User login
Ulcerative colitis: Tofacitinib more effective than vedolizumab in patients refractory to anti-TNF
Key clinical point: Tofacitinib showed higher efficacy and comparable safety to vedolizumab in patients with ulcerative colitis (UC) with prior treatment failure with anti-tumor necrosis factor (anti-TNF) therapy.
Major finding: Patients treated with tofacitinib vs. vedolizumab were more likely to achieve corticosteroid-free remission at weeks 12 (odds ratio [OR] 6.33; P < .01), 24 (OR 3.02; P < .01), and 52 (OR 1.86; P = .01). The overall risk for adverse events (AE) was higher in patients treated with vedolizumab (OR 1.83; P = .02), whereas the risk for serious AE was similar in both the groups.
Study details: This was a prospective cohort study of 148 patients with UC from the Initiative on Crohn and Colitis (ICC) registry who were treated with vedolizumab (n = 83) or tofacitinib (n = 65) after the failure of treatment with at least one anti-TNF agent.
Disclosures: The ICC fellowship was sponsored by AbbVie, Pfizer, and others. Some authors reported receiving grants, consulting fees, speakers’ fees, presentation fees from, or serving on advisory boards for various sources, including the sponsors of the ICC Fellowship.
Source: Straatmijer T et al. Superior effectiveness of tofacitinib compared to vedolizumab in anti-TNF experienced ulcerative colitis patients: A nationwide Dutch Registry study. Clin Gastroenterol Hepatol. 2022 (May 26). Doi: 10.1016/j.cgh.2022.04.038
Key clinical point: Tofacitinib showed higher efficacy and comparable safety to vedolizumab in patients with ulcerative colitis (UC) with prior treatment failure with anti-tumor necrosis factor (anti-TNF) therapy.
Major finding: Patients treated with tofacitinib vs. vedolizumab were more likely to achieve corticosteroid-free remission at weeks 12 (odds ratio [OR] 6.33; P < .01), 24 (OR 3.02; P < .01), and 52 (OR 1.86; P = .01). The overall risk for adverse events (AE) was higher in patients treated with vedolizumab (OR 1.83; P = .02), whereas the risk for serious AE was similar in both the groups.
Study details: This was a prospective cohort study of 148 patients with UC from the Initiative on Crohn and Colitis (ICC) registry who were treated with vedolizumab (n = 83) or tofacitinib (n = 65) after the failure of treatment with at least one anti-TNF agent.
Disclosures: The ICC fellowship was sponsored by AbbVie, Pfizer, and others. Some authors reported receiving grants, consulting fees, speakers’ fees, presentation fees from, or serving on advisory boards for various sources, including the sponsors of the ICC Fellowship.
Source: Straatmijer T et al. Superior effectiveness of tofacitinib compared to vedolizumab in anti-TNF experienced ulcerative colitis patients: A nationwide Dutch Registry study. Clin Gastroenterol Hepatol. 2022 (May 26). Doi: 10.1016/j.cgh.2022.04.038
Key clinical point: Tofacitinib showed higher efficacy and comparable safety to vedolizumab in patients with ulcerative colitis (UC) with prior treatment failure with anti-tumor necrosis factor (anti-TNF) therapy.
Major finding: Patients treated with tofacitinib vs. vedolizumab were more likely to achieve corticosteroid-free remission at weeks 12 (odds ratio [OR] 6.33; P < .01), 24 (OR 3.02; P < .01), and 52 (OR 1.86; P = .01). The overall risk for adverse events (AE) was higher in patients treated with vedolizumab (OR 1.83; P = .02), whereas the risk for serious AE was similar in both the groups.
Study details: This was a prospective cohort study of 148 patients with UC from the Initiative on Crohn and Colitis (ICC) registry who were treated with vedolizumab (n = 83) or tofacitinib (n = 65) after the failure of treatment with at least one anti-TNF agent.
Disclosures: The ICC fellowship was sponsored by AbbVie, Pfizer, and others. Some authors reported receiving grants, consulting fees, speakers’ fees, presentation fees from, or serving on advisory boards for various sources, including the sponsors of the ICC Fellowship.
Source: Straatmijer T et al. Superior effectiveness of tofacitinib compared to vedolizumab in anti-TNF experienced ulcerative colitis patients: A nationwide Dutch Registry study. Clin Gastroenterol Hepatol. 2022 (May 26). Doi: 10.1016/j.cgh.2022.04.038
Risankizumab induction therapy safe and effective in moderate-to-severe Crohn’s disease
Key Clinical Point: Intravenous risankizumab induction therapy is safe and effective in patients with moderate-to-severe Crohn’s disease (CD).
Major finding: In the ADVANCE trial, Crohn’s Disease Activity Index clinical remission at week 12 was higher with 600 mg risankizumab (adjusted difference [Δ] 21%) and 1200 mg (Δ 17%) vs. placebo, with the endoscopic response being higher with 600 mg risankizumab (Δ 28%) and 1200 mg (Δ 20%; all P < .0001) vs. placebo. The MOTIVATE trial reported similar findings. The incidence of adverse events was similar across all treatment groups.
Study details: This study included patients with moderate-to-severe CD and intolerance/inadequate response to biologics or conventional therapy from the phase 3 ADVANCE (n=931) and MOTIVATE (n = 618) trials who were randomly assigned to receive risankizumab (600 or 1200 mg) or placebo.
Disclosures: This study was funded by AbbVie. Some authors declared being employees or holding stocks at AbbVie, and other authors reported receiving grants, speaker’s fees, or consulting fees or serving as advisory board members for various sources, including AbbVie.
Source: D’Haens G et al. Risankizumab as induction therapy for Crohn's disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030 (May 28). Doi: 10.1016/S0140-6736(22)00467-6
Key Clinical Point: Intravenous risankizumab induction therapy is safe and effective in patients with moderate-to-severe Crohn’s disease (CD).
Major finding: In the ADVANCE trial, Crohn’s Disease Activity Index clinical remission at week 12 was higher with 600 mg risankizumab (adjusted difference [Δ] 21%) and 1200 mg (Δ 17%) vs. placebo, with the endoscopic response being higher with 600 mg risankizumab (Δ 28%) and 1200 mg (Δ 20%; all P < .0001) vs. placebo. The MOTIVATE trial reported similar findings. The incidence of adverse events was similar across all treatment groups.
Study details: This study included patients with moderate-to-severe CD and intolerance/inadequate response to biologics or conventional therapy from the phase 3 ADVANCE (n=931) and MOTIVATE (n = 618) trials who were randomly assigned to receive risankizumab (600 or 1200 mg) or placebo.
Disclosures: This study was funded by AbbVie. Some authors declared being employees or holding stocks at AbbVie, and other authors reported receiving grants, speaker’s fees, or consulting fees or serving as advisory board members for various sources, including AbbVie.
Source: D’Haens G et al. Risankizumab as induction therapy for Crohn's disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030 (May 28). Doi: 10.1016/S0140-6736(22)00467-6
Key Clinical Point: Intravenous risankizumab induction therapy is safe and effective in patients with moderate-to-severe Crohn’s disease (CD).
Major finding: In the ADVANCE trial, Crohn’s Disease Activity Index clinical remission at week 12 was higher with 600 mg risankizumab (adjusted difference [Δ] 21%) and 1200 mg (Δ 17%) vs. placebo, with the endoscopic response being higher with 600 mg risankizumab (Δ 28%) and 1200 mg (Δ 20%; all P < .0001) vs. placebo. The MOTIVATE trial reported similar findings. The incidence of adverse events was similar across all treatment groups.
Study details: This study included patients with moderate-to-severe CD and intolerance/inadequate response to biologics or conventional therapy from the phase 3 ADVANCE (n=931) and MOTIVATE (n = 618) trials who were randomly assigned to receive risankizumab (600 or 1200 mg) or placebo.
Disclosures: This study was funded by AbbVie. Some authors declared being employees or holding stocks at AbbVie, and other authors reported receiving grants, speaker’s fees, or consulting fees or serving as advisory board members for various sources, including AbbVie.
Source: D’Haens G et al. Risankizumab as induction therapy for Crohn's disease: Results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015-2030 (May 28). Doi: 10.1016/S0140-6736(22)00467-6
Gaps in ulcerative colitis care expectations, perceptions
Gaps in priorities and perceptions about managing disease exist between physicians and patients with ulcerative colitis (UC), according to survey findings recently published in Therapeutic Advances in Gastroenterology.
The results – which come from the Ulcerative Colitis Narrative Survey from Japan – point to the ongoing need to foster a good relationship between physicians and patients, even as treatment methods for UC become more sophisticated, wrote the researchers led by Kenji Watanabe, MD, PhD, professor of internal medicine at Keio (Japan) University.
“While adjustments of the treatment regimens according to the results of objective monitoring in the treat-to-target strategy have led to improvements in UC management, the importance of patient-physician communication should not be neglected, as shared decision-making is a major driver of treatment satisfaction,” they wrote.
The UC Narrative is a multinational initiative sponsored by Pfizer meant to identify barriers to care and find solutions to those barriers. A total of 210 patients and 151 physicians completed the survey.
Overall, 65% of patients said they wished they had more time at appointments with their physicians, and 52% said their physician rarely had time to address all their questions and concerns. The majority of physicians (79%) also said they wish they had more time at appointments.
About half of patients (54%) ranked avoiding toileting accidents as a top priority more than any other concern, but physicians perceived this as less of a concern, with just 28% saying it was a top priority. For physicians, healing of mucosa was the second-highest ranked concern, with 59% saying it was a top priority, compared with just 29% for patients, and they also overestimated the importance of avoiding hospitalization among patients (56% vs. 38%).
Most patients (72%) said they felt comfortable raising concerns with their physician. But of those, 66% said they wished they had talked more about their fears of medical treatments, 53% said they worried that they would be seen as difficult if they asked too many questions, and 51% said their physician rarely had time to address all of their concerns.
Still, 85% of patients said they were satisfied overall with the communication they have with their physician, while physicians underestimated patient satisfaction, thinking that just 71% of their patients are satisfied with the communication.
The survey also found that physicians were more likely to discuss treatment-related topics than quality of life topics. And 52% of patients said they felt their doctor could do better in explaining the hereditary nature of UC, and just over half (52%) said their physician could do better in helping them access information and support from patient advocacy organizations.
The survey also found problems with patients’ knowledge of ulcerative colitis. About 26% said they thought that if their symptoms were under control then their disease was not active, and 23% said they didn’t know it was important to keep their disease under control to reduce long-term complications.
The majority of patients (82%) said their UC was mentally exhausting, and 64% said they felt they would be more successful if they didn’t have UC.
“This survey highlights the importance of regularly monitoring patients’ mental health,” the researchers wrote.
Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute at the Cleveland Clinic, said the findings illustrate the need for, and point to the challenges of establishing, quality communication between patients and physicians, which he said is “vitally important.”
“I find that physicians who listen, ask questions, and pause to hear the answers with honest dialogue on quality of life, have a physician-patient relationship that allows [them] to probe important topics of quality of life, depression, anxiety, stress,” he said. “As the Japanese study found, physicians may focus on the objective outcomes of UC treatment” – for example, mucosal healing – “but not always ask about the ‘whole person’ issues of UC,” such as quality of life.
According to Dr. Regueiro, Cleveland Clinic has developed an “IBD (inflammatory bowel disease) home” that includes doctors, dietitians, psychologists, nurses, and others that allows them to consider and manage many factors associated with the illness, not just the clinical picture.
“The team allows for the ‘How does UC impact you?’ discussions on a regular basis, and we have found that this whole-person approach is greatly appreciated by patients,” he said.
He suggested that physicians ask open-ended questions, such as: “What are two to three things that are bothering you or that you want out of the visit?”
“Even though one physician may not be able to address all of the problems,” he said, “the physician can start the narrative.”
The survey was sponsored by Pfizer. Dr. Watanabe reported receiving research funding or consulting fees from several companies including Pfizer, as well as Asahi Kasei Medical, Mitsubishi Tanabe, AbbVie Japan, Janssen, Takeda, and others. Dr. Regueiro reported advisory board work or consulting for AbbVie, Janssen, Takeda, Pfizer, Celgene, and other companies.
Gaps in priorities and perceptions about managing disease exist between physicians and patients with ulcerative colitis (UC), according to survey findings recently published in Therapeutic Advances in Gastroenterology.
The results – which come from the Ulcerative Colitis Narrative Survey from Japan – point to the ongoing need to foster a good relationship between physicians and patients, even as treatment methods for UC become more sophisticated, wrote the researchers led by Kenji Watanabe, MD, PhD, professor of internal medicine at Keio (Japan) University.
“While adjustments of the treatment regimens according to the results of objective monitoring in the treat-to-target strategy have led to improvements in UC management, the importance of patient-physician communication should not be neglected, as shared decision-making is a major driver of treatment satisfaction,” they wrote.
The UC Narrative is a multinational initiative sponsored by Pfizer meant to identify barriers to care and find solutions to those barriers. A total of 210 patients and 151 physicians completed the survey.
Overall, 65% of patients said they wished they had more time at appointments with their physicians, and 52% said their physician rarely had time to address all their questions and concerns. The majority of physicians (79%) also said they wish they had more time at appointments.
About half of patients (54%) ranked avoiding toileting accidents as a top priority more than any other concern, but physicians perceived this as less of a concern, with just 28% saying it was a top priority. For physicians, healing of mucosa was the second-highest ranked concern, with 59% saying it was a top priority, compared with just 29% for patients, and they also overestimated the importance of avoiding hospitalization among patients (56% vs. 38%).
Most patients (72%) said they felt comfortable raising concerns with their physician. But of those, 66% said they wished they had talked more about their fears of medical treatments, 53% said they worried that they would be seen as difficult if they asked too many questions, and 51% said their physician rarely had time to address all of their concerns.
Still, 85% of patients said they were satisfied overall with the communication they have with their physician, while physicians underestimated patient satisfaction, thinking that just 71% of their patients are satisfied with the communication.
The survey also found that physicians were more likely to discuss treatment-related topics than quality of life topics. And 52% of patients said they felt their doctor could do better in explaining the hereditary nature of UC, and just over half (52%) said their physician could do better in helping them access information and support from patient advocacy organizations.
The survey also found problems with patients’ knowledge of ulcerative colitis. About 26% said they thought that if their symptoms were under control then their disease was not active, and 23% said they didn’t know it was important to keep their disease under control to reduce long-term complications.
The majority of patients (82%) said their UC was mentally exhausting, and 64% said they felt they would be more successful if they didn’t have UC.
“This survey highlights the importance of regularly monitoring patients’ mental health,” the researchers wrote.
Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute at the Cleveland Clinic, said the findings illustrate the need for, and point to the challenges of establishing, quality communication between patients and physicians, which he said is “vitally important.”
“I find that physicians who listen, ask questions, and pause to hear the answers with honest dialogue on quality of life, have a physician-patient relationship that allows [them] to probe important topics of quality of life, depression, anxiety, stress,” he said. “As the Japanese study found, physicians may focus on the objective outcomes of UC treatment” – for example, mucosal healing – “but not always ask about the ‘whole person’ issues of UC,” such as quality of life.
According to Dr. Regueiro, Cleveland Clinic has developed an “IBD (inflammatory bowel disease) home” that includes doctors, dietitians, psychologists, nurses, and others that allows them to consider and manage many factors associated with the illness, not just the clinical picture.
“The team allows for the ‘How does UC impact you?’ discussions on a regular basis, and we have found that this whole-person approach is greatly appreciated by patients,” he said.
He suggested that physicians ask open-ended questions, such as: “What are two to three things that are bothering you or that you want out of the visit?”
“Even though one physician may not be able to address all of the problems,” he said, “the physician can start the narrative.”
The survey was sponsored by Pfizer. Dr. Watanabe reported receiving research funding or consulting fees from several companies including Pfizer, as well as Asahi Kasei Medical, Mitsubishi Tanabe, AbbVie Japan, Janssen, Takeda, and others. Dr. Regueiro reported advisory board work or consulting for AbbVie, Janssen, Takeda, Pfizer, Celgene, and other companies.
Gaps in priorities and perceptions about managing disease exist between physicians and patients with ulcerative colitis (UC), according to survey findings recently published in Therapeutic Advances in Gastroenterology.
The results – which come from the Ulcerative Colitis Narrative Survey from Japan – point to the ongoing need to foster a good relationship between physicians and patients, even as treatment methods for UC become more sophisticated, wrote the researchers led by Kenji Watanabe, MD, PhD, professor of internal medicine at Keio (Japan) University.
“While adjustments of the treatment regimens according to the results of objective monitoring in the treat-to-target strategy have led to improvements in UC management, the importance of patient-physician communication should not be neglected, as shared decision-making is a major driver of treatment satisfaction,” they wrote.
The UC Narrative is a multinational initiative sponsored by Pfizer meant to identify barriers to care and find solutions to those barriers. A total of 210 patients and 151 physicians completed the survey.
Overall, 65% of patients said they wished they had more time at appointments with their physicians, and 52% said their physician rarely had time to address all their questions and concerns. The majority of physicians (79%) also said they wish they had more time at appointments.
About half of patients (54%) ranked avoiding toileting accidents as a top priority more than any other concern, but physicians perceived this as less of a concern, with just 28% saying it was a top priority. For physicians, healing of mucosa was the second-highest ranked concern, with 59% saying it was a top priority, compared with just 29% for patients, and they also overestimated the importance of avoiding hospitalization among patients (56% vs. 38%).
Most patients (72%) said they felt comfortable raising concerns with their physician. But of those, 66% said they wished they had talked more about their fears of medical treatments, 53% said they worried that they would be seen as difficult if they asked too many questions, and 51% said their physician rarely had time to address all of their concerns.
Still, 85% of patients said they were satisfied overall with the communication they have with their physician, while physicians underestimated patient satisfaction, thinking that just 71% of their patients are satisfied with the communication.
The survey also found that physicians were more likely to discuss treatment-related topics than quality of life topics. And 52% of patients said they felt their doctor could do better in explaining the hereditary nature of UC, and just over half (52%) said their physician could do better in helping them access information and support from patient advocacy organizations.
The survey also found problems with patients’ knowledge of ulcerative colitis. About 26% said they thought that if their symptoms were under control then their disease was not active, and 23% said they didn’t know it was important to keep their disease under control to reduce long-term complications.
The majority of patients (82%) said their UC was mentally exhausting, and 64% said they felt they would be more successful if they didn’t have UC.
“This survey highlights the importance of regularly monitoring patients’ mental health,” the researchers wrote.
Miguel Regueiro, MD, chair of the Digestive Disease & Surgery Institute at the Cleveland Clinic, said the findings illustrate the need for, and point to the challenges of establishing, quality communication between patients and physicians, which he said is “vitally important.”
“I find that physicians who listen, ask questions, and pause to hear the answers with honest dialogue on quality of life, have a physician-patient relationship that allows [them] to probe important topics of quality of life, depression, anxiety, stress,” he said. “As the Japanese study found, physicians may focus on the objective outcomes of UC treatment” – for example, mucosal healing – “but not always ask about the ‘whole person’ issues of UC,” such as quality of life.
According to Dr. Regueiro, Cleveland Clinic has developed an “IBD (inflammatory bowel disease) home” that includes doctors, dietitians, psychologists, nurses, and others that allows them to consider and manage many factors associated with the illness, not just the clinical picture.
“The team allows for the ‘How does UC impact you?’ discussions on a regular basis, and we have found that this whole-person approach is greatly appreciated by patients,” he said.
He suggested that physicians ask open-ended questions, such as: “What are two to three things that are bothering you or that you want out of the visit?”
“Even though one physician may not be able to address all of the problems,” he said, “the physician can start the narrative.”
The survey was sponsored by Pfizer. Dr. Watanabe reported receiving research funding or consulting fees from several companies including Pfizer, as well as Asahi Kasei Medical, Mitsubishi Tanabe, AbbVie Japan, Janssen, Takeda, and others. Dr. Regueiro reported advisory board work or consulting for AbbVie, Janssen, Takeda, Pfizer, Celgene, and other companies.
FROM THERAPEUTIC ADVANCES IN GASTROENTEROLOGY
AGA Clinical Practice Guidelines: Pharmacologic treatment of IBS
The American Gastroenterological Association has issued new guidelines for the medical treatment of irritable bowel syndrome (IBS).
The guidelines, which are separated into one publication for IBS with constipation (IBS-C) and another for IBS with diarrhea (IBS-D), are the first to advise clinicians in the usage of new, old, and over-the-counter drugs for IBS, according to a press release from the AGA.
“With more treatments available, physicians can tailor a personalized approach based on the symptoms a patient with IBS is experiencing,” AGA said.
Published simultaneously in Gastroenterology, the two guidelines describe a shared rationale for their creation, noting how the treatment landscape has changed since the AGA last issued IBS guidelines in 2014.
“New pharmacological treatments have become available and new evidence has accumulated about established treatments,” both guidelines stated. “The purpose of these guidelines is to provide evidence-based recommendations for the pharmacologic management” of individuals with IBS “based on a systematic and comprehensive synthesis of the literature.”
IBS-C
In the IBS-C guidelines, co–first authors Lin Chang, MD, AGAF, of the University of Los Angeles, and Shahnaz Sultan, MD, MHSc, AGAF, of the Minneapolis Veterans Affairs Healthcare System, noted that IBS-C accounts for “more than a third of IBS cases,” with patients frequently reporting “feeling self-conscious, avoiding sex, difficulty concentrating, [and] not feeling able to reach one’s full potential.”
They offered nine pharmacologic recommendations, eight of which are conditional, with certainty in evidence ranging from low to high.
The only strong recommendation with a high certainty in evidence is for linaclotide.
“Across four RCTs [randomized controlled trials], linaclotide improved global assessment of IBS-C symptoms (FDA responder), abdominal pain, complete spontaneous bowel movement response, as well as adequate global response,” Dr. Chang and colleagues wrote.
Conditional recommendations with moderate certainty in evidence are provided for tenapanor, plecanatide, tegaserod, and lubiprostone. Recommendations for polyethylene glycol laxatives, tricyclic antidepressants and antispasmodics are conditional and based on low-certainty evidence, as well as a conditional recommendation against selective serotonin reuptake inhibitors, also based on low-certainty evidence.
IBS-D
The IBS-D guidelines, led by co–first authors Anthony Lembo, MD, AGAF, of Beth Israel Deaconess Medical Center, Boston, and Dr. Sultan, includes eight conditional recommendations with certainty in evidence ranging from very low to moderate.
Drugs recommended based on moderate-certainty evidence include eluxadoline, alosetron, and rifaximin, with the added note that patients who respond to rifaximin but have recurrence should be treated again with rifaximin. Low-certainty evidence supported recommendations for tricyclic antidepressants, and antispasmodics. Very low–certainty evidence stands behind a recommendation for loperamide. Again, the panel made a conditional recommendation against SSRIs, also based on low-certainty evidence.
Shared decision-making
Both publications concluded with similar statements about the importance of shared decision-making, plus a practical mindset, in management of IBS.
“Acknowledging that multimodal treatments that include dietary and behavioral approaches in conjunction with drug therapy may provide maximal benefits and that treatment choices may be influenced by patient preferences, practitioners should engage in shared decision-making with patients when choosing the best therapy,” Dr. Lembo and colleagues wrote. “The importance of the patient-physician relationship is paramount in caring for individuals with IBS, and understanding patient preferences (for side-effect tolerability as well as cost) is valuable in choosing the right therapy.”
Both guidelines noted that some newer drugs for IBS have no generic alternative, and preauthorization may be required. Payer approval may depend on previous treatment failure with generic alternatives, they added.
The guidelines were commissioned and funded by the AGA Institute. The authors disclosed relationships with Ardelyx, Immunic, Protagonist, and others.
The American Gastroenterological Association has issued new guidelines for the medical treatment of irritable bowel syndrome (IBS).
The guidelines, which are separated into one publication for IBS with constipation (IBS-C) and another for IBS with diarrhea (IBS-D), are the first to advise clinicians in the usage of new, old, and over-the-counter drugs for IBS, according to a press release from the AGA.
“With more treatments available, physicians can tailor a personalized approach based on the symptoms a patient with IBS is experiencing,” AGA said.
Published simultaneously in Gastroenterology, the two guidelines describe a shared rationale for their creation, noting how the treatment landscape has changed since the AGA last issued IBS guidelines in 2014.
“New pharmacological treatments have become available and new evidence has accumulated about established treatments,” both guidelines stated. “The purpose of these guidelines is to provide evidence-based recommendations for the pharmacologic management” of individuals with IBS “based on a systematic and comprehensive synthesis of the literature.”
IBS-C
In the IBS-C guidelines, co–first authors Lin Chang, MD, AGAF, of the University of Los Angeles, and Shahnaz Sultan, MD, MHSc, AGAF, of the Minneapolis Veterans Affairs Healthcare System, noted that IBS-C accounts for “more than a third of IBS cases,” with patients frequently reporting “feeling self-conscious, avoiding sex, difficulty concentrating, [and] not feeling able to reach one’s full potential.”
They offered nine pharmacologic recommendations, eight of which are conditional, with certainty in evidence ranging from low to high.
The only strong recommendation with a high certainty in evidence is for linaclotide.
“Across four RCTs [randomized controlled trials], linaclotide improved global assessment of IBS-C symptoms (FDA responder), abdominal pain, complete spontaneous bowel movement response, as well as adequate global response,” Dr. Chang and colleagues wrote.
Conditional recommendations with moderate certainty in evidence are provided for tenapanor, plecanatide, tegaserod, and lubiprostone. Recommendations for polyethylene glycol laxatives, tricyclic antidepressants and antispasmodics are conditional and based on low-certainty evidence, as well as a conditional recommendation against selective serotonin reuptake inhibitors, also based on low-certainty evidence.
IBS-D
The IBS-D guidelines, led by co–first authors Anthony Lembo, MD, AGAF, of Beth Israel Deaconess Medical Center, Boston, and Dr. Sultan, includes eight conditional recommendations with certainty in evidence ranging from very low to moderate.
Drugs recommended based on moderate-certainty evidence include eluxadoline, alosetron, and rifaximin, with the added note that patients who respond to rifaximin but have recurrence should be treated again with rifaximin. Low-certainty evidence supported recommendations for tricyclic antidepressants, and antispasmodics. Very low–certainty evidence stands behind a recommendation for loperamide. Again, the panel made a conditional recommendation against SSRIs, also based on low-certainty evidence.
Shared decision-making
Both publications concluded with similar statements about the importance of shared decision-making, plus a practical mindset, in management of IBS.
“Acknowledging that multimodal treatments that include dietary and behavioral approaches in conjunction with drug therapy may provide maximal benefits and that treatment choices may be influenced by patient preferences, practitioners should engage in shared decision-making with patients when choosing the best therapy,” Dr. Lembo and colleagues wrote. “The importance of the patient-physician relationship is paramount in caring for individuals with IBS, and understanding patient preferences (for side-effect tolerability as well as cost) is valuable in choosing the right therapy.”
Both guidelines noted that some newer drugs for IBS have no generic alternative, and preauthorization may be required. Payer approval may depend on previous treatment failure with generic alternatives, they added.
The guidelines were commissioned and funded by the AGA Institute. The authors disclosed relationships with Ardelyx, Immunic, Protagonist, and others.
The American Gastroenterological Association has issued new guidelines for the medical treatment of irritable bowel syndrome (IBS).
The guidelines, which are separated into one publication for IBS with constipation (IBS-C) and another for IBS with diarrhea (IBS-D), are the first to advise clinicians in the usage of new, old, and over-the-counter drugs for IBS, according to a press release from the AGA.
“With more treatments available, physicians can tailor a personalized approach based on the symptoms a patient with IBS is experiencing,” AGA said.
Published simultaneously in Gastroenterology, the two guidelines describe a shared rationale for their creation, noting how the treatment landscape has changed since the AGA last issued IBS guidelines in 2014.
“New pharmacological treatments have become available and new evidence has accumulated about established treatments,” both guidelines stated. “The purpose of these guidelines is to provide evidence-based recommendations for the pharmacologic management” of individuals with IBS “based on a systematic and comprehensive synthesis of the literature.”
IBS-C
In the IBS-C guidelines, co–first authors Lin Chang, MD, AGAF, of the University of Los Angeles, and Shahnaz Sultan, MD, MHSc, AGAF, of the Minneapolis Veterans Affairs Healthcare System, noted that IBS-C accounts for “more than a third of IBS cases,” with patients frequently reporting “feeling self-conscious, avoiding sex, difficulty concentrating, [and] not feeling able to reach one’s full potential.”
They offered nine pharmacologic recommendations, eight of which are conditional, with certainty in evidence ranging from low to high.
The only strong recommendation with a high certainty in evidence is for linaclotide.
“Across four RCTs [randomized controlled trials], linaclotide improved global assessment of IBS-C symptoms (FDA responder), abdominal pain, complete spontaneous bowel movement response, as well as adequate global response,” Dr. Chang and colleagues wrote.
Conditional recommendations with moderate certainty in evidence are provided for tenapanor, plecanatide, tegaserod, and lubiprostone. Recommendations for polyethylene glycol laxatives, tricyclic antidepressants and antispasmodics are conditional and based on low-certainty evidence, as well as a conditional recommendation against selective serotonin reuptake inhibitors, also based on low-certainty evidence.
IBS-D
The IBS-D guidelines, led by co–first authors Anthony Lembo, MD, AGAF, of Beth Israel Deaconess Medical Center, Boston, and Dr. Sultan, includes eight conditional recommendations with certainty in evidence ranging from very low to moderate.
Drugs recommended based on moderate-certainty evidence include eluxadoline, alosetron, and rifaximin, with the added note that patients who respond to rifaximin but have recurrence should be treated again with rifaximin. Low-certainty evidence supported recommendations for tricyclic antidepressants, and antispasmodics. Very low–certainty evidence stands behind a recommendation for loperamide. Again, the panel made a conditional recommendation against SSRIs, also based on low-certainty evidence.
Shared decision-making
Both publications concluded with similar statements about the importance of shared decision-making, plus a practical mindset, in management of IBS.
“Acknowledging that multimodal treatments that include dietary and behavioral approaches in conjunction with drug therapy may provide maximal benefits and that treatment choices may be influenced by patient preferences, practitioners should engage in shared decision-making with patients when choosing the best therapy,” Dr. Lembo and colleagues wrote. “The importance of the patient-physician relationship is paramount in caring for individuals with IBS, and understanding patient preferences (for side-effect tolerability as well as cost) is valuable in choosing the right therapy.”
Both guidelines noted that some newer drugs for IBS have no generic alternative, and preauthorization may be required. Payer approval may depend on previous treatment failure with generic alternatives, they added.
The guidelines were commissioned and funded by the AGA Institute. The authors disclosed relationships with Ardelyx, Immunic, Protagonist, and others.
FROM GASTROENTEROLOGY
FDA approves risankizumab (Skyrizi) for Crohn’s disease
The U.S. Food and Drug Administration – making it the first specific anti–interleukin-23 monoclonal antibody indicated for Crohn’s disease.
The safety and efficacy of risankizumab in Crohn’s disease is supported by data from two induction clinical trials (ADVANCE and MOTIVATE) and one maintenance clinical trial (FORTIFY).
Results of the three studies were presented at the annual scientific meeting of the American College of Gastroenterology in 2021.
“In both the induction and maintenance clinical trials, a significantly greater number of adult patients saw few or no symptoms and a meaningful reduction of visible signs of intestinal inflammation, compared to placebo,” Marla Dubinsky, MD, gastroenterologist with the Mount Sinai Health System and codirector of the IBD Center at Mount Sinai, New York, said in a news release from AbbVie.
“This approval provides health care professionals with a greatly needed additional option for treating the disruptive symptoms of Crohn’s disease,” Dr. Dubinsky said.
For the treatment of Crohn’s disease, risankizumab is dosed at 600 mg administered by intravenous infusion over at least 1 hour at week 0, 4, and 8, followed by 360 mg self-administered by subcutaneous injection at week 12, and every 8 weeks thereafter.
Risankizumab is already approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration – making it the first specific anti–interleukin-23 monoclonal antibody indicated for Crohn’s disease.
The safety and efficacy of risankizumab in Crohn’s disease is supported by data from two induction clinical trials (ADVANCE and MOTIVATE) and one maintenance clinical trial (FORTIFY).
Results of the three studies were presented at the annual scientific meeting of the American College of Gastroenterology in 2021.
“In both the induction and maintenance clinical trials, a significantly greater number of adult patients saw few or no symptoms and a meaningful reduction of visible signs of intestinal inflammation, compared to placebo,” Marla Dubinsky, MD, gastroenterologist with the Mount Sinai Health System and codirector of the IBD Center at Mount Sinai, New York, said in a news release from AbbVie.
“This approval provides health care professionals with a greatly needed additional option for treating the disruptive symptoms of Crohn’s disease,” Dr. Dubinsky said.
For the treatment of Crohn’s disease, risankizumab is dosed at 600 mg administered by intravenous infusion over at least 1 hour at week 0, 4, and 8, followed by 360 mg self-administered by subcutaneous injection at week 12, and every 8 weeks thereafter.
Risankizumab is already approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration – making it the first specific anti–interleukin-23 monoclonal antibody indicated for Crohn’s disease.
The safety and efficacy of risankizumab in Crohn’s disease is supported by data from two induction clinical trials (ADVANCE and MOTIVATE) and one maintenance clinical trial (FORTIFY).
Results of the three studies were presented at the annual scientific meeting of the American College of Gastroenterology in 2021.
“In both the induction and maintenance clinical trials, a significantly greater number of adult patients saw few or no symptoms and a meaningful reduction of visible signs of intestinal inflammation, compared to placebo,” Marla Dubinsky, MD, gastroenterologist with the Mount Sinai Health System and codirector of the IBD Center at Mount Sinai, New York, said in a news release from AbbVie.
“This approval provides health care professionals with a greatly needed additional option for treating the disruptive symptoms of Crohn’s disease,” Dr. Dubinsky said.
For the treatment of Crohn’s disease, risankizumab is dosed at 600 mg administered by intravenous infusion over at least 1 hour at week 0, 4, and 8, followed by 360 mg self-administered by subcutaneous injection at week 12, and every 8 weeks thereafter.
Risankizumab is already approved in the United States for the treatment of adults with active psoriatic arthritis and moderate to severe plaque psoriasis.
A version of this article first appeared on Medscape.com.
Endoscopic management of duodenal and ampullary adenomas
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.
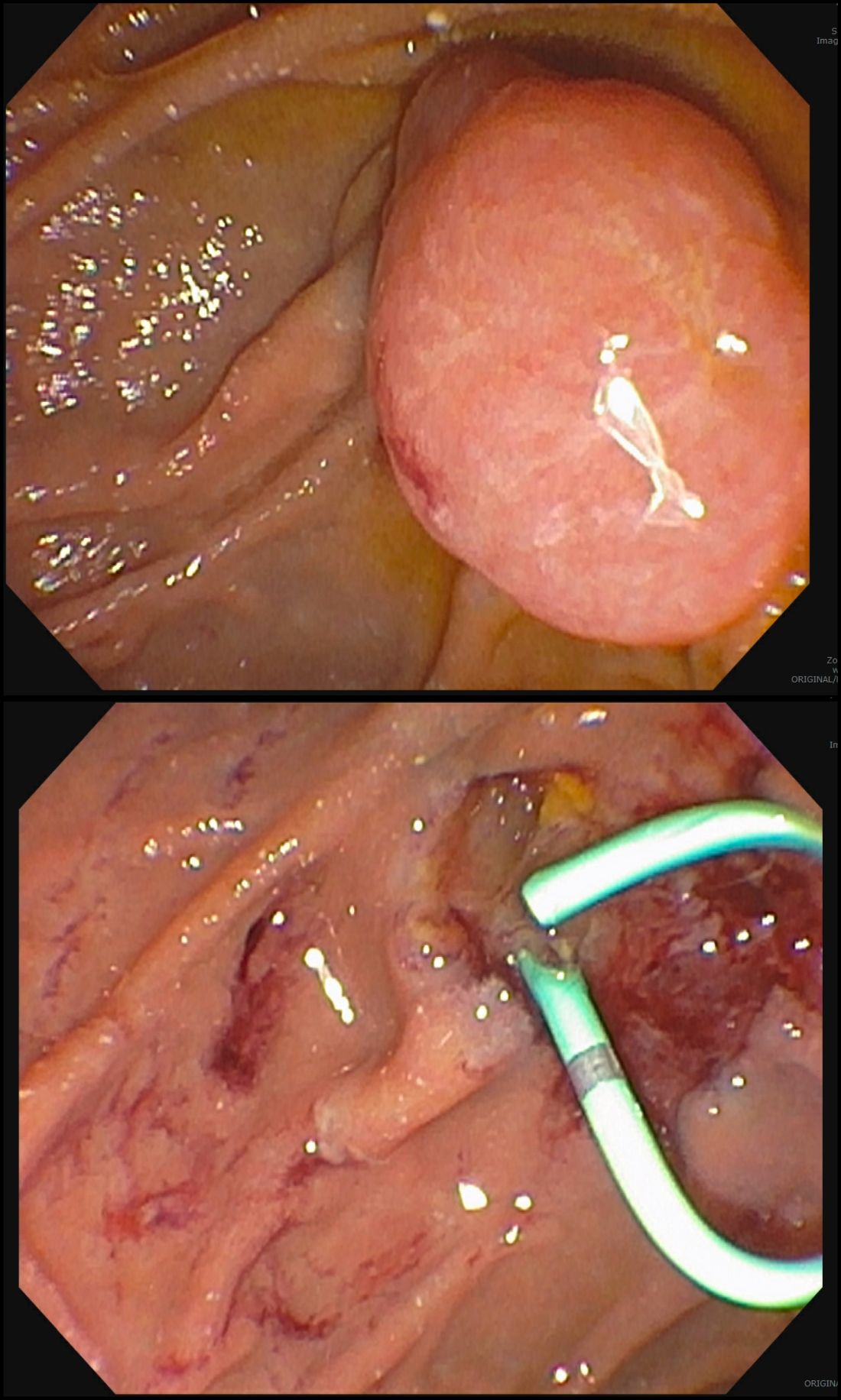
The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.

The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Duodenal polyps are a relatively rare entity with a reported incidence of 0.3%-4.6%.1 There are three major types of duodenal adenomas: sporadic, nonampullary duodenal adenomas (SNDAs), adenomas in familial adenomatous polyposis syndrome, and ampullary adenomas. It is important to distinguish between the different types of duodenal polyps as the management may differ depending on the etiology.
SNDAs constitute <10% of all duodenal polyps, most commonly located in the second portion of the duodenum, and up to 85% have been shown to have malignant transformation over time.2 Most of the studies of SNDAs are small series, and there are no consensus guidelines for management. Villous features increase malignancy risk, thus resection of SNDAs is advised.3-7 It has also been shown that 72% of patients with SNDAs also have colon polyps,8 and therefore these patients should be up to date on colonoscopy screening.
Ampullary adenomas are less common, but up to half may be associated with familial adenomatous polyposis (FAP), and some may be surveyed.9 However, those that are larger than 10 mm or have villous features may raise concern for malignancy with up to half harboring small foci of adenocarcinoma.10,11 These require ERCP with ampullectomy. For the purposes of this paper, we will focus on endoscopic resection of SNDAs and ampullary adenomas.
Endoscopic mucosal resection (EMR) of duodenal polyps can be technically challenging. There are considerations specific to the duodenum: thin muscle layer, increased motility, and significant vascular supply including two major arterial supplies – the gastroduodenal artery from the celiac branch and the inferior pancreaticoduodenal artery from the superior mesenteric artery. These factors may explain higher reported rates of perforation and bleeding compared to colon EMR.
After a detailed inspection is performed to define the duodenal polyp in terms of size, location, and position relative to the ampulla, a submucosal injection is performed using a dye solution. Once adequate lift is achieved, the lesion is resected using stiff monofilament snares. If possible, resection sites are closed with hemostatic clips, although their utility in preventing delayed complications may be less than that in the colon because of increased motility causing them to become dislodged more easily. We avoid using snares larger than 2 cm given increased risk of perforation. Intraprocedural bleeding may be controlled with coagulation graspers on soft coagulation setting; using a bipolar electrocoagulation therapy or argon plasma coagulation is avoided, as these have been shown to increase rates of complications. Figure 1 provides examples of duodenal adenomas that have been resected.

The ampullectomy technique is slightly different from duodenal EMRs and carries the additional risk of pancreatitis.12,13 In our opinion, there is low utility for submucosal injection unless there is a laterally spreading component onto the duodenal wall, as injection of the ampulla itself does not lift well and simply distorts views. Typically, both the common bile duct and the pancreatic duct are injected with contrast, and we typically perform a biliary sphincterotomy prior to ampullectomy. Based on endoscopist preference, one can also leave a guide wire in the pancreatic duct (PD) and pass the snare over it to perform resection to maintain access for a subsequent stent placement. This technique has the advantage of never losing pancreatic duct access, which can occur after resection from edema or bleeding and allows easy PD stent placement. The snare should be opened in a line corresponding to the long axis of the mound with the snare tip anchored above the apex of the papilla and snare opened and drawn down over the papilla. After resection, the PD must be stented to minimize pancreatitis risk.14 Figure 2 shows an ampullary adenoma pre and post EMR, with a PD stent.
Recurrence of duodenal adenomas, both SNDAs and ampullary, can be quite high, with reports up to 39%.15-18 Risk factors include histology and size, but interestingly were not shown to be associated with en-bloc resection.15,19 On the other hand, intraprocedural bleeding also occurs in up to 43% of patients and is associated with size, number of resections, and procedure time.20 Per 2015 ASGE Standards of Practice, duodenal lesions warrant short follow-up at 3- to 6-month intervals given high recurrence rates, then at 6- to 12-month intervals for 2-5 years thereafter.21
When a duodenal polyp is detected, it is important to determine which type of adenoma it is to guide management. There are various techniques utilized to perform duodenal EMR and ampullectomy with some highlighted in this article. It is important to understand how to recognize, prevent, and manage associated adverse events, as well as to have a surveillance plan given the risk of recurrence.
Dr. Kim has no disclosures. Dr. Siddiqui has financial relationships with Boston Scientific (research support, consulting fees, speaking honoraria); Cook, Medtronic, ConMed (consulting fees, speaking honoraria); and Pinnacle Biologic, Ovesco (speaking honoraria).
Dr. Kim is a GI fellow, section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago. Dr. Siddiqui is a professor of medicine and the director of the Center for Endoscopic Research and Therapeutics (CERT), section of gastroenterology, hepatology, and nutrition, department of internal medicine, University of Chicago.
References
1. Jepsen JM et al. Scand J Gastroenterol. Jun 1994;29(6):483-7.
2. Sellner F. Cancer. 1990 Aug 15;66(4):702-15.
3. Witteman BJ et al. Neth J Med. 1993 Feb;42(1-2):5-11.
4. Reddy RR et al. J Clin Gastroenterol. 1981 Jun;3(2):139-47.
5. Sakorafas GH et al. Scand J Gastroenterol. 2000 Apr;35(4):337-44.
6. Galandiuk S et al. Ann Surg. 1988 Mar;207(3):234-9.
7. Farnell MB et al. J Gastrointest Surg. 2000 Jan-Feb;4(1):13-21, discussion 22-3.
8. Apel D et al. Gastrointest Endosc. 2004 Sep;60(3):397-9.
9. Kashiwagi H et al. Lancet. 1994 Dec 3;344(8936):1582.
10. Clary BM et al. Surgery. 2000 Jun;127(6):628-33.
11. Posner S et al. Surgery. 2000 Oct;128(4):694-701.
12. Harewood GC et al. Gastrointest Endosc. 2005 Sep;62(3):367-70.
13. Chini P et al. World J Gastrointest Endosc. 2011 Dec 16;3(12):241-7.
14. Chang WI et al. Gut Liver. May 2014;8(3):306-12.
15. Hoibian S et al. Ann Gastroenterol. 2021;34(2):169-176. doi: 10.20524/aog.2021.0581.
16. Kakushima N et al. World J Gastroenterol. 2014 Sep 21;20(35):12501-8.
17. Lienert A and Bagshaw PF. ANZ J Surg. 2007 May;77(5):371-3.
18. Singh A et al. Gastrointest Endosc. 2016 Oct;84(4):700-8.
19. Tomizawa Y and Ginsberg GG. Gastrointest Endosc. 2018 May;87(5):1270-8.
20. Klein A et al. Gastrointest Endosc. 2016 Oct;84(4):688-96.
21. Chathadi KV et al. Gastrointest Endosc. 2015 Nov;82(5):773-81.
Do myenteric neurons replicate in small intestine?
A new study contradicts controversial findings from a 2017 study that had suggested around two-thirds of myenteric neurons replicate within 1 week under normal conditions, which – if true – would have an impact on research into several GI diseases and pathologies.
Previous research had suggested that enteric nerve cells, which help control peristalsis throughout the digestive tract, do not replicate in the small intestine under normal conditions, with some limited potential for it observed only after injury, wrote Heikki Virtanen, MD, of the University of Helsinki (Finland), and colleagues. Their report is in Cellular and Molecular Gastroenterology and Hepatology. However, a study by Subhash Kulkarni, PhD, published in 2017, “challenged this dogma, suggesting that almost 70% of myenteric neurons are replaced within 1 week under normal physiological conditions.” These findings were reportedly considered controversial and presented “possibly far-reaching impact on future research,” Dr. Virtanen and colleagues explained.
According to the researchers, the difference between the controversial study findings and other research results may be partially explained by differences in methodology such as DNA labeling times, antigen retrieval methods, and analyzed portions of the small intestine. Dr. Virtanen and colleagues initiated the current study because no systematic evaluation of those potential confounding variables or attempt at independently replicating the findings had been undertaken.
For example, Dr. Virtanen and colleagues administered the nucleoside analogue 5-iodo-2’-deoxyuridine (IdU) in drinking water with the same concentration and labeling period, DNA denaturation steps, and antibodies as Dr. Kulkarni’s 2017 study had used. However, they also examined additional areas of the small intestine, employed paraffin embedding, performed parallel analysis using “click chemistry”-based detection of 5-ethynyl-2’-deoxyuridine (EdU), and more.
The gut’s epithelial cells turn over within 1 week “and serve as an internal positive control for DNA replication,” the researchers noted. In this study, IdU-positive enteric nerve cells were not revealed in microscopic analysis of immunohistochemically labeled small intestines of both cryosections and paraffin-embedded sections or in measurement of 300 ganglia in the small intestine. In contrast, the researchers wrote that the epithelium demonstrated label retention.
In their discussion section of their paper, Dr. Virtanen and colleagues wrote that while “proliferating epithelial cells were readily detectable” in the study, they were unable to detect enteric neuronal proliferation. Although noting that they could not identify reasons for the observations by Kulkarni and colleagues, Dr. Virtanen and colleagues continued to suspect unnoticed variables in the 2017 study affected its findings.
“The fact that the repeat of exactly the same experiment with the same reagents and methods did not reproduce the finding, not even partially, supports this interpretation and is further supported by the same conclusion using EdU-based click chemistry data and previous studies.”
The authors disclose no conflicts.
The enteric nervous system (ENS) is composed of neurons and glia along the GI tract that are responsible for coordinating its motility, absorption, secretion, and other essential functions. While new neurons are formed during gut development, enteric neurogenesis in adult animals has been a subject of controversy but is of fundamental importance to understanding ENS biology and pathophysiology.
To settle the debate, Virtanen et al. replicated the Kulkarni study using the same methods, with the addition of EdU-based click chemistry, and found no replicating neurons. The bulk of evidence thus supports the concept that enteric neurons in the adult gut are a stable population that undergo minimal turnover. Enteric neuronal progenitors, however, are present in the adult gut and can undergo neurogenesis in response to injury. Further research is needed to identify the signals that activate that neurogenic response and to understand how it can be leveraged to treat neurointestinal diseases.
Allan M. Goldstein, MD, is chief of pediatric surgery at Massachusetts General Hospital, professor of surgery at Harvard Medical School, principal investigator in the Pediatric Surgery Research Laboratories, and codirector of the Massachusetts General Center for Neurointestinal Health, all in Boston. He has no relevant conflicts.
The enteric nervous system (ENS) is composed of neurons and glia along the GI tract that are responsible for coordinating its motility, absorption, secretion, and other essential functions. While new neurons are formed during gut development, enteric neurogenesis in adult animals has been a subject of controversy but is of fundamental importance to understanding ENS biology and pathophysiology.
To settle the debate, Virtanen et al. replicated the Kulkarni study using the same methods, with the addition of EdU-based click chemistry, and found no replicating neurons. The bulk of evidence thus supports the concept that enteric neurons in the adult gut are a stable population that undergo minimal turnover. Enteric neuronal progenitors, however, are present in the adult gut and can undergo neurogenesis in response to injury. Further research is needed to identify the signals that activate that neurogenic response and to understand how it can be leveraged to treat neurointestinal diseases.
Allan M. Goldstein, MD, is chief of pediatric surgery at Massachusetts General Hospital, professor of surgery at Harvard Medical School, principal investigator in the Pediatric Surgery Research Laboratories, and codirector of the Massachusetts General Center for Neurointestinal Health, all in Boston. He has no relevant conflicts.
The enteric nervous system (ENS) is composed of neurons and glia along the GI tract that are responsible for coordinating its motility, absorption, secretion, and other essential functions. While new neurons are formed during gut development, enteric neurogenesis in adult animals has been a subject of controversy but is of fundamental importance to understanding ENS biology and pathophysiology.
To settle the debate, Virtanen et al. replicated the Kulkarni study using the same methods, with the addition of EdU-based click chemistry, and found no replicating neurons. The bulk of evidence thus supports the concept that enteric neurons in the adult gut are a stable population that undergo minimal turnover. Enteric neuronal progenitors, however, are present in the adult gut and can undergo neurogenesis in response to injury. Further research is needed to identify the signals that activate that neurogenic response and to understand how it can be leveraged to treat neurointestinal diseases.
Allan M. Goldstein, MD, is chief of pediatric surgery at Massachusetts General Hospital, professor of surgery at Harvard Medical School, principal investigator in the Pediatric Surgery Research Laboratories, and codirector of the Massachusetts General Center for Neurointestinal Health, all in Boston. He has no relevant conflicts.
A new study contradicts controversial findings from a 2017 study that had suggested around two-thirds of myenteric neurons replicate within 1 week under normal conditions, which – if true – would have an impact on research into several GI diseases and pathologies.
Previous research had suggested that enteric nerve cells, which help control peristalsis throughout the digestive tract, do not replicate in the small intestine under normal conditions, with some limited potential for it observed only after injury, wrote Heikki Virtanen, MD, of the University of Helsinki (Finland), and colleagues. Their report is in Cellular and Molecular Gastroenterology and Hepatology. However, a study by Subhash Kulkarni, PhD, published in 2017, “challenged this dogma, suggesting that almost 70% of myenteric neurons are replaced within 1 week under normal physiological conditions.” These findings were reportedly considered controversial and presented “possibly far-reaching impact on future research,” Dr. Virtanen and colleagues explained.
According to the researchers, the difference between the controversial study findings and other research results may be partially explained by differences in methodology such as DNA labeling times, antigen retrieval methods, and analyzed portions of the small intestine. Dr. Virtanen and colleagues initiated the current study because no systematic evaluation of those potential confounding variables or attempt at independently replicating the findings had been undertaken.
For example, Dr. Virtanen and colleagues administered the nucleoside analogue 5-iodo-2’-deoxyuridine (IdU) in drinking water with the same concentration and labeling period, DNA denaturation steps, and antibodies as Dr. Kulkarni’s 2017 study had used. However, they also examined additional areas of the small intestine, employed paraffin embedding, performed parallel analysis using “click chemistry”-based detection of 5-ethynyl-2’-deoxyuridine (EdU), and more.
The gut’s epithelial cells turn over within 1 week “and serve as an internal positive control for DNA replication,” the researchers noted. In this study, IdU-positive enteric nerve cells were not revealed in microscopic analysis of immunohistochemically labeled small intestines of both cryosections and paraffin-embedded sections or in measurement of 300 ganglia in the small intestine. In contrast, the researchers wrote that the epithelium demonstrated label retention.
In their discussion section of their paper, Dr. Virtanen and colleagues wrote that while “proliferating epithelial cells were readily detectable” in the study, they were unable to detect enteric neuronal proliferation. Although noting that they could not identify reasons for the observations by Kulkarni and colleagues, Dr. Virtanen and colleagues continued to suspect unnoticed variables in the 2017 study affected its findings.
“The fact that the repeat of exactly the same experiment with the same reagents and methods did not reproduce the finding, not even partially, supports this interpretation and is further supported by the same conclusion using EdU-based click chemistry data and previous studies.”
The authors disclose no conflicts.
A new study contradicts controversial findings from a 2017 study that had suggested around two-thirds of myenteric neurons replicate within 1 week under normal conditions, which – if true – would have an impact on research into several GI diseases and pathologies.
Previous research had suggested that enteric nerve cells, which help control peristalsis throughout the digestive tract, do not replicate in the small intestine under normal conditions, with some limited potential for it observed only after injury, wrote Heikki Virtanen, MD, of the University of Helsinki (Finland), and colleagues. Their report is in Cellular and Molecular Gastroenterology and Hepatology. However, a study by Subhash Kulkarni, PhD, published in 2017, “challenged this dogma, suggesting that almost 70% of myenteric neurons are replaced within 1 week under normal physiological conditions.” These findings were reportedly considered controversial and presented “possibly far-reaching impact on future research,” Dr. Virtanen and colleagues explained.
According to the researchers, the difference between the controversial study findings and other research results may be partially explained by differences in methodology such as DNA labeling times, antigen retrieval methods, and analyzed portions of the small intestine. Dr. Virtanen and colleagues initiated the current study because no systematic evaluation of those potential confounding variables or attempt at independently replicating the findings had been undertaken.
For example, Dr. Virtanen and colleagues administered the nucleoside analogue 5-iodo-2’-deoxyuridine (IdU) in drinking water with the same concentration and labeling period, DNA denaturation steps, and antibodies as Dr. Kulkarni’s 2017 study had used. However, they also examined additional areas of the small intestine, employed paraffin embedding, performed parallel analysis using “click chemistry”-based detection of 5-ethynyl-2’-deoxyuridine (EdU), and more.
The gut’s epithelial cells turn over within 1 week “and serve as an internal positive control for DNA replication,” the researchers noted. In this study, IdU-positive enteric nerve cells were not revealed in microscopic analysis of immunohistochemically labeled small intestines of both cryosections and paraffin-embedded sections or in measurement of 300 ganglia in the small intestine. In contrast, the researchers wrote that the epithelium demonstrated label retention.
In their discussion section of their paper, Dr. Virtanen and colleagues wrote that while “proliferating epithelial cells were readily detectable” in the study, they were unable to detect enteric neuronal proliferation. Although noting that they could not identify reasons for the observations by Kulkarni and colleagues, Dr. Virtanen and colleagues continued to suspect unnoticed variables in the 2017 study affected its findings.
“The fact that the repeat of exactly the same experiment with the same reagents and methods did not reproduce the finding, not even partially, supports this interpretation and is further supported by the same conclusion using EdU-based click chemistry data and previous studies.”
The authors disclose no conflicts.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Psychological intervention looks promising in Crohn’s disease
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.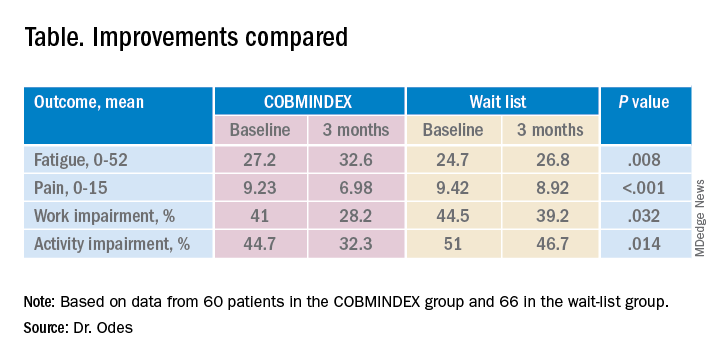
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
AT DDW 2022
Eosinophilic diseases often overlap, raising costs
Eosinophilic GI diseases (EGIDs) often overlap with other eosinophil-associated diseases (EADs), which leads to greater health care costs, according to an analysis of the U.S. Optum Clinformatics claims database.
EADs have gained increased attention in recent years. They include eosinophilic esophagitis (EoE), eosinophilic asthma, bullous pemphigoid, eosinophilic granulomatosis with polyangiitis, eosinophilic gastritis/gastroenteritis (EG/EGE), and a subset of non–cystic fibrosis bronchiectasis. All involve infiltration of eosinophils, but the exact immune mechanisms behind them seem to vary and are poorly understood, according to Justin Kwiatek, PharmD, who presented the results at the annual Digestive Disease Week® (DDW).
“We do know that the suitable course of treatment is dependent on the organs impacted. From this study, we also know that EoE mostly exists on its own, with only a small portion also being diagnosed with asthma, while overlap with other EGIDs tends to be higher. This could be because EoE appears to be pathologically different from other EGIDs in the gastrointestinal tract such as eosinophilic gastritis in the stomach or eosinophilic gastroenteritis in the stomach and small bowel. Eosinophils are not normally present in the esophagus but are often found in the stomach or small bowel without inflammation,” said Dr. Kwiatek, who is senior global medical affairs leader, respiratory & immunology, at AstraZeneca.
The study is important, said Dhyanesh Patel, MD, who was asked to comment on the study. “There’s been a lot of interest in eosinophilic gastrointestinal diseases recently because there is lack of a clear definition. We need to define it better because we need to figure out treatment options for the patients,” said Dr. Patel, who is an assistant professor of medicine at Vanderbilt University, Nashville, Tenn.
“It highlights that a lot of the patients that have one eosinophilic disease might have other concomitant atopic diseases. [It may be that] you can use one drug to treat all of them together, so I think it’s important to have a multidisciplinary approach where you work with an allergist and you work with an immunologist and treat their eosinophilic gastritis and their asthma together with one drug. That may help reduce medication burden,” said Dr. Patel.
The researchers analyzed records from 1,326,645 diagnosed patients with at least one EAD and at least 2 years following treatment. There were 13,872 patients with EoE, 38.4% of whom had at least one overlapping EAD. Of 1,365 patients with EG/EGE, 57.9% had at least one overlapping EAD.
EADs were associated with higher Charlson Comorbidity Index scores and high blood eosinophil levels (≥ 300 cells/mcL) among EoE patients, but not among EG/EGE patients. Within the EoE group, female gender was linked to more EAD comorbidities: 35% of patients with only EoE were female; 45% of patients with one comorbidity were female, as were 55% of those with two comorbidities and 57% of those with three or more comorbidities. There was no such trend among patients with EG/EGE.
Total health care costs were lower in the absence of one overlapping EAD among both EoE ($2,061 vs. $3,766 per patient per month) and EG/EGE patients ($2,860 vs. $4,053). Costs went up with more overlap: $8,572 for EoE and three or more other EADs, and $10,397 for EG/EGE and three or more other EADs. These costs were largely driven by outpatient care.
“The data shows that patients with eosinophilic gastritis and eosinophilic gastroenteritis are more likely to have overlapping eosinophilic conditions, such as asthma. When diagnosing a patient with EG or EGE, it’s important to monitor any new symptoms closely and to educate them about the risk factors. This is particularly true for patients with elevated blood eosinophil counts. Accounting for comorbidities and establishing a treatment plan early can help to manage the higher health care spend for patients with overlapping conditions,” said Dr. Kwiatek.
Dr. Kwiatek is an employee and stockholder of AstraZeneca, which funded the study and developed benralizumab, a drug that has been granted orphan drug status for EG/EGE and EoE. Optum Clinformatics is a longitudinal database of deidentified data formed by UnitedHealth Group. Dr. Patel has no relevant financial disclosures.
Eosinophilic GI diseases (EGIDs) often overlap with other eosinophil-associated diseases (EADs), which leads to greater health care costs, according to an analysis of the U.S. Optum Clinformatics claims database.
EADs have gained increased attention in recent years. They include eosinophilic esophagitis (EoE), eosinophilic asthma, bullous pemphigoid, eosinophilic granulomatosis with polyangiitis, eosinophilic gastritis/gastroenteritis (EG/EGE), and a subset of non–cystic fibrosis bronchiectasis. All involve infiltration of eosinophils, but the exact immune mechanisms behind them seem to vary and are poorly understood, according to Justin Kwiatek, PharmD, who presented the results at the annual Digestive Disease Week® (DDW).
“We do know that the suitable course of treatment is dependent on the organs impacted. From this study, we also know that EoE mostly exists on its own, with only a small portion also being diagnosed with asthma, while overlap with other EGIDs tends to be higher. This could be because EoE appears to be pathologically different from other EGIDs in the gastrointestinal tract such as eosinophilic gastritis in the stomach or eosinophilic gastroenteritis in the stomach and small bowel. Eosinophils are not normally present in the esophagus but are often found in the stomach or small bowel without inflammation,” said Dr. Kwiatek, who is senior global medical affairs leader, respiratory & immunology, at AstraZeneca.
The study is important, said Dhyanesh Patel, MD, who was asked to comment on the study. “There’s been a lot of interest in eosinophilic gastrointestinal diseases recently because there is lack of a clear definition. We need to define it better because we need to figure out treatment options for the patients,” said Dr. Patel, who is an assistant professor of medicine at Vanderbilt University, Nashville, Tenn.
“It highlights that a lot of the patients that have one eosinophilic disease might have other concomitant atopic diseases. [It may be that] you can use one drug to treat all of them together, so I think it’s important to have a multidisciplinary approach where you work with an allergist and you work with an immunologist and treat their eosinophilic gastritis and their asthma together with one drug. That may help reduce medication burden,” said Dr. Patel.
The researchers analyzed records from 1,326,645 diagnosed patients with at least one EAD and at least 2 years following treatment. There were 13,872 patients with EoE, 38.4% of whom had at least one overlapping EAD. Of 1,365 patients with EG/EGE, 57.9% had at least one overlapping EAD.
EADs were associated with higher Charlson Comorbidity Index scores and high blood eosinophil levels (≥ 300 cells/mcL) among EoE patients, but not among EG/EGE patients. Within the EoE group, female gender was linked to more EAD comorbidities: 35% of patients with only EoE were female; 45% of patients with one comorbidity were female, as were 55% of those with two comorbidities and 57% of those with three or more comorbidities. There was no such trend among patients with EG/EGE.
Total health care costs were lower in the absence of one overlapping EAD among both EoE ($2,061 vs. $3,766 per patient per month) and EG/EGE patients ($2,860 vs. $4,053). Costs went up with more overlap: $8,572 for EoE and three or more other EADs, and $10,397 for EG/EGE and three or more other EADs. These costs were largely driven by outpatient care.
“The data shows that patients with eosinophilic gastritis and eosinophilic gastroenteritis are more likely to have overlapping eosinophilic conditions, such as asthma. When diagnosing a patient with EG or EGE, it’s important to monitor any new symptoms closely and to educate them about the risk factors. This is particularly true for patients with elevated blood eosinophil counts. Accounting for comorbidities and establishing a treatment plan early can help to manage the higher health care spend for patients with overlapping conditions,” said Dr. Kwiatek.
Dr. Kwiatek is an employee and stockholder of AstraZeneca, which funded the study and developed benralizumab, a drug that has been granted orphan drug status for EG/EGE and EoE. Optum Clinformatics is a longitudinal database of deidentified data formed by UnitedHealth Group. Dr. Patel has no relevant financial disclosures.
Eosinophilic GI diseases (EGIDs) often overlap with other eosinophil-associated diseases (EADs), which leads to greater health care costs, according to an analysis of the U.S. Optum Clinformatics claims database.
EADs have gained increased attention in recent years. They include eosinophilic esophagitis (EoE), eosinophilic asthma, bullous pemphigoid, eosinophilic granulomatosis with polyangiitis, eosinophilic gastritis/gastroenteritis (EG/EGE), and a subset of non–cystic fibrosis bronchiectasis. All involve infiltration of eosinophils, but the exact immune mechanisms behind them seem to vary and are poorly understood, according to Justin Kwiatek, PharmD, who presented the results at the annual Digestive Disease Week® (DDW).
“We do know that the suitable course of treatment is dependent on the organs impacted. From this study, we also know that EoE mostly exists on its own, with only a small portion also being diagnosed with asthma, while overlap with other EGIDs tends to be higher. This could be because EoE appears to be pathologically different from other EGIDs in the gastrointestinal tract such as eosinophilic gastritis in the stomach or eosinophilic gastroenteritis in the stomach and small bowel. Eosinophils are not normally present in the esophagus but are often found in the stomach or small bowel without inflammation,” said Dr. Kwiatek, who is senior global medical affairs leader, respiratory & immunology, at AstraZeneca.
The study is important, said Dhyanesh Patel, MD, who was asked to comment on the study. “There’s been a lot of interest in eosinophilic gastrointestinal diseases recently because there is lack of a clear definition. We need to define it better because we need to figure out treatment options for the patients,” said Dr. Patel, who is an assistant professor of medicine at Vanderbilt University, Nashville, Tenn.
“It highlights that a lot of the patients that have one eosinophilic disease might have other concomitant atopic diseases. [It may be that] you can use one drug to treat all of them together, so I think it’s important to have a multidisciplinary approach where you work with an allergist and you work with an immunologist and treat their eosinophilic gastritis and their asthma together with one drug. That may help reduce medication burden,” said Dr. Patel.
The researchers analyzed records from 1,326,645 diagnosed patients with at least one EAD and at least 2 years following treatment. There were 13,872 patients with EoE, 38.4% of whom had at least one overlapping EAD. Of 1,365 patients with EG/EGE, 57.9% had at least one overlapping EAD.
EADs were associated with higher Charlson Comorbidity Index scores and high blood eosinophil levels (≥ 300 cells/mcL) among EoE patients, but not among EG/EGE patients. Within the EoE group, female gender was linked to more EAD comorbidities: 35% of patients with only EoE were female; 45% of patients with one comorbidity were female, as were 55% of those with two comorbidities and 57% of those with three or more comorbidities. There was no such trend among patients with EG/EGE.
Total health care costs were lower in the absence of one overlapping EAD among both EoE ($2,061 vs. $3,766 per patient per month) and EG/EGE patients ($2,860 vs. $4,053). Costs went up with more overlap: $8,572 for EoE and three or more other EADs, and $10,397 for EG/EGE and three or more other EADs. These costs were largely driven by outpatient care.
“The data shows that patients with eosinophilic gastritis and eosinophilic gastroenteritis are more likely to have overlapping eosinophilic conditions, such as asthma. When diagnosing a patient with EG or EGE, it’s important to monitor any new symptoms closely and to educate them about the risk factors. This is particularly true for patients with elevated blood eosinophil counts. Accounting for comorbidities and establishing a treatment plan early can help to manage the higher health care spend for patients with overlapping conditions,” said Dr. Kwiatek.
Dr. Kwiatek is an employee and stockholder of AstraZeneca, which funded the study and developed benralizumab, a drug that has been granted orphan drug status for EG/EGE and EoE. Optum Clinformatics is a longitudinal database of deidentified data formed by UnitedHealth Group. Dr. Patel has no relevant financial disclosures.
FROM DDW 2022
H. pylori antibiotics briefly disrupt gut microbiome
SAN DIEGO – Treatments to eradicate Helicobacter pylori (H. pylori) infections do increase the antibiotic resistance of the gut microbiota, but for only a few months, researchers reported at Digestive Disease Week® (DDW).
The finding applies similarly to levofloxacin quadruple therapy and bismuth quadruple therapy, both of which are equally efficacious as second-line treatments, said Jyh-Ming Liou, MD, PhD, clinical professor of internal medicine at National Taiwan University in Taipei.
This provides some reassurance that increased use of antibiotics to treat these infections won’t cause long-term disruptions to the patients’ microbiomes, said Dr. Liou.
“Maybe if we have indications for antibiotic treatment, then we don’t worry about the emergence of resistance in our bodies,” he said. “But the accumulation of antibodies in the environment may induce bacteria to mutate, so maybe we still need cautious use of antibiotics.”
H. pylori infections are becoming harder to treat as more strains develop resistance to antibiotics, leading physicians to use regimens with multiple agents. This in turn has raised concerns that gut microbiota could be disrupted, with pathogens potentially developing their own resistance.
To explore these risks, Dr. Liou and colleagues recruited adults whose H. pylori infections were not successfully eradicated.
They randomly assigned 280 patients each to one of two second-line therapies, levofloxacin quadruple or bismuth quadruple. At baseline, the researchers could not find any statistically significant differences in the two groups’ demographics, cigarette and alcohol use, or ulcers, as well as antibiotic resistance in patients’ microbiome between the groups.
Levofloxacin quadruple therapy consisted of esomeprazole 40 mg and amoxicillin 1 g for the first 7 days, followed by esomeprazole 40 mg, metronidazole 500 mg, and levofloxacin 250 mg for another 7 days (all twice daily).
Bismuth quadruple therapy consisted of esomeprazole 40 mg twice daily, bismuth tripotassium dicitrate 300 mg four times a day, tetracycline 500 mg four times a day, and metronidazole 500 mg three times a day, for 10 days.
The researchers collected stool samples at baseline, week 2, week 8, and 1 year after eradication therapy and analyzed them for microbiota diversity and antibiotic susceptibility.
The H. pylori eradication rates were almost the same in the two second-line therapies: 87.9% for levofloxacin quadruple and 87.5% for bismuth quadruple. When they were used as third-line (rescue) therapies, the success rates were also statistically the same, and the cumulative second-line and third-line eradication rate was 95.6% for levofloxacin quadruple and 96.6% for bismuth quadruple.
The two treatments did differ in adverse events with 48.4% for levofloxacin quadruple and 77.3% for bismuth quadruple, which was statistically significant (P < .0001).
After a year, H. pylori reinfected 2.5% of the levofloxacin group and 3% of the bismuth quadruple group.
The researchers used metagenomic sequencing to examine the bacteria in the patients’ microbiome for antibiotic resistance. Using 16S rRNA sequencing, they found that the proportion of genera and species with significant changes in abundance at 2 weeks after treatment compared with baseline was 52.4% for levofloxacin quadruple therapy versus 45.1% for bismuth quadruple therapy.
However, 8 weeks after treatment, the proportion with significant changes had dropped to 5.8% for the levofloxacin group and 21.5% for the bismuth group. And at the end of a year, they had further dropped to 0.9% for the levofloxacin group and 8.4% for the bismuth group.
“It was generally reassuring that, even after giving these combinations of different antibiotics, eventually it doesn’t seem to affect the resistance pattern in bacteria lower down in the gut,” said session moderator Steven Moss, MD, professor of medicine at Brown University in Providence, R.I.
Still, continuing to pile on more and more antibiotics to treat H. pylori infections won’t work forever because H. pylori strains are themselves developing resistance so rapidly, he said. “We’re certainly going to have worse eradications in the future unless we can come up with new tricks.”
A hopeful development are new techniques to test H. pylori for resistance to specific antibiotics before initiating treatment, said Dr. Moss.
Dr. Moss consults with companies developing H. pylori therapies and diagnostics. Dr. Liou reported no relevant financial interests.
SAN DIEGO – Treatments to eradicate Helicobacter pylori (H. pylori) infections do increase the antibiotic resistance of the gut microbiota, but for only a few months, researchers reported at Digestive Disease Week® (DDW).
The finding applies similarly to levofloxacin quadruple therapy and bismuth quadruple therapy, both of which are equally efficacious as second-line treatments, said Jyh-Ming Liou, MD, PhD, clinical professor of internal medicine at National Taiwan University in Taipei.
This provides some reassurance that increased use of antibiotics to treat these infections won’t cause long-term disruptions to the patients’ microbiomes, said Dr. Liou.
“Maybe if we have indications for antibiotic treatment, then we don’t worry about the emergence of resistance in our bodies,” he said. “But the accumulation of antibodies in the environment may induce bacteria to mutate, so maybe we still need cautious use of antibiotics.”
H. pylori infections are becoming harder to treat as more strains develop resistance to antibiotics, leading physicians to use regimens with multiple agents. This in turn has raised concerns that gut microbiota could be disrupted, with pathogens potentially developing their own resistance.
To explore these risks, Dr. Liou and colleagues recruited adults whose H. pylori infections were not successfully eradicated.
They randomly assigned 280 patients each to one of two second-line therapies, levofloxacin quadruple or bismuth quadruple. At baseline, the researchers could not find any statistically significant differences in the two groups’ demographics, cigarette and alcohol use, or ulcers, as well as antibiotic resistance in patients’ microbiome between the groups.
Levofloxacin quadruple therapy consisted of esomeprazole 40 mg and amoxicillin 1 g for the first 7 days, followed by esomeprazole 40 mg, metronidazole 500 mg, and levofloxacin 250 mg for another 7 days (all twice daily).
Bismuth quadruple therapy consisted of esomeprazole 40 mg twice daily, bismuth tripotassium dicitrate 300 mg four times a day, tetracycline 500 mg four times a day, and metronidazole 500 mg three times a day, for 10 days.
The researchers collected stool samples at baseline, week 2, week 8, and 1 year after eradication therapy and analyzed them for microbiota diversity and antibiotic susceptibility.
The H. pylori eradication rates were almost the same in the two second-line therapies: 87.9% for levofloxacin quadruple and 87.5% for bismuth quadruple. When they were used as third-line (rescue) therapies, the success rates were also statistically the same, and the cumulative second-line and third-line eradication rate was 95.6% for levofloxacin quadruple and 96.6% for bismuth quadruple.
The two treatments did differ in adverse events with 48.4% for levofloxacin quadruple and 77.3% for bismuth quadruple, which was statistically significant (P < .0001).
After a year, H. pylori reinfected 2.5% of the levofloxacin group and 3% of the bismuth quadruple group.
The researchers used metagenomic sequencing to examine the bacteria in the patients’ microbiome for antibiotic resistance. Using 16S rRNA sequencing, they found that the proportion of genera and species with significant changes in abundance at 2 weeks after treatment compared with baseline was 52.4% for levofloxacin quadruple therapy versus 45.1% for bismuth quadruple therapy.
However, 8 weeks after treatment, the proportion with significant changes had dropped to 5.8% for the levofloxacin group and 21.5% for the bismuth group. And at the end of a year, they had further dropped to 0.9% for the levofloxacin group and 8.4% for the bismuth group.
“It was generally reassuring that, even after giving these combinations of different antibiotics, eventually it doesn’t seem to affect the resistance pattern in bacteria lower down in the gut,” said session moderator Steven Moss, MD, professor of medicine at Brown University in Providence, R.I.
Still, continuing to pile on more and more antibiotics to treat H. pylori infections won’t work forever because H. pylori strains are themselves developing resistance so rapidly, he said. “We’re certainly going to have worse eradications in the future unless we can come up with new tricks.”
A hopeful development are new techniques to test H. pylori for resistance to specific antibiotics before initiating treatment, said Dr. Moss.
Dr. Moss consults with companies developing H. pylori therapies and diagnostics. Dr. Liou reported no relevant financial interests.
SAN DIEGO – Treatments to eradicate Helicobacter pylori (H. pylori) infections do increase the antibiotic resistance of the gut microbiota, but for only a few months, researchers reported at Digestive Disease Week® (DDW).
The finding applies similarly to levofloxacin quadruple therapy and bismuth quadruple therapy, both of which are equally efficacious as second-line treatments, said Jyh-Ming Liou, MD, PhD, clinical professor of internal medicine at National Taiwan University in Taipei.
This provides some reassurance that increased use of antibiotics to treat these infections won’t cause long-term disruptions to the patients’ microbiomes, said Dr. Liou.
“Maybe if we have indications for antibiotic treatment, then we don’t worry about the emergence of resistance in our bodies,” he said. “But the accumulation of antibodies in the environment may induce bacteria to mutate, so maybe we still need cautious use of antibiotics.”
H. pylori infections are becoming harder to treat as more strains develop resistance to antibiotics, leading physicians to use regimens with multiple agents. This in turn has raised concerns that gut microbiota could be disrupted, with pathogens potentially developing their own resistance.
To explore these risks, Dr. Liou and colleagues recruited adults whose H. pylori infections were not successfully eradicated.
They randomly assigned 280 patients each to one of two second-line therapies, levofloxacin quadruple or bismuth quadruple. At baseline, the researchers could not find any statistically significant differences in the two groups’ demographics, cigarette and alcohol use, or ulcers, as well as antibiotic resistance in patients’ microbiome between the groups.
Levofloxacin quadruple therapy consisted of esomeprazole 40 mg and amoxicillin 1 g for the first 7 days, followed by esomeprazole 40 mg, metronidazole 500 mg, and levofloxacin 250 mg for another 7 days (all twice daily).
Bismuth quadruple therapy consisted of esomeprazole 40 mg twice daily, bismuth tripotassium dicitrate 300 mg four times a day, tetracycline 500 mg four times a day, and metronidazole 500 mg three times a day, for 10 days.
The researchers collected stool samples at baseline, week 2, week 8, and 1 year after eradication therapy and analyzed them for microbiota diversity and antibiotic susceptibility.
The H. pylori eradication rates were almost the same in the two second-line therapies: 87.9% for levofloxacin quadruple and 87.5% for bismuth quadruple. When they were used as third-line (rescue) therapies, the success rates were also statistically the same, and the cumulative second-line and third-line eradication rate was 95.6% for levofloxacin quadruple and 96.6% for bismuth quadruple.
The two treatments did differ in adverse events with 48.4% for levofloxacin quadruple and 77.3% for bismuth quadruple, which was statistically significant (P < .0001).
After a year, H. pylori reinfected 2.5% of the levofloxacin group and 3% of the bismuth quadruple group.
The researchers used metagenomic sequencing to examine the bacteria in the patients’ microbiome for antibiotic resistance. Using 16S rRNA sequencing, they found that the proportion of genera and species with significant changes in abundance at 2 weeks after treatment compared with baseline was 52.4% for levofloxacin quadruple therapy versus 45.1% for bismuth quadruple therapy.
However, 8 weeks after treatment, the proportion with significant changes had dropped to 5.8% for the levofloxacin group and 21.5% for the bismuth group. And at the end of a year, they had further dropped to 0.9% for the levofloxacin group and 8.4% for the bismuth group.
“It was generally reassuring that, even after giving these combinations of different antibiotics, eventually it doesn’t seem to affect the resistance pattern in bacteria lower down in the gut,” said session moderator Steven Moss, MD, professor of medicine at Brown University in Providence, R.I.
Still, continuing to pile on more and more antibiotics to treat H. pylori infections won’t work forever because H. pylori strains are themselves developing resistance so rapidly, he said. “We’re certainly going to have worse eradications in the future unless we can come up with new tricks.”
A hopeful development are new techniques to test H. pylori for resistance to specific antibiotics before initiating treatment, said Dr. Moss.
Dr. Moss consults with companies developing H. pylori therapies and diagnostics. Dr. Liou reported no relevant financial interests.
AT DDW 2022








