User login
IMF denounces report on newer MM drugs

Photo by Bill Branson
A report assessing the value of newer multiple myeloma (MM) treatments “dangerously oversimplifies” a complex issue and could limit patients’ access to treatment, according to the International Myeloma Foundation (IMF).
The report, which was drafted by the Institute for Clinical and Economic Review (ICER), is scheduled to be discussed at the inaugural meeting of the Midwest Comparative Effectiveness Public Advisory Council (Midwest CEPAC) in St. Louis, Missouri, on May 26.
The main conclusion of ICER’s report was that newer second- and third-line treatment regimens for MM appear to confer clinical benefits, but the estimated cost-effectiveness of these regimens exceeds commonly cited thresholds.
For example, ICER said that, based on the available data, there was “moderate certainty” that carfilzomib (CFZ), ixazomib (IX), or elotuzumab (ELO) given in combination with lenalidomide and dexamethasone (LEN+DEX) can provide an incremental or better net health benefit for second-line, third-line, or subsequent therapy in adults with relapsed/refractory MM, relative to LEN+DEX alone.
However, the estimated cost-effectiveness, compared to LEN+DEX, was $200,000 per quality-adjusted life year (QALY) gained for CFZ+LEN+DEX, $428,000 for ELO+LEN+DEX, and $434,000 for IX+LEN+DEX. All of these exceed commonly cited thresholds of $50,000 to $150,000 per QALY.
ICER said achieving levels of value more closely aligned with patient benefit would require substantial discounts from the list price in many cases. In other cases, there is no realistic price for the newest agents that would achieve these thresholds.
IMF’s response
IMF said ICER’s report has a few flaws—namely, the absence of many newer MM drugs and combinations, the use of inaccurate data, and an underestimation of QALYs.
Furthermore, IMF said it is concerned that, if ICER's recommendations were to be adopted by the Centers for Medicare & Medicaid Services, patients might be required to “fail first” before other, possibly more effective drugs would be an option.
“We believe that the IMF’s research body, the International Myeloma Working Group (IMWG), will produce superior patient-centered and research-supported guidelines to effectively impact drug costs at our annual summit in June,” said IMF Chairman Brian Durie.
IMWG plans to focus on healthcare cost containment in a special session at the 2016 IMWG Summit, which is scheduled to take place June 7-9 in Copenhagen, Denmark.
IMF said the guidelines resulting from this session should be available in about 2 months. And they will spell out primary and secondary recommendations that allow for individualized therapy choices based on unique features of the disease, patient and/or physician preference, and local and/or regional access issues. ![]()

Photo by Bill Branson
A report assessing the value of newer multiple myeloma (MM) treatments “dangerously oversimplifies” a complex issue and could limit patients’ access to treatment, according to the International Myeloma Foundation (IMF).
The report, which was drafted by the Institute for Clinical and Economic Review (ICER), is scheduled to be discussed at the inaugural meeting of the Midwest Comparative Effectiveness Public Advisory Council (Midwest CEPAC) in St. Louis, Missouri, on May 26.
The main conclusion of ICER’s report was that newer second- and third-line treatment regimens for MM appear to confer clinical benefits, but the estimated cost-effectiveness of these regimens exceeds commonly cited thresholds.
For example, ICER said that, based on the available data, there was “moderate certainty” that carfilzomib (CFZ), ixazomib (IX), or elotuzumab (ELO) given in combination with lenalidomide and dexamethasone (LEN+DEX) can provide an incremental or better net health benefit for second-line, third-line, or subsequent therapy in adults with relapsed/refractory MM, relative to LEN+DEX alone.
However, the estimated cost-effectiveness, compared to LEN+DEX, was $200,000 per quality-adjusted life year (QALY) gained for CFZ+LEN+DEX, $428,000 for ELO+LEN+DEX, and $434,000 for IX+LEN+DEX. All of these exceed commonly cited thresholds of $50,000 to $150,000 per QALY.
ICER said achieving levels of value more closely aligned with patient benefit would require substantial discounts from the list price in many cases. In other cases, there is no realistic price for the newest agents that would achieve these thresholds.
IMF’s response
IMF said ICER’s report has a few flaws—namely, the absence of many newer MM drugs and combinations, the use of inaccurate data, and an underestimation of QALYs.
Furthermore, IMF said it is concerned that, if ICER's recommendations were to be adopted by the Centers for Medicare & Medicaid Services, patients might be required to “fail first” before other, possibly more effective drugs would be an option.
“We believe that the IMF’s research body, the International Myeloma Working Group (IMWG), will produce superior patient-centered and research-supported guidelines to effectively impact drug costs at our annual summit in June,” said IMF Chairman Brian Durie.
IMWG plans to focus on healthcare cost containment in a special session at the 2016 IMWG Summit, which is scheduled to take place June 7-9 in Copenhagen, Denmark.
IMF said the guidelines resulting from this session should be available in about 2 months. And they will spell out primary and secondary recommendations that allow for individualized therapy choices based on unique features of the disease, patient and/or physician preference, and local and/or regional access issues. ![]()

Photo by Bill Branson
A report assessing the value of newer multiple myeloma (MM) treatments “dangerously oversimplifies” a complex issue and could limit patients’ access to treatment, according to the International Myeloma Foundation (IMF).
The report, which was drafted by the Institute for Clinical and Economic Review (ICER), is scheduled to be discussed at the inaugural meeting of the Midwest Comparative Effectiveness Public Advisory Council (Midwest CEPAC) in St. Louis, Missouri, on May 26.
The main conclusion of ICER’s report was that newer second- and third-line treatment regimens for MM appear to confer clinical benefits, but the estimated cost-effectiveness of these regimens exceeds commonly cited thresholds.
For example, ICER said that, based on the available data, there was “moderate certainty” that carfilzomib (CFZ), ixazomib (IX), or elotuzumab (ELO) given in combination with lenalidomide and dexamethasone (LEN+DEX) can provide an incremental or better net health benefit for second-line, third-line, or subsequent therapy in adults with relapsed/refractory MM, relative to LEN+DEX alone.
However, the estimated cost-effectiveness, compared to LEN+DEX, was $200,000 per quality-adjusted life year (QALY) gained for CFZ+LEN+DEX, $428,000 for ELO+LEN+DEX, and $434,000 for IX+LEN+DEX. All of these exceed commonly cited thresholds of $50,000 to $150,000 per QALY.
ICER said achieving levels of value more closely aligned with patient benefit would require substantial discounts from the list price in many cases. In other cases, there is no realistic price for the newest agents that would achieve these thresholds.
IMF’s response
IMF said ICER’s report has a few flaws—namely, the absence of many newer MM drugs and combinations, the use of inaccurate data, and an underestimation of QALYs.
Furthermore, IMF said it is concerned that, if ICER's recommendations were to be adopted by the Centers for Medicare & Medicaid Services, patients might be required to “fail first” before other, possibly more effective drugs would be an option.
“We believe that the IMF’s research body, the International Myeloma Working Group (IMWG), will produce superior patient-centered and research-supported guidelines to effectively impact drug costs at our annual summit in June,” said IMF Chairman Brian Durie.
IMWG plans to focus on healthcare cost containment in a special session at the 2016 IMWG Summit, which is scheduled to take place June 7-9 in Copenhagen, Denmark.
IMF said the guidelines resulting from this session should be available in about 2 months. And they will spell out primary and secondary recommendations that allow for individualized therapy choices based on unique features of the disease, patient and/or physician preference, and local and/or regional access issues. ![]()
EC grants drug conditional approval to treat MM

Photo courtesy of Janssen
The European Commission (EC) has granted conditional marketing authorization for daratumumab (Darzalex), a monoclonal antibody targeting CD38.
The conditional approval is for daratumumab as monotherapy in adults with relapsed and refractory multiple myeloma (MM) who progressed on their last therapy and have received treatment with a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is the first human CD38 monoclonal antibody approved for use in Europe.
About conditional marketing authorization
A product may receive conditional marketing authorization if the EC finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells. In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The EC’s decision to grant conditional marketing authorization for daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies of daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen Biotech, Inc., has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()

Photo courtesy of Janssen
The European Commission (EC) has granted conditional marketing authorization for daratumumab (Darzalex), a monoclonal antibody targeting CD38.
The conditional approval is for daratumumab as monotherapy in adults with relapsed and refractory multiple myeloma (MM) who progressed on their last therapy and have received treatment with a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is the first human CD38 monoclonal antibody approved for use in Europe.
About conditional marketing authorization
A product may receive conditional marketing authorization if the EC finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells. In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The EC’s decision to grant conditional marketing authorization for daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies of daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen Biotech, Inc., has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()

Photo courtesy of Janssen
The European Commission (EC) has granted conditional marketing authorization for daratumumab (Darzalex), a monoclonal antibody targeting CD38.
The conditional approval is for daratumumab as monotherapy in adults with relapsed and refractory multiple myeloma (MM) who progressed on their last therapy and have received treatment with a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is the first human CD38 monoclonal antibody approved for use in Europe.
About conditional marketing authorization
A product may receive conditional marketing authorization if the EC finds that, although comprehensive clinical data on the safety and efficacy of the product are not available, all of the following requirements are met:
- The risk-benefit balance of the product is positive
- The company developing the product will likely be in a position to provide comprehensive clinical data in the future
- Unmet medical needs will be fulfilled
- The benefit to public health of the immediate availability of the product outweighs the risk inherent in the fact that additional data are still required.
Conditional marketing authorizations are valid for 1 year, on a renewable basis. The holder will be required to complete ongoing studies or to conduct new studies with a view to confirming that the benefit-risk balance of a product is positive. In addition, specific obligations may be imposed in relation to the collection of pharmacovigilance data.
About daratumumab
Daratumumab works by binding to CD38, a signaling molecule highly expressed on the surface of MM cells. In binding to CD38, daratumumab triggers the patient’s own immune system to attack the cancer cells, resulting in tumor cell death through multiple, immune-mediated mechanisms of action and through immunomodulatory effects, in addition to direct tumor cell death via apoptosis.
The EC’s decision to grant conditional marketing authorization for daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and 3 additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies of daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the drug’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen Biotech, Inc., has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. Janssen licensed daratumumab from Genmab A/S in August 2012. ![]()
Study reveals how BET inhibitors kill cancer cells

Image courtesy of PNAS
Researchers say they have determined how BET inhibitors fight hematologic malignancies.
Previous studies showed that BET inhibitors are effective at halting tumor growth, but it wasn’t clear whether the drugs kill cancer cells outright or merely pause their growth.
The new study provides an answer and reveals potential ways in which cancer cells may develop resistance to BET inhibitors.
The findings have been published in Leukaemia.
Researchers tested the BET inhibitors JQ1 and IBET151 in a range of hematopoietic cancer cell lines (leukemias, lymphomas, and multiple myeloma) and in mice (with and without malignancy).
The team found that JQ1’s ability to kill cancer cells principally relies on the activation of BAX/BAK-dependent mitochondrial apoptosis. They said this is largely triggered by upregulation of the protein BIM when BET inhibitors suppress miR-17-92, a post-transcriptional repressor of BIM expression.
“We found that when apoptosis was impaired—for instance, by loss of BIM—the BET inhibitors were no longer effective,” said study author Zhen Xu, PhD, of Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“This suggests that cancer cells that acquire mutations in genes that drive apoptosis will lose sensitivity to BET inhibitors and thus will be able to survive treatment, leading to disease relapse.”
The researchers also found that BET inhibitors could induce apoptosis in normal hematopoietic cells, particularly those of lymphoid origin. The team said this suggests the cells’ susceptibility to BET inhibitors did not arise from oncogenic transformation.
These findings could help researchers improve strategies for using BET inhibitors to treat cancers, according to study author Stefan Glaser, PhD, of the Walter and Eliza Hall Institute of Medical Research.
“Understanding how the drugs work gives us the opportunity to investigate new treatments—for example, by using combination therapies or altering the dosage and timing of treatment to prevent drug resistance from emerging,” Dr Glaser said. ![]()

Image courtesy of PNAS
Researchers say they have determined how BET inhibitors fight hematologic malignancies.
Previous studies showed that BET inhibitors are effective at halting tumor growth, but it wasn’t clear whether the drugs kill cancer cells outright or merely pause their growth.
The new study provides an answer and reveals potential ways in which cancer cells may develop resistance to BET inhibitors.
The findings have been published in Leukaemia.
Researchers tested the BET inhibitors JQ1 and IBET151 in a range of hematopoietic cancer cell lines (leukemias, lymphomas, and multiple myeloma) and in mice (with and without malignancy).
The team found that JQ1’s ability to kill cancer cells principally relies on the activation of BAX/BAK-dependent mitochondrial apoptosis. They said this is largely triggered by upregulation of the protein BIM when BET inhibitors suppress miR-17-92, a post-transcriptional repressor of BIM expression.
“We found that when apoptosis was impaired—for instance, by loss of BIM—the BET inhibitors were no longer effective,” said study author Zhen Xu, PhD, of Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“This suggests that cancer cells that acquire mutations in genes that drive apoptosis will lose sensitivity to BET inhibitors and thus will be able to survive treatment, leading to disease relapse.”
The researchers also found that BET inhibitors could induce apoptosis in normal hematopoietic cells, particularly those of lymphoid origin. The team said this suggests the cells’ susceptibility to BET inhibitors did not arise from oncogenic transformation.
These findings could help researchers improve strategies for using BET inhibitors to treat cancers, according to study author Stefan Glaser, PhD, of the Walter and Eliza Hall Institute of Medical Research.
“Understanding how the drugs work gives us the opportunity to investigate new treatments—for example, by using combination therapies or altering the dosage and timing of treatment to prevent drug resistance from emerging,” Dr Glaser said. ![]()

Image courtesy of PNAS
Researchers say they have determined how BET inhibitors fight hematologic malignancies.
Previous studies showed that BET inhibitors are effective at halting tumor growth, but it wasn’t clear whether the drugs kill cancer cells outright or merely pause their growth.
The new study provides an answer and reveals potential ways in which cancer cells may develop resistance to BET inhibitors.
The findings have been published in Leukaemia.
Researchers tested the BET inhibitors JQ1 and IBET151 in a range of hematopoietic cancer cell lines (leukemias, lymphomas, and multiple myeloma) and in mice (with and without malignancy).
The team found that JQ1’s ability to kill cancer cells principally relies on the activation of BAX/BAK-dependent mitochondrial apoptosis. They said this is largely triggered by upregulation of the protein BIM when BET inhibitors suppress miR-17-92, a post-transcriptional repressor of BIM expression.
“We found that when apoptosis was impaired—for instance, by loss of BIM—the BET inhibitors were no longer effective,” said study author Zhen Xu, PhD, of Walter and Eliza Hall Institute of Medical Research in Melbourne, Victoria, Australia.
“This suggests that cancer cells that acquire mutations in genes that drive apoptosis will lose sensitivity to BET inhibitors and thus will be able to survive treatment, leading to disease relapse.”
The researchers also found that BET inhibitors could induce apoptosis in normal hematopoietic cells, particularly those of lymphoid origin. The team said this suggests the cells’ susceptibility to BET inhibitors did not arise from oncogenic transformation.
These findings could help researchers improve strategies for using BET inhibitors to treat cancers, according to study author Stefan Glaser, PhD, of the Walter and Eliza Hall Institute of Medical Research.
“Understanding how the drugs work gives us the opportunity to investigate new treatments—for example, by using combination therapies or altering the dosage and timing of treatment to prevent drug resistance from emerging,” Dr Glaser said. ![]()
Fertility concerns of female cancer survivors

Photo by Vera Kratochvil
A new study indicates that many young adult female cancer survivors do not receive adequate information about their fertility as part of their survivorship care, despite having concerns about their ability to bear children in the future.
The research, published in Cancer, suggests a need for better resources to support cancer survivors in making informed decisions about their reproductive options after they complete treatment.
To conduct this study, Catherine Benedict, PhD, of North Shore-Long Island Jewish Medical Center in Manhasset, New York, and her colleagues asked female cancer survivors to complete a web-based, anonymous survey.
There were 346 participants. They had an average age of 29.9 and had completed treatment an average of 4.9 years earlier.
The investigators focused on a subgroup of 179 women with uncertain fertility status who had not previously undergone or attempted fertility preservation, either before or after their cancer treatment, and who either wanted future children or were unsure.
Many of these women said they did not have enough information concerning their risk of infertility (58%), risk of early menopause (60%), options to assess their fertility (62%), options to preserve their fertility (51%), or options for alternative family building (43%).
The women’s greatest reproductive concerns were potential fertility problems and the health of a future child. Sixty-four percent of the women said they were concerned about not being able to have children (or more children), and 59% were worried about passing the risk of cancer on to their future children.
Only 13% of women said they were well informed about options for preserving fertility, and 74% were unclear about their personal values regarding fertility preservation.
Seventy percent of the women said they hadn’t received enough advice on fertility preservation, and 35% said they didn’t have enough support to make a decision about fertility preservation.
The investigators found a significant association between greater unmet information needs and higher levels of decisional conflict about fertility preservation (P<0.001).
On the other hand, having undergone a fertility evaluation after treatment was associated with lower decisional conflict (P=0.02).
The investigators said these findings establish the need for support services to help young female cancer survivors make decisions about fertility preservation and family-building as part of survivorship care.
The literature has largely focused on the clinical and support needs of women making fertility decisions before their treatment begins, but most patients do not preserve their fertility before treatment for a number of reasons, despite wanting children in the future.
“The potential loss of fertility has been described in the literature as being almost as painful, if not more so, than the cancer diagnosis itself,” Dr Benedict said.
“Failure to provide information and address concerns with respect to fertility-related decisions may have lasting consequences for young women who hope to move on from their cancer experience to achieve important life goals such as having children. For women at risk for early menopause, delaying fertility-related decisions may cause them to miss their narrowed window of opportunity to preserve their fertility, if desired.” ![]()

Photo by Vera Kratochvil
A new study indicates that many young adult female cancer survivors do not receive adequate information about their fertility as part of their survivorship care, despite having concerns about their ability to bear children in the future.
The research, published in Cancer, suggests a need for better resources to support cancer survivors in making informed decisions about their reproductive options after they complete treatment.
To conduct this study, Catherine Benedict, PhD, of North Shore-Long Island Jewish Medical Center in Manhasset, New York, and her colleagues asked female cancer survivors to complete a web-based, anonymous survey.
There were 346 participants. They had an average age of 29.9 and had completed treatment an average of 4.9 years earlier.
The investigators focused on a subgroup of 179 women with uncertain fertility status who had not previously undergone or attempted fertility preservation, either before or after their cancer treatment, and who either wanted future children or were unsure.
Many of these women said they did not have enough information concerning their risk of infertility (58%), risk of early menopause (60%), options to assess their fertility (62%), options to preserve their fertility (51%), or options for alternative family building (43%).
The women’s greatest reproductive concerns were potential fertility problems and the health of a future child. Sixty-four percent of the women said they were concerned about not being able to have children (or more children), and 59% were worried about passing the risk of cancer on to their future children.
Only 13% of women said they were well informed about options for preserving fertility, and 74% were unclear about their personal values regarding fertility preservation.
Seventy percent of the women said they hadn’t received enough advice on fertility preservation, and 35% said they didn’t have enough support to make a decision about fertility preservation.
The investigators found a significant association between greater unmet information needs and higher levels of decisional conflict about fertility preservation (P<0.001).
On the other hand, having undergone a fertility evaluation after treatment was associated with lower decisional conflict (P=0.02).
The investigators said these findings establish the need for support services to help young female cancer survivors make decisions about fertility preservation and family-building as part of survivorship care.
The literature has largely focused on the clinical and support needs of women making fertility decisions before their treatment begins, but most patients do not preserve their fertility before treatment for a number of reasons, despite wanting children in the future.
“The potential loss of fertility has been described in the literature as being almost as painful, if not more so, than the cancer diagnosis itself,” Dr Benedict said.
“Failure to provide information and address concerns with respect to fertility-related decisions may have lasting consequences for young women who hope to move on from their cancer experience to achieve important life goals such as having children. For women at risk for early menopause, delaying fertility-related decisions may cause them to miss their narrowed window of opportunity to preserve their fertility, if desired.” ![]()

Photo by Vera Kratochvil
A new study indicates that many young adult female cancer survivors do not receive adequate information about their fertility as part of their survivorship care, despite having concerns about their ability to bear children in the future.
The research, published in Cancer, suggests a need for better resources to support cancer survivors in making informed decisions about their reproductive options after they complete treatment.
To conduct this study, Catherine Benedict, PhD, of North Shore-Long Island Jewish Medical Center in Manhasset, New York, and her colleagues asked female cancer survivors to complete a web-based, anonymous survey.
There were 346 participants. They had an average age of 29.9 and had completed treatment an average of 4.9 years earlier.
The investigators focused on a subgroup of 179 women with uncertain fertility status who had not previously undergone or attempted fertility preservation, either before or after their cancer treatment, and who either wanted future children or were unsure.
Many of these women said they did not have enough information concerning their risk of infertility (58%), risk of early menopause (60%), options to assess their fertility (62%), options to preserve their fertility (51%), or options for alternative family building (43%).
The women’s greatest reproductive concerns were potential fertility problems and the health of a future child. Sixty-four percent of the women said they were concerned about not being able to have children (or more children), and 59% were worried about passing the risk of cancer on to their future children.
Only 13% of women said they were well informed about options for preserving fertility, and 74% were unclear about their personal values regarding fertility preservation.
Seventy percent of the women said they hadn’t received enough advice on fertility preservation, and 35% said they didn’t have enough support to make a decision about fertility preservation.
The investigators found a significant association between greater unmet information needs and higher levels of decisional conflict about fertility preservation (P<0.001).
On the other hand, having undergone a fertility evaluation after treatment was associated with lower decisional conflict (P=0.02).
The investigators said these findings establish the need for support services to help young female cancer survivors make decisions about fertility preservation and family-building as part of survivorship care.
The literature has largely focused on the clinical and support needs of women making fertility decisions before their treatment begins, but most patients do not preserve their fertility before treatment for a number of reasons, despite wanting children in the future.
“The potential loss of fertility has been described in the literature as being almost as painful, if not more so, than the cancer diagnosis itself,” Dr Benedict said.
“Failure to provide information and address concerns with respect to fertility-related decisions may have lasting consequences for young women who hope to move on from their cancer experience to achieve important life goals such as having children. For women at risk for early menopause, delaying fertility-related decisions may cause them to miss their narrowed window of opportunity to preserve their fertility, if desired.” ![]()
Upfront ASCT still preferred for young MM patients

Photo by Chad McNeeley
CHICAGO—An interim analysis of a large, phase 3 study has confirmed that upfront autologous stem cell transplantation (ASCT) is still the preferred
treatment for newly diagnosed, young multiple myeloma (MM) patients, even in the age of novel agents such as bortezomib.
Investigators compared 4 cycles of bortezomib-melphalan-prednisone (VMP) with high-dose melphalan (HDM) and single or double ASCT, depending upon the policy of the treating institution.
At a median follow-up of 24 months, the 3-year progression-free survival (PFS) was significantly better for patients who had received ASCT.
Michele Cavo, MD, of Seràgnoli Institute of Hematology in Bologna, Italy, reported the results of this first interim analysis of the European Myeloma Network trial (EMN/HO95 MM) at a press briefing preceding the 2016 ASCO Annual Meeting. More details will be presented at the meeting itself (abstract 8000).
Study investigators enrolled 1503 patients from February 2011 through April 2014. They performed the specified interim analysis in January 2016.
Patients were 65 years or younger, and all received bortezomib-based induction therapy followed by stem cell collection. Investigators then randomized 1266 patients to receive either VMP (n=754) or HDM plus single or double ASCT (n=512).
Patients underwent a second randomization to either 2 cycles of bortezomib-based consolidation or no consolidation therapy.
All patients received lenalidomide maintenance until disease progression. The primary endpoint was PFS after the first randomization.
Results
PFS was significantly longer in patients who had received a transplant, with a hazard ratio (HR) of 0.76, 95% confidence interval (CI) of 0.61-0.94, and P value of 0.01.
This benefit held true for patients with revised ISS stage III (HR=0.52, 95% CI 0.32-0.84, P=0.01).
And patients with high-risk cytogenetics also retained the benefit (HR=0.72, 95% CI 0.54-0.97, P=0.03). High-risk was defined as t(4;14), del(17p), del(1p), or gain of 1q.
Investigators also performed a multivariate analysis and found randomization to the HDM arm to be an independent predictor of prolonged PFS (HR=0.61, 95% CI 0.45-0.82, P=0.001).
There was no significant difference between the 2 arms in terms of stringent complete response and complete response.
However, when very good partial response was included in the best-response analysis, patients in the transplant arm fared significantly better (P<0.0001) than patients in the VMP arm—84% and 74%, respectively.
Investigators have not yet completed the interim data analysis related to the second randomization. The study is ongoing, and future analyses will include overall survival, toxicity, quality of life, and other measures.
This study was funded by the Haemato Oncology Foundation for Adults in the Netherlands (HOVON). ![]()

Photo by Chad McNeeley
CHICAGO—An interim analysis of a large, phase 3 study has confirmed that upfront autologous stem cell transplantation (ASCT) is still the preferred
treatment for newly diagnosed, young multiple myeloma (MM) patients, even in the age of novel agents such as bortezomib.
Investigators compared 4 cycles of bortezomib-melphalan-prednisone (VMP) with high-dose melphalan (HDM) and single or double ASCT, depending upon the policy of the treating institution.
At a median follow-up of 24 months, the 3-year progression-free survival (PFS) was significantly better for patients who had received ASCT.
Michele Cavo, MD, of Seràgnoli Institute of Hematology in Bologna, Italy, reported the results of this first interim analysis of the European Myeloma Network trial (EMN/HO95 MM) at a press briefing preceding the 2016 ASCO Annual Meeting. More details will be presented at the meeting itself (abstract 8000).
Study investigators enrolled 1503 patients from February 2011 through April 2014. They performed the specified interim analysis in January 2016.
Patients were 65 years or younger, and all received bortezomib-based induction therapy followed by stem cell collection. Investigators then randomized 1266 patients to receive either VMP (n=754) or HDM plus single or double ASCT (n=512).
Patients underwent a second randomization to either 2 cycles of bortezomib-based consolidation or no consolidation therapy.
All patients received lenalidomide maintenance until disease progression. The primary endpoint was PFS after the first randomization.
Results
PFS was significantly longer in patients who had received a transplant, with a hazard ratio (HR) of 0.76, 95% confidence interval (CI) of 0.61-0.94, and P value of 0.01.
This benefit held true for patients with revised ISS stage III (HR=0.52, 95% CI 0.32-0.84, P=0.01).
And patients with high-risk cytogenetics also retained the benefit (HR=0.72, 95% CI 0.54-0.97, P=0.03). High-risk was defined as t(4;14), del(17p), del(1p), or gain of 1q.
Investigators also performed a multivariate analysis and found randomization to the HDM arm to be an independent predictor of prolonged PFS (HR=0.61, 95% CI 0.45-0.82, P=0.001).
There was no significant difference between the 2 arms in terms of stringent complete response and complete response.
However, when very good partial response was included in the best-response analysis, patients in the transplant arm fared significantly better (P<0.0001) than patients in the VMP arm—84% and 74%, respectively.
Investigators have not yet completed the interim data analysis related to the second randomization. The study is ongoing, and future analyses will include overall survival, toxicity, quality of life, and other measures.
This study was funded by the Haemato Oncology Foundation for Adults in the Netherlands (HOVON). ![]()

Photo by Chad McNeeley
CHICAGO—An interim analysis of a large, phase 3 study has confirmed that upfront autologous stem cell transplantation (ASCT) is still the preferred
treatment for newly diagnosed, young multiple myeloma (MM) patients, even in the age of novel agents such as bortezomib.
Investigators compared 4 cycles of bortezomib-melphalan-prednisone (VMP) with high-dose melphalan (HDM) and single or double ASCT, depending upon the policy of the treating institution.
At a median follow-up of 24 months, the 3-year progression-free survival (PFS) was significantly better for patients who had received ASCT.
Michele Cavo, MD, of Seràgnoli Institute of Hematology in Bologna, Italy, reported the results of this first interim analysis of the European Myeloma Network trial (EMN/HO95 MM) at a press briefing preceding the 2016 ASCO Annual Meeting. More details will be presented at the meeting itself (abstract 8000).
Study investigators enrolled 1503 patients from February 2011 through April 2014. They performed the specified interim analysis in January 2016.
Patients were 65 years or younger, and all received bortezomib-based induction therapy followed by stem cell collection. Investigators then randomized 1266 patients to receive either VMP (n=754) or HDM plus single or double ASCT (n=512).
Patients underwent a second randomization to either 2 cycles of bortezomib-based consolidation or no consolidation therapy.
All patients received lenalidomide maintenance until disease progression. The primary endpoint was PFS after the first randomization.
Results
PFS was significantly longer in patients who had received a transplant, with a hazard ratio (HR) of 0.76, 95% confidence interval (CI) of 0.61-0.94, and P value of 0.01.
This benefit held true for patients with revised ISS stage III (HR=0.52, 95% CI 0.32-0.84, P=0.01).
And patients with high-risk cytogenetics also retained the benefit (HR=0.72, 95% CI 0.54-0.97, P=0.03). High-risk was defined as t(4;14), del(17p), del(1p), or gain of 1q.
Investigators also performed a multivariate analysis and found randomization to the HDM arm to be an independent predictor of prolonged PFS (HR=0.61, 95% CI 0.45-0.82, P=0.001).
There was no significant difference between the 2 arms in terms of stringent complete response and complete response.
However, when very good partial response was included in the best-response analysis, patients in the transplant arm fared significantly better (P<0.0001) than patients in the VMP arm—84% and 74%, respectively.
Investigators have not yet completed the interim data analysis related to the second randomization. The study is ongoing, and future analyses will include overall survival, toxicity, quality of life, and other measures.
This study was funded by the Haemato Oncology Foundation for Adults in the Netherlands (HOVON). ![]()
Physical activity may lower risk of some cancers

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts. ![]()

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts. ![]()

Photo by K. Johansson
Being physically active during leisure time may lower a person’s risk of certain cancers, according to a new study.
A high level of physical activity was associated with a 20% lower risk of myeloid leukemia, a 17% lower risk of myeloma, a 9% lower risk of non-Hodgkin lymphoma, and a 7% lower risk of cancer in general.
On the other hand, a high level of physical activity was also associated with a higher risk of malignant melanoma and prostate cancer.
Steven C. Moore, PhD, of the National Cancer Institute in Bethesda, Maryland, and his colleagues reported these findings in JAMA Internal Medicine.
The researchers pooled data from 12 US and European study cohorts with self-reported physical activity (1987-2004). And they analyzed associations between physical activity and 26 types of cancer.
The study included 1.4 million participants, and 186,932 cancers were identified during a median of 11 years of follow-up.
Compared with the lowest level of leisure-time physical activity (10th percentile), the highest level of activity (90th percentile) had strong inverse associations (a 20% or greater reduction in risk) for 7 cancer types:
- Myeloid leukemia (hazard ratio [HR]=0.80 [95% CI, 0.70-0.92])
- Esophageal adenocarcinoma (HR=0.58 [95% CI, 0.37-0.89])
- Liver cancer (HR=0.73 [95% CI, 0.55-0.98])
- Lung cancer (HR=0.74 [95% CI, 0.71-0.77])
- Kidney cancer (HR=0.77 [95% CI, 0.70-0.85])
- Gastric cardia (HR=0.78 [95% CI, 0.64-0.95])
- Endometrial cancer (HR=0.79 [95% CI, 0.68-0.92]).
There were moderate inverse associations (a 10% to 20% reduction in risk) between the highest level of activity and 6 cancers:
- Myeloma (HR=0.83 [95% CI, 0.72-0.95])
- Colon cancer (HR=0.84 [95% CI, 0.77-0.91])
- Head and neck cancer (HR=0.85 [95% CI, 0.78-0.93])
- Rectal cancer (HR=0.87 [95% CI, 0.80-0.95])
- Bladder cancer (HR=0.87 [95% CI, 0.82-0.92])
- Breast cancer (HR=0.90 [95% CI, 0.87-0.93]).
And there were suggestive inverse associations between the highest level of activity and 3 cancers:
- Non-Hodgkin lymphoma (HR=0.91 [95% CI, 0.83-1.00])
- Gallbladder cancer (HR=0.72 [95% CI, 0.51-1.01])
- Small intestine cancer (HR=0.78 [95% CI, 0.60-1.00]).
However, the highest level of activity was also associated with an increased risk of prostate cancer (HR=1.05 [95% CI, 1.03-1.08]) and malignant melanoma (HR=1.27 [95% CI, 1.16-1.40]).
The researchers said the main limitation of this study is that they cannot fully exclude the possibility that diet, smoking, and other factors may have affected these results. Also, the study used self-reported physical activity, which can mean errors in recall.
Still, the team said these findings support promoting physical activity as a key component of population-wide cancer prevention and control efforts. ![]()
FDA warns of counterfeit BiCNU
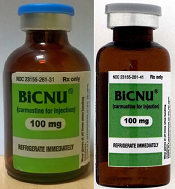
shown on the left and a
counterfeit vial on the right
Photo courtesy of the FDA
The US Food and Drug Administration (FDA) is warning healthcare professionals that a counterfeit version of BiCNU (carmustine for injection) 100 mg has been detected in some foreign countries.
The agency said there is no indication that counterfeit BiCNU has entered the legitimate US drug supply chain and no indication that any US patients have received counterfeit BiCNU.
Still, the FDA is advising that healthcare professionals inspect BiCNU vials as an added precaution to ensure the product administered to patients is authentic.
BiCNU is approved to treat brain cancers, multiple myeloma, and lymphoma. It is manufactured by Emcure Pharmaceuticals Ltd. and distributed in the US by Heritage Pharmaceuticals Inc.
Heritage previously announced that counterfeit BiCNU had been found in India, Ireland, and Israel.
How to identify counterfeit BiCNU
BiCNU is available as a vial of BiCNU and dehydrated alcohol co-packaged together.
While the NDC on the outer package of the authentic and counterfeit versions might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging. The authentic product has a blue flip top, while the counterfeit product may have a gray flip top.
The product may also be counterfeit if the vial displays the following lot numbers, batch numbers, manufacturing dates, and expiration dates.
| Product | Expiration
date |
Manufacturing
date |
Lot number | Batch number |
| BiCNU | 01/18 | 2/16 | BCEM771322 | EM/BC20161990 |
| Diluent | 01/18 | 2/16 | SBCDA224736 | EM/BCD2220 |
| BiCNU | 12/17 | 1/16 | BCEM771318 | EM/BC20151896 |
| Diluent | 12/17 | 1/16 | SBCDA224732 | EM/BCD2216 |
| BiCNU | 10/17 | 11/15 | BCEM771317 | EM/BC20151895 |
| Diluent | 10/17 | 11/15 | SBCDA224731 | EM/BCD2215 |
The FDA urges healthcare professionals to purchase drug products only from legitimate suppliers.
Healthcare professionals are encouraged to report sales solicitation of suspect drug products by calling the FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, reporting via OCI’s website, or emailing [email protected].
Healthcare professionals and patients should report adverse events related to the use of any suspect medications to the FDA’s MedWatch Adverse Event Reporting Program. ![]()

shown on the left and a
counterfeit vial on the right
Photo courtesy of the FDA
The US Food and Drug Administration (FDA) is warning healthcare professionals that a counterfeit version of BiCNU (carmustine for injection) 100 mg has been detected in some foreign countries.
The agency said there is no indication that counterfeit BiCNU has entered the legitimate US drug supply chain and no indication that any US patients have received counterfeit BiCNU.
Still, the FDA is advising that healthcare professionals inspect BiCNU vials as an added precaution to ensure the product administered to patients is authentic.
BiCNU is approved to treat brain cancers, multiple myeloma, and lymphoma. It is manufactured by Emcure Pharmaceuticals Ltd. and distributed in the US by Heritage Pharmaceuticals Inc.
Heritage previously announced that counterfeit BiCNU had been found in India, Ireland, and Israel.
How to identify counterfeit BiCNU
BiCNU is available as a vial of BiCNU and dehydrated alcohol co-packaged together.
While the NDC on the outer package of the authentic and counterfeit versions might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging. The authentic product has a blue flip top, while the counterfeit product may have a gray flip top.
The product may also be counterfeit if the vial displays the following lot numbers, batch numbers, manufacturing dates, and expiration dates.
| Product | Expiration
date |
Manufacturing
date |
Lot number | Batch number |
| BiCNU | 01/18 | 2/16 | BCEM771322 | EM/BC20161990 |
| Diluent | 01/18 | 2/16 | SBCDA224736 | EM/BCD2220 |
| BiCNU | 12/17 | 1/16 | BCEM771318 | EM/BC20151896 |
| Diluent | 12/17 | 1/16 | SBCDA224732 | EM/BCD2216 |
| BiCNU | 10/17 | 11/15 | BCEM771317 | EM/BC20151895 |
| Diluent | 10/17 | 11/15 | SBCDA224731 | EM/BCD2215 |
The FDA urges healthcare professionals to purchase drug products only from legitimate suppliers.
Healthcare professionals are encouraged to report sales solicitation of suspect drug products by calling the FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, reporting via OCI’s website, or emailing [email protected].
Healthcare professionals and patients should report adverse events related to the use of any suspect medications to the FDA’s MedWatch Adverse Event Reporting Program. ![]()

shown on the left and a
counterfeit vial on the right
Photo courtesy of the FDA
The US Food and Drug Administration (FDA) is warning healthcare professionals that a counterfeit version of BiCNU (carmustine for injection) 100 mg has been detected in some foreign countries.
The agency said there is no indication that counterfeit BiCNU has entered the legitimate US drug supply chain and no indication that any US patients have received counterfeit BiCNU.
Still, the FDA is advising that healthcare professionals inspect BiCNU vials as an added precaution to ensure the product administered to patients is authentic.
BiCNU is approved to treat brain cancers, multiple myeloma, and lymphoma. It is manufactured by Emcure Pharmaceuticals Ltd. and distributed in the US by Heritage Pharmaceuticals Inc.
Heritage previously announced that counterfeit BiCNU had been found in India, Ireland, and Israel.
How to identify counterfeit BiCNU
BiCNU is available as a vial of BiCNU and dehydrated alcohol co-packaged together.
While the NDC on the outer package of the authentic and counterfeit versions might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging. The authentic product has a blue flip top, while the counterfeit product may have a gray flip top.
The product may also be counterfeit if the vial displays the following lot numbers, batch numbers, manufacturing dates, and expiration dates.
| Product | Expiration
date |
Manufacturing
date |
Lot number | Batch number |
| BiCNU | 01/18 | 2/16 | BCEM771322 | EM/BC20161990 |
| Diluent | 01/18 | 2/16 | SBCDA224736 | EM/BCD2220 |
| BiCNU | 12/17 | 1/16 | BCEM771318 | EM/BC20151896 |
| Diluent | 12/17 | 1/16 | SBCDA224732 | EM/BCD2216 |
| BiCNU | 10/17 | 11/15 | BCEM771317 | EM/BC20151895 |
| Diluent | 10/17 | 11/15 | SBCDA224731 | EM/BCD2215 |
The FDA urges healthcare professionals to purchase drug products only from legitimate suppliers.
Healthcare professionals are encouraged to report sales solicitation of suspect drug products by calling the FDA’s Office of Criminal Investigations (OCI) at 800-551-3989, reporting via OCI’s website, or emailing [email protected].
Healthcare professionals and patients should report adverse events related to the use of any suspect medications to the FDA’s MedWatch Adverse Event Reporting Program.
FDA issues warning about counterfeit BiCNU
A counterfeit version of BiCNU has been detected in foreign countries, the Food and Drug Administration reports.
BiCNU is approved to treat different types of brain cancer, multiple myeloma, and lymphoma (Hodgkin’s and non-Hodgkin’s), manufactured by Emcure Pharmaceuticals, and distributed by Heritage Pharmaceuticals.
There has been no counterfeit BiCNU detected in the United States, but the FDA encourages health care professionals to diligently inspect BiCNU vials before administering the drug to patients.
“While the [National Drug Code] on the outer package of the authentic and counterfeit version might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging,” the FDA reported in a written statement, which includes a list of the counterfeit lots.
To report sales solicitation of suspect drugs call the FDA’s office of criminal investigations at 800-551-3989 or e-mail [email protected]. To report adverse events related to suspect medications, submit a report online at www.fda.gov/medwatch/report.htm.
On Twitter @jess_craig94
A counterfeit version of BiCNU has been detected in foreign countries, the Food and Drug Administration reports.
BiCNU is approved to treat different types of brain cancer, multiple myeloma, and lymphoma (Hodgkin’s and non-Hodgkin’s), manufactured by Emcure Pharmaceuticals, and distributed by Heritage Pharmaceuticals.
There has been no counterfeit BiCNU detected in the United States, but the FDA encourages health care professionals to diligently inspect BiCNU vials before administering the drug to patients.
“While the [National Drug Code] on the outer package of the authentic and counterfeit version might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging,” the FDA reported in a written statement, which includes a list of the counterfeit lots.
To report sales solicitation of suspect drugs call the FDA’s office of criminal investigations at 800-551-3989 or e-mail [email protected]. To report adverse events related to suspect medications, submit a report online at www.fda.gov/medwatch/report.htm.
On Twitter @jess_craig94
A counterfeit version of BiCNU has been detected in foreign countries, the Food and Drug Administration reports.
BiCNU is approved to treat different types of brain cancer, multiple myeloma, and lymphoma (Hodgkin’s and non-Hodgkin’s), manufactured by Emcure Pharmaceuticals, and distributed by Heritage Pharmaceuticals.
There has been no counterfeit BiCNU detected in the United States, but the FDA encourages health care professionals to diligently inspect BiCNU vials before administering the drug to patients.
“While the [National Drug Code] on the outer package of the authentic and counterfeit version might match, the best way to distinguish a counterfeit is to look at the BiCNU vial inside the packaging,” the FDA reported in a written statement, which includes a list of the counterfeit lots.
To report sales solicitation of suspect drugs call the FDA’s office of criminal investigations at 800-551-3989 or e-mail [email protected]. To report adverse events related to suspect medications, submit a report online at www.fda.gov/medwatch/report.htm.
On Twitter @jess_craig94
EC approves first immunostimulatory antibody to treat MM

Photo courtesy of
Bristol-Myers Squibb
The European Commission (EC) has approved elotuzumab (Empliciti) for use in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received at least one prior therapy.
Elotuzumab is an immunostimulatory antibody that specifically targets signaling lymphocyte activation molecule family member 7 (SLAMF7), a cell-surface glycoprotein expressed on myeloma cells, natural killer (NK) cells, plasma cells, and specific immune cell subsets of differentiated cells in the hematopoietic lineage.
Elotuzumab has a dual mechanism of action. It directly activates the immune system through NK cells via the SLAMF7 pathway, and it targets SLAMF7 on myeloma cells, tagging them for NK-cell-mediated destruction via antibody-dependent cellular toxicity.
Elotuzumab is the first immunostimulatory antibody approved to treat MM in the European Union.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities.
Phase 3 trial
The EC approved elotuzumab based on data from the phase 3 ELOQUENT-2 trial, which were presented at ASCO 2015 and published in NEJM.
The trial included 646 MM patients who had received 1 to 3 prior therapies.
The patients were randomized 1:1 to receive either elotuzumab at 10 mg/kg in combination with lenalidomide and dexamethasone (len-dex) or len-dex alone in 4-week cycles until disease progression or unacceptable toxicity.
Baseline patient demographics and disease characteristics were well balanced between treatment arms.
The minimum follow-up for all study subjects was 24 months. The co-primary endpoints were progression-free survival (PFS) and overall response rate.
The overall response rate was 78.5% in the elotuzumab arm and 65.5% in the len-dex arm (P=0.0002).
The median PFS was 19.4 months in the elotuzumab arm and 14.9 months in the len-dex arm (P=0.0004). At 1 year, the PFS was 68% in the elotuzumab arm and 57% in the len-dex arm. At 2 years, the PFS was 41% and 27%, respectively.
The most common adverse events in the elotuzumab arm and len-dex arm, respectively, were fatigue (61.6% vs 51.7%), diarrhea (46.9% vs 36.0%), pyrexia (37.4% vs 24.6%), constipation (35.5% vs 27.1%), cough (34.3% vs 18.9%), peripheral neuropathy (26.7% vs 20.8%), nasopharyngitis (24.5% vs 19.2%), upper respiratory tract infection (22.6% vs 17.4%), decreased appetite (20.8% vs 12.6%), and pneumonia (20.1% vs 14.2%).
Serious adverse events occurred in 65.4% of patients in the elotuzumab arm and 56.5% in the len-dex arm. The most frequent events were pneumonia, pyrexia, respiratory tract infection, anemia, pulmonary embolism, and acute renal failure.

Photo courtesy of
Bristol-Myers Squibb
The European Commission (EC) has approved elotuzumab (Empliciti) for use in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received at least one prior therapy.
Elotuzumab is an immunostimulatory antibody that specifically targets signaling lymphocyte activation molecule family member 7 (SLAMF7), a cell-surface glycoprotein expressed on myeloma cells, natural killer (NK) cells, plasma cells, and specific immune cell subsets of differentiated cells in the hematopoietic lineage.
Elotuzumab has a dual mechanism of action. It directly activates the immune system through NK cells via the SLAMF7 pathway, and it targets SLAMF7 on myeloma cells, tagging them for NK-cell-mediated destruction via antibody-dependent cellular toxicity.
Elotuzumab is the first immunostimulatory antibody approved to treat MM in the European Union.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities.
Phase 3 trial
The EC approved elotuzumab based on data from the phase 3 ELOQUENT-2 trial, which were presented at ASCO 2015 and published in NEJM.
The trial included 646 MM patients who had received 1 to 3 prior therapies.
The patients were randomized 1:1 to receive either elotuzumab at 10 mg/kg in combination with lenalidomide and dexamethasone (len-dex) or len-dex alone in 4-week cycles until disease progression or unacceptable toxicity.
Baseline patient demographics and disease characteristics were well balanced between treatment arms.
The minimum follow-up for all study subjects was 24 months. The co-primary endpoints were progression-free survival (PFS) and overall response rate.
The overall response rate was 78.5% in the elotuzumab arm and 65.5% in the len-dex arm (P=0.0002).
The median PFS was 19.4 months in the elotuzumab arm and 14.9 months in the len-dex arm (P=0.0004). At 1 year, the PFS was 68% in the elotuzumab arm and 57% in the len-dex arm. At 2 years, the PFS was 41% and 27%, respectively.
The most common adverse events in the elotuzumab arm and len-dex arm, respectively, were fatigue (61.6% vs 51.7%), diarrhea (46.9% vs 36.0%), pyrexia (37.4% vs 24.6%), constipation (35.5% vs 27.1%), cough (34.3% vs 18.9%), peripheral neuropathy (26.7% vs 20.8%), nasopharyngitis (24.5% vs 19.2%), upper respiratory tract infection (22.6% vs 17.4%), decreased appetite (20.8% vs 12.6%), and pneumonia (20.1% vs 14.2%).
Serious adverse events occurred in 65.4% of patients in the elotuzumab arm and 56.5% in the len-dex arm. The most frequent events were pneumonia, pyrexia, respiratory tract infection, anemia, pulmonary embolism, and acute renal failure.

Photo courtesy of
Bristol-Myers Squibb
The European Commission (EC) has approved elotuzumab (Empliciti) for use in combination with lenalidomide and dexamethasone to treat patients with multiple myeloma (MM) who have received at least one prior therapy.
Elotuzumab is an immunostimulatory antibody that specifically targets signaling lymphocyte activation molecule family member 7 (SLAMF7), a cell-surface glycoprotein expressed on myeloma cells, natural killer (NK) cells, plasma cells, and specific immune cell subsets of differentiated cells in the hematopoietic lineage.
Elotuzumab has a dual mechanism of action. It directly activates the immune system through NK cells via the SLAMF7 pathway, and it targets SLAMF7 on myeloma cells, tagging them for NK-cell-mediated destruction via antibody-dependent cellular toxicity.
Elotuzumab is the first immunostimulatory antibody approved to treat MM in the European Union.
Bristol-Myers Squibb and AbbVie are co-developing elotuzumab, with Bristol-Myers Squibb solely responsible for commercial activities.
Phase 3 trial
The EC approved elotuzumab based on data from the phase 3 ELOQUENT-2 trial, which were presented at ASCO 2015 and published in NEJM.
The trial included 646 MM patients who had received 1 to 3 prior therapies.
The patients were randomized 1:1 to receive either elotuzumab at 10 mg/kg in combination with lenalidomide and dexamethasone (len-dex) or len-dex alone in 4-week cycles until disease progression or unacceptable toxicity.
Baseline patient demographics and disease characteristics were well balanced between treatment arms.
The minimum follow-up for all study subjects was 24 months. The co-primary endpoints were progression-free survival (PFS) and overall response rate.
The overall response rate was 78.5% in the elotuzumab arm and 65.5% in the len-dex arm (P=0.0002).
The median PFS was 19.4 months in the elotuzumab arm and 14.9 months in the len-dex arm (P=0.0004). At 1 year, the PFS was 68% in the elotuzumab arm and 57% in the len-dex arm. At 2 years, the PFS was 41% and 27%, respectively.
The most common adverse events in the elotuzumab arm and len-dex arm, respectively, were fatigue (61.6% vs 51.7%), diarrhea (46.9% vs 36.0%), pyrexia (37.4% vs 24.6%), constipation (35.5% vs 27.1%), cough (34.3% vs 18.9%), peripheral neuropathy (26.7% vs 20.8%), nasopharyngitis (24.5% vs 19.2%), upper respiratory tract infection (22.6% vs 17.4%), decreased appetite (20.8% vs 12.6%), and pneumonia (20.1% vs 14.2%).
Serious adverse events occurred in 65.4% of patients in the elotuzumab arm and 56.5% in the len-dex arm. The most frequent events were pneumonia, pyrexia, respiratory tract infection, anemia, pulmonary embolism, and acute renal failure.
Company warns of counterfeit drug

Photo by Bill Branson
Heritage Pharmaceuticals Inc., has announced the existence of a counterfeit drug product labeled as BiCNU® (carmustine for injection) 100 mg.
The company said that, to the best of its knowledge, the counterfeit product has only been distributed in India, Ireland, and Israel.
However, Heritage is consulting with the US Food and Drug Administration (FDA) to aid the agency’s evaluations of this product, assist with determining the source of the counterfeit drug, and prevent the further distribution of this product or its introduction into the US.
BiCNU® is primarily used for chemotherapy in the treatment of lymphomas, multiple myeloma, and brain cancers. But the drug is also used for immunosuppression before organ transplant or hematopoietic stem cell transplant.
Heritage said it has directly notified all customers and provided detailed information that will help them identify a counterfeit BiCNU® product. Customers have been instructed to examine their inventory immediately and to quarantine, discontinue distribution of, and return any suspected counterfeit product.
Any customers who may have recently distributed the BiCNU® products to their own customers have been asked to convey this information to their customers so they will be able to carefully examine all BiCNU® products before use and identify the characteristics of a suspected counterfeit product.
Any end users who believe they may have received a counterfeit drug should return the product to the pharmacy that dispensed the medicine.
Any US health practitioners who determine they are in possession of a counterfeit product should contact the FDA through MedWatch. Instructions for such reporting are available on the FDA website.
Anyone with questions about the counterfeit product should contact the Heritage customer call center directly at (866) 901-3784, which is open Monday through Friday, from 9 am to 5 pm EST.

Photo by Bill Branson
Heritage Pharmaceuticals Inc., has announced the existence of a counterfeit drug product labeled as BiCNU® (carmustine for injection) 100 mg.
The company said that, to the best of its knowledge, the counterfeit product has only been distributed in India, Ireland, and Israel.
However, Heritage is consulting with the US Food and Drug Administration (FDA) to aid the agency’s evaluations of this product, assist with determining the source of the counterfeit drug, and prevent the further distribution of this product or its introduction into the US.
BiCNU® is primarily used for chemotherapy in the treatment of lymphomas, multiple myeloma, and brain cancers. But the drug is also used for immunosuppression before organ transplant or hematopoietic stem cell transplant.
Heritage said it has directly notified all customers and provided detailed information that will help them identify a counterfeit BiCNU® product. Customers have been instructed to examine their inventory immediately and to quarantine, discontinue distribution of, and return any suspected counterfeit product.
Any customers who may have recently distributed the BiCNU® products to their own customers have been asked to convey this information to their customers so they will be able to carefully examine all BiCNU® products before use and identify the characteristics of a suspected counterfeit product.
Any end users who believe they may have received a counterfeit drug should return the product to the pharmacy that dispensed the medicine.
Any US health practitioners who determine they are in possession of a counterfeit product should contact the FDA through MedWatch. Instructions for such reporting are available on the FDA website.
Anyone with questions about the counterfeit product should contact the Heritage customer call center directly at (866) 901-3784, which is open Monday through Friday, from 9 am to 5 pm EST.

Photo by Bill Branson
Heritage Pharmaceuticals Inc., has announced the existence of a counterfeit drug product labeled as BiCNU® (carmustine for injection) 100 mg.
The company said that, to the best of its knowledge, the counterfeit product has only been distributed in India, Ireland, and Israel.
However, Heritage is consulting with the US Food and Drug Administration (FDA) to aid the agency’s evaluations of this product, assist with determining the source of the counterfeit drug, and prevent the further distribution of this product or its introduction into the US.
BiCNU® is primarily used for chemotherapy in the treatment of lymphomas, multiple myeloma, and brain cancers. But the drug is also used for immunosuppression before organ transplant or hematopoietic stem cell transplant.
Heritage said it has directly notified all customers and provided detailed information that will help them identify a counterfeit BiCNU® product. Customers have been instructed to examine their inventory immediately and to quarantine, discontinue distribution of, and return any suspected counterfeit product.
Any customers who may have recently distributed the BiCNU® products to their own customers have been asked to convey this information to their customers so they will be able to carefully examine all BiCNU® products before use and identify the characteristics of a suspected counterfeit product.
Any end users who believe they may have received a counterfeit drug should return the product to the pharmacy that dispensed the medicine.
Any US health practitioners who determine they are in possession of a counterfeit product should contact the FDA through MedWatch. Instructions for such reporting are available on the FDA website.
Anyone with questions about the counterfeit product should contact the Heritage customer call center directly at (866) 901-3784, which is open Monday through Friday, from 9 am to 5 pm EST.