User login
Social isolation, loneliness tied to death, MI, stroke: AHA
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
People who are socially isolated or lonely have an increased risk for myocardial infarction, stroke, and death, independent of other factors, the American Heart Association concludes in a new scientific statement.
More than 4 decades of research have “clearly demonstrated that social isolation and loneliness are both associated with adverse health outcomes,” writing group chair Crystal Wiley Cené, MD, University of California San Diego Health, said in a news release.
“Given the prevalence of social disconnectedness across the United States, the public health impact is quite significant,” Dr. Cené added.
The writing group says more research is needed to develop, implement, and test interventions to improve cardiovascular (CV) and brain health in people who are socially isolated or lonely.
The scientific statement was published online in the Journal of the American Heart Association.
Common and potentially deadly
Social isolation is defined as having infrequent in-person contact with people and loneliness is when a person feels he or she is alone or has less connection with others than desired.
It’s estimated that one-quarter of community-dwelling Americans 65 years and older are socially isolated, with even more experiencing loneliness.
The problem is not limited to older adults, however. Research suggests that younger adults also experience social isolation and loneliness, which might be attributed to more social media use and less frequent in-person activities.
Dr. Cené and colleagues reviewed observational and intervention research on social isolation published through July 2021 to examine the impact of social isolation and loneliness on CV and brain health.
The evidence is most consistent for a direct association between social isolation, loneliness, and death from coronary heart disease (CHD) and stroke, they reported.
For example, one meta-analysis of 19 studies showed that social isolation and loneliness increase the risk for CHD by 29%; most of these studies focused on acute MI and/or CHD death as the measure of CHD.
A meta-analysis of eight longitudinal observational studies showed social isolation and loneliness were associated with a 32% increased risk for stroke, after adjustment for age, sex, and socioeconomic status.
The literature also suggests social isolation and loneliness are associated with worse prognoses in adults with existing CHD or history of stroke.
One systematic review showed that socially isolated people with CHD had a two- to threefold increase in illness and death over 6 years, independent of cardiac risk factors.
Other research suggests that socially isolated adults with three or fewer social contacts per month have a 40% increased risk for recurrent stroke or MI.
There are fewer and less robust data on the association between social isolation and loneliness with heart failure (HF), dementia, and cognitive impairment, the writing group noted.
It’s also unclear whether actually being isolated (social isolation) or feeling isolated (loneliness) matters most for cardiovascular and brain health, because only a few studies have examined both in the same sample, they pointed out.
However, a study published in Neurology in June showed that older adults who reported feeling socially isolated had worse cognitive function at baseline than did those who did not report social isolation, and were 26% more likely to have dementia at follow-up, as reported by this news organization.
Urgent need for interventions
“There is an urgent need to develop, implement, and evaluate programs and strategies to reduce the negative effects of social isolation and loneliness on cardiovascular and brain health, particularly for at-risk populations,” Dr. Cené said in the news release.
She encourages clinicians to ask patients about their social life and whether they are satisfied with their level of interactions with friends and family, and to be prepared to refer patients who are socially isolated or lonely, especially those with a history of CHD or stroke, to community resources to help them connect with others.
Fitness programs and recreational activities at senior centers, as well as interventions that address negative thoughts of self-worth and other negative thinking, have shown promise in reducing isolation and loneliness, the writing group said.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Social Determinants of Health Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Quality of Care and Outcomes Research; the Prevention Science Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing; the Council on Arteriosclerosis, Thrombosis, and Vascular Biology; and the Stroke Council.
This research had no commercial funding. Members of the writing group have disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Onset and awareness of hypertension varies by race, ethnicity
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.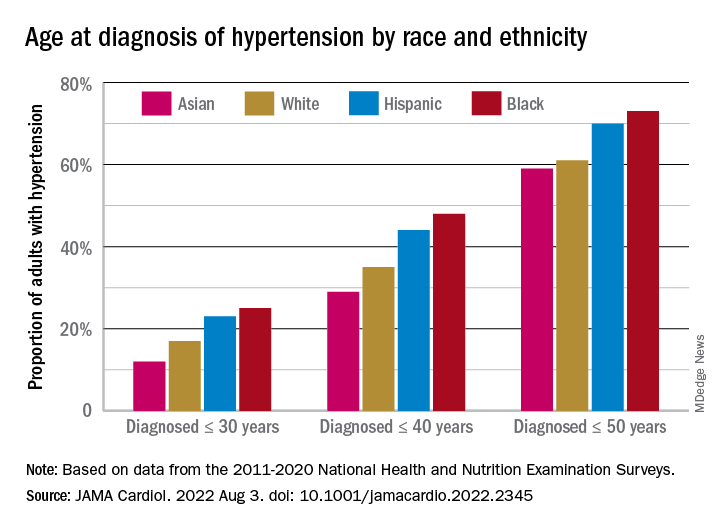
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
Black and Hispanic adults are diagnosed with hypertension at a significantly younger age than are white adults, and they also are more likely than Whites to be unaware of undiagnosed high blood pressure, based on national survey data collected from 2011 to 2020.
“Earlier hypertension onset in Black and Hispanic adults may contribute to racial and ethnic CVD disparities,” Xiaoning Huang, PhD, and associates wrote in JAMA Cardiology, also noting that “lower hypertension awareness among racial and ethnic minoritized groups suggests potential for underestimating differences in age at onset.”
Overall mean age at diagnosis was 46 years for the overall study sample of 9,627 participants in the National Health and Nutrition Examination Surveys over the 10 years covered in the analysis. Black adults, with a median age of 42 years, and Hispanic adults (median, 43 years) were significantly younger at diagnosis than White adults, who had a median age of 47 years, the investigators reported.
“Earlier age at hypertension onset may mean greater cumulative exposure to high blood pressure across the life course, which is associated with increased risk of [cardiovascular disease] and may contribute to racial disparities in hypertension-related outcomes,” said Dr. Huang and associates at Northwestern University, Chicago.
The increased cumulative exposure can be seen when age at diagnosis is stratified “across the life course.” Black/Hispanic adults were significantly more likely than White/Asian adults to be diagnosed at or before 30 years of age, and that difference continued to at least age 50 years, the investigators said.
Many adults unaware of their hypertension
There was a somewhat different trend among those in the study population who reported BP at or above 140/90 mm Hg but did not report a hypertension diagnosis. Black, Hispanic, and Asian adults all were significantly more likely than White adults to be unaware of their hypertension, the survey data showed.
Overall, 18% of those who did not report a hypertension diagnosis had a BP of 140/90 mm Hg or higher and 38% had a BP of 130/80 mm Hg or more. Broken down by race and ethnicity, 16% and 36% of Whites reporting no hypertension had BPs of 140/90 and 130/80 mm Hg, respectively; those proportions were 21% and 42% for Hispanics, 24% and 44% for Asians, and 28% and 51% for Blacks, with all of the differences between Whites and the others significant, the research team reported.
One investigator is an associate editor for JAMA Cardiology and reported receiving grants from the American Heart Association and the National Institutes of Health during the conduct of the study. None of the other investigators reported any conflicts.
FROM JAMA CARDIOLOGY
‘Staggering’ CVD rise projected in U.S., especially in minorities
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.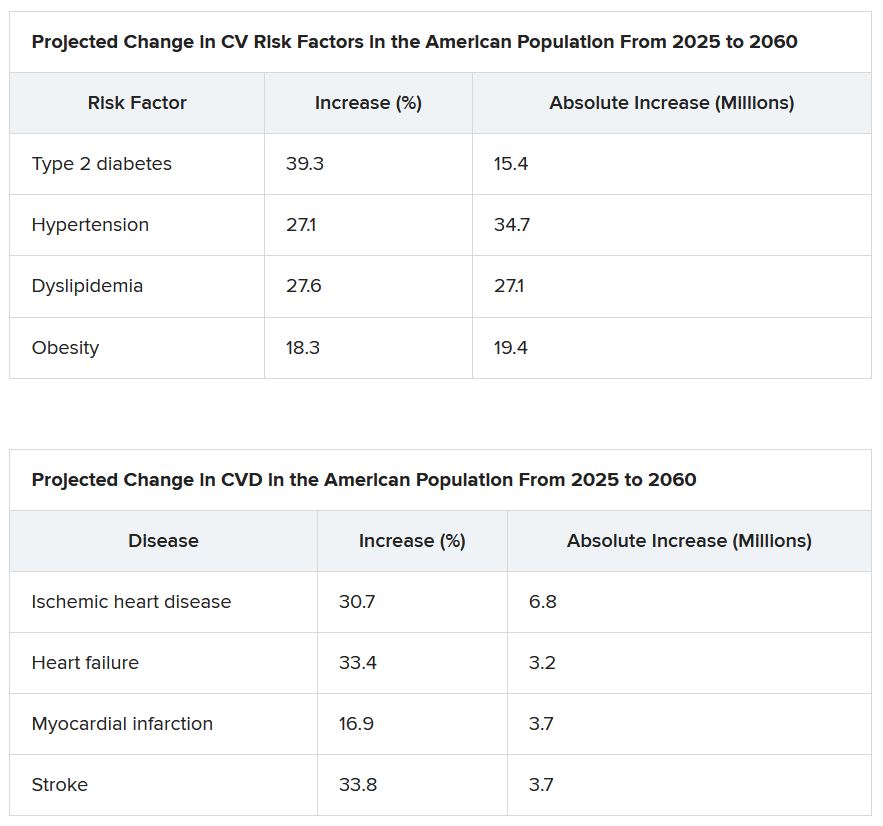
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
A new analysis projects steep increases by 2060 in the prevalence of cardiovascular (CV) risk factors and disease that will disproportionately affect non-White populations who have limited access to health care.
The study by Reza Mohebi, MD, Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues was published in the Journal of the American College of Cardiology.
“Even though several assumptions underlie these projections, the importance of this work cannot be overestimated,” Andreas P. Kalogeropoulos, MD, MPH, PhD, and Javed Butler, MD, MPH, MBA, wrote in an accompanying editorial. “The absolute numbers are staggering.”
From 2025 to 2060, the number of people with any one of four CV risk factors – type 2 diabetes, hypertension, dyslipidemia, and obesity – is projected to increase by 15.4 million, to 34.7 million.
And the number of people with of any one of four CV disease types – ischemic heart disease, heart failure, MI, and stroke – is projected to increase by 3.2 million, to 6.8 million.
Although the model predicts that the prevalence of CV risk factors will gradually decrease among White Americans, the highest prevalence of CV risk factors will be among the White population because of its overall size.
Conversely, the projected prevalence of CV risk factors is expected to increase in Black, Hispanic, Asian, and other race/ethnicity populations.
In parallel, the prevalence of CV disease is projected to decrease in the White population and increase among all other race/ethnicities, particularly in the Black and Hispanic populations.
“Our results project a worrisome increase with a particularly ominous increase in risk factors and disease in our most vulnerable patients, including Blacks and Hispanics,” senior author James L. Januzzi Jr., MD, summarized in a video issued by the society.
“The steep rise in CV risk factors and disease reflects the generally higher prevalence in populations projected to increase in the United States, owing to immigration and growth, including Black or Hispanic individuals,” Dr. Januzzi, also from Massachusetts General and Harvard, said in an interview.
“The disproportionate size of the risk is expected in a sense, as minority populations are disproportionately disadvantaged with respect to their health care,” he said. “But whether it is expected or not, the increase in projected prevalence is, nonetheless, concerning and a call to action.”
This study identifies “areas of opportunity for change in the U.S. health care system,” he continued. “Business as usual will result in us encountering a huge number of individuals with CV risk factors and diseases.”
The results from the current analysis assume there will be no modification in health care policies or changes in access to care for at-risk populations, Dr. Mohebi and colleagues noted.
To “stem the rising tide of CV disease in at-risk individuals,” would require strategies such as “emphasis on education regarding CV risk factors, improving access to quality healthcare, and facilitating lower-cost access to effective therapies for treatment of CV risk factors,” according to the researchers.
“Such advances need to be applied in a more equitable way throughout the United States, however,” they cautioned.
Census plus NHANES data
The researchers used 2020 U.S. census data and projected growth and 2013-2018 U.S. National Health and Nutrition Survey data to estimate the number of people with CV risk factors and CV disease from 2025 to 2060.
The estimates are based on a growing population and a fixed frequency.
The projected changes in CV risk factors and disease over time were similar in men and women.
The researchers acknowledge that study limitations include the assumption that the prevalence patterns for CV risk factors and disease will be stable.
“To the extent the frequency of risk factors and disease are not likely to remain static, that assumption may reduce the accuracy of the projections,” Dr. Januzzi said. “However, we would point out that the goals of our analysis were to set general trends, and not to seek to project exact figures.”
Also, they did not take into account the effect of COVID-19. CV diseases were also based on self-report and CV risk factors could have been underestimated in minority populations that do not access health care.
Changing demographic landscape
It is “striking” that the numbers of non-White individuals with CV risk factors is projected to surpass the number of White individuals over time, and the number of non-White individuals with CV disease will be almost as many as White individuals by the year 2060, the editorialists noted.
“From a policy perspective, this means that unless appropriate, targeted action is taken, disparities in the burden of cardiovascular disease are only going to be exacerbated over time,” wrote Dr. Kalogeropoulos, from Stony Brook (N.Y.) University, and Dr. Butler, from Baylor College of Medicine, Dallas.
“On the positive side,” they continued, “the absolute increase in the percent prevalence of cardiovascular risk factors and conditions is projected to lie within a manageable range,” assuming that specific prevention policies are implemented.
“This is an opportunity for professional societies, including the cardiovascular care community, to re-evaluate priorities and strategies, for both training and practice, to best match the growing demands of a changing demographic landscape in the United States,” Dr. Kalogeropoulos and Dr. Butler concluded.
Dr. Mohebi is supported by the Barry Fellowship. Dr. Januzzi is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Abbott Diagnostics, Applied Therapeutics, Innolife, and Novartis; has received consulting income from Abbott Diagnostics, Boehringer Ingelheim, Janssen, Novartis, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for AbbVie, Siemens, Takeda, and Vifor. Dr. Kalogeropoulos has received research funding from the National Heart, Lung, and Blood Institute; the American Heart Association; and the Centers for Disease Control and Prevention. Dr. Butler has been a consultant for numerous pharmaceutical companies.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF AMERICAN COLLEGE OF CARDIOLOGY
RADIANCE II: Positive signal for ultrasound renal denervation
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Top-line results released on July 26 from the RADIANCE II trial show the Paradise ultrasound renal denervation system significantly reduces daytime ambulatory systolic blood pressure, compared with a sham procedure at 2 months in patients with mild to moderate uncontrolled hypertension.
The trial was conducted in 224 patients who were previously treated with up to two medications and were randomized while off medication at more than 60 centers in 8 countries. No further details or results were provided.
The pivotal RADIANCE II trial, required for FDA approval, is the third and largest randomized, sham-controlled study following positive results reported by RADIANCE-HTN SOLO and RADIANCE-HTN TRIO, ReCor Medical and its subsidiary Otsuka Medical Devices noted in the announcement.
The field of renal denervation fell out of favor after the largest trial in 535 patients, SYMPLICITY HTN-3, failed to show a significant reduction in systolic blood pressure at 6 months, compared with sham control in resistant hypertension.
A version of this article first appeared on Medscape.com.
Moms using frozen embryos carry higher hypertensive risk
Women who become pregnant during in vitro fertilization (IVF) from previously frozen embryos have a significantly higher chance of developing hypertensive disorders such as preeclampsia than do women who become pregnant through natural conception, researchers have found.
The new findings come from a study presented at the 2022 annual meeting of the European Society of Human Reproduction and Embryology. In the study, which will soon be published in Hypertension, researchers analyzed more than 4.5 million pregnancies from Denmark, Norway, and Sweden.
“Our findings are significant because frozen embryo transfers are increasingly common all over the world, partly due to the elective freezing of all embryos,” said Sindre Hoff Petersen, PhD, a fellow in the department of public health and nursing at the Norwegian University of Science and Technology, Trondheim, who led the study.
More than 320,000 IVF procedures were performed in the United States in 2020, according to preliminary data from the Centers for Disease Control and Prevention.
Of those, more than 123,000 eggs or embryos were frozen for future use.
The use of assisted reproductive technology, which includes IVF, has more than doubled during the past decade, the CDC reports. Roughly 2% of all babies born in the United States each year are conceived through assisted reproductive technology.
Dr. Petersen and his colleagues compared maternal complications in sibling pregnancies. Women who became pregnant following the transfer of a frozen embryo were 74% more likely to develop a hypertensive disorder than women who became pregnant following natural conception (7.4% vs. 4.3%; adjusted odds ratio, 1.74; 95% confidence interval, P < .001). The difference was even higher with respect to sibling births: Women who became pregnant using frozen embryos were 102% more likely than women who became pregnant using natural conception to develop a hypertensive disorder (adjusted odds ratio 2.02; 95% CI, 1.72-2.39, P < .001).
The researchers found no difference in the risk of hypertensive disorders between women who used fresh embryos during IVF and women who used natural conception (5.9% vs. 4.3%, 95% CI, P = .382).
“When we find that the association between frozen embryo transfer and hypertensive disorders in pregnancy persists in sibling comparisons, we believe we have strong indications that treatment factors might in fact contribute to the higher risk,” Dr. Petersen told this news organization.
Women in the study who became pregnant after natural conception had a 4.3% chance of developing hypertensive disorders. That effect persisted after controlling for maternal body mass index, smoking, and time between deliveries, he said.
The findings can add to discussions between patients and doctors on the potential benefits and harms of freezing embryos on an elective basis if there is no clinical indication, Dr. Petersen said. The frozen method is most often used to transfer a single embryo in order to reduce the incidence of multiple pregnancies, such as twins and triplets, which in turn reduces pregnancy complications.
“The vast majority of IVF pregnancies, including frozen embryo transfer, are healthy and uncomplicated, and both short- and long-term outcomes for both the mother and the children are very reassuring,” Dr. Petersen said.
Women who become pregnant through use of frozen embryos should be more closely monitored for potential hypertensive disorders, although more work is needed to determine the reasons for the association, said Elizabeth S. Ginsburg, MD, at Brigham and Women’s Hospital and professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, both in Boston.
“This is something general ob.gyns. need to be aware of, but it’s not clear which subpopulations of patients are going to be affected,” Dr. Ginsburg said. “More investigation is needed to determine if this is caused by the way the uterus is readied for the embryo transfer or if it’s patient population etiology.”
Some studies have suggested that the absence of a hormone-producing cyst, which forms on the ovary during each menstrual cycle, could explain the link between frozen embryo transfer and heightened preeclampsia risk.
Dr. Petersen and Dr. Ginsburg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who become pregnant during in vitro fertilization (IVF) from previously frozen embryos have a significantly higher chance of developing hypertensive disorders such as preeclampsia than do women who become pregnant through natural conception, researchers have found.
The new findings come from a study presented at the 2022 annual meeting of the European Society of Human Reproduction and Embryology. In the study, which will soon be published in Hypertension, researchers analyzed more than 4.5 million pregnancies from Denmark, Norway, and Sweden.
“Our findings are significant because frozen embryo transfers are increasingly common all over the world, partly due to the elective freezing of all embryos,” said Sindre Hoff Petersen, PhD, a fellow in the department of public health and nursing at the Norwegian University of Science and Technology, Trondheim, who led the study.
More than 320,000 IVF procedures were performed in the United States in 2020, according to preliminary data from the Centers for Disease Control and Prevention.
Of those, more than 123,000 eggs or embryos were frozen for future use.
The use of assisted reproductive technology, which includes IVF, has more than doubled during the past decade, the CDC reports. Roughly 2% of all babies born in the United States each year are conceived through assisted reproductive technology.
Dr. Petersen and his colleagues compared maternal complications in sibling pregnancies. Women who became pregnant following the transfer of a frozen embryo were 74% more likely to develop a hypertensive disorder than women who became pregnant following natural conception (7.4% vs. 4.3%; adjusted odds ratio, 1.74; 95% confidence interval, P < .001). The difference was even higher with respect to sibling births: Women who became pregnant using frozen embryos were 102% more likely than women who became pregnant using natural conception to develop a hypertensive disorder (adjusted odds ratio 2.02; 95% CI, 1.72-2.39, P < .001).
The researchers found no difference in the risk of hypertensive disorders between women who used fresh embryos during IVF and women who used natural conception (5.9% vs. 4.3%, 95% CI, P = .382).
“When we find that the association between frozen embryo transfer and hypertensive disorders in pregnancy persists in sibling comparisons, we believe we have strong indications that treatment factors might in fact contribute to the higher risk,” Dr. Petersen told this news organization.
Women in the study who became pregnant after natural conception had a 4.3% chance of developing hypertensive disorders. That effect persisted after controlling for maternal body mass index, smoking, and time between deliveries, he said.
The findings can add to discussions between patients and doctors on the potential benefits and harms of freezing embryos on an elective basis if there is no clinical indication, Dr. Petersen said. The frozen method is most often used to transfer a single embryo in order to reduce the incidence of multiple pregnancies, such as twins and triplets, which in turn reduces pregnancy complications.
“The vast majority of IVF pregnancies, including frozen embryo transfer, are healthy and uncomplicated, and both short- and long-term outcomes for both the mother and the children are very reassuring,” Dr. Petersen said.
Women who become pregnant through use of frozen embryos should be more closely monitored for potential hypertensive disorders, although more work is needed to determine the reasons for the association, said Elizabeth S. Ginsburg, MD, at Brigham and Women’s Hospital and professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, both in Boston.
“This is something general ob.gyns. need to be aware of, but it’s not clear which subpopulations of patients are going to be affected,” Dr. Ginsburg said. “More investigation is needed to determine if this is caused by the way the uterus is readied for the embryo transfer or if it’s patient population etiology.”
Some studies have suggested that the absence of a hormone-producing cyst, which forms on the ovary during each menstrual cycle, could explain the link between frozen embryo transfer and heightened preeclampsia risk.
Dr. Petersen and Dr. Ginsburg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Women who become pregnant during in vitro fertilization (IVF) from previously frozen embryos have a significantly higher chance of developing hypertensive disorders such as preeclampsia than do women who become pregnant through natural conception, researchers have found.
The new findings come from a study presented at the 2022 annual meeting of the European Society of Human Reproduction and Embryology. In the study, which will soon be published in Hypertension, researchers analyzed more than 4.5 million pregnancies from Denmark, Norway, and Sweden.
“Our findings are significant because frozen embryo transfers are increasingly common all over the world, partly due to the elective freezing of all embryos,” said Sindre Hoff Petersen, PhD, a fellow in the department of public health and nursing at the Norwegian University of Science and Technology, Trondheim, who led the study.
More than 320,000 IVF procedures were performed in the United States in 2020, according to preliminary data from the Centers for Disease Control and Prevention.
Of those, more than 123,000 eggs or embryos were frozen for future use.
The use of assisted reproductive technology, which includes IVF, has more than doubled during the past decade, the CDC reports. Roughly 2% of all babies born in the United States each year are conceived through assisted reproductive technology.
Dr. Petersen and his colleagues compared maternal complications in sibling pregnancies. Women who became pregnant following the transfer of a frozen embryo were 74% more likely to develop a hypertensive disorder than women who became pregnant following natural conception (7.4% vs. 4.3%; adjusted odds ratio, 1.74; 95% confidence interval, P < .001). The difference was even higher with respect to sibling births: Women who became pregnant using frozen embryos were 102% more likely than women who became pregnant using natural conception to develop a hypertensive disorder (adjusted odds ratio 2.02; 95% CI, 1.72-2.39, P < .001).
The researchers found no difference in the risk of hypertensive disorders between women who used fresh embryos during IVF and women who used natural conception (5.9% vs. 4.3%, 95% CI, P = .382).
“When we find that the association between frozen embryo transfer and hypertensive disorders in pregnancy persists in sibling comparisons, we believe we have strong indications that treatment factors might in fact contribute to the higher risk,” Dr. Petersen told this news organization.
Women in the study who became pregnant after natural conception had a 4.3% chance of developing hypertensive disorders. That effect persisted after controlling for maternal body mass index, smoking, and time between deliveries, he said.
The findings can add to discussions between patients and doctors on the potential benefits and harms of freezing embryos on an elective basis if there is no clinical indication, Dr. Petersen said. The frozen method is most often used to transfer a single embryo in order to reduce the incidence of multiple pregnancies, such as twins and triplets, which in turn reduces pregnancy complications.
“The vast majority of IVF pregnancies, including frozen embryo transfer, are healthy and uncomplicated, and both short- and long-term outcomes for both the mother and the children are very reassuring,” Dr. Petersen said.
Women who become pregnant through use of frozen embryos should be more closely monitored for potential hypertensive disorders, although more work is needed to determine the reasons for the association, said Elizabeth S. Ginsburg, MD, at Brigham and Women’s Hospital and professor of obstetrics, gynecology, and reproductive biology at Harvard Medical School, both in Boston.
“This is something general ob.gyns. need to be aware of, but it’s not clear which subpopulations of patients are going to be affected,” Dr. Ginsburg said. “More investigation is needed to determine if this is caused by the way the uterus is readied for the embryo transfer or if it’s patient population etiology.”
Some studies have suggested that the absence of a hormone-producing cyst, which forms on the ovary during each menstrual cycle, could explain the link between frozen embryo transfer and heightened preeclampsia risk.
Dr. Petersen and Dr. Ginsburg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metabolic syndrome raises dementia risk in under-60s
The more components of metabolic syndrome a person has in midlife seems to raise their risk of dementia, although that relationship seems to go away after age 70, a post hoc analysis of data from a major European cohort study has found.
A team of European researchers reported online in the journal Diabetes Care that the follow-up of the Whitehall II cohort study, a study of more than 10,000 civil servants in London that was established in the late 1980s, also found that cardiovascular disease (CVD) may only partially contribute to the risk of dementia in study participants.
They found that each additional metabolic syndrome component before age 60 years was linked to a 13% rise in the risk of dementia (hazard ratio, 1.13; 95% confidence interval [CI], 1.05-1.23) and, from age 60 to 70, the risk rose 8% (HR, 1.08; 95% CI, 1.00-1.16). However, in people aged 70 years and older, the relationship wasn’t statistically significant (HR, 1.04; 95% CI, 0.96-1.13]).
The study used “the latest harmonized definition” of metabolic syndrome; that is, participants were classified as having metabolic syndrome if they had three or more of the five components. As lead author Marcos D. Machado-Fragua, PhD, noted in an email interview, those components are abdominal obesity, high triglycerides, low HDL cholesterol levels, high blood pressure, and high fasting glucose.
“Our research question was on the association between metabolic syndrome and late-life dementia. We found that the presence of one metabolic syndrome component and the presence of metabolic risk before age 60, but not after, is associated with higher risk of dementia,” said Dr. Machado-Fragua, a post-doctoral researcher at the French Institute for Health and Medical Research in Paris.
The study cohort consisted of 10,308 London-based civil servants aged 35-55 years. Every 4-5 years after enrollment, from 1991 through 2016, they completed a questionnaire and had a clinical examination. The U.K. National Health Service electronic health record system tracked outcomes for all but 10 participants through March 2019.
The study identified the individual metabolic syndrome components that posed the highest risk for dementia in these three age groups:
- Age < 60 years: elevated waist circumference (HR 1.39 [95% CI 1.07, 1.81]), low HDL-C, (HR 1.30 [95% CI 1.02, 1.66]), and elevated blood pressure (HR 1.34 [95% CI 1.09, 1.63]).
- Age 60-70 years: low HDL-C (HR 1.26 [95% CI 1.02, 1.57]) and elevated fasting glucose (HR 1.40 [95% CI 1.12, 1.74]).
- Age >70 years: elevated fasting glucose (HR 1.38 [95% CI 1.07, 1.79]).
The study found that the dementia risk was significantly high in study participants under age 60 who had at least one (HR 1.99 [95% CI 1.08, 3.66]) or two (HR 1.69 [95% CI 1.12, 2.56]) metabolic syndrome components even when they didn’t have CVD.
“The present study adds to the understanding of the association between metabolic syndrome and dementia due to three novel features,” Dr. Machado-Fragua said. “First, we tested alternative thresholds to define ‘high metabolic risk,’ and findings show increased risk of dementia to start with the presence of one metabolic syndrome component. Second, assessment of metabolic syndrome components in midlife and later life allowed the examination of the role of age at prevalence of metabolic risk for incident dementia at older ages. Third, our findings showed high dementia risk in those free of cardiovascular disease during follow-up, suggesting that the association between high metabolic risk and incident dementia is not fully explained by cardiovascular disease.”
Dr. Machado-Fragua added, “For now, a cure for dementia remains elusive, making it important to think of prevention strategies. Our findings support targeting the components of the metabolic syndrome in midlife, even in those who have fewer than three of the metabolic syndrome components.”
Applicability ‘confusing’
In an interview, Yehuda Handelsman, MD, questioned the applicability of the study findings in the clinic. “Metabolic syndrome is a clinical manifestation of insulin resistance,” he said. “The more metabolic syndrome criteria a person has, the more insulin resistant that person will be. There is literature that is [suggesting] that insulin resistance is an important cause of dementia.”
The finding of a higher dementia risk before age 70, compared to afterward, makes the applicability “even more confusing,” he said. The results are even more muddled for U.S. physicians, who have moved away from the term metabolic syndrome in favor of cardiometabolic syndrome, said Dr. Handelsman, medical director and principal investigator at the Metabolic Institute of America and president of the Diabetes CardioRenal & Metabolism Institute, both in Tarzana, Calif.
Confusion also surrounds one of the components of metabolic syndrome: Waist circumference, per the harmonized definition the study used, and body mass index, which the more traditional definition uses.
Nonetheless, metabolic syndrome can be used as “kind of a risk calculator” for CVD, diabetes, and dementia, he said. One strength of the study, Dr. Handelsman said, is its size and scope, following 28 years of data. But a weakness was its observational design. “It doesn’t evaluate any true intervention to modify risk,” he said.
Dr. Machado-Fragua and coauthors have no disclosures.
The more components of metabolic syndrome a person has in midlife seems to raise their risk of dementia, although that relationship seems to go away after age 70, a post hoc analysis of data from a major European cohort study has found.
A team of European researchers reported online in the journal Diabetes Care that the follow-up of the Whitehall II cohort study, a study of more than 10,000 civil servants in London that was established in the late 1980s, also found that cardiovascular disease (CVD) may only partially contribute to the risk of dementia in study participants.
They found that each additional metabolic syndrome component before age 60 years was linked to a 13% rise in the risk of dementia (hazard ratio, 1.13; 95% confidence interval [CI], 1.05-1.23) and, from age 60 to 70, the risk rose 8% (HR, 1.08; 95% CI, 1.00-1.16). However, in people aged 70 years and older, the relationship wasn’t statistically significant (HR, 1.04; 95% CI, 0.96-1.13]).
The study used “the latest harmonized definition” of metabolic syndrome; that is, participants were classified as having metabolic syndrome if they had three or more of the five components. As lead author Marcos D. Machado-Fragua, PhD, noted in an email interview, those components are abdominal obesity, high triglycerides, low HDL cholesterol levels, high blood pressure, and high fasting glucose.
“Our research question was on the association between metabolic syndrome and late-life dementia. We found that the presence of one metabolic syndrome component and the presence of metabolic risk before age 60, but not after, is associated with higher risk of dementia,” said Dr. Machado-Fragua, a post-doctoral researcher at the French Institute for Health and Medical Research in Paris.
The study cohort consisted of 10,308 London-based civil servants aged 35-55 years. Every 4-5 years after enrollment, from 1991 through 2016, they completed a questionnaire and had a clinical examination. The U.K. National Health Service electronic health record system tracked outcomes for all but 10 participants through March 2019.
The study identified the individual metabolic syndrome components that posed the highest risk for dementia in these three age groups:
- Age < 60 years: elevated waist circumference (HR 1.39 [95% CI 1.07, 1.81]), low HDL-C, (HR 1.30 [95% CI 1.02, 1.66]), and elevated blood pressure (HR 1.34 [95% CI 1.09, 1.63]).
- Age 60-70 years: low HDL-C (HR 1.26 [95% CI 1.02, 1.57]) and elevated fasting glucose (HR 1.40 [95% CI 1.12, 1.74]).
- Age >70 years: elevated fasting glucose (HR 1.38 [95% CI 1.07, 1.79]).
The study found that the dementia risk was significantly high in study participants under age 60 who had at least one (HR 1.99 [95% CI 1.08, 3.66]) or two (HR 1.69 [95% CI 1.12, 2.56]) metabolic syndrome components even when they didn’t have CVD.
“The present study adds to the understanding of the association between metabolic syndrome and dementia due to three novel features,” Dr. Machado-Fragua said. “First, we tested alternative thresholds to define ‘high metabolic risk,’ and findings show increased risk of dementia to start with the presence of one metabolic syndrome component. Second, assessment of metabolic syndrome components in midlife and later life allowed the examination of the role of age at prevalence of metabolic risk for incident dementia at older ages. Third, our findings showed high dementia risk in those free of cardiovascular disease during follow-up, suggesting that the association between high metabolic risk and incident dementia is not fully explained by cardiovascular disease.”
Dr. Machado-Fragua added, “For now, a cure for dementia remains elusive, making it important to think of prevention strategies. Our findings support targeting the components of the metabolic syndrome in midlife, even in those who have fewer than three of the metabolic syndrome components.”
Applicability ‘confusing’
In an interview, Yehuda Handelsman, MD, questioned the applicability of the study findings in the clinic. “Metabolic syndrome is a clinical manifestation of insulin resistance,” he said. “The more metabolic syndrome criteria a person has, the more insulin resistant that person will be. There is literature that is [suggesting] that insulin resistance is an important cause of dementia.”
The finding of a higher dementia risk before age 70, compared to afterward, makes the applicability “even more confusing,” he said. The results are even more muddled for U.S. physicians, who have moved away from the term metabolic syndrome in favor of cardiometabolic syndrome, said Dr. Handelsman, medical director and principal investigator at the Metabolic Institute of America and president of the Diabetes CardioRenal & Metabolism Institute, both in Tarzana, Calif.
Confusion also surrounds one of the components of metabolic syndrome: Waist circumference, per the harmonized definition the study used, and body mass index, which the more traditional definition uses.
Nonetheless, metabolic syndrome can be used as “kind of a risk calculator” for CVD, diabetes, and dementia, he said. One strength of the study, Dr. Handelsman said, is its size and scope, following 28 years of data. But a weakness was its observational design. “It doesn’t evaluate any true intervention to modify risk,” he said.
Dr. Machado-Fragua and coauthors have no disclosures.
The more components of metabolic syndrome a person has in midlife seems to raise their risk of dementia, although that relationship seems to go away after age 70, a post hoc analysis of data from a major European cohort study has found.
A team of European researchers reported online in the journal Diabetes Care that the follow-up of the Whitehall II cohort study, a study of more than 10,000 civil servants in London that was established in the late 1980s, also found that cardiovascular disease (CVD) may only partially contribute to the risk of dementia in study participants.
They found that each additional metabolic syndrome component before age 60 years was linked to a 13% rise in the risk of dementia (hazard ratio, 1.13; 95% confidence interval [CI], 1.05-1.23) and, from age 60 to 70, the risk rose 8% (HR, 1.08; 95% CI, 1.00-1.16). However, in people aged 70 years and older, the relationship wasn’t statistically significant (HR, 1.04; 95% CI, 0.96-1.13]).
The study used “the latest harmonized definition” of metabolic syndrome; that is, participants were classified as having metabolic syndrome if they had three or more of the five components. As lead author Marcos D. Machado-Fragua, PhD, noted in an email interview, those components are abdominal obesity, high triglycerides, low HDL cholesterol levels, high blood pressure, and high fasting glucose.
“Our research question was on the association between metabolic syndrome and late-life dementia. We found that the presence of one metabolic syndrome component and the presence of metabolic risk before age 60, but not after, is associated with higher risk of dementia,” said Dr. Machado-Fragua, a post-doctoral researcher at the French Institute for Health and Medical Research in Paris.
The study cohort consisted of 10,308 London-based civil servants aged 35-55 years. Every 4-5 years after enrollment, from 1991 through 2016, they completed a questionnaire and had a clinical examination. The U.K. National Health Service electronic health record system tracked outcomes for all but 10 participants through March 2019.
The study identified the individual metabolic syndrome components that posed the highest risk for dementia in these three age groups:
- Age < 60 years: elevated waist circumference (HR 1.39 [95% CI 1.07, 1.81]), low HDL-C, (HR 1.30 [95% CI 1.02, 1.66]), and elevated blood pressure (HR 1.34 [95% CI 1.09, 1.63]).
- Age 60-70 years: low HDL-C (HR 1.26 [95% CI 1.02, 1.57]) and elevated fasting glucose (HR 1.40 [95% CI 1.12, 1.74]).
- Age >70 years: elevated fasting glucose (HR 1.38 [95% CI 1.07, 1.79]).
The study found that the dementia risk was significantly high in study participants under age 60 who had at least one (HR 1.99 [95% CI 1.08, 3.66]) or two (HR 1.69 [95% CI 1.12, 2.56]) metabolic syndrome components even when they didn’t have CVD.
“The present study adds to the understanding of the association between metabolic syndrome and dementia due to three novel features,” Dr. Machado-Fragua said. “First, we tested alternative thresholds to define ‘high metabolic risk,’ and findings show increased risk of dementia to start with the presence of one metabolic syndrome component. Second, assessment of metabolic syndrome components in midlife and later life allowed the examination of the role of age at prevalence of metabolic risk for incident dementia at older ages. Third, our findings showed high dementia risk in those free of cardiovascular disease during follow-up, suggesting that the association between high metabolic risk and incident dementia is not fully explained by cardiovascular disease.”
Dr. Machado-Fragua added, “For now, a cure for dementia remains elusive, making it important to think of prevention strategies. Our findings support targeting the components of the metabolic syndrome in midlife, even in those who have fewer than three of the metabolic syndrome components.”
Applicability ‘confusing’
In an interview, Yehuda Handelsman, MD, questioned the applicability of the study findings in the clinic. “Metabolic syndrome is a clinical manifestation of insulin resistance,” he said. “The more metabolic syndrome criteria a person has, the more insulin resistant that person will be. There is literature that is [suggesting] that insulin resistance is an important cause of dementia.”
The finding of a higher dementia risk before age 70, compared to afterward, makes the applicability “even more confusing,” he said. The results are even more muddled for U.S. physicians, who have moved away from the term metabolic syndrome in favor of cardiometabolic syndrome, said Dr. Handelsman, medical director and principal investigator at the Metabolic Institute of America and president of the Diabetes CardioRenal & Metabolism Institute, both in Tarzana, Calif.
Confusion also surrounds one of the components of metabolic syndrome: Waist circumference, per the harmonized definition the study used, and body mass index, which the more traditional definition uses.
Nonetheless, metabolic syndrome can be used as “kind of a risk calculator” for CVD, diabetes, and dementia, he said. One strength of the study, Dr. Handelsman said, is its size and scope, following 28 years of data. But a weakness was its observational design. “It doesn’t evaluate any true intervention to modify risk,” he said.
Dr. Machado-Fragua and coauthors have no disclosures.
FROM DIABETES CARE
Boosting hypertension screening, treatment would cut global mortality 7%
If 80% of individuals with hypertension were screened, 80% received treatment, and 80% then reached guideline-specified targets, up to 200 million cases of cardiovascular disease (CVD) and 130 million deaths could be averted by 2050, a modeling study suggests.
Achievement of the 80-80-80 target “could be one of the single most important global public health accomplishments of the coming decades,” according to the authors.
“We need to reprioritize hypertension care in our practices,” principal investigator David A. Watkins, MD, MPH, University of Washington, Seattle, told this news organization. “Only about one in five persons with hypertension around the world has their blood pressure well controlled. Oftentimes, clinicians are focused on addressing patients’ other health needs, many of which can be pressing in the short term, and we forget to talk about blood pressure, which has more than earned its reputation as ‘the silent killer.’ ”
The modeling study was published online in Nature Medicine, with lead author Sarah J. Pickersgill, MPH, also from the University of Washington.
Two interventions, three scenarios
Dr. Watkins and colleagues based their analysis on two approaches to blood pressure (BP) control shown to be beneficial: drug treatment to a systolic BP of either 130 mm Hg or 140 mm Hg or less, depending on local guidelines, and dietary sodium reduction, as recommended by the World Health Organization.
The team modeled the impacts of these interventions in 182 countries according to three scenarios:
- Business as usual (control): allowing hypertension to increase at historic rates of change and mean sodium intake to remain at current levels
- Progress: matching historically high-performing countries (for example, accelerating hypertension control by about 3% per year at intermediate levels of intervention coverage) while lowering mean sodium intake by 15% by 2030
- Aspirational: hypertension control achieved faster than historically high-performing countries (about 4% per year) and mean sodium intake decreased by 30% by 2027
The analysis suggests that in the progressive scenario, all countries could achieve 80-80-80 targets by 2050 and most countries by 2040; the aspirational scenario would have all countries meeting them by 2040. That would result in reductions in all-cause mortality of 4%-7% (76 million to 130 million deaths averted) with progressive and aspirational interventions, respectively, compared with the control scenario.
There would also be a slower rise in expected CVD from population growth and aging (110 million to 200 million cases averted). That is, the probability of dying from any CVD cause between the ages of 30 and 80 years would be reduced by 16% in the progressive scenario and 26% in the aspirational scenario.
Of note, about 83%-85% of the potential mortality reductions would result from scaling up hypertension treatment in the progressive and aspirational scenarios, respectively, with the remaining 15%-17% coming from sodium reduction, the researchers state.
Further, they propose, scaling up BP interventions could reduce CVD inequalities across countries, with low-income and lower-middle-income countries likely experiencing the largest reductions in disease rates and mortality.
Implementation barriers
“Health systems in many low- and middle-income countries have not traditionally been set up to succeed in chronic disease management in primary care,” Dr. Watkins noted. For interventions to be successful, he said, “several barriers need to be addressed, including: low population awareness of chronic diseases like hypertension and diabetes, which leads to low rates of screening and treatment; high out-of-pocket cost and low availability of medicines for chronic diseases; and need for adherence support and provider incentives for improving quality of chronic disease care in primary care settings.”
“Based on the analysis, achieving the 80-80-80 seems feasible, though actually getting there may be much more complicated. I wonder whether countries have the resources to implement the needed policies,” Rodrigo M. Carrillo-Larco, MD, researcher, department of epidemiology and biostatistics, School of Public Health, Imperial College London, told this news organization.
“It may be challenging, particularly after COVID-19, which revealed deficiencies in many health care systems, and care for hypertension may have been disturbed,” said Dr. Carrillo-Larco, who is not connected with the analysis.
That said, simplified BP screening approaches could help maximize the number of people screened overall, potentially identifying those with hypertension and raising awareness, he proposed. His team’s recent study showed that such approaches vary from country to country but are generally reliable and can be used effectively for population screening.
In addition, Dr. Carrillo-Larco said, any efforts by clinicians to improve adherence and help patients achieve BP control “would also have positive effects at the population level.”
The study was supported by a grant from the Bill & Melinda Gates Foundation, with additional funding by a grant to Dr. Watkins from Resolve to Save Lives. No conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
If 80% of individuals with hypertension were screened, 80% received treatment, and 80% then reached guideline-specified targets, up to 200 million cases of cardiovascular disease (CVD) and 130 million deaths could be averted by 2050, a modeling study suggests.
Achievement of the 80-80-80 target “could be one of the single most important global public health accomplishments of the coming decades,” according to the authors.
“We need to reprioritize hypertension care in our practices,” principal investigator David A. Watkins, MD, MPH, University of Washington, Seattle, told this news organization. “Only about one in five persons with hypertension around the world has their blood pressure well controlled. Oftentimes, clinicians are focused on addressing patients’ other health needs, many of which can be pressing in the short term, and we forget to talk about blood pressure, which has more than earned its reputation as ‘the silent killer.’ ”
The modeling study was published online in Nature Medicine, with lead author Sarah J. Pickersgill, MPH, also from the University of Washington.
Two interventions, three scenarios
Dr. Watkins and colleagues based their analysis on two approaches to blood pressure (BP) control shown to be beneficial: drug treatment to a systolic BP of either 130 mm Hg or 140 mm Hg or less, depending on local guidelines, and dietary sodium reduction, as recommended by the World Health Organization.
The team modeled the impacts of these interventions in 182 countries according to three scenarios:
- Business as usual (control): allowing hypertension to increase at historic rates of change and mean sodium intake to remain at current levels
- Progress: matching historically high-performing countries (for example, accelerating hypertension control by about 3% per year at intermediate levels of intervention coverage) while lowering mean sodium intake by 15% by 2030
- Aspirational: hypertension control achieved faster than historically high-performing countries (about 4% per year) and mean sodium intake decreased by 30% by 2027
The analysis suggests that in the progressive scenario, all countries could achieve 80-80-80 targets by 2050 and most countries by 2040; the aspirational scenario would have all countries meeting them by 2040. That would result in reductions in all-cause mortality of 4%-7% (76 million to 130 million deaths averted) with progressive and aspirational interventions, respectively, compared with the control scenario.
There would also be a slower rise in expected CVD from population growth and aging (110 million to 200 million cases averted). That is, the probability of dying from any CVD cause between the ages of 30 and 80 years would be reduced by 16% in the progressive scenario and 26% in the aspirational scenario.
Of note, about 83%-85% of the potential mortality reductions would result from scaling up hypertension treatment in the progressive and aspirational scenarios, respectively, with the remaining 15%-17% coming from sodium reduction, the researchers state.
Further, they propose, scaling up BP interventions could reduce CVD inequalities across countries, with low-income and lower-middle-income countries likely experiencing the largest reductions in disease rates and mortality.
Implementation barriers
“Health systems in many low- and middle-income countries have not traditionally been set up to succeed in chronic disease management in primary care,” Dr. Watkins noted. For interventions to be successful, he said, “several barriers need to be addressed, including: low population awareness of chronic diseases like hypertension and diabetes, which leads to low rates of screening and treatment; high out-of-pocket cost and low availability of medicines for chronic diseases; and need for adherence support and provider incentives for improving quality of chronic disease care in primary care settings.”
“Based on the analysis, achieving the 80-80-80 seems feasible, though actually getting there may be much more complicated. I wonder whether countries have the resources to implement the needed policies,” Rodrigo M. Carrillo-Larco, MD, researcher, department of epidemiology and biostatistics, School of Public Health, Imperial College London, told this news organization.
“It may be challenging, particularly after COVID-19, which revealed deficiencies in many health care systems, and care for hypertension may have been disturbed,” said Dr. Carrillo-Larco, who is not connected with the analysis.
That said, simplified BP screening approaches could help maximize the number of people screened overall, potentially identifying those with hypertension and raising awareness, he proposed. His team’s recent study showed that such approaches vary from country to country but are generally reliable and can be used effectively for population screening.
In addition, Dr. Carrillo-Larco said, any efforts by clinicians to improve adherence and help patients achieve BP control “would also have positive effects at the population level.”
The study was supported by a grant from the Bill & Melinda Gates Foundation, with additional funding by a grant to Dr. Watkins from Resolve to Save Lives. No conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
If 80% of individuals with hypertension were screened, 80% received treatment, and 80% then reached guideline-specified targets, up to 200 million cases of cardiovascular disease (CVD) and 130 million deaths could be averted by 2050, a modeling study suggests.
Achievement of the 80-80-80 target “could be one of the single most important global public health accomplishments of the coming decades,” according to the authors.
“We need to reprioritize hypertension care in our practices,” principal investigator David A. Watkins, MD, MPH, University of Washington, Seattle, told this news organization. “Only about one in five persons with hypertension around the world has their blood pressure well controlled. Oftentimes, clinicians are focused on addressing patients’ other health needs, many of which can be pressing in the short term, and we forget to talk about blood pressure, which has more than earned its reputation as ‘the silent killer.’ ”
The modeling study was published online in Nature Medicine, with lead author Sarah J. Pickersgill, MPH, also from the University of Washington.
Two interventions, three scenarios
Dr. Watkins and colleagues based their analysis on two approaches to blood pressure (BP) control shown to be beneficial: drug treatment to a systolic BP of either 130 mm Hg or 140 mm Hg or less, depending on local guidelines, and dietary sodium reduction, as recommended by the World Health Organization.
The team modeled the impacts of these interventions in 182 countries according to three scenarios:
- Business as usual (control): allowing hypertension to increase at historic rates of change and mean sodium intake to remain at current levels
- Progress: matching historically high-performing countries (for example, accelerating hypertension control by about 3% per year at intermediate levels of intervention coverage) while lowering mean sodium intake by 15% by 2030
- Aspirational: hypertension control achieved faster than historically high-performing countries (about 4% per year) and mean sodium intake decreased by 30% by 2027
The analysis suggests that in the progressive scenario, all countries could achieve 80-80-80 targets by 2050 and most countries by 2040; the aspirational scenario would have all countries meeting them by 2040. That would result in reductions in all-cause mortality of 4%-7% (76 million to 130 million deaths averted) with progressive and aspirational interventions, respectively, compared with the control scenario.
There would also be a slower rise in expected CVD from population growth and aging (110 million to 200 million cases averted). That is, the probability of dying from any CVD cause between the ages of 30 and 80 years would be reduced by 16% in the progressive scenario and 26% in the aspirational scenario.
Of note, about 83%-85% of the potential mortality reductions would result from scaling up hypertension treatment in the progressive and aspirational scenarios, respectively, with the remaining 15%-17% coming from sodium reduction, the researchers state.
Further, they propose, scaling up BP interventions could reduce CVD inequalities across countries, with low-income and lower-middle-income countries likely experiencing the largest reductions in disease rates and mortality.
Implementation barriers
“Health systems in many low- and middle-income countries have not traditionally been set up to succeed in chronic disease management in primary care,” Dr. Watkins noted. For interventions to be successful, he said, “several barriers need to be addressed, including: low population awareness of chronic diseases like hypertension and diabetes, which leads to low rates of screening and treatment; high out-of-pocket cost and low availability of medicines for chronic diseases; and need for adherence support and provider incentives for improving quality of chronic disease care in primary care settings.”
“Based on the analysis, achieving the 80-80-80 seems feasible, though actually getting there may be much more complicated. I wonder whether countries have the resources to implement the needed policies,” Rodrigo M. Carrillo-Larco, MD, researcher, department of epidemiology and biostatistics, School of Public Health, Imperial College London, told this news organization.
“It may be challenging, particularly after COVID-19, which revealed deficiencies in many health care systems, and care for hypertension may have been disturbed,” said Dr. Carrillo-Larco, who is not connected with the analysis.
That said, simplified BP screening approaches could help maximize the number of people screened overall, potentially identifying those with hypertension and raising awareness, he proposed. His team’s recent study showed that such approaches vary from country to country but are generally reliable and can be used effectively for population screening.
In addition, Dr. Carrillo-Larco said, any efforts by clinicians to improve adherence and help patients achieve BP control “would also have positive effects at the population level.”
The study was supported by a grant from the Bill & Melinda Gates Foundation, with additional funding by a grant to Dr. Watkins from Resolve to Save Lives. No conflicts of interest were declared.
A version of this article first appeared on Medscape.com.
The next blood pressure breakthrough: temporary tattoos
As scientists work on wearable technology that promises to revolutionize health care, researchers from the University of Texas at Austin and Texas A&M University, College Station, are reporting a big win in the pursuit of one highly popular target: a noninvasive solution for continuous blood pressure monitoring at home.
Not only that, but this development comes in the surprising form of a temporary tattoo. That’s right: Just like the kind that children like to wear.
the researchers report in their new study.
“With this new technology, we are going to have an opportunity to understand how our blood pressure fluctuates during the day. We will be able to quantify how stress is impacting us,” says Roozbeh Jafari, PhD, a professor of biomedical engineering, electrical engineering, and computer science at Texas A&M, College Station, and a coauthor of the study.
Revealing the whole picture, not just dots
At-home blood pressure monitors have been around for many years now. They work just like the blood pressure machines doctors use at their office: You place your arm inside a cuff, press a button, feel a squeeze on your arm, and get a reading.
While results from this method are accurate, they are also just a moment in time. Our blood pressure can vary greatly throughout the day – especially among people who have labile hypertension, where blood pressure changes from one extreme to the other. So, looking at point-in-time readings is a bit like focusing on a few dots inside of a pointillism painting – one might miss the bigger picture.
Doctors may also find continuous monitoring useful for getting rid of false readings from “white coat syndrome.” Basically, this means a person’s blood pressure rises due to the anxiety of being in a doctor’s office but is not true hypertension.
Bottom line: The ability to monitor a person’s blood pressure continuously for hours or even days can provide clearer, and more accurate, insights into a person’s health.
How do health monitoring tattoos work?
Electronic tattoos for health monitoring are not completely new. John A. Rogers, PhD, of Northwestern University, Chicago, first put forth the idea of monitoring through temporary tattoos 12 years ago. Some concepts, such as UV monitoring tattoos, had already been adopted by scientists and put on the market. But the existing models weren’t suitable for monitoring blood pressure, according to Deji Akinwande, PhD, a professor of electrical and computer engineering at the University of Texas at Austin and another coauthor of the study.
“[UV monitoring tattoos] are very thick,” he says. “They create too much movement when used to measure blood pressure because they slide around.”
So, the Texas-based research team worked to develop an option that was slimmer and more stable.
“The key ingredient within e-tattoos is graphene,” says Dr. Akinwande.
Graphene is carbon that’s similar to what’s inside your graphite pencil. The material is conductive, meaning it can conduct small electrical currents through the skin. For blood pressure monitoring, graphene promotes bioelectrical impedance analysis (BIA), which is like the technology used in smart scales that measure body fat.
With e-tattoos, the thin layers of graphene stick to the skin and do not slide around, getting rid of “artifacts,” or bad data. The graphene e-tattoos can be worn on the skin for about a week – or roughly as long as the temporary tattoos kids love.
Once the graphene captures the raw data, a machine learning algorithm interprets the information and provides results in units used for measuring blood pressure: millimeters of mercury (mmHg), commonly referred to as blood pressure “points.”
How accurate are the results? The tests measured blood pressure within 0.2 ± 5.8 mmHg (systolic), 0.2 ± 4.5 mmHg (diastolic), and 0.1 ± 5.3 mmHg (mean arterial pressure). In other words: If this were a basketball player shooting baskets, the great majority of shots taken would be swishes and occasionally a few would hit the rim. That means good accuracy.
When will e-tattoos be available?
The teams of Dr. Jafari and Dr. Akinwande are working on a second generation of their e-tattoo that they expect to be available in the next 5 years.
The upgrade they envision will be smaller and compatible with smartwatches and phones that use Bluetooth technology and near-field communication (NFC) to transfer data and give it power. With these updates, e-tattoos for continuous blood pressure monitoring will be ready for clinical trials and mainstream use soon after.
“Everyone can benefit from knowing their blood pressure recordings,” Dr. Akinwande says. “It is not just for people at risk for hypertension but for others to proactively monitor their health, for stress and other factors.”
A version of this article first appeared on WebMD.com.
As scientists work on wearable technology that promises to revolutionize health care, researchers from the University of Texas at Austin and Texas A&M University, College Station, are reporting a big win in the pursuit of one highly popular target: a noninvasive solution for continuous blood pressure monitoring at home.
Not only that, but this development comes in the surprising form of a temporary tattoo. That’s right: Just like the kind that children like to wear.
the researchers report in their new study.
“With this new technology, we are going to have an opportunity to understand how our blood pressure fluctuates during the day. We will be able to quantify how stress is impacting us,” says Roozbeh Jafari, PhD, a professor of biomedical engineering, electrical engineering, and computer science at Texas A&M, College Station, and a coauthor of the study.
Revealing the whole picture, not just dots
At-home blood pressure monitors have been around for many years now. They work just like the blood pressure machines doctors use at their office: You place your arm inside a cuff, press a button, feel a squeeze on your arm, and get a reading.
While results from this method are accurate, they are also just a moment in time. Our blood pressure can vary greatly throughout the day – especially among people who have labile hypertension, where blood pressure changes from one extreme to the other. So, looking at point-in-time readings is a bit like focusing on a few dots inside of a pointillism painting – one might miss the bigger picture.
Doctors may also find continuous monitoring useful for getting rid of false readings from “white coat syndrome.” Basically, this means a person’s blood pressure rises due to the anxiety of being in a doctor’s office but is not true hypertension.
Bottom line: The ability to monitor a person’s blood pressure continuously for hours or even days can provide clearer, and more accurate, insights into a person’s health.
How do health monitoring tattoos work?
Electronic tattoos for health monitoring are not completely new. John A. Rogers, PhD, of Northwestern University, Chicago, first put forth the idea of monitoring through temporary tattoos 12 years ago. Some concepts, such as UV monitoring tattoos, had already been adopted by scientists and put on the market. But the existing models weren’t suitable for monitoring blood pressure, according to Deji Akinwande, PhD, a professor of electrical and computer engineering at the University of Texas at Austin and another coauthor of the study.
“[UV monitoring tattoos] are very thick,” he says. “They create too much movement when used to measure blood pressure because they slide around.”
So, the Texas-based research team worked to develop an option that was slimmer and more stable.
“The key ingredient within e-tattoos is graphene,” says Dr. Akinwande.
Graphene is carbon that’s similar to what’s inside your graphite pencil. The material is conductive, meaning it can conduct small electrical currents through the skin. For blood pressure monitoring, graphene promotes bioelectrical impedance analysis (BIA), which is like the technology used in smart scales that measure body fat.
With e-tattoos, the thin layers of graphene stick to the skin and do not slide around, getting rid of “artifacts,” or bad data. The graphene e-tattoos can be worn on the skin for about a week – or roughly as long as the temporary tattoos kids love.
Once the graphene captures the raw data, a machine learning algorithm interprets the information and provides results in units used for measuring blood pressure: millimeters of mercury (mmHg), commonly referred to as blood pressure “points.”
How accurate are the results? The tests measured blood pressure within 0.2 ± 5.8 mmHg (systolic), 0.2 ± 4.5 mmHg (diastolic), and 0.1 ± 5.3 mmHg (mean arterial pressure). In other words: If this were a basketball player shooting baskets, the great majority of shots taken would be swishes and occasionally a few would hit the rim. That means good accuracy.
When will e-tattoos be available?
The teams of Dr. Jafari and Dr. Akinwande are working on a second generation of their e-tattoo that they expect to be available in the next 5 years.
The upgrade they envision will be smaller and compatible with smartwatches and phones that use Bluetooth technology and near-field communication (NFC) to transfer data and give it power. With these updates, e-tattoos for continuous blood pressure monitoring will be ready for clinical trials and mainstream use soon after.
“Everyone can benefit from knowing their blood pressure recordings,” Dr. Akinwande says. “It is not just for people at risk for hypertension but for others to proactively monitor their health, for stress and other factors.”
A version of this article first appeared on WebMD.com.
As scientists work on wearable technology that promises to revolutionize health care, researchers from the University of Texas at Austin and Texas A&M University, College Station, are reporting a big win in the pursuit of one highly popular target: a noninvasive solution for continuous blood pressure monitoring at home.
Not only that, but this development comes in the surprising form of a temporary tattoo. That’s right: Just like the kind that children like to wear.
the researchers report in their new study.
“With this new technology, we are going to have an opportunity to understand how our blood pressure fluctuates during the day. We will be able to quantify how stress is impacting us,” says Roozbeh Jafari, PhD, a professor of biomedical engineering, electrical engineering, and computer science at Texas A&M, College Station, and a coauthor of the study.
Revealing the whole picture, not just dots
At-home blood pressure monitors have been around for many years now. They work just like the blood pressure machines doctors use at their office: You place your arm inside a cuff, press a button, feel a squeeze on your arm, and get a reading.
While results from this method are accurate, they are also just a moment in time. Our blood pressure can vary greatly throughout the day – especially among people who have labile hypertension, where blood pressure changes from one extreme to the other. So, looking at point-in-time readings is a bit like focusing on a few dots inside of a pointillism painting – one might miss the bigger picture.
Doctors may also find continuous monitoring useful for getting rid of false readings from “white coat syndrome.” Basically, this means a person’s blood pressure rises due to the anxiety of being in a doctor’s office but is not true hypertension.
Bottom line: The ability to monitor a person’s blood pressure continuously for hours or even days can provide clearer, and more accurate, insights into a person’s health.
How do health monitoring tattoos work?
Electronic tattoos for health monitoring are not completely new. John A. Rogers, PhD, of Northwestern University, Chicago, first put forth the idea of monitoring through temporary tattoos 12 years ago. Some concepts, such as UV monitoring tattoos, had already been adopted by scientists and put on the market. But the existing models weren’t suitable for monitoring blood pressure, according to Deji Akinwande, PhD, a professor of electrical and computer engineering at the University of Texas at Austin and another coauthor of the study.
“[UV monitoring tattoos] are very thick,” he says. “They create too much movement when used to measure blood pressure because they slide around.”
So, the Texas-based research team worked to develop an option that was slimmer and more stable.
“The key ingredient within e-tattoos is graphene,” says Dr. Akinwande.
Graphene is carbon that’s similar to what’s inside your graphite pencil. The material is conductive, meaning it can conduct small electrical currents through the skin. For blood pressure monitoring, graphene promotes bioelectrical impedance analysis (BIA), which is like the technology used in smart scales that measure body fat.
With e-tattoos, the thin layers of graphene stick to the skin and do not slide around, getting rid of “artifacts,” or bad data. The graphene e-tattoos can be worn on the skin for about a week – or roughly as long as the temporary tattoos kids love.
Once the graphene captures the raw data, a machine learning algorithm interprets the information and provides results in units used for measuring blood pressure: millimeters of mercury (mmHg), commonly referred to as blood pressure “points.”
How accurate are the results? The tests measured blood pressure within 0.2 ± 5.8 mmHg (systolic), 0.2 ± 4.5 mmHg (diastolic), and 0.1 ± 5.3 mmHg (mean arterial pressure). In other words: If this were a basketball player shooting baskets, the great majority of shots taken would be swishes and occasionally a few would hit the rim. That means good accuracy.
When will e-tattoos be available?
The teams of Dr. Jafari and Dr. Akinwande are working on a second generation of their e-tattoo that they expect to be available in the next 5 years.
The upgrade they envision will be smaller and compatible with smartwatches and phones that use Bluetooth technology and near-field communication (NFC) to transfer data and give it power. With these updates, e-tattoos for continuous blood pressure monitoring will be ready for clinical trials and mainstream use soon after.
“Everyone can benefit from knowing their blood pressure recordings,” Dr. Akinwande says. “It is not just for people at risk for hypertension but for others to proactively monitor their health, for stress and other factors.”
A version of this article first appeared on WebMD.com.
Hypertension heightens risk for severe COVID-19, even in the fully vaxxed
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
Adults with hypertension who were vaccinated for COVID-19 with at least one booster were more than twice as likely as vaccinated and boosted individuals without hypertension to be hospitalized for severe COVID-19, according to data from more than 900 individuals.
“We were surprised to learn that many people who were hospitalized with COVID-19 had hypertension and no other risk factors,” said Susan Cheng, MD, MPH, director of the Institute for Research on Healthy Aging in the department of cardiology at the Smidt Heart Institute, Los Angeles, and a senior author of the study. “This is concerning when you consider that almost half of American adults have high blood pressure.”
COVID-19 vaccines demonstrated ability to reduce death and some of the most severe side effects from the infection in the early stages of the pandemic. Although the Omicron surge prompted recommendations for a third mRNA vaccine dose, “a proportion of individuals who received three mRNA vaccine doses still required hospitalization for COVID-19 during the Omicron surge,” and the characteristics associated with severe illness in vaccinated and boosted patients have not been explored, Joseph Ebinger, MD, of Cedars-Sinai Medical Center, Los Angeles, and colleagues wrote.
Previous research has shown an association between high blood pressure an increased risk for developing severe COVID-19 compared to several other chronic health conditions, including kidney disease, type 2 diabetes, chronic obstructive pulmonary disease, and heart failure, the researchers noted.
In a study published in Hypertension, the researchers identified 912 adults who received at least three doses of mRNA COVID-19 vaccine and were later diagnosed with COVID-19 during the surge in infections from the Omicron variant between December 2021 and April 2022.
A total of 145 of the individuals were hospitalized (16%); of these, 125 (86%) had hypertension.
Patients with hypertension were the most likely to be hospitalized, with an odds ratio of 2.9. In addition to high blood pressure, factors including older age (OR, 1.3), chronic kidney disease (OR, 2.2), prior myocardial infarction or heart failure (OR, 2.2), and longer time since the last vaccination and COVID-19 infection were associated with increased risk of hospitalization in a multivariate analysis.
However, the increased risk of severe illness and hospitalization associated with high blood pressure persisted, with an OR of 2.6, in the absence of comorbid conditions such as type 2 diabetes, kidney disease, and heart failure, the researchers emphasized.
“Although the mechanism for hypertension-associated COVID-19 risk remains unclear, prior studies have identified delayed SARS-CoV-2 viral clearance and prolonged inflammatory response among hypertensive patients, which may contribute to greater disease severity,” they wrote.
The findings were limited by several factors, including the use of data from a single center and lack of information on which Omicron variants and subvariants were behind the infections, the researchers noted.
However, the results highlight the need for more research on how to reduce the risks of severe COVID-19 in vulnerable populations, and on the mechanism for a potential connection between high blood pressure and severe COVID-19, they said.
Given the high prevalence of hypertension worldwide, increased understanding of the hypertension-specific risks and identification of individual and population-level risk reduction strategies will be important to the transition of COVID-19 from pandemic to endemic, they concluded.
Omicron changes the game
“When the pandemic initially started, many conditions were seen to increase risk for more severe COVID illness, and hypertension was one of those factors – and then things changed,” lead author Dr. Ebinger said in an interview. “First, vaccines arrived on the scene and substantially reduced risk of severe COVID for everyone who received them. Second, Omicron arrived and, while more transmissible, this variant has been less likely to cause severe COVID. On the one hand, we have vaccines and boosters that we want to think of as ‘the great equalizer’ when it comes to preexisting conditions. On the other hand, we have a dominant set of SARS-CoV-2 subvariants that seem less virulent in most people.
“Taken together, we have been hoping and even assuming that we have been doing pretty well with minimizing risks. Unfortunately, our study results indicate this is not exactly the case,” he said.
“Although vaccines and boosters appear to have equalized or minimized the risks of severe COVID for some people, this has not happened for others – even in the setting of the milder Omicron variant. Of individuals who were fully vaccinated and boosted, having hypertension increased the odds of needing to be hospitalized after getting infected with Omicron by 2.6-fold, even when accounting for or in the absence of having any major chronic disease that might otherwise predispose to more severe COVID-19 illness,” Dr. Ebinger added.
“So, while the originally seen risks of having obesity or diabetes seem to have been minimized during this current era of pandemic, the risk of having hypertension has persisted. We found this both surprising and concerning, because hypertension is very common and present in over half of people over age 50.”
Surprisingly, “we found that a fair number of people, even after being fully vaccinated plus a having gotten a booster, will not only catch Omicron but get sick enough to need hospital care,” Dr. Ebinger emphasized. “Moreover, it is not just older adults with major comorbid conditions who are vulnerable. Our data show that this can happen to an adult of any age and especially if a person has only hypertension and otherwise no major chronic disease.”
The first takeaway message for clinicians at this time is to raise awareness, Dr. Ebinger stressed in the interview. “We need to raise understanding around the fact that receiving three doses of vaccine may not prevent severe COVID-19 illness in everyone, even when the circulating viral variant is presumed to be causing only mild disease in most people. Moreover, the people who are most at risk are not whom we might think they are. They are not the sickest of the sick. They include people who might not have major conditions such as heart disease or kidney disease, but they do have hypertension.”
Second, “we need more research to understand out why there is this link between hypertension and excess risk for the more severe forms of COVID-19, despite it arising from a supposedly milder variant,” said Dr. Ebinger.
“Third, we need to determine how to reduce these risks, whether through more tailored vaccine regimens or novel therapeutics or a combination approach,” he said.
Looking ahead, “the biological mechanism underpinning the association between hypertension and severe COVID-19 remains underexplored. Future work should focus on understanding the factors linking hypertension to severe COVID-19, as this may elucidate both information on how SARS-CoV-2 effects the body and potential targets for intervention,” Dr. Ebinger added.
The study was supported in part by Cedars-Sinai Medical Center, the Erika J. Glazer Family Foundation and the National Institutes of Health. The researchers had no financial conflicts to disclose.
FROM HYPERTENSION
Nurses’ cohort study: Endometriosis elevates stroke risk
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
Women who’ve had endometriosis carry an elevated risk of stroke with them for the rest of their lives, with the greatest risk found in women who’ve had a hysterectomy with an oophorectomy, according to a cohort study of the Nurses’ Health Study.
“This is yet additional evidence that those girls and women with endometriosis are having effects across their lives and in multiple aspects of their health and well-being,” senior study author Stacey A. Missmer, ScD, of the Michigan State University, East Lansing, said in an interview. “This is not, in quotes ‘just a gynecologic condition,’ ” Dr. Missmer added. “It is not strictly about the pelvic pain or infertility, but it really is about the whole health across the life course.”
The study included 112,056 women in the NHSII cohort study who were followed from 1989 to June 2017, documenting 893 incident cases of stroke among them – an incidence of less than 1%. Endometriosis was reported in 5,244 women, and 93% of the cohort were White.
Multivariate adjusted models showed that women who had laparoscopically confirmed endometriosis had a 34% greater risk of stroke than women without a history of endometriosis. Leslie V. Farland, ScD, of the University of Arizona, Tucson, was lead author of the study.
While previous studies have demonstrated an increased risk of cardiovascular disease, heart attack, angina, and atherosclerosis in women who’ve had endometriosis, this is the first study that has confirmed an additional increased risk of stroke, Dr. Missmer said.
Another novel finding, Dr. Missmer said, is that while the CVD risks for these women “seem to peak at an earlier age,” the study found no age differences for stroke risk. “That also reinforces that these stroke events are often happening in an age range typical for stroke, which is further removed from when women are thinking about their gynecologic health specifically.”
These findings don’t translate into a significantly greater risk for stroke overall in women who’ve had endometriosis, Dr. Missmer said. She characterized the risk as “not negligible, but it’s not a huge increased risk.” The absolute risk is still fairly low, she said.
“We don’t want to give the impression that all women with endometriosis need to be panicked or fearful about stroke, she said. “Rather, the messaging is that this yet another bit of evidence that whole health care for those with endometriosis is important.”
Women who’ve had endometriosis and their primary care providers need to be attuned to stroke risk, she said. “This is a critical condition that primary care physicians need to engage around, and perhaps if symptoms related to cardiovascular and cerebrovascular disease emerge in their patients, they need to be engaging cardiology and similar types of support. This is not just about the gynecologists.”
The study also explored other factors that may contribute to stroke risk, with the most significant being hysterectomy with bilateral oophorectomy, Dr. Missmer said.
This study was unique because it used laparoscopically confirmed rather than self-reported endometriosis, said Louise D. McCullough, MD, neurology chair at the University of Texas Health Science Center, Houston. Another strength of the study she noted was its longitudinal design, although the cohort study design yielded a low number of stroke patients.
“Regardless, I do think it was a very important study because we have a growing recognition about how women’s health and factors such as pregnancy, infertility, parity, complications, and gonadal hormones such as estrogen can influence a woman’s stroke risk much later in life,” Dr. McCullough said in an interview.
Future studies into the relationship between endometriosis and CVD and stroke risk should focus on the mechanism behind the inflammation that occurs in endometriosis, Dr. McCullough said. “Part of it is probably the loss of hormones if a patient has to have an oophorectomy, but part of it is just what do these diseases do for a woman’s later risk – and for primary care physicians, ob.gyns., and stroke neurologists to recognize that these are questions we should ask: Have you ever had eclampsia or preeclampsia? Did you have endometriosis? Have you had miscarriages?”
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute for Neurological Disorders and Stroke. Dr. Missmer disclosed relationships with Shanghai Huilun Biotechnology, Roche, and AbbVie. Dr. McCullough has no relevant disclosures.
FROM STROKE











