User login
ESC says continue hypertension meds despite COVID-19 concern
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The European Society of Cardiology (ESC) has issued a statement urging physicians and patients to continue treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), in light of a newly described theory that those agents could increase the risk of developing COVID-19 and/or worsen its severity.
The concern arises from the observation that the new coronavirus SARS-CoV-2 causing COVID-19 binds to angiotensin-converting enzyme 2 (ACE2) to infect cells, and both ACE inhibitors and ARBs increase ACE2 levels.
This mechanism has been theorized as a possible risk factor for facilitating the acquisition of COVID-19 infection and worsening its severity. However, paradoxically, it has also been hypothesized to protect against acute lung injury from the disease.
Meanwhile, a Lancet Respiratory Medicine article was published March 11 entitled, “Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?”
“We ... hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” said the authors.
This prompted some media coverage in the United Kingdom and “social media-related amplification,” leading to concern and, in some cases, discontinuation of the drugs by patients.
But on March 13, the ESC Council on Hypertension dismissed the concerns as entirely speculative, in a statement posted to the ESC website.
It said that the council “strongly recommend that physicians and patients should continue treatment with their usual antihypertensive therapy because there is no clinical or scientific evidence to suggest that treatment with ACE inhibitors or ARBs should be discontinued because of the COVID-19 infection.”
The statement, signed by Council Chair Professor Giovanni de Simone, MD, on behalf of the nucleus members, also says that in regard to the theorized protective effect against serious lung complications in individuals with COVID-19, the data come only from animal, and not human, studies.
“Speculation about the safety of ACE-inhibitor or ARB treatment in relation to COVID-19 does not have a sound scientific basis or evidence to support it,” the ESC panel concludes.
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The European Society of Cardiology (ESC) has issued a statement urging physicians and patients to continue treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), in light of a newly described theory that those agents could increase the risk of developing COVID-19 and/or worsen its severity.
The concern arises from the observation that the new coronavirus SARS-CoV-2 causing COVID-19 binds to angiotensin-converting enzyme 2 (ACE2) to infect cells, and both ACE inhibitors and ARBs increase ACE2 levels.
This mechanism has been theorized as a possible risk factor for facilitating the acquisition of COVID-19 infection and worsening its severity. However, paradoxically, it has also been hypothesized to protect against acute lung injury from the disease.
Meanwhile, a Lancet Respiratory Medicine article was published March 11 entitled, “Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?”
“We ... hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” said the authors.
This prompted some media coverage in the United Kingdom and “social media-related amplification,” leading to concern and, in some cases, discontinuation of the drugs by patients.
But on March 13, the ESC Council on Hypertension dismissed the concerns as entirely speculative, in a statement posted to the ESC website.
It said that the council “strongly recommend that physicians and patients should continue treatment with their usual antihypertensive therapy because there is no clinical or scientific evidence to suggest that treatment with ACE inhibitors or ARBs should be discontinued because of the COVID-19 infection.”
The statement, signed by Council Chair Professor Giovanni de Simone, MD, on behalf of the nucleus members, also says that in regard to the theorized protective effect against serious lung complications in individuals with COVID-19, the data come only from animal, and not human, studies.
“Speculation about the safety of ACE-inhibitor or ARB treatment in relation to COVID-19 does not have a sound scientific basis or evidence to support it,” the ESC panel concludes.
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The European Society of Cardiology (ESC) has issued a statement urging physicians and patients to continue treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), in light of a newly described theory that those agents could increase the risk of developing COVID-19 and/or worsen its severity.
The concern arises from the observation that the new coronavirus SARS-CoV-2 causing COVID-19 binds to angiotensin-converting enzyme 2 (ACE2) to infect cells, and both ACE inhibitors and ARBs increase ACE2 levels.
This mechanism has been theorized as a possible risk factor for facilitating the acquisition of COVID-19 infection and worsening its severity. However, paradoxically, it has also been hypothesized to protect against acute lung injury from the disease.
Meanwhile, a Lancet Respiratory Medicine article was published March 11 entitled, “Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?”
“We ... hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” said the authors.
This prompted some media coverage in the United Kingdom and “social media-related amplification,” leading to concern and, in some cases, discontinuation of the drugs by patients.
But on March 13, the ESC Council on Hypertension dismissed the concerns as entirely speculative, in a statement posted to the ESC website.
It said that the council “strongly recommend that physicians and patients should continue treatment with their usual antihypertensive therapy because there is no clinical or scientific evidence to suggest that treatment with ACE inhibitors or ARBs should be discontinued because of the COVID-19 infection.”
The statement, signed by Council Chair Professor Giovanni de Simone, MD, on behalf of the nucleus members, also says that in regard to the theorized protective effect against serious lung complications in individuals with COVID-19, the data come only from animal, and not human, studies.
“Speculation about the safety of ACE-inhibitor or ARB treatment in relation to COVID-19 does not have a sound scientific basis or evidence to support it,” the ESC panel concludes.
This article first appeared on Medscape.com.
CV health in pregnancy improves outcomes for mother and infant
PHOENIX – according to results from a multinational cohort study.
“Over the past 10 years, cardiovascular health [CVH] has been characterized across most of the life course and is associated with a variety of health outcomes, but CVH as a whole has not been well studied during pregnancy,” Amanda M. Perak, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
In an effort to examine the associations of maternal gestational CVH with adverse maternal and newborn outcomes, Dr. Perak of the departments of pediatrics and preventive medicine at Northwestern University and Lurie Children’s Hospital, both in Chicago, and colleagues drew from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study, which examined pregnant women at a target of 28 weeks’ gestation and assessed the associations of glycemia with pregnancy outcomes. The researchers analyzed data from an ancillary study of 2,230 mother-child dyads to characterize clinical gestational CVH with use of five metrics: body mass index, blood pressure, cholesterol, glucose, and smoking. The study excluded women with prepregnancy diabetes, preterm births, and cases of fetal death/major malformations.
Each maternal CVH metric was classified as ideal, intermediate, or poor according to modified definitions based on pregnancy guidelines. “For lipids, it’s known that levels change substantially during pregnancy, but there are no pregnancy guidelines,” Dr. Perak said. “We and others have also shown that higher triglycerides in pregnancy are associated with adverse pregnancy outcomes. We selected thresholds of less than 250 mg/dL for ideal and at least 500 mg/dL for poor, based on triglyceride distribution and clinical relevance.”
Total CVH was scored by assigning 2 points for ideal, 1 for intermediate, and 0 for each poor metric, for a total possible 10 points, with 10 being most favorable. They also created four CVH categories, ranging from all ideal to two or more poor metrics. Maternal adverse pregnancy outcomes included preeclampsia and unplanned primary cesarean section. Newborn adverse pregnancy outcomes included birth weight above the 90th percentile and a cord blood insulin sensitivity index lower than the 10th percentile.
The researchers used logistic and multinomial logistic regression of pregnancy outcomes on maternal gestational CVH in two adjusted models. Secondarily, they examined associations of individual CVH metrics with outcomes, with adjustment for the other metrics.
The cohort comprised mother-child dyads from nine field centers in six countries: the United States (25%), Barbados (23%), United Kingdom (21%), China (18%), Thailand (7%), and Canada (7%). The mothers’ mean age was 30 years, and the mean gestational age was 28 weeks. The mean gestational CVH score was 8.8 out of 10. Nearly half of mothers (42%) had ideal metrics, while 4% had two or more poor metrics. Delivery occurred at a mean of 39.8 weeks, and adverse pregnancy outcomes occurred in 4.7%-17.9% of pregnancies.
In the fully adjusted model, which accounted for maternal age, height, alcohol use, gestational age at pregnancy exam, maternal parity, and newborn sex and race/ethnicity, odds ratios per 1-point higher (better) CVH score were 0.61 (95% confidence interval, 0.53-0.70) for preeclampsia, 0.85 (95% CI, 0.76-0.95) for unplanned primary cesarean section (among primiparous mothers), 0.83 (95% CI, 0.77-0.91) for large for gestational age infant, and 0.79 (95% CI, 0.72-0.87) for infant insulin sensitivity index below the 10th percentile. CVH categories were also associated with outcomes. For example, odds ratios for preeclampsia were 4.61 (95% CI, 2.13-11.14) for mothers with one or more intermediate metrics, 7.62 (95% CI, 3.60-18.13) for mothers with one poor metric, and 12.02 (95% CI, 4.70-32.50) for mothers with two or more poor metrics, compared with mothers with all metrics ideal.
“Except for smoking, each CVH metric was independently associated with adverse outcomes,” Dr. Perak said. “However, total CVH was associated with a wider range of outcomes than any single metric. This suggests that CVH provides health insights beyond single risk factors.”
Strengths of the study, she continued, included geographic and racial diversity of participants and high-quality research measurements of CVH. Limitations were that the cohort excluded prepregnancy diabetes and preterm births. “Diet and exercise data were not available, and CVH was measured once at 28 weeks,” she said. “Further study is needed across pregnancy and in other settings, but this study provides the first data on the relevance of gestational CVH for pregnancy outcomes.”
In an interview, Stephen S. Rich, PhD, who directs the Center for Public Health Genomics at the University of Virginia, said that the data “provide strong epidemiologic support to focus on the full range of cardiovascular health. In my view, the primary limitation of the study is that there may be significant differences in how one achieves ideal CHV across a single country, not to mention across the world, particularly in absence of a highly controlled, research environment. It is not clear that the approach used in this study at nine selected sites in six relatively highly developed countries could be translated into primary care – particularly in the U.S. with different regulatory and reimbursement plans and payers. Nonetheless, the evidence suggests a way to reduce adverse outcomes in pregnancy and the area deserves greater research.”
According to Dr. Perak, gestational diabetes is associated with a twofold higher maternal risk for cardiovascular disease (Diabetologia. 2019;62:905-14), while diabetes is also associated with higher offspring risk for CVD (BMJ. 2019;367:16398). However, a paucity of data exists on gestational CVH. In one report, better gestational CVH was associated with less subclinical CVD for the mother 10 years later (J Am Heart Assoc. 2019 Jul 23. doi:10.1161/JAHA.118.011394). In a separate analysis, Dr. Perak and her colleagues found that better gestational CVH was associated with better offspring CVH in childhood. “Unfortunately, we also reported that, among pregnant women in the United States, fewer than 1 in 10 had high CVH,” she said (J Am Heart Assoc. 2020 Feb 17. doi:10.1161/JAHA.119.015123). “However, the relevance of gestational CVH for pregnancy outcomes is unknown, but a it’s key question when considering CVH monitoring in prenatal care.”
Dr. Perak reported having received grant support from the National Heart, Lung, and Blood Institute, the American Heart Association, and Northwestern University. The HAPO Study was supported by NHLBI and the National Institute of Diabetes and Digestive and Kidney Diseases.
The meeting was sponsored by the American Heart Association.
SOURCE: Perak A et al. Epi/Lifestyle 2020, Abstract 33.
PHOENIX – according to results from a multinational cohort study.
“Over the past 10 years, cardiovascular health [CVH] has been characterized across most of the life course and is associated with a variety of health outcomes, but CVH as a whole has not been well studied during pregnancy,” Amanda M. Perak, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
In an effort to examine the associations of maternal gestational CVH with adverse maternal and newborn outcomes, Dr. Perak of the departments of pediatrics and preventive medicine at Northwestern University and Lurie Children’s Hospital, both in Chicago, and colleagues drew from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study, which examined pregnant women at a target of 28 weeks’ gestation and assessed the associations of glycemia with pregnancy outcomes. The researchers analyzed data from an ancillary study of 2,230 mother-child dyads to characterize clinical gestational CVH with use of five metrics: body mass index, blood pressure, cholesterol, glucose, and smoking. The study excluded women with prepregnancy diabetes, preterm births, and cases of fetal death/major malformations.
Each maternal CVH metric was classified as ideal, intermediate, or poor according to modified definitions based on pregnancy guidelines. “For lipids, it’s known that levels change substantially during pregnancy, but there are no pregnancy guidelines,” Dr. Perak said. “We and others have also shown that higher triglycerides in pregnancy are associated with adverse pregnancy outcomes. We selected thresholds of less than 250 mg/dL for ideal and at least 500 mg/dL for poor, based on triglyceride distribution and clinical relevance.”
Total CVH was scored by assigning 2 points for ideal, 1 for intermediate, and 0 for each poor metric, for a total possible 10 points, with 10 being most favorable. They also created four CVH categories, ranging from all ideal to two or more poor metrics. Maternal adverse pregnancy outcomes included preeclampsia and unplanned primary cesarean section. Newborn adverse pregnancy outcomes included birth weight above the 90th percentile and a cord blood insulin sensitivity index lower than the 10th percentile.
The researchers used logistic and multinomial logistic regression of pregnancy outcomes on maternal gestational CVH in two adjusted models. Secondarily, they examined associations of individual CVH metrics with outcomes, with adjustment for the other metrics.
The cohort comprised mother-child dyads from nine field centers in six countries: the United States (25%), Barbados (23%), United Kingdom (21%), China (18%), Thailand (7%), and Canada (7%). The mothers’ mean age was 30 years, and the mean gestational age was 28 weeks. The mean gestational CVH score was 8.8 out of 10. Nearly half of mothers (42%) had ideal metrics, while 4% had two or more poor metrics. Delivery occurred at a mean of 39.8 weeks, and adverse pregnancy outcomes occurred in 4.7%-17.9% of pregnancies.
In the fully adjusted model, which accounted for maternal age, height, alcohol use, gestational age at pregnancy exam, maternal parity, and newborn sex and race/ethnicity, odds ratios per 1-point higher (better) CVH score were 0.61 (95% confidence interval, 0.53-0.70) for preeclampsia, 0.85 (95% CI, 0.76-0.95) for unplanned primary cesarean section (among primiparous mothers), 0.83 (95% CI, 0.77-0.91) for large for gestational age infant, and 0.79 (95% CI, 0.72-0.87) for infant insulin sensitivity index below the 10th percentile. CVH categories were also associated with outcomes. For example, odds ratios for preeclampsia were 4.61 (95% CI, 2.13-11.14) for mothers with one or more intermediate metrics, 7.62 (95% CI, 3.60-18.13) for mothers with one poor metric, and 12.02 (95% CI, 4.70-32.50) for mothers with two or more poor metrics, compared with mothers with all metrics ideal.
“Except for smoking, each CVH metric was independently associated with adverse outcomes,” Dr. Perak said. “However, total CVH was associated with a wider range of outcomes than any single metric. This suggests that CVH provides health insights beyond single risk factors.”
Strengths of the study, she continued, included geographic and racial diversity of participants and high-quality research measurements of CVH. Limitations were that the cohort excluded prepregnancy diabetes and preterm births. “Diet and exercise data were not available, and CVH was measured once at 28 weeks,” she said. “Further study is needed across pregnancy and in other settings, but this study provides the first data on the relevance of gestational CVH for pregnancy outcomes.”
In an interview, Stephen S. Rich, PhD, who directs the Center for Public Health Genomics at the University of Virginia, said that the data “provide strong epidemiologic support to focus on the full range of cardiovascular health. In my view, the primary limitation of the study is that there may be significant differences in how one achieves ideal CHV across a single country, not to mention across the world, particularly in absence of a highly controlled, research environment. It is not clear that the approach used in this study at nine selected sites in six relatively highly developed countries could be translated into primary care – particularly in the U.S. with different regulatory and reimbursement plans and payers. Nonetheless, the evidence suggests a way to reduce adverse outcomes in pregnancy and the area deserves greater research.”
According to Dr. Perak, gestational diabetes is associated with a twofold higher maternal risk for cardiovascular disease (Diabetologia. 2019;62:905-14), while diabetes is also associated with higher offspring risk for CVD (BMJ. 2019;367:16398). However, a paucity of data exists on gestational CVH. In one report, better gestational CVH was associated with less subclinical CVD for the mother 10 years later (J Am Heart Assoc. 2019 Jul 23. doi:10.1161/JAHA.118.011394). In a separate analysis, Dr. Perak and her colleagues found that better gestational CVH was associated with better offspring CVH in childhood. “Unfortunately, we also reported that, among pregnant women in the United States, fewer than 1 in 10 had high CVH,” she said (J Am Heart Assoc. 2020 Feb 17. doi:10.1161/JAHA.119.015123). “However, the relevance of gestational CVH for pregnancy outcomes is unknown, but a it’s key question when considering CVH monitoring in prenatal care.”
Dr. Perak reported having received grant support from the National Heart, Lung, and Blood Institute, the American Heart Association, and Northwestern University. The HAPO Study was supported by NHLBI and the National Institute of Diabetes and Digestive and Kidney Diseases.
The meeting was sponsored by the American Heart Association.
SOURCE: Perak A et al. Epi/Lifestyle 2020, Abstract 33.
PHOENIX – according to results from a multinational cohort study.
“Over the past 10 years, cardiovascular health [CVH] has been characterized across most of the life course and is associated with a variety of health outcomes, but CVH as a whole has not been well studied during pregnancy,” Amanda M. Perak, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
In an effort to examine the associations of maternal gestational CVH with adverse maternal and newborn outcomes, Dr. Perak of the departments of pediatrics and preventive medicine at Northwestern University and Lurie Children’s Hospital, both in Chicago, and colleagues drew from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study, which examined pregnant women at a target of 28 weeks’ gestation and assessed the associations of glycemia with pregnancy outcomes. The researchers analyzed data from an ancillary study of 2,230 mother-child dyads to characterize clinical gestational CVH with use of five metrics: body mass index, blood pressure, cholesterol, glucose, and smoking. The study excluded women with prepregnancy diabetes, preterm births, and cases of fetal death/major malformations.
Each maternal CVH metric was classified as ideal, intermediate, or poor according to modified definitions based on pregnancy guidelines. “For lipids, it’s known that levels change substantially during pregnancy, but there are no pregnancy guidelines,” Dr. Perak said. “We and others have also shown that higher triglycerides in pregnancy are associated with adverse pregnancy outcomes. We selected thresholds of less than 250 mg/dL for ideal and at least 500 mg/dL for poor, based on triglyceride distribution and clinical relevance.”
Total CVH was scored by assigning 2 points for ideal, 1 for intermediate, and 0 for each poor metric, for a total possible 10 points, with 10 being most favorable. They also created four CVH categories, ranging from all ideal to two or more poor metrics. Maternal adverse pregnancy outcomes included preeclampsia and unplanned primary cesarean section. Newborn adverse pregnancy outcomes included birth weight above the 90th percentile and a cord blood insulin sensitivity index lower than the 10th percentile.
The researchers used logistic and multinomial logistic regression of pregnancy outcomes on maternal gestational CVH in two adjusted models. Secondarily, they examined associations of individual CVH metrics with outcomes, with adjustment for the other metrics.
The cohort comprised mother-child dyads from nine field centers in six countries: the United States (25%), Barbados (23%), United Kingdom (21%), China (18%), Thailand (7%), and Canada (7%). The mothers’ mean age was 30 years, and the mean gestational age was 28 weeks. The mean gestational CVH score was 8.8 out of 10. Nearly half of mothers (42%) had ideal metrics, while 4% had two or more poor metrics. Delivery occurred at a mean of 39.8 weeks, and adverse pregnancy outcomes occurred in 4.7%-17.9% of pregnancies.
In the fully adjusted model, which accounted for maternal age, height, alcohol use, gestational age at pregnancy exam, maternal parity, and newborn sex and race/ethnicity, odds ratios per 1-point higher (better) CVH score were 0.61 (95% confidence interval, 0.53-0.70) for preeclampsia, 0.85 (95% CI, 0.76-0.95) for unplanned primary cesarean section (among primiparous mothers), 0.83 (95% CI, 0.77-0.91) for large for gestational age infant, and 0.79 (95% CI, 0.72-0.87) for infant insulin sensitivity index below the 10th percentile. CVH categories were also associated with outcomes. For example, odds ratios for preeclampsia were 4.61 (95% CI, 2.13-11.14) for mothers with one or more intermediate metrics, 7.62 (95% CI, 3.60-18.13) for mothers with one poor metric, and 12.02 (95% CI, 4.70-32.50) for mothers with two or more poor metrics, compared with mothers with all metrics ideal.
“Except for smoking, each CVH metric was independently associated with adverse outcomes,” Dr. Perak said. “However, total CVH was associated with a wider range of outcomes than any single metric. This suggests that CVH provides health insights beyond single risk factors.”
Strengths of the study, she continued, included geographic and racial diversity of participants and high-quality research measurements of CVH. Limitations were that the cohort excluded prepregnancy diabetes and preterm births. “Diet and exercise data were not available, and CVH was measured once at 28 weeks,” she said. “Further study is needed across pregnancy and in other settings, but this study provides the first data on the relevance of gestational CVH for pregnancy outcomes.”
In an interview, Stephen S. Rich, PhD, who directs the Center for Public Health Genomics at the University of Virginia, said that the data “provide strong epidemiologic support to focus on the full range of cardiovascular health. In my view, the primary limitation of the study is that there may be significant differences in how one achieves ideal CHV across a single country, not to mention across the world, particularly in absence of a highly controlled, research environment. It is not clear that the approach used in this study at nine selected sites in six relatively highly developed countries could be translated into primary care – particularly in the U.S. with different regulatory and reimbursement plans and payers. Nonetheless, the evidence suggests a way to reduce adverse outcomes in pregnancy and the area deserves greater research.”
According to Dr. Perak, gestational diabetes is associated with a twofold higher maternal risk for cardiovascular disease (Diabetologia. 2019;62:905-14), while diabetes is also associated with higher offspring risk for CVD (BMJ. 2019;367:16398). However, a paucity of data exists on gestational CVH. In one report, better gestational CVH was associated with less subclinical CVD for the mother 10 years later (J Am Heart Assoc. 2019 Jul 23. doi:10.1161/JAHA.118.011394). In a separate analysis, Dr. Perak and her colleagues found that better gestational CVH was associated with better offspring CVH in childhood. “Unfortunately, we also reported that, among pregnant women in the United States, fewer than 1 in 10 had high CVH,” she said (J Am Heart Assoc. 2020 Feb 17. doi:10.1161/JAHA.119.015123). “However, the relevance of gestational CVH for pregnancy outcomes is unknown, but a it’s key question when considering CVH monitoring in prenatal care.”
Dr. Perak reported having received grant support from the National Heart, Lung, and Blood Institute, the American Heart Association, and Northwestern University. The HAPO Study was supported by NHLBI and the National Institute of Diabetes and Digestive and Kidney Diseases.
The meeting was sponsored by the American Heart Association.
SOURCE: Perak A et al. Epi/Lifestyle 2020, Abstract 33.
REPORTING FROM EPI/LIFESTYLE 2020
Risk factors for death from COVID-19 identified in Wuhan patients
Patients who did not survive hospitalization for COVID-19 in Wuhan were more likely to be older, have comorbidities, and elevated D-dimer, according to the first study to examine risk factors associated with death among adults hospitalized with COVID-19. “Older age, showing signs of sepsis on admission, underlying diseases like high blood pressure and diabetes, and the prolonged use of noninvasive ventilation were important factors in the deaths of these patients,” coauthor Zhibo Liu said in a news release. Abnormal blood clotting was part of the clinical picture too.
Fei Zhou, MD, from the Chinese Academy of Medical Sciences, and colleagues conducted a retrospective, observational, multicenter cohort study of 191 patients, 137 of whom were discharged and 54 of whom died in the hospital.
The study, published online today in The Lancet, included all adult inpatients with laboratory-confirmed COVID-19 from Jinyintan Hospital and Wuhan Pulmonary Hospital who had been discharged or died by January 31 of this year. Severely ill patients in the province were transferred to these hospitals until February 1.
The researchers compared demographic, clinical, treatment, and laboratory data from electronic medical records between survivors and those who succumbed to the disease. The analysis also tested serial samples for viral RNA. Overall, 91 (48%) of the 191 patients had comorbidity. Most common was hypertension (30%), followed by diabetes (19%) and coronary heart disease (8%).
The odds of dying in the hospital increased with age (odds ratio 1.10; 95% confidence interval, 1.03-1.17; per year increase in age), higher Sequential Organ Failure Assessment (SOFA) score (5.65, 2.61-12.23; P < .0001), and D-dimer level exceeding 1 mcg/L on admission. The SOFA was previously called the “sepsis-related organ failure assessment score” and assesses rate of organ failure in intensive care units. Elevated D-dimer indicates increased risk of abnormal blood clotting, such as deep vein thrombosis.
Nonsurvivors compared with survivors had higher frequencies of respiratory failure (98% vs 36%), sepsis (100%, vs 42%), and secondary infections (50% vs 1%).
The average age of survivors was 52 years compared to 69 for those who died. Liu cited weakening of the immune system and increased inflammation, which damages organs and also promotes viral replication, as explanations for the age effect.
From the time of initial symptoms, median time to discharge from the hospital was 22 days. Average time to death was 18.5 days.
Fever persisted for a median of 12 days among all patients, and cough persisted for a median 19 days; 45% of the survivors were still coughing on discharge. In survivors, shortness of breath improved after 13 days, but persisted until death in the others.
Viral shedding persisted for a median duration of 20 days in survivors, ranging from 8 to 37. The virus (SARS-CoV-2) was detectable in nonsurvivors until death. Antiviral treatment did not curtail viral shedding.
But the viral shedding data come with a caveat. “The extended viral shedding noted in our study has important implications for guiding decisions around isolation precautions and antiviral treatment in patients with confirmed COVID-19 infection. However, we need to be clear that viral shedding time should not be confused with other self-isolation guidance for people who may have been exposed to COVID-19 but do not have symptoms, as this guidance is based on the incubation time of the virus,” explained colead author Bin Cao.
“Older age, elevated D-dimer levels, and high SOFA score could help clinicians to identify at an early stage those patients with COVID-19 who have poor prognosis. Prolonged viral shedding provides the rationale for a strategy of isolation of infected patients and optimal antiviral interventions in the future,” the researchers conclude.
A limitation in interpreting the findings of the study is that hospitalized patients do not represent the entire infected population. The researchers caution that “the number of deaths does not reflect the true mortality of COVID-19.” They also note that they did not have enough genetic material to accurately assess duration of viral shedding.
This article first appeared on Medscape.com.
Patients who did not survive hospitalization for COVID-19 in Wuhan were more likely to be older, have comorbidities, and elevated D-dimer, according to the first study to examine risk factors associated with death among adults hospitalized with COVID-19. “Older age, showing signs of sepsis on admission, underlying diseases like high blood pressure and diabetes, and the prolonged use of noninvasive ventilation were important factors in the deaths of these patients,” coauthor Zhibo Liu said in a news release. Abnormal blood clotting was part of the clinical picture too.
Fei Zhou, MD, from the Chinese Academy of Medical Sciences, and colleagues conducted a retrospective, observational, multicenter cohort study of 191 patients, 137 of whom were discharged and 54 of whom died in the hospital.
The study, published online today in The Lancet, included all adult inpatients with laboratory-confirmed COVID-19 from Jinyintan Hospital and Wuhan Pulmonary Hospital who had been discharged or died by January 31 of this year. Severely ill patients in the province were transferred to these hospitals until February 1.
The researchers compared demographic, clinical, treatment, and laboratory data from electronic medical records between survivors and those who succumbed to the disease. The analysis also tested serial samples for viral RNA. Overall, 91 (48%) of the 191 patients had comorbidity. Most common was hypertension (30%), followed by diabetes (19%) and coronary heart disease (8%).
The odds of dying in the hospital increased with age (odds ratio 1.10; 95% confidence interval, 1.03-1.17; per year increase in age), higher Sequential Organ Failure Assessment (SOFA) score (5.65, 2.61-12.23; P < .0001), and D-dimer level exceeding 1 mcg/L on admission. The SOFA was previously called the “sepsis-related organ failure assessment score” and assesses rate of organ failure in intensive care units. Elevated D-dimer indicates increased risk of abnormal blood clotting, such as deep vein thrombosis.
Nonsurvivors compared with survivors had higher frequencies of respiratory failure (98% vs 36%), sepsis (100%, vs 42%), and secondary infections (50% vs 1%).
The average age of survivors was 52 years compared to 69 for those who died. Liu cited weakening of the immune system and increased inflammation, which damages organs and also promotes viral replication, as explanations for the age effect.
From the time of initial symptoms, median time to discharge from the hospital was 22 days. Average time to death was 18.5 days.
Fever persisted for a median of 12 days among all patients, and cough persisted for a median 19 days; 45% of the survivors were still coughing on discharge. In survivors, shortness of breath improved after 13 days, but persisted until death in the others.
Viral shedding persisted for a median duration of 20 days in survivors, ranging from 8 to 37. The virus (SARS-CoV-2) was detectable in nonsurvivors until death. Antiviral treatment did not curtail viral shedding.
But the viral shedding data come with a caveat. “The extended viral shedding noted in our study has important implications for guiding decisions around isolation precautions and antiviral treatment in patients with confirmed COVID-19 infection. However, we need to be clear that viral shedding time should not be confused with other self-isolation guidance for people who may have been exposed to COVID-19 but do not have symptoms, as this guidance is based on the incubation time of the virus,” explained colead author Bin Cao.
“Older age, elevated D-dimer levels, and high SOFA score could help clinicians to identify at an early stage those patients with COVID-19 who have poor prognosis. Prolonged viral shedding provides the rationale for a strategy of isolation of infected patients and optimal antiviral interventions in the future,” the researchers conclude.
A limitation in interpreting the findings of the study is that hospitalized patients do not represent the entire infected population. The researchers caution that “the number of deaths does not reflect the true mortality of COVID-19.” They also note that they did not have enough genetic material to accurately assess duration of viral shedding.
This article first appeared on Medscape.com.
Patients who did not survive hospitalization for COVID-19 in Wuhan were more likely to be older, have comorbidities, and elevated D-dimer, according to the first study to examine risk factors associated with death among adults hospitalized with COVID-19. “Older age, showing signs of sepsis on admission, underlying diseases like high blood pressure and diabetes, and the prolonged use of noninvasive ventilation were important factors in the deaths of these patients,” coauthor Zhibo Liu said in a news release. Abnormal blood clotting was part of the clinical picture too.
Fei Zhou, MD, from the Chinese Academy of Medical Sciences, and colleagues conducted a retrospective, observational, multicenter cohort study of 191 patients, 137 of whom were discharged and 54 of whom died in the hospital.
The study, published online today in The Lancet, included all adult inpatients with laboratory-confirmed COVID-19 from Jinyintan Hospital and Wuhan Pulmonary Hospital who had been discharged or died by January 31 of this year. Severely ill patients in the province were transferred to these hospitals until February 1.
The researchers compared demographic, clinical, treatment, and laboratory data from electronic medical records between survivors and those who succumbed to the disease. The analysis also tested serial samples for viral RNA. Overall, 91 (48%) of the 191 patients had comorbidity. Most common was hypertension (30%), followed by diabetes (19%) and coronary heart disease (8%).
The odds of dying in the hospital increased with age (odds ratio 1.10; 95% confidence interval, 1.03-1.17; per year increase in age), higher Sequential Organ Failure Assessment (SOFA) score (5.65, 2.61-12.23; P < .0001), and D-dimer level exceeding 1 mcg/L on admission. The SOFA was previously called the “sepsis-related organ failure assessment score” and assesses rate of organ failure in intensive care units. Elevated D-dimer indicates increased risk of abnormal blood clotting, such as deep vein thrombosis.
Nonsurvivors compared with survivors had higher frequencies of respiratory failure (98% vs 36%), sepsis (100%, vs 42%), and secondary infections (50% vs 1%).
The average age of survivors was 52 years compared to 69 for those who died. Liu cited weakening of the immune system and increased inflammation, which damages organs and also promotes viral replication, as explanations for the age effect.
From the time of initial symptoms, median time to discharge from the hospital was 22 days. Average time to death was 18.5 days.
Fever persisted for a median of 12 days among all patients, and cough persisted for a median 19 days; 45% of the survivors were still coughing on discharge. In survivors, shortness of breath improved after 13 days, but persisted until death in the others.
Viral shedding persisted for a median duration of 20 days in survivors, ranging from 8 to 37. The virus (SARS-CoV-2) was detectable in nonsurvivors until death. Antiviral treatment did not curtail viral shedding.
But the viral shedding data come with a caveat. “The extended viral shedding noted in our study has important implications for guiding decisions around isolation precautions and antiviral treatment in patients with confirmed COVID-19 infection. However, we need to be clear that viral shedding time should not be confused with other self-isolation guidance for people who may have been exposed to COVID-19 but do not have symptoms, as this guidance is based on the incubation time of the virus,” explained colead author Bin Cao.
“Older age, elevated D-dimer levels, and high SOFA score could help clinicians to identify at an early stage those patients with COVID-19 who have poor prognosis. Prolonged viral shedding provides the rationale for a strategy of isolation of infected patients and optimal antiviral interventions in the future,” the researchers conclude.
A limitation in interpreting the findings of the study is that hospitalized patients do not represent the entire infected population. The researchers caution that “the number of deaths does not reflect the true mortality of COVID-19.” They also note that they did not have enough genetic material to accurately assess duration of viral shedding.
This article first appeared on Medscape.com.
Know the 15% rule in scleroderma
MAUI, HAWAII – The 15% rule in scleroderma is a handy tool that raises awareness of the disease’s associated prevalence of various severe organ complications so clinicians can screen appropriately, Janet Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Dr. Pope and colleagues in the Canadian Scleroderma Research Group developed the 15% rule because they recognized that scleroderma is rare enough that most physicians practicing outside of a few specialized centers don’t see many affected patients. The systemic autoimmune disease is marked by numerous possible expressions of vascular inflammation and malfunction, fibrosis, and autoimmunity in different organ systems.
“A lot of clinicians do not know how common this stuff is,” according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Center in London, Ont.
Basically, the 15% rule holds that, at any given time, a patient with scleroderma has roughly a 15% chance – or one in six – of having any of an extensive array of severe organ complications. That means a 15% chance of having prevalent clinically significant pulmonary hypertension as defined by a systolic pulmonary artery pressure of 45 mm Hg or more on Doppler echocardiography, a 15% likelihood of interstitial lung disease or clinically significant pulmonary fibrosis as suggested by a forced vital capacity less than 70% of predicted, a 15% prevalence of Sjögren’s syndrome, a 15% likelihood of having pulmonary artery hypertension upon right heart catheterization, a 15% chance of inflammatory arthritis, and a one-in-six chance of having a myopathy or myositis. Also, diastolic dysfunction, 15%. Ditto symptomatic arrhythmias.
“It’s a good little rule of thumb,” Dr. Pope commented.
The odds of having a current digital ulcer on any given day? Again, about 15%. In addition, scleroderma patients have a 15% lifetime risk of developing a complicated digital ulcer requiring hospitalization and/or amputation, she continued.
And while the prevalence of scleroderma renal crisis in the overall population with scleroderma is low, at 3%, in the subgroup with diffuse cutaneous systemic sclerosis, it climbs to 12%-15%.
Every rule has its exceptions. The 15% rule doesn’t apply to Raynaud’s phenomenon, which is present in nearly all patients with scleroderma, nor to gastroesophageal reflux disease or dysphagia, present in roughly 80% of patients.
Dr. Pope and coinvestigators developed the 15% rule pertaining to the prevalence of serious organ complications in scleroderma by conducting a systematic review of 69 published studies, each including a minimum of 50 scleroderma patients. The detailed results of the systematic review have been published.
Dr. Pope reported receiving research grants from and/or serving as a consultant to more than a dozen pharmaceutical companies.
MAUI, HAWAII – The 15% rule in scleroderma is a handy tool that raises awareness of the disease’s associated prevalence of various severe organ complications so clinicians can screen appropriately, Janet Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Dr. Pope and colleagues in the Canadian Scleroderma Research Group developed the 15% rule because they recognized that scleroderma is rare enough that most physicians practicing outside of a few specialized centers don’t see many affected patients. The systemic autoimmune disease is marked by numerous possible expressions of vascular inflammation and malfunction, fibrosis, and autoimmunity in different organ systems.
“A lot of clinicians do not know how common this stuff is,” according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Center in London, Ont.
Basically, the 15% rule holds that, at any given time, a patient with scleroderma has roughly a 15% chance – or one in six – of having any of an extensive array of severe organ complications. That means a 15% chance of having prevalent clinically significant pulmonary hypertension as defined by a systolic pulmonary artery pressure of 45 mm Hg or more on Doppler echocardiography, a 15% likelihood of interstitial lung disease or clinically significant pulmonary fibrosis as suggested by a forced vital capacity less than 70% of predicted, a 15% prevalence of Sjögren’s syndrome, a 15% likelihood of having pulmonary artery hypertension upon right heart catheterization, a 15% chance of inflammatory arthritis, and a one-in-six chance of having a myopathy or myositis. Also, diastolic dysfunction, 15%. Ditto symptomatic arrhythmias.
“It’s a good little rule of thumb,” Dr. Pope commented.
The odds of having a current digital ulcer on any given day? Again, about 15%. In addition, scleroderma patients have a 15% lifetime risk of developing a complicated digital ulcer requiring hospitalization and/or amputation, she continued.
And while the prevalence of scleroderma renal crisis in the overall population with scleroderma is low, at 3%, in the subgroup with diffuse cutaneous systemic sclerosis, it climbs to 12%-15%.
Every rule has its exceptions. The 15% rule doesn’t apply to Raynaud’s phenomenon, which is present in nearly all patients with scleroderma, nor to gastroesophageal reflux disease or dysphagia, present in roughly 80% of patients.
Dr. Pope and coinvestigators developed the 15% rule pertaining to the prevalence of serious organ complications in scleroderma by conducting a systematic review of 69 published studies, each including a minimum of 50 scleroderma patients. The detailed results of the systematic review have been published.
Dr. Pope reported receiving research grants from and/or serving as a consultant to more than a dozen pharmaceutical companies.
MAUI, HAWAII – The 15% rule in scleroderma is a handy tool that raises awareness of the disease’s associated prevalence of various severe organ complications so clinicians can screen appropriately, Janet Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
Dr. Pope and colleagues in the Canadian Scleroderma Research Group developed the 15% rule because they recognized that scleroderma is rare enough that most physicians practicing outside of a few specialized centers don’t see many affected patients. The systemic autoimmune disease is marked by numerous possible expressions of vascular inflammation and malfunction, fibrosis, and autoimmunity in different organ systems.
“A lot of clinicians do not know how common this stuff is,” according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Center in London, Ont.
Basically, the 15% rule holds that, at any given time, a patient with scleroderma has roughly a 15% chance – or one in six – of having any of an extensive array of severe organ complications. That means a 15% chance of having prevalent clinically significant pulmonary hypertension as defined by a systolic pulmonary artery pressure of 45 mm Hg or more on Doppler echocardiography, a 15% likelihood of interstitial lung disease or clinically significant pulmonary fibrosis as suggested by a forced vital capacity less than 70% of predicted, a 15% prevalence of Sjögren’s syndrome, a 15% likelihood of having pulmonary artery hypertension upon right heart catheterization, a 15% chance of inflammatory arthritis, and a one-in-six chance of having a myopathy or myositis. Also, diastolic dysfunction, 15%. Ditto symptomatic arrhythmias.
“It’s a good little rule of thumb,” Dr. Pope commented.
The odds of having a current digital ulcer on any given day? Again, about 15%. In addition, scleroderma patients have a 15% lifetime risk of developing a complicated digital ulcer requiring hospitalization and/or amputation, she continued.
And while the prevalence of scleroderma renal crisis in the overall population with scleroderma is low, at 3%, in the subgroup with diffuse cutaneous systemic sclerosis, it climbs to 12%-15%.
Every rule has its exceptions. The 15% rule doesn’t apply to Raynaud’s phenomenon, which is present in nearly all patients with scleroderma, nor to gastroesophageal reflux disease or dysphagia, present in roughly 80% of patients.
Dr. Pope and coinvestigators developed the 15% rule pertaining to the prevalence of serious organ complications in scleroderma by conducting a systematic review of 69 published studies, each including a minimum of 50 scleroderma patients. The detailed results of the systematic review have been published.
Dr. Pope reported receiving research grants from and/or serving as a consultant to more than a dozen pharmaceutical companies.
REPORTING FROM RWCS 2020
Prescription cascade more likely after CCBs than other hypertension meds
Elderly adults with hypertension who are newly prescribed a calcium-channel blocker (CCB), compared to other antihypertensive agents, are at least twice as likely to be given a loop diuretic over the following months, a large cohort study suggests.
The likelihood remained elevated for as long as a year after the start of a CCB and was more pronounced when comparing CCBs to any other kind of medication.
“Our findings suggest that many older adults who begin taking a CCB may subsequently experience a prescribing cascade” when loop diuretics are prescribed for peripheral edema, a known CCB adverse effect, that is misinterpreted as a new medical condition, Rachel D. Savage, PhD, Women’s College Hospital, Toronto, Canada, told theheart.org/Medscape Cardiology.
Edema caused by CCBs is caused by fluid redistribution, not overload, and “treating euvolemic individuals with a diuretic places them at increased risk of overdiuresis, leading to falls, urinary incontinence, acute kidney injury, electrolyte imbalances, and a cascade of other downstream consequences to which older adults are especially vulnerable,” explain Savage and coauthors of the analysis published online February 24 in JAMA Internal Medicine.
However, 1.4% of the cohort had been prescribed a loop diuretic, and 4.5% had been given any diuretic within 90 days after the start of CCBs. The corresponding rates were 0.7% and 3.4%, respectively, for patients who had started on ACE inhibitors or angiotensin receptor blocker (ARB) rather than a CCB.
Also, Savage observed, “the likelihood of being prescribed a loop diuretic following initiation of a CCB changed over time and was greatest 61 to 90 days postinitiation.” At that point, it was increased 2.4 times compared with initiation of an ACE inhibitor or an ARB in an adjusted analysis and increased almost 4 times compared with starting on any non-CCB agent.
Importantly, the actual prevalence of peripheral edema among those started on CCBs, ACE inhibitors, ARBs, or any non-CCB medication was not available in the data sets.
However, “the main message for clinicians is to consider medication side effects as a potential cause for new symptoms when patients present. We also encourage patients to ask prescribers about whether new symptoms could be caused by a medication,” senior author Lisa M. McCarthy, PharmD, told theheart.org/Medscape Cardiology.
“If a patient experiences peripheral edema while taking a CCB, we would encourage clinicians to consider whether the calcium-channel blocker is still necessary, whether it could be discontinued or the dose reduced, or whether the patient can be switched to another therapy,” she said.
Based on the current analysis, if the rate of CCB-induced peripheral edema is assumed to be 10%, which is consistent with the literature, then “potentially 7% to 14% of people who develop edema while taking a calcium channel blocker may then receive a loop diuretic,” an accompanying editorial notes.
“Patients with polypharmacy are at heightened risk of being exposed to [a] series of prescribing cascades if their current use of medications is not carefully discussed before the decision to add a new antihypertensive,” observe Timothy S. Anderson, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, and Michael A. Steinman, MD, San Francisco Veterans Affairs Medical Center and University of California, San Francisco.
“The initial prescribing cascade can set off many other negative consequences, including adverse drug events, potentially avoidable diagnostic testing, and hospitalizations,” the editorialists caution.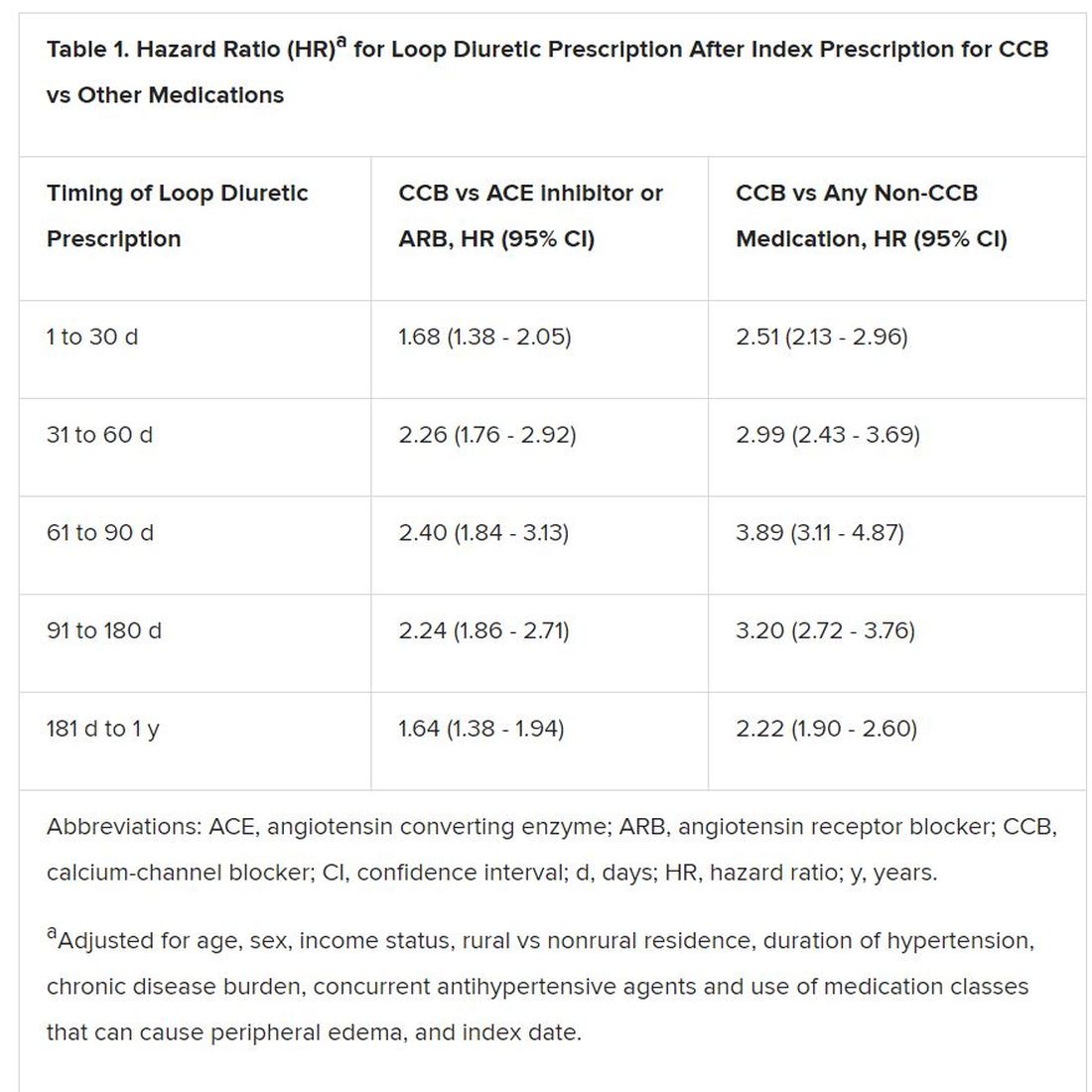
“Identifying prescribing cascades and their consequences is an important step to stem the tide of polypharmacy and inform deprescribing efforts.”
The analysis was based on administrative data from almost 340,000 adults in the community aged 66 years or older with hypertension and new drug prescriptions over 5 years ending in September 2016, the report notes. Their mean age was 74.5 years and 56.5% were women.
The data set included 41,086 patients who were newly prescribed a CCB; 66,494 who were newly prescribed an ACE inhibitor or ARB; and 231,439 newly prescribed any medication other than a CCB. The prescribed CCB was amlodipine in 79.6% of patients.
Although loop diuretics could possibly have been prescribed sometimes as a second-tier antihypertensive in the absence of peripheral edema, “we made efforts, through the design of our study, to limit this where possible,” Savage said in an interview.
For example, the focus was on loop diuretics, which aren’t generally recommended for blood-pressure lowering. Also, patients with heart failure and those with a recent history of diuretic or other antihypertensive medication use had been excluded, she said.
“As such, our cohort comprised individuals with new-onset or milder hypertension for whom diuretics would unlikely to be prescribed as part of guideline-based hypertension management.”
Although amlodipine was the most commonly prescribed CCB, the potential for a prescribing cascade seemed to be a class effect and to apply at a range of dosages.
That was unexpected, McCarthy observed, because “peripheral edema occurs more commonly in people taking dihydropyridine CCBs, like amlodipine, compared to non–dihydropyridine CCBs, such as verapamil and diltiazem.”
Savage, McCarthy, their coauthors, and the editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Elderly adults with hypertension who are newly prescribed a calcium-channel blocker (CCB), compared to other antihypertensive agents, are at least twice as likely to be given a loop diuretic over the following months, a large cohort study suggests.
The likelihood remained elevated for as long as a year after the start of a CCB and was more pronounced when comparing CCBs to any other kind of medication.
“Our findings suggest that many older adults who begin taking a CCB may subsequently experience a prescribing cascade” when loop diuretics are prescribed for peripheral edema, a known CCB adverse effect, that is misinterpreted as a new medical condition, Rachel D. Savage, PhD, Women’s College Hospital, Toronto, Canada, told theheart.org/Medscape Cardiology.
Edema caused by CCBs is caused by fluid redistribution, not overload, and “treating euvolemic individuals with a diuretic places them at increased risk of overdiuresis, leading to falls, urinary incontinence, acute kidney injury, electrolyte imbalances, and a cascade of other downstream consequences to which older adults are especially vulnerable,” explain Savage and coauthors of the analysis published online February 24 in JAMA Internal Medicine.
However, 1.4% of the cohort had been prescribed a loop diuretic, and 4.5% had been given any diuretic within 90 days after the start of CCBs. The corresponding rates were 0.7% and 3.4%, respectively, for patients who had started on ACE inhibitors or angiotensin receptor blocker (ARB) rather than a CCB.
Also, Savage observed, “the likelihood of being prescribed a loop diuretic following initiation of a CCB changed over time and was greatest 61 to 90 days postinitiation.” At that point, it was increased 2.4 times compared with initiation of an ACE inhibitor or an ARB in an adjusted analysis and increased almost 4 times compared with starting on any non-CCB agent.
Importantly, the actual prevalence of peripheral edema among those started on CCBs, ACE inhibitors, ARBs, or any non-CCB medication was not available in the data sets.
However, “the main message for clinicians is to consider medication side effects as a potential cause for new symptoms when patients present. We also encourage patients to ask prescribers about whether new symptoms could be caused by a medication,” senior author Lisa M. McCarthy, PharmD, told theheart.org/Medscape Cardiology.
“If a patient experiences peripheral edema while taking a CCB, we would encourage clinicians to consider whether the calcium-channel blocker is still necessary, whether it could be discontinued or the dose reduced, or whether the patient can be switched to another therapy,” she said.
Based on the current analysis, if the rate of CCB-induced peripheral edema is assumed to be 10%, which is consistent with the literature, then “potentially 7% to 14% of people who develop edema while taking a calcium channel blocker may then receive a loop diuretic,” an accompanying editorial notes.
“Patients with polypharmacy are at heightened risk of being exposed to [a] series of prescribing cascades if their current use of medications is not carefully discussed before the decision to add a new antihypertensive,” observe Timothy S. Anderson, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, and Michael A. Steinman, MD, San Francisco Veterans Affairs Medical Center and University of California, San Francisco.
“The initial prescribing cascade can set off many other negative consequences, including adverse drug events, potentially avoidable diagnostic testing, and hospitalizations,” the editorialists caution.
“Identifying prescribing cascades and their consequences is an important step to stem the tide of polypharmacy and inform deprescribing efforts.”
The analysis was based on administrative data from almost 340,000 adults in the community aged 66 years or older with hypertension and new drug prescriptions over 5 years ending in September 2016, the report notes. Their mean age was 74.5 years and 56.5% were women.
The data set included 41,086 patients who were newly prescribed a CCB; 66,494 who were newly prescribed an ACE inhibitor or ARB; and 231,439 newly prescribed any medication other than a CCB. The prescribed CCB was amlodipine in 79.6% of patients.
Although loop diuretics could possibly have been prescribed sometimes as a second-tier antihypertensive in the absence of peripheral edema, “we made efforts, through the design of our study, to limit this where possible,” Savage said in an interview.
For example, the focus was on loop diuretics, which aren’t generally recommended for blood-pressure lowering. Also, patients with heart failure and those with a recent history of diuretic or other antihypertensive medication use had been excluded, she said.
“As such, our cohort comprised individuals with new-onset or milder hypertension for whom diuretics would unlikely to be prescribed as part of guideline-based hypertension management.”
Although amlodipine was the most commonly prescribed CCB, the potential for a prescribing cascade seemed to be a class effect and to apply at a range of dosages.
That was unexpected, McCarthy observed, because “peripheral edema occurs more commonly in people taking dihydropyridine CCBs, like amlodipine, compared to non–dihydropyridine CCBs, such as verapamil and diltiazem.”
Savage, McCarthy, their coauthors, and the editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Elderly adults with hypertension who are newly prescribed a calcium-channel blocker (CCB), compared to other antihypertensive agents, are at least twice as likely to be given a loop diuretic over the following months, a large cohort study suggests.
The likelihood remained elevated for as long as a year after the start of a CCB and was more pronounced when comparing CCBs to any other kind of medication.
“Our findings suggest that many older adults who begin taking a CCB may subsequently experience a prescribing cascade” when loop diuretics are prescribed for peripheral edema, a known CCB adverse effect, that is misinterpreted as a new medical condition, Rachel D. Savage, PhD, Women’s College Hospital, Toronto, Canada, told theheart.org/Medscape Cardiology.
Edema caused by CCBs is caused by fluid redistribution, not overload, and “treating euvolemic individuals with a diuretic places them at increased risk of overdiuresis, leading to falls, urinary incontinence, acute kidney injury, electrolyte imbalances, and a cascade of other downstream consequences to which older adults are especially vulnerable,” explain Savage and coauthors of the analysis published online February 24 in JAMA Internal Medicine.
However, 1.4% of the cohort had been prescribed a loop diuretic, and 4.5% had been given any diuretic within 90 days after the start of CCBs. The corresponding rates were 0.7% and 3.4%, respectively, for patients who had started on ACE inhibitors or angiotensin receptor blocker (ARB) rather than a CCB.
Also, Savage observed, “the likelihood of being prescribed a loop diuretic following initiation of a CCB changed over time and was greatest 61 to 90 days postinitiation.” At that point, it was increased 2.4 times compared with initiation of an ACE inhibitor or an ARB in an adjusted analysis and increased almost 4 times compared with starting on any non-CCB agent.
Importantly, the actual prevalence of peripheral edema among those started on CCBs, ACE inhibitors, ARBs, or any non-CCB medication was not available in the data sets.
However, “the main message for clinicians is to consider medication side effects as a potential cause for new symptoms when patients present. We also encourage patients to ask prescribers about whether new symptoms could be caused by a medication,” senior author Lisa M. McCarthy, PharmD, told theheart.org/Medscape Cardiology.
“If a patient experiences peripheral edema while taking a CCB, we would encourage clinicians to consider whether the calcium-channel blocker is still necessary, whether it could be discontinued or the dose reduced, or whether the patient can be switched to another therapy,” she said.
Based on the current analysis, if the rate of CCB-induced peripheral edema is assumed to be 10%, which is consistent with the literature, then “potentially 7% to 14% of people who develop edema while taking a calcium channel blocker may then receive a loop diuretic,” an accompanying editorial notes.
“Patients with polypharmacy are at heightened risk of being exposed to [a] series of prescribing cascades if their current use of medications is not carefully discussed before the decision to add a new antihypertensive,” observe Timothy S. Anderson, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts, and Michael A. Steinman, MD, San Francisco Veterans Affairs Medical Center and University of California, San Francisco.
“The initial prescribing cascade can set off many other negative consequences, including adverse drug events, potentially avoidable diagnostic testing, and hospitalizations,” the editorialists caution.
“Identifying prescribing cascades and their consequences is an important step to stem the tide of polypharmacy and inform deprescribing efforts.”
The analysis was based on administrative data from almost 340,000 adults in the community aged 66 years or older with hypertension and new drug prescriptions over 5 years ending in September 2016, the report notes. Their mean age was 74.5 years and 56.5% were women.
The data set included 41,086 patients who were newly prescribed a CCB; 66,494 who were newly prescribed an ACE inhibitor or ARB; and 231,439 newly prescribed any medication other than a CCB. The prescribed CCB was amlodipine in 79.6% of patients.
Although loop diuretics could possibly have been prescribed sometimes as a second-tier antihypertensive in the absence of peripheral edema, “we made efforts, through the design of our study, to limit this where possible,” Savage said in an interview.
For example, the focus was on loop diuretics, which aren’t generally recommended for blood-pressure lowering. Also, patients with heart failure and those with a recent history of diuretic or other antihypertensive medication use had been excluded, she said.
“As such, our cohort comprised individuals with new-onset or milder hypertension for whom diuretics would unlikely to be prescribed as part of guideline-based hypertension management.”
Although amlodipine was the most commonly prescribed CCB, the potential for a prescribing cascade seemed to be a class effect and to apply at a range of dosages.
That was unexpected, McCarthy observed, because “peripheral edema occurs more commonly in people taking dihydropyridine CCBs, like amlodipine, compared to non–dihydropyridine CCBs, such as verapamil and diltiazem.”
Savage, McCarthy, their coauthors, and the editorialists have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FDA promises rigorous review of new renal denervation trials
NATIONAL HARBOR, MD. – Just a month before results from the first of several new pivotal trials with a renal denervation device are to be presented, a Food and Drug Administration medical officer speaking at CRT 2020 sponsored by MedStar Heart & Vascular Institute explained which data will most attract the scrutiny of regulators.
“The FDA is very interested in these devices. We recognize that there is a clinical need, but a reasonable benefit-to-risk relationship has to be established,” said Meir Shinnar, MD, PhD, who works in the division of cardiac devices in the FDA’s Office of Device Evaluation.
The field of renal denervation is expected to heat up again if the results of the SPYRAL HTN OFF MED pivotal trial, planned as a late-breaking presentation at the annual meeting of the American College of Cardiology in March 2020, are positive. However, long-term safety will remain a concern, and positive results will not diminish the rigor with which the relative safety and efficacy of other devices in late stages of clinical testing are evaluated.
“The safety profile is unique to the device design and the procedural technique,” Dr. Shinnar said. For example, vascular injury from the energy employed for denervation, whether radiofrequency or another modality, is an important theoretical risk. A minor initial injury might have no immediate consequences but pose major risks if it leads to altered kidney function over time.
“Most of the follow-up data we have now [with renal denervation devices] is about 1-3 years, but I think long-term safety requires a minimum of 5 years of safety data,” Dr. Shinnar said. “We do not expect all that data to be available at the time of approval, but postmarketing studies will be needed.”
Almost 6 years after the SYMPLICITY HTN-3 trial failed to show a significant reduction in blood pressure among patients with resistant hypertension treated with renal denervation rather than a sham procedure (N Engl J Med 2014;370:1393-401), this treatment is again considered promising. The surprising SYMPLICITY HTN-3 result led to several revisions in technique based on the suspicion that denervation was inadequate.
However, the basic principles remain unchanged. For renal denervation, SPYRAL HTN OFF MED, like the SYMPLICITY HTN 3 study, is employing the Symplicity (Medtronic) device, which has been approved in 50 countries but not in the United States, Canada, or Japan.
SPYRAL HTN OFF MED is designed to provide a very straightforward test of efficacy. Unlike SYMPLICITY HTN-3, which permitted patients to remain on their antihypertensive medications, patients in SPYRAL HTN OFF MED will be tested in the absence of drug therapy (a trial with adjunctive antihypertensive drugs, SPYRAL HTN ON MED, is ongoing). This is a design feature that is relevant to regulatory evaluation.
Although not speaking about the SPYRAL HTN OFF MED trial specifically, Dr. Shinnar noted that “the bar is considered to be higher for a first-line indication than when a device is used as an adjunctive to drug therapy.”
Whether used with or without medications, devices are not likely to receive approval without showing a durable benefit. Dr. Shinnar, citing the surgical studies in which blood pressure control was lost 1-2 years after denervation, said 12 months is now considered a “preferred” length of follow-up to confirm efficacy.
If renal denervation moves forward as a result of the new wave of phase 3 trials, there will still be many unanswered questions, according to Dr. Shinnar, who noted that the FDA convened an advisory committee in December 2018 to gather expert opinion about meaningful safety as well as efficacy endpoints for this modality. One will be determining which populations, defined by age, gender, or phenotype, most benefit.
It also remains unclear whether the first approval will create a standard to which subsequent devices should be compared, according to Dr. Shinnar. Although the FDA recognizes blood pressure reductions as an acceptable endpoint, he believes that documentation of the impact on clinical events will be sought in postmarketing analyses.
“All of the denervation modalities involve class 3 devices that require significant data,” Dr. Shinnar cautioned.
Even if the SPYRAL HTN OFF MED trial is positive on the basis of efficacy, it does not guarantee regulatory approval. Dr. Shinnar described a multifaceted approach to defining an acceptable risk-to-benefit ratio from approved devices, and warned that several points regarding the evaluation of renal denervation devices by the FDA are still being debated internally.
Dr. Shinnar reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Just a month before results from the first of several new pivotal trials with a renal denervation device are to be presented, a Food and Drug Administration medical officer speaking at CRT 2020 sponsored by MedStar Heart & Vascular Institute explained which data will most attract the scrutiny of regulators.
“The FDA is very interested in these devices. We recognize that there is a clinical need, but a reasonable benefit-to-risk relationship has to be established,” said Meir Shinnar, MD, PhD, who works in the division of cardiac devices in the FDA’s Office of Device Evaluation.
The field of renal denervation is expected to heat up again if the results of the SPYRAL HTN OFF MED pivotal trial, planned as a late-breaking presentation at the annual meeting of the American College of Cardiology in March 2020, are positive. However, long-term safety will remain a concern, and positive results will not diminish the rigor with which the relative safety and efficacy of other devices in late stages of clinical testing are evaluated.
“The safety profile is unique to the device design and the procedural technique,” Dr. Shinnar said. For example, vascular injury from the energy employed for denervation, whether radiofrequency or another modality, is an important theoretical risk. A minor initial injury might have no immediate consequences but pose major risks if it leads to altered kidney function over time.
“Most of the follow-up data we have now [with renal denervation devices] is about 1-3 years, but I think long-term safety requires a minimum of 5 years of safety data,” Dr. Shinnar said. “We do not expect all that data to be available at the time of approval, but postmarketing studies will be needed.”
Almost 6 years after the SYMPLICITY HTN-3 trial failed to show a significant reduction in blood pressure among patients with resistant hypertension treated with renal denervation rather than a sham procedure (N Engl J Med 2014;370:1393-401), this treatment is again considered promising. The surprising SYMPLICITY HTN-3 result led to several revisions in technique based on the suspicion that denervation was inadequate.
However, the basic principles remain unchanged. For renal denervation, SPYRAL HTN OFF MED, like the SYMPLICITY HTN 3 study, is employing the Symplicity (Medtronic) device, which has been approved in 50 countries but not in the United States, Canada, or Japan.
SPYRAL HTN OFF MED is designed to provide a very straightforward test of efficacy. Unlike SYMPLICITY HTN-3, which permitted patients to remain on their antihypertensive medications, patients in SPYRAL HTN OFF MED will be tested in the absence of drug therapy (a trial with adjunctive antihypertensive drugs, SPYRAL HTN ON MED, is ongoing). This is a design feature that is relevant to regulatory evaluation.
Although not speaking about the SPYRAL HTN OFF MED trial specifically, Dr. Shinnar noted that “the bar is considered to be higher for a first-line indication than when a device is used as an adjunctive to drug therapy.”
Whether used with or without medications, devices are not likely to receive approval without showing a durable benefit. Dr. Shinnar, citing the surgical studies in which blood pressure control was lost 1-2 years after denervation, said 12 months is now considered a “preferred” length of follow-up to confirm efficacy.
If renal denervation moves forward as a result of the new wave of phase 3 trials, there will still be many unanswered questions, according to Dr. Shinnar, who noted that the FDA convened an advisory committee in December 2018 to gather expert opinion about meaningful safety as well as efficacy endpoints for this modality. One will be determining which populations, defined by age, gender, or phenotype, most benefit.
It also remains unclear whether the first approval will create a standard to which subsequent devices should be compared, according to Dr. Shinnar. Although the FDA recognizes blood pressure reductions as an acceptable endpoint, he believes that documentation of the impact on clinical events will be sought in postmarketing analyses.
“All of the denervation modalities involve class 3 devices that require significant data,” Dr. Shinnar cautioned.
Even if the SPYRAL HTN OFF MED trial is positive on the basis of efficacy, it does not guarantee regulatory approval. Dr. Shinnar described a multifaceted approach to defining an acceptable risk-to-benefit ratio from approved devices, and warned that several points regarding the evaluation of renal denervation devices by the FDA are still being debated internally.
Dr. Shinnar reported no potential financial conflicts of interest.
NATIONAL HARBOR, MD. – Just a month before results from the first of several new pivotal trials with a renal denervation device are to be presented, a Food and Drug Administration medical officer speaking at CRT 2020 sponsored by MedStar Heart & Vascular Institute explained which data will most attract the scrutiny of regulators.
“The FDA is very interested in these devices. We recognize that there is a clinical need, but a reasonable benefit-to-risk relationship has to be established,” said Meir Shinnar, MD, PhD, who works in the division of cardiac devices in the FDA’s Office of Device Evaluation.
The field of renal denervation is expected to heat up again if the results of the SPYRAL HTN OFF MED pivotal trial, planned as a late-breaking presentation at the annual meeting of the American College of Cardiology in March 2020, are positive. However, long-term safety will remain a concern, and positive results will not diminish the rigor with which the relative safety and efficacy of other devices in late stages of clinical testing are evaluated.
“The safety profile is unique to the device design and the procedural technique,” Dr. Shinnar said. For example, vascular injury from the energy employed for denervation, whether radiofrequency or another modality, is an important theoretical risk. A minor initial injury might have no immediate consequences but pose major risks if it leads to altered kidney function over time.
“Most of the follow-up data we have now [with renal denervation devices] is about 1-3 years, but I think long-term safety requires a minimum of 5 years of safety data,” Dr. Shinnar said. “We do not expect all that data to be available at the time of approval, but postmarketing studies will be needed.”
Almost 6 years after the SYMPLICITY HTN-3 trial failed to show a significant reduction in blood pressure among patients with resistant hypertension treated with renal denervation rather than a sham procedure (N Engl J Med 2014;370:1393-401), this treatment is again considered promising. The surprising SYMPLICITY HTN-3 result led to several revisions in technique based on the suspicion that denervation was inadequate.
However, the basic principles remain unchanged. For renal denervation, SPYRAL HTN OFF MED, like the SYMPLICITY HTN 3 study, is employing the Symplicity (Medtronic) device, which has been approved in 50 countries but not in the United States, Canada, or Japan.
SPYRAL HTN OFF MED is designed to provide a very straightforward test of efficacy. Unlike SYMPLICITY HTN-3, which permitted patients to remain on their antihypertensive medications, patients in SPYRAL HTN OFF MED will be tested in the absence of drug therapy (a trial with adjunctive antihypertensive drugs, SPYRAL HTN ON MED, is ongoing). This is a design feature that is relevant to regulatory evaluation.
Although not speaking about the SPYRAL HTN OFF MED trial specifically, Dr. Shinnar noted that “the bar is considered to be higher for a first-line indication than when a device is used as an adjunctive to drug therapy.”
Whether used with or without medications, devices are not likely to receive approval without showing a durable benefit. Dr. Shinnar, citing the surgical studies in which blood pressure control was lost 1-2 years after denervation, said 12 months is now considered a “preferred” length of follow-up to confirm efficacy.
If renal denervation moves forward as a result of the new wave of phase 3 trials, there will still be many unanswered questions, according to Dr. Shinnar, who noted that the FDA convened an advisory committee in December 2018 to gather expert opinion about meaningful safety as well as efficacy endpoints for this modality. One will be determining which populations, defined by age, gender, or phenotype, most benefit.
It also remains unclear whether the first approval will create a standard to which subsequent devices should be compared, according to Dr. Shinnar. Although the FDA recognizes blood pressure reductions as an acceptable endpoint, he believes that documentation of the impact on clinical events will be sought in postmarketing analyses.
“All of the denervation modalities involve class 3 devices that require significant data,” Dr. Shinnar cautioned.
Even if the SPYRAL HTN OFF MED trial is positive on the basis of efficacy, it does not guarantee regulatory approval. Dr. Shinnar described a multifaceted approach to defining an acceptable risk-to-benefit ratio from approved devices, and warned that several points regarding the evaluation of renal denervation devices by the FDA are still being debated internally.
Dr. Shinnar reported no potential financial conflicts of interest.
EXPERT ANALYSIS FROM CRT 2020
Key to denervation response for hypertension may be in carotid body
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
NATIONAL HARBOR, MD. – Of characteristics that might predict which patients with resistant hypertension will respond to carotid body ablation, the relative activity of the carotid body organ itself might be critical for moving this technology forward, an investigator on a first-in-man study suggests.
The study, first presented in 2018 at the European Society of Cardiology congress, showed carotid body ablation resulted in significant but modest reductions in blood pressure in patients with resistant hypertension.
For many reasons, the carotid body is an attractive target for sustained or indefinite control of resistant hypertension, but median systolic blood pressure reductions following ultrasound ablation were highly variable in the first-in-man study, according to Felix Mahfoud, MD, of Saarland University Hospital in Homburg, Germany, a coinvestigator on the study who discussed the findings at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
Of several strategies being pursued to separate those most likely to gain a major benefit, simply measuring carotid body activity is now emerging as particularly promising.
“Patients with a high degree of carotid body activity had a significantly larger fall in blood pressure versus all other methods of grouping patients,” Dr. Mahfoud reported.
The carotid body is a “grain-size” organ of about 2 mm in size that sits on the carotid bifurcation. It communicates directly with the brain to alter sympathetic activity in response to changing levels of such physiologic variable as oxygen, carbon dioxide, and pH, according to Dr. Mahfoud.
In a 2016 proof-of-principle study conducted in resistant hypertension patients, surgical resection of the carotid body was associated with a median 18–mm Hg reduction in systolic blood pressure (SBP) on 24-hour ambulatory monitoring that was sustained through 24 months (Narkiewicz K et al. JACC Basic Transl Sci. 2016;29:313-24).
Subsequently, ultrasound ablation of the carotid body was by way of a transcatheter approach. Dr. Mahfoud’s unpublished first-in-man study, conducted in 2018 enrolled 38 patients with resistant hypertension. The median reductions from baseline of 7-8 mm Hg at 1, 3, and 6 months were significant (P less than .01), but the benefit was disappointingly modest.
However, the variability was large. Some patients achieved SBP reductions of up to 20 mm Hg at 6 months, prompting additional analyses to understand if the best responders could be identified. When compared to the mean reduction of 7 mm Hg at 6 months, this represented a 13–mm Hg additional reduction. There are now several potential approaches being considered.
In addition to carotid body activity, which is readily measured and has substantial potential to serve as a routine selection criterion, isolated systolic hypertension (ISH) was also found to be a discriminator for response. For those with ISH, which Dr. Mahfoud noted is also characterized as “stiff arteries,” SBP reductions at 6 months were negligible, but in those without ISH, the median reduction from baseline was 11 mm Hg.
Further investigations are now planned to evaluate potential predictors of response, according to Dr. Mahfoud. He believes carotid body ablation might have advantages over alternatives, including other experimental therapies, in at least some patients.
To deliver ultrasound ablation in the first-in-man study, a propriety catheter (Cibiem transvenous system) was advanced through the jugular vein guided with intravascular ultrasound. When the carotid body was reached, two to three ultrasound ablations of 8-12 seconds each were applied. The procedure time was 20-30 minutes.
Initially, an arterial approach to the carotid body was used. However, after a transient ischemic attack early in the series, the approach was switched to the jugular vein. There have been no serious subsequent procedural-related complications since.
Although the median SBP reductions were modest, they were not insignificant in a population selected for severe resistant hypertension. The median SBP at entry was 180 mm Hg in patients taking a median of 4.5 antihypertensive drugs, according to Dr. Mahfoud.
In other words, this approach still retains promise for selected patients if larger studies demonstrate that response can be predicted, and the data continue to support tolerability.
A venous approach to the carotid body with intravascular ultrasound guidance and therapeutic ultrasound appears “to offer a safe and effective treatment option in resistant hypertension,” according to Dr. Mahfoud. “A companion diagnostic test is being developed to determine whether patients are likely to respond to this therapy.”
Dr. Mahfoud reports financial relationships with Medtronic, St. Jude, and ReCor.
This article was updated to clarify the study details and correct misspellings of the presenter's name.
SOURCE: CRT 2020.
REPORTING FROM CRT 2020
Study implicates gut bacteria in PAH
Model finds microbiota highly predictive
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
Model finds microbiota highly predictive
Model finds microbiota highly predictive
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
A unique collection of bacteria in the gut may have a strong association with pulmonary arterial hypertension and could be highly predictive of the disease in undiagnosed patients, according to a study published in the journal Hypertension.
This is the first study to show that people with PAH have a common specific gut microbiota profile, wrote lead study author Mohan Raizada, PhD, distinguished professor in the department of physiology and functional genomics at the University of Florida, Gainesville.
The findings have the potential to change how cardiologists diagnose and treat PAH, he added. “While current PAH treatments focus on the lungs, looking at the lung/gut axis could open the door to new therapies centered in the digestive system,” Dr. Raizada said.
The researchers developed a model that found the specific microbiota profile was 83% accurate in predicting the presence or absence of PAH. If a larger study can validate the findings, the researchers wrote, this could lead to a new test for diagnosing PAH that’s less invasive than cardiac catheterization. It could also lead to new treatments that target the gut microbiome.
Study investigators collected stool samples from 18 PAH patients and 12 people without a history of cardiopulmonary disease. The microbiota DNA from the stool samples were isolated and sequenced. The analysis revealed that PAH patients had reduced richness and evenness of the gut bacteria, known as alpha diversity. They had increased levels of bacteria associated with atherosclerosis, and healthy patients had increased levels of bacteria that produced short-chain fatty acids.
Although recent studies have begun to show potential associations between the gut microbiome and cardiovascular diseases, this research is in its infancy, Mariell Jessup, MD, commented. “Even though the study by Dr. Raizada and colleagues predicted pulmonary arterial hypertension based on an individual’s microbiome with some accuracy, it is an observational study, so it does not prove cause and effect. Many other factors, especially diet, affect the gut microbiome,” added Dr. Jessup, Chief Science and Medical Officer for the American Heart Association.
She stressed that, “In addition, even if studies confirm an association between the gut microbiome and cardiovascular diseases such as PAH, more research is needed to determine if improving gut microbiota could directly impact PAH or other cardiovascular diseases. The findings of this study will not impact clinical practice.”
Dr. Raizada and his coinvestigators offered two possible mechanisms through which the gut microbiome influences pulmonary physiology. One is that lower levels of bacteria that produce the short-chain fatty acid butyrate, such as Coprococcus, Butyrivibrio, Lachnospiraceae, and Eubacterium, along with Clostridia in the gut of PAH patients, may increase gut permeability. Reduced butyrate weakens gut barrier function and can induce inflammation and leakage. This can allow microbial metabolites to enter the circulatory system, disrupting metabolism and immunity and affecting pulmonary vessels.
The second potential mechanism is that increased Collinsella in the PAH cohort may be the culprit that increases gut permeability, resulting in the ensuing gut barrier dysfunction and inflammation. The study noted Collinsella contributed most of the increased genes for the biosynthesis on the amino acid proline in these patients, and that a previous study implicated Collinsella and its parent, Cariobacteriales, in trimethylamine/trimethylamine N-oxide production (TMA/TMAO) in atherosclerosis (Cell. 2015;163[7]:1585-95). The non-PAH patients had higher levels of bacteria that had a low correlation with TMA/TMAO.
“We were very surprised to see such an association within a small group of study subjects,” wrote Dr. Raizada and associates. “It usually requires hundreds of patients to achieve such significance.”
More research is needed to determine if the specific microbiota associated with PAH causes the disease or is a result of it, they concluded.
The study was funded by grants from the National Institutes of Health, the NIH National Center for Research Resources, and the U.S. Department of Defense. Dr. Raizada and coauthors reported no relevant financial relationships.
SOURCE: Raizada MK et al. Hypertension. 2020. doi: 10.1161/HYPERTENSIONAHA.119.14294.
FROM HYPERTENSION
Exercise PH poised for comeback as new definition takes hold
Patients with a pulmonary artery pressure/cardiac output slope greater than 3 mm Hg/L/min on cardiopulmonary exercise tests have more than double the risk of cardiovascular hospitalization and all-cause mortality, according to a prospective study of 714 subjects with exertional dyspnea but preserved ejection fractions.
The findings “suggest that across a wide range of individuals with chronic dyspnea, exercise can unmask abnormal pulmonary vascular responses that in turn bear significant clinical implications. These findings, coupled with a growing body of work ... suggest that reintroduction of an exercise based definition of [pulmonary hypertension (PH)] in PH guidelines” – using the pulmonary artery pressure/cardiac output slope – “merits consideration,” wrote Jennifer Ho, MD, a heart failure and transplantation cardiologist at Massachusetts General Hospital, Boston, and colleagues (J Am Coll Cardiol. 2020 Jan 7;75[1]:17-26. doi: 10.1016/j.jacc.2019.10.048).
A new definition takes hold
The slope captures the steepness of pulmonary artery pressure increase as cardiac output goes up, giving a measure of overall pulmonary resistance. A value above 3 mm Hg/L/min means that pulmonary artery pressure (PAP) is too high for a given cardiac output (CO). The slope “is preferable to using a single absolute cut point value for exercise PAP” to define exercise pulmonary hypertension.“ Indeed, we confirm that in the absence of elevated PAP/CO, an absolute exercise PAP [above] 30 mm Hg” – the definition of exercise-induced pulmonary hypertension in years past – “does not portend worse outcomes,” Dr. Ho and her team noted.
In an accompanying editorial titled, “Exercise Pulmonary Hypertension Is Back,” Marius Hoeper, MD, a senior physician in the department of respiratory medicine at Hannover (Germany) Medical School, explained that the findings likely signal the revival of exercise pulmonary hypertension as a useful clinical concept (J Am Coll Cardiol. 2020 Jan 7;75[1]:27-8. doi: 10.1016/j.jacc.2019.11.010).
The standalone 30 mm Hg cut point was largely abandoned about a decade ago when it was realized that pressures above that mark were “not necessarily abnormal in certain subjects, for instance in athletes or elderly individuals,” he said.
But it’s become clear in recent years, and now confirmed by Dr. Ho and her team, that what matters is not the stand-alone measurement, but it’s relationship to cardiac output. “There is now sufficient evidence to define exercise PH by an abnormal [mean]PAP/CO slope [above] 3 mm Hg/L/min,” Dr. Hoeper said.
Abnormal slopes in over 40%
Each subject in the Massachusetts General study had an average of 10 paired PAP and CO measurements taken by invasive hemodynamic monitoring, including pulmonary artery catheterization via the internal jugular vein, while they road a stationary bicycle. The measurements were used to calculate the PAP/CO slope. A slope greater than 3 mm Hg/L/min was defined as abnormal based on previous research.
Results of the one-time assessment were correlated with the study’s primary outcome – cardiovascular hospitalization or all-cause death – over a mean follow up of 3.7 years. Subjects were 57 years old, on average, and 59% were women; just 2% had a previous diagnosis of pulmonary hypertension. Overall, 41% of the subjects had abnormal PAP/CO slopes, 26% had abnormal slopes without resting pulmonary hypertension, and 208 subjects (29%) met the primary outcome.
After adjustments for age, sex, and cardiopulmonary comorbidities, abnormal slopes more than doubled the risk of the primary outcome (hazard ratio [HR] 2.03; 95% confidence interval [CI]: 1.48-2.78; P less than .001). The risk remained elevated even in the absence of resting pulmonary hypertension (HR 1.75, 95% CI 1.21-2.54, P = .003), and in people with only mildly elevated resting PAPs of 21-29 mm Hg.
Older people were more likely to have abnormally elevated slopes, as well as were those with cardiopulmonary comorbidities, lower exercise tolerance, lower peak oxygen uptake, and more severely impaired right ventricular function. Diabetes, prior heart failure, chronic obstructive pulmonary disease, and interstitial lung disease were more prevalent in the elevated slope group, and their median N-terminal pro–B type natriuretic peptide level was 154 pg/mL, versus 52 pg/mL among people with normal slopes.
A simpler test is needed
In his editorial, Dr. Hoeper noted that diagnosing exercise PH by elevated slope “will occasionally help physicians and patients to better understand exertional dyspnea and to detect early pulmonary vascular disease in patients at risk,” but for the most part, the new definition “will have little immediate [effect] on clinical practice, as evidence-based treatments for this condition are not yet available.”
Even so, “having a globally accepted gold standard” for exercise PH based on the PAP/CO slope might well spur development of “simpler, noninvasive” ways to measure it so it can be used outside of specialty settings.
Dr. Ho and her team agreed. “These findings should prompt additional work using less invasive measurement modalities such as exercise echocardiography to evaluate” exercise PAP/CO slopes, they said.
The work was funded by the National Institutes of Health, Gilead Sciences, the American Heart Association, and the Massachusetts General Hospital Heart Failure Research Innovation Fund. The investigators had no relevant disclosures. Dr. Hoeper reported lecture and consultation fees from Actelion, Bayer, Merck Sharp and Dohme, and Pfizer.
SOURCE: Ho JE et al., J Am Coll Cardiol. 2020 Jan 7;75(1):17-26. doi: 10.1016/j.jacc.2019.10.048.
Patients with a pulmonary artery pressure/cardiac output slope greater than 3 mm Hg/L/min on cardiopulmonary exercise tests have more than double the risk of cardiovascular hospitalization and all-cause mortality, according to a prospective study of 714 subjects with exertional dyspnea but preserved ejection fractions.
The findings “suggest that across a wide range of individuals with chronic dyspnea, exercise can unmask abnormal pulmonary vascular responses that in turn bear significant clinical implications. These findings, coupled with a growing body of work ... suggest that reintroduction of an exercise based definition of [pulmonary hypertension (PH)] in PH guidelines” – using the pulmonary artery pressure/cardiac output slope – “merits consideration,” wrote Jennifer Ho, MD, a heart failure and transplantation cardiologist at Massachusetts General Hospital, Boston, and colleagues (J Am Coll Cardiol. 2020 Jan 7;75[1]:17-26. doi: 10.1016/j.jacc.2019.10.048).
A new definition takes hold
The slope captures the steepness of pulmonary artery pressure increase as cardiac output goes up, giving a measure of overall pulmonary resistance. A value above 3 mm Hg/L/min means that pulmonary artery pressure (PAP) is too high for a given cardiac output (CO). The slope “is preferable to using a single absolute cut point value for exercise PAP” to define exercise pulmonary hypertension.“ Indeed, we confirm that in the absence of elevated PAP/CO, an absolute exercise PAP [above] 30 mm Hg” – the definition of exercise-induced pulmonary hypertension in years past – “does not portend worse outcomes,” Dr. Ho and her team noted.
In an accompanying editorial titled, “Exercise Pulmonary Hypertension Is Back,” Marius Hoeper, MD, a senior physician in the department of respiratory medicine at Hannover (Germany) Medical School, explained that the findings likely signal the revival of exercise pulmonary hypertension as a useful clinical concept (J Am Coll Cardiol. 2020 Jan 7;75[1]:27-8. doi: 10.1016/j.jacc.2019.11.010).
The standalone 30 mm Hg cut point was largely abandoned about a decade ago when it was realized that pressures above that mark were “not necessarily abnormal in certain subjects, for instance in athletes or elderly individuals,” he said.
But it’s become clear in recent years, and now confirmed by Dr. Ho and her team, that what matters is not the stand-alone measurement, but it’s relationship to cardiac output. “There is now sufficient evidence to define exercise PH by an abnormal [mean]PAP/CO slope [above] 3 mm Hg/L/min,” Dr. Hoeper said.
Abnormal slopes in over 40%
Each subject in the Massachusetts General study had an average of 10 paired PAP and CO measurements taken by invasive hemodynamic monitoring, including pulmonary artery catheterization via the internal jugular vein, while they road a stationary bicycle. The measurements were used to calculate the PAP/CO slope. A slope greater than 3 mm Hg/L/min was defined as abnormal based on previous research.
Results of the one-time assessment were correlated with the study’s primary outcome – cardiovascular hospitalization or all-cause death – over a mean follow up of 3.7 years. Subjects were 57 years old, on average, and 59% were women; just 2% had a previous diagnosis of pulmonary hypertension. Overall, 41% of the subjects had abnormal PAP/CO slopes, 26% had abnormal slopes without resting pulmonary hypertension, and 208 subjects (29%) met the primary outcome.
After adjustments for age, sex, and cardiopulmonary comorbidities, abnormal slopes more than doubled the risk of the primary outcome (hazard ratio [HR] 2.03; 95% confidence interval [CI]: 1.48-2.78; P less than .001). The risk remained elevated even in the absence of resting pulmonary hypertension (HR 1.75, 95% CI 1.21-2.54, P = .003), and in people with only mildly elevated resting PAPs of 21-29 mm Hg.
Older people were more likely to have abnormally elevated slopes, as well as were those with cardiopulmonary comorbidities, lower exercise tolerance, lower peak oxygen uptake, and more severely impaired right ventricular function. Diabetes, prior heart failure, chronic obstructive pulmonary disease, and interstitial lung disease were more prevalent in the elevated slope group, and their median N-terminal pro–B type natriuretic peptide level was 154 pg/mL, versus 52 pg/mL among people with normal slopes.
A simpler test is needed
In his editorial, Dr. Hoeper noted that diagnosing exercise PH by elevated slope “will occasionally help physicians and patients to better understand exertional dyspnea and to detect early pulmonary vascular disease in patients at risk,” but for the most part, the new definition “will have little immediate [effect] on clinical practice, as evidence-based treatments for this condition are not yet available.”
Even so, “having a globally accepted gold standard” for exercise PH based on the PAP/CO slope might well spur development of “simpler, noninvasive” ways to measure it so it can be used outside of specialty settings.
Dr. Ho and her team agreed. “These findings should prompt additional work using less invasive measurement modalities such as exercise echocardiography to evaluate” exercise PAP/CO slopes, they said.
The work was funded by the National Institutes of Health, Gilead Sciences, the American Heart Association, and the Massachusetts General Hospital Heart Failure Research Innovation Fund. The investigators had no relevant disclosures. Dr. Hoeper reported lecture and consultation fees from Actelion, Bayer, Merck Sharp and Dohme, and Pfizer.
SOURCE: Ho JE et al., J Am Coll Cardiol. 2020 Jan 7;75(1):17-26. doi: 10.1016/j.jacc.2019.10.048.
Patients with a pulmonary artery pressure/cardiac output slope greater than 3 mm Hg/L/min on cardiopulmonary exercise tests have more than double the risk of cardiovascular hospitalization and all-cause mortality, according to a prospective study of 714 subjects with exertional dyspnea but preserved ejection fractions.
The findings “suggest that across a wide range of individuals with chronic dyspnea, exercise can unmask abnormal pulmonary vascular responses that in turn bear significant clinical implications. These findings, coupled with a growing body of work ... suggest that reintroduction of an exercise based definition of [pulmonary hypertension (PH)] in PH guidelines” – using the pulmonary artery pressure/cardiac output slope – “merits consideration,” wrote Jennifer Ho, MD, a heart failure and transplantation cardiologist at Massachusetts General Hospital, Boston, and colleagues (J Am Coll Cardiol. 2020 Jan 7;75[1]:17-26. doi: 10.1016/j.jacc.2019.10.048).
A new definition takes hold
The slope captures the steepness of pulmonary artery pressure increase as cardiac output goes up, giving a measure of overall pulmonary resistance. A value above 3 mm Hg/L/min means that pulmonary artery pressure (PAP) is too high for a given cardiac output (CO). The slope “is preferable to using a single absolute cut point value for exercise PAP” to define exercise pulmonary hypertension.“ Indeed, we confirm that in the absence of elevated PAP/CO, an absolute exercise PAP [above] 30 mm Hg” – the definition of exercise-induced pulmonary hypertension in years past – “does not portend worse outcomes,” Dr. Ho and her team noted.
In an accompanying editorial titled, “Exercise Pulmonary Hypertension Is Back,” Marius Hoeper, MD, a senior physician in the department of respiratory medicine at Hannover (Germany) Medical School, explained that the findings likely signal the revival of exercise pulmonary hypertension as a useful clinical concept (J Am Coll Cardiol. 2020 Jan 7;75[1]:27-8. doi: 10.1016/j.jacc.2019.11.010).
The standalone 30 mm Hg cut point was largely abandoned about a decade ago when it was realized that pressures above that mark were “not necessarily abnormal in certain subjects, for instance in athletes or elderly individuals,” he said.
But it’s become clear in recent years, and now confirmed by Dr. Ho and her team, that what matters is not the stand-alone measurement, but it’s relationship to cardiac output. “There is now sufficient evidence to define exercise PH by an abnormal [mean]PAP/CO slope [above] 3 mm Hg/L/min,” Dr. Hoeper said.
Abnormal slopes in over 40%
Each subject in the Massachusetts General study had an average of 10 paired PAP and CO measurements taken by invasive hemodynamic monitoring, including pulmonary artery catheterization via the internal jugular vein, while they road a stationary bicycle. The measurements were used to calculate the PAP/CO slope. A slope greater than 3 mm Hg/L/min was defined as abnormal based on previous research.
Results of the one-time assessment were correlated with the study’s primary outcome – cardiovascular hospitalization or all-cause death – over a mean follow up of 3.7 years. Subjects were 57 years old, on average, and 59% were women; just 2% had a previous diagnosis of pulmonary hypertension. Overall, 41% of the subjects had abnormal PAP/CO slopes, 26% had abnormal slopes without resting pulmonary hypertension, and 208 subjects (29%) met the primary outcome.
After adjustments for age, sex, and cardiopulmonary comorbidities, abnormal slopes more than doubled the risk of the primary outcome (hazard ratio [HR] 2.03; 95% confidence interval [CI]: 1.48-2.78; P less than .001). The risk remained elevated even in the absence of resting pulmonary hypertension (HR 1.75, 95% CI 1.21-2.54, P = .003), and in people with only mildly elevated resting PAPs of 21-29 mm Hg.
Older people were more likely to have abnormally elevated slopes, as well as were those with cardiopulmonary comorbidities, lower exercise tolerance, lower peak oxygen uptake, and more severely impaired right ventricular function. Diabetes, prior heart failure, chronic obstructive pulmonary disease, and interstitial lung disease were more prevalent in the elevated slope group, and their median N-terminal pro–B type natriuretic peptide level was 154 pg/mL, versus 52 pg/mL among people with normal slopes.
A simpler test is needed
In his editorial, Dr. Hoeper noted that diagnosing exercise PH by elevated slope “will occasionally help physicians and patients to better understand exertional dyspnea and to detect early pulmonary vascular disease in patients at risk,” but for the most part, the new definition “will have little immediate [effect] on clinical practice, as evidence-based treatments for this condition are not yet available.”
Even so, “having a globally accepted gold standard” for exercise PH based on the PAP/CO slope might well spur development of “simpler, noninvasive” ways to measure it so it can be used outside of specialty settings.
Dr. Ho and her team agreed. “These findings should prompt additional work using less invasive measurement modalities such as exercise echocardiography to evaluate” exercise PAP/CO slopes, they said.
The work was funded by the National Institutes of Health, Gilead Sciences, the American Heart Association, and the Massachusetts General Hospital Heart Failure Research Innovation Fund. The investigators had no relevant disclosures. Dr. Hoeper reported lecture and consultation fees from Actelion, Bayer, Merck Sharp and Dohme, and Pfizer.
SOURCE: Ho JE et al., J Am Coll Cardiol. 2020 Jan 7;75(1):17-26. doi: 10.1016/j.jacc.2019.10.048.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Tools for preventing heart failure
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
SNOWMASS, COLO. – If ever there was a major chronic disease that’s teed up and ready to be stamped into submission through diligent application of preventive medicine, it’s the epidemic of heart failure.
“The best way to treat heart failure is to prevent it in the first place. There will be more than 1 million new cases of heart failure this year, and the vast majority of them could have been prevented,” Gregg C. Fonarow, MD, asserted at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
Using firmly evidence-based, guideline-directed therapies, it’s often possible to prevent patients at high risk for developing heart failure (HF) from actually doing so. Or, in the terminology of the ACC/American Heart Association heart failure guidelines coauthored by Dr. Fonarow, the goal is to keep patients who are stage A – that is, pre-HF but at high risk because of hypertension, coronary artery disease, diabetes, family history of cardiomyopathy, or other reasons – from progressing to stage B, marked by asymptomatic left ventricular dysfunction, a prior MI, or asymptomatic valvular disease; and blocking those who are stage B from then moving on to stage C, the classic symptomatic form of HF; and thence to end-stage stage D disease.
Heart failure is an enormous public health problem, and one of the most expensive of all diseases. The prognostic impact of newly diagnosed HF is profound, with 10-15 years of life lost, compared with the general population. Even today, roughly one in five newly diagnosed patients won’t survive for a year, and the 5-year mortality is about 50%, said Dr. Fonarow, who is professor of cardiovascular medicine and chief of the division of cardiology at the University of California, Los Angeles, and director of the Ahmanson-UCLA Cardiomyopathy Center, also in Los Angeles.
Symptomatic stage C is “the tip of the iceberg,” the cardiologist stressed. Vastly more patients are in stages A and B. In order to keep them from progressing to stage C, it’s first necessary to identify them. That’s why the 2013 guidelines give a class IC recommendation for periodic evaluation for signs and symptoms of HF in patients who are at high risk, and for a noninvasive assessment of left ventricular ejection fraction in those with a strong family history of cardiomyopathy or who are on cardiotoxic drugs (J Am Coll Cardiol. 2013 Oct 15;62[16]:e147-239).
The two biggest risk factors for the development of symptomatic stage C HF are hypertension and atherosclerotic cardiovascular disease. Close to 80% of patients presenting with heart failure have prevalent hypertension, and a history of ischemic heart disease is nearly as common.
Other major modifiable risk factors are diabetes, overweight and obesity, metabolic syndrome, dyslipidemia, smoking, valvular heart disease, and chronic kidney disease.
Hypertension
Most patients with high blood pressure believe they’re on antihypertensive medication to prevent MI and stroke, but in reality the largest benefit is what Dr. Fonarow termed the “phenomenal” reduction in the risk of developing HF, which amounted to a 52% relative risk reduction in one meta-analysis of older randomized trials. In the contemporary era, the landmark SPRINT trial of close to 10,000 randomized hypertensive patients showed that more-intensive blood pressure lowering to a target systolic BP of less than 120 mm Hg resulted in a 38% reduction in the risk of new-onset HF, compared with standard treatment to a target of less than 140 mm Hg. That’s why the 2017 focused update of the HF guidelines gives a strong class IB recommendation for a target blood pressure of less than 130/80 mm Hg in hypertensive patients with stage A HF (J Am Coll Cardiol. 2017 Aug 8;70[6]:776-803).
Atherosclerotic cardiovascular disease
Within 6 years after diagnosis of an MI, 22% of men and 46% of women will develop symptomatic heart failure. Intensive statin therapy gets a strong recommendation post MI in the guidelines, not only because in a meta-analysis of four major randomized trials it resulted in a further 64% reduction in the risk of coronary death or recurrent MI, compared with moderate statin therapy, but also because of the 27% relative risk reduction in new-onset HF. ACE inhibitors get a class IA recommendation for prevention of symptomatic HF in patients who are stage A with a history of atherosclerotic disease, diabetes, or hypertension. Angiotensin receptor blockers get a class IC recommendation.
Diabetes
Diabetes markedly increases the risk of developing HF: by two to four times overall and by four to eight times in younger diabetes patients. The two chronic diseases are highly comorbid, with roughly 45% of patients with HF also having diabetes. Moreover, diabetes in HF patients is associated with a substantially worse prognosis, even when standard HF therapies are applied.
Choices regarding glycemic management can markedly affect HF risk and outcomes. Randomized trials show that the peroxisome proliferator-activated receptor agonists double the risk of HF. The glucagonlike peptide–1 receptor agonists are absolutely neutral with regard to HF outcomes. Similarly, the dipeptidyl peptidase–4 inhibitors have no impact on the risks of major adverse cardiovascular events or HF. Intensive glycemic control has no impact on the risk of new-onset HF. Insulin therapy, too, is neutral on this score.
“Depressingly, even lifestyle modification with weight loss, once you have type 2 diabetes, does not lower the risk,” Dr. Fonarow continued.
In contrast, the sodium-glucose transporter 2 (SGLT2) inhibitors have impressive cardiovascular and renal protective benefits in patients with type 2 diabetes, as demonstrated in a meta-analysis of more than 34,000 participants in the randomized trials of empagliflozin (Jardiance) in EMPA-REG OUTCOME, canagliflozin (Invokana) in CANVAS/CANVAS-R, and dapagliflozin (Farxiga) in DECLARE-TIMI 58. The SGLT2 inhibitors collectively reduced the risk of HF hospitalization by 21% in participants with no baseline history of the disease and by 29% in those with a history of HF. Moreover, the risk of progression of renal disease was reduced by 45% (Lancet. 2019 Jan 5;393[10166]:31-9).
More recently, the landmark DAPA-HF trial established SGLT2 inhibitor therapy as part of standard-of-care, guideline-directed medical therapy for patients with HF with reduced ejection fraction regardless of whether they have comorbid type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008).
These are remarkable medications, generally very well tolerated, and it’s critical that cardiologists get on board in prescribing them, Dr. Fonarow emphasized. He alerted his colleagues to what he called an “incredibly helpful” review article that provides practical guidance for cardiologists in how to start using the SGLT2 inhibitors (JACC Heart Fail. 2019 Feb;7[2]:169-72).
“It’s pretty straightforward,” according to Dr. Fonarow. “If you’re comfortable enough in using ACE inhibitors, angiotensin receptor blockers, and beta-blockers, I think you’ll find these medications fit similarly when you actually get experience in utilizing them.”
He reported serving as a consultant to 10 pharmaceutical or medical device companies.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020