User login
Lowering portal pressure boosts cirrhosis outcomes
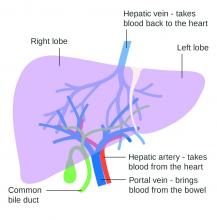
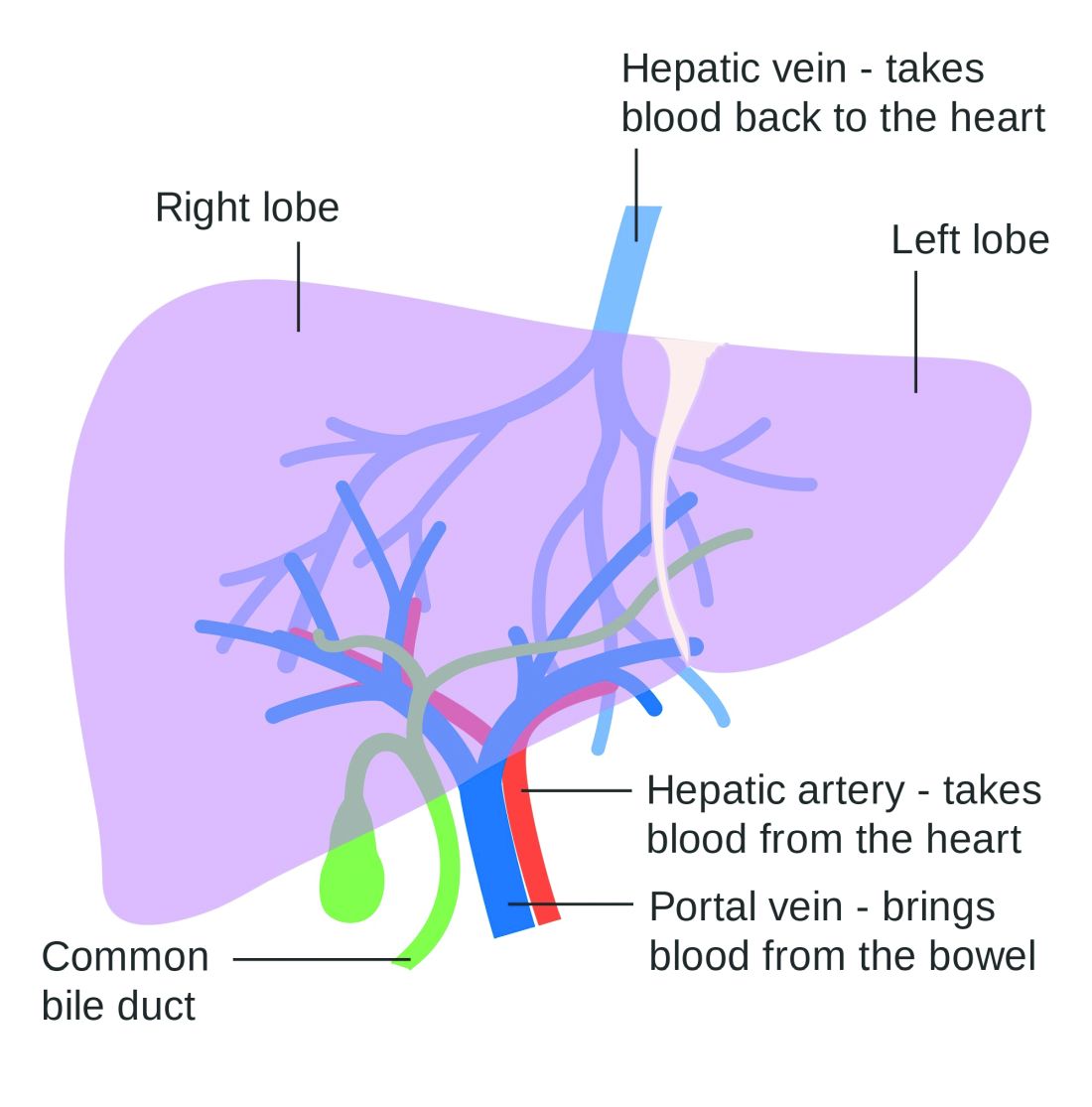
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
Use of nonselective beta-blockers to reduce portal pressure in cirrhosis improved outcomes in adults with or without ascites, based on data from a meta-analysis of more than 1,000 patients.
Previous research has suggested that nonselective beta-blockers (NSBBs) might have a negative effect on patients with refractory ascites, but the effect on patients with and without ascites has not been assessed, wrote Laura Turco, MD, of the University of Modena and Reggio Emilia, Emilia-Romagna, Italy, and colleagues.
In a study published in Clinical Gastroenterology and Hepatology, the researchers analyzed 1,113 cirrhosis patients including 452 with ascites. Overall, 968 patients had received treatment with NSBBs. Response to pressure reduction was defined as a decrease of more than 20% from baseline or a decrease to less than 12 mm Hg.
A total of 329 of the 661 patients without ascites (50%) met the definition as responders. These responders had significantly lower odds than did nonresponders of a combination of clinical events including ascites, variceal hemorrhage, or encephalopathy (odds ratio, 0.35) and also had significantly lower odds than nonresponders of liver transplantation or death (OR, 0.50).
A total of 188 of the 452 patients with ascites were responders (42%). These responders had significantly lower odds than those of nonresponders (OR, 0.27) of variceal hemorrhage, refractory ascites, spontaneous bacterial peritonitis, or hepatorenal syndrome. The responders also had significantly lower odds of liver transplantation or death (OR, 0.47).
The results are important in light of concerns about the impact of NSBBs on renal function and mortality in cirrhosis patients with ascites, the researchers said.
The study findings were limited by several factors, including the use of retrospective data from prospective studies, and the incomplete collection of data on the variables of comorbidities, hepatocellular carcinoma, and other predictive scores; alcohol use or abstinence was a potential confounder as well, the researchers noted.
However, “By showing that reductions in portal pressure induced by NSBB-based pharmacologic therapy improve outcomes and decrease mortality, our study supports the use of NSBB in all clinical settings (primary or secondary prophylaxis) and in both patients with or without ascites,” they concluded. They found no heterogeneity among the studies included in the analysis.
The study was supported by multiple sources including the University of Modena and Reggio Emilia, Yale Liver Center, National Institutes of Health, and Instituto de Salud Carlos III, and was cofunded by the European Union. The researchers had no financial conflicts to disclose.
SOURCE: Turco L et al. Clin Gastroenterol Hepatol. 2019 June 5. doi: 10.1016/j.cgh.2019.05.050.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Statins hamper hepatocellular carcinoma in viral hepatitis patients
Lipophilic statin therapy significantly reduced the incidence and mortality of hepatocellular carcinoma in adults with viral hepatitis, based on data from 16,668 patients.
The mortality rates for hepatocellular carcinoma in the United States and Europe have been on the rise for decades, and the risk may persist in severe cases despite the use of hepatitis B virus suppression or hepatitis C virus eradication, wrote Tracey G. Simon, MD, of Harvard Medical School, Boston, and colleagues. Previous studies suggest that statins might reduce HCC risk in viral hepatitis patients, but evidence supporting one type of statin over another for HCC prevention is limited, they said.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a national registry of hepatitis patients in Sweden to assess the effect of lipophilic or hydrophilic statin use on HCC incidence and mortality.
They found a significant reduction in 10-year HCC risk for lipophilic statin users, compared with nonusers (8.1% vs. 3.3%. However, the difference was not significant for hydrophilic statin users vs. nonusers (8.0% vs. 6.8%). The effect of lipophilic statin use was dose dependent; the largest effect on reduction in HCC risk occurred with 600 or more lipophilic statin cumulative daily doses in users, compared with nonusers (8.4% vs. 2.5%).
The study population included 6,554 lipophilic statin users and 1,780 hydrophilic statin users, matched with 8,334 nonusers. Patient demographics were similar between both types of statin user and nonuser groups.
In addition, 10-year mortality was significantly lower for lipophilic statin users compared with nonusers (15.2% vs. 7.3%) and also for hydrophilic statin users, compared with nonusers (16.0% vs. 11.5%).
In a small number of patients with liver disease (462), liver-specific mortality was significantly reduced in lipophilic statin users, compared with nonusers (adjusted hazard ratio, 0.76 vs. 0.98).
“Of note, our findings were robust across several sensitivity analyses and were similar in all predefined subgroups, including among men and women and persons with and without cirrhosis or antiviral therapy use,” the researchers noted.
The study findings were limited by several factors including the potential confounding from variables such as smoking, hepatitis B viral DNA, hepatitis C virus eradication, stage of fibrosis, and HCC screening, as well as a lack of laboratory data to assess cholesterol levels’ impact on statin use, the researchers said. In addition, the study did not compare lipophilic and hydrophilic statins.
However, the results suggest potential distinct benefits of lipophilic statins to reduce HCC risk and support the need for further research, the researchers concluded.
Dr. Simon had no financial conflicts to disclose, but disclosed support from a North American Training Grant from the American College of Gastroenterology. Several coauthors disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck Sharp & Dohme. The study was supported in part by the American College of Gastroenterology, the American Association for the Study of Liver Diseases, the Boston Nutrition Obesity Research Center, the National Institutes of Health, Nyckelfonden, Region Orebro (Sweden) County, and the Karolinska Institutet.
SOURCE: Simon TG et al. Ann Intern Med. 2019 Aug 19. doi: 10.7326/M18-2753.
Lipophilic statin therapy significantly reduced the incidence and mortality of hepatocellular carcinoma in adults with viral hepatitis, based on data from 16,668 patients.
The mortality rates for hepatocellular carcinoma in the United States and Europe have been on the rise for decades, and the risk may persist in severe cases despite the use of hepatitis B virus suppression or hepatitis C virus eradication, wrote Tracey G. Simon, MD, of Harvard Medical School, Boston, and colleagues. Previous studies suggest that statins might reduce HCC risk in viral hepatitis patients, but evidence supporting one type of statin over another for HCC prevention is limited, they said.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a national registry of hepatitis patients in Sweden to assess the effect of lipophilic or hydrophilic statin use on HCC incidence and mortality.
They found a significant reduction in 10-year HCC risk for lipophilic statin users, compared with nonusers (8.1% vs. 3.3%. However, the difference was not significant for hydrophilic statin users vs. nonusers (8.0% vs. 6.8%). The effect of lipophilic statin use was dose dependent; the largest effect on reduction in HCC risk occurred with 600 or more lipophilic statin cumulative daily doses in users, compared with nonusers (8.4% vs. 2.5%).
The study population included 6,554 lipophilic statin users and 1,780 hydrophilic statin users, matched with 8,334 nonusers. Patient demographics were similar between both types of statin user and nonuser groups.
In addition, 10-year mortality was significantly lower for lipophilic statin users compared with nonusers (15.2% vs. 7.3%) and also for hydrophilic statin users, compared with nonusers (16.0% vs. 11.5%).
In a small number of patients with liver disease (462), liver-specific mortality was significantly reduced in lipophilic statin users, compared with nonusers (adjusted hazard ratio, 0.76 vs. 0.98).
“Of note, our findings were robust across several sensitivity analyses and were similar in all predefined subgroups, including among men and women and persons with and without cirrhosis or antiviral therapy use,” the researchers noted.
The study findings were limited by several factors including the potential confounding from variables such as smoking, hepatitis B viral DNA, hepatitis C virus eradication, stage of fibrosis, and HCC screening, as well as a lack of laboratory data to assess cholesterol levels’ impact on statin use, the researchers said. In addition, the study did not compare lipophilic and hydrophilic statins.
However, the results suggest potential distinct benefits of lipophilic statins to reduce HCC risk and support the need for further research, the researchers concluded.
Dr. Simon had no financial conflicts to disclose, but disclosed support from a North American Training Grant from the American College of Gastroenterology. Several coauthors disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck Sharp & Dohme. The study was supported in part by the American College of Gastroenterology, the American Association for the Study of Liver Diseases, the Boston Nutrition Obesity Research Center, the National Institutes of Health, Nyckelfonden, Region Orebro (Sweden) County, and the Karolinska Institutet.
SOURCE: Simon TG et al. Ann Intern Med. 2019 Aug 19. doi: 10.7326/M18-2753.
Lipophilic statin therapy significantly reduced the incidence and mortality of hepatocellular carcinoma in adults with viral hepatitis, based on data from 16,668 patients.
The mortality rates for hepatocellular carcinoma in the United States and Europe have been on the rise for decades, and the risk may persist in severe cases despite the use of hepatitis B virus suppression or hepatitis C virus eradication, wrote Tracey G. Simon, MD, of Harvard Medical School, Boston, and colleagues. Previous studies suggest that statins might reduce HCC risk in viral hepatitis patients, but evidence supporting one type of statin over another for HCC prevention is limited, they said.
In a study published in the Annals of Internal Medicine, the researchers reviewed data from a national registry of hepatitis patients in Sweden to assess the effect of lipophilic or hydrophilic statin use on HCC incidence and mortality.
They found a significant reduction in 10-year HCC risk for lipophilic statin users, compared with nonusers (8.1% vs. 3.3%. However, the difference was not significant for hydrophilic statin users vs. nonusers (8.0% vs. 6.8%). The effect of lipophilic statin use was dose dependent; the largest effect on reduction in HCC risk occurred with 600 or more lipophilic statin cumulative daily doses in users, compared with nonusers (8.4% vs. 2.5%).
The study population included 6,554 lipophilic statin users and 1,780 hydrophilic statin users, matched with 8,334 nonusers. Patient demographics were similar between both types of statin user and nonuser groups.
In addition, 10-year mortality was significantly lower for lipophilic statin users compared with nonusers (15.2% vs. 7.3%) and also for hydrophilic statin users, compared with nonusers (16.0% vs. 11.5%).
In a small number of patients with liver disease (462), liver-specific mortality was significantly reduced in lipophilic statin users, compared with nonusers (adjusted hazard ratio, 0.76 vs. 0.98).
“Of note, our findings were robust across several sensitivity analyses and were similar in all predefined subgroups, including among men and women and persons with and without cirrhosis or antiviral therapy use,” the researchers noted.
The study findings were limited by several factors including the potential confounding from variables such as smoking, hepatitis B viral DNA, hepatitis C virus eradication, stage of fibrosis, and HCC screening, as well as a lack of laboratory data to assess cholesterol levels’ impact on statin use, the researchers said. In addition, the study did not compare lipophilic and hydrophilic statins.
However, the results suggest potential distinct benefits of lipophilic statins to reduce HCC risk and support the need for further research, the researchers concluded.
Dr. Simon had no financial conflicts to disclose, but disclosed support from a North American Training Grant from the American College of Gastroenterology. Several coauthors disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck Sharp & Dohme. The study was supported in part by the American College of Gastroenterology, the American Association for the Study of Liver Diseases, the Boston Nutrition Obesity Research Center, the National Institutes of Health, Nyckelfonden, Region Orebro (Sweden) County, and the Karolinska Institutet.
SOURCE: Simon TG et al. Ann Intern Med. 2019 Aug 19. doi: 10.7326/M18-2753.
FROM THE ANNALS OF INTERNAL MEDICINE
Key clinical point: Use of lipophilic statins significantly reduced incidence and mortality of hepatocellular cancer in adults with viral hepatitis.
Major finding: The 10-year risk of HCC was 8.1% among patients taking lipophilic statins, compared with 3.3% among those not on statins.
Study details: The data come from a population-based cohort study of 16,668 adult with viral hepatitis from a national registry in Sweden.
Disclosures: Dr. Simon had no financial conflicts to disclose, but disclosed support from a North American Training Grant from the American College of Gastroenterology. Several coauthors disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and MSD.
Source: Simon TG et al. Ann Intern Med. 2019 Aug 19. doi: 10.7326/M18-2753.
The postgraduate course on liver, pancreas, and biliary tract
The course was framed with the theme, “The Practice of Gastroenterology: The Literature and the Art,” with each speaker highlighting not only the relevant updates in the literature, but also sharing the insights into the art of medical practice. The course incorporated an audience response system to fully utilize the available educational technology and increase participant engagement.
Manal Abdelmalek, MD, provided an update on the hot topic of nonalcoholic fatty liver disease, including new developments in pharmacotherapy. The AGA President-elect, Hashem El-Serag, MD, MPH, AGAF, delivered a state-of-the-art presentation on the burgeoning burden of hepatocellular carcinoma and cutting-edge multidisciplinary management. Vijay Shah, MD, then reminded us of the persistent presence of alcoholic liver disease in the United States and the controversies surrounding liver transplantation in this setting. Steven Flamm, MD, completed the liver session by sharing the secrets of managing the complications of cirrhosis.
The second session, on the pancreas and biliary tract, was headed by Timothy Gardner, MD, who shared the pearls of the management of pancreatitis. Michelle Kim, MD, provided fresh and up-to-date insights on the management of pancreatic and biliary cancer, including updated technological options. Finally, Marcia Canto, MD, discussed the hot topic of pancreatic cancer and whether screening should be instituted. Both of these sessions had designated time set aside for panel discussions with questions from the audience.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Ahn, MD, MS, MBA, is professor of medicine and director of clinical hepatology at Oregon Health & Science University, Portland. He has no relevant conflicts of interest.
The course was framed with the theme, “The Practice of Gastroenterology: The Literature and the Art,” with each speaker highlighting not only the relevant updates in the literature, but also sharing the insights into the art of medical practice. The course incorporated an audience response system to fully utilize the available educational technology and increase participant engagement.
Manal Abdelmalek, MD, provided an update on the hot topic of nonalcoholic fatty liver disease, including new developments in pharmacotherapy. The AGA President-elect, Hashem El-Serag, MD, MPH, AGAF, delivered a state-of-the-art presentation on the burgeoning burden of hepatocellular carcinoma and cutting-edge multidisciplinary management. Vijay Shah, MD, then reminded us of the persistent presence of alcoholic liver disease in the United States and the controversies surrounding liver transplantation in this setting. Steven Flamm, MD, completed the liver session by sharing the secrets of managing the complications of cirrhosis.
The second session, on the pancreas and biliary tract, was headed by Timothy Gardner, MD, who shared the pearls of the management of pancreatitis. Michelle Kim, MD, provided fresh and up-to-date insights on the management of pancreatic and biliary cancer, including updated technological options. Finally, Marcia Canto, MD, discussed the hot topic of pancreatic cancer and whether screening should be instituted. Both of these sessions had designated time set aside for panel discussions with questions from the audience.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Ahn, MD, MS, MBA, is professor of medicine and director of clinical hepatology at Oregon Health & Science University, Portland. He has no relevant conflicts of interest.
The course was framed with the theme, “The Practice of Gastroenterology: The Literature and the Art,” with each speaker highlighting not only the relevant updates in the literature, but also sharing the insights into the art of medical practice. The course incorporated an audience response system to fully utilize the available educational technology and increase participant engagement.
Manal Abdelmalek, MD, provided an update on the hot topic of nonalcoholic fatty liver disease, including new developments in pharmacotherapy. The AGA President-elect, Hashem El-Serag, MD, MPH, AGAF, delivered a state-of-the-art presentation on the burgeoning burden of hepatocellular carcinoma and cutting-edge multidisciplinary management. Vijay Shah, MD, then reminded us of the persistent presence of alcoholic liver disease in the United States and the controversies surrounding liver transplantation in this setting. Steven Flamm, MD, completed the liver session by sharing the secrets of managing the complications of cirrhosis.
The second session, on the pancreas and biliary tract, was headed by Timothy Gardner, MD, who shared the pearls of the management of pancreatitis. Michelle Kim, MD, provided fresh and up-to-date insights on the management of pancreatic and biliary cancer, including updated technological options. Finally, Marcia Canto, MD, discussed the hot topic of pancreatic cancer and whether screening should be instituted. Both of these sessions had designated time set aside for panel discussions with questions from the audience.
This is a summary provided by the moderator of one of the AGA Postgraduate Course sessions held at DDW 2019. Dr. Ahn, MD, MS, MBA, is professor of medicine and director of clinical hepatology at Oregon Health & Science University, Portland. He has no relevant conflicts of interest.
Inflammation diminishes quality of life in NAFLD, not fibrosis
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
A variety of demographic and disease-related factors contribute to poorer quality of life in patients with nonalcoholic fatty liver disease (NAFLD), based on questionnaires involving 304 European patients.
In contrast with previous research, lobular inflammation, but not hepatic fibrosis, was associated with worse quality of life, reported to lead author Yvonne Huber, MD, of Johannes Gutenberg University in Mainz, Germany, and colleagues. Women and those with advanced disease or comorbidities had the lowest health-related quality of life (HRQL) scores. The investigators suggested that these findings could be used for treatment planning at a population and patient level.
“With the emergence of medical therapy for [nonalcoholic steatohepatitis (NASH)], it will be of importance to identify patients with the highest unmet need for treatment,” the investigators wrote in Clinical Gastroenterology and Hepatology, emphasizing that therapies targeting inflammation could provide the greatest relief.
To determine which patients with NAFLD were most affected by their condition, the investigators used the Chronic Liver Disease Questionnaire (CLDQ), which assesses physical, mental, social, and emotional function, with lower scores indicating poorer health-related quality of life. “[The CLDQ] more specifically addresses symptoms of patients with chronic liver disease, including extrahepatic manifestations, compared with traditional HRQL measures such as the [Short Form–36 (SF-36)] Health Survey Questionnaire,” the investigators explained. Recent research has used the CLDQ to reveal a variety of findings, the investigators noted, such as a 2016 study by Alt and colleagues outlining the most common symptoms in noninfectious chronic liver disease (abdominal discomfort, fatigue, and anxiety), and two studies by Younossi and colleagues describing quality of life improvements after curing hepatitis C virus, and negative impacts of viremia and hepatic inflammation in patients with hepatitis B.
The current study involved 304 patients with histologically confirmed NAFLD who were prospectively entered into the European NAFLD registry via centers in Germany (n = 133), the United Kingdom (n = 154), and Spain (n = 17). Patient data included demographic factors, laboratory findings, and histologic features. Within 6 months of liver biopsy, patients completed the CLDQ.
The mean patient age was 52.3 years, with slightly more men than women (53.3% vs. 46.7%). Most patients (75%) were obese, leading to a median body mass index of 33.3 kg/m2. More than two-thirds of patients (69.1%) had NASH, while approximately half of the population (51.4%) had moderate steatosis, no or low-grade fibrosis (F0-2, 58.2%), and no or low-grade lobular inflammation (grade 0 or 1, 54.7%). The three countries had significantly different population profiles; for example, the United Kingdom had an approximately 10% higher prevalence of type 2 diabetes and obesity compared with the entire cohort, but a decreased arterial hypertension rate of a similar magnitude. The United Kingdom also had a significantly lower mean CLDQ score than that of the study population as a whole (4.73 vs. 4.99).
Analysis of the entire cohort revealed that a variety of demographic and disease-related factors negatively impacted health-related quality of life. Women had a significantly lower mean CLDQ score than that of men (5.31 vs. 4.62; P less than .001), more often reporting abdominal symptoms, fatigue, systemic symptoms, reduced activity, diminished emotional functioning, and worry. CLDQ overall score was negatively influenced by obesity (4.83 vs. 5.46), type 2 diabetes (4.74 vs. 5.25), and hyperlipidemia (4.84 vs. 5.24), but not hypertension. Laboratory findings that negatively correlated with CLDQ included aspartate transaminase (AST) and HbA1c, whereas ferritin was positively correlated.
Generally, patients with NASH reported worse quality of life than that of those with just NAFLD (4.85 vs. 5.31). Factors contributing most to this disparity were fatigue, systemic symptoms, activity, and worry. On a histologic level, hepatic steatosis, ballooning, and lobular inflammation predicted poorer quality of life; although advanced fibrosis and compensated cirrhosis were associated with a trend toward reduced quality of life, this pattern lacked statistical significance. Multivariate analysis, which accounted for age, sex, body mass index, country, and type 2 diabetes, revealed independent associations between reduced quality of life and type 2 diabetes, sex, age, body mass index, and hepatic inflammation, but not fibrosis.
“The striking finding of the current analysis in this well-characterized European cohort was that, in contrast to the published data on predictors of overall and liver-specific mortality, lobular inflammation correlated independently with HRQL,” the investigators wrote. “These results differ from the NASH [Clinical Research Network] cohort, which found lower HRQL using the generic [SF-36 Health Survey Questionnaire] in NASH compared with a healthy U.S. population and a significant effect in cirrhosis only.” The investigators suggested that mechanistic differences in disease progression could explain this discordance.
Although hepatic fibrosis has been tied with quality of life by some studies, the investigators pointed out that patients with chronic hepatitis B or C have reported improved quality of life after viral elimination or suppression, which reduce inflammation, but not fibrosis. “On the basis of the current analysis, it can be expected that improvement of steatohepatitis, and in particular lobular inflammation, will have measurable influence on HRQL even independently of fibrosis improvement,” the investigators concluded.
The study was funded by H2020. The investigators reported no conflicts of interest.
SOURCE: Huber Y et al. CGH. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.016.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Spleen/liver stiffness ratio differentiates HCV, ALD
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
The spleen stiffness (SS) to liver stiffness (LS) ratio was significantly higher in patients with hepatitis C virus infection (HCV) than in patients with alcohol-related liver disease (ALD), according to the results of a multicenter prospective study. In addition, long-term outcome and complications differed dramatically between HCV and ALD. Variceal bleeding was the most common sign of decompensation and cause of death in patients with HCV, while jaundice was the most common sign of decompensation in patients with ALD.
Omar Elshaarawy, MSc, of the University of Heidelberg (Germany) and colleagues reported on their prospective study of 411 patients with HCV (220 patients) or ALD (191 patients) that were assessed for both LS and SS using the Fibroscan device. They also discussed their retrospective analysis of LS and spleen length (SL) from a separate, retrospective cohort of 449 patients (267 with HCV, 182 with ALD) for whom long-term data on decompensation/death were available.
The researchers found that SS was significantly higher in HCV patients, compared with those with ALD (42.0 vs. 32.6 kPa; P less than .0001), as was SL (15.6 vs. 11.9 cm, P less than .0001); this was despite a lower mean LS in HCV. As a result, the SS/LS ratio and the SL/LS ratio were both significantly higher in HCV (3.8 vs. 1.72 and 1.46 vs. 0.86, P less than .0001) through all fibrosis stages.
They also found that patients with ALD had higher LS values (30.5 vs. 21.3 kPa) and predominantly presented with jaundice (65.2%), with liver failure as the major cause of death (P less than .01). In contrast, in HCV, spleens were larger (17.6 vs. 12.1 cm) while variceal bleeding was the major cause of decompensation (73.2%) and death (P less than .001).
“We have demonstrated the disease-specific differences in SS/LS and SL/LS ratio between HCV and ALD. They underscore the role of the intrahepatic histological site of inflammation/fibrosis. We suggest that the SS/LS ratio could be used to confirm the disease etiology and predict disease-specific complications,” the researchers concluded.
The study was supported by the Dietmar Hopp Foundation. The authors reported they had no conflicts.
SOURCE: Elshaarawy O et al. J Hepatol Reports. 2019 Jun 20. doi: 10.1016/j.jhepr.2019.05.003.
FROM JHEP REPORTS
USPSTF updates, reaffirms recommendation for HBV screening in pregnant women
The according to task force member Douglas K. Owens, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System and other members of the task force.
The recommendation statement, published in JAMA, was based on an evidence report and systematic review also published in JAMA. In that review, two studies of fair quality were identified; one included 155,081 infants born to HBV-positive women identified for case management through the national Perinatal Hepatitis B Prevention Program from 1994 to 2008, and the other included 4,446 infants born in a large, regional health care organization in the United States between 1997 and 2010. In both, low rates of perinatal transmission were reported for those periods – between 0.5% and 1.9% – with the rate falling over time.
In the 2009 recommendation, the USPSTF found adequate evidence that serologic testing for hepatitis B surface antigen accurately identifies HBV infection, and that interventions were effective in preventing perinatal transmission. That recommendation has been reaffirmed in the current update, with HBV screening receiving a grade of A, which is the strongest the USPSTF offers.
In a related editorial, Neil S. Silverman, MD, of the Center for Fetal Medicine and Women’s Ultrasound in Los Angeles noted several improvements in maternal HBV therapy since the publication of the original 2009 recommendation, including maternal HBV-targeted antiviral therapy during pregnancy as an adjunct to neonatal immunoprophylaxis and the ability to refer women for chronic treatment of their HBV disease to prevent long-term infection complications. The task forces also noted that HBV screening of all pregnant women is mandated by law in 26 states.
One member of the task force reported receiving grants and/or personal fees from Healthwise, another member reported receiving personal fees from UpToDate; a third reported participating in the American Association for the Study of Liver Diseases’ Hepatitis B Guidance and Hepatitis B Systematic Review Group. The evidence and review study was funded by the Agency for Healthcare Research and Quality. Dr. Silverman reported no disclosures.
SOURCes: Owens DK et al. JAMA. 2019 Jul 23. doi: 10.1001/jama.2019.9365; Henderson JT et al. JAMA. 2019 Jul 23;32(4):360-2; Silverman NS. JAMA. 2019 Jul 23;32(4):312-14.
The according to task force member Douglas K. Owens, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System and other members of the task force.
The recommendation statement, published in JAMA, was based on an evidence report and systematic review also published in JAMA. In that review, two studies of fair quality were identified; one included 155,081 infants born to HBV-positive women identified for case management through the national Perinatal Hepatitis B Prevention Program from 1994 to 2008, and the other included 4,446 infants born in a large, regional health care organization in the United States between 1997 and 2010. In both, low rates of perinatal transmission were reported for those periods – between 0.5% and 1.9% – with the rate falling over time.
In the 2009 recommendation, the USPSTF found adequate evidence that serologic testing for hepatitis B surface antigen accurately identifies HBV infection, and that interventions were effective in preventing perinatal transmission. That recommendation has been reaffirmed in the current update, with HBV screening receiving a grade of A, which is the strongest the USPSTF offers.
In a related editorial, Neil S. Silverman, MD, of the Center for Fetal Medicine and Women’s Ultrasound in Los Angeles noted several improvements in maternal HBV therapy since the publication of the original 2009 recommendation, including maternal HBV-targeted antiviral therapy during pregnancy as an adjunct to neonatal immunoprophylaxis and the ability to refer women for chronic treatment of their HBV disease to prevent long-term infection complications. The task forces also noted that HBV screening of all pregnant women is mandated by law in 26 states.
One member of the task force reported receiving grants and/or personal fees from Healthwise, another member reported receiving personal fees from UpToDate; a third reported participating in the American Association for the Study of Liver Diseases’ Hepatitis B Guidance and Hepatitis B Systematic Review Group. The evidence and review study was funded by the Agency for Healthcare Research and Quality. Dr. Silverman reported no disclosures.
SOURCes: Owens DK et al. JAMA. 2019 Jul 23. doi: 10.1001/jama.2019.9365; Henderson JT et al. JAMA. 2019 Jul 23;32(4):360-2; Silverman NS. JAMA. 2019 Jul 23;32(4):312-14.
The according to task force member Douglas K. Owens, MD, of the Veterans Affairs Palo Alto (Calif.) Health Care System and other members of the task force.
The recommendation statement, published in JAMA, was based on an evidence report and systematic review also published in JAMA. In that review, two studies of fair quality were identified; one included 155,081 infants born to HBV-positive women identified for case management through the national Perinatal Hepatitis B Prevention Program from 1994 to 2008, and the other included 4,446 infants born in a large, regional health care organization in the United States between 1997 and 2010. In both, low rates of perinatal transmission were reported for those periods – between 0.5% and 1.9% – with the rate falling over time.
In the 2009 recommendation, the USPSTF found adequate evidence that serologic testing for hepatitis B surface antigen accurately identifies HBV infection, and that interventions were effective in preventing perinatal transmission. That recommendation has been reaffirmed in the current update, with HBV screening receiving a grade of A, which is the strongest the USPSTF offers.
In a related editorial, Neil S. Silverman, MD, of the Center for Fetal Medicine and Women’s Ultrasound in Los Angeles noted several improvements in maternal HBV therapy since the publication of the original 2009 recommendation, including maternal HBV-targeted antiviral therapy during pregnancy as an adjunct to neonatal immunoprophylaxis and the ability to refer women for chronic treatment of their HBV disease to prevent long-term infection complications. The task forces also noted that HBV screening of all pregnant women is mandated by law in 26 states.
One member of the task force reported receiving grants and/or personal fees from Healthwise, another member reported receiving personal fees from UpToDate; a third reported participating in the American Association for the Study of Liver Diseases’ Hepatitis B Guidance and Hepatitis B Systematic Review Group. The evidence and review study was funded by the Agency for Healthcare Research and Quality. Dr. Silverman reported no disclosures.
SOURCes: Owens DK et al. JAMA. 2019 Jul 23. doi: 10.1001/jama.2019.9365; Henderson JT et al. JAMA. 2019 Jul 23;32(4):360-2; Silverman NS. JAMA. 2019 Jul 23;32(4):312-14.
FROM JAMA
Microbiome – Impact on health and disease
The gut microbiota influences our biology through our mucosal immune system as well as by leading to the production of bioactive small molecules. I’ll describe how gut microbiota influences colon cancer, liver disease, the production of bioactive compounds, as well as the current status and future prospects of microbiota therapeutics.
The gut microbiota may be a factor in colon cancer. Studies have shown that bacterial biofilms are associated with right-sided colon cancers in humans. More recently, a study has shown that mucosal biofilm formation is carcinogenic in an animal model, suggesting that such biofilms may play a role in the disease pathogenesis. From the standpoint of the liver, the microbiome may be a biomarker for diseases such as cirrhosis and fibrosis in patients with nonalcoholic steatohepatitis. Therapeutically, a recent study suggests that the function of gut microbiota can be altered by introducing an engineered Escherichia coli bacterial strain to treat hyperammonemia by modifying its metabolism to overproduce arginine, thereby sequestering ammonia produced by gut bacteria into the amino acids (Sci Transl Med. 2019 Jan 16;11[475]. doi: 10.1126/scitranslmed.aau7975). Drug metabolism also can be influenced by the gut microbiota and vice versa. For example, drugs such as metformin have effects on the composition of the gut microbiota in humans. In turn, the gut microbiota and its metabolites can have an influence on hepatic drug metabolism, thereby altering xenobiotic pharmacokinetics and pharmacodynamics.
The production of bioactive small molecules by bacterial metabolism is a topic of intense interest in the microbiome field. Such small molecules have been shown to act as antibiotics, neurotransmitters, immune modulators, and ligands for host receptors. Some of these small metabolites are generated through the dietary aromatic amino acids in which the bacterial enzymatic pathways are being elucidated. Such small molecules have a myriad of functions. For example, indole propionic acid, a bacterial metabolite of tryptophan, can activate the pregnane X receptor to fortify intestinal epithelial barrier function, a pathway that may have relevance to inflammatory bowel disease.
Probiotics that are found in dietary supplements represent our currently available strategy for the prevention and/or treatment of disease through the delivery of specific live microbes. However, there are limitations to their effectiveness since none have been approved for the prevention or treatment of any disease process. Via an intensive human subject study, (Cell. 2018 Sep 6;174[6]:1388-405) investigators have shown that the mucosally associated microbiota was a better biomarker for probiotic engraftment than stool was, where the response was very personalized. It’s possible that the personalized nature of probiotic engraftment may indicate that “one size may not fit all.” There will be a technical review and guideline document published by the American Gastroenterological Association early in 2020.
Currently, the only effective therapeutic modality for the treatment of a human disease by deeply altering the composition of the gut microbiota is the use of fecal microbiota transplantation (FMT) for the treatment of recurrent Clostridium difficile infection (CDI). However, there is now early evidence that FMT might have efficacy in the treatment of a disease other than recurrent CDI, namely ulcerative colitis. Although the short-term risks for FMT are low and quantifiable and long-term risks are largely hypothetical, there is a need for caution and regulation in the practice of FMT. Indeed, long-term engraftment of bacterial strains from the donor into the recipient has been demonstrated. Ultimately, as the science in the microbiota field moves forward together with product development, more sophisticated microbiota-based therapeutics will be generated. During this interim period, the AGA and partner national societies have developed an FMT National Registry to gather information on FMT practice, assess effectiveness as well as short- and long-term safety, and promote scientific investigation.
In conclusion, the field of gut microbiome research is very dynamic and exciting with tremendous opportunities at the intersection between fabulous science and technology, clinical practice, and federal regulation involving the practice of FMT, concurrent in a significant interest in intellectual property and business.
Dr. Wu is the Ferdinand G. Weisbrod Professor in Gastroenterology at the University of Pennsylvania, Philadelphia. He has received research funding from Seres Therapeutics, Intercept Pharmaceuticals, and Takeda; is on the scientific advisory board for Danone and Biocodex; and does consulting for Hitachi High-Technologies. Dr. Wu made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
The gut microbiota influences our biology through our mucosal immune system as well as by leading to the production of bioactive small molecules. I’ll describe how gut microbiota influences colon cancer, liver disease, the production of bioactive compounds, as well as the current status and future prospects of microbiota therapeutics.
The gut microbiota may be a factor in colon cancer. Studies have shown that bacterial biofilms are associated with right-sided colon cancers in humans. More recently, a study has shown that mucosal biofilm formation is carcinogenic in an animal model, suggesting that such biofilms may play a role in the disease pathogenesis. From the standpoint of the liver, the microbiome may be a biomarker for diseases such as cirrhosis and fibrosis in patients with nonalcoholic steatohepatitis. Therapeutically, a recent study suggests that the function of gut microbiota can be altered by introducing an engineered Escherichia coli bacterial strain to treat hyperammonemia by modifying its metabolism to overproduce arginine, thereby sequestering ammonia produced by gut bacteria into the amino acids (Sci Transl Med. 2019 Jan 16;11[475]. doi: 10.1126/scitranslmed.aau7975). Drug metabolism also can be influenced by the gut microbiota and vice versa. For example, drugs such as metformin have effects on the composition of the gut microbiota in humans. In turn, the gut microbiota and its metabolites can have an influence on hepatic drug metabolism, thereby altering xenobiotic pharmacokinetics and pharmacodynamics.
The production of bioactive small molecules by bacterial metabolism is a topic of intense interest in the microbiome field. Such small molecules have been shown to act as antibiotics, neurotransmitters, immune modulators, and ligands for host receptors. Some of these small metabolites are generated through the dietary aromatic amino acids in which the bacterial enzymatic pathways are being elucidated. Such small molecules have a myriad of functions. For example, indole propionic acid, a bacterial metabolite of tryptophan, can activate the pregnane X receptor to fortify intestinal epithelial barrier function, a pathway that may have relevance to inflammatory bowel disease.
Probiotics that are found in dietary supplements represent our currently available strategy for the prevention and/or treatment of disease through the delivery of specific live microbes. However, there are limitations to their effectiveness since none have been approved for the prevention or treatment of any disease process. Via an intensive human subject study, (Cell. 2018 Sep 6;174[6]:1388-405) investigators have shown that the mucosally associated microbiota was a better biomarker for probiotic engraftment than stool was, where the response was very personalized. It’s possible that the personalized nature of probiotic engraftment may indicate that “one size may not fit all.” There will be a technical review and guideline document published by the American Gastroenterological Association early in 2020.
Currently, the only effective therapeutic modality for the treatment of a human disease by deeply altering the composition of the gut microbiota is the use of fecal microbiota transplantation (FMT) for the treatment of recurrent Clostridium difficile infection (CDI). However, there is now early evidence that FMT might have efficacy in the treatment of a disease other than recurrent CDI, namely ulcerative colitis. Although the short-term risks for FMT are low and quantifiable and long-term risks are largely hypothetical, there is a need for caution and regulation in the practice of FMT. Indeed, long-term engraftment of bacterial strains from the donor into the recipient has been demonstrated. Ultimately, as the science in the microbiota field moves forward together with product development, more sophisticated microbiota-based therapeutics will be generated. During this interim period, the AGA and partner national societies have developed an FMT National Registry to gather information on FMT practice, assess effectiveness as well as short- and long-term safety, and promote scientific investigation.
In conclusion, the field of gut microbiome research is very dynamic and exciting with tremendous opportunities at the intersection between fabulous science and technology, clinical practice, and federal regulation involving the practice of FMT, concurrent in a significant interest in intellectual property and business.
Dr. Wu is the Ferdinand G. Weisbrod Professor in Gastroenterology at the University of Pennsylvania, Philadelphia. He has received research funding from Seres Therapeutics, Intercept Pharmaceuticals, and Takeda; is on the scientific advisory board for Danone and Biocodex; and does consulting for Hitachi High-Technologies. Dr. Wu made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
The gut microbiota influences our biology through our mucosal immune system as well as by leading to the production of bioactive small molecules. I’ll describe how gut microbiota influences colon cancer, liver disease, the production of bioactive compounds, as well as the current status and future prospects of microbiota therapeutics.
The gut microbiota may be a factor in colon cancer. Studies have shown that bacterial biofilms are associated with right-sided colon cancers in humans. More recently, a study has shown that mucosal biofilm formation is carcinogenic in an animal model, suggesting that such biofilms may play a role in the disease pathogenesis. From the standpoint of the liver, the microbiome may be a biomarker for diseases such as cirrhosis and fibrosis in patients with nonalcoholic steatohepatitis. Therapeutically, a recent study suggests that the function of gut microbiota can be altered by introducing an engineered Escherichia coli bacterial strain to treat hyperammonemia by modifying its metabolism to overproduce arginine, thereby sequestering ammonia produced by gut bacteria into the amino acids (Sci Transl Med. 2019 Jan 16;11[475]. doi: 10.1126/scitranslmed.aau7975). Drug metabolism also can be influenced by the gut microbiota and vice versa. For example, drugs such as metformin have effects on the composition of the gut microbiota in humans. In turn, the gut microbiota and its metabolites can have an influence on hepatic drug metabolism, thereby altering xenobiotic pharmacokinetics and pharmacodynamics.
The production of bioactive small molecules by bacterial metabolism is a topic of intense interest in the microbiome field. Such small molecules have been shown to act as antibiotics, neurotransmitters, immune modulators, and ligands for host receptors. Some of these small metabolites are generated through the dietary aromatic amino acids in which the bacterial enzymatic pathways are being elucidated. Such small molecules have a myriad of functions. For example, indole propionic acid, a bacterial metabolite of tryptophan, can activate the pregnane X receptor to fortify intestinal epithelial barrier function, a pathway that may have relevance to inflammatory bowel disease.
Probiotics that are found in dietary supplements represent our currently available strategy for the prevention and/or treatment of disease through the delivery of specific live microbes. However, there are limitations to their effectiveness since none have been approved for the prevention or treatment of any disease process. Via an intensive human subject study, (Cell. 2018 Sep 6;174[6]:1388-405) investigators have shown that the mucosally associated microbiota was a better biomarker for probiotic engraftment than stool was, where the response was very personalized. It’s possible that the personalized nature of probiotic engraftment may indicate that “one size may not fit all.” There will be a technical review and guideline document published by the American Gastroenterological Association early in 2020.
Currently, the only effective therapeutic modality for the treatment of a human disease by deeply altering the composition of the gut microbiota is the use of fecal microbiota transplantation (FMT) for the treatment of recurrent Clostridium difficile infection (CDI). However, there is now early evidence that FMT might have efficacy in the treatment of a disease other than recurrent CDI, namely ulcerative colitis. Although the short-term risks for FMT are low and quantifiable and long-term risks are largely hypothetical, there is a need for caution and regulation in the practice of FMT. Indeed, long-term engraftment of bacterial strains from the donor into the recipient has been demonstrated. Ultimately, as the science in the microbiota field moves forward together with product development, more sophisticated microbiota-based therapeutics will be generated. During this interim period, the AGA and partner national societies have developed an FMT National Registry to gather information on FMT practice, assess effectiveness as well as short- and long-term safety, and promote scientific investigation.
In conclusion, the field of gut microbiome research is very dynamic and exciting with tremendous opportunities at the intersection between fabulous science and technology, clinical practice, and federal regulation involving the practice of FMT, concurrent in a significant interest in intellectual property and business.
Dr. Wu is the Ferdinand G. Weisbrod Professor in Gastroenterology at the University of Pennsylvania, Philadelphia. He has received research funding from Seres Therapeutics, Intercept Pharmaceuticals, and Takeda; is on the scientific advisory board for Danone and Biocodex; and does consulting for Hitachi High-Technologies. Dr. Wu made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
The changing epidemiology of hepatocellular carcinoma
Three main changes characterize the secular trends in the incidence of hepatocellular carcinoma (HCC) in the United States. First, the overall incidence and mortality rates of HCC have been rising for the past 3 decades. Second, Hispanics are disproportionately affected by the HCC increase and have recently surpassed Asian Americans as the racial/ethnic group at highest HCC risk. Third, Southern and Western states have registered higher incidence rates of HCC than did the rest of the country, with Texas having the highest rates.
There are significant racial/ethnic differences in the population distribution of HCC risk factors, notably the disproportionately high prevalence of metabolic syndrome (e.g., obesity, abdominal obesity, and diabetes) and nonalcoholic fatty liver disease (NAFLD) in Hispanics. This observation may explain some of the findings in the secular trends of HCC described above. Most, but not all, studies have reported modest increases in relative risk of HCC in persons with obesity as measured by body mass index. However, studies investigating more specific obesity measures such as obesity in early adulthood or abdominal obesity reported higher and more consistent HCC risk than did those using body mass index. Hispanics have been shown to have a higher proportion of abdominal, especially visceral fat, than African Americans. The prevalence of NAFLD in the United States has doubled over the last 2 decades, and is estimated to affect 15%-20% of adults overall, but up to 30% in adult Texas Hispanics. Recently, a large cohort study including 296,707 patients with NAFLD and an equal number of matched controls without NAFLD from 130 facilities of the Department of Veterans Affairs found that patients with NAFLD had several-fold higher HCC risk than controls. The study also reported that HCC incidence rates for patients with NAFLD ranged from 1.6 to 23.7 per 1000 person-years, with the highest risk among older Hispanic patients with cirrhosis. Approximately 20% of patients with NAFLD and HCC had no evidence of cirrhosis. Lastly, type 2 diabetes, a condition that also is disproportionately higher in Hispanics than in other racial/ethnic groups in the United States has been consistently associated with an approximately twofold increase in the risk of HCC.
Risk factors for cirrhosis and HCC in contemporary clinical practice, and to a lesser extent, in the general population have shifted from active viral hepatitis to resolved hepatitis C infection or adequately suppressed hepatitis B infection as well as alcoholic liver disease and NAFLD. The shift from uncommon risk factors that carry a considerable increased risk of cirrhosis and HCC (active hepatitis C virus, hepatitis B virus) to more common but weaker risk factors (alcohol, NAFLD) is likely to result in a larger pool of chronic liver disease patients at risk for developing cirrhosis and HCC. However, given that the relative risk of HCC is lower with these emerging risk factors, it also will become increasingly difficult to define the highest-risk groups in need of interventions or monitoring. Therefore, there is a clear need for risk-stratification tools for cirrhosis and HCC in patients with HCV and a sustained virologic response, adequate HBV suppression, alcoholic liver disease, and NAFLD.
Dr. El-Serag is with the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. Dr. El-Serag made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
Three main changes characterize the secular trends in the incidence of hepatocellular carcinoma (HCC) in the United States. First, the overall incidence and mortality rates of HCC have been rising for the past 3 decades. Second, Hispanics are disproportionately affected by the HCC increase and have recently surpassed Asian Americans as the racial/ethnic group at highest HCC risk. Third, Southern and Western states have registered higher incidence rates of HCC than did the rest of the country, with Texas having the highest rates.
There are significant racial/ethnic differences in the population distribution of HCC risk factors, notably the disproportionately high prevalence of metabolic syndrome (e.g., obesity, abdominal obesity, and diabetes) and nonalcoholic fatty liver disease (NAFLD) in Hispanics. This observation may explain some of the findings in the secular trends of HCC described above. Most, but not all, studies have reported modest increases in relative risk of HCC in persons with obesity as measured by body mass index. However, studies investigating more specific obesity measures such as obesity in early adulthood or abdominal obesity reported higher and more consistent HCC risk than did those using body mass index. Hispanics have been shown to have a higher proportion of abdominal, especially visceral fat, than African Americans. The prevalence of NAFLD in the United States has doubled over the last 2 decades, and is estimated to affect 15%-20% of adults overall, but up to 30% in adult Texas Hispanics. Recently, a large cohort study including 296,707 patients with NAFLD and an equal number of matched controls without NAFLD from 130 facilities of the Department of Veterans Affairs found that patients with NAFLD had several-fold higher HCC risk than controls. The study also reported that HCC incidence rates for patients with NAFLD ranged from 1.6 to 23.7 per 1000 person-years, with the highest risk among older Hispanic patients with cirrhosis. Approximately 20% of patients with NAFLD and HCC had no evidence of cirrhosis. Lastly, type 2 diabetes, a condition that also is disproportionately higher in Hispanics than in other racial/ethnic groups in the United States has been consistently associated with an approximately twofold increase in the risk of HCC.
Risk factors for cirrhosis and HCC in contemporary clinical practice, and to a lesser extent, in the general population have shifted from active viral hepatitis to resolved hepatitis C infection or adequately suppressed hepatitis B infection as well as alcoholic liver disease and NAFLD. The shift from uncommon risk factors that carry a considerable increased risk of cirrhosis and HCC (active hepatitis C virus, hepatitis B virus) to more common but weaker risk factors (alcohol, NAFLD) is likely to result in a larger pool of chronic liver disease patients at risk for developing cirrhosis and HCC. However, given that the relative risk of HCC is lower with these emerging risk factors, it also will become increasingly difficult to define the highest-risk groups in need of interventions or monitoring. Therefore, there is a clear need for risk-stratification tools for cirrhosis and HCC in patients with HCV and a sustained virologic response, adequate HBV suppression, alcoholic liver disease, and NAFLD.
Dr. El-Serag is with the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. Dr. El-Serag made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
Three main changes characterize the secular trends in the incidence of hepatocellular carcinoma (HCC) in the United States. First, the overall incidence and mortality rates of HCC have been rising for the past 3 decades. Second, Hispanics are disproportionately affected by the HCC increase and have recently surpassed Asian Americans as the racial/ethnic group at highest HCC risk. Third, Southern and Western states have registered higher incidence rates of HCC than did the rest of the country, with Texas having the highest rates.
There are significant racial/ethnic differences in the population distribution of HCC risk factors, notably the disproportionately high prevalence of metabolic syndrome (e.g., obesity, abdominal obesity, and diabetes) and nonalcoholic fatty liver disease (NAFLD) in Hispanics. This observation may explain some of the findings in the secular trends of HCC described above. Most, but not all, studies have reported modest increases in relative risk of HCC in persons with obesity as measured by body mass index. However, studies investigating more specific obesity measures such as obesity in early adulthood or abdominal obesity reported higher and more consistent HCC risk than did those using body mass index. Hispanics have been shown to have a higher proportion of abdominal, especially visceral fat, than African Americans. The prevalence of NAFLD in the United States has doubled over the last 2 decades, and is estimated to affect 15%-20% of adults overall, but up to 30% in adult Texas Hispanics. Recently, a large cohort study including 296,707 patients with NAFLD and an equal number of matched controls without NAFLD from 130 facilities of the Department of Veterans Affairs found that patients with NAFLD had several-fold higher HCC risk than controls. The study also reported that HCC incidence rates for patients with NAFLD ranged from 1.6 to 23.7 per 1000 person-years, with the highest risk among older Hispanic patients with cirrhosis. Approximately 20% of patients with NAFLD and HCC had no evidence of cirrhosis. Lastly, type 2 diabetes, a condition that also is disproportionately higher in Hispanics than in other racial/ethnic groups in the United States has been consistently associated with an approximately twofold increase in the risk of HCC.
Risk factors for cirrhosis and HCC in contemporary clinical practice, and to a lesser extent, in the general population have shifted from active viral hepatitis to resolved hepatitis C infection or adequately suppressed hepatitis B infection as well as alcoholic liver disease and NAFLD. The shift from uncommon risk factors that carry a considerable increased risk of cirrhosis and HCC (active hepatitis C virus, hepatitis B virus) to more common but weaker risk factors (alcohol, NAFLD) is likely to result in a larger pool of chronic liver disease patients at risk for developing cirrhosis and HCC. However, given that the relative risk of HCC is lower with these emerging risk factors, it also will become increasingly difficult to define the highest-risk groups in need of interventions or monitoring. Therefore, there is a clear need for risk-stratification tools for cirrhosis and HCC in patients with HCV and a sustained virologic response, adequate HBV suppression, alcoholic liver disease, and NAFLD.
Dr. El-Serag is with the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. Dr. El-Serag made these comments during the AGA Institute Presidential Plenary at the annual Digestive Disease Week®.
Louisiana HCV program cuts costs – and hassles
Beginning July 15, physicians will no longer have to seek prior authorization or preauthorization to prescribe the authorized generic version of Epclusa (sofosbuvir/velpatasvir) to any Medicaid patient with hepatitis C. There will be no forms to file.
The change comes as part of a supplemental rebate agreement approved June 26 by CMS. That same day, Louisiana announced a deal with Asegua Therapeutics, a wholly owned subsidiary of Epclusa maker Gilead, that essentially caps the annual cost to the state for treating hepatitis C in incarcerated patients and Medicaid recipients.
State officials estimate about 39,000 Louisianans fit those criteria; the goal of the program is to cure at least 31,000 of them by the time the 5-year agreement expires.
“This new model has the potential to save many lives and improve the health of our citizens,” Louisiana Gov. John Bel Edwards (D) said in a statement. “Asegua was willing to come to the table to work with us to help Louisiana residents and we are pleased to initiate this 5-year partnership. Ultimately our goal is to eliminate this disease in Louisiana, and we have taken a big step forward in that effort.”
The agreement was designed to change very little in terms of the mechanics of how Medicaid managed care organizations, which cover most of the state’s Medicaid population and handle coverage and claims. The biggest change is that, when a spending cap is reached, Asegua will rebate 100% excess costs to the state. Louisiana officials did not disclose what the annual financial caps were as part of the agreement.
“We really thought it was important to leave the system – as much as possible – intact because we think that is going to make us most successful,” Alex Billioux, MD, of the Louisiana Department of Health said in an interview. “We think it leverages existing patient relationships and existing [Medicaid managed care organization] care management responsibilities.”
He added that, by keeping current processes unchanged, “it takes what is an otherwise very complicated arrangement with the state and makes it a little simpler.”
Patients will see no change in terms of copayments for the approved generic topping out at $3 depending on income level as they would have prior to the agreement. The biggest difference for them is that “people who couldn’t be treated are now going to have access to those prescriptions,” Dr. Billioux said.
Some cautious optimism surrounds this kind of arrangement and the potential effect it can have on the affected population.
“Innovation geared to improve access to hepatitis C treatment is critical, particularly in areas like Louisiana where treatment rates for Medicaid patients have been very low,” Robert Brown, MD, member of the American Liver Foundation’s National Medical Advisory Committee and hepatologist at Weill Cornell Medical College, New York, said. “If we can enhance patient access to treatment, we know we will improve health outcomes. However, it is too early to tell if this innovation will be a success. At the end of the day, the number of additional patients cured will determine if this was the right approach.”
Visit the AGA GI Patient Center for information to share with your patients about hepatitis C at https://www.gastro.org/practice-guidance/gi-patient-center/topic/hepatitis-c-hcv
Beginning July 15, physicians will no longer have to seek prior authorization or preauthorization to prescribe the authorized generic version of Epclusa (sofosbuvir/velpatasvir) to any Medicaid patient with hepatitis C. There will be no forms to file.
The change comes as part of a supplemental rebate agreement approved June 26 by CMS. That same day, Louisiana announced a deal with Asegua Therapeutics, a wholly owned subsidiary of Epclusa maker Gilead, that essentially caps the annual cost to the state for treating hepatitis C in incarcerated patients and Medicaid recipients.
State officials estimate about 39,000 Louisianans fit those criteria; the goal of the program is to cure at least 31,000 of them by the time the 5-year agreement expires.
“This new model has the potential to save many lives and improve the health of our citizens,” Louisiana Gov. John Bel Edwards (D) said in a statement. “Asegua was willing to come to the table to work with us to help Louisiana residents and we are pleased to initiate this 5-year partnership. Ultimately our goal is to eliminate this disease in Louisiana, and we have taken a big step forward in that effort.”
The agreement was designed to change very little in terms of the mechanics of how Medicaid managed care organizations, which cover most of the state’s Medicaid population and handle coverage and claims. The biggest change is that, when a spending cap is reached, Asegua will rebate 100% excess costs to the state. Louisiana officials did not disclose what the annual financial caps were as part of the agreement.
“We really thought it was important to leave the system – as much as possible – intact because we think that is going to make us most successful,” Alex Billioux, MD, of the Louisiana Department of Health said in an interview. “We think it leverages existing patient relationships and existing [Medicaid managed care organization] care management responsibilities.”
He added that, by keeping current processes unchanged, “it takes what is an otherwise very complicated arrangement with the state and makes it a little simpler.”
Patients will see no change in terms of copayments for the approved generic topping out at $3 depending on income level as they would have prior to the agreement. The biggest difference for them is that “people who couldn’t be treated are now going to have access to those prescriptions,” Dr. Billioux said.
Some cautious optimism surrounds this kind of arrangement and the potential effect it can have on the affected population.
“Innovation geared to improve access to hepatitis C treatment is critical, particularly in areas like Louisiana where treatment rates for Medicaid patients have been very low,” Robert Brown, MD, member of the American Liver Foundation’s National Medical Advisory Committee and hepatologist at Weill Cornell Medical College, New York, said. “If we can enhance patient access to treatment, we know we will improve health outcomes. However, it is too early to tell if this innovation will be a success. At the end of the day, the number of additional patients cured will determine if this was the right approach.”
Visit the AGA GI Patient Center for information to share with your patients about hepatitis C at https://www.gastro.org/practice-guidance/gi-patient-center/topic/hepatitis-c-hcv
Beginning July 15, physicians will no longer have to seek prior authorization or preauthorization to prescribe the authorized generic version of Epclusa (sofosbuvir/velpatasvir) to any Medicaid patient with hepatitis C. There will be no forms to file.
The change comes as part of a supplemental rebate agreement approved June 26 by CMS. That same day, Louisiana announced a deal with Asegua Therapeutics, a wholly owned subsidiary of Epclusa maker Gilead, that essentially caps the annual cost to the state for treating hepatitis C in incarcerated patients and Medicaid recipients.
State officials estimate about 39,000 Louisianans fit those criteria; the goal of the program is to cure at least 31,000 of them by the time the 5-year agreement expires.
“This new model has the potential to save many lives and improve the health of our citizens,” Louisiana Gov. John Bel Edwards (D) said in a statement. “Asegua was willing to come to the table to work with us to help Louisiana residents and we are pleased to initiate this 5-year partnership. Ultimately our goal is to eliminate this disease in Louisiana, and we have taken a big step forward in that effort.”
The agreement was designed to change very little in terms of the mechanics of how Medicaid managed care organizations, which cover most of the state’s Medicaid population and handle coverage and claims. The biggest change is that, when a spending cap is reached, Asegua will rebate 100% excess costs to the state. Louisiana officials did not disclose what the annual financial caps were as part of the agreement.
“We really thought it was important to leave the system – as much as possible – intact because we think that is going to make us most successful,” Alex Billioux, MD, of the Louisiana Department of Health said in an interview. “We think it leverages existing patient relationships and existing [Medicaid managed care organization] care management responsibilities.”
He added that, by keeping current processes unchanged, “it takes what is an otherwise very complicated arrangement with the state and makes it a little simpler.”
Patients will see no change in terms of copayments for the approved generic topping out at $3 depending on income level as they would have prior to the agreement. The biggest difference for them is that “people who couldn’t be treated are now going to have access to those prescriptions,” Dr. Billioux said.
Some cautious optimism surrounds this kind of arrangement and the potential effect it can have on the affected population.
“Innovation geared to improve access to hepatitis C treatment is critical, particularly in areas like Louisiana where treatment rates for Medicaid patients have been very low,” Robert Brown, MD, member of the American Liver Foundation’s National Medical Advisory Committee and hepatologist at Weill Cornell Medical College, New York, said. “If we can enhance patient access to treatment, we know we will improve health outcomes. However, it is too early to tell if this innovation will be a success. At the end of the day, the number of additional patients cured will determine if this was the right approach.”
Visit the AGA GI Patient Center for information to share with your patients about hepatitis C at https://www.gastro.org/practice-guidance/gi-patient-center/topic/hepatitis-c-hcv
HCC surveillance after anti-HCV therapy cost effective only for patients with cirrhosis
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
For patients with hepatitis C virus (HCV)–related cirrhosis (F4), but not those with advanced fibrosis (F3), hepatocellular carcinoma (HCC) surveillance after a sustained virologic response (SVR) is cost effective, according to investigators.
Current international guidelines call for HCC surveillance among all patients with advanced fibrosis (F3) or cirrhosis (F4) who have achieved SVR, but this is “very unlikely to be cost effective,” reported lead author Hooman Farhang Zangneh, MD, of Toronto General Hospital and colleagues. “HCV-related HCC rarely occurs in patients without cirrhosis,” the investigators explained in Clinical Gastroenterology and Hepatology. “With cirrhosis present, HCC incidence is 1.4% to 4.9% per year. If found early, options for curative therapy include radiofrequency ablation (RFA), surgical resection, and liver transplantation.”
The investigators developed a Markov model to determine which at-risk patients could undergo surveillance while remaining below willingness-to-pay thresholds. Specifically, cost-effectiveness was assessed for ultrasound screenings annually (every year) or biannually (twice a year) among patients with advanced fibrosis (F3) or compensated cirrhosis (F4) who were aged 50 years and had an SVR. Relevant data were drawn from expert opinions, medical literature, and Canada Life Tables. Various HCC incidence rates were tested, including a constant annual rate, rates based on type of antiviral treatment (direct-acting and interferon-based therapies), others based on stage of fibrosis, and another that increased with age. The model was validated by applying it to patients with F3 or F4 fibrosis who had not yet achieved an SVR. All monetary values were reported in 2015 Canadian dollars.
Representative of current guidelines, the investigators first tested costs when conducting surveillance among all patients with F3 or F4 fibrosis with an assumed constant HCC annual incidence rate of 0.5%. Biannual ultrasound surveillance after SVR caught more cases of HCC still in a curable stage (78%) than no surveillance (29%); however, false-positives were relatively common at 21.8% and 15.7% for biannual and annual surveillance, respectively. The investigators noted that in the real world, some of these false-positives are not detected by more advanced imaging, so patients go on to receive unnecessary RFA, which incurs additional costs. Partly for this reason, while biannual surveillance was more effective, it was also more expensive, with an incremental cost-effectiveness ratio (ICER) of $106,792 per quality-adjusted life-years (QALY), compared with $72,105 per QALY for annual surveillance.
Including only patients with F3 fibrosis after interferon-based therapy, using an HCC incidence of 0.23%, biannual and annual ICERs rose to $484,160 and $204,708 per QALY, respectively, both of which exceed standard willingness-to-pay thresholds. In comparison, annual and biannual ICERs were at most $55,850 and $42,305 per QALY, respectively, among patients with cirrhosis before interferon-induced SVR, using an HCC incidence rate of up to 1.39% per year.
“These results suggest that biannual (or annual) HCC surveillance is likely to be cost effective for patients with cirrhosis, but not for patients with F3 fibrosis before SVR,” the investigators wrote.
Costs for HCC surveillance among cirrhosis patients after direct-acting antiviral-induced SVR were still lower, at $43,229 and $34,307 per QALY, which were far lower than costs for patients with F3 fibrosis, which were $188,157 and $111,667 per QALY.
Focusing on the evident savings associated with surveillance of patients with cirrhosis, the investigators tested two diagnostic thresholds within this population with the aim of reducing costs further. They found that surveillance of patients with a pretreatment aspartate aminotransferase to platelet ratio index (APRI) greater than 2.0 (HCC incidence, 0.89%) was associated with biannual and annual ICERs of $48,729 and $37,806 per QALY, respectively, but when APRI was less than 2.0 (HCC incidence, 0.093%), surveillance was less effective and more expensive than no surveillance at all. A similar trend was found for an FIB-4 threshold of 3.25.
Employment of age-stratified risk of HCC also reduced costs of screening for patients with cirrhosis. With this strategy, ICER was $48,432 per QALY for biannual surveillance and $37,201 per QALY for annual surveillance.
“These data suggest that, if we assume HCC incidence increases with age, biannual or annual surveillance will be cost effective for the vast majority, if not all, patients with cirrhosis before SVR,” the investigators wrote.
“Our analysis suggests that HCC surveillance is very unlikely to be cost effective in patients with F3 fibrosis, whereas both annual and biannual modalities are likely to be cost effective at standard willingness-to-pay thresholds for patients with cirrhosis compared with no surveillance,” the investigators wrote.
“Additional long-term follow-up data are required to help identify patients at highest risk of HCC after SVR to tailor surveillance guidelines,” the investigators concluded.
The study was funded by the Toronto Centre for Liver Disease. The investigators declared no conflicts of interest.
This story was updated on 7/12/2019.
SOURCE: Zangneh et al. Clin Gastroenterol Hepatol. 2018 Dec 20. doi: 10.1016/j.cgh.2018.12.018.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY