User login
Hypertension goes unmedicated in 40% of adults
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.
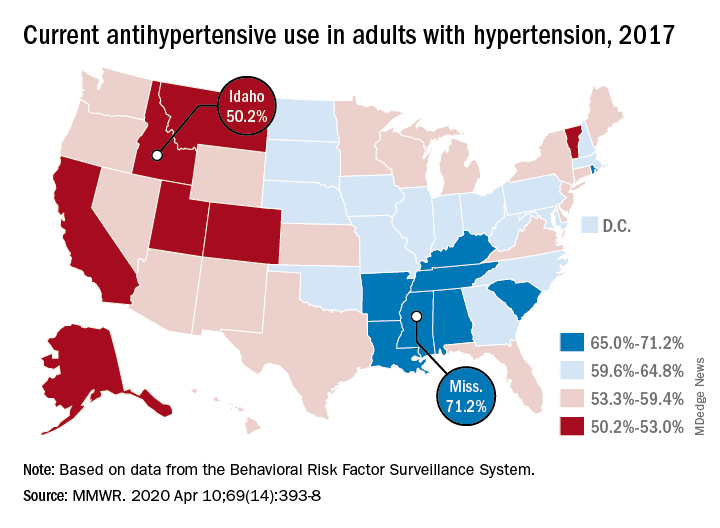
There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
FROM THE MMWR
BPA analogs increase blood pressure in animal study
findings in a new study have shown.
Researchers tested exposures to BPA, as well as bisphenol-S (BPS) and bisphenol-F (BPF), which have been introduced in recent years as BPA alternatives and are now increasingly detectable in human and animal tissues. BPS and BPF are often found in products labeled as “BPA free.”
BPS and BPF have similar physiochemical properties to BPA, and there is concern over their effects.
But their physiological impact is not yet clear, according to Puliyur MohanKumar, DVM, PhD, of the University of Georgia Regenerative Bioscience Center, Athens. “We are exposed to BPA and related compounds on a regular basis, and the important thing is that BPA and related compounds easily cross the placental barrier,” Dr. MohanKumar said during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. MohanKumar and colleagues exposed pregnant rats to BPA, BPS, or BPF. When the offspring reached adulthood, the researchers implanted them with radiotelemetry devices to track systolic and diastolic blood pressure, which they measured every 10 minutes over a 24-hour period. This was repeated once a week for 11 weeks.
“The female offspring had elevated systolic as well as diastolic blood pressure, and this was an increase of about 8 mm [Hg] higher than the control animals. That was pretty significant. Keeping these animals at such a prehypertensive state for such a long period of time is going to [lead to] lots of cardiovascular issues later on,” said Dr. MohanKumar.
Robert Sargis, MD, PhD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, noted that, although animal studies don’t necessarily translate to similar outcomes in humans, the results are cause for concern.
“What’s particularly interesting, is that there is whole area of essential hypertension, where people develop hypertension and we don’t really know why. We just treat it,” he said in an interview. “But thinking about biological origins [of hypertension] is potentially interesting for a couple of reasons. These bisphenol compounds are really common. Most Americans are exposed to bisphenol A, and it’s been associated with other adverse metabolic effects, including alterations to body weight and glucose homeostasis.
“[These findings] feed into a whole series of studies that have begun to look at the BPA replacements and the fact that they may be, at best, as bad as BPA, and at worst, possibly slightly worse, depending on which outcomes you’re looking at,” Dr. Sargis added.
In the study, seven pregnant rats were orally exposed to saline, four pregnant rats to 5 mcg/kg BPA, four to 5 mcg/kg BPS, and five to 1 mcg/kg BPF during days 6-21 of pregnancy. The lower dose of BPF was used because a dose of 5 mcg/kg proved too toxic. When the offspring reached adulthood, the researchers implanted radiotelemetry devices in the offspring’s femoral artery.
Mean daytime systolic BP was highest in the BPA group (133.3 mg Hg; P < .05), followed by BPS (132.5 mm Hg; P < .05) and BPF (129.2 mm Hg; nonsignificant), compared with 125.2 mm Hg in controls. Nighttime systolic BP was again highest in the BPA group (134.2 mm Hg; P < .01), followed by BPS (133.2 mm Hg; P < .05) and BPF (129.6 mm Hg; nonsignificant), compared with 125.1 mm Hg in controls.
During the day, diastolic BP was highest in the BPS group (91.3 mm Hg; P < .01), followed by BPA (88.8 mm Hg; nonsignificant) and BPF (88.6 mm Hg; nonsignificant), compared with 84.1 mm Hg in controls. At night, diastolic BP was highest in the BPS group (89.7 mm Hg; P < .01), followed by BPA (89.6 mm Hg; P < .01) and BPF (88.6 mm Hg; P < .01), compared with 83.3 mm Hg in controls.
During the day, mean arterial pressure was highest in the BPA group (110.5 mm Hg; P < .01), followed by BPS (108.9 mm Hg; P < .01) and BPF (105.2 mm Hg; nonsignificant), compared with 102.6 mm Hg in controls. At night, mean arterial pressure was highest in BPS (108.6 mm Hg; P < .05), followed by BPA (107.5 mm Hg; nonsignificant) and BPF (105.7 mm Hg; nonsignificant), compared with 101.8 mm Hg in controls.
“These results indicate that prenatal exposure to low levels of BPA analogs has a profound effect on hypertension” in the offspring of pregnant rats exposed to bisphenols, Dr. MohanKumar and colleagues wrote in the abstract.
He noted during his presentation that he and his colleagues plan to repeat the study in male offspring to determine if there are sex differences.
Dr. MohanKumar and colleagues reported having no relevant financial disclosures. Dr. Sargis also reported no conflicts of interest.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: MohanKumar P et al. ENDO 2020, Abstract 719.
This article was updated on 4/17/2020.
findings in a new study have shown.
Researchers tested exposures to BPA, as well as bisphenol-S (BPS) and bisphenol-F (BPF), which have been introduced in recent years as BPA alternatives and are now increasingly detectable in human and animal tissues. BPS and BPF are often found in products labeled as “BPA free.”
BPS and BPF have similar physiochemical properties to BPA, and there is concern over their effects.
But their physiological impact is not yet clear, according to Puliyur MohanKumar, DVM, PhD, of the University of Georgia Regenerative Bioscience Center, Athens. “We are exposed to BPA and related compounds on a regular basis, and the important thing is that BPA and related compounds easily cross the placental barrier,” Dr. MohanKumar said during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. MohanKumar and colleagues exposed pregnant rats to BPA, BPS, or BPF. When the offspring reached adulthood, the researchers implanted them with radiotelemetry devices to track systolic and diastolic blood pressure, which they measured every 10 minutes over a 24-hour period. This was repeated once a week for 11 weeks.
“The female offspring had elevated systolic as well as diastolic blood pressure, and this was an increase of about 8 mm [Hg] higher than the control animals. That was pretty significant. Keeping these animals at such a prehypertensive state for such a long period of time is going to [lead to] lots of cardiovascular issues later on,” said Dr. MohanKumar.
Robert Sargis, MD, PhD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, noted that, although animal studies don’t necessarily translate to similar outcomes in humans, the results are cause for concern.
“What’s particularly interesting, is that there is whole area of essential hypertension, where people develop hypertension and we don’t really know why. We just treat it,” he said in an interview. “But thinking about biological origins [of hypertension] is potentially interesting for a couple of reasons. These bisphenol compounds are really common. Most Americans are exposed to bisphenol A, and it’s been associated with other adverse metabolic effects, including alterations to body weight and glucose homeostasis.
“[These findings] feed into a whole series of studies that have begun to look at the BPA replacements and the fact that they may be, at best, as bad as BPA, and at worst, possibly slightly worse, depending on which outcomes you’re looking at,” Dr. Sargis added.
In the study, seven pregnant rats were orally exposed to saline, four pregnant rats to 5 mcg/kg BPA, four to 5 mcg/kg BPS, and five to 1 mcg/kg BPF during days 6-21 of pregnancy. The lower dose of BPF was used because a dose of 5 mcg/kg proved too toxic. When the offspring reached adulthood, the researchers implanted radiotelemetry devices in the offspring’s femoral artery.
Mean daytime systolic BP was highest in the BPA group (133.3 mg Hg; P < .05), followed by BPS (132.5 mm Hg; P < .05) and BPF (129.2 mm Hg; nonsignificant), compared with 125.2 mm Hg in controls. Nighttime systolic BP was again highest in the BPA group (134.2 mm Hg; P < .01), followed by BPS (133.2 mm Hg; P < .05) and BPF (129.6 mm Hg; nonsignificant), compared with 125.1 mm Hg in controls.
During the day, diastolic BP was highest in the BPS group (91.3 mm Hg; P < .01), followed by BPA (88.8 mm Hg; nonsignificant) and BPF (88.6 mm Hg; nonsignificant), compared with 84.1 mm Hg in controls. At night, diastolic BP was highest in the BPS group (89.7 mm Hg; P < .01), followed by BPA (89.6 mm Hg; P < .01) and BPF (88.6 mm Hg; P < .01), compared with 83.3 mm Hg in controls.
During the day, mean arterial pressure was highest in the BPA group (110.5 mm Hg; P < .01), followed by BPS (108.9 mm Hg; P < .01) and BPF (105.2 mm Hg; nonsignificant), compared with 102.6 mm Hg in controls. At night, mean arterial pressure was highest in BPS (108.6 mm Hg; P < .05), followed by BPA (107.5 mm Hg; nonsignificant) and BPF (105.7 mm Hg; nonsignificant), compared with 101.8 mm Hg in controls.
“These results indicate that prenatal exposure to low levels of BPA analogs has a profound effect on hypertension” in the offspring of pregnant rats exposed to bisphenols, Dr. MohanKumar and colleagues wrote in the abstract.
He noted during his presentation that he and his colleagues plan to repeat the study in male offspring to determine if there are sex differences.
Dr. MohanKumar and colleagues reported having no relevant financial disclosures. Dr. Sargis also reported no conflicts of interest.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: MohanKumar P et al. ENDO 2020, Abstract 719.
This article was updated on 4/17/2020.
findings in a new study have shown.
Researchers tested exposures to BPA, as well as bisphenol-S (BPS) and bisphenol-F (BPF), which have been introduced in recent years as BPA alternatives and are now increasingly detectable in human and animal tissues. BPS and BPF are often found in products labeled as “BPA free.”
BPS and BPF have similar physiochemical properties to BPA, and there is concern over their effects.
But their physiological impact is not yet clear, according to Puliyur MohanKumar, DVM, PhD, of the University of Georgia Regenerative Bioscience Center, Athens. “We are exposed to BPA and related compounds on a regular basis, and the important thing is that BPA and related compounds easily cross the placental barrier,” Dr. MohanKumar said during a virtual news conference held by the Endocrine Society. The study had been slated for presentation during ENDO 2020, the society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. MohanKumar and colleagues exposed pregnant rats to BPA, BPS, or BPF. When the offspring reached adulthood, the researchers implanted them with radiotelemetry devices to track systolic and diastolic blood pressure, which they measured every 10 minutes over a 24-hour period. This was repeated once a week for 11 weeks.
“The female offspring had elevated systolic as well as diastolic blood pressure, and this was an increase of about 8 mm [Hg] higher than the control animals. That was pretty significant. Keeping these animals at such a prehypertensive state for such a long period of time is going to [lead to] lots of cardiovascular issues later on,” said Dr. MohanKumar.
Robert Sargis, MD, PhD, professor of endocrinology, diabetes, and metabolism at the University of Illinois at Chicago, noted that, although animal studies don’t necessarily translate to similar outcomes in humans, the results are cause for concern.
“What’s particularly interesting, is that there is whole area of essential hypertension, where people develop hypertension and we don’t really know why. We just treat it,” he said in an interview. “But thinking about biological origins [of hypertension] is potentially interesting for a couple of reasons. These bisphenol compounds are really common. Most Americans are exposed to bisphenol A, and it’s been associated with other adverse metabolic effects, including alterations to body weight and glucose homeostasis.
“[These findings] feed into a whole series of studies that have begun to look at the BPA replacements and the fact that they may be, at best, as bad as BPA, and at worst, possibly slightly worse, depending on which outcomes you’re looking at,” Dr. Sargis added.
In the study, seven pregnant rats were orally exposed to saline, four pregnant rats to 5 mcg/kg BPA, four to 5 mcg/kg BPS, and five to 1 mcg/kg BPF during days 6-21 of pregnancy. The lower dose of BPF was used because a dose of 5 mcg/kg proved too toxic. When the offspring reached adulthood, the researchers implanted radiotelemetry devices in the offspring’s femoral artery.
Mean daytime systolic BP was highest in the BPA group (133.3 mg Hg; P < .05), followed by BPS (132.5 mm Hg; P < .05) and BPF (129.2 mm Hg; nonsignificant), compared with 125.2 mm Hg in controls. Nighttime systolic BP was again highest in the BPA group (134.2 mm Hg; P < .01), followed by BPS (133.2 mm Hg; P < .05) and BPF (129.6 mm Hg; nonsignificant), compared with 125.1 mm Hg in controls.
During the day, diastolic BP was highest in the BPS group (91.3 mm Hg; P < .01), followed by BPA (88.8 mm Hg; nonsignificant) and BPF (88.6 mm Hg; nonsignificant), compared with 84.1 mm Hg in controls. At night, diastolic BP was highest in the BPS group (89.7 mm Hg; P < .01), followed by BPA (89.6 mm Hg; P < .01) and BPF (88.6 mm Hg; P < .01), compared with 83.3 mm Hg in controls.
During the day, mean arterial pressure was highest in the BPA group (110.5 mm Hg; P < .01), followed by BPS (108.9 mm Hg; P < .01) and BPF (105.2 mm Hg; nonsignificant), compared with 102.6 mm Hg in controls. At night, mean arterial pressure was highest in BPS (108.6 mm Hg; P < .05), followed by BPA (107.5 mm Hg; nonsignificant) and BPF (105.7 mm Hg; nonsignificant), compared with 101.8 mm Hg in controls.
“These results indicate that prenatal exposure to low levels of BPA analogs has a profound effect on hypertension” in the offspring of pregnant rats exposed to bisphenols, Dr. MohanKumar and colleagues wrote in the abstract.
He noted during his presentation that he and his colleagues plan to repeat the study in male offspring to determine if there are sex differences.
Dr. MohanKumar and colleagues reported having no relevant financial disclosures. Dr. Sargis also reported no conflicts of interest.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: MohanKumar P et al. ENDO 2020, Abstract 719.
This article was updated on 4/17/2020.
FROM ENDO 2020
Renal denervation shown safe and effective in pivotal trial
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
REPORTING FROM ACC 20
Primordial cardiovascular prevention draws closer
A powerful genetic predisposition to cardiovascular disease was overcome by low lifetime exposure to LDL cholesterol and systolic blood pressure in a naturalistic study conducted in nearly half a million people, Brian A. Ference, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This novel finding potentially opens the door to primordial cardiovascular prevention, the earliest possible form of primary prevention, in which cardiovascular risk factors are curtailed before they can become established.
“It’s important to note that the trajectories of lifetime risk for cardiovascular disease predicted by a PGS [polygenic risk score] are not fixed. At the same level of a PGS for coronary artery disease, participants with lower lifetime exposure to LDL and systolic blood pressure had a lower trajectory of risk for cardiovascular disease. This finding implies that the trajectory of cardiovascular risk predicted by a PGS can be reduced by lowering LDL and blood pressure,” observed Dr. Ference, professor of translational therapeutics and executive director of the Center for Naturally Randomised Trials at the University of Cambridge (England).
Together with an international team of coinvestigators, he analyzed lifetime cardiovascular risk as predicted by a PGS derived by genomic testing in relation to lifetime LDL and systolic blood pressure levels in 445,566 participants in the UK Biobank. Subjects had a mean age of 57.2 years at enrollment and 65.2 years at last follow-up. The primary study outcome, a first major coronary event (MCE) as defined by a fatal or nonfatal MI or coronary revascularization, occurred in 23,032 subjects.
The investigators found a stepwise increase in MCE risk across increasing quintiles of genetic risk as reflected in the PGS, such that participants in the top PGS quintile were at 2.8-fold greater risk of an MCE than those in the first quintile. The risk was essentially the same in men and women.
A key finding was that, at any level of lifetime MCE risk as defined by PGS, the actual event rate varied 10-fold depending upon lifetime exposure to LDL cholesterol and systolic blood pressure (SBP). For example, men in the top PGS quintile with high lifetime SBP and LDL cholesterol had a 93% lifetime MCE risk, but that MCE risk plummeted to 8% in those in the top quintile but with low lifetime SBP and LDL cholesterol.
Small differences in those two cardiovascular risk factors over the course of many decades had a big impact. For example, it took only a 10-mg/dL lower lifetime exposure to LDL cholesterol and a 2–mm Hg lower SBP to blunt the trajectory of lifetime risk for MCE in individuals in the middle quintile of PGS to the more favorable trajectory of those in the lowest PGS quintile. Conversely, with a 10-mg/dL increase in LDL cholesterol and 2–mm Hg greater SBP over the course of a lifetime, the trajectory of risk for people in the middle quintile of PGS became essentially superimposable upon the trajectory associated with the highest PGS quintile, the cardiologist explained.
“Participants with low lifetime exposure to LDL and blood pressure had a low lifetime risk of cardiovascular disease at all levels of PGS for coronary disease. This implies that LDL and blood pressure, which are modifiable, may be more powerful determinants of lifetime risk than polygenic predisposition,” Dr. Ference declared.
Discussant Vera Bittner, MD, professor of medicine at the University of Alabama, Birmingham, said that for her this study carried a heartening take-home message: “The polygenic risk score can stratify the population into different risk groups and, at the same time, lifetime exposure to LDL and blood pressure significantly modifies the risk, suggesting that genetics is not destiny, and we may be able to intervene.”
“To be able to know what your cardiovascular risk is from an early age and to plan therapies to prevent cardiovascular disease would be incredible,” agreed session chair B. Hadley Wilson, MD, of the Sanger Heart and Vascular Institute in Charlotte, N.C.
Sekar Kathiresan, MD, said the study introduces the PGS as a new risk factor for coronary artery disease. Focusing efforts to achieve lifelong low exposure to LDL cholesterol and blood pressure in those individuals in the top 10%-20% in PGS should provide a great absolute reduction in MCE risk.
“It potentially can give you a 30- or 40-year head start in understanding who’s at risk because the factor can be measured as early as birth,” observed Dr. Kathiresan, a cardiologist who is director of the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
“It’s also very inexpensive: You get the information once, bank it, and use it throughout life,” noted Paul M. Ridker, MD, director of the Center for Cardiovascular Disease Prevention and professor of medicine at Harvard Medical School, Boston.
“A genome-wide scan will give us information not just on cardiovascular risk, but on cancer risk, on risk of kidney disease, and on the risk of a host of other issues. It’s a very different way of thinking about risk presentation across a whole variety of endpoints,” Dr. Ridker added.
Dr. Ference reported receiving fees and/or research grants from Merck, Amgen, Regeneron, Sanofi, Novartis, Pfizer, Eli Lilly, NovoNordisk, The Medicines Company, Mylan, Daiichi Sankyo, Silence Therapeutics, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, KrKa Pharmaceuticals, Medtronic, and Celera.
A powerful genetic predisposition to cardiovascular disease was overcome by low lifetime exposure to LDL cholesterol and systolic blood pressure in a naturalistic study conducted in nearly half a million people, Brian A. Ference, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This novel finding potentially opens the door to primordial cardiovascular prevention, the earliest possible form of primary prevention, in which cardiovascular risk factors are curtailed before they can become established.
“It’s important to note that the trajectories of lifetime risk for cardiovascular disease predicted by a PGS [polygenic risk score] are not fixed. At the same level of a PGS for coronary artery disease, participants with lower lifetime exposure to LDL and systolic blood pressure had a lower trajectory of risk for cardiovascular disease. This finding implies that the trajectory of cardiovascular risk predicted by a PGS can be reduced by lowering LDL and blood pressure,” observed Dr. Ference, professor of translational therapeutics and executive director of the Center for Naturally Randomised Trials at the University of Cambridge (England).
Together with an international team of coinvestigators, he analyzed lifetime cardiovascular risk as predicted by a PGS derived by genomic testing in relation to lifetime LDL and systolic blood pressure levels in 445,566 participants in the UK Biobank. Subjects had a mean age of 57.2 years at enrollment and 65.2 years at last follow-up. The primary study outcome, a first major coronary event (MCE) as defined by a fatal or nonfatal MI or coronary revascularization, occurred in 23,032 subjects.
The investigators found a stepwise increase in MCE risk across increasing quintiles of genetic risk as reflected in the PGS, such that participants in the top PGS quintile were at 2.8-fold greater risk of an MCE than those in the first quintile. The risk was essentially the same in men and women.
A key finding was that, at any level of lifetime MCE risk as defined by PGS, the actual event rate varied 10-fold depending upon lifetime exposure to LDL cholesterol and systolic blood pressure (SBP). For example, men in the top PGS quintile with high lifetime SBP and LDL cholesterol had a 93% lifetime MCE risk, but that MCE risk plummeted to 8% in those in the top quintile but with low lifetime SBP and LDL cholesterol.
Small differences in those two cardiovascular risk factors over the course of many decades had a big impact. For example, it took only a 10-mg/dL lower lifetime exposure to LDL cholesterol and a 2–mm Hg lower SBP to blunt the trajectory of lifetime risk for MCE in individuals in the middle quintile of PGS to the more favorable trajectory of those in the lowest PGS quintile. Conversely, with a 10-mg/dL increase in LDL cholesterol and 2–mm Hg greater SBP over the course of a lifetime, the trajectory of risk for people in the middle quintile of PGS became essentially superimposable upon the trajectory associated with the highest PGS quintile, the cardiologist explained.
“Participants with low lifetime exposure to LDL and blood pressure had a low lifetime risk of cardiovascular disease at all levels of PGS for coronary disease. This implies that LDL and blood pressure, which are modifiable, may be more powerful determinants of lifetime risk than polygenic predisposition,” Dr. Ference declared.
Discussant Vera Bittner, MD, professor of medicine at the University of Alabama, Birmingham, said that for her this study carried a heartening take-home message: “The polygenic risk score can stratify the population into different risk groups and, at the same time, lifetime exposure to LDL and blood pressure significantly modifies the risk, suggesting that genetics is not destiny, and we may be able to intervene.”
“To be able to know what your cardiovascular risk is from an early age and to plan therapies to prevent cardiovascular disease would be incredible,” agreed session chair B. Hadley Wilson, MD, of the Sanger Heart and Vascular Institute in Charlotte, N.C.
Sekar Kathiresan, MD, said the study introduces the PGS as a new risk factor for coronary artery disease. Focusing efforts to achieve lifelong low exposure to LDL cholesterol and blood pressure in those individuals in the top 10%-20% in PGS should provide a great absolute reduction in MCE risk.
“It potentially can give you a 30- or 40-year head start in understanding who’s at risk because the factor can be measured as early as birth,” observed Dr. Kathiresan, a cardiologist who is director of the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
“It’s also very inexpensive: You get the information once, bank it, and use it throughout life,” noted Paul M. Ridker, MD, director of the Center for Cardiovascular Disease Prevention and professor of medicine at Harvard Medical School, Boston.
“A genome-wide scan will give us information not just on cardiovascular risk, but on cancer risk, on risk of kidney disease, and on the risk of a host of other issues. It’s a very different way of thinking about risk presentation across a whole variety of endpoints,” Dr. Ridker added.
Dr. Ference reported receiving fees and/or research grants from Merck, Amgen, Regeneron, Sanofi, Novartis, Pfizer, Eli Lilly, NovoNordisk, The Medicines Company, Mylan, Daiichi Sankyo, Silence Therapeutics, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, KrKa Pharmaceuticals, Medtronic, and Celera.
A powerful genetic predisposition to cardiovascular disease was overcome by low lifetime exposure to LDL cholesterol and systolic blood pressure in a naturalistic study conducted in nearly half a million people, Brian A. Ference, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
This novel finding potentially opens the door to primordial cardiovascular prevention, the earliest possible form of primary prevention, in which cardiovascular risk factors are curtailed before they can become established.
“It’s important to note that the trajectories of lifetime risk for cardiovascular disease predicted by a PGS [polygenic risk score] are not fixed. At the same level of a PGS for coronary artery disease, participants with lower lifetime exposure to LDL and systolic blood pressure had a lower trajectory of risk for cardiovascular disease. This finding implies that the trajectory of cardiovascular risk predicted by a PGS can be reduced by lowering LDL and blood pressure,” observed Dr. Ference, professor of translational therapeutics and executive director of the Center for Naturally Randomised Trials at the University of Cambridge (England).
Together with an international team of coinvestigators, he analyzed lifetime cardiovascular risk as predicted by a PGS derived by genomic testing in relation to lifetime LDL and systolic blood pressure levels in 445,566 participants in the UK Biobank. Subjects had a mean age of 57.2 years at enrollment and 65.2 years at last follow-up. The primary study outcome, a first major coronary event (MCE) as defined by a fatal or nonfatal MI or coronary revascularization, occurred in 23,032 subjects.
The investigators found a stepwise increase in MCE risk across increasing quintiles of genetic risk as reflected in the PGS, such that participants in the top PGS quintile were at 2.8-fold greater risk of an MCE than those in the first quintile. The risk was essentially the same in men and women.
A key finding was that, at any level of lifetime MCE risk as defined by PGS, the actual event rate varied 10-fold depending upon lifetime exposure to LDL cholesterol and systolic blood pressure (SBP). For example, men in the top PGS quintile with high lifetime SBP and LDL cholesterol had a 93% lifetime MCE risk, but that MCE risk plummeted to 8% in those in the top quintile but with low lifetime SBP and LDL cholesterol.
Small differences in those two cardiovascular risk factors over the course of many decades had a big impact. For example, it took only a 10-mg/dL lower lifetime exposure to LDL cholesterol and a 2–mm Hg lower SBP to blunt the trajectory of lifetime risk for MCE in individuals in the middle quintile of PGS to the more favorable trajectory of those in the lowest PGS quintile. Conversely, with a 10-mg/dL increase in LDL cholesterol and 2–mm Hg greater SBP over the course of a lifetime, the trajectory of risk for people in the middle quintile of PGS became essentially superimposable upon the trajectory associated with the highest PGS quintile, the cardiologist explained.
“Participants with low lifetime exposure to LDL and blood pressure had a low lifetime risk of cardiovascular disease at all levels of PGS for coronary disease. This implies that LDL and blood pressure, which are modifiable, may be more powerful determinants of lifetime risk than polygenic predisposition,” Dr. Ference declared.
Discussant Vera Bittner, MD, professor of medicine at the University of Alabama, Birmingham, said that for her this study carried a heartening take-home message: “The polygenic risk score can stratify the population into different risk groups and, at the same time, lifetime exposure to LDL and blood pressure significantly modifies the risk, suggesting that genetics is not destiny, and we may be able to intervene.”
“To be able to know what your cardiovascular risk is from an early age and to plan therapies to prevent cardiovascular disease would be incredible,” agreed session chair B. Hadley Wilson, MD, of the Sanger Heart and Vascular Institute in Charlotte, N.C.
Sekar Kathiresan, MD, said the study introduces the PGS as a new risk factor for coronary artery disease. Focusing efforts to achieve lifelong low exposure to LDL cholesterol and blood pressure in those individuals in the top 10%-20% in PGS should provide a great absolute reduction in MCE risk.
“It potentially can give you a 30- or 40-year head start in understanding who’s at risk because the factor can be measured as early as birth,” observed Dr. Kathiresan, a cardiologist who is director of the Center for Genomic Medicine at Massachusetts General Hospital, Boston.
“It’s also very inexpensive: You get the information once, bank it, and use it throughout life,” noted Paul M. Ridker, MD, director of the Center for Cardiovascular Disease Prevention and professor of medicine at Harvard Medical School, Boston.
“A genome-wide scan will give us information not just on cardiovascular risk, but on cancer risk, on risk of kidney disease, and on the risk of a host of other issues. It’s a very different way of thinking about risk presentation across a whole variety of endpoints,” Dr. Ridker added.
Dr. Ference reported receiving fees and/or research grants from Merck, Amgen, Regeneron, Sanofi, Novartis, Pfizer, Eli Lilly, NovoNordisk, The Medicines Company, Mylan, Daiichi Sankyo, Silence Therapeutics, Ionis Pharmaceuticals, dalCOR, CiVi Pharma, KrKa Pharmaceuticals, Medtronic, and Celera.
REPORTING FROM ACC 20
Dramatic rise in hypertension-related deaths in the United States
There has been a dramatic rise in hypertension-related deaths in the United States between 2007 and 2017, a new study shows. The authors, led by Lakshmi Nambiar, MD, Larner College of Medicine, University of Vermont, Burlington, analyzed data from the Centers for Disease Control and Prevention, which collates information from every death certificate in the country, amounting to more than 10 million deaths.
They found that age-adjusted hypertension-related deaths had increased from 18.3 per 100,000 in 2007 to 23.0 per 100,000 in 2017 (P < .001 for decade-long temporal trend).
Nambiar reported results of the study at an American College of Cardiology 2020/World Congress of Cardiology press conference on March 19. It was also published online on the same day in the Journal of the American College of Cardiology.
She noted that death rates due to cardiovascular disease have been falling over the past 20 years largely attributable to statins to treat high cholesterol and stents to treat coronary artery disease. But since 2011, the rate of decline in cardiovascular deaths has slowed. One contributing factor is an increase in heart failure-related deaths but there hasn’t been any data in recent years on hypertension-related deaths.
“Our data show an increase in hypertension-related deaths in all age groups, in all regions of the United States, and in both sexes. These findings are alarming and warrant further investigation, as well as preventative efforts,” Nambiar said. “This is a public health emergency that has not been fully recognized,” she added.
“We were surprised to see how dramatically these deaths were increasing, and we think this is related to the rise in diabetes, obesity, and the aging of the population. We need targeted public health measures to address some of those factors,” Nambiar told Medscape Medical News.
“We are winning the battle against coronary artery disease with statins and stents but we are not winning the battle against hypertension,” she added.
Worst Figures in Rural South
Results showed that hypertension-related deaths increased in both rural and urban regions, but the increase was much steeper in rural areas — a 72% increase over the decade compared with a 20% increase in urban areas.
The highest death risk was identified in the rural South, which demonstrated an age-adjusted 2.5-fold higher death rate compared with other regions (P < .001).
The urban South also demonstrated increasing hypertension-related cardiovascular death rates over time: age-adjusted death rates in the urban South increased by 27% compared with all other urban regions (P < .001).
But the absolute mortality rates and slope of the curves demonstrate the highest risk in patients in the rural South, the researchers report. Age-adjusted hypertension-related death rates increased in the rural South from 23.9 deaths per 100,000 in 2007 to 39.5 deaths per 100,000 in 2017.
Nambiar said the trends in the rural South could be related to social factors and lack of access to healthcare in the area, which has been exacerbated by failure to adopt Medicaid expansion in many of the states in this region.
“When it comes to the management of hypertension you need to be seen regularly by a primary care doctor to get the best treatment and regular assessments,” she stressed.
Chair of the ACC press conference at which the data were presented, Martha Gulati, MD, University of Arizona School of Medicine, Phoenix, said: “In this day and time, there is less smoking, which should translate into lower rates of hypertension, but these trends reported here are very different from what we would expect and are probably associated with the rise in other risk factors such as diabetes and obesity, especially in the rural South.”
Nambiar praised the new ACC/AHA hypertension guidelines that recommend a lower diagnostic threshold, “so more people now fit the criteria for raised blood pressure and need treatment.”
“It is important for all primary care physicians and cardiologists to recognize the new threshold and treat people accordingly,” she said. “High blood pressure is the leading cause of cardiovascular disease. If we can control it better, we may be able to control some of this increased mortality we are seeing.”
This article first appeared on Medscape.com.
There has been a dramatic rise in hypertension-related deaths in the United States between 2007 and 2017, a new study shows. The authors, led by Lakshmi Nambiar, MD, Larner College of Medicine, University of Vermont, Burlington, analyzed data from the Centers for Disease Control and Prevention, which collates information from every death certificate in the country, amounting to more than 10 million deaths.
They found that age-adjusted hypertension-related deaths had increased from 18.3 per 100,000 in 2007 to 23.0 per 100,000 in 2017 (P < .001 for decade-long temporal trend).
Nambiar reported results of the study at an American College of Cardiology 2020/World Congress of Cardiology press conference on March 19. It was also published online on the same day in the Journal of the American College of Cardiology.
She noted that death rates due to cardiovascular disease have been falling over the past 20 years largely attributable to statins to treat high cholesterol and stents to treat coronary artery disease. But since 2011, the rate of decline in cardiovascular deaths has slowed. One contributing factor is an increase in heart failure-related deaths but there hasn’t been any data in recent years on hypertension-related deaths.
“Our data show an increase in hypertension-related deaths in all age groups, in all regions of the United States, and in both sexes. These findings are alarming and warrant further investigation, as well as preventative efforts,” Nambiar said. “This is a public health emergency that has not been fully recognized,” she added.
“We were surprised to see how dramatically these deaths were increasing, and we think this is related to the rise in diabetes, obesity, and the aging of the population. We need targeted public health measures to address some of those factors,” Nambiar told Medscape Medical News.
“We are winning the battle against coronary artery disease with statins and stents but we are not winning the battle against hypertension,” she added.
Worst Figures in Rural South
Results showed that hypertension-related deaths increased in both rural and urban regions, but the increase was much steeper in rural areas — a 72% increase over the decade compared with a 20% increase in urban areas.
The highest death risk was identified in the rural South, which demonstrated an age-adjusted 2.5-fold higher death rate compared with other regions (P < .001).
The urban South also demonstrated increasing hypertension-related cardiovascular death rates over time: age-adjusted death rates in the urban South increased by 27% compared with all other urban regions (P < .001).
But the absolute mortality rates and slope of the curves demonstrate the highest risk in patients in the rural South, the researchers report. Age-adjusted hypertension-related death rates increased in the rural South from 23.9 deaths per 100,000 in 2007 to 39.5 deaths per 100,000 in 2017.
Nambiar said the trends in the rural South could be related to social factors and lack of access to healthcare in the area, which has been exacerbated by failure to adopt Medicaid expansion in many of the states in this region.
“When it comes to the management of hypertension you need to be seen regularly by a primary care doctor to get the best treatment and regular assessments,” she stressed.
Chair of the ACC press conference at which the data were presented, Martha Gulati, MD, University of Arizona School of Medicine, Phoenix, said: “In this day and time, there is less smoking, which should translate into lower rates of hypertension, but these trends reported here are very different from what we would expect and are probably associated with the rise in other risk factors such as diabetes and obesity, especially in the rural South.”
Nambiar praised the new ACC/AHA hypertension guidelines that recommend a lower diagnostic threshold, “so more people now fit the criteria for raised blood pressure and need treatment.”
“It is important for all primary care physicians and cardiologists to recognize the new threshold and treat people accordingly,” she said. “High blood pressure is the leading cause of cardiovascular disease. If we can control it better, we may be able to control some of this increased mortality we are seeing.”
This article first appeared on Medscape.com.
There has been a dramatic rise in hypertension-related deaths in the United States between 2007 and 2017, a new study shows. The authors, led by Lakshmi Nambiar, MD, Larner College of Medicine, University of Vermont, Burlington, analyzed data from the Centers for Disease Control and Prevention, which collates information from every death certificate in the country, amounting to more than 10 million deaths.
They found that age-adjusted hypertension-related deaths had increased from 18.3 per 100,000 in 2007 to 23.0 per 100,000 in 2017 (P < .001 for decade-long temporal trend).
Nambiar reported results of the study at an American College of Cardiology 2020/World Congress of Cardiology press conference on March 19. It was also published online on the same day in the Journal of the American College of Cardiology.
She noted that death rates due to cardiovascular disease have been falling over the past 20 years largely attributable to statins to treat high cholesterol and stents to treat coronary artery disease. But since 2011, the rate of decline in cardiovascular deaths has slowed. One contributing factor is an increase in heart failure-related deaths but there hasn’t been any data in recent years on hypertension-related deaths.
“Our data show an increase in hypertension-related deaths in all age groups, in all regions of the United States, and in both sexes. These findings are alarming and warrant further investigation, as well as preventative efforts,” Nambiar said. “This is a public health emergency that has not been fully recognized,” she added.
“We were surprised to see how dramatically these deaths were increasing, and we think this is related to the rise in diabetes, obesity, and the aging of the population. We need targeted public health measures to address some of those factors,” Nambiar told Medscape Medical News.
“We are winning the battle against coronary artery disease with statins and stents but we are not winning the battle against hypertension,” she added.
Worst Figures in Rural South
Results showed that hypertension-related deaths increased in both rural and urban regions, but the increase was much steeper in rural areas — a 72% increase over the decade compared with a 20% increase in urban areas.
The highest death risk was identified in the rural South, which demonstrated an age-adjusted 2.5-fold higher death rate compared with other regions (P < .001).
The urban South also demonstrated increasing hypertension-related cardiovascular death rates over time: age-adjusted death rates in the urban South increased by 27% compared with all other urban regions (P < .001).
But the absolute mortality rates and slope of the curves demonstrate the highest risk in patients in the rural South, the researchers report. Age-adjusted hypertension-related death rates increased in the rural South from 23.9 deaths per 100,000 in 2007 to 39.5 deaths per 100,000 in 2017.
Nambiar said the trends in the rural South could be related to social factors and lack of access to healthcare in the area, which has been exacerbated by failure to adopt Medicaid expansion in many of the states in this region.
“When it comes to the management of hypertension you need to be seen regularly by a primary care doctor to get the best treatment and regular assessments,” she stressed.
Chair of the ACC press conference at which the data were presented, Martha Gulati, MD, University of Arizona School of Medicine, Phoenix, said: “In this day and time, there is less smoking, which should translate into lower rates of hypertension, but these trends reported here are very different from what we would expect and are probably associated with the rise in other risk factors such as diabetes and obesity, especially in the rural South.”
Nambiar praised the new ACC/AHA hypertension guidelines that recommend a lower diagnostic threshold, “so more people now fit the criteria for raised blood pressure and need treatment.”
“It is important for all primary care physicians and cardiologists to recognize the new threshold and treat people accordingly,” she said. “High blood pressure is the leading cause of cardiovascular disease. If we can control it better, we may be able to control some of this increased mortality we are seeing.”
This article first appeared on Medscape.com.
Cardiovascular risk varies between black ethnic subgroups
PHOENIX, ARIZ. – Cardiovascular disease risk factors differ significantly between three black ethnic subgroups in the United States, compared with whites, results from a large, long-term cross-sectional study show.
“Race alone does not account for health disparities in CVD risk factors,” lead author Diana Baptiste, DNP, RN, CNE, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “We must consider the environmental, psychosocial, and social factors that may play a larger role in CVD risk among these populations.”
Dr. Baptiste, of the Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care in Baltimore, noted that blacks bear a disproportionately greater burden of CVD than that of any other racial group. “Blacks living in the U.S. are not monolithic and include different ethnic subgroups: African Americans, Afro-Caribbeans, defined as black persons who are born in the Caribbean islands, and African immigrants, defined as black persons who are born in Africa,” she said. “It is unclear how Afro-Caribbeans and African immigrants compare to African Americans and whites with regard to CVD risk factors.”
To examine trends in CVD risk factors among the three black ethnic subgroups compared with whites, she and her colleagues performed a cross-sectional analysis of 452,997 adults who participated in the 2010-2018 National Health Interview Survey (NHIS). Of these, 82% were white and 18% were black. Among blacks, 89% were African Americans, 6% were Afro-Caribbeans, and 5% were African immigrants. Outcomes of interest were four self-reported CVD risk factors: hypertension, diabetes, overweight/obesity, and smoking. The researchers used generalized linear models with Poisson distribution to calculate predictive probabilities of CVD risk factors, adjusted for age and sex.
Dr. Baptiste reported that African immigrants represented the youngest subgroup, with an average age of 41 years, compared with an average age of 50 among whites. They were also less likely to have health insurance (76%), compared with Afro-Caribbeans (81%), African Americans (83%), and whites (91%; P < .001). Disparities were observed in the proportion of individuals living below the poverty level. This was led by African Americans (24%), followed by African immigrants (22%), Afro-Caribbeans (18%), and whites (9%).
African immigrants were most likely to be college educated (36%), compared with whites (32%), Afro-Caribbeans (23%), and African Americans (17%; P =.001). In addition, only 33% of African Americans were married, compared with more than 50% of participants in the other ethnic groups.
African Americans had the highest prevalence of hypertension over the time period (from 44% in 2010 to 42% in 2018), while African immigrants had the lowest (from 19% to 17%). African Americans also had the highest prevalence of diabetes over the time period (from 14% to 15%), while African immigrants had the lowest (from 9% to 7%). The prevalence of overweight and obesity was highest among African Americans (from 74% to 76%), while African immigrants had the lowest (63% to 60%). Finally, smoking prevalence was highest in whites and African Americans compared with African immigrants and Afro-Caribbeans, but the prevalence decreased significantly between 2010 and 2018 (P for trend < .001).
In an interview, one of the meeting session’s moderators, Sherry-Ann Brown, MD, PhD, said that the study’s findings underscore the importance of heterogeneity when counseling patients about CVD risk factors. “Everybody comes from a different cultural background,” said Dr. Brown, a cardiologist and physician scientist at Mayo Clinic, Rochester, Minn. “Cultural backgrounds have an impact on when people eat, how they eat, who they eat with, when they exercise, and whether obesity is valued or not. It’s important to recognize that those cultural underpinnings can contribute to heterogeneity. Other factors – whether they are psychosocial or socioeconomic or environmental – also contribute.”
Strengths of the study, Dr. Baptiste said, included the use of a large, nationally representative dataset. Limitations included its cross-sectional design and the National Health Interview Survey’s reliance on self-reported data. “There were also small sample sizes for African immigrants and Afro-Caribbeans,” she said.
The study was supported by Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care. Dr. Baptiste reported having no financial disclosures.
The meeting was sponsored by the American Heart Association.
SOURCE: Baptiste D et al. EPI/Lifestyle 2020, Session 4, Abstract 8.
PHOENIX, ARIZ. – Cardiovascular disease risk factors differ significantly between three black ethnic subgroups in the United States, compared with whites, results from a large, long-term cross-sectional study show.
“Race alone does not account for health disparities in CVD risk factors,” lead author Diana Baptiste, DNP, RN, CNE, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “We must consider the environmental, psychosocial, and social factors that may play a larger role in CVD risk among these populations.”
Dr. Baptiste, of the Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care in Baltimore, noted that blacks bear a disproportionately greater burden of CVD than that of any other racial group. “Blacks living in the U.S. are not monolithic and include different ethnic subgroups: African Americans, Afro-Caribbeans, defined as black persons who are born in the Caribbean islands, and African immigrants, defined as black persons who are born in Africa,” she said. “It is unclear how Afro-Caribbeans and African immigrants compare to African Americans and whites with regard to CVD risk factors.”
To examine trends in CVD risk factors among the three black ethnic subgroups compared with whites, she and her colleagues performed a cross-sectional analysis of 452,997 adults who participated in the 2010-2018 National Health Interview Survey (NHIS). Of these, 82% were white and 18% were black. Among blacks, 89% were African Americans, 6% were Afro-Caribbeans, and 5% were African immigrants. Outcomes of interest were four self-reported CVD risk factors: hypertension, diabetes, overweight/obesity, and smoking. The researchers used generalized linear models with Poisson distribution to calculate predictive probabilities of CVD risk factors, adjusted for age and sex.
Dr. Baptiste reported that African immigrants represented the youngest subgroup, with an average age of 41 years, compared with an average age of 50 among whites. They were also less likely to have health insurance (76%), compared with Afro-Caribbeans (81%), African Americans (83%), and whites (91%; P < .001). Disparities were observed in the proportion of individuals living below the poverty level. This was led by African Americans (24%), followed by African immigrants (22%), Afro-Caribbeans (18%), and whites (9%).
African immigrants were most likely to be college educated (36%), compared with whites (32%), Afro-Caribbeans (23%), and African Americans (17%; P =.001). In addition, only 33% of African Americans were married, compared with more than 50% of participants in the other ethnic groups.
African Americans had the highest prevalence of hypertension over the time period (from 44% in 2010 to 42% in 2018), while African immigrants had the lowest (from 19% to 17%). African Americans also had the highest prevalence of diabetes over the time period (from 14% to 15%), while African immigrants had the lowest (from 9% to 7%). The prevalence of overweight and obesity was highest among African Americans (from 74% to 76%), while African immigrants had the lowest (63% to 60%). Finally, smoking prevalence was highest in whites and African Americans compared with African immigrants and Afro-Caribbeans, but the prevalence decreased significantly between 2010 and 2018 (P for trend < .001).
In an interview, one of the meeting session’s moderators, Sherry-Ann Brown, MD, PhD, said that the study’s findings underscore the importance of heterogeneity when counseling patients about CVD risk factors. “Everybody comes from a different cultural background,” said Dr. Brown, a cardiologist and physician scientist at Mayo Clinic, Rochester, Minn. “Cultural backgrounds have an impact on when people eat, how they eat, who they eat with, when they exercise, and whether obesity is valued or not. It’s important to recognize that those cultural underpinnings can contribute to heterogeneity. Other factors – whether they are psychosocial or socioeconomic or environmental – also contribute.”
Strengths of the study, Dr. Baptiste said, included the use of a large, nationally representative dataset. Limitations included its cross-sectional design and the National Health Interview Survey’s reliance on self-reported data. “There were also small sample sizes for African immigrants and Afro-Caribbeans,” she said.
The study was supported by Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care. Dr. Baptiste reported having no financial disclosures.
The meeting was sponsored by the American Heart Association.
SOURCE: Baptiste D et al. EPI/Lifestyle 2020, Session 4, Abstract 8.
PHOENIX, ARIZ. – Cardiovascular disease risk factors differ significantly between three black ethnic subgroups in the United States, compared with whites, results from a large, long-term cross-sectional study show.
“Race alone does not account for health disparities in CVD risk factors,” lead author Diana Baptiste, DNP, RN, CNE, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “We must consider the environmental, psychosocial, and social factors that may play a larger role in CVD risk among these populations.”
Dr. Baptiste, of the Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care in Baltimore, noted that blacks bear a disproportionately greater burden of CVD than that of any other racial group. “Blacks living in the U.S. are not monolithic and include different ethnic subgroups: African Americans, Afro-Caribbeans, defined as black persons who are born in the Caribbean islands, and African immigrants, defined as black persons who are born in Africa,” she said. “It is unclear how Afro-Caribbeans and African immigrants compare to African Americans and whites with regard to CVD risk factors.”
To examine trends in CVD risk factors among the three black ethnic subgroups compared with whites, she and her colleagues performed a cross-sectional analysis of 452,997 adults who participated in the 2010-2018 National Health Interview Survey (NHIS). Of these, 82% were white and 18% were black. Among blacks, 89% were African Americans, 6% were Afro-Caribbeans, and 5% were African immigrants. Outcomes of interest were four self-reported CVD risk factors: hypertension, diabetes, overweight/obesity, and smoking. The researchers used generalized linear models with Poisson distribution to calculate predictive probabilities of CVD risk factors, adjusted for age and sex.
Dr. Baptiste reported that African immigrants represented the youngest subgroup, with an average age of 41 years, compared with an average age of 50 among whites. They were also less likely to have health insurance (76%), compared with Afro-Caribbeans (81%), African Americans (83%), and whites (91%; P < .001). Disparities were observed in the proportion of individuals living below the poverty level. This was led by African Americans (24%), followed by African immigrants (22%), Afro-Caribbeans (18%), and whites (9%).
African immigrants were most likely to be college educated (36%), compared with whites (32%), Afro-Caribbeans (23%), and African Americans (17%; P =.001). In addition, only 33% of African Americans were married, compared with more than 50% of participants in the other ethnic groups.
African Americans had the highest prevalence of hypertension over the time period (from 44% in 2010 to 42% in 2018), while African immigrants had the lowest (from 19% to 17%). African Americans also had the highest prevalence of diabetes over the time period (from 14% to 15%), while African immigrants had the lowest (from 9% to 7%). The prevalence of overweight and obesity was highest among African Americans (from 74% to 76%), while African immigrants had the lowest (63% to 60%). Finally, smoking prevalence was highest in whites and African Americans compared with African immigrants and Afro-Caribbeans, but the prevalence decreased significantly between 2010 and 2018 (P for trend < .001).
In an interview, one of the meeting session’s moderators, Sherry-Ann Brown, MD, PhD, said that the study’s findings underscore the importance of heterogeneity when counseling patients about CVD risk factors. “Everybody comes from a different cultural background,” said Dr. Brown, a cardiologist and physician scientist at Mayo Clinic, Rochester, Minn. “Cultural backgrounds have an impact on when people eat, how they eat, who they eat with, when they exercise, and whether obesity is valued or not. It’s important to recognize that those cultural underpinnings can contribute to heterogeneity. Other factors – whether they are psychosocial or socioeconomic or environmental – also contribute.”
Strengths of the study, Dr. Baptiste said, included the use of a large, nationally representative dataset. Limitations included its cross-sectional design and the National Health Interview Survey’s reliance on self-reported data. “There were also small sample sizes for African immigrants and Afro-Caribbeans,” she said.
The study was supported by Johns Hopkins University School of Nursing Center for Cardiovascular and Chronic Care. Dr. Baptiste reported having no financial disclosures.
The meeting was sponsored by the American Heart Association.
SOURCE: Baptiste D et al. EPI/Lifestyle 2020, Session 4, Abstract 8.
REPORTING FROM EPI/LIFESTYLE 2020
COVID-19: U.S. cardiology groups reaffirm continued use of RAAS-active drugs
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
Controversy continued over the potential effect of drugs that interfere with the renin-angiotensin-aldosterone system via the angiotensin-converting enzymes (ACE) may have on exacerbating infection with the SARS-CoV-2 virus that causes COVID-19.
A joint statement from the American Heart Association, American College of Cardiology, and the Heart Failure Society of America on March 17 gave full, unqualified support to maintaining patients on drugs that work this way, specifically the ACE inhibitors and angiotensin-receptor blockers (ARBs), which together form a long-standing cornerstone of treatment for hypertension, heart failure, and ischemic heart disease.
The three societies “recommend continuation” of ACE inhibitors or ARBs “for all patients already prescribed.” The statement went on to say that patients already diagnosed with a COVID-19 infection “should be fully evaluated before adding or removing any treatments, and any changes to their treatment should be based on the latest scientific evidence and shared decision making with their physician and health care team.”
“We understand the concern – as it has become clear that people with cardiovascular disease are at much higher risk of serious complications including death from COVID-19. However, we have reviewed the latest research – the evidence does not confirm the need to discontinue ACE inhibitors or ARBs, and we strongly recommend all physicians to consider the individual needs of each patient before making any changes to ACE-inhibitor or ARB treatment regimens,” said Robert A. Harrington, MD, president of the American Heart Association and professor and chair of medicine at Stanford (Calif.) University, in the statement.
“There are no experimental or clinical data demonstrating beneficial or adverse outcomes among COVID-19 patients using ACE-inhibitor or ARB medications,” added Richard J. Kovacs, MD, president of the American College of Cardiology and professor of cardiology at Indiana University in Indianapolis.
The “latest research” referred to in the statement likely focuses on a report that had appeared less than a week earlier in a British journal that hypothesized a possible increase in the susceptibility of human epithelial cells of the lungs, intestine, kidneys, and blood vessels exposed to these or certain other drugs, like the thiazolidinedione oral diabetes drugs or ibuprofen, because they cause up-regulation of the ACE2 protein in cell membranes, and ACE2 is the primary cell-surface receptor that allows the SARS-CoV-2 virus to enter.
“We therefore hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” wrote Michael Roth, MD, and his associates in their recent article (Lancet Resp Med. 2020 Mar 11. doi: 10.1016/S2213-2600[20]30116-8). While the potential clinical impact of an increase in the number of ACE2 molecules in a cell’s surface membrane remains uninvestigated, the risk this phenomenon poses should mean that patients taking these drugs should receive heightened monitoring for COVID-19 disease, suggested Dr. Roth, a professor of biomedicine who specializes in studying inflammatory lung diseases including asthma, and associates.
However, others who have considered the impact that ACE inhibitors and ARBs might have on ACE2 and COVID-19 infections have noted that the picture is not simple. “Higher ACE2 expression following chronically medicating SARS‐CoV‐2 infected patients with AT1R [angiotensin receptor 1] blockers, while seemingly paradoxical, may protect them against acute lung injury rather than putting them at higher risk to develop SARS. This may be accounted for by two complementary mechanisms: blocking the excessive angiotensin‐mediated AT1R activation caused by the viral infection, as well as up-regulating ACE2, thereby reducing angiotensin production by ACE and increasing the production” of a vasodilating form of angiotensin, wrote David Gurwitz, PhD, in a recently published editorial (Drug Dev Res. 2020 Mar 4. doi: 10.1002/ddr.21656). A data-mining approach may allow researchers to determine whether patients who received drugs that interfere with angiotensin 1 function prior to being diagnosed with a COVID-19 infection had a better disease outcome, suggested Dr. Gurwitz, a molecular geneticist at Tel Aviv University in Jerusalem.
The statement from the three U.S. cardiology societies came a few days following a similar statement of support for ongoing use of ACE inhibitors and ARBs from the European Society of Cardiology’s Council on Hypertension.
Dr. Harrington, Dr. Kovacs, Dr. Roth, and Dr. Gurwitz had no relevant disclosures.
PARAGON-HF: Optimal systolic pressure in HFpEF is 120-129 mm Hg
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
A target systolic blood pressure (SBP) of 120-129 mm Hg in patients with heart failure with preserved ejection fraction proved to be the sweet spot with the lowest rates of major adverse cardiovascular and renal events in a new analysis from the landmark PARAGON-HF trial.
This finding from the largest-ever randomized, controlled study in heart failure with preserved ejection fraction (HFpEF) strengthens support for current U.S. joint hypertension guidelines, which call for a target SBP less than 130 mm Hg in patients with HFpEF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803), a recommendation based upon weak evidence until now. That’s because the SPRINT trial, the major impetus for adoption of intensive blood pressure control in the current guidelines, excluded patients with symptomatic HF, Scott D. Solomon, MD, and coinvestigators noted in their new analysis. The study was published in the Journal of the American College of Cardiology and had been planned for presentation during the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
The new analysis from PARAGON-HF (Prospective Comparison of ARNI with ARB Global Outcomes in HFpEF) also ruled out the SBP-lowering effect of sacubitril/valsartan (Entresto) as the explanation for the combination drug’s demonstrated beneficial impact on outcomes in the subgroup with an SBP of 120-129 mm Hg. That wasn’t actually a surprise. Indeed, the new study had two hypotheses: one, that the relationship between SBP and cardiovascular and renal outcomes in HFpEF would follow a J-shaped curve, and two, that sacubitril/valsartan’s blood pressure–lowering effect would not account for the drug’s outcome benefits in the subset of HFpEF patients with an SBP in the sweet spot of 120-129 mm Hg. Both hypotheses were borne out, noted Dr. Solomon, professor of medicine at Harvard Medical School and director of noninvasive cardiology at Brigham and Women’s Hospital, both in Boston.
“These data strongly support that additional mechanisms other than blood pressure–lowering account for the benefit. But this is not surprising. The same can be said for most of the therapies that work in heart failure,” he said in an interview.
Take, for example, spironolactone. In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), another major trial in which Dr. Solomon played a leadership role, the beneficial effect of spironolactone on clinical outcomes also proved unrelated to the drug’s blood pressure–lowering effect.
Other known effects of sacubitril/valsartan, a novel angiotensin receptor–neprilysin inhibitor, or ARNI, might in theory account for the observed clinical benefits in ARNI-treated patients with an on-treatment SBP of 120-129 mm Hg in PARAGON-HF. These include improved left atrial remodeling, an increase in natriuretic peptides, and improved myocardial relaxation. However, the current lack of understanding of the basic mechanistic processes underlying the varied clinical expressions of HFpEF is a major factor contributing to the lack of any proven-effective therapy for this extremely common and costly disorder, according to Dr. Solomon and coinvestigators.
In contrast to HFpEF, for which to date there is no proven treatment, heart failure with reduced ejection fraction sacubitril/valsartan has a class I recommendation on the strength of its performance in significantly reducing cardiovascular deaths and heart failure hospitalizations in the PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371:993-1004).
PARAGON-HF included 4,822 patients with symptomatic HFpEF who were randomized to sacubitril/valsartan at 97/103 mg b.i.d. or valsartan at 160 mg b.i.d. As previously reported (N Engl J Med. 2019 Oct 24;381[17]:1609-20), at an average follow-up of 35 months, the primary outcome – a composite of total hospitalizations for heart failure and cardiovascular death – occurred at a rate of 12.8 events per 100 patient-years in the sacubitril/valsartan group and 14.6 per 100 patient-years in the valsartan arm, for a 13% relative risk reduction that narrowly missed statistical significance (P = .059).
However, sacubitril/valsartan showed significant benefit on some prespecified secondary endpoints, including worsening renal function, change in New York Heart Association class, and quality of life. Women, who notably accounted for 52% of study participants, appeared to benefit from sacubitril/valsartan more than men as evidenced by their 27% relative risk reduction in the primary endpoint. Also, in the roughly half of PARAGON-HF participants with a baseline left ventricular ejection fraction of 45%-57%, treatment with sacubitril/valsartan resulted in a statistically significant 22% relative risk reduction in the primary endpoint, compared with valsartan alone.
SBP and cardiovascular outcomes in HFpEF
In the new analysis, Dr. Solomon and coworkers examined outcomes based on baseline and mean achieved SBP quartiles regardless of treatment arm. In an unadjusted analysis, the primary composite endpoint occurred at a rate of 15.2 events/100 patient-years in HFpEF patients with an achieved SBP below 120 mm Hg, 11.4/100 patient-years at 120-129 mm Hg, 12.2/100 patient-years at 130-139 mm Hg, and 15.6/100 patient-years at 140 mm Hg or more. Further, in a multivariate regression analysis extensively adjusted for atrial fibrillation, sex, race, and numerous other potential confounders, the group with an achieved SBP of 120-129 mm Hg continued to fare best. The adjusted risks for the primary endpoint were 11% and 21% higher in patients in the first and third quartiles of achieved SBP, compared with those at 120-129 mm Hg, although neither trend reached statistical significance. But patients in the top quartile, with an achieved SBP of 140 mm Hg or more, had a highly significant 56% increase in risk, compared with patients in the second-lowest SBP quartile.
Change in blood pressure from baseline to week 48 had no impact on quality of life or high-sensitivity troponin T. However, each 10–mm Hg lowering of SBP was associated with a modest 2.1% reduction in log-transformed N-terminal of the prohormone brain natriuretic peptide.
Sacubitril/valsartan reduced SBP by an average of 5.2 mm Hg more than valsartan alone at 4 weeks regardless of baseline SBP. And the combo drug had a significantly greater SBP-lowering effect in women than men, by a margin of 6.3 mm Hg versus 4.0 mm Hg. But a Cox regression analysis showed that in women, as in the study population as a whole, sacubitril/valsartan’s SBP-lowering effects didn’t account for the drug’s impact on outcomes.
In an editorial accompanying publication of the new PARAGON-HF blood pressure analysis (J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.024), Hector O. Ventura, MD, and colleagues at the Ochsner Clinic in New Orleans observed that the study results “lend some credence to the prognostic relationship of blood pressure in HFpEF, but whether they should serve as a therapeutic target or are merely a prognostic surrogate determined by other pathogenic factors, such as vascular ventricular uncoupling or aortic stiffness on one hand when blood pressure is greater than 140 mm Hg, or a reduced cardiac performance indicated by reduced blood pressure to less than 120 mm Hg, remains uncertain.”
“What is certain, however, is that the relationship and contributions of hypertension in manifest HFpEF are complex, multifactorial and likely go well beyond a simplistic framework of hemodynamic influences,” they added.
Dr. Solomon has received research grants from and serves as a consultant to Novartis, which funded PARAGON-HF, and has similar financial relationships with more than a dozen other pharmaceutical companies. Dr. Ventura reported having no relevant financial interests.
SOURCE: Solomon SD et al. J Am Coll Cardiol. 2020 Mar 16. doi: 10.1016/j.jacc.2020.02.009.
FROM ACC 2020
High CV event risk seen in SLE patients with ACC/AHA-defined hypertension
, results of a retrospective, single-center investigation suggest.
Risk of atherosclerotic vascular events was increased by 73% for patients with systemic lupus erythematosus (SLE) who sustained a mean blood pressure of 130/80 to 139/89 mm Hg over 2 years in the study, which included 1,532 patients treated at a clinic in Toronto.
Management of hypertension in SLE patients should start early, and should aim to achieve levels below 130/80 mm Hg, according to the investigators, led by Konstantinos Tselios, MD, PhD, of the Centre for Prognosis Studies in the Rheumatic Diseases at the University of Toronto.
“The findings of the present study support that the target BP should be less than 130/80 mm Hg in all patients with lupus in order to minimize their cardiovascular risk,” Dr. Tselios and coauthors said in their study, which appears in Annals of the Rheumatic Diseases.
Despite the limitations inherent in a retrospective, observational study, this work by Dr. Tselios and colleagues may help inform the care of patients with SLE, according to C. Michael Stein, MBChB, professor of medicine at Vanderbilt University in Nashville, Tenn.
“It’s really interesting data that’s important and helps us think in terms of figuring out what may be reasonable to do for a particular patient,” Dr. Stein said in an interview.
Starting antihypertensive management early and aiming at levels below 130/80 mm Hg is a strategy that should be “reasonable” for most patients with SLE, said Dr. Stein, adding that the approach specified in the 2017 American College of Cardiology/American Heart Association (ACC/AHA) hypertension guidelines are appropriate to follow. In those guidelines, the threshold for diagnosis of hypertension was lowered to 130/80 mm Hg.
“You can start with risk factor modification, in terms of losing weight, exercising, stopping alcohol, and decreasing salt in the diet to see if you can get the blood pressure down, though it may come down to drug therapy for many patients, I believe,” Dr. Stein said.
Authors of those 2017 ACC/AHA guidelines made no recommendations for patients with SLE or other connective tissue diseases, despite including a section devoted to specific patient subgroups and comorbidities of interest, Dr. Tselios and coauthors noted in their report.
Management of hypertension in patients with lupus may be “delayed” in patients with blood pressures reaching the current hypertension threshold, according to Dr. Tselios and colleagues, due in part to difficulties in cardiovascular risk calculation in SLE patients, as well as current risk considerations outlined in the guidelines.
“On the basis of the recent guidelines, the patient with typical lupus (young female with no traditional atherosclerotic risk factors) would be considered as a low-risk individual and not offered treatment for a BP of 130-139/80-89 mm Hg,” they said in their report.
Accordingly, Dr. Tselios and colleagues sought to determine whether the new hypertension definition predicted atherosclerotic vascular events, including new-onset angina, acute myocardial infarction, cerebrovascular events, revascularization procedures, heart failure, or peripheral vascular disease requiring angioplasty, in patients with SLE treated at a Canadian clinic.
Their analysis comprised 1,532 patients with SLE who had at least 2 years of follow-up and had no prior atherosclerotic events on record. Over a mean follow-up of nearly 11 years, there were 124 such events documented in those patients.
With a mean follow-up of nearly 11 years, the incidence of atherosclerotic events was 18.9 per 1,000 patient-years for patients with blood pressure ≥ 140/90 mm Hg, 11.5 per 1,000 patient-years for the 130-139/80-89 mm Hg group, and 4.5 per 1,000 patient-years for those with blood pressures of 130/80 mm Hg or lower, with differences that were statistically significant between groups, according to the report.
An adjusted blood pressure of 130-139/80-89 mm Hg over the first 2 years since enrollment in the clinic was independently associated with the occurrence of an atherosclerotic event, with a hazard ratio of 1.73 (95% confidence interval, 1.13-2.69, P = 0.011), according to results of a multivariable analysis.
Those findings support targeting a blood pressure below 130/80 mm Hg in all patients with lupus, according to Dr. Tselios and coauthors.
“It seems reasonable that clinicians should not rely on CV risk calculators in SLE and commence treatment as soon as possible in cases of sustained BP elevation above the threshold of 130/80 mm Hg,” they wrote in their report.
How low to go remains unclear, however, as targeting lower levels of blood pressure might be unsafe in certain groups, such as those SLE patients with prior heart disease or heart failure; nevertheless, recent observational data from non-SLE populations suggest that effective treatment to levels lower than 130/80 mm Hg would “further reduce the incidence of atherosclerotic events in SLE,” the authors said in a discussion of their results.
Dr. Tselios and coauthors said they had no competing interests relative to the study. They reported funding for the University of Toronto Lupus Clinic from the University Health Network, Lou & Marissa Rocca, Mark & Diana Bozzo, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. Ann Rheum Dis. 2020 Mar 10. doi: 10.1136/annrheumdis-2019-216764
, results of a retrospective, single-center investigation suggest.
Risk of atherosclerotic vascular events was increased by 73% for patients with systemic lupus erythematosus (SLE) who sustained a mean blood pressure of 130/80 to 139/89 mm Hg over 2 years in the study, which included 1,532 patients treated at a clinic in Toronto.
Management of hypertension in SLE patients should start early, and should aim to achieve levels below 130/80 mm Hg, according to the investigators, led by Konstantinos Tselios, MD, PhD, of the Centre for Prognosis Studies in the Rheumatic Diseases at the University of Toronto.
“The findings of the present study support that the target BP should be less than 130/80 mm Hg in all patients with lupus in order to minimize their cardiovascular risk,” Dr. Tselios and coauthors said in their study, which appears in Annals of the Rheumatic Diseases.
Despite the limitations inherent in a retrospective, observational study, this work by Dr. Tselios and colleagues may help inform the care of patients with SLE, according to C. Michael Stein, MBChB, professor of medicine at Vanderbilt University in Nashville, Tenn.
“It’s really interesting data that’s important and helps us think in terms of figuring out what may be reasonable to do for a particular patient,” Dr. Stein said in an interview.
Starting antihypertensive management early and aiming at levels below 130/80 mm Hg is a strategy that should be “reasonable” for most patients with SLE, said Dr. Stein, adding that the approach specified in the 2017 American College of Cardiology/American Heart Association (ACC/AHA) hypertension guidelines are appropriate to follow. In those guidelines, the threshold for diagnosis of hypertension was lowered to 130/80 mm Hg.
“You can start with risk factor modification, in terms of losing weight, exercising, stopping alcohol, and decreasing salt in the diet to see if you can get the blood pressure down, though it may come down to drug therapy for many patients, I believe,” Dr. Stein said.
Authors of those 2017 ACC/AHA guidelines made no recommendations for patients with SLE or other connective tissue diseases, despite including a section devoted to specific patient subgroups and comorbidities of interest, Dr. Tselios and coauthors noted in their report.
Management of hypertension in patients with lupus may be “delayed” in patients with blood pressures reaching the current hypertension threshold, according to Dr. Tselios and colleagues, due in part to difficulties in cardiovascular risk calculation in SLE patients, as well as current risk considerations outlined in the guidelines.
“On the basis of the recent guidelines, the patient with typical lupus (young female with no traditional atherosclerotic risk factors) would be considered as a low-risk individual and not offered treatment for a BP of 130-139/80-89 mm Hg,” they said in their report.
Accordingly, Dr. Tselios and colleagues sought to determine whether the new hypertension definition predicted atherosclerotic vascular events, including new-onset angina, acute myocardial infarction, cerebrovascular events, revascularization procedures, heart failure, or peripheral vascular disease requiring angioplasty, in patients with SLE treated at a Canadian clinic.
Their analysis comprised 1,532 patients with SLE who had at least 2 years of follow-up and had no prior atherosclerotic events on record. Over a mean follow-up of nearly 11 years, there were 124 such events documented in those patients.
With a mean follow-up of nearly 11 years, the incidence of atherosclerotic events was 18.9 per 1,000 patient-years for patients with blood pressure ≥ 140/90 mm Hg, 11.5 per 1,000 patient-years for the 130-139/80-89 mm Hg group, and 4.5 per 1,000 patient-years for those with blood pressures of 130/80 mm Hg or lower, with differences that were statistically significant between groups, according to the report.
An adjusted blood pressure of 130-139/80-89 mm Hg over the first 2 years since enrollment in the clinic was independently associated with the occurrence of an atherosclerotic event, with a hazard ratio of 1.73 (95% confidence interval, 1.13-2.69, P = 0.011), according to results of a multivariable analysis.
Those findings support targeting a blood pressure below 130/80 mm Hg in all patients with lupus, according to Dr. Tselios and coauthors.
“It seems reasonable that clinicians should not rely on CV risk calculators in SLE and commence treatment as soon as possible in cases of sustained BP elevation above the threshold of 130/80 mm Hg,” they wrote in their report.
How low to go remains unclear, however, as targeting lower levels of blood pressure might be unsafe in certain groups, such as those SLE patients with prior heart disease or heart failure; nevertheless, recent observational data from non-SLE populations suggest that effective treatment to levels lower than 130/80 mm Hg would “further reduce the incidence of atherosclerotic events in SLE,” the authors said in a discussion of their results.
Dr. Tselios and coauthors said they had no competing interests relative to the study. They reported funding for the University of Toronto Lupus Clinic from the University Health Network, Lou & Marissa Rocca, Mark & Diana Bozzo, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. Ann Rheum Dis. 2020 Mar 10. doi: 10.1136/annrheumdis-2019-216764
, results of a retrospective, single-center investigation suggest.
Risk of atherosclerotic vascular events was increased by 73% for patients with systemic lupus erythematosus (SLE) who sustained a mean blood pressure of 130/80 to 139/89 mm Hg over 2 years in the study, which included 1,532 patients treated at a clinic in Toronto.
Management of hypertension in SLE patients should start early, and should aim to achieve levels below 130/80 mm Hg, according to the investigators, led by Konstantinos Tselios, MD, PhD, of the Centre for Prognosis Studies in the Rheumatic Diseases at the University of Toronto.
“The findings of the present study support that the target BP should be less than 130/80 mm Hg in all patients with lupus in order to minimize their cardiovascular risk,” Dr. Tselios and coauthors said in their study, which appears in Annals of the Rheumatic Diseases.
Despite the limitations inherent in a retrospective, observational study, this work by Dr. Tselios and colleagues may help inform the care of patients with SLE, according to C. Michael Stein, MBChB, professor of medicine at Vanderbilt University in Nashville, Tenn.
“It’s really interesting data that’s important and helps us think in terms of figuring out what may be reasonable to do for a particular patient,” Dr. Stein said in an interview.
Starting antihypertensive management early and aiming at levels below 130/80 mm Hg is a strategy that should be “reasonable” for most patients with SLE, said Dr. Stein, adding that the approach specified in the 2017 American College of Cardiology/American Heart Association (ACC/AHA) hypertension guidelines are appropriate to follow. In those guidelines, the threshold for diagnosis of hypertension was lowered to 130/80 mm Hg.
“You can start with risk factor modification, in terms of losing weight, exercising, stopping alcohol, and decreasing salt in the diet to see if you can get the blood pressure down, though it may come down to drug therapy for many patients, I believe,” Dr. Stein said.
Authors of those 2017 ACC/AHA guidelines made no recommendations for patients with SLE or other connective tissue diseases, despite including a section devoted to specific patient subgroups and comorbidities of interest, Dr. Tselios and coauthors noted in their report.
Management of hypertension in patients with lupus may be “delayed” in patients with blood pressures reaching the current hypertension threshold, according to Dr. Tselios and colleagues, due in part to difficulties in cardiovascular risk calculation in SLE patients, as well as current risk considerations outlined in the guidelines.
“On the basis of the recent guidelines, the patient with typical lupus (young female with no traditional atherosclerotic risk factors) would be considered as a low-risk individual and not offered treatment for a BP of 130-139/80-89 mm Hg,” they said in their report.
Accordingly, Dr. Tselios and colleagues sought to determine whether the new hypertension definition predicted atherosclerotic vascular events, including new-onset angina, acute myocardial infarction, cerebrovascular events, revascularization procedures, heart failure, or peripheral vascular disease requiring angioplasty, in patients with SLE treated at a Canadian clinic.
Their analysis comprised 1,532 patients with SLE who had at least 2 years of follow-up and had no prior atherosclerotic events on record. Over a mean follow-up of nearly 11 years, there were 124 such events documented in those patients.
With a mean follow-up of nearly 11 years, the incidence of atherosclerotic events was 18.9 per 1,000 patient-years for patients with blood pressure ≥ 140/90 mm Hg, 11.5 per 1,000 patient-years for the 130-139/80-89 mm Hg group, and 4.5 per 1,000 patient-years for those with blood pressures of 130/80 mm Hg or lower, with differences that were statistically significant between groups, according to the report.
An adjusted blood pressure of 130-139/80-89 mm Hg over the first 2 years since enrollment in the clinic was independently associated with the occurrence of an atherosclerotic event, with a hazard ratio of 1.73 (95% confidence interval, 1.13-2.69, P = 0.011), according to results of a multivariable analysis.
Those findings support targeting a blood pressure below 130/80 mm Hg in all patients with lupus, according to Dr. Tselios and coauthors.
“It seems reasonable that clinicians should not rely on CV risk calculators in SLE and commence treatment as soon as possible in cases of sustained BP elevation above the threshold of 130/80 mm Hg,” they wrote in their report.
How low to go remains unclear, however, as targeting lower levels of blood pressure might be unsafe in certain groups, such as those SLE patients with prior heart disease or heart failure; nevertheless, recent observational data from non-SLE populations suggest that effective treatment to levels lower than 130/80 mm Hg would “further reduce the incidence of atherosclerotic events in SLE,” the authors said in a discussion of their results.
Dr. Tselios and coauthors said they had no competing interests relative to the study. They reported funding for the University of Toronto Lupus Clinic from the University Health Network, Lou & Marissa Rocca, Mark & Diana Bozzo, and the Lupus Foundation of Ontario.
SOURCE: Tselios K et al. Ann Rheum Dis. 2020 Mar 10. doi: 10.1136/annrheumdis-2019-216764
FROM ANNALS OF THE RHEUMATIC DISEASES
Trump to governors: Don’t wait for feds on medical supplies
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.