User login
MM patients with t(11;14) benefit from venetoclax

SAN DIEGO—Venetoclax, the oral BCL-2 inhibitor approved by the US Food and Drug Administration to treat chronic lymphocytic leukemia (CLL) patients with 17p deletion, is also showing activity in multiple myeloma (MM) patients, particularly those with t(11;14).
Final results of a phase 1 study showed venetoclax to be safe as monotherapy in relapsed or refractory MM, producing a response rate of 40% in patients with the translocation and 21% overall.
Preliminary results of the study were presented at the 2015 ASH Annual Meeting, and final results were presented at the 2016 ASH Annual Meeting.
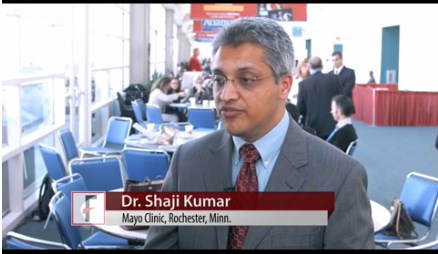
“So I think we have a drug that potentially can change the outcome of a lot of patients with myeloma,” Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, said during the presentation of the findings at ASH (abstract 488*).
“[It] also opens the possibility of being combined with a variety of other therapeutics that we have in this disease today.”
Venetoclax induces cell death in MM cell lines, particularly those positive for t(11;14). The translocation correlates with higher ratios of BCL-2 to MCL-1 and BCL-2 to MCL-2L1 (BCL-XL) mRNA. BCL-2 and MCL-1 promote survival of MM cells.
Study design and enrollment
The phase 1, open-label, multicenter study was designed to determine the best tolerated dose of venetoclax.
Secondary and exploratory objectives included overall response rate (ORR), time to progression, duration of response, and predictive biomarkers.
Patients had to have previously treated MM with measurable disease, ECOG status of 0 or 1, and adequate organ function.
They were excluded if they had an active infection, a history of significant renal, neurologic, psychiatric, endocrine, immunologic, cardiovascular, or hepatic disease within 6 months of study entry, or a history of other active malignancies within 3 years of study entry.
The study called for a 2-week lead-in period of venetoclax with weekly dose escalation. Four different dose cohorts were evaluated—300 mg, 600 mg, 900 mg, and 1200 mg.
Thirty patients were enrolled during the lead-in period, and 36 additional patients enrolled at the maximum evaluated dose of 1200 mg in the safety expansion cohort, for a total of 66 patients.
Patients were treated on a 21-day cycle with daily venetoclax. They could also receive dexamethasone to continue on the study if they progressed while receiving the monotherapy.
Patient characteristics
Patient characteristics were “similar to what you would see in relapsed/refractory multiple myloma,” Dr Kumar said.
Median age was 63 (range, 31–79), and most (62%) were ISS stage II/III.
“I want to draw your attention to two features here,” Dr Kumar said.
“Thirty patients, or 46% of the patients, had 11;14 translocation, and that reflects the interest in this drug for this particular class of patients.”
Twelve patients (18%) had 17p deletion, 32 (48%) had 13q deletion, and 27 (41%) were hyperdiploid.
“What is most striking in this cohort of patients,” Dr Kumar added, “is the fact that the median number of prior lines of therapy was 5, with some as high as 15 prior lines of therapy.”
Seventy percent were refractory to bortezomib, 77% refractory to lenalidomide, and 61% refractory to both. Fifty-two patients (79%) were refractory to their last prior therapy.
Patient disposition
At the time of data cutoff on August 19, 2016, 11 patients (17%) were still active on the study.
The median time on study was 3.3 months (range, 0.2–27), median time on venetoclax monotherapy was 2.5 months (range, 0.2–25), and median time on venetoclax plus dexamethasone was 1.4 months (range, 1–13). Seventeen patients received the combination after disease progression.
Fifty-five patients (83%) discontinued treatment, 41 (62%) because of disease progression, 5 (8%) because of adverse events, 2 (3%) withdrew consent, 1 (2%) was lost to follow-up, and 6 (9%) for unspecified reasons.
The 5 adverse events leading to withdrawal included renal failure (n=2), worsening pulmonary disorder (n=1), paralyzing sciatica (n=1), and shortness of breath and pain (n=1).
“Eight patients died on study,” Dr Kumar said, “none thought to be related to the drug.”
Adverse events
The toxicity profile was primarily hematologic and gastrointestinal.
All patients experienced an adverse event of any grade, and 45 (68%) had a grade 3 or 4 event.
“I wanted to highlight that the majority of the gastrointestinal and non-hematologic toxicity we saw were grades 1 and 2,” Dr Kumar pointed out, “and could be managed symptomatically or with dose modifications.”
Grade 3-4 hematologic adverse events included thrombocytopenia (26%), neutropenia (21%), anemia (14%), leukopenia (14%), and lymphopenia (15%).
Grade 3-4 non-hematologic adverse events included nausea (3%), diarrhea (3%), fatigue (5%), back pain (8%), and vomiting (3%).
Serious adverse events occurring in 2% or more of patients included pneumonia (8%), sepsis (5%), pain, pyrexia, cough, and hypotension (3% each).
Two patients had dose-limiting toxicities of abdominal pain and nausea at the 600 mg dose.
No events of tumor lysis syndrome (TLS) were reported. Dr Kumar explained that this may have been the case because patients thought to be at high risk for TLS were mandated to be in the hospital and observed for early tumor lysis in the initial part of the study.
Response
The ORR was 21% in all patients, including a stringent complete response (sCR) of 3% and a CR of 4%.
“But what was really striking was the response rate that we observed in the 30 patients with translocation 11;14,” Dr Kumar said. “The overall response rate was 40%, with 14% of the patients having complete response or better [stringent CR] and 13% of the patients with very good partial response.”
The 36 patients without t(11;14) had a 6% ORR, 3% sCR, and 3% very good partial response.
“If you look at the response rates based on the type of therapy they were coming off or the drugs they were refractory to, the response rate is very similar across all these patient subgroups, irrespective of what groups of drugs they were refractory to,” he added.
Time to progression for all patients was about 2.5 months. For patients with the translocation, it was about 6.6 months.
“Responses were fairly durable among those who had a response,” Dr Kumar said, “considering these are patients with a median of 5 prior lines of therapy.”
Duration of response for patients with t(11;14) was close to 10 months.
Biomarker analysis
The underlying biology for the response was the BCL-2 to BCL-2L1 ratio, as the investigators had observed in the cell lines.
So they analyzed the BCL-2 gene expression ratio in 24 of the 30 patients with t(11;14).
The investigators used droplet digital PCR performed on CD138-selected bone marrow mononuclear cells collected at baseline.
Nine patients had a high ratio, and their ORR was 88%. Fifteen patients had a low ratio, and their ORR was 20%.
Median time to progression for patients with a high ratio was about 12 months. For those with a low ratio, it was about 9 months.
Median change in M protein for patients with t(11;14) was –53%, compared to +11% in the patients without the translocation.
The investigators recommend additional studies with venetoclax in MM, including those with alternative combination therapies.
Venetoclax is being developed by AbbVie, in partnership with Genentech and Roche. This study was sponsored by AbbVie. ![]()
*Data in the abstract differ from the presentation.

SAN DIEGO—Venetoclax, the oral BCL-2 inhibitor approved by the US Food and Drug Administration to treat chronic lymphocytic leukemia (CLL) patients with 17p deletion, is also showing activity in multiple myeloma (MM) patients, particularly those with t(11;14).
Final results of a phase 1 study showed venetoclax to be safe as monotherapy in relapsed or refractory MM, producing a response rate of 40% in patients with the translocation and 21% overall.
Preliminary results of the study were presented at the 2015 ASH Annual Meeting, and final results were presented at the 2016 ASH Annual Meeting.
“So I think we have a drug that potentially can change the outcome of a lot of patients with myeloma,” Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, said during the presentation of the findings at ASH (abstract 488*).
“[It] also opens the possibility of being combined with a variety of other therapeutics that we have in this disease today.”
Venetoclax induces cell death in MM cell lines, particularly those positive for t(11;14). The translocation correlates with higher ratios of BCL-2 to MCL-1 and BCL-2 to MCL-2L1 (BCL-XL) mRNA. BCL-2 and MCL-1 promote survival of MM cells.
Study design and enrollment
The phase 1, open-label, multicenter study was designed to determine the best tolerated dose of venetoclax.
Secondary and exploratory objectives included overall response rate (ORR), time to progression, duration of response, and predictive biomarkers.
Patients had to have previously treated MM with measurable disease, ECOG status of 0 or 1, and adequate organ function.
They were excluded if they had an active infection, a history of significant renal, neurologic, psychiatric, endocrine, immunologic, cardiovascular, or hepatic disease within 6 months of study entry, or a history of other active malignancies within 3 years of study entry.
The study called for a 2-week lead-in period of venetoclax with weekly dose escalation. Four different dose cohorts were evaluated—300 mg, 600 mg, 900 mg, and 1200 mg.
Thirty patients were enrolled during the lead-in period, and 36 additional patients enrolled at the maximum evaluated dose of 1200 mg in the safety expansion cohort, for a total of 66 patients.
Patients were treated on a 21-day cycle with daily venetoclax. They could also receive dexamethasone to continue on the study if they progressed while receiving the monotherapy.
Patient characteristics
Patient characteristics were “similar to what you would see in relapsed/refractory multiple myloma,” Dr Kumar said.
Median age was 63 (range, 31–79), and most (62%) were ISS stage II/III.
“I want to draw your attention to two features here,” Dr Kumar said.
“Thirty patients, or 46% of the patients, had 11;14 translocation, and that reflects the interest in this drug for this particular class of patients.”
Twelve patients (18%) had 17p deletion, 32 (48%) had 13q deletion, and 27 (41%) were hyperdiploid.
“What is most striking in this cohort of patients,” Dr Kumar added, “is the fact that the median number of prior lines of therapy was 5, with some as high as 15 prior lines of therapy.”
Seventy percent were refractory to bortezomib, 77% refractory to lenalidomide, and 61% refractory to both. Fifty-two patients (79%) were refractory to their last prior therapy.
Patient disposition
At the time of data cutoff on August 19, 2016, 11 patients (17%) were still active on the study.
The median time on study was 3.3 months (range, 0.2–27), median time on venetoclax monotherapy was 2.5 months (range, 0.2–25), and median time on venetoclax plus dexamethasone was 1.4 months (range, 1–13). Seventeen patients received the combination after disease progression.
Fifty-five patients (83%) discontinued treatment, 41 (62%) because of disease progression, 5 (8%) because of adverse events, 2 (3%) withdrew consent, 1 (2%) was lost to follow-up, and 6 (9%) for unspecified reasons.
The 5 adverse events leading to withdrawal included renal failure (n=2), worsening pulmonary disorder (n=1), paralyzing sciatica (n=1), and shortness of breath and pain (n=1).
“Eight patients died on study,” Dr Kumar said, “none thought to be related to the drug.”
Adverse events
The toxicity profile was primarily hematologic and gastrointestinal.
All patients experienced an adverse event of any grade, and 45 (68%) had a grade 3 or 4 event.
“I wanted to highlight that the majority of the gastrointestinal and non-hematologic toxicity we saw were grades 1 and 2,” Dr Kumar pointed out, “and could be managed symptomatically or with dose modifications.”
Grade 3-4 hematologic adverse events included thrombocytopenia (26%), neutropenia (21%), anemia (14%), leukopenia (14%), and lymphopenia (15%).
Grade 3-4 non-hematologic adverse events included nausea (3%), diarrhea (3%), fatigue (5%), back pain (8%), and vomiting (3%).
Serious adverse events occurring in 2% or more of patients included pneumonia (8%), sepsis (5%), pain, pyrexia, cough, and hypotension (3% each).
Two patients had dose-limiting toxicities of abdominal pain and nausea at the 600 mg dose.
No events of tumor lysis syndrome (TLS) were reported. Dr Kumar explained that this may have been the case because patients thought to be at high risk for TLS were mandated to be in the hospital and observed for early tumor lysis in the initial part of the study.
Response
The ORR was 21% in all patients, including a stringent complete response (sCR) of 3% and a CR of 4%.
“But what was really striking was the response rate that we observed in the 30 patients with translocation 11;14,” Dr Kumar said. “The overall response rate was 40%, with 14% of the patients having complete response or better [stringent CR] and 13% of the patients with very good partial response.”
The 36 patients without t(11;14) had a 6% ORR, 3% sCR, and 3% very good partial response.
“If you look at the response rates based on the type of therapy they were coming off or the drugs they were refractory to, the response rate is very similar across all these patient subgroups, irrespective of what groups of drugs they were refractory to,” he added.
Time to progression for all patients was about 2.5 months. For patients with the translocation, it was about 6.6 months.
“Responses were fairly durable among those who had a response,” Dr Kumar said, “considering these are patients with a median of 5 prior lines of therapy.”
Duration of response for patients with t(11;14) was close to 10 months.
Biomarker analysis
The underlying biology for the response was the BCL-2 to BCL-2L1 ratio, as the investigators had observed in the cell lines.
So they analyzed the BCL-2 gene expression ratio in 24 of the 30 patients with t(11;14).
The investigators used droplet digital PCR performed on CD138-selected bone marrow mononuclear cells collected at baseline.
Nine patients had a high ratio, and their ORR was 88%. Fifteen patients had a low ratio, and their ORR was 20%.
Median time to progression for patients with a high ratio was about 12 months. For those with a low ratio, it was about 9 months.
Median change in M protein for patients with t(11;14) was –53%, compared to +11% in the patients without the translocation.
The investigators recommend additional studies with venetoclax in MM, including those with alternative combination therapies.
Venetoclax is being developed by AbbVie, in partnership with Genentech and Roche. This study was sponsored by AbbVie. ![]()
*Data in the abstract differ from the presentation.

SAN DIEGO—Venetoclax, the oral BCL-2 inhibitor approved by the US Food and Drug Administration to treat chronic lymphocytic leukemia (CLL) patients with 17p deletion, is also showing activity in multiple myeloma (MM) patients, particularly those with t(11;14).
Final results of a phase 1 study showed venetoclax to be safe as monotherapy in relapsed or refractory MM, producing a response rate of 40% in patients with the translocation and 21% overall.
Preliminary results of the study were presented at the 2015 ASH Annual Meeting, and final results were presented at the 2016 ASH Annual Meeting.
“So I think we have a drug that potentially can change the outcome of a lot of patients with myeloma,” Shaji Kumar, MD, of the Mayo Clinic in Rochester, Minnesota, said during the presentation of the findings at ASH (abstract 488*).
“[It] also opens the possibility of being combined with a variety of other therapeutics that we have in this disease today.”
Venetoclax induces cell death in MM cell lines, particularly those positive for t(11;14). The translocation correlates with higher ratios of BCL-2 to MCL-1 and BCL-2 to MCL-2L1 (BCL-XL) mRNA. BCL-2 and MCL-1 promote survival of MM cells.
Study design and enrollment
The phase 1, open-label, multicenter study was designed to determine the best tolerated dose of venetoclax.
Secondary and exploratory objectives included overall response rate (ORR), time to progression, duration of response, and predictive biomarkers.
Patients had to have previously treated MM with measurable disease, ECOG status of 0 or 1, and adequate organ function.
They were excluded if they had an active infection, a history of significant renal, neurologic, psychiatric, endocrine, immunologic, cardiovascular, or hepatic disease within 6 months of study entry, or a history of other active malignancies within 3 years of study entry.
The study called for a 2-week lead-in period of venetoclax with weekly dose escalation. Four different dose cohorts were evaluated—300 mg, 600 mg, 900 mg, and 1200 mg.
Thirty patients were enrolled during the lead-in period, and 36 additional patients enrolled at the maximum evaluated dose of 1200 mg in the safety expansion cohort, for a total of 66 patients.
Patients were treated on a 21-day cycle with daily venetoclax. They could also receive dexamethasone to continue on the study if they progressed while receiving the monotherapy.
Patient characteristics
Patient characteristics were “similar to what you would see in relapsed/refractory multiple myloma,” Dr Kumar said.
Median age was 63 (range, 31–79), and most (62%) were ISS stage II/III.
“I want to draw your attention to two features here,” Dr Kumar said.
“Thirty patients, or 46% of the patients, had 11;14 translocation, and that reflects the interest in this drug for this particular class of patients.”
Twelve patients (18%) had 17p deletion, 32 (48%) had 13q deletion, and 27 (41%) were hyperdiploid.
“What is most striking in this cohort of patients,” Dr Kumar added, “is the fact that the median number of prior lines of therapy was 5, with some as high as 15 prior lines of therapy.”
Seventy percent were refractory to bortezomib, 77% refractory to lenalidomide, and 61% refractory to both. Fifty-two patients (79%) were refractory to their last prior therapy.
Patient disposition
At the time of data cutoff on August 19, 2016, 11 patients (17%) were still active on the study.
The median time on study was 3.3 months (range, 0.2–27), median time on venetoclax monotherapy was 2.5 months (range, 0.2–25), and median time on venetoclax plus dexamethasone was 1.4 months (range, 1–13). Seventeen patients received the combination after disease progression.
Fifty-five patients (83%) discontinued treatment, 41 (62%) because of disease progression, 5 (8%) because of adverse events, 2 (3%) withdrew consent, 1 (2%) was lost to follow-up, and 6 (9%) for unspecified reasons.
The 5 adverse events leading to withdrawal included renal failure (n=2), worsening pulmonary disorder (n=1), paralyzing sciatica (n=1), and shortness of breath and pain (n=1).
“Eight patients died on study,” Dr Kumar said, “none thought to be related to the drug.”
Adverse events
The toxicity profile was primarily hematologic and gastrointestinal.
All patients experienced an adverse event of any grade, and 45 (68%) had a grade 3 or 4 event.
“I wanted to highlight that the majority of the gastrointestinal and non-hematologic toxicity we saw were grades 1 and 2,” Dr Kumar pointed out, “and could be managed symptomatically or with dose modifications.”
Grade 3-4 hematologic adverse events included thrombocytopenia (26%), neutropenia (21%), anemia (14%), leukopenia (14%), and lymphopenia (15%).
Grade 3-4 non-hematologic adverse events included nausea (3%), diarrhea (3%), fatigue (5%), back pain (8%), and vomiting (3%).
Serious adverse events occurring in 2% or more of patients included pneumonia (8%), sepsis (5%), pain, pyrexia, cough, and hypotension (3% each).
Two patients had dose-limiting toxicities of abdominal pain and nausea at the 600 mg dose.
No events of tumor lysis syndrome (TLS) were reported. Dr Kumar explained that this may have been the case because patients thought to be at high risk for TLS were mandated to be in the hospital and observed for early tumor lysis in the initial part of the study.
Response
The ORR was 21% in all patients, including a stringent complete response (sCR) of 3% and a CR of 4%.
“But what was really striking was the response rate that we observed in the 30 patients with translocation 11;14,” Dr Kumar said. “The overall response rate was 40%, with 14% of the patients having complete response or better [stringent CR] and 13% of the patients with very good partial response.”
The 36 patients without t(11;14) had a 6% ORR, 3% sCR, and 3% very good partial response.
“If you look at the response rates based on the type of therapy they were coming off or the drugs they were refractory to, the response rate is very similar across all these patient subgroups, irrespective of what groups of drugs they were refractory to,” he added.
Time to progression for all patients was about 2.5 months. For patients with the translocation, it was about 6.6 months.
“Responses were fairly durable among those who had a response,” Dr Kumar said, “considering these are patients with a median of 5 prior lines of therapy.”
Duration of response for patients with t(11;14) was close to 10 months.
Biomarker analysis
The underlying biology for the response was the BCL-2 to BCL-2L1 ratio, as the investigators had observed in the cell lines.
So they analyzed the BCL-2 gene expression ratio in 24 of the 30 patients with t(11;14).
The investigators used droplet digital PCR performed on CD138-selected bone marrow mononuclear cells collected at baseline.
Nine patients had a high ratio, and their ORR was 88%. Fifteen patients had a low ratio, and their ORR was 20%.
Median time to progression for patients with a high ratio was about 12 months. For those with a low ratio, it was about 9 months.
Median change in M protein for patients with t(11;14) was –53%, compared to +11% in the patients without the translocation.
The investigators recommend additional studies with venetoclax in MM, including those with alternative combination therapies.
Venetoclax is being developed by AbbVie, in partnership with Genentech and Roche. This study was sponsored by AbbVie. ![]()
*Data in the abstract differ from the presentation.
‘Unprecedented’ MRD negativity with daratumumab in MM

© Todd Buchanan 2016
SAN DIEGO—Daratumumab added to standard of care regimens drives deep clinical responses beyond complete response (CR), a magnitude that is “unprecedented” in the relapsed/refractory multiple myeloma (MM) setting, according to a speaker at the 2016 ASH Annual Meeting.
Investigators added daratumumab to lenalidomide/dexamethasone in the POLLUX trial and to bortezomib/dexamethasone in the CASTOR trial.
In both phase 3 trials, the addition of daratumumab resulted in significant improvements in progression-free survival (PFS), overall response rate, and minimal residual disease (MRD) negativity when compared to control groups.
“The magnitude of daratumumab-induced MRD negativity in the relapsed setting is unprecedented and, for me, was not expected,” said Hervé Avet-Loiseau, MD, of Centre Hospitalier Universitaire Rangueil, Unité de Genomique du Myelome in Toulouse, France.
Dr Avet-Loiseau presented the MRD findings from CASTOR and POLLUX at the ASH Annual Meeting as abstract 246.*
He noted that, based on these studies, daratumumab received US Food and Drug Administration approvals for use in combination with standard of care regimens for MM patients who had received 1 or more prior lines of treatment.
Daratumumab had been previously approved as monotherapy for relapsed or refractory MM.
Study designs and findings from the POLLUX and CASTOR trials have been described earlier in Hematology Times.
Dr Avet-Loiseau provided updated PFS figures for the 2 studies.
At 18 months’ follow-up in the POLLUX study, the PFS rate for patients treated with daratumumab/lenalidomide/dexamethasone was 76%, compared to 49% in the lenalidomide/dexamethasone arm (P<0.0001).
At 12 months’ follow-up in the CASTOR study, the PFS with daratumumab was 60%, compared to 22% for bortezomib/dexamethasone (P<0.0001).
MRD criteria
In both studies, MRD assessments were conducted at suspected complete response (CR). Assessments were also conducted at 3 months and 6 months after CR in the POLLUX study and at 6 months and 12 months after the first study dose in the CASTOR study.
For the assessment of MRD, investigators used bone marrow aspirate samples and the ClonoSEQTM NGS-based assay.
Investigators evaluated MRD at 3 sensitivity thresholds: 10-4, 10-5, and 10-6.
And they used a stringent, unbiased evaluation, Dr Avet-Loiseau said. Any patient in the intent-to-treat population who was not assessed to be MRD negative was scored as MRD positive.
And the minimum cell input equivalent to the sensitivity threshold was required to determine MRD negativity.
MRD results
In the POLLUX study, 24.8% of patients achieved MRD negativity at the 10-5 cutoff, and 11.9% achieved MRD negativity at the 10-6 cutoff with the daratumumab combination.
This compared to 5.7% and 2.5% MRD negativity at the 10-5 and 10-6 cutoffs, respectively, without daratumumab (P<0.0001).
In the CASTOR study, the daratumumab-treated patients achieved 10.4% and 4.4% MRD negativity at the 10-5 and 10-6 cutoffs, respectively.
This compared to 2.4% and 0.8% MRD negativity in the control arm at the 10-5 and 10-6 cutoffs (P<0.005 and P<0.05), respectively.
“So, definitely, the addition of daratumumab improved the MRD negativity rate in both studies,” Dr Avet-Loiseau said.
“If you just look at the patients who did achieve CR in the POLLUX study, almost 50% of the patients [treated with daratumumab] achieved CR, and half of them were MRD negative at the cutoff of 10-5.”
In the CASTOR study, 25% of the patients treated with daratumumab achieved a CR. The MRD negativity rate was one-third in these patients.
“So again, we have consistently higher MRD negative rates in patients who achieve CR when they were treated in the daratumumab arms,” Dr Avet-Loiseau said.
“What is interesting, I think, is that the achievement of molecular CR was very rapid. [A]t 3 months, some patients did already achieve MRD negativity, and so we continued to see an improvement. [W]e still continue to see some achievement of MRD negativity.”
Investigators continue to follow the patients annually.
The investigators also analyzed MRD at 10-5 by cytogenetic risk and did not observe any MRD negativity in the control arm in either the POLLUX or CASTOR study.
“In contrast, we did observe some significant MRD negativity in the experimental arm with daratumumab—18% (POLLUX) and 14% (CASTOR) in high-risk patients,” Dr Avet-Loiseau said. “The most important prognostic factor is to achieve MRD negativity.”
However, even for patients who did not achieve MRD negativity, the PFS was much better in the experimental arms than in the control arms, he added.
This study, presented as a “Best of ASH” abstract, was funded by Janssen Research & Development, LLC. ![]()
*Information in the abstract differs from that presented at the meeting.

© Todd Buchanan 2016
SAN DIEGO—Daratumumab added to standard of care regimens drives deep clinical responses beyond complete response (CR), a magnitude that is “unprecedented” in the relapsed/refractory multiple myeloma (MM) setting, according to a speaker at the 2016 ASH Annual Meeting.
Investigators added daratumumab to lenalidomide/dexamethasone in the POLLUX trial and to bortezomib/dexamethasone in the CASTOR trial.
In both phase 3 trials, the addition of daratumumab resulted in significant improvements in progression-free survival (PFS), overall response rate, and minimal residual disease (MRD) negativity when compared to control groups.
“The magnitude of daratumumab-induced MRD negativity in the relapsed setting is unprecedented and, for me, was not expected,” said Hervé Avet-Loiseau, MD, of Centre Hospitalier Universitaire Rangueil, Unité de Genomique du Myelome in Toulouse, France.
Dr Avet-Loiseau presented the MRD findings from CASTOR and POLLUX at the ASH Annual Meeting as abstract 246.*
He noted that, based on these studies, daratumumab received US Food and Drug Administration approvals for use in combination with standard of care regimens for MM patients who had received 1 or more prior lines of treatment.
Daratumumab had been previously approved as monotherapy for relapsed or refractory MM.
Study designs and findings from the POLLUX and CASTOR trials have been described earlier in Hematology Times.
Dr Avet-Loiseau provided updated PFS figures for the 2 studies.
At 18 months’ follow-up in the POLLUX study, the PFS rate for patients treated with daratumumab/lenalidomide/dexamethasone was 76%, compared to 49% in the lenalidomide/dexamethasone arm (P<0.0001).
At 12 months’ follow-up in the CASTOR study, the PFS with daratumumab was 60%, compared to 22% for bortezomib/dexamethasone (P<0.0001).
MRD criteria
In both studies, MRD assessments were conducted at suspected complete response (CR). Assessments were also conducted at 3 months and 6 months after CR in the POLLUX study and at 6 months and 12 months after the first study dose in the CASTOR study.
For the assessment of MRD, investigators used bone marrow aspirate samples and the ClonoSEQTM NGS-based assay.
Investigators evaluated MRD at 3 sensitivity thresholds: 10-4, 10-5, and 10-6.
And they used a stringent, unbiased evaluation, Dr Avet-Loiseau said. Any patient in the intent-to-treat population who was not assessed to be MRD negative was scored as MRD positive.
And the minimum cell input equivalent to the sensitivity threshold was required to determine MRD negativity.
MRD results
In the POLLUX study, 24.8% of patients achieved MRD negativity at the 10-5 cutoff, and 11.9% achieved MRD negativity at the 10-6 cutoff with the daratumumab combination.
This compared to 5.7% and 2.5% MRD negativity at the 10-5 and 10-6 cutoffs, respectively, without daratumumab (P<0.0001).
In the CASTOR study, the daratumumab-treated patients achieved 10.4% and 4.4% MRD negativity at the 10-5 and 10-6 cutoffs, respectively.
This compared to 2.4% and 0.8% MRD negativity in the control arm at the 10-5 and 10-6 cutoffs (P<0.005 and P<0.05), respectively.
“So, definitely, the addition of daratumumab improved the MRD negativity rate in both studies,” Dr Avet-Loiseau said.
“If you just look at the patients who did achieve CR in the POLLUX study, almost 50% of the patients [treated with daratumumab] achieved CR, and half of them were MRD negative at the cutoff of 10-5.”
In the CASTOR study, 25% of the patients treated with daratumumab achieved a CR. The MRD negativity rate was one-third in these patients.
“So again, we have consistently higher MRD negative rates in patients who achieve CR when they were treated in the daratumumab arms,” Dr Avet-Loiseau said.
“What is interesting, I think, is that the achievement of molecular CR was very rapid. [A]t 3 months, some patients did already achieve MRD negativity, and so we continued to see an improvement. [W]e still continue to see some achievement of MRD negativity.”
Investigators continue to follow the patients annually.
The investigators also analyzed MRD at 10-5 by cytogenetic risk and did not observe any MRD negativity in the control arm in either the POLLUX or CASTOR study.
“In contrast, we did observe some significant MRD negativity in the experimental arm with daratumumab—18% (POLLUX) and 14% (CASTOR) in high-risk patients,” Dr Avet-Loiseau said. “The most important prognostic factor is to achieve MRD negativity.”
However, even for patients who did not achieve MRD negativity, the PFS was much better in the experimental arms than in the control arms, he added.
This study, presented as a “Best of ASH” abstract, was funded by Janssen Research & Development, LLC. ![]()
*Information in the abstract differs from that presented at the meeting.

© Todd Buchanan 2016
SAN DIEGO—Daratumumab added to standard of care regimens drives deep clinical responses beyond complete response (CR), a magnitude that is “unprecedented” in the relapsed/refractory multiple myeloma (MM) setting, according to a speaker at the 2016 ASH Annual Meeting.
Investigators added daratumumab to lenalidomide/dexamethasone in the POLLUX trial and to bortezomib/dexamethasone in the CASTOR trial.
In both phase 3 trials, the addition of daratumumab resulted in significant improvements in progression-free survival (PFS), overall response rate, and minimal residual disease (MRD) negativity when compared to control groups.
“The magnitude of daratumumab-induced MRD negativity in the relapsed setting is unprecedented and, for me, was not expected,” said Hervé Avet-Loiseau, MD, of Centre Hospitalier Universitaire Rangueil, Unité de Genomique du Myelome in Toulouse, France.
Dr Avet-Loiseau presented the MRD findings from CASTOR and POLLUX at the ASH Annual Meeting as abstract 246.*
He noted that, based on these studies, daratumumab received US Food and Drug Administration approvals for use in combination with standard of care regimens for MM patients who had received 1 or more prior lines of treatment.
Daratumumab had been previously approved as monotherapy for relapsed or refractory MM.
Study designs and findings from the POLLUX and CASTOR trials have been described earlier in Hematology Times.
Dr Avet-Loiseau provided updated PFS figures for the 2 studies.
At 18 months’ follow-up in the POLLUX study, the PFS rate for patients treated with daratumumab/lenalidomide/dexamethasone was 76%, compared to 49% in the lenalidomide/dexamethasone arm (P<0.0001).
At 12 months’ follow-up in the CASTOR study, the PFS with daratumumab was 60%, compared to 22% for bortezomib/dexamethasone (P<0.0001).
MRD criteria
In both studies, MRD assessments were conducted at suspected complete response (CR). Assessments were also conducted at 3 months and 6 months after CR in the POLLUX study and at 6 months and 12 months after the first study dose in the CASTOR study.
For the assessment of MRD, investigators used bone marrow aspirate samples and the ClonoSEQTM NGS-based assay.
Investigators evaluated MRD at 3 sensitivity thresholds: 10-4, 10-5, and 10-6.
And they used a stringent, unbiased evaluation, Dr Avet-Loiseau said. Any patient in the intent-to-treat population who was not assessed to be MRD negative was scored as MRD positive.
And the minimum cell input equivalent to the sensitivity threshold was required to determine MRD negativity.
MRD results
In the POLLUX study, 24.8% of patients achieved MRD negativity at the 10-5 cutoff, and 11.9% achieved MRD negativity at the 10-6 cutoff with the daratumumab combination.
This compared to 5.7% and 2.5% MRD negativity at the 10-5 and 10-6 cutoffs, respectively, without daratumumab (P<0.0001).
In the CASTOR study, the daratumumab-treated patients achieved 10.4% and 4.4% MRD negativity at the 10-5 and 10-6 cutoffs, respectively.
This compared to 2.4% and 0.8% MRD negativity in the control arm at the 10-5 and 10-6 cutoffs (P<0.005 and P<0.05), respectively.
“So, definitely, the addition of daratumumab improved the MRD negativity rate in both studies,” Dr Avet-Loiseau said.
“If you just look at the patients who did achieve CR in the POLLUX study, almost 50% of the patients [treated with daratumumab] achieved CR, and half of them were MRD negative at the cutoff of 10-5.”
In the CASTOR study, 25% of the patients treated with daratumumab achieved a CR. The MRD negativity rate was one-third in these patients.
“So again, we have consistently higher MRD negative rates in patients who achieve CR when they were treated in the daratumumab arms,” Dr Avet-Loiseau said.
“What is interesting, I think, is that the achievement of molecular CR was very rapid. [A]t 3 months, some patients did already achieve MRD negativity, and so we continued to see an improvement. [W]e still continue to see some achievement of MRD negativity.”
Investigators continue to follow the patients annually.
The investigators also analyzed MRD at 10-5 by cytogenetic risk and did not observe any MRD negativity in the control arm in either the POLLUX or CASTOR study.
“In contrast, we did observe some significant MRD negativity in the experimental arm with daratumumab—18% (POLLUX) and 14% (CASTOR) in high-risk patients,” Dr Avet-Loiseau said. “The most important prognostic factor is to achieve MRD negativity.”
However, even for patients who did not achieve MRD negativity, the PFS was much better in the experimental arms than in the control arms, he added.
This study, presented as a “Best of ASH” abstract, was funded by Janssen Research & Development, LLC. ![]()
*Information in the abstract differs from that presented at the meeting.
Autologous stem cell transplantation beat bortezomib regimen in myeloma
SAN DIEGO – Autologous stem cell transplantation outperformed bortezomib-based intensification in fit patients younger than 66 years of age with newly diagnosed multiple myeloma, based on a prespecified interim analysis of 1,192 patients from a randomized phase III trial.
After a median follow-up of 32 months, median progression-free survival (PFS) had not been reached among patients who received high-dose melphalan plus single or double autologous stem cell transplantation, but was 42.5 months among patients who instead received standard-dose bortezomib-melphalan-prednisone (VMP), Michele Cavo, MD, reported at the annual meeting of the American Society of Hematology. Three-year rates of progression free survival were 65% with ASCT and 57% with VMP (hazard ratio, 0.73; 95% confidence interval, 0.61-0.88; P = .001), he reported.
There was a trend toward better outcomes with double ASCT instead of single ASCT, said Dr. Cavo of Bologna (Italy) University. At 3 years, PFS rates were 74% with double ASCT and 62% with single ASCT (HR, 0.7; P = .05).
The effect was stronger among patients with high-risk cytogenetics, for whom 3-year PFS rates were 65% and 41% (HR, 0.49; P = .046). Those patients had median PFS times of 47 months and 27 months, respectively, Dr. Cavo said. In a multivariable analysis, double ASCT also reduced the chances of death or progression by about 35% compared with single ASCT, even after controlling for high-risk cytogenetics, age, and other risk factors for poor prognosis (HR, 0.65; P = .03).
This is the first trial of its type to prospectively compare single and double ASCT with a novel myeloma regimen, according to Dr. Cavo. The data are not yet mature enough to support firm conclusions, but do highlight the role of ASCT in the bortezomib era and the potential for double ASCT to benefit patients with poor prognostic risk factors, particularly high-risk cytogenetics, he said.
The EMN02/HO95 trial enrolled more than 1,500 patients aged 18-65 years with symptomatic, newly diagnosed multiple myeloma. Patients underwent induction therapy with three to four cycles of bortezomib plus cyclophosphamide and dexamethasone (VCD), and then were randomly assigned to either high-dose melphalan (200 mg/m2) plus single or double ASCT, or to four cycles of bortezomib (1.3 mg/m2), melphalan (9 mg/m2), and prednisone (60 mg/m2; VMP). Patients were then re-randomized to receive lenalidomide maintenance alone or after consolidation with two cycles of bortezomib, lenalidomide, and dexamethasone (VRD).
This prespecified analysis was triggered in early November 2016, when 33% of required events occurred. Future analyses will examine the effects of consolidation as well as safety, toxicity, and quality of life, Dr. Cavo noted.
Celgene and Janssen provided funding for the study. Dr. Cavo disclosed ties to Celgene, Janssen, Takeda, Bristol-Myers Squibb, and Amgen.
SAN DIEGO – Autologous stem cell transplantation outperformed bortezomib-based intensification in fit patients younger than 66 years of age with newly diagnosed multiple myeloma, based on a prespecified interim analysis of 1,192 patients from a randomized phase III trial.
After a median follow-up of 32 months, median progression-free survival (PFS) had not been reached among patients who received high-dose melphalan plus single or double autologous stem cell transplantation, but was 42.5 months among patients who instead received standard-dose bortezomib-melphalan-prednisone (VMP), Michele Cavo, MD, reported at the annual meeting of the American Society of Hematology. Three-year rates of progression free survival were 65% with ASCT and 57% with VMP (hazard ratio, 0.73; 95% confidence interval, 0.61-0.88; P = .001), he reported.
There was a trend toward better outcomes with double ASCT instead of single ASCT, said Dr. Cavo of Bologna (Italy) University. At 3 years, PFS rates were 74% with double ASCT and 62% with single ASCT (HR, 0.7; P = .05).
The effect was stronger among patients with high-risk cytogenetics, for whom 3-year PFS rates were 65% and 41% (HR, 0.49; P = .046). Those patients had median PFS times of 47 months and 27 months, respectively, Dr. Cavo said. In a multivariable analysis, double ASCT also reduced the chances of death or progression by about 35% compared with single ASCT, even after controlling for high-risk cytogenetics, age, and other risk factors for poor prognosis (HR, 0.65; P = .03).
This is the first trial of its type to prospectively compare single and double ASCT with a novel myeloma regimen, according to Dr. Cavo. The data are not yet mature enough to support firm conclusions, but do highlight the role of ASCT in the bortezomib era and the potential for double ASCT to benefit patients with poor prognostic risk factors, particularly high-risk cytogenetics, he said.
The EMN02/HO95 trial enrolled more than 1,500 patients aged 18-65 years with symptomatic, newly diagnosed multiple myeloma. Patients underwent induction therapy with three to four cycles of bortezomib plus cyclophosphamide and dexamethasone (VCD), and then were randomly assigned to either high-dose melphalan (200 mg/m2) plus single or double ASCT, or to four cycles of bortezomib (1.3 mg/m2), melphalan (9 mg/m2), and prednisone (60 mg/m2; VMP). Patients were then re-randomized to receive lenalidomide maintenance alone or after consolidation with two cycles of bortezomib, lenalidomide, and dexamethasone (VRD).
This prespecified analysis was triggered in early November 2016, when 33% of required events occurred. Future analyses will examine the effects of consolidation as well as safety, toxicity, and quality of life, Dr. Cavo noted.
Celgene and Janssen provided funding for the study. Dr. Cavo disclosed ties to Celgene, Janssen, Takeda, Bristol-Myers Squibb, and Amgen.
SAN DIEGO – Autologous stem cell transplantation outperformed bortezomib-based intensification in fit patients younger than 66 years of age with newly diagnosed multiple myeloma, based on a prespecified interim analysis of 1,192 patients from a randomized phase III trial.
After a median follow-up of 32 months, median progression-free survival (PFS) had not been reached among patients who received high-dose melphalan plus single or double autologous stem cell transplantation, but was 42.5 months among patients who instead received standard-dose bortezomib-melphalan-prednisone (VMP), Michele Cavo, MD, reported at the annual meeting of the American Society of Hematology. Three-year rates of progression free survival were 65% with ASCT and 57% with VMP (hazard ratio, 0.73; 95% confidence interval, 0.61-0.88; P = .001), he reported.
There was a trend toward better outcomes with double ASCT instead of single ASCT, said Dr. Cavo of Bologna (Italy) University. At 3 years, PFS rates were 74% with double ASCT and 62% with single ASCT (HR, 0.7; P = .05).
The effect was stronger among patients with high-risk cytogenetics, for whom 3-year PFS rates were 65% and 41% (HR, 0.49; P = .046). Those patients had median PFS times of 47 months and 27 months, respectively, Dr. Cavo said. In a multivariable analysis, double ASCT also reduced the chances of death or progression by about 35% compared with single ASCT, even after controlling for high-risk cytogenetics, age, and other risk factors for poor prognosis (HR, 0.65; P = .03).
This is the first trial of its type to prospectively compare single and double ASCT with a novel myeloma regimen, according to Dr. Cavo. The data are not yet mature enough to support firm conclusions, but do highlight the role of ASCT in the bortezomib era and the potential for double ASCT to benefit patients with poor prognostic risk factors, particularly high-risk cytogenetics, he said.
The EMN02/HO95 trial enrolled more than 1,500 patients aged 18-65 years with symptomatic, newly diagnosed multiple myeloma. Patients underwent induction therapy with three to four cycles of bortezomib plus cyclophosphamide and dexamethasone (VCD), and then were randomly assigned to either high-dose melphalan (200 mg/m2) plus single or double ASCT, or to four cycles of bortezomib (1.3 mg/m2), melphalan (9 mg/m2), and prednisone (60 mg/m2; VMP). Patients were then re-randomized to receive lenalidomide maintenance alone or after consolidation with two cycles of bortezomib, lenalidomide, and dexamethasone (VRD).
This prespecified analysis was triggered in early November 2016, when 33% of required events occurred. Future analyses will examine the effects of consolidation as well as safety, toxicity, and quality of life, Dr. Cavo noted.
Celgene and Janssen provided funding for the study. Dr. Cavo disclosed ties to Celgene, Janssen, Takeda, Bristol-Myers Squibb, and Amgen.
AT ASH 2016
Key clinical point: Autologous stem cell transplantation outperformed bortezomib-based intensification in patients with newly diagnosed multiple myeloma.
Major finding: Progression-free survival at 3 years was 65% with melphalan plus ASCT and 57% with bortezomib, melphalan, and prednisone (HR, 0.73; P = .001).
Data source: An interim analysis of a phase III study of 1,510 patients with newly diagnosed multiple myeloma.
Disclosures: Celgene and Janssen provided funding. Dr. Cavo disclosed ties to Celgene, Janssen, Takeda, Bristol-Myers Squibb, and Amgen.
Company terminates study of drug for MM

multiple myeloma
BioInvent International has decided to terminate its phase 2 trial of the antibody BI-505 in patients with multiple myeloma (MM).
The decision follows a review and discussion with the US Food and Drug Administration (FDA), which put the trial on full clinical hold in November due to an adverse cardiopulmonary event.
The trial was designed to determine if BI-505 could deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
The termination of this trial may not mean the end of BI-505. BioInvent is currently in discussions with the FDA about the potential to develop the drug for use in other patient populations.
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
The development strategy for BI-505 has been focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
BI-505 has orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency.
Results of a phase 1 trial of BI-505 in MM patients were published in Clinical Cancer Research in June 2015. ![]()

multiple myeloma
BioInvent International has decided to terminate its phase 2 trial of the antibody BI-505 in patients with multiple myeloma (MM).
The decision follows a review and discussion with the US Food and Drug Administration (FDA), which put the trial on full clinical hold in November due to an adverse cardiopulmonary event.
The trial was designed to determine if BI-505 could deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
The termination of this trial may not mean the end of BI-505. BioInvent is currently in discussions with the FDA about the potential to develop the drug for use in other patient populations.
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
The development strategy for BI-505 has been focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
BI-505 has orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency.
Results of a phase 1 trial of BI-505 in MM patients were published in Clinical Cancer Research in June 2015. ![]()

multiple myeloma
BioInvent International has decided to terminate its phase 2 trial of the antibody BI-505 in patients with multiple myeloma (MM).
The decision follows a review and discussion with the US Food and Drug Administration (FDA), which put the trial on full clinical hold in November due to an adverse cardiopulmonary event.
The trial was designed to determine if BI-505 could deepen therapeutic response and thereby prevent or delay relapse in MM patients undergoing autologous stem cell transplant with high-dose melphalan.
The termination of this trial may not mean the end of BI-505. BioInvent is currently in discussions with the FDA about the potential to develop the drug for use in other patient populations.
BI-505 is a human antibody targeting ICAM-1, a protein that is elevated in MM cells. BI-505 has been shown to attack MM in 2 ways—by inducing apoptosis in MM cells and by engaging macrophages to attack and kill MM cells.
The development strategy for BI-505 has been focused on eliminating residual disease by combining the antibody with modern standard-of-care drugs used to treat MM.
BI-505 has orphan drug designation as a treatment for MM from both the FDA and the European Medicines Agency.
Results of a phase 1 trial of BI-505 in MM patients were published in Clinical Cancer Research in June 2015. ![]()
Study reinforces lenalidomide maintenance in newly diagnosed multiple myeloma
SAN DIEGO – Maintenance therapy with lenalidomide significantly improved progression-free survival in patients of all ages with myeloma, regardless of their risk or response status at the end of induction, Gareth Morgan, MD, PhD, said during an oral session at the annual meeting of the American Society of Hematology.
“The very important point is that maintenance therapy with lenalidomide worked across a range of different risk groups,” said Dr. Morgan, director of the Myeloma Institute at the University of Arkansas for Medical Sciences in Little Rock. “It worked independent of gender, age, [International Staging System] disease stage, and response at baseline,” he added. ‘It also worked irrespective of genetic risk status, which is contrary to what you hear very frequently. All of the curves are consistent with better outcomes if you continue lenalidomide long-term.”
In the overall cohort analysis, half of the patients who received lenalidomide (Revlimid) maintenance were alive and progression-free after 36 months (95% confidence interval, 31-39 months), twice the median PFS of observation-only patients, for a hazard ratio of 0.45 (95% CI, 0.39-0.52; P less than .0001).
This effect held up across numerous subgroups. For example, among 828 transplant-eligible patients, median PFS was 50 months with lenalidomide maintenance and 28 months with observation only (HR, 0.47; P less than .0001). Among 724 transplant-ineligible patients, median PFS was 24 months with lenalidomide and 11 months with observation only (HR, 0.42; P less than .0001), Dr. Morgan reported.
Lenalidomide maintenance did not fully overcome the effects of high-risk cytogenetics but still increased PFS by a median of 10 months, compared with no maintenance (median PFS, 23 months vs. 13 months, respectively; P less than .0001). For patients with standard-risk cytogenetics, median PFS was 44 months on lenalidomide maintenance and 25 months otherwise (P less than .0001).
When patients had minimal residual disease after induction, their median PFS on lenalidomide was 17 months longer if they received maintenance lenalidomide (30 vs. 13 months; P less than .0001). Not surprisingly, the best overall outcomes occurred in MRD-negative patients who received lenalidomide maintenance (median PFS, 44 months, vs. 31 months without lenalidomide; P less than .0001), he said.
Responses also were more likely to deepen over time if patients received lenalidomide maintenance (HR, 1.74; 95% CI, 1.2-2.6; P = .004). “This continued down to about 24 months, which is compatible with conventional response rates,” Dr. Morgan noted.
Safety results reflected prior studies and were unremarkable, he added. “I treat a lot of people with lenalidomide for long periods of time, and the worst thing I usually see is some fatigue.” About one-third of patients developed grade 3-4 neutropenia on lenalidomide maintenance, but less than 5% developed grade 3-4 thrombocytopenia, anemia, deep vein thromboses, or neuropathies. Rates of primary and second malignancies were no worse with maintenance than without it. “All investigators are now in agreement on this finding,” Dr. Morgan emphasized.
The researchers also performed a whole exosome study of 70 paired specimens collected when patients were randomized and again when they relapsed. They found no evidence that lenalidomide induced excess mutations and no significant difference between groups in mutational patterns or genomic copy number variants that alter risk status.
Dr. Morgan and his associates will present overall survival data when the number of events reaches 458, he said. For now, the PFS data reinforce lenalidomide as the standard of care for patients of all ages with newly diagnosed multiple myeloma, he concluded.
The Myeloma XI trial is funded by Cancer Research UK, the Experimental Cancer Medicine Centre, NIHR Clinical Research Network: Cancer, and the University of Leeds. Dr. Morgan disclosed consulting and other relationships with Celgene, the maker of lenalidomide.
SAN DIEGO – Maintenance therapy with lenalidomide significantly improved progression-free survival in patients of all ages with myeloma, regardless of their risk or response status at the end of induction, Gareth Morgan, MD, PhD, said during an oral session at the annual meeting of the American Society of Hematology.
“The very important point is that maintenance therapy with lenalidomide worked across a range of different risk groups,” said Dr. Morgan, director of the Myeloma Institute at the University of Arkansas for Medical Sciences in Little Rock. “It worked independent of gender, age, [International Staging System] disease stage, and response at baseline,” he added. ‘It also worked irrespective of genetic risk status, which is contrary to what you hear very frequently. All of the curves are consistent with better outcomes if you continue lenalidomide long-term.”
In the overall cohort analysis, half of the patients who received lenalidomide (Revlimid) maintenance were alive and progression-free after 36 months (95% confidence interval, 31-39 months), twice the median PFS of observation-only patients, for a hazard ratio of 0.45 (95% CI, 0.39-0.52; P less than .0001).
This effect held up across numerous subgroups. For example, among 828 transplant-eligible patients, median PFS was 50 months with lenalidomide maintenance and 28 months with observation only (HR, 0.47; P less than .0001). Among 724 transplant-ineligible patients, median PFS was 24 months with lenalidomide and 11 months with observation only (HR, 0.42; P less than .0001), Dr. Morgan reported.
Lenalidomide maintenance did not fully overcome the effects of high-risk cytogenetics but still increased PFS by a median of 10 months, compared with no maintenance (median PFS, 23 months vs. 13 months, respectively; P less than .0001). For patients with standard-risk cytogenetics, median PFS was 44 months on lenalidomide maintenance and 25 months otherwise (P less than .0001).
When patients had minimal residual disease after induction, their median PFS on lenalidomide was 17 months longer if they received maintenance lenalidomide (30 vs. 13 months; P less than .0001). Not surprisingly, the best overall outcomes occurred in MRD-negative patients who received lenalidomide maintenance (median PFS, 44 months, vs. 31 months without lenalidomide; P less than .0001), he said.
Responses also were more likely to deepen over time if patients received lenalidomide maintenance (HR, 1.74; 95% CI, 1.2-2.6; P = .004). “This continued down to about 24 months, which is compatible with conventional response rates,” Dr. Morgan noted.
Safety results reflected prior studies and were unremarkable, he added. “I treat a lot of people with lenalidomide for long periods of time, and the worst thing I usually see is some fatigue.” About one-third of patients developed grade 3-4 neutropenia on lenalidomide maintenance, but less than 5% developed grade 3-4 thrombocytopenia, anemia, deep vein thromboses, or neuropathies. Rates of primary and second malignancies were no worse with maintenance than without it. “All investigators are now in agreement on this finding,” Dr. Morgan emphasized.
The researchers also performed a whole exosome study of 70 paired specimens collected when patients were randomized and again when they relapsed. They found no evidence that lenalidomide induced excess mutations and no significant difference between groups in mutational patterns or genomic copy number variants that alter risk status.
Dr. Morgan and his associates will present overall survival data when the number of events reaches 458, he said. For now, the PFS data reinforce lenalidomide as the standard of care for patients of all ages with newly diagnosed multiple myeloma, he concluded.
The Myeloma XI trial is funded by Cancer Research UK, the Experimental Cancer Medicine Centre, NIHR Clinical Research Network: Cancer, and the University of Leeds. Dr. Morgan disclosed consulting and other relationships with Celgene, the maker of lenalidomide.
SAN DIEGO – Maintenance therapy with lenalidomide significantly improved progression-free survival in patients of all ages with myeloma, regardless of their risk or response status at the end of induction, Gareth Morgan, MD, PhD, said during an oral session at the annual meeting of the American Society of Hematology.
“The very important point is that maintenance therapy with lenalidomide worked across a range of different risk groups,” said Dr. Morgan, director of the Myeloma Institute at the University of Arkansas for Medical Sciences in Little Rock. “It worked independent of gender, age, [International Staging System] disease stage, and response at baseline,” he added. ‘It also worked irrespective of genetic risk status, which is contrary to what you hear very frequently. All of the curves are consistent with better outcomes if you continue lenalidomide long-term.”
In the overall cohort analysis, half of the patients who received lenalidomide (Revlimid) maintenance were alive and progression-free after 36 months (95% confidence interval, 31-39 months), twice the median PFS of observation-only patients, for a hazard ratio of 0.45 (95% CI, 0.39-0.52; P less than .0001).
This effect held up across numerous subgroups. For example, among 828 transplant-eligible patients, median PFS was 50 months with lenalidomide maintenance and 28 months with observation only (HR, 0.47; P less than .0001). Among 724 transplant-ineligible patients, median PFS was 24 months with lenalidomide and 11 months with observation only (HR, 0.42; P less than .0001), Dr. Morgan reported.
Lenalidomide maintenance did not fully overcome the effects of high-risk cytogenetics but still increased PFS by a median of 10 months, compared with no maintenance (median PFS, 23 months vs. 13 months, respectively; P less than .0001). For patients with standard-risk cytogenetics, median PFS was 44 months on lenalidomide maintenance and 25 months otherwise (P less than .0001).
When patients had minimal residual disease after induction, their median PFS on lenalidomide was 17 months longer if they received maintenance lenalidomide (30 vs. 13 months; P less than .0001). Not surprisingly, the best overall outcomes occurred in MRD-negative patients who received lenalidomide maintenance (median PFS, 44 months, vs. 31 months without lenalidomide; P less than .0001), he said.
Responses also were more likely to deepen over time if patients received lenalidomide maintenance (HR, 1.74; 95% CI, 1.2-2.6; P = .004). “This continued down to about 24 months, which is compatible with conventional response rates,” Dr. Morgan noted.
Safety results reflected prior studies and were unremarkable, he added. “I treat a lot of people with lenalidomide for long periods of time, and the worst thing I usually see is some fatigue.” About one-third of patients developed grade 3-4 neutropenia on lenalidomide maintenance, but less than 5% developed grade 3-4 thrombocytopenia, anemia, deep vein thromboses, or neuropathies. Rates of primary and second malignancies were no worse with maintenance than without it. “All investigators are now in agreement on this finding,” Dr. Morgan emphasized.
The researchers also performed a whole exosome study of 70 paired specimens collected when patients were randomized and again when they relapsed. They found no evidence that lenalidomide induced excess mutations and no significant difference between groups in mutational patterns or genomic copy number variants that alter risk status.
Dr. Morgan and his associates will present overall survival data when the number of events reaches 458, he said. For now, the PFS data reinforce lenalidomide as the standard of care for patients of all ages with newly diagnosed multiple myeloma, he concluded.
The Myeloma XI trial is funded by Cancer Research UK, the Experimental Cancer Medicine Centre, NIHR Clinical Research Network: Cancer, and the University of Leeds. Dr. Morgan disclosed consulting and other relationships with Celgene, the maker of lenalidomide.
AT ASH 2016
Key clinical point: Maintenance therapy with lenalidomide significantly improved progression-free survival in patients of all ages with myeloma, regardless of response to induction or baseline risk status.
Major finding: Median PFS for patients on lenalidomide maintenance was 36 months (95% confidence interval, 31-39 months), twice that of observation-only patients (hazard ratio, 0.45; P less than .0001).
Data source: A phase III, multicenter, open-label, parallel-group, randomized controlled trial of 1,551 patients with newly diagnosed multiple myeloma.
Disclosures: The Myeloma XI trial is funded by Cancer Research UK, the Experimental Cancer Medicine Centre, NIHR Clinical Research Network: Cancer, and the University of Leeds. Dr. Morgan disclosed consulting and other relationships with Celgene, the maker of lenalidomide.
Group estimates global cancer cases, deaths in 2015

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()

receiving chemotherapy
Photo by Rhoda Baer
Researchers have estimated the global incidence of 32 cancer types and deaths related to these malignancies in 2015.
The group’s data, published in JAMA Oncology, suggest there were 17.5 million cancer cases and 8.7 million cancer deaths last year.
There were 78,000 cases of Hodgkin lymphoma and 24,000 deaths from the disease, as well as 666,000 cases of non-Hodgkin lymphoma (NHL) and 231,000 NHL deaths.
There were 154,000 cases of multiple myeloma and 101,000 deaths from the disease.
And there were 606,000 cases of leukemia, with 353,000 leukemia deaths. This included 161,000 cases of acute lymphoid leukemia (110,000 deaths), 191,000 cases of chronic lymphoid leukemia (61,000 deaths), 190,000 cases of acute myeloid leukemia (147,000 deaths), and 64,000 cases of chronic myeloid leukemia (35,000 deaths).
The data also show that, between 2005 and 2015, cancer cases increased by 33%, mostly due to population aging and growth, plus changes in age-specific cancer rates.
Globally, the odds of developing cancer during a lifetime were 1 in 3 for men and 1 in 4 for women in 2015.
Prostate cancer was the most common cancer in men (1.6 million cases), although tracheal, bronchus, and lung cancer was the leading cause of cancer deaths for men.
Breast cancer was the most common cancer for women (2.4 million cases) and the leading cause of cancer deaths in women.
The most common childhood cancers were leukemia, “other neoplasms,” NHL, and brain and nervous system cancers. ![]()
Second transplant, consolidation don’t add benefit in upfront multiple myeloma therapy
SAN DIEGO – It took a clinical trial with a byzantine design to prove it, but neither posttransplant consolidation therapy nor second transplant offered any additional survival benefits to patients with multiple myeloma, including patients with high-risk disease who were treated with an upfront thalidomide analogue and a proteasome inhibitor, followed by stem cell transplant and lenalidomide maintenance.
Among 758 patients with multiple myeloma who underwent standard induction therapy, followed by melphalan conditioning and autologous stem cell transplant (ASCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) among patients assigned to follow-on therapy with either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant, followed by lenalidomide maintenance, reported Edward A. Stadtmauer, MD, coleader of the hematologic malignancies program at the Abramson Cancer Center, and chief of the section of hematologic malignancies, University of Pennsylvania, Philadelphia.
Investigators in the STAMINA (Stem Cell Transplant With Lenalidomide Maintenance in Patients With Multiple Myeloma) trial (BMT CTN 0702) hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and ASCT would improve survival, compared with a second ASCT.
To test this idea, they enrolled 758 patients and randomized them to one of the three aforementioned posttransplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and ASCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
Slightly more than half of patients received RVD upfront; about 13% received cyclophosphamide, bortezomib, and dexamethasone (CyBorD); roughly 10% received lenalidomide dexamethasone; 12% were treated with bortezomib/dexamethasone; and about 8% received other, unspecified combinations.
At a median follow-up time of 37.8 months, the PFS rate, which was the primary endpoint, was 56.5% for the second transplant arm, 56.7% for the RVD arm, and 52.2% for the maintenance-only arm. The differences were not statistically significant.
Similarly, there were no among-arm differences in PFS for patients with standard-risk disease (60.9%, 59.5%, and 55.9%) or for those with high-risk myeloma (42.2%, 48.3%, and 40.2%)
Overall survival, a secondary endpoint, was also not significantly different among the groups, at 82%, 85.7%, and 83.4%, respectively.
Encouragingly, however, despite lower PFS rates, patients with high-risk disease had high OS rates, with 79.6% of patients in the double-transplant arm, 77.5% of those in the RVD consolidation arm, and 79.5% of those in the lenalidomide maintenance-alone arm still alive at 38 months.
Secondary malignancies occurred among 5.1% of patients overall: 14 in the dual-transplant arm, 15 in the consolidation arm, and 10 in the maintenance-only arm. The most frequently reported second malignancies were leukemia, which occurred in 3 of 14 patients with second cancers after second transplant and in 9 of 15 patients with second cancers after consolidation, and solid tumors, which occurred most frequently among second cancers in the maintenance arm.
The investigators are continuing to parse the data by study arm to see whether response assessment correlates with outcomes and with complete remissions. They also plan to examine minimal residual disease via flow cytometry and sequencing, and to obtain long-term data on survival, toxicities, and second primary malignancies.
The trial was funded by the National Institutes of Health with support from Celgene and Millennium/Takeda. Dr. Stadtmauer disclosed consulting for Takeda and travel expenses from Celgene.
SAN DIEGO – It took a clinical trial with a byzantine design to prove it, but neither posttransplant consolidation therapy nor second transplant offered any additional survival benefits to patients with multiple myeloma, including patients with high-risk disease who were treated with an upfront thalidomide analogue and a proteasome inhibitor, followed by stem cell transplant and lenalidomide maintenance.
Among 758 patients with multiple myeloma who underwent standard induction therapy, followed by melphalan conditioning and autologous stem cell transplant (ASCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) among patients assigned to follow-on therapy with either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant, followed by lenalidomide maintenance, reported Edward A. Stadtmauer, MD, coleader of the hematologic malignancies program at the Abramson Cancer Center, and chief of the section of hematologic malignancies, University of Pennsylvania, Philadelphia.
Investigators in the STAMINA (Stem Cell Transplant With Lenalidomide Maintenance in Patients With Multiple Myeloma) trial (BMT CTN 0702) hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and ASCT would improve survival, compared with a second ASCT.
To test this idea, they enrolled 758 patients and randomized them to one of the three aforementioned posttransplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and ASCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
Slightly more than half of patients received RVD upfront; about 13% received cyclophosphamide, bortezomib, and dexamethasone (CyBorD); roughly 10% received lenalidomide dexamethasone; 12% were treated with bortezomib/dexamethasone; and about 8% received other, unspecified combinations.
At a median follow-up time of 37.8 months, the PFS rate, which was the primary endpoint, was 56.5% for the second transplant arm, 56.7% for the RVD arm, and 52.2% for the maintenance-only arm. The differences were not statistically significant.
Similarly, there were no among-arm differences in PFS for patients with standard-risk disease (60.9%, 59.5%, and 55.9%) or for those with high-risk myeloma (42.2%, 48.3%, and 40.2%)
Overall survival, a secondary endpoint, was also not significantly different among the groups, at 82%, 85.7%, and 83.4%, respectively.
Encouragingly, however, despite lower PFS rates, patients with high-risk disease had high OS rates, with 79.6% of patients in the double-transplant arm, 77.5% of those in the RVD consolidation arm, and 79.5% of those in the lenalidomide maintenance-alone arm still alive at 38 months.
Secondary malignancies occurred among 5.1% of patients overall: 14 in the dual-transplant arm, 15 in the consolidation arm, and 10 in the maintenance-only arm. The most frequently reported second malignancies were leukemia, which occurred in 3 of 14 patients with second cancers after second transplant and in 9 of 15 patients with second cancers after consolidation, and solid tumors, which occurred most frequently among second cancers in the maintenance arm.
The investigators are continuing to parse the data by study arm to see whether response assessment correlates with outcomes and with complete remissions. They also plan to examine minimal residual disease via flow cytometry and sequencing, and to obtain long-term data on survival, toxicities, and second primary malignancies.
The trial was funded by the National Institutes of Health with support from Celgene and Millennium/Takeda. Dr. Stadtmauer disclosed consulting for Takeda and travel expenses from Celgene.
SAN DIEGO – It took a clinical trial with a byzantine design to prove it, but neither posttransplant consolidation therapy nor second transplant offered any additional survival benefits to patients with multiple myeloma, including patients with high-risk disease who were treated with an upfront thalidomide analogue and a proteasome inhibitor, followed by stem cell transplant and lenalidomide maintenance.
Among 758 patients with multiple myeloma who underwent standard induction therapy, followed by melphalan conditioning and autologous stem cell transplant (ASCT), there were no differences in either progression-free survival (PFS) or overall survival (OS) among patients assigned to follow-on therapy with either lenalidomide (Revlimid) maintenance alone; consolidation therapy with four cycles of lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVD), followed by lenalidomide maintenance; or second transplant, followed by lenalidomide maintenance, reported Edward A. Stadtmauer, MD, coleader of the hematologic malignancies program at the Abramson Cancer Center, and chief of the section of hematologic malignancies, University of Pennsylvania, Philadelphia.
Investigators in the STAMINA (Stem Cell Transplant With Lenalidomide Maintenance in Patients With Multiple Myeloma) trial (BMT CTN 0702) hypothesized that the use of thalidomide analogues and proteasome inhibitors used in first-line therapy, consolidation, and long-term maintenance after high-dose melphalan and ASCT would improve survival, compared with a second ASCT.
To test this idea, they enrolled 758 patients and randomized them to one of the three aforementioned posttransplant strategies prior to transplant conditioning with high-dose melphalan (200 mg/m2) and ASCT.
Roughly 25% of patients in each treatment arm had high-risk disease, defined as beta2 microglobulin levels greater than 5.5 mg/L, high-risk cytogenetics, and deletion 13 detected by standard cytogenetics only. The remaining patients in each arm had standard-risk disease.
Slightly more than half of patients received RVD upfront; about 13% received cyclophosphamide, bortezomib, and dexamethasone (CyBorD); roughly 10% received lenalidomide dexamethasone; 12% were treated with bortezomib/dexamethasone; and about 8% received other, unspecified combinations.
At a median follow-up time of 37.8 months, the PFS rate, which was the primary endpoint, was 56.5% for the second transplant arm, 56.7% for the RVD arm, and 52.2% for the maintenance-only arm. The differences were not statistically significant.
Similarly, there were no among-arm differences in PFS for patients with standard-risk disease (60.9%, 59.5%, and 55.9%) or for those with high-risk myeloma (42.2%, 48.3%, and 40.2%)
Overall survival, a secondary endpoint, was also not significantly different among the groups, at 82%, 85.7%, and 83.4%, respectively.
Encouragingly, however, despite lower PFS rates, patients with high-risk disease had high OS rates, with 79.6% of patients in the double-transplant arm, 77.5% of those in the RVD consolidation arm, and 79.5% of those in the lenalidomide maintenance-alone arm still alive at 38 months.
Secondary malignancies occurred among 5.1% of patients overall: 14 in the dual-transplant arm, 15 in the consolidation arm, and 10 in the maintenance-only arm. The most frequently reported second malignancies were leukemia, which occurred in 3 of 14 patients with second cancers after second transplant and in 9 of 15 patients with second cancers after consolidation, and solid tumors, which occurred most frequently among second cancers in the maintenance arm.
The investigators are continuing to parse the data by study arm to see whether response assessment correlates with outcomes and with complete remissions. They also plan to examine minimal residual disease via flow cytometry and sequencing, and to obtain long-term data on survival, toxicities, and second primary malignancies.
The trial was funded by the National Institutes of Health with support from Celgene and Millennium/Takeda. Dr. Stadtmauer disclosed consulting for Takeda and travel expenses from Celgene.
AT ASH 2016
Key clinical point: Three posttransplant strategies for patients with previously untreated myeloma were comparably effective.
Major finding: There were no differences in PFS or OS among patients treated with upfront therapy and transplant followed by either second transplant, consolidation, or lenalidomide maintenance alone.
Data source: Randomized prospective trial of 758 patients with multiple myeloma treated with a thalidomide analogue, proteasome inhibitor, and autologous stem cell transplant.
Disclosures: The trial was funded by the National Institutes of Health with support from Celgene and Millennium/Takeda. Dr. Stadtmauer disclosed consulting for Takeda and travel expenses from Celgene.
VIDEO: Venetoclax shows early good results in multiple myeloma
SAN DIEGO – Venetoclax has shown preliminary good results as an investigational monotherapy in patients with relapsed/refractory multiple myeloma, based on phase I data reported at the annual meeting of the American Society of Hematology.
Shaji Kumar, MD, of the Mayo Clinic, Rochester, Minn., reported that venetoclax monotherapy had anti-myeloma activity in a dose-finding study among patients treated with a median of five previous therapies. As would be expected, the best responses to the small-molecule BCL-2 inhibitor were seen primarily in patients with t(11;14) chromosomal aberrations and high BCL-2, low BCL-XL and low MCL-1 expression levels.
In a video interview, Dr. Kumar discussed the results of this early-stage research as well as ongoing studies that are beginning to examine venetoclax in combination regimens for multiple myeloma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Venetoclax is approved for the treatment of patients with chronic lymphocytic leukemia with 17p deletion who have received at least one prior treatment. Dr. Kumar receives research funding from Abbvie, the maker of venetoclax (Venclexta), and is a consultant to and receives research funding from several other drug companies.
[email protected]
On Twitter @maryjodales
SAN DIEGO – Venetoclax has shown preliminary good results as an investigational monotherapy in patients with relapsed/refractory multiple myeloma, based on phase I data reported at the annual meeting of the American Society of Hematology.
Shaji Kumar, MD, of the Mayo Clinic, Rochester, Minn., reported that venetoclax monotherapy had anti-myeloma activity in a dose-finding study among patients treated with a median of five previous therapies. As would be expected, the best responses to the small-molecule BCL-2 inhibitor were seen primarily in patients with t(11;14) chromosomal aberrations and high BCL-2, low BCL-XL and low MCL-1 expression levels.
In a video interview, Dr. Kumar discussed the results of this early-stage research as well as ongoing studies that are beginning to examine venetoclax in combination regimens for multiple myeloma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Venetoclax is approved for the treatment of patients with chronic lymphocytic leukemia with 17p deletion who have received at least one prior treatment. Dr. Kumar receives research funding from Abbvie, the maker of venetoclax (Venclexta), and is a consultant to and receives research funding from several other drug companies.
[email protected]
On Twitter @maryjodales
SAN DIEGO – Venetoclax has shown preliminary good results as an investigational monotherapy in patients with relapsed/refractory multiple myeloma, based on phase I data reported at the annual meeting of the American Society of Hematology.
Shaji Kumar, MD, of the Mayo Clinic, Rochester, Minn., reported that venetoclax monotherapy had anti-myeloma activity in a dose-finding study among patients treated with a median of five previous therapies. As would be expected, the best responses to the small-molecule BCL-2 inhibitor were seen primarily in patients with t(11;14) chromosomal aberrations and high BCL-2, low BCL-XL and low MCL-1 expression levels.
In a video interview, Dr. Kumar discussed the results of this early-stage research as well as ongoing studies that are beginning to examine venetoclax in combination regimens for multiple myeloma.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Venetoclax is approved for the treatment of patients with chronic lymphocytic leukemia with 17p deletion who have received at least one prior treatment. Dr. Kumar receives research funding from Abbvie, the maker of venetoclax (Venclexta), and is a consultant to and receives research funding from several other drug companies.
[email protected]
On Twitter @maryjodales
AT ASH 2016
NCCN releases new guidelines for cancer patients

Nausea and Vomiting
©NCCN® 2016
The National Comprehensive Cancer Network (NCCN) has released new educational materials designed to help cancer patients combat nausea and vomiting.
The NCCN Guidelines for Patients® for Nausea and Vomiting and NCCN Quick Guide™ for Nausea and Vomiting are the first patient resources from NCCN to focus specifically on supportive care.
The resources are available on NCCN.org/patients and via the NCCN Patient Guides for Cancer mobile app.
NCCN Guidelines for Patients are patient-friendly translations of the NCCN Clinical Practice Guidelines in Oncology. Each resource features guidance from US cancer centers designed to help people living with cancer talk with their physicians about the best treatment options for their disease.
NCCN Quick Guide™ sheets are 1-page summaries of key points in the patient guidelines. They include elements such as “questions to ask your doctor,” a glossary of terms, and medical illustrations of anatomy, tests, and treatments.
The NCCN Guidelines for Patients for Nausea and Vomiting:
- Explain how these side effects are related to cancer treatment
- List cancer treatments that can cause nausea and vomiting
- Detail methods of preventing and treating these side effects
- Outline methods of coping with nausea and vomiting
- Provide a list of resources for information and support.
“At NCCN, our mission is to improve the lives of patients with cancer, and we are excited to be able to provide the information that will help patients better understand this common side effect of cancer treatment,” said Marcie R. Reeder, executive director of the NCCN Foundation.
“The NCCN Guidelines for Patients for Nausea and Vomiting are the first of a highly anticipated library of supportive care resources that provide patients with the same information their doctors use.” ![]()

Nausea and Vomiting
©NCCN® 2016
The National Comprehensive Cancer Network (NCCN) has released new educational materials designed to help cancer patients combat nausea and vomiting.
The NCCN Guidelines for Patients® for Nausea and Vomiting and NCCN Quick Guide™ for Nausea and Vomiting are the first patient resources from NCCN to focus specifically on supportive care.
The resources are available on NCCN.org/patients and via the NCCN Patient Guides for Cancer mobile app.
NCCN Guidelines for Patients are patient-friendly translations of the NCCN Clinical Practice Guidelines in Oncology. Each resource features guidance from US cancer centers designed to help people living with cancer talk with their physicians about the best treatment options for their disease.
NCCN Quick Guide™ sheets are 1-page summaries of key points in the patient guidelines. They include elements such as “questions to ask your doctor,” a glossary of terms, and medical illustrations of anatomy, tests, and treatments.
The NCCN Guidelines for Patients for Nausea and Vomiting:
- Explain how these side effects are related to cancer treatment
- List cancer treatments that can cause nausea and vomiting
- Detail methods of preventing and treating these side effects
- Outline methods of coping with nausea and vomiting
- Provide a list of resources for information and support.
“At NCCN, our mission is to improve the lives of patients with cancer, and we are excited to be able to provide the information that will help patients better understand this common side effect of cancer treatment,” said Marcie R. Reeder, executive director of the NCCN Foundation.
“The NCCN Guidelines for Patients for Nausea and Vomiting are the first of a highly anticipated library of supportive care resources that provide patients with the same information their doctors use.” ![]()

Nausea and Vomiting
©NCCN® 2016
The National Comprehensive Cancer Network (NCCN) has released new educational materials designed to help cancer patients combat nausea and vomiting.
The NCCN Guidelines for Patients® for Nausea and Vomiting and NCCN Quick Guide™ for Nausea and Vomiting are the first patient resources from NCCN to focus specifically on supportive care.
The resources are available on NCCN.org/patients and via the NCCN Patient Guides for Cancer mobile app.
NCCN Guidelines for Patients are patient-friendly translations of the NCCN Clinical Practice Guidelines in Oncology. Each resource features guidance from US cancer centers designed to help people living with cancer talk with their physicians about the best treatment options for their disease.
NCCN Quick Guide™ sheets are 1-page summaries of key points in the patient guidelines. They include elements such as “questions to ask your doctor,” a glossary of terms, and medical illustrations of anatomy, tests, and treatments.
The NCCN Guidelines for Patients for Nausea and Vomiting:
- Explain how these side effects are related to cancer treatment
- List cancer treatments that can cause nausea and vomiting
- Detail methods of preventing and treating these side effects
- Outline methods of coping with nausea and vomiting
- Provide a list of resources for information and support.
“At NCCN, our mission is to improve the lives of patients with cancer, and we are excited to be able to provide the information that will help patients better understand this common side effect of cancer treatment,” said Marcie R. Reeder, executive director of the NCCN Foundation.
“The NCCN Guidelines for Patients for Nausea and Vomiting are the first of a highly anticipated library of supportive care resources that provide patients with the same information their doctors use.” ![]()
NICE recommends pomalidomide for routine use
Photo from Business Wire
The National Institute for Health and Care Excellence (NICE) has issued a final appraisal determination recommending that pomalidomide be made available through the National Health Service (NHS).
NICE is recommending pomalidomide be available for use in combination with low-dose dexamethasone to treat adults with multiple myeloma who have received at least 3 previous treatments, including lenalidomide and bortezomib.
NICE previously evaluated pomalidomide in 2015 and said it could not recommend the drug, as analyses suggested pomalidomide doesn’t provide enough benefit to justify its high price.
Since that time, a committee advising NICE has reviewed additional data on pomalidomide.
And Celgene, the company that makes pomalidomide, has agreed to provide the NHS with a discount.
The cost of pomalidomide is £8884 per 21-tablet pack (excluding tax). The average cost of a course of treatment is £44,420 (excluding tax).
The discount Celgene will provide to the NHS is confidential.
NICE’s final appraisal determination on pomalidomide is now with consultees who have the opportunity to appeal against it. If there is no appeal, or an appeal is not upheld, the final appraisal determination is issued by NICE as a guidance.
The final guidance is expected in January 2017. Once NICE issues a final guidance on pomalidomide, the NHS must make the drug available within 3 months. ![]()

Photo from Business Wire
The National Institute for Health and Care Excellence (NICE) has issued a final appraisal determination recommending that pomalidomide be made available through the National Health Service (NHS).
NICE is recommending pomalidomide be available for use in combination with low-dose dexamethasone to treat adults with multiple myeloma who have received at least 3 previous treatments, including lenalidomide and bortezomib.
NICE previously evaluated pomalidomide in 2015 and said it could not recommend the drug, as analyses suggested pomalidomide doesn’t provide enough benefit to justify its high price.
Since that time, a committee advising NICE has reviewed additional data on pomalidomide.
And Celgene, the company that makes pomalidomide, has agreed to provide the NHS with a discount.
The cost of pomalidomide is £8884 per 21-tablet pack (excluding tax). The average cost of a course of treatment is £44,420 (excluding tax).
The discount Celgene will provide to the NHS is confidential.
NICE’s final appraisal determination on pomalidomide is now with consultees who have the opportunity to appeal against it. If there is no appeal, or an appeal is not upheld, the final appraisal determination is issued by NICE as a guidance.
The final guidance is expected in January 2017. Once NICE issues a final guidance on pomalidomide, the NHS must make the drug available within 3 months. ![]()

Photo from Business Wire
The National Institute for Health and Care Excellence (NICE) has issued a final appraisal determination recommending that pomalidomide be made available through the National Health Service (NHS).
NICE is recommending pomalidomide be available for use in combination with low-dose dexamethasone to treat adults with multiple myeloma who have received at least 3 previous treatments, including lenalidomide and bortezomib.
NICE previously evaluated pomalidomide in 2015 and said it could not recommend the drug, as analyses suggested pomalidomide doesn’t provide enough benefit to justify its high price.
Since that time, a committee advising NICE has reviewed additional data on pomalidomide.
And Celgene, the company that makes pomalidomide, has agreed to provide the NHS with a discount.
The cost of pomalidomide is £8884 per 21-tablet pack (excluding tax). The average cost of a course of treatment is £44,420 (excluding tax).
The discount Celgene will provide to the NHS is confidential.
NICE’s final appraisal determination on pomalidomide is now with consultees who have the opportunity to appeal against it. If there is no appeal, or an appeal is not upheld, the final appraisal determination is issued by NICE as a guidance.
The final guidance is expected in January 2017. Once NICE issues a final guidance on pomalidomide, the NHS must make the drug available within 3 months. ![]()