User login
DIAMOND: Adding patiromer helps optimize HF meds, foils hyperkalemia
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.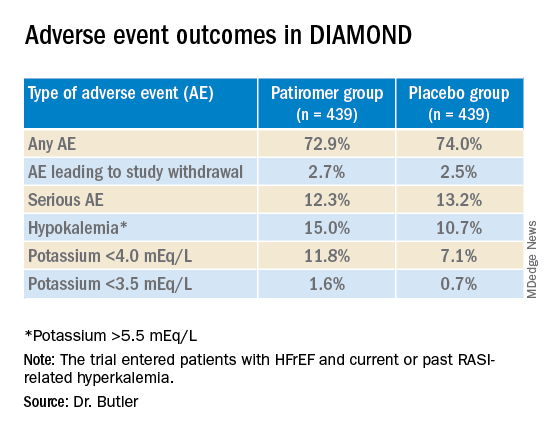
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
Several of the core medications for patients with heart failure with reduced ejection fraction (HFrEF) come with a well-known risk of causing hyperkalemia, to which many clinicians respond by pulling back on dosing or withdrawing the culprit drug.
But accompanying renin-angiotensin system–inhibiting agents with the potassium-sequestrant patiromer (Veltassa, Vifor Pharma) appears to shield patients against hyperkalemia enough that they can take more RASI medications at higher doses, suggests a randomized, a controlled study.
The DIAMOND trial’s HFrEF patients, who had current or a history of RASI-related hyperkalemia, added either patiromer or placebo to their guideline-directed medical therapy (GDMT), which includes, even emphasizes, the culprit medication. They include ACE inhibitors, angiotensin-receptor blockers (ARBs), angiotensin-receptor/neprilysin inhibitors (ARNIs), and mineralocorticoid receptor antagonists (MRAs).
Those taking patiromer tolerated more intense RASI therapy – including MRAs, which are especially prone to causing hyperkalemia – than the patients assigned to placebo. They also maintained lower potassium concentrations and experienced fewer clinically important hyperkalemia episodes, reported Javed Butler, MD, MPH, MBA, Baylor Scott and White Research Institute, Dallas, at the annual scientific sessions of the American College of Cardiology.
The apparent benefit from patiromer came in part from an advantage for a composite hyperkalemia-event endpoint that included mortality, Dr. Butler noted. That advantage seemed to hold regardless of age, sex, body mass index, HFrEF symptom severity, or initial natriuretic peptide levels.
Patients who took patiromer, compared with those who took placebo, showed a 37% reduction in risk for hyperkalemia (P = .006), defined as potassium levels exceeding 5.5 mEq/L, over a median follow-up of 27 weeks. They were 38% less likely to have their MRA dosage reduced to below target level (P = .006).
More patients in the patiromer group than in the control group attained at least 50% of target dosage for MRAs and ACE inhibitors, ARBs, or ARNIs (92% vs. 87%; P = .015).
Patients with HFrEF are unlikely to achieve best possible outcomes without GDMT optimization, but failure to optimize is often attributed to hyperkalemia concerns. DIAMOND, Dr. Butler said, suggests that, by adding the potassium sequestrant to GDMT, “you can simultaneously control potassium and optimize RASI therapy.” Many clinicians seem to believe they can achieve only one or the other.
DIAMOND was too underpowered to show whether preventing hyperkalemia with patiromer could improve clinical outcomes. But failure to optimize RASI medication in HFrEF can worsen risk for heart failure events and death. So “it stands to reason that optimization of RASI therapy without a concomitant risk of hyperkalemia may, in the long run, lead to better outcomes for these patients,” Dr. Butler said in an interview.
Given the drug’s ability to keep potassium levels in check during RASI therapy, Dr. Butler said, “hypokalemia should not be a reason for suboptimal therapy.”
Patiromer and other potassium sequestrants have been available in the United States and Europe for 4-6 years, but their value as adjuncts to RASI medication in HFrEF or other heart failure has been unclear.
“There’s a good opportunity to expand the use of the drug. The question is, in whom and when?” James L. Januzzi, MD, Massachusetts General Hospital, Boston, said in an interview.
Some HFrEF patients on GDMT “should be treated with patiromer. The bigger question is, should we give someone who has a history of hyperkalemia another chance at GDMT before we treat them with patiromer? Because they may not necessarily develop hyperkalemia a second time,” said Dr. Januzzi, who was on the DIAMOND endpoint-adjudication committee.
Among the most notable findings of the trial, he said, is that the number of people who developed hyperkalemia on RASI medication, although significantly elevated, “wasn’t as high as they expected it would be,” he said. “The data from DIAMOND argue that if a really significant majority does not become hyperkalemic on rechallenge, jumping straight to a potassium-binding drug may be premature.”
Physicians across specialties can differ in how they interpret potassium-level elevation and can use various cut points to flag when to stop RASI medication or at least hold back on up-titration, Dr. Butler observed. “Cardiologists have a different threshold of potassium that they tolerate than say, for instance, a nephrologist.”
Useful, then, might be a way to tell which patients are most likely to develop hyperkalemia with RASI up-titration and so might benefit from a potassium-binding agent right away. But DIAMOND, Dr. Butler said, “does not necessarily define any patient phenotype or any potassium level where we would say that you should use a potassium binder.”
The trial entered 1,642 patients with HFrEF and current or past RASI-related hyperkalemia to a 12-week run-in phase for optimization of GDMT with patiromer. The trial was conducted at nearly 400 centers in 21 countries.
RASI medication could be optimized in 85% of the cohort, from which 878 patients were randomly assigned either to continue optimized GDMT with patiromer or to have the potassium-sequestrant replaced with a placebo.
The patients on patiromer showed a 0.03-mEq/L mean rise in serum potassium levels from randomization to the end of the study, the primary endpoint, compared with a 0.13 mEq/L mean increase for those in the control group (P < .001), Dr. Butler reported.
The win ratio for a RASI-use score hierarchically featuring cardiovascular death and CV hospitalization for hyperkalemia at several levels of severity was 1.25 (95% confidence interval, 1.003-1.564; P = .048), favoring the patiromer group. The win ratio solely for hyperkalemia-related events also favored patients on patiromer, at 1.53 (95% CI, 1.23-1.91; P < .001).
Patiromer also seemed well tolerated, Dr. Butler said.
Hyperkalemia is “one of the most common excuses” from clinicians for failing to up-titrate RASI medicine in patients with heart failure, Dr. Januzzi said. DIAMOND was less about patiromer itself than about ways “to facilitate better GDMT, where we’re really falling short of the mark. During the run-in phase they were able to get the vast majority of individuals to target, which to me is a critically important point, and emblematic of the need for things that facilitate this kind of excellent care.”
DIAMOND was funded by Vifor Pharma. Dr. Butler disclosed receiving consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Sequana Medical, and Vifor Pharma. Dr. Januzzi disclosed receiving consultant fees or honoraria from Abbott Laboratories, Imbria, Jana Care, Novartis, Prevencio, and Roche Diagnostics; serving on a data safety monitoring board for AbbVie, Amgen, Bayer Healthcare Pharmaceuticals, Beyer, CVRx, and Takeda Pharmaceuticals North America; and receiving research grants from Abbott Laboratories, Janssen, and Vifor Pharma.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Extraction of infected implanted cardiac devices rare, despite guidelines
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
The rates of infection involving cardiac implanted electronic devices (CIEDs), like pacemakers and cardioverter defibrillators (ICDs), are substantial, but only a minority of patients in the United States receive the guideline-directed recommendation of device removal, according to data from a Medicare population.
The study was conducted on the hypothesis that adherence to guidelines were low, “but we were surprised by how low the extraction rates turned out to be,” Sean D. Pokorney, MD, an electrophysiologist at the Duke Clinical Research Institute, Durham, N.C., reported at the annual scientific sessions of the American College of Cardiology.
The major U.S. and European guidelines are uniform in recommending complete extraction for a CIED infection. The American Heart Association and the Heart Rhythm Society and two out of the three other guidelines cited by Dr. Pokorney not only recommend extraction but specify prompt extraction.
Neither complete extraction nor prompt extraction are typical.
Of the 11,619 CIED infection cases identified in the Medicare database, 18.2% underwent extraction within 30 days of diagnosis. Only 13% were extracted within 6 days.
Lack of extraction may cause avoidable mortality
The result is likely to be avoidable mortality. Among those with extraction within 30 days, 80% were still alive 1 year later. Survival at 1 year fell to 67.6% in those without an extraction within this time frame.
This translated to a 22% lower rate of death at 1 year (hazard ratio, 0.78; P = .008) in those who underwent extraction within 30 days.
For those in whom the device was extracted within 7 days, the associated HR for death at 1 year was more than 40% lower (HR, 0.59; P < .001), reported Dr. Pokorney, who characterized these reductions as occurring in “a dose-response fashion.”
The very high risk of relapse despite antibiotics is the reason that “there is a class 1 indication for complete hardware removal,” Dr. Pokorney. He cited five studies that addressed this question. With partial device removal or medical therapy alone, relapse was consistently 50% or greater. In one study, it was 67%. In another it was 100%.
With complete removal, the rate of infection relapse was 1% or lower in four. In the fifth, the rate was 4.2%.
Infections can occur early or late after implantation, but cases accumulate over time. In the Medicare data sample, infection rates climbed from 0.3% at 1 year to 0.6% at 2 years and then to 1.1% at 3 years, Dr. Pokorney reported.
Other studies have also shown a steady increase in the proportion of implanted devices associated with infection over time. In a cohort study conducted in Olmstead County, Minnesota, the cumulative probability of a CIED infection reached 6.2% after 15 years and 11.7% after 25 years. While about half of these were infections localized to the device pocket, the others were potentially life-threatening systemic infections, according to Dr. Pokorney, who cited this study.
In his analysis of the Medicare data, all fee-for-service patients receiving a first CIED implant over a period of 14 years were included. The 14-year period ended just before the COVID-19 epidemic.
The more than 11,000 CIED infections were identified in 1,065,549 total CIED patients. Most (72%) had received a pacemaker. Of the others , more than half received an ICD and the others received a cardiac resynchronization device. The median age was 78 years.
Female and Black patients even less likely to undergo extraction
About half (49.1%) of the overall study population was female, but females represented only about 40% of those who developed an infection. Blacks represented just under 8% of the population but nearly 16% of the CIED infections. Both females and Blacks were significantly less likely than the overall study population to undergo extraction for their infection (P < .001 for both).
Perhaps predictably, patients with comorbidities were more likely to develop CIED infections. For example, 87% of those with infection, versus only 64.9% of the overall population, were in heart failure at the time of implantation. Diabetes (68.3% vs. 49.3%), ischemic heart disease (91.9% vs. 79.4%), renal disease (70.5% vs. 37.9%), and chronic obstructive pulmonary disease (70.6% vs. 55.0%) were also more common at baseline in those who went on to a CIED infection than in the overall population.
Based on the evidence that there is a large unmet need to improve adherence to the guidelines, Dr. Pokorney called for care pathways and other quality initiatives to address the problem.
The reasons that so many patients are not undergoing prompt device extraction at the time of infection is unclear, but Dr. Pokorney offered some hypotheses.
“There appears to be a false belief in the efficacy of antibiotics for treating CIED infections,” Dr. Pokorney said.
Comorbidities shouldn’t delay extraction
It is also possible that clinicians are concerned about performing extractions in patients with multiple comorbidities. If clinicians are delaying extractions for this reason, Dr. Pokorney suggested this behavior is misdirected given the fact that delays appear to increase mortality risk.
Several experts, including Rachel Lambert, MD, an electrophysiologist and professor of medicine at Yale University, New Haven, Conn., agreed that these data deserve a response.
“I was not surprised by the mortality data, but I was surprised at this low extraction rate,” said Dr. Lambert, who concurs with the guidelines. She indicated this study provides teeth to prompt action.
“It is great to have these data about the increased mortality risk to back up the guidelines,” she said.
More information is needed to understand exactly why CIED infection is not now leading to guideline-directed care. Dr. Pokorney said: “Where do we go from here is a key question.”
While several different types of initiatives might be needed, Dr. Pokorney called for regionalization of care to address the fact that not every center that places CIEDs has the capability to perform extractions.
“Extraction is not available at every center, and it probably should not be available at every center, so mechanisms are need to get patients with infection to the specialized centers that provide care,” he said.
Dr. Pokorney has financial relationships with Boston Scientific, Bristol-Myers Squibb, Gilead, Janssen, Medtronic, Pfizer, and Philips. Dr. Lambert reported financial relationships with Abbott, Amgen, and Medtronic.
FROM ACC 2022
Flu vaccines cut seasonal death in heart failure patients
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
WASHINGTON – Patients with heart failure who received an annual influenza vaccine for 3 years running had significantly fewer all-cause hospitalizations and significantly fewer cases of pneumonia during that time, compared with placebo-treated patients with heart failure, in a prospective, randomized, global trial with 5,129 participants.
Although the results failed to show a significant reduction in all-cause deaths linked to influenza vaccination, compared with controls during the entire 3 years of the study, the results did show a significant 21% relative mortality-risk reduction by vaccination during periods of peak influenza circulation, and a significant 23% reduction in cardiovascular deaths, compared with controls during peak seasons.
“This is the first randomized, controlled trial of influenza vaccine in patients with heart failure, and we showed that vaccination reduces deaths” during peak influenza seasons, Mark Loeb, MD, said during a press briefing at the annual scientific sessions of the American College of Cardiology. The results send “an important global message that patients with heart failure should receive the influenza vaccine,” said Dr. Loeb, a professor at McMaster University, Hamilton, Ont., who specializes in clinical epidemiology and infectious diseases.
Dr. Loeb admitted that he and his associates erred when they picked the time window to assess the two primary endpoints for the trial: the combined rate of cardiovascular death, nonfatal MI, and nonfatal stroke, and this combined endpoint plus hospitalizations for heart failure.
The time window they selected was the entirety of all 3 years following three annual immunizations. That was a mistake.
No flu vaccine benefit outside flu season
“We know that the influenza vaccine will not have any effect outside of when influenza is circulating. In retrospect, we should have done that,” Dr. Loeb bemoaned during his talk. He chalked up the bad choice to concern over collecting enough endpoints to see a significant between-group difference when the researchers designed the study.
For the entire 3 years of follow-up, influenza vaccination was tied to a nonsignificant 7% relative risk reduction for the first primary endpoint, and a nonsignificant 9% relative risk reduction for the second primary endpoint, he reported.
But Dr. Loeb lobbied for the relevance of several significant secondary endpoints that collectively showed a compelling pattern of benefit during his talk. These included, for the full 3-years of follow-up, important, significant reductions relative to placebo of 16% for first all-cause hospitalizations (P = .01), and a 42% relative risk reduction in first cases of pneumonia (P = .0006).
Then there were the benefits that appeared during influenza season. In that analysis, first events for the first primary endpoint fell after vaccination by a significant 18% relative to placebo. The in-season analysis also showed the significant cuts in both all-cause and cardiovascular deaths.
Despite the neutral primary endpoints, “if you look at these data as a whole I think they speak to the importance of vaccinating patients with heart failure against influenza,” Dr. Loeb maintained.
‘Totality of evidence supports vaccination’
“I agree that the totality of evidence supports influenza vaccination,” commented Mark H. Drazner, MD, professor and clinical chief of cardiology at the University of Texas Southwestern Medical Center, Dallas, who was designated discussant for the report.
“The message should be to offer influenza vaccine to patients with heart failure,” Dr. Drazner said in an interview. “Previous data on influenza vaccine in patients with heart failure were largely observational. This was a randomized, prospective, placebo-controlled trial. That’s a step forward. Proving efficacy in a randomized trial is important.”
Dr Drazner added that his institution already promotes a “strong mandate” to vaccinate patients with heart failure against influenza.
“The influenza vaccine is a very effective and cost-efficient public health measure. Preventing hospitalizations of patients with heart failure has so many benefits,” commented Craig Beavers, PharmD, vice president of professional services at Baptist Health in Paducah, Ky., and a discussant during the press briefing.
The Influenza Vaccine To Prevent Adverse Vascular Events (IVVE) trial enrolled people with heart failure in New York Heart Association functional class II, III, or IV from any of 10 low- and middle-income countries including China, India, the Philippines, and multiple countries from Africa and the Middle East. They averaged 57 years of age, and slightly more than half were women.
IVVE was sponsored by McMaster University; the only commercial support that IVVE received was a free supply of influenza vaccine from Sanofi Pasteur. Dr. Loeb, Dr. Drazner, and Dr. Beavers had no disclosures.
AT ACC 2022
Anticoagulation not routinely needed after TAVR: ADAPT-TAVR
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
FDA approves leadless, single-chamber pacemaker system
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.
New HF guidelines feature ‘quad’ therapy, tweaked terminology
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
The new heart failure (HF) guidelines released by three North American societies had a lot of catching up to do given the significant, even paradigm-shifting, additions to available treatment options in the last few years.
The landscape now includes both new and repurposed drug therapies that benefit almost without regard to ejection fraction (EF), and evidence-based urgency to engage patients early on with at least four core medication classes, so-called quadruple therapy.
The guideline document offers a roadmap for navigating those key issues and many others and uses some creative tactics. They include the introduction of generalist-friendly labels for the traditional but obscurely named four stages of HF severity that, it is hoped, will have wider reach and expand the use of effective therapies.
It introduces additional disease-staging terminology that characterizes the syndrome as a continuum:
- “At risk for HF” for stage A, applied to asymptomatic patients with risk factors such as diabetes or hypertension but no known cardiac changes.
- “Pre-HF” for stage B, which adds cardiac structural changes or elevated natriuretic peptides, still in the absence of symptoms.
- “Symptomatic HF” for stage C, that is, structural disease with current or previous symptoms.
- “Advanced HF” for stage D, characterized by severe debilitating symptoms or repeated hospitalizations even with guideline-directed medical therapy (GDMT).
The new terms should be “easier for primary care physicians as well as nonspecialists” to remember and use effectively “and easier to translate to the patients,” compared with the solely alphabetical staging labels appearing in the guidelines for more than 15 years, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
An emphasis on “at risk for HF” and “pre-HF” in the new document may help efforts to expand primary prevention of HF and management of preclinical HF. The guideline, Dr. Bozkurt said, includes specific treatment recommendations for those early stages.
The document also updates and sometimes introduces “recommendations for advanced heart failure, acute heart failure, and comorbidities – specifically for atrial fibrillation, iron deficiency, sleep apnea, coronary artery disease, and valvular heart disease,” Dr. Bozkurt observed, as well as for cardiomyopathy and HF related to pregnancy and cancer chemotherapy. “So, it’s a very comprehensive guideline.”
Dr. Bozkurt is vice chair of the guideline writing committee and helped introduce the guideline at the annual scientific sessions of the American College of Cardiology. The document, developed by the ACC, the American Heart Association, and the Heart Failure Society of America, was published April 1, 2022, in the societies’ flagship journals, Journal of the American College of Cardiology, Circulation, and the Journal of Cardiac Failure, respectively. It replaces the 2013 guideline from the ACC and AHA and the ACC/AHA/HFSA–focused update from 2017.
“We really need to treat early, and then we need to treat appropriately,” Douglas L. Mann, MD, Washington University in St. Louis, said in an interview. Dr. Mann, who was not involved in development of the new guideline, said he is “enthusiastic” about the new staging terminology.
“I think it makes it easier to convey the message that these people do need medicines, will benefit from medicines, and in some cases heart failure can be preventable,” he said. “I’m in favor of anything that simplifies it and makes it more readily interpretable by busy doctors who aren’t specialists.”
With the new staging terminology and in other ways, the guideline seems to appreciate cardiomyopathy as a journey from preclinical to advanced symptomatic stages – the preclinical “at-risk” stage tightening focus on primary prevention – and updated thinking on classification of HF by EF.
For example, there is new consideration of “HF with improved ejection fraction” (HFimpEF), which suggests the patient may be evolving from HF with reduced EF (HFrEF) to HF with EF that is preserved or mildly reduced, or vice versa.
With HFimpEF, which identifies patients previously with an EF of 40% or lower that improves to beyond 40% at follow-up testing, patients should continue on the medications they had been previously taking for HFrEF, Dr. Bozkurt said.
Patients at risk for HF, in stage A by the older terminology, are characterized by one or more significant HF risk factors, such as hypertension, diabetes, or coronary disease, as they have been in prior guidelines. But the new document, Dr. Bozkurt observed, adds genetic cardiomyopathies and exposure to cardiotoxic agents to the list.
Perhaps surprisingly, the guideline also includes elevated natriuretic peptides as an indicator of “at risk for HF,” with implications for screening. The evidence suggests that, “for patients who are at risk for heart failure, natriuretic peptide-based screening, followed by team-based care, can prevent development of left ventricular dysfunction in heart failure,” Dr. Bozkurt said.
Persons at risk for HF realistically encompass a huge swath of the population given the world prevalence of high blood pressure, obesity, and diabetes. Management of stage A, therefore, focuses on established tenets of primary cardiovascular prevention, such as weight and BP control, exercise, and healthy dietary choices.
They may well be eligible for treatment with sodium-glucose transporter 2 (SGLT2) inhibitors, which have been “game changers,” Dr. Mann said. “Now you can give them to diabetics and it’s going to prevent heart failure and [cardiovascular] events. We didn’t have a drug like that before, so I think that places a lot of emphasis on aggressive treatment of diabetes.”
For patients with symptomatic HF, the document touts multidisciplinary care and early initiation of drugs from each of four drug classes. Such quadruple therapy includes an SGLT2 inhibitor along with a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin system (RAS) inhibitor: the “core foundational therapies” for patients with HFrEF, Dr. Bozkurt observed.
Of note, she said, the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan (Entresto, Novartis) is the preferred RAS inhibitor. But “if the ARNI cannot be used, then use ACE inhibitors.” If the patient is intolerant of ACE inhibitors because of cough or angioedema, then the choice should be an angiotensin-receptor blocker.
“We have very effective therapies offering survival and morbidity benefits as well as improvements in quality of life and reverse remodeling,” Dr. Bozkurt observed. “The most important message is that optimization of therapies, including all of these medication classes, saves lives.”
The guideline also includes, for the first time, a series of “value statements” on cost-effectiveness of different therapies that assign a “high-value” rating to MRAs, hydralazine, and isosorbide dinitrate in otherwise optimally treated self-identified African Americans, and device therapy in appropriately selected patients. The statements hold SGLT2 inhibitors in chronic symptomatic HF and cardiac transplantation in advanced GDMT-resistant HF to be of “intermediate” value.
The value statements, Dr. Bozkurt noted, “are included throughout the document when there is evidence; when there is a high-quality cost-effectiveness study published.”
Dr. Bozkurt disclosed receiving honoraria or consulting fees from Amgen, AstraZeneca, Baxter International, Bristol-Myers Squibb, Sanofi-Aventis, scPharmaceuticals, and Vifor Pharma; serving on a data safety monitoring board for LivaNova USA; and holding other relationships with Abbott Laboratories and Relypsa. Dr. Mann disclosed receiving honoraria or consulting fees from MyoKardia, Novartis, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Mavacamten controlled hypertrophic cardiomyopathy for over 1 year
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
AT ACC 2022
VALOR-HCM: Novel drug may delay, avert invasive therapy in OHCM
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.
The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.

The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.

The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
SCORED: Sotagliflozin shows robust MACE benefit
WASHINGTON – Results from new analyses further fleshed out the potent effect by the investigational SGLT1&2 inhibitor sotagliflozin on major cardiovascular adverse events in patients with type 2 diabetes, chronic kidney disease, and at high risk for cardiovascular disease in the SCORED trial that randomized more than 10,000 patients.
In prespecified, secondary analyses of the SCORED results, treatment with sotagliflozin during a median of 16 months was linked to a significant 21% risk reduction relative to placebo for the combined incidence of total major adverse cardiovascular events (MACE), which included cardiovascular death, first and recurrent episodes of nonfatal MI, and nonfatal stroke among the 5,144 randomized patients who entered the trial with a history of cardiovascular disease (CVD), Deepak L. Bhatt, MD, said at the annual scientific sessions of the American College of Cardiology.

Among the 5,440 patients in the study who did not have a history of CVD (although they did have at least one major risk factor or at least two minor risk factors), treatment with sotagliflozin was linked to a significant 26% relative risk reduction in total MACE events.
Part of these overall MACE benefits resulted from similar improvements from sotagliflozin treatment on the individual outcomes of total nonfatal MI and total nonfatal strokes. Treatment with sotagliflozin cut these MIs by a significant 31% in patients with a history of CVD relative to patients who received placebo, and by a relative 34% in those without a CVD event in their history, a difference compared with placebo that fell short of significance, said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Health, both in Boston.
Treatment with sotagliflozin also cut total nonfatal strokes by 31% relative to placebo in patients with a history of CVD, and by a relative 38% in those without a CVD history. Both differences fell short of significance.
An early MACE benefit and a stroke benefit
“This stroke benefit has not been clearly seen” with any agent from the closely related sodium-glucose cotransport-2 (SGLT2) inhibitor class, and “the MACE benefit appeared very early,” within 3 months from the start of sotagliflozin treatment, “which may be because of the SGLT1 inhibition,” Dr. Bhatt said during his report.
The SGLT1 receptor is the primary mechanism cells in the gut use to absorb glucose and galactose in the human gastrointestinal tract, Dr. Bhatt explained, while the SGLT2 receptor appears on kidney cells and is the major player in the reabsorption of filtered glucose. The SGLT2 inhibitor class includes the agents canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), while sotagliflozin inhibits both SGLT1 and SGLT2.
Main results from SCORED appeared in a report first released in late 2020, and showed that for the study’s primary endpoint treatment with sotagliflozin linked with a significant 26% relative risk reduction for the composite of cardiovascular deaths, hospitalizations for heart failure, and urgent visits for heart failure (N Engl J Med. 2021 Jan 14;384[2]:129-39). Patient follow-up in SCORED was not as long as originally planned when the study stopped early due to a loss of funding from a sponsor that was triggered by the COVID-19 pandemic.
MACE results ‘heterogeneous’ from SGLT2 inhibitors
Sotagliflozin and agents from the SGLT2 inhibitor class “have been consistent” in their benefits for reducing cardiovascular death and hospitalization for heart failure, but for MACE, the results from the SGLT2 inhibitors “have been more heterogeneous,” and the effect of sotagliflozin on MACE “were different in SCORED,” commented Michelle L. O’Donoghue, MD, MPH, a cardiologist at Brigham and Women’s Hospital in Boston who was not involved with this work.

“The results suggest a benefit [from sotagliflozin] on atherosclerotic events, which could be a potential advantage” compared with the SGLT2 inhibitors, “but the heterogeneity of this effect” among these agents means that more confirmatory data are needed for sotagliflozin, Dr. O’Donoghue said in an interview.
“There is a lot of enthusiasm for the concept” of combined inhibition of the SGLT1 and 2 receptors, and if more evidence for unique benefits of this effect accumulate “it may lead to increased enthusiasm for sotagliflozin,” she said. “A lot will also depend on pricing decisions” for sotagliflozin, if it receives U.S. marketing approval from the Food and Drug Administration. Decisions about which agent from the SGLT2 inhibitor class to prescribe “are often being made based on price right now,” Dr. O’Donoghue said.
Lexicon Pharmaceuticals, the company developing sotagliflozin, has announced plans to resubmit its new drug application for sotagliflozin to the FDA later in 2022, with the agency’s approval decision likely occurring late in 2022 or sometime during 2023. In February, the company withdrew its December 2021 application to correct a “technical issue” it had found.
An additional analysis reported by Dr. Bhatt used combined data from SCORED as well as several additional randomized trials of sotagliflozin involving a total of more than 20,000 patients that showed a significant 21% reduction in the incidence of MACE compared with placebo.
During his talk, Dr. Bhatt said that sotagliflozin was potentially superior to the agents that inhibit only SGLT2. In an interview, he based this tentative assessment on at least four attributes of sotagliflozin that have emerged from trial results:
- The drug’s ability to significantly reduce MACE and to have this effect apparent within a few months of treatment onset;
- The significantly reduced rate of stroke with sotagliflozin (when patients are not subdivided into those with or without a history of CVD) that has not yet been seen with any SGLT2 inhibitor;
- The ability of sotagliflozin to reduce hemoglobin A1c levels in patients with type 2 diabetes even when their estimated glomerular filtration rate is less than 30 mL/min per 1.73 m2, an effect not seen with SGLT2 inhibitors and possibly explained by sotagliflozin having an effect on gut absorption of glucose in addition to its SGLT2 inhibitory effect in the kidney;
- And the proven ability of sotagliflozin to be safe and effective when initiated in patients hospitalized for heart failure, a property that so far has only also been shown for the SGLT2 inhibitor empagliflozin in the EMPULSE trial (Nature Med. 2022 Mar;28: 568-74).
SCORED was sponsored by Sanofi and Lexicon Pharmaceuticals, the companies originally developing sotagliflozin, although with the withdrawal of Sanofi’s support, further development is now sponsored entirely by Lexicon. Dr. Bhatt received research funding from Sanofi and Lexicon that was paid to Brigham and Women’s Health, and he has been an advisor to numerous companies. Dr. O’Donoghue has been a consultant to Amgen, Janssen, and Novartis, and has received research funding from Amgen, AZ MedImmune, Intarcia, Janssen, Merck, and Novartis.
WASHINGTON – Results from new analyses further fleshed out the potent effect by the investigational SGLT1&2 inhibitor sotagliflozin on major cardiovascular adverse events in patients with type 2 diabetes, chronic kidney disease, and at high risk for cardiovascular disease in the SCORED trial that randomized more than 10,000 patients.
In prespecified, secondary analyses of the SCORED results, treatment with sotagliflozin during a median of 16 months was linked to a significant 21% risk reduction relative to placebo for the combined incidence of total major adverse cardiovascular events (MACE), which included cardiovascular death, first and recurrent episodes of nonfatal MI, and nonfatal stroke among the 5,144 randomized patients who entered the trial with a history of cardiovascular disease (CVD), Deepak L. Bhatt, MD, said at the annual scientific sessions of the American College of Cardiology.

Among the 5,440 patients in the study who did not have a history of CVD (although they did have at least one major risk factor or at least two minor risk factors), treatment with sotagliflozin was linked to a significant 26% relative risk reduction in total MACE events.
Part of these overall MACE benefits resulted from similar improvements from sotagliflozin treatment on the individual outcomes of total nonfatal MI and total nonfatal strokes. Treatment with sotagliflozin cut these MIs by a significant 31% in patients with a history of CVD relative to patients who received placebo, and by a relative 34% in those without a CVD event in their history, a difference compared with placebo that fell short of significance, said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Health, both in Boston.
Treatment with sotagliflozin also cut total nonfatal strokes by 31% relative to placebo in patients with a history of CVD, and by a relative 38% in those without a CVD history. Both differences fell short of significance.
An early MACE benefit and a stroke benefit
“This stroke benefit has not been clearly seen” with any agent from the closely related sodium-glucose cotransport-2 (SGLT2) inhibitor class, and “the MACE benefit appeared very early,” within 3 months from the start of sotagliflozin treatment, “which may be because of the SGLT1 inhibition,” Dr. Bhatt said during his report.
The SGLT1 receptor is the primary mechanism cells in the gut use to absorb glucose and galactose in the human gastrointestinal tract, Dr. Bhatt explained, while the SGLT2 receptor appears on kidney cells and is the major player in the reabsorption of filtered glucose. The SGLT2 inhibitor class includes the agents canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), while sotagliflozin inhibits both SGLT1 and SGLT2.
Main results from SCORED appeared in a report first released in late 2020, and showed that for the study’s primary endpoint treatment with sotagliflozin linked with a significant 26% relative risk reduction for the composite of cardiovascular deaths, hospitalizations for heart failure, and urgent visits for heart failure (N Engl J Med. 2021 Jan 14;384[2]:129-39). Patient follow-up in SCORED was not as long as originally planned when the study stopped early due to a loss of funding from a sponsor that was triggered by the COVID-19 pandemic.
MACE results ‘heterogeneous’ from SGLT2 inhibitors
Sotagliflozin and agents from the SGLT2 inhibitor class “have been consistent” in their benefits for reducing cardiovascular death and hospitalization for heart failure, but for MACE, the results from the SGLT2 inhibitors “have been more heterogeneous,” and the effect of sotagliflozin on MACE “were different in SCORED,” commented Michelle L. O’Donoghue, MD, MPH, a cardiologist at Brigham and Women’s Hospital in Boston who was not involved with this work.

“The results suggest a benefit [from sotagliflozin] on atherosclerotic events, which could be a potential advantage” compared with the SGLT2 inhibitors, “but the heterogeneity of this effect” among these agents means that more confirmatory data are needed for sotagliflozin, Dr. O’Donoghue said in an interview.
“There is a lot of enthusiasm for the concept” of combined inhibition of the SGLT1 and 2 receptors, and if more evidence for unique benefits of this effect accumulate “it may lead to increased enthusiasm for sotagliflozin,” she said. “A lot will also depend on pricing decisions” for sotagliflozin, if it receives U.S. marketing approval from the Food and Drug Administration. Decisions about which agent from the SGLT2 inhibitor class to prescribe “are often being made based on price right now,” Dr. O’Donoghue said.
Lexicon Pharmaceuticals, the company developing sotagliflozin, has announced plans to resubmit its new drug application for sotagliflozin to the FDA later in 2022, with the agency’s approval decision likely occurring late in 2022 or sometime during 2023. In February, the company withdrew its December 2021 application to correct a “technical issue” it had found.
An additional analysis reported by Dr. Bhatt used combined data from SCORED as well as several additional randomized trials of sotagliflozin involving a total of more than 20,000 patients that showed a significant 21% reduction in the incidence of MACE compared with placebo.
During his talk, Dr. Bhatt said that sotagliflozin was potentially superior to the agents that inhibit only SGLT2. In an interview, he based this tentative assessment on at least four attributes of sotagliflozin that have emerged from trial results:
- The drug’s ability to significantly reduce MACE and to have this effect apparent within a few months of treatment onset;
- The significantly reduced rate of stroke with sotagliflozin (when patients are not subdivided into those with or without a history of CVD) that has not yet been seen with any SGLT2 inhibitor;
- The ability of sotagliflozin to reduce hemoglobin A1c levels in patients with type 2 diabetes even when their estimated glomerular filtration rate is less than 30 mL/min per 1.73 m2, an effect not seen with SGLT2 inhibitors and possibly explained by sotagliflozin having an effect on gut absorption of glucose in addition to its SGLT2 inhibitory effect in the kidney;
- And the proven ability of sotagliflozin to be safe and effective when initiated in patients hospitalized for heart failure, a property that so far has only also been shown for the SGLT2 inhibitor empagliflozin in the EMPULSE trial (Nature Med. 2022 Mar;28: 568-74).
SCORED was sponsored by Sanofi and Lexicon Pharmaceuticals, the companies originally developing sotagliflozin, although with the withdrawal of Sanofi’s support, further development is now sponsored entirely by Lexicon. Dr. Bhatt received research funding from Sanofi and Lexicon that was paid to Brigham and Women’s Health, and he has been an advisor to numerous companies. Dr. O’Donoghue has been a consultant to Amgen, Janssen, and Novartis, and has received research funding from Amgen, AZ MedImmune, Intarcia, Janssen, Merck, and Novartis.
WASHINGTON – Results from new analyses further fleshed out the potent effect by the investigational SGLT1&2 inhibitor sotagliflozin on major cardiovascular adverse events in patients with type 2 diabetes, chronic kidney disease, and at high risk for cardiovascular disease in the SCORED trial that randomized more than 10,000 patients.
In prespecified, secondary analyses of the SCORED results, treatment with sotagliflozin during a median of 16 months was linked to a significant 21% risk reduction relative to placebo for the combined incidence of total major adverse cardiovascular events (MACE), which included cardiovascular death, first and recurrent episodes of nonfatal MI, and nonfatal stroke among the 5,144 randomized patients who entered the trial with a history of cardiovascular disease (CVD), Deepak L. Bhatt, MD, said at the annual scientific sessions of the American College of Cardiology.

Among the 5,440 patients in the study who did not have a history of CVD (although they did have at least one major risk factor or at least two minor risk factors), treatment with sotagliflozin was linked to a significant 26% relative risk reduction in total MACE events.
Part of these overall MACE benefits resulted from similar improvements from sotagliflozin treatment on the individual outcomes of total nonfatal MI and total nonfatal strokes. Treatment with sotagliflozin cut these MIs by a significant 31% in patients with a history of CVD relative to patients who received placebo, and by a relative 34% in those without a CVD event in their history, a difference compared with placebo that fell short of significance, said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Health, both in Boston.
Treatment with sotagliflozin also cut total nonfatal strokes by 31% relative to placebo in patients with a history of CVD, and by a relative 38% in those without a CVD history. Both differences fell short of significance.
An early MACE benefit and a stroke benefit
“This stroke benefit has not been clearly seen” with any agent from the closely related sodium-glucose cotransport-2 (SGLT2) inhibitor class, and “the MACE benefit appeared very early,” within 3 months from the start of sotagliflozin treatment, “which may be because of the SGLT1 inhibition,” Dr. Bhatt said during his report.
The SGLT1 receptor is the primary mechanism cells in the gut use to absorb glucose and galactose in the human gastrointestinal tract, Dr. Bhatt explained, while the SGLT2 receptor appears on kidney cells and is the major player in the reabsorption of filtered glucose. The SGLT2 inhibitor class includes the agents canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), while sotagliflozin inhibits both SGLT1 and SGLT2.
Main results from SCORED appeared in a report first released in late 2020, and showed that for the study’s primary endpoint treatment with sotagliflozin linked with a significant 26% relative risk reduction for the composite of cardiovascular deaths, hospitalizations for heart failure, and urgent visits for heart failure (N Engl J Med. 2021 Jan 14;384[2]:129-39). Patient follow-up in SCORED was not as long as originally planned when the study stopped early due to a loss of funding from a sponsor that was triggered by the COVID-19 pandemic.
MACE results ‘heterogeneous’ from SGLT2 inhibitors
Sotagliflozin and agents from the SGLT2 inhibitor class “have been consistent” in their benefits for reducing cardiovascular death and hospitalization for heart failure, but for MACE, the results from the SGLT2 inhibitors “have been more heterogeneous,” and the effect of sotagliflozin on MACE “were different in SCORED,” commented Michelle L. O’Donoghue, MD, MPH, a cardiologist at Brigham and Women’s Hospital in Boston who was not involved with this work.

“The results suggest a benefit [from sotagliflozin] on atherosclerotic events, which could be a potential advantage” compared with the SGLT2 inhibitors, “but the heterogeneity of this effect” among these agents means that more confirmatory data are needed for sotagliflozin, Dr. O’Donoghue said in an interview.
“There is a lot of enthusiasm for the concept” of combined inhibition of the SGLT1 and 2 receptors, and if more evidence for unique benefits of this effect accumulate “it may lead to increased enthusiasm for sotagliflozin,” she said. “A lot will also depend on pricing decisions” for sotagliflozin, if it receives U.S. marketing approval from the Food and Drug Administration. Decisions about which agent from the SGLT2 inhibitor class to prescribe “are often being made based on price right now,” Dr. O’Donoghue said.
Lexicon Pharmaceuticals, the company developing sotagliflozin, has announced plans to resubmit its new drug application for sotagliflozin to the FDA later in 2022, with the agency’s approval decision likely occurring late in 2022 or sometime during 2023. In February, the company withdrew its December 2021 application to correct a “technical issue” it had found.
An additional analysis reported by Dr. Bhatt used combined data from SCORED as well as several additional randomized trials of sotagliflozin involving a total of more than 20,000 patients that showed a significant 21% reduction in the incidence of MACE compared with placebo.
During his talk, Dr. Bhatt said that sotagliflozin was potentially superior to the agents that inhibit only SGLT2. In an interview, he based this tentative assessment on at least four attributes of sotagliflozin that have emerged from trial results:
- The drug’s ability to significantly reduce MACE and to have this effect apparent within a few months of treatment onset;
- The significantly reduced rate of stroke with sotagliflozin (when patients are not subdivided into those with or without a history of CVD) that has not yet been seen with any SGLT2 inhibitor;
- The ability of sotagliflozin to reduce hemoglobin A1c levels in patients with type 2 diabetes even when their estimated glomerular filtration rate is less than 30 mL/min per 1.73 m2, an effect not seen with SGLT2 inhibitors and possibly explained by sotagliflozin having an effect on gut absorption of glucose in addition to its SGLT2 inhibitory effect in the kidney;
- And the proven ability of sotagliflozin to be safe and effective when initiated in patients hospitalized for heart failure, a property that so far has only also been shown for the SGLT2 inhibitor empagliflozin in the EMPULSE trial (Nature Med. 2022 Mar;28: 568-74).
SCORED was sponsored by Sanofi and Lexicon Pharmaceuticals, the companies originally developing sotagliflozin, although with the withdrawal of Sanofi’s support, further development is now sponsored entirely by Lexicon. Dr. Bhatt received research funding from Sanofi and Lexicon that was paid to Brigham and Women’s Health, and he has been an advisor to numerous companies. Dr. O’Donoghue has been a consultant to Amgen, Janssen, and Novartis, and has received research funding from Amgen, AZ MedImmune, Intarcia, Janssen, Merck, and Novartis.
AT ACC 2022
Low-sodium diet did not cut clinical events in heart failure trial
A low-sodium diet was not associated with a reduction in future clinical events in a new study in ambulatory patients with heart failure. But there was a moderate benefit on quality of life and New York Heart Association (NYHA) functional class.
The results of the SODIUM-HF trial were presented April 2 at the annual scientific sessions of the American College of Cardiology, conducted virtually and in person in Washington. They were also simultaneously published online in the Lancet.
The study found that a strategy to reduce dietary sodium intake to less than 1,500 mg daily was not more effective than usual care in reducing the primary endpoint of risk for hospitalization or emergency department visits due to cardiovascular causes or all-cause death at 12 months.
“This is the largest and longest trial to look at the question of reducing dietary sodium in heart failure patients,” lead author Justin Ezekowitz, MBBCh, from the Canadian VIGOUR Center at the University of Alberta, Edmonton, Canada, told this news organization.
But he pointed out that there were fewer events than expected in the study, which was stopped early because of a combination of futility and practical difficulties caused by the COVID pandemic, so it could have been underpowered. Dr. Ezekowitz also suggested that a greater reduction in sodium than achieved in this study or a longer follow-up may be required to show an effect on clinical events.
“We hope others will do additional studies of sodium as well as other dietary recommendations as part of a comprehensive diet for heart failure patients,” he commented.
Dr. Ezekowitz said that the study results did not allow blanket recommendations to be made on reducing sodium intake in heart failure.
But he added: “I don’t think we should write off sodium reduction in this population. I think we can tell patients that reducing dietary sodium may potentially improve symptoms and quality of life, and I will continue to recommend reducing sodium as part of an overall healthy diet. We don’t want to throw the baby out with the bathwater.”
Dr. Ezekowitz noted that heart failure is associated with neurohormonal activation and abnormalities in autonomic control that lead to sodium and water retention; thus, dietary restriction of sodium has been historically endorsed as a mechanism to prevent fluid overload and subsequent clinical outcomes; however, clinical trials so far have shown mixed results.
“The guidelines used to strongly recommend a reduction in sodium intake in heart failure patients, but this advice has backed off in recent years because of the lack of data. Most heart failure guidelines now do not make any recommendations on dietary sodium,” he said.
SODIUM-HF was a pragmatic, multinational, open-label, randomized trial conducted in six countries (Australia, Canada, Chile, Colombia, Mexico, and New Zealand), which included 809 patients (median age, 67 years) with chronic heart failure (NYHA functional class II–III) who were receiving optimally tolerated guideline-directed medical treatment. They were randomly assigned to usual care according to local guidelines or a low-sodium diet of less than 100 mmol (<1,500 mg/day). Patients with a baseline sodium intake of less than 1,500 mg/day were excluded.
In the intervention group, patients were asked to follow low-sodium menus developed by dietitians localized to each region. They also received behavioral counseling by trained dietitians or physicians or nurses.
Dietary sodium intake was assessed by using a 3-day food record (including 1 weekend day) at baseline, 6 months, and 12 months in both groups and, for the intervention group, also at 3 and 9 months to monitor and support dietary adherence.
Dr. Ezekowitz explained that although the best method for measuring sodium levels would normally be a 24-hour urine sodium, this would be impractical in a large clinical trial. In addition, he pointed out that urinary sodium is not an accurate measure of actual sodium levels in patients taking diuretics, so it is not a good measure to use in a heart failure population.
“The food record method of assessing sodium levels has been well validated; I think we measured it as accurately as we could have done,” he added.
Results showed that between baseline and 12 months, the median sodium intake decreased from 2,286 mg/day to 1,658 mg/day in the low-sodium group and from 2,119 mg/day to 2,073 mg/day in the usual care group. The median difference between groups was 415 mg/day at 12 months.
By 12 months, events comprising the primary outcome (hospitalization or emergency department visits due to cardiovascular causes or all-cause death) had occurred in 15% of patients in the low-sodium diet group and 17% of those in the usual care group (hazard ratio [HR], 0.89 [95% CI, 0.63 - 1.26]; P = .53).
All-cause death occurred in 6% of patients in the low-sodium diet group and 4% of those in the usual care group (HR, 1.38; P = .32). Cardiovascular-related hospitalization occurred in 10% of the low-sodium group and 12% of the usual care group (HR, 0.82; P = .36), and cardiovascular-related emergency department visits occurred in 4% of both groups (HR, 1.21; P = .60).
The absence of treatment effect for the primary outcome was consistent across most prespecified subgroups, including those with higher vs lower baseline sodium intake. But there was a suggestion of a greater reduction in the primary outcome in individuals younger than age 65 years than in those age 65 years and older.
Quality-of-life measures on the Kansas City Cardiomyopathy Questionnaire (KCCQ) suggested a benefit in the low-sodium group, with mean between-group differences in the change from baseline to 12 months of 3.38 points in the overall summary score, 3.29 points in the clinical summary score, and 3.77 points in the physical limitation score (all differences were statistically significant).
There was no significant difference in 6-minute-walk distance at 12 months between the low-sodium diet group and the usual care group.
NYHA functional class at 12 months differed significantly between groups; the low-sodium diet group had a greater likelihood of improving by one NYHA class than the usual care group (odds ratio, 0.59; P = .0061).
No safety events related to the study treatment were reported in either group.
Dr. Ezekowitz said that to investigate whether longer follow-up may show a difference in events, further analyses are planned at 2 years and 5 years.
Questions on food recall and blinding
Commenting on the findings at the late-breaking clinical trials session at the ACC meeting, Biykem Bozkurt, MD, professor of medicine at Baylor College of Medicine, Houston, congratulated Dr. Ezekowitz on conducting this trial.
“We have been chasing the holy grail of sodium reduction in heart failure for a very long time, so I have to commend you and your team for taking on this challenge, especially during the pandemic,” she said.
But Dr. Bozkurt questioned whether the intervention group actually had a meaningful sodium reduction given that this was measured by food recall and this may have been accounted for by under-reporting of certain food intakes.
Dr. Ezekowitz responded that patients acted as their own controls in that calorie intake, fluid intake, and weight were also assessed and did not change. “So I think we did have a meaningful reduction in sodium,” he said.
Dr. Bozkurt also queried whether the improvements in quality of life and functional status were reliable given that this was an unblinded study.
To this point, Dr. Ezekowitz pointed out that the KCCQ quality-of-life measure was a highly validated instrument and that improvements were seen in these measures at 3, 6, and 12 months. “It is not like these were spurious findings, so I think we have to look at this as a real result,” he argued.
Commenting on the study at an ACC press conference, Mary Norine Walsh, MD, director of the heart failure and cardiac transplantation programs at St. Vincent Heart Center in Indianapolis, said the trial had answered two important questions: that sodium reduction in heart failure may not reduce heart failure hospitalization/death but that patients feel better.
“I think we can safely tell patients that if they slip up a bit they may not end up in hospital,” she added.
This study was funded by the Canadian Institutes of Health Research and the University Hospital Foundation (Edmonton, Alberta, Canada) and the Health Research Council of New Zealand. Dr. Ezekowitz reports research grants from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, eko.ai, US2.ai, Merck, Novartis, Otsuka, Sanofi, and Servier and consulting fees from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Merck, Novartis, Otsuka, Sanofi, and Servier.
A version of this article first appeared on Medscape.com.
A low-sodium diet was not associated with a reduction in future clinical events in a new study in ambulatory patients with heart failure. But there was a moderate benefit on quality of life and New York Heart Association (NYHA) functional class.
The results of the SODIUM-HF trial were presented April 2 at the annual scientific sessions of the American College of Cardiology, conducted virtually and in person in Washington. They were also simultaneously published online in the Lancet.
The study found that a strategy to reduce dietary sodium intake to less than 1,500 mg daily was not more effective than usual care in reducing the primary endpoint of risk for hospitalization or emergency department visits due to cardiovascular causes or all-cause death at 12 months.
“This is the largest and longest trial to look at the question of reducing dietary sodium in heart failure patients,” lead author Justin Ezekowitz, MBBCh, from the Canadian VIGOUR Center at the University of Alberta, Edmonton, Canada, told this news organization.
But he pointed out that there were fewer events than expected in the study, which was stopped early because of a combination of futility and practical difficulties caused by the COVID pandemic, so it could have been underpowered. Dr. Ezekowitz also suggested that a greater reduction in sodium than achieved in this study or a longer follow-up may be required to show an effect on clinical events.
“We hope others will do additional studies of sodium as well as other dietary recommendations as part of a comprehensive diet for heart failure patients,” he commented.
Dr. Ezekowitz said that the study results did not allow blanket recommendations to be made on reducing sodium intake in heart failure.
But he added: “I don’t think we should write off sodium reduction in this population. I think we can tell patients that reducing dietary sodium may potentially improve symptoms and quality of life, and I will continue to recommend reducing sodium as part of an overall healthy diet. We don’t want to throw the baby out with the bathwater.”
Dr. Ezekowitz noted that heart failure is associated with neurohormonal activation and abnormalities in autonomic control that lead to sodium and water retention; thus, dietary restriction of sodium has been historically endorsed as a mechanism to prevent fluid overload and subsequent clinical outcomes; however, clinical trials so far have shown mixed results.
“The guidelines used to strongly recommend a reduction in sodium intake in heart failure patients, but this advice has backed off in recent years because of the lack of data. Most heart failure guidelines now do not make any recommendations on dietary sodium,” he said.
SODIUM-HF was a pragmatic, multinational, open-label, randomized trial conducted in six countries (Australia, Canada, Chile, Colombia, Mexico, and New Zealand), which included 809 patients (median age, 67 years) with chronic heart failure (NYHA functional class II–III) who were receiving optimally tolerated guideline-directed medical treatment. They were randomly assigned to usual care according to local guidelines or a low-sodium diet of less than 100 mmol (<1,500 mg/day). Patients with a baseline sodium intake of less than 1,500 mg/day were excluded.
In the intervention group, patients were asked to follow low-sodium menus developed by dietitians localized to each region. They also received behavioral counseling by trained dietitians or physicians or nurses.
Dietary sodium intake was assessed by using a 3-day food record (including 1 weekend day) at baseline, 6 months, and 12 months in both groups and, for the intervention group, also at 3 and 9 months to monitor and support dietary adherence.
Dr. Ezekowitz explained that although the best method for measuring sodium levels would normally be a 24-hour urine sodium, this would be impractical in a large clinical trial. In addition, he pointed out that urinary sodium is not an accurate measure of actual sodium levels in patients taking diuretics, so it is not a good measure to use in a heart failure population.
“The food record method of assessing sodium levels has been well validated; I think we measured it as accurately as we could have done,” he added.
Results showed that between baseline and 12 months, the median sodium intake decreased from 2,286 mg/day to 1,658 mg/day in the low-sodium group and from 2,119 mg/day to 2,073 mg/day in the usual care group. The median difference between groups was 415 mg/day at 12 months.
By 12 months, events comprising the primary outcome (hospitalization or emergency department visits due to cardiovascular causes or all-cause death) had occurred in 15% of patients in the low-sodium diet group and 17% of those in the usual care group (hazard ratio [HR], 0.89 [95% CI, 0.63 - 1.26]; P = .53).
All-cause death occurred in 6% of patients in the low-sodium diet group and 4% of those in the usual care group (HR, 1.38; P = .32). Cardiovascular-related hospitalization occurred in 10% of the low-sodium group and 12% of the usual care group (HR, 0.82; P = .36), and cardiovascular-related emergency department visits occurred in 4% of both groups (HR, 1.21; P = .60).
The absence of treatment effect for the primary outcome was consistent across most prespecified subgroups, including those with higher vs lower baseline sodium intake. But there was a suggestion of a greater reduction in the primary outcome in individuals younger than age 65 years than in those age 65 years and older.
Quality-of-life measures on the Kansas City Cardiomyopathy Questionnaire (KCCQ) suggested a benefit in the low-sodium group, with mean between-group differences in the change from baseline to 12 months of 3.38 points in the overall summary score, 3.29 points in the clinical summary score, and 3.77 points in the physical limitation score (all differences were statistically significant).
There was no significant difference in 6-minute-walk distance at 12 months between the low-sodium diet group and the usual care group.
NYHA functional class at 12 months differed significantly between groups; the low-sodium diet group had a greater likelihood of improving by one NYHA class than the usual care group (odds ratio, 0.59; P = .0061).
No safety events related to the study treatment were reported in either group.
Dr. Ezekowitz said that to investigate whether longer follow-up may show a difference in events, further analyses are planned at 2 years and 5 years.
Questions on food recall and blinding
Commenting on the findings at the late-breaking clinical trials session at the ACC meeting, Biykem Bozkurt, MD, professor of medicine at Baylor College of Medicine, Houston, congratulated Dr. Ezekowitz on conducting this trial.
“We have been chasing the holy grail of sodium reduction in heart failure for a very long time, so I have to commend you and your team for taking on this challenge, especially during the pandemic,” she said.
But Dr. Bozkurt questioned whether the intervention group actually had a meaningful sodium reduction given that this was measured by food recall and this may have been accounted for by under-reporting of certain food intakes.
Dr. Ezekowitz responded that patients acted as their own controls in that calorie intake, fluid intake, and weight were also assessed and did not change. “So I think we did have a meaningful reduction in sodium,” he said.
Dr. Bozkurt also queried whether the improvements in quality of life and functional status were reliable given that this was an unblinded study.
To this point, Dr. Ezekowitz pointed out that the KCCQ quality-of-life measure was a highly validated instrument and that improvements were seen in these measures at 3, 6, and 12 months. “It is not like these were spurious findings, so I think we have to look at this as a real result,” he argued.
Commenting on the study at an ACC press conference, Mary Norine Walsh, MD, director of the heart failure and cardiac transplantation programs at St. Vincent Heart Center in Indianapolis, said the trial had answered two important questions: that sodium reduction in heart failure may not reduce heart failure hospitalization/death but that patients feel better.
“I think we can safely tell patients that if they slip up a bit they may not end up in hospital,” she added.
This study was funded by the Canadian Institutes of Health Research and the University Hospital Foundation (Edmonton, Alberta, Canada) and the Health Research Council of New Zealand. Dr. Ezekowitz reports research grants from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, eko.ai, US2.ai, Merck, Novartis, Otsuka, Sanofi, and Servier and consulting fees from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Merck, Novartis, Otsuka, Sanofi, and Servier.
A version of this article first appeared on Medscape.com.
A low-sodium diet was not associated with a reduction in future clinical events in a new study in ambulatory patients with heart failure. But there was a moderate benefit on quality of life and New York Heart Association (NYHA) functional class.
The results of the SODIUM-HF trial were presented April 2 at the annual scientific sessions of the American College of Cardiology, conducted virtually and in person in Washington. They were also simultaneously published online in the Lancet.
The study found that a strategy to reduce dietary sodium intake to less than 1,500 mg daily was not more effective than usual care in reducing the primary endpoint of risk for hospitalization or emergency department visits due to cardiovascular causes or all-cause death at 12 months.
“This is the largest and longest trial to look at the question of reducing dietary sodium in heart failure patients,” lead author Justin Ezekowitz, MBBCh, from the Canadian VIGOUR Center at the University of Alberta, Edmonton, Canada, told this news organization.
But he pointed out that there were fewer events than expected in the study, which was stopped early because of a combination of futility and practical difficulties caused by the COVID pandemic, so it could have been underpowered. Dr. Ezekowitz also suggested that a greater reduction in sodium than achieved in this study or a longer follow-up may be required to show an effect on clinical events.
“We hope others will do additional studies of sodium as well as other dietary recommendations as part of a comprehensive diet for heart failure patients,” he commented.
Dr. Ezekowitz said that the study results did not allow blanket recommendations to be made on reducing sodium intake in heart failure.
But he added: “I don’t think we should write off sodium reduction in this population. I think we can tell patients that reducing dietary sodium may potentially improve symptoms and quality of life, and I will continue to recommend reducing sodium as part of an overall healthy diet. We don’t want to throw the baby out with the bathwater.”
Dr. Ezekowitz noted that heart failure is associated with neurohormonal activation and abnormalities in autonomic control that lead to sodium and water retention; thus, dietary restriction of sodium has been historically endorsed as a mechanism to prevent fluid overload and subsequent clinical outcomes; however, clinical trials so far have shown mixed results.
“The guidelines used to strongly recommend a reduction in sodium intake in heart failure patients, but this advice has backed off in recent years because of the lack of data. Most heart failure guidelines now do not make any recommendations on dietary sodium,” he said.
SODIUM-HF was a pragmatic, multinational, open-label, randomized trial conducted in six countries (Australia, Canada, Chile, Colombia, Mexico, and New Zealand), which included 809 patients (median age, 67 years) with chronic heart failure (NYHA functional class II–III) who were receiving optimally tolerated guideline-directed medical treatment. They were randomly assigned to usual care according to local guidelines or a low-sodium diet of less than 100 mmol (<1,500 mg/day). Patients with a baseline sodium intake of less than 1,500 mg/day were excluded.
In the intervention group, patients were asked to follow low-sodium menus developed by dietitians localized to each region. They also received behavioral counseling by trained dietitians or physicians or nurses.
Dietary sodium intake was assessed by using a 3-day food record (including 1 weekend day) at baseline, 6 months, and 12 months in both groups and, for the intervention group, also at 3 and 9 months to monitor and support dietary adherence.
Dr. Ezekowitz explained that although the best method for measuring sodium levels would normally be a 24-hour urine sodium, this would be impractical in a large clinical trial. In addition, he pointed out that urinary sodium is not an accurate measure of actual sodium levels in patients taking diuretics, so it is not a good measure to use in a heart failure population.
“The food record method of assessing sodium levels has been well validated; I think we measured it as accurately as we could have done,” he added.
Results showed that between baseline and 12 months, the median sodium intake decreased from 2,286 mg/day to 1,658 mg/day in the low-sodium group and from 2,119 mg/day to 2,073 mg/day in the usual care group. The median difference between groups was 415 mg/day at 12 months.
By 12 months, events comprising the primary outcome (hospitalization or emergency department visits due to cardiovascular causes or all-cause death) had occurred in 15% of patients in the low-sodium diet group and 17% of those in the usual care group (hazard ratio [HR], 0.89 [95% CI, 0.63 - 1.26]; P = .53).
All-cause death occurred in 6% of patients in the low-sodium diet group and 4% of those in the usual care group (HR, 1.38; P = .32). Cardiovascular-related hospitalization occurred in 10% of the low-sodium group and 12% of the usual care group (HR, 0.82; P = .36), and cardiovascular-related emergency department visits occurred in 4% of both groups (HR, 1.21; P = .60).
The absence of treatment effect for the primary outcome was consistent across most prespecified subgroups, including those with higher vs lower baseline sodium intake. But there was a suggestion of a greater reduction in the primary outcome in individuals younger than age 65 years than in those age 65 years and older.
Quality-of-life measures on the Kansas City Cardiomyopathy Questionnaire (KCCQ) suggested a benefit in the low-sodium group, with mean between-group differences in the change from baseline to 12 months of 3.38 points in the overall summary score, 3.29 points in the clinical summary score, and 3.77 points in the physical limitation score (all differences were statistically significant).
There was no significant difference in 6-minute-walk distance at 12 months between the low-sodium diet group and the usual care group.
NYHA functional class at 12 months differed significantly between groups; the low-sodium diet group had a greater likelihood of improving by one NYHA class than the usual care group (odds ratio, 0.59; P = .0061).
No safety events related to the study treatment were reported in either group.
Dr. Ezekowitz said that to investigate whether longer follow-up may show a difference in events, further analyses are planned at 2 years and 5 years.
Questions on food recall and blinding
Commenting on the findings at the late-breaking clinical trials session at the ACC meeting, Biykem Bozkurt, MD, professor of medicine at Baylor College of Medicine, Houston, congratulated Dr. Ezekowitz on conducting this trial.
“We have been chasing the holy grail of sodium reduction in heart failure for a very long time, so I have to commend you and your team for taking on this challenge, especially during the pandemic,” she said.
But Dr. Bozkurt questioned whether the intervention group actually had a meaningful sodium reduction given that this was measured by food recall and this may have been accounted for by under-reporting of certain food intakes.
Dr. Ezekowitz responded that patients acted as their own controls in that calorie intake, fluid intake, and weight were also assessed and did not change. “So I think we did have a meaningful reduction in sodium,” he said.
Dr. Bozkurt also queried whether the improvements in quality of life and functional status were reliable given that this was an unblinded study.
To this point, Dr. Ezekowitz pointed out that the KCCQ quality-of-life measure was a highly validated instrument and that improvements were seen in these measures at 3, 6, and 12 months. “It is not like these were spurious findings, so I think we have to look at this as a real result,” he argued.
Commenting on the study at an ACC press conference, Mary Norine Walsh, MD, director of the heart failure and cardiac transplantation programs at St. Vincent Heart Center in Indianapolis, said the trial had answered two important questions: that sodium reduction in heart failure may not reduce heart failure hospitalization/death but that patients feel better.
“I think we can safely tell patients that if they slip up a bit they may not end up in hospital,” she added.
This study was funded by the Canadian Institutes of Health Research and the University Hospital Foundation (Edmonton, Alberta, Canada) and the Health Research Council of New Zealand. Dr. Ezekowitz reports research grants from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, eko.ai, US2.ai, Merck, Novartis, Otsuka, Sanofi, and Servier and consulting fees from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Merck, Novartis, Otsuka, Sanofi, and Servier.
A version of this article first appeared on Medscape.com.
FROM ACC 2022

















