User login
Tenofovir didn’t prevent hepatitis B transmission to newborns
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
The trial by Jourdain et al. – although described by the authors as negative – “puts down an intriguing marker attesting to the possibility that rapidly phasing in the timely administration of a safe monovalent HBV vaccine within a few hours after birth could contribute to the interruption of mother-to-child transmission and avert preventable HBV infections in childhood,” Geoffrey Dusheiko, MD, wrote in an accompanying editorial (N Engl J Med. 2018;378:952-3).
The World Health Organization supports HBV vaccine schedules of three or four doses, which are usually given as part of a combination immunization protocol beginning at 6 weeks of age. “Currently, HBV vaccination is most frequently administered as a pentavalent or hexavalent vaccine as part of the Expanded Program on Immunization, typically in combination with vaccines against diphtheria, tetanus, pertussis, polio, and Haemophilus influenzae type B,” wrote Dr. Dusheiko. “Paradoxically, support for combination vaccines within an integrated EPI schedule has unwittingly but undesirably shifted thinking and policy away from HBV vaccination at birth. This gap in vaccine strategy is disadvantageous.”
While the study doesn’t support tenofovir for maternal prophylaxis, it does imply value for treating the infant with immune globulin and HBV vaccination soon after birth. Delivering this kind of care globally will be challenging, but it’s entirely feasible, Dr. Dusheiko said.
“It is necessary to analyze regional data to assess the requirements for implementing vaccination at birth, including ... the training of otherwise unskilled birth attendants to deliver monovalent HBV vaccine at the same time as the vaccines against polio and bacille Calmette–Guérin. Importantly, the use of monovalent HBV vaccine would also require governmental or nongovernmental support. HBV vaccination at birth, despite the challenges for poverty-affected countries to deliver vaccination in rural and isolated locales, is feasible.”
Dr. Dusheiko is a hepatologist at the University College London School of Medicine and King’s College Hospital, London.
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
Prenatal tenofovir didn’t reduce the rate of hepatitis B among infants born to women infected with the virus.
Among 322 6-month-olds, the rate of HBV transmission was 0 in those whose mothers received the antiviral during pregnancy and 2% among those whose mothers received placebo – not a statistically significant difference, Gonzague Jourdain, MD, and his colleagues reported in the New England Journal of Medicine.
The study randomized 331 pregnant women with proven HBV infections to either tenofovir or placebo from 28 weeks’ gestation to 2 months post partum. All infants received HBV immune globulin at birth, and HBV vaccine at birth and at 1, 2, 4, and 6 months. The primary endpoint was confirmed HBV infection in the infant at 6 months.
Women were a mean of 28 weeks pregnant at baseline, with a mean viral load of 8.0 log10 IU/mL. Most women (about 90% of each group) had an HBV DNA of more than 200,000 IU/mL – a level associated with an increased risk of perinatal HBV infection despite vaccination.
There were 322 deliveries, resulting in 319 singletons, two pairs of twins, and one stillbirth. Postpartum infant treatment was quick, with a median of 1.3 hours from birth to administration of immune globulin and a median of 1.2 hours to administration of the first dose of the vaccine.
At 6 months, there were no HBV infections in the tenofovir-exposed group and 3 (2%) in the placebo group – a nonsignificant difference (P = .12).
Tenofovir was safe for both mother and fetus, with no significant adverse events in either group. The incidence of elevated maternal alanine aminotransferase level (more than 300 IU/L) was 6% in the tenofovir group and 3% in the placebo group, also a nonsignificant finding.
Dr. Jourdain and his colleagues noted that the 2% transmission rate in the placebo group is considerably lower than the 7% seen in similar studies and could be related to the rapid postpartum administration of HBV immune globulin and vaccine. If this is the case, prenatal antivirals could be more effective in countries where postpartum treatment is delayed or inconsistent.
“Maternal use of tenofovir may prevent transmissions that would occur when the birth dose is delayed, but its exact timing has not been reported consistently in previous perinatal studies,” the team said.
Another question is whether the stringent, 5-dose infant HBV vaccine series required in Thailand is simply more effective than schedules that have fewer doses or are combined with other vaccines and delivered later.
“It remains unclear whether the administration of more vaccine doses is more efficacious than the administration of the three vaccine doses that is recommended in the United States and by the World Health Organization.”
Dr. Jourdain had no financial disclosures relevant to the study, which was sponsored by the National Institute of Child Health and Human Development.
SOURCE: Jourdain G et al. N Engl J Med. 2018;378:911-23.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tenofovir was no different than placebo in preventing HBV transmission to newborns.
Major finding: The transmission rate was 0% in the tenofovir group and 2% in the placebo group (P = .12).
Study details: The study randomized 331 pregnant women to tenofovir or placebo from gestational week 28 to 2 months postpartum.
Disclosures: Dr. Jourdain had no financial disclosures; the National Institute of Child Health and Development sponsored the study.
Source: Jourdain G et al. N Engl J Med. 2018;378:911-23.
HCV infection tied to premature ovarian senescence and a high miscarriage rate
Premenopausal women with hepatitis C virus (HCV) showed increased ovarian senescence, which was associated with a lower chance of live birth. Such women also had a greater risk of infertility, as reported in the Journal of Hepatology.
Researchers examined three cohort studies, which comprised an age-matched prospectively enrolled cohort study of 100 women who were HCV positive and had chronic liver disease, 50 women who were HBV positive and had CLD, and 100 healthy women; 1,998 HCV-infected women enrolled in the Platform for the Study of Viral Hepatitis Therapies (PITER) trial from Italy; and 6,085 women infected with HCV plus 20,415 uninfected women from a United States database, according to Aimilia Karampatou, MD, of the University of Bologna, Modena, Italy, and colleagues.
In the second group examined, the women from the PITER trial, miscarriages occurred in 42% of the HCV-infected women with 44.6% of these women experiencing multiple miscarriages. The total fertility rate, defined as the average number of children that would be born in a lifetime, was 0.7 for the HCV-infected women, compared with 1.37 in the general Italian population.
Infertility data from the large U.S. study was assessed from a total of 27,525 women (20,415 HCV negative and HIV negative; 6,805 HCV positive; and 305 HCV positive/HIV positive). Women with HCV showed a significantly higher probability of infertility compared with uninfected controls (odds ratio, 2.44), and those women dually infected with HCV and HIV were affected even more (OR, 3.64).
Primarily based on the observations of AMH, which in many of the HCV-positive women fell into the menopausal range, the researchers suggested that “the reduced reproductive capacity of women who are HCV positive is related to failing ovarian function and subsequent follicular depletion in the context of a more generalized dysfunction of other fertility-related factors.”
With regard to the effect of antiviral therapy, AMH levels remained stable in women who attained a sustained virologic response but continued to fall in those for whom the therapy was a failure.
“HCV infection significantly and negatively affects many aspects of fertility. It remains to be assessed whether antiviral therapy at a very early age can positively influence the occurrence of miscarriages and can prevent ovarian senescence because the latter has broader health implications than simply preserving fertility,” the researchers concluded.
The authors reported that they had no conflicts of interest.
AGA provides resources and education for your patients about hepatitis C at www.gastro.org/HCV
SOURCE: Karampatou A et al. J Hepatology. 2018;68:33-41.
Premenopausal women with hepatitis C virus (HCV) showed increased ovarian senescence, which was associated with a lower chance of live birth. Such women also had a greater risk of infertility, as reported in the Journal of Hepatology.
Researchers examined three cohort studies, which comprised an age-matched prospectively enrolled cohort study of 100 women who were HCV positive and had chronic liver disease, 50 women who were HBV positive and had CLD, and 100 healthy women; 1,998 HCV-infected women enrolled in the Platform for the Study of Viral Hepatitis Therapies (PITER) trial from Italy; and 6,085 women infected with HCV plus 20,415 uninfected women from a United States database, according to Aimilia Karampatou, MD, of the University of Bologna, Modena, Italy, and colleagues.
In the second group examined, the women from the PITER trial, miscarriages occurred in 42% of the HCV-infected women with 44.6% of these women experiencing multiple miscarriages. The total fertility rate, defined as the average number of children that would be born in a lifetime, was 0.7 for the HCV-infected women, compared with 1.37 in the general Italian population.
Infertility data from the large U.S. study was assessed from a total of 27,525 women (20,415 HCV negative and HIV negative; 6,805 HCV positive; and 305 HCV positive/HIV positive). Women with HCV showed a significantly higher probability of infertility compared with uninfected controls (odds ratio, 2.44), and those women dually infected with HCV and HIV were affected even more (OR, 3.64).
Primarily based on the observations of AMH, which in many of the HCV-positive women fell into the menopausal range, the researchers suggested that “the reduced reproductive capacity of women who are HCV positive is related to failing ovarian function and subsequent follicular depletion in the context of a more generalized dysfunction of other fertility-related factors.”
With regard to the effect of antiviral therapy, AMH levels remained stable in women who attained a sustained virologic response but continued to fall in those for whom the therapy was a failure.
“HCV infection significantly and negatively affects many aspects of fertility. It remains to be assessed whether antiviral therapy at a very early age can positively influence the occurrence of miscarriages and can prevent ovarian senescence because the latter has broader health implications than simply preserving fertility,” the researchers concluded.
The authors reported that they had no conflicts of interest.
AGA provides resources and education for your patients about hepatitis C at www.gastro.org/HCV
SOURCE: Karampatou A et al. J Hepatology. 2018;68:33-41.
Premenopausal women with hepatitis C virus (HCV) showed increased ovarian senescence, which was associated with a lower chance of live birth. Such women also had a greater risk of infertility, as reported in the Journal of Hepatology.
Researchers examined three cohort studies, which comprised an age-matched prospectively enrolled cohort study of 100 women who were HCV positive and had chronic liver disease, 50 women who were HBV positive and had CLD, and 100 healthy women; 1,998 HCV-infected women enrolled in the Platform for the Study of Viral Hepatitis Therapies (PITER) trial from Italy; and 6,085 women infected with HCV plus 20,415 uninfected women from a United States database, according to Aimilia Karampatou, MD, of the University of Bologna, Modena, Italy, and colleagues.
In the second group examined, the women from the PITER trial, miscarriages occurred in 42% of the HCV-infected women with 44.6% of these women experiencing multiple miscarriages. The total fertility rate, defined as the average number of children that would be born in a lifetime, was 0.7 for the HCV-infected women, compared with 1.37 in the general Italian population.
Infertility data from the large U.S. study was assessed from a total of 27,525 women (20,415 HCV negative and HIV negative; 6,805 HCV positive; and 305 HCV positive/HIV positive). Women with HCV showed a significantly higher probability of infertility compared with uninfected controls (odds ratio, 2.44), and those women dually infected with HCV and HIV were affected even more (OR, 3.64).
Primarily based on the observations of AMH, which in many of the HCV-positive women fell into the menopausal range, the researchers suggested that “the reduced reproductive capacity of women who are HCV positive is related to failing ovarian function and subsequent follicular depletion in the context of a more generalized dysfunction of other fertility-related factors.”
With regard to the effect of antiviral therapy, AMH levels remained stable in women who attained a sustained virologic response but continued to fall in those for whom the therapy was a failure.
“HCV infection significantly and negatively affects many aspects of fertility. It remains to be assessed whether antiviral therapy at a very early age can positively influence the occurrence of miscarriages and can prevent ovarian senescence because the latter has broader health implications than simply preserving fertility,” the researchers concluded.
The authors reported that they had no conflicts of interest.
AGA provides resources and education for your patients about hepatitis C at www.gastro.org/HCV
SOURCE: Karampatou A et al. J Hepatology. 2018;68:33-41.
FROM THE JOURNAL OF HEPATOLOGY
Key clinical point: HCV-positive women appear to undergo increased ovarian senescence.
Major finding: The fertility rate of HCV-positive women was 0.7 vs. 1.37 in the general population.
Study details: Three separate studies together comprising more than 30,000 HCV-infected and -uninfected women.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Karampatou A et al. J Hepatology. 2018;68:33-41.
Racial disparities by region persist despite multiple liver transplant allocation schemes
Racial and regional disparities in liver transplant allocation persist despite multiple allocation schemes as identified in a large dataset spanning at least 30 years, according to a study.
The Model for End-Stage Liver Disease (MELD) score was put forth in 1998 by the Organ Procurement and Transplantation Network under the guidance of the Centers for Medicare & Medicaid Services and the Department of Health & Human Services, to indicate that organs should be allocated to the sickest patients as interpreted by the MELD score. Since then, four separate liver allocation systems are being followed across the country owing to disagreements over a universal allocation scheme. These include MELD score, the initial three-tier UNOS (United Network for Organ Sharing) Status, Share 35, and now MELD Na.
Unfavorable supply and demand ratios for liver allograft allocation persist across the country with differential and limited access to care, which leads to decreased wellness, lower life expectancies, and higher baseline morbidity and mortality rates in some areas. Furthermore, a short cold storage capacity of liver allografts limits greater allocation and distribution schema.
Dominique J. Monlezun, MD, PhD, MPH, and his colleagues at the Tulane Transplant Institute at Tulane University, New Orleans, evaluated the effect of MELD and other allocation schemes on the incidence of racial and regional disparities in a study published in Surgery. They performed fixed-effects multivariate logistic regression augmented by modified forward and backward stepwise-regression of transplanted patients from the United Network for Organ Sharing Standard Transplant Analysis and Research database (1985-2016) to assess causal inference of such disparities.
“Significant disparities in the odds of receiving a liver were found: African Americans, odds ratio, 1.12 (95% confidence interval, 1.08-1.17); Asians, 1.12 (95% CI, 1.07-1.18); females, 0.80 (95% CI, 0.78-0.83); and malignancy 1.18 (95% CI, 1.13-1.22). Significant racial disparities by region were identified using Caucasian Region 7 (Ill., Minn., N.D., S.D., and Wisc.) as the reference: Hispanic Region 9 (N.Y., West Vt.) 1.22 (1.02-1.45), Hispanic Region 1 (New England) 1.26 (1.01-1.57), Hispanic Region 4 (Ok., Tex.) 1.23 (1.05-1.43), and Asian Region 4 (Ok., Tex.) 1.35 (1.05-1.73).” Since the transplantation rate in Region 7 closely approximated the sex and race-matched rate of the national post–Share 35 average, it was used as a reference in the study.
“Although traditional disparities as with African Americans and [whites] have been improved during the past 30 years, new disparities as with Hispanics and Asians have developed in certain regions,” stated the authors.
They acknowledged the limitations in the observational nature of the study and those of the statistical analyses, which could only approximate, rather than perfectly replicate, a randomized trial. Big Data tools such as artificial intelligence–based machine learning can provide real-time analysis of large heterogeneous datasets for patients across different regions.
The authors reported no conflicts of interest.
SOURCE: Monlezun DJ et al. Surgery. 2018 doi: 10.1016/j.surg.2017.10.009.
Racial and regional disparities in liver transplant allocation persist despite multiple allocation schemes as identified in a large dataset spanning at least 30 years, according to a study.
The Model for End-Stage Liver Disease (MELD) score was put forth in 1998 by the Organ Procurement and Transplantation Network under the guidance of the Centers for Medicare & Medicaid Services and the Department of Health & Human Services, to indicate that organs should be allocated to the sickest patients as interpreted by the MELD score. Since then, four separate liver allocation systems are being followed across the country owing to disagreements over a universal allocation scheme. These include MELD score, the initial three-tier UNOS (United Network for Organ Sharing) Status, Share 35, and now MELD Na.
Unfavorable supply and demand ratios for liver allograft allocation persist across the country with differential and limited access to care, which leads to decreased wellness, lower life expectancies, and higher baseline morbidity and mortality rates in some areas. Furthermore, a short cold storage capacity of liver allografts limits greater allocation and distribution schema.
Dominique J. Monlezun, MD, PhD, MPH, and his colleagues at the Tulane Transplant Institute at Tulane University, New Orleans, evaluated the effect of MELD and other allocation schemes on the incidence of racial and regional disparities in a study published in Surgery. They performed fixed-effects multivariate logistic regression augmented by modified forward and backward stepwise-regression of transplanted patients from the United Network for Organ Sharing Standard Transplant Analysis and Research database (1985-2016) to assess causal inference of such disparities.
“Significant disparities in the odds of receiving a liver were found: African Americans, odds ratio, 1.12 (95% confidence interval, 1.08-1.17); Asians, 1.12 (95% CI, 1.07-1.18); females, 0.80 (95% CI, 0.78-0.83); and malignancy 1.18 (95% CI, 1.13-1.22). Significant racial disparities by region were identified using Caucasian Region 7 (Ill., Minn., N.D., S.D., and Wisc.) as the reference: Hispanic Region 9 (N.Y., West Vt.) 1.22 (1.02-1.45), Hispanic Region 1 (New England) 1.26 (1.01-1.57), Hispanic Region 4 (Ok., Tex.) 1.23 (1.05-1.43), and Asian Region 4 (Ok., Tex.) 1.35 (1.05-1.73).” Since the transplantation rate in Region 7 closely approximated the sex and race-matched rate of the national post–Share 35 average, it was used as a reference in the study.
“Although traditional disparities as with African Americans and [whites] have been improved during the past 30 years, new disparities as with Hispanics and Asians have developed in certain regions,” stated the authors.
They acknowledged the limitations in the observational nature of the study and those of the statistical analyses, which could only approximate, rather than perfectly replicate, a randomized trial. Big Data tools such as artificial intelligence–based machine learning can provide real-time analysis of large heterogeneous datasets for patients across different regions.
The authors reported no conflicts of interest.
SOURCE: Monlezun DJ et al. Surgery. 2018 doi: 10.1016/j.surg.2017.10.009.
Racial and regional disparities in liver transplant allocation persist despite multiple allocation schemes as identified in a large dataset spanning at least 30 years, according to a study.
The Model for End-Stage Liver Disease (MELD) score was put forth in 1998 by the Organ Procurement and Transplantation Network under the guidance of the Centers for Medicare & Medicaid Services and the Department of Health & Human Services, to indicate that organs should be allocated to the sickest patients as interpreted by the MELD score. Since then, four separate liver allocation systems are being followed across the country owing to disagreements over a universal allocation scheme. These include MELD score, the initial three-tier UNOS (United Network for Organ Sharing) Status, Share 35, and now MELD Na.
Unfavorable supply and demand ratios for liver allograft allocation persist across the country with differential and limited access to care, which leads to decreased wellness, lower life expectancies, and higher baseline morbidity and mortality rates in some areas. Furthermore, a short cold storage capacity of liver allografts limits greater allocation and distribution schema.
Dominique J. Monlezun, MD, PhD, MPH, and his colleagues at the Tulane Transplant Institute at Tulane University, New Orleans, evaluated the effect of MELD and other allocation schemes on the incidence of racial and regional disparities in a study published in Surgery. They performed fixed-effects multivariate logistic regression augmented by modified forward and backward stepwise-regression of transplanted patients from the United Network for Organ Sharing Standard Transplant Analysis and Research database (1985-2016) to assess causal inference of such disparities.
“Significant disparities in the odds of receiving a liver were found: African Americans, odds ratio, 1.12 (95% confidence interval, 1.08-1.17); Asians, 1.12 (95% CI, 1.07-1.18); females, 0.80 (95% CI, 0.78-0.83); and malignancy 1.18 (95% CI, 1.13-1.22). Significant racial disparities by region were identified using Caucasian Region 7 (Ill., Minn., N.D., S.D., and Wisc.) as the reference: Hispanic Region 9 (N.Y., West Vt.) 1.22 (1.02-1.45), Hispanic Region 1 (New England) 1.26 (1.01-1.57), Hispanic Region 4 (Ok., Tex.) 1.23 (1.05-1.43), and Asian Region 4 (Ok., Tex.) 1.35 (1.05-1.73).” Since the transplantation rate in Region 7 closely approximated the sex and race-matched rate of the national post–Share 35 average, it was used as a reference in the study.
“Although traditional disparities as with African Americans and [whites] have been improved during the past 30 years, new disparities as with Hispanics and Asians have developed in certain regions,” stated the authors.
They acknowledged the limitations in the observational nature of the study and those of the statistical analyses, which could only approximate, rather than perfectly replicate, a randomized trial. Big Data tools such as artificial intelligence–based machine learning can provide real-time analysis of large heterogeneous datasets for patients across different regions.
The authors reported no conflicts of interest.
SOURCE: Monlezun DJ et al. Surgery. 2018 doi: 10.1016/j.surg.2017.10.009.
FROM SURGERY
Key clinical point: The existence of racial disparity in liver allograft distribution is undisputed. Disagreements persist over optimal allocation schemes, with centers using different schemes.
Major finding: A rigorous causal inference statistic on a large national dataset spanning at least 30 years showed that racial disparities by region persist despite multiple allocation schemes.
Study details: Patients from the United Network for Organ Sharing Standard Transplant Analysis and Research database (1985-2016) were used to assess causal inference of racial and regional disparities.
Disclosures: None reported.
Source: Monlezun DJ et al. Surgery. 2018. doi: 10.1016/j.surg.2017.10.009.
Engineered liver models to study human hepatotropic pathogens
Recently, exciting clinical progress has been made in the study of hepatotropic pathogens in the context of liver-dependent infectious diseases. This is crucial for the development and validation of therapeutic interventions, such as drug and vaccine candidates that may act on the liver cells. The engineered models range from two-dimensional (2-D) cultures of primary human hepatocytes (HH) and stem cell–derived progeny to three-dimensional (3-D) organoid cultures and humanized rodent models. A review by Nil Gural and colleagues, published in Cellular and Molecular Gastroenterology and Hepatology, described these unique models. Furthermore, the progress made in combining individual approaches and pairing the most appropriate model system and readout modality was discussed.
The major human hepatotropic pathogens include hepatitis C virus (HCV), hepatitis B virus (HBV), and the protozoan parasites Plasmodium falciparum and P. vivax. While HBV and HCV can cause chronic liver diseases such as cirrhosis and hepatocellular carcinoma, Plasmodium parasites cause malaria. The use of cancer cell lines and animal models to study host-pathogen interactions is limited by uncontrolled proliferation, abnormal liver-specific functions, and stringent host dependency of the hepatotropic pathogens. HHs are thus the only ideal system to study these pathogens, however, maintaining these cells ex vivo is challenging.
For instance, 2D monolayers of human hepatoma-derived cell lines (such as HepG2-A16 and HepaRG) are easier to maintain, to amplify for scaling up, and to use for drug screening, thus representing a renewable alternative to primary hepatocytes. These model systems have been useful to study short-term infections of human Plasmodium parasites (P. vivax and P. falciparum); other hepatotropic pathogens such as Ebola, Lassa, human cytomegalovirus, and dengue viruses; and to generate virion stocks (HCV, HBV). For long-term scientific analyses and cultures, as well as clinical isolates of pathogens that do not infect hepatoma cells, immortalized cell lines have been engineered to differentiate and maintain HH functions for a longer duration. Additionally, cocultivation of primary hepatocytes with nonparenchymal cells or hepatocytes with mouse fibroblasts preserves hepatocyte phenotype. The latter is a self-assembling coculture system that could potentially maintain an infection for over 30 days and be used for testing anti-HBV drugs. A micropatterned coculture system, in which hepatocytes are positioned in “islands” via photolithographic patterning of collagen, surrounded by mouse embryonic fibroblasts, can maintain hepatocyte phenotypes for 4-6 weeks, and remain permissive to P. falciparum, P. vivax, HBV, and HCV infections. Furthermore, micropatterned coculture systems support full developmental liver stages of both P. falciparum and P. vivax, with the release of merozoites from hepatocytes and their subsequent infection of overlaid human red blood cells.
Alternatively, embryonic stem cells and induced pluripotent stem cells of human origin can be differentiated into hepatocytelike cells that enable investigation of host genetics within the context of host-pathogen interactions, and can also be used for target identification for drug development. However, stem cell cultures require significant culture expertise and may not represent a fully differentiated adult hepatocyte phenotype.
Although 2D cultures offer ease of use and monitoring of infection, they often lack the complexity of the liver microenvironment and impact of different cell types on liver infections. A 3D radial-flow bioreactor (cylindrical matrix) was able to maintain and amplify human hepatoma cells (for example, Huh7 cells), by providing sufficient oxygen and nutrient supply, supporting productive HCV infection for months. Other 3D cultures of hepatoma cells using polyethylene glycol–based hydrogels, thermoreversible gelatin polymers, alginate, galactosylated cellulosic sponges, matrigel, and collagen have been developed and shown to be permissive to HCV or HBV infections. Although 3D coculture systems exhibit better hepatic function and differential gene expression profiles in comparison to 2D counterparts, they require a large quantity of cells and are a challenge to scale up. Recently, several liver-on-a-chip models have been created that mimic shear stress, blood flow, and the extracellular environment within a tissue, holding great potential for modeling liver-specific pathogens.
Humanized mouse models with ectopic human liver structures have been developed in which primary HHs are transplanted following liver injury. Chimeric mouse models including Alb-uPA/SCID (HHs transplanted into urokinase-type plasminogen activator-transgenic severe combined immunodeficient mice), FNRG/FRG (HHs transplanted into Fah[-/-], Rag2[-/-], and Il2rg[-/-] mice with or without a nonobese diabetic background), and TK-NOG (HHs transplanted into herpes simplex virus type-1 thymidine kinase mice) were validated for HCV, HBV, P. falciparum, and P. vivax infections. It is, however, laborious to create and maintain chimeric mouse models and monitor infection processes in them.
It is important to note that the selection of model system and the readout modality to monitor infection will vary based on the experimental question at hand. Tissue engineering has thus far made significant contributions to the knowledge of hepatotropic pathogens; a continued effort to develop better liver models is envisioned.
Gural et al. present a timely and outstanding review of the advances made in the engineering of human-relevant liver culture platforms for investigating the molecular mechanisms of infectious diseases (e.g., hepatitis B/C viruses and Plasmodium parasites that cause malaria) and developing better drugs or vaccines against such diseases. The authors cover a continuum of platforms with increasing physiological complexity, such as 2-D hepatocyte monocultures on collagen-coated plastic, 2-D cocultures of hepatocytes and nonparenchymal cells, (both randomly distributed and patterned into microdomains to optimize cell-cell contact), 3-D cultures/cocultures housed in biomaterial-based scaffolds, perfusion-based bioreactors to induce cell growth and phenotypic stability, and finally rodents with humanized livers. Cell sourcing considerations for building human-relevant platforms are discussed, including cancerous cell lines, primary human hepatocytes, and stem cell–derived hepatocytes (e.g., induced pluripotent stem cells).
From the discussions of various studies, it is clear that this field has benefitted tremendously from advances in tissue engineering, including microfabrication tools adapted from the semiconductor industry, to construct human liver platforms that last for several weeks in vitro, can be infected with hepatitis B/C virus and Plasmodium parasites with high efficiencies, and are very useful for high-throughput and high-content drug screening applications. The latest protocols in isolating and cryopreserving primary human hepatocytes and differentiating stem cells into hepatocyte-like cells with adult functions help reduce the reliance on abnormal or cancerous cell lines for building platforms with higher relevance to the clinic. Ultimately, continued advances in microfabricated human liver platforms can aid our understanding of liver infections and spur further drug/vaccine development.
Salman R. Khetani, PhD, is associate professor, department of bioengineering, University of Illinois at Chicago. He has no conflicts of interest.
Gural et al. present a timely and outstanding review of the advances made in the engineering of human-relevant liver culture platforms for investigating the molecular mechanisms of infectious diseases (e.g., hepatitis B/C viruses and Plasmodium parasites that cause malaria) and developing better drugs or vaccines against such diseases. The authors cover a continuum of platforms with increasing physiological complexity, such as 2-D hepatocyte monocultures on collagen-coated plastic, 2-D cocultures of hepatocytes and nonparenchymal cells, (both randomly distributed and patterned into microdomains to optimize cell-cell contact), 3-D cultures/cocultures housed in biomaterial-based scaffolds, perfusion-based bioreactors to induce cell growth and phenotypic stability, and finally rodents with humanized livers. Cell sourcing considerations for building human-relevant platforms are discussed, including cancerous cell lines, primary human hepatocytes, and stem cell–derived hepatocytes (e.g., induced pluripotent stem cells).
From the discussions of various studies, it is clear that this field has benefitted tremendously from advances in tissue engineering, including microfabrication tools adapted from the semiconductor industry, to construct human liver platforms that last for several weeks in vitro, can be infected with hepatitis B/C virus and Plasmodium parasites with high efficiencies, and are very useful for high-throughput and high-content drug screening applications. The latest protocols in isolating and cryopreserving primary human hepatocytes and differentiating stem cells into hepatocyte-like cells with adult functions help reduce the reliance on abnormal or cancerous cell lines for building platforms with higher relevance to the clinic. Ultimately, continued advances in microfabricated human liver platforms can aid our understanding of liver infections and spur further drug/vaccine development.
Salman R. Khetani, PhD, is associate professor, department of bioengineering, University of Illinois at Chicago. He has no conflicts of interest.
Gural et al. present a timely and outstanding review of the advances made in the engineering of human-relevant liver culture platforms for investigating the molecular mechanisms of infectious diseases (e.g., hepatitis B/C viruses and Plasmodium parasites that cause malaria) and developing better drugs or vaccines against such diseases. The authors cover a continuum of platforms with increasing physiological complexity, such as 2-D hepatocyte monocultures on collagen-coated plastic, 2-D cocultures of hepatocytes and nonparenchymal cells, (both randomly distributed and patterned into microdomains to optimize cell-cell contact), 3-D cultures/cocultures housed in biomaterial-based scaffolds, perfusion-based bioreactors to induce cell growth and phenotypic stability, and finally rodents with humanized livers. Cell sourcing considerations for building human-relevant platforms are discussed, including cancerous cell lines, primary human hepatocytes, and stem cell–derived hepatocytes (e.g., induced pluripotent stem cells).
From the discussions of various studies, it is clear that this field has benefitted tremendously from advances in tissue engineering, including microfabrication tools adapted from the semiconductor industry, to construct human liver platforms that last for several weeks in vitro, can be infected with hepatitis B/C virus and Plasmodium parasites with high efficiencies, and are very useful for high-throughput and high-content drug screening applications. The latest protocols in isolating and cryopreserving primary human hepatocytes and differentiating stem cells into hepatocyte-like cells with adult functions help reduce the reliance on abnormal or cancerous cell lines for building platforms with higher relevance to the clinic. Ultimately, continued advances in microfabricated human liver platforms can aid our understanding of liver infections and spur further drug/vaccine development.
Salman R. Khetani, PhD, is associate professor, department of bioengineering, University of Illinois at Chicago. He has no conflicts of interest.
Recently, exciting clinical progress has been made in the study of hepatotropic pathogens in the context of liver-dependent infectious diseases. This is crucial for the development and validation of therapeutic interventions, such as drug and vaccine candidates that may act on the liver cells. The engineered models range from two-dimensional (2-D) cultures of primary human hepatocytes (HH) and stem cell–derived progeny to three-dimensional (3-D) organoid cultures and humanized rodent models. A review by Nil Gural and colleagues, published in Cellular and Molecular Gastroenterology and Hepatology, described these unique models. Furthermore, the progress made in combining individual approaches and pairing the most appropriate model system and readout modality was discussed.
The major human hepatotropic pathogens include hepatitis C virus (HCV), hepatitis B virus (HBV), and the protozoan parasites Plasmodium falciparum and P. vivax. While HBV and HCV can cause chronic liver diseases such as cirrhosis and hepatocellular carcinoma, Plasmodium parasites cause malaria. The use of cancer cell lines and animal models to study host-pathogen interactions is limited by uncontrolled proliferation, abnormal liver-specific functions, and stringent host dependency of the hepatotropic pathogens. HHs are thus the only ideal system to study these pathogens, however, maintaining these cells ex vivo is challenging.
For instance, 2D monolayers of human hepatoma-derived cell lines (such as HepG2-A16 and HepaRG) are easier to maintain, to amplify for scaling up, and to use for drug screening, thus representing a renewable alternative to primary hepatocytes. These model systems have been useful to study short-term infections of human Plasmodium parasites (P. vivax and P. falciparum); other hepatotropic pathogens such as Ebola, Lassa, human cytomegalovirus, and dengue viruses; and to generate virion stocks (HCV, HBV). For long-term scientific analyses and cultures, as well as clinical isolates of pathogens that do not infect hepatoma cells, immortalized cell lines have been engineered to differentiate and maintain HH functions for a longer duration. Additionally, cocultivation of primary hepatocytes with nonparenchymal cells or hepatocytes with mouse fibroblasts preserves hepatocyte phenotype. The latter is a self-assembling coculture system that could potentially maintain an infection for over 30 days and be used for testing anti-HBV drugs. A micropatterned coculture system, in which hepatocytes are positioned in “islands” via photolithographic patterning of collagen, surrounded by mouse embryonic fibroblasts, can maintain hepatocyte phenotypes for 4-6 weeks, and remain permissive to P. falciparum, P. vivax, HBV, and HCV infections. Furthermore, micropatterned coculture systems support full developmental liver stages of both P. falciparum and P. vivax, with the release of merozoites from hepatocytes and their subsequent infection of overlaid human red blood cells.
Alternatively, embryonic stem cells and induced pluripotent stem cells of human origin can be differentiated into hepatocytelike cells that enable investigation of host genetics within the context of host-pathogen interactions, and can also be used for target identification for drug development. However, stem cell cultures require significant culture expertise and may not represent a fully differentiated adult hepatocyte phenotype.
Although 2D cultures offer ease of use and monitoring of infection, they often lack the complexity of the liver microenvironment and impact of different cell types on liver infections. A 3D radial-flow bioreactor (cylindrical matrix) was able to maintain and amplify human hepatoma cells (for example, Huh7 cells), by providing sufficient oxygen and nutrient supply, supporting productive HCV infection for months. Other 3D cultures of hepatoma cells using polyethylene glycol–based hydrogels, thermoreversible gelatin polymers, alginate, galactosylated cellulosic sponges, matrigel, and collagen have been developed and shown to be permissive to HCV or HBV infections. Although 3D coculture systems exhibit better hepatic function and differential gene expression profiles in comparison to 2D counterparts, they require a large quantity of cells and are a challenge to scale up. Recently, several liver-on-a-chip models have been created that mimic shear stress, blood flow, and the extracellular environment within a tissue, holding great potential for modeling liver-specific pathogens.
Humanized mouse models with ectopic human liver structures have been developed in which primary HHs are transplanted following liver injury. Chimeric mouse models including Alb-uPA/SCID (HHs transplanted into urokinase-type plasminogen activator-transgenic severe combined immunodeficient mice), FNRG/FRG (HHs transplanted into Fah[-/-], Rag2[-/-], and Il2rg[-/-] mice with or without a nonobese diabetic background), and TK-NOG (HHs transplanted into herpes simplex virus type-1 thymidine kinase mice) were validated for HCV, HBV, P. falciparum, and P. vivax infections. It is, however, laborious to create and maintain chimeric mouse models and monitor infection processes in them.
It is important to note that the selection of model system and the readout modality to monitor infection will vary based on the experimental question at hand. Tissue engineering has thus far made significant contributions to the knowledge of hepatotropic pathogens; a continued effort to develop better liver models is envisioned.
Recently, exciting clinical progress has been made in the study of hepatotropic pathogens in the context of liver-dependent infectious diseases. This is crucial for the development and validation of therapeutic interventions, such as drug and vaccine candidates that may act on the liver cells. The engineered models range from two-dimensional (2-D) cultures of primary human hepatocytes (HH) and stem cell–derived progeny to three-dimensional (3-D) organoid cultures and humanized rodent models. A review by Nil Gural and colleagues, published in Cellular and Molecular Gastroenterology and Hepatology, described these unique models. Furthermore, the progress made in combining individual approaches and pairing the most appropriate model system and readout modality was discussed.
The major human hepatotropic pathogens include hepatitis C virus (HCV), hepatitis B virus (HBV), and the protozoan parasites Plasmodium falciparum and P. vivax. While HBV and HCV can cause chronic liver diseases such as cirrhosis and hepatocellular carcinoma, Plasmodium parasites cause malaria. The use of cancer cell lines and animal models to study host-pathogen interactions is limited by uncontrolled proliferation, abnormal liver-specific functions, and stringent host dependency of the hepatotropic pathogens. HHs are thus the only ideal system to study these pathogens, however, maintaining these cells ex vivo is challenging.
For instance, 2D monolayers of human hepatoma-derived cell lines (such as HepG2-A16 and HepaRG) are easier to maintain, to amplify for scaling up, and to use for drug screening, thus representing a renewable alternative to primary hepatocytes. These model systems have been useful to study short-term infections of human Plasmodium parasites (P. vivax and P. falciparum); other hepatotropic pathogens such as Ebola, Lassa, human cytomegalovirus, and dengue viruses; and to generate virion stocks (HCV, HBV). For long-term scientific analyses and cultures, as well as clinical isolates of pathogens that do not infect hepatoma cells, immortalized cell lines have been engineered to differentiate and maintain HH functions for a longer duration. Additionally, cocultivation of primary hepatocytes with nonparenchymal cells or hepatocytes with mouse fibroblasts preserves hepatocyte phenotype. The latter is a self-assembling coculture system that could potentially maintain an infection for over 30 days and be used for testing anti-HBV drugs. A micropatterned coculture system, in which hepatocytes are positioned in “islands” via photolithographic patterning of collagen, surrounded by mouse embryonic fibroblasts, can maintain hepatocyte phenotypes for 4-6 weeks, and remain permissive to P. falciparum, P. vivax, HBV, and HCV infections. Furthermore, micropatterned coculture systems support full developmental liver stages of both P. falciparum and P. vivax, with the release of merozoites from hepatocytes and their subsequent infection of overlaid human red blood cells.
Alternatively, embryonic stem cells and induced pluripotent stem cells of human origin can be differentiated into hepatocytelike cells that enable investigation of host genetics within the context of host-pathogen interactions, and can also be used for target identification for drug development. However, stem cell cultures require significant culture expertise and may not represent a fully differentiated adult hepatocyte phenotype.
Although 2D cultures offer ease of use and monitoring of infection, they often lack the complexity of the liver microenvironment and impact of different cell types on liver infections. A 3D radial-flow bioreactor (cylindrical matrix) was able to maintain and amplify human hepatoma cells (for example, Huh7 cells), by providing sufficient oxygen and nutrient supply, supporting productive HCV infection for months. Other 3D cultures of hepatoma cells using polyethylene glycol–based hydrogels, thermoreversible gelatin polymers, alginate, galactosylated cellulosic sponges, matrigel, and collagen have been developed and shown to be permissive to HCV or HBV infections. Although 3D coculture systems exhibit better hepatic function and differential gene expression profiles in comparison to 2D counterparts, they require a large quantity of cells and are a challenge to scale up. Recently, several liver-on-a-chip models have been created that mimic shear stress, blood flow, and the extracellular environment within a tissue, holding great potential for modeling liver-specific pathogens.
Humanized mouse models with ectopic human liver structures have been developed in which primary HHs are transplanted following liver injury. Chimeric mouse models including Alb-uPA/SCID (HHs transplanted into urokinase-type plasminogen activator-transgenic severe combined immunodeficient mice), FNRG/FRG (HHs transplanted into Fah[-/-], Rag2[-/-], and Il2rg[-/-] mice with or without a nonobese diabetic background), and TK-NOG (HHs transplanted into herpes simplex virus type-1 thymidine kinase mice) were validated for HCV, HBV, P. falciparum, and P. vivax infections. It is, however, laborious to create and maintain chimeric mouse models and monitor infection processes in them.
It is important to note that the selection of model system and the readout modality to monitor infection will vary based on the experimental question at hand. Tissue engineering has thus far made significant contributions to the knowledge of hepatotropic pathogens; a continued effort to develop better liver models is envisioned.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Sofosbuvir/ledipasvir looks good in HBV coinfected patients
For patients or death in a phase 3b, multicenter, open-label study.
“Although we observed increases in HBV DNA in most patients, these increases were [usually] not associated with ALT [alanine amino transferase] flares or clinical complications,” reported Chun-Jen Liu, MD, of National Taiwan University College of Medicine and Hospital, Taipei, and his associates. Although nearly two-thirds of patients developed HBV reactivation, less than 5% developed alanine aminotransferase rises at least twice the upper limit of normal, and only one patient had symptomatic HBV reactivation, which entecavir therapy resolved. This study was the first to prospectively evaluate the risk of HBV reactivation during HCV treatment, the researchers wrote in the March issue of Gastroenterology.
Because chronic hepatitis C virus infection tends to suppress HBV replication, peginterferon/ribavirin or direct-acting anti-HCV treatment can reactivate HBV infection, especially in patients who test positive for hepatitis B surface antigen (HBsAg). Left untreated, reactivated HBV can lead to fulminant hepatitis, liver failure, and death, as noted on recently mandated boxed warnings.
Accordingly, guidelines recommend testing patients for HBV infection before starting HCV treatment. The study enrolled 111 coinfected patients; about two-thirds were female, and 16% had compensated cirrhosis. All tested positive for HBsAg at screening, and all but one also tested positive at baseline. Mean baseline HBV DNA levels were 2.1 log10 IU/mL. Patients received 90 mg ledipasvir plus 400 mg sofosbuvir for 12 weeks, and levels of HCV RNA, HBV DNA, and HBsAg were tested at weeks 1, 2, 4, 8, 12, posttreatment week 4, and then every 12 weeks until posttreatment week 108.
In all, 70 (63%) patients developed HBV reactivation, including 84% of the 37 patients with undetectable HBV DNA at baseline. During treatment, none of these patients had ALT rise more than twice the upper limit of normal. By 48 weeks post treatment, however, 77% still had quantifiable HBV DNA, and two had marked ALT rises. Furthermore, by posttreatment week 53, one of these patients developed bilirubinemia and symptomatic HBV infection (malaise, anorexia, sclera jaundice, and nausea), which resolved after treatment with entecavir.
A total of 74 patients had quantifiable baseline HBV DNA (at least 20 IU/mL). Three received entecavir or tenofovir disoproxil fumarate based on confirmed HBV reactivation with a concomitant ALT rise of at least twice the upper limit of normal. All were asymptomatic. There were no cases of liver failure or death.
“Regardless of HBV DNA and/or ALT elevations, no patient had signs of liver failure,” the researchers wrote. “Our results support the recommendations put forth in clinical treatment guidelines: HCV-infected patients should be evaluated for HBV infection prior to HCV treatment with direct-acting antivirals. Those who are HBsAg positive should be monitored during and after treatment for HBV reactivation, and treatment should be initiated in accordance with existing guidelines.”
Gilead funded the study. Dr. Liu and 12 coinvestigators reported having no conflicts of interest. Nine coinvestigators reported being employees and shareholders of Gilead, and one coinvestigator reporting consulting for Gilead. The senior author disclosed ties to Roche, Bristol-Myers Squibb, Johnson & Johnson, Bayer, MSD, and Taiha.
SOURCE: Lui C-J et al. Gastroenterology. 2017 Nov 21. doi: 10.1053/j.gastro.2017.11.011.
For patients or death in a phase 3b, multicenter, open-label study.
“Although we observed increases in HBV DNA in most patients, these increases were [usually] not associated with ALT [alanine amino transferase] flares or clinical complications,” reported Chun-Jen Liu, MD, of National Taiwan University College of Medicine and Hospital, Taipei, and his associates. Although nearly two-thirds of patients developed HBV reactivation, less than 5% developed alanine aminotransferase rises at least twice the upper limit of normal, and only one patient had symptomatic HBV reactivation, which entecavir therapy resolved. This study was the first to prospectively evaluate the risk of HBV reactivation during HCV treatment, the researchers wrote in the March issue of Gastroenterology.
Because chronic hepatitis C virus infection tends to suppress HBV replication, peginterferon/ribavirin or direct-acting anti-HCV treatment can reactivate HBV infection, especially in patients who test positive for hepatitis B surface antigen (HBsAg). Left untreated, reactivated HBV can lead to fulminant hepatitis, liver failure, and death, as noted on recently mandated boxed warnings.
Accordingly, guidelines recommend testing patients for HBV infection before starting HCV treatment. The study enrolled 111 coinfected patients; about two-thirds were female, and 16% had compensated cirrhosis. All tested positive for HBsAg at screening, and all but one also tested positive at baseline. Mean baseline HBV DNA levels were 2.1 log10 IU/mL. Patients received 90 mg ledipasvir plus 400 mg sofosbuvir for 12 weeks, and levels of HCV RNA, HBV DNA, and HBsAg were tested at weeks 1, 2, 4, 8, 12, posttreatment week 4, and then every 12 weeks until posttreatment week 108.
In all, 70 (63%) patients developed HBV reactivation, including 84% of the 37 patients with undetectable HBV DNA at baseline. During treatment, none of these patients had ALT rise more than twice the upper limit of normal. By 48 weeks post treatment, however, 77% still had quantifiable HBV DNA, and two had marked ALT rises. Furthermore, by posttreatment week 53, one of these patients developed bilirubinemia and symptomatic HBV infection (malaise, anorexia, sclera jaundice, and nausea), which resolved after treatment with entecavir.
A total of 74 patients had quantifiable baseline HBV DNA (at least 20 IU/mL). Three received entecavir or tenofovir disoproxil fumarate based on confirmed HBV reactivation with a concomitant ALT rise of at least twice the upper limit of normal. All were asymptomatic. There were no cases of liver failure or death.
“Regardless of HBV DNA and/or ALT elevations, no patient had signs of liver failure,” the researchers wrote. “Our results support the recommendations put forth in clinical treatment guidelines: HCV-infected patients should be evaluated for HBV infection prior to HCV treatment with direct-acting antivirals. Those who are HBsAg positive should be monitored during and after treatment for HBV reactivation, and treatment should be initiated in accordance with existing guidelines.”
Gilead funded the study. Dr. Liu and 12 coinvestigators reported having no conflicts of interest. Nine coinvestigators reported being employees and shareholders of Gilead, and one coinvestigator reporting consulting for Gilead. The senior author disclosed ties to Roche, Bristol-Myers Squibb, Johnson & Johnson, Bayer, MSD, and Taiha.
SOURCE: Lui C-J et al. Gastroenterology. 2017 Nov 21. doi: 10.1053/j.gastro.2017.11.011.
For patients or death in a phase 3b, multicenter, open-label study.
“Although we observed increases in HBV DNA in most patients, these increases were [usually] not associated with ALT [alanine amino transferase] flares or clinical complications,” reported Chun-Jen Liu, MD, of National Taiwan University College of Medicine and Hospital, Taipei, and his associates. Although nearly two-thirds of patients developed HBV reactivation, less than 5% developed alanine aminotransferase rises at least twice the upper limit of normal, and only one patient had symptomatic HBV reactivation, which entecavir therapy resolved. This study was the first to prospectively evaluate the risk of HBV reactivation during HCV treatment, the researchers wrote in the March issue of Gastroenterology.
Because chronic hepatitis C virus infection tends to suppress HBV replication, peginterferon/ribavirin or direct-acting anti-HCV treatment can reactivate HBV infection, especially in patients who test positive for hepatitis B surface antigen (HBsAg). Left untreated, reactivated HBV can lead to fulminant hepatitis, liver failure, and death, as noted on recently mandated boxed warnings.
Accordingly, guidelines recommend testing patients for HBV infection before starting HCV treatment. The study enrolled 111 coinfected patients; about two-thirds were female, and 16% had compensated cirrhosis. All tested positive for HBsAg at screening, and all but one also tested positive at baseline. Mean baseline HBV DNA levels were 2.1 log10 IU/mL. Patients received 90 mg ledipasvir plus 400 mg sofosbuvir for 12 weeks, and levels of HCV RNA, HBV DNA, and HBsAg were tested at weeks 1, 2, 4, 8, 12, posttreatment week 4, and then every 12 weeks until posttreatment week 108.
In all, 70 (63%) patients developed HBV reactivation, including 84% of the 37 patients with undetectable HBV DNA at baseline. During treatment, none of these patients had ALT rise more than twice the upper limit of normal. By 48 weeks post treatment, however, 77% still had quantifiable HBV DNA, and two had marked ALT rises. Furthermore, by posttreatment week 53, one of these patients developed bilirubinemia and symptomatic HBV infection (malaise, anorexia, sclera jaundice, and nausea), which resolved after treatment with entecavir.
A total of 74 patients had quantifiable baseline HBV DNA (at least 20 IU/mL). Three received entecavir or tenofovir disoproxil fumarate based on confirmed HBV reactivation with a concomitant ALT rise of at least twice the upper limit of normal. All were asymptomatic. There were no cases of liver failure or death.
“Regardless of HBV DNA and/or ALT elevations, no patient had signs of liver failure,” the researchers wrote. “Our results support the recommendations put forth in clinical treatment guidelines: HCV-infected patients should be evaluated for HBV infection prior to HCV treatment with direct-acting antivirals. Those who are HBsAg positive should be monitored during and after treatment for HBV reactivation, and treatment should be initiated in accordance with existing guidelines.”
Gilead funded the study. Dr. Liu and 12 coinvestigators reported having no conflicts of interest. Nine coinvestigators reported being employees and shareholders of Gilead, and one coinvestigator reporting consulting for Gilead. The senior author disclosed ties to Roche, Bristol-Myers Squibb, Johnson & Johnson, Bayer, MSD, and Taiha.
SOURCE: Lui C-J et al. Gastroenterology. 2017 Nov 21. doi: 10.1053/j.gastro.2017.11.011.
FROM GASTROENTEROLOGY
Key clinical point: Combination therapy with sofosbuvir/ledipasvir effectively treated chronic hepatitis C infection in hepatitis B coinfected patients.
Major finding: The rate of sustained viral response was 100% at 12 weeks. Most (63%) of patients had an increase in hepatitis B viral DNA, but only 5% of patients had a concomitant increase in alanine aminotransferase. There were no cases of liver failure or death.
Data source: A phase 3b, multicenter, single-arm, open-label study of 111 coinfected patients.
Disclosures: Gilead funded the study. Dr. Liu and 12 coinvestigators reported having no conflicts of interest. Nine coinvestigators reported being employees and shareholders of Gilead, and one coinvestigator reporting consulting for Gilead. The senior author disclosed ties to Roche, Bristol-Myers Squibb, Johnson & Johnson, Bayer, MSD, and Taiha.
Source: Lui C-J et al. Gastroenterology. 2017 Nov 21. doi: 10.1053/j.gastro.2017.11.011.
NASH rapidly overtaking hepatitis C as cause of liver cancer
Researchers reported on their analysis of past prevalence of HCV, NASH, and alcoholic cirrhosis and prediction of future trends and their effect on hepatocellular carcinoma in the Feb. 24 online edition of the Journal of Clinical and Experimental Hepatology.
The analysis, based on data from the National Health and Nutrition Examination Survey and the Organ Procurement and Transplantation Network, shows that the prevalence of HCV has been in steady decline since 2005 and that decline is forecast to continue. From a prevalence of 3.22 million cases in 2005, researchers have forecasted a decline to 1.06 million cases by 2025.
At the same time, even a conservative linear model for the changing prevalence of NASH forecast a rapid increase from 1.37 million cases in 2005 to 17.95 million in 2025. The exponential model suggested an increase from 2.41 million in 2005 to 42.34 million in 2025.
In terms of the effect on the prevalence of hepatocellular carcinoma (HCC), the modeling suggested cases of HCV-related liver cancer were predicted to peak at around 29,000 cases in 2015 then decline to fewer than 18,000 cases by 2025. In contrast, the prevalence of HCC from NASH is forecast to increase from between 5000 and 6000 cases in 2005 to 45,000 in 2025 by the conservative linear model or even as high as 106,000 cases according to the exponential model. It overtook HCV infection as a cause of liver cancer by around 2015.
“Despite the lack of existing data off of which to work, the general trends of our prediction models are consistent with the documented trends of liver transplant etiology, as well as 2010 insurance data indicating nonalcoholic fatty liver disease/NASH as the leading etiology associated with HCC,” wrote Osmanuddin Ahmed, MD, from the Rush University Medical Center in Chicago and his coauthors.
The study used liver transplant data as a proxy for the prevalence of hepatocellular carcinoma and also took into account the natural history of the disease. Between 5% and 20% of untreated HCV infections will go on to develop into cirrhosis, and of patients with HCV-related cirrhosis, around 15% will develop HCC within 10 years. In the case of NASH, the authors cited research suggesting that around 35% of patients go on to develop progressive fibrosis, that progression to cirrhosis takes around 29 years, and that the risk of progression to HCC ranged from 2.4% over 7 years to 12.8% over 3 years.
“A higher proportion of patients with NASH develop cirrhosis, but of those who develop cirrhosis, the probability of developing HCC is higher in patients with HCV,” the authors wrote. “In contrast, HCV progression to HCC rarely occurs in noncirrhotic patients.”
The authors wrote that it was important to explore projected trends in the etiology of hepatocellular carcinoma to inform the development of screening, diagnostic, and treatment approaches, particularly given potential differences in the pathology, natural history, and treatment options for NASH-related and HCV-related liver cancer.
“Histologically, NASH shares characteristics with alcoholic liver disease, primarily proinflammatory fat accumulation in parenchymal cells, [and] key players in NASH progression to HCC are suggested to include genetic modifications, proinflammatory high-fat and/or high-fructose diets, and oxidative and endoplasmic cellular stresses,” they wrote. “In HCV progression to HCC, the presence of the HCV core protein may induce HCC without the prerequisite load of genetic errors normally required for cancer development, skipping or accelerating some of the classic steps of cancer induction.”
The authors did note that their model represented a base scenario that assumed the environmental and genetic factors driving NASH would continue along the path of current trends.
“Therefore, the possibility exists that our models underestimate the response of the medical community in addressing the rising nonalcoholic fatty liver disease/NASH epidemic.”
No funding sources or conflicts of interest were declared.
SOURCE: Ahmed O et al. J Clin Exp Hepatology. 2018 Feb 24. doi: 10.1016/j.jceh.2018.02.006.
Researchers reported on their analysis of past prevalence of HCV, NASH, and alcoholic cirrhosis and prediction of future trends and their effect on hepatocellular carcinoma in the Feb. 24 online edition of the Journal of Clinical and Experimental Hepatology.
The analysis, based on data from the National Health and Nutrition Examination Survey and the Organ Procurement and Transplantation Network, shows that the prevalence of HCV has been in steady decline since 2005 and that decline is forecast to continue. From a prevalence of 3.22 million cases in 2005, researchers have forecasted a decline to 1.06 million cases by 2025.
At the same time, even a conservative linear model for the changing prevalence of NASH forecast a rapid increase from 1.37 million cases in 2005 to 17.95 million in 2025. The exponential model suggested an increase from 2.41 million in 2005 to 42.34 million in 2025.
In terms of the effect on the prevalence of hepatocellular carcinoma (HCC), the modeling suggested cases of HCV-related liver cancer were predicted to peak at around 29,000 cases in 2015 then decline to fewer than 18,000 cases by 2025. In contrast, the prevalence of HCC from NASH is forecast to increase from between 5000 and 6000 cases in 2005 to 45,000 in 2025 by the conservative linear model or even as high as 106,000 cases according to the exponential model. It overtook HCV infection as a cause of liver cancer by around 2015.
“Despite the lack of existing data off of which to work, the general trends of our prediction models are consistent with the documented trends of liver transplant etiology, as well as 2010 insurance data indicating nonalcoholic fatty liver disease/NASH as the leading etiology associated with HCC,” wrote Osmanuddin Ahmed, MD, from the Rush University Medical Center in Chicago and his coauthors.
The study used liver transplant data as a proxy for the prevalence of hepatocellular carcinoma and also took into account the natural history of the disease. Between 5% and 20% of untreated HCV infections will go on to develop into cirrhosis, and of patients with HCV-related cirrhosis, around 15% will develop HCC within 10 years. In the case of NASH, the authors cited research suggesting that around 35% of patients go on to develop progressive fibrosis, that progression to cirrhosis takes around 29 years, and that the risk of progression to HCC ranged from 2.4% over 7 years to 12.8% over 3 years.
“A higher proportion of patients with NASH develop cirrhosis, but of those who develop cirrhosis, the probability of developing HCC is higher in patients with HCV,” the authors wrote. “In contrast, HCV progression to HCC rarely occurs in noncirrhotic patients.”
The authors wrote that it was important to explore projected trends in the etiology of hepatocellular carcinoma to inform the development of screening, diagnostic, and treatment approaches, particularly given potential differences in the pathology, natural history, and treatment options for NASH-related and HCV-related liver cancer.
“Histologically, NASH shares characteristics with alcoholic liver disease, primarily proinflammatory fat accumulation in parenchymal cells, [and] key players in NASH progression to HCC are suggested to include genetic modifications, proinflammatory high-fat and/or high-fructose diets, and oxidative and endoplasmic cellular stresses,” they wrote. “In HCV progression to HCC, the presence of the HCV core protein may induce HCC without the prerequisite load of genetic errors normally required for cancer development, skipping or accelerating some of the classic steps of cancer induction.”
The authors did note that their model represented a base scenario that assumed the environmental and genetic factors driving NASH would continue along the path of current trends.
“Therefore, the possibility exists that our models underestimate the response of the medical community in addressing the rising nonalcoholic fatty liver disease/NASH epidemic.”
No funding sources or conflicts of interest were declared.
SOURCE: Ahmed O et al. J Clin Exp Hepatology. 2018 Feb 24. doi: 10.1016/j.jceh.2018.02.006.
Researchers reported on their analysis of past prevalence of HCV, NASH, and alcoholic cirrhosis and prediction of future trends and their effect on hepatocellular carcinoma in the Feb. 24 online edition of the Journal of Clinical and Experimental Hepatology.
The analysis, based on data from the National Health and Nutrition Examination Survey and the Organ Procurement and Transplantation Network, shows that the prevalence of HCV has been in steady decline since 2005 and that decline is forecast to continue. From a prevalence of 3.22 million cases in 2005, researchers have forecasted a decline to 1.06 million cases by 2025.
At the same time, even a conservative linear model for the changing prevalence of NASH forecast a rapid increase from 1.37 million cases in 2005 to 17.95 million in 2025. The exponential model suggested an increase from 2.41 million in 2005 to 42.34 million in 2025.
In terms of the effect on the prevalence of hepatocellular carcinoma (HCC), the modeling suggested cases of HCV-related liver cancer were predicted to peak at around 29,000 cases in 2015 then decline to fewer than 18,000 cases by 2025. In contrast, the prevalence of HCC from NASH is forecast to increase from between 5000 and 6000 cases in 2005 to 45,000 in 2025 by the conservative linear model or even as high as 106,000 cases according to the exponential model. It overtook HCV infection as a cause of liver cancer by around 2015.
“Despite the lack of existing data off of which to work, the general trends of our prediction models are consistent with the documented trends of liver transplant etiology, as well as 2010 insurance data indicating nonalcoholic fatty liver disease/NASH as the leading etiology associated with HCC,” wrote Osmanuddin Ahmed, MD, from the Rush University Medical Center in Chicago and his coauthors.
The study used liver transplant data as a proxy for the prevalence of hepatocellular carcinoma and also took into account the natural history of the disease. Between 5% and 20% of untreated HCV infections will go on to develop into cirrhosis, and of patients with HCV-related cirrhosis, around 15% will develop HCC within 10 years. In the case of NASH, the authors cited research suggesting that around 35% of patients go on to develop progressive fibrosis, that progression to cirrhosis takes around 29 years, and that the risk of progression to HCC ranged from 2.4% over 7 years to 12.8% over 3 years.
“A higher proportion of patients with NASH develop cirrhosis, but of those who develop cirrhosis, the probability of developing HCC is higher in patients with HCV,” the authors wrote. “In contrast, HCV progression to HCC rarely occurs in noncirrhotic patients.”
The authors wrote that it was important to explore projected trends in the etiology of hepatocellular carcinoma to inform the development of screening, diagnostic, and treatment approaches, particularly given potential differences in the pathology, natural history, and treatment options for NASH-related and HCV-related liver cancer.
“Histologically, NASH shares characteristics with alcoholic liver disease, primarily proinflammatory fat accumulation in parenchymal cells, [and] key players in NASH progression to HCC are suggested to include genetic modifications, proinflammatory high-fat and/or high-fructose diets, and oxidative and endoplasmic cellular stresses,” they wrote. “In HCV progression to HCC, the presence of the HCV core protein may induce HCC without the prerequisite load of genetic errors normally required for cancer development, skipping or accelerating some of the classic steps of cancer induction.”
The authors did note that their model represented a base scenario that assumed the environmental and genetic factors driving NASH would continue along the path of current trends.
“Therefore, the possibility exists that our models underestimate the response of the medical community in addressing the rising nonalcoholic fatty liver disease/NASH epidemic.”
No funding sources or conflicts of interest were declared.
SOURCE: Ahmed O et al. J Clin Exp Hepatology. 2018 Feb 24. doi: 10.1016/j.jceh.2018.02.006.
FROM THE JOURNAL OF CLINICAL AND EXPERIMENTAL HEPATOLOGY
Key clinical point: NASH is rapidly eclipsing HCV infection as the leading contributor to liver cancer in the United States.
Major finding: The prevalence of HCV infection is forecast to decline to 1.06 million cases by 2025 while the prevalence of NASH is projected to increase to as many as 42.34 million cases by 2025.
Data source: Analysis based on data from the National Health and Nutrition Examination Survey and the Organ Procurement and Transplantation Network.
Disclosures: No funding sources or conflicts of interest were declared.
Source: Ahmed O et al. J Clin Exp Hepatology. 2018 Feb 24. doi: 10.1016/j.jceh.2018.02.006.
ACIP unanimously recommends HEPLISAV-B
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
REPORTING FROM AN ACIP MEETING
Liver cancer deaths expected to increase again in 2018
Liver cancer mortality for 2018 is expected to be lowest in Utah and highest in Hawaii.
A total of 30,200 deaths from liver and intrahepatic bile duct cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That’s up from the 28,920 predicted by the ACS for 2017.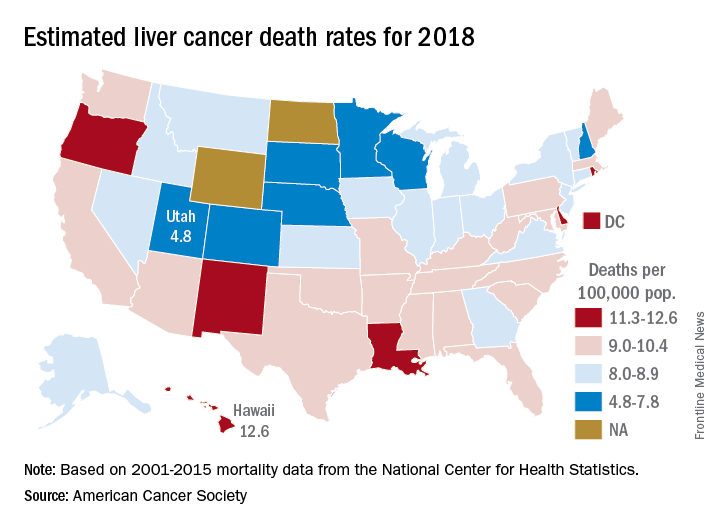
Mortality from liver cancer has been rising since the early 1980s, and in the last 10 years for which data are available (2006-2015), it increased by 2.5% per year. Over almost the same period (2005-2014), incidence rose by approximately 3% a year, and 42,220 new cases are expected in 2018, the ACS noted.
Over the most recent 5 years with available data (2011-2015), racial and ethnic disparities put American Indian/Alaska Natives at the highest mortality risk – 14.8 per 100,000 for men and 7.0 for women – followed by Asian/Pacific Islanders at 14.0 and 6.0, respectively. Non-Hispanic whites had the lowest rates: 8.2 for men and 3.4 for women, according to the ACS report.
Read more about the ACS’s research and estimates here.
Liver cancer mortality for 2018 is expected to be lowest in Utah and highest in Hawaii.
A total of 30,200 deaths from liver and intrahepatic bile duct cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That’s up from the 28,920 predicted by the ACS for 2017.
Mortality from liver cancer has been rising since the early 1980s, and in the last 10 years for which data are available (2006-2015), it increased by 2.5% per year. Over almost the same period (2005-2014), incidence rose by approximately 3% a year, and 42,220 new cases are expected in 2018, the ACS noted.
Over the most recent 5 years with available data (2011-2015), racial and ethnic disparities put American Indian/Alaska Natives at the highest mortality risk – 14.8 per 100,000 for men and 7.0 for women – followed by Asian/Pacific Islanders at 14.0 and 6.0, respectively. Non-Hispanic whites had the lowest rates: 8.2 for men and 3.4 for women, according to the ACS report.
Read more about the ACS’s research and estimates here.
Liver cancer mortality for 2018 is expected to be lowest in Utah and highest in Hawaii.
A total of 30,200 deaths from liver and intrahepatic bile duct cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That’s up from the 28,920 predicted by the ACS for 2017.
Mortality from liver cancer has been rising since the early 1980s, and in the last 10 years for which data are available (2006-2015), it increased by 2.5% per year. Over almost the same period (2005-2014), incidence rose by approximately 3% a year, and 42,220 new cases are expected in 2018, the ACS noted.
Over the most recent 5 years with available data (2011-2015), racial and ethnic disparities put American Indian/Alaska Natives at the highest mortality risk – 14.8 per 100,000 for men and 7.0 for women – followed by Asian/Pacific Islanders at 14.0 and 6.0, respectively. Non-Hispanic whites had the lowest rates: 8.2 for men and 3.4 for women, according to the ACS report.
Read more about the ACS’s research and estimates here.
Obesity affects the ability to diagnose liver fibrosis
Body mass index accounts for a 43.7% discordance in fibrosis findings between magnetic resonance elastography (MRE) and transient elastography (TE), according to a study from the University of California, San Diego.
“This study demonstrates that BMI is a significant factor of discordancy between MRE and TE for the stage of significant fibrosis (2-4 vs. 0-1),” wrote Cyrielle Caussy, MD, and her colleagues (Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037). “Furthermore, this study showed that the grade of obesity is also a significant predictor of discordancy between MRE and TE because the discordance rate between MRE and TE increases with the increase in BMI.”
Dr. Caussy of the University of California, San Diego, and her colleagues had noted that MRE and TE had discordant findings in obese patients. To ascertain under what conditions TE and MRE produce the same readings, Dr. Caussy and her associates conducted a cross-sectional study of two cohorts with nonalcoholic fatty liver disease (NAFLD) who underwent contemporaneous MRE, TE, and liver biopsy. TE utilized both M and XL probes during imaging. The training cohort involved 119 adult patients undergoing NAFLD testing from October 2011 through January 2017. The validation cohort, consisting of 75 adults with NAFLD undergoing liver imaging from March 2010 through May 2013, was formed to validate the findings of the training cohort.
The study revealed that BMI was a significant predictor of the difference between MRE and TE results and made it difficult to assess the stage of liver fibrosis (2-4 vs. 0-1). After adjustment for age and sex, BMI accounted for a 5-unit increase of 1.694 (95% confidence interval, 1.145-2.507; P = .008). This was not a static relationship, and as BMI increased, so did the discordance between MRE and TE (P = .0309). Interestingly, the discordance rate was significantly higher in participants with BMIs greater than 35 kg/m2, compared with participants with BMIs below 35 (63.0% vs. 38.0%; P = .022), the investigators reported.
While the study revealed valuable information, it had both strengths and limitations. A strength of the study was the use of two cohorts, specifically the validation cohort. The use of the liver biopsy as a reference, which is the standard for assessing fibrosis, was also a strength of the study. A limitation was that the study was conducted at specialized, tertiary care centers using advanced imaging techniques that may not be available at other clinics. Additionally, the cohorts included a small number of patients with advanced fibrosis.
“The integration of the BMI in the screening strategy for the noninvasive detection of liver fibrosis in NAFLD should be considered, and this parameter would help to determine when MRE is not needed in future guidelines” wrote Dr. Caussy and her associates. “Further cost-effectiveness studies are necessary to evaluate the clinical utility of MRE, TE, and/or liver biopsy to develop optimal screening strategies for diagnosing NAFLD-associated fibrosis.”
Jun Chen, MD, Meng Yin, MD, and Richard L. Ehman, MD, all have intellectual property rights and financial interests in elastography technology. Dr. Ehman also serves as an noncompensated CEO of Resoundant. Claude B. Sirlin, MD, has served as a consultant to Bayer and GE Healthcare. All other authors did not disclose any conflicts.
The AGA Obesity Practice Guide provides a comprehensive, multi-disciplinary process to personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
SOURCE: Caussy C et al. Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037.
Body mass index accounts for a 43.7% discordance in fibrosis findings between magnetic resonance elastography (MRE) and transient elastography (TE), according to a study from the University of California, San Diego.
“This study demonstrates that BMI is a significant factor of discordancy between MRE and TE for the stage of significant fibrosis (2-4 vs. 0-1),” wrote Cyrielle Caussy, MD, and her colleagues (Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037). “Furthermore, this study showed that the grade of obesity is also a significant predictor of discordancy between MRE and TE because the discordance rate between MRE and TE increases with the increase in BMI.”
Dr. Caussy of the University of California, San Diego, and her colleagues had noted that MRE and TE had discordant findings in obese patients. To ascertain under what conditions TE and MRE produce the same readings, Dr. Caussy and her associates conducted a cross-sectional study of two cohorts with nonalcoholic fatty liver disease (NAFLD) who underwent contemporaneous MRE, TE, and liver biopsy. TE utilized both M and XL probes during imaging. The training cohort involved 119 adult patients undergoing NAFLD testing from October 2011 through January 2017. The validation cohort, consisting of 75 adults with NAFLD undergoing liver imaging from March 2010 through May 2013, was formed to validate the findings of the training cohort.
The study revealed that BMI was a significant predictor of the difference between MRE and TE results and made it difficult to assess the stage of liver fibrosis (2-4 vs. 0-1). After adjustment for age and sex, BMI accounted for a 5-unit increase of 1.694 (95% confidence interval, 1.145-2.507; P = .008). This was not a static relationship, and as BMI increased, so did the discordance between MRE and TE (P = .0309). Interestingly, the discordance rate was significantly higher in participants with BMIs greater than 35 kg/m2, compared with participants with BMIs below 35 (63.0% vs. 38.0%; P = .022), the investigators reported.
While the study revealed valuable information, it had both strengths and limitations. A strength of the study was the use of two cohorts, specifically the validation cohort. The use of the liver biopsy as a reference, which is the standard for assessing fibrosis, was also a strength of the study. A limitation was that the study was conducted at specialized, tertiary care centers using advanced imaging techniques that may not be available at other clinics. Additionally, the cohorts included a small number of patients with advanced fibrosis.
“The integration of the BMI in the screening strategy for the noninvasive detection of liver fibrosis in NAFLD should be considered, and this parameter would help to determine when MRE is not needed in future guidelines” wrote Dr. Caussy and her associates. “Further cost-effectiveness studies are necessary to evaluate the clinical utility of MRE, TE, and/or liver biopsy to develop optimal screening strategies for diagnosing NAFLD-associated fibrosis.”
Jun Chen, MD, Meng Yin, MD, and Richard L. Ehman, MD, all have intellectual property rights and financial interests in elastography technology. Dr. Ehman also serves as an noncompensated CEO of Resoundant. Claude B. Sirlin, MD, has served as a consultant to Bayer and GE Healthcare. All other authors did not disclose any conflicts.
The AGA Obesity Practice Guide provides a comprehensive, multi-disciplinary process to personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
SOURCE: Caussy C et al. Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037.
Body mass index accounts for a 43.7% discordance in fibrosis findings between magnetic resonance elastography (MRE) and transient elastography (TE), according to a study from the University of California, San Diego.
“This study demonstrates that BMI is a significant factor of discordancy between MRE and TE for the stage of significant fibrosis (2-4 vs. 0-1),” wrote Cyrielle Caussy, MD, and her colleagues (Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037). “Furthermore, this study showed that the grade of obesity is also a significant predictor of discordancy between MRE and TE because the discordance rate between MRE and TE increases with the increase in BMI.”
Dr. Caussy of the University of California, San Diego, and her colleagues had noted that MRE and TE had discordant findings in obese patients. To ascertain under what conditions TE and MRE produce the same readings, Dr. Caussy and her associates conducted a cross-sectional study of two cohorts with nonalcoholic fatty liver disease (NAFLD) who underwent contemporaneous MRE, TE, and liver biopsy. TE utilized both M and XL probes during imaging. The training cohort involved 119 adult patients undergoing NAFLD testing from October 2011 through January 2017. The validation cohort, consisting of 75 adults with NAFLD undergoing liver imaging from March 2010 through May 2013, was formed to validate the findings of the training cohort.
The study revealed that BMI was a significant predictor of the difference between MRE and TE results and made it difficult to assess the stage of liver fibrosis (2-4 vs. 0-1). After adjustment for age and sex, BMI accounted for a 5-unit increase of 1.694 (95% confidence interval, 1.145-2.507; P = .008). This was not a static relationship, and as BMI increased, so did the discordance between MRE and TE (P = .0309). Interestingly, the discordance rate was significantly higher in participants with BMIs greater than 35 kg/m2, compared with participants with BMIs below 35 (63.0% vs. 38.0%; P = .022), the investigators reported.
While the study revealed valuable information, it had both strengths and limitations. A strength of the study was the use of two cohorts, specifically the validation cohort. The use of the liver biopsy as a reference, which is the standard for assessing fibrosis, was also a strength of the study. A limitation was that the study was conducted at specialized, tertiary care centers using advanced imaging techniques that may not be available at other clinics. Additionally, the cohorts included a small number of patients with advanced fibrosis.
“The integration of the BMI in the screening strategy for the noninvasive detection of liver fibrosis in NAFLD should be considered, and this parameter would help to determine when MRE is not needed in future guidelines” wrote Dr. Caussy and her associates. “Further cost-effectiveness studies are necessary to evaluate the clinical utility of MRE, TE, and/or liver biopsy to develop optimal screening strategies for diagnosing NAFLD-associated fibrosis.”
Jun Chen, MD, Meng Yin, MD, and Richard L. Ehman, MD, all have intellectual property rights and financial interests in elastography technology. Dr. Ehman also serves as an noncompensated CEO of Resoundant. Claude B. Sirlin, MD, has served as a consultant to Bayer and GE Healthcare. All other authors did not disclose any conflicts.
The AGA Obesity Practice Guide provides a comprehensive, multi-disciplinary process to personalize innovative obesity care for safe and effective weight management. Learn more at www.gastro.org/obesity.
SOURCE: Caussy C et al. Clin Gastrolenterol Hepatol. 2018 Jan 15. doi: 10.1016/j.cgh.2017.10.037.
Psychiatric issues common among hepatitis C inpatients
Adult inpatients with hepatitis C are much more likely to have mental health comorbidities, compared with those who do not have hepatitis C, according to the Agency for Healthcare Research and Quality.
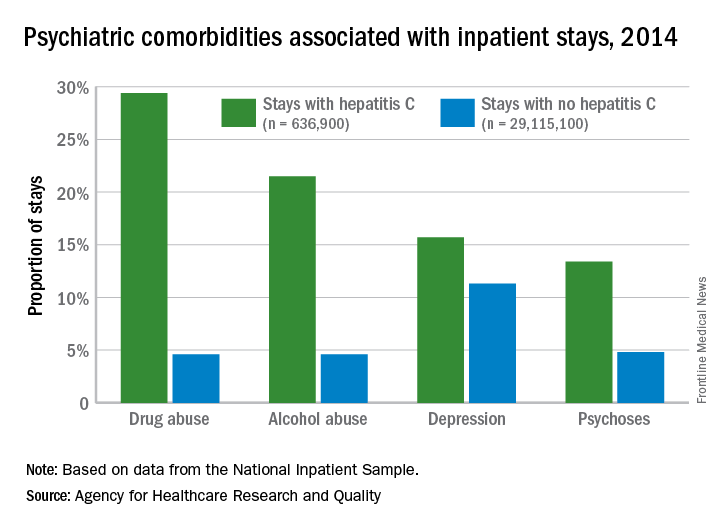
All four comorbidities skewed younger, and the oldest patients (73 years and older) with hepatitis C presented with each condition at about the same rate as the non–hepatitis C population. The proportions of hepatitis C–related inpatient stays with alcohol abuse by age, for example, were 20.5% for 18-51 years, 23.3% for those aged 52-72, and 5.8% for the 73-and-older group, according to data from the National Inpatient Sample, which includes more than 95% of all discharges from community (short-term, nonfederal, nonrehabilitation) hospitals in the United States.
Adult inpatients with hepatitis C are much more likely to have mental health comorbidities, compared with those who do not have hepatitis C, according to the Agency for Healthcare Research and Quality.

All four comorbidities skewed younger, and the oldest patients (73 years and older) with hepatitis C presented with each condition at about the same rate as the non–hepatitis C population. The proportions of hepatitis C–related inpatient stays with alcohol abuse by age, for example, were 20.5% for 18-51 years, 23.3% for those aged 52-72, and 5.8% for the 73-and-older group, according to data from the National Inpatient Sample, which includes more than 95% of all discharges from community (short-term, nonfederal, nonrehabilitation) hospitals in the United States.
Adult inpatients with hepatitis C are much more likely to have mental health comorbidities, compared with those who do not have hepatitis C, according to the Agency for Healthcare Research and Quality.

All four comorbidities skewed younger, and the oldest patients (73 years and older) with hepatitis C presented with each condition at about the same rate as the non–hepatitis C population. The proportions of hepatitis C–related inpatient stays with alcohol abuse by age, for example, were 20.5% for 18-51 years, 23.3% for those aged 52-72, and 5.8% for the 73-and-older group, according to data from the National Inpatient Sample, which includes more than 95% of all discharges from community (short-term, nonfederal, nonrehabilitation) hospitals in the United States.