User login
Death follows a normal EKG ...Kidney failure after multiple meds
Death follows a normal EKG
MID-CHEST DISCOMFORT, A COUGH, AND SWEATING brought a 59-year-old man to his primary care physician. The patient had normal vital signs and reported that belching relieved the chest discomfort. He had a history of severe coronary artery disease and had undergone angioplasty and stenting several years earlier.
The primary care physician performed an electrocardiogram (EKG), which was normal and unchanged from one done the year before. The doctor suspected bronchitis, but prescribed omeprazole because the patient had previously been diagnosed with gastroesophageal reflux disease. He ordered a chemical stress test to be performed within a month and a chest radiograph to be done if the patient’s symptoms didn’t improve.
Two hours after returning home, the patient called an ambulance. He told paramedics that he’d been having chest pain for an hour. While they were putting the patient into the ambulance, he went into cardiac arrest. Four defibrillation attempts en route to the hospital and additional resuscitation attempts in the ED failed; he was pronounced dead 3½ hours after leaving his physician’s office.
No autopsy was performed. The patient’s widow found the omeprazole bottle, with one pill missing, and fast-food hamburger wrappers on the kitchen table.
PLAINTIFF’S CLAIM The primary care physician should have sent the patient to the ED to determine whether the chest pain had a cardiac cause; the patient was suffering from acute cardiac syndrome when the doctor saw him.
THE DEFENSE The patient’s normal EKG and vital signs and the fact that belching relieved his chest symptoms indicated that the complaints did not arise from cardiac causes or require emergency assessment. The patient didn’t report chest pain at the office visit; the later cardiac arrest probably resulted from a sudden plaque rupture unrelated to the earlier chest discomfort.
VERDICT $1.5 million Illinois verdict.
COMMENT I hope most doctors won’t have to learn this lesson from their own experience. A normal EKG does not rule out acute ischemia in a high-risk patient with chest pain and sweating. Admit such patients immediately to a cardiac observation unit.
Kidney failure after multiple meds
A MAN WAS TAKING MULTIPLE MEDICATIONS: 3 blood pressure drugs prescribed by his primary care physician, an NSAID prescribed by another doctor, and sizable doses of BC Powder, an over-the-counter analgesic containing aspirin, salicylamide, and caffeine. After 4 years on this medication regimen, the patient’s kidneys failed.
PLAINTIFF’S CLAIM The primary care physician failed to properly monitor kidney function with blood and urine tests while his patient was taking the medications. Proper testing would have resulted in a diagnosis of kidney disease before the patient’s kidneys failed completely. In addition, the primary care physician failed to explain the risks and side effects of the medications to the patient.
THE DEFENSE The patient refused kidney function testing and did not follow medical advice. He consumed excessive amounts of alcohol against medical advice, did not tell the primary care physician about other drugs he was taking, and had allowed his supply of blood pressure medication to run out.
VERDICT $2 million gross verdict in Georgia, with a finding of 47% comparative negligence.
COMMENT This case offers several lessons: First, each BC Powder packet contains the equivalent of 2 aspirin. Second, chronic, high-dose NSAIDs can cause renal failure, especially in patients whose renal function is compromised by hypertension. Third, all patients with hypertension should undergo periodic monitoring of renal function.
Death follows a normal EKG
MID-CHEST DISCOMFORT, A COUGH, AND SWEATING brought a 59-year-old man to his primary care physician. The patient had normal vital signs and reported that belching relieved the chest discomfort. He had a history of severe coronary artery disease and had undergone angioplasty and stenting several years earlier.
The primary care physician performed an electrocardiogram (EKG), which was normal and unchanged from one done the year before. The doctor suspected bronchitis, but prescribed omeprazole because the patient had previously been diagnosed with gastroesophageal reflux disease. He ordered a chemical stress test to be performed within a month and a chest radiograph to be done if the patient’s symptoms didn’t improve.
Two hours after returning home, the patient called an ambulance. He told paramedics that he’d been having chest pain for an hour. While they were putting the patient into the ambulance, he went into cardiac arrest. Four defibrillation attempts en route to the hospital and additional resuscitation attempts in the ED failed; he was pronounced dead 3½ hours after leaving his physician’s office.
No autopsy was performed. The patient’s widow found the omeprazole bottle, with one pill missing, and fast-food hamburger wrappers on the kitchen table.
PLAINTIFF’S CLAIM The primary care physician should have sent the patient to the ED to determine whether the chest pain had a cardiac cause; the patient was suffering from acute cardiac syndrome when the doctor saw him.
THE DEFENSE The patient’s normal EKG and vital signs and the fact that belching relieved his chest symptoms indicated that the complaints did not arise from cardiac causes or require emergency assessment. The patient didn’t report chest pain at the office visit; the later cardiac arrest probably resulted from a sudden plaque rupture unrelated to the earlier chest discomfort.
VERDICT $1.5 million Illinois verdict.
COMMENT I hope most doctors won’t have to learn this lesson from their own experience. A normal EKG does not rule out acute ischemia in a high-risk patient with chest pain and sweating. Admit such patients immediately to a cardiac observation unit.
Kidney failure after multiple meds
A MAN WAS TAKING MULTIPLE MEDICATIONS: 3 blood pressure drugs prescribed by his primary care physician, an NSAID prescribed by another doctor, and sizable doses of BC Powder, an over-the-counter analgesic containing aspirin, salicylamide, and caffeine. After 4 years on this medication regimen, the patient’s kidneys failed.
PLAINTIFF’S CLAIM The primary care physician failed to properly monitor kidney function with blood and urine tests while his patient was taking the medications. Proper testing would have resulted in a diagnosis of kidney disease before the patient’s kidneys failed completely. In addition, the primary care physician failed to explain the risks and side effects of the medications to the patient.
THE DEFENSE The patient refused kidney function testing and did not follow medical advice. He consumed excessive amounts of alcohol against medical advice, did not tell the primary care physician about other drugs he was taking, and had allowed his supply of blood pressure medication to run out.
VERDICT $2 million gross verdict in Georgia, with a finding of 47% comparative negligence.
COMMENT This case offers several lessons: First, each BC Powder packet contains the equivalent of 2 aspirin. Second, chronic, high-dose NSAIDs can cause renal failure, especially in patients whose renal function is compromised by hypertension. Third, all patients with hypertension should undergo periodic monitoring of renal function.
Death follows a normal EKG
MID-CHEST DISCOMFORT, A COUGH, AND SWEATING brought a 59-year-old man to his primary care physician. The patient had normal vital signs and reported that belching relieved the chest discomfort. He had a history of severe coronary artery disease and had undergone angioplasty and stenting several years earlier.
The primary care physician performed an electrocardiogram (EKG), which was normal and unchanged from one done the year before. The doctor suspected bronchitis, but prescribed omeprazole because the patient had previously been diagnosed with gastroesophageal reflux disease. He ordered a chemical stress test to be performed within a month and a chest radiograph to be done if the patient’s symptoms didn’t improve.
Two hours after returning home, the patient called an ambulance. He told paramedics that he’d been having chest pain for an hour. While they were putting the patient into the ambulance, he went into cardiac arrest. Four defibrillation attempts en route to the hospital and additional resuscitation attempts in the ED failed; he was pronounced dead 3½ hours after leaving his physician’s office.
No autopsy was performed. The patient’s widow found the omeprazole bottle, with one pill missing, and fast-food hamburger wrappers on the kitchen table.
PLAINTIFF’S CLAIM The primary care physician should have sent the patient to the ED to determine whether the chest pain had a cardiac cause; the patient was suffering from acute cardiac syndrome when the doctor saw him.
THE DEFENSE The patient’s normal EKG and vital signs and the fact that belching relieved his chest symptoms indicated that the complaints did not arise from cardiac causes or require emergency assessment. The patient didn’t report chest pain at the office visit; the later cardiac arrest probably resulted from a sudden plaque rupture unrelated to the earlier chest discomfort.
VERDICT $1.5 million Illinois verdict.
COMMENT I hope most doctors won’t have to learn this lesson from their own experience. A normal EKG does not rule out acute ischemia in a high-risk patient with chest pain and sweating. Admit such patients immediately to a cardiac observation unit.
Kidney failure after multiple meds
A MAN WAS TAKING MULTIPLE MEDICATIONS: 3 blood pressure drugs prescribed by his primary care physician, an NSAID prescribed by another doctor, and sizable doses of BC Powder, an over-the-counter analgesic containing aspirin, salicylamide, and caffeine. After 4 years on this medication regimen, the patient’s kidneys failed.
PLAINTIFF’S CLAIM The primary care physician failed to properly monitor kidney function with blood and urine tests while his patient was taking the medications. Proper testing would have resulted in a diagnosis of kidney disease before the patient’s kidneys failed completely. In addition, the primary care physician failed to explain the risks and side effects of the medications to the patient.
THE DEFENSE The patient refused kidney function testing and did not follow medical advice. He consumed excessive amounts of alcohol against medical advice, did not tell the primary care physician about other drugs he was taking, and had allowed his supply of blood pressure medication to run out.
VERDICT $2 million gross verdict in Georgia, with a finding of 47% comparative negligence.
COMMENT This case offers several lessons: First, each BC Powder packet contains the equivalent of 2 aspirin. Second, chronic, high-dose NSAIDs can cause renal failure, especially in patients whose renal function is compromised by hypertension. Third, all patients with hypertension should undergo periodic monitoring of renal function.
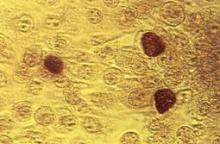
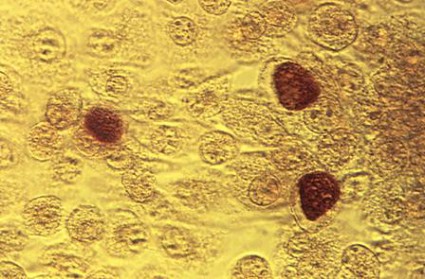
Nongonococcal urethritis: Time to ditch azithromycin?
PRAGUE – Recent evidence dictates the need to reassess the roles of doxycycline and azithromycin in treating Chlamydia trachomatis nongonococcal urethritis, according to Dr. Michel Janier.
Current first-line therapy for nongonococcal urethritis (NGU), as recommended by the Centers for Disease Control and Prevention and other major groups, features a choice: a single 1-g dose of azithromycin, or doxycycline at 100 mg b.i.d. for 7 days. Single-dose azithromycin is a popular option, given its convenience and likely better adherence.
But a couple of important head-to-head comparative trials published recently are making waves in the infectious disease world. Doxycycline proved significantly more effective than azithromycin in clearing chlamydial NGU in one study and had a markedly lower C. trachomatis persistence rate 45 days post treatment in the other, said Dr. Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections–Europe.
One study was a phase IIb randomized, double-blind trial involving 305 men with NGU at sexually transmitted disease clinics in four U.S. cities. They were assigned to guideline-recommended treatment with either azithromycin or doxycycline alone or with a single 2-g dose of tinidazole, an antitrichomonal agent, in order to test the hypothesis that adding the second agent would boost cure rates. As it turned out, it did not.
Among the 43% of men with C. trachomatis NGU, the chlamydial clearance rate was 94.8% in the doxycycline arm compared with 77.4% with azithromycin.
While doxycycline outperformed azithromycin in men with C. trachomatis NGU, the converse was true among the 31% of participants with Mycoplasma genitalium NGU. The clearance rate was 30.8% in the doxycycline arm compared with 66.7% in the azithromycin arm (Clin. Infect. Dis. 2011;52:163-70).
The same group of investigators recently analyzed data on post-treatment persistence of NGU in a study involving 293 heterosexual men treated for NGU at STD clinics. Among the 129 men with C. trachomatis NGU, persistent C. trachomatis infection was detected via nucleic acid amplification testing 4 weeks post treatment in 23% of those who received azithromycin compared with just 5% treated with doxycycline (J. Infect. Dis. 2012;206:357-65).
The explanation for the observed higher failure rate with azithromycin compared with doxycycline in treating chlamydial NGU is probably twofold: homotypic resistance of the organism to a single dose of a bacteriostatic antibiotic, coupled with heterotopic resistance stemming from a persistent subpopulation of cells having a reduced growth rate within the larger pathogen load, Dr. Janier said.
He added that while it’s important not to overreact to a couple of studies carried out by a single group, there is intense interest on the part of many infectious disease experts in taking a closer look at the possibility that azithromycin may need to be dethroned as first-line therapy.
The dermatologist also highlighted another recent study that bears on the treatment of uncomplicated C. trachomatis NGU. The double-blind, randomized, double-dummy multicenter trial included 323 men and nonpregnant women with urogenital chlamydia. They were randomized to 7 days of once-daily delayed-release doxycycline (Doryx) at 200 mg or to 100 mg b.i.d. of standard-release doxycycline (Vibramycin).
The primary outcome, microbial cure by nucleic acid amplification testing on day 28, occurred in 95.5% of the delayed-release doxycycline group and a virtually identical 95.2% on Vibramycin. However, the delayed-release formulation was significantly better tolerated, with a 13% nausea rate compared with 21% in the Vibramycin group. Vomiting occurred in 8% of patients on delayed-release doxycycline, a significantly lower rate than the 12% with Vibramycin.
The investigators concluded that delayed-release doxycycline, with its once-daily dosing and better tolerability, could improve treatment adherence (Clin. Infect. Dis. 2012; 55: 82-8).
Dr. Janier reported having no relevant financial disclosures.
PRAGUE – Recent evidence dictates the need to reassess the roles of doxycycline and azithromycin in treating Chlamydia trachomatis nongonococcal urethritis, according to Dr. Michel Janier.
Current first-line therapy for nongonococcal urethritis (NGU), as recommended by the Centers for Disease Control and Prevention and other major groups, features a choice: a single 1-g dose of azithromycin, or doxycycline at 100 mg b.i.d. for 7 days. Single-dose azithromycin is a popular option, given its convenience and likely better adherence.
But a couple of important head-to-head comparative trials published recently are making waves in the infectious disease world. Doxycycline proved significantly more effective than azithromycin in clearing chlamydial NGU in one study and had a markedly lower C. trachomatis persistence rate 45 days post treatment in the other, said Dr. Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections–Europe.
One study was a phase IIb randomized, double-blind trial involving 305 men with NGU at sexually transmitted disease clinics in four U.S. cities. They were assigned to guideline-recommended treatment with either azithromycin or doxycycline alone or with a single 2-g dose of tinidazole, an antitrichomonal agent, in order to test the hypothesis that adding the second agent would boost cure rates. As it turned out, it did not.
Among the 43% of men with C. trachomatis NGU, the chlamydial clearance rate was 94.8% in the doxycycline arm compared with 77.4% with azithromycin.
While doxycycline outperformed azithromycin in men with C. trachomatis NGU, the converse was true among the 31% of participants with Mycoplasma genitalium NGU. The clearance rate was 30.8% in the doxycycline arm compared with 66.7% in the azithromycin arm (Clin. Infect. Dis. 2011;52:163-70).
The same group of investigators recently analyzed data on post-treatment persistence of NGU in a study involving 293 heterosexual men treated for NGU at STD clinics. Among the 129 men with C. trachomatis NGU, persistent C. trachomatis infection was detected via nucleic acid amplification testing 4 weeks post treatment in 23% of those who received azithromycin compared with just 5% treated with doxycycline (J. Infect. Dis. 2012;206:357-65).
The explanation for the observed higher failure rate with azithromycin compared with doxycycline in treating chlamydial NGU is probably twofold: homotypic resistance of the organism to a single dose of a bacteriostatic antibiotic, coupled with heterotopic resistance stemming from a persistent subpopulation of cells having a reduced growth rate within the larger pathogen load, Dr. Janier said.
He added that while it’s important not to overreact to a couple of studies carried out by a single group, there is intense interest on the part of many infectious disease experts in taking a closer look at the possibility that azithromycin may need to be dethroned as first-line therapy.
The dermatologist also highlighted another recent study that bears on the treatment of uncomplicated C. trachomatis NGU. The double-blind, randomized, double-dummy multicenter trial included 323 men and nonpregnant women with urogenital chlamydia. They were randomized to 7 days of once-daily delayed-release doxycycline (Doryx) at 200 mg or to 100 mg b.i.d. of standard-release doxycycline (Vibramycin).
The primary outcome, microbial cure by nucleic acid amplification testing on day 28, occurred in 95.5% of the delayed-release doxycycline group and a virtually identical 95.2% on Vibramycin. However, the delayed-release formulation was significantly better tolerated, with a 13% nausea rate compared with 21% in the Vibramycin group. Vomiting occurred in 8% of patients on delayed-release doxycycline, a significantly lower rate than the 12% with Vibramycin.
The investigators concluded that delayed-release doxycycline, with its once-daily dosing and better tolerability, could improve treatment adherence (Clin. Infect. Dis. 2012; 55: 82-8).
Dr. Janier reported having no relevant financial disclosures.
PRAGUE – Recent evidence dictates the need to reassess the roles of doxycycline and azithromycin in treating Chlamydia trachomatis nongonococcal urethritis, according to Dr. Michel Janier.
Current first-line therapy for nongonococcal urethritis (NGU), as recommended by the Centers for Disease Control and Prevention and other major groups, features a choice: a single 1-g dose of azithromycin, or doxycycline at 100 mg b.i.d. for 7 days. Single-dose azithromycin is a popular option, given its convenience and likely better adherence.
But a couple of important head-to-head comparative trials published recently are making waves in the infectious disease world. Doxycycline proved significantly more effective than azithromycin in clearing chlamydial NGU in one study and had a markedly lower C. trachomatis persistence rate 45 days post treatment in the other, said Dr. Janier, head of dermatology at Saint Joseph Hospital in Paris and the French representative to the International Union Against Sexually Transmitted Infections–Europe.
One study was a phase IIb randomized, double-blind trial involving 305 men with NGU at sexually transmitted disease clinics in four U.S. cities. They were assigned to guideline-recommended treatment with either azithromycin or doxycycline alone or with a single 2-g dose of tinidazole, an antitrichomonal agent, in order to test the hypothesis that adding the second agent would boost cure rates. As it turned out, it did not.
Among the 43% of men with C. trachomatis NGU, the chlamydial clearance rate was 94.8% in the doxycycline arm compared with 77.4% with azithromycin.
While doxycycline outperformed azithromycin in men with C. trachomatis NGU, the converse was true among the 31% of participants with Mycoplasma genitalium NGU. The clearance rate was 30.8% in the doxycycline arm compared with 66.7% in the azithromycin arm (Clin. Infect. Dis. 2011;52:163-70).
The same group of investigators recently analyzed data on post-treatment persistence of NGU in a study involving 293 heterosexual men treated for NGU at STD clinics. Among the 129 men with C. trachomatis NGU, persistent C. trachomatis infection was detected via nucleic acid amplification testing 4 weeks post treatment in 23% of those who received azithromycin compared with just 5% treated with doxycycline (J. Infect. Dis. 2012;206:357-65).
The explanation for the observed higher failure rate with azithromycin compared with doxycycline in treating chlamydial NGU is probably twofold: homotypic resistance of the organism to a single dose of a bacteriostatic antibiotic, coupled with heterotopic resistance stemming from a persistent subpopulation of cells having a reduced growth rate within the larger pathogen load, Dr. Janier said.
He added that while it’s important not to overreact to a couple of studies carried out by a single group, there is intense interest on the part of many infectious disease experts in taking a closer look at the possibility that azithromycin may need to be dethroned as first-line therapy.
The dermatologist also highlighted another recent study that bears on the treatment of uncomplicated C. trachomatis NGU. The double-blind, randomized, double-dummy multicenter trial included 323 men and nonpregnant women with urogenital chlamydia. They were randomized to 7 days of once-daily delayed-release doxycycline (Doryx) at 200 mg or to 100 mg b.i.d. of standard-release doxycycline (Vibramycin).
The primary outcome, microbial cure by nucleic acid amplification testing on day 28, occurred in 95.5% of the delayed-release doxycycline group and a virtually identical 95.2% on Vibramycin. However, the delayed-release formulation was significantly better tolerated, with a 13% nausea rate compared with 21% in the Vibramycin group. Vomiting occurred in 8% of patients on delayed-release doxycycline, a significantly lower rate than the 12% with Vibramycin.
The investigators concluded that delayed-release doxycycline, with its once-daily dosing and better tolerability, could improve treatment adherence (Clin. Infect. Dis. 2012; 55: 82-8).
Dr. Janier reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Sugary Beverages Linked to Kidney Stone Formation
SAN DIEGO – Regular consumption of sugar-sweetened sodas and punch are linked with an increased risk of kidney stone formation while consumption of coffee, tea, and other beverages may be protective, results from a large analysis demonstrated.
The findings confirm earlier reports of beverages believed to be associated with a reduced risk of kidney stone formation, Dr. Pietro Manuel Ferraro said in an interview during a poster session Kidney Week 2012. "Patients with a previous kidney stone are advised to ingest at least two liters of fluid per day, but not all fluids are equally beneficial," said Dr. Ferraro, a nephrologist at Catholic University of the Sacred Heart, Rome. "What we can say from this analysis is that it’s best to reduce consumption of sugar-sweetened beverages in these patients."
For the study, which Dr. Ferraro and his associates conducted over the past year at the Channing Division of Network Medicine in Boston, the researchers analyzed data from three large ongoing cohort studies: the Health Professionals Follow-Up Study, and the Nurses’ Health Study I and II. They used a Cox model to assess the risk of developing kidney stones associated with each beverage and adjusted for covariates including age, race, physical activity, body mass index, diabetes, high blood pressure, gout, use of diuretics and intake of calcium, potassium, animal protein, phytate, vitamin C, total energy, and alcohol.
Dr. Ferraro reported data from 194,095 participants in the pooled analysis, which represented 2,643,708 person-years of follow-up. Five categories of beverage consumption were evaluated: less than 1 beverage/week (the reference category), 1/week, 2-4/week, 5-6/week, and 1 or more/day. The researchers found that consumption of sugar-sweetened cola was significantly associated with kidney stone formation (hazard ratio of 1.07 for 1/week; HR, 1.19 for 2-4/week; HR, 1.28 for 5-6/week; and HR, 1.23 for 1 or more/day, compared with the less than 1/week category; P = .02), as was consumption of sugar-sweetened non-cola (HR, 1.17, 1.07, 1.22, and 1.33, respectively; P = .003) and sugar-sweetened punch (HR, 1.10, 1.15, 1.21, and 1.18, respectively; P = .04).
At the same time, consumption of certain beverages were found to be inversely associated with kidney stone formation, including coffee (P less than .001), tea (P = .02), red wine (P = .004), white wine (P = .002), beer (P less than .001), and orange juice (P = .004).
The study, which is the largest of its kind, was supported by a grant from the National Institutes of Health. Dr. Ferraro said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Regular consumption of sugar-sweetened sodas and punch are linked with an increased risk of kidney stone formation while consumption of coffee, tea, and other beverages may be protective, results from a large analysis demonstrated.
The findings confirm earlier reports of beverages believed to be associated with a reduced risk of kidney stone formation, Dr. Pietro Manuel Ferraro said in an interview during a poster session Kidney Week 2012. "Patients with a previous kidney stone are advised to ingest at least two liters of fluid per day, but not all fluids are equally beneficial," said Dr. Ferraro, a nephrologist at Catholic University of the Sacred Heart, Rome. "What we can say from this analysis is that it’s best to reduce consumption of sugar-sweetened beverages in these patients."
For the study, which Dr. Ferraro and his associates conducted over the past year at the Channing Division of Network Medicine in Boston, the researchers analyzed data from three large ongoing cohort studies: the Health Professionals Follow-Up Study, and the Nurses’ Health Study I and II. They used a Cox model to assess the risk of developing kidney stones associated with each beverage and adjusted for covariates including age, race, physical activity, body mass index, diabetes, high blood pressure, gout, use of diuretics and intake of calcium, potassium, animal protein, phytate, vitamin C, total energy, and alcohol.
Dr. Ferraro reported data from 194,095 participants in the pooled analysis, which represented 2,643,708 person-years of follow-up. Five categories of beverage consumption were evaluated: less than 1 beverage/week (the reference category), 1/week, 2-4/week, 5-6/week, and 1 or more/day. The researchers found that consumption of sugar-sweetened cola was significantly associated with kidney stone formation (hazard ratio of 1.07 for 1/week; HR, 1.19 for 2-4/week; HR, 1.28 for 5-6/week; and HR, 1.23 for 1 or more/day, compared with the less than 1/week category; P = .02), as was consumption of sugar-sweetened non-cola (HR, 1.17, 1.07, 1.22, and 1.33, respectively; P = .003) and sugar-sweetened punch (HR, 1.10, 1.15, 1.21, and 1.18, respectively; P = .04).
At the same time, consumption of certain beverages were found to be inversely associated with kidney stone formation, including coffee (P less than .001), tea (P = .02), red wine (P = .004), white wine (P = .002), beer (P less than .001), and orange juice (P = .004).
The study, which is the largest of its kind, was supported by a grant from the National Institutes of Health. Dr. Ferraro said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Regular consumption of sugar-sweetened sodas and punch are linked with an increased risk of kidney stone formation while consumption of coffee, tea, and other beverages may be protective, results from a large analysis demonstrated.
The findings confirm earlier reports of beverages believed to be associated with a reduced risk of kidney stone formation, Dr. Pietro Manuel Ferraro said in an interview during a poster session Kidney Week 2012. "Patients with a previous kidney stone are advised to ingest at least two liters of fluid per day, but not all fluids are equally beneficial," said Dr. Ferraro, a nephrologist at Catholic University of the Sacred Heart, Rome. "What we can say from this analysis is that it’s best to reduce consumption of sugar-sweetened beverages in these patients."
For the study, which Dr. Ferraro and his associates conducted over the past year at the Channing Division of Network Medicine in Boston, the researchers analyzed data from three large ongoing cohort studies: the Health Professionals Follow-Up Study, and the Nurses’ Health Study I and II. They used a Cox model to assess the risk of developing kidney stones associated with each beverage and adjusted for covariates including age, race, physical activity, body mass index, diabetes, high blood pressure, gout, use of diuretics and intake of calcium, potassium, animal protein, phytate, vitamin C, total energy, and alcohol.
Dr. Ferraro reported data from 194,095 participants in the pooled analysis, which represented 2,643,708 person-years of follow-up. Five categories of beverage consumption were evaluated: less than 1 beverage/week (the reference category), 1/week, 2-4/week, 5-6/week, and 1 or more/day. The researchers found that consumption of sugar-sweetened cola was significantly associated with kidney stone formation (hazard ratio of 1.07 for 1/week; HR, 1.19 for 2-4/week; HR, 1.28 for 5-6/week; and HR, 1.23 for 1 or more/day, compared with the less than 1/week category; P = .02), as was consumption of sugar-sweetened non-cola (HR, 1.17, 1.07, 1.22, and 1.33, respectively; P = .003) and sugar-sweetened punch (HR, 1.10, 1.15, 1.21, and 1.18, respectively; P = .04).
At the same time, consumption of certain beverages were found to be inversely associated with kidney stone formation, including coffee (P less than .001), tea (P = .02), red wine (P = .004), white wine (P = .002), beer (P less than .001), and orange juice (P = .004).
The study, which is the largest of its kind, was supported by a grant from the National Institutes of Health. Dr. Ferraro said that he had no relevant financial conflicts to disclose.
AT KIDNEY WEEK 2012
Major Finding: Consumption of sugar-sweetened cola was significantly associated with kidney stone formation (hazard ratio, 1.07 for 1 drink/week; HR, 1.19 for 2-4 drinks/week; HR, 1.28 for 5-6 drinks/week; and HR, 1.23 for 1 or more drinks/day, compared with the less than 1 drink/week category P = .02), as was consumption of sugar-sweetened non-cola and sugar-sweetened punch.
Data Source: Results were taken from a study of 194,095 people who participated in the Health Professionals Follow-up Study or in the Nurses’ Health Study I and II.
Disclosures: The study was supported by a grant from the National Institutes of Health. Dr. Ferraro said that he had no relevant financial conflicts to disclose.
Healthy Lifestyle Cut Cardiac Risks in CKD
SAN DIEGO – A healthy lifestyle cut the risk of cardiovascular events and death in chronic kidney disease, but it had no significant impact on the risk of renal events, preliminary results from an ongoing study have demonstrated.
"The impact of a healthy lifestyle has been studied most often in the general population, but lifestyle as a predictor of adverse outcomes has not been previously evaluated in individuals with CKD," Dr. Ana C. Ricardo said in an interview during a poster session at Kidney Week 2012.
"There have been studies looking at individual risk factors such as smoking and chronic kidney disease progression alone, and exercise and mortality alone; but none have examined the impact of adherence to multiple lifestyle factors."
The findings come from 4 years of follow-up in 3,670 men and women with mild to moderate kidney disease who are enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study, a multicenter, nationwide study supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to examine the epidemiology, management, and outcomes of CKD.
Dr. Ricardo, a nephrologist with the University of Illinois at Chicago, and her colleagues evaluated the association of a healthy lifestyle with clinical outcomes based on each participant’s healthy lifestyle score. This was calculated by allocating one point for each of the following factors measured at study entry: not currently smoking, engaged in moderate exercise (defined as 150 minutes or greater per week), engaged in vigorous exercise (defined as 75 minutes or greater per week), and having a urinary sodium output of less than 100 mEq/day.
Outcomes of interest were progression of CKD (defined as 50% or greater estimated glomerular filtration rate loss or end-stage renal disease), the development of cardiovascular events (defined as myocardial infarction, stroke, heart failure, or peripheral arterial disease), or death. The researchers used multivariable Cox proportional hazards regression models to determine the impact of the lifestyle factors on these outcomes.
Dr. Ricardo reported that 86% of participants adhered to one or two healthy lifestyle factors. Women, non-Hispanic whites, and college graduates were more likely to have a healthy lifestyle score of 3. Participants with a healthy lifestyle score of 1 had a 35% reduced risk of cardiovascular events or death. This risk was reduced further for those with a score of 2 or 3 (45% and 44%, respectively).
The researchers also found that patients with a healthy lifestyle score of 1 had a 30% reduced risk of CKD progression – but this risk reduction did not reach statistical significance, and risk was not reduced further among those with a score of 2 or 3 (24% and 7%, respectively). "We will explore this in further analyses," Dr. Ricardo said.
She acknowledged certain limitations of the study, including its observational design. "This is a work in progress," she said of the work. "We have more analysis to do. This is just the beginning."
Kidney Week 2012 was sponsored by the American Society of Nephrology. The CRIC study was funded by the NIDDK. Dr. Ricardo said that she had no relevant financial conflicts to disclose.
SAN DIEGO – A healthy lifestyle cut the risk of cardiovascular events and death in chronic kidney disease, but it had no significant impact on the risk of renal events, preliminary results from an ongoing study have demonstrated.
"The impact of a healthy lifestyle has been studied most often in the general population, but lifestyle as a predictor of adverse outcomes has not been previously evaluated in individuals with CKD," Dr. Ana C. Ricardo said in an interview during a poster session at Kidney Week 2012.
"There have been studies looking at individual risk factors such as smoking and chronic kidney disease progression alone, and exercise and mortality alone; but none have examined the impact of adherence to multiple lifestyle factors."
The findings come from 4 years of follow-up in 3,670 men and women with mild to moderate kidney disease who are enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study, a multicenter, nationwide study supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to examine the epidemiology, management, and outcomes of CKD.
Dr. Ricardo, a nephrologist with the University of Illinois at Chicago, and her colleagues evaluated the association of a healthy lifestyle with clinical outcomes based on each participant’s healthy lifestyle score. This was calculated by allocating one point for each of the following factors measured at study entry: not currently smoking, engaged in moderate exercise (defined as 150 minutes or greater per week), engaged in vigorous exercise (defined as 75 minutes or greater per week), and having a urinary sodium output of less than 100 mEq/day.
Outcomes of interest were progression of CKD (defined as 50% or greater estimated glomerular filtration rate loss or end-stage renal disease), the development of cardiovascular events (defined as myocardial infarction, stroke, heart failure, or peripheral arterial disease), or death. The researchers used multivariable Cox proportional hazards regression models to determine the impact of the lifestyle factors on these outcomes.
Dr. Ricardo reported that 86% of participants adhered to one or two healthy lifestyle factors. Women, non-Hispanic whites, and college graduates were more likely to have a healthy lifestyle score of 3. Participants with a healthy lifestyle score of 1 had a 35% reduced risk of cardiovascular events or death. This risk was reduced further for those with a score of 2 or 3 (45% and 44%, respectively).
The researchers also found that patients with a healthy lifestyle score of 1 had a 30% reduced risk of CKD progression – but this risk reduction did not reach statistical significance, and risk was not reduced further among those with a score of 2 or 3 (24% and 7%, respectively). "We will explore this in further analyses," Dr. Ricardo said.
She acknowledged certain limitations of the study, including its observational design. "This is a work in progress," she said of the work. "We have more analysis to do. This is just the beginning."
Kidney Week 2012 was sponsored by the American Society of Nephrology. The CRIC study was funded by the NIDDK. Dr. Ricardo said that she had no relevant financial conflicts to disclose.
SAN DIEGO – A healthy lifestyle cut the risk of cardiovascular events and death in chronic kidney disease, but it had no significant impact on the risk of renal events, preliminary results from an ongoing study have demonstrated.
"The impact of a healthy lifestyle has been studied most often in the general population, but lifestyle as a predictor of adverse outcomes has not been previously evaluated in individuals with CKD," Dr. Ana C. Ricardo said in an interview during a poster session at Kidney Week 2012.
"There have been studies looking at individual risk factors such as smoking and chronic kidney disease progression alone, and exercise and mortality alone; but none have examined the impact of adherence to multiple lifestyle factors."
The findings come from 4 years of follow-up in 3,670 men and women with mild to moderate kidney disease who are enrolled in the Chronic Renal Insufficiency Cohort (CRIC) study, a multicenter, nationwide study supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to examine the epidemiology, management, and outcomes of CKD.
Dr. Ricardo, a nephrologist with the University of Illinois at Chicago, and her colleagues evaluated the association of a healthy lifestyle with clinical outcomes based on each participant’s healthy lifestyle score. This was calculated by allocating one point for each of the following factors measured at study entry: not currently smoking, engaged in moderate exercise (defined as 150 minutes or greater per week), engaged in vigorous exercise (defined as 75 minutes or greater per week), and having a urinary sodium output of less than 100 mEq/day.
Outcomes of interest were progression of CKD (defined as 50% or greater estimated glomerular filtration rate loss or end-stage renal disease), the development of cardiovascular events (defined as myocardial infarction, stroke, heart failure, or peripheral arterial disease), or death. The researchers used multivariable Cox proportional hazards regression models to determine the impact of the lifestyle factors on these outcomes.
Dr. Ricardo reported that 86% of participants adhered to one or two healthy lifestyle factors. Women, non-Hispanic whites, and college graduates were more likely to have a healthy lifestyle score of 3. Participants with a healthy lifestyle score of 1 had a 35% reduced risk of cardiovascular events or death. This risk was reduced further for those with a score of 2 or 3 (45% and 44%, respectively).
The researchers also found that patients with a healthy lifestyle score of 1 had a 30% reduced risk of CKD progression – but this risk reduction did not reach statistical significance, and risk was not reduced further among those with a score of 2 or 3 (24% and 7%, respectively). "We will explore this in further analyses," Dr. Ricardo said.
She acknowledged certain limitations of the study, including its observational design. "This is a work in progress," she said of the work. "We have more analysis to do. This is just the beginning."
Kidney Week 2012 was sponsored by the American Society of Nephrology. The CRIC study was funded by the NIDDK. Dr. Ricardo said that she had no relevant financial conflicts to disclose.
AT KIDNEY WEEK 2012
Major Finding: Men and women with chronic kidney disease who had a healthy lifestyle score of 1 based on a three-point scale had a 35% reduced risk of cardiovascular events or death. This risk was further reduced for those with a score of 2 or 3 (45% and 44%, respectively).
Data Source: This was a study of 3,670 individuals with mild to moderate kidney disease who are enrolled in the federally funded Chronic Renal Insufficiency Cohort (CRIC) Study.
Disclosures: Kidney Week 2012 was sponsored by the American Society of Nephrology. The CRIC study was funded by the NIDDK. Dr. Ricardo said that she had no relevant financial conflicts to disclose.
Kidneys Benefited From Everolimus After Liver Transplant
BOSTON – Everolimus given with a reduced dose of tacrolimus to liver transplant patients yielded similar rates of acute rejection, graft loss, and death, but better kidney function than standard-dose tacrolimus alone at 2 years in a randomized, open-label, multicenter, controlled trial.
These 2-year results confirm and build on the recently published results of the trial at 1 year (Am. J. Transpl. 2012;12:3008-20), lead investigator Dr. Faouzi Saliba of Paul Brousse Hospital, Villejuif, France, reported at the annual meeting of the American Association for the Study of Liver Diseases.
Since the first study was published 9 years ago documenting a cumulative incidence of chronic renal failure of 28% after 10 years of tacrolimus treatment after liver transplant (N. Engl. J. Med. 2003;349:931-40), another study has reported estimated glomerular filtration rates (eGFRs) of less than 60 mL/min per 1.73 m2 (stage 3 chronic kidney failure) for 58% of liver transplant recipients after 5 years of treatment with tacrolimus (Liver Transpl. 2009:15:1083-91).
On the basis of the effectiveness of everolimus in reducing the dose of calcineurin inhibitor needed for immunosuppression without reducing efficacy in patients with de novo kidney transplantation (Am. J. Transpl. 2010;10:1401-13), Dr. Saliba and his coinvestigators conducted the current trial.
Following a 30-day run-in period in which liver transplant recipients received tacrolimus with or without mycophenolate mofetil and prednisone, the investigators randomized 719 patients to three arms: two arms with everolimus 3-8 ng/mL, tacrolimus reduced to 3-5 ng/mL, and prednisone, and a control arm with tacrolimus dosed to a standard 8-12 ng/mL plus prednisone. After 4 months, in one of the reduced-dose arms, tacrolimus was withdrawn and the everolimus dose was increased to 8-10 ng/mL (231 patients), whereas the dose of everolimus was kept constant in the other reduced-dose arm (245 patients) and the dose of tacrolimus was kept the same in the control arm (243 patients). Prednisone could be eliminated at 4 months in any arm. However, enrollment into the tacrolimus withdrawal arm was stopped at the time of conversion to everolimus alone because of a high rate of rejection episodes, and the trial’s protocol was amended to compare efficacy between only the reduced-dose and control arms.
By 2 years, the number of patients who completed the study was similar in both the reduced-dose and control arms (82% vs. 84%, respectively), and similar percentages in each group remained on the study medications at 2 years (58% vs. 68%, respectively).
In each group, recipients were mostly men (74%) and white (80%-86%), with a mean age of 54 years and mean donor age of 49 years. They had a Model for End-Stage Liver Disease score of 19, and eGFRs of about 80 mL/min per 1.73 m2.
Mean levels of tacrolimus dropped from 10.5 ng/mL at randomization to 4 ng/mL at 2 years in the dose-reduction arm, compared with 10 ng/mL to 7 ng/mL in the control arm.
At 2 years in the intent-to-treat population, the groups had similar rates of the composite primary end point of treated biopsy-proven acute rejection, graft failure, and death (10.5% in the dose-reduction arm and 12.5% in the control arm). Biopsy-proven acute rejection occurred at a significantly lower rate among dose-reduced patients (6%) than among control patients (13%). All of the episodes of acute rejection in the tacrolimus dose-reduced patients were borderline or mild based on the Banff rejection activity index. However, liver biopsies at 1 and 2 years were only part of the trial’s protocol for hepatitis C virus (HCV)-infected patients. The decision to biopsy was otherwise left up to the physicians of each center.
At 1 year, there appeared to be less fibrosis in patients who received everolimus, Dr. Saliba said in response to a question from the audience. In about half of the 75 HCV-infected patients in the dose-reduction arm, liver biopsies showed less fibrosis of at least stage 1 than in patients with HCV infection in the other group. The investigators are now analyzing 2-year data, he said.
The dose-reduced group maintained significantly better eGFR than the control group, through the duration of the trial, finishing with levels of 66 vs. 78 mL/min per 1.73 m2.
Several types of adverse events with an incidence of at least 10% occurred more often in the dose-reduction arm than in the control arm, including leucopenia (13% vs. 5%), peripheral edema (20% vs. 13%), and hypercholesterolemia (11% vs. 4%).
Proteinuria of less than 0.5 g/24 hours occurred in 92%-93% of the patients; none of the patients had severe proteinuria of 3 g/24 hours or more.
One audience member noted that the most important patients to study in this clinical population are those on the borderline of renal failure with low eGFR and elevated creatinine, who would benefit most from improved renal function. Dr. Saliba said that at the time of randomization, the investigators looked at levels of eGFR in each group and over the course of the 2 years, more patients in the standard-dose tacrolimus arm had worsened renal function, whereas many in the dose-reduction arm had improved or at least stable renal function, and few had worsened function.
The study was sponsored by Novartis, which manufactures everolimus. Dr. Saliba reported financial ties to Novartis and Astellas Pharma (manufacturer of tacrolimus), as well as other companies that manufacture drugs used by liver transplant patients. Several other investigators reported financial ties to companies that manufacture antirejection drugs used in liver transplant patients, including Novartis. Three study investigators are employees of Novartis.
BOSTON – Everolimus given with a reduced dose of tacrolimus to liver transplant patients yielded similar rates of acute rejection, graft loss, and death, but better kidney function than standard-dose tacrolimus alone at 2 years in a randomized, open-label, multicenter, controlled trial.
These 2-year results confirm and build on the recently published results of the trial at 1 year (Am. J. Transpl. 2012;12:3008-20), lead investigator Dr. Faouzi Saliba of Paul Brousse Hospital, Villejuif, France, reported at the annual meeting of the American Association for the Study of Liver Diseases.
Since the first study was published 9 years ago documenting a cumulative incidence of chronic renal failure of 28% after 10 years of tacrolimus treatment after liver transplant (N. Engl. J. Med. 2003;349:931-40), another study has reported estimated glomerular filtration rates (eGFRs) of less than 60 mL/min per 1.73 m2 (stage 3 chronic kidney failure) for 58% of liver transplant recipients after 5 years of treatment with tacrolimus (Liver Transpl. 2009:15:1083-91).
On the basis of the effectiveness of everolimus in reducing the dose of calcineurin inhibitor needed for immunosuppression without reducing efficacy in patients with de novo kidney transplantation (Am. J. Transpl. 2010;10:1401-13), Dr. Saliba and his coinvestigators conducted the current trial.
Following a 30-day run-in period in which liver transplant recipients received tacrolimus with or without mycophenolate mofetil and prednisone, the investigators randomized 719 patients to three arms: two arms with everolimus 3-8 ng/mL, tacrolimus reduced to 3-5 ng/mL, and prednisone, and a control arm with tacrolimus dosed to a standard 8-12 ng/mL plus prednisone. After 4 months, in one of the reduced-dose arms, tacrolimus was withdrawn and the everolimus dose was increased to 8-10 ng/mL (231 patients), whereas the dose of everolimus was kept constant in the other reduced-dose arm (245 patients) and the dose of tacrolimus was kept the same in the control arm (243 patients). Prednisone could be eliminated at 4 months in any arm. However, enrollment into the tacrolimus withdrawal arm was stopped at the time of conversion to everolimus alone because of a high rate of rejection episodes, and the trial’s protocol was amended to compare efficacy between only the reduced-dose and control arms.
By 2 years, the number of patients who completed the study was similar in both the reduced-dose and control arms (82% vs. 84%, respectively), and similar percentages in each group remained on the study medications at 2 years (58% vs. 68%, respectively).
In each group, recipients were mostly men (74%) and white (80%-86%), with a mean age of 54 years and mean donor age of 49 years. They had a Model for End-Stage Liver Disease score of 19, and eGFRs of about 80 mL/min per 1.73 m2.
Mean levels of tacrolimus dropped from 10.5 ng/mL at randomization to 4 ng/mL at 2 years in the dose-reduction arm, compared with 10 ng/mL to 7 ng/mL in the control arm.
At 2 years in the intent-to-treat population, the groups had similar rates of the composite primary end point of treated biopsy-proven acute rejection, graft failure, and death (10.5% in the dose-reduction arm and 12.5% in the control arm). Biopsy-proven acute rejection occurred at a significantly lower rate among dose-reduced patients (6%) than among control patients (13%). All of the episodes of acute rejection in the tacrolimus dose-reduced patients were borderline or mild based on the Banff rejection activity index. However, liver biopsies at 1 and 2 years were only part of the trial’s protocol for hepatitis C virus (HCV)-infected patients. The decision to biopsy was otherwise left up to the physicians of each center.
At 1 year, there appeared to be less fibrosis in patients who received everolimus, Dr. Saliba said in response to a question from the audience. In about half of the 75 HCV-infected patients in the dose-reduction arm, liver biopsies showed less fibrosis of at least stage 1 than in patients with HCV infection in the other group. The investigators are now analyzing 2-year data, he said.
The dose-reduced group maintained significantly better eGFR than the control group, through the duration of the trial, finishing with levels of 66 vs. 78 mL/min per 1.73 m2.
Several types of adverse events with an incidence of at least 10% occurred more often in the dose-reduction arm than in the control arm, including leucopenia (13% vs. 5%), peripheral edema (20% vs. 13%), and hypercholesterolemia (11% vs. 4%).
Proteinuria of less than 0.5 g/24 hours occurred in 92%-93% of the patients; none of the patients had severe proteinuria of 3 g/24 hours or more.
One audience member noted that the most important patients to study in this clinical population are those on the borderline of renal failure with low eGFR and elevated creatinine, who would benefit most from improved renal function. Dr. Saliba said that at the time of randomization, the investigators looked at levels of eGFR in each group and over the course of the 2 years, more patients in the standard-dose tacrolimus arm had worsened renal function, whereas many in the dose-reduction arm had improved or at least stable renal function, and few had worsened function.
The study was sponsored by Novartis, which manufactures everolimus. Dr. Saliba reported financial ties to Novartis and Astellas Pharma (manufacturer of tacrolimus), as well as other companies that manufacture drugs used by liver transplant patients. Several other investigators reported financial ties to companies that manufacture antirejection drugs used in liver transplant patients, including Novartis. Three study investigators are employees of Novartis.
BOSTON – Everolimus given with a reduced dose of tacrolimus to liver transplant patients yielded similar rates of acute rejection, graft loss, and death, but better kidney function than standard-dose tacrolimus alone at 2 years in a randomized, open-label, multicenter, controlled trial.
These 2-year results confirm and build on the recently published results of the trial at 1 year (Am. J. Transpl. 2012;12:3008-20), lead investigator Dr. Faouzi Saliba of Paul Brousse Hospital, Villejuif, France, reported at the annual meeting of the American Association for the Study of Liver Diseases.
Since the first study was published 9 years ago documenting a cumulative incidence of chronic renal failure of 28% after 10 years of tacrolimus treatment after liver transplant (N. Engl. J. Med. 2003;349:931-40), another study has reported estimated glomerular filtration rates (eGFRs) of less than 60 mL/min per 1.73 m2 (stage 3 chronic kidney failure) for 58% of liver transplant recipients after 5 years of treatment with tacrolimus (Liver Transpl. 2009:15:1083-91).
On the basis of the effectiveness of everolimus in reducing the dose of calcineurin inhibitor needed for immunosuppression without reducing efficacy in patients with de novo kidney transplantation (Am. J. Transpl. 2010;10:1401-13), Dr. Saliba and his coinvestigators conducted the current trial.
Following a 30-day run-in period in which liver transplant recipients received tacrolimus with or without mycophenolate mofetil and prednisone, the investigators randomized 719 patients to three arms: two arms with everolimus 3-8 ng/mL, tacrolimus reduced to 3-5 ng/mL, and prednisone, and a control arm with tacrolimus dosed to a standard 8-12 ng/mL plus prednisone. After 4 months, in one of the reduced-dose arms, tacrolimus was withdrawn and the everolimus dose was increased to 8-10 ng/mL (231 patients), whereas the dose of everolimus was kept constant in the other reduced-dose arm (245 patients) and the dose of tacrolimus was kept the same in the control arm (243 patients). Prednisone could be eliminated at 4 months in any arm. However, enrollment into the tacrolimus withdrawal arm was stopped at the time of conversion to everolimus alone because of a high rate of rejection episodes, and the trial’s protocol was amended to compare efficacy between only the reduced-dose and control arms.
By 2 years, the number of patients who completed the study was similar in both the reduced-dose and control arms (82% vs. 84%, respectively), and similar percentages in each group remained on the study medications at 2 years (58% vs. 68%, respectively).
In each group, recipients were mostly men (74%) and white (80%-86%), with a mean age of 54 years and mean donor age of 49 years. They had a Model for End-Stage Liver Disease score of 19, and eGFRs of about 80 mL/min per 1.73 m2.
Mean levels of tacrolimus dropped from 10.5 ng/mL at randomization to 4 ng/mL at 2 years in the dose-reduction arm, compared with 10 ng/mL to 7 ng/mL in the control arm.
At 2 years in the intent-to-treat population, the groups had similar rates of the composite primary end point of treated biopsy-proven acute rejection, graft failure, and death (10.5% in the dose-reduction arm and 12.5% in the control arm). Biopsy-proven acute rejection occurred at a significantly lower rate among dose-reduced patients (6%) than among control patients (13%). All of the episodes of acute rejection in the tacrolimus dose-reduced patients were borderline or mild based on the Banff rejection activity index. However, liver biopsies at 1 and 2 years were only part of the trial’s protocol for hepatitis C virus (HCV)-infected patients. The decision to biopsy was otherwise left up to the physicians of each center.
At 1 year, there appeared to be less fibrosis in patients who received everolimus, Dr. Saliba said in response to a question from the audience. In about half of the 75 HCV-infected patients in the dose-reduction arm, liver biopsies showed less fibrosis of at least stage 1 than in patients with HCV infection in the other group. The investigators are now analyzing 2-year data, he said.
The dose-reduced group maintained significantly better eGFR than the control group, through the duration of the trial, finishing with levels of 66 vs. 78 mL/min per 1.73 m2.
Several types of adverse events with an incidence of at least 10% occurred more often in the dose-reduction arm than in the control arm, including leucopenia (13% vs. 5%), peripheral edema (20% vs. 13%), and hypercholesterolemia (11% vs. 4%).
Proteinuria of less than 0.5 g/24 hours occurred in 92%-93% of the patients; none of the patients had severe proteinuria of 3 g/24 hours or more.
One audience member noted that the most important patients to study in this clinical population are those on the borderline of renal failure with low eGFR and elevated creatinine, who would benefit most from improved renal function. Dr. Saliba said that at the time of randomization, the investigators looked at levels of eGFR in each group and over the course of the 2 years, more patients in the standard-dose tacrolimus arm had worsened renal function, whereas many in the dose-reduction arm had improved or at least stable renal function, and few had worsened function.
The study was sponsored by Novartis, which manufactures everolimus. Dr. Saliba reported financial ties to Novartis and Astellas Pharma (manufacturer of tacrolimus), as well as other companies that manufacture drugs used by liver transplant patients. Several other investigators reported financial ties to companies that manufacture antirejection drugs used in liver transplant patients, including Novartis. Three study investigators are employees of Novartis.
AT THE ANNUAL MEETING OF THE AMERICAN ASSOCIATION FOR THE STUDY OF LIVER DISEASES
Major Finding: At 2 years in the intent-to-treat population, the groups had similar rates of the composite primary end point of treated biopsy-proven acute rejection, graft failure, and death (10.5% in the dose-reduction arm and 12.5% in the control arm).
Data Source: This was a randomized, open-label, multicenter, controlled trial of 719 liver transplant recipients testing the addition of everolimus with or without the withdrawal of tacrolimus (4 months after transplant) or tacrolimus alone.
Disclosures: The study was sponsored by Novartis, which manufactures everolimus. Dr. Saliba reported financial ties to Novartis and Astellas Pharma (manufacturer of tacrolimus), as well as other companies that manufacture drugs used by liver transplant patients. Several other investigators reported financial ties to companies that manufacture antirejection drugs used in liver transplant patients, including Novartis. Three study investigators are employees of Novartis.
Serum Creatinine Elevations: Red Flag After Noncardiac Surgery
SAN DIEGO – Patients who have minor elevations in serum creatinine after noncardiac surgery may be more likely to require a longer postoperative hospital stay and face a twofold increased risk of dying during that stay, preliminary data from a German study have shown.
"This is a big problem, because minor kidney dysfunction may not be noticed postoperatively," Dr. Felix Kork said in an interview during a poster session at Kidney Week 2012 "About 2% of people in general have a small increase in serum creatinine. They are at a greater risk of dying and staying longer in the hospital. Therapeutic options are needed to prevent this minor kidney dysfunction perioperatively."
Dr. Kork of the department of anesthesiology and intensive care medicine at Charité Hospital in Berlin and his associates retrospectively studied the records of 27,616 patients who underwent noncardiac surgery at Charité between 2006 and 2012. The researchers evaluated perioperative renal function by serum creatinine level.
After doing a multivariate analysis that adjusted for age, comorbidities, renal function, high-risk surgery, and postoperative admission to the ICU, the researchers observed that minor elevations in serum creatinine (defined as a range from 0.25 to 0.50 mg/dL) were independently associated with a prolonged hospital length of stay (HR for early discharge, 0.81) and a twofold increased risk of death during the postoperative hospital stay (OR, 1.99) compared with patients without an increase in serum creatinine level. Both findings were statistically significant.
"While adjusting for covariates, we also found that having received radio contrast agent before surgery is independently associated with a greater risk of mortality and hospital length of stay, whether there was kidney dysfunction after the radio contrast agent or not," Dr. Kork added. "We’re still looking into that [association]. It could be that those patients were sicker."
He acknowledged that the study’s retrospective design is a limitation. Because of this "we can only show the association between the serum creatinine increase and the outcome," he said. "We are planning a prospective study right now." Dr. Kork explained that the current study has been submitted for publication in an undisclosed journal, which will contain more detail about these findings.
Dr. Kork said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Patients who have minor elevations in serum creatinine after noncardiac surgery may be more likely to require a longer postoperative hospital stay and face a twofold increased risk of dying during that stay, preliminary data from a German study have shown.
"This is a big problem, because minor kidney dysfunction may not be noticed postoperatively," Dr. Felix Kork said in an interview during a poster session at Kidney Week 2012 "About 2% of people in general have a small increase in serum creatinine. They are at a greater risk of dying and staying longer in the hospital. Therapeutic options are needed to prevent this minor kidney dysfunction perioperatively."
Dr. Kork of the department of anesthesiology and intensive care medicine at Charité Hospital in Berlin and his associates retrospectively studied the records of 27,616 patients who underwent noncardiac surgery at Charité between 2006 and 2012. The researchers evaluated perioperative renal function by serum creatinine level.
After doing a multivariate analysis that adjusted for age, comorbidities, renal function, high-risk surgery, and postoperative admission to the ICU, the researchers observed that minor elevations in serum creatinine (defined as a range from 0.25 to 0.50 mg/dL) were independently associated with a prolonged hospital length of stay (HR for early discharge, 0.81) and a twofold increased risk of death during the postoperative hospital stay (OR, 1.99) compared with patients without an increase in serum creatinine level. Both findings were statistically significant.
"While adjusting for covariates, we also found that having received radio contrast agent before surgery is independently associated with a greater risk of mortality and hospital length of stay, whether there was kidney dysfunction after the radio contrast agent or not," Dr. Kork added. "We’re still looking into that [association]. It could be that those patients were sicker."
He acknowledged that the study’s retrospective design is a limitation. Because of this "we can only show the association between the serum creatinine increase and the outcome," he said. "We are planning a prospective study right now." Dr. Kork explained that the current study has been submitted for publication in an undisclosed journal, which will contain more detail about these findings.
Dr. Kork said that he had no relevant financial conflicts to disclose.
SAN DIEGO – Patients who have minor elevations in serum creatinine after noncardiac surgery may be more likely to require a longer postoperative hospital stay and face a twofold increased risk of dying during that stay, preliminary data from a German study have shown.
"This is a big problem, because minor kidney dysfunction may not be noticed postoperatively," Dr. Felix Kork said in an interview during a poster session at Kidney Week 2012 "About 2% of people in general have a small increase in serum creatinine. They are at a greater risk of dying and staying longer in the hospital. Therapeutic options are needed to prevent this minor kidney dysfunction perioperatively."
Dr. Kork of the department of anesthesiology and intensive care medicine at Charité Hospital in Berlin and his associates retrospectively studied the records of 27,616 patients who underwent noncardiac surgery at Charité between 2006 and 2012. The researchers evaluated perioperative renal function by serum creatinine level.
After doing a multivariate analysis that adjusted for age, comorbidities, renal function, high-risk surgery, and postoperative admission to the ICU, the researchers observed that minor elevations in serum creatinine (defined as a range from 0.25 to 0.50 mg/dL) were independently associated with a prolonged hospital length of stay (HR for early discharge, 0.81) and a twofold increased risk of death during the postoperative hospital stay (OR, 1.99) compared with patients without an increase in serum creatinine level. Both findings were statistically significant.
"While adjusting for covariates, we also found that having received radio contrast agent before surgery is independently associated with a greater risk of mortality and hospital length of stay, whether there was kidney dysfunction after the radio contrast agent or not," Dr. Kork added. "We’re still looking into that [association]. It could be that those patients were sicker."
He acknowledged that the study’s retrospective design is a limitation. Because of this "we can only show the association between the serum creatinine increase and the outcome," he said. "We are planning a prospective study right now." Dr. Kork explained that the current study has been submitted for publication in an undisclosed journal, which will contain more detail about these findings.
Dr. Kork said that he had no relevant financial conflicts to disclose.
AT KIDNEY WEEK 2012
Major Finding: Patients who experienced minor elevations in serum creatinine after noncardiac surgery had an increased risk of a prolonged hospital length of stay (HR for early discharge, 0.81; P less than .01) and a twofold increased risk of death during the postoperative hospital stay (OR, 1.99; P less than .01).
Data Source: A study of 27,616 patients who underwent noncardiac surgery at Charité Hospital in Berlin between 2006 and 2012.
Disclosures: Dr. Kork said he had no relevant financial conflicts to disclose.
ACE Inhibitors Up Mortality Risk When Given Prior to Scleroderma Renal Crisis
WASHINGTON – Exposure to angiotensin-converting enzyme inhibitors prior to the onset of renal crisis in patients with scleroderma increases the risk of death, according to 1-year findings from the prospective observational International Scleroderma Renal Crisis Survey.
The findings, which contrast with those from a preliminary analysis reported last year, suggest that clinicians caring for patients with systemic scleroderma should exercise caution when prescribing angiotensin-converting enzyme (ACE) inhibitors, Dr. Marie Hudson said at the annual meeting of the American College of Rheumatology, where she reported the survey results.
Of 75 patients who experienced scleroderma renal crisis (SRC) in the course of the study, 16 were taking an ACE inhibitor prior to onset of SRC. At the 1-year follow-up, 27 (36%) of the patients had died and 25% remained on dialysis. After adjusting for differences in prednisone exposure and history of systemic hypertension, ACE inhibitor exposure prior to SRC, compared with no such exposure, was associated with significantly increased risk of death (adjusted hazard ratio, 2.52), said Dr. Hudson of the division of rheumatology at McGill University, Montreal.
SRC is a rare but life-threatening complication of systemic sclerosis that typically presents with malignant hypertension and acute renal failure.
"Prior to the advent of ACE inhibitors, it was almost a universally deadly complication of scleroderma, but since the advent of ACE inhibitors, the outcomes of patients with scleroderma renal crisis have improved tremendously," Dr. Hudson said, noting also that some evidence suggest that the incidence of SRC has decreased over the past two decades as well – due, perhaps, to more liberal use of ACE inhibitors.
"So, given the benefits of ACE inhibitors to treat SRC along with this perceived decrease in the incidence of SRC, the prophylactic use of ACE inhibitors to prevent SRC has been considered. However, some would argue that there’s no clear physiologic rationale for this," she said, explaining that most patients with SRC are not hyperreninemic prior to renal crisis and that prophylactic treatment could mask hypertension and delay diagnosis, thus leading to worse outcomes in those who develop SRC.
In fact, recent retrospective data support the idea that those exposed prior to SRC may have worse outcomes, she noted.
The findings of this survey, which are important given the widespread availability of ACE inhibitors, confirm that, she said.
Patients included in the study were identified by physicians from numerous practices around the world who had agreed to participate in the survey. A total of 589 physicians were asked biweekly if they had made a diagnosis of SRC, and if so, they filled out a short case report form at that time and then submitted another 1 year later.
The patients had a mean age of 52 years, and 67% were women. Most (76%) had diffuse systemic scleroderma with a median disease duration of 1.5 years. SRC was hypertensive in 71 patients; only 5 patients had normotensive SRC, she noted.
The rate of prednisone use was surprisingly high at nearly 50% overall, but, more importantly, the mean daily dose was twice as high in the group of patients who were not exposed to ACE inhibitors prior to SRC (mean dose of 18 mg/day vs. 9 mg/day in the exposed group), Dr. Hudson said.
However, even after adjusting for prednisone use, the risk of death in exposed patients was more than twice that of nonexposed patients, and the difference was statistically significant, she said.
Although the precise risk of death after SRC remains uncertain, it does appear that caution when using ACE inhibitors in these patients is warranted, especially early in disease when the risk of SRC is the greatest, she said.
Dr. Hudson had no disclosures to report.
WASHINGTON – Exposure to angiotensin-converting enzyme inhibitors prior to the onset of renal crisis in patients with scleroderma increases the risk of death, according to 1-year findings from the prospective observational International Scleroderma Renal Crisis Survey.
The findings, which contrast with those from a preliminary analysis reported last year, suggest that clinicians caring for patients with systemic scleroderma should exercise caution when prescribing angiotensin-converting enzyme (ACE) inhibitors, Dr. Marie Hudson said at the annual meeting of the American College of Rheumatology, where she reported the survey results.
Of 75 patients who experienced scleroderma renal crisis (SRC) in the course of the study, 16 were taking an ACE inhibitor prior to onset of SRC. At the 1-year follow-up, 27 (36%) of the patients had died and 25% remained on dialysis. After adjusting for differences in prednisone exposure and history of systemic hypertension, ACE inhibitor exposure prior to SRC, compared with no such exposure, was associated with significantly increased risk of death (adjusted hazard ratio, 2.52), said Dr. Hudson of the division of rheumatology at McGill University, Montreal.
SRC is a rare but life-threatening complication of systemic sclerosis that typically presents with malignant hypertension and acute renal failure.
"Prior to the advent of ACE inhibitors, it was almost a universally deadly complication of scleroderma, but since the advent of ACE inhibitors, the outcomes of patients with scleroderma renal crisis have improved tremendously," Dr. Hudson said, noting also that some evidence suggest that the incidence of SRC has decreased over the past two decades as well – due, perhaps, to more liberal use of ACE inhibitors.
"So, given the benefits of ACE inhibitors to treat SRC along with this perceived decrease in the incidence of SRC, the prophylactic use of ACE inhibitors to prevent SRC has been considered. However, some would argue that there’s no clear physiologic rationale for this," she said, explaining that most patients with SRC are not hyperreninemic prior to renal crisis and that prophylactic treatment could mask hypertension and delay diagnosis, thus leading to worse outcomes in those who develop SRC.
In fact, recent retrospective data support the idea that those exposed prior to SRC may have worse outcomes, she noted.
The findings of this survey, which are important given the widespread availability of ACE inhibitors, confirm that, she said.
Patients included in the study were identified by physicians from numerous practices around the world who had agreed to participate in the survey. A total of 589 physicians were asked biweekly if they had made a diagnosis of SRC, and if so, they filled out a short case report form at that time and then submitted another 1 year later.
The patients had a mean age of 52 years, and 67% were women. Most (76%) had diffuse systemic scleroderma with a median disease duration of 1.5 years. SRC was hypertensive in 71 patients; only 5 patients had normotensive SRC, she noted.
The rate of prednisone use was surprisingly high at nearly 50% overall, but, more importantly, the mean daily dose was twice as high in the group of patients who were not exposed to ACE inhibitors prior to SRC (mean dose of 18 mg/day vs. 9 mg/day in the exposed group), Dr. Hudson said.
However, even after adjusting for prednisone use, the risk of death in exposed patients was more than twice that of nonexposed patients, and the difference was statistically significant, she said.
Although the precise risk of death after SRC remains uncertain, it does appear that caution when using ACE inhibitors in these patients is warranted, especially early in disease when the risk of SRC is the greatest, she said.
Dr. Hudson had no disclosures to report.
WASHINGTON – Exposure to angiotensin-converting enzyme inhibitors prior to the onset of renal crisis in patients with scleroderma increases the risk of death, according to 1-year findings from the prospective observational International Scleroderma Renal Crisis Survey.
The findings, which contrast with those from a preliminary analysis reported last year, suggest that clinicians caring for patients with systemic scleroderma should exercise caution when prescribing angiotensin-converting enzyme (ACE) inhibitors, Dr. Marie Hudson said at the annual meeting of the American College of Rheumatology, where she reported the survey results.
Of 75 patients who experienced scleroderma renal crisis (SRC) in the course of the study, 16 were taking an ACE inhibitor prior to onset of SRC. At the 1-year follow-up, 27 (36%) of the patients had died and 25% remained on dialysis. After adjusting for differences in prednisone exposure and history of systemic hypertension, ACE inhibitor exposure prior to SRC, compared with no such exposure, was associated with significantly increased risk of death (adjusted hazard ratio, 2.52), said Dr. Hudson of the division of rheumatology at McGill University, Montreal.
SRC is a rare but life-threatening complication of systemic sclerosis that typically presents with malignant hypertension and acute renal failure.
"Prior to the advent of ACE inhibitors, it was almost a universally deadly complication of scleroderma, but since the advent of ACE inhibitors, the outcomes of patients with scleroderma renal crisis have improved tremendously," Dr. Hudson said, noting also that some evidence suggest that the incidence of SRC has decreased over the past two decades as well – due, perhaps, to more liberal use of ACE inhibitors.
"So, given the benefits of ACE inhibitors to treat SRC along with this perceived decrease in the incidence of SRC, the prophylactic use of ACE inhibitors to prevent SRC has been considered. However, some would argue that there’s no clear physiologic rationale for this," she said, explaining that most patients with SRC are not hyperreninemic prior to renal crisis and that prophylactic treatment could mask hypertension and delay diagnosis, thus leading to worse outcomes in those who develop SRC.
In fact, recent retrospective data support the idea that those exposed prior to SRC may have worse outcomes, she noted.
The findings of this survey, which are important given the widespread availability of ACE inhibitors, confirm that, she said.
Patients included in the study were identified by physicians from numerous practices around the world who had agreed to participate in the survey. A total of 589 physicians were asked biweekly if they had made a diagnosis of SRC, and if so, they filled out a short case report form at that time and then submitted another 1 year later.
The patients had a mean age of 52 years, and 67% were women. Most (76%) had diffuse systemic scleroderma with a median disease duration of 1.5 years. SRC was hypertensive in 71 patients; only 5 patients had normotensive SRC, she noted.
The rate of prednisone use was surprisingly high at nearly 50% overall, but, more importantly, the mean daily dose was twice as high in the group of patients who were not exposed to ACE inhibitors prior to SRC (mean dose of 18 mg/day vs. 9 mg/day in the exposed group), Dr. Hudson said.
However, even after adjusting for prednisone use, the risk of death in exposed patients was more than twice that of nonexposed patients, and the difference was statistically significant, she said.
Although the precise risk of death after SRC remains uncertain, it does appear that caution when using ACE inhibitors in these patients is warranted, especially early in disease when the risk of SRC is the greatest, she said.
Dr. Hudson had no disclosures to report.
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF RHEUMATOLOGY
Major Finding: ACE inhibitor exposure prior to SRC, compared with no such exposure, was found to be associated with significantly increased risk of death (adjusted hazard ratio, 2.52).
Data Source: This finding comes from a prospective international cohort study (International Scleroderma Renal Crisis Survey).
Disclosures: Dr. Hudson had no disclosures to report.
Sildenafil Protects Sexual Function Following Prostate Radiation
BOSTON – The drugs that have revitalized the love lives of millions of aging men may also help preserve sexual function in men undergoing radiation therapy for prostate cancer, investigators reported at the annual meeting of the American Society for Radiation Oncology.
Men who took sildenafil citrate (Viagra) before, during, and for 6 months after radiotherapy for prostate cancer had better sexual function and reported better overall satisfaction than did men who took placebo in a randomized double-blind trial, said Dr. Michael J. Zelefsky, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York City.
Men who were also treated with androgen deprivation, however, did not appear to experience a benefit from sildenafil and were excluded from the analysis.
"We believe our study is a very important one, for it demonstrates proof of principle that penile rehabilitation is important in the population of radiotherapy patients treated for prostate cancer, and demonstrates a significant benefit for improved sexual function outcomes," Dr. Zelefsky said at a plenary session.
Studies in animal models have suggested that phosphodiesterase-5 (PDE5) inhibitors such as sildenafil, vardenafil (Levitra), and tadalafil (Cialis) could help to preserve or rehabilitate penile function by protecting the vascular endothelium of the corpus cavernosum of the penis and smooth muscle tissue involved in erections.
Dr. Zelefsky pointed to a European randomized trial showing that patients who had undergone bilateral nerve-sparing prostatectomy and were randomized to vardenafil had improved spontaneous erections compared with placebo-taking controls (Eur. Urol. 2008;54:924-31).
Pretreatment Potency Assessed
The current study enrolled 295 men who had excellent sexual function (defined as a score of 17 or greater on the International Index of Erectile Function 5 [IIEF-5]) and were scheduled to undergo radiotherapy to the prostate with either external-beam radiation (EBRT) or brachytherapy. They were randomly assigned on a 2:1 basis to sildenafil or placebo, respectively.
Sildenafil was given in a 50-mg dose starting 3 days before therapy and continuing out to 6 months. Patients were followed with the patient-derived IIEF-5 (including domains of erectile function, orgasmic function, intercourse satisfaction, and overall satisfaction), International Prostate Symptom Score (IPSS), and a quality of life questionnaire every 3 months for the first year, and then every 6 months up to 2 years.
"Thirty one patients were treated with androgen-deprivation therapy, and when we looked at erectile function scores over time, there were no significant differences or improvements noted with the use of daily sildenafil compared to the placebo group, suggesting that there was no apparent benefit among this cohort of patients. For this reason, we excluded this cohort and turned our attention to a group of patients who did not receive androgen deprivation therapy, leaving us with an evaluable cohort of 142 patients," Dr. Zelefsky said. The analysis included patients who completed surveys before treatment and at least one additional time period.
There were no significant between-group differences at baseline in factors that might affect erectile function, such as smoking history, diabetes, or hypertension.
Overall total IEFF-5 scores were significantly higher among patients in the sildenafil arm at 6 (P = .006), 12 (P=.02) and 24 months (P = .04) after therapy. However, at 24 months, there were significant differences in favor of sildenafil only in the IEFF-5 domains of sexual desire (P = .001) and overall satisfaction (P = .04).
The investigators also noted that the differences between the treatment groups became less apparent beyond 12 months.
Does This Set a New Standard?
The study had a few minor limitations, including variations in treatment, once-daily rather than more frequent dosing, and the lack of information on a relationship or partner effect, but these do not detract from the conclusion, said invited discussant Dr. Thomas M. Pisansky, professor of radiation oncology at Mayo Clinic, Rochester, Minn.
"Nonetheless, this does serve as an additional test of proof of principle of early PDE5 inhibitor use. Does this represent a new standard? I believe that for the time being it certainly does, but additional study is warranted, and that is ongoing," he said.
Dr. Pisansky added that it is incumbent upon radiation oncologists, when evaluating patients for radiotherapy to the prostate, to incorporate a validated instrument of sexual function such as the IIEF-5.
Dr. Zelefsky and a coauthor disclosed receiving grants from Pfizer, maker of Viagra. Dr. Pisansky reported no conflicts of interest.
BOSTON – The drugs that have revitalized the love lives of millions of aging men may also help preserve sexual function in men undergoing radiation therapy for prostate cancer, investigators reported at the annual meeting of the American Society for Radiation Oncology.
Men who took sildenafil citrate (Viagra) before, during, and for 6 months after radiotherapy for prostate cancer had better sexual function and reported better overall satisfaction than did men who took placebo in a randomized double-blind trial, said Dr. Michael J. Zelefsky, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York City.
Men who were also treated with androgen deprivation, however, did not appear to experience a benefit from sildenafil and were excluded from the analysis.
"We believe our study is a very important one, for it demonstrates proof of principle that penile rehabilitation is important in the population of radiotherapy patients treated for prostate cancer, and demonstrates a significant benefit for improved sexual function outcomes," Dr. Zelefsky said at a plenary session.
Studies in animal models have suggested that phosphodiesterase-5 (PDE5) inhibitors such as sildenafil, vardenafil (Levitra), and tadalafil (Cialis) could help to preserve or rehabilitate penile function by protecting the vascular endothelium of the corpus cavernosum of the penis and smooth muscle tissue involved in erections.
Dr. Zelefsky pointed to a European randomized trial showing that patients who had undergone bilateral nerve-sparing prostatectomy and were randomized to vardenafil had improved spontaneous erections compared with placebo-taking controls (Eur. Urol. 2008;54:924-31).
Pretreatment Potency Assessed
The current study enrolled 295 men who had excellent sexual function (defined as a score of 17 or greater on the International Index of Erectile Function 5 [IIEF-5]) and were scheduled to undergo radiotherapy to the prostate with either external-beam radiation (EBRT) or brachytherapy. They were randomly assigned on a 2:1 basis to sildenafil or placebo, respectively.
Sildenafil was given in a 50-mg dose starting 3 days before therapy and continuing out to 6 months. Patients were followed with the patient-derived IIEF-5 (including domains of erectile function, orgasmic function, intercourse satisfaction, and overall satisfaction), International Prostate Symptom Score (IPSS), and a quality of life questionnaire every 3 months for the first year, and then every 6 months up to 2 years.
"Thirty one patients were treated with androgen-deprivation therapy, and when we looked at erectile function scores over time, there were no significant differences or improvements noted with the use of daily sildenafil compared to the placebo group, suggesting that there was no apparent benefit among this cohort of patients. For this reason, we excluded this cohort and turned our attention to a group of patients who did not receive androgen deprivation therapy, leaving us with an evaluable cohort of 142 patients," Dr. Zelefsky said. The analysis included patients who completed surveys before treatment and at least one additional time period.
There were no significant between-group differences at baseline in factors that might affect erectile function, such as smoking history, diabetes, or hypertension.
Overall total IEFF-5 scores were significantly higher among patients in the sildenafil arm at 6 (P = .006), 12 (P=.02) and 24 months (P = .04) after therapy. However, at 24 months, there were significant differences in favor of sildenafil only in the IEFF-5 domains of sexual desire (P = .001) and overall satisfaction (P = .04).
The investigators also noted that the differences between the treatment groups became less apparent beyond 12 months.
Does This Set a New Standard?
The study had a few minor limitations, including variations in treatment, once-daily rather than more frequent dosing, and the lack of information on a relationship or partner effect, but these do not detract from the conclusion, said invited discussant Dr. Thomas M. Pisansky, professor of radiation oncology at Mayo Clinic, Rochester, Minn.
"Nonetheless, this does serve as an additional test of proof of principle of early PDE5 inhibitor use. Does this represent a new standard? I believe that for the time being it certainly does, but additional study is warranted, and that is ongoing," he said.
Dr. Pisansky added that it is incumbent upon radiation oncologists, when evaluating patients for radiotherapy to the prostate, to incorporate a validated instrument of sexual function such as the IIEF-5.
Dr. Zelefsky and a coauthor disclosed receiving grants from Pfizer, maker of Viagra. Dr. Pisansky reported no conflicts of interest.
BOSTON – The drugs that have revitalized the love lives of millions of aging men may also help preserve sexual function in men undergoing radiation therapy for prostate cancer, investigators reported at the annual meeting of the American Society for Radiation Oncology.
Men who took sildenafil citrate (Viagra) before, during, and for 6 months after radiotherapy for prostate cancer had better sexual function and reported better overall satisfaction than did men who took placebo in a randomized double-blind trial, said Dr. Michael J. Zelefsky, a radiation oncologist at Memorial Sloan-Kettering Cancer Center in New York City.
Men who were also treated with androgen deprivation, however, did not appear to experience a benefit from sildenafil and were excluded from the analysis.
"We believe our study is a very important one, for it demonstrates proof of principle that penile rehabilitation is important in the population of radiotherapy patients treated for prostate cancer, and demonstrates a significant benefit for improved sexual function outcomes," Dr. Zelefsky said at a plenary session.
Studies in animal models have suggested that phosphodiesterase-5 (PDE5) inhibitors such as sildenafil, vardenafil (Levitra), and tadalafil (Cialis) could help to preserve or rehabilitate penile function by protecting the vascular endothelium of the corpus cavernosum of the penis and smooth muscle tissue involved in erections.
Dr. Zelefsky pointed to a European randomized trial showing that patients who had undergone bilateral nerve-sparing prostatectomy and were randomized to vardenafil had improved spontaneous erections compared with placebo-taking controls (Eur. Urol. 2008;54:924-31).
Pretreatment Potency Assessed
The current study enrolled 295 men who had excellent sexual function (defined as a score of 17 or greater on the International Index of Erectile Function 5 [IIEF-5]) and were scheduled to undergo radiotherapy to the prostate with either external-beam radiation (EBRT) or brachytherapy. They were randomly assigned on a 2:1 basis to sildenafil or placebo, respectively.
Sildenafil was given in a 50-mg dose starting 3 days before therapy and continuing out to 6 months. Patients were followed with the patient-derived IIEF-5 (including domains of erectile function, orgasmic function, intercourse satisfaction, and overall satisfaction), International Prostate Symptom Score (IPSS), and a quality of life questionnaire every 3 months for the first year, and then every 6 months up to 2 years.
"Thirty one patients were treated with androgen-deprivation therapy, and when we looked at erectile function scores over time, there were no significant differences or improvements noted with the use of daily sildenafil compared to the placebo group, suggesting that there was no apparent benefit among this cohort of patients. For this reason, we excluded this cohort and turned our attention to a group of patients who did not receive androgen deprivation therapy, leaving us with an evaluable cohort of 142 patients," Dr. Zelefsky said. The analysis included patients who completed surveys before treatment and at least one additional time period.
There were no significant between-group differences at baseline in factors that might affect erectile function, such as smoking history, diabetes, or hypertension.
Overall total IEFF-5 scores were significantly higher among patients in the sildenafil arm at 6 (P = .006), 12 (P=.02) and 24 months (P = .04) after therapy. However, at 24 months, there were significant differences in favor of sildenafil only in the IEFF-5 domains of sexual desire (P = .001) and overall satisfaction (P = .04).
The investigators also noted that the differences between the treatment groups became less apparent beyond 12 months.
Does This Set a New Standard?
The study had a few minor limitations, including variations in treatment, once-daily rather than more frequent dosing, and the lack of information on a relationship or partner effect, but these do not detract from the conclusion, said invited discussant Dr. Thomas M. Pisansky, professor of radiation oncology at Mayo Clinic, Rochester, Minn.
"Nonetheless, this does serve as an additional test of proof of principle of early PDE5 inhibitor use. Does this represent a new standard? I believe that for the time being it certainly does, but additional study is warranted, and that is ongoing," he said.
Dr. Pisansky added that it is incumbent upon radiation oncologists, when evaluating patients for radiotherapy to the prostate, to incorporate a validated instrument of sexual function such as the IIEF-5.
Dr. Zelefsky and a coauthor disclosed receiving grants from Pfizer, maker of Viagra. Dr. Pisansky reported no conflicts of interest.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY FOR RADIATION ONCOLOGY
Major Finding: Overall total International Index of Erectile Function scores were significantly higher among men who took sildenafil at 6 (P = .006), 12 (P=.02), and 24 months (P = .04) after radiation therapy to the prostate.
Data Source: This was a randomized double-blind placebo controlled trial.
Disclosures: Dr. Zelefsky and a coauthor disclosed receiving grants from Pfizer, maker of Viagra. Dr. Pisansky reported no conflicts of interest.
Kidney Disease a Risk Factor for Death in Pregnancy
SAN DIEGO – Pregnant women with kidney disease face an increased risk of adverse maternal outcomes including maternal mortality independent of underlying comorbid conditions that can occur with kidney disease, according to Dr. Shailendra Sharma.
"Any degree of kidney disease during pregnancy should be recognized and should be treated promptly with respect because we now know that can lead to bad outcomes down the road," Dr. Sharma said in an interview during a poster session at the Kidney Week 2012. "This is not something that should be underestimated."
Dr. Sharma, a second-year renal fellow at the University of Colorado, Aurora, and his associates retrospectively studied the records of 646 women with kidney disease who gave birth in Colorado and Utah between 2000 and 2011 at facilities operated by Intermountain Health Care. For comparison, the researchers randomly selected the records of 62,757 pregnancies from women without kidney disease.
Kidney disease was defined by ICD-9 code, and adverse maternal outcomes were defined as preterm delivery (prior to 37 weeks’ gestation), delivery by cesarean section, length of hospital stay, and maternal death. The researchers used multivariate logistic regression analysis to examine the association between kidney disease and adverse maternal outcomes. Covariates included in the fully adjusted model were maternal age, race, history of diabetes, chronic hypertension, liver disease, and connective tissue disorders.
The mean age of patients was 28 years. Compared with women who did not have kidney disease, those who did were significantly more likely to have comorbid conditions including diabetes (12% vs. 1%, respectively); chronic hypertension (2% vs. 7%); liver disease (9% vs. 1%); and connective tissue disorders (7% vs. 0.4%). They also were more likely to have preeclampsia/eclampsia (11% vs. 3%), to have a longer hospital stay (a mean of 3 vs. 2 days), and to give birth to a lower-weight infant (a mean of 3,067 g vs. 3,325 g).
After the investigators adjusted for age, race, history of diabetes, hypertension, liver disease, and connective tissue disorders, Dr. Sharma and his associates found that pregnant women with kidney disease had a significantly increased risk of death (OR, 3.38); preterm delivery (OR, 1.95); delivery via C-section (OR, 1.38); and longer length of hospital stay (OR, 1.39). "The most striking finding was the association of kidney disease with maternal mortality," Dr. Sharma said at the meeting, which was sponsored by the American Society of Nephrology. "The magnitude of this association surprised us."
He said that the retrospective design of the study is a limitation. "If there’s a prospective study moving forward, specifically designed to answer these questions, then it probably would help us establish the causality."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Sharma said he had no relevant financial conflicts to disclose.
SAN DIEGO – Pregnant women with kidney disease face an increased risk of adverse maternal outcomes including maternal mortality independent of underlying comorbid conditions that can occur with kidney disease, according to Dr. Shailendra Sharma.
"Any degree of kidney disease during pregnancy should be recognized and should be treated promptly with respect because we now know that can lead to bad outcomes down the road," Dr. Sharma said in an interview during a poster session at the Kidney Week 2012. "This is not something that should be underestimated."
Dr. Sharma, a second-year renal fellow at the University of Colorado, Aurora, and his associates retrospectively studied the records of 646 women with kidney disease who gave birth in Colorado and Utah between 2000 and 2011 at facilities operated by Intermountain Health Care. For comparison, the researchers randomly selected the records of 62,757 pregnancies from women without kidney disease.
Kidney disease was defined by ICD-9 code, and adverse maternal outcomes were defined as preterm delivery (prior to 37 weeks’ gestation), delivery by cesarean section, length of hospital stay, and maternal death. The researchers used multivariate logistic regression analysis to examine the association between kidney disease and adverse maternal outcomes. Covariates included in the fully adjusted model were maternal age, race, history of diabetes, chronic hypertension, liver disease, and connective tissue disorders.
The mean age of patients was 28 years. Compared with women who did not have kidney disease, those who did were significantly more likely to have comorbid conditions including diabetes (12% vs. 1%, respectively); chronic hypertension (2% vs. 7%); liver disease (9% vs. 1%); and connective tissue disorders (7% vs. 0.4%). They also were more likely to have preeclampsia/eclampsia (11% vs. 3%), to have a longer hospital stay (a mean of 3 vs. 2 days), and to give birth to a lower-weight infant (a mean of 3,067 g vs. 3,325 g).
After the investigators adjusted for age, race, history of diabetes, hypertension, liver disease, and connective tissue disorders, Dr. Sharma and his associates found that pregnant women with kidney disease had a significantly increased risk of death (OR, 3.38); preterm delivery (OR, 1.95); delivery via C-section (OR, 1.38); and longer length of hospital stay (OR, 1.39). "The most striking finding was the association of kidney disease with maternal mortality," Dr. Sharma said at the meeting, which was sponsored by the American Society of Nephrology. "The magnitude of this association surprised us."
He said that the retrospective design of the study is a limitation. "If there’s a prospective study moving forward, specifically designed to answer these questions, then it probably would help us establish the causality."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Sharma said he had no relevant financial conflicts to disclose.
SAN DIEGO – Pregnant women with kidney disease face an increased risk of adverse maternal outcomes including maternal mortality independent of underlying comorbid conditions that can occur with kidney disease, according to Dr. Shailendra Sharma.
"Any degree of kidney disease during pregnancy should be recognized and should be treated promptly with respect because we now know that can lead to bad outcomes down the road," Dr. Sharma said in an interview during a poster session at the Kidney Week 2012. "This is not something that should be underestimated."
Dr. Sharma, a second-year renal fellow at the University of Colorado, Aurora, and his associates retrospectively studied the records of 646 women with kidney disease who gave birth in Colorado and Utah between 2000 and 2011 at facilities operated by Intermountain Health Care. For comparison, the researchers randomly selected the records of 62,757 pregnancies from women without kidney disease.
Kidney disease was defined by ICD-9 code, and adverse maternal outcomes were defined as preterm delivery (prior to 37 weeks’ gestation), delivery by cesarean section, length of hospital stay, and maternal death. The researchers used multivariate logistic regression analysis to examine the association between kidney disease and adverse maternal outcomes. Covariates included in the fully adjusted model were maternal age, race, history of diabetes, chronic hypertension, liver disease, and connective tissue disorders.
The mean age of patients was 28 years. Compared with women who did not have kidney disease, those who did were significantly more likely to have comorbid conditions including diabetes (12% vs. 1%, respectively); chronic hypertension (2% vs. 7%); liver disease (9% vs. 1%); and connective tissue disorders (7% vs. 0.4%). They also were more likely to have preeclampsia/eclampsia (11% vs. 3%), to have a longer hospital stay (a mean of 3 vs. 2 days), and to give birth to a lower-weight infant (a mean of 3,067 g vs. 3,325 g).
After the investigators adjusted for age, race, history of diabetes, hypertension, liver disease, and connective tissue disorders, Dr. Sharma and his associates found that pregnant women with kidney disease had a significantly increased risk of death (OR, 3.38); preterm delivery (OR, 1.95); delivery via C-section (OR, 1.38); and longer length of hospital stay (OR, 1.39). "The most striking finding was the association of kidney disease with maternal mortality," Dr. Sharma said at the meeting, which was sponsored by the American Society of Nephrology. "The magnitude of this association surprised us."
He said that the retrospective design of the study is a limitation. "If there’s a prospective study moving forward, specifically designed to answer these questions, then it probably would help us establish the causality."
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Sharma said he had no relevant financial conflicts to disclose.
AT KIDNEY WEEK 2012
Major Finding: Pregnant women with kidney disease had a significantly increased risk of death (OR, 3.38), preterm delivery (OR, 1.95), delivery via C-section (OR, 1.38), and longer length of hospital stay (OR, 1.39), compared with pregnant women who did not have kidney disease.
Data Source: Data are from a retrospective study comparing 646 women with kidney disease who gave birth in Colorado and Utah between 2000 and 2011 with 62,757 pregnancies from women without kidney disease. The women all gave birth at Intermountain Health Care.
Disclosures: The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Sharma said he had no relevant financial conflicts to disclose.
Recent Evidence Challenges Four Inpatient Management Habits
Why did you order that test? Dr. Leonard S. Feldman wants you to turn off the autopilot and consider the evidence from the medical literature on the following four practices:
• Is the best target hematocrit for a cardiac patient 30% (or a hemoglobin level of 10 g/dL)?
• Should nasogastric lavage be routine in patients with suspected GI bleeds?
• Is it helpful to measure the fractional excretion of sodium or fractional excretion of urea nitrogen when evaluating acute kidney injury?
• Are daily chest x-rays a good idea in patients on mechanical ventilation in an ICU or step-down unit?
"Many of us do all of these things regularly on a reflexive basis," said Dr. Feldman, director of the general medicine comprehensive consult service at Johns Hopkins University, Baltimore.
Yet recent studies challenge the value of these approaches:
Transfusions
Three studies influenced the AABB (formerly the American Association of Blood Banks) to publish a clinical practice guideline this year recommending a restrictive strategy when considering blood transfusions in hospitalized patients with preexisting cardiovascular disease. The AABB says not to transfuse if the hemoglobin level is above 7-8 g/dL and to consider transfusing patients who are symptomatic or who have a hemoglobin level of less than 8 g/dL, the AABB says (Ann. Intern. Med. 2012;157:49-58).
One recent study randomized 502 patients undergoing coronary artery bypass grafting (CABG) or valve replacement who were on cardiac bypass support to either a liberal strategy aiming to maintain a hematocrit of at or above 30% from the start of surgery until discharge from the ICU or a restrictive strategy that aimed to keep the hematocrit at or above 24%.
Both groups were able to meet these goals. The two groups did not differ significantly, however, in a composite end point of 30-day all-cause mortality and severe morbidity occurring during hospitalization (JAMA 2011;304:1559-67).
There was a trend toward higher risk of death with the restrictive strategy, with a hazard ratio of 1.28, but the ratio ranged from 0.6 to 2.7, so the risk was not statistically significant. Analysis of a slew of secondary outcomes also found no significant differences between groups.
Another study randomized 2,016 patients aged 50 years or older who either had a history of cardiovascular disease or cardiac risk factors and who had a hemoglobin level below 10 g/dL after surgery for hip fracture. The liberal strategy triggered transfusion in all patients with a hemoglobin below 10 g/dL. The restrictive strategy transfused only if the patient showed symptoms of anemia or at the physician’s discretion in patients with a hemoglobin level less than 8 g/dL.
Patients received a lot more blood under the liberal strategy compared with the restrictive strategy – 1,866 vs. 652 units of blood, respectively – but the groups did not differ significantly in death rates or the ability to walk across a room without human assistance at 30 and 60 days after surgery (N. Engl. J. Med. 2011;365:2453-62). Sixty days after surgery, 35% of patients in each group had died or were unable to walk across a room unaided.
"This is a trial that you should be showing all of your orthopedists," Dr. Feldman said. "When the orthopedist says that we need to give a patient blood so the patient will be able to do better in rehab" after hip surgery, point to the data showing that this isn’t necessarily true, he suggested.
An earlier trial of 838 critically ill patients who had hemoglobin levels below 9 g/dL within 72 hours of admission to the ICU found 30-day mortality rates of 19% in those randomized to a restrictive transfusion strategy and 23% in patients randomized to a liberal transfusion strategy, a difference that was not statistically significant (N. Engl. J. Med. 1999;340:409-17).
The liberal strategy transfused when hemoglobin levels fell below 10 g/dL and maintained the hemoglobin at 10-12 g/dL. The restrictive strategy waited until hemoglobin levels fell below 7 g/dL before transfusing and maintained hemoglobin at 7-9 g/dL.
"So, we have three different studies here that really seem to indicate that for transfusion of those patients who are at highest risk – cardiac patients – it did not seem to make a difference in their outcomes, particularly in mortality, if you were restrictive or liberal" in setting thresholds for transfusion, Dr. Feldman said. "This is not the same, though, for patients who are having an acute coronary syndrome. We don’t have any good data for them," and the AABB doesn’t recommend for or against liberal or restrictive transfusion thresholds, he added.
The evidence behind the AABB recommendation is only of moderate quality because there are only the three studies. Although the literature generally favors a restrictive versus a liberal approach, there’s no robust evidence for any particular transfusion threshold, said Dr. Daniel S. VanderEnde, a hospitalist and member of the joint transfusion committee at Emory University, Atlanta.
Pointing to the limited amount of research, the AABB itself calls the recommendation’s quality of evidence "very low" and the strength of the recommendation "uncertain."
"Subpar" evidence is one reason that Dr. VanderEnde’s institution leaves transfusion decisions to individual clinicians, he said in an interview. One ICU may transfuse at a hemoglobin threshold "in the mid-7s, and another ICU will have a transfusion threshold in the mid-8s."
Emory is starting a computer order entry protocol requiring physicians to tell why they’re transfusing blood, compared with no previous oversight. "It doesn’t stop them from transfusing for any reason. It is just trying to collect data, in the hopes that maybe they will be more restrictive in their use rather than liberal," he said.
Anecdotally, transfusion practices do seem to be shifting, at least among newly-trained physicians, Dr. VanderEnde added. Five years ago when he would ask medical students about transfusion thresholds, many hewed to "the old 10/30 rule," but far fewer do so today, he said. "The younger orthopedists tend to not transfuse as much as the older orthopedists."
Nasogastric Lavage
Few procedures performed in emergency departments are more painful for patients than nasogastric intubation, and there’s a study to prove that (Ann. Emerg. Med. 1999;33:652-8).
"Patients think nasogastric lavage and nasogastric intubation really stink, so we need to have a good reason to do it," Dr. Feldman said.
And, like all invasive procedures, there are risks involved, Dr. Chad T. Whelan said in an interview. There are only modestly convincing data suggesting that nasogastric lavage can provide some prognostic or "localizing" information (such as differentiating upper vs. lower bleed). "Therefore, the risk/benefit ratio of routinely performing them for all patients has shifted with our increasing understanding of their risks and benefits," said Dr. Whelan, a hospitalist at the University of Chicago.
International consensus recommendations on the management of patients with nonvariceal upper GI bleeding suggest that physicians consider placing a nasogastric tube in selected patients because the findings may have prognostic value – not very helpful advice in decision making, Dr. Feldman said. (Ann. Intern. Med. 2010;152:101-13).
The rationale until now has been that patients with bloody aspirate on nasogastric lavage are significantly more likely to have high-risk GI lesions on endoscopy, compared with patients with clear or bilious aspirates on lavage. But does knowing this improve outcomes?
One review of the literature on how to determine if a patient has a severe upper GI bleed confirmed that a bloody aspirate on nasogastric lavage increases the likelihood of an upper GI bleed but there’s only a mildly increased likelihood of a severe bleed, "and the negative likelihood ratio is not unimpressive," Dr. Feldman said (JAMA 2012;307:1072-19).
Results of a separate propensity-matched retrospective analysis of data on 632 patients admitted with GI bleeding are "as good as we’re going to get on this topic," he said. The study found that getting or not getting nasogastric lavage did not change 30-day mortality, mean length of stay, transfusion requirements, or emergency surgery rates (Gastrointest. Endosc. 2011;74:971-80).
The only things that nasogastric lavage did change were an increase in the rate of patients undergoing endoscopy, a shorter interval to endoscopy, and a shorter length of stay among patients who had endoscopy.
That suggests that there was an individual-provider confounder that the study could not measure. Perhaps emergency physicians or gastroenterologists who order nasogastric lavage are simply more aggressive, Dr. Feldman said. "This is information that you might want to take to your emergency department," he said.
In a joint editorial accompanying the study, an emergency physician and an endoscopist concluded that the practice of nasogastric lavage in patients with acute upper GI bleeding is "antiquated."
Dr. Whelan said the role of nasogastric lavage "is in transition rather than antiquated." As upper GI bleeding epidemiology evolves and endoscopic interventions improve, "the widespread use of nasogastric lavage as a universal piece of the upper GI bleed protocol should decrease. Whether nasogastric lavage ultimately becomes a completely unnecessary procedure remains to be seen," he said.
Physicians at his institution no longer routinely perform nasogastric lavage when evaluating suspected upper GI bleeding, but "it has not completely disappeared from practice, either," he said. That’s less a factor of "aggressive" physicians and more a result of how practice changes and environmental factors, Dr. Whelan added. "Not all emergency rooms have access to full-service endoscopy on site, so emergency room physicians may have a different set of risk/benefit tradeoffs to consider."
Acute Kidney Injury
Can the fractional excretion of sodium (FENa) or fractional excretion of urea nitrogen (FEUN) help narrow the differential diagnosis in acute kidney disease? Widespread use of these measures began after a 17-person study in 1976 suggested that patients with prerenal azotemia had a FENa of less than 1 and patients with acute tubular necrosis had a FENa greater than 3 (JAMA 1976;236:579-81).
The FENa is not perfect, because many intrinsic kidney disorders can cause low FENa and the FENa can be elevated when diuretic use contributes to prerenal states, so a few studies looked at adding the FEUN to the diagnostic tools. Their results were contradictory.
One study of 102 patients in the ICU found that incorporating FEUN was 85% sensitive and 92% specific in detecting prerenal injury, but the study excluded patients with acute glomerulonephritis and obstructive nephropathy, "so you have to make sure that you exclude those patients if you’re going to use FEUN," Dr. Feldman said (Kidney Int. 2002;62:2223-9).
In a separate study of 99 patients, however, the FENa and FEUN were much less impressive in patients with or without diuretics. In patients on diuretics, FEUN had a sensitivity for distinguishing transient from persistent acute kidney injury of 79% and a specificity of 33%, and in patients not on diuretics the sensitivity was 48% and the specificity was 75% (Am. J. Kidney Dis. 2007;50:568-73).
A recent analysis reviewed the literature to provide some guidance for clinicians, but the end result is confusing, Dr. Feldman said. Under best-case scenarios, these two measures would be likely to make a difference in diagnosing the cause of acute kidney injury, but under worst-case scenarios, "they really stink," he said (Cleve. Clin. J. Med. 2012;79:121-6).
The authors cautioned that a single index calculated at a specific time often is insufficient to properly characterize the pathogenesis of acute kidney injury. "In the end, probably FENa and FEUN really don’t help you very much to decide" the reason behind acute kidney injury, Dr. Feldman said.
Chest X-Rays
Routine chest x-rays in mechanically ventilated patients in ICUs provide, well, too many unneeded x-rays, recent data show.
A crossover study that randomized 21 French ICUs to either routine daily chest x-rays for these patients or x-rays on demand found that the on-demand strategy reduced the number of x-rays by 32% without affecting the number of days on ventilation, length of ICU stay, or mortality. With the daily x-ray strategy, 424 patients got 4,607 x-rays, compared with 3,148 x-rays in 425 patients under the on-demand strategy (Lancet 2009;374:1687-93).
Patients had their ventilators changed more often under the on-demand strategy, probably as clinicians were troubleshooting potential problems, but the number of interventions did not differ significantly by x-ray strategy, Dr. Feldman noted.
A meta-analysis this year of eight trials including 7,078 adult ICU patients concluded that routine daily x-rays can be eliminated without increasing adverse outcomes (Radiology 2012;255:386-95).
Dr. Feldman suggested specific goals for these four scenarios, which he presented at the annual meeting of the Society of Hospitalist Medicine.
"Do not reflexively transfuse cardiac patients to hematocrits of 30%. Do not do routine daily chest x-rays. Do not reflexively NG [nasogastric] lavage our patients. And spend more time doing a really great history and physical and thinking about why your patient has acute renal failure than trying to use indices that don’t actually help us very much," he said.
He added a personal goal: "If I can make the residents at Johns Hopkins change, that will be a real feat, because they love to order tests on everybody."
Dr. Feldman, Dr. Whelan, and Dr. VanderEnde reported having no financial disclosures.
Why did you order that test? Dr. Leonard S. Feldman wants you to turn off the autopilot and consider the evidence from the medical literature on the following four practices:
• Is the best target hematocrit for a cardiac patient 30% (or a hemoglobin level of 10 g/dL)?
• Should nasogastric lavage be routine in patients with suspected GI bleeds?
• Is it helpful to measure the fractional excretion of sodium or fractional excretion of urea nitrogen when evaluating acute kidney injury?
• Are daily chest x-rays a good idea in patients on mechanical ventilation in an ICU or step-down unit?
"Many of us do all of these things regularly on a reflexive basis," said Dr. Feldman, director of the general medicine comprehensive consult service at Johns Hopkins University, Baltimore.
Yet recent studies challenge the value of these approaches:
Transfusions
Three studies influenced the AABB (formerly the American Association of Blood Banks) to publish a clinical practice guideline this year recommending a restrictive strategy when considering blood transfusions in hospitalized patients with preexisting cardiovascular disease. The AABB says not to transfuse if the hemoglobin level is above 7-8 g/dL and to consider transfusing patients who are symptomatic or who have a hemoglobin level of less than 8 g/dL, the AABB says (Ann. Intern. Med. 2012;157:49-58).
One recent study randomized 502 patients undergoing coronary artery bypass grafting (CABG) or valve replacement who were on cardiac bypass support to either a liberal strategy aiming to maintain a hematocrit of at or above 30% from the start of surgery until discharge from the ICU or a restrictive strategy that aimed to keep the hematocrit at or above 24%.
Both groups were able to meet these goals. The two groups did not differ significantly, however, in a composite end point of 30-day all-cause mortality and severe morbidity occurring during hospitalization (JAMA 2011;304:1559-67).
There was a trend toward higher risk of death with the restrictive strategy, with a hazard ratio of 1.28, but the ratio ranged from 0.6 to 2.7, so the risk was not statistically significant. Analysis of a slew of secondary outcomes also found no significant differences between groups.
Another study randomized 2,016 patients aged 50 years or older who either had a history of cardiovascular disease or cardiac risk factors and who had a hemoglobin level below 10 g/dL after surgery for hip fracture. The liberal strategy triggered transfusion in all patients with a hemoglobin below 10 g/dL. The restrictive strategy transfused only if the patient showed symptoms of anemia or at the physician’s discretion in patients with a hemoglobin level less than 8 g/dL.
Patients received a lot more blood under the liberal strategy compared with the restrictive strategy – 1,866 vs. 652 units of blood, respectively – but the groups did not differ significantly in death rates or the ability to walk across a room without human assistance at 30 and 60 days after surgery (N. Engl. J. Med. 2011;365:2453-62). Sixty days after surgery, 35% of patients in each group had died or were unable to walk across a room unaided.
"This is a trial that you should be showing all of your orthopedists," Dr. Feldman said. "When the orthopedist says that we need to give a patient blood so the patient will be able to do better in rehab" after hip surgery, point to the data showing that this isn’t necessarily true, he suggested.
An earlier trial of 838 critically ill patients who had hemoglobin levels below 9 g/dL within 72 hours of admission to the ICU found 30-day mortality rates of 19% in those randomized to a restrictive transfusion strategy and 23% in patients randomized to a liberal transfusion strategy, a difference that was not statistically significant (N. Engl. J. Med. 1999;340:409-17).
The liberal strategy transfused when hemoglobin levels fell below 10 g/dL and maintained the hemoglobin at 10-12 g/dL. The restrictive strategy waited until hemoglobin levels fell below 7 g/dL before transfusing and maintained hemoglobin at 7-9 g/dL.
"So, we have three different studies here that really seem to indicate that for transfusion of those patients who are at highest risk – cardiac patients – it did not seem to make a difference in their outcomes, particularly in mortality, if you were restrictive or liberal" in setting thresholds for transfusion, Dr. Feldman said. "This is not the same, though, for patients who are having an acute coronary syndrome. We don’t have any good data for them," and the AABB doesn’t recommend for or against liberal or restrictive transfusion thresholds, he added.
The evidence behind the AABB recommendation is only of moderate quality because there are only the three studies. Although the literature generally favors a restrictive versus a liberal approach, there’s no robust evidence for any particular transfusion threshold, said Dr. Daniel S. VanderEnde, a hospitalist and member of the joint transfusion committee at Emory University, Atlanta.
Pointing to the limited amount of research, the AABB itself calls the recommendation’s quality of evidence "very low" and the strength of the recommendation "uncertain."
"Subpar" evidence is one reason that Dr. VanderEnde’s institution leaves transfusion decisions to individual clinicians, he said in an interview. One ICU may transfuse at a hemoglobin threshold "in the mid-7s, and another ICU will have a transfusion threshold in the mid-8s."
Emory is starting a computer order entry protocol requiring physicians to tell why they’re transfusing blood, compared with no previous oversight. "It doesn’t stop them from transfusing for any reason. It is just trying to collect data, in the hopes that maybe they will be more restrictive in their use rather than liberal," he said.
Anecdotally, transfusion practices do seem to be shifting, at least among newly-trained physicians, Dr. VanderEnde added. Five years ago when he would ask medical students about transfusion thresholds, many hewed to "the old 10/30 rule," but far fewer do so today, he said. "The younger orthopedists tend to not transfuse as much as the older orthopedists."
Nasogastric Lavage
Few procedures performed in emergency departments are more painful for patients than nasogastric intubation, and there’s a study to prove that (Ann. Emerg. Med. 1999;33:652-8).
"Patients think nasogastric lavage and nasogastric intubation really stink, so we need to have a good reason to do it," Dr. Feldman said.
And, like all invasive procedures, there are risks involved, Dr. Chad T. Whelan said in an interview. There are only modestly convincing data suggesting that nasogastric lavage can provide some prognostic or "localizing" information (such as differentiating upper vs. lower bleed). "Therefore, the risk/benefit ratio of routinely performing them for all patients has shifted with our increasing understanding of their risks and benefits," said Dr. Whelan, a hospitalist at the University of Chicago.
International consensus recommendations on the management of patients with nonvariceal upper GI bleeding suggest that physicians consider placing a nasogastric tube in selected patients because the findings may have prognostic value – not very helpful advice in decision making, Dr. Feldman said. (Ann. Intern. Med. 2010;152:101-13).
The rationale until now has been that patients with bloody aspirate on nasogastric lavage are significantly more likely to have high-risk GI lesions on endoscopy, compared with patients with clear or bilious aspirates on lavage. But does knowing this improve outcomes?
One review of the literature on how to determine if a patient has a severe upper GI bleed confirmed that a bloody aspirate on nasogastric lavage increases the likelihood of an upper GI bleed but there’s only a mildly increased likelihood of a severe bleed, "and the negative likelihood ratio is not unimpressive," Dr. Feldman said (JAMA 2012;307:1072-19).
Results of a separate propensity-matched retrospective analysis of data on 632 patients admitted with GI bleeding are "as good as we’re going to get on this topic," he said. The study found that getting or not getting nasogastric lavage did not change 30-day mortality, mean length of stay, transfusion requirements, or emergency surgery rates (Gastrointest. Endosc. 2011;74:971-80).
The only things that nasogastric lavage did change were an increase in the rate of patients undergoing endoscopy, a shorter interval to endoscopy, and a shorter length of stay among patients who had endoscopy.
That suggests that there was an individual-provider confounder that the study could not measure. Perhaps emergency physicians or gastroenterologists who order nasogastric lavage are simply more aggressive, Dr. Feldman said. "This is information that you might want to take to your emergency department," he said.
In a joint editorial accompanying the study, an emergency physician and an endoscopist concluded that the practice of nasogastric lavage in patients with acute upper GI bleeding is "antiquated."
Dr. Whelan said the role of nasogastric lavage "is in transition rather than antiquated." As upper GI bleeding epidemiology evolves and endoscopic interventions improve, "the widespread use of nasogastric lavage as a universal piece of the upper GI bleed protocol should decrease. Whether nasogastric lavage ultimately becomes a completely unnecessary procedure remains to be seen," he said.
Physicians at his institution no longer routinely perform nasogastric lavage when evaluating suspected upper GI bleeding, but "it has not completely disappeared from practice, either," he said. That’s less a factor of "aggressive" physicians and more a result of how practice changes and environmental factors, Dr. Whelan added. "Not all emergency rooms have access to full-service endoscopy on site, so emergency room physicians may have a different set of risk/benefit tradeoffs to consider."
Acute Kidney Injury
Can the fractional excretion of sodium (FENa) or fractional excretion of urea nitrogen (FEUN) help narrow the differential diagnosis in acute kidney disease? Widespread use of these measures began after a 17-person study in 1976 suggested that patients with prerenal azotemia had a FENa of less than 1 and patients with acute tubular necrosis had a FENa greater than 3 (JAMA 1976;236:579-81).
The FENa is not perfect, because many intrinsic kidney disorders can cause low FENa and the FENa can be elevated when diuretic use contributes to prerenal states, so a few studies looked at adding the FEUN to the diagnostic tools. Their results were contradictory.
One study of 102 patients in the ICU found that incorporating FEUN was 85% sensitive and 92% specific in detecting prerenal injury, but the study excluded patients with acute glomerulonephritis and obstructive nephropathy, "so you have to make sure that you exclude those patients if you’re going to use FEUN," Dr. Feldman said (Kidney Int. 2002;62:2223-9).
In a separate study of 99 patients, however, the FENa and FEUN were much less impressive in patients with or without diuretics. In patients on diuretics, FEUN had a sensitivity for distinguishing transient from persistent acute kidney injury of 79% and a specificity of 33%, and in patients not on diuretics the sensitivity was 48% and the specificity was 75% (Am. J. Kidney Dis. 2007;50:568-73).
A recent analysis reviewed the literature to provide some guidance for clinicians, but the end result is confusing, Dr. Feldman said. Under best-case scenarios, these two measures would be likely to make a difference in diagnosing the cause of acute kidney injury, but under worst-case scenarios, "they really stink," he said (Cleve. Clin. J. Med. 2012;79:121-6).
The authors cautioned that a single index calculated at a specific time often is insufficient to properly characterize the pathogenesis of acute kidney injury. "In the end, probably FENa and FEUN really don’t help you very much to decide" the reason behind acute kidney injury, Dr. Feldman said.
Chest X-Rays
Routine chest x-rays in mechanically ventilated patients in ICUs provide, well, too many unneeded x-rays, recent data show.
A crossover study that randomized 21 French ICUs to either routine daily chest x-rays for these patients or x-rays on demand found that the on-demand strategy reduced the number of x-rays by 32% without affecting the number of days on ventilation, length of ICU stay, or mortality. With the daily x-ray strategy, 424 patients got 4,607 x-rays, compared with 3,148 x-rays in 425 patients under the on-demand strategy (Lancet 2009;374:1687-93).
Patients had their ventilators changed more often under the on-demand strategy, probably as clinicians were troubleshooting potential problems, but the number of interventions did not differ significantly by x-ray strategy, Dr. Feldman noted.
A meta-analysis this year of eight trials including 7,078 adult ICU patients concluded that routine daily x-rays can be eliminated without increasing adverse outcomes (Radiology 2012;255:386-95).
Dr. Feldman suggested specific goals for these four scenarios, which he presented at the annual meeting of the Society of Hospitalist Medicine.
"Do not reflexively transfuse cardiac patients to hematocrits of 30%. Do not do routine daily chest x-rays. Do not reflexively NG [nasogastric] lavage our patients. And spend more time doing a really great history and physical and thinking about why your patient has acute renal failure than trying to use indices that don’t actually help us very much," he said.
He added a personal goal: "If I can make the residents at Johns Hopkins change, that will be a real feat, because they love to order tests on everybody."
Dr. Feldman, Dr. Whelan, and Dr. VanderEnde reported having no financial disclosures.
Why did you order that test? Dr. Leonard S. Feldman wants you to turn off the autopilot and consider the evidence from the medical literature on the following four practices:
• Is the best target hematocrit for a cardiac patient 30% (or a hemoglobin level of 10 g/dL)?
• Should nasogastric lavage be routine in patients with suspected GI bleeds?
• Is it helpful to measure the fractional excretion of sodium or fractional excretion of urea nitrogen when evaluating acute kidney injury?
• Are daily chest x-rays a good idea in patients on mechanical ventilation in an ICU or step-down unit?
"Many of us do all of these things regularly on a reflexive basis," said Dr. Feldman, director of the general medicine comprehensive consult service at Johns Hopkins University, Baltimore.
Yet recent studies challenge the value of these approaches:
Transfusions
Three studies influenced the AABB (formerly the American Association of Blood Banks) to publish a clinical practice guideline this year recommending a restrictive strategy when considering blood transfusions in hospitalized patients with preexisting cardiovascular disease. The AABB says not to transfuse if the hemoglobin level is above 7-8 g/dL and to consider transfusing patients who are symptomatic or who have a hemoglobin level of less than 8 g/dL, the AABB says (Ann. Intern. Med. 2012;157:49-58).
One recent study randomized 502 patients undergoing coronary artery bypass grafting (CABG) or valve replacement who were on cardiac bypass support to either a liberal strategy aiming to maintain a hematocrit of at or above 30% from the start of surgery until discharge from the ICU or a restrictive strategy that aimed to keep the hematocrit at or above 24%.
Both groups were able to meet these goals. The two groups did not differ significantly, however, in a composite end point of 30-day all-cause mortality and severe morbidity occurring during hospitalization (JAMA 2011;304:1559-67).
There was a trend toward higher risk of death with the restrictive strategy, with a hazard ratio of 1.28, but the ratio ranged from 0.6 to 2.7, so the risk was not statistically significant. Analysis of a slew of secondary outcomes also found no significant differences between groups.
Another study randomized 2,016 patients aged 50 years or older who either had a history of cardiovascular disease or cardiac risk factors and who had a hemoglobin level below 10 g/dL after surgery for hip fracture. The liberal strategy triggered transfusion in all patients with a hemoglobin below 10 g/dL. The restrictive strategy transfused only if the patient showed symptoms of anemia or at the physician’s discretion in patients with a hemoglobin level less than 8 g/dL.
Patients received a lot more blood under the liberal strategy compared with the restrictive strategy – 1,866 vs. 652 units of blood, respectively – but the groups did not differ significantly in death rates or the ability to walk across a room without human assistance at 30 and 60 days after surgery (N. Engl. J. Med. 2011;365:2453-62). Sixty days after surgery, 35% of patients in each group had died or were unable to walk across a room unaided.
"This is a trial that you should be showing all of your orthopedists," Dr. Feldman said. "When the orthopedist says that we need to give a patient blood so the patient will be able to do better in rehab" after hip surgery, point to the data showing that this isn’t necessarily true, he suggested.
An earlier trial of 838 critically ill patients who had hemoglobin levels below 9 g/dL within 72 hours of admission to the ICU found 30-day mortality rates of 19% in those randomized to a restrictive transfusion strategy and 23% in patients randomized to a liberal transfusion strategy, a difference that was not statistically significant (N. Engl. J. Med. 1999;340:409-17).
The liberal strategy transfused when hemoglobin levels fell below 10 g/dL and maintained the hemoglobin at 10-12 g/dL. The restrictive strategy waited until hemoglobin levels fell below 7 g/dL before transfusing and maintained hemoglobin at 7-9 g/dL.
"So, we have three different studies here that really seem to indicate that for transfusion of those patients who are at highest risk – cardiac patients – it did not seem to make a difference in their outcomes, particularly in mortality, if you were restrictive or liberal" in setting thresholds for transfusion, Dr. Feldman said. "This is not the same, though, for patients who are having an acute coronary syndrome. We don’t have any good data for them," and the AABB doesn’t recommend for or against liberal or restrictive transfusion thresholds, he added.
The evidence behind the AABB recommendation is only of moderate quality because there are only the three studies. Although the literature generally favors a restrictive versus a liberal approach, there’s no robust evidence for any particular transfusion threshold, said Dr. Daniel S. VanderEnde, a hospitalist and member of the joint transfusion committee at Emory University, Atlanta.
Pointing to the limited amount of research, the AABB itself calls the recommendation’s quality of evidence "very low" and the strength of the recommendation "uncertain."
"Subpar" evidence is one reason that Dr. VanderEnde’s institution leaves transfusion decisions to individual clinicians, he said in an interview. One ICU may transfuse at a hemoglobin threshold "in the mid-7s, and another ICU will have a transfusion threshold in the mid-8s."
Emory is starting a computer order entry protocol requiring physicians to tell why they’re transfusing blood, compared with no previous oversight. "It doesn’t stop them from transfusing for any reason. It is just trying to collect data, in the hopes that maybe they will be more restrictive in their use rather than liberal," he said.
Anecdotally, transfusion practices do seem to be shifting, at least among newly-trained physicians, Dr. VanderEnde added. Five years ago when he would ask medical students about transfusion thresholds, many hewed to "the old 10/30 rule," but far fewer do so today, he said. "The younger orthopedists tend to not transfuse as much as the older orthopedists."
Nasogastric Lavage
Few procedures performed in emergency departments are more painful for patients than nasogastric intubation, and there’s a study to prove that (Ann. Emerg. Med. 1999;33:652-8).
"Patients think nasogastric lavage and nasogastric intubation really stink, so we need to have a good reason to do it," Dr. Feldman said.
And, like all invasive procedures, there are risks involved, Dr. Chad T. Whelan said in an interview. There are only modestly convincing data suggesting that nasogastric lavage can provide some prognostic or "localizing" information (such as differentiating upper vs. lower bleed). "Therefore, the risk/benefit ratio of routinely performing them for all patients has shifted with our increasing understanding of their risks and benefits," said Dr. Whelan, a hospitalist at the University of Chicago.
International consensus recommendations on the management of patients with nonvariceal upper GI bleeding suggest that physicians consider placing a nasogastric tube in selected patients because the findings may have prognostic value – not very helpful advice in decision making, Dr. Feldman said. (Ann. Intern. Med. 2010;152:101-13).
The rationale until now has been that patients with bloody aspirate on nasogastric lavage are significantly more likely to have high-risk GI lesions on endoscopy, compared with patients with clear or bilious aspirates on lavage. But does knowing this improve outcomes?
One review of the literature on how to determine if a patient has a severe upper GI bleed confirmed that a bloody aspirate on nasogastric lavage increases the likelihood of an upper GI bleed but there’s only a mildly increased likelihood of a severe bleed, "and the negative likelihood ratio is not unimpressive," Dr. Feldman said (JAMA 2012;307:1072-19).
Results of a separate propensity-matched retrospective analysis of data on 632 patients admitted with GI bleeding are "as good as we’re going to get on this topic," he said. The study found that getting or not getting nasogastric lavage did not change 30-day mortality, mean length of stay, transfusion requirements, or emergency surgery rates (Gastrointest. Endosc. 2011;74:971-80).
The only things that nasogastric lavage did change were an increase in the rate of patients undergoing endoscopy, a shorter interval to endoscopy, and a shorter length of stay among patients who had endoscopy.
That suggests that there was an individual-provider confounder that the study could not measure. Perhaps emergency physicians or gastroenterologists who order nasogastric lavage are simply more aggressive, Dr. Feldman said. "This is information that you might want to take to your emergency department," he said.
In a joint editorial accompanying the study, an emergency physician and an endoscopist concluded that the practice of nasogastric lavage in patients with acute upper GI bleeding is "antiquated."
Dr. Whelan said the role of nasogastric lavage "is in transition rather than antiquated." As upper GI bleeding epidemiology evolves and endoscopic interventions improve, "the widespread use of nasogastric lavage as a universal piece of the upper GI bleed protocol should decrease. Whether nasogastric lavage ultimately becomes a completely unnecessary procedure remains to be seen," he said.
Physicians at his institution no longer routinely perform nasogastric lavage when evaluating suspected upper GI bleeding, but "it has not completely disappeared from practice, either," he said. That’s less a factor of "aggressive" physicians and more a result of how practice changes and environmental factors, Dr. Whelan added. "Not all emergency rooms have access to full-service endoscopy on site, so emergency room physicians may have a different set of risk/benefit tradeoffs to consider."
Acute Kidney Injury
Can the fractional excretion of sodium (FENa) or fractional excretion of urea nitrogen (FEUN) help narrow the differential diagnosis in acute kidney disease? Widespread use of these measures began after a 17-person study in 1976 suggested that patients with prerenal azotemia had a FENa of less than 1 and patients with acute tubular necrosis had a FENa greater than 3 (JAMA 1976;236:579-81).
The FENa is not perfect, because many intrinsic kidney disorders can cause low FENa and the FENa can be elevated when diuretic use contributes to prerenal states, so a few studies looked at adding the FEUN to the diagnostic tools. Their results were contradictory.
One study of 102 patients in the ICU found that incorporating FEUN was 85% sensitive and 92% specific in detecting prerenal injury, but the study excluded patients with acute glomerulonephritis and obstructive nephropathy, "so you have to make sure that you exclude those patients if you’re going to use FEUN," Dr. Feldman said (Kidney Int. 2002;62:2223-9).
In a separate study of 99 patients, however, the FENa and FEUN were much less impressive in patients with or without diuretics. In patients on diuretics, FEUN had a sensitivity for distinguishing transient from persistent acute kidney injury of 79% and a specificity of 33%, and in patients not on diuretics the sensitivity was 48% and the specificity was 75% (Am. J. Kidney Dis. 2007;50:568-73).
A recent analysis reviewed the literature to provide some guidance for clinicians, but the end result is confusing, Dr. Feldman said. Under best-case scenarios, these two measures would be likely to make a difference in diagnosing the cause of acute kidney injury, but under worst-case scenarios, "they really stink," he said (Cleve. Clin. J. Med. 2012;79:121-6).
The authors cautioned that a single index calculated at a specific time often is insufficient to properly characterize the pathogenesis of acute kidney injury. "In the end, probably FENa and FEUN really don’t help you very much to decide" the reason behind acute kidney injury, Dr. Feldman said.
Chest X-Rays
Routine chest x-rays in mechanically ventilated patients in ICUs provide, well, too many unneeded x-rays, recent data show.
A crossover study that randomized 21 French ICUs to either routine daily chest x-rays for these patients or x-rays on demand found that the on-demand strategy reduced the number of x-rays by 32% without affecting the number of days on ventilation, length of ICU stay, or mortality. With the daily x-ray strategy, 424 patients got 4,607 x-rays, compared with 3,148 x-rays in 425 patients under the on-demand strategy (Lancet 2009;374:1687-93).
Patients had their ventilators changed more often under the on-demand strategy, probably as clinicians were troubleshooting potential problems, but the number of interventions did not differ significantly by x-ray strategy, Dr. Feldman noted.
A meta-analysis this year of eight trials including 7,078 adult ICU patients concluded that routine daily x-rays can be eliminated without increasing adverse outcomes (Radiology 2012;255:386-95).
Dr. Feldman suggested specific goals for these four scenarios, which he presented at the annual meeting of the Society of Hospitalist Medicine.
"Do not reflexively transfuse cardiac patients to hematocrits of 30%. Do not do routine daily chest x-rays. Do not reflexively NG [nasogastric] lavage our patients. And spend more time doing a really great history and physical and thinking about why your patient has acute renal failure than trying to use indices that don’t actually help us very much," he said.
He added a personal goal: "If I can make the residents at Johns Hopkins change, that will be a real feat, because they love to order tests on everybody."
Dr. Feldman, Dr. Whelan, and Dr. VanderEnde reported having no financial disclosures.