User login
Flu activity declines for second straight week
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.
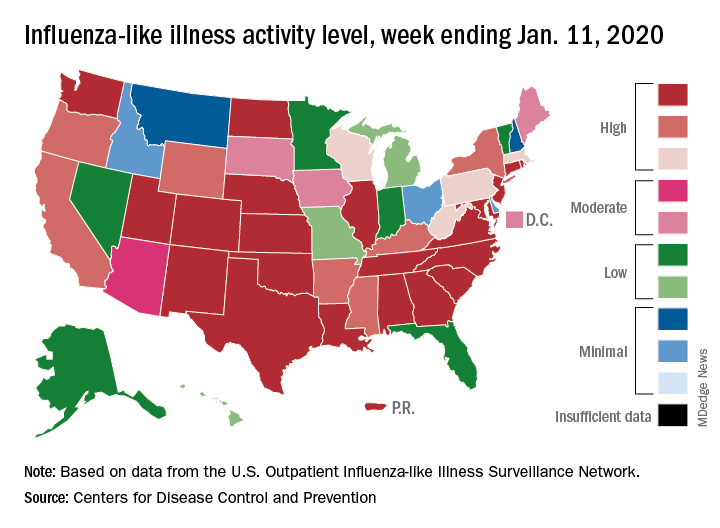
Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.

Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Flu activity dropped nationally for a second consecutive week, but the changing predominance in type from influenza B to A suggests that “it is too early to know whether the season has peaked,” the Centers for Disease Control and Prevention said Jan. 17.

Patients with influenza-like illness (ILI) dropped from 5.7% to 4.7% of all visits to outpatient providers for the week ending Jan. 11, and the proportion of respiratory specimens positive for influenza decreased from 23.6% the week before to 22.9%, the CDC’s influenza division reported.
Despite that overall drop in positive specimens, however, “the percent positive for influenza A viruses increased and some regions are seeing increases in the proportion of influenza A(H1N1)pdm09 viruses compared to other influenza viruses,” the influenza division noted.
Outpatient activity on the state level also was down for the week. There were 23 jurisdictions – 21 states, New York City, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Jan. 11, compared with 33 the previous week, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Indicators of ILI severity have not risen to high levels. “The percentage of deaths attributed to pneumonia and influenza increased from 6.0% to 6.9% but remains below the epidemic threshold” of 7.0% for the week, and the hospitalization rate remains at a fairly typical level for this time of year, the influenza division said.
For the week ending Jan. 11, 7 new ILI-related pediatric deaths were reported, which brings the total to 39 for the 2019-2020 season. Children aged 0-4 years are the second-most likely age group to be hospitalized with the flu (34.4/100,000 population) after adults aged 65 years and older, who have a cumulative rate of 47.6/100,000 for the season, the CDC reported.
Drop in flu activity may not signal seasonal peak
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.
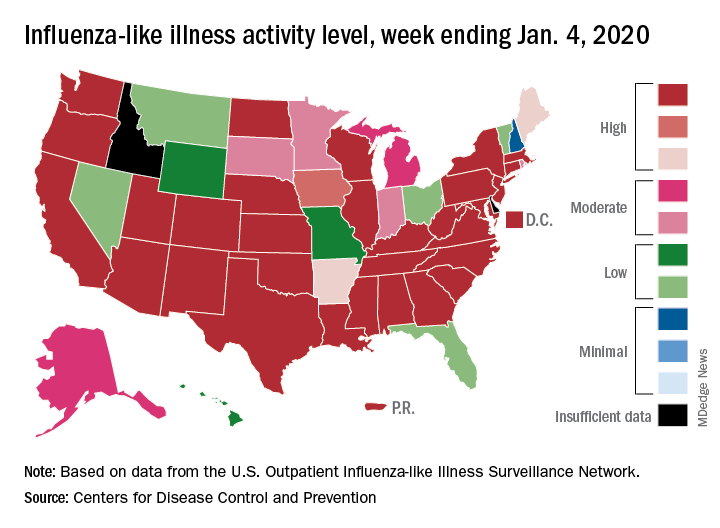
Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.

Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
A key indicator of flu activity dropped but remains high, but measures of severity have not yet shown any unusual increases, according to the Centers for Disease Control and Prevention.

Patients with influenza-like illness (ILI) made up an estimated 5.8% of the visits to outpatient providers during the week ending Jan. 4, and that’s a decline from 7.0% for the last full week of 2019, the CDC’s influenza division reported.
That 7.0% outpatient ILI visit rate was the highest seen in December since 2003, but “hospitalization rates and percent of deaths due to pneumonia and influenza remain low,” the influenza division said in its weekly report.
Influenza B/Victoria and influenza A(H1N1)pdm09 viruses have been the predominant strains so far this season, and they “are more likely to affect children and younger adults than the elderly. Because the majority of hospitalizations and deaths occur among people age 65 and older, with fewer illnesses among that group, we expect, on a population level, to see less impact in flu-related hospitalizations and deaths,” the CDC said.
Last year, there was a similar drop in the outpatient ILI rate in early January after visits rose through December. The rate then increased for another 5 weeks before peaking at 5.0% in February. A similar pattern also occurred during the 2016-2017 and 2015-2016 seasons, CDC data show.
The nationwide ILI hospitalization rate, which is cumulative through the season, was up to 14.6 per 100,000 population for the week ending Jan. 4, the CDC said. Here are the corresponding rates for each of the last five seasons:
- 11.6 (2018-2019).
- 30.5 (2017-2018).
- 12.2 (2016-2017).
- 1.8 (2015-2016).
- 38.3 (2014-2015).
There were five new ILI-related pediatric deaths reported for the week ending Jan. 4, two of which occurred the week before. The total is now up to 32 for the 2019-2020 season, the CDC said in the weekly report. Last season, there were 21 pediatric deaths through the first January report, compared with 42 during the 2017-2018 season and 13 in 2016-2017.
Flu records most active December since 2003
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.
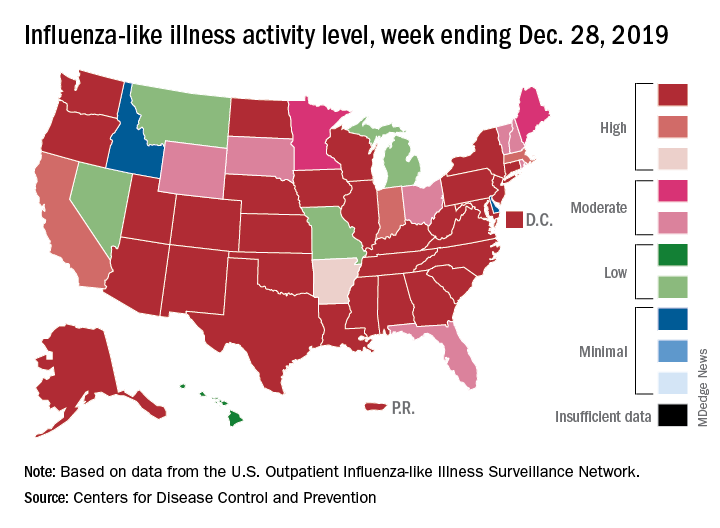
For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.

For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
The 2019-2020 flu season took a big jump in severity during the last full week of 2019, according to the Centers for Disease Control and Prevention.

For the week ending Dec. 28, 6.9% of all outpatient visits to health care providers were for influenza-like illness (ILI), the CDC’s influenza division reported Jan. 3. That is up from 5.1% the previous week and is the highest rate recorded in December since 2003. During the flu pandemic season of 2009-2010, the rate peaked in October and dropped to relatively normal levels by the end of November, CDC data show.
This marks the eighth consecutive week that the outpatient visit rate has been at or above the nation’s baseline level of 2.4%, but the data for this week “may in part be influenced by changes in healthcare-seeking behavior that can occur during the holidays,” the CDC suggested.
All those outpatient visits mean that the ILI activity map is getting quite red. Thirty states, as well as the District of Columbia and Puerto Rico, were at the highest level on the CDC’s 1-10 activity scale during the week ending Dec. 28, compared with 20 the week before. Four states were categorized in the “high” range with activity levels of 8 and 9.
There have been approximately 6.4 million flu illnesses so far this season, the CDC estimated, along with 55,000 hospitalizations, although the ILI admission rate of 9.2 per 100,000 population is fairly typical for this time of year.
The week of Dec. 28 also brought reports of five more ILI-related pediatric deaths, which all occurred in the two previous weeks. A total of 27 children have died from the flu so far during the 2019-2020 season, the CDC said.
Early increase in flu activity shows no signs of slowing
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.

reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.

reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
An important measure of U.S. flu activity for the 2019-2020 season has already surpassed last season’s high, and more than half the states are experiencing high levels of activity, according to the Centers for Disease Control and Prevention.

reported Dec. 27.
The last time the outpatient visit rate was higher than that was in February of the 2017-2018 season, when it peaked at 7.5%. The peak month of flu activity occurs most often – about once every 3 years – in February, and the odds of a December peak are about one in five, the CDC has said.
Outpatient illness activity also increased at the state level during the week ending Dec. 21. There were 20 jurisdictions – 18 states, the District of Columbia, and Puerto Rico – at level 10 on the CDC’s 1-10 scale of activity, compared with 13 the previous week, and the number of jurisdictions in the “high” range (levels 8-10) jumped from 21 to 28, the CDC data show.
The influenza division estimated that there have been 4.6 million flu illnesses so far this season, nearly a million more than the total after last week, along with 39,000 hospitalizations. The overall hospitalization rate for the season is up to 6.6 per 100,000 population, which is about average at this point. The proportion of deaths attributed to pneumonia and influenza increased to 5.7%, which is below the epidemic threshold, the CDC said.
Three pediatric deaths related to influenza-like illness were reported during the week ending Dec. 21, two of which occurred in an earlier week. For the 2019-2020 season so far, a total of 22 pediatric deaths have been reported to the CDC.
Influenza activity continues to be unusually high
The 2019-2020 flu season continues its unusually early rise in activity, with the Centers for Disease Control and Prevention estimating that 3.7 million cases have occurred through Dec. 14.
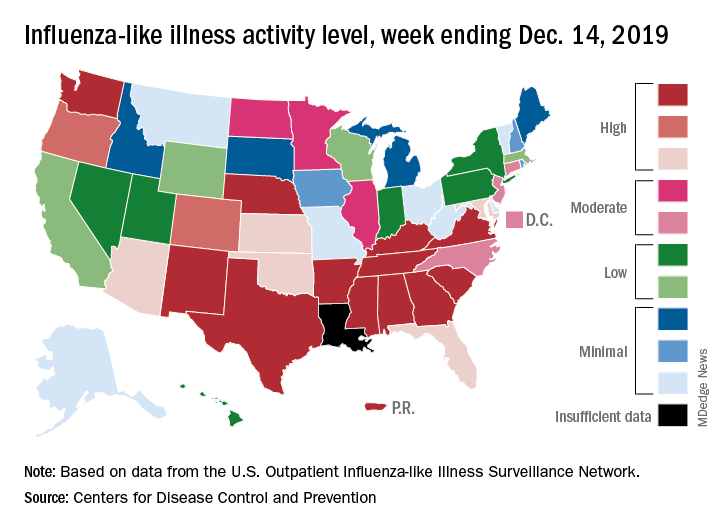
which is up from 3.2% the previous week and is the sixth consecutive week that the United States has been at or above the national baseline of 2.4%, the CDC reported Dec. 20. This year’s 3.9% is the highest mid-December rate recorded since 2003, when it reached almost 7.4%.
Most of the influenza activity so far this season is being driven by influenza B/Victoria viruses. Nationwide testing puts influenza B prevalence at 68.5% of all positive specimens, exactly the same as last week, but A(H1N1) viruses “are increasing in proportion relative to other influenza viruses in some regions,” the CDC’s influenza division said.
A look at this week’s activity map shows that 21 states, compared with 12 last week, were in the “high” range of activity – that’s levels 8-10 on the CDC’s 1-10 scale. Twelve of those states, along with Puerto Rico, were at level 10, which was up from nine a week earlier, the CDC said.
The overall hospitalization rate through the week of Dec. 8-14 (5.5 per 100,000 population) “is similar to what has been seen at this time during recent seasons,” the CDC noted. The highest rates are occurring among adults over age 65 years (12.7 per 100,000) and children aged 0-4 years (10.9 per 100,000).
Three ILI-related deaths among children that occurred last week were reported, which brings the total for the 2019-2020 season to 19, the CDC said.
The 2019-2020 flu season continues its unusually early rise in activity, with the Centers for Disease Control and Prevention estimating that 3.7 million cases have occurred through Dec. 14.

which is up from 3.2% the previous week and is the sixth consecutive week that the United States has been at or above the national baseline of 2.4%, the CDC reported Dec. 20. This year’s 3.9% is the highest mid-December rate recorded since 2003, when it reached almost 7.4%.
Most of the influenza activity so far this season is being driven by influenza B/Victoria viruses. Nationwide testing puts influenza B prevalence at 68.5% of all positive specimens, exactly the same as last week, but A(H1N1) viruses “are increasing in proportion relative to other influenza viruses in some regions,” the CDC’s influenza division said.
A look at this week’s activity map shows that 21 states, compared with 12 last week, were in the “high” range of activity – that’s levels 8-10 on the CDC’s 1-10 scale. Twelve of those states, along with Puerto Rico, were at level 10, which was up from nine a week earlier, the CDC said.
The overall hospitalization rate through the week of Dec. 8-14 (5.5 per 100,000 population) “is similar to what has been seen at this time during recent seasons,” the CDC noted. The highest rates are occurring among adults over age 65 years (12.7 per 100,000) and children aged 0-4 years (10.9 per 100,000).
Three ILI-related deaths among children that occurred last week were reported, which brings the total for the 2019-2020 season to 19, the CDC said.
The 2019-2020 flu season continues its unusually early rise in activity, with the Centers for Disease Control and Prevention estimating that 3.7 million cases have occurred through Dec. 14.

which is up from 3.2% the previous week and is the sixth consecutive week that the United States has been at or above the national baseline of 2.4%, the CDC reported Dec. 20. This year’s 3.9% is the highest mid-December rate recorded since 2003, when it reached almost 7.4%.
Most of the influenza activity so far this season is being driven by influenza B/Victoria viruses. Nationwide testing puts influenza B prevalence at 68.5% of all positive specimens, exactly the same as last week, but A(H1N1) viruses “are increasing in proportion relative to other influenza viruses in some regions,” the CDC’s influenza division said.
A look at this week’s activity map shows that 21 states, compared with 12 last week, were in the “high” range of activity – that’s levels 8-10 on the CDC’s 1-10 scale. Twelve of those states, along with Puerto Rico, were at level 10, which was up from nine a week earlier, the CDC said.
The overall hospitalization rate through the week of Dec. 8-14 (5.5 per 100,000 population) “is similar to what has been seen at this time during recent seasons,” the CDC noted. The highest rates are occurring among adults over age 65 years (12.7 per 100,000) and children aged 0-4 years (10.9 per 100,000).
Three ILI-related deaths among children that occurred last week were reported, which brings the total for the 2019-2020 season to 19, the CDC said.
Flu activity dropped in early December
according to the Centers for Disease Control and Prevention.
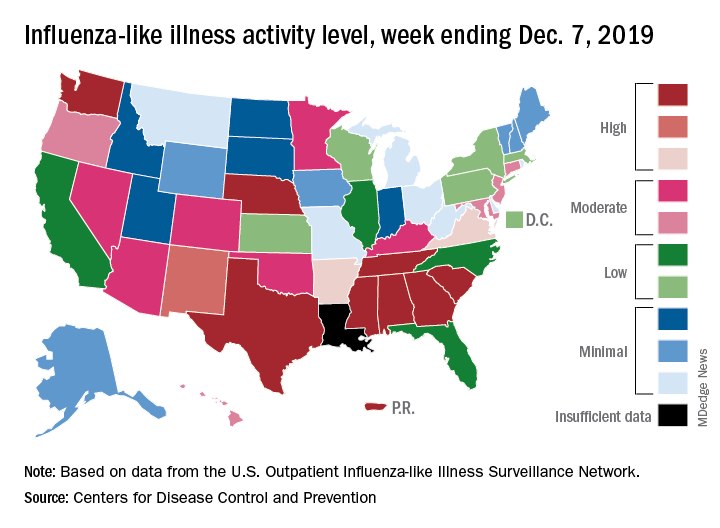
Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
according to the Centers for Disease Control and Prevention.

Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
according to the Centers for Disease Control and Prevention.

Nationally, 3.2% of outpatient visits were for influenza-like illness (ILI) during the week of Dec. 1-7, the CDC reported. That is down from 3.4% the week before, which was the highest November rate in 10 years. The national baseline rate is 2.4%, and the current 3.2% marks the fifth consecutive week that the outpatient ILI rate has been at or above the baseline level, the CDC report noted.
The drop in activity “may be influenced in part by a reduction in routine healthcare visits surrounding the Thanksgiving holiday. … as has occurred during previous seasons,” the CDC influenza division said Dec. 13 in its weekly flu report.
The early spike in “activity is being caused mostly by influenza B/Victoria viruses, which is unusual for this time of year,” the report said. Since the beginning of the 2019-2020 season a little over 2 months ago, almost 70% of specimens that have been positive for influenza have been identified as type B.
The nationwide decline in activity doesn’t, however, show up at the state level. For the week ending Dec. 7, there were eight states along with Puerto Rico at level 10 on the CDC’s 1-10 scale of flu activity, as there were the previous week. Washington state moved up from 9 to 10, but Louisiana, which was at level 10 last week, had insufficient data to be included this week, the CDC data show.
There were four flu-related pediatric deaths reported to the CDC during the week ending Dec. 7, all occurring in previous weeks, which brings the total to 10 for the season. In 2018-2019, there were 143 pediatric deaths caused by influenza, the CDC said.
Many Americans planning to avoid flu vaccination
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.
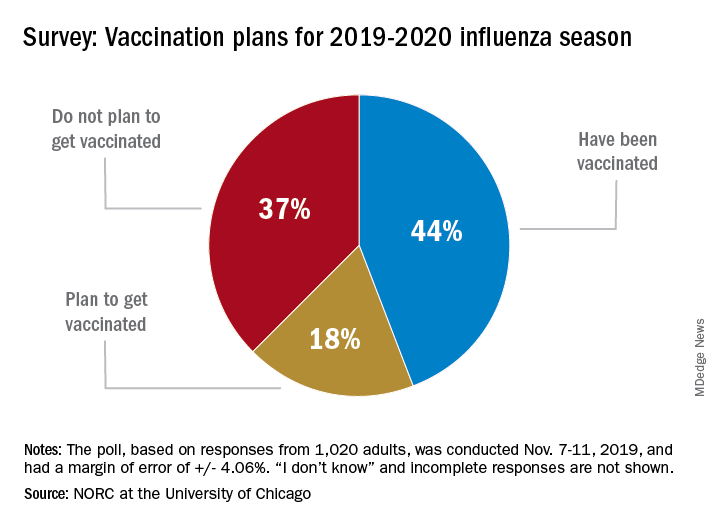
Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.

Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
As the 2019-20 flu season got underway, more than half of American adults had not yet been vaccinated, according to a survey from the research organization NORC at the University of Chicago.

Only 44% of the 1,020 adults surveyed said that they had already received the vaccine as of Nov. 7-11, when the poll was conducted. Another the NORC reported. About 1% of those surveyed said they didn’t know or skipped the question.
Age was a strong determinant of vaccination status: 35% of those aged 18-29 years had gotten their flu shot, along with 36% of respondents aged 30-44 years and 34% of those aged 45- 59 years, compared with 65% of those aged 60 years and older. Of the respondents with children under age 18 years, 43% said that they were not planning to have the children vaccinated, the NORC said.
Concern about side effects, mentioned by 37% of those who were not planning to get vaccinated, was the most common reason given to avoid a flu shot, followed by belief that the vaccine doesn’t work very well (36%) and “never get the flu” (26%), the survey results showed.
“Widespread misconceptions exist regarding the safety and efficacy of flu shots. Because of the way the flu spreads in a community, failing to get a vaccination not only puts you at risk but also others for whom the consequences of the flu can be severe. Policymakers should focus on changing erroneous beliefs about immunizing against the flu,” said Caitlin Oppenheimer, who is senior vice president of public health research for the NORC, which has conducted the National Immunization Survey for the Centers for Disease Control and Prevention since 2005.
Influenza already in midseason form
It’s been a decade since flu activity levels were this high this early in the season.
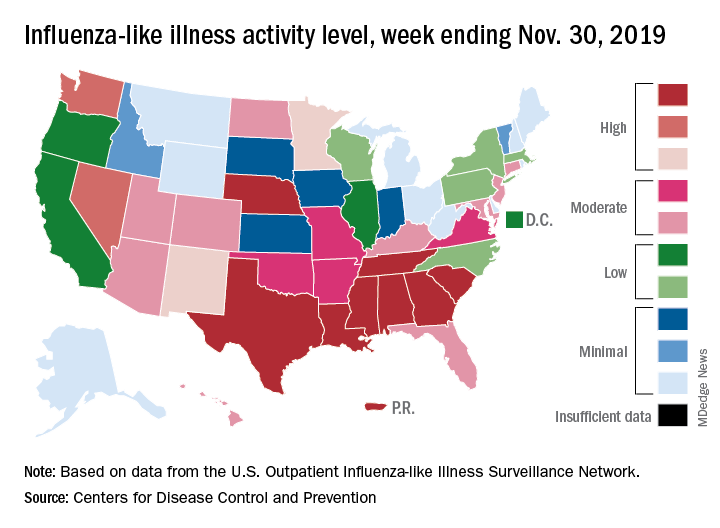
For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
It’s been a decade since flu activity levels were this high this early in the season.

For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
It’s been a decade since flu activity levels were this high this early in the season.

For the week ending Nov. 30, outpatient visits for influenza-like illness reached 3.5% of all visits to health care providers, the Centers for Disease Control and Prevention reported Dec. 6. That is the highest pre-December rate since the pandemic of 2009-2010, when the rate peaked at 7.7% in mid-October, CDC data show.
For the last week of November, eight states and Puerto Rico reported activity levels at the high point of the CDC’s 1-10 scale, which is at least five more states than any of the past five flu seasons. Three of the last five seasons had no states at level 10 this early in the season.
Another 4 states at levels 8 and 9 put a total of 13 jurisdictions in the “high” range of flu activity, with another 14 states in the “moderate” range of levels 6 and 7. Geographically speaking, 24 jurisdictions are experiencing regional or widespread activity, which is up from the 15 reported last week, the CDC’s influenza division said.
The hospitalization rate to date for the 2019-2020 season – 2.7 per 100,000 population – is “similar to what has been seen at this time during other recent seasons,” the CDC said.
One influenza-related pediatric death was reported during the week ending Nov. 30, which brings the total for the season to six, according to the CDC report.
2019-2020 flu season starts off full throttle
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.
For the week ending Nov. 23, there were five states, along with Puerto Rico, at the highest level of the Centers for Disease Control and Prevention’s 1-10 scale of flu activity. That’s more than any year since 2012, including the pandemic season of 2017-2018, according to CDC data, and may suggest either an early peak or the beginning of a particularly bad winter.
“Nationally, ILI [influenza-like illness] activity has been at or above baseline for 3 weeks; however, the amount of influenza activity across the country varies with the south and parts of the west seeing elevated activity while other parts of the country are still seeing low activity,” the CDC’s influenza division said in its weekly FluView report.
The five highest-activity states – Alabama, Georgia, Louisiana, Mississippi, and Texas – are all at level 10, and they join two others – South Carolina and Tennessee, which are at level 8 – in the “high” range from 8-10 on the ILI activity scale; Puerto Rico also is at level 10. ILI is defined as “fever (temperature of 100° F [37.8° C] or greater) and a cough and/or a sore throat without a known cause other than influenza,” the CDC said.
The activity scale is based on the percentage of outpatient visits for ILI in each state, which is reported to the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) each week. The national rate for the week ending Nov. 23 was 2.9%, which is above the new-for-this-season baseline rate of 2.4%. For the three previous flu seasons, the national baseline was 2.2%, having been raised from its previous level of 2.1% in 2015-2016, CDC data show.
The peak month of flu activity occurs most often in February – 15 times from 1982-1983 to 2017-2018 – but there were seven peaks in December and six each in January and March over that time period, along with one peak each in October and November, the CDC said. The October peak occurred during the H1N1 pandemic year of 2009, when the national outpatient ILI rate climbed to just over 7.7%.
Flu vaccine cuts infection severity in kids and adults
WASHINGTON –
During recent U.S. flu seasons, children and adults who contracted influenza despite vaccination had significantly fewer severe infections and infection complications, compared with unimmunized people, according to two separate reports from CDC researchers presented at an annual scientific meeting on infectious diseases.
One of the reports tracked the impact of flu vaccine in children using data that the CDC collected at seven medical centers that participated in the agency’s New Vaccine Surveillance Network, which provided information on children aged 6 months to 17 years who were hospitalized for an acute respiratory illness, including more than 1,700 children during the 2016-2017 flu season and more than 1,900 during the 2017-2018 season. Roughly 10% of these children tested positive for influenza, and the subsequent analysis focused on these cases and compared incidence rates among children who had been vaccinated during the index season and those who had remained unvaccinated.
Combined data from both seasons showed that vaccinated children were 50% less likely to have been hospitalized for an acute influenza infection, compared with unvaccinated kids, a pattern consistently seen both in children aged 6 months to 8 years and in those aged 9-17 years. The pattern of vaccine effectiveness also held regardless of which flu strain caused the infections, reported Angela P. Campbell, MD, a CDC medical officer.
“We saw a nice benefit from vaccination, both in previously healthy children and in those with an underlying medical condition,” a finding that adds to existing evidence of vaccine effectiveness, Dr. Campbell said in a video interview. The results confirmed that flu vaccination does not just prevent infections but also cuts the rate of more severe infections that lead to hospitalization, she explained.
Another CDC study looked at data collected by the agency’s Influenza Hospitalization Surveillance Network from adults at least 18 years old who were hospitalized for a laboratory-confirmed influenza infection during five flu seasons, 2013-2014 through 2017-18. The data, which came from more than 250 acute-care hospitals in 13 states, included more than 43,000 people hospitalized for an identified influenza strain and with a known vaccination history who were not institutionalized and had not received any antiviral treatment.
After propensity-weighted adjustment to create better parity between the vaccinated and unvaccinated patients, the results showed that people 18-64 years old with vaccination had statistically significant decreases in mortality of a relative 36%, need for mechanical ventilation of 34%, pneumonia of 20%, and need for ICU admission of a relative 19%, as well as an 18% drop in average ICU length of stay, Shikha Garg, MD, said at the meeting. The propensity-weighted analysis of data from people at least 65 years old showed statistically significant relative reductions linked with vaccination: 46% reduction in the need for mechanical ventilation, 28% reduction in ICU admissions, and 9% reduction in hospitalized length of stay.
Further analysis of these outcomes by the strains that caused these influenza infections showed that the statistically significant benefits from vaccination were seen only in patients infected with an H1N1 strain. Statistically significant effects on these severe outcomes were not apparent among people infected with the H3N2 or B strains, said Dr. Garg, a medical epidemiologist at the CDC.
“All adults should receive an annual flu vaccination as it can improve outcomes among those who develop influenza despite vaccination,” she concluded.
Results from a third CDC study reported at the meeting examined the importance of two vaccine doses (administered at least 4 weeks apart) given to children aged 6 months to 8 years for the first season they receive flu vaccination, which is the immunization approach for flu recommended by the CDC. The findings from a total of more than 7,500 children immunized during the 2014-2018 seasons showed a clear increment in vaccine protection among kids who received two doses during their first season vaccinated, especially in children who were 2 years old or younger. In that age group, administration of two doses produced vaccine effectiveness of 53% versus a 23% vaccine effectiveness after a single vaccine dose, reported Jessie Chung, a CDC epidemiologist.
WASHINGTON –
During recent U.S. flu seasons, children and adults who contracted influenza despite vaccination had significantly fewer severe infections and infection complications, compared with unimmunized people, according to two separate reports from CDC researchers presented at an annual scientific meeting on infectious diseases.
One of the reports tracked the impact of flu vaccine in children using data that the CDC collected at seven medical centers that participated in the agency’s New Vaccine Surveillance Network, which provided information on children aged 6 months to 17 years who were hospitalized for an acute respiratory illness, including more than 1,700 children during the 2016-2017 flu season and more than 1,900 during the 2017-2018 season. Roughly 10% of these children tested positive for influenza, and the subsequent analysis focused on these cases and compared incidence rates among children who had been vaccinated during the index season and those who had remained unvaccinated.
Combined data from both seasons showed that vaccinated children were 50% less likely to have been hospitalized for an acute influenza infection, compared with unvaccinated kids, a pattern consistently seen both in children aged 6 months to 8 years and in those aged 9-17 years. The pattern of vaccine effectiveness also held regardless of which flu strain caused the infections, reported Angela P. Campbell, MD, a CDC medical officer.
“We saw a nice benefit from vaccination, both in previously healthy children and in those with an underlying medical condition,” a finding that adds to existing evidence of vaccine effectiveness, Dr. Campbell said in a video interview. The results confirmed that flu vaccination does not just prevent infections but also cuts the rate of more severe infections that lead to hospitalization, she explained.
Another CDC study looked at data collected by the agency’s Influenza Hospitalization Surveillance Network from adults at least 18 years old who were hospitalized for a laboratory-confirmed influenza infection during five flu seasons, 2013-2014 through 2017-18. The data, which came from more than 250 acute-care hospitals in 13 states, included more than 43,000 people hospitalized for an identified influenza strain and with a known vaccination history who were not institutionalized and had not received any antiviral treatment.
After propensity-weighted adjustment to create better parity between the vaccinated and unvaccinated patients, the results showed that people 18-64 years old with vaccination had statistically significant decreases in mortality of a relative 36%, need for mechanical ventilation of 34%, pneumonia of 20%, and need for ICU admission of a relative 19%, as well as an 18% drop in average ICU length of stay, Shikha Garg, MD, said at the meeting. The propensity-weighted analysis of data from people at least 65 years old showed statistically significant relative reductions linked with vaccination: 46% reduction in the need for mechanical ventilation, 28% reduction in ICU admissions, and 9% reduction in hospitalized length of stay.
Further analysis of these outcomes by the strains that caused these influenza infections showed that the statistically significant benefits from vaccination were seen only in patients infected with an H1N1 strain. Statistically significant effects on these severe outcomes were not apparent among people infected with the H3N2 or B strains, said Dr. Garg, a medical epidemiologist at the CDC.
“All adults should receive an annual flu vaccination as it can improve outcomes among those who develop influenza despite vaccination,” she concluded.
Results from a third CDC study reported at the meeting examined the importance of two vaccine doses (administered at least 4 weeks apart) given to children aged 6 months to 8 years for the first season they receive flu vaccination, which is the immunization approach for flu recommended by the CDC. The findings from a total of more than 7,500 children immunized during the 2014-2018 seasons showed a clear increment in vaccine protection among kids who received two doses during their first season vaccinated, especially in children who were 2 years old or younger. In that age group, administration of two doses produced vaccine effectiveness of 53% versus a 23% vaccine effectiveness after a single vaccine dose, reported Jessie Chung, a CDC epidemiologist.
WASHINGTON –
During recent U.S. flu seasons, children and adults who contracted influenza despite vaccination had significantly fewer severe infections and infection complications, compared with unimmunized people, according to two separate reports from CDC researchers presented at an annual scientific meeting on infectious diseases.
One of the reports tracked the impact of flu vaccine in children using data that the CDC collected at seven medical centers that participated in the agency’s New Vaccine Surveillance Network, which provided information on children aged 6 months to 17 years who were hospitalized for an acute respiratory illness, including more than 1,700 children during the 2016-2017 flu season and more than 1,900 during the 2017-2018 season. Roughly 10% of these children tested positive for influenza, and the subsequent analysis focused on these cases and compared incidence rates among children who had been vaccinated during the index season and those who had remained unvaccinated.
Combined data from both seasons showed that vaccinated children were 50% less likely to have been hospitalized for an acute influenza infection, compared with unvaccinated kids, a pattern consistently seen both in children aged 6 months to 8 years and in those aged 9-17 years. The pattern of vaccine effectiveness also held regardless of which flu strain caused the infections, reported Angela P. Campbell, MD, a CDC medical officer.
“We saw a nice benefit from vaccination, both in previously healthy children and in those with an underlying medical condition,” a finding that adds to existing evidence of vaccine effectiveness, Dr. Campbell said in a video interview. The results confirmed that flu vaccination does not just prevent infections but also cuts the rate of more severe infections that lead to hospitalization, she explained.
Another CDC study looked at data collected by the agency’s Influenza Hospitalization Surveillance Network from adults at least 18 years old who were hospitalized for a laboratory-confirmed influenza infection during five flu seasons, 2013-2014 through 2017-18. The data, which came from more than 250 acute-care hospitals in 13 states, included more than 43,000 people hospitalized for an identified influenza strain and with a known vaccination history who were not institutionalized and had not received any antiviral treatment.
After propensity-weighted adjustment to create better parity between the vaccinated and unvaccinated patients, the results showed that people 18-64 years old with vaccination had statistically significant decreases in mortality of a relative 36%, need for mechanical ventilation of 34%, pneumonia of 20%, and need for ICU admission of a relative 19%, as well as an 18% drop in average ICU length of stay, Shikha Garg, MD, said at the meeting. The propensity-weighted analysis of data from people at least 65 years old showed statistically significant relative reductions linked with vaccination: 46% reduction in the need for mechanical ventilation, 28% reduction in ICU admissions, and 9% reduction in hospitalized length of stay.
Further analysis of these outcomes by the strains that caused these influenza infections showed that the statistically significant benefits from vaccination were seen only in patients infected with an H1N1 strain. Statistically significant effects on these severe outcomes were not apparent among people infected with the H3N2 or B strains, said Dr. Garg, a medical epidemiologist at the CDC.
“All adults should receive an annual flu vaccination as it can improve outcomes among those who develop influenza despite vaccination,” she concluded.
Results from a third CDC study reported at the meeting examined the importance of two vaccine doses (administered at least 4 weeks apart) given to children aged 6 months to 8 years for the first season they receive flu vaccination, which is the immunization approach for flu recommended by the CDC. The findings from a total of more than 7,500 children immunized during the 2014-2018 seasons showed a clear increment in vaccine protection among kids who received two doses during their first season vaccinated, especially in children who were 2 years old or younger. In that age group, administration of two doses produced vaccine effectiveness of 53% versus a 23% vaccine effectiveness after a single vaccine dose, reported Jessie Chung, a CDC epidemiologist.
REPORTING FROM ID WEEK 2019

