User login
Abdominal pain and constipation
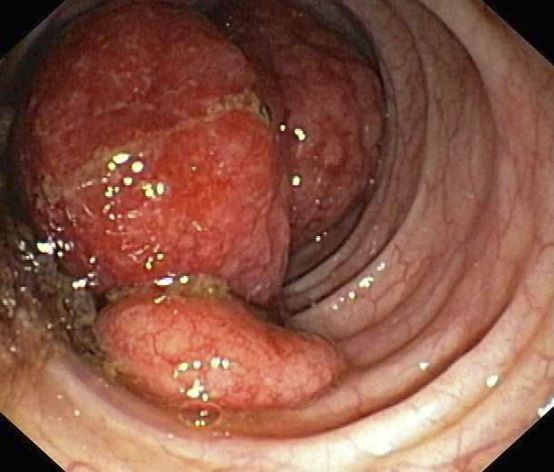
This patient's clinical presentation and endoscopy findings are consistent with a diagnosis of recurrent MCL presenting as a colonic mass.
MCL is an aggressive type of non-Hodgkin lymphoma that accounts for approximately 5%-7% of all lymphomas. Nearly 80% of patients have extranodal involvement at initial presentation, occurring in sites such as the bone marrow, spleen, Waldeyer ring, and the gastrointestinal (GI) tract. Secondary GI involvement in MCL (involving nodal and/or other extranodal tissue) is common and may be detected at diagnosis and/or relapse. In several retrospective studies, the prevalence of secondary GI involvement in MCL ranged from 15% to 30%. However, in later studies, routine endoscopies in patients with untreated MCL showed GI involvement in up to 90% of patients, despite most patients not reporting GI symptoms.
The colon is the most commonly involved GI site; however, both the upper and lower GI tract from the stomach to the colon can be involved. Lymphomatous polyposis is the most common endoscopic presentation of MCL, but polyp, mass, or even normal-appearing mucosa may also be seen.
New and emerging treatment options are helping to improve survival in patients with relapsed/refractory MCL. According to National Comprehensive Cancer Network guidelines, the preferred second-line and subsequent regimens are:
• Bruton tyrosine kinase (BTK) inhibitors:
o Acalabrutinib
o Ibrutinib ± rituximab
o Zanubrutinib
• Lenalidomide + rituximab (if BTK inhibitor is contraindicated)
Other regimens that may be useful in certain circumstances are:
• Bendamustine + rituximab (if not previously given)
• Bendamustine + rituximab + cytarabine (RBAC500) (if not previously given)
• Bortezomib ± rituximab
• RDHA (rituximab, dexamethasone, cytarabine) + platinum (carboplatin, cisplatin, or oxaliplatin) (if not previously given)
• GemOx (gemcitabine, oxaliplatin) + rituximab
• Ibrutinib, lenalidomide, rituximab (category 2B)
• Ibrutinib + venetoclax
• Venetoclax, lenalidomide, rituximab (category 2B)
• Venetoclax ± rituximab
Brexucabtagene autoleucel is suggested as third-line therapy, after chemoimmunotherapy and treatment with a BTK inhibitor.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation and endoscopy findings are consistent with a diagnosis of recurrent MCL presenting as a colonic mass.
MCL is an aggressive type of non-Hodgkin lymphoma that accounts for approximately 5%-7% of all lymphomas. Nearly 80% of patients have extranodal involvement at initial presentation, occurring in sites such as the bone marrow, spleen, Waldeyer ring, and the gastrointestinal (GI) tract. Secondary GI involvement in MCL (involving nodal and/or other extranodal tissue) is common and may be detected at diagnosis and/or relapse. In several retrospective studies, the prevalence of secondary GI involvement in MCL ranged from 15% to 30%. However, in later studies, routine endoscopies in patients with untreated MCL showed GI involvement in up to 90% of patients, despite most patients not reporting GI symptoms.
The colon is the most commonly involved GI site; however, both the upper and lower GI tract from the stomach to the colon can be involved. Lymphomatous polyposis is the most common endoscopic presentation of MCL, but polyp, mass, or even normal-appearing mucosa may also be seen.
New and emerging treatment options are helping to improve survival in patients with relapsed/refractory MCL. According to National Comprehensive Cancer Network guidelines, the preferred second-line and subsequent regimens are:
• Bruton tyrosine kinase (BTK) inhibitors:
o Acalabrutinib
o Ibrutinib ± rituximab
o Zanubrutinib
• Lenalidomide + rituximab (if BTK inhibitor is contraindicated)
Other regimens that may be useful in certain circumstances are:
• Bendamustine + rituximab (if not previously given)
• Bendamustine + rituximab + cytarabine (RBAC500) (if not previously given)
• Bortezomib ± rituximab
• RDHA (rituximab, dexamethasone, cytarabine) + platinum (carboplatin, cisplatin, or oxaliplatin) (if not previously given)
• GemOx (gemcitabine, oxaliplatin) + rituximab
• Ibrutinib, lenalidomide, rituximab (category 2B)
• Ibrutinib + venetoclax
• Venetoclax, lenalidomide, rituximab (category 2B)
• Venetoclax ± rituximab
Brexucabtagene autoleucel is suggested as third-line therapy, after chemoimmunotherapy and treatment with a BTK inhibitor.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation and endoscopy findings are consistent with a diagnosis of recurrent MCL presenting as a colonic mass.
MCL is an aggressive type of non-Hodgkin lymphoma that accounts for approximately 5%-7% of all lymphomas. Nearly 80% of patients have extranodal involvement at initial presentation, occurring in sites such as the bone marrow, spleen, Waldeyer ring, and the gastrointestinal (GI) tract. Secondary GI involvement in MCL (involving nodal and/or other extranodal tissue) is common and may be detected at diagnosis and/or relapse. In several retrospective studies, the prevalence of secondary GI involvement in MCL ranged from 15% to 30%. However, in later studies, routine endoscopies in patients with untreated MCL showed GI involvement in up to 90% of patients, despite most patients not reporting GI symptoms.
The colon is the most commonly involved GI site; however, both the upper and lower GI tract from the stomach to the colon can be involved. Lymphomatous polyposis is the most common endoscopic presentation of MCL, but polyp, mass, or even normal-appearing mucosa may also be seen.
New and emerging treatment options are helping to improve survival in patients with relapsed/refractory MCL. According to National Comprehensive Cancer Network guidelines, the preferred second-line and subsequent regimens are:
• Bruton tyrosine kinase (BTK) inhibitors:
o Acalabrutinib
o Ibrutinib ± rituximab
o Zanubrutinib
• Lenalidomide + rituximab (if BTK inhibitor is contraindicated)
Other regimens that may be useful in certain circumstances are:
• Bendamustine + rituximab (if not previously given)
• Bendamustine + rituximab + cytarabine (RBAC500) (if not previously given)
• Bortezomib ± rituximab
• RDHA (rituximab, dexamethasone, cytarabine) + platinum (carboplatin, cisplatin, or oxaliplatin) (if not previously given)
• GemOx (gemcitabine, oxaliplatin) + rituximab
• Ibrutinib, lenalidomide, rituximab (category 2B)
• Ibrutinib + venetoclax
• Venetoclax, lenalidomide, rituximab (category 2B)
• Venetoclax ± rituximab
Brexucabtagene autoleucel is suggested as third-line therapy, after chemoimmunotherapy and treatment with a BTK inhibitor.
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 55-year-old White woman presents with complaints of left-sided abdominal pain and constipation of 10-day duration. The patient's prior medical history is notable for mantle cell lymphoma (MCL) treated 2 years earlier with RDHA (rituximab, dexamethasone, cytarabine) + platinum (carboplatin, cisplatin, or oxaliplatin) followed by autologous stem cell transplantation. No lymphadenopathy is noted on physical examination. Abdominal examination reveals abdominal distension, normal bowel sounds, and left lower quadrant tenderness to palpation without guarding, rigidity, or hepatosplenomegaly. Laboratory test results including CBC are within normal range. Endoscopy reveals a growth in the colon, as shown in the image.
Fatigue and sporadic fever
This patient's findings are consistent with a diagnosis of malignant mantle cell lymphoma (MCL).
MCL is a rare and aggressive form of non-Hodgkin lymphoma that accounts for approximately 5%-7% of all lymphomas. MCL has a characteristic immunophenotype (ie, CD5+, CD10−, Bcl-2+, Bcl-6−, CD20+), with the t(11;14)(q13;q32) chromosomal translocation, and expression of cyclin D1. The median age at diagnosis is between 60 and 70 years. Approximately 70% of all cases occur in men.
The clinical presentation of MCL can vary. Patients may have asymptomatic monoclonal MCL type lymphocytosis or nonbulky nodal/extra nodal disease with minimal symptoms, or they may present with significant symptoms, progressive generalized lymphadenopathy, cytopenia, splenomegaly, and extranodal disease, including gastrointestinal involvement (lymphomatous polyposis), kidney involvement, involvement of other organs, or, rarely, central nervous system involvement. Disease involving multiple lymph nodes and other sites of the body is seen in most patients. Approximately 70% of patients present with stage IV disease requiring systemic treatment.
According to 2022 guidelines from the National Comprehensive Cancer Network (NCCN), essential components in the workup for MCL include:
• Physical examination, with attention to node-bearing areas, including Waldeyer ring, and to size of liver and spleen
• Assessment of performance status and B symptoms (ie, fever > 100.4°F [may be sporadic], drenching night sweats, unintentional weight loss of > 10% of body weight over 6 months or less)
• CBC with differential
• Comprehensive metabolic panel
• Serum lactate dehydrogenase (LDH) level (an important prognostic marker)
• PET/CT scan (including neck)
• Hepatitis B testing if treatment with rituximab is being contemplated
• Echocardiogram or multigated acquisition (MUGA) scan if anthracycline or anthracenedione-based regimen is indicated
• Pregnancy testing in women of childbearing age (if chemotherapy or radiation therapy is planned)
Additional testing may be indicated in specific circumstances, such as colonoscopy/endoscopy.
MCL remains challenging to treat. While 50%-90% of patients with MCL respond to combination chemotherapy, only 30% achieve a complete response. Median time to treatment failure is < 18 months.
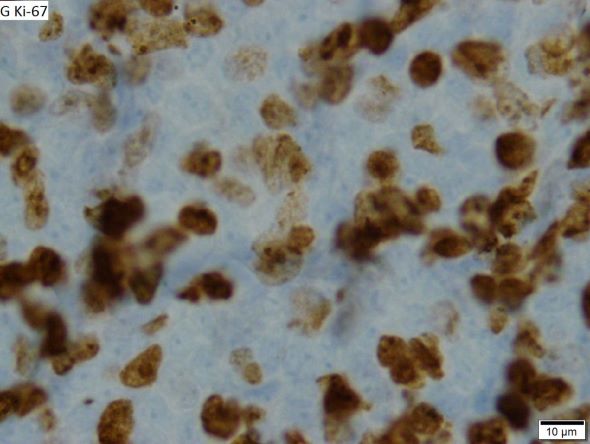
When selecting systemic treatment for patients with MCL, clinicians should consider the availability of clinical trials for subsets of patients, eligibility for stem cell transplant (SCT), high-risk status (ie, blastoid MCL, high Ki-67% > 30%, or central nervous system involvement), age, and performance status. The addition of radiation to chemotherapy may be beneficial for patients with limited-stage, nonbulky disease, although this has not been confirmed in large, randomized studies. Outside of clinical trials, the usual approach for frontline treatment of MCL is chemoimmunotherapy with/without autologous SCT and with/without maintenance therapy.
Available options for primary MCL therapy in patients who require systemic therapy include:
• Single alkylating agents
• CVP (cyclophosphamide, vincristine, prednisone)
• CHOP (cyclophosphamide, doxorubicin [hydroxydaunorubicin], vincristine [Oncovin], prednisone)
• Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) with or without rituximab
• R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone)
• Lenalidomide plus rituximab
• Hyper-CVAD with autologous SCT
Options for relapsed or refractory MCL include:
• R-hyper-CVAD
• Hyper-CVAD with or without rituximab followed by autologous SCT
• Nucleoside analogues and combinations
• Salvage chemotherapy combinations followed by autologous SCT
• Bortezomib
• Lenalidomide
• Ibrutinib
• Radioimmunotherapy
• Rituximab
• Rituximab and thalidomide combination
• Acalabrutinib
• High-dose chemotherapy with autologous bone marrow or SCT
• Brexucabtagene autoleucel
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's findings are consistent with a diagnosis of malignant mantle cell lymphoma (MCL).
MCL is a rare and aggressive form of non-Hodgkin lymphoma that accounts for approximately 5%-7% of all lymphomas. MCL has a characteristic immunophenotype (ie, CD5+, CD10−, Bcl-2+, Bcl-6−, CD20+), with the t(11;14)(q13;q32) chromosomal translocation, and expression of cyclin D1. The median age at diagnosis is between 60 and 70 years. Approximately 70% of all cases occur in men.
The clinical presentation of MCL can vary. Patients may have asymptomatic monoclonal MCL type lymphocytosis or nonbulky nodal/extra nodal disease with minimal symptoms, or they may present with significant symptoms, progressive generalized lymphadenopathy, cytopenia, splenomegaly, and extranodal disease, including gastrointestinal involvement (lymphomatous polyposis), kidney involvement, involvement of other organs, or, rarely, central nervous system involvement. Disease involving multiple lymph nodes and other sites of the body is seen in most patients. Approximately 70% of patients present with stage IV disease requiring systemic treatment.
According to 2022 guidelines from the National Comprehensive Cancer Network (NCCN), essential components in the workup for MCL include:
• Physical examination, with attention to node-bearing areas, including Waldeyer ring, and to size of liver and spleen
• Assessment of performance status and B symptoms (ie, fever > 100.4°F [may be sporadic], drenching night sweats, unintentional weight loss of > 10% of body weight over 6 months or less)
• CBC with differential
• Comprehensive metabolic panel
• Serum lactate dehydrogenase (LDH) level (an important prognostic marker)
• PET/CT scan (including neck)
• Hepatitis B testing if treatment with rituximab is being contemplated
• Echocardiogram or multigated acquisition (MUGA) scan if anthracycline or anthracenedione-based regimen is indicated
• Pregnancy testing in women of childbearing age (if chemotherapy or radiation therapy is planned)
Additional testing may be indicated in specific circumstances, such as colonoscopy/endoscopy.
MCL remains challenging to treat. While 50%-90% of patients with MCL respond to combination chemotherapy, only 30% achieve a complete response. Median time to treatment failure is < 18 months.
When selecting systemic treatment for patients with MCL, clinicians should consider the availability of clinical trials for subsets of patients, eligibility for stem cell transplant (SCT), high-risk status (ie, blastoid MCL, high Ki-67% > 30%, or central nervous system involvement), age, and performance status. The addition of radiation to chemotherapy may be beneficial for patients with limited-stage, nonbulky disease, although this has not been confirmed in large, randomized studies. Outside of clinical trials, the usual approach for frontline treatment of MCL is chemoimmunotherapy with/without autologous SCT and with/without maintenance therapy.
Available options for primary MCL therapy in patients who require systemic therapy include:
• Single alkylating agents
• CVP (cyclophosphamide, vincristine, prednisone)
• CHOP (cyclophosphamide, doxorubicin [hydroxydaunorubicin], vincristine [Oncovin], prednisone)
• Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) with or without rituximab
• R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone)
• Lenalidomide plus rituximab
• Hyper-CVAD with autologous SCT
Options for relapsed or refractory MCL include:
• R-hyper-CVAD
• Hyper-CVAD with or without rituximab followed by autologous SCT
• Nucleoside analogues and combinations
• Salvage chemotherapy combinations followed by autologous SCT
• Bortezomib
• Lenalidomide
• Ibrutinib
• Radioimmunotherapy
• Rituximab
• Rituximab and thalidomide combination
• Acalabrutinib
• High-dose chemotherapy with autologous bone marrow or SCT
• Brexucabtagene autoleucel
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's findings are consistent with a diagnosis of malignant mantle cell lymphoma (MCL).
MCL is a rare and aggressive form of non-Hodgkin lymphoma that accounts for approximately 5%-7% of all lymphomas. MCL has a characteristic immunophenotype (ie, CD5+, CD10−, Bcl-2+, Bcl-6−, CD20+), with the t(11;14)(q13;q32) chromosomal translocation, and expression of cyclin D1. The median age at diagnosis is between 60 and 70 years. Approximately 70% of all cases occur in men.
The clinical presentation of MCL can vary. Patients may have asymptomatic monoclonal MCL type lymphocytosis or nonbulky nodal/extra nodal disease with minimal symptoms, or they may present with significant symptoms, progressive generalized lymphadenopathy, cytopenia, splenomegaly, and extranodal disease, including gastrointestinal involvement (lymphomatous polyposis), kidney involvement, involvement of other organs, or, rarely, central nervous system involvement. Disease involving multiple lymph nodes and other sites of the body is seen in most patients. Approximately 70% of patients present with stage IV disease requiring systemic treatment.
According to 2022 guidelines from the National Comprehensive Cancer Network (NCCN), essential components in the workup for MCL include:
• Physical examination, with attention to node-bearing areas, including Waldeyer ring, and to size of liver and spleen
• Assessment of performance status and B symptoms (ie, fever > 100.4°F [may be sporadic], drenching night sweats, unintentional weight loss of > 10% of body weight over 6 months or less)
• CBC with differential
• Comprehensive metabolic panel
• Serum lactate dehydrogenase (LDH) level (an important prognostic marker)
• PET/CT scan (including neck)
• Hepatitis B testing if treatment with rituximab is being contemplated
• Echocardiogram or multigated acquisition (MUGA) scan if anthracycline or anthracenedione-based regimen is indicated
• Pregnancy testing in women of childbearing age (if chemotherapy or radiation therapy is planned)
Additional testing may be indicated in specific circumstances, such as colonoscopy/endoscopy.
MCL remains challenging to treat. While 50%-90% of patients with MCL respond to combination chemotherapy, only 30% achieve a complete response. Median time to treatment failure is < 18 months.
When selecting systemic treatment for patients with MCL, clinicians should consider the availability of clinical trials for subsets of patients, eligibility for stem cell transplant (SCT), high-risk status (ie, blastoid MCL, high Ki-67% > 30%, or central nervous system involvement), age, and performance status. The addition of radiation to chemotherapy may be beneficial for patients with limited-stage, nonbulky disease, although this has not been confirmed in large, randomized studies. Outside of clinical trials, the usual approach for frontline treatment of MCL is chemoimmunotherapy with/without autologous SCT and with/without maintenance therapy.
Available options for primary MCL therapy in patients who require systemic therapy include:
• Single alkylating agents
• CVP (cyclophosphamide, vincristine, prednisone)
• CHOP (cyclophosphamide, doxorubicin [hydroxydaunorubicin], vincristine [Oncovin], prednisone)
• Hyper-CVAD (hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone) with or without rituximab
• R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone)
• Lenalidomide plus rituximab
• Hyper-CVAD with autologous SCT
Options for relapsed or refractory MCL include:
• R-hyper-CVAD
• Hyper-CVAD with or without rituximab followed by autologous SCT
• Nucleoside analogues and combinations
• Salvage chemotherapy combinations followed by autologous SCT
• Bortezomib
• Lenalidomide
• Ibrutinib
• Radioimmunotherapy
• Rituximab
• Rituximab and thalidomide combination
• Acalabrutinib
• High-dose chemotherapy with autologous bone marrow or SCT
• Brexucabtagene autoleucel
Timothy J. Voorhees, MD, MSCR, Assistant Professor of Internal Medicine - Clinical, Division of Hematology, The Ohio State University James Comprehensive Cancer Center, Columbus, OH.
Timothy J. Voorhees, MD, MSCR, has disclosed the following relevant financial relationships:
Received research grant from: AstraZeneca; Morphosys; Incyte; Recordati.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
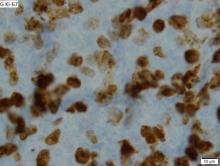
A 64-year-old Black man with a history of hypertension and hyperlipidemia presents with complaints of fatigue, sporadic fever > 100.4° F, and mild abdominal pain. The patient has lost 12 lb since he was last seen 9 months earlier. When questioned, he states that he simply doesn't have the appetite he once had. Physical examination reveals pallor; abdominal distension; lymphadenopathy in the anterior cervical, inguinal, and axillary regions; and palpable spleen and liver. CBC findings include RBC 4.4 x 106/µL; WBC 2400/μL; PLT 148,000/dL; MCV 57.8 fL; hematocrit 38%; and ALC 4200/µL. Immunophenotyping by flow cytometry and immunohistochemistry was positive for CD5 and CD19, with no expression of CD10 or CD23. Cyclin D1 was overexpressed.
MCL Presentation
MCL Overview
MCL: Ibrutinib could become the ‘new standard’
First-line patients fared well on ibrutinib, a Bruton’s tyrosine kinase inhibitor, according to the findings.
“Based on the results so far ... at least for the majority of patients, ibrutinib early will become the new standard,” said study lead author Martin Dreyling, MD, associate professor of medicine at Ludwig Maximilian University (LMU) Munich. Dr. Dreyling spoke in a news briefing and at a separate presentation at the annual meeting of the American Society of Hematology. “It might well be that specific subsets of patients may benefit from autologous transplant.”
MCL is a rare form of non-Hodgkin’s lymphoma that strikes cells in the mantle zone of lymph nodes. It is usually diagnosed in older men and often presents at an advanced stage. Multiple available treatments include rituximab/bendamustine, CAR-T cell therapy, stem cell transplants, and Bruton’s tyrosine kinase inhibitors. Ibrutinib is approved by the Food and Drug Administration only for refractory/relapsed cases, however.
Dr. Dreyling was a pioneer in confirming benefit from stem-cell transplants for MCL. “However,” he said, “no one likes autologous transplant because it also has side effects.”
For the new open-label study, Dr. Dreyling and colleagues in the European MCL Network in 2016 began recruiting patients with newly diagnosed, advanced stage II-IV MCL. The patients were younger than 65.
The subjects were randomly assigned to three trial arms: Standard treatment (high-dose cytarabine followed by autologous stem cell transplant and rituximab maintenance, n = 288), the standard treatment plus ibrutinib (n = 292), and ibrutinib without stem cell transplant (n = 290). The median age was 57, and 76% of patients were male.
The primary endpoint was failure-free survival at 31 months. Standard therapy was not superior to the ibrutinib without transplant group (72% vs. 86%, respectively, P = .9979). However, standard therapy with ibrutinib was superior to the standard therapy group (88% vs. 72%, respectively, P = .0008). The researchers haven’t finished their analysis of standard therapy with ibrutinib vs. ibrutinib without transplant.
Subjects in the standard therapy plus ibrutinib arm had more grade 3-5 adverse events than did the standard therapy and ibrutinib without transplant groups: Neutropenia, 44%, 17%, and 23%, respectively; leukopenia, 4%, 2%, and 2%; febrile neutropenia, 6%, 3%, and 3%; infections and infestations, 25%, 13%, and 19%; and cardiac disorders, 3%, 1%, 4%. P values were not provided.
In an interview, Ohio State University hematologist Narendranath Epperla, MD, MS, who was not involved in the study, said that this research reflects efforts to understand how novel agents such as ibrutinib and cellular therapies fit into MCL treatment. “We are trying to incorporate them in the frontline setting with either chemo backbone or with other targeted agents to improve outcomes and minimize toxicity. We are also trying to understand in whom auto-HCT can be precluded.”
The results of the new study appear promising, Dr. Epperla said, but he questioned the primary endpoint (failure-free survival instead of progress-free survival) and the short duration of the trial.
“I would like to see how the patients with high-risk features such as TP53 mutation, complex cytogenetics, and blastoid/pleomorphic variants did on the three arms,” Dr. Epperla said. “And I would like to see longer follow-up data before adapting this – [addition] of ibrutinib to the chemotherapy backbone without auto-HCT – into clinical practice.”
What’s next? Dr. Dreyling said that upcoming data will provide further insight into ibrutinib vs. stem-cell transplantation. And “within the next half year or so,” he said, “there will be a next generation of studies challenging chemotherapy overall in mantle cell lymphoma and substituting targeted treatment, hopefully achieving much better tolerability.”
Funding information was not provided. Dr. Dreyling disclosed ties with Lilly/Loxo, AstraZeneca, Novartis, Amgen, Roche, Janssen, Gilead/Kite, BMS/Celgene, Bayer, Abbvie, and Beigene. The other study authors reported various disclosures. Dr. Epperla disclosed a relationship with Pharmacyclics.
First-line patients fared well on ibrutinib, a Bruton’s tyrosine kinase inhibitor, according to the findings.
“Based on the results so far ... at least for the majority of patients, ibrutinib early will become the new standard,” said study lead author Martin Dreyling, MD, associate professor of medicine at Ludwig Maximilian University (LMU) Munich. Dr. Dreyling spoke in a news briefing and at a separate presentation at the annual meeting of the American Society of Hematology. “It might well be that specific subsets of patients may benefit from autologous transplant.”
MCL is a rare form of non-Hodgkin’s lymphoma that strikes cells in the mantle zone of lymph nodes. It is usually diagnosed in older men and often presents at an advanced stage. Multiple available treatments include rituximab/bendamustine, CAR-T cell therapy, stem cell transplants, and Bruton’s tyrosine kinase inhibitors. Ibrutinib is approved by the Food and Drug Administration only for refractory/relapsed cases, however.
Dr. Dreyling was a pioneer in confirming benefit from stem-cell transplants for MCL. “However,” he said, “no one likes autologous transplant because it also has side effects.”
For the new open-label study, Dr. Dreyling and colleagues in the European MCL Network in 2016 began recruiting patients with newly diagnosed, advanced stage II-IV MCL. The patients were younger than 65.
The subjects were randomly assigned to three trial arms: Standard treatment (high-dose cytarabine followed by autologous stem cell transplant and rituximab maintenance, n = 288), the standard treatment plus ibrutinib (n = 292), and ibrutinib without stem cell transplant (n = 290). The median age was 57, and 76% of patients were male.
The primary endpoint was failure-free survival at 31 months. Standard therapy was not superior to the ibrutinib without transplant group (72% vs. 86%, respectively, P = .9979). However, standard therapy with ibrutinib was superior to the standard therapy group (88% vs. 72%, respectively, P = .0008). The researchers haven’t finished their analysis of standard therapy with ibrutinib vs. ibrutinib without transplant.
Subjects in the standard therapy plus ibrutinib arm had more grade 3-5 adverse events than did the standard therapy and ibrutinib without transplant groups: Neutropenia, 44%, 17%, and 23%, respectively; leukopenia, 4%, 2%, and 2%; febrile neutropenia, 6%, 3%, and 3%; infections and infestations, 25%, 13%, and 19%; and cardiac disorders, 3%, 1%, 4%. P values were not provided.
In an interview, Ohio State University hematologist Narendranath Epperla, MD, MS, who was not involved in the study, said that this research reflects efforts to understand how novel agents such as ibrutinib and cellular therapies fit into MCL treatment. “We are trying to incorporate them in the frontline setting with either chemo backbone or with other targeted agents to improve outcomes and minimize toxicity. We are also trying to understand in whom auto-HCT can be precluded.”
The results of the new study appear promising, Dr. Epperla said, but he questioned the primary endpoint (failure-free survival instead of progress-free survival) and the short duration of the trial.
“I would like to see how the patients with high-risk features such as TP53 mutation, complex cytogenetics, and blastoid/pleomorphic variants did on the three arms,” Dr. Epperla said. “And I would like to see longer follow-up data before adapting this – [addition] of ibrutinib to the chemotherapy backbone without auto-HCT – into clinical practice.”
What’s next? Dr. Dreyling said that upcoming data will provide further insight into ibrutinib vs. stem-cell transplantation. And “within the next half year or so,” he said, “there will be a next generation of studies challenging chemotherapy overall in mantle cell lymphoma and substituting targeted treatment, hopefully achieving much better tolerability.”
Funding information was not provided. Dr. Dreyling disclosed ties with Lilly/Loxo, AstraZeneca, Novartis, Amgen, Roche, Janssen, Gilead/Kite, BMS/Celgene, Bayer, Abbvie, and Beigene. The other study authors reported various disclosures. Dr. Epperla disclosed a relationship with Pharmacyclics.
First-line patients fared well on ibrutinib, a Bruton’s tyrosine kinase inhibitor, according to the findings.
“Based on the results so far ... at least for the majority of patients, ibrutinib early will become the new standard,” said study lead author Martin Dreyling, MD, associate professor of medicine at Ludwig Maximilian University (LMU) Munich. Dr. Dreyling spoke in a news briefing and at a separate presentation at the annual meeting of the American Society of Hematology. “It might well be that specific subsets of patients may benefit from autologous transplant.”
MCL is a rare form of non-Hodgkin’s lymphoma that strikes cells in the mantle zone of lymph nodes. It is usually diagnosed in older men and often presents at an advanced stage. Multiple available treatments include rituximab/bendamustine, CAR-T cell therapy, stem cell transplants, and Bruton’s tyrosine kinase inhibitors. Ibrutinib is approved by the Food and Drug Administration only for refractory/relapsed cases, however.
Dr. Dreyling was a pioneer in confirming benefit from stem-cell transplants for MCL. “However,” he said, “no one likes autologous transplant because it also has side effects.”
For the new open-label study, Dr. Dreyling and colleagues in the European MCL Network in 2016 began recruiting patients with newly diagnosed, advanced stage II-IV MCL. The patients were younger than 65.
The subjects were randomly assigned to three trial arms: Standard treatment (high-dose cytarabine followed by autologous stem cell transplant and rituximab maintenance, n = 288), the standard treatment plus ibrutinib (n = 292), and ibrutinib without stem cell transplant (n = 290). The median age was 57, and 76% of patients were male.
The primary endpoint was failure-free survival at 31 months. Standard therapy was not superior to the ibrutinib without transplant group (72% vs. 86%, respectively, P = .9979). However, standard therapy with ibrutinib was superior to the standard therapy group (88% vs. 72%, respectively, P = .0008). The researchers haven’t finished their analysis of standard therapy with ibrutinib vs. ibrutinib without transplant.
Subjects in the standard therapy plus ibrutinib arm had more grade 3-5 adverse events than did the standard therapy and ibrutinib without transplant groups: Neutropenia, 44%, 17%, and 23%, respectively; leukopenia, 4%, 2%, and 2%; febrile neutropenia, 6%, 3%, and 3%; infections and infestations, 25%, 13%, and 19%; and cardiac disorders, 3%, 1%, 4%. P values were not provided.
In an interview, Ohio State University hematologist Narendranath Epperla, MD, MS, who was not involved in the study, said that this research reflects efforts to understand how novel agents such as ibrutinib and cellular therapies fit into MCL treatment. “We are trying to incorporate them in the frontline setting with either chemo backbone or with other targeted agents to improve outcomes and minimize toxicity. We are also trying to understand in whom auto-HCT can be precluded.”
The results of the new study appear promising, Dr. Epperla said, but he questioned the primary endpoint (failure-free survival instead of progress-free survival) and the short duration of the trial.
“I would like to see how the patients with high-risk features such as TP53 mutation, complex cytogenetics, and blastoid/pleomorphic variants did on the three arms,” Dr. Epperla said. “And I would like to see longer follow-up data before adapting this – [addition] of ibrutinib to the chemotherapy backbone without auto-HCT – into clinical practice.”
What’s next? Dr. Dreyling said that upcoming data will provide further insight into ibrutinib vs. stem-cell transplantation. And “within the next half year or so,” he said, “there will be a next generation of studies challenging chemotherapy overall in mantle cell lymphoma and substituting targeted treatment, hopefully achieving much better tolerability.”
Funding information was not provided. Dr. Dreyling disclosed ties with Lilly/Loxo, AstraZeneca, Novartis, Amgen, Roche, Janssen, Gilead/Kite, BMS/Celgene, Bayer, Abbvie, and Beigene. The other study authors reported various disclosures. Dr. Epperla disclosed a relationship with Pharmacyclics.
AT ASH 2022
ASH 2022: New clinical data challenge long-held assumptions
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
FROM ASH 2022
High cost and demand for old cancer drug sparks crisis
At Oregon Health and Science University, for example, an extensive algorithm now offers guidance through a thicket of alternative options, from adjusting doses and using substitutes to delaying treatment. Meanwhile, some institutions have enlisted ethicists and attorneys to guide their decisions on which patients will have to wait for potentially life-saving treatment.
Even as surgeons turn to alternatives, advocates for transplantation in hematology have warned about the potential for harm.
“This continued fludarabine shortage is forcing centers to use non–[Food and Drug Administration] approved lymphodepleting regimens that may negatively impact the success of a possibly lifesaving CAR-T therapy,” Brenda Sandmaier, MD, president of the Transplantation and Cellular Therapy American Society, and Jeffery Auletta, MD, a senior vice president with the National Marrow Donor, said in a June 30 letter to the FDA. The physicians added that they “request the FDA to take immediate action on this critical shortage. Many centers currently have no ability to purchase fludarabine through their suppliers and have no estimated time frame for return of availability. Other centers are limited to mere weeks of supply, with continued uncertainty of future availability.”
In October, less than 4 months after that letter was sent, one of the manufacturers of fludarabine – Areva Pharmaceuticals – marked up the price of fludarabine to $2,736 per vial, 10-20 times that of two other makers of the drug.
In new treatment era, fludarabine remains crucial
In 2015, ASH Clinical News – a publication of the American Society of Hematology – invited a pair of hematologists to discuss whether fludarabine is “dead” as a front-line treatment for chronic lymphocytic leukemia (CLL). “Fludarabine is not dead yet, but the data from those and other long-term trials may be the final nail in its coffin,” said Mitchell Smith, MD, PhD, who was then with Cleveland Clinic and now works for George Washington University.
Seven years later, the role of fludarabine as a long-term chemotherapeutic agent in blood cancer has definitely evolved. Just as oncologists predicted back in 2015, “the use of fludarabine declined for the primary management of CLL and other B cell malignancies, due to the development of targeted therapies such as BTK inhibitors, venetoclax, and other agents,” Memorial Sloan Kettering hematologic oncologist Anthony Mato, MD, said in an interview.
But the drug “remains a critical agent for conditioning the immune system for cellular therapies such as allogeneic stem cell transplantation and CAR-T cells,” Dr. Mato said.
Nirav Shah, MD, a hematologic oncologist at the Medical College of Wisconsin, explained in an interview that “conditioning” in the stem-cell transplant context refers to “wiping out” the immune system, allowing the donor’s stem cells to avoid rejection. “It’s a commonly used drug,” he said, “and shortage was not really a concern that people faced until this year.”
As shortage continues, price hike brings yet another hit
The first reports of fludarabine being in short supply surfaced about a year ago. According to a Nov. 2 update from the American Society of Health-System Pharmacists, five companies now manufacture fludarabine, and all of them report shortages. Areva, which dramatically raised its price, is accepting direct orders. Leucadia and Teva don’t know when the drug will be available; and Fresenius Kabi and Sagent expect availability in early 2023.
Areva, Leucadia, and Teva didn’t provide reasons for their shortages. Fresenius Kabi blamed increased demand, and Sagent pointed to manufacturing delays. Pfizer, another manufacturer, had a tiny market share and stopped making fludarabine in 2020, according to the pharmacist society.
In a May 12 press release, a company called Lannett announced it would take over U.S. distribution of fludarabine for Areva and suggested that the supply shortage would be lucrative: “While total U.S. sales for the 12 months ended March 2022 of Fludarabine Phosphate for injection, USP, 50 mg/2mL were approximately $4.9 million, according to IQVIA, the current market value is believed to be higher due to the recent market disruptions.”
“We were all shocked and outraged when Areva came out with the new, dramatically higher prices,” Bill Greene, PharmD, chief pharmaceutical officer at St. Jude Children’s Research Hospital, said in a recent interview.
In a prior interview, conducted during the summer of 2022, Dr. Greene addressed the topic of hematologic drug shortages. Back then he noted that he was seeking emergency supplies of fludarabine, since all five manufacturers reported having no stock available.
Interviewed again in November 2022, Dr. Greene noted that the hospital “had been able to stay ahead of the need and meet the needs of our patients” through arrangements with Teva and Fresenius Kabi. “In cases of patient need, we certainly are willing to pay a higher product price if that’s what it takes to get it – assuming the product is a quality product.”
The Medical College of Wisconsin’s Dr. Shah said insurers may refuse to cover the higher price, sticking medical institutions with the bill.
Alternatives abound, but do they suffice?
There is some good news on the fludarabine shortage front. Areva recently alerted providers that it was releasing fludarabine from non-FDA-approved suppliers with the agency’s permission, and Accord Healthcare said it received permission to sell fludarabine that was marketed in Canada.
Another option – oral fludarabine instead of the standard IV version – remains unavailable in the United States. According to the June letter to the FDA from the American Society for Transplantation and Cellular Therapy and National Marrow Donor Program, it “might be an appropriate alternative” and is available in Europe, Canada and Australia.
The letter warns that “transplant centers have also been forced to move away from fludarabine-based regimens and use alternative drugs such as cladribine or clofarabine, which are both significantly less studied and rely on single-center experience or limited phase II data. ... The limited availability of fludarabine is leading to the use of alternative regimens that are known to be more toxic or understudied alternatives with unknown long-term clinical effects or harms to patients.”
In a November 2022 report published in Transplantation and Cellular Therapy, Dr. Shah and colleagues noted that institutions are adopting strategies such as “(1) pharmacy dose banding and rounding down to save vials, even if a >5% reduction was required; (2) administering all dosing of fludarabine based not on actual body weight but on adjusted body weight; and (3) switching the billing of fludarabine from single-dose vials to billing by dose delivery.”
If the shortage continues, “it becomes necessary for centers to establish algorithms for management now,” they wrote. “Substitution of such agents as bendamustine and cladribine can be considered ... [and] another acceptable solution could be the substitution of clofarabine for fludarabine.”
Still, there are many unanswered questions. “The challenge is that these alternative regimens have not been extensively studied in a large population,” Dr. Shah said. “You have to be more mindful of potential side effects and risks, and the biggest concern is efficacy. Is changing the drug going to be detrimental to a patient’s outcome? To be honest, we don’t know the answer to that.”
Dr. Mato disclosed ties with TG Therapeutics, Pharmacyclics, AbbVie, Acerta, Adaptive Biotechnologies, AstraZeneca, BeiGene, BioPharma, BMS, Curio, Dava, DTRM, Genentech, Genmab, Janssen, Johnson & Johnson, LOXO, Medscape, Nurix, Octapharma, PER, PerView, and Pfizer. Dr. Greene and Dr. Shah have no disclosures.
At Oregon Health and Science University, for example, an extensive algorithm now offers guidance through a thicket of alternative options, from adjusting doses and using substitutes to delaying treatment. Meanwhile, some institutions have enlisted ethicists and attorneys to guide their decisions on which patients will have to wait for potentially life-saving treatment.
Even as surgeons turn to alternatives, advocates for transplantation in hematology have warned about the potential for harm.
“This continued fludarabine shortage is forcing centers to use non–[Food and Drug Administration] approved lymphodepleting regimens that may negatively impact the success of a possibly lifesaving CAR-T therapy,” Brenda Sandmaier, MD, president of the Transplantation and Cellular Therapy American Society, and Jeffery Auletta, MD, a senior vice president with the National Marrow Donor, said in a June 30 letter to the FDA. The physicians added that they “request the FDA to take immediate action on this critical shortage. Many centers currently have no ability to purchase fludarabine through their suppliers and have no estimated time frame for return of availability. Other centers are limited to mere weeks of supply, with continued uncertainty of future availability.”
In October, less than 4 months after that letter was sent, one of the manufacturers of fludarabine – Areva Pharmaceuticals – marked up the price of fludarabine to $2,736 per vial, 10-20 times that of two other makers of the drug.
In new treatment era, fludarabine remains crucial
In 2015, ASH Clinical News – a publication of the American Society of Hematology – invited a pair of hematologists to discuss whether fludarabine is “dead” as a front-line treatment for chronic lymphocytic leukemia (CLL). “Fludarabine is not dead yet, but the data from those and other long-term trials may be the final nail in its coffin,” said Mitchell Smith, MD, PhD, who was then with Cleveland Clinic and now works for George Washington University.
Seven years later, the role of fludarabine as a long-term chemotherapeutic agent in blood cancer has definitely evolved. Just as oncologists predicted back in 2015, “the use of fludarabine declined for the primary management of CLL and other B cell malignancies, due to the development of targeted therapies such as BTK inhibitors, venetoclax, and other agents,” Memorial Sloan Kettering hematologic oncologist Anthony Mato, MD, said in an interview.
But the drug “remains a critical agent for conditioning the immune system for cellular therapies such as allogeneic stem cell transplantation and CAR-T cells,” Dr. Mato said.
Nirav Shah, MD, a hematologic oncologist at the Medical College of Wisconsin, explained in an interview that “conditioning” in the stem-cell transplant context refers to “wiping out” the immune system, allowing the donor’s stem cells to avoid rejection. “It’s a commonly used drug,” he said, “and shortage was not really a concern that people faced until this year.”
As shortage continues, price hike brings yet another hit
The first reports of fludarabine being in short supply surfaced about a year ago. According to a Nov. 2 update from the American Society of Health-System Pharmacists, five companies now manufacture fludarabine, and all of them report shortages. Areva, which dramatically raised its price, is accepting direct orders. Leucadia and Teva don’t know when the drug will be available; and Fresenius Kabi and Sagent expect availability in early 2023.
Areva, Leucadia, and Teva didn’t provide reasons for their shortages. Fresenius Kabi blamed increased demand, and Sagent pointed to manufacturing delays. Pfizer, another manufacturer, had a tiny market share and stopped making fludarabine in 2020, according to the pharmacist society.
In a May 12 press release, a company called Lannett announced it would take over U.S. distribution of fludarabine for Areva and suggested that the supply shortage would be lucrative: “While total U.S. sales for the 12 months ended March 2022 of Fludarabine Phosphate for injection, USP, 50 mg/2mL were approximately $4.9 million, according to IQVIA, the current market value is believed to be higher due to the recent market disruptions.”
“We were all shocked and outraged when Areva came out with the new, dramatically higher prices,” Bill Greene, PharmD, chief pharmaceutical officer at St. Jude Children’s Research Hospital, said in a recent interview.
In a prior interview, conducted during the summer of 2022, Dr. Greene addressed the topic of hematologic drug shortages. Back then he noted that he was seeking emergency supplies of fludarabine, since all five manufacturers reported having no stock available.
Interviewed again in November 2022, Dr. Greene noted that the hospital “had been able to stay ahead of the need and meet the needs of our patients” through arrangements with Teva and Fresenius Kabi. “In cases of patient need, we certainly are willing to pay a higher product price if that’s what it takes to get it – assuming the product is a quality product.”
The Medical College of Wisconsin’s Dr. Shah said insurers may refuse to cover the higher price, sticking medical institutions with the bill.
Alternatives abound, but do they suffice?
There is some good news on the fludarabine shortage front. Areva recently alerted providers that it was releasing fludarabine from non-FDA-approved suppliers with the agency’s permission, and Accord Healthcare said it received permission to sell fludarabine that was marketed in Canada.
Another option – oral fludarabine instead of the standard IV version – remains unavailable in the United States. According to the June letter to the FDA from the American Society for Transplantation and Cellular Therapy and National Marrow Donor Program, it “might be an appropriate alternative” and is available in Europe, Canada and Australia.
The letter warns that “transplant centers have also been forced to move away from fludarabine-based regimens and use alternative drugs such as cladribine or clofarabine, which are both significantly less studied and rely on single-center experience or limited phase II data. ... The limited availability of fludarabine is leading to the use of alternative regimens that are known to be more toxic or understudied alternatives with unknown long-term clinical effects or harms to patients.”
In a November 2022 report published in Transplantation and Cellular Therapy, Dr. Shah and colleagues noted that institutions are adopting strategies such as “(1) pharmacy dose banding and rounding down to save vials, even if a >5% reduction was required; (2) administering all dosing of fludarabine based not on actual body weight but on adjusted body weight; and (3) switching the billing of fludarabine from single-dose vials to billing by dose delivery.”
If the shortage continues, “it becomes necessary for centers to establish algorithms for management now,” they wrote. “Substitution of such agents as bendamustine and cladribine can be considered ... [and] another acceptable solution could be the substitution of clofarabine for fludarabine.”
Still, there are many unanswered questions. “The challenge is that these alternative regimens have not been extensively studied in a large population,” Dr. Shah said. “You have to be more mindful of potential side effects and risks, and the biggest concern is efficacy. Is changing the drug going to be detrimental to a patient’s outcome? To be honest, we don’t know the answer to that.”
Dr. Mato disclosed ties with TG Therapeutics, Pharmacyclics, AbbVie, Acerta, Adaptive Biotechnologies, AstraZeneca, BeiGene, BioPharma, BMS, Curio, Dava, DTRM, Genentech, Genmab, Janssen, Johnson & Johnson, LOXO, Medscape, Nurix, Octapharma, PER, PerView, and Pfizer. Dr. Greene and Dr. Shah have no disclosures.
At Oregon Health and Science University, for example, an extensive algorithm now offers guidance through a thicket of alternative options, from adjusting doses and using substitutes to delaying treatment. Meanwhile, some institutions have enlisted ethicists and attorneys to guide their decisions on which patients will have to wait for potentially life-saving treatment.
Even as surgeons turn to alternatives, advocates for transplantation in hematology have warned about the potential for harm.
“This continued fludarabine shortage is forcing centers to use non–[Food and Drug Administration] approved lymphodepleting regimens that may negatively impact the success of a possibly lifesaving CAR-T therapy,” Brenda Sandmaier, MD, president of the Transplantation and Cellular Therapy American Society, and Jeffery Auletta, MD, a senior vice president with the National Marrow Donor, said in a June 30 letter to the FDA. The physicians added that they “request the FDA to take immediate action on this critical shortage. Many centers currently have no ability to purchase fludarabine through their suppliers and have no estimated time frame for return of availability. Other centers are limited to mere weeks of supply, with continued uncertainty of future availability.”
In October, less than 4 months after that letter was sent, one of the manufacturers of fludarabine – Areva Pharmaceuticals – marked up the price of fludarabine to $2,736 per vial, 10-20 times that of two other makers of the drug.
In new treatment era, fludarabine remains crucial
In 2015, ASH Clinical News – a publication of the American Society of Hematology – invited a pair of hematologists to discuss whether fludarabine is “dead” as a front-line treatment for chronic lymphocytic leukemia (CLL). “Fludarabine is not dead yet, but the data from those and other long-term trials may be the final nail in its coffin,” said Mitchell Smith, MD, PhD, who was then with Cleveland Clinic and now works for George Washington University.
Seven years later, the role of fludarabine as a long-term chemotherapeutic agent in blood cancer has definitely evolved. Just as oncologists predicted back in 2015, “the use of fludarabine declined for the primary management of CLL and other B cell malignancies, due to the development of targeted therapies such as BTK inhibitors, venetoclax, and other agents,” Memorial Sloan Kettering hematologic oncologist Anthony Mato, MD, said in an interview.
But the drug “remains a critical agent for conditioning the immune system for cellular therapies such as allogeneic stem cell transplantation and CAR-T cells,” Dr. Mato said.
Nirav Shah, MD, a hematologic oncologist at the Medical College of Wisconsin, explained in an interview that “conditioning” in the stem-cell transplant context refers to “wiping out” the immune system, allowing the donor’s stem cells to avoid rejection. “It’s a commonly used drug,” he said, “and shortage was not really a concern that people faced until this year.”
As shortage continues, price hike brings yet another hit
The first reports of fludarabine being in short supply surfaced about a year ago. According to a Nov. 2 update from the American Society of Health-System Pharmacists, five companies now manufacture fludarabine, and all of them report shortages. Areva, which dramatically raised its price, is accepting direct orders. Leucadia and Teva don’t know when the drug will be available; and Fresenius Kabi and Sagent expect availability in early 2023.
Areva, Leucadia, and Teva didn’t provide reasons for their shortages. Fresenius Kabi blamed increased demand, and Sagent pointed to manufacturing delays. Pfizer, another manufacturer, had a tiny market share and stopped making fludarabine in 2020, according to the pharmacist society.
In a May 12 press release, a company called Lannett announced it would take over U.S. distribution of fludarabine for Areva and suggested that the supply shortage would be lucrative: “While total U.S. sales for the 12 months ended March 2022 of Fludarabine Phosphate for injection, USP, 50 mg/2mL were approximately $4.9 million, according to IQVIA, the current market value is believed to be higher due to the recent market disruptions.”
“We were all shocked and outraged when Areva came out with the new, dramatically higher prices,” Bill Greene, PharmD, chief pharmaceutical officer at St. Jude Children’s Research Hospital, said in a recent interview.
In a prior interview, conducted during the summer of 2022, Dr. Greene addressed the topic of hematologic drug shortages. Back then he noted that he was seeking emergency supplies of fludarabine, since all five manufacturers reported having no stock available.
Interviewed again in November 2022, Dr. Greene noted that the hospital “had been able to stay ahead of the need and meet the needs of our patients” through arrangements with Teva and Fresenius Kabi. “In cases of patient need, we certainly are willing to pay a higher product price if that’s what it takes to get it – assuming the product is a quality product.”
The Medical College of Wisconsin’s Dr. Shah said insurers may refuse to cover the higher price, sticking medical institutions with the bill.
Alternatives abound, but do they suffice?
There is some good news on the fludarabine shortage front. Areva recently alerted providers that it was releasing fludarabine from non-FDA-approved suppliers with the agency’s permission, and Accord Healthcare said it received permission to sell fludarabine that was marketed in Canada.
Another option – oral fludarabine instead of the standard IV version – remains unavailable in the United States. According to the June letter to the FDA from the American Society for Transplantation and Cellular Therapy and National Marrow Donor Program, it “might be an appropriate alternative” and is available in Europe, Canada and Australia.
The letter warns that “transplant centers have also been forced to move away from fludarabine-based regimens and use alternative drugs such as cladribine or clofarabine, which are both significantly less studied and rely on single-center experience or limited phase II data. ... The limited availability of fludarabine is leading to the use of alternative regimens that are known to be more toxic or understudied alternatives with unknown long-term clinical effects or harms to patients.”
In a November 2022 report published in Transplantation and Cellular Therapy, Dr. Shah and colleagues noted that institutions are adopting strategies such as “(1) pharmacy dose banding and rounding down to save vials, even if a >5% reduction was required; (2) administering all dosing of fludarabine based not on actual body weight but on adjusted body weight; and (3) switching the billing of fludarabine from single-dose vials to billing by dose delivery.”
If the shortage continues, “it becomes necessary for centers to establish algorithms for management now,” they wrote. “Substitution of such agents as bendamustine and cladribine can be considered ... [and] another acceptable solution could be the substitution of clofarabine for fludarabine.”
Still, there are many unanswered questions. “The challenge is that these alternative regimens have not been extensively studied in a large population,” Dr. Shah said. “You have to be more mindful of potential side effects and risks, and the biggest concern is efficacy. Is changing the drug going to be detrimental to a patient’s outcome? To be honest, we don’t know the answer to that.”
Dr. Mato disclosed ties with TG Therapeutics, Pharmacyclics, AbbVie, Acerta, Adaptive Biotechnologies, AstraZeneca, BeiGene, BioPharma, BMS, Curio, Dava, DTRM, Genentech, Genmab, Janssen, Johnson & Johnson, LOXO, Medscape, Nurix, Octapharma, PER, PerView, and Pfizer. Dr. Greene and Dr. Shah have no disclosures.
Transplant provides no clear survival benefit in real-world MCL study
In younger patients with mantle cell lymphoma treated in U.S. community oncology settings in recent years, use of autologous transplant was not associated with improved survival, results of a large observational study show.
Autologous stem-cell transplant (ASCT) use was not linked overall survival (OS), according to the authors of the retrospective analysis of patients diagnosed with mantle cell lymphoma (MCL) between 2011 and 2021.
This lack of a clear survival benefit with use of ASCT is an “apparent contradiction” with prospective data from earlier clinical trials, authors wrote in the Journal of Clinical Oncology
However, they added, the finding is consistent with several recent registry analyses that also do not support a link between ASCT and overall survival in patients with MCL.
Although these findings are limited by the retrospective nature of the study, the results at least suggest that it is ethical to do research that doesn’t involve ASCT, study author Peter Martin, MD, said in an interview.
Furthermore, emerging data from the randomized TRIANGLE study from the European MCL Network suggest the potential for ASCT to be replaced by maintenance therapy with the Bruton's tyrosine kinase inhibitor ibrutinib, according to Dr. Martin, associate professor with Weill Cornell Medicine, New York.
“There are probably a lot of questions that will come up there, but essentially the barriers to research that do not include ASCT have been moved away, and we can go ahead and study non-ASCT (approaches) in younger patients,” Dr. Martin said.
No clear OS benefit
In current guidelines, recommended initial therapy for MCL patients younger than 65 years includes use of high-dose cytarabine-containing chemoimmunotherapy induction, followed by ASCT as consolidation, and then rituximab maintenance, Dr. Martin and coauthors say in their report.
Their primary analysis was based on the Flatiron Health database, which is derived from electronic medical records, mostly in U.S. community oncology practices, according to the report.
The researchers identified 1,274 patients under the age of 65 with a record of first-line treatment for MCL, and of those, 962 (or 76%) were considered eligible for ASCT.
Among ASCT-eligible patients, there was no significant association between receipt of ASCT and OS, with a hazard ratio of 0.86 (95% confidence interval, 0.63-1.18). The 3-year OS was 88% for patients receiving ASCT and similarly, 84% for those who did not, according to authors.
Likewise, there was no association between ASCT and real-world time to next treatment, an endpoint defined as time from start of first-line therapy to subsequent treatment or death, the report says.
Findings in perspective
The lack of clear survival benefit with ASCT in this and other recent observational studies may be explained in part by improvements in induction regimens, according to Timothy Fenske, MD, professor in the department of medicine at the Medical College of Wisconsin, Milwaukee.
“As our induction regimens have improved, it is very possible that the benefit for autologous transplantation will become less apparent,” Dr. Fenske said in an interview.
The discussion over ASCT in MCL is expected to evolve further in light of findings from TRIANGLE and EA4151, a randomized phase 3 trial of rituximab with or without ASCT specifically in patients with minimal residual disease (MRD)–negative MCL in first complete remission.
“If that study shows that the MRD-negative patients do not have much benefit from autologous transplantation,” Dr. Fenske said, “I think these studies will all be giving the same message – that autologous transplantation was beneficial back when induction regimens were poor (for example, CHOP without rituximab), but will have much less benefit in patients receiving modern inductions, which by and large will get more patients to be MRD negative.”
However, subgroup analyses of those TRIANGLE will be important, he added, since some patients may still benefit from ASCT, such as younger patients who remain MRD positive, or who have certain other high-risk molecular features.
Dr. Martin reported consulting or advisory roles with Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, and Takeda. He reported institutional research funding from Karyopharm Therapeutics.
In younger patients with mantle cell lymphoma treated in U.S. community oncology settings in recent years, use of autologous transplant was not associated with improved survival, results of a large observational study show.
Autologous stem-cell transplant (ASCT) use was not linked overall survival (OS), according to the authors of the retrospective analysis of patients diagnosed with mantle cell lymphoma (MCL) between 2011 and 2021.
This lack of a clear survival benefit with use of ASCT is an “apparent contradiction” with prospective data from earlier clinical trials, authors wrote in the Journal of Clinical Oncology
However, they added, the finding is consistent with several recent registry analyses that also do not support a link between ASCT and overall survival in patients with MCL.
Although these findings are limited by the retrospective nature of the study, the results at least suggest that it is ethical to do research that doesn’t involve ASCT, study author Peter Martin, MD, said in an interview.
Furthermore, emerging data from the randomized TRIANGLE study from the European MCL Network suggest the potential for ASCT to be replaced by maintenance therapy with the Bruton's tyrosine kinase inhibitor ibrutinib, according to Dr. Martin, associate professor with Weill Cornell Medicine, New York.
“There are probably a lot of questions that will come up there, but essentially the barriers to research that do not include ASCT have been moved away, and we can go ahead and study non-ASCT (approaches) in younger patients,” Dr. Martin said.
No clear OS benefit
In current guidelines, recommended initial therapy for MCL patients younger than 65 years includes use of high-dose cytarabine-containing chemoimmunotherapy induction, followed by ASCT as consolidation, and then rituximab maintenance, Dr. Martin and coauthors say in their report.
Their primary analysis was based on the Flatiron Health database, which is derived from electronic medical records, mostly in U.S. community oncology practices, according to the report.
The researchers identified 1,274 patients under the age of 65 with a record of first-line treatment for MCL, and of those, 962 (or 76%) were considered eligible for ASCT.
Among ASCT-eligible patients, there was no significant association between receipt of ASCT and OS, with a hazard ratio of 0.86 (95% confidence interval, 0.63-1.18). The 3-year OS was 88% for patients receiving ASCT and similarly, 84% for those who did not, according to authors.
Likewise, there was no association between ASCT and real-world time to next treatment, an endpoint defined as time from start of first-line therapy to subsequent treatment or death, the report says.
Findings in perspective
The lack of clear survival benefit with ASCT in this and other recent observational studies may be explained in part by improvements in induction regimens, according to Timothy Fenske, MD, professor in the department of medicine at the Medical College of Wisconsin, Milwaukee.
“As our induction regimens have improved, it is very possible that the benefit for autologous transplantation will become less apparent,” Dr. Fenske said in an interview.
The discussion over ASCT in MCL is expected to evolve further in light of findings from TRIANGLE and EA4151, a randomized phase 3 trial of rituximab with or without ASCT specifically in patients with minimal residual disease (MRD)–negative MCL in first complete remission.
“If that study shows that the MRD-negative patients do not have much benefit from autologous transplantation,” Dr. Fenske said, “I think these studies will all be giving the same message – that autologous transplantation was beneficial back when induction regimens were poor (for example, CHOP without rituximab), but will have much less benefit in patients receiving modern inductions, which by and large will get more patients to be MRD negative.”
However, subgroup analyses of those TRIANGLE will be important, he added, since some patients may still benefit from ASCT, such as younger patients who remain MRD positive, or who have certain other high-risk molecular features.
Dr. Martin reported consulting or advisory roles with Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, and Takeda. He reported institutional research funding from Karyopharm Therapeutics.
In younger patients with mantle cell lymphoma treated in U.S. community oncology settings in recent years, use of autologous transplant was not associated with improved survival, results of a large observational study show.
Autologous stem-cell transplant (ASCT) use was not linked overall survival (OS), according to the authors of the retrospective analysis of patients diagnosed with mantle cell lymphoma (MCL) between 2011 and 2021.
This lack of a clear survival benefit with use of ASCT is an “apparent contradiction” with prospective data from earlier clinical trials, authors wrote in the Journal of Clinical Oncology
However, they added, the finding is consistent with several recent registry analyses that also do not support a link between ASCT and overall survival in patients with MCL.
Although these findings are limited by the retrospective nature of the study, the results at least suggest that it is ethical to do research that doesn’t involve ASCT, study author Peter Martin, MD, said in an interview.
Furthermore, emerging data from the randomized TRIANGLE study from the European MCL Network suggest the potential for ASCT to be replaced by maintenance therapy with the Bruton's tyrosine kinase inhibitor ibrutinib, according to Dr. Martin, associate professor with Weill Cornell Medicine, New York.
“There are probably a lot of questions that will come up there, but essentially the barriers to research that do not include ASCT have been moved away, and we can go ahead and study non-ASCT (approaches) in younger patients,” Dr. Martin said.
No clear OS benefit
In current guidelines, recommended initial therapy for MCL patients younger than 65 years includes use of high-dose cytarabine-containing chemoimmunotherapy induction, followed by ASCT as consolidation, and then rituximab maintenance, Dr. Martin and coauthors say in their report.
Their primary analysis was based on the Flatiron Health database, which is derived from electronic medical records, mostly in U.S. community oncology practices, according to the report.
The researchers identified 1,274 patients under the age of 65 with a record of first-line treatment for MCL, and of those, 962 (or 76%) were considered eligible for ASCT.
Among ASCT-eligible patients, there was no significant association between receipt of ASCT and OS, with a hazard ratio of 0.86 (95% confidence interval, 0.63-1.18). The 3-year OS was 88% for patients receiving ASCT and similarly, 84% for those who did not, according to authors.
Likewise, there was no association between ASCT and real-world time to next treatment, an endpoint defined as time from start of first-line therapy to subsequent treatment or death, the report says.
Findings in perspective
The lack of clear survival benefit with ASCT in this and other recent observational studies may be explained in part by improvements in induction regimens, according to Timothy Fenske, MD, professor in the department of medicine at the Medical College of Wisconsin, Milwaukee.
“As our induction regimens have improved, it is very possible that the benefit for autologous transplantation will become less apparent,” Dr. Fenske said in an interview.
The discussion over ASCT in MCL is expected to evolve further in light of findings from TRIANGLE and EA4151, a randomized phase 3 trial of rituximab with or without ASCT specifically in patients with minimal residual disease (MRD)–negative MCL in first complete remission.
“If that study shows that the MRD-negative patients do not have much benefit from autologous transplantation,” Dr. Fenske said, “I think these studies will all be giving the same message – that autologous transplantation was beneficial back when induction regimens were poor (for example, CHOP without rituximab), but will have much less benefit in patients receiving modern inductions, which by and large will get more patients to be MRD negative.”
However, subgroup analyses of those TRIANGLE will be important, he added, since some patients may still benefit from ASCT, such as younger patients who remain MRD positive, or who have certain other high-risk molecular features.
Dr. Martin reported consulting or advisory roles with Janssen, BeiGene, Karyopharm Therapeutics, Kite/Gilead, Verastem, ADC Therapeutics, Bristol Myers Squibb/Celgene, Epizyme, Merck, MorphoSys, and Takeda. He reported institutional research funding from Karyopharm Therapeutics.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Blame MCL, not transplantation, for late effects?
In patients with mantle cell lymphoma, rates of respiratory disease, blood disorders, and infectious diseases do not vary according to the intensity of treatment given, the results of a large retrospective analysis suggested.
The rate of hospitalization among MCL patients was also high, but again, did not differ between ASCT and non-ASCT subgroups in the study, which included adult patients younger than age 70 with MCL who were treated in Sweden between 2000 and 2014.
Late effects independent of ASCT
These findings may have implications for clinicians tempted to avoid intensive first-line treatment including ASCT because it is “demanding” and may cause late effects, study authors wrote in a research article that appeared in Blood Advances.
In fact, the great majority of long-term health care needs in patients with MCL appear to be related to the lymphoma in itself, according to study senior author Ingrid Glimelius, MD, PhD, senior consultant and professor in oncology in the department of immunology, genetics, and pathology at Uppsala University in Sweden.
“You do have to keep your eyes open for complications like blood disorders, infections, and respiratory (disorders),” Dr. Glimelius said in an interview. “But it’s not the transplant that adds to the extra toxicity. So don’t be afraid of giving that, if you think that can prolong your patient’s remission.”
Whither transplantation?
While these data may advance the discussion over the relative safety of ASCT, she added, the paradigm is changing to ask a different question: Does the patient need a transplant, or not?
Dr. Glimelius said she was looking forward to results of TRIANGLE, a randomized, open-label, three-arm study initiated by the European MCL Network. This study compares standard first-line treatment including ASCT to the kinase inhibitor ibrutinib, which the U.S. Food and Drug Administration approved in 2013 for patients previously treated for MCL.
In the TRIANGLE study, younger patients with MCL were randomized to the standard first-line treatment, standard treatment plus ibrutinib, or ibrutinib alone.
A preliminary report on the study stated that the current standard is “not superior” to the new ibrutinib-containing regimen without ASCT, though more follow-up is needed.
Full results of the study are expected to be presented at the American Society of Hematology meeting on December 11.
“In my opinion, our data will be practice-changing,” said lead investigator Martin Dreyling, MD, PhD, professor of medicine and head of the lymphoma program at the University of Munich Hospital.
Little known about late effects
In the meantime, clinicians may be reassured by the current data from Dr. Glimelius and coauthors, which showed that late effects varied little by treatment choice.
That’s important, Dr. Glimelius said, because even as survival is improving and novel targeted drugs are taking the stage, knowledge about the late effects of MCL remains limited.
Their population-based study included all 620 patients with MCL in the Swedish Lymphoma Register who were 18-69 years of age and diagnosed between 2000 and 2014. Records were found for 620 patients, of whom 247 received high-dose chemotherapy with ASCT.
Compared with healthy individuals with no MCL, the patients with MCL had a high rate of specialist visits and hospital visits, according to the report. The MCL patients also had high risks of infections, respiratory complications, and blood disorders relative to the healthy subjects.
Lack of differences between arms
The key finding of the report, though, is the lack of significant differences in the rate of complications between the ASCT and non–ASCT-treated patients.
Relative to healthy subjects, patients undergoing ASCT and not undergoing ASCT had a higher risk of infections, with hazard ratios of 5.62 (95% confidence interval, 4.20-7.52) and 4.66 (95% CI, 3.62-5.00), respectively.
Relative risks of respiratory complications were also similar, with HRs of 4.38 and 5.26, respectively, and overlapping CIs. Likewise, the risk of blood disorders was not statistically different, with HRs of 9.84 and 5.80, respectively, but again with overlapping CIs.
Outpatient visits, inpatient visits, and bed days were likewise similar between ASCT and non-ASCT arms.
In fact, most patients died of their lymphoma, rather than a treatment complication or another cause of death, the investigators noted in their report.
Dr. Glimelius reported receiving honoraria from Janssen. Coauthors on the paper reported disclosures related to Janssen, Gilead, Celgene, Roche, Acerta. and AbbVie.
Correction, 11/21/22: The photo caption misstated Dr. Ingrid Glimelius' name.
In patients with mantle cell lymphoma, rates of respiratory disease, blood disorders, and infectious diseases do not vary according to the intensity of treatment given, the results of a large retrospective analysis suggested.
The rate of hospitalization among MCL patients was also high, but again, did not differ between ASCT and non-ASCT subgroups in the study, which included adult patients younger than age 70 with MCL who were treated in Sweden between 2000 and 2014.
Late effects independent of ASCT
These findings may have implications for clinicians tempted to avoid intensive first-line treatment including ASCT because it is “demanding” and may cause late effects, study authors wrote in a research article that appeared in Blood Advances.
In fact, the great majority of long-term health care needs in patients with MCL appear to be related to the lymphoma in itself, according to study senior author Ingrid Glimelius, MD, PhD, senior consultant and professor in oncology in the department of immunology, genetics, and pathology at Uppsala University in Sweden.
“You do have to keep your eyes open for complications like blood disorders, infections, and respiratory (disorders),” Dr. Glimelius said in an interview. “But it’s not the transplant that adds to the extra toxicity. So don’t be afraid of giving that, if you think that can prolong your patient’s remission.”
Whither transplantation?
While these data may advance the discussion over the relative safety of ASCT, she added, the paradigm is changing to ask a different question: Does the patient need a transplant, or not?
Dr. Glimelius said she was looking forward to results of TRIANGLE, a randomized, open-label, three-arm study initiated by the European MCL Network. This study compares standard first-line treatment including ASCT to the kinase inhibitor ibrutinib, which the U.S. Food and Drug Administration approved in 2013 for patients previously treated for MCL.
In the TRIANGLE study, younger patients with MCL were randomized to the standard first-line treatment, standard treatment plus ibrutinib, or ibrutinib alone.
A preliminary report on the study stated that the current standard is “not superior” to the new ibrutinib-containing regimen without ASCT, though more follow-up is needed.
Full results of the study are expected to be presented at the American Society of Hematology meeting on December 11.
“In my opinion, our data will be practice-changing,” said lead investigator Martin Dreyling, MD, PhD, professor of medicine and head of the lymphoma program at the University of Munich Hospital.
Little known about late effects
In the meantime, clinicians may be reassured by the current data from Dr. Glimelius and coauthors, which showed that late effects varied little by treatment choice.
That’s important, Dr. Glimelius said, because even as survival is improving and novel targeted drugs are taking the stage, knowledge about the late effects of MCL remains limited.
Their population-based study included all 620 patients with MCL in the Swedish Lymphoma Register who were 18-69 years of age and diagnosed between 2000 and 2014. Records were found for 620 patients, of whom 247 received high-dose chemotherapy with ASCT.
Compared with healthy individuals with no MCL, the patients with MCL had a high rate of specialist visits and hospital visits, according to the report. The MCL patients also had high risks of infections, respiratory complications, and blood disorders relative to the healthy subjects.
Lack of differences between arms
The key finding of the report, though, is the lack of significant differences in the rate of complications between the ASCT and non–ASCT-treated patients.
Relative to healthy subjects, patients undergoing ASCT and not undergoing ASCT had a higher risk of infections, with hazard ratios of 5.62 (95% confidence interval, 4.20-7.52) and 4.66 (95% CI, 3.62-5.00), respectively.
Relative risks of respiratory complications were also similar, with HRs of 4.38 and 5.26, respectively, and overlapping CIs. Likewise, the risk of blood disorders was not statistically different, with HRs of 9.84 and 5.80, respectively, but again with overlapping CIs.
Outpatient visits, inpatient visits, and bed days were likewise similar between ASCT and non-ASCT arms.
In fact, most patients died of their lymphoma, rather than a treatment complication or another cause of death, the investigators noted in their report.
Dr. Glimelius reported receiving honoraria from Janssen. Coauthors on the paper reported disclosures related to Janssen, Gilead, Celgene, Roche, Acerta. and AbbVie.
Correction, 11/21/22: The photo caption misstated Dr. Ingrid Glimelius' name.
In patients with mantle cell lymphoma, rates of respiratory disease, blood disorders, and infectious diseases do not vary according to the intensity of treatment given, the results of a large retrospective analysis suggested.
The rate of hospitalization among MCL patients was also high, but again, did not differ between ASCT and non-ASCT subgroups in the study, which included adult patients younger than age 70 with MCL who were treated in Sweden between 2000 and 2014.
Late effects independent of ASCT
These findings may have implications for clinicians tempted to avoid intensive first-line treatment including ASCT because it is “demanding” and may cause late effects, study authors wrote in a research article that appeared in Blood Advances.
In fact, the great majority of long-term health care needs in patients with MCL appear to be related to the lymphoma in itself, according to study senior author Ingrid Glimelius, MD, PhD, senior consultant and professor in oncology in the department of immunology, genetics, and pathology at Uppsala University in Sweden.
“You do have to keep your eyes open for complications like blood disorders, infections, and respiratory (disorders),” Dr. Glimelius said in an interview. “But it’s not the transplant that adds to the extra toxicity. So don’t be afraid of giving that, if you think that can prolong your patient’s remission.”
Whither transplantation?
While these data may advance the discussion over the relative safety of ASCT, she added, the paradigm is changing to ask a different question: Does the patient need a transplant, or not?
Dr. Glimelius said she was looking forward to results of TRIANGLE, a randomized, open-label, three-arm study initiated by the European MCL Network. This study compares standard first-line treatment including ASCT to the kinase inhibitor ibrutinib, which the U.S. Food and Drug Administration approved in 2013 for patients previously treated for MCL.
In the TRIANGLE study, younger patients with MCL were randomized to the standard first-line treatment, standard treatment plus ibrutinib, or ibrutinib alone.
A preliminary report on the study stated that the current standard is “not superior” to the new ibrutinib-containing regimen without ASCT, though more follow-up is needed.
Full results of the study are expected to be presented at the American Society of Hematology meeting on December 11.
“In my opinion, our data will be practice-changing,” said lead investigator Martin Dreyling, MD, PhD, professor of medicine and head of the lymphoma program at the University of Munich Hospital.
Little known about late effects
In the meantime, clinicians may be reassured by the current data from Dr. Glimelius and coauthors, which showed that late effects varied little by treatment choice.
That’s important, Dr. Glimelius said, because even as survival is improving and novel targeted drugs are taking the stage, knowledge about the late effects of MCL remains limited.
Their population-based study included all 620 patients with MCL in the Swedish Lymphoma Register who were 18-69 years of age and diagnosed between 2000 and 2014. Records were found for 620 patients, of whom 247 received high-dose chemotherapy with ASCT.
Compared with healthy individuals with no MCL, the patients with MCL had a high rate of specialist visits and hospital visits, according to the report. The MCL patients also had high risks of infections, respiratory complications, and blood disorders relative to the healthy subjects.
Lack of differences between arms
The key finding of the report, though, is the lack of significant differences in the rate of complications between the ASCT and non–ASCT-treated patients.
Relative to healthy subjects, patients undergoing ASCT and not undergoing ASCT had a higher risk of infections, with hazard ratios of 5.62 (95% confidence interval, 4.20-7.52) and 4.66 (95% CI, 3.62-5.00), respectively.
Relative risks of respiratory complications were also similar, with HRs of 4.38 and 5.26, respectively, and overlapping CIs. Likewise, the risk of blood disorders was not statistically different, with HRs of 9.84 and 5.80, respectively, but again with overlapping CIs.
Outpatient visits, inpatient visits, and bed days were likewise similar between ASCT and non-ASCT arms.
In fact, most patients died of their lymphoma, rather than a treatment complication or another cause of death, the investigators noted in their report.
Dr. Glimelius reported receiving honoraria from Janssen. Coauthors on the paper reported disclosures related to Janssen, Gilead, Celgene, Roche, Acerta. and AbbVie.
Correction, 11/21/22: The photo caption misstated Dr. Ingrid Glimelius' name.
FROM BLOOD ADVANCES
Phase 3 trial yields better way to predict MCL outcomes
Single assessments of minimal residual disease (MRD) effectively prognosticated relapse risks in patients with MCL treated with chemoimmunotherapy and transplantation, according to investigators.
However, by evaluating MRD status over time, disease progression could be predicted with greater certainty, according to study lead author Simone Ferrero, MD, assistant professor of hematology at the University of Torino (Italy).
“The most important message is that MRD is predictive, and then in particular, if you want to study MRD, you have to do a repeated MRD analysis in order to have a better picture of the disease course and to determine the prognosis of each single patient,” Dr. Ferrero said in an interview.
Predictive power of MRD
Piers Blombery, MBBS, hematologist and medical lead of the Molecular Hematology Laboratory at Peter MacCallum Cancer Centre in Melbourne, said these study findings illustrate how the predictive power of MRD is enhanced when it is evaluated longitudinally, over the course of a patient’s disease.
“We need to be prepared to have models of MRD that take into account changes in values across time,” Dr. Blombery said in an interview.
“Conventionally, we have looked at single time points in disease, such as end of induction therapy or post autologous stem cell transplant,” said Dr. Blombery, “but we may need now to have more complex models that can integrate values over multiple time points to guide intervention.”
Dr. Blombery, who was not involved in the study, coauthored an independent commentary on the results that was also published in Blood.
Tools to predict relapse
Although initial MCL treatment is often effective, most patients will relapse. Accordingly, there have been considerable efforts to develop tools that identify patients at high risk of relapse, according to Dr. Ferrero and coauthors.
Among these tools is the Mantle Cell Lymphoma International Prognostic Index (MIPI), which risk-stratifies patients on the basis of age, performance status, leukocyte count, and lactate dehydrogenase.
More recently, in several published studies, MRD has been demonstrated a high predictive value in MCL. However, the best way to evaluate MRD has yet to be worked out. There are differences in testing methods, sampling (that is, bone marrow versus peripheral blood), and time points that are evaluated.
In some recent studies, investigators have pooled MRD samples taken at different time points, according to Dr. Ferrero and colleagues. Furthermore, there had been no prior systematic attempts to compare the prognostic performance of MRD data evaluated over time, as compared with MRD at a single time point.
Exploiting MRD data
The analysis by Dr. Ferrero and coauthors, which appears in the journal Blood, is a substudy of FIL MCL0208, a recent multicenter, randomized, phase 3 clinical trial that demonstrated the benefit of lenalidomide maintenance over observation following autologous stem cell transplantation (ASCT) among 300 patients with MCL in Italy and Portugal.
In the study, investigators monitored MRD in both peripheral blood and bone marrow at 10 fixed time points throughout treatment (including induction, consolidation, post ASCT, and then every 6 months during maintenance and follow-up), yielding 4,351 individual MRD results.
Individual MRD analyses were prognostic in MCL, according to the investigators, with results that were relevant to disease progression right after ASCT, and were most predictive starting at 6 months after ASCT.
However, the best way to exploit the bulk of MRD information that had been collected, investigators said, was a model that combined regularly updated MRD results with MIPI scores.
This MIPI-adjusted model, based on MRD assessed in bone marrow by real-time quantitative polymerase chain reaction (RQ-PCR), outperformed both MIPI and a MIPI-adjusted single time-point analysis in terms of predicting time to progression.
The predictive power of the MIPI-adjusted bone marrow RQ-PCR analysis was illustrated by an area under the curve of 0.85-0.87, compared with an AUC of just 0.60-0.63 for classic MIPI analysis, 0.62-0.65 for MIPI-adjusted MRD at a post-ASCT time-point analysis, and 0.74-0.77 for MIPI-adjusted MRD at 6 months from transplant.
Peripheral blood ‘easier to collect’
Although bone marrow analysis performed best in the single-point MRD evaluations, looking at MRD over time greatly improved the predictive power of the peripheral blood MRD results, yielding an AUC up to 0.81, according to the report.
This enhancement of peripheral blood performance is a finding that may have important clinical implications, according to Dr. Ferrero, considering that peripheral blood monitoring is more practical for long-term, repeated MRD monitoring.
“It is interesting, because peripheral blood is easier to collect than bone marrow,” Dr. Ferrero said. “So if you plan in your analysis to monitor MRD in peripheral blood every 6 months, then you can have a predictive value similar to that of one single point in bone marrow.”
Dr. Ferrero provided disclosures related to Janssen, EUSA Pharma, Gilead, Morphosys, Incyte, Clinigen, Servier, and Gentili. The other authors reported no relevant conflicts of interest.
Single assessments of minimal residual disease (MRD) effectively prognosticated relapse risks in patients with MCL treated with chemoimmunotherapy and transplantation, according to investigators.
However, by evaluating MRD status over time, disease progression could be predicted with greater certainty, according to study lead author Simone Ferrero, MD, assistant professor of hematology at the University of Torino (Italy).
“The most important message is that MRD is predictive, and then in particular, if you want to study MRD, you have to do a repeated MRD analysis in order to have a better picture of the disease course and to determine the prognosis of each single patient,” Dr. Ferrero said in an interview.
Predictive power of MRD
Piers Blombery, MBBS, hematologist and medical lead of the Molecular Hematology Laboratory at Peter MacCallum Cancer Centre in Melbourne, said these study findings illustrate how the predictive power of MRD is enhanced when it is evaluated longitudinally, over the course of a patient’s disease.
“We need to be prepared to have models of MRD that take into account changes in values across time,” Dr. Blombery said in an interview.
“Conventionally, we have looked at single time points in disease, such as end of induction therapy or post autologous stem cell transplant,” said Dr. Blombery, “but we may need now to have more complex models that can integrate values over multiple time points to guide intervention.”
Dr. Blombery, who was not involved in the study, coauthored an independent commentary on the results that was also published in Blood.
Tools to predict relapse
Although initial MCL treatment is often effective, most patients will relapse. Accordingly, there have been considerable efforts to develop tools that identify patients at high risk of relapse, according to Dr. Ferrero and coauthors.
Among these tools is the Mantle Cell Lymphoma International Prognostic Index (MIPI), which risk-stratifies patients on the basis of age, performance status, leukocyte count, and lactate dehydrogenase.
More recently, in several published studies, MRD has been demonstrated a high predictive value in MCL. However, the best way to evaluate MRD has yet to be worked out. There are differences in testing methods, sampling (that is, bone marrow versus peripheral blood), and time points that are evaluated.
In some recent studies, investigators have pooled MRD samples taken at different time points, according to Dr. Ferrero and colleagues. Furthermore, there had been no prior systematic attempts to compare the prognostic performance of MRD data evaluated over time, as compared with MRD at a single time point.
Exploiting MRD data
The analysis by Dr. Ferrero and coauthors, which appears in the journal Blood, is a substudy of FIL MCL0208, a recent multicenter, randomized, phase 3 clinical trial that demonstrated the benefit of lenalidomide maintenance over observation following autologous stem cell transplantation (ASCT) among 300 patients with MCL in Italy and Portugal.
In the study, investigators monitored MRD in both peripheral blood and bone marrow at 10 fixed time points throughout treatment (including induction, consolidation, post ASCT, and then every 6 months during maintenance and follow-up), yielding 4,351 individual MRD results.
Individual MRD analyses were prognostic in MCL, according to the investigators, with results that were relevant to disease progression right after ASCT, and were most predictive starting at 6 months after ASCT.
However, the best way to exploit the bulk of MRD information that had been collected, investigators said, was a model that combined regularly updated MRD results with MIPI scores.
This MIPI-adjusted model, based on MRD assessed in bone marrow by real-time quantitative polymerase chain reaction (RQ-PCR), outperformed both MIPI and a MIPI-adjusted single time-point analysis in terms of predicting time to progression.
The predictive power of the MIPI-adjusted bone marrow RQ-PCR analysis was illustrated by an area under the curve of 0.85-0.87, compared with an AUC of just 0.60-0.63 for classic MIPI analysis, 0.62-0.65 for MIPI-adjusted MRD at a post-ASCT time-point analysis, and 0.74-0.77 for MIPI-adjusted MRD at 6 months from transplant.
Peripheral blood ‘easier to collect’
Although bone marrow analysis performed best in the single-point MRD evaluations, looking at MRD over time greatly improved the predictive power of the peripheral blood MRD results, yielding an AUC up to 0.81, according to the report.
This enhancement of peripheral blood performance is a finding that may have important clinical implications, according to Dr. Ferrero, considering that peripheral blood monitoring is more practical for long-term, repeated MRD monitoring.
“It is interesting, because peripheral blood is easier to collect than bone marrow,” Dr. Ferrero said. “So if you plan in your analysis to monitor MRD in peripheral blood every 6 months, then you can have a predictive value similar to that of one single point in bone marrow.”
Dr. Ferrero provided disclosures related to Janssen, EUSA Pharma, Gilead, Morphosys, Incyte, Clinigen, Servier, and Gentili. The other authors reported no relevant conflicts of interest.
Single assessments of minimal residual disease (MRD) effectively prognosticated relapse risks in patients with MCL treated with chemoimmunotherapy and transplantation, according to investigators.
However, by evaluating MRD status over time, disease progression could be predicted with greater certainty, according to study lead author Simone Ferrero, MD, assistant professor of hematology at the University of Torino (Italy).
“The most important message is that MRD is predictive, and then in particular, if you want to study MRD, you have to do a repeated MRD analysis in order to have a better picture of the disease course and to determine the prognosis of each single patient,” Dr. Ferrero said in an interview.
Predictive power of MRD
Piers Blombery, MBBS, hematologist and medical lead of the Molecular Hematology Laboratory at Peter MacCallum Cancer Centre in Melbourne, said these study findings illustrate how the predictive power of MRD is enhanced when it is evaluated longitudinally, over the course of a patient’s disease.
“We need to be prepared to have models of MRD that take into account changes in values across time,” Dr. Blombery said in an interview.
“Conventionally, we have looked at single time points in disease, such as end of induction therapy or post autologous stem cell transplant,” said Dr. Blombery, “but we may need now to have more complex models that can integrate values over multiple time points to guide intervention.”
Dr. Blombery, who was not involved in the study, coauthored an independent commentary on the results that was also published in Blood.
Tools to predict relapse
Although initial MCL treatment is often effective, most patients will relapse. Accordingly, there have been considerable efforts to develop tools that identify patients at high risk of relapse, according to Dr. Ferrero and coauthors.
Among these tools is the Mantle Cell Lymphoma International Prognostic Index (MIPI), which risk-stratifies patients on the basis of age, performance status, leukocyte count, and lactate dehydrogenase.
More recently, in several published studies, MRD has been demonstrated a high predictive value in MCL. However, the best way to evaluate MRD has yet to be worked out. There are differences in testing methods, sampling (that is, bone marrow versus peripheral blood), and time points that are evaluated.
In some recent studies, investigators have pooled MRD samples taken at different time points, according to Dr. Ferrero and colleagues. Furthermore, there had been no prior systematic attempts to compare the prognostic performance of MRD data evaluated over time, as compared with MRD at a single time point.
Exploiting MRD data
The analysis by Dr. Ferrero and coauthors, which appears in the journal Blood, is a substudy of FIL MCL0208, a recent multicenter, randomized, phase 3 clinical trial that demonstrated the benefit of lenalidomide maintenance over observation following autologous stem cell transplantation (ASCT) among 300 patients with MCL in Italy and Portugal.
In the study, investigators monitored MRD in both peripheral blood and bone marrow at 10 fixed time points throughout treatment (including induction, consolidation, post ASCT, and then every 6 months during maintenance and follow-up), yielding 4,351 individual MRD results.
Individual MRD analyses were prognostic in MCL, according to the investigators, with results that were relevant to disease progression right after ASCT, and were most predictive starting at 6 months after ASCT.
However, the best way to exploit the bulk of MRD information that had been collected, investigators said, was a model that combined regularly updated MRD results with MIPI scores.
This MIPI-adjusted model, based on MRD assessed in bone marrow by real-time quantitative polymerase chain reaction (RQ-PCR), outperformed both MIPI and a MIPI-adjusted single time-point analysis in terms of predicting time to progression.
The predictive power of the MIPI-adjusted bone marrow RQ-PCR analysis was illustrated by an area under the curve of 0.85-0.87, compared with an AUC of just 0.60-0.63 for classic MIPI analysis, 0.62-0.65 for MIPI-adjusted MRD at a post-ASCT time-point analysis, and 0.74-0.77 for MIPI-adjusted MRD at 6 months from transplant.
Peripheral blood ‘easier to collect’
Although bone marrow analysis performed best in the single-point MRD evaluations, looking at MRD over time greatly improved the predictive power of the peripheral blood MRD results, yielding an AUC up to 0.81, according to the report.
This enhancement of peripheral blood performance is a finding that may have important clinical implications, according to Dr. Ferrero, considering that peripheral blood monitoring is more practical for long-term, repeated MRD monitoring.
“It is interesting, because peripheral blood is easier to collect than bone marrow,” Dr. Ferrero said. “So if you plan in your analysis to monitor MRD in peripheral blood every 6 months, then you can have a predictive value similar to that of one single point in bone marrow.”
Dr. Ferrero provided disclosures related to Janssen, EUSA Pharma, Gilead, Morphosys, Incyte, Clinigen, Servier, and Gentili. The other authors reported no relevant conflicts of interest.
FROM BLOOD