User login
Flu activity levels down, but outpatient visits highest since 1998-99
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.
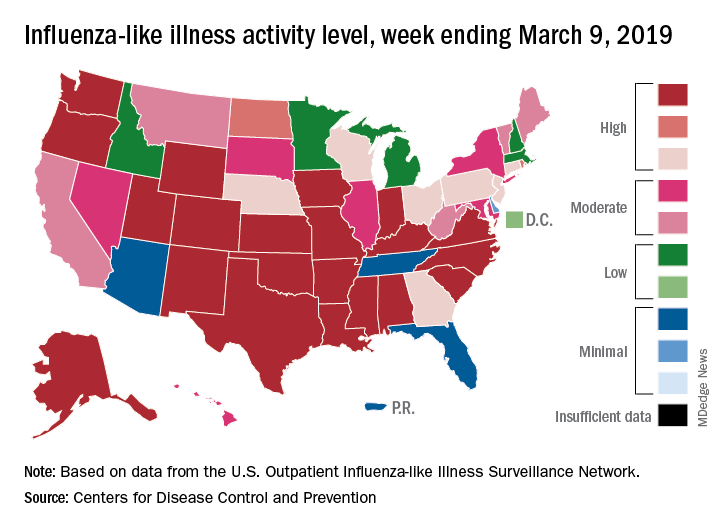
For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.

For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.
Influenza activity measures declined for a third consecutive week, but levels are higher than usual at this point in the flu season, according to the Centers for Disease Control and Prevention.

For the week ending March 9, an estimated 4.5% of outpatient visits were for influenza-like illness (ILI), which was down from 4.6% the previous week, the CDC’s influenza division reported March 15, but that is higher than the comparable week for any year since 1998-1999. During last year’s very severe flu season, the outpatient visit rate was just under 3.2% for the week ending March 10.
Although the number of states at level 10 on the CDC’s 1-10 scale remained at 21, the activity map actually looks more red than last week since Rhode Island and West Virgina were replaced by the much larger states of Iowa and Washington. The number of states in the high range (8-10), did go down from 32 to 30, data from the CDC’s Outpatient ILI Surveillance Network show.
Of those four deaths, only one occurred during the most recent reporting week, the CDC said.
Flu activity down for a second straight week
A second straight week of reduced influenza activity suggests that the 2018-2019 flu season is on the decline, according to the most recent data from the Centers for Disease Control and Prevention.
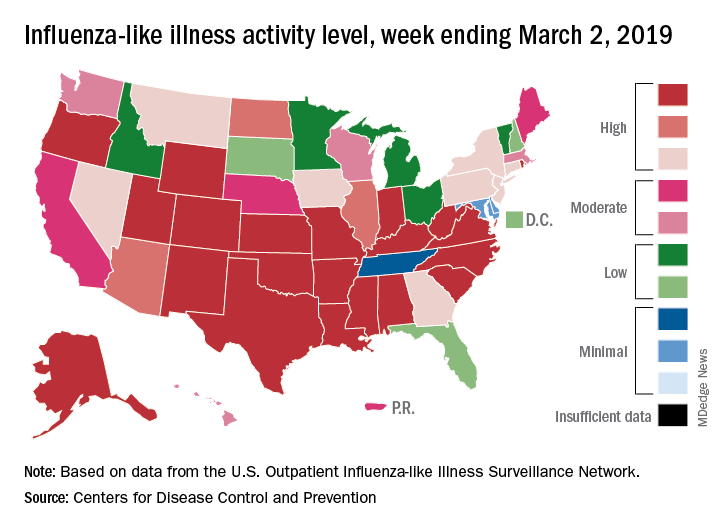
The proportion of outpatient visits for influenza-like illness (ILI) was 4.7% during the week ending March 2, which means that, thanks to a revision of the number for the previous week (Feb. 23) from 5.0% down to 4.9%, there have been two straight weeks of declines since outpatient visits reached a season-high 5.0% for the week ending Feb. 16, the CDC’s influenza division said March 8. The national baseline level is 2.2%.
This marks the second 2-week drop in ILI visits for the 2018-2019 season, as there was similar dip in the beginning of January before activity started rising again.
This compares with 24 the week before; 32 states were in the high range of 8-10, compared with the 33 reported last week, based on data from the Outpatient ILI Surveillance Network.
There were nine flu-related pediatric deaths reported during the week, with three occurring in the week ending March 2. To underscore the preliminary nature of these data, one of the deaths reported this week occurred in 2016. A total of 64 deaths in children have been associated with influenza so far for the 2018-2019 season, and the total for the 2015-2016 season is now 95, the CDC said.
A second straight week of reduced influenza activity suggests that the 2018-2019 flu season is on the decline, according to the most recent data from the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 4.7% during the week ending March 2, which means that, thanks to a revision of the number for the previous week (Feb. 23) from 5.0% down to 4.9%, there have been two straight weeks of declines since outpatient visits reached a season-high 5.0% for the week ending Feb. 16, the CDC’s influenza division said March 8. The national baseline level is 2.2%.
This marks the second 2-week drop in ILI visits for the 2018-2019 season, as there was similar dip in the beginning of January before activity started rising again.
This compares with 24 the week before; 32 states were in the high range of 8-10, compared with the 33 reported last week, based on data from the Outpatient ILI Surveillance Network.
There were nine flu-related pediatric deaths reported during the week, with three occurring in the week ending March 2. To underscore the preliminary nature of these data, one of the deaths reported this week occurred in 2016. A total of 64 deaths in children have been associated with influenza so far for the 2018-2019 season, and the total for the 2015-2016 season is now 95, the CDC said.
A second straight week of reduced influenza activity suggests that the 2018-2019 flu season is on the decline, according to the most recent data from the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 4.7% during the week ending March 2, which means that, thanks to a revision of the number for the previous week (Feb. 23) from 5.0% down to 4.9%, there have been two straight weeks of declines since outpatient visits reached a season-high 5.0% for the week ending Feb. 16, the CDC’s influenza division said March 8. The national baseline level is 2.2%.
This marks the second 2-week drop in ILI visits for the 2018-2019 season, as there was similar dip in the beginning of January before activity started rising again.
This compares with 24 the week before; 32 states were in the high range of 8-10, compared with the 33 reported last week, based on data from the Outpatient ILI Surveillance Network.
There were nine flu-related pediatric deaths reported during the week, with three occurring in the week ending March 2. To underscore the preliminary nature of these data, one of the deaths reported this week occurred in 2016. A total of 64 deaths in children have been associated with influenza so far for the 2018-2019 season, and the total for the 2015-2016 season is now 95, the CDC said.
Flu season shows signs of peaking
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.
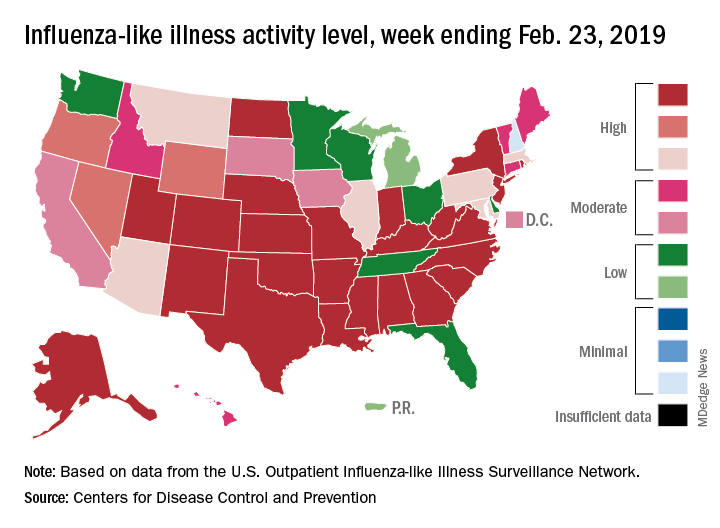
Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.

Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
The 2018-2019 flu season may have peaked as the major nationwide measure of influenza activity held steady for the week ending Feb. 23, according to the Centers for Disease Control and Prevention. The proportion of outpatient visits for influenza-like illness (ILI) was 5.0% for the most recent reporting week, the CDC’s influenza division said in its March 1 report. The previous week’s outpatient visit rate, originally reported as 5.1%, was revised this week to 5.0% as well, suggesting that flu activity is no longer increasing.

Activity at the state level was more mixed. The number of states at level 10 on the CDC’s 1-10 scale of ILI activity stayed at 24 as Indiana and North Dakota replaced Tennessee and Wyoming, but the number of states in the high range (8-10) of the activity scale increased from 30 to 33, CDC data show.
The signs of plateauing ILI activity did not, however, extend to flu-related deaths, with 15 reported among children – the highest weekly number for the 2018-2019 season, although 11 actually occurred in previous weeks – during the week ending Feb. 23 and 289 deaths among all ages for the week ending Feb. 16, which is already more than the 268 listed the week before despite less complete reporting (82% vs. 97%), the CDC reported. Total flu-related deaths in children are now up to 56, compared with 138 at the corresponding point in the 2017-2018 season.
New BinaxNOW influenza test gets CLIA waiver
The Food and Drug Administration has granted the reformulated BinaxNOW Influenza A & B Card 2 waived status under the Clinical Laboratory Improvements Amendments for use with Abbott’s DIGIVAL reader, which means the system is relatively simple and has a low risk of erroneous results.
According to Abbott’s press release, the DIGIVAL reader (formerly known as the Alere Reader) automatically interprets this rapid influenza diagnostic test’s results in seconds and is intended to remove subjectivity from the diagnostic process. The BinaxNOW Influenza Card A & B Card 2 is a Class II assay and complies with the FDA’s new reclassification requirements for rapid influenza diagnostic tests.
The Food and Drug Administration has granted the reformulated BinaxNOW Influenza A & B Card 2 waived status under the Clinical Laboratory Improvements Amendments for use with Abbott’s DIGIVAL reader, which means the system is relatively simple and has a low risk of erroneous results.
According to Abbott’s press release, the DIGIVAL reader (formerly known as the Alere Reader) automatically interprets this rapid influenza diagnostic test’s results in seconds and is intended to remove subjectivity from the diagnostic process. The BinaxNOW Influenza Card A & B Card 2 is a Class II assay and complies with the FDA’s new reclassification requirements for rapid influenza diagnostic tests.
The Food and Drug Administration has granted the reformulated BinaxNOW Influenza A & B Card 2 waived status under the Clinical Laboratory Improvements Amendments for use with Abbott’s DIGIVAL reader, which means the system is relatively simple and has a low risk of erroneous results.
According to Abbott’s press release, the DIGIVAL reader (formerly known as the Alere Reader) automatically interprets this rapid influenza diagnostic test’s results in seconds and is intended to remove subjectivity from the diagnostic process. The BinaxNOW Influenza Card A & B Card 2 is a Class II assay and complies with the FDA’s new reclassification requirements for rapid influenza diagnostic tests.
Influenza activity continues to increase
The 2018-2019 flu season is showing no signs of decline as activity measures continued to increase into mid-February, according to the Centers for Disease Control and Prevention.
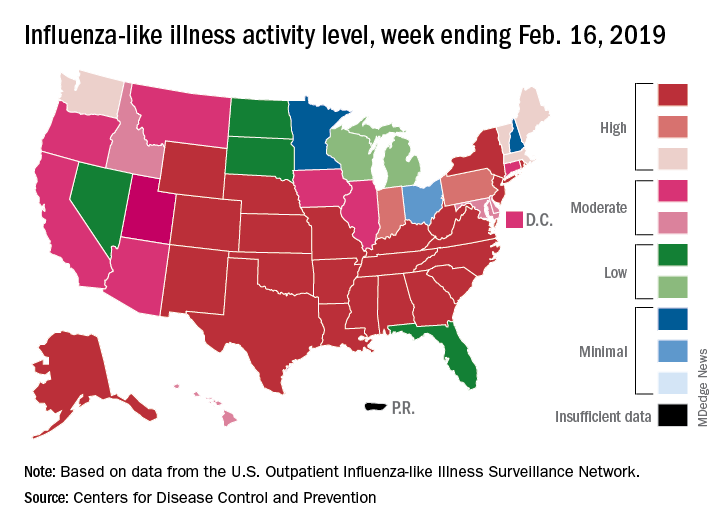
Eight of the last 10 flu seasons had already reached their peak before mid-February, but another rise brought the proportion of outpatient visits for influenza-like illness (ILI) to 5.1% for the week ending Feb. 16, compared with 4.8% the week before, the CDC’s influenza division reported Feb. 22. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
The week also brought more ILI to more states, as the number reporting an activity level of 10 on the CDC’s 1-10 scale rose from 21 to 24 and the number in the high range of 8-10 increased from 26 to 30. Another seven states – including California, which was at level 5 the previous week – and the District of Columbia were at level 7 for the current reporting week, the CDC said.
Two flu-related pediatric deaths occurred during the week ending Feb. 16 and another five were reported from previous weeks, which brings the total to 41 for the 2018-2019 season. Data for influenza deaths at all ages, which are reported a week later, show that 205 occurred in the week ending Feb. 9, with reporting 75% complete. There were 236 total deaths for the week ending Feb. 2 (94% reporting) and 218 deaths during the week ending Jan. 26 (99% reporting), the CDC said.
The 2018-2019 flu season is showing no signs of decline as activity measures continued to increase into mid-February, according to the Centers for Disease Control and Prevention.

Eight of the last 10 flu seasons had already reached their peak before mid-February, but another rise brought the proportion of outpatient visits for influenza-like illness (ILI) to 5.1% for the week ending Feb. 16, compared with 4.8% the week before, the CDC’s influenza division reported Feb. 22. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
The week also brought more ILI to more states, as the number reporting an activity level of 10 on the CDC’s 1-10 scale rose from 21 to 24 and the number in the high range of 8-10 increased from 26 to 30. Another seven states – including California, which was at level 5 the previous week – and the District of Columbia were at level 7 for the current reporting week, the CDC said.
Two flu-related pediatric deaths occurred during the week ending Feb. 16 and another five were reported from previous weeks, which brings the total to 41 for the 2018-2019 season. Data for influenza deaths at all ages, which are reported a week later, show that 205 occurred in the week ending Feb. 9, with reporting 75% complete. There were 236 total deaths for the week ending Feb. 2 (94% reporting) and 218 deaths during the week ending Jan. 26 (99% reporting), the CDC said.
The 2018-2019 flu season is showing no signs of decline as activity measures continued to increase into mid-February, according to the Centers for Disease Control and Prevention.

Eight of the last 10 flu seasons had already reached their peak before mid-February, but another rise brought the proportion of outpatient visits for influenza-like illness (ILI) to 5.1% for the week ending Feb. 16, compared with 4.8% the week before, the CDC’s influenza division reported Feb. 22. ILI is defined as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.
The week also brought more ILI to more states, as the number reporting an activity level of 10 on the CDC’s 1-10 scale rose from 21 to 24 and the number in the high range of 8-10 increased from 26 to 30. Another seven states – including California, which was at level 5 the previous week – and the District of Columbia were at level 7 for the current reporting week, the CDC said.
Two flu-related pediatric deaths occurred during the week ending Feb. 16 and another five were reported from previous weeks, which brings the total to 41 for the 2018-2019 season. Data for influenza deaths at all ages, which are reported a week later, show that 205 occurred in the week ending Feb. 9, with reporting 75% complete. There were 236 total deaths for the week ending Feb. 2 (94% reporting) and 218 deaths during the week ending Jan. 26 (99% reporting), the CDC said.
Flu season showing its staying power
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.
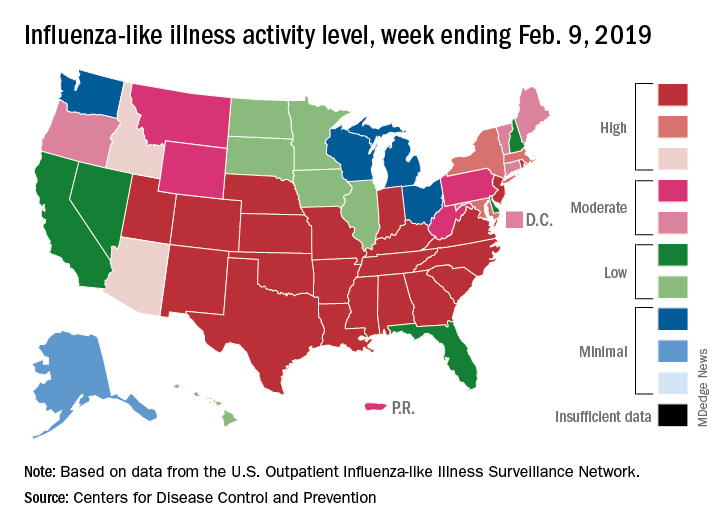
There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.

There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Like an unwelcome guest, the 2018-2019 flu season seems to be settling in for a lengthy stay as three more states have reached the highest level of influenza-like illness (ILI) activity, according to the Centers for Disease Control and Prevention.

There are now 21 states at level 10 on the CDC’s 1-10 scale, with the South showing up almost solidly red on the flu activity map for the week ending Feb. 9. Another five states are at levels 8 and 9, bringing the total in the high range to 26 for the week, compared with 24 the previous week, the CDC’s influenza division reported Feb. 15.
National activity, reflected in the proportion of outpatient visits involving ILI, took a step up from 4.3% the week before to 4.8% for the week ending Feb. 9. The national baseline rate is 2.2% for ILI, which the CDC defines “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Two flu-related pediatric deaths occurred during the week ending Feb. 9, and another four were reported from earlier weeks, which brings the total for the 2018-2019 season to 34, the CDC said. At the same point in last year’s flu season, there had been 84 flu-related deaths in children.
In a separate report, the CDC said that, based on data collected from Nov. 23, 2018 to Feb. 2, 2019, “the influenza vaccine has been 47% effective in preventing medically attended acute respiratory virus infection across all age groups and specifically was 46% effective in preventing medical visits associated with influenza A(H1N1)pdm09.” The effectiveness of the vaccine was 61% for children aged 6 months to 17 years, the CDC said (MMWR. 2019 Feb 15;68[6];135-9).
Flu vaccination during the 2017-2018 season prevented 7.1 million illnesses, 3.7 million medical visits, 109,000 hospitalizations, and 8,000 flu-related deaths, the CDC said, adding that “vaccination has been found to reduce deaths, intensive care unit admissions and length of stay, and overall duration of hospitalization among hospitalized influenza patients.”
Forecasts for the rest of the 2018-2019 season “predict that elevated influenza activity in parts of the United States will continue for several more weeks,” the CDC said.
Flu activity hits seasonal high
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.
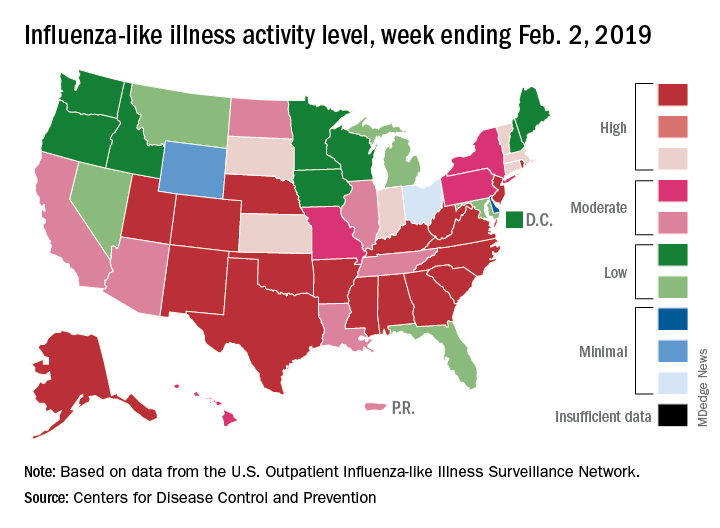
The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Influenza activity increased for the third consecutive week and has now reached its highest point for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) hit 4.3% for the week ending Feb. 2, which topped the previous high of 4.0% that was reached in late December (the national baseline rate is 2.2%). Outpatient ILI visits then dipped down to 3.1% after 2 weeks of decreases before rising again in mid-January, the CDC’s influenza division reported Feb. 8.
Season-high activity also was seen at the state level for the week ending Feb. 2. There were 18 states at level 10 on the CDC’s 1-10 scale of ILI activity, which was up from 16 the week before, and a total of 24 states were in the high range from 8-10, compared with 23 for the previous week. The geographic spread of influenza was reported as widespread in 47 states and Puerto Rico, the CDC said.
Four flu-related pediatric deaths were reported during the week ending Feb. 2, two of which occurred the previous week, which brings the total for the 2018-2019 season to 28, the CDC said.
There were 158 flu-related deaths among all ages during the week ending Jan. 26 – the latest for which such data are available – with reporting almost 75% complete. The previous week saw 177 overall flu deaths, with reporting for that week over 90% complete. During the corresponding weeks of the very severe 2017-2018 flu season, the overall death totals were 1,448 and 1,626, CDC data show.
Flu activity ticks up for second week in a row
Influenza activity increased for a second straight week after a 2-week drop and by one measure has topped the high reached in late December, according to the Centers for Disease Control and Prevention.
For the week ending Jan. 26, 2019, there were 16 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, compared with 12 states during the week ending Dec. 29. With another seven states at levels 8 and 9, that makes 23 in the high range for the week ending Jan. 26, again putting it above the 19 reported for Dec. 29, the CDC’s influenza division reported Feb. 1.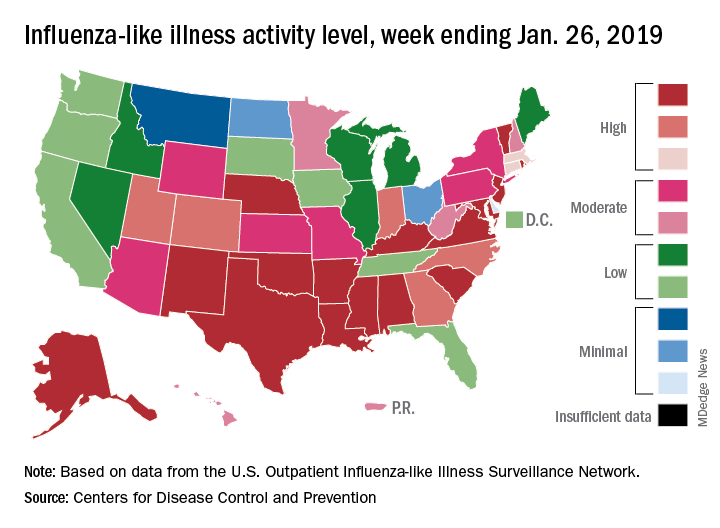
By another measure, however, that December peak in activity remains the seasonal high. The proportion of outpatient visits for ILI that week was 4.0%, compared with the 3.8% reported for Jan. 26. That’s up from 3.3% the week before and 3.1% the week before that, which in turn was the second week of a 2-week decline in activity in early January, CDC data show.
Two flu-related pediatric deaths were reported during the week ending Jan. 26, but both occurred the previous week. For the 2018-2019 flu season so far, a total of 24 pediatric flu deaths have been reported, the CDC said. At the same point in the 2017-2018 flu season, there had been 84 such deaths, according to the CDC’s Influenza-Associated Pediatric Mortality Surveillance System.
There were 143 overall flu-related deaths during the week of Jan. 19, which is the most recent week available. That is down from 189 the week before, but the Jan. 19 reporting is only 75% complete, data from the National Center for Health Statistics show.
Influenza activity increased for a second straight week after a 2-week drop and by one measure has topped the high reached in late December, according to the Centers for Disease Control and Prevention.
For the week ending Jan. 26, 2019, there were 16 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, compared with 12 states during the week ending Dec. 29. With another seven states at levels 8 and 9, that makes 23 in the high range for the week ending Jan. 26, again putting it above the 19 reported for Dec. 29, the CDC’s influenza division reported Feb. 1.
By another measure, however, that December peak in activity remains the seasonal high. The proportion of outpatient visits for ILI that week was 4.0%, compared with the 3.8% reported for Jan. 26. That’s up from 3.3% the week before and 3.1% the week before that, which in turn was the second week of a 2-week decline in activity in early January, CDC data show.
Two flu-related pediatric deaths were reported during the week ending Jan. 26, but both occurred the previous week. For the 2018-2019 flu season so far, a total of 24 pediatric flu deaths have been reported, the CDC said. At the same point in the 2017-2018 flu season, there had been 84 such deaths, according to the CDC’s Influenza-Associated Pediatric Mortality Surveillance System.
There were 143 overall flu-related deaths during the week of Jan. 19, which is the most recent week available. That is down from 189 the week before, but the Jan. 19 reporting is only 75% complete, data from the National Center for Health Statistics show.
Influenza activity increased for a second straight week after a 2-week drop and by one measure has topped the high reached in late December, according to the Centers for Disease Control and Prevention.
For the week ending Jan. 26, 2019, there were 16 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, compared with 12 states during the week ending Dec. 29. With another seven states at levels 8 and 9, that makes 23 in the high range for the week ending Jan. 26, again putting it above the 19 reported for Dec. 29, the CDC’s influenza division reported Feb. 1.
By another measure, however, that December peak in activity remains the seasonal high. The proportion of outpatient visits for ILI that week was 4.0%, compared with the 3.8% reported for Jan. 26. That’s up from 3.3% the week before and 3.1% the week before that, which in turn was the second week of a 2-week decline in activity in early January, CDC data show.
Two flu-related pediatric deaths were reported during the week ending Jan. 26, but both occurred the previous week. For the 2018-2019 flu season so far, a total of 24 pediatric flu deaths have been reported, the CDC said. At the same point in the 2017-2018 flu season, there had been 84 such deaths, according to the CDC’s Influenza-Associated Pediatric Mortality Surveillance System.
There were 143 overall flu-related deaths during the week of Jan. 19, which is the most recent week available. That is down from 189 the week before, but the Jan. 19 reporting is only 75% complete, data from the National Center for Health Statistics show.
Flu activity increases after 2 weeks of declines
according to the Centers for Disease Control and Prevention.
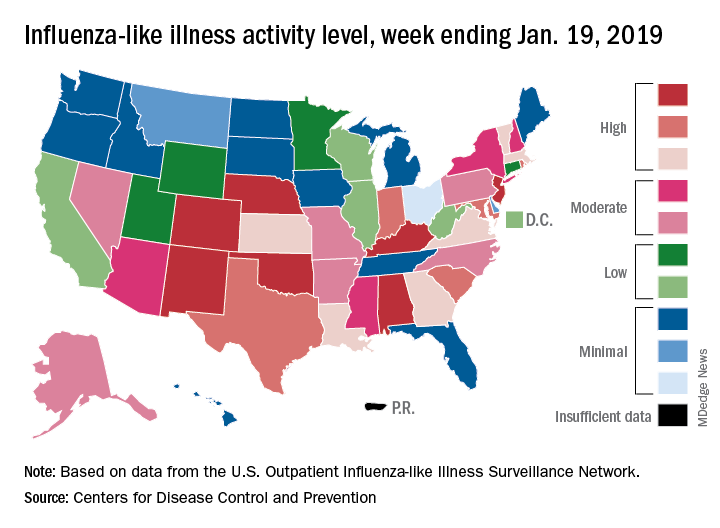
The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.3% for the most recent measurement period, the CDC’s influenza division reported Jan 25. The previous 2-week decline had seen ILI visits dip down to 3.1% for the week ending Jan. 12 after hitting a season high of 4%.
To go along with the national increase in visits, more states reported high levels of flu activity. For the week ending Jan. 19, seven states were at level 10 on the CDC’s 1-10 scale, compared with four the previous week, and there were 18 states in the high range (levels 8-10), compared with 9 the week before, the CDC said.
Three flu-related pediatric deaths were reported in the week ending Jan. 19, although all three occurred during earlier weeks. The total number of pediatric deaths for the 2018-2019 season is now up to 22. Deaths among all ages, which are reported a week later, totaled 118 for the week ending Jan. 12, with 63% of reporting complete. There were 144 deaths during the week ending Jan. 5, with reporting 86% complete. During the second full week of 2018, in the middle of the very severe 2017-2018 season, there were 1,537 flu-related deaths, CDC data show.
New study determines factors that can send flu patients to the ICU
Numerous independent factors – including a history of obstructive/central sleep apnea syndrome (OSAS/CSAS) or myocardial infarction, along with a body mass index greater than 30 g/m2 – could be related to ICU admission and subsequent high mortality rates in influenza patients, according to an analysis of patients in the Netherlands who were treated during the influenza epidemic of 2015-2016.
Along with determining these factors, lead author M.C. Beumer, of Radboud University Medical Center, the Netherlands, and his coauthors found that “coinfections with bacterial, fungal, and viral pathogens developed more often in patients who were admitted to the ICU.” The study was published in the Journal of Critical Care.
The coauthors reviewed 199 influenza patients who were admitted to two medical centers in the Netherlands during October 2015–April 2016. Of those patients, 45 (23%) were admitted to the ICU, primarily because of respiratory failure, and their mortality rate was 17/45 (38%) versus an overall mortality rate of 18/199 (9%).
Compared with patients in the normal ward, patients admitted to the ICU more frequently had a history of OSAS/CSAS (11% vs. 3%; P = .03) and MI (20% vs. 6%; P = .007), along with a BMI higher than 30 g/m2 (30% vs. 15%; P = .04) and dyspnea as a symptom (77% vs. 48%,; P = .001). In addition, more ICU-admitted patients had influenza A rather than influenza B, compared with those not admitted (87% vs. 66%; P = .009).
Pulmonary coinfections – including bacterial, fungal, and viral pathogens – were also proportionally higher among the 45 ICU patients (56% vs. 20%; P less than .0001). The most common bacterial pathogens were Staphylococcus aureus (11%) and Streptococcus pneumoniae (7%) while Aspergillus fumigatus (18%) and Pneumocystis jirovecii (7%) topped the fungal pathogens.
Mr. Beumer and his colleagues noted potential limitations of their work, including the selection of patients from among the “most severely ill” contributing to an ICU admission rate that surpassed the 5%-10% described elsewhere. They also admitted that their study relied on a “relatively small sample size,” focusing on one seasonal influenza outbreak. However, “despite the limited validity,” they reiterated that “the identified factors may contribute to a complicated disease course and could represent a tool for early recognition of the influenza patients at risk for a complicated disease course.”
The authors reported no conflicts of interest.
SOURCE: Beumer MC et al. J Crit Care. 2019;50:59-65.
.
Numerous independent factors – including a history of obstructive/central sleep apnea syndrome (OSAS/CSAS) or myocardial infarction, along with a body mass index greater than 30 g/m2 – could be related to ICU admission and subsequent high mortality rates in influenza patients, according to an analysis of patients in the Netherlands who were treated during the influenza epidemic of 2015-2016.
Along with determining these factors, lead author M.C. Beumer, of Radboud University Medical Center, the Netherlands, and his coauthors found that “coinfections with bacterial, fungal, and viral pathogens developed more often in patients who were admitted to the ICU.” The study was published in the Journal of Critical Care.
The coauthors reviewed 199 influenza patients who were admitted to two medical centers in the Netherlands during October 2015–April 2016. Of those patients, 45 (23%) were admitted to the ICU, primarily because of respiratory failure, and their mortality rate was 17/45 (38%) versus an overall mortality rate of 18/199 (9%).
Compared with patients in the normal ward, patients admitted to the ICU more frequently had a history of OSAS/CSAS (11% vs. 3%; P = .03) and MI (20% vs. 6%; P = .007), along with a BMI higher than 30 g/m2 (30% vs. 15%; P = .04) and dyspnea as a symptom (77% vs. 48%,; P = .001). In addition, more ICU-admitted patients had influenza A rather than influenza B, compared with those not admitted (87% vs. 66%; P = .009).
Pulmonary coinfections – including bacterial, fungal, and viral pathogens – were also proportionally higher among the 45 ICU patients (56% vs. 20%; P less than .0001). The most common bacterial pathogens were Staphylococcus aureus (11%) and Streptococcus pneumoniae (7%) while Aspergillus fumigatus (18%) and Pneumocystis jirovecii (7%) topped the fungal pathogens.
Mr. Beumer and his colleagues noted potential limitations of their work, including the selection of patients from among the “most severely ill” contributing to an ICU admission rate that surpassed the 5%-10% described elsewhere. They also admitted that their study relied on a “relatively small sample size,” focusing on one seasonal influenza outbreak. However, “despite the limited validity,” they reiterated that “the identified factors may contribute to a complicated disease course and could represent a tool for early recognition of the influenza patients at risk for a complicated disease course.”
The authors reported no conflicts of interest.
SOURCE: Beumer MC et al. J Crit Care. 2019;50:59-65.
.
Numerous independent factors – including a history of obstructive/central sleep apnea syndrome (OSAS/CSAS) or myocardial infarction, along with a body mass index greater than 30 g/m2 – could be related to ICU admission and subsequent high mortality rates in influenza patients, according to an analysis of patients in the Netherlands who were treated during the influenza epidemic of 2015-2016.
Along with determining these factors, lead author M.C. Beumer, of Radboud University Medical Center, the Netherlands, and his coauthors found that “coinfections with bacterial, fungal, and viral pathogens developed more often in patients who were admitted to the ICU.” The study was published in the Journal of Critical Care.
The coauthors reviewed 199 influenza patients who were admitted to two medical centers in the Netherlands during October 2015–April 2016. Of those patients, 45 (23%) were admitted to the ICU, primarily because of respiratory failure, and their mortality rate was 17/45 (38%) versus an overall mortality rate of 18/199 (9%).
Compared with patients in the normal ward, patients admitted to the ICU more frequently had a history of OSAS/CSAS (11% vs. 3%; P = .03) and MI (20% vs. 6%; P = .007), along with a BMI higher than 30 g/m2 (30% vs. 15%; P = .04) and dyspnea as a symptom (77% vs. 48%,; P = .001). In addition, more ICU-admitted patients had influenza A rather than influenza B, compared with those not admitted (87% vs. 66%; P = .009).
Pulmonary coinfections – including bacterial, fungal, and viral pathogens – were also proportionally higher among the 45 ICU patients (56% vs. 20%; P less than .0001). The most common bacterial pathogens were Staphylococcus aureus (11%) and Streptococcus pneumoniae (7%) while Aspergillus fumigatus (18%) and Pneumocystis jirovecii (7%) topped the fungal pathogens.
Mr. Beumer and his colleagues noted potential limitations of their work, including the selection of patients from among the “most severely ill” contributing to an ICU admission rate that surpassed the 5%-10% described elsewhere. They also admitted that their study relied on a “relatively small sample size,” focusing on one seasonal influenza outbreak. However, “despite the limited validity,” they reiterated that “the identified factors may contribute to a complicated disease course and could represent a tool for early recognition of the influenza patients at risk for a complicated disease course.”
The authors reported no conflicts of interest.
SOURCE: Beumer MC et al. J Crit Care. 2019;50:59-65.
.
FROM THE JOURNAL OF CRITICAL CARE
Key clinical point:
Major finding: Flu patients in the ICU more frequently had a history of obstructive/central sleep apnea syndrome (11% vs. 3%; P = .03) and MI (20% vs. 6%; P = .007), compared with non-ICU flu patients.
Study details: A retrospective cohort study of 199 flu patients who were admitted to two academic hospitals in the Netherlands.
Disclosures: The authors reported no conflicts of interest.
Source: Beumer MC et al. J Crit Care. 2019; 50:59-65.

