and Takotsubo-type mechanism, you’d think it would be worse if it was your kid that died. That shocked me a bit.”
The strong association between mortality and the loss of a spouse or partner was not surprising, given that they’re an important source of mutual social support, he added.
“If it’s a 75-year-old whose spouse dies, we need to make sure that we have the children’s phone number or other people that we can reach out to and say: ‘Can you check on them?’ ” he said. “And we need to make sure that somebody else is coming in with them because I would guess that probably at least half of what patients hear in a clinic visit goes in one ear and out the other and it’s going to make that much better. So we need to find who that new support person is for the patient.”
Asked whether there are efforts underway to incorporate psychosocial factors into current U.S. guidelines, Dr. Russell replied, “certainly within heart failure, I don’t think we’re really discussing it and, that may be the best part of this paper. It really makes us think about a different way of approaching these older patients.”
Dr. László said that future studies are needed to investigate whether less severe sources of stress may also contribute to poor HF prognosis.
“In our population, 12% of patients were affected, which is quite high, but there are patients with heart failure who experience on a daily basis other sources of stress, which are less severe but chronic and affect large numbers,” she said. “This may also have important public health implications and will be an important next step.”
The authors noted that they were unable to eliminate residual confounding by genetic factors or unmeasured socioeconomic-, lifestyle-, or health-related factors shared by family members. Other limitations are limited power to detect a modest effect in some of the subanalyses and that the findings may be generalizable only to countries with social and cultural contexts and health-related factors similar to those of Sweden.
The study was supported by grants from the Swedish Council for Working Life and Social Research, the Karolinska Institutet’s Research Foundation, and the China Scholarship Council. Dr. László is also supported by a grant from the Heart and Lung Foundation. All other authors and Dr. Russell reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
that points to the need for greater integration of psychosocial risk factors in the treatment of HF.
The adjusted relative risk of dying was nearly 30% higher among bereaved patients with HF (1.29; 95% confidence interval, 1.27-1.30) and slightly higher for those grieving the loss of more than one family member (RR, 1.35).
The highest risk was in the first week after the loss (RR, 1.78) but persisted after 5 years of follow-up (RR, 1.30).
“Heart failure is a very difficult condition and has a very poor prognosis comparable to many, many cancers,” senior author Krisztina László, PhD, Karolinska Institutet, Stockholm, said in an interview. “So it’s important for us to be aware of these increased risks and to understand them better.”
The early risk for death could be related to stress-induced cardiomyopathy, or Takotsubo syndrome, as well as activation of the hypothalamic-pituitary-adrenal axis, the renin-angiotensin-aldosterone system, and sympathetic nervous system, she explained. Higher long-term risks may reflect chronic stress, leading to poorly managed disease and an unhealthy lifestyle.
“If we understand better the underlying mechanisms maybe we can give more specific advice,” Dr. László said. “At this stage, I think having an awareness of the risk and trying to follow patients or at least not let them fall out of usual care, asking questions, trying to understand what their needs are, maybe that is what we can do well.”
A recent position paper by the European Association of Preventive Cardiology pointed out that psychosocial risk factors, like depression and social isolation, can exacerbate heart failure and calls for better integration of psychosocial factors in the treatment of patients with chronic HF.
“We don’t do a very good job of it, but I think they are very important,” observed Stuart D. Russell, MD, a professor of medicine who specializes in advanced HF at Duke University, Durham, N.C., and was not involved in the study.
“When we hear about a spouse dying, we might call and give condolences, but it’s probably a group of patients that for the next 6 months or so we need to watch more closely and see if there are things we can impact both medically as well as socially to perhaps prevent some of this increase in mortality,” he told this news organization.
Although several studies have linked bereavement with adverse health outcomes, this is just one of two studies to look specifically at its role in HF prognosis, Dr. László noted. A 2013 study of 66,000 male veterans reported that widowers had nearly a 38% higher all-cause mortality risk than did married veterans.
The present study extends those findings to 490,527 patients in the Swedish Heart Failure Registry between 2000 and 2018 and/or in the Swedish Patient Register with a primary diagnosis of HF between 1987 and 2018. During a mean follow-up of 3.7 years, 12% of participants had a family member die, and 383,674 participants died.
Results showed the HF mortality risk increased 10% after the death of a child, 20% with the death of a spouse/partner, 13% with a sibling’s death, and 5% with the death of a grandchild.
No increased risk was seen after the death of a parent, which is likely owed to a median patient age of about 75 years and “is in line with our expectations of the life cycle,” Dr. László said.
An association between bereavement and mortality risk was observed in cases of loss caused by cardiovascular disease (RR, 1.34) and other natural causes (RR, 1.27) but also in cases of unnatural deaths, such as suicide (RR, 1.13).
The overall findings were similar regardless of left ventricular ejection fraction and New York Heart Association functional class and were not affected by sex or country of birth.
Dr. Russell agreed that the death of a parent would be expected among these older patients with HF but said that “if the mechanism of this truly is kind of this increased stress hormones and Takotsubo-type mechanism, you’d think it would be worse if it was your kid that died. That shocked me a bit.”
The strong association between mortality and the loss of a spouse or partner was not surprising, given that they’re an important source of mutual social support, he added.
“If it’s a 75-year-old whose spouse dies, we need to make sure that we have the children’s phone number or other people that we can reach out to and say: ‘Can you check on them?’ ” he said. “And we need to make sure that somebody else is coming in with them because I would guess that probably at least half of what patients hear in a clinic visit goes in one ear and out the other and it’s going to make that much better. So we need to find who that new support person is for the patient.”
Asked whether there are efforts underway to incorporate psychosocial factors into current U.S. guidelines, Dr. Russell replied, “certainly within heart failure, I don’t think we’re really discussing it and, that may be the best part of this paper. It really makes us think about a different way of approaching these older patients.”
Dr. László said that future studies are needed to investigate whether less severe sources of stress may also contribute to poor HF prognosis.
“In our population, 12% of patients were affected, which is quite high, but there are patients with heart failure who experience on a daily basis other sources of stress, which are less severe but chronic and affect large numbers,” she said. “This may also have important public health implications and will be an important next step.”
The authors noted that they were unable to eliminate residual confounding by genetic factors or unmeasured socioeconomic-, lifestyle-, or health-related factors shared by family members. Other limitations are limited power to detect a modest effect in some of the subanalyses and that the findings may be generalizable only to countries with social and cultural contexts and health-related factors similar to those of Sweden.
The study was supported by grants from the Swedish Council for Working Life and Social Research, the Karolinska Institutet’s Research Foundation, and the China Scholarship Council. Dr. László is also supported by a grant from the Heart and Lung Foundation. All other authors and Dr. Russell reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Transplanted pig hearts functioned normally in deceased persons on ventilator support
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.
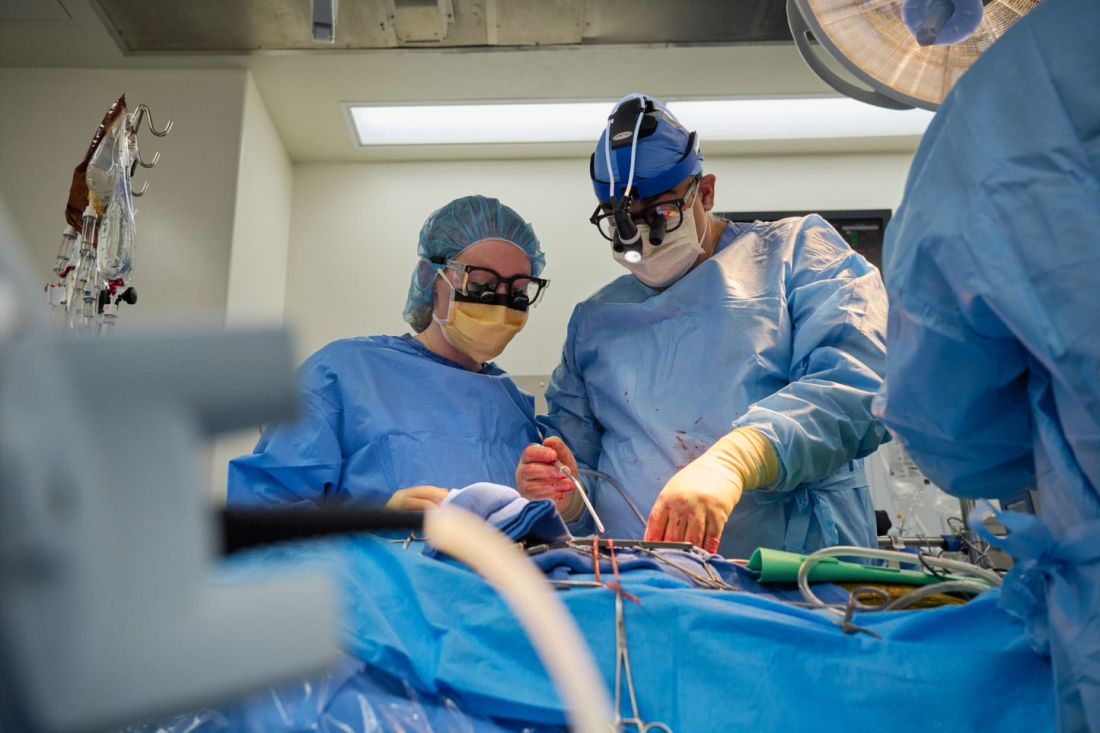
Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).
The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.

Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.

Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.