User login
Fatty liver disease drives rise in liver cancer deaths
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
Cardiologists concerned for patient safety after abortion ruling
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Pregnancy termination for medical reasons had been part of the fabric of everyday health care in the United States since the Supreme Court’s 1973 Roe v. Wade decision, which the current high court overturned in a ruling announced on June 24.
That means many clinicians across specialties are entering uncharted territory with the country’s new patchwork of abortion legality. Some specialties, cardiology among them, may feel the impact more than others.
“We know that the rising maternal mortality rate is predominantly driven by cardiovascular disease, women having children at older ages, and ... risk factors like hypertension, diabetes, and obesity,” Jennifer H. Haythe, MD, told this news organization.
So the high court’s decision in Dobbs v. Jackson Women’s Health Organization, which overturned Roe v. Wade and leaves the legality of abortion up to the 50 separate state legislatures, “is very relevant to cardiologists specifically,” said Dr. Haythe, who is director of cardiology in the cardio-obstetrics program at New York-Presbyterian/Columbia University Irving Medical Center, New York.
The ruling “is going to have a huge effect on women who may not be able to tolerate pregnancy,” she said. Whether to terminate a pregnancy “is a relatively common discussion I have with women with bad heart failure about their risk of further decompensation, death, or needing a heart transplant or heart pump after delivery, or the risk of death in women with pulmonary hypertension.”
The high court’s decision “is a direct attack on the practice of medicine and really the sanctity of the patient-clinician relationship,” Rachel M. Bond, MD, director of Women’s Heart Health Systems Dignity Health of Arizona, told this news organization.
Physicians take an oath “that we should do no harm to our patients, and once the law or governance impacts that, it places us in a very vulnerable situation,” Dr. Bond said. “As a cardiologist who focuses a lot on high-risk pregnancies, I am worried and hesitant to give guidance to many of these patients in the states that may not have access to something that is a medical right, which at times is an abortion.”
She has colleagues in obstetrics in states where abortion is newly illegal who “don’t know what to do,” Dr. Bond said. Many have sought guidance from their legal teams, she said, “and many of them are now trying to figure out what is the best path.”
Pregnancy is “a very significant cardiovascular stress test, and women who may tolerate certain conditions reasonably well outside of the setting of pregnancy may have severe issues, not just for the mother, but for the baby as well,” Ki Park, MD, University of Florida Health, Gainesville, said in an interview.
“As clinicians, none of us like recommending a medically indicated abortion. But it is health care, just like any other medication or treatment that we advise to our patients in cases where the risk of the mother is excessively high and mortality risk is elevated,” said Dr. Park, who is cochair of the American College of Cardiology Cardio-Obstetrics Work Group.
Some conditions, such as pulmonary hypertension and severe aortic valve stenosis, during pregnancy are well recognized as very high risk, and there are various scoring systems to help clinicians with risk stratification, she observed. “But there are also a lot of gray areas where patients don’t necessarily fit into these risk scores that we use.”
So physician-patient discussions in high-risk pregnancies “are already complicated,” Dr. Park said. “Patients want to have options, and they look to us as physicians for guidance with regard to their risks. And if abortion is not available as an option, then part of our toolbox is no longer available to help us care for the mother.”
In the new legal climate, clinicians in states where abortion is illegal may well want to put more emphasis on preconception counseling, so more of their patients with high-risk conditions are aware of the new barriers to pregnancy termination.
“Unfortunately,” Dr. Haythe said, “many of the states that are going to make or have made abortion illegal are not providing that kind of preconception counseling or good prenatal care to women.”
Cardiologists can provide such counseling to their female patients of childbearing age who have high-risk cardiac conditions, “but not everybody knows that they have a heart problem when they get pregnant, and not everybody is getting screened for heart problems when they’re of childbearing age,” Dr. Haythe said.
“Sometimes it’s not clear whether the problems could have been picked up until a woman is pregnant and has started to have symptoms.” For example, “a lot of women with poor access to health care have rheumatic heart disease. They may have no idea that they have severe aortic stenosis, and it’s not until their second trimester that they start to feel really short of breath.” Often that can be treated in the cath lab, “but again, that’s putting the woman and the baby at risk.”
Cardiologists in states where abortion is illegal will still present the option to their patients with high-risk pregnancies, noted Dr. Haythe. But the conversation may sound something like, “you are at very high risk, termination of the pregnancy takes that risk away, but you’ll have to find a state where it’s legal to do that.”
Dr. Park said such a situation, when abortion is recommended but locally unavailable, is much like any other in cardiology for which the patient may want a second opinion. If a center “doesn’t have the capability or the technology to offer a certain treatment, the patient can opt to seek another opinion at another center,” she said. “Patients will often travel out of state to get the care they need.”
A requirement for out-of-state travel to obtain abortions is likely to worsen socioeconomic disparities in health care, Dr. Bond observed, “because we know that those who are low-income won’t be able to afford that travel.”
Dr. Bond is cosignatory on a statement from the Association of Black Cardiologists (ABC) responding to the high court’s ruling in Dobbs v. Jackson. “This decision will isolate the poor, socioeconomically disadvantaged, and minority populations specifically, widening the already large gaps in health care for our most vulnerable communities,” it states.
“The loss of broad protections supporting the medical and often lifesaving procedure of abortions is likely to have a real impact on the maternal mortality rate, especially in those with congenital and/or acquired cardiovascular conditions where evidence-based guidelines advise at times on termination of such high-risk pregnancies.”
The ABC, it states, “believes that every woman, and every person, should be afforded the right to safe, accessible, legal, timely, patient-centered, equitable, and affordable health care.”
The American College of Cardiology (ACC) released a statement on the matter June 24, signed by its president, Edward T.A. Fry, MD, along with five former ACC presidents. “While the ACC has no official policy on abortion, clinical practice guidelines and other clinical guidance tools address the dangers of pregnancy in certain patient populations at higher risk of death or serious cardiac events.”
The college, it states, is “deeply concerned about the potential implications of the Supreme Court decision regarding Roe vs. Wade on the ability of patients and clinicians to engage in important shared discussions about maternal health, or to remove previously available health care options.”
Dr. Bond proposed that a “vocal stance” from medical societies involved in women’s health, “perhaps even a collective stance from our cardiovascular societies and our obstetrics societies,” would also perhaps reach “the masses of doctors in private practice who are dealing with these patients.”
A version of this article first appeared on Medscape.com.
Evidence still lacking that vitamins prevent CVD, cancer: USPSTF
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
There is not enough evidence to recommend for or against taking most vitamin and mineral supplements to prevent heart disease, stroke, and cancer, a new report by the U.S. Preventive Services Task Force concludes.
However, there are two vitamins – vitamin E and beta-carotene – that the task force recommends against for the prevention of heart disease, stroke, and cancer. Evidence shows that there is no benefit to taking vitamin E and that beta-carotene can increase the risk for lung cancer in people already at risk, such as smokers and those with occupational exposure to asbestos.
These are the main findings of the USPSTF’s final recommendation statement on vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer. The statement was published in JAMA.
“This is essentially the same recommendation that the task force made in 2014,” USPSTF member John Wong, MD, professor of medicine at Tufts University, Boston, said in an interview.
“We recognize that over half of people in the U.S. take a vitamin supplement of some sort every day and 30% take a vitamin/mineral combination. We wanted to review the evidence again to see if there was any benefit in terms of reducing the risk of cardiovascular disease or cancer or increasing the chances of living longer,” Dr. Wong explained.
“We looked hard for evidence, reviewing 84 studies in total. But we did not find sufficient evidence in favor of taking or not taking vitamins, with the two exceptions of beta-carotene and vitamin E, which we recommend against taking,” he noted.
Although there is evidence of some harm with beta-carotene, the main reason behind the recommendation against taking vitamin E is the consistent evidence of no benefit, Dr. Wong explained.
“While the evidence for some other vitamins is conflicting, there is more consistent evidence of no benefit for vitamin E,” he said.
The bulk of new evidence since the last review in 2014 was predominately for vitamin D supplementation, but despite the inclusion of 32 new randomized, controlled trials and two cohort studies, pooled estimates for all-cause mortality were similar to those in the previous review, with confidence intervals only slightly crossing 1, and point estimates that suggest at most a very small benefit, the task force noted.
“Apart from beta-carotene and vitamin E, after reviewing 84 studies – including 78 randomized controlled trials – in over a million patients, we can find no clear demonstration of benefit or harm of taking vitamins in terms of developing cardiovascular disease or cancer or the effect on all-cause mortality. So, we don’t know whether people should take vitamins or not, and we need more research,” Dr. Wong added.
On the use of a multivitamin supplement, Dr. Wong noted that the complete body of evidence did not find any benefit of taking a multivitamin on cardiovascular or cancer mortality. But there was a small reduction in cancer incidence.
However, he pointed out that the three studies that suggested a reduction in cancer incidence all had issues regarding generalizability.
“The recently published COSMOS trial had an average follow-up of only 3.6 years, which isn’t really long enough when thinking about the prevention of cancer, one of the other studies only used antioxidants, and the third study was conducted only in U.S. male physicians. So those limitations regarding generalizability limited our confidence in making recommendations about multivitamins,” Dr. Wong explained.
But he noted that the task force did not find any significant harms from taking multivitamins.
“There are possible harms from taking high doses of vitamin A and vitamin D, but generally the doses contained in a multivitamin tablet are lower than these. But if the goal for taking a multivitamin is to lower your risk of cancer or cardiovascular disease, we didn’t find sufficient evidence to be able to make a recommendation,” he said.
Asked what he would say to all the people currently taking multivitamins, Dr. Wong responded that he would advise them to have a conversation with a trusted health care professional about their particular circumstances.
“Our statement has quite a narrow focus. It is directed toward community-dwelling, nonpregnant adults. This recommendation does not apply to children, persons who are pregnant or may become pregnant, or persons who are chronically ill, are hospitalized, or have a known nutritional deficiency,” he commented.
‘Any benefit likely to be small’
In an editorial accompanying the publication of the USPSTF statement, Jenny Jia, MD; Natalie Cameron, MD; and Jeffrey Linder, MD – all from Northwestern University, Chicago – noted that the current evidence base includes 52 additional studies not available when the last USPSTF recommendation on this topic was published in 2014.
The editorialists pointed out that for multivitamins, proving the absence of a benefit is challenging, but at best, current evidence suggests that any potential benefits of a multivitamin to reduce mortality are likely to be small.
They gave an example of a healthy 65-year-old woman with a 9-year estimated mortality risk of about 8%, and note that taking a multivitamin for 5-10 years might reduce her estimated mortality risk to 7.5% (based on an odds ratio of 0.94).
“In addition to showing small potential benefit, this estimate is based on imperfect evidence, is imprecise, and is highly sensitive to how the data are interpreted and analyzed,” they said.
The editorialists recommended that lifestyle counseling to prevent chronic diseases should continue to focus on evidence-based approaches, including balanced diets that are high in fruits and vegetables and physical activity.
However, they added that healthy eating can be a challenge when the American industrialized food system does not prioritize health, and healthy foods tend to be more expensive, leading to access problems and food insecurity.
The editorialists suggested that, rather than focusing money, time, and attention on supplements, it would be better to emphasize lower-risk, higher-benefit activities, such as getting exercise, maintaining a healthy weight, and avoiding smoking, in addition to following a healthful diet.
Possible benefit for older adults?
Commenting on the USPSTF statement, JoAnn Manson, MD, chief, division of preventive medicine, Brigham and Women’s Hospital, Boston, who led the recent COSMOS study, said that vitamin and mineral supplements should not be perceived as a substitute for a healthful diet.
“The emphasis needs to be on getting nutritional needs from a healthy diet that is high in plant-based and whole foods that don’t strip the vitamins and minerals through excessive processing,” she said. “Although it’s easier to pop a pill each day than to focus on healthful dietary patterns, the mixture of phytochemicals, fiber, and all the other nutrients in actual foods just can’t be packaged into a pill. Also, vitamins and minerals tend to be better absorbed from food than from supplements and healthy foods can replace calories from less healthy foods, such as red meat and processed foods.”
However, Dr. Manson noted that the evidence is mounting that taking a tablet containing moderate doses of a wide range of vitamins and minerals is safe and may actually have benefits for some people.
She pointed out that the COSMOS and COSMOS-Mind studies showed benefits of multivitamins in slowing cognitive decline in older adults, but the findings need to be replicated.
“The USPSTF did see a statistically significant 7% reduction in cancer with multivitamins in their meta-analysis of four randomized trials and a borderline 6% reduction in all-cause mortality,” she noted. “Plus, multivitamins have been shown to be quite safe in several large and long-term randomized trials. I agree the evidence is not sufficient to make a blanket recommendation for everyone to take multivitamins, but the evidence is mounting that this would be a prudent approach for many older adults,” Dr. Manson said.
“Many people view multivitamins as a form of insurance, as a way to hedge their bets,” she added. “Although this is a rational approach, especially for those who have concerns about the adequacy of their diet, it’s important that this mindset not lead to complacency about following healthy lifestyle practices, including healthy eating, regular physical activity, not smoking, making sure that blood pressure and cholesterol levels are well controlled, and many other practices that critically important for health but are more challenging than simply popping a pill each day.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Bone density loss in lean male runners parallels similar issue in women
Similar to a phenomenon already well documented in women, inadequate nutrition appears to be linked to hormonal abnormalities and potentially preventable tibial cortical bone density loss in athletic men, according to results of a small, prospective study.
Based on these findings, “we suspect that a subset of male runners might not be fueling their bodies with enough nutrition and calories for their physical activity,” reported Melanie S. Haines, MD, at the annual meeting of the Endocrine Society.
This is not the first study to suggest male athletes are at risk of a condition equivalent to what has been commonly referred to as the female athlete triad, but it enlarges the objective data that the phenomenon is real, and it makes insufficient availability of energy the likely cause.
In women, the triad is described as a lack of adequate stored energy, irregular menses, and bone density loss. In men, menstrual cycles are not relevant, of course, but this study like others suggests a link between the failure to maintain adequate stores of energy, disturbances in hormone function, and decreased bone density in both men and women, Dr. Haines explained.
RED-S vs. male or female athlete triad
“There is now a move away from the term female athlete triad or male athlete triad,” Dr. Haines reported. Rather the factors of failing to maintain adequate energy for metabolic demands, hormonal disturbances, and bone density loss appear to be relevant to both sexes, according to Dr. Haines, an endocrinologist at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. She said several groups, including the International Olympic Committee (IOC), have transitioned to the term RED-S to apply to both sexes.
“RED-S is an acronym for relative energy deficiency in sport, and it appears to be gaining traction,” Dr. Haines said in an interview.
According to her study and others, excessive lean body mass from failure to supply sufficient energy for physiological needs “negatively affects hormones and bone,” Dr. Haines explained. In men and women, endocrine disturbances are triggered when insufficient calories lead to inadequate macro- and micronutrients.
In this study, 31 men aged 16-30 years were evaluated. Fifteen were in the athlete group, defined by running at least 30 miles per week for at least the previous 6 months. There were 16 control subjects; all exercised less than 2 hours per week and did not participate in team sports, but they were not permitted in the study if their body mass index exceeded 27.5 kg/m2.
Athletes vs. otherwise healthy controls
Conditions that affect bone health were exclusion criteria in both groups, and neither group was permitted to take medications affecting bone health other than dietary calcium or vitamin D supplements for 2 months prior to the study.
Tibial cortical porosity was significantly greater – signaling deterioration in microarchitecture – in athletes, compared with control subjects (P = .003), according to quantitative computed tomography measurements. There was also significantly lower tibial cortical bone mineral density (P = .008) among athletes relative to controls.
Conversely, tibial trabecular measures of bone density and architecture were better among athletes than controls, but this was expected and did not contradict the hypothesis of the study.
“Trabecular bone refers to the inner part of the bone, which increases with weight-bearing exercise, but cortical bone is the outer shell, and the source of stress fractures,” Dr. Haines explained.
The median age of both the athletes and the controls was 24 years. Baseline measurements were similar. Body mass index, fat mass, estradiol, and leptin were all numerically lower in the athletes than controls, but none were significant, although there was a trend for the difference in leptin (P = .085).
Hormones correlated with tibial failure load
When these characteristics were evaluated in the context of mean tibial failure load, a metric related to strength, there was a strongly significant positive association with lean body mass (R = 0.85; P < 0.001) and estradiol level (R = 0.66; P = .007). The relationship with leptin also reached significance (R = 0.59; P = .046).
Unexpectedly, there was no relationship between testosterone and tibial failure load. The reason is unclear, but Dr. Haines’s interpretation is that the relationship between specific hormonal disturbances and bone density loss “might not be as simple” as once hypothesized.
The next step is a longitudinal evaluation of the same group of athletes to follow changes in the relationship between these variables over time, according to Dr. Haines.
Eventually, with evidence that there is a causal relationship between nutrition, hormonal changes, and bone loss, the research in this area will focus on better detection of risk and prophylactic strategies.
“Intervention trials to show that we can prevent stress factors will be difficult to perform,” Dr. Haines acknowledged, but she said that preventing adverse changes in bone at relatively young ages could have implications for long-term bone health, including protection from osteoporosis later in life.
The research presented by Dr. Haines is consistent with an area of research that is several decades old, at least in females, according to Siobhan M. Statuta, MD, a sports medicine primary care specialist at the University of Virginia, Charlottesville. The evidence that the same phenomenon occurs in men is more recent, but she said that it is now well accepted the there is a parallel hormonal issue in men and women.
“It is not a question of not eating enough. Often, athletes continue to consume the same diet, but their activity increases,” Dr. Statuta explained. “The problem is that they are not supplying enough of the calories they need to sustain the energy they are expending. You might say they are not fueling their engines appropriately.”
In 2014, the International Olympic Committee published a consensus statement on RED-S. They described this as a condition in which a state of energy deficiency leads to numerous complications in athletes, not just osteoporosis. Rather, a host of physiological systems, ranging from gastrointestinal complaints to cardiovascular events, were described.
RED-S addresses health beyond bones
“The RED-S theory is better described as a spoke-and-wheel concept rather than a triad. While inadequate energy availability is important to both, RED-S places this at the center of the wheel with spokes leading to all the possible complications rather than as a first event in a limited triad,” Dr. Statuta said in an interview.
However, she noted that the term RED-S is not yet appropriate to replace that of the male and female athlete triad.
“More research is required to hash out the relationship of a body in a state of energy deficiency and how it affects the entire body, which is the principle of RED-S,” Dr. Statuta said. “There likely are scientific effects, and we are currently investigating these relationships more.”
“These are really quite similar entities but have different foci,” she added. Based on data collected over several decades, “the triad narrows in on two body systems affected by low energy – the reproductive system and bones. RED-S incorporates these same systems yet adds on many more organ systems.
The original group of researchers have remained loyal to the concept of the triad that involves inadequate availability of energy followed by hormonal irregularities and osteoporosis. This group, the Female and Male Athlete Triad Coalition, has issued publications on this topic several times. Consensus statements were updated last year.
“The premise is that the triad leading to bone loss is shared by both men and women, even if the clinical manifestations differ,” said Dr. Statuta. The most notable difference is that men do not experience menstrual irregularities, but Dr. Statuta suggested that the clinical consequences are not necessarily any less.
“Males do not have menstrual cycles as an outward marker of an endocrine disturbance, so it is harder to recognize clinically, but I think there is agreement that not having enough energy available is the trigger of endocrine changes and then bone loss is relevant to both sexes,” she said. She said this is supported by a growing body of evidence, including the data presented by Dr. Haines at the Endocrine Society meeting.
Dr. Haines and Dr. Statuta report no potential conflicts of interest.
Similar to a phenomenon already well documented in women, inadequate nutrition appears to be linked to hormonal abnormalities and potentially preventable tibial cortical bone density loss in athletic men, according to results of a small, prospective study.
Based on these findings, “we suspect that a subset of male runners might not be fueling their bodies with enough nutrition and calories for their physical activity,” reported Melanie S. Haines, MD, at the annual meeting of the Endocrine Society.
This is not the first study to suggest male athletes are at risk of a condition equivalent to what has been commonly referred to as the female athlete triad, but it enlarges the objective data that the phenomenon is real, and it makes insufficient availability of energy the likely cause.
In women, the triad is described as a lack of adequate stored energy, irregular menses, and bone density loss. In men, menstrual cycles are not relevant, of course, but this study like others suggests a link between the failure to maintain adequate stores of energy, disturbances in hormone function, and decreased bone density in both men and women, Dr. Haines explained.
RED-S vs. male or female athlete triad
“There is now a move away from the term female athlete triad or male athlete triad,” Dr. Haines reported. Rather the factors of failing to maintain adequate energy for metabolic demands, hormonal disturbances, and bone density loss appear to be relevant to both sexes, according to Dr. Haines, an endocrinologist at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. She said several groups, including the International Olympic Committee (IOC), have transitioned to the term RED-S to apply to both sexes.
“RED-S is an acronym for relative energy deficiency in sport, and it appears to be gaining traction,” Dr. Haines said in an interview.
According to her study and others, excessive lean body mass from failure to supply sufficient energy for physiological needs “negatively affects hormones and bone,” Dr. Haines explained. In men and women, endocrine disturbances are triggered when insufficient calories lead to inadequate macro- and micronutrients.
In this study, 31 men aged 16-30 years were evaluated. Fifteen were in the athlete group, defined by running at least 30 miles per week for at least the previous 6 months. There were 16 control subjects; all exercised less than 2 hours per week and did not participate in team sports, but they were not permitted in the study if their body mass index exceeded 27.5 kg/m2.
Athletes vs. otherwise healthy controls
Conditions that affect bone health were exclusion criteria in both groups, and neither group was permitted to take medications affecting bone health other than dietary calcium or vitamin D supplements for 2 months prior to the study.
Tibial cortical porosity was significantly greater – signaling deterioration in microarchitecture – in athletes, compared with control subjects (P = .003), according to quantitative computed tomography measurements. There was also significantly lower tibial cortical bone mineral density (P = .008) among athletes relative to controls.
Conversely, tibial trabecular measures of bone density and architecture were better among athletes than controls, but this was expected and did not contradict the hypothesis of the study.
“Trabecular bone refers to the inner part of the bone, which increases with weight-bearing exercise, but cortical bone is the outer shell, and the source of stress fractures,” Dr. Haines explained.
The median age of both the athletes and the controls was 24 years. Baseline measurements were similar. Body mass index, fat mass, estradiol, and leptin were all numerically lower in the athletes than controls, but none were significant, although there was a trend for the difference in leptin (P = .085).
Hormones correlated with tibial failure load
When these characteristics were evaluated in the context of mean tibial failure load, a metric related to strength, there was a strongly significant positive association with lean body mass (R = 0.85; P < 0.001) and estradiol level (R = 0.66; P = .007). The relationship with leptin also reached significance (R = 0.59; P = .046).
Unexpectedly, there was no relationship between testosterone and tibial failure load. The reason is unclear, but Dr. Haines’s interpretation is that the relationship between specific hormonal disturbances and bone density loss “might not be as simple” as once hypothesized.
The next step is a longitudinal evaluation of the same group of athletes to follow changes in the relationship between these variables over time, according to Dr. Haines.
Eventually, with evidence that there is a causal relationship between nutrition, hormonal changes, and bone loss, the research in this area will focus on better detection of risk and prophylactic strategies.
“Intervention trials to show that we can prevent stress factors will be difficult to perform,” Dr. Haines acknowledged, but she said that preventing adverse changes in bone at relatively young ages could have implications for long-term bone health, including protection from osteoporosis later in life.
The research presented by Dr. Haines is consistent with an area of research that is several decades old, at least in females, according to Siobhan M. Statuta, MD, a sports medicine primary care specialist at the University of Virginia, Charlottesville. The evidence that the same phenomenon occurs in men is more recent, but she said that it is now well accepted the there is a parallel hormonal issue in men and women.
“It is not a question of not eating enough. Often, athletes continue to consume the same diet, but their activity increases,” Dr. Statuta explained. “The problem is that they are not supplying enough of the calories they need to sustain the energy they are expending. You might say they are not fueling their engines appropriately.”
In 2014, the International Olympic Committee published a consensus statement on RED-S. They described this as a condition in which a state of energy deficiency leads to numerous complications in athletes, not just osteoporosis. Rather, a host of physiological systems, ranging from gastrointestinal complaints to cardiovascular events, were described.
RED-S addresses health beyond bones
“The RED-S theory is better described as a spoke-and-wheel concept rather than a triad. While inadequate energy availability is important to both, RED-S places this at the center of the wheel with spokes leading to all the possible complications rather than as a first event in a limited triad,” Dr. Statuta said in an interview.
However, she noted that the term RED-S is not yet appropriate to replace that of the male and female athlete triad.
“More research is required to hash out the relationship of a body in a state of energy deficiency and how it affects the entire body, which is the principle of RED-S,” Dr. Statuta said. “There likely are scientific effects, and we are currently investigating these relationships more.”
“These are really quite similar entities but have different foci,” she added. Based on data collected over several decades, “the triad narrows in on two body systems affected by low energy – the reproductive system and bones. RED-S incorporates these same systems yet adds on many more organ systems.
The original group of researchers have remained loyal to the concept of the triad that involves inadequate availability of energy followed by hormonal irregularities and osteoporosis. This group, the Female and Male Athlete Triad Coalition, has issued publications on this topic several times. Consensus statements were updated last year.
“The premise is that the triad leading to bone loss is shared by both men and women, even if the clinical manifestations differ,” said Dr. Statuta. The most notable difference is that men do not experience menstrual irregularities, but Dr. Statuta suggested that the clinical consequences are not necessarily any less.
“Males do not have menstrual cycles as an outward marker of an endocrine disturbance, so it is harder to recognize clinically, but I think there is agreement that not having enough energy available is the trigger of endocrine changes and then bone loss is relevant to both sexes,” she said. She said this is supported by a growing body of evidence, including the data presented by Dr. Haines at the Endocrine Society meeting.
Dr. Haines and Dr. Statuta report no potential conflicts of interest.
Similar to a phenomenon already well documented in women, inadequate nutrition appears to be linked to hormonal abnormalities and potentially preventable tibial cortical bone density loss in athletic men, according to results of a small, prospective study.
Based on these findings, “we suspect that a subset of male runners might not be fueling their bodies with enough nutrition and calories for their physical activity,” reported Melanie S. Haines, MD, at the annual meeting of the Endocrine Society.
This is not the first study to suggest male athletes are at risk of a condition equivalent to what has been commonly referred to as the female athlete triad, but it enlarges the objective data that the phenomenon is real, and it makes insufficient availability of energy the likely cause.
In women, the triad is described as a lack of adequate stored energy, irregular menses, and bone density loss. In men, menstrual cycles are not relevant, of course, but this study like others suggests a link between the failure to maintain adequate stores of energy, disturbances in hormone function, and decreased bone density in both men and women, Dr. Haines explained.
RED-S vs. male or female athlete triad
“There is now a move away from the term female athlete triad or male athlete triad,” Dr. Haines reported. Rather the factors of failing to maintain adequate energy for metabolic demands, hormonal disturbances, and bone density loss appear to be relevant to both sexes, according to Dr. Haines, an endocrinologist at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. She said several groups, including the International Olympic Committee (IOC), have transitioned to the term RED-S to apply to both sexes.
“RED-S is an acronym for relative energy deficiency in sport, and it appears to be gaining traction,” Dr. Haines said in an interview.
According to her study and others, excessive lean body mass from failure to supply sufficient energy for physiological needs “negatively affects hormones and bone,” Dr. Haines explained. In men and women, endocrine disturbances are triggered when insufficient calories lead to inadequate macro- and micronutrients.
In this study, 31 men aged 16-30 years were evaluated. Fifteen were in the athlete group, defined by running at least 30 miles per week for at least the previous 6 months. There were 16 control subjects; all exercised less than 2 hours per week and did not participate in team sports, but they were not permitted in the study if their body mass index exceeded 27.5 kg/m2.
Athletes vs. otherwise healthy controls
Conditions that affect bone health were exclusion criteria in both groups, and neither group was permitted to take medications affecting bone health other than dietary calcium or vitamin D supplements for 2 months prior to the study.
Tibial cortical porosity was significantly greater – signaling deterioration in microarchitecture – in athletes, compared with control subjects (P = .003), according to quantitative computed tomography measurements. There was also significantly lower tibial cortical bone mineral density (P = .008) among athletes relative to controls.
Conversely, tibial trabecular measures of bone density and architecture were better among athletes than controls, but this was expected and did not contradict the hypothesis of the study.
“Trabecular bone refers to the inner part of the bone, which increases with weight-bearing exercise, but cortical bone is the outer shell, and the source of stress fractures,” Dr. Haines explained.
The median age of both the athletes and the controls was 24 years. Baseline measurements were similar. Body mass index, fat mass, estradiol, and leptin were all numerically lower in the athletes than controls, but none were significant, although there was a trend for the difference in leptin (P = .085).
Hormones correlated with tibial failure load
When these characteristics were evaluated in the context of mean tibial failure load, a metric related to strength, there was a strongly significant positive association with lean body mass (R = 0.85; P < 0.001) and estradiol level (R = 0.66; P = .007). The relationship with leptin also reached significance (R = 0.59; P = .046).
Unexpectedly, there was no relationship between testosterone and tibial failure load. The reason is unclear, but Dr. Haines’s interpretation is that the relationship between specific hormonal disturbances and bone density loss “might not be as simple” as once hypothesized.
The next step is a longitudinal evaluation of the same group of athletes to follow changes in the relationship between these variables over time, according to Dr. Haines.
Eventually, with evidence that there is a causal relationship between nutrition, hormonal changes, and bone loss, the research in this area will focus on better detection of risk and prophylactic strategies.
“Intervention trials to show that we can prevent stress factors will be difficult to perform,” Dr. Haines acknowledged, but she said that preventing adverse changes in bone at relatively young ages could have implications for long-term bone health, including protection from osteoporosis later in life.
The research presented by Dr. Haines is consistent with an area of research that is several decades old, at least in females, according to Siobhan M. Statuta, MD, a sports medicine primary care specialist at the University of Virginia, Charlottesville. The evidence that the same phenomenon occurs in men is more recent, but she said that it is now well accepted the there is a parallel hormonal issue in men and women.
“It is not a question of not eating enough. Often, athletes continue to consume the same diet, but their activity increases,” Dr. Statuta explained. “The problem is that they are not supplying enough of the calories they need to sustain the energy they are expending. You might say they are not fueling their engines appropriately.”
In 2014, the International Olympic Committee published a consensus statement on RED-S. They described this as a condition in which a state of energy deficiency leads to numerous complications in athletes, not just osteoporosis. Rather, a host of physiological systems, ranging from gastrointestinal complaints to cardiovascular events, were described.
RED-S addresses health beyond bones
“The RED-S theory is better described as a spoke-and-wheel concept rather than a triad. While inadequate energy availability is important to both, RED-S places this at the center of the wheel with spokes leading to all the possible complications rather than as a first event in a limited triad,” Dr. Statuta said in an interview.
However, she noted that the term RED-S is not yet appropriate to replace that of the male and female athlete triad.
“More research is required to hash out the relationship of a body in a state of energy deficiency and how it affects the entire body, which is the principle of RED-S,” Dr. Statuta said. “There likely are scientific effects, and we are currently investigating these relationships more.”
“These are really quite similar entities but have different foci,” she added. Based on data collected over several decades, “the triad narrows in on two body systems affected by low energy – the reproductive system and bones. RED-S incorporates these same systems yet adds on many more organ systems.
The original group of researchers have remained loyal to the concept of the triad that involves inadequate availability of energy followed by hormonal irregularities and osteoporosis. This group, the Female and Male Athlete Triad Coalition, has issued publications on this topic several times. Consensus statements were updated last year.
“The premise is that the triad leading to bone loss is shared by both men and women, even if the clinical manifestations differ,” said Dr. Statuta. The most notable difference is that men do not experience menstrual irregularities, but Dr. Statuta suggested that the clinical consequences are not necessarily any less.
“Males do not have menstrual cycles as an outward marker of an endocrine disturbance, so it is harder to recognize clinically, but I think there is agreement that not having enough energy available is the trigger of endocrine changes and then bone loss is relevant to both sexes,” she said. She said this is supported by a growing body of evidence, including the data presented by Dr. Haines at the Endocrine Society meeting.
Dr. Haines and Dr. Statuta report no potential conflicts of interest.
FROM ENDO 2022
Remnant cholesterol improves CV risk prediction
, a new study suggests.
The study, which followed almost 42,000 Danish individuals without a history of ischemic cardiovascular disease, diabetes, or statin use for more than 10 years, found that elevated remnant cholesterol appropriately reclassified up to 40% of those who later experienced myocardial infarction and ischemic heart disease.
“The clinical implications of our study include that doctors and patients should be aware of remnant cholesterol levels to prevent future risk of MI and ischemic heart disease,” the authors conclude.
They suggest that the development of a cardiovascular risk algorithm, including remnant cholesterol together with LDL cholesterol, would help to better identify high-risk individuals who could be candidates for statins in a primary prevention setting.
They note that physicians are encouraged to evaluate non-HDL cholesterol and/or apolipoprotein B rather than LDL cholesterol and certainly not yet remnant cholesterol, possibly because of the limited availability of remnant cholesterol values in some parts of the world.
However, they point out that remnant cholesterol can be calculated with a standard lipid profile without additional cost, which is currently already the standard procedure in the greater Copenhagen area.
“This means that the use of remnant cholesterol is easy to introduce into daily clinical practice,” they say.
The study was published online in the Journal of the American College of Cardiology.
The authors, Takahito Doi, MD, Anne Langsted, MD, and Børge Nordestgaard, from Copenhagen University Hospital, Denmark, explain that remnant cholesterol is total cholesterol minus LDL-cholesterol minus HDL-cholesterol and includes the cholesterol content of the triglyceride-rich very-low-density lipoproteins, intermediate-density lipoproteins, and chylomicron remnants in the nonfasting state.
“When these particles enter the arterial wall, they are taken up by macrophages to produce foam cells, and therefore elevated remnant cholesterol likely enhance accumulation of cholesterol in the arterial wall, leading to progression of atherosclerosis and in consequence ischemic heart disease,” they note.
They point out that most guidelines for assessment of the 10-year risk of ischemic heart and atherosclerotic cardiovascular disease include levels of total and HDL cholesterol, but remnant cholesterol levels are not included.
They conducted the current study to investigate whether elevated remnant cholesterol would lead to appropriate reclassification of individuals who later experienced MI or ischemic heart disease.
The researchers analyzed data from the Copenhagen General Population Study, which recruited individuals from the White Danish general population from 2003-2015 and followed them until 2018. Information on lifestyle, health, and medication, including statin therapy, was obtained through a questionnaire, and participants underwent physical examinations and had nonfasting blood samples drawn for biochemical measurements.
For the current study, they included 41,928 individuals aged 40-100 years enrolled before 2009 without a history of ischemic cardiovascular disease, diabetes, and statin use at baseline. The median follow-up time was 12 years. Information on diagnoses of MI and ischemic heart disease was collected from the national Danish Causes of Death Registry and all hospital admissions and diagnoses entered in the national Danish Patient Registry.
During the first 10 years of follow-up there were 1,063 MIs and 1,460 ischemic heart disease events (death of ischemic heart disease, nonfatal MI, and coronary revascularization).
Results showed that in models based on conventional risk factors estimating risk of heart disease of above or below 5% in 10 years, adding remnant cholesterol at levels above the 95th percentile, appropriately reclassified 23% of individuals who had an MI and 21% of individuals who had an ischemic heart disease event.
Using remnant cholesterol levels above the 75th percentile appropriately reclassified 10% of those who had an MI and 8% of those who had an ischemic heart disease event. No events were reclassified incorrectly.
Using measurements of remnant cholesterol also improved reclassification of individuals with heart disease risk above or below 7.5% or 10% in 10 years.
When reclassifications were combined from below to above 5%, 7.5%, and 10% risk of events, 42% of individuals with MI and 41% with ischemic heart disease events were reclassified appropriately.
In an editorial accompanying publication of the study in JACC, Peter Wilson, MD, Emory University School of Medicine, Atlanta, and Alan Remaley, MD, National Heart, Lung, and Blood Institute, say these findings rekindle interest in atherogenic nonfasting lipid measurements and emphasize an important role for elevated nonfasting remnant cholesterol as a value-added predictor of ischemic events.
The editorialists note that both fasting and nonfasting lipid values provide useful information for atherosclerotic cardiovascular disease (ASCVD) risk estimation, and elevated nonfasting remnant cholesterol appears to help identify persons at greater risk for an initial cardiovascular ischemic event.
They add that very elevated levels (above the 75th percentile) of nonfasting remnant cholesterol deserve further evaluation as a potentially valuable “modifier of ASCVD risk,” and replication of the results could move these findings forward to potentially improve prognostication and care for patients at risk for ischemic heart disease events.
An indirect measure of triglycerides
Dr. Wilson explained that remnant cholesterol is an indirect measure of triglycerides beyond LDL levels, and it is thus including a new lipid measurement in risk prediction.
“We are completely focused on LDL cholesterol,” he said. “This opens it up a bit by adding in another measure that takes into account triglycerides as well as LDL.”
He also pointed out that use of a nonfasting sample is another advantage of measuring remnant cholesterol.
“An accurate measure of LDL needs a fasting sample, which is a nuisance, whereas remnant cholesterol can be measured in a nonfasting blood sample, so it is more convenient,” Dr. Wilson said.
While this study shows this measure is helpful for risk prediction in the primary prevention population, Dr. Wilson believes remnant cholesterol could be most useful in helping to guide further medication choice in patients who are already taking statins.
“Statins mainly target LDL, but if we can also measure nonfasting triglycerides this will be helpful. It may help us select some patients who may need a different type of drug to use in addition to statins that lowers triglycerides,” he said.
This work was supported by the Global Excellence Programme, the Research Fund for the Capital Region of Denmark, the Japanese College of Cardiology Overseas Research Fellowship, and the Scandinavia Japan Sasakawa Foundation. Mr. Nordestgaard has reported consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Amarin, Kowa, Denka, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. Dr. Doi has reported talks sponsored by MSD.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The study, which followed almost 42,000 Danish individuals without a history of ischemic cardiovascular disease, diabetes, or statin use for more than 10 years, found that elevated remnant cholesterol appropriately reclassified up to 40% of those who later experienced myocardial infarction and ischemic heart disease.
“The clinical implications of our study include that doctors and patients should be aware of remnant cholesterol levels to prevent future risk of MI and ischemic heart disease,” the authors conclude.
They suggest that the development of a cardiovascular risk algorithm, including remnant cholesterol together with LDL cholesterol, would help to better identify high-risk individuals who could be candidates for statins in a primary prevention setting.
They note that physicians are encouraged to evaluate non-HDL cholesterol and/or apolipoprotein B rather than LDL cholesterol and certainly not yet remnant cholesterol, possibly because of the limited availability of remnant cholesterol values in some parts of the world.
However, they point out that remnant cholesterol can be calculated with a standard lipid profile without additional cost, which is currently already the standard procedure in the greater Copenhagen area.
“This means that the use of remnant cholesterol is easy to introduce into daily clinical practice,” they say.
The study was published online in the Journal of the American College of Cardiology.
The authors, Takahito Doi, MD, Anne Langsted, MD, and Børge Nordestgaard, from Copenhagen University Hospital, Denmark, explain that remnant cholesterol is total cholesterol minus LDL-cholesterol minus HDL-cholesterol and includes the cholesterol content of the triglyceride-rich very-low-density lipoproteins, intermediate-density lipoproteins, and chylomicron remnants in the nonfasting state.
“When these particles enter the arterial wall, they are taken up by macrophages to produce foam cells, and therefore elevated remnant cholesterol likely enhance accumulation of cholesterol in the arterial wall, leading to progression of atherosclerosis and in consequence ischemic heart disease,” they note.
They point out that most guidelines for assessment of the 10-year risk of ischemic heart and atherosclerotic cardiovascular disease include levels of total and HDL cholesterol, but remnant cholesterol levels are not included.
They conducted the current study to investigate whether elevated remnant cholesterol would lead to appropriate reclassification of individuals who later experienced MI or ischemic heart disease.
The researchers analyzed data from the Copenhagen General Population Study, which recruited individuals from the White Danish general population from 2003-2015 and followed them until 2018. Information on lifestyle, health, and medication, including statin therapy, was obtained through a questionnaire, and participants underwent physical examinations and had nonfasting blood samples drawn for biochemical measurements.
For the current study, they included 41,928 individuals aged 40-100 years enrolled before 2009 without a history of ischemic cardiovascular disease, diabetes, and statin use at baseline. The median follow-up time was 12 years. Information on diagnoses of MI and ischemic heart disease was collected from the national Danish Causes of Death Registry and all hospital admissions and diagnoses entered in the national Danish Patient Registry.
During the first 10 years of follow-up there were 1,063 MIs and 1,460 ischemic heart disease events (death of ischemic heart disease, nonfatal MI, and coronary revascularization).
Results showed that in models based on conventional risk factors estimating risk of heart disease of above or below 5% in 10 years, adding remnant cholesterol at levels above the 95th percentile, appropriately reclassified 23% of individuals who had an MI and 21% of individuals who had an ischemic heart disease event.
Using remnant cholesterol levels above the 75th percentile appropriately reclassified 10% of those who had an MI and 8% of those who had an ischemic heart disease event. No events were reclassified incorrectly.
Using measurements of remnant cholesterol also improved reclassification of individuals with heart disease risk above or below 7.5% or 10% in 10 years.
When reclassifications were combined from below to above 5%, 7.5%, and 10% risk of events, 42% of individuals with MI and 41% with ischemic heart disease events were reclassified appropriately.
In an editorial accompanying publication of the study in JACC, Peter Wilson, MD, Emory University School of Medicine, Atlanta, and Alan Remaley, MD, National Heart, Lung, and Blood Institute, say these findings rekindle interest in atherogenic nonfasting lipid measurements and emphasize an important role for elevated nonfasting remnant cholesterol as a value-added predictor of ischemic events.
The editorialists note that both fasting and nonfasting lipid values provide useful information for atherosclerotic cardiovascular disease (ASCVD) risk estimation, and elevated nonfasting remnant cholesterol appears to help identify persons at greater risk for an initial cardiovascular ischemic event.
They add that very elevated levels (above the 75th percentile) of nonfasting remnant cholesterol deserve further evaluation as a potentially valuable “modifier of ASCVD risk,” and replication of the results could move these findings forward to potentially improve prognostication and care for patients at risk for ischemic heart disease events.
An indirect measure of triglycerides
Dr. Wilson explained that remnant cholesterol is an indirect measure of triglycerides beyond LDL levels, and it is thus including a new lipid measurement in risk prediction.
“We are completely focused on LDL cholesterol,” he said. “This opens it up a bit by adding in another measure that takes into account triglycerides as well as LDL.”
He also pointed out that use of a nonfasting sample is another advantage of measuring remnant cholesterol.
“An accurate measure of LDL needs a fasting sample, which is a nuisance, whereas remnant cholesterol can be measured in a nonfasting blood sample, so it is more convenient,” Dr. Wilson said.
While this study shows this measure is helpful for risk prediction in the primary prevention population, Dr. Wilson believes remnant cholesterol could be most useful in helping to guide further medication choice in patients who are already taking statins.
“Statins mainly target LDL, but if we can also measure nonfasting triglycerides this will be helpful. It may help us select some patients who may need a different type of drug to use in addition to statins that lowers triglycerides,” he said.
This work was supported by the Global Excellence Programme, the Research Fund for the Capital Region of Denmark, the Japanese College of Cardiology Overseas Research Fellowship, and the Scandinavia Japan Sasakawa Foundation. Mr. Nordestgaard has reported consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Amarin, Kowa, Denka, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. Dr. Doi has reported talks sponsored by MSD.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The study, which followed almost 42,000 Danish individuals without a history of ischemic cardiovascular disease, diabetes, or statin use for more than 10 years, found that elevated remnant cholesterol appropriately reclassified up to 40% of those who later experienced myocardial infarction and ischemic heart disease.
“The clinical implications of our study include that doctors and patients should be aware of remnant cholesterol levels to prevent future risk of MI and ischemic heart disease,” the authors conclude.
They suggest that the development of a cardiovascular risk algorithm, including remnant cholesterol together with LDL cholesterol, would help to better identify high-risk individuals who could be candidates for statins in a primary prevention setting.
They note that physicians are encouraged to evaluate non-HDL cholesterol and/or apolipoprotein B rather than LDL cholesterol and certainly not yet remnant cholesterol, possibly because of the limited availability of remnant cholesterol values in some parts of the world.
However, they point out that remnant cholesterol can be calculated with a standard lipid profile without additional cost, which is currently already the standard procedure in the greater Copenhagen area.
“This means that the use of remnant cholesterol is easy to introduce into daily clinical practice,” they say.
The study was published online in the Journal of the American College of Cardiology.
The authors, Takahito Doi, MD, Anne Langsted, MD, and Børge Nordestgaard, from Copenhagen University Hospital, Denmark, explain that remnant cholesterol is total cholesterol minus LDL-cholesterol minus HDL-cholesterol and includes the cholesterol content of the triglyceride-rich very-low-density lipoproteins, intermediate-density lipoproteins, and chylomicron remnants in the nonfasting state.
“When these particles enter the arterial wall, they are taken up by macrophages to produce foam cells, and therefore elevated remnant cholesterol likely enhance accumulation of cholesterol in the arterial wall, leading to progression of atherosclerosis and in consequence ischemic heart disease,” they note.
They point out that most guidelines for assessment of the 10-year risk of ischemic heart and atherosclerotic cardiovascular disease include levels of total and HDL cholesterol, but remnant cholesterol levels are not included.
They conducted the current study to investigate whether elevated remnant cholesterol would lead to appropriate reclassification of individuals who later experienced MI or ischemic heart disease.
The researchers analyzed data from the Copenhagen General Population Study, which recruited individuals from the White Danish general population from 2003-2015 and followed them until 2018. Information on lifestyle, health, and medication, including statin therapy, was obtained through a questionnaire, and participants underwent physical examinations and had nonfasting blood samples drawn for biochemical measurements.
For the current study, they included 41,928 individuals aged 40-100 years enrolled before 2009 without a history of ischemic cardiovascular disease, diabetes, and statin use at baseline. The median follow-up time was 12 years. Information on diagnoses of MI and ischemic heart disease was collected from the national Danish Causes of Death Registry and all hospital admissions and diagnoses entered in the national Danish Patient Registry.
During the first 10 years of follow-up there were 1,063 MIs and 1,460 ischemic heart disease events (death of ischemic heart disease, nonfatal MI, and coronary revascularization).
Results showed that in models based on conventional risk factors estimating risk of heart disease of above or below 5% in 10 years, adding remnant cholesterol at levels above the 95th percentile, appropriately reclassified 23% of individuals who had an MI and 21% of individuals who had an ischemic heart disease event.
Using remnant cholesterol levels above the 75th percentile appropriately reclassified 10% of those who had an MI and 8% of those who had an ischemic heart disease event. No events were reclassified incorrectly.
Using measurements of remnant cholesterol also improved reclassification of individuals with heart disease risk above or below 7.5% or 10% in 10 years.
When reclassifications were combined from below to above 5%, 7.5%, and 10% risk of events, 42% of individuals with MI and 41% with ischemic heart disease events were reclassified appropriately.
In an editorial accompanying publication of the study in JACC, Peter Wilson, MD, Emory University School of Medicine, Atlanta, and Alan Remaley, MD, National Heart, Lung, and Blood Institute, say these findings rekindle interest in atherogenic nonfasting lipid measurements and emphasize an important role for elevated nonfasting remnant cholesterol as a value-added predictor of ischemic events.
The editorialists note that both fasting and nonfasting lipid values provide useful information for atherosclerotic cardiovascular disease (ASCVD) risk estimation, and elevated nonfasting remnant cholesterol appears to help identify persons at greater risk for an initial cardiovascular ischemic event.
They add that very elevated levels (above the 75th percentile) of nonfasting remnant cholesterol deserve further evaluation as a potentially valuable “modifier of ASCVD risk,” and replication of the results could move these findings forward to potentially improve prognostication and care for patients at risk for ischemic heart disease events.
An indirect measure of triglycerides
Dr. Wilson explained that remnant cholesterol is an indirect measure of triglycerides beyond LDL levels, and it is thus including a new lipid measurement in risk prediction.
“We are completely focused on LDL cholesterol,” he said. “This opens it up a bit by adding in another measure that takes into account triglycerides as well as LDL.”
He also pointed out that use of a nonfasting sample is another advantage of measuring remnant cholesterol.
“An accurate measure of LDL needs a fasting sample, which is a nuisance, whereas remnant cholesterol can be measured in a nonfasting blood sample, so it is more convenient,” Dr. Wilson said.
While this study shows this measure is helpful for risk prediction in the primary prevention population, Dr. Wilson believes remnant cholesterol could be most useful in helping to guide further medication choice in patients who are already taking statins.
“Statins mainly target LDL, but if we can also measure nonfasting triglycerides this will be helpful. It may help us select some patients who may need a different type of drug to use in addition to statins that lowers triglycerides,” he said.
This work was supported by the Global Excellence Programme, the Research Fund for the Capital Region of Denmark, the Japanese College of Cardiology Overseas Research Fellowship, and the Scandinavia Japan Sasakawa Foundation. Mr. Nordestgaard has reported consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Amarin, Kowa, Denka, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. Dr. Doi has reported talks sponsored by MSD.
A version of this article first appeared on Medscape.com.
FDA approves setmelanotide for obesity in Bardet-Biedl syndrome
The Food and Drug Administration has approved a supplemental indication for setmelanotide (Imcivree, Rhythm Pharmaceuticals) injection for chronic weight management in adults and pediatric patients age 6 and older with obesity due to Bardet-Biedl Syndrome (BBS).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for BBS, a rare genetic disorder that impairs a hunger signal along the melanocortin-4 receptor (MC4R) pathway.
BBS affects an estimated 1,500-2,500 people in the United States.
Individuals with BBS typically have obesity that starts at age 1 along with insatiable hunger (hyperphagia). Available weight management options are generally unsuccessful.
Other symptoms may include retinal degeneration, reduced kidney function, or extra digits of the hands or feet.
Setmelanotide received priority review, orphan drug designation, and breakthrough designation for this new indication.
As previously reported, in November 2020, the FDA approved setmelanotide for weight management in adults and children as young as 6 years with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing – who also have impaired hunger signaling from the brain.
These individuals have a normal weight at birth but develop persistent, severe obesity within months due to hyperphagia.
The FDA approval of Imcivree for BBS “represents a significant milestone for Rhythm [Pharmaceuticals], validating our strategy of developing Imcivree for people with hyperphagia and severe obesity caused by rare MC4R-pathway diseases and allowing us to provide our precision therapy to an established community of patients living with BBS and their families who are eagerly awaiting a new treatment option,” said David Meeker, MD, chair, president and CEO of Rhythm, in a press release.
Safety, effectiveness in 66-week trial in 44 patients
The safety and effectiveness of setmelanotidewas evaluated in a 66-week phase 3 clinical trial that enrolled 44 patients age 6 and older who had a diagnosis of BBS and obesity – defined as a body mass index greater than or equal to 30 kg/m2 or greater than or equal to 97th percentile for pediatric patients.
After an initial 14-week, randomized, double-blind, placebo-controlled treatment period, patients entered a 52-week, open-label period.
The trial met its primary endpoint and all key secondary endpoints, with statistically significant reductions in weight and hunger at 52 weeks on therapy.
- After 52 weeks of treatment, patients taking setmelanotide lost, on average, 7.9% of their initial BMI.
- 61% of patients lost 5% or more of their initial BMI, and 39% lost 10% or more of their initial BMI.
- In the 14-week, placebo-controlled treatment, on average, BMI dropped by 4.6% in the 22 patients treated with the study drug and dropped 0.1% in the 22 patients treated with placebo.
- At 52 weeks, the 14 patients aged 12 and older who were able to self-report their hunger had a significant –2.1 mean change in hunger score.
Setmelanotide is associated with the following warnings and precautions:
- Spontaneous penile erections in males and sexual adverse reactions in females. Instruct males with erection lasting longer than 4 hours to seek emergency medical attention.
- Depression and suicidal ideation. Monitor patients for new onset or worsening depression or suicidal thoughts or behaviors. Consider discontinuing the drug if patients have suicidal thoughts or behaviors or clinically significant or persistent depression symptoms.
- Skin pigmentation and darkening of preexisting nevi (moles). Examine skin before and during treatment.
- Setmelanotide is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low-birth-weight infants treated with benzyl alcohol-preserved drugs.
The most common adverse reactions (with an incidence greater than or equal to 20%) included skin hyperpigmentation, injection site reactions, nausea, headache, diarrhea, abdominal pain, vomiting, depression, and spontaneous penile erection.
The FDA did not approve the company’s supplemental new drug application for setmelanotide in Alström syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a supplemental indication for setmelanotide (Imcivree, Rhythm Pharmaceuticals) injection for chronic weight management in adults and pediatric patients age 6 and older with obesity due to Bardet-Biedl Syndrome (BBS).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for BBS, a rare genetic disorder that impairs a hunger signal along the melanocortin-4 receptor (MC4R) pathway.
BBS affects an estimated 1,500-2,500 people in the United States.
Individuals with BBS typically have obesity that starts at age 1 along with insatiable hunger (hyperphagia). Available weight management options are generally unsuccessful.
Other symptoms may include retinal degeneration, reduced kidney function, or extra digits of the hands or feet.
Setmelanotide received priority review, orphan drug designation, and breakthrough designation for this new indication.
As previously reported, in November 2020, the FDA approved setmelanotide for weight management in adults and children as young as 6 years with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing – who also have impaired hunger signaling from the brain.
These individuals have a normal weight at birth but develop persistent, severe obesity within months due to hyperphagia.
The FDA approval of Imcivree for BBS “represents a significant milestone for Rhythm [Pharmaceuticals], validating our strategy of developing Imcivree for people with hyperphagia and severe obesity caused by rare MC4R-pathway diseases and allowing us to provide our precision therapy to an established community of patients living with BBS and their families who are eagerly awaiting a new treatment option,” said David Meeker, MD, chair, president and CEO of Rhythm, in a press release.
Safety, effectiveness in 66-week trial in 44 patients
The safety and effectiveness of setmelanotidewas evaluated in a 66-week phase 3 clinical trial that enrolled 44 patients age 6 and older who had a diagnosis of BBS and obesity – defined as a body mass index greater than or equal to 30 kg/m2 or greater than or equal to 97th percentile for pediatric patients.
After an initial 14-week, randomized, double-blind, placebo-controlled treatment period, patients entered a 52-week, open-label period.
The trial met its primary endpoint and all key secondary endpoints, with statistically significant reductions in weight and hunger at 52 weeks on therapy.
- After 52 weeks of treatment, patients taking setmelanotide lost, on average, 7.9% of their initial BMI.
- 61% of patients lost 5% or more of their initial BMI, and 39% lost 10% or more of their initial BMI.
- In the 14-week, placebo-controlled treatment, on average, BMI dropped by 4.6% in the 22 patients treated with the study drug and dropped 0.1% in the 22 patients treated with placebo.
- At 52 weeks, the 14 patients aged 12 and older who were able to self-report their hunger had a significant –2.1 mean change in hunger score.
Setmelanotide is associated with the following warnings and precautions:
- Spontaneous penile erections in males and sexual adverse reactions in females. Instruct males with erection lasting longer than 4 hours to seek emergency medical attention.
- Depression and suicidal ideation. Monitor patients for new onset or worsening depression or suicidal thoughts or behaviors. Consider discontinuing the drug if patients have suicidal thoughts or behaviors or clinically significant or persistent depression symptoms.
- Skin pigmentation and darkening of preexisting nevi (moles). Examine skin before and during treatment.
- Setmelanotide is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low-birth-weight infants treated with benzyl alcohol-preserved drugs.
The most common adverse reactions (with an incidence greater than or equal to 20%) included skin hyperpigmentation, injection site reactions, nausea, headache, diarrhea, abdominal pain, vomiting, depression, and spontaneous penile erection.
The FDA did not approve the company’s supplemental new drug application for setmelanotide in Alström syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a supplemental indication for setmelanotide (Imcivree, Rhythm Pharmaceuticals) injection for chronic weight management in adults and pediatric patients age 6 and older with obesity due to Bardet-Biedl Syndrome (BBS).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for BBS, a rare genetic disorder that impairs a hunger signal along the melanocortin-4 receptor (MC4R) pathway.
BBS affects an estimated 1,500-2,500 people in the United States.
Individuals with BBS typically have obesity that starts at age 1 along with insatiable hunger (hyperphagia). Available weight management options are generally unsuccessful.
Other symptoms may include retinal degeneration, reduced kidney function, or extra digits of the hands or feet.
Setmelanotide received priority review, orphan drug designation, and breakthrough designation for this new indication.
As previously reported, in November 2020, the FDA approved setmelanotide for weight management in adults and children as young as 6 years with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing – who also have impaired hunger signaling from the brain.
These individuals have a normal weight at birth but develop persistent, severe obesity within months due to hyperphagia.
The FDA approval of Imcivree for BBS “represents a significant milestone for Rhythm [Pharmaceuticals], validating our strategy of developing Imcivree for people with hyperphagia and severe obesity caused by rare MC4R-pathway diseases and allowing us to provide our precision therapy to an established community of patients living with BBS and their families who are eagerly awaiting a new treatment option,” said David Meeker, MD, chair, president and CEO of Rhythm, in a press release.
Safety, effectiveness in 66-week trial in 44 patients
The safety and effectiveness of setmelanotidewas evaluated in a 66-week phase 3 clinical trial that enrolled 44 patients age 6 and older who had a diagnosis of BBS and obesity – defined as a body mass index greater than or equal to 30 kg/m2 or greater than or equal to 97th percentile for pediatric patients.
After an initial 14-week, randomized, double-blind, placebo-controlled treatment period, patients entered a 52-week, open-label period.
The trial met its primary endpoint and all key secondary endpoints, with statistically significant reductions in weight and hunger at 52 weeks on therapy.
- After 52 weeks of treatment, patients taking setmelanotide lost, on average, 7.9% of their initial BMI.
- 61% of patients lost 5% or more of their initial BMI, and 39% lost 10% or more of their initial BMI.
- In the 14-week, placebo-controlled treatment, on average, BMI dropped by 4.6% in the 22 patients treated with the study drug and dropped 0.1% in the 22 patients treated with placebo.
- At 52 weeks, the 14 patients aged 12 and older who were able to self-report their hunger had a significant –2.1 mean change in hunger score.
Setmelanotide is associated with the following warnings and precautions:
- Spontaneous penile erections in males and sexual adverse reactions in females. Instruct males with erection lasting longer than 4 hours to seek emergency medical attention.
- Depression and suicidal ideation. Monitor patients for new onset or worsening depression or suicidal thoughts or behaviors. Consider discontinuing the drug if patients have suicidal thoughts or behaviors or clinically significant or persistent depression symptoms.
- Skin pigmentation and darkening of preexisting nevi (moles). Examine skin before and during treatment.
- Setmelanotide is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low-birth-weight infants treated with benzyl alcohol-preserved drugs.
The most common adverse reactions (with an incidence greater than or equal to 20%) included skin hyperpigmentation, injection site reactions, nausea, headache, diarrhea, abdominal pain, vomiting, depression, and spontaneous penile erection.
The FDA did not approve the company’s supplemental new drug application for setmelanotide in Alström syndrome.
A version of this article first appeared on Medscape.com.
Heart failure: Medicare cost sharing may put quadruple therapy out of reach
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.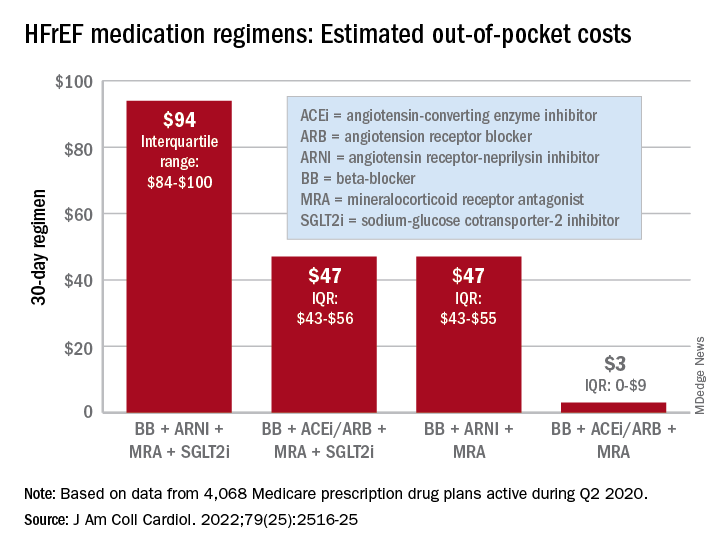
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
FROM THE JOURNAL Of the AMERICAN COLLEGE OF CARDIOLOGY
New National Lipid Association statement on statin intolerance
The U.S. National Lipid Association has issued a new scientific statement on the management of patients with statin intolerance, which recommends different strategies to help patients stay on statin medications, and also suggests alternatives that can be used in patients who really cannot tolerate statin drugs.
The statement was published online in the Journal of Clinical Lipidology.
It notes that, although statins are generally well tolerated, statin intolerance is reported in 5%-30% of patients and contributes to reduced statin adherence and persistence, as well as higher risk for adverse cardiovascular outcomes.
The statement acknowledges the importance of identifying modifiable risk factors for statin intolerance and recognizes the possibility of a “nocebo” effect, basically the patient expectation of harm resulting in perceived side effects.
To identify a tolerable statin regimen, it recommends that clinicians consider using several different strategies (different statin, dose, and/or dosing frequency), and to classify a patient as having statin intolerance, a minimum of two statins should have been attempted, including at least one at the lowest-approved daily dosage.
The statement says that nonstatin therapy may be required for patients who cannot reach therapeutic objectives with lifestyle and maximal tolerated statin therapy, and in these cases, therapies with outcomes data from randomized trials showing reduced cardiovascular events are favored.
In high and very high-risk patients who are statin intolerant, clinicians should consider initiating nonstatin therapy while additional attempts are made to identify a tolerable statin in order to limit the time of exposure to elevated levels of atherogenic lipoproteins, it suggests.
“There is strong evidence that statins reduce risk of cardiovascular events particularly in patients with atherosclerotic cardiovascular disease, but recent research shows that only about half of patients with ASCVD are on a statin,” Kevin C. Maki, PhD, coauthor of the statement and current president of the National Lipid Association, said in an interview.
“There is an urgent problem with underutilization of statins and undertreatment of ASCVD. And we know that perceived side effects associated with statins are a common reason for discontinuation of these drugs and the consequent failure to manage ASCVD adequately,” he said.
Dr. Maki noted that the NLA’s first message is that, when experiencing symptoms taking statins, a large majority of patients can still tolerate a statin. “They can try a different agent or a different dose. But for those who still can’t tolerate a statin, we then recommend nonstatin therapies and we favor those therapies with evidence from randomized trials.”
He pointed out that many patients who believe they are experiencing side effects from taking statins still experience the same effects on a placebo, a condition known as the nocebo effect.
“Several studies have shown that the nocebo effect is very common and accounts for more than half of perceived statin side effects. It is therefore estimated that many of the complaints of statin intolerance are probably not directly related to the pharmacodynamic actions of the drugs,” Dr. Maki said.
One recent study on the nocebo effect, the SAMSON study, suggested that 90% of symptoms attributed to statins were elicited by placebo tablets too.
But Dr. Maki added that it can be a losing battle for the clinician if patients think their symptoms are related to taking a statin.
“We suggest that clinicians inform patients that most people can tolerate a statin – maybe with a different agent or an alternative dose – and it is really important to lower LDL cholesterol as that will lower the risk of MI and stroke, so we need to find a regimen that works for each individual,” he said. “Most people can find a regimen that works. If this means taking a lower dose of a statin, they can take some additional therapy as well. This is a better situation than stopping taking statins altogether and allowing ASCVD to progress.”
Dr. Maki stressed that statins should still be the first choice as they are effective, taken orally, and inexpensive.
“Other medications do not have all these advantages. For example, PCSK9 inhibitors are very effective but they are expensive and injectable,” he noted. “And while ezetimibe [Zetia] is now generic so inexpensive, it has a more modest effect on LDL-lowering compared to statins, so by itself it is not normally enough for most patients to get to their target LDL, but it is an option for use in combination with a statin.”
He added that the NLA message is to do everything possible to keep patients on a statin, especially patients with preexisting ASCVD.
“We would like these patients to be on high-intensity statins. If they really can’t tolerate this, then they could be on a low-intensity statin plus an additional agent.”
Commenting on the NLA statement, SAMSON study coauthor James Howard, MB BChir, PhD, Imperial College London, said he had reservations about some of the recommendations.
“Whilst I think it is great news that the existence and importance of the nocebo effect is increasingly recognized in international guidelines and statements, I think we need to be very careful about recommending reduced doses and frequencies of statins,” Dr. Howard said.
“Studies such as SAMSON and StatinWISE indicate the vast majority of side effects reported by patients taking statins are not caused by the statin molecule, but instead are caused by either the nocebo effect, or ever-present background symptoms that are wrongly attributed to the statins,” he commented. “Therefore, to recommend that the correct approach in a patient with a history of MI suffering symptoms on 80 mg of atorvastatin is to reduce the dose or try alternate daily dosing. This reinforces the view that these drugs are side-effect prone and need to be carefully titrated.”
Dr. Howard suggested that patients should be educated on the possibility of the nocebo effect or background symptoms and encouraged to retrial statins at the same dose. “If that doesn’t work, then formal recording with a symptom diary might help patients recognize background symptoms,” he added.
Dr. Howard noted that, if symptoms still persist, an “n-of-1” trial could be conducted, in which the patient rotates between multiple periods of taking a statin and a placebo, but he acknowledged that this is expensive and time consuming.
Also commenting, Steve Nissen, MD, Cleveland Clinic, said he thought the NLA statement was “reasonable and thoughtful.”
“Regardless of whether the symptoms are due to the nocebo effect or not, some patients will just not take a statin no matter how hard you try to convince them to persevere, so we do need alternatives,” Dr. Nissen said.
He noted that current alternatives would include the PCSK9 inhibitors and ezetimibe, but a future candidate could be the oral bempedoic acid (Nexletol), which is currently being evaluated in a large outcomes trial (CLEAR Outcomes).
A version of this article first appeared on Medscape.com.
The U.S. National Lipid Association has issued a new scientific statement on the management of patients with statin intolerance, which recommends different strategies to help patients stay on statin medications, and also suggests alternatives that can be used in patients who really cannot tolerate statin drugs.
The statement was published online in the Journal of Clinical Lipidology.
It notes that, although statins are generally well tolerated, statin intolerance is reported in 5%-30% of patients and contributes to reduced statin adherence and persistence, as well as higher risk for adverse cardiovascular outcomes.
The statement acknowledges the importance of identifying modifiable risk factors for statin intolerance and recognizes the possibility of a “nocebo” effect, basically the patient expectation of harm resulting in perceived side effects.
To identify a tolerable statin regimen, it recommends that clinicians consider using several different strategies (different statin, dose, and/or dosing frequency), and to classify a patient as having statin intolerance, a minimum of two statins should have been attempted, including at least one at the lowest-approved daily dosage.
The statement says that nonstatin therapy may be required for patients who cannot reach therapeutic objectives with lifestyle and maximal tolerated statin therapy, and in these cases, therapies with outcomes data from randomized trials showing reduced cardiovascular events are favored.
In high and very high-risk patients who are statin intolerant, clinicians should consider initiating nonstatin therapy while additional attempts are made to identify a tolerable statin in order to limit the time of exposure to elevated levels of atherogenic lipoproteins, it suggests.
“There is strong evidence that statins reduce risk of cardiovascular events particularly in patients with atherosclerotic cardiovascular disease, but recent research shows that only about half of patients with ASCVD are on a statin,” Kevin C. Maki, PhD, coauthor of the statement and current president of the National Lipid Association, said in an interview.
“There is an urgent problem with underutilization of statins and undertreatment of ASCVD. And we know that perceived side effects associated with statins are a common reason for discontinuation of these drugs and the consequent failure to manage ASCVD adequately,” he said.
Dr. Maki noted that the NLA’s first message is that, when experiencing symptoms taking statins, a large majority of patients can still tolerate a statin. “They can try a different agent or a different dose. But for those who still can’t tolerate a statin, we then recommend nonstatin therapies and we favor those therapies with evidence from randomized trials.”
He pointed out that many patients who believe they are experiencing side effects from taking statins still experience the same effects on a placebo, a condition known as the nocebo effect.
“Several studies have shown that the nocebo effect is very common and accounts for more than half of perceived statin side effects. It is therefore estimated that many of the complaints of statin intolerance are probably not directly related to the pharmacodynamic actions of the drugs,” Dr. Maki said.
One recent study on the nocebo effect, the SAMSON study, suggested that 90% of symptoms attributed to statins were elicited by placebo tablets too.
But Dr. Maki added that it can be a losing battle for the clinician if patients think their symptoms are related to taking a statin.
“We suggest that clinicians inform patients that most people can tolerate a statin – maybe with a different agent or an alternative dose – and it is really important to lower LDL cholesterol as that will lower the risk of MI and stroke, so we need to find a regimen that works for each individual,” he said. “Most people can find a regimen that works. If this means taking a lower dose of a statin, they can take some additional therapy as well. This is a better situation than stopping taking statins altogether and allowing ASCVD to progress.”
Dr. Maki stressed that statins should still be the first choice as they are effective, taken orally, and inexpensive.
“Other medications do not have all these advantages. For example, PCSK9 inhibitors are very effective but they are expensive and injectable,” he noted. “And while ezetimibe [Zetia] is now generic so inexpensive, it has a more modest effect on LDL-lowering compared to statins, so by itself it is not normally enough for most patients to get to their target LDL, but it is an option for use in combination with a statin.”
He added that the NLA message is to do everything possible to keep patients on a statin, especially patients with preexisting ASCVD.
“We would like these patients to be on high-intensity statins. If they really can’t tolerate this, then they could be on a low-intensity statin plus an additional agent.”
Commenting on the NLA statement, SAMSON study coauthor James Howard, MB BChir, PhD, Imperial College London, said he had reservations about some of the recommendations.
“Whilst I think it is great news that the existence and importance of the nocebo effect is increasingly recognized in international guidelines and statements, I think we need to be very careful about recommending reduced doses and frequencies of statins,” Dr. Howard said.
“Studies such as SAMSON and StatinWISE indicate the vast majority of side effects reported by patients taking statins are not caused by the statin molecule, but instead are caused by either the nocebo effect, or ever-present background symptoms that are wrongly attributed to the statins,” he commented. “Therefore, to recommend that the correct approach in a patient with a history of MI suffering symptoms on 80 mg of atorvastatin is to reduce the dose or try alternate daily dosing. This reinforces the view that these drugs are side-effect prone and need to be carefully titrated.”
Dr. Howard suggested that patients should be educated on the possibility of the nocebo effect or background symptoms and encouraged to retrial statins at the same dose. “If that doesn’t work, then formal recording with a symptom diary might help patients recognize background symptoms,” he added.
Dr. Howard noted that, if symptoms still persist, an “n-of-1” trial could be conducted, in which the patient rotates between multiple periods of taking a statin and a placebo, but he acknowledged that this is expensive and time consuming.
Also commenting, Steve Nissen, MD, Cleveland Clinic, said he thought the NLA statement was “reasonable and thoughtful.”
“Regardless of whether the symptoms are due to the nocebo effect or not, some patients will just not take a statin no matter how hard you try to convince them to persevere, so we do need alternatives,” Dr. Nissen said.
He noted that current alternatives would include the PCSK9 inhibitors and ezetimibe, but a future candidate could be the oral bempedoic acid (Nexletol), which is currently being evaluated in a large outcomes trial (CLEAR Outcomes).
A version of this article first appeared on Medscape.com.
The U.S. National Lipid Association has issued a new scientific statement on the management of patients with statin intolerance, which recommends different strategies to help patients stay on statin medications, and also suggests alternatives that can be used in patients who really cannot tolerate statin drugs.
The statement was published online in the Journal of Clinical Lipidology.
It notes that, although statins are generally well tolerated, statin intolerance is reported in 5%-30% of patients and contributes to reduced statin adherence and persistence, as well as higher risk for adverse cardiovascular outcomes.
The statement acknowledges the importance of identifying modifiable risk factors for statin intolerance and recognizes the possibility of a “nocebo” effect, basically the patient expectation of harm resulting in perceived side effects.
To identify a tolerable statin regimen, it recommends that clinicians consider using several different strategies (different statin, dose, and/or dosing frequency), and to classify a patient as having statin intolerance, a minimum of two statins should have been attempted, including at least one at the lowest-approved daily dosage.
The statement says that nonstatin therapy may be required for patients who cannot reach therapeutic objectives with lifestyle and maximal tolerated statin therapy, and in these cases, therapies with outcomes data from randomized trials showing reduced cardiovascular events are favored.
In high and very high-risk patients who are statin intolerant, clinicians should consider initiating nonstatin therapy while additional attempts are made to identify a tolerable statin in order to limit the time of exposure to elevated levels of atherogenic lipoproteins, it suggests.
“There is strong evidence that statins reduce risk of cardiovascular events particularly in patients with atherosclerotic cardiovascular disease, but recent research shows that only about half of patients with ASCVD are on a statin,” Kevin C. Maki, PhD, coauthor of the statement and current president of the National Lipid Association, said in an interview.
“There is an urgent problem with underutilization of statins and undertreatment of ASCVD. And we know that perceived side effects associated with statins are a common reason for discontinuation of these drugs and the consequent failure to manage ASCVD adequately,” he said.
Dr. Maki noted that the NLA’s first message is that, when experiencing symptoms taking statins, a large majority of patients can still tolerate a statin. “They can try a different agent or a different dose. But for those who still can’t tolerate a statin, we then recommend nonstatin therapies and we favor those therapies with evidence from randomized trials.”
He pointed out that many patients who believe they are experiencing side effects from taking statins still experience the same effects on a placebo, a condition known as the nocebo effect.
“Several studies have shown that the nocebo effect is very common and accounts for more than half of perceived statin side effects. It is therefore estimated that many of the complaints of statin intolerance are probably not directly related to the pharmacodynamic actions of the drugs,” Dr. Maki said.
One recent study on the nocebo effect, the SAMSON study, suggested that 90% of symptoms attributed to statins were elicited by placebo tablets too.
But Dr. Maki added that it can be a losing battle for the clinician if patients think their symptoms are related to taking a statin.
“We suggest that clinicians inform patients that most people can tolerate a statin – maybe with a different agent or an alternative dose – and it is really important to lower LDL cholesterol as that will lower the risk of MI and stroke, so we need to find a regimen that works for each individual,” he said. “Most people can find a regimen that works. If this means taking a lower dose of a statin, they can take some additional therapy as well. This is a better situation than stopping taking statins altogether and allowing ASCVD to progress.”
Dr. Maki stressed that statins should still be the first choice as they are effective, taken orally, and inexpensive.
“Other medications do not have all these advantages. For example, PCSK9 inhibitors are very effective but they are expensive and injectable,” he noted. “And while ezetimibe [Zetia] is now generic so inexpensive, it has a more modest effect on LDL-lowering compared to statins, so by itself it is not normally enough for most patients to get to their target LDL, but it is an option for use in combination with a statin.”
He added that the NLA message is to do everything possible to keep patients on a statin, especially patients with preexisting ASCVD.
“We would like these patients to be on high-intensity statins. If they really can’t tolerate this, then they could be on a low-intensity statin plus an additional agent.”
Commenting on the NLA statement, SAMSON study coauthor James Howard, MB BChir, PhD, Imperial College London, said he had reservations about some of the recommendations.
“Whilst I think it is great news that the existence and importance of the nocebo effect is increasingly recognized in international guidelines and statements, I think we need to be very careful about recommending reduced doses and frequencies of statins,” Dr. Howard said.
“Studies such as SAMSON and StatinWISE indicate the vast majority of side effects reported by patients taking statins are not caused by the statin molecule, but instead are caused by either the nocebo effect, or ever-present background symptoms that are wrongly attributed to the statins,” he commented. “Therefore, to recommend that the correct approach in a patient with a history of MI suffering symptoms on 80 mg of atorvastatin is to reduce the dose or try alternate daily dosing. This reinforces the view that these drugs are side-effect prone and need to be carefully titrated.”
Dr. Howard suggested that patients should be educated on the possibility of the nocebo effect or background symptoms and encouraged to retrial statins at the same dose. “If that doesn’t work, then formal recording with a symptom diary might help patients recognize background symptoms,” he added.
Dr. Howard noted that, if symptoms still persist, an “n-of-1” trial could be conducted, in which the patient rotates between multiple periods of taking a statin and a placebo, but he acknowledged that this is expensive and time consuming.
Also commenting, Steve Nissen, MD, Cleveland Clinic, said he thought the NLA statement was “reasonable and thoughtful.”
“Regardless of whether the symptoms are due to the nocebo effect or not, some patients will just not take a statin no matter how hard you try to convince them to persevere, so we do need alternatives,” Dr. Nissen said.
He noted that current alternatives would include the PCSK9 inhibitors and ezetimibe, but a future candidate could be the oral bempedoic acid (Nexletol), which is currently being evaluated in a large outcomes trial (CLEAR Outcomes).
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL LIPIDOLOGY
Prediabetes is linked independently to myocardial infarction
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
Prediabetes is not only a predictor of diabetes and the cardiovascular complications that ensue, but it is also a risk factor by itself for myocardial infarction, according to data drawn from almost 1.8 million patients hospitalized for MI.
“Our study serves as a wakeup call for clinicians and patients to shift the focus to preventing prediabetes, and not just diabetes, said Geethika Thota, MD, at the annual meeting of the Endocrine Society.
There are plenty of data suggesting that prediabetes places patients on a trajectory toward cardiovascular disease. In a meta-analysis of 129 studies published 2 years ago, prediabetes was not only associated with a statistically significant 16% increase in coronary heart disease, but also a 13% increased risk of all-cause mortality relative to those with normoglycemia.
Data drawn from 1.8 million patients
In this study, 1,794,149 weighted patient hospitalizations for MI were drawn from the National Inpatient Sample database. Excluding patients who eventually developed diabetes, roughly 1% of these patients had a history of prediabetes in the past, according to a search of ICD-10 codes.
Before adjustment for other risk factors, prediabetes was linked to a greater than 40% increased odds of MI (odds ratio, 1.41; P < .01). After adjustment for a large array of known MI risk factors – including prior history of MI, dyslipidemia, hypertension, nicotine dependence, and obesity – prediabetes remained an independent risk factor, corresponding with a 25% increased risk of MI (OR, 1.25; P < .01).
A history of prediabetes was also an independent risk factor for percutaneous intervention and coronary artery bypass grafting, with increased risk of 45% and 95%, respectively.
As a retrospective study looking at prediabetes as a risk factor in those who already had a MI, it is possible that not all patients with prediabetes were properly coded, but Dr. Thota said that was unlikely to have been an issue of sufficient magnitude to have affected the major conclusions.
Relevance seen for community care
Although the study was drawn from hospitalized patients, its relevance is for the community setting, where screening and intervention for prediabetes has the potential to alter the risk, according to Dr. Thota.
Most clinicians are likely aware of the value of screening for prediabetes, which was defined in this study as a hemoglobin A1c of 5.7%-6.4%, but Dr. Thota suggested that many might not fully grasp the full scope of goals. Early detection and prevention will prevent diabetes and, by extension, cardiovascular disease, but her data suggest that control of prediabetes with lower cardiovascular risk by a more direct route.
“Despite mounting evidence, many clinicians are unaware that prediabetes is also a major risk factor for atherosclerotic cardiovascular disease,” said Dr. Thota, an internal medicine resident at Saint Peter’s University Hospital, New Brunswick, N.J.
Like diabetes, the prevalence of prediabetes is growing rapidly, according to data from the Centers for Disease Control that Dr. Thota cited. In 2020, the Centers for Disease Control and Prevention estimated that 38% of the adult population have prediabetes. By 2030, one model predicts a further 25% growth.
Screening for hyperglycemia is part of routine patient evaluations at Dr. Thota’s center. In an interview, she said that once a diagnosis of prediabetes is entered in the electronic medical record, the history is carried forward so that changes in status are continually monitored.
Worsening prediabetes should be addressed
“Prediabetes is not treated with medication, at least initially,” Dr. Thota explained. Rather, patients are educated about important lifestyle changes, such as diet and physical activity, that can reverse the diagnosis. However, patients who remain on a path of worsening hyperglycemia are candidates for more intensive lifestyle intervention and might be considered selectively for metformin.
“Early recognition of prediabetes through screening is important,” Dr. Thota emphasized. The benefit for preventing patients from progressing to diabetes is well recognized, but these data provide the basis for incentivizing lifestyle changes in patients with prediabetes by telling them that it can reduce their risk for MI.
These data have an important message, but they are not surprising, according to Deepak L. Bhatt, MD, executive director, interventional cardiovascular programs, Brigham and Women’s Hospital Heart & Vascular Center, Boston.
“In fact, in daily practice we see a substantial percentage of patients with MI who have prediabetes that had not been previously recognized or formally diagnosed,” Dr. Bhatt said in an interview.
“Identifying these patients – preferably prior to coming in with cardiovascular complications – is important both to reduce cardiovascular risk but also to try and prevent progression at diabetes,” he added.
Dr. Bhatt went on to say that this large analysis, confirming that prediabetes is independently associated with MI, should prompt clinicians to screen patients rigorously for this condition.
“At a minimum, such patients would be candidates for intensive lifestyle modification aimed at weight loss and treatment of frequent coexistent conditions, such as hypertension and dyslipidemia,” Dr. Bhatt said.
Dr. Thota reports no potential conflicts of interest. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, many of which make products relevant to the management of diabetes and cardiovascular disease.
FROM ENDO 2022
Avexitide promising for hypoglycemia after weight-loss surgery
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
Avexitide (Eiger Biopharmaceuticals), a first-in-class glucagonlike peptide (GLP)–1 receptor blocker, significantly reduced hypoglycemia in patients with refractory postbariatric hypoglycemia, new research finds.
Postbariatric hypoglycemia is a complication of bariatric surgery that is estimated to occur in about 29%-34% of people who undergo Roux-en-Y gastric bypass and in 11%-23% of those who undergo vertical sleeve gastrectomy. It typically manifests about 1-3 hours after meals and can lead to severe neuroglycopenic symptoms including blurred vision, confusion, drowsiness, and incoordination.
In addition, more than one-third (37%) with the condition have hypoglycemic unawareness. This can lead to seizures in about 59%, loss of consciousness and hospitalization in 50%, motor vehicle accidents, and even death. More than 90% with the condition consider themselves disabled, and 41% report being unable to work.
There are no currently approved medical treatments for postbariatric hypoglycemia. The standard of care is medical nutrition therapy involving a low-carbohydrate diet with carb restriction and small, frequent mixed meals. If this doesn’t work, off-label stepped pharmacotherapy has been tried, including acarbose (Precose), octreotide (Sandostatin), and diazoxide (Proglycem).
But “these are limited by efficacy and tolerability,” said Marilyn Tan, MD, who presented the findings from the phase 2 trial of avexitide at the annual meeting of the Endocrine Society.
In very severe cases, gastrostomy tubes or bypass reversal are options but those lead to weight regain and incomplete efficacy. “Safe, effective, and targeted therapies are needed urgently for postbariatric hypoglycemia,” said Dr. Tan, of the department of endocrinology at Stanford (Calif.) University.
The pathophysiology isn’t fully understood, but there appears to be an exaggerated GLP-1 response that leads to abnormal insulin secretion and symptomatic hyperinsulinemic hypoglycemia. Avexitide (formerly exendin 9-39), blocks the GLP-1 receptor and mitigates the excessive GLP-1 response, she explained.
Asked to comment, session moderator Michelle Van Name, MD, told this news organization, “This is a problem and it’s important for us to understand more about it and to identify different treatment options so these patients can continue to live their full, healthy lives post bariatric surgery.”
And, avexitide also holds potential for treating congenital hyperinsulinism, “which is a very challenging disease to treat in babies,” noted Dr. Van Name, a pediatric endocrinologist at Yale University, New Haven, Conn.
Drug reduced all levels of hypoglycemia, across surgery types
The study enrolled 14 women and 2 men with severe refractory postbariatric surgery hypoglycemia despite medical nutrition therapy. A majority (9) had undergone Roux-en-Y gastric bypass, 4 had vertical sleeve gastrectomy, 2 gastrectomy, and 1 had Nissen fundoplication. Seven patients (43.7%) had experienced loss of consciousness from hyperinsulinemic hypoglycemia. None had diabetes.
They were randomly assigned to either subcutaneous 45 mg of avexitide twice daily or 90 mg once daily for 14 days each, with a 2-day washout period followed by a switch to the other dose.
Both doses resulted in significant reductions in hypoglycemia as measured by self–blood glucose monitoring. The once-daily dose reduced level 1 hypoglycemia (glucose < 70 mg/dL) by 67.5% and it reduced level 2 (< 54 mg/dL) by 53.3% (P = .0043).
Even greater reductions were seen in severe hypoglycemia (that is, altered mental status/requiring assistance) – by 67.5% for the twice-daily dose (P = .0003) and by 66.1% with the once-daily dose (P = .0003).
“This is consistent with what we’ve seen in prior avexitide trials,” Dr. Tan noted.
More hypoglycemic events were captured using blinded continuous glucose monitoring (CGM), since it picked up episodes of which the patient was unaware. There were significant reductions in percentage time spent in level 1 and level 2 hypoglycemia, as well as in absolute number of hypoglycemic events over 14 days.
Here, the effect was greater with the once-daily 90 mg dose, with reductions of up to 65% in time spent and number of events, but results for the twice-daily dose were also significant, Dr. Tan said.
The drug was effective across all surgical subtypes. Patients who underwent vertical sleeve gastrectomy/gastrectomy had greater rates of hypoglycemia at baseline and “robust responses to avexitide subcutaneous injections. This supports the critical role of GLP-1,” Dr. Tan said.
There were no severe adverse events. The most commonly reported adverse events were diarrhea, headache, bloating, and injection-site reaction/bruising. All were mild and self-limited and resolved without treatment. No participants withdrew from the study.
Eiger Biopharmaceuticals is currently working with the U.S. Food and Drug Administration and the European Medicines Agency to design a phase 3 trial.
The study is an investor-initiated trial with funding from Eiger Biopharmaceuticals. Dr. Tan receives research support from Eiger Biopharmaceuticals as a site investigator. Dr. Van Name is an investigator for a trial sponsored by Provention Bio.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022















