User login
Accelerated Prolonged Exposure Therapy for Posttraumatic Stress Disorder in a Veterans Health Administration System
Accelerated Prolonged Exposure Therapy for Posttraumatic Stress Disorder in a Veterans Health Administration System
Evidence-based psychotherapy (EBP) for posttraumatic stress disorder (PTSD), such as prolonged exposure (PE), is supported by multiple clinical practice guidelines and is expected to be available to veterans served by the Veterans Health Administration (VHA).1-5 However, traditional models of EBP delivery with 1 or 2 sessions weekly have high dropout rates.6,7 Few veterans who could benefit from such EBPs receive them, and those who do have low completion rates.8,9 Over a 15-year period, VHA records review of > 265,500 veterans with PTSD showed only 9.1% completed EBP treatment that included but was not limited to PE.10
One empirically supported solution that has yet to be widely implemented is delivering EBPs for PTSD in a massed or accelerated format of ≥ 3 sessions weekly.11 While these massed models of EBP delivery for PTSD are promising, their implementation is limited in federal health care settings, such as the VHA.12 PE therapy is a first-line treatment for PTSD that has been evaluated in numerous clinical trials since the early 1990s and in a wide range of trauma populations.13,14 Massed PE is effective and PE has been found to be effective both in-person and via telehealth.11,15,16
Another approach to accelerated PE is the inclusion of a massed PE course within a broader treatment context that includes augmentation of the massed PE with additional services, this is referred to as an intensive outpatient model (IOP).17 PE-IOP has also been shown to be feasible, acceptable, and effective with increased completion rates in comparison to the traditional (1 or 2 sessions weekly) model of PE.12,16,18,19 Ragsdale et al describe a 2-week IOP with multiple treatment tracks, including a PTSD track. The PTSD treatment track includes massed PE and additional standard services including case management, wellness services, family services, and a single session effective behaviors group. Additional augmentation services are available when clinically indicated (eg, repetitive transcranial magnetic stimulation, transcranial direct current stimulation treatment, psychoeducation, motivational interviewing, and/or relapse prevention).17
Rauch et al studied the first 80 patients completing an IOP program that consisted of PE (5 sessions weekly) and complementary interventions (eg, mindfulness and yoga) and reported a 96% retention rate, significant reductions of self-reported PTSD symptoms, significant reduction in self-reported co-occurring depression symptoms, and significant increase in self-reported satisfaction with social functioning. 18 In another study, Sherril et al explored patient reactions to participation in massed PE (5 sessions weekly) and found that patients reported significantly more positive than negative reactions. Sherrill et al noted that according to patients, the benefits of massed PE included a structured format that limits avoidance and distraction. The resulting fast pace of progress enhanced motivation; however, drawbacks included short-term discomfort and time demands.19 Yamokoski et al explored the feasibility of massed PE in a larger study of PTSD treatment in an intensive outpatient track (IOT) in a VHA PTSD clinic with minimal staffing. The 48 patients who completed IOT PTSD treatment in 2 or 4 weeks (including 35 patients who received massed PE) had high retention rates (85%), reported high satisfaction, and had significantly reduced PTSD and depression symptoms.12
The massed IOT PE model implemented by Yamokoski et al included the primary EBP intervention of massed PE with adjunctive groups. The addition of these groups increased both retention and patient-reported satisfaction. The PE-IOP model implemented by Rauch et al and Sherrill et al also included wellness and educational groups, as well as access to complementary interventions such as mindfulness and yoga.18,19 The addition of wellness education along with a primary EBP aligned with the VHA focus on whole health well-being and wellness. The whole health approach includes understanding the factors that motivate a patient toward health and well-being, provision of health education, and providing access to complementary interventions such as mindfulness.20 Dryden et al describe the whole health transformation within VHA as a proactive approach to addressing employee and patient wellness and health. Their research found that the whole health model promoted well-being in patients and staff and was sustained even during the COVID-19 pandemic.21 Dryden et al also noted that use of virtual technologies facilitated and promoted continued whole health implementation. The literature illustrates that: (1) massed PE can be provided with complementary education and wellness offerings, and that such offerings may increase both retention and satisfaction by enriching the massed PE treatment (eg, delivering PE-IOP); (2) whole health including wellness education and complementary interventions (eg, mindfulness, motivational enhancement) promotes well-being in both patients and mental health professionals; and (3) whole health education and complementary interventions can be delivered virtually.
Health Care Need
Prior to the implementation of a massed EBP for PTSD program at US Department of Veterans Affairs (VA) Pacific Islands Health Care System (VAPIHCS), our setting included a traditional outpatient program for treatment of PTSD and a 12- bed residential program for treatment of PTSD for male-identified (self-identified and identified as male in the electronic medical record) veterans via a cohort model with an 8- or 9-week length of stay. Both programs were located on Oahu. Thus, veterans who received care at VAPIHCS had access to PE in both outpatient and residential settings and via in-person and telehealth modalities. However, their access to PE was limited to the traditional models of PE delivery (eg, 1 or 2 session per week) and very few veterans outside of the island of Oahu had accessed PE treatment for PTSD. Moreover, when looking at PE reach within VAPIHCS, in the fiscal year prior to the implementation of the massed EBP program, only 32 of the > 5000 eligible veterans with a PTSD diagnosis had received PE. VAPIHCS serves veterans in a catchment area across the Pacific Basin which includes 3 time zones: Hawaii Standard Time (HST), Chamorro Standard Time (ChST), and Samoa Standard Time (SST). ChST is 20 hours ahead of HST, making service delivery that is inclusive for patients in Guam and Saipan especially challenging when providing care from Hawaii or other US states or territories. Given all of this, implementation of a new program offering accelerated PE virtually to any veterans with PTSD within the VAPIHCS would increase access to and reduce barriers to receiving PE.
PROGRAM DESCRIPTION
The Intensive Virtual EBP Team (iVET) for PTSD consists of an accelerated course of PE therapy and whole health education provided via VA Video Connect (VVC). iVET is a 3-week program and includes 3 parts: (1) massed individual PE therapy for PTSD; (2) group whole health and wellness classes; and (3) individual health coaching to address personal wellness goals. Programming is offered over 10-hour days to increase access across multiple time zones, especially to allow for participation in Guam and Saipan.
When a patient is referred to the iVET, their first contact is a video (or telephone) appointment with a registered nurse (RN) for a screening session. The screening session is designed to educate the patient about the program, including interventions, time commitment, and resources required for participation. In addition, following the educational discussion, the RN completes screening for safety with the patient including suicidal ideation and risk, as well as intimate partner violence risk. If urgent safety concerns are present, a licensed social worker or psychologist will join the screening to complete further assessment of risk and to address any safety concerns. Following screening, patients are scheduled for a VVC intake with a licensed therapist (social worker or psychologist) to complete the Clinician-Administered PTSD Scale (CAPS-5) for the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition), a clinical interview for PTSD assessment. Patients are also sent a secure link to complete a measurement-based care (MBC) battery of self-report measures including measures assessing demographics, PTSD symptoms, anxiety symptoms, depression symptoms, substance use, quality of life (QOL), and satisfaction with mental health care. The results of the CAPS-5 and self-report measures are discussed with the patient during the intake session when planning next steps and engaging in shared decision-making. This initial VVC intake not only allows for diagnostic goodness of fit but also provides the opportunity to troubleshoot any technical difficulties the patients might have with the virtual platforms.
There are minimal exclusion criteria for participation in iVET, which include active unmanaged psychosis or manic symptoms, recent suicidal crises (attempt within 8 weeks), active nonsuicidal self-injurious behaviors (within 8 weeks), and moderate-to-severe cognitive impairment. Following intake, patients are scheduled to begin their course of care with iVET. Upon completion of intake, patients are sent program materials for their individual and group classes, asked to obtain or request a recording device, and told they will receive email links for all VVC appointments. Patients are admitted to the iVET in a rolling admission fashion, thereby increasing access when compared to closed group and/or cohort models of care.
Patients receiving care in iVET attend 2 or 3 telehealth appointments daily with practice exercises daily between telehealth sessions. The primary EBP intervention in the iVET for PTSD program is a massed or accelerated course of PE, which includes 4 primary components: psychoeducation, in-vivo exposure, imaginal exposure, and breathing retraining. Specifically, PE is delivered in 4 90-minute individual sessions weekly allowing completion of the full PE protocol, to fidelity, in 3 weeks. In addition to receiving this primary intervention, patients also participate in four 50-minute group sessions per week of a whole health and wellness education class and have access to one 30- to 60-minute session weekly of individual health coaching should they wish to set wellness goals and receive coaching in support of attaining wellness goals. During iVET, patients are invited to complete MBC batteries of selfreport measures including measures assessing PTSD symptoms, anxiety symptoms, depression symptoms, substance use, QOL, and satisfaction with mental health care at sessions 1, 5, 9, and the final session of PE. Following discharge from the iVET, patients are offered 1-month, 3-month, and 6-month individual postdischarge check-up sessions with a therapist where they are invited to complete MBC measures and review relapse prevention and maintenance of treatment gains. Likewise, they are offered 1-month, 3-month, and 6-month postdischarge check-up sessions with an RN focused on maintaining wellness gains.
The iVET for PTSD staff includes 3 therapists (psychologists or social workers) and an RN. Additionally, the iVET for PTSD is supported by a program manager and a program support assistant. The primary cost of the program is salary for staff. Additional iVET for PTSD resources included computer equipment for staff and minimal supplies. Due to the virtual environment of care, iVET staff telework and do not require physical space within VAPIHCS.
OUTCOMES
All veterans receiving care in iVET for PTSD are invited to complete a MBC at multiple timepoints including pretreatment, during PE treatment, and posttreatment. The MBC measures included self-reported demographics, a 2-item measure of satisfaction with mental health services, the Brief Addiction Monitor-Intensive Outpatient Program questionnaire,22 the Generalized Anxiety Disorder-7 scale,23, the Patient Health Questionnaire (PHQ-9),24 the QOL Enjoyment and Satisfaction Questionnaire- Short Form,25 and the PTSD Checklist for DSM-5 (PCL-5), both weekly and monthly versions. 26,27
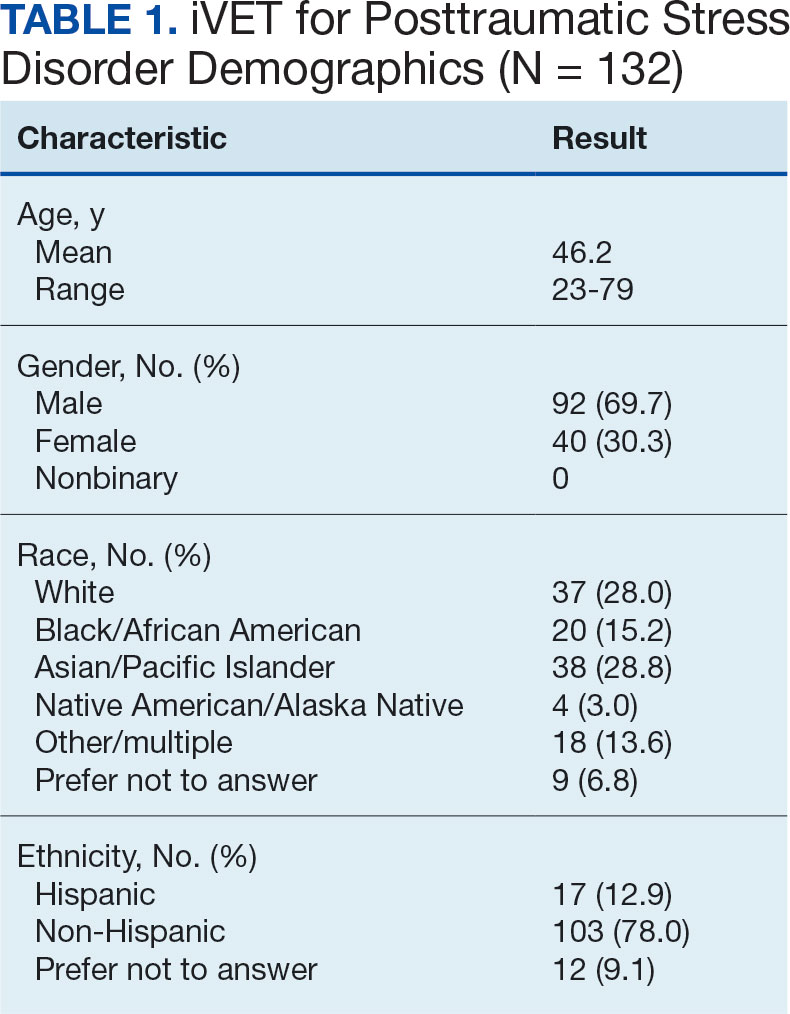
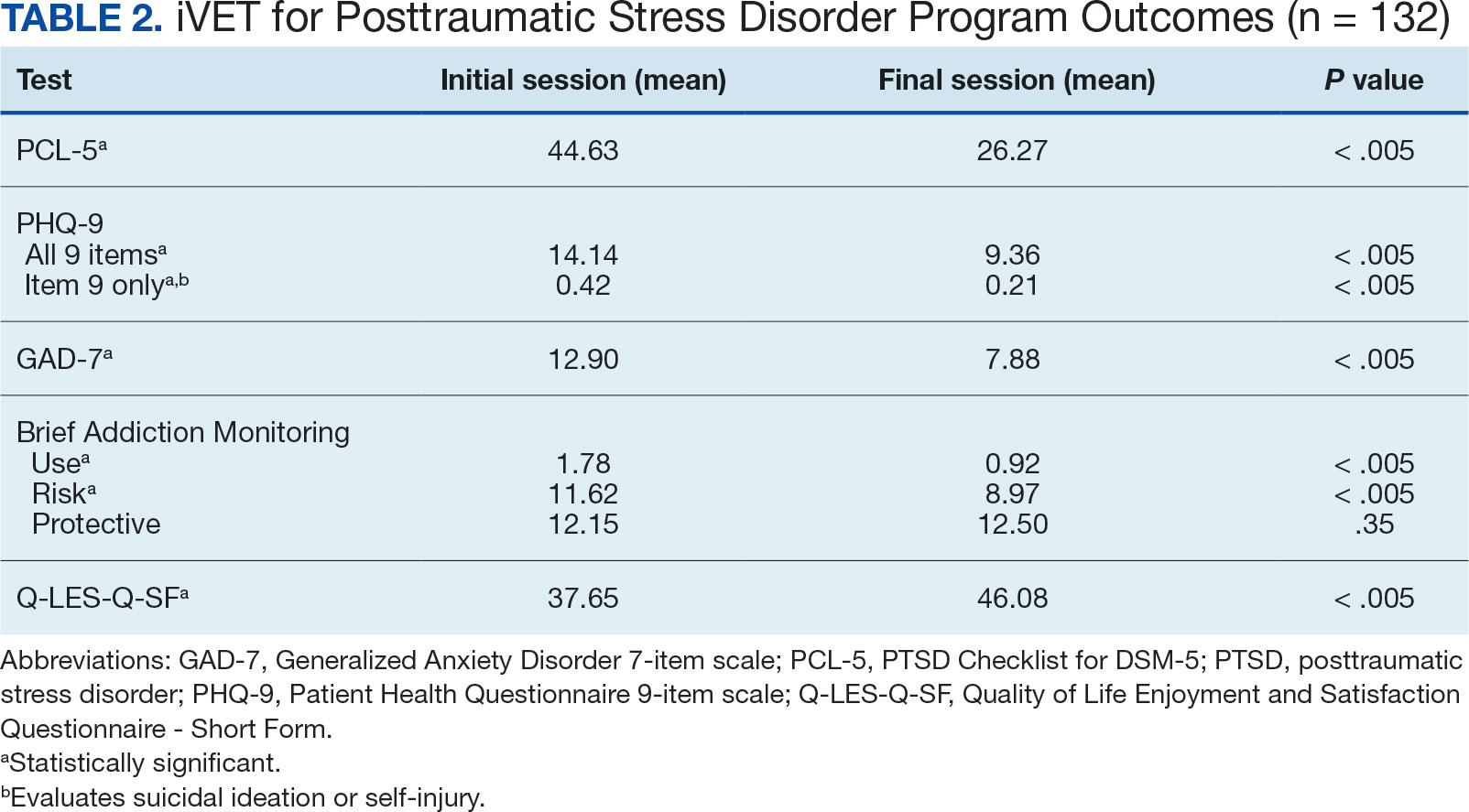
The retention rate has averaged 81% since the iVET for PTSD opened in 2022. To date, 132 veterans have completed the iVET for PTSD program, including a full course of massed PE (Table 1). Veterans experienced reduced PTSD (P < .005), depression (P < .005), anxiety (P < .005), and substance use risk (P < .005). Veterans experienced improved QOL (P < .005) and reported high satisfaction with mental health care in iVET for PTSD (Table 2). Veterans also experienced reduced thoughts of death or suicidal ideation (SI) based on PHQ-9 item 9 responses. When looking categorically at presence or absence of SI on PHQ-9 item 9, a significant relationship was found between the absence of suicidal ideation and completion of a course of massed PE: X2 (1, N = 132) = 13.75, P < .001. In addition, veterans who completed the program showed a significant decrease in severity of SI as measured continuously (range, 0-3) on PHQ-9 item 9 (P < .005).
Another important aspect to consider when implementing massed models of EBP is the impact on employee well-being and job satisfaction. The impact of EBP on staff was assessed following the initial EBP project. To explore this further, all staff members in the iVET for PTSD were invited to engage in a small program evaluation. iVET staff were guided through a visualization meditation intended to recall a typical workday 1 month prior to starting their new position with iVET. After the visualization meditation, staff completed the Professional Quality of Life (ProQOL) scale, a 30-item, self-reported questionnaire for health care workers that evaluates compassion satisfaction, perceived support, burnout, secondary traumatic stress, and moral distress.28 One week later, staff were asked to complete the ProQOL again to capture their state after the first 6 months into their tenure as iVET staff. iVET employees experienced significantly increased perceived support (P < .05), reduced burnout (P < .05), reduced secondary traumatic stress (P < .05), and reduced moral distress (P < .05). Team members also remarked on the rewarding nature of the work and care model.
Future Directions
Future research should aim to sustain these outcomes as the iVET program continues to serve more veterans. Another important line of inquiry is longer-term follow-up, as exploring if outcomes are maintained over time is an important question that has not been answered in this article. In addition, we hope to see the accelerated model of care applied to treatment of other presenting concerns in mental health treatment (eg, anxiety, depression, insomnia). Expansion of accelerated mental health treatment into other federal and non-federal health care settings is another worthy direction. Finally, while short term (6 months) assessment of staff satisfaction in iVET was promising, ongoing assessment staff satisfaction over a longer timeframe (1-5 years) is also important.
CONCLUSIONS
PE for PTSD has been demonstrated to be effective and improve functioning and is supported by multiple clinical practice guidelines.1-5 However, as federal practitioners, we must consider the reality that many of the individuals who could benefit are not engaging in PE and there is a high dropout rate for those that do. It is vital that we envision a future state where access to PE for PTSD is equitable and inclusive, retention rates are dramatically improved, and clinicians providing PE do not experience high rates of burnout.
We must continue exploring how we can better care for our patients and colleagues. We posit that the development of programs, or tracks within existing programs, that provide massed or accelerated PE for PTSD with virtual delivery options is an imperative step toward improved care. Federal health care settings treating trauma-exposed patients with PTSD, such as those within the US Department of Defense, Indian Health Services, Federal Bureau of Prisons, and VA, are well positioned to implement programs like iVET. We believe this model of care has great merit and foresee a future where all patients seeking PTSD treatment have the option to complete an accelerated or massed course of PE should they so desire. The experiences outlined in this article illustrate the feasibility, acceptability, and sustainability of such programs without requiring substantial staffing and financial resources.
- American Psychological Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. February 24, 2017. Accessed February 27, 2025. https://www.apa.org/ptsd-guideline/ptsd.pdf
- US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. Veterans Health Administration (VHA) Handbook 1160.01. September 11, 2008. Accessed February 27, 2025. https://www.mentalhealth.va.gov/providers/sud/docs/UniformServicesHandbook1160-01.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3. 2017. Accessed February 27, 2025. https://www.healthquality.va.gov/guidelines/MH/ptsd/VA-DoD-CPG-PTSD-Full-CPG-Edited-11162024.pdf
- Hamblen JL, Bernardy NC, Sherrieb K, et al. VA PTSD clinic director perspectives: How perceptions of readiness influence delivery of evidence-based PTSD treatment. Prof Psychol Res Pract. 2015;46(2): 90-96. doi:10.1037/a0038535
- Schnurr PP, Chard KM, Ruzek JI, et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2136921. doi:10.1001/jamanetworkopen. 2021.36921
- Kehle-Forbes SM, Meis LA, Spoont MR, Polusny MA. Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma. 2016;8(1):107-114. doi:10.1037/tra0000065
- Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273. doi:10.1002/jts.21927
- Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder. Adm Policy Ment Health. 2013;40(4):311-318. doi:10.1007/s10488-012-0421-0
- Maguen S, Holder N, Madden E, et al. Evidence-based psychotherapy trends among posttraumatic stress disorder patients in a national healthcare system, 2001-2014. Depress Anxiety. 2020;37(4):356-364. doi:10.1002/da.22983
- Maguen S, Li Y, Madden E, et al. Factors associated with completing evidence-based psychotherapy for PTSD among veterans in a national healthcare system. Psychiatry Res. 2019;274:112-128. doi:10.1016/j.psychres.2019.02.027
- Foa EB, McLean CP, Zang Y, et al. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA. 2018;319(4):354-364. doi:10.1001/jama.2017.21242
- Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration. Psychol Serv. 2023;20(3):506-515. doi:10.1037/ser0000628
- McLean CP, Foa EB. State of the Science: Prolonged exposure therapy for the treatment of posttraumatic stress disorder. J Trauma Stress. 2024;37(4):535-550. doi:10.1002/jts.23046
- McLean CP, Levy HC, Miller ML, Tolin DF. Exposure therapy for PTSD: A meta-analysis. Clin Psychol Rev. 2022;91:102115. doi:10.1016/j.cpr.2021.102115
- Wells SY, Morland LA, Wilhite ER, et al. Delivering Prolonged Exposure Therapy via Videoconferencing During the COVID-19 Pandemic: An Overview of the Research and Special Considerations for Providers. J Trauma Stress. 2020;33(4):380-390. doi:10.1002/jts.22573
- Peterson AL, Blount TH, Foa EB, et al. Massed vs intensive outpatient prolonged exposure for combat-related posttraumatic stress disorder: a randomized clinical trial. JAMA Netw Open. 2023;6(1):e2249422. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.49422
- Ragsdale KA, Nichols AA, Mehta M, et al. Comorbid treatment of traumatic brain injury and mental health disorders. NeuroRehabilitation. 2024;55(3):375-384. doi:10.3233/NRE-230235
- Rauch SAM, Yasinski CW, Post LM, et al. An intensive outpatient program with prolonged exposure for veterans with posttraumatic stress disorder: retention, predictors, and patterns of change. Psychol Serv. 2021;18(4):606-618. doi:10.1037/ser0000422
- Sherrill AM, Maples-Keller JL, Yasinski CW, Loucks LA, Rothbaum BO, Rauch SAM. Perceived benefits and drawbacks of massed prolonged exposure: qualitative thematic analysis of reactions from treatment completers. Psychol Trauma. 2022;14(5):862-870. doi:10.1037/tra0000548
- Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
- Dryden EM, Bolton RE, Bokhour BG, et al. Leaning Into whole health: sustaining system transformation while supporting patients and employees during COVID-19. Glob Adv Health Med. 2021;10:21649561211021047. doi:10.1177/21649561211021047
- Cacciola JS, Alterman AI, Dephilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treat. 2013;44(3):256-263. doi:10.1016/j.jsat.2012.07.013
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
- Kroenke K, Spi tze r RL , Wi l l i ams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Stevanovic D. Quality of Life Enjoyment and Satisfaction Questionnaire-short form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18(8):744-750. doi:10.1111/j.1365-2850.2011.01735.x
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL- 5). National Center for PTSD. Updated August 29, 2023. Accessed February 27, 2025. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.pdf
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Stamm BH. The Concise ProQOL Manual. 2nd ed. Pro- QOL.org; 2010.
Evidence-based psychotherapy (EBP) for posttraumatic stress disorder (PTSD), such as prolonged exposure (PE), is supported by multiple clinical practice guidelines and is expected to be available to veterans served by the Veterans Health Administration (VHA).1-5 However, traditional models of EBP delivery with 1 or 2 sessions weekly have high dropout rates.6,7 Few veterans who could benefit from such EBPs receive them, and those who do have low completion rates.8,9 Over a 15-year period, VHA records review of > 265,500 veterans with PTSD showed only 9.1% completed EBP treatment that included but was not limited to PE.10
One empirically supported solution that has yet to be widely implemented is delivering EBPs for PTSD in a massed or accelerated format of ≥ 3 sessions weekly.11 While these massed models of EBP delivery for PTSD are promising, their implementation is limited in federal health care settings, such as the VHA.12 PE therapy is a first-line treatment for PTSD that has been evaluated in numerous clinical trials since the early 1990s and in a wide range of trauma populations.13,14 Massed PE is effective and PE has been found to be effective both in-person and via telehealth.11,15,16
Another approach to accelerated PE is the inclusion of a massed PE course within a broader treatment context that includes augmentation of the massed PE with additional services, this is referred to as an intensive outpatient model (IOP).17 PE-IOP has also been shown to be feasible, acceptable, and effective with increased completion rates in comparison to the traditional (1 or 2 sessions weekly) model of PE.12,16,18,19 Ragsdale et al describe a 2-week IOP with multiple treatment tracks, including a PTSD track. The PTSD treatment track includes massed PE and additional standard services including case management, wellness services, family services, and a single session effective behaviors group. Additional augmentation services are available when clinically indicated (eg, repetitive transcranial magnetic stimulation, transcranial direct current stimulation treatment, psychoeducation, motivational interviewing, and/or relapse prevention).17
Rauch et al studied the first 80 patients completing an IOP program that consisted of PE (5 sessions weekly) and complementary interventions (eg, mindfulness and yoga) and reported a 96% retention rate, significant reductions of self-reported PTSD symptoms, significant reduction in self-reported co-occurring depression symptoms, and significant increase in self-reported satisfaction with social functioning. 18 In another study, Sherril et al explored patient reactions to participation in massed PE (5 sessions weekly) and found that patients reported significantly more positive than negative reactions. Sherrill et al noted that according to patients, the benefits of massed PE included a structured format that limits avoidance and distraction. The resulting fast pace of progress enhanced motivation; however, drawbacks included short-term discomfort and time demands.19 Yamokoski et al explored the feasibility of massed PE in a larger study of PTSD treatment in an intensive outpatient track (IOT) in a VHA PTSD clinic with minimal staffing. The 48 patients who completed IOT PTSD treatment in 2 or 4 weeks (including 35 patients who received massed PE) had high retention rates (85%), reported high satisfaction, and had significantly reduced PTSD and depression symptoms.12
The massed IOT PE model implemented by Yamokoski et al included the primary EBP intervention of massed PE with adjunctive groups. The addition of these groups increased both retention and patient-reported satisfaction. The PE-IOP model implemented by Rauch et al and Sherrill et al also included wellness and educational groups, as well as access to complementary interventions such as mindfulness and yoga.18,19 The addition of wellness education along with a primary EBP aligned with the VHA focus on whole health well-being and wellness. The whole health approach includes understanding the factors that motivate a patient toward health and well-being, provision of health education, and providing access to complementary interventions such as mindfulness.20 Dryden et al describe the whole health transformation within VHA as a proactive approach to addressing employee and patient wellness and health. Their research found that the whole health model promoted well-being in patients and staff and was sustained even during the COVID-19 pandemic.21 Dryden et al also noted that use of virtual technologies facilitated and promoted continued whole health implementation. The literature illustrates that: (1) massed PE can be provided with complementary education and wellness offerings, and that such offerings may increase both retention and satisfaction by enriching the massed PE treatment (eg, delivering PE-IOP); (2) whole health including wellness education and complementary interventions (eg, mindfulness, motivational enhancement) promotes well-being in both patients and mental health professionals; and (3) whole health education and complementary interventions can be delivered virtually.
Health Care Need
Prior to the implementation of a massed EBP for PTSD program at US Department of Veterans Affairs (VA) Pacific Islands Health Care System (VAPIHCS), our setting included a traditional outpatient program for treatment of PTSD and a 12- bed residential program for treatment of PTSD for male-identified (self-identified and identified as male in the electronic medical record) veterans via a cohort model with an 8- or 9-week length of stay. Both programs were located on Oahu. Thus, veterans who received care at VAPIHCS had access to PE in both outpatient and residential settings and via in-person and telehealth modalities. However, their access to PE was limited to the traditional models of PE delivery (eg, 1 or 2 session per week) and very few veterans outside of the island of Oahu had accessed PE treatment for PTSD. Moreover, when looking at PE reach within VAPIHCS, in the fiscal year prior to the implementation of the massed EBP program, only 32 of the > 5000 eligible veterans with a PTSD diagnosis had received PE. VAPIHCS serves veterans in a catchment area across the Pacific Basin which includes 3 time zones: Hawaii Standard Time (HST), Chamorro Standard Time (ChST), and Samoa Standard Time (SST). ChST is 20 hours ahead of HST, making service delivery that is inclusive for patients in Guam and Saipan especially challenging when providing care from Hawaii or other US states or territories. Given all of this, implementation of a new program offering accelerated PE virtually to any veterans with PTSD within the VAPIHCS would increase access to and reduce barriers to receiving PE.
PROGRAM DESCRIPTION
The Intensive Virtual EBP Team (iVET) for PTSD consists of an accelerated course of PE therapy and whole health education provided via VA Video Connect (VVC). iVET is a 3-week program and includes 3 parts: (1) massed individual PE therapy for PTSD; (2) group whole health and wellness classes; and (3) individual health coaching to address personal wellness goals. Programming is offered over 10-hour days to increase access across multiple time zones, especially to allow for participation in Guam and Saipan.
When a patient is referred to the iVET, their first contact is a video (or telephone) appointment with a registered nurse (RN) for a screening session. The screening session is designed to educate the patient about the program, including interventions, time commitment, and resources required for participation. In addition, following the educational discussion, the RN completes screening for safety with the patient including suicidal ideation and risk, as well as intimate partner violence risk. If urgent safety concerns are present, a licensed social worker or psychologist will join the screening to complete further assessment of risk and to address any safety concerns. Following screening, patients are scheduled for a VVC intake with a licensed therapist (social worker or psychologist) to complete the Clinician-Administered PTSD Scale (CAPS-5) for the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition), a clinical interview for PTSD assessment. Patients are also sent a secure link to complete a measurement-based care (MBC) battery of self-report measures including measures assessing demographics, PTSD symptoms, anxiety symptoms, depression symptoms, substance use, quality of life (QOL), and satisfaction with mental health care. The results of the CAPS-5 and self-report measures are discussed with the patient during the intake session when planning next steps and engaging in shared decision-making. This initial VVC intake not only allows for diagnostic goodness of fit but also provides the opportunity to troubleshoot any technical difficulties the patients might have with the virtual platforms.
There are minimal exclusion criteria for participation in iVET, which include active unmanaged psychosis or manic symptoms, recent suicidal crises (attempt within 8 weeks), active nonsuicidal self-injurious behaviors (within 8 weeks), and moderate-to-severe cognitive impairment. Following intake, patients are scheduled to begin their course of care with iVET. Upon completion of intake, patients are sent program materials for their individual and group classes, asked to obtain or request a recording device, and told they will receive email links for all VVC appointments. Patients are admitted to the iVET in a rolling admission fashion, thereby increasing access when compared to closed group and/or cohort models of care.
Patients receiving care in iVET attend 2 or 3 telehealth appointments daily with practice exercises daily between telehealth sessions. The primary EBP intervention in the iVET for PTSD program is a massed or accelerated course of PE, which includes 4 primary components: psychoeducation, in-vivo exposure, imaginal exposure, and breathing retraining. Specifically, PE is delivered in 4 90-minute individual sessions weekly allowing completion of the full PE protocol, to fidelity, in 3 weeks. In addition to receiving this primary intervention, patients also participate in four 50-minute group sessions per week of a whole health and wellness education class and have access to one 30- to 60-minute session weekly of individual health coaching should they wish to set wellness goals and receive coaching in support of attaining wellness goals. During iVET, patients are invited to complete MBC batteries of selfreport measures including measures assessing PTSD symptoms, anxiety symptoms, depression symptoms, substance use, QOL, and satisfaction with mental health care at sessions 1, 5, 9, and the final session of PE. Following discharge from the iVET, patients are offered 1-month, 3-month, and 6-month individual postdischarge check-up sessions with a therapist where they are invited to complete MBC measures and review relapse prevention and maintenance of treatment gains. Likewise, they are offered 1-month, 3-month, and 6-month postdischarge check-up sessions with an RN focused on maintaining wellness gains.
The iVET for PTSD staff includes 3 therapists (psychologists or social workers) and an RN. Additionally, the iVET for PTSD is supported by a program manager and a program support assistant. The primary cost of the program is salary for staff. Additional iVET for PTSD resources included computer equipment for staff and minimal supplies. Due to the virtual environment of care, iVET staff telework and do not require physical space within VAPIHCS.
OUTCOMES
All veterans receiving care in iVET for PTSD are invited to complete a MBC at multiple timepoints including pretreatment, during PE treatment, and posttreatment. The MBC measures included self-reported demographics, a 2-item measure of satisfaction with mental health services, the Brief Addiction Monitor-Intensive Outpatient Program questionnaire,22 the Generalized Anxiety Disorder-7 scale,23, the Patient Health Questionnaire (PHQ-9),24 the QOL Enjoyment and Satisfaction Questionnaire- Short Form,25 and the PTSD Checklist for DSM-5 (PCL-5), both weekly and monthly versions. 26,27
The retention rate has averaged 81% since the iVET for PTSD opened in 2022. To date, 132 veterans have completed the iVET for PTSD program, including a full course of massed PE (Table 1). Veterans experienced reduced PTSD (P < .005), depression (P < .005), anxiety (P < .005), and substance use risk (P < .005). Veterans experienced improved QOL (P < .005) and reported high satisfaction with mental health care in iVET for PTSD (Table 2). Veterans also experienced reduced thoughts of death or suicidal ideation (SI) based on PHQ-9 item 9 responses. When looking categorically at presence or absence of SI on PHQ-9 item 9, a significant relationship was found between the absence of suicidal ideation and completion of a course of massed PE: X2 (1, N = 132) = 13.75, P < .001. In addition, veterans who completed the program showed a significant decrease in severity of SI as measured continuously (range, 0-3) on PHQ-9 item 9 (P < .005).


Another important aspect to consider when implementing massed models of EBP is the impact on employee well-being and job satisfaction. The impact of EBP on staff was assessed following the initial EBP project. To explore this further, all staff members in the iVET for PTSD were invited to engage in a small program evaluation. iVET staff were guided through a visualization meditation intended to recall a typical workday 1 month prior to starting their new position with iVET. After the visualization meditation, staff completed the Professional Quality of Life (ProQOL) scale, a 30-item, self-reported questionnaire for health care workers that evaluates compassion satisfaction, perceived support, burnout, secondary traumatic stress, and moral distress.28 One week later, staff were asked to complete the ProQOL again to capture their state after the first 6 months into their tenure as iVET staff. iVET employees experienced significantly increased perceived support (P < .05), reduced burnout (P < .05), reduced secondary traumatic stress (P < .05), and reduced moral distress (P < .05). Team members also remarked on the rewarding nature of the work and care model.
Future Directions
Future research should aim to sustain these outcomes as the iVET program continues to serve more veterans. Another important line of inquiry is longer-term follow-up, as exploring if outcomes are maintained over time is an important question that has not been answered in this article. In addition, we hope to see the accelerated model of care applied to treatment of other presenting concerns in mental health treatment (eg, anxiety, depression, insomnia). Expansion of accelerated mental health treatment into other federal and non-federal health care settings is another worthy direction. Finally, while short term (6 months) assessment of staff satisfaction in iVET was promising, ongoing assessment staff satisfaction over a longer timeframe (1-5 years) is also important.
CONCLUSIONS
PE for PTSD has been demonstrated to be effective and improve functioning and is supported by multiple clinical practice guidelines.1-5 However, as federal practitioners, we must consider the reality that many of the individuals who could benefit are not engaging in PE and there is a high dropout rate for those that do. It is vital that we envision a future state where access to PE for PTSD is equitable and inclusive, retention rates are dramatically improved, and clinicians providing PE do not experience high rates of burnout.
We must continue exploring how we can better care for our patients and colleagues. We posit that the development of programs, or tracks within existing programs, that provide massed or accelerated PE for PTSD with virtual delivery options is an imperative step toward improved care. Federal health care settings treating trauma-exposed patients with PTSD, such as those within the US Department of Defense, Indian Health Services, Federal Bureau of Prisons, and VA, are well positioned to implement programs like iVET. We believe this model of care has great merit and foresee a future where all patients seeking PTSD treatment have the option to complete an accelerated or massed course of PE should they so desire. The experiences outlined in this article illustrate the feasibility, acceptability, and sustainability of such programs without requiring substantial staffing and financial resources.
Evidence-based psychotherapy (EBP) for posttraumatic stress disorder (PTSD), such as prolonged exposure (PE), is supported by multiple clinical practice guidelines and is expected to be available to veterans served by the Veterans Health Administration (VHA).1-5 However, traditional models of EBP delivery with 1 or 2 sessions weekly have high dropout rates.6,7 Few veterans who could benefit from such EBPs receive them, and those who do have low completion rates.8,9 Over a 15-year period, VHA records review of > 265,500 veterans with PTSD showed only 9.1% completed EBP treatment that included but was not limited to PE.10
One empirically supported solution that has yet to be widely implemented is delivering EBPs for PTSD in a massed or accelerated format of ≥ 3 sessions weekly.11 While these massed models of EBP delivery for PTSD are promising, their implementation is limited in federal health care settings, such as the VHA.12 PE therapy is a first-line treatment for PTSD that has been evaluated in numerous clinical trials since the early 1990s and in a wide range of trauma populations.13,14 Massed PE is effective and PE has been found to be effective both in-person and via telehealth.11,15,16
Another approach to accelerated PE is the inclusion of a massed PE course within a broader treatment context that includes augmentation of the massed PE with additional services, this is referred to as an intensive outpatient model (IOP).17 PE-IOP has also been shown to be feasible, acceptable, and effective with increased completion rates in comparison to the traditional (1 or 2 sessions weekly) model of PE.12,16,18,19 Ragsdale et al describe a 2-week IOP with multiple treatment tracks, including a PTSD track. The PTSD treatment track includes massed PE and additional standard services including case management, wellness services, family services, and a single session effective behaviors group. Additional augmentation services are available when clinically indicated (eg, repetitive transcranial magnetic stimulation, transcranial direct current stimulation treatment, psychoeducation, motivational interviewing, and/or relapse prevention).17
Rauch et al studied the first 80 patients completing an IOP program that consisted of PE (5 sessions weekly) and complementary interventions (eg, mindfulness and yoga) and reported a 96% retention rate, significant reductions of self-reported PTSD symptoms, significant reduction in self-reported co-occurring depression symptoms, and significant increase in self-reported satisfaction with social functioning. 18 In another study, Sherril et al explored patient reactions to participation in massed PE (5 sessions weekly) and found that patients reported significantly more positive than negative reactions. Sherrill et al noted that according to patients, the benefits of massed PE included a structured format that limits avoidance and distraction. The resulting fast pace of progress enhanced motivation; however, drawbacks included short-term discomfort and time demands.19 Yamokoski et al explored the feasibility of massed PE in a larger study of PTSD treatment in an intensive outpatient track (IOT) in a VHA PTSD clinic with minimal staffing. The 48 patients who completed IOT PTSD treatment in 2 or 4 weeks (including 35 patients who received massed PE) had high retention rates (85%), reported high satisfaction, and had significantly reduced PTSD and depression symptoms.12
The massed IOT PE model implemented by Yamokoski et al included the primary EBP intervention of massed PE with adjunctive groups. The addition of these groups increased both retention and patient-reported satisfaction. The PE-IOP model implemented by Rauch et al and Sherrill et al also included wellness and educational groups, as well as access to complementary interventions such as mindfulness and yoga.18,19 The addition of wellness education along with a primary EBP aligned with the VHA focus on whole health well-being and wellness. The whole health approach includes understanding the factors that motivate a patient toward health and well-being, provision of health education, and providing access to complementary interventions such as mindfulness.20 Dryden et al describe the whole health transformation within VHA as a proactive approach to addressing employee and patient wellness and health. Their research found that the whole health model promoted well-being in patients and staff and was sustained even during the COVID-19 pandemic.21 Dryden et al also noted that use of virtual technologies facilitated and promoted continued whole health implementation. The literature illustrates that: (1) massed PE can be provided with complementary education and wellness offerings, and that such offerings may increase both retention and satisfaction by enriching the massed PE treatment (eg, delivering PE-IOP); (2) whole health including wellness education and complementary interventions (eg, mindfulness, motivational enhancement) promotes well-being in both patients and mental health professionals; and (3) whole health education and complementary interventions can be delivered virtually.
Health Care Need
Prior to the implementation of a massed EBP for PTSD program at US Department of Veterans Affairs (VA) Pacific Islands Health Care System (VAPIHCS), our setting included a traditional outpatient program for treatment of PTSD and a 12- bed residential program for treatment of PTSD for male-identified (self-identified and identified as male in the electronic medical record) veterans via a cohort model with an 8- or 9-week length of stay. Both programs were located on Oahu. Thus, veterans who received care at VAPIHCS had access to PE in both outpatient and residential settings and via in-person and telehealth modalities. However, their access to PE was limited to the traditional models of PE delivery (eg, 1 or 2 session per week) and very few veterans outside of the island of Oahu had accessed PE treatment for PTSD. Moreover, when looking at PE reach within VAPIHCS, in the fiscal year prior to the implementation of the massed EBP program, only 32 of the > 5000 eligible veterans with a PTSD diagnosis had received PE. VAPIHCS serves veterans in a catchment area across the Pacific Basin which includes 3 time zones: Hawaii Standard Time (HST), Chamorro Standard Time (ChST), and Samoa Standard Time (SST). ChST is 20 hours ahead of HST, making service delivery that is inclusive for patients in Guam and Saipan especially challenging when providing care from Hawaii or other US states or territories. Given all of this, implementation of a new program offering accelerated PE virtually to any veterans with PTSD within the VAPIHCS would increase access to and reduce barriers to receiving PE.
PROGRAM DESCRIPTION
The Intensive Virtual EBP Team (iVET) for PTSD consists of an accelerated course of PE therapy and whole health education provided via VA Video Connect (VVC). iVET is a 3-week program and includes 3 parts: (1) massed individual PE therapy for PTSD; (2) group whole health and wellness classes; and (3) individual health coaching to address personal wellness goals. Programming is offered over 10-hour days to increase access across multiple time zones, especially to allow for participation in Guam and Saipan.
When a patient is referred to the iVET, their first contact is a video (or telephone) appointment with a registered nurse (RN) for a screening session. The screening session is designed to educate the patient about the program, including interventions, time commitment, and resources required for participation. In addition, following the educational discussion, the RN completes screening for safety with the patient including suicidal ideation and risk, as well as intimate partner violence risk. If urgent safety concerns are present, a licensed social worker or psychologist will join the screening to complete further assessment of risk and to address any safety concerns. Following screening, patients are scheduled for a VVC intake with a licensed therapist (social worker or psychologist) to complete the Clinician-Administered PTSD Scale (CAPS-5) for the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition), a clinical interview for PTSD assessment. Patients are also sent a secure link to complete a measurement-based care (MBC) battery of self-report measures including measures assessing demographics, PTSD symptoms, anxiety symptoms, depression symptoms, substance use, quality of life (QOL), and satisfaction with mental health care. The results of the CAPS-5 and self-report measures are discussed with the patient during the intake session when planning next steps and engaging in shared decision-making. This initial VVC intake not only allows for diagnostic goodness of fit but also provides the opportunity to troubleshoot any technical difficulties the patients might have with the virtual platforms.
There are minimal exclusion criteria for participation in iVET, which include active unmanaged psychosis or manic symptoms, recent suicidal crises (attempt within 8 weeks), active nonsuicidal self-injurious behaviors (within 8 weeks), and moderate-to-severe cognitive impairment. Following intake, patients are scheduled to begin their course of care with iVET. Upon completion of intake, patients are sent program materials for their individual and group classes, asked to obtain or request a recording device, and told they will receive email links for all VVC appointments. Patients are admitted to the iVET in a rolling admission fashion, thereby increasing access when compared to closed group and/or cohort models of care.
Patients receiving care in iVET attend 2 or 3 telehealth appointments daily with practice exercises daily between telehealth sessions. The primary EBP intervention in the iVET for PTSD program is a massed or accelerated course of PE, which includes 4 primary components: psychoeducation, in-vivo exposure, imaginal exposure, and breathing retraining. Specifically, PE is delivered in 4 90-minute individual sessions weekly allowing completion of the full PE protocol, to fidelity, in 3 weeks. In addition to receiving this primary intervention, patients also participate in four 50-minute group sessions per week of a whole health and wellness education class and have access to one 30- to 60-minute session weekly of individual health coaching should they wish to set wellness goals and receive coaching in support of attaining wellness goals. During iVET, patients are invited to complete MBC batteries of selfreport measures including measures assessing PTSD symptoms, anxiety symptoms, depression symptoms, substance use, QOL, and satisfaction with mental health care at sessions 1, 5, 9, and the final session of PE. Following discharge from the iVET, patients are offered 1-month, 3-month, and 6-month individual postdischarge check-up sessions with a therapist where they are invited to complete MBC measures and review relapse prevention and maintenance of treatment gains. Likewise, they are offered 1-month, 3-month, and 6-month postdischarge check-up sessions with an RN focused on maintaining wellness gains.
The iVET for PTSD staff includes 3 therapists (psychologists or social workers) and an RN. Additionally, the iVET for PTSD is supported by a program manager and a program support assistant. The primary cost of the program is salary for staff. Additional iVET for PTSD resources included computer equipment for staff and minimal supplies. Due to the virtual environment of care, iVET staff telework and do not require physical space within VAPIHCS.
OUTCOMES
All veterans receiving care in iVET for PTSD are invited to complete a MBC at multiple timepoints including pretreatment, during PE treatment, and posttreatment. The MBC measures included self-reported demographics, a 2-item measure of satisfaction with mental health services, the Brief Addiction Monitor-Intensive Outpatient Program questionnaire,22 the Generalized Anxiety Disorder-7 scale,23, the Patient Health Questionnaire (PHQ-9),24 the QOL Enjoyment and Satisfaction Questionnaire- Short Form,25 and the PTSD Checklist for DSM-5 (PCL-5), both weekly and monthly versions. 26,27
The retention rate has averaged 81% since the iVET for PTSD opened in 2022. To date, 132 veterans have completed the iVET for PTSD program, including a full course of massed PE (Table 1). Veterans experienced reduced PTSD (P < .005), depression (P < .005), anxiety (P < .005), and substance use risk (P < .005). Veterans experienced improved QOL (P < .005) and reported high satisfaction with mental health care in iVET for PTSD (Table 2). Veterans also experienced reduced thoughts of death or suicidal ideation (SI) based on PHQ-9 item 9 responses. When looking categorically at presence or absence of SI on PHQ-9 item 9, a significant relationship was found between the absence of suicidal ideation and completion of a course of massed PE: X2 (1, N = 132) = 13.75, P < .001. In addition, veterans who completed the program showed a significant decrease in severity of SI as measured continuously (range, 0-3) on PHQ-9 item 9 (P < .005).


Another important aspect to consider when implementing massed models of EBP is the impact on employee well-being and job satisfaction. The impact of EBP on staff was assessed following the initial EBP project. To explore this further, all staff members in the iVET for PTSD were invited to engage in a small program evaluation. iVET staff were guided through a visualization meditation intended to recall a typical workday 1 month prior to starting their new position with iVET. After the visualization meditation, staff completed the Professional Quality of Life (ProQOL) scale, a 30-item, self-reported questionnaire for health care workers that evaluates compassion satisfaction, perceived support, burnout, secondary traumatic stress, and moral distress.28 One week later, staff were asked to complete the ProQOL again to capture their state after the first 6 months into their tenure as iVET staff. iVET employees experienced significantly increased perceived support (P < .05), reduced burnout (P < .05), reduced secondary traumatic stress (P < .05), and reduced moral distress (P < .05). Team members also remarked on the rewarding nature of the work and care model.
Future Directions
Future research should aim to sustain these outcomes as the iVET program continues to serve more veterans. Another important line of inquiry is longer-term follow-up, as exploring if outcomes are maintained over time is an important question that has not been answered in this article. In addition, we hope to see the accelerated model of care applied to treatment of other presenting concerns in mental health treatment (eg, anxiety, depression, insomnia). Expansion of accelerated mental health treatment into other federal and non-federal health care settings is another worthy direction. Finally, while short term (6 months) assessment of staff satisfaction in iVET was promising, ongoing assessment staff satisfaction over a longer timeframe (1-5 years) is also important.
CONCLUSIONS
PE for PTSD has been demonstrated to be effective and improve functioning and is supported by multiple clinical practice guidelines.1-5 However, as federal practitioners, we must consider the reality that many of the individuals who could benefit are not engaging in PE and there is a high dropout rate for those that do. It is vital that we envision a future state where access to PE for PTSD is equitable and inclusive, retention rates are dramatically improved, and clinicians providing PE do not experience high rates of burnout.
We must continue exploring how we can better care for our patients and colleagues. We posit that the development of programs, or tracks within existing programs, that provide massed or accelerated PE for PTSD with virtual delivery options is an imperative step toward improved care. Federal health care settings treating trauma-exposed patients with PTSD, such as those within the US Department of Defense, Indian Health Services, Federal Bureau of Prisons, and VA, are well positioned to implement programs like iVET. We believe this model of care has great merit and foresee a future where all patients seeking PTSD treatment have the option to complete an accelerated or massed course of PE should they so desire. The experiences outlined in this article illustrate the feasibility, acceptability, and sustainability of such programs without requiring substantial staffing and financial resources.
- American Psychological Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. February 24, 2017. Accessed February 27, 2025. https://www.apa.org/ptsd-guideline/ptsd.pdf
- US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. Veterans Health Administration (VHA) Handbook 1160.01. September 11, 2008. Accessed February 27, 2025. https://www.mentalhealth.va.gov/providers/sud/docs/UniformServicesHandbook1160-01.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3. 2017. Accessed February 27, 2025. https://www.healthquality.va.gov/guidelines/MH/ptsd/VA-DoD-CPG-PTSD-Full-CPG-Edited-11162024.pdf
- Hamblen JL, Bernardy NC, Sherrieb K, et al. VA PTSD clinic director perspectives: How perceptions of readiness influence delivery of evidence-based PTSD treatment. Prof Psychol Res Pract. 2015;46(2): 90-96. doi:10.1037/a0038535
- Schnurr PP, Chard KM, Ruzek JI, et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2136921. doi:10.1001/jamanetworkopen. 2021.36921
- Kehle-Forbes SM, Meis LA, Spoont MR, Polusny MA. Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma. 2016;8(1):107-114. doi:10.1037/tra0000065
- Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273. doi:10.1002/jts.21927
- Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder. Adm Policy Ment Health. 2013;40(4):311-318. doi:10.1007/s10488-012-0421-0
- Maguen S, Holder N, Madden E, et al. Evidence-based psychotherapy trends among posttraumatic stress disorder patients in a national healthcare system, 2001-2014. Depress Anxiety. 2020;37(4):356-364. doi:10.1002/da.22983
- Maguen S, Li Y, Madden E, et al. Factors associated with completing evidence-based psychotherapy for PTSD among veterans in a national healthcare system. Psychiatry Res. 2019;274:112-128. doi:10.1016/j.psychres.2019.02.027
- Foa EB, McLean CP, Zang Y, et al. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA. 2018;319(4):354-364. doi:10.1001/jama.2017.21242
- Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration. Psychol Serv. 2023;20(3):506-515. doi:10.1037/ser0000628
- McLean CP, Foa EB. State of the Science: Prolonged exposure therapy for the treatment of posttraumatic stress disorder. J Trauma Stress. 2024;37(4):535-550. doi:10.1002/jts.23046
- McLean CP, Levy HC, Miller ML, Tolin DF. Exposure therapy for PTSD: A meta-analysis. Clin Psychol Rev. 2022;91:102115. doi:10.1016/j.cpr.2021.102115
- Wells SY, Morland LA, Wilhite ER, et al. Delivering Prolonged Exposure Therapy via Videoconferencing During the COVID-19 Pandemic: An Overview of the Research and Special Considerations for Providers. J Trauma Stress. 2020;33(4):380-390. doi:10.1002/jts.22573
- Peterson AL, Blount TH, Foa EB, et al. Massed vs intensive outpatient prolonged exposure for combat-related posttraumatic stress disorder: a randomized clinical trial. JAMA Netw Open. 2023;6(1):e2249422. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.49422
- Ragsdale KA, Nichols AA, Mehta M, et al. Comorbid treatment of traumatic brain injury and mental health disorders. NeuroRehabilitation. 2024;55(3):375-384. doi:10.3233/NRE-230235
- Rauch SAM, Yasinski CW, Post LM, et al. An intensive outpatient program with prolonged exposure for veterans with posttraumatic stress disorder: retention, predictors, and patterns of change. Psychol Serv. 2021;18(4):606-618. doi:10.1037/ser0000422
- Sherrill AM, Maples-Keller JL, Yasinski CW, Loucks LA, Rothbaum BO, Rauch SAM. Perceived benefits and drawbacks of massed prolonged exposure: qualitative thematic analysis of reactions from treatment completers. Psychol Trauma. 2022;14(5):862-870. doi:10.1037/tra0000548
- Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
- Dryden EM, Bolton RE, Bokhour BG, et al. Leaning Into whole health: sustaining system transformation while supporting patients and employees during COVID-19. Glob Adv Health Med. 2021;10:21649561211021047. doi:10.1177/21649561211021047
- Cacciola JS, Alterman AI, Dephilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treat. 2013;44(3):256-263. doi:10.1016/j.jsat.2012.07.013
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
- Kroenke K, Spi tze r RL , Wi l l i ams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Stevanovic D. Quality of Life Enjoyment and Satisfaction Questionnaire-short form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18(8):744-750. doi:10.1111/j.1365-2850.2011.01735.x
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL- 5). National Center for PTSD. Updated August 29, 2023. Accessed February 27, 2025. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.pdf
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Stamm BH. The Concise ProQOL Manual. 2nd ed. Pro- QOL.org; 2010.
- American Psychological Association. Clinical Practice Guideline for the Treatment of Posttraumatic Stress Disorder (PTSD) in Adults. February 24, 2017. Accessed February 27, 2025. https://www.apa.org/ptsd-guideline/ptsd.pdf
- US Department of Veterans Affairs, Veterans Health Administration. Uniform mental health services in VA medical centers and clinics. Veterans Health Administration (VHA) Handbook 1160.01. September 11, 2008. Accessed February 27, 2025. https://www.mentalhealth.va.gov/providers/sud/docs/UniformServicesHandbook1160-01.pdf
- US Department of Veterans Affairs, US Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3. 2017. Accessed February 27, 2025. https://www.healthquality.va.gov/guidelines/MH/ptsd/VA-DoD-CPG-PTSD-Full-CPG-Edited-11162024.pdf
- Hamblen JL, Bernardy NC, Sherrieb K, et al. VA PTSD clinic director perspectives: How perceptions of readiness influence delivery of evidence-based PTSD treatment. Prof Psychol Res Pract. 2015;46(2): 90-96. doi:10.1037/a0038535
- Schnurr PP, Chard KM, Ruzek JI, et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among US veterans: a randomized clinical trial. JAMA Netw Open. 2022;5(1):e2136921. doi:10.1001/jamanetworkopen. 2021.36921
- Kehle-Forbes SM, Meis LA, Spoont MR, Polusny MA. Treatment initiation and dropout from prolonged exposure and cognitive processing therapy in a VA outpatient clinic. Psychol Trauma. 2016;8(1):107-114. doi:10.1037/tra0000065
- Mott JM, Mondragon S, Hundt NE, Beason-Smith M, Grady RH, Teng EJ. Characteristics of U.S. veterans who begin and complete prolonged exposure and cognitive processing therapy for PTSD. J Trauma Stress. 2014;27(3):265-273. doi:10.1002/jts.21927
- Shiner B, D’Avolio LW, Nguyen TM, et al. Measuring use of evidence based psychotherapy for posttraumatic stress disorder. Adm Policy Ment Health. 2013;40(4):311-318. doi:10.1007/s10488-012-0421-0
- Maguen S, Holder N, Madden E, et al. Evidence-based psychotherapy trends among posttraumatic stress disorder patients in a national healthcare system, 2001-2014. Depress Anxiety. 2020;37(4):356-364. doi:10.1002/da.22983
- Maguen S, Li Y, Madden E, et al. Factors associated with completing evidence-based psychotherapy for PTSD among veterans in a national healthcare system. Psychiatry Res. 2019;274:112-128. doi:10.1016/j.psychres.2019.02.027
- Foa EB, McLean CP, Zang Y, et al. Effect of prolonged exposure therapy delivered over 2 weeks vs 8 weeks vs present-centered therapy on PTSD symptom severity in military personnel: a randomized clinical trial. JAMA. 2018;319(4):354-364. doi:10.1001/jama.2017.21242
- Yamokoski C, Flores H, Facemire V, Maieritsch K, Perez S, Fedynich A. Feasibility of an intensive outpatient treatment program for posttraumatic stress disorder within the veterans health care administration. Psychol Serv. 2023;20(3):506-515. doi:10.1037/ser0000628
- McLean CP, Foa EB. State of the Science: Prolonged exposure therapy for the treatment of posttraumatic stress disorder. J Trauma Stress. 2024;37(4):535-550. doi:10.1002/jts.23046
- McLean CP, Levy HC, Miller ML, Tolin DF. Exposure therapy for PTSD: A meta-analysis. Clin Psychol Rev. 2022;91:102115. doi:10.1016/j.cpr.2021.102115
- Wells SY, Morland LA, Wilhite ER, et al. Delivering Prolonged Exposure Therapy via Videoconferencing During the COVID-19 Pandemic: An Overview of the Research and Special Considerations for Providers. J Trauma Stress. 2020;33(4):380-390. doi:10.1002/jts.22573
- Peterson AL, Blount TH, Foa EB, et al. Massed vs intensive outpatient prolonged exposure for combat-related posttraumatic stress disorder: a randomized clinical trial. JAMA Netw Open. 2023;6(1):e2249422. Published 2023 Jan 3. doi:10.1001/jamanetworkopen.2022.49422
- Ragsdale KA, Nichols AA, Mehta M, et al. Comorbid treatment of traumatic brain injury and mental health disorders. NeuroRehabilitation. 2024;55(3):375-384. doi:10.3233/NRE-230235
- Rauch SAM, Yasinski CW, Post LM, et al. An intensive outpatient program with prolonged exposure for veterans with posttraumatic stress disorder: retention, predictors, and patterns of change. Psychol Serv. 2021;18(4):606-618. doi:10.1037/ser0000422
- Sherrill AM, Maples-Keller JL, Yasinski CW, Loucks LA, Rothbaum BO, Rauch SAM. Perceived benefits and drawbacks of massed prolonged exposure: qualitative thematic analysis of reactions from treatment completers. Psychol Trauma. 2022;14(5):862-870. doi:10.1037/tra0000548
- Gaudet T, Kligler B. Whole health in the whole system of the Veterans Administration: how will we know we have reached this future state? J Altern Complement Med. 2019;25(S1):S7-S11. doi:10.1089/acm.2018.29061.gau
- Dryden EM, Bolton RE, Bokhour BG, et al. Leaning Into whole health: sustaining system transformation while supporting patients and employees during COVID-19. Glob Adv Health Med. 2021;10:21649561211021047. doi:10.1177/21649561211021047
- Cacciola JS, Alterman AI, Dephilippis D, et al. Development and initial evaluation of the Brief Addiction Monitor (BAM). J Subst Abuse Treat. 2013;44(3):256-263. doi:10.1016/j.jsat.2012.07.013
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi:10.1001/archinte.166.10.1092
- Kroenke K, Spi tze r RL , Wi l l i ams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Stevanovic D. Quality of Life Enjoyment and Satisfaction Questionnaire-short form for quality of life assessments in clinical practice: a psychometric study. J Psychiatr Ment Health Nurs. 2011;18(8):744-750. doi:10.1111/j.1365-2850.2011.01735.x
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for DSM-5 (PCL- 5). National Center for PTSD. Updated August 29, 2023. Accessed February 27, 2025. https://www.ptsd.va.gov/professional/assessment/documents/PCL5_Standard_form.pdf
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Stamm BH. The Concise ProQOL Manual. 2nd ed. Pro- QOL.org; 2010.
Accelerated Prolonged Exposure Therapy for Posttraumatic Stress Disorder in a Veterans Health Administration System
Accelerated Prolonged Exposure Therapy for Posttraumatic Stress Disorder in a Veterans Health Administration System
VA is a Leader in Mental Health and Social Service Research and Operations
VA is a Leader in Mental Health and Social Service Research and Operations
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
The US Department of Veterans Affairs (VA) mission is defined by President Abraham Lincoln’s promise “to care for him who shall have borne the battle, and for his widow, and his orphan.” Critically, the biopsychosocial needs of veterans differ from the needs of civilians due to the nature of military service.1 Veterans commonly experience traumatic brain injury (TBI) due to combat- or training-related injuries.2 Psychologically, veterans are disproportionately likely to be diagnosed with mental health conditions, such as posttraumatic stress disorder (PTSD), often linked to military exposures.3 Spiritually, veterans frequently express moral injury after living through circumstances when they perpetrate, fail to prevent, or witness events that contradict moral beliefs/ expectations.4 Veterans also have significant social challenges, including high rates of homelessness. 5 A critical strength of the VA mission is its awareness of these complex sequelae and its ability to provide well-informed treatment and social services to meet veterans’ unique needs.
Foundational to a well-informed health care system is a robust research and operational quality improvement infrastructure. The VA Office of Research and Development (ORD) has worked tirelessly to understand and address the unique, idiographic needs of veterans. In 2024 the ORD had a budget of $2.4 billion, excluding quality improvement initiatives enhancing VA operations.6
The integrated VA health care system is a major strength for providing state-of-the-science to inform veterans’ treatment and social service needs. The VA features medical centers and clinics capable of synergistically leveraging extant infrastructure to facilitate collaborations and centralized procedures across sites. The VA also has dedicated research centers, such as the National Center for PTSD, Centers of Excellence, Centers of Innovation, and Mental Illness, Research, Education and Clinical Centers that focus on PTSD, suicide prevention, TBI, and other high-priority areas. These centers recruit, train, and invest in experts dedicated to improving veterans’ lives. The VA Corporate Data Warehouse provides a national, system-wide repository for patient-level data, allowing for advanced analysis of large datasets.7
This special issue is a showcase of the strengths of VA mental health and social service research, aligning with the current strategic priorities of VA research. Topics focus on the unique needs of veterans, including sequelae (eg, PTSD, homelessness, moral injury), with particular attention to veterans. Manuscripts highlight the strengths of collaborations, including those between specialized research centers and national VA operational partners. Analyses highlight the VA research approach, leveraging data and perspectives from inside and outside the VA, and studying new and established approaches to care. This issue highlights the distinct advantages that VA research provides: experts with the tools, experience, and dedication to addressing the unique needs of veterans. Given the passion for veteran care among VA researchers, including those featured in this issue, we strongly believe the VA will continue to be a leader in this research.
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
- Oster C, Morello A, Venning A, Redpath P, Lawn S. The health and wellbeing needs of veterans: a rapid review. BMC Psychiatry. 2017;17(1):414. doi:10.1186/s12888-017-1547-0
- Cypel YS, Vogt D, Maguen S, et al. Physical health of Post- 9/11 U.S. military veterans in the context of Healthy People 2020 targeted topic areas: results from the Comparative Health Assessment Interview Research Study. Prev Med Rep. 2023;32:102122. doi:10.1016/j.pmedr.2023.102122
- Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and veteran Status. Am J Prev Med. 2018;54(1):e1-e9. doi:10.1016/j.amepre.2017.09.008
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Tsai J, Pietrzak RH, Szymkowiak D. The problem of veteran homelessness: an update for the new decade. Am J Prev Med. 2021;60(6):774-780. doi:10.1016/j.amepre.2020.12.012
- US Department of Veterans Affairs, Office of Research and Development. About the office of research & development. Updated January 22, 2025. Accessed March 18, 2025. https://www.research.va.gov/about/default.cfm
- Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi:10.1377/hlthaff.2014.0054
VA is a Leader in Mental Health and Social Service Research and Operations
VA is a Leader in Mental Health and Social Service Research and Operations
VA Shake-up Disrupts Mental Health Services for Some US Veterans
SAN FRANCISCO (Reuters) — Joey Cortez, who served 24 years in the US Air Force, had been waiting since August to see a mental health specialist from the Department of Veterans’ Affairs, when he experienced a fresh jolt of anxiety.
Cortez was fired last month from his human resources job at the agency - one of about 2400 employees who lost their jobs at Veterans’ Affairs (VA) in the first wave of President Donald Trump’s efforts to shrink the federal workforce.
“Once the firings happened and I was terminated, I started having panic attacks to the point where I black out,” Cortez, who suffers from post-traumatic stress disorder, told Reuters. The layoff is also making it harder to maintain his sobriety, as a recovering alcoholic.
“Not a day has gone by since I was fired that I haven’t thought about picking up a bottle,” said Cortez.
After losing his job, Cortez asked the VA to expedite his wait for a therapist and was told there was no record of his request, he said. After a month of calls to the agency, he got an appointment for this August, one year after he started the process. Then the VA offered him an appointment next week because another patient had canceled.
The VA provides health care to 9.31 million US veterans at hundreds of medical centers, clinics, and nursing homes across the country.
It also faces complex problems.
“The VA has bloat. There are redundancies. There are places where we have questioned the administration of care and asked, does it need to be the way it is?” Pat Murray, the legislative director for the Veterans of Foreign Wars, which represents Americans who have fought overseas, said in an interview.
The Trump administration plans additional cuts to the VA of more than 80,000 personnel, according to an internal memo obtained by Reuters. The agency has also announced it is phasing out telework.
Reuters spoke to nine current and former VA employees in California, Oregon, Texas, and the Washington D.C. area who said the changes were further disrupting some mental health services and fueling anxieties among those who provide and rely on them.
The VA employees — who include six mental health professionals and three people in leadership positions — described cancellations of some in-person and telehealth appointments; confusion over staffing of a crisis hot-line; and professionals conducting telehealth visits in makeshift meeting rooms inside VA buildings.
They spoke on the condition of anonymity, because they were not authorized to speak with the media.
STAFFING SHORTAGES
A former employee at the VA’s Office of Inspector General, who is also a veteran, said any future large-scale staffing cuts would likely worsen shortages and impact the quality of care.
“There’s no way to take a scalpel and do it appropriately that quickly,” he said.
VA spokesperson Peter Kasperowicz told Reuters mental health professionals, such as psychologists and social workers, were not included in February’s staffing cuts, and the agency is working to recruit mental health providers and improve wait times.
He did not specify how many support staff for these providers had been affected.
Last week, two federal judges ordered the VA and other federal agencies to reinstate thousands of fired probationary workers. Cortez’s pay was reinstated but he was told not to return to work.
The Veterans Health Administration, the branch of the VA that provides healthcare, has experienced severe staffing shortages since 2015, especially among mental health professionals, according to an OIG report last year.
Veterans often benefit from specialized services to treat anxiety, trauma, depression and substance abuse. The proportion of veterans receiving mental health services rose to 31% in 2022 from 20% in 2007, according to the VA. Suicide among veterans is twice the rate of Americans overall.
The VFW’s Murray said his organization supports a thorough review of the VA’s mental health services, but it needs to be done carefully, “not with a chainsaw.”
‘THE MOOD IS SO LOW’
In recent years, the agency had encouraged remote work to help expand access to telehealth services and reduce wait times, especially in rural areas where recruiting providers is difficult.
The VA’s Kasperowicz said that, while providers will need to return to VA facilities, veterans will be able to access telehealth appointments.
He did not directly address questions about why mental health providers needed to return to the office.
“The VA will make accommodations as needed to ensure employees have enough space to work and will always ensure that Veterans’ access to benefits and services remains uninterrupted as employees return to in-person work,” Kasperowicz said.
In the last few weeks, demand for services among veterans who are VA employees has also risen, one of the mental health professionals, a social worker, told Reuters. A quarter of VA employees are veterans.
The social worker said he is meeting with two to three VA employees a week who are seeking access to mental health care, citing stress and the fear that they will lose their jobs.
“People are calling out sick. People are ill with stress and worry. The mood is so low.”
A mental health supervisor in California described scrambling to cover the caseload of a remote worker who had to cancel appointments with more than a dozen veterans, because she could not access a VA facility.
VA employees in the Washington area and in Oregon said mental health professionals were unsure if they were allowed to answer calls from the VA’s crisis hot-line if they were not physically in an office, because they had been instructed not to conduct work outside of a facility.
“People are nervous to be on-call,” said a supervisor of mental health providers in the Washington area. “The system is under a lot of duress.”
The VA told Reuters that crisis line workers are exempt from the return-to-office policy, and that staff continue to respond quickly to nearly 3000 calls daily.
Therapists returning to the office are struggling to find private meeting rooms at some VA facilities, according to four of the mental health professionals interviewed by Reuters.
They described medical and mental health professionals converting closets and conference rooms into offices to comply with the mandate to conduct telehealth visits from VA facilities. They expressed concerns that the crowded rooms could violate patient privacy rights.
“We are scrambling to find space,” said a provider in California. “Veterans are going without until we can find spaces for these providers.”
Reuters was unable to independently verify the accounts of overcrowding. Kasperowicz said the agency’s “policy is to bring as many employees back to the office as space permits.”
(Reporting by Robin Respaut in San Francisco; additional reporting by Julia Harte in New York and Gabriella Borter in D.C.; Editing by Michele Gershberg and Suzanne Goldenberg)
SAN FRANCISCO (Reuters) — Joey Cortez, who served 24 years in the US Air Force, had been waiting since August to see a mental health specialist from the Department of Veterans’ Affairs, when he experienced a fresh jolt of anxiety.
Cortez was fired last month from his human resources job at the agency - one of about 2400 employees who lost their jobs at Veterans’ Affairs (VA) in the first wave of President Donald Trump’s efforts to shrink the federal workforce.
“Once the firings happened and I was terminated, I started having panic attacks to the point where I black out,” Cortez, who suffers from post-traumatic stress disorder, told Reuters. The layoff is also making it harder to maintain his sobriety, as a recovering alcoholic.
“Not a day has gone by since I was fired that I haven’t thought about picking up a bottle,” said Cortez.
After losing his job, Cortez asked the VA to expedite his wait for a therapist and was told there was no record of his request, he said. After a month of calls to the agency, he got an appointment for this August, one year after he started the process. Then the VA offered him an appointment next week because another patient had canceled.
The VA provides health care to 9.31 million US veterans at hundreds of medical centers, clinics, and nursing homes across the country.
It also faces complex problems.
“The VA has bloat. There are redundancies. There are places where we have questioned the administration of care and asked, does it need to be the way it is?” Pat Murray, the legislative director for the Veterans of Foreign Wars, which represents Americans who have fought overseas, said in an interview.
The Trump administration plans additional cuts to the VA of more than 80,000 personnel, according to an internal memo obtained by Reuters. The agency has also announced it is phasing out telework.
Reuters spoke to nine current and former VA employees in California, Oregon, Texas, and the Washington D.C. area who said the changes were further disrupting some mental health services and fueling anxieties among those who provide and rely on them.
The VA employees — who include six mental health professionals and three people in leadership positions — described cancellations of some in-person and telehealth appointments; confusion over staffing of a crisis hot-line; and professionals conducting telehealth visits in makeshift meeting rooms inside VA buildings.
They spoke on the condition of anonymity, because they were not authorized to speak with the media.
STAFFING SHORTAGES
A former employee at the VA’s Office of Inspector General, who is also a veteran, said any future large-scale staffing cuts would likely worsen shortages and impact the quality of care.
“There’s no way to take a scalpel and do it appropriately that quickly,” he said.
VA spokesperson Peter Kasperowicz told Reuters mental health professionals, such as psychologists and social workers, were not included in February’s staffing cuts, and the agency is working to recruit mental health providers and improve wait times.
He did not specify how many support staff for these providers had been affected.
Last week, two federal judges ordered the VA and other federal agencies to reinstate thousands of fired probationary workers. Cortez’s pay was reinstated but he was told not to return to work.
The Veterans Health Administration, the branch of the VA that provides healthcare, has experienced severe staffing shortages since 2015, especially among mental health professionals, according to an OIG report last year.
Veterans often benefit from specialized services to treat anxiety, trauma, depression and substance abuse. The proportion of veterans receiving mental health services rose to 31% in 2022 from 20% in 2007, according to the VA. Suicide among veterans is twice the rate of Americans overall.
The VFW’s Murray said his organization supports a thorough review of the VA’s mental health services, but it needs to be done carefully, “not with a chainsaw.”
‘THE MOOD IS SO LOW’
In recent years, the agency had encouraged remote work to help expand access to telehealth services and reduce wait times, especially in rural areas where recruiting providers is difficult.
The VA’s Kasperowicz said that, while providers will need to return to VA facilities, veterans will be able to access telehealth appointments.
He did not directly address questions about why mental health providers needed to return to the office.
“The VA will make accommodations as needed to ensure employees have enough space to work and will always ensure that Veterans’ access to benefits and services remains uninterrupted as employees return to in-person work,” Kasperowicz said.
In the last few weeks, demand for services among veterans who are VA employees has also risen, one of the mental health professionals, a social worker, told Reuters. A quarter of VA employees are veterans.
The social worker said he is meeting with two to three VA employees a week who are seeking access to mental health care, citing stress and the fear that they will lose their jobs.
“People are calling out sick. People are ill with stress and worry. The mood is so low.”
A mental health supervisor in California described scrambling to cover the caseload of a remote worker who had to cancel appointments with more than a dozen veterans, because she could not access a VA facility.
VA employees in the Washington area and in Oregon said mental health professionals were unsure if they were allowed to answer calls from the VA’s crisis hot-line if they were not physically in an office, because they had been instructed not to conduct work outside of a facility.
“People are nervous to be on-call,” said a supervisor of mental health providers in the Washington area. “The system is under a lot of duress.”
The VA told Reuters that crisis line workers are exempt from the return-to-office policy, and that staff continue to respond quickly to nearly 3000 calls daily.
Therapists returning to the office are struggling to find private meeting rooms at some VA facilities, according to four of the mental health professionals interviewed by Reuters.
They described medical and mental health professionals converting closets and conference rooms into offices to comply with the mandate to conduct telehealth visits from VA facilities. They expressed concerns that the crowded rooms could violate patient privacy rights.
“We are scrambling to find space,” said a provider in California. “Veterans are going without until we can find spaces for these providers.”
Reuters was unable to independently verify the accounts of overcrowding. Kasperowicz said the agency’s “policy is to bring as many employees back to the office as space permits.”
(Reporting by Robin Respaut in San Francisco; additional reporting by Julia Harte in New York and Gabriella Borter in D.C.; Editing by Michele Gershberg and Suzanne Goldenberg)
SAN FRANCISCO (Reuters) — Joey Cortez, who served 24 years in the US Air Force, had been waiting since August to see a mental health specialist from the Department of Veterans’ Affairs, when he experienced a fresh jolt of anxiety.
Cortez was fired last month from his human resources job at the agency - one of about 2400 employees who lost their jobs at Veterans’ Affairs (VA) in the first wave of President Donald Trump’s efforts to shrink the federal workforce.
“Once the firings happened and I was terminated, I started having panic attacks to the point where I black out,” Cortez, who suffers from post-traumatic stress disorder, told Reuters. The layoff is also making it harder to maintain his sobriety, as a recovering alcoholic.
“Not a day has gone by since I was fired that I haven’t thought about picking up a bottle,” said Cortez.
After losing his job, Cortez asked the VA to expedite his wait for a therapist and was told there was no record of his request, he said. After a month of calls to the agency, he got an appointment for this August, one year after he started the process. Then the VA offered him an appointment next week because another patient had canceled.
The VA provides health care to 9.31 million US veterans at hundreds of medical centers, clinics, and nursing homes across the country.
It also faces complex problems.
“The VA has bloat. There are redundancies. There are places where we have questioned the administration of care and asked, does it need to be the way it is?” Pat Murray, the legislative director for the Veterans of Foreign Wars, which represents Americans who have fought overseas, said in an interview.
The Trump administration plans additional cuts to the VA of more than 80,000 personnel, according to an internal memo obtained by Reuters. The agency has also announced it is phasing out telework.
Reuters spoke to nine current and former VA employees in California, Oregon, Texas, and the Washington D.C. area who said the changes were further disrupting some mental health services and fueling anxieties among those who provide and rely on them.
The VA employees — who include six mental health professionals and three people in leadership positions — described cancellations of some in-person and telehealth appointments; confusion over staffing of a crisis hot-line; and professionals conducting telehealth visits in makeshift meeting rooms inside VA buildings.
They spoke on the condition of anonymity, because they were not authorized to speak with the media.
STAFFING SHORTAGES
A former employee at the VA’s Office of Inspector General, who is also a veteran, said any future large-scale staffing cuts would likely worsen shortages and impact the quality of care.
“There’s no way to take a scalpel and do it appropriately that quickly,” he said.
VA spokesperson Peter Kasperowicz told Reuters mental health professionals, such as psychologists and social workers, were not included in February’s staffing cuts, and the agency is working to recruit mental health providers and improve wait times.
He did not specify how many support staff for these providers had been affected.
Last week, two federal judges ordered the VA and other federal agencies to reinstate thousands of fired probationary workers. Cortez’s pay was reinstated but he was told not to return to work.
The Veterans Health Administration, the branch of the VA that provides healthcare, has experienced severe staffing shortages since 2015, especially among mental health professionals, according to an OIG report last year.
Veterans often benefit from specialized services to treat anxiety, trauma, depression and substance abuse. The proportion of veterans receiving mental health services rose to 31% in 2022 from 20% in 2007, according to the VA. Suicide among veterans is twice the rate of Americans overall.
The VFW’s Murray said his organization supports a thorough review of the VA’s mental health services, but it needs to be done carefully, “not with a chainsaw.”
‘THE MOOD IS SO LOW’
In recent years, the agency had encouraged remote work to help expand access to telehealth services and reduce wait times, especially in rural areas where recruiting providers is difficult.
The VA’s Kasperowicz said that, while providers will need to return to VA facilities, veterans will be able to access telehealth appointments.
He did not directly address questions about why mental health providers needed to return to the office.
“The VA will make accommodations as needed to ensure employees have enough space to work and will always ensure that Veterans’ access to benefits and services remains uninterrupted as employees return to in-person work,” Kasperowicz said.
In the last few weeks, demand for services among veterans who are VA employees has also risen, one of the mental health professionals, a social worker, told Reuters. A quarter of VA employees are veterans.
The social worker said he is meeting with two to three VA employees a week who are seeking access to mental health care, citing stress and the fear that they will lose their jobs.
“People are calling out sick. People are ill with stress and worry. The mood is so low.”
A mental health supervisor in California described scrambling to cover the caseload of a remote worker who had to cancel appointments with more than a dozen veterans, because she could not access a VA facility.
VA employees in the Washington area and in Oregon said mental health professionals were unsure if they were allowed to answer calls from the VA’s crisis hot-line if they were not physically in an office, because they had been instructed not to conduct work outside of a facility.
“People are nervous to be on-call,” said a supervisor of mental health providers in the Washington area. “The system is under a lot of duress.”
The VA told Reuters that crisis line workers are exempt from the return-to-office policy, and that staff continue to respond quickly to nearly 3000 calls daily.
Therapists returning to the office are struggling to find private meeting rooms at some VA facilities, according to four of the mental health professionals interviewed by Reuters.
They described medical and mental health professionals converting closets and conference rooms into offices to comply with the mandate to conduct telehealth visits from VA facilities. They expressed concerns that the crowded rooms could violate patient privacy rights.
“We are scrambling to find space,” said a provider in California. “Veterans are going without until we can find spaces for these providers.”
Reuters was unable to independently verify the accounts of overcrowding. Kasperowicz said the agency’s “policy is to bring as many employees back to the office as space permits.”
(Reporting by Robin Respaut in San Francisco; additional reporting by Julia Harte in New York and Gabriella Borter in D.C.; Editing by Michele Gershberg and Suzanne Goldenberg)
Trump Administration Review of Psychiatric Meds Raises Concerns
The Trump administration’s plans to study the “threat” posed by psychiatric medications in children have medical societies and mental health professionals concerned that the administration may be considering restrictions on the use of psychotropic drugs in pediatric patients.
An executive order signed last week created the “Make American Healthy Again Commission” to investigate the nation’s “escalating health crisis,” particularly in child health. Recently confirmed Secretary of the US Department of Health and Human Services Robert F. Kennedy Jr. will chair the effort.
As part of its investigation, the executive order directed the commission to assess “the prevalence of and threat posed by the prescription of selective serotonin reuptake inhibitors (SSRIs), antipsychotics, mood stabilizers, stimulants, and weight-loss drugs.”
A report on the commission’s findings is due in a little less than 100 days. Eighty days later, the commission must submit recommendations for federal action.
Although who the commission members are and the scope of its work is unclear, the language in the executive order — namely the implication that the Trump administration views psychotropic medication as a “threat” to children — was enough to prompt psychiatrists from across the country to contact the American Psychiatric Association (APA) about possible limitations on the use of psychotropic medications in pediatric patients.
“It’s concerning and surprising that some of our nation’s most vulnerable children who need these treatments to participate fully in life would be under scrutiny in this way,” Marketa Wills, MD, MBA, chief executive officer and medical director for the APA, told this news organization.
“If these medications are under threat and children decompensate that would not be good from a public health perspective, for the healthcare system or for the families we serve,” Wills said.
Past Comments Fuel Distress
Past comments by the commission chair have only fueled distress over the commission’s goals. Kennedy has long expressed skepticism about antidepressants, especially (SSRIs), questioning their safety and suggesting they are as addictive as heroin.
“I know people, including members of my family, who’ve had a much worse time getting off of SSRIs than they have getting off of heroin,” Kennedy said during his Senate confirmation hearing in late January.
But there is no evidence to suggest SSRIs or other antidepressants are addictive, Leslie A. Hulvershorn, MD, chair and associate professor of psychiatry at Indiana University School of Medicine, told this news organization.
“They don’t work in the systems of the brain that drive addiction. A large amount of research suggests that they are safe to take for a long time,” she said. “I suspect the confusion comes from the difference between it not being wise to come off of the medication, because of a concern for relapse of a psychiatric illness, and some transient discomfort from abruptly stopping SSRIs without tapering them off versus being addicted to it, like heroin.”
During the hearing, Kennedy was also asked to respond to comments he made during a 2023 livestream on X in which he claimed that the use of antidepressants have contributed to the increase in school shootings in the United States.
“I am also going to look very closely at the role of psychiatric drugs in these events and there are no good studies right now that should have been done years ago on this issue because there is a tremendous circumstantial evidence that SSRIs and benzos and other drugs are doing this,” he said in the livestream.
Research has shown that there is no link between school shootings and antidepressant use.
In a 2024 interview on the Latino Capitalist podcast, Kennedy said that he wanted create “wellness farms” for adults addicted to illicit drugs and children who take antidepressants or stimulants for ADHD could be “reparented.”
“The views on those wellness farms are concerning for us here at the American Psychiatric Association. It remains to be seen if he brings that back up in his new role at HHS. There is currently no evidence of their efficacy,” Wills said.
Fear Is a ‘Real Concern’
These controversial comments, combined with the commission’s charge to investigate the potential “threat” psychotropic medications pose to children, worry clinicians and families fear that access to medication could be restricted.
“Psychiatrists and patients are very concerned about the risk these statements may pose,” Hulvershorn said.
“Certainly, there is evidence that psychotropic medications are overprescribed, particularly in children who are in state care — like wards of the state — and who are part of Medicaid programs, but there is tremendous overall benefit associated with psychotropic medications in youth and adults. They are lifesaving and game changing in many instances,” she added.
Psychiatrists who’ve contacted the APA since last week’s announcement echo Hulvershorn’s comments.
“The fear is the real concern,” Wills said. “No parent takes the decision lightly to put their child on medication. With all interventions, particularly with children, there are risks and benefits that must be carefully weighed. The best person to weigh those risks and benefits is the child and adolescent psychiatrist, in conjunction with the child’s parents.”
The focus on medication also overlooks the fact that psychosocial interventions — not medication — are first-line treatment for children with mental health issues and that guidelines recommend medication be used alongside nonpharmacological therapy.
“Extensive research, including large national multi-site studies, have examined the most effective ways to reduce psychological symptoms among youth, including anxiety, depression, and ADHD. Results consistently reveal that both psychotropic medications and psychological interventions can offer significant improvements, often in combination,” Mitch Prinstein, PhD, chief of psychology strategy and integration at the American Psychological Association, told this news organization.
“Given the substantial challenges for many in gaining access to psychotherapy and a national shortage of licensed psychologists, reducing access to medications would undoubtedly have a debilitating effect of the already concerning youth mental health crisis,” Prinstein said.
A Seat at The Table
While the launch of the commission has left some feeling uneasy, experts agree that a national focus on children’s mental health is needed.
The APA would “welcome an opportunity to be part of this national conversation following the evidence base, following settled science that shows when and how these medications are effective and helpful for children and families,” said Wills. “We also think it’s very important that child and adolescent psychiatrists be at the table for this national conversation on behalf of the families they serve.”
In a joint letter with the APA, officials with the American Academy of Child and Adolescent Psychiatry also expressed interest in playing a role in the commission’s work.
“We are in the middle of a mental health crisis, with a record number of Americans struggling with mental health and substance use disorders. We strongly urge you to prioritize strengthening the ability to respond to an increasing demand for psychiatric services, especially for children,” the letter stated.
Indeed, looking beyond just the use of psychotropic medications is vital to the success of any strategy to address the youth mental health crisis, Hulvershorn noted.
“There are already many programs underway to examine the overprescribing. In my view, the lack of supports by payors for behavioral interventions, such as evidence-based family interventions, psychotherapies, etc., is the major driver for overuse of medications,” she said.
“Every pediatrician and child psychiatrist I know would rather try a behavioral intervention with a family first, but those are services that our systems do not financially support well and are, thus, underdeveloped, and very difficult to access,” Hulvershorn added.
More funding for evidence-based interventions — both behavioral and pharmacological — is desperately needed, she said. Support for workforce development should also be a part of any proposed solution.
“Adequate and responsible funding in all of those areas is needed, but we have some low hanging fruit in terms of figuring out how to just deliver the interventions that science has shown us do work,” Hulvershorn said. “Many of those interventions don’t involve medication and I think every expert in the field would be glad to see more effort put into system reform to better deliver interventions that work to youth and their families.”
A version of this article first appeared on Medscape.com.
The Trump administration’s plans to study the “threat” posed by psychiatric medications in children have medical societies and mental health professionals concerned that the administration may be considering restrictions on the use of psychotropic drugs in pediatric patients.
An executive order signed last week created the “Make American Healthy Again Commission” to investigate the nation’s “escalating health crisis,” particularly in child health. Recently confirmed Secretary of the US Department of Health and Human Services Robert F. Kennedy Jr. will chair the effort.
As part of its investigation, the executive order directed the commission to assess “the prevalence of and threat posed by the prescription of selective serotonin reuptake inhibitors (SSRIs), antipsychotics, mood stabilizers, stimulants, and weight-loss drugs.”
A report on the commission’s findings is due in a little less than 100 days. Eighty days later, the commission must submit recommendations for federal action.
Although who the commission members are and the scope of its work is unclear, the language in the executive order — namely the implication that the Trump administration views psychotropic medication as a “threat” to children — was enough to prompt psychiatrists from across the country to contact the American Psychiatric Association (APA) about possible limitations on the use of psychotropic medications in pediatric patients.
“It’s concerning and surprising that some of our nation’s most vulnerable children who need these treatments to participate fully in life would be under scrutiny in this way,” Marketa Wills, MD, MBA, chief executive officer and medical director for the APA, told this news organization.
“If these medications are under threat and children decompensate that would not be good from a public health perspective, for the healthcare system or for the families we serve,” Wills said.
Past Comments Fuel Distress
Past comments by the commission chair have only fueled distress over the commission’s goals. Kennedy has long expressed skepticism about antidepressants, especially (SSRIs), questioning their safety and suggesting they are as addictive as heroin.
“I know people, including members of my family, who’ve had a much worse time getting off of SSRIs than they have getting off of heroin,” Kennedy said during his Senate confirmation hearing in late January.
But there is no evidence to suggest SSRIs or other antidepressants are addictive, Leslie A. Hulvershorn, MD, chair and associate professor of psychiatry at Indiana University School of Medicine, told this news organization.
“They don’t work in the systems of the brain that drive addiction. A large amount of research suggests that they are safe to take for a long time,” she said. “I suspect the confusion comes from the difference between it not being wise to come off of the medication, because of a concern for relapse of a psychiatric illness, and some transient discomfort from abruptly stopping SSRIs without tapering them off versus being addicted to it, like heroin.”
During the hearing, Kennedy was also asked to respond to comments he made during a 2023 livestream on X in which he claimed that the use of antidepressants have contributed to the increase in school shootings in the United States.
“I am also going to look very closely at the role of psychiatric drugs in these events and there are no good studies right now that should have been done years ago on this issue because there is a tremendous circumstantial evidence that SSRIs and benzos and other drugs are doing this,” he said in the livestream.
Research has shown that there is no link between school shootings and antidepressant use.
In a 2024 interview on the Latino Capitalist podcast, Kennedy said that he wanted create “wellness farms” for adults addicted to illicit drugs and children who take antidepressants or stimulants for ADHD could be “reparented.”
“The views on those wellness farms are concerning for us here at the American Psychiatric Association. It remains to be seen if he brings that back up in his new role at HHS. There is currently no evidence of their efficacy,” Wills said.
Fear Is a ‘Real Concern’
These controversial comments, combined with the commission’s charge to investigate the potential “threat” psychotropic medications pose to children, worry clinicians and families fear that access to medication could be restricted.
“Psychiatrists and patients are very concerned about the risk these statements may pose,” Hulvershorn said.
“Certainly, there is evidence that psychotropic medications are overprescribed, particularly in children who are in state care — like wards of the state — and who are part of Medicaid programs, but there is tremendous overall benefit associated with psychotropic medications in youth and adults. They are lifesaving and game changing in many instances,” she added.
Psychiatrists who’ve contacted the APA since last week’s announcement echo Hulvershorn’s comments.
“The fear is the real concern,” Wills said. “No parent takes the decision lightly to put their child on medication. With all interventions, particularly with children, there are risks and benefits that must be carefully weighed. The best person to weigh those risks and benefits is the child and adolescent psychiatrist, in conjunction with the child’s parents.”
The focus on medication also overlooks the fact that psychosocial interventions — not medication — are first-line treatment for children with mental health issues and that guidelines recommend medication be used alongside nonpharmacological therapy.
“Extensive research, including large national multi-site studies, have examined the most effective ways to reduce psychological symptoms among youth, including anxiety, depression, and ADHD. Results consistently reveal that both psychotropic medications and psychological interventions can offer significant improvements, often in combination,” Mitch Prinstein, PhD, chief of psychology strategy and integration at the American Psychological Association, told this news organization.
“Given the substantial challenges for many in gaining access to psychotherapy and a national shortage of licensed psychologists, reducing access to medications would undoubtedly have a debilitating effect of the already concerning youth mental health crisis,” Prinstein said.
A Seat at The Table
While the launch of the commission has left some feeling uneasy, experts agree that a national focus on children’s mental health is needed.
The APA would “welcome an opportunity to be part of this national conversation following the evidence base, following settled science that shows when and how these medications are effective and helpful for children and families,” said Wills. “We also think it’s very important that child and adolescent psychiatrists be at the table for this national conversation on behalf of the families they serve.”
In a joint letter with the APA, officials with the American Academy of Child and Adolescent Psychiatry also expressed interest in playing a role in the commission’s work.
“We are in the middle of a mental health crisis, with a record number of Americans struggling with mental health and substance use disorders. We strongly urge you to prioritize strengthening the ability to respond to an increasing demand for psychiatric services, especially for children,” the letter stated.
Indeed, looking beyond just the use of psychotropic medications is vital to the success of any strategy to address the youth mental health crisis, Hulvershorn noted.
“There are already many programs underway to examine the overprescribing. In my view, the lack of supports by payors for behavioral interventions, such as evidence-based family interventions, psychotherapies, etc., is the major driver for overuse of medications,” she said.
“Every pediatrician and child psychiatrist I know would rather try a behavioral intervention with a family first, but those are services that our systems do not financially support well and are, thus, underdeveloped, and very difficult to access,” Hulvershorn added.
More funding for evidence-based interventions — both behavioral and pharmacological — is desperately needed, she said. Support for workforce development should also be a part of any proposed solution.
“Adequate and responsible funding in all of those areas is needed, but we have some low hanging fruit in terms of figuring out how to just deliver the interventions that science has shown us do work,” Hulvershorn said. “Many of those interventions don’t involve medication and I think every expert in the field would be glad to see more effort put into system reform to better deliver interventions that work to youth and their families.”
A version of this article first appeared on Medscape.com.
The Trump administration’s plans to study the “threat” posed by psychiatric medications in children have medical societies and mental health professionals concerned that the administration may be considering restrictions on the use of psychotropic drugs in pediatric patients.
An executive order signed last week created the “Make American Healthy Again Commission” to investigate the nation’s “escalating health crisis,” particularly in child health. Recently confirmed Secretary of the US Department of Health and Human Services Robert F. Kennedy Jr. will chair the effort.
As part of its investigation, the executive order directed the commission to assess “the prevalence of and threat posed by the prescription of selective serotonin reuptake inhibitors (SSRIs), antipsychotics, mood stabilizers, stimulants, and weight-loss drugs.”
A report on the commission’s findings is due in a little less than 100 days. Eighty days later, the commission must submit recommendations for federal action.
Although who the commission members are and the scope of its work is unclear, the language in the executive order — namely the implication that the Trump administration views psychotropic medication as a “threat” to children — was enough to prompt psychiatrists from across the country to contact the American Psychiatric Association (APA) about possible limitations on the use of psychotropic medications in pediatric patients.
“It’s concerning and surprising that some of our nation’s most vulnerable children who need these treatments to participate fully in life would be under scrutiny in this way,” Marketa Wills, MD, MBA, chief executive officer and medical director for the APA, told this news organization.
“If these medications are under threat and children decompensate that would not be good from a public health perspective, for the healthcare system or for the families we serve,” Wills said.
Past Comments Fuel Distress
Past comments by the commission chair have only fueled distress over the commission’s goals. Kennedy has long expressed skepticism about antidepressants, especially (SSRIs), questioning their safety and suggesting they are as addictive as heroin.
“I know people, including members of my family, who’ve had a much worse time getting off of SSRIs than they have getting off of heroin,” Kennedy said during his Senate confirmation hearing in late January.
But there is no evidence to suggest SSRIs or other antidepressants are addictive, Leslie A. Hulvershorn, MD, chair and associate professor of psychiatry at Indiana University School of Medicine, told this news organization.
“They don’t work in the systems of the brain that drive addiction. A large amount of research suggests that they are safe to take for a long time,” she said. “I suspect the confusion comes from the difference between it not being wise to come off of the medication, because of a concern for relapse of a psychiatric illness, and some transient discomfort from abruptly stopping SSRIs without tapering them off versus being addicted to it, like heroin.”
During the hearing, Kennedy was also asked to respond to comments he made during a 2023 livestream on X in which he claimed that the use of antidepressants have contributed to the increase in school shootings in the United States.
“I am also going to look very closely at the role of psychiatric drugs in these events and there are no good studies right now that should have been done years ago on this issue because there is a tremendous circumstantial evidence that SSRIs and benzos and other drugs are doing this,” he said in the livestream.
Research has shown that there is no link between school shootings and antidepressant use.
In a 2024 interview on the Latino Capitalist podcast, Kennedy said that he wanted create “wellness farms” for adults addicted to illicit drugs and children who take antidepressants or stimulants for ADHD could be “reparented.”
“The views on those wellness farms are concerning for us here at the American Psychiatric Association. It remains to be seen if he brings that back up in his new role at HHS. There is currently no evidence of their efficacy,” Wills said.
Fear Is a ‘Real Concern’
These controversial comments, combined with the commission’s charge to investigate the potential “threat” psychotropic medications pose to children, worry clinicians and families fear that access to medication could be restricted.
“Psychiatrists and patients are very concerned about the risk these statements may pose,” Hulvershorn said.
“Certainly, there is evidence that psychotropic medications are overprescribed, particularly in children who are in state care — like wards of the state — and who are part of Medicaid programs, but there is tremendous overall benefit associated with psychotropic medications in youth and adults. They are lifesaving and game changing in many instances,” she added.
Psychiatrists who’ve contacted the APA since last week’s announcement echo Hulvershorn’s comments.
“The fear is the real concern,” Wills said. “No parent takes the decision lightly to put their child on medication. With all interventions, particularly with children, there are risks and benefits that must be carefully weighed. The best person to weigh those risks and benefits is the child and adolescent psychiatrist, in conjunction with the child’s parents.”
The focus on medication also overlooks the fact that psychosocial interventions — not medication — are first-line treatment for children with mental health issues and that guidelines recommend medication be used alongside nonpharmacological therapy.
“Extensive research, including large national multi-site studies, have examined the most effective ways to reduce psychological symptoms among youth, including anxiety, depression, and ADHD. Results consistently reveal that both psychotropic medications and psychological interventions can offer significant improvements, often in combination,” Mitch Prinstein, PhD, chief of psychology strategy and integration at the American Psychological Association, told this news organization.
“Given the substantial challenges for many in gaining access to psychotherapy and a national shortage of licensed psychologists, reducing access to medications would undoubtedly have a debilitating effect of the already concerning youth mental health crisis,” Prinstein said.
A Seat at The Table
While the launch of the commission has left some feeling uneasy, experts agree that a national focus on children’s mental health is needed.
The APA would “welcome an opportunity to be part of this national conversation following the evidence base, following settled science that shows when and how these medications are effective and helpful for children and families,” said Wills. “We also think it’s very important that child and adolescent psychiatrists be at the table for this national conversation on behalf of the families they serve.”
In a joint letter with the APA, officials with the American Academy of Child and Adolescent Psychiatry also expressed interest in playing a role in the commission’s work.
“We are in the middle of a mental health crisis, with a record number of Americans struggling with mental health and substance use disorders. We strongly urge you to prioritize strengthening the ability to respond to an increasing demand for psychiatric services, especially for children,” the letter stated.
Indeed, looking beyond just the use of psychotropic medications is vital to the success of any strategy to address the youth mental health crisis, Hulvershorn noted.
“There are already many programs underway to examine the overprescribing. In my view, the lack of supports by payors for behavioral interventions, such as evidence-based family interventions, psychotherapies, etc., is the major driver for overuse of medications,” she said.
“Every pediatrician and child psychiatrist I know would rather try a behavioral intervention with a family first, but those are services that our systems do not financially support well and are, thus, underdeveloped, and very difficult to access,” Hulvershorn added.
More funding for evidence-based interventions — both behavioral and pharmacological — is desperately needed, she said. Support for workforce development should also be a part of any proposed solution.
“Adequate and responsible funding in all of those areas is needed, but we have some low hanging fruit in terms of figuring out how to just deliver the interventions that science has shown us do work,” Hulvershorn said. “Many of those interventions don’t involve medication and I think every expert in the field would be glad to see more effort put into system reform to better deliver interventions that work to youth and their families.”
A version of this article first appeared on Medscape.com.
Headache Strongly Linked to Attempted, Completed Suicide
Headache, including migraine, tension-type, trigeminal autonomic cephalalgia (TAC), and posttraumatic stress headache are significantly associated with both attempted and completed suicide, results of a large study suggested.
The risk for attempted and completed suicide was more than threefold higher for individuals with posttraumatic headache and about twofold higher for those with TAC than their counterparts without headache.
Even those with tension-type headache, one of the milder headache types, carried nearly a twofold increased risk for attempted suicide vs the comparison group with no headache.
First author Holly Elser, MD, MPH, PhD, a resident physician in the Department of Neurology at the University of Pennsylvania, Philadelphia, told Medscape Medical News that the findings were “quite striking” and underscore the importance of screening for suicide risk even in patients with mild headache.
The findings were published online on February 3 in JAMA Neurology.
Common, Disabling
With an estimated global lifetime prevalence of 67%, headache disorders are a leading cause of productivity loss, work absences, and short-term disability.
The mechanisms linking headache disorders to suicide remain unclear for several reasons, the investigators noted.
First, the relationship between headache and psychiatric comorbidities may be complex and bidirectional, with psychiatric symptoms potentially exacerbating headache severity and frequency, the investigators noted.
Secondly, research has shown a consistent link between chronic pain and suicidality, even after adjusting for comorbid psychiatric conditions. Finally, disruptions in serotonergic pathways and increased production of inflammatory cytokines may contribute to both headache disorders and psychiatric symptoms, suggesting a shared biological basis.
The mechanisms linking headache disorders to suicide remain unclear for several reasons, the investigators noted.
First, the relationship between headache and psychiatric comorbidities may be complex and bidirectional, with psychiatric symptoms potentially exacerbating headache severity and frequency, the investigators noted.
Secondly, research has shown a consistent link between chronic pain and suicidality, even after adjusting for comorbid psychiatric conditions. Finally, disruptions in serotonergic pathways and increased production of inflammatory cytokines may contribute to both headache disorders and psychiatric symptoms, suggesting a shared biological basis.
“Patients diagnosed with headache with comorbid psychiatric symptoms may benefit in particular from comanagement with behavioral health specialists,” she added.
Not ‘Just Headaches’
In an interview with Medscape Medical News, Fred Cohen, MD, an assistant professor of medicine and neurology at the Icahn School of Medicine at Mount Sinai Hospital, New York City, shared his perspective on the findings. Cohen, who was not involved in the study agreed with Elser’s point and incorporated screening into his practice.
“As part of my routine at every new patient appointment, I conduct screenings for depression and suicide risk. If a patient responds affirmatively to any of these questions, I make sure they get connected to the mental health resources they need,” Cohen said.
At least one of his patients per week screens positive for depression, he noted.
“Primary headaches, including migraine and trigeminal autonomic cephalalgias, are a significant source of disability and suffering,” said Cohen, adding that migraine, in particular, is the leading cause of disability worldwide among women aged 18-50 years. “These conditions are often misunderstood and dismissed as ‘just headaches,’ when in reality, they are much more complex and debilitating.”
Given that depression and anxiety are common co-occurring conditions with primary headache disorders, he said, “depression screenings should be standard practice when evaluating patients with headaches.”
The study’s limitations include dependence on diagnosis codes, which are prone to misclassification, and lack of information about headache chronicity and severity, which could have affected the findings.
There was no information provided about study funding. Elser and Cohen reported no relevant financial relationships.
Headache, including migraine, tension-type, trigeminal autonomic cephalalgia (TAC), and posttraumatic stress headache are significantly associated with both attempted and completed suicide, results of a large study suggested.
The risk for attempted and completed suicide was more than threefold higher for individuals with posttraumatic headache and about twofold higher for those with TAC than their counterparts without headache.
Even those with tension-type headache, one of the milder headache types, carried nearly a twofold increased risk for attempted suicide vs the comparison group with no headache.
First author Holly Elser, MD, MPH, PhD, a resident physician in the Department of Neurology at the University of Pennsylvania, Philadelphia, told Medscape Medical News that the findings were “quite striking” and underscore the importance of screening for suicide risk even in patients with mild headache.
The findings were published online on February 3 in JAMA Neurology.
Common, Disabling
With an estimated global lifetime prevalence of 67%, headache disorders are a leading cause of productivity loss, work absences, and short-term disability.
The mechanisms linking headache disorders to suicide remain unclear for several reasons, the investigators noted.
First, the relationship between headache and psychiatric comorbidities may be complex and bidirectional, with psychiatric symptoms potentially exacerbating headache severity and frequency, the investigators noted.
Secondly, research has shown a consistent link between chronic pain and suicidality, even after adjusting for comorbid psychiatric conditions. Finally, disruptions in serotonergic pathways and increased production of inflammatory cytokines may contribute to both headache disorders and psychiatric symptoms, suggesting a shared biological basis.
The mechanisms linking headache disorders to suicide remain unclear for several reasons, the investigators noted.
First, the relationship between headache and psychiatric comorbidities may be complex and bidirectional, with psychiatric symptoms potentially exacerbating headache severity and frequency, the investigators noted.
Secondly, research has shown a consistent link between chronic pain and suicidality, even after adjusting for comorbid psychiatric conditions. Finally, disruptions in serotonergic pathways and increased production of inflammatory cytokines may contribute to both headache disorders and psychiatric symptoms, suggesting a shared biological basis.
“Patients diagnosed with headache with comorbid psychiatric symptoms may benefit in particular from comanagement with behavioral health specialists,” she added.
Not ‘Just Headaches’
In an interview with Medscape Medical News, Fred Cohen, MD, an assistant professor of medicine and neurology at the Icahn School of Medicine at Mount Sinai Hospital, New York City, shared his perspective on the findings. Cohen, who was not involved in the study agreed with Elser’s point and incorporated screening into his practice.
“As part of my routine at every new patient appointment, I conduct screenings for depression and suicide risk. If a patient responds affirmatively to any of these questions, I make sure they get connected to the mental health resources they need,” Cohen said.
At least one of his patients per week screens positive for depression, he noted.
“Primary headaches, including migraine and trigeminal autonomic cephalalgias, are a significant source of disability and suffering,” said Cohen, adding that migraine, in particular, is the leading cause of disability worldwide among women aged 18-50 years. “These conditions are often misunderstood and dismissed as ‘just headaches,’ when in reality, they are much more complex and debilitating.”
Given that depression and anxiety are common co-occurring conditions with primary headache disorders, he said, “depression screenings should be standard practice when evaluating patients with headaches.”
The study’s limitations include dependence on diagnosis codes, which are prone to misclassification, and lack of information about headache chronicity and severity, which could have affected the findings.
There was no information provided about study funding. Elser and Cohen reported no relevant financial relationships.
Headache, including migraine, tension-type, trigeminal autonomic cephalalgia (TAC), and posttraumatic stress headache are significantly associated with both attempted and completed suicide, results of a large study suggested.
The risk for attempted and completed suicide was more than threefold higher for individuals with posttraumatic headache and about twofold higher for those with TAC than their counterparts without headache.
Even those with tension-type headache, one of the milder headache types, carried nearly a twofold increased risk for attempted suicide vs the comparison group with no headache.
First author Holly Elser, MD, MPH, PhD, a resident physician in the Department of Neurology at the University of Pennsylvania, Philadelphia, told Medscape Medical News that the findings were “quite striking” and underscore the importance of screening for suicide risk even in patients with mild headache.
The findings were published online on February 3 in JAMA Neurology.
Common, Disabling
With an estimated global lifetime prevalence of 67%, headache disorders are a leading cause of productivity loss, work absences, and short-term disability.
The mechanisms linking headache disorders to suicide remain unclear for several reasons, the investigators noted.
First, the relationship between headache and psychiatric comorbidities may be complex and bidirectional, with psychiatric symptoms potentially exacerbating headache severity and frequency, the investigators noted.
Secondly, research has shown a consistent link between chronic pain and suicidality, even after adjusting for comorbid psychiatric conditions. Finally, disruptions in serotonergic pathways and increased production of inflammatory cytokines may contribute to both headache disorders and psychiatric symptoms, suggesting a shared biological basis.
The mechanisms linking headache disorders to suicide remain unclear for several reasons, the investigators noted.
First, the relationship between headache and psychiatric comorbidities may be complex and bidirectional, with psychiatric symptoms potentially exacerbating headache severity and frequency, the investigators noted.
Secondly, research has shown a consistent link between chronic pain and suicidality, even after adjusting for comorbid psychiatric conditions. Finally, disruptions in serotonergic pathways and increased production of inflammatory cytokines may contribute to both headache disorders and psychiatric symptoms, suggesting a shared biological basis.
“Patients diagnosed with headache with comorbid psychiatric symptoms may benefit in particular from comanagement with behavioral health specialists,” she added.
Not ‘Just Headaches’
In an interview with Medscape Medical News, Fred Cohen, MD, an assistant professor of medicine and neurology at the Icahn School of Medicine at Mount Sinai Hospital, New York City, shared his perspective on the findings. Cohen, who was not involved in the study agreed with Elser’s point and incorporated screening into his practice.
“As part of my routine at every new patient appointment, I conduct screenings for depression and suicide risk. If a patient responds affirmatively to any of these questions, I make sure they get connected to the mental health resources they need,” Cohen said.
At least one of his patients per week screens positive for depression, he noted.
“Primary headaches, including migraine and trigeminal autonomic cephalalgias, are a significant source of disability and suffering,” said Cohen, adding that migraine, in particular, is the leading cause of disability worldwide among women aged 18-50 years. “These conditions are often misunderstood and dismissed as ‘just headaches,’ when in reality, they are much more complex and debilitating.”
Given that depression and anxiety are common co-occurring conditions with primary headache disorders, he said, “depression screenings should be standard practice when evaluating patients with headaches.”
The study’s limitations include dependence on diagnosis codes, which are prone to misclassification, and lack of information about headache chronicity and severity, which could have affected the findings.
There was no information provided about study funding. Elser and Cohen reported no relevant financial relationships.
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
If I can stop one heart from breaking, I shall not live in vain.
Emily Dickinson1
The celebration of Valentine’s Day has made the association of hearts with the month of February almost automatic. There is, though, another commemoration of hearts in the second month of the year with special significance for federal practice: American Heart Month. President Lyndon B. Johnson proclaimed February as American Heart Month in 1964 to raise awareness of the enormous human and economic cost of cardiovascular diseases (CVD) that impact many Americans in their prime.
The Centers for Disease Control and Prevention estimates that 1 in 5 deaths in the United States is due to CVD, which includes coronary artery disease, heart failure, heart attack, and stroke.2 American Heart Month aims to increase public attention to heart disease prevention and promote research to develop better diagnostic treatment methods for the leading cause of death in most populations.
Forty years after this proclamation, the American Heart Association launched Go Red for Women. On the first Friday of American Heart Month, Americans are encouraged to wear red to draw attention to CVD as the leading cause of death among women as well as men.2,3 A 2024 report from the American Heart Institute and McKinsey Health Institute attributed at least one-third of the overall health care disparities between men and women to inequities in CVD care. These detrimental differences in the management of heart disease in women encompass both diagnostic misadventures and failure to promptly employ effective therapeutics. CVD morbidity and mortality data for Black women are even higher due to multiple and overlapping social determinants of health.4
Higher rates of hypertension, hyperlipidemia, and smoking in women veterans compared with civilians have resulted in an increased risk of heart disease and a 26% higher rate of CVD-related mortality. One in 10 women enrolled in US Department of Veterans Affairs (VA) health care has CVD. Research shows that these women are less likely compared to male veterans to receive counseling about exercise or to be prescribed medications such as statins, even when evidence-based treatment guidelines are followed. The increased rates of heart disease and its complications in women veterans are in part due to risk factors related to military service such as posttraumatic stress disorder (PTSD) and depression, which exceed the rates of nonveteran women.5
The heart has a long association with psychological health. For millennia, philosophers and physicians alike believed the heart was the center of the self and the locus of sentience. Even William Harvey, whose discovery of the circulation of blood earned him the title of the father of cardiology, viewed the heart as the life force.6 The heart has been explicitly linked to American military trauma since the Civil War era diagnosis of Soldier’s Heart. More recently, mutual genetic vulnerabilities to PTSD and CVD have been posited.7 Indeed, research with male combat veterans helped establish the association.
Until recently, there has been a dearth of research to establish the same connection between CVD and PTSD in women veterans, who have elevated rates of PTSD in part due to higher rates of homelessness and military sexual trauma.5 Due in large part to the work of a group of VA and US Department of Defense (DoD) researchers, this is starting to change. A research group conducted a retrospective longitudinal study using electronic health record data from nearly 400,000 women veterans to determine the propensity scores of associations between a PTSD diagnosis and the incidence of heart disease over nearly 5 years. The hazard ratio (HR) for the incidence of CVD in women with trauma was 1.44 (compared with matched controls) and even higher in younger women (HR, 1.72).8 Researchers also compared CVD mortality in civilian and veteran women and found a concerning trend: not only were mortality rates higher in veterans, but they also did not benefit from an overall improved trend in deaths from heart disease over the past 20 years.9
Two years later, the same VA/DoD research group conducted additional analysis on the dataset used in the prior study to examine potential mechanisms underlying the epidemiological link between CVD and PTSD in women veterans. Women with and without PTSD were matched on age and traditional CVD risk factor parameters. The findings demonstrated an association of PTSD with higher risks of diabetes, hypertension, hyperlipidemia, and smoking. However, these traditional risk factors only accounted for one-fourth of the total association. About 34% of the risk was attributed to depression, anxiety, and substance use disorders, as well as obesity and neuroendocrine disorders. This leaves slightly more than half of the elevated risk of CVD unexplained.10
This research, along with other studies, have identified several mechanisms elucidating the link. Promising translational research may lead to new diagnostic techniques or improved treatment modalities for CVD in women. The most established etiology is that veterans with PTSD have a higher prevalence of multiple CVD risk factors, including smoking, substance use disorders, obesity, poor diet, sleep disorders, depression, and inactivity. There is also increased recognition that PTSD involves neuroendocrine dysfunction in the stress-response that triggers a cascade of metabolic responses (eg, chronic inflammation) that contribute to the onset and progression of heart disease.11
This burgeoning scientific work on CVD and its close association with PTSD and the role of both traditional and nontraditional risk factors can inform VA efforts to educate frontline VA and DoD clinicians, leading to better care for women veterans. Whether a practitioner provides primary, specialty, or mental health care, this new knowledge can inform efforts to optimize prevention and treatment for both PTSD and CVD. For example, the VA/DoD researchers recommend prescribing antidepressants that are less likely to cause or worsen hypertension and to employ psychotherapies known to reduce the harmful CVD effects of increased stress acting through the hypothalamic-pituitary axis. These studies empower VA clinicians to realize Emily Dickinson’s aspiration to prevent trauma and reduce damage to both the psyche and the soma. The health of every veteran’s heart and mind matters, as does every effort of federal practitioners to protect and heal it.
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
- Dickinson E. The Complete Poems of Emily Dickinson. Back Bay Books; 1976.
- Centers for Disease Control. Heart disease facts. Updated October 24, 2024. Accessed January 27, 2025. https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html
- American Heart Association. Historical timeline of the American Heart Association. Accessed January 27, 2025. https:// www.heart.org/-/media/files/about-us/history/history-of-the-american-heart-association.pdf
- McKinsey Health Institute in Collaboration with the American Heart Association. The state of US women’s heart health: a path to improved health and financial outcomes. June 2024. Accessed January 27, 2025. https://www.goredforwomen.org/-/media/GRFW-Files/About-Heart-Disease-in-Women/The-state-of-US-womens-heart-health-report.pdf?sc_lang=en
- Han JK, Yano EM, Watson KE, Ebrahimi R. Cardiovascular Care in women veterans. Circulation. 2019;139(8):1102-1109. doi:10.1161/CIRCULATIONAHA.118.037748
- Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995:335-338.
- Bremner JD, Wittbrodt MT, Shah AJ, et al. Confederates in the attic: posttraumatic stress disorder, cardiovascular disease, and the return of soldier’s heart. J Nerv Ment Dis. 2020;208(3):171-180. doi:10.1097/NMD.0000000000001100
- Ebrahimi R, Lynch KE, Beckham JC, et al. Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiol. 2021;6(6):642-651. doi:10.1001/jamacardio.2021.0227
- Ebrahimi R, Yano EM, Alvarez CA, et al. Trends in cardiovascular disease mortality in US women veterans vs civilians. JAMA Netw Open. 2023;6(10):e2340242. doi:10.1001/jamanetworkopen.2023.40242
- Ebrahimi R, Dennis PA, Shroyer ALW, et al. Pathways linking post-traumatic stress disorder to incident ischemic heart disease in women: call to action. JACC Adv. 2023;3(1):100744. doi:10.1016/j.jacadv.2023.100744
- Arenson M, Cohen B. Posttraumatic Stress Disorder and Cardiovascular Disease. National Center for PTSD. PTSD Res Q. 2017;28(1):1-3. Accessed January 27, 2025. https://www.ptsd.va.gov/publications/rq_docs/V28N1.pdf
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
The Heart Matters: Women Veterans, Cardiovascular Disease, and PTSD
Reports Find Room for Improvement in VA Suicide-Risk Screening
About 18 veterans die by suicide daily, and while many received health care services in the year prior to their death, half did not receive a mental health diagnosis.
To address this, the Veterans Health Administration (VHA) has updated or initiated programs and policies aimed at identifying at-risk veterans. Since May 2018, the VHA introduced the Suicide Risk Identification Strategy (Risk ID) program, which includes screening patients using the Columbia-Suicide Severity Rating Scale (C-SSRS). Positive screenings call for a licensed independent clinician to document a comprehensive suicide risk evaluation.
Despite these measures, challenges persist in implementation and effectiveness, outlined in reports issue by the VA Office of Inspector General (OIG) during the Biden Administration. Michael Missal, who had served as VA Inspector General since 2016 was recently dismissed by President Trump.
Risk ID
The OIG report surveyed 137 facilities regarding Risk ID processes, training, and monitoring. Findings from that review revealed gaps in training: suicide prevention training does not adequately address Risk ID requirements, leaving staff unprepared to conduct screenings and evaluations. Although the VHA has developed additional training related to Risk ID, the training is not required and the VHA does not monitor staff training completion.
The VHA requires annual screening for all patients and has established a screening clinical reminder in patients’ electronic health records. Despite this, the national screening metric remained below 60% in 2023. Conversely, same-day evaluations after positive screenings reached 82%, though this metric excludes patients who were not screened. In 2024, the VHA added Risk ID evaluation metrics to leadership performance plans, aiming to clarify standards and promote adherence.
Mental Health Treatment Coordinators
A second OIG investigation from December 2024 reviewed VHA requirements related to suicide risk identification processes and also evaluated national compliance with mental health treatment coordinator (MHTC) role requirements.
Suicide risks peaks after discharge from mental health units, with 40% of suicidal behaviors occurring within 90 days. The VHA requires suicide risk screening within 24 hours of discharge and safety plans for high-risk patients using the C-SSRS, but the OIG found adherence issues. In a review of 200 patients discharged between October 2019 and September 2020, staff failed to complete the required screening for 27% of patients and safety plans for 12% of patients.
The VHA also requires clinicians to develop a safety plan with patients who recently attempted suicide or expressed suicidal ideation, are at risk of suicide prior to mental health unit discharge, or are determined to be at “high or intermediate acute or chronic risk” of suicide. For those patients, staff must flag the electronic health record.
OIG also found that over half of surveyed patients with an assigned MHTC were not able to identify the MHTC or another VHA staff member to contact for help with care. One-third of assigned MHTCs did not participate in patients’ transitions from inpatient to outpatient care. Despite the VHA no longer requiring 7-day follow-up appointments as of 2023, the OIG emphasized the need for guidance on scheduling postdischarge mental health appointments to promote engagement.
Consistent with VHA’s discontinuation of a required 7-day follow-up appointment, the OIG recognizes that postdischarge follow-up appointments are most effectively scheduled in consideration of a patient’s treatment needs, preferences, and availability rather than an arbitrary timeliness expectation. Patients flagged as high-risk must attend 4 mental health visits within 30 days of discharge. However, the OIG found that only 48% met this requirement, while 34% attended 1 to 3 appointments, and 18% attended none. Among surveyed patients, self-motivation and encouragement from family or friends were key drivers of attendance.
The OIG concluded that failures in suicide risk identification and care coordination could lead to underestimated suicide risk, overestimated discharge readiness, and unmitigated risks. Inadequate safety planning may also leave patients ill-equipped to manage crises. While the VHA has updated guidelines for MHTC involvement, these measures have not significantly improved continuity of care.
About 18 veterans die by suicide daily, and while many received health care services in the year prior to their death, half did not receive a mental health diagnosis.
To address this, the Veterans Health Administration (VHA) has updated or initiated programs and policies aimed at identifying at-risk veterans. Since May 2018, the VHA introduced the Suicide Risk Identification Strategy (Risk ID) program, which includes screening patients using the Columbia-Suicide Severity Rating Scale (C-SSRS). Positive screenings call for a licensed independent clinician to document a comprehensive suicide risk evaluation.
Despite these measures, challenges persist in implementation and effectiveness, outlined in reports issue by the VA Office of Inspector General (OIG) during the Biden Administration. Michael Missal, who had served as VA Inspector General since 2016 was recently dismissed by President Trump.
Risk ID
The OIG report surveyed 137 facilities regarding Risk ID processes, training, and monitoring. Findings from that review revealed gaps in training: suicide prevention training does not adequately address Risk ID requirements, leaving staff unprepared to conduct screenings and evaluations. Although the VHA has developed additional training related to Risk ID, the training is not required and the VHA does not monitor staff training completion.
The VHA requires annual screening for all patients and has established a screening clinical reminder in patients’ electronic health records. Despite this, the national screening metric remained below 60% in 2023. Conversely, same-day evaluations after positive screenings reached 82%, though this metric excludes patients who were not screened. In 2024, the VHA added Risk ID evaluation metrics to leadership performance plans, aiming to clarify standards and promote adherence.
Mental Health Treatment Coordinators
A second OIG investigation from December 2024 reviewed VHA requirements related to suicide risk identification processes and also evaluated national compliance with mental health treatment coordinator (MHTC) role requirements.
Suicide risks peaks after discharge from mental health units, with 40% of suicidal behaviors occurring within 90 days. The VHA requires suicide risk screening within 24 hours of discharge and safety plans for high-risk patients using the C-SSRS, but the OIG found adherence issues. In a review of 200 patients discharged between October 2019 and September 2020, staff failed to complete the required screening for 27% of patients and safety plans for 12% of patients.
The VHA also requires clinicians to develop a safety plan with patients who recently attempted suicide or expressed suicidal ideation, are at risk of suicide prior to mental health unit discharge, or are determined to be at “high or intermediate acute or chronic risk” of suicide. For those patients, staff must flag the electronic health record.
OIG also found that over half of surveyed patients with an assigned MHTC were not able to identify the MHTC or another VHA staff member to contact for help with care. One-third of assigned MHTCs did not participate in patients’ transitions from inpatient to outpatient care. Despite the VHA no longer requiring 7-day follow-up appointments as of 2023, the OIG emphasized the need for guidance on scheduling postdischarge mental health appointments to promote engagement.
Consistent with VHA’s discontinuation of a required 7-day follow-up appointment, the OIG recognizes that postdischarge follow-up appointments are most effectively scheduled in consideration of a patient’s treatment needs, preferences, and availability rather than an arbitrary timeliness expectation. Patients flagged as high-risk must attend 4 mental health visits within 30 days of discharge. However, the OIG found that only 48% met this requirement, while 34% attended 1 to 3 appointments, and 18% attended none. Among surveyed patients, self-motivation and encouragement from family or friends were key drivers of attendance.
The OIG concluded that failures in suicide risk identification and care coordination could lead to underestimated suicide risk, overestimated discharge readiness, and unmitigated risks. Inadequate safety planning may also leave patients ill-equipped to manage crises. While the VHA has updated guidelines for MHTC involvement, these measures have not significantly improved continuity of care.
About 18 veterans die by suicide daily, and while many received health care services in the year prior to their death, half did not receive a mental health diagnosis.
To address this, the Veterans Health Administration (VHA) has updated or initiated programs and policies aimed at identifying at-risk veterans. Since May 2018, the VHA introduced the Suicide Risk Identification Strategy (Risk ID) program, which includes screening patients using the Columbia-Suicide Severity Rating Scale (C-SSRS). Positive screenings call for a licensed independent clinician to document a comprehensive suicide risk evaluation.
Despite these measures, challenges persist in implementation and effectiveness, outlined in reports issue by the VA Office of Inspector General (OIG) during the Biden Administration. Michael Missal, who had served as VA Inspector General since 2016 was recently dismissed by President Trump.
Risk ID
The OIG report surveyed 137 facilities regarding Risk ID processes, training, and monitoring. Findings from that review revealed gaps in training: suicide prevention training does not adequately address Risk ID requirements, leaving staff unprepared to conduct screenings and evaluations. Although the VHA has developed additional training related to Risk ID, the training is not required and the VHA does not monitor staff training completion.
The VHA requires annual screening for all patients and has established a screening clinical reminder in patients’ electronic health records. Despite this, the national screening metric remained below 60% in 2023. Conversely, same-day evaluations after positive screenings reached 82%, though this metric excludes patients who were not screened. In 2024, the VHA added Risk ID evaluation metrics to leadership performance plans, aiming to clarify standards and promote adherence.
Mental Health Treatment Coordinators
A second OIG investigation from December 2024 reviewed VHA requirements related to suicide risk identification processes and also evaluated national compliance with mental health treatment coordinator (MHTC) role requirements.
Suicide risks peaks after discharge from mental health units, with 40% of suicidal behaviors occurring within 90 days. The VHA requires suicide risk screening within 24 hours of discharge and safety plans for high-risk patients using the C-SSRS, but the OIG found adherence issues. In a review of 200 patients discharged between October 2019 and September 2020, staff failed to complete the required screening for 27% of patients and safety plans for 12% of patients.
The VHA also requires clinicians to develop a safety plan with patients who recently attempted suicide or expressed suicidal ideation, are at risk of suicide prior to mental health unit discharge, or are determined to be at “high or intermediate acute or chronic risk” of suicide. For those patients, staff must flag the electronic health record.
OIG also found that over half of surveyed patients with an assigned MHTC were not able to identify the MHTC or another VHA staff member to contact for help with care. One-third of assigned MHTCs did not participate in patients’ transitions from inpatient to outpatient care. Despite the VHA no longer requiring 7-day follow-up appointments as of 2023, the OIG emphasized the need for guidance on scheduling postdischarge mental health appointments to promote engagement.
Consistent with VHA’s discontinuation of a required 7-day follow-up appointment, the OIG recognizes that postdischarge follow-up appointments are most effectively scheduled in consideration of a patient’s treatment needs, preferences, and availability rather than an arbitrary timeliness expectation. Patients flagged as high-risk must attend 4 mental health visits within 30 days of discharge. However, the OIG found that only 48% met this requirement, while 34% attended 1 to 3 appointments, and 18% attended none. Among surveyed patients, self-motivation and encouragement from family or friends were key drivers of attendance.
The OIG concluded that failures in suicide risk identification and care coordination could lead to underestimated suicide risk, overestimated discharge readiness, and unmitigated risks. Inadequate safety planning may also leave patients ill-equipped to manage crises. While the VHA has updated guidelines for MHTC involvement, these measures have not significantly improved continuity of care.
GLP-1s Have Real-World Benefits and Risks In Large Scale VA Study
A study of more than 2 million veterans with diabetes builds on evidence of broad-ranging benefits and risks of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in the clinical setting, providing an “atlas” mapping extensive outcomes and some new insights to potentially explore in more rigorous clinical trials.
“This is the largest study on GLP-1 receptor agonists,” first author Ziyad Al-Aly, MD, chief of research and development at the US Department of Veterans Affairs (VA) St. Louis Healthcare System, in St. Louis, told this news organization regarding the research, published this week, in Nature Medicine.
“The [study] reflects the real experiences of people using GLP-1 RAs [in the VA] clinical setting,” he said.
“Altogether, our discovery approach confirms previous studies and clinical trials and also uncovers previously unreported benefits and risks of GLP-1 RAs,” the authors wrote.
For the comprehensive study, Al-Aly and his colleagues evaluated data from the US Department of Veterans Affairs on more than 2 million veterans treated for diabetes between October 2017 and December 2023, assessing GLP-1 RA treatment in comparison with other diabetes therapies regarding a striking 175 clinical outcomes.
Of the patients, 215,970 initiated treatment with GLP-1 RAs; 159,465 started sulfonylureas, 117,989 dipeptidyl peptidase 4 inhibitors, and 258,614 were initiated on sodium-glucose cotransporter-2 inhibitors.
The study also included a composite group of the latter three drug groups (n = 536,068), and a control group of 1,203,097 of patients receiving usual care, who were compared with usual care with the addition of GLP-1 RAs.
After inverse probability weighting, the groups were well-balanced in terms of their baseline characteristics. While the majority in the VA cohort overall were White men, the study adjusted for gender, age, race, comorbidities, and an extensive array of covariates.
With an average follow-up of 3.68 years, after the multivariate adjustment, GLP-1 RAs showed “effectiveness and risks that extended beyond those currently recognized,” in comparison with each of the treatment groups and with the main control group of usual care, the authors reported.
For the largest comparison with the main control group of usual care alone, the addition of GLP-1 RAs was associated with a decreased risk in 24% of the outcomes evaluated, and an increased risk in 10.86% of outcomes, with no significant difference for the remaining 65.14% of outcomes.
Of the various benefits, key improvements included a reduced risk for several substance use disorders including alcohol (hazard ratio [HR], 0.89) and opioid (HR, 0.87) use, suicidal ideation, attempt or intentional self-harm (HR, 0.90), seizures (HR, 0.90), neurocognitive disorders including Alzheimer disease (HR, 0.88) and dementia (HR, 0.92), coagulation and clotting disorders (HR, 0.92), and cardiac arrest (HR, 0.78).
Further benefits vs usual care alone included a reduced risk for infectious illnesses (HR, 0.88), acute kidney injury (HR, 0.88), and chronic kidney disease (CKD) (HR, 0.97; P <.05 for all the outcomes).
In terms of risks associated with GLP-1 RAs, in addition to the well-known risks for nausea and vomiting, additional increased risks vs usual care included gastrointestinal disorders such as noninfectious gastroenteritis (HR, 1.12), hypotension (HR, 1.06), arthritis (HR, 1.11), tendinitis and synovitis (HR, 1.10), interstitial nephritis (HR, 1.06), nephrolithiasis (HR, 1.15), and the known risk for drug-induced acute pancreatitis (HR, 2.46).
Neuropsychiatric Effects
Among the various benefits in the study, Al-Aly said some of the most intriguing are those involving the brain.
“I am struck by the consistent effects on many neuropsychiatric disorders — this aligns with data showing the presence of GLP-1 receptors in the brain and evidence showing that GLP-1s permeates through the blood brain barrier and acts on the brain to reduce inflammation and oxidative stress, improve neuroplasticity, etc.,” he said.
“Clearly, there is a neurotropic effect. There is also the possibility of an effect on the immune system/fighting infection — with reduced risks of infections, sepsis, etc.”
The reductions in suicidal ideation are encouraging after earlier reports of suicidal thoughts and self-injury among young users of GLP-1 RAs prompted concerns, including a 2023 review of the drug use by the European Medicines Agency that ultimately found no causal association, the authors added. The US Food and Drug Administration also found no association with GLP-1s and suicide risk.
The reductions in addictive behaviors are also encouraging and are consistent with the role of GLP-1 receptors in the brain in terms of impulse control and reward signaling that can relate to addictive behaviors, Al-Aly explained.
The reduced risks for dementia and Alzheimer disease are likewise consistent with preclinical studies in animal models of Alzheimer disease, as well as clinical studies showing a reduced risk for dementia in patients with type 2 diabetes, the authors noted.
The observed reduced risk for seizures further “adds to an emerging body of knowledge, both mechanistic and early clinical data, indicative of the anticonvulsant properties of GLP-1 RA use,” they added.
“GLP-1 RAs should be further evaluated in future studies as potential adjuvant therapeutics for epilepsy and its associated comorbidities,” the authors suggested.
Kidneys
While the findings support evidence of protective effects of GLP-1 RAs on the kidneys and a reduction in CKD risk, notable risks observed, also involving the kidneys, include nephrolithiasis or kidney stones.
Al-Aly noted the mechanisms with kidney stone formation are very different from CKD, and he speculated that the risk for the former could in fact stem from potentially low hydration with GLP-1 RA use.
“When patients are on GLP-1 RAs, they definitely eat a lot less to lose weight, but they also hydrate themselves less,” he explained in a press briefing. “They drink less water because they feel full very quickly after eating, and I’m just theorizing, but perhaps chronic dehydration [is behind] the increased risk of kidney stones.”
Modest Effects?
While, overall, the benefits of GLP-1 RA drugs showed modest benefits ranging between a 10% and 20% reduction for most outcomes, Al-Aly said those effects are still important.
“The modest effect does not negate the potential value of these drugs, especially for conditions where few effective treatment options exist, for example, dementia,” he said in the press statement.
“This may also imply that these drugs are most beneficial when used in conjunction with other interventions, such as lifestyle changes or other medications.”
Potential Confounders A Concern
Commenting on the study, David M. Nathan, MD, founder of the MGH Diabetes Center and a professor of medicine at Harvard Medical School, in Boston, Massachusetts, noted that, while the study is hypothesis-generating, the key limitation is its observational nature.
“The authors did a perfectly respectable job of doing all you can do to adjust for [confounders], but with these kinds of studies, as much as you try to statistically account for differences in the populations before they were put on the drug, you can never truly adjust for all the potential confounders that may influence the results,” he told this news organization.
In addition, the 3.8-year follow-up time of the study, as the authors acknowledge, is especially short considering that GLP-1 RAs are generally recommended to be taken indefinitely.
“You have to take these drugs presumably for a lifetime and we have no idea what the longer-term benefits and risks are,” Nathan said.
Nathan, who was among the first investigators to evaluate GLP-1 RAs about 30 years ago, underscored that “I do think that these drugs are generally really spectacular; they’ve taken over the world and they are probably the single greatest pharmaceutical story of the 21st century.”
“But much more rigorous randomized trials would be needed to prove study results that haven’t already been established in previous clinical trials,” he said.
“The types of [randomized] trials that are necessary are very expensive and require a huge amount of work, but at the end of the day, they provide proof as to what does and doesn’t work, and what the true risks are,” he added. “Whether the GLP-RAs will cure all ills and bring about world peace needs to be proved.”
In further comments provided through the Science Media Center, Stephen O’Rahilly, FRS, a professor of clinical biochemistry and medicine and director of the Wellcome-MRC Institute of Metabolic Science-Metabolic Research Laboratories, University of Cambridge, Cambridge, England, echoed Nathan’s concern that “studies such as these have to be interpreted very cautiously as the people studied have not been randomly allocated to GLP-1 RA treatment, so any difference between those taking and not taking the class of drug could potentially be attributable to factors other than the drug.”
He noted, however, that “the study provides useful reassurance about the safety of this class of drugs. The expected benefits on heart disease, stroke and other cardiovascular and most kidney diseases are clearly seen.”
Al-Aly reported being an uncompensated consultant for Pfizer. Nathan, who has previously conducted clinical trials on GLP-1 RAs, currently has no relationships to report. O’Rahilly reported receiving remuneration from several pharmaceutical companies for scientific advice relating to the development of drugs for metabolic diseases, but none involving GLP-1 RAs in the past 3 years.
A version of this article first appeared on Medscape.com.
A study of more than 2 million veterans with diabetes builds on evidence of broad-ranging benefits and risks of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in the clinical setting, providing an “atlas” mapping extensive outcomes and some new insights to potentially explore in more rigorous clinical trials.
“This is the largest study on GLP-1 receptor agonists,” first author Ziyad Al-Aly, MD, chief of research and development at the US Department of Veterans Affairs (VA) St. Louis Healthcare System, in St. Louis, told this news organization regarding the research, published this week, in Nature Medicine.
“The [study] reflects the real experiences of people using GLP-1 RAs [in the VA] clinical setting,” he said.
“Altogether, our discovery approach confirms previous studies and clinical trials and also uncovers previously unreported benefits and risks of GLP-1 RAs,” the authors wrote.
For the comprehensive study, Al-Aly and his colleagues evaluated data from the US Department of Veterans Affairs on more than 2 million veterans treated for diabetes between October 2017 and December 2023, assessing GLP-1 RA treatment in comparison with other diabetes therapies regarding a striking 175 clinical outcomes.
Of the patients, 215,970 initiated treatment with GLP-1 RAs; 159,465 started sulfonylureas, 117,989 dipeptidyl peptidase 4 inhibitors, and 258,614 were initiated on sodium-glucose cotransporter-2 inhibitors.
The study also included a composite group of the latter three drug groups (n = 536,068), and a control group of 1,203,097 of patients receiving usual care, who were compared with usual care with the addition of GLP-1 RAs.
After inverse probability weighting, the groups were well-balanced in terms of their baseline characteristics. While the majority in the VA cohort overall were White men, the study adjusted for gender, age, race, comorbidities, and an extensive array of covariates.
With an average follow-up of 3.68 years, after the multivariate adjustment, GLP-1 RAs showed “effectiveness and risks that extended beyond those currently recognized,” in comparison with each of the treatment groups and with the main control group of usual care, the authors reported.
For the largest comparison with the main control group of usual care alone, the addition of GLP-1 RAs was associated with a decreased risk in 24% of the outcomes evaluated, and an increased risk in 10.86% of outcomes, with no significant difference for the remaining 65.14% of outcomes.
Of the various benefits, key improvements included a reduced risk for several substance use disorders including alcohol (hazard ratio [HR], 0.89) and opioid (HR, 0.87) use, suicidal ideation, attempt or intentional self-harm (HR, 0.90), seizures (HR, 0.90), neurocognitive disorders including Alzheimer disease (HR, 0.88) and dementia (HR, 0.92), coagulation and clotting disorders (HR, 0.92), and cardiac arrest (HR, 0.78).
Further benefits vs usual care alone included a reduced risk for infectious illnesses (HR, 0.88), acute kidney injury (HR, 0.88), and chronic kidney disease (CKD) (HR, 0.97; P <.05 for all the outcomes).
In terms of risks associated with GLP-1 RAs, in addition to the well-known risks for nausea and vomiting, additional increased risks vs usual care included gastrointestinal disorders such as noninfectious gastroenteritis (HR, 1.12), hypotension (HR, 1.06), arthritis (HR, 1.11), tendinitis and synovitis (HR, 1.10), interstitial nephritis (HR, 1.06), nephrolithiasis (HR, 1.15), and the known risk for drug-induced acute pancreatitis (HR, 2.46).
Neuropsychiatric Effects
Among the various benefits in the study, Al-Aly said some of the most intriguing are those involving the brain.
“I am struck by the consistent effects on many neuropsychiatric disorders — this aligns with data showing the presence of GLP-1 receptors in the brain and evidence showing that GLP-1s permeates through the blood brain barrier and acts on the brain to reduce inflammation and oxidative stress, improve neuroplasticity, etc.,” he said.
“Clearly, there is a neurotropic effect. There is also the possibility of an effect on the immune system/fighting infection — with reduced risks of infections, sepsis, etc.”
The reductions in suicidal ideation are encouraging after earlier reports of suicidal thoughts and self-injury among young users of GLP-1 RAs prompted concerns, including a 2023 review of the drug use by the European Medicines Agency that ultimately found no causal association, the authors added. The US Food and Drug Administration also found no association with GLP-1s and suicide risk.
The reductions in addictive behaviors are also encouraging and are consistent with the role of GLP-1 receptors in the brain in terms of impulse control and reward signaling that can relate to addictive behaviors, Al-Aly explained.
The reduced risks for dementia and Alzheimer disease are likewise consistent with preclinical studies in animal models of Alzheimer disease, as well as clinical studies showing a reduced risk for dementia in patients with type 2 diabetes, the authors noted.
The observed reduced risk for seizures further “adds to an emerging body of knowledge, both mechanistic and early clinical data, indicative of the anticonvulsant properties of GLP-1 RA use,” they added.
“GLP-1 RAs should be further evaluated in future studies as potential adjuvant therapeutics for epilepsy and its associated comorbidities,” the authors suggested.
Kidneys
While the findings support evidence of protective effects of GLP-1 RAs on the kidneys and a reduction in CKD risk, notable risks observed, also involving the kidneys, include nephrolithiasis or kidney stones.
Al-Aly noted the mechanisms with kidney stone formation are very different from CKD, and he speculated that the risk for the former could in fact stem from potentially low hydration with GLP-1 RA use.
“When patients are on GLP-1 RAs, they definitely eat a lot less to lose weight, but they also hydrate themselves less,” he explained in a press briefing. “They drink less water because they feel full very quickly after eating, and I’m just theorizing, but perhaps chronic dehydration [is behind] the increased risk of kidney stones.”
Modest Effects?
While, overall, the benefits of GLP-1 RA drugs showed modest benefits ranging between a 10% and 20% reduction for most outcomes, Al-Aly said those effects are still important.
“The modest effect does not negate the potential value of these drugs, especially for conditions where few effective treatment options exist, for example, dementia,” he said in the press statement.
“This may also imply that these drugs are most beneficial when used in conjunction with other interventions, such as lifestyle changes or other medications.”
Potential Confounders A Concern
Commenting on the study, David M. Nathan, MD, founder of the MGH Diabetes Center and a professor of medicine at Harvard Medical School, in Boston, Massachusetts, noted that, while the study is hypothesis-generating, the key limitation is its observational nature.
“The authors did a perfectly respectable job of doing all you can do to adjust for [confounders], but with these kinds of studies, as much as you try to statistically account for differences in the populations before they were put on the drug, you can never truly adjust for all the potential confounders that may influence the results,” he told this news organization.
In addition, the 3.8-year follow-up time of the study, as the authors acknowledge, is especially short considering that GLP-1 RAs are generally recommended to be taken indefinitely.
“You have to take these drugs presumably for a lifetime and we have no idea what the longer-term benefits and risks are,” Nathan said.
Nathan, who was among the first investigators to evaluate GLP-1 RAs about 30 years ago, underscored that “I do think that these drugs are generally really spectacular; they’ve taken over the world and they are probably the single greatest pharmaceutical story of the 21st century.”
“But much more rigorous randomized trials would be needed to prove study results that haven’t already been established in previous clinical trials,” he said.
“The types of [randomized] trials that are necessary are very expensive and require a huge amount of work, but at the end of the day, they provide proof as to what does and doesn’t work, and what the true risks are,” he added. “Whether the GLP-RAs will cure all ills and bring about world peace needs to be proved.”
In further comments provided through the Science Media Center, Stephen O’Rahilly, FRS, a professor of clinical biochemistry and medicine and director of the Wellcome-MRC Institute of Metabolic Science-Metabolic Research Laboratories, University of Cambridge, Cambridge, England, echoed Nathan’s concern that “studies such as these have to be interpreted very cautiously as the people studied have not been randomly allocated to GLP-1 RA treatment, so any difference between those taking and not taking the class of drug could potentially be attributable to factors other than the drug.”
He noted, however, that “the study provides useful reassurance about the safety of this class of drugs. The expected benefits on heart disease, stroke and other cardiovascular and most kidney diseases are clearly seen.”
Al-Aly reported being an uncompensated consultant for Pfizer. Nathan, who has previously conducted clinical trials on GLP-1 RAs, currently has no relationships to report. O’Rahilly reported receiving remuneration from several pharmaceutical companies for scientific advice relating to the development of drugs for metabolic diseases, but none involving GLP-1 RAs in the past 3 years.
A version of this article first appeared on Medscape.com.
A study of more than 2 million veterans with diabetes builds on evidence of broad-ranging benefits and risks of glucagon-like peptide 1 receptor agonists (GLP-1 RAs) in the clinical setting, providing an “atlas” mapping extensive outcomes and some new insights to potentially explore in more rigorous clinical trials.
“This is the largest study on GLP-1 receptor agonists,” first author Ziyad Al-Aly, MD, chief of research and development at the US Department of Veterans Affairs (VA) St. Louis Healthcare System, in St. Louis, told this news organization regarding the research, published this week, in Nature Medicine.
“The [study] reflects the real experiences of people using GLP-1 RAs [in the VA] clinical setting,” he said.
“Altogether, our discovery approach confirms previous studies and clinical trials and also uncovers previously unreported benefits and risks of GLP-1 RAs,” the authors wrote.
For the comprehensive study, Al-Aly and his colleagues evaluated data from the US Department of Veterans Affairs on more than 2 million veterans treated for diabetes between October 2017 and December 2023, assessing GLP-1 RA treatment in comparison with other diabetes therapies regarding a striking 175 clinical outcomes.
Of the patients, 215,970 initiated treatment with GLP-1 RAs; 159,465 started sulfonylureas, 117,989 dipeptidyl peptidase 4 inhibitors, and 258,614 were initiated on sodium-glucose cotransporter-2 inhibitors.
The study also included a composite group of the latter three drug groups (n = 536,068), and a control group of 1,203,097 of patients receiving usual care, who were compared with usual care with the addition of GLP-1 RAs.
After inverse probability weighting, the groups were well-balanced in terms of their baseline characteristics. While the majority in the VA cohort overall were White men, the study adjusted for gender, age, race, comorbidities, and an extensive array of covariates.
With an average follow-up of 3.68 years, after the multivariate adjustment, GLP-1 RAs showed “effectiveness and risks that extended beyond those currently recognized,” in comparison with each of the treatment groups and with the main control group of usual care, the authors reported.
For the largest comparison with the main control group of usual care alone, the addition of GLP-1 RAs was associated with a decreased risk in 24% of the outcomes evaluated, and an increased risk in 10.86% of outcomes, with no significant difference for the remaining 65.14% of outcomes.
Of the various benefits, key improvements included a reduced risk for several substance use disorders including alcohol (hazard ratio [HR], 0.89) and opioid (HR, 0.87) use, suicidal ideation, attempt or intentional self-harm (HR, 0.90), seizures (HR, 0.90), neurocognitive disorders including Alzheimer disease (HR, 0.88) and dementia (HR, 0.92), coagulation and clotting disorders (HR, 0.92), and cardiac arrest (HR, 0.78).
Further benefits vs usual care alone included a reduced risk for infectious illnesses (HR, 0.88), acute kidney injury (HR, 0.88), and chronic kidney disease (CKD) (HR, 0.97; P <.05 for all the outcomes).
In terms of risks associated with GLP-1 RAs, in addition to the well-known risks for nausea and vomiting, additional increased risks vs usual care included gastrointestinal disorders such as noninfectious gastroenteritis (HR, 1.12), hypotension (HR, 1.06), arthritis (HR, 1.11), tendinitis and synovitis (HR, 1.10), interstitial nephritis (HR, 1.06), nephrolithiasis (HR, 1.15), and the known risk for drug-induced acute pancreatitis (HR, 2.46).
Neuropsychiatric Effects
Among the various benefits in the study, Al-Aly said some of the most intriguing are those involving the brain.
“I am struck by the consistent effects on many neuropsychiatric disorders — this aligns with data showing the presence of GLP-1 receptors in the brain and evidence showing that GLP-1s permeates through the blood brain barrier and acts on the brain to reduce inflammation and oxidative stress, improve neuroplasticity, etc.,” he said.
“Clearly, there is a neurotropic effect. There is also the possibility of an effect on the immune system/fighting infection — with reduced risks of infections, sepsis, etc.”
The reductions in suicidal ideation are encouraging after earlier reports of suicidal thoughts and self-injury among young users of GLP-1 RAs prompted concerns, including a 2023 review of the drug use by the European Medicines Agency that ultimately found no causal association, the authors added. The US Food and Drug Administration also found no association with GLP-1s and suicide risk.
The reductions in addictive behaviors are also encouraging and are consistent with the role of GLP-1 receptors in the brain in terms of impulse control and reward signaling that can relate to addictive behaviors, Al-Aly explained.
The reduced risks for dementia and Alzheimer disease are likewise consistent with preclinical studies in animal models of Alzheimer disease, as well as clinical studies showing a reduced risk for dementia in patients with type 2 diabetes, the authors noted.
The observed reduced risk for seizures further “adds to an emerging body of knowledge, both mechanistic and early clinical data, indicative of the anticonvulsant properties of GLP-1 RA use,” they added.
“GLP-1 RAs should be further evaluated in future studies as potential adjuvant therapeutics for epilepsy and its associated comorbidities,” the authors suggested.
Kidneys
While the findings support evidence of protective effects of GLP-1 RAs on the kidneys and a reduction in CKD risk, notable risks observed, also involving the kidneys, include nephrolithiasis or kidney stones.
Al-Aly noted the mechanisms with kidney stone formation are very different from CKD, and he speculated that the risk for the former could in fact stem from potentially low hydration with GLP-1 RA use.
“When patients are on GLP-1 RAs, they definitely eat a lot less to lose weight, but they also hydrate themselves less,” he explained in a press briefing. “They drink less water because they feel full very quickly after eating, and I’m just theorizing, but perhaps chronic dehydration [is behind] the increased risk of kidney stones.”
Modest Effects?
While, overall, the benefits of GLP-1 RA drugs showed modest benefits ranging between a 10% and 20% reduction for most outcomes, Al-Aly said those effects are still important.
“The modest effect does not negate the potential value of these drugs, especially for conditions where few effective treatment options exist, for example, dementia,” he said in the press statement.
“This may also imply that these drugs are most beneficial when used in conjunction with other interventions, such as lifestyle changes or other medications.”
Potential Confounders A Concern
Commenting on the study, David M. Nathan, MD, founder of the MGH Diabetes Center and a professor of medicine at Harvard Medical School, in Boston, Massachusetts, noted that, while the study is hypothesis-generating, the key limitation is its observational nature.
“The authors did a perfectly respectable job of doing all you can do to adjust for [confounders], but with these kinds of studies, as much as you try to statistically account for differences in the populations before they were put on the drug, you can never truly adjust for all the potential confounders that may influence the results,” he told this news organization.
In addition, the 3.8-year follow-up time of the study, as the authors acknowledge, is especially short considering that GLP-1 RAs are generally recommended to be taken indefinitely.
“You have to take these drugs presumably for a lifetime and we have no idea what the longer-term benefits and risks are,” Nathan said.
Nathan, who was among the first investigators to evaluate GLP-1 RAs about 30 years ago, underscored that “I do think that these drugs are generally really spectacular; they’ve taken over the world and they are probably the single greatest pharmaceutical story of the 21st century.”
“But much more rigorous randomized trials would be needed to prove study results that haven’t already been established in previous clinical trials,” he said.
“The types of [randomized] trials that are necessary are very expensive and require a huge amount of work, but at the end of the day, they provide proof as to what does and doesn’t work, and what the true risks are,” he added. “Whether the GLP-RAs will cure all ills and bring about world peace needs to be proved.”
In further comments provided through the Science Media Center, Stephen O’Rahilly, FRS, a professor of clinical biochemistry and medicine and director of the Wellcome-MRC Institute of Metabolic Science-Metabolic Research Laboratories, University of Cambridge, Cambridge, England, echoed Nathan’s concern that “studies such as these have to be interpreted very cautiously as the people studied have not been randomly allocated to GLP-1 RA treatment, so any difference between those taking and not taking the class of drug could potentially be attributable to factors other than the drug.”
He noted, however, that “the study provides useful reassurance about the safety of this class of drugs. The expected benefits on heart disease, stroke and other cardiovascular and most kidney diseases are clearly seen.”
Al-Aly reported being an uncompensated consultant for Pfizer. Nathan, who has previously conducted clinical trials on GLP-1 RAs, currently has no relationships to report. O’Rahilly reported receiving remuneration from several pharmaceutical companies for scientific advice relating to the development of drugs for metabolic diseases, but none involving GLP-1 RAs in the past 3 years.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Jumping Jacks and Cold Water: How Pediatricians Are Stepping up in the Youth Mental Health Crisis
A young boy with a habit of screaming when he didn’t get his way is among the patients Joannie Yeh, MD, a primary care physician at Nemours Children’s Health in Media, Pennsylvania, has helped in her practice.
Yeh taught the boy to stretch out his hands into the shape of a starfish, then trace around the edges of his fingers while breathing slowly and deeply. His parents later reported that after using the strategy at home, their son was no longer taking his rage out on his younger siblings.
Interventions like breathing exercises are just a few techniques Yeh hopes more primary care clinicians will teach young patients as mental health issues among this population soar to a national state of emergency, major medical groups say. But many children go without treatment because of shortages of mental health clinicians and long wait-lists for appointments.
“Knowledge of different types of interventions allows pediatricians to offer more options to families — more than just medication alone,” Yeh said. “There are some strategies, like cognitive behavioral therapy, that a therapist is equipped to deliver, but we can help explain them or teach simple skills that borrow from principles of higher-level techniques and can help patients and families while they wait to see a therapist.”
, said Theresa Nguyen, MD, chair of pediatrics at Greater Baltimore Medical Center, Baltimore.
“It kind of sucks if you come in worried and then your doctor says, ‘Okay, let me send you to a psychiatrist who you can’t see for 6 months; let me send you to a therapist who’s going to take a couple of weeks to get in with,’” Nguyen said.
Yeh said over the past few years she has cared for more youth coming in as follow-ups after an emergency department visit for a mental health episode.
“Oftentimes, this is the first time we become aware that the child is struggling,” Yeh said. “We are seeing issues like intentional medication overdose, referrals after other self-harm actions, or even the discovery of a note indicating the intention to do harm to self.”
Suicide deaths among 10- to 14-year-olds tripled between 2007 and 2018 and held steady through 2021, with rates climbing even among children as young as 8 years, according to a research in JAMA Network Open. Meanwhile, one in five high school students seriously contemplated suicide in 2023 (27% girls, 14% boys).
Mental Health Strategies for Kids in Primary Care
While pediatricians cannot replace a mental health professional, they have the unique advantage of maintaining a long-term relationship with patients. Experts said clinicians should take an active role in supporting the mental health of patients through a variety of evidence-based strategies.
Changing Thought Patterns
Cognitive-behavioral therapy (CBT) involves identifying and challenging automatic negative thoughts, which can affect a child’s emotional state and lead to behaviors like withdrawal or lashing out.
Yeh recommended asking a child about what is bothering them, pointing out unhelpful and negative thoughts, and then offering a different, positive one instead.
She also often draws a picture of the CBT chart, which is a visual representation of how feelings lead to thoughts, and then behaviors.
“I draw this diagram because it helps give the patients a visual understanding of how their feelings and emotions are connected,” Yeh said.
Tools to Tolerate Stressful Situations
Simple tools like breathing exercises, body scanning, and physical exercise can help children better tolerate distress.
Pediatricians can also recommend families use guided meditations, which have been shown to lower anxiety and increase positive social behavior, said Mollie Grow, MD, an associate professor of pediatrics at the University of Washington Medicine and Seattle Children’s Hospital, both in Seattle.
But a child might first need to get negative energy out before they can become calm.
“So I’m like, ‘okay, let’s do actual physical exercise. Give me 10 jumping jacks.’ No one’s nervous after those jumping jacks,” Nguyen said. “When you’ve already been triggered, your nerves have gotten going, and you’re starting to spiral, you can’t slow yourself down enough to do a breathing exercise.”
Nguyen also said that cold water quickly calms the nervous system.
“I’ll run cold water in the office and have them put their hand in it until it’s almost frozen,” and the child or teen is able to think more clearly, Nguyen said. “It’s a real physiological response. It works.”
The Origin of a Feeling
Explaining how symptoms of anxiety, depression, or ADHD work can help children and teens better understand that what they are experiencing is normal and better cope, Yeh said.
Clinicians might teach patients about how shallow breathing — a symptom of anxiety — is a result of the brain scanning for danger, and how slowing breathing tricks the brain into feeling safe again.
Barriers Abound
The use of these interventions in pediatric settings is not yet widespread, Grow said.
But starting in July 2025, the Accreditation Council for Graduate Medical Education will require pediatric residencies to include 4 weeks of mental health training. How that requirement is fulfilled will be up to residencies, said Brian Alverson, MD, pediatric program director and vice-chair of education at Nemours Children’s Hospital in Wilmington, Delaware.
Even with training, many pediatricians lack the time to address mental health issues during an office visit, said Carlos Lerner, MD, a professor of clinical pediatrics at University of California, Los Angeles Health. And despite low or sometimes no reimbursement for discussing these issues with patients, “the reality is we end up doing it anyway.”
Treating issues like anxiety and depression “is a daily, constant part of the care that I provide for my patients,” said Lerner. “Whether the pandemic or social media exacerbated it, we are absolutely seeing a rise in mental health issues.”
A version of this article first appeared on Medscape.com.
A young boy with a habit of screaming when he didn’t get his way is among the patients Joannie Yeh, MD, a primary care physician at Nemours Children’s Health in Media, Pennsylvania, has helped in her practice.
Yeh taught the boy to stretch out his hands into the shape of a starfish, then trace around the edges of his fingers while breathing slowly and deeply. His parents later reported that after using the strategy at home, their son was no longer taking his rage out on his younger siblings.
Interventions like breathing exercises are just a few techniques Yeh hopes more primary care clinicians will teach young patients as mental health issues among this population soar to a national state of emergency, major medical groups say. But many children go without treatment because of shortages of mental health clinicians and long wait-lists for appointments.
“Knowledge of different types of interventions allows pediatricians to offer more options to families — more than just medication alone,” Yeh said. “There are some strategies, like cognitive behavioral therapy, that a therapist is equipped to deliver, but we can help explain them or teach simple skills that borrow from principles of higher-level techniques and can help patients and families while they wait to see a therapist.”
, said Theresa Nguyen, MD, chair of pediatrics at Greater Baltimore Medical Center, Baltimore.
“It kind of sucks if you come in worried and then your doctor says, ‘Okay, let me send you to a psychiatrist who you can’t see for 6 months; let me send you to a therapist who’s going to take a couple of weeks to get in with,’” Nguyen said.
Yeh said over the past few years she has cared for more youth coming in as follow-ups after an emergency department visit for a mental health episode.
“Oftentimes, this is the first time we become aware that the child is struggling,” Yeh said. “We are seeing issues like intentional medication overdose, referrals after other self-harm actions, or even the discovery of a note indicating the intention to do harm to self.”
Suicide deaths among 10- to 14-year-olds tripled between 2007 and 2018 and held steady through 2021, with rates climbing even among children as young as 8 years, according to a research in JAMA Network Open. Meanwhile, one in five high school students seriously contemplated suicide in 2023 (27% girls, 14% boys).
Mental Health Strategies for Kids in Primary Care
While pediatricians cannot replace a mental health professional, they have the unique advantage of maintaining a long-term relationship with patients. Experts said clinicians should take an active role in supporting the mental health of patients through a variety of evidence-based strategies.
Changing Thought Patterns
Cognitive-behavioral therapy (CBT) involves identifying and challenging automatic negative thoughts, which can affect a child’s emotional state and lead to behaviors like withdrawal or lashing out.
Yeh recommended asking a child about what is bothering them, pointing out unhelpful and negative thoughts, and then offering a different, positive one instead.
She also often draws a picture of the CBT chart, which is a visual representation of how feelings lead to thoughts, and then behaviors.
“I draw this diagram because it helps give the patients a visual understanding of how their feelings and emotions are connected,” Yeh said.
Tools to Tolerate Stressful Situations
Simple tools like breathing exercises, body scanning, and physical exercise can help children better tolerate distress.
Pediatricians can also recommend families use guided meditations, which have been shown to lower anxiety and increase positive social behavior, said Mollie Grow, MD, an associate professor of pediatrics at the University of Washington Medicine and Seattle Children’s Hospital, both in Seattle.
But a child might first need to get negative energy out before they can become calm.
“So I’m like, ‘okay, let’s do actual physical exercise. Give me 10 jumping jacks.’ No one’s nervous after those jumping jacks,” Nguyen said. “When you’ve already been triggered, your nerves have gotten going, and you’re starting to spiral, you can’t slow yourself down enough to do a breathing exercise.”
Nguyen also said that cold water quickly calms the nervous system.
“I’ll run cold water in the office and have them put their hand in it until it’s almost frozen,” and the child or teen is able to think more clearly, Nguyen said. “It’s a real physiological response. It works.”
The Origin of a Feeling
Explaining how symptoms of anxiety, depression, or ADHD work can help children and teens better understand that what they are experiencing is normal and better cope, Yeh said.
Clinicians might teach patients about how shallow breathing — a symptom of anxiety — is a result of the brain scanning for danger, and how slowing breathing tricks the brain into feeling safe again.
Barriers Abound
The use of these interventions in pediatric settings is not yet widespread, Grow said.
But starting in July 2025, the Accreditation Council for Graduate Medical Education will require pediatric residencies to include 4 weeks of mental health training. How that requirement is fulfilled will be up to residencies, said Brian Alverson, MD, pediatric program director and vice-chair of education at Nemours Children’s Hospital in Wilmington, Delaware.
Even with training, many pediatricians lack the time to address mental health issues during an office visit, said Carlos Lerner, MD, a professor of clinical pediatrics at University of California, Los Angeles Health. And despite low or sometimes no reimbursement for discussing these issues with patients, “the reality is we end up doing it anyway.”
Treating issues like anxiety and depression “is a daily, constant part of the care that I provide for my patients,” said Lerner. “Whether the pandemic or social media exacerbated it, we are absolutely seeing a rise in mental health issues.”
A version of this article first appeared on Medscape.com.
A young boy with a habit of screaming when he didn’t get his way is among the patients Joannie Yeh, MD, a primary care physician at Nemours Children’s Health in Media, Pennsylvania, has helped in her practice.
Yeh taught the boy to stretch out his hands into the shape of a starfish, then trace around the edges of his fingers while breathing slowly and deeply. His parents later reported that after using the strategy at home, their son was no longer taking his rage out on his younger siblings.
Interventions like breathing exercises are just a few techniques Yeh hopes more primary care clinicians will teach young patients as mental health issues among this population soar to a national state of emergency, major medical groups say. But many children go without treatment because of shortages of mental health clinicians and long wait-lists for appointments.
“Knowledge of different types of interventions allows pediatricians to offer more options to families — more than just medication alone,” Yeh said. “There are some strategies, like cognitive behavioral therapy, that a therapist is equipped to deliver, but we can help explain them or teach simple skills that borrow from principles of higher-level techniques and can help patients and families while they wait to see a therapist.”
, said Theresa Nguyen, MD, chair of pediatrics at Greater Baltimore Medical Center, Baltimore.
“It kind of sucks if you come in worried and then your doctor says, ‘Okay, let me send you to a psychiatrist who you can’t see for 6 months; let me send you to a therapist who’s going to take a couple of weeks to get in with,’” Nguyen said.
Yeh said over the past few years she has cared for more youth coming in as follow-ups after an emergency department visit for a mental health episode.
“Oftentimes, this is the first time we become aware that the child is struggling,” Yeh said. “We are seeing issues like intentional medication overdose, referrals after other self-harm actions, or even the discovery of a note indicating the intention to do harm to self.”
Suicide deaths among 10- to 14-year-olds tripled between 2007 and 2018 and held steady through 2021, with rates climbing even among children as young as 8 years, according to a research in JAMA Network Open. Meanwhile, one in five high school students seriously contemplated suicide in 2023 (27% girls, 14% boys).
Mental Health Strategies for Kids in Primary Care
While pediatricians cannot replace a mental health professional, they have the unique advantage of maintaining a long-term relationship with patients. Experts said clinicians should take an active role in supporting the mental health of patients through a variety of evidence-based strategies.
Changing Thought Patterns
Cognitive-behavioral therapy (CBT) involves identifying and challenging automatic negative thoughts, which can affect a child’s emotional state and lead to behaviors like withdrawal or lashing out.
Yeh recommended asking a child about what is bothering them, pointing out unhelpful and negative thoughts, and then offering a different, positive one instead.
She also often draws a picture of the CBT chart, which is a visual representation of how feelings lead to thoughts, and then behaviors.
“I draw this diagram because it helps give the patients a visual understanding of how their feelings and emotions are connected,” Yeh said.
Tools to Tolerate Stressful Situations
Simple tools like breathing exercises, body scanning, and physical exercise can help children better tolerate distress.
Pediatricians can also recommend families use guided meditations, which have been shown to lower anxiety and increase positive social behavior, said Mollie Grow, MD, an associate professor of pediatrics at the University of Washington Medicine and Seattle Children’s Hospital, both in Seattle.
But a child might first need to get negative energy out before they can become calm.
“So I’m like, ‘okay, let’s do actual physical exercise. Give me 10 jumping jacks.’ No one’s nervous after those jumping jacks,” Nguyen said. “When you’ve already been triggered, your nerves have gotten going, and you’re starting to spiral, you can’t slow yourself down enough to do a breathing exercise.”
Nguyen also said that cold water quickly calms the nervous system.
“I’ll run cold water in the office and have them put their hand in it until it’s almost frozen,” and the child or teen is able to think more clearly, Nguyen said. “It’s a real physiological response. It works.”
The Origin of a Feeling
Explaining how symptoms of anxiety, depression, or ADHD work can help children and teens better understand that what they are experiencing is normal and better cope, Yeh said.
Clinicians might teach patients about how shallow breathing — a symptom of anxiety — is a result of the brain scanning for danger, and how slowing breathing tricks the brain into feeling safe again.
Barriers Abound
The use of these interventions in pediatric settings is not yet widespread, Grow said.
But starting in July 2025, the Accreditation Council for Graduate Medical Education will require pediatric residencies to include 4 weeks of mental health training. How that requirement is fulfilled will be up to residencies, said Brian Alverson, MD, pediatric program director and vice-chair of education at Nemours Children’s Hospital in Wilmington, Delaware.
Even with training, many pediatricians lack the time to address mental health issues during an office visit, said Carlos Lerner, MD, a professor of clinical pediatrics at University of California, Los Angeles Health. And despite low or sometimes no reimbursement for discussing these issues with patients, “the reality is we end up doing it anyway.”
Treating issues like anxiety and depression “is a daily, constant part of the care that I provide for my patients,” said Lerner. “Whether the pandemic or social media exacerbated it, we are absolutely seeing a rise in mental health issues.”
A version of this article first appeared on Medscape.com.
Murthy Offers Hope as Tenure Ends
In his parting words as US Surgeon General, Vivek Murthy, MD, MBA, urges togetherness as it works through current and future issues, as opposed to continuing down the path of divisiveness.
“Today, we are faced with a profound choice: do we continue with the status quo, marked by pain, disconnection, and division? Or do we choose a different path—one of joy, health, and fulfillment where we turn toward each other instead of away from each other, where we choose love over fear; where we recognize community as the irreplaceable foundation for our well-being?” Murthy writes in his Jan. 7 valedictory essay. “As I finish my tenure as Surgeon General, this is my parting prescription, my final wish for all of us: choose community.”
Murthy based his essay on personal and professional experiences from his tenures as the 19th and 21st US Surgeon General. He outlines his individual perspective on the root causes of widespread pain and unhappiness he has seen across America and offers a prescription for how we can “cultivate health and fulfillment.”
The core pillars of community—relationships, service, and purpose—are powerful drivers of fulfillment, Murthy writes, because “community is a powerful source of life satisfaction and life expectancy.” In his essay, he describes how these elements affect our health.
Relationships can be a powerful source of joy and support. They can act as buffers to stress and break down the barriers of loneliness and improve your overall health. According to Murthy, one-third of adults and one-half of young people experience loneliness; and social disconnectedness increases the risk of heart disease, dementia, depression, anxiety, and premature death.
Service comprises the actions we take that benefit others. Research shows that sustained service efforts can reduce the risk of hypertension, stroke, early death, depression, and cognitive decline.
And purpose is the feeling of having an overarching life aim to guide our decisions and actions. Simply, it is the “why” we do something, and according to Murthy, a high sense of individual purpose may reduce the risk of early death as well as stroke, lung disease, and dementia.
Building community isn’t always easy, Murthy wrote. It requires “rethinking and, in some cases, rejecting the conventional wisdom that tells us what defines success and a good life.” At the conclusion of his essay, Murthy notes how choices we make now must be made with an eye toward the future.
“The choice we make to build community has the power to change lives and transform society,” he writes. “Let us never forget that good people with hearts full of love can change the world.”
In his parting words as US Surgeon General, Vivek Murthy, MD, MBA, urges togetherness as it works through current and future issues, as opposed to continuing down the path of divisiveness.
“Today, we are faced with a profound choice: do we continue with the status quo, marked by pain, disconnection, and division? Or do we choose a different path—one of joy, health, and fulfillment where we turn toward each other instead of away from each other, where we choose love over fear; where we recognize community as the irreplaceable foundation for our well-being?” Murthy writes in his Jan. 7 valedictory essay. “As I finish my tenure as Surgeon General, this is my parting prescription, my final wish for all of us: choose community.”
Murthy based his essay on personal and professional experiences from his tenures as the 19th and 21st US Surgeon General. He outlines his individual perspective on the root causes of widespread pain and unhappiness he has seen across America and offers a prescription for how we can “cultivate health and fulfillment.”
The core pillars of community—relationships, service, and purpose—are powerful drivers of fulfillment, Murthy writes, because “community is a powerful source of life satisfaction and life expectancy.” In his essay, he describes how these elements affect our health.
Relationships can be a powerful source of joy and support. They can act as buffers to stress and break down the barriers of loneliness and improve your overall health. According to Murthy, one-third of adults and one-half of young people experience loneliness; and social disconnectedness increases the risk of heart disease, dementia, depression, anxiety, and premature death.
Service comprises the actions we take that benefit others. Research shows that sustained service efforts can reduce the risk of hypertension, stroke, early death, depression, and cognitive decline.
And purpose is the feeling of having an overarching life aim to guide our decisions and actions. Simply, it is the “why” we do something, and according to Murthy, a high sense of individual purpose may reduce the risk of early death as well as stroke, lung disease, and dementia.
Building community isn’t always easy, Murthy wrote. It requires “rethinking and, in some cases, rejecting the conventional wisdom that tells us what defines success and a good life.” At the conclusion of his essay, Murthy notes how choices we make now must be made with an eye toward the future.
“The choice we make to build community has the power to change lives and transform society,” he writes. “Let us never forget that good people with hearts full of love can change the world.”
In his parting words as US Surgeon General, Vivek Murthy, MD, MBA, urges togetherness as it works through current and future issues, as opposed to continuing down the path of divisiveness.
“Today, we are faced with a profound choice: do we continue with the status quo, marked by pain, disconnection, and division? Or do we choose a different path—one of joy, health, and fulfillment where we turn toward each other instead of away from each other, where we choose love over fear; where we recognize community as the irreplaceable foundation for our well-being?” Murthy writes in his Jan. 7 valedictory essay. “As I finish my tenure as Surgeon General, this is my parting prescription, my final wish for all of us: choose community.”
Murthy based his essay on personal and professional experiences from his tenures as the 19th and 21st US Surgeon General. He outlines his individual perspective on the root causes of widespread pain and unhappiness he has seen across America and offers a prescription for how we can “cultivate health and fulfillment.”
The core pillars of community—relationships, service, and purpose—are powerful drivers of fulfillment, Murthy writes, because “community is a powerful source of life satisfaction and life expectancy.” In his essay, he describes how these elements affect our health.
Relationships can be a powerful source of joy and support. They can act as buffers to stress and break down the barriers of loneliness and improve your overall health. According to Murthy, one-third of adults and one-half of young people experience loneliness; and social disconnectedness increases the risk of heart disease, dementia, depression, anxiety, and premature death.
Service comprises the actions we take that benefit others. Research shows that sustained service efforts can reduce the risk of hypertension, stroke, early death, depression, and cognitive decline.
And purpose is the feeling of having an overarching life aim to guide our decisions and actions. Simply, it is the “why” we do something, and according to Murthy, a high sense of individual purpose may reduce the risk of early death as well as stroke, lung disease, and dementia.
Building community isn’t always easy, Murthy wrote. It requires “rethinking and, in some cases, rejecting the conventional wisdom that tells us what defines success and a good life.” At the conclusion of his essay, Murthy notes how choices we make now must be made with an eye toward the future.
“The choice we make to build community has the power to change lives and transform society,” he writes. “Let us never forget that good people with hearts full of love can change the world.”