User login
Manufactured graft deemed safe in blood cancer patients
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
LISBON—Phase 1 results suggest a programmed cellular therapy is safe for use in patients with hematologic malignancies.
The therapy, ProTmune, is being developed as a next-generation allogeneic graft intended to reduce the incidence and severity of acute graft-versus-host disease (GVHD) after hematopoietic stem cell transplant (HSCT).
Three of 7 patients who received ProTmune in this trial did develop acute GVHD, and 2 patients died.
However, the remaining 5 patients were still alive and disease-free at last follow-up.
There were no serious adverse events (AEs) attributed to ProTmune. The most common AEs were nausea, vomiting, and chest pain.
These results were presented at the 44th Annual Meeting of the EBMT (abstract A401*).
The trial, known as PROTECT, is sponsored by Fate Therapeutics, the company developing ProTmune.
The phase 1 portion of PROTECT enrolled 7 adults with hematologic malignancies—1 with myelodysplastic syndrome, 3 with acute lymphoblastic leukemia, and 3 with acute myeloid leukemia.
Patients were set to undergo matched, unrelated donor HSCT and received ProTmune as the graft. ProTmune is manufactured by modulating a mobilized peripheral blood graft with 2 small molecules, FT1050 and FT4145.
The patients ranged in age from 34 to 69, and most (n=5) were female. For conditioning, patients received fludarabine/busulfan (n=1), busulfan/cyclophosphamide (n=1), fludarabine/melphalan (n=3), or cyclophosphamide/total body irradiation (n=2).
Results
The data cut-off was February 26, 2018. The median time on study was 228 days (range, 151 to 353).
None of the patients had graft failure. The median time to neutrophil engraftment was 18 days (range, 14 to 22).
Three patients had acute GVHD at day 100 after HSCT. Two patients had grade 2 skin GVHD, and 1 had grade 3 GVHD in the skin and gut.
All 3 patients responded to steroid treatment. GVHD resolved in 5 days for the patient with grade 3 GVHD. For the grade 2 patients, GVHD resolved in 7 days and 8 days, respectively.
None of the patients relapsed, but 2 died—1 of pulmonary edema and 1 of atrial fibrillation.
AEs related to ProTmune included grade 1 vomiting (n=2), grade 2 nausea (n=2), and grade 2 chest pain (n=1).
Phase 2
The phase 2 portion of PROTECT is ongoing. This is a randomized, controlled, double-blinded trial designed to assess the safety and efficacy of ProTmune in up to 60 adults with hematologic malignancies undergoing matched, unrelated donor HSCT following myeloablative conditioning.
Patients are being randomized, in a 1:1 ratio, to receive either ProTmune or a conventional, mobilized peripheral blood cell graft from a matched, unrelated donor.
The primary efficacy endpoint is the cumulative incidence of grade 2-4 acute GVHD by day 100 post-HSCT. Rates of chronic GVHD, cancer relapse, disease-free survival, and overall survival are also being assessed.
*Some data in the abstract differ from the presentation.
Metabolic changes in T cells may limit CAR potential in kids
Researchers analyzed peripheral blood T cells from 157 pediatric cancer patients at diagnosis and after chemotherapy and found the potential to produce effective chimeric antigen receptor (CAR) T cells declined with each cycle of chemotherapy.
This was also true for acute lymphoblastic leukemia (ALL) and Wilms’ tumor, which had high CAR T-cell manufacturing potential in the pre-chemotherapy samples.
Children younger than 3 years particularly showed a significant decline in CAR T-cell potential with cumulative cycles of chemotherapy.
“Everybody knows that chemotherapy is really bad for your T cells, and the more chemo you get, the less likely you are to have healthy T cells,” David M. Barrett, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania, said at a press preview of research to be presented at the AACR Annual Meeting 2018.
“We know a lot about what a highly active, highly successful CAR T cell looks like right before it goes back into the patient after it’s finished manufacturing,” Dr Barrett added.
But he and his colleagues wanted to determine what goes into producing high-quality cells from a patient and the difference between cells that were good starting material and cells that weren’t.
The investigators analyzed blood samples from pediatric patients with ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms’ tumor, Hodgkin lymphoma, chronic myeloid leukemia, and Ewing sarcoma. The team collected samples at diagnosis and after every cycle of chemotherapy.
Using flow cytometry, they quantified the CD3+ cell population and expanded the T cells using CD3 and CD28 stimulatory beads, “the backbone of pretty much every center’s way to make CAR T cells in the lab,” Dr Barrett said.
And the researchers found poor CAR T-cell manufacturing potential in all tumor types at diagnosis except for ALL and Wilms’ tumor. In standard-risk and high-risk ALL, more than 90% of patients had high-quality T cells at diagnosis.
The team report the findings in abstract 1631, which is scheduled to be presented at the AACR Annual Meeting on April 15.
“This may have played into why pediatric ALL is one of the great successes with CAR T-cell therapy,” Dr Barrett explained. “We may have actually been working with uniquely well-suited, good starting material to build a CAR T cell.”
T cells from lymphoma patients—Burkitt lymphoma, diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, and Hodgkin lymphoma—were actually quite poor in their potential to become good CAR T cells, Dr Barrett noted.
“This may be reflected clinically in pediatrics at Children’s Hospital of Philadelphia,” he said. “We’ve only been able to successfully treat 3 children with lymphoma, as opposed to more than 200 children with leukemia.”
The only other type of tumor that seemed to have good CAR T potential was Wilms’ tumor.
“I don’t have a CAR T cell for Wilms’ tumor yet,” Dr Barrett said, “but, if I wanted to make one, I would at least have a degree of confidence that the cells gotten from a patient would at least be able to be successfully made into a highly functional T cell that can go back into a patient.”
The investigators also observed that cumulative chemotherapy alters the metabolic profile in T cells, “gradually turning them by cycle 6 into something that doesn’t work anymore,” Dr Barrett said.
The researchers then looked into what differences there were in the quality of collected T cells and found that metabolic changes varied with tumor and treatment.
T cells with poor CAR T-cell potential were biased toward using glycolysis as their energy source instead of using fatty acids.
“Normal, healthy donor T cells cluster together in terms of metabolic pathways that are active or inactive,” Dr Barrett explained.
“[P]atients who had leukemia and the Wilms’ tumor patients could make successful CAR T cells from those samples. On the other hand, solid tumors and a Hodgkin disease patient look like they have a very different metabolic profile. And that is associated with failure to make a good CAR T cell.”
The investigators were able to get the T cells to shift metabolic pathways by “essentially force-feeding T cells things like fatty acids so they don’t use as much glucose,” Dr Barrett said.
“We’ve had some success in force-feeding them essentially neutral amino acids and others like arginine. And so you can actually potentially provide a T cell with an attractive alternative fuel source.”
Dr Barrett noted that the findings have already altered practice for children at his institution.
They now collect T cells early even if the patient is not eligible for a CAR trial, “simply because we know that cumulative chemotherapy is going to progressively deteriorate the likelihood that those cells will make a functional CAR product, and we’ve been recommending that to other centers,” Dr Barrett said.
“We’re trying to understand what goes into making the best starting material so that we can alter our approaches to make sure that we make a highly functional CAR T-cell product not only for kids with leukemia and CART19, but also potentially for solid tumor CARs as we try to develop those in the future.”
Researchers analyzed peripheral blood T cells from 157 pediatric cancer patients at diagnosis and after chemotherapy and found the potential to produce effective chimeric antigen receptor (CAR) T cells declined with each cycle of chemotherapy.
This was also true for acute lymphoblastic leukemia (ALL) and Wilms’ tumor, which had high CAR T-cell manufacturing potential in the pre-chemotherapy samples.
Children younger than 3 years particularly showed a significant decline in CAR T-cell potential with cumulative cycles of chemotherapy.
“Everybody knows that chemotherapy is really bad for your T cells, and the more chemo you get, the less likely you are to have healthy T cells,” David M. Barrett, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania, said at a press preview of research to be presented at the AACR Annual Meeting 2018.
“We know a lot about what a highly active, highly successful CAR T cell looks like right before it goes back into the patient after it’s finished manufacturing,” Dr Barrett added.
But he and his colleagues wanted to determine what goes into producing high-quality cells from a patient and the difference between cells that were good starting material and cells that weren’t.
The investigators analyzed blood samples from pediatric patients with ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms’ tumor, Hodgkin lymphoma, chronic myeloid leukemia, and Ewing sarcoma. The team collected samples at diagnosis and after every cycle of chemotherapy.
Using flow cytometry, they quantified the CD3+ cell population and expanded the T cells using CD3 and CD28 stimulatory beads, “the backbone of pretty much every center’s way to make CAR T cells in the lab,” Dr Barrett said.
And the researchers found poor CAR T-cell manufacturing potential in all tumor types at diagnosis except for ALL and Wilms’ tumor. In standard-risk and high-risk ALL, more than 90% of patients had high-quality T cells at diagnosis.
The team report the findings in abstract 1631, which is scheduled to be presented at the AACR Annual Meeting on April 15.
“This may have played into why pediatric ALL is one of the great successes with CAR T-cell therapy,” Dr Barrett explained. “We may have actually been working with uniquely well-suited, good starting material to build a CAR T cell.”
T cells from lymphoma patients—Burkitt lymphoma, diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, and Hodgkin lymphoma—were actually quite poor in their potential to become good CAR T cells, Dr Barrett noted.
“This may be reflected clinically in pediatrics at Children’s Hospital of Philadelphia,” he said. “We’ve only been able to successfully treat 3 children with lymphoma, as opposed to more than 200 children with leukemia.”
The only other type of tumor that seemed to have good CAR T potential was Wilms’ tumor.
“I don’t have a CAR T cell for Wilms’ tumor yet,” Dr Barrett said, “but, if I wanted to make one, I would at least have a degree of confidence that the cells gotten from a patient would at least be able to be successfully made into a highly functional T cell that can go back into a patient.”
The investigators also observed that cumulative chemotherapy alters the metabolic profile in T cells, “gradually turning them by cycle 6 into something that doesn’t work anymore,” Dr Barrett said.
The researchers then looked into what differences there were in the quality of collected T cells and found that metabolic changes varied with tumor and treatment.
T cells with poor CAR T-cell potential were biased toward using glycolysis as their energy source instead of using fatty acids.
“Normal, healthy donor T cells cluster together in terms of metabolic pathways that are active or inactive,” Dr Barrett explained.
“[P]atients who had leukemia and the Wilms’ tumor patients could make successful CAR T cells from those samples. On the other hand, solid tumors and a Hodgkin disease patient look like they have a very different metabolic profile. And that is associated with failure to make a good CAR T cell.”
The investigators were able to get the T cells to shift metabolic pathways by “essentially force-feeding T cells things like fatty acids so they don’t use as much glucose,” Dr Barrett said.
“We’ve had some success in force-feeding them essentially neutral amino acids and others like arginine. And so you can actually potentially provide a T cell with an attractive alternative fuel source.”
Dr Barrett noted that the findings have already altered practice for children at his institution.
They now collect T cells early even if the patient is not eligible for a CAR trial, “simply because we know that cumulative chemotherapy is going to progressively deteriorate the likelihood that those cells will make a functional CAR product, and we’ve been recommending that to other centers,” Dr Barrett said.
“We’re trying to understand what goes into making the best starting material so that we can alter our approaches to make sure that we make a highly functional CAR T-cell product not only for kids with leukemia and CART19, but also potentially for solid tumor CARs as we try to develop those in the future.”
Researchers analyzed peripheral blood T cells from 157 pediatric cancer patients at diagnosis and after chemotherapy and found the potential to produce effective chimeric antigen receptor (CAR) T cells declined with each cycle of chemotherapy.
This was also true for acute lymphoblastic leukemia (ALL) and Wilms’ tumor, which had high CAR T-cell manufacturing potential in the pre-chemotherapy samples.
Children younger than 3 years particularly showed a significant decline in CAR T-cell potential with cumulative cycles of chemotherapy.
“Everybody knows that chemotherapy is really bad for your T cells, and the more chemo you get, the less likely you are to have healthy T cells,” David M. Barrett, MD, PhD, of Children’s Hospital of Philadelphia in Pennsylvania, said at a press preview of research to be presented at the AACR Annual Meeting 2018.
“We know a lot about what a highly active, highly successful CAR T cell looks like right before it goes back into the patient after it’s finished manufacturing,” Dr Barrett added.
But he and his colleagues wanted to determine what goes into producing high-quality cells from a patient and the difference between cells that were good starting material and cells that weren’t.
The investigators analyzed blood samples from pediatric patients with ALL, non-Hodgkin lymphoma, neuroblastoma, osteosarcoma, rhabdomyosarcoma, Wilms’ tumor, Hodgkin lymphoma, chronic myeloid leukemia, and Ewing sarcoma. The team collected samples at diagnosis and after every cycle of chemotherapy.
Using flow cytometry, they quantified the CD3+ cell population and expanded the T cells using CD3 and CD28 stimulatory beads, “the backbone of pretty much every center’s way to make CAR T cells in the lab,” Dr Barrett said.
And the researchers found poor CAR T-cell manufacturing potential in all tumor types at diagnosis except for ALL and Wilms’ tumor. In standard-risk and high-risk ALL, more than 90% of patients had high-quality T cells at diagnosis.
The team report the findings in abstract 1631, which is scheduled to be presented at the AACR Annual Meeting on April 15.
“This may have played into why pediatric ALL is one of the great successes with CAR T-cell therapy,” Dr Barrett explained. “We may have actually been working with uniquely well-suited, good starting material to build a CAR T cell.”
T cells from lymphoma patients—Burkitt lymphoma, diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, and Hodgkin lymphoma—were actually quite poor in their potential to become good CAR T cells, Dr Barrett noted.
“This may be reflected clinically in pediatrics at Children’s Hospital of Philadelphia,” he said. “We’ve only been able to successfully treat 3 children with lymphoma, as opposed to more than 200 children with leukemia.”
The only other type of tumor that seemed to have good CAR T potential was Wilms’ tumor.
“I don’t have a CAR T cell for Wilms’ tumor yet,” Dr Barrett said, “but, if I wanted to make one, I would at least have a degree of confidence that the cells gotten from a patient would at least be able to be successfully made into a highly functional T cell that can go back into a patient.”
The investigators also observed that cumulative chemotherapy alters the metabolic profile in T cells, “gradually turning them by cycle 6 into something that doesn’t work anymore,” Dr Barrett said.
The researchers then looked into what differences there were in the quality of collected T cells and found that metabolic changes varied with tumor and treatment.
T cells with poor CAR T-cell potential were biased toward using glycolysis as their energy source instead of using fatty acids.
“Normal, healthy donor T cells cluster together in terms of metabolic pathways that are active or inactive,” Dr Barrett explained.
“[P]atients who had leukemia and the Wilms’ tumor patients could make successful CAR T cells from those samples. On the other hand, solid tumors and a Hodgkin disease patient look like they have a very different metabolic profile. And that is associated with failure to make a good CAR T cell.”
The investigators were able to get the T cells to shift metabolic pathways by “essentially force-feeding T cells things like fatty acids so they don’t use as much glucose,” Dr Barrett said.
“We’ve had some success in force-feeding them essentially neutral amino acids and others like arginine. And so you can actually potentially provide a T cell with an attractive alternative fuel source.”
Dr Barrett noted that the findings have already altered practice for children at his institution.
They now collect T cells early even if the patient is not eligible for a CAR trial, “simply because we know that cumulative chemotherapy is going to progressively deteriorate the likelihood that those cells will make a functional CAR product, and we’ve been recommending that to other centers,” Dr Barrett said.
“We’re trying to understand what goes into making the best starting material so that we can alter our approaches to make sure that we make a highly functional CAR T-cell product not only for kids with leukemia and CART19, but also potentially for solid tumor CARs as we try to develop those in the future.”
More evidence links increased BMI to higher multiple myeloma risk
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
A high body mass index in both early and later adulthood increases the risk for developing multiple myeloma (MM), according to a prospective analysis.
“This association did not significantly differ by gender but was nonetheless slightly stronger in men,” wrote Catherine R. Marinac, PhD, of the Dana-Farber Cancer Institute, Boston, and her colleagues. “MM risk was significantly positively associated with weight change and suggestive of a positive association for change in BMI since young adulthood. In contrast, we did not observe statistically significant associations of cumulative average physical activity or walking with MM risk.”
Dr. Marinac and her associates analyzed participants from the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Women’s Health Study (WHS) with a pooled total of 575 MM cases and more than 5 million person-years of follow-up. From all of those databases, a combined baseline total of 49,374 men and 153,260 women were included in the analyses. Participants in all three of the cohorts were predominately white.
Each participant was required to report height and weight on a baseline questionnaire and updated weights on subsequent questionnaires. Using that height and weight information, the researchers calculated BMI. Physical activity also was reported using questionnaires, beginning in 1986 in the HPFS and NHS groups and at baseline for WHS, with all groups providing updates every 2-4 years. The researchers used the physical activity information to calculate the total metabolic equivalent (MET) hours of all activity and of walking per week.
Dr. Marinac and her team identified a total of 205 men from the HPFS cohort and 370 women (325 NHS, 45 WHS) with confirmed diagnoses of MM. The BMIs of those participants ranged from 23.8-25.8 kg/m2 at baseline and from 21.3-23.0 kg/m2 in young adulthood. Across all cohorts, each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17% (hazard ratio, 1.17; 95% confidence interval, 1.05-1.29).
In addition, the MM risk rose almost 30% for every 5 kg/m2 increase in young adult BMI (HR, 1.28; 95% CI, 1.12-1.47). Increased risk was not strictly related to changes in BMI but to incremental weight gain since young adulthood. (pooled HR, 1.04; 95% CI, 1.00-1.08; P = 0.03).
The study confirmed correlations between weight gain and increased MM risk, however, it also had certain limitations. For example, much of the data concerning weight, height, and physical activity were all self-reported. Another limitation is the sociodemographic heterogeneity of the study population.
Despite those limitations, Dr. Marinac emphasized that the study results add to evidence concerning weight gain and MM risk.
“Our findings support the growing body of literature demonstrating that a high BMI both early and later in adulthood is associated with the risk of MM, and suggest that maintaining a healthy body weight throughout life may be an important component to a much-needed MM prevention strategy,” wrote Dr. Marinac, who also is affiliated with the Harvard T.H. Chan School of Public Health, also in Boston.
“Further larger-scale studies aimed at clarifying the influence of obesity timing and duration and at directly evaluating the role of weight loss, ideally conducted in diverse prospective study populations and in [monoclonal gammopathy of undetermined significance] patients, will be important for elaborating the role of weight maintenance in MM prevention and for identifying high risk subgroups of patients that may benefit from weight loss.”
None of the researchers had competing financial interests to disclose.
SOURCE: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
FROM BRITISH JOURNAL OF CANCER
Key clinical point: Moderate increases in body mass index (BMI) can dramatically increase the risk of developing multiple myeloma (MM).
Major finding: Each 5 kg/m2 increase in cumulative average adult BMI significantly increased the risk of MM by 17%.
Study details: Prospective analysis of 49,374 men and 153,260 women from three databases.
Disclosures: None of the researchers had competing financial interests to disclose.
Source: Marinac CR et al. Br J Cancer. 2018 Mar 12. doi: 10.1038/s41416-018-0010-4.
Atraumatic splenic rupture as an initial presentation of chronic myelogenous leukemia
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
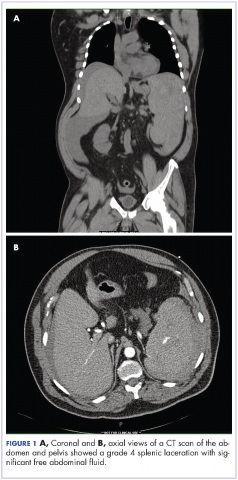
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
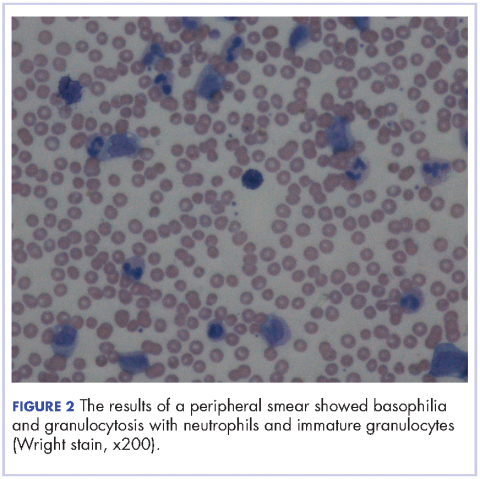
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
Chronic myelogenous leukemia (CML) is a myeloproliferative neoplasm associated with the fusion of the BCR gene located on chromosome 22 and the ABL1 gene on chromosome 9. The fusion results in a reciprocal translocation between chromosomes 9 and 22, leading to the formation of the Philadelphia (Ph) chromosome found in 90%-95% of patients with CML. The incidence of CML is 1.5 per 100,000 people per year, with a male predominance and an average age at diagnosis of 64.1
About 85%-90% of newly diagnosed patients present in the chronic phase and therefore many of them are asymptomatic at the time of diagnosis. If symptoms are present, they often include fatigue, malaise, unintentional weight loss, early satiety, or left upper quadrant pain. Progression of the disease is associated with worsening symptoms such as unexplained fever, significant weight loss, bone or joint pain, bleeding, thrombosis, and infections suggestive of transformation to the accelerated phase or blast crisis. Physical exam findings most commonly include splenomegaly and occasionally mild hepatomegaly.
Atraumatic splenic rupture is a rare complication of this hematologic malignancy, and there are almost no reported cases of CML as the underlying cause.2-4 Here we present the case of a man with sudden-onset generalized abdominal pain and leukocytosis. A computed-tomography scan showed splenic rupture, and the patient was taken for emergency splenectomy. The patient was subsequently positive for t(9,22)(q34;q11.2).
Case presentation and summary
A 59-year-old white man with a history of hypertension and kidney stones presented to a community emergency department with a chief complaint of abdominal pain. About 30 minutes before his arrival, the patient had woken up from sleep with generalized, nonradiating, abdominal pain, which he described as “like my previous kidney stones.” He also reported worsening dyspnea, nausea without vomiting, and lightheadedness without loss of consciousness. The remainder of the review of systems was negative. A physical exam revealed that he was in moderate distress with clear lung fields and had tachycardia without murmur, no CVA tenderness, and a diffusely tender abdomen.
Complete blood count with differential showed leukocytosis (109.1 x 103/uL), normocytic anemia (8.1 g/dL), thrombocytopenia (100,000 cells/uL), neutrophils (71.06 cells/uL), bands (27.13 cells/uL), and monocytes (11.63 cells/uL). A CT scan of the abdomen and pelvis showed a grade 4 splenic laceration with significant free abdominal fluid (Figure 1).
The patient was taken to the operating room where he underwent a splenectomy which was complicated by partial gastrectomy and partial omentectomy. He remained intubated on mechanical ventilation in the intensive care for 7 days. His progress was complicated by profound hypotension that required significant fluid administration and ultimately multiple pressors for blood pressure support. Hypotensive shock was beginning to improve on day 3 and was completely resolved by day 5. The patient underwent continuous positive airway pressure (CPAP) trials on day 6 and was successfully extubated on day 7.
After extubation a more thorough history could be obtained from the patient. He denied any history of weight loss, night sweats, or fatigue. Patient denied any known family history of hematologic malignancies. His peripheral smear showed basophilia and granulocytosis with neutrophils and immature granulocytes (Figure 2). The patient was evaluated by the hematology service and was started on allopurinol and hydroxyurea for presumed hematologic malignancy. He was given the meningococcus and streptococcus pneumoniae vaccine and was discharged home in stable condition on day eleven. Patient was subsequently positive for t(9,22)(q34;q11.2) and was started on imatinib. He has continued to follow in the clinic and is currently in remission.
Discussion
CML has a triphasic clinical course and treatment is based on the specific disease phase. The 3 phases of the disease include the chronic (more indolent) phase, accelerated (more aggressive) phase, and blast crisis. If the disease is left untreated, it will inevitably transition from a chronic to an accelerated phase and finally to blast crisis within a median time of 4 years.
The chronic phase is the most common, representing 85% of diagnoses. Patients can be asymptomatic and many in this phase will be diagnosed by routine lab testing.5 According to the World Health Organization, the accelerated phase is defined as CML patients with one of the following: 10%-19% blasts, basophils ≥20%, platelets <100,000/microL or >1,000,000/microL, unresponsive to therapy, splenomegaly unresponsive to therapy, an increasing white cell count unresponsive to therapy, or cytogenetic evolution.6 Blast crisis is the most aggressive phase and is usually defined by ≥20% blasts, large foci or clusters of blasts on the bone marrow biopsy, or the presence of extramedullary blastic infiltrates.7,8
The diagnosis of CML should be suspected in the presence of distinct lab abnormalities in the peripheral blood. These include elevated white blood cell counts with a median count of 100,000 cells/microL, elevated platelet counts, and a mild normocytic normochromic anemia. Platelet counts of 600,000 or greater have been seen in 15%-30% of patients at the time of diagnosis. The white count differential can show a variety of cells but there will be a notably greater percentage of myelocytes than metamyelocytes. Bone marrow biopsy will reveal increased cellularity, normal to slightly elevated percentage of blasts, and reticulin fibrosis. The diagnosis should be confirmed by the presence of the Philadelphia chromosome either by cytogenetics, fluorescence in situ hybridization, or reverse-transcription polymerase chain reaction (RT-PCR). The Philadelphia chromosome is found in 90%-95% of patients with CML. Most of the remaining patients will have other translocations, but a small minority will have no detectable genetic abnormalities and those patients are known as Ph-negative.9
Treatment options for CML include potential cure with allogeneic hematopoietic stem-cell transplant (HSCT) or disease control using tyrosine kinase inhibitors (TKIs). TKIs are the initial treatment of choice for newly diagnosed patients and are able to produce long-term remission in most patients. The drugs in this category include imatinib, dasatinib, and nilotinib. They work by inhibiting the Bcr-Abl tyrosine kinase, thereby blocking proliferation and inducing apoptosis in Bcr-Abl-positive cells. The majority of patients with chronic-phase CML will have an excellent response to initial treatment with a TKI. It is critical to follow these patients on a regular basis and monitor their disease status. Although the gold standard for assessing cytogenetic response is cytogenetic analysis of a bone marrow biopsy, more sensitive methods such as quantitative PCR using peripheral blood are now available, thereby minimizing the need for bone marrow biopsy. Patients in the accelerated phase are more difficult to manage because they are resistant to most forms of treatment and have short-lived responses to TKI therapy. These patients should strongly be considered for transplantation. Patients in blast crisis have aggressive disease that is more complex and requires more extensive testing. These patients should ideally be treated at tertiary care centers and treatment often involves chemotherapy in addition to TKI therapy usually followed by HSCT.
Atraumatic splenic rupture (ASR) presents similarly to traumatic splenic rupture with typical symptoms being acute onset of upper abdominal, left chest wall, or left shoulder pain (Kehr’s sign) but without a known history of trauma. Quick recognition and surgical intervention represent the best means of definitive care.10 Renzulli and colleagues conducted a literature review for all ASR cases from 1980-2008, examining 632 publications representing 845 cases. They examined the cases using logistic regression analysis to better define the clinicopathology behind ASR. The reported causes of ASR are neoplastic processes (30.3%), infectious (27.3%), inflammatory noninfectious (20.0%), drug- and treatment-related (9.2%), mechanical (6.8%), and normal spleen (6.4%). Treatment included total splenectomy in 84.1% of cases, organ-preserving surgery in 1.2%, and conservative measures in 14.7%. They reported an ASR-related mortality of 12.2%, with being older than 40 and neoplastic disorders associated with increased mortality – although male sex and splenomegaly have also been reported.11-13 Thomas and colleagues have reported on 48 cases of ASR related to hematologic malignancy showing acute myeloid leukemia being the most common cause (21%), followed by acute lymphoblastic leukemia (19%).2
Hematologic malignancies commonly cause splenic engorgement and pain although splenic rupture is an extremely rare event. Recent literature review has shown fewer than a thousand reported cases since 1980.4 There far fewer reported cases of ASR being related to CML, with most being reported as a complication.3,14 Based on our review, we could identify only a handful cases of CML with ASR being the initial symptom. These include a patient with Ph-negative CML and ASR following blast crisis, a patient with Phil-negative BCR-ABL-positive essential thrombocythemia, several cases in which the patient ultimately died, and 1 in which the patient survived into remission.4,14-16 Our case is different because the patient was ultimately positive for t(9,22)(q34;q11.2) and although he experienced multiple complications, he is currently functioning at his baseline and in remission. We hope this case will remind others that CML should be considered in the differential diagnosis of patients ASR.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
1. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, Ga: American Cancer Society; 2015.
2. Bauer TW, Haskins GE, Armitage JO. Splenic rupture in patients with hematologic malignancies. Cancer. 1981;48:2729-2733.
3. Giagounidis AA, Burk M, Meckenstock G, Koch AJ, Schneider W. Pathologic rupture of the spleen in hematologic malignancies: two additional cases. Ann Hematol. 1996;73(6):297-302.
4. Goodard SL, Chesney AE, Reis MD, et al. Pathologic splenic rupture: a rare complication of chronic myelomonocytic leukemia. Am J Hematology. 2007;82:405-408.
5. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
6. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
7. Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292-2302.
8. Kantarjian HM, O’Brien S, Cortes J, et al. Results of decitabine (5-aza-2’deoxycytidine) therapy in 130 patients with chronic myelogenous leukemia. Cancer.2003; 98:522-528.
9. Swerdlow SH, Campo E, Harris NL, et al, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC Press; 2008.
10. Maung A, KaplanL. Management of splenic injury in the adult trauma patient. In: UpToDate, Basow DS (ed), Waltham, MA, 2013.
11. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;8(10):1114-1121.
12. Hehlmann R, Heimpel H, Hasford J, et al. Randomized comparison of interferon-alpha with busulfan and hydroxyurea in chronic myelogenous leukemia. The German CML Study Group. Blood. 1994;84:4064-4077.
13. Cortes J, Kantarjian H. How I treat newly diagnosed chronic phase CML. Blood. 2012;120:1390-1397.
14. Nestok BR, Goldstein JD, Lipkovic P. Splenic rupture as a cause of sudden death in undiagnosed chronic myelogenous leukemia. Am J Forensic Med Pathol. 1988;9:241-245.
15. Sachithanandan A, Gleadhil I, Alexander HD, Morris TC. Spontaneous splenic rupture in atypical (Philadelphia chromosome negative) chronic myeloid leukemia following blastic crisis. Ir Med J. 2003;96(6):181-182.
16. Chim CS, Kwong YL, Shek TW, Ma SK, Ooi GC. Splenic rupture as the presenting symptom of blastic crisis in a patient with Philadelphia-negative, BCR-ABL-positive ET. Am J Hematology. 2001;66:70-71.
AML patients may fare better at NCI centers
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
New research suggests patients with acute myeloid leukemia (AML) may have a lower risk of early mortality if they receive treatment at a National Cancer Institute (NCI) cancer center.
In a study of AML patients in California, the risk of 60-day mortality was 53% lower among patients treated at NCI cancer centers than among those treated at other centers.
These findings were reported in Cancer.
“We found the early mortality, deaths less than 60 days after diagnosis, was significantly lower at the NCI-designated cancer centers compared to non-NCI-designated cancer centers in California,” said study author Brian Jonas, MD, PhD, of the University of California at Davis School of Medicine in Sacramento, California.
To conduct this study, Dr Jonas and his colleagues analyzed data from the California Cancer Registry and the California Office of Statewide Health Planning and Development Patient Discharge Database.
The California Cancer Registry provides sociodemographic and clinical data for all California cancer patients. The California Office of Statewide Health Planning and Development Patient Discharge Database has data on diagnoses and procedures for all hospital patients in California, excluding 14 Veterans Affairs and military hospitals.
Patients
The study included data on AML patients 18 and older who received inpatient chemotherapy between 1999 and 2014. There were 7007 patients, 1762 (25%) of whom were treated at NCI-designated cancer centers.
The median number of new AML patients per year was 13.5 (range, 0-43) at the NCI centers and 2 (range, 1-17) at non-NCI centers that admitted at least 1 patient with AML. More than half of the non-NCI centers had a median of 0 new AML patients per year.
NCI patients were more likely to be younger (≤65) than non-NCI patients (P<0.0001), to live in neighborhoods with higher socioeconomic status (P<0.0001), have fewer comorbidities (P<0.0001), and have public health insurance (P<0.0001).
Results
There were several types of complications that differed significantly between center types.
Patients treated at NCI centers were significantly more likely to have leukapheresis (5.5% vs 2.7%; P<0.001) and renal failure (22.8% vs 19.9%; P=0.010).
But they were significantly less likely to have respiratory failure (11.6% vs 14.3%; P=0.003) and cardiac arrest (1.1% vs 2.0%; P=0.014).
Sixty-day survival was significantly higher among NCI patients (88.0% vs 76.3%; P<0.001).
In an inverse-probability-weighted analysis adjusted for sociodemographic factors and comorbidities, treatment at an NCI center was associated with significantly lower early mortality, with an odds ratio (OR) of 0.46 (P<0.001).
This analysis also revealed a significant association between increased early mortality and major bleeding (OR=1.79, P<0.001), renal failure (OR=2.33, P<0.001), respiratory failure (OR=6.46, P<0.001), and cardiac arrest (OR=13.33, P<0.001).
For the most part, the impact of complications on early mortality did not differ significantly by treatment center.
The exception was respiratory failure. Patients with respiratory failure had a significantly greater risk of early mortality if they were treated at a non-NCI center (OR=9.48) than at an NCI center (OR=4.20).
Potential explanations
The researchers believe the variations in early mortality they observed point to inconsistent supportive care. However, more work must be done to fully understand the differences in care driving these issues.
“This is clearly provocative data that makes you want to understand exactly why,” Dr Jonas said. “We’re going to have to dive into that question in a more significant way.”
In the absence of data that could identify the exact causes, the researchers noted that other studies have shown higher patient volumes may contribute to better care.
“I see 60 or more AML cases per year,” Dr Jonas said. “High volume/low volume must play a role.”
The researchers believe other potential contributing factors could be access to clinical trials, better nursing ratios, and more sophisticated intensive care units.
The team hopes this research will spawn more intensive efforts to identify the causes that underlie variations in early mortality between hospital sites.
“This is a provocative and hopeful paper in terms of improving outcomes,” Dr Jonas said. “It sends a positive message that there are things we could probably do that could help everyone.”
CAR T before transplant yields durable remission in B-cell malignancies
SALT LAKE CITY – Chimeric antigen receptor (CAR) T-cell therapy may be an effective bridge to hematopoietic cell transplant (HCT) for high-risk B-cell malignancies, according to a systematic analysis of patient data from the National Cancer Institute.
Additionally, patients who have received CAR T-cell therapy are likely to enter HCT with a minimal residual disease (MRD)–negative complete response, which raises the possibility of a significantly less intense conditioning regimen that could omit total body irradiation (TBI), Haneen Shalabi, DO, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Patients who underwent HCT post–CAR T therapy did not have increased transplant-related morbidity or mortality,” said Dr. Shalabi, a pediatric oncologist in the hematologic diseases division of the National Cancer Institute’s pediatric oncology branch.
The combined approach also overcomes the frequent relapses seen after CAR T-cell therapy in this population. Of the 45 patients who received CAR T-cell therapy and achieved MRD-negative complete response as measured by flow cytometry, 20 did not go on to receive HCT. Of the 20 who didn’t receive HCT, 16 (80%) relapsed; 19 of the 20 (95%) had received prior HCT, said Dr. Shalabi.
However, of the 25 patients who proceeded on to receive HCT, 15 (60%) were in ongoing remission, with a median duration of 35 months (range, 11-55 months). Six patients (24%) experienced transplant-related mortality; four of these patients had no prior HCT. Ten patients (40%) experienced acute graft-versus-host disease (GVHD); two of these patients experienced grade 4 GVHD, and one experienced grade 3 GVHD.
Of the 25 patients who went on to HCT, 19 were receiving their first transplant, with a median time to transplant after CAR T-cell therapy of 57 days. Five patients (20%) had primary refractory disease. Most patients (n = 18; 72%) had TBI-based conditioning prior to their post–CAR T-cell therapy HCT. The median patient age was 15 (range, 5-30) years.
The systematic review included patients from two phase 1 studies; one was of CD19-28z CAR T-cell therapy for children and young adults with B-cell leukemia or lymphoma, and the other was of CD22-41BB CAR T-cell therapy for children and young adults with recurrent or refractory B-cell malignancies expressing CD22.
To weigh the benefit of the combined CAR T-cell therapy/HCT approach, Dr. Shalabi and her colleagues used a competing risk analysis to determine the risk of relapse post-HCT versus the risk of transplant-related mortality. Among patients undergoing their first HCT, the researchers found a 12-month cumulative incidence of relapse of 5.3% with the combined CAR T-cell therapy/HCT approach (95% confidence interval, 0.3%-22.1%). The 24-month cumulative incidence of relapse was 11.3% (95% CI, 1.7%-31.1%).
The analysis also showed the value of next-generation sequencing (NGS). “As we think about utilizing CAR T therapy as a bridge to transplant, we wanted to study the depth of CAR T–induced remission by next-gen sequencing,” Dr. Shalabi said.
Eight patients on the CD22 CAR trial had MRD analyses based on both flow cytometry and NGS. According to flow cytometry, all eight were MRD negative by 1 month; however, according to NGS, two did have detectable disease, which decreased with time. “Next-gen sequencing can identify earlier time points for relapse or ongoing remission” than flow cytometry can, she said.
An additional finding was that two-thirds of the patients who received the CD19/CD28z CAR T cells had no detectable CAR T cells when the pre-HCT conditioning regimen was initiated, said Dr. Shalabi. “CAR persistence – or lack thereof – didn’t impact post-HCT outcomes,” she said, adding that shorter-acting CAR T cells may actually be preferable when HCT is readily available as an option.
“The impact of CAR persistence peritransplant requires further analysis,” Dr. Shalabi said. It’s possible, though, that “consolidative HCT following CAR may synergistically improve event-free and overall survival for this high-risk population.”
Looking forward, Dr. Shalabi and her team are asking bigger questions: “For future directions – and this is a very big question that those in the room would probably like to know – by inducing NGS-negativity, can CAR T therapy allow for HCT conditioning deintensification, potentially reducing the risk of TRM [transplant-related mortality] and long term comorbidities?”
A future trial will explore outcomes for a conditioning regimen that omits TBI for patients who are MRD-negative by NGS, said Dr. Shalabi.
Another direction for her team’s research is to see whether introducing CAR T-cell therapy earlier in a very-high-risk population may improve outcomes; the current study population was heavily pretreated, Dr. Shalabi said.
Dr. Shalabi is employed by the National Cancer Institute. She reported no conflicts of interest.
SOURCE: Shalabi H et al. 2018 BMT Tandem Meetings, Abstract 6.
SALT LAKE CITY – Chimeric antigen receptor (CAR) T-cell therapy may be an effective bridge to hematopoietic cell transplant (HCT) for high-risk B-cell malignancies, according to a systematic analysis of patient data from the National Cancer Institute.
Additionally, patients who have received CAR T-cell therapy are likely to enter HCT with a minimal residual disease (MRD)–negative complete response, which raises the possibility of a significantly less intense conditioning regimen that could omit total body irradiation (TBI), Haneen Shalabi, DO, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Patients who underwent HCT post–CAR T therapy did not have increased transplant-related morbidity or mortality,” said Dr. Shalabi, a pediatric oncologist in the hematologic diseases division of the National Cancer Institute’s pediatric oncology branch.
The combined approach also overcomes the frequent relapses seen after CAR T-cell therapy in this population. Of the 45 patients who received CAR T-cell therapy and achieved MRD-negative complete response as measured by flow cytometry, 20 did not go on to receive HCT. Of the 20 who didn’t receive HCT, 16 (80%) relapsed; 19 of the 20 (95%) had received prior HCT, said Dr. Shalabi.
However, of the 25 patients who proceeded on to receive HCT, 15 (60%) were in ongoing remission, with a median duration of 35 months (range, 11-55 months). Six patients (24%) experienced transplant-related mortality; four of these patients had no prior HCT. Ten patients (40%) experienced acute graft-versus-host disease (GVHD); two of these patients experienced grade 4 GVHD, and one experienced grade 3 GVHD.
Of the 25 patients who went on to HCT, 19 were receiving their first transplant, with a median time to transplant after CAR T-cell therapy of 57 days. Five patients (20%) had primary refractory disease. Most patients (n = 18; 72%) had TBI-based conditioning prior to their post–CAR T-cell therapy HCT. The median patient age was 15 (range, 5-30) years.
The systematic review included patients from two phase 1 studies; one was of CD19-28z CAR T-cell therapy for children and young adults with B-cell leukemia or lymphoma, and the other was of CD22-41BB CAR T-cell therapy for children and young adults with recurrent or refractory B-cell malignancies expressing CD22.
To weigh the benefit of the combined CAR T-cell therapy/HCT approach, Dr. Shalabi and her colleagues used a competing risk analysis to determine the risk of relapse post-HCT versus the risk of transplant-related mortality. Among patients undergoing their first HCT, the researchers found a 12-month cumulative incidence of relapse of 5.3% with the combined CAR T-cell therapy/HCT approach (95% confidence interval, 0.3%-22.1%). The 24-month cumulative incidence of relapse was 11.3% (95% CI, 1.7%-31.1%).
The analysis also showed the value of next-generation sequencing (NGS). “As we think about utilizing CAR T therapy as a bridge to transplant, we wanted to study the depth of CAR T–induced remission by next-gen sequencing,” Dr. Shalabi said.
Eight patients on the CD22 CAR trial had MRD analyses based on both flow cytometry and NGS. According to flow cytometry, all eight were MRD negative by 1 month; however, according to NGS, two did have detectable disease, which decreased with time. “Next-gen sequencing can identify earlier time points for relapse or ongoing remission” than flow cytometry can, she said.
An additional finding was that two-thirds of the patients who received the CD19/CD28z CAR T cells had no detectable CAR T cells when the pre-HCT conditioning regimen was initiated, said Dr. Shalabi. “CAR persistence – or lack thereof – didn’t impact post-HCT outcomes,” she said, adding that shorter-acting CAR T cells may actually be preferable when HCT is readily available as an option.
“The impact of CAR persistence peritransplant requires further analysis,” Dr. Shalabi said. It’s possible, though, that “consolidative HCT following CAR may synergistically improve event-free and overall survival for this high-risk population.”
Looking forward, Dr. Shalabi and her team are asking bigger questions: “For future directions – and this is a very big question that those in the room would probably like to know – by inducing NGS-negativity, can CAR T therapy allow for HCT conditioning deintensification, potentially reducing the risk of TRM [transplant-related mortality] and long term comorbidities?”
A future trial will explore outcomes for a conditioning regimen that omits TBI for patients who are MRD-negative by NGS, said Dr. Shalabi.
Another direction for her team’s research is to see whether introducing CAR T-cell therapy earlier in a very-high-risk population may improve outcomes; the current study population was heavily pretreated, Dr. Shalabi said.
Dr. Shalabi is employed by the National Cancer Institute. She reported no conflicts of interest.
SOURCE: Shalabi H et al. 2018 BMT Tandem Meetings, Abstract 6.
SALT LAKE CITY – Chimeric antigen receptor (CAR) T-cell therapy may be an effective bridge to hematopoietic cell transplant (HCT) for high-risk B-cell malignancies, according to a systematic analysis of patient data from the National Cancer Institute.
Additionally, patients who have received CAR T-cell therapy are likely to enter HCT with a minimal residual disease (MRD)–negative complete response, which raises the possibility of a significantly less intense conditioning regimen that could omit total body irradiation (TBI), Haneen Shalabi, DO, said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“Patients who underwent HCT post–CAR T therapy did not have increased transplant-related morbidity or mortality,” said Dr. Shalabi, a pediatric oncologist in the hematologic diseases division of the National Cancer Institute’s pediatric oncology branch.
The combined approach also overcomes the frequent relapses seen after CAR T-cell therapy in this population. Of the 45 patients who received CAR T-cell therapy and achieved MRD-negative complete response as measured by flow cytometry, 20 did not go on to receive HCT. Of the 20 who didn’t receive HCT, 16 (80%) relapsed; 19 of the 20 (95%) had received prior HCT, said Dr. Shalabi.
However, of the 25 patients who proceeded on to receive HCT, 15 (60%) were in ongoing remission, with a median duration of 35 months (range, 11-55 months). Six patients (24%) experienced transplant-related mortality; four of these patients had no prior HCT. Ten patients (40%) experienced acute graft-versus-host disease (GVHD); two of these patients experienced grade 4 GVHD, and one experienced grade 3 GVHD.
Of the 25 patients who went on to HCT, 19 were receiving their first transplant, with a median time to transplant after CAR T-cell therapy of 57 days. Five patients (20%) had primary refractory disease. Most patients (n = 18; 72%) had TBI-based conditioning prior to their post–CAR T-cell therapy HCT. The median patient age was 15 (range, 5-30) years.
The systematic review included patients from two phase 1 studies; one was of CD19-28z CAR T-cell therapy for children and young adults with B-cell leukemia or lymphoma, and the other was of CD22-41BB CAR T-cell therapy for children and young adults with recurrent or refractory B-cell malignancies expressing CD22.
To weigh the benefit of the combined CAR T-cell therapy/HCT approach, Dr. Shalabi and her colleagues used a competing risk analysis to determine the risk of relapse post-HCT versus the risk of transplant-related mortality. Among patients undergoing their first HCT, the researchers found a 12-month cumulative incidence of relapse of 5.3% with the combined CAR T-cell therapy/HCT approach (95% confidence interval, 0.3%-22.1%). The 24-month cumulative incidence of relapse was 11.3% (95% CI, 1.7%-31.1%).
The analysis also showed the value of next-generation sequencing (NGS). “As we think about utilizing CAR T therapy as a bridge to transplant, we wanted to study the depth of CAR T–induced remission by next-gen sequencing,” Dr. Shalabi said.
Eight patients on the CD22 CAR trial had MRD analyses based on both flow cytometry and NGS. According to flow cytometry, all eight were MRD negative by 1 month; however, according to NGS, two did have detectable disease, which decreased with time. “Next-gen sequencing can identify earlier time points for relapse or ongoing remission” than flow cytometry can, she said.
An additional finding was that two-thirds of the patients who received the CD19/CD28z CAR T cells had no detectable CAR T cells when the pre-HCT conditioning regimen was initiated, said Dr. Shalabi. “CAR persistence – or lack thereof – didn’t impact post-HCT outcomes,” she said, adding that shorter-acting CAR T cells may actually be preferable when HCT is readily available as an option.
“The impact of CAR persistence peritransplant requires further analysis,” Dr. Shalabi said. It’s possible, though, that “consolidative HCT following CAR may synergistically improve event-free and overall survival for this high-risk population.”
Looking forward, Dr. Shalabi and her team are asking bigger questions: “For future directions – and this is a very big question that those in the room would probably like to know – by inducing NGS-negativity, can CAR T therapy allow for HCT conditioning deintensification, potentially reducing the risk of TRM [transplant-related mortality] and long term comorbidities?”
A future trial will explore outcomes for a conditioning regimen that omits TBI for patients who are MRD-negative by NGS, said Dr. Shalabi.
Another direction for her team’s research is to see whether introducing CAR T-cell therapy earlier in a very-high-risk population may improve outcomes; the current study population was heavily pretreated, Dr. Shalabi said.
Dr. Shalabi is employed by the National Cancer Institute. She reported no conflicts of interest.
SOURCE: Shalabi H et al. 2018 BMT Tandem Meetings, Abstract 6.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Of 20 patients receiving CAR T before HCT, 15 (60%) were in ongoing remission of a median 35 months.
Study details: Systematic analysis of 42 patients with B-cell malignancies receiving CAR T-cell therapy at the National Cancer Institute.
Disclosures: The study was conducted at the National Cancer Institute, where Dr. Shalabi is employed.
Source: Shalabi H et al. 2018 BMT Tandem Meetings, Abstract 6.
A global snapshot of leukemia incidence
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.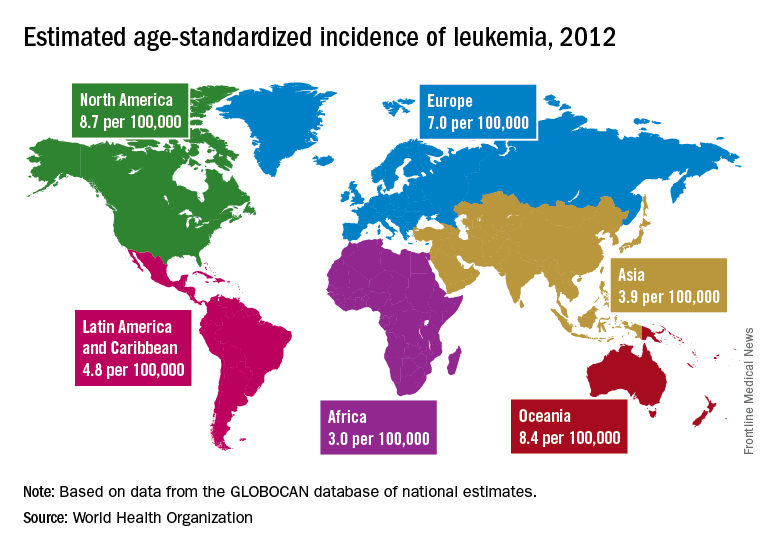
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
, according to an analysis of World Health Organization cancer databases.
Incidence also is generally higher in males, with a global male to female ratio of 1.4. For men, the highest regional leukemia rate – estimated at 11.3 per 100,000 population for 2012 – was found in Australia and New Zealand, with northern America (the United States and Canada) next at 10.5 per 100,000. Australia/New Zealand and northern America had the highest rate for women at 7.2 per 100,000, followed by western Europe and northern Europe at 6.0 per 100,000, reported Adalberto Miranda-Filho, PhD, of the WHO’s International Agency for Research on Cancer in Lyon, France, and his associates.
The lowest regional rates for women were found in western Africa (1.2 per 100,000), middle Africa (1.8), and Micronesia/Polynesia (2.1). For men, leukemia incidence was lowest in western Africa (1.4 per 100,000), middle Africa (2.6), and south-central Asia (3.4), according to data from the WHO’s GLOBOCAN database. The report was published in The Lancet Haematology.
Estimates for leukemia subtypes in 2003-2007 – calculated for 54 countries, not regions – also showed a great deal of variation. For acute lymphoblastic leukemia, Ecuador had the highest rates for both males (2.8 per 100,000) and females (3.3), with high rates seen in Costa Rica, Columbia, and Cyprus. Rates in the United States were near the top: 2.1 for males and 1.6 for females. Rates were lowest for men in Jamaica (0.4) and Serbia (0.6) and for women in India (0.5) and Serbia and Cuba (0.6), Dr. Miranda-Filho and his associates said.
Incidence rates for acute myeloid leukemia were highest in Australia for men (2.8 per 100,000) and Austria for women (2.2), with the United States near the top for both men (2.6) and women (1.9). The lowest rates occurred in Cuba and Egypt for men (0.9 per 100,000) and Cuba for women (0.4), data from the WHO’s Cancer Incidence in Five Continents Volume X show.
Chronic lymphocytic leukemia incidence was highest for men in Canada (4.5 per 100,000), Ireland and Lithuania (4.4), and Slovakia (4.3). The incidence was highest for women in Lithuania (2.5), Canada (2.3), and Slovakia and Denmark (2.1). Incidence in the United States was 3.5 for men and 1.8 for women. At the other end of the scale, the lowest rates for both men and women were in Japan and Malaysia (0.1), the investigators’ analysis showed.
Chronic myeloid leukemia rates were the lowest of the subtypes, and Tunisia was the lowest for men at 0.4 per 100,000 and tied for lowest with Serbia, Slovenia, and Puerto Rico for women at 0.3. Incidence was highest for men in Australia at 1.8 per 100,000 and highest for women in Uruguay at 1.1. Rates in the United States were 1.3 for men and 0.8 for women, Dr. Miranda-Filho and his associates said.
“The higher incidence of acute lymphoblastic leukaemia in parts of South America, as well as of chronic lymphocytic leukaemia in populations across North America and Oceania, alongside a lower incidence in Asia, might be important markers for further epidemiological study, and a means to better understand the underlying factors to support future cancer prevention strategies,” the investigators wrote.
SOURCE: Miranda-Filho A et al. Lancet Haematol. 2018;5:e14-24.
FROM THE LANCET HAEMATOLOGY
AYA cancer survivors have better social support than peers
Researchers have developed a new method to measure social networks of adolescent and young adult (AYA) cancer survivors.
This method indicated that AYA cancer survivors often have stronger social networks than their non-cancer peers.
However, the strength of the social network varied by diagnosis, with the lymphoma and leukemia survivors having the greatest support.
These findings were published in Cancer.
“Cancer survivors need healthy social connections, and, to the best of our knowledge, this is the first published study to quantify social networks of adolescent and young adult cancer survivors compared to their peers,” said study author I-Chan Huang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The study introduces a method we developed and validated for evaluating social networks of these cancer survivors.”
The method, called the functional social network index (FSNI), measures marital status, contact frequency with friends and relatives, and available resources for health support/advice, which includes emotional support, tangible support, physical activity advice, and weight management advice.
The researchers compared the FSNI to a pair of traditional social network indices—density and betweenness centrality.
Density represents the ratio of the existing relationships/connections within a network to all possible relationships/connections. And betweenness centrality represents the ratio of the existing shortest paths between 2 friends/relatives of the study participants to the shortest possible paths between 2 friends/relatives.
Subjects
The researchers used the 3 social network indices to analyze 102 AYA cancer survivors, ages 18 to 30, and 102 young adults with no cancer history who were matched to the survivors by age, sex, and race.
Subjects were recruited from a commercial national Internet survey panel. They reported detailed social connection information with up to 25 friends and relatives.
The cancer survivors were between 15 and 30 years old when their cancer was diagnosed, and all had completed treatment at least 5 years prior.
Results
Neither the density index nor the betweenness centrality index demonstrated significant differences between cancer survivors and controls (all P values were less than 0.05).
However, according to the FSNI, cancer survivors had more available resources for emotional support (beta [b]=3.02; P=0.003), tangible support (b=4.17; P<0.001), physical activity advice (b=3.94; P<0.001), and weight management advice (b=4.10; P<0.001).
“This makes sense,” Dr Huang said. “Because of their cancer, survivors often have strong networks of physicians, friends, and relatives to provide advice and support.”
However, the FSNI showed the strength of cancer survivors’ support network varied by diagnosis.
Lymphoma survivors ranked highest on the FSNI (b=2.765; P=0.02), followed by survivors of leukemia (b=2.542; P=0.03) and solid tumors (b=2.178; P=0.047), with central nervous system malignancies as the reference.
The researchers also found a higher FSNI was associated with better coping skills, including using emotional support (b=0.08; P=0.04), using instrumental support (b=0.12; P<0.001), venting of emotions (b=0.10; P=0.004), positive reframing (b=0.12; P=0.003), planning for the future (b=0.08; P=0.03), participating in religious activities (b=0.16; P<0.001), and less denial (b=0.10; P=0.01) and destructive behavior (b=0.08; P=0.04).
The researchers said long-term follow-up is needed to understand how social networks and social support may change over time.
“Adolescents and young adult cancer survivors are in a transitory stage of independence from parents,” Dr Huang said. “While this study suggests that survivors often report strong social connections, our previous studies have reported that childhood cancer survivors are more likely than their peers to struggle mentally and physically and report issues like distress and loneliness.”
Dr Huang and his colleagues are working to streamline the FSNI to make it easier for healthcare providers to assess support available to cancer survivors of any age.
Meanwhile, researchers are working to better understand how social connections affect health outcomes in order to design interventions to foster those connections.
“A lack of social connections with friends and relatives is associated with poor quality of life, risky health behaviors, chronic health conditions, and premature death,” Dr Huang said. “Once we identify the mechanism between social connections and health outcomes, we can start designing interventions to use social networks to improve health outcomes of cancer survivors.”
Researchers have developed a new method to measure social networks of adolescent and young adult (AYA) cancer survivors.
This method indicated that AYA cancer survivors often have stronger social networks than their non-cancer peers.
However, the strength of the social network varied by diagnosis, with the lymphoma and leukemia survivors having the greatest support.
These findings were published in Cancer.
“Cancer survivors need healthy social connections, and, to the best of our knowledge, this is the first published study to quantify social networks of adolescent and young adult cancer survivors compared to their peers,” said study author I-Chan Huang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The study introduces a method we developed and validated for evaluating social networks of these cancer survivors.”
The method, called the functional social network index (FSNI), measures marital status, contact frequency with friends and relatives, and available resources for health support/advice, which includes emotional support, tangible support, physical activity advice, and weight management advice.
The researchers compared the FSNI to a pair of traditional social network indices—density and betweenness centrality.
Density represents the ratio of the existing relationships/connections within a network to all possible relationships/connections. And betweenness centrality represents the ratio of the existing shortest paths between 2 friends/relatives of the study participants to the shortest possible paths between 2 friends/relatives.
Subjects
The researchers used the 3 social network indices to analyze 102 AYA cancer survivors, ages 18 to 30, and 102 young adults with no cancer history who were matched to the survivors by age, sex, and race.
Subjects were recruited from a commercial national Internet survey panel. They reported detailed social connection information with up to 25 friends and relatives.
The cancer survivors were between 15 and 30 years old when their cancer was diagnosed, and all had completed treatment at least 5 years prior.
Results
Neither the density index nor the betweenness centrality index demonstrated significant differences between cancer survivors and controls (all P values were less than 0.05).
However, according to the FSNI, cancer survivors had more available resources for emotional support (beta [b]=3.02; P=0.003), tangible support (b=4.17; P<0.001), physical activity advice (b=3.94; P<0.001), and weight management advice (b=4.10; P<0.001).
“This makes sense,” Dr Huang said. “Because of their cancer, survivors often have strong networks of physicians, friends, and relatives to provide advice and support.”
However, the FSNI showed the strength of cancer survivors’ support network varied by diagnosis.
Lymphoma survivors ranked highest on the FSNI (b=2.765; P=0.02), followed by survivors of leukemia (b=2.542; P=0.03) and solid tumors (b=2.178; P=0.047), with central nervous system malignancies as the reference.
The researchers also found a higher FSNI was associated with better coping skills, including using emotional support (b=0.08; P=0.04), using instrumental support (b=0.12; P<0.001), venting of emotions (b=0.10; P=0.004), positive reframing (b=0.12; P=0.003), planning for the future (b=0.08; P=0.03), participating in religious activities (b=0.16; P<0.001), and less denial (b=0.10; P=0.01) and destructive behavior (b=0.08; P=0.04).
The researchers said long-term follow-up is needed to understand how social networks and social support may change over time.
“Adolescents and young adult cancer survivors are in a transitory stage of independence from parents,” Dr Huang said. “While this study suggests that survivors often report strong social connections, our previous studies have reported that childhood cancer survivors are more likely than their peers to struggle mentally and physically and report issues like distress and loneliness.”
Dr Huang and his colleagues are working to streamline the FSNI to make it easier for healthcare providers to assess support available to cancer survivors of any age.
Meanwhile, researchers are working to better understand how social connections affect health outcomes in order to design interventions to foster those connections.
“A lack of social connections with friends and relatives is associated with poor quality of life, risky health behaviors, chronic health conditions, and premature death,” Dr Huang said. “Once we identify the mechanism between social connections and health outcomes, we can start designing interventions to use social networks to improve health outcomes of cancer survivors.”
Researchers have developed a new method to measure social networks of adolescent and young adult (AYA) cancer survivors.
This method indicated that AYA cancer survivors often have stronger social networks than their non-cancer peers.
However, the strength of the social network varied by diagnosis, with the lymphoma and leukemia survivors having the greatest support.
These findings were published in Cancer.
“Cancer survivors need healthy social connections, and, to the best of our knowledge, this is the first published study to quantify social networks of adolescent and young adult cancer survivors compared to their peers,” said study author I-Chan Huang, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The study introduces a method we developed and validated for evaluating social networks of these cancer survivors.”
The method, called the functional social network index (FSNI), measures marital status, contact frequency with friends and relatives, and available resources for health support/advice, which includes emotional support, tangible support, physical activity advice, and weight management advice.
The researchers compared the FSNI to a pair of traditional social network indices—density and betweenness centrality.
Density represents the ratio of the existing relationships/connections within a network to all possible relationships/connections. And betweenness centrality represents the ratio of the existing shortest paths between 2 friends/relatives of the study participants to the shortest possible paths between 2 friends/relatives.
Subjects
The researchers used the 3 social network indices to analyze 102 AYA cancer survivors, ages 18 to 30, and 102 young adults with no cancer history who were matched to the survivors by age, sex, and race.
Subjects were recruited from a commercial national Internet survey panel. They reported detailed social connection information with up to 25 friends and relatives.
The cancer survivors were between 15 and 30 years old when their cancer was diagnosed, and all had completed treatment at least 5 years prior.
Results
Neither the density index nor the betweenness centrality index demonstrated significant differences between cancer survivors and controls (all P values were less than 0.05).
However, according to the FSNI, cancer survivors had more available resources for emotional support (beta [b]=3.02; P=0.003), tangible support (b=4.17; P<0.001), physical activity advice (b=3.94; P<0.001), and weight management advice (b=4.10; P<0.001).
“This makes sense,” Dr Huang said. “Because of their cancer, survivors often have strong networks of physicians, friends, and relatives to provide advice and support.”
However, the FSNI showed the strength of cancer survivors’ support network varied by diagnosis.
Lymphoma survivors ranked highest on the FSNI (b=2.765; P=0.02), followed by survivors of leukemia (b=2.542; P=0.03) and solid tumors (b=2.178; P=0.047), with central nervous system malignancies as the reference.
The researchers also found a higher FSNI was associated with better coping skills, including using emotional support (b=0.08; P=0.04), using instrumental support (b=0.12; P<0.001), venting of emotions (b=0.10; P=0.004), positive reframing (b=0.12; P=0.003), planning for the future (b=0.08; P=0.03), participating in religious activities (b=0.16; P<0.001), and less denial (b=0.10; P=0.01) and destructive behavior (b=0.08; P=0.04).
The researchers said long-term follow-up is needed to understand how social networks and social support may change over time.
“Adolescents and young adult cancer survivors are in a transitory stage of independence from parents,” Dr Huang said. “While this study suggests that survivors often report strong social connections, our previous studies have reported that childhood cancer survivors are more likely than their peers to struggle mentally and physically and report issues like distress and loneliness.”
Dr Huang and his colleagues are working to streamline the FSNI to make it easier for healthcare providers to assess support available to cancer survivors of any age.
Meanwhile, researchers are working to better understand how social connections affect health outcomes in order to design interventions to foster those connections.
“A lack of social connections with friends and relatives is associated with poor quality of life, risky health behaviors, chronic health conditions, and premature death,” Dr Huang said. “Once we identify the mechanism between social connections and health outcomes, we can start designing interventions to use social networks to improve health outcomes of cancer survivors.”
TFR achievable with second-line nilotinib for chronic CML
Second-line nilotinib may lead to maintained molecular response and treatment-free remission that can last 48 weeks or longer for patients with chronic myeloid leukemia (CML), findings from a phase 2 study suggest.
Treatment-free remission (TFR) is an emerging treatment goal for patients with CML in the chronic phase, according to François-Xavier Mahon, MD, PhD, of the University of Bordeaux (France) and his colleagues. “Potential motivators and benefits of achieving TFR may include relief of treatment side effects, reduced risk for long-term [tyrosine kinase inhibitor] toxicity, and the ability to plan a family,” they wrote. “When TFR is a treatment goal, achievement of [deep molecular response] is a key prerequisite.”
Established molecular response benchmarks include major molecular response (MMR), MR4 and MR4.5, according to the researchers.
In an open-label phase 2 study, the researchers enrolled patients with Philadelphia chromosome-positive CML who received nilotinib for 2 years or longer after having received imatinib for longer than 4 weeks. The other key criterion for enrollment was achieving MR4.5 during treatment with nilotinib, according to the study, published in Annals of Internal Medicine.
In total, 163 patients were enrolled and entered the 1-year consolidation phase. Of those patients, 126 were eligible for the TFR phase during which nilotinib treatment was stopped.
Dr. Mahon and his colleagues reported that 73 (58%) patients in the TFR phase maintained TFR at 48 weeks, 67 of whom had MR4.5. Of the seven patients who had a loss of MR4.5, four did not have a loss of MMR or confirmed loss of MR4, according to the researchers.
While the primary endpoint was TFR at 48 weeks, the researchers reported that 53% of patients maintained TFR at 96 weeks. Some patients had reinitiated nilotinib by the 96-week cutoff. Of those patients, the study showed that 93% regained MR4 and MR4.5.
The researchers noted that the safety findings were consistent with previously published data of nilotinib. “Improvements in quality of life have been cited as a motivator for stopping treatment,” they wrote. “Minimal changes in quality of life were seen with treatment cessation, possibly because the patients in this study already had a relatively high quality of life, given that they had tolerated at least 3 years of nilotinib therapy before stopping treatment.”
Novartis Pharmaceuticals funded the study. Dr. Mahon and other researchers reported financial ties to several pharmaceutical companies, including Novartis.
SOURCE: Mahon FX et al. Ann Intern Med. 2018 Feb 20. doi: 10.7326/M17-1094.
Second-line nilotinib may lead to maintained molecular response and treatment-free remission that can last 48 weeks or longer for patients with chronic myeloid leukemia (CML), findings from a phase 2 study suggest.
Treatment-free remission (TFR) is an emerging treatment goal for patients with CML in the chronic phase, according to François-Xavier Mahon, MD, PhD, of the University of Bordeaux (France) and his colleagues. “Potential motivators and benefits of achieving TFR may include relief of treatment side effects, reduced risk for long-term [tyrosine kinase inhibitor] toxicity, and the ability to plan a family,” they wrote. “When TFR is a treatment goal, achievement of [deep molecular response] is a key prerequisite.”
Established molecular response benchmarks include major molecular response (MMR), MR4 and MR4.5, according to the researchers.
In an open-label phase 2 study, the researchers enrolled patients with Philadelphia chromosome-positive CML who received nilotinib for 2 years or longer after having received imatinib for longer than 4 weeks. The other key criterion for enrollment was achieving MR4.5 during treatment with nilotinib, according to the study, published in Annals of Internal Medicine.
In total, 163 patients were enrolled and entered the 1-year consolidation phase. Of those patients, 126 were eligible for the TFR phase during which nilotinib treatment was stopped.
Dr. Mahon and his colleagues reported that 73 (58%) patients in the TFR phase maintained TFR at 48 weeks, 67 of whom had MR4.5. Of the seven patients who had a loss of MR4.5, four did not have a loss of MMR or confirmed loss of MR4, according to the researchers.
While the primary endpoint was TFR at 48 weeks, the researchers reported that 53% of patients maintained TFR at 96 weeks. Some patients had reinitiated nilotinib by the 96-week cutoff. Of those patients, the study showed that 93% regained MR4 and MR4.5.
The researchers noted that the safety findings were consistent with previously published data of nilotinib. “Improvements in quality of life have been cited as a motivator for stopping treatment,” they wrote. “Minimal changes in quality of life were seen with treatment cessation, possibly because the patients in this study already had a relatively high quality of life, given that they had tolerated at least 3 years of nilotinib therapy before stopping treatment.”
Novartis Pharmaceuticals funded the study. Dr. Mahon and other researchers reported financial ties to several pharmaceutical companies, including Novartis.
SOURCE: Mahon FX et al. Ann Intern Med. 2018 Feb 20. doi: 10.7326/M17-1094.
Second-line nilotinib may lead to maintained molecular response and treatment-free remission that can last 48 weeks or longer for patients with chronic myeloid leukemia (CML), findings from a phase 2 study suggest.
Treatment-free remission (TFR) is an emerging treatment goal for patients with CML in the chronic phase, according to François-Xavier Mahon, MD, PhD, of the University of Bordeaux (France) and his colleagues. “Potential motivators and benefits of achieving TFR may include relief of treatment side effects, reduced risk for long-term [tyrosine kinase inhibitor] toxicity, and the ability to plan a family,” they wrote. “When TFR is a treatment goal, achievement of [deep molecular response] is a key prerequisite.”
Established molecular response benchmarks include major molecular response (MMR), MR4 and MR4.5, according to the researchers.
In an open-label phase 2 study, the researchers enrolled patients with Philadelphia chromosome-positive CML who received nilotinib for 2 years or longer after having received imatinib for longer than 4 weeks. The other key criterion for enrollment was achieving MR4.5 during treatment with nilotinib, according to the study, published in Annals of Internal Medicine.
In total, 163 patients were enrolled and entered the 1-year consolidation phase. Of those patients, 126 were eligible for the TFR phase during which nilotinib treatment was stopped.
Dr. Mahon and his colleagues reported that 73 (58%) patients in the TFR phase maintained TFR at 48 weeks, 67 of whom had MR4.5. Of the seven patients who had a loss of MR4.5, four did not have a loss of MMR or confirmed loss of MR4, according to the researchers.
While the primary endpoint was TFR at 48 weeks, the researchers reported that 53% of patients maintained TFR at 96 weeks. Some patients had reinitiated nilotinib by the 96-week cutoff. Of those patients, the study showed that 93% regained MR4 and MR4.5.
The researchers noted that the safety findings were consistent with previously published data of nilotinib. “Improvements in quality of life have been cited as a motivator for stopping treatment,” they wrote. “Minimal changes in quality of life were seen with treatment cessation, possibly because the patients in this study already had a relatively high quality of life, given that they had tolerated at least 3 years of nilotinib therapy before stopping treatment.”
Novartis Pharmaceuticals funded the study. Dr. Mahon and other researchers reported financial ties to several pharmaceutical companies, including Novartis.
SOURCE: Mahon FX et al. Ann Intern Med. 2018 Feb 20. doi: 10.7326/M17-1094.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point:
Major finding: In total, 58% of patients who switched to nilotinib experienced treatment-free remission at 48 weeks.
Study details: A single-group, open-label phase 2 study.
Disclosures: Novartis Pharmaceuticals funded the study. The researchers reported financial ties to Novartis and other pharmaceutical companies.
Source: Mahon FX et al. Ann Intern Med. 2018 Feb 20. doi: 10.7326/M17-1094.
CCSs have greater risk of cardiovascular disease
Childhood cancer survivors (CCSs) have an increased risk of premature cardiovascular disease in adulthood, according to a new study.
Researchers found a nearly 2-fold increased risk of cardiovascular diseases in CCSs compared to the general population.
Cardiovascular disease was identified in 4.5% of CCSs and occurred in most before they reached the age of 40, nearly 8 years earlier than in the general population.
“Our results show that these survivors of childhood cancer have a substantially elevated burden of prematurely occurring traditional cardiovascular risk factors and cardiovascular diseases,” said study author Joerg Faber, MD, PhD, of the Johannes Gutenberg University Mainz in Germany.
Dr Faber and his colleagues reported these results in the European Heart Journal.
The researchers evaluated 951 adult CCSs (ages 23 to 48), referred to as the CVSS cohort (Cardiac and Vascular Late Sequelae in Long-Term Survivors of Childhood Cancer Study).
The patients had been diagnosed with cancer between 1980 and 1990. The most common diagnoses were leukemia (43.5%), central nervous system tumors (12.8%), and lymphoma (9.9%).
For this study, the patients underwent standardized clinical and laboratory cardiovascular screening. The mean time from cancer diagnosis to cardiovascular screening was 28.4 years (range, 23–36).
The researchers compared the incidence of cardiovascular risk factors and cardiovascular disease in the CVSS cohort and subjects from the Gutenberg Health Study (GHS), a population-based study including more than 15,000 subjects.
Risk factors
The CVSS cohort had a greater risk of 2 cardiovascular risk factors—arterial hypertension and dyslipidemia—than the GHS cohort. In the CVSS cohort, the incidence of dyslipidemia was 28.3%, and the incidence of hypertension was 23.0%.
CVSS subjects had an age-adjusted 38% increase in risk for hypertension (relative risk [RR]=1.38) and a 26% increase in risk for dyslipidemia (RR=1.26).
Hypertension occurred about 6 years earlier in CVSS subjects than GHS subjects (rate advancement period estimator [RAP]=5.75). And dyslipidemia occurred about 8 years earlier in the CVSS cohort (RAP=8.16).
“[T]he premature onset of high blood pressure and blood lipid disorders may play an important role in the development of severe cardiovascular conditions, such as heart disease and stroke, in the long-term,” said study author Philipp S. Wild, MD, of the German Center for Cardiovascular Research (DZHK) in Mainz, Germany.
“We also found that a remarkable number [of CVSS subjects] attended their clinical examination for this study with previously unidentified cardiovascular risk factors and cardiovascular disease. For example, only 62 out of 269 were aware of having dyslipidemia. Consequently, 207, approximately 80%, were only diagnosed at that point.”
Disease
In the CVSS cohort, 4.5% of patients had at least 1 type of cardiovascular disease. This included venous thromboembolism (2.0%), congestive heart failure (1.2%), stroke (0.5%), peripheral artery disease (0.5%), atrial fibrillation (0.4%), and coronary heart disease (0.3%).
CVSS subjects had nearly twice the risk of cardiovascular disease as GHS subjects. The age-adjusted RR was 1.89. And CVSS subjects developed cardiovascular disease roughly 8 years earlier than GHS subjects (RAP=7.9).
In the CVSS cohort, the probability of developing cardiovascular disease was estimated as 2.9% at age 30 and 9.6% at age 45.
The researchers said these findings show that CCSs have a greater risk of cardiovascular disease that continues to increase with age. This, in turn, means CCSs may be more likely to die earlier. However, this might be preventable, according to Dr Wild.
“Early systematic screening, particularly focusing on blood pressure and lipid measurements, might be suggested in all childhood cancer survivors irrespective of the type of cancer or treatment they had had,” Dr Wild said. “This might help to prevent long-term cardiovascular diseases by intervening early—for instance, by modifying lifestyles and having treatment for high blood pressure.”
“Usually, survivors are followed for only 5 to 10 years after completion of therapy, and this is focused on the risk of the cancer returning and the acute adverse effects of their treatment, rather than on other conditions,” added Dr Faber.
“Current guidelines recommend cardiovascular assessments only for subgroups known to be at risk, such as for patients who were treated with anthracycline therapy and/or radiation therapy. However, further investigations are needed to answer questions about the best follow-up care.”
Childhood cancer survivors (CCSs) have an increased risk of premature cardiovascular disease in adulthood, according to a new study.
Researchers found a nearly 2-fold increased risk of cardiovascular diseases in CCSs compared to the general population.
Cardiovascular disease was identified in 4.5% of CCSs and occurred in most before they reached the age of 40, nearly 8 years earlier than in the general population.
“Our results show that these survivors of childhood cancer have a substantially elevated burden of prematurely occurring traditional cardiovascular risk factors and cardiovascular diseases,” said study author Joerg Faber, MD, PhD, of the Johannes Gutenberg University Mainz in Germany.
Dr Faber and his colleagues reported these results in the European Heart Journal.
The researchers evaluated 951 adult CCSs (ages 23 to 48), referred to as the CVSS cohort (Cardiac and Vascular Late Sequelae in Long-Term Survivors of Childhood Cancer Study).
The patients had been diagnosed with cancer between 1980 and 1990. The most common diagnoses were leukemia (43.5%), central nervous system tumors (12.8%), and lymphoma (9.9%).
For this study, the patients underwent standardized clinical and laboratory cardiovascular screening. The mean time from cancer diagnosis to cardiovascular screening was 28.4 years (range, 23–36).
The researchers compared the incidence of cardiovascular risk factors and cardiovascular disease in the CVSS cohort and subjects from the Gutenberg Health Study (GHS), a population-based study including more than 15,000 subjects.
Risk factors
The CVSS cohort had a greater risk of 2 cardiovascular risk factors—arterial hypertension and dyslipidemia—than the GHS cohort. In the CVSS cohort, the incidence of dyslipidemia was 28.3%, and the incidence of hypertension was 23.0%.
CVSS subjects had an age-adjusted 38% increase in risk for hypertension (relative risk [RR]=1.38) and a 26% increase in risk for dyslipidemia (RR=1.26).
Hypertension occurred about 6 years earlier in CVSS subjects than GHS subjects (rate advancement period estimator [RAP]=5.75). And dyslipidemia occurred about 8 years earlier in the CVSS cohort (RAP=8.16).
“[T]he premature onset of high blood pressure and blood lipid disorders may play an important role in the development of severe cardiovascular conditions, such as heart disease and stroke, in the long-term,” said study author Philipp S. Wild, MD, of the German Center for Cardiovascular Research (DZHK) in Mainz, Germany.
“We also found that a remarkable number [of CVSS subjects] attended their clinical examination for this study with previously unidentified cardiovascular risk factors and cardiovascular disease. For example, only 62 out of 269 were aware of having dyslipidemia. Consequently, 207, approximately 80%, were only diagnosed at that point.”
Disease
In the CVSS cohort, 4.5% of patients had at least 1 type of cardiovascular disease. This included venous thromboembolism (2.0%), congestive heart failure (1.2%), stroke (0.5%), peripheral artery disease (0.5%), atrial fibrillation (0.4%), and coronary heart disease (0.3%).
CVSS subjects had nearly twice the risk of cardiovascular disease as GHS subjects. The age-adjusted RR was 1.89. And CVSS subjects developed cardiovascular disease roughly 8 years earlier than GHS subjects (RAP=7.9).
In the CVSS cohort, the probability of developing cardiovascular disease was estimated as 2.9% at age 30 and 9.6% at age 45.
The researchers said these findings show that CCSs have a greater risk of cardiovascular disease that continues to increase with age. This, in turn, means CCSs may be more likely to die earlier. However, this might be preventable, according to Dr Wild.
“Early systematic screening, particularly focusing on blood pressure and lipid measurements, might be suggested in all childhood cancer survivors irrespective of the type of cancer or treatment they had had,” Dr Wild said. “This might help to prevent long-term cardiovascular diseases by intervening early—for instance, by modifying lifestyles and having treatment for high blood pressure.”
“Usually, survivors are followed for only 5 to 10 years after completion of therapy, and this is focused on the risk of the cancer returning and the acute adverse effects of their treatment, rather than on other conditions,” added Dr Faber.
“Current guidelines recommend cardiovascular assessments only for subgroups known to be at risk, such as for patients who were treated with anthracycline therapy and/or radiation therapy. However, further investigations are needed to answer questions about the best follow-up care.”
Childhood cancer survivors (CCSs) have an increased risk of premature cardiovascular disease in adulthood, according to a new study.
Researchers found a nearly 2-fold increased risk of cardiovascular diseases in CCSs compared to the general population.
Cardiovascular disease was identified in 4.5% of CCSs and occurred in most before they reached the age of 40, nearly 8 years earlier than in the general population.
“Our results show that these survivors of childhood cancer have a substantially elevated burden of prematurely occurring traditional cardiovascular risk factors and cardiovascular diseases,” said study author Joerg Faber, MD, PhD, of the Johannes Gutenberg University Mainz in Germany.
Dr Faber and his colleagues reported these results in the European Heart Journal.
The researchers evaluated 951 adult CCSs (ages 23 to 48), referred to as the CVSS cohort (Cardiac and Vascular Late Sequelae in Long-Term Survivors of Childhood Cancer Study).
The patients had been diagnosed with cancer between 1980 and 1990. The most common diagnoses were leukemia (43.5%), central nervous system tumors (12.8%), and lymphoma (9.9%).
For this study, the patients underwent standardized clinical and laboratory cardiovascular screening. The mean time from cancer diagnosis to cardiovascular screening was 28.4 years (range, 23–36).
The researchers compared the incidence of cardiovascular risk factors and cardiovascular disease in the CVSS cohort and subjects from the Gutenberg Health Study (GHS), a population-based study including more than 15,000 subjects.
Risk factors
The CVSS cohort had a greater risk of 2 cardiovascular risk factors—arterial hypertension and dyslipidemia—than the GHS cohort. In the CVSS cohort, the incidence of dyslipidemia was 28.3%, and the incidence of hypertension was 23.0%.
CVSS subjects had an age-adjusted 38% increase in risk for hypertension (relative risk [RR]=1.38) and a 26% increase in risk for dyslipidemia (RR=1.26).
Hypertension occurred about 6 years earlier in CVSS subjects than GHS subjects (rate advancement period estimator [RAP]=5.75). And dyslipidemia occurred about 8 years earlier in the CVSS cohort (RAP=8.16).
“[T]he premature onset of high blood pressure and blood lipid disorders may play an important role in the development of severe cardiovascular conditions, such as heart disease and stroke, in the long-term,” said study author Philipp S. Wild, MD, of the German Center for Cardiovascular Research (DZHK) in Mainz, Germany.
“We also found that a remarkable number [of CVSS subjects] attended their clinical examination for this study with previously unidentified cardiovascular risk factors and cardiovascular disease. For example, only 62 out of 269 were aware of having dyslipidemia. Consequently, 207, approximately 80%, were only diagnosed at that point.”
Disease
In the CVSS cohort, 4.5% of patients had at least 1 type of cardiovascular disease. This included venous thromboembolism (2.0%), congestive heart failure (1.2%), stroke (0.5%), peripheral artery disease (0.5%), atrial fibrillation (0.4%), and coronary heart disease (0.3%).
CVSS subjects had nearly twice the risk of cardiovascular disease as GHS subjects. The age-adjusted RR was 1.89. And CVSS subjects developed cardiovascular disease roughly 8 years earlier than GHS subjects (RAP=7.9).
In the CVSS cohort, the probability of developing cardiovascular disease was estimated as 2.9% at age 30 and 9.6% at age 45.
The researchers said these findings show that CCSs have a greater risk of cardiovascular disease that continues to increase with age. This, in turn, means CCSs may be more likely to die earlier. However, this might be preventable, according to Dr Wild.
“Early systematic screening, particularly focusing on blood pressure and lipid measurements, might be suggested in all childhood cancer survivors irrespective of the type of cancer or treatment they had had,” Dr Wild said. “This might help to prevent long-term cardiovascular diseases by intervening early—for instance, by modifying lifestyles and having treatment for high blood pressure.”
“Usually, survivors are followed for only 5 to 10 years after completion of therapy, and this is focused on the risk of the cancer returning and the acute adverse effects of their treatment, rather than on other conditions,” added Dr Faber.
“Current guidelines recommend cardiovascular assessments only for subgroups known to be at risk, such as for patients who were treated with anthracycline therapy and/or radiation therapy. However, further investigations are needed to answer questions about the best follow-up care.”