User login
Adding T-vec might help surmount PD-1 resistance in melanoma
Almost two-thirds of patients with advanced melanoma responded to combination therapy with pembrolizumab and talimogene laherparepvec (T-vec) in a small phase 1b trial, investigators reported.
A third of patients achieved a complete response and median progression-free and overall survival were not reached after typically 18.6 (range, 17.7 to 20.8) months of follow-up, said Antoni Ribas, MD, of the University of California, Los Angeles, and his coinvestigators. In contrast to single-agent pembrolizumab therapy, responders to the combination regimen included patients with very low levels of CD8+ T cell infiltrates or negative interferon-gamma (IFN-gamma) gene signatures in baseline tumor biopsies, suggesting that oncolytic virotherapy might make anti-PD-1 therapy more effective by altering the tumor microenvironment, the researchers concluded. Serious adverse events were uncommon in this study, and there were no dose-limiting toxicities, they wrote (Cell. 2017 Sept. 7 doi: 10.1016/j.cell.2017.08.027).
To see if attracting CD8+ T cells into tumors helped surmount this obstacle, the researchers treated 21 patients with advanced melanoma with pembrolizumab and T-vec, an intratumorally administered, genetically modified clinical herpes simplex virus-1 strain approved for treating melanoma. Patients first received up to 4 mL T-vec (106 plaque-forming units [pfu] per mL) to induce a protective immune response. Three weeks later, they started receiving to 4 mL (108 pfu/mL) T-vec plus 200 mg intravenous pembrolizumab every 2 weeks.
Thirteen patients (62%) showed at least a partial response, and seven (33%) had a complete response based on immune criteria. Notably, 9 of 13 (69%) patients with baseline tumor CD8+ densities below 1,000 cells/mm2 responded to combination treatment, as did three of five patients with low baseline IFN-gamma signatures.
“There was only one baseline biopsy that was scored as PD-L1 negative, but that patient went on to have a complete response to the combined therapy,” the researchers wrote. “Patients who responded to combination therapy had increased CD8+ T cells, elevated PD-L1 protein expression, [and] IFN-gamma gene expression on several cell subsets in tumors after [T-vec] treatment. Response to combination therapy did not appear to be associated with baseline CD8+ T cell infiltration or baseline IFN-gamma signature.” Increased levels of circulating immune cells and shrinkage of untreated tumors both suggested that intratumoral T-vec injections led to systemic effects, they added.
Amgen and Merck provided funding. Dr. Ribas disclosed consulting fees from both companies.
Almost two-thirds of patients with advanced melanoma responded to combination therapy with pembrolizumab and talimogene laherparepvec (T-vec) in a small phase 1b trial, investigators reported.
A third of patients achieved a complete response and median progression-free and overall survival were not reached after typically 18.6 (range, 17.7 to 20.8) months of follow-up, said Antoni Ribas, MD, of the University of California, Los Angeles, and his coinvestigators. In contrast to single-agent pembrolizumab therapy, responders to the combination regimen included patients with very low levels of CD8+ T cell infiltrates or negative interferon-gamma (IFN-gamma) gene signatures in baseline tumor biopsies, suggesting that oncolytic virotherapy might make anti-PD-1 therapy more effective by altering the tumor microenvironment, the researchers concluded. Serious adverse events were uncommon in this study, and there were no dose-limiting toxicities, they wrote (Cell. 2017 Sept. 7 doi: 10.1016/j.cell.2017.08.027).

To see if attracting CD8+ T cells into tumors helped surmount this obstacle, the researchers treated 21 patients with advanced melanoma with pembrolizumab and T-vec, an intratumorally administered, genetically modified clinical herpes simplex virus-1 strain approved for treating melanoma. Patients first received up to 4 mL T-vec (106 plaque-forming units [pfu] per mL) to induce a protective immune response. Three weeks later, they started receiving to 4 mL (108 pfu/mL) T-vec plus 200 mg intravenous pembrolizumab every 2 weeks.
Thirteen patients (62%) showed at least a partial response, and seven (33%) had a complete response based on immune criteria. Notably, 9 of 13 (69%) patients with baseline tumor CD8+ densities below 1,000 cells/mm2 responded to combination treatment, as did three of five patients with low baseline IFN-gamma signatures.
“There was only one baseline biopsy that was scored as PD-L1 negative, but that patient went on to have a complete response to the combined therapy,” the researchers wrote. “Patients who responded to combination therapy had increased CD8+ T cells, elevated PD-L1 protein expression, [and] IFN-gamma gene expression on several cell subsets in tumors after [T-vec] treatment. Response to combination therapy did not appear to be associated with baseline CD8+ T cell infiltration or baseline IFN-gamma signature.” Increased levels of circulating immune cells and shrinkage of untreated tumors both suggested that intratumoral T-vec injections led to systemic effects, they added.
Amgen and Merck provided funding. Dr. Ribas disclosed consulting fees from both companies.
Almost two-thirds of patients with advanced melanoma responded to combination therapy with pembrolizumab and talimogene laherparepvec (T-vec) in a small phase 1b trial, investigators reported.
A third of patients achieved a complete response and median progression-free and overall survival were not reached after typically 18.6 (range, 17.7 to 20.8) months of follow-up, said Antoni Ribas, MD, of the University of California, Los Angeles, and his coinvestigators. In contrast to single-agent pembrolizumab therapy, responders to the combination regimen included patients with very low levels of CD8+ T cell infiltrates or negative interferon-gamma (IFN-gamma) gene signatures in baseline tumor biopsies, suggesting that oncolytic virotherapy might make anti-PD-1 therapy more effective by altering the tumor microenvironment, the researchers concluded. Serious adverse events were uncommon in this study, and there were no dose-limiting toxicities, they wrote (Cell. 2017 Sept. 7 doi: 10.1016/j.cell.2017.08.027).

To see if attracting CD8+ T cells into tumors helped surmount this obstacle, the researchers treated 21 patients with advanced melanoma with pembrolizumab and T-vec, an intratumorally administered, genetically modified clinical herpes simplex virus-1 strain approved for treating melanoma. Patients first received up to 4 mL T-vec (106 plaque-forming units [pfu] per mL) to induce a protective immune response. Three weeks later, they started receiving to 4 mL (108 pfu/mL) T-vec plus 200 mg intravenous pembrolizumab every 2 weeks.
Thirteen patients (62%) showed at least a partial response, and seven (33%) had a complete response based on immune criteria. Notably, 9 of 13 (69%) patients with baseline tumor CD8+ densities below 1,000 cells/mm2 responded to combination treatment, as did three of five patients with low baseline IFN-gamma signatures.
“There was only one baseline biopsy that was scored as PD-L1 negative, but that patient went on to have a complete response to the combined therapy,” the researchers wrote. “Patients who responded to combination therapy had increased CD8+ T cells, elevated PD-L1 protein expression, [and] IFN-gamma gene expression on several cell subsets in tumors after [T-vec] treatment. Response to combination therapy did not appear to be associated with baseline CD8+ T cell infiltration or baseline IFN-gamma signature.” Increased levels of circulating immune cells and shrinkage of untreated tumors both suggested that intratumoral T-vec injections led to systemic effects, they added.
Amgen and Merck provided funding. Dr. Ribas disclosed consulting fees from both companies.
FROM CELL
Key clinical point: Adding talimogene laherparepvec (T-vec) might help overcome resistance to anti-PD-1 antibodies in patients with advanced melanoma.
Major finding: In all, 62% of patients had at least a partial response and 33% had a complete response. Median progression-free and overall survival were not reached after a median of 18.6 weeks of follow-up.
Data source: A phase 1b clinical trial of 21 adults with advanced melanoma who received T-vec and pembrolizumab.
Disclosures: Amgen and Merck provided funding. Dr. Ribas disclosed consulting fees from both companies.
An ASCO 2017 recap: significant advances continue
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
As we head into vacation season and the dog days of summer, let’s reflect for a few minutes on some of the very important advances we heard about at this year’s annual meeting of the American Society of Clinical Oncology in Chicago. Nearly 40,000 individuals registered for the conference, an indication of both the interest and the excitement around the new agents and the emerging clinical trial data. Scientific sessions dedicated to the use of combination immunotherapy, the role of antibody drug conjugates, and targeting molecular aberrations with small molecules were among the most popular (p. e236).
In the setting of metastatic breast cancer, several trials produced highly significant results that will positively affect the duration and quality of life for our patients. The use of PARP inhibitors in BRCA-mutated cancers has been shown to be effective in a few areas, particularly advanced ovarian cancer. The OlympiAD study evaluated olaparib monotherapy and a physician’s choice arm (capecitabine, eribulin, or vinorelbine) in BRCA-mutated, HER2-negative metastatic breast cancer. The 2:1 design enrolled 302 patients and demonstrated a 3-month improvement in progression-free survival (PFS) for olaparib compared with the control arm (7.0 vs 4.2 months, respectively; P = .0009). The patient population for this BRCA-mutated trial was relatively young, with a median age of 45 years, and 50% of the women were hormone positive and 30%, platinum resistant.
The CDK4/6 inhibitors continue to be impressive, with the recently reported results from the MONARCH 2 trial showing encouraging PFS and overall response rate results with the addition of the CDK4/6 inhibitor abemaciclib to fulvestrant, a selective estrogen-receptor degrader. In this study, hormone-positive, HER2-negative women who had progressed on previous endocrine therapy were randomized 2:1 to abemaciclib plus fulvestrant or placebo plus fulvestrant. A total of 669 patients were accrued, and after a median follow-up of 19 months, a highly significant PFS difference of 7 months between the abemaciclib–fulvestrant and fulvestrant–only groups was observed (16.4 vs 9.3 months, respectively; P < .0000001) along with an overall response rate of 48.1 months, compared with 21.3 months. Previous findings have demonstrated monotherapy activity for abemaciclib, and the comparisons with palbociclib and ribociclib will be forthcoming, although no comparative trials are underway. These agents will be extensively assessed in a variety of settings, including adjuvantly.
The results of the much anticipated APHINITY study, which evaluated the addition of pertuzumab to trastuzumab in the adjuvant HER2-positive setting, were met with mixed reviews. Patients were included if they had node-positive invasive breast cancer or node-negative tumors of >1.0 cm. A total of 4,804 patients (37% node negative) were enrolled in the study. The intent-to-treat primary endpoint of invasive disease-free survival (DFS) was statistically positive (P = .045), although the 3-year absolute percentages for the pertuzumab–trastuzumab and trastuzumab-only groups were 94.1% and 93.2%, respectively. It should be noted that the planned statistical assumption was for a delta of 2.6% – 91.8% and 89.2%, respectively. Thus, both arms actually did better than had been planned, which was based on historical comparisons, and the node-positive and hormone-negative subgroups trended toward a greater benefit with the addition of pertuzumab. There was, and will continue to be, much debate around the cost–benefit ratio and which patients should be offered the combination. The outstanding results with the addition of pertuzumab in the neoadjuvant setting will continue to be the setting in which the greatest absolute clinical benefit will be seen. It is unusual in this era to see trials this large planned to identify a small difference, and it is likely that resource constraints will make such studies a thing of the past.
The very active hormonal therapies, abiraterone and enzalutimide, for castrate-resistant prostate cancer remain of high interest in the area of clinical trials. The LATITUDE study evaluated a straightforward design that compared abiraterone with placebo in patients who were newly diagnosed with high-risk, metastatic hormone-naïve prostate cancer. Patients in both arms received androgen-deprivation therapy and high risk was defined by having 2 of 3 criteria: a Gleason score of ≥8; 3 or more bone lesions; or visceral disease. Of note is that 1,199 patients were enrolled before publication of the CHAARTED or STAMPEDE results, which established docetaxel as a standard for these patients. The median age in the LATITUDE trial was 68 years, with 17% of patients having visceral disease and 48% having nodal disease, making it a similar patient population to those in the docetaxel studies. The results favoring abiraterone were strikingly positive, with a 38% reduction in the risk of death (P < .0001) and a 53% reduction in the risk of radiographic progression or death (P < .0001). The regimen was well tolerated overall, and it is clear that this option will be widely considered by physicians and their patients.
Two studies addressing the importance of managing symptoms and improving outcomes were also part of the plenary session. The IDEA Collaboration conducted a prospective pooled analysis of 6 phase 3 studies that assessed 3 and 6 months of oxaliplatin-based regimens for stage 3 colon cancer. FOLFOX and CAPOX given to 12,834 patients in 6 studies from the United States, European Union, Canada, Australia, New Zealand, and Japan were evaluated for DFS, treatment compliance, and adverse events. As would be anticipated, fewer side effects, particularly neurotoxicity, and greater compliance were observed in the 3-month group. Although DFS noninferiority for 3 months of therapy was not established statistically, the overall data led the investigators to issue a consensus statement advocating for a risk-based approach in deciding the duration of therapy and recommending 3 months of therapy for patients with stage 3, T1-3N1 disease, and consideration of 6 months therapy for T4 and/ or N2 disease. The investigators also acknowledged the leader and creator of IDEA, the late Daniel Sargent, PhD, of the Mayo Clinic, who passed away far too young after a brief illness last fall (1970-2016).
The second symptom-based study was performed at Memorial Sloan Kettering Cancer Center (MSKCC) in New York and designed by a group of investigators from the Dana-Farber Cancer Institute in Boston; the Mayo Clinic in Rochester, Minnesota; the University of North Carolina in Chapel Hill; and MSKCC (p. e236). The hypothesis was simply that proactive symptom monitoring during chemotherapy would improve symptom management and lead to better outcomes. For the study, 766 patients with advanced solid tumors who were receiving outpatient chemotherapy were randomized to a control arm with standard follow-up or to the intervention arm, on which patients self-reported on 12 common symptoms before and between visits using a web-based tool and received weekly e-mail reminders and nursing alerts. At 6 months, and compared with baseline, the self-reporting patients in the intervention arm experienced an improved quality of life (P < .001). In addition, 7% fewer of the self-reporting patients visited the emergency department (P = .02), and they experienced longer survival by 5 months compared with the standard follow-up group (31.2 vs 26.0 months, respectively; P = .03). Although there are limitations to such a study, the growth in technological advances should create the opportunity to expand on this strategy in further trials and in practice. With such an emphasis in the Medicare Oncology Home Model on decreasing hospital admissions and visits to the emergency department, there should great motivation for all involved to consider incorporating self-reporting into their patterns of care.
A continued emphasis on molecular profiling, personalized and/or precision medicine, and identifying or matching the patient to the best possible therapy or the most appropriate clinical trial remains vital to improving outcomes. Just before the ASCO meeting, the US Food and Drug Administration approved pembrolizumab for the treatment of patients with high-level microsatellite instability (MSI-H) and mismatch-repair deficient (dMMR) cancers, regardless of the site of origin. The approval was based on data from 149 patients with MSI-H or dMMR cancers, which showed a 40% response rate in this group of patients, two-thirds of whom had previously treated colon cancer. This landmark approval of a cancer therapy for a specific molecular profile and not the site of the disease, will certainly shape the future of oncology drug development. One of the highlighted stories at ASCO was the success of the larotrectinib (LOXO 101) tropomyosin receptor kinase inhibitor in patients with the TRK fusion mutations (p. e237). The data, including waterfall charts, swimmer plots, and computed-tomography scans, were impressive in this targeted population with a 76% response rate and a 91% duration of response at 6 months with a mild side effect profile.
In summary, across a variety of cancers, with treatment strategies of an equally diverse nature, we saw practice-changing data from the ASCO meeting that will benefit our patients. Continuing to seek out clinical trial options for patients will be critical in answering the many questions that have emerged and the substantial number of studies that are ongoing with combination immunotherapies, targeted small molecules, and a growing armamentarium of monoclonal antibodies.
Adverse effects of PD-1/PD-L1 inhibitors varied by tumor type in systematic review
The immune-related adverse effects of inhibitors of programmed cell death protein 1 (PD-1) and its ligand varied by tumor type in a large systematic review and meta-analysis.
Patients with melanoma were significantly more likely to develop colitis (odds ratio, 4.2; 95% confidence interval, 1.3 to 14.0), diarrhea (OR, 1.9), pruritus (OR, 2.4), and rash (OR, 1.8) compared with patients with non–small cell lung cancer, who were significantly more likely to develop pneumonitis, reported Leila Khoja, MBChB, PhD, of AstraZeneca UK, Melbourn, England, and associates. Patients with melanoma also were significantly more likely to develop arthralgia, hypothyroidism, rash, pruritus, and diarrhea compared with patients with renal cell carcinoma, who were more likely to develop pneumonitis and dyspnea.
“In light of this study, we should be mindful that different tumor types may have different immune-related adverse effect patterns when treated with the same immune checkpoint inhibitor,” the reviewers noted (Ann Oncol. 2017 Aug 8. doi: 10.1093/annonc/mdx286).
The review included 48 trials of nearly 7,000 patients with solid tumors who received CTLA-4 inhibitors (26 studies), PD-1 inhibitors (17 studies), PD-1 ligand (PD-L1) inhibitors (two trials), or both CTLA-4 and PD-1 inhibitors (three trials). The reviewers identified the studies by searching the Medline, EMBASE, and COCHRANE databases for prospective trials published from 2003 through November 2015.
Severe or life-threatening immune-related adverse effects developed in 31% of patients who received CTLA-4 inhibitors and 10% of patients who received PD-1 inhibitors. Inhibitors of CTLA-4 were significantly more likely to cause all grades of colitis (OR, 8.7), hypophysitis (OR, 6.5), and rash (OR, 2.0), while PD-1 inhibitors were more strongly linked with pneumonitis (OR 6.4), hypothyroidism (OR 4.3), arthralgia (OR, 3.5), and vitiligo (OR, 3.5).
The reviewers also looked for significant predictors of immune-related colitis and pneumonitis, because these are potentially fatal. They found that pneumonitis was significantly linked to PD-1/PD-L1 inhibitor therapy (P less than .001) and colitis to CTLA-4 treatment (P = .04), even after accounting for therapeutic dose and tumor type. No other factors reached significance in this multivariable model.
“Clearly, a more thorough understanding of the mechanisms of immune-related adverse effects is needed, which may lead to the identification of biomarkers to predict the occurrence of toxicity in patients or predict those who have immune-related adverse effects that are unlikely to respond to corticosteroids,” the reviewers concluded. Researchers should also study whether clinical factors such as treatment history or comorbidities affect the risk of immune-related adverse effects from immune checkpoint inhibitors, they said.
The reviewers reported having no funding sources and no relevant conflicts of interest.
The immune-related adverse effects of inhibitors of programmed cell death protein 1 (PD-1) and its ligand varied by tumor type in a large systematic review and meta-analysis.
Patients with melanoma were significantly more likely to develop colitis (odds ratio, 4.2; 95% confidence interval, 1.3 to 14.0), diarrhea (OR, 1.9), pruritus (OR, 2.4), and rash (OR, 1.8) compared with patients with non–small cell lung cancer, who were significantly more likely to develop pneumonitis, reported Leila Khoja, MBChB, PhD, of AstraZeneca UK, Melbourn, England, and associates. Patients with melanoma also were significantly more likely to develop arthralgia, hypothyroidism, rash, pruritus, and diarrhea compared with patients with renal cell carcinoma, who were more likely to develop pneumonitis and dyspnea.
“In light of this study, we should be mindful that different tumor types may have different immune-related adverse effect patterns when treated with the same immune checkpoint inhibitor,” the reviewers noted (Ann Oncol. 2017 Aug 8. doi: 10.1093/annonc/mdx286).
The review included 48 trials of nearly 7,000 patients with solid tumors who received CTLA-4 inhibitors (26 studies), PD-1 inhibitors (17 studies), PD-1 ligand (PD-L1) inhibitors (two trials), or both CTLA-4 and PD-1 inhibitors (three trials). The reviewers identified the studies by searching the Medline, EMBASE, and COCHRANE databases for prospective trials published from 2003 through November 2015.
Severe or life-threatening immune-related adverse effects developed in 31% of patients who received CTLA-4 inhibitors and 10% of patients who received PD-1 inhibitors. Inhibitors of CTLA-4 were significantly more likely to cause all grades of colitis (OR, 8.7), hypophysitis (OR, 6.5), and rash (OR, 2.0), while PD-1 inhibitors were more strongly linked with pneumonitis (OR 6.4), hypothyroidism (OR 4.3), arthralgia (OR, 3.5), and vitiligo (OR, 3.5).
The reviewers also looked for significant predictors of immune-related colitis and pneumonitis, because these are potentially fatal. They found that pneumonitis was significantly linked to PD-1/PD-L1 inhibitor therapy (P less than .001) and colitis to CTLA-4 treatment (P = .04), even after accounting for therapeutic dose and tumor type. No other factors reached significance in this multivariable model.
“Clearly, a more thorough understanding of the mechanisms of immune-related adverse effects is needed, which may lead to the identification of biomarkers to predict the occurrence of toxicity in patients or predict those who have immune-related adverse effects that are unlikely to respond to corticosteroids,” the reviewers concluded. Researchers should also study whether clinical factors such as treatment history or comorbidities affect the risk of immune-related adverse effects from immune checkpoint inhibitors, they said.
The reviewers reported having no funding sources and no relevant conflicts of interest.
The immune-related adverse effects of inhibitors of programmed cell death protein 1 (PD-1) and its ligand varied by tumor type in a large systematic review and meta-analysis.
Patients with melanoma were significantly more likely to develop colitis (odds ratio, 4.2; 95% confidence interval, 1.3 to 14.0), diarrhea (OR, 1.9), pruritus (OR, 2.4), and rash (OR, 1.8) compared with patients with non–small cell lung cancer, who were significantly more likely to develop pneumonitis, reported Leila Khoja, MBChB, PhD, of AstraZeneca UK, Melbourn, England, and associates. Patients with melanoma also were significantly more likely to develop arthralgia, hypothyroidism, rash, pruritus, and diarrhea compared with patients with renal cell carcinoma, who were more likely to develop pneumonitis and dyspnea.
“In light of this study, we should be mindful that different tumor types may have different immune-related adverse effect patterns when treated with the same immune checkpoint inhibitor,” the reviewers noted (Ann Oncol. 2017 Aug 8. doi: 10.1093/annonc/mdx286).
The review included 48 trials of nearly 7,000 patients with solid tumors who received CTLA-4 inhibitors (26 studies), PD-1 inhibitors (17 studies), PD-1 ligand (PD-L1) inhibitors (two trials), or both CTLA-4 and PD-1 inhibitors (three trials). The reviewers identified the studies by searching the Medline, EMBASE, and COCHRANE databases for prospective trials published from 2003 through November 2015.
Severe or life-threatening immune-related adverse effects developed in 31% of patients who received CTLA-4 inhibitors and 10% of patients who received PD-1 inhibitors. Inhibitors of CTLA-4 were significantly more likely to cause all grades of colitis (OR, 8.7), hypophysitis (OR, 6.5), and rash (OR, 2.0), while PD-1 inhibitors were more strongly linked with pneumonitis (OR 6.4), hypothyroidism (OR 4.3), arthralgia (OR, 3.5), and vitiligo (OR, 3.5).
The reviewers also looked for significant predictors of immune-related colitis and pneumonitis, because these are potentially fatal. They found that pneumonitis was significantly linked to PD-1/PD-L1 inhibitor therapy (P less than .001) and colitis to CTLA-4 treatment (P = .04), even after accounting for therapeutic dose and tumor type. No other factors reached significance in this multivariable model.
“Clearly, a more thorough understanding of the mechanisms of immune-related adverse effects is needed, which may lead to the identification of biomarkers to predict the occurrence of toxicity in patients or predict those who have immune-related adverse effects that are unlikely to respond to corticosteroids,” the reviewers concluded. Researchers should also study whether clinical factors such as treatment history or comorbidities affect the risk of immune-related adverse effects from immune checkpoint inhibitors, they said.
The reviewers reported having no funding sources and no relevant conflicts of interest.
FROM ANNALS OF ONCOLOGY
Key clinical point: Immune-related adverse effects varied by tumor type in patients receiving programmed cell death protein 1 (PD-1) and PD-L1 inhibitors.
Major finding: Patients with melanoma who received PD-1/PD-L1 inhibitors were significantly more likely to develop colitis (odds ratio, 4.2; 95% confidence interval, 1.3 to 14.0), diarrhea (OR, 1.9), pruritus (OR, 2.4), and rash (OR, 1.8), compared with patients with non-small cell lung cancer, who were significantly more likely to develop pneumonitis.
Data source: A systematic review and meta-analysis of 48 prospective trials of immune checkpoint inhibitors in of 6,938 adults with solid tumors.
Disclosures: The reviewers reported having no funding sources and no relevant conflicts of interest.
FDA approves nivolumab for metastatic CRC
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor nivolumab for the treatment of patients with mismatch repair deficient (dMMR) and microsatellite instability high (MSI-H) metastatic colorectal cancer (CRC) that has progressed following treatment with fluoropyrimidine, oxaliplatin, and irinotecan.
The indication covers patients aged 12 years and older. Efficacy for adolescent patients with MSI-H or dMMR metastatic CRC is extrapolated from the results in the respective adult population, the FDA said in a statement.
Approval of nivolumab in the adult population was based on an objective response rate of 28% in CHECKMATE 142, an open-label, single-arm study of 53 patients with locally determined dMMR or MSI-H metastatic CRC who had disease progression during, after, or were intolerant to prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
The most common adverse reactions to nivolumab, marketed as Opdivo by Bristol-Myers Squibb, include fatigue, rash, musculoskeletal pain, pruritus, diarrhea, nausea, asthenia, cough, dyspnea, constipation, decreased appetite, back pain, arthralgia, upper respiratory tract infection, and pyrexia, the FDA said.
The recommended nivolumab dose is 240 mg every 2 weeks.
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor nivolumab for the treatment of patients with mismatch repair deficient (dMMR) and microsatellite instability high (MSI-H) metastatic colorectal cancer (CRC) that has progressed following treatment with fluoropyrimidine, oxaliplatin, and irinotecan.
The indication covers patients aged 12 years and older. Efficacy for adolescent patients with MSI-H or dMMR metastatic CRC is extrapolated from the results in the respective adult population, the FDA said in a statement.
Approval of nivolumab in the adult population was based on an objective response rate of 28% in CHECKMATE 142, an open-label, single-arm study of 53 patients with locally determined dMMR or MSI-H metastatic CRC who had disease progression during, after, or were intolerant to prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
The most common adverse reactions to nivolumab, marketed as Opdivo by Bristol-Myers Squibb, include fatigue, rash, musculoskeletal pain, pruritus, diarrhea, nausea, asthenia, cough, dyspnea, constipation, decreased appetite, back pain, arthralgia, upper respiratory tract infection, and pyrexia, the FDA said.
The recommended nivolumab dose is 240 mg every 2 weeks.
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor nivolumab for the treatment of patients with mismatch repair deficient (dMMR) and microsatellite instability high (MSI-H) metastatic colorectal cancer (CRC) that has progressed following treatment with fluoropyrimidine, oxaliplatin, and irinotecan.
The indication covers patients aged 12 years and older. Efficacy for adolescent patients with MSI-H or dMMR metastatic CRC is extrapolated from the results in the respective adult population, the FDA said in a statement.
Approval of nivolumab in the adult population was based on an objective response rate of 28% in CHECKMATE 142, an open-label, single-arm study of 53 patients with locally determined dMMR or MSI-H metastatic CRC who had disease progression during, after, or were intolerant to prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy.
The most common adverse reactions to nivolumab, marketed as Opdivo by Bristol-Myers Squibb, include fatigue, rash, musculoskeletal pain, pruritus, diarrhea, nausea, asthenia, cough, dyspnea, constipation, decreased appetite, back pain, arthralgia, upper respiratory tract infection, and pyrexia, the FDA said.
The recommended nivolumab dose is 240 mg every 2 weeks.
New SU2C translational team aims to apply CAR T-cell therapy to pancreatic cancer
Stand Up To Cancer (SU2C) is supporting a new translational research team to explore how chimeric antigen receptor T-cell (CAR T-cell) therapy can be applied to pancreatic cancer.
The Stand Up To Cancer–Lustgarten Foundation CAR T Translational Research Team will be directed by three investigators at the University of Pennsylvania’s Perelman School of Medicine who have been pioneers in CAR T-cell therapy development: Carl H. June, MD, the Richard W. Vague professor in immunotherapy; Shelley L. Berger, PhD, the Daniel S. Och university professor; and E. John Wherry, PhD, Richard and Barbara Schiffrin president’s distinguished professor of microbiology, and director, Institute for Immunology, according to a press release from the American Association for Cancer Research, SU2C’s Scientific Partner.
The team, which will receive a total of $2 million in funding from both SU2C and the Lustgarten Foundation for Pancreatic Cancer Research, will focus on epigenetics; a phase 1 trial will help identify epigenetic changes that are common to patients who don’t respond to immunotherapy, compared to those who do.
The team will also explore the use of CAR T cells to target mesothelin, a protein that is overexpressed in pancreatic cancer, according to the press release.
The Food and Drug Administration’s Oncologic Drugs Advisory Committee recently gave a thumbs up to a version of CAR T-cell therapy for the treatment of advanced acute lymphoblastic leukemia.This new SU2C translational research team will meet twice a year with the three other SU2C-sponsored research teams addressing pancreatic cancer to share progress and data.
Stand Up To Cancer (SU2C) is supporting a new translational research team to explore how chimeric antigen receptor T-cell (CAR T-cell) therapy can be applied to pancreatic cancer.
The Stand Up To Cancer–Lustgarten Foundation CAR T Translational Research Team will be directed by three investigators at the University of Pennsylvania’s Perelman School of Medicine who have been pioneers in CAR T-cell therapy development: Carl H. June, MD, the Richard W. Vague professor in immunotherapy; Shelley L. Berger, PhD, the Daniel S. Och university professor; and E. John Wherry, PhD, Richard and Barbara Schiffrin president’s distinguished professor of microbiology, and director, Institute for Immunology, according to a press release from the American Association for Cancer Research, SU2C’s Scientific Partner.
The team, which will receive a total of $2 million in funding from both SU2C and the Lustgarten Foundation for Pancreatic Cancer Research, will focus on epigenetics; a phase 1 trial will help identify epigenetic changes that are common to patients who don’t respond to immunotherapy, compared to those who do.
The team will also explore the use of CAR T cells to target mesothelin, a protein that is overexpressed in pancreatic cancer, according to the press release.
The Food and Drug Administration’s Oncologic Drugs Advisory Committee recently gave a thumbs up to a version of CAR T-cell therapy for the treatment of advanced acute lymphoblastic leukemia.This new SU2C translational research team will meet twice a year with the three other SU2C-sponsored research teams addressing pancreatic cancer to share progress and data.
Stand Up To Cancer (SU2C) is supporting a new translational research team to explore how chimeric antigen receptor T-cell (CAR T-cell) therapy can be applied to pancreatic cancer.
The Stand Up To Cancer–Lustgarten Foundation CAR T Translational Research Team will be directed by three investigators at the University of Pennsylvania’s Perelman School of Medicine who have been pioneers in CAR T-cell therapy development: Carl H. June, MD, the Richard W. Vague professor in immunotherapy; Shelley L. Berger, PhD, the Daniel S. Och university professor; and E. John Wherry, PhD, Richard and Barbara Schiffrin president’s distinguished professor of microbiology, and director, Institute for Immunology, according to a press release from the American Association for Cancer Research, SU2C’s Scientific Partner.
The team, which will receive a total of $2 million in funding from both SU2C and the Lustgarten Foundation for Pancreatic Cancer Research, will focus on epigenetics; a phase 1 trial will help identify epigenetic changes that are common to patients who don’t respond to immunotherapy, compared to those who do.
The team will also explore the use of CAR T cells to target mesothelin, a protein that is overexpressed in pancreatic cancer, according to the press release.
The Food and Drug Administration’s Oncologic Drugs Advisory Committee recently gave a thumbs up to a version of CAR T-cell therapy for the treatment of advanced acute lymphoblastic leukemia.This new SU2C translational research team will meet twice a year with the three other SU2C-sponsored research teams addressing pancreatic cancer to share progress and data.
Pancreatitis associated with newer classes of antineoplastic therapies
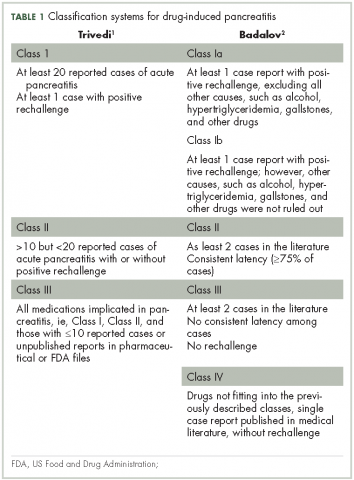
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
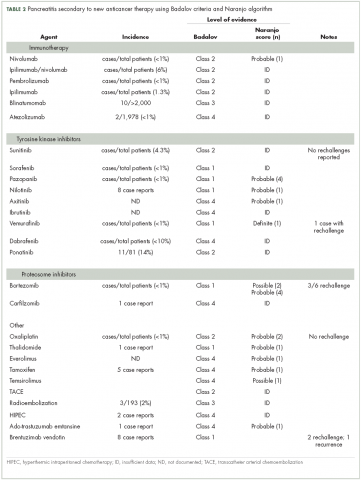
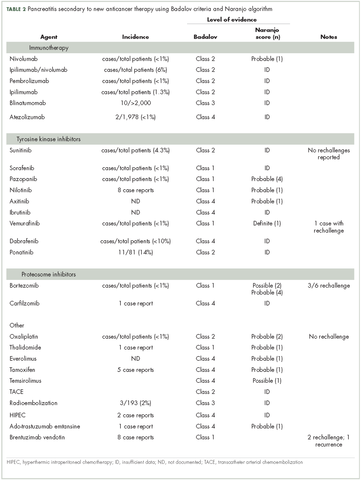
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
Patients with advanced malignancies may develop pancreatitis during therapy for their cancer. Acute pancreatitis is inflammation of the pancreas. Common symptoms include abdominal pain, nausea, vomiting, shortness of breath, dehydration. Laboratory evidence of acute pancreatitis includes elevations of the amylase and lipase. Mild pancreatitis occurs when there is no organ dysfunction, moderate pancreatitis is associated with one organ dysfunction, and severe pancreatitis is complicated by multiple organ dysfunction. Hypotension, hypocalcemia, or anemia suggest a more severe course of the pancreatitis. In some instances, the pancreatitis may be an adverse reaction to the therapy being given. However, other causes such as hypercalcemia, hypertriglyceridemia, cholelithiasis, and underlying malignancy must be ruled out before ascribing pancreatitis to a specific drug. To date, two classifications systems have been proposed by Trivedi1 and Badalov2 to evaluate the degree to which a drug is responsible for pancreatitis (Table 1). Furthermore, Naranjo and colleagues have proposed a more general method of assessing the causal relationship between drugs and adverse events.3 The Naranjo algorithm is not specific for pancreatitis. Jones and colleagues4 reported that 0.1%-2% of acute pancreatitis cases were owing to drugs. In 2015, they listed the older chemotherapy agents associated with pancreatitis. However, more recently, many new agents have been approved for the management of cancers. The newer classes of antineoplastic agents including proteasome inhibitors, immune-modulating agents, tyrosine kinase inhibitors, monoclonal antibodies against programmed cell death-1 (PD-1) and its ligand PD-L1 and antibody-toxin conjugates are now associated with acute pancreatitis.
Methods
We conducted a search in PubMed, Google Scholar, and Micromedex for pancreatitis related to antineoplastic agents, including proteasome inhibitors, immune checkpoint inhibitors, monoclonal antibodies, immune-modulating agents, drug-induced pancreatitis. Terms used for the searches included each specific agent and pancreatitis, immunotherapy and pancreatitis, tyrosine kinase inhibitors and pancreatitis, auto immune pancreatitis, and toxicities of molecular target therapies. Reference lists from the identified manuscripts were reviewed for further studies of pancreatitis as a result of antineoplastic therapy. The most recent search date was February 15, 2017.
The degree to which each agent was associated with inducing pancreatitis was evaluated using the Badalov classification system2 in addition to the Naranjo Adverse Drug Reaction (ADR) Probability Scale.3 The Naranjo scale consists of 10 questions with values assigned to each answer. Total scores range from -4 to 13, where 13-9 indicates the reaction is considered definitely attributable to the drug; 8-5, probably attributable; 4-1, possibly attributable; and ≤0, doubtful if attributable.
A total of 67 manuscripts and abstracts were identified. Four manuscripts and 3 abstracts were excluded because they had insufficient information about possible pancreatitis or there was a presence of multiple other agents or conditions that might have caused pancreatitis. In total, 60 publications met inclusion criteria and were evaluated.
Results
Immune checkpoint inhibitors
In a review of toxicities of anti-programmed cell death-1 (PD-1) therapy, pancreatitis was reported to occur in about 1.8% of patients who received nivolumab or pembrolizumab.5 The 9 patients with pancreatitis attributed to an immune etiology were treated with corticosteroids. Pancreatitis was grade 2 in 3 patients (1.5-2 times upper limit of normal [ULN]), grade 3 in 4 patients (>2-5 ULN), and grade 4 ( >5 ULN) in 2 patients.
In asymptomatic individuals, pancreatitis has been detected on a positron-emission tomography–computed tomography (CT) scan after anti-PD-1 therapy.5 By contrast, there was a case report of a patient treated with nivolumab for lung cancer who developed anorexia, vomiting, and back pain on day 18 of therapy with an elevation of the amylase and lipase levels, but a negative CT.6 Later the patient developed a swollen pancreas on CT. Autoimmune pancreatitis comes in two forms. The most common relates to elevated levels of immunoglobulin G4 (IgG4; normal, 135 mg/dL ULN)7 The mechanism of immune pancreatitis associated with anti-PD-1 therapy is unknown.
Ipilimumab (an anti-CTLA4 antibody) has been approved by the US Food and Drug Administration (FDA) for the treatment of melanoma. Pancreatitis occurred in 1 patient in a phase 1 trial in pediatric patients.9 In a summary of 14 phase 1-3 trials of ipilimumab in advanced melanoma, pancreatitis was reported in fewer than 1% of the patients.10 In management guidelines for therapy with ipilimumab, pancreatitis may present as an asymptomatic increase in the levels of amylase and lipase, or with fevers, malaise, or abdominal pain. Oral prednisone or dexamethasone were given for the immune pancreatitis, but the decline in enzymes was slow, often taking months.11 In a preclinical model of autoimmune pancreatitis due to the blocking of CTLA4, there was suppression of regulatory T-cell function. The autoimmune pancreatitis responded to cyclosporin or rapamycin but there are no clinical data for these agents.12 The anti-PD-L1 agent atezolizumab has been associated with acute pancreatitis in 2 of 1,978 patients (0.1%).13 A review by Champiat and colleagues on dysimmune toxcities related to immune checkpoint inhibitors includes pancreatitis as an autoimmune complication of such therapies.14
Blinatumomab is an anti-CD19–directed CD3 T-cell engager that has been approved by the FDA for refractory B-cell acute lymphoblastic leukemia. In August 2016, the maker of the drug, Amgen, advised hematologists and oncologists that since February 2016, 10 patients out of more than 2,000 treated with blinatumomab had developed pancreatitis.15 Other medications the patients were receiving such as high-dose steroids might have caused or contributed to the pancreatitis. In one case, the pancreatitis improved with stopping blinatumomab but worsened with re-challenge. It is possible that the mechanism of the associated pancreatitis relates to a change in immune checkpoint inhibition. CD19-positive, CD24-high, CD27-positive regulatory B cells are decreased in autoimmune pancreatitis.16 Treatment with blinatumomab may decrease the CD19-positive cells.
Molecularly targeted agents, including TKIs
Molecularly targeted agents such as tyrosine kinase inhibitors (TKIs) or other kinase inhibitors have been associated with pancreatitis.17, 18 In a retrospective study by Tiruman and colleagues,19 the investigators found 91 patients with pancreatitis on imaging, of whom 15 were receiving molecularly target drugs. The pancreatitis was asymptomatic in 2 patients, but 13 had abdominal pain, many with nausea. Four of the patients also had gallstones, but the drug was deemed to be the cause of the pancreatitis. In 4 of the 9 patients in whom a rechallenge was done with the TKI, the pancreatitis relapsed. The pancreatitis resolved in 14 of the 15 patients; 1 patient died because of progressive cancer before the pancreatitis resolved. The pancreatitis was mild, 7 of the 15 patients had normal pancreatic enzymes and the pancreatitis was diagnosed by radiology.
Ghatlia and colleagues17 performed a meta-analysis of trials of TKI. They found 9 cases of pancreatitis in patients on sunitinib therapy. Of those, 4 patients were on sunitinib alone, and 5 were on other chemotherapy agents in combination with sunitinib. Eight cases of pancreatitis due to sorafenib were found. Three of the patients were on sorafenib alone, and 5 were on other chemotherapy including 1 on transcatheter embolization (TACE). Three cases of pancreatitis were associated with vandetanib; 2 of those patients had other concurrent chemotherapy. One case of axitinib induced pancreatitis was described.
Pancreatitis was reported in the phase 1 trials of sorafenib and sunitinib. In all, 3 of 69 patients treated with sorafenib had grade 3 pancreatitis and asymptomatic elevations of amylase and lipase levels were present in about 5% of patients receiving sunitinib.18,19
Other TKIs associated with pancreatitis include pazopanib,20,21 axitinib,22 and nilotinib.23 Pezzilli and coleagues24 described 5 patients with pancreatitis on sorafenib, 3 on sunitinib, 6 on nilotinib. It is possible that some of these cases appeared in other reviews. Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, caused a single case of pancreatitis in 9 patients.25
Vemurafenib, a BRAF kinase inhibitor, was associated with pancreatitis in one case. In this case, the pancreatitis resolved on stopping the medication but recurred when vemurafenib rechallenge was attempted.26 There is a report of dabrafenib being associated with pancreatitis in 1 patient.27
Agents that inhibit the TKIs associated with BCR-ABL in chronic myelogenous leukemia are associated with acute pancreatitis. Imatinib-induced pancreatitis was reported in a small number of cases.28 Nilotinib has caused amylase/lipase elevations with and without symptomatic pancreatitis.29,30 Ponatinib, an inhibitor of BCR-ABL tyrosine kinase, is associated with pancreatitis.31 Pancreatitis occurred in 11 of 81 patients treated with ponatinib, and in 8 patients it was described as serious. Further elevation of amylase or lipase levels without clinical pancreatitis was noted in 7 other patients.
Proteosome inhibitors
In 2010, Elouni and colleagues32 reported a case of IV bortezomib-induced pancreatitis, which recurred on rechallenge with bortezomib. This same patient was also reported in an abstract in 2009.33 In 2009, there was an editorial comment which was added to the end of the abstract that the World Health Organization Adverse Drug Reaction database had 11 reports of bortezomib associated pancreatitis. Talamo and colleagues34 reported a case of bortezomib-induced pancreatitis due to bortezomib that had been administered subcutaneously. At that time, they also summarized 7 previous reports of bortezomib-associated pancreatitis. The mechanism of bortezomib-induced pancreatitis is not known.35-37
Fotoh and colleagues reported a patient with myeloma who had elevated triglyceride levels after bortezomib therapy.38 In one case of bortezomib-associated pancreatitis, the patient had an elevated triglyceride level, but it was not extremely high.39 Multiple myeloma itself may be associated with hyperlipidemia but only rarely.40 Gozetti and colleagues reported a patient who developed hyperlipidemia after two courses of bortezomib;41 stopping bisphosphonates may be associated with a rise in triglycerides. There was one case of carfilzomib causing pancreatitis during a phase 1 trial.42
Older chemotherapy agents
Reviews of drug-induced pancreatitis have listed many chemotherapy agents which may cause pancreatitis.1,43 The agent most frequently associated with acute pancreatitis has been asparaginase,44 with 2%-16% of patients undergoing asparaginase therapy developing pancreatitis. Asparaginase-related pancreatitis is grade 3 or 4 in 5%-10% of patients, and recurs in 63% of patients on rechallenge. Other chemotherapy agents associated with pancreatitis include: mercaptopurine, cytosine arabinoside, cisplatin, interferon alfa-2b, doxorubicin, tamoxifen, gefitinib, vinorelbine, oxaliplatin, levamisole, methotrexate, azathioprine, 5-fluorouracil, capecitabine, ifosfamide, paclitaxel, and all-trans retinoic acid.
Oxaliplatin carries a 0.1%-2% incidence of drug-induced pancreatitis. In one series of 6 patients, cessation of the agent allowed for resolution of symptoms and decrease in serum lipase and amylase levels.45 With capecitabine there are 2 case reports of pancreatitis.46 Cases of pancreatitis associated with trifluridine or tipiracil were not present in the literature.
Thalidomide caused severe pancreatitis in a patient when it was used to treat chronic graft-versus-host disease.47 This patient suffered recurrent pancreatitis on retreatment with the thalidomide. The authors further referenced two other suspected cases of thalidomide-induced, acute pancreatitis. However, in view of the extensive use of thalidomide for multiple myeloma before the development of lenalidomide, thalidomide-associated pancreatitis would be <1% of patients.
Agents that cause hypertriglyceridemia may cause pancreatitis. This mechanism has been reported as the cause of pancreatitis for everolimus48 and tamoxifen.49,50-52 Everolimus causes elevated triglycerides in 30%-50% of patients. There are case reports and a review of tamoxifen-associated pancreatitis caused by elevated triglycerides.52 There has also been a case of temsirolimus-associated pancreatitis,53 another agent that elevates triglycerides.
Pancreatitis associated with hepatic embolization or HIPEC
TACE leads to symptomatic acute pancreatitis in 0.4%-2% of patients, but nonselective TACE (into the hepatic artery and not just feeder vessels), may lead to elevated amylase levels in 15%-40% of patients.54-56 The risk of pancreatitis would depend on which chemotherapy drug is being infused into the liver. It would also be greater if the chemotherapy has to be infused into a larger part of the liver than into a small portion of the liver. In one patient, severe pancreatitis secondary to TACE occurred after two previous embolizations; prior embolization may have led to occlusion of the previously infused vessels.57 Radioembolization with 90Y microspheres was associated with one case of pancreatitis in 112 consecutive patients.58 The postembolization syndrome in the first 24 hours after the procedure may involve fever, abdominal pain, nausea, and vomiting due to acute pancreatitis in some instances.
Acute pancreatitis has also been described as a complication of hyperthermic intraperitoneal chemotherapy (HIPEC).59,60 Two of 13 patients receiving HIPEC for gastric cancer developed pancreatitis.59 In 25 patients with colon cancer who were treated with HIPEC, 1 patient had pancreatitis.60
Antibody-drug conjugates
Muzaffar and colleagues reported a patient with acute pancreatitis 3 days after starting therapy with ado-trastuzumab emtansine.61 Urru and colleagues62 reported a patient who developed acute pancreatitis after brentuximab vedotin therapy. Ghandi and colleagues63 identified 2 cases of fatal acute pancreatitis with brentuximab vedotin and 6 cases of nonfatal pancreatitis. Two of the nonfatal patients were rechallenged, and 1 developed recurrent pancreatitis. Because abdominal pain may occur in up to 18% of patients receiving brentuximab vedotin, the incidence of pancreatitis may be underestimated with this agent.64
In Table 2, ado-trastuzumab emtansine and brentuximab vedotin are listed with incidence and level of association given by the Baldavo2 and Naranjo.3 With greater awareness, the incidence of pancreatitis associated with these agents may rise or fall as more data is accumulated. In many instances, there are insufficient numbers of reported cases or insufficient information in single-case reports to complete the entire table.
Discussion
Acute pancreatitis is an uncommon complication of tyrosine kinase inhibitors, other kinase inhibitors, proteasome inhibitors, monoclonal antibody-drug conjugates and anti-PD-1 immunotherapies. As nausea, abdominal pain, emesis are common in patients with cancer on antineoplastic therapy, some patients may have acute pancreatitis which is undiagnosed. It is not clear whether a patient with pancreatitis secondary to a TKI can be safely switched to a different TKI. As more molecularly targeted agents and more monoclonal antibodies targeting PD-L1 and PD-1 are under development, screening for amylase and lipase levels during phase 1/2 testing may prove helpful.
The natural history of cancer-drug–associated pancreatitis may depend on which agent is the cause. Although there are descriptions of the course of autoimmune pancreatitis, these studies have not included pancreatitis associated with anti-PD-L1 or -PD-1 therapies.65 It is possible that once an autoimmune pancreatitis has developed, simply stopping the inciting anti-PD-L1 or -PD-1 antibody may not lead to immediate resolution. Therapy with combined immune checkpoint blockade agents (eg, nivolumab and ipilimumab) may cause a higher incidence of pancreatitis than therapy with a single agent.66
In a report of 119 patients with melanoma who were treated with nivolumab and ipilimumab, there were 2 cases of acute pancreatitis, though 20% of patients had a grade 3 or higher amylase level, and just over 6% had a grade 3 or higher lipase.67 Stopping this type of immunotherapy early for grade 1,2, or 3 rises in pancreatic enzymes might prevent symptomatic pancreatitis from developing, but would stop potentially curative therapy for many patients who would have never developed clinically serious pancreatitis. Patients who suffer immune toxicities with anti-PD-1 therapies may be more apt to obtain some clinical benefit. The development of immune-related toxicities in patients treated with ipilimumab ( an anti CTLA4 antibody) seemed to correlate the tumor regression.68 This has also been suggested by the fact that the development of vitiligo correlates with clinical response in melanoma patients treated with nivolumab.69 Although clinically significant pancreatitis might be averted by stopping immune therapies earlier, stopping before it is deemed necessary might prevent cancer patients from receiving life-prolonging therapy.
Acute pancreatitis in general is severe in about 25% of cases and is associated with a significant risk of death. Scoring systems such as Ranson criteria and Apache 2 are used to assess the severity of pancreatitis although their utility is debated.70 Asparaginase is the chemotherapy agent most frequently associated with pancreatitis. It has been used to treat acute lymphoblastic leukemia for more than 30 years. This allowed for a study of 5,185 children and young adults who received asparaginase to determine what clinical factors and genomic factors were associated with the development of acute pancreatitis in 117 individuals.71 Further information gathered from programs such as the FDA and the adverse drug reaction program at Northwestern University in Chicago, coupled with the publication of other cases of pancreatitis associated with newer cancer agents may provide more insight into the mechanism causing pancreatitis due to a specific agent. With more cases being published, it may also become possible to determine if there are specific predisposing factors based on the clearance or metabolism of the offending agent or any genetic predisposition for drug-related pancreatitis.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
1. Trivedi CD, Pitchumoni CS. Drug-induced pancreatitis: an update. J Clin Gastroenterol. 2005;29:709-716.
2. Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroeneterol Hepatol. 2007;5:648-661.
3. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
4. Jones MR, Hall OM, Kaye AM, et al. Drug-induced acute pancreatitis: a review. Oschner J. 2015;15:45-51.
5. Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190-209.
6. Alabed YZ, Aghayev A, Sakellis C, et al. Pancreatitis secondary to anti-programmed death receptor 1 immunotherapy diagnosed by FDG PET/CT. Clin Nucl Med. 2015;40:e528-529.
7. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer. 2016;90:148-150.
8. Webster GJ. Autoimmune pancreatitis – a riddle wrapped in an enigma. Dig Dis. 2016;34:532-539.
9. Merchant MS, Baird K, Wexler L, et al. Ipilimumab: first results of a phase I trial in pediatric patients with advanced solid tumors. J Clin Oncol. 2012;30:abstract 9545.
10. Ibrahim RA, Berman DM, Depril V, et al. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29:abstract 8583.
11. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691-2697.
12. Mayerle J, van den Brandt C, Schwaiger T, et al. Blockage of CTLA-4 suggests that autoimmune pancreatitis is a T-cell mediated disease responsive to ciclosporin A and rapamycin . Pancreatology. 2012;12:579(abstract S8-3).
13. Tecentriq (package insert). South San Francisco, CA: Genentech Inc; 2016.
14. Champiat S, Lambotte E, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2015;27:559-574.
15. Amgen. New safety information for Blincyto (blinatumomab) Risk of pancreatitis. August 2016 and update to Micromedex 2016.
16. Sumimoto K, Uchida K, KusudaT, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high, CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis . Pancreatology. 2014;14:193-200.
17. Ghatalia P, Morgan CJ, Choueiri TK, et al. Pancreatitis with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol. 2015;94:136-145.
18. Sevin A, Chen A, Atkinson B. Tyrosine kinase inhibitor induced pancreatitis . J Oncol Pharm Pract. 2012;19:257-260.
19. Tirumani SH, Jagannathan JP, Shinagare AB, et al. Acute pancreatitis associated with molecular targeted therapies: a retrospective review of the clinico-radiological features, management and outcome. Pancreatology . 2013;13:461-467.
20. Russano M, Vincenzi B, Benditti O, et al. Pazopanib and pancreatic toxicity: a case report. BMC Res notes. 2015;8:196-198.
21. Kawakubo K, Hata H, Kawakami H, et al. Pazopanib induced severe acute pancreatitis. Case Rep Oncol. 2015;8:356-358.
22. Peron J, Khenifer S, Potier V, et al. Axitinib induced acute pancreatitis: a case report . Anticancer Drugs. 2014;25:478-479.
23. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis . Ann Pharmaco. 2013;37:33.
24. Pezzilli R, Corinaldesi R, Morselli-LabateAM. Tyrosine kinase inhibitors and acute pancreatitis. http://www.serena.unina.it/index.php/jop/article/view/3836/4278. Published May 5, 2010. Accessed May 22 , 2017.
25. Blum KA, Christian B, Flynn JM, et al. A phase I trial of the Bruton’s tyrosine kinase inhibitor, ibrutinib, in combination with rituximab and bendamustine in patients with relapsed/refractory non Hodgkin’s lymphoma. Blood. 2012;120:abstract 1643.
26. Muluneh B, Buie LW, Collichio F. Vemurafenib-associated pancreatitis: a case report. Pharmacotherapy. 2013;33:e43-e44.
27. Dabrafenib. In Life-Sciences-Europe.com from Tafinlar. EU Summary of Product Characteristics. 30 August 2013.
28. Varma MR, Mathew S, Krishnadas D, et al. Imatinib-induced pancreatitis. Indian J Pharmacol. 2010;42:50-52.
29. Palandri F, Castagnetti F, Soverinie S, et al. Pancreatic enzyme elevation in chronic myeloid leukemia patients treated with nilotinib after imatinib failure. Haematologica. 2009;94:1758-1761.
30. Engel T, Justo D, Amitai M, et al. Nilotinib-associated acute pancreatitis. Ann of Pharmacother. 2013;47:e.3
31. Cortesk JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadephia chromosome-positive leukemias. New Engl J Med. 2012;367:2075-2088.
32. Elouni B, Ben Salem C, Zamy M, et al. Bortezomib-induced acute pancreatitis [Letter]. J Pancreas. 2010;119:275-276.
33. Elouni B. Acute pancreatitis induced by Velcade ( bortezomib) with positive rechallenge. 9th Annual meeting of the International Society of Pharmacovigilance. Oct 2009 abstract 74.
34. Talamo G, Sikik J, Pandey MK, et al. Bortezomib-induced acute pancreatitis. Case report and review of the literature . J Oncol Pharm Prac. 2016;22:332-334.
35. SolakogluT, Akyol P, Guney T, et al. Acute pancreatitis caused by bortezomib. Pancreatology. 2013;13:189-190.
36. Mihaila RG. A possible rare complication of bortezomib treatment, acute pancreatitis. Acta Medica Transilvanica. 2013;2:269-171
37. Gupta H, Bansal R, Khanna S, et al. An unusual complication of bortezomib therapy: acute pancreatitis. Indian J Nephr. 2014;24:135-136.
38. Fotoh M, KitaharaT, Sakuta J, et al. Multiple lipoma with hyperlipidemia in a multiple myeloma patient treated with bortezomib/dexamethasone. Leuk Res. 2010;34:e120-121.
39. Wang HH, Tsui J, Wang XY, et al. Bortezomib induced acute pancreatitis in a patient with multiple myeloma. Leuk Lymphoma. 2014;55:1404-1405.
40. Misselwitz B, Goede JS, Pestalozzi BC, et al. Hyperlipidemic myeloma: review of 53 cases. Ann Hematol. 2010;89:569-577.
41. Gozzetti A, Fabbri A, Defina M, et al. Hyperlipidemia in a myeloma patient after bortezomib treatment. Leuk Research. 2010;34:e250.
42. Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121:893-897.
43. Runzi M, Layer P. Drug-associated pancreatitis: facts and fiction. Pancreas. 1996;13:100-109.
44. Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748-757.
45. Butt W, Saadati H, Wasif- Saif M. Oxaliplatin-induced pancreatitis: a case series. Anticancer Res. 2010;30:5113-5115.
46. Yucel H, Warmerdam LV. Capecitabine-induced pancreatitis. J Onc Pharm Pract. 2010;16:133-134.
47. Chung LW, Yeh S-P, Hsieh C-Y, et al. Life-threatening acute pancreatitis due to thalidomide therapy for chronic graft-versus-host disease. Ann Hematol. 2008;87:421-424.
48. Subramaniam S, Zell JA, Kunz PL. Everolimus causing severe hypertriglyceridemia and acute pancreatitis. J Natl Compr Canc Netw. 2013;11:5-9.
49. Wadood A, Chesner R, Mirza M, et al. Tamoxifen precipitation of familial hypertriglyceridaemia: a rare cause of acute pancreatitis. BMJ Case Rep. Published August 3, 2016. doi: 10.1136/bcr-2016-214837.
50. Sakhri J, BenSalem C, Fathallah H, et al. Severe pancreatitis due to tamoxifen induced hypertriglyceridemia with positive rechallenge. J Pancreas. 2010;11:382-384.
51. Elisaf MS, Nakou K, Liamis G, et al. Tamoxifen-induced severe hypertriglyceridemia and pancreatitis. Ann Oncol. 2000;11:1067-1069.
52. Artac M, Sari R, Altunbas J, et al. Asymptomatic acute pancreatitis due to tamoxifen-induced hypertriglyceridemia in a patient with diabetes mellitus and breast cancer. J Chemother. 2002;14:309-311.
53. [Author name not available]. Acute pancreatitis: 15 case reports. React Wkly. 2015;1546:29.
54. Ozcinar B, Guven K, Poylani A, et al. Necrotizing pancreatitis after transcatheter embolization for hepatocellular carcinoma. Diagn In
56. She WH, Chan ACY, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int. 2016;15:93-98.
57. Bae SI, Yeon JE, Lee JM, et al. A case of necrotizing pancreatitis subsequent to transcatheter arterial chemoembolization in a patient with hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:321-325.
58. Peterson JL, Vallow LA, Johnson DW, et al. Complications after 90Y microsphere radioembolization for unresectable hepatic tumors: an evaluation of 112 patients. Brachytherapy. 2013;12:573-579.
59. Piso P, Glockzin G, Schlitt HJ. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal carcinomatosis arising from gastric cancer. J Clin Oncol. 2011;29(suppl 4):abstract 132.
60. Sammartino P, Sibio S, Biacchi D, et al. Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3356888/?report=reader. Published 2012. Accessed May 23, 2017.
61. Muzaffar M, Jia J, Liles D, et al. Acute pancreatitis associated with ado-trastuzumab emtansine. Am J Ther. 2016;23:e572-574.
62. Urru SA, Mariotti E, Carta P, et al. Acute pancreatitis following brentuzimab vedotin therapy for refractory Hodgkin lymphoma: a case report. Drugs R D. 2014;14:9-11.
63. Gandhi MD, Evens AM, Fenske TS, et al. Pancreatitis in patients treated with brentuximab vedotin: a previously unrecognized serious adverse event. Blood. 2014;123:2895-2897.
64. Brentuximab vedotin in Micromedex solutions, Truven Health Analytics. 2016.
65. Okazaki K, Uchida K. Autoimmune pancreatitis: the past, present and future. Pancreas. 2015;44:1006-1016.
66. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab and ipilimumab in advanced melanoma. New Engl J Med. 2013;369:122-133.
67. Friedman CF, Clark V, Raikhel AV, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab and ipilimumab. J Natl Cancer Inst. 2017;109:[page numbers not available].
68. Day D, Hansen AR. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs. 2016;30:571-584.
69. Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multi-institutional retrospective study. J Dermatol. 2017;44:117-122.
70 . Di MY, Liu H, Zu-Yao Y, et al. Prediction models of mortality in acute pancreatitis in adults. A systematic review. Ann Int Med. 2016;165:482-490.
71. Liu C, Yang W, Devidas M, et al. Clinical and genetic risk factors for acute pancreatitis in patients with acute lymphoblastic leukemia. J Clin Oncol. 2016;18:2133-2140.
Targeted Therapy and Immunotherapy in the Treatment of Metastatic Cutaneous Melanoma
INTRODUCTION
The incidence of cutaneous melanoma has increased over the past 2 decades, with SEER estimates indicating that the number of new cases of melanoma diagnosed annually rose from 38,300 in 1996 to 76,000 in 2016.1 Among persons younger than 50 years, the incidence is higher in females, and younger women (aged 15–39 years) are especially vulnerable.2 Among persons older than 50, melanoma incidence in men is nearly twice that of women, in whom melanomas are often thicker and often associated with worse outcomes.1,2 Approximately 85% of melanomas are diagnosed at early stages when surgery is curative, but the lifetime probability of developing invasive disease is 3% in men and 2% in women.
Prior to the advent of effective immunotherapies and targeted therapies, melanoma was often managed with chemotherapy, which had dismal response rates and commensurately poor outcomes. Advances in the understanding of the molecular etiopathogenesis and immune escape responses of cutaneous metastatic melanoma have transformed therapeutic approaches. Specifically, improved understanding of the genetic mutations driving melanoma tumorigenesis coupled with insights into mechanisms of tumor-mediated immune evasion resulted in development of inhibitors of mitogen-activated protein kinases (MAPK; BRAF and MEK) along with inhibitors of negative regulatory immune checkpoints (cytotoxic T lymphocyte–associated antigen 4 [CTLA-4] and programmed cell death-1 [PD-1]). In this review, we discuss the role of immune therapy, targeted therapy, and combinations of these in the treatment of metastatic cutaneous melanoma. We limit the immuno-therapy discussion to approved CTLA-4/PD-1 inhibitors and the targeted therapy discussion to approved BRAF/NRAS/MEK inhibitors and do not discuss non-checkpoint immunotherapies including cytokines (HD IL-2), vaccines, or adoptive T-cell approaches. Interested readers are directed to other excellent works covering these important topics.26–29
DEVELOPMENT OF TARGETED AND NOVEL IMMUNE THERAPIES
For many years the degree of ultraviolet (UV) light exposure was considered the sole major risk factor for melanoma oncogenesis, even though its mechanism was largely unknown.3 However, clinical observations regarding the occurrence of melanoma on less exposed areas (trunk and limbs) in individuals with intermittent sun exposure led to the proposition that melanomas that arose in younger patients with intermittent sun exposure were distinct from melanomas that arose in older patients in association with markers of chronic sun exposure—the “divergent pathway” hypothesis.3 Critical to this understanding were whole-exome sequencing data from multiple groups, including The Cancer Genome Atlas, that identified patterns of mutations in oncogenic drivers that were distinct in patients with and without chronically sun-damaged (CSD) skin.4–7 It is now clear that based on its association with CSD skin, melanoma can be subclassified into CSD or non-CSD melanoma. CSD and non-CSD melanoma have distinct clinico-pathological characteristics and are associated with different driver mutations. CSD melanomas typically arise in older patients on sun-exposed areas (head/neck, dorsal surfaces of distal extremities) and are associated with particular driver mutations (BRAF non-V600E, NRAS, NF1, or KIT) and genetic signatures of UV-induced DNA damage (G > T [UVA] or C > T [UVB]) transitions. Conversely, non-CSD melanomas typically arise in younger (< 55 years) patients on intermittently sun-exposed areas (trunk, proximal extremities) and are associated with BRAF V600E/K driver mutations and often lack genetic signatures of UV mutagenesis.
Identification of driver mutations in components of the MAPK pathway, including BRAF and NRAS, facilitated the development of targeted inhibitors. The BRAF inhibitors vemurafenib and dabrafenib have been shown in pivotal phase 3 studies to significantly improve overall and progression-free survival in patients with metastatic melanoma compared with chemotherapy and garnered regulatory approval (vemurafenib, BRIM-3;8,9 dabrafenib, BREAK-310). Concomitant MEK and BRAF inhibition extends the duration of benefit by preventing downstream kinase activation in the MAPK pathway. Notably, concomitant MEK inhibition alters the side-effect profile of BRAF inhibitors, with reduced incidence of keratoacanthomas and cutaneous squamous cell carcinomas that are attributable to on-target, off-tumor effects of BRAF inhibitors. Combined BRAF and MEK inhibition (vemurafenib/cobimetinib and dabrafenib/trametinib) further improved overall and progression-free survival compared to single-agent BRAF inhibition in phase 3 studies (COMBI-d,11 COMBI-v,12 and coBRIM13). Although often deep, the responses seen with the use of targeted kinase inhibitors are not often durable, with the vast majority of patients progressing after 12 to 15 months of therapy.In parallel, work primarily done in murine models of chronic viral infection uncovered the role played by co-inhibitory or co-excitatory immune checkpoints in mediating T-cell immune responses. These efforts clarified that tumor-mediated immune suppression primarily occurs through enhancement of inhibitory signals via the negative T-cell immune checkpoints CTLA-4 or PD-1.14,15 Blockade of negative T-cell immune checkpoints resulted in activation of the adaptive immune system, resulting in durable anti-tumor responses as demonstrated in studies of the CTLA-4 inhibitor ipilimumab (CA184-02016 and CA184-02417) and the PD-1 inhibitors nivolumab (CA209-003,18 CheckMate 037,19 and CheckMate 06620) and pembrolizumab (KEYNOTE-00121 and KEYNOTE-00622). Compared to the deep but short-lived responses seen with targeted kinase inhibitors, patients treated with CTLA-4 or PD-1 immune checkpoint blockade often developed durable responses that persisted even after completion of therapy. Combined CTLA-4 and PD-1 blockade results in greater magnitude of response with proportionately increased toxicity.23–25
IMMUNOTHERAPY
CTLA-4 AND PD-1 IMMUNE CHECKPOINT INHIBITORS
The novel success of immunotherapy in recent decades is largely attributable to improved understanding of adaptive immune physiology, specifically T-cell activation and regulation. T-cell activation requires 2 independent signaling events: it is initiated upon recognition of the antigen-MHC class II-receptor complex on antigen-presenting cells (APC), and requires a secondary co-stimulatory interaction of CD80/CD86 (B7.1/B7.2) on APCs and CD28 molecule on T-cells; without this second event, T-cells enter an anergic state.30–32 Upon successful signaling and co-stimulation, newly activated T-cells upregulate CTLA-4, which can bind to B7 molecules with a nearly 100-fold greater affinity than CD28.33,34 Unlike CD28, CTLA-4 engagement negatively regulates T-cell activation. The opposing signals produced by CD28 and CTLA-4 are integrated by the T-cell to determine eventual response to activation, and provide a means by which T-cell activation is homeostatically regulated to prevent exaggerated physiologic immune responses.35 It was hypothesized that CTLA-4 blockade would permit T-cell activation, which is thwarted in the tumor microenvironment by tumor-mediated CTLA-4 engagement, thereby unleashing an anti-tumor immune response.36
PD-1 is a member of the CD28 and CTLA-4 immunoglobulin super family and, similar to CTLA-4, binds activated T-cells. PD-1 has 2 ligands on activated T-cells: PD-L1 and PD-L2.37 PD-L1 is constitutively expressed by a variety of immune and non-immune cells, particularly in inflammatory environments including tumor microenvironments, in response to the release of inflammatory cytokines such as interferon (IFN)-γ.37,38 Conversely, PD-L2 is only minimally expressed constitutively, although its expression on immune and non-immune cells can be induced by similar cues from inflammatory microenvironments. PD-L1 and PD-L2 cross-compete for binding to PD-1, with PD-L2 exhibiting 2- to 6-fold greater relative affinity than PD-L1.39 PD-L1/PD-1 binding results in phosphorylation of 2 tyrosinases in the intracellular portion of PD-1, which contains immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM). PD-1 ITSM subsequently recruits either of 2 SH2-domain–containing protein tyrosine phosphatases: SHP-1 and SHP-2. SHP-2 signaling suppresses PI3K/Akt activation, down-regulates Bcl-xL, and suppresses expression of multiple transcription factors that mediate T-cell effector function including GATA-3, Eomes, and T-bet.40–42 The net effect of PD-L1/PD-1 engagement is to suppress T-cell proliferation, cytokine production, cytolytic function, and survival. Unlike CTLA-4, which primarily affects the priming phase of naive T-cell activation, PD-1 chiefly regulates the effector phase of T-cell function. Furthermore, because PD-L1/PD-L2 expression is limited to inflammatory microenvironments, the effects of PD-1 are less generalized than those of CTLA-4.
SINGLE AGENT ACTIVITY OF CTLA-4 AND PD-1 INHIBITORS
Ipilimumab (MDX-010) is a human IgG1 monoclonal antibody shown to inhibit CTLA-4.43 Early studies tested different formulations (transfectoma-derived and hybridoma-derived), doses, and schedules of ipilimumab primarily in patients with advanced refractory melanoma.44–46 Although responses were infrequent, responding patients experienced durable remissions at 1- and 2-year time points. Notably, in a foreshadowing of changes to response criteria used to evaluate these agents, several treated patients who initially had radiographically stable disease upon completion of therapy subsequently experienced a gradual decline in tumor burden.
Ipilimumab was subsequently evaluated in 2 phase 3 trials. The first study (MDX010-020/CA184-020), which involved 676 HLA-A*0201–positive patients with advanced melanoma, compared ipilimumab 3 mg/kg every 3 weeks for 4 doses either singly or in combination with gp100 vaccine with a gp100-only control arm.16 Ipilimumab administration resulted in objective responses in 11% of patients and improved progression-free and overall survival compared to gp100 alone. Of note, ipilimumab monotherapy was superior to ipilimumab/gp100 combination, possibly related to timing of vaccine in relation to ipilimumab. A confirmatory study (CA184-024) compared a higher dose of ipilimumab (10 mg/kg) in combination with dacarbazine to dacarbazine monotherapy in previously untreated melanoma and was positive.17 Given the lack of augmented efficacy with the higher (10 mg/kg) dose, ipilimumab received regulatory approval in 2011 for the treatment of melanoma at the lower dose: 3 mg/kg administered every 3 weeks for 4 doses (Table 1). Survival data was strikingly similar to patterns observed in prior phase 2 studies, with survival curves plateauing after 2 years at 23.5% to 28.5% of treated patients. Pooled survival data from prospective and retrospective studies of ipilimumab corroborate the plateau of 22% (26% treated; 20% untreated) reached at year 3 regardless of prior therapy or ipilimumab dose, underscoring the durability of long-term survival in ipilimumab-treated patients.47 Ipilimumab administration resulted in an unusual spectrum of toxicities including diarrhea, rash, hepatitis, and hypophysitis (termed immune-related adverse events, or irAEs) in up to a third of patients.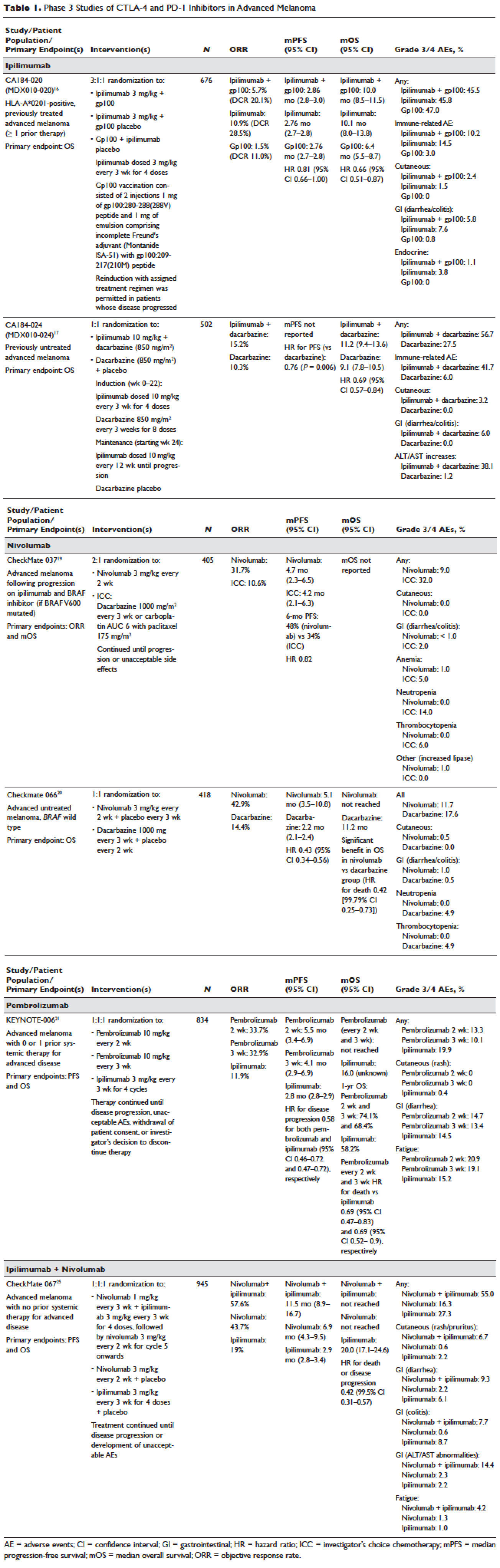
Pembrolizumab and nivolumab are humanized IgG4 monoclonal antibodies that target the PD-1 receptor found on activated T cells, B cells, and myeloid cells. Pembrolizumab and nivolumab are engineered similarly: by immunizing transgenic mice with recombinant human PD-1-Fc protein and subsequently screening murine splenic cells fused with myeloma cells for hybridomas producing antibodies reactive to PD-1-Fc.48,49 Unlike IgG1, the IgG4 moiety neither engages Fc receptors nor activates complement, avoiding cytotoxic effects of the antibody upon binding to the T cells that it is intended to activate. Both pembrolizumab and nivolumab bind PD-1 with high affinity and specificity, effectively inhibiting the interaction between PD-1 and ligands PD-L1 and PD-L2.
Nivolumab was first studied in a phase 1 study (CA209-003) of 296 patients with advanced cancers who received 1, 3, or 10 mg/kg administered every 2 weeks.18 Histologies tested included melanoma, non–small-cell lung cancer (NSCLC), renal-cell cancer (RCC), castration-resistant prostate cancer (CRPC), and colorectal cancer (CRC). Responses were seen in melanoma and RCC and unusually in NSCLC, including in both squamous and non-squamous tumors. Objective responses were noted in 41% of the 107 melanoma patients treated at 3 mg/kg. Survival was improved, with 1- and 2-year survival rates of 62% and 43% at extended follow up.50
Subsequently, nivolumab was compared to chemotherapy in a pair of phase 3 studies involving both previously untreated (Checkmate 066) and ipilimumab/BRAF inhibitor–refractory (CheckMate 037) patients.19,20 In both studies, nivolumab produced durable responses in 32% to 34% of patients and improved survival over chemotherapy. Compared to ipilimumab, the incidence of irAEs was much lower with nivolumab. The depth and magnitude of responses observed led to regulatory approval for nivolumab in both indications (untreated and ipilimumab/BRAF inhibitor–treated melanoma) in 2014. Data from both studies are summarized in Table 1.
Pembrolizumab was first evaluated in a phase 1 study of 30 patients with a variety of solid organ malignancies in which no dose-limiting toxicities were observed and no defined maximal tolerated dose was reached.51 Per protocol, maximal administered dose was 10 mg/kg every 2 weeks. Following startling responses including 2 complete responses of long duration, pembrolizumab was evaluated in a large phase 1 study (KEYNOTE-001) of 1260 patients that evaluated 3 doses (10 mg/kg every 2 weeks, 10 mg/kg every 3 weeks, and 2 mg/kg every 3 weeks) in separate melanoma and NSCLC substudies.21 Both ipilimumab-naïve and ipilimumab-treated patients were enrolled in the melanoma substudy. Objective responses were seen in 38% ofpatients across all 3 dosing schedules and were similar in both ipilimumab-naïve and ipilimumab-treated patients. Similar to nivolumab, most responders experienced durable remissions.
Pembrolizumab was subsequently compared to ipilimumab in untreated patients (KEYNOTE-006) in which patients were randomly assigned to receive either ipilimumab or pembrolizumab at 1 of 2 doses: 10 mg/kg every 2 weeks and pembrolizumab 10 mg/kg every 3 weeks.22 Response rates were greater with pembrolizumab than ipilimumab, with commensurately greater 1-year survival rates. Rates of treatment-related adverse events requiring discontinuation of study drug were much lower with pembrolizumab than ipilimumab. This trial was instrumental in proving the superior profile of pembrolizumab over ipilimumab. The US Food and Drug Administration (FDA) granted pembrolizumab accelerated approval for second-line treatment of melanoma in 2014, and updated this to include a first-line indication in 2015 (Table 1).
EFFICACY OF COMBINED CTLA-4 AND PD-1 INHIBITION
Preclinical studies demonstrated that PD-1 blockade was more effective than CTLA-4 blockade and combination PD-1/CTLA-4 blockade was synergistic, with complete rejection of tumors in approximately half of the treated animals.14 This hypothesis was evaluated in a phase 1 study that explored both concurrent and sequential combinations of ipilimumab and nivolumab along with increasing doses of both agents in PD-1/CTLA-4–naïve advanced melanoma.23 Responses were greater in the concurrent arm (40%) than in the sequential arm (20%) across dose-levels with a small fraction of patients treated in the concurrent arm experiencing a profound reduction (80%) in tumor burden.
The superiority of ipilimumab/nivolumab combination to ipilimumab monotherapy was demonstrated in a randomized blinded phase 2 study (CheckMate 069).24 Of the 4 different ipilimumab/nivolumab doses explored in the phase 1 study (3 mg/kg and 0.3 mg/kg, 3 mg/kg and 1 mg/kg, 1 mg/kg and 3 mg/kg, 3 mg/kg and 3 mg/kg), ipilimumab 3 mg/kg and nivolumab 1 mg/kg (followed by nivolumab 3 mg/kg) was compared to ipilimumab and nivolumab-matched placebo. Responses were significantly greater with dual PD-1/CTLA-4 blockade compared to CTLA-4 blockade alone (59% versus 11%). Concurrently, a 3-arm randomized phase 3 study compared the same dose of ipilimumab/nivolumab to ipilimumab and nivolumab in previously untreated advanced melanoma (CheckMate 067).25 Similar to CheckMate 069, CheckMate 067 demonstrated that ipilimumab/nivolumab combination resulted in more profound responses (58%) than either ipilimumab (19%) or nivolumab (44%) alone. Toxicity, primarily diarrhea, fatigue, pruritus, and rash, was considerable in the combination arm (55% grade 3/4 adverse events) and resulted in treatment discontinuation in 30% of patients. The profound and durable responses observed led to accelerated approval of ipilimumab/nivolumab combination in 2015 (Table 1).
Efforts to improve the toxicity/benefit ratio of ipilimumab/nivolumab combination have centered around studying lower doses and/or extended dosing schedules of ipilimumab, including ipilimumab 1 mg/kg every 6 or 12 weeks with nivolumab dosed at 3 mg/kg every 2 weeks or 480 mg every 4 weeks. Promising data from a first-line study in NSCLC (CheckMate 012) support the evaluation of nivolumab in combination with lower-dosed ipilimumab (1 mg/kg every 6 or 12 weeks).52 This approach is being tested against platinum doublet chemotherapy in a confirmatory phase 3 study in NSCLC (CheckMate 227).
TARGETED THERAPY
MAPK KINASE PATHWAY IN MELANOMA TUMORIGENESIS
The MAPK pathway mediates cellular responses to growth signals. RAF kinases are central mediators in the MAPK pathway and exert their effect primarily through MEK phosphorylation and activation following dimerization (hetero- or homo-) of RAF molecules. As a result, RAF is integral to multiple cellular processes, including transcriptional regulation, cellular differentiation, and cell proliferation. MAPK pathway activation is a common event in many cancers, primarily due to activating mutations in BRAF or RAS. Alternatively, MAPK pathway activation can occur in the absence of activating mutations in BRAF or NRAS through down-regulation of MAPK pathway inhibitory proteins (RAF-1 inhibitory protein or SPRY-2), C-MET overexpression, or activating mutations in non-BRAF/NRAS kinases including CRAF, HRAS, and NRAS.53,54
Somatic point mutations in BRAF are frequently observed (37%–50%) in malignant melanomas and at lower frequency in a range of human cancers including NSCLC, colorectal cancer, papillary thyroid cancer, ovarian cancer, glioma, and gastrointestinal stromal tumor.6,55,56 BRAF mutations in melanoma typically occur within the activation segment of the kinase domain (exon 15). Between 80% and 90% of activating mutations result in an amino acid substitution of glutamate (E) for valine (V) at position 600: V600E.57,58 V600E mutations are true oncogenic drivers, resulting in increased kinase activity with demonstrable transformational capacity in vitro. BRAF mutations are usually mutually exclusive, with tumors typically containing no other driver mutations in NRAS, KIT, NF1, or other genes.
NRAS mutations are less common than BRAF mutations, having a reported frequency of 13% to 25% in melanoma.4 NRAS mutations generally occur within the P-loop region of the G domain (exon 2), or less commonly in the switch II region of the G domain (exon 3). Most NRAS exon 2 mutations comprise amino acid substitutions at position 61 from glutamine (Q) to arginine (R; 35%), lysine (K; 34%) and less often to glutamate (E), leucine (L), or proline (P). Preclinical data suggest that NRAS mutations paradoxically stimulate the MAPK pathway and thus enhance tumor growth in vitro.59,60 Several important phenotypic differences distinguish NRAS- from BRAF-mutated melanoma. NRAS-mutated tumors are typically associated with increasing age and CSD skin, while BRAF-mutated tumors arise in younger patients in non-CSD skin. A large population-based study suggested that NRAS-mutated melanomas were associated with mitoses and lower tumor infiltrating lymphocytes (TIL) grade, and arose in anatomic sites other than the head/neck, while BRAF-mutated tumors were associated with mitoses and superficial spreading histology.61 Although the lower TIL grade seen with NRAS-mutated melanomas suggests a more immunosuppressed microenvironment and argues for poorer responses to immune therapies, clinical studies comparing responses to immunotherapies in various categories of driver mutations provide conflicting results for the prognostic role of NRAS mutations in relation to immune checkpoint blockade and other immune therapies.62–64
NF1 represents the third known driver in cutaneous melanoma, with mutations reported in 12% of cases.6,7 NF1 encodes neurofibromin, which has GTPase activity and regulates RAS proteins; NF1 loss results in increased RAS.65 Unlike BRAF or NRAS, which are usually mutually exclusive, NF1 mutations in melanoma can occur singly or in combination with either BRAF or NRAS mutations. In these settings, NF1 mutations are associated with RAS activation, MEK-dependence, and resistance to RAF inhibition.66
MAPK PATHWAY INHIBITION SINGLY AND IN COMBINATION
Although multiple MEK 1/2 inhibitors (AS703026, AZD8330/ARRY-704, AZD6244, CH5126766, CI-1040, GSK1120212, PD0325901, RDEA119, and XL518) and RAF inhibitors (ARQ 680, GDC-0879, GSK2118436, PLX4032, RAF265, sorafenib, XL281/BMS-908662) were developed, the initial evaluation of MAPK pathway inhibitors in advanced human cancers began with CI-1040. Preclinical data suggested that CI-1040 potently and selectively inhibited both MEK1 and MEK2, but phase 1 and 2 human trial results were disappointing, likely because these trials were not selectively enriched for NRAS/BRAF–mutated tumors or cancers in which these oncogenic mutations were most commonly detected, such as melanoma.67,68 The subsequent evaluation of selumetinib (AZD6244/ARRY-142886) in a phase 2 study was also negative. Although investigators enrolled a presumably enriched population (cutaneous melanoma), the incidence of NRAS/BRAF–mutated tumors was not ascertained to determine this, but rather assumed, which led to a discrepancy between the assumed (prestudy) and observed (on-study) proportions of BRAF/NRAS mutations that was not accounted for in power calculations.69,70 Lessons learned from these earlier misadventures informed the current paradigm of targeted therapy development: (1) identification of a highly specific and potent inhibitor through high-throughput screening; (2) establishment of maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) in unselected patients; (3) confirmation of RP2D in selected tumor types enriched for target of interest; and (4) confirmatory study against standard comparator to seek regulatory approval.
Vemurafenib and dabrafenib were evaluated in this tiered fashion in phase 1 dose-finding studies comprising unselected patients, followed by phase 2 studies in advanced BRAF V600E–mutated melanoma. Both were subsequently evaluated in randomized phase 3 trials (vemurafenib, BRIM-38; dabrafenib, BREAK-310) that compared them with dacarbazine (1000 mg/m2 intravenously every 3 weeks) in the treatment of advanced BRAF V600E–mutated melanoma. Response kinetics for both agents were remarkably similar: single-agent BRAF inhibitors resulted in rapid (time to response 2–3 months), profound (approximately 50% objective responses) reductions in tumor burden that lasted 6 to 7 months. Adverse events common to both agents included rash, fatigue, and arthralgia, although clinically significant photosensitivity was more common with vemurafenib and clinically significant pyrexia was more common with dabrafenib. Class-specific adverse events included the development of cutaneous squamous-cell carcinomas and keratoacanthomas secondary to paradoxical activation of MAPK pathway signaling either through activating mutations in HRAS or mutations or amplifications in receptor tyrosine kinases upstream of BRAF, resulting in elevated levels of RAS–guanosine triphosphate complexes.71 Results of these studies resulted in regulatory approval of single-agent BRAF inhibitors for the treatment of BRAF V600E (and later V600K)–mutated melanoma (vemurafenib in 2011; dabrafenib in 2013). Details regarding trial populations, study interventions, efficacy, and adverse events are summarized in Table 2.
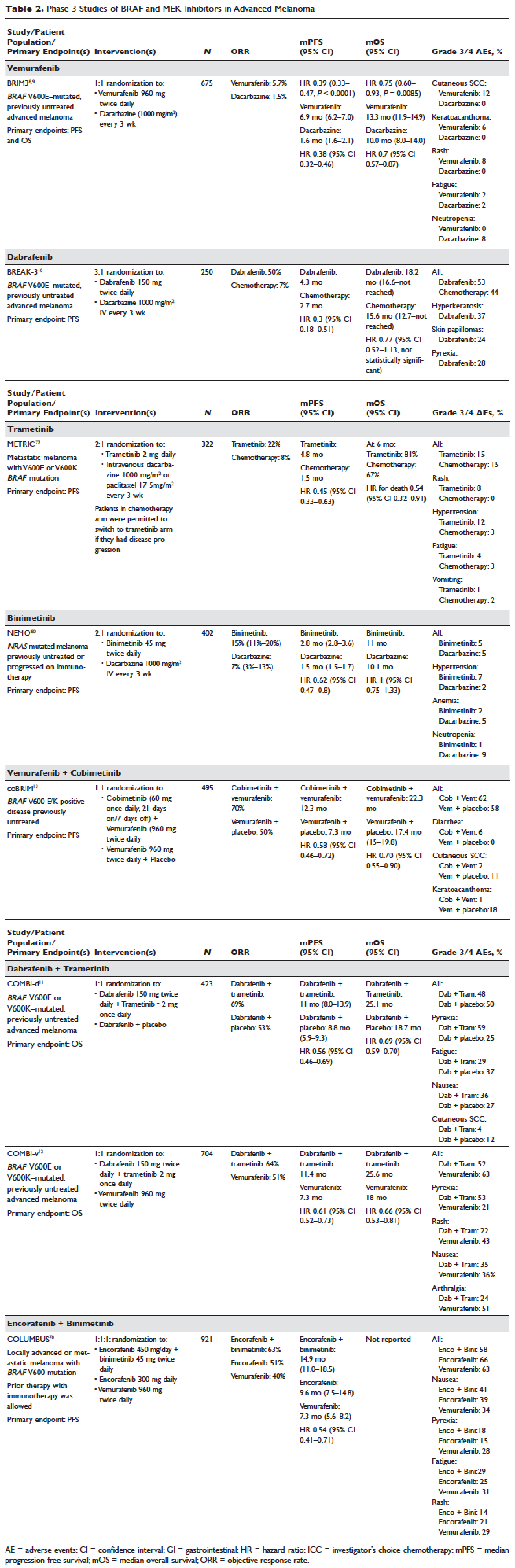
Responses to BRAF inhibitors are typically profound but temporary. Mechanisms of acquired resistance are diverse and include reactivation of MAPK pathway–dependent signaling (RAS activation or increased RAF expression), and development of MAPK pathway–independent signaling (COT overexpression; increased PI3K or AKT signaling) that permits bypass of inhibited BRAF signaling within the MAPK pathway.72–76 These findings suggested that upfront inhibition of both MEK and mutant BRAF may produce more durable responses than BRAF inhibition alone. Three pivotal phase 3 studies established the superiority of combination BRAF and MEK inhibition over BRAF inhibition alone: COMBI-d11 (dabrafenib/trametinib versus dabrafenib/placebo), COMBI-v12 (dabrafenib/trametinib versus vemurafenib), and coBRIM13 (vemurafenib/cobimetinib versus vemurafenib/placebo). As expected, compared to BRAF inhibitor monotherapy, combination BRAF and MEK inhibition produced greater responses and improved progression-free and overall survival (Table 2). Interestingly, the rate of cutaneous squamous-cell carcinomas was much lower with combination therapy, reflecting the more profound degree of MAPK pathway inhibition achieved with combination BRAF and MEK inhibition. Based on these results, FDA approval was granted for both dabrafenib/trametinib and vemurafenib/cobimetinib combinations in 2015. Although the dabrafenib/trametinib combination was only approved in 2015, trametinib had independently gained FDA approval in 2013 for the treatment of BRAF V600E/K–mutated melanoma on the basis of the phase 3 METRIC study.77
Encorafenib (LGX818) and binimetinib (MEK162, ARRY-162, ARRY-438162) are new BRAF and MEK inhibitors currently being evaluated in clinical trials. Encorafenib/binimetinib combination was first evaluated in a phase 3 study (COLUMBUS) that compared it with vemurafenib monotherapy in BRAF-mutant melanoma.78 Unsurprisingly, encorafenib/binimetinib combination produced greater and more durable responses compared to vemurafenib monotherapy. The median progression-free survival of the encorafenib/binimetinib combination (14.9 months) was greater than vemurafenib monotherapy (7.3 months) in this study, and intriguingly greater than that seen with vemurafenib/cobimetinib (coBRIM 9.9 months) and dabrafenib/trametinib (COMBI-d 9.3 months; COMBI-v 11.4 months). Of note, although encorafenib has an IC50 midway between dabrafenib and vemurafenib in cell-free assays (0.8 nM dabrafenib, 4 nM encorafenib, and 31 nM vemurafenib), it has an extremely slower off-rate from BRAF V600E, which results in significantly greater target inhibition in cells following drug wash-out.79 This may account for the significantly greater clinical benefit seen with encorafenib/binimetinib in clinical trials. Final study data are eagerly awaited. Regulatory approval has been sought, and is pending at this time.
Binimetinib has been compared to dacarbazine in a phase 3 study (NEMO) of patients with NRAS-mutant melanoma, most of whom had been previously treated with immunotherapy.80 Response rates were low in both arms, although slightly greater with binimetinib than dacarbazine (15% versus 9%), commensurate with a modest improvement in progression-free survival. FDA approval has been sought and remains pending at this time.
KIT INHIBITION SINGLY AND IN COMBINATION
The KIT receptor protein tyrosine kinase is a transmembrane protein consisting of extracellular and intracellular domains. Activating KIT mutations occur in 2% to 8% of all melanoma patients and may be found in all melanoma subtypes but are commonest in acral melanomas (10%–20%) and mucosal melanomas (15%–20%). Activating KIT mutations primarily occur in exons 11 and 13, which code for the juxtamembrane and kinase domains, respectively.5,81–83
Imatinib mesylate is a tyrosine kinase inhibitor of the 2-phenyl amino pyrimidine class that occupies the tyrosine kinase active site with resultant blocking of tyrosine kinase activity. Imatinib mesylate is known to block KIT and has been extensively studied in patients with gastrointestinal stromal tumors (GIST), 80% of whom harbor KIT mutations, in both the adjuvant and the metastatic settings. In melanoma, imatinib mesylate was studied in a Chinese open-label, phase 2 study of imatinib mesylate monotherapy in metastatic melanoma harboring KIT mutation or amplification; 25% of the study patients had mucosal disease and the rest had cutaneous disease, with acral involvement in 50% of all patients.84 Overall response rate was 23%, while 51% of patients remained alive at 1 year with no differences in response rate and/or survival being noted between patients with either KIT mutations or amplifications. In a separate study of imatinib mesylate at 400 mg daily or 400 mg twice daily in Caucasian patients with KIT-mutated/amplified melanoma, similar response and survival rates were reported, although patients with KIT mutations did nonsignificantly better than those with KIT amplifications.85
Other novel studies evaluating KIT inhibitors include KIT inhibition in combination with the VEGF inhibitor bevacizumab and a study of selective BCR-ABL kinase inhibitor nilotinib in imatinib-resistant melanoma. In the former phase 1/2 study, Flaherty and colleagues studied imatinib 800 mg daily and bevacizumab at 10 mg/kg every 2 weeks in 63 patients with advanced tumors, including 23 with metastatic melanoma. Although the combination was relatively nontoxic, no significant efficacy signal was seen and further accrual to the phase 2 portion was halted after the first stage was completed.86 Nilotinib is a BCR-ABL1 tyrosine kinase inhibitor intelligently designed based on the structure of the ABL-imatinib complex that is 10 to 30 times more potent than imatinib in inhibiting BCR-ABL1 tyrosine kinase activity. Nilotinib is approved for the treatment of imatinib-resistant chronic myelogenous leukemia (CML), with reported efficacy in patients with central nervous system (CNS) involvement.87,88 Nilotinib has been studied in a single study of KIT-mutated/amplified melanoma that included patients with imatinib-resistance and those with treated CNS disease. Nilotinib appeared to be active in imatinib-resistant melanoma, although no responses were seen in the CNS disease cohort.89 Overall, the response rates observed with KIT inhibition in melanoma are much lower than those observed in CML and GIST.
CONCLUSION AND FUTURE DIRECTIONS
Prior to 2011, the only approved agents for the treatment of advanced melanoma were dacarbazine and high-dose interleukin-2. Since 2011, drug approvals in melanoma have proceeded at a frenetic pace unmatched in any other disease. The primary events underlying this are advances in our understanding of the gene mutation landscape driving melanoma tumorigenesis, accompanied by insights into the means by which tumors circumvent the induction of effective anti-tumor T-cell responses. These insights have resulted in the development of inhibitors targeting MAPK pathway kinases BRAF, MEK, and NRAS), KIT, and regulatory immune checkpoints (CTLA-4 and PD-1). Although BRAF/MEK inhibition results in profound reductions and even occasional complete responses in patients, these responses are typically short lived, rarely lasting more than 9 to 11 months; the encorafenib/binimetinib combination may improve that duration marginally. However, the signature therapeutic advance in melanoma of the past decade is immunotherapy, particularly the development of inhibitors of CTLA-4 and PD-1 immune checkpoints. With these agents, significant proportions of treated patients remain free of progression off-therapy (ipilimumab 23%; nivolumab 34%; pembrolizumab 35%; ipilimumab/nivolumab 64%), and some patients can be successfully re-induced after delayed progression. Separately, the high response rates observed with the use of KIT inhibitors in CML and GIST have not been observed in KIT mutated/amplified melanoma and development of agents in this space has been limited. The challenges ahead center around identifying predictive biomarkers and circumventing primary or acquired resistance, with the eventual goal of producing durable remissions in the majority of treated patients.
Our improved understanding of the mechanisms of acquired resistance to BRAF/MEK inhibitors suggests that anti-tumor activity may be achieved by targeting multiple pathways, possibly with combination regimens comprising other inhibitors and/or immunotherapy. Preclinical data supports the use of combination strategies targeting both ERK and PI3K/mTOR to circumvent acquired resistance.90 Ongoing studies are evaluating combinations with biguanides (metformin: NCT02143050 and NCT01638676; phenformin: NCT03026517), HSP90 inhibitors (XL888: NCT02721459; AT13387: NCT02097225), and decitabine (NCT01876641).
One complexity affecting management of resistance in the targeted therapy landscape remains tumor heterogeneity, particularly intra- and intertumoral heterogeneity, which may explain the apparent contradiction between continued efficacy of BRAF inhibitors in BRAF-resistant tumors and preclinical data predicting slower progression of resistant tumors on cessation of BRAF inhibitors.91–94 These data provide a rationale to investigate intermittent dosing regimens with BRAF/MEK inhibitors; several studies exploring this approach are ongoing (NCT01894672 and NCT02583516).
Given the specificity, adaptability, and memory response associated with immunotherapy, it is likely that these agents will be used to treat the majority of patients regardless of mutational status. Hence, identifying predictive biomarkers of response to immune checkpoint inhibitors is vital. The presence of CD8+ T-cell infiltrate and IFN-γ gene signature, which indicate an “inflamed” tumor microenvironment, are highly predictive of clinical benefit from PD-1 inhibitors.95,96 However, not all PD-1 responders have “inflamed” tumor microenvironments, and not all patients with an “inflamed” tumor microenvironment respond to immune checkpoint inhibitors. The complexity of the immune system is reflected in the multiple non-redundant immunologic pathways, both positive and negative, with checkpoints and ligands that emerge dynamically in response to treatment. Given the dynamic nature of the immune response, it is unlikely that any single immunologic biomarker identified pre-treatment will be completely predictive. Rather, the complexity of the biomarker approach must match the complexity of the immune response elicited, and will likely incorporate multifarious elements including CD8+ T-cell infiltrate, IFN-γ gene signature, and additional elements including microbiome, genetic polymorphisms, and tumor mutation load. The goal is to use multiple markers to guide development of combinations and then, depending on initial response, to examine tumors for alterations to guide decisions about additional treatment(s) to improve responses, with the eventual goal being durable clinical responses for all patients.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30.
- Guy GP, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982-2030. MMWR Morb Mortal Wkly Rep 2015;64:591–6.
- Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer 2009;115:4176–85.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–47.
- Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006;24:4340–6.
- Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251–63.
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681–96.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16.
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323–32.
- Hauschild A, Grob J-J, Demidov LV, et al. DaBRAFenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358–65.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–88.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined daBRAFenib and trametinib. N Engl J Med 2015;372:30–9.
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867–76.
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275–80.
- Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014;515:577–81.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–26.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54.
- Weber JS, Angelo SP D’, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–44.
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34.
- Eggermont AMM. Therapeutic vaccines in solid tumours: can they be harmful? Eur J Cancer 2009;45:2087–90.
- Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med 2012;4:127ps8.
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451–8.
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–8.
- Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 1992;356:607–9.
- Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol 1998;18:389–418.
- Sharpe AH, Abbas AK. T-cell costimulation--biology, therapeutic potential, and challenges. N Engl J Med 2006;355:973–5.
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001;19:565–94.
- Collins AV, Brodie DW, Gilbert RJC, et al. The interaction properties of costimulatory molecules revisited. Immunity 2002;17:201–10.
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459–65.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–6.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002;169:5538–45.
- Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun 2003;307:672–7.
- Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004;173:945–54.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543–53.
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009;229:114–25.
- Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 2013;1291:1–13.
- Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008;26:5950–6.
- Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008;105:3005–10.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155–64.
- Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94.
- Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014;2:846–56.
- Poole RM. Pembrolizumab: first global approval. Drugs 2014;74:1973–81.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–30.
- Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015;21:4286–93.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41.
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am 2009;23:529–45, ix.
- Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 2003;63:756–9.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54.
- Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012;44:1006–14.
- Rubinstein JC, Sznol M, Pavlick AC, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med 2010;8:67.
- Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS ONE 2012;7:e35309.
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010;464:431–5.
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010;464:427–30.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncology 2015;1:359–68.
- Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother 2012;35:66–72.
- Johnson DB, Lovly CM, Flavin M, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res 2015;3:288–95.
- Johnson DB, Frampton GM, Rioth MJ, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res 2016;4:959–67.
- Kiuru M, Busam KJ. The NF1 gene in tumor syndromes and melanoma. Lab Invest 2017;97:146–57.
- Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res 2014;74:2340–50.
- Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol 2005;23:5281–93.
- Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol 2004;22:4456–62.
- Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res 2012;18:555–67.
- Davar D, Kirkwood JM. CCR 20th anniversary commentary: MAPK/ERK pathway inhibition in melanoma-kinase inhibition redux. Clin Cancer Res 2015;21:5412–4.
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012;366:207–15.
- Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010;468:968–72.
- Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010;468:973–7.
- Shi H, Hong A, Kong X, et al. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discov 2014;4:69–79.
- Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4:80–93.
- Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 2014;4:94–109.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107–14.
- Dummer R, Ascierto PA, Gogas HJ, et al. Results of COLUMBUS Part 1: a phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in BRAF-mutant melanoma. Presented at Society for Melanoma Research 2016 Congress. November 6-9, 2016. Boston (MA).
- Adelmann CH, Ching G, Du L, et al. Comparative profiles of BRAF inhibitors: the paradox index as a predictor of clinical toxicity. Oncotarget 2016;7:30453–60.
- Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:435–45.
- Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol 2005;36:486–93.
- Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res 2008;14:6821–8.
- Handolias D, Salemi R, Murray W, et al. Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment Cell Melanoma Res 2010;23:210–5.
- Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011;29:2904–9.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 2013;31:3182–90.
- Flaherty KT, Hamilton BK, Rosen MA, et al. Phase I/II trial of imatinib and bevacizumab in patients with advanced melanoma and other advanced cancers. Oncologist 2015;20:952–9.
- Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia 2013;27:107–12.
- Reinwald M, Schleyer E, Kiewe P, et al. Efficacy and pharmacologic data of second-generation tyrosine kinase inhibitor nilotinib in BCR-ABL-positive leukemia patients with central nervous system relapse after allogeneic stem cell transplantation. Biomed Res Int 2014;2014:637059.
- Carvajal RD, Lawrence DP, Weber JS, et al. Phase II study of nilotinib in melanoma harboring KIT alterations following progression to prior KIT inhibition. Clin Cancer Res 2015;21:2289–96.
- Carlino MS, Todd JR, Gowrishankar K, et al. Differential activity of MEK and ERK inhibitors in BRAF inhibitor resistant melanoma. Mol Oncol 2014;8:544–54.
- Carlino MS, Gowrishankar K, Saunders CAB, et al. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther 2013;12:1332–42.
- Chan MMK, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer 2014;120:3142–53.
- Thakur M Das, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013;494:251–5.
- Thakur M Das, Stuart DD. Molecular pathways: response and resistance to BRAF and MEK inhibitors in BRAF(V600E) tumors. Clin Cancer Res 2014;20:1074–80.
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71.
- Ayers M, Lunceford J, Nebozhyn M, et al. Relationship between immune gene signatures and clinical response to PD-1 blockade with pembrolizumab (MK-3475) in patients with advanced solid tumors. J Immunotherapy Cancer 2015;3(Suppl 2):P80.
INTRODUCTION
The incidence of cutaneous melanoma has increased over the past 2 decades, with SEER estimates indicating that the number of new cases of melanoma diagnosed annually rose from 38,300 in 1996 to 76,000 in 2016.1 Among persons younger than 50 years, the incidence is higher in females, and younger women (aged 15–39 years) are especially vulnerable.2 Among persons older than 50, melanoma incidence in men is nearly twice that of women, in whom melanomas are often thicker and often associated with worse outcomes.1,2 Approximately 85% of melanomas are diagnosed at early stages when surgery is curative, but the lifetime probability of developing invasive disease is 3% in men and 2% in women.
Prior to the advent of effective immunotherapies and targeted therapies, melanoma was often managed with chemotherapy, which had dismal response rates and commensurately poor outcomes. Advances in the understanding of the molecular etiopathogenesis and immune escape responses of cutaneous metastatic melanoma have transformed therapeutic approaches. Specifically, improved understanding of the genetic mutations driving melanoma tumorigenesis coupled with insights into mechanisms of tumor-mediated immune evasion resulted in development of inhibitors of mitogen-activated protein kinases (MAPK; BRAF and MEK) along with inhibitors of negative regulatory immune checkpoints (cytotoxic T lymphocyte–associated antigen 4 [CTLA-4] and programmed cell death-1 [PD-1]). In this review, we discuss the role of immune therapy, targeted therapy, and combinations of these in the treatment of metastatic cutaneous melanoma. We limit the immuno-therapy discussion to approved CTLA-4/PD-1 inhibitors and the targeted therapy discussion to approved BRAF/NRAS/MEK inhibitors and do not discuss non-checkpoint immunotherapies including cytokines (HD IL-2), vaccines, or adoptive T-cell approaches. Interested readers are directed to other excellent works covering these important topics.26–29
DEVELOPMENT OF TARGETED AND NOVEL IMMUNE THERAPIES
For many years the degree of ultraviolet (UV) light exposure was considered the sole major risk factor for melanoma oncogenesis, even though its mechanism was largely unknown.3 However, clinical observations regarding the occurrence of melanoma on less exposed areas (trunk and limbs) in individuals with intermittent sun exposure led to the proposition that melanomas that arose in younger patients with intermittent sun exposure were distinct from melanomas that arose in older patients in association with markers of chronic sun exposure—the “divergent pathway” hypothesis.3 Critical to this understanding were whole-exome sequencing data from multiple groups, including The Cancer Genome Atlas, that identified patterns of mutations in oncogenic drivers that were distinct in patients with and without chronically sun-damaged (CSD) skin.4–7 It is now clear that based on its association with CSD skin, melanoma can be subclassified into CSD or non-CSD melanoma. CSD and non-CSD melanoma have distinct clinico-pathological characteristics and are associated with different driver mutations. CSD melanomas typically arise in older patients on sun-exposed areas (head/neck, dorsal surfaces of distal extremities) and are associated with particular driver mutations (BRAF non-V600E, NRAS, NF1, or KIT) and genetic signatures of UV-induced DNA damage (G > T [UVA] or C > T [UVB]) transitions. Conversely, non-CSD melanomas typically arise in younger (< 55 years) patients on intermittently sun-exposed areas (trunk, proximal extremities) and are associated with BRAF V600E/K driver mutations and often lack genetic signatures of UV mutagenesis.
Identification of driver mutations in components of the MAPK pathway, including BRAF and NRAS, facilitated the development of targeted inhibitors. The BRAF inhibitors vemurafenib and dabrafenib have been shown in pivotal phase 3 studies to significantly improve overall and progression-free survival in patients with metastatic melanoma compared with chemotherapy and garnered regulatory approval (vemurafenib, BRIM-3;8,9 dabrafenib, BREAK-310). Concomitant MEK and BRAF inhibition extends the duration of benefit by preventing downstream kinase activation in the MAPK pathway. Notably, concomitant MEK inhibition alters the side-effect profile of BRAF inhibitors, with reduced incidence of keratoacanthomas and cutaneous squamous cell carcinomas that are attributable to on-target, off-tumor effects of BRAF inhibitors. Combined BRAF and MEK inhibition (vemurafenib/cobimetinib and dabrafenib/trametinib) further improved overall and progression-free survival compared to single-agent BRAF inhibition in phase 3 studies (COMBI-d,11 COMBI-v,12 and coBRIM13). Although often deep, the responses seen with the use of targeted kinase inhibitors are not often durable, with the vast majority of patients progressing after 12 to 15 months of therapy.In parallel, work primarily done in murine models of chronic viral infection uncovered the role played by co-inhibitory or co-excitatory immune checkpoints in mediating T-cell immune responses. These efforts clarified that tumor-mediated immune suppression primarily occurs through enhancement of inhibitory signals via the negative T-cell immune checkpoints CTLA-4 or PD-1.14,15 Blockade of negative T-cell immune checkpoints resulted in activation of the adaptive immune system, resulting in durable anti-tumor responses as demonstrated in studies of the CTLA-4 inhibitor ipilimumab (CA184-02016 and CA184-02417) and the PD-1 inhibitors nivolumab (CA209-003,18 CheckMate 037,19 and CheckMate 06620) and pembrolizumab (KEYNOTE-00121 and KEYNOTE-00622). Compared to the deep but short-lived responses seen with targeted kinase inhibitors, patients treated with CTLA-4 or PD-1 immune checkpoint blockade often developed durable responses that persisted even after completion of therapy. Combined CTLA-4 and PD-1 blockade results in greater magnitude of response with proportionately increased toxicity.23–25
IMMUNOTHERAPY
CTLA-4 AND PD-1 IMMUNE CHECKPOINT INHIBITORS
The novel success of immunotherapy in recent decades is largely attributable to improved understanding of adaptive immune physiology, specifically T-cell activation and regulation. T-cell activation requires 2 independent signaling events: it is initiated upon recognition of the antigen-MHC class II-receptor complex on antigen-presenting cells (APC), and requires a secondary co-stimulatory interaction of CD80/CD86 (B7.1/B7.2) on APCs and CD28 molecule on T-cells; without this second event, T-cells enter an anergic state.30–32 Upon successful signaling and co-stimulation, newly activated T-cells upregulate CTLA-4, which can bind to B7 molecules with a nearly 100-fold greater affinity than CD28.33,34 Unlike CD28, CTLA-4 engagement negatively regulates T-cell activation. The opposing signals produced by CD28 and CTLA-4 are integrated by the T-cell to determine eventual response to activation, and provide a means by which T-cell activation is homeostatically regulated to prevent exaggerated physiologic immune responses.35 It was hypothesized that CTLA-4 blockade would permit T-cell activation, which is thwarted in the tumor microenvironment by tumor-mediated CTLA-4 engagement, thereby unleashing an anti-tumor immune response.36
PD-1 is a member of the CD28 and CTLA-4 immunoglobulin super family and, similar to CTLA-4, binds activated T-cells. PD-1 has 2 ligands on activated T-cells: PD-L1 and PD-L2.37 PD-L1 is constitutively expressed by a variety of immune and non-immune cells, particularly in inflammatory environments including tumor microenvironments, in response to the release of inflammatory cytokines such as interferon (IFN)-γ.37,38 Conversely, PD-L2 is only minimally expressed constitutively, although its expression on immune and non-immune cells can be induced by similar cues from inflammatory microenvironments. PD-L1 and PD-L2 cross-compete for binding to PD-1, with PD-L2 exhibiting 2- to 6-fold greater relative affinity than PD-L1.39 PD-L1/PD-1 binding results in phosphorylation of 2 tyrosinases in the intracellular portion of PD-1, which contains immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM). PD-1 ITSM subsequently recruits either of 2 SH2-domain–containing protein tyrosine phosphatases: SHP-1 and SHP-2. SHP-2 signaling suppresses PI3K/Akt activation, down-regulates Bcl-xL, and suppresses expression of multiple transcription factors that mediate T-cell effector function including GATA-3, Eomes, and T-bet.40–42 The net effect of PD-L1/PD-1 engagement is to suppress T-cell proliferation, cytokine production, cytolytic function, and survival. Unlike CTLA-4, which primarily affects the priming phase of naive T-cell activation, PD-1 chiefly regulates the effector phase of T-cell function. Furthermore, because PD-L1/PD-L2 expression is limited to inflammatory microenvironments, the effects of PD-1 are less generalized than those of CTLA-4.
SINGLE AGENT ACTIVITY OF CTLA-4 AND PD-1 INHIBITORS
Ipilimumab (MDX-010) is a human IgG1 monoclonal antibody shown to inhibit CTLA-4.43 Early studies tested different formulations (transfectoma-derived and hybridoma-derived), doses, and schedules of ipilimumab primarily in patients with advanced refractory melanoma.44–46 Although responses were infrequent, responding patients experienced durable remissions at 1- and 2-year time points. Notably, in a foreshadowing of changes to response criteria used to evaluate these agents, several treated patients who initially had radiographically stable disease upon completion of therapy subsequently experienced a gradual decline in tumor burden.
Ipilimumab was subsequently evaluated in 2 phase 3 trials. The first study (MDX010-020/CA184-020), which involved 676 HLA-A*0201–positive patients with advanced melanoma, compared ipilimumab 3 mg/kg every 3 weeks for 4 doses either singly or in combination with gp100 vaccine with a gp100-only control arm.16 Ipilimumab administration resulted in objective responses in 11% of patients and improved progression-free and overall survival compared to gp100 alone. Of note, ipilimumab monotherapy was superior to ipilimumab/gp100 combination, possibly related to timing of vaccine in relation to ipilimumab. A confirmatory study (CA184-024) compared a higher dose of ipilimumab (10 mg/kg) in combination with dacarbazine to dacarbazine monotherapy in previously untreated melanoma and was positive.17 Given the lack of augmented efficacy with the higher (10 mg/kg) dose, ipilimumab received regulatory approval in 2011 for the treatment of melanoma at the lower dose: 3 mg/kg administered every 3 weeks for 4 doses (Table 1). Survival data was strikingly similar to patterns observed in prior phase 2 studies, with survival curves plateauing after 2 years at 23.5% to 28.5% of treated patients. Pooled survival data from prospective and retrospective studies of ipilimumab corroborate the plateau of 22% (26% treated; 20% untreated) reached at year 3 regardless of prior therapy or ipilimumab dose, underscoring the durability of long-term survival in ipilimumab-treated patients.47 Ipilimumab administration resulted in an unusual spectrum of toxicities including diarrhea, rash, hepatitis, and hypophysitis (termed immune-related adverse events, or irAEs) in up to a third of patients.
Pembrolizumab and nivolumab are humanized IgG4 monoclonal antibodies that target the PD-1 receptor found on activated T cells, B cells, and myeloid cells. Pembrolizumab and nivolumab are engineered similarly: by immunizing transgenic mice with recombinant human PD-1-Fc protein and subsequently screening murine splenic cells fused with myeloma cells for hybridomas producing antibodies reactive to PD-1-Fc.48,49 Unlike IgG1, the IgG4 moiety neither engages Fc receptors nor activates complement, avoiding cytotoxic effects of the antibody upon binding to the T cells that it is intended to activate. Both pembrolizumab and nivolumab bind PD-1 with high affinity and specificity, effectively inhibiting the interaction between PD-1 and ligands PD-L1 and PD-L2.
Nivolumab was first studied in a phase 1 study (CA209-003) of 296 patients with advanced cancers who received 1, 3, or 10 mg/kg administered every 2 weeks.18 Histologies tested included melanoma, non–small-cell lung cancer (NSCLC), renal-cell cancer (RCC), castration-resistant prostate cancer (CRPC), and colorectal cancer (CRC). Responses were seen in melanoma and RCC and unusually in NSCLC, including in both squamous and non-squamous tumors. Objective responses were noted in 41% of the 107 melanoma patients treated at 3 mg/kg. Survival was improved, with 1- and 2-year survival rates of 62% and 43% at extended follow up.50
Subsequently, nivolumab was compared to chemotherapy in a pair of phase 3 studies involving both previously untreated (Checkmate 066) and ipilimumab/BRAF inhibitor–refractory (CheckMate 037) patients.19,20 In both studies, nivolumab produced durable responses in 32% to 34% of patients and improved survival over chemotherapy. Compared to ipilimumab, the incidence of irAEs was much lower with nivolumab. The depth and magnitude of responses observed led to regulatory approval for nivolumab in both indications (untreated and ipilimumab/BRAF inhibitor–treated melanoma) in 2014. Data from both studies are summarized in Table 1.
Pembrolizumab was first evaluated in a phase 1 study of 30 patients with a variety of solid organ malignancies in which no dose-limiting toxicities were observed and no defined maximal tolerated dose was reached.51 Per protocol, maximal administered dose was 10 mg/kg every 2 weeks. Following startling responses including 2 complete responses of long duration, pembrolizumab was evaluated in a large phase 1 study (KEYNOTE-001) of 1260 patients that evaluated 3 doses (10 mg/kg every 2 weeks, 10 mg/kg every 3 weeks, and 2 mg/kg every 3 weeks) in separate melanoma and NSCLC substudies.21 Both ipilimumab-naïve and ipilimumab-treated patients were enrolled in the melanoma substudy. Objective responses were seen in 38% ofpatients across all 3 dosing schedules and were similar in both ipilimumab-naïve and ipilimumab-treated patients. Similar to nivolumab, most responders experienced durable remissions.
Pembrolizumab was subsequently compared to ipilimumab in untreated patients (KEYNOTE-006) in which patients were randomly assigned to receive either ipilimumab or pembrolizumab at 1 of 2 doses: 10 mg/kg every 2 weeks and pembrolizumab 10 mg/kg every 3 weeks.22 Response rates were greater with pembrolizumab than ipilimumab, with commensurately greater 1-year survival rates. Rates of treatment-related adverse events requiring discontinuation of study drug were much lower with pembrolizumab than ipilimumab. This trial was instrumental in proving the superior profile of pembrolizumab over ipilimumab. The US Food and Drug Administration (FDA) granted pembrolizumab accelerated approval for second-line treatment of melanoma in 2014, and updated this to include a first-line indication in 2015 (Table 1).
EFFICACY OF COMBINED CTLA-4 AND PD-1 INHIBITION
Preclinical studies demonstrated that PD-1 blockade was more effective than CTLA-4 blockade and combination PD-1/CTLA-4 blockade was synergistic, with complete rejection of tumors in approximately half of the treated animals.14 This hypothesis was evaluated in a phase 1 study that explored both concurrent and sequential combinations of ipilimumab and nivolumab along with increasing doses of both agents in PD-1/CTLA-4–naïve advanced melanoma.23 Responses were greater in the concurrent arm (40%) than in the sequential arm (20%) across dose-levels with a small fraction of patients treated in the concurrent arm experiencing a profound reduction (80%) in tumor burden.
The superiority of ipilimumab/nivolumab combination to ipilimumab monotherapy was demonstrated in a randomized blinded phase 2 study (CheckMate 069).24 Of the 4 different ipilimumab/nivolumab doses explored in the phase 1 study (3 mg/kg and 0.3 mg/kg, 3 mg/kg and 1 mg/kg, 1 mg/kg and 3 mg/kg, 3 mg/kg and 3 mg/kg), ipilimumab 3 mg/kg and nivolumab 1 mg/kg (followed by nivolumab 3 mg/kg) was compared to ipilimumab and nivolumab-matched placebo. Responses were significantly greater with dual PD-1/CTLA-4 blockade compared to CTLA-4 blockade alone (59% versus 11%). Concurrently, a 3-arm randomized phase 3 study compared the same dose of ipilimumab/nivolumab to ipilimumab and nivolumab in previously untreated advanced melanoma (CheckMate 067).25 Similar to CheckMate 069, CheckMate 067 demonstrated that ipilimumab/nivolumab combination resulted in more profound responses (58%) than either ipilimumab (19%) or nivolumab (44%) alone. Toxicity, primarily diarrhea, fatigue, pruritus, and rash, was considerable in the combination arm (55% grade 3/4 adverse events) and resulted in treatment discontinuation in 30% of patients. The profound and durable responses observed led to accelerated approval of ipilimumab/nivolumab combination in 2015 (Table 1).
Efforts to improve the toxicity/benefit ratio of ipilimumab/nivolumab combination have centered around studying lower doses and/or extended dosing schedules of ipilimumab, including ipilimumab 1 mg/kg every 6 or 12 weeks with nivolumab dosed at 3 mg/kg every 2 weeks or 480 mg every 4 weeks. Promising data from a first-line study in NSCLC (CheckMate 012) support the evaluation of nivolumab in combination with lower-dosed ipilimumab (1 mg/kg every 6 or 12 weeks).52 This approach is being tested against platinum doublet chemotherapy in a confirmatory phase 3 study in NSCLC (CheckMate 227).
TARGETED THERAPY
MAPK KINASE PATHWAY IN MELANOMA TUMORIGENESIS
The MAPK pathway mediates cellular responses to growth signals. RAF kinases are central mediators in the MAPK pathway and exert their effect primarily through MEK phosphorylation and activation following dimerization (hetero- or homo-) of RAF molecules. As a result, RAF is integral to multiple cellular processes, including transcriptional regulation, cellular differentiation, and cell proliferation. MAPK pathway activation is a common event in many cancers, primarily due to activating mutations in BRAF or RAS. Alternatively, MAPK pathway activation can occur in the absence of activating mutations in BRAF or NRAS through down-regulation of MAPK pathway inhibitory proteins (RAF-1 inhibitory protein or SPRY-2), C-MET overexpression, or activating mutations in non-BRAF/NRAS kinases including CRAF, HRAS, and NRAS.53,54
Somatic point mutations in BRAF are frequently observed (37%–50%) in malignant melanomas and at lower frequency in a range of human cancers including NSCLC, colorectal cancer, papillary thyroid cancer, ovarian cancer, glioma, and gastrointestinal stromal tumor.6,55,56 BRAF mutations in melanoma typically occur within the activation segment of the kinase domain (exon 15). Between 80% and 90% of activating mutations result in an amino acid substitution of glutamate (E) for valine (V) at position 600: V600E.57,58 V600E mutations are true oncogenic drivers, resulting in increased kinase activity with demonstrable transformational capacity in vitro. BRAF mutations are usually mutually exclusive, with tumors typically containing no other driver mutations in NRAS, KIT, NF1, or other genes.
NRAS mutations are less common than BRAF mutations, having a reported frequency of 13% to 25% in melanoma.4 NRAS mutations generally occur within the P-loop region of the G domain (exon 2), or less commonly in the switch II region of the G domain (exon 3). Most NRAS exon 2 mutations comprise amino acid substitutions at position 61 from glutamine (Q) to arginine (R; 35%), lysine (K; 34%) and less often to glutamate (E), leucine (L), or proline (P). Preclinical data suggest that NRAS mutations paradoxically stimulate the MAPK pathway and thus enhance tumor growth in vitro.59,60 Several important phenotypic differences distinguish NRAS- from BRAF-mutated melanoma. NRAS-mutated tumors are typically associated with increasing age and CSD skin, while BRAF-mutated tumors arise in younger patients in non-CSD skin. A large population-based study suggested that NRAS-mutated melanomas were associated with mitoses and lower tumor infiltrating lymphocytes (TIL) grade, and arose in anatomic sites other than the head/neck, while BRAF-mutated tumors were associated with mitoses and superficial spreading histology.61 Although the lower TIL grade seen with NRAS-mutated melanomas suggests a more immunosuppressed microenvironment and argues for poorer responses to immune therapies, clinical studies comparing responses to immunotherapies in various categories of driver mutations provide conflicting results for the prognostic role of NRAS mutations in relation to immune checkpoint blockade and other immune therapies.62–64
NF1 represents the third known driver in cutaneous melanoma, with mutations reported in 12% of cases.6,7 NF1 encodes neurofibromin, which has GTPase activity and regulates RAS proteins; NF1 loss results in increased RAS.65 Unlike BRAF or NRAS, which are usually mutually exclusive, NF1 mutations in melanoma can occur singly or in combination with either BRAF or NRAS mutations. In these settings, NF1 mutations are associated with RAS activation, MEK-dependence, and resistance to RAF inhibition.66
MAPK PATHWAY INHIBITION SINGLY AND IN COMBINATION
Although multiple MEK 1/2 inhibitors (AS703026, AZD8330/ARRY-704, AZD6244, CH5126766, CI-1040, GSK1120212, PD0325901, RDEA119, and XL518) and RAF inhibitors (ARQ 680, GDC-0879, GSK2118436, PLX4032, RAF265, sorafenib, XL281/BMS-908662) were developed, the initial evaluation of MAPK pathway inhibitors in advanced human cancers began with CI-1040. Preclinical data suggested that CI-1040 potently and selectively inhibited both MEK1 and MEK2, but phase 1 and 2 human trial results were disappointing, likely because these trials were not selectively enriched for NRAS/BRAF–mutated tumors or cancers in which these oncogenic mutations were most commonly detected, such as melanoma.67,68 The subsequent evaluation of selumetinib (AZD6244/ARRY-142886) in a phase 2 study was also negative. Although investigators enrolled a presumably enriched population (cutaneous melanoma), the incidence of NRAS/BRAF–mutated tumors was not ascertained to determine this, but rather assumed, which led to a discrepancy between the assumed (prestudy) and observed (on-study) proportions of BRAF/NRAS mutations that was not accounted for in power calculations.69,70 Lessons learned from these earlier misadventures informed the current paradigm of targeted therapy development: (1) identification of a highly specific and potent inhibitor through high-throughput screening; (2) establishment of maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) in unselected patients; (3) confirmation of RP2D in selected tumor types enriched for target of interest; and (4) confirmatory study against standard comparator to seek regulatory approval.
Vemurafenib and dabrafenib were evaluated in this tiered fashion in phase 1 dose-finding studies comprising unselected patients, followed by phase 2 studies in advanced BRAF V600E–mutated melanoma. Both were subsequently evaluated in randomized phase 3 trials (vemurafenib, BRIM-38; dabrafenib, BREAK-310) that compared them with dacarbazine (1000 mg/m2 intravenously every 3 weeks) in the treatment of advanced BRAF V600E–mutated melanoma. Response kinetics for both agents were remarkably similar: single-agent BRAF inhibitors resulted in rapid (time to response 2–3 months), profound (approximately 50% objective responses) reductions in tumor burden that lasted 6 to 7 months. Adverse events common to both agents included rash, fatigue, and arthralgia, although clinically significant photosensitivity was more common with vemurafenib and clinically significant pyrexia was more common with dabrafenib. Class-specific adverse events included the development of cutaneous squamous-cell carcinomas and keratoacanthomas secondary to paradoxical activation of MAPK pathway signaling either through activating mutations in HRAS or mutations or amplifications in receptor tyrosine kinases upstream of BRAF, resulting in elevated levels of RAS–guanosine triphosphate complexes.71 Results of these studies resulted in regulatory approval of single-agent BRAF inhibitors for the treatment of BRAF V600E (and later V600K)–mutated melanoma (vemurafenib in 2011; dabrafenib in 2013). Details regarding trial populations, study interventions, efficacy, and adverse events are summarized in Table 2.

Responses to BRAF inhibitors are typically profound but temporary. Mechanisms of acquired resistance are diverse and include reactivation of MAPK pathway–dependent signaling (RAS activation or increased RAF expression), and development of MAPK pathway–independent signaling (COT overexpression; increased PI3K or AKT signaling) that permits bypass of inhibited BRAF signaling within the MAPK pathway.72–76 These findings suggested that upfront inhibition of both MEK and mutant BRAF may produce more durable responses than BRAF inhibition alone. Three pivotal phase 3 studies established the superiority of combination BRAF and MEK inhibition over BRAF inhibition alone: COMBI-d11 (dabrafenib/trametinib versus dabrafenib/placebo), COMBI-v12 (dabrafenib/trametinib versus vemurafenib), and coBRIM13 (vemurafenib/cobimetinib versus vemurafenib/placebo). As expected, compared to BRAF inhibitor monotherapy, combination BRAF and MEK inhibition produced greater responses and improved progression-free and overall survival (Table 2). Interestingly, the rate of cutaneous squamous-cell carcinomas was much lower with combination therapy, reflecting the more profound degree of MAPK pathway inhibition achieved with combination BRAF and MEK inhibition. Based on these results, FDA approval was granted for both dabrafenib/trametinib and vemurafenib/cobimetinib combinations in 2015. Although the dabrafenib/trametinib combination was only approved in 2015, trametinib had independently gained FDA approval in 2013 for the treatment of BRAF V600E/K–mutated melanoma on the basis of the phase 3 METRIC study.77
Encorafenib (LGX818) and binimetinib (MEK162, ARRY-162, ARRY-438162) are new BRAF and MEK inhibitors currently being evaluated in clinical trials. Encorafenib/binimetinib combination was first evaluated in a phase 3 study (COLUMBUS) that compared it with vemurafenib monotherapy in BRAF-mutant melanoma.78 Unsurprisingly, encorafenib/binimetinib combination produced greater and more durable responses compared to vemurafenib monotherapy. The median progression-free survival of the encorafenib/binimetinib combination (14.9 months) was greater than vemurafenib monotherapy (7.3 months) in this study, and intriguingly greater than that seen with vemurafenib/cobimetinib (coBRIM 9.9 months) and dabrafenib/trametinib (COMBI-d 9.3 months; COMBI-v 11.4 months). Of note, although encorafenib has an IC50 midway between dabrafenib and vemurafenib in cell-free assays (0.8 nM dabrafenib, 4 nM encorafenib, and 31 nM vemurafenib), it has an extremely slower off-rate from BRAF V600E, which results in significantly greater target inhibition in cells following drug wash-out.79 This may account for the significantly greater clinical benefit seen with encorafenib/binimetinib in clinical trials. Final study data are eagerly awaited. Regulatory approval has been sought, and is pending at this time.
Binimetinib has been compared to dacarbazine in a phase 3 study (NEMO) of patients with NRAS-mutant melanoma, most of whom had been previously treated with immunotherapy.80 Response rates were low in both arms, although slightly greater with binimetinib than dacarbazine (15% versus 9%), commensurate with a modest improvement in progression-free survival. FDA approval has been sought and remains pending at this time.
KIT INHIBITION SINGLY AND IN COMBINATION
The KIT receptor protein tyrosine kinase is a transmembrane protein consisting of extracellular and intracellular domains. Activating KIT mutations occur in 2% to 8% of all melanoma patients and may be found in all melanoma subtypes but are commonest in acral melanomas (10%–20%) and mucosal melanomas (15%–20%). Activating KIT mutations primarily occur in exons 11 and 13, which code for the juxtamembrane and kinase domains, respectively.5,81–83
Imatinib mesylate is a tyrosine kinase inhibitor of the 2-phenyl amino pyrimidine class that occupies the tyrosine kinase active site with resultant blocking of tyrosine kinase activity. Imatinib mesylate is known to block KIT and has been extensively studied in patients with gastrointestinal stromal tumors (GIST), 80% of whom harbor KIT mutations, in both the adjuvant and the metastatic settings. In melanoma, imatinib mesylate was studied in a Chinese open-label, phase 2 study of imatinib mesylate monotherapy in metastatic melanoma harboring KIT mutation or amplification; 25% of the study patients had mucosal disease and the rest had cutaneous disease, with acral involvement in 50% of all patients.84 Overall response rate was 23%, while 51% of patients remained alive at 1 year with no differences in response rate and/or survival being noted between patients with either KIT mutations or amplifications. In a separate study of imatinib mesylate at 400 mg daily or 400 mg twice daily in Caucasian patients with KIT-mutated/amplified melanoma, similar response and survival rates were reported, although patients with KIT mutations did nonsignificantly better than those with KIT amplifications.85
Other novel studies evaluating KIT inhibitors include KIT inhibition in combination with the VEGF inhibitor bevacizumab and a study of selective BCR-ABL kinase inhibitor nilotinib in imatinib-resistant melanoma. In the former phase 1/2 study, Flaherty and colleagues studied imatinib 800 mg daily and bevacizumab at 10 mg/kg every 2 weeks in 63 patients with advanced tumors, including 23 with metastatic melanoma. Although the combination was relatively nontoxic, no significant efficacy signal was seen and further accrual to the phase 2 portion was halted after the first stage was completed.86 Nilotinib is a BCR-ABL1 tyrosine kinase inhibitor intelligently designed based on the structure of the ABL-imatinib complex that is 10 to 30 times more potent than imatinib in inhibiting BCR-ABL1 tyrosine kinase activity. Nilotinib is approved for the treatment of imatinib-resistant chronic myelogenous leukemia (CML), with reported efficacy in patients with central nervous system (CNS) involvement.87,88 Nilotinib has been studied in a single study of KIT-mutated/amplified melanoma that included patients with imatinib-resistance and those with treated CNS disease. Nilotinib appeared to be active in imatinib-resistant melanoma, although no responses were seen in the CNS disease cohort.89 Overall, the response rates observed with KIT inhibition in melanoma are much lower than those observed in CML and GIST.
CONCLUSION AND FUTURE DIRECTIONS
Prior to 2011, the only approved agents for the treatment of advanced melanoma were dacarbazine and high-dose interleukin-2. Since 2011, drug approvals in melanoma have proceeded at a frenetic pace unmatched in any other disease. The primary events underlying this are advances in our understanding of the gene mutation landscape driving melanoma tumorigenesis, accompanied by insights into the means by which tumors circumvent the induction of effective anti-tumor T-cell responses. These insights have resulted in the development of inhibitors targeting MAPK pathway kinases BRAF, MEK, and NRAS), KIT, and regulatory immune checkpoints (CTLA-4 and PD-1). Although BRAF/MEK inhibition results in profound reductions and even occasional complete responses in patients, these responses are typically short lived, rarely lasting more than 9 to 11 months; the encorafenib/binimetinib combination may improve that duration marginally. However, the signature therapeutic advance in melanoma of the past decade is immunotherapy, particularly the development of inhibitors of CTLA-4 and PD-1 immune checkpoints. With these agents, significant proportions of treated patients remain free of progression off-therapy (ipilimumab 23%; nivolumab 34%; pembrolizumab 35%; ipilimumab/nivolumab 64%), and some patients can be successfully re-induced after delayed progression. Separately, the high response rates observed with the use of KIT inhibitors in CML and GIST have not been observed in KIT mutated/amplified melanoma and development of agents in this space has been limited. The challenges ahead center around identifying predictive biomarkers and circumventing primary or acquired resistance, with the eventual goal of producing durable remissions in the majority of treated patients.
Our improved understanding of the mechanisms of acquired resistance to BRAF/MEK inhibitors suggests that anti-tumor activity may be achieved by targeting multiple pathways, possibly with combination regimens comprising other inhibitors and/or immunotherapy. Preclinical data supports the use of combination strategies targeting both ERK and PI3K/mTOR to circumvent acquired resistance.90 Ongoing studies are evaluating combinations with biguanides (metformin: NCT02143050 and NCT01638676; phenformin: NCT03026517), HSP90 inhibitors (XL888: NCT02721459; AT13387: NCT02097225), and decitabine (NCT01876641).
One complexity affecting management of resistance in the targeted therapy landscape remains tumor heterogeneity, particularly intra- and intertumoral heterogeneity, which may explain the apparent contradiction between continued efficacy of BRAF inhibitors in BRAF-resistant tumors and preclinical data predicting slower progression of resistant tumors on cessation of BRAF inhibitors.91–94 These data provide a rationale to investigate intermittent dosing regimens with BRAF/MEK inhibitors; several studies exploring this approach are ongoing (NCT01894672 and NCT02583516).
Given the specificity, adaptability, and memory response associated with immunotherapy, it is likely that these agents will be used to treat the majority of patients regardless of mutational status. Hence, identifying predictive biomarkers of response to immune checkpoint inhibitors is vital. The presence of CD8+ T-cell infiltrate and IFN-γ gene signature, which indicate an “inflamed” tumor microenvironment, are highly predictive of clinical benefit from PD-1 inhibitors.95,96 However, not all PD-1 responders have “inflamed” tumor microenvironments, and not all patients with an “inflamed” tumor microenvironment respond to immune checkpoint inhibitors. The complexity of the immune system is reflected in the multiple non-redundant immunologic pathways, both positive and negative, with checkpoints and ligands that emerge dynamically in response to treatment. Given the dynamic nature of the immune response, it is unlikely that any single immunologic biomarker identified pre-treatment will be completely predictive. Rather, the complexity of the biomarker approach must match the complexity of the immune response elicited, and will likely incorporate multifarious elements including CD8+ T-cell infiltrate, IFN-γ gene signature, and additional elements including microbiome, genetic polymorphisms, and tumor mutation load. The goal is to use multiple markers to guide development of combinations and then, depending on initial response, to examine tumors for alterations to guide decisions about additional treatment(s) to improve responses, with the eventual goal being durable clinical responses for all patients.
INTRODUCTION
The incidence of cutaneous melanoma has increased over the past 2 decades, with SEER estimates indicating that the number of new cases of melanoma diagnosed annually rose from 38,300 in 1996 to 76,000 in 2016.1 Among persons younger than 50 years, the incidence is higher in females, and younger women (aged 15–39 years) are especially vulnerable.2 Among persons older than 50, melanoma incidence in men is nearly twice that of women, in whom melanomas are often thicker and often associated with worse outcomes.1,2 Approximately 85% of melanomas are diagnosed at early stages when surgery is curative, but the lifetime probability of developing invasive disease is 3% in men and 2% in women.
Prior to the advent of effective immunotherapies and targeted therapies, melanoma was often managed with chemotherapy, which had dismal response rates and commensurately poor outcomes. Advances in the understanding of the molecular etiopathogenesis and immune escape responses of cutaneous metastatic melanoma have transformed therapeutic approaches. Specifically, improved understanding of the genetic mutations driving melanoma tumorigenesis coupled with insights into mechanisms of tumor-mediated immune evasion resulted in development of inhibitors of mitogen-activated protein kinases (MAPK; BRAF and MEK) along with inhibitors of negative regulatory immune checkpoints (cytotoxic T lymphocyte–associated antigen 4 [CTLA-4] and programmed cell death-1 [PD-1]). In this review, we discuss the role of immune therapy, targeted therapy, and combinations of these in the treatment of metastatic cutaneous melanoma. We limit the immuno-therapy discussion to approved CTLA-4/PD-1 inhibitors and the targeted therapy discussion to approved BRAF/NRAS/MEK inhibitors and do not discuss non-checkpoint immunotherapies including cytokines (HD IL-2), vaccines, or adoptive T-cell approaches. Interested readers are directed to other excellent works covering these important topics.26–29
DEVELOPMENT OF TARGETED AND NOVEL IMMUNE THERAPIES
For many years the degree of ultraviolet (UV) light exposure was considered the sole major risk factor for melanoma oncogenesis, even though its mechanism was largely unknown.3 However, clinical observations regarding the occurrence of melanoma on less exposed areas (trunk and limbs) in individuals with intermittent sun exposure led to the proposition that melanomas that arose in younger patients with intermittent sun exposure were distinct from melanomas that arose in older patients in association with markers of chronic sun exposure—the “divergent pathway” hypothesis.3 Critical to this understanding were whole-exome sequencing data from multiple groups, including The Cancer Genome Atlas, that identified patterns of mutations in oncogenic drivers that were distinct in patients with and without chronically sun-damaged (CSD) skin.4–7 It is now clear that based on its association with CSD skin, melanoma can be subclassified into CSD or non-CSD melanoma. CSD and non-CSD melanoma have distinct clinico-pathological characteristics and are associated with different driver mutations. CSD melanomas typically arise in older patients on sun-exposed areas (head/neck, dorsal surfaces of distal extremities) and are associated with particular driver mutations (BRAF non-V600E, NRAS, NF1, or KIT) and genetic signatures of UV-induced DNA damage (G > T [UVA] or C > T [UVB]) transitions. Conversely, non-CSD melanomas typically arise in younger (< 55 years) patients on intermittently sun-exposed areas (trunk, proximal extremities) and are associated with BRAF V600E/K driver mutations and often lack genetic signatures of UV mutagenesis.
Identification of driver mutations in components of the MAPK pathway, including BRAF and NRAS, facilitated the development of targeted inhibitors. The BRAF inhibitors vemurafenib and dabrafenib have been shown in pivotal phase 3 studies to significantly improve overall and progression-free survival in patients with metastatic melanoma compared with chemotherapy and garnered regulatory approval (vemurafenib, BRIM-3;8,9 dabrafenib, BREAK-310). Concomitant MEK and BRAF inhibition extends the duration of benefit by preventing downstream kinase activation in the MAPK pathway. Notably, concomitant MEK inhibition alters the side-effect profile of BRAF inhibitors, with reduced incidence of keratoacanthomas and cutaneous squamous cell carcinomas that are attributable to on-target, off-tumor effects of BRAF inhibitors. Combined BRAF and MEK inhibition (vemurafenib/cobimetinib and dabrafenib/trametinib) further improved overall and progression-free survival compared to single-agent BRAF inhibition in phase 3 studies (COMBI-d,11 COMBI-v,12 and coBRIM13). Although often deep, the responses seen with the use of targeted kinase inhibitors are not often durable, with the vast majority of patients progressing after 12 to 15 months of therapy.In parallel, work primarily done in murine models of chronic viral infection uncovered the role played by co-inhibitory or co-excitatory immune checkpoints in mediating T-cell immune responses. These efforts clarified that tumor-mediated immune suppression primarily occurs through enhancement of inhibitory signals via the negative T-cell immune checkpoints CTLA-4 or PD-1.14,15 Blockade of negative T-cell immune checkpoints resulted in activation of the adaptive immune system, resulting in durable anti-tumor responses as demonstrated in studies of the CTLA-4 inhibitor ipilimumab (CA184-02016 and CA184-02417) and the PD-1 inhibitors nivolumab (CA209-003,18 CheckMate 037,19 and CheckMate 06620) and pembrolizumab (KEYNOTE-00121 and KEYNOTE-00622). Compared to the deep but short-lived responses seen with targeted kinase inhibitors, patients treated with CTLA-4 or PD-1 immune checkpoint blockade often developed durable responses that persisted even after completion of therapy. Combined CTLA-4 and PD-1 blockade results in greater magnitude of response with proportionately increased toxicity.23–25
IMMUNOTHERAPY
CTLA-4 AND PD-1 IMMUNE CHECKPOINT INHIBITORS
The novel success of immunotherapy in recent decades is largely attributable to improved understanding of adaptive immune physiology, specifically T-cell activation and regulation. T-cell activation requires 2 independent signaling events: it is initiated upon recognition of the antigen-MHC class II-receptor complex on antigen-presenting cells (APC), and requires a secondary co-stimulatory interaction of CD80/CD86 (B7.1/B7.2) on APCs and CD28 molecule on T-cells; without this second event, T-cells enter an anergic state.30–32 Upon successful signaling and co-stimulation, newly activated T-cells upregulate CTLA-4, which can bind to B7 molecules with a nearly 100-fold greater affinity than CD28.33,34 Unlike CD28, CTLA-4 engagement negatively regulates T-cell activation. The opposing signals produced by CD28 and CTLA-4 are integrated by the T-cell to determine eventual response to activation, and provide a means by which T-cell activation is homeostatically regulated to prevent exaggerated physiologic immune responses.35 It was hypothesized that CTLA-4 blockade would permit T-cell activation, which is thwarted in the tumor microenvironment by tumor-mediated CTLA-4 engagement, thereby unleashing an anti-tumor immune response.36
PD-1 is a member of the CD28 and CTLA-4 immunoglobulin super family and, similar to CTLA-4, binds activated T-cells. PD-1 has 2 ligands on activated T-cells: PD-L1 and PD-L2.37 PD-L1 is constitutively expressed by a variety of immune and non-immune cells, particularly in inflammatory environments including tumor microenvironments, in response to the release of inflammatory cytokines such as interferon (IFN)-γ.37,38 Conversely, PD-L2 is only minimally expressed constitutively, although its expression on immune and non-immune cells can be induced by similar cues from inflammatory microenvironments. PD-L1 and PD-L2 cross-compete for binding to PD-1, with PD-L2 exhibiting 2- to 6-fold greater relative affinity than PD-L1.39 PD-L1/PD-1 binding results in phosphorylation of 2 tyrosinases in the intracellular portion of PD-1, which contains immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM). PD-1 ITSM subsequently recruits either of 2 SH2-domain–containing protein tyrosine phosphatases: SHP-1 and SHP-2. SHP-2 signaling suppresses PI3K/Akt activation, down-regulates Bcl-xL, and suppresses expression of multiple transcription factors that mediate T-cell effector function including GATA-3, Eomes, and T-bet.40–42 The net effect of PD-L1/PD-1 engagement is to suppress T-cell proliferation, cytokine production, cytolytic function, and survival. Unlike CTLA-4, which primarily affects the priming phase of naive T-cell activation, PD-1 chiefly regulates the effector phase of T-cell function. Furthermore, because PD-L1/PD-L2 expression is limited to inflammatory microenvironments, the effects of PD-1 are less generalized than those of CTLA-4.
SINGLE AGENT ACTIVITY OF CTLA-4 AND PD-1 INHIBITORS
Ipilimumab (MDX-010) is a human IgG1 monoclonal antibody shown to inhibit CTLA-4.43 Early studies tested different formulations (transfectoma-derived and hybridoma-derived), doses, and schedules of ipilimumab primarily in patients with advanced refractory melanoma.44–46 Although responses were infrequent, responding patients experienced durable remissions at 1- and 2-year time points. Notably, in a foreshadowing of changes to response criteria used to evaluate these agents, several treated patients who initially had radiographically stable disease upon completion of therapy subsequently experienced a gradual decline in tumor burden.
Ipilimumab was subsequently evaluated in 2 phase 3 trials. The first study (MDX010-020/CA184-020), which involved 676 HLA-A*0201–positive patients with advanced melanoma, compared ipilimumab 3 mg/kg every 3 weeks for 4 doses either singly or in combination with gp100 vaccine with a gp100-only control arm.16 Ipilimumab administration resulted in objective responses in 11% of patients and improved progression-free and overall survival compared to gp100 alone. Of note, ipilimumab monotherapy was superior to ipilimumab/gp100 combination, possibly related to timing of vaccine in relation to ipilimumab. A confirmatory study (CA184-024) compared a higher dose of ipilimumab (10 mg/kg) in combination with dacarbazine to dacarbazine monotherapy in previously untreated melanoma and was positive.17 Given the lack of augmented efficacy with the higher (10 mg/kg) dose, ipilimumab received regulatory approval in 2011 for the treatment of melanoma at the lower dose: 3 mg/kg administered every 3 weeks for 4 doses (Table 1). Survival data was strikingly similar to patterns observed in prior phase 2 studies, with survival curves plateauing after 2 years at 23.5% to 28.5% of treated patients. Pooled survival data from prospective and retrospective studies of ipilimumab corroborate the plateau of 22% (26% treated; 20% untreated) reached at year 3 regardless of prior therapy or ipilimumab dose, underscoring the durability of long-term survival in ipilimumab-treated patients.47 Ipilimumab administration resulted in an unusual spectrum of toxicities including diarrhea, rash, hepatitis, and hypophysitis (termed immune-related adverse events, or irAEs) in up to a third of patients.
Pembrolizumab and nivolumab are humanized IgG4 monoclonal antibodies that target the PD-1 receptor found on activated T cells, B cells, and myeloid cells. Pembrolizumab and nivolumab are engineered similarly: by immunizing transgenic mice with recombinant human PD-1-Fc protein and subsequently screening murine splenic cells fused with myeloma cells for hybridomas producing antibodies reactive to PD-1-Fc.48,49 Unlike IgG1, the IgG4 moiety neither engages Fc receptors nor activates complement, avoiding cytotoxic effects of the antibody upon binding to the T cells that it is intended to activate. Both pembrolizumab and nivolumab bind PD-1 with high affinity and specificity, effectively inhibiting the interaction between PD-1 and ligands PD-L1 and PD-L2.
Nivolumab was first studied in a phase 1 study (CA209-003) of 296 patients with advanced cancers who received 1, 3, or 10 mg/kg administered every 2 weeks.18 Histologies tested included melanoma, non–small-cell lung cancer (NSCLC), renal-cell cancer (RCC), castration-resistant prostate cancer (CRPC), and colorectal cancer (CRC). Responses were seen in melanoma and RCC and unusually in NSCLC, including in both squamous and non-squamous tumors. Objective responses were noted in 41% of the 107 melanoma patients treated at 3 mg/kg. Survival was improved, with 1- and 2-year survival rates of 62% and 43% at extended follow up.50
Subsequently, nivolumab was compared to chemotherapy in a pair of phase 3 studies involving both previously untreated (Checkmate 066) and ipilimumab/BRAF inhibitor–refractory (CheckMate 037) patients.19,20 In both studies, nivolumab produced durable responses in 32% to 34% of patients and improved survival over chemotherapy. Compared to ipilimumab, the incidence of irAEs was much lower with nivolumab. The depth and magnitude of responses observed led to regulatory approval for nivolumab in both indications (untreated and ipilimumab/BRAF inhibitor–treated melanoma) in 2014. Data from both studies are summarized in Table 1.
Pembrolizumab was first evaluated in a phase 1 study of 30 patients with a variety of solid organ malignancies in which no dose-limiting toxicities were observed and no defined maximal tolerated dose was reached.51 Per protocol, maximal administered dose was 10 mg/kg every 2 weeks. Following startling responses including 2 complete responses of long duration, pembrolizumab was evaluated in a large phase 1 study (KEYNOTE-001) of 1260 patients that evaluated 3 doses (10 mg/kg every 2 weeks, 10 mg/kg every 3 weeks, and 2 mg/kg every 3 weeks) in separate melanoma and NSCLC substudies.21 Both ipilimumab-naïve and ipilimumab-treated patients were enrolled in the melanoma substudy. Objective responses were seen in 38% ofpatients across all 3 dosing schedules and were similar in both ipilimumab-naïve and ipilimumab-treated patients. Similar to nivolumab, most responders experienced durable remissions.
Pembrolizumab was subsequently compared to ipilimumab in untreated patients (KEYNOTE-006) in which patients were randomly assigned to receive either ipilimumab or pembrolizumab at 1 of 2 doses: 10 mg/kg every 2 weeks and pembrolizumab 10 mg/kg every 3 weeks.22 Response rates were greater with pembrolizumab than ipilimumab, with commensurately greater 1-year survival rates. Rates of treatment-related adverse events requiring discontinuation of study drug were much lower with pembrolizumab than ipilimumab. This trial was instrumental in proving the superior profile of pembrolizumab over ipilimumab. The US Food and Drug Administration (FDA) granted pembrolizumab accelerated approval for second-line treatment of melanoma in 2014, and updated this to include a first-line indication in 2015 (Table 1).
EFFICACY OF COMBINED CTLA-4 AND PD-1 INHIBITION
Preclinical studies demonstrated that PD-1 blockade was more effective than CTLA-4 blockade and combination PD-1/CTLA-4 blockade was synergistic, with complete rejection of tumors in approximately half of the treated animals.14 This hypothesis was evaluated in a phase 1 study that explored both concurrent and sequential combinations of ipilimumab and nivolumab along with increasing doses of both agents in PD-1/CTLA-4–naïve advanced melanoma.23 Responses were greater in the concurrent arm (40%) than in the sequential arm (20%) across dose-levels with a small fraction of patients treated in the concurrent arm experiencing a profound reduction (80%) in tumor burden.
The superiority of ipilimumab/nivolumab combination to ipilimumab monotherapy was demonstrated in a randomized blinded phase 2 study (CheckMate 069).24 Of the 4 different ipilimumab/nivolumab doses explored in the phase 1 study (3 mg/kg and 0.3 mg/kg, 3 mg/kg and 1 mg/kg, 1 mg/kg and 3 mg/kg, 3 mg/kg and 3 mg/kg), ipilimumab 3 mg/kg and nivolumab 1 mg/kg (followed by nivolumab 3 mg/kg) was compared to ipilimumab and nivolumab-matched placebo. Responses were significantly greater with dual PD-1/CTLA-4 blockade compared to CTLA-4 blockade alone (59% versus 11%). Concurrently, a 3-arm randomized phase 3 study compared the same dose of ipilimumab/nivolumab to ipilimumab and nivolumab in previously untreated advanced melanoma (CheckMate 067).25 Similar to CheckMate 069, CheckMate 067 demonstrated that ipilimumab/nivolumab combination resulted in more profound responses (58%) than either ipilimumab (19%) or nivolumab (44%) alone. Toxicity, primarily diarrhea, fatigue, pruritus, and rash, was considerable in the combination arm (55% grade 3/4 adverse events) and resulted in treatment discontinuation in 30% of patients. The profound and durable responses observed led to accelerated approval of ipilimumab/nivolumab combination in 2015 (Table 1).
Efforts to improve the toxicity/benefit ratio of ipilimumab/nivolumab combination have centered around studying lower doses and/or extended dosing schedules of ipilimumab, including ipilimumab 1 mg/kg every 6 or 12 weeks with nivolumab dosed at 3 mg/kg every 2 weeks or 480 mg every 4 weeks. Promising data from a first-line study in NSCLC (CheckMate 012) support the evaluation of nivolumab in combination with lower-dosed ipilimumab (1 mg/kg every 6 or 12 weeks).52 This approach is being tested against platinum doublet chemotherapy in a confirmatory phase 3 study in NSCLC (CheckMate 227).
TARGETED THERAPY
MAPK KINASE PATHWAY IN MELANOMA TUMORIGENESIS
The MAPK pathway mediates cellular responses to growth signals. RAF kinases are central mediators in the MAPK pathway and exert their effect primarily through MEK phosphorylation and activation following dimerization (hetero- or homo-) of RAF molecules. As a result, RAF is integral to multiple cellular processes, including transcriptional regulation, cellular differentiation, and cell proliferation. MAPK pathway activation is a common event in many cancers, primarily due to activating mutations in BRAF or RAS. Alternatively, MAPK pathway activation can occur in the absence of activating mutations in BRAF or NRAS through down-regulation of MAPK pathway inhibitory proteins (RAF-1 inhibitory protein or SPRY-2), C-MET overexpression, or activating mutations in non-BRAF/NRAS kinases including CRAF, HRAS, and NRAS.53,54
Somatic point mutations in BRAF are frequently observed (37%–50%) in malignant melanomas and at lower frequency in a range of human cancers including NSCLC, colorectal cancer, papillary thyroid cancer, ovarian cancer, glioma, and gastrointestinal stromal tumor.6,55,56 BRAF mutations in melanoma typically occur within the activation segment of the kinase domain (exon 15). Between 80% and 90% of activating mutations result in an amino acid substitution of glutamate (E) for valine (V) at position 600: V600E.57,58 V600E mutations are true oncogenic drivers, resulting in increased kinase activity with demonstrable transformational capacity in vitro. BRAF mutations are usually mutually exclusive, with tumors typically containing no other driver mutations in NRAS, KIT, NF1, or other genes.
NRAS mutations are less common than BRAF mutations, having a reported frequency of 13% to 25% in melanoma.4 NRAS mutations generally occur within the P-loop region of the G domain (exon 2), or less commonly in the switch II region of the G domain (exon 3). Most NRAS exon 2 mutations comprise amino acid substitutions at position 61 from glutamine (Q) to arginine (R; 35%), lysine (K; 34%) and less often to glutamate (E), leucine (L), or proline (P). Preclinical data suggest that NRAS mutations paradoxically stimulate the MAPK pathway and thus enhance tumor growth in vitro.59,60 Several important phenotypic differences distinguish NRAS- from BRAF-mutated melanoma. NRAS-mutated tumors are typically associated with increasing age and CSD skin, while BRAF-mutated tumors arise in younger patients in non-CSD skin. A large population-based study suggested that NRAS-mutated melanomas were associated with mitoses and lower tumor infiltrating lymphocytes (TIL) grade, and arose in anatomic sites other than the head/neck, while BRAF-mutated tumors were associated with mitoses and superficial spreading histology.61 Although the lower TIL grade seen with NRAS-mutated melanomas suggests a more immunosuppressed microenvironment and argues for poorer responses to immune therapies, clinical studies comparing responses to immunotherapies in various categories of driver mutations provide conflicting results for the prognostic role of NRAS mutations in relation to immune checkpoint blockade and other immune therapies.62–64
NF1 represents the third known driver in cutaneous melanoma, with mutations reported in 12% of cases.6,7 NF1 encodes neurofibromin, which has GTPase activity and regulates RAS proteins; NF1 loss results in increased RAS.65 Unlike BRAF or NRAS, which are usually mutually exclusive, NF1 mutations in melanoma can occur singly or in combination with either BRAF or NRAS mutations. In these settings, NF1 mutations are associated with RAS activation, MEK-dependence, and resistance to RAF inhibition.66
MAPK PATHWAY INHIBITION SINGLY AND IN COMBINATION
Although multiple MEK 1/2 inhibitors (AS703026, AZD8330/ARRY-704, AZD6244, CH5126766, CI-1040, GSK1120212, PD0325901, RDEA119, and XL518) and RAF inhibitors (ARQ 680, GDC-0879, GSK2118436, PLX4032, RAF265, sorafenib, XL281/BMS-908662) were developed, the initial evaluation of MAPK pathway inhibitors in advanced human cancers began with CI-1040. Preclinical data suggested that CI-1040 potently and selectively inhibited both MEK1 and MEK2, but phase 1 and 2 human trial results were disappointing, likely because these trials were not selectively enriched for NRAS/BRAF–mutated tumors or cancers in which these oncogenic mutations were most commonly detected, such as melanoma.67,68 The subsequent evaluation of selumetinib (AZD6244/ARRY-142886) in a phase 2 study was also negative. Although investigators enrolled a presumably enriched population (cutaneous melanoma), the incidence of NRAS/BRAF–mutated tumors was not ascertained to determine this, but rather assumed, which led to a discrepancy between the assumed (prestudy) and observed (on-study) proportions of BRAF/NRAS mutations that was not accounted for in power calculations.69,70 Lessons learned from these earlier misadventures informed the current paradigm of targeted therapy development: (1) identification of a highly specific and potent inhibitor through high-throughput screening; (2) establishment of maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) in unselected patients; (3) confirmation of RP2D in selected tumor types enriched for target of interest; and (4) confirmatory study against standard comparator to seek regulatory approval.
Vemurafenib and dabrafenib were evaluated in this tiered fashion in phase 1 dose-finding studies comprising unselected patients, followed by phase 2 studies in advanced BRAF V600E–mutated melanoma. Both were subsequently evaluated in randomized phase 3 trials (vemurafenib, BRIM-38; dabrafenib, BREAK-310) that compared them with dacarbazine (1000 mg/m2 intravenously every 3 weeks) in the treatment of advanced BRAF V600E–mutated melanoma. Response kinetics for both agents were remarkably similar: single-agent BRAF inhibitors resulted in rapid (time to response 2–3 months), profound (approximately 50% objective responses) reductions in tumor burden that lasted 6 to 7 months. Adverse events common to both agents included rash, fatigue, and arthralgia, although clinically significant photosensitivity was more common with vemurafenib and clinically significant pyrexia was more common with dabrafenib. Class-specific adverse events included the development of cutaneous squamous-cell carcinomas and keratoacanthomas secondary to paradoxical activation of MAPK pathway signaling either through activating mutations in HRAS or mutations or amplifications in receptor tyrosine kinases upstream of BRAF, resulting in elevated levels of RAS–guanosine triphosphate complexes.71 Results of these studies resulted in regulatory approval of single-agent BRAF inhibitors for the treatment of BRAF V600E (and later V600K)–mutated melanoma (vemurafenib in 2011; dabrafenib in 2013). Details regarding trial populations, study interventions, efficacy, and adverse events are summarized in Table 2.

Responses to BRAF inhibitors are typically profound but temporary. Mechanisms of acquired resistance are diverse and include reactivation of MAPK pathway–dependent signaling (RAS activation or increased RAF expression), and development of MAPK pathway–independent signaling (COT overexpression; increased PI3K or AKT signaling) that permits bypass of inhibited BRAF signaling within the MAPK pathway.72–76 These findings suggested that upfront inhibition of both MEK and mutant BRAF may produce more durable responses than BRAF inhibition alone. Three pivotal phase 3 studies established the superiority of combination BRAF and MEK inhibition over BRAF inhibition alone: COMBI-d11 (dabrafenib/trametinib versus dabrafenib/placebo), COMBI-v12 (dabrafenib/trametinib versus vemurafenib), and coBRIM13 (vemurafenib/cobimetinib versus vemurafenib/placebo). As expected, compared to BRAF inhibitor monotherapy, combination BRAF and MEK inhibition produced greater responses and improved progression-free and overall survival (Table 2). Interestingly, the rate of cutaneous squamous-cell carcinomas was much lower with combination therapy, reflecting the more profound degree of MAPK pathway inhibition achieved with combination BRAF and MEK inhibition. Based on these results, FDA approval was granted for both dabrafenib/trametinib and vemurafenib/cobimetinib combinations in 2015. Although the dabrafenib/trametinib combination was only approved in 2015, trametinib had independently gained FDA approval in 2013 for the treatment of BRAF V600E/K–mutated melanoma on the basis of the phase 3 METRIC study.77
Encorafenib (LGX818) and binimetinib (MEK162, ARRY-162, ARRY-438162) are new BRAF and MEK inhibitors currently being evaluated in clinical trials. Encorafenib/binimetinib combination was first evaluated in a phase 3 study (COLUMBUS) that compared it with vemurafenib monotherapy in BRAF-mutant melanoma.78 Unsurprisingly, encorafenib/binimetinib combination produced greater and more durable responses compared to vemurafenib monotherapy. The median progression-free survival of the encorafenib/binimetinib combination (14.9 months) was greater than vemurafenib monotherapy (7.3 months) in this study, and intriguingly greater than that seen with vemurafenib/cobimetinib (coBRIM 9.9 months) and dabrafenib/trametinib (COMBI-d 9.3 months; COMBI-v 11.4 months). Of note, although encorafenib has an IC50 midway between dabrafenib and vemurafenib in cell-free assays (0.8 nM dabrafenib, 4 nM encorafenib, and 31 nM vemurafenib), it has an extremely slower off-rate from BRAF V600E, which results in significantly greater target inhibition in cells following drug wash-out.79 This may account for the significantly greater clinical benefit seen with encorafenib/binimetinib in clinical trials. Final study data are eagerly awaited. Regulatory approval has been sought, and is pending at this time.
Binimetinib has been compared to dacarbazine in a phase 3 study (NEMO) of patients with NRAS-mutant melanoma, most of whom had been previously treated with immunotherapy.80 Response rates were low in both arms, although slightly greater with binimetinib than dacarbazine (15% versus 9%), commensurate with a modest improvement in progression-free survival. FDA approval has been sought and remains pending at this time.
KIT INHIBITION SINGLY AND IN COMBINATION
The KIT receptor protein tyrosine kinase is a transmembrane protein consisting of extracellular and intracellular domains. Activating KIT mutations occur in 2% to 8% of all melanoma patients and may be found in all melanoma subtypes but are commonest in acral melanomas (10%–20%) and mucosal melanomas (15%–20%). Activating KIT mutations primarily occur in exons 11 and 13, which code for the juxtamembrane and kinase domains, respectively.5,81–83
Imatinib mesylate is a tyrosine kinase inhibitor of the 2-phenyl amino pyrimidine class that occupies the tyrosine kinase active site with resultant blocking of tyrosine kinase activity. Imatinib mesylate is known to block KIT and has been extensively studied in patients with gastrointestinal stromal tumors (GIST), 80% of whom harbor KIT mutations, in both the adjuvant and the metastatic settings. In melanoma, imatinib mesylate was studied in a Chinese open-label, phase 2 study of imatinib mesylate monotherapy in metastatic melanoma harboring KIT mutation or amplification; 25% of the study patients had mucosal disease and the rest had cutaneous disease, with acral involvement in 50% of all patients.84 Overall response rate was 23%, while 51% of patients remained alive at 1 year with no differences in response rate and/or survival being noted between patients with either KIT mutations or amplifications. In a separate study of imatinib mesylate at 400 mg daily or 400 mg twice daily in Caucasian patients with KIT-mutated/amplified melanoma, similar response and survival rates were reported, although patients with KIT mutations did nonsignificantly better than those with KIT amplifications.85
Other novel studies evaluating KIT inhibitors include KIT inhibition in combination with the VEGF inhibitor bevacizumab and a study of selective BCR-ABL kinase inhibitor nilotinib in imatinib-resistant melanoma. In the former phase 1/2 study, Flaherty and colleagues studied imatinib 800 mg daily and bevacizumab at 10 mg/kg every 2 weeks in 63 patients with advanced tumors, including 23 with metastatic melanoma. Although the combination was relatively nontoxic, no significant efficacy signal was seen and further accrual to the phase 2 portion was halted after the first stage was completed.86 Nilotinib is a BCR-ABL1 tyrosine kinase inhibitor intelligently designed based on the structure of the ABL-imatinib complex that is 10 to 30 times more potent than imatinib in inhibiting BCR-ABL1 tyrosine kinase activity. Nilotinib is approved for the treatment of imatinib-resistant chronic myelogenous leukemia (CML), with reported efficacy in patients with central nervous system (CNS) involvement.87,88 Nilotinib has been studied in a single study of KIT-mutated/amplified melanoma that included patients with imatinib-resistance and those with treated CNS disease. Nilotinib appeared to be active in imatinib-resistant melanoma, although no responses were seen in the CNS disease cohort.89 Overall, the response rates observed with KIT inhibition in melanoma are much lower than those observed in CML and GIST.
CONCLUSION AND FUTURE DIRECTIONS
Prior to 2011, the only approved agents for the treatment of advanced melanoma were dacarbazine and high-dose interleukin-2. Since 2011, drug approvals in melanoma have proceeded at a frenetic pace unmatched in any other disease. The primary events underlying this are advances in our understanding of the gene mutation landscape driving melanoma tumorigenesis, accompanied by insights into the means by which tumors circumvent the induction of effective anti-tumor T-cell responses. These insights have resulted in the development of inhibitors targeting MAPK pathway kinases BRAF, MEK, and NRAS), KIT, and regulatory immune checkpoints (CTLA-4 and PD-1). Although BRAF/MEK inhibition results in profound reductions and even occasional complete responses in patients, these responses are typically short lived, rarely lasting more than 9 to 11 months; the encorafenib/binimetinib combination may improve that duration marginally. However, the signature therapeutic advance in melanoma of the past decade is immunotherapy, particularly the development of inhibitors of CTLA-4 and PD-1 immune checkpoints. With these agents, significant proportions of treated patients remain free of progression off-therapy (ipilimumab 23%; nivolumab 34%; pembrolizumab 35%; ipilimumab/nivolumab 64%), and some patients can be successfully re-induced after delayed progression. Separately, the high response rates observed with the use of KIT inhibitors in CML and GIST have not been observed in KIT mutated/amplified melanoma and development of agents in this space has been limited. The challenges ahead center around identifying predictive biomarkers and circumventing primary or acquired resistance, with the eventual goal of producing durable remissions in the majority of treated patients.
Our improved understanding of the mechanisms of acquired resistance to BRAF/MEK inhibitors suggests that anti-tumor activity may be achieved by targeting multiple pathways, possibly with combination regimens comprising other inhibitors and/or immunotherapy. Preclinical data supports the use of combination strategies targeting both ERK and PI3K/mTOR to circumvent acquired resistance.90 Ongoing studies are evaluating combinations with biguanides (metformin: NCT02143050 and NCT01638676; phenformin: NCT03026517), HSP90 inhibitors (XL888: NCT02721459; AT13387: NCT02097225), and decitabine (NCT01876641).
One complexity affecting management of resistance in the targeted therapy landscape remains tumor heterogeneity, particularly intra- and intertumoral heterogeneity, which may explain the apparent contradiction between continued efficacy of BRAF inhibitors in BRAF-resistant tumors and preclinical data predicting slower progression of resistant tumors on cessation of BRAF inhibitors.91–94 These data provide a rationale to investigate intermittent dosing regimens with BRAF/MEK inhibitors; several studies exploring this approach are ongoing (NCT01894672 and NCT02583516).
Given the specificity, adaptability, and memory response associated with immunotherapy, it is likely that these agents will be used to treat the majority of patients regardless of mutational status. Hence, identifying predictive biomarkers of response to immune checkpoint inhibitors is vital. The presence of CD8+ T-cell infiltrate and IFN-γ gene signature, which indicate an “inflamed” tumor microenvironment, are highly predictive of clinical benefit from PD-1 inhibitors.95,96 However, not all PD-1 responders have “inflamed” tumor microenvironments, and not all patients with an “inflamed” tumor microenvironment respond to immune checkpoint inhibitors. The complexity of the immune system is reflected in the multiple non-redundant immunologic pathways, both positive and negative, with checkpoints and ligands that emerge dynamically in response to treatment. Given the dynamic nature of the immune response, it is unlikely that any single immunologic biomarker identified pre-treatment will be completely predictive. Rather, the complexity of the biomarker approach must match the complexity of the immune response elicited, and will likely incorporate multifarious elements including CD8+ T-cell infiltrate, IFN-γ gene signature, and additional elements including microbiome, genetic polymorphisms, and tumor mutation load. The goal is to use multiple markers to guide development of combinations and then, depending on initial response, to examine tumors for alterations to guide decisions about additional treatment(s) to improve responses, with the eventual goal being durable clinical responses for all patients.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30.
- Guy GP, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982-2030. MMWR Morb Mortal Wkly Rep 2015;64:591–6.
- Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer 2009;115:4176–85.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–47.
- Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006;24:4340–6.
- Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251–63.
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681–96.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16.
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323–32.
- Hauschild A, Grob J-J, Demidov LV, et al. DaBRAFenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358–65.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–88.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined daBRAFenib and trametinib. N Engl J Med 2015;372:30–9.
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867–76.
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275–80.
- Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014;515:577–81.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–26.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54.
- Weber JS, Angelo SP D’, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–44.
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34.
- Eggermont AMM. Therapeutic vaccines in solid tumours: can they be harmful? Eur J Cancer 2009;45:2087–90.
- Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med 2012;4:127ps8.
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451–8.
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–8.
- Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 1992;356:607–9.
- Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol 1998;18:389–418.
- Sharpe AH, Abbas AK. T-cell costimulation--biology, therapeutic potential, and challenges. N Engl J Med 2006;355:973–5.
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001;19:565–94.
- Collins AV, Brodie DW, Gilbert RJC, et al. The interaction properties of costimulatory molecules revisited. Immunity 2002;17:201–10.
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459–65.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–6.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002;169:5538–45.
- Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun 2003;307:672–7.
- Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004;173:945–54.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543–53.
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009;229:114–25.
- Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 2013;1291:1–13.
- Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008;26:5950–6.
- Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008;105:3005–10.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155–64.
- Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94.
- Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014;2:846–56.
- Poole RM. Pembrolizumab: first global approval. Drugs 2014;74:1973–81.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–30.
- Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015;21:4286–93.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41.
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am 2009;23:529–45, ix.
- Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 2003;63:756–9.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54.
- Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012;44:1006–14.
- Rubinstein JC, Sznol M, Pavlick AC, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med 2010;8:67.
- Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS ONE 2012;7:e35309.
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010;464:431–5.
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010;464:427–30.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncology 2015;1:359–68.
- Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother 2012;35:66–72.
- Johnson DB, Lovly CM, Flavin M, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res 2015;3:288–95.
- Johnson DB, Frampton GM, Rioth MJ, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res 2016;4:959–67.
- Kiuru M, Busam KJ. The NF1 gene in tumor syndromes and melanoma. Lab Invest 2017;97:146–57.
- Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res 2014;74:2340–50.
- Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol 2005;23:5281–93.
- Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol 2004;22:4456–62.
- Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res 2012;18:555–67.
- Davar D, Kirkwood JM. CCR 20th anniversary commentary: MAPK/ERK pathway inhibition in melanoma-kinase inhibition redux. Clin Cancer Res 2015;21:5412–4.
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012;366:207–15.
- Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010;468:968–72.
- Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010;468:973–7.
- Shi H, Hong A, Kong X, et al. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discov 2014;4:69–79.
- Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4:80–93.
- Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 2014;4:94–109.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107–14.
- Dummer R, Ascierto PA, Gogas HJ, et al. Results of COLUMBUS Part 1: a phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in BRAF-mutant melanoma. Presented at Society for Melanoma Research 2016 Congress. November 6-9, 2016. Boston (MA).
- Adelmann CH, Ching G, Du L, et al. Comparative profiles of BRAF inhibitors: the paradox index as a predictor of clinical toxicity. Oncotarget 2016;7:30453–60.
- Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:435–45.
- Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol 2005;36:486–93.
- Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res 2008;14:6821–8.
- Handolias D, Salemi R, Murray W, et al. Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment Cell Melanoma Res 2010;23:210–5.
- Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011;29:2904–9.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 2013;31:3182–90.
- Flaherty KT, Hamilton BK, Rosen MA, et al. Phase I/II trial of imatinib and bevacizumab in patients with advanced melanoma and other advanced cancers. Oncologist 2015;20:952–9.
- Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia 2013;27:107–12.
- Reinwald M, Schleyer E, Kiewe P, et al. Efficacy and pharmacologic data of second-generation tyrosine kinase inhibitor nilotinib in BCR-ABL-positive leukemia patients with central nervous system relapse after allogeneic stem cell transplantation. Biomed Res Int 2014;2014:637059.
- Carvajal RD, Lawrence DP, Weber JS, et al. Phase II study of nilotinib in melanoma harboring KIT alterations following progression to prior KIT inhibition. Clin Cancer Res 2015;21:2289–96.
- Carlino MS, Todd JR, Gowrishankar K, et al. Differential activity of MEK and ERK inhibitors in BRAF inhibitor resistant melanoma. Mol Oncol 2014;8:544–54.
- Carlino MS, Gowrishankar K, Saunders CAB, et al. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther 2013;12:1332–42.
- Chan MMK, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer 2014;120:3142–53.
- Thakur M Das, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013;494:251–5.
- Thakur M Das, Stuart DD. Molecular pathways: response and resistance to BRAF and MEK inhibitors in BRAF(V600E) tumors. Clin Cancer Res 2014;20:1074–80.
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71.
- Ayers M, Lunceford J, Nebozhyn M, et al. Relationship between immune gene signatures and clinical response to PD-1 blockade with pembrolizumab (MK-3475) in patients with advanced solid tumors. J Immunotherapy Cancer 2015;3(Suppl 2):P80.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30.
- Guy GP, Thomas CC, Thompson T, et al. Vital signs: melanoma incidence and mortality trends and projections - United States, 1982-2030. MMWR Morb Mortal Wkly Rep 2015;64:591–6.
- Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer 2009;115:4176–85.
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135–47.
- Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006;24:4340–6.
- Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251–63.
- Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 2015;161:1681–96.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507–16.
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323–32.
- Hauschild A, Grob J-J, Demidov LV, et al. DaBRAFenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358–65.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877–88.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined daBRAFenib and trametinib. N Engl J Med 2015;372:30–9.
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867–76.
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275–80.
- Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014;515:577–81.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23.
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517–26.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–54.
- Weber JS, Angelo SP D’, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375–84.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134–44.
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–32.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–33.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34.
- Eggermont AMM. Therapeutic vaccines in solid tumours: can they be harmful? Eur J Cancer 2009;45:2087–90.
- Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med 2012;4:127ps8.
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451–8.
- Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–8.
- Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 1992;356:607–9.
- Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol 1998;18:389–418.
- Sharpe AH, Abbas AK. T-cell costimulation--biology, therapeutic potential, and challenges. N Engl J Med 2006;355:973–5.
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol 2001;19:565–94.
- Collins AV, Brodie DW, Gilbert RJC, et al. The interaction properties of costimulatory molecules revisited. Immunity 2002;17:201–10.
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459–65.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–6.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002;169:5538–45.
- Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun 2003;307:672–7.
- Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 2004;173:945–54.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543–53.
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009;229:114–25.
- Wolchok JD, Hodi FS, Weber JS, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci 2013;1291:1–13.
- Weber JS, O’Day S, Urba W, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol 2008;26:5950–6.
- Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008;105:3005–10.
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155–64.
- Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015;33:1889–94.
- Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014;2:846–56.
- Poole RM. Pembrolizumab: first global approval. Drugs 2014;74:1973–81.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–30.
- Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. Clin Cancer Res 2015;21:4286–93.
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31–41.
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am 2009;23:529–45, ix.
- Satyamoorthy K, Li G, Gerrero MR, et al. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 2003;63:756–9.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–54.
- Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012;44:1006–14.
- Rubinstein JC, Sznol M, Pavlick AC, et al. Incidence of the V600K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med 2010;8:67.
- Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS ONE 2012;7:e35309.
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010;464:431–5.
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010;464:427–30.
- Thomas NE, Edmiston SN, Alexander A, et al. Association between NRAS and BRAF mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncology 2015;1:359–68.
- Joseph RW, Sullivan RJ, Harrell R, et al. Correlation of NRAS mutations with clinical response to high-dose IL-2 in patients with advanced melanoma. J Immunother 2012;35:66–72.
- Johnson DB, Lovly CM, Flavin M, et al. Impact of NRAS mutations for patients with advanced melanoma treated with immune therapies. Cancer Immunol Res 2015;3:288–95.
- Johnson DB, Frampton GM, Rioth MJ, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res 2016;4:959–67.
- Kiuru M, Busam KJ. The NF1 gene in tumor syndromes and melanoma. Lab Invest 2017;97:146–57.
- Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res 2014;74:2340–50.
- Lorusso PM, Adjei AA, Varterasian M, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol 2005;23:5281–93.
- Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol 2004;22:4456–62.
- Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res 2012;18:555–67.
- Davar D, Kirkwood JM. CCR 20th anniversary commentary: MAPK/ERK pathway inhibition in melanoma-kinase inhibition redux. Clin Cancer Res 2015;21:5412–4.
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 2012;366:207–15.
- Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010;468:968–72.
- Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010;468:973–7.
- Shi H, Hong A, Kong X, et al. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discov 2014;4:69–79.
- Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4:80–93.
- Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 2014;4:94–109.
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107–14.
- Dummer R, Ascierto PA, Gogas HJ, et al. Results of COLUMBUS Part 1: a phase 3 trial of encorafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in BRAF-mutant melanoma. Presented at Society for Melanoma Research 2016 Congress. November 6-9, 2016. Boston (MA).
- Adelmann CH, Ching G, Du L, et al. Comparative profiles of BRAF inhibitors: the paradox index as a predictor of clinical toxicity. Oncotarget 2016;7:30453–60.
- Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2017;18:435–45.
- Willmore-Payne C, Holden JA, Tripp S, Layfield LJ. Human malignant melanoma: detection of BRAF- and c-kit-activating mutations by high-resolution amplicon melting analysis. Hum Pathol 2005;36:486–93.
- Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res 2008;14:6821–8.
- Handolias D, Salemi R, Murray W, et al. Mutations in KIT occur at low frequency in melanomas arising from anatomical sites associated with chronic and intermittent sun exposure. Pigment Cell Melanoma Res 2010;23:210–5.
- Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011;29:2904–9.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 2013;31:3182–90.
- Flaherty KT, Hamilton BK, Rosen MA, et al. Phase I/II trial of imatinib and bevacizumab in patients with advanced melanoma and other advanced cancers. Oncologist 2015;20:952–9.
- Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia 2013;27:107–12.
- Reinwald M, Schleyer E, Kiewe P, et al. Efficacy and pharmacologic data of second-generation tyrosine kinase inhibitor nilotinib in BCR-ABL-positive leukemia patients with central nervous system relapse after allogeneic stem cell transplantation. Biomed Res Int 2014;2014:637059.
- Carvajal RD, Lawrence DP, Weber JS, et al. Phase II study of nilotinib in melanoma harboring KIT alterations following progression to prior KIT inhibition. Clin Cancer Res 2015;21:2289–96.
- Carlino MS, Todd JR, Gowrishankar K, et al. Differential activity of MEK and ERK inhibitors in BRAF inhibitor resistant melanoma. Mol Oncol 2014;8:544–54.
- Carlino MS, Gowrishankar K, Saunders CAB, et al. Antiproliferative effects of continued mitogen-activated protein kinase pathway inhibition following acquired resistance to BRAF and/or MEK inhibition in melanoma. Mol Cancer Ther 2013;12:1332–42.
- Chan MMK, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer 2014;120:3142–53.
- Thakur M Das, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013;494:251–5.
- Thakur M Das, Stuart DD. Molecular pathways: response and resistance to BRAF and MEK inhibitors in BRAF(V600E) tumors. Clin Cancer Res 2014;20:1074–80.
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71.
- Ayers M, Lunceford J, Nebozhyn M, et al. Relationship between immune gene signatures and clinical response to PD-1 blockade with pembrolizumab (MK-3475) in patients with advanced solid tumors. J Immunotherapy Cancer 2015;3(Suppl 2):P80.
VIDEO: Cancer immunotherapies activate rheumatologic adverse effects
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
MADRID – The introduction of immune checkpoint inhibitor drugs has “been great for cancer but bad for rheumatology.”
That’s the gist of the immunologic adverse effect fallout from the immunomodulatory revolution that’s recently swept oncology, Leonard Calabrese, DO, said in a video interview during the European Congress of Rheumatology.
Results from a recent survey of U.S. rheumatologists run by Dr. Calabrese and his associates showed that “more than a quarter” now have seen at least one patient who experienced activation of a rheumatologic disease after starting treatment with an immune checkpoint inhibitor, said Dr. Calabrese, head of the section of clinical immunology at the Cleveland Clinic in Ohio.
Unlike most other immunological adverse effects caused by immune checkpoint inhibitors, the rheumatologic complications usually don’t resolve when treatment stops, he added.
These adverse effects represent a new wrinkle for the practice of rheumatology and are now something that clinicians must familiarize themselves with, Dr. Calabrese advised.
Dr. Calabrese reported that he is a consultant to Bristol-Myers Squibb.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE EULAR 2017 CONGRESS
Pembrolizumab + rituximab boost response rates in relapsed follicular lymphoma
LUGANO, SWITZERLAND – A novel combination of the anti-programmed death 1 (PD-1) checkpoint inhibitor pembrolizumab (Keytruda) and the anti-CD20 monoclonal antibody rituximab was associated with a high overall response rate (ORR) in patients with relapsed follicular lymphoma in a phase II clinical trial.
Among 20 patients evaluable for efficacy, the overall response rate to the combination was 65%, including 50% complete responses (CR) reported Loretta J. Nastoupil, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Follicular lymphoma is probably one of the best examples of targeting the immune system and also one of the earliest examples. Over the last few years we’ve learned a great deal about the different mechanisms of not only negative impact on infiltrating T cells, but also immune escape and T-cell exhaustion,” she said at the International Conference on Malignant Lymphoma.
Although biopsies of follicular lymphoma tumors have demonstrated infiltration of anti-tumor T cells, these cells are typically impeded by immune checkpoints, including PD-1 and its ligand (PD-L1).
The use of anti-PD-1 checkpoint inhibitors such as pembrolizumab has been shown to enhance the function of antitumor T cells in follicular lymphoma, and blocking PD-1 on natural killer cells enhances the antibody-dependent cell-mediated cytotoxicity of the natural killer cells, she said.
Because rituximab, a mainstay of therapy for non-Hodgkin lymphomas, induces antibody-dependent cell-mediated cytotoxicity, the investigators reasoned that combining it with pembrolizumab would simultaneously and synergistically stimulate activation of innate and adaptive immunity.
They designed a phase II, single-arm study in 30 patients with relapsed follicular lymphoma following one or more prior lines of therapy. The patients also had to have rituximab-sensitive disease, defined as a complete response (CR) or partial response lasting for at least 6 months following the most recent rituximab-containing therapy.
The patients were treated with rituximab 375 mg/m2 IV on days 1, 8, 15, and 22 of cycle 1, and pembrolizumab 200 mg IV every 3 weeks for up to 16 cycles starting on day 2 of cycle 1.
The investigators expected that the combination would improve ORR, the primary endpoint, to at least 60%, compared with 40% for historical controls treated with repeat courses of rituximab.
At the data cutoff for the interim analysis, 32 patients had been enrolled, 30 were evaluable for safety, and 20 for efficacy after a median follow-up of 8.2 months.
Among the 20 patients (median age 64) in the efficacy analysis, 10 (50%) had a CR, and 3 (15%) had a partial response, for an ORR of 65%. Three additional patients had stable disease, and four had disease progression as best responses.
Among the patients with CRs, the duration of response ranged from nearly 275 days to more than 600 days.
“This does appear to be durable, and it is time dependent in terms of response. We did see early response, and we also saw deepening of response over time,” Dr. Nastoupil said.
Four patients were discontinued from the study because of immune-related adverse events. All four patients had achieved a CR at the time of study removal, and all four have ongoing CRs.
Among the 30 patients evaluable for safety, there were no grade 4 adverse events, no deaths, and few grade 3 events. Most events were grade 1 or 2, and included fatigue, eye pain/blurred vision/watery eye, nausea and vomiting, diarrhea dyspnea, rash, cough, and lymphopenia.
The investigators also looked at potential biomarkers for response, including PD-L1 expression in tumors prior to treatment. They found in samples from three patients who went on to achieve CRs that PD-L1 expression in tumor cells was low, ranging from 0% to 8%, suggesting that PD-L1 expression may not be necessary to generate a response with the combination.
They then looked at the association between CD8-positive T effector cells and responses in 12 patients, and found that patients with higher levels of expression had better ORR and CR rates.
“These interim results warrant further investigation of this combination in follicular lymphoma, and an expansion to include patients with refractory follicular lymphoma is planned,” Dr. Nastoupil concluded.
The Leukemia & Lymphoma Society supported the study. Dr Nastoupil has disclosed consulting fees from Celgene and contracted research for Abbvie, Janssen, and TG Therapeutics.
LUGANO, SWITZERLAND – A novel combination of the anti-programmed death 1 (PD-1) checkpoint inhibitor pembrolizumab (Keytruda) and the anti-CD20 monoclonal antibody rituximab was associated with a high overall response rate (ORR) in patients with relapsed follicular lymphoma in a phase II clinical trial.
Among 20 patients evaluable for efficacy, the overall response rate to the combination was 65%, including 50% complete responses (CR) reported Loretta J. Nastoupil, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Follicular lymphoma is probably one of the best examples of targeting the immune system and also one of the earliest examples. Over the last few years we’ve learned a great deal about the different mechanisms of not only negative impact on infiltrating T cells, but also immune escape and T-cell exhaustion,” she said at the International Conference on Malignant Lymphoma.
Although biopsies of follicular lymphoma tumors have demonstrated infiltration of anti-tumor T cells, these cells are typically impeded by immune checkpoints, including PD-1 and its ligand (PD-L1).
The use of anti-PD-1 checkpoint inhibitors such as pembrolizumab has been shown to enhance the function of antitumor T cells in follicular lymphoma, and blocking PD-1 on natural killer cells enhances the antibody-dependent cell-mediated cytotoxicity of the natural killer cells, she said.
Because rituximab, a mainstay of therapy for non-Hodgkin lymphomas, induces antibody-dependent cell-mediated cytotoxicity, the investigators reasoned that combining it with pembrolizumab would simultaneously and synergistically stimulate activation of innate and adaptive immunity.
They designed a phase II, single-arm study in 30 patients with relapsed follicular lymphoma following one or more prior lines of therapy. The patients also had to have rituximab-sensitive disease, defined as a complete response (CR) or partial response lasting for at least 6 months following the most recent rituximab-containing therapy.
The patients were treated with rituximab 375 mg/m2 IV on days 1, 8, 15, and 22 of cycle 1, and pembrolizumab 200 mg IV every 3 weeks for up to 16 cycles starting on day 2 of cycle 1.
The investigators expected that the combination would improve ORR, the primary endpoint, to at least 60%, compared with 40% for historical controls treated with repeat courses of rituximab.
At the data cutoff for the interim analysis, 32 patients had been enrolled, 30 were evaluable for safety, and 20 for efficacy after a median follow-up of 8.2 months.
Among the 20 patients (median age 64) in the efficacy analysis, 10 (50%) had a CR, and 3 (15%) had a partial response, for an ORR of 65%. Three additional patients had stable disease, and four had disease progression as best responses.
Among the patients with CRs, the duration of response ranged from nearly 275 days to more than 600 days.
“This does appear to be durable, and it is time dependent in terms of response. We did see early response, and we also saw deepening of response over time,” Dr. Nastoupil said.
Four patients were discontinued from the study because of immune-related adverse events. All four patients had achieved a CR at the time of study removal, and all four have ongoing CRs.
Among the 30 patients evaluable for safety, there were no grade 4 adverse events, no deaths, and few grade 3 events. Most events were grade 1 or 2, and included fatigue, eye pain/blurred vision/watery eye, nausea and vomiting, diarrhea dyspnea, rash, cough, and lymphopenia.
The investigators also looked at potential biomarkers for response, including PD-L1 expression in tumors prior to treatment. They found in samples from three patients who went on to achieve CRs that PD-L1 expression in tumor cells was low, ranging from 0% to 8%, suggesting that PD-L1 expression may not be necessary to generate a response with the combination.
They then looked at the association between CD8-positive T effector cells and responses in 12 patients, and found that patients with higher levels of expression had better ORR and CR rates.
“These interim results warrant further investigation of this combination in follicular lymphoma, and an expansion to include patients with refractory follicular lymphoma is planned,” Dr. Nastoupil concluded.
The Leukemia & Lymphoma Society supported the study. Dr Nastoupil has disclosed consulting fees from Celgene and contracted research for Abbvie, Janssen, and TG Therapeutics.
LUGANO, SWITZERLAND – A novel combination of the anti-programmed death 1 (PD-1) checkpoint inhibitor pembrolizumab (Keytruda) and the anti-CD20 monoclonal antibody rituximab was associated with a high overall response rate (ORR) in patients with relapsed follicular lymphoma in a phase II clinical trial.
Among 20 patients evaluable for efficacy, the overall response rate to the combination was 65%, including 50% complete responses (CR) reported Loretta J. Nastoupil, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“Follicular lymphoma is probably one of the best examples of targeting the immune system and also one of the earliest examples. Over the last few years we’ve learned a great deal about the different mechanisms of not only negative impact on infiltrating T cells, but also immune escape and T-cell exhaustion,” she said at the International Conference on Malignant Lymphoma.
Although biopsies of follicular lymphoma tumors have demonstrated infiltration of anti-tumor T cells, these cells are typically impeded by immune checkpoints, including PD-1 and its ligand (PD-L1).
The use of anti-PD-1 checkpoint inhibitors such as pembrolizumab has been shown to enhance the function of antitumor T cells in follicular lymphoma, and blocking PD-1 on natural killer cells enhances the antibody-dependent cell-mediated cytotoxicity of the natural killer cells, she said.
Because rituximab, a mainstay of therapy for non-Hodgkin lymphomas, induces antibody-dependent cell-mediated cytotoxicity, the investigators reasoned that combining it with pembrolizumab would simultaneously and synergistically stimulate activation of innate and adaptive immunity.
They designed a phase II, single-arm study in 30 patients with relapsed follicular lymphoma following one or more prior lines of therapy. The patients also had to have rituximab-sensitive disease, defined as a complete response (CR) or partial response lasting for at least 6 months following the most recent rituximab-containing therapy.
The patients were treated with rituximab 375 mg/m2 IV on days 1, 8, 15, and 22 of cycle 1, and pembrolizumab 200 mg IV every 3 weeks for up to 16 cycles starting on day 2 of cycle 1.
The investigators expected that the combination would improve ORR, the primary endpoint, to at least 60%, compared with 40% for historical controls treated with repeat courses of rituximab.
At the data cutoff for the interim analysis, 32 patients had been enrolled, 30 were evaluable for safety, and 20 for efficacy after a median follow-up of 8.2 months.
Among the 20 patients (median age 64) in the efficacy analysis, 10 (50%) had a CR, and 3 (15%) had a partial response, for an ORR of 65%. Three additional patients had stable disease, and four had disease progression as best responses.
Among the patients with CRs, the duration of response ranged from nearly 275 days to more than 600 days.
“This does appear to be durable, and it is time dependent in terms of response. We did see early response, and we also saw deepening of response over time,” Dr. Nastoupil said.
Four patients were discontinued from the study because of immune-related adverse events. All four patients had achieved a CR at the time of study removal, and all four have ongoing CRs.
Among the 30 patients evaluable for safety, there were no grade 4 adverse events, no deaths, and few grade 3 events. Most events were grade 1 or 2, and included fatigue, eye pain/blurred vision/watery eye, nausea and vomiting, diarrhea dyspnea, rash, cough, and lymphopenia.
The investigators also looked at potential biomarkers for response, including PD-L1 expression in tumors prior to treatment. They found in samples from three patients who went on to achieve CRs that PD-L1 expression in tumor cells was low, ranging from 0% to 8%, suggesting that PD-L1 expression may not be necessary to generate a response with the combination.
They then looked at the association between CD8-positive T effector cells and responses in 12 patients, and found that patients with higher levels of expression had better ORR and CR rates.
“These interim results warrant further investigation of this combination in follicular lymphoma, and an expansion to include patients with refractory follicular lymphoma is planned,” Dr. Nastoupil concluded.
The Leukemia & Lymphoma Society supported the study. Dr Nastoupil has disclosed consulting fees from Celgene and contracted research for Abbvie, Janssen, and TG Therapeutics.
AT 14-ICML
Key clinical point: The combination of pembrolizumab and rituximab increased responses compared with repeat rituximab in patients with relapsed follicular lymphoma.
Major finding: The overall response rate with the combination was 65%, including 50% complete responses.
Data source: Open-label, phase II, single-arm study in 32 patients with relapsed follicular lymphoma (20 for efficacy, 30 for safety analysis).
Disclosures: The Leukemia & Lymphoma Society supported the study. Dr Nastoupil has disclosed consulting fees from Celgene and contracted research for Abbvie, Janssen, and TG Therapeutics.
Studies provide insight into link between cancer immunotherapy and autoimmune disease
MADRID – Rheumatologists all over the world are beginning to find that the new class of anticancer immune checkpoint inhibitor therapies have the potential to elicit symptoms of rheumatoid arthritis (RA) and other rheumatic diseases in patients with no previous history of them, and two reports from the European Congress of Rheumatology provide typical examples.
These immune checkpoint inhibitor (ICI) agents, which include ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda), target regulatory pathways in T cells to boost antitumor immune responses, leading to improved survival for many cancer patients, but the induction of rheumatic disease can sometimes lead to the suspension of the agents, according to investigators.
“This phenomenon was unknown to me and my group before [February 2016], when we started noting referrals of patients from oncology,” Dr. Calabrese said. “We were seeing symptoms of everything from Sjögren’s syndrome to inflammatory arthritis and myositis in patients being treated with these drugs for their cancer.” The same year, Dr. Calabrese and her team began coordinating an ongoing study to assess these patients.
Dr. Calabrese said that the cohort has shown so far that patients who develop autoimmune disease after immune checkpoint inhibitors “require much higher doses – of steroids in particular – to treat their symptoms,” and this can all too often result in being taken out of a clinical trial or having to stop cancer treatment.
Most of the patients in the cohort were treated with steroids only, while three patients received biologic agents, and four received methotrexate or antimalarials.
Dr. Calabrese said that the serology results were available for all the patients in the cohort and “were largely unremarkable.”
She noted that the rheumatic symptoms did not always resolve after pausing or stopping the cancer treatment. “We have some patients that have been off their checkpoint inhibitors for over a year and still have symptoms, so it’s looking like it might be a more long-term effect,” she said.
“In my unit, we also manage patients with myeloma, and I developed a weekly consultation with a cancer center,” Dr. Belkhir said. In 2015, she saw her first patient with RA and no previous history who had been treated with checkpoint inhibitors. That patient’s symptoms resolved after treatment with nonsteroidal anti-inflammatory drugs alone.
Dr. Belkhir is sharing results from this and five other patients presenting with symptoms of RA after their cancer treatment with immune checkpoint inhibitors, taken from a larger cohort of patients (n = 13) with a spectrum of rheumatic disease–like adverse effects. None of the six patients in this study had a previous clinical history of RA. They manifested their RA symptoms after a median of 1 month on cancer immunotherapy.
Some were able to continue their checkpoint inhibitors and be treated simultaneously for RA with steroids, antimalarials, methotrexate, and NSAIDs, Dr. Belkhir said. None received biologic agents, and each medication strategy, she said, was arrived at in consultation with the treating oncologist.
Dr. Belkhir’s team also looked closely at serology and found all six patients to be at least weakly, and mostly strongly, seropositive for RA. Three patients underwent testing for anticyclic citrullinated protein antibodies prior to starting cancer immunotherapy and two of these three were anti-CCP positive. Now, she said, the oncologists she’s working with are testing for anticyclic citrullinated peptides and rheumatoid factor prior to initiating cancer immunotherapy, so that this relationship is better understood.
“It is possible that antibodies were already present and that the anti-PD1 immunotherapy,” one type of immune checkpoint inhibitor, “acted as a trigger for the disease.” Animal studies have suggested a role for PD1 in the development of autoimmune disease, “but it’s not well investigated,” Dr. Belkhir said.
Dr. Belkhir and Dr. Calabrese both acknowledged that the understanding of checkpoint inhibitor–induced autoimmune disease is in its infancy. Clinical trials largely missed the phenomenon, the researchers said, because the trials were not designed to capture musculoskeletal adverse effects with the same granularity as other serious adverse events.
“This will be a long discussion in the months and the years ahead with oncologists,” Dr. Belkhir said.
Neither Dr. Calabrese nor Dr. Belkhir reported having any relevant conflicts of interest.
MADRID – Rheumatologists all over the world are beginning to find that the new class of anticancer immune checkpoint inhibitor therapies have the potential to elicit symptoms of rheumatoid arthritis (RA) and other rheumatic diseases in patients with no previous history of them, and two reports from the European Congress of Rheumatology provide typical examples.
These immune checkpoint inhibitor (ICI) agents, which include ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda), target regulatory pathways in T cells to boost antitumor immune responses, leading to improved survival for many cancer patients, but the induction of rheumatic disease can sometimes lead to the suspension of the agents, according to investigators.
“This phenomenon was unknown to me and my group before [February 2016], when we started noting referrals of patients from oncology,” Dr. Calabrese said. “We were seeing symptoms of everything from Sjögren’s syndrome to inflammatory arthritis and myositis in patients being treated with these drugs for their cancer.” The same year, Dr. Calabrese and her team began coordinating an ongoing study to assess these patients.
Dr. Calabrese said that the cohort has shown so far that patients who develop autoimmune disease after immune checkpoint inhibitors “require much higher doses – of steroids in particular – to treat their symptoms,” and this can all too often result in being taken out of a clinical trial or having to stop cancer treatment.
Most of the patients in the cohort were treated with steroids only, while three patients received biologic agents, and four received methotrexate or antimalarials.
Dr. Calabrese said that the serology results were available for all the patients in the cohort and “were largely unremarkable.”
She noted that the rheumatic symptoms did not always resolve after pausing or stopping the cancer treatment. “We have some patients that have been off their checkpoint inhibitors for over a year and still have symptoms, so it’s looking like it might be a more long-term effect,” she said.
“In my unit, we also manage patients with myeloma, and I developed a weekly consultation with a cancer center,” Dr. Belkhir said. In 2015, she saw her first patient with RA and no previous history who had been treated with checkpoint inhibitors. That patient’s symptoms resolved after treatment with nonsteroidal anti-inflammatory drugs alone.
Dr. Belkhir is sharing results from this and five other patients presenting with symptoms of RA after their cancer treatment with immune checkpoint inhibitors, taken from a larger cohort of patients (n = 13) with a spectrum of rheumatic disease–like adverse effects. None of the six patients in this study had a previous clinical history of RA. They manifested their RA symptoms after a median of 1 month on cancer immunotherapy.
Some were able to continue their checkpoint inhibitors and be treated simultaneously for RA with steroids, antimalarials, methotrexate, and NSAIDs, Dr. Belkhir said. None received biologic agents, and each medication strategy, she said, was arrived at in consultation with the treating oncologist.
Dr. Belkhir’s team also looked closely at serology and found all six patients to be at least weakly, and mostly strongly, seropositive for RA. Three patients underwent testing for anticyclic citrullinated protein antibodies prior to starting cancer immunotherapy and two of these three were anti-CCP positive. Now, she said, the oncologists she’s working with are testing for anticyclic citrullinated peptides and rheumatoid factor prior to initiating cancer immunotherapy, so that this relationship is better understood.
“It is possible that antibodies were already present and that the anti-PD1 immunotherapy,” one type of immune checkpoint inhibitor, “acted as a trigger for the disease.” Animal studies have suggested a role for PD1 in the development of autoimmune disease, “but it’s not well investigated,” Dr. Belkhir said.
Dr. Belkhir and Dr. Calabrese both acknowledged that the understanding of checkpoint inhibitor–induced autoimmune disease is in its infancy. Clinical trials largely missed the phenomenon, the researchers said, because the trials were not designed to capture musculoskeletal adverse effects with the same granularity as other serious adverse events.
“This will be a long discussion in the months and the years ahead with oncologists,” Dr. Belkhir said.
Neither Dr. Calabrese nor Dr. Belkhir reported having any relevant conflicts of interest.
MADRID – Rheumatologists all over the world are beginning to find that the new class of anticancer immune checkpoint inhibitor therapies have the potential to elicit symptoms of rheumatoid arthritis (RA) and other rheumatic diseases in patients with no previous history of them, and two reports from the European Congress of Rheumatology provide typical examples.
These immune checkpoint inhibitor (ICI) agents, which include ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda), target regulatory pathways in T cells to boost antitumor immune responses, leading to improved survival for many cancer patients, but the induction of rheumatic disease can sometimes lead to the suspension of the agents, according to investigators.
“This phenomenon was unknown to me and my group before [February 2016], when we started noting referrals of patients from oncology,” Dr. Calabrese said. “We were seeing symptoms of everything from Sjögren’s syndrome to inflammatory arthritis and myositis in patients being treated with these drugs for their cancer.” The same year, Dr. Calabrese and her team began coordinating an ongoing study to assess these patients.
Dr. Calabrese said that the cohort has shown so far that patients who develop autoimmune disease after immune checkpoint inhibitors “require much higher doses – of steroids in particular – to treat their symptoms,” and this can all too often result in being taken out of a clinical trial or having to stop cancer treatment.
Most of the patients in the cohort were treated with steroids only, while three patients received biologic agents, and four received methotrexate or antimalarials.
Dr. Calabrese said that the serology results were available for all the patients in the cohort and “were largely unremarkable.”
She noted that the rheumatic symptoms did not always resolve after pausing or stopping the cancer treatment. “We have some patients that have been off their checkpoint inhibitors for over a year and still have symptoms, so it’s looking like it might be a more long-term effect,” she said.
“In my unit, we also manage patients with myeloma, and I developed a weekly consultation with a cancer center,” Dr. Belkhir said. In 2015, she saw her first patient with RA and no previous history who had been treated with checkpoint inhibitors. That patient’s symptoms resolved after treatment with nonsteroidal anti-inflammatory drugs alone.
Dr. Belkhir is sharing results from this and five other patients presenting with symptoms of RA after their cancer treatment with immune checkpoint inhibitors, taken from a larger cohort of patients (n = 13) with a spectrum of rheumatic disease–like adverse effects. None of the six patients in this study had a previous clinical history of RA. They manifested their RA symptoms after a median of 1 month on cancer immunotherapy.
Some were able to continue their checkpoint inhibitors and be treated simultaneously for RA with steroids, antimalarials, methotrexate, and NSAIDs, Dr. Belkhir said. None received biologic agents, and each medication strategy, she said, was arrived at in consultation with the treating oncologist.
Dr. Belkhir’s team also looked closely at serology and found all six patients to be at least weakly, and mostly strongly, seropositive for RA. Three patients underwent testing for anticyclic citrullinated protein antibodies prior to starting cancer immunotherapy and two of these three were anti-CCP positive. Now, she said, the oncologists she’s working with are testing for anticyclic citrullinated peptides and rheumatoid factor prior to initiating cancer immunotherapy, so that this relationship is better understood.
“It is possible that antibodies were already present and that the anti-PD1 immunotherapy,” one type of immune checkpoint inhibitor, “acted as a trigger for the disease.” Animal studies have suggested a role for PD1 in the development of autoimmune disease, “but it’s not well investigated,” Dr. Belkhir said.
Dr. Belkhir and Dr. Calabrese both acknowledged that the understanding of checkpoint inhibitor–induced autoimmune disease is in its infancy. Clinical trials largely missed the phenomenon, the researchers said, because the trials were not designed to capture musculoskeletal adverse effects with the same granularity as other serious adverse events.
“This will be a long discussion in the months and the years ahead with oncologists,” Dr. Belkhir said.
Neither Dr. Calabrese nor Dr. Belkhir reported having any relevant conflicts of interest.
AT THE EULAR 2017 CONGRESS
Key clinical point:
Major finding: Rheumatic symptoms did not always resolve after pausing or stopping the cancer treatment, and some were able to continue their checkpoint inhibitors and be treated simultaneously for RA.
Data source: Two retrospective cohort reviews of patients on immune checkpoint inhibitors.
Disclosures: Neither Dr. Calabrese nor Dr. Belkhir reported having any relevant conflicts of interest.