User login
Hepatitis C vaccine alters viral trajectory, but fails in chronic infection protection
BOSTON – A prime-boost hepatitis C virus (HCV) vaccine regimen did not protect against chronic infection, but it did evoke immune responses and differences in viral trajectory, according to investigators in what is believed to be the first randomized, placebo-controlled efficacy trial in this setting.
There were no apparent safety concerns with the vaccine according to investigators, led by Kimberly Page, PhD, MPH, of the University of New Mexico, Albuquerque.
“A safe and effective vaccine to prevent chronic hepatitis C virus infection is essential to reduce transmission,” Dr. Page and coauthors said in a late-breaking abstract of the study results, which will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase 1/2 trial described by Dr. Page and colleagues included 455 adults at risk of HCV infection because of injection drug use. They were randomized to vaccine, which consisted of a recombinant chimpanzee adenovirus-3 vectored vaccine prime plus a recombinant Modified Vaccinia virus Ankara boost, or to two doses of placebo at days 0 and 56 of the study.
There was no difference in chronic HCV infection at 6 months, the primary endpoint of the study. There were 14 chronically infected participants in the vaccine group, as well as 14 in the placebo group, for an overall incidence of infection of 13.0/100 person-years, Dr. Page and coauthors reported in the abstract.
However, there were significant differences in HCV RNA geometric mean peak at 1 month, which was 193,795 IU/L in the vaccine group and 1,078,092 IU/L in the placebo group, according to investigators. Similarly, geometric mean fold rise after infection was 0.2 in the vaccine group and 13.5 in the placebo group.
A total of 78% of vaccinated individuals had T-cell responses to at least one vaccine antigen pool, investigators said, adding that the vaccine was safe, well tolerated, and not associated with any serious adverse events.
Dr. Page had no disclosures related to the abstract.
SOURCE: Page K et al. The Liver Meeting 2019. Abstract LP17.
BOSTON – A prime-boost hepatitis C virus (HCV) vaccine regimen did not protect against chronic infection, but it did evoke immune responses and differences in viral trajectory, according to investigators in what is believed to be the first randomized, placebo-controlled efficacy trial in this setting.
There were no apparent safety concerns with the vaccine according to investigators, led by Kimberly Page, PhD, MPH, of the University of New Mexico, Albuquerque.
“A safe and effective vaccine to prevent chronic hepatitis C virus infection is essential to reduce transmission,” Dr. Page and coauthors said in a late-breaking abstract of the study results, which will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase 1/2 trial described by Dr. Page and colleagues included 455 adults at risk of HCV infection because of injection drug use. They were randomized to vaccine, which consisted of a recombinant chimpanzee adenovirus-3 vectored vaccine prime plus a recombinant Modified Vaccinia virus Ankara boost, or to two doses of placebo at days 0 and 56 of the study.
There was no difference in chronic HCV infection at 6 months, the primary endpoint of the study. There were 14 chronically infected participants in the vaccine group, as well as 14 in the placebo group, for an overall incidence of infection of 13.0/100 person-years, Dr. Page and coauthors reported in the abstract.
However, there were significant differences in HCV RNA geometric mean peak at 1 month, which was 193,795 IU/L in the vaccine group and 1,078,092 IU/L in the placebo group, according to investigators. Similarly, geometric mean fold rise after infection was 0.2 in the vaccine group and 13.5 in the placebo group.
A total of 78% of vaccinated individuals had T-cell responses to at least one vaccine antigen pool, investigators said, adding that the vaccine was safe, well tolerated, and not associated with any serious adverse events.
Dr. Page had no disclosures related to the abstract.
SOURCE: Page K et al. The Liver Meeting 2019. Abstract LP17.
BOSTON – A prime-boost hepatitis C virus (HCV) vaccine regimen did not protect against chronic infection, but it did evoke immune responses and differences in viral trajectory, according to investigators in what is believed to be the first randomized, placebo-controlled efficacy trial in this setting.
There were no apparent safety concerns with the vaccine according to investigators, led by Kimberly Page, PhD, MPH, of the University of New Mexico, Albuquerque.
“A safe and effective vaccine to prevent chronic hepatitis C virus infection is essential to reduce transmission,” Dr. Page and coauthors said in a late-breaking abstract of the study results, which will be presented at the annual meeting of the American Association for the Study of Liver Diseases.
The phase 1/2 trial described by Dr. Page and colleagues included 455 adults at risk of HCV infection because of injection drug use. They were randomized to vaccine, which consisted of a recombinant chimpanzee adenovirus-3 vectored vaccine prime plus a recombinant Modified Vaccinia virus Ankara boost, or to two doses of placebo at days 0 and 56 of the study.
There was no difference in chronic HCV infection at 6 months, the primary endpoint of the study. There were 14 chronically infected participants in the vaccine group, as well as 14 in the placebo group, for an overall incidence of infection of 13.0/100 person-years, Dr. Page and coauthors reported in the abstract.
However, there were significant differences in HCV RNA geometric mean peak at 1 month, which was 193,795 IU/L in the vaccine group and 1,078,092 IU/L in the placebo group, according to investigators. Similarly, geometric mean fold rise after infection was 0.2 in the vaccine group and 13.5 in the placebo group.
A total of 78% of vaccinated individuals had T-cell responses to at least one vaccine antigen pool, investigators said, adding that the vaccine was safe, well tolerated, and not associated with any serious adverse events.
Dr. Page had no disclosures related to the abstract.
SOURCE: Page K et al. The Liver Meeting 2019. Abstract LP17.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: A prime-boost HCV vaccine altered viral trajectory but did not protect against chronic infection.
Major finding: At 6 months after vaccination, there were 14 chronically infected participants in the vaccine group, and 14 in the placebo group.
Study details: A randomized, placebo controlled phase 1/2 trial including 455 adults at risk of HCV infection.
Disclosures: The first author reported no disclosures.
Source: Page K et al. The Liver Meeting 2019. Abstract LP17.
Fenofibrate fights increased triglycerides in NASH
BOSTON – Fenofibrate is safe and effective for limiting triglyceride increases in patients with nonalcoholic steatohepatitis and advanced fibrosis, based on data from 31 adults.
Treatment of NASH with acetyl-CoA carboxylase inhibitors has been shown to improve liver fat and other liver conditions but may be associated with hyperlipidemia, according to Eric J. Lawitz, MD, of the University of Texas Health, San Antonio, and colleagues. The researchers examined the safety and effectiveness of fenofibrate to mitigate serum triglyceride increases in a study be presented in a late-breaking session at the annual meeting of the American Association for the Study of Liver Diseases.
The researchers randomized 15 patients to treatment with 48 mg of fenofibrate or 145 mg of fenofibrate once daily for 2 weeks, followed by a combination of the fenofibrate doses plus 20 mg of ACC inhibitor firsocostat daily for 24 weeks.
The median fasting triglycerides (TG) in the 48-mg and 145-mg fenofibrate groups were 218 mg/dL and 202 mg/dL, respectively. After 2 weeks, the median change in TG was +2 mg/dL in the 48-mg group and –42 mg/dL in the 145-mg group. After 24 weeks of combination therapy, TG levels were not significantly different from baseline in either group (+19 mg/dL in 48-mg group and +6 mg/dL in the 145-mg group). Significant reductions in serum alanine aminotransferase from baseline to week 24 occurred in the combined groups (median of 39 U/L vs. 27 U/L, respectively). In addition, 43% of patients overall showed at least a 30% reduction in protein density fat fraction.
Both firsocostat and fenofibrate were well tolerated, the researchers said. One treatment-emergent grade 3 TG elevation (defined as greater than 500 mg/dL) occurred in the 48-mg group at week 24. No hepatotoxicity was noted, no patients discontinued therapy because of adverse events, and no other grade 3 or 4 adverse events were reported.
“The combination of firsocostat and fenofibrate led to improvements in hepatic fat, liver biochemistry, and markers of fibrosis,” the researchers concluded in their abstract.
Lead author Dr. Lawitz disclosed financial relationships with Allergan, Akcea Therapeutics, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Madrigal Pharmaceuticals, and Novartis.
The AGA GI Patient Center provides education to help your patients understand NASH at https://www.gastro.org/practice-guidance/gi-patient-center/topic/nonalcoholic-steatohepatitis-nash.
SOURCE: Lawitz E et al. The Liver Meeting 2019. Presentation LP5.
BOSTON – Fenofibrate is safe and effective for limiting triglyceride increases in patients with nonalcoholic steatohepatitis and advanced fibrosis, based on data from 31 adults.
Treatment of NASH with acetyl-CoA carboxylase inhibitors has been shown to improve liver fat and other liver conditions but may be associated with hyperlipidemia, according to Eric J. Lawitz, MD, of the University of Texas Health, San Antonio, and colleagues. The researchers examined the safety and effectiveness of fenofibrate to mitigate serum triglyceride increases in a study be presented in a late-breaking session at the annual meeting of the American Association for the Study of Liver Diseases.
The researchers randomized 15 patients to treatment with 48 mg of fenofibrate or 145 mg of fenofibrate once daily for 2 weeks, followed by a combination of the fenofibrate doses plus 20 mg of ACC inhibitor firsocostat daily for 24 weeks.
The median fasting triglycerides (TG) in the 48-mg and 145-mg fenofibrate groups were 218 mg/dL and 202 mg/dL, respectively. After 2 weeks, the median change in TG was +2 mg/dL in the 48-mg group and –42 mg/dL in the 145-mg group. After 24 weeks of combination therapy, TG levels were not significantly different from baseline in either group (+19 mg/dL in 48-mg group and +6 mg/dL in the 145-mg group). Significant reductions in serum alanine aminotransferase from baseline to week 24 occurred in the combined groups (median of 39 U/L vs. 27 U/L, respectively). In addition, 43% of patients overall showed at least a 30% reduction in protein density fat fraction.
Both firsocostat and fenofibrate were well tolerated, the researchers said. One treatment-emergent grade 3 TG elevation (defined as greater than 500 mg/dL) occurred in the 48-mg group at week 24. No hepatotoxicity was noted, no patients discontinued therapy because of adverse events, and no other grade 3 or 4 adverse events were reported.
“The combination of firsocostat and fenofibrate led to improvements in hepatic fat, liver biochemistry, and markers of fibrosis,” the researchers concluded in their abstract.
Lead author Dr. Lawitz disclosed financial relationships with Allergan, Akcea Therapeutics, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Madrigal Pharmaceuticals, and Novartis.
The AGA GI Patient Center provides education to help your patients understand NASH at https://www.gastro.org/practice-guidance/gi-patient-center/topic/nonalcoholic-steatohepatitis-nash.
SOURCE: Lawitz E et al. The Liver Meeting 2019. Presentation LP5.
BOSTON – Fenofibrate is safe and effective for limiting triglyceride increases in patients with nonalcoholic steatohepatitis and advanced fibrosis, based on data from 31 adults.
Treatment of NASH with acetyl-CoA carboxylase inhibitors has been shown to improve liver fat and other liver conditions but may be associated with hyperlipidemia, according to Eric J. Lawitz, MD, of the University of Texas Health, San Antonio, and colleagues. The researchers examined the safety and effectiveness of fenofibrate to mitigate serum triglyceride increases in a study be presented in a late-breaking session at the annual meeting of the American Association for the Study of Liver Diseases.
The researchers randomized 15 patients to treatment with 48 mg of fenofibrate or 145 mg of fenofibrate once daily for 2 weeks, followed by a combination of the fenofibrate doses plus 20 mg of ACC inhibitor firsocostat daily for 24 weeks.
The median fasting triglycerides (TG) in the 48-mg and 145-mg fenofibrate groups were 218 mg/dL and 202 mg/dL, respectively. After 2 weeks, the median change in TG was +2 mg/dL in the 48-mg group and –42 mg/dL in the 145-mg group. After 24 weeks of combination therapy, TG levels were not significantly different from baseline in either group (+19 mg/dL in 48-mg group and +6 mg/dL in the 145-mg group). Significant reductions in serum alanine aminotransferase from baseline to week 24 occurred in the combined groups (median of 39 U/L vs. 27 U/L, respectively). In addition, 43% of patients overall showed at least a 30% reduction in protein density fat fraction.
Both firsocostat and fenofibrate were well tolerated, the researchers said. One treatment-emergent grade 3 TG elevation (defined as greater than 500 mg/dL) occurred in the 48-mg group at week 24. No hepatotoxicity was noted, no patients discontinued therapy because of adverse events, and no other grade 3 or 4 adverse events were reported.
“The combination of firsocostat and fenofibrate led to improvements in hepatic fat, liver biochemistry, and markers of fibrosis,” the researchers concluded in their abstract.
Lead author Dr. Lawitz disclosed financial relationships with Allergan, Akcea Therapeutics, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Madrigal Pharmaceuticals, and Novartis.
The AGA GI Patient Center provides education to help your patients understand NASH at https://www.gastro.org/practice-guidance/gi-patient-center/topic/nonalcoholic-steatohepatitis-nash.
SOURCE: Lawitz E et al. The Liver Meeting 2019. Presentation LP5.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: Fenofibrate mitigated serum triglyceride increases in patients with NASH.
Major finding: After 24 weeks of fenofibrate treatment, 43% of patients showed a relative reduction of at least 30% in protein density fat fraction with an average of 40% at a 48 mg-dose and 47% at a 145-mg dose.
Study details: The data come from a 24-week study of 31 adults with advanced fibrosis caused by NASH.
Disclosures: Lead author Dr. Lawitz disclosed financial relationships with Allergan, Akcea Therapeutics, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Madrigal Pharmaceuticals, and Novartis.
Source: Lawitz E et al. The Liver Meeting 2019. Presentation LP5.
Terlipressin reversed hepatorenal syndrome in large prospective study
BOSTON – Terlipressin, an investigational vasopressin analogue, improved renal function and reversed hepatorenal syndrome type 1 (HRS-1) among patients with progressive advanced liver disease in a randomized trial, according to investigators.
Compared with albumin alone, terlipressin plus albumin significantly reversed worsening of renal function in cirrhotic patients, including those meeting systemic inflammatory response syndrome (SIRS) criteria, said investigators, led by Florence Wong, MD, from the University of Toronto.
“This response was durable and associated with less need for early renal replacement therapy,” said Dr. Wong and coauthors of an abstract describing the study, which will be presented in a late-breaking study session here at the annual meeting of the American Association for the Study of Liver Diseases.
The North American randomized, controlled trial, known as CONFIRM, was designed to confirm the safety and efficacy of terlipressin/albumin as a treatment for HRS-1, a serious but potentially reversible type of acute kidney injury seen in patients with cirrhosis and ascites, investigators said in their communication.
Patients in CONFIRM had “well-defined” HRS-1, based on diagnostic criteria as outlined by the International Club of Ascites, investigators said.
A total of 300 participants were randomized, 199 to terlipressin 1 mg IV every 6 hours and 101 to placebo, with both groups also receiving albumin, for up to 14 days of treatment. According to the report, 132 subjects (44%) met SIRS criteria.
Verified HRS reversal was documented in 29.1% of terlipressin-treated patients versus just 15.8% of the placebo group (P less than .012), investigators reported in their abstract. Among the SIRS patients, verified HRS reversal was seen in 33.3% and 6.3% of the terlipressin- and placebo-treated groups, respectively (P less than .001).
As the primary endpoint of the study, verified HRS reversal is an outcome that combines improvement in renal function, short-term survival following improvement, and avoidance of renal replacement therapy, investigators explained in their report.
Liver transplantation occurred in 23.1% of the terlipressin group and 28.7% of the placebo group, investigators also reported in their abstract.
Ischemia-associated adverse events were seen in 4.5% of the terlipressin arm and 0% for placebo, though in general the rate of adverse events were similar for the treatment and control arms, Dr. Wong and colleagues noted in their report.
The CONFIRM study is supported by Mallinckrodt. Dr. Wong provided disclosures related to Mallinckrodt, Ferring, and Sequana.
BOSTON – Terlipressin, an investigational vasopressin analogue, improved renal function and reversed hepatorenal syndrome type 1 (HRS-1) among patients with progressive advanced liver disease in a randomized trial, according to investigators.
Compared with albumin alone, terlipressin plus albumin significantly reversed worsening of renal function in cirrhotic patients, including those meeting systemic inflammatory response syndrome (SIRS) criteria, said investigators, led by Florence Wong, MD, from the University of Toronto.
“This response was durable and associated with less need for early renal replacement therapy,” said Dr. Wong and coauthors of an abstract describing the study, which will be presented in a late-breaking study session here at the annual meeting of the American Association for the Study of Liver Diseases.
The North American randomized, controlled trial, known as CONFIRM, was designed to confirm the safety and efficacy of terlipressin/albumin as a treatment for HRS-1, a serious but potentially reversible type of acute kidney injury seen in patients with cirrhosis and ascites, investigators said in their communication.
Patients in CONFIRM had “well-defined” HRS-1, based on diagnostic criteria as outlined by the International Club of Ascites, investigators said.
A total of 300 participants were randomized, 199 to terlipressin 1 mg IV every 6 hours and 101 to placebo, with both groups also receiving albumin, for up to 14 days of treatment. According to the report, 132 subjects (44%) met SIRS criteria.
Verified HRS reversal was documented in 29.1% of terlipressin-treated patients versus just 15.8% of the placebo group (P less than .012), investigators reported in their abstract. Among the SIRS patients, verified HRS reversal was seen in 33.3% and 6.3% of the terlipressin- and placebo-treated groups, respectively (P less than .001).
As the primary endpoint of the study, verified HRS reversal is an outcome that combines improvement in renal function, short-term survival following improvement, and avoidance of renal replacement therapy, investigators explained in their report.
Liver transplantation occurred in 23.1% of the terlipressin group and 28.7% of the placebo group, investigators also reported in their abstract.
Ischemia-associated adverse events were seen in 4.5% of the terlipressin arm and 0% for placebo, though in general the rate of adverse events were similar for the treatment and control arms, Dr. Wong and colleagues noted in their report.
The CONFIRM study is supported by Mallinckrodt. Dr. Wong provided disclosures related to Mallinckrodt, Ferring, and Sequana.
BOSTON – Terlipressin, an investigational vasopressin analogue, improved renal function and reversed hepatorenal syndrome type 1 (HRS-1) among patients with progressive advanced liver disease in a randomized trial, according to investigators.
Compared with albumin alone, terlipressin plus albumin significantly reversed worsening of renal function in cirrhotic patients, including those meeting systemic inflammatory response syndrome (SIRS) criteria, said investigators, led by Florence Wong, MD, from the University of Toronto.
“This response was durable and associated with less need for early renal replacement therapy,” said Dr. Wong and coauthors of an abstract describing the study, which will be presented in a late-breaking study session here at the annual meeting of the American Association for the Study of Liver Diseases.
The North American randomized, controlled trial, known as CONFIRM, was designed to confirm the safety and efficacy of terlipressin/albumin as a treatment for HRS-1, a serious but potentially reversible type of acute kidney injury seen in patients with cirrhosis and ascites, investigators said in their communication.
Patients in CONFIRM had “well-defined” HRS-1, based on diagnostic criteria as outlined by the International Club of Ascites, investigators said.
A total of 300 participants were randomized, 199 to terlipressin 1 mg IV every 6 hours and 101 to placebo, with both groups also receiving albumin, for up to 14 days of treatment. According to the report, 132 subjects (44%) met SIRS criteria.
Verified HRS reversal was documented in 29.1% of terlipressin-treated patients versus just 15.8% of the placebo group (P less than .012), investigators reported in their abstract. Among the SIRS patients, verified HRS reversal was seen in 33.3% and 6.3% of the terlipressin- and placebo-treated groups, respectively (P less than .001).
As the primary endpoint of the study, verified HRS reversal is an outcome that combines improvement in renal function, short-term survival following improvement, and avoidance of renal replacement therapy, investigators explained in their report.
Liver transplantation occurred in 23.1% of the terlipressin group and 28.7% of the placebo group, investigators also reported in their abstract.
Ischemia-associated adverse events were seen in 4.5% of the terlipressin arm and 0% for placebo, though in general the rate of adverse events were similar for the treatment and control arms, Dr. Wong and colleagues noted in their report.
The CONFIRM study is supported by Mallinckrodt. Dr. Wong provided disclosures related to Mallinckrodt, Ferring, and Sequana.
REPORTING FROM THE LIVER MEETING 2019
Key clinical point: Terlipressin, an investigational vasopressin analogue, improved renal function and reversed hepatorenal syndrome type 1 (HRS-1) among patients with progressive advanced liver disease.
Major finding: Verified HRS reversal was documented in 29.1% of patients treated with terlipressin/albumin versus 15.8% with placebo/albumin (P less than .012).
Study details: CONFIRM, a randomized, controlled trial including 300 patients with HRS-1.
Disclosures: The CONFIRM study is supported by Mallinckrodt. Dr. Wong provided disclosures related to Mallinckrodt, Ferring, and Sequana.
Source: Wong F et al. The Liver Meeting 2019, Presentation LO5.
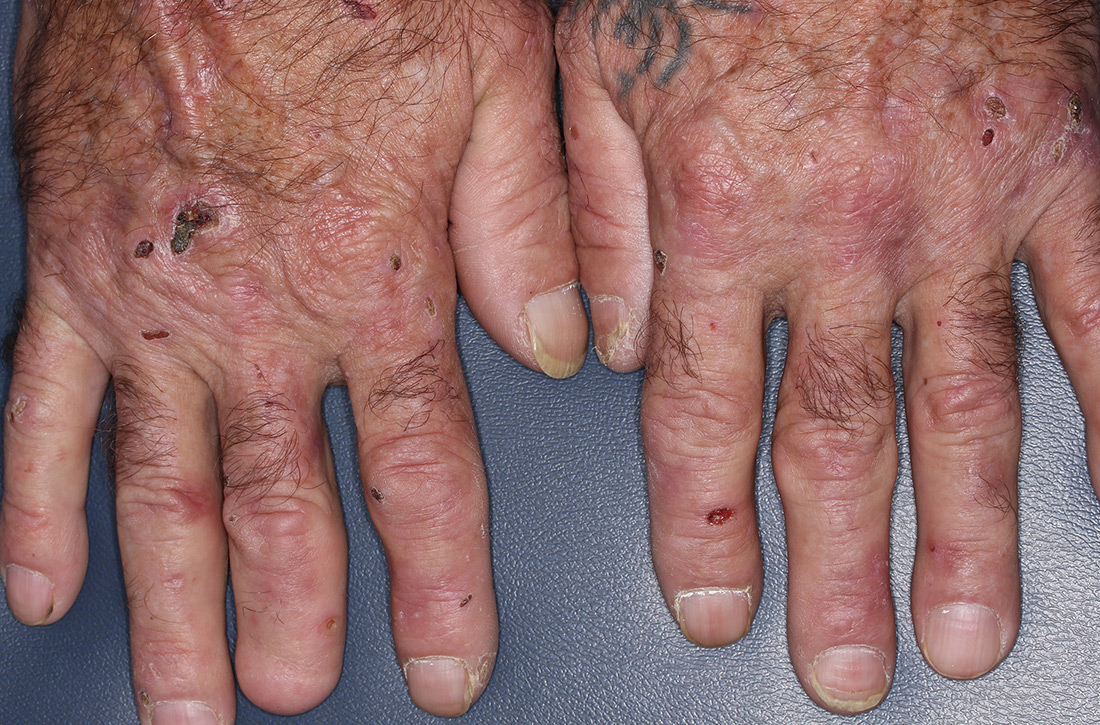
Chronic blistering rash on hands
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
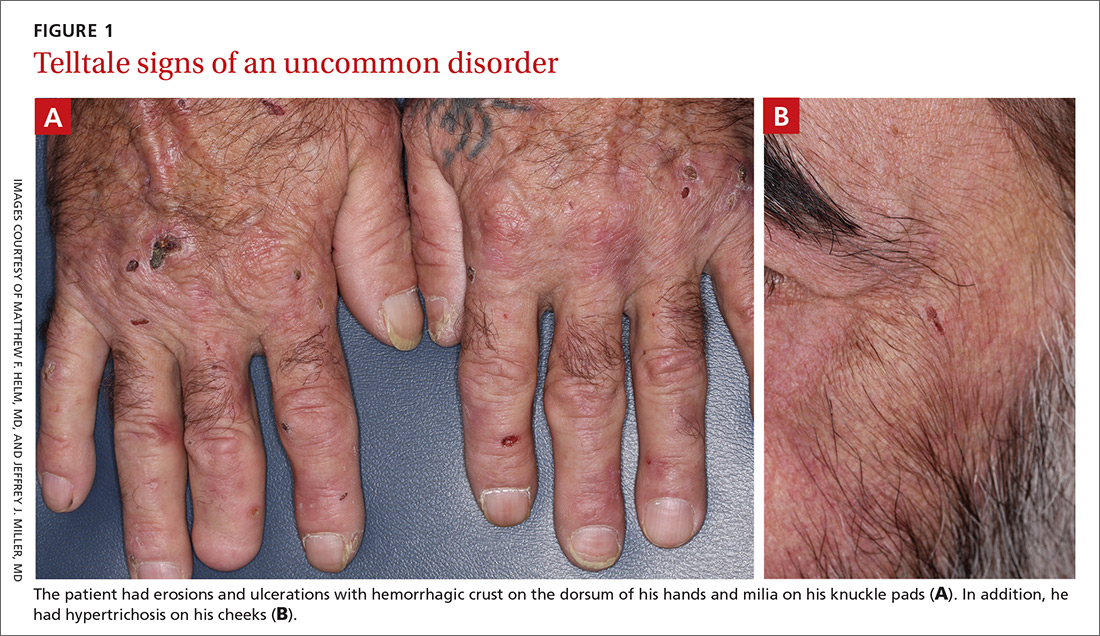
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; [email protected]
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; [email protected]
A 60-year-old man presented to our dermatology clinic with a chronic, recurrent pruritic rash on his hands and neck. He noted that the rash developed into blisters, which he would pick until they scabbed over. The rash only manifested on sun-exposed areas.
The patient did not take any medications. He admitted to drinking alcohol (4 beers/d on average) and had roughly a 50-pack year history of smoking. There was no family history of similar symptoms.
On physical exam, we noted erosions and ulcerations with hemorrhagic crust on the dorsal aspect of his hands, along with milia on the knuckle pads (FIGURE 1A). Further skin examination revealed hypopigmented scars on his shoulders and lower extremities bilaterally, with hypertrichosis of the cheeks (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Porphyria cutanea tarda
Based on the clinical presentation and the patient’s history of smoking and alcohol consumption, we suspected that this was a case of porphyria cutanea tarda (PCT). Laboratory studies, including a complete blood count, basic metabolic panel, iron studies, and liver function tests, were ordered. These revealed elevated levels of serum alanine transaminase (116 IU/L; reference range, 20-60 IU/L), aspartate aminotransferase (184 IU/L; reference range, 6-34 IU/L in men), and ferritin (1594 ng/mL; reference range, 12-300 ng/mL in men), consistent with PCT. Total porphyrins were then measured and found to be elevated (128.5 mcg/dL; reference range, 0 to 1 mcg/dL), which confirmed the diagnosis. Further testing revealed that the patient was positive for both hepatitis C virus (HCV) and hepatitis B virus infection.
While PCT is the most common porphyria worldwide, it is nonetheless a rare disorder that results from deficient activity (< 20% of normal) of uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme synthetic pathway.1,2 It is typically (~75% cases) an acquired disorder of mid- to late adulthood and more commonly affects males.1 In the remainder of cases, patients have a genetic predisposition—a mutation of the UROD or HFE gene. Patients with a genetic predisposition may present earlier.2,3 Susceptibility factors for both forms of PCT include chronic alcohol use, HCV and/or human immunodeficiency virus (HIV) infection, estrogen therapy, and a history of chronic/heavy smoking.1,4
Cutaneous manifestations of PCT are caused by the accumulation of porphyrins, which are photo-oxidized in the skin.1 Findings include photosensitivity, skin fragility, blistering, scarring, hypo- or hyperpigmentation, and milia in sun-exposed areas, such as the dorsum of the hands, forearms, face, ears, neck, and feet.1,2 Hypertrichosis can occur, particularly on the cheeks and forearms.1 Elevated transaminases often accompany cutaneous findings, due to porphyrin accumulation in hepatocytes and the hepatotoxic effects of alcohol, HCV infection, or iron overload.5 Iron overload, in part due to dysregulation of hepcidin, can lead to increased serum ferritin, iron, and transferrin saturation.1
Differential includes autoimmune and autosomal conditions
Diseases that manifest with blistering, elevated porphyrins or porphyrin precursors, and iron overload should be included in the differential diagnosis.
Bullous pemphigoid is an autoimmune subepithelial blistering disorder that occurs when antibodies attack hemidesmosomes in the epidermis. It commonly manifests in the elderly and classically presents with tense bullae, typically on the trunk, abdomen, and proximal extremities. Serologic testing and biopsy can confirm the diagnosis.6
Continue to: Pseudoporphyria...
Pseudoporphyria has a similar presentation to PCT but with no abnormalities in porphyrin metabolism. Risk factors include UV radiation exposure; use of medications such as nonsteroidal anti-inflammatory drugs, diuretics, and retinoids; chronic renal failure; and hemodialysis.7
Acute intermittent porphyria is an autosomal dominant disorder due to deficiency of porphobilinogen deaminase, a heme biosynthetic enzyme. Clinical manifestations usually arise in adulthood and include neurovisceral attacks (eg, abdominal pain, vomiting, muscle weakness). Diagnosis during an acute attack can be made by measuring urinary 5-aminolaevulinc acid and porphobilinogen.1
Hereditary hemochromatosis is an autosomal recessive disorder most commonly due to mutations in the HFE gene. Patients typically have iron overload and abnormal liver function test results. The main cutaneous finding is skin hyperpigmentation. Patients also may develop diabetes mellitus, arthropathy, cardiac disease, and hypopituitarism, although most are diagnosed with asymptomatic disease following routine laboratory studies.8
Confirm the diagnosis with total porphyrin measurement
The preferred initial test to confirm the diagnosis of PCT is measurement of plasma or urine total porphyrins, which will be elevated.1 Further testing is then performed to discern PCT from the other, less common cutaneous porphyrias.1 If needed, biopsy can be done to exclude other diagnoses. Testing for HIV and viral hepatitis infection may be performed when clinical suspicion is high.1 Testing for UROD and HFE mutations may also be advised.1
Treatment choice is guided by iron levels
For patients with normal iron levels, low-dose hydroxychloroquine 100 mg or chloroquine 125 mg twice per week can be used until restoration of normal plasma or urine porphyrin levels has been achieved for several months.1 For those with iron excess (serum ferritin > 600 ng/dL), repeat phlebotomy is the preferred treatment; a unit of blood (350-500 mL) is typically removed, as tolerated, until iron stores return to normal.1 In severe cases of PCT, these therapies can be used in combination.1 Clinical remission with these methods can be expected within 6 to 9 months.9
Continue to: In addition...
In addition, it is important to provide patient education regarding proper sun protection and risk factor modification.1 Underlying HIV and viral hepatitis infection should be managed appropriately by the relevant specialists.
Our patient was counseled on proper sun protection and encouraged to cease alcohol consumption and smoking. We subsequently referred him to Hepatology for the treatment of his liver disease. Given that the patient’s ferritin level was so high (1594 ng/mL), serial phlebotomy was initiated twice monthly until levels reached the lower limit of normal. He was also started on direct-acting antiviral therapy with Epclusa (sofosbuvir/velpatasvir) for 12 weeks for treatment of his HCV and is currently in remission.
CORRESPONDENCE
Christopher G. Bazewicz, MD, Department of Dermatology, Penn State Milton S. Hershey Medical Center, 500 University Drive, Hershey, PA 17033; [email protected]
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
1. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
2. Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene. Br J Dermatol. 2007;157:501-507.
3. Brady JJ, Jackson HA, Roberts AG, et al. Co-inheritance of mutations in the uroporphyrinogen decarboxylase and hemochromatosis genes accelerates the onset of porphyria cutanea tarda. J Invest Dermatol. 2000;115:868-874.
4. Jalil S, Grady JJ, Lee C, et al. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297-302, 302.e1.
5. Gisbert JP, García-Buey L, Alonso A, et al. Hepatocellular carcinoma risk in patients with porphyria cutanea tarda. Eur J Gastroenterol Hepatol. 2004;16:689-692.
6. Di Zenzo G, Della Torre R, Zambruno G, et al. Bullous pemphigoid: from the clinic to the bench. Clin Dermatol. 2012;30:3-16.
7. Green JJ, Manders SM. Pseudoporphyria. J Am Acad Dermatol. 2001;44:100-108.
8. Crownover BK, Covey CJ. Hereditary hemochromatosis. Am Fam Physician. 2013;87:183-190.
9. Sarkany RP. The management of porphyria cutanea tarda. Clin Exp Dermatol. 2001;26:225-232.
Liver abnormalities, disease common in patients with psoriatic arthritis
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
FROM THE JOURNAL OF RHEUMATOLOGY
AGA Clinical Practice Update: Surveillance for hepatobiliary cancers in primary sclerosing cholangitis
All adult patients with primary sclerosing cholangitis should be screened at least annually for cholangiocarcinoma and gallbladder cancer, particularly in the first year after their diagnosis, according to a clinical practice update published in Clinical Gastroenterology and Hepatology.
Individuals with primary sclerosing cholangitis have a 400-fold higher risk of cholangiocarcinoma, compared with the general population, and around one-third of cancers are detected within 1 year of the cholangitis diagnosis, Christopher L. Bowlus, MD, of the University of California, Davis, and coauthors wrote.
The clinical practice update from the American Gastroenterological Association was in response to the observation that, while there is significant evidence for an increasing incidence of cirrhosis, hepatic decompensation, hepatocellular carcinoma, and liver transplant listing among patients with primary sclerosing cholangitis, there is a lack of good evidence to guide cholangiocarcinoma surveillance in these patients.
“The low prevalence and long duration of PSC [primary sclerosing cholangitis] present substantial barriers to better understanding risk stratification, developing biomarkers, and measuring the impact surveillance has on clinical outcomes,” they wrote.
The first recommendation was that surveillance for cholangiocarcinoma and gallbladder cancer should be considered in all adult patients with primary sclerosing cholangitis, regardless of their disease stage. The authors especially emphasized the importance of surveillance in the first year after a diagnosis of primary sclerosing cholangitis, in patients who also have ulcerative colitis, and in those diagnosed at an older age.
They cited one study that found regular surveillance of patients with primary sclerosing cholangitis was associated with significantly higher 5-year survival rates, compared with those no regular screening (68% vs. 20%; P less than .0061).
In terms of surveillance modalities, the update suggested 6- to 12-monthly imaging of the biliary tree with ultrasound computed tomography, computed tomography, or magnetic resonance imaging – with or without serum carbohydrate antigen 19-9. However the authors wrote that MRI was often preferred to CT because of its superior sensitivity.
They advised against endoscopic retrograde cholangiopancreatography with brush cytology for routine surveillance because of procedural risks. On the other hand, they suggested this procedure, with or without fluorescence in situ hybridization analysis and/or cholangioscopy, could be used for investigation.
“In addition to ERCP [endoscopic retrograde cholangiopancreatography] with brushings, endoscopic ultrasound, intraductal ultrasonography, and cholangioscopy may be used to direct biopsy sampling,” they wrote. Symptoms such as increasing cholestatic biochemistry values, jaundice, fever, right upper quadrant pain, or pruritus should trigger evaluation for cholangiocarcinoma.
However the authors advised “great caution” with the use of fine-needle aspiration of perihilar biliary strictures in liver transplant candidates because of the risk of tumor seeding if the lesion turned out to be cholangiocarcinoma.
On the question of cholangiocarcinoma surveillance in pediatric patients and those with small-duct primary sclerosing cholangitis, the authors wrote that cholangiocarcinoma was so rare in these patients that routine cholangiocarcinoma surveillance was not required.
The clinical update also looked at the prevalence and risk factors for gallbladder cancer, which affects around 2% of individuals with primary sclerosing cholangitis. Two studies found gallbladder polyps in 10%-17% of patients, but the authors noted that “the optimal modality for diagnosis of gallbladder polyps in PSC remains unknown”.
“Because of the high risk of malignancy in gallbladder mass lesions and a 5-year survival rate of 5% to 10% for gallbladder cancer, patients should undergo annual US [ultrasound] screening,” they wrote.
They said the question of whether to perform a cholecystectomy in patients with gallbladder polyps should be guided by the size and growth of the polyps because there is an increased risk of gallbladder cancer in polyps larger than 8 mm and by the clinical status of the patient.
Finally, the update examined the issue of hepatocellular carcinoma in patients with primary sclerosing cholangitis, which – while rare – may increase with the presence of cirrhosis.
The authors advised that patients with primary sclerosing cholangitis and cirrhosis should undergo surveillance for hepatocellular carcinoma every 6 months with ultrasound, CT, or MRI.
“We anticipate that with the development of large patient cohorts, advances in uncovering genetic and other risk factors for cholangiocarcinoma, and development of effective treatments for PSC, further refinement of this practice update will be required.”
Two authors declared consultancies, grants and research contracts with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Bowlus C et al. Clin Gastroenterol Hepatol. 2019 Jul 12. doi 10.1016/j.cgh.2019.07.011.
All adult patients with primary sclerosing cholangitis should be screened at least annually for cholangiocarcinoma and gallbladder cancer, particularly in the first year after their diagnosis, according to a clinical practice update published in Clinical Gastroenterology and Hepatology.
Individuals with primary sclerosing cholangitis have a 400-fold higher risk of cholangiocarcinoma, compared with the general population, and around one-third of cancers are detected within 1 year of the cholangitis diagnosis, Christopher L. Bowlus, MD, of the University of California, Davis, and coauthors wrote.
The clinical practice update from the American Gastroenterological Association was in response to the observation that, while there is significant evidence for an increasing incidence of cirrhosis, hepatic decompensation, hepatocellular carcinoma, and liver transplant listing among patients with primary sclerosing cholangitis, there is a lack of good evidence to guide cholangiocarcinoma surveillance in these patients.
“The low prevalence and long duration of PSC [primary sclerosing cholangitis] present substantial barriers to better understanding risk stratification, developing biomarkers, and measuring the impact surveillance has on clinical outcomes,” they wrote.
The first recommendation was that surveillance for cholangiocarcinoma and gallbladder cancer should be considered in all adult patients with primary sclerosing cholangitis, regardless of their disease stage. The authors especially emphasized the importance of surveillance in the first year after a diagnosis of primary sclerosing cholangitis, in patients who also have ulcerative colitis, and in those diagnosed at an older age.
They cited one study that found regular surveillance of patients with primary sclerosing cholangitis was associated with significantly higher 5-year survival rates, compared with those no regular screening (68% vs. 20%; P less than .0061).
In terms of surveillance modalities, the update suggested 6- to 12-monthly imaging of the biliary tree with ultrasound computed tomography, computed tomography, or magnetic resonance imaging – with or without serum carbohydrate antigen 19-9. However the authors wrote that MRI was often preferred to CT because of its superior sensitivity.
They advised against endoscopic retrograde cholangiopancreatography with brush cytology for routine surveillance because of procedural risks. On the other hand, they suggested this procedure, with or without fluorescence in situ hybridization analysis and/or cholangioscopy, could be used for investigation.
“In addition to ERCP [endoscopic retrograde cholangiopancreatography] with brushings, endoscopic ultrasound, intraductal ultrasonography, and cholangioscopy may be used to direct biopsy sampling,” they wrote. Symptoms such as increasing cholestatic biochemistry values, jaundice, fever, right upper quadrant pain, or pruritus should trigger evaluation for cholangiocarcinoma.
However the authors advised “great caution” with the use of fine-needle aspiration of perihilar biliary strictures in liver transplant candidates because of the risk of tumor seeding if the lesion turned out to be cholangiocarcinoma.
On the question of cholangiocarcinoma surveillance in pediatric patients and those with small-duct primary sclerosing cholangitis, the authors wrote that cholangiocarcinoma was so rare in these patients that routine cholangiocarcinoma surveillance was not required.
The clinical update also looked at the prevalence and risk factors for gallbladder cancer, which affects around 2% of individuals with primary sclerosing cholangitis. Two studies found gallbladder polyps in 10%-17% of patients, but the authors noted that “the optimal modality for diagnosis of gallbladder polyps in PSC remains unknown”.
“Because of the high risk of malignancy in gallbladder mass lesions and a 5-year survival rate of 5% to 10% for gallbladder cancer, patients should undergo annual US [ultrasound] screening,” they wrote.
They said the question of whether to perform a cholecystectomy in patients with gallbladder polyps should be guided by the size and growth of the polyps because there is an increased risk of gallbladder cancer in polyps larger than 8 mm and by the clinical status of the patient.
Finally, the update examined the issue of hepatocellular carcinoma in patients with primary sclerosing cholangitis, which – while rare – may increase with the presence of cirrhosis.
The authors advised that patients with primary sclerosing cholangitis and cirrhosis should undergo surveillance for hepatocellular carcinoma every 6 months with ultrasound, CT, or MRI.
“We anticipate that with the development of large patient cohorts, advances in uncovering genetic and other risk factors for cholangiocarcinoma, and development of effective treatments for PSC, further refinement of this practice update will be required.”
Two authors declared consultancies, grants and research contracts with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Bowlus C et al. Clin Gastroenterol Hepatol. 2019 Jul 12. doi 10.1016/j.cgh.2019.07.011.
All adult patients with primary sclerosing cholangitis should be screened at least annually for cholangiocarcinoma and gallbladder cancer, particularly in the first year after their diagnosis, according to a clinical practice update published in Clinical Gastroenterology and Hepatology.
Individuals with primary sclerosing cholangitis have a 400-fold higher risk of cholangiocarcinoma, compared with the general population, and around one-third of cancers are detected within 1 year of the cholangitis diagnosis, Christopher L. Bowlus, MD, of the University of California, Davis, and coauthors wrote.
The clinical practice update from the American Gastroenterological Association was in response to the observation that, while there is significant evidence for an increasing incidence of cirrhosis, hepatic decompensation, hepatocellular carcinoma, and liver transplant listing among patients with primary sclerosing cholangitis, there is a lack of good evidence to guide cholangiocarcinoma surveillance in these patients.
“The low prevalence and long duration of PSC [primary sclerosing cholangitis] present substantial barriers to better understanding risk stratification, developing biomarkers, and measuring the impact surveillance has on clinical outcomes,” they wrote.
The first recommendation was that surveillance for cholangiocarcinoma and gallbladder cancer should be considered in all adult patients with primary sclerosing cholangitis, regardless of their disease stage. The authors especially emphasized the importance of surveillance in the first year after a diagnosis of primary sclerosing cholangitis, in patients who also have ulcerative colitis, and in those diagnosed at an older age.
They cited one study that found regular surveillance of patients with primary sclerosing cholangitis was associated with significantly higher 5-year survival rates, compared with those no regular screening (68% vs. 20%; P less than .0061).
In terms of surveillance modalities, the update suggested 6- to 12-monthly imaging of the biliary tree with ultrasound computed tomography, computed tomography, or magnetic resonance imaging – with or without serum carbohydrate antigen 19-9. However the authors wrote that MRI was often preferred to CT because of its superior sensitivity.
They advised against endoscopic retrograde cholangiopancreatography with brush cytology for routine surveillance because of procedural risks. On the other hand, they suggested this procedure, with or without fluorescence in situ hybridization analysis and/or cholangioscopy, could be used for investigation.
“In addition to ERCP [endoscopic retrograde cholangiopancreatography] with brushings, endoscopic ultrasound, intraductal ultrasonography, and cholangioscopy may be used to direct biopsy sampling,” they wrote. Symptoms such as increasing cholestatic biochemistry values, jaundice, fever, right upper quadrant pain, or pruritus should trigger evaluation for cholangiocarcinoma.
However the authors advised “great caution” with the use of fine-needle aspiration of perihilar biliary strictures in liver transplant candidates because of the risk of tumor seeding if the lesion turned out to be cholangiocarcinoma.
On the question of cholangiocarcinoma surveillance in pediatric patients and those with small-duct primary sclerosing cholangitis, the authors wrote that cholangiocarcinoma was so rare in these patients that routine cholangiocarcinoma surveillance was not required.
The clinical update also looked at the prevalence and risk factors for gallbladder cancer, which affects around 2% of individuals with primary sclerosing cholangitis. Two studies found gallbladder polyps in 10%-17% of patients, but the authors noted that “the optimal modality for diagnosis of gallbladder polyps in PSC remains unknown”.
“Because of the high risk of malignancy in gallbladder mass lesions and a 5-year survival rate of 5% to 10% for gallbladder cancer, patients should undergo annual US [ultrasound] screening,” they wrote.
They said the question of whether to perform a cholecystectomy in patients with gallbladder polyps should be guided by the size and growth of the polyps because there is an increased risk of gallbladder cancer in polyps larger than 8 mm and by the clinical status of the patient.
Finally, the update examined the issue of hepatocellular carcinoma in patients with primary sclerosing cholangitis, which – while rare – may increase with the presence of cirrhosis.
The authors advised that patients with primary sclerosing cholangitis and cirrhosis should undergo surveillance for hepatocellular carcinoma every 6 months with ultrasound, CT, or MRI.
“We anticipate that with the development of large patient cohorts, advances in uncovering genetic and other risk factors for cholangiocarcinoma, and development of effective treatments for PSC, further refinement of this practice update will be required.”
Two authors declared consultancies, grants and research contracts with the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Bowlus C et al. Clin Gastroenterol Hepatol. 2019 Jul 12. doi 10.1016/j.cgh.2019.07.011.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Fibrosis severity and cirrhosis drive patient-reported outcomes with NASH
Patients with nonalcoholic steatohepatitis (NASH) and advanced fibrosis reported lower quality of life that is worsened in those who develop cirrhosis, based on data from 1,667 individuals.
NASH is becoming an increasingly common cause of liver disease, cirrhosis, and hepatocellular carcinoma and can have a negative effect on patients’ quality of life and other patient-reported outcomes (PROs), but studies of the impact on PROs in these patients are limited, wrote Zobair M. Younossi, MD, of the Inova Health System, Falls Church, Va., and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers reviewed data from 870 adults with NASH cirrhosis and 797 with NASH and bridging fibrosis. The average age of the patients was 58 years, 73% were white, 40% were male, 52% had cirrhosis, and 74% had diabetes.
The researchers used four tools to assess quality of life: the SF-36 (36-Item Short Form Health Survey), the EQ-5D (Euroqol, a generic health questionnaire), the CLDQ-NASH (Chronic Liver Disease Questionnaire-NASH), and the WPAI:SHP (Work Productivity and Activity Impairment: Specific Health Problem).
The SF-36 score is based on eight domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health.
Overall, patients with NASH and cirrhosis had significantly lower scores on domains of the SF-36 that related to physical function, compared with bridging fibrosis patients (70.3 vs. 73.6), as well as role physical (71.6 vs. 75.4) and bodily pain (65.0 vs. 68.6). The other areas of significant impairment in NASH patients with cirrhosis, compared with NASH patients with fibrosis, appeared in four domains of the disease-specific CLDQ-NASH: activity, emotional, fatigue, and worry. In addition, the EQ-5D utility score was significantly lower in cirrhosis patients, compared with fibrosis patients.
Older age, male sex, Asian race, and U.S. location of study enrollment were independent predictors of higher PRO scores in a multivariate analysis, while black race, history of smoking, history of diabetes, higher body mass index, cirrhosis, and history of comorbidities that were gastrointestinal, musculoskeletal, psychiatric, or neurologic were independent predictors of lower PRO scores in patients with advanced fibrosis and NASH.
WPAI:SHP scores, which focused on work productivity impairment and absenteeism, were not significantly different between the groups.
The study findings were limited by several factors including the specific nature of the study population and absence of non-NASH controls, the potential of false positives because of the use of self-reports, and the lack of longitudinal data, the researchers said. The results should be verified in a larger, diverse patient population, the researchers noted, but the data highlight the impairment in quality of life and productivity among patients with NASH and “can inform patients, clinicians, payers, and policymakers about the total burden of the disease and also the comprehensive benefit of treatment,” they concluded.
The study was supported by Gilead Sciences. Dr. Younossi disclosed relationships with Gilead Sciences, as well as Intercept, NovoNordisk, BMS, Allergan/Tobira, Terns, Viking, AbbVie, Novartis, and Quest Diagnostics.
SOURCE: Younossi ZM et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.02.024.
Patients with nonalcoholic steatohepatitis (NASH) and advanced fibrosis reported lower quality of life that is worsened in those who develop cirrhosis, based on data from 1,667 individuals.
NASH is becoming an increasingly common cause of liver disease, cirrhosis, and hepatocellular carcinoma and can have a negative effect on patients’ quality of life and other patient-reported outcomes (PROs), but studies of the impact on PROs in these patients are limited, wrote Zobair M. Younossi, MD, of the Inova Health System, Falls Church, Va., and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers reviewed data from 870 adults with NASH cirrhosis and 797 with NASH and bridging fibrosis. The average age of the patients was 58 years, 73% were white, 40% were male, 52% had cirrhosis, and 74% had diabetes.
The researchers used four tools to assess quality of life: the SF-36 (36-Item Short Form Health Survey), the EQ-5D (Euroqol, a generic health questionnaire), the CLDQ-NASH (Chronic Liver Disease Questionnaire-NASH), and the WPAI:SHP (Work Productivity and Activity Impairment: Specific Health Problem).
The SF-36 score is based on eight domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health.
Overall, patients with NASH and cirrhosis had significantly lower scores on domains of the SF-36 that related to physical function, compared with bridging fibrosis patients (70.3 vs. 73.6), as well as role physical (71.6 vs. 75.4) and bodily pain (65.0 vs. 68.6). The other areas of significant impairment in NASH patients with cirrhosis, compared with NASH patients with fibrosis, appeared in four domains of the disease-specific CLDQ-NASH: activity, emotional, fatigue, and worry. In addition, the EQ-5D utility score was significantly lower in cirrhosis patients, compared with fibrosis patients.
Older age, male sex, Asian race, and U.S. location of study enrollment were independent predictors of higher PRO scores in a multivariate analysis, while black race, history of smoking, history of diabetes, higher body mass index, cirrhosis, and history of comorbidities that were gastrointestinal, musculoskeletal, psychiatric, or neurologic were independent predictors of lower PRO scores in patients with advanced fibrosis and NASH.
WPAI:SHP scores, which focused on work productivity impairment and absenteeism, were not significantly different between the groups.
The study findings were limited by several factors including the specific nature of the study population and absence of non-NASH controls, the potential of false positives because of the use of self-reports, and the lack of longitudinal data, the researchers said. The results should be verified in a larger, diverse patient population, the researchers noted, but the data highlight the impairment in quality of life and productivity among patients with NASH and “can inform patients, clinicians, payers, and policymakers about the total burden of the disease and also the comprehensive benefit of treatment,” they concluded.
The study was supported by Gilead Sciences. Dr. Younossi disclosed relationships with Gilead Sciences, as well as Intercept, NovoNordisk, BMS, Allergan/Tobira, Terns, Viking, AbbVie, Novartis, and Quest Diagnostics.
SOURCE: Younossi ZM et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.02.024.
Patients with nonalcoholic steatohepatitis (NASH) and advanced fibrosis reported lower quality of life that is worsened in those who develop cirrhosis, based on data from 1,667 individuals.
NASH is becoming an increasingly common cause of liver disease, cirrhosis, and hepatocellular carcinoma and can have a negative effect on patients’ quality of life and other patient-reported outcomes (PROs), but studies of the impact on PROs in these patients are limited, wrote Zobair M. Younossi, MD, of the Inova Health System, Falls Church, Va., and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers reviewed data from 870 adults with NASH cirrhosis and 797 with NASH and bridging fibrosis. The average age of the patients was 58 years, 73% were white, 40% were male, 52% had cirrhosis, and 74% had diabetes.
The researchers used four tools to assess quality of life: the SF-36 (36-Item Short Form Health Survey), the EQ-5D (Euroqol, a generic health questionnaire), the CLDQ-NASH (Chronic Liver Disease Questionnaire-NASH), and the WPAI:SHP (Work Productivity and Activity Impairment: Specific Health Problem).
The SF-36 score is based on eight domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health.
Overall, patients with NASH and cirrhosis had significantly lower scores on domains of the SF-36 that related to physical function, compared with bridging fibrosis patients (70.3 vs. 73.6), as well as role physical (71.6 vs. 75.4) and bodily pain (65.0 vs. 68.6). The other areas of significant impairment in NASH patients with cirrhosis, compared with NASH patients with fibrosis, appeared in four domains of the disease-specific CLDQ-NASH: activity, emotional, fatigue, and worry. In addition, the EQ-5D utility score was significantly lower in cirrhosis patients, compared with fibrosis patients.
Older age, male sex, Asian race, and U.S. location of study enrollment were independent predictors of higher PRO scores in a multivariate analysis, while black race, history of smoking, history of diabetes, higher body mass index, cirrhosis, and history of comorbidities that were gastrointestinal, musculoskeletal, psychiatric, or neurologic were independent predictors of lower PRO scores in patients with advanced fibrosis and NASH.
WPAI:SHP scores, which focused on work productivity impairment and absenteeism, were not significantly different between the groups.
The study findings were limited by several factors including the specific nature of the study population and absence of non-NASH controls, the potential of false positives because of the use of self-reports, and the lack of longitudinal data, the researchers said. The results should be verified in a larger, diverse patient population, the researchers noted, but the data highlight the impairment in quality of life and productivity among patients with NASH and “can inform patients, clinicians, payers, and policymakers about the total burden of the disease and also the comprehensive benefit of treatment,” they concluded.
The study was supported by Gilead Sciences. Dr. Younossi disclosed relationships with Gilead Sciences, as well as Intercept, NovoNordisk, BMS, Allergan/Tobira, Terns, Viking, AbbVie, Novartis, and Quest Diagnostics.
SOURCE: Younossi ZM et al. Clin Gastroenterol Hepatol. 2019. doi: 10.1016/j.cgh.2019.02.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Direct-acting antiviral therapy boosts survival for infected HCC patients
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
FROM GASTROENTEROLOGY
Increased levels of a bacterial strain may cause nonalcoholic fatty liver disease
A new study involving both human patients and mice has confirmed a long-believed association between nonalcoholic fatty liver disease (NAFLD) and an alteration in the gut microbiome that produces high levels of alcohol.
The study was initiated after the treatment of a rare case: a patient who presented with severe nonalcoholic steatohepatitis (NASH) plus auto-brewery syndrome. The patient had a very high blood alcohol concentration but an alcohol-free, high-carbohydrate diet. It was determined that strains of high alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) rather than a fungal infection were the catalyst for the high blood alcohol level. As such, Jing Yuan of the Capital Institute of Pediatrics in Beijing and coauthors attempted to “connect these commensal HiAlc Kpn to the pathogenesis of hepatic damage” through this study, which was published in Cell Metabolism.
The researchers began by examining 43 patients with NAFLD and 48 healthy controls. Among the patients with NAFLD, 11 had nonalcoholic fatty liver and 32 had NASH. Specifically, they analyzed the presence and effects of HiAlc Kpn, determining that the abundance of Klebsiella pneumoniae was slightly higher in the feces of NAFLD patients, compared with healthy patients, but that their alcohol-producing ability in NAFLD patients was significantly stronger. Of the patients with NAFLD, 61% carried HiAlc and medium-alcohol-producing Kpn, compared with 6.25% of the controls.
Another phase of their study involved feeding specific pathogen-free mice either HiAlc Kpn, ethanol, or yeast extract peptone dextrose medium (pair-fed) for 4, 6, and 8 weeks. The mice that were fed HiAlc Kpn or ethanol showed clear microsteatosis and macrosteatosis in their livers at 4 and 8 weeks, compared with the pair-fed mice. In addition, the HiAlc-Kpn-fed and ethanol-fed mice had increased levels of aspartate transaminase and alanine transaminase in their serum and increased levels of triglycerides and thiobarbituric acid reactive substances in their liver. The results overall indicated that the HiAlc-Kpn-fed mice had developed hepatic steatosis.
An additional phase included the intestinal flora from a NASH patient with a specific Kpn strain being fed to germ-free mice. At the same time, two types of intestinal flora from mice with NAFLD were transplanted into healthy mice: one induced by two other specific Kpn strains and one in which those strains had been selectively eliminated. The results saw obvious steatosis in the mice who received the flora from either mice with NAFLD induced by Kpn or the NASH patient at 4 weeks and 8 weeks, respectively. The mice who received the flora win which Kpn had been eliminated saw no fat-related changes in the liver. “These results further suggest that HiAlc Kpn might be one of the major causes of NAFLD development,” the researchers wrote.
The authors acknowledged the study’s limitations, chiefly including the lack of a clinical cohort of individuals with auto brewery syndrome but without NAFLD that could be used as a control. However, they also noted a belief that “causality was shown by the transfer experiments of HiAlc Kpn” while adding that “the further analysis of the impact of ethanol in ABS [auto brewery syndrome] patients should be investigated.”
The study was funded by grants from the National Natural Science Foundation for Key Programs of China, the National Natural Science Foundation of China, Megaprojects of Science and Technology Research of China, and CAMS Innovation Fund for Medical Sciences. The authors reported no conflicts of interest.
SOURCE: Yuan J et al. Cell Metab. 2019 Sep 19. doi: 10.1016/j.cmet.2019.08.018.
A new study involving both human patients and mice has confirmed a long-believed association between nonalcoholic fatty liver disease (NAFLD) and an alteration in the gut microbiome that produces high levels of alcohol.
The study was initiated after the treatment of a rare case: a patient who presented with severe nonalcoholic steatohepatitis (NASH) plus auto-brewery syndrome. The patient had a very high blood alcohol concentration but an alcohol-free, high-carbohydrate diet. It was determined that strains of high alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) rather than a fungal infection were the catalyst for the high blood alcohol level. As such, Jing Yuan of the Capital Institute of Pediatrics in Beijing and coauthors attempted to “connect these commensal HiAlc Kpn to the pathogenesis of hepatic damage” through this study, which was published in Cell Metabolism.
The researchers began by examining 43 patients with NAFLD and 48 healthy controls. Among the patients with NAFLD, 11 had nonalcoholic fatty liver and 32 had NASH. Specifically, they analyzed the presence and effects of HiAlc Kpn, determining that the abundance of Klebsiella pneumoniae was slightly higher in the feces of NAFLD patients, compared with healthy patients, but that their alcohol-producing ability in NAFLD patients was significantly stronger. Of the patients with NAFLD, 61% carried HiAlc and medium-alcohol-producing Kpn, compared with 6.25% of the controls.
Another phase of their study involved feeding specific pathogen-free mice either HiAlc Kpn, ethanol, or yeast extract peptone dextrose medium (pair-fed) for 4, 6, and 8 weeks. The mice that were fed HiAlc Kpn or ethanol showed clear microsteatosis and macrosteatosis in their livers at 4 and 8 weeks, compared with the pair-fed mice. In addition, the HiAlc-Kpn-fed and ethanol-fed mice had increased levels of aspartate transaminase and alanine transaminase in their serum and increased levels of triglycerides and thiobarbituric acid reactive substances in their liver. The results overall indicated that the HiAlc-Kpn-fed mice had developed hepatic steatosis.
An additional phase included the intestinal flora from a NASH patient with a specific Kpn strain being fed to germ-free mice. At the same time, two types of intestinal flora from mice with NAFLD were transplanted into healthy mice: one induced by two other specific Kpn strains and one in which those strains had been selectively eliminated. The results saw obvious steatosis in the mice who received the flora from either mice with NAFLD induced by Kpn or the NASH patient at 4 weeks and 8 weeks, respectively. The mice who received the flora win which Kpn had been eliminated saw no fat-related changes in the liver. “These results further suggest that HiAlc Kpn might be one of the major causes of NAFLD development,” the researchers wrote.
The authors acknowledged the study’s limitations, chiefly including the lack of a clinical cohort of individuals with auto brewery syndrome but without NAFLD that could be used as a control. However, they also noted a belief that “causality was shown by the transfer experiments of HiAlc Kpn” while adding that “the further analysis of the impact of ethanol in ABS [auto brewery syndrome] patients should be investigated.”
The study was funded by grants from the National Natural Science Foundation for Key Programs of China, the National Natural Science Foundation of China, Megaprojects of Science and Technology Research of China, and CAMS Innovation Fund for Medical Sciences. The authors reported no conflicts of interest.
SOURCE: Yuan J et al. Cell Metab. 2019 Sep 19. doi: 10.1016/j.cmet.2019.08.018.
A new study involving both human patients and mice has confirmed a long-believed association between nonalcoholic fatty liver disease (NAFLD) and an alteration in the gut microbiome that produces high levels of alcohol.
The study was initiated after the treatment of a rare case: a patient who presented with severe nonalcoholic steatohepatitis (NASH) plus auto-brewery syndrome. The patient had a very high blood alcohol concentration but an alcohol-free, high-carbohydrate diet. It was determined that strains of high alcohol-producing Klebsiella pneumoniae (HiAlc Kpn) rather than a fungal infection were the catalyst for the high blood alcohol level. As such, Jing Yuan of the Capital Institute of Pediatrics in Beijing and coauthors attempted to “connect these commensal HiAlc Kpn to the pathogenesis of hepatic damage” through this study, which was published in Cell Metabolism.
The researchers began by examining 43 patients with NAFLD and 48 healthy controls. Among the patients with NAFLD, 11 had nonalcoholic fatty liver and 32 had NASH. Specifically, they analyzed the presence and effects of HiAlc Kpn, determining that the abundance of Klebsiella pneumoniae was slightly higher in the feces of NAFLD patients, compared with healthy patients, but that their alcohol-producing ability in NAFLD patients was significantly stronger. Of the patients with NAFLD, 61% carried HiAlc and medium-alcohol-producing Kpn, compared with 6.25% of the controls.
Another phase of their study involved feeding specific pathogen-free mice either HiAlc Kpn, ethanol, or yeast extract peptone dextrose medium (pair-fed) for 4, 6, and 8 weeks. The mice that were fed HiAlc Kpn or ethanol showed clear microsteatosis and macrosteatosis in their livers at 4 and 8 weeks, compared with the pair-fed mice. In addition, the HiAlc-Kpn-fed and ethanol-fed mice had increased levels of aspartate transaminase and alanine transaminase in their serum and increased levels of triglycerides and thiobarbituric acid reactive substances in their liver. The results overall indicated that the HiAlc-Kpn-fed mice had developed hepatic steatosis.
An additional phase included the intestinal flora from a NASH patient with a specific Kpn strain being fed to germ-free mice. At the same time, two types of intestinal flora from mice with NAFLD were transplanted into healthy mice: one induced by two other specific Kpn strains and one in which those strains had been selectively eliminated. The results saw obvious steatosis in the mice who received the flora from either mice with NAFLD induced by Kpn or the NASH patient at 4 weeks and 8 weeks, respectively. The mice who received the flora win which Kpn had been eliminated saw no fat-related changes in the liver. “These results further suggest that HiAlc Kpn might be one of the major causes of NAFLD development,” the researchers wrote.
The authors acknowledged the study’s limitations, chiefly including the lack of a clinical cohort of individuals with auto brewery syndrome but without NAFLD that could be used as a control. However, they also noted a belief that “causality was shown by the transfer experiments of HiAlc Kpn” while adding that “the further analysis of the impact of ethanol in ABS [auto brewery syndrome] patients should be investigated.”
The study was funded by grants from the National Natural Science Foundation for Key Programs of China, the National Natural Science Foundation of China, Megaprojects of Science and Technology Research of China, and CAMS Innovation Fund for Medical Sciences. The authors reported no conflicts of interest.
SOURCE: Yuan J et al. Cell Metab. 2019 Sep 19. doi: 10.1016/j.cmet.2019.08.018.
FROM CELL METABOLISM
Key clinical point: Nonalcoholic fatty liver disease can be caused or exacerbated by excess levels of a high-alcohol-producing bacterial strain.
Major finding: 61% of patients with NAFLD carried high alcohol- and medium alcohol-producing Klebsiella pneumoniae (HiAlc Kpn), compared to 6.25% of healthy controls.
Study details: A multiphase study that included analysis of a 43-patient cohort with nonalcoholic fatty liver disease as well as experiments with mice and HiAlc Kpn.
Disclosures: The study was funded by grants from the National Natural Science Foundation for Key Programs of China, the National Natural Science Foundation of China, Megaprojects of Science and Technology Research of China, and CAMS Innovation Fund for Medical Sciences. The authors reported no conflicts of interest.
Source: Yuan J et al. Cell Metab. 2019 Sep 19. doi: 10.1016/j.cmet.2019.08.018.
Three factors predict 6-month mortality in patients with DILI
Medical comorbidity burden is significantly associated with 6-month and overall mortality in individuals with suspected drug-induced liver injury (DILI). In addition,
Those are key findings from a study which set out to investigate the association between comorbidity burden and outcomes of patients with DILI and to develop a model to calculate risk of death within 6 months.
“Drug-induced liver injury is an important cause of liver-related morbidity and mortality that is likely under-recognized,” investigators led by Marwan S. Ghabril, MD, of the division of gastroenterology and hepatology at Indiana University, Indianapolis, wrote in a study published in Gastroenterology. “Its diagnosis depends on high index of suspicion, compatible temporal relationship, and thorough exclusion of competing etiologies. DILI by an implicated drug commonly occurs in patients with one or several comorbid conditions such as hypertension, diabetes mellitus, cardiovascular disease, renal disease, and malignancy. However, the impact of comorbidity burden on mortality in patients with suspected DILI has not been previously investigated.”
For the current analysis and model development, the researchers drew from 306 patients enrolled in the multicenter Drug-Induced Liver Injury Network Prospective Study at Indiana University between 2003 and 2017 (discovery cohort; Drug Saf. 2009;32:55-68). To validate their model, they used data from 247 patients who were enrolled in the same study at the University of North Carolina (validation cohort). The primary outcome of interest was mortality within 6 months of onset of liver injury.
The mean ages of the discovery and validation cohorts were 49 years and 51 years, respectively. Dr. Ghabril and colleagues found that 6-month mortality was 8.5% in the discovery cohort and 4.5% in the validation cohort. “The most common class of implicated agent was antimicrobials with no significant differences between groups,” they wrote. “However, herbal and dietary supplements were predominantly implicated in patients with none to mild comorbidity, while cardiovascular agents were predominantly implicated in patients with significant comorbidity.”
Among patients in the discovery cohort, the presence of significant comorbidities, defined as a Charlson Comorbidity Index score greater than 2, was independently associated with 6-month mortality (odds ratio, 5.22), as was model for end-stage liver disease score (OR, 1.11) and serum level of albumin at presentation (OR, 0.39). When the researchers created a morbidity risk model based on those three clinical variables, it performed well, identifying patients who died within 6 months with a C statistic value of 0.89 in the discovery cohort and 0.91 in the validation cohort. This spurred the development of a web-based risk calculator, which clinicians can access at http://gihep.com/calculators/hepatology/dili-cam/.
“Since DILI is not a unique cause of liver injury, it is conceivable that models incorporating comorbidity burden and severity of liver injury could prove useful in improving the prediction of mortality in a variety of liver injuries and diseases, and as such warrants further studies,” the researchers wrote.
The study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ghabril reported having no financial disclosures, but two coauthors reported having numerous financial ties to industry.
SOURCE: Ghabril M et al. Gastroenterology. 2019 Jul 11. doi: 10/1053/j.gastro.2019.07.006.
Medical comorbidity burden is significantly associated with 6-month and overall mortality in individuals with suspected drug-induced liver injury (DILI). In addition,
Those are key findings from a study which set out to investigate the association between comorbidity burden and outcomes of patients with DILI and to develop a model to calculate risk of death within 6 months.
“Drug-induced liver injury is an important cause of liver-related morbidity and mortality that is likely under-recognized,” investigators led by Marwan S. Ghabril, MD, of the division of gastroenterology and hepatology at Indiana University, Indianapolis, wrote in a study published in Gastroenterology. “Its diagnosis depends on high index of suspicion, compatible temporal relationship, and thorough exclusion of competing etiologies. DILI by an implicated drug commonly occurs in patients with one or several comorbid conditions such as hypertension, diabetes mellitus, cardiovascular disease, renal disease, and malignancy. However, the impact of comorbidity burden on mortality in patients with suspected DILI has not been previously investigated.”
For the current analysis and model development, the researchers drew from 306 patients enrolled in the multicenter Drug-Induced Liver Injury Network Prospective Study at Indiana University between 2003 and 2017 (discovery cohort; Drug Saf. 2009;32:55-68). To validate their model, they used data from 247 patients who were enrolled in the same study at the University of North Carolina (validation cohort). The primary outcome of interest was mortality within 6 months of onset of liver injury.
The mean ages of the discovery and validation cohorts were 49 years and 51 years, respectively. Dr. Ghabril and colleagues found that 6-month mortality was 8.5% in the discovery cohort and 4.5% in the validation cohort. “The most common class of implicated agent was antimicrobials with no significant differences between groups,” they wrote. “However, herbal and dietary supplements were predominantly implicated in patients with none to mild comorbidity, while cardiovascular agents were predominantly implicated in patients with significant comorbidity.”
Among patients in the discovery cohort, the presence of significant comorbidities, defined as a Charlson Comorbidity Index score greater than 2, was independently associated with 6-month mortality (odds ratio, 5.22), as was model for end-stage liver disease score (OR, 1.11) and serum level of albumin at presentation (OR, 0.39). When the researchers created a morbidity risk model based on those three clinical variables, it performed well, identifying patients who died within 6 months with a C statistic value of 0.89 in the discovery cohort and 0.91 in the validation cohort. This spurred the development of a web-based risk calculator, which clinicians can access at http://gihep.com/calculators/hepatology/dili-cam/.
“Since DILI is not a unique cause of liver injury, it is conceivable that models incorporating comorbidity burden and severity of liver injury could prove useful in improving the prediction of mortality in a variety of liver injuries and diseases, and as such warrants further studies,” the researchers wrote.
The study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ghabril reported having no financial disclosures, but two coauthors reported having numerous financial ties to industry.
SOURCE: Ghabril M et al. Gastroenterology. 2019 Jul 11. doi: 10/1053/j.gastro.2019.07.006.
Medical comorbidity burden is significantly associated with 6-month and overall mortality in individuals with suspected drug-induced liver injury (DILI). In addition,
Those are key findings from a study which set out to investigate the association between comorbidity burden and outcomes of patients with DILI and to develop a model to calculate risk of death within 6 months.
“Drug-induced liver injury is an important cause of liver-related morbidity and mortality that is likely under-recognized,” investigators led by Marwan S. Ghabril, MD, of the division of gastroenterology and hepatology at Indiana University, Indianapolis, wrote in a study published in Gastroenterology. “Its diagnosis depends on high index of suspicion, compatible temporal relationship, and thorough exclusion of competing etiologies. DILI by an implicated drug commonly occurs in patients with one or several comorbid conditions such as hypertension, diabetes mellitus, cardiovascular disease, renal disease, and malignancy. However, the impact of comorbidity burden on mortality in patients with suspected DILI has not been previously investigated.”
For the current analysis and model development, the researchers drew from 306 patients enrolled in the multicenter Drug-Induced Liver Injury Network Prospective Study at Indiana University between 2003 and 2017 (discovery cohort; Drug Saf. 2009;32:55-68). To validate their model, they used data from 247 patients who were enrolled in the same study at the University of North Carolina (validation cohort). The primary outcome of interest was mortality within 6 months of onset of liver injury.
The mean ages of the discovery and validation cohorts were 49 years and 51 years, respectively. Dr. Ghabril and colleagues found that 6-month mortality was 8.5% in the discovery cohort and 4.5% in the validation cohort. “The most common class of implicated agent was antimicrobials with no significant differences between groups,” they wrote. “However, herbal and dietary supplements were predominantly implicated in patients with none to mild comorbidity, while cardiovascular agents were predominantly implicated in patients with significant comorbidity.”
Among patients in the discovery cohort, the presence of significant comorbidities, defined as a Charlson Comorbidity Index score greater than 2, was independently associated with 6-month mortality (odds ratio, 5.22), as was model for end-stage liver disease score (OR, 1.11) and serum level of albumin at presentation (OR, 0.39). When the researchers created a morbidity risk model based on those three clinical variables, it performed well, identifying patients who died within 6 months with a C statistic value of 0.89 in the discovery cohort and 0.91 in the validation cohort. This spurred the development of a web-based risk calculator, which clinicians can access at http://gihep.com/calculators/hepatology/dili-cam/.
“Since DILI is not a unique cause of liver injury, it is conceivable that models incorporating comorbidity burden and severity of liver injury could prove useful in improving the prediction of mortality in a variety of liver injuries and diseases, and as such warrants further studies,” the researchers wrote.
The study was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Ghabril reported having no financial disclosures, but two coauthors reported having numerous financial ties to industry.
SOURCE: Ghabril M et al. Gastroenterology. 2019 Jul 11. doi: 10/1053/j.gastro.2019.07.006.
FROM GASTROENTEROLOGY