User login
Flu activity down for second consecutive week
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.
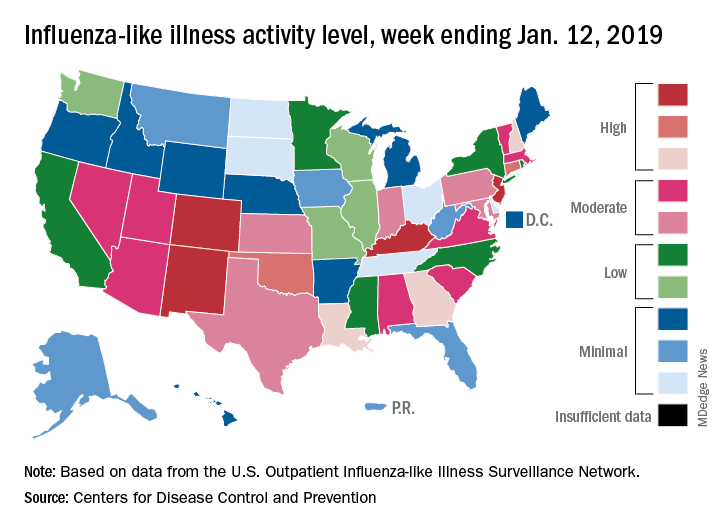
The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
The second week of the new year brought a second straight week of declining activity for the 2018-2019 flu season, according to the Centers for Disease Control and Prevention.

The proportion of outpatient visits for influenza-like illness (ILI) was 3.1% for the week ending Jan. 12, 2019, down from 3.5% the previous week but still above the national baseline level of 2.2%, the CDC’s influenza division reported Jan. 18.
Activity was also down at the state level. There were 4 states – Colorado, Kentucky, New Jersey, and New Mexico – at level 10 on the CDC’s 1-10 scale for ILI activity, compared with 10 the week before, and a total of 9 were in the high range from 8 to 10, compared with 15 the previous week, data from the influenza division show.
Reports of total influenza deaths, which lag a week behind other measures, continue to rise: 111 for the week ending Jan. 5, although reporting is only 72% complete. There were 89 deaths during the previous week, with reporting 82% complete so far. Total flu-related deaths among children are up to 19 for the 2018-2019 season after three more were reported during the week ending Jan. 12, the CDC said. Influenza deaths from the comparable weeks of the much more severe 2017-2018 season were 1,163 for all ages and 10 for children.
Flu season showing signs of decline
The 2018-2019 flu season may have peaked as measures of influenza-like illness (ILI) activity dropped in the first week of the new year, according to the U.S. Centers for Disease Control and Prevention.

The proportion of outpatients visits for ILI dropped to 3.5% for the week ending Jan. 5, 2019, after reaching 4.0% the previous week. Outpatient ILI visits first topped the national baseline of 2.2% during the week ending Dec. 8, 2018, and have remained above that value for 5 consecutive weeks, the CDC’s influenza division said on Jan. 11.
Flu activity reported by the states reflects the national drop: 10 states came in at level 10 on the CDC’s 1-10 scale of activity for the week ending Jan. 5 – down from 12 the week before – and a total of 15 were in the high range from 8 to 10, compared with 19 the previous week, the CDC said. Two states, Mississippi and Texas, dropped from level 10 to level 7, which the CDC categorizes as moderate activity.
A total of 73 ILI-related deaths were reported during the week ending Dec. 29 (the latest with data available; reporting less than 68% complete), which already exceeds the 71 deaths reported for the week ending Dec. 22 (reporting 85% complete). Flu deaths totaled 437 through the first 13 weeks of the 2018-2019 season, compared with the 1,659 that occurred during weeks 1-13 of the very severe 2017-2018 season, CDC data show.
For the week ending Jan. 5, the CDC received reports of three flu-related pediatric deaths, all of which occurred the previous week. For the season so far, there have been 16 pediatric deaths, compared with 20 at this point in the 2017-2018 season.
Estimates released during the flu season for the first time show that between 6 and 7 million Americans have been infected since Oct. 1, 2018, and that 69,000-84,000 people have been hospitalized with the flu through Jan. 5, 2019. These cumulative totals have previously been available only at the end of the season, the CDC noted.
The 2018-2019 flu season may have peaked as measures of influenza-like illness (ILI) activity dropped in the first week of the new year, according to the U.S. Centers for Disease Control and Prevention.

The proportion of outpatients visits for ILI dropped to 3.5% for the week ending Jan. 5, 2019, after reaching 4.0% the previous week. Outpatient ILI visits first topped the national baseline of 2.2% during the week ending Dec. 8, 2018, and have remained above that value for 5 consecutive weeks, the CDC’s influenza division said on Jan. 11.
Flu activity reported by the states reflects the national drop: 10 states came in at level 10 on the CDC’s 1-10 scale of activity for the week ending Jan. 5 – down from 12 the week before – and a total of 15 were in the high range from 8 to 10, compared with 19 the previous week, the CDC said. Two states, Mississippi and Texas, dropped from level 10 to level 7, which the CDC categorizes as moderate activity.
A total of 73 ILI-related deaths were reported during the week ending Dec. 29 (the latest with data available; reporting less than 68% complete), which already exceeds the 71 deaths reported for the week ending Dec. 22 (reporting 85% complete). Flu deaths totaled 437 through the first 13 weeks of the 2018-2019 season, compared with the 1,659 that occurred during weeks 1-13 of the very severe 2017-2018 season, CDC data show.
For the week ending Jan. 5, the CDC received reports of three flu-related pediatric deaths, all of which occurred the previous week. For the season so far, there have been 16 pediatric deaths, compared with 20 at this point in the 2017-2018 season.
Estimates released during the flu season for the first time show that between 6 and 7 million Americans have been infected since Oct. 1, 2018, and that 69,000-84,000 people have been hospitalized with the flu through Jan. 5, 2019. These cumulative totals have previously been available only at the end of the season, the CDC noted.
The 2018-2019 flu season may have peaked as measures of influenza-like illness (ILI) activity dropped in the first week of the new year, according to the U.S. Centers for Disease Control and Prevention.

The proportion of outpatients visits for ILI dropped to 3.5% for the week ending Jan. 5, 2019, after reaching 4.0% the previous week. Outpatient ILI visits first topped the national baseline of 2.2% during the week ending Dec. 8, 2018, and have remained above that value for 5 consecutive weeks, the CDC’s influenza division said on Jan. 11.
Flu activity reported by the states reflects the national drop: 10 states came in at level 10 on the CDC’s 1-10 scale of activity for the week ending Jan. 5 – down from 12 the week before – and a total of 15 were in the high range from 8 to 10, compared with 19 the previous week, the CDC said. Two states, Mississippi and Texas, dropped from level 10 to level 7, which the CDC categorizes as moderate activity.
A total of 73 ILI-related deaths were reported during the week ending Dec. 29 (the latest with data available; reporting less than 68% complete), which already exceeds the 71 deaths reported for the week ending Dec. 22 (reporting 85% complete). Flu deaths totaled 437 through the first 13 weeks of the 2018-2019 season, compared with the 1,659 that occurred during weeks 1-13 of the very severe 2017-2018 season, CDC data show.
For the week ending Jan. 5, the CDC received reports of three flu-related pediatric deaths, all of which occurred the previous week. For the season so far, there have been 16 pediatric deaths, compared with 20 at this point in the 2017-2018 season.
Estimates released during the flu season for the first time show that between 6 and 7 million Americans have been infected since Oct. 1, 2018, and that 69,000-84,000 people have been hospitalized with the flu through Jan. 5, 2019. These cumulative totals have previously been available only at the end of the season, the CDC noted.
Children who are coughing: Is it flu or bacterial pneumonia?
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
We are in the middle of flu season, and many of our patients are coughing. Is it the flu or might the child have a secondary bacterial pneumonia? Let’s start with the history for a tip off. The course of flu and respiratory viral infections in general involves a typical pattern of timing for fever and cough.
A late-developing fever or fever that subsides then recurs should raise concern. A prolonged cough or cough that subsides then recurs also should raise concern. The respiratory rate and chest retractions are key physical findings that can aid in distinguishing children with bacterial pneumonia. Rales and decreased breath sounds in lung segments are best heard with deep breaths.
What diagnostic laboratory and imaging tests should be used
Fortunately, rapid tests to detect influenza are available, and many providers have added those to their laboratory evaluation. A complete blood count and differential may be helpful. If a pulse oximeter is available, checking oxygen saturation might be helpful. The American Academy of Pediatrics community pneumonia guideline states that routine chest radiographs are not necessary for the confirmation of suspected community-acquired pneumonia (CAP) in patients well enough to be treated in the outpatient setting (Clin Inf Dis. 2011 Oct;53[7]:e25–e76). Blood cultures should not be performed routinely in nontoxic, fully immunized children with CAP managed in the outpatient setting.
What antibiotic should be used
Antimicrobial therapy is not routinely required for preschool-aged children with cough, even cough caused by CAP, because viral pathogens are responsible for the great majority of clinical disease. If the diagnosis of CAP is made, the AAP endorses amoxicillin as first-line therapy for previously healthy, appropriately immunized infants and preschool children with mild to moderate CAP suspected to be of bacterial origin. For previously healthy, appropriately immunized school-aged children and adolescents with mild to moderate CAP, amoxicillin is recommended for treatment of Streptococcus pneumoniae, the most prominent invasive bacterial pathogen.
However, the treatment paradigm is complicated because Mycoplasma pneumoniae also should be considered in management decisions. Children with signs and symptoms suspicious for M. pneumoniae should be tested to help guide antibiotic selection. This may be a simple bedside cold agglutinin test. The highest incidence of Mycoplasma pneumonia is in 5- to 20-year-olds (51% in 5- to 9-year-olds, 74% in 9- to 15-year-olds, and 3%-18% in adults with pneumonia), but 9% of CAP occurs in patients younger than 5 years old. The clinical features of Mycoplasma pneumonia resemble influenza: The patient has gradual onset of headache, malaise, fever, sore throat, and cough. Mycoplasma pneumonia has a similar incidence of productive cough, rales, and diarrhea as pneumococcal CAP, but with more frequent upper respiratory symptoms and a normal leukocyte count. Mycoplasma bronchopneumonia occurs 30 times more frequently than Mycoplasma lobar pneumonia. The radiologic features of Mycoplasma is typical of a bronchopneumonia, usually involving a single lobe, subsegmental atelectasis, peribronchial thickening, and streaky interstitial densities. While Mycoplasma pneumonia is usually self-limited, the duration of illness is shortened by oral treatment with doxycycline, erythromycin, clarithromycin, or azithromycin.
What is the appropriate duration of antimicrobial therapy
Recommendations by the AAP for CAP note that treatment courses of 10 days have been best studied, although shorter courses may be just as effective, particularly for mild disease managed on an outpatient basis.
When should children be hospitalized
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He had no conflicts to declare. Email him at [email protected].
LAIV4 was less effective for children than IIV against influenza A/H1N1pdm09
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
There are many explanations for the decline in effectiveness of the live attenuated influenza vaccine (LAIV4), but the data are complicated by conflicting information from studies outside the United States indicating “reasonable protection” against influenza A/H1N1pdm09, A/H3N2, and influenza B, compared with the inactivated influenza virus (IIV), Pedro A. Piedra, MD, wrote in an accompanying editorial.
In 2016, the World Health Organization met to discuss LAIV effectiveness and highlighted factors such as methodological study differences, inadequate vaccine handling at distribution centers, intrinsic virological differences of the A/H1N1pdm09 virus, and increased preexisting population immunity in the United States since 2010 as potential explanations. During the transition from LAIV3 to LAIV4 for the 2013-2014 influenza season, viral interference may have also occurred when the influenza B strain was introduced into the vaccine, he added.
According to the CDC’s Advisory Committee on Immunization Practices (ACIP), viral growth properties of A/H1N1pdm09 has improved in LAIV4, and viral shedding also has improved for children between 2 years and 4 years of age. Although effectiveness numbers were not available for the ACIP recommendation, an interim analysis from Public Health England for the 2017-2018 influenza season found a vaccine effectiveness of 90.3% (95% confidence interval, 16.4%-98.9%).
“This early result is encouraging and supports the reintroduction of LAIV4 in the United States as an option for the control of seasonal influenza,” he said. “It also highlights the need for annual influenza vaccine effectiveness estimates and the importance of the U.S. Influenza Vaccine Effectiveness Network in providing updated information for ACIP recommendations.”
Dr. Piedra is from the departments of molecular virology and microbiology and pediatrics, Baylor College of Medicine, Houston. He reports being a consultant for AstraZeneca, Sanofi Pasteur, GlaxoSmithKline, and Merck Sharp and Dohme, and he has received travel support to present at an influenza seminar supported by Seqirus. His comments are from an editorial accompanying the article by Chung and colleagues ( Pediatrics. 2019. doi: 10.1542/peds.2018- 3290 ).
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
The live attenuated influenza vaccine was less effective against the influenza A/H1N1pdm09 virus in children and adolescents across multiple influenza seasons between 2013 and 2016, compared with the inactivated influenza vaccine, according to research published in the journal Pediatrics.
Jessie R. Chung, MPH, from the influenza division at the Centers for Disease Control and Prevention in Atlanta, and her colleagues performed an analysis of five different studies where vaccine effectiveness (VE) was examined for quadrivalent live attenuated vaccine (LAIV4) and inactivated influenza vaccine (IIV) in children and adolescents aged 2-17 years from 42 states.
The analysis included data from the U.S. Influenza Vaccine Effectiveness Network (6,793 patients), a study from the Louisiana State University Health Sciences Center (3,822 patients), the Influenza Clinical Investigation for Children (3,521 patients), Department of Defense Global, Laboratory-based, Influenza Surveillance Program (1,935 patients), and the Influenza Incidence Surveillance Project (1,102 patients) between the periods of 2013-2014 and 2015-2016. The researchers sourced current and previous season vaccination history from electronic medical records and immunization registries.
Of patients who were vaccinated across all seasons, there was 67% effectiveness against influenza A/H1N1pdm09 (95% confidence interval, 62%-72%) for those who received the IIV and 20% (95% CI, −6%-39%) for LAIV4. Among patients who received the LAIV4 vaccination, there was a significantly higher likelihood of developing influenza A/H1N1pdm09 (odds ratio, 2.66; 95% CI, 2.06-3.44) compared with patients who received the IIV vaccination.
With regard to other strains, there was similar effectiveness against influenza A/H3N2 and influenza B with LAIV4 and IIV vaccinations.
“In contrast to findings of reduced LAIV4 effectiveness against influenza A/H1N1pdm09 viruses, our results suggest a possible but nonsignificant benefit of LAIV4 over IIV against influenza B viruses, which has been described previously,” the investigators wrote.
Limitations of the study included having data only one season prior to enrollment and little available demographic information beyond age, gender, and geographic location.
The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
SOURCE: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
FROM PEDIATRICS
Key clinical point: The live attenuated influenza vaccine (LAIV4) was significantly less effective than was the inactivated influenza vaccine (IIV) for children against the influenza A/H1N1pdm09 virus across multiple flu seasons.
Major finding:
Study details: A combined analysis of five studies in the United States between the periods of 2013-2014 and 2015-2016 from the U.S. Influenza Vaccine Effectiveness Network.
Disclosures: The Influenza Clinical Investigation for Children was funded by MedImmune, a member of the AstraZeneca Group. Two of the researchers are employees of AstraZeneca. The other authors reported having no conflicts of interest. The U.S. Influenza Vaccine Effectiveness Network was supported by the CDC through cooperative agreements with the University of Michigan, Kaiser Permanente Washington Health Research Institute, Marshfield Clinic Research Institute, University of Pittsburgh, and Baylor Scott & White Health. At the University of Pittsburgh, the project also was supported by the National Institutes of Health.
Source: Chung JR et al. Pediatrics. 2018. doi: 10.1542/peds.2018-2094.
Flu season intensifies: High activity now in 19 states
The effects of the flu became much more widespread in the last full week of 2018 as the number of states with a high level of influenza activity more than doubled from the week before, according to the Centers for Disease Control and Prevention.
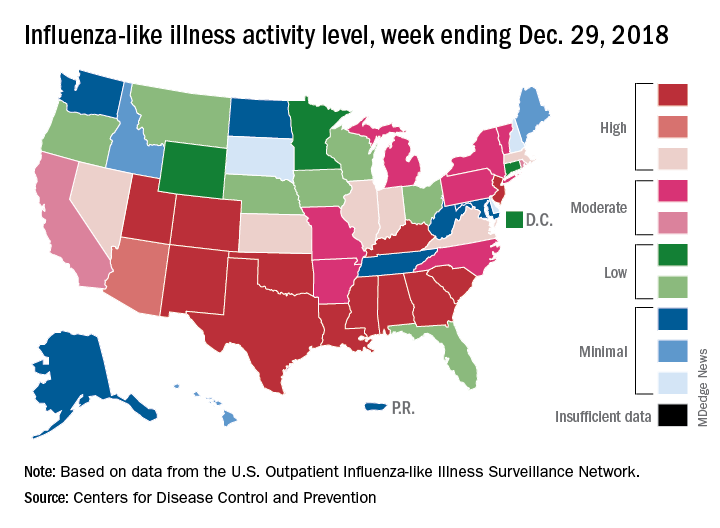
A total of 19 states were in the high range (8-10) on the CDC’s 1-10 scale of influenza-like illness (ILI) activity for the week ending Dec. 29, compared with 9 states the week before, the CDC’s influenza division reported Jan. 4. Of those 19 most-affected states, 12 were at level 10, 1 was at level 9, and 6 were at level 8. Geographic distribution of the virus was reported to be widespread in 24 states, the CDC said.
The proportion of outpatient visits for ILI – defined as fever (temperature of 100° F or greater) and cough and/or sore throat – rose to 4.1% for the week, which was up from 3.3% the previous week and well above the national baseline of 2.2%.
“The increase in the percentage of patient visits for ILI may be influenced in part by a reduction in routine health care visits during the winter holidays,” the report noted. There were 38 influenza deaths reported for the most recent week with available data (the week ending Dec. 22), although reporting for that week was just over 54% complete as of Jan. 4. For the previous weeks, 39 flu-related deaths occurred during the week ending Dec. 15 (reporting 84% complete) and 43 deaths during the week ending Dec. 8 (reporting 94% complete). For the respective weeks of last year’s flu season, total deaths were 359, 165, and 118, CDC data show.
For the week ending Dec. 29, two pediatric deaths were reported, one of which occurred the week before. For the 2018-2019 season so far, 13 flu-related pediatric deaths have been reported, the CDC said.
The effects of the flu became much more widespread in the last full week of 2018 as the number of states with a high level of influenza activity more than doubled from the week before, according to the Centers for Disease Control and Prevention.

A total of 19 states were in the high range (8-10) on the CDC’s 1-10 scale of influenza-like illness (ILI) activity for the week ending Dec. 29, compared with 9 states the week before, the CDC’s influenza division reported Jan. 4. Of those 19 most-affected states, 12 were at level 10, 1 was at level 9, and 6 were at level 8. Geographic distribution of the virus was reported to be widespread in 24 states, the CDC said.
The proportion of outpatient visits for ILI – defined as fever (temperature of 100° F or greater) and cough and/or sore throat – rose to 4.1% for the week, which was up from 3.3% the previous week and well above the national baseline of 2.2%.
“The increase in the percentage of patient visits for ILI may be influenced in part by a reduction in routine health care visits during the winter holidays,” the report noted. There were 38 influenza deaths reported for the most recent week with available data (the week ending Dec. 22), although reporting for that week was just over 54% complete as of Jan. 4. For the previous weeks, 39 flu-related deaths occurred during the week ending Dec. 15 (reporting 84% complete) and 43 deaths during the week ending Dec. 8 (reporting 94% complete). For the respective weeks of last year’s flu season, total deaths were 359, 165, and 118, CDC data show.
For the week ending Dec. 29, two pediatric deaths were reported, one of which occurred the week before. For the 2018-2019 season so far, 13 flu-related pediatric deaths have been reported, the CDC said.
The effects of the flu became much more widespread in the last full week of 2018 as the number of states with a high level of influenza activity more than doubled from the week before, according to the Centers for Disease Control and Prevention.

A total of 19 states were in the high range (8-10) on the CDC’s 1-10 scale of influenza-like illness (ILI) activity for the week ending Dec. 29, compared with 9 states the week before, the CDC’s influenza division reported Jan. 4. Of those 19 most-affected states, 12 were at level 10, 1 was at level 9, and 6 were at level 8. Geographic distribution of the virus was reported to be widespread in 24 states, the CDC said.
The proportion of outpatient visits for ILI – defined as fever (temperature of 100° F or greater) and cough and/or sore throat – rose to 4.1% for the week, which was up from 3.3% the previous week and well above the national baseline of 2.2%.
“The increase in the percentage of patient visits for ILI may be influenced in part by a reduction in routine health care visits during the winter holidays,” the report noted. There were 38 influenza deaths reported for the most recent week with available data (the week ending Dec. 22), although reporting for that week was just over 54% complete as of Jan. 4. For the previous weeks, 39 flu-related deaths occurred during the week ending Dec. 15 (reporting 84% complete) and 43 deaths during the week ending Dec. 8 (reporting 94% complete). For the respective weeks of last year’s flu season, total deaths were 359, 165, and 118, CDC data show.
For the week ending Dec. 29, two pediatric deaths were reported, one of which occurred the week before. For the 2018-2019 season so far, 13 flu-related pediatric deaths have been reported, the CDC said.
CDC: Flu activity ‘high’ in nine states
according to the Centers for Disease Control and Prevention.
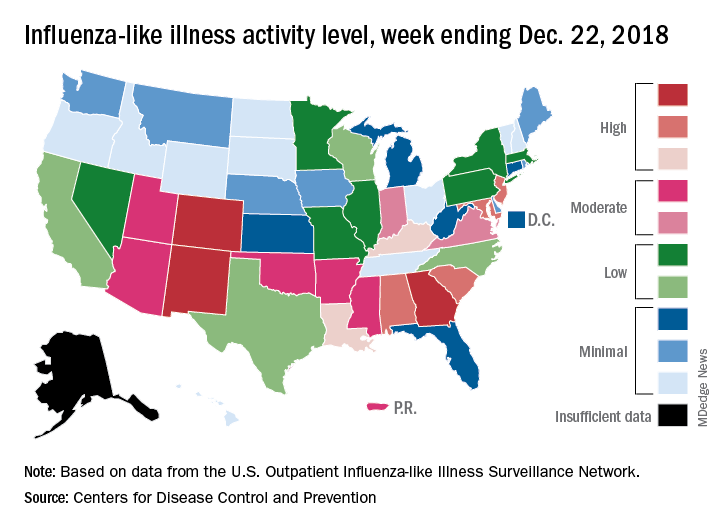
Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
according to the Centers for Disease Control and Prevention.

Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
according to the Centers for Disease Control and Prevention.

Patients with ILI made up an estimated 3.3% of outpatient visits for the week, which is up from 2.7% the previous week and well above the baseline rate of 2.2%, which the 2018-2019 flu season has now exceeded for the past 3 weeks, the CDC reported Dec. 28. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Three states – Colorado, Georgia, and New Mexico – are now at the highest level of flu activity on the CDC’s 1-10 scale, and nine states are in the “high” range (8-10), compared with two states in high range (both at level 10) for the week ending Dec. 15. Another seven states and Puerto Rico are now in the “moderate” range of 6-7, data from the CDC’s Outpatient ILI Surveillance Network show.
Four flu-related deaths in children were reported during the week ending Dec. 22, two of which occurred in previous weeks, which brings the total to 11 for the 2018-2019 season, the CDC reported.
2018-2019 flu season starts in earnest
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.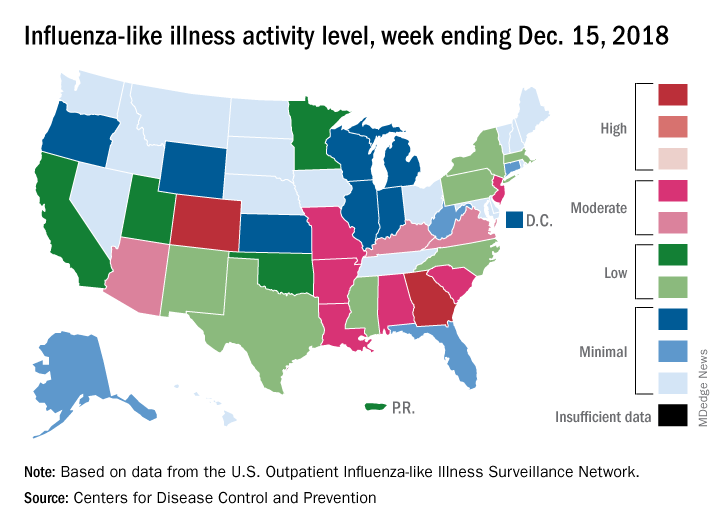
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
National flu activity moved solidly into above-average territory during the week ending Dec. 15, as Colorado and Georgia took the lead with the highest activity levels in the country, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) was 2.7% for the week, which was up from 2.3% the previous week and above the national baseline of 2.2%, the CDC reported. ILI is defined “as fever (temperature of 100°F [37.8°C] or greater) and cough and/or sore throat.”
Colorado and Georgia both reported ILI activity of 10 on the CDC’s 1-10 scale, making them the only states in the “high” range (8-10). Nine states and New York City had activity levels in the “moderate” range (6-7), Puerto Rico and 11 states were in the “low” range (4-5), and 28 states and the District of Columbia were in the “minimal” range (1-3), the CDC said.
During the comparable period of last year’s high-severity flu season, which ultimately resulted in 900,000 flu-related hospitalizations and 80,000 deaths (185 pediatric), nine states were already at level 10. For the 2018-2019 season so far, there have been seven ILI-related pediatric deaths, CDC data show.
Pregnant women commonly refuse the influenza vaccine
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women make up 1% of the population but accounted for 5% of all influenza deaths during the 2009 H1N1 pandemic, which makes the common vaccine refusals reported by the nation’s ob.gyns. all the more serious, according to Sonja A. Rasmussen, MD, MS, of the University of Florida in Gainesville and Denise J. Jamieson, MD, MPH, of Emory University in Atlanta.
After the 2009 pandemic, vaccination coverage for pregnant woman during flu season leapt from less than 30% to 54%, according to data from a 2016-2017 Internet panel survey. This was in large part because of the committed work of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists, who emphasized the importance of the influenza vaccine. But coverage rates have stagnated since then, and these two coauthors wrote that “the 2017-2018 severe influenza season was a stern reminder that influenza should not be underestimated.”
These last 2 years saw the highest-documented rate of hospitalizations for influenza since 2005-2006, but given that there’s been very little specific information available on hospitalizations of pregnant women, Dr. Rasmussen and Dr. Jamieson fear the onset of “complacency among health care providers, pregnant women, and the general public” when it comes to the effects of influenza.
They insisted that, as 2009 drifts even further into memory, “obstetric providers should not become complacent regarding influenza.” Strategies to improve coverage are necessary to break that 50% barrier, and “pregnant women and their infants deserve our best efforts to protect them from influenza.”
These comments are adapted from an accompanying editorial (Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003040). No conflicts of interest were reported.
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
Pregnant women commonly refuse vaccines, and refusal of influenza vaccine is more common than refusal of Tdap vaccine, according to a nationally representative survey of obstetrician/gynecologists.
“It appears vaccine refusal among pregnant women may be more common than parental refusal of childhood vaccines,” Sean T. O’Leary, MD, MPH, director of the Colorado Children’s Outcomes Network at the University of Colorado in Aurora, and his coauthors wrote in Obstetrics & Gynecology.
The survey was sent to 477 ob.gyns. via both email and mail between March and June 2016. The response rate was 69%, and almost all respondents reported recommending both influenza (97%) and Tdap (95%) vaccines to pregnant women.
However, respondents also reported that refusal of both vaccines was common, with more refusals of influenza vaccine than Tdap vaccine. Of ob.gyns. who responded, 62% reported that 10% or greater of their pregnant patients refused the influenza vaccine, compared with 32% reporting this for Tdap vaccine (P greater than .001; x2, less than 10% vs. 10% or greater). Of those refusing the vaccine, 48% believed influenza vaccine would make them sick; 38% felt they were unlikely to get a vaccine-preventable disease; and 32% had general worries about vaccines overall. In addition, the only strategy perceived as “very effective” in convincing a vaccine refuser to choose otherwise was “explaining that not getting the vaccine puts the fetus or newborn at risk.”
The authors shared potential limitations of their study, including the fact that they examined reported practices and perceptions, not observed practices, along with the potential that the attitudes and practices of respondents may differ from those of nonrespondents. However, they noted that this is unlikely given prior work and that next steps should consider responses to refusal while also sympathizing with the patients’ concerns. “Future work should focus on testing evidence-based strategies for addressing vaccine refusal in the obstetric setting and understanding how the unique concerns of pregnant women influence the effectiveness of such strategies,” they wrote.
The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
SOURCE: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Although almost all ob.gyns. recommend the influenza and Tdap vaccines for pregnant women, both commonly are refused.
Major finding: A total of 62% of ob.gyns. reported that 10% or greater of their pregnant patients refused the influenza vaccine; 32% reported this for Tdap vaccine.
Study details: An email and mail survey sent to a national network of ob.gyns. between March and June 2016.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. No conflicts of interest were reported.
Source: O’Leary ST et al. Obstet Gynecol. 2018 Dec. doi: 10.1097/AOG.0000000000003005.
The powerful virus inflammatory response
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Inflammation is a double-edged sword. Controlled and modest proinflammatory responses can enhance host immunity against viruses and decrease bacterial colonization and infection, whereas excessive uncontrolled proinflammatory responses may increase the susceptibility to bacterial colonization and secondary infection to facilitate disease pathogenesis. The immune system produces both proinflammatory and anti-inflammatory cytokines and chemokines. It is a balanced response that is key to maintaining good health.
Viral upper respiratory tract infections (URIs) are caused by rhinoviruses, coronaviruses, enteroviruses, respiratory syncytial viruses, influenza A and B viruses, parainfluenza viruses, adenoviruses, and human metapneumoviruses. Viruses are powerful. In the nose, they induce hypersecretion of mucus, slow cilia beating, up-regulate nasal epithelial cell receptors to facilitate bacterial attachment, suppress neutrophil function, and cause increased release of proinflammatory cytokines and chemokines. All these actions by respiratory viruses promote bacterial overgrowth in the nasopharynx and thereby facilitate bacterial superinfections. In fact, progression in pathogenesis of the common bacterial respiratory infections – acute otitis media, acute sinusitis, acute conjunctivitis, and pneumonia – almost always is preceded by a viral URI. Viruses activate multiple target cells in the upper respiratory tract to produce an array of proinflammatory cytokines and chemokines. The symptoms of a viral URI resolve coinciding with an anti-inflammatory response and adaptive immunity.
In recent work, we found a higher frequency of viral URIs in children who experienced more frequent acute otitis media (AOM). We sought to understand why this might occur by comparing levels of inflammatory cytokines/chemokines in the nose during viral URI that did not precipitate AOM versus when a viral URI precipitated an AOM episode. When a child had a viral URI but did not go on to experience an AOM, the child had higher proinflammatory responses than when the viral URI precipitated an AOM. When differences of levels of proinflammatory cytokines/chemokines were compared in otitis-prone and non–otitis-prone children, lower nasal responses were associated with higher otitis-prone classification frequency (Clin Infect Dis. 2018. doi: 10.1093/cid/ciy750).
The powerful virus and the inflammatory response it can induce also play a major role in allergy and asthma. Viral URIs enhance allergic sensitization to respiratory viruses, such as influenza and respiratory syncytial virus, cause cytopathic damage to airway epithelium, promote excessive proinflammatory cytokine/chemokine production, and increase the exposure of allergens and irritants to antigen-presenting cells. Viral infections also may induce the release of epithelial mediators and cytokines that may propagate eosinophilia. Viral URIs, particularly with respiratory syncytial virus and rhinovirus, are the most common causes of wheezing in children, and they have important influences on the development of asthma. Studies have shown that viral infections trigger up to 85% of asthma exacerbations in school-aged children.
Because this column is being published during the winter, a brief discussion of influenza as a powerful virus is appropriate. Influenza occurs in winter outbreaks of varying extent every year. The severity of the influenza season reflects the changing nature of the antigenic properties of influenza viruses, and their spread depends on susceptibility of the population. Influenza outbreaks typically peak over a 2-3 week period and last for 2-3 months. Most outbreaks have attack rates of 10%-20% in children. There may be variations in disease severity caused by different influenza virus types. The symptoms are caused by excessive proinflammatory cytokine/chemokine production in the nose and lung.
Influenza and other viruses can precipitate the systemic inflammatory response syndrome (SIRS), a manifestation of extreme immune dysregulation resulting in organ dysfunction that clinically resembles bacterial sepsis. In this syndrome, tissues remote from the original insult display the cardinal signs of inflammation, including vasodilation, increased microvascular permeability, and leukocyte accumulation. SIRS is another example of the double-edged sword of inflammation.
The onset and progression of SIRS occurs because of dysregulation of the normal inflammatory response, usually with an increase in both proinflammatory and anti-inflammatory cytokines and chemokines, initiating a chain of events that leads to organ failure.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at [email protected].
Full-dose quadrivalent flu vaccine shows increased efficacy in children
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
according to data from a randomized trial of nearly 2,000 children aged 6-35 months.
Data from previous studies have suggested that a full dose of vaccine may be more immunogenic in young children compared with a half dose, and Sanofi Pasteur has submitted a supplemental Biologics License Application to the Food and Drug Administration to allow use of the full 0.5-mL dose in children as young as 6 months, Monica Mercer, MD, of Sanofi Pasteur, said at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices in Atlanta.
Dr. Mercer presented findings from a phase IV randomized, observer-blinded study, in which the researchers assigned healthy children aged 6-35 months to receive Fluzone quadrivalent vaccine at a dose of 0.25 mL or 0.5 mL.
A total of 1,941 children (949 for the 0.25-mL dose and 992 for the 0.5-mL dose) were included in the safety analysis.
The most important safety outcome was to compare the rate of any fever, Dr. Mercer said at the meeting.
Overall, at 7 days after vaccination, the rate of fever was 11% for the half dose and 12% for the full dose, she said. The resulting difference of 0.84% met the criteria for noninferiority (less than 5%), she added.
In terms of safety, tenderness was the most frequently reported injection site reaction, noted in 47% of the half-dose group and 50% of the full-dose group. The rates of unsolicited adverse events were similar in both groups, the most common included diarrhea and cough, Dr. Mercer said.
No subjects in the full-dose group and three in the half-dose group discontinued the study because of adverse events. The only reported serious adverse event was one case of chronic urticaria in the half-dose group; no deaths were reported in either group.
As for efficacy, the full dose demonstrated noninferiority, compared with the half dose, against each of four strains: influenza A H1N1, influenza A H3N2, influenza B Victoria, and influenza B Yamagata. The geometric mean titers of the full and half doses for each of the four strains were, respectively, 310 and 214, 332 and 221, 348 and 261, and 349 and 243.
The potential action date for the supplemental Biologics License Application is January 2019, noted Dr. Mercer, who is employed by Sanofi Pasteur.
REPORTING FROM AN ACIP MEETING