User login
Ch4 Density Is a Potential Imaging Biomarker of Cognition in Early Parkinson’s Disease
Increasing Ch4 density is associated with higher scores on various cognitive measurements.
MIAMI—Reduced cholinergic nucleus 4 (Ch4) density in Parkinson’s disease, as measured with MRI, is associated with deficits in attention, processing speed, and visuospatial function, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Ch4 density may serve as a surrogate imaging biomarker of cognition in early Parkinson’s disease, said the researchers.
Degeneration of the nucleus basalis of Meynert (NBM) contributes to dementia in Parkinson’s disease through a loss of cholinergic innervation to the neocortex. Cholinergic neurons of the NBM are in Ch4, a structure that can be measured with MRI techniques using cytoarchitectonic maps.
Evaluating Ch4 Density and Cognitive Performance
To determine whether Ch4 density, a proxy measure for NBM volume, is associated with cognitive test performance in de novo Parkinson’s disease, Cody S. Freeman, MD, a fellow at the University of Virginia School of Medicine in Charlottesville, and colleagues analyzed baseline brain MRIs and neuropsychologic test scores for 228 patients with Parkinson’s disease and 101 healthy controls from the Parkinson’s Progression Markers Initiative (PPMI). They also analyzed brain MRIs and neuropsychologic test scores at four years for a subset of 92 participants with Parkinson’s disease in the PPMI.
Neuropsychologic testing included the Montreal Cognitive Assessment (MoCA), Hopkins Verbal Learning Test (HVLT), Judgment of Line Orientation (JLO), Letter Number Sequencing (LNS), Symbol Digit Modalities Test (SDMT), and semantic fluency (animals).
The researchers used MP-RAGE T1 sequences and a probabilistic atlas from the reference Montreal Neurological Institute single subject brain to apply voxel-based morphometry methods to determine Ch4 density. In addition, they used correlation coefficients and linear regression models to analyze relationships between Ch4 density and cognitive scores.
Ch4 Density Was Significantly Associated With Higher MoCA Scores
At baseline, 33.7% of healthy controls and 38.2% of patients with Parkinson’s disease were female. The mean age at neurologic testing was 59.5 among healthy controls and 61.0 in the Parkinson’s disease cohort. The median MoCA score was 28 for controls and patients with Parkinson’s disease at baseline. The mean Ch4 density was 87.9 in the control group and 86.4 in the Parkinson’s disease cohort.
At baseline, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT scores. In a linear regression model adjusted for age and sex, Ch4 density was significantly associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores. The researchers observed no associations between Ch4 density and JLO and semantic fluency in linear regression models adjusted for sex.
For the subset of participants with Parkinson’s disease with brain MRI and neuropsychologic testing available at four years, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT. In a linear regression model adjusted for age and sex, increasing Ch4 density was associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores.
Increasing Ch4 density is associated with higher scores on various cognitive measurements.
Increasing Ch4 density is associated with higher scores on various cognitive measurements.
MIAMI—Reduced cholinergic nucleus 4 (Ch4) density in Parkinson’s disease, as measured with MRI, is associated with deficits in attention, processing speed, and visuospatial function, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Ch4 density may serve as a surrogate imaging biomarker of cognition in early Parkinson’s disease, said the researchers.
Degeneration of the nucleus basalis of Meynert (NBM) contributes to dementia in Parkinson’s disease through a loss of cholinergic innervation to the neocortex. Cholinergic neurons of the NBM are in Ch4, a structure that can be measured with MRI techniques using cytoarchitectonic maps.
Evaluating Ch4 Density and Cognitive Performance
To determine whether Ch4 density, a proxy measure for NBM volume, is associated with cognitive test performance in de novo Parkinson’s disease, Cody S. Freeman, MD, a fellow at the University of Virginia School of Medicine in Charlottesville, and colleagues analyzed baseline brain MRIs and neuropsychologic test scores for 228 patients with Parkinson’s disease and 101 healthy controls from the Parkinson’s Progression Markers Initiative (PPMI). They also analyzed brain MRIs and neuropsychologic test scores at four years for a subset of 92 participants with Parkinson’s disease in the PPMI.
Neuropsychologic testing included the Montreal Cognitive Assessment (MoCA), Hopkins Verbal Learning Test (HVLT), Judgment of Line Orientation (JLO), Letter Number Sequencing (LNS), Symbol Digit Modalities Test (SDMT), and semantic fluency (animals).
The researchers used MP-RAGE T1 sequences and a probabilistic atlas from the reference Montreal Neurological Institute single subject brain to apply voxel-based morphometry methods to determine Ch4 density. In addition, they used correlation coefficients and linear regression models to analyze relationships between Ch4 density and cognitive scores.
Ch4 Density Was Significantly Associated With Higher MoCA Scores
At baseline, 33.7% of healthy controls and 38.2% of patients with Parkinson’s disease were female. The mean age at neurologic testing was 59.5 among healthy controls and 61.0 in the Parkinson’s disease cohort. The median MoCA score was 28 for controls and patients with Parkinson’s disease at baseline. The mean Ch4 density was 87.9 in the control group and 86.4 in the Parkinson’s disease cohort.
At baseline, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT scores. In a linear regression model adjusted for age and sex, Ch4 density was significantly associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores. The researchers observed no associations between Ch4 density and JLO and semantic fluency in linear regression models adjusted for sex.
For the subset of participants with Parkinson’s disease with brain MRI and neuropsychologic testing available at four years, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT. In a linear regression model adjusted for age and sex, increasing Ch4 density was associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores.
MIAMI—Reduced cholinergic nucleus 4 (Ch4) density in Parkinson’s disease, as measured with MRI, is associated with deficits in attention, processing speed, and visuospatial function, according to research described at the Second Pan American Parkinson’s Disease and Movement Disorders Congress. Ch4 density may serve as a surrogate imaging biomarker of cognition in early Parkinson’s disease, said the researchers.
Degeneration of the nucleus basalis of Meynert (NBM) contributes to dementia in Parkinson’s disease through a loss of cholinergic innervation to the neocortex. Cholinergic neurons of the NBM are in Ch4, a structure that can be measured with MRI techniques using cytoarchitectonic maps.
Evaluating Ch4 Density and Cognitive Performance
To determine whether Ch4 density, a proxy measure for NBM volume, is associated with cognitive test performance in de novo Parkinson’s disease, Cody S. Freeman, MD, a fellow at the University of Virginia School of Medicine in Charlottesville, and colleagues analyzed baseline brain MRIs and neuropsychologic test scores for 228 patients with Parkinson’s disease and 101 healthy controls from the Parkinson’s Progression Markers Initiative (PPMI). They also analyzed brain MRIs and neuropsychologic test scores at four years for a subset of 92 participants with Parkinson’s disease in the PPMI.
Neuropsychologic testing included the Montreal Cognitive Assessment (MoCA), Hopkins Verbal Learning Test (HVLT), Judgment of Line Orientation (JLO), Letter Number Sequencing (LNS), Symbol Digit Modalities Test (SDMT), and semantic fluency (animals).
The researchers used MP-RAGE T1 sequences and a probabilistic atlas from the reference Montreal Neurological Institute single subject brain to apply voxel-based morphometry methods to determine Ch4 density. In addition, they used correlation coefficients and linear regression models to analyze relationships between Ch4 density and cognitive scores.
Ch4 Density Was Significantly Associated With Higher MoCA Scores
At baseline, 33.7% of healthy controls and 38.2% of patients with Parkinson’s disease were female. The mean age at neurologic testing was 59.5 among healthy controls and 61.0 in the Parkinson’s disease cohort. The median MoCA score was 28 for controls and patients with Parkinson’s disease at baseline. The mean Ch4 density was 87.9 in the control group and 86.4 in the Parkinson’s disease cohort.
At baseline, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT scores. In a linear regression model adjusted for age and sex, Ch4 density was significantly associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores. The researchers observed no associations between Ch4 density and JLO and semantic fluency in linear regression models adjusted for sex.
For the subset of participants with Parkinson’s disease with brain MRI and neuropsychologic testing available at four years, Ch4 density was significantly correlated with MoCA, JLO, LNS, and SDMT. In a linear regression model adjusted for age and sex, increasing Ch4 density was associated with higher MoCA scores in patients with Parkinson’s disease. In linear regression models adjusted for sex, increasing Ch4 density was associated with higher JLO, LNS, and SDMT scores.
What Is the Prevalence of Sleep Disorders in Neurologic Populations?
A retrospective study finds that insomnia may be associated with worse neurologic status in patients with movement disorders and patients with epilepsy.
LOS ANGELES—About one-third of neurologic patients has a high risk of obstructive sleep apnea (OSA), and approximately one-quarter has significant symptoms of insomnia, according to data presented at the 70th Annual Meeting of the American Academy of Neurology. The presence of insomnia symptoms is associated with worse neurologic status in movement disorders and epilepsy populations, researchers said.
“Given the high prevalence of sleep disorder symptoms, further investigation into the role of sleep therapies on disease-specific outcomes in neurologic populations is warranted,” said Thapanee Somboon, MD, a neurologist at Prasat Neurological Institute in Bangkok, Thailand, and research fellow at the Cleveland Clinic Sleep Disorders Center, and colleagues.
Analyzing STOP and Insomnia Severity Index Scores
OSA and insomnia are highly prevalent in the general population and may be more common in patients with neurologic conditions. To examine the association between sleep instrument scores and disease-specific outcomes in neurologic populations, Dr. Somboon and colleagues conducted a retrospective analysis of data from 19,052 adult initial visits to the psychiatry, brain tumor, movement disorders, cerebrovascular, and epilepsy centers at the Cleveland Clinic between March 2015 and October 2016.
In all, 7,762 patients had completed the snoring, tiredness, observed apnea, and high blood pressure (STOP) questionnaire, and 8,530 patients had completed the Insomnia Severity Index. A STOP score of 2 or greater predicted a high risk of OSA, and an Insomnia Severity Index score of 15 or greater indicated significant insomnia symptoms.
The crude prevalence of high-risk OSA was 47.9% in the cerebrovascular center, 44.1% in the movement disorders center, 34% in the brain tumor center, 33% in the epilepsy center, 29.8% in the psychiatry center, and 36.7% overall.
The crude prevalence of significant insomnia symptoms was 33.6% in the psychiatry center, 26.1% in the epilepsy center, 20.7% in the brain tumor center, 20% in the movement disorders center, 19.5% in the cerebrovascular center, and 25.5% overall.
Disease-Specific Outcomes
The researchers used regression models to adjust for patients’ age, sex, race, marital status, self-reported sleep duration, income, tobacco use, and comorbid conditions. Multivariate models evaluated the associations between abnormal sleep scores and scores on the Patient Health Questionnaire-9 (PHQ-9; from all centers), Karnofsky Performance Status (from the brain tumor center), Unified Parkinson’s Disease Rating Scale (UPDRS II; from the movement disorders center), modified Rankin Scale (from the cerebrovascular center), and Liverpool Seizure Severity Scale (from the epilepsy center).
Patients with a STOP score of 2 or greater were older, more likely to be male, more likely to be a current or former smoker, had greater PHQ-9 scores, and had more comorbidities.
Patients with Insomnia Severity Index scores of 15 or greater were younger, more likely to be female, more likely to be a current or former smoker, and had a higher prevalence of depression.
OSA and insomnia were significantly associated with PHQ-9 scores. In addition, insomnia symptoms were significantly associated with Liverpool Seizure Severity Scale and UPDRS II scores.
—Jake Remaly
A retrospective study finds that insomnia may be associated with worse neurologic status in patients with movement disorders and patients with epilepsy.
A retrospective study finds that insomnia may be associated with worse neurologic status in patients with movement disorders and patients with epilepsy.
LOS ANGELES—About one-third of neurologic patients has a high risk of obstructive sleep apnea (OSA), and approximately one-quarter has significant symptoms of insomnia, according to data presented at the 70th Annual Meeting of the American Academy of Neurology. The presence of insomnia symptoms is associated with worse neurologic status in movement disorders and epilepsy populations, researchers said.
“Given the high prevalence of sleep disorder symptoms, further investigation into the role of sleep therapies on disease-specific outcomes in neurologic populations is warranted,” said Thapanee Somboon, MD, a neurologist at Prasat Neurological Institute in Bangkok, Thailand, and research fellow at the Cleveland Clinic Sleep Disorders Center, and colleagues.
Analyzing STOP and Insomnia Severity Index Scores
OSA and insomnia are highly prevalent in the general population and may be more common in patients with neurologic conditions. To examine the association between sleep instrument scores and disease-specific outcomes in neurologic populations, Dr. Somboon and colleagues conducted a retrospective analysis of data from 19,052 adult initial visits to the psychiatry, brain tumor, movement disorders, cerebrovascular, and epilepsy centers at the Cleveland Clinic between March 2015 and October 2016.
In all, 7,762 patients had completed the snoring, tiredness, observed apnea, and high blood pressure (STOP) questionnaire, and 8,530 patients had completed the Insomnia Severity Index. A STOP score of 2 or greater predicted a high risk of OSA, and an Insomnia Severity Index score of 15 or greater indicated significant insomnia symptoms.
The crude prevalence of high-risk OSA was 47.9% in the cerebrovascular center, 44.1% in the movement disorders center, 34% in the brain tumor center, 33% in the epilepsy center, 29.8% in the psychiatry center, and 36.7% overall.
The crude prevalence of significant insomnia symptoms was 33.6% in the psychiatry center, 26.1% in the epilepsy center, 20.7% in the brain tumor center, 20% in the movement disorders center, 19.5% in the cerebrovascular center, and 25.5% overall.
Disease-Specific Outcomes
The researchers used regression models to adjust for patients’ age, sex, race, marital status, self-reported sleep duration, income, tobacco use, and comorbid conditions. Multivariate models evaluated the associations between abnormal sleep scores and scores on the Patient Health Questionnaire-9 (PHQ-9; from all centers), Karnofsky Performance Status (from the brain tumor center), Unified Parkinson’s Disease Rating Scale (UPDRS II; from the movement disorders center), modified Rankin Scale (from the cerebrovascular center), and Liverpool Seizure Severity Scale (from the epilepsy center).
Patients with a STOP score of 2 or greater were older, more likely to be male, more likely to be a current or former smoker, had greater PHQ-9 scores, and had more comorbidities.
Patients with Insomnia Severity Index scores of 15 or greater were younger, more likely to be female, more likely to be a current or former smoker, and had a higher prevalence of depression.
OSA and insomnia were significantly associated with PHQ-9 scores. In addition, insomnia symptoms were significantly associated with Liverpool Seizure Severity Scale and UPDRS II scores.
—Jake Remaly
LOS ANGELES—About one-third of neurologic patients has a high risk of obstructive sleep apnea (OSA), and approximately one-quarter has significant symptoms of insomnia, according to data presented at the 70th Annual Meeting of the American Academy of Neurology. The presence of insomnia symptoms is associated with worse neurologic status in movement disorders and epilepsy populations, researchers said.
“Given the high prevalence of sleep disorder symptoms, further investigation into the role of sleep therapies on disease-specific outcomes in neurologic populations is warranted,” said Thapanee Somboon, MD, a neurologist at Prasat Neurological Institute in Bangkok, Thailand, and research fellow at the Cleveland Clinic Sleep Disorders Center, and colleagues.
Analyzing STOP and Insomnia Severity Index Scores
OSA and insomnia are highly prevalent in the general population and may be more common in patients with neurologic conditions. To examine the association between sleep instrument scores and disease-specific outcomes in neurologic populations, Dr. Somboon and colleagues conducted a retrospective analysis of data from 19,052 adult initial visits to the psychiatry, brain tumor, movement disorders, cerebrovascular, and epilepsy centers at the Cleveland Clinic between March 2015 and October 2016.
In all, 7,762 patients had completed the snoring, tiredness, observed apnea, and high blood pressure (STOP) questionnaire, and 8,530 patients had completed the Insomnia Severity Index. A STOP score of 2 or greater predicted a high risk of OSA, and an Insomnia Severity Index score of 15 or greater indicated significant insomnia symptoms.
The crude prevalence of high-risk OSA was 47.9% in the cerebrovascular center, 44.1% in the movement disorders center, 34% in the brain tumor center, 33% in the epilepsy center, 29.8% in the psychiatry center, and 36.7% overall.
The crude prevalence of significant insomnia symptoms was 33.6% in the psychiatry center, 26.1% in the epilepsy center, 20.7% in the brain tumor center, 20% in the movement disorders center, 19.5% in the cerebrovascular center, and 25.5% overall.
Disease-Specific Outcomes
The researchers used regression models to adjust for patients’ age, sex, race, marital status, self-reported sleep duration, income, tobacco use, and comorbid conditions. Multivariate models evaluated the associations between abnormal sleep scores and scores on the Patient Health Questionnaire-9 (PHQ-9; from all centers), Karnofsky Performance Status (from the brain tumor center), Unified Parkinson’s Disease Rating Scale (UPDRS II; from the movement disorders center), modified Rankin Scale (from the cerebrovascular center), and Liverpool Seizure Severity Scale (from the epilepsy center).
Patients with a STOP score of 2 or greater were older, more likely to be male, more likely to be a current or former smoker, had greater PHQ-9 scores, and had more comorbidities.
Patients with Insomnia Severity Index scores of 15 or greater were younger, more likely to be female, more likely to be a current or former smoker, and had a higher prevalence of depression.
OSA and insomnia were significantly associated with PHQ-9 scores. In addition, insomnia symptoms were significantly associated with Liverpool Seizure Severity Scale and UPDRS II scores.
—Jake Remaly
What Are the Best Therapeutic Options for Parkinson’s Disease?
Levodopa remains the most effective treatment, and techniques for deep brain stimulation are improving.
LOS ANGELES—Physicians who treat patients with Parkinson’s disease have many decisions to make based on therapeutic efficacy and desired outcomes. At the 70th Annual Meeting of the American Academy of Neurology, Melissa J. Nirenberg, MD, PhD, outlined the current landscape of Parkinson’s disease therapeutics, including data about symptom control, timing of treatment, and new therapies.
Initial Therapy: No Benefit to Levodopa Sparing
Levodopa, along with dopamine agonists and monoamine oxidase B (MAO-B) inhibitors, has Level A evidence as initial symptomatic therapy for Parkinson’s disease. “There is no question that levodopa is the most effective treatment for Parkinson’s disease,” said Dr. Nirenberg, Chief Medical Officer of the New York Stem Cell Foundation Research Institute and Adjunct Professor of Neurology at NYU Langone Health in New York City. “However, after people have been taking levodopa for a number of years, its therapeutic effect lasts for shorter periods of time, and patients spend an increasing amount of time in the off state, rather than in the on state.”
In addition to this wearing-off effect, levodopa treatment is associated with dyskinesias. This association and the wearing-off effect have prompted many physicians to adopt levodopa-sparing strategies, such as using dopamine agonists as initial treatment. However, dopamine agonists have other serious side effects, and research shows that in the long run, starting with a dopamine agonist does not improve outcomes.
In one study, data were compared between a large cohort of patients with Parkinson’s disease in Ghana, where levodopa therapy was initiated after a mean of 4.2 years’ disease duration, and patients with Parkinson’s disease in Italy, where levodopa was initiated at a mean of 2.4 years’ disease duration. “Disease duration and medication dosage, rather than the duration of levodopa therapy, affected the likelihood of dyskinesia,” Dr. Nirenberg said. “When you start levodopa late, you miss the honeymoon period,” she said, referring to the period during which patients experience the benefits of levodopa therapy before developing motor complications. “Simply put, levodopa as initial treatment works better [and] has fewer short- and long-term adverse effects [than dopamine agonists].”
Other Therapies
Dopamine agonists are highly efficacious as add-on treatment, but they also can have serious adverse effects. “Neurogenic orthostatic hypotension, psychosis, and sleepiness are adverse effects that are worse with dopamine agonists than with levodopa,” Dr. Nirenberg noted. “Another common adverse effect associated with dopamine agonists is impulse control disorders—pathologic gambling, compulsive eating, compulsive shopping, and hypersexuality.”
MAO-B inhibitors are also commonly used, well-tolerated medications that can be administered alone or in combination with levodopa or other medications. Of these drugs, selegiline and rasagiline can be used as monotherapy, Dr. Nirenberg noted, but a newer MAO-B inhibitor, safinamide, is not effective as monotherapy and should only be used as an adjunctive therapy with levodopa.
Extended release (ER) carbidopa–levodopa capsules, which contain immediate-release and ER beads to provide initial and extended levodopa plasma concentrations, have been effective in reducing wearing off between doses of levodopa, but conversion to this formulation from immediate-release levodopa is not straightforward. Rather than using the suggested conversion table in the package insert, neurologists might try the approach suggested by investigators who participated in the original clinical trials, said Dr. Nirenberg. Extended-release “capsules can be twisted open, and the beads poured into applesauce for people who have trouble swallowing,” she added.
Amantadine and anticholinergics are second-line medications that can be used as initial or adjunctive therapy. “They are weaker than the first-line drugs and have unfavorable adverse-effect profiles,” said Dr. Nirenberg. Amantadine, of which a newly approved extended-release formulation is available, can reduce dyskinesias.
New and Investigational Treatments
Deep brain stimulation (DBS) techniques are advancing, said Dr. Nirenberg. With DBS, a device implanted in the chest sends electrical pulses to electrodes inserted into targeted areas of the brain. “Recent studies are looking at closed-loop systems that provide direct feedback from the brain to the pacemaker so that stimulation is adjusted in real time.”
Continuous enteral infusion of carbidopa–levodopa intestinal gel over 16 hours via percutaneous endoscopic gastrojejunostomy is an option for people for whom DBS is being considered, but who have contraindications such as cognitive impairment or psychosis. “This [treatment] should only be prescribed to someone who has a good caregiver, because the pump has to be flushed often, removed before bathing, and checked to make sure there are no hardware problems or infections associated with its use.”
Droxidopa, a synthetic amino acid precursor of noradrenaline, received orphan-product designation for treatment of
Pimavanserin, a first-in-class drug approved in 2016 to treat hallucinations and delusions associated with Parkinson’s disease psychosis, is an atypical antipsychotic with a serotonergic mechanism of action. While the prospect of having such a treatment option initially generated excitement in the medical community, there have been recent concerns about adverse events in patients taking pimavanserin, including deaths, falls, insomnia, and nausea, in addition to continued hallucinations.
Focused ultrasound is approved for essential tremor and is investigational for Parkinson’s disease, Dr. Nirenberg noted. During the procedure, which can be performed on an outpatient basis, focused beams of ultrasonic energy are trained on targets deep in the brain to destroy diseased tissue without damaging surrounding normal tissue. Because of the lack of long-term follow-up of these patients, neurologists “do not know where this ultimately will fit in with Parkinson’s disease management,” said Dr. Nirenberg. Focused ultrasound is mainly being investigated as unilateral treatment because of concerns about the safety of bilateral ablative therapy.
To date, research on oral cannabinoids has not shown evidence of benefit for Parkinson’s disease, said Dr. Nirenberg. Neurologists have concerns about potential drug interactions and side effects such as imbalance, falls, cognitive impairment, and psychosis, which are of particular concern in people with Parkinson’s disease.
—Adriene Marshall
Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(Pt 10):2731-2742.
Tetrud J, Nausieda P, Kreitzman D, et al. Conversion to carbidopa and levodopa extended-release (IPX066) followed by its extended use in patients previously taking controlled-release carbidopa-levodopa for advanced Parkinson’s disease. J Neurol Sci. 2017;373:116-123.
Levodopa remains the most effective treatment, and techniques for deep brain stimulation are improving.
Levodopa remains the most effective treatment, and techniques for deep brain stimulation are improving.
LOS ANGELES—Physicians who treat patients with Parkinson’s disease have many decisions to make based on therapeutic efficacy and desired outcomes. At the 70th Annual Meeting of the American Academy of Neurology, Melissa J. Nirenberg, MD, PhD, outlined the current landscape of Parkinson’s disease therapeutics, including data about symptom control, timing of treatment, and new therapies.
Initial Therapy: No Benefit to Levodopa Sparing
Levodopa, along with dopamine agonists and monoamine oxidase B (MAO-B) inhibitors, has Level A evidence as initial symptomatic therapy for Parkinson’s disease. “There is no question that levodopa is the most effective treatment for Parkinson’s disease,” said Dr. Nirenberg, Chief Medical Officer of the New York Stem Cell Foundation Research Institute and Adjunct Professor of Neurology at NYU Langone Health in New York City. “However, after people have been taking levodopa for a number of years, its therapeutic effect lasts for shorter periods of time, and patients spend an increasing amount of time in the off state, rather than in the on state.”
In addition to this wearing-off effect, levodopa treatment is associated with dyskinesias. This association and the wearing-off effect have prompted many physicians to adopt levodopa-sparing strategies, such as using dopamine agonists as initial treatment. However, dopamine agonists have other serious side effects, and research shows that in the long run, starting with a dopamine agonist does not improve outcomes.
In one study, data were compared between a large cohort of patients with Parkinson’s disease in Ghana, where levodopa therapy was initiated after a mean of 4.2 years’ disease duration, and patients with Parkinson’s disease in Italy, where levodopa was initiated at a mean of 2.4 years’ disease duration. “Disease duration and medication dosage, rather than the duration of levodopa therapy, affected the likelihood of dyskinesia,” Dr. Nirenberg said. “When you start levodopa late, you miss the honeymoon period,” she said, referring to the period during which patients experience the benefits of levodopa therapy before developing motor complications. “Simply put, levodopa as initial treatment works better [and] has fewer short- and long-term adverse effects [than dopamine agonists].”
Other Therapies
Dopamine agonists are highly efficacious as add-on treatment, but they also can have serious adverse effects. “Neurogenic orthostatic hypotension, psychosis, and sleepiness are adverse effects that are worse with dopamine agonists than with levodopa,” Dr. Nirenberg noted. “Another common adverse effect associated with dopamine agonists is impulse control disorders—pathologic gambling, compulsive eating, compulsive shopping, and hypersexuality.”
MAO-B inhibitors are also commonly used, well-tolerated medications that can be administered alone or in combination with levodopa or other medications. Of these drugs, selegiline and rasagiline can be used as monotherapy, Dr. Nirenberg noted, but a newer MAO-B inhibitor, safinamide, is not effective as monotherapy and should only be used as an adjunctive therapy with levodopa.
Extended release (ER) carbidopa–levodopa capsules, which contain immediate-release and ER beads to provide initial and extended levodopa plasma concentrations, have been effective in reducing wearing off between doses of levodopa, but conversion to this formulation from immediate-release levodopa is not straightforward. Rather than using the suggested conversion table in the package insert, neurologists might try the approach suggested by investigators who participated in the original clinical trials, said Dr. Nirenberg. Extended-release “capsules can be twisted open, and the beads poured into applesauce for people who have trouble swallowing,” she added.
Amantadine and anticholinergics are second-line medications that can be used as initial or adjunctive therapy. “They are weaker than the first-line drugs and have unfavorable adverse-effect profiles,” said Dr. Nirenberg. Amantadine, of which a newly approved extended-release formulation is available, can reduce dyskinesias.
New and Investigational Treatments
Deep brain stimulation (DBS) techniques are advancing, said Dr. Nirenberg. With DBS, a device implanted in the chest sends electrical pulses to electrodes inserted into targeted areas of the brain. “Recent studies are looking at closed-loop systems that provide direct feedback from the brain to the pacemaker so that stimulation is adjusted in real time.”
Continuous enteral infusion of carbidopa–levodopa intestinal gel over 16 hours via percutaneous endoscopic gastrojejunostomy is an option for people for whom DBS is being considered, but who have contraindications such as cognitive impairment or psychosis. “This [treatment] should only be prescribed to someone who has a good caregiver, because the pump has to be flushed often, removed before bathing, and checked to make sure there are no hardware problems or infections associated with its use.”
Droxidopa, a synthetic amino acid precursor of noradrenaline, received orphan-product designation for treatment of
Pimavanserin, a first-in-class drug approved in 2016 to treat hallucinations and delusions associated with Parkinson’s disease psychosis, is an atypical antipsychotic with a serotonergic mechanism of action. While the prospect of having such a treatment option initially generated excitement in the medical community, there have been recent concerns about adverse events in patients taking pimavanserin, including deaths, falls, insomnia, and nausea, in addition to continued hallucinations.
Focused ultrasound is approved for essential tremor and is investigational for Parkinson’s disease, Dr. Nirenberg noted. During the procedure, which can be performed on an outpatient basis, focused beams of ultrasonic energy are trained on targets deep in the brain to destroy diseased tissue without damaging surrounding normal tissue. Because of the lack of long-term follow-up of these patients, neurologists “do not know where this ultimately will fit in with Parkinson’s disease management,” said Dr. Nirenberg. Focused ultrasound is mainly being investigated as unilateral treatment because of concerns about the safety of bilateral ablative therapy.
To date, research on oral cannabinoids has not shown evidence of benefit for Parkinson’s disease, said Dr. Nirenberg. Neurologists have concerns about potential drug interactions and side effects such as imbalance, falls, cognitive impairment, and psychosis, which are of particular concern in people with Parkinson’s disease.
—Adriene Marshall
Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(Pt 10):2731-2742.
Tetrud J, Nausieda P, Kreitzman D, et al. Conversion to carbidopa and levodopa extended-release (IPX066) followed by its extended use in patients previously taking controlled-release carbidopa-levodopa for advanced Parkinson’s disease. J Neurol Sci. 2017;373:116-123.
LOS ANGELES—Physicians who treat patients with Parkinson’s disease have many decisions to make based on therapeutic efficacy and desired outcomes. At the 70th Annual Meeting of the American Academy of Neurology, Melissa J. Nirenberg, MD, PhD, outlined the current landscape of Parkinson’s disease therapeutics, including data about symptom control, timing of treatment, and new therapies.
Initial Therapy: No Benefit to Levodopa Sparing
Levodopa, along with dopamine agonists and monoamine oxidase B (MAO-B) inhibitors, has Level A evidence as initial symptomatic therapy for Parkinson’s disease. “There is no question that levodopa is the most effective treatment for Parkinson’s disease,” said Dr. Nirenberg, Chief Medical Officer of the New York Stem Cell Foundation Research Institute and Adjunct Professor of Neurology at NYU Langone Health in New York City. “However, after people have been taking levodopa for a number of years, its therapeutic effect lasts for shorter periods of time, and patients spend an increasing amount of time in the off state, rather than in the on state.”
In addition to this wearing-off effect, levodopa treatment is associated with dyskinesias. This association and the wearing-off effect have prompted many physicians to adopt levodopa-sparing strategies, such as using dopamine agonists as initial treatment. However, dopamine agonists have other serious side effects, and research shows that in the long run, starting with a dopamine agonist does not improve outcomes.
In one study, data were compared between a large cohort of patients with Parkinson’s disease in Ghana, where levodopa therapy was initiated after a mean of 4.2 years’ disease duration, and patients with Parkinson’s disease in Italy, where levodopa was initiated at a mean of 2.4 years’ disease duration. “Disease duration and medication dosage, rather than the duration of levodopa therapy, affected the likelihood of dyskinesia,” Dr. Nirenberg said. “When you start levodopa late, you miss the honeymoon period,” she said, referring to the period during which patients experience the benefits of levodopa therapy before developing motor complications. “Simply put, levodopa as initial treatment works better [and] has fewer short- and long-term adverse effects [than dopamine agonists].”
Other Therapies
Dopamine agonists are highly efficacious as add-on treatment, but they also can have serious adverse effects. “Neurogenic orthostatic hypotension, psychosis, and sleepiness are adverse effects that are worse with dopamine agonists than with levodopa,” Dr. Nirenberg noted. “Another common adverse effect associated with dopamine agonists is impulse control disorders—pathologic gambling, compulsive eating, compulsive shopping, and hypersexuality.”
MAO-B inhibitors are also commonly used, well-tolerated medications that can be administered alone or in combination with levodopa or other medications. Of these drugs, selegiline and rasagiline can be used as monotherapy, Dr. Nirenberg noted, but a newer MAO-B inhibitor, safinamide, is not effective as monotherapy and should only be used as an adjunctive therapy with levodopa.
Extended release (ER) carbidopa–levodopa capsules, which contain immediate-release and ER beads to provide initial and extended levodopa plasma concentrations, have been effective in reducing wearing off between doses of levodopa, but conversion to this formulation from immediate-release levodopa is not straightforward. Rather than using the suggested conversion table in the package insert, neurologists might try the approach suggested by investigators who participated in the original clinical trials, said Dr. Nirenberg. Extended-release “capsules can be twisted open, and the beads poured into applesauce for people who have trouble swallowing,” she added.
Amantadine and anticholinergics are second-line medications that can be used as initial or adjunctive therapy. “They are weaker than the first-line drugs and have unfavorable adverse-effect profiles,” said Dr. Nirenberg. Amantadine, of which a newly approved extended-release formulation is available, can reduce dyskinesias.
New and Investigational Treatments
Deep brain stimulation (DBS) techniques are advancing, said Dr. Nirenberg. With DBS, a device implanted in the chest sends electrical pulses to electrodes inserted into targeted areas of the brain. “Recent studies are looking at closed-loop systems that provide direct feedback from the brain to the pacemaker so that stimulation is adjusted in real time.”
Continuous enteral infusion of carbidopa–levodopa intestinal gel over 16 hours via percutaneous endoscopic gastrojejunostomy is an option for people for whom DBS is being considered, but who have contraindications such as cognitive impairment or psychosis. “This [treatment] should only be prescribed to someone who has a good caregiver, because the pump has to be flushed often, removed before bathing, and checked to make sure there are no hardware problems or infections associated with its use.”
Droxidopa, a synthetic amino acid precursor of noradrenaline, received orphan-product designation for treatment of
Pimavanserin, a first-in-class drug approved in 2016 to treat hallucinations and delusions associated with Parkinson’s disease psychosis, is an atypical antipsychotic with a serotonergic mechanism of action. While the prospect of having such a treatment option initially generated excitement in the medical community, there have been recent concerns about adverse events in patients taking pimavanserin, including deaths, falls, insomnia, and nausea, in addition to continued hallucinations.
Focused ultrasound is approved for essential tremor and is investigational for Parkinson’s disease, Dr. Nirenberg noted. During the procedure, which can be performed on an outpatient basis, focused beams of ultrasonic energy are trained on targets deep in the brain to destroy diseased tissue without damaging surrounding normal tissue. Because of the lack of long-term follow-up of these patients, neurologists “do not know where this ultimately will fit in with Parkinson’s disease management,” said Dr. Nirenberg. Focused ultrasound is mainly being investigated as unilateral treatment because of concerns about the safety of bilateral ablative therapy.
To date, research on oral cannabinoids has not shown evidence of benefit for Parkinson’s disease, said Dr. Nirenberg. Neurologists have concerns about potential drug interactions and side effects such as imbalance, falls, cognitive impairment, and psychosis, which are of particular concern in people with Parkinson’s disease.
—Adriene Marshall
Cilia R, Akpalu A, Sarfo FS, et al. The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain. 2014;137(Pt 10):2731-2742.
Tetrud J, Nausieda P, Kreitzman D, et al. Conversion to carbidopa and levodopa extended-release (IPX066) followed by its extended use in patients previously taking controlled-release carbidopa-levodopa for advanced Parkinson’s disease. J Neurol Sci. 2017;373:116-123.
Impulse control disorders in Parkinson’s patients may be higher than thought
Nearly half of patients with Parkinson’s disease who were taking dopamine agonist treatment experienced impulse control disorders over a follow-up of 5 years, according to recently published results of a longitudinal study.
The 5-year cumulative incidence of impulse control disorders was approximately 45% in the study, which included 411 patients with a high prevalence of dopamine agonist use and disease duration of 5 years or less at baseline.
There was a strong association between dopamine agonist use and impulse control disorders in the study, which was conducted by Jean-Christophe Corvol, MD, of Publique Hôpitaux de Paris and his coinvestigators.
Impulse disorders increased in incidence with both duration and dose of dopamine agonists and resolved progressively after discontinuation of those agents, the investigators reported online June 20 in Neurology. The investigators used item 1.6 of part I of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale to determine the presence of an impulse control disorder.
“Given the high cumulative incidence of impulse control disorders in patients with Parkinson’s disease, these adverse effects should be carefully monitored in patients ever treated with dopamine agonists,” Dr. Corvol and his coauthors wrote.
The results came from the ongoing Drug Interaction With Genes in Parkinson’s Disease (DIGPD) study, a longitudinal cohort study including Parkinson’s disease patients consecutively recruited between 2009 and 2013 at eight French hospitals. All patients had no more than 5 years of disease duration at recruitment, and follow-up included annual evaluations by movement disorder specialists.
At baseline, the majority of patients (302, or 73.5%) had taken dopamine agonists within the past 12 months.
Over the course of 5 years, the prevalence of impulse control disorders increased from 19.7% at baseline to 32.8%, Dr. Corvol and his colleagues reported.
Among 306 patients with no impulse control disorders at baseline, 94 developed one, for a 5-year cumulative incidence of 46.1%, they added. Only 4 of the 94 new cases occurred in patients who never used dopamine agonists.
Dopamine agonist use in the previous 12 months was associated with a 2.23-fold higher prevalence of impulse control disorders (P less than .001), with prevalence increasing along with average daily dose and cumulative dose duration over that time period, according to the investigators.
These findings suggests tools are needed to screen for impulse control disorders and identify high-risk patients, they said.
“Further studies are needed to understand the mechanisms involved in the relation between [dopamine agonists] and [impulse control disorders], in particular the role of apathy, anxiety, and depression,” they added.
The study was funded by grants from the French Ministry of Health and from the French Drug Agency. Dr. Corvol and many of his colleagues reported financial disclosures with many pharmaceutical companies.
SOURCE: Corvol J-C et al. Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005816.
Data from the study by Dr. Corvol and colleagues are robust and suggest neurologists may be “missing the boat or even harming patients” by underestimating the adverse effects associated with dopamine agonists, the authors of an editorial wrote.
The 5-year incidence of impulse control disorders approaching 50% suggests they are even more common than previously reported, according to editorial authors Laura S. Boylan, MD, and Vladimir S. Kostic, MD, PhD.Compulsive gambling, shopping, eating, sexual behaviors and other impulse control disorders at their worst can ruin finances, disrupt families, and have legal implications, Dr. Boylan and Dr. Kostic said in their editorial.
Neurologists are “often uncomfortable” with psychiatric disorders, they added, even though they are the ones managing movement disorder medications.
There is an absence of high-quality evidence on how to treat impulse control disorders, though one common approach, switching to levodopa, is in the wheelhouse of neurologists. However, “levodopaphobia” persists in some circles despite evidence debunking the notion that the medication is neurotoxic, according to Dr. Boylan and Dr. Kostic.
“Practice change in medicine, as in other areas, can be like turning a cruise ship,” they wrote, “but this study may help give a little push to the boat and, we hope, promote further basic and clinical research on nonmotor aspects of PD and other movement disorders.”
Dr. Boylan is with Essentia Health, Duluth, Minn., Albany-Stratton VA Medical Center, Albany, N.Y., and Bellevue Hospital/New York University. Dr. Kosticis with the Institute of Neurology CCS, School of Medicine University of Belgrade (Serbia). Dr. Kostic reported receiving speaker honoraria from Novartis, Teva, and Salveo. Their editorial accompanied Dr. Corvol and colleagues’ report (Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005806 ).
Data from the study by Dr. Corvol and colleagues are robust and suggest neurologists may be “missing the boat or even harming patients” by underestimating the adverse effects associated with dopamine agonists, the authors of an editorial wrote.
The 5-year incidence of impulse control disorders approaching 50% suggests they are even more common than previously reported, according to editorial authors Laura S. Boylan, MD, and Vladimir S. Kostic, MD, PhD.Compulsive gambling, shopping, eating, sexual behaviors and other impulse control disorders at their worst can ruin finances, disrupt families, and have legal implications, Dr. Boylan and Dr. Kostic said in their editorial.
Neurologists are “often uncomfortable” with psychiatric disorders, they added, even though they are the ones managing movement disorder medications.
There is an absence of high-quality evidence on how to treat impulse control disorders, though one common approach, switching to levodopa, is in the wheelhouse of neurologists. However, “levodopaphobia” persists in some circles despite evidence debunking the notion that the medication is neurotoxic, according to Dr. Boylan and Dr. Kostic.
“Practice change in medicine, as in other areas, can be like turning a cruise ship,” they wrote, “but this study may help give a little push to the boat and, we hope, promote further basic and clinical research on nonmotor aspects of PD and other movement disorders.”
Dr. Boylan is with Essentia Health, Duluth, Minn., Albany-Stratton VA Medical Center, Albany, N.Y., and Bellevue Hospital/New York University. Dr. Kosticis with the Institute of Neurology CCS, School of Medicine University of Belgrade (Serbia). Dr. Kostic reported receiving speaker honoraria from Novartis, Teva, and Salveo. Their editorial accompanied Dr. Corvol and colleagues’ report (Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005806 ).
Data from the study by Dr. Corvol and colleagues are robust and suggest neurologists may be “missing the boat or even harming patients” by underestimating the adverse effects associated with dopamine agonists, the authors of an editorial wrote.
The 5-year incidence of impulse control disorders approaching 50% suggests they are even more common than previously reported, according to editorial authors Laura S. Boylan, MD, and Vladimir S. Kostic, MD, PhD.Compulsive gambling, shopping, eating, sexual behaviors and other impulse control disorders at their worst can ruin finances, disrupt families, and have legal implications, Dr. Boylan and Dr. Kostic said in their editorial.
Neurologists are “often uncomfortable” with psychiatric disorders, they added, even though they are the ones managing movement disorder medications.
There is an absence of high-quality evidence on how to treat impulse control disorders, though one common approach, switching to levodopa, is in the wheelhouse of neurologists. However, “levodopaphobia” persists in some circles despite evidence debunking the notion that the medication is neurotoxic, according to Dr. Boylan and Dr. Kostic.
“Practice change in medicine, as in other areas, can be like turning a cruise ship,” they wrote, “but this study may help give a little push to the boat and, we hope, promote further basic and clinical research on nonmotor aspects of PD and other movement disorders.”
Dr. Boylan is with Essentia Health, Duluth, Minn., Albany-Stratton VA Medical Center, Albany, N.Y., and Bellevue Hospital/New York University. Dr. Kosticis with the Institute of Neurology CCS, School of Medicine University of Belgrade (Serbia). Dr. Kostic reported receiving speaker honoraria from Novartis, Teva, and Salveo. Their editorial accompanied Dr. Corvol and colleagues’ report (Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005806 ).
Nearly half of patients with Parkinson’s disease who were taking dopamine agonist treatment experienced impulse control disorders over a follow-up of 5 years, according to recently published results of a longitudinal study.
The 5-year cumulative incidence of impulse control disorders was approximately 45% in the study, which included 411 patients with a high prevalence of dopamine agonist use and disease duration of 5 years or less at baseline.
There was a strong association between dopamine agonist use and impulse control disorders in the study, which was conducted by Jean-Christophe Corvol, MD, of Publique Hôpitaux de Paris and his coinvestigators.
Impulse disorders increased in incidence with both duration and dose of dopamine agonists and resolved progressively after discontinuation of those agents, the investigators reported online June 20 in Neurology. The investigators used item 1.6 of part I of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale to determine the presence of an impulse control disorder.
“Given the high cumulative incidence of impulse control disorders in patients with Parkinson’s disease, these adverse effects should be carefully monitored in patients ever treated with dopamine agonists,” Dr. Corvol and his coauthors wrote.
The results came from the ongoing Drug Interaction With Genes in Parkinson’s Disease (DIGPD) study, a longitudinal cohort study including Parkinson’s disease patients consecutively recruited between 2009 and 2013 at eight French hospitals. All patients had no more than 5 years of disease duration at recruitment, and follow-up included annual evaluations by movement disorder specialists.
At baseline, the majority of patients (302, or 73.5%) had taken dopamine agonists within the past 12 months.
Over the course of 5 years, the prevalence of impulse control disorders increased from 19.7% at baseline to 32.8%, Dr. Corvol and his colleagues reported.
Among 306 patients with no impulse control disorders at baseline, 94 developed one, for a 5-year cumulative incidence of 46.1%, they added. Only 4 of the 94 new cases occurred in patients who never used dopamine agonists.
Dopamine agonist use in the previous 12 months was associated with a 2.23-fold higher prevalence of impulse control disorders (P less than .001), with prevalence increasing along with average daily dose and cumulative dose duration over that time period, according to the investigators.
These findings suggests tools are needed to screen for impulse control disorders and identify high-risk patients, they said.
“Further studies are needed to understand the mechanisms involved in the relation between [dopamine agonists] and [impulse control disorders], in particular the role of apathy, anxiety, and depression,” they added.
The study was funded by grants from the French Ministry of Health and from the French Drug Agency. Dr. Corvol and many of his colleagues reported financial disclosures with many pharmaceutical companies.
SOURCE: Corvol J-C et al. Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005816.
Nearly half of patients with Parkinson’s disease who were taking dopamine agonist treatment experienced impulse control disorders over a follow-up of 5 years, according to recently published results of a longitudinal study.
The 5-year cumulative incidence of impulse control disorders was approximately 45% in the study, which included 411 patients with a high prevalence of dopamine agonist use and disease duration of 5 years or less at baseline.
There was a strong association between dopamine agonist use and impulse control disorders in the study, which was conducted by Jean-Christophe Corvol, MD, of Publique Hôpitaux de Paris and his coinvestigators.
Impulse disorders increased in incidence with both duration and dose of dopamine agonists and resolved progressively after discontinuation of those agents, the investigators reported online June 20 in Neurology. The investigators used item 1.6 of part I of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale to determine the presence of an impulse control disorder.
“Given the high cumulative incidence of impulse control disorders in patients with Parkinson’s disease, these adverse effects should be carefully monitored in patients ever treated with dopamine agonists,” Dr. Corvol and his coauthors wrote.
The results came from the ongoing Drug Interaction With Genes in Parkinson’s Disease (DIGPD) study, a longitudinal cohort study including Parkinson’s disease patients consecutively recruited between 2009 and 2013 at eight French hospitals. All patients had no more than 5 years of disease duration at recruitment, and follow-up included annual evaluations by movement disorder specialists.
At baseline, the majority of patients (302, or 73.5%) had taken dopamine agonists within the past 12 months.
Over the course of 5 years, the prevalence of impulse control disorders increased from 19.7% at baseline to 32.8%, Dr. Corvol and his colleagues reported.
Among 306 patients with no impulse control disorders at baseline, 94 developed one, for a 5-year cumulative incidence of 46.1%, they added. Only 4 of the 94 new cases occurred in patients who never used dopamine agonists.
Dopamine agonist use in the previous 12 months was associated with a 2.23-fold higher prevalence of impulse control disorders (P less than .001), with prevalence increasing along with average daily dose and cumulative dose duration over that time period, according to the investigators.
These findings suggests tools are needed to screen for impulse control disorders and identify high-risk patients, they said.
“Further studies are needed to understand the mechanisms involved in the relation between [dopamine agonists] and [impulse control disorders], in particular the role of apathy, anxiety, and depression,” they added.
The study was funded by grants from the French Ministry of Health and from the French Drug Agency. Dr. Corvol and many of his colleagues reported financial disclosures with many pharmaceutical companies.
SOURCE: Corvol J-C et al. Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005816.
FROM NEUROLOGY
Key clinical point: Nearly half of Parkinson’s disease patients reported having an impulse control disorder during a 5-year period, the great majority of whom were receiving dopamine agonist treatment.
Major finding: The 5-year cumulative incidence of impulse control disorders was approximately 45%, with increased risk correlating with dose and duration of dopamine agonist treatment.
Study details: Analysis of a multicenter, longitudinal cohort including 5 years of follow-up on 411 consecutive patients with Parkinson’s disease and a disease duration of 5 years or less at baseline.
Disclosures: The study was funded by grants from the French Ministry of Health and from the French Drug Agency. Many of the authors reported financial disclosures with many pharmaceutical companies.
Source: Corvol J-C et al. Neurology. 2018 Jun 20. doi: 10.1212/WNL.0000000000005816.
Monoclonal antibody reduced alpha-synuclein levels in Parkinson’s patients
Adults with mild to moderate Parkinson’s disease showed reductions in free serum alpha-synuclein levels without notable side effects after intravenous treatment with a monoclonal antibody known as PRX002.
“Pathologically, PD [Parkinson’s disease] is typically associated with an accumulation of aggregated alpha-synuclein protein in the central nervous system and the peripheral nervous system,” making alpha-synuclein a target for treatment in preclinical studies, wrote Joseph Jankovic, MD, of Baylor College of Medicine, Houston, and his colleagues.
“Notably, rapid and robust reductions in free serum alpha-synuclein levels were achieved without seriously affecting safety,” the researchers said. Overall, reductions in free serum alpha-synuclein occurred quickly and were similar throughout the study period, and treatment with PRX002 was safe, well tolerated, and effective at doses up to 60 mg/kg.
The most relevant adverse events were mild to moderate infusion-related reactions in four patients in the highest-dose group; two of these patients discontinued the study. No anti-PRX002 antibodies were seen, and no serious adverse events or deaths occurred during the study period.
Statistically significant reductions from baseline were noted at 1 and 4 hours after the first and third infusion in all dose groups, compared with placebo, and these reductions lasted longer after the higher doses.
Over the longer term, statistically significant reductions after the third infusion were noted at day 64 for the 1.0-mg/kg through 60-mg/kg dose groups, day 71 for the 1.0-mg/kg through 60-mg/kg dose groups, and at day 85 for the 3-mg/kg through 60-mg/kg dose groups.
The study findings were limited by several factors, including the relatively small sample size, short period of exposure to the treatment, homogeneous population, and lack of imaging to monitor brain pathology, the researchers noted. However, the results support the safety of PRX002 and the progression of the follow-up phase 2 study known as PASADENA.
The study was funded by Prothena Biosciences and F. Hoffmann-LaRoche. Lead author Dr. Jankovich disclosed relationships with both of those companies and has received funding from the Parkinson’s Foundation. Many of the other authors are employees of Prothena Biosciences or F. Hoffmann-LaRoche or a subsidiary.
SOURCE: Jankovic J et al. JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.1487.
The study results met the endpoints for safety and tolerance; however, “the question remains: To what extent does this process reflect the role of alpha-synuclein in the causal mechanisms of Parkinson disease?” wrote Fredric P. Manfredsson, PhD; Malú G. Tansey, PhD; and Todd E. Golde, PhD, in an accompanying editorial (JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.0346).
The trio noted that the potential of alpha-synuclein for cell-to-cell transmission and disease propagation and progression remains unknown and the research behind the passive immunization technique remains limited and controversial at the preclinical level. In addition, they emphasized the need to consider the potential for neurotoxicity with the removal of soluble alpha-synuclein from neurons.
“Thus, the potential negative consequences following sustained treatment with PRX002 must also be heavily scrutinized before it can be said to be safe for long-term use in elderly individuals,” they wrote.
The study also lacked data on whether the antibody directly engaged its target in the CNS, they said.
“Although the PRX002 trial met its primary goals and is now poised to move forward into efficacy trials, it is clear that progress within the synuclein basic science field needs to follow suit,” they concluded.
Dr. Manfredsson is affiliated with Michigan State University in Grand Rapids. Dr. Tansey is affiliated with Emory University in Atlanta. Dr. Golde is affiliated with the University of Florida, Gainesville. Dr. Tansey disclosed relationships with INmune Bio, Above and Beyond, Hygieia Sciences, UCB, and the Michael J. Fox Foundation for Parkinson’s Research.
The study results met the endpoints for safety and tolerance; however, “the question remains: To what extent does this process reflect the role of alpha-synuclein in the causal mechanisms of Parkinson disease?” wrote Fredric P. Manfredsson, PhD; Malú G. Tansey, PhD; and Todd E. Golde, PhD, in an accompanying editorial (JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.0346).
The trio noted that the potential of alpha-synuclein for cell-to-cell transmission and disease propagation and progression remains unknown and the research behind the passive immunization technique remains limited and controversial at the preclinical level. In addition, they emphasized the need to consider the potential for neurotoxicity with the removal of soluble alpha-synuclein from neurons.
“Thus, the potential negative consequences following sustained treatment with PRX002 must also be heavily scrutinized before it can be said to be safe for long-term use in elderly individuals,” they wrote.
The study also lacked data on whether the antibody directly engaged its target in the CNS, they said.
“Although the PRX002 trial met its primary goals and is now poised to move forward into efficacy trials, it is clear that progress within the synuclein basic science field needs to follow suit,” they concluded.
Dr. Manfredsson is affiliated with Michigan State University in Grand Rapids. Dr. Tansey is affiliated with Emory University in Atlanta. Dr. Golde is affiliated with the University of Florida, Gainesville. Dr. Tansey disclosed relationships with INmune Bio, Above and Beyond, Hygieia Sciences, UCB, and the Michael J. Fox Foundation for Parkinson’s Research.
The study results met the endpoints for safety and tolerance; however, “the question remains: To what extent does this process reflect the role of alpha-synuclein in the causal mechanisms of Parkinson disease?” wrote Fredric P. Manfredsson, PhD; Malú G. Tansey, PhD; and Todd E. Golde, PhD, in an accompanying editorial (JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.0346).
The trio noted that the potential of alpha-synuclein for cell-to-cell transmission and disease propagation and progression remains unknown and the research behind the passive immunization technique remains limited and controversial at the preclinical level. In addition, they emphasized the need to consider the potential for neurotoxicity with the removal of soluble alpha-synuclein from neurons.
“Thus, the potential negative consequences following sustained treatment with PRX002 must also be heavily scrutinized before it can be said to be safe for long-term use in elderly individuals,” they wrote.
The study also lacked data on whether the antibody directly engaged its target in the CNS, they said.
“Although the PRX002 trial met its primary goals and is now poised to move forward into efficacy trials, it is clear that progress within the synuclein basic science field needs to follow suit,” they concluded.
Dr. Manfredsson is affiliated with Michigan State University in Grand Rapids. Dr. Tansey is affiliated with Emory University in Atlanta. Dr. Golde is affiliated with the University of Florida, Gainesville. Dr. Tansey disclosed relationships with INmune Bio, Above and Beyond, Hygieia Sciences, UCB, and the Michael J. Fox Foundation for Parkinson’s Research.
Adults with mild to moderate Parkinson’s disease showed reductions in free serum alpha-synuclein levels without notable side effects after intravenous treatment with a monoclonal antibody known as PRX002.
“Pathologically, PD [Parkinson’s disease] is typically associated with an accumulation of aggregated alpha-synuclein protein in the central nervous system and the peripheral nervous system,” making alpha-synuclein a target for treatment in preclinical studies, wrote Joseph Jankovic, MD, of Baylor College of Medicine, Houston, and his colleagues.
“Notably, rapid and robust reductions in free serum alpha-synuclein levels were achieved without seriously affecting safety,” the researchers said. Overall, reductions in free serum alpha-synuclein occurred quickly and were similar throughout the study period, and treatment with PRX002 was safe, well tolerated, and effective at doses up to 60 mg/kg.
The most relevant adverse events were mild to moderate infusion-related reactions in four patients in the highest-dose group; two of these patients discontinued the study. No anti-PRX002 antibodies were seen, and no serious adverse events or deaths occurred during the study period.
Statistically significant reductions from baseline were noted at 1 and 4 hours after the first and third infusion in all dose groups, compared with placebo, and these reductions lasted longer after the higher doses.
Over the longer term, statistically significant reductions after the third infusion were noted at day 64 for the 1.0-mg/kg through 60-mg/kg dose groups, day 71 for the 1.0-mg/kg through 60-mg/kg dose groups, and at day 85 for the 3-mg/kg through 60-mg/kg dose groups.
The study findings were limited by several factors, including the relatively small sample size, short period of exposure to the treatment, homogeneous population, and lack of imaging to monitor brain pathology, the researchers noted. However, the results support the safety of PRX002 and the progression of the follow-up phase 2 study known as PASADENA.
The study was funded by Prothena Biosciences and F. Hoffmann-LaRoche. Lead author Dr. Jankovich disclosed relationships with both of those companies and has received funding from the Parkinson’s Foundation. Many of the other authors are employees of Prothena Biosciences or F. Hoffmann-LaRoche or a subsidiary.
SOURCE: Jankovic J et al. JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.1487.
Adults with mild to moderate Parkinson’s disease showed reductions in free serum alpha-synuclein levels without notable side effects after intravenous treatment with a monoclonal antibody known as PRX002.
“Pathologically, PD [Parkinson’s disease] is typically associated with an accumulation of aggregated alpha-synuclein protein in the central nervous system and the peripheral nervous system,” making alpha-synuclein a target for treatment in preclinical studies, wrote Joseph Jankovic, MD, of Baylor College of Medicine, Houston, and his colleagues.
“Notably, rapid and robust reductions in free serum alpha-synuclein levels were achieved without seriously affecting safety,” the researchers said. Overall, reductions in free serum alpha-synuclein occurred quickly and were similar throughout the study period, and treatment with PRX002 was safe, well tolerated, and effective at doses up to 60 mg/kg.
The most relevant adverse events were mild to moderate infusion-related reactions in four patients in the highest-dose group; two of these patients discontinued the study. No anti-PRX002 antibodies were seen, and no serious adverse events or deaths occurred during the study period.
Statistically significant reductions from baseline were noted at 1 and 4 hours after the first and third infusion in all dose groups, compared with placebo, and these reductions lasted longer after the higher doses.
Over the longer term, statistically significant reductions after the third infusion were noted at day 64 for the 1.0-mg/kg through 60-mg/kg dose groups, day 71 for the 1.0-mg/kg through 60-mg/kg dose groups, and at day 85 for the 3-mg/kg through 60-mg/kg dose groups.
The study findings were limited by several factors, including the relatively small sample size, short period of exposure to the treatment, homogeneous population, and lack of imaging to monitor brain pathology, the researchers noted. However, the results support the safety of PRX002 and the progression of the follow-up phase 2 study known as PASADENA.
The study was funded by Prothena Biosciences and F. Hoffmann-LaRoche. Lead author Dr. Jankovich disclosed relationships with both of those companies and has received funding from the Parkinson’s Foundation. Many of the other authors are employees of Prothena Biosciences or F. Hoffmann-LaRoche or a subsidiary.
SOURCE: Jankovic J et al. JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.1487.
FROM JAMA NEUROLOGY
Key clinical point: Treatment with a monoclonal antibody for alpha-synuclein known as PRX002 was safe and effective in a preliminary study of Parkinson’s disease patients.
Major finding: Significant reductions in free serum alpha-synuclein levels persisted at 85 days after the third infusion in the 3-mg/kg through 60-mg/kg dose groups.
Study details: The data come from a randomized, phase 1b trial of 80 adults aged 40-80 years with Parkinson’s disease.
Disclosures: The study was funded by Prothena Biosciences and F. Hoffmann-LaRoche. Lead author Dr. Jankovich disclosed relationships with both of those companies and has received funding from the Parkinson’s Foundation. Many of the other authors are employees of Prothena Biosciences or F. Hoffmann-LaRoche or a subsidiary.
Source: Jankovic J et al. JAMA Neurol. 2018 June 18. doi: 10.1001/jamaneurol.2018.1487.
Experimental Therapy Shows Promise in Early-Stage Huntington’s Disease
LOS ANGELES—IONIS-HTTRx, an antisense oligonucleotide (ASO), effectively reduced levels of the protein responsible for Huntington’s disease in early-stage patients, according to findings presented at the American Academy of Neurology’s 70th Annual Meeting.
“ASO technology has the potential to provide disease-modifying benefits to patients with neurodegenerative diseases,” said lead author Sarah Tabrizi, BSc, MBChB, PhD, Professor of Clinical Neurology at the University College London Institute of Neurology, and colleagues. “In this phase I/IIa trial in patients with early-stage Huntington’s disease, IONIS-HTTRx delivered via intrathecal injection was well tolerated with no study-drug-related adverse safety signals during the treatment or follow-up periods.” In addition, significant dose-dependent reductions in CSF mutant huntingtin protein (mHTT) were observed, suggesting that IONIS-HTTRx is a promising therapeutic for the treatment of Huntington’s disease.
Huntington’s disease is caused by CAG repeat expansion in the HTT gene resulting in polyglutamine expansion in the mHTT with a toxic gain-of-function disease mechanism. A comprehensive drug discovery effort, including extensive preclinical testing, was undertaken to design a well-tolerated ASO with high specificity to human HTT mRNA that could potently suppresses mHTT production.
In the present study—a first-in-human, multicenter, double-blind clinical trial—46 patients were randomized (3:1) to receive four doses of IONIS-HTTRx or placebo by monthly bolus intrathecal injection, followed by a four-month untreated period. Five ascending-dose cohorts were enrolled with independent Data Safety Monitoring Board review of safety, pharmacokinetics, and target engagement prior to dose escalation.
Dr. Tabrizi reported that IONIS-HTTRx was well tolerated at all doses tested. Adverse events were mostly mild and unrelated to the study drug. There were no adverse trends in laboratory parameters. No patients prematurely discontinued the treatment. ASO was measurable in CSF and plasma, and concentrations were generally aligned with predictions from a linked pharmacokinetic/pharmacodynamic preclinical model. Significant, dose-dependent reductions in CSF mHTT were observed. At the highest dose of the ASO, 40% to 60% reductions in CSF mHTT were observed. “When we looked more carefully at the data with exploratory analyses, we found a link between lowering of CSF mHTT and improvement in total motor scores and neurologic function,” Dr. Tabrizi said.
LOS ANGELES—IONIS-HTTRx, an antisense oligonucleotide (ASO), effectively reduced levels of the protein responsible for Huntington’s disease in early-stage patients, according to findings presented at the American Academy of Neurology’s 70th Annual Meeting.
“ASO technology has the potential to provide disease-modifying benefits to patients with neurodegenerative diseases,” said lead author Sarah Tabrizi, BSc, MBChB, PhD, Professor of Clinical Neurology at the University College London Institute of Neurology, and colleagues. “In this phase I/IIa trial in patients with early-stage Huntington’s disease, IONIS-HTTRx delivered via intrathecal injection was well tolerated with no study-drug-related adverse safety signals during the treatment or follow-up periods.” In addition, significant dose-dependent reductions in CSF mutant huntingtin protein (mHTT) were observed, suggesting that IONIS-HTTRx is a promising therapeutic for the treatment of Huntington’s disease.
Huntington’s disease is caused by CAG repeat expansion in the HTT gene resulting in polyglutamine expansion in the mHTT with a toxic gain-of-function disease mechanism. A comprehensive drug discovery effort, including extensive preclinical testing, was undertaken to design a well-tolerated ASO with high specificity to human HTT mRNA that could potently suppresses mHTT production.
In the present study—a first-in-human, multicenter, double-blind clinical trial—46 patients were randomized (3:1) to receive four doses of IONIS-HTTRx or placebo by monthly bolus intrathecal injection, followed by a four-month untreated period. Five ascending-dose cohorts were enrolled with independent Data Safety Monitoring Board review of safety, pharmacokinetics, and target engagement prior to dose escalation.
Dr. Tabrizi reported that IONIS-HTTRx was well tolerated at all doses tested. Adverse events were mostly mild and unrelated to the study drug. There were no adverse trends in laboratory parameters. No patients prematurely discontinued the treatment. ASO was measurable in CSF and plasma, and concentrations were generally aligned with predictions from a linked pharmacokinetic/pharmacodynamic preclinical model. Significant, dose-dependent reductions in CSF mHTT were observed. At the highest dose of the ASO, 40% to 60% reductions in CSF mHTT were observed. “When we looked more carefully at the data with exploratory analyses, we found a link between lowering of CSF mHTT and improvement in total motor scores and neurologic function,” Dr. Tabrizi said.
LOS ANGELES—IONIS-HTTRx, an antisense oligonucleotide (ASO), effectively reduced levels of the protein responsible for Huntington’s disease in early-stage patients, according to findings presented at the American Academy of Neurology’s 70th Annual Meeting.
“ASO technology has the potential to provide disease-modifying benefits to patients with neurodegenerative diseases,” said lead author Sarah Tabrizi, BSc, MBChB, PhD, Professor of Clinical Neurology at the University College London Institute of Neurology, and colleagues. “In this phase I/IIa trial in patients with early-stage Huntington’s disease, IONIS-HTTRx delivered via intrathecal injection was well tolerated with no study-drug-related adverse safety signals during the treatment or follow-up periods.” In addition, significant dose-dependent reductions in CSF mutant huntingtin protein (mHTT) were observed, suggesting that IONIS-HTTRx is a promising therapeutic for the treatment of Huntington’s disease.
Huntington’s disease is caused by CAG repeat expansion in the HTT gene resulting in polyglutamine expansion in the mHTT with a toxic gain-of-function disease mechanism. A comprehensive drug discovery effort, including extensive preclinical testing, was undertaken to design a well-tolerated ASO with high specificity to human HTT mRNA that could potently suppresses mHTT production.
In the present study—a first-in-human, multicenter, double-blind clinical trial—46 patients were randomized (3:1) to receive four doses of IONIS-HTTRx or placebo by monthly bolus intrathecal injection, followed by a four-month untreated period. Five ascending-dose cohorts were enrolled with independent Data Safety Monitoring Board review of safety, pharmacokinetics, and target engagement prior to dose escalation.
Dr. Tabrizi reported that IONIS-HTTRx was well tolerated at all doses tested. Adverse events were mostly mild and unrelated to the study drug. There were no adverse trends in laboratory parameters. No patients prematurely discontinued the treatment. ASO was measurable in CSF and plasma, and concentrations were generally aligned with predictions from a linked pharmacokinetic/pharmacodynamic preclinical model. Significant, dose-dependent reductions in CSF mHTT were observed. At the highest dose of the ASO, 40% to 60% reductions in CSF mHTT were observed. “When we looked more carefully at the data with exploratory analyses, we found a link between lowering of CSF mHTT and improvement in total motor scores and neurologic function,” Dr. Tabrizi said.
Repetitive transcranial magnetic stimulation for tic disorders
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31
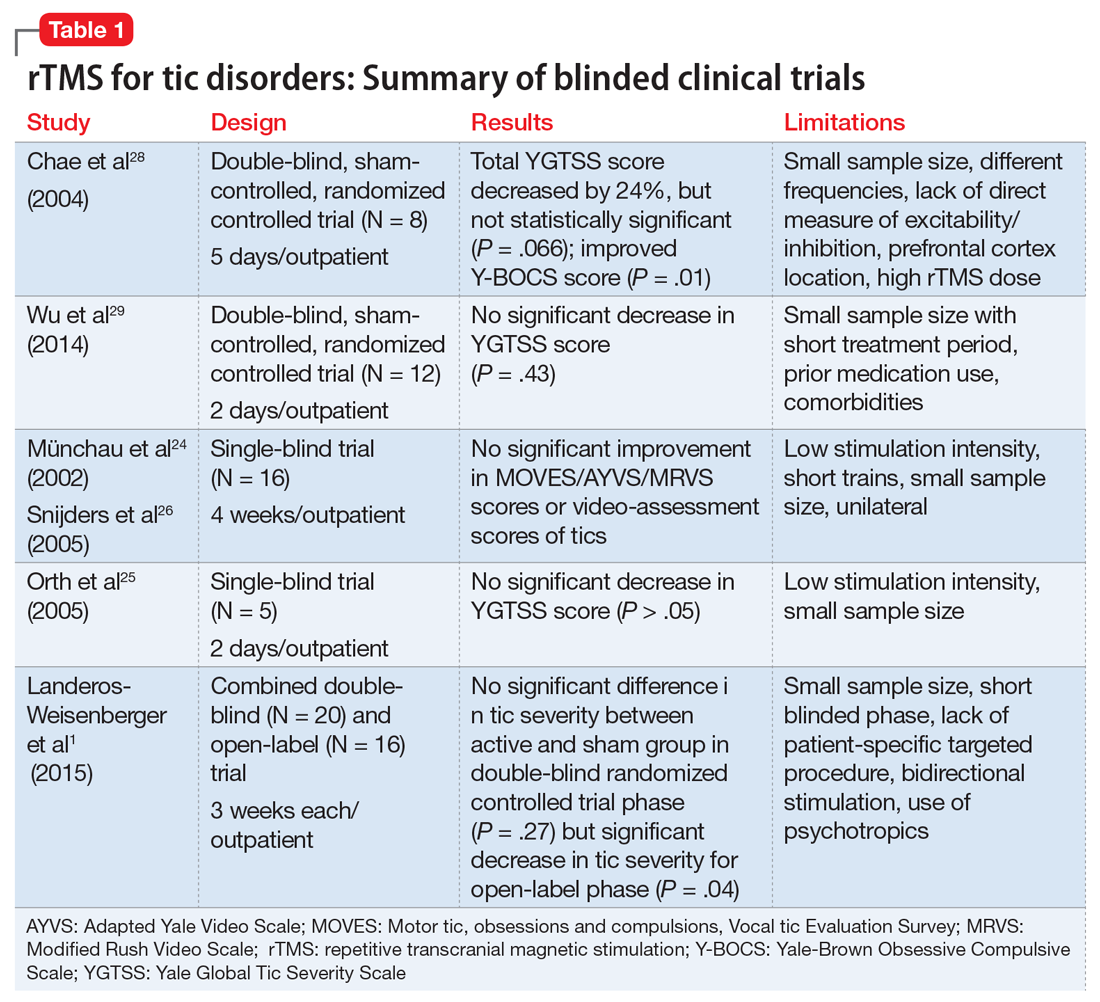
Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31
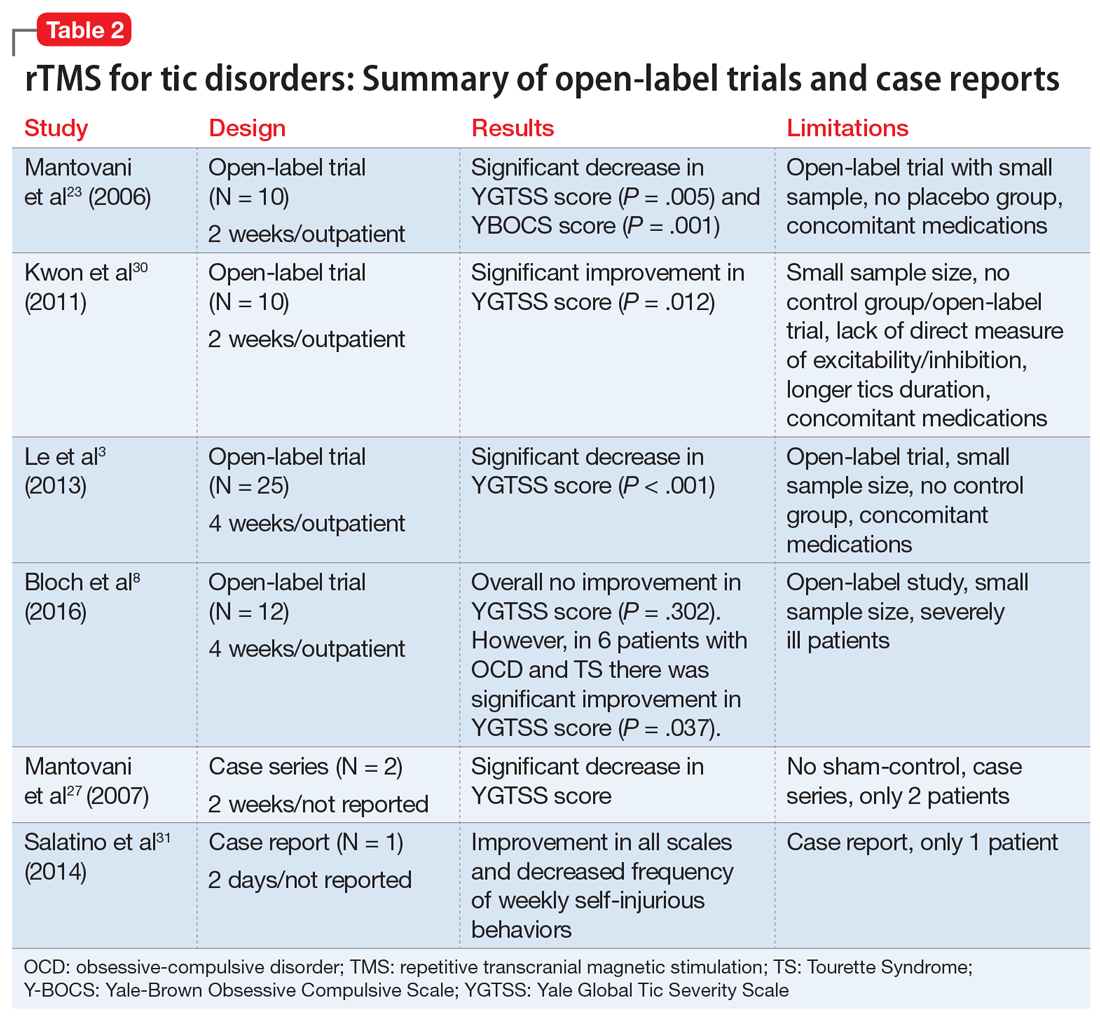
Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31

Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31

Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31

Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31

Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.
Tear Proteins May Be Biomarkers for Parkinson’s Disease
LOS ANGELES—Tears may hold diagnostic clues as to whether someone has Parkinson’s disease, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting. “We believe our research is the first to show that tears may be a reliable, inexpensive, and noninvasive biologic marker of Parkinson’s disease,” said study author Mark Floyd Lew, MD, Professor of Clinical Neurology, and Joseph P. Van Der Meulen, MD, Chair in Parkinson’s Disease Research in Honor of Robert J. Pasarow, and Vice Chair, in the Department of Neurology at the Keck School of Medicine of the University of Southern California in Los Angeles.
Nonmotor features of Parkinson’s disease occur years prior to motor dysfunction and represent a well-suited platform to investigate for a possible biomarker. Lacrimal glands are highly innervated by cholinergic neurons, and tear fluid secreted by lacrimal glands is greatly stimulated by cholinergic neurons. The production, packaging, and secretion of specific proteins into tears may be regulated by changes in nerve function to lacrimal glands. According to the researchers, analysis of any alteration in the secretion of proteins into tears may identify a reliable and noninvasive biomarker for Parkinson’s disease.
For the study, tear samples were collected from 55 patients with Parkinson’s disease of varying severity and 27 age- and gender-matched controls without Parkinson’s disease. In addition, tears were analyzed for the levels of four proteins—total alpha synuclein, CC chemokine ligand 2 (CCL-2), DJ-1 (Parkinson’s disease protein 7), and oligomeric alpha synuclein.
The researchers found differences in the levels of a total alpha-synuclein in the tears of patients with Parkinson’s disease, compared with those of controls. Additionally, levels of oligomeric alpha-synuclein, which is alpha-synuclein that has formed aggregates that are implicated in nerve damage in Parkinson’s disease, were also significantly different, compared with controls. It is also possible that the tear gland secretory cells themselves produce these different forms of alpha-synuclein that can be directly secreted into tears, the researchers said.
Total levels of alpha-synuclein were decreased in patients with Parkinson’s disease, with an average of 423 picograms of that protein per milligram (pg/mg) compared with 704 pg/mg in healthy controls. However, levels of oligomeric alpha-synuclein were increased in patients with Parkinson’s disease, with an average of 1.45 nanograms per milligram of tear protein (ng/mg), compared with 0.27 ng/mg in controls. While detectable in tears, neither CCL-2 nor DJ-1 varied between patients with Parkinson’s disease and controls.
“Knowing that something as simple as tears could help neurologists differentiate between people who have Parkinson’s disease and those who do not in a noninvasive manner is exciting,” said Dr. Lew. “And because the Parkinson’s disease process can begin years or decades before symptoms appear, a biologic marker like this could be useful in diagnosing, or even treating, the disease earlier.”
More research needs to be done in larger groups of people to investigate whether these protein changes can be detected in tears in the earliest presymptomatic stages of the disease, said the researchers.
The study was supported by the Michael J. Fox Foundation for Parkinson’s Research and the Plotkin Foundation.
LOS ANGELES—Tears may hold diagnostic clues as to whether someone has Parkinson’s disease, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting. “We believe our research is the first to show that tears may be a reliable, inexpensive, and noninvasive biologic marker of Parkinson’s disease,” said study author Mark Floyd Lew, MD, Professor of Clinical Neurology, and Joseph P. Van Der Meulen, MD, Chair in Parkinson’s Disease Research in Honor of Robert J. Pasarow, and Vice Chair, in the Department of Neurology at the Keck School of Medicine of the University of Southern California in Los Angeles.
Nonmotor features of Parkinson’s disease occur years prior to motor dysfunction and represent a well-suited platform to investigate for a possible biomarker. Lacrimal glands are highly innervated by cholinergic neurons, and tear fluid secreted by lacrimal glands is greatly stimulated by cholinergic neurons. The production, packaging, and secretion of specific proteins into tears may be regulated by changes in nerve function to lacrimal glands. According to the researchers, analysis of any alteration in the secretion of proteins into tears may identify a reliable and noninvasive biomarker for Parkinson’s disease.
For the study, tear samples were collected from 55 patients with Parkinson’s disease of varying severity and 27 age- and gender-matched controls without Parkinson’s disease. In addition, tears were analyzed for the levels of four proteins—total alpha synuclein, CC chemokine ligand 2 (CCL-2), DJ-1 (Parkinson’s disease protein 7), and oligomeric alpha synuclein.
The researchers found differences in the levels of a total alpha-synuclein in the tears of patients with Parkinson’s disease, compared with those of controls. Additionally, levels of oligomeric alpha-synuclein, which is alpha-synuclein that has formed aggregates that are implicated in nerve damage in Parkinson’s disease, were also significantly different, compared with controls. It is also possible that the tear gland secretory cells themselves produce these different forms of alpha-synuclein that can be directly secreted into tears, the researchers said.
Total levels of alpha-synuclein were decreased in patients with Parkinson’s disease, with an average of 423 picograms of that protein per milligram (pg/mg) compared with 704 pg/mg in healthy controls. However, levels of oligomeric alpha-synuclein were increased in patients with Parkinson’s disease, with an average of 1.45 nanograms per milligram of tear protein (ng/mg), compared with 0.27 ng/mg in controls. While detectable in tears, neither CCL-2 nor DJ-1 varied between patients with Parkinson’s disease and controls.
“Knowing that something as simple as tears could help neurologists differentiate between people who have Parkinson’s disease and those who do not in a noninvasive manner is exciting,” said Dr. Lew. “And because the Parkinson’s disease process can begin years or decades before symptoms appear, a biologic marker like this could be useful in diagnosing, or even treating, the disease earlier.”
More research needs to be done in larger groups of people to investigate whether these protein changes can be detected in tears in the earliest presymptomatic stages of the disease, said the researchers.
The study was supported by the Michael J. Fox Foundation for Parkinson’s Research and the Plotkin Foundation.
LOS ANGELES—Tears may hold diagnostic clues as to whether someone has Parkinson’s disease, according to a preliminary study presented at the American Academy of Neurology’s 70th Annual Meeting. “We believe our research is the first to show that tears may be a reliable, inexpensive, and noninvasive biologic marker of Parkinson’s disease,” said study author Mark Floyd Lew, MD, Professor of Clinical Neurology, and Joseph P. Van Der Meulen, MD, Chair in Parkinson’s Disease Research in Honor of Robert J. Pasarow, and Vice Chair, in the Department of Neurology at the Keck School of Medicine of the University of Southern California in Los Angeles.
Nonmotor features of Parkinson’s disease occur years prior to motor dysfunction and represent a well-suited platform to investigate for a possible biomarker. Lacrimal glands are highly innervated by cholinergic neurons, and tear fluid secreted by lacrimal glands is greatly stimulated by cholinergic neurons. The production, packaging, and secretion of specific proteins into tears may be regulated by changes in nerve function to lacrimal glands. According to the researchers, analysis of any alteration in the secretion of proteins into tears may identify a reliable and noninvasive biomarker for Parkinson’s disease.
For the study, tear samples were collected from 55 patients with Parkinson’s disease of varying severity and 27 age- and gender-matched controls without Parkinson’s disease. In addition, tears were analyzed for the levels of four proteins—total alpha synuclein, CC chemokine ligand 2 (CCL-2), DJ-1 (Parkinson’s disease protein 7), and oligomeric alpha synuclein.
The researchers found differences in the levels of a total alpha-synuclein in the tears of patients with Parkinson’s disease, compared with those of controls. Additionally, levels of oligomeric alpha-synuclein, which is alpha-synuclein that has formed aggregates that are implicated in nerve damage in Parkinson’s disease, were also significantly different, compared with controls. It is also possible that the tear gland secretory cells themselves produce these different forms of alpha-synuclein that can be directly secreted into tears, the researchers said.
Total levels of alpha-synuclein were decreased in patients with Parkinson’s disease, with an average of 423 picograms of that protein per milligram (pg/mg) compared with 704 pg/mg in healthy controls. However, levels of oligomeric alpha-synuclein were increased in patients with Parkinson’s disease, with an average of 1.45 nanograms per milligram of tear protein (ng/mg), compared with 0.27 ng/mg in controls. While detectable in tears, neither CCL-2 nor DJ-1 varied between patients with Parkinson’s disease and controls.
“Knowing that something as simple as tears could help neurologists differentiate between people who have Parkinson’s disease and those who do not in a noninvasive manner is exciting,” said Dr. Lew. “And because the Parkinson’s disease process can begin years or decades before symptoms appear, a biologic marker like this could be useful in diagnosing, or even treating, the disease earlier.”
More research needs to be done in larger groups of people to investigate whether these protein changes can be detected in tears in the earliest presymptomatic stages of the disease, said the researchers.
The study was supported by the Michael J. Fox Foundation for Parkinson’s Research and the Plotkin Foundation.
Staging System Classifies Nearly All Patients With Lewy Body Synucleinopathy
LOS ANGELES—The Unified Staging System for Lewy Body Disorders (USSLB) enables the categorization of almost all brains with Lewy body synucleinopathy, according to research described at the 70th Annual Meeting of the American Academy of Neurology. The USSLB’s stages correlate significantly with motor and nonmotor findings. “Wider use of the USSLB would help standardize research in synucleinopathies,” said Charles H. Adler, MD, PhD, Professor of Neurology at Mayo Clinic in Scottsdale, Arizona.
Investigators have developed several neuropathologic staging systems for Lewy body disorders, but many focus on specific diseases, such as Parkinson’s disease or dementia with Lewy bodies. They thus do not allow the classification of all patients with Lewy body disorders. In addition, the literature contains few data about how well these systems’ stages correlate with clinical and pathologic findings.
The Emergence of the USSLB
Dr. Adler and colleagues proposed the USSLB in research published in Acta Neuropathologica in 2009. Their goal was to enable the classification of patients with Lewy body disorders, regardless of their specific diagnoses. The USSLB includes four stages. Stage I denotes pathology limited to the olfactory bulb. Stage IIa denotes predominantly brainstem involvement. Stage IIb refers to pathology predominantly in the limbic system, rather than the brainstem. Stage III denotes pathology in the brainstem and limbic system. Stage IV represents neocortical pathology.
To examine the correlation between patients’ motor and nonmotor findings, including cognitive measures, and the extent of Lewy-type synucleinopathy, as categorized by the USSLB, Dr. Adler and others examined data from the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND). That study includes participants in the Banner Sun Health Research Institute brain and body donation program. Participants undergo annual clinical exams that include movement testing, cognitive testing, sleep and autonomic questionnaires, and a smell test.
The investigators searched the AZSAND database for patients who presented from January 1997 through December 2015. They identified 641 autopsies. Clinical data and information on Lewy-type synucleinopathy were available for 280 of the cases. The population included cases with Lewy bodies and those with synuclein pathology within the neuropil and fibers. The population’s mean age at death was 83. Greater severity of synucleinopathy was associated with younger age at death.
Braak Staging Could Not Characterize Some Patients
The researchers classified 8.6% of cases as Stage I, 15.4% as Stage IIa, 13.6% as Stage IIb, 31.8% as Stage III, and 30.7% as Stage IV. Cognition was normal in 25.7% of the cases, 8.6% had mild cognitive impairment, and 65.7% had dementia.
Multiple measures of motor parkinsonism and cognitive impairment, as well as of hyposmia and probable REM sleep behavior disorder, correlated significantly with increasing USSLB stage. A few clinical features had no correlation with USSLB stage.
Dr. Adler and colleagues also applied the Braak staging criteria to the cases. To classify all cases, the investigators added an olfactory-bulb-only stage to the Braak criteria. Of the initial cohort, 70 cases could not be assigned a Braak stage. When the researchers removed cases with Alzheimer’s disease, 21% of cases could not be staged.
—Erik Greb
LOS ANGELES—The Unified Staging System for Lewy Body Disorders (USSLB) enables the categorization of almost all brains with Lewy body synucleinopathy, according to research described at the 70th Annual Meeting of the American Academy of Neurology. The USSLB’s stages correlate significantly with motor and nonmotor findings. “Wider use of the USSLB would help standardize research in synucleinopathies,” said Charles H. Adler, MD, PhD, Professor of Neurology at Mayo Clinic in Scottsdale, Arizona.
Investigators have developed several neuropathologic staging systems for Lewy body disorders, but many focus on specific diseases, such as Parkinson’s disease or dementia with Lewy bodies. They thus do not allow the classification of all patients with Lewy body disorders. In addition, the literature contains few data about how well these systems’ stages correlate with clinical and pathologic findings.
The Emergence of the USSLB
Dr. Adler and colleagues proposed the USSLB in research published in Acta Neuropathologica in 2009. Their goal was to enable the classification of patients with Lewy body disorders, regardless of their specific diagnoses. The USSLB includes four stages. Stage I denotes pathology limited to the olfactory bulb. Stage IIa denotes predominantly brainstem involvement. Stage IIb refers to pathology predominantly in the limbic system, rather than the brainstem. Stage III denotes pathology in the brainstem and limbic system. Stage IV represents neocortical pathology.
To examine the correlation between patients’ motor and nonmotor findings, including cognitive measures, and the extent of Lewy-type synucleinopathy, as categorized by the USSLB, Dr. Adler and others examined data from the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND). That study includes participants in the Banner Sun Health Research Institute brain and body donation program. Participants undergo annual clinical exams that include movement testing, cognitive testing, sleep and autonomic questionnaires, and a smell test.
The investigators searched the AZSAND database for patients who presented from January 1997 through December 2015. They identified 641 autopsies. Clinical data and information on Lewy-type synucleinopathy were available for 280 of the cases. The population included cases with Lewy bodies and those with synuclein pathology within the neuropil and fibers. The population’s mean age at death was 83. Greater severity of synucleinopathy was associated with younger age at death.
Braak Staging Could Not Characterize Some Patients
The researchers classified 8.6% of cases as Stage I, 15.4% as Stage IIa, 13.6% as Stage IIb, 31.8% as Stage III, and 30.7% as Stage IV. Cognition was normal in 25.7% of the cases, 8.6% had mild cognitive impairment, and 65.7% had dementia.
Multiple measures of motor parkinsonism and cognitive impairment, as well as of hyposmia and probable REM sleep behavior disorder, correlated significantly with increasing USSLB stage. A few clinical features had no correlation with USSLB stage.
Dr. Adler and colleagues also applied the Braak staging criteria to the cases. To classify all cases, the investigators added an olfactory-bulb-only stage to the Braak criteria. Of the initial cohort, 70 cases could not be assigned a Braak stage. When the researchers removed cases with Alzheimer’s disease, 21% of cases could not be staged.
—Erik Greb
LOS ANGELES—The Unified Staging System for Lewy Body Disorders (USSLB) enables the categorization of almost all brains with Lewy body synucleinopathy, according to research described at the 70th Annual Meeting of the American Academy of Neurology. The USSLB’s stages correlate significantly with motor and nonmotor findings. “Wider use of the USSLB would help standardize research in synucleinopathies,” said Charles H. Adler, MD, PhD, Professor of Neurology at Mayo Clinic in Scottsdale, Arizona.
Investigators have developed several neuropathologic staging systems for Lewy body disorders, but many focus on specific diseases, such as Parkinson’s disease or dementia with Lewy bodies. They thus do not allow the classification of all patients with Lewy body disorders. In addition, the literature contains few data about how well these systems’ stages correlate with clinical and pathologic findings.
The Emergence of the USSLB
Dr. Adler and colleagues proposed the USSLB in research published in Acta Neuropathologica in 2009. Their goal was to enable the classification of patients with Lewy body disorders, regardless of their specific diagnoses. The USSLB includes four stages. Stage I denotes pathology limited to the olfactory bulb. Stage IIa denotes predominantly brainstem involvement. Stage IIb refers to pathology predominantly in the limbic system, rather than the brainstem. Stage III denotes pathology in the brainstem and limbic system. Stage IV represents neocortical pathology.
To examine the correlation between patients’ motor and nonmotor findings, including cognitive measures, and the extent of Lewy-type synucleinopathy, as categorized by the USSLB, Dr. Adler and others examined data from the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND). That study includes participants in the Banner Sun Health Research Institute brain and body donation program. Participants undergo annual clinical exams that include movement testing, cognitive testing, sleep and autonomic questionnaires, and a smell test.
The investigators searched the AZSAND database for patients who presented from January 1997 through December 2015. They identified 641 autopsies. Clinical data and information on Lewy-type synucleinopathy were available for 280 of the cases. The population included cases with Lewy bodies and those with synuclein pathology within the neuropil and fibers. The population’s mean age at death was 83. Greater severity of synucleinopathy was associated with younger age at death.
Braak Staging Could Not Characterize Some Patients
The researchers classified 8.6% of cases as Stage I, 15.4% as Stage IIa, 13.6% as Stage IIb, 31.8% as Stage III, and 30.7% as Stage IV. Cognition was normal in 25.7% of the cases, 8.6% had mild cognitive impairment, and 65.7% had dementia.
Multiple measures of motor parkinsonism and cognitive impairment, as well as of hyposmia and probable REM sleep behavior disorder, correlated significantly with increasing USSLB stage. A few clinical features had no correlation with USSLB stage.
Dr. Adler and colleagues also applied the Braak staging criteria to the cases. To classify all cases, the investigators added an olfactory-bulb-only stage to the Braak criteria. Of the initial cohort, 70 cases could not be assigned a Braak stage. When the researchers removed cases with Alzheimer’s disease, 21% of cases could not be staged.
—Erik Greb
Valbenazine Provides Long-Term Benefits for Tardive Dyskinesia
LOS ANGELES—Once-daily treatment with valbenazine for 48 weeks provides substantial improvements on clinician- and patient-reported outcomes in adults with tardive dyskinesia, according to data described at the 70th Annual Meeting of the American Academy of Neurology. The results are consistent with those of previous trials, said the researchers. Valbenazine is well tolerated and does not raise significant safety concerns.
Valbenazine was approved as a treatment for tardive dyskinesia on the basis of several short-term placebo-controlled trials, a blinded extension study, and the long-term KINECT 4 study. Stewart Factor, DO, Professor of Neurology at Emory University School of Medicine in Atlanta, and colleagues conducted a study to evaluate the long-term effects of once-daily valbenazine on tardive dyskinesia.
Eligible participants were adults with de novo tardive dyskinesia and those who had participated in prior trials of valbenazine. All participants received 48 weeks of open-label treatment with valbenazine. The initial dose was 40 mg. If an investigator judged that a patient had inadequate clinical response at week four, the dose was increased to 80 mg, based on tolerability. For patients who could not tolerate the 80-mg dose, the dose was decreased to 40 mg.
Dr. Factor and colleagues used the change from baseline in the Abnormal Involuntary Movement Scale (AIMS) total score to assess changes in tardive dyskinesia. Other efficacy assessments included the Patient Global Impression of Change (PGIC) and Clinical Global Impression of Change-Tardive Dyskinesia (CGI-TD) scales. The investigators applied standard safety methods, including treatment-emergent adverse event reporting.
The safety population included 163 participants. Of this group, 107 participants received and tolerated the 80-mg dose, 45 did not require escalation from the 40-mg dose, and 11 were escalated to 80-mg dose, but later required reduction to the 40-mg dose. The mean change from baseline to week 48 in AIMS total score indicated improvements in tardive dyskinesia in all dose groups. The 80-mg group had a decrease of 11.0 points, the 40-mg group had a decrease of 10.2 points, and the group whose dose was decreased from 80 mg to 40 mg had a decrease of 7.2 points.
At week 48, more than 75% of participants in each study arm had a PGIC score of 2 or lower (ie, much improved or very much improved). This outcome was achieved in 89.2% of the 80-mg group, 90.0% of the 40-mg group, and 77.8% of the group whose dose was decreased from 80 mg to 40 mg. Mean CGI-TD scores at week 48 were 1.6 for the 80-mg group, 1.7 for the 40-mg group, and 2.3 for the group whose dose was reduced from 80 mg to 40 mg. These scores indicated clinically meaningful long-term improvement for all dose groups.
Less than 15% of all participants had a serious treatment-emergent adverse event (12.9%) or treatment-emergent adverse event leading to discontinuation (14.7%).
The study was funded by Neurocrine Biosciences, the manufacturer of valbenazine.
LOS ANGELES—Once-daily treatment with valbenazine for 48 weeks provides substantial improvements on clinician- and patient-reported outcomes in adults with tardive dyskinesia, according to data described at the 70th Annual Meeting of the American Academy of Neurology. The results are consistent with those of previous trials, said the researchers. Valbenazine is well tolerated and does not raise significant safety concerns.
Valbenazine was approved as a treatment for tardive dyskinesia on the basis of several short-term placebo-controlled trials, a blinded extension study, and the long-term KINECT 4 study. Stewart Factor, DO, Professor of Neurology at Emory University School of Medicine in Atlanta, and colleagues conducted a study to evaluate the long-term effects of once-daily valbenazine on tardive dyskinesia.
Eligible participants were adults with de novo tardive dyskinesia and those who had participated in prior trials of valbenazine. All participants received 48 weeks of open-label treatment with valbenazine. The initial dose was 40 mg. If an investigator judged that a patient had inadequate clinical response at week four, the dose was increased to 80 mg, based on tolerability. For patients who could not tolerate the 80-mg dose, the dose was decreased to 40 mg.
Dr. Factor and colleagues used the change from baseline in the Abnormal Involuntary Movement Scale (AIMS) total score to assess changes in tardive dyskinesia. Other efficacy assessments included the Patient Global Impression of Change (PGIC) and Clinical Global Impression of Change-Tardive Dyskinesia (CGI-TD) scales. The investigators applied standard safety methods, including treatment-emergent adverse event reporting.
The safety population included 163 participants. Of this group, 107 participants received and tolerated the 80-mg dose, 45 did not require escalation from the 40-mg dose, and 11 were escalated to 80-mg dose, but later required reduction to the 40-mg dose. The mean change from baseline to week 48 in AIMS total score indicated improvements in tardive dyskinesia in all dose groups. The 80-mg group had a decrease of 11.0 points, the 40-mg group had a decrease of 10.2 points, and the group whose dose was decreased from 80 mg to 40 mg had a decrease of 7.2 points.
At week 48, more than 75% of participants in each study arm had a PGIC score of 2 or lower (ie, much improved or very much improved). This outcome was achieved in 89.2% of the 80-mg group, 90.0% of the 40-mg group, and 77.8% of the group whose dose was decreased from 80 mg to 40 mg. Mean CGI-TD scores at week 48 were 1.6 for the 80-mg group, 1.7 for the 40-mg group, and 2.3 for the group whose dose was reduced from 80 mg to 40 mg. These scores indicated clinically meaningful long-term improvement for all dose groups.
Less than 15% of all participants had a serious treatment-emergent adverse event (12.9%) or treatment-emergent adverse event leading to discontinuation (14.7%).
The study was funded by Neurocrine Biosciences, the manufacturer of valbenazine.
LOS ANGELES—Once-daily treatment with valbenazine for 48 weeks provides substantial improvements on clinician- and patient-reported outcomes in adults with tardive dyskinesia, according to data described at the 70th Annual Meeting of the American Academy of Neurology. The results are consistent with those of previous trials, said the researchers. Valbenazine is well tolerated and does not raise significant safety concerns.
Valbenazine was approved as a treatment for tardive dyskinesia on the basis of several short-term placebo-controlled trials, a blinded extension study, and the long-term KINECT 4 study. Stewart Factor, DO, Professor of Neurology at Emory University School of Medicine in Atlanta, and colleagues conducted a study to evaluate the long-term effects of once-daily valbenazine on tardive dyskinesia.
Eligible participants were adults with de novo tardive dyskinesia and those who had participated in prior trials of valbenazine. All participants received 48 weeks of open-label treatment with valbenazine. The initial dose was 40 mg. If an investigator judged that a patient had inadequate clinical response at week four, the dose was increased to 80 mg, based on tolerability. For patients who could not tolerate the 80-mg dose, the dose was decreased to 40 mg.
Dr. Factor and colleagues used the change from baseline in the Abnormal Involuntary Movement Scale (AIMS) total score to assess changes in tardive dyskinesia. Other efficacy assessments included the Patient Global Impression of Change (PGIC) and Clinical Global Impression of Change-Tardive Dyskinesia (CGI-TD) scales. The investigators applied standard safety methods, including treatment-emergent adverse event reporting.
The safety population included 163 participants. Of this group, 107 participants received and tolerated the 80-mg dose, 45 did not require escalation from the 40-mg dose, and 11 were escalated to 80-mg dose, but later required reduction to the 40-mg dose. The mean change from baseline to week 48 in AIMS total score indicated improvements in tardive dyskinesia in all dose groups. The 80-mg group had a decrease of 11.0 points, the 40-mg group had a decrease of 10.2 points, and the group whose dose was decreased from 80 mg to 40 mg had a decrease of 7.2 points.
At week 48, more than 75% of participants in each study arm had a PGIC score of 2 or lower (ie, much improved or very much improved). This outcome was achieved in 89.2% of the 80-mg group, 90.0% of the 40-mg group, and 77.8% of the group whose dose was decreased from 80 mg to 40 mg. Mean CGI-TD scores at week 48 were 1.6 for the 80-mg group, 1.7 for the 40-mg group, and 2.3 for the group whose dose was reduced from 80 mg to 40 mg. These scores indicated clinically meaningful long-term improvement for all dose groups.
Less than 15% of all participants had a serious treatment-emergent adverse event (12.9%) or treatment-emergent adverse event leading to discontinuation (14.7%).
The study was funded by Neurocrine Biosciences, the manufacturer of valbenazine.
















