User login
Fidaxomicin favored over vancomycin in real-world C. diff study
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
AT DDW 2022
Vibrating pill counters constipation
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
A swallowable, vibrating capsule improved symptoms among patients with chronic idiopathic constipation in a phase 3 multicenter, randomized, controlled trial. The method represents a mechanical approach to the treatment of constipation.
The swallowable pill acts by vibrating during passage through the gut, where it is thought to augment colonic biorhythm and peristalsis. Traditional treatments for constipation generally increase motility or secretion.
“That’s how we have been managing constipation since time immemorial. Now we have come up with this novel approach, where there’s a pill that is designed to increase local oscillations, and probably induce local contractions of the colon to mimic what happens normally,” said Satish Rao, MD, PhD, who presented the results of the trial at the annual Digestive Disease Week® (DDW).
“We’re now seeing that local stimulation works, and the other neat thing seems to be a lack of side effects, which is really a huge plus. I think it will benefit people with both occasional or chronic constipation,” said Dr. Rao, professor of medicine at Medical College of Georgia, Augusta.
The capsules activate for two stimulation cycles, each lasting for about 2 hours. The cycle includes 3 seconds of vibration followed by 16 seconds of rest.
The researchers conducted a study with two active arms and a placebo. It included 312 patients age 22 or older who had an average of between 1 and 2.5 spontaneous bowel movements (SBM) per week.
Treatment lasted for 8 weeks, with patients ingesting a capsule between 9 and 10 p.m. In one treatment group, the device was activated at 6 a.m., and in the other group at about 2 p.m.
The placebo and treatment groups had similar baseline characteristics, except for a longer duration of constipation in the treatment groups (17.9 versus 14.5 years; P = .0253). In an intention-to-treat analysis, the treatment groups were more likely to achieve an increase of one complete SBM per week (39.26% versus 22.15%; P < .0001) and an increase of two complete SBMs per week (22.7% versus 11.41%; P < .0006).
The capsules also improved straining score, stool consistency, and quality of life. There was no significant difference between treatment and placebo groups with respect to bloating or rescue medication use.
The product had few adverse effects. The most common was a vibrating sensation or discomfort (11.0%, versus none in the placebo group).
Dr. Rao expects that the treatment could be widely applicable since many constipation patients don’t gain sufficient benefit from existing treatments, or find side effects intolerable.
Another benefit is that the therapy’s mimicry of natural cycles appears to grant patients more control of bowel movements. Laxatives and other pharmaceutical interventions may prompt the patient to go to the bathroom within an hour or two, but patients in the trial reported bowel movements at predictable times.
However, he noted that the pill is nondissolvable, which would make it contraindicated for patients who have had previous gut surgeries or narrowing of the gut. He noted that the sponsoring company, Vibrant Gastro, expects to obtain Food and Drug Administration approval by the end of 2022.
The results of the study were well received. “I think it’s an exciting new approach for managing patients with chronic constipation. It was a large sample size, and the treatment seems to be well tolerated. It may offer a promising option for patients who have not responded to many other medications,” said Adil E. Bharucha, MD, who comoderated the session where the research was presented.
However, he pointed out that the presentation did not indicate how many of the patients had previously tried other therapies. “We’d like to see the full paper, which will provide a better understanding of the role of this treatment in practice down the road,” said Dr. Bharucha, professor of medicine in the division of gastroenterology and hepatology and director of the office of clinical trials at Mayo Clinic, Rochester, Minn.
The capsule may not work for everyone, said Dr. Bharucha. He suspects that many refractory patients have an issue with pelvic floor muscles, which may restrict stool evacuation. “You wouldn’t expect those people to respond optimally to a laxative and I suspect perhaps not to a capsule, either. I think defecatory disorders are substantially underdiagnosed in patients who don’t respond to laxatives,” said Dr. Bharucha.
Asked why the capsule might benefit patients who don’t improve with laxatives, Dr. Bharucha responded: “I think we need more studies to understand how the capsule works.”
Dr. Rao consults for Vibrant Gastro. Dr. Bharucha has no relevant financial disclosures.
FROM DDW 2022
Videos may not increase vaccinations in IBD
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
SAN DIEGO – Video and text messaging may not increase the proportion of people with inflammatory bowel disease (IBD) who get influenza vaccinations.
Although patients who received the messages expressed greater intention to get the vaccinations in a trial of the two methods, they didn’t follow through and get the shots, said Keren Appel, MD, a pediatric gastroenterologist at Children’s Hospital of Orange County in Orange, Calif.
“We found there was no difference in the uptake of the influenza vaccine between the two groups,” she said in an interview. Dr. Appel, who participated in the research while at Cedars-Sinai Medical Center in Los Angeles, presented the finding at the annual Digestive Diseases Week® (DDW) 2022.
People with IBD run an increased risk of complications such as infection, bone fractures, and cancer, said Dr. Appel. Previous research has suggested many people with IBD lack understanding or awareness or are skeptical of immunizations.
A previous trial with text-based email reminders did not result in more immunizations, according to Dr. Appel, so she and her colleagues decided to try promoting health prevention with videos. With feedback from patients, they created a series of animations encouraging patients to get influenza, pneumococcal, and zoster vaccinations and screening for bone health and skin cancer.
They randomly assigned 511 to receive videos and 545 patients to receive texts as a control group. After 6 months, 345 patients remained in the text group and 322 remained in the video group. The two groups had similar demographics, health status, and preventive health behaviors. They were mostly educated White women whose IBD was in remission.
The percentage of those who got flu vaccines increased from 59% (for the 2018-2019 season) to 63% (for the 2019-2020 flu season) in the group that watched the videos. However this change did not quite reach statistical significance (P = .07). The change in the text group, from 55% to 57%, was also not significant (P = .23).
The subjects did express more intention to get flu vaccines. The percentage with this intention increased from 59 to 75 in the video group, and from 55 to 72 in the text group. Both changes were statistically significant (P < .001).
Intentions to receive pneumonia and shingles vaccines, and bone and skin cancer screening, were not statistically different between the groups.
The researchers looked at age, immunosuppression, gender, and education to see if these factors could predict who was most likely to get the flu vaccine, but the only significant predictor was having received a previous flu shot.
Dr. Appel speculated that the videos might have been more effective in a more racially diverse, less educated population, or one where fewer people had previously received vaccinations.
“While we didn’t see a difference in this study, I think it opens up a lot of other questions that we can explore and answer,” she said. “It’s possible that patients may not have a one size fits all on their response. Some may respond better to video. Some may respond to text. Some may need more frequent reminders. Some might need to hear it from their doctor directly.”
Session comoderator Alyse Bedell, PhD, an assistant professor of psychiatry and behavioral neuroscience at the University of Chicago, agreed that a different patient population might have responded differently. “A population that may have lower access to educational resources, or has less educational attainment, or may have fewer people in their communities that are already receiving vaccines – those I think are going to be the populations where we’re going to be more likely to see the effects of an intervention like this,” she said in an interview.
Neither Dr. Appel nor Dr. Bedell reported any relevant financial interests. The study was funded by Pfizer.
AT DDW 2022
Adsorbent offers promise for irritable bowel syndrome diarrhea
SAN DIEGO – An intestinal adsorbent, polymethylsiloxane polyhydrate (PMSPH), may relieve the diarrhea associated with irritable bowel syndrome (IBS), researchers say.
The adsorbent reduced abdominal pain, improved stool consistency, and won praise from patients, said Yan Yiannakou, MBChB, a consultant in gastroenterology at County Durham and Darlington (England) National Health Service Foundation Trust.
“It’s great to have something new for patients to try,” he told MDEdge. “And it’s great that this treatment is so safe, and easy to use.”
Dr. Yiannakou presented the finding at the annual Digestive Diseases Week® (DDW).
Many people with irritable bowel syndrome find the currently available treatments and diets difficult to use or ineffective.
First developed 30 years ago in Eastern Europe, PMSPH is marketed over the counter in 30 European countries under the name Enterosgel as a treatment for diarrhea, said Dr. Yiannakou. It received conformité européenne (CE) mark in 2011.
Since PMSPH is not adsorbed by the body, it has been approved as a medical device rather than as a drug, said Dr. Yiannakou. Although its manufacturer is not yet marketing it in the United States, websites there are offering it as a dietary supplement for "toxin binding" and "cleansing the gut."*
Since the etiology of IBS is poorly understood, it is also not clear exactly how PMSPH improves IBS symptoms, Dr. Yiannakou said. “I think this is binding a whole range of molecules which are either irritant or induce diarrhea through secretion.” Fat, bile salts, immune chemicals, and bacterial breakdown products are possibilities, he said.
PMSPH’s approval in Europe rests largely on trials for other forms of diarrhea; it did not undergo a high-quality randomized, placebo-controlled trial for IBS, Dr. Yiannakou said.
To fill that gap, he and his colleagues recruited 440 people with IBS, aged 16-75 years, from 28 sites in England. They randomly assigned 219 to receive PMSPH and 221 to receive a placebo for 8 weeks. Following this blinded phase, both groups received PMSPH for another 8 weeks (a phase requested by the patients who helped design the trial). The investigators then followed up with a phone call 8 weeks later to those who responded to the treatment.
The subjects recorded their symptoms in an e-diary and completed questionnaires. Because of COVID-19 constraints imposed after the trial began, the researchers collected some of the data through virtual visits and online questionnaires.
On a U.S. Food and Drug Administration–recommended composite score for abdominal pain and stool consistency, 37.4% of the patients receiving PMSPH were defined as responders versus 24.3% of the patients receiving the placebo, a statistically significant difference.
However, that score does not accurately reflect the main concerns of people with IBS diarrhea, said Dr. Yiannakou. More important is how often they have diarrhea, and by that measure the difference between the placebo and treatment groups was larger.
There were also statistically significant differences in favor of the PMSPH group in separate scores for abdominal pain, stool frequency, bloating, and urgency.
Surveyed between week 5 and week 8, 69% of patients taking PMSPH reported that they were getting adequate relief, compared with 30% of those taking the placebo. Among the responders surveyed 8 weeks after the open-label phase ended, 74% said they were still benefiting from the treatment. And 81% said they were still using PMSPH, even though they had to buy it for themselves.
Only a handful of patients experienced any adverse events, and there were no significant differences in the number of these events between those taking the placebo and those taking PMSPH.
“I think we’re going to be eager to learn which patients that have irritable bowel syndrome would benefit from this particular treatment,” said session comoderator Eric Shah, MD, MBA, director of gastrointestinal motility at Dartmouth University in Hanover, N.H., who was not involved in the study. He also wanted to know how PMSPH compares to similar binding agents on the market.
Session comoderator Nikrad Shahnavaz, MD, an associate professor of medicine in the division of digestive diseases, department of medicine, at Emory University, Atlanta, said his patients complain two binding agents now prescribed for IBS in the United States, cholestyramine and colestipol, cause nausea and vomiting. That could be an advantage for PMSPH, he said. “It’s good to add to your tools.”
Dr. Yiannakou, Dr. Shahnavaz, and Dr. Shah reported no relevant financial interests.
*An earlier version of this article misstated PMSPH's mechanism of action. It is not adsorbed by the body. Additionally, the marketing status of PMSPH was misstated; it is not currently on the U.S. market.
SAN DIEGO – An intestinal adsorbent, polymethylsiloxane polyhydrate (PMSPH), may relieve the diarrhea associated with irritable bowel syndrome (IBS), researchers say.
The adsorbent reduced abdominal pain, improved stool consistency, and won praise from patients, said Yan Yiannakou, MBChB, a consultant in gastroenterology at County Durham and Darlington (England) National Health Service Foundation Trust.
“It’s great to have something new for patients to try,” he told MDEdge. “And it’s great that this treatment is so safe, and easy to use.”
Dr. Yiannakou presented the finding at the annual Digestive Diseases Week® (DDW).
Many people with irritable bowel syndrome find the currently available treatments and diets difficult to use or ineffective.
First developed 30 years ago in Eastern Europe, PMSPH is marketed over the counter in 30 European countries under the name Enterosgel as a treatment for diarrhea, said Dr. Yiannakou. It received conformité européenne (CE) mark in 2011.
Since PMSPH is not adsorbed by the body, it has been approved as a medical device rather than as a drug, said Dr. Yiannakou. Although its manufacturer is not yet marketing it in the United States, websites there are offering it as a dietary supplement for "toxin binding" and "cleansing the gut."*
Since the etiology of IBS is poorly understood, it is also not clear exactly how PMSPH improves IBS symptoms, Dr. Yiannakou said. “I think this is binding a whole range of molecules which are either irritant or induce diarrhea through secretion.” Fat, bile salts, immune chemicals, and bacterial breakdown products are possibilities, he said.
PMSPH’s approval in Europe rests largely on trials for other forms of diarrhea; it did not undergo a high-quality randomized, placebo-controlled trial for IBS, Dr. Yiannakou said.
To fill that gap, he and his colleagues recruited 440 people with IBS, aged 16-75 years, from 28 sites in England. They randomly assigned 219 to receive PMSPH and 221 to receive a placebo for 8 weeks. Following this blinded phase, both groups received PMSPH for another 8 weeks (a phase requested by the patients who helped design the trial). The investigators then followed up with a phone call 8 weeks later to those who responded to the treatment.
The subjects recorded their symptoms in an e-diary and completed questionnaires. Because of COVID-19 constraints imposed after the trial began, the researchers collected some of the data through virtual visits and online questionnaires.
On a U.S. Food and Drug Administration–recommended composite score for abdominal pain and stool consistency, 37.4% of the patients receiving PMSPH were defined as responders versus 24.3% of the patients receiving the placebo, a statistically significant difference.
However, that score does not accurately reflect the main concerns of people with IBS diarrhea, said Dr. Yiannakou. More important is how often they have diarrhea, and by that measure the difference between the placebo and treatment groups was larger.
There were also statistically significant differences in favor of the PMSPH group in separate scores for abdominal pain, stool frequency, bloating, and urgency.
Surveyed between week 5 and week 8, 69% of patients taking PMSPH reported that they were getting adequate relief, compared with 30% of those taking the placebo. Among the responders surveyed 8 weeks after the open-label phase ended, 74% said they were still benefiting from the treatment. And 81% said they were still using PMSPH, even though they had to buy it for themselves.
Only a handful of patients experienced any adverse events, and there were no significant differences in the number of these events between those taking the placebo and those taking PMSPH.
“I think we’re going to be eager to learn which patients that have irritable bowel syndrome would benefit from this particular treatment,” said session comoderator Eric Shah, MD, MBA, director of gastrointestinal motility at Dartmouth University in Hanover, N.H., who was not involved in the study. He also wanted to know how PMSPH compares to similar binding agents on the market.
Session comoderator Nikrad Shahnavaz, MD, an associate professor of medicine in the division of digestive diseases, department of medicine, at Emory University, Atlanta, said his patients complain two binding agents now prescribed for IBS in the United States, cholestyramine and colestipol, cause nausea and vomiting. That could be an advantage for PMSPH, he said. “It’s good to add to your tools.”
Dr. Yiannakou, Dr. Shahnavaz, and Dr. Shah reported no relevant financial interests.
*An earlier version of this article misstated PMSPH's mechanism of action. It is not adsorbed by the body. Additionally, the marketing status of PMSPH was misstated; it is not currently on the U.S. market.
SAN DIEGO – An intestinal adsorbent, polymethylsiloxane polyhydrate (PMSPH), may relieve the diarrhea associated with irritable bowel syndrome (IBS), researchers say.
The adsorbent reduced abdominal pain, improved stool consistency, and won praise from patients, said Yan Yiannakou, MBChB, a consultant in gastroenterology at County Durham and Darlington (England) National Health Service Foundation Trust.
“It’s great to have something new for patients to try,” he told MDEdge. “And it’s great that this treatment is so safe, and easy to use.”
Dr. Yiannakou presented the finding at the annual Digestive Diseases Week® (DDW).
Many people with irritable bowel syndrome find the currently available treatments and diets difficult to use or ineffective.
First developed 30 years ago in Eastern Europe, PMSPH is marketed over the counter in 30 European countries under the name Enterosgel as a treatment for diarrhea, said Dr. Yiannakou. It received conformité européenne (CE) mark in 2011.
Since PMSPH is not adsorbed by the body, it has been approved as a medical device rather than as a drug, said Dr. Yiannakou. Although its manufacturer is not yet marketing it in the United States, websites there are offering it as a dietary supplement for "toxin binding" and "cleansing the gut."*
Since the etiology of IBS is poorly understood, it is also not clear exactly how PMSPH improves IBS symptoms, Dr. Yiannakou said. “I think this is binding a whole range of molecules which are either irritant or induce diarrhea through secretion.” Fat, bile salts, immune chemicals, and bacterial breakdown products are possibilities, he said.
PMSPH’s approval in Europe rests largely on trials for other forms of diarrhea; it did not undergo a high-quality randomized, placebo-controlled trial for IBS, Dr. Yiannakou said.
To fill that gap, he and his colleagues recruited 440 people with IBS, aged 16-75 years, from 28 sites in England. They randomly assigned 219 to receive PMSPH and 221 to receive a placebo for 8 weeks. Following this blinded phase, both groups received PMSPH for another 8 weeks (a phase requested by the patients who helped design the trial). The investigators then followed up with a phone call 8 weeks later to those who responded to the treatment.
The subjects recorded their symptoms in an e-diary and completed questionnaires. Because of COVID-19 constraints imposed after the trial began, the researchers collected some of the data through virtual visits and online questionnaires.
On a U.S. Food and Drug Administration–recommended composite score for abdominal pain and stool consistency, 37.4% of the patients receiving PMSPH were defined as responders versus 24.3% of the patients receiving the placebo, a statistically significant difference.
However, that score does not accurately reflect the main concerns of people with IBS diarrhea, said Dr. Yiannakou. More important is how often they have diarrhea, and by that measure the difference between the placebo and treatment groups was larger.
There were also statistically significant differences in favor of the PMSPH group in separate scores for abdominal pain, stool frequency, bloating, and urgency.
Surveyed between week 5 and week 8, 69% of patients taking PMSPH reported that they were getting adequate relief, compared with 30% of those taking the placebo. Among the responders surveyed 8 weeks after the open-label phase ended, 74% said they were still benefiting from the treatment. And 81% said they were still using PMSPH, even though they had to buy it for themselves.
Only a handful of patients experienced any adverse events, and there were no significant differences in the number of these events between those taking the placebo and those taking PMSPH.
“I think we’re going to be eager to learn which patients that have irritable bowel syndrome would benefit from this particular treatment,” said session comoderator Eric Shah, MD, MBA, director of gastrointestinal motility at Dartmouth University in Hanover, N.H., who was not involved in the study. He also wanted to know how PMSPH compares to similar binding agents on the market.
Session comoderator Nikrad Shahnavaz, MD, an associate professor of medicine in the division of digestive diseases, department of medicine, at Emory University, Atlanta, said his patients complain two binding agents now prescribed for IBS in the United States, cholestyramine and colestipol, cause nausea and vomiting. That could be an advantage for PMSPH, he said. “It’s good to add to your tools.”
Dr. Yiannakou, Dr. Shahnavaz, and Dr. Shah reported no relevant financial interests.
*An earlier version of this article misstated PMSPH's mechanism of action. It is not adsorbed by the body. Additionally, the marketing status of PMSPH was misstated; it is not currently on the U.S. market.
AT DDW 2022
More first-degree relatives develop celiac than expected
Children who have first-degree relatives with celiac disease (CD) may be at higher risk of developing CD before age 10 than previously thought, according to results of an analysis of 10-year follow-up data. This analysis also yielded a web-based prediction model to help with screening for CD in children.
Screening is recommended for children who have a first-degree relative with celiac disease (CD) because they have a much higher risk for CD, but it has been unclear when to screen such children and how frequently. The researchers, led by Caroline Meijer, MD, in the department of pediatrics and medical statistics at Leiden (the Netherlands) University Medical Center, wrote in Gastroenterology that a prediction model they developed can help parents and providers with those questions.
The researchers analyzed prospective data from 10 years of follow-up from the PreventCD birth cohort with 944 children genetically predisposed to celiac disease with at least one first-degree relative affected. The children were enrolled at birth between 2007 and 2010 in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain.
“The data of the follow-up of the PreventCD cohort at the mean age of 10 years offers a unique opportunity to study the natural development of CD in children from high risk families,” the authors wrote.
Children were assessed regularly from birth for CD development at specific intervals, including seven times during the first 3 years of age and once a year after that or at least once between March 2016 and March 2019.
The researchers monitored parent-reported health status, recorded weight and height and gluten consumption and serum IgA against anti-transglutaminase. They found that risk for CD differs by gender, age, and human leukocyte antigen (HLA-DQ) genes. Variables that significantly affected the risk were combined to develop a risk score.
The noted that CD affects as many as 1%-3% of the general population. Until recently, the lifetime risk of CD when a child has a first-degree CD connection was considered to be 5%-10%, with one review indicating a pooled prevalence of 7.5%. However, the investigators found that the cumulative incidence of CD was 7.5%, 16.6%, and 17.5% at 3, 8, and 10 years of age, respectively.
“Our data show that, at the age of 8 years, this is as high as 17%, emphasizing the importance of a sound advice for early screening,” Dr. Meijer and coauthors wrote. “We also confirm that CD develops in children with affected FDR at a very young age, as the mean age of diagnosis in our cohort was 4 years of age.”
They found that CD developed more often in girls (P = .0005). Data show that, at the age of 10 years, girls in the cohort have a 7.7% higher cumulative incidence compared to boys (21.5% vs 13.8%). CD also developed more often in HLA-DQ2 homozygous participants than in other HLA-risk groups (8-year cumulative incidence of 35.4% vs. the maximum of the other groups of 18.2%; P < .001).
Benjamin Lebwohl, MD, MS, said the study provides data that can help parents of a child with a family history of celiac disease.
“The results of the study include an estimate of celiac disease risk over time, based on the child’s age, number of affected relatives, and genetic test results. Using this information, the web-based prediction model provides advice on how often to test the child for celiac disease, based on the child’s risk of developing the condition in the years ahead,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University Medical Center, New York, said in an interview.
“Overall,” he said, “the study advances our understanding of the natural history of celiac disease among children with a family history and gives clinicians and families practical advice on when to test.”
Validation of the prediction model was done with data from the independent NeoCel cohort. In that cohort, all children were assessed regularly from birth for CD development at predefined intervals, in a similar way as in the PreventCD cohort.
The investigators concluded that children should be screened early in life and that screening should include HLA-DQ2/8-typing. If children are genetically predisposed to CD, they should get further personalized screening advice using the web-based prediction “app” developed by the investigators.
“If the prediction for CD development [according to the app] is higher than 10% in the next 2 years, we advice to repeat the screening after 6 months. If the prediction is between 5%-10%, the advice is to repeat the screening after 1 year and if the prediction is lower than 5%, to repeat the screening after 2 years.”
The study authors reported no relevant financial relationships. The work is supported by funding from the European Commission; the Azrieli Foundation; Deutsche Zöliakie Gesellschaft; Eurospital; Fondazione Celiachia; Fria Bröd Sweden; Instituto de Salud Carlos III; Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Komitet Badań Naukowych; Fundacja Nutricia; Hungarian Scientific Research Funds; and TAMOP; Stichting Coeliakie Onderzoek Nederland; Thermo Fisher Scientific; and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Dr. Lebwohl reported having no relevant disclosures.
AGA offers guidance on celiac disease to help patients maintain a gluten free diet in the AGA GI Patient Center: www.gastro.org/celiac
Children who have first-degree relatives with celiac disease (CD) may be at higher risk of developing CD before age 10 than previously thought, according to results of an analysis of 10-year follow-up data. This analysis also yielded a web-based prediction model to help with screening for CD in children.
Screening is recommended for children who have a first-degree relative with celiac disease (CD) because they have a much higher risk for CD, but it has been unclear when to screen such children and how frequently. The researchers, led by Caroline Meijer, MD, in the department of pediatrics and medical statistics at Leiden (the Netherlands) University Medical Center, wrote in Gastroenterology that a prediction model they developed can help parents and providers with those questions.
The researchers analyzed prospective data from 10 years of follow-up from the PreventCD birth cohort with 944 children genetically predisposed to celiac disease with at least one first-degree relative affected. The children were enrolled at birth between 2007 and 2010 in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain.
“The data of the follow-up of the PreventCD cohort at the mean age of 10 years offers a unique opportunity to study the natural development of CD in children from high risk families,” the authors wrote.
Children were assessed regularly from birth for CD development at specific intervals, including seven times during the first 3 years of age and once a year after that or at least once between March 2016 and March 2019.
The researchers monitored parent-reported health status, recorded weight and height and gluten consumption and serum IgA against anti-transglutaminase. They found that risk for CD differs by gender, age, and human leukocyte antigen (HLA-DQ) genes. Variables that significantly affected the risk were combined to develop a risk score.
The noted that CD affects as many as 1%-3% of the general population. Until recently, the lifetime risk of CD when a child has a first-degree CD connection was considered to be 5%-10%, with one review indicating a pooled prevalence of 7.5%. However, the investigators found that the cumulative incidence of CD was 7.5%, 16.6%, and 17.5% at 3, 8, and 10 years of age, respectively.
“Our data show that, at the age of 8 years, this is as high as 17%, emphasizing the importance of a sound advice for early screening,” Dr. Meijer and coauthors wrote. “We also confirm that CD develops in children with affected FDR at a very young age, as the mean age of diagnosis in our cohort was 4 years of age.”
They found that CD developed more often in girls (P = .0005). Data show that, at the age of 10 years, girls in the cohort have a 7.7% higher cumulative incidence compared to boys (21.5% vs 13.8%). CD also developed more often in HLA-DQ2 homozygous participants than in other HLA-risk groups (8-year cumulative incidence of 35.4% vs. the maximum of the other groups of 18.2%; P < .001).
Benjamin Lebwohl, MD, MS, said the study provides data that can help parents of a child with a family history of celiac disease.
“The results of the study include an estimate of celiac disease risk over time, based on the child’s age, number of affected relatives, and genetic test results. Using this information, the web-based prediction model provides advice on how often to test the child for celiac disease, based on the child’s risk of developing the condition in the years ahead,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University Medical Center, New York, said in an interview.
“Overall,” he said, “the study advances our understanding of the natural history of celiac disease among children with a family history and gives clinicians and families practical advice on when to test.”
Validation of the prediction model was done with data from the independent NeoCel cohort. In that cohort, all children were assessed regularly from birth for CD development at predefined intervals, in a similar way as in the PreventCD cohort.
The investigators concluded that children should be screened early in life and that screening should include HLA-DQ2/8-typing. If children are genetically predisposed to CD, they should get further personalized screening advice using the web-based prediction “app” developed by the investigators.
“If the prediction for CD development [according to the app] is higher than 10% in the next 2 years, we advice to repeat the screening after 6 months. If the prediction is between 5%-10%, the advice is to repeat the screening after 1 year and if the prediction is lower than 5%, to repeat the screening after 2 years.”
The study authors reported no relevant financial relationships. The work is supported by funding from the European Commission; the Azrieli Foundation; Deutsche Zöliakie Gesellschaft; Eurospital; Fondazione Celiachia; Fria Bröd Sweden; Instituto de Salud Carlos III; Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Komitet Badań Naukowych; Fundacja Nutricia; Hungarian Scientific Research Funds; and TAMOP; Stichting Coeliakie Onderzoek Nederland; Thermo Fisher Scientific; and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Dr. Lebwohl reported having no relevant disclosures.
AGA offers guidance on celiac disease to help patients maintain a gluten free diet in the AGA GI Patient Center: www.gastro.org/celiac
Children who have first-degree relatives with celiac disease (CD) may be at higher risk of developing CD before age 10 than previously thought, according to results of an analysis of 10-year follow-up data. This analysis also yielded a web-based prediction model to help with screening for CD in children.
Screening is recommended for children who have a first-degree relative with celiac disease (CD) because they have a much higher risk for CD, but it has been unclear when to screen such children and how frequently. The researchers, led by Caroline Meijer, MD, in the department of pediatrics and medical statistics at Leiden (the Netherlands) University Medical Center, wrote in Gastroenterology that a prediction model they developed can help parents and providers with those questions.
The researchers analyzed prospective data from 10 years of follow-up from the PreventCD birth cohort with 944 children genetically predisposed to celiac disease with at least one first-degree relative affected. The children were enrolled at birth between 2007 and 2010 in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain.
“The data of the follow-up of the PreventCD cohort at the mean age of 10 years offers a unique opportunity to study the natural development of CD in children from high risk families,” the authors wrote.
Children were assessed regularly from birth for CD development at specific intervals, including seven times during the first 3 years of age and once a year after that or at least once between March 2016 and March 2019.
The researchers monitored parent-reported health status, recorded weight and height and gluten consumption and serum IgA against anti-transglutaminase. They found that risk for CD differs by gender, age, and human leukocyte antigen (HLA-DQ) genes. Variables that significantly affected the risk were combined to develop a risk score.
The noted that CD affects as many as 1%-3% of the general population. Until recently, the lifetime risk of CD when a child has a first-degree CD connection was considered to be 5%-10%, with one review indicating a pooled prevalence of 7.5%. However, the investigators found that the cumulative incidence of CD was 7.5%, 16.6%, and 17.5% at 3, 8, and 10 years of age, respectively.
“Our data show that, at the age of 8 years, this is as high as 17%, emphasizing the importance of a sound advice for early screening,” Dr. Meijer and coauthors wrote. “We also confirm that CD develops in children with affected FDR at a very young age, as the mean age of diagnosis in our cohort was 4 years of age.”
They found that CD developed more often in girls (P = .0005). Data show that, at the age of 10 years, girls in the cohort have a 7.7% higher cumulative incidence compared to boys (21.5% vs 13.8%). CD also developed more often in HLA-DQ2 homozygous participants than in other HLA-risk groups (8-year cumulative incidence of 35.4% vs. the maximum of the other groups of 18.2%; P < .001).
Benjamin Lebwohl, MD, MS, said the study provides data that can help parents of a child with a family history of celiac disease.
“The results of the study include an estimate of celiac disease risk over time, based on the child’s age, number of affected relatives, and genetic test results. Using this information, the web-based prediction model provides advice on how often to test the child for celiac disease, based on the child’s risk of developing the condition in the years ahead,” said Dr. Lebwohl, director of clinical research at the Celiac Disease Center at Columbia University Medical Center, New York, said in an interview.
“Overall,” he said, “the study advances our understanding of the natural history of celiac disease among children with a family history and gives clinicians and families practical advice on when to test.”
Validation of the prediction model was done with data from the independent NeoCel cohort. In that cohort, all children were assessed regularly from birth for CD development at predefined intervals, in a similar way as in the PreventCD cohort.
The investigators concluded that children should be screened early in life and that screening should include HLA-DQ2/8-typing. If children are genetically predisposed to CD, they should get further personalized screening advice using the web-based prediction “app” developed by the investigators.
“If the prediction for CD development [according to the app] is higher than 10% in the next 2 years, we advice to repeat the screening after 6 months. If the prediction is between 5%-10%, the advice is to repeat the screening after 1 year and if the prediction is lower than 5%, to repeat the screening after 2 years.”
The study authors reported no relevant financial relationships. The work is supported by funding from the European Commission; the Azrieli Foundation; Deutsche Zöliakie Gesellschaft; Eurospital; Fondazione Celiachia; Fria Bröd Sweden; Instituto de Salud Carlos III; Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Komitet Badań Naukowych; Fundacja Nutricia; Hungarian Scientific Research Funds; and TAMOP; Stichting Coeliakie Onderzoek Nederland; Thermo Fisher Scientific; and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Dr. Lebwohl reported having no relevant disclosures.
AGA offers guidance on celiac disease to help patients maintain a gluten free diet in the AGA GI Patient Center: www.gastro.org/celiac
FROM GASTROENTEROLOGY
Bacterial cocktail, spores counter recurrent C. diff
SAN DIEGO – A novel combination of eight human commensal bacteria has shown efficacy in preventing recurrent Clostridioides difficile infections in high-risk populations. The cocktail of bacterial strains (VE303), produced under tightly-controlled conditions, is delivered in powdered form over a period of 14 days.
The approach, sponsored by Vedanta Biosciences, is one of several efforts to use carefully defined microbial populations instead of fecal microbiota transplantation (FMT) to treat or prevent C. diff infections.
The key issue is that not all of the bacteria found in FMTs are needed to provide a therapeutic effect, according to Thomas Louie, MD, professor of medicine at the University of Calgary (Alta.). “You don’t need all the bugs. You don’t need raw [stool]. You can take only the good parts,” said Dr. Louie, who presented the results of the phase 2 study at the annual Digestive Disease Week® (DDW). In fact, FMT carries the risk of infection of pathogenic bacteria.
The strains found in VE303 were consistently identified in patients’ microbiota following successful FMTs, though they were absent before the transplant. Animal and human studies then showed that the microbes could repopulate microbiota.
Among 78 patients included in the efficacy analysis of the study, after 8 weeks, 13.8% of the VE303 group experienced a recurrent C. diff infection, versus 45.5% of the placebo group, amounting to more than an 80% reduction in risk (odds ratio, 0.192; P = .0077). Adverse events were mild and similar across both groups, with no treatment-related serious adverse events reported.
The same session included a post hoc analysis of a phase 3 study sponsored by Seres Therapeutics, which showed that the company’s oral product SER-109, composed of purified Firmicutes spores, reduced the risk of recurrent C. diff infection after 8 weeks compared to placebo (12.4% versus 39.8%; P < .001).
The new analysis examined short-, medium-, and branch-chained fatty acids in patient stools. After just 1 week of treatment, there was an increase in the short-chain fatty acid butyrate and medium-chain fatty acids valerate and hexanoate. They continued to be higher in weeks 2 and 8 in the treatment arm. The results suggest that increased fatty acid production might boost clinical outcomes, according to Kevin Litcofsky of Seres, who presented the results.
Both approaches have potential, according to Melinda Engevik, PhD, who comoderated the session where the study was presented. “I think that they’re both interesting ideas. The spores [from Seres], I think, are going to be better at passing through the stomach and a little bit more resistant, but then they have to germinate and engraft, whereas if you give the lyophilized bacteria [from Vedanta], you might lose some more, but they’re already primed and ready to go. So I think they’re both very different approaches, but the data from both seem to support that they worked and probably in different ways,” said Dr. Engevik, assistant professor at the Medical University of South Carolina, Charleston.
“Patients that have recurrent [C. diff], they are desperate to be able to break the cycle of recurrence. I think that they’ve shown a lot of safety with this, which is an issue for FMT. Both of the talks seemed like there is a path moving forward to help those patients. I was encouraged,” said Dr. Engevik.
Comoderator Anoop Kumar, PhD, assistant professor of gastroenterology and hepatology at University of Illinois, Chicago, agreed and noted the advantage of such treatments over FMT during the COVID-19 pandemic, which has disrupted FMT delivery.
Previous studies have looked at probiotics, but results so far have been mixed, said Dr. Engevik. She suspects these two approaches, containing more bacterial strains, are likely to have better success. “I think you really have to have a complex gut microbiota community, at least minimally complex, to be able to get the effects. I think it’s the wave of the future,” she said.
Dr. Engevik also suggested that the benefits might not stop at C. diff. She highlighted research in other gastrointestinal diseases such as inflammatory bowel disease, and even efforts underway to enhance responses to checkpoint inhibitors in the treatment of cancer. “Gut microbes are master regulators, so they have these wide-reaching effects. I think that a lot of human health will be started to be targeted by looking at the gut microbiota,” she said.
Dr. Louie also highlighted the potential for more applications. “C. diff is low-hanging fruit. I think these bugs will have some usefulness for [irritable bowel syndrome]. I’ve transplanted some patients with IBS and it seemed to work. I haven’t had time to design and do an IBS trial, but the future is these bugs.”
Dr. Louie also participated in the Seres study. He has been on the advisory board for Vedanta, Seres, Finch Therapeutics, and Artugen Therapeutics. Dr. Engevik and Dr. Kumar have no relevant financial disclosures.
SAN DIEGO – A novel combination of eight human commensal bacteria has shown efficacy in preventing recurrent Clostridioides difficile infections in high-risk populations. The cocktail of bacterial strains (VE303), produced under tightly-controlled conditions, is delivered in powdered form over a period of 14 days.
The approach, sponsored by Vedanta Biosciences, is one of several efforts to use carefully defined microbial populations instead of fecal microbiota transplantation (FMT) to treat or prevent C. diff infections.
The key issue is that not all of the bacteria found in FMTs are needed to provide a therapeutic effect, according to Thomas Louie, MD, professor of medicine at the University of Calgary (Alta.). “You don’t need all the bugs. You don’t need raw [stool]. You can take only the good parts,” said Dr. Louie, who presented the results of the phase 2 study at the annual Digestive Disease Week® (DDW). In fact, FMT carries the risk of infection of pathogenic bacteria.
The strains found in VE303 were consistently identified in patients’ microbiota following successful FMTs, though they were absent before the transplant. Animal and human studies then showed that the microbes could repopulate microbiota.
Among 78 patients included in the efficacy analysis of the study, after 8 weeks, 13.8% of the VE303 group experienced a recurrent C. diff infection, versus 45.5% of the placebo group, amounting to more than an 80% reduction in risk (odds ratio, 0.192; P = .0077). Adverse events were mild and similar across both groups, with no treatment-related serious adverse events reported.
The same session included a post hoc analysis of a phase 3 study sponsored by Seres Therapeutics, which showed that the company’s oral product SER-109, composed of purified Firmicutes spores, reduced the risk of recurrent C. diff infection after 8 weeks compared to placebo (12.4% versus 39.8%; P < .001).
The new analysis examined short-, medium-, and branch-chained fatty acids in patient stools. After just 1 week of treatment, there was an increase in the short-chain fatty acid butyrate and medium-chain fatty acids valerate and hexanoate. They continued to be higher in weeks 2 and 8 in the treatment arm. The results suggest that increased fatty acid production might boost clinical outcomes, according to Kevin Litcofsky of Seres, who presented the results.
Both approaches have potential, according to Melinda Engevik, PhD, who comoderated the session where the study was presented. “I think that they’re both interesting ideas. The spores [from Seres], I think, are going to be better at passing through the stomach and a little bit more resistant, but then they have to germinate and engraft, whereas if you give the lyophilized bacteria [from Vedanta], you might lose some more, but they’re already primed and ready to go. So I think they’re both very different approaches, but the data from both seem to support that they worked and probably in different ways,” said Dr. Engevik, assistant professor at the Medical University of South Carolina, Charleston.
“Patients that have recurrent [C. diff], they are desperate to be able to break the cycle of recurrence. I think that they’ve shown a lot of safety with this, which is an issue for FMT. Both of the talks seemed like there is a path moving forward to help those patients. I was encouraged,” said Dr. Engevik.
Comoderator Anoop Kumar, PhD, assistant professor of gastroenterology and hepatology at University of Illinois, Chicago, agreed and noted the advantage of such treatments over FMT during the COVID-19 pandemic, which has disrupted FMT delivery.
Previous studies have looked at probiotics, but results so far have been mixed, said Dr. Engevik. She suspects these two approaches, containing more bacterial strains, are likely to have better success. “I think you really have to have a complex gut microbiota community, at least minimally complex, to be able to get the effects. I think it’s the wave of the future,” she said.
Dr. Engevik also suggested that the benefits might not stop at C. diff. She highlighted research in other gastrointestinal diseases such as inflammatory bowel disease, and even efforts underway to enhance responses to checkpoint inhibitors in the treatment of cancer. “Gut microbes are master regulators, so they have these wide-reaching effects. I think that a lot of human health will be started to be targeted by looking at the gut microbiota,” she said.
Dr. Louie also highlighted the potential for more applications. “C. diff is low-hanging fruit. I think these bugs will have some usefulness for [irritable bowel syndrome]. I’ve transplanted some patients with IBS and it seemed to work. I haven’t had time to design and do an IBS trial, but the future is these bugs.”
Dr. Louie also participated in the Seres study. He has been on the advisory board for Vedanta, Seres, Finch Therapeutics, and Artugen Therapeutics. Dr. Engevik and Dr. Kumar have no relevant financial disclosures.
SAN DIEGO – A novel combination of eight human commensal bacteria has shown efficacy in preventing recurrent Clostridioides difficile infections in high-risk populations. The cocktail of bacterial strains (VE303), produced under tightly-controlled conditions, is delivered in powdered form over a period of 14 days.
The approach, sponsored by Vedanta Biosciences, is one of several efforts to use carefully defined microbial populations instead of fecal microbiota transplantation (FMT) to treat or prevent C. diff infections.
The key issue is that not all of the bacteria found in FMTs are needed to provide a therapeutic effect, according to Thomas Louie, MD, professor of medicine at the University of Calgary (Alta.). “You don’t need all the bugs. You don’t need raw [stool]. You can take only the good parts,” said Dr. Louie, who presented the results of the phase 2 study at the annual Digestive Disease Week® (DDW). In fact, FMT carries the risk of infection of pathogenic bacteria.
The strains found in VE303 were consistently identified in patients’ microbiota following successful FMTs, though they were absent before the transplant. Animal and human studies then showed that the microbes could repopulate microbiota.
Among 78 patients included in the efficacy analysis of the study, after 8 weeks, 13.8% of the VE303 group experienced a recurrent C. diff infection, versus 45.5% of the placebo group, amounting to more than an 80% reduction in risk (odds ratio, 0.192; P = .0077). Adverse events were mild and similar across both groups, with no treatment-related serious adverse events reported.
The same session included a post hoc analysis of a phase 3 study sponsored by Seres Therapeutics, which showed that the company’s oral product SER-109, composed of purified Firmicutes spores, reduced the risk of recurrent C. diff infection after 8 weeks compared to placebo (12.4% versus 39.8%; P < .001).
The new analysis examined short-, medium-, and branch-chained fatty acids in patient stools. After just 1 week of treatment, there was an increase in the short-chain fatty acid butyrate and medium-chain fatty acids valerate and hexanoate. They continued to be higher in weeks 2 and 8 in the treatment arm. The results suggest that increased fatty acid production might boost clinical outcomes, according to Kevin Litcofsky of Seres, who presented the results.
Both approaches have potential, according to Melinda Engevik, PhD, who comoderated the session where the study was presented. “I think that they’re both interesting ideas. The spores [from Seres], I think, are going to be better at passing through the stomach and a little bit more resistant, but then they have to germinate and engraft, whereas if you give the lyophilized bacteria [from Vedanta], you might lose some more, but they’re already primed and ready to go. So I think they’re both very different approaches, but the data from both seem to support that they worked and probably in different ways,” said Dr. Engevik, assistant professor at the Medical University of South Carolina, Charleston.
“Patients that have recurrent [C. diff], they are desperate to be able to break the cycle of recurrence. I think that they’ve shown a lot of safety with this, which is an issue for FMT. Both of the talks seemed like there is a path moving forward to help those patients. I was encouraged,” said Dr. Engevik.
Comoderator Anoop Kumar, PhD, assistant professor of gastroenterology and hepatology at University of Illinois, Chicago, agreed and noted the advantage of such treatments over FMT during the COVID-19 pandemic, which has disrupted FMT delivery.
Previous studies have looked at probiotics, but results so far have been mixed, said Dr. Engevik. She suspects these two approaches, containing more bacterial strains, are likely to have better success. “I think you really have to have a complex gut microbiota community, at least minimally complex, to be able to get the effects. I think it’s the wave of the future,” she said.
Dr. Engevik also suggested that the benefits might not stop at C. diff. She highlighted research in other gastrointestinal diseases such as inflammatory bowel disease, and even efforts underway to enhance responses to checkpoint inhibitors in the treatment of cancer. “Gut microbes are master regulators, so they have these wide-reaching effects. I think that a lot of human health will be started to be targeted by looking at the gut microbiota,” she said.
Dr. Louie also highlighted the potential for more applications. “C. diff is low-hanging fruit. I think these bugs will have some usefulness for [irritable bowel syndrome]. I’ve transplanted some patients with IBS and it seemed to work. I haven’t had time to design and do an IBS trial, but the future is these bugs.”
Dr. Louie also participated in the Seres study. He has been on the advisory board for Vedanta, Seres, Finch Therapeutics, and Artugen Therapeutics. Dr. Engevik and Dr. Kumar have no relevant financial disclosures.
AT DDW 2022
How social determinants of health impact disparities in IBD care, outcomes
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
A version of this article first appeared on Medscape.com.
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
A version of this article first appeared on Medscape.com.
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Steroid use associated with higher relapse risk in some UC
Patients who have histologically active ulcerative colitis (UC) with a Mayo endoscopic subscore (MES) of 1 and a history of steroid use may be at increased risk for clinical relapse, according to a new single-center, retrospective analysis.
In recent years, treat-to-target approaches in UC have incorporated clinician and patient-reported outcomes, along with endoscopic remission, defined as MES 1 or less. However, additional studies showed a higher risk of relapse with MES 1, and the STRIDE-II (Selecting Therapeutic Targets in Inflammatory Bowel Disease) guidelines released in 2021 suggest that only MES 0 indicates endoscopic healing.
Nevertheless, there is concern that striving to achieve MES 0 could lead to overtreatment, since MES values are somewhat subjective, and patients with MES 1 sometimes report few or no symptoms. This led to the current study by Gyeol Seong, MD, and colleagues, published in Inflammatory Bowel Diseases.
Commenting on the study, Miguel Regueiro, MD, chair for the Digestive Disease and Surgery Institute and a professor of medicine at the Cleveland Clinic said “the question has always been, if the patient is in endoscopic remission and clinically feels well, how important is it to have histologically normal mucosa?”
In their retrospective study, Dr. Seong and colleagues analyzed data from 492 patients. The median age was 48, and 51.6% were male. The median duration of disease was 78 months. During a median follow-up of 549 days, 18.7% experienced a relapse, defined as “change or escalation of medication, hospitalization due to the aggravation of UC, or total colectomy.” Of these patients, 39.4% had used steroids and 51.4% had a MES score of 0 at the index endoscopy, and 70.5% had histologic improvement, as defined by a Geboes score of less than 3.1.
A Geboes score of 3.1 or higher and a history of steroid use was associated with an increased cumulative incidence of clinical relapse, compared with a Geboes score lower than 3.1 and no steroid use (P = .001). In a multivariate analysis, histologic activity alone was not a predictor of relapse. Among patients with an MES score of 1, a history of steroid use predicted risk of relapse (adjusted hazard ratio, 2.102; P = .006).
Although Dr. Regueiro said the new findings would not change his current practice, he does incorporate histopathology in clinical decision-making. In cases where a patient is in endoscopic remission but has histologic activity, he will consider adjusting the current medication rather than changing to a different therapeutic. “I wouldn’t recommend that we switch patients off one therapy to another just because of histologic activity.”
That’s because patients often improve dramatically after effective therapy. Dr. Regueiro said: “The one concern is whether the next medicine works as well as what the patient is already on? I’m also worried that we burn through our biologics too quickly. We go from one to another and another, sometimes I think we just need to optimize the one they’re on.”
The study does have the potential to influence surveillance for dysplasia, according to Dr. Regueiro, noting that a recent retrospective analysis showed an increased risk of dysplasia in UC with persistent histologic activity. “This suggests the need for a colonoscopy in a patient who has had ulcerative colitis for a number of years, even if there’s improvement but, on biopsy, there’s evidence of inflammation.”
According to Dr. Regueiro, such patients might benefit from a fecal calprotectin measurement in 3-6 months to monitor for colonic inflammation. Increased levels might be a sign that the patient is skipping medication doses, although other factors, like respiratory infections, can also explain the results. If the patient’s medication adherence is good, another therapy can be added or the dose increased, and the next endoscopy can be pushed up.
A key limitation to the study by Dr. Seong and colleagues is that it was based in South Korea. “Would this be the same in different countries and different populations? That is still a question,” said Dr. Regueiro.
Dr. Regueiro has received unrestricted educational grants from Abbvie, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, Bristol-Myers Squibb, and Lilly. He has been on advisory boards or consulted for numerous companies. He also has relationships with the following CME companies: CME Outfitters, Imedex, GI Health Foundation, Cornerstones, Remedy, MJH Life Sciences, Medscape, MDEducation, WebMD, and HMPGlobal.
Patients who have histologically active ulcerative colitis (UC) with a Mayo endoscopic subscore (MES) of 1 and a history of steroid use may be at increased risk for clinical relapse, according to a new single-center, retrospective analysis.
In recent years, treat-to-target approaches in UC have incorporated clinician and patient-reported outcomes, along with endoscopic remission, defined as MES 1 or less. However, additional studies showed a higher risk of relapse with MES 1, and the STRIDE-II (Selecting Therapeutic Targets in Inflammatory Bowel Disease) guidelines released in 2021 suggest that only MES 0 indicates endoscopic healing.
Nevertheless, there is concern that striving to achieve MES 0 could lead to overtreatment, since MES values are somewhat subjective, and patients with MES 1 sometimes report few or no symptoms. This led to the current study by Gyeol Seong, MD, and colleagues, published in Inflammatory Bowel Diseases.
Commenting on the study, Miguel Regueiro, MD, chair for the Digestive Disease and Surgery Institute and a professor of medicine at the Cleveland Clinic said “the question has always been, if the patient is in endoscopic remission and clinically feels well, how important is it to have histologically normal mucosa?”
In their retrospective study, Dr. Seong and colleagues analyzed data from 492 patients. The median age was 48, and 51.6% were male. The median duration of disease was 78 months. During a median follow-up of 549 days, 18.7% experienced a relapse, defined as “change or escalation of medication, hospitalization due to the aggravation of UC, or total colectomy.” Of these patients, 39.4% had used steroids and 51.4% had a MES score of 0 at the index endoscopy, and 70.5% had histologic improvement, as defined by a Geboes score of less than 3.1.
A Geboes score of 3.1 or higher and a history of steroid use was associated with an increased cumulative incidence of clinical relapse, compared with a Geboes score lower than 3.1 and no steroid use (P = .001). In a multivariate analysis, histologic activity alone was not a predictor of relapse. Among patients with an MES score of 1, a history of steroid use predicted risk of relapse (adjusted hazard ratio, 2.102; P = .006).
Although Dr. Regueiro said the new findings would not change his current practice, he does incorporate histopathology in clinical decision-making. In cases where a patient is in endoscopic remission but has histologic activity, he will consider adjusting the current medication rather than changing to a different therapeutic. “I wouldn’t recommend that we switch patients off one therapy to another just because of histologic activity.”
That’s because patients often improve dramatically after effective therapy. Dr. Regueiro said: “The one concern is whether the next medicine works as well as what the patient is already on? I’m also worried that we burn through our biologics too quickly. We go from one to another and another, sometimes I think we just need to optimize the one they’re on.”
The study does have the potential to influence surveillance for dysplasia, according to Dr. Regueiro, noting that a recent retrospective analysis showed an increased risk of dysplasia in UC with persistent histologic activity. “This suggests the need for a colonoscopy in a patient who has had ulcerative colitis for a number of years, even if there’s improvement but, on biopsy, there’s evidence of inflammation.”
According to Dr. Regueiro, such patients might benefit from a fecal calprotectin measurement in 3-6 months to monitor for colonic inflammation. Increased levels might be a sign that the patient is skipping medication doses, although other factors, like respiratory infections, can also explain the results. If the patient’s medication adherence is good, another therapy can be added or the dose increased, and the next endoscopy can be pushed up.
A key limitation to the study by Dr. Seong and colleagues is that it was based in South Korea. “Would this be the same in different countries and different populations? That is still a question,” said Dr. Regueiro.
Dr. Regueiro has received unrestricted educational grants from Abbvie, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, Bristol-Myers Squibb, and Lilly. He has been on advisory boards or consulted for numerous companies. He also has relationships with the following CME companies: CME Outfitters, Imedex, GI Health Foundation, Cornerstones, Remedy, MJH Life Sciences, Medscape, MDEducation, WebMD, and HMPGlobal.
Patients who have histologically active ulcerative colitis (UC) with a Mayo endoscopic subscore (MES) of 1 and a history of steroid use may be at increased risk for clinical relapse, according to a new single-center, retrospective analysis.
In recent years, treat-to-target approaches in UC have incorporated clinician and patient-reported outcomes, along with endoscopic remission, defined as MES 1 or less. However, additional studies showed a higher risk of relapse with MES 1, and the STRIDE-II (Selecting Therapeutic Targets in Inflammatory Bowel Disease) guidelines released in 2021 suggest that only MES 0 indicates endoscopic healing.
Nevertheless, there is concern that striving to achieve MES 0 could lead to overtreatment, since MES values are somewhat subjective, and patients with MES 1 sometimes report few or no symptoms. This led to the current study by Gyeol Seong, MD, and colleagues, published in Inflammatory Bowel Diseases.
Commenting on the study, Miguel Regueiro, MD, chair for the Digestive Disease and Surgery Institute and a professor of medicine at the Cleveland Clinic said “the question has always been, if the patient is in endoscopic remission and clinically feels well, how important is it to have histologically normal mucosa?”
In their retrospective study, Dr. Seong and colleagues analyzed data from 492 patients. The median age was 48, and 51.6% were male. The median duration of disease was 78 months. During a median follow-up of 549 days, 18.7% experienced a relapse, defined as “change or escalation of medication, hospitalization due to the aggravation of UC, or total colectomy.” Of these patients, 39.4% had used steroids and 51.4% had a MES score of 0 at the index endoscopy, and 70.5% had histologic improvement, as defined by a Geboes score of less than 3.1.
A Geboes score of 3.1 or higher and a history of steroid use was associated with an increased cumulative incidence of clinical relapse, compared with a Geboes score lower than 3.1 and no steroid use (P = .001). In a multivariate analysis, histologic activity alone was not a predictor of relapse. Among patients with an MES score of 1, a history of steroid use predicted risk of relapse (adjusted hazard ratio, 2.102; P = .006).
Although Dr. Regueiro said the new findings would not change his current practice, he does incorporate histopathology in clinical decision-making. In cases where a patient is in endoscopic remission but has histologic activity, he will consider adjusting the current medication rather than changing to a different therapeutic. “I wouldn’t recommend that we switch patients off one therapy to another just because of histologic activity.”
That’s because patients often improve dramatically after effective therapy. Dr. Regueiro said: “The one concern is whether the next medicine works as well as what the patient is already on? I’m also worried that we burn through our biologics too quickly. We go from one to another and another, sometimes I think we just need to optimize the one they’re on.”
The study does have the potential to influence surveillance for dysplasia, according to Dr. Regueiro, noting that a recent retrospective analysis showed an increased risk of dysplasia in UC with persistent histologic activity. “This suggests the need for a colonoscopy in a patient who has had ulcerative colitis for a number of years, even if there’s improvement but, on biopsy, there’s evidence of inflammation.”
According to Dr. Regueiro, such patients might benefit from a fecal calprotectin measurement in 3-6 months to monitor for colonic inflammation. Increased levels might be a sign that the patient is skipping medication doses, although other factors, like respiratory infections, can also explain the results. If the patient’s medication adherence is good, another therapy can be added or the dose increased, and the next endoscopy can be pushed up.
A key limitation to the study by Dr. Seong and colleagues is that it was based in South Korea. “Would this be the same in different countries and different populations? That is still a question,” said Dr. Regueiro.
Dr. Regueiro has received unrestricted educational grants from Abbvie, Janssen, UCB, Pfizer, Takeda, Celgene, Genentech, Gilead, Bristol-Myers Squibb, and Lilly. He has been on advisory boards or consulted for numerous companies. He also has relationships with the following CME companies: CME Outfitters, Imedex, GI Health Foundation, Cornerstones, Remedy, MJH Life Sciences, Medscape, MDEducation, WebMD, and HMPGlobal.
FROM INFLAMMATORY BOWEL DISEASES
IBD after age 60: More evidence antibiotics play a role
Most studies to date have assessed a link between antibiotics and IBD in younger patients, lead researcher Adam S. Faye, MD, said during a media briefing that previewed select research for the annual Digestive Disease Week® (DDW).
The impact of antibiotic use on the incidence of IBD in older adults is really unknown, he added.
In contrast to younger people with IBD, who tend to have a strong family history or genetic predisposition to developing Crohn’s disease or ulcerative colitis, the cause is likely different in older populations.
“There’s clearly something in the environment that’s driving this new older-onset IBD,” said Dr. Faye, who is an assistant professor of medicine and population health at New York University.
Antibiotics as a contributing link
Dr. Faye and colleagues took a closer look at antibiotics as contributing to this link. They studied 2.3 million patient records in Denmark’s national medical registry from 2000 to 2018. They identified people aged 60 years and older who were newly diagnosed with IBD, and they then assessed the number, frequency, and timing of any antibiotic prescriptions.
They found that IBD was 27% more likely in this age group if the patients had received any antibiotic prescription.
They also found that the chance of developing IBD was higher as the number of antibiotic prescriptions increased. For example, IBD was 55% more likely if a person had received two prescriptions, and it was 96% more likely with four prescriptions. The risk really jumped with five or more antibiotic prescriptions – a person with this many prescriptions was more than 2.3 times (236%) more likely to be diagnosed with IBD than those who had not been prescribed antibiotics in the prior 5 years.
Not all antibiotics were equal, however. For example, the investigators found no link with nitrofurantoin, an antibiotic commonly prescribed for urinary tract infections. In contrast, all other antibiotic agents that were evaluated, and especially fluoroquinolones, nitroimidazoles, and macrolides, were associated with IBD.
Timing made some difference.
“The risk was highest if antibiotics were prescribed within the 1- to 2-year period before diagnosis, and it declined as you go farther out. But the risks persist,” Dr. Faye said. He noted that they even found that risk was elevated 10 years out.
The investigators also considered whether the antibiotic agent or infection was behind the association.
Dr. Faye cited previous research, again in younger people with IBD, that revealed that “infections plus antibiotics substantially increase the odds or risk of developing IBD more than the infection alone.
“So there really does seem to be something that the antibiotics are doing here,” Dr. Faye said.
A leading theory is that antibiotics disrupt the gut microbiota and increase the risk for developing IBD. “But, obviously, it’s quite complicated,” he said.
Clinical implications
The findings suggest that older people who may have IBD should be screened for prior antibiotic use, Dr. Faye said.
“This is a result that really has important implications for diagnosing older adults with new gastrointestinal symptoms,” he said. “Inflammatory bowel disease often can be overlooked in older adults because there’s a lot of different diagnoses you’re thinking of.”
IBD “should be considered, especially if you have a patient who’s reporting multiple courses of antibiotics in the last few years,” he added.
The results suggest another reason that antimicrobial stewardship programs should promote judicial use of these agents beyond concerns about resistance.
“We think of antibiotic stewardship to prevent the development of multidrug-resistant organisms, but we should be thinking about it to also prevent the development of inflammatory bowel disease,” Dr. Faye said.
Although this study adds to the evidence implicating antibiotics and expands the concept to an older population, “we really don’t have a great handle on what all of the environmental and other factors are,” he said.
Some researchers point to smoking and diet, among other factors, but the interplay remains unknown, Dr. Faye added.
The study is important because the incidence of IBD is increasing within the older population, “and this is one of the first studies to look at it,” he said.
Dr. Faye and colleagues plan to start a new study to evaluate other environmental factors.
“Hopefully, we’ll have more within the next few years to report,” he said.
Shedding more light on older-onset IBD worldwide
“It’s a well-done study,” Aline Charabaty, MD, said in a comment. “We are seeing that there’s an increase of incidence of IBD in the entire population, but even more so in the elderly.”
IBD is likely caused by a combination of factors, including genetics, environmental influences, and dysfunction of the gut immune system, agreed Dr. Charabaty, who is an assistant clinical professor in the division of gastroenterology at Johns Hopkins University, Baltimore.
The research “goes along with other studies that we’ve done in the pediatric and adult populations that show antibiotics exposure increases the risk of developing inflammatory bowel disease,” she said.
For a broader perspective of the study’s findings, Dr. Faye was asked during the media briefing if the results of this Danish registry study would be generalizable to the U.S. population.
“The simplest answer is we’ll need to redo this study within the U.S. to make absolutely sure,” Dr. Faye said. She noted that prior studies in the United States and elsewhere have found a risk associated with antibiotics, although again these studies focused on younger patients.
Dr. Charabaty was more certain that the findings were meaningful outside of Denmark.
“I definitely think this will apply to our U.S. population,” added Dr. Charabaty, who is also the clinical director of the IBD Center at Johns Hopkins–Sibley Memorial Hospital, Washington. “We have very similar practices in terms of how we approach antibiotic use.
“This could be one of the risk factors that’s promoting an increase in IBD everywhere,” she added.
The study was conducted in partnership with the Danish National Center of Excellence PREDICT Program. Dr. Faye and Dr. Charabaty did not report any conflicts of interest related to this study.
A version of this article first appeared on Medscape.com.
Most studies to date have assessed a link between antibiotics and IBD in younger patients, lead researcher Adam S. Faye, MD, said during a media briefing that previewed select research for the annual Digestive Disease Week® (DDW).
The impact of antibiotic use on the incidence of IBD in older adults is really unknown, he added.
In contrast to younger people with IBD, who tend to have a strong family history or genetic predisposition to developing Crohn’s disease or ulcerative colitis, the cause is likely different in older populations.
“There’s clearly something in the environment that’s driving this new older-onset IBD,” said Dr. Faye, who is an assistant professor of medicine and population health at New York University.
Antibiotics as a contributing link
Dr. Faye and colleagues took a closer look at antibiotics as contributing to this link. They studied 2.3 million patient records in Denmark’s national medical registry from 2000 to 2018. They identified people aged 60 years and older who were newly diagnosed with IBD, and they then assessed the number, frequency, and timing of any antibiotic prescriptions.
They found that IBD was 27% more likely in this age group if the patients had received any antibiotic prescription.
They also found that the chance of developing IBD was higher as the number of antibiotic prescriptions increased. For example, IBD was 55% more likely if a person had received two prescriptions, and it was 96% more likely with four prescriptions. The risk really jumped with five or more antibiotic prescriptions – a person with this many prescriptions was more than 2.3 times (236%) more likely to be diagnosed with IBD than those who had not been prescribed antibiotics in the prior 5 years.
Not all antibiotics were equal, however. For example, the investigators found no link with nitrofurantoin, an antibiotic commonly prescribed for urinary tract infections. In contrast, all other antibiotic agents that were evaluated, and especially fluoroquinolones, nitroimidazoles, and macrolides, were associated with IBD.
Timing made some difference.
“The risk was highest if antibiotics were prescribed within the 1- to 2-year period before diagnosis, and it declined as you go farther out. But the risks persist,” Dr. Faye said. He noted that they even found that risk was elevated 10 years out.
The investigators also considered whether the antibiotic agent or infection was behind the association.
Dr. Faye cited previous research, again in younger people with IBD, that revealed that “infections plus antibiotics substantially increase the odds or risk of developing IBD more than the infection alone.
“So there really does seem to be something that the antibiotics are doing here,” Dr. Faye said.
A leading theory is that antibiotics disrupt the gut microbiota and increase the risk for developing IBD. “But, obviously, it’s quite complicated,” he said.
Clinical implications
The findings suggest that older people who may have IBD should be screened for prior antibiotic use, Dr. Faye said.
“This is a result that really has important implications for diagnosing older adults with new gastrointestinal symptoms,” he said. “Inflammatory bowel disease often can be overlooked in older adults because there’s a lot of different diagnoses you’re thinking of.”
IBD “should be considered, especially if you have a patient who’s reporting multiple courses of antibiotics in the last few years,” he added.
The results suggest another reason that antimicrobial stewardship programs should promote judicial use of these agents beyond concerns about resistance.
“We think of antibiotic stewardship to prevent the development of multidrug-resistant organisms, but we should be thinking about it to also prevent the development of inflammatory bowel disease,” Dr. Faye said.
Although this study adds to the evidence implicating antibiotics and expands the concept to an older population, “we really don’t have a great handle on what all of the environmental and other factors are,” he said.
Some researchers point to smoking and diet, among other factors, but the interplay remains unknown, Dr. Faye added.
The study is important because the incidence of IBD is increasing within the older population, “and this is one of the first studies to look at it,” he said.
Dr. Faye and colleagues plan to start a new study to evaluate other environmental factors.
“Hopefully, we’ll have more within the next few years to report,” he said.
Shedding more light on older-onset IBD worldwide
“It’s a well-done study,” Aline Charabaty, MD, said in a comment. “We are seeing that there’s an increase of incidence of IBD in the entire population, but even more so in the elderly.”
IBD is likely caused by a combination of factors, including genetics, environmental influences, and dysfunction of the gut immune system, agreed Dr. Charabaty, who is an assistant clinical professor in the division of gastroenterology at Johns Hopkins University, Baltimore.
The research “goes along with other studies that we’ve done in the pediatric and adult populations that show antibiotics exposure increases the risk of developing inflammatory bowel disease,” she said.
For a broader perspective of the study’s findings, Dr. Faye was asked during the media briefing if the results of this Danish registry study would be generalizable to the U.S. population.
“The simplest answer is we’ll need to redo this study within the U.S. to make absolutely sure,” Dr. Faye said. She noted that prior studies in the United States and elsewhere have found a risk associated with antibiotics, although again these studies focused on younger patients.
Dr. Charabaty was more certain that the findings were meaningful outside of Denmark.
“I definitely think this will apply to our U.S. population,” added Dr. Charabaty, who is also the clinical director of the IBD Center at Johns Hopkins–Sibley Memorial Hospital, Washington. “We have very similar practices in terms of how we approach antibiotic use.
“This could be one of the risk factors that’s promoting an increase in IBD everywhere,” she added.
The study was conducted in partnership with the Danish National Center of Excellence PREDICT Program. Dr. Faye and Dr. Charabaty did not report any conflicts of interest related to this study.
A version of this article first appeared on Medscape.com.
Most studies to date have assessed a link between antibiotics and IBD in younger patients, lead researcher Adam S. Faye, MD, said during a media briefing that previewed select research for the annual Digestive Disease Week® (DDW).
The impact of antibiotic use on the incidence of IBD in older adults is really unknown, he added.
In contrast to younger people with IBD, who tend to have a strong family history or genetic predisposition to developing Crohn’s disease or ulcerative colitis, the cause is likely different in older populations.
“There’s clearly something in the environment that’s driving this new older-onset IBD,” said Dr. Faye, who is an assistant professor of medicine and population health at New York University.
Antibiotics as a contributing link
Dr. Faye and colleagues took a closer look at antibiotics as contributing to this link. They studied 2.3 million patient records in Denmark’s national medical registry from 2000 to 2018. They identified people aged 60 years and older who were newly diagnosed with IBD, and they then assessed the number, frequency, and timing of any antibiotic prescriptions.
They found that IBD was 27% more likely in this age group if the patients had received any antibiotic prescription.
They also found that the chance of developing IBD was higher as the number of antibiotic prescriptions increased. For example, IBD was 55% more likely if a person had received two prescriptions, and it was 96% more likely with four prescriptions. The risk really jumped with five or more antibiotic prescriptions – a person with this many prescriptions was more than 2.3 times (236%) more likely to be diagnosed with IBD than those who had not been prescribed antibiotics in the prior 5 years.
Not all antibiotics were equal, however. For example, the investigators found no link with nitrofurantoin, an antibiotic commonly prescribed for urinary tract infections. In contrast, all other antibiotic agents that were evaluated, and especially fluoroquinolones, nitroimidazoles, and macrolides, were associated with IBD.
Timing made some difference.
“The risk was highest if antibiotics were prescribed within the 1- to 2-year period before diagnosis, and it declined as you go farther out. But the risks persist,” Dr. Faye said. He noted that they even found that risk was elevated 10 years out.
The investigators also considered whether the antibiotic agent or infection was behind the association.
Dr. Faye cited previous research, again in younger people with IBD, that revealed that “infections plus antibiotics substantially increase the odds or risk of developing IBD more than the infection alone.
“So there really does seem to be something that the antibiotics are doing here,” Dr. Faye said.
A leading theory is that antibiotics disrupt the gut microbiota and increase the risk for developing IBD. “But, obviously, it’s quite complicated,” he said.
Clinical implications
The findings suggest that older people who may have IBD should be screened for prior antibiotic use, Dr. Faye said.
“This is a result that really has important implications for diagnosing older adults with new gastrointestinal symptoms,” he said. “Inflammatory bowel disease often can be overlooked in older adults because there’s a lot of different diagnoses you’re thinking of.”
IBD “should be considered, especially if you have a patient who’s reporting multiple courses of antibiotics in the last few years,” he added.
The results suggest another reason that antimicrobial stewardship programs should promote judicial use of these agents beyond concerns about resistance.
“We think of antibiotic stewardship to prevent the development of multidrug-resistant organisms, but we should be thinking about it to also prevent the development of inflammatory bowel disease,” Dr. Faye said.
Although this study adds to the evidence implicating antibiotics and expands the concept to an older population, “we really don’t have a great handle on what all of the environmental and other factors are,” he said.
Some researchers point to smoking and diet, among other factors, but the interplay remains unknown, Dr. Faye added.
The study is important because the incidence of IBD is increasing within the older population, “and this is one of the first studies to look at it,” he said.
Dr. Faye and colleagues plan to start a new study to evaluate other environmental factors.
“Hopefully, we’ll have more within the next few years to report,” he said.
Shedding more light on older-onset IBD worldwide
“It’s a well-done study,” Aline Charabaty, MD, said in a comment. “We are seeing that there’s an increase of incidence of IBD in the entire population, but even more so in the elderly.”
IBD is likely caused by a combination of factors, including genetics, environmental influences, and dysfunction of the gut immune system, agreed Dr. Charabaty, who is an assistant clinical professor in the division of gastroenterology at Johns Hopkins University, Baltimore.
The research “goes along with other studies that we’ve done in the pediatric and adult populations that show antibiotics exposure increases the risk of developing inflammatory bowel disease,” she said.
For a broader perspective of the study’s findings, Dr. Faye was asked during the media briefing if the results of this Danish registry study would be generalizable to the U.S. population.
“The simplest answer is we’ll need to redo this study within the U.S. to make absolutely sure,” Dr. Faye said. She noted that prior studies in the United States and elsewhere have found a risk associated with antibiotics, although again these studies focused on younger patients.
Dr. Charabaty was more certain that the findings were meaningful outside of Denmark.
“I definitely think this will apply to our U.S. population,” added Dr. Charabaty, who is also the clinical director of the IBD Center at Johns Hopkins–Sibley Memorial Hospital, Washington. “We have very similar practices in terms of how we approach antibiotic use.
“This could be one of the risk factors that’s promoting an increase in IBD everywhere,” she added.
The study was conducted in partnership with the Danish National Center of Excellence PREDICT Program. Dr. Faye and Dr. Charabaty did not report any conflicts of interest related to this study.
A version of this article first appeared on Medscape.com.
FROM DDW 2022
TNF blockers beat newer biologics in Crohn’s disease: Meta-analysis
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.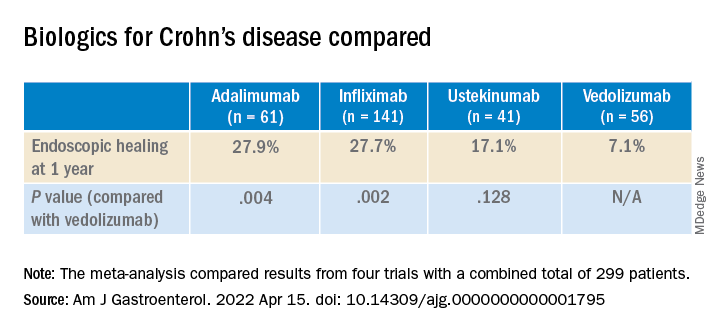
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY




