User login
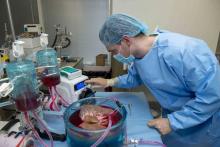
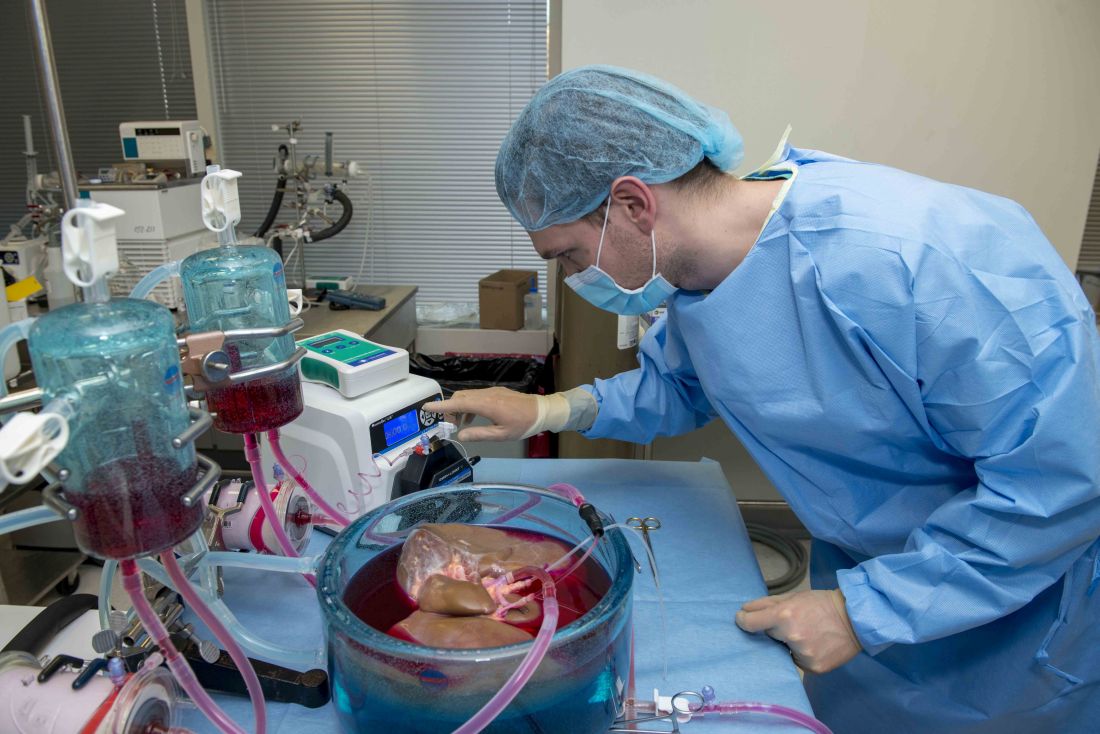
Supercooling extends donor liver viability by 27 hours
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
Standard cooling to 4°C provides just 12 hours of organ preservation, but laboratory testing showed that supercooling to –4°C added 27 hours of viability, reported lead author Reinier J. de Vries, MD, of Harvard Medical School and Massachusetts General Hospital in Boston, and colleagues.
“The absence of technology to preserve organs for more than a few hours is one of the fundamental causes of the donor organ–shortage crisis,” the investigators wrote in Nature Biotechnology.
Supercooling organs to high-subzero temperatures has been shown to prolong organ life while avoiding ice-mediated injury, but techniques that are successful for rat livers have been difficult to translate to human livers because of their larger size, which increases the risk of ice formation, the investigators explained.
Three strategies were employed to overcome this problem: minimization of air-liquid interfaces, development of a new supercooling-preservation solution, and hypothermic machine perfusion to more evenly distribute preservation solution throughout the liver tissue. For recovery of organs after supercooling, the investigators used subnormothermic machine perfusion, which has been used effectively in rat transplants.
In order to measure the impact of this process on organ viability, the investigators first measured adenylate energy content, both before supercooling and after recovery.
“Adenylate energy content, and, particularly, the organ’s ability to recover it during (re)perfusion, is considered the most representative metric for liver viability,” they wrote.
The difference between pre- and postsupercooling energy charge was less than 20%; in comparison, failed liver transplants in large animals and clinical trials have typically involved an energy-charge loss of 40% or more.
To further test organ viability, the investigators measured pre- and postsupercooling levels of bile production, oxygen uptake, and vascular resistance. All of these parameters have been shown to predict transplant success in rats, and bile production has additional precedent from human studies.
On average, bile production, portal resistance, and arterial resistance were not significantly affected by supercooling. Although portal vein resistance was 20% higher after supercooling, this compared favorably with increases of 100%-150% that have been measured in nonviable livers. Similarly, oxygen uptake increased by a mean of 17%, but this was three times lower than changes that have been observed in livers with impaired viability, at 51%.
Additional measures of hepatocellular injury, including AST and ALT, were also supportive of viability after supercooling. Histopathology confirmed these findings by showing preserved tissue architecture.
“In summary, we find that the human livers tested displayed no substantial difference in viability before and after extended subzero supercooling preservation,” the investigators wrote.
To simulate transplantation, the investigators reperfused the organs with blood at a normal temperature, including platelets, complement, and white blood cells, which are drivers of ischemia reperfusion injury. During this process, energy charge remained stable, which indicates preserved mitochondrial function. While energy charge held steady, lactate metabolism increased with bile and urea production, suggesting increased liver function. Bile pH and HCO3– levels fell within range for viability. Although bile glucose exceeded proposed criteria, the investigators pointed out that levels still fell within parameters for research-quality livers. Lactate levels also rose within the first hour of reperfusion, but the investigators suggested that this finding should be interpreted with appropriate context.
“It should be considered that the livers in this study were initially rejected for transplantation,” they wrote, “and the confidence intervals of the lactate concentration at the end of reperfusion largely overlap with time-matched values reported by others during [normothermic machine perfusion] of rejected human livers.”
Hepatocellular injury and histology also were evaluated during and after simulated transplantation, respectively, with favorable results. Although sites of preexisting hepatic injury were aggravated by the process, and rates of apoptosis increased, the investigators considered these changes were clinically insignificant.
Looking to the future, the investigators suggested that further refinement of the process could facilitate even-lower storage temperatures while better preserving liver viability.
“The use of human livers makes this study clinically relevant and promotes the translation of subzero organ preservation to the clinic,” the investigators concluded. “However, long-term survival experiments of transplanted supercooled livers in swine or an alternative large animal model will be needed before clinical translation.”
The study was funded by the National Institutes of Health and the Department of Defense. Dr. de Vries and four other coauthors have provisional patent applications related to the study, and one coauthor disclosed a financial relationship with Organ Solutions.
SOURCE: de Vries RJ et al. Nature Biotechnol. 2019 Sep 9. doi: 10.1038/s41587-019-0223-y.
FROM NATURE BIOTECHNOLOGY
Patients with viral hepatitis are living longer, increasing risk of extrahepatic mortality
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Chronic liver disease is one of the leading causes of death in the United States. Whereas mortality from other causes (e.g., heart disease and cancer) has declined, age-adjusted mortality from chronic liver disease has continued to increase. There have been a few major advances in the treatment of several chronic liver diseases in recent years. These include nucleos(t)ide analogues for hepatitis B virus (HBV) and direct-acting antiviral agents for the treatment of hepatitis C virus infection (HCV). Many studies show that these treatments are highly effective in improving patient outcomes, including patient survival. However, whether these individual-level benefits have translated into population-level improvements remains unclear.
Overall, the results were mixed; they were encouraging for viral hepatitis but concerning for alcoholic and nonalcoholic liver disease. Specifically, all-cause mortality from HCV was on an upward trajectory in the first 7 years (from 2007 to 2014) but the trend shifted from 2014 onward. Importantly, this inflection point coincided with the timing of the new HCV treatments. Most of this positive shift post 2014 was related to a strong downward trend in liver-related mortality. In contrast, upward trends in mortality related to extrahepatic causes (such as cardiovascular mortality) continued unabated. The authors found similar results for HBV. The story, however, was different for alcohol and nonalcohol-related liver disease – both conditions lacking effective treatments; liver-related mortality for both continued to increase during the study period.
Although we cannot make causal inferences from this study, overall, the results are good news. They suggest that HBV and HCV treatments have reached enough infected people to result in tangible improvements in the burden of chronic liver disease. We may now need to shift the focus of secondary prevention efforts from liver to nonliver (extrahepatic) morbidity in the newer cohorts of patients with treated HCV and HBV.
Fasiha Kanwal, MD, MSHS, is an investigator in the clinical epidemiology and comparative effectiveness program for the Center for Innovations in Quality, Effectiveness, and Safety in collaboration with the Michael E. DeBakey VA Medical Center, as well as an associate professor of medicine in gastroenterology and hepatology at Baylor College of Medicine in Houston. She has no conflicts of interest.
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
Patients with viral hepatitis may live longer after treatment with direct-acting antiviral agents (DAAs), but their risk of extrahepatic causes of death may rise as a result, according to investigators.
Importantly, this increasing rate of extrahepatic mortality shouldn’t be seen as a causal link with DAA use, cautioned lead author Donghee Kim, MD, PhD, of Stanford (Calif.) University, and colleagues. Instead, the upward trend is more likely because of successful treatment with DAAs, which can increase lifespan, and with it, time for susceptibility to extrahepatic conditions.
This was just one finding from a retrospective study that used U.S. Census and National Center for Health Statistics mortality records to evaluate almost 28 million deaths that occurred between 2007 and 2017. The investigators looked for mortality trends among patients with common chronic liver diseases, including viral hepatitis, alcoholic liver disease (ALD), and nonalcoholic fatty liver disease (NAFLD), noting that each of these conditions is associated with extrahepatic complications. The study included deaths due to extrahepatic cancer, cardiovascular disease, and diabetes.
While the efficacy of therapy for viral hepatitis has improved markedly since 2014, treatments for ALD and NAFLD have remained static, the investigators noted.
“Unfortunately, there have been no significant breakthroughs in the treatment of [ALD] over the last 2 decades, resulting in an increase in estimated global mortality to 3.8%,” the investigators wrote in Gastroenterology.
“[NAFLD] is the most common chronic liver disease in the world,” they added. “The leading cause of death in individuals with NAFLD is cardiovascular disease, followed by extrahepatic malignancies, and then liver-related mortality. However, recent trends in ALD and NAFLD-related extrahepatic complications in comparison to viral hepatitis have not been studied.”
The results of the current study supported the positive impact of DAAs, which began to see widespread use in 2014. Age-standardized mortality among patients with hepatitis C virus rose until 2014 (2.2% per year) and dropped thereafter (–6.5% per year). Mortality among those with hepatitis B virus steadily decreased over the study period (–1.2% per year).
Of note, while deaths because of HCV-related liver disease dropped from 2014 to 2017, extrahepatic causes of death didn’t follow suit. Age-standardized mortality for cardiovascular disease and diabetes increased at average annual rates of 1.9% and 3.3%, respectively, while the rate of extrahepatic cancer-related deaths held steady.
“The widespread use, higher efficacy and durable response to DAA agents in individuals with HCV infection may have resulted in a paradigm shift in the clinical progression of coexisting disease entities following response to DAA agents in the virus-free environment,” the investigators wrote. “These findings suggest assessment and identification of risk and risk factors for extrahepatic cancer, cardiovascular disease, and diabetes in individuals who have been successfully treated and cured of HCV infection.”
In sharp contrast with the viral hepatitis findings, mortality rates among patients with ALD and NAFLD increased at an accelerating rate over the 11-year study period.
Among patients with ALD, all-cause mortality increased by an average of 3.4% per year, at a higher rate in the second half of the study than the first (4.6% vs 2.1%). Liver disease–related mortality rose at a similar, accelerating rate. In the same group, deaths due to cardiovascular disease increased at an average annual rate of 2.1%, which was accelerating, while extrahepatic cancer-related deaths increased at a more constant rate of 3.6%.
For patients with NAFLD, all-cause mortality increased by 8.1% per year, accelerating from 6.1% in the first half of the study to 11.2% in the second. Deaths from liver disease increased at an average rate of 12.6% per year, while extrahepatic deaths increased significantly for all three included types: cardiovascular disease (2.0%), extrahepatic cancer (15.1%), and diabetes (9.7%).
Concerning the worsening rates of mortality among patients with ALD and NAFLD, the investigators cited a lack of progress in treatments, and suggested that “the quest for newer therapies must remain the cornerstone in our efforts.”
The investigators reported no external funding or conflicts of interest.
SOURCE: Kim D et al. Gastroenterology. 2019 Jun 25. doi: 10.1053/j.gastro.2019.06.026.
FROM GASTROENTEROLOGY
Type of renal dysfunction affects liver cirrhosis mortality risk
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
Cirrhotic patients with renal failure have a sevenfold increase in mortality compared with those without renal failure. Acute kidney injury (AKI) is common in cirrhosis; increasingly, cirrhotic patients awaiting liver transplantation have or are also at risk for CKD. They are sicker, older, and have more comorbidities such as obesity and diabetes. In this study, the cumulative incidence of death on the wait list was much more pronounced for any form of AKI, with those with AKI on CKD having the highest cumulative incidence of wait list mortality compared with those with normal renal function. The study notably raises several important issues. First, AKI exerts a greater influence in risk of mortality on CKD than it does on those with normal renal function. This is relevant given the increasing prevalence of CKD in this population. Second, it emphasizes the need to effectively measure renal function. All serum creatinine-based equations overestimate glomerular filtration rate in the presence of renal dysfunction. Finally, the study highlights the importance of extrahepatic factors in determining mortality on the wait list. While in all comers, a mathematical model such as the MELDNa score may be able to predict mortality, for a specific patient the presence of comorbid conditions, malnutrition and sarcopenia, infections, critical illness, and now pattern of renal dysfunction, may all play a role.
Sumeet K. Asrani, MD, MSc, is a hepatologist affiliated with Baylor University Medical Center, Dallas. He has no conflicts of interest.
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
For non–status 1 patients with cirrhosis who are awaiting liver transplantation, type of renal dysfunction may be a key determinant of mortality risk, based on a retrospective analysis of more than 22,000 patients.
Risk of death was greatest for patients with acute on chronic kidney disease (AKI on CKD), followed by AKI alone, then CKD alone, reported lead author Giuseppe Cullaro, MD, of the University of California, San Francisco, and colleagues.
Although it is well known that renal dysfunction worsens outcomes among patients with liver cirrhosis, the impact of different types of kidney pathology on mortality risk has been minimally researched, the investigators wrote in Clinical Gastroenterology and Hepatology. “To date, studies evaluating the impact of renal dysfunction on prognosis in patients with cirrhosis have mostly focused on AKI.”
To learn more, the investigators performed a retrospective study involving acute, chronic, and acute on chronic kidney disease among patients with cirrhosis. They included data from 22,680 non–status 1 adults who were awaiting liver transplantation between 2007 and 2014, with at least 90 days on the wait list. Information was gathered from the Organ Procurement and Transplantation Network registry.
AKI was defined by fewer than 72 days of hemodialysis, or an increase in creatinine of at least 0.3 mg/dL or at least 50% in the last 7 days. CKD was identified by more than 72 days of hemodialysis, or an estimated glomerular filtration rate less than 60 mL/min/1.73 m2 for 90 days with a final rate of at least 30 mL/min/1.73 m2. Using these criteria, the researchers put patients into four possible categories: AKI on CKD, AKI, CKD, or normal renal function. The primary outcome was wait list mortality, which was defined as death, or removal from the wait list for illness. Follow-up started at the time of addition to the wait list and continued until transplant, removal from the wait list, or death.
Multivariate analysis, which accounted for final MELD-Na score and other confounders, showed that patients with AKI on CKD fared worst, with a 2.86-fold higher mortality risk (subhazard [SHR] ratio, 2.86) than that of patients with normal renal function. The mortality risk for acute on chronic kidney disease was followed closely by patients with AKI alone (SHR, 2.42), and more distantly by patients with CKD alone (SHR, 1.56). Further analysis showed that the disparity between mortality risks of each subgroup became more pronounced with increased MELD-Na score. In addition, evaluation of receiver operating characteristic curves for 6-month wait list mortality showed that the addition of renal function to MELD-Na score increased the accuracy of prognosis from an area under the curve of 0.71 to 0.80 (P less than .001).
“This suggests that incorporating the pattern of renal function could provide an opportunity to better prognosticate risk of mortality in the patients with cirrhosis who are the sickest,” the investigators concluded.
They also speculated about why outcomes may vary by type of kidney dysfunction.
“We suspect that those patients who experience AKI and AKI on CKD in our cohort likely had a triggering event – infection, bleeding, hypovolemia – that put these patients at greater risk for waitlist mortality,” the investigators wrote. “These events inherently carry more risk than stable nonliver-related elevations in serum creatinine that are seen in patients with CKD. Because of this heterogeneity of etiology in renal dysfunction in patients with cirrhosis, it is perhaps not surprising that unique renal function patterns variably impact mortality.”
The investigators noted that the findings from the study have “important implications for clinical practice,” and suggested that including type of renal dysfunction would have the most significant affect on accuracy of prognoses among patients at greatest risk of mortality.
The study was funded by a Paul B. Beeson Career Development Award and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Verna disclosed relationships with Salix, Merck, and Gilead.
SOURCE: Cullaro et al. Clin Gastroenterol Hepatol. 2019 Feb 1. doi: 10.1016/j.cgh.2019.01.043.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Some HCV medications associated with serious liver injury
Many of the affected patients had signs or symptoms of moderate to severe liver impairment (Child-Pugh class B or C), and given that these medications – glecaprevir/pibrentasvir (Mavyret), elbasvir/grazoprevir (Zepatier), and sofosbuvir/velpatasvir/voxilaprevir (Vosevi) – are not indicated for such patients, they should not have been prescribed in the first place, the FDA noted in the drug safety communication. Some cases had other preexisting risk factors, such as liver cancer, alcohol abuse, or serious medical illnesses associated with liver problems.
In most cases, impairment or decompensation occurred within the first 4 weeks of starting treatment, and symptoms resolved or new-onset worsening of liver function improved after stopping. These medicines have been widely used and, among patients with no or mild liver impairment, have been shown to be safe and effective.
Health care professionals should continue prescribing these medicines as indicated; they should assess patients at baseline for severity of liver disease and other risk factors and closely monitor these patients after for signs and symptoms of worsening liver function. Patients should be aware that the risk of injury is rare and continue taking prescribed medicines; if they develop fatigue, weakness, loss of appetite, nausea and vomiting, yellow eyes or skin, or light-colored stools, they should talk with their health care professional but should continue taking the medications in question until instructed to do otherwise.
The full communication is available on the FDA website and includes more facts about these drugs and information for patients and health care professionals.
Many of the affected patients had signs or symptoms of moderate to severe liver impairment (Child-Pugh class B or C), and given that these medications – glecaprevir/pibrentasvir (Mavyret), elbasvir/grazoprevir (Zepatier), and sofosbuvir/velpatasvir/voxilaprevir (Vosevi) – are not indicated for such patients, they should not have been prescribed in the first place, the FDA noted in the drug safety communication. Some cases had other preexisting risk factors, such as liver cancer, alcohol abuse, or serious medical illnesses associated with liver problems.
In most cases, impairment or decompensation occurred within the first 4 weeks of starting treatment, and symptoms resolved or new-onset worsening of liver function improved after stopping. These medicines have been widely used and, among patients with no or mild liver impairment, have been shown to be safe and effective.
Health care professionals should continue prescribing these medicines as indicated; they should assess patients at baseline for severity of liver disease and other risk factors and closely monitor these patients after for signs and symptoms of worsening liver function. Patients should be aware that the risk of injury is rare and continue taking prescribed medicines; if they develop fatigue, weakness, loss of appetite, nausea and vomiting, yellow eyes or skin, or light-colored stools, they should talk with their health care professional but should continue taking the medications in question until instructed to do otherwise.
The full communication is available on the FDA website and includes more facts about these drugs and information for patients and health care professionals.
Many of the affected patients had signs or symptoms of moderate to severe liver impairment (Child-Pugh class B or C), and given that these medications – glecaprevir/pibrentasvir (Mavyret), elbasvir/grazoprevir (Zepatier), and sofosbuvir/velpatasvir/voxilaprevir (Vosevi) – are not indicated for such patients, they should not have been prescribed in the first place, the FDA noted in the drug safety communication. Some cases had other preexisting risk factors, such as liver cancer, alcohol abuse, or serious medical illnesses associated with liver problems.
In most cases, impairment or decompensation occurred within the first 4 weeks of starting treatment, and symptoms resolved or new-onset worsening of liver function improved after stopping. These medicines have been widely used and, among patients with no or mild liver impairment, have been shown to be safe and effective.
Health care professionals should continue prescribing these medicines as indicated; they should assess patients at baseline for severity of liver disease and other risk factors and closely monitor these patients after for signs and symptoms of worsening liver function. Patients should be aware that the risk of injury is rare and continue taking prescribed medicines; if they develop fatigue, weakness, loss of appetite, nausea and vomiting, yellow eyes or skin, or light-colored stools, they should talk with their health care professional but should continue taking the medications in question until instructed to do otherwise.
The full communication is available on the FDA website and includes more facts about these drugs and information for patients and health care professionals.
USPSTF issues draft recommendation statement for HCV screening in adults
and now suggests that all adults aged 18-79 years receive screening.
This proposal represents an update and expansion of its current recommendation for screening for HCV infection. The draft recommendation incorporates new evidence and would replace the recommendation made in 2013, which calls for screening in persons at high risk for infection and one-time screening in adults born between 1945 and 1965 (Grade B).
“Today, more people are infected with hepatitis C than there were a decade ago, but there are now better treatments available. The evidence now shows more people can benefit from screening; therefore, we are recommending to screen all adults ages 18-79 for hepatitis C,” task force chair Douglas K. Owens, MD, MS, said in a bulletin issued by the USPSTF.
To update the previous recommendation, the USPSTF conducted a systematic review that included a total of 97 studies. No direct evidence was found regarding the benefits of HCV screening versus no screening or repeat versus one-time screening, and no new studies analyzed the psychological and social consequences of HCV screening.
Evidence concerning direct-acting antiviral (DAA) treatment was more compelling given that 49 trials found DAA therapy to be associated with pooled sustained virologic response (SVR) rates between 95.5% and 98.9% across genotypes. The rate of serious adverse events caused by DAA treatment was 1.9%, and the discontinuation of treatment because of adverse events was 0.4%. In seven trials involving adolescents, SVR rates after antiviral treatment were similar to those in adults.
Achieving an SVR after DAA treatment was associated with a decreased risk in those treated of all-cause mortality (hazard ratio, 0.40; 95% confidence interval, 0.28-0.56), liver mortality (HR, 0.11; 95% CI, 0.04-0.27), cirrhosis (HR, 0.36; 95% CI, 0.33-0.40), and hepatocellular carcinoma (HR, 0.29; 95% CI, 0.23-0.38), compared with those who did not respond.
Because of the evidence collected, the USPSTF issued a B recommendation for HCV screening in adults and recommended screening for all people aged 18-79 years in the draft recommendation statement. “Clinicians may want to consider screening in adolescents younger than age 18 years and in adults older than age 79 years who are at high risk [for HCV],” the proposal says.
The draft recommendation statement and evidence review is available at www.uspreventiveservicestaskforce.org. The public comment period will last until Sept. 23, 2019.
and now suggests that all adults aged 18-79 years receive screening.
This proposal represents an update and expansion of its current recommendation for screening for HCV infection. The draft recommendation incorporates new evidence and would replace the recommendation made in 2013, which calls for screening in persons at high risk for infection and one-time screening in adults born between 1945 and 1965 (Grade B).
“Today, more people are infected with hepatitis C than there were a decade ago, but there are now better treatments available. The evidence now shows more people can benefit from screening; therefore, we are recommending to screen all adults ages 18-79 for hepatitis C,” task force chair Douglas K. Owens, MD, MS, said in a bulletin issued by the USPSTF.
To update the previous recommendation, the USPSTF conducted a systematic review that included a total of 97 studies. No direct evidence was found regarding the benefits of HCV screening versus no screening or repeat versus one-time screening, and no new studies analyzed the psychological and social consequences of HCV screening.
Evidence concerning direct-acting antiviral (DAA) treatment was more compelling given that 49 trials found DAA therapy to be associated with pooled sustained virologic response (SVR) rates between 95.5% and 98.9% across genotypes. The rate of serious adverse events caused by DAA treatment was 1.9%, and the discontinuation of treatment because of adverse events was 0.4%. In seven trials involving adolescents, SVR rates after antiviral treatment were similar to those in adults.
Achieving an SVR after DAA treatment was associated with a decreased risk in those treated of all-cause mortality (hazard ratio, 0.40; 95% confidence interval, 0.28-0.56), liver mortality (HR, 0.11; 95% CI, 0.04-0.27), cirrhosis (HR, 0.36; 95% CI, 0.33-0.40), and hepatocellular carcinoma (HR, 0.29; 95% CI, 0.23-0.38), compared with those who did not respond.
Because of the evidence collected, the USPSTF issued a B recommendation for HCV screening in adults and recommended screening for all people aged 18-79 years in the draft recommendation statement. “Clinicians may want to consider screening in adolescents younger than age 18 years and in adults older than age 79 years who are at high risk [for HCV],” the proposal says.
The draft recommendation statement and evidence review is available at www.uspreventiveservicestaskforce.org. The public comment period will last until Sept. 23, 2019.
and now suggests that all adults aged 18-79 years receive screening.
This proposal represents an update and expansion of its current recommendation for screening for HCV infection. The draft recommendation incorporates new evidence and would replace the recommendation made in 2013, which calls for screening in persons at high risk for infection and one-time screening in adults born between 1945 and 1965 (Grade B).
“Today, more people are infected with hepatitis C than there were a decade ago, but there are now better treatments available. The evidence now shows more people can benefit from screening; therefore, we are recommending to screen all adults ages 18-79 for hepatitis C,” task force chair Douglas K. Owens, MD, MS, said in a bulletin issued by the USPSTF.
To update the previous recommendation, the USPSTF conducted a systematic review that included a total of 97 studies. No direct evidence was found regarding the benefits of HCV screening versus no screening or repeat versus one-time screening, and no new studies analyzed the psychological and social consequences of HCV screening.
Evidence concerning direct-acting antiviral (DAA) treatment was more compelling given that 49 trials found DAA therapy to be associated with pooled sustained virologic response (SVR) rates between 95.5% and 98.9% across genotypes. The rate of serious adverse events caused by DAA treatment was 1.9%, and the discontinuation of treatment because of adverse events was 0.4%. In seven trials involving adolescents, SVR rates after antiviral treatment were similar to those in adults.
Achieving an SVR after DAA treatment was associated with a decreased risk in those treated of all-cause mortality (hazard ratio, 0.40; 95% confidence interval, 0.28-0.56), liver mortality (HR, 0.11; 95% CI, 0.04-0.27), cirrhosis (HR, 0.36; 95% CI, 0.33-0.40), and hepatocellular carcinoma (HR, 0.29; 95% CI, 0.23-0.38), compared with those who did not respond.
Because of the evidence collected, the USPSTF issued a B recommendation for HCV screening in adults and recommended screening for all people aged 18-79 years in the draft recommendation statement. “Clinicians may want to consider screening in adolescents younger than age 18 years and in adults older than age 79 years who are at high risk [for HCV],” the proposal says.
The draft recommendation statement and evidence review is available at www.uspreventiveservicestaskforce.org. The public comment period will last until Sept. 23, 2019.
Hidradenitis suppurativa linked to higher NAFLD risk
independent of other metabolic risk factors, a study has found.
The results of the case-control study of 70 individuals with hidradenitis suppurativa (HS) and 150 age- and gender-matched controls were published in the Journal of the European Academy of Dermatology and Venereology. Using hepatic ultrasonography and transient elastography, the investigators found that 51 (72.9%) the participants with HS also had nonalcoholic fatty liver disease (NAFLD), compared with 37 (24.7%) of the controls (P less than .001).
Those with HS and NAFLD were more likely to be obese, have more central adiposity, and meet more of the criteria for metabolic syndrome than those with HS but without NAFLD. They also showed higher serum ALT levels, higher triglycerides, and higher controlled attenuation parameter scores, which is a surrogate marker of liver steatosis.
However, the HS plus NAFLD group had similar rates of active smoking, diabetes, dyslipidemia, hypertension, and cardiovascular events, compared with those who had HS only. They also showed no differences in hemoglobin A1c, serum insulin, insulin resistance, or liver stiffness, compared with the HS-only group.
When researchers compared the participants with HS plus NAFLD with controls with NAFLD, they found the HS group had significantly higher levels of liver stiffness measurement, which is a surrogate marker of liver fibrosis and severity, but there were no differences in the degree of hepatic steatosis.
The individuals with HS plus NAFLD had significantly lower serum albumin, but significantly higher serum gamma–glutamyl transpeptidase and ferritin, compared with controls who had NAFLD. They were also more likely to have metabolic risk factors such as hypertension, dyslipidemia, and metabolic syndrome.
The multivariate analysis also showed that male sex was a protective factor, because the prevalence of obesity was higher in women.
After adjusting for classic cardiovascular and steatosis risk factors, the researchers calculated that HS was a significant and independent risk factor for NAFLD, with an odds ratio of 7.75 (P less than .001). The results provide “the first evidence that patients with HS have a significant high prevalence of NAFLD, which is independent of classic metabolic risk factors and, according to our results, probably not related to the severity of the disease,” wrote Carlos Durán-Vian, MD, from the department of dermatology at the University of Cantabria, Santander, Spain, and coauthors.
“We think that our findings might have potential clinical implications, and physicians involved in the care of patients with HS should be aware of the link between this entity and NAFLD, in order to improve the overall management of these patients,” they wrote, noting that HS is often associated with the same metabolic disorders that can promote fatty liver disease, such as obesity and metabolic syndrome. But the discovery that it is an independent risk factor demands other hypotheses to explain the association between the two conditions.
“In this sense, a possible explanation to deeper understanding the link between HS and NAFLD could be the presence of chronic inflammation due to persistent and abnormal secretion of adipokines (i.e. adiponectin, leptin, resistin) and several proinflammatory cytokines,” the authors wrote, pointing out that NAFLD is also common among people with immune-mediated inflammatory disorders.
The study had the limitation of being an observational, cross-sectional design, and the authors acknowledged that the cohort was relatively small. They also were unable to use liver biopsies to confirm the NAFLD diagnosis.
No funding or conflicts of interest were reported.
SOURCE: Durán-Vian C et al. J Eur Acad Dermatol Venereol. 2019 Jul 1. doi: 10.1111/jdv.15764.
independent of other metabolic risk factors, a study has found.
The results of the case-control study of 70 individuals with hidradenitis suppurativa (HS) and 150 age- and gender-matched controls were published in the Journal of the European Academy of Dermatology and Venereology. Using hepatic ultrasonography and transient elastography, the investigators found that 51 (72.9%) the participants with HS also had nonalcoholic fatty liver disease (NAFLD), compared with 37 (24.7%) of the controls (P less than .001).
Those with HS and NAFLD were more likely to be obese, have more central adiposity, and meet more of the criteria for metabolic syndrome than those with HS but without NAFLD. They also showed higher serum ALT levels, higher triglycerides, and higher controlled attenuation parameter scores, which is a surrogate marker of liver steatosis.
However, the HS plus NAFLD group had similar rates of active smoking, diabetes, dyslipidemia, hypertension, and cardiovascular events, compared with those who had HS only. They also showed no differences in hemoglobin A1c, serum insulin, insulin resistance, or liver stiffness, compared with the HS-only group.
When researchers compared the participants with HS plus NAFLD with controls with NAFLD, they found the HS group had significantly higher levels of liver stiffness measurement, which is a surrogate marker of liver fibrosis and severity, but there were no differences in the degree of hepatic steatosis.
The individuals with HS plus NAFLD had significantly lower serum albumin, but significantly higher serum gamma–glutamyl transpeptidase and ferritin, compared with controls who had NAFLD. They were also more likely to have metabolic risk factors such as hypertension, dyslipidemia, and metabolic syndrome.
The multivariate analysis also showed that male sex was a protective factor, because the prevalence of obesity was higher in women.
After adjusting for classic cardiovascular and steatosis risk factors, the researchers calculated that HS was a significant and independent risk factor for NAFLD, with an odds ratio of 7.75 (P less than .001). The results provide “the first evidence that patients with HS have a significant high prevalence of NAFLD, which is independent of classic metabolic risk factors and, according to our results, probably not related to the severity of the disease,” wrote Carlos Durán-Vian, MD, from the department of dermatology at the University of Cantabria, Santander, Spain, and coauthors.
“We think that our findings might have potential clinical implications, and physicians involved in the care of patients with HS should be aware of the link between this entity and NAFLD, in order to improve the overall management of these patients,” they wrote, noting that HS is often associated with the same metabolic disorders that can promote fatty liver disease, such as obesity and metabolic syndrome. But the discovery that it is an independent risk factor demands other hypotheses to explain the association between the two conditions.
“In this sense, a possible explanation to deeper understanding the link between HS and NAFLD could be the presence of chronic inflammation due to persistent and abnormal secretion of adipokines (i.e. adiponectin, leptin, resistin) and several proinflammatory cytokines,” the authors wrote, pointing out that NAFLD is also common among people with immune-mediated inflammatory disorders.
The study had the limitation of being an observational, cross-sectional design, and the authors acknowledged that the cohort was relatively small. They also were unable to use liver biopsies to confirm the NAFLD diagnosis.
No funding or conflicts of interest were reported.
SOURCE: Durán-Vian C et al. J Eur Acad Dermatol Venereol. 2019 Jul 1. doi: 10.1111/jdv.15764.
independent of other metabolic risk factors, a study has found.
The results of the case-control study of 70 individuals with hidradenitis suppurativa (HS) and 150 age- and gender-matched controls were published in the Journal of the European Academy of Dermatology and Venereology. Using hepatic ultrasonography and transient elastography, the investigators found that 51 (72.9%) the participants with HS also had nonalcoholic fatty liver disease (NAFLD), compared with 37 (24.7%) of the controls (P less than .001).
Those with HS and NAFLD were more likely to be obese, have more central adiposity, and meet more of the criteria for metabolic syndrome than those with HS but without NAFLD. They also showed higher serum ALT levels, higher triglycerides, and higher controlled attenuation parameter scores, which is a surrogate marker of liver steatosis.
However, the HS plus NAFLD group had similar rates of active smoking, diabetes, dyslipidemia, hypertension, and cardiovascular events, compared with those who had HS only. They also showed no differences in hemoglobin A1c, serum insulin, insulin resistance, or liver stiffness, compared with the HS-only group.
When researchers compared the participants with HS plus NAFLD with controls with NAFLD, they found the HS group had significantly higher levels of liver stiffness measurement, which is a surrogate marker of liver fibrosis and severity, but there were no differences in the degree of hepatic steatosis.
The individuals with HS plus NAFLD had significantly lower serum albumin, but significantly higher serum gamma–glutamyl transpeptidase and ferritin, compared with controls who had NAFLD. They were also more likely to have metabolic risk factors such as hypertension, dyslipidemia, and metabolic syndrome.
The multivariate analysis also showed that male sex was a protective factor, because the prevalence of obesity was higher in women.
After adjusting for classic cardiovascular and steatosis risk factors, the researchers calculated that HS was a significant and independent risk factor for NAFLD, with an odds ratio of 7.75 (P less than .001). The results provide “the first evidence that patients with HS have a significant high prevalence of NAFLD, which is independent of classic metabolic risk factors and, according to our results, probably not related to the severity of the disease,” wrote Carlos Durán-Vian, MD, from the department of dermatology at the University of Cantabria, Santander, Spain, and coauthors.
“We think that our findings might have potential clinical implications, and physicians involved in the care of patients with HS should be aware of the link between this entity and NAFLD, in order to improve the overall management of these patients,” they wrote, noting that HS is often associated with the same metabolic disorders that can promote fatty liver disease, such as obesity and metabolic syndrome. But the discovery that it is an independent risk factor demands other hypotheses to explain the association between the two conditions.
“In this sense, a possible explanation to deeper understanding the link between HS and NAFLD could be the presence of chronic inflammation due to persistent and abnormal secretion of adipokines (i.e. adiponectin, leptin, resistin) and several proinflammatory cytokines,” the authors wrote, pointing out that NAFLD is also common among people with immune-mediated inflammatory disorders.
The study had the limitation of being an observational, cross-sectional design, and the authors acknowledged that the cohort was relatively small. They also were unable to use liver biopsies to confirm the NAFLD diagnosis.
No funding or conflicts of interest were reported.
SOURCE: Durán-Vian C et al. J Eur Acad Dermatol Venereol. 2019 Jul 1. doi: 10.1111/jdv.15764.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Lowering portal pressure boosts cirrhosis outcomes
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Statins hamper hepatocellular carcinoma in viral hepatitis patients
Lipophilic statin therapy significantly reduced the incidence and mortality of hepatocellular carcinoma in adults with viral hepatitis, based on data from 16,668 patients.
The mortality rates for hepatocellular carcinoma in the United States and Europe have been on the rise for decades, and the risk may persist in severe cases despite the use of hepatitis B virus suppression or hepatitis C virus eradication, wrote Tracey G. Simon, MD, of Harvard Medical School, Boston, and colleagues. Previous studies suggest that statins might reduce HCC risk in viral hepatitis patients, but evidence supporting one type of statin over another for HCC prevention is limited, they said.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a national registry of hepatitis patients in Sweden to assess the effect of lipophilic or hydrophilic statin use on HCC incidence and mortality.
They found a significant reduction in 10-year HCC risk for lipophilic statin users, compared with nonusers (8.1% vs. 3.3%. However, the difference was not significant for hydrophilic statin users vs. nonusers (8.0% vs. 6.8%). The effect of lipophilic statin use was dose dependent; the largest effect on reduction in HCC risk occurred with 600 or more lipophilic statin cumulative daily doses in users, compared with nonusers (8.4% vs. 2.5%).
The study population included 6,554 lipophilic statin users and 1,780 hydrophilic statin users, matched with 8,334 nonusers. Patient demographics were similar between both types of statin user and nonuser groups.
In addition, 10-year mortality was significantly lower for lipophilic statin users compared with nonusers (15.2% vs. 7.3%) and also for hydrophilic statin users, compared with nonusers (16.0% vs. 11.5%).
In a small number of patients with liver disease (462), liver-specific mortality was significantly reduced in lipophilic statin users, compared with nonusers (adjusted hazard ratio, 0.76 vs. 0.98).
“Of note, our findings were robust across several sensitivity analyses and were similar in all predefined subgroups, including among men and women and persons with and without cirrhosis or antiviral therapy use,” the researchers noted.
The study findings were limited by several factors including the potential confounding from variables such as smoking, hepatitis B viral DNA, hepatitis C virus eradication, stage of fibrosis, and HCC screening, as well as a lack of laboratory data to assess cholesterol levels’ impact on statin use, the researchers said. In addition, the study did not compare lipophilic and hydrophilic statins.
However, the results suggest potential distinct benefits of lipophilic statins to reduce HCC risk and support the need for further research, the researchers concluded.
Dr. Simon had no financial conflicts to disclose, but disclosed support from a North American Training Grant from the American College of Gastroenterology. Several coauthors disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck Sharp & Dohme. The study was supported in part by the American College of Gastroenterology, the American Association for the Study of Liver Diseases, the Boston Nutrition Obesity Research Center, the National Institutes of Health, Nyckelfonden, Region Orebro (Sweden) County, and the Karolinska Institutet.
SOURCE: Simon TG et al. Ann Intern Med. 2019 Aug 19. doi: 10.7326/M18-2753.
Lipophilic statin therapy significantly reduced the incidence and mortality of hepatocellular carcinoma in adults with viral hepatitis, based on data from 16,668 patients.
The mortality rates for hepatocellular carcinoma in the United States and Europe have been on the rise for decades, and the risk may persist in severe cases despite the use of hepatitis B virus suppression or hepatitis C virus eradication, wrote Tracey G. Simon, MD, of Harvard Medical School, Boston, and colleagues. Previous studies suggest that statins might reduce HCC risk in viral hepatitis patients, but evidence supporting one type of statin over another for HCC prevention is limited, they said.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a national registry of hepatitis patients in Sweden to assess the effect of lipophilic or hydrophilic statin use on HCC incidence and mortality.
They found a significant reduction in 10-year HCC risk for lipophilic statin users, compared with nonusers (8.1% vs. 3.3%. However, the difference was not significant for hydrophilic statin users vs. nonusers (8.0% vs. 6.8%). The effect of lipophilic statin use was dose dependent; the largest effect on reduction in HCC risk occurred with 600 or more lipophilic statin cumulative daily doses in users, compared with nonusers (8.4% vs. 2.5%).
The study population included 6,554 lipophilic statin users and 1,780 hydrophilic statin users, matched with 8,334 nonusers. Patient demographics were similar between both types of statin user and nonuser groups.
In addition, 10-year mortality was significantly lower for lipophilic statin users compared with nonusers (15.2% vs. 7.3%) and also for hydrophilic statin users, compared with nonusers (16.0% vs. 11.5%).
In a small number of patients with liver disease (462), liver-specific mortality was significantly reduced in lipophilic statin users, compared with nonusers (adjusted hazard ratio, 0.76 vs. 0.98).
“Of note, our findings were robust across several sensitivity analyses and were similar in all predefined subgroups, including among men and women and persons with and without cirrhosis or antiviral therapy use,” the researchers noted.
The study findings were limited by several factors including the potential confounding from variables such as smoking, hepatitis B viral DNA, hepatitis C virus eradication, stage of fibrosis, and HCC screening, as well as a lack of laboratory data to assess cholesterol levels’ impact on statin use, the researchers said. In addition, the study did not compare lipophilic and hydrophilic statins.
However, the results suggest potential distinct benefits of lipophilic statins to reduce HCC risk and support the need for further research, the researchers concluded.
Dr. Simon had no financial conflicts to disclose, but disclosed support from a North American Training Grant from the American College of Gastroenterology. Several coauthors disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck Sharp & Dohme. The study was supported in part by the American College of Gastroenterology, the American Association for the Study of Liver Diseases, the Boston Nutrition Obesity Research Center, the National Institutes of Health, Nyckelfonden, Region Orebro (Sweden) County, and the Karolinska Institutet.
SOURCE: Simon TG et al. Ann Intern Med. 2019 Aug 19. doi: 10.7326/M18-2753.
Lipophilic statin therapy significantly reduced the incidence and mortality of hepatocellular carcinoma in adults with viral hepatitis, based on data from 16,668 patients.
The mortality rates for hepatocellular carcinoma in the United States and Europe have been on the rise for decades, and the risk may persist in severe cases despite the use of hepatitis B virus suppression or hepatitis C virus eradication, wrote Tracey G. Simon, MD, of Harvard Medical School, Boston, and colleagues. Previous studies suggest that statins might reduce HCC risk in viral hepatitis patients, but evidence supporting one type of statin over another for HCC prevention is limited, they said.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a national registry of hepatitis patients in Sweden to assess the effect of lipophilic or hydrophilic statin use on HCC incidence and mortality.
They found a significant reduction in 10-year HCC risk for lipophilic statin users, compared with nonusers (8.1% vs. 3.3%. However, the difference was not significant for hydrophilic statin users vs. nonusers (8.0% vs. 6.8%). The effect of lipophilic statin use was dose dependent; the largest effect on reduction in HCC risk occurred with 600 or more lipophilic statin cumulative daily doses in users, compared with nonusers (8.4% vs. 2.5%).
The study population included 6,554 lipophilic statin users and 1,780 hydrophilic statin users, matched with 8,334 nonusers. Patient demographics were similar between both types of statin user and nonuser groups.
In addition, 10-year mortality was significantly lower for lipophilic statin users compared with nonusers (15.2% vs. 7.3%) and also for hydrophilic statin users, compared with nonusers (16.0% vs. 11.5%).
In a small number of patients with liver disease (462), liver-specific mortality was significantly reduced in lipophilic statin users, compared with nonusers (adjusted hazard ratio, 0.76 vs. 0.98).
“Of note, our findings were robust across several sensitivity analyses and were similar in all predefined subgroups, including among men and women and persons with and without cirrhosis or antiviral therapy use,” the researchers noted.
The study findings were limited by several factors including the potential confounding from variables such as smoking, hepatitis B viral DNA, hepatitis C virus eradication, stage of fibrosis, and HCC screening, as well as a lack of laboratory data to assess cholesterol levels’ impact on statin use, the researchers said. In addition, the study did not compare lipophilic and hydrophilic statins.
However, the results suggest potential distinct benefits of lipophilic statins to reduce HCC risk and support the need for further research, the researchers concluded.
Dr. Simon had no financial conflicts to disclose, but disclosed support from a North American Training Grant from the American College of Gastroenterology. Several coauthors disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck Sharp & Dohme. The study was supported in part by the American College of Gastroenterology, the American Association for the Study of Liver Diseases, the Boston Nutrition Obesity Research Center, the National Institutes of Health, Nyckelfonden, Region Orebro (Sweden) County, and the Karolinska Institutet.
SOURCE: Simon TG et al. Ann Intern Med. 2019 Aug 19. doi: 10.7326/M18-2753.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: Use of lipophilic statins significantly reduced incidence and mortality of hepatocellular cancer in adults with viral hepatitis.
Major finding: The 10-year risk of HCC was 8.1% among patients taking lipophilic statins, compared with 3.3% among those not on statins.
Study details: The data come from a population-based cohort study of 16,668 adult with viral hepatitis from a national registry in Sweden.
Disclosures: Dr. Simon had no financial conflicts to disclose, but disclosed support from a North American Training Grant from the American College of Gastroenterology. Several coauthors disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and MSD.
Source: Simon TG et al. Ann Intern Med. 2019 Aug 19. doi: 10.7326/M18-2753.
NAFLD unchecked is a ‘harbinger of deadly dysmetabolism’
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
SAN DIEGO – When it comes to metabolic and endocrine health, nonalcoholic fatty liver disease (NAFLD) is the furthest thing from a nonissue – it’s “a harbinger of deadly dysmetabolism,” said Christine Kessler, MN, ANP-BC, CNS, BC-ADM, FAANP, a nurse practitioner and founder of Metabolic Medicine Associates, King George, Va.
“I chase it, I follow it, I worry about it. Never look at it again as a benign thing,” Ms. Kessler said in a presentation at the Metabolic & Endocrine Disease Summit by Global Academy for Medical Education. “It’s the most common chronic liver disease in the United States – move over, hep C ... and it’ll be the number one cause of liver transplant within 20 years.”
But the news isn’t all grim: NAFLD can be reversible, because the liver is one organ that can “take a licking and keep on ticking,” she said.
An estimated 30%-40% of adults in the United States have NAFLD, according to the National Institute of Diabetes and Digestive and Kidney Diseases. The most severe form of the disease, nonalcoholic steatohepatitis (NASH), causes liver cell damage and affects an estimated 3%-12% of adults.
Why worry about NAFLD? Because it can boost cardiovascular risk (especially in conjunction with metabolic syndrome) and the risk for liver cancer, said Ms. Kessler.
Among the risk factors for NAFLD are obesity, type 2 diabetes, metabolic syndrome, polycystic ovary syndrome, and many others, including medications such as methotrexate, corticosteroids, and tetracyclines. Men, and Latino and Asian individuals are especially vulnerable, whereas black individuals may have protection against it.
Researchers are exploring the possibility that NAFLD is a “multihit” condition that is linked to multiple causes, possibly including overgrowth of bacteria in the gut, Ms. Kessler noted. It is not clear, however, whether regulation of gut microbiota would be helpful in preventing the condition.
Ms. Kessler urged her colleagues to consider workups in the following situations: when an incidental finding is noticed during imaging, when liver enzymes are abnormal (although they can misleadingly appear normal), and when there are overt symptoms of liver diseases. Causes such as alcohol use, medications, and hepatitis must first be ruled out, she said, and patients should be referred to a gastroenterologist if NAFLD is confirmed.
In regard to treatment, weight and diet control are crucial because they can have a significant impact in a patient with NAFLD. “You don’t come down with NAFLD, and then NASH, and then cirrhosis,” she explained. “It goes back and forth. You can go from normal liver to fatty liver, and back to normal. We’ve all seen it.”
Reduce weight, blood pressure, and blood sugar, she said, “and you’ll see NASH go to fatty liver, and fatty liver go over to normal. If you can have someone lose between 9% and 10% of their weight, you can turn around NASH. This is huge.”
As for medications, she said, “there is no one drug for fatty liver disease.” No medication has been approved by the Food and Drug Administration for the treatment of NAFLD or NASH, but there are several treatments that seem to be helpful, she said.
They include statins, though not for patients with decompensated cirrhosis, and some of the diabetes drugs – pioglitazone (Actos; to treat steatohepatitis in patients with or without type 2 diabetes who have biopsy-proven NASH); metformin (only in patients with diabetes); and the glucagon-like peptide-1 receptor agonists.
Also included among the therapies are vitamin E 800 IU/day and omega-3 fatty acids for patients who have a high levels of triglycerides, as well as lower-dose vitamin E (600 IU/day) and vitamin C (500 mg/day), which are best when used with lifestyle changes; increased choline intake – which supports liver health in menopausal women – from foods such as eggs; and milk thistle, which helps decrease liver inflammation.
Patients without chronic liver disease may find another helpful preventive tool on the shelves of their local liquor store: red wine, but with moderation, Ms. Kessler cautioned.
Global Academy and this news organization are owned by the same parent company. Ms. Kessler disclosed relationships as an adviser and speaker with Novo Nordisk, and with Clarion Brands.
EXPERT ANALYSIS FROM MEDS 2019
HCV-infected people who inject drugs also have substantial alcohol use
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
Curing hepatitis C virus (HCV) infection without addressing the high rate of alcohol use disorder in many patients may undermine the benefits of treatment to long-term liver health, according to the results of a large cohort study.
Because excess alcohol use is known to accelerate liver disease progression, researchers Risha Irvin, MD, and her colleagues from Johns Hopkins University, Baltimore, examined the prevalence of alcohol use in HCV-infected people who inject drugs (PWID). Their study examined the prevalence and associated correlates of alcohol use (Addictive Behaviors 2019;96:56-61).
They followed a large cohort of 1,623 HCV-antibody positive PWID from 2005 to 2013 from the AIDS Linked to the Intravenous Experience (ALIVE) study. They characterized alcohol use with the Alcohol Use Disorders Identification Test (AUDIT-C) questionnaire. Multivariable logistic regression with generalized estimated equations was used to examine sociodemographic, clinical, and substance use correlates of alcohol use.
At baseline, the median age was 47 years, 67% were men, 81% were black, and 34% were HIV positive. The majority (60%) reported injection drug use in the prior 6 months, while 46% reported noninjection cocaine or heroin, 31% reported street-acquired prescription drugs, and 22% reported marijuana use in the same time period. According to the AUDIT-C results, 41% of the patients reported no alcohol use, 21% reported moderate alcohol use, and 38% reported heavy alcohol use at their baseline visit.
The factors that were significantly associated with heavy alcohol use included male sex, black race, income of $5,000 or less, a Center for Epidemiologic Studies Depression Scale (range 0-60) score of 23 or greater, being homeless, being incarcerated, marijuana use, use of street-acquired prescription drugs, noninjection cocaine/heroin, injection drug use, and cigarette smoking. In a model that included the composite summary variable for substance use intensity, one drug type (adjusted odds ratio, 1.92), two drug types (AOR, 2.93), and three drug types (AOR, 3.65) were significantly associated with heavy alcohol use.
“While clinicians are undoubtedly concerned about any level of alcohol use in the setting of HCV infection due to the acceleration of liver fibrosis, there is particular concern for individuals with heavy alcohol use and their increased risk for cirrhosis and liver failure even after HCV cure. Without intervention, alcohol use will persist after HCV is cured with the potential to undermine the benefit of HCV cure. Therefore, our data point to the need to invest in and develop programs that effectively address alcohol use and co-occurring substance use in this population of PWID with HCV,” the researchers concluded.
The study was supported by the U.S. National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, and the National Institute on Alcohol Abuse and Alcoholism. The authors declared that they had no conflicts.
SOURCE: Irvin R et al. Addictive Behaviors. 2019;96:56-61.
FROM ADDICTIVE BEHAVIORS