User login
Arginine deficiency implicated in novel hemorrhagic fever fatality
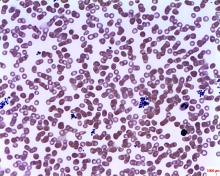
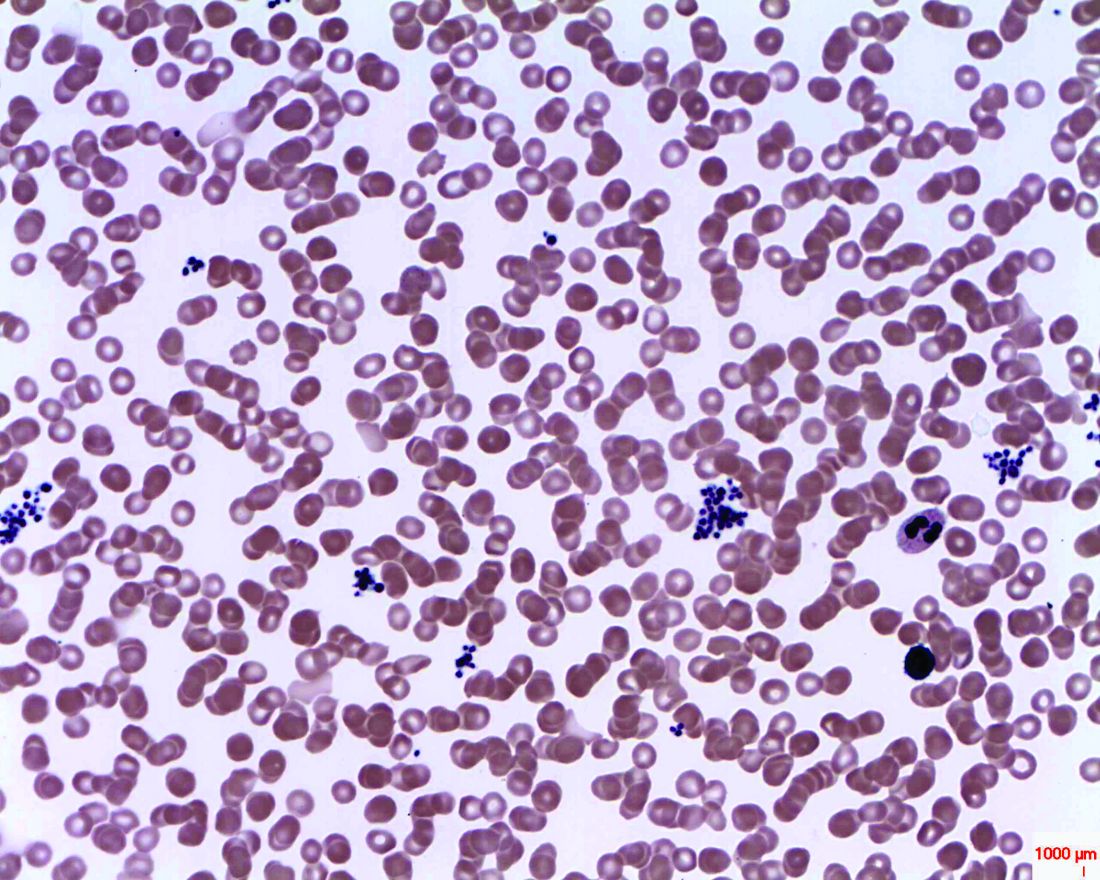
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Low arginine bioavailability was associated with increased risk for severe fever with thrombocytopenia syndrome (SFTS) fatality.
Major finding: Arginine bioavailability had an area under the receiver operating curve of 0.713 for predicting fatality.
Study details: A prospective cohort metabolomics study of 242 serum samples from patients with and without SFTS and a randomized, double-blind, placebo-controlled clinical trial of 113 patients given intravenous arginine supplementation or vehicle alone, in conjunction with supportive care.
Disclosures: The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
Source: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
UN aims to eradicate TB by 2030
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.
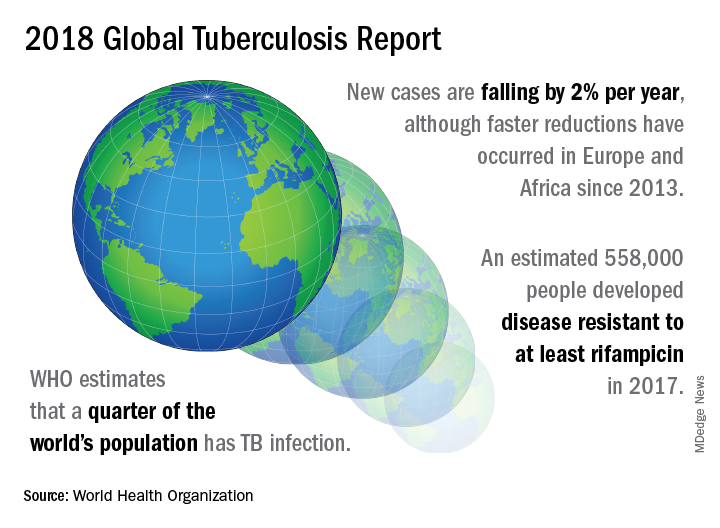
On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
FROM A WORLD HEALTH ORGANIZATION PRESS CONFERENCE
Cutaneous lesions? Consider C. diphtheriae in those with foreign travel
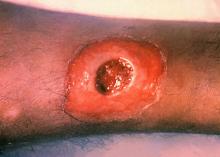
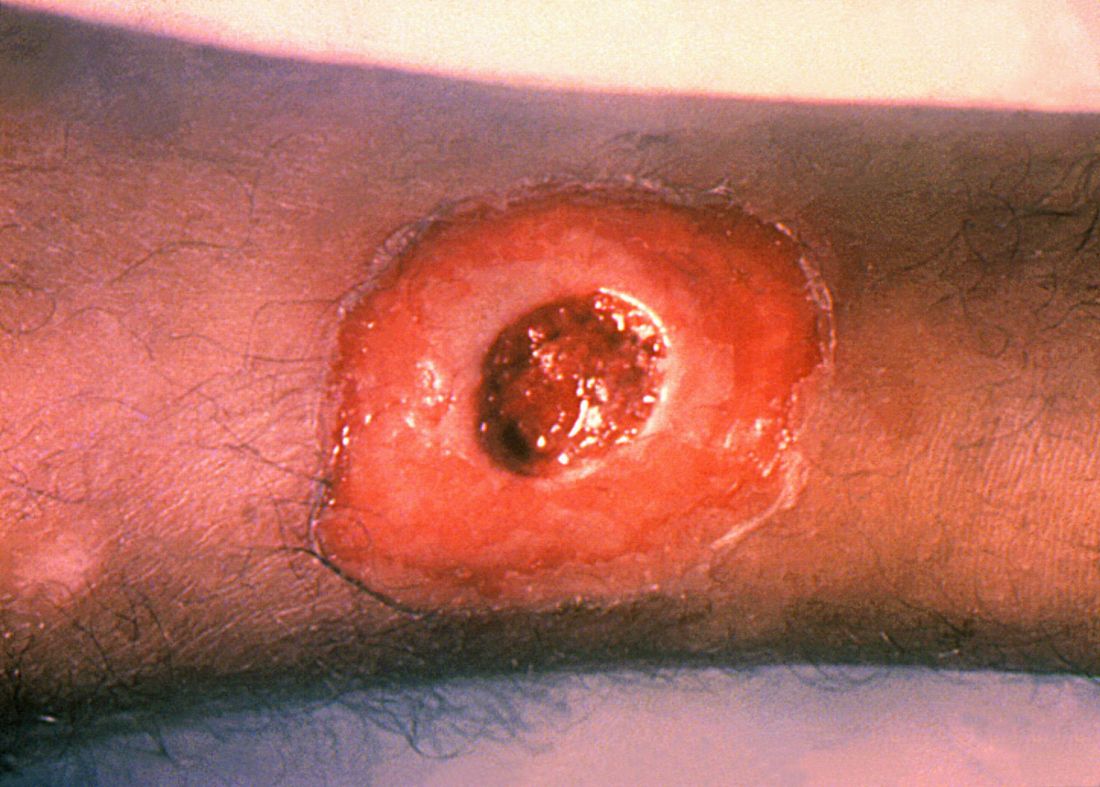
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
ATLANTA – Seven cases of imported Corynebacterium diphtheriae in Minnesota highlight the importance of maintaining suspicion that cutaneous lesions in individuals with recent travel to endemic countries might be associated with C. diphtheriae infection.
The cases also underscore the importance of referring C. diphtheriae isolates to state health departments for confirmatory testing, Jayne Griffith, of the Minnesota Department of Health, and her colleagues reported in a poster at the International Conference on Emerging Infectious Diseases.
“C. diphtheriae infections was not clinically suspected in any of these case-patients. All infections were initially identified solely by [matrix-assisted laser desorption/ionization time-of-flight spectrometry] testing performed at the clinical institutions,” the investigators wrote. “Confirmation and further toxigenicity testing allowed for prompt case investigation and public health response, preventing disease spread.”
Infections caused by toxigenic C. diphtheriae are rare in the United States because of widespread vaccination, but remain endemic in countries with suboptimal vaccine coverage. For this reason, infection is a concern for unvaccinated individuals traveling to diphtheria-endemic countries as well as for those who have contact with people from these areas. The investigators noted that “infections are primarily respiratory or cutaneous; respiratory infections can be life-threatening and cutaneous wounds may serve as a reservoir from which bacteria can be transmitted to susceptible contacts.”
The Minnesota cases involved patients who presented with cutaneous ulcers between 2014 and 2017. The Minnesota Department of Health confirmed C. diphtheriae status by culture after the initial identification at private institutions or providers using matrix-assisted laser desorption/ionization time-of-flight spectrometry. Isolates were sent to the Centers for Disease Control and Prevention Pertussis and Diphtheria Laboratory for biotyping and confirmation of toxigenicity.
The CDC confirmed that isolates from two patients were toxigenic C. diphtheriae biotype mitis. The remaining cases were nontoxigenic diphtheria, including C. diphtheriae mitis (three case-patients, including one who also had Staphylococcus aureus, and another who also had methicillin-resistant S. aureus) and two case-patients with C. diphtheriae belfanti.
The patients with toxigenic infection included a 35-year-old woman who developed an abdominal boil after spending months in Somalia, and a 48-year-old man who cut his leg while in Ethiopia.
The patients with nontoxigenic infection included four foreign-born individuals ranging in age from 7 to 66 years and one 24-year-old man from the United States. One of the foreign-born individuals had immigrated from a diphtheria-endemic country 3 months prior to his diagnosis, but none of the remaining four had traveled outside the United States in the 6 months prior to infection onset. One, however, lived in a home with family members who traveled frequently to eastern Africa. The vaccination status of these patients was unknown.
Contact tracing was conducted and prophylactic antibiotics were recommended as appropriate. Vaccination was recommended when a case-patient and/or contact was inadequately immunized.
Both patients with toxigenic infection experienced wound healing after appropriate antibiotic therapy.
Ms. Griffith reported having no disclosures.
REPORTING FROM ICEID 2018
Key clinical point: Corynebacterium diphtheriae should be considered in individuals who present with cutaneous lesions after traveling to diphtheria-endemic countries.
Major finding: Refer suspect C. diphtheriae isolates to state health departments.
Study details: A review of seven C. diphtheriae cases.
Disclosures: Ms. Griffith reported having no disclosures.
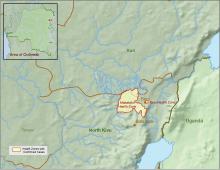
CDC supports Ebola response in DRC
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
The Centers for Disease Control and Prevention has been working with the Ministry of Health of the Democratic Republic of the Congo (DRC) on a new Ebola outbreak reported on Aug, 1, 2018, in North Kivu province.
“For the current outbreak, CDC has deployed experienced Ebola experts to DRC and the World Health Organization [WHO] to provide guidance on coordination of outbreak response, laboratory testing, disease contact tracing, infection control, and health communication,” according to a CDC press release.
The CDC’s online response also provides a Traveler’s Health notice of Watch Level 1 for the DRC, which advises standard precautions and avoiding infected individuals, but not an advisory against travel.
The Ebola virus associated with the current outbreak is Zaire ebolavirus, according to genetic testing by scientists in the DRC. This is the same species that caused an outbreak earlier this year in Equateur province in northwestern DRC, although differences between the genes of the viruses suggest the two outbreaks are not linked, according to the CDC media announcement.
As of Aug. 12, 2018, the following statistics were reported by the WHO on the outbreak:
- Confirmed cases: 30
- Probable cases: 27
- Total cases: 57
- Deaths: 41 (14 confirmed, 27 probable)
The outbreak is of particular concern because of the instability of the area, which hampers relief and quarantine efforts. The outbreak is in a part of the country identified by the U.S. State Department as a “restricted travel” zone due to armed conflict and violence targeting civilians, according to the CDC.
FDA: Krintafel approved as ‘radical cure’ for preventing malaria relapse
The Food and Drug Administration has approved, under priority review, single-dose tafenoquine (to be marketed as Krintafel) for the prevention of relapse in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute Plasmodium vivax infection, according to an announcement by research partners GSK and the nonprofit Medicines for Malaria Venture.
Clinical efficacy and safety of the 300-mg single-dose tablet was provided by three randomized, double-blind studies: DETECTIVE Part 1 and Part 2 (TAF112582) and GATHER (TAF116564). The results of the two phase III studies were announced in June 2017.
Tafenoquine is referred to as a “radical cure,” because it targets the dormant liver forms of P. vivax and is coadministered with currently available antimalarials such as chloroquine or artemisinin-based combination therapies.
“The world has waited decades for a new medicine to counter P. vivax malaria relapse. Today, we can say the wait is over. Moreover, as the first ever single-dose for this indication, Krintafel will help improve patient compliance,” David Reddy, MD, CEO of MMV stated.
The FDA approval letter and the Krintafel label information are available online.
The Food and Drug Administration has approved, under priority review, single-dose tafenoquine (to be marketed as Krintafel) for the prevention of relapse in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute Plasmodium vivax infection, according to an announcement by research partners GSK and the nonprofit Medicines for Malaria Venture.
Clinical efficacy and safety of the 300-mg single-dose tablet was provided by three randomized, double-blind studies: DETECTIVE Part 1 and Part 2 (TAF112582) and GATHER (TAF116564). The results of the two phase III studies were announced in June 2017.
Tafenoquine is referred to as a “radical cure,” because it targets the dormant liver forms of P. vivax and is coadministered with currently available antimalarials such as chloroquine or artemisinin-based combination therapies.
“The world has waited decades for a new medicine to counter P. vivax malaria relapse. Today, we can say the wait is over. Moreover, as the first ever single-dose for this indication, Krintafel will help improve patient compliance,” David Reddy, MD, CEO of MMV stated.
The FDA approval letter and the Krintafel label information are available online.
The Food and Drug Administration has approved, under priority review, single-dose tafenoquine (to be marketed as Krintafel) for the prevention of relapse in patients aged 16 years and older who are receiving appropriate antimalarial therapy for acute Plasmodium vivax infection, according to an announcement by research partners GSK and the nonprofit Medicines for Malaria Venture.
Clinical efficacy and safety of the 300-mg single-dose tablet was provided by three randomized, double-blind studies: DETECTIVE Part 1 and Part 2 (TAF112582) and GATHER (TAF116564). The results of the two phase III studies were announced in June 2017.
Tafenoquine is referred to as a “radical cure,” because it targets the dormant liver forms of P. vivax and is coadministered with currently available antimalarials such as chloroquine or artemisinin-based combination therapies.
“The world has waited decades for a new medicine to counter P. vivax malaria relapse. Today, we can say the wait is over. Moreover, as the first ever single-dose for this indication, Krintafel will help improve patient compliance,” David Reddy, MD, CEO of MMV stated.
The FDA approval letter and the Krintafel label information are available online.
Female survivor transmits Ebola virus 1 year after outbreak
Molecular analysis showed that a Liberian woman who survived Ebola virus disease in 2014 had viral persistence or recurrent disease and transmitted the virus to other family members a year later, according to a study published online in The Lancet Infectious Diseases.
Although the original 2014-2015 Ebola virus disease epidemic in West Africa had been contained, subsequent clusters of infection continued to occur in the region, according to researchers. A particular cluster in Liberia in November 2015 was identified after a 15-year-old boy in Monrovia tested positive.
Based on serology and epidemiological and genomic data, the researchers concluded that this cluster was caused by a woman who survived Ebola virus disease in 2014 and transmitted the virus to three family members a year later.
Ebola transmission from persistently infected male survivors is well documented, but this is the first confirmed evidence for Ebola transmission from a persistently infected female survivor, according to Emily Kainne Dokubo, MD, of the Centers for Disease Control and Prevention and her colleagues.
“The findings from this and recent Ebola virus disease clusters highlight the risk of Ebola virus disease flare-ups even after an outbreak is declared over. Risk assessment and focused prevention efforts are needed for Ebola survivors and their close contacts,” Dr. Dokubo and her colleagues concluded.
The study was funded by the CDC, Defense Threat Reduction Agency, and WHO.
SOURCE: Dokubo EK et al. The Lancet Infectious Diseases, July 23, 2018.
Molecular analysis showed that a Liberian woman who survived Ebola virus disease in 2014 had viral persistence or recurrent disease and transmitted the virus to other family members a year later, according to a study published online in The Lancet Infectious Diseases.
Although the original 2014-2015 Ebola virus disease epidemic in West Africa had been contained, subsequent clusters of infection continued to occur in the region, according to researchers. A particular cluster in Liberia in November 2015 was identified after a 15-year-old boy in Monrovia tested positive.
Based on serology and epidemiological and genomic data, the researchers concluded that this cluster was caused by a woman who survived Ebola virus disease in 2014 and transmitted the virus to three family members a year later.
Ebola transmission from persistently infected male survivors is well documented, but this is the first confirmed evidence for Ebola transmission from a persistently infected female survivor, according to Emily Kainne Dokubo, MD, of the Centers for Disease Control and Prevention and her colleagues.
“The findings from this and recent Ebola virus disease clusters highlight the risk of Ebola virus disease flare-ups even after an outbreak is declared over. Risk assessment and focused prevention efforts are needed for Ebola survivors and their close contacts,” Dr. Dokubo and her colleagues concluded.
The study was funded by the CDC, Defense Threat Reduction Agency, and WHO.
SOURCE: Dokubo EK et al. The Lancet Infectious Diseases, July 23, 2018.
Molecular analysis showed that a Liberian woman who survived Ebola virus disease in 2014 had viral persistence or recurrent disease and transmitted the virus to other family members a year later, according to a study published online in The Lancet Infectious Diseases.
Although the original 2014-2015 Ebola virus disease epidemic in West Africa had been contained, subsequent clusters of infection continued to occur in the region, according to researchers. A particular cluster in Liberia in November 2015 was identified after a 15-year-old boy in Monrovia tested positive.
Based on serology and epidemiological and genomic data, the researchers concluded that this cluster was caused by a woman who survived Ebola virus disease in 2014 and transmitted the virus to three family members a year later.
Ebola transmission from persistently infected male survivors is well documented, but this is the first confirmed evidence for Ebola transmission from a persistently infected female survivor, according to Emily Kainne Dokubo, MD, of the Centers for Disease Control and Prevention and her colleagues.
“The findings from this and recent Ebola virus disease clusters highlight the risk of Ebola virus disease flare-ups even after an outbreak is declared over. Risk assessment and focused prevention efforts are needed for Ebola survivors and their close contacts,” Dr. Dokubo and her colleagues concluded.
The study was funded by the CDC, Defense Threat Reduction Agency, and WHO.
SOURCE: Dokubo EK et al. The Lancet Infectious Diseases, July 23, 2018.
FROM THE LANCET INFECTIOUS DISEASES
Mosaic HIV-1 vaccine stimulates broad antigenicity, enters phase 2b human efficacy trial
A mosaic adenovirus human immunodeficiency virus-1 (HIV-1) vaccine induced robust immune responses in humans and rhesus monkeys, and significantly protected the monkeys against repetitive simian/HIV (SHIV) mosaic challenges in rhesus monkeys. This vaccine concept is currently being evaluated in a phase 2b clinical efficacy study in sub-Saharan Africa, according to a report published online in The Lancet.
Because a mosaic combination of antigens can induce an immunogenic response to a broad geographic range of viral subtypes, such a vaccine can offer the theoretical possibility of developing a global HIV-1 vaccine, according to Dan H. Barouch, MD, of Harvard Medical School, Boston, and his colleagues.
The researchers conducted a multicenter, randomized, double-blind, placebo-controlled, phase 1/2a trial (APPROACH) with 393 healthy, HIV-1-uninfected participants (aged 18-50 years) who were considered at low risk for HIV-1 infection and were recruited from 12 clinics in East Africa, South Africa, Thailand, and the United States. Participants were primed at weeks 0 and 12 with Ad26.Mos.HIV (5 × 1010 viral particles per 0.5 mL) expressing mosaic HIV-1 envelope (Env)/Gag/Pol antigens and given boosters at weeks 24 and 48 with Ad26.Mos.HIV or modified vaccinia Ankara (MVA; 108 plaque-forming units per 0.5 mL) vectors with or without adjuvanted Env gp140 protein. The placebo group received 0.9% saline.
Eligible participants were randomly assigned to one of eight study groups: Ad26/Ad26 plus high-dose gp140; Ad26/Ad26 plus low-dose gp140; Ad26/Ad26; Ad26/MVA plus high-dose gp140; Ad26/MVA plus low-dose gp140; Ad26/MVA; Ad26/high-dose gp140; and placebo. “Overall, no substantial differences in safety or tolerability of any of the seven active vaccine groups were observed,” according to Dr. Barouch and his colleagues.
In addition, the researchers vaccinated 72 Indian-origin rhesus monkeys (Macaca mulatta) using a study design that was similar to that of the human APPROACH clinical study.
The mosaic HIV-1 vaccine “induced robust humoral and cellular immune responses in both humans and rhesus monkeys,” which were similar in both species in “magnitude, durability, and phenotype,” the investigators reported. In addition, the vaccine “provided 67% protection against acquisition of six intrarectal SHIV challenges in rhesus monkeys.”
Based on these results, the mosaic Ad26/Ad26 plus gp140 HIV-1 vaccine sufficiently met “pre-established safety and immunogenicity criteria to advance into a phase 2b clinical efficacy study in sub-Saharan Africa, which is now underway (NCT03060629),” according to the authors.
“Previous HIV-1 vaccine candidates have typically been limited to specific regions of the world. Optimized mosaic antigens offer the theoretical possibility of developing a global HIV-1 vaccine,” Dr. Barouch and his colleagues concluded.
This study was funded in part by Janssen Vaccines & Prevention BV and the National Institutes of Health. Dr. Barouch has received grant funding from Janssen Vaccines & Prevention BV and is a co-inventor on HIV-1 vaccine antigen patents that have been licensed to Janssen Vaccines & Prevention.
SOURCE: Barouch DH et al., The Lancet. 2018 July 6. doi: 10.1016/S0140-6736(18)31364-3.
A mosaic adenovirus human immunodeficiency virus-1 (HIV-1) vaccine induced robust immune responses in humans and rhesus monkeys, and significantly protected the monkeys against repetitive simian/HIV (SHIV) mosaic challenges in rhesus monkeys. This vaccine concept is currently being evaluated in a phase 2b clinical efficacy study in sub-Saharan Africa, according to a report published online in The Lancet.
Because a mosaic combination of antigens can induce an immunogenic response to a broad geographic range of viral subtypes, such a vaccine can offer the theoretical possibility of developing a global HIV-1 vaccine, according to Dan H. Barouch, MD, of Harvard Medical School, Boston, and his colleagues.
The researchers conducted a multicenter, randomized, double-blind, placebo-controlled, phase 1/2a trial (APPROACH) with 393 healthy, HIV-1-uninfected participants (aged 18-50 years) who were considered at low risk for HIV-1 infection and were recruited from 12 clinics in East Africa, South Africa, Thailand, and the United States. Participants were primed at weeks 0 and 12 with Ad26.Mos.HIV (5 × 1010 viral particles per 0.5 mL) expressing mosaic HIV-1 envelope (Env)/Gag/Pol antigens and given boosters at weeks 24 and 48 with Ad26.Mos.HIV or modified vaccinia Ankara (MVA; 108 plaque-forming units per 0.5 mL) vectors with or without adjuvanted Env gp140 protein. The placebo group received 0.9% saline.
Eligible participants were randomly assigned to one of eight study groups: Ad26/Ad26 plus high-dose gp140; Ad26/Ad26 plus low-dose gp140; Ad26/Ad26; Ad26/MVA plus high-dose gp140; Ad26/MVA plus low-dose gp140; Ad26/MVA; Ad26/high-dose gp140; and placebo. “Overall, no substantial differences in safety or tolerability of any of the seven active vaccine groups were observed,” according to Dr. Barouch and his colleagues.
In addition, the researchers vaccinated 72 Indian-origin rhesus monkeys (Macaca mulatta) using a study design that was similar to that of the human APPROACH clinical study.
The mosaic HIV-1 vaccine “induced robust humoral and cellular immune responses in both humans and rhesus monkeys,” which were similar in both species in “magnitude, durability, and phenotype,” the investigators reported. In addition, the vaccine “provided 67% protection against acquisition of six intrarectal SHIV challenges in rhesus monkeys.”
Based on these results, the mosaic Ad26/Ad26 plus gp140 HIV-1 vaccine sufficiently met “pre-established safety and immunogenicity criteria to advance into a phase 2b clinical efficacy study in sub-Saharan Africa, which is now underway (NCT03060629),” according to the authors.
“Previous HIV-1 vaccine candidates have typically been limited to specific regions of the world. Optimized mosaic antigens offer the theoretical possibility of developing a global HIV-1 vaccine,” Dr. Barouch and his colleagues concluded.
This study was funded in part by Janssen Vaccines & Prevention BV and the National Institutes of Health. Dr. Barouch has received grant funding from Janssen Vaccines & Prevention BV and is a co-inventor on HIV-1 vaccine antigen patents that have been licensed to Janssen Vaccines & Prevention.
SOURCE: Barouch DH et al., The Lancet. 2018 July 6. doi: 10.1016/S0140-6736(18)31364-3.
A mosaic adenovirus human immunodeficiency virus-1 (HIV-1) vaccine induced robust immune responses in humans and rhesus monkeys, and significantly protected the monkeys against repetitive simian/HIV (SHIV) mosaic challenges in rhesus monkeys. This vaccine concept is currently being evaluated in a phase 2b clinical efficacy study in sub-Saharan Africa, according to a report published online in The Lancet.
Because a mosaic combination of antigens can induce an immunogenic response to a broad geographic range of viral subtypes, such a vaccine can offer the theoretical possibility of developing a global HIV-1 vaccine, according to Dan H. Barouch, MD, of Harvard Medical School, Boston, and his colleagues.
The researchers conducted a multicenter, randomized, double-blind, placebo-controlled, phase 1/2a trial (APPROACH) with 393 healthy, HIV-1-uninfected participants (aged 18-50 years) who were considered at low risk for HIV-1 infection and were recruited from 12 clinics in East Africa, South Africa, Thailand, and the United States. Participants were primed at weeks 0 and 12 with Ad26.Mos.HIV (5 × 1010 viral particles per 0.5 mL) expressing mosaic HIV-1 envelope (Env)/Gag/Pol antigens and given boosters at weeks 24 and 48 with Ad26.Mos.HIV or modified vaccinia Ankara (MVA; 108 plaque-forming units per 0.5 mL) vectors with or without adjuvanted Env gp140 protein. The placebo group received 0.9% saline.
Eligible participants were randomly assigned to one of eight study groups: Ad26/Ad26 plus high-dose gp140; Ad26/Ad26 plus low-dose gp140; Ad26/Ad26; Ad26/MVA plus high-dose gp140; Ad26/MVA plus low-dose gp140; Ad26/MVA; Ad26/high-dose gp140; and placebo. “Overall, no substantial differences in safety or tolerability of any of the seven active vaccine groups were observed,” according to Dr. Barouch and his colleagues.
In addition, the researchers vaccinated 72 Indian-origin rhesus monkeys (Macaca mulatta) using a study design that was similar to that of the human APPROACH clinical study.
The mosaic HIV-1 vaccine “induced robust humoral and cellular immune responses in both humans and rhesus monkeys,” which were similar in both species in “magnitude, durability, and phenotype,” the investigators reported. In addition, the vaccine “provided 67% protection against acquisition of six intrarectal SHIV challenges in rhesus monkeys.”
Based on these results, the mosaic Ad26/Ad26 plus gp140 HIV-1 vaccine sufficiently met “pre-established safety and immunogenicity criteria to advance into a phase 2b clinical efficacy study in sub-Saharan Africa, which is now underway (NCT03060629),” according to the authors.
“Previous HIV-1 vaccine candidates have typically been limited to specific regions of the world. Optimized mosaic antigens offer the theoretical possibility of developing a global HIV-1 vaccine,” Dr. Barouch and his colleagues concluded.
This study was funded in part by Janssen Vaccines & Prevention BV and the National Institutes of Health. Dr. Barouch has received grant funding from Janssen Vaccines & Prevention BV and is a co-inventor on HIV-1 vaccine antigen patents that have been licensed to Janssen Vaccines & Prevention.
SOURCE: Barouch DH et al., The Lancet. 2018 July 6. doi: 10.1016/S0140-6736(18)31364-3.
FROM THE LANCET
Primary efficacy not met by new M. tuberculosis vaccine strategies
Vaccination may have reduced the rate of sustained Mycobacterium tuberculosis infection in a recent randomized, placebo-controlled clinical trial conducted in a high-risk setting for tuberculosis transmission, despite not meeting the primary endpoint of the study.
In adolescents who had received the bacille Calmette-Guérin (BCG) vaccine in infancy, BCG revaccination reduced the rate of sustained conversion of QuantiFERON-TB Gold In-Tube assay (QFT), a test that is thought to reflect sustained M. tuberculosis infection.
The study also evaluated a candidate subunit vaccine, H4:IC31, which also reduced the rate of sustained QFT conversion, though the efficacy estimate did not reach statistical significance, investigators reported.
Neither H4:IC31 nor BCG revaccination prevented initial QFT conversion, the primary endpoint of the study; however, both vaccines were immunogenic, they said.
Moreover, the significantly reduced rate of sustained conversion with BCG revaccination provides a “promising signal,” study authors said in the New England Journal of Medicine.
“The durability of this important finding and potential public health significance for protection against tuberculosis disease warrants epidemiologic modeling and further clinical evaluation,” wrote Elisa Nemes, PhD, of the South African Tuberculosis Vaccine Initiative, which is part of the Institute of Infectious Disease and Molecular Medicine at the University of Cape Town (South Africa), and her coauthors.
Similarly, the nonsignificantly reduced rate of sustained QFT conversion seen with H4:IC31 suggested that subunit vaccines can have a biologic effect in this setting, which may inform development of new tuberculosis vaccines, Dr. Nemes and her colleagues added.
The phase 2 trial included 990 adolescents in South Africa who had undergone neonatal BCG vaccination. They were randomly assigned to receive BCG revaccination, H4:IC31 vaccine, or placebo.
Neither vaccine met the primary efficacy criterion based on initial QFT conversion rates, which were 13.1% for BCG revaccination, 14.3% for H4:IC31 vaccine, and 15.8% for placebo.
For the secondary endpoint of sustained QFT conversion, the efficacy of BCG revaccination was 45.4% (95% confidence interval, 6.4%-68.1%; P = .03), while the efficacy of H4:IC31 vaccine was 34.2% (95% CI, –10.4% to 60.7%; P = .11).
“These encouraging findings provide an impetus to reevaluate the use of BCG revaccination of populations that are free of M. tuberculosis infection for the prevention of disease,” Dr. Nemes and her coauthors wrote in their report.
Revaccination with BCG was associated with more adverse events, compared with the other groups, although adverse events in the trial were predominantly injection-site reactions that were mild to moderate in severity, investigators reported. There were no serious adverse events judged by investigators to be related to trial vaccine.
Taken together, these results raise important questions regarding the potential benefits of vaccine-mediated prevention of M. tuberculosis infection for control of tuberculosis disease, according to Dr. Nemes and her coauthors.
However, interpretation of the findings is limited because there is no definitive test for M. tuberculosis infection.
Recent infection diagnosed by tuberculin skin test or QFT conversion has been associated with higher risk of disease, compared with nonconversion, according to investigators, while reversion to a negative tuberculin skin test correlates with infection containment and lower risk of tuberculosis.
“Although the clinical significance of QFT reversion remains to be established, we propose that sustained QFT conversion more likely represents sustained M. tuberculosis infection and a higher risk of progression to disease than transient QFT conversion,” they wrote.
The study was supported by Aeras, Sanofi Pasteur, the Bill & Melinda Gates Foundation, the Government of the Netherlands Directorate-General for International Cooperation and Development, and the United Kingdom Department for International Development. Study authors reported disclosures related to GlaxoSmithKline, Sanofi Pasteur, and Aeras.
SOURCE: Nemes E et al. N Engl J Med. 2018;379:138-49.
Vaccination may have reduced the rate of sustained Mycobacterium tuberculosis infection in a recent randomized, placebo-controlled clinical trial conducted in a high-risk setting for tuberculosis transmission, despite not meeting the primary endpoint of the study.
In adolescents who had received the bacille Calmette-Guérin (BCG) vaccine in infancy, BCG revaccination reduced the rate of sustained conversion of QuantiFERON-TB Gold In-Tube assay (QFT), a test that is thought to reflect sustained M. tuberculosis infection.
The study also evaluated a candidate subunit vaccine, H4:IC31, which also reduced the rate of sustained QFT conversion, though the efficacy estimate did not reach statistical significance, investigators reported.
Neither H4:IC31 nor BCG revaccination prevented initial QFT conversion, the primary endpoint of the study; however, both vaccines were immunogenic, they said.
Moreover, the significantly reduced rate of sustained conversion with BCG revaccination provides a “promising signal,” study authors said in the New England Journal of Medicine.
“The durability of this important finding and potential public health significance for protection against tuberculosis disease warrants epidemiologic modeling and further clinical evaluation,” wrote Elisa Nemes, PhD, of the South African Tuberculosis Vaccine Initiative, which is part of the Institute of Infectious Disease and Molecular Medicine at the University of Cape Town (South Africa), and her coauthors.
Similarly, the nonsignificantly reduced rate of sustained QFT conversion seen with H4:IC31 suggested that subunit vaccines can have a biologic effect in this setting, which may inform development of new tuberculosis vaccines, Dr. Nemes and her colleagues added.
The phase 2 trial included 990 adolescents in South Africa who had undergone neonatal BCG vaccination. They were randomly assigned to receive BCG revaccination, H4:IC31 vaccine, or placebo.
Neither vaccine met the primary efficacy criterion based on initial QFT conversion rates, which were 13.1% for BCG revaccination, 14.3% for H4:IC31 vaccine, and 15.8% for placebo.
For the secondary endpoint of sustained QFT conversion, the efficacy of BCG revaccination was 45.4% (95% confidence interval, 6.4%-68.1%; P = .03), while the efficacy of H4:IC31 vaccine was 34.2% (95% CI, –10.4% to 60.7%; P = .11).
“These encouraging findings provide an impetus to reevaluate the use of BCG revaccination of populations that are free of M. tuberculosis infection for the prevention of disease,” Dr. Nemes and her coauthors wrote in their report.
Revaccination with BCG was associated with more adverse events, compared with the other groups, although adverse events in the trial were predominantly injection-site reactions that were mild to moderate in severity, investigators reported. There were no serious adverse events judged by investigators to be related to trial vaccine.
Taken together, these results raise important questions regarding the potential benefits of vaccine-mediated prevention of M. tuberculosis infection for control of tuberculosis disease, according to Dr. Nemes and her coauthors.
However, interpretation of the findings is limited because there is no definitive test for M. tuberculosis infection.
Recent infection diagnosed by tuberculin skin test or QFT conversion has been associated with higher risk of disease, compared with nonconversion, according to investigators, while reversion to a negative tuberculin skin test correlates with infection containment and lower risk of tuberculosis.
“Although the clinical significance of QFT reversion remains to be established, we propose that sustained QFT conversion more likely represents sustained M. tuberculosis infection and a higher risk of progression to disease than transient QFT conversion,” they wrote.
The study was supported by Aeras, Sanofi Pasteur, the Bill & Melinda Gates Foundation, the Government of the Netherlands Directorate-General for International Cooperation and Development, and the United Kingdom Department for International Development. Study authors reported disclosures related to GlaxoSmithKline, Sanofi Pasteur, and Aeras.
SOURCE: Nemes E et al. N Engl J Med. 2018;379:138-49.
Vaccination may have reduced the rate of sustained Mycobacterium tuberculosis infection in a recent randomized, placebo-controlled clinical trial conducted in a high-risk setting for tuberculosis transmission, despite not meeting the primary endpoint of the study.
In adolescents who had received the bacille Calmette-Guérin (BCG) vaccine in infancy, BCG revaccination reduced the rate of sustained conversion of QuantiFERON-TB Gold In-Tube assay (QFT), a test that is thought to reflect sustained M. tuberculosis infection.
The study also evaluated a candidate subunit vaccine, H4:IC31, which also reduced the rate of sustained QFT conversion, though the efficacy estimate did not reach statistical significance, investigators reported.
Neither H4:IC31 nor BCG revaccination prevented initial QFT conversion, the primary endpoint of the study; however, both vaccines were immunogenic, they said.
Moreover, the significantly reduced rate of sustained conversion with BCG revaccination provides a “promising signal,” study authors said in the New England Journal of Medicine.
“The durability of this important finding and potential public health significance for protection against tuberculosis disease warrants epidemiologic modeling and further clinical evaluation,” wrote Elisa Nemes, PhD, of the South African Tuberculosis Vaccine Initiative, which is part of the Institute of Infectious Disease and Molecular Medicine at the University of Cape Town (South Africa), and her coauthors.
Similarly, the nonsignificantly reduced rate of sustained QFT conversion seen with H4:IC31 suggested that subunit vaccines can have a biologic effect in this setting, which may inform development of new tuberculosis vaccines, Dr. Nemes and her colleagues added.
The phase 2 trial included 990 adolescents in South Africa who had undergone neonatal BCG vaccination. They were randomly assigned to receive BCG revaccination, H4:IC31 vaccine, or placebo.
Neither vaccine met the primary efficacy criterion based on initial QFT conversion rates, which were 13.1% for BCG revaccination, 14.3% for H4:IC31 vaccine, and 15.8% for placebo.
For the secondary endpoint of sustained QFT conversion, the efficacy of BCG revaccination was 45.4% (95% confidence interval, 6.4%-68.1%; P = .03), while the efficacy of H4:IC31 vaccine was 34.2% (95% CI, –10.4% to 60.7%; P = .11).
“These encouraging findings provide an impetus to reevaluate the use of BCG revaccination of populations that are free of M. tuberculosis infection for the prevention of disease,” Dr. Nemes and her coauthors wrote in their report.
Revaccination with BCG was associated with more adverse events, compared with the other groups, although adverse events in the trial were predominantly injection-site reactions that were mild to moderate in severity, investigators reported. There were no serious adverse events judged by investigators to be related to trial vaccine.
Taken together, these results raise important questions regarding the potential benefits of vaccine-mediated prevention of M. tuberculosis infection for control of tuberculosis disease, according to Dr. Nemes and her coauthors.
However, interpretation of the findings is limited because there is no definitive test for M. tuberculosis infection.
Recent infection diagnosed by tuberculin skin test or QFT conversion has been associated with higher risk of disease, compared with nonconversion, according to investigators, while reversion to a negative tuberculin skin test correlates with infection containment and lower risk of tuberculosis.
“Although the clinical significance of QFT reversion remains to be established, we propose that sustained QFT conversion more likely represents sustained M. tuberculosis infection and a higher risk of progression to disease than transient QFT conversion,” they wrote.
The study was supported by Aeras, Sanofi Pasteur, the Bill & Melinda Gates Foundation, the Government of the Netherlands Directorate-General for International Cooperation and Development, and the United Kingdom Department for International Development. Study authors reported disclosures related to GlaxoSmithKline, Sanofi Pasteur, and Aeras.
SOURCE: Nemes E et al. N Engl J Med. 2018;379:138-49.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Neither H4:IC31 nor BCG revaccination prevented initial QFT conversion, the primary endpoint; however, both vaccines were immunogenic.
Major finding: For the secondary endpoint of sustained QuantiFERON-TB Gold In-Tube Assay (QFT) conversion, efficacy was 45.4% (P = .03) for BCG revaccination and 34.2% (P = .11) for H4:IC31, a candidate subunit vaccine.
Study details: A phase 2, randomized, placebo-controlled trial including 990 adolescents in South Africa who had received BCG vaccine in infancy.
Disclosures: The study was supported by Aeras, Sanofi Pasteur, the Bill & Melinda Gates Foundation, the Government of the Netherlands Directorate-General for International Cooperation and Development, and the United Kingdom Department for International Development. Study authors reported disclosures related to GlaxoSmithKline, Sanofi Pasteur, and Aeras.
Source: Nemes E et al. N Engl J Med. 2018;379:138-49.
Cost is high for Japanese encephalitis vaccinations
Vaccination costs surrounding Japanese encephalitis is high, according to an economic analysis presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The analytic horizon for this research was 6 years, but productivity losses were evaluated over average life expectancy.
The researchers analyzed their data from two analytic perspectives: societal and traveler perspective. Factors analyzed included vaccine per dose, vaccine administration cost, and vaccine adverse events costs per vaccine.
The researchers assessed the risk to travelers based upon disease incidence among different groups. The highest-risk travelers (Group 1) – those who planned to spend a month or more in JE-endemic areas – had an incidence rate of 0.53/1 million travelers. Group 2 travelers – those who planned to stay less than a month and more than a fifth of their time outdoors – had an incidence rate of 0.25/1 million. Group 3 travelers, at lowest risk, had the lowest incidence rate at 0.04/1 million.
The calculated societal perspective cost per outcome averted was quite high for each risk group. For Group 1, the cost was $596 million per case averted. This cost rose to $1.3 billion for each case of long-term sequelae averted, and rose even higher to avert death, to $1.8 billion per death averted. These costs nearly doubled for Group 2, and in Group 3, the cost ballooned to $7.9 billion to avert one case of JE, $17 billion per prevention of long-term sequelae, and $23 billion per death averted.
The individual costs for JE vaccination are $292 per dose, with an administration fee of $46. Short-term treatment of JE costs nearly $30,000, and long-term treatment of JE also comes with a large bill of $8,437.
These costs are not simply monetary but are also felt in lost economic productivity. The cost of complete short-term recovery is nearly $60,000. Over an individual’s lifetime, this number rose to more than $1.5 million based on total loss of productivity.
Of the 67% of patients who survive JE, 32% recover completely while 68% deal with long-term sequelae. Of those, 28% experience mild symptoms while the remaining 72% have severe sequelae.
JE vaccine effectiveness does not appear to be an issue, with a 0.91 proportion of neutralizing antibodies seen after 1 year. With booster doses, vaccine effectiveness is 0.96.
There are limitations to this study, such as results being affected by the uncertainty of JE incidence. “The single most important variable is incidence,” stated Dr. Meltzer.
Vaccination costs surrounding Japanese encephalitis is high, according to an economic analysis presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The analytic horizon for this research was 6 years, but productivity losses were evaluated over average life expectancy.
The researchers analyzed their data from two analytic perspectives: societal and traveler perspective. Factors analyzed included vaccine per dose, vaccine administration cost, and vaccine adverse events costs per vaccine.
The researchers assessed the risk to travelers based upon disease incidence among different groups. The highest-risk travelers (Group 1) – those who planned to spend a month or more in JE-endemic areas – had an incidence rate of 0.53/1 million travelers. Group 2 travelers – those who planned to stay less than a month and more than a fifth of their time outdoors – had an incidence rate of 0.25/1 million. Group 3 travelers, at lowest risk, had the lowest incidence rate at 0.04/1 million.
The calculated societal perspective cost per outcome averted was quite high for each risk group. For Group 1, the cost was $596 million per case averted. This cost rose to $1.3 billion for each case of long-term sequelae averted, and rose even higher to avert death, to $1.8 billion per death averted. These costs nearly doubled for Group 2, and in Group 3, the cost ballooned to $7.9 billion to avert one case of JE, $17 billion per prevention of long-term sequelae, and $23 billion per death averted.
The individual costs for JE vaccination are $292 per dose, with an administration fee of $46. Short-term treatment of JE costs nearly $30,000, and long-term treatment of JE also comes with a large bill of $8,437.
These costs are not simply monetary but are also felt in lost economic productivity. The cost of complete short-term recovery is nearly $60,000. Over an individual’s lifetime, this number rose to more than $1.5 million based on total loss of productivity.
Of the 67% of patients who survive JE, 32% recover completely while 68% deal with long-term sequelae. Of those, 28% experience mild symptoms while the remaining 72% have severe sequelae.
JE vaccine effectiveness does not appear to be an issue, with a 0.91 proportion of neutralizing antibodies seen after 1 year. With booster doses, vaccine effectiveness is 0.96.
There are limitations to this study, such as results being affected by the uncertainty of JE incidence. “The single most important variable is incidence,” stated Dr. Meltzer.
Vaccination costs surrounding Japanese encephalitis is high, according to an economic analysis presented at a meeting of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
The analytic horizon for this research was 6 years, but productivity losses were evaluated over average life expectancy.
The researchers analyzed their data from two analytic perspectives: societal and traveler perspective. Factors analyzed included vaccine per dose, vaccine administration cost, and vaccine adverse events costs per vaccine.
The researchers assessed the risk to travelers based upon disease incidence among different groups. The highest-risk travelers (Group 1) – those who planned to spend a month or more in JE-endemic areas – had an incidence rate of 0.53/1 million travelers. Group 2 travelers – those who planned to stay less than a month and more than a fifth of their time outdoors – had an incidence rate of 0.25/1 million. Group 3 travelers, at lowest risk, had the lowest incidence rate at 0.04/1 million.
The calculated societal perspective cost per outcome averted was quite high for each risk group. For Group 1, the cost was $596 million per case averted. This cost rose to $1.3 billion for each case of long-term sequelae averted, and rose even higher to avert death, to $1.8 billion per death averted. These costs nearly doubled for Group 2, and in Group 3, the cost ballooned to $7.9 billion to avert one case of JE, $17 billion per prevention of long-term sequelae, and $23 billion per death averted.
The individual costs for JE vaccination are $292 per dose, with an administration fee of $46. Short-term treatment of JE costs nearly $30,000, and long-term treatment of JE also comes with a large bill of $8,437.
These costs are not simply monetary but are also felt in lost economic productivity. The cost of complete short-term recovery is nearly $60,000. Over an individual’s lifetime, this number rose to more than $1.5 million based on total loss of productivity.
Of the 67% of patients who survive JE, 32% recover completely while 68% deal with long-term sequelae. Of those, 28% experience mild symptoms while the remaining 72% have severe sequelae.
JE vaccine effectiveness does not appear to be an issue, with a 0.91 proportion of neutralizing antibodies seen after 1 year. With booster doses, vaccine effectiveness is 0.96.
There are limitations to this study, such as results being affected by the uncertainty of JE incidence. “The single most important variable is incidence,” stated Dr. Meltzer.
REPORTING FROM AN ACIP MEETING
Oral tecovirimat for smallpox shows efficacy in animals, safety in humans
Tecovirimat, an oral antiviral agent, is being advanced as a treatment for smallpox based on recently reported studies showing efficacy data in animals and safety and pharmacokinetic data in humans.
“The aggregation of the results from these multiple studies involving animals and humans supports tecovirimat as potential smallpox antiviral drug,” Douglas W. Grosenbach, PhD, of SIGA Technologies and his coauthors reported in the New England Journal of Medicine.
Tecovirimat inhibits p37, a protein that is present in all orthopoxviruses, to prevent “formation and egress of enveloped virions, which are essential for virulence,” Dr. Grosenbach and his coauthors wrote in the report.
Smallpox, caused by the variola virus, was eradicated in 1980. However, the disease remains concerning because of the potential for intentional release of variola virus in an act of bioterrorism or biowarfare, according to the authors.
“A single case of smallpox anywhere in the world would be a global health emergency,” Dr. Grosenbach and his colleagues wrote.
Available vaccines are not used because of the risk of side effects and would not be used except in the case of known or suspected variola virus exposure, they added.
Since it would be unethical to intentionally expose humans to variola virus, tecovirimat is being developed in line with the Food and Drug Administration Animal Efficacy Rule, which gives the agency the authority to approve drugs based on animal data for diseases that have a low or nonexistent rate of natural occurrence.
The first treatment developed under the Animal Efficacy Rule, was raxibacumab, a biologic approved by the FDA in December 2012 for treatment of inhalation anthrax. Subsequently, several other agents for prophylaxis or treatment of anthrax and botulism have been developed based on the rule and approved.
Tecovirimat has demonstrated protective efficacy in pilot studies conducted in rabbits infected with rabbitpox virus and nonhuman primates infected with monkeypox virus, Dr. Grosenbach and his colleagues explained in the current report.
In nonhuman primate studies, doses of 3-10 mg/kilogram provided nearly full protection from death with a survival rate of approximately 95% versus 5% for placebo, along with reduced lesion counts and viral loads, according to investigators.
In a tecovirimat clinical trial, 452 volunteers were randomized to receive the antiviral agent twice daily at 600 mg or matching placebo for 14 days. Adverse events occurred in 37.3% of tecovirimat-treated and 33.3% of placebo-treated participants, with events of grade 3 or higher occurring in 1.1% of patients in both groups.
There was one death on the tecovirimat arm, which was caused by a pulmonary embolism in a participant who had a history of recurrent deep-vein thrombosis but was not receiving anticoagulant treatment, investigators said.
Dr. Grosenbach and colleagues also presented pharmacokinetic profiles and exposures for 48 volunteers in a fed state in their report in the New England Journal of Medicine, along with pharmacokinetic data from the animal studies.
The FDA has set a target date of Aug. 8, 2018, for final action on a new drug application submitted for oral tecovirimat for treatment of smallpox, SIGA Technologies said in a May 2018 news release.
Dr. Grosenbach and his coauthors reported a disclosure related to Biomedical Advanced Research and Development Authority (BARDA), grants from NIH during the conduct of the study, and personal fees from SIGA Technologies outside the submitted work.
SOURCE: Grosenbach DW et al. N Engl J Med. 2018 Jul 5;379(1):44-53.
Tecovirimat, an oral antiviral agent, is being advanced as a treatment for smallpox based on recently reported studies showing efficacy data in animals and safety and pharmacokinetic data in humans.
“The aggregation of the results from these multiple studies involving animals and humans supports tecovirimat as potential smallpox antiviral drug,” Douglas W. Grosenbach, PhD, of SIGA Technologies and his coauthors reported in the New England Journal of Medicine.
Tecovirimat inhibits p37, a protein that is present in all orthopoxviruses, to prevent “formation and egress of enveloped virions, which are essential for virulence,” Dr. Grosenbach and his coauthors wrote in the report.
Smallpox, caused by the variola virus, was eradicated in 1980. However, the disease remains concerning because of the potential for intentional release of variola virus in an act of bioterrorism or biowarfare, according to the authors.
“A single case of smallpox anywhere in the world would be a global health emergency,” Dr. Grosenbach and his colleagues wrote.
Available vaccines are not used because of the risk of side effects and would not be used except in the case of known or suspected variola virus exposure, they added.
Since it would be unethical to intentionally expose humans to variola virus, tecovirimat is being developed in line with the Food and Drug Administration Animal Efficacy Rule, which gives the agency the authority to approve drugs based on animal data for diseases that have a low or nonexistent rate of natural occurrence.
The first treatment developed under the Animal Efficacy Rule, was raxibacumab, a biologic approved by the FDA in December 2012 for treatment of inhalation anthrax. Subsequently, several other agents for prophylaxis or treatment of anthrax and botulism have been developed based on the rule and approved.
Tecovirimat has demonstrated protective efficacy in pilot studies conducted in rabbits infected with rabbitpox virus and nonhuman primates infected with monkeypox virus, Dr. Grosenbach and his colleagues explained in the current report.
In nonhuman primate studies, doses of 3-10 mg/kilogram provided nearly full protection from death with a survival rate of approximately 95% versus 5% for placebo, along with reduced lesion counts and viral loads, according to investigators.
In a tecovirimat clinical trial, 452 volunteers were randomized to receive the antiviral agent twice daily at 600 mg or matching placebo for 14 days. Adverse events occurred in 37.3% of tecovirimat-treated and 33.3% of placebo-treated participants, with events of grade 3 or higher occurring in 1.1% of patients in both groups.
There was one death on the tecovirimat arm, which was caused by a pulmonary embolism in a participant who had a history of recurrent deep-vein thrombosis but was not receiving anticoagulant treatment, investigators said.
Dr. Grosenbach and colleagues also presented pharmacokinetic profiles and exposures for 48 volunteers in a fed state in their report in the New England Journal of Medicine, along with pharmacokinetic data from the animal studies.
The FDA has set a target date of Aug. 8, 2018, for final action on a new drug application submitted for oral tecovirimat for treatment of smallpox, SIGA Technologies said in a May 2018 news release.
Dr. Grosenbach and his coauthors reported a disclosure related to Biomedical Advanced Research and Development Authority (BARDA), grants from NIH during the conduct of the study, and personal fees from SIGA Technologies outside the submitted work.
SOURCE: Grosenbach DW et al. N Engl J Med. 2018 Jul 5;379(1):44-53.
Tecovirimat, an oral antiviral agent, is being advanced as a treatment for smallpox based on recently reported studies showing efficacy data in animals and safety and pharmacokinetic data in humans.
“The aggregation of the results from these multiple studies involving animals and humans supports tecovirimat as potential smallpox antiviral drug,” Douglas W. Grosenbach, PhD, of SIGA Technologies and his coauthors reported in the New England Journal of Medicine.
Tecovirimat inhibits p37, a protein that is present in all orthopoxviruses, to prevent “formation and egress of enveloped virions, which are essential for virulence,” Dr. Grosenbach and his coauthors wrote in the report.
Smallpox, caused by the variola virus, was eradicated in 1980. However, the disease remains concerning because of the potential for intentional release of variola virus in an act of bioterrorism or biowarfare, according to the authors.
“A single case of smallpox anywhere in the world would be a global health emergency,” Dr. Grosenbach and his colleagues wrote.
Available vaccines are not used because of the risk of side effects and would not be used except in the case of known or suspected variola virus exposure, they added.
Since it would be unethical to intentionally expose humans to variola virus, tecovirimat is being developed in line with the Food and Drug Administration Animal Efficacy Rule, which gives the agency the authority to approve drugs based on animal data for diseases that have a low or nonexistent rate of natural occurrence.
The first treatment developed under the Animal Efficacy Rule, was raxibacumab, a biologic approved by the FDA in December 2012 for treatment of inhalation anthrax. Subsequently, several other agents for prophylaxis or treatment of anthrax and botulism have been developed based on the rule and approved.
Tecovirimat has demonstrated protective efficacy in pilot studies conducted in rabbits infected with rabbitpox virus and nonhuman primates infected with monkeypox virus, Dr. Grosenbach and his colleagues explained in the current report.
In nonhuman primate studies, doses of 3-10 mg/kilogram provided nearly full protection from death with a survival rate of approximately 95% versus 5% for placebo, along with reduced lesion counts and viral loads, according to investigators.
In a tecovirimat clinical trial, 452 volunteers were randomized to receive the antiviral agent twice daily at 600 mg or matching placebo for 14 days. Adverse events occurred in 37.3% of tecovirimat-treated and 33.3% of placebo-treated participants, with events of grade 3 or higher occurring in 1.1% of patients in both groups.
There was one death on the tecovirimat arm, which was caused by a pulmonary embolism in a participant who had a history of recurrent deep-vein thrombosis but was not receiving anticoagulant treatment, investigators said.
Dr. Grosenbach and colleagues also presented pharmacokinetic profiles and exposures for 48 volunteers in a fed state in their report in the New England Journal of Medicine, along with pharmacokinetic data from the animal studies.
The FDA has set a target date of Aug. 8, 2018, for final action on a new drug application submitted for oral tecovirimat for treatment of smallpox, SIGA Technologies said in a May 2018 news release.
Dr. Grosenbach and his coauthors reported a disclosure related to Biomedical Advanced Research and Development Authority (BARDA), grants from NIH during the conduct of the study, and personal fees from SIGA Technologies outside the submitted work.
SOURCE: Grosenbach DW et al. N Engl J Med. 2018 Jul 5;379(1):44-53.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tecovirimat, an oral antiviral agent, is being advanced as a treatment for smallpox based on animal and human study data.
Major finding: The survival rate was approximately 95% in monkeypox-infected primates receiving doses of 3-10 mg/kilogram. In humans, the serious adverse event rate was 1.1% for both tecovirimat and placebo.
Study details: A report on multiple studies in small animals and nonhuman primates, along with a randomized safety trial involving 452 healthy human volunteers.
Disclosures: Study authors reported a disclosure related to Biomedical Advanced Research and Development Authority (BARDA), grants from NIH during the conduct of the study, and personal fees from SIGA Technologies outside the submitted work.
Source: Grosenbach DW et al. N Engl J Med. 2018 Jul 5;379(1):44-53.