User login
Data Trends 2025: Mental Health
Data Trends 2025: Mental Health
Click to view more from Federal Health Care Data Trends 2025.
- US Department of Veterans Affairs, Office of Suicide Prevention. 2024 National Veteran Suicide Prevention Annual Report. 2024. https://www.mentalhealth.va.gov/suicide_prevention/data.asp.
- Tenso K, et al. JAMA Netw Open. 2024;7(11):e2443054. doi:10.1001/jamanetworkopen.2024.43054
- Saulnier KG, et al. JAMA Netw Open. 2024;7(12):e2452144. doi:10.1001/jamanetworkopen.2024.52144
- Elser H, et al. Am J Epidemiol. 2025;194(2):123-132. doi:10.1093/aje/kwaf002
Click to view more from Federal Health Care Data Trends 2025.
Click to view more from Federal Health Care Data Trends 2025.
- US Department of Veterans Affairs, Office of Suicide Prevention. 2024 National Veteran Suicide Prevention Annual Report. 2024. https://www.mentalhealth.va.gov/suicide_prevention/data.asp.
- Tenso K, et al. JAMA Netw Open. 2024;7(11):e2443054. doi:10.1001/jamanetworkopen.2024.43054
- Saulnier KG, et al. JAMA Netw Open. 2024;7(12):e2452144. doi:10.1001/jamanetworkopen.2024.52144
- Elser H, et al. Am J Epidemiol. 2025;194(2):123-132. doi:10.1093/aje/kwaf002
- US Department of Veterans Affairs, Office of Suicide Prevention. 2024 National Veteran Suicide Prevention Annual Report. 2024. https://www.mentalhealth.va.gov/suicide_prevention/data.asp.
- Tenso K, et al. JAMA Netw Open. 2024;7(11):e2443054. doi:10.1001/jamanetworkopen.2024.43054
- Saulnier KG, et al. JAMA Netw Open. 2024;7(12):e2452144. doi:10.1001/jamanetworkopen.2024.52144
- Elser H, et al. Am J Epidemiol. 2025;194(2):123-132. doi:10.1093/aje/kwaf002
Data Trends 2025: Mental Health
Data Trends 2025: Mental Health
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
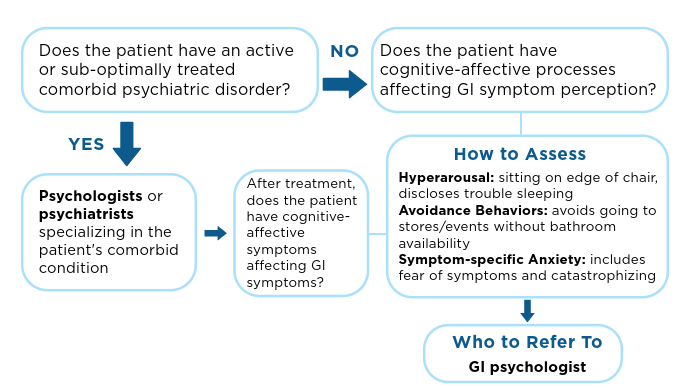
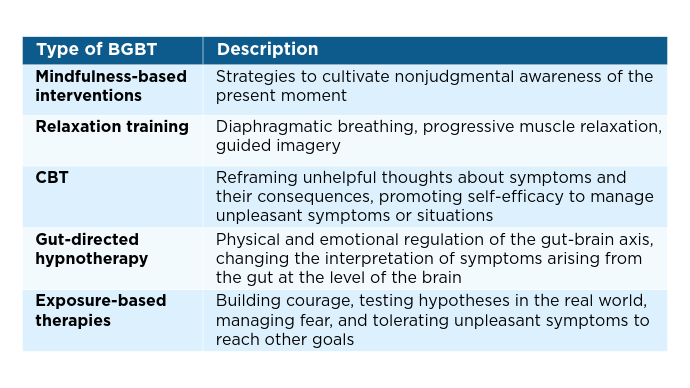
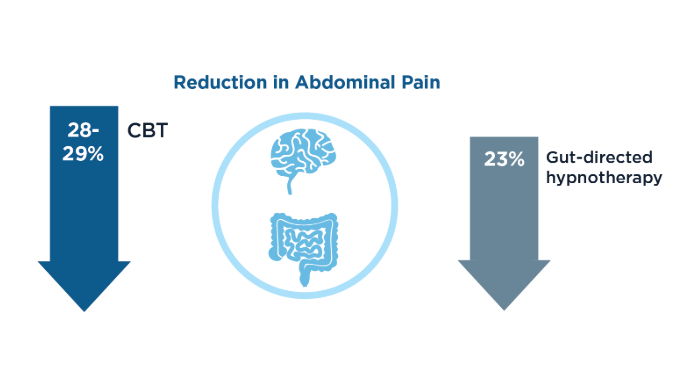
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.
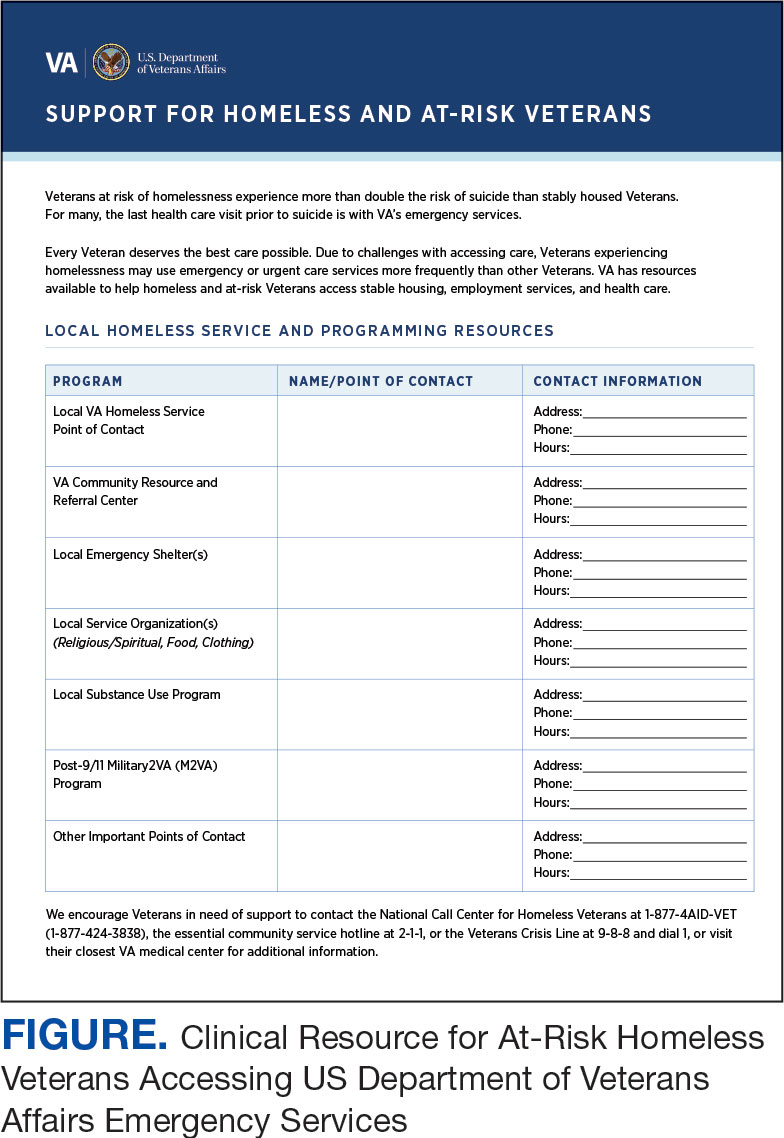
Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.

Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
Veterans experiencing homelessness are at an elevated risk for adverse health outcomes, including suicide. This population also experiences chronic health conditions (eg, cardiovascular disease and sexually transmitted infections) and psychiatric conditions (eg, substance use disorders and posttraumatic stress disorder) with a greater propensity than veterans without history of homelessness.1,2 Similarly, veterans experiencing homelessness often report concurrent stressors, such as justice involvement and unemployment, which further impact social functioning.3
The US Department of Veterans Affairs (VA) offers a range of health and social services to veterans experiencing homelessness. These programs are designed to respond to the multifactorial challenges faced by this population and are aimed at achieving sustained, permanent housing.4 To facilitate this effort, these programs provide targeted and tailored health (eg, primary care) and social (eg, case management and vocational rehabilitation) services to address barriers to housing stability (eg, substance use, serious mental illness, interacting with the criminal legal system, and unemployment).
Despite the availability of these programs, engaging veterans in VA services—whether in general or tailored for those experiencing or at risk for homelessness—remains challenging. Many veterans at risk for or experiencing homelessness overuse service settings that provide immediate care, such as urgent care or emergency departments (EDs).5,6 These individuals often visit an ED to augment or complement medical care they received in an outpatient setting, which can result in an elevated health care burden as well as impacted provision of treatment, especially surrounding care for chronic conditions (eg, cardiovascular health or serious mental illness).7-9
VA EDs offer urgent care and emergency services and often serve as a point of entry for veterans experiencing homelessness.10 They offer veterans expedient access to care that can address immediate needs (eg, substance use withdrawal, pain management, and suicide risk). EDs may be easier to access given they have longer hours of operation and patients can present without a scheduled appointment. VA EDs are an important point to identify homelessness and connect individuals to social service resources and outpatient health care referrals (eg, primary care and mental health).4,11
Some clinicians experience uncertainty in navigating or providing care for veterans experiencing or at risk for homelessness. A qualitative study conducted outside the VA found many clinicians did not know how to approach clinical conversations among unstably housed individuals, particularly when they discussed how to manage care for complex health conditions in the context of ongoing case management challenges, such as discharge planning.12 Another study found that clinicians working with individuals experiencing homelessness may have limited prior training or experience treating these patients.13 As a result, these clinicians may be unaware of available social services or unknowingly have biases that negatively impact care. Research remains limited surrounding beliefs about and methods of enhancing care among VA clinicians working with veterans experiencing homelessness in the ED.
This multiphase pilot study sought to understand service delivery processes and gaps in VA ED settings. Phase 1 examined ED clinician perceptions of care, facilitators, and barriers to providing care (including suicide risk assessments) and making postdischarge outpatient referrals among VA ED clinicians who regularly work with veterans experiencing homelessness. Phase 2 used this information to develop a clinical psychoeducational resource to enhance post-ED access to care for veterans experiencing or at risk for homelessness.
QUALITATIVE INTERVIEWS
Semistructured qualitative interviews were conducted with 11 VA ED clinicians from 6 Veteran Integrated Service Networks between August 2022 and February 2023. Clinicians were eligible if they currently worked within a VA ED setting (including urgent care) and indicated that some of their patients were veterans experiencing homelessness. All health care practitioners (HCPs) participated in an interview and a postinterview self-report survey that assessed demographic and job-related characteristics. Eight HCPs identified as female and 3 identified as male. All clinicians identified as White and 3 as Hispanic or Latino. Eight clinicians were licensed clinical social workers, 2 were ED nurses, and 1 was an ED physician.
After each clinician provided informed consent, they were invited to complete a telephone or Microsoft Teams interview. All interviews were recorded and subsequently transcribed. Interviews explored clinicians’ experiences caring for veterans experiencing homelessness, with a focus on services provided within the ED, as well as mandated ED screenings such as a suicide risk assessment. Interview questions also addressed postdischarge knowledge and experiences with referrals to VA health services (eg, primary care, mental health) and social services (eg, housing programs). Interviews lasted 30 to 90 minutes.
Recruitment ended after attaining sufficient thematic data, accomplished via an information power approach to sampling. This occurred when the study aims, sample characteristics, existing theory, and depth and quality of interviews dynamically informed the decision to cease recruitment of additional participants.14,15 Given the scope of study (examining service delivery and knowledge gaps), the specificity of the targeted sample (VA ED clinicians providing care to veterans experiencing homelessness), the level of pre-existing theoretical background informing the study aims, and depth and quality of interview dialogue, this information power approach provides justification for attaining small sample sizes. Following the interview, HCPs completed a demographic questionnaire. Participants were not compensated.
Data Analysis
Directed content analysis was used to analyze qualitative data, with the framework method employed as an analytic instrument to facilitate analysis.16-18 Analysts engaged in bracketing and discussed reflexivity before data analysis to reflect on personal subjectivities and reduce potential bias.19,20
A prototype coding framework was developed that enabled coders to meaningfully summarize and condense data within transcripts into varying domains, categories, or topics found within the interview guide. Domain examples included clinical backgrounds, suicide risk and assessment protocols among veterans experiencing homelessness, beliefs about service delivery for veterans experiencing homelessness, and barriers and facilitators that may impact their ability to provide post-ED discharge care. Coders discussed the findings and if there was a need to modify templates. All transcripts were double coded. Once complete, individual templates were merged into a unified Microsoft Excel sheet, which allowed for more discrete analyses, enabling analysts to examine trends across content areas within the dataset.
Clinical Resource Development
HCPs were queried regarding available outpatient resources for post-ED care (eg, printed discharge paperwork and best practice alerts or automated workflows within the electronic health record). Resources used by participants were examined, as well as which resources clinicians thought would help them care for veterans experiencing homelessness. Noted gaps were used to develop a tailored resource for clinicians who treat veterans experiencing homelessness in the ED. This resource was created with the intention it could inform all ED clinicians, with the option for personalization to align with the needs of local services, based on needed content areas identified (eg, emergency shelters and suicide prevention resources).
Resource development followed an information systems research (ISR) framework that used a 3-pronged process of identifying circumstances for how a tool is developed, the problems it aims to address, and the knowledge that informs its development, implementation, and evaluation.21,22 Initial wireframes of the resource were provided via email to 10 subject matter experts (SMEs) in veteran suicide prevention, emergency medicine, and homeless programs. SMEs were identified via professional listservs, VA program office leadership, literature searches of similar research, and snowball sampling. Solicited feedback on the resource from the SMEs included its design, language, tone, flow, format, and content (ideation and prototyping). The feedback was collated and used to revise the resource. SMEs then reviewed and provided feedback on the revised resource. This iterative cycle (prototype review, commentary, ideation, prototype review) continued until the SMEs offered no additional edits to the resource. In total, 7 iterations of the resource were developed, critiqued, and revised.
INTERVIEW RESULTS
Compassion Fatigue
Many participants expressed concerns about compassion fatigue among VA ED clinicians. Those interviewed indicated that treating veterans experiencing homelessness sometimes led to the development of what they described as a “callus,” a “sixth sense,” or an inherent sense of “suspicion” or distrust. These feelings resulted from concerns about an individual’s secondary gain or potential hidden agenda (eg, a veteran reporting suicidal ideation to attain shelter on a cold night), with clinicians not wanting to feel as if they were taken advantage of or deceived.
Many clinicians noted that compassion fatigue resulted from witnessing the same veterans experiencing homelessness routinely use emergency services for nonemergent or nonmedical needs. Some also expressed that over time this may result in them becoming less empathetic when caring for veterans experiencing homelessness. They hypothesized that clinicians may experience burnout, which could potentially result in a lack of curiosity and concern about a veteran’s risk for suicide or need for social services. Others may “take things for granted,” leading them to discount stressors that are “very real to the patient, this person.”
Clinicians indicated that such sentiments may impact overall care. Potential negative consequences included stigmatization of veterans experiencing homelessness, incomplete or partial suicide risk screenings with this population, inattentive or impersonal care, and expedited discharge from the ED without appropriate safety planning or social service referrals. Clinicians interviewed intended to find ways to combat compassion fatigue and maintain a commitment to provide comprehensive care to all veterans, including those experiencing homelessness. They felt conflict between a lack of empathy for individuals experiencing homelessness and becoming numb to the problem due to overexposure. However, these clinicians remained committed to providing care to these veterans and fighting to maintain the purpose of recovery-focused care.
Knowledge Gaps on Available Services
While many clinicians knew of general resources available to veterans experiencing homelessness, few had detailed information on where to seek consults for other homeless programs, who to contact regarding these services, when they were available, or how to refer to them. Many reported feeling uneasy when discharging veterans experiencing homelessness from care, often being unable to provide local, comprehensive referrals to support their needs and ensure their well-being. These sentiments were compounded when the veteran reported suicidal thoughts or recent suicidal behavior; clinicians felt concerned about the methods to engage these individuals into evidence-based mental health care within the context of unstable housing arrangements.
Some clinicians appeared to lack awareness of the wide array of VA homeless programming. Most could acknowledge at least some aspects of available programming (eg, the US Department of Housing and Urban Development– VA Supportive Housing program), while others were unaware of services tailored to the needs of those experiencing homelessness (eg, homeless patient aligned care teams), or of services targeting concurrent psychosocial stressors (eg, Veterans Justice Programs). Interviewees hypothesized this as being particularly notable among clinicians who are new to the VA or those who work in VA settings as part of their graduate or medical school training. Those aware of the services were uncertain of the referral process, relying on a single social worker or nurse to connect individuals experiencing homelessness to health and social services.
Interviewed clinicians noted that suicide risk screening of veterans experiencing homelessness was only performed by a limited number of individuals within the ED. Some did not feel sufficiently trained, comfortable, or knowledgeable about how to navigate care for veterans experiencing homelessness and at risk of suicide. Clinicians described “an uncomfortableness about suicidal ideation, where people just freeze up” and “don’t know what to do and don’t know what to say.”
Lack of Tangible Resources, Trainings, and Referrals
HCPs reported occasionally lacking the necessary clinical resources and information in the ED to properly support veterans experiencing homelessness and suicidal ideation. Common concerns included case management and discharge planning, as well as navigating health factors, such as elevated suicide risk. Some HCPs felt the local resources they do have access to—discharge packets or other forms of patient information—were not always tailored for the needs (eg, transportation) or abilities of veterans experiencing homelessness. One noted: “We give them a sheet of paper with some resources, which they don’t have the skills to follow up [with] anyway.”
Many interviewees wished for additional training in working with veterans experiencing homelessness. They reported that prior training from the VA Talent Management System or through unit-based programming could assist in educating clinicians on homeless services and suicide risk assessment. When queried on what training they had received, many noted there was “no formal training on what the VA offers homeless vets,” leading many to describe it as on-the-job training. This appeared especially among newer clinicians, who reported they were reliant upon learning from other, more senior staff within the ED.
The absence of training further illustrates the issue of institutional knowledge on these services and referrals, which was often confined to a single individual or team. Not having readily accessible resources, training, or information appropriate for all skill levels and positions within the ED hindered the ability of HCPs to connect veterans experiencing homelessness with social services to ensure their health and safety postdischarge: “If we had a better knowledge base of what the VA offers and the steps to go through in order to get the veteran set up for those things, it would be helpful.”
CLINICAL RESOURCE
A psychoeducational resource was developed for HCPs treating veterans experiencing homelessness (Figure). The resource was designed to mitigate compassion fatigue and recenter attention on the VA commitment to care while emphasizing the need to be responsive to the concerns of these individuals. Initial wireframes of the resource were developed by a small group of authors in review and appraisal of qualitative findings (EP, RH). These wireframes were developed to broadly illustrate the arrangement/structure of content, range of resources to potentially include (eg, available VA homeless programs or consultation resources), and to draft initial wording and phrasing. Subject matter expert feedback refined these wireframes, providing commentary on specific programs to include or exclude, changes and alterations to the design and flow of the resource, and edits to language, word choice, and tone over numerous iterations.

Given that many ED HCPs presented concerns surrounding secondary gain in the context of suicide risk, this resource focused on suicide risk. At the top of the resource, it states “Veterans at risk for homelessness experience more than double the risk for suicide than stably housed veterans.”23 Also at the top, the resource states: “For many, the last health care visit prior to suicide is often with VA emergency services."24 The goal of these statements was to educate users on the elevated risk for suicide in veterans experiencing homelessness and their role in preventing such deaths.
Text in this section emphasizes that every veteran deserves the best care possible and recenters HCP attention on providing quality, comprehensive care regardless of housing status. The inclusion of this material was prioritized given the concerns expressed regarding compassion fatigue and suspicions of secondary gain (eg, a veteran reporting suicidal ideation to attain shelter or respite from outside conditions).
The resource also attempts to address high rates of emergency service by veterans experiencing homelessness: “Due to challenges with accessing care, Veterans experiencing homelessness may use emergency or urgent care services more frequently than other Veterans.”25 The resource also indicates that VA resources are available to help homeless and at-risk veterans to acquire stable housing, employment, and engage in healthcare, which are outlined with specific contact information. Given the breadth of local and VA services, a portion of the resource is dedicated to local health and social services available for veterans experiencing homelessness. HCPs complete the first page, which is devoted to local homeless service and program resources.
Following SME consultation, the list of programs provided underwent a series of iterations. The program types listed are deemed to be of greatest benefit to veterans experiencing homelessness and most consulted by HCPs. Including VA and non-VA emergency shelters allows clinicians flexible options if a particular shelter is full, closed, or would not meet the veteran’s needs or preference (eg, lack of childcare or does not allow pets). The second column of this section is left intentionally blank; here, the HCP is to list a local point-of- contact at each program. This encourages clinical teams to seek out and make direct contact with these programs and establish (in)formal relationships with them. The HCP then completes the third column with contact information.
Once completed, the resource acts as a living document. Clinicians and SMEs consulted for this study expressed the desire to have an easily accessible resource that can be updated based on necessary changes (eg, emergency shelter address or hours of operation). The resource can be housed within each local VA emergency or urgent care service setting alongside other available clinical tools.
While local resources are the primary focus, interviewees also suggested that some HCPs are not aware of the available VA services . This material, found on the back of the resource, provides a general overview of services available through VA homeless programs. SME consultation and discussion led to selecting the 5 listed categories: housing services, health care services, case management, employment services, and justice-related programming, each with a brief description.
Information for the National Call Center for Homeless Veterans, community service hotline, and Veterans Crisis Line are included on the front page. These hotlines and phone numbers are always available for veterans experiencing homelessness, enabling them to make these connections themselves, if desired. Additionally, given the challenges noted by some HCPs in performing suicide risk screening, evaluation, and intervention, a prompt for the VA Suicide Risk Management Consultation service was also included on the back page.
Creating a Shared and Local Resource
This clinical resource was developed to establish a centralized, shared, local resource available to VA ED HCPs who lacked knowledge of available services or reported discomfort conducting suicide risk screening for veterans experiencing homelessness. In many cases, ED referrals to homeless programs and suicide prevention care was assigned to a single individual, often a nurse or social worker. As a result, an undue amount of work and strain was placed on these individuals, as this forced them to act as the sole bridge between care in the ED and postdischarge social (eg, homeless programs) and mental health (eg, suicide prevention) services. The creation of a unified, easily accessible document aimed to distribute this responsibility more equitably across ED staff.
DISCUSSION
This project intended to develop a clinician resource to support VA ED clinicians caring for veterans experiencing homelessness and their access to services postdischarge. Qualitative interviews provided insights into the burnout and compassion fatigue present in these settings, as well as the challenges and needs regarding knowledge of local and VA services. Emphasis was placed on leveraging extant resources and subject matter expertise to develop a resource capable of providing brief and informative guidance.
This resource is particularly relevant for HCPs new to the VA, including trainees and new hires, who may be less aware of VA and local social services. It has the potential to reduce the burden on VA ED staff to provide guidance and recommendations surrounding postdischarge social services. The resource acknowledges homeless programming focused on social determinants of health that can destabilize housing (eg, legal or occupational challenges). This can incentivize clinicians to discuss these programs with veterans to facilitate their ability to navigate complex health and psychosocial challenges.
HCPs interviewed for this study indicated their apprehension regarding suicide risk screening and evaluation, a process currently mandated within VA ED settings.26 This may be compounded among HCPs with minimal mental health training or those who have worked in community-based settings where such screening and evaluation efforts are not required. The resource reminds clinicians of available VA consultation services, which can provide additional training, clinical guidance, and review of existing local ED processes.
While the resource was directly informed by qualitative interviews conducted with VA emergency service HCPs and developed through an iterative process with SMEs, further research is necessary to determine its effectiveness at increasing access to health and social services among veterans experiencing homelessness. The resource has not been used by HCPs working in these settings to examine uptake or sustained use, nor clinicians’ perceptions of its utility, including acceptability and feasibility; these are important next steps to understand if the resource is functioning as intended.
Compassion fatigue, as well as associated sequelae (eg, burnout, distress, and psychiatric symptoms), is well-documented among individuals working with individuals experiencing homelessness, including VA HCPs.27-30 Such experiences are likely driven by several factors, including the clinical complexity and service needs of this veteran population. Although compassion fatigue was noted by many clinicians interviewed for this study, it is unclear if the resource alone would address factors driving compassion fatigue, or if additional programming or services may be necessary.
Limitations
The resource requires local HCPs to routinely update its content (eg, establishment of a new emergency shelter in the community or change in hours or contact information of an existing one), which may be challenging. This is especially true as it relates to community resources, which may be more likely to change than national VA programming.
This resource was initially developed following qualitative interviews with a small sample of VA HCPs (explicitly those working within ED settings) and may not be representative of all HCPs engaged in VA care with veterans experiencing homelessness. The perspectives and experiences of those interviewed do not represent the views of all VA ED HCPs and may differ from the perspectives of those in regions with unique cultural and regional considerations.31
Given that most of the interviewees were social workers in EDs engaged in care for veterans experiencing homelessness, these findings and informational needs may differ among other types of HCPs who provide services for veterans experiencing homelessness in other settings. Content in the resource was included based on clinician input, and may not reflect the perspectives of veterans, who may perceive some resources as more important (eg, access to primary care or dental services).28
CONCLUSIONS
This project represents the culmination of qualitative interviews and SME input to develop a free-to-use clinician resource to facilitate service delivery and connection to services following discharge from VA EDs for veterans experiencing homelessness. Serving as a template, this resource can be customized to increase knowledge of local VA and community resources to support these individuals. Continued refinement and piloting of this resource to evaluate acceptability, implementation barriers, and use remains warranted.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
- Holliday R, Kinney AR, Smith AA, et al. A latent class analysis to identify subgroups of VHA using homeless veterans at greater risk for suicide mortality. J Affect Disord. 2022;315:162-167. doi:10.1016/j.jad.2022.07.062
- Weber J, Lee RC, Martsolf D. Understanding the health of veterans who are homeless: a review of the literature. Public Health Nurs. 2017;34(5):505-511. doi:10.1111/phn.12338
- Holliday R, Desai A, Stimmel M, Liu S, Monteith LL, Stewart KE. Meeting the health and social service needs of veterans who interact with the criminal justice system and experience homelessness: a holistic conceptualization and recommendations for tailoring care. Curr Treat Options Psychiatry. 2022;9(3):174-185. doi:10.1007/s40501-022-00275-1
- Holliday R, Desai A, Gerard G, Liu S, Stimmel M. Understanding the intersection of homelessness and justice involvement: enhancing veteran suicide prevention through VA programming. Fed Pract. 2022;39(1):8-11. doi:10.12788/fp.0216
- Kushel MB, Perry S, Bangsberg D, Clark R, Moss AR. Emergency department use among the homeless and marginally housed: results from a community-based study. Am J Public Health. 2002;92(5):778-784. doi:10.2105/ajph.92.5.778
- Tsai J, Doran KM, Rosenheck RA. When health insurance is not a factor: national comparison of homeless and nonhomeless US veterans who use Veterans Affairs emergency departments. Am J Public Health. 2013;103(Suppl 2):S225-S231. doi:10.2105/AJPH.2013.301307
- Doran KM, Raven MC, Rosenheck RA. What drives frequent emergency department use in an integrated health system? National data from the Veterans Health Administration. Ann Emerg Med. 2013;62(2):151-159. doi:10.1016/j.annemergmed.2013.02.016
- Tsai J, Rosenheck RA. Risk factors for ED use among homeless veterans. Am J Emerg Med. 2013;31(5):855-858. doi:10.1016/j.ajem.2013.02.046
- Nelson RE, Suo Y, Pettey W, et al. Costs associated with health care services accessed through VA and in the community through Medicare for veterans experiencing homelessness. Health Serv Res. 2018;53(Suppl 3):5352-5374. doi:10.1111/1475-6773.13054
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Decker H, Raguram M, Kanzaria HK, Duke M, Wick E. Provider perceptions of challenges and facilitators to surgical care in unhoused patients: a qualitative analysis. Surgery. 2024;175(4):1095-1102. doi:10.1016/j.surg.2023.11.009
- Panushka KA, Kozlowski Z, Dalessandro C, Sanders JN, Millar MM, Gawron LM. “It’s not a top priority”: a qualitative analysis of provider views on barriers to reproductive healthcare provision for homeless women in the United States. Soc Work Public Health. 2023;38(5 -8):428-436. doi:10.1080/19371918.2024.2315180
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52:1893-1907. doi:10.1007/s11135-017-0574-8
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016;26(13):1753-1760. doi:10.1177/1049732315617444
- Assarroudi A, Heshmati Nabavi F, Armat MR, Ebadi A, Vaismoradi M. Directed qualitative content analysis: the description and elaboration of its underpinning methods and data analysis process. J Res Nurs. 2018;23(1):42-55. doi:10.1177/1744987117741667
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288.
- Goldsmith LJ. Using Framework Analysis in Applied Qualitative Research. Qual Rep. 2021;26(6):2061-2076. doi:10.46743/2160-3715/2021.5011
- Tufford L, Newman P. Bracketing in qualitative research. Qual Soc Work. 2012;11(1):80-96.
- Dodgson JE. Reflexivity in Qualitative Research. J Hum Lact. 2019;35(2):220-222. doi:10.1177/0890334419830990
- Hevner AR. A three cycle view of design science research. Scand J Inf Syst. 2007;19(2):4.
- Farao J, Malila B, Conrad N, Mutsvangwa T, Rangaka MX, Douglas TS. A user-centred design frame work for mHealth. PLOS ONE. 2020;15(8):e0237910. doi:10.1371/journal.pone.0237910
- Hoffberg AS, Spitzer E, Mackelprang JL, Farro SA, Brenner LA. Suicidal Self-Directed Violence Among Homeless US Veterans: A Systematic Review. Suicide Life Threat Behav. 2018;48(4):481-498. doi:10.1111/sltb.12369
- Larkin GL, Beautrais AL. Emergency departments are underutilized sites for suicide prevention. Crisis. 2010;31(1):1- 6. doi:10.1027/0227-5910/a000001
- Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and lowincome Veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461. doi:10.1097/MLR.0000000000000112
- Holliday R, Hostetter T, Brenner LA, Bahraini N, Tsai J. Suicide risk screening and evaluation among patients accessing VHA services and identified as being newly homeless. Health Serv Res. 2024;59(5):e14301. doi:10.1111/1475-6773.14301
- Waegemakers Schiff J, Lane AM. PTSD symptoms, vicarious traumatization, and burnout in front line workers in the homeless sector. Community Ment Health J. 2019;55(3):454-462. doi:10.1007/s10597-018-00364-7
- Steenekamp BL, Barker SL. Exploring the experiences of compassion fatigue amongst peer support workers in homelessness services. Community Ment Health J. 2024;60(4):772-783. doi:10.1007/s10597-024-01234-1
- Perez S, Kerman N, Dej E, et al. When I can’t help, I suffer: a scoping review of moral distress in service providers working with persons experiencing homelessness. J Ment Health. Published online 2024:1-16. doi:10.1080/09638237.2024.2426986
- Monteith LL, Holliday R, Christe’An DI, Sherrill A, Brenner LA, Hoffmire CA. Suicide risk and prevention in Guam: clinical and research considerations and a call to action. Asian J Psychiatry. 2023;83:103546. doi:10.1016/j.ajp.2023.103546
- Surís A, Holliday R, Hooshyar D, et al. Development and implementation of a homeless mobile medical/mental veteran intervention. Fed Pract. 2017;34(9):18.
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Development of a VA Clinician Resource to Facilitate Care Among Veterans Experiencing Homelessness
Suicide Prevention Grant Program Reauthorized
Suicide Prevention Grant Program Reauthorized
Community-based organizations that provide suicide-prevention services can now access about $52.5 million in US Department of Veterans Affairs (VA) grants. The grant is part of the 3-year Staff Sergeant Fox Suicide Prevention Grant Program, which honors Parker Gordon Fox, a sniper instructor at the U.S. Army Infantry School at Fort Benning, Georgia, who died by suicide in 2020. In consecutive Congressional hearings, lawmakers called for the reauthorization of the program to address gaps in VA care.
“It has been a game-changer for so many veterans,” Sen. Richard Blumenthal (D-CT) said.
The money provides or coordinates primarily nonclinical suicide prevention services, including outreach and linkage to VA and community resources. Services also may include baseline mental health screenings, case management and peer support, education on suicide risk, VA benefits assistance, and emergency clinical services.
Since its inception in 2022, the program has awarded $157.5 million to 95 organizations in 43 states, US territories, and tribal lands. Speaking before the House Committee on Veterans’ Affairs on May 15, VA Secretary Doug Collins praised the Fox program for bringing “different voices into the conversation,” but added it wasn’t enough. He noted that the veteran suicide rate has not changed since 2008, despite the VA annually spending $588 million on suicide prevention over the past few years.
In an op-ed, Russell Lemle, a senior policy analyst at the Veterans Healthcare Policy Institute, disputed Collins' characterization of veteran suicides. Between 2008 and 2022 (the last year for which complete data is available), US deaths by suicide increased 37% while the number of veteran deaths by suicide fell 2%. “This data collection was the single best part of the program,” he argued, calling for reauthorization to continue requiring data-targeted solutions.
According to a 2024 VA interim report on the Fox grant program, grantees had completed > 16,590 outreach contacts and engaged 3204 participants as of September 30, 2023. An additional 864 individuals were onboarding at the time of the report.
The current version of the grant program requires grantees to use validated tools, including the VA Data Collection Tool, and other assessments furnished by VA to determine the effectiveness of the suicide prevention services. They must also provide each participant with a satisfaction survey and submit periodic and annual financial and performance reports.
Despite the Trump administration’s cuts and cancellations to the federal workforce and federal programs, Collins told the Senate committee he is firmly on the side of working with community-based organizations like the Fox grant program to broaden the VA’s reach: “I want to use grants and programs like [the Fox grant program] to reach out beyond the scope of where we’re currently reaching, to say how can we actually touch the veteran that’s not being touched right now by these programs,” Collins said. “We’ve got to do better at using the grants, using our programs to go outside the normal bubble and use others to help get the word out.”
Grant applications are due in July and VA will choose awardees in September. Organizations can apply for grants worth up to $750,000 and may apply to renew awards from year to year throughout the length of the program.
Community-based organizations that provide suicide-prevention services can now access about $52.5 million in US Department of Veterans Affairs (VA) grants. The grant is part of the 3-year Staff Sergeant Fox Suicide Prevention Grant Program, which honors Parker Gordon Fox, a sniper instructor at the U.S. Army Infantry School at Fort Benning, Georgia, who died by suicide in 2020. In consecutive Congressional hearings, lawmakers called for the reauthorization of the program to address gaps in VA care.
“It has been a game-changer for so many veterans,” Sen. Richard Blumenthal (D-CT) said.
The money provides or coordinates primarily nonclinical suicide prevention services, including outreach and linkage to VA and community resources. Services also may include baseline mental health screenings, case management and peer support, education on suicide risk, VA benefits assistance, and emergency clinical services.
Since its inception in 2022, the program has awarded $157.5 million to 95 organizations in 43 states, US territories, and tribal lands. Speaking before the House Committee on Veterans’ Affairs on May 15, VA Secretary Doug Collins praised the Fox program for bringing “different voices into the conversation,” but added it wasn’t enough. He noted that the veteran suicide rate has not changed since 2008, despite the VA annually spending $588 million on suicide prevention over the past few years.
In an op-ed, Russell Lemle, a senior policy analyst at the Veterans Healthcare Policy Institute, disputed Collins' characterization of veteran suicides. Between 2008 and 2022 (the last year for which complete data is available), US deaths by suicide increased 37% while the number of veteran deaths by suicide fell 2%. “This data collection was the single best part of the program,” he argued, calling for reauthorization to continue requiring data-targeted solutions.
According to a 2024 VA interim report on the Fox grant program, grantees had completed > 16,590 outreach contacts and engaged 3204 participants as of September 30, 2023. An additional 864 individuals were onboarding at the time of the report.
The current version of the grant program requires grantees to use validated tools, including the VA Data Collection Tool, and other assessments furnished by VA to determine the effectiveness of the suicide prevention services. They must also provide each participant with a satisfaction survey and submit periodic and annual financial and performance reports.
Despite the Trump administration’s cuts and cancellations to the federal workforce and federal programs, Collins told the Senate committee he is firmly on the side of working with community-based organizations like the Fox grant program to broaden the VA’s reach: “I want to use grants and programs like [the Fox grant program] to reach out beyond the scope of where we’re currently reaching, to say how can we actually touch the veteran that’s not being touched right now by these programs,” Collins said. “We’ve got to do better at using the grants, using our programs to go outside the normal bubble and use others to help get the word out.”
Grant applications are due in July and VA will choose awardees in September. Organizations can apply for grants worth up to $750,000 and may apply to renew awards from year to year throughout the length of the program.
Community-based organizations that provide suicide-prevention services can now access about $52.5 million in US Department of Veterans Affairs (VA) grants. The grant is part of the 3-year Staff Sergeant Fox Suicide Prevention Grant Program, which honors Parker Gordon Fox, a sniper instructor at the U.S. Army Infantry School at Fort Benning, Georgia, who died by suicide in 2020. In consecutive Congressional hearings, lawmakers called for the reauthorization of the program to address gaps in VA care.
“It has been a game-changer for so many veterans,” Sen. Richard Blumenthal (D-CT) said.
The money provides or coordinates primarily nonclinical suicide prevention services, including outreach and linkage to VA and community resources. Services also may include baseline mental health screenings, case management and peer support, education on suicide risk, VA benefits assistance, and emergency clinical services.
Since its inception in 2022, the program has awarded $157.5 million to 95 organizations in 43 states, US territories, and tribal lands. Speaking before the House Committee on Veterans’ Affairs on May 15, VA Secretary Doug Collins praised the Fox program for bringing “different voices into the conversation,” but added it wasn’t enough. He noted that the veteran suicide rate has not changed since 2008, despite the VA annually spending $588 million on suicide prevention over the past few years.
In an op-ed, Russell Lemle, a senior policy analyst at the Veterans Healthcare Policy Institute, disputed Collins' characterization of veteran suicides. Between 2008 and 2022 (the last year for which complete data is available), US deaths by suicide increased 37% while the number of veteran deaths by suicide fell 2%. “This data collection was the single best part of the program,” he argued, calling for reauthorization to continue requiring data-targeted solutions.
According to a 2024 VA interim report on the Fox grant program, grantees had completed > 16,590 outreach contacts and engaged 3204 participants as of September 30, 2023. An additional 864 individuals were onboarding at the time of the report.
The current version of the grant program requires grantees to use validated tools, including the VA Data Collection Tool, and other assessments furnished by VA to determine the effectiveness of the suicide prevention services. They must also provide each participant with a satisfaction survey and submit periodic and annual financial and performance reports.
Despite the Trump administration’s cuts and cancellations to the federal workforce and federal programs, Collins told the Senate committee he is firmly on the side of working with community-based organizations like the Fox grant program to broaden the VA’s reach: “I want to use grants and programs like [the Fox grant program] to reach out beyond the scope of where we’re currently reaching, to say how can we actually touch the veteran that’s not being touched right now by these programs,” Collins said. “We’ve got to do better at using the grants, using our programs to go outside the normal bubble and use others to help get the word out.”
Grant applications are due in July and VA will choose awardees in September. Organizations can apply for grants worth up to $750,000 and may apply to renew awards from year to year throughout the length of the program.
Suicide Prevention Grant Program Reauthorized
Suicide Prevention Grant Program Reauthorized
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19
A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).
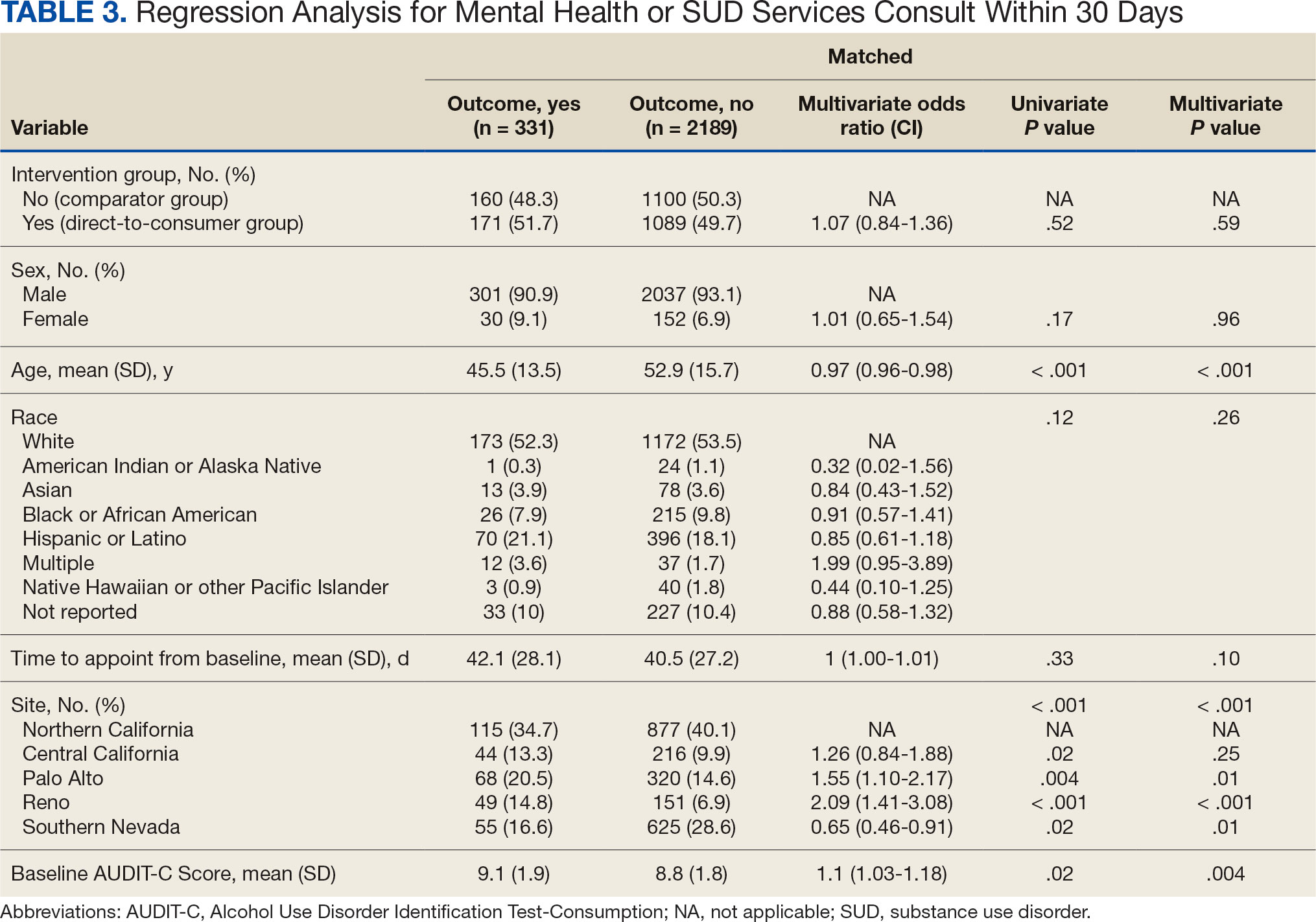
DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19

A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).



DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19

A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).



DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
IBS: Mental Health Factors and Comorbidities
IBS: Mental Health Factors and Comorbidities
Click to view more from Gastroenterology Data Trends 2025.
- Staudacher HM, Black CJ, Teasdale SB, Mikocka-Walus A, Keefer L. Irritable bowel syndrome and mental health comorbidity - approach to multidisciplinary management. Nat Rev Gastroenterol Hepatol. 2023;20(9):582-596. doi:10.1038/s41575-023-00794-z
- Ballou S, Vasant DH, Guadagnoli L, et al. A primer for the gastroenterology provider on psychosocial assessment of patients with disorders of gut-brain interaction. Neurogastroenterol Motil. 2024;36(12):e14894. doi:10.1111/nmo.14894
- Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome Working Team Report on Brain-Gut Behavior Therapies for Disorders of Gut-Brain Interaction. Gastroenterology. 2022;162(1):300-315. doi:10.1053/j.gastro.2021.09.015
- Goodoory VC, Khasawneh M, Thakur ER, et al. Effect of Brain-Gut Behavioral Treatments on Abdominal Pain in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gastroenterology. 2024;167(5):934-943.e5. doi:10.1053/j.gastro.2024.05.010
- Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology. 2022;163(1):118-136. doi:10.1053/j.gastro.2022.04.016
- Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018;154(4):1140-1171.e1. doi:10.1053/j.gastro.2017.11.279
- Hasan SS, Ballou S, Keefer L, Vasant DH. Improving access to gut-directed hypnotherapy for irritable bowel syndrome in the digital therapeutics’ era: Are mobile applications a “smart” solution? Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Tarar ZI, Farooq U, Zafar Y, et al. Burden of anxiety and depression among hospitalized patients with irritable bowel syndrome: a nationwide analysis. Ir J Med Sci. 2023;192(5):2159-2166. doi:10.1007/s11845-022-03258-6
- Barbara G, Aziz I, Ballou S, et al. Rome Foundation Working Team Report on overlap in disorders of gut-brain interaction. Nat Rev Gastroenterol Hepatol. doi:10.1038/s41575-024-01033-9
- Thakur ER, Kunik M, Jarbrink-Sehgal ME, Lackner J, Dindo L, El-Serag H. Behavior Medicine Management of Irritable Bowel Syndrome: A Referral Toolkit for Gastroenterology Providers. 2018. Accessed February 19, 2025. https://www.mirecc.va.gov/VISN16/docs/ibs-referral-toolkit.pdf
- Irritable bowel syndrome (IBS). Johns Hopkins Medicine website. Accessed February 19, 2025. https://www.hopkinsmedicine.org/health/conditionsanddiseases/irritable-bowel-syndrome-ibs
- Burton-Murray H, Guadagnoli L, Kamp K, et al. Rome Foundation Working Team Report: Consensus Statement on the Design and Conduct of Behavioural Clinical Trials for Disorders of Gut-Brain Interaction. Aliment Pharmacol Ther. 2025;61(5):787-802. doi:10.1111/apt.18482
- Rome GastroPsych. Rome Foundation website. Accessed February 19, 2025. https://romegipsych.org/
- Scarlata K, Riehl M. Mind Your Gut: The Science-based, Whole-body Guide to Living Well with IBS. Hachette Book Group, 2025.
- IFFGD International Foundation for Gastrointestinal Disorders. IFFGD website. January 10, 2025. Accessed February 19, 2025. https://iffgd.org/
- GI Psychology: Mind Your Gut website. April 2, 2024. Accessed February 19, 2025. https://www.gipsychology.com/
- Drossman DA, Ruddy J. Gut Feelings: Disorders of the Gut-Brain Interaction (DGBI) and the Patient-Doctor Relationship. DrossmanCare Chapel Hill, 2020.
- Tuesday Night IBS website. Accessed February 19, 2025. https://www.tuesdaynightibs.com/
Click to view more from Gastroenterology Data Trends 2025.
Click to view more from Gastroenterology Data Trends 2025.
- Staudacher HM, Black CJ, Teasdale SB, Mikocka-Walus A, Keefer L. Irritable bowel syndrome and mental health comorbidity - approach to multidisciplinary management. Nat Rev Gastroenterol Hepatol. 2023;20(9):582-596. doi:10.1038/s41575-023-00794-z
- Ballou S, Vasant DH, Guadagnoli L, et al. A primer for the gastroenterology provider on psychosocial assessment of patients with disorders of gut-brain interaction. Neurogastroenterol Motil. 2024;36(12):e14894. doi:10.1111/nmo.14894
- Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome Working Team Report on Brain-Gut Behavior Therapies for Disorders of Gut-Brain Interaction. Gastroenterology. 2022;162(1):300-315. doi:10.1053/j.gastro.2021.09.015
- Goodoory VC, Khasawneh M, Thakur ER, et al. Effect of Brain-Gut Behavioral Treatments on Abdominal Pain in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gastroenterology. 2024;167(5):934-943.e5. doi:10.1053/j.gastro.2024.05.010
- Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology. 2022;163(1):118-136. doi:10.1053/j.gastro.2022.04.016
- Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018;154(4):1140-1171.e1. doi:10.1053/j.gastro.2017.11.279
- Hasan SS, Ballou S, Keefer L, Vasant DH. Improving access to gut-directed hypnotherapy for irritable bowel syndrome in the digital therapeutics’ era: Are mobile applications a “smart” solution? Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Tarar ZI, Farooq U, Zafar Y, et al. Burden of anxiety and depression among hospitalized patients with irritable bowel syndrome: a nationwide analysis. Ir J Med Sci. 2023;192(5):2159-2166. doi:10.1007/s11845-022-03258-6
- Barbara G, Aziz I, Ballou S, et al. Rome Foundation Working Team Report on overlap in disorders of gut-brain interaction. Nat Rev Gastroenterol Hepatol. doi:10.1038/s41575-024-01033-9
- Thakur ER, Kunik M, Jarbrink-Sehgal ME, Lackner J, Dindo L, El-Serag H. Behavior Medicine Management of Irritable Bowel Syndrome: A Referral Toolkit for Gastroenterology Providers. 2018. Accessed February 19, 2025. https://www.mirecc.va.gov/VISN16/docs/ibs-referral-toolkit.pdf
- Irritable bowel syndrome (IBS). Johns Hopkins Medicine website. Accessed February 19, 2025. https://www.hopkinsmedicine.org/health/conditionsanddiseases/irritable-bowel-syndrome-ibs
- Burton-Murray H, Guadagnoli L, Kamp K, et al. Rome Foundation Working Team Report: Consensus Statement on the Design and Conduct of Behavioural Clinical Trials for Disorders of Gut-Brain Interaction. Aliment Pharmacol Ther. 2025;61(5):787-802. doi:10.1111/apt.18482
- Rome GastroPsych. Rome Foundation website. Accessed February 19, 2025. https://romegipsych.org/
- Scarlata K, Riehl M. Mind Your Gut: The Science-based, Whole-body Guide to Living Well with IBS. Hachette Book Group, 2025.
- IFFGD International Foundation for Gastrointestinal Disorders. IFFGD website. January 10, 2025. Accessed February 19, 2025. https://iffgd.org/
- GI Psychology: Mind Your Gut website. April 2, 2024. Accessed February 19, 2025. https://www.gipsychology.com/
- Drossman DA, Ruddy J. Gut Feelings: Disorders of the Gut-Brain Interaction (DGBI) and the Patient-Doctor Relationship. DrossmanCare Chapel Hill, 2020.
- Tuesday Night IBS website. Accessed February 19, 2025. https://www.tuesdaynightibs.com/
- Staudacher HM, Black CJ, Teasdale SB, Mikocka-Walus A, Keefer L. Irritable bowel syndrome and mental health comorbidity - approach to multidisciplinary management. Nat Rev Gastroenterol Hepatol. 2023;20(9):582-596. doi:10.1038/s41575-023-00794-z
- Ballou S, Vasant DH, Guadagnoli L, et al. A primer for the gastroenterology provider on psychosocial assessment of patients with disorders of gut-brain interaction. Neurogastroenterol Motil. 2024;36(12):e14894. doi:10.1111/nmo.14894
- Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome Working Team Report on Brain-Gut Behavior Therapies for Disorders of Gut-Brain Interaction. Gastroenterology. 2022;162(1):300-315. doi:10.1053/j.gastro.2021.09.015
- Goodoory VC, Khasawneh M, Thakur ER, et al. Effect of Brain-Gut Behavioral Treatments on Abdominal Pain in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gastroenterology. 2024;167(5):934-943.e5. doi:10.1053/j.gastro.2024.05.010
- Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology. 2022;163(1):118-136. doi:10.1053/j.gastro.2022.04.016
- Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, Van Oudenhove L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018;154(4):1140-1171.e1. doi:10.1053/j.gastro.2017.11.279
- Hasan SS, Ballou S, Keefer L, Vasant DH. Improving access to gut-directed hypnotherapy for irritable bowel syndrome in the digital therapeutics’ era: Are mobile applications a “smart” solution? Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Tarar ZI, Farooq U, Zafar Y, et al. Burden of anxiety and depression among hospitalized patients with irritable bowel syndrome: a nationwide analysis. Ir J Med Sci. 2023;192(5):2159-2166. doi:10.1007/s11845-022-03258-6
- Barbara G, Aziz I, Ballou S, et al. Rome Foundation Working Team Report on overlap in disorders of gut-brain interaction. Nat Rev Gastroenterol Hepatol. doi:10.1038/s41575-024-01033-9
- Thakur ER, Kunik M, Jarbrink-Sehgal ME, Lackner J, Dindo L, El-Serag H. Behavior Medicine Management of Irritable Bowel Syndrome: A Referral Toolkit for Gastroenterology Providers. 2018. Accessed February 19, 2025. https://www.mirecc.va.gov/VISN16/docs/ibs-referral-toolkit.pdf
- Irritable bowel syndrome (IBS). Johns Hopkins Medicine website. Accessed February 19, 2025. https://www.hopkinsmedicine.org/health/conditionsanddiseases/irritable-bowel-syndrome-ibs
- Burton-Murray H, Guadagnoli L, Kamp K, et al. Rome Foundation Working Team Report: Consensus Statement on the Design and Conduct of Behavioural Clinical Trials for Disorders of Gut-Brain Interaction. Aliment Pharmacol Ther. 2025;61(5):787-802. doi:10.1111/apt.18482
- Rome GastroPsych. Rome Foundation website. Accessed February 19, 2025. https://romegipsych.org/
- Scarlata K, Riehl M. Mind Your Gut: The Science-based, Whole-body Guide to Living Well with IBS. Hachette Book Group, 2025.
- IFFGD International Foundation for Gastrointestinal Disorders. IFFGD website. January 10, 2025. Accessed February 19, 2025. https://iffgd.org/
- GI Psychology: Mind Your Gut website. April 2, 2024. Accessed February 19, 2025. https://www.gipsychology.com/
- Drossman DA, Ruddy J. Gut Feelings: Disorders of the Gut-Brain Interaction (DGBI) and the Patient-Doctor Relationship. DrossmanCare Chapel Hill, 2020.
- Tuesday Night IBS website. Accessed February 19, 2025. https://www.tuesdaynightibs.com/
IBS: Mental Health Factors and Comorbidities
IBS: Mental Health Factors and Comorbidities
Clinicians Should Have Private Spaces for Telehealth According to VA Memo
US Department of Veterans Affairs (VA) officials are insisting that when remote telehealth clinicians return to an office setting, they must have private workspaces “that foster trusted, confidential, and therapeutic relationships with veterans,” according to an April internal memo reported on by NPR.
The return-to-office mandate followed a Trump Administration executive order in February indicated that mental health clinicians at the US Department of Veterans Affairs (VA) must physically return to their workplace by May 5. For some, the deadline came as early as April 14; however, that order, like many others, may now be being revised or reconsidered due to concerns that have been raised. Many mental health clinicians were hired specifically to work remotely. They worried there would simply not be enough space for them, particularly to provide confidential counseling.
Millions of veterans use telehealth to access VA care. More than 98% of VA mental health clinicians have conducted ≥ 1 video visit to screen and treat patients for anxiety, depression, posttraumatic stress disorder, and more. Telehealth has been particularly important for veterans living in rural communities.
The April VA memo stipulated that “spaces used to deliver synchronous telehealth services should offer the same level of privacy and therapeutic environment applicable to an in-person visit in the same space.”
Therapists, patients, advocacy groups, and lawmakers have expressed concern about the potential impacts the policy change could have on patient care for veterans and, above all, about what it could mean for privacy. On Mar. 27, the American Psychological Association issued a statement noting that the change “resulted in providers being asked to conduct sensitive therapy sessions in open office environments, cubicles, or shared spaces that fail to meet basic confidentiality and privacy requirements for the delivery of mental health care services.”
Twenty Democrats in the House of Representatives sent a letter to VA Secretary Doug Collins expressing concern with the return to office policy. According to the letter a VA social worker supervisor reported managing their caseload while sharing a 100 ft2 shower space with another supervisor. It also reported that Clinical Resource Hub employees were being told to report to buildings where federal employees from other agencies work. “We have heard from countless stakeholders, veterans, and Department of Veterans Affairs (VA) employees that by carrying out President Trump’s blanket return-to-office policy your administration is damaging veteran and employee trust in VA, disrupting and impeding veterans’ access to care, and creating untenable and inefficient conditions for both veterans and the VA workforce,” the letter stated.
“This is a clear violation of veterans’ privacy and VA’s obligation to protect veterans’ private health information, and risks violation of the Health Insurance Portability and Accountability Act (HIPAA),” the letter added.
The lawmakers noted that, as of March 10, the VA was exempting Veterans Crisis Line workers, most of whom had been working remotely for the past 5 years, responding to more than 10 million calls, texts, and chats. That move, they said, indicated “that you understand there will be negative impacts to veterans’ care due to the return-to-office order and that these must be mitigated.”
VA spokesperson Peter Kasperowicz called the privacy concerns “nonsensical” and blamed “fear mongering from the media.” The VA, he said, “is no longer a place where the status quo for employees is to simply phone it in from home.” He also claimed that “the small number of employees who are desperate to avoid returning to the office will do more to drive away staff and patients than VA’s commonsense return-to-office policy ever will.”
VA care, he said, would continue uninterrupted and the “VA will ensure that employees have a workspace that is appropriate for the work they do.”
US Department of Veterans Affairs (VA) officials are insisting that when remote telehealth clinicians return to an office setting, they must have private workspaces “that foster trusted, confidential, and therapeutic relationships with veterans,” according to an April internal memo reported on by NPR.
The return-to-office mandate followed a Trump Administration executive order in February indicated that mental health clinicians at the US Department of Veterans Affairs (VA) must physically return to their workplace by May 5. For some, the deadline came as early as April 14; however, that order, like many others, may now be being revised or reconsidered due to concerns that have been raised. Many mental health clinicians were hired specifically to work remotely. They worried there would simply not be enough space for them, particularly to provide confidential counseling.
Millions of veterans use telehealth to access VA care. More than 98% of VA mental health clinicians have conducted ≥ 1 video visit to screen and treat patients for anxiety, depression, posttraumatic stress disorder, and more. Telehealth has been particularly important for veterans living in rural communities.
The April VA memo stipulated that “spaces used to deliver synchronous telehealth services should offer the same level of privacy and therapeutic environment applicable to an in-person visit in the same space.”
Therapists, patients, advocacy groups, and lawmakers have expressed concern about the potential impacts the policy change could have on patient care for veterans and, above all, about what it could mean for privacy. On Mar. 27, the American Psychological Association issued a statement noting that the change “resulted in providers being asked to conduct sensitive therapy sessions in open office environments, cubicles, or shared spaces that fail to meet basic confidentiality and privacy requirements for the delivery of mental health care services.”
Twenty Democrats in the House of Representatives sent a letter to VA Secretary Doug Collins expressing concern with the return to office policy. According to the letter a VA social worker supervisor reported managing their caseload while sharing a 100 ft2 shower space with another supervisor. It also reported that Clinical Resource Hub employees were being told to report to buildings where federal employees from other agencies work. “We have heard from countless stakeholders, veterans, and Department of Veterans Affairs (VA) employees that by carrying out President Trump’s blanket return-to-office policy your administration is damaging veteran and employee trust in VA, disrupting and impeding veterans’ access to care, and creating untenable and inefficient conditions for both veterans and the VA workforce,” the letter stated.
“This is a clear violation of veterans’ privacy and VA’s obligation to protect veterans’ private health information, and risks violation of the Health Insurance Portability and Accountability Act (HIPAA),” the letter added.
The lawmakers noted that, as of March 10, the VA was exempting Veterans Crisis Line workers, most of whom had been working remotely for the past 5 years, responding to more than 10 million calls, texts, and chats. That move, they said, indicated “that you understand there will be negative impacts to veterans’ care due to the return-to-office order and that these must be mitigated.”
VA spokesperson Peter Kasperowicz called the privacy concerns “nonsensical” and blamed “fear mongering from the media.” The VA, he said, “is no longer a place where the status quo for employees is to simply phone it in from home.” He also claimed that “the small number of employees who are desperate to avoid returning to the office will do more to drive away staff and patients than VA’s commonsense return-to-office policy ever will.”
VA care, he said, would continue uninterrupted and the “VA will ensure that employees have a workspace that is appropriate for the work they do.”
US Department of Veterans Affairs (VA) officials are insisting that when remote telehealth clinicians return to an office setting, they must have private workspaces “that foster trusted, confidential, and therapeutic relationships with veterans,” according to an April internal memo reported on by NPR.
The return-to-office mandate followed a Trump Administration executive order in February indicated that mental health clinicians at the US Department of Veterans Affairs (VA) must physically return to their workplace by May 5. For some, the deadline came as early as April 14; however, that order, like many others, may now be being revised or reconsidered due to concerns that have been raised. Many mental health clinicians were hired specifically to work remotely. They worried there would simply not be enough space for them, particularly to provide confidential counseling.
Millions of veterans use telehealth to access VA care. More than 98% of VA mental health clinicians have conducted ≥ 1 video visit to screen and treat patients for anxiety, depression, posttraumatic stress disorder, and more. Telehealth has been particularly important for veterans living in rural communities.
The April VA memo stipulated that “spaces used to deliver synchronous telehealth services should offer the same level of privacy and therapeutic environment applicable to an in-person visit in the same space.”
Therapists, patients, advocacy groups, and lawmakers have expressed concern about the potential impacts the policy change could have on patient care for veterans and, above all, about what it could mean for privacy. On Mar. 27, the American Psychological Association issued a statement noting that the change “resulted in providers being asked to conduct sensitive therapy sessions in open office environments, cubicles, or shared spaces that fail to meet basic confidentiality and privacy requirements for the delivery of mental health care services.”
Twenty Democrats in the House of Representatives sent a letter to VA Secretary Doug Collins expressing concern with the return to office policy. According to the letter a VA social worker supervisor reported managing their caseload while sharing a 100 ft2 shower space with another supervisor. It also reported that Clinical Resource Hub employees were being told to report to buildings where federal employees from other agencies work. “We have heard from countless stakeholders, veterans, and Department of Veterans Affairs (VA) employees that by carrying out President Trump’s blanket return-to-office policy your administration is damaging veteran and employee trust in VA, disrupting and impeding veterans’ access to care, and creating untenable and inefficient conditions for both veterans and the VA workforce,” the letter stated.
“This is a clear violation of veterans’ privacy and VA’s obligation to protect veterans’ private health information, and risks violation of the Health Insurance Portability and Accountability Act (HIPAA),” the letter added.
The lawmakers noted that, as of March 10, the VA was exempting Veterans Crisis Line workers, most of whom had been working remotely for the past 5 years, responding to more than 10 million calls, texts, and chats. That move, they said, indicated “that you understand there will be negative impacts to veterans’ care due to the return-to-office order and that these must be mitigated.”
VA spokesperson Peter Kasperowicz called the privacy concerns “nonsensical” and blamed “fear mongering from the media.” The VA, he said, “is no longer a place where the status quo for employees is to simply phone it in from home.” He also claimed that “the small number of employees who are desperate to avoid returning to the office will do more to drive away staff and patients than VA’s commonsense return-to-office policy ever will.”
VA care, he said, would continue uninterrupted and the “VA will ensure that employees have a workspace that is appropriate for the work they do.”
The Cruelty of April: Suicide in Spring
The Cruelty of April: Suicide in Spring
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
April is the cruellest month, breeding
Lilacs out of the dead land, mixing
Memory and desire, stirring
Dull roots with spring rain.
T.S. Eliot1
The epigraph for this column is from The Waste Land, T.S. Eliot’s postmodern poem that, in part, reflects his experience of the destruction of an entire way of living and a generation of young men in the wake of the First World War. The terrible contemporary toll suicide has taken on veterans and the active-duty military makes it easy to forget that suicide is an inveterate and disturbing aftermath of all wars.2
There is a profound and elemental connection in the human mind between Spring and renewal. In almost every culture and religion, across nearly every historical epoch and location, Spring is associated with themes of growth, returning life, light, and hope. On a more prosaic modern level, almost all of us—us—especially those in Northern climates—look forward to warmer weather, more time spent outdoors, and the simple joys of seeing perennials return in the garden and birds nest in blooming trees.
It is a paradox of human life that suicide is more common in the season of rebirth than in the season of decline. The bare trees, freezing temperatures, and icy darkness that accompany winter in much of the world inherently lead us to contemplate our mortality. The counterintuitive finding that individuals, many of them veterans, take their own lives more often in Spring creates a cognitive dissonance to be explored in this editorial.
As a layperson, I too assumed there were more suicides in winter, especially around the holidays when the expectation of belonging, privilege, and pleasure painfully reminds the alienated, lonely, homeless, and ailing of all they lack and all they have lost. As a psychiatric intern, I anticipated that the inpatient US Department of Veterans Affairs (VA) ward where I was training would empty with the arrival of nicer weather. Instead, I was mystified when the opposite occurred and the unit was overflowing with manic and suicidal patients.
The Centers for Disease Control and Prevention National Center for Health Statistics ranked suicide by month from 1999 to 2010. Contrary to popular belief, more suicides occurred in late Spring and Summer than any other season.3 A 2023 study of systematic reviews of seasonal variation in mood disorders, suicide risk, and health care utilization found that suicide was 11% to 23% higher, suicide attempts resulting in emergency department visits showed an increase of 1.2% to 1.7%, and hospital admissions for mania rose 7.4% to 16.0% in Spring and Summer, compared with Fall and Winter.4 This general population finding is also seen in veteran and military cohorts. A recent study analyzed VA and US Department of Defense (DoD) data from 133,867 veteran suicides from 2001 to 2021. Results showed that veteran suicides were highest in Summer.5
The rise of suicide in the Spring was first observed in the 17th century and has been the object of scientific study for at least 3 decades. That research has produced several different hypotheses from a variety of disciplines, none of which are conclusive as of this writing. Cho and Lee note that the phrase “Spring fever” is a much more serious illness for those with a predisposition or diagnosis of unipolar or bipolar disorder than the quotidian irritant that afflicts those without affective disorders.6 In residency, I learned that longer exposure to light in Spring led to an imbalance in neurotransmitters that triggered manias. This is a simplistic version of the complex circadian interactions of temperature, climate, light, and other environmental variables causing dysregulation or misalignment of our natural biological cycles and those of nature proposed by chronobiologists.7
Sociological and criminal justice scholars underscore that an increase in temperature may exacerbate violent tendencies, especially in older males—a demographic profile more frequently found in veterans—and those already prone to acting out their frustrations with firearms.8 Psychologists have hypothesized that individuals with depressions persevere through Winter by telling themselves they will feel relief in the Spring. Too often the coming of Spring brings not reprieve but a deadly combination of deeper mental desperation coupled with the release from winter lassitude that energizes the now hopeless person to put ideation into action.4,9 The elevation of suicide rates in Spring is likely multidetermined with all these putative causes contributing in different variations to every individual who tragically dies by suicide.
Yet despite decades of public education, this dangerous fiction stubbornly persists in the educated public and even among many health care professionals, in part due to misguided media. For years, the Annenberg Public Policy Center (APPC) has made busting this myth of holiday suicides in the media an organizational initiative. A 2023 APPC survey found that 4 of 5 Americans picked December as the month when suicide rates were highest. The organization has been analyzing holiday—related media reports for decades; those results show some improvement, with the most recent analysis of media reports somewhat better and 40% communicating erroneous information. 10
APPC believes the opinion that suicide is more common around the holidays will persuade those struggling with an exacerbation of a mental health condition or an acute crisis to attempt or die by suicide, believing it to be a reasonable social response. While recognizing there is a real risk of such contagion behavior, I believe the reverse problem is more concerning. As I observed during my internship, the acceptance of the fiction that everyone is happy in Spring may even blind health care professionals from detecting clues that patients and even our loved ones are contemplating suicide. Our relief that Winter has passed and enjoyment of Spring activities can fool us into believing everyone else is also feeling fine and doing well and miss an opportunity to intervene and treat mania or depression to save a life—the medical manifestation of renewal.
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
- Elliot TS, North M. The Waste Land and Other Poems: A Norton Critical Edition. W.W. Norton & Company; 2022.
- Lester D. Suicide rates before, during, and after the world wars. Eur Psychiatry. 1994;9(5):262-264. doi:10.1017/S092493380000362X
- Centers for Disease Control Center and Prevention. National Center for Health Statistics. Fact or fiction: suicides increase during the holiday season and winter months. January 10, 2014. Accessed March 27, 2025. https://blogs.cdc.gov/nchs/2014/01/10/1121/
- Della DF, Allison S, Bidargaddi N, Wa SK, Bastiampillai T. An umbrella systematic review of seasonality in mood disorders and suicide risk: the impact on demand for primary behavioral health care and acute psychiatric services. Prim Care Companion CNS Disord. 2023;25(3):22r03395. doi:10.4088/PCC.22r03395
- Gold SA, Goodrich M, Morley SW, Stephens B, McCarthy JF. Temporal patterns of veteran suicide: variation by season, day of the week, and holidays. Suicide Life Threat Behav. 2025;55(2):e13148. doi:10.1111/sltb.13148
- Cho CH, Lee HJ. Why do mania and suicide occur most often in the Spring? Psychiatry Investig. 2018;15(3):232-234. doi:10.30773/pi.2017.12.20
- Postolache TT, Mortensen PB, Tonelli LH, et al. Seasonal spring peaks of suicide in victims with and without prior history of hospitalization for mood disorders. J Affect Disord. 2010;121(1-2):88-93. doi:10.1016/j.jad.2009.05.015
- Christodoulou C, Efstathiou V, Bouras G, Korkoliakou P, Lykouras L. Seasonal variation of suicide: a brief review. Encephalos. 2012;49:73-79.
- Shapiro M. Suicide rates spike in spring, not winter. Dome. May/June 2019. Accessed March 28, 2025. https://www.hopkinsmedicine.org/news/articles/2019/05/suicide-rates-spike-in-spring-not-winter
- Annenberg Public Policy Center. Suicides don’t spike around the holiday season, but Americans think they do. December 6, 2023. Accessed March 27, 2025. https:// www.asc.upenn.edu/news-events/news/suicides-dont-spike-around-holiday-season-americans-think-they-do
The Cruelty of April: Suicide in Spring
The Cruelty of April: Suicide in Spring
Examining Moral Injury in Legal-Involved Veterans: Psychometric Properties of the Moral Injury Events Scale
Examining Moral Injury in Legal-Involved Veterans: Psychometric Properties of the Moral Injury Events Scale
Following exposure to potentially morally injurious events (PMIEs), some individuals may experience moral injury, which represents negative psychological, social, behavioral, and occasionally spiritual impacts.1 The consequences of PMIE exposure and moral injury are well documented. Individuals may begin to question the goodness and trustworthiness of oneself, others, or the world.1 Examples of other sequelae include guilt, demoralization, spiritual pain, loss of trust in the self or others, and difficulties with forgiveness.2-6 In addition, prior studies have found that moral injury is associated with an increased risk of suicidal thoughts and behaviors, posttraumatic stress disorder (PTSD) symptoms, spiritual distress, and interpersonal difficulties.7-11
Moral injury was first conceptualized in relation to combat trauma. However in recent years it has been examined in other groups such as health care practitioners, educators, refugees, and law enforcement personnel.12-17 Furthermore, there has been a recent call for the study of moral injury in other understudied groups. One such group is legal-involved individuals, defined as those who are currently involved or previously involved in the criminal justice system (ie, arrests, incarceration, parole, and probation).1,18-22
Many veterans are also involved with the legal system. Specifically, veterans currently comprise about 8% of the incarcerated US population, with an estimated > 180,000 veterans in prisons or jails and even more on parole or probation.23,24 Legal-involved veterans may be at heightened risk for homelessness, suicide, unemployment, and high prevalence rates of psychiatric diagnoses.25-28
Limited research has explored exposure to PMIEs as part of the legal process and the resulting expression of moral injury. The circumstances leading to incarceration, interactions with the US legal system, the environment of prison itself, and the subsequent challenges faced by legal-involved individuals after release all provide ample opportunity for PMIEs to occur.18 For example, engaging in a criminal act may represent a PMIE, particularly in violent offenses that involve harm to another individual. Moreover, the process of being convicted and charged with an offense may serve as a powerful reminder of the PMIE and tie this event to the individual’s identity and future. Furthermore, the physical and social environment of prison itself (eg, being surrounded by other offenders, witnessing the perpetration of violence, participating in violence for survival) presents a myriad of opportunities for PMIEs to occur.18
The consequences of PMIEs in the context of legal involvement may also have bearing on a touchstone of moral injury: changes in one’s schema of the self and world.4 At a societal level, legal-involved individuals are, by definition, deemed “guilty” and held culpable for their offense, which may reinforce a negative change in one’s view of self and the world.29 In line with identity theory, external negative appraisals about legal-involved individuals (eg, they are a danger to society, they cannot be trusted to do the right thing) may influence their self-perception.30 Furthermore, the affective characteristics often found in the context of moral injury (eg, guilt, shame, anger, contempt) may be exacerbated by legal involvement.29 Personal feelings of guilt and shame may be reinforced by receiving a verdict and sentence, as well as the negative perceptions of individuals around them (eg, disapproval from prior sources of social support). Additionally, feelings of betrayal and distrust towards the legal system may arise.
In sum, legal-involved veterans incur increased risk of moral injury due to the potential for exposure to PMIEs across multiple time points (eg, prior to military service, during military service, during arrest/sentencing, during imprisonment, and postincarceration). The stigma that accompanies legal involvement may limit access to treatment or a willingness to seek treatment for distress related to moral injury.29 Additionally, repeated exposure to PMIEs and resulting moral injury may compound over time, potentially exacerbating psychosocial functioning and increasing the risk for psychosocial stressors (eg, homelessness, unemployment) and mental health disorders (eg, depression, substance misuse).31
Although numerous measures of moral injury have been developed, most require that respondents consider a specific context (eg, military experiences).32 Therefore, study of legal-related moral injury requires adaptation of existing instruments to the legal context. The original and most commonly used scale of moral injury is the Moral Injury Events Scale (MIES).33 The MIES scales was originally developed to measure moral injury in military-related contexts but has since been adapted as a measure of exposure to context-specific PMIEs.34
Unfortunately, there are no validated measures for assessing legal-related moral injury. Such a gap in understanding is problematic, as it may impact measurement of the prevalence of PMIEs in both clinical and research settings for this at-risk population. The goal of this study was to conduct a psychometric evaluation of an adapted version of the MIES for legal-involved persons (MIES-LIP).
METHODS
A total of 177 veterans from the US Department of Veterans Affairs (VA) North Texas Health Care System were contacted for study enrollment between November 2020 and June 2021, yielding a final sample of 100 legal-involved veteran participants. Adults aged ≥ 18 years who were US military veterans and had ≥ 1 prior felony conviction resulting in incarceration were included. Participants were excluded if they had symptoms of psychosis that would preclude meaningful participation.
The study collected data on participants’ demographic and clinical characteristics using a semistructured survey instrument. Each participant completed an instructor-led questionnaire in a session that lasted about 1.5 hours. Participants who completed the visit in person received a $50 cash voucher for their time. Participants who were unable to meet with the study coordinator in person were able to complete the visit via telephone and received a $25 digital gift card. Of the total 100 participants, 79 participants completed the interview in person, and 21 completed by telephone. No significant differences were found in assessment measures between administration methods. Written informed consent was obtained during all in-person visits. For those completing via telephone, a waiver of written informed consent was obtained. This study was approved by the VA North Texas Health Care System’s Institutional Review Board.
Measures
The Moral Injury Events Scale (MIES) is a 9-item self-report measure that assesses exposure to PMIEs.33 Respondents rate their agreement with each item on a 6-point Likert scale (strongly disagree to strongly agree), with higher scores indicating greater moral injury. The MIES has a 2-factor structure: Factor 1 has 6 items on perceived transgressions and Factor 2 has 3 items on perceived betrayals.33
Creation of Legal-Involved Moral Injury Measure. To create the MIES-LIP, items and instructions from the MIES were modified to address moral injury in the context of legal involvement.33 Adaptations were finalized following consultation and approval by the authors of the original measure. Specifically, the instructions were changed to: “Please respond to these items based specifically in the context of your involvement with the legal system.” The instructions clarified that legal involvement could include experiences related to committing an offense, legal proceedings and sentencing, incarceration, or transitioning out of the legal system. This differs from the original measure, which focused on military experiences, with instructions stating: “Please respond to these items based specifically in the context of your military service (ie, events and experiences during enlistment, deployment, combat, etc).”
Other measures. The study collected data on demographic characteristics including sex, race and ethnicity, marital status, military service, combat experience, and legal involvement. PTSD symptom severity, based on the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), was assessed using the PTSD Checklist for DSM-5 (PCL-5).35,36 The PCL-5 is a 20-item self-report measure in which item scores are summed to create a total score. The PCL-5 has demonstrated strong psychometric properties, including good internal consistency, test-retest reliability convergent validity, and discriminant validity.37,38
Depressive symptom severity was measured using the Personal Health Questionnaire-9 (PHQ-9).39 The PHQ-9 is a 9-item self-report measure where item scores summed to create a total score. The PHQ-9 has demonstrated strong psychometric properties, including internal consistency and test-retest reliability.39
STATISTICAL METHODS
Descriptive statistics (mean and standard deviation for continuous variables; frequencies and percentages for categorical variables) were used to describe the study sample. Factor analysis was conducted to evaluate the psychometric properties of the MIES-LIP. Confirmatory factor analysis (CFA) was used to determine whether the MEIS-LIP had a similar factor structure to the MIES.40 Criteria for fit indices used for CFA include the Comparative Fit Index (CFI; values of > 0.95 suggest good fit), Tucker-Lewis index (TLI; values of > 0.95 suggest a good fit), root mean square error of approximation (RMSEA; values of ≥ 0.06 suggest good fit), and standardized root mean square residual (SRMR; values of ≥ 0.08 suggest good fit). With insufficient fit, subsequent exploratory factor analysis was conducted using maximum likelihood estimation with an Oblimin rotation. The Kaiser rule and a scree plot were considered when defining the factor structure. Reliability was evaluated using the McDonald omega coefficient test. Convergent validity was assessed through the association between adapted measures and other clinical measures (ie, PCL-5, PHQ-9). In addition, associations between the PCL-5 and PHQ-9 were examined as they related to the MIES and MIES-LIP.
RESULTS
Table 1 describes demographic characteristics of the study sample. Rates of potentially morally injurious experiences and the expression of moral injury in the legal context are presented in Table 2. Witnessing PMIEs while in the legal system was nearly ubiquitous, with > 90% of the sample endorsing this experience. More than half of the sample also endorsed engaging in morally injurious behavior by commission or omission, as well as experiencing betrayal while involved with the legal system.
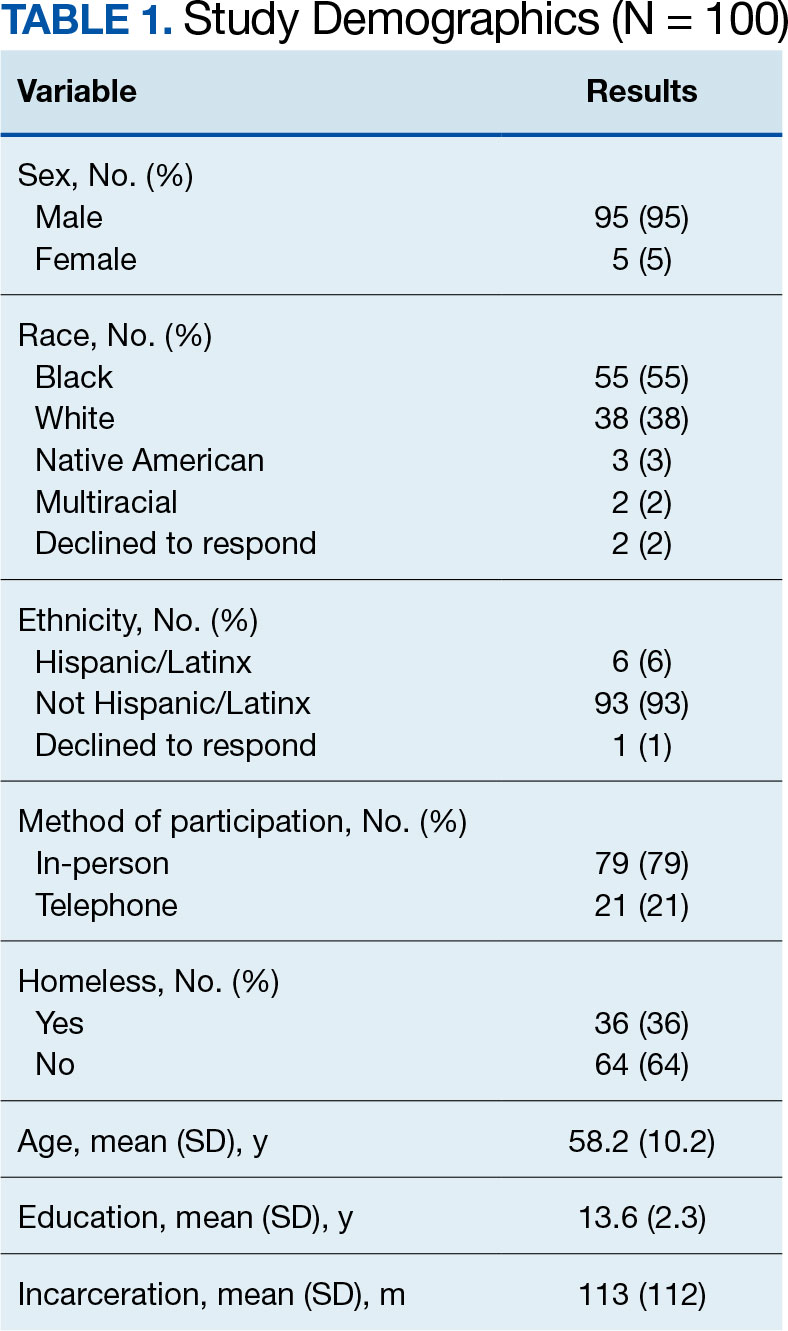
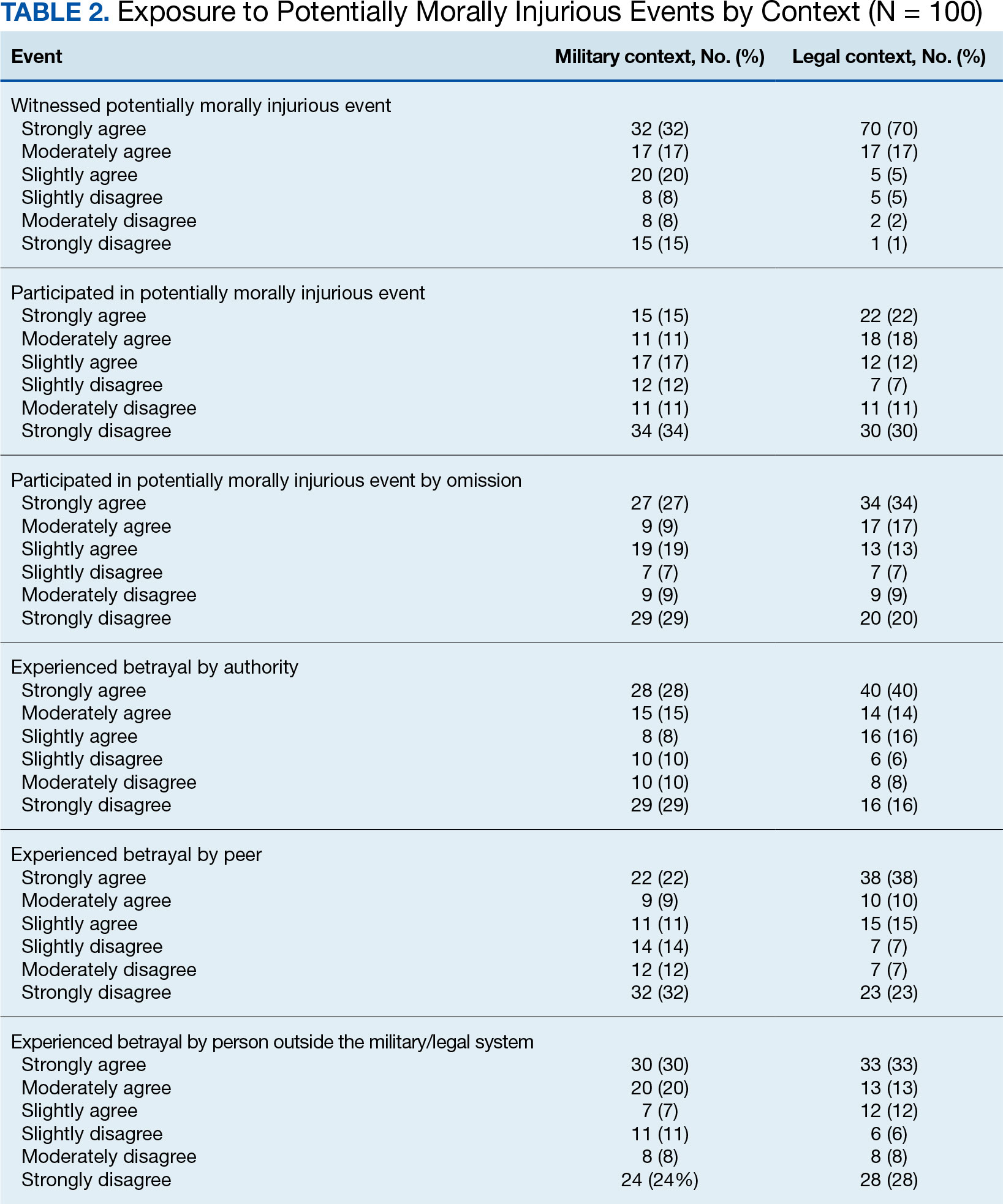
Factor Analysis
Confirmatory factor analysis (CFA) was utilized to test the factor structure of the adapted MIES-LIP in our sample compared to the published factor structures of the MIES.33 Results did not support the established factor structure. Analysis yielded unacceptable CFI (0.79), TLI (0.70), SRMR (0.14), and RMSEA (0.21). The unsatisfactory results of CFA warranted follow-up exploratory factor analysis (EFA) to examine the factor structure of the moral injury scales in this sample.
EFA of MIES-LIP
The factor structure of the MIES-LIP was examined using EFA. The factorability of the data was examined using the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO value = 0.75) and Bartlett Test of Sphericity (X2 = 525.41; P < .001), both of which suggested that the data were appropriate for factor analysis. The number of factors to retain was selected based on the Kaiser criterion.41 After extraction, an Oblimin rotation was applied, given that we expected factors to be correlated. A 2-factor solution was found, explaining 65.76% of the common variance. All 9 items were retained as they had factor loadings > 0.30. Factor 1, comprised self-directed moral injury questions (3-6). Factor 2 comprised other directed moral injury questions (1, 2, 7-9) (Table 3). The factor correlation coefficient between Factor 1 and Factor 2 was 0.34, which supports utilizing an oblique rotation.
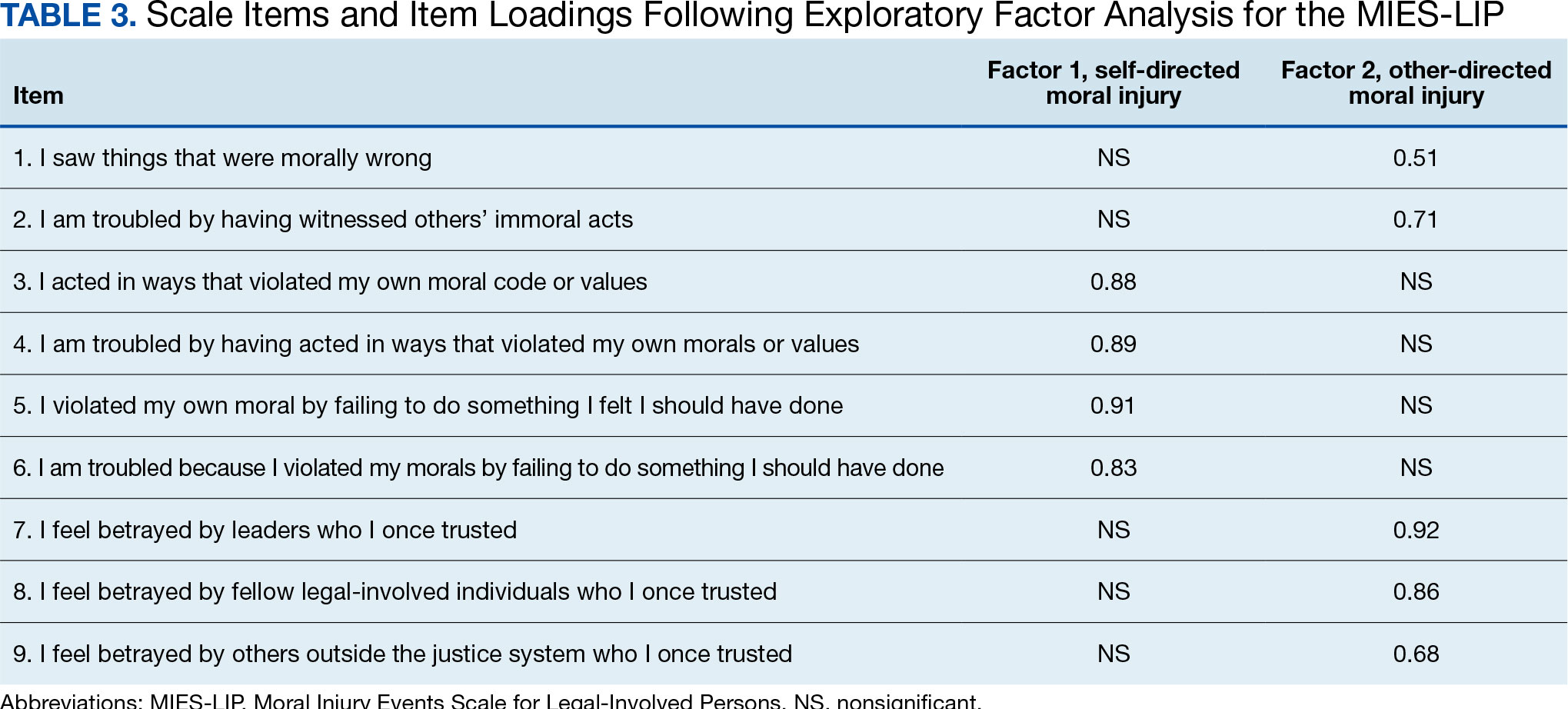
Reliability. We examined the reliability of the adapted MIES-LIP using measures of internal consistency, with both MIES-LIP factors demonstrating good reliability. The internal consistency of both factors of the MIES-LIP were found to be good (self-directed moral injury: Ω = 0.89; other-directed moral injury: Ω = 0.83).
Convergent Validity
Association between moral injury scales. A significant, moderate correlation was observed between all subscales of the MIES and MIES-LIP. Specifically, the self-directed moral injury factor of the MIES-LIP was associated with both the perceived transgressions (r = 0.41, P < .001) and the MIES perceived betrayals factors (r = 0.25, P < .05). Similarly, the other-directed moral injury factor of the MIES-LIP was associated with both the MIES perceived transgressions (r = 0.45, P < .001) and the MIES perceived betrayals factors (r = 0.45, P < .001).
Association with PTSD symptoms. All subscales of both the MIES and MIES-LIP were associated with PTSD symptom severity. The MIES perceived transgressions factor (r = 0.43, P < .001) and the perceived betrayals factor of the MIES (r = 0.39, P < .001) were moderately associated with the PCL-5. Mirroring this, the “self-directed moral injury” factor of the MIESLIP (r = 0.44, P < .001) and the “other-directed moral injury” factor of the MIES-LIP (r = 0.42, P < .001) were also positively associated with PCL-5.
Association with depression symptoms. All subscales of the MIES and MIES-LIP were also associated with depressive symptoms. The MIES perceived transgressions factor (r = 0.27, P < .01) and the MIES perceived betrayals factor (r = 0.23, P < .05) had a small association with the PHQ-9. In addition, the self-directed moral injury factor of the MIES-LIP (r = 0.40, P < .001) and the other-directed moral injury factor of the MIES-LIP (r = 0.31, P < .01) had small to moderate associations with the PCL-5.
DISCUSSION
Potentially morally injurious events appear to be a salient factor affecting legal-involved veterans. Among our sample, the vast majority of legal-involved veterans endorsed experiencing both legal- and military-related PMIEs. Witnessing or participating in a legal-related PMIE appears to be widespread among those who have experienced incarceration. The MIES-LIP yielded a 2-factor structure: self-directed moral injury and other-directed moral injury, in the evaluated population. The MIES-LIP showed similar psychometric performance to the MIES in our sample. Specifically, the MIES-LIP had good reliability and adequate convergent validity. While CFA did not confirm the anticipated factor structure of the MIES-LIP within our sample, EFA showed similarities in factor structure between the original and adapted measures. While further research and validation are needed, preliminary results show promise of the MIES-LIP in assessing legal-related moral injury.
Originally, the MIES was found to have a 2-factor structure, defined by perceived transgressions and perceived betrayals.33 However, additional research has identified a 3-factor structure, where the betrayal factor is maintained, and the transgressions factor is divided into transgressions by others and by self.8 The factor structure of the MIES-LIP was more closely related to the factor structure, with transgressions by others and betrayal mapped onto the same factor (ie, other-directed moral injury).8 While further research is needed, it is possible that the nature of morally injurious events experienced in legal contexts are experienced more in terms of self vs other, compared to morally injurious events experienced by veterans or active-duty service members.
Accurately identifying the types of moral injury experienced in a legal context may be important for determining the differences in drivers of legal-related moral injury compared to military-related moral injury. For example, self-directed moral injury in legal contexts may include a variety of actions the individual initiated that led to conviction and incarceration (eg, a criminal offense), as well as behaviors performed or witnessed while incarcerated (eg, engaging in violence). Inconsistent with military populations where other-directed moral injury clusters with self-directed moral injury, other-directed moral injury clustered with betrayal in legal contexts in our sample. This discrepancy may result from differences in identification with the military vs legal system. When veterans witness fellow service members engaging in PMIEs (eg, physical violence towards civilians in a military setting), this may be similar to self-directed moral injury due to the veteran’s identification with the same military system as the perpetrator.42 When legal-involved veterans witness other incarcerated individuals engaging in PMIEs (eg, physical violence toward other inmates), this may be experienced as similar to betrayal due to lack of personal identification with the criminal-legal system. Additional research is needed to better understand how self- and other-related moral injury are associated with betrayal in legal contexts.
Another potential driver of legal-related moral injury may be culpability. In order for moral injury to occur, an individual must perceive that something has taken place that deeply violated their sense of right and wrong.1 In terms of criminal offenses or even engaging in violent behavior while incarcerated, the potential for moral injury may differ based on whether an individual views themselves as culpable for the act(s).29 This may further distinguish between self-directed and other-directed moral injury in legal contexts. In situations where the individual views themselves as culpable, self-directed moral injury may be higher. In situations where the individual does not view themselves as culpable, other-directed moral injury may be higher based on the perception that the legal system is unfairly punishing them. Further research is needed to clarify how an individual’s view of their culpability relates to moral injury, as well as to elucidate which aspects of military service and legal involvement are most closely associated with moral injury among legal-involved veterans.
While this study treated legal-related and military-related moral injury as distinct, it is possible moral injury may have a cumulative effect over time with individuals experiencing morally injurious events across different contexts (eg, military, legal involvement). This, in turn, may compound risk for moral injury. These cumulative experiences may result in increased negative outcomes such as exacerbated psychiatric symptoms, substance misuse, and elevated suicide risk. Future studies should examine differences between groups who have experienced moral injury in differing contexts, as well as those with multiple sources of moral injury.
Limitations
The sample for this study included only veterans. The number of veterans incarcerated is large and the focus on veterans also allowed for a more robust comparison of moral injury related to the legal system and the more traditional military-related moral injury. However, the generalizability of the findings to nonveterans cannot be assured. The study used a relatively small sample (N = 100), which was overwhelmingly male. Although the PCL-5 was utilized to examine traumatic stress symptoms, this measure was not anchored to a specific criterion A trauma nor was it anchored specifically to a morally injurious experience. For all participants, their most recent military service preceded their most recent legal involvement which could affect the associations between variables. Furthermore, while all participants endorsed prior legal involvement, many participants reported no combat exposure.
CONCLUSIONS
This study resulted in several key findings. First, legal-involved veterans endorsed high rates of experiencing legal-related morally injurious experiences. Second, our adapted measure displayed adequate psychometric strength and suggests that legal-related moral injury is a salient and distinct phenomenon affecting legal-involved veterans. These items may not capture all the nuances of legal-related moral injury. Qualitative interviews with legal-involved persons may help identify relevant areas of legal-related moral injury not reflected in the current instrument. The MIES-LIP represents a practical measure that may help clinicians identify and address legal-related moral injury when working with legal-involved veterans. Given the high prevalence of PMIEs among legal-involved veterans, further examination of whether current interventions for moral injury and novel treatments being developed are effective for this population is needed.
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Currier JM, Holland JM, Malott J. Moral injury, meaning making, and mental health in returning veterans. J Clin Psychol. 2015;71(3):229-240. doi:10.1002/jclp.22134
- Jinkerson JD. Defining and assessing moral injury: a syndrome perspective. Traumatology. 2016;22(2):122-130. doi:10.1037/trm0000069
- Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
- Maguen S, Litz B. Moral injury in veterans of war. PTSD Res Q. 2012;23(1):1-6. www.vva1071.org/uploads/3/4/4/6/34460116/moral_injury_in_veterans_of_war.pdf
- Drescher KD, Foy DW, Kelly C, Leshner A, Schutz K, Litz B. An exploration of the viability and usefulness of the construct of moral injury in war veterans. Traumatology. 2011;17(1):8-13. doi:10.1177/1534765610395615
- Wisco BE, Marx BP, May CL, et al. Moral injury in U.S. combat veterans: results from the national health and resilience in veterans study. Depress Anxiety. 2017; 34(4):340-347. doi:10.1002/da.22614
- Bryan CJ, Bryan AO, Anestis MD, et al. Measuring moral injury: psychometric properties of the moral injury events scale in two military samples. Assessment. 2016;23(5):557- 570. doi:10.1177/1073191115590855
- Currier JM, Smith PN, Kuhlman S. Assessing the unique role of religious coping in suicidal behavior among U.S. Iraq and Afghanistan veterans. Psychol Relig Spiritual. 2017;9(1):118-123. doi:10.1037/rel0000055
- Kopacz MS, Connery AL, Bishop TM, et al. Moral injury: a new challenge for complementary and alternative medicine. Complement Ther Med. 2016;24:29-33. doi:10.1016/j.ctim.2015.11.003
- Vargas AF, Hanson T, Kraus D, Drescher K, Foy D. Moral injury themes in combat veterans’ narrative responses from the national vietnam veterans’ readjustment study. Traumatology. 2013;19(3):243-250. doi:10.1177/1534765613476099
- Borges LM, Barnes SM, Farnsworth JK, Bahraini NH, Brenner LA. A commentary on moral injury among health care providers during the COVID-19 pandemic. Psychol Trauma. 2020;12(S1):S138-S140. doi:10.1037/tra0000698
- Borges LM, Holliday R, Barnes SM, et al. A longitudinal analysis of the role of potentially morally injurious events on COVID-19-related psychosocial functioning among healthcare providers. PLoS One. 2021;16(11):e0260033. doi:10.1371/journal.pone.0260033
- Currier JM, Holland JM, Rojas-Flores L, Herrera S, Foy D. Morally injurious experiences and meaning in Salvadorian teachers exposed to violence. Psychol Trauma. 2015;7(1):24-33. doi:10.1037/a0034092
- Nickerson A, Schnyder U, Bryant RA, Schick M, Mueller J, Morina N. Moral injury in traumatized refugees. Psychother Psychosom. 2015;84(2):122-123. doi:10.1159/000369353
- Papazoglou K, Chopko B. The role of moral suffering (moral distress and moral injury) in police compassion fatigue and PTSD: An unexplored topic. Front Psychol. 2017;8:1999. doi:10.3389/fpsyg.2017.01999
- Papazoglou K, Blumberg DM, Chiongbian VB, et al. The role of moral injury in PTSD among law enforcement officers: a brief report. Front Psychol. 2020;11:310. doi:10.3389/fpsyg.2020.00310
- Martin WB, Holliday R, LePage JP. Trauma and diversity: moral injury among justice involved veterans: an understudied clinical concern. Stresspoints. 2020;33(5).
- Currier JM, Drescher KD, Nieuwsma J. Future directions for addressing moral injury in clinical practice: concluding comments. In: Currier JM, Drescher KD, Nieuwsma J, eds. Addressing Moral Injury in Clinical Practice. American Psychological Association; 2021:261-271. doi:10.1037/0000204-015
- Alexander AR, Mendez L, Kerig PK. Moral injury as a transdiagnostic risk factor for mental health problems in detained youth. Crim Justice Behav. 2023;51(2):194-212. doi:10.1177/00938548231208203
- DeCaro JB, Straka K, Malek N, Zalta AK. Sentenced to shame: moral injury exposure in former lifers. Psychol Trauma. 2024; 15(5):722-730. doi:10.1037/tra0001400
- Orak U, Kelton K, Vaughn MG, Tsai J, Pietrzak RH. Homelessness and contact with the criminal legal system among U.S. combat veterans: an exploration of potential mediating factors. Crim Justice Behav. 2022;50(3):392-409. doi:10.1177/00938548221140352
- Bronson J, Carson EA, Noonan M. Veterans in Prison and Jail, 2011-12. US Department of Justice, Bureau of Justice Statistics; Published December 2015. Accessed March 4, 2025. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
- Maruschak LM, Bronson J, Alper M. Veterans in Prison: Survey of Prison Inmates, 2016. US Department of Justice, Bureau of Justice Statistics; March 2021. Accessed March 4, 2025. https://bjs.ojp.gov/redirect-legacy/content/pub/pdf/vpspi16st.pdf
- Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justiceinvolved veterans. Epidemiol Rev. 2015;37:163-176. doi:10.1093/epirev/mxu003
- Finlay AK, Owens MD, Taylor E, et al. A scoping review of military veterans involved in the criminal justice system and their health and healthcare. Health Justice. 2019;7(1):6. doi:10.1186/s40352-019-0086-9
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Wortzel HS, Binswanger IA, Anderson CA, Adler LE. Suicide among incarcerated veterans. J Am Acad Psychiatry Law. 2009;37(1):82-91.
- Desai A, Holliday R, Borges LM, et al. Facilitating successful reentry among justice-involved veterans: the role of veteran and offender identity. J Psychiatr Pract. 2021;27(1):52-60. doi:10.1097/PRA.0000000000000520
- Asencio EK, Burke PJ. Does incarceration change the criminal identity? A synthesis of labeling and identity theory perspectives on identity change. Sociol Perspect. 2011;54(2):163-182. doi:10.1525/sop.2011.54.2.163
- Borges LM, Desai A, Barnes SM, Johnson JPS. The role of social determinants of health in moral injury: implications and future directions. Curr Treat Options Psychiatry. 2022;9(3):202-214. doi:10.1007/s40501-022-00272-4
- Houle SA, Ein N, Gervasio J, et al. Measuring moral distress and moral injury: a systematic review and content analysis of existing scales. Clin Psychol Rev. 2024;108:102377. doi:10.1016/j.cpr.2023.102377
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the moral injury events scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Zerach G, Ben-Yehuda A, Levi-Belz Y. Prospective associations between psychological factors, potentially morally injurious events, and psychiatric symptoms among Israeli combatants: the roles of ethical leadership and ethical preparation. Psychol Trauma. 2023;15(8):1367-1377. doi:10.1037/tra0001466
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013.
- Weathers FW, Litz BT, Keane TM, Palmeri PA, Marx BP. The PTSD Checklist for DSM-5 (PCL-5). National Center for PTSD. Accessed March 4, 2025. www.ptsd.va.gov
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379-1391. doi:10.1037/pas0000254
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The osttraumatic stress disorder checklist for DSM-5 (PCL- 5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Kroenke K, Spi tzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Brown TA. Confirmatory Factor Analysis for Applied Research. 2nd ed. Guilford Press; 2015.
- Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20(1):141-151. doi:10.1177/001316446002000116
- Schorr Y, Stein NR, Maguen S, Barnes JB, Bosch J, Litz BT. Sources of moral injury among war veterans: a qualitative evaluation. J Clin Psychol. 2018;74(12):2203-2218. doi:10.1002/jclp.22660
Following exposure to potentially morally injurious events (PMIEs), some individuals may experience moral injury, which represents negative psychological, social, behavioral, and occasionally spiritual impacts.1 The consequences of PMIE exposure and moral injury are well documented. Individuals may begin to question the goodness and trustworthiness of oneself, others, or the world.1 Examples of other sequelae include guilt, demoralization, spiritual pain, loss of trust in the self or others, and difficulties with forgiveness.2-6 In addition, prior studies have found that moral injury is associated with an increased risk of suicidal thoughts and behaviors, posttraumatic stress disorder (PTSD) symptoms, spiritual distress, and interpersonal difficulties.7-11
Moral injury was first conceptualized in relation to combat trauma. However in recent years it has been examined in other groups such as health care practitioners, educators, refugees, and law enforcement personnel.12-17 Furthermore, there has been a recent call for the study of moral injury in other understudied groups. One such group is legal-involved individuals, defined as those who are currently involved or previously involved in the criminal justice system (ie, arrests, incarceration, parole, and probation).1,18-22
Many veterans are also involved with the legal system. Specifically, veterans currently comprise about 8% of the incarcerated US population, with an estimated > 180,000 veterans in prisons or jails and even more on parole or probation.23,24 Legal-involved veterans may be at heightened risk for homelessness, suicide, unemployment, and high prevalence rates of psychiatric diagnoses.25-28
Limited research has explored exposure to PMIEs as part of the legal process and the resulting expression of moral injury. The circumstances leading to incarceration, interactions with the US legal system, the environment of prison itself, and the subsequent challenges faced by legal-involved individuals after release all provide ample opportunity for PMIEs to occur.18 For example, engaging in a criminal act may represent a PMIE, particularly in violent offenses that involve harm to another individual. Moreover, the process of being convicted and charged with an offense may serve as a powerful reminder of the PMIE and tie this event to the individual’s identity and future. Furthermore, the physical and social environment of prison itself (eg, being surrounded by other offenders, witnessing the perpetration of violence, participating in violence for survival) presents a myriad of opportunities for PMIEs to occur.18
The consequences of PMIEs in the context of legal involvement may also have bearing on a touchstone of moral injury: changes in one’s schema of the self and world.4 At a societal level, legal-involved individuals are, by definition, deemed “guilty” and held culpable for their offense, which may reinforce a negative change in one’s view of self and the world.29 In line with identity theory, external negative appraisals about legal-involved individuals (eg, they are a danger to society, they cannot be trusted to do the right thing) may influence their self-perception.30 Furthermore, the affective characteristics often found in the context of moral injury (eg, guilt, shame, anger, contempt) may be exacerbated by legal involvement.29 Personal feelings of guilt and shame may be reinforced by receiving a verdict and sentence, as well as the negative perceptions of individuals around them (eg, disapproval from prior sources of social support). Additionally, feelings of betrayal and distrust towards the legal system may arise.
In sum, legal-involved veterans incur increased risk of moral injury due to the potential for exposure to PMIEs across multiple time points (eg, prior to military service, during military service, during arrest/sentencing, during imprisonment, and postincarceration). The stigma that accompanies legal involvement may limit access to treatment or a willingness to seek treatment for distress related to moral injury.29 Additionally, repeated exposure to PMIEs and resulting moral injury may compound over time, potentially exacerbating psychosocial functioning and increasing the risk for psychosocial stressors (eg, homelessness, unemployment) and mental health disorders (eg, depression, substance misuse).31
Although numerous measures of moral injury have been developed, most require that respondents consider a specific context (eg, military experiences).32 Therefore, study of legal-related moral injury requires adaptation of existing instruments to the legal context. The original and most commonly used scale of moral injury is the Moral Injury Events Scale (MIES).33 The MIES scales was originally developed to measure moral injury in military-related contexts but has since been adapted as a measure of exposure to context-specific PMIEs.34
Unfortunately, there are no validated measures for assessing legal-related moral injury. Such a gap in understanding is problematic, as it may impact measurement of the prevalence of PMIEs in both clinical and research settings for this at-risk population. The goal of this study was to conduct a psychometric evaluation of an adapted version of the MIES for legal-involved persons (MIES-LIP).
METHODS
A total of 177 veterans from the US Department of Veterans Affairs (VA) North Texas Health Care System were contacted for study enrollment between November 2020 and June 2021, yielding a final sample of 100 legal-involved veteran participants. Adults aged ≥ 18 years who were US military veterans and had ≥ 1 prior felony conviction resulting in incarceration were included. Participants were excluded if they had symptoms of psychosis that would preclude meaningful participation.
The study collected data on participants’ demographic and clinical characteristics using a semistructured survey instrument. Each participant completed an instructor-led questionnaire in a session that lasted about 1.5 hours. Participants who completed the visit in person received a $50 cash voucher for their time. Participants who were unable to meet with the study coordinator in person were able to complete the visit via telephone and received a $25 digital gift card. Of the total 100 participants, 79 participants completed the interview in person, and 21 completed by telephone. No significant differences were found in assessment measures between administration methods. Written informed consent was obtained during all in-person visits. For those completing via telephone, a waiver of written informed consent was obtained. This study was approved by the VA North Texas Health Care System’s Institutional Review Board.
Measures
The Moral Injury Events Scale (MIES) is a 9-item self-report measure that assesses exposure to PMIEs.33 Respondents rate their agreement with each item on a 6-point Likert scale (strongly disagree to strongly agree), with higher scores indicating greater moral injury. The MIES has a 2-factor structure: Factor 1 has 6 items on perceived transgressions and Factor 2 has 3 items on perceived betrayals.33
Creation of Legal-Involved Moral Injury Measure. To create the MIES-LIP, items and instructions from the MIES were modified to address moral injury in the context of legal involvement.33 Adaptations were finalized following consultation and approval by the authors of the original measure. Specifically, the instructions were changed to: “Please respond to these items based specifically in the context of your involvement with the legal system.” The instructions clarified that legal involvement could include experiences related to committing an offense, legal proceedings and sentencing, incarceration, or transitioning out of the legal system. This differs from the original measure, which focused on military experiences, with instructions stating: “Please respond to these items based specifically in the context of your military service (ie, events and experiences during enlistment, deployment, combat, etc).”
Other measures. The study collected data on demographic characteristics including sex, race and ethnicity, marital status, military service, combat experience, and legal involvement. PTSD symptom severity, based on the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), was assessed using the PTSD Checklist for DSM-5 (PCL-5).35,36 The PCL-5 is a 20-item self-report measure in which item scores are summed to create a total score. The PCL-5 has demonstrated strong psychometric properties, including good internal consistency, test-retest reliability convergent validity, and discriminant validity.37,38
Depressive symptom severity was measured using the Personal Health Questionnaire-9 (PHQ-9).39 The PHQ-9 is a 9-item self-report measure where item scores summed to create a total score. The PHQ-9 has demonstrated strong psychometric properties, including internal consistency and test-retest reliability.39
STATISTICAL METHODS
Descriptive statistics (mean and standard deviation for continuous variables; frequencies and percentages for categorical variables) were used to describe the study sample. Factor analysis was conducted to evaluate the psychometric properties of the MIES-LIP. Confirmatory factor analysis (CFA) was used to determine whether the MEIS-LIP had a similar factor structure to the MIES.40 Criteria for fit indices used for CFA include the Comparative Fit Index (CFI; values of > 0.95 suggest good fit), Tucker-Lewis index (TLI; values of > 0.95 suggest a good fit), root mean square error of approximation (RMSEA; values of ≥ 0.06 suggest good fit), and standardized root mean square residual (SRMR; values of ≥ 0.08 suggest good fit). With insufficient fit, subsequent exploratory factor analysis was conducted using maximum likelihood estimation with an Oblimin rotation. The Kaiser rule and a scree plot were considered when defining the factor structure. Reliability was evaluated using the McDonald omega coefficient test. Convergent validity was assessed through the association between adapted measures and other clinical measures (ie, PCL-5, PHQ-9). In addition, associations between the PCL-5 and PHQ-9 were examined as they related to the MIES and MIES-LIP.
RESULTS
Table 1 describes demographic characteristics of the study sample. Rates of potentially morally injurious experiences and the expression of moral injury in the legal context are presented in Table 2. Witnessing PMIEs while in the legal system was nearly ubiquitous, with > 90% of the sample endorsing this experience. More than half of the sample also endorsed engaging in morally injurious behavior by commission or omission, as well as experiencing betrayal while involved with the legal system.


Factor Analysis
Confirmatory factor analysis (CFA) was utilized to test the factor structure of the adapted MIES-LIP in our sample compared to the published factor structures of the MIES.33 Results did not support the established factor structure. Analysis yielded unacceptable CFI (0.79), TLI (0.70), SRMR (0.14), and RMSEA (0.21). The unsatisfactory results of CFA warranted follow-up exploratory factor analysis (EFA) to examine the factor structure of the moral injury scales in this sample.
EFA of MIES-LIP
The factor structure of the MIES-LIP was examined using EFA. The factorability of the data was examined using the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO value = 0.75) and Bartlett Test of Sphericity (X2 = 525.41; P < .001), both of which suggested that the data were appropriate for factor analysis. The number of factors to retain was selected based on the Kaiser criterion.41 After extraction, an Oblimin rotation was applied, given that we expected factors to be correlated. A 2-factor solution was found, explaining 65.76% of the common variance. All 9 items were retained as they had factor loadings > 0.30. Factor 1, comprised self-directed moral injury questions (3-6). Factor 2 comprised other directed moral injury questions (1, 2, 7-9) (Table 3). The factor correlation coefficient between Factor 1 and Factor 2 was 0.34, which supports utilizing an oblique rotation.

Reliability. We examined the reliability of the adapted MIES-LIP using measures of internal consistency, with both MIES-LIP factors demonstrating good reliability. The internal consistency of both factors of the MIES-LIP were found to be good (self-directed moral injury: Ω = 0.89; other-directed moral injury: Ω = 0.83).
Convergent Validity
Association between moral injury scales. A significant, moderate correlation was observed between all subscales of the MIES and MIES-LIP. Specifically, the self-directed moral injury factor of the MIES-LIP was associated with both the perceived transgressions (r = 0.41, P < .001) and the MIES perceived betrayals factors (r = 0.25, P < .05). Similarly, the other-directed moral injury factor of the MIES-LIP was associated with both the MIES perceived transgressions (r = 0.45, P < .001) and the MIES perceived betrayals factors (r = 0.45, P < .001).
Association with PTSD symptoms. All subscales of both the MIES and MIES-LIP were associated with PTSD symptom severity. The MIES perceived transgressions factor (r = 0.43, P < .001) and the perceived betrayals factor of the MIES (r = 0.39, P < .001) were moderately associated with the PCL-5. Mirroring this, the “self-directed moral injury” factor of the MIESLIP (r = 0.44, P < .001) and the “other-directed moral injury” factor of the MIES-LIP (r = 0.42, P < .001) were also positively associated with PCL-5.
Association with depression symptoms. All subscales of the MIES and MIES-LIP were also associated with depressive symptoms. The MIES perceived transgressions factor (r = 0.27, P < .01) and the MIES perceived betrayals factor (r = 0.23, P < .05) had a small association with the PHQ-9. In addition, the self-directed moral injury factor of the MIES-LIP (r = 0.40, P < .001) and the other-directed moral injury factor of the MIES-LIP (r = 0.31, P < .01) had small to moderate associations with the PCL-5.
DISCUSSION
Potentially morally injurious events appear to be a salient factor affecting legal-involved veterans. Among our sample, the vast majority of legal-involved veterans endorsed experiencing both legal- and military-related PMIEs. Witnessing or participating in a legal-related PMIE appears to be widespread among those who have experienced incarceration. The MIES-LIP yielded a 2-factor structure: self-directed moral injury and other-directed moral injury, in the evaluated population. The MIES-LIP showed similar psychometric performance to the MIES in our sample. Specifically, the MIES-LIP had good reliability and adequate convergent validity. While CFA did not confirm the anticipated factor structure of the MIES-LIP within our sample, EFA showed similarities in factor structure between the original and adapted measures. While further research and validation are needed, preliminary results show promise of the MIES-LIP in assessing legal-related moral injury.
Originally, the MIES was found to have a 2-factor structure, defined by perceived transgressions and perceived betrayals.33 However, additional research has identified a 3-factor structure, where the betrayal factor is maintained, and the transgressions factor is divided into transgressions by others and by self.8 The factor structure of the MIES-LIP was more closely related to the factor structure, with transgressions by others and betrayal mapped onto the same factor (ie, other-directed moral injury).8 While further research is needed, it is possible that the nature of morally injurious events experienced in legal contexts are experienced more in terms of self vs other, compared to morally injurious events experienced by veterans or active-duty service members.
Accurately identifying the types of moral injury experienced in a legal context may be important for determining the differences in drivers of legal-related moral injury compared to military-related moral injury. For example, self-directed moral injury in legal contexts may include a variety of actions the individual initiated that led to conviction and incarceration (eg, a criminal offense), as well as behaviors performed or witnessed while incarcerated (eg, engaging in violence). Inconsistent with military populations where other-directed moral injury clusters with self-directed moral injury, other-directed moral injury clustered with betrayal in legal contexts in our sample. This discrepancy may result from differences in identification with the military vs legal system. When veterans witness fellow service members engaging in PMIEs (eg, physical violence towards civilians in a military setting), this may be similar to self-directed moral injury due to the veteran’s identification with the same military system as the perpetrator.42 When legal-involved veterans witness other incarcerated individuals engaging in PMIEs (eg, physical violence toward other inmates), this may be experienced as similar to betrayal due to lack of personal identification with the criminal-legal system. Additional research is needed to better understand how self- and other-related moral injury are associated with betrayal in legal contexts.
Another potential driver of legal-related moral injury may be culpability. In order for moral injury to occur, an individual must perceive that something has taken place that deeply violated their sense of right and wrong.1 In terms of criminal offenses or even engaging in violent behavior while incarcerated, the potential for moral injury may differ based on whether an individual views themselves as culpable for the act(s).29 This may further distinguish between self-directed and other-directed moral injury in legal contexts. In situations where the individual views themselves as culpable, self-directed moral injury may be higher. In situations where the individual does not view themselves as culpable, other-directed moral injury may be higher based on the perception that the legal system is unfairly punishing them. Further research is needed to clarify how an individual’s view of their culpability relates to moral injury, as well as to elucidate which aspects of military service and legal involvement are most closely associated with moral injury among legal-involved veterans.
While this study treated legal-related and military-related moral injury as distinct, it is possible moral injury may have a cumulative effect over time with individuals experiencing morally injurious events across different contexts (eg, military, legal involvement). This, in turn, may compound risk for moral injury. These cumulative experiences may result in increased negative outcomes such as exacerbated psychiatric symptoms, substance misuse, and elevated suicide risk. Future studies should examine differences between groups who have experienced moral injury in differing contexts, as well as those with multiple sources of moral injury.
Limitations
The sample for this study included only veterans. The number of veterans incarcerated is large and the focus on veterans also allowed for a more robust comparison of moral injury related to the legal system and the more traditional military-related moral injury. However, the generalizability of the findings to nonveterans cannot be assured. The study used a relatively small sample (N = 100), which was overwhelmingly male. Although the PCL-5 was utilized to examine traumatic stress symptoms, this measure was not anchored to a specific criterion A trauma nor was it anchored specifically to a morally injurious experience. For all participants, their most recent military service preceded their most recent legal involvement which could affect the associations between variables. Furthermore, while all participants endorsed prior legal involvement, many participants reported no combat exposure.
CONCLUSIONS
This study resulted in several key findings. First, legal-involved veterans endorsed high rates of experiencing legal-related morally injurious experiences. Second, our adapted measure displayed adequate psychometric strength and suggests that legal-related moral injury is a salient and distinct phenomenon affecting legal-involved veterans. These items may not capture all the nuances of legal-related moral injury. Qualitative interviews with legal-involved persons may help identify relevant areas of legal-related moral injury not reflected in the current instrument. The MIES-LIP represents a practical measure that may help clinicians identify and address legal-related moral injury when working with legal-involved veterans. Given the high prevalence of PMIEs among legal-involved veterans, further examination of whether current interventions for moral injury and novel treatments being developed are effective for this population is needed.
Following exposure to potentially morally injurious events (PMIEs), some individuals may experience moral injury, which represents negative psychological, social, behavioral, and occasionally spiritual impacts.1 The consequences of PMIE exposure and moral injury are well documented. Individuals may begin to question the goodness and trustworthiness of oneself, others, or the world.1 Examples of other sequelae include guilt, demoralization, spiritual pain, loss of trust in the self or others, and difficulties with forgiveness.2-6 In addition, prior studies have found that moral injury is associated with an increased risk of suicidal thoughts and behaviors, posttraumatic stress disorder (PTSD) symptoms, spiritual distress, and interpersonal difficulties.7-11
Moral injury was first conceptualized in relation to combat trauma. However in recent years it has been examined in other groups such as health care practitioners, educators, refugees, and law enforcement personnel.12-17 Furthermore, there has been a recent call for the study of moral injury in other understudied groups. One such group is legal-involved individuals, defined as those who are currently involved or previously involved in the criminal justice system (ie, arrests, incarceration, parole, and probation).1,18-22
Many veterans are also involved with the legal system. Specifically, veterans currently comprise about 8% of the incarcerated US population, with an estimated > 180,000 veterans in prisons or jails and even more on parole or probation.23,24 Legal-involved veterans may be at heightened risk for homelessness, suicide, unemployment, and high prevalence rates of psychiatric diagnoses.25-28
Limited research has explored exposure to PMIEs as part of the legal process and the resulting expression of moral injury. The circumstances leading to incarceration, interactions with the US legal system, the environment of prison itself, and the subsequent challenges faced by legal-involved individuals after release all provide ample opportunity for PMIEs to occur.18 For example, engaging in a criminal act may represent a PMIE, particularly in violent offenses that involve harm to another individual. Moreover, the process of being convicted and charged with an offense may serve as a powerful reminder of the PMIE and tie this event to the individual’s identity and future. Furthermore, the physical and social environment of prison itself (eg, being surrounded by other offenders, witnessing the perpetration of violence, participating in violence for survival) presents a myriad of opportunities for PMIEs to occur.18
The consequences of PMIEs in the context of legal involvement may also have bearing on a touchstone of moral injury: changes in one’s schema of the self and world.4 At a societal level, legal-involved individuals are, by definition, deemed “guilty” and held culpable for their offense, which may reinforce a negative change in one’s view of self and the world.29 In line with identity theory, external negative appraisals about legal-involved individuals (eg, they are a danger to society, they cannot be trusted to do the right thing) may influence their self-perception.30 Furthermore, the affective characteristics often found in the context of moral injury (eg, guilt, shame, anger, contempt) may be exacerbated by legal involvement.29 Personal feelings of guilt and shame may be reinforced by receiving a verdict and sentence, as well as the negative perceptions of individuals around them (eg, disapproval from prior sources of social support). Additionally, feelings of betrayal and distrust towards the legal system may arise.
In sum, legal-involved veterans incur increased risk of moral injury due to the potential for exposure to PMIEs across multiple time points (eg, prior to military service, during military service, during arrest/sentencing, during imprisonment, and postincarceration). The stigma that accompanies legal involvement may limit access to treatment or a willingness to seek treatment for distress related to moral injury.29 Additionally, repeated exposure to PMIEs and resulting moral injury may compound over time, potentially exacerbating psychosocial functioning and increasing the risk for psychosocial stressors (eg, homelessness, unemployment) and mental health disorders (eg, depression, substance misuse).31
Although numerous measures of moral injury have been developed, most require that respondents consider a specific context (eg, military experiences).32 Therefore, study of legal-related moral injury requires adaptation of existing instruments to the legal context. The original and most commonly used scale of moral injury is the Moral Injury Events Scale (MIES).33 The MIES scales was originally developed to measure moral injury in military-related contexts but has since been adapted as a measure of exposure to context-specific PMIEs.34
Unfortunately, there are no validated measures for assessing legal-related moral injury. Such a gap in understanding is problematic, as it may impact measurement of the prevalence of PMIEs in both clinical and research settings for this at-risk population. The goal of this study was to conduct a psychometric evaluation of an adapted version of the MIES for legal-involved persons (MIES-LIP).
METHODS
A total of 177 veterans from the US Department of Veterans Affairs (VA) North Texas Health Care System were contacted for study enrollment between November 2020 and June 2021, yielding a final sample of 100 legal-involved veteran participants. Adults aged ≥ 18 years who were US military veterans and had ≥ 1 prior felony conviction resulting in incarceration were included. Participants were excluded if they had symptoms of psychosis that would preclude meaningful participation.
The study collected data on participants’ demographic and clinical characteristics using a semistructured survey instrument. Each participant completed an instructor-led questionnaire in a session that lasted about 1.5 hours. Participants who completed the visit in person received a $50 cash voucher for their time. Participants who were unable to meet with the study coordinator in person were able to complete the visit via telephone and received a $25 digital gift card. Of the total 100 participants, 79 participants completed the interview in person, and 21 completed by telephone. No significant differences were found in assessment measures between administration methods. Written informed consent was obtained during all in-person visits. For those completing via telephone, a waiver of written informed consent was obtained. This study was approved by the VA North Texas Health Care System’s Institutional Review Board.
Measures
The Moral Injury Events Scale (MIES) is a 9-item self-report measure that assesses exposure to PMIEs.33 Respondents rate their agreement with each item on a 6-point Likert scale (strongly disagree to strongly agree), with higher scores indicating greater moral injury. The MIES has a 2-factor structure: Factor 1 has 6 items on perceived transgressions and Factor 2 has 3 items on perceived betrayals.33
Creation of Legal-Involved Moral Injury Measure. To create the MIES-LIP, items and instructions from the MIES were modified to address moral injury in the context of legal involvement.33 Adaptations were finalized following consultation and approval by the authors of the original measure. Specifically, the instructions were changed to: “Please respond to these items based specifically in the context of your involvement with the legal system.” The instructions clarified that legal involvement could include experiences related to committing an offense, legal proceedings and sentencing, incarceration, or transitioning out of the legal system. This differs from the original measure, which focused on military experiences, with instructions stating: “Please respond to these items based specifically in the context of your military service (ie, events and experiences during enlistment, deployment, combat, etc).”
Other measures. The study collected data on demographic characteristics including sex, race and ethnicity, marital status, military service, combat experience, and legal involvement. PTSD symptom severity, based on the criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), was assessed using the PTSD Checklist for DSM-5 (PCL-5).35,36 The PCL-5 is a 20-item self-report measure in which item scores are summed to create a total score. The PCL-5 has demonstrated strong psychometric properties, including good internal consistency, test-retest reliability convergent validity, and discriminant validity.37,38
Depressive symptom severity was measured using the Personal Health Questionnaire-9 (PHQ-9).39 The PHQ-9 is a 9-item self-report measure where item scores summed to create a total score. The PHQ-9 has demonstrated strong psychometric properties, including internal consistency and test-retest reliability.39
STATISTICAL METHODS
Descriptive statistics (mean and standard deviation for continuous variables; frequencies and percentages for categorical variables) were used to describe the study sample. Factor analysis was conducted to evaluate the psychometric properties of the MIES-LIP. Confirmatory factor analysis (CFA) was used to determine whether the MEIS-LIP had a similar factor structure to the MIES.40 Criteria for fit indices used for CFA include the Comparative Fit Index (CFI; values of > 0.95 suggest good fit), Tucker-Lewis index (TLI; values of > 0.95 suggest a good fit), root mean square error of approximation (RMSEA; values of ≥ 0.06 suggest good fit), and standardized root mean square residual (SRMR; values of ≥ 0.08 suggest good fit). With insufficient fit, subsequent exploratory factor analysis was conducted using maximum likelihood estimation with an Oblimin rotation. The Kaiser rule and a scree plot were considered when defining the factor structure. Reliability was evaluated using the McDonald omega coefficient test. Convergent validity was assessed through the association between adapted measures and other clinical measures (ie, PCL-5, PHQ-9). In addition, associations between the PCL-5 and PHQ-9 were examined as they related to the MIES and MIES-LIP.
RESULTS
Table 1 describes demographic characteristics of the study sample. Rates of potentially morally injurious experiences and the expression of moral injury in the legal context are presented in Table 2. Witnessing PMIEs while in the legal system was nearly ubiquitous, with > 90% of the sample endorsing this experience. More than half of the sample also endorsed engaging in morally injurious behavior by commission or omission, as well as experiencing betrayal while involved with the legal system.


Factor Analysis
Confirmatory factor analysis (CFA) was utilized to test the factor structure of the adapted MIES-LIP in our sample compared to the published factor structures of the MIES.33 Results did not support the established factor structure. Analysis yielded unacceptable CFI (0.79), TLI (0.70), SRMR (0.14), and RMSEA (0.21). The unsatisfactory results of CFA warranted follow-up exploratory factor analysis (EFA) to examine the factor structure of the moral injury scales in this sample.
EFA of MIES-LIP
The factor structure of the MIES-LIP was examined using EFA. The factorability of the data was examined using the Kaiser-Meyer-Olkin Measure of Sampling Adequacy (KMO value = 0.75) and Bartlett Test of Sphericity (X2 = 525.41; P < .001), both of which suggested that the data were appropriate for factor analysis. The number of factors to retain was selected based on the Kaiser criterion.41 After extraction, an Oblimin rotation was applied, given that we expected factors to be correlated. A 2-factor solution was found, explaining 65.76% of the common variance. All 9 items were retained as they had factor loadings > 0.30. Factor 1, comprised self-directed moral injury questions (3-6). Factor 2 comprised other directed moral injury questions (1, 2, 7-9) (Table 3). The factor correlation coefficient between Factor 1 and Factor 2 was 0.34, which supports utilizing an oblique rotation.

Reliability. We examined the reliability of the adapted MIES-LIP using measures of internal consistency, with both MIES-LIP factors demonstrating good reliability. The internal consistency of both factors of the MIES-LIP were found to be good (self-directed moral injury: Ω = 0.89; other-directed moral injury: Ω = 0.83).
Convergent Validity
Association between moral injury scales. A significant, moderate correlation was observed between all subscales of the MIES and MIES-LIP. Specifically, the self-directed moral injury factor of the MIES-LIP was associated with both the perceived transgressions (r = 0.41, P < .001) and the MIES perceived betrayals factors (r = 0.25, P < .05). Similarly, the other-directed moral injury factor of the MIES-LIP was associated with both the MIES perceived transgressions (r = 0.45, P < .001) and the MIES perceived betrayals factors (r = 0.45, P < .001).
Association with PTSD symptoms. All subscales of both the MIES and MIES-LIP were associated with PTSD symptom severity. The MIES perceived transgressions factor (r = 0.43, P < .001) and the perceived betrayals factor of the MIES (r = 0.39, P < .001) were moderately associated with the PCL-5. Mirroring this, the “self-directed moral injury” factor of the MIESLIP (r = 0.44, P < .001) and the “other-directed moral injury” factor of the MIES-LIP (r = 0.42, P < .001) were also positively associated with PCL-5.
Association with depression symptoms. All subscales of the MIES and MIES-LIP were also associated with depressive symptoms. The MIES perceived transgressions factor (r = 0.27, P < .01) and the MIES perceived betrayals factor (r = 0.23, P < .05) had a small association with the PHQ-9. In addition, the self-directed moral injury factor of the MIES-LIP (r = 0.40, P < .001) and the other-directed moral injury factor of the MIES-LIP (r = 0.31, P < .01) had small to moderate associations with the PCL-5.
DISCUSSION
Potentially morally injurious events appear to be a salient factor affecting legal-involved veterans. Among our sample, the vast majority of legal-involved veterans endorsed experiencing both legal- and military-related PMIEs. Witnessing or participating in a legal-related PMIE appears to be widespread among those who have experienced incarceration. The MIES-LIP yielded a 2-factor structure: self-directed moral injury and other-directed moral injury, in the evaluated population. The MIES-LIP showed similar psychometric performance to the MIES in our sample. Specifically, the MIES-LIP had good reliability and adequate convergent validity. While CFA did not confirm the anticipated factor structure of the MIES-LIP within our sample, EFA showed similarities in factor structure between the original and adapted measures. While further research and validation are needed, preliminary results show promise of the MIES-LIP in assessing legal-related moral injury.
Originally, the MIES was found to have a 2-factor structure, defined by perceived transgressions and perceived betrayals.33 However, additional research has identified a 3-factor structure, where the betrayal factor is maintained, and the transgressions factor is divided into transgressions by others and by self.8 The factor structure of the MIES-LIP was more closely related to the factor structure, with transgressions by others and betrayal mapped onto the same factor (ie, other-directed moral injury).8 While further research is needed, it is possible that the nature of morally injurious events experienced in legal contexts are experienced more in terms of self vs other, compared to morally injurious events experienced by veterans or active-duty service members.
Accurately identifying the types of moral injury experienced in a legal context may be important for determining the differences in drivers of legal-related moral injury compared to military-related moral injury. For example, self-directed moral injury in legal contexts may include a variety of actions the individual initiated that led to conviction and incarceration (eg, a criminal offense), as well as behaviors performed or witnessed while incarcerated (eg, engaging in violence). Inconsistent with military populations where other-directed moral injury clusters with self-directed moral injury, other-directed moral injury clustered with betrayal in legal contexts in our sample. This discrepancy may result from differences in identification with the military vs legal system. When veterans witness fellow service members engaging in PMIEs (eg, physical violence towards civilians in a military setting), this may be similar to self-directed moral injury due to the veteran’s identification with the same military system as the perpetrator.42 When legal-involved veterans witness other incarcerated individuals engaging in PMIEs (eg, physical violence toward other inmates), this may be experienced as similar to betrayal due to lack of personal identification with the criminal-legal system. Additional research is needed to better understand how self- and other-related moral injury are associated with betrayal in legal contexts.
Another potential driver of legal-related moral injury may be culpability. In order for moral injury to occur, an individual must perceive that something has taken place that deeply violated their sense of right and wrong.1 In terms of criminal offenses or even engaging in violent behavior while incarcerated, the potential for moral injury may differ based on whether an individual views themselves as culpable for the act(s).29 This may further distinguish between self-directed and other-directed moral injury in legal contexts. In situations where the individual views themselves as culpable, self-directed moral injury may be higher. In situations where the individual does not view themselves as culpable, other-directed moral injury may be higher based on the perception that the legal system is unfairly punishing them. Further research is needed to clarify how an individual’s view of their culpability relates to moral injury, as well as to elucidate which aspects of military service and legal involvement are most closely associated with moral injury among legal-involved veterans.
While this study treated legal-related and military-related moral injury as distinct, it is possible moral injury may have a cumulative effect over time with individuals experiencing morally injurious events across different contexts (eg, military, legal involvement). This, in turn, may compound risk for moral injury. These cumulative experiences may result in increased negative outcomes such as exacerbated psychiatric symptoms, substance misuse, and elevated suicide risk. Future studies should examine differences between groups who have experienced moral injury in differing contexts, as well as those with multiple sources of moral injury.
Limitations
The sample for this study included only veterans. The number of veterans incarcerated is large and the focus on veterans also allowed for a more robust comparison of moral injury related to the legal system and the more traditional military-related moral injury. However, the generalizability of the findings to nonveterans cannot be assured. The study used a relatively small sample (N = 100), which was overwhelmingly male. Although the PCL-5 was utilized to examine traumatic stress symptoms, this measure was not anchored to a specific criterion A trauma nor was it anchored specifically to a morally injurious experience. For all participants, their most recent military service preceded their most recent legal involvement which could affect the associations between variables. Furthermore, while all participants endorsed prior legal involvement, many participants reported no combat exposure.
CONCLUSIONS
This study resulted in several key findings. First, legal-involved veterans endorsed high rates of experiencing legal-related morally injurious experiences. Second, our adapted measure displayed adequate psychometric strength and suggests that legal-related moral injury is a salient and distinct phenomenon affecting legal-involved veterans. These items may not capture all the nuances of legal-related moral injury. Qualitative interviews with legal-involved persons may help identify relevant areas of legal-related moral injury not reflected in the current instrument. The MIES-LIP represents a practical measure that may help clinicians identify and address legal-related moral injury when working with legal-involved veterans. Given the high prevalence of PMIEs among legal-involved veterans, further examination of whether current interventions for moral injury and novel treatments being developed are effective for this population is needed.
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Currier JM, Holland JM, Malott J. Moral injury, meaning making, and mental health in returning veterans. J Clin Psychol. 2015;71(3):229-240. doi:10.1002/jclp.22134
- Jinkerson JD. Defining and assessing moral injury: a syndrome perspective. Traumatology. 2016;22(2):122-130. doi:10.1037/trm0000069
- Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
- Maguen S, Litz B. Moral injury in veterans of war. PTSD Res Q. 2012;23(1):1-6. www.vva1071.org/uploads/3/4/4/6/34460116/moral_injury_in_veterans_of_war.pdf
- Drescher KD, Foy DW, Kelly C, Leshner A, Schutz K, Litz B. An exploration of the viability and usefulness of the construct of moral injury in war veterans. Traumatology. 2011;17(1):8-13. doi:10.1177/1534765610395615
- Wisco BE, Marx BP, May CL, et al. Moral injury in U.S. combat veterans: results from the national health and resilience in veterans study. Depress Anxiety. 2017; 34(4):340-347. doi:10.1002/da.22614
- Bryan CJ, Bryan AO, Anestis MD, et al. Measuring moral injury: psychometric properties of the moral injury events scale in two military samples. Assessment. 2016;23(5):557- 570. doi:10.1177/1073191115590855
- Currier JM, Smith PN, Kuhlman S. Assessing the unique role of religious coping in suicidal behavior among U.S. Iraq and Afghanistan veterans. Psychol Relig Spiritual. 2017;9(1):118-123. doi:10.1037/rel0000055
- Kopacz MS, Connery AL, Bishop TM, et al. Moral injury: a new challenge for complementary and alternative medicine. Complement Ther Med. 2016;24:29-33. doi:10.1016/j.ctim.2015.11.003
- Vargas AF, Hanson T, Kraus D, Drescher K, Foy D. Moral injury themes in combat veterans’ narrative responses from the national vietnam veterans’ readjustment study. Traumatology. 2013;19(3):243-250. doi:10.1177/1534765613476099
- Borges LM, Barnes SM, Farnsworth JK, Bahraini NH, Brenner LA. A commentary on moral injury among health care providers during the COVID-19 pandemic. Psychol Trauma. 2020;12(S1):S138-S140. doi:10.1037/tra0000698
- Borges LM, Holliday R, Barnes SM, et al. A longitudinal analysis of the role of potentially morally injurious events on COVID-19-related psychosocial functioning among healthcare providers. PLoS One. 2021;16(11):e0260033. doi:10.1371/journal.pone.0260033
- Currier JM, Holland JM, Rojas-Flores L, Herrera S, Foy D. Morally injurious experiences and meaning in Salvadorian teachers exposed to violence. Psychol Trauma. 2015;7(1):24-33. doi:10.1037/a0034092
- Nickerson A, Schnyder U, Bryant RA, Schick M, Mueller J, Morina N. Moral injury in traumatized refugees. Psychother Psychosom. 2015;84(2):122-123. doi:10.1159/000369353
- Papazoglou K, Chopko B. The role of moral suffering (moral distress and moral injury) in police compassion fatigue and PTSD: An unexplored topic. Front Psychol. 2017;8:1999. doi:10.3389/fpsyg.2017.01999
- Papazoglou K, Blumberg DM, Chiongbian VB, et al. The role of moral injury in PTSD among law enforcement officers: a brief report. Front Psychol. 2020;11:310. doi:10.3389/fpsyg.2020.00310
- Martin WB, Holliday R, LePage JP. Trauma and diversity: moral injury among justice involved veterans: an understudied clinical concern. Stresspoints. 2020;33(5).
- Currier JM, Drescher KD, Nieuwsma J. Future directions for addressing moral injury in clinical practice: concluding comments. In: Currier JM, Drescher KD, Nieuwsma J, eds. Addressing Moral Injury in Clinical Practice. American Psychological Association; 2021:261-271. doi:10.1037/0000204-015
- Alexander AR, Mendez L, Kerig PK. Moral injury as a transdiagnostic risk factor for mental health problems in detained youth. Crim Justice Behav. 2023;51(2):194-212. doi:10.1177/00938548231208203
- DeCaro JB, Straka K, Malek N, Zalta AK. Sentenced to shame: moral injury exposure in former lifers. Psychol Trauma. 2024; 15(5):722-730. doi:10.1037/tra0001400
- Orak U, Kelton K, Vaughn MG, Tsai J, Pietrzak RH. Homelessness and contact with the criminal legal system among U.S. combat veterans: an exploration of potential mediating factors. Crim Justice Behav. 2022;50(3):392-409. doi:10.1177/00938548221140352
- Bronson J, Carson EA, Noonan M. Veterans in Prison and Jail, 2011-12. US Department of Justice, Bureau of Justice Statistics; Published December 2015. Accessed March 4, 2025. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
- Maruschak LM, Bronson J, Alper M. Veterans in Prison: Survey of Prison Inmates, 2016. US Department of Justice, Bureau of Justice Statistics; March 2021. Accessed March 4, 2025. https://bjs.ojp.gov/redirect-legacy/content/pub/pdf/vpspi16st.pdf
- Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justiceinvolved veterans. Epidemiol Rev. 2015;37:163-176. doi:10.1093/epirev/mxu003
- Finlay AK, Owens MD, Taylor E, et al. A scoping review of military veterans involved in the criminal justice system and their health and healthcare. Health Justice. 2019;7(1):6. doi:10.1186/s40352-019-0086-9
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Wortzel HS, Binswanger IA, Anderson CA, Adler LE. Suicide among incarcerated veterans. J Am Acad Psychiatry Law. 2009;37(1):82-91.
- Desai A, Holliday R, Borges LM, et al. Facilitating successful reentry among justice-involved veterans: the role of veteran and offender identity. J Psychiatr Pract. 2021;27(1):52-60. doi:10.1097/PRA.0000000000000520
- Asencio EK, Burke PJ. Does incarceration change the criminal identity? A synthesis of labeling and identity theory perspectives on identity change. Sociol Perspect. 2011;54(2):163-182. doi:10.1525/sop.2011.54.2.163
- Borges LM, Desai A, Barnes SM, Johnson JPS. The role of social determinants of health in moral injury: implications and future directions. Curr Treat Options Psychiatry. 2022;9(3):202-214. doi:10.1007/s40501-022-00272-4
- Houle SA, Ein N, Gervasio J, et al. Measuring moral distress and moral injury: a systematic review and content analysis of existing scales. Clin Psychol Rev. 2024;108:102377. doi:10.1016/j.cpr.2023.102377
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the moral injury events scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Zerach G, Ben-Yehuda A, Levi-Belz Y. Prospective associations between psychological factors, potentially morally injurious events, and psychiatric symptoms among Israeli combatants: the roles of ethical leadership and ethical preparation. Psychol Trauma. 2023;15(8):1367-1377. doi:10.1037/tra0001466
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013.
- Weathers FW, Litz BT, Keane TM, Palmeri PA, Marx BP. The PTSD Checklist for DSM-5 (PCL-5). National Center for PTSD. Accessed March 4, 2025. www.ptsd.va.gov
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379-1391. doi:10.1037/pas0000254
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The osttraumatic stress disorder checklist for DSM-5 (PCL- 5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Kroenke K, Spi tzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Brown TA. Confirmatory Factor Analysis for Applied Research. 2nd ed. Guilford Press; 2015.
- Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20(1):141-151. doi:10.1177/001316446002000116
- Schorr Y, Stein NR, Maguen S, Barnes JB, Bosch J, Litz BT. Sources of moral injury among war veterans: a qualitative evaluation. J Clin Psychol. 2018;74(12):2203-2218. doi:10.1002/jclp.22660
- Griffin BJ, Purcell N, Burkman K, et al. Moral injury: an integrative review. J Trauma Stress. 2019;32(3):350-362. doi:10.1002/jts.22362
- Currier JM, Holland JM, Malott J. Moral injury, meaning making, and mental health in returning veterans. J Clin Psychol. 2015;71(3):229-240. doi:10.1002/jclp.22134
- Jinkerson JD. Defining and assessing moral injury: a syndrome perspective. Traumatology. 2016;22(2):122-130. doi:10.1037/trm0000069
- Litz BT, Stein N, Delaney E, et al. Moral injury and moral repair in war veterans: a preliminary model and intervention strategy. Clin Psychol Rev. 2009;29(8):695-706. doi:10.1016/j.cpr.2009.07.003
- Maguen S, Litz B. Moral injury in veterans of war. PTSD Res Q. 2012;23(1):1-6. www.vva1071.org/uploads/3/4/4/6/34460116/moral_injury_in_veterans_of_war.pdf
- Drescher KD, Foy DW, Kelly C, Leshner A, Schutz K, Litz B. An exploration of the viability and usefulness of the construct of moral injury in war veterans. Traumatology. 2011;17(1):8-13. doi:10.1177/1534765610395615
- Wisco BE, Marx BP, May CL, et al. Moral injury in U.S. combat veterans: results from the national health and resilience in veterans study. Depress Anxiety. 2017; 34(4):340-347. doi:10.1002/da.22614
- Bryan CJ, Bryan AO, Anestis MD, et al. Measuring moral injury: psychometric properties of the moral injury events scale in two military samples. Assessment. 2016;23(5):557- 570. doi:10.1177/1073191115590855
- Currier JM, Smith PN, Kuhlman S. Assessing the unique role of religious coping in suicidal behavior among U.S. Iraq and Afghanistan veterans. Psychol Relig Spiritual. 2017;9(1):118-123. doi:10.1037/rel0000055
- Kopacz MS, Connery AL, Bishop TM, et al. Moral injury: a new challenge for complementary and alternative medicine. Complement Ther Med. 2016;24:29-33. doi:10.1016/j.ctim.2015.11.003
- Vargas AF, Hanson T, Kraus D, Drescher K, Foy D. Moral injury themes in combat veterans’ narrative responses from the national vietnam veterans’ readjustment study. Traumatology. 2013;19(3):243-250. doi:10.1177/1534765613476099
- Borges LM, Barnes SM, Farnsworth JK, Bahraini NH, Brenner LA. A commentary on moral injury among health care providers during the COVID-19 pandemic. Psychol Trauma. 2020;12(S1):S138-S140. doi:10.1037/tra0000698
- Borges LM, Holliday R, Barnes SM, et al. A longitudinal analysis of the role of potentially morally injurious events on COVID-19-related psychosocial functioning among healthcare providers. PLoS One. 2021;16(11):e0260033. doi:10.1371/journal.pone.0260033
- Currier JM, Holland JM, Rojas-Flores L, Herrera S, Foy D. Morally injurious experiences and meaning in Salvadorian teachers exposed to violence. Psychol Trauma. 2015;7(1):24-33. doi:10.1037/a0034092
- Nickerson A, Schnyder U, Bryant RA, Schick M, Mueller J, Morina N. Moral injury in traumatized refugees. Psychother Psychosom. 2015;84(2):122-123. doi:10.1159/000369353
- Papazoglou K, Chopko B. The role of moral suffering (moral distress and moral injury) in police compassion fatigue and PTSD: An unexplored topic. Front Psychol. 2017;8:1999. doi:10.3389/fpsyg.2017.01999
- Papazoglou K, Blumberg DM, Chiongbian VB, et al. The role of moral injury in PTSD among law enforcement officers: a brief report. Front Psychol. 2020;11:310. doi:10.3389/fpsyg.2020.00310
- Martin WB, Holliday R, LePage JP. Trauma and diversity: moral injury among justice involved veterans: an understudied clinical concern. Stresspoints. 2020;33(5).
- Currier JM, Drescher KD, Nieuwsma J. Future directions for addressing moral injury in clinical practice: concluding comments. In: Currier JM, Drescher KD, Nieuwsma J, eds. Addressing Moral Injury in Clinical Practice. American Psychological Association; 2021:261-271. doi:10.1037/0000204-015
- Alexander AR, Mendez L, Kerig PK. Moral injury as a transdiagnostic risk factor for mental health problems in detained youth. Crim Justice Behav. 2023;51(2):194-212. doi:10.1177/00938548231208203
- DeCaro JB, Straka K, Malek N, Zalta AK. Sentenced to shame: moral injury exposure in former lifers. Psychol Trauma. 2024; 15(5):722-730. doi:10.1037/tra0001400
- Orak U, Kelton K, Vaughn MG, Tsai J, Pietrzak RH. Homelessness and contact with the criminal legal system among U.S. combat veterans: an exploration of potential mediating factors. Crim Justice Behav. 2022;50(3):392-409. doi:10.1177/00938548221140352
- Bronson J, Carson EA, Noonan M. Veterans in Prison and Jail, 2011-12. US Department of Justice, Bureau of Justice Statistics; Published December 2015. Accessed March 4, 2025. https://bjs.ojp.gov/content/pub/pdf/vpj1112.pdf
- Maruschak LM, Bronson J, Alper M. Veterans in Prison: Survey of Prison Inmates, 2016. US Department of Justice, Bureau of Justice Statistics; March 2021. Accessed March 4, 2025. https://bjs.ojp.gov/redirect-legacy/content/pub/pdf/vpspi16st.pdf
- Blodgett JC, Avoundjian T, Finlay AK, et al. Prevalence of mental health disorders among justiceinvolved veterans. Epidemiol Rev. 2015;37:163-176. doi:10.1093/epirev/mxu003
- Finlay AK, Owens MD, Taylor E, et al. A scoping review of military veterans involved in the criminal justice system and their health and healthcare. Health Justice. 2019;7(1):6. doi:10.1186/s40352-019-0086-9
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Wortzel HS, Binswanger IA, Anderson CA, Adler LE. Suicide among incarcerated veterans. J Am Acad Psychiatry Law. 2009;37(1):82-91.
- Desai A, Holliday R, Borges LM, et al. Facilitating successful reentry among justice-involved veterans: the role of veteran and offender identity. J Psychiatr Pract. 2021;27(1):52-60. doi:10.1097/PRA.0000000000000520
- Asencio EK, Burke PJ. Does incarceration change the criminal identity? A synthesis of labeling and identity theory perspectives on identity change. Sociol Perspect. 2011;54(2):163-182. doi:10.1525/sop.2011.54.2.163
- Borges LM, Desai A, Barnes SM, Johnson JPS. The role of social determinants of health in moral injury: implications and future directions. Curr Treat Options Psychiatry. 2022;9(3):202-214. doi:10.1007/s40501-022-00272-4
- Houle SA, Ein N, Gervasio J, et al. Measuring moral distress and moral injury: a systematic review and content analysis of existing scales. Clin Psychol Rev. 2024;108:102377. doi:10.1016/j.cpr.2023.102377
- Nash WP, Marino Carper TL, Mills MA, Au T, Goldsmith A, Litz BT. Psychometric evaluation of the moral injury events scale. Mil Med. 2013;178(6):646-652. doi:10.7205/MILMED-D-13-00017
- Zerach G, Ben-Yehuda A, Levi-Belz Y. Prospective associations between psychological factors, potentially morally injurious events, and psychiatric symptoms among Israeli combatants: the roles of ethical leadership and ethical preparation. Psychol Trauma. 2023;15(8):1367-1377. doi:10.1037/tra0001466
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; 2013.
- Weathers FW, Litz BT, Keane TM, Palmeri PA, Marx BP. The PTSD Checklist for DSM-5 (PCL-5). National Center for PTSD. Accessed March 4, 2025. www.ptsd.va.gov
- Bovin MJ, Marx BP, Weathers FW, et al. Psychometric properties of the PTSD checklist for diagnostic and statistical manual of mental disorders-fifth edition (PCL-5) in veterans. Psychol Assess. 2016;28(11):1379-1391. doi:10.1037/pas0000254
- Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The osttraumatic stress disorder checklist for DSM-5 (PCL- 5): development and initial psychometric evaluation. J Trauma Stress. 2015;28(6):489-498. doi:10.1002/jts.22059
- Kroenke K, Spi tzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Brown TA. Confirmatory Factor Analysis for Applied Research. 2nd ed. Guilford Press; 2015.
- Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Meas. 1960;20(1):141-151. doi:10.1177/001316446002000116
- Schorr Y, Stein NR, Maguen S, Barnes JB, Bosch J, Litz BT. Sources of moral injury among war veterans: a qualitative evaluation. J Clin Psychol. 2018;74(12):2203-2218. doi:10.1002/jclp.22660
Examining Moral Injury in Legal-Involved Veterans: Psychometric Properties of the Moral Injury Events Scale
Examining Moral Injury in Legal-Involved Veterans: Psychometric Properties of the Moral Injury Events Scale
Leveraging Community Asset Mapping to Improve Suicide Prevention for Veterans
Leveraging Community Asset Mapping to Improve Suicide Prevention for Veterans
Suicide prevention is the leading clinical priority for the US Department of Veterans Affairs (VA).1 An average of 18 veterans died by suicide each day in 2021.2 Numerous risk factors for veteran suicide have been identified, including mental health disorders, comorbidities, access to firearms, and potentially lethal medications.3-5 To better understand groups of patients at risk of suicide in medical settings, the authors have previously compared demographic and clinical risk factors between patients who died by suicide by using firearms or other means with matched patients who did not die by suicide (control group) to examine the impact of lack of social support, financial stress,6 legal problems,7 homelessness,8 and discrimination.9 The number of cooccurring risk factors a veteran experiences is associated with a greater likelihood of suicide attempts over time.10 In addition, some risk factors are social and environmental risk factors known as social determinants of health (SDoH), including financial stability and access to health care, food, housing, and education. 11 SDoH may influence health outcomes more broadly and are associated with greater risk of suicide.12,13
The VA offers programming to address suicide risk factors. However, not all veterans are eligible for VA care. Further, some veterans prefer to obtain non-VA services in their communities. Providing veterans with community resources that address risk factors, particularly SDoH, may be a worthwhile strategy for reducing suicide. Such resources have demonstrated success; for example, greater use of housing services was associated with a reduced risk for suicide-related mortality among unhoused veterans.12
The challenges that veterans experience can go beyond the scope of services the VA provides. For example, while the VA provides some services related to homelessness, justice involvement, and assistance with home loans, these services are often limited. Other services for veterans to address SDoH may require access to community resources, including food banks, employment assistance, respite and childcare services, and transportation assistance. Some veterans also may have experienced institutional betrayal, which could be a barrier to VA care and may motivate veterans to address their needs in the community.14 Veterans therefore may need a range of services beyond those within the VA. Leveraging community resources for veterans at risk for suicide is critical, as these resources may help to mitigate suicide risk.
An emerging emphasis of the VA is improving coordination with community partners to prevent veteran suicide. In 2019, the VA launched an improved Veterans Community Care Program, which implemented portions of the VA MISSION Act of 2018 to create additional connection to community care for VA-enrolled veterans. This includes assisting veterans in gaining access to specialty services not offered at a local VA medical center (VAMC), getting access to services sooner, and receiving care if they do not live near a VAMC.15 In addition, the COMPACT Act allows veterans in acute suicidal crisis to receive emergency health care through either VA or non-VA facilities at no cost.16 The VA National Strategy for Preventing Veteran Suicide 2018-2028 is a 10-year plan to reduce veteran suicide rates that includes initiatives to increase connections between VA and community agencies.17 A suicide prevention community toolkit is available online for health care professionals (HCPs) (and others, including employers) outside of the VA who may be unfamiliar with best practices for working with veterans at risk for suicide.18
The challenge, however, is that there is often a lack of “connectedness” between VA suicide prevention coordinators and community resources to address suicide risk factors and related social determinants of health. These services include, but are not limited to suicide prevention, mental health counseling (particularly no/low-cost services), unemployment resources, financial assistance and counseling, housing assistance, and identity-related supportive spaces. A major stumbling block in connecting resources with veterans (regardless of discharge status) who need them is there is no single, national organization with a comprehensive, community-based network that can serve in this intermediary role.
Community asset mapping (CAM), also known as asset mapping or environmental scanning, is a way to bridge the gap.19 CAM provides a method for identifying and aligning community resources relative to a specific need.20 CAM may be used to build community relationships in service of veteran suicide prevention. This process can help individuals learn about and make use of organizations and services within their communities. CAM also helps connect HCPs so they can network, exchange ideas, and collaborate with an eye toward increasing the availability of services and enhancing care coordination. CAM also allows community members (eg, leaders, organizations, individuals) to identify possible gaps in services that address suicide risk factors and solve these problems.
This article details CAM for suicide prevention, which can be utilized by the VA and community organizations alike. Within the VA, CAM can be used by HCPs and administrators, such as VA community engagement and partnership coordinators, to identify potential partnering organizations. For those who serve veterans outside of the VA, CAM can be used to connect at-risk individuals to resources that can enhance their care. This process can help increase the overall knowledge of, and access to, community resources.
COMMUNITY ASSET MAPPING
The University of California, Los Angeles Center for Health Policy Research provides 6 steps for the CAM process.21 These steps include: (1) defining the boundaries of people and places that comprise the community; (2) identifying people and organizations who share similar interests and goals; (3) determining the assets to include; (4) creating an inventory of all organizations’ assets; (5) creating an inventory of individuals’ assets; and (6) organizing the assets on a map. To address the needs of the veteran population, we’ve taken these 6 steps and adapted them to create a CAM for veterans at risk for suicide (Figure). The discussion that follows details how these steps can be implemented to identify community resources that address social determinants of health that may contribute to suicide risk. The goal is to prevent veteran suicide.
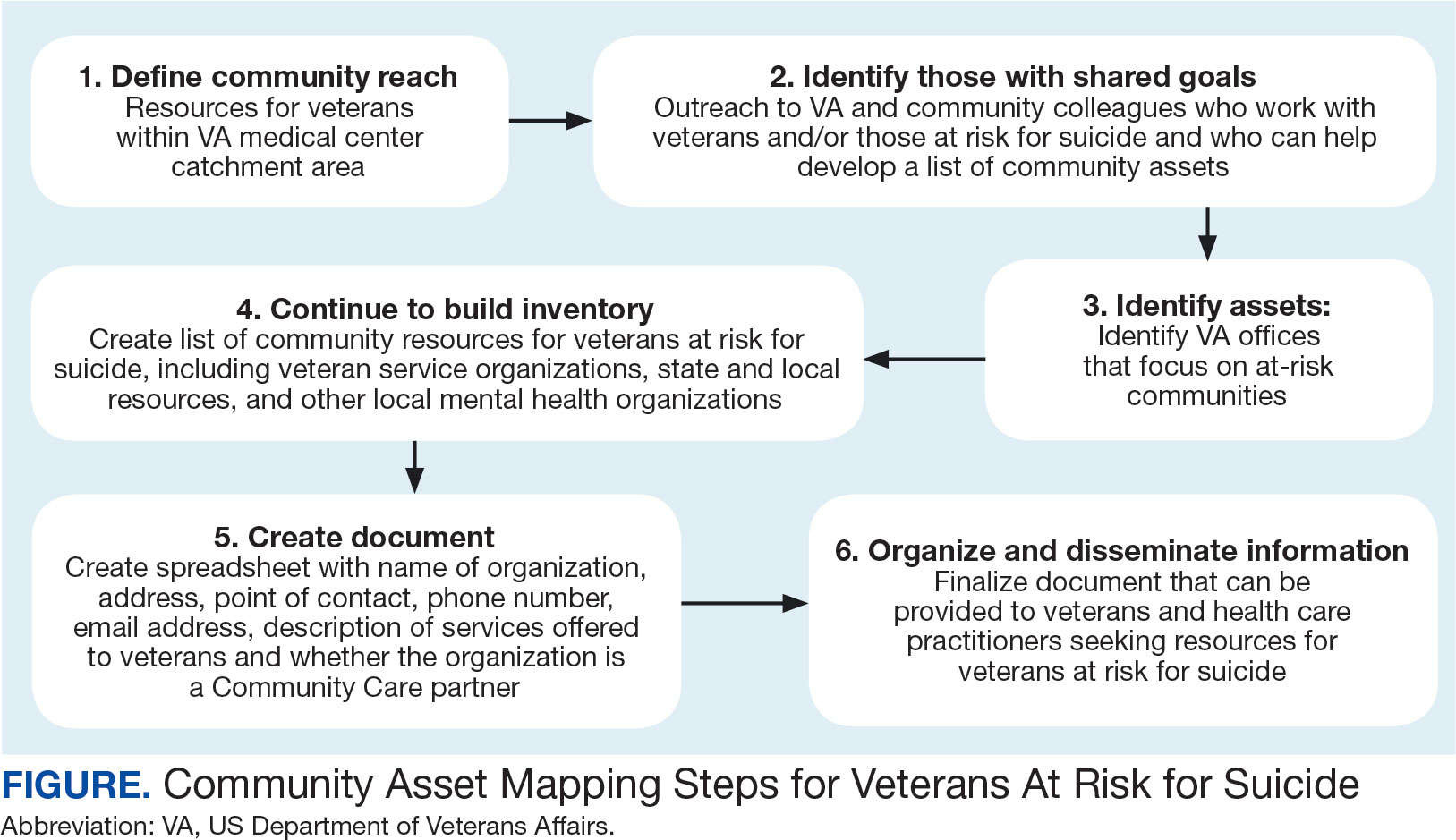
Step 1: Define Community Reach. The first step is to identify the geographical boundaries of the community. This may include all veterans within a catchment area (eg, veterans within 60 miles of a VAMC). Defining the geographical parameters of the community will provide structure to the effort so that the resource list is as comprehensive as possible.
Steps 2 and 3: Identify Community Members with Shared Goals; Identify Assets. It is important to identify community members who share similar interests and goals, including people with specific knowledge and skills, organizations with particular goals, and community partners with a broad reach. To begin building a list of referrals, reach out to colleagues within the VA system who are familiar with community resources for those with suicide risk factors. The local VA Transition and Care Management (TCM) office is a resource that connects those transitioning from military to civilian sectors with needed resources, and thus may be a helpful resource while building a CAM. Additionally, each office has a transition patient advocate, who is trained to resolve care-related concerns and may be familiar with community resources.
VA HCPs that can assist include Community Engagement and Partnership Coordinators, Suicide Prevention Coordinators, Local Recovery Coordinators, and substance abuse counselors. In addition, VA patient services, patient safety, and public affairs office staff—as well as VA Homeless Programs—may be good resources. Every VA health care system has care coordinators focused on military sexual trauma, intimate partner violence, and lesbian, gay, bisexual, transgender, queer+ care. These care coordinators may be able to provide information on community resources that address social determinants of health (eg, discrimination, violence).
Reaching out to key community resources and asking for recommendations of other groups that provide assistance to veterans can also be productive. You can start by connecting with veterans service organizations (VSOs), Vet Centers, Veterans Experience Offices (VEO), and Community Veterans Engagement Boards (CVEBs). The VEO is an office designed around VA and community engagement efforts. This office utilizes the CVEBs to foster a 2-way communication feedback loop between veterans and local VA facilities regarding community engagement efforts and outreach.22 CVEBs are particularly valuable sources of information because veterans directly contribute to the conversation about community engagement by describing the difficulties and successes they’ve experienced. Veteran feedback about how a particular resource met their needs can inform which community services are prioritized for inclusion in the resource list. In addition, CVEBs may have a listing of local government, military, and/or community resources that provide services for veterans. Consider, too, organizations that are unrelated to an individual’s veteran status, but speak to their race/ethnicity, sexual orientation, gender identity, spirituality, socioeconomic status, or disability.
Step 4: Continue to Build Inventory. Use online searches to identify additional resources in the community that are known to have local relationships. These include state suicide prevention coordinators, mental health organizations, and other resources that address social determinants of health (eg, public health and human service organizations, faith-based organizations, collegial organizations). A list of links and search tips are available in the Table.
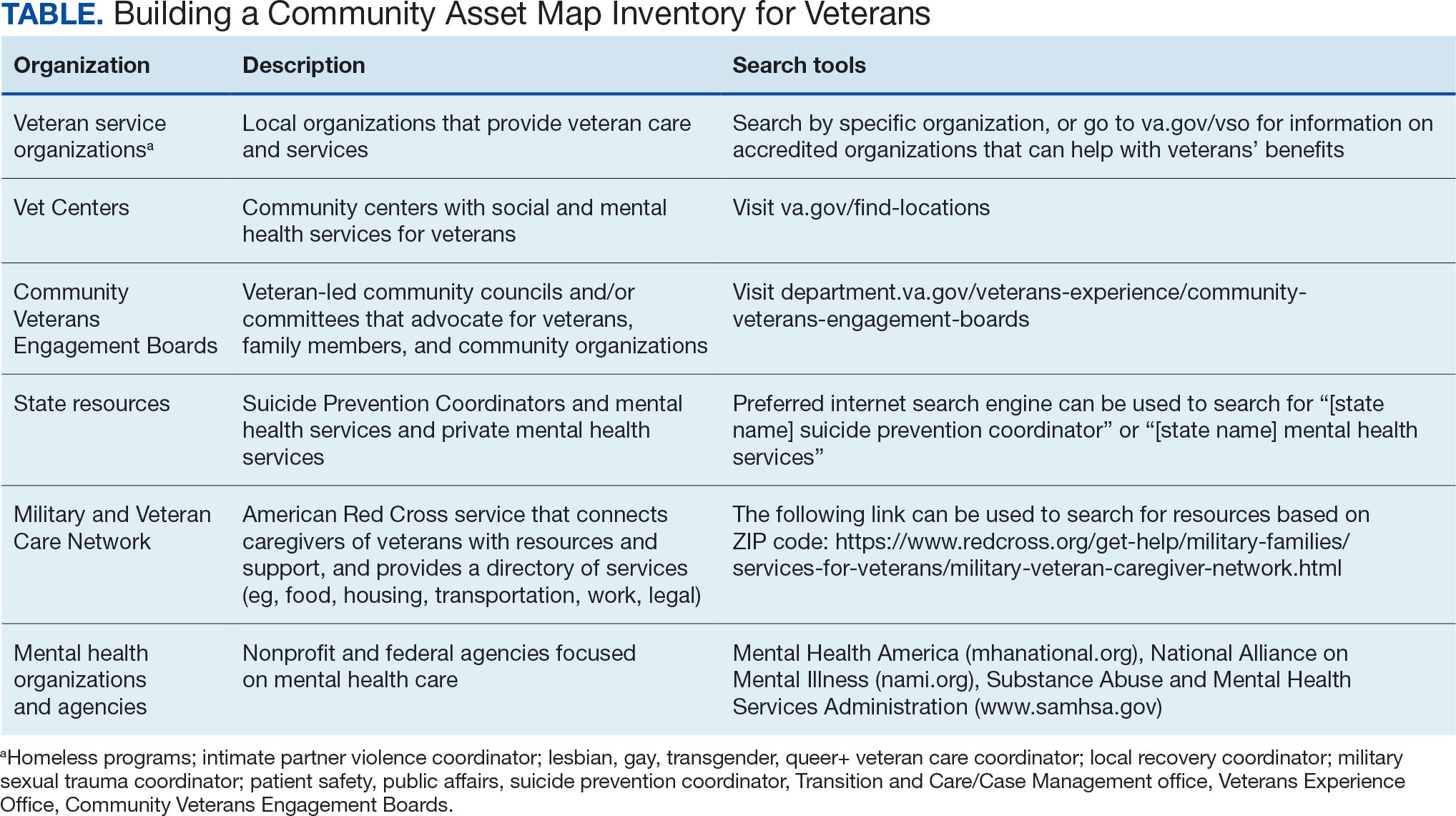
Steps 5 and 6: Create Document; Organize and Disseminate Information. A spreadsheet can be used to document organization information (Appendix). It is critical to record: (1) the name of the organization or individual; (2) the local address and a point of contact with contact information; (3) services offered to veterans; (4) services specific to suicide prevention, or that address risk factors for suicide; and (5) whether the referral organization is partnered with the VA Community Care Network, which is comprised of contracted HCPs who contract with the VA to provide care to veterans.23
Once a document is created, it can be disseminated through VA offices and among community partners who work with veterans at risk for suicide. It should also be stored in a centralized location such as a shared folder so that it can be continuously updated.
Regularly updating the list is vital so the resource list can continue to be helpful in addressing veterans’ needs and reducing suicide risk factors. Continued collaboration with those in the community can help ensure the resource list is up to date with all available services and pertinent contact information. It can also go far in strengthening collaborative bonds.
IMPLEMENTATION
To illustrate the use of CAM for veteran suicide prevention, we offer a case example of CAM conducted by the VA Patient Safety Center of Inquiry — Suicide Prevention Collaborative (VA PSCI-SPC) team, consisting of 4 team members. A veteran was included as a team member and assisted with the CAM process.
The VA PSCI-SPC sought to identify community services for veterans in Colorado who were not enrolled in VA health care and had risk factors for suicide. Next, the team reached out to colleagues and asked about community organizations that work with individuals at risk for suicide. VA PSCI-SPC outreach resulted in a list of assets that included resources to address mental health, legal concerns, employment, homelessness/housing, finances, religion, peer support, food insecurity, exercise, intimate partner violence, sexual and gender identity needs, and peer support. VSOs and CVEBs were also added to the list.
Next, the team continued to build on the inventory and identified state suicide prevention coordinators; health care systems; regional suicide prevention commissions; Colorado Department of Health and Human Services; program coordinators for Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and their Families; veterans councils; universities (eg, counseling clinics, legal clinics); and foundations devoted to general and veteran-specific suicide prevention within the region.
All the identified resources were inventoried. Details were gathered about each of the organizations, including addresses, points of contact and phone numbers, descriptions of services offered for veterans, descriptions of suicide prevention services offered, whether or not organizations were not-for-profit, the mission of the organizations, and whether or not the organizations were under contract for VA Community Care. Finally, the resource spreadsheet was created and disseminated among stakeholders to be used to enhance veteran suicide care. Stakeholders included social workers, psychologists, and nurse practitioners working with veterans. The list was circulated to VA and community partners as needed.
The VA PSCI-SPC resource document was only 1 benefit of CAM. The asset mapping also resulted in the creation of a learning collaborative comprised of VA and community partners, designed to share knowledge of best practices in suicide prevention and create an established referral network for veterans at risk for suicide.24 Ultimately, the goal of the CAM and the creation of the learning collaborative was to better connect veterans to care in order to decrease suicide risk. A secondary benefit of this community connectedness is that the list of resources produced by CAM became a living document that was, and continues to be, updated as the network became aware of new resources and resources that were no longer available. The VA PSCI-SPC learning collaborative met quarterly to discuss implementation of suicide prevention best practices within their organization.
Data from the VA PSCI-SPC learning collaborative via CAM revealed that organizations felt more efficacious in implementing suicide prevention best practices, noticed increased connections and collaborations with community organizations with the goal of providing services to veterans, and resulted in staff training that improved services provided to veterans.24 This is supported by other findings of a literature review of suicide prevention interventions, which indicated that programs with an established community support network were more effective at reducing suicide rates.25 CAM therefore may be a process through which greater community connection and increased knowledge of resources may help prevent suicide among veterans.
It seems reasonable that the CAM processes used by the VA PSCI-SPC can be implemented within the regional Veterans Integrated Service Networks to identify assets in a specific geographical area to address challenges with social determinants of health and potentially decrease veteran suicide risk.
CONCLUSIONS
CAM can be used to identify and build relationships with community resources that address the stressors that place veterans at risk for suicide. Six proposed steps to CAM for veterans at risk for suicide include: defining community reach (the map); identifying community members and organizations with shared goals; identifying assets within the community; continuing to build inventory; creating a document; and organizing and disseminating the information (while continuing to update the resources).21
CAM can be used to connect veterans with resources to address needs related to adverse social determinants of health that may heighten their risk for suicide. For example, veterans facing legal challenges can connect with a legal clinic; those having difficulties paying bills can obtain financial assistance; those who need help completing their VA claims can connect with the Veterans Benefits Administration or VSOs to assist them with their claims; and those experiencing discrimination can connect with organizations where they may experience acceptance, safety, and support. Broad community support surrounding suicide risk factors can be critical for effective suicide prevention.25
CAM may also be helpful for HCPs and others involved in veteran health care. For example, community mapping can be utilized by newly hired community engagement and partnership coordinators as a tool for outlining resources available for veterans in their community and as a framework to continually update their resource network. CAM develops community awareness, integrates resources, and enhances service utilization, which may assist in veteran suicide prevention by increasing care coordination.17 Finally, mapping community resources can create awareness of the many resources available to help veterans, even before suicide becomes a consideration.
- Rice L. VA Secretary Robert Wilkie says suicide prevention is his agency’s top ‘clinical’ priority. June 17, 2019. Accessed January 30, 2025. https://www.kut.org/post/va-secretary-robert-wilkie-says-suicide-prevention-his-agencys-top-clinical-priority
- US Department of Veterans Affairs. 2023 national veteran suicide prevention annual report. November 2023. Accessed January 30, 2025. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- DeBeer BB, Meyer EC, Kimbrel NA, Kittel JA, Gulliver SB, Morissette SB. Psychological inflexibility predicts of suicidal ideation over time in veterans of the conflicts in Iraq and Afghanistan. Suicide Life Threat Behav. 2018;48(6):627–641. doi:10.1111/sltb.12388
- Ilgen MA, Bohnert ASB, Ignacio RV, et al. Psychiatric diagnoses and risk of suicide in veterans. Arch Gen Psychiatry. 2010;67(11):1152–1158. doi:10.1001/archgenpsychiatry.2010.129
- Kimbrel NA, Meyer EC, DeBeer BB, Gulliver SB, Morissette SB. A 12-month prospective study of the effects of PTSD-depression comorbidity on suicidal behavior in Iraq/ Afghanistan-era veterans. Psychiatry Res. 2016;243:97–99. doi:10.1016/j.psychres.2016.06.011
- Hoffmire CA, Borowski S, Vogt D. Contribution of veterans’ initial post-separation vocational, financial, and social experiences to their suicidal ideation trajectories following military service. Suicide Life Threat Behav. 2023;53(3):443- 456. doi:10.1111/sltb.12955
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Holliday R, Liu S, Brenner LA, et al. Preventing suicide among homeless veterans: a consensus statement by the Veterans Affairs suicide prevention among veterans experiencing homelessness workgroup. Med Care. 2021;59(Suppl 2):S103- S105. doi:10.1097/MLR.0000000000001399
- Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: The role of social supsupport and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
- Lee DJ, Kearns JC, Wisco BE, et al. A longitudinal study of risk factors for suicide attempts among Operation Enduring Freedom and Operation Iraqi Freedom veterans. Depress Anxiety. 2018;35(7): 609-618. doi:10.1002/da.22736
- Center for Disease Control and Prevention. Social determinants of health (SDOH). Accessed January 30, 2025. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
- Montgomery AE, Dichter M, Byrne T, Blosnich J. Intervention to address homelessness and all-cause and suicide mortality among unstably housed US veterans, 2012- 2016. J Epidemiol Community Health. 2021;75:380-386. doi: 10.1136/jech-2020-214664
- Llamocca EN, Yeh HH, Miller-Matero LR, et al. Association between adverse social determinants of health and suicide death. Med Care. 2023;61(11):744-749. doi:10.1097/MLR.0000000000001918
- Monteith LL, Holliday R, Schneider AL, et al. Institutional betrayal and help-seeking among women survivors of military sexual trauma. Psychol Trauma. 2021;13(7):814-823. doi:10.1037/tra0001027
- VA launches new health care options under MISSION Act. News release. US Department of Veterans Affairs. June 6, 2019. Accessed January 31, 2025. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5264
- COMPACT Act expands free emergency suicide care for veterans. News release. US Department of Veterans Affairs. February 1, 2023. Accessed January 31,2025. https://www.va.gov/poplar-bluff-health-care/news-releases/compact-act-expands-free-emergency-suicide-care-for-veterans/
- US Department of Veterans Affairs. National strategy for preventing Veteran suicide 2018-2028. 2018. Accessed January 31, 2025. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. Veteran outreach toolkit: preventing veteran suicide is everyone’s business. A community call to action. Accessed February 3, 2025. https://floridavets.org/wp-content/uploads/2022/06/VA-Suicide-Prevention-Community-Outreach-Toolkit.pdf
- Crane K, Mooney M. Essential tools: community resource mapping. 2005. Accessed February 3, 2025. https://conservancy.umn.edu/bitstream/handle/11299/172995/NCSET_EssentialTools_ResourceMapping.pdf
- Community Tool Box. 2. Assessing Community Needs and Resources. Accessed February 3, 2025. https://ctb.ku.edu/en/assessing-community-needs-and-resources
- UCLA Center for Health Policy Research. Section 1: asset mapping. 2012. Accessed February 3, 2025. https://healthpolicy.ucla.edu/programs/healthdata/trainings/documents/tw_cba20.pdf
- US Department of Veterans Affairs, Veterans Experience Office. 4th quarter 2018 community engagement news. October 2, 2018. Accessed February 4, 2025. https://content.govdelivery.com/accounts/USVAVEO/bulletins/211836e
- US Department of Veterans Affairs. About our VA community care network and covered services. Accessed February 6, 2025. https://www.va.gov/resources/aboutour-va-community-care-network-and-covered-services/
- DeBeer B, Mignogna J, Borah E, et al. A pilot of a veteran suicide prevention learning collaborative among community organizations: Initial results and outcomes. Suicide Life Threat Behav. 2023;53(4):628-641. doi:10.1111/sltb.12969
- Fountoulakis KN, Gonda X, Rihmer Z. Suicide prevention programs through community intervention. J Affect Disord. 2011;130(1-2):10–16. doi:10.1016/j.jad.2010.06.009
Suicide prevention is the leading clinical priority for the US Department of Veterans Affairs (VA).1 An average of 18 veterans died by suicide each day in 2021.2 Numerous risk factors for veteran suicide have been identified, including mental health disorders, comorbidities, access to firearms, and potentially lethal medications.3-5 To better understand groups of patients at risk of suicide in medical settings, the authors have previously compared demographic and clinical risk factors between patients who died by suicide by using firearms or other means with matched patients who did not die by suicide (control group) to examine the impact of lack of social support, financial stress,6 legal problems,7 homelessness,8 and discrimination.9 The number of cooccurring risk factors a veteran experiences is associated with a greater likelihood of suicide attempts over time.10 In addition, some risk factors are social and environmental risk factors known as social determinants of health (SDoH), including financial stability and access to health care, food, housing, and education. 11 SDoH may influence health outcomes more broadly and are associated with greater risk of suicide.12,13
The VA offers programming to address suicide risk factors. However, not all veterans are eligible for VA care. Further, some veterans prefer to obtain non-VA services in their communities. Providing veterans with community resources that address risk factors, particularly SDoH, may be a worthwhile strategy for reducing suicide. Such resources have demonstrated success; for example, greater use of housing services was associated with a reduced risk for suicide-related mortality among unhoused veterans.12
The challenges that veterans experience can go beyond the scope of services the VA provides. For example, while the VA provides some services related to homelessness, justice involvement, and assistance with home loans, these services are often limited. Other services for veterans to address SDoH may require access to community resources, including food banks, employment assistance, respite and childcare services, and transportation assistance. Some veterans also may have experienced institutional betrayal, which could be a barrier to VA care and may motivate veterans to address their needs in the community.14 Veterans therefore may need a range of services beyond those within the VA. Leveraging community resources for veterans at risk for suicide is critical, as these resources may help to mitigate suicide risk.
An emerging emphasis of the VA is improving coordination with community partners to prevent veteran suicide. In 2019, the VA launched an improved Veterans Community Care Program, which implemented portions of the VA MISSION Act of 2018 to create additional connection to community care for VA-enrolled veterans. This includes assisting veterans in gaining access to specialty services not offered at a local VA medical center (VAMC), getting access to services sooner, and receiving care if they do not live near a VAMC.15 In addition, the COMPACT Act allows veterans in acute suicidal crisis to receive emergency health care through either VA or non-VA facilities at no cost.16 The VA National Strategy for Preventing Veteran Suicide 2018-2028 is a 10-year plan to reduce veteran suicide rates that includes initiatives to increase connections between VA and community agencies.17 A suicide prevention community toolkit is available online for health care professionals (HCPs) (and others, including employers) outside of the VA who may be unfamiliar with best practices for working with veterans at risk for suicide.18
The challenge, however, is that there is often a lack of “connectedness” between VA suicide prevention coordinators and community resources to address suicide risk factors and related social determinants of health. These services include, but are not limited to suicide prevention, mental health counseling (particularly no/low-cost services), unemployment resources, financial assistance and counseling, housing assistance, and identity-related supportive spaces. A major stumbling block in connecting resources with veterans (regardless of discharge status) who need them is there is no single, national organization with a comprehensive, community-based network that can serve in this intermediary role.
Community asset mapping (CAM), also known as asset mapping or environmental scanning, is a way to bridge the gap.19 CAM provides a method for identifying and aligning community resources relative to a specific need.20 CAM may be used to build community relationships in service of veteran suicide prevention. This process can help individuals learn about and make use of organizations and services within their communities. CAM also helps connect HCPs so they can network, exchange ideas, and collaborate with an eye toward increasing the availability of services and enhancing care coordination. CAM also allows community members (eg, leaders, organizations, individuals) to identify possible gaps in services that address suicide risk factors and solve these problems.
This article details CAM for suicide prevention, which can be utilized by the VA and community organizations alike. Within the VA, CAM can be used by HCPs and administrators, such as VA community engagement and partnership coordinators, to identify potential partnering organizations. For those who serve veterans outside of the VA, CAM can be used to connect at-risk individuals to resources that can enhance their care. This process can help increase the overall knowledge of, and access to, community resources.
COMMUNITY ASSET MAPPING
The University of California, Los Angeles Center for Health Policy Research provides 6 steps for the CAM process.21 These steps include: (1) defining the boundaries of people and places that comprise the community; (2) identifying people and organizations who share similar interests and goals; (3) determining the assets to include; (4) creating an inventory of all organizations’ assets; (5) creating an inventory of individuals’ assets; and (6) organizing the assets on a map. To address the needs of the veteran population, we’ve taken these 6 steps and adapted them to create a CAM for veterans at risk for suicide (Figure). The discussion that follows details how these steps can be implemented to identify community resources that address social determinants of health that may contribute to suicide risk. The goal is to prevent veteran suicide.

Step 1: Define Community Reach. The first step is to identify the geographical boundaries of the community. This may include all veterans within a catchment area (eg, veterans within 60 miles of a VAMC). Defining the geographical parameters of the community will provide structure to the effort so that the resource list is as comprehensive as possible.
Steps 2 and 3: Identify Community Members with Shared Goals; Identify Assets. It is important to identify community members who share similar interests and goals, including people with specific knowledge and skills, organizations with particular goals, and community partners with a broad reach. To begin building a list of referrals, reach out to colleagues within the VA system who are familiar with community resources for those with suicide risk factors. The local VA Transition and Care Management (TCM) office is a resource that connects those transitioning from military to civilian sectors with needed resources, and thus may be a helpful resource while building a CAM. Additionally, each office has a transition patient advocate, who is trained to resolve care-related concerns and may be familiar with community resources.
VA HCPs that can assist include Community Engagement and Partnership Coordinators, Suicide Prevention Coordinators, Local Recovery Coordinators, and substance abuse counselors. In addition, VA patient services, patient safety, and public affairs office staff—as well as VA Homeless Programs—may be good resources. Every VA health care system has care coordinators focused on military sexual trauma, intimate partner violence, and lesbian, gay, bisexual, transgender, queer+ care. These care coordinators may be able to provide information on community resources that address social determinants of health (eg, discrimination, violence).
Reaching out to key community resources and asking for recommendations of other groups that provide assistance to veterans can also be productive. You can start by connecting with veterans service organizations (VSOs), Vet Centers, Veterans Experience Offices (VEO), and Community Veterans Engagement Boards (CVEBs). The VEO is an office designed around VA and community engagement efforts. This office utilizes the CVEBs to foster a 2-way communication feedback loop between veterans and local VA facilities regarding community engagement efforts and outreach.22 CVEBs are particularly valuable sources of information because veterans directly contribute to the conversation about community engagement by describing the difficulties and successes they’ve experienced. Veteran feedback about how a particular resource met their needs can inform which community services are prioritized for inclusion in the resource list. In addition, CVEBs may have a listing of local government, military, and/or community resources that provide services for veterans. Consider, too, organizations that are unrelated to an individual’s veteran status, but speak to their race/ethnicity, sexual orientation, gender identity, spirituality, socioeconomic status, or disability.
Step 4: Continue to Build Inventory. Use online searches to identify additional resources in the community that are known to have local relationships. These include state suicide prevention coordinators, mental health organizations, and other resources that address social determinants of health (eg, public health and human service organizations, faith-based organizations, collegial organizations). A list of links and search tips are available in the Table.

Steps 5 and 6: Create Document; Organize and Disseminate Information. A spreadsheet can be used to document organization information (Appendix). It is critical to record: (1) the name of the organization or individual; (2) the local address and a point of contact with contact information; (3) services offered to veterans; (4) services specific to suicide prevention, or that address risk factors for suicide; and (5) whether the referral organization is partnered with the VA Community Care Network, which is comprised of contracted HCPs who contract with the VA to provide care to veterans.23

Once a document is created, it can be disseminated through VA offices and among community partners who work with veterans at risk for suicide. It should also be stored in a centralized location such as a shared folder so that it can be continuously updated.
Regularly updating the list is vital so the resource list can continue to be helpful in addressing veterans’ needs and reducing suicide risk factors. Continued collaboration with those in the community can help ensure the resource list is up to date with all available services and pertinent contact information. It can also go far in strengthening collaborative bonds.
IMPLEMENTATION
To illustrate the use of CAM for veteran suicide prevention, we offer a case example of CAM conducted by the VA Patient Safety Center of Inquiry — Suicide Prevention Collaborative (VA PSCI-SPC) team, consisting of 4 team members. A veteran was included as a team member and assisted with the CAM process.
The VA PSCI-SPC sought to identify community services for veterans in Colorado who were not enrolled in VA health care and had risk factors for suicide. Next, the team reached out to colleagues and asked about community organizations that work with individuals at risk for suicide. VA PSCI-SPC outreach resulted in a list of assets that included resources to address mental health, legal concerns, employment, homelessness/housing, finances, religion, peer support, food insecurity, exercise, intimate partner violence, sexual and gender identity needs, and peer support. VSOs and CVEBs were also added to the list.
Next, the team continued to build on the inventory and identified state suicide prevention coordinators; health care systems; regional suicide prevention commissions; Colorado Department of Health and Human Services; program coordinators for Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and their Families; veterans councils; universities (eg, counseling clinics, legal clinics); and foundations devoted to general and veteran-specific suicide prevention within the region.
All the identified resources were inventoried. Details were gathered about each of the organizations, including addresses, points of contact and phone numbers, descriptions of services offered for veterans, descriptions of suicide prevention services offered, whether or not organizations were not-for-profit, the mission of the organizations, and whether or not the organizations were under contract for VA Community Care. Finally, the resource spreadsheet was created and disseminated among stakeholders to be used to enhance veteran suicide care. Stakeholders included social workers, psychologists, and nurse practitioners working with veterans. The list was circulated to VA and community partners as needed.
The VA PSCI-SPC resource document was only 1 benefit of CAM. The asset mapping also resulted in the creation of a learning collaborative comprised of VA and community partners, designed to share knowledge of best practices in suicide prevention and create an established referral network for veterans at risk for suicide.24 Ultimately, the goal of the CAM and the creation of the learning collaborative was to better connect veterans to care in order to decrease suicide risk. A secondary benefit of this community connectedness is that the list of resources produced by CAM became a living document that was, and continues to be, updated as the network became aware of new resources and resources that were no longer available. The VA PSCI-SPC learning collaborative met quarterly to discuss implementation of suicide prevention best practices within their organization.
Data from the VA PSCI-SPC learning collaborative via CAM revealed that organizations felt more efficacious in implementing suicide prevention best practices, noticed increased connections and collaborations with community organizations with the goal of providing services to veterans, and resulted in staff training that improved services provided to veterans.24 This is supported by other findings of a literature review of suicide prevention interventions, which indicated that programs with an established community support network were more effective at reducing suicide rates.25 CAM therefore may be a process through which greater community connection and increased knowledge of resources may help prevent suicide among veterans.
It seems reasonable that the CAM processes used by the VA PSCI-SPC can be implemented within the regional Veterans Integrated Service Networks to identify assets in a specific geographical area to address challenges with social determinants of health and potentially decrease veteran suicide risk.
CONCLUSIONS
CAM can be used to identify and build relationships with community resources that address the stressors that place veterans at risk for suicide. Six proposed steps to CAM for veterans at risk for suicide include: defining community reach (the map); identifying community members and organizations with shared goals; identifying assets within the community; continuing to build inventory; creating a document; and organizing and disseminating the information (while continuing to update the resources).21
CAM can be used to connect veterans with resources to address needs related to adverse social determinants of health that may heighten their risk for suicide. For example, veterans facing legal challenges can connect with a legal clinic; those having difficulties paying bills can obtain financial assistance; those who need help completing their VA claims can connect with the Veterans Benefits Administration or VSOs to assist them with their claims; and those experiencing discrimination can connect with organizations where they may experience acceptance, safety, and support. Broad community support surrounding suicide risk factors can be critical for effective suicide prevention.25
CAM may also be helpful for HCPs and others involved in veteran health care. For example, community mapping can be utilized by newly hired community engagement and partnership coordinators as a tool for outlining resources available for veterans in their community and as a framework to continually update their resource network. CAM develops community awareness, integrates resources, and enhances service utilization, which may assist in veteran suicide prevention by increasing care coordination.17 Finally, mapping community resources can create awareness of the many resources available to help veterans, even before suicide becomes a consideration.
Suicide prevention is the leading clinical priority for the US Department of Veterans Affairs (VA).1 An average of 18 veterans died by suicide each day in 2021.2 Numerous risk factors for veteran suicide have been identified, including mental health disorders, comorbidities, access to firearms, and potentially lethal medications.3-5 To better understand groups of patients at risk of suicide in medical settings, the authors have previously compared demographic and clinical risk factors between patients who died by suicide by using firearms or other means with matched patients who did not die by suicide (control group) to examine the impact of lack of social support, financial stress,6 legal problems,7 homelessness,8 and discrimination.9 The number of cooccurring risk factors a veteran experiences is associated with a greater likelihood of suicide attempts over time.10 In addition, some risk factors are social and environmental risk factors known as social determinants of health (SDoH), including financial stability and access to health care, food, housing, and education. 11 SDoH may influence health outcomes more broadly and are associated with greater risk of suicide.12,13
The VA offers programming to address suicide risk factors. However, not all veterans are eligible for VA care. Further, some veterans prefer to obtain non-VA services in their communities. Providing veterans with community resources that address risk factors, particularly SDoH, may be a worthwhile strategy for reducing suicide. Such resources have demonstrated success; for example, greater use of housing services was associated with a reduced risk for suicide-related mortality among unhoused veterans.12
The challenges that veterans experience can go beyond the scope of services the VA provides. For example, while the VA provides some services related to homelessness, justice involvement, and assistance with home loans, these services are often limited. Other services for veterans to address SDoH may require access to community resources, including food banks, employment assistance, respite and childcare services, and transportation assistance. Some veterans also may have experienced institutional betrayal, which could be a barrier to VA care and may motivate veterans to address their needs in the community.14 Veterans therefore may need a range of services beyond those within the VA. Leveraging community resources for veterans at risk for suicide is critical, as these resources may help to mitigate suicide risk.
An emerging emphasis of the VA is improving coordination with community partners to prevent veteran suicide. In 2019, the VA launched an improved Veterans Community Care Program, which implemented portions of the VA MISSION Act of 2018 to create additional connection to community care for VA-enrolled veterans. This includes assisting veterans in gaining access to specialty services not offered at a local VA medical center (VAMC), getting access to services sooner, and receiving care if they do not live near a VAMC.15 In addition, the COMPACT Act allows veterans in acute suicidal crisis to receive emergency health care through either VA or non-VA facilities at no cost.16 The VA National Strategy for Preventing Veteran Suicide 2018-2028 is a 10-year plan to reduce veteran suicide rates that includes initiatives to increase connections between VA and community agencies.17 A suicide prevention community toolkit is available online for health care professionals (HCPs) (and others, including employers) outside of the VA who may be unfamiliar with best practices for working with veterans at risk for suicide.18
The challenge, however, is that there is often a lack of “connectedness” between VA suicide prevention coordinators and community resources to address suicide risk factors and related social determinants of health. These services include, but are not limited to suicide prevention, mental health counseling (particularly no/low-cost services), unemployment resources, financial assistance and counseling, housing assistance, and identity-related supportive spaces. A major stumbling block in connecting resources with veterans (regardless of discharge status) who need them is there is no single, national organization with a comprehensive, community-based network that can serve in this intermediary role.
Community asset mapping (CAM), also known as asset mapping or environmental scanning, is a way to bridge the gap.19 CAM provides a method for identifying and aligning community resources relative to a specific need.20 CAM may be used to build community relationships in service of veteran suicide prevention. This process can help individuals learn about and make use of organizations and services within their communities. CAM also helps connect HCPs so they can network, exchange ideas, and collaborate with an eye toward increasing the availability of services and enhancing care coordination. CAM also allows community members (eg, leaders, organizations, individuals) to identify possible gaps in services that address suicide risk factors and solve these problems.
This article details CAM for suicide prevention, which can be utilized by the VA and community organizations alike. Within the VA, CAM can be used by HCPs and administrators, such as VA community engagement and partnership coordinators, to identify potential partnering organizations. For those who serve veterans outside of the VA, CAM can be used to connect at-risk individuals to resources that can enhance their care. This process can help increase the overall knowledge of, and access to, community resources.
COMMUNITY ASSET MAPPING
The University of California, Los Angeles Center for Health Policy Research provides 6 steps for the CAM process.21 These steps include: (1) defining the boundaries of people and places that comprise the community; (2) identifying people and organizations who share similar interests and goals; (3) determining the assets to include; (4) creating an inventory of all organizations’ assets; (5) creating an inventory of individuals’ assets; and (6) organizing the assets on a map. To address the needs of the veteran population, we’ve taken these 6 steps and adapted them to create a CAM for veterans at risk for suicide (Figure). The discussion that follows details how these steps can be implemented to identify community resources that address social determinants of health that may contribute to suicide risk. The goal is to prevent veteran suicide.

Step 1: Define Community Reach. The first step is to identify the geographical boundaries of the community. This may include all veterans within a catchment area (eg, veterans within 60 miles of a VAMC). Defining the geographical parameters of the community will provide structure to the effort so that the resource list is as comprehensive as possible.
Steps 2 and 3: Identify Community Members with Shared Goals; Identify Assets. It is important to identify community members who share similar interests and goals, including people with specific knowledge and skills, organizations with particular goals, and community partners with a broad reach. To begin building a list of referrals, reach out to colleagues within the VA system who are familiar with community resources for those with suicide risk factors. The local VA Transition and Care Management (TCM) office is a resource that connects those transitioning from military to civilian sectors with needed resources, and thus may be a helpful resource while building a CAM. Additionally, each office has a transition patient advocate, who is trained to resolve care-related concerns and may be familiar with community resources.
VA HCPs that can assist include Community Engagement and Partnership Coordinators, Suicide Prevention Coordinators, Local Recovery Coordinators, and substance abuse counselors. In addition, VA patient services, patient safety, and public affairs office staff—as well as VA Homeless Programs—may be good resources. Every VA health care system has care coordinators focused on military sexual trauma, intimate partner violence, and lesbian, gay, bisexual, transgender, queer+ care. These care coordinators may be able to provide information on community resources that address social determinants of health (eg, discrimination, violence).
Reaching out to key community resources and asking for recommendations of other groups that provide assistance to veterans can also be productive. You can start by connecting with veterans service organizations (VSOs), Vet Centers, Veterans Experience Offices (VEO), and Community Veterans Engagement Boards (CVEBs). The VEO is an office designed around VA and community engagement efforts. This office utilizes the CVEBs to foster a 2-way communication feedback loop between veterans and local VA facilities regarding community engagement efforts and outreach.22 CVEBs are particularly valuable sources of information because veterans directly contribute to the conversation about community engagement by describing the difficulties and successes they’ve experienced. Veteran feedback about how a particular resource met their needs can inform which community services are prioritized for inclusion in the resource list. In addition, CVEBs may have a listing of local government, military, and/or community resources that provide services for veterans. Consider, too, organizations that are unrelated to an individual’s veteran status, but speak to their race/ethnicity, sexual orientation, gender identity, spirituality, socioeconomic status, or disability.
Step 4: Continue to Build Inventory. Use online searches to identify additional resources in the community that are known to have local relationships. These include state suicide prevention coordinators, mental health organizations, and other resources that address social determinants of health (eg, public health and human service organizations, faith-based organizations, collegial organizations). A list of links and search tips are available in the Table.

Steps 5 and 6: Create Document; Organize and Disseminate Information. A spreadsheet can be used to document organization information (Appendix). It is critical to record: (1) the name of the organization or individual; (2) the local address and a point of contact with contact information; (3) services offered to veterans; (4) services specific to suicide prevention, or that address risk factors for suicide; and (5) whether the referral organization is partnered with the VA Community Care Network, which is comprised of contracted HCPs who contract with the VA to provide care to veterans.23

Once a document is created, it can be disseminated through VA offices and among community partners who work with veterans at risk for suicide. It should also be stored in a centralized location such as a shared folder so that it can be continuously updated.
Regularly updating the list is vital so the resource list can continue to be helpful in addressing veterans’ needs and reducing suicide risk factors. Continued collaboration with those in the community can help ensure the resource list is up to date with all available services and pertinent contact information. It can also go far in strengthening collaborative bonds.
IMPLEMENTATION
To illustrate the use of CAM for veteran suicide prevention, we offer a case example of CAM conducted by the VA Patient Safety Center of Inquiry — Suicide Prevention Collaborative (VA PSCI-SPC) team, consisting of 4 team members. A veteran was included as a team member and assisted with the CAM process.
The VA PSCI-SPC sought to identify community services for veterans in Colorado who were not enrolled in VA health care and had risk factors for suicide. Next, the team reached out to colleagues and asked about community organizations that work with individuals at risk for suicide. VA PSCI-SPC outreach resulted in a list of assets that included resources to address mental health, legal concerns, employment, homelessness/housing, finances, religion, peer support, food insecurity, exercise, intimate partner violence, sexual and gender identity needs, and peer support. VSOs and CVEBs were also added to the list.
Next, the team continued to build on the inventory and identified state suicide prevention coordinators; health care systems; regional suicide prevention commissions; Colorado Department of Health and Human Services; program coordinators for Governor’s and Mayor’s Challenges to Prevent Suicide Among Service Members, Veterans, and their Families; veterans councils; universities (eg, counseling clinics, legal clinics); and foundations devoted to general and veteran-specific suicide prevention within the region.
All the identified resources were inventoried. Details were gathered about each of the organizations, including addresses, points of contact and phone numbers, descriptions of services offered for veterans, descriptions of suicide prevention services offered, whether or not organizations were not-for-profit, the mission of the organizations, and whether or not the organizations were under contract for VA Community Care. Finally, the resource spreadsheet was created and disseminated among stakeholders to be used to enhance veteran suicide care. Stakeholders included social workers, psychologists, and nurse practitioners working with veterans. The list was circulated to VA and community partners as needed.
The VA PSCI-SPC resource document was only 1 benefit of CAM. The asset mapping also resulted in the creation of a learning collaborative comprised of VA and community partners, designed to share knowledge of best practices in suicide prevention and create an established referral network for veterans at risk for suicide.24 Ultimately, the goal of the CAM and the creation of the learning collaborative was to better connect veterans to care in order to decrease suicide risk. A secondary benefit of this community connectedness is that the list of resources produced by CAM became a living document that was, and continues to be, updated as the network became aware of new resources and resources that were no longer available. The VA PSCI-SPC learning collaborative met quarterly to discuss implementation of suicide prevention best practices within their organization.
Data from the VA PSCI-SPC learning collaborative via CAM revealed that organizations felt more efficacious in implementing suicide prevention best practices, noticed increased connections and collaborations with community organizations with the goal of providing services to veterans, and resulted in staff training that improved services provided to veterans.24 This is supported by other findings of a literature review of suicide prevention interventions, which indicated that programs with an established community support network were more effective at reducing suicide rates.25 CAM therefore may be a process through which greater community connection and increased knowledge of resources may help prevent suicide among veterans.
It seems reasonable that the CAM processes used by the VA PSCI-SPC can be implemented within the regional Veterans Integrated Service Networks to identify assets in a specific geographical area to address challenges with social determinants of health and potentially decrease veteran suicide risk.
CONCLUSIONS
CAM can be used to identify and build relationships with community resources that address the stressors that place veterans at risk for suicide. Six proposed steps to CAM for veterans at risk for suicide include: defining community reach (the map); identifying community members and organizations with shared goals; identifying assets within the community; continuing to build inventory; creating a document; and organizing and disseminating the information (while continuing to update the resources).21
CAM can be used to connect veterans with resources to address needs related to adverse social determinants of health that may heighten their risk for suicide. For example, veterans facing legal challenges can connect with a legal clinic; those having difficulties paying bills can obtain financial assistance; those who need help completing their VA claims can connect with the Veterans Benefits Administration or VSOs to assist them with their claims; and those experiencing discrimination can connect with organizations where they may experience acceptance, safety, and support. Broad community support surrounding suicide risk factors can be critical for effective suicide prevention.25
CAM may also be helpful for HCPs and others involved in veteran health care. For example, community mapping can be utilized by newly hired community engagement and partnership coordinators as a tool for outlining resources available for veterans in their community and as a framework to continually update their resource network. CAM develops community awareness, integrates resources, and enhances service utilization, which may assist in veteran suicide prevention by increasing care coordination.17 Finally, mapping community resources can create awareness of the many resources available to help veterans, even before suicide becomes a consideration.
- Rice L. VA Secretary Robert Wilkie says suicide prevention is his agency’s top ‘clinical’ priority. June 17, 2019. Accessed January 30, 2025. https://www.kut.org/post/va-secretary-robert-wilkie-says-suicide-prevention-his-agencys-top-clinical-priority
- US Department of Veterans Affairs. 2023 national veteran suicide prevention annual report. November 2023. Accessed January 30, 2025. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- DeBeer BB, Meyer EC, Kimbrel NA, Kittel JA, Gulliver SB, Morissette SB. Psychological inflexibility predicts of suicidal ideation over time in veterans of the conflicts in Iraq and Afghanistan. Suicide Life Threat Behav. 2018;48(6):627–641. doi:10.1111/sltb.12388
- Ilgen MA, Bohnert ASB, Ignacio RV, et al. Psychiatric diagnoses and risk of suicide in veterans. Arch Gen Psychiatry. 2010;67(11):1152–1158. doi:10.1001/archgenpsychiatry.2010.129
- Kimbrel NA, Meyer EC, DeBeer BB, Gulliver SB, Morissette SB. A 12-month prospective study of the effects of PTSD-depression comorbidity on suicidal behavior in Iraq/ Afghanistan-era veterans. Psychiatry Res. 2016;243:97–99. doi:10.1016/j.psychres.2016.06.011
- Hoffmire CA, Borowski S, Vogt D. Contribution of veterans’ initial post-separation vocational, financial, and social experiences to their suicidal ideation trajectories following military service. Suicide Life Threat Behav. 2023;53(3):443- 456. doi:10.1111/sltb.12955
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Holliday R, Liu S, Brenner LA, et al. Preventing suicide among homeless veterans: a consensus statement by the Veterans Affairs suicide prevention among veterans experiencing homelessness workgroup. Med Care. 2021;59(Suppl 2):S103- S105. doi:10.1097/MLR.0000000000001399
- Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: The role of social supsupport and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
- Lee DJ, Kearns JC, Wisco BE, et al. A longitudinal study of risk factors for suicide attempts among Operation Enduring Freedom and Operation Iraqi Freedom veterans. Depress Anxiety. 2018;35(7): 609-618. doi:10.1002/da.22736
- Center for Disease Control and Prevention. Social determinants of health (SDOH). Accessed January 30, 2025. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
- Montgomery AE, Dichter M, Byrne T, Blosnich J. Intervention to address homelessness and all-cause and suicide mortality among unstably housed US veterans, 2012- 2016. J Epidemiol Community Health. 2021;75:380-386. doi: 10.1136/jech-2020-214664
- Llamocca EN, Yeh HH, Miller-Matero LR, et al. Association between adverse social determinants of health and suicide death. Med Care. 2023;61(11):744-749. doi:10.1097/MLR.0000000000001918
- Monteith LL, Holliday R, Schneider AL, et al. Institutional betrayal and help-seeking among women survivors of military sexual trauma. Psychol Trauma. 2021;13(7):814-823. doi:10.1037/tra0001027
- VA launches new health care options under MISSION Act. News release. US Department of Veterans Affairs. June 6, 2019. Accessed January 31, 2025. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5264
- COMPACT Act expands free emergency suicide care for veterans. News release. US Department of Veterans Affairs. February 1, 2023. Accessed January 31,2025. https://www.va.gov/poplar-bluff-health-care/news-releases/compact-act-expands-free-emergency-suicide-care-for-veterans/
- US Department of Veterans Affairs. National strategy for preventing Veteran suicide 2018-2028. 2018. Accessed January 31, 2025. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. Veteran outreach toolkit: preventing veteran suicide is everyone’s business. A community call to action. Accessed February 3, 2025. https://floridavets.org/wp-content/uploads/2022/06/VA-Suicide-Prevention-Community-Outreach-Toolkit.pdf
- Crane K, Mooney M. Essential tools: community resource mapping. 2005. Accessed February 3, 2025. https://conservancy.umn.edu/bitstream/handle/11299/172995/NCSET_EssentialTools_ResourceMapping.pdf
- Community Tool Box. 2. Assessing Community Needs and Resources. Accessed February 3, 2025. https://ctb.ku.edu/en/assessing-community-needs-and-resources
- UCLA Center for Health Policy Research. Section 1: asset mapping. 2012. Accessed February 3, 2025. https://healthpolicy.ucla.edu/programs/healthdata/trainings/documents/tw_cba20.pdf
- US Department of Veterans Affairs, Veterans Experience Office. 4th quarter 2018 community engagement news. October 2, 2018. Accessed February 4, 2025. https://content.govdelivery.com/accounts/USVAVEO/bulletins/211836e
- US Department of Veterans Affairs. About our VA community care network and covered services. Accessed February 6, 2025. https://www.va.gov/resources/aboutour-va-community-care-network-and-covered-services/
- DeBeer B, Mignogna J, Borah E, et al. A pilot of a veteran suicide prevention learning collaborative among community organizations: Initial results and outcomes. Suicide Life Threat Behav. 2023;53(4):628-641. doi:10.1111/sltb.12969
- Fountoulakis KN, Gonda X, Rihmer Z. Suicide prevention programs through community intervention. J Affect Disord. 2011;130(1-2):10–16. doi:10.1016/j.jad.2010.06.009
- Rice L. VA Secretary Robert Wilkie says suicide prevention is his agency’s top ‘clinical’ priority. June 17, 2019. Accessed January 30, 2025. https://www.kut.org/post/va-secretary-robert-wilkie-says-suicide-prevention-his-agencys-top-clinical-priority
- US Department of Veterans Affairs. 2023 national veteran suicide prevention annual report. November 2023. Accessed January 30, 2025. https://www.mentalhealth.va.gov/docs/data-sheets/2023/2023-National-Veteran-Suicide-Prevention-Annual-Report-FINAL-508.pdf
- DeBeer BB, Meyer EC, Kimbrel NA, Kittel JA, Gulliver SB, Morissette SB. Psychological inflexibility predicts of suicidal ideation over time in veterans of the conflicts in Iraq and Afghanistan. Suicide Life Threat Behav. 2018;48(6):627–641. doi:10.1111/sltb.12388
- Ilgen MA, Bohnert ASB, Ignacio RV, et al. Psychiatric diagnoses and risk of suicide in veterans. Arch Gen Psychiatry. 2010;67(11):1152–1158. doi:10.1001/archgenpsychiatry.2010.129
- Kimbrel NA, Meyer EC, DeBeer BB, Gulliver SB, Morissette SB. A 12-month prospective study of the effects of PTSD-depression comorbidity on suicidal behavior in Iraq/ Afghanistan-era veterans. Psychiatry Res. 2016;243:97–99. doi:10.1016/j.psychres.2016.06.011
- Hoffmire CA, Borowski S, Vogt D. Contribution of veterans’ initial post-separation vocational, financial, and social experiences to their suicidal ideation trajectories following military service. Suicide Life Threat Behav. 2023;53(3):443- 456. doi:10.1111/sltb.12955
- Holliday R, Martin WB, Monteith LL, Clark SC, LePage JP. Suicide among justice-involved veterans: a brief overview of extant research, theoretical conceptualization, and recommendations for future research. J Soc Distress Homeless. 2020;30(1):41-49. doi:10.1080/10530789.2019.1711306
- Holliday R, Liu S, Brenner LA, et al. Preventing suicide among homeless veterans: a consensus statement by the Veterans Affairs suicide prevention among veterans experiencing homelessness workgroup. Med Care. 2021;59(Suppl 2):S103- S105. doi:10.1097/MLR.0000000000001399
- Carter SP, Allred KM, Tucker RP, Simpson TL, Shipherd JC, Lehavot K. Discrimination and suicidal ideation among transgender veterans: The role of social supsupport and connection. LGBT Health. 2019;6(2):43-50. doi:10.1089/lgbt.2018.0239
- Lee DJ, Kearns JC, Wisco BE, et al. A longitudinal study of risk factors for suicide attempts among Operation Enduring Freedom and Operation Iraqi Freedom veterans. Depress Anxiety. 2018;35(7): 609-618. doi:10.1002/da.22736
- Center for Disease Control and Prevention. Social determinants of health (SDOH). Accessed January 30, 2025. https://odphp.health.gov/healthypeople/priority-areas/social-determinants-health
- Montgomery AE, Dichter M, Byrne T, Blosnich J. Intervention to address homelessness and all-cause and suicide mortality among unstably housed US veterans, 2012- 2016. J Epidemiol Community Health. 2021;75:380-386. doi: 10.1136/jech-2020-214664
- Llamocca EN, Yeh HH, Miller-Matero LR, et al. Association between adverse social determinants of health and suicide death. Med Care. 2023;61(11):744-749. doi:10.1097/MLR.0000000000001918
- Monteith LL, Holliday R, Schneider AL, et al. Institutional betrayal and help-seeking among women survivors of military sexual trauma. Psychol Trauma. 2021;13(7):814-823. doi:10.1037/tra0001027
- VA launches new health care options under MISSION Act. News release. US Department of Veterans Affairs. June 6, 2019. Accessed January 31, 2025. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5264
- COMPACT Act expands free emergency suicide care for veterans. News release. US Department of Veterans Affairs. February 1, 2023. Accessed January 31,2025. https://www.va.gov/poplar-bluff-health-care/news-releases/compact-act-expands-free-emergency-suicide-care-for-veterans/
- US Department of Veterans Affairs. National strategy for preventing Veteran suicide 2018-2028. 2018. Accessed January 31, 2025. https://www.mentalhealth.va.gov/suicide_prevention/docs/Office-of-Mental-Health-and-Suicide-Prevention-National-Strategy-for-Preventing-Veterans-Suicide.pdf
- US Department of Veterans Affairs. Veteran outreach toolkit: preventing veteran suicide is everyone’s business. A community call to action. Accessed February 3, 2025. https://floridavets.org/wp-content/uploads/2022/06/VA-Suicide-Prevention-Community-Outreach-Toolkit.pdf
- Crane K, Mooney M. Essential tools: community resource mapping. 2005. Accessed February 3, 2025. https://conservancy.umn.edu/bitstream/handle/11299/172995/NCSET_EssentialTools_ResourceMapping.pdf
- Community Tool Box. 2. Assessing Community Needs and Resources. Accessed February 3, 2025. https://ctb.ku.edu/en/assessing-community-needs-and-resources
- UCLA Center for Health Policy Research. Section 1: asset mapping. 2012. Accessed February 3, 2025. https://healthpolicy.ucla.edu/programs/healthdata/trainings/documents/tw_cba20.pdf
- US Department of Veterans Affairs, Veterans Experience Office. 4th quarter 2018 community engagement news. October 2, 2018. Accessed February 4, 2025. https://content.govdelivery.com/accounts/USVAVEO/bulletins/211836e
- US Department of Veterans Affairs. About our VA community care network and covered services. Accessed February 6, 2025. https://www.va.gov/resources/aboutour-va-community-care-network-and-covered-services/
- DeBeer B, Mignogna J, Borah E, et al. A pilot of a veteran suicide prevention learning collaborative among community organizations: Initial results and outcomes. Suicide Life Threat Behav. 2023;53(4):628-641. doi:10.1111/sltb.12969
- Fountoulakis KN, Gonda X, Rihmer Z. Suicide prevention programs through community intervention. J Affect Disord. 2011;130(1-2):10–16. doi:10.1016/j.jad.2010.06.009
Leveraging Community Asset Mapping to Improve Suicide Prevention for Veterans
Leveraging Community Asset Mapping to Improve Suicide Prevention for Veterans
Needs of Veterans With Personality Disorder Diagnoses in Community-Based Mental Health Care
Needs of Veterans With Personality Disorder Diagnoses in Community-Based Mental Health Care
Personality disorders (PDs) are enduring patterns of internal experience and behavior that differ from cultural norms and expectations, are inflexible and pervasive, have their onset in adolescence or early adulthood, and lead to distress or impairment. Ten PDs are included in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition): paranoid, schizoid, schizotypal, borderline, antisocial, histrionic, narcissistic, avoidant, dependent, and obsessive-compulsive.1 These disorders impose a high burden on patients, families, health care systems, and broader economic systems.2,3 Up to 1 in 7 persons in the community and 50% of those receiving outpatient mental health treatment experience a PD.4,5 These conditions are associated with an increased risk of adverse events, including suicide attempt and death by suicide, criminal-legal involvement, homelessness, substance use, underemployment, relational issues, and high utilization of psychiatric services.6-9 PDs are routinely underassessed, underdocumented, and undertreated in clinical settings, and consistently receive less research funding than other, less prevalent forms of psychopathology. 10-12 As a result, there is limited understanding of clinical needs of individuals experiencing PDs.
MILITARY VETERANS WITH PERSONALITY DISORDERS
Underacknowledgment of PDs and their associated difficulties may be especially pronounced in veteran populations. Due to longstanding etiological theories that implicate childhood trauma and adolescent onset in pathology development, PDs are traditionally considered pre-existing conditions or developmental abnormalities by the US Department of Defense and US Department of Veterans Affairs (VA). As a result, PDs are therefore deemed incompatible with military service and ineligible for service-connected disability benefits.13-15 Such determinations allowed PD pathology to be used as grounds for discharge for 26,000 service members from 2001 to 2007, or 2.6% of total enlisted discharges during that period.13,15,16
Despite this structural discrimination, recent research suggests veterans may be more likely to experience PD pathology than the general population.17 For example, a 2021 epidemiological survey in a community-based veteran sample found elevated rates of borderline, antisocial, and schizotypal PDs (6%-13%).6 In contrast, only 0.8% to 5.0% of veteran electronic health records (EHRs) have a documented PD diagnosis.8,18,19 Such elevations in PD pathology within veteran samples imply either a disproportionately high prevalence among enlistees (and therefore missed during recruitment procedures) or onset following military service, possibly due to exposure to traumatic events and/ or occupational stress.17 Due to the relative infancy of research in this area and a lack of longitudinal studies, etiology and course of illness for personality pathology in veterans remains largely unclear.
Structural underacknowledgment of PDs among military personnel has contributed to their underrepresentation in research on veteran populations. PD-focused research with veterans is rare, despite a rapid increase in broader empirical attention paid to these conditions in nonveteran samples.20 A recent meta-analysis of veterans with PDs identified 27 studies that included basic prevalence statistics. PDs were rarely a primary focus for these studies, and most were limited to veterans seen in Veterans Health Administration (VHA) settings.17 The literature also paints a bleak picture, suggesting veterans who experience PDs are at higher risk for suicide attempt and death by suicide, criminal-legal involvement, and homelessness. They also tend to experience more severe comorbid psychopathological symptoms and more often use high-intensity mental health services (eg, care within emergency departments or psychiatric inpatient settings) than veterans without PD pathology.6,8,18,19,21 However, PD pathology does not appear to impede the effectiveness of treatment for veterans.22-24 The implications of PD pathology on broader psychosocial functioning and health care needs certify a need for additional research that examines patterns of personality pathology, particularly in veterans outside the VHA.
METHODS
This study aims to enhance understanding of veterans affected by PDs and offer insight and guidance for treatment of these conditions in federal and nonfederal treatment settings. Previous research has been largely limited to VHA care-receiving samples; the longstanding stigma against PDs by the US military and VA may contribute to biased diagnosis and documentation of PDs in these settings. A large sample of veterans receiving community-based mental health care was therefore used to explore aims of the current study. This study specifically examined demographic patterns, diagnostic comorbidity, psychosocial outcomes, and treatment care settings among veterans with and without a PD diagnosis. Consistent with previous research, we hypothesized that veterans with a PD diagnosis would have more severe mental health comorbidities, poorer psychosocial outcomes, and receive care in higher intensity settings relative to veterans without a diagnosis.
Data for the sample were drawn from the Mental Health Client-Level Data, a publicly available national dataset of nearly 7 million patients who received mental health treatment services provided or funded through state mental health agencies in 2022.25 The analytic sample included about 2.5 million patients for whom veteran status and data around the presence or absence of a PD diagnosis were available. Of these patients, 104,198 were identified as veterans. Veteran patients were identified as predominantly male (63%), White (71%), non-Hispanic (90%), and never married (54%).
Measures
The parent dataset included demographic, clinical, and psychosocial outcome information reported by treatment facilities to individual state administrative systems for each patient who received services. To protect patient privacy, only nonprotected health information is included, and efforts were made throughout compilation of the parent dataset to ensure patient privacy (eg, limiting detail of information disseminated for public access). Because the parent dataset does not include protected health information, studies using these data are considered exempt from institutional review board oversight.
Demographic information. This study reviewed veteran status, sex, race, ethnicity, age, education, and marital status. Veteran status was defined by whether the patient was aged ≥ 18 years and had previously served (but was not currently serving) in the military. Patients with a history of service in the National Guard or Military Reserves were only classified as veterans if they had been called or ordered to active duty while serving. Sex was operationalized dichotomously as male or female; no patients were identified as intersex, transgender, or other gender identities.
Clinical information. Up to 3 mental health diagnoses were reported for each patient and included the following disorders: personality, trauma and attention-deficit/hyperactivity, stressor, anxiety, conduct, delirium/dementia, bipolar, depressive, oppositional defiant, pervasive developmental, schizophrenia or other psychotic, and alcohol or substance use. Mental health diagnosis categories were generated for the parent dataset by grouping diagnostic codes corresponding to each category. To protect patient privacy, more detailed diagnostic information was not available as part of the parent dataset. Although the American Psychiatric Association recognizes 10 distinct PDs, the exact nature of PD diagnoses was not included within the parent dataset. PD diagnoses were coded to reflect the presence or absence of any such diagnosis.
A substance use problem designation was also provided for patients according to various identification methods, including substance use disorder (SUD) diagnosis, substance use screening results, enrollment in a substance use program, substance use survey, service claims information, and other related sources of information. A severe mental illness or serious emotional disturbance designation was provided for patients meeting state definitions of these designations. Context(s) of service provision were coded as inpatient state psychiatric hospital, community-based program, residential treatment center, judicial institution, or other psychiatric inpatient setting.
Psychosocial outcome information. Patient employment and residential status were also included in analyses. Each reflected status at the time of discharge from services or end of reporting period; employment status was only provided for patients receiving treatment in community-based programs.
Data Analysis
Descriptive statistics and X2 analyses were used to compare demographic, clinical, and psychosocial outcome variables between patients with and without PD diagnoses. These analyses were calculated for both the 104,198 veterans and the 2,222,306 nonveterans aged ≥ 18 years in the dataset. Given the sample size, a conservative α of .01 was used to determine statistical significance.
RESULTS
In this sample of persons receiving state-funded mental health care, veterans were significantly less likely than nonveterans to have a documented PD diagnosis (2.1% vs 3.6%, X2 [1] = 647.49; P < .01). PD diagnoses were more common among White (risk ratio [RR], 1.11), non-Hispanic (RR, 1.03) veterans who were in middle to late adulthood (RR, 1.16-1.40), more educated (RR, 1.35), and divorced or widowed (RR, 1.43), and less common among Black/African American (RR, 0.78) or Puerto Rican (RR, 0.32) veterans who were in early adulthood (RR, 0.31-0.79), less educated (RR, 0.64-0.89), and currently married (RR, 0.89) or never married (RR, 0.86). Veteran men and women were equally likely to have a PD diagnosis (RR, 1.03) (Table 1). Among nonveterans, men were less likely than women to have a PD diagnosis (RR, 0.79), and PD diagnoses were most common among persons in middle adulthood (RR, 1.06-1.15) (eAppendix 1).
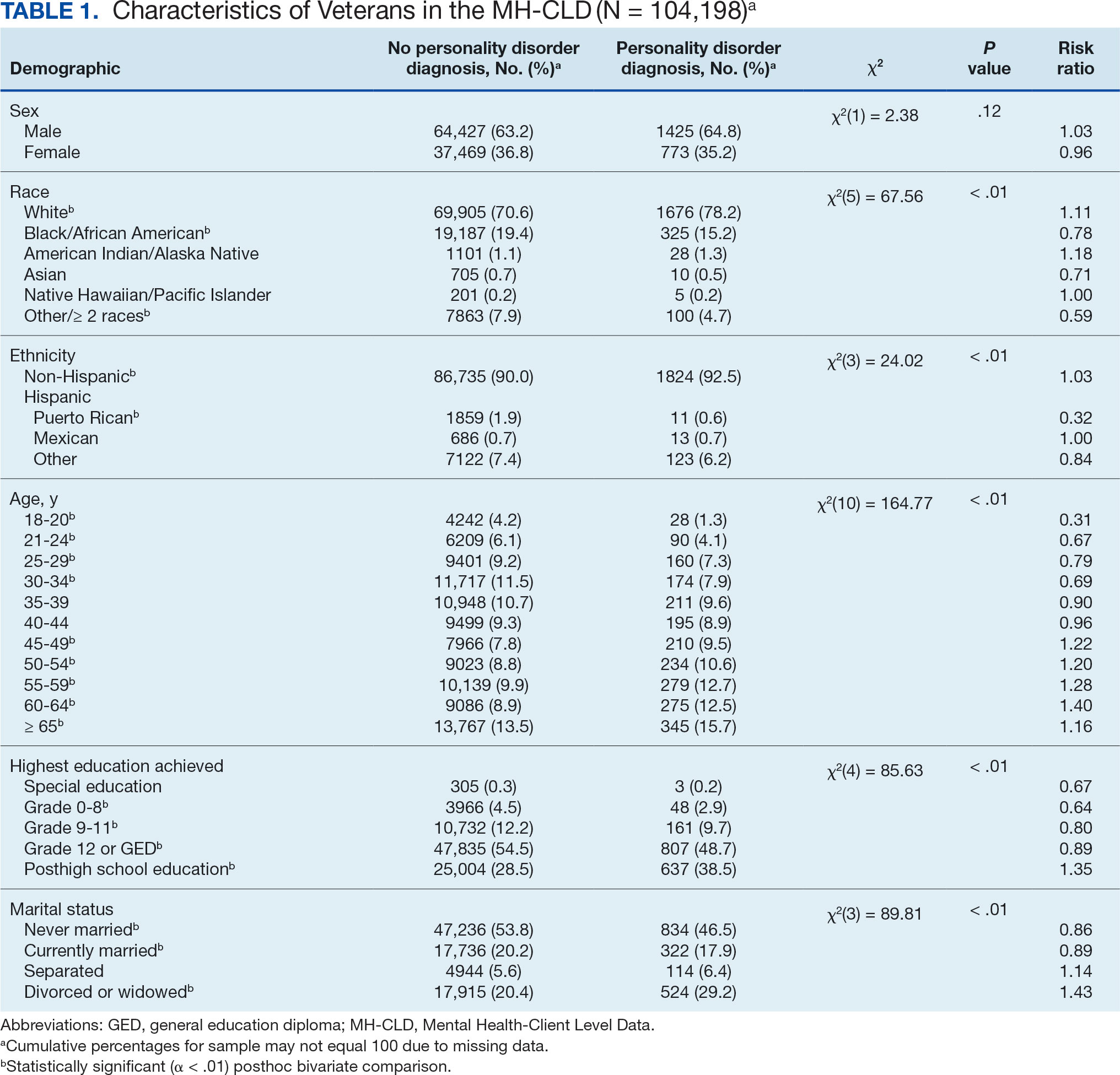
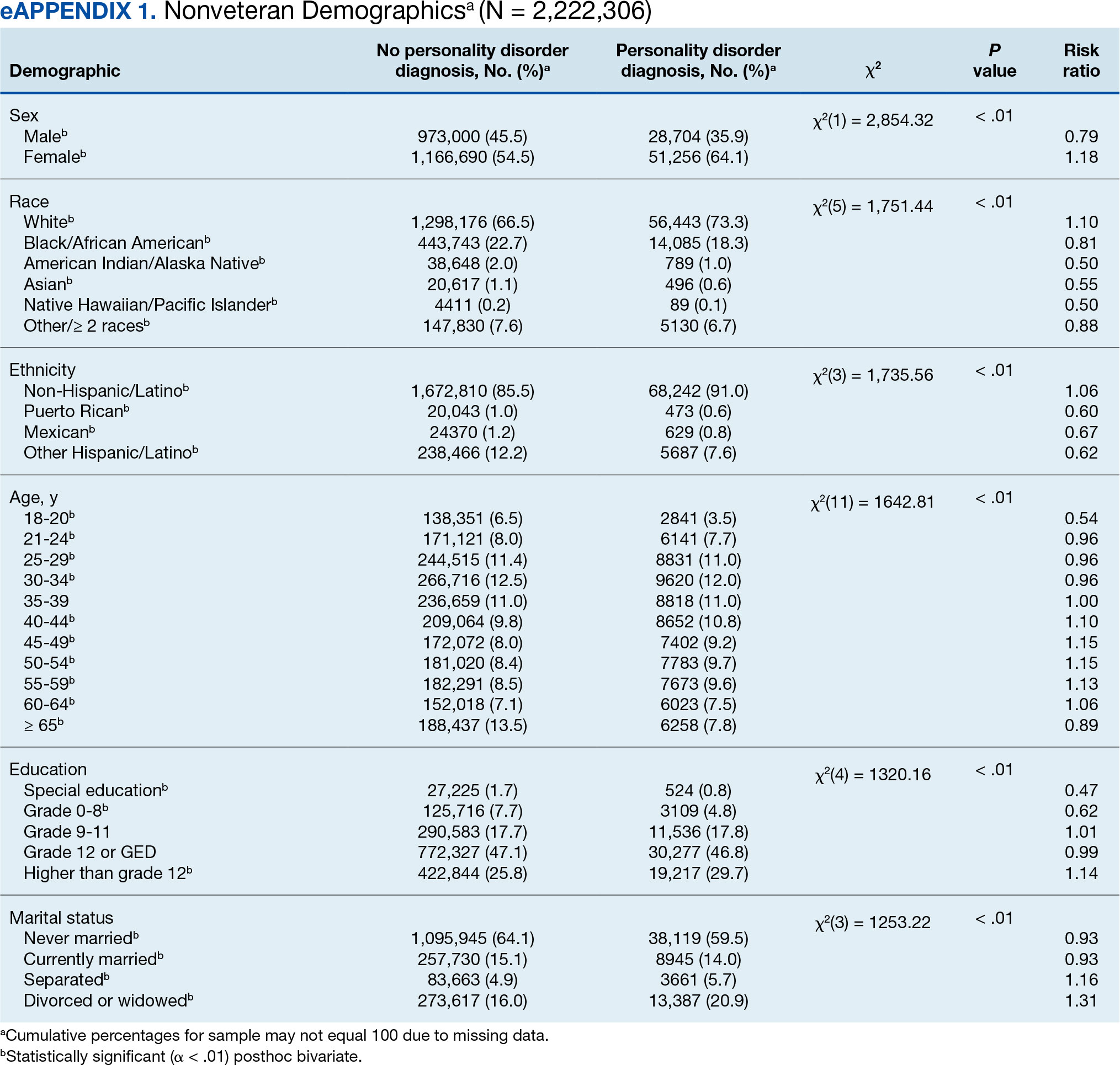
Veterans with a PD diagnosis were more likely than those without a diagnosis to have more diagnoses (RR, 2.96-8.49) and to have comorbid trauma or related stressor (RR, 1.33), or bipolar (RR, 1.56) or psychotic (RR, 1.15) disorder diagnoses, but less likely to have comorbid depressive disorder (RR, 0.82). Although veterans with and without a PD diagnosis were similarly likely to have a comorbid SUD (RR, 1.13), those with a PD diagnosis were significantly less likely to be assigned a substance use problem designation (RR, 0.78). PD diagnosis was also more common among veterans who received services in state psychiatric hospitals (RR, 3.05), community-based clinics (RR, 1.06), and judicial institutions (RR, 6.33) and less common among those who received services in other psychiatric inpatient settings (RR, 0.30). No differences were observed for residential treatment settings (RR, 0.79). Among nonveterans, a PD diagnosis was associated with slightly greater odds of a substance use designation (RR, 1.03) (eAppendix 2).
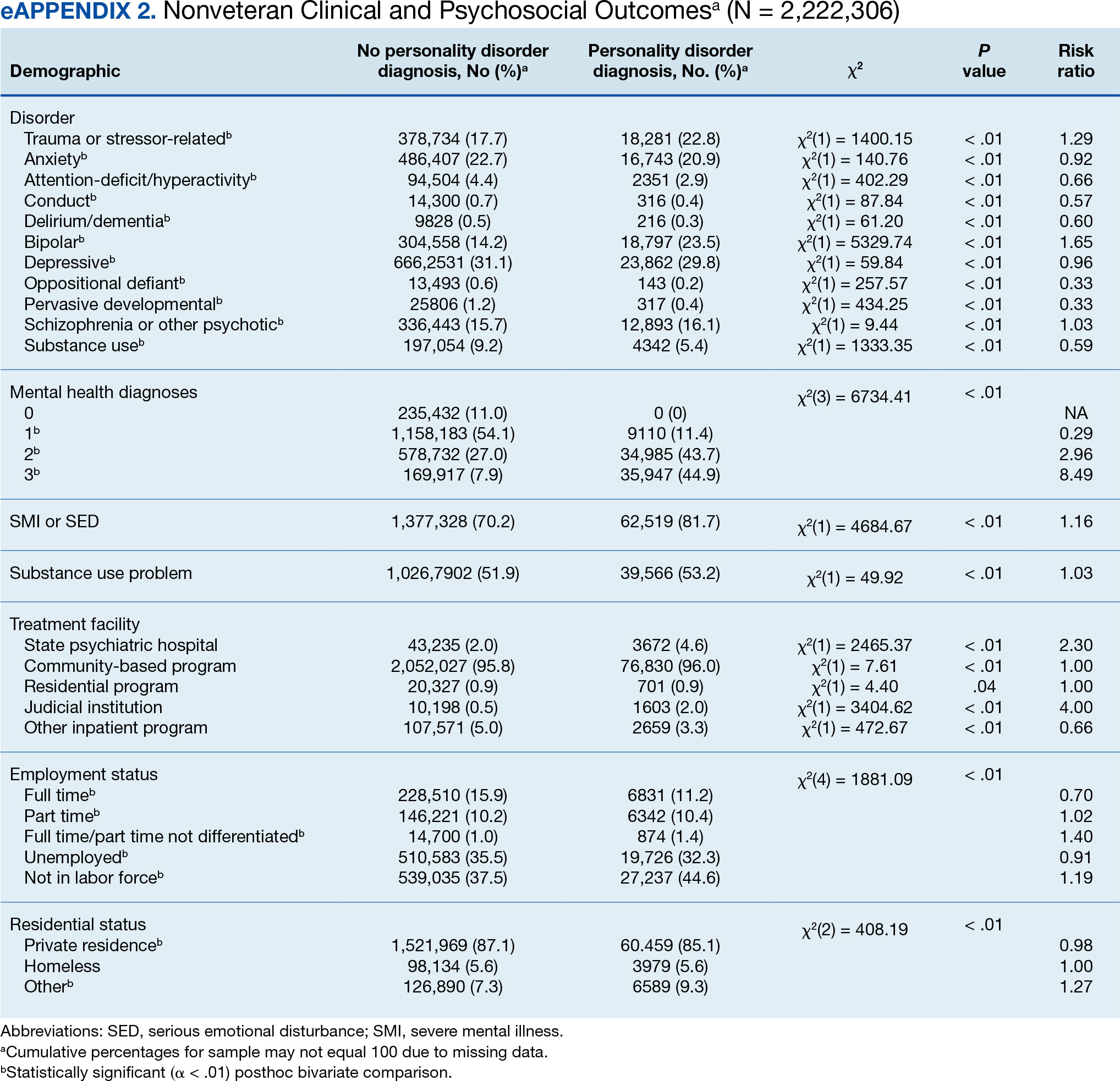
Veterans with a PD diagnosis were also less likely to have full-time employment (RR, 0.73) and more likely to have undifferentiated employment (RR, 2.00) or to be removed from the labor force (RR, 1.35). Veterans with a PD diagnosis were also more likely to reside in nontraditional living conditions (RR, 1.42) and less likely to be residing in a private residence (RR, 0.98), compared with those without PD diagnosis. The rates of homelessness were similar for veterans with and without a PD diagnosis (RR, 0.90) (Table 2). These patterns were similar among nonveterans.
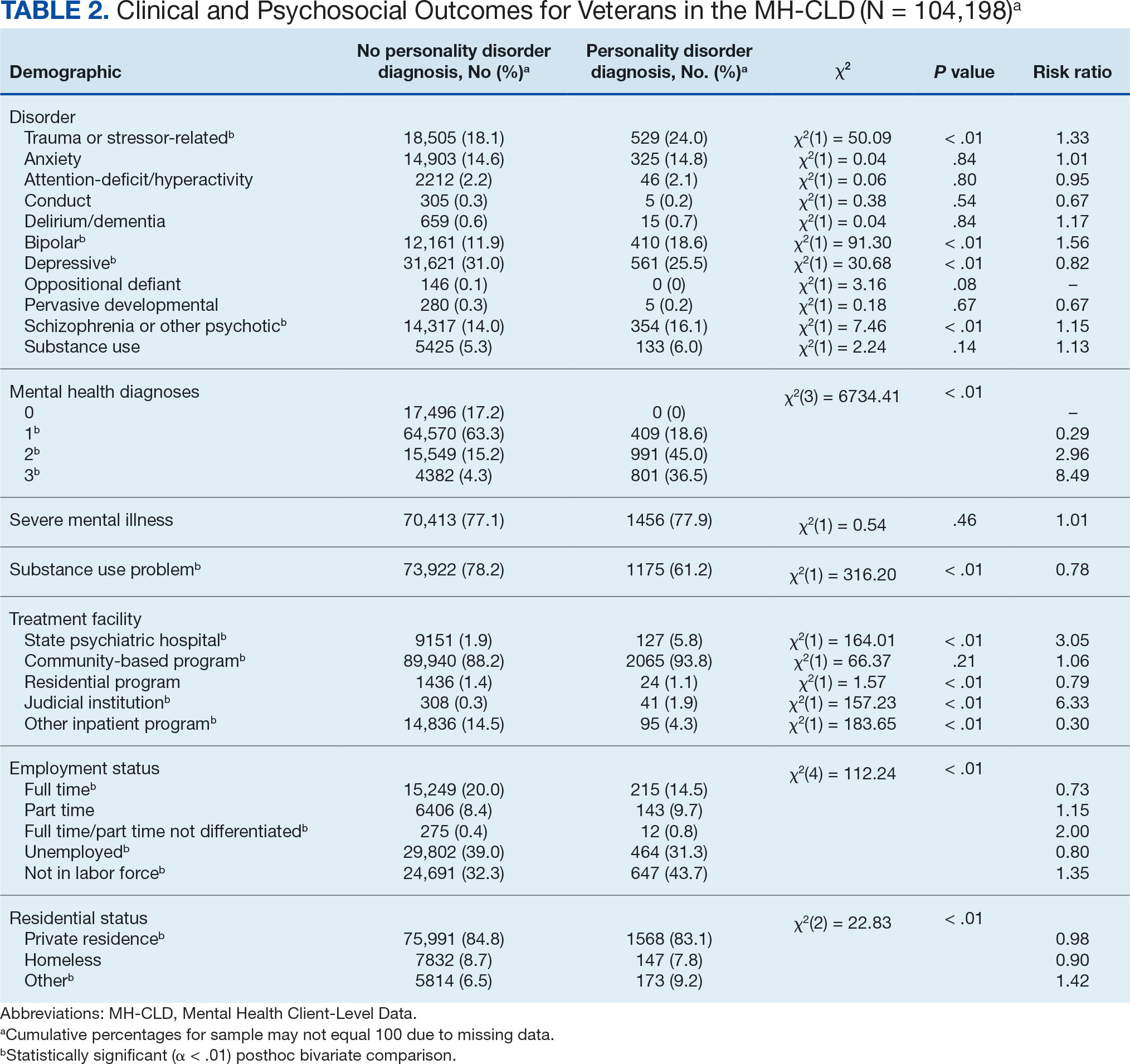
DISCUSSION
This study examined the rate and correlates of PD diagnosis among a large, community-based sample of veterans receiving state-funded mental health care. About 2% of veterans in this sample had a PD diagnosis, with diagnoses more common among veterans who were White, non-Hispanic, aged ≥ 45 years, with higher education, divorced or widowed, also diagnosed with trauma-related, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in state psychiatric hospital, community-based clinic, or judicial system settings.
The observed rate of PD diagnosis in this study aligns with what is typically observed in VHA EHRs.8,18,19 However, the rate is notably lower than prevalence estimates for psychiatric outpatient settings (about 50%) and in meta-analyses of prevalence among veterans (0.8%-23% for each of the 10 PDs).4,17,26 Longstanding stigma against PDs may contribute to underdiagnosis. For example, many clinicians are concerned that documentation or disclosure of a PD will interfere with the patient’s ability to access treatment due to stigma and discrimination.27,28 These fears are not unfounded; even among clinicians, PDs are commonly considered untreatable, and many individuals with PDs are denied access to evidence-based treatments due to the diagnosis.29 In a 2016 survey of community psychiatrists, nearly 1 in 4 reported that they avoid taking patients with a borderline PD diagnosis in their caseloads.28 To date, no studies have been conducted to explore clinicians’ willingness to accept patients with other PDs or, specifically, among veterans.
Despite such widespread stigma, research suggests clinicians' negative attitudes toward PDs can be decreased through antistigma campaigns.30 However, it remains unclear if such efforts also contribute to an increase in clinicians’ willingness to document PD diagnoses. Without accurate identification and documentation, the field’s understanding of PDs will remain limited.
In the current study, veterans with PD diagnoses tended to present with more complex and severe psychiatric comorbidities compared to veterans without such diagnoses. Observed comorbidity of PDs (particularly borderline PD) with trauma-related and bipolar disorders is well established.8 Conversely, co-occurring personality and psychotic disorders—which comprise 16% of veterans with a PD diagnosis in the sample in this study—are not consistently examined in the literature. A 2022 examination of veterans receiving VHA care suggested 12% and 13% of those with a PD diagnosis documented in their EHR also had documented schizophrenia or another psychotic disorder, respectively. PD diagnoses were associated with 6.88- and 9.80-fold increases in risk for comorbid schizophrenia and other psychotic disorder diagnoses, respectively.8 Similarly, a recent longitudinal study of nearly 2 million Swedish individuals suggested borderline PD is specifically associated with a > 24-times greater risk of having a comorbid psychotic disorder.31 It is therefore possible that the comorbidity between personality and psychotic disorders is quite common despite its relative lack of attention in empirical research.
Veterans with PD diagnoses in this study were also more likely to experience substandard housing, employment challenges, and receive treatment through judicial institutions than those without a PD diagnosis. Such findings are consistent with previous research demonstrating the substantial psychosocial challenges associated with PD diagnosis, even after controlling for comorbid conditions.7,9 Veterans with PDs may benefit from specialized case management and support to facilitate stable housing and employment and to mitigate the risk of judicial involvement. Some research suggests veterans with PDs may be less likely to gain competitive employment after participating in VA therapeutic and supportive employment services programs, suggesting standard programming may be less suitable for this population.32 Similarly, other research suggests individuals with PDs may benefit more from specialized, intensive services than standard clinical case management.33 Future research may therefore benefit from clarifying the degree to which adaptations to standard programming could yield beneficial effects for persons with PD diagnoses.
Implications
Cumulatively, the results of this study attest to the necessity for transdiagnostic treatment planning that includes close collaboration between psychotherapeutic, pharmacological, and case management services. Some psychotherapy models for PDs, such as dialectical behavior therapy (DBT), which includes a combination of group skills training, individual therapy, as-needed phone coaching, and therapist consultation, may be successfully adapted to include this collaboration.34-36 However, implementation of such comprehensive programming often requires extensive clinician training and coordination of resources, which poses implementation challenges.37-39 In 2021, the VHA began large-scale implementation of PD-specific psychotherapy for veterans with recent suicidal self-directed violence and borderline PD, including DBT, though to date results remain unclear.40 Generalist approaches, such as good psychiatric management (GPM), which emphasizes emotional validation, practical problem solving, realistic goal setting, and relationship functioning within the context of standard care appointments, may be more easily implemented in community care settings due to lesser training and resource requirements and can also be adapted to include needed elements of care coordination.41,42 Both DBT and GPM were initially developed for the treatment of borderline PD. Although DBT has also demonstrated some effectiveness in the treatment of antisocial PD, potential applications of DBT and GPM to other PDs remain largely underdeveloped.43-46
There are no widely accepted medications for the treatment of PDs. Pharmacotherapy for these conditions typically consists of individualized approaches informed by personal experience that attempt to balance targeting of specific symptoms while minimizing polypharmacy and potential risks (eg, overdose or addiction).47,48 Despite this, pharmacotherapy is often considered a necessary component in the treatment of bipolar and psychotic disorders, both common comorbidities of PDs found in veterans in this study.49,50 Careful consideration of complex comorbidities and pharmacotherapy needs is warranted in the treatment of veterans with PDs. Future research may benefit from clarifying clinical guidelines around pharmacotherapy, particularly for observed comorbidities of PDs to trauma, bipolar, and psychotic disorders.
It is important to note the discrepancies in the results of this study surrounding patient substance use. The results suggest a negligible or inverse association between the likelihood of a PD diagnosis and difficulties with substance use among the veterans in this study. However, the unexpectedly low rate of SUD diagnoses (< 6%) suggests that they were likely underdocumented. Research suggests a strong association between personality and SUDs in both veteran and civilian samples.6,51 Results suggesting a lower prevalence of substance use difficulties among treatment-seeking veterans with PDs should be interpreted with great caution.
Demographically, PD diagnoses were more common among veterans who were White, non-Hispanic, and aged ≥ 45 years, and less common among veterans who were Black/ African American, mixed/unspecified race, Puerto Rican or other non-Mexican Hispanic ethnicity, or aged < 35 years. No significant sex-based differences were observed. These patterns are consistent with research suggesting individuals who identify as Black may be less likely than individuals who identify as White to report PD symptoms, meet criteria for a PD, and have a PD diagnosed even when it is warranted.52
The findings observed in this study with respect to age, however, are notably inconsistent with the literature. Previous research typically suggests a negative association between age and PD pathology; however, a 2020 review of PDs in older adults by Penders et al suggests a prevalence of 11% to 15% in this population.53,54 Research into PDs most often focuses on adolescent and early adulthood developmental periods, limiting insight into the phenomenology of PDs in middle to late adulthood.55 Further, most research into PDs among geriatric populations has focused on psychometric assessment rather than practical treatment guidance.54 However, in this study, elevated risk for PD diagnoses was salient throughout middle to late adulthood among veterans; similar, albeit less pronounced patterns were also observed for elevated risk of PD diagnosis in middle adulthood among nonveterans. Such findings suggest clarifying the phenomenology and treatment needs of individuals with PDs in middle to late adulthood may have particularly salient implications for the mental health care of veterans affected by these conditions. As the veteran population advances in age, these needs will present unique challenges if health care systems are unprepared to effectively address them.
Limitations
This study is characterized by several strengths, most notably its use of a large dataset recently collected on a national scale. Few studies outside of the VHA system include samples of > 100,000 treatment-seeking veterans collected on a national scale. Nevertheless, results should be understood within the context of several methodological limitations. However, the dataset was limited to the first 3 diagnoses documented in patients’ EHRs, and many patients had no listed diagnoses. Patients with complex comorbidities may have > 3 diagnoses; for these individuals, data provided an incomplete picture of clinical presentation. This is especially relevant for individuals with PDs, who tend to meet criteria for a range of comorbid conditions.8,10 The now dated practice of listing PDs on Axis II also increases the chance of clinicians listing PDs after conditions traditionally listed on Axis I (eg, major depressive disorder) in patient charts.56 This study’s inclusion of only the first 3 listed diagnoses likely underestimated true PD diagnosis prevalence.
The results of this study must be interpreted as reflecting the prevalence and correlates of receiving a PD diagnosis rather than meeting diagnostic criteria for a PD. Relatedly, PD diagnoses were reported as a single construct, limiting insight into prevalence and correlates of individual PD diagnoses (eg, borderline vs paranoid PDs). Meta-analyses estimates suggest PD prevalence among veterans is likely much higher than observed in this study.17 Stigma continues to discourage clinicians from documenting and disclosing PD diagnoses even when warranted.27,28 Continued research should aim to clarify conditions (eg, patient presentation, stigma, or institutional culture) contributing to documentation of PD diagnoses. Given the cross-sectional nature of this study, results cannot speak to longitudinal treatment outcomes or prognosis of persons receiving a PD diagnosis.
Despite its large sample size and national representation, the sampling strategy of this study could have contributed to idiosyncrasies in the dataset. Restriction of data to the persons receiving state-funded mental health services introduces a notable bias to the composition of the sample, which is likely comprised of a disproportionately high number of Medicaid recipients, students, and individuals with chronic illnesses and underrepresentation of persons who pay for mental health services using private insurance or private pay arrangements. As such, although socioeconomic information was not provided within this dataset, one can presume a generally lower socioeconomic status among study participants compared to the community at large. This study also included a proportionally small sample of veterans (3.6% compared to about 6.2% in the broader US population), suggesting veterans may have been underrepresented or underidentified in surveyed mental health care settings.57 This study also did not include data around service in active-duty military, national guard, or military reserves; a greater proportion of the sample likely had a history of military service than was represented by veteran status designation. Further, the proportionally high sample of individuals with severe mental illness suggests a likely overrepresentation of such conditions in surveyed settings.
Institutional differences in the practice of assigning diagnoses likely limited statistical power to detect potentially meaningful associations and effects. Structural influences, such as stigma and institutional culture, may have notable effects on documentation practices, particularly for PDs. Future research should aim to replicate observed associations using more controlled diagnostic procedures.
Lastly, even with the use of a more conservative α and a focus on effect sizes to guide interpretation of results, use of multiple bivariate analyses can be presumed to have increased the likelihood of type I error. Given the limited prior research in this area, an exploratory approach to statistical analysis was considered warranted to maximize opportunity for identifying areas in need of additional empirical attention. Continued research using more conservative statistical approaches (eg, multivariate analyses) is needed to determine replicability and generalizability of observed results.
CONCLUSIONS
This study examined the prevalence and correlates of PD diagnoses in a national sample of veterans receiving community-based, state-funded mental health care. About 2% received a PD diagnosis, with diagnoses most common among veterans who were White, non-Hispanic, aged ≥ 45 years, also diagnosed with trauma-based, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in a state psychiatric hospital or judicial system setting. The results attest to a necessity for transdiagnostic treatment planning and care coordination for this population, with particular attention to psychosocial stressors.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
- Hastrup LH, Jennum P, Ibsen R, Kjellberg J, Simonsen E. Societal costs of borderline personality disorders: a matched-controlled nationwide study of patients and spouses. Acta Psychiatr Scand. 2019;140(5):458-467. doi:10.1111/acps.13094
- Sveen CA, Pedersen G, Ulvestad DA, Zahl KE, Wilberg T, Kvarstein EH. Societal costs of personality disorders: a cross-sectional multicenter study of treatment-seeking patients in mental health services in Norway. J Clin Psychol. 2023;79(8):1752-1769. doi:10.1002/jclp.23504
- Beckwith H, Moran PF, Reilly J. Personality disorder prevalence in psychiatric outpatients: a systematic literature review. Personal Ment Health. 2014;8(2):91-101. doi:10.1002/pmh.1252
- Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc. 2017.09.001
- Edwards ER, Barnes S, Govindarajulu U, Geraci J, Tsai J. Mental health and substance use patterns associated with lifetime suicide attempt, incarceration, and homelessness: a latent class analysis of a nationally representative sample of U.S. veterans. Psychol Serv. 2021;18(4):619-631. doi:10.1037/ser0000488
- Moran P, Romaniuk H, Coffey C, et al. The influence of personality disorder on the future mental health and social adjustment of young adults: a population-based cohort study. Lancet Psychiatry. 2016;3(7):636-645. doi:10.1016/S2215-0366(16)30029-3
- Nelson SM, Griffin CA, Hein TC, Bowersox N, McCarthy JF. Personality disorder and suicide risk among patients in the Veterans Affairs health system. Personal Disord. 2022;13(6):563-571. doi:10.1037/per0000521
- Skodol AE. Impact of personality pathology on psychosocial functioning. Curr Opin Psychol. 2018;21;33-38. doi:10.1016/j.copsyc.2017.09.006
- Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015;385(9969):717-726. doi:10.1016/S0140-6736(14)61995-4
- Fitzpatrick S, Goss S, Di Bartolomeo A, Varma S, Tissera T, Earle E. Follow the money: is borderline personality disorder research underfunded in Canada? Can Psychol. 2024;65(1):46-57. doi:10.1037/cap0000375
- Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944. doi:10.1016/j.psychres.2014.09.021
- Leroux TC. U.S. military discharges and pre-existing personality disorders: a health policy review. Adm Policy Ment Health. 2015;42(6):748-755. doi:10.1007/s10488-014-0611-z
- Monahan MC, Keener JK. Fitness-for-duty evaluations. In Kennedy CH, Zillmer EA, eds. Military Psychology: Clinical and Operational Applications. 2nd ed. Guilford Publications; 2012:25-49.
- Hearing Before the Committee on Veterans’ Affairs, 111th Congress 2nd Sess (2010). Personality disorder discharges: impact on veterans benefits. Accessed March 4, 2025. https://www.govinfo.gov/content/pkg/CHRG-111hhrg61755/html/CHRG-111hhrg61755.htm
- Ader M, Cuthbert R, Hoechst K, Simon EH, Strassburger Z, Wishnie M. Casting troops aside: the United States military’s illegal personality disorder discharge problem. Vietnam Veterans of America. March 2012. Accessed February 28, 2025. https://law.yale.edu/sites/default/files/documents/pdf/Clinics/VLSC_CastingTroopsAside.pdf
- Edwards ER, Tran H, Wrobleski J, Rabhan Y, Yin J, Chiodi C, Goodman M, Geraci J. Prevalence of personality disorders across veteran samples: A meta-analysis. J Pers Disord. 2022;36(3):339-358. doi:10.1521/ pedi.2022.36.3.339
- Holliday R, Desai A, Edwards E, Borges L. Personal i ty disorder diagnosis among just ice -involved veterans: an investigation of VA-using veterans. J Nerv Ment Dis. 2023;211(5):402-406 doi:10.1097/ NMD.0000000000001627
- McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942. doi:10.2105/AJPH.2015.302737
- Liu Y, Chen C, Zhou Y, Zhang N, Liu S. Twenty years of research on borderline personality disorder: a scientometric analysis of hotspots, bursts, and research trends. Front Psych. 2024;15:1361535. doi:10.3389/ fpsyt.2024.1361535
- Williams R, Holliday R, Clem M, Anderson E, Morris EE, Surís A. Borderline personality disorder and military sexual trauma: analysis of previous traumatization and current psychiatric presentation. J Interpers Violence. 2017;32(15):2223-2236. doi:10.1177/0886260515596149
- Holder N, Holliday R, Pai A, Surís A. Role of borderline personality disorder in the treatment of military sexual trauma-related posttraumatic stress disorder with cognitive processing therapy. Behav Med. 2017;43(3):184-190. doi:10.1080/08964289.2016.1276430
- Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of personality disorders on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. Am J Addict. 2007;16(6):443- 449. doi:10.1080/10550490701643336
- Walter KH, Bolte TA, Owens GP, Chard KM. The impact of personality disorders on treatment outcome for veterans in a posttraumatic stress disorder residential treatment program. Cognit Ther Res. 2012;36(5):576-584. doi:10.1007/s10608-011-9393-8
- Substance Abuse and Mental Health Services. Mental health client-level data (MH-CLD), 2022. Accessed February 28, 2025. https://www.datafiles.samhsa.gov/dataset/mental-health-client-level-data-2022-mh-cld-2022-ds0001
- Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918. doi:10.1176/appi.ajp.162.10.1911
- Campbell K, Clarke KA, Massey D, Lakeman R. Borderline personality disorder: To diagnose or not to diagnose? That is the question. Int J Mental Health Nurs. 2020;29(5):972-981. doi:10.1111/inm.12737
- Sisti D, Segal AG, Siegel AM, Johnson R, Gunderson J. Diagnosing, disclosing, and documenting borderline personality disorder: a survey of psychiatrists’ practices. J Pers Disord. 2016;30(6):848-856. doi:10.1521/ pedi_2015_29_228
- Klein P, Fairweather AK, Lawn S. Structural stigma and its impact on healthcare for borderline personality disorder: a scoping review. Int J Ment Health Syst. 2022;16(1):48. doi:10.1186/s13033-022-00558-3
- Knaak S, Szeto AC, Fitch K, Modgill G, Patten S. Stigma towards borderline personality disorder: effectiveness and generalizability of an anti-stigma program for healthcare providers using a pre-post randomized design. Borderline Personal Disord Emot Dysregul. 2015;2:9. doi:10.1186/s40479-015-0030-0
- Tate AE, Sahlin H, Liu S, et al. Borderline personality disorder: associations with psychiatric disorders, somatic illnesses, trauma, and adverse behaviors. Mol Psychiatry. 2022;27:2514-2521. doi:10.1038/s41380- 022-01503-z
- Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA Therapeutic and Supported Employment Services programs. Psychiatr Serv. 2017;68(9)938-946. doi:10.1176/appi. ps201600412
- Frisman LK, Mueser KT, Covell NH, et al. Use of integrated dual disorder treatment via Assertive Community Treatment versus clinical case management for persons with co-occurring disorders and antisocial personality disorder. J Nerv Ment Dis. 2009;197(11):822-828. doi:10.1097/NMD.0b013e3181beac52
- Edwards ER, Kober H, Rinne GR, Griffin SA, Axelrod S, Cooney EB. Skills]homework completion and phone coaching as predictors of therapeutic change and outcomes in completers of a DBT intensive outpatient programme. Psychol Psychother. 2021;94(3):504-522. doi:10.1111/papt.12325
- Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67(1):13-26. doi:10.1016/s0376-8716(02)00011-x
- Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. JAMA Psychiatry. 2015;72(5):475-482.doi:10.1001 /jamapsychiatry.2014.3039
- Carmel A, Rose ML, Fruzzetti AE. Barriers and solutions to implementing dialectical behavior therapy in a public behavioral health system. Adm Policy Ment Health. 2014;41(5):608-614. doi:10.1007/s10488-013-0504-6
- Decker SE, Matthieu MM, Smith BN, Landes SJ. Barriers and facilitators to dialectical behavior therapy skills groups in the Veterans Health Administration. Mil Med. 2024;189(5-6):1055-1063. doi:10.1093/milmed/ usad123
- Landes SJ, Rodriguez AL, Smith BN, et al. Barriers, facilitators, and benefits of implementation of dialectical behavior therapy in routine care: results from a national program evaluation survey in the Veterans Health Administration. Transl Behav Med. 2017;7(4):832-844. doi:10.1007/s13142-017-0465-5
- Walker J, Betthauser LM, Green K, Landes SJ, Stacy M. Suicide Prevention 2.0 Clinical Telehealth Program: Evidence- Based Treatment in the Veterans Health Administration. April 28, 2024. Accessed February 28, 2025. https://www.youtube.com/watch?v=fFsDzkg0SR0
- Gunderson J, Masland S, Choi-Kain L. Good psychiatric management: a review. Curr Opin Psychol. 2018;21:127- 131. doi:10.1016/j.copsyc.2017.12.006
- Kramer U. Good-enough therapy: a review of the empirical basis of good psychiatric management. Am J Psychother. 2025;78(1): 11-15. doi:10.1176/appi .psychotherapy.20230041
- Visdómine-Lozano JC. Contextualist perspectives in the treatment of antisocial behaviors and offending: a comparative review of FAP, ACT, DBT, and MDT. Trauma Violence Abuse. 2022;23(1):241-254. doi:10.1177/1524838020939509
- Drago A, Marogna C, Jørgen Søgaard H. A review of characteristics and treatments of the avoidant personality disorder. Could the DBT be an option? Int J Psychol Psychoanal. 2016;2(1):013.
- Finch EF, Choi-Kain LW, Iliakis EA, Eisen JL, Pinto A. Good psychiatric management for obsessive–compulsive personality disorder. Curr Behav Neurosci Rep. 2021;8:160-171. doi:10.1007/s40473-021-00239-4
- Miller TW, Kraus RF. Modified dialectical behavior therapy and problem solving for obsessive-compulsive personality disorder. Journal Contemp Psychother. 2007;37:79-85. doi:10.1007/s10879-006-9039-4
- Bozzatello P, Rocca P, De Rosa ML, Bellino S. Current and emerging medications for borderline personality disorder: is pharmacotherapy alone enough? Expert Opin Pharmacother. 2020;21(1):47-61.doi:10.1080/14656566 .2019.1686482
- Sand P, Derviososki E, Kollia S, Strand J, Di Leone F. Psychiatrists’ perspectives on prescription decisions for patients with personality disorders. J Pers Disord. 2024;38(3):225-240. doi:10.1521/pedi.2024.38.3.225
- Kane JM, Leucht S, Carpenter D, Docherty JP; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: Methods, commentary, and summary. J Clin Psychiatry. 2003;64 Suppl 12:5-19.
- Nierenberg AA, Agustini B, Köhler-Forsberg O, et al. Diagnosis and treatment of bipolar disorder: a review. JAMA. 2023;330(14):1370-1380. doi:10.1001 /jama.2023.18588
- Köck P, Walter M. Personality disorder and substance use disorder–an update. Ment Health Prev. 2018;12:82- 89. doi:10.1016/J.MHP.2018.10.003
- Garb HN. Race bias and gender bias in the diagnosis of psychological disorders. Clin Psych Rev. 2021;90:102087. doi:10.1016/j.cpr.2021.102087
- Debast I, van Alphen SPJ, Rossi G, et al. Personality traits and personality disorders in late middle and old age: do they remain stable? A literature review. Clin Gerontol. 2014;37(3):253-271.doi:10.1080/07317115 .2014.885917
- Penders KAP, Peeters IGP, Metsemakers JFM, van Alphen SPJ. Personality disorders in older adults: a review of epidemiology, assessment, and treatment. Curr Psychiatry Rep. 2020;22(3):1-14. doi:10.1007/s11920-020- 1133-x
- Videler AC, Hutsebaut J, Schulkens JEM, Sobczak S, van Alphen SPJ. A life span perspective on borderline personality disorder. Curr Psychiatry Rep. 2019;21(7) :1-8. doi:10.1007/s11920-019-1040-1
- Wakefield JC. DSM-5 and the general definition of personality disorder. Clin Soc Work J. 2013;41(2):168-183. doi:10.1007/s10615-012-0402-5
- US Census Bureau. 2022 American Community Survey 1-year. Accessed February 28, 2025. https://data.census.gov/table/ACSST1Y2022.S2101?q=Veterans&y=2022comparison
Personality disorders (PDs) are enduring patterns of internal experience and behavior that differ from cultural norms and expectations, are inflexible and pervasive, have their onset in adolescence or early adulthood, and lead to distress or impairment. Ten PDs are included in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition): paranoid, schizoid, schizotypal, borderline, antisocial, histrionic, narcissistic, avoidant, dependent, and obsessive-compulsive.1 These disorders impose a high burden on patients, families, health care systems, and broader economic systems.2,3 Up to 1 in 7 persons in the community and 50% of those receiving outpatient mental health treatment experience a PD.4,5 These conditions are associated with an increased risk of adverse events, including suicide attempt and death by suicide, criminal-legal involvement, homelessness, substance use, underemployment, relational issues, and high utilization of psychiatric services.6-9 PDs are routinely underassessed, underdocumented, and undertreated in clinical settings, and consistently receive less research funding than other, less prevalent forms of psychopathology. 10-12 As a result, there is limited understanding of clinical needs of individuals experiencing PDs.
MILITARY VETERANS WITH PERSONALITY DISORDERS
Underacknowledgment of PDs and their associated difficulties may be especially pronounced in veteran populations. Due to longstanding etiological theories that implicate childhood trauma and adolescent onset in pathology development, PDs are traditionally considered pre-existing conditions or developmental abnormalities by the US Department of Defense and US Department of Veterans Affairs (VA). As a result, PDs are therefore deemed incompatible with military service and ineligible for service-connected disability benefits.13-15 Such determinations allowed PD pathology to be used as grounds for discharge for 26,000 service members from 2001 to 2007, or 2.6% of total enlisted discharges during that period.13,15,16
Despite this structural discrimination, recent research suggests veterans may be more likely to experience PD pathology than the general population.17 For example, a 2021 epidemiological survey in a community-based veteran sample found elevated rates of borderline, antisocial, and schizotypal PDs (6%-13%).6 In contrast, only 0.8% to 5.0% of veteran electronic health records (EHRs) have a documented PD diagnosis.8,18,19 Such elevations in PD pathology within veteran samples imply either a disproportionately high prevalence among enlistees (and therefore missed during recruitment procedures) or onset following military service, possibly due to exposure to traumatic events and/ or occupational stress.17 Due to the relative infancy of research in this area and a lack of longitudinal studies, etiology and course of illness for personality pathology in veterans remains largely unclear.
Structural underacknowledgment of PDs among military personnel has contributed to their underrepresentation in research on veteran populations. PD-focused research with veterans is rare, despite a rapid increase in broader empirical attention paid to these conditions in nonveteran samples.20 A recent meta-analysis of veterans with PDs identified 27 studies that included basic prevalence statistics. PDs were rarely a primary focus for these studies, and most were limited to veterans seen in Veterans Health Administration (VHA) settings.17 The literature also paints a bleak picture, suggesting veterans who experience PDs are at higher risk for suicide attempt and death by suicide, criminal-legal involvement, and homelessness. They also tend to experience more severe comorbid psychopathological symptoms and more often use high-intensity mental health services (eg, care within emergency departments or psychiatric inpatient settings) than veterans without PD pathology.6,8,18,19,21 However, PD pathology does not appear to impede the effectiveness of treatment for veterans.22-24 The implications of PD pathology on broader psychosocial functioning and health care needs certify a need for additional research that examines patterns of personality pathology, particularly in veterans outside the VHA.
METHODS
This study aims to enhance understanding of veterans affected by PDs and offer insight and guidance for treatment of these conditions in federal and nonfederal treatment settings. Previous research has been largely limited to VHA care-receiving samples; the longstanding stigma against PDs by the US military and VA may contribute to biased diagnosis and documentation of PDs in these settings. A large sample of veterans receiving community-based mental health care was therefore used to explore aims of the current study. This study specifically examined demographic patterns, diagnostic comorbidity, psychosocial outcomes, and treatment care settings among veterans with and without a PD diagnosis. Consistent with previous research, we hypothesized that veterans with a PD diagnosis would have more severe mental health comorbidities, poorer psychosocial outcomes, and receive care in higher intensity settings relative to veterans without a diagnosis.
Data for the sample were drawn from the Mental Health Client-Level Data, a publicly available national dataset of nearly 7 million patients who received mental health treatment services provided or funded through state mental health agencies in 2022.25 The analytic sample included about 2.5 million patients for whom veteran status and data around the presence or absence of a PD diagnosis were available. Of these patients, 104,198 were identified as veterans. Veteran patients were identified as predominantly male (63%), White (71%), non-Hispanic (90%), and never married (54%).
Measures
The parent dataset included demographic, clinical, and psychosocial outcome information reported by treatment facilities to individual state administrative systems for each patient who received services. To protect patient privacy, only nonprotected health information is included, and efforts were made throughout compilation of the parent dataset to ensure patient privacy (eg, limiting detail of information disseminated for public access). Because the parent dataset does not include protected health information, studies using these data are considered exempt from institutional review board oversight.
Demographic information. This study reviewed veteran status, sex, race, ethnicity, age, education, and marital status. Veteran status was defined by whether the patient was aged ≥ 18 years and had previously served (but was not currently serving) in the military. Patients with a history of service in the National Guard or Military Reserves were only classified as veterans if they had been called or ordered to active duty while serving. Sex was operationalized dichotomously as male or female; no patients were identified as intersex, transgender, or other gender identities.
Clinical information. Up to 3 mental health diagnoses were reported for each patient and included the following disorders: personality, trauma and attention-deficit/hyperactivity, stressor, anxiety, conduct, delirium/dementia, bipolar, depressive, oppositional defiant, pervasive developmental, schizophrenia or other psychotic, and alcohol or substance use. Mental health diagnosis categories were generated for the parent dataset by grouping diagnostic codes corresponding to each category. To protect patient privacy, more detailed diagnostic information was not available as part of the parent dataset. Although the American Psychiatric Association recognizes 10 distinct PDs, the exact nature of PD diagnoses was not included within the parent dataset. PD diagnoses were coded to reflect the presence or absence of any such diagnosis.
A substance use problem designation was also provided for patients according to various identification methods, including substance use disorder (SUD) diagnosis, substance use screening results, enrollment in a substance use program, substance use survey, service claims information, and other related sources of information. A severe mental illness or serious emotional disturbance designation was provided for patients meeting state definitions of these designations. Context(s) of service provision were coded as inpatient state psychiatric hospital, community-based program, residential treatment center, judicial institution, or other psychiatric inpatient setting.
Psychosocial outcome information. Patient employment and residential status were also included in analyses. Each reflected status at the time of discharge from services or end of reporting period; employment status was only provided for patients receiving treatment in community-based programs.
Data Analysis
Descriptive statistics and X2 analyses were used to compare demographic, clinical, and psychosocial outcome variables between patients with and without PD diagnoses. These analyses were calculated for both the 104,198 veterans and the 2,222,306 nonveterans aged ≥ 18 years in the dataset. Given the sample size, a conservative α of .01 was used to determine statistical significance.
RESULTS
In this sample of persons receiving state-funded mental health care, veterans were significantly less likely than nonveterans to have a documented PD diagnosis (2.1% vs 3.6%, X2 [1] = 647.49; P < .01). PD diagnoses were more common among White (risk ratio [RR], 1.11), non-Hispanic (RR, 1.03) veterans who were in middle to late adulthood (RR, 1.16-1.40), more educated (RR, 1.35), and divorced or widowed (RR, 1.43), and less common among Black/African American (RR, 0.78) or Puerto Rican (RR, 0.32) veterans who were in early adulthood (RR, 0.31-0.79), less educated (RR, 0.64-0.89), and currently married (RR, 0.89) or never married (RR, 0.86). Veteran men and women were equally likely to have a PD diagnosis (RR, 1.03) (Table 1). Among nonveterans, men were less likely than women to have a PD diagnosis (RR, 0.79), and PD diagnoses were most common among persons in middle adulthood (RR, 1.06-1.15) (eAppendix 1).


Veterans with a PD diagnosis were more likely than those without a diagnosis to have more diagnoses (RR, 2.96-8.49) and to have comorbid trauma or related stressor (RR, 1.33), or bipolar (RR, 1.56) or psychotic (RR, 1.15) disorder diagnoses, but less likely to have comorbid depressive disorder (RR, 0.82). Although veterans with and without a PD diagnosis were similarly likely to have a comorbid SUD (RR, 1.13), those with a PD diagnosis were significantly less likely to be assigned a substance use problem designation (RR, 0.78). PD diagnosis was also more common among veterans who received services in state psychiatric hospitals (RR, 3.05), community-based clinics (RR, 1.06), and judicial institutions (RR, 6.33) and less common among those who received services in other psychiatric inpatient settings (RR, 0.30). No differences were observed for residential treatment settings (RR, 0.79). Among nonveterans, a PD diagnosis was associated with slightly greater odds of a substance use designation (RR, 1.03) (eAppendix 2).

Veterans with a PD diagnosis were also less likely to have full-time employment (RR, 0.73) and more likely to have undifferentiated employment (RR, 2.00) or to be removed from the labor force (RR, 1.35). Veterans with a PD diagnosis were also more likely to reside in nontraditional living conditions (RR, 1.42) and less likely to be residing in a private residence (RR, 0.98), compared with those without PD diagnosis. The rates of homelessness were similar for veterans with and without a PD diagnosis (RR, 0.90) (Table 2). These patterns were similar among nonveterans.

DISCUSSION
This study examined the rate and correlates of PD diagnosis among a large, community-based sample of veterans receiving state-funded mental health care. About 2% of veterans in this sample had a PD diagnosis, with diagnoses more common among veterans who were White, non-Hispanic, aged ≥ 45 years, with higher education, divorced or widowed, also diagnosed with trauma-related, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in state psychiatric hospital, community-based clinic, or judicial system settings.
The observed rate of PD diagnosis in this study aligns with what is typically observed in VHA EHRs.8,18,19 However, the rate is notably lower than prevalence estimates for psychiatric outpatient settings (about 50%) and in meta-analyses of prevalence among veterans (0.8%-23% for each of the 10 PDs).4,17,26 Longstanding stigma against PDs may contribute to underdiagnosis. For example, many clinicians are concerned that documentation or disclosure of a PD will interfere with the patient’s ability to access treatment due to stigma and discrimination.27,28 These fears are not unfounded; even among clinicians, PDs are commonly considered untreatable, and many individuals with PDs are denied access to evidence-based treatments due to the diagnosis.29 In a 2016 survey of community psychiatrists, nearly 1 in 4 reported that they avoid taking patients with a borderline PD diagnosis in their caseloads.28 To date, no studies have been conducted to explore clinicians’ willingness to accept patients with other PDs or, specifically, among veterans.
Despite such widespread stigma, research suggests clinicians' negative attitudes toward PDs can be decreased through antistigma campaigns.30 However, it remains unclear if such efforts also contribute to an increase in clinicians’ willingness to document PD diagnoses. Without accurate identification and documentation, the field’s understanding of PDs will remain limited.
In the current study, veterans with PD diagnoses tended to present with more complex and severe psychiatric comorbidities compared to veterans without such diagnoses. Observed comorbidity of PDs (particularly borderline PD) with trauma-related and bipolar disorders is well established.8 Conversely, co-occurring personality and psychotic disorders—which comprise 16% of veterans with a PD diagnosis in the sample in this study—are not consistently examined in the literature. A 2022 examination of veterans receiving VHA care suggested 12% and 13% of those with a PD diagnosis documented in their EHR also had documented schizophrenia or another psychotic disorder, respectively. PD diagnoses were associated with 6.88- and 9.80-fold increases in risk for comorbid schizophrenia and other psychotic disorder diagnoses, respectively.8 Similarly, a recent longitudinal study of nearly 2 million Swedish individuals suggested borderline PD is specifically associated with a > 24-times greater risk of having a comorbid psychotic disorder.31 It is therefore possible that the comorbidity between personality and psychotic disorders is quite common despite its relative lack of attention in empirical research.
Veterans with PD diagnoses in this study were also more likely to experience substandard housing, employment challenges, and receive treatment through judicial institutions than those without a PD diagnosis. Such findings are consistent with previous research demonstrating the substantial psychosocial challenges associated with PD diagnosis, even after controlling for comorbid conditions.7,9 Veterans with PDs may benefit from specialized case management and support to facilitate stable housing and employment and to mitigate the risk of judicial involvement. Some research suggests veterans with PDs may be less likely to gain competitive employment after participating in VA therapeutic and supportive employment services programs, suggesting standard programming may be less suitable for this population.32 Similarly, other research suggests individuals with PDs may benefit more from specialized, intensive services than standard clinical case management.33 Future research may therefore benefit from clarifying the degree to which adaptations to standard programming could yield beneficial effects for persons with PD diagnoses.
Implications
Cumulatively, the results of this study attest to the necessity for transdiagnostic treatment planning that includes close collaboration between psychotherapeutic, pharmacological, and case management services. Some psychotherapy models for PDs, such as dialectical behavior therapy (DBT), which includes a combination of group skills training, individual therapy, as-needed phone coaching, and therapist consultation, may be successfully adapted to include this collaboration.34-36 However, implementation of such comprehensive programming often requires extensive clinician training and coordination of resources, which poses implementation challenges.37-39 In 2021, the VHA began large-scale implementation of PD-specific psychotherapy for veterans with recent suicidal self-directed violence and borderline PD, including DBT, though to date results remain unclear.40 Generalist approaches, such as good psychiatric management (GPM), which emphasizes emotional validation, practical problem solving, realistic goal setting, and relationship functioning within the context of standard care appointments, may be more easily implemented in community care settings due to lesser training and resource requirements and can also be adapted to include needed elements of care coordination.41,42 Both DBT and GPM were initially developed for the treatment of borderline PD. Although DBT has also demonstrated some effectiveness in the treatment of antisocial PD, potential applications of DBT and GPM to other PDs remain largely underdeveloped.43-46
There are no widely accepted medications for the treatment of PDs. Pharmacotherapy for these conditions typically consists of individualized approaches informed by personal experience that attempt to balance targeting of specific symptoms while minimizing polypharmacy and potential risks (eg, overdose or addiction).47,48 Despite this, pharmacotherapy is often considered a necessary component in the treatment of bipolar and psychotic disorders, both common comorbidities of PDs found in veterans in this study.49,50 Careful consideration of complex comorbidities and pharmacotherapy needs is warranted in the treatment of veterans with PDs. Future research may benefit from clarifying clinical guidelines around pharmacotherapy, particularly for observed comorbidities of PDs to trauma, bipolar, and psychotic disorders.
It is important to note the discrepancies in the results of this study surrounding patient substance use. The results suggest a negligible or inverse association between the likelihood of a PD diagnosis and difficulties with substance use among the veterans in this study. However, the unexpectedly low rate of SUD diagnoses (< 6%) suggests that they were likely underdocumented. Research suggests a strong association between personality and SUDs in both veteran and civilian samples.6,51 Results suggesting a lower prevalence of substance use difficulties among treatment-seeking veterans with PDs should be interpreted with great caution.
Demographically, PD diagnoses were more common among veterans who were White, non-Hispanic, and aged ≥ 45 years, and less common among veterans who were Black/ African American, mixed/unspecified race, Puerto Rican or other non-Mexican Hispanic ethnicity, or aged < 35 years. No significant sex-based differences were observed. These patterns are consistent with research suggesting individuals who identify as Black may be less likely than individuals who identify as White to report PD symptoms, meet criteria for a PD, and have a PD diagnosed even when it is warranted.52
The findings observed in this study with respect to age, however, are notably inconsistent with the literature. Previous research typically suggests a negative association between age and PD pathology; however, a 2020 review of PDs in older adults by Penders et al suggests a prevalence of 11% to 15% in this population.53,54 Research into PDs most often focuses on adolescent and early adulthood developmental periods, limiting insight into the phenomenology of PDs in middle to late adulthood.55 Further, most research into PDs among geriatric populations has focused on psychometric assessment rather than practical treatment guidance.54 However, in this study, elevated risk for PD diagnoses was salient throughout middle to late adulthood among veterans; similar, albeit less pronounced patterns were also observed for elevated risk of PD diagnosis in middle adulthood among nonveterans. Such findings suggest clarifying the phenomenology and treatment needs of individuals with PDs in middle to late adulthood may have particularly salient implications for the mental health care of veterans affected by these conditions. As the veteran population advances in age, these needs will present unique challenges if health care systems are unprepared to effectively address them.
Limitations
This study is characterized by several strengths, most notably its use of a large dataset recently collected on a national scale. Few studies outside of the VHA system include samples of > 100,000 treatment-seeking veterans collected on a national scale. Nevertheless, results should be understood within the context of several methodological limitations. However, the dataset was limited to the first 3 diagnoses documented in patients’ EHRs, and many patients had no listed diagnoses. Patients with complex comorbidities may have > 3 diagnoses; for these individuals, data provided an incomplete picture of clinical presentation. This is especially relevant for individuals with PDs, who tend to meet criteria for a range of comorbid conditions.8,10 The now dated practice of listing PDs on Axis II also increases the chance of clinicians listing PDs after conditions traditionally listed on Axis I (eg, major depressive disorder) in patient charts.56 This study’s inclusion of only the first 3 listed diagnoses likely underestimated true PD diagnosis prevalence.
The results of this study must be interpreted as reflecting the prevalence and correlates of receiving a PD diagnosis rather than meeting diagnostic criteria for a PD. Relatedly, PD diagnoses were reported as a single construct, limiting insight into prevalence and correlates of individual PD diagnoses (eg, borderline vs paranoid PDs). Meta-analyses estimates suggest PD prevalence among veterans is likely much higher than observed in this study.17 Stigma continues to discourage clinicians from documenting and disclosing PD diagnoses even when warranted.27,28 Continued research should aim to clarify conditions (eg, patient presentation, stigma, or institutional culture) contributing to documentation of PD diagnoses. Given the cross-sectional nature of this study, results cannot speak to longitudinal treatment outcomes or prognosis of persons receiving a PD diagnosis.
Despite its large sample size and national representation, the sampling strategy of this study could have contributed to idiosyncrasies in the dataset. Restriction of data to the persons receiving state-funded mental health services introduces a notable bias to the composition of the sample, which is likely comprised of a disproportionately high number of Medicaid recipients, students, and individuals with chronic illnesses and underrepresentation of persons who pay for mental health services using private insurance or private pay arrangements. As such, although socioeconomic information was not provided within this dataset, one can presume a generally lower socioeconomic status among study participants compared to the community at large. This study also included a proportionally small sample of veterans (3.6% compared to about 6.2% in the broader US population), suggesting veterans may have been underrepresented or underidentified in surveyed mental health care settings.57 This study also did not include data around service in active-duty military, national guard, or military reserves; a greater proportion of the sample likely had a history of military service than was represented by veteran status designation. Further, the proportionally high sample of individuals with severe mental illness suggests a likely overrepresentation of such conditions in surveyed settings.
Institutional differences in the practice of assigning diagnoses likely limited statistical power to detect potentially meaningful associations and effects. Structural influences, such as stigma and institutional culture, may have notable effects on documentation practices, particularly for PDs. Future research should aim to replicate observed associations using more controlled diagnostic procedures.
Lastly, even with the use of a more conservative α and a focus on effect sizes to guide interpretation of results, use of multiple bivariate analyses can be presumed to have increased the likelihood of type I error. Given the limited prior research in this area, an exploratory approach to statistical analysis was considered warranted to maximize opportunity for identifying areas in need of additional empirical attention. Continued research using more conservative statistical approaches (eg, multivariate analyses) is needed to determine replicability and generalizability of observed results.
CONCLUSIONS
This study examined the prevalence and correlates of PD diagnoses in a national sample of veterans receiving community-based, state-funded mental health care. About 2% received a PD diagnosis, with diagnoses most common among veterans who were White, non-Hispanic, aged ≥ 45 years, also diagnosed with trauma-based, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in a state psychiatric hospital or judicial system setting. The results attest to a necessity for transdiagnostic treatment planning and care coordination for this population, with particular attention to psychosocial stressors.
Personality disorders (PDs) are enduring patterns of internal experience and behavior that differ from cultural norms and expectations, are inflexible and pervasive, have their onset in adolescence or early adulthood, and lead to distress or impairment. Ten PDs are included in the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition): paranoid, schizoid, schizotypal, borderline, antisocial, histrionic, narcissistic, avoidant, dependent, and obsessive-compulsive.1 These disorders impose a high burden on patients, families, health care systems, and broader economic systems.2,3 Up to 1 in 7 persons in the community and 50% of those receiving outpatient mental health treatment experience a PD.4,5 These conditions are associated with an increased risk of adverse events, including suicide attempt and death by suicide, criminal-legal involvement, homelessness, substance use, underemployment, relational issues, and high utilization of psychiatric services.6-9 PDs are routinely underassessed, underdocumented, and undertreated in clinical settings, and consistently receive less research funding than other, less prevalent forms of psychopathology. 10-12 As a result, there is limited understanding of clinical needs of individuals experiencing PDs.
MILITARY VETERANS WITH PERSONALITY DISORDERS
Underacknowledgment of PDs and their associated difficulties may be especially pronounced in veteran populations. Due to longstanding etiological theories that implicate childhood trauma and adolescent onset in pathology development, PDs are traditionally considered pre-existing conditions or developmental abnormalities by the US Department of Defense and US Department of Veterans Affairs (VA). As a result, PDs are therefore deemed incompatible with military service and ineligible for service-connected disability benefits.13-15 Such determinations allowed PD pathology to be used as grounds for discharge for 26,000 service members from 2001 to 2007, or 2.6% of total enlisted discharges during that period.13,15,16
Despite this structural discrimination, recent research suggests veterans may be more likely to experience PD pathology than the general population.17 For example, a 2021 epidemiological survey in a community-based veteran sample found elevated rates of borderline, antisocial, and schizotypal PDs (6%-13%).6 In contrast, only 0.8% to 5.0% of veteran electronic health records (EHRs) have a documented PD diagnosis.8,18,19 Such elevations in PD pathology within veteran samples imply either a disproportionately high prevalence among enlistees (and therefore missed during recruitment procedures) or onset following military service, possibly due to exposure to traumatic events and/ or occupational stress.17 Due to the relative infancy of research in this area and a lack of longitudinal studies, etiology and course of illness for personality pathology in veterans remains largely unclear.
Structural underacknowledgment of PDs among military personnel has contributed to their underrepresentation in research on veteran populations. PD-focused research with veterans is rare, despite a rapid increase in broader empirical attention paid to these conditions in nonveteran samples.20 A recent meta-analysis of veterans with PDs identified 27 studies that included basic prevalence statistics. PDs were rarely a primary focus for these studies, and most were limited to veterans seen in Veterans Health Administration (VHA) settings.17 The literature also paints a bleak picture, suggesting veterans who experience PDs are at higher risk for suicide attempt and death by suicide, criminal-legal involvement, and homelessness. They also tend to experience more severe comorbid psychopathological symptoms and more often use high-intensity mental health services (eg, care within emergency departments or psychiatric inpatient settings) than veterans without PD pathology.6,8,18,19,21 However, PD pathology does not appear to impede the effectiveness of treatment for veterans.22-24 The implications of PD pathology on broader psychosocial functioning and health care needs certify a need for additional research that examines patterns of personality pathology, particularly in veterans outside the VHA.
METHODS
This study aims to enhance understanding of veterans affected by PDs and offer insight and guidance for treatment of these conditions in federal and nonfederal treatment settings. Previous research has been largely limited to VHA care-receiving samples; the longstanding stigma against PDs by the US military and VA may contribute to biased diagnosis and documentation of PDs in these settings. A large sample of veterans receiving community-based mental health care was therefore used to explore aims of the current study. This study specifically examined demographic patterns, diagnostic comorbidity, psychosocial outcomes, and treatment care settings among veterans with and without a PD diagnosis. Consistent with previous research, we hypothesized that veterans with a PD diagnosis would have more severe mental health comorbidities, poorer psychosocial outcomes, and receive care in higher intensity settings relative to veterans without a diagnosis.
Data for the sample were drawn from the Mental Health Client-Level Data, a publicly available national dataset of nearly 7 million patients who received mental health treatment services provided or funded through state mental health agencies in 2022.25 The analytic sample included about 2.5 million patients for whom veteran status and data around the presence or absence of a PD diagnosis were available. Of these patients, 104,198 were identified as veterans. Veteran patients were identified as predominantly male (63%), White (71%), non-Hispanic (90%), and never married (54%).
Measures
The parent dataset included demographic, clinical, and psychosocial outcome information reported by treatment facilities to individual state administrative systems for each patient who received services. To protect patient privacy, only nonprotected health information is included, and efforts were made throughout compilation of the parent dataset to ensure patient privacy (eg, limiting detail of information disseminated for public access). Because the parent dataset does not include protected health information, studies using these data are considered exempt from institutional review board oversight.
Demographic information. This study reviewed veteran status, sex, race, ethnicity, age, education, and marital status. Veteran status was defined by whether the patient was aged ≥ 18 years and had previously served (but was not currently serving) in the military. Patients with a history of service in the National Guard or Military Reserves were only classified as veterans if they had been called or ordered to active duty while serving. Sex was operationalized dichotomously as male or female; no patients were identified as intersex, transgender, or other gender identities.
Clinical information. Up to 3 mental health diagnoses were reported for each patient and included the following disorders: personality, trauma and attention-deficit/hyperactivity, stressor, anxiety, conduct, delirium/dementia, bipolar, depressive, oppositional defiant, pervasive developmental, schizophrenia or other psychotic, and alcohol or substance use. Mental health diagnosis categories were generated for the parent dataset by grouping diagnostic codes corresponding to each category. To protect patient privacy, more detailed diagnostic information was not available as part of the parent dataset. Although the American Psychiatric Association recognizes 10 distinct PDs, the exact nature of PD diagnoses was not included within the parent dataset. PD diagnoses were coded to reflect the presence or absence of any such diagnosis.
A substance use problem designation was also provided for patients according to various identification methods, including substance use disorder (SUD) diagnosis, substance use screening results, enrollment in a substance use program, substance use survey, service claims information, and other related sources of information. A severe mental illness or serious emotional disturbance designation was provided for patients meeting state definitions of these designations. Context(s) of service provision were coded as inpatient state psychiatric hospital, community-based program, residential treatment center, judicial institution, or other psychiatric inpatient setting.
Psychosocial outcome information. Patient employment and residential status were also included in analyses. Each reflected status at the time of discharge from services or end of reporting period; employment status was only provided for patients receiving treatment in community-based programs.
Data Analysis
Descriptive statistics and X2 analyses were used to compare demographic, clinical, and psychosocial outcome variables between patients with and without PD diagnoses. These analyses were calculated for both the 104,198 veterans and the 2,222,306 nonveterans aged ≥ 18 years in the dataset. Given the sample size, a conservative α of .01 was used to determine statistical significance.
RESULTS
In this sample of persons receiving state-funded mental health care, veterans were significantly less likely than nonveterans to have a documented PD diagnosis (2.1% vs 3.6%, X2 [1] = 647.49; P < .01). PD diagnoses were more common among White (risk ratio [RR], 1.11), non-Hispanic (RR, 1.03) veterans who were in middle to late adulthood (RR, 1.16-1.40), more educated (RR, 1.35), and divorced or widowed (RR, 1.43), and less common among Black/African American (RR, 0.78) or Puerto Rican (RR, 0.32) veterans who were in early adulthood (RR, 0.31-0.79), less educated (RR, 0.64-0.89), and currently married (RR, 0.89) or never married (RR, 0.86). Veteran men and women were equally likely to have a PD diagnosis (RR, 1.03) (Table 1). Among nonveterans, men were less likely than women to have a PD diagnosis (RR, 0.79), and PD diagnoses were most common among persons in middle adulthood (RR, 1.06-1.15) (eAppendix 1).


Veterans with a PD diagnosis were more likely than those without a diagnosis to have more diagnoses (RR, 2.96-8.49) and to have comorbid trauma or related stressor (RR, 1.33), or bipolar (RR, 1.56) or psychotic (RR, 1.15) disorder diagnoses, but less likely to have comorbid depressive disorder (RR, 0.82). Although veterans with and without a PD diagnosis were similarly likely to have a comorbid SUD (RR, 1.13), those with a PD diagnosis were significantly less likely to be assigned a substance use problem designation (RR, 0.78). PD diagnosis was also more common among veterans who received services in state psychiatric hospitals (RR, 3.05), community-based clinics (RR, 1.06), and judicial institutions (RR, 6.33) and less common among those who received services in other psychiatric inpatient settings (RR, 0.30). No differences were observed for residential treatment settings (RR, 0.79). Among nonveterans, a PD diagnosis was associated with slightly greater odds of a substance use designation (RR, 1.03) (eAppendix 2).

Veterans with a PD diagnosis were also less likely to have full-time employment (RR, 0.73) and more likely to have undifferentiated employment (RR, 2.00) or to be removed from the labor force (RR, 1.35). Veterans with a PD diagnosis were also more likely to reside in nontraditional living conditions (RR, 1.42) and less likely to be residing in a private residence (RR, 0.98), compared with those without PD diagnosis. The rates of homelessness were similar for veterans with and without a PD diagnosis (RR, 0.90) (Table 2). These patterns were similar among nonveterans.

DISCUSSION
This study examined the rate and correlates of PD diagnosis among a large, community-based sample of veterans receiving state-funded mental health care. About 2% of veterans in this sample had a PD diagnosis, with diagnoses more common among veterans who were White, non-Hispanic, aged ≥ 45 years, with higher education, divorced or widowed, also diagnosed with trauma-related, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in state psychiatric hospital, community-based clinic, or judicial system settings.
The observed rate of PD diagnosis in this study aligns with what is typically observed in VHA EHRs.8,18,19 However, the rate is notably lower than prevalence estimates for psychiatric outpatient settings (about 50%) and in meta-analyses of prevalence among veterans (0.8%-23% for each of the 10 PDs).4,17,26 Longstanding stigma against PDs may contribute to underdiagnosis. For example, many clinicians are concerned that documentation or disclosure of a PD will interfere with the patient’s ability to access treatment due to stigma and discrimination.27,28 These fears are not unfounded; even among clinicians, PDs are commonly considered untreatable, and many individuals with PDs are denied access to evidence-based treatments due to the diagnosis.29 In a 2016 survey of community psychiatrists, nearly 1 in 4 reported that they avoid taking patients with a borderline PD diagnosis in their caseloads.28 To date, no studies have been conducted to explore clinicians’ willingness to accept patients with other PDs or, specifically, among veterans.
Despite such widespread stigma, research suggests clinicians' negative attitudes toward PDs can be decreased through antistigma campaigns.30 However, it remains unclear if such efforts also contribute to an increase in clinicians’ willingness to document PD diagnoses. Without accurate identification and documentation, the field’s understanding of PDs will remain limited.
In the current study, veterans with PD diagnoses tended to present with more complex and severe psychiatric comorbidities compared to veterans without such diagnoses. Observed comorbidity of PDs (particularly borderline PD) with trauma-related and bipolar disorders is well established.8 Conversely, co-occurring personality and psychotic disorders—which comprise 16% of veterans with a PD diagnosis in the sample in this study—are not consistently examined in the literature. A 2022 examination of veterans receiving VHA care suggested 12% and 13% of those with a PD diagnosis documented in their EHR also had documented schizophrenia or another psychotic disorder, respectively. PD diagnoses were associated with 6.88- and 9.80-fold increases in risk for comorbid schizophrenia and other psychotic disorder diagnoses, respectively.8 Similarly, a recent longitudinal study of nearly 2 million Swedish individuals suggested borderline PD is specifically associated with a > 24-times greater risk of having a comorbid psychotic disorder.31 It is therefore possible that the comorbidity between personality and psychotic disorders is quite common despite its relative lack of attention in empirical research.
Veterans with PD diagnoses in this study were also more likely to experience substandard housing, employment challenges, and receive treatment through judicial institutions than those without a PD diagnosis. Such findings are consistent with previous research demonstrating the substantial psychosocial challenges associated with PD diagnosis, even after controlling for comorbid conditions.7,9 Veterans with PDs may benefit from specialized case management and support to facilitate stable housing and employment and to mitigate the risk of judicial involvement. Some research suggests veterans with PDs may be less likely to gain competitive employment after participating in VA therapeutic and supportive employment services programs, suggesting standard programming may be less suitable for this population.32 Similarly, other research suggests individuals with PDs may benefit more from specialized, intensive services than standard clinical case management.33 Future research may therefore benefit from clarifying the degree to which adaptations to standard programming could yield beneficial effects for persons with PD diagnoses.
Implications
Cumulatively, the results of this study attest to the necessity for transdiagnostic treatment planning that includes close collaboration between psychotherapeutic, pharmacological, and case management services. Some psychotherapy models for PDs, such as dialectical behavior therapy (DBT), which includes a combination of group skills training, individual therapy, as-needed phone coaching, and therapist consultation, may be successfully adapted to include this collaboration.34-36 However, implementation of such comprehensive programming often requires extensive clinician training and coordination of resources, which poses implementation challenges.37-39 In 2021, the VHA began large-scale implementation of PD-specific psychotherapy for veterans with recent suicidal self-directed violence and borderline PD, including DBT, though to date results remain unclear.40 Generalist approaches, such as good psychiatric management (GPM), which emphasizes emotional validation, practical problem solving, realistic goal setting, and relationship functioning within the context of standard care appointments, may be more easily implemented in community care settings due to lesser training and resource requirements and can also be adapted to include needed elements of care coordination.41,42 Both DBT and GPM were initially developed for the treatment of borderline PD. Although DBT has also demonstrated some effectiveness in the treatment of antisocial PD, potential applications of DBT and GPM to other PDs remain largely underdeveloped.43-46
There are no widely accepted medications for the treatment of PDs. Pharmacotherapy for these conditions typically consists of individualized approaches informed by personal experience that attempt to balance targeting of specific symptoms while minimizing polypharmacy and potential risks (eg, overdose or addiction).47,48 Despite this, pharmacotherapy is often considered a necessary component in the treatment of bipolar and psychotic disorders, both common comorbidities of PDs found in veterans in this study.49,50 Careful consideration of complex comorbidities and pharmacotherapy needs is warranted in the treatment of veterans with PDs. Future research may benefit from clarifying clinical guidelines around pharmacotherapy, particularly for observed comorbidities of PDs to trauma, bipolar, and psychotic disorders.
It is important to note the discrepancies in the results of this study surrounding patient substance use. The results suggest a negligible or inverse association between the likelihood of a PD diagnosis and difficulties with substance use among the veterans in this study. However, the unexpectedly low rate of SUD diagnoses (< 6%) suggests that they were likely underdocumented. Research suggests a strong association between personality and SUDs in both veteran and civilian samples.6,51 Results suggesting a lower prevalence of substance use difficulties among treatment-seeking veterans with PDs should be interpreted with great caution.
Demographically, PD diagnoses were more common among veterans who were White, non-Hispanic, and aged ≥ 45 years, and less common among veterans who were Black/ African American, mixed/unspecified race, Puerto Rican or other non-Mexican Hispanic ethnicity, or aged < 35 years. No significant sex-based differences were observed. These patterns are consistent with research suggesting individuals who identify as Black may be less likely than individuals who identify as White to report PD symptoms, meet criteria for a PD, and have a PD diagnosed even when it is warranted.52
The findings observed in this study with respect to age, however, are notably inconsistent with the literature. Previous research typically suggests a negative association between age and PD pathology; however, a 2020 review of PDs in older adults by Penders et al suggests a prevalence of 11% to 15% in this population.53,54 Research into PDs most often focuses on adolescent and early adulthood developmental periods, limiting insight into the phenomenology of PDs in middle to late adulthood.55 Further, most research into PDs among geriatric populations has focused on psychometric assessment rather than practical treatment guidance.54 However, in this study, elevated risk for PD diagnoses was salient throughout middle to late adulthood among veterans; similar, albeit less pronounced patterns were also observed for elevated risk of PD diagnosis in middle adulthood among nonveterans. Such findings suggest clarifying the phenomenology and treatment needs of individuals with PDs in middle to late adulthood may have particularly salient implications for the mental health care of veterans affected by these conditions. As the veteran population advances in age, these needs will present unique challenges if health care systems are unprepared to effectively address them.
Limitations
This study is characterized by several strengths, most notably its use of a large dataset recently collected on a national scale. Few studies outside of the VHA system include samples of > 100,000 treatment-seeking veterans collected on a national scale. Nevertheless, results should be understood within the context of several methodological limitations. However, the dataset was limited to the first 3 diagnoses documented in patients’ EHRs, and many patients had no listed diagnoses. Patients with complex comorbidities may have > 3 diagnoses; for these individuals, data provided an incomplete picture of clinical presentation. This is especially relevant for individuals with PDs, who tend to meet criteria for a range of comorbid conditions.8,10 The now dated practice of listing PDs on Axis II also increases the chance of clinicians listing PDs after conditions traditionally listed on Axis I (eg, major depressive disorder) in patient charts.56 This study’s inclusion of only the first 3 listed diagnoses likely underestimated true PD diagnosis prevalence.
The results of this study must be interpreted as reflecting the prevalence and correlates of receiving a PD diagnosis rather than meeting diagnostic criteria for a PD. Relatedly, PD diagnoses were reported as a single construct, limiting insight into prevalence and correlates of individual PD diagnoses (eg, borderline vs paranoid PDs). Meta-analyses estimates suggest PD prevalence among veterans is likely much higher than observed in this study.17 Stigma continues to discourage clinicians from documenting and disclosing PD diagnoses even when warranted.27,28 Continued research should aim to clarify conditions (eg, patient presentation, stigma, or institutional culture) contributing to documentation of PD diagnoses. Given the cross-sectional nature of this study, results cannot speak to longitudinal treatment outcomes or prognosis of persons receiving a PD diagnosis.
Despite its large sample size and national representation, the sampling strategy of this study could have contributed to idiosyncrasies in the dataset. Restriction of data to the persons receiving state-funded mental health services introduces a notable bias to the composition of the sample, which is likely comprised of a disproportionately high number of Medicaid recipients, students, and individuals with chronic illnesses and underrepresentation of persons who pay for mental health services using private insurance or private pay arrangements. As such, although socioeconomic information was not provided within this dataset, one can presume a generally lower socioeconomic status among study participants compared to the community at large. This study also included a proportionally small sample of veterans (3.6% compared to about 6.2% in the broader US population), suggesting veterans may have been underrepresented or underidentified in surveyed mental health care settings.57 This study also did not include data around service in active-duty military, national guard, or military reserves; a greater proportion of the sample likely had a history of military service than was represented by veteran status designation. Further, the proportionally high sample of individuals with severe mental illness suggests a likely overrepresentation of such conditions in surveyed settings.
Institutional differences in the practice of assigning diagnoses likely limited statistical power to detect potentially meaningful associations and effects. Structural influences, such as stigma and institutional culture, may have notable effects on documentation practices, particularly for PDs. Future research should aim to replicate observed associations using more controlled diagnostic procedures.
Lastly, even with the use of a more conservative α and a focus on effect sizes to guide interpretation of results, use of multiple bivariate analyses can be presumed to have increased the likelihood of type I error. Given the limited prior research in this area, an exploratory approach to statistical analysis was considered warranted to maximize opportunity for identifying areas in need of additional empirical attention. Continued research using more conservative statistical approaches (eg, multivariate analyses) is needed to determine replicability and generalizability of observed results.
CONCLUSIONS
This study examined the prevalence and correlates of PD diagnoses in a national sample of veterans receiving community-based, state-funded mental health care. About 2% received a PD diagnosis, with diagnoses most common among veterans who were White, non-Hispanic, aged ≥ 45 years, also diagnosed with trauma-based, bipolar, and/or psychotic disorders, underemployed, nontraditionally housed, and receiving treatment in a state psychiatric hospital or judicial system setting. The results attest to a necessity for transdiagnostic treatment planning and care coordination for this population, with particular attention to psychosocial stressors.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
- Hastrup LH, Jennum P, Ibsen R, Kjellberg J, Simonsen E. Societal costs of borderline personality disorders: a matched-controlled nationwide study of patients and spouses. Acta Psychiatr Scand. 2019;140(5):458-467. doi:10.1111/acps.13094
- Sveen CA, Pedersen G, Ulvestad DA, Zahl KE, Wilberg T, Kvarstein EH. Societal costs of personality disorders: a cross-sectional multicenter study of treatment-seeking patients in mental health services in Norway. J Clin Psychol. 2023;79(8):1752-1769. doi:10.1002/jclp.23504
- Beckwith H, Moran PF, Reilly J. Personality disorder prevalence in psychiatric outpatients: a systematic literature review. Personal Ment Health. 2014;8(2):91-101. doi:10.1002/pmh.1252
- Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc. 2017.09.001
- Edwards ER, Barnes S, Govindarajulu U, Geraci J, Tsai J. Mental health and substance use patterns associated with lifetime suicide attempt, incarceration, and homelessness: a latent class analysis of a nationally representative sample of U.S. veterans. Psychol Serv. 2021;18(4):619-631. doi:10.1037/ser0000488
- Moran P, Romaniuk H, Coffey C, et al. The influence of personality disorder on the future mental health and social adjustment of young adults: a population-based cohort study. Lancet Psychiatry. 2016;3(7):636-645. doi:10.1016/S2215-0366(16)30029-3
- Nelson SM, Griffin CA, Hein TC, Bowersox N, McCarthy JF. Personality disorder and suicide risk among patients in the Veterans Affairs health system. Personal Disord. 2022;13(6):563-571. doi:10.1037/per0000521
- Skodol AE. Impact of personality pathology on psychosocial functioning. Curr Opin Psychol. 2018;21;33-38. doi:10.1016/j.copsyc.2017.09.006
- Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015;385(9969):717-726. doi:10.1016/S0140-6736(14)61995-4
- Fitzpatrick S, Goss S, Di Bartolomeo A, Varma S, Tissera T, Earle E. Follow the money: is borderline personality disorder research underfunded in Canada? Can Psychol. 2024;65(1):46-57. doi:10.1037/cap0000375
- Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944. doi:10.1016/j.psychres.2014.09.021
- Leroux TC. U.S. military discharges and pre-existing personality disorders: a health policy review. Adm Policy Ment Health. 2015;42(6):748-755. doi:10.1007/s10488-014-0611-z
- Monahan MC, Keener JK. Fitness-for-duty evaluations. In Kennedy CH, Zillmer EA, eds. Military Psychology: Clinical and Operational Applications. 2nd ed. Guilford Publications; 2012:25-49.
- Hearing Before the Committee on Veterans’ Affairs, 111th Congress 2nd Sess (2010). Personality disorder discharges: impact on veterans benefits. Accessed March 4, 2025. https://www.govinfo.gov/content/pkg/CHRG-111hhrg61755/html/CHRG-111hhrg61755.htm
- Ader M, Cuthbert R, Hoechst K, Simon EH, Strassburger Z, Wishnie M. Casting troops aside: the United States military’s illegal personality disorder discharge problem. Vietnam Veterans of America. March 2012. Accessed February 28, 2025. https://law.yale.edu/sites/default/files/documents/pdf/Clinics/VLSC_CastingTroopsAside.pdf
- Edwards ER, Tran H, Wrobleski J, Rabhan Y, Yin J, Chiodi C, Goodman M, Geraci J. Prevalence of personality disorders across veteran samples: A meta-analysis. J Pers Disord. 2022;36(3):339-358. doi:10.1521/ pedi.2022.36.3.339
- Holliday R, Desai A, Edwards E, Borges L. Personal i ty disorder diagnosis among just ice -involved veterans: an investigation of VA-using veterans. J Nerv Ment Dis. 2023;211(5):402-406 doi:10.1097/ NMD.0000000000001627
- McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942. doi:10.2105/AJPH.2015.302737
- Liu Y, Chen C, Zhou Y, Zhang N, Liu S. Twenty years of research on borderline personality disorder: a scientometric analysis of hotspots, bursts, and research trends. Front Psych. 2024;15:1361535. doi:10.3389/ fpsyt.2024.1361535
- Williams R, Holliday R, Clem M, Anderson E, Morris EE, Surís A. Borderline personality disorder and military sexual trauma: analysis of previous traumatization and current psychiatric presentation. J Interpers Violence. 2017;32(15):2223-2236. doi:10.1177/0886260515596149
- Holder N, Holliday R, Pai A, Surís A. Role of borderline personality disorder in the treatment of military sexual trauma-related posttraumatic stress disorder with cognitive processing therapy. Behav Med. 2017;43(3):184-190. doi:10.1080/08964289.2016.1276430
- Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of personality disorders on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. Am J Addict. 2007;16(6):443- 449. doi:10.1080/10550490701643336
- Walter KH, Bolte TA, Owens GP, Chard KM. The impact of personality disorders on treatment outcome for veterans in a posttraumatic stress disorder residential treatment program. Cognit Ther Res. 2012;36(5):576-584. doi:10.1007/s10608-011-9393-8
- Substance Abuse and Mental Health Services. Mental health client-level data (MH-CLD), 2022. Accessed February 28, 2025. https://www.datafiles.samhsa.gov/dataset/mental-health-client-level-data-2022-mh-cld-2022-ds0001
- Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918. doi:10.1176/appi.ajp.162.10.1911
- Campbell K, Clarke KA, Massey D, Lakeman R. Borderline personality disorder: To diagnose or not to diagnose? That is the question. Int J Mental Health Nurs. 2020;29(5):972-981. doi:10.1111/inm.12737
- Sisti D, Segal AG, Siegel AM, Johnson R, Gunderson J. Diagnosing, disclosing, and documenting borderline personality disorder: a survey of psychiatrists’ practices. J Pers Disord. 2016;30(6):848-856. doi:10.1521/ pedi_2015_29_228
- Klein P, Fairweather AK, Lawn S. Structural stigma and its impact on healthcare for borderline personality disorder: a scoping review. Int J Ment Health Syst. 2022;16(1):48. doi:10.1186/s13033-022-00558-3
- Knaak S, Szeto AC, Fitch K, Modgill G, Patten S. Stigma towards borderline personality disorder: effectiveness and generalizability of an anti-stigma program for healthcare providers using a pre-post randomized design. Borderline Personal Disord Emot Dysregul. 2015;2:9. doi:10.1186/s40479-015-0030-0
- Tate AE, Sahlin H, Liu S, et al. Borderline personality disorder: associations with psychiatric disorders, somatic illnesses, trauma, and adverse behaviors. Mol Psychiatry. 2022;27:2514-2521. doi:10.1038/s41380- 022-01503-z
- Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA Therapeutic and Supported Employment Services programs. Psychiatr Serv. 2017;68(9)938-946. doi:10.1176/appi. ps201600412
- Frisman LK, Mueser KT, Covell NH, et al. Use of integrated dual disorder treatment via Assertive Community Treatment versus clinical case management for persons with co-occurring disorders and antisocial personality disorder. J Nerv Ment Dis. 2009;197(11):822-828. doi:10.1097/NMD.0b013e3181beac52
- Edwards ER, Kober H, Rinne GR, Griffin SA, Axelrod S, Cooney EB. Skills]homework completion and phone coaching as predictors of therapeutic change and outcomes in completers of a DBT intensive outpatient programme. Psychol Psychother. 2021;94(3):504-522. doi:10.1111/papt.12325
- Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67(1):13-26. doi:10.1016/s0376-8716(02)00011-x
- Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. JAMA Psychiatry. 2015;72(5):475-482.doi:10.1001 /jamapsychiatry.2014.3039
- Carmel A, Rose ML, Fruzzetti AE. Barriers and solutions to implementing dialectical behavior therapy in a public behavioral health system. Adm Policy Ment Health. 2014;41(5):608-614. doi:10.1007/s10488-013-0504-6
- Decker SE, Matthieu MM, Smith BN, Landes SJ. Barriers and facilitators to dialectical behavior therapy skills groups in the Veterans Health Administration. Mil Med. 2024;189(5-6):1055-1063. doi:10.1093/milmed/ usad123
- Landes SJ, Rodriguez AL, Smith BN, et al. Barriers, facilitators, and benefits of implementation of dialectical behavior therapy in routine care: results from a national program evaluation survey in the Veterans Health Administration. Transl Behav Med. 2017;7(4):832-844. doi:10.1007/s13142-017-0465-5
- Walker J, Betthauser LM, Green K, Landes SJ, Stacy M. Suicide Prevention 2.0 Clinical Telehealth Program: Evidence- Based Treatment in the Veterans Health Administration. April 28, 2024. Accessed February 28, 2025. https://www.youtube.com/watch?v=fFsDzkg0SR0
- Gunderson J, Masland S, Choi-Kain L. Good psychiatric management: a review. Curr Opin Psychol. 2018;21:127- 131. doi:10.1016/j.copsyc.2017.12.006
- Kramer U. Good-enough therapy: a review of the empirical basis of good psychiatric management. Am J Psychother. 2025;78(1): 11-15. doi:10.1176/appi .psychotherapy.20230041
- Visdómine-Lozano JC. Contextualist perspectives in the treatment of antisocial behaviors and offending: a comparative review of FAP, ACT, DBT, and MDT. Trauma Violence Abuse. 2022;23(1):241-254. doi:10.1177/1524838020939509
- Drago A, Marogna C, Jørgen Søgaard H. A review of characteristics and treatments of the avoidant personality disorder. Could the DBT be an option? Int J Psychol Psychoanal. 2016;2(1):013.
- Finch EF, Choi-Kain LW, Iliakis EA, Eisen JL, Pinto A. Good psychiatric management for obsessive–compulsive personality disorder. Curr Behav Neurosci Rep. 2021;8:160-171. doi:10.1007/s40473-021-00239-4
- Miller TW, Kraus RF. Modified dialectical behavior therapy and problem solving for obsessive-compulsive personality disorder. Journal Contemp Psychother. 2007;37:79-85. doi:10.1007/s10879-006-9039-4
- Bozzatello P, Rocca P, De Rosa ML, Bellino S. Current and emerging medications for borderline personality disorder: is pharmacotherapy alone enough? Expert Opin Pharmacother. 2020;21(1):47-61.doi:10.1080/14656566 .2019.1686482
- Sand P, Derviososki E, Kollia S, Strand J, Di Leone F. Psychiatrists’ perspectives on prescription decisions for patients with personality disorders. J Pers Disord. 2024;38(3):225-240. doi:10.1521/pedi.2024.38.3.225
- Kane JM, Leucht S, Carpenter D, Docherty JP; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: Methods, commentary, and summary. J Clin Psychiatry. 2003;64 Suppl 12:5-19.
- Nierenberg AA, Agustini B, Köhler-Forsberg O, et al. Diagnosis and treatment of bipolar disorder: a review. JAMA. 2023;330(14):1370-1380. doi:10.1001 /jama.2023.18588
- Köck P, Walter M. Personality disorder and substance use disorder–an update. Ment Health Prev. 2018;12:82- 89. doi:10.1016/J.MHP.2018.10.003
- Garb HN. Race bias and gender bias in the diagnosis of psychological disorders. Clin Psych Rev. 2021;90:102087. doi:10.1016/j.cpr.2021.102087
- Debast I, van Alphen SPJ, Rossi G, et al. Personality traits and personality disorders in late middle and old age: do they remain stable? A literature review. Clin Gerontol. 2014;37(3):253-271.doi:10.1080/07317115 .2014.885917
- Penders KAP, Peeters IGP, Metsemakers JFM, van Alphen SPJ. Personality disorders in older adults: a review of epidemiology, assessment, and treatment. Curr Psychiatry Rep. 2020;22(3):1-14. doi:10.1007/s11920-020- 1133-x
- Videler AC, Hutsebaut J, Schulkens JEM, Sobczak S, van Alphen SPJ. A life span perspective on borderline personality disorder. Curr Psychiatry Rep. 2019;21(7) :1-8. doi:10.1007/s11920-019-1040-1
- Wakefield JC. DSM-5 and the general definition of personality disorder. Clin Soc Work J. 2013;41(2):168-183. doi:10.1007/s10615-012-0402-5
- US Census Bureau. 2022 American Community Survey 1-year. Accessed February 28, 2025. https://data.census.gov/table/ACSST1Y2022.S2101?q=Veterans&y=2022comparison
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
- Hastrup LH, Jennum P, Ibsen R, Kjellberg J, Simonsen E. Societal costs of borderline personality disorders: a matched-controlled nationwide study of patients and spouses. Acta Psychiatr Scand. 2019;140(5):458-467. doi:10.1111/acps.13094
- Sveen CA, Pedersen G, Ulvestad DA, Zahl KE, Wilberg T, Kvarstein EH. Societal costs of personality disorders: a cross-sectional multicenter study of treatment-seeking patients in mental health services in Norway. J Clin Psychol. 2023;79(8):1752-1769. doi:10.1002/jclp.23504
- Beckwith H, Moran PF, Reilly J. Personality disorder prevalence in psychiatric outpatients: a systematic literature review. Personal Ment Health. 2014;8(2):91-101. doi:10.1002/pmh.1252
- Eaton NR, Greene AL. Personality disorders: community prevalence and socio-demographic correlates. Curr Opin Psychol. 2018;21:28-32. doi:10.1016/j.copsyc. 2017.09.001
- Edwards ER, Barnes S, Govindarajulu U, Geraci J, Tsai J. Mental health and substance use patterns associated with lifetime suicide attempt, incarceration, and homelessness: a latent class analysis of a nationally representative sample of U.S. veterans. Psychol Serv. 2021;18(4):619-631. doi:10.1037/ser0000488
- Moran P, Romaniuk H, Coffey C, et al. The influence of personality disorder on the future mental health and social adjustment of young adults: a population-based cohort study. Lancet Psychiatry. 2016;3(7):636-645. doi:10.1016/S2215-0366(16)30029-3
- Nelson SM, Griffin CA, Hein TC, Bowersox N, McCarthy JF. Personality disorder and suicide risk among patients in the Veterans Affairs health system. Personal Disord. 2022;13(6):563-571. doi:10.1037/per0000521
- Skodol AE. Impact of personality pathology on psychosocial functioning. Curr Opin Psychol. 2018;21;33-38. doi:10.1016/j.copsyc.2017.09.006
- Tyrer P, Reed GM, Crawford MJ. Classification, assessment, prevalence, and effect of personality disorder. Lancet. 2015;385(9969):717-726. doi:10.1016/S0140-6736(14)61995-4
- Fitzpatrick S, Goss S, Di Bartolomeo A, Varma S, Tissera T, Earle E. Follow the money: is borderline personality disorder research underfunded in Canada? Can Psychol. 2024;65(1):46-57. doi:10.1037/cap0000375
- Zimmerman M, Gazarian D. Is research on borderline personality disorder underfunded by the National Institute of Health? Psychiatry Res. 2014;220(3):941-944. doi:10.1016/j.psychres.2014.09.021
- Leroux TC. U.S. military discharges and pre-existing personality disorders: a health policy review. Adm Policy Ment Health. 2015;42(6):748-755. doi:10.1007/s10488-014-0611-z
- Monahan MC, Keener JK. Fitness-for-duty evaluations. In Kennedy CH, Zillmer EA, eds. Military Psychology: Clinical and Operational Applications. 2nd ed. Guilford Publications; 2012:25-49.
- Hearing Before the Committee on Veterans’ Affairs, 111th Congress 2nd Sess (2010). Personality disorder discharges: impact on veterans benefits. Accessed March 4, 2025. https://www.govinfo.gov/content/pkg/CHRG-111hhrg61755/html/CHRG-111hhrg61755.htm
- Ader M, Cuthbert R, Hoechst K, Simon EH, Strassburger Z, Wishnie M. Casting troops aside: the United States military’s illegal personality disorder discharge problem. Vietnam Veterans of America. March 2012. Accessed February 28, 2025. https://law.yale.edu/sites/default/files/documents/pdf/Clinics/VLSC_CastingTroopsAside.pdf
- Edwards ER, Tran H, Wrobleski J, Rabhan Y, Yin J, Chiodi C, Goodman M, Geraci J. Prevalence of personality disorders across veteran samples: A meta-analysis. J Pers Disord. 2022;36(3):339-358. doi:10.1521/ pedi.2022.36.3.339
- Holliday R, Desai A, Edwards E, Borges L. Personal i ty disorder diagnosis among just ice -involved veterans: an investigation of VA-using veterans. J Nerv Ment Dis. 2023;211(5):402-406 doi:10.1097/ NMD.0000000000001627
- McCarthy JF, Bossarte RM, Katz IR, et al. Predictive modeling and concentration of the risk of suicide: implications for preventive interventions in the US Department of Veterans Affairs. Am J Public Health. 2015;105(9):1935-1942. doi:10.2105/AJPH.2015.302737
- Liu Y, Chen C, Zhou Y, Zhang N, Liu S. Twenty years of research on borderline personality disorder: a scientometric analysis of hotspots, bursts, and research trends. Front Psych. 2024;15:1361535. doi:10.3389/ fpsyt.2024.1361535
- Williams R, Holliday R, Clem M, Anderson E, Morris EE, Surís A. Borderline personality disorder and military sexual trauma: analysis of previous traumatization and current psychiatric presentation. J Interpers Violence. 2017;32(15):2223-2236. doi:10.1177/0886260515596149
- Holder N, Holliday R, Pai A, Surís A. Role of borderline personality disorder in the treatment of military sexual trauma-related posttraumatic stress disorder with cognitive processing therapy. Behav Med. 2017;43(3):184-190. doi:10.1080/08964289.2016.1276430
- Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of personality disorders on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. Am J Addict. 2007;16(6):443- 449. doi:10.1080/10550490701643336
- Walter KH, Bolte TA, Owens GP, Chard KM. The impact of personality disorders on treatment outcome for veterans in a posttraumatic stress disorder residential treatment program. Cognit Ther Res. 2012;36(5):576-584. doi:10.1007/s10608-011-9393-8
- Substance Abuse and Mental Health Services. Mental health client-level data (MH-CLD), 2022. Accessed February 28, 2025. https://www.datafiles.samhsa.gov/dataset/mental-health-client-level-data-2022-mh-cld-2022-ds0001
- Zimmerman M, Rothschild L, Chelminski I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am J Psychiatry. 2005;162(10):1911-1918. doi:10.1176/appi.ajp.162.10.1911
- Campbell K, Clarke KA, Massey D, Lakeman R. Borderline personality disorder: To diagnose or not to diagnose? That is the question. Int J Mental Health Nurs. 2020;29(5):972-981. doi:10.1111/inm.12737
- Sisti D, Segal AG, Siegel AM, Johnson R, Gunderson J. Diagnosing, disclosing, and documenting borderline personality disorder: a survey of psychiatrists’ practices. J Pers Disord. 2016;30(6):848-856. doi:10.1521/ pedi_2015_29_228
- Klein P, Fairweather AK, Lawn S. Structural stigma and its impact on healthcare for borderline personality disorder: a scoping review. Int J Ment Health Syst. 2022;16(1):48. doi:10.1186/s13033-022-00558-3
- Knaak S, Szeto AC, Fitch K, Modgill G, Patten S. Stigma towards borderline personality disorder: effectiveness and generalizability of an anti-stigma program for healthcare providers using a pre-post randomized design. Borderline Personal Disord Emot Dysregul. 2015;2:9. doi:10.1186/s40479-015-0030-0
- Tate AE, Sahlin H, Liu S, et al. Borderline personality disorder: associations with psychiatric disorders, somatic illnesses, trauma, and adverse behaviors. Mol Psychiatry. 2022;27:2514-2521. doi:10.1038/s41380- 022-01503-z
- Abraham KM, Yosef M, Resnick SG, Zivin K. Competitive employment outcomes among veterans in VHA Therapeutic and Supported Employment Services programs. Psychiatr Serv. 2017;68(9)938-946. doi:10.1176/appi. ps201600412
- Frisman LK, Mueser KT, Covell NH, et al. Use of integrated dual disorder treatment via Assertive Community Treatment versus clinical case management for persons with co-occurring disorders and antisocial personality disorder. J Nerv Ment Dis. 2009;197(11):822-828. doi:10.1097/NMD.0b013e3181beac52
- Edwards ER, Kober H, Rinne GR, Griffin SA, Axelrod S, Cooney EB. Skills]homework completion and phone coaching as predictors of therapeutic change and outcomes in completers of a DBT intensive outpatient programme. Psychol Psychother. 2021;94(3):504-522. doi:10.1111/papt.12325
- Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend. 2002;67(1):13-26. doi:10.1016/s0376-8716(02)00011-x
- Linehan MM, Korslund KE, Harned MS, et al. Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: a randomized clinical trial and component analysis. JAMA Psychiatry. 2015;72(5):475-482.doi:10.1001 /jamapsychiatry.2014.3039
- Carmel A, Rose ML, Fruzzetti AE. Barriers and solutions to implementing dialectical behavior therapy in a public behavioral health system. Adm Policy Ment Health. 2014;41(5):608-614. doi:10.1007/s10488-013-0504-6
- Decker SE, Matthieu MM, Smith BN, Landes SJ. Barriers and facilitators to dialectical behavior therapy skills groups in the Veterans Health Administration. Mil Med. 2024;189(5-6):1055-1063. doi:10.1093/milmed/ usad123
- Landes SJ, Rodriguez AL, Smith BN, et al. Barriers, facilitators, and benefits of implementation of dialectical behavior therapy in routine care: results from a national program evaluation survey in the Veterans Health Administration. Transl Behav Med. 2017;7(4):832-844. doi:10.1007/s13142-017-0465-5
- Walker J, Betthauser LM, Green K, Landes SJ, Stacy M. Suicide Prevention 2.0 Clinical Telehealth Program: Evidence- Based Treatment in the Veterans Health Administration. April 28, 2024. Accessed February 28, 2025. https://www.youtube.com/watch?v=fFsDzkg0SR0
- Gunderson J, Masland S, Choi-Kain L. Good psychiatric management: a review. Curr Opin Psychol. 2018;21:127- 131. doi:10.1016/j.copsyc.2017.12.006
- Kramer U. Good-enough therapy: a review of the empirical basis of good psychiatric management. Am J Psychother. 2025;78(1): 11-15. doi:10.1176/appi .psychotherapy.20230041
- Visdómine-Lozano JC. Contextualist perspectives in the treatment of antisocial behaviors and offending: a comparative review of FAP, ACT, DBT, and MDT. Trauma Violence Abuse. 2022;23(1):241-254. doi:10.1177/1524838020939509
- Drago A, Marogna C, Jørgen Søgaard H. A review of characteristics and treatments of the avoidant personality disorder. Could the DBT be an option? Int J Psychol Psychoanal. 2016;2(1):013.
- Finch EF, Choi-Kain LW, Iliakis EA, Eisen JL, Pinto A. Good psychiatric management for obsessive–compulsive personality disorder. Curr Behav Neurosci Rep. 2021;8:160-171. doi:10.1007/s40473-021-00239-4
- Miller TW, Kraus RF. Modified dialectical behavior therapy and problem solving for obsessive-compulsive personality disorder. Journal Contemp Psychother. 2007;37:79-85. doi:10.1007/s10879-006-9039-4
- Bozzatello P, Rocca P, De Rosa ML, Bellino S. Current and emerging medications for borderline personality disorder: is pharmacotherapy alone enough? Expert Opin Pharmacother. 2020;21(1):47-61.doi:10.1080/14656566 .2019.1686482
- Sand P, Derviososki E, Kollia S, Strand J, Di Leone F. Psychiatrists’ perspectives on prescription decisions for patients with personality disorders. J Pers Disord. 2024;38(3):225-240. doi:10.1521/pedi.2024.38.3.225
- Kane JM, Leucht S, Carpenter D, Docherty JP; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: Methods, commentary, and summary. J Clin Psychiatry. 2003;64 Suppl 12:5-19.
- Nierenberg AA, Agustini B, Köhler-Forsberg O, et al. Diagnosis and treatment of bipolar disorder: a review. JAMA. 2023;330(14):1370-1380. doi:10.1001 /jama.2023.18588
- Köck P, Walter M. Personality disorder and substance use disorder–an update. Ment Health Prev. 2018;12:82- 89. doi:10.1016/J.MHP.2018.10.003
- Garb HN. Race bias and gender bias in the diagnosis of psychological disorders. Clin Psych Rev. 2021;90:102087. doi:10.1016/j.cpr.2021.102087
- Debast I, van Alphen SPJ, Rossi G, et al. Personality traits and personality disorders in late middle and old age: do they remain stable? A literature review. Clin Gerontol. 2014;37(3):253-271.doi:10.1080/07317115 .2014.885917
- Penders KAP, Peeters IGP, Metsemakers JFM, van Alphen SPJ. Personality disorders in older adults: a review of epidemiology, assessment, and treatment. Curr Psychiatry Rep. 2020;22(3):1-14. doi:10.1007/s11920-020- 1133-x
- Videler AC, Hutsebaut J, Schulkens JEM, Sobczak S, van Alphen SPJ. A life span perspective on borderline personality disorder. Curr Psychiatry Rep. 2019;21(7) :1-8. doi:10.1007/s11920-019-1040-1
- Wakefield JC. DSM-5 and the general definition of personality disorder. Clin Soc Work J. 2013;41(2):168-183. doi:10.1007/s10615-012-0402-5
- US Census Bureau. 2022 American Community Survey 1-year. Accessed February 28, 2025. https://data.census.gov/table/ACSST1Y2022.S2101?q=Veterans&y=2022comparison
Needs of Veterans With Personality Disorder Diagnoses in Community-Based Mental Health Care
Needs of Veterans With Personality Disorder Diagnoses in Community-Based Mental Health Care