User login
Hepatitis A Outbreak in a Childcare Facility
In young children, hepatitis A is usually asymptomatic. So a childcare facility (CCF) in Ireland surprised by an outbreak of hepatitis A that infected 7 adults and 5 children, hospitalizing 6 of the adults. By the time the investigation and interventions were over, > 554 contacts had been followed up, and it had all cost > €45,000 ($53,000).
The outbreak was traced to a man with hepatitis A whose child had been unwell for 3 weeks with fever, fatigue, abdominal pain, diarrhea, pale stools, and possible jaundice. The child (and an infected cousin) attended a local CCF but because several other cases seemed to be limited to the family and their friends, no one immediately considered the CCF as a possible source of infection. However, approximately 10 days after the first 2 cases were reported, an outbreak was officially declared.
At the time, 93 children were attending the CCF. All 7 adults were household contacts of children in the CCF. None of the 23-member CCF staff developed symptoms of hepatitis A.
As many as 70% of infections are asymptomatic in children under age 6, the researchers note, but that group is often a source of transmission due to suboptimal hygiene. Transmission is usually fecal-oral and person-to-person. Although the initial source of outbreak was not identified, the subsequent transmission suggested person-to-person spread. The researchers say the distribution of cases suggests that the transmission probably happened in the school, with asymptomatic children infecting their families, highlighting the fact that symptomatic cases of hepatitis A only represent a portion of the cases in an outbreak.
A preschool inspection report that preceded the outbreak highlighted deficiencies in the staff’s handwashing practices. An infection control audit undertaken because of the outbreak found a number of deficits, including lack of foot-operated bins and the use of cloth covers on furnishings rather than waterproof material. Medical expenses, including hospitalization, serology, and vaccine, cost between €43,400 - €47, 400 ($51,000 - $56,000).
The researchers say the delayed notification to public health of the first case probably contributed to the extent of the outbreak. Medical professionals, they note, should be aware that although uncommon, hepatitis A still occurs. Prompt recognition and notification can mitigate the significant morbidity associated with the infection.
Source:
O'Connor L, McGovern E, O'Meara M, et al. Epidemiol Infect. 2018;146(6):705-711.
In young children, hepatitis A is usually asymptomatic. So a childcare facility (CCF) in Ireland surprised by an outbreak of hepatitis A that infected 7 adults and 5 children, hospitalizing 6 of the adults. By the time the investigation and interventions were over, > 554 contacts had been followed up, and it had all cost > €45,000 ($53,000).
The outbreak was traced to a man with hepatitis A whose child had been unwell for 3 weeks with fever, fatigue, abdominal pain, diarrhea, pale stools, and possible jaundice. The child (and an infected cousin) attended a local CCF but because several other cases seemed to be limited to the family and their friends, no one immediately considered the CCF as a possible source of infection. However, approximately 10 days after the first 2 cases were reported, an outbreak was officially declared.
At the time, 93 children were attending the CCF. All 7 adults were household contacts of children in the CCF. None of the 23-member CCF staff developed symptoms of hepatitis A.
As many as 70% of infections are asymptomatic in children under age 6, the researchers note, but that group is often a source of transmission due to suboptimal hygiene. Transmission is usually fecal-oral and person-to-person. Although the initial source of outbreak was not identified, the subsequent transmission suggested person-to-person spread. The researchers say the distribution of cases suggests that the transmission probably happened in the school, with asymptomatic children infecting their families, highlighting the fact that symptomatic cases of hepatitis A only represent a portion of the cases in an outbreak.
A preschool inspection report that preceded the outbreak highlighted deficiencies in the staff’s handwashing practices. An infection control audit undertaken because of the outbreak found a number of deficits, including lack of foot-operated bins and the use of cloth covers on furnishings rather than waterproof material. Medical expenses, including hospitalization, serology, and vaccine, cost between €43,400 - €47, 400 ($51,000 - $56,000).
The researchers say the delayed notification to public health of the first case probably contributed to the extent of the outbreak. Medical professionals, they note, should be aware that although uncommon, hepatitis A still occurs. Prompt recognition and notification can mitigate the significant morbidity associated with the infection.
Source:
O'Connor L, McGovern E, O'Meara M, et al. Epidemiol Infect. 2018;146(6):705-711.
In young children, hepatitis A is usually asymptomatic. So a childcare facility (CCF) in Ireland surprised by an outbreak of hepatitis A that infected 7 adults and 5 children, hospitalizing 6 of the adults. By the time the investigation and interventions were over, > 554 contacts had been followed up, and it had all cost > €45,000 ($53,000).
The outbreak was traced to a man with hepatitis A whose child had been unwell for 3 weeks with fever, fatigue, abdominal pain, diarrhea, pale stools, and possible jaundice. The child (and an infected cousin) attended a local CCF but because several other cases seemed to be limited to the family and their friends, no one immediately considered the CCF as a possible source of infection. However, approximately 10 days after the first 2 cases were reported, an outbreak was officially declared.
At the time, 93 children were attending the CCF. All 7 adults were household contacts of children in the CCF. None of the 23-member CCF staff developed symptoms of hepatitis A.
As many as 70% of infections are asymptomatic in children under age 6, the researchers note, but that group is often a source of transmission due to suboptimal hygiene. Transmission is usually fecal-oral and person-to-person. Although the initial source of outbreak was not identified, the subsequent transmission suggested person-to-person spread. The researchers say the distribution of cases suggests that the transmission probably happened in the school, with asymptomatic children infecting their families, highlighting the fact that symptomatic cases of hepatitis A only represent a portion of the cases in an outbreak.
A preschool inspection report that preceded the outbreak highlighted deficiencies in the staff’s handwashing practices. An infection control audit undertaken because of the outbreak found a number of deficits, including lack of foot-operated bins and the use of cloth covers on furnishings rather than waterproof material. Medical expenses, including hospitalization, serology, and vaccine, cost between €43,400 - €47, 400 ($51,000 - $56,000).
The researchers say the delayed notification to public health of the first case probably contributed to the extent of the outbreak. Medical professionals, they note, should be aware that although uncommon, hepatitis A still occurs. Prompt recognition and notification can mitigate the significant morbidity associated with the infection.
Source:
O'Connor L, McGovern E, O'Meara M, et al. Epidemiol Infect. 2018;146(6):705-711.
CDC warns of hepatitis A outbreaks in injection drug users
From January 2017 to April 2018, more than 2,500 cases of hepatitis A infection associated with person-to-person transmission were reported to the CDC; of the 1,900 cases where risk factors are known, 68% were related to drug use, homelessness, or both. Various state responses caused a shortage in hepatitis A vaccine during this time, however, because of improvements in controlling outbreaks and an increased vaccine supply, the vaccine has become more available.
Usage of contaminated needles or other injection paraphernalia increase risk of hepatitis A infection, and transience, economic instability, limited health care access, distrust of public officials and public messages, and frequent lack of follow-up contact information in the population who regularly inject drugs make them difficult to reach with preventative services, such as vaccination, use of sterile injection equipment, and case management and contact tracing.
“These challenges make outbreaks among these groups difficult to control,” the CDC said in a statement.
The CDC recommends health departments ensure people who report drug use are vaccinated for hepatitis A, and consider programs to educate at-risk populations, as well as to provide vaccinations in places where at-risk populations may seek treatment. Health care providers should encourage patients who report drug use to be vaccinated for the disease.
For health care professionals, the CDC recommends considering a diagnosis of hepatitis A in any patient with jaundice and clinically compatible symptoms. The agency also recommends one dose of single-antigen hepatitis A vaccine or immune globulin within 2 weeks of exposure for unvaccinated patients who have been exposed to hepatitis A virus.
Find the full Health Advisory on the CDC website.
From January 2017 to April 2018, more than 2,500 cases of hepatitis A infection associated with person-to-person transmission were reported to the CDC; of the 1,900 cases where risk factors are known, 68% were related to drug use, homelessness, or both. Various state responses caused a shortage in hepatitis A vaccine during this time, however, because of improvements in controlling outbreaks and an increased vaccine supply, the vaccine has become more available.
Usage of contaminated needles or other injection paraphernalia increase risk of hepatitis A infection, and transience, economic instability, limited health care access, distrust of public officials and public messages, and frequent lack of follow-up contact information in the population who regularly inject drugs make them difficult to reach with preventative services, such as vaccination, use of sterile injection equipment, and case management and contact tracing.
“These challenges make outbreaks among these groups difficult to control,” the CDC said in a statement.
The CDC recommends health departments ensure people who report drug use are vaccinated for hepatitis A, and consider programs to educate at-risk populations, as well as to provide vaccinations in places where at-risk populations may seek treatment. Health care providers should encourage patients who report drug use to be vaccinated for the disease.
For health care professionals, the CDC recommends considering a diagnosis of hepatitis A in any patient with jaundice and clinically compatible symptoms. The agency also recommends one dose of single-antigen hepatitis A vaccine or immune globulin within 2 weeks of exposure for unvaccinated patients who have been exposed to hepatitis A virus.
Find the full Health Advisory on the CDC website.
From January 2017 to April 2018, more than 2,500 cases of hepatitis A infection associated with person-to-person transmission were reported to the CDC; of the 1,900 cases where risk factors are known, 68% were related to drug use, homelessness, or both. Various state responses caused a shortage in hepatitis A vaccine during this time, however, because of improvements in controlling outbreaks and an increased vaccine supply, the vaccine has become more available.
Usage of contaminated needles or other injection paraphernalia increase risk of hepatitis A infection, and transience, economic instability, limited health care access, distrust of public officials and public messages, and frequent lack of follow-up contact information in the population who regularly inject drugs make them difficult to reach with preventative services, such as vaccination, use of sterile injection equipment, and case management and contact tracing.
“These challenges make outbreaks among these groups difficult to control,” the CDC said in a statement.
The CDC recommends health departments ensure people who report drug use are vaccinated for hepatitis A, and consider programs to educate at-risk populations, as well as to provide vaccinations in places where at-risk populations may seek treatment. Health care providers should encourage patients who report drug use to be vaccinated for the disease.
For health care professionals, the CDC recommends considering a diagnosis of hepatitis A in any patient with jaundice and clinically compatible symptoms. The agency also recommends one dose of single-antigen hepatitis A vaccine or immune globulin within 2 weeks of exposure for unvaccinated patients who have been exposed to hepatitis A virus.
Find the full Health Advisory on the CDC website.
VIDEO: Hepatitis C eradication cuts nonliver cancer rate
WASHINGTON – Treatment of hepatitis C infection with a direct-acting antiviral drug strongly linked with a rapid, 14% drop in the incidence of all nonhepatic cancers, based on analysis of data from more than 30,000 U.S. patients.
The data also showed compared with infected patients who had been treated with an interferon-based regimen during the period immediately preceding the availability of DAAs in late 2013. This included a 45% cut in lung cancers, a 49% cut in bladder cancer, a 62% relative risk reduction in leukemia, and a 29% drop in prostate cancer, Michael B. Charlton, MD, said at the annual Digestive Disease Week.®
The relative reductions in nonhepatic cancer incidence appeared soon after DAA treatment. The data Dr. Charlton reported reflected a median follow-up of 1 year for DAA-treated patients and 2.6 years for the hepatitis C–infected patients who had received interferon and did not get a DAA. A major difference between these two regimens is their efficacy, with DAA regimens producing sustained virologic response rates of 90% or better, while the interferon regimens produced substantially lower eradication rates.
“The most obvious hypothesis” to explain the observed effects is that “hepatitis C is a potent carcinogen,” possibly acting by inhibiting immune surveillance for new cancers in infected people, Dr. Charlton said in a video interview.
The study he reported used insurance-claims data from more than 146 million U.S. residents during 2007-2017 in the IQVIA PharMetrics Plus database, which included more than 367,000 adults infected with hepatitis C. Dr. Charlton and his associates pulled from this claims data on 10,989 of the infected patients who received interferon during January 2007-May 2011 (and followed through November 2013), and 22,894 infected patients treated with any type of DAA during December 2013 through March 2017. They used these two discrete time windows to completely separate the patients who received a DAA from those who did not.
The primary analysis calculated a hazard ratio for the development of any nonhepatic cancer after adjustment for a number of demographic and clinical covariates including age, smoking history, and weight, and also applied propensity-score weighting to the data. The Kaplan-Meier analysis of the data showed clear separation of the cancer-free survival curves of the two subgroups by 6 months of follow-up, and then showed steady further separation over time suggesting an ongoing carcinogenic effect from continued hepatitis C infection in patients who had received the less effective antiviral regimen. The analysis was able to reveal this effect because it had data from many thousands of treated hepatitis C patients, far more than had been enrolled in the pivotal trials for the DAAs, noted Dr. Charlton, professor and director of the Center for Liver Diseases at the University of Chicago.
The Centers for Disease Control and Prevention estimates that 3.5 million Americans have a chronic hepatitis C infection. Dr. Charlton believed the number today might be more like 1-2 million remaining chronic U.S. cases because of the strong impact of DAA treatment. These chronic infections largely remain because hepatitis C is mostly silent and many clinicians fail to act on screening recommendations. The new findings provide even greater incentive for more rigorous screening and treatment, Dr. Charlton suggested.
“As if you needed another reason to get rid of hepatitis C, lowering your cancer risk is now added to the list,” he said.
WASHINGTON – Treatment of hepatitis C infection with a direct-acting antiviral drug strongly linked with a rapid, 14% drop in the incidence of all nonhepatic cancers, based on analysis of data from more than 30,000 U.S. patients.
The data also showed compared with infected patients who had been treated with an interferon-based regimen during the period immediately preceding the availability of DAAs in late 2013. This included a 45% cut in lung cancers, a 49% cut in bladder cancer, a 62% relative risk reduction in leukemia, and a 29% drop in prostate cancer, Michael B. Charlton, MD, said at the annual Digestive Disease Week.®
The relative reductions in nonhepatic cancer incidence appeared soon after DAA treatment. The data Dr. Charlton reported reflected a median follow-up of 1 year for DAA-treated patients and 2.6 years for the hepatitis C–infected patients who had received interferon and did not get a DAA. A major difference between these two regimens is their efficacy, with DAA regimens producing sustained virologic response rates of 90% or better, while the interferon regimens produced substantially lower eradication rates.
“The most obvious hypothesis” to explain the observed effects is that “hepatitis C is a potent carcinogen,” possibly acting by inhibiting immune surveillance for new cancers in infected people, Dr. Charlton said in a video interview.
The study he reported used insurance-claims data from more than 146 million U.S. residents during 2007-2017 in the IQVIA PharMetrics Plus database, which included more than 367,000 adults infected with hepatitis C. Dr. Charlton and his associates pulled from this claims data on 10,989 of the infected patients who received interferon during January 2007-May 2011 (and followed through November 2013), and 22,894 infected patients treated with any type of DAA during December 2013 through March 2017. They used these two discrete time windows to completely separate the patients who received a DAA from those who did not.
The primary analysis calculated a hazard ratio for the development of any nonhepatic cancer after adjustment for a number of demographic and clinical covariates including age, smoking history, and weight, and also applied propensity-score weighting to the data. The Kaplan-Meier analysis of the data showed clear separation of the cancer-free survival curves of the two subgroups by 6 months of follow-up, and then showed steady further separation over time suggesting an ongoing carcinogenic effect from continued hepatitis C infection in patients who had received the less effective antiviral regimen. The analysis was able to reveal this effect because it had data from many thousands of treated hepatitis C patients, far more than had been enrolled in the pivotal trials for the DAAs, noted Dr. Charlton, professor and director of the Center for Liver Diseases at the University of Chicago.
The Centers for Disease Control and Prevention estimates that 3.5 million Americans have a chronic hepatitis C infection. Dr. Charlton believed the number today might be more like 1-2 million remaining chronic U.S. cases because of the strong impact of DAA treatment. These chronic infections largely remain because hepatitis C is mostly silent and many clinicians fail to act on screening recommendations. The new findings provide even greater incentive for more rigorous screening and treatment, Dr. Charlton suggested.
“As if you needed another reason to get rid of hepatitis C, lowering your cancer risk is now added to the list,” he said.
WASHINGTON – Treatment of hepatitis C infection with a direct-acting antiviral drug strongly linked with a rapid, 14% drop in the incidence of all nonhepatic cancers, based on analysis of data from more than 30,000 U.S. patients.
The data also showed compared with infected patients who had been treated with an interferon-based regimen during the period immediately preceding the availability of DAAs in late 2013. This included a 45% cut in lung cancers, a 49% cut in bladder cancer, a 62% relative risk reduction in leukemia, and a 29% drop in prostate cancer, Michael B. Charlton, MD, said at the annual Digestive Disease Week.®
The relative reductions in nonhepatic cancer incidence appeared soon after DAA treatment. The data Dr. Charlton reported reflected a median follow-up of 1 year for DAA-treated patients and 2.6 years for the hepatitis C–infected patients who had received interferon and did not get a DAA. A major difference between these two regimens is their efficacy, with DAA regimens producing sustained virologic response rates of 90% or better, while the interferon regimens produced substantially lower eradication rates.
“The most obvious hypothesis” to explain the observed effects is that “hepatitis C is a potent carcinogen,” possibly acting by inhibiting immune surveillance for new cancers in infected people, Dr. Charlton said in a video interview.
The study he reported used insurance-claims data from more than 146 million U.S. residents during 2007-2017 in the IQVIA PharMetrics Plus database, which included more than 367,000 adults infected with hepatitis C. Dr. Charlton and his associates pulled from this claims data on 10,989 of the infected patients who received interferon during January 2007-May 2011 (and followed through November 2013), and 22,894 infected patients treated with any type of DAA during December 2013 through March 2017. They used these two discrete time windows to completely separate the patients who received a DAA from those who did not.
The primary analysis calculated a hazard ratio for the development of any nonhepatic cancer after adjustment for a number of demographic and clinical covariates including age, smoking history, and weight, and also applied propensity-score weighting to the data. The Kaplan-Meier analysis of the data showed clear separation of the cancer-free survival curves of the two subgroups by 6 months of follow-up, and then showed steady further separation over time suggesting an ongoing carcinogenic effect from continued hepatitis C infection in patients who had received the less effective antiviral regimen. The analysis was able to reveal this effect because it had data from many thousands of treated hepatitis C patients, far more than had been enrolled in the pivotal trials for the DAAs, noted Dr. Charlton, professor and director of the Center for Liver Diseases at the University of Chicago.
The Centers for Disease Control and Prevention estimates that 3.5 million Americans have a chronic hepatitis C infection. Dr. Charlton believed the number today might be more like 1-2 million remaining chronic U.S. cases because of the strong impact of DAA treatment. These chronic infections largely remain because hepatitis C is mostly silent and many clinicians fail to act on screening recommendations. The new findings provide even greater incentive for more rigorous screening and treatment, Dr. Charlton suggested.
“As if you needed another reason to get rid of hepatitis C, lowering your cancer risk is now added to the list,” he said.
REPORTING FROM DDW 2018
Key clinical point: Eradicating hepatitis C with direct-acting antivirals significantly cut the incidence of many nonliver cancers.
Major finding: Direct-acting antiviral treatment linked with a 14% drop in nonhepatic cancers, compared with patients not getting this treatment.
Study details: Analysis of 33,883 Americans treated for hepatitis C during 2007-2017 in an insurance claims database.
Disclosures: The study was funded by Gilead, a company that markets direct-acting antiviral drugs for hepatitis C virus. Dr. Charlton has been a consultant to and has received research funding from Gilead and several other companies that market drugs from this class.
Heart Transplantation Outcomes in Patients With Hepatitis C Virus Infection: Potential Impact of Newer Antiviral Treatments After Transplantation (FULL)
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
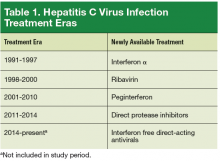
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
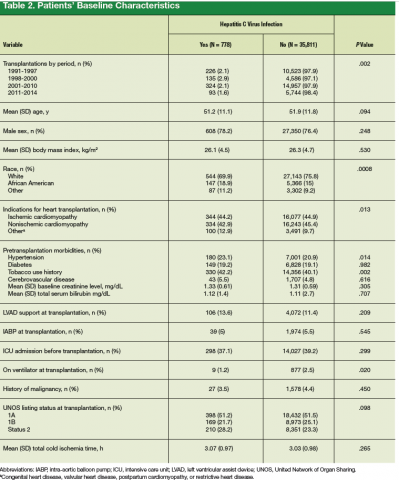
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
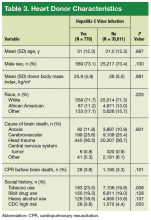
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
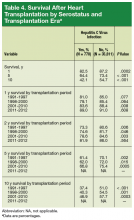
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
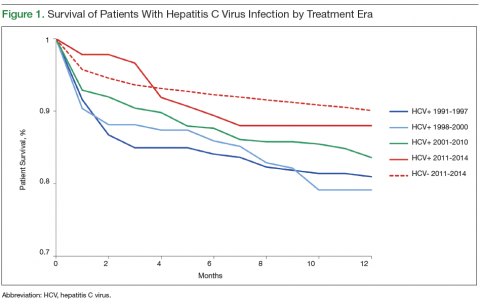
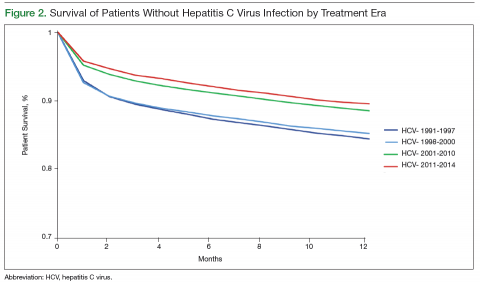
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
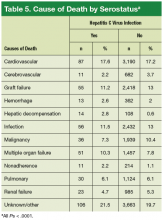
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.
Open Clinical Trials for Patients With HIV and/or Hepatitis B and C (FULL)
Web-Based Intervention to Reduce Alcohol Use in Veterans With Hepatitis C
The primary objective of this study is to implement and evaluate a web-based brief alcohol intervention (BAI) for treating veterans with hepatitis C virus (HCV) and seeking care at 2 VA HCV clinics–VAPAHCS and SFVAMC. This study will have 3 aims: First, the investigators plan to assess patient, provider, and system factors that may impact the initial adoption of this intervention in 2 VA HCV clinics. These data will result in the development of a protocol for the initial implementation of the web-based BAI at the investigators’ 2 study sites. A secondary aim will involve obtaining patient and provider feedback on an existing web-based BAI (see www.bmi-aft.org, VA intranet only) to help inform its redesign for use with this population. Second, the investigators will implement and examine the effectiveness of a web-based BAI in 2 HCV clinics to reduce alcohol consumption in veterans with HCV at 3- and 6-months post-treatment. Third, the investigators will conduct a budget impact analysis to estimate the short-term costs (1-3 years) of adoption and diffusion of the web-based BAI and the trajectory of health care spending for study participants.
ID: NCT01707030
Sponsor: VA Office of Research and Development
Location (contact): VA Palo Alto Health Care System, California (Keith Humphreys); San Francisco VAMC, California (Alex Monto)
Pilot Study of the Effect of Rifaximin On B-Cell Dysregulation in Cirrhosis
Hepatitis C is the leading cause of chronic liver disease and cirrhosis in U.S. veterans. Cirrhosis is associated with impaired antibody responses and increased risk of bacterial infections. We have recently identified that cirrhosis is associated with abnormalities of memory B-cells, cells that make antibodies and help protect against bacterial infections. We have identified that chemicals associated with gut bacteria might play a role in causing these B-cell abnormalities. It is well known that gut bacteria have increased access to the blood in individuals with cirrhosis, a process called bacterial translocation. We hypothesize that reducing bacteria counts in the gut by using poorly-absorbed antibiotics (also known as selective gut decontamination) will partially reverse losses of memory B-cells in cirrhosis by reducing bacterial translocation.
ID: NCT01951209
Sponsor: David E. Kaplan, MD MS
Location (contact): Corporal Michael J. Crescenz VAMC, Philadelphia, Pennsylvania (David Kaplan)
Long-Term Study of Liver Disease in People With Hepatitis B and/or Hepatitis C With or Without HIV Infection
The primary objective of the proposed study is to characterize viral liver disease and factors affecting the natural history of viral liver disease in persons with and without HIV with an emphasis on those living in the Washington DC metropolitan area. There are few longitudinal research cohorts of participants with viral hepatitis and HIV coinfection, especially at integrated medical care centers. The study, including a participant questionnaire for HCV infected participants only and phlebotomy, will be administered on-site at clinical facilities in the District of Columbia and at the National Institutes of Health. The cohort will be designed to study research questions with respect to liver disease, disease pathogenesis using genomics, proteomics, and immunologic disease models. Secondary objectives include study of the immunopathogenesis of hepatitis B virus (HBV) and HCV disease progression in HIV-infected subjects. In addition, this is an invaluable opportunity to determine the prevalence and risk factors associated with the development of hepatocellular carcinoma, the long-term effects of HCV clearance with direct-acting antivirals (DAAs), along with biomarker profile(s) for diagnosis and outcome. Moreover, this will serve as a catchment protocol to select appropriate participants for novel HBV and HCV therapeutic trials.
ID: NCT01350648
Sponsor: National Institutes of Health Clinical Center
Location: Washington DC VAMC; National Institutes of Health Clinical Center, Bethesda, Maryland
Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults (REPRIEVE)
Currently, there are few strategies to prevent cardiovascular disease (CVD) in HIV-infected people, even though they are at high risk for developing CVD. Statin medications are used to lower cholesterol and may be effective at reducing the risk of CVD in people infected with HIV. The purpose of this study is to evaluate the use of pitavastatin to reduce the risk of CVD in adults infected with HIV who are on antiretroviral therapy (ART). This study will enroll adults infected with HIV who are on any ART regimen (ART is not provided by the study) for at least 6 months before study entry considered low-to-moderate risk using the 2013 American College of Cardiology/American Heart Association guideline thresholds forecommended statin initiation. Total study duration will be approximately 72 months from the time the first participant is enrolled.
ID: NCT02344290
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Location (contact): VA West Los Angeles Medical Center CRS, California (Wendy Rossen); VA Connecticut Healthcare System CRS, West Haven (Laurie Andrews); Washington DC VAMC; Malcom Randall VA Medical Center CRS, Gainesville, Florida (Evan Waters); VA New York Harbor Healthcare System (Christine Reel-Brander); Dallas VAMC, Texas (Ashley Liggion-Turk); Michael E. DeBakey VAMC, Houston, Texas (Mahwish Mushtaq)
Development of a City-Wide Cohort of HIVInfected Persons in Care in the District of Columbia: The DC Cohort
All major community and academic clinics treating HIVinfected persons in the District of Columbia (DC) will initially be included in the development of a city-wide “DC Cohort” of HIV-infected persons in care, with consideration to be given subsequently to the inclusion of large private physician practices. Socio-demographics, risk factors, treatments, diagnoses, labs and procedures documented in outpatient medical record systems will be included in the DC Cohort database. Routine reports will be generated every 6 months for sites comparing their participants’ socio-demographics, clinical status, treatments, and outcomes to all other data in the DC Cohort database, and other comparisons specifically requested by sites. All sites will be provided analytic support in research areas of interest.
ID: NCT01206920
Sponsor: George Washington University
Location: Washington DC VAMC
Study of Immune Responses Induced by a HIV Vaccine
The primary purpose of this study is to define in HIV-uninfected volunteers the innate, cell-mediated and humoral responses induced by AIDSVAX B/E in the systemic and mucosal compartments and to characterize B cell functional specificities in peripheral blood, bone marrow and sigmoid compartments.
ID: NCT01933685
Sponsor: U.S. Army Medical Research and Materiel Command
Strength Training and Endurance Exercise for LIFE (STEEL)
The objective of this study is to determine the effect of exercise training on the central (cardiovascular) and peripheral (muscular) impairments underlying poor physical function by comparing older HIV-infected veterans randomized to combine aerobic and resistance exercise training versus usual care. The study hypothesis is that a progressive aerobic and resistance rehabilitation program will increase aerobic capacity and muscle strength, which will be mediated by improved diastolic function, increased muscle mass, and decreased systemic inflammation. To test this hypothesis, investigators will conduct a randomized 16-week trial of progressive aerobic and resistance training versus usual care control in 40 sedentary older (50+ years) HIVinfected veterans. The study will determine the effects of exercise training on aerobic capacity and diastolic function, and their relationship to changes in biomarkers of systemic inflammation and cardiac fibrosis (AIM 1). The study also will determine the effect of exercise training on strength and muscle mass, and their relationship to changes in biomarkers of systemic inflammation (AIM 2).
ID: NCT02101060
Sponsor: VA Office of Research and Development
Location (contact): Salem VA Medical Center, Virginia (Carolyn Jones, Tracy A Hicks)
Click here to read the digital edition.
Web-Based Intervention to Reduce Alcohol Use in Veterans With Hepatitis C
The primary objective of this study is to implement and evaluate a web-based brief alcohol intervention (BAI) for treating veterans with hepatitis C virus (HCV) and seeking care at 2 VA HCV clinics–VAPAHCS and SFVAMC. This study will have 3 aims: First, the investigators plan to assess patient, provider, and system factors that may impact the initial adoption of this intervention in 2 VA HCV clinics. These data will result in the development of a protocol for the initial implementation of the web-based BAI at the investigators’ 2 study sites. A secondary aim will involve obtaining patient and provider feedback on an existing web-based BAI (see www.bmi-aft.org, VA intranet only) to help inform its redesign for use with this population. Second, the investigators will implement and examine the effectiveness of a web-based BAI in 2 HCV clinics to reduce alcohol consumption in veterans with HCV at 3- and 6-months post-treatment. Third, the investigators will conduct a budget impact analysis to estimate the short-term costs (1-3 years) of adoption and diffusion of the web-based BAI and the trajectory of health care spending for study participants.
ID: NCT01707030
Sponsor: VA Office of Research and Development
Location (contact): VA Palo Alto Health Care System, California (Keith Humphreys); San Francisco VAMC, California (Alex Monto)
Pilot Study of the Effect of Rifaximin On B-Cell Dysregulation in Cirrhosis
Hepatitis C is the leading cause of chronic liver disease and cirrhosis in U.S. veterans. Cirrhosis is associated with impaired antibody responses and increased risk of bacterial infections. We have recently identified that cirrhosis is associated with abnormalities of memory B-cells, cells that make antibodies and help protect against bacterial infections. We have identified that chemicals associated with gut bacteria might play a role in causing these B-cell abnormalities. It is well known that gut bacteria have increased access to the blood in individuals with cirrhosis, a process called bacterial translocation. We hypothesize that reducing bacteria counts in the gut by using poorly-absorbed antibiotics (also known as selective gut decontamination) will partially reverse losses of memory B-cells in cirrhosis by reducing bacterial translocation.
ID: NCT01951209
Sponsor: David E. Kaplan, MD MS
Location (contact): Corporal Michael J. Crescenz VAMC, Philadelphia, Pennsylvania (David Kaplan)
Long-Term Study of Liver Disease in People With Hepatitis B and/or Hepatitis C With or Without HIV Infection
The primary objective of the proposed study is to characterize viral liver disease and factors affecting the natural history of viral liver disease in persons with and without HIV with an emphasis on those living in the Washington DC metropolitan area. There are few longitudinal research cohorts of participants with viral hepatitis and HIV coinfection, especially at integrated medical care centers. The study, including a participant questionnaire for HCV infected participants only and phlebotomy, will be administered on-site at clinical facilities in the District of Columbia and at the National Institutes of Health. The cohort will be designed to study research questions with respect to liver disease, disease pathogenesis using genomics, proteomics, and immunologic disease models. Secondary objectives include study of the immunopathogenesis of hepatitis B virus (HBV) and HCV disease progression in HIV-infected subjects. In addition, this is an invaluable opportunity to determine the prevalence and risk factors associated with the development of hepatocellular carcinoma, the long-term effects of HCV clearance with direct-acting antivirals (DAAs), along with biomarker profile(s) for diagnosis and outcome. Moreover, this will serve as a catchment protocol to select appropriate participants for novel HBV and HCV therapeutic trials.
ID: NCT01350648
Sponsor: National Institutes of Health Clinical Center
Location: Washington DC VAMC; National Institutes of Health Clinical Center, Bethesda, Maryland
Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults (REPRIEVE)
Currently, there are few strategies to prevent cardiovascular disease (CVD) in HIV-infected people, even though they are at high risk for developing CVD. Statin medications are used to lower cholesterol and may be effective at reducing the risk of CVD in people infected with HIV. The purpose of this study is to evaluate the use of pitavastatin to reduce the risk of CVD in adults infected with HIV who are on antiretroviral therapy (ART). This study will enroll adults infected with HIV who are on any ART regimen (ART is not provided by the study) for at least 6 months before study entry considered low-to-moderate risk using the 2013 American College of Cardiology/American Heart Association guideline thresholds forecommended statin initiation. Total study duration will be approximately 72 months from the time the first participant is enrolled.
ID: NCT02344290
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Location (contact): VA West Los Angeles Medical Center CRS, California (Wendy Rossen); VA Connecticut Healthcare System CRS, West Haven (Laurie Andrews); Washington DC VAMC; Malcom Randall VA Medical Center CRS, Gainesville, Florida (Evan Waters); VA New York Harbor Healthcare System (Christine Reel-Brander); Dallas VAMC, Texas (Ashley Liggion-Turk); Michael E. DeBakey VAMC, Houston, Texas (Mahwish Mushtaq)
Development of a City-Wide Cohort of HIVInfected Persons in Care in the District of Columbia: The DC Cohort
All major community and academic clinics treating HIVinfected persons in the District of Columbia (DC) will initially be included in the development of a city-wide “DC Cohort” of HIV-infected persons in care, with consideration to be given subsequently to the inclusion of large private physician practices. Socio-demographics, risk factors, treatments, diagnoses, labs and procedures documented in outpatient medical record systems will be included in the DC Cohort database. Routine reports will be generated every 6 months for sites comparing their participants’ socio-demographics, clinical status, treatments, and outcomes to all other data in the DC Cohort database, and other comparisons specifically requested by sites. All sites will be provided analytic support in research areas of interest.
ID: NCT01206920
Sponsor: George Washington University
Location: Washington DC VAMC
Study of Immune Responses Induced by a HIV Vaccine
The primary purpose of this study is to define in HIV-uninfected volunteers the innate, cell-mediated and humoral responses induced by AIDSVAX B/E in the systemic and mucosal compartments and to characterize B cell functional specificities in peripheral blood, bone marrow and sigmoid compartments.
ID: NCT01933685
Sponsor: U.S. Army Medical Research and Materiel Command
Strength Training and Endurance Exercise for LIFE (STEEL)
The objective of this study is to determine the effect of exercise training on the central (cardiovascular) and peripheral (muscular) impairments underlying poor physical function by comparing older HIV-infected veterans randomized to combine aerobic and resistance exercise training versus usual care. The study hypothesis is that a progressive aerobic and resistance rehabilitation program will increase aerobic capacity and muscle strength, which will be mediated by improved diastolic function, increased muscle mass, and decreased systemic inflammation. To test this hypothesis, investigators will conduct a randomized 16-week trial of progressive aerobic and resistance training versus usual care control in 40 sedentary older (50+ years) HIVinfected veterans. The study will determine the effects of exercise training on aerobic capacity and diastolic function, and their relationship to changes in biomarkers of systemic inflammation and cardiac fibrosis (AIM 1). The study also will determine the effect of exercise training on strength and muscle mass, and their relationship to changes in biomarkers of systemic inflammation (AIM 2).
ID: NCT02101060
Sponsor: VA Office of Research and Development
Location (contact): Salem VA Medical Center, Virginia (Carolyn Jones, Tracy A Hicks)
Click here to read the digital edition.
Web-Based Intervention to Reduce Alcohol Use in Veterans With Hepatitis C
The primary objective of this study is to implement and evaluate a web-based brief alcohol intervention (BAI) for treating veterans with hepatitis C virus (HCV) and seeking care at 2 VA HCV clinics–VAPAHCS and SFVAMC. This study will have 3 aims: First, the investigators plan to assess patient, provider, and system factors that may impact the initial adoption of this intervention in 2 VA HCV clinics. These data will result in the development of a protocol for the initial implementation of the web-based BAI at the investigators’ 2 study sites. A secondary aim will involve obtaining patient and provider feedback on an existing web-based BAI (see www.bmi-aft.org, VA intranet only) to help inform its redesign for use with this population. Second, the investigators will implement and examine the effectiveness of a web-based BAI in 2 HCV clinics to reduce alcohol consumption in veterans with HCV at 3- and 6-months post-treatment. Third, the investigators will conduct a budget impact analysis to estimate the short-term costs (1-3 years) of adoption and diffusion of the web-based BAI and the trajectory of health care spending for study participants.
ID: NCT01707030
Sponsor: VA Office of Research and Development
Location (contact): VA Palo Alto Health Care System, California (Keith Humphreys); San Francisco VAMC, California (Alex Monto)
Pilot Study of the Effect of Rifaximin On B-Cell Dysregulation in Cirrhosis
Hepatitis C is the leading cause of chronic liver disease and cirrhosis in U.S. veterans. Cirrhosis is associated with impaired antibody responses and increased risk of bacterial infections. We have recently identified that cirrhosis is associated with abnormalities of memory B-cells, cells that make antibodies and help protect against bacterial infections. We have identified that chemicals associated with gut bacteria might play a role in causing these B-cell abnormalities. It is well known that gut bacteria have increased access to the blood in individuals with cirrhosis, a process called bacterial translocation. We hypothesize that reducing bacteria counts in the gut by using poorly-absorbed antibiotics (also known as selective gut decontamination) will partially reverse losses of memory B-cells in cirrhosis by reducing bacterial translocation.
ID: NCT01951209
Sponsor: David E. Kaplan, MD MS
Location (contact): Corporal Michael J. Crescenz VAMC, Philadelphia, Pennsylvania (David Kaplan)
Long-Term Study of Liver Disease in People With Hepatitis B and/or Hepatitis C With or Without HIV Infection
The primary objective of the proposed study is to characterize viral liver disease and factors affecting the natural history of viral liver disease in persons with and without HIV with an emphasis on those living in the Washington DC metropolitan area. There are few longitudinal research cohorts of participants with viral hepatitis and HIV coinfection, especially at integrated medical care centers. The study, including a participant questionnaire for HCV infected participants only and phlebotomy, will be administered on-site at clinical facilities in the District of Columbia and at the National Institutes of Health. The cohort will be designed to study research questions with respect to liver disease, disease pathogenesis using genomics, proteomics, and immunologic disease models. Secondary objectives include study of the immunopathogenesis of hepatitis B virus (HBV) and HCV disease progression in HIV-infected subjects. In addition, this is an invaluable opportunity to determine the prevalence and risk factors associated with the development of hepatocellular carcinoma, the long-term effects of HCV clearance with direct-acting antivirals (DAAs), along with biomarker profile(s) for diagnosis and outcome. Moreover, this will serve as a catchment protocol to select appropriate participants for novel HBV and HCV therapeutic trials.
ID: NCT01350648
Sponsor: National Institutes of Health Clinical Center
Location: Washington DC VAMC; National Institutes of Health Clinical Center, Bethesda, Maryland
Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults (REPRIEVE)
Currently, there are few strategies to prevent cardiovascular disease (CVD) in HIV-infected people, even though they are at high risk for developing CVD. Statin medications are used to lower cholesterol and may be effective at reducing the risk of CVD in people infected with HIV. The purpose of this study is to evaluate the use of pitavastatin to reduce the risk of CVD in adults infected with HIV who are on antiretroviral therapy (ART). This study will enroll adults infected with HIV who are on any ART regimen (ART is not provided by the study) for at least 6 months before study entry considered low-to-moderate risk using the 2013 American College of Cardiology/American Heart Association guideline thresholds forecommended statin initiation. Total study duration will be approximately 72 months from the time the first participant is enrolled.
ID: NCT02344290
Sponsor: National Institute of Allergy and Infectious Diseases (NIAID)
Location (contact): VA West Los Angeles Medical Center CRS, California (Wendy Rossen); VA Connecticut Healthcare System CRS, West Haven (Laurie Andrews); Washington DC VAMC; Malcom Randall VA Medical Center CRS, Gainesville, Florida (Evan Waters); VA New York Harbor Healthcare System (Christine Reel-Brander); Dallas VAMC, Texas (Ashley Liggion-Turk); Michael E. DeBakey VAMC, Houston, Texas (Mahwish Mushtaq)
Development of a City-Wide Cohort of HIVInfected Persons in Care in the District of Columbia: The DC Cohort
All major community and academic clinics treating HIVinfected persons in the District of Columbia (DC) will initially be included in the development of a city-wide “DC Cohort” of HIV-infected persons in care, with consideration to be given subsequently to the inclusion of large private physician practices. Socio-demographics, risk factors, treatments, diagnoses, labs and procedures documented in outpatient medical record systems will be included in the DC Cohort database. Routine reports will be generated every 6 months for sites comparing their participants’ socio-demographics, clinical status, treatments, and outcomes to all other data in the DC Cohort database, and other comparisons specifically requested by sites. All sites will be provided analytic support in research areas of interest.
ID: NCT01206920
Sponsor: George Washington University
Location: Washington DC VAMC
Study of Immune Responses Induced by a HIV Vaccine
The primary purpose of this study is to define in HIV-uninfected volunteers the innate, cell-mediated and humoral responses induced by AIDSVAX B/E in the systemic and mucosal compartments and to characterize B cell functional specificities in peripheral blood, bone marrow and sigmoid compartments.
ID: NCT01933685
Sponsor: U.S. Army Medical Research and Materiel Command
Strength Training and Endurance Exercise for LIFE (STEEL)
The objective of this study is to determine the effect of exercise training on the central (cardiovascular) and peripheral (muscular) impairments underlying poor physical function by comparing older HIV-infected veterans randomized to combine aerobic and resistance exercise training versus usual care. The study hypothesis is that a progressive aerobic and resistance rehabilitation program will increase aerobic capacity and muscle strength, which will be mediated by improved diastolic function, increased muscle mass, and decreased systemic inflammation. To test this hypothesis, investigators will conduct a randomized 16-week trial of progressive aerobic and resistance training versus usual care control in 40 sedentary older (50+ years) HIVinfected veterans. The study will determine the effects of exercise training on aerobic capacity and diastolic function, and their relationship to changes in biomarkers of systemic inflammation and cardiac fibrosis (AIM 1). The study also will determine the effect of exercise training on strength and muscle mass, and their relationship to changes in biomarkers of systemic inflammation (AIM 2).
ID: NCT02101060
Sponsor: VA Office of Research and Development
Location (contact): Salem VA Medical Center, Virginia (Carolyn Jones, Tracy A Hicks)
Click here to read the digital edition.
Scoring system quantified chances of HCV treatment benefit
A new scoring system predicted which patients with decompensated cirrhosis caused by hepatitis C virus (HCV) infection were most likely to experience meaningful benefits from direct-acting antiviral (DAA) therapy.
Dubbed BEA3, their scoring system assigns one point each for body mass index under 25 kg/m2, absence of encephalopathy, absence of ascites, ALT more than 1.5 times the upper limit of normal, and albumin above 3.5 g/dL. Patients who scored 4 or 5 were more than 50 times more likely to improve to Child-Pugh Turcotte (CPT) class A (compensated) cirrhosis with DAA therapy than were patients who scored 0 (hazard ratio, 52.3; 95% confidence interval, 15.2-179.7; P less than .001), wrote Omar El-Sherif, MB, BCh, of St. James’s Hospital, Dublin, together with his associates in the June issue of Gastroenterology.
Eradicating HCV does not necessarily improve the odds of transplant-free survival in the setting of decompensated cirrhosis, the researchers noted. Patients can end up in “MELD [Model for End-Stage Liver Disease] purgatory,” meaning they are still decompensated despite achieving sustained virologic response and improved MELD scores. Such patients can face longer waits for liver transplantation than if they had foregone DAA therapy. “There is an urgent need for data to refine our understanding of the reversibility of hepatic decompensation with viral eradication, and, ultimately, define the “point of no return,” the degree of liver dysfunction at which HCV therapy does not yield any meaningful clinical benefit, the researchers wrote.
Their study included 622 patients from the SOLAR-1, SOLAR-2, ASTRAL-4, and GS-US-334-0125 trials, which evaluated interferon-free sofosbuvir-based DAA therapy in patients with chronic hepatitis C virus infection and advanced liver disease. Patients received 12 or 24 weeks of therapy with ledipasvir, sofosbuvir, and ribavirin or velpatasvir, sofosbuvir, and/or ribavirin, or 48 weeks of treatment with sofosbuvir and ribavirin.
A total of 32% of patients with CPT class B cirrhosis improved to class A, as did 12% of patients with class C cirrhosis. Each factor in the scoring system independently affected the chances of reaching CPT class A cirrhosis, even after accounting for SVR.
Notably, patients with intermediate BEA3 scores of 1, 2, or 3 were significantly more likely to reach CPT class A cirrhosis than were patients with scores of 0, with hazard ratios ranging from 4.2 (for a score of 1) to 21.2 (for a score of 3). Most patients had scores of 0 (106 individuals), 1 (219 individuals), or 2 (180 individuals), and only 23 patients scored a 4 or a 5.
CPT score reflects prothrombin time, serum albumin and bilirubin, and the presence or severity of ascites. The investigators called the new scoring system “a tool that can enhance shared decision making at the point of care, quantifying the potential benefits of DAA therapy for patients with decompensated cirrhosis in the pretransplant setting.”Dr. El-Sherif disclosed ties to Gilead Sciences, Bristol-Myers Squibb, and the Health Research Board of Ireland. Four coinvestigators disclosed employment with Gilead, and several other coinvestigators disclosed ties to Gilead, BMS, AbbVie, and other companies.
SOURCE: El-Sherif O et al. Gastroenterology. 2018 Mar 10. doi: 10.1053/j.gastro.2018.03.022.
Patients with decompensated cirrhosis are now able to receive antiviral therapy without risk of worsening symptoms of decompensation. More clinics are able to offer DAA therapy to patients with hepatitis C, without the need for expertise in managing the side effects of interferon-based therapy.
The study by El-Sherif et al. summarizes well the benefits and potential pitfalls of treatment of hepatitis C in patients with decompensated cirrhosis. Their scoring system is largely intuitive and mirrors the traditional Child-Turcotte-Pugh score in that patients with low serum albumin, hepatic encephalopathy, and ascites are at risk of failing to improve clinically. Patients can have their hepatitis C successfully treated but can be trapped in “MELD purgatory,” a state of significant symptoms of liver disease, without the objective priority points necessary to be candidates for liver transplantation.
As experience is gained in the use of DAA medications for HCV, it is incumbent on physicians to gather knowledge that will further refine their understanding of which patients with signs of liver decompensation might benefit. It is also clear that patients with decompensated cirrhosis should be managed by clinicians who have experience in liver transplantation, to ensure that patients are counseled regarding not just the benefits, but potential risks of DAA therapy for hepatitis C.
Roman E. Perri, MD, is assistant professor of medicine, division of gastroenterology and hepatology, Medical Director for Liver Transplantion, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
Patients with decompensated cirrhosis are now able to receive antiviral therapy without risk of worsening symptoms of decompensation. More clinics are able to offer DAA therapy to patients with hepatitis C, without the need for expertise in managing the side effects of interferon-based therapy.
The study by El-Sherif et al. summarizes well the benefits and potential pitfalls of treatment of hepatitis C in patients with decompensated cirrhosis. Their scoring system is largely intuitive and mirrors the traditional Child-Turcotte-Pugh score in that patients with low serum albumin, hepatic encephalopathy, and ascites are at risk of failing to improve clinically. Patients can have their hepatitis C successfully treated but can be trapped in “MELD purgatory,” a state of significant symptoms of liver disease, without the objective priority points necessary to be candidates for liver transplantation.
As experience is gained in the use of DAA medications for HCV, it is incumbent on physicians to gather knowledge that will further refine their understanding of which patients with signs of liver decompensation might benefit. It is also clear that patients with decompensated cirrhosis should be managed by clinicians who have experience in liver transplantation, to ensure that patients are counseled regarding not just the benefits, but potential risks of DAA therapy for hepatitis C.
Roman E. Perri, MD, is assistant professor of medicine, division of gastroenterology and hepatology, Medical Director for Liver Transplantion, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
Patients with decompensated cirrhosis are now able to receive antiviral therapy without risk of worsening symptoms of decompensation. More clinics are able to offer DAA therapy to patients with hepatitis C, without the need for expertise in managing the side effects of interferon-based therapy.
The study by El-Sherif et al. summarizes well the benefits and potential pitfalls of treatment of hepatitis C in patients with decompensated cirrhosis. Their scoring system is largely intuitive and mirrors the traditional Child-Turcotte-Pugh score in that patients with low serum albumin, hepatic encephalopathy, and ascites are at risk of failing to improve clinically. Patients can have their hepatitis C successfully treated but can be trapped in “MELD purgatory,” a state of significant symptoms of liver disease, without the objective priority points necessary to be candidates for liver transplantation.
As experience is gained in the use of DAA medications for HCV, it is incumbent on physicians to gather knowledge that will further refine their understanding of which patients with signs of liver decompensation might benefit. It is also clear that patients with decompensated cirrhosis should be managed by clinicians who have experience in liver transplantation, to ensure that patients are counseled regarding not just the benefits, but potential risks of DAA therapy for hepatitis C.
Roman E. Perri, MD, is assistant professor of medicine, division of gastroenterology and hepatology, Medical Director for Liver Transplantion, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
A new scoring system predicted which patients with decompensated cirrhosis caused by hepatitis C virus (HCV) infection were most likely to experience meaningful benefits from direct-acting antiviral (DAA) therapy.
Dubbed BEA3, their scoring system assigns one point each for body mass index under 25 kg/m2, absence of encephalopathy, absence of ascites, ALT more than 1.5 times the upper limit of normal, and albumin above 3.5 g/dL. Patients who scored 4 or 5 were more than 50 times more likely to improve to Child-Pugh Turcotte (CPT) class A (compensated) cirrhosis with DAA therapy than were patients who scored 0 (hazard ratio, 52.3; 95% confidence interval, 15.2-179.7; P less than .001), wrote Omar El-Sherif, MB, BCh, of St. James’s Hospital, Dublin, together with his associates in the June issue of Gastroenterology.
Eradicating HCV does not necessarily improve the odds of transplant-free survival in the setting of decompensated cirrhosis, the researchers noted. Patients can end up in “MELD [Model for End-Stage Liver Disease] purgatory,” meaning they are still decompensated despite achieving sustained virologic response and improved MELD scores. Such patients can face longer waits for liver transplantation than if they had foregone DAA therapy. “There is an urgent need for data to refine our understanding of the reversibility of hepatic decompensation with viral eradication, and, ultimately, define the “point of no return,” the degree of liver dysfunction at which HCV therapy does not yield any meaningful clinical benefit, the researchers wrote.
Their study included 622 patients from the SOLAR-1, SOLAR-2, ASTRAL-4, and GS-US-334-0125 trials, which evaluated interferon-free sofosbuvir-based DAA therapy in patients with chronic hepatitis C virus infection and advanced liver disease. Patients received 12 or 24 weeks of therapy with ledipasvir, sofosbuvir, and ribavirin or velpatasvir, sofosbuvir, and/or ribavirin, or 48 weeks of treatment with sofosbuvir and ribavirin.
A total of 32% of patients with CPT class B cirrhosis improved to class A, as did 12% of patients with class C cirrhosis. Each factor in the scoring system independently affected the chances of reaching CPT class A cirrhosis, even after accounting for SVR.
Notably, patients with intermediate BEA3 scores of 1, 2, or 3 were significantly more likely to reach CPT class A cirrhosis than were patients with scores of 0, with hazard ratios ranging from 4.2 (for a score of 1) to 21.2 (for a score of 3). Most patients had scores of 0 (106 individuals), 1 (219 individuals), or 2 (180 individuals), and only 23 patients scored a 4 or a 5.
CPT score reflects prothrombin time, serum albumin and bilirubin, and the presence or severity of ascites. The investigators called the new scoring system “a tool that can enhance shared decision making at the point of care, quantifying the potential benefits of DAA therapy for patients with decompensated cirrhosis in the pretransplant setting.”Dr. El-Sherif disclosed ties to Gilead Sciences, Bristol-Myers Squibb, and the Health Research Board of Ireland. Four coinvestigators disclosed employment with Gilead, and several other coinvestigators disclosed ties to Gilead, BMS, AbbVie, and other companies.
SOURCE: El-Sherif O et al. Gastroenterology. 2018 Mar 10. doi: 10.1053/j.gastro.2018.03.022.
A new scoring system predicted which patients with decompensated cirrhosis caused by hepatitis C virus (HCV) infection were most likely to experience meaningful benefits from direct-acting antiviral (DAA) therapy.
Dubbed BEA3, their scoring system assigns one point each for body mass index under 25 kg/m2, absence of encephalopathy, absence of ascites, ALT more than 1.5 times the upper limit of normal, and albumin above 3.5 g/dL. Patients who scored 4 or 5 were more than 50 times more likely to improve to Child-Pugh Turcotte (CPT) class A (compensated) cirrhosis with DAA therapy than were patients who scored 0 (hazard ratio, 52.3; 95% confidence interval, 15.2-179.7; P less than .001), wrote Omar El-Sherif, MB, BCh, of St. James’s Hospital, Dublin, together with his associates in the June issue of Gastroenterology.
Eradicating HCV does not necessarily improve the odds of transplant-free survival in the setting of decompensated cirrhosis, the researchers noted. Patients can end up in “MELD [Model for End-Stage Liver Disease] purgatory,” meaning they are still decompensated despite achieving sustained virologic response and improved MELD scores. Such patients can face longer waits for liver transplantation than if they had foregone DAA therapy. “There is an urgent need for data to refine our understanding of the reversibility of hepatic decompensation with viral eradication, and, ultimately, define the “point of no return,” the degree of liver dysfunction at which HCV therapy does not yield any meaningful clinical benefit, the researchers wrote.
Their study included 622 patients from the SOLAR-1, SOLAR-2, ASTRAL-4, and GS-US-334-0125 trials, which evaluated interferon-free sofosbuvir-based DAA therapy in patients with chronic hepatitis C virus infection and advanced liver disease. Patients received 12 or 24 weeks of therapy with ledipasvir, sofosbuvir, and ribavirin or velpatasvir, sofosbuvir, and/or ribavirin, or 48 weeks of treatment with sofosbuvir and ribavirin.
A total of 32% of patients with CPT class B cirrhosis improved to class A, as did 12% of patients with class C cirrhosis. Each factor in the scoring system independently affected the chances of reaching CPT class A cirrhosis, even after accounting for SVR.
Notably, patients with intermediate BEA3 scores of 1, 2, or 3 were significantly more likely to reach CPT class A cirrhosis than were patients with scores of 0, with hazard ratios ranging from 4.2 (for a score of 1) to 21.2 (for a score of 3). Most patients had scores of 0 (106 individuals), 1 (219 individuals), or 2 (180 individuals), and only 23 patients scored a 4 or a 5.
CPT score reflects prothrombin time, serum albumin and bilirubin, and the presence or severity of ascites. The investigators called the new scoring system “a tool that can enhance shared decision making at the point of care, quantifying the potential benefits of DAA therapy for patients with decompensated cirrhosis in the pretransplant setting.”Dr. El-Sherif disclosed ties to Gilead Sciences, Bristol-Myers Squibb, and the Health Research Board of Ireland. Four coinvestigators disclosed employment with Gilead, and several other coinvestigators disclosed ties to Gilead, BMS, AbbVie, and other companies.
SOURCE: El-Sherif O et al. Gastroenterology. 2018 Mar 10. doi: 10.1053/j.gastro.2018.03.022.
FROM GASTROENTEROLOGY
Alcohol abuse untreated in HCV patients, including HIV coinfected
Nearly 4% of Veterans Affairs patients who screened positive for unhealthy alcohol use were infected with hepatitis C virus, and 64% of these patients were diagnosed with alcohol use disorder, according to the results of a large database analysis.
Despite the fact that alcohol use at all levels can compound the adverse effects of HCV and lead to heightened risks of mortality, particularly among those coinfected with HIV, the majority of these patients did not receive specialty addiction treatment, according to Mandy D. Owens, PhD, and her colleagues at the VA Puget Sound Health Care System, Seattle.
In their study, published in Drug and Alcohol Dependence, the researchers queried the national VA health care system database, which is made up of 139 large facilities and more than 900 clinics throughout the United States, for all patients with a documented outpatient appointment between October 2009 and May 2013 to identify those with one or more with positive screens on the AUDIT-C (Alcohol Use Disorders Identification Test-Consumption) questionnaire. Those with AUDIT-C scores greater than or equal to 5 were considered positive, and each positive screen was tracked for up to 1 year to assess alcohol-related care outcomes. The four alcohol-related care outcomes measured were: receipt of brief intervention, specialty addiction treatment, alcohol use disorder (AUD) pharmacotherapy, and a composite measure of receiving any of these services.
Patients also were compared across HCV status in the entire sample of patients with positive screening as well as in the subsample with a clinically documented AUD.
During the study period, 830,825 VA patients screened positive for unhealthy alcohol use. Among those, 31,841 (3.8%) patients had a documented diagnosis for HCV, and of these 20,320 (64%) had an AUD. Two-thirds of these AUD patients did not receive specialty addiction treatment, and more than 90% did not receive pharmacotherapy that is approved by the Food and Drug Administration to treat AUD, according to the researchers. “These rates are concerning given the negative impact alcohol use can have on HCV,” they wrote.
They reiterated the importance of the 2016 change in policy adopted by the VA Health System, which updated its treatment guidelines to recommend that all patients with HCV be considered for treatment, regardless of substance use, and explicitly stated that alcohol use and length of abstinence should not be disqualifiers for receiving HCV treatment.
“All patients with HCV should be receiving evidence-based alcohol-related care given the risks of alcohol use in this population, particularly among those coinfected with HIV,” the researchers concluded.
The research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism. The authors reported that they had no conflicts of interest.
SOURCE: Owens MD et al. Drug Alcohol Depend. 2018;188:79-85.
Nearly 4% of Veterans Affairs patients who screened positive for unhealthy alcohol use were infected with hepatitis C virus, and 64% of these patients were diagnosed with alcohol use disorder, according to the results of a large database analysis.
Despite the fact that alcohol use at all levels can compound the adverse effects of HCV and lead to heightened risks of mortality, particularly among those coinfected with HIV, the majority of these patients did not receive specialty addiction treatment, according to Mandy D. Owens, PhD, and her colleagues at the VA Puget Sound Health Care System, Seattle.
In their study, published in Drug and Alcohol Dependence, the researchers queried the national VA health care system database, which is made up of 139 large facilities and more than 900 clinics throughout the United States, for all patients with a documented outpatient appointment between October 2009 and May 2013 to identify those with one or more with positive screens on the AUDIT-C (Alcohol Use Disorders Identification Test-Consumption) questionnaire. Those with AUDIT-C scores greater than or equal to 5 were considered positive, and each positive screen was tracked for up to 1 year to assess alcohol-related care outcomes. The four alcohol-related care outcomes measured were: receipt of brief intervention, specialty addiction treatment, alcohol use disorder (AUD) pharmacotherapy, and a composite measure of receiving any of these services.
Patients also were compared across HCV status in the entire sample of patients with positive screening as well as in the subsample with a clinically documented AUD.
During the study period, 830,825 VA patients screened positive for unhealthy alcohol use. Among those, 31,841 (3.8%) patients had a documented diagnosis for HCV, and of these 20,320 (64%) had an AUD. Two-thirds of these AUD patients did not receive specialty addiction treatment, and more than 90% did not receive pharmacotherapy that is approved by the Food and Drug Administration to treat AUD, according to the researchers. “These rates are concerning given the negative impact alcohol use can have on HCV,” they wrote.
They reiterated the importance of the 2016 change in policy adopted by the VA Health System, which updated its treatment guidelines to recommend that all patients with HCV be considered for treatment, regardless of substance use, and explicitly stated that alcohol use and length of abstinence should not be disqualifiers for receiving HCV treatment.
“All patients with HCV should be receiving evidence-based alcohol-related care given the risks of alcohol use in this population, particularly among those coinfected with HIV,” the researchers concluded.
The research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism. The authors reported that they had no conflicts of interest.
SOURCE: Owens MD et al. Drug Alcohol Depend. 2018;188:79-85.
Nearly 4% of Veterans Affairs patients who screened positive for unhealthy alcohol use were infected with hepatitis C virus, and 64% of these patients were diagnosed with alcohol use disorder, according to the results of a large database analysis.
Despite the fact that alcohol use at all levels can compound the adverse effects of HCV and lead to heightened risks of mortality, particularly among those coinfected with HIV, the majority of these patients did not receive specialty addiction treatment, according to Mandy D. Owens, PhD, and her colleagues at the VA Puget Sound Health Care System, Seattle.
In their study, published in Drug and Alcohol Dependence, the researchers queried the national VA health care system database, which is made up of 139 large facilities and more than 900 clinics throughout the United States, for all patients with a documented outpatient appointment between October 2009 and May 2013 to identify those with one or more with positive screens on the AUDIT-C (Alcohol Use Disorders Identification Test-Consumption) questionnaire. Those with AUDIT-C scores greater than or equal to 5 were considered positive, and each positive screen was tracked for up to 1 year to assess alcohol-related care outcomes. The four alcohol-related care outcomes measured were: receipt of brief intervention, specialty addiction treatment, alcohol use disorder (AUD) pharmacotherapy, and a composite measure of receiving any of these services.
Patients also were compared across HCV status in the entire sample of patients with positive screening as well as in the subsample with a clinically documented AUD.
During the study period, 830,825 VA patients screened positive for unhealthy alcohol use. Among those, 31,841 (3.8%) patients had a documented diagnosis for HCV, and of these 20,320 (64%) had an AUD. Two-thirds of these AUD patients did not receive specialty addiction treatment, and more than 90% did not receive pharmacotherapy that is approved by the Food and Drug Administration to treat AUD, according to the researchers. “These rates are concerning given the negative impact alcohol use can have on HCV,” they wrote.
They reiterated the importance of the 2016 change in policy adopted by the VA Health System, which updated its treatment guidelines to recommend that all patients with HCV be considered for treatment, regardless of substance use, and explicitly stated that alcohol use and length of abstinence should not be disqualifiers for receiving HCV treatment.
“All patients with HCV should be receiving evidence-based alcohol-related care given the risks of alcohol use in this population, particularly among those coinfected with HIV,” the researchers concluded.
The research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism. The authors reported that they had no conflicts of interest.
SOURCE: Owens MD et al. Drug Alcohol Depend. 2018;188:79-85.
FROM DRUG AND ALCOHOL DEPENDENCE
Key clinical point: Alcohol-use disorder therapy is underdelivered to patients with HCV who would benefit.
Major finding: Only 27% of patients with HCV plus alcohol-abuse disorder received AUD therapy.
Study details: National VA health care system database of 830,825 patients who screened positive for unhealthy alcohol use.
Disclosures: The research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism. The authors reported that they had no conflicts of interest.
Source: Owens MD et al. Drug Alcohol Depend. 2018;188:79-85.
CKD triples risk of bad outcomes in HIV
BOSTON – A lot of people do well with HIV thanks to potent antiretrovirals, but there’s still at least one group that needs extra attention: HIV patients with chronic kidney disease (CKD), according to Lene Ryom, MD, PhD, an HIV researcher at the University of Copenhagen.
She was the lead investigator on a review of 2,467 HIV patients with CKD – which is becoming more common in HIV as patients live longer – and 33,427 HIV patients without CKD.
The incidence of serious clinical events following CKD diagnosis was 68.9 events per 1,000 patient-years. Among the HIV patients without CKD, the incidence was 23 events per 1,000 patient-years.
“In an era when many HIV patients require much less management due to effective antiretroviral treatment, those living with CKD have a much higher burden of serious clinical events and require much closer monitoring. Modifiable risk factors ... play a central role in CKD morbidity and mortality, highlighting the need for increased awareness, effective treatment, and preventative measures. In particular, smoking seems to be quite important for all” serious adverse outcomes, “so that’s a good place to start,” Dr. Ryom said at the Conference on Retroviruses & Opportunistic Infections.
Most of the 2,467 HIV patients with CKD were white men who have sex with men. At baseline, the median age was 60 years, and median CD4 cell count was above 500. One in three were smokers, 22.4% were HCV positive, and most had viral loads below 400 copies/mL. More than half of the patients were estimated to have died within 5 years of CKD diagnosis.
CKD was defined as two estimated glomerular filtration rates at or below 60 mL/min per 1.73 m2 taken at least 3 months apart, or a 25% decrease in eGFR when patients entered the study at that level.
The subjects were all participants in the D:A:D project [Data Collection on Adverse Events of Anti-HIV Drugs], an ongoing international cohort study based at the University of Copenhagen, and funded by pharmaceutical companies, among others.
Dr. Ryom had no disclosures.
SOURCE: Ryom L et al. CROI, Abstract 75.
BOSTON – A lot of people do well with HIV thanks to potent antiretrovirals, but there’s still at least one group that needs extra attention: HIV patients with chronic kidney disease (CKD), according to Lene Ryom, MD, PhD, an HIV researcher at the University of Copenhagen.
She was the lead investigator on a review of 2,467 HIV patients with CKD – which is becoming more common in HIV as patients live longer – and 33,427 HIV patients without CKD.
The incidence of serious clinical events following CKD diagnosis was 68.9 events per 1,000 patient-years. Among the HIV patients without CKD, the incidence was 23 events per 1,000 patient-years.
“In an era when many HIV patients require much less management due to effective antiretroviral treatment, those living with CKD have a much higher burden of serious clinical events and require much closer monitoring. Modifiable risk factors ... play a central role in CKD morbidity and mortality, highlighting the need for increased awareness, effective treatment, and preventative measures. In particular, smoking seems to be quite important for all” serious adverse outcomes, “so that’s a good place to start,” Dr. Ryom said at the Conference on Retroviruses & Opportunistic Infections.
Most of the 2,467 HIV patients with CKD were white men who have sex with men. At baseline, the median age was 60 years, and median CD4 cell count was above 500. One in three were smokers, 22.4% were HCV positive, and most had viral loads below 400 copies/mL. More than half of the patients were estimated to have died within 5 years of CKD diagnosis.
CKD was defined as two estimated glomerular filtration rates at or below 60 mL/min per 1.73 m2 taken at least 3 months apart, or a 25% decrease in eGFR when patients entered the study at that level.
The subjects were all participants in the D:A:D project [Data Collection on Adverse Events of Anti-HIV Drugs], an ongoing international cohort study based at the University of Copenhagen, and funded by pharmaceutical companies, among others.
Dr. Ryom had no disclosures.
SOURCE: Ryom L et al. CROI, Abstract 75.
BOSTON – A lot of people do well with HIV thanks to potent antiretrovirals, but there’s still at least one group that needs extra attention: HIV patients with chronic kidney disease (CKD), according to Lene Ryom, MD, PhD, an HIV researcher at the University of Copenhagen.
She was the lead investigator on a review of 2,467 HIV patients with CKD – which is becoming more common in HIV as patients live longer – and 33,427 HIV patients without CKD.
The incidence of serious clinical events following CKD diagnosis was 68.9 events per 1,000 patient-years. Among the HIV patients without CKD, the incidence was 23 events per 1,000 patient-years.
“In an era when many HIV patients require much less management due to effective antiretroviral treatment, those living with CKD have a much higher burden of serious clinical events and require much closer monitoring. Modifiable risk factors ... play a central role in CKD morbidity and mortality, highlighting the need for increased awareness, effective treatment, and preventative measures. In particular, smoking seems to be quite important for all” serious adverse outcomes, “so that’s a good place to start,” Dr. Ryom said at the Conference on Retroviruses & Opportunistic Infections.
Most of the 2,467 HIV patients with CKD were white men who have sex with men. At baseline, the median age was 60 years, and median CD4 cell count was above 500. One in three were smokers, 22.4% were HCV positive, and most had viral loads below 400 copies/mL. More than half of the patients were estimated to have died within 5 years of CKD diagnosis.
CKD was defined as two estimated glomerular filtration rates at or below 60 mL/min per 1.73 m2 taken at least 3 months apart, or a 25% decrease in eGFR when patients entered the study at that level.
The subjects were all participants in the D:A:D project [Data Collection on Adverse Events of Anti-HIV Drugs], an ongoing international cohort study based at the University of Copenhagen, and funded by pharmaceutical companies, among others.
Dr. Ryom had no disclosures.
SOURCE: Ryom L et al. CROI, Abstract 75.
REPORTING FROM CROI
Key clinical point: Smoking, diabetes, dyslipidemia, low body mass index, and poor HIV control increase the risk of poor outcomes in HIV patients who have chronic kidney disease.
Major finding: In HIV patients with CKD, the incidence of a serious clinical event is 68.9 per 1,000 patient-years; in HIV patients without CKD, it’s 23 events per 1,000 patient-years.
Study details: Review of nearly 36,000 HIV patients.
Disclosures: The lead investigator had no disclosures. Funding came from pharmaceutical companies, among others.
Source: Ryom L et al. CROI, Abstract 75.
HCV Patient on Tacrolimus? Keep an Eye on RBCs
When interpreting tacrolimus levels, it is important to monitor changes in red blood cells (RBC). A patient who had hepatitis C virus (HCV) genotype 3A lesson reinforced that lesson for clinicians in the dialysis unit at Fraser Health in Abbotsford, Canada who authored a recent case report. Ribavirin-induced anemia reduced the level of tacrolimus in the patien, the authors reported.
The patient, a 37-year-old man, developed renal failure and received a kidney transplant, which subsequently failed (due to complications likely related to HCV). He had been started on tacrolimus while waiting to go back on the transplant list. Eight years later, the patient restarted hemodialysis (HD), with a regimen of sofosbuvir and ribavirin (SOF/RBV). His whole-blood tacrolimus level was 6.6 ng/mL (target 4–6); his hemoglobin (Hb) level was 10.3 g/dL. Two months later, the patient left for a 1-month vacation. When he returned, his Hb was “dramatically low,” at 3.7 g/dL.
The clinicians put the ribavirin on hold and increased darbepoetin. The patient was given packed RBCs; a bone marrow biopsy ruled out myeloproliferative disorder. Two weeks later, the tacrolimus level was 1.0 mg/mL and then dropped to an undetectable level 5 days later. The clinicians increased the dose. During the next month, the patient’s Hb “gradually bounced back,” to > 10 g/dL, whereupon the clinicians restarted RBV. When the tacrolimus level reached 7.2 ng/mL, the dosages were gradually cut back. Sofosbuvir and RBV were stopped a couple of months later. The patient’s HCV RNA level was undetectable 12 weeks after therapy finished.
Practitioners are always facing the dilemma of risk (anemia) vs benefit (efficacy) in deciding whether and how much RBV should be reduced in renal impairment, the case report authors say. They note that the guidelines for treatment-naïve genotype 3 patients have changed. The recommendation for SOF/RBV has been replaced by one for glecaprevir/pibrentasvir or SOF/velpatasvir.
With the availability of newer direct antivirals, they add, the case again supports the abandoning of RBV-containing regimens. In the “very unlikely instance” that RBV is warranted in a patient on HD, the clinicians advise careful monitoring of Hb, especially 1 month after RBV therapy has started. Consider increasing the dosage of erythropoietin-stimulating agents if the patient’s Hb is at the lower end of the target range.
The decision to increase tacrolimus dosage based on a lower tacrolimus whole-blood concentration induced by anemia is “debatable,” the clinicians say. Nevertheless, this case reminds practitioners to take account of changes in RBC counts when interpreting tacrolimus levels—especially when a drastic change is not expected.
Source:
Liu HY, Cheung CYS, Cooper SE. BMJ Case Rep. 2018;2018. pii: bcr-2017-222477.
doi: 10.1136/bcr-2017-222477.
When interpreting tacrolimus levels, it is important to monitor changes in red blood cells (RBC). A patient who had hepatitis C virus (HCV) genotype 3A lesson reinforced that lesson for clinicians in the dialysis unit at Fraser Health in Abbotsford, Canada who authored a recent case report. Ribavirin-induced anemia reduced the level of tacrolimus in the patien, the authors reported.
The patient, a 37-year-old man, developed renal failure and received a kidney transplant, which subsequently failed (due to complications likely related to HCV). He had been started on tacrolimus while waiting to go back on the transplant list. Eight years later, the patient restarted hemodialysis (HD), with a regimen of sofosbuvir and ribavirin (SOF/RBV). His whole-blood tacrolimus level was 6.6 ng/mL (target 4–6); his hemoglobin (Hb) level was 10.3 g/dL. Two months later, the patient left for a 1-month vacation. When he returned, his Hb was “dramatically low,” at 3.7 g/dL.
The clinicians put the ribavirin on hold and increased darbepoetin. The patient was given packed RBCs; a bone marrow biopsy ruled out myeloproliferative disorder. Two weeks later, the tacrolimus level was 1.0 mg/mL and then dropped to an undetectable level 5 days later. The clinicians increased the dose. During the next month, the patient’s Hb “gradually bounced back,” to > 10 g/dL, whereupon the clinicians restarted RBV. When the tacrolimus level reached 7.2 ng/mL, the dosages were gradually cut back. Sofosbuvir and RBV were stopped a couple of months later. The patient’s HCV RNA level was undetectable 12 weeks after therapy finished.
Practitioners are always facing the dilemma of risk (anemia) vs benefit (efficacy) in deciding whether and how much RBV should be reduced in renal impairment, the case report authors say. They note that the guidelines for treatment-naïve genotype 3 patients have changed. The recommendation for SOF/RBV has been replaced by one for glecaprevir/pibrentasvir or SOF/velpatasvir.
With the availability of newer direct antivirals, they add, the case again supports the abandoning of RBV-containing regimens. In the “very unlikely instance” that RBV is warranted in a patient on HD, the clinicians advise careful monitoring of Hb, especially 1 month after RBV therapy has started. Consider increasing the dosage of erythropoietin-stimulating agents if the patient’s Hb is at the lower end of the target range.
The decision to increase tacrolimus dosage based on a lower tacrolimus whole-blood concentration induced by anemia is “debatable,” the clinicians say. Nevertheless, this case reminds practitioners to take account of changes in RBC counts when interpreting tacrolimus levels—especially when a drastic change is not expected.
Source:
Liu HY, Cheung CYS, Cooper SE. BMJ Case Rep. 2018;2018. pii: bcr-2017-222477.
doi: 10.1136/bcr-2017-222477.
When interpreting tacrolimus levels, it is important to monitor changes in red blood cells (RBC). A patient who had hepatitis C virus (HCV) genotype 3A lesson reinforced that lesson for clinicians in the dialysis unit at Fraser Health in Abbotsford, Canada who authored a recent case report. Ribavirin-induced anemia reduced the level of tacrolimus in the patien, the authors reported.
The patient, a 37-year-old man, developed renal failure and received a kidney transplant, which subsequently failed (due to complications likely related to HCV). He had been started on tacrolimus while waiting to go back on the transplant list. Eight years later, the patient restarted hemodialysis (HD), with a regimen of sofosbuvir and ribavirin (SOF/RBV). His whole-blood tacrolimus level was 6.6 ng/mL (target 4–6); his hemoglobin (Hb) level was 10.3 g/dL. Two months later, the patient left for a 1-month vacation. When he returned, his Hb was “dramatically low,” at 3.7 g/dL.
The clinicians put the ribavirin on hold and increased darbepoetin. The patient was given packed RBCs; a bone marrow biopsy ruled out myeloproliferative disorder. Two weeks later, the tacrolimus level was 1.0 mg/mL and then dropped to an undetectable level 5 days later. The clinicians increased the dose. During the next month, the patient’s Hb “gradually bounced back,” to > 10 g/dL, whereupon the clinicians restarted RBV. When the tacrolimus level reached 7.2 ng/mL, the dosages were gradually cut back. Sofosbuvir and RBV were stopped a couple of months later. The patient’s HCV RNA level was undetectable 12 weeks after therapy finished.
Practitioners are always facing the dilemma of risk (anemia) vs benefit (efficacy) in deciding whether and how much RBV should be reduced in renal impairment, the case report authors say. They note that the guidelines for treatment-naïve genotype 3 patients have changed. The recommendation for SOF/RBV has been replaced by one for glecaprevir/pibrentasvir or SOF/velpatasvir.
With the availability of newer direct antivirals, they add, the case again supports the abandoning of RBV-containing regimens. In the “very unlikely instance” that RBV is warranted in a patient on HD, the clinicians advise careful monitoring of Hb, especially 1 month after RBV therapy has started. Consider increasing the dosage of erythropoietin-stimulating agents if the patient’s Hb is at the lower end of the target range.
The decision to increase tacrolimus dosage based on a lower tacrolimus whole-blood concentration induced by anemia is “debatable,” the clinicians say. Nevertheless, this case reminds practitioners to take account of changes in RBC counts when interpreting tacrolimus levels—especially when a drastic change is not expected.
Source:
Liu HY, Cheung CYS, Cooper SE. BMJ Case Rep. 2018;2018. pii: bcr-2017-222477.
doi: 10.1136/bcr-2017-222477.
How to choose between highly effective HBV therapies
MAUI, HAWAII – With three highly effective, guideline-recommended nucleoside/tide therapies for chronic hepatitis B virus (HBV) infection now available, the selection of the optimal antiviral agent for a given patient can seem daunting.
Norah Terrault, MD, offered guidance on this score at the Gastroenterology Updates, IBD, Liver Disease meeting.
As the current guidelines of the American Association for the Study of Liver Disease and European Association for the Study of the Liver make clear, these agents are to be used in targeting patients with active disease, defined by elevated alanine aminotransferase and HBV DNA levels and/or advanced fibrosis. These antivirals aim at long-term HBV control rather than disease cure.
Although numerous investigational drugs are in development with a focus on cure, the day of curative therapy has not yet arrived, noted Dr. Terrault, a professor of medicine and the director of the viral hepatitis center at the University of California, San Francisco.
Randomized trials presented at the 2017 annual meeting of European Association for the Study of the Liver demonstrated that the newest agent, tenofovir alafenamide (TAF), was as effective as tenofovir disoproxil fumarate (TDF) at suppressing HBV DNA through 96 weeks in both hepatitis B e-antigen (HBeAg)–negative and HBeAg-positive patients.
Moreover, TAF had a small but statistically significant advantage in terms of seroconversion rate at week 96 in the HBeAg-positive group, by a margin of 18%-12%. The alanine aminotransferase normalization rate was also significantly higher in the TAF group, by a margin of 81%-71% in HBeAg-negative patients and 75%-68% in HBeAg-positive patients.
“This [TAF] is a very good drug, a nice one to have at our disposal,” according to Dr. Terrault. “The primary reason we wanted it has to do with its safety profile.”
Its potential benefit in this regard was illustrated in a pair of multinational phase 3 clinical trials totaling nearly 1,300 patients with chronic HBV presented at the 2017 meeting of American Association for the Study of Liver Disease. After completing 96 weeks of double-blind treatment with TAF or TDF, everyone was switched to open-label TAF.
“There was a nice rebound in creatinine clearance and bone density when the switch was made from TDF to TAF,” she observed. “This is encouraging data for us, in that if you have a patient on TDF and you are concerned about renal or bone safety, you can do the switch to TAF and very quickly see some recovery in those abnormalities.”
Dr. Terrault offered the following guidance in selecting HBV therapy: For most patients with no comorbidities, monotherapy with either TAF, TDF, or entecavir is an excellent approach.
However, in a patient who has preexisting bone or renal disease – or who is at increased risk for such disease based upon age greater than 60 years, a history of fragility fractures, chronic systemic corticosteroid therapy, or renal abnormalities – entecavir or TAF is a better option than TDF.
TAF becomes the preferred choice over entecavir if the patient has previously been exposed to a nucleoside or if the patient is coinfected with HIV because the drug is approved for treatment of HIV and HBV, while entecavir is not.
Also, no dose adjustment of TAF is needed so long as the creatinine clearance is at least 15 mL/min, whereas the dose-adjustment threshold is 50 mL/min for both entecavir and TDF.
Conversely, entecavir, an older and less expensive drug than TAF, deserves priority in a patient with no prior exposure to a nucleoside, no HIV coinfection, or in an individual with a creatinine clearance below 15 mL/min after dose adjustment. TAF hasn’t been studied in patients with a creatinine clearance that low.
Also, TAF has not been studied in pregnant women or in patients with decompensated cirrhosis, the hepatologist noted.
Dr. Terrault serves as a consultant to AbbVie, Biotest, CoCrystal Pharmaceuticals, and Gilead Sciences.
MAUI, HAWAII – With three highly effective, guideline-recommended nucleoside/tide therapies for chronic hepatitis B virus (HBV) infection now available, the selection of the optimal antiviral agent for a given patient can seem daunting.
Norah Terrault, MD, offered guidance on this score at the Gastroenterology Updates, IBD, Liver Disease meeting.
As the current guidelines of the American Association for the Study of Liver Disease and European Association for the Study of the Liver make clear, these agents are to be used in targeting patients with active disease, defined by elevated alanine aminotransferase and HBV DNA levels and/or advanced fibrosis. These antivirals aim at long-term HBV control rather than disease cure.
Although numerous investigational drugs are in development with a focus on cure, the day of curative therapy has not yet arrived, noted Dr. Terrault, a professor of medicine and the director of the viral hepatitis center at the University of California, San Francisco.
Randomized trials presented at the 2017 annual meeting of European Association for the Study of the Liver demonstrated that the newest agent, tenofovir alafenamide (TAF), was as effective as tenofovir disoproxil fumarate (TDF) at suppressing HBV DNA through 96 weeks in both hepatitis B e-antigen (HBeAg)–negative and HBeAg-positive patients.
Moreover, TAF had a small but statistically significant advantage in terms of seroconversion rate at week 96 in the HBeAg-positive group, by a margin of 18%-12%. The alanine aminotransferase normalization rate was also significantly higher in the TAF group, by a margin of 81%-71% in HBeAg-negative patients and 75%-68% in HBeAg-positive patients.
“This [TAF] is a very good drug, a nice one to have at our disposal,” according to Dr. Terrault. “The primary reason we wanted it has to do with its safety profile.”
Its potential benefit in this regard was illustrated in a pair of multinational phase 3 clinical trials totaling nearly 1,300 patients with chronic HBV presented at the 2017 meeting of American Association for the Study of Liver Disease. After completing 96 weeks of double-blind treatment with TAF or TDF, everyone was switched to open-label TAF.
“There was a nice rebound in creatinine clearance and bone density when the switch was made from TDF to TAF,” she observed. “This is encouraging data for us, in that if you have a patient on TDF and you are concerned about renal or bone safety, you can do the switch to TAF and very quickly see some recovery in those abnormalities.”
Dr. Terrault offered the following guidance in selecting HBV therapy: For most patients with no comorbidities, monotherapy with either TAF, TDF, or entecavir is an excellent approach.
However, in a patient who has preexisting bone or renal disease – or who is at increased risk for such disease based upon age greater than 60 years, a history of fragility fractures, chronic systemic corticosteroid therapy, or renal abnormalities – entecavir or TAF is a better option than TDF.
TAF becomes the preferred choice over entecavir if the patient has previously been exposed to a nucleoside or if the patient is coinfected with HIV because the drug is approved for treatment of HIV and HBV, while entecavir is not.
Also, no dose adjustment of TAF is needed so long as the creatinine clearance is at least 15 mL/min, whereas the dose-adjustment threshold is 50 mL/min for both entecavir and TDF.
Conversely, entecavir, an older and less expensive drug than TAF, deserves priority in a patient with no prior exposure to a nucleoside, no HIV coinfection, or in an individual with a creatinine clearance below 15 mL/min after dose adjustment. TAF hasn’t been studied in patients with a creatinine clearance that low.
Also, TAF has not been studied in pregnant women or in patients with decompensated cirrhosis, the hepatologist noted.
Dr. Terrault serves as a consultant to AbbVie, Biotest, CoCrystal Pharmaceuticals, and Gilead Sciences.
MAUI, HAWAII – With three highly effective, guideline-recommended nucleoside/tide therapies for chronic hepatitis B virus (HBV) infection now available, the selection of the optimal antiviral agent for a given patient can seem daunting.
Norah Terrault, MD, offered guidance on this score at the Gastroenterology Updates, IBD, Liver Disease meeting.
As the current guidelines of the American Association for the Study of Liver Disease and European Association for the Study of the Liver make clear, these agents are to be used in targeting patients with active disease, defined by elevated alanine aminotransferase and HBV DNA levels and/or advanced fibrosis. These antivirals aim at long-term HBV control rather than disease cure.
Although numerous investigational drugs are in development with a focus on cure, the day of curative therapy has not yet arrived, noted Dr. Terrault, a professor of medicine and the director of the viral hepatitis center at the University of California, San Francisco.
Randomized trials presented at the 2017 annual meeting of European Association for the Study of the Liver demonstrated that the newest agent, tenofovir alafenamide (TAF), was as effective as tenofovir disoproxil fumarate (TDF) at suppressing HBV DNA through 96 weeks in both hepatitis B e-antigen (HBeAg)–negative and HBeAg-positive patients.
Moreover, TAF had a small but statistically significant advantage in terms of seroconversion rate at week 96 in the HBeAg-positive group, by a margin of 18%-12%. The alanine aminotransferase normalization rate was also significantly higher in the TAF group, by a margin of 81%-71% in HBeAg-negative patients and 75%-68% in HBeAg-positive patients.
“This [TAF] is a very good drug, a nice one to have at our disposal,” according to Dr. Terrault. “The primary reason we wanted it has to do with its safety profile.”
Its potential benefit in this regard was illustrated in a pair of multinational phase 3 clinical trials totaling nearly 1,300 patients with chronic HBV presented at the 2017 meeting of American Association for the Study of Liver Disease. After completing 96 weeks of double-blind treatment with TAF or TDF, everyone was switched to open-label TAF.
“There was a nice rebound in creatinine clearance and bone density when the switch was made from TDF to TAF,” she observed. “This is encouraging data for us, in that if you have a patient on TDF and you are concerned about renal or bone safety, you can do the switch to TAF and very quickly see some recovery in those abnormalities.”
Dr. Terrault offered the following guidance in selecting HBV therapy: For most patients with no comorbidities, monotherapy with either TAF, TDF, or entecavir is an excellent approach.
However, in a patient who has preexisting bone or renal disease – or who is at increased risk for such disease based upon age greater than 60 years, a history of fragility fractures, chronic systemic corticosteroid therapy, or renal abnormalities – entecavir or TAF is a better option than TDF.
TAF becomes the preferred choice over entecavir if the patient has previously been exposed to a nucleoside or if the patient is coinfected with HIV because the drug is approved for treatment of HIV and HBV, while entecavir is not.
Also, no dose adjustment of TAF is needed so long as the creatinine clearance is at least 15 mL/min, whereas the dose-adjustment threshold is 50 mL/min for both entecavir and TDF.
Conversely, entecavir, an older and less expensive drug than TAF, deserves priority in a patient with no prior exposure to a nucleoside, no HIV coinfection, or in an individual with a creatinine clearance below 15 mL/min after dose adjustment. TAF hasn’t been studied in patients with a creatinine clearance that low.
Also, TAF has not been studied in pregnant women or in patients with decompensated cirrhosis, the hepatologist noted.
Dr. Terrault serves as a consultant to AbbVie, Biotest, CoCrystal Pharmaceuticals, and Gilead Sciences.
EXPERT ANALYSIS FROM GUILD 2018