User login
AGA Clinical Practice Update: Expert review of dietary options for IBS
The American Gastroenterological Association has published a clinical practice update on dietary interventions for patients with irritable bowel syndrome (IBS). The topics range from identification of suitable candidates for dietary interventions, to levels of evidence for specific diets, which are becoming increasingly recognized for their key role in managing patients with IBS, according to lead author William D. Chey, MD, of the University of Michigan, Ann Arbor, and colleagues.
“Most medical therapies for IBS improve global symptoms in fewer than one-half of patients, with a therapeutic gain of 7%-15% over placebo,” the researchers wrote in Gastroenterology. “Most patients with IBS associate their GI symptoms with eating food.”
According to Dr. Chey and colleagues, clinicians who are considering dietary modifications for treating IBS should first recognize the inherent challenges presented by this process and be aware that new diets won’t work for everyone.
“Specialty diets require planning and preparation, which may be impractical for some patients,” they wrote, noting that individuals with “decreased cognitive abilities and significant psychiatric disease” may be unable to follow diets or interpret their own responses to specific foods. Special diets may also be inappropriate for patients with financial constraints, and “should be avoided in patients with an eating disorder.”
Because of the challenges involved in dietary interventions, Dr. Chey and colleagues advised clinical support from a registered dietitian nutritionist or other resource.
Patients who are suitable candidates for intervention and willing to try a new diet should first provide information about their current eating habits. A food trial can then be personalized and implemented for a predetermined amount of time. If the patient does not respond, then the diet should be stopped and changed to a new diet or another intervention.
Dr. Chey and colleagues discussed three specific dietary interventions and their corresponding levels of evidence: soluble fiber; the low-fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet; and a gluten-free diet.
“Soluble fiber is efficacious in treating global symptoms of IBS,” they wrote, citing 15 randomized controlled trials. Soluble fiber is most suitable for patients with constipation-predominant IBS, and different soluble fibers may yield different outcomes based on characteristics such as rate of fermentation and viscosity. In contrast, insoluble fiber is unlikely to help with IBS, and may worsen abdominal pain and bloating.
The low-FODMAP diet is “currently the most evidence-based diet intervention for IBS,” especially for patients with diarrhea-predominant IBS. Dr. Chey and colleagues offered a clear roadmap for employing the diet. First, patients should eat only low-FODMAP foods for 4-6 weeks. If symptoms don’t improve, the diet should be stopped. If symptoms do improve, foods containing a single FODMAP should be reintroduced one at a time, each in increasing quantities over 3 days, alongside documentation of symptom responses. Finally, the diet should be personalized based on these responses. The majority of patients (close to 80%) “can liberalize” a low-FODMAP diet based on their responses.
In contrast with the low-FODMAP diet, which has a relatively solid body of supporting evidence, efficacy data are still limited for treating IBS with a gluten-free diet. “Although observational studies found that most patients with IBS improve with a gluten-free diet, randomized controlled trials have yielded mixed results,” Dr. Chey and colleagues explained.
Their report cited a recent monograph on the topic that concluded that gluten-free eating offered no significant benefit over placebo (relative risk, 0.46; 95% confidence interval, 0.16-1.28). While some studies have documented positive results with a gluten-free diet, Dr. Chey and colleagues suggested that confounding variables such as the nocebo effect and the impact of other dietary factors have yet to be ruled out. “At present, it remains unclear whether a gluten-free diet is of benefit to patients with IBS.”
Dr. Chey and colleagues also explored IBS biomarkers. While some early data have shown that biomarkers may predict dietary responses, “there is insufficient evidence to support their routine use in clinical practice. ... Further efforts to identify and validate biomarkers that predict response to dietary interventions are needed to deliver ‘personalized nutrition.’ ”
The clinical practice update was commissioned and approved by the AGA CPU Committee and the AGA Governing Board. The researchers disclosed relationships with Biomerica, Salix, Mauna Kea Technologies, and others.
This article was updated May 19, 2022.
The American Gastroenterological Association has published a clinical practice update on dietary interventions for patients with irritable bowel syndrome (IBS). The topics range from identification of suitable candidates for dietary interventions, to levels of evidence for specific diets, which are becoming increasingly recognized for their key role in managing patients with IBS, according to lead author William D. Chey, MD, of the University of Michigan, Ann Arbor, and colleagues.
“Most medical therapies for IBS improve global symptoms in fewer than one-half of patients, with a therapeutic gain of 7%-15% over placebo,” the researchers wrote in Gastroenterology. “Most patients with IBS associate their GI symptoms with eating food.”
According to Dr. Chey and colleagues, clinicians who are considering dietary modifications for treating IBS should first recognize the inherent challenges presented by this process and be aware that new diets won’t work for everyone.
“Specialty diets require planning and preparation, which may be impractical for some patients,” they wrote, noting that individuals with “decreased cognitive abilities and significant psychiatric disease” may be unable to follow diets or interpret their own responses to specific foods. Special diets may also be inappropriate for patients with financial constraints, and “should be avoided in patients with an eating disorder.”
Because of the challenges involved in dietary interventions, Dr. Chey and colleagues advised clinical support from a registered dietitian nutritionist or other resource.
Patients who are suitable candidates for intervention and willing to try a new diet should first provide information about their current eating habits. A food trial can then be personalized and implemented for a predetermined amount of time. If the patient does not respond, then the diet should be stopped and changed to a new diet or another intervention.
Dr. Chey and colleagues discussed three specific dietary interventions and their corresponding levels of evidence: soluble fiber; the low-fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet; and a gluten-free diet.
“Soluble fiber is efficacious in treating global symptoms of IBS,” they wrote, citing 15 randomized controlled trials. Soluble fiber is most suitable for patients with constipation-predominant IBS, and different soluble fibers may yield different outcomes based on characteristics such as rate of fermentation and viscosity. In contrast, insoluble fiber is unlikely to help with IBS, and may worsen abdominal pain and bloating.
The low-FODMAP diet is “currently the most evidence-based diet intervention for IBS,” especially for patients with diarrhea-predominant IBS. Dr. Chey and colleagues offered a clear roadmap for employing the diet. First, patients should eat only low-FODMAP foods for 4-6 weeks. If symptoms don’t improve, the diet should be stopped. If symptoms do improve, foods containing a single FODMAP should be reintroduced one at a time, each in increasing quantities over 3 days, alongside documentation of symptom responses. Finally, the diet should be personalized based on these responses. The majority of patients (close to 80%) “can liberalize” a low-FODMAP diet based on their responses.
In contrast with the low-FODMAP diet, which has a relatively solid body of supporting evidence, efficacy data are still limited for treating IBS with a gluten-free diet. “Although observational studies found that most patients with IBS improve with a gluten-free diet, randomized controlled trials have yielded mixed results,” Dr. Chey and colleagues explained.
Their report cited a recent monograph on the topic that concluded that gluten-free eating offered no significant benefit over placebo (relative risk, 0.46; 95% confidence interval, 0.16-1.28). While some studies have documented positive results with a gluten-free diet, Dr. Chey and colleagues suggested that confounding variables such as the nocebo effect and the impact of other dietary factors have yet to be ruled out. “At present, it remains unclear whether a gluten-free diet is of benefit to patients with IBS.”
Dr. Chey and colleagues also explored IBS biomarkers. While some early data have shown that biomarkers may predict dietary responses, “there is insufficient evidence to support their routine use in clinical practice. ... Further efforts to identify and validate biomarkers that predict response to dietary interventions are needed to deliver ‘personalized nutrition.’ ”
The clinical practice update was commissioned and approved by the AGA CPU Committee and the AGA Governing Board. The researchers disclosed relationships with Biomerica, Salix, Mauna Kea Technologies, and others.
This article was updated May 19, 2022.
The American Gastroenterological Association has published a clinical practice update on dietary interventions for patients with irritable bowel syndrome (IBS). The topics range from identification of suitable candidates for dietary interventions, to levels of evidence for specific diets, which are becoming increasingly recognized for their key role in managing patients with IBS, according to lead author William D. Chey, MD, of the University of Michigan, Ann Arbor, and colleagues.
“Most medical therapies for IBS improve global symptoms in fewer than one-half of patients, with a therapeutic gain of 7%-15% over placebo,” the researchers wrote in Gastroenterology. “Most patients with IBS associate their GI symptoms with eating food.”
According to Dr. Chey and colleagues, clinicians who are considering dietary modifications for treating IBS should first recognize the inherent challenges presented by this process and be aware that new diets won’t work for everyone.
“Specialty diets require planning and preparation, which may be impractical for some patients,” they wrote, noting that individuals with “decreased cognitive abilities and significant psychiatric disease” may be unable to follow diets or interpret their own responses to specific foods. Special diets may also be inappropriate for patients with financial constraints, and “should be avoided in patients with an eating disorder.”
Because of the challenges involved in dietary interventions, Dr. Chey and colleagues advised clinical support from a registered dietitian nutritionist or other resource.
Patients who are suitable candidates for intervention and willing to try a new diet should first provide information about their current eating habits. A food trial can then be personalized and implemented for a predetermined amount of time. If the patient does not respond, then the diet should be stopped and changed to a new diet or another intervention.
Dr. Chey and colleagues discussed three specific dietary interventions and their corresponding levels of evidence: soluble fiber; the low-fermentable oligo-, di-, and monosaccharides and polyols (FODMAP) diet; and a gluten-free diet.
“Soluble fiber is efficacious in treating global symptoms of IBS,” they wrote, citing 15 randomized controlled trials. Soluble fiber is most suitable for patients with constipation-predominant IBS, and different soluble fibers may yield different outcomes based on characteristics such as rate of fermentation and viscosity. In contrast, insoluble fiber is unlikely to help with IBS, and may worsen abdominal pain and bloating.
The low-FODMAP diet is “currently the most evidence-based diet intervention for IBS,” especially for patients with diarrhea-predominant IBS. Dr. Chey and colleagues offered a clear roadmap for employing the diet. First, patients should eat only low-FODMAP foods for 4-6 weeks. If symptoms don’t improve, the diet should be stopped. If symptoms do improve, foods containing a single FODMAP should be reintroduced one at a time, each in increasing quantities over 3 days, alongside documentation of symptom responses. Finally, the diet should be personalized based on these responses. The majority of patients (close to 80%) “can liberalize” a low-FODMAP diet based on their responses.
In contrast with the low-FODMAP diet, which has a relatively solid body of supporting evidence, efficacy data are still limited for treating IBS with a gluten-free diet. “Although observational studies found that most patients with IBS improve with a gluten-free diet, randomized controlled trials have yielded mixed results,” Dr. Chey and colleagues explained.
Their report cited a recent monograph on the topic that concluded that gluten-free eating offered no significant benefit over placebo (relative risk, 0.46; 95% confidence interval, 0.16-1.28). While some studies have documented positive results with a gluten-free diet, Dr. Chey and colleagues suggested that confounding variables such as the nocebo effect and the impact of other dietary factors have yet to be ruled out. “At present, it remains unclear whether a gluten-free diet is of benefit to patients with IBS.”
Dr. Chey and colleagues also explored IBS biomarkers. While some early data have shown that biomarkers may predict dietary responses, “there is insufficient evidence to support their routine use in clinical practice. ... Further efforts to identify and validate biomarkers that predict response to dietary interventions are needed to deliver ‘personalized nutrition.’ ”
The clinical practice update was commissioned and approved by the AGA CPU Committee and the AGA Governing Board. The researchers disclosed relationships with Biomerica, Salix, Mauna Kea Technologies, and others.
This article was updated May 19, 2022.
FROM GASTROENTEROLOGY
Steroids show less effectiveness in older-onset UC
Intravenous steroids were less effective in ulcerative colitis patients with older-onset disease, compared with younger-onset patients, according to data from nearly 500 individuals.
A combination of rising ulcerative colitis rates and an aging population has driven an increase in older-onset UC worldwide, Shinji Okabayashi, MD, of Kyoto University, and colleagues wrote in a study published in Alimentary Pharmacology & Therapeutics.
Data on differences in disease history between younger- and older-onset cases have been inconsistent, but one meta-analysis suggested a higher rate of surgery in older-onset cases, the authors of the current study wrote. “The higher risk of surgery may be due to the difference in effectiveness of intravenous steroid treatment, which is one of the important treatment options to avoid surgery for a severe course of UC,” but data on the effectiveness of IV steroids for older-onset UC are lacking.
The researchers reviewed data from 467 adults with ulcerative colitis at 27 centers in Japan. The participants were hospitalized and received their initial intravenous steroids between April 2014 and July 2019. The treatment was a daily dose of 40 mg or more of IV prednisolone or its equivalent, with dosing according to current guidelines. The primary outcome was clinical remission after 30 days.
The study population included 83 patients with older-onset UC and 384 with younger-onset UC. No cutoff currently exists to classify UC by age; the researchers defined younger onset as patients diagnosed at younger than 60 years and older onset as those diagnosed at age 60 years and older. The median age of onset was 32 years in the younger-onset patients and 68 in the older-onset group.
Overall, 51.8% of older-onset patients and 65.6% of younger-onset patients had clinical remission at 30 days (adjusted risk ratio, 0.74; P = .009). The incidence of colectomy at 30 days was significantly higher in older-onset patients, compared with younger-onset patients (15.7% vs. 1.8%; P < .001).
The researchers also assessed risk of surgery and adverse events at 90 days as secondary outcomes. The risk of surgery was significantly higher in older-onset patients compared with younger-onset patients (20.5% vs. 3.1%; ARR, 8.92) as was the risk of adverse events (25.3% vs. 9.1%, ARR, 2.19). A total of four deaths occurred during the study period, all in older-onset patients.
In addition, the researchers found that clinical remission rates at 30 days in older patients with younger-onset UC was similar to that of younger patients with younger-onset UC.
Potential contributors to the lower effectiveness of intravenous steroids in older-onset UC include genetic susceptibility, gut microbiota, and environmental factors, the researchers noted. The dysregulated immune response in older-onset UC also might play a role in limiting the effectiveness of intravenous steroids in these patients.
The study findings were limited by several factors including the lack of data on genetic susceptibility, environmental factors, and gut microbiota, as well as the inclusion only of patients with moderate to severe disease, which might have contributed to the higher risk of surgery in older patients, the researchers said. Other potential limitations include the potential confounders of concomitant drugs and nutritional status, and the use of symptom-based scoring to determine clinical remission.
However, the overall results reflect data from a recent meta-analysis, and the current study “clearly suggests that one of the reasons for the poor prognosis in patients with older-onset UC is the lower effectiveness of intravenous steroid treatment, which is one of the important treatment options for a severe course of UC,” the researchers wrote.
“Further research is warranted to establish the optimal treatment strategies for moderate to severe older-onset UC,” they concluded.
Findings have value for high-risk patients
The study is of interest to clinicians in practice, Hamed Khalili, MD, of Massachusetts General Hospital, Boston, said in an interview. “The findings are largely consistent with prior studies that have shown older-onset IBD patients have higher risk of surgery,” said Dr. Khalili, who was not involved with the study.
“This study focuses on a smaller subset of patients with IBD who present with acute severe UC,” Dr. Khalili noted. “Since this is a higher-risk patient population, the findings that older-onset UC is associated with lower response to intravenous steroids could have direct clinical implications.”
The study was supported by the Japanese Society for Inflammatory Bowel Disease. The researchers had no relevant financial conflicts to disclose. Dr. Khalili had no financial conflicts to disclose.
Intravenous steroids were less effective in ulcerative colitis patients with older-onset disease, compared with younger-onset patients, according to data from nearly 500 individuals.
A combination of rising ulcerative colitis rates and an aging population has driven an increase in older-onset UC worldwide, Shinji Okabayashi, MD, of Kyoto University, and colleagues wrote in a study published in Alimentary Pharmacology & Therapeutics.
Data on differences in disease history between younger- and older-onset cases have been inconsistent, but one meta-analysis suggested a higher rate of surgery in older-onset cases, the authors of the current study wrote. “The higher risk of surgery may be due to the difference in effectiveness of intravenous steroid treatment, which is one of the important treatment options to avoid surgery for a severe course of UC,” but data on the effectiveness of IV steroids for older-onset UC are lacking.
The researchers reviewed data from 467 adults with ulcerative colitis at 27 centers in Japan. The participants were hospitalized and received their initial intravenous steroids between April 2014 and July 2019. The treatment was a daily dose of 40 mg or more of IV prednisolone or its equivalent, with dosing according to current guidelines. The primary outcome was clinical remission after 30 days.
The study population included 83 patients with older-onset UC and 384 with younger-onset UC. No cutoff currently exists to classify UC by age; the researchers defined younger onset as patients diagnosed at younger than 60 years and older onset as those diagnosed at age 60 years and older. The median age of onset was 32 years in the younger-onset patients and 68 in the older-onset group.
Overall, 51.8% of older-onset patients and 65.6% of younger-onset patients had clinical remission at 30 days (adjusted risk ratio, 0.74; P = .009). The incidence of colectomy at 30 days was significantly higher in older-onset patients, compared with younger-onset patients (15.7% vs. 1.8%; P < .001).
The researchers also assessed risk of surgery and adverse events at 90 days as secondary outcomes. The risk of surgery was significantly higher in older-onset patients compared with younger-onset patients (20.5% vs. 3.1%; ARR, 8.92) as was the risk of adverse events (25.3% vs. 9.1%, ARR, 2.19). A total of four deaths occurred during the study period, all in older-onset patients.
In addition, the researchers found that clinical remission rates at 30 days in older patients with younger-onset UC was similar to that of younger patients with younger-onset UC.
Potential contributors to the lower effectiveness of intravenous steroids in older-onset UC include genetic susceptibility, gut microbiota, and environmental factors, the researchers noted. The dysregulated immune response in older-onset UC also might play a role in limiting the effectiveness of intravenous steroids in these patients.
The study findings were limited by several factors including the lack of data on genetic susceptibility, environmental factors, and gut microbiota, as well as the inclusion only of patients with moderate to severe disease, which might have contributed to the higher risk of surgery in older patients, the researchers said. Other potential limitations include the potential confounders of concomitant drugs and nutritional status, and the use of symptom-based scoring to determine clinical remission.
However, the overall results reflect data from a recent meta-analysis, and the current study “clearly suggests that one of the reasons for the poor prognosis in patients with older-onset UC is the lower effectiveness of intravenous steroid treatment, which is one of the important treatment options for a severe course of UC,” the researchers wrote.
“Further research is warranted to establish the optimal treatment strategies for moderate to severe older-onset UC,” they concluded.
Findings have value for high-risk patients
The study is of interest to clinicians in practice, Hamed Khalili, MD, of Massachusetts General Hospital, Boston, said in an interview. “The findings are largely consistent with prior studies that have shown older-onset IBD patients have higher risk of surgery,” said Dr. Khalili, who was not involved with the study.
“This study focuses on a smaller subset of patients with IBD who present with acute severe UC,” Dr. Khalili noted. “Since this is a higher-risk patient population, the findings that older-onset UC is associated with lower response to intravenous steroids could have direct clinical implications.”
The study was supported by the Japanese Society for Inflammatory Bowel Disease. The researchers had no relevant financial conflicts to disclose. Dr. Khalili had no financial conflicts to disclose.
Intravenous steroids were less effective in ulcerative colitis patients with older-onset disease, compared with younger-onset patients, according to data from nearly 500 individuals.
A combination of rising ulcerative colitis rates and an aging population has driven an increase in older-onset UC worldwide, Shinji Okabayashi, MD, of Kyoto University, and colleagues wrote in a study published in Alimentary Pharmacology & Therapeutics.
Data on differences in disease history between younger- and older-onset cases have been inconsistent, but one meta-analysis suggested a higher rate of surgery in older-onset cases, the authors of the current study wrote. “The higher risk of surgery may be due to the difference in effectiveness of intravenous steroid treatment, which is one of the important treatment options to avoid surgery for a severe course of UC,” but data on the effectiveness of IV steroids for older-onset UC are lacking.
The researchers reviewed data from 467 adults with ulcerative colitis at 27 centers in Japan. The participants were hospitalized and received their initial intravenous steroids between April 2014 and July 2019. The treatment was a daily dose of 40 mg or more of IV prednisolone or its equivalent, with dosing according to current guidelines. The primary outcome was clinical remission after 30 days.
The study population included 83 patients with older-onset UC and 384 with younger-onset UC. No cutoff currently exists to classify UC by age; the researchers defined younger onset as patients diagnosed at younger than 60 years and older onset as those diagnosed at age 60 years and older. The median age of onset was 32 years in the younger-onset patients and 68 in the older-onset group.
Overall, 51.8% of older-onset patients and 65.6% of younger-onset patients had clinical remission at 30 days (adjusted risk ratio, 0.74; P = .009). The incidence of colectomy at 30 days was significantly higher in older-onset patients, compared with younger-onset patients (15.7% vs. 1.8%; P < .001).
The researchers also assessed risk of surgery and adverse events at 90 days as secondary outcomes. The risk of surgery was significantly higher in older-onset patients compared with younger-onset patients (20.5% vs. 3.1%; ARR, 8.92) as was the risk of adverse events (25.3% vs. 9.1%, ARR, 2.19). A total of four deaths occurred during the study period, all in older-onset patients.
In addition, the researchers found that clinical remission rates at 30 days in older patients with younger-onset UC was similar to that of younger patients with younger-onset UC.
Potential contributors to the lower effectiveness of intravenous steroids in older-onset UC include genetic susceptibility, gut microbiota, and environmental factors, the researchers noted. The dysregulated immune response in older-onset UC also might play a role in limiting the effectiveness of intravenous steroids in these patients.
The study findings were limited by several factors including the lack of data on genetic susceptibility, environmental factors, and gut microbiota, as well as the inclusion only of patients with moderate to severe disease, which might have contributed to the higher risk of surgery in older patients, the researchers said. Other potential limitations include the potential confounders of concomitant drugs and nutritional status, and the use of symptom-based scoring to determine clinical remission.
However, the overall results reflect data from a recent meta-analysis, and the current study “clearly suggests that one of the reasons for the poor prognosis in patients with older-onset UC is the lower effectiveness of intravenous steroid treatment, which is one of the important treatment options for a severe course of UC,” the researchers wrote.
“Further research is warranted to establish the optimal treatment strategies for moderate to severe older-onset UC,” they concluded.
Findings have value for high-risk patients
The study is of interest to clinicians in practice, Hamed Khalili, MD, of Massachusetts General Hospital, Boston, said in an interview. “The findings are largely consistent with prior studies that have shown older-onset IBD patients have higher risk of surgery,” said Dr. Khalili, who was not involved with the study.
“This study focuses on a smaller subset of patients with IBD who present with acute severe UC,” Dr. Khalili noted. “Since this is a higher-risk patient population, the findings that older-onset UC is associated with lower response to intravenous steroids could have direct clinical implications.”
The study was supported by the Japanese Society for Inflammatory Bowel Disease. The researchers had no relevant financial conflicts to disclose. Dr. Khalili had no financial conflicts to disclose.
FROM ALIMENTARY PHARMACOLOGY & THERAPEUTICS
Management of gastroparesis in 2022
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.
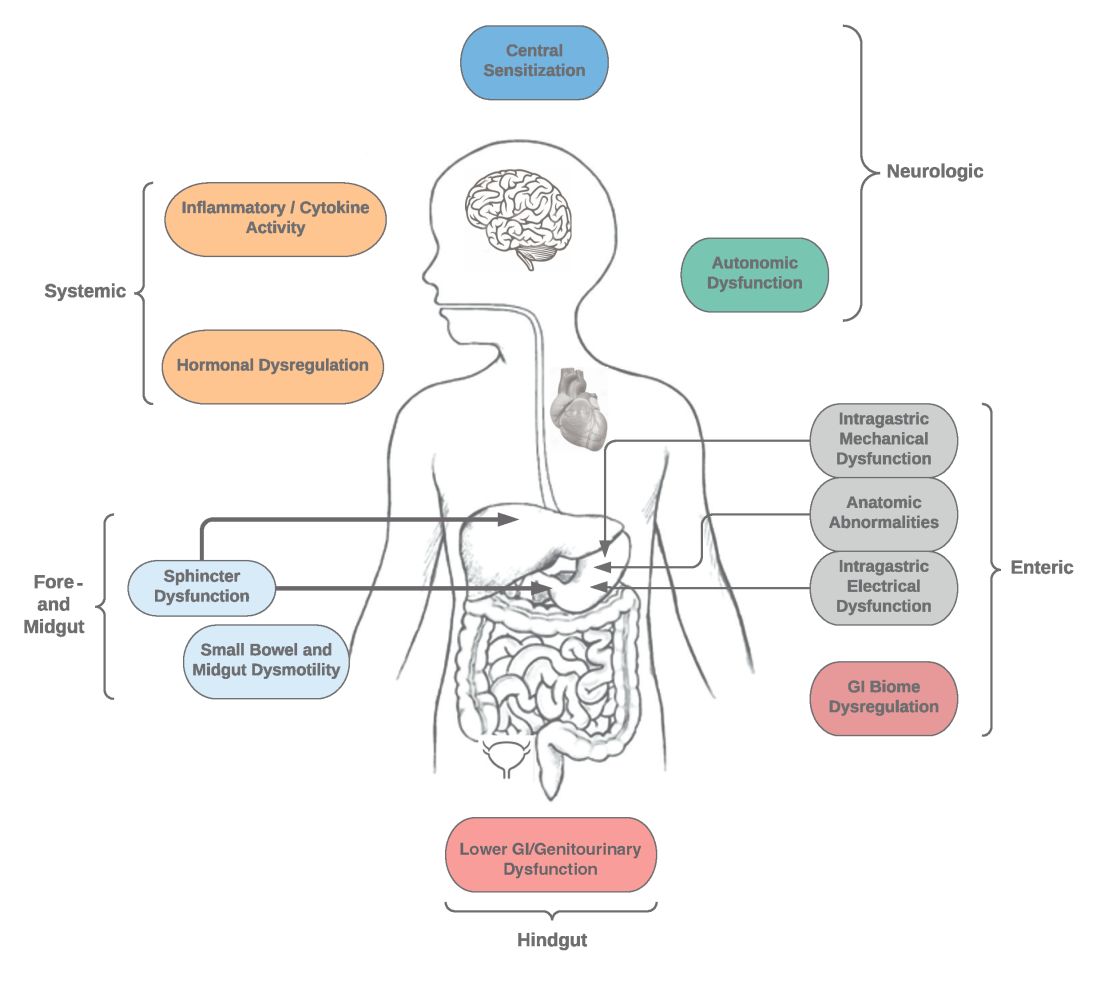
Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.
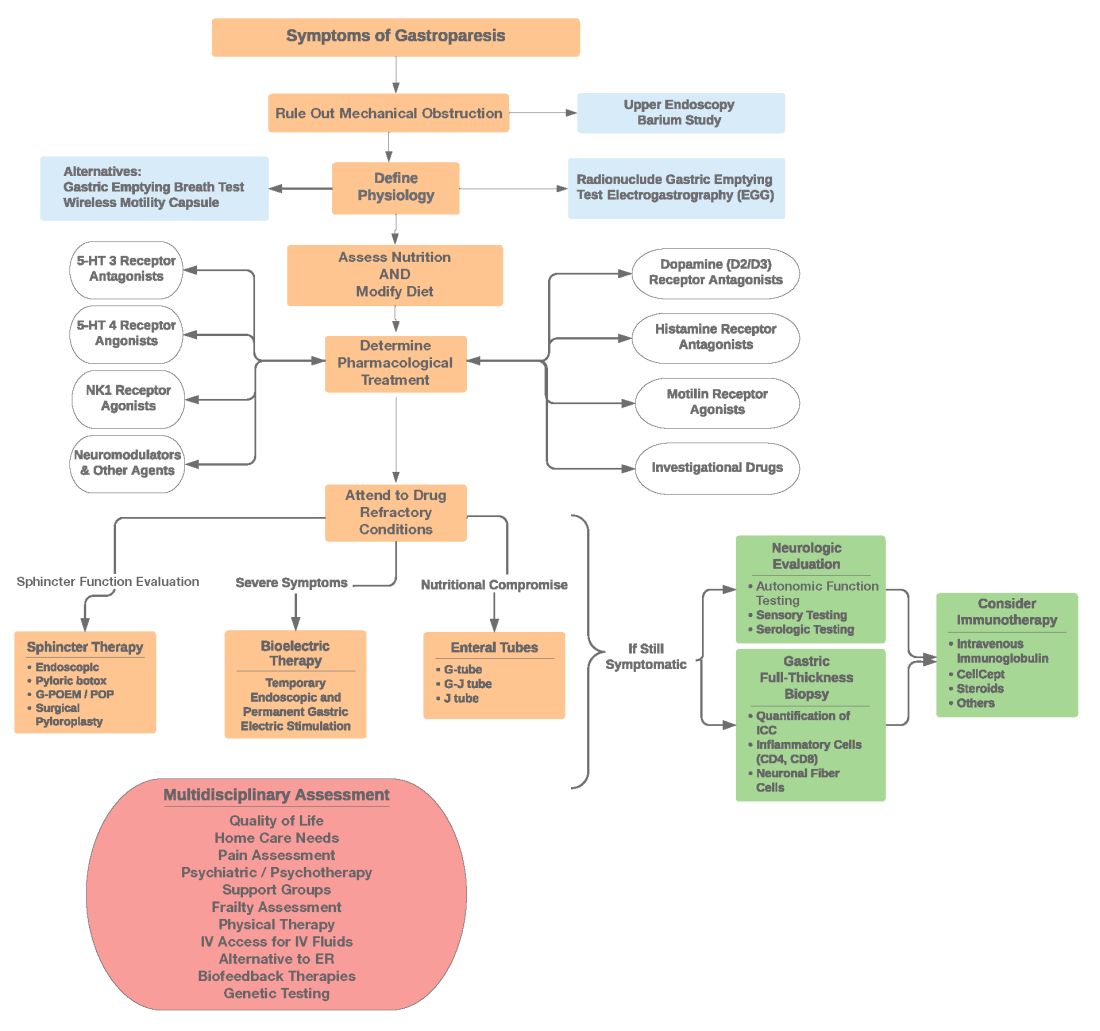
Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.

Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.

Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
Introduction
Patients presenting with the symptoms of gastroparesis (Gp) are commonly seen in gastroenterology practice.
Presentation
Patients with foregut symptoms of Gp have characteristic presentations, with nausea, vomiting/retching, and abdominal pain often associated with bloating and distension, early satiety, anorexia, and heartburn. Mid- and hindgut gastrointestinal and/or urinary symptoms may be seen in patients with Gp as well.
The precise epidemiology of gastroparesis syndromes (GpS) is unknown. Classic gastroparesis, defined as delayed gastric emptying without known mechanical obstruction, has a prevalence of about 10 per 100,000 population in men and 30 per 100,000 in women with women being affected 3 to 4 times more than men.1,2 Some risk factors for GpS, such as diabetes mellitus (DM) in up to 5% of patients with Type 1 DM, are known.3 Caucasians have the highest prevalence of GpS, followed by African Americans.4,5
The classic definition of Gp has blurred with the realization that patients may have symptoms of Gp without delayed solid gastric emptying. Some patients have been described as having chronic unexplained nausea and vomiting or gastroparesis like syndrome.6 More recently the NIH Gastroparesis Consortium has proposed that disorders like functional dyspepsia may be a spectrum of the two disorders and classic Gp.7 Using this broadened definition, the number of patients with Gp symptoms is much greater, found in 10% or more of the U.S. population.8 For this discussion, GpS is used to encompass this spectrum of disorders.
The etiology of GpS is often unknown for a given patient, but clues to etiology exist in what is known about pathophysiology. Types of Gp are described as being idiopathic, diabetic, or postsurgical, each of which may have varying pathophysiology. Many patients with mild-to-moderate GpS symptoms are effectively treated with out-patient therapies; other patients may be refractory to available treatments. Refractory GpS patients have a high burden of illness affecting them, their families, providers, hospitals, and payers.
Pathophysiology
Specific types of gastroparesis syndromes have variable pathophysiology (Figure 1). In some cases, like GpS associated with DM, pathophysiology is partially related to diabetic autonomic dysfunction. GpS are multifactorial, however, and rather than focusing on subtypes, this discussion focuses on shared pathophysiology. Understanding pathophysiology is key to determining treatment options and potential future targets for therapy.

Intragastric mechanical dysfunction, both proximal (fundic relaxation and accommodation and/or lack of fundic contractility) and distal stomach (antral hypomotility) may be involved. Additionally, intragastric electrical disturbances in frequency, amplitude, and propagation of gastric electrical waves can be seen with low/high resolution gastric mapping.
Both gastroesophageal and gastropyloric sphincter dysfunction may be seen. Esophageal dysfunction is frequently seen but is not always categorized in GpS. Pyloric dysfunction is increasingly a focus of both diagnosis and therapy. GI anatomic abnormalities can be identified with gastric biopsies of full thickness muscle and mucosa. CD117/interstitial cells of Cajal, neural fibers, inflammatory and other cells can be evaluated by light microscopy, electron microscopy, and special staining techniques.
Small bowel, mid-, and hindgut dysmotility involvement has often been associated with pathologies of intragastric motility. Not only GI but genitourinary dysfunction may be associated with fore- and mid-gut dysfunction in GpS. Equally well described are abnormalities of the autonomic and sensory nervous system, which have recently been better quantified. Serologic measures, such as channelopathies and other antibody mediated abnormalities, have been recently noted.
Suspected for many years, immune dysregulation has now been documented in patients with GpS. Further investigation, including genetic dysregulation of immune measures, is ongoing. Other mechanisms include systemic and local inflammation, hormonal abnormalities, macro- and micronutrient deficiencies, dysregulation in GI microbiome, and physical frailty. The above factors may play a role in the pathophysiology of GpS, and it is likely that many of these are involved with a given patient presenting for care.9
Diagnosis of GpS
Diagnosis of GpS is often delayed and can be challenging; various tools have been developed, but not all are used. A diagnostic approach for patients with symptoms of Gp is listed below, and Figure 2 details a diagnostic approach and treatment options for symptomatic patients.

Symptom Assessment: Initially Gp symptoms can be assessed using Food and Drug Administration–approved patient-reported outcomes, including frequency and severity of nausea, vomiting, anorexia/early satiety, bloating/distention, and abdominal pain on a 0-4, 0-5 or 0-10 scale. The Gastrointestinal Cardinal Symptom Index or visual analog scales can also be used. It is also important to evaluate midgut and hindgut symptoms.9-11
Mechanical obstruction assessment: Mechanical obstruction can be ruled out using upper endoscopy or barium studies.
Physiologic testing: The most common is radionuclide gastric emptying testing (GET). Compliance with guidelines, standardization, and consistency of GETs is vital to help with an accurate diagnosis. Currently, two consensus recommendations for the standardized performance of GETs exist.12,13 Breath testing is FDA approved in the United States and can be used as an alternative. Wireless motility capsule testing can be complimentary.
Gastric dysrhythmias assessment: Assessment of gastric dysrhythmias can be performed in outpatient settings using cutaneous electrogastrogram, currently available in many referral centers. Most patients with GpS have an underlying gastric electrical abnormality.14,15
Sphincter dysfunction assessment: Both proximal and distal sphincter abnormalities have been described for many years and are of particular interest recently. Use of the functional luminal imaging probe (FLIP) shows patients with GpS may have decreased sphincter distensibility when examining the comparisons of the cross-sectional area relative to pressure Using this information, sphincter therapies can be offered.16-18
Other testing: Neurologic and autonomic testing, along with psychosocial, genetic and frailty assessments, are helpful to explore.19 Nutritional evaluation can be done using standardized scales, such as subjective global assessment and serologic testing for micronutrient deficiency or electrical impedance.20
Treatment of GpS
Therapies for GpS can be viewed as the five D’s: Diet, Drug, Disruption, Devices, and Details.
Diet and nutrition: The mainstay treatment of GpS remains dietary modification. The most common recommendation is to limit meal size, often with increased meal frequency, as well as nutrient composition, in areas that may retard gastric emptying. In addition, some patients with GpS report intolerances of specific foods, such as specific carbohydrates. Nutritional consultation can assist patients with meals tailored for their current nutritional needs. Nutritional supplementation is widely used for patients with GpS.20
Pharmacological treatment: The next tier of treatment for GpS is drugs. Review of a patient’s medications is important to minimize drugs that may retard gastric emptying such as opiates and GLP-1 agonists. A full discussion of medications is beyond the scope of this article, but classes of drugs available include: prokinetics, antiemetics, neuromodulators, and investigational agents.
There is only one approved prokinetic medication for gastroparesis – the dopamine blocker metoclopramide – and most providers are aware of metoclopramide’s limitations in terms of potential side effects, such as the risk of tardive dyskinesia and labeling on duration of therapy, with a maximum of 12 weeks recommended. Alternative prokinetics, such as domperidone, are not easily available in the United States; some mediations approved for other indications, such as the 5-HT drug prucalopride, are sometimes given for GpS off-label. Antiemetics such as promethazine and ondansetron are frequently used for symptomatic control in GpS. Despite lack of positive controlled trials in Gp, neuromodulator drugs, such as tricyclic or tetracyclic antidepressants like amitriptyline or mirtazapine are often used; their efficacy is more proven in the functional dyspepsia area. Other drugs such as the NK-1 drug aprepitant have been studied in Gp and are sometimes used off-label. Drugs such as scopolamine and related compounds can also provide symptomatic relief, as can the tetrahydrocannabinol-containing drug, dronabinol. New pharmacologic agents for GpS include investigational drugs such as ghrelin agonists and several novel compounds, none of which are currently FDA approved.21,22
Fortunately, the majority of patients with GpS respond to conservative therapies, such as dietary changes and/or medications. The last part of the section on treatment of GpS includes patients that are diet and drug refractory. Patients in this group are often referred to gastroenterologists and can be complex, time consuming, and frustrating to provide care for. Many of these patients are eventually seen in referral centers, and some travel great distances and have considerable medical expenses.
Pylorus-directed therapies: The recent renewed interest in pyloric dysfunction in patients with Gp symptoms has led to a great deal of clinical activity. Gastropyloric dysfunction in Gp has been documented for decades, originally in diabetic patients with autonomic and enteric neuropathy. The use of botulinum toxin in upper- and lower-gastric sphincters has led to continuing use of this therapy for patients with GpS. Despite initial negative controlled trials of botulinum toxin in the pyloric sphincter, newer studies indicate that physiologic measures, such as the FLIP, may help with patient selection. Other disruptive pyloric therapies, including pyloromyotomy, per oral pyloromyotomy, and gastric peroral endoscopic myotomy, are supported by open-label use, despite a lack of published positive controlled trials.17
Bioelectric therapy: Another approach for patients with symptomatic drug refractory GpS is bioelectric device therapies, which can be delivered several ways, including directly to the stomach or to the spinal cord or the vagus nerve in the neck or ear, as well as by electro-acupuncture. High-frequency, low-energy gastric electrical stimulation (GES) is the best studied. First done in 1992 as an experimental therapy, GES was investigational from 1995 to 2000, when it became FDA approved as a humanitarian-use device. GES has been used in over 10,000 patients worldwide; only a small number (greater than 700 study patients) have been in controlled trials. Nine controlled trials of GES have been primarily positive, and durability for over 10 years has been shown. Temporary GES can also be performed endoscopically, although that is an off-label procedure. It has been shown to predict long-term therapy outcome.23-26
Nutritional support: Nutritional abnormalities in some cases of GpS lead to consideration of enteral tubes, starting with a trial of feeding with an N-J tube placed endoscopically. An N-J trial is most often performed in patients who have macro-malnutrition and weight loss but can be considered for other highly symptomatic patients. Other endoscopic tubes can be PEG or PEG-J or direct PEJ tubes. Some patients may require surgical placement of enteral tubes, presenting an opportunity for a small bowel or gastric full-thickness biopsy. Enteral tubes are sometimes used for decompression in highly symptomatic patients.27
For patients presenting with neurological symptoms, findings and serologic abnormalities have led to interest in immunotherapies. One is intravenous immunoglobulin, given parenterally. Several open-label studies have been published, the most recent one with 47 patients showing better response if glutamic acid decarboxylase–65 antibodies were present and with longer therapeutic dosing.28 Drawbacks to immunotherapies like intravenous immunoglobulin are cost and requiring parenteral access.
Other evaluation/treatments for drug refractory patients can be detailed as follows: First, an overall quality of life assessment can be helpful, especially one that includes impact of GpS on the patients and family. Nutritional considerations, which may not have been fully assessed, can be examined in more detail. Frailty assessments may show the need for physical therapy. Assessment for home care needs may indicate, in severe patients, needs for IV fluids at home, either enteral or parenteral, if nutrition is not adequate. Psychosocial and/or psychiatric assessments may lead to the need for medications, psychotherapy, and/or support groups. Lastly, an assessment of overall health status may lead to approaches for minimizing visits to emergency rooms and hospitalizations.29,30
Conclusion
Patients with Gp symptoms are becoming increasingly recognized and referred to gastroenterologists. Better understandings of the pathophysiology of the spectrum of gastroparesis syndromes, assisted by innovations in diagnosis, have led to expansion of existing and new therapeutic approaches. Fortunately, most patients can benefit from a standardized diagnostic approach and directed noninvasive therapies. Patients with refractory gastroparesis symptoms, often with complex issues referred to gastroenterologists, remain a challenge, and novel approaches may improve their quality of life.
Dr. Mathur is a GI motility research fellow at the University of Louisville, Ky. He reports no conflicts of interest. Dr. Abell is the Arthur M. Schoen, MD, Chair in Gastroenterology at the University of Louisville. His main funding is NIH GpCRC and NIH Definitive Evaluation of Gastric Dysrhythmia. He is an investigator for Cindome, Vanda, Allergan, and Neurogastrx; a consultant for Censa, Nuvaira, and Takeda; a speaker for Takeda and Medtronic; and a reviewer for UpToDate. He is also the founder of ADEPT-GI, which holds IP related to mucosal stimulation and autonomic and enteric profiling.
References
1. Jung HK et al. Gastroenterology. 2009;136(4):1225-33.
2. Ye Y et al. Gut. 2021;70(4):644-53.
3. Oshima T et al. J Neurogastroenterol Motil. 2021;27(1):46-54.
4. Soykan I et al. Dig Dis Sci. 1998;43(11):2398-404.
5. Syed AR et al. J Clin Gastroenterol. 2020;54(1):50-4.
6.Pasricha PJ et al. Clin Gastroenterol Hepatol. 2011;9(7):567-76.e1-4.
7. Pasricha PJ et al. Gastroenterology. 2021;160(6):2006-17.
8. Almario CV et al. Am J Gastroenterol. 2018;113(11):1701-10.
9. Abell TL et al. Dig Dis Sci. 2021 Apr;66(4):1127-41.
10. Abell TL et al. Neurogastroenterol Motil. 2019;31(3):e13534.
11. Elmasry M et al. Neurogastroenterol Motil. 2021 Oct 26;e14274.
12. Maurer AH et al. J Nucl Med. 2020;61(3):11N-7N.
13. Abell TL et al. J Nucl Med Technol. 2008 Mar;36(1):44-54.
14. Shine A et al. Neuromodulation. 2022 Feb 16;S1094-7159(21)06986-5.
15. O’Grady G et al. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G527-g42.
16. Saadi M et al. Rev Gastroenterol Mex (Engl Ed). Oct-Dec 2018;83(4):375-84.
17. Kamal F et al. Aliment Pharmacol Ther. 2022;55(2):168-77.
18. Harberson J et al. Dig Dis Sci. 2010;55(2):359-70.
19. Winston J. Gastrointestinal Disorders. 2021;3(2):78-83.
20. Parkman HP et al. Gastroenterology. 2011;141(2):486-98, 98.e1-7.
21. Heckroth M et al. J Clin Gastroenterol. 2021;55(4):279-99.
22. Camilleri M. Clin Gastroenterol Hepatol. 2022;20(1):19-24.
23. Payne SC et al. Nat Rev Gastroenterol Hepatol. 2019;16(2):89-105.
24. Ducrotte P et al. Gastroenterology. 2020;158(3):506-14.e2.
25. Burlen J et al. Gastroenterology Res. 2018;11(5):349-54.
26. Hedjoudje A et al. Neurogastroenterol Motil. 2020;32(11):e13949.
27. Petrov RV et al. Gastroenterol Clin North Am. 2020;49(3):539-56.
28. Gala K et al. J Clin Gastroenterol. 2021 Dec 31. doi: 10.1097/MCG.0000000000001655.
29. Abell TL et al. Neurogastroenterol Motil. 2006;18(4):263-83.
30. Camilleri M et al. Am J Gastroenterol. 2013;108(1):18-37.
TNF inhibitors prior to surgery safe in patients with IBD: Study
Patients with inflammatory bowel disease (IBD) can safely take tumor necrosis factor inhibitors (TNFi) prior to abdominal surgery, a prospective, multicenter, observational study confirms.
The researchers found that exposure to TNFi in the 12 weeks prior to surgery was not associated with an increased risk of either overall infections or surgical site infections (SSI).
The findings should be “very reassuring” for clinicians, lead author Benjamin L. Cohen, MD, Cleveland Clinic Foundation, told this news organization. “In the past, when clinicians were unsure about the safety of using these drugs in the perioperative period, they may have delayed surgeries or stopped medications unnecessarily.”
“For me, the key take-home point of this study is that we need to plan the timing and management of medications around surgery based on factors other than the use of tumor necrosis factor inhibitors in most patients,” Dr. Cohen continued.
Ultimately, “we will help change practice in how we manage patients with IBD having surgery,” he said.
The research was published online in Gastroenterology.
No increased postop infection risk
The Prospective Cohort of Ulcerative Colitis and Crohn’s Disease Patients Undergoing Surgery to Identify Risk Factors for Post-Operative Infection I (PUCCINI) trial enrolled patients with IBD from 17 sites participating in the Crohn’s and Colitis Foundation Clinical Research Alliance between September 2014 and June 2017.
Patients had Crohn’s disease, ulcerative colitis, or indeterminate colitis, as determined by standard criteria, and planned to undergo intra-abdominal surgery or had undergone intra-abdominal surgery in the preceding 4 days.
Among the 947 patients enrolled, 47.8% were women. All were aged 18 years or older. The median disease duration was 10 years; 34.4% of patients had undergone prior bowel resection, and a further 17.5% had undergone other abdominal surgery.
Systemic corticosteroid use within 2 weeks of surgery was reported by 40.9% of patients, and 42.3% had used antibiotics.
TNFi exposure within the 12 weeks prior to surgery was reported by 40.3% of patients. Adalimumab and infliximab were the most commonly used drugs. Among those who had not used TNFi prior to surgery, 23.7% were TNFi-naive, and 36.0% had used them in the past.
The researchers report that there was no significant difference in the rate of postoperative infections between patients who reported using TNFi in the 12 weeks prior to surgery and those who did not (18.1% vs. 20.2%; P = .469). There was also no difference in SSI, as defined using the Centers for Disease Control and Prevention criteria, between the two groups (12.0% vs 12.6%; P = .889).
Multivariate analysis revealed that current TNFi exposure was not associated with any infection, at an odds ratio versus no exposure of 1.050 (P = .80), or with SSI, at an odds ratio of 1.249 (P = .34).
In contrast, preoperative corticosteroid exposure, prior bowel resection, and current smoking were associated with any infection and with SSI.
Approached for comment, Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, said that the current findings are consistent with those of previous studies and that their relevance extends beyond abdominal surgery.
In the past, when surgeons were “confronted with a patient on a TNF blocker, even if it’s orthopedic or plastic surgery, they recommended against using a TNF blocker or operating at the end of the cycle when the drug levels are low,” he told this news organization.
Dr. Hanauer said such practice gets clinicians into a “bind because you’ve got a patient, for instance, who’s got a blockage with Crohn’s disease ... but the only way you could manage them when the TNFi was out of their system was with steroids, which is worse” in terms of postoperative infection risk, he explained.
Prospective studies important
The researchers note that up to 50% of patients with IBD are exposed to TNFi prior to their first surgery. They also note that there is concern that preoperative treatment with these and other immunosuppressive medications may increase the risk of postoperative infections.
However, the evidence is inconsistent, they write, so whether to continue or stop the drugs prior to surgery remains controversial.
“A lot of the initial studies in the perioperative population were single-center and retrospective for the most part,” Dr. Cohen said, adding that the studies used different modes of assessment and followed different time frames.
“So, there’s a lot of heterogeneity,” he said.
In addition, early studies of TNFi were often conducted with patients who were very ill and who had started receiving the drug right before surgery, and they sometimes had a complication Dr. Cohen said. “But you don’t know if that’s because of the drug itself or because of many other factors associated with them being very sick, such as being on steroids, being very malnourished, or having other complications of disease.”
It is difficult to control for such risk factors in retrospective analyses because the information is not always available from medical records, he said. “That’s why it’s so important to study clinical questions like this in a prospective manner.”
Dr. Cohen added that it is important that studies such as theirs continue to be undertaken as new drugs become available.
“We’re entering an era of rapidly expanding drug discovery, so we’re going to have new medications available for use in our patients with IBD,” he explained. “It’s important that we continue to build prospective cohorts to look at questions such as the safety of medications in the perioperative period, rather than solely relying on retrospective data.”
The study was funded by a Crohn’s & Colitis Foundation Senior Research Award. Dr. Cohen reports relationships with AbbVie, Celgene, Bristol-Myers Squibb, Pfizer, Sublimity Therapeutics, Target RWE, Janssen, Ferring, AlphaSigma, and Takeda. Other authors report numerous financial relationships. Dr. Hanauer reports relationships with Janssen, AbbVie, Pfizer, Amgen, Genentech, and Merck.
A version of this article first appeared on Medscape.com.
Patients with inflammatory bowel disease (IBD) can safely take tumor necrosis factor inhibitors (TNFi) prior to abdominal surgery, a prospective, multicenter, observational study confirms.
The researchers found that exposure to TNFi in the 12 weeks prior to surgery was not associated with an increased risk of either overall infections or surgical site infections (SSI).
The findings should be “very reassuring” for clinicians, lead author Benjamin L. Cohen, MD, Cleveland Clinic Foundation, told this news organization. “In the past, when clinicians were unsure about the safety of using these drugs in the perioperative period, they may have delayed surgeries or stopped medications unnecessarily.”
“For me, the key take-home point of this study is that we need to plan the timing and management of medications around surgery based on factors other than the use of tumor necrosis factor inhibitors in most patients,” Dr. Cohen continued.
Ultimately, “we will help change practice in how we manage patients with IBD having surgery,” he said.
The research was published online in Gastroenterology.
No increased postop infection risk
The Prospective Cohort of Ulcerative Colitis and Crohn’s Disease Patients Undergoing Surgery to Identify Risk Factors for Post-Operative Infection I (PUCCINI) trial enrolled patients with IBD from 17 sites participating in the Crohn’s and Colitis Foundation Clinical Research Alliance between September 2014 and June 2017.
Patients had Crohn’s disease, ulcerative colitis, or indeterminate colitis, as determined by standard criteria, and planned to undergo intra-abdominal surgery or had undergone intra-abdominal surgery in the preceding 4 days.
Among the 947 patients enrolled, 47.8% were women. All were aged 18 years or older. The median disease duration was 10 years; 34.4% of patients had undergone prior bowel resection, and a further 17.5% had undergone other abdominal surgery.
Systemic corticosteroid use within 2 weeks of surgery was reported by 40.9% of patients, and 42.3% had used antibiotics.
TNFi exposure within the 12 weeks prior to surgery was reported by 40.3% of patients. Adalimumab and infliximab were the most commonly used drugs. Among those who had not used TNFi prior to surgery, 23.7% were TNFi-naive, and 36.0% had used them in the past.
The researchers report that there was no significant difference in the rate of postoperative infections between patients who reported using TNFi in the 12 weeks prior to surgery and those who did not (18.1% vs. 20.2%; P = .469). There was also no difference in SSI, as defined using the Centers for Disease Control and Prevention criteria, between the two groups (12.0% vs 12.6%; P = .889).
Multivariate analysis revealed that current TNFi exposure was not associated with any infection, at an odds ratio versus no exposure of 1.050 (P = .80), or with SSI, at an odds ratio of 1.249 (P = .34).
In contrast, preoperative corticosteroid exposure, prior bowel resection, and current smoking were associated with any infection and with SSI.
Approached for comment, Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, said that the current findings are consistent with those of previous studies and that their relevance extends beyond abdominal surgery.
In the past, when surgeons were “confronted with a patient on a TNF blocker, even if it’s orthopedic or plastic surgery, they recommended against using a TNF blocker or operating at the end of the cycle when the drug levels are low,” he told this news organization.
Dr. Hanauer said such practice gets clinicians into a “bind because you’ve got a patient, for instance, who’s got a blockage with Crohn’s disease ... but the only way you could manage them when the TNFi was out of their system was with steroids, which is worse” in terms of postoperative infection risk, he explained.
Prospective studies important
The researchers note that up to 50% of patients with IBD are exposed to TNFi prior to their first surgery. They also note that there is concern that preoperative treatment with these and other immunosuppressive medications may increase the risk of postoperative infections.
However, the evidence is inconsistent, they write, so whether to continue or stop the drugs prior to surgery remains controversial.
“A lot of the initial studies in the perioperative population were single-center and retrospective for the most part,” Dr. Cohen said, adding that the studies used different modes of assessment and followed different time frames.
“So, there’s a lot of heterogeneity,” he said.
In addition, early studies of TNFi were often conducted with patients who were very ill and who had started receiving the drug right before surgery, and they sometimes had a complication Dr. Cohen said. “But you don’t know if that’s because of the drug itself or because of many other factors associated with them being very sick, such as being on steroids, being very malnourished, or having other complications of disease.”
It is difficult to control for such risk factors in retrospective analyses because the information is not always available from medical records, he said. “That’s why it’s so important to study clinical questions like this in a prospective manner.”
Dr. Cohen added that it is important that studies such as theirs continue to be undertaken as new drugs become available.
“We’re entering an era of rapidly expanding drug discovery, so we’re going to have new medications available for use in our patients with IBD,” he explained. “It’s important that we continue to build prospective cohorts to look at questions such as the safety of medications in the perioperative period, rather than solely relying on retrospective data.”
The study was funded by a Crohn’s & Colitis Foundation Senior Research Award. Dr. Cohen reports relationships with AbbVie, Celgene, Bristol-Myers Squibb, Pfizer, Sublimity Therapeutics, Target RWE, Janssen, Ferring, AlphaSigma, and Takeda. Other authors report numerous financial relationships. Dr. Hanauer reports relationships with Janssen, AbbVie, Pfizer, Amgen, Genentech, and Merck.
A version of this article first appeared on Medscape.com.
Patients with inflammatory bowel disease (IBD) can safely take tumor necrosis factor inhibitors (TNFi) prior to abdominal surgery, a prospective, multicenter, observational study confirms.
The researchers found that exposure to TNFi in the 12 weeks prior to surgery was not associated with an increased risk of either overall infections or surgical site infections (SSI).
The findings should be “very reassuring” for clinicians, lead author Benjamin L. Cohen, MD, Cleveland Clinic Foundation, told this news organization. “In the past, when clinicians were unsure about the safety of using these drugs in the perioperative period, they may have delayed surgeries or stopped medications unnecessarily.”
“For me, the key take-home point of this study is that we need to plan the timing and management of medications around surgery based on factors other than the use of tumor necrosis factor inhibitors in most patients,” Dr. Cohen continued.
Ultimately, “we will help change practice in how we manage patients with IBD having surgery,” he said.
The research was published online in Gastroenterology.
No increased postop infection risk
The Prospective Cohort of Ulcerative Colitis and Crohn’s Disease Patients Undergoing Surgery to Identify Risk Factors for Post-Operative Infection I (PUCCINI) trial enrolled patients with IBD from 17 sites participating in the Crohn’s and Colitis Foundation Clinical Research Alliance between September 2014 and June 2017.
Patients had Crohn’s disease, ulcerative colitis, or indeterminate colitis, as determined by standard criteria, and planned to undergo intra-abdominal surgery or had undergone intra-abdominal surgery in the preceding 4 days.
Among the 947 patients enrolled, 47.8% were women. All were aged 18 years or older. The median disease duration was 10 years; 34.4% of patients had undergone prior bowel resection, and a further 17.5% had undergone other abdominal surgery.
Systemic corticosteroid use within 2 weeks of surgery was reported by 40.9% of patients, and 42.3% had used antibiotics.
TNFi exposure within the 12 weeks prior to surgery was reported by 40.3% of patients. Adalimumab and infliximab were the most commonly used drugs. Among those who had not used TNFi prior to surgery, 23.7% were TNFi-naive, and 36.0% had used them in the past.
The researchers report that there was no significant difference in the rate of postoperative infections between patients who reported using TNFi in the 12 weeks prior to surgery and those who did not (18.1% vs. 20.2%; P = .469). There was also no difference in SSI, as defined using the Centers for Disease Control and Prevention criteria, between the two groups (12.0% vs 12.6%; P = .889).
Multivariate analysis revealed that current TNFi exposure was not associated with any infection, at an odds ratio versus no exposure of 1.050 (P = .80), or with SSI, at an odds ratio of 1.249 (P = .34).
In contrast, preoperative corticosteroid exposure, prior bowel resection, and current smoking were associated with any infection and with SSI.
Approached for comment, Stephen B. Hanauer, MD, medical director of the Digestive Health Center at Northwestern University, Chicago, said that the current findings are consistent with those of previous studies and that their relevance extends beyond abdominal surgery.
In the past, when surgeons were “confronted with a patient on a TNF blocker, even if it’s orthopedic or plastic surgery, they recommended against using a TNF blocker or operating at the end of the cycle when the drug levels are low,” he told this news organization.
Dr. Hanauer said such practice gets clinicians into a “bind because you’ve got a patient, for instance, who’s got a blockage with Crohn’s disease ... but the only way you could manage them when the TNFi was out of their system was with steroids, which is worse” in terms of postoperative infection risk, he explained.
Prospective studies important
The researchers note that up to 50% of patients with IBD are exposed to TNFi prior to their first surgery. They also note that there is concern that preoperative treatment with these and other immunosuppressive medications may increase the risk of postoperative infections.
However, the evidence is inconsistent, they write, so whether to continue or stop the drugs prior to surgery remains controversial.
“A lot of the initial studies in the perioperative population were single-center and retrospective for the most part,” Dr. Cohen said, adding that the studies used different modes of assessment and followed different time frames.
“So, there’s a lot of heterogeneity,” he said.
In addition, early studies of TNFi were often conducted with patients who were very ill and who had started receiving the drug right before surgery, and they sometimes had a complication Dr. Cohen said. “But you don’t know if that’s because of the drug itself or because of many other factors associated with them being very sick, such as being on steroids, being very malnourished, or having other complications of disease.”
It is difficult to control for such risk factors in retrospective analyses because the information is not always available from medical records, he said. “That’s why it’s so important to study clinical questions like this in a prospective manner.”
Dr. Cohen added that it is important that studies such as theirs continue to be undertaken as new drugs become available.
“We’re entering an era of rapidly expanding drug discovery, so we’re going to have new medications available for use in our patients with IBD,” he explained. “It’s important that we continue to build prospective cohorts to look at questions such as the safety of medications in the perioperative period, rather than solely relying on retrospective data.”
The study was funded by a Crohn’s & Colitis Foundation Senior Research Award. Dr. Cohen reports relationships with AbbVie, Celgene, Bristol-Myers Squibb, Pfizer, Sublimity Therapeutics, Target RWE, Janssen, Ferring, AlphaSigma, and Takeda. Other authors report numerous financial relationships. Dr. Hanauer reports relationships with Janssen, AbbVie, Pfizer, Amgen, Genentech, and Merck.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
Ultraprocessed foods: Large study finds link with Crohn’s disease
Higher consumption of ultraprocessed foods was linked with a significantly higher risk of Crohn’s disease (CD), but not ulcerative colitis, in a large prospective cohort study published online in Clinical Gastroenterology and Hepatology.
Researchers, led by Chun-Han Lo, MD, of Massachusetts General Hospital, Boston, defined ultraprocessed foods “as ready-to-consume formulations of ingredients, typically created by [a] series industrial techniques and processes. They frequently involve the incorporation of additives, such as sweeteners, preservatives, emulsifiers, thickeners, and flavors, which aid in food preservation and produce hyperpalatable products.”
The rising global incidence of inflammatory bowel disease (IBD) in regions undergoing Westernization has overlapped with rising increase in consumption of ultraprocessed food (UPF) over the past few decades, according to the authors. Previous studies have focused on links with individual nutrients and IBD, but this study focuses on the processing role itself. This study comprised 245,112 participants (203,516 women and 41,596 men) and more than 5,468,444 person-years of follow-up, taken from three cohorts: Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study.
In the highest quartile, UPFs made up on average nearly half (46.4%) of participants’ total energy consumption, compared with 21% in the lowest quartile.
The researchers found that compared with participants in the lowest quartile of simple updated UPF consumption, those in the highest quartile had a significantly increased risk of CD (adjusted hazard ratio, 1.70; 95% confidence interval, 1.23-2.35).
In addition, “a secondary analysis across different CD locations demonstrated that participants in the highest quartile of simple updated UPF intake had the highest risk of ileal, colonic, and ileocolonic CD,” the authors wrote.
Three groups of processed foods driving risk increase
Three groups of UPFs appeared to drive the increased risk of CD: ultraprocessed breads and breakfast foods; frozen or shelf-stable ready-to-eat/heat meals; and sauces, cheeses, spreads, and gravies.
Just as with overall consumption, researchers did not find an association between any of those three subgroups and UC risk.
The authors suggested several reasons for the link with Crohn’s disease. Among them were that higher UPF consumption may mean those foods are taking the place of unprocessed or minimally processed foods, such as those rich in fiber. Second, UPFs contain additives, such as salt, that may promote intestinal inflammation. Third, artificial sweeteners in UPFs may predispose the gut to inflammation, as supported by supplementing sucralose/maltodextrins in mice models of spontaneous ileitis.
As for why CD, but not UC, the authors said diet may be more relevant and have a stronger effect biologically in CD compared with UC. Another potential reason, they said, is that results “may reflect the greater specificity of dietary ligands and metabolites on the small intestine compared with the colon.”
Data from three large, prospective cohorts
Researchers used data from three ongoing, prospective nationwide cohorts of health professionals in the United States – the Nurses’ Health Study (1986-2014); the Nurses’ Health Study II (1991-2017); and the Health Professionals Follow-up Study (1986-2012).
In all three cohorts, participants filled in questionnaires at enrollment and every 2 years thereafter with information such as medical history and lifestyle factors. Diet was assessed via validated semi-quantitative food frequency questionnaires.
They used Cox proportional hazards models, adjusting for confounders to estimate the hazard ratios (HRs) and 95% confidence intervals for Crohn’s disease and ulcerative colitis, according to participants’ self-reports of their consumption of ultraprocessed foods.
Further studies could help determine which UPF components are driving the higher risk for Crohn’s disease and whether risk differs by length of exposure to UPFs.
“By avoiding UPF consumption, individuals might substantially lower their risk of developing CD in addition to gaining other health benefits,” the authors wrote.
A coauthor, James M. Richter, MD, is a consultant for Policy Analysis Inc and Takeda Pharmaceuticals. Andrew T. Chan, MD, serves as a consultant for Janssen Pharmaceuticals, Pfizer Inc, and Bayer Pharma AG. Ashwin N. Ananthakrishnan, MD, has served as a scientific advisory board member for Abbvie, Gilead, and Kyn Therapeutics, and received research grants from Pfizer and Merck. The remaining authors disclosed no conflicts. This work was supported by the National Institutes of Health; the Beker Foundation, the Chleck Family Foundation, and the Crohn’s and Colitis Foundation.
Because consumption of industrially manufactured foods has risen in parallel with the incidence of autoimmune diseases, modern diets are hypothesized to contribute to the development of inflammatory bowel disease. In this study, Lo and colleagues conducted a retrospective cohort study to determine if individuals who reported higher levels of ultraprocessed food intake had higher rates of developing IBD. In their adjusted analysis, the authors report that the rate of developing Crohn’s disease was 70% higher for individuals who consumed the highest quartile of ultraprocessed foods; there was no association seen with ulcerative colitis.
While we await clarification about which ingredients are responsible, we should continue to encourage our patients to incorporate whole foods into their diets for both gastrointestinal and cardiometabolic health. At the same time, we must remain empathetic to systemic barriers to accessing and preparing high-quality, minimally processed foods. As such, we should advocate for policies and programs that mitigate food deserts. If food policy remains status quo, this study illustrates a frightening possibility of how disparities in gastrointestinal health equity could worsen in the future.
Ravy K. Vajravelu, MD, MSCE, is an assistant professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Pittsburgh Center for Health Equity Research and Promotion and the VA Pittsburgh Healthcare System. This commentary does not represent the views of the U.S. Department of Veterans Affairs or the United States Government. Dr. Vajravelu reports no relevant disclosures.
Because consumption of industrially manufactured foods has risen in parallel with the incidence of autoimmune diseases, modern diets are hypothesized to contribute to the development of inflammatory bowel disease. In this study, Lo and colleagues conducted a retrospective cohort study to determine if individuals who reported higher levels of ultraprocessed food intake had higher rates of developing IBD. In their adjusted analysis, the authors report that the rate of developing Crohn’s disease was 70% higher for individuals who consumed the highest quartile of ultraprocessed foods; there was no association seen with ulcerative colitis.
While we await clarification about which ingredients are responsible, we should continue to encourage our patients to incorporate whole foods into their diets for both gastrointestinal and cardiometabolic health. At the same time, we must remain empathetic to systemic barriers to accessing and preparing high-quality, minimally processed foods. As such, we should advocate for policies and programs that mitigate food deserts. If food policy remains status quo, this study illustrates a frightening possibility of how disparities in gastrointestinal health equity could worsen in the future.
Ravy K. Vajravelu, MD, MSCE, is an assistant professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Pittsburgh Center for Health Equity Research and Promotion and the VA Pittsburgh Healthcare System. This commentary does not represent the views of the U.S. Department of Veterans Affairs or the United States Government. Dr. Vajravelu reports no relevant disclosures.
Because consumption of industrially manufactured foods has risen in parallel with the incidence of autoimmune diseases, modern diets are hypothesized to contribute to the development of inflammatory bowel disease. In this study, Lo and colleagues conducted a retrospective cohort study to determine if individuals who reported higher levels of ultraprocessed food intake had higher rates of developing IBD. In their adjusted analysis, the authors report that the rate of developing Crohn’s disease was 70% higher for individuals who consumed the highest quartile of ultraprocessed foods; there was no association seen with ulcerative colitis.
While we await clarification about which ingredients are responsible, we should continue to encourage our patients to incorporate whole foods into their diets for both gastrointestinal and cardiometabolic health. At the same time, we must remain empathetic to systemic barriers to accessing and preparing high-quality, minimally processed foods. As such, we should advocate for policies and programs that mitigate food deserts. If food policy remains status quo, this study illustrates a frightening possibility of how disparities in gastrointestinal health equity could worsen in the future.
Ravy K. Vajravelu, MD, MSCE, is an assistant professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Pittsburgh Center for Health Equity Research and Promotion and the VA Pittsburgh Healthcare System. This commentary does not represent the views of the U.S. Department of Veterans Affairs or the United States Government. Dr. Vajravelu reports no relevant disclosures.
Higher consumption of ultraprocessed foods was linked with a significantly higher risk of Crohn’s disease (CD), but not ulcerative colitis, in a large prospective cohort study published online in Clinical Gastroenterology and Hepatology.
Researchers, led by Chun-Han Lo, MD, of Massachusetts General Hospital, Boston, defined ultraprocessed foods “as ready-to-consume formulations of ingredients, typically created by [a] series industrial techniques and processes. They frequently involve the incorporation of additives, such as sweeteners, preservatives, emulsifiers, thickeners, and flavors, which aid in food preservation and produce hyperpalatable products.”
The rising global incidence of inflammatory bowel disease (IBD) in regions undergoing Westernization has overlapped with rising increase in consumption of ultraprocessed food (UPF) over the past few decades, according to the authors. Previous studies have focused on links with individual nutrients and IBD, but this study focuses on the processing role itself. This study comprised 245,112 participants (203,516 women and 41,596 men) and more than 5,468,444 person-years of follow-up, taken from three cohorts: Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study.
In the highest quartile, UPFs made up on average nearly half (46.4%) of participants’ total energy consumption, compared with 21% in the lowest quartile.
The researchers found that compared with participants in the lowest quartile of simple updated UPF consumption, those in the highest quartile had a significantly increased risk of CD (adjusted hazard ratio, 1.70; 95% confidence interval, 1.23-2.35).
In addition, “a secondary analysis across different CD locations demonstrated that participants in the highest quartile of simple updated UPF intake had the highest risk of ileal, colonic, and ileocolonic CD,” the authors wrote.
Three groups of processed foods driving risk increase
Three groups of UPFs appeared to drive the increased risk of CD: ultraprocessed breads and breakfast foods; frozen or shelf-stable ready-to-eat/heat meals; and sauces, cheeses, spreads, and gravies.
Just as with overall consumption, researchers did not find an association between any of those three subgroups and UC risk.
The authors suggested several reasons for the link with Crohn’s disease. Among them were that higher UPF consumption may mean those foods are taking the place of unprocessed or minimally processed foods, such as those rich in fiber. Second, UPFs contain additives, such as salt, that may promote intestinal inflammation. Third, artificial sweeteners in UPFs may predispose the gut to inflammation, as supported by supplementing sucralose/maltodextrins in mice models of spontaneous ileitis.
As for why CD, but not UC, the authors said diet may be more relevant and have a stronger effect biologically in CD compared with UC. Another potential reason, they said, is that results “may reflect the greater specificity of dietary ligands and metabolites on the small intestine compared with the colon.”
Data from three large, prospective cohorts
Researchers used data from three ongoing, prospective nationwide cohorts of health professionals in the United States – the Nurses’ Health Study (1986-2014); the Nurses’ Health Study II (1991-2017); and the Health Professionals Follow-up Study (1986-2012).
In all three cohorts, participants filled in questionnaires at enrollment and every 2 years thereafter with information such as medical history and lifestyle factors. Diet was assessed via validated semi-quantitative food frequency questionnaires.
They used Cox proportional hazards models, adjusting for confounders to estimate the hazard ratios (HRs) and 95% confidence intervals for Crohn’s disease and ulcerative colitis, according to participants’ self-reports of their consumption of ultraprocessed foods.
Further studies could help determine which UPF components are driving the higher risk for Crohn’s disease and whether risk differs by length of exposure to UPFs.
“By avoiding UPF consumption, individuals might substantially lower their risk of developing CD in addition to gaining other health benefits,” the authors wrote.
A coauthor, James M. Richter, MD, is a consultant for Policy Analysis Inc and Takeda Pharmaceuticals. Andrew T. Chan, MD, serves as a consultant for Janssen Pharmaceuticals, Pfizer Inc, and Bayer Pharma AG. Ashwin N. Ananthakrishnan, MD, has served as a scientific advisory board member for Abbvie, Gilead, and Kyn Therapeutics, and received research grants from Pfizer and Merck. The remaining authors disclosed no conflicts. This work was supported by the National Institutes of Health; the Beker Foundation, the Chleck Family Foundation, and the Crohn’s and Colitis Foundation.
Higher consumption of ultraprocessed foods was linked with a significantly higher risk of Crohn’s disease (CD), but not ulcerative colitis, in a large prospective cohort study published online in Clinical Gastroenterology and Hepatology.
Researchers, led by Chun-Han Lo, MD, of Massachusetts General Hospital, Boston, defined ultraprocessed foods “as ready-to-consume formulations of ingredients, typically created by [a] series industrial techniques and processes. They frequently involve the incorporation of additives, such as sweeteners, preservatives, emulsifiers, thickeners, and flavors, which aid in food preservation and produce hyperpalatable products.”
The rising global incidence of inflammatory bowel disease (IBD) in regions undergoing Westernization has overlapped with rising increase in consumption of ultraprocessed food (UPF) over the past few decades, according to the authors. Previous studies have focused on links with individual nutrients and IBD, but this study focuses on the processing role itself. This study comprised 245,112 participants (203,516 women and 41,596 men) and more than 5,468,444 person-years of follow-up, taken from three cohorts: Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study.
In the highest quartile, UPFs made up on average nearly half (46.4%) of participants’ total energy consumption, compared with 21% in the lowest quartile.
The researchers found that compared with participants in the lowest quartile of simple updated UPF consumption, those in the highest quartile had a significantly increased risk of CD (adjusted hazard ratio, 1.70; 95% confidence interval, 1.23-2.35).
In addition, “a secondary analysis across different CD locations demonstrated that participants in the highest quartile of simple updated UPF intake had the highest risk of ileal, colonic, and ileocolonic CD,” the authors wrote.
Three groups of processed foods driving risk increase
Three groups of UPFs appeared to drive the increased risk of CD: ultraprocessed breads and breakfast foods; frozen or shelf-stable ready-to-eat/heat meals; and sauces, cheeses, spreads, and gravies.
Just as with overall consumption, researchers did not find an association between any of those three subgroups and UC risk.
The authors suggested several reasons for the link with Crohn’s disease. Among them were that higher UPF consumption may mean those foods are taking the place of unprocessed or minimally processed foods, such as those rich in fiber. Second, UPFs contain additives, such as salt, that may promote intestinal inflammation. Third, artificial sweeteners in UPFs may predispose the gut to inflammation, as supported by supplementing sucralose/maltodextrins in mice models of spontaneous ileitis.
As for why CD, but not UC, the authors said diet may be more relevant and have a stronger effect biologically in CD compared with UC. Another potential reason, they said, is that results “may reflect the greater specificity of dietary ligands and metabolites on the small intestine compared with the colon.”
Data from three large, prospective cohorts
Researchers used data from three ongoing, prospective nationwide cohorts of health professionals in the United States – the Nurses’ Health Study (1986-2014); the Nurses’ Health Study II (1991-2017); and the Health Professionals Follow-up Study (1986-2012).
In all three cohorts, participants filled in questionnaires at enrollment and every 2 years thereafter with information such as medical history and lifestyle factors. Diet was assessed via validated semi-quantitative food frequency questionnaires.
They used Cox proportional hazards models, adjusting for confounders to estimate the hazard ratios (HRs) and 95% confidence intervals for Crohn’s disease and ulcerative colitis, according to participants’ self-reports of their consumption of ultraprocessed foods.
Further studies could help determine which UPF components are driving the higher risk for Crohn’s disease and whether risk differs by length of exposure to UPFs.
“By avoiding UPF consumption, individuals might substantially lower their risk of developing CD in addition to gaining other health benefits,” the authors wrote.
A coauthor, James M. Richter, MD, is a consultant for Policy Analysis Inc and Takeda Pharmaceuticals. Andrew T. Chan, MD, serves as a consultant for Janssen Pharmaceuticals, Pfizer Inc, and Bayer Pharma AG. Ashwin N. Ananthakrishnan, MD, has served as a scientific advisory board member for Abbvie, Gilead, and Kyn Therapeutics, and received research grants from Pfizer and Merck. The remaining authors disclosed no conflicts. This work was supported by the National Institutes of Health; the Beker Foundation, the Chleck Family Foundation, and the Crohn’s and Colitis Foundation.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Diet’s impact on the microbiome: It’s real, and broad
WASHINGTON – – including amino acid metabolites – that may modify health, said Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia.
During the 2022 Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility, Dr. Wu led a plenary session in which the impact of diet on the microbiome was characterized as important, rapid, personalized, likely modest relative to other contributing ecological factors, influenced by the process of cooking, and exceedingly difficult to tease apart and characterize in human studies.
In a human study published in 2021, Dr. Wu and coinvestigators performed a controlled feeding experiment with 30 healthy volunteers, randomizing them to several weeks of a vegan diet, an omnivore diet (a typical American diet), and an exclusive enteral nutrition diet (EEN) devoid of dietary fiber.
They compared the composition and metabolic function of the gut microbiome during three phases: an initial dietary phase (days 1-5), a purge phase in which antibiotics and polyethylene glycol were administered to transiently reduce bacterial load in the gut (days 6-8), and a recovery phase (days 9-15).
Diversity of the gut microbiota recovered from the purge phase in both vegans and omnivores, but not in those receiving EEN. “The EEN diet was having a profound effect on the [short-term] recovery of microbiota,” said Dr. Wu, the Ferdinand G. Weisbrod Professor in Gastroenterology, in describing the Food And Resulting Microbial Metabolites study.
Using genetic sequencing, microbial culturing, and bioinformatics processing, the researchers also determined that EEN subsequently led to metabolites that were distinct from omnivores and vegans. Unexpectedly, bacterial metabolites of amino acid origin – not only carbohydrate origin – were altered in the EEN group, suggesting a broad impact of dietary fiber on the bacterial metabolome. EEN-induced alterations in the microbiome and metabolome resolved after the study period, he noted.
In other words, “depriving or supplying the gut microbiome with one dietary component (i.e., fiber) can directly impact metabolites of an unrelated portion of the diet (i.e., amino acids) via the induction of specific gut bacterial taxa,” Dr. Wu and colleagues wrote.
Clinically, the results as a whole suggest that the combination of antibiotics with EEN may be less effective in patients with Crohn’s disease than EEN alone, and can be potentially harmful, they said.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Wu said that, for patients in the ICU on EEN treatment and antibiotics, “we do need to think carefully about microbiota reconstitution because it could have a very significant effect not only on short-chain fatty acid metabolites but on amino acid metabolites that may be good or bad in the setting of disease.”
The scientific rationale for the effectiveness of EEN for IBD is still not well understood, he noted. “All I can say is that EEN works in IBD, but there are aspects about the microbiota and diet and IBD that we don’t understand.”
Dietary impact through the immigration lens
In another presentation, Dan Knights, PhD, of the University of Minnesota, Minneapolis, described his lab’s findings on the association of U.S. immigration with loss of gut microbiome diversity, and the role of diet.
As part of the Immigration Microbiome Project reported several years ago, his team collected stool, dietary recalls, and anthropometrics from 550 Hmong and Karen individuals living in Thailand and the United States, including first- and second-generation immigrants, as well as some U.S.-born European American individuals. They found that the gut microbiome of immigrants changed within months of arriving in the United States, and that immigration status had a stronger effect on the microbiome than obesity status.
“By the time people were in their second generation, their microbiomes were roughly on the same order of diversity as U.S. controls,” said Dr. Knights, associate professor in the Biotechnology Institute and the department of computer science and engineering.
Dietary changes only partly explained microbiome variation, however. “By the second generation, the microbiome tended to be fully Westernized, but the diet was only partly Westernized,” he said. “Diet is only part of the story.”
Other research from his lab, including one study that performed daily fecal shotgun sequencing on 34 people, has found that effects of diet on the microbiome are likely to be observable within days, and that microbial responses to food are highly personalized. Diet appears to explain about 6% of the daily microbiome variation within an individual, and “an average diet explains about 4% of microbiome variation between people,” Dr. Knights said.
The impact of cooking
“The gut microbiota responds to food and to its form,” said Rachel N. Carmody, PhD, of the department of human evolutionary biology at Harvard University, Cambridge, during the plenary session. Her research has shown that, in mice, a plant diet served raw versus cooked quickly reshaped the gut microbiome and disrupted gut microbial physiology. Notably, shifts in gut microbiota modulated host energy status – one of the many areas that begs further research.
The effects of cooking have also been detectable in human pilot studies. “We saw different changes in the microbiome when [study participants] were eating the same plant items either raw or cooked,” she said. “Some microbes were affected only on the raw diet, other were affected only on the cooked diet.”
Other research in animal models and humans has demonstrated a significant amount of plasticity in the microbiome in response to diet. “In mice you get the microbiome signatures to shift within 24 hours by feeding them a new diet,” Dr. Carmody said.
In an interview about the plenary session, Eugene B. Chang, MD, the Martin Boyer Distinguished Professor of Medicine at the University of Chicago, said that he was struck both by the resiliency of individual gut microbiomes overall and by findings that, “in animal models where conditions and diets can be carefully controlled, diet and environment are major drivers of gut microbial membership and function.”
Dr. Chang coled a separate workshop on “defining a healthy gut microbiome” – a task that he said remains “a challenge [and is not yet] resolved, at least with general consensus.”
Dr. Chang, Dr. Wu, and Dr. Carmody reported no relevant disclosures. Dr. Knights disclosed that he is a paid adviser to Diversigen, a company involved with the commercialization of microbiome analysis.
The 2022 Gut Microbiota for Health World Summit was supported by sponsorships from Danone, Ferring Pharmaceuticals, Aimmune Therapeutics and Seres Therapeutics, Sanofi, and Intrinsic Medicine Inc. with additional support from educational grants provided by Ferring Pharmaceuticals and Salix Pharmaceuticals.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/
WASHINGTON – – including amino acid metabolites – that may modify health, said Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia.
During the 2022 Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility, Dr. Wu led a plenary session in which the impact of diet on the microbiome was characterized as important, rapid, personalized, likely modest relative to other contributing ecological factors, influenced by the process of cooking, and exceedingly difficult to tease apart and characterize in human studies.
In a human study published in 2021, Dr. Wu and coinvestigators performed a controlled feeding experiment with 30 healthy volunteers, randomizing them to several weeks of a vegan diet, an omnivore diet (a typical American diet), and an exclusive enteral nutrition diet (EEN) devoid of dietary fiber.
They compared the composition and metabolic function of the gut microbiome during three phases: an initial dietary phase (days 1-5), a purge phase in which antibiotics and polyethylene glycol were administered to transiently reduce bacterial load in the gut (days 6-8), and a recovery phase (days 9-15).
Diversity of the gut microbiota recovered from the purge phase in both vegans and omnivores, but not in those receiving EEN. “The EEN diet was having a profound effect on the [short-term] recovery of microbiota,” said Dr. Wu, the Ferdinand G. Weisbrod Professor in Gastroenterology, in describing the Food And Resulting Microbial Metabolites study.
Using genetic sequencing, microbial culturing, and bioinformatics processing, the researchers also determined that EEN subsequently led to metabolites that were distinct from omnivores and vegans. Unexpectedly, bacterial metabolites of amino acid origin – not only carbohydrate origin – were altered in the EEN group, suggesting a broad impact of dietary fiber on the bacterial metabolome. EEN-induced alterations in the microbiome and metabolome resolved after the study period, he noted.
In other words, “depriving or supplying the gut microbiome with one dietary component (i.e., fiber) can directly impact metabolites of an unrelated portion of the diet (i.e., amino acids) via the induction of specific gut bacterial taxa,” Dr. Wu and colleagues wrote.
Clinically, the results as a whole suggest that the combination of antibiotics with EEN may be less effective in patients with Crohn’s disease than EEN alone, and can be potentially harmful, they said.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Wu said that, for patients in the ICU on EEN treatment and antibiotics, “we do need to think carefully about microbiota reconstitution because it could have a very significant effect not only on short-chain fatty acid metabolites but on amino acid metabolites that may be good or bad in the setting of disease.”
The scientific rationale for the effectiveness of EEN for IBD is still not well understood, he noted. “All I can say is that EEN works in IBD, but there are aspects about the microbiota and diet and IBD that we don’t understand.”
Dietary impact through the immigration lens
In another presentation, Dan Knights, PhD, of the University of Minnesota, Minneapolis, described his lab’s findings on the association of U.S. immigration with loss of gut microbiome diversity, and the role of diet.
As part of the Immigration Microbiome Project reported several years ago, his team collected stool, dietary recalls, and anthropometrics from 550 Hmong and Karen individuals living in Thailand and the United States, including first- and second-generation immigrants, as well as some U.S.-born European American individuals. They found that the gut microbiome of immigrants changed within months of arriving in the United States, and that immigration status had a stronger effect on the microbiome than obesity status.
“By the time people were in their second generation, their microbiomes were roughly on the same order of diversity as U.S. controls,” said Dr. Knights, associate professor in the Biotechnology Institute and the department of computer science and engineering.
Dietary changes only partly explained microbiome variation, however. “By the second generation, the microbiome tended to be fully Westernized, but the diet was only partly Westernized,” he said. “Diet is only part of the story.”
Other research from his lab, including one study that performed daily fecal shotgun sequencing on 34 people, has found that effects of diet on the microbiome are likely to be observable within days, and that microbial responses to food are highly personalized. Diet appears to explain about 6% of the daily microbiome variation within an individual, and “an average diet explains about 4% of microbiome variation between people,” Dr. Knights said.
The impact of cooking
“The gut microbiota responds to food and to its form,” said Rachel N. Carmody, PhD, of the department of human evolutionary biology at Harvard University, Cambridge, during the plenary session. Her research has shown that, in mice, a plant diet served raw versus cooked quickly reshaped the gut microbiome and disrupted gut microbial physiology. Notably, shifts in gut microbiota modulated host energy status – one of the many areas that begs further research.
The effects of cooking have also been detectable in human pilot studies. “We saw different changes in the microbiome when [study participants] were eating the same plant items either raw or cooked,” she said. “Some microbes were affected only on the raw diet, other were affected only on the cooked diet.”
Other research in animal models and humans has demonstrated a significant amount of plasticity in the microbiome in response to diet. “In mice you get the microbiome signatures to shift within 24 hours by feeding them a new diet,” Dr. Carmody said.
In an interview about the plenary session, Eugene B. Chang, MD, the Martin Boyer Distinguished Professor of Medicine at the University of Chicago, said that he was struck both by the resiliency of individual gut microbiomes overall and by findings that, “in animal models where conditions and diets can be carefully controlled, diet and environment are major drivers of gut microbial membership and function.”
Dr. Chang coled a separate workshop on “defining a healthy gut microbiome” – a task that he said remains “a challenge [and is not yet] resolved, at least with general consensus.”
Dr. Chang, Dr. Wu, and Dr. Carmody reported no relevant disclosures. Dr. Knights disclosed that he is a paid adviser to Diversigen, a company involved with the commercialization of microbiome analysis.
The 2022 Gut Microbiota for Health World Summit was supported by sponsorships from Danone, Ferring Pharmaceuticals, Aimmune Therapeutics and Seres Therapeutics, Sanofi, and Intrinsic Medicine Inc. with additional support from educational grants provided by Ferring Pharmaceuticals and Salix Pharmaceuticals.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/
WASHINGTON – – including amino acid metabolites – that may modify health, said Gary D. Wu, MD, of the University of Pennsylvania, Philadelphia.
During the 2022 Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association and the European Society of Neurogastroenterology and Motility, Dr. Wu led a plenary session in which the impact of diet on the microbiome was characterized as important, rapid, personalized, likely modest relative to other contributing ecological factors, influenced by the process of cooking, and exceedingly difficult to tease apart and characterize in human studies.
In a human study published in 2021, Dr. Wu and coinvestigators performed a controlled feeding experiment with 30 healthy volunteers, randomizing them to several weeks of a vegan diet, an omnivore diet (a typical American diet), and an exclusive enteral nutrition diet (EEN) devoid of dietary fiber.
They compared the composition and metabolic function of the gut microbiome during three phases: an initial dietary phase (days 1-5), a purge phase in which antibiotics and polyethylene glycol were administered to transiently reduce bacterial load in the gut (days 6-8), and a recovery phase (days 9-15).
Diversity of the gut microbiota recovered from the purge phase in both vegans and omnivores, but not in those receiving EEN. “The EEN diet was having a profound effect on the [short-term] recovery of microbiota,” said Dr. Wu, the Ferdinand G. Weisbrod Professor in Gastroenterology, in describing the Food And Resulting Microbial Metabolites study.
Using genetic sequencing, microbial culturing, and bioinformatics processing, the researchers also determined that EEN subsequently led to metabolites that were distinct from omnivores and vegans. Unexpectedly, bacterial metabolites of amino acid origin – not only carbohydrate origin – were altered in the EEN group, suggesting a broad impact of dietary fiber on the bacterial metabolome. EEN-induced alterations in the microbiome and metabolome resolved after the study period, he noted.
In other words, “depriving or supplying the gut microbiome with one dietary component (i.e., fiber) can directly impact metabolites of an unrelated portion of the diet (i.e., amino acids) via the induction of specific gut bacterial taxa,” Dr. Wu and colleagues wrote.
Clinically, the results as a whole suggest that the combination of antibiotics with EEN may be less effective in patients with Crohn’s disease than EEN alone, and can be potentially harmful, they said.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Wu said that, for patients in the ICU on EEN treatment and antibiotics, “we do need to think carefully about microbiota reconstitution because it could have a very significant effect not only on short-chain fatty acid metabolites but on amino acid metabolites that may be good or bad in the setting of disease.”
The scientific rationale for the effectiveness of EEN for IBD is still not well understood, he noted. “All I can say is that EEN works in IBD, but there are aspects about the microbiota and diet and IBD that we don’t understand.”
Dietary impact through the immigration lens
In another presentation, Dan Knights, PhD, of the University of Minnesota, Minneapolis, described his lab’s findings on the association of U.S. immigration with loss of gut microbiome diversity, and the role of diet.
As part of the Immigration Microbiome Project reported several years ago, his team collected stool, dietary recalls, and anthropometrics from 550 Hmong and Karen individuals living in Thailand and the United States, including first- and second-generation immigrants, as well as some U.S.-born European American individuals. They found that the gut microbiome of immigrants changed within months of arriving in the United States, and that immigration status had a stronger effect on the microbiome than obesity status.
“By the time people were in their second generation, their microbiomes were roughly on the same order of diversity as U.S. controls,” said Dr. Knights, associate professor in the Biotechnology Institute and the department of computer science and engineering.
Dietary changes only partly explained microbiome variation, however. “By the second generation, the microbiome tended to be fully Westernized, but the diet was only partly Westernized,” he said. “Diet is only part of the story.”
Other research from his lab, including one study that performed daily fecal shotgun sequencing on 34 people, has found that effects of diet on the microbiome are likely to be observable within days, and that microbial responses to food are highly personalized. Diet appears to explain about 6% of the daily microbiome variation within an individual, and “an average diet explains about 4% of microbiome variation between people,” Dr. Knights said.
The impact of cooking
“The gut microbiota responds to food and to its form,” said Rachel N. Carmody, PhD, of the department of human evolutionary biology at Harvard University, Cambridge, during the plenary session. Her research has shown that, in mice, a plant diet served raw versus cooked quickly reshaped the gut microbiome and disrupted gut microbial physiology. Notably, shifts in gut microbiota modulated host energy status – one of the many areas that begs further research.
The effects of cooking have also been detectable in human pilot studies. “We saw different changes in the microbiome when [study participants] were eating the same plant items either raw or cooked,” she said. “Some microbes were affected only on the raw diet, other were affected only on the cooked diet.”
Other research in animal models and humans has demonstrated a significant amount of plasticity in the microbiome in response to diet. “In mice you get the microbiome signatures to shift within 24 hours by feeding them a new diet,” Dr. Carmody said.
In an interview about the plenary session, Eugene B. Chang, MD, the Martin Boyer Distinguished Professor of Medicine at the University of Chicago, said that he was struck both by the resiliency of individual gut microbiomes overall and by findings that, “in animal models where conditions and diets can be carefully controlled, diet and environment are major drivers of gut microbial membership and function.”
Dr. Chang coled a separate workshop on “defining a healthy gut microbiome” – a task that he said remains “a challenge [and is not yet] resolved, at least with general consensus.”
Dr. Chang, Dr. Wu, and Dr. Carmody reported no relevant disclosures. Dr. Knights disclosed that he is a paid adviser to Diversigen, a company involved with the commercialization of microbiome analysis.
The 2022 Gut Microbiota for Health World Summit was supported by sponsorships from Danone, Ferring Pharmaceuticals, Aimmune Therapeutics and Seres Therapeutics, Sanofi, and Intrinsic Medicine Inc. with additional support from educational grants provided by Ferring Pharmaceuticals and Salix Pharmaceuticals.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/
AT GMFH 2022
Researchers present cellular atlas of the human gut
New research sheds light on how different cell types behave across all intestinal regions and demonstrates variations in gene expression between these cells across three independent organ donors.
Research led by Joseph Burclaff, PhD, of the University of North Carolina at Chapel Hill, explained that the regional differences observed in the study “highlight the importance of regional selection when studying the gut.” Dr. Burclaff and colleagues, whose findings were published online in Cellular and Molecular Gastroenterology and Hepatology, wrote that they hope their “database serves as a resource to understand how drugs affect the intestinal epithelium and as guidance for future precision medicine approaches.”
In the study, Dr. Burclaff and colleagues performed single-cell transcriptomics that covered the duodenum, jejunum, ileum, as well as ascending, descending, and transverse colon from three independently processed organ donors. The donors varied in age, race, and body mass index.
The investigators evaluated 12,590 single epithelial cells for organ-specific lineage biomarkers, differentially regulated genes, receptors, and drug targets. The focus of the analyses was on intrinsic cell properties and their capacity for response to extrinsic signals found along the gut axis.
The research group assigned cells to 25 epithelial lineage clusters. According to the researchers, multiple accepted intestinal cell markers did not specifically mark all intestinal stem cells. In addition, the investigators explained that lysozyme expression was not unique to Paneth cells, and these cells lacked expression of certain “expected niche factors.” In fact, the researchers demonstrated lysozyme’s insufficiency for marking human Paneth cells.
Bestrophin-4þ (BEST4þ) cells, which expressed neuropeptide Y, demonstrated maturational differences between the colon and small intestine, suggesting organ-specific maturation for tuft and BEST4+ cells. In addition, the data from Dr. Burclaff and colleagues suggest BEST4+ cells are engaged in “diverse roles within the intestinal epithelium, laying the groundwork for functional studies.”
The researchers noted that “tuft cells possess a broad ability to interact with the innate and adaptive immune systems through previously unreported receptors.” Specifically, the researchers found these cells exhibit genes believed to be important for taste signaling, monitoring intestinal content, and signaling the immune system.
Certain classes of cell junctions, hormones, mucins, and nutrient absorption genes demonstrated “unappreciated regional expression differences across lineages,” the researchers wrote. The investigators added that the differential expression of receptors as well as drug targets across lineages demonstrated “biological variation and the potential for variegated responses.”
The researchers noted that while the regional differences identified in their study show the importance of regional selection during gut investigations, several previous colonic single-cell RNA sequencing studies did not specify the sample region or explain “if pooled samples are from consistent regions.”
In the study, the investigators also assessed how drugs may affect the intestinal epithelium and why certain side effects associated with pharmacologic agents occur. The researchers identified 498 drugs approved by the Food and Drug Administration that had 232 primary gene targets expressed in the gut epithelial dataset.
In their analysis, the researchers found that carboxylesterase-2, which metabolizes the drug irinotecan into biologically active SN-38, is the highest expressed phase 1 metabolism gene in the small intestine. Phase 2 enzyme UGT1A1, which inactivates SN-38, features low gut epithelial expression. The researchers explained that this finding suggests the cancer drug irinotecan may feature prolonged gut activation, supporting the notion that the orally administered agent may have efficacy against cancers of the intestine.
The researchers concluded their “database provides a foundation for understanding individual contributions of diverse epithelial cells across the length of the human intestine and colon to maintain physiologic function.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
Single cell transcriptomics has revolutionized our understanding of complex tissues, as this technology enables the identification of rare and/or novel cell types. Gastrointestinal science has benefited greatly from these technical advances, with multiple studies profiling liver, pancreas, stomach and intestine in health and disease, both in mouse and human samples.
In sum, this study is the “final answer” for GI biologists needing a complete compendium of all genes active in the multitude of specialized human intestinal epithelial cells.
Klaus H. Kaestner, PhD, MS, is with the department of genetics and the Center for Molecular Studies in Digestive and Liver Diseases at the University of Pennsylvania, Philadelphia. He declares having no conflicts of interest.
Single cell transcriptomics has revolutionized our understanding of complex tissues, as this technology enables the identification of rare and/or novel cell types. Gastrointestinal science has benefited greatly from these technical advances, with multiple studies profiling liver, pancreas, stomach and intestine in health and disease, both in mouse and human samples.
In sum, this study is the “final answer” for GI biologists needing a complete compendium of all genes active in the multitude of specialized human intestinal epithelial cells.
Klaus H. Kaestner, PhD, MS, is with the department of genetics and the Center for Molecular Studies in Digestive and Liver Diseases at the University of Pennsylvania, Philadelphia. He declares having no conflicts of interest.
Single cell transcriptomics has revolutionized our understanding of complex tissues, as this technology enables the identification of rare and/or novel cell types. Gastrointestinal science has benefited greatly from these technical advances, with multiple studies profiling liver, pancreas, stomach and intestine in health and disease, both in mouse and human samples.
In sum, this study is the “final answer” for GI biologists needing a complete compendium of all genes active in the multitude of specialized human intestinal epithelial cells.
Klaus H. Kaestner, PhD, MS, is with the department of genetics and the Center for Molecular Studies in Digestive and Liver Diseases at the University of Pennsylvania, Philadelphia. He declares having no conflicts of interest.
New research sheds light on how different cell types behave across all intestinal regions and demonstrates variations in gene expression between these cells across three independent organ donors.
Research led by Joseph Burclaff, PhD, of the University of North Carolina at Chapel Hill, explained that the regional differences observed in the study “highlight the importance of regional selection when studying the gut.” Dr. Burclaff and colleagues, whose findings were published online in Cellular and Molecular Gastroenterology and Hepatology, wrote that they hope their “database serves as a resource to understand how drugs affect the intestinal epithelium and as guidance for future precision medicine approaches.”
In the study, Dr. Burclaff and colleagues performed single-cell transcriptomics that covered the duodenum, jejunum, ileum, as well as ascending, descending, and transverse colon from three independently processed organ donors. The donors varied in age, race, and body mass index.
The investigators evaluated 12,590 single epithelial cells for organ-specific lineage biomarkers, differentially regulated genes, receptors, and drug targets. The focus of the analyses was on intrinsic cell properties and their capacity for response to extrinsic signals found along the gut axis.
The research group assigned cells to 25 epithelial lineage clusters. According to the researchers, multiple accepted intestinal cell markers did not specifically mark all intestinal stem cells. In addition, the investigators explained that lysozyme expression was not unique to Paneth cells, and these cells lacked expression of certain “expected niche factors.” In fact, the researchers demonstrated lysozyme’s insufficiency for marking human Paneth cells.
Bestrophin-4þ (BEST4þ) cells, which expressed neuropeptide Y, demonstrated maturational differences between the colon and small intestine, suggesting organ-specific maturation for tuft and BEST4+ cells. In addition, the data from Dr. Burclaff and colleagues suggest BEST4+ cells are engaged in “diverse roles within the intestinal epithelium, laying the groundwork for functional studies.”
The researchers noted that “tuft cells possess a broad ability to interact with the innate and adaptive immune systems through previously unreported receptors.” Specifically, the researchers found these cells exhibit genes believed to be important for taste signaling, monitoring intestinal content, and signaling the immune system.
Certain classes of cell junctions, hormones, mucins, and nutrient absorption genes demonstrated “unappreciated regional expression differences across lineages,” the researchers wrote. The investigators added that the differential expression of receptors as well as drug targets across lineages demonstrated “biological variation and the potential for variegated responses.”
The researchers noted that while the regional differences identified in their study show the importance of regional selection during gut investigations, several previous colonic single-cell RNA sequencing studies did not specify the sample region or explain “if pooled samples are from consistent regions.”
In the study, the investigators also assessed how drugs may affect the intestinal epithelium and why certain side effects associated with pharmacologic agents occur. The researchers identified 498 drugs approved by the Food and Drug Administration that had 232 primary gene targets expressed in the gut epithelial dataset.
In their analysis, the researchers found that carboxylesterase-2, which metabolizes the drug irinotecan into biologically active SN-38, is the highest expressed phase 1 metabolism gene in the small intestine. Phase 2 enzyme UGT1A1, which inactivates SN-38, features low gut epithelial expression. The researchers explained that this finding suggests the cancer drug irinotecan may feature prolonged gut activation, supporting the notion that the orally administered agent may have efficacy against cancers of the intestine.
The researchers concluded their “database provides a foundation for understanding individual contributions of diverse epithelial cells across the length of the human intestine and colon to maintain physiologic function.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
New research sheds light on how different cell types behave across all intestinal regions and demonstrates variations in gene expression between these cells across three independent organ donors.
Research led by Joseph Burclaff, PhD, of the University of North Carolina at Chapel Hill, explained that the regional differences observed in the study “highlight the importance of regional selection when studying the gut.” Dr. Burclaff and colleagues, whose findings were published online in Cellular and Molecular Gastroenterology and Hepatology, wrote that they hope their “database serves as a resource to understand how drugs affect the intestinal epithelium and as guidance for future precision medicine approaches.”
In the study, Dr. Burclaff and colleagues performed single-cell transcriptomics that covered the duodenum, jejunum, ileum, as well as ascending, descending, and transverse colon from three independently processed organ donors. The donors varied in age, race, and body mass index.
The investigators evaluated 12,590 single epithelial cells for organ-specific lineage biomarkers, differentially regulated genes, receptors, and drug targets. The focus of the analyses was on intrinsic cell properties and their capacity for response to extrinsic signals found along the gut axis.
The research group assigned cells to 25 epithelial lineage clusters. According to the researchers, multiple accepted intestinal cell markers did not specifically mark all intestinal stem cells. In addition, the investigators explained that lysozyme expression was not unique to Paneth cells, and these cells lacked expression of certain “expected niche factors.” In fact, the researchers demonstrated lysozyme’s insufficiency for marking human Paneth cells.
Bestrophin-4þ (BEST4þ) cells, which expressed neuropeptide Y, demonstrated maturational differences between the colon and small intestine, suggesting organ-specific maturation for tuft and BEST4+ cells. In addition, the data from Dr. Burclaff and colleagues suggest BEST4+ cells are engaged in “diverse roles within the intestinal epithelium, laying the groundwork for functional studies.”
The researchers noted that “tuft cells possess a broad ability to interact with the innate and adaptive immune systems through previously unreported receptors.” Specifically, the researchers found these cells exhibit genes believed to be important for taste signaling, monitoring intestinal content, and signaling the immune system.
Certain classes of cell junctions, hormones, mucins, and nutrient absorption genes demonstrated “unappreciated regional expression differences across lineages,” the researchers wrote. The investigators added that the differential expression of receptors as well as drug targets across lineages demonstrated “biological variation and the potential for variegated responses.”
The researchers noted that while the regional differences identified in their study show the importance of regional selection during gut investigations, several previous colonic single-cell RNA sequencing studies did not specify the sample region or explain “if pooled samples are from consistent regions.”
In the study, the investigators also assessed how drugs may affect the intestinal epithelium and why certain side effects associated with pharmacologic agents occur. The researchers identified 498 drugs approved by the Food and Drug Administration that had 232 primary gene targets expressed in the gut epithelial dataset.
In their analysis, the researchers found that carboxylesterase-2, which metabolizes the drug irinotecan into biologically active SN-38, is the highest expressed phase 1 metabolism gene in the small intestine. Phase 2 enzyme UGT1A1, which inactivates SN-38, features low gut epithelial expression. The researchers explained that this finding suggests the cancer drug irinotecan may feature prolonged gut activation, supporting the notion that the orally administered agent may have efficacy against cancers of the intestine.
The researchers concluded their “database provides a foundation for understanding individual contributions of diverse epithelial cells across the length of the human intestine and colon to maintain physiologic function.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The study received no industry funding.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
IBD: Patients struggle with presenteeism, mental health
Anxiety and depression are common among individuals with inflammatory bowel disease (IBD) who are in remission, and the conditions are linked to unemployment and presenteeism, according to a prospective study.
Previous studies have found a heightened risk of anxiety and depression in IBD, as well as an association with poor treatment compliance and greater morbidity. Presenteeism, defined as reduced productivity due to a physical or mental condition, is increasingly recognized as an indirect economic cost of chronic conditions that may exact a higher cost than absenteeism.
More than one-third of patients with IBD experienced presenteeism in one study. Other studies have examined exercise in chronic diseases, and most find an association between more exercise and lower rates of anxiety and depression. To date, few studies have examined a combination of physical activity, mental health, and presenteeism in the context of IBD.
The new study, published in the Journal of Crohn’s and Colitis, “is very important, as presenteeism is not commonly discussed in any formal, measurable way in the IBD space,” said Laurie Keefer, PhD, professor of medicine and a gastropsychologist at Icahn School of Medicine, who was asked to comment on the research. “While conducted in Europe and Israel, the study also has relevance in the U.S. since, here, employers typically pay for health care – so absenteeism, presenteeism, and health care cost are all intertwined.”
“The study confirmed high rates of depression and anxiety after a diagnosis of IBD. One high-quality aspect of this study was that the diagnosis of depression and anxiety was confirmed by a medical practitioner,” said Dr. Keefer.
The results suggest that current efforts to screen IBD patients for depression and anxiety may not be enough, according to Stephen Lupe, PsyD, who was also asked to comment on the study. He noted that almost half of the patients had symptoms of anxiety or depression as measured by the Hospital Anxiety and Depression Score (HADS). “We’re missing all kinds of people,” said Dr. Lupe, who is director of behavioral medicine for the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. He cited other data that show that, if someone develops depression, they are more likely to have a surgical outcome, and they are more likely to have problems with medication adherence. They likely will have more flares and less time in remission. “We need to be screening for it as part of care,” he said.
The researchers prospectively studied 585 IBD patients who were in clinical remission at 8 centers in Europe and Israel between September 2020 and March 2021. Participants filled out the HADS and The Stanford Presenteeism scale (SPS-6). A total of 62.2% of the participants had CD; 53.0% were male. Participants’ mean age was 39 years. Among the group, 10.8% had a pre existing diagnosis of anxiety or depression at the time of IBD diagnosis, and an additional 14.2% were later diagnosed with anxiety or depression.
Less than half, 46.1%, of IBD patients had a score 8 or higher in the HADS-anxiety (HADS-A) or HADS-depression (HADS-D) subscale, a cutoff that suggests evidence of clinical depression or anxiety. A total of 27.4% had a score of 11 or higher, indicating a mood disorder. High HADS-A score was associated with female gender (odds ratio, 1.91; P < 0.05), long duration of disease (OR, 1.04; P < 0.01), and perianal disease (P < 0.023). The authors speculate that the latter result may be due to a higher burden of symptoms. Three-quarters, 74.5%, of patients were employed; 34.0% experienced presenteeism as defined by SPS-6 score less than or equal to 18.
The researchers found that 23.0% of the patients were sedentary, and this was more common among individuals with HADS-A or HADS-D scores greater than or equal to 8. Among those experiencing presenteeism, 50% were sedentary, 29.4% were active, and 20.6% were moderately active (P < 0.01). Individuals with higher HADS-A or HADS-D scores had a greater likelihood of being sedentary (P < 0.05).
One limitation of the study was that the questionnaires were translated into the respective languages and scores were taken at only one time point.
“Rather than relying on the patient to come forward and seek help,” physicians should familiarize themselves with these validated screening tools “in order to increase the diagnostic rate of such pathologies and enable a better holistic care for the IBD patients,” the authors concluded. “Active involvement of a psychologist and/or a psychiatrist, as part of the IBD team, should be pursued to further improve the patients’ quality of life, which has emerged as one of the top priority outcomes in IBD.”
Dr. Lupe said that the findings regarding presenteeism are consistent with his experience. He pointed out that IBD patients must be more aware of their body and vigilant in managing symptoms, and he speculated that that could detract from concentration at work. He said that the study shows the need for a holistic approach to treatment. “When someone is coping with a chronic disease, like ulcerative colitis or Crohn’s disease, it affects the whole person,” including psychologically, professionally, and personally. “These are bidirectional relationships, so that if someone’s social life starts falling down, it’s more contributory to the development of something like depression and anxiety, and maybe that’s contributory to complications that come up in a disease state like Crohn’s disease or ulcerative colitis.”
The study did not receive funding, but two authors disclosed relations with AbbVie, Janssen, Pfizer, and other companies. Dr. Keefer is a cofounder and has equity ownership In Trellus Health. Dr. Lupe has no relevant financial disclosures.
Anxiety and depression are common among individuals with inflammatory bowel disease (IBD) who are in remission, and the conditions are linked to unemployment and presenteeism, according to a prospective study.
Previous studies have found a heightened risk of anxiety and depression in IBD, as well as an association with poor treatment compliance and greater morbidity. Presenteeism, defined as reduced productivity due to a physical or mental condition, is increasingly recognized as an indirect economic cost of chronic conditions that may exact a higher cost than absenteeism.
More than one-third of patients with IBD experienced presenteeism in one study. Other studies have examined exercise in chronic diseases, and most find an association between more exercise and lower rates of anxiety and depression. To date, few studies have examined a combination of physical activity, mental health, and presenteeism in the context of IBD.
The new study, published in the Journal of Crohn’s and Colitis, “is very important, as presenteeism is not commonly discussed in any formal, measurable way in the IBD space,” said Laurie Keefer, PhD, professor of medicine and a gastropsychologist at Icahn School of Medicine, who was asked to comment on the research. “While conducted in Europe and Israel, the study also has relevance in the U.S. since, here, employers typically pay for health care – so absenteeism, presenteeism, and health care cost are all intertwined.”
“The study confirmed high rates of depression and anxiety after a diagnosis of IBD. One high-quality aspect of this study was that the diagnosis of depression and anxiety was confirmed by a medical practitioner,” said Dr. Keefer.
The results suggest that current efforts to screen IBD patients for depression and anxiety may not be enough, according to Stephen Lupe, PsyD, who was also asked to comment on the study. He noted that almost half of the patients had symptoms of anxiety or depression as measured by the Hospital Anxiety and Depression Score (HADS). “We’re missing all kinds of people,” said Dr. Lupe, who is director of behavioral medicine for the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. He cited other data that show that, if someone develops depression, they are more likely to have a surgical outcome, and they are more likely to have problems with medication adherence. They likely will have more flares and less time in remission. “We need to be screening for it as part of care,” he said.
The researchers prospectively studied 585 IBD patients who were in clinical remission at 8 centers in Europe and Israel between September 2020 and March 2021. Participants filled out the HADS and The Stanford Presenteeism scale (SPS-6). A total of 62.2% of the participants had CD; 53.0% were male. Participants’ mean age was 39 years. Among the group, 10.8% had a pre existing diagnosis of anxiety or depression at the time of IBD diagnosis, and an additional 14.2% were later diagnosed with anxiety or depression.
Less than half, 46.1%, of IBD patients had a score 8 or higher in the HADS-anxiety (HADS-A) or HADS-depression (HADS-D) subscale, a cutoff that suggests evidence of clinical depression or anxiety. A total of 27.4% had a score of 11 or higher, indicating a mood disorder. High HADS-A score was associated with female gender (odds ratio, 1.91; P < 0.05), long duration of disease (OR, 1.04; P < 0.01), and perianal disease (P < 0.023). The authors speculate that the latter result may be due to a higher burden of symptoms. Three-quarters, 74.5%, of patients were employed; 34.0% experienced presenteeism as defined by SPS-6 score less than or equal to 18.
The researchers found that 23.0% of the patients were sedentary, and this was more common among individuals with HADS-A or HADS-D scores greater than or equal to 8. Among those experiencing presenteeism, 50% were sedentary, 29.4% were active, and 20.6% were moderately active (P < 0.01). Individuals with higher HADS-A or HADS-D scores had a greater likelihood of being sedentary (P < 0.05).
One limitation of the study was that the questionnaires were translated into the respective languages and scores were taken at only one time point.
“Rather than relying on the patient to come forward and seek help,” physicians should familiarize themselves with these validated screening tools “in order to increase the diagnostic rate of such pathologies and enable a better holistic care for the IBD patients,” the authors concluded. “Active involvement of a psychologist and/or a psychiatrist, as part of the IBD team, should be pursued to further improve the patients’ quality of life, which has emerged as one of the top priority outcomes in IBD.”
Dr. Lupe said that the findings regarding presenteeism are consistent with his experience. He pointed out that IBD patients must be more aware of their body and vigilant in managing symptoms, and he speculated that that could detract from concentration at work. He said that the study shows the need for a holistic approach to treatment. “When someone is coping with a chronic disease, like ulcerative colitis or Crohn’s disease, it affects the whole person,” including psychologically, professionally, and personally. “These are bidirectional relationships, so that if someone’s social life starts falling down, it’s more contributory to the development of something like depression and anxiety, and maybe that’s contributory to complications that come up in a disease state like Crohn’s disease or ulcerative colitis.”
The study did not receive funding, but two authors disclosed relations with AbbVie, Janssen, Pfizer, and other companies. Dr. Keefer is a cofounder and has equity ownership In Trellus Health. Dr. Lupe has no relevant financial disclosures.
Anxiety and depression are common among individuals with inflammatory bowel disease (IBD) who are in remission, and the conditions are linked to unemployment and presenteeism, according to a prospective study.
Previous studies have found a heightened risk of anxiety and depression in IBD, as well as an association with poor treatment compliance and greater morbidity. Presenteeism, defined as reduced productivity due to a physical or mental condition, is increasingly recognized as an indirect economic cost of chronic conditions that may exact a higher cost than absenteeism.
More than one-third of patients with IBD experienced presenteeism in one study. Other studies have examined exercise in chronic diseases, and most find an association between more exercise and lower rates of anxiety and depression. To date, few studies have examined a combination of physical activity, mental health, and presenteeism in the context of IBD.
The new study, published in the Journal of Crohn’s and Colitis, “is very important, as presenteeism is not commonly discussed in any formal, measurable way in the IBD space,” said Laurie Keefer, PhD, professor of medicine and a gastropsychologist at Icahn School of Medicine, who was asked to comment on the research. “While conducted in Europe and Israel, the study also has relevance in the U.S. since, here, employers typically pay for health care – so absenteeism, presenteeism, and health care cost are all intertwined.”
“The study confirmed high rates of depression and anxiety after a diagnosis of IBD. One high-quality aspect of this study was that the diagnosis of depression and anxiety was confirmed by a medical practitioner,” said Dr. Keefer.
The results suggest that current efforts to screen IBD patients for depression and anxiety may not be enough, according to Stephen Lupe, PsyD, who was also asked to comment on the study. He noted that almost half of the patients had symptoms of anxiety or depression as measured by the Hospital Anxiety and Depression Score (HADS). “We’re missing all kinds of people,” said Dr. Lupe, who is director of behavioral medicine for the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic. He cited other data that show that, if someone develops depression, they are more likely to have a surgical outcome, and they are more likely to have problems with medication adherence. They likely will have more flares and less time in remission. “We need to be screening for it as part of care,” he said.
The researchers prospectively studied 585 IBD patients who were in clinical remission at 8 centers in Europe and Israel between September 2020 and March 2021. Participants filled out the HADS and The Stanford Presenteeism scale (SPS-6). A total of 62.2% of the participants had CD; 53.0% were male. Participants’ mean age was 39 years. Among the group, 10.8% had a pre existing diagnosis of anxiety or depression at the time of IBD diagnosis, and an additional 14.2% were later diagnosed with anxiety or depression.
Less than half, 46.1%, of IBD patients had a score 8 or higher in the HADS-anxiety (HADS-A) or HADS-depression (HADS-D) subscale, a cutoff that suggests evidence of clinical depression or anxiety. A total of 27.4% had a score of 11 or higher, indicating a mood disorder. High HADS-A score was associated with female gender (odds ratio, 1.91; P < 0.05), long duration of disease (OR, 1.04; P < 0.01), and perianal disease (P < 0.023). The authors speculate that the latter result may be due to a higher burden of symptoms. Three-quarters, 74.5%, of patients were employed; 34.0% experienced presenteeism as defined by SPS-6 score less than or equal to 18.
The researchers found that 23.0% of the patients were sedentary, and this was more common among individuals with HADS-A or HADS-D scores greater than or equal to 8. Among those experiencing presenteeism, 50% were sedentary, 29.4% were active, and 20.6% were moderately active (P < 0.01). Individuals with higher HADS-A or HADS-D scores had a greater likelihood of being sedentary (P < 0.05).
One limitation of the study was that the questionnaires were translated into the respective languages and scores were taken at only one time point.
“Rather than relying on the patient to come forward and seek help,” physicians should familiarize themselves with these validated screening tools “in order to increase the diagnostic rate of such pathologies and enable a better holistic care for the IBD patients,” the authors concluded. “Active involvement of a psychologist and/or a psychiatrist, as part of the IBD team, should be pursued to further improve the patients’ quality of life, which has emerged as one of the top priority outcomes in IBD.”
Dr. Lupe said that the findings regarding presenteeism are consistent with his experience. He pointed out that IBD patients must be more aware of their body and vigilant in managing symptoms, and he speculated that that could detract from concentration at work. He said that the study shows the need for a holistic approach to treatment. “When someone is coping with a chronic disease, like ulcerative colitis or Crohn’s disease, it affects the whole person,” including psychologically, professionally, and personally. “These are bidirectional relationships, so that if someone’s social life starts falling down, it’s more contributory to the development of something like depression and anxiety, and maybe that’s contributory to complications that come up in a disease state like Crohn’s disease or ulcerative colitis.”
The study did not receive funding, but two authors disclosed relations with AbbVie, Janssen, Pfizer, and other companies. Dr. Keefer is a cofounder and has equity ownership In Trellus Health. Dr. Lupe has no relevant financial disclosures.
REPORTING FROM JOURNAL OF CROHN'S AND COLITIS
Guselkumab found promising for Crohn’s in phase 2 study
according to phase 2 trial data
Conventional first-line therapies for Crohn’s disease (CD) often are not effective for maintaining clinical remission and are associated with significant toxicity concerns, wrote study investigator William J. Sandborn, MD, of the University of California, San Diego, and colleagues. Guselkumab is a human monoclonal antibody that selectively inhibits the p19 subunit of interleukin 23, a cytokine that plays an important role in gut inflammation, the researchers wrote. Their report was published online Feb. 5 in Gastroenterology.
In the phase 2 GALAXI-1 study, Dr. Sandborn and colleagues evaluated the safety and efficacy of guselkumab in 309 patients with moderate to severe CD for at least 3 months. All patients previously had experienced either an inadequate response or intolerance to convention treatment or biologic agents.
Patients were randomly assigned to either placebo (n = 61); intravenous guselkumab at doses of 200 mg (n = 61), 600 mg (n = 63), or 1,200 mg at weeks 0, 4, and 8 (n = 61); or a reference arm comprising ustekinumab approximately 6 mg/kg IV at week 0 and subcutaneous 90 mg at week 8 (n = 63).
The study’s primary endpoint included the change from baseline to 12 weeks in the CD Activity Index score. The mean age of the population was 38.8 years and the mean duration of CD was 8.8 years.
There were patients in the primary efficacy analysis set who discontinued the study through week 12. At one point the study was paused to assess a serious adverse event of toxic hepatitis in a guselkumab-treated patient. Fifty-one patients were discontinued from the study because their induction treatment was paused during the adverse event evaluation; however, these patients were included in the safety analyses.
At the 12-week follow-up assessment, patients assigned to all doses of guselkumab experienced significantly greater reductions in the CD Activity Index from baseline when compared with placebo (least squares mean: 200 mg: –160.4; 600 mg: –138.9; and 1,200 mg: –144.9 vs. placebo: –36.2; all P < .05). In addition, a significantly greater proportion of patients in each guselkumab arm achieved clinical remission compared with the placebo group (CD Activity Index < 150; 57.4%, 55.6%, and 45.9% vs. 16.4%; all P < .05).
Among the patients who had an inadequate response or intolerance to prior biologic therapy, 47.5% of those in the combined guselkumab arm and 10.0% in the placebo arm met the criteria for clinical remission at 12 weeks. In addition, 62.4% of patients in the combined guselkumab group and 20% in the placebo group within the prior biologic therapy subgroup achieved clinical response at week 12.
Of patients with inadequate response or intolerance to prior conventional therapy, approximately 60% treated with guselkumab at all doses vs. 22.6% of the placebo group had clinical remission by 12 weeks. Also within this subgroup, 70.2% of patients in the combined guselkumab arm and 29% in the placebo arm had clinical response.
Finally, among the 360 patients in the safety analysis set, the proportions of patients with at least one adverse event were similar across the treatment groups during the treatment period (60% for placebo; 45.7% for guselkumab combined; 50.7% for ustekinumab).
There was no observable relationship between the dose of guselkumab and the proportion of patients with adverse events. Infection rates were 21.4% in the placebo arm, 15.1% in the combined guselkumab group, and 12.7% in the ustekinumab arm. Approximately 3.7% of patients in the combined guselkumab arm, 5.7% of patients in the placebo arm, and 5.6% of patients in the ustekinumab arm experienced at least one serious adverse event.
Greater proportions of patients receiving guselkumab achieved clinical response, Patient Reported Outcomes–2 remission, clinical-biomarker response, and endoscopic response at week 12 vs. placebo. Efficacy of ustekinumab vs. placebo was demonstrated. Safety event rates were generally similar across treatment groups.
Limitations of the study included the small number of patients in the overall dataset and the relatively short treatment period of 12 weeks. The researchers noted that phase 3 studies of guselkumab for the treatment of Crohn’s disease are underway.
Several of the researchers reported conflicts of interest with the pharmaceutical industry. The study received funding from Janssen Research & Development, LLC.
Over the last 20 years, multiple targeted therapies have been developed for Crohn’s disease (CD) and have changed the management landscape for this chronic disease. Despite many successes, a proportion of patients still experience treatment failure or intolerance to the currently available biologics, and the need for ongoing development of new therapies remains. This study by Sandborn and colleagues highlights the development of a novel therapy for Crohn’s disease patients. The novel therapy, guselkumab, targets a more specific interleukin pathway (IL-23p19 inhibition) than is currently available. In the study, guselkumab was found to be effective at improving multiple clinical parameters such as Crohn’s Disease Activity Index and Patient-Reported Outcome–2 as well as objective parameters including biomarker response and endoscopic response in patients with moderate to severe CD. There was no apparent exposure response observed over multiple dose regimens. Guselkumab also demonstrated a favorable safety profile.
Robin Dalal, MD, is an assistant professor of medicine, director of IBD education, and director of the advanced IBD fellowship at Vanderbilt University Medical Center in Nashville, Tenn. She reported being a consultant for AbbVie.
Over the last 20 years, multiple targeted therapies have been developed for Crohn’s disease (CD) and have changed the management landscape for this chronic disease. Despite many successes, a proportion of patients still experience treatment failure or intolerance to the currently available biologics, and the need for ongoing development of new therapies remains. This study by Sandborn and colleagues highlights the development of a novel therapy for Crohn’s disease patients. The novel therapy, guselkumab, targets a more specific interleukin pathway (IL-23p19 inhibition) than is currently available. In the study, guselkumab was found to be effective at improving multiple clinical parameters such as Crohn’s Disease Activity Index and Patient-Reported Outcome–2 as well as objective parameters including biomarker response and endoscopic response in patients with moderate to severe CD. There was no apparent exposure response observed over multiple dose regimens. Guselkumab also demonstrated a favorable safety profile.
Robin Dalal, MD, is an assistant professor of medicine, director of IBD education, and director of the advanced IBD fellowship at Vanderbilt University Medical Center in Nashville, Tenn. She reported being a consultant for AbbVie.
Over the last 20 years, multiple targeted therapies have been developed for Crohn’s disease (CD) and have changed the management landscape for this chronic disease. Despite many successes, a proportion of patients still experience treatment failure or intolerance to the currently available biologics, and the need for ongoing development of new therapies remains. This study by Sandborn and colleagues highlights the development of a novel therapy for Crohn’s disease patients. The novel therapy, guselkumab, targets a more specific interleukin pathway (IL-23p19 inhibition) than is currently available. In the study, guselkumab was found to be effective at improving multiple clinical parameters such as Crohn’s Disease Activity Index and Patient-Reported Outcome–2 as well as objective parameters including biomarker response and endoscopic response in patients with moderate to severe CD. There was no apparent exposure response observed over multiple dose regimens. Guselkumab also demonstrated a favorable safety profile.
Robin Dalal, MD, is an assistant professor of medicine, director of IBD education, and director of the advanced IBD fellowship at Vanderbilt University Medical Center in Nashville, Tenn. She reported being a consultant for AbbVie.
according to phase 2 trial data
Conventional first-line therapies for Crohn’s disease (CD) often are not effective for maintaining clinical remission and are associated with significant toxicity concerns, wrote study investigator William J. Sandborn, MD, of the University of California, San Diego, and colleagues. Guselkumab is a human monoclonal antibody that selectively inhibits the p19 subunit of interleukin 23, a cytokine that plays an important role in gut inflammation, the researchers wrote. Their report was published online Feb. 5 in Gastroenterology.
In the phase 2 GALAXI-1 study, Dr. Sandborn and colleagues evaluated the safety and efficacy of guselkumab in 309 patients with moderate to severe CD for at least 3 months. All patients previously had experienced either an inadequate response or intolerance to convention treatment or biologic agents.
Patients were randomly assigned to either placebo (n = 61); intravenous guselkumab at doses of 200 mg (n = 61), 600 mg (n = 63), or 1,200 mg at weeks 0, 4, and 8 (n = 61); or a reference arm comprising ustekinumab approximately 6 mg/kg IV at week 0 and subcutaneous 90 mg at week 8 (n = 63).
The study’s primary endpoint included the change from baseline to 12 weeks in the CD Activity Index score. The mean age of the population was 38.8 years and the mean duration of CD was 8.8 years.
There were patients in the primary efficacy analysis set who discontinued the study through week 12. At one point the study was paused to assess a serious adverse event of toxic hepatitis in a guselkumab-treated patient. Fifty-one patients were discontinued from the study because their induction treatment was paused during the adverse event evaluation; however, these patients were included in the safety analyses.
At the 12-week follow-up assessment, patients assigned to all doses of guselkumab experienced significantly greater reductions in the CD Activity Index from baseline when compared with placebo (least squares mean: 200 mg: –160.4; 600 mg: –138.9; and 1,200 mg: –144.9 vs. placebo: –36.2; all P < .05). In addition, a significantly greater proportion of patients in each guselkumab arm achieved clinical remission compared with the placebo group (CD Activity Index < 150; 57.4%, 55.6%, and 45.9% vs. 16.4%; all P < .05).
Among the patients who had an inadequate response or intolerance to prior biologic therapy, 47.5% of those in the combined guselkumab arm and 10.0% in the placebo arm met the criteria for clinical remission at 12 weeks. In addition, 62.4% of patients in the combined guselkumab group and 20% in the placebo group within the prior biologic therapy subgroup achieved clinical response at week 12.
Of patients with inadequate response or intolerance to prior conventional therapy, approximately 60% treated with guselkumab at all doses vs. 22.6% of the placebo group had clinical remission by 12 weeks. Also within this subgroup, 70.2% of patients in the combined guselkumab arm and 29% in the placebo arm had clinical response.
Finally, among the 360 patients in the safety analysis set, the proportions of patients with at least one adverse event were similar across the treatment groups during the treatment period (60% for placebo; 45.7% for guselkumab combined; 50.7% for ustekinumab).
There was no observable relationship between the dose of guselkumab and the proportion of patients with adverse events. Infection rates were 21.4% in the placebo arm, 15.1% in the combined guselkumab group, and 12.7% in the ustekinumab arm. Approximately 3.7% of patients in the combined guselkumab arm, 5.7% of patients in the placebo arm, and 5.6% of patients in the ustekinumab arm experienced at least one serious adverse event.
Greater proportions of patients receiving guselkumab achieved clinical response, Patient Reported Outcomes–2 remission, clinical-biomarker response, and endoscopic response at week 12 vs. placebo. Efficacy of ustekinumab vs. placebo was demonstrated. Safety event rates were generally similar across treatment groups.
Limitations of the study included the small number of patients in the overall dataset and the relatively short treatment period of 12 weeks. The researchers noted that phase 3 studies of guselkumab for the treatment of Crohn’s disease are underway.
Several of the researchers reported conflicts of interest with the pharmaceutical industry. The study received funding from Janssen Research & Development, LLC.
according to phase 2 trial data
Conventional first-line therapies for Crohn’s disease (CD) often are not effective for maintaining clinical remission and are associated with significant toxicity concerns, wrote study investigator William J. Sandborn, MD, of the University of California, San Diego, and colleagues. Guselkumab is a human monoclonal antibody that selectively inhibits the p19 subunit of interleukin 23, a cytokine that plays an important role in gut inflammation, the researchers wrote. Their report was published online Feb. 5 in Gastroenterology.
In the phase 2 GALAXI-1 study, Dr. Sandborn and colleagues evaluated the safety and efficacy of guselkumab in 309 patients with moderate to severe CD for at least 3 months. All patients previously had experienced either an inadequate response or intolerance to convention treatment or biologic agents.
Patients were randomly assigned to either placebo (n = 61); intravenous guselkumab at doses of 200 mg (n = 61), 600 mg (n = 63), or 1,200 mg at weeks 0, 4, and 8 (n = 61); or a reference arm comprising ustekinumab approximately 6 mg/kg IV at week 0 and subcutaneous 90 mg at week 8 (n = 63).
The study’s primary endpoint included the change from baseline to 12 weeks in the CD Activity Index score. The mean age of the population was 38.8 years and the mean duration of CD was 8.8 years.
There were patients in the primary efficacy analysis set who discontinued the study through week 12. At one point the study was paused to assess a serious adverse event of toxic hepatitis in a guselkumab-treated patient. Fifty-one patients were discontinued from the study because their induction treatment was paused during the adverse event evaluation; however, these patients were included in the safety analyses.
At the 12-week follow-up assessment, patients assigned to all doses of guselkumab experienced significantly greater reductions in the CD Activity Index from baseline when compared with placebo (least squares mean: 200 mg: –160.4; 600 mg: –138.9; and 1,200 mg: –144.9 vs. placebo: –36.2; all P < .05). In addition, a significantly greater proportion of patients in each guselkumab arm achieved clinical remission compared with the placebo group (CD Activity Index < 150; 57.4%, 55.6%, and 45.9% vs. 16.4%; all P < .05).
Among the patients who had an inadequate response or intolerance to prior biologic therapy, 47.5% of those in the combined guselkumab arm and 10.0% in the placebo arm met the criteria for clinical remission at 12 weeks. In addition, 62.4% of patients in the combined guselkumab group and 20% in the placebo group within the prior biologic therapy subgroup achieved clinical response at week 12.
Of patients with inadequate response or intolerance to prior conventional therapy, approximately 60% treated with guselkumab at all doses vs. 22.6% of the placebo group had clinical remission by 12 weeks. Also within this subgroup, 70.2% of patients in the combined guselkumab arm and 29% in the placebo arm had clinical response.
Finally, among the 360 patients in the safety analysis set, the proportions of patients with at least one adverse event were similar across the treatment groups during the treatment period (60% for placebo; 45.7% for guselkumab combined; 50.7% for ustekinumab).
There was no observable relationship between the dose of guselkumab and the proportion of patients with adverse events. Infection rates were 21.4% in the placebo arm, 15.1% in the combined guselkumab group, and 12.7% in the ustekinumab arm. Approximately 3.7% of patients in the combined guselkumab arm, 5.7% of patients in the placebo arm, and 5.6% of patients in the ustekinumab arm experienced at least one serious adverse event.
Greater proportions of patients receiving guselkumab achieved clinical response, Patient Reported Outcomes–2 remission, clinical-biomarker response, and endoscopic response at week 12 vs. placebo. Efficacy of ustekinumab vs. placebo was demonstrated. Safety event rates were generally similar across treatment groups.
Limitations of the study included the small number of patients in the overall dataset and the relatively short treatment period of 12 weeks. The researchers noted that phase 3 studies of guselkumab for the treatment of Crohn’s disease are underway.
Several of the researchers reported conflicts of interest with the pharmaceutical industry. The study received funding from Janssen Research & Development, LLC.
FROM GASTROENTEROLOGY
New index predicts histologic remission for UC: Study
A new score to gauge histologic remission in ulcerative colitis (UC), based simply on the presence or absence of neutrophils, is effective and easier to use than other indices, according to authors of a study published online in Gut.
Researchers, led by Xianyong Gui, MD, a surgical pathologist at the University of Washington, Seattle, developed the index, called the Paddington International Virtual Chromoendoscopy Score (PICaSSO) Histologic Remission Index (PHRI). They wrote that, when the index was plugged into an artificial intelligence (AI) model, the algorithm accurately determined histologic remission.
“Our preliminary AI algorithm differentiated active from quiescent UC with 78% sensitivity, 91.7% specificity, and 86% accuracy,” the authors noted.
Histologic remission has been previously proposed as a treatment target for UC and many indices have been developed to score disease activity, but they haven’t been widely used because of their complexity, the authors wrote. They believe this index can be applied easily and efficiently in clinical practice. “Since a pathologist needs only to identify neutrophils, which is a part of routine in reading biopsy slides as clinical histopathological evaluation, one can have the PHRI score immediately without making additional effort and spending extra time. Thus, the PHRI score can also be easily included into the pathology reports.”
The researchers found that the index correlates strongly with endoscopic activity and predicts UC clinical outcomes, including hospitalization, colectomy, and initiation or changes in treatment caused by UC flare-up.
Dr. Gui’s team developed the index using 614 biopsies from 307 patients with UC from 11 centers in Europe and North America who were prospectively enrolled in the PICaSSO study.
The index was a collaboration between pathologists and endoscopists who wanted a histologic score that would align with the endoscopic score and go beyond endoscopic evaluation. It eliminates consideration of ulceration/erosion, often a factor in other indices because variability can be high without contributing much to accuracy.
Keen interest in histologic remission
David T. Rubin, MD, chief of gastroenterology, hepatology and nutrition and the codirector of the Digestive Diseases Center at the University of Chicago, noted continued interest in whether histologic findings (biopsies) of the mucosa are a clinically important and reachable treatment goal with UC.
“It’s important to acknowledge that it is not yet a target of treatment, in part because of a variety of challenges and unknowns related to it,” he said.
The current study addresses a major barrier to incorporation of histology in the clinical management of patients with UC: individual interpretation. “Development of this simplified novel scoring approach with artificial intelligence could be a major step forward. We are hopeful that this type of AI approach will eliminate some of the barriers to use of histology as a marker of treatment control. It is of interest to note that this novel score correlated to the endoscopic appearance, but didn’t necessarily demonstrate superiority to it. This is important, since we have hypothesized that histology may provide more information about outcomes than endoscopy alone,” Dr. Rubin said.
He added that PHRI will need broader validation and incorporation into meaningful interventions before it can be incorporated into clinical practice, but that “this type of technological innovation is what the field needs in order to move forward.”
The authors and Dr. Rubin declared no relevant financial conflicts. Two coauthors are funded by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham in the United Kingdom.
A new score to gauge histologic remission in ulcerative colitis (UC), based simply on the presence or absence of neutrophils, is effective and easier to use than other indices, according to authors of a study published online in Gut.
Researchers, led by Xianyong Gui, MD, a surgical pathologist at the University of Washington, Seattle, developed the index, called the Paddington International Virtual Chromoendoscopy Score (PICaSSO) Histologic Remission Index (PHRI). They wrote that, when the index was plugged into an artificial intelligence (AI) model, the algorithm accurately determined histologic remission.
“Our preliminary AI algorithm differentiated active from quiescent UC with 78% sensitivity, 91.7% specificity, and 86% accuracy,” the authors noted.
Histologic remission has been previously proposed as a treatment target for UC and many indices have been developed to score disease activity, but they haven’t been widely used because of their complexity, the authors wrote. They believe this index can be applied easily and efficiently in clinical practice. “Since a pathologist needs only to identify neutrophils, which is a part of routine in reading biopsy slides as clinical histopathological evaluation, one can have the PHRI score immediately without making additional effort and spending extra time. Thus, the PHRI score can also be easily included into the pathology reports.”
The researchers found that the index correlates strongly with endoscopic activity and predicts UC clinical outcomes, including hospitalization, colectomy, and initiation or changes in treatment caused by UC flare-up.
Dr. Gui’s team developed the index using 614 biopsies from 307 patients with UC from 11 centers in Europe and North America who were prospectively enrolled in the PICaSSO study.
The index was a collaboration between pathologists and endoscopists who wanted a histologic score that would align with the endoscopic score and go beyond endoscopic evaluation. It eliminates consideration of ulceration/erosion, often a factor in other indices because variability can be high without contributing much to accuracy.
Keen interest in histologic remission
David T. Rubin, MD, chief of gastroenterology, hepatology and nutrition and the codirector of the Digestive Diseases Center at the University of Chicago, noted continued interest in whether histologic findings (biopsies) of the mucosa are a clinically important and reachable treatment goal with UC.
“It’s important to acknowledge that it is not yet a target of treatment, in part because of a variety of challenges and unknowns related to it,” he said.
The current study addresses a major barrier to incorporation of histology in the clinical management of patients with UC: individual interpretation. “Development of this simplified novel scoring approach with artificial intelligence could be a major step forward. We are hopeful that this type of AI approach will eliminate some of the barriers to use of histology as a marker of treatment control. It is of interest to note that this novel score correlated to the endoscopic appearance, but didn’t necessarily demonstrate superiority to it. This is important, since we have hypothesized that histology may provide more information about outcomes than endoscopy alone,” Dr. Rubin said.
He added that PHRI will need broader validation and incorporation into meaningful interventions before it can be incorporated into clinical practice, but that “this type of technological innovation is what the field needs in order to move forward.”
The authors and Dr. Rubin declared no relevant financial conflicts. Two coauthors are funded by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham in the United Kingdom.
A new score to gauge histologic remission in ulcerative colitis (UC), based simply on the presence or absence of neutrophils, is effective and easier to use than other indices, according to authors of a study published online in Gut.
Researchers, led by Xianyong Gui, MD, a surgical pathologist at the University of Washington, Seattle, developed the index, called the Paddington International Virtual Chromoendoscopy Score (PICaSSO) Histologic Remission Index (PHRI). They wrote that, when the index was plugged into an artificial intelligence (AI) model, the algorithm accurately determined histologic remission.
“Our preliminary AI algorithm differentiated active from quiescent UC with 78% sensitivity, 91.7% specificity, and 86% accuracy,” the authors noted.
Histologic remission has been previously proposed as a treatment target for UC and many indices have been developed to score disease activity, but they haven’t been widely used because of their complexity, the authors wrote. They believe this index can be applied easily and efficiently in clinical practice. “Since a pathologist needs only to identify neutrophils, which is a part of routine in reading biopsy slides as clinical histopathological evaluation, one can have the PHRI score immediately without making additional effort and spending extra time. Thus, the PHRI score can also be easily included into the pathology reports.”
The researchers found that the index correlates strongly with endoscopic activity and predicts UC clinical outcomes, including hospitalization, colectomy, and initiation or changes in treatment caused by UC flare-up.
Dr. Gui’s team developed the index using 614 biopsies from 307 patients with UC from 11 centers in Europe and North America who were prospectively enrolled in the PICaSSO study.
The index was a collaboration between pathologists and endoscopists who wanted a histologic score that would align with the endoscopic score and go beyond endoscopic evaluation. It eliminates consideration of ulceration/erosion, often a factor in other indices because variability can be high without contributing much to accuracy.
Keen interest in histologic remission
David T. Rubin, MD, chief of gastroenterology, hepatology and nutrition and the codirector of the Digestive Diseases Center at the University of Chicago, noted continued interest in whether histologic findings (biopsies) of the mucosa are a clinically important and reachable treatment goal with UC.
“It’s important to acknowledge that it is not yet a target of treatment, in part because of a variety of challenges and unknowns related to it,” he said.
The current study addresses a major barrier to incorporation of histology in the clinical management of patients with UC: individual interpretation. “Development of this simplified novel scoring approach with artificial intelligence could be a major step forward. We are hopeful that this type of AI approach will eliminate some of the barriers to use of histology as a marker of treatment control. It is of interest to note that this novel score correlated to the endoscopic appearance, but didn’t necessarily demonstrate superiority to it. This is important, since we have hypothesized that histology may provide more information about outcomes than endoscopy alone,” Dr. Rubin said.
He added that PHRI will need broader validation and incorporation into meaningful interventions before it can be incorporated into clinical practice, but that “this type of technological innovation is what the field needs in order to move forward.”
The authors and Dr. Rubin declared no relevant financial conflicts. Two coauthors are funded by the NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham in the United Kingdom.
FROM GUT