User login
VIDEO: Immune therapy effective, durable in treatment-naive melanoma brain metastases
CHICAGO – Immune therapy shows promise for use in the treatment of melanoma brain metastases, especially for treatment-naive patients, judging from the findings of a new phase II randomized study.
For patients with asymptomatic brain metastases from melanoma who had not had prior treatment, nivolumab combined with ipilimumab produced a 50% intracranial response rate after at least 12 weeks of therapy. When nivolumab alone was given to untreated patients, the intracranial response rate was 21%, Georgina Long MD, PhD, co–medical director of the Melanoma Institute Australia , said during a video interview at the annual meeting of the American Society of Clinical Oncology.
“If you look at progression-free survival by response, none of our complete responders have progressed,” said Dr. Long. “And this is with a median follow-up of 16.4 months.” The partial responders have also done well, with little progression, she said. “Remember, these patients usually survive only a few weeks.”
The Anti-PD1 Brain Collaboration study, a phase II clinical trial, enrolled patients with melanoma brain metastases at least 5 mm but less than 40 mm in diameter who had not received previous anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), anti-programmed cell death protein 1 (anti-PD-1), or anti-programmed death-ligand 1 (anti-PD-L1) therapies. Patients were permitted to have had previous BRAF and MEK inhibitor therapies. Asymptomatic patients who had no previous local brain therapy (i.e., radiation treatment or surgery) were randomized 1:1 to receive nivolumab alone, or nivolumab plus ipilimumab.
The nivolumab arm received 3 mg/kg by intravenous infusion every 2 weeks. The combination arm began with nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for four doses. After this, they also received nivolumab 3 mg/kg monotherapy every 2 weeks.
The third cohort – a small group of 15 patients who received nivolumab alone – either had symptomatic brain metastases or leptomeningeal disease and could have had previous brain surgery or radiotherapy. Unlike the first two cohorts, they were also permitted to be on up to 10 mg/day of prednisone; these patients received nivolumab alone at 3 mg/kg every 2 weeks.
For all patients, immune therapy was given until the disease progressed, consent was withdrawn, or patients experienced unacceptable toxicity or they died.
“We were most interested in the randomized cohorts,” said Dr. Long. Interestingly, she said, ipilimumab became available in Australia when 27 patients were enrolled in the nivolumab arm and 26 to the combination arm. “So we stopped the monotherapy arm, and the rest of the 60 patients to be recruited all went into the combination arm,” she said. A total of 76 patients were recruited, 33 into the combination arm, 27 to the asymptomatic nivolumab monotherapy arm, and 16 to the symptomatic and/or previously treated arm.
Data analysis from the point of the data cut included 67 patients who were followed for a period ranging from 5 to 34 months. Intracranial disease was evaluated by gadolinium-enhanced MRI and modified Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.
“The results of the trial were very interesting,” said Dr. Long. The nivolumab plus ipilimumab combo resulted in an overall 42% intracranial response rate, while nivolumab alone produced an overall intracranial response rate of 21%. However, patients in either arm who had prior BRAF or MEK inhibitor exposure “didn’t do too well on immunotherapy,” said Dr. Long, noting that the response rate was just 16% for these patients. These were, she said, “small numbers, but still, an interesting signal there.”
When comparing the secondary endpoint of extracranial response to intracranial response on a per-patient basis, Dr. Long and her collaborators could see that “the intracranial and extracranial results were mostly concordant.”
Analysis of the additional secondary endpoints of progression-free survival (PFS) and overall survival (OS) also showed an interesting pattern, said Dr. Long. After an initial drop-off period of about 5 months, the curves for patients in all arms have stabilized, so that patients who were responders are maintaining that response. The overall 6-month PFS rate for the combination cohort was 47%, with a durable response: “If you look at the curve, it’s flattened out since that stage, and we haven’t had any progression since that time,” said Dr. Long. The PFS rate was 29% for the cohort receiving nivolumab alone. “Activity is highest when nivolumab and ipilimumab are given upfront,” said Dr. Long.
For asymptomatic patients pretreated with BRAF or MEK inhibitors, “activity is low,” said Dr. Long, with an intracranial response rate of 16% in both cohorts.
Symptomatic patients who were more heavily pretreated fared even worse: “The activity of nivolumab monotherapy is low after multiple modality therapy or in leptomeningeal melanoma,” said Dr. Long. The intracranial response rate in the third cohort was just 6%.
The combination therapy cohort had the most treatment-related adverse events, with 96% of patients experiencing some adverse event. About half (12/26, 46%) had grade 3 or 4 events, and the same number had a serious adverse event. Seven patients (27%) discontinued therapy because of treatment-related adverse events in the combination study arm. However, said Dr. Long, this side effect profile is in keeping with what has been seen in other studies of combination therapy with nivolumab and ipilimumab. “There were not unexpected adverse events,” she said.
Dr. Long reported relationships with Bristol-Myers Squibb, Merck, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
CHICAGO – Immune therapy shows promise for use in the treatment of melanoma brain metastases, especially for treatment-naive patients, judging from the findings of a new phase II randomized study.
For patients with asymptomatic brain metastases from melanoma who had not had prior treatment, nivolumab combined with ipilimumab produced a 50% intracranial response rate after at least 12 weeks of therapy. When nivolumab alone was given to untreated patients, the intracranial response rate was 21%, Georgina Long MD, PhD, co–medical director of the Melanoma Institute Australia , said during a video interview at the annual meeting of the American Society of Clinical Oncology.
“If you look at progression-free survival by response, none of our complete responders have progressed,” said Dr. Long. “And this is with a median follow-up of 16.4 months.” The partial responders have also done well, with little progression, she said. “Remember, these patients usually survive only a few weeks.”
The Anti-PD1 Brain Collaboration study, a phase II clinical trial, enrolled patients with melanoma brain metastases at least 5 mm but less than 40 mm in diameter who had not received previous anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), anti-programmed cell death protein 1 (anti-PD-1), or anti-programmed death-ligand 1 (anti-PD-L1) therapies. Patients were permitted to have had previous BRAF and MEK inhibitor therapies. Asymptomatic patients who had no previous local brain therapy (i.e., radiation treatment or surgery) were randomized 1:1 to receive nivolumab alone, or nivolumab plus ipilimumab.
The nivolumab arm received 3 mg/kg by intravenous infusion every 2 weeks. The combination arm began with nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for four doses. After this, they also received nivolumab 3 mg/kg monotherapy every 2 weeks.
The third cohort – a small group of 15 patients who received nivolumab alone – either had symptomatic brain metastases or leptomeningeal disease and could have had previous brain surgery or radiotherapy. Unlike the first two cohorts, they were also permitted to be on up to 10 mg/day of prednisone; these patients received nivolumab alone at 3 mg/kg every 2 weeks.
For all patients, immune therapy was given until the disease progressed, consent was withdrawn, or patients experienced unacceptable toxicity or they died.
“We were most interested in the randomized cohorts,” said Dr. Long. Interestingly, she said, ipilimumab became available in Australia when 27 patients were enrolled in the nivolumab arm and 26 to the combination arm. “So we stopped the monotherapy arm, and the rest of the 60 patients to be recruited all went into the combination arm,” she said. A total of 76 patients were recruited, 33 into the combination arm, 27 to the asymptomatic nivolumab monotherapy arm, and 16 to the symptomatic and/or previously treated arm.
Data analysis from the point of the data cut included 67 patients who were followed for a period ranging from 5 to 34 months. Intracranial disease was evaluated by gadolinium-enhanced MRI and modified Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.
“The results of the trial were very interesting,” said Dr. Long. The nivolumab plus ipilimumab combo resulted in an overall 42% intracranial response rate, while nivolumab alone produced an overall intracranial response rate of 21%. However, patients in either arm who had prior BRAF or MEK inhibitor exposure “didn’t do too well on immunotherapy,” said Dr. Long, noting that the response rate was just 16% for these patients. These were, she said, “small numbers, but still, an interesting signal there.”
When comparing the secondary endpoint of extracranial response to intracranial response on a per-patient basis, Dr. Long and her collaborators could see that “the intracranial and extracranial results were mostly concordant.”
Analysis of the additional secondary endpoints of progression-free survival (PFS) and overall survival (OS) also showed an interesting pattern, said Dr. Long. After an initial drop-off period of about 5 months, the curves for patients in all arms have stabilized, so that patients who were responders are maintaining that response. The overall 6-month PFS rate for the combination cohort was 47%, with a durable response: “If you look at the curve, it’s flattened out since that stage, and we haven’t had any progression since that time,” said Dr. Long. The PFS rate was 29% for the cohort receiving nivolumab alone. “Activity is highest when nivolumab and ipilimumab are given upfront,” said Dr. Long.
For asymptomatic patients pretreated with BRAF or MEK inhibitors, “activity is low,” said Dr. Long, with an intracranial response rate of 16% in both cohorts.
Symptomatic patients who were more heavily pretreated fared even worse: “The activity of nivolumab monotherapy is low after multiple modality therapy or in leptomeningeal melanoma,” said Dr. Long. The intracranial response rate in the third cohort was just 6%.
The combination therapy cohort had the most treatment-related adverse events, with 96% of patients experiencing some adverse event. About half (12/26, 46%) had grade 3 or 4 events, and the same number had a serious adverse event. Seven patients (27%) discontinued therapy because of treatment-related adverse events in the combination study arm. However, said Dr. Long, this side effect profile is in keeping with what has been seen in other studies of combination therapy with nivolumab and ipilimumab. “There were not unexpected adverse events,” she said.
Dr. Long reported relationships with Bristol-Myers Squibb, Merck, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
CHICAGO – Immune therapy shows promise for use in the treatment of melanoma brain metastases, especially for treatment-naive patients, judging from the findings of a new phase II randomized study.
For patients with asymptomatic brain metastases from melanoma who had not had prior treatment, nivolumab combined with ipilimumab produced a 50% intracranial response rate after at least 12 weeks of therapy. When nivolumab alone was given to untreated patients, the intracranial response rate was 21%, Georgina Long MD, PhD, co–medical director of the Melanoma Institute Australia , said during a video interview at the annual meeting of the American Society of Clinical Oncology.
“If you look at progression-free survival by response, none of our complete responders have progressed,” said Dr. Long. “And this is with a median follow-up of 16.4 months.” The partial responders have also done well, with little progression, she said. “Remember, these patients usually survive only a few weeks.”
The Anti-PD1 Brain Collaboration study, a phase II clinical trial, enrolled patients with melanoma brain metastases at least 5 mm but less than 40 mm in diameter who had not received previous anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), anti-programmed cell death protein 1 (anti-PD-1), or anti-programmed death-ligand 1 (anti-PD-L1) therapies. Patients were permitted to have had previous BRAF and MEK inhibitor therapies. Asymptomatic patients who had no previous local brain therapy (i.e., radiation treatment or surgery) were randomized 1:1 to receive nivolumab alone, or nivolumab plus ipilimumab.
The nivolumab arm received 3 mg/kg by intravenous infusion every 2 weeks. The combination arm began with nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for four doses. After this, they also received nivolumab 3 mg/kg monotherapy every 2 weeks.
The third cohort – a small group of 15 patients who received nivolumab alone – either had symptomatic brain metastases or leptomeningeal disease and could have had previous brain surgery or radiotherapy. Unlike the first two cohorts, they were also permitted to be on up to 10 mg/day of prednisone; these patients received nivolumab alone at 3 mg/kg every 2 weeks.
For all patients, immune therapy was given until the disease progressed, consent was withdrawn, or patients experienced unacceptable toxicity or they died.
“We were most interested in the randomized cohorts,” said Dr. Long. Interestingly, she said, ipilimumab became available in Australia when 27 patients were enrolled in the nivolumab arm and 26 to the combination arm. “So we stopped the monotherapy arm, and the rest of the 60 patients to be recruited all went into the combination arm,” she said. A total of 76 patients were recruited, 33 into the combination arm, 27 to the asymptomatic nivolumab monotherapy arm, and 16 to the symptomatic and/or previously treated arm.
Data analysis from the point of the data cut included 67 patients who were followed for a period ranging from 5 to 34 months. Intracranial disease was evaluated by gadolinium-enhanced MRI and modified Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.
“The results of the trial were very interesting,” said Dr. Long. The nivolumab plus ipilimumab combo resulted in an overall 42% intracranial response rate, while nivolumab alone produced an overall intracranial response rate of 21%. However, patients in either arm who had prior BRAF or MEK inhibitor exposure “didn’t do too well on immunotherapy,” said Dr. Long, noting that the response rate was just 16% for these patients. These were, she said, “small numbers, but still, an interesting signal there.”
When comparing the secondary endpoint of extracranial response to intracranial response on a per-patient basis, Dr. Long and her collaborators could see that “the intracranial and extracranial results were mostly concordant.”
Analysis of the additional secondary endpoints of progression-free survival (PFS) and overall survival (OS) also showed an interesting pattern, said Dr. Long. After an initial drop-off period of about 5 months, the curves for patients in all arms have stabilized, so that patients who were responders are maintaining that response. The overall 6-month PFS rate for the combination cohort was 47%, with a durable response: “If you look at the curve, it’s flattened out since that stage, and we haven’t had any progression since that time,” said Dr. Long. The PFS rate was 29% for the cohort receiving nivolumab alone. “Activity is highest when nivolumab and ipilimumab are given upfront,” said Dr. Long.
For asymptomatic patients pretreated with BRAF or MEK inhibitors, “activity is low,” said Dr. Long, with an intracranial response rate of 16% in both cohorts.
Symptomatic patients who were more heavily pretreated fared even worse: “The activity of nivolumab monotherapy is low after multiple modality therapy or in leptomeningeal melanoma,” said Dr. Long. The intracranial response rate in the third cohort was just 6%.
The combination therapy cohort had the most treatment-related adverse events, with 96% of patients experiencing some adverse event. About half (12/26, 46%) had grade 3 or 4 events, and the same number had a serious adverse event. Seven patients (27%) discontinued therapy because of treatment-related adverse events in the combination study arm. However, said Dr. Long, this side effect profile is in keeping with what has been seen in other studies of combination therapy with nivolumab and ipilimumab. “There were not unexpected adverse events,” she said.
Dr. Long reported relationships with Bristol-Myers Squibb, Merck, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
AT ASCO 2017
VIDEO: Combined immunotherapy strategy shows promise in advanced solid tumors
CHICAGO – Adding an experimental immune-enhancing agent to a checkpoint inhibitor was safe and showed early promise of activity against advanced solid tumors in a phase I/IIa clinical trial.
BMS-986156 is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucorticoid-induced tumor necrosis factor receptor-related gene. The drug acts synergistically with the programmed-death 1 inhibitor (PD-1) nivolumab (Opdivo) by increasing survival of T effector cells, promoting regulatory T-cell depletion and reduction, and reducing regulatory T cell suppression of T effector cells to produce a more robust antitumor immune response.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Lillian Siu, MD, from the Princess Margaret Hospital, Toronto, describes how the combination has induced durable partial responses in patients with tumors thought to be insensitive to immunotherapy, as well as patients who had disease progression while on a PD-1 inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Adding an experimental immune-enhancing agent to a checkpoint inhibitor was safe and showed early promise of activity against advanced solid tumors in a phase I/IIa clinical trial.
BMS-986156 is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucorticoid-induced tumor necrosis factor receptor-related gene. The drug acts synergistically with the programmed-death 1 inhibitor (PD-1) nivolumab (Opdivo) by increasing survival of T effector cells, promoting regulatory T-cell depletion and reduction, and reducing regulatory T cell suppression of T effector cells to produce a more robust antitumor immune response.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Lillian Siu, MD, from the Princess Margaret Hospital, Toronto, describes how the combination has induced durable partial responses in patients with tumors thought to be insensitive to immunotherapy, as well as patients who had disease progression while on a PD-1 inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Adding an experimental immune-enhancing agent to a checkpoint inhibitor was safe and showed early promise of activity against advanced solid tumors in a phase I/IIa clinical trial.
BMS-986156 is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucorticoid-induced tumor necrosis factor receptor-related gene. The drug acts synergistically with the programmed-death 1 inhibitor (PD-1) nivolumab (Opdivo) by increasing survival of T effector cells, promoting regulatory T-cell depletion and reduction, and reducing regulatory T cell suppression of T effector cells to produce a more robust antitumor immune response.
In this video interview at the annual meeting of the American Society of Clinical Oncology, Lillian Siu, MD, from the Princess Margaret Hospital, Toronto, describes how the combination has induced durable partial responses in patients with tumors thought to be insensitive to immunotherapy, as well as patients who had disease progression while on a PD-1 inhibitor.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASCO 2017
Immune-agonist combo has activity against several tumor types
CHICAGO – A combination of the programmed death 1 (PD-1) inhibitor nivolumab (Opdivo) with an experimental immune-enhancing monoclonal antibody induced clinical responses in patients with several different solid tumor types, including some patients who had disease progression on a PD-1 inhibitor, investigators reported.
The investigational agent, euphoniously named BMS-986156 (986156), is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucocorticoid-induced tumor necrosis factor receptor–related gene (GITR).
BMS-986156156 “induces potent antitumor immunity by several mechanisms. First, it increases T-effector cell survival and function. Second, it promotes T-regulatory cell depletion and reduction through its conversion to other immune cells. As well, it reduces T-reg-mediated suppression of T-effector cells,” said Lillian L Siu, MD, from the Princess Margaret Hospital in Toronto.
In preclinical studies, the combination of an anti-GITR and an anti-PD-1 agent showed synergistic activity against murine tumor models.
Dr. Siu and colleagues conducted a phase I/IIa study of BMS-986156 with or without nivolumab in 66 patients with advanced solid tumors.
The 29 patients assigned to BMS-986156 monotherapy were started at 10 mg every 2 weeks, which was gradually titrated upward to find the maximum tolerated dose of 240 mg Q2 weeks.
The 37 patients assigned to the combination were started on a dose of 30-mg nivolumab and 240-mg BMS-986156. The nivolumab dose but not the BMS-986156 dose was then titrated upward to a maximum tolerated dose of 240 mg for each agent. This dose was based on pharmacodynamic and pharmacokinetic studies.
Tumor types included melanoma, cervical, colon, breast, renal, pancreatic, and ovarian cancers and cholangiocarcinoma.
Approximately one-third of patients in the monotherapy arm and nearly half of those in the combination arm had undergone three or more prior therapies for cancer. Seven patients in the monotherapy group and five in the combination group had previously received a PD-1 or PD-L1 inhibitor.
The median duration of treatment ranged from 7 to 15.5 weeks for 156 monotherapy and 8 to 18 weeks for the combination.
Safe and well tolerated
There were no dose-limiting toxicities or treatment-related deaths in either study arm, and patients tolerated both BMS-986156 monotherapy and the combination well. There were no grade 3 or 4 adverse events in the monotherapy arm.
“In the combination arm, the toxicity is very consistent with that observed with nivolumab monotherapy alone,” Dr. Siu said.
The only grade 4 event in this group was an increase in blood creatine phosphokinase. In this group, there were six grade 3 adverse events, including one each of colitis, dehydration, fatigue and increases in hepatic enzymes, lipase increase, and lung infection.
In pharmacokinetic studies, the action of the combinations was linear, with dose-related increases in exposure, and the combination had low immunogenicity, with no patients developing persistent antidrug antibodies.
The combination was also associated with increases in natural killer and CD8 cells in peripheral blood. Immunophenotyping of patients treated with the 240/240-mg dose of the combination showed increased proliferation and activation of CD8 effector cells, central memory cells, and CD4 cells.
Early promise
Dr. Siu reviewed interim efficacy results for the five patients treated with the combination who had responses.
For example, a 44-year-old woman with metastatic cervical cancer – a tumor type known to have high levels of GITR expression – had received more than three prior lines of therapy, including chemotherapy with a vascular endothelial growth factor inhibitor. She had a partial response with the combination, with an approximately 62% reduction in tumor burden. She had an ongoing response to the combination at the time of data cutoff in March 2017.
Two other patients had partial responses after progression on an anti-PD-1 agents, including one with nasopharyngeal cancer who had received three prior lines of therapy, including chemotherapy and a PD-1 inhibitor. This patient had an approximately 43% reduction in tumor burden, with a 17-week duration of response and ongoing response at data cutoff.
The other patient was a 59-year-old with malignant melanoma that had advanced on pembrolizumab (Keytruda). This patient too had received three prior lines of therapy, including a BRAF inhibitor, anti-PD-1, and BRAF/MEK inhibitor combination.
This patient had a response of 24-week duration at the time of data cutoff. It is ongoing, Dr. Liu said.
“This combination of immune agonists was safe with a low incidence of severe toxicity, and there was no maximum tolerated dose; however, the maximum administered dose may not be the most effective dose to move forward,” commented Siwen Hu-Lieskovan MD, PhD, from the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, the invited discussant.
She noted that activity of the combination has been seen in a wide range of tumor histologies but added that further biomarker studies will be critical for identifying patients who are likely to respond.
The study was funded by Bristol-Myers Squibb. Dr. Siu disclosed research funding from the company and others and consulting/advising several different companies. Dr. Hu-Lieskovan disclosed institutional research funding from BMS and other companies, as well as honoraria and consulting and serving in an advisory capacity for companies other than BMS. Several coauthors are employees of the company.
CHICAGO – A combination of the programmed death 1 (PD-1) inhibitor nivolumab (Opdivo) with an experimental immune-enhancing monoclonal antibody induced clinical responses in patients with several different solid tumor types, including some patients who had disease progression on a PD-1 inhibitor, investigators reported.
The investigational agent, euphoniously named BMS-986156 (986156), is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucocorticoid-induced tumor necrosis factor receptor–related gene (GITR).
BMS-986156156 “induces potent antitumor immunity by several mechanisms. First, it increases T-effector cell survival and function. Second, it promotes T-regulatory cell depletion and reduction through its conversion to other immune cells. As well, it reduces T-reg-mediated suppression of T-effector cells,” said Lillian L Siu, MD, from the Princess Margaret Hospital in Toronto.
In preclinical studies, the combination of an anti-GITR and an anti-PD-1 agent showed synergistic activity against murine tumor models.
Dr. Siu and colleagues conducted a phase I/IIa study of BMS-986156 with or without nivolumab in 66 patients with advanced solid tumors.
The 29 patients assigned to BMS-986156 monotherapy were started at 10 mg every 2 weeks, which was gradually titrated upward to find the maximum tolerated dose of 240 mg Q2 weeks.
The 37 patients assigned to the combination were started on a dose of 30-mg nivolumab and 240-mg BMS-986156. The nivolumab dose but not the BMS-986156 dose was then titrated upward to a maximum tolerated dose of 240 mg for each agent. This dose was based on pharmacodynamic and pharmacokinetic studies.
Tumor types included melanoma, cervical, colon, breast, renal, pancreatic, and ovarian cancers and cholangiocarcinoma.
Approximately one-third of patients in the monotherapy arm and nearly half of those in the combination arm had undergone three or more prior therapies for cancer. Seven patients in the monotherapy group and five in the combination group had previously received a PD-1 or PD-L1 inhibitor.
The median duration of treatment ranged from 7 to 15.5 weeks for 156 monotherapy and 8 to 18 weeks for the combination.
Safe and well tolerated
There were no dose-limiting toxicities or treatment-related deaths in either study arm, and patients tolerated both BMS-986156 monotherapy and the combination well. There were no grade 3 or 4 adverse events in the monotherapy arm.
“In the combination arm, the toxicity is very consistent with that observed with nivolumab monotherapy alone,” Dr. Siu said.
The only grade 4 event in this group was an increase in blood creatine phosphokinase. In this group, there were six grade 3 adverse events, including one each of colitis, dehydration, fatigue and increases in hepatic enzymes, lipase increase, and lung infection.
In pharmacokinetic studies, the action of the combinations was linear, with dose-related increases in exposure, and the combination had low immunogenicity, with no patients developing persistent antidrug antibodies.
The combination was also associated with increases in natural killer and CD8 cells in peripheral blood. Immunophenotyping of patients treated with the 240/240-mg dose of the combination showed increased proliferation and activation of CD8 effector cells, central memory cells, and CD4 cells.
Early promise
Dr. Siu reviewed interim efficacy results for the five patients treated with the combination who had responses.
For example, a 44-year-old woman with metastatic cervical cancer – a tumor type known to have high levels of GITR expression – had received more than three prior lines of therapy, including chemotherapy with a vascular endothelial growth factor inhibitor. She had a partial response with the combination, with an approximately 62% reduction in tumor burden. She had an ongoing response to the combination at the time of data cutoff in March 2017.
Two other patients had partial responses after progression on an anti-PD-1 agents, including one with nasopharyngeal cancer who had received three prior lines of therapy, including chemotherapy and a PD-1 inhibitor. This patient had an approximately 43% reduction in tumor burden, with a 17-week duration of response and ongoing response at data cutoff.
The other patient was a 59-year-old with malignant melanoma that had advanced on pembrolizumab (Keytruda). This patient too had received three prior lines of therapy, including a BRAF inhibitor, anti-PD-1, and BRAF/MEK inhibitor combination.
This patient had a response of 24-week duration at the time of data cutoff. It is ongoing, Dr. Liu said.
“This combination of immune agonists was safe with a low incidence of severe toxicity, and there was no maximum tolerated dose; however, the maximum administered dose may not be the most effective dose to move forward,” commented Siwen Hu-Lieskovan MD, PhD, from the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, the invited discussant.
She noted that activity of the combination has been seen in a wide range of tumor histologies but added that further biomarker studies will be critical for identifying patients who are likely to respond.
The study was funded by Bristol-Myers Squibb. Dr. Siu disclosed research funding from the company and others and consulting/advising several different companies. Dr. Hu-Lieskovan disclosed institutional research funding from BMS and other companies, as well as honoraria and consulting and serving in an advisory capacity for companies other than BMS. Several coauthors are employees of the company.
CHICAGO – A combination of the programmed death 1 (PD-1) inhibitor nivolumab (Opdivo) with an experimental immune-enhancing monoclonal antibody induced clinical responses in patients with several different solid tumor types, including some patients who had disease progression on a PD-1 inhibitor, investigators reported.
The investigational agent, euphoniously named BMS-986156 (986156), is a fully human immunoglobulin G1 agonist monoclonal antibody with high affinity binding for the glucocorticoid-induced tumor necrosis factor receptor–related gene (GITR).
BMS-986156156 “induces potent antitumor immunity by several mechanisms. First, it increases T-effector cell survival and function. Second, it promotes T-regulatory cell depletion and reduction through its conversion to other immune cells. As well, it reduces T-reg-mediated suppression of T-effector cells,” said Lillian L Siu, MD, from the Princess Margaret Hospital in Toronto.
In preclinical studies, the combination of an anti-GITR and an anti-PD-1 agent showed synergistic activity against murine tumor models.
Dr. Siu and colleagues conducted a phase I/IIa study of BMS-986156 with or without nivolumab in 66 patients with advanced solid tumors.
The 29 patients assigned to BMS-986156 monotherapy were started at 10 mg every 2 weeks, which was gradually titrated upward to find the maximum tolerated dose of 240 mg Q2 weeks.
The 37 patients assigned to the combination were started on a dose of 30-mg nivolumab and 240-mg BMS-986156. The nivolumab dose but not the BMS-986156 dose was then titrated upward to a maximum tolerated dose of 240 mg for each agent. This dose was based on pharmacodynamic and pharmacokinetic studies.
Tumor types included melanoma, cervical, colon, breast, renal, pancreatic, and ovarian cancers and cholangiocarcinoma.
Approximately one-third of patients in the monotherapy arm and nearly half of those in the combination arm had undergone three or more prior therapies for cancer. Seven patients in the monotherapy group and five in the combination group had previously received a PD-1 or PD-L1 inhibitor.
The median duration of treatment ranged from 7 to 15.5 weeks for 156 monotherapy and 8 to 18 weeks for the combination.
Safe and well tolerated
There were no dose-limiting toxicities or treatment-related deaths in either study arm, and patients tolerated both BMS-986156 monotherapy and the combination well. There were no grade 3 or 4 adverse events in the monotherapy arm.
“In the combination arm, the toxicity is very consistent with that observed with nivolumab monotherapy alone,” Dr. Siu said.
The only grade 4 event in this group was an increase in blood creatine phosphokinase. In this group, there were six grade 3 adverse events, including one each of colitis, dehydration, fatigue and increases in hepatic enzymes, lipase increase, and lung infection.
In pharmacokinetic studies, the action of the combinations was linear, with dose-related increases in exposure, and the combination had low immunogenicity, with no patients developing persistent antidrug antibodies.
The combination was also associated with increases in natural killer and CD8 cells in peripheral blood. Immunophenotyping of patients treated with the 240/240-mg dose of the combination showed increased proliferation and activation of CD8 effector cells, central memory cells, and CD4 cells.
Early promise
Dr. Siu reviewed interim efficacy results for the five patients treated with the combination who had responses.
For example, a 44-year-old woman with metastatic cervical cancer – a tumor type known to have high levels of GITR expression – had received more than three prior lines of therapy, including chemotherapy with a vascular endothelial growth factor inhibitor. She had a partial response with the combination, with an approximately 62% reduction in tumor burden. She had an ongoing response to the combination at the time of data cutoff in March 2017.
Two other patients had partial responses after progression on an anti-PD-1 agents, including one with nasopharyngeal cancer who had received three prior lines of therapy, including chemotherapy and a PD-1 inhibitor. This patient had an approximately 43% reduction in tumor burden, with a 17-week duration of response and ongoing response at data cutoff.
The other patient was a 59-year-old with malignant melanoma that had advanced on pembrolizumab (Keytruda). This patient too had received three prior lines of therapy, including a BRAF inhibitor, anti-PD-1, and BRAF/MEK inhibitor combination.
This patient had a response of 24-week duration at the time of data cutoff. It is ongoing, Dr. Liu said.
“This combination of immune agonists was safe with a low incidence of severe toxicity, and there was no maximum tolerated dose; however, the maximum administered dose may not be the most effective dose to move forward,” commented Siwen Hu-Lieskovan MD, PhD, from the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles, the invited discussant.
She noted that activity of the combination has been seen in a wide range of tumor histologies but added that further biomarker studies will be critical for identifying patients who are likely to respond.
The study was funded by Bristol-Myers Squibb. Dr. Siu disclosed research funding from the company and others and consulting/advising several different companies. Dr. Hu-Lieskovan disclosed institutional research funding from BMS and other companies, as well as honoraria and consulting and serving in an advisory capacity for companies other than BMS. Several coauthors are employees of the company.
AT ASCO 2017
Key clinical point: A combination of a GITR-agonist and anti-PD-1 agent was safe and produced partial responses in patients with heavily pretreated advanced cancers.
Major finding: Two patients with cancers that had progression on a PD-1 inhibitor had durable partial responses.
Data source: A phase I/IIa dose-finding and safety study of BMS986156 alone or in combination with nivolumab (Opdivo).
Disclosures: The study was funded by Bristol-Myers Squibb. Dr. Siu disclosed research funding from the company and others and consulting/advising for several different companies. Dr. Hu-Lieskovan disclosed institutional research funding from BMS and other companies, as well as honoraria and consulting and serving in an advisory capacity for companies other than BMS. Several coauthors are employees of the company.
Palmoplantar exacerbation of psoriasis after nivolumab for lung cancer
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
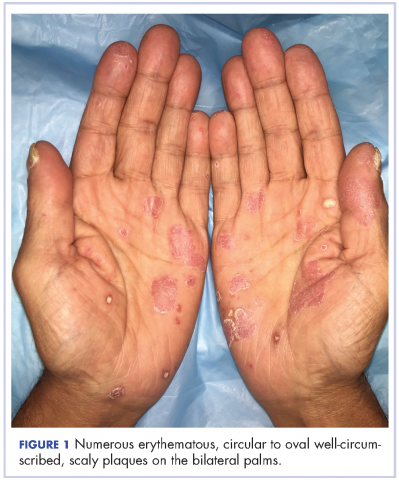
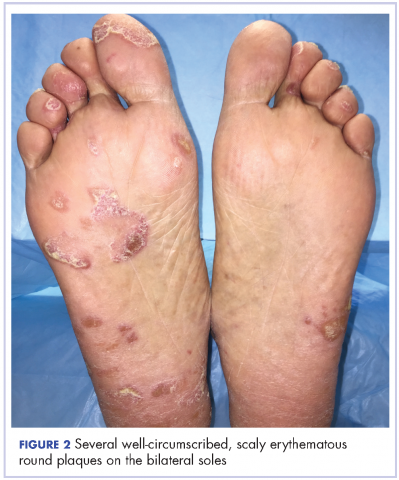
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
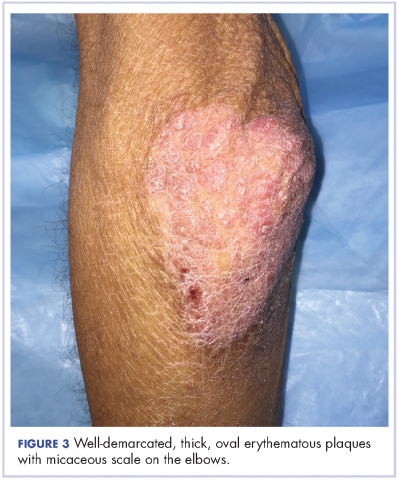
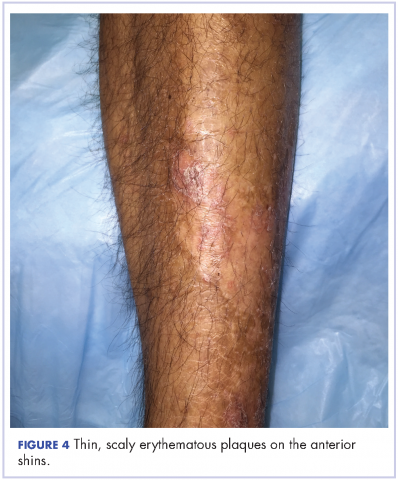
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
Nivolumab is a full human immunoglobulin antibody to the programmed cell death 1 (PD-1) immune checkpoint receptor on T cells. This programmed cell death inhibitor is a targeted immunotherapy used to treat patients with melanoma, among other malignancies.1 More recently, nivolumab has been used for advanced non-small-cell lung cancer (NSCLC) after failure of previous chemotherapeutic agents. It was approved by the US Food and Drug Administration for the NSCLC indication in 2015.2
PD-1 inhibitors are efficacious in treating advanced malignancies, although their immune-mediated functions can lead to undesirable side effects. Patients treated with nivolumab have been reported to develop thyroid disease,1,3,4 diabetes,3 hypophysitis,1,3 hypopituitarism,3 and pneumonitis,4,2 as well as other autoimmune conditions.3 Although nivolumab is often used to treat skin diseases such as melanoma, it can have many cutaneous side effects including pruritus,1,3-6 rash,1,3,4,6,7 vitiligo,1,3,7,6 mouth sores,3 injection site reactions,3,6 and alopecia.5 Herein, we describe a patient who was treated with nivolumab and developed an exacerbation of pre-existing psoriasis.
Case presentation and summary
A 57-year-old man with metastatic NSCLC and a history of plaque psoriasis presented to the dermatology clinic for evaluation of new lesions on his palms and soles. The patient had been previously treated with numerous therapies for NSCLC, including chemotherapy and radiation. Previous chemotherapeutic agents included the cisplatin plus etoposide combination, with doxetaxel and pemetrexed. The patient was not able to tolerate the chemotherapy and instead opted for hospice care. After several months, he chose to restart therapy, and was started on the programmed cell death (PD)-1 inhibitor, nivolumab, at a dose of 3 mg/kg for a total of 6 cycles. He received his first dose 5 weeks before his current presentation to the clinic, and his second dose 2 weeks before.
The patient reported a 20-year history of plaque psoriasis, characterized by psoriatic plaques on the elbows and shins and for which he was treated with topical therapies with good effect. Every few months, he would develop one or two small plaques of psoriasis on his palms and soles. The lesions were inconsequential to the patient, as he never experienced more than one or two small palmoplantar lesions at a time. One week after his second cycle of nivolumab, the patient developed an eruption of lesions on his palms and soles. He observed that the lesions seemed to be similar to his previous palmoplantar psoriatic plaques but with significantly greater skin involvement. The patient denied any new-onset joint pain.
The results of a physical examination revealed a cachectic man in no acute distress, with more than 30 erythematous circular to oval circumscribed plaques with yellow to whitish scales on the bilateral palms (Figure 1) and soles (Figure 2).
The patient also had well-demarcated, thick oval erythematous plaques with micaceous scales on the bilateral elbows (Figure 3), and thin scaly erythematous plaques on the anterior shins (Figure 4). There were no psoriatic plaques on the remainder of the trunk or extremities. Mucosal surfaces, scalp, and nails were uninvolved.
A clinical diagnosis of exacerbation of pre-existing psoriasis owing to nivolumab therapy was made. The patient was started on clobetasol 0.05% ointment twice daily under occlusion with plastic wrap to the affected areas, and he was continued on nivolumab for his NSCLC.
Discussion
Treatment with nivolumab can lead to a range of autoimmune side effects, and as shown in this case, psoriasis is one of the cutaneous findings that could be exacerbated by treatment with nivolumab. To date, two cases of exacerbation of psoriasis in patients treated with nivolumab for melanoma have been reported in the literature.8,9 In the first case, the patient had well-controlled plaque psoriasis at baseline and he subsequently developed psoriatic plaques on the trunk and extremities after the second infusion of nivolumab for metastatic melanoma. A biopsy showed regular acanthosis with hyperkeratosis and parakeratosis in addition to dilated vessels in the papillary dermis.8 In the second case, the patient had a history of psoriasis vulgaris with no active lesions. Three weeks after his first course of nivolumab for metastatic oral mucosal melanoma, he developed new, well-circumscribed erythematous scaly plaques on the trunk and extremities that were clinically diagnosed as psoriasis.9 In a third case, a patient without a prior history of psoriasis experienced a psoriasiform eruption on the trunk and extremities after the fourth dose of nivolumab for oral mucosal melanoma.10 Thus, our case is the third reported case of exacerbation of preexisting psoriasis in a patient treated with nivolumab. Furthermore, our patient is the first reported case of a patient treated with nivolumab for NSCLC to develop this adverse event. Whereas the previously reported cases were characterized by widespread trunk and extremity involvement, our patient developed focal exacerbation of the palmoplantar areas.
Additional studies are needed to more clearly characterize the specific cutaneous toxicities of nivolumab and to determine if particular skin reactions may indicate a better response to the anticancer agent. Side effects such as psoriasis can often be managed with topical therapies and may not require withdrawal of the medication. We encourage the collaboration of dermatologists and oncologists to enhance the diagnosis and management of these cutaneous side effects in cancer patients.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
1. Larkin J, Lao CD, Urba WJ, et al. Efficacy and safety of Nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433-440.
2. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33(18):2004-2012.
3. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454.
4. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257-265.
5. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-384.
6. Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311-4318.
7. Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber J. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2015.
8. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96(2):259-260.
9. Kato Y, Otsuka A, Miyachi Y, Kabashima K. Exacerbation of psoriasis vulgaris during nivolumab for oral mucosal melanoma. J Eur Acad Dermatol Venereol. 2016;30(10):e89-e91.
10. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during nivolumab therapy for primary oral mucosal melanoma. JAMA Dermatol. 2015;151(7):797-799.
FDA approves pembrolizumab for first-line advanced NSCLC
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor pembrolizumab in combination with pemetrexed and carboplatin for the treatment of patients with previously untreated metastatic nonsquamous non–small cell lung cancer (NSCLC).
The immunotherapy pembrolizumab was approved as a second-line treatment for metastatic NSCLC in 2015.
First-line approval was based on an improved overall response rate (ORR) and progression-free survival (PFS) in a cohort of 123 patients within an open-label, multicohort study (KEYNOTE-21). Enrollees in cohort G1 had locally advanced or metastatic NSCLC and no prior systemic treatment for metastatic disease. They were randomized to receive either pembrolizumab, in combination with pemetrexed and carboplatin (PC) for four cycles followed by pembrolizumab for a maximum of 24 months (n = 60) or PC alone (n = 63). Randomization was stratified by PD-L1 tumor expression (tumor proportion score [TPS] less than 1% vs. TPS greater than or equal to 1%).
The hazard ratio for PFS was 0.53 (95% CI: 0.31, 0.91, P = .0205). The median PFS was 13.0 months for the pembrolizumab plus PC arm and 8.9 months for the PC-alone arm. In the TPS less than 1% subgroup, the ORR was 57% and 13% in the pembrolizumab-plus-PC and in the PC-alone arms, respectively. In the TPS greater-than-or-equal-to-1% subgroup, the ORR was 54% in the pembrolizumab-plus-PC arm and 38% in the pembrolizumab-plus-PC arm, the FDA said.
There were serious adverse events in 41% of the patients in the pembrolizumab-plus-PC arm compared with 28% in the PC-alone arm. Pembrolizumab was discontinued for adverse reactions in 10% of patients, most commonly due to acute kidney injury. The most common grade 3-4 adverse reactions were fatigue, dyspnea, nausea, vomiting, diarrhea, and rash.
The FDA cautioned that immune-mediated adverse reactions can occur with pembrolizumab including pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis. Based on the severity of the adverse reaction, pembrolizumab should be withheld or discontinued and corticosteroids administered when appropriate. The recommended dose and schedule for NSCLC is 200 mg as an intravenous infusion every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab is marketed as Keytruda by Merck.
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor pembrolizumab in combination with pemetrexed and carboplatin for the treatment of patients with previously untreated metastatic nonsquamous non–small cell lung cancer (NSCLC).
The immunotherapy pembrolizumab was approved as a second-line treatment for metastatic NSCLC in 2015.
First-line approval was based on an improved overall response rate (ORR) and progression-free survival (PFS) in a cohort of 123 patients within an open-label, multicohort study (KEYNOTE-21). Enrollees in cohort G1 had locally advanced or metastatic NSCLC and no prior systemic treatment for metastatic disease. They were randomized to receive either pembrolizumab, in combination with pemetrexed and carboplatin (PC) for four cycles followed by pembrolizumab for a maximum of 24 months (n = 60) or PC alone (n = 63). Randomization was stratified by PD-L1 tumor expression (tumor proportion score [TPS] less than 1% vs. TPS greater than or equal to 1%).
The hazard ratio for PFS was 0.53 (95% CI: 0.31, 0.91, P = .0205). The median PFS was 13.0 months for the pembrolizumab plus PC arm and 8.9 months for the PC-alone arm. In the TPS less than 1% subgroup, the ORR was 57% and 13% in the pembrolizumab-plus-PC and in the PC-alone arms, respectively. In the TPS greater-than-or-equal-to-1% subgroup, the ORR was 54% in the pembrolizumab-plus-PC arm and 38% in the pembrolizumab-plus-PC arm, the FDA said.
There were serious adverse events in 41% of the patients in the pembrolizumab-plus-PC arm compared with 28% in the PC-alone arm. Pembrolizumab was discontinued for adverse reactions in 10% of patients, most commonly due to acute kidney injury. The most common grade 3-4 adverse reactions were fatigue, dyspnea, nausea, vomiting, diarrhea, and rash.
The FDA cautioned that immune-mediated adverse reactions can occur with pembrolizumab including pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis. Based on the severity of the adverse reaction, pembrolizumab should be withheld or discontinued and corticosteroids administered when appropriate. The recommended dose and schedule for NSCLC is 200 mg as an intravenous infusion every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab is marketed as Keytruda by Merck.
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor pembrolizumab in combination with pemetrexed and carboplatin for the treatment of patients with previously untreated metastatic nonsquamous non–small cell lung cancer (NSCLC).
The immunotherapy pembrolizumab was approved as a second-line treatment for metastatic NSCLC in 2015.
First-line approval was based on an improved overall response rate (ORR) and progression-free survival (PFS) in a cohort of 123 patients within an open-label, multicohort study (KEYNOTE-21). Enrollees in cohort G1 had locally advanced or metastatic NSCLC and no prior systemic treatment for metastatic disease. They were randomized to receive either pembrolizumab, in combination with pemetrexed and carboplatin (PC) for four cycles followed by pembrolizumab for a maximum of 24 months (n = 60) or PC alone (n = 63). Randomization was stratified by PD-L1 tumor expression (tumor proportion score [TPS] less than 1% vs. TPS greater than or equal to 1%).
The hazard ratio for PFS was 0.53 (95% CI: 0.31, 0.91, P = .0205). The median PFS was 13.0 months for the pembrolizumab plus PC arm and 8.9 months for the PC-alone arm. In the TPS less than 1% subgroup, the ORR was 57% and 13% in the pembrolizumab-plus-PC and in the PC-alone arms, respectively. In the TPS greater-than-or-equal-to-1% subgroup, the ORR was 54% in the pembrolizumab-plus-PC arm and 38% in the pembrolizumab-plus-PC arm, the FDA said.
There were serious adverse events in 41% of the patients in the pembrolizumab-plus-PC arm compared with 28% in the PC-alone arm. Pembrolizumab was discontinued for adverse reactions in 10% of patients, most commonly due to acute kidney injury. The most common grade 3-4 adverse reactions were fatigue, dyspnea, nausea, vomiting, diarrhea, and rash.
The FDA cautioned that immune-mediated adverse reactions can occur with pembrolizumab including pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis. Based on the severity of the adverse reaction, pembrolizumab should be withheld or discontinued and corticosteroids administered when appropriate. The recommended dose and schedule for NSCLC is 200 mg as an intravenous infusion every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab is marketed as Keytruda by Merck.
Flu shots may spark immune adverse events in PD-1 blockade for NSCLC
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
GENEVA – The influenza vaccine may interact with immune checkpoint inhibitors in patients with lung cancer, results of a small study suggest.
Among 23 patients with non–small cell lung cancer (NSCLC) treated with a drug targeted against programmed death-1 (PD-1), the seasonal flu vaccine appeared to produce good serologic protection against infection, but at the possible cost of an increase in the rate of immune-related adverse events (IrAE), reported Sacha Rothschild, MD, PhD, of University Hospital Basel (Switzerland) at the European Lung Cancer Conference.
Among 23 patients with lung cancer treated with a PD-1 inhibitor, 12 (52.2%) had one or more IrAEs. In contrast, the most frequent IrAE in a key registration trial for nivolumab (Opdivo) was skin rash, which occurred in 9% of patients (N Engl J Med. 2015 Jul 9;373:1627-39).
“It’s a very small study, but it raises some concern that there might be an interaction between the vaccine and PD-1 blockade,” Dr. Rothschild said.
To see whether blocking the PD-1/PD–ligand-1 (PD-L1) axis might induce an overactive immune response, the investigators prospectively studied 23 patients with NSCLC who were undergoing treatment with a PD-1 inhibitor – 22 with nivolumab and 1 with pembrolizumab (Keytruda) – who were also vaccinated with a trivalent influenza vaccine in October or November 2015. They used the partners of the patients, also vaccinated, for an age-matched cohort of healthy controls.
The investigators looked at antibody titers against flu strains covered by the vaccine, measured inflammatory chemokines and assessed the vaccine’s safety and the frequency of IrAEs.
None of the patients came down with the flu during the 2015-2016 season. There were no major differences over time in the generation of antibodies against all three viral strains tested.
However, at both 30 and 60 days after vaccination, a hemagglutination inhibition assay showed slightly elevated antibody titers among patients, compared with controls. Antibody titers against H1N1 virus also appeared to increase somewhat more rapidly among patients than among controls, the authors found.
The patients appeared to tolerate the vaccine well, and no serious adverse events were reported within 30 days of vaccination.
When they looked at the incidence of IrAEs, however, the investigators found that six patients had grade 1 or 2 IrAEs, and six had grade 3 or 4 events.
The events included skin rash and arthritis in three patients each, colitis and encephalitis in two patients each, and hypothyroidism, pneumonitis, and neuropathy in one patient each.
“We looked into inflammatory chemokines to understand if there was a high rate of systemic inflammation, and we didn’t find any differences in this regard. So far, we have no clue about why the immune-related adverse event rate in this group is higher,” Dr. Rothschild said.
Although the sample size was small, the IrAE effect they saw was large enough to warrant concern, and it should be studied in a larger population sample, he said.
Egbert Smit, MD, PhD, of the Netherlands Cancer Institute in Amsterdam, who was not involved in the study, commented that “it shows how much we still have to learn about the optimal use of checkpoint inhibitors in lung cancer patients. The study is important as it is the first to investigate the impact of influenza vaccination in such patients, and there is a hint that we actually put them at increased risk for serious toxicities, including encephalitis. However, until we have data on a larger cohort, preferably in a controlled, prospective study, in my institution, we advocate influenza vaccination irrespective of concurrent treatment with immune-checkpoint inhibitors.”
The study was supported by institutional funding. The investigators and Dr. Smit reported no relevant conflicts of interest.
AT ELCC
Key clinical point:
Major finding: Of 23 patients who were vaccinated, 12 developed IrAEs, including 6 grade 1/2 and 6 grade 3/4 events.
Data source: Prospective study of 23 patients with NSCLC and 23 healthy controls.
Disclosures: The study was supported by institutional funding. The investigators reported no relevant conflicts of interest.
Modifying CAR-T with IL-15 improved activity in glioma models
MONTREAL – Adding an immunostimulatory cytokine to chimeric antigen receptor–T cells (CAR-T) improved the adaptive immunotherapy’s activity against aggressive pediatric brain malignancies both in vitro and in animal models, an investigator reported.
CAR-T cells engineered to express interleukin-15 (IL-15), an inducer of T-cell proliferation and survival, were associated with significantly longer progression-free survival (PFS) and overall survival in mouse models of glioma, compared with regular CAR-T cells, said Giedre Krenciute, PhD, of Baylor College of Medicine in Houston.
The improved T-cell persistence, however, still resulted in the eventual loss of both targeted and nontargeted tumor-associated antigens and tumor recurrence, Dr. Krenciute noted.
The results suggest that T-cell persistence is critical for antitumor activity, and that it will be necessary to perform antigen profiling of recurrent tumors in order to develop follow-on therapies targeting multiple tumor-associated antigens, she said.
Her team had previously reported on the development of a CAR-T cell directed specifically against the IL-13 receptor alpha-2, which is expressed at high frequency in diffuse intrinsic pontine glioma and glioblastoma tumors but not in normal brain tissues.
In preclinical models, the construct had potent antiglioblastoma activity. However, the T-cells had only limited persistence, and tumors positive for IL-13 receptor alpha-2 recurred.
To see whether they could improve on T-cell persistence, they took their CARs back to the shop and modified them with a retroviral vector to express IL-15 transgenes. They then tested the altered cells in vitro using standard assays and found that the addition of IL-15 did not change the T-cell phenotype or affect the cells’ cytotoxicity.
The addition of IL-15 significantly improved the persistence of the T cells when they were injected into the tumors of mice with human glioblastoma xenografts (P less than .05), and this persistence translated into a near-doubling of PFS, compared with regular CAR-T cells (media, 84 days vs. 49 days; P = .008), as well as improved overall survival (P = .02).
Of 10 mice that received the IL-15–expressing CAR-Ts, 4 were free of glioma through at least 80 days of follow-up.
Of five mice with recurring gliomas, three had down-regulated expression of the IL-13 receptor alpha-2 target, indicating immune escape, and all recurring tumors had reduced expression of the human epidermal growth factor receptor-2 antigen, which is associated with gliomas.
The investigators are currently test-driving a new CD20-targeted CAR, transduced to express IL-15, and have seen good expansion and persistence of the T cells for up to 15 days in culture, with in vivo tests in the planning stage, Dr. Krenciute said.
The work is supported by the National Institutes of Health, Alex’s Lemonade Stand Foundation, the James S. McDonnell Foundation, and the American Brain Tumor Association. The laboratory, the Center for Cell and Gene Therapy at Baylor, has or had research collaborations with Celgene, Bluebird Bio, and Tessa Therapeutics, and investigators at the center hold or have applied for patents in T-cell and gene-modified T-cell therapies for cancer.
MONTREAL – Adding an immunostimulatory cytokine to chimeric antigen receptor–T cells (CAR-T) improved the adaptive immunotherapy’s activity against aggressive pediatric brain malignancies both in vitro and in animal models, an investigator reported.
CAR-T cells engineered to express interleukin-15 (IL-15), an inducer of T-cell proliferation and survival, were associated with significantly longer progression-free survival (PFS) and overall survival in mouse models of glioma, compared with regular CAR-T cells, said Giedre Krenciute, PhD, of Baylor College of Medicine in Houston.
The improved T-cell persistence, however, still resulted in the eventual loss of both targeted and nontargeted tumor-associated antigens and tumor recurrence, Dr. Krenciute noted.
The results suggest that T-cell persistence is critical for antitumor activity, and that it will be necessary to perform antigen profiling of recurrent tumors in order to develop follow-on therapies targeting multiple tumor-associated antigens, she said.
Her team had previously reported on the development of a CAR-T cell directed specifically against the IL-13 receptor alpha-2, which is expressed at high frequency in diffuse intrinsic pontine glioma and glioblastoma tumors but not in normal brain tissues.
In preclinical models, the construct had potent antiglioblastoma activity. However, the T-cells had only limited persistence, and tumors positive for IL-13 receptor alpha-2 recurred.
To see whether they could improve on T-cell persistence, they took their CARs back to the shop and modified them with a retroviral vector to express IL-15 transgenes. They then tested the altered cells in vitro using standard assays and found that the addition of IL-15 did not change the T-cell phenotype or affect the cells’ cytotoxicity.
The addition of IL-15 significantly improved the persistence of the T cells when they were injected into the tumors of mice with human glioblastoma xenografts (P less than .05), and this persistence translated into a near-doubling of PFS, compared with regular CAR-T cells (media, 84 days vs. 49 days; P = .008), as well as improved overall survival (P = .02).
Of 10 mice that received the IL-15–expressing CAR-Ts, 4 were free of glioma through at least 80 days of follow-up.
Of five mice with recurring gliomas, three had down-regulated expression of the IL-13 receptor alpha-2 target, indicating immune escape, and all recurring tumors had reduced expression of the human epidermal growth factor receptor-2 antigen, which is associated with gliomas.
The investigators are currently test-driving a new CD20-targeted CAR, transduced to express IL-15, and have seen good expansion and persistence of the T cells for up to 15 days in culture, with in vivo tests in the planning stage, Dr. Krenciute said.
The work is supported by the National Institutes of Health, Alex’s Lemonade Stand Foundation, the James S. McDonnell Foundation, and the American Brain Tumor Association. The laboratory, the Center for Cell and Gene Therapy at Baylor, has or had research collaborations with Celgene, Bluebird Bio, and Tessa Therapeutics, and investigators at the center hold or have applied for patents in T-cell and gene-modified T-cell therapies for cancer.
MONTREAL – Adding an immunostimulatory cytokine to chimeric antigen receptor–T cells (CAR-T) improved the adaptive immunotherapy’s activity against aggressive pediatric brain malignancies both in vitro and in animal models, an investigator reported.
CAR-T cells engineered to express interleukin-15 (IL-15), an inducer of T-cell proliferation and survival, were associated with significantly longer progression-free survival (PFS) and overall survival in mouse models of glioma, compared with regular CAR-T cells, said Giedre Krenciute, PhD, of Baylor College of Medicine in Houston.
The improved T-cell persistence, however, still resulted in the eventual loss of both targeted and nontargeted tumor-associated antigens and tumor recurrence, Dr. Krenciute noted.
The results suggest that T-cell persistence is critical for antitumor activity, and that it will be necessary to perform antigen profiling of recurrent tumors in order to develop follow-on therapies targeting multiple tumor-associated antigens, she said.
Her team had previously reported on the development of a CAR-T cell directed specifically against the IL-13 receptor alpha-2, which is expressed at high frequency in diffuse intrinsic pontine glioma and glioblastoma tumors but not in normal brain tissues.
In preclinical models, the construct had potent antiglioblastoma activity. However, the T-cells had only limited persistence, and tumors positive for IL-13 receptor alpha-2 recurred.
To see whether they could improve on T-cell persistence, they took their CARs back to the shop and modified them with a retroviral vector to express IL-15 transgenes. They then tested the altered cells in vitro using standard assays and found that the addition of IL-15 did not change the T-cell phenotype or affect the cells’ cytotoxicity.
The addition of IL-15 significantly improved the persistence of the T cells when they were injected into the tumors of mice with human glioblastoma xenografts (P less than .05), and this persistence translated into a near-doubling of PFS, compared with regular CAR-T cells (media, 84 days vs. 49 days; P = .008), as well as improved overall survival (P = .02).
Of 10 mice that received the IL-15–expressing CAR-Ts, 4 were free of glioma through at least 80 days of follow-up.
Of five mice with recurring gliomas, three had down-regulated expression of the IL-13 receptor alpha-2 target, indicating immune escape, and all recurring tumors had reduced expression of the human epidermal growth factor receptor-2 antigen, which is associated with gliomas.
The investigators are currently test-driving a new CD20-targeted CAR, transduced to express IL-15, and have seen good expansion and persistence of the T cells for up to 15 days in culture, with in vivo tests in the planning stage, Dr. Krenciute said.
The work is supported by the National Institutes of Health, Alex’s Lemonade Stand Foundation, the James S. McDonnell Foundation, and the American Brain Tumor Association. The laboratory, the Center for Cell and Gene Therapy at Baylor, has or had research collaborations with Celgene, Bluebird Bio, and Tessa Therapeutics, and investigators at the center hold or have applied for patents in T-cell and gene-modified T-cell therapies for cancer.
FROM ASPHO
Key clinical point: CAR-T cells, modified to express IL-15, have improved activity against aggressive pediatric gliomas.
Major finding: IL-15 expressed T cells were associated with improved progression-free and overall survival in animal models of glioblastoma.
Data source: In vitro and in vivo experiments of CAR-T cells modified to improve T-cell persistence and clinical efficacy.
Disclosures: The work is supported by the National Institutes of Health, Alex’s Lemonade Stand Foundation, the James S. McDonnell Foundation, and the American Brain Tumor Association. The laboratory, the Center for Cell and Gene Therapy at Baylor, has or had research collaborations with Celgene, Bluebird Bio, and Tessa Therapeutics, and investigators at the center hold or have applied for patents in T-cell and gene-modified T-cell therapies for cancer.
VIDEO: Blinatumomab, inotuzumab reshape relapsed ALL treatment
NEW YORK – A pair of new monoclonal antibodies have dramatically changed treatment for patients with acute lymphoblastic leukemia to prepare them for a stem cell transplant, Daniel J. DeAngelo, MD, said at a conference held by Imedex.
“We don’t use standard chemotherapy for reinduction anymore; we use blinatumomab or inotuzumab,” said Dr. DeAngelo, a hematologist oncologist at Dana-Farber Cancer Institute in Boston.
Blinatumomab (Blincyto), approved by the Food and Drug Administration in 2014, has produced “exceptional” response rates, becoming “standard of care” for patients with relapsed acute lymphoblastic leukemia (ALL) that does not have a Philadelphia chromosome, Dr. DeAngelo said in a video interview.
Approved based on results from a phase II study, blinatumomab’s efficacy and safety were recently further delineated in results from the first phase III trial (N Engl J Med. 2017 Mar 2;376[9]:836-74), with 376 treated patients. In that trial, blinatumomab more than doubled the complete remission rate, compared with control patients (34% vs. 16%), and nearly doubled median overall survival – 7.7 months with blinatumomab, compared with 4.0 months for control patients treated with standard chemotherapy.
These findings “further substantiated” blinatumomab’s role, he said.
Blinatumomab’s big limitations are certain adverse effects and the logistics of its dosing. The major adverse effect is “cytokine release syndrome,” which manifests as fever, low blood pressure, and neurologic toxicities that can range from tremors to encephalopathy and seizure. These are manageable by close observation of patients by experienced nurses, Dr. DeAngelo said.
Dosing involves 4 weeks of continuous infusion, starting with 10 days done entirely in the hospital, with the remaining 18 days with patients going home but needing to return every 48 hours to have their infusion bag changed. “Depending on how far the patient lives from the clinic, it can be a logistical challenge,” he said.
A second new antibody he has used on many patients is inotuzumab, which was accepted for review for approval by the FDA in February 2017, with action expected by August.
Dr. DeAngelo served as a coinvestigator in a phase III trial reported in 2016 with 218 evaluable patients. In that trial, investigators reported an 81% complete remission rate with inotuzumab treatment, compared with a 29% among control patients on chemotherapy (N Engl J Med. 2016 Aug 25;375[8]:740-53).
Inotuzumab was effective against patients with Philadelphia chromosome positive ALL, but it will not work for the roughly 5%-10% of ALL patients who lack CD-22 expression in their B-cell ALL.
Inotuzumab is easier to administer than blinatumomab, requiring a once a week infusion, and causes little immediate toxicity – although thrombocytopenia and liver-function abnormalities can occur with continued use, and the risk of veno-occlusive disease is increased when patients later receive a stem cell transplant, Dr. DeAngelo said.
“It’s nice to have options” when choosing antibody-based treatment, he said. Blinatumomab is a good choice for patients with a lower tumor burden – either patients with early relapse or with minimal residual disease – while inotuzumab works better for patients with more bulky disease, as well as those who are not able to accommodate the logistic demands of blinatumomab infusions.
Dr. DeAngelo also highlighted several trials now underway that are testing the efficacy of both antibodies when used as part of first-line treatment.
Dr. DeAngelo has been a consultant to Amgen, the company that markets blinatumomab (Blincyto); to Pfizer, the company developing inotuzumab; and to Ariad, InCyte, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
NEW YORK – A pair of new monoclonal antibodies have dramatically changed treatment for patients with acute lymphoblastic leukemia to prepare them for a stem cell transplant, Daniel J. DeAngelo, MD, said at a conference held by Imedex.
“We don’t use standard chemotherapy for reinduction anymore; we use blinatumomab or inotuzumab,” said Dr. DeAngelo, a hematologist oncologist at Dana-Farber Cancer Institute in Boston.
Blinatumomab (Blincyto), approved by the Food and Drug Administration in 2014, has produced “exceptional” response rates, becoming “standard of care” for patients with relapsed acute lymphoblastic leukemia (ALL) that does not have a Philadelphia chromosome, Dr. DeAngelo said in a video interview.
Approved based on results from a phase II study, blinatumomab’s efficacy and safety were recently further delineated in results from the first phase III trial (N Engl J Med. 2017 Mar 2;376[9]:836-74), with 376 treated patients. In that trial, blinatumomab more than doubled the complete remission rate, compared with control patients (34% vs. 16%), and nearly doubled median overall survival – 7.7 months with blinatumomab, compared with 4.0 months for control patients treated with standard chemotherapy.
These findings “further substantiated” blinatumomab’s role, he said.
Blinatumomab’s big limitations are certain adverse effects and the logistics of its dosing. The major adverse effect is “cytokine release syndrome,” which manifests as fever, low blood pressure, and neurologic toxicities that can range from tremors to encephalopathy and seizure. These are manageable by close observation of patients by experienced nurses, Dr. DeAngelo said.
Dosing involves 4 weeks of continuous infusion, starting with 10 days done entirely in the hospital, with the remaining 18 days with patients going home but needing to return every 48 hours to have their infusion bag changed. “Depending on how far the patient lives from the clinic, it can be a logistical challenge,” he said.
A second new antibody he has used on many patients is inotuzumab, which was accepted for review for approval by the FDA in February 2017, with action expected by August.
Dr. DeAngelo served as a coinvestigator in a phase III trial reported in 2016 with 218 evaluable patients. In that trial, investigators reported an 81% complete remission rate with inotuzumab treatment, compared with a 29% among control patients on chemotherapy (N Engl J Med. 2016 Aug 25;375[8]:740-53).
Inotuzumab was effective against patients with Philadelphia chromosome positive ALL, but it will not work for the roughly 5%-10% of ALL patients who lack CD-22 expression in their B-cell ALL.
Inotuzumab is easier to administer than blinatumomab, requiring a once a week infusion, and causes little immediate toxicity – although thrombocytopenia and liver-function abnormalities can occur with continued use, and the risk of veno-occlusive disease is increased when patients later receive a stem cell transplant, Dr. DeAngelo said.
“It’s nice to have options” when choosing antibody-based treatment, he said. Blinatumomab is a good choice for patients with a lower tumor burden – either patients with early relapse or with minimal residual disease – while inotuzumab works better for patients with more bulky disease, as well as those who are not able to accommodate the logistic demands of blinatumomab infusions.
Dr. DeAngelo also highlighted several trials now underway that are testing the efficacy of both antibodies when used as part of first-line treatment.
Dr. DeAngelo has been a consultant to Amgen, the company that markets blinatumomab (Blincyto); to Pfizer, the company developing inotuzumab; and to Ariad, InCyte, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
NEW YORK – A pair of new monoclonal antibodies have dramatically changed treatment for patients with acute lymphoblastic leukemia to prepare them for a stem cell transplant, Daniel J. DeAngelo, MD, said at a conference held by Imedex.
“We don’t use standard chemotherapy for reinduction anymore; we use blinatumomab or inotuzumab,” said Dr. DeAngelo, a hematologist oncologist at Dana-Farber Cancer Institute in Boston.
Blinatumomab (Blincyto), approved by the Food and Drug Administration in 2014, has produced “exceptional” response rates, becoming “standard of care” for patients with relapsed acute lymphoblastic leukemia (ALL) that does not have a Philadelphia chromosome, Dr. DeAngelo said in a video interview.
Approved based on results from a phase II study, blinatumomab’s efficacy and safety were recently further delineated in results from the first phase III trial (N Engl J Med. 2017 Mar 2;376[9]:836-74), with 376 treated patients. In that trial, blinatumomab more than doubled the complete remission rate, compared with control patients (34% vs. 16%), and nearly doubled median overall survival – 7.7 months with blinatumomab, compared with 4.0 months for control patients treated with standard chemotherapy.
These findings “further substantiated” blinatumomab’s role, he said.
Blinatumomab’s big limitations are certain adverse effects and the logistics of its dosing. The major adverse effect is “cytokine release syndrome,” which manifests as fever, low blood pressure, and neurologic toxicities that can range from tremors to encephalopathy and seizure. These are manageable by close observation of patients by experienced nurses, Dr. DeAngelo said.
Dosing involves 4 weeks of continuous infusion, starting with 10 days done entirely in the hospital, with the remaining 18 days with patients going home but needing to return every 48 hours to have their infusion bag changed. “Depending on how far the patient lives from the clinic, it can be a logistical challenge,” he said.
A second new antibody he has used on many patients is inotuzumab, which was accepted for review for approval by the FDA in February 2017, with action expected by August.
Dr. DeAngelo served as a coinvestigator in a phase III trial reported in 2016 with 218 evaluable patients. In that trial, investigators reported an 81% complete remission rate with inotuzumab treatment, compared with a 29% among control patients on chemotherapy (N Engl J Med. 2016 Aug 25;375[8]:740-53).
Inotuzumab was effective against patients with Philadelphia chromosome positive ALL, but it will not work for the roughly 5%-10% of ALL patients who lack CD-22 expression in their B-cell ALL.
Inotuzumab is easier to administer than blinatumomab, requiring a once a week infusion, and causes little immediate toxicity – although thrombocytopenia and liver-function abnormalities can occur with continued use, and the risk of veno-occlusive disease is increased when patients later receive a stem cell transplant, Dr. DeAngelo said.
“It’s nice to have options” when choosing antibody-based treatment, he said. Blinatumomab is a good choice for patients with a lower tumor burden – either patients with early relapse or with minimal residual disease – while inotuzumab works better for patients with more bulky disease, as well as those who are not able to accommodate the logistic demands of blinatumomab infusions.
Dr. DeAngelo also highlighted several trials now underway that are testing the efficacy of both antibodies when used as part of first-line treatment.
Dr. DeAngelo has been a consultant to Amgen, the company that markets blinatumomab (Blincyto); to Pfizer, the company developing inotuzumab; and to Ariad, InCyte, and Novartis.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM A MEETING ON HEMATOLOGIC MALIGNANCIES
Atezolizumab improved survival in triple-negative breast cancer
Treatment with the anti-PD-L1 cancer immunotherapy atezolizumab produced a durable clinical benefit in patients with metastatic triple-negative breast cancer who responded to treatment, according to results from a phase I study.
Overall survival (OS) rates were 41% at 1 year and 22% at both year 2 and year 3. Patients with PD-L1 on 5% or more of tumor-infiltrating immune cells (IC2/3) achieved even better clinical outcomes: Their OS rates at 1, 2, and 3 years were 45%, 28%, and 28%.
The findings from this early phase I trial, which were presented at the annual meeting of the American Association for Cancer Research, also demonstrated that response rates were higher in the first-line setting, and an exploratory biomarker analysis suggested that higher CD8 T cell and tumor-infiltrating lymphocyte counts also contributed to a better response.
“We have no targeted therapy at the moment for triple-negative breast cancer,” said study lead author Dr. Peter Schmid, director of the St. Bartholomew’s Breast Centre at St. Bartholomew’s Hospital and Barts Cancer Institute in London, during a media briefing. “The treatment we have is chemotherapy, and most patients develop resistance relatively quickly.”
Dr. Schmid noted that the median survival for these patients is still relatively short – about 9-12 months – so, the data from this trial need to be seen in that context.
“On the other hand, triple-negative breast cancer is probably the best subtype of breast cancer in terms of selecting patients for immune therapy,” said Dr. Schmid. “This is based on a high degree of genetic instability, a high rate of mutations, higher levels of PD-L1 expression, and tumor infiltrating lymphocytes inside the tumor.”
Atezolizumab is a humanized monoclonal antibody that disrupts the PD pathway, inhibits the binding of PD-L1 to PD-1 and B7.1, and, in doing so, restores tumor-specific T-cell immunity.
In this study, Dr Schmid and colleagues recruited patients with metastatic triple-negative breast cancer to one of the expansion cohorts of a phase I trial. A total of 112 patients were evaluable for response. Of this group, 19 received atezolizumab as first-line treatment, and 93 had received at least two lines of prior therapy.
Atezolizumab was administered every 3 weeks at 15 mg/kg or 20 mg/kg, and the level of PD-L1 expression on tumor-infiltrating immune cells was evaluated. The primary endpoint of the study was safety, with overall response rate, duration of response, and progression-free survival as key secondary endpoints.
The 1- and 2-year overall survival rates for responders were 100%, but that dropped to 33% and 11%, respectively, for nonresponders. Of the 11 responders, 5 received atezolizumab as first-line therapy, while 9 had high PD-L1 expression (IC2/3).
For patients who received atezolizumab in the first-line setting, 1-year overall survival was 63%, and 2-year overall survival was 47%. The rates were lower for second-line and beyond; 37% and 18%, respectively.
For IC2/3 patients, 1-year overall survival was 45%, compared with 37% for those with low to no PD-L1 expression (IC0/1).
Only 11% of patients experienced treatment-related grade 3 or greater adverse events, and side effects led to treatment discontinuation in 3% of patients.
A key message was that the duration of response had a median of 21 months, and that is significant in this disease setting, explained Dr. Schmid. Another important point was that “overall survival was significantly longer that what we see with chemotherapy.”
Genentech funded the study. Dr Schmid’s spouse is a consultant to Roche/Genentech.
Treatment with the anti-PD-L1 cancer immunotherapy atezolizumab produced a durable clinical benefit in patients with metastatic triple-negative breast cancer who responded to treatment, according to results from a phase I study.
Overall survival (OS) rates were 41% at 1 year and 22% at both year 2 and year 3. Patients with PD-L1 on 5% or more of tumor-infiltrating immune cells (IC2/3) achieved even better clinical outcomes: Their OS rates at 1, 2, and 3 years were 45%, 28%, and 28%.
The findings from this early phase I trial, which were presented at the annual meeting of the American Association for Cancer Research, also demonstrated that response rates were higher in the first-line setting, and an exploratory biomarker analysis suggested that higher CD8 T cell and tumor-infiltrating lymphocyte counts also contributed to a better response.
“We have no targeted therapy at the moment for triple-negative breast cancer,” said study lead author Dr. Peter Schmid, director of the St. Bartholomew’s Breast Centre at St. Bartholomew’s Hospital and Barts Cancer Institute in London, during a media briefing. “The treatment we have is chemotherapy, and most patients develop resistance relatively quickly.”
Dr. Schmid noted that the median survival for these patients is still relatively short – about 9-12 months – so, the data from this trial need to be seen in that context.
“On the other hand, triple-negative breast cancer is probably the best subtype of breast cancer in terms of selecting patients for immune therapy,” said Dr. Schmid. “This is based on a high degree of genetic instability, a high rate of mutations, higher levels of PD-L1 expression, and tumor infiltrating lymphocytes inside the tumor.”
Atezolizumab is a humanized monoclonal antibody that disrupts the PD pathway, inhibits the binding of PD-L1 to PD-1 and B7.1, and, in doing so, restores tumor-specific T-cell immunity.
In this study, Dr Schmid and colleagues recruited patients with metastatic triple-negative breast cancer to one of the expansion cohorts of a phase I trial. A total of 112 patients were evaluable for response. Of this group, 19 received atezolizumab as first-line treatment, and 93 had received at least two lines of prior therapy.
Atezolizumab was administered every 3 weeks at 15 mg/kg or 20 mg/kg, and the level of PD-L1 expression on tumor-infiltrating immune cells was evaluated. The primary endpoint of the study was safety, with overall response rate, duration of response, and progression-free survival as key secondary endpoints.
The 1- and 2-year overall survival rates for responders were 100%, but that dropped to 33% and 11%, respectively, for nonresponders. Of the 11 responders, 5 received atezolizumab as first-line therapy, while 9 had high PD-L1 expression (IC2/3).
For patients who received atezolizumab in the first-line setting, 1-year overall survival was 63%, and 2-year overall survival was 47%. The rates were lower for second-line and beyond; 37% and 18%, respectively.
For IC2/3 patients, 1-year overall survival was 45%, compared with 37% for those with low to no PD-L1 expression (IC0/1).
Only 11% of patients experienced treatment-related grade 3 or greater adverse events, and side effects led to treatment discontinuation in 3% of patients.
A key message was that the duration of response had a median of 21 months, and that is significant in this disease setting, explained Dr. Schmid. Another important point was that “overall survival was significantly longer that what we see with chemotherapy.”
Genentech funded the study. Dr Schmid’s spouse is a consultant to Roche/Genentech.
Treatment with the anti-PD-L1 cancer immunotherapy atezolizumab produced a durable clinical benefit in patients with metastatic triple-negative breast cancer who responded to treatment, according to results from a phase I study.
Overall survival (OS) rates were 41% at 1 year and 22% at both year 2 and year 3. Patients with PD-L1 on 5% or more of tumor-infiltrating immune cells (IC2/3) achieved even better clinical outcomes: Their OS rates at 1, 2, and 3 years were 45%, 28%, and 28%.
The findings from this early phase I trial, which were presented at the annual meeting of the American Association for Cancer Research, also demonstrated that response rates were higher in the first-line setting, and an exploratory biomarker analysis suggested that higher CD8 T cell and tumor-infiltrating lymphocyte counts also contributed to a better response.
“We have no targeted therapy at the moment for triple-negative breast cancer,” said study lead author Dr. Peter Schmid, director of the St. Bartholomew’s Breast Centre at St. Bartholomew’s Hospital and Barts Cancer Institute in London, during a media briefing. “The treatment we have is chemotherapy, and most patients develop resistance relatively quickly.”
Dr. Schmid noted that the median survival for these patients is still relatively short – about 9-12 months – so, the data from this trial need to be seen in that context.
“On the other hand, triple-negative breast cancer is probably the best subtype of breast cancer in terms of selecting patients for immune therapy,” said Dr. Schmid. “This is based on a high degree of genetic instability, a high rate of mutations, higher levels of PD-L1 expression, and tumor infiltrating lymphocytes inside the tumor.”
Atezolizumab is a humanized monoclonal antibody that disrupts the PD pathway, inhibits the binding of PD-L1 to PD-1 and B7.1, and, in doing so, restores tumor-specific T-cell immunity.
In this study, Dr Schmid and colleagues recruited patients with metastatic triple-negative breast cancer to one of the expansion cohorts of a phase I trial. A total of 112 patients were evaluable for response. Of this group, 19 received atezolizumab as first-line treatment, and 93 had received at least two lines of prior therapy.
Atezolizumab was administered every 3 weeks at 15 mg/kg or 20 mg/kg, and the level of PD-L1 expression on tumor-infiltrating immune cells was evaluated. The primary endpoint of the study was safety, with overall response rate, duration of response, and progression-free survival as key secondary endpoints.
The 1- and 2-year overall survival rates for responders were 100%, but that dropped to 33% and 11%, respectively, for nonresponders. Of the 11 responders, 5 received atezolizumab as first-line therapy, while 9 had high PD-L1 expression (IC2/3).
For patients who received atezolizumab in the first-line setting, 1-year overall survival was 63%, and 2-year overall survival was 47%. The rates were lower for second-line and beyond; 37% and 18%, respectively.
For IC2/3 patients, 1-year overall survival was 45%, compared with 37% for those with low to no PD-L1 expression (IC0/1).
Only 11% of patients experienced treatment-related grade 3 or greater adverse events, and side effects led to treatment discontinuation in 3% of patients.
A key message was that the duration of response had a median of 21 months, and that is significant in this disease setting, explained Dr. Schmid. Another important point was that “overall survival was significantly longer that what we see with chemotherapy.”
Genentech funded the study. Dr Schmid’s spouse is a consultant to Roche/Genentech.
FROM THE AACR ANNUAL MEETING
Key clinical point: In a phase I trial, the immunotherapy agent atezolizumab improved survival in triple-negative breast cancer.
Major finding: Overall survival rates were 41% at 1 year and 22% at both year 2 and year 3.
Data source: A phase I trial with a total of 112 patients who were evaluable for response.
Disclosures: Genentech funded the study. Dr Schmid’s spouse is a consultant to Roche/Genentech.
Get ready for cancer immunotherapy-induced rheumatic diseases
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
SNOWMASS, COLO. – Physicians can expect to encounter more and more patients with inflammatory arthritis and other rheumatic adverse events induced by immune checkpoint inhibitors as a result of anticipated exponential growth in the use of these drugs to treat an expanding list of cancers, Clifton O. Bingham III, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
These cancer immunotherapy–induced rheumatic diseases may superficially look like the classic forms of familiar autoimmune diseases, but they have highly atypical features that will affect treatment decisions.
“What we’ve seen consistently is that the normal doses of prednisone we would use to treat an inflammatory arthritis are really ineffective in most of these patients. We’ve had to use super doses – up to 120 mg/day – for initial control, and then 7.5-40 mg daily for maintenance of response,” according to Dr. Bingham, professor of medicine and director of the Johns Hopkins Arthritis Center in Baltimore.
To date, only limited data from case series are available on rheumatic IRAEs. There are no prospective patient registries logging accurate data on the incidence of these rheumatic adverse events among cancer patients treated with immune checkpoint inhibitors (ICIs). These IRAEs, which lie at the intersection of rheumatology and oncology, are of special interest to Dr. Bingham – he and his coinvestigators have published five articles on the topic over the course of a single year.
In a wide-ranging talk at the symposium, he touched on the phenotypic spectrum of rheumatologic IRAEs, his conviction that they are greatly underdiagnosed, why physicians can expect to encounter them much more frequently, rheumatologic IRAE treatment issues, and the risks of prescribing ICIs in patients with known preexisting rheumatologic disease.
Rheumatologic IRAE presentations
Inflammatory arthritis is the most common form of rheumatologic IRAE, followed by sicca syndrome. At the Johns Hopkins Arthritis Center, Dr. Bingham and his coworkers have 25 well-characterized patients with inflammatory arthritis resulting from an ICI, only 1 of whom is HLA-B27-positive.
“Also, just one is autoantibody-positive, even though they all look for all the world as though they have rheumatoid arthritis,” the rheumatologist observed.
This ICI-induced inflammatory arthritis initially presents most commonly in the midsize and large joints – knees, ankles, elbows – then expands to include small joints such as the wrists, proximal interphalangeal joints, and the metacarpophalangeal joints.
Notably, the Hopkins group also has three patients with classic reactive arthritis marked by conjunctivitis, urethritis, arthritis, and dactylitis.
“I don’t know about you, but, in our general rheumatology practice, we see maybe one case of reactive arthritis in several years, so this is something that has struck us as really quite interesting,” said Dr. Bingham, who is also director of research in the division of rheumatology at Johns Hopkins.
The arthritis center is also managing a group of patients with ICI-induced sicca syndrome, which is uniformly extremely severe and treatment resistant, as well as a couple of patients with myositis IRAE, one with polymyalgia rheumatica, and two with crystal disease that is highly inflammatory in nature, difficult to treat, and includes an inflammatory polyarthritis component not typical in patients with crystal arthritis.
Why physicians will see more rheumatologic IRAEs
ICIs have dramatically transformed the treatment of selected advanced-stage cancers. For example, whereas patients with metastatic melanoma historically had a 2-year survival rate of 5%, combination therapy with the ICIs ipilimumab (Yervoy) and nivolumab (Opdivo) resulted in a 60% rate of partial or complete remission in a landmark clinical trial.
The basis of cancer immunotherapy is the discovery that, in order for cancer cells to thrive, they emit blocking signals that downregulate the native ability of T cells to recognize and kill them. This is true for both solid tumors and hematologic malignancies. The ICIs inhibit these blocking signals, which include cytotoxic T-lymphocyte–associated protein 4 (CTLA4), programmed death-1 (PD-1), and programmed death ligand-1 (PDL-1), thereby freeing up the T cells for tumor fighting.
Because of the nonspecific mechanism of this T-cell activation, however, ICIs have, as their main toxicities, T-cell–mediated autoimmune inflammatory tissue damage, which gets lumped under the umbrella term IRAEs. It can affect almost every organ system. Skin rashes are the most common, colitis second. Other commonly encountered IRAEs include thyroiditis, hypophysitis, hepatitis, peripheral neuropathy, and pneumonitis.
In addition to the four currently approved ICIs – ipilimumab, nivolumab, pembrolizumab (Keytruda), and atezolizumab (Tecentriq) – investigational ICIs targeting CTLA4, PD-1, and/or PDL-1 are in development. Plus, new ICIs targeting other blocking signals, including lymphocyte activation gene-3, CD137, and T-cell immunoglobulin and mucin domain-3, are now in clinical trials.
Clinical trials aimed at expanding the indications of existing ICIs and using ICIs in earlier-stage cancers in an effort to improve rates of lasting remission are also underway.
All told, probably at least 400 clinical trials of ICIs are ongoing worldwide, the rheumatologist estimated.
“More people will be exposed to these drugs, and we’ll see more and more of these rheumatologic IRAEs,” Dr. Bingham predicted.
Rheumatologic IRAEs are seriously underdiagnosed
Back in the pre-ICI days, Dr. Bingham was coauthor of a major study which concluded that clinical trialists in oncology consistently downgrade the severity of rheumatologic adverse events, often by 1 or 2 grades (J Rheumatol. 2007 Jun;34[6]:1401-14).
Unpublished details of ICI clinical trials in melanoma that he obtained from Bristol-Myers Squibb suggest that the true rate of rheumatologic IRAEs is about 20%, or roughly double that reported in the studies. That’s because the adverse events–grading system used in oncology undercalls the severity of arthritis and autoimmune disorders.
Indeed, the National Cancer Institute’s Common Terminology Criteria for Adverse Events, used in oncology clinical trials, is confusing on the topic of musculoskeletal and connective tissue disorders as treatment-emergent adverse events, according to Dr. Bingham. He noted that an oncologist can code a swollen joint in three different ways – joint effusion, arthritis, or arthralgia – and it takes disabling interference with self-care in activities of daily living for that swollen joint to rise to the level of a Grade 3 adverse event. From a rheumatology trialist’s perspective, that would be a Grade 4 disability.
Plus, neither the product labeling nor the patient information guides for the approved immunotherapy drugs mention the importance of monitoring for rheumatologic IRAEs or their management.
“There is poor awareness of musculoskeletal and rheumatic IRAEs in the general oncology community,” Dr. Bingham asserted. “But, if you talk with any oncology nurses who work in a clinical trial, they will tell you they’re seeing these events with significant frequency and severity.”
Treatment and response
It’s critical to gain control of rheumatologic IRAEs quickly so that patients can get on with their cancer immunotherapy. Dr. Bingham uses intra-articular steroid injections for patients with oligoarthritis and high-dose oral prednisone for polyarticular disease. He starts methotrexate and/or leflunomide early because the conventional disease-modifying antirheumatic drugs have roughly a 2-month delay in onset of action. He has had several patients who are unable to taper steroids despite background methotrexate.
In the most severely affected patients, he has turned to biologic agents in consultation with their oncologists. Tumor necrosis factor (TNF) inhibitors are the ones he and other rheumatologists have used most often.
“Notably, we have not been able to taper down very well. We have patients who are out more than 2 years now who still require their TNF inhibitor to treat their inflammatory arthritis, and these are patients on conventional disease–modifying antirheumatic drugs as well. As soon as it’s tapered, the arthritis begins to come back,” according to Dr. Bingham.
In marked contrast, colitis as an IRAE typically clears in response to just one or two doses of a TNF inhibitor.
One audience member related that she’d encountered a roadblock in trying to get authorization for a TNF inhibitor for a patient with a rheumatologic IRAE secondary to ICI treatment for metastatic melanoma because the labeling states these agents are relatively contraindicated in melanoma patients. Dr. Bingham offered a tip: Collaborate with the patient’s oncologist.
“In most cases, oncologists can get infliximab for these patients and administer it in their infusion centers. They are able to get things authorized with very little trouble,” he said.
Besides, most of these patients with severe inflammatory arthritis meet conventional criteria for TNF inhibitor therapy, based on their number of infected joints and elevated acute phase reactants for longer than 6 weeks, Dr. Bingham noted.
“We’ve had some very interesting conversations with patients. It’s impressive to see the impact arthritis can have on people. A lot of patients have said, ‘I don’t care if I die. Get me functional right now.’ That’s pretty profound. Quality of life is still very important for people, even when dealing with life-threatening diseases,” he observed.
Oncologists are actually eager for their patients to get on steroid-sparing therapy because of concern that high doses of steroids may reduce the efficacy of cancer immunotherapy. That’s not an issue with the TNF inhibitors, the rheumatologist continued.
Turning to the utility of other classes of biologic agents, Dr. Bingham advised avoiding abatacept (Orencia) because its mechanism of action is likely to cause interference with the cancer immunotherapy. Rituximab (Rituxan) takes too long to act. Anakinra (Kineret), tofacitinib (Xeljanz), and tocilizumab (Actemra), on the other hand, are agents he is interested in using as alternatives to TNF inhibitors, although he hasn’t done so yet.
Use of ICIs in patients with preexisting autoimmune disease
The experience here is entirely anecdotal, since such patients have been excluded from ICI clinical trials, but the available evidence suggests physicians should be prepared for higher rheumatologic IRAE rates in this setting. Investigators at Vanderbilt University reported that 8 of 30 cancer patients with known preexisting autoimmune disease experienced flares of that disease when treated with ipilimumab, and 10 developed a new IRAE (Therap Adv Gastroenterol. 2016 Jul;9[4]:457-62).
The Hopkins group has three patients with preexisting rheumatoid arthritis and two with preexisting scleroderma who have received ICIs. All three rheumatoid arthritis patients flared. Rheumatologists are trying to manage these flares so the patients can continue on their ICI. One of the scleroderma patients experienced no change in that disease while on an ICI, while the other showed a definite improvement in scleroderma symptoms.
“I think the jury’s still out in terms of what you do about ICI therapy in patients with preexisting autoimmunity. The data would say that there’s maybe a 50-50 chance of the autoimmune disease becoming worse, but, if patients have an otherwise fatal cancer, I think it’s probably worth the chance,” Dr. Bingham said.
Anecdotal reports suggest that more severe IRAEs may be a favorable prognostic sign in terms of cancer eradication, but a lot more patient experience will be needed in order to be sure, the rheumatologist said.
Dr. Bingham reported serving as a consultant to Bristol-Myers Squibb.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM