User login
Aliskiren Didn't Protect Heart, Kidneys in Diabetes
Adding the renin inhibitor aliskiren to standard hypertension treatment in type 2 diabetes patients with comorbid kidney or cardiovascular disease did not reduce serious cardiovascular or renal events, according to a multinational study of more than 8,000 patients.
The findings from ALTITUDE (Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints) were presented at Kidney Week 2012 and simultaneously published online Nov. 3 in the New England Journal of Medicine.
Data from previous studies suggest that the direct renin inhibitor aliskiren, when combined with an angiotensin-receptor blocker (ARB), was associated with a greater decrease in albuminuria than ARB treatment alone in patients with diabetic renal disease, said Dr. Hans-Henrik Parving of the University of Copenhagen and his colleagues. However, the effect on renal and cardiovascular outcomes of combining aliskiren with an ARB or an angiotensin-converting–enzyme (ACE) inhibitor is unknown, they said.
To determine the safety of a dual renin–angiotensin–aldosterone system (RAAS) blockade, the researchers randomized 8,606 adults at 838 centers in 36 countries to receive standard treatment plus aliskiren or a placebo. Complete data were available for 8,561 patients enrolled between October 2007 and June 2010. Eligible patients had type 2 diabetes and were taking either an ARB or ACE inhibitor (N. Engl. J. Med. 2012, Nov. 3 [doi:10.1056/NEJMoa1208799]).
The average age of the patients was 65 years, and approximately one-third were female. Baseline demographics were similar between the two groups. After 2 months, 84% of the aliskiren patients were taking the maximum dose of 300 mg.
The primary composite outcome included death from cardiovascular causes, cardiac arrest with resuscitation, myocardial infarction (fatal or nonfatal), stroke (fatal or nonfatal), unplanned hospitalization for heart failure, end-stage renal disease (death attributable to kidney failure or loss of kidney function), and doubling of baseline serum creatinine. Any one patient "may have had multiple cardiovascular or renal events of different types," the researchers noted.
After a median follow-up of approximately 2.5 years, 783 patients in the aliskiren group (18%) and 732 in the placebo group (17%) met the primary end point, though the difference was not statistically significant (P = .12)
Aliskiren patients showed significantly lower systolic and diastolic blood pressures and higher mean reductions in urinary albumin-to-creatinine ratios after 6 months than placebo patients. However, significantly more aliskiren patients than placebo patients had hyperkalemia (39% vs. 29%, respectively) and hypotension (12% vs. 8%, respectively).
The number of deaths from any cause was not significantly different between the aliskiren and placebo groups (119 and 102, respectively).
Significantly more aliskiren patients than placebo patients discontinued the study drug due to an adverse event (13% vs. 10%). The most common adverse event was hyperkalemia, followed by renal impairment and hypotension.
"The overall lack of benefit with regard to the primary composite cardiovascular and renal outcome was observed across all the predefined subgroups," the researchers said.
Two trials of aliskiren in combination with another renin-angiotensin system blocker in heart failure patients are ongoing, the researchers said.
However, "the present study documented more adverse events in the aliskiren group than in the placebo group without clinical benefits to offset them, which underscores the need to go beyond surrogate biomarkers and obtain risk-benefit data from clinical end-point trials to better inform clinical decisions," they said.
Novartis supported the study. Dr. Parving has received funding from Novartis, served on the speakers bureau for Novartis and Sanofi, and has served as a consultant for Abbott, Reata, and Takeda.
Adding the renin inhibitor aliskiren to standard hypertension treatment in type 2 diabetes patients with comorbid kidney or cardiovascular disease did not reduce serious cardiovascular or renal events, according to a multinational study of more than 8,000 patients.
The findings from ALTITUDE (Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints) were presented at Kidney Week 2012 and simultaneously published online Nov. 3 in the New England Journal of Medicine.
Data from previous studies suggest that the direct renin inhibitor aliskiren, when combined with an angiotensin-receptor blocker (ARB), was associated with a greater decrease in albuminuria than ARB treatment alone in patients with diabetic renal disease, said Dr. Hans-Henrik Parving of the University of Copenhagen and his colleagues. However, the effect on renal and cardiovascular outcomes of combining aliskiren with an ARB or an angiotensin-converting–enzyme (ACE) inhibitor is unknown, they said.
To determine the safety of a dual renin–angiotensin–aldosterone system (RAAS) blockade, the researchers randomized 8,606 adults at 838 centers in 36 countries to receive standard treatment plus aliskiren or a placebo. Complete data were available for 8,561 patients enrolled between October 2007 and June 2010. Eligible patients had type 2 diabetes and were taking either an ARB or ACE inhibitor (N. Engl. J. Med. 2012, Nov. 3 [doi:10.1056/NEJMoa1208799]).
The average age of the patients was 65 years, and approximately one-third were female. Baseline demographics were similar between the two groups. After 2 months, 84% of the aliskiren patients were taking the maximum dose of 300 mg.
The primary composite outcome included death from cardiovascular causes, cardiac arrest with resuscitation, myocardial infarction (fatal or nonfatal), stroke (fatal or nonfatal), unplanned hospitalization for heart failure, end-stage renal disease (death attributable to kidney failure or loss of kidney function), and doubling of baseline serum creatinine. Any one patient "may have had multiple cardiovascular or renal events of different types," the researchers noted.
After a median follow-up of approximately 2.5 years, 783 patients in the aliskiren group (18%) and 732 in the placebo group (17%) met the primary end point, though the difference was not statistically significant (P = .12)
Aliskiren patients showed significantly lower systolic and diastolic blood pressures and higher mean reductions in urinary albumin-to-creatinine ratios after 6 months than placebo patients. However, significantly more aliskiren patients than placebo patients had hyperkalemia (39% vs. 29%, respectively) and hypotension (12% vs. 8%, respectively).
The number of deaths from any cause was not significantly different between the aliskiren and placebo groups (119 and 102, respectively).
Significantly more aliskiren patients than placebo patients discontinued the study drug due to an adverse event (13% vs. 10%). The most common adverse event was hyperkalemia, followed by renal impairment and hypotension.
"The overall lack of benefit with regard to the primary composite cardiovascular and renal outcome was observed across all the predefined subgroups," the researchers said.
Two trials of aliskiren in combination with another renin-angiotensin system blocker in heart failure patients are ongoing, the researchers said.
However, "the present study documented more adverse events in the aliskiren group than in the placebo group without clinical benefits to offset them, which underscores the need to go beyond surrogate biomarkers and obtain risk-benefit data from clinical end-point trials to better inform clinical decisions," they said.
Novartis supported the study. Dr. Parving has received funding from Novartis, served on the speakers bureau for Novartis and Sanofi, and has served as a consultant for Abbott, Reata, and Takeda.
Adding the renin inhibitor aliskiren to standard hypertension treatment in type 2 diabetes patients with comorbid kidney or cardiovascular disease did not reduce serious cardiovascular or renal events, according to a multinational study of more than 8,000 patients.
The findings from ALTITUDE (Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints) were presented at Kidney Week 2012 and simultaneously published online Nov. 3 in the New England Journal of Medicine.
Data from previous studies suggest that the direct renin inhibitor aliskiren, when combined with an angiotensin-receptor blocker (ARB), was associated with a greater decrease in albuminuria than ARB treatment alone in patients with diabetic renal disease, said Dr. Hans-Henrik Parving of the University of Copenhagen and his colleagues. However, the effect on renal and cardiovascular outcomes of combining aliskiren with an ARB or an angiotensin-converting–enzyme (ACE) inhibitor is unknown, they said.
To determine the safety of a dual renin–angiotensin–aldosterone system (RAAS) blockade, the researchers randomized 8,606 adults at 838 centers in 36 countries to receive standard treatment plus aliskiren or a placebo. Complete data were available for 8,561 patients enrolled between October 2007 and June 2010. Eligible patients had type 2 diabetes and were taking either an ARB or ACE inhibitor (N. Engl. J. Med. 2012, Nov. 3 [doi:10.1056/NEJMoa1208799]).
The average age of the patients was 65 years, and approximately one-third were female. Baseline demographics were similar between the two groups. After 2 months, 84% of the aliskiren patients were taking the maximum dose of 300 mg.
The primary composite outcome included death from cardiovascular causes, cardiac arrest with resuscitation, myocardial infarction (fatal or nonfatal), stroke (fatal or nonfatal), unplanned hospitalization for heart failure, end-stage renal disease (death attributable to kidney failure or loss of kidney function), and doubling of baseline serum creatinine. Any one patient "may have had multiple cardiovascular or renal events of different types," the researchers noted.
After a median follow-up of approximately 2.5 years, 783 patients in the aliskiren group (18%) and 732 in the placebo group (17%) met the primary end point, though the difference was not statistically significant (P = .12)
Aliskiren patients showed significantly lower systolic and diastolic blood pressures and higher mean reductions in urinary albumin-to-creatinine ratios after 6 months than placebo patients. However, significantly more aliskiren patients than placebo patients had hyperkalemia (39% vs. 29%, respectively) and hypotension (12% vs. 8%, respectively).
The number of deaths from any cause was not significantly different between the aliskiren and placebo groups (119 and 102, respectively).
Significantly more aliskiren patients than placebo patients discontinued the study drug due to an adverse event (13% vs. 10%). The most common adverse event was hyperkalemia, followed by renal impairment and hypotension.
"The overall lack of benefit with regard to the primary composite cardiovascular and renal outcome was observed across all the predefined subgroups," the researchers said.
Two trials of aliskiren in combination with another renin-angiotensin system blocker in heart failure patients are ongoing, the researchers said.
However, "the present study documented more adverse events in the aliskiren group than in the placebo group without clinical benefits to offset them, which underscores the need to go beyond surrogate biomarkers and obtain risk-benefit data from clinical end-point trials to better inform clinical decisions," they said.
Novartis supported the study. Dr. Parving has received funding from Novartis, served on the speakers bureau for Novartis and Sanofi, and has served as a consultant for Abbott, Reata, and Takeda.
FROM KIDNEY WEEK 2012, SPONSORED BY THE AMERICAN SOCIETY OF NEPHROLOGY
Major Finding: After 33 months’ follow-up, 18% of patients taking aliskiren and 17% of placebo patients experienced a serious cardiovascular or renal event, or death. The trial was discontinued after its second interim efficacy analysis.
Data Source: The data come from a randomized, double-blind, placebo-controlled trial of 8,561 patients.
Disclosures: Novartis supported the study. Lead author Dr. Parving has received funding from Novartis, served on the speakers’ bureau for Novartis and Sanofi, and has served as a consultant for Abbott, Reata, and Takeda.
Many May Not Need Vitamin D Supplements
Vitamin D levels above 20 ng/mL were not associated with lower mortality rates in patients with and without kidney disease; however, levels below 12 ng/mL were associated with higher mortality rates in these patients, according to the results of a study published Oct. 24 in the online journal PLoS One.
The minimal difference in mortality rates for individuals with vitamin D levels between 20 ng/mL and 30 ng/mL suggests that vitamin D supplements may not be necessary for approximately 3 million adults with chronic kidney disease and 75 million adults without kidney disease, said Dr. Holly Kramer of Loyola University Medical Center in Maywood, Ill., and her colleagues.
To examine the impact of vitamin D levels on mortality, the researchers reviewed data from 15,099 adults who were part of the Third National Health and Nutrition Examination Study (NHANES III). The study population included 1,097 adults with chronic kidney disease, which was defined as an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2.
In order for mortality rates to be compared, the researchers divided the study population into groups based on vitamin D levels, ranging from less than 12 ng/mL to greater than 40 ng/mL, using the 24- to 29.9-ng/mL group as the reference group.
"This group was selected as the referent, because it includes 25[OH]D levels which are above the threshold for ‘risk of insufficiency,’ defined by the Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, yet below the thresholds defined as ‘insufficient’ in previous analyses," the researchers wrote.
Overall, about one-third of the adults with kidney disease (35%) and of those without kidney disease (30%) had insufficient levels of vitamin D, based on Institute of Medicine recommendations.
The median vitamin D levels for each of the groups were 10.0 ng/mL, 14.1 ng/mL, 18.0 ng/mL, 21.9 ng/mL, 26.5 ng/mL, 33.9 ng/mL, and 43.6 ng/mL.
After the investigators controlled for risk factors including age, race, sex, smoking status, and comorbid conditions, the all-cause mortality rate was determined for the patients with kidney disease. The mortality rate was 153/1,000 person-years for those with vitamin D levels less than 12 ng/mL, 121/1,000 person-years for those in the 12- to 16-ng/mL group, and 108/1,000 person years for those in the 24- to 29.9-ng/mL range.
Mortality rates were similar for those with kidney disease and vitamin D levels greater than 20 ng/mL, the researchers noted, with the lowest mortality rate of 97/1,000 person-years seen in those in the highest vitamin D group.
Among patients without kidney disease, the adjusted all-cause mortality rate was 17/1,000 person-years among those with vitamin D levels less than 12 ng/mL, compared with 13 for those in the 24- to 29.9-ng/mL range and 12 among patients with vitamin D levels greater than 40 ng/mL.
The study was limited by its observational design, and the results may not be generalizable to nursing home residents, individuals on dialysis, or anyone who has had a kidney transplant, the researchers noted.
Vitamin D supplementation has been linked to an increased risk of cancer and kidney stones, and clinical trials are needed to assess the risks versus benefits in individuals with and without kidney disease, the researchers added.
The National Institutes of Health supported the study.
Vitamin D levels above 20 ng/mL were not associated with lower mortality rates in patients with and without kidney disease; however, levels below 12 ng/mL were associated with higher mortality rates in these patients, according to the results of a study published Oct. 24 in the online journal PLoS One.
The minimal difference in mortality rates for individuals with vitamin D levels between 20 ng/mL and 30 ng/mL suggests that vitamin D supplements may not be necessary for approximately 3 million adults with chronic kidney disease and 75 million adults without kidney disease, said Dr. Holly Kramer of Loyola University Medical Center in Maywood, Ill., and her colleagues.
To examine the impact of vitamin D levels on mortality, the researchers reviewed data from 15,099 adults who were part of the Third National Health and Nutrition Examination Study (NHANES III). The study population included 1,097 adults with chronic kidney disease, which was defined as an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2.
In order for mortality rates to be compared, the researchers divided the study population into groups based on vitamin D levels, ranging from less than 12 ng/mL to greater than 40 ng/mL, using the 24- to 29.9-ng/mL group as the reference group.
"This group was selected as the referent, because it includes 25[OH]D levels which are above the threshold for ‘risk of insufficiency,’ defined by the Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, yet below the thresholds defined as ‘insufficient’ in previous analyses," the researchers wrote.
Overall, about one-third of the adults with kidney disease (35%) and of those without kidney disease (30%) had insufficient levels of vitamin D, based on Institute of Medicine recommendations.
The median vitamin D levels for each of the groups were 10.0 ng/mL, 14.1 ng/mL, 18.0 ng/mL, 21.9 ng/mL, 26.5 ng/mL, 33.9 ng/mL, and 43.6 ng/mL.
After the investigators controlled for risk factors including age, race, sex, smoking status, and comorbid conditions, the all-cause mortality rate was determined for the patients with kidney disease. The mortality rate was 153/1,000 person-years for those with vitamin D levels less than 12 ng/mL, 121/1,000 person-years for those in the 12- to 16-ng/mL group, and 108/1,000 person years for those in the 24- to 29.9-ng/mL range.
Mortality rates were similar for those with kidney disease and vitamin D levels greater than 20 ng/mL, the researchers noted, with the lowest mortality rate of 97/1,000 person-years seen in those in the highest vitamin D group.
Among patients without kidney disease, the adjusted all-cause mortality rate was 17/1,000 person-years among those with vitamin D levels less than 12 ng/mL, compared with 13 for those in the 24- to 29.9-ng/mL range and 12 among patients with vitamin D levels greater than 40 ng/mL.
The study was limited by its observational design, and the results may not be generalizable to nursing home residents, individuals on dialysis, or anyone who has had a kidney transplant, the researchers noted.
Vitamin D supplementation has been linked to an increased risk of cancer and kidney stones, and clinical trials are needed to assess the risks versus benefits in individuals with and without kidney disease, the researchers added.
The National Institutes of Health supported the study.
Vitamin D levels above 20 ng/mL were not associated with lower mortality rates in patients with and without kidney disease; however, levels below 12 ng/mL were associated with higher mortality rates in these patients, according to the results of a study published Oct. 24 in the online journal PLoS One.
The minimal difference in mortality rates for individuals with vitamin D levels between 20 ng/mL and 30 ng/mL suggests that vitamin D supplements may not be necessary for approximately 3 million adults with chronic kidney disease and 75 million adults without kidney disease, said Dr. Holly Kramer of Loyola University Medical Center in Maywood, Ill., and her colleagues.
To examine the impact of vitamin D levels on mortality, the researchers reviewed data from 15,099 adults who were part of the Third National Health and Nutrition Examination Study (NHANES III). The study population included 1,097 adults with chronic kidney disease, which was defined as an estimated glomerular filtration rate of less than 60 mL/min per 1.73 m2.
In order for mortality rates to be compared, the researchers divided the study population into groups based on vitamin D levels, ranging from less than 12 ng/mL to greater than 40 ng/mL, using the 24- to 29.9-ng/mL group as the reference group.
"This group was selected as the referent, because it includes 25[OH]D levels which are above the threshold for ‘risk of insufficiency,’ defined by the Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, yet below the thresholds defined as ‘insufficient’ in previous analyses," the researchers wrote.
Overall, about one-third of the adults with kidney disease (35%) and of those without kidney disease (30%) had insufficient levels of vitamin D, based on Institute of Medicine recommendations.
The median vitamin D levels for each of the groups were 10.0 ng/mL, 14.1 ng/mL, 18.0 ng/mL, 21.9 ng/mL, 26.5 ng/mL, 33.9 ng/mL, and 43.6 ng/mL.
After the investigators controlled for risk factors including age, race, sex, smoking status, and comorbid conditions, the all-cause mortality rate was determined for the patients with kidney disease. The mortality rate was 153/1,000 person-years for those with vitamin D levels less than 12 ng/mL, 121/1,000 person-years for those in the 12- to 16-ng/mL group, and 108/1,000 person years for those in the 24- to 29.9-ng/mL range.
Mortality rates were similar for those with kidney disease and vitamin D levels greater than 20 ng/mL, the researchers noted, with the lowest mortality rate of 97/1,000 person-years seen in those in the highest vitamin D group.
Among patients without kidney disease, the adjusted all-cause mortality rate was 17/1,000 person-years among those with vitamin D levels less than 12 ng/mL, compared with 13 for those in the 24- to 29.9-ng/mL range and 12 among patients with vitamin D levels greater than 40 ng/mL.
The study was limited by its observational design, and the results may not be generalizable to nursing home residents, individuals on dialysis, or anyone who has had a kidney transplant, the researchers noted.
Vitamin D supplementation has been linked to an increased risk of cancer and kidney stones, and clinical trials are needed to assess the risks versus benefits in individuals with and without kidney disease, the researchers added.
The National Institutes of Health supported the study.
FROM PLoS ONE
Kidney Failure in the 21st Century
The Initiating Dialysis Early and Late (IDEAL) study in Australia and New Zealand1 examined the optimal time to initiate dialysis. This well-designed, randomized, controlled trial gave the nephrology community guidelines for treating patients as they progress through chronic kidney disease to stage 5 (CKD 5). The IDEAL investigators demonstrated that planned early initiation of dialysis did not enhance survival—and in some cases, it hastened death.1
Although most patients have a nephrology provider by the time they reach CKD 5 (ie, kidney failure), primary care providers can be instrumental in preparing the patient for what lies ahead as kidney failure progresses. Presented here is an overview of diagnosis and management of the patient with CKD 5 in the 21st century.
CASE PRESENTATION
A 70-year-old woman with an extensive history of diabetes and hypertension presents to her primary care clinician complaining of a lack of energy. She has just returned from a trip to Disney World, where she says she was unable to keep up with her grandchildren. She sat in the shade while they enjoyed Space Mountain and other attractions, and because of uncustomary fatigue, she required a nap every afternoon.
Physical examination shows an elderly female in no acute distress. Cardiac exam shows a regular heart rate and rhythm, the patient’s lungs are clear, and she has 1+ pitting leg edema bilaterally. The patient’s blood pressure is slightly elevated at 142/92 mm Hg, and no protein is detected on spot urine testing.
Blood work is ordered, including a complete blood count, a comprehensive metabolic panel, and an A1C. Results include a hemoglobin level of 8.7 g/dL (reference range for women, 12.0 to 16.0 g/dL), a serum creatinine level (SCr) of 3 mg/dL (range, 0.6 to 1.2 mg/dL; estimated glomerular filtration rate [eGFR], 15 mL/min/1.73 m2), and an A1C value of 7.5% (normal, & < 7%).
The patient is told that she is in kidney failure and is referred immediately to a nephrology practice, where she is seen the following day. After her appointment, she returns to the primary care office. When she is asked when she will be starting dialysis, she seems surprised, saying, “They told me I was doing well. I need some shots for my blood, but they didn’t seem worried at all.”
Is this patient being managed correctly by the nephrology practitioner?
EPIDEMIOLOGY
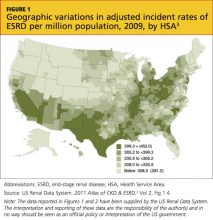
Presently, more than 1 million persons in the United States have CKD 5, and 500,000 are undergoing dialysis. The number of patients with CDK 5 who are on dialysis has doubled since 1994 and is projected to reach 774,000 by 20202,3 (see Figure 13 and Figure 23).
CKD 5 is defined as an eGFR below 15 mL/min/1.73 m2, according to the Modification of Diet in Renal Disease (MDRD) study group formula; or as a creatinine clearance of less than 15 mL/min, using the Cockcroft-Gault formula.4-6 Both formulas have limitations, since they are not fully accurate at extremes of age, with variations in weight, for some racial mixes, or for the very malnourished patient.7,8 However, they do correct for normal age-related loss of kidney function, gender, and SCr, and they are currently the generally accepted formulas.4
A rise in SCr is exponential; for each doubling of the SCr, a reduction in kidney function of approximately 50% occurs. This means that a rise in SCr from 4 to 8 mg/dL is equivalent (in the proportion of loss of kidney function) to a rise of SCr from 0.5 to 1 mg/dL. Commonly, patients are not referred to nephrology until the SCr doubles to 4 or 6 mg/dL—whereas the rise in SCr from 0.5 to 1 mg/dL should be of greatest concern to the primary care provider, prompting a referral.9 Early recognition of reduced renal function allows for identification of potentially reversible etiologies and the slowing of CKD progression.
INDICATIONS FOR RENAL REPLACEMENT THERAPY
Not all patients with an eGFR below 15 mL/min/1.73 m2 are started on dialysis immediately. The decision to initiate dialysis is guided by assessment of a constellation of uremic manifestations—not eGFR alone. Newer data suggest that early initiation of dialysis may be associated with increased mortality.10-15
The IDEAL study,1 a well-designed, randomized, controlled trial of all patients who started dialysis in Australia and New Zealand over a multiyear period, was designed to determine the optimal time to initiate dialysis. Patients were randomized to start dialysis (hemodialysis or peritoneal dialysis) at an eGFR between 10 and 14 mL/min/1.73 m2 or between 5 and 7 mL/min/1.73 m2. While there was some overlap and some patients were started on dialysis outside their randomized eGFR (eg, earlier than the allotted time because symptoms developed), what the IDEAL investigators found surprised the entire nephrology community: Early dialysis starts did not enhance survival and, in some cases, it hastened death.1
The indications for initiation of dialysis often develop long after the patient has progressed within CKD 5, commonly with an eGFR of 10 mL/min/1.73 m2 or less. Medicare has acknowledged this by reimbursing dialysis only in eligible patients whose eGFR is below 10 mL/min/1.73 m2.16 There are also acute indications for initiation of dialysis, such as uremic pericarditis, hyperkalemia, bleeding related to uremic platelet dysfunction, and metabolic encephalopathy related to uremia (and reimbursement for dialysis can be justified), but these are uncommon.
RENAL REPLACEMENT THERAPY CHOICES
The choices of treatment for kidney failure (note: treatment, not cure) are medical management, hemodialysis, peritoneal dialysis, and transplant. Each choice has its advantages and disadvantages, and all patients should receive clear explanations of what they can expect from each modality.
While younger, higher-functioning individuals are likely to benefit from dialysis, patients with extensive comorbid illnesses and/or low functional status tend to respond poorly17; medical management may be the best choice for these patients. In one recent study, the functional status of residents in skilled nursing homes was examined, before and after initiation of dialysis. At one year, 58% of the nursing home residents who underwent dialysis had died, and only 13% had maintained their pre-dialysis functional status.18
Another research team recently compared conservative management of CKD 5 (ie, medical therapy without dialysis) with dialysis in elderly patients. For patients 75 and older with extensive comorbid illness, the researchers found no statistically significant survival benefit to dialysis.19
Dialysis, whether administered as hemodialysis or peritoneal dialysis, is a rigorous, intensive medical therapy. Dialysis does not necessarily prolong life in patients with extensive comorbidities, and it does not always enhance quality of life.18,20-23 The decision to undergo dialysis is personal and individual, and both the patient and family should be actively involved in making it. Primary care providers should be the nephrologist’s ally in the discussion of therapy for renal failure; often, they have cared for the patient for years, and they understand the patient and family dynamics.
An important message the nephrology practitioner must communicate is that choosing medical management without dialysis is not withholding care; sometimes it is a more humane choice.24 Dying of kidney failure can be a peaceful and comfortable death: As the uremic toxins build up (with eGFR ≤ 2 mL/min/1.73 m2, although this can take many years), the patient becomes confused and slowly slips into a sleepiness that leads to death.25 Hospice is usually involved to support the patient and family.
Hemodialysis
Hemodialysis is the most common, best-known treatment modality for CKD in the US, with about 94% of patients choosing it.3 In this process, blood is removed from the body (approximately 500 cc at a time) and filtered through a semipermeable membrane that removes uremic toxins and excess fluid, normalizing the metabolic and electrolyte derangements. The filtered blood is then returned to the patient.
The average dialysis session is four hours long and is conducted three times per week, following recommendations from the 2002 Hemodialysis Study (HEMO).26 Most patients come to a free-standing dialysis center on a Monday/Wednesday/Friday or a Tuesday/Thursday/Saturday schedule.
In theory, there is no such thing as too much dialysis (since the kidneys work 24 hours per day, seven days per week); thus, researchers have recently examined lengthening dialysis in an attempt to extend survival.27-29 According to study results, patients who undergo longer dialysis times generally enjoy better nutrition with a more liberal diet, require fewer medications, have reduced incidence of increased left ventricular mass (a marker for coronary artery disease), and report better quality of life, all in addition to a survival benefit; however, the latter was not considered statistically significant in any of the studies.27-29 Additionally, this survival benefit may not extend to daily hemodialysis; in a recent publication, daily hemodialysis was associated with a 60% higher death rate.30
A number of dialysis units have begun to offer nocturnal dialysis. In this option, patients sleep at the unit for eight hours, three nights per week, for a total of 24 hours of dialysis (vs the typical 12 hours per week). Some patients receive dialysis at home, allowing them to dialyze for six weekly sessions of two to three hours. This strategy attempts to mimic a more “natural” state.
One of the primary challenges associated with hemodialysis is establishing and maintaining a vascular access. An arteriovenous (AV) fistula is the access of choice because its use reduces the likelihood of clotting, improves access survival, and increases clearances during dialysis.31 However, the AV fistula is most effective if it is placed a minimum of six months before use.32
For many patients, an AV fistula is created surgically when the eGFR falls to 15 mL/min/1.73 m2. Fistula placement in preparation for the initiation of hemodialysis is important to reduce the need for hemodialysis catheters, which are associated with higher risk for infection and poorer outcomes.33
Peritoneal Dialysis
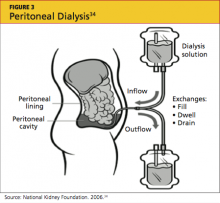
Peritoneal dialysis (PD) filters out uremic toxins and normalizes the metabolic and electrolyte derangements by using the patient’s peritoneal membrane as a filter. Dialysate is instilled through a catheter into the peritoneal cavity where it is allowed to dwell, often for four to six hours (see Figure 334). Exchanges can be performed three to four times per day (allowing six to eight hours of dwell time per exchange for the dialysate), or by way of a cycler at night.35,36
Performing exchanges by way of a cycler requires the patient to be connected via the PD catheter for eight hours; thus the advantage of performing the exchange during sleep. The cycler has a soft, whooshing sound that many patients describe as “white noise” that does not disturb their sleep.
The principal advantage of peritoneal dialysis is extended survival of the patient.37-39 PD also allows the patient a more liberal diet. Although PD must be performed every day, exchanges can be adjusted to the patient’s timing preference. PD reduces cost and time, since the patient need not travel to a dialysis center. PD also preserves residual renal function,39 which is associated with a survival benefit and contributes to the patient’s overall health and well-being. The use of PD requires a committed, competent patient in a clean home environment.
This intervention may not be suitable for patients with a history of abdominal surgeries. However, as a therapy considered gentler than hemodialysis, PD is an excellent choice for the patient with congestive heart failure.40 The PD catheter is not placed until two to four weeks before it will be needed.41
Kidney Transplantation
Last, but certainly not least, is transplant. As entire books have been written on this modality, only the highlights will be addressed here.42 Survival rates following transplantation are reported to exceed those associated with any other treatment modality, even when controlling for comorbidities and patient selection bias.43 A patient can receive a kidney from either a living or a deceased donor. Apart from the perioperative risks associated with transplantation surgery, long-term surgical or medical problems are not common for the living kidney donor.44-46
Since 2002, as a result of a policy change from the United Network for Organ Sharing (UNOS),47 the donor pool has been expanded by including kidneys from what are commonly referred to as extended criteria donors: those who are older than 60, or who are 50 to 59 and have two of the following factors: cerebrovascular accident as the cause of death; preexisting hypertension; or an SCr level exceeding 1.5 mg/dL. These kidneys may be given to any recipients but are primarily used for those age 50 or older.47
Graft survival is statistically shorter in a cadaver kidney than in a living donor kidney.48 However, the larger problem is the long wait time for a cadaver transplant—seven to eight years (or possibly longer) for some centers and some blood types.49 A patient can be referred to a transplant center when the eGFR falls below 25 mL/min/1.73 m2; he or she will then be actively listed for transplant when eGFR reaches 20 mL/min/1.73 m2 or lower.
Early referral for transplant listing buys the patient time before dialysis becomes essential. Patients can receive preemptive transplants (ie, transplantation before dialysis is initiated); these are becoming increasingly popular because they generally extend survival for the recipient.50,51
UNOS (www.unos.org) has set few limits on transplant recipients: patients are required to undergo an extensive medical work-up, but there are no upper age limits (although some centers do set their own) and usually no limits on patients infected with HIV, hepatitis B, or hepatitis C (although there may be separate listing requirements). Patients who are not US citizens are not denied.
Because more than 100,000 patients are currently on the wait list, domino kidney transplants are now being offered at many centers.52-54 These organ exchanges involve altruistic donors who do not match their targeted kidney recipients. Since publication of the first article describing this procedure at Johns Hopkins Medical Center,53 there have been two-way, three-way, and up to 32-way domino transplants. However, patients wishing to engage in this type of trade require a donor; not every patient has access to an altruistic donor.
CKD PATIENT EDUCATION
When Congress passed the Medicare Improvements for Patients and Providers Act of 2008,55 a component little noted outside the nephrology community was the offer of kidney disease education (KDE) classes to Medicare-eligible patients with CKD 4.56-58 Medicare patients with an eGFR of 15 to 30 mL/min/1.73 m2 are eligible to attend KDE classes presented by a physician, an NP, a PA, or a clinical nurse specialist. Medicare will reimburse for six hours of education.
This groundbreaking educational benefit, the first such program paid for by Medicare, was championed by the National Kidney Foundation and other nephrology groups. Many nephrology practices now offer these classes to all their CKD patients, regardless of health insurance status. Instructors educate patients on the choices of renal replacement therapy and help patients select their best option.
KDE classes can be conducted in the office setting or at the patient’s home, and they can be billed on the same day as an evaluation and management (E/M) visit.56,57 This may help explain why, in 2010, gerontologists billed for more home KDE classes than did nephrologists.58
FUTURE TRENDS AND ONGOING TRIALS
The overarching goal of therapy for CKD patients is to diagnose accurately and treat effectively in order to slow or prevent progression to end-stage renal disease (ESRD). Following is a brief review of a selection of new diagnostic tools and therapeutic interventions that may impact the management of CKD in the coming years.
The QxMD Kidney Failure Risk Equation is a newly developed, well-validated predictive model that offers relatively accurate prediction of a patient’s likelihood of progression to ESRD, based on age, sex, eGFR, and levels of albuminuria, SCr, serum phosphorus, serum bicarbonate, and serum albumin59 (see table59). This tool can be accessed online at www.qxmd.com/calculate-online/nephrology/kidney-failure-risk-equation.
Increased awareness of the role that phosphorus plays in the development of vascular calcifications has led to an emphasis on earlier, more aggressive control of dietary phosphorus. Historically, dietary phosphorus control and phosphorus binders were recommended only when the patient’s serum phosphorus exceeded normal limits. Dietary phosphorus control may now be advised as early as CKD 3, based on the understanding that phosphorus is a key component in driving the development of vascular calcifications—which in turn contribute to the high incidence of cardiovascular disease in patients with CKD.60
Bardoxolone is a new medication developed for treatment of diabetic nephropathy.61,62 It works by inhibiting proinflammatory mediators and moderating the effects of oxidative stress, thus interrupting the cascade of inflammation and cellular injury that result in diabetic nephropathy.62 Early clinical trials examining this agent have shown promise in terms of raising eGFR and reducing serum creatinine, but tolerability and toxicity profiles have been an issue.61 Additionally, some reduction in serum creatinine may have been attributable to weight loss as opposed to true improvement of renal function.62
Data have recently been released regarding the use of the herb silymarin for treatment of patients with macroalbuminuria related to diabetic nephropathy. Results from a small (n = 60), randomized, nonblinded clinical trial by Fallahzadeh et al63 indicate that silymarin decreases proteinuria when used in combination with an ACE inhibitor or an angiotensin receptor blocker (ARB). Silymarin, an extract from milk thistle, has been used medicinally for centuries for its antioxidant, anti-inflammatory, and antiviral properties in those with liver “ailments.” In this clinical trial, silymarin was well tolerated and associated with a reduction in pro-inflammatory markers.63
AST-120 is another agent intended to slow progression of CKD. It promotes intestinal adsorption and fecal excretion of the uremic toxin indoxyl sulfate.64 A recently published study demonstrated an association between use of AST-120 and a delay in required initiation of hemodialysis, but no survival benefit.65 This was a nonrandomized trial, and previous research showed no effect of AST-120 on progression of CKD.66 However, patients close to requiring dialysis are likely to welcome a means to delay it.
A growing body of evidence suggests that reversal of CKD-associated acidemia by administering sodium bicarbonate may actually forestall progression of CKD.67 However, treatment with sodium bicarbonate can lead to complications of hypertension and fluid volume overload. In one study, patients at risk for these complications derived equivalent benefit by increasing dietary fruit and vegetable intake to reduce renal acid load.68 However, providers must be mindful of patients’ serum potassium levels, especially patients who are taking an ACE inhibitor or an ARB.
The Provider’s Current Role
Despite the hope offered by new and novel therapies, the mainstay of treatment for CKD continues to be aggressive management of diabetes, hypertension, and the cardiovascular risk profile. As a result of vigorous preventive strategies to address the cardiovascular risks inherent in this patient population, the patient with type 2 diabetes is now more likely to progress to ESRD than to die of a cardiovascular event.69
The well-informed clinician can be instrumental in providing evidence-based medical therapy and excellent patient education from the time of initial CKD diagnosis through CKD 5.
PATIENT OUTCOME
The case patient was started on injections of epoietin alfa for her anemia. She attended KDE classes led by an advanced practitioner in her nephrology group and decided to undergo peritoneal dialysis, since it would allow her to maintain her travel schedule. When last heard from, the patient was preparing for a trip to see the Great Barrier Reef in Australia.
CONCLUSION
The decision to begin renal replacement therapy—whether a form of dialysis, or another management option—depends not on SCr or eGFR alone. Rather, a number of uremic manifestations, comorbidities, lifestyle factors, and other variables must be carefully weighed before the patient, the family, and the clinicians involved can decide on the management plan most likely to enhance the patient’s quality of life and extend survival.
1. Cooper BA, Branley P, Bulfone L, et al; IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010; 363(7):609-619.
2. Olan G. Policy Update: ASN to CDC: data collection of creatinine levels will advance research. ASN Kidney News. 2012;4(6):16. www.asn-online.org/publications/kidneynews/archives/2012/KN_jun2012.pdf. Accessed August 27, 2012.
3. US Renal Data System, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. 2011 Atlas of CKD & ESRD. Vol 2. Chap 1: Incidence, prevalence, patient characteristics, and modalities. www.usrds.org/2011/pdf/v2_ch01_11.pdf. Accessed September 26, 2012.
4. National Kidney Foundation. Kidney Disease Outcomes Quality Initiative (KDOQI). Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. ?Part 4. Definition and classification of the stages of chronic kidney disease. 2002:43-80. www.kidney.org/professionals/kdoqi/pdf/ckd_evalua tion_classification_stratification.pdf. Accessed August 27, 2012.
5. Levey AS, Bosch JP, Lewis JB, et al; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Internal Med. 1999;130 (6):461-470.
6. Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763-773.
7. Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16(5):1413-1419.
8. Zuo L, Ma YC, Zhou YH, et al. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45(3):463-472.
9. Stevens LA, Stoycheff N, Levey AS. Staging and management of chronic kidney disease. In: Greenberg A, ed. Primer of Kidney Diseases: Expert Consult. 5th ed. Philadelphia, PA: WB Saunders; 2009:436-445.
10. Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47-53.
11. Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14(9):2305-2312.
12. Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887-896.
13. Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77(8):700-707.
14. Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24 (10):3175-3182.
15. Traynor JP, Simpson K, Geddes CC, et al. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13(8):2125-2132.
16. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. CMS Form 2728. End-Stage Renal Disease Medical Evidence Report: Medicare Entitlement and/or Patient Registration. www.usrds.org/2008/rg/forms/02_2728_1965.pdf. Accessed September 25, 2012.
17. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline. 2nd ed. Rockville, MD: Renal Physicians Association; Oct 2010:1-12.
18. Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
19. Chandna SM, Da Silva-Gane M, Marshall C, et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614.
20. Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95:c40-c46.
21. Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356:1543-1550.
22. O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012; 15(2):228-235.
23. Jassal SV, Trpeski L, Zhu N, et al. Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ. 2007;177(9):?1033-1038.
24. Schmidt RJ. Informing our elders about dialysis: is an age-attuned approach warranted? Clin J Am Soc Nephrol. 2012;7(1):185-191.
25. Holley JL. Providing optimal care before and after discontinuation. Semin Dial. 2012;25(1):?33-34.
26. Eknoyan G, Beck GJ, Cheung AK, et al; Hemodialysis (HEMO) Study Group. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):?2010-2019.
27. Lacson E, Lazarus M. Dialysis time: does it matter? A reappraisal of existing literature. Curr Opin Nephrol Hypertens. 2011;20(2):189-194.
28. Chertow GM, Levin NW, Beck GJ, et al; FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):22878-2300.
29. Lacson E Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23(4):687-695.
30. Suri RS, Lindsay RM, Bieber BA, et al. A multinational cohort study of in-center daily hemodialysis and patient survival. Kidney Int. 2012 Sep 12. [Epub ahead of print]
31. Sidawy AN, Spergel LM, Besarab A, et al; Society for Vascular Surgery. Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. ?J Vasc Surg. 2008;48(5 Suppl):2S-25S.
32. Heaf JG. Algorithm for optimal dialysis access timing. Clin Nephrol. 2007;67(2):96-104.
33. Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60(1):1-13.
34. National Kidney Foundation. Peritoneal dialysis: what you need to know (2006;11-50-0215). www.kidney.org/atoz/pdf/PeritonealDialysis.pdf. Accessed September 24, 2012.
35. Davison SN, Ghangri GS, Jindal K, Pannu N. Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2009;4(6):1044-1050.
36. Juergensen P, Eras J, McClure B, et al. The impact of various cycling regimens on phosphorus removal in chronic peritoneal dialysis patients. Int J Artif Organs. 2005;28(12):1219-1223.
37. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006 Nov;(103):S3-S11.
38. Heaf J, Løkkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(1):112-117.
39. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726-1732.
40. Cnossen N, Kooman JP, Konings CJ, et al. Peritoneal dialysis in patients with congestive heart failure. Nephrol Dial Transplant. 2006;21 suppl 2: ii63-ii66.
The Initiating Dialysis Early and Late (IDEAL) study in Australia and New Zealand1 examined the optimal time to initiate dialysis. This well-designed, randomized, controlled trial gave the nephrology community guidelines for treating patients as they progress through chronic kidney disease to stage 5 (CKD 5). The IDEAL investigators demonstrated that planned early initiation of dialysis did not enhance survival—and in some cases, it hastened death.1
Although most patients have a nephrology provider by the time they reach CKD 5 (ie, kidney failure), primary care providers can be instrumental in preparing the patient for what lies ahead as kidney failure progresses. Presented here is an overview of diagnosis and management of the patient with CKD 5 in the 21st century.
CASE PRESENTATION
A 70-year-old woman with an extensive history of diabetes and hypertension presents to her primary care clinician complaining of a lack of energy. She has just returned from a trip to Disney World, where she says she was unable to keep up with her grandchildren. She sat in the shade while they enjoyed Space Mountain and other attractions, and because of uncustomary fatigue, she required a nap every afternoon.
Physical examination shows an elderly female in no acute distress. Cardiac exam shows a regular heart rate and rhythm, the patient’s lungs are clear, and she has 1+ pitting leg edema bilaterally. The patient’s blood pressure is slightly elevated at 142/92 mm Hg, and no protein is detected on spot urine testing.
Blood work is ordered, including a complete blood count, a comprehensive metabolic panel, and an A1C. Results include a hemoglobin level of 8.7 g/dL (reference range for women, 12.0 to 16.0 g/dL), a serum creatinine level (SCr) of 3 mg/dL (range, 0.6 to 1.2 mg/dL; estimated glomerular filtration rate [eGFR], 15 mL/min/1.73 m2), and an A1C value of 7.5% (normal, & < 7%).
The patient is told that she is in kidney failure and is referred immediately to a nephrology practice, where she is seen the following day. After her appointment, she returns to the primary care office. When she is asked when she will be starting dialysis, she seems surprised, saying, “They told me I was doing well. I need some shots for my blood, but they didn’t seem worried at all.”
Is this patient being managed correctly by the nephrology practitioner?
EPIDEMIOLOGY
Presently, more than 1 million persons in the United States have CKD 5, and 500,000 are undergoing dialysis. The number of patients with CDK 5 who are on dialysis has doubled since 1994 and is projected to reach 774,000 by 20202,3 (see Figure 13 and Figure 23).
CKD 5 is defined as an eGFR below 15 mL/min/1.73 m2, according to the Modification of Diet in Renal Disease (MDRD) study group formula; or as a creatinine clearance of less than 15 mL/min, using the Cockcroft-Gault formula.4-6 Both formulas have limitations, since they are not fully accurate at extremes of age, with variations in weight, for some racial mixes, or for the very malnourished patient.7,8 However, they do correct for normal age-related loss of kidney function, gender, and SCr, and they are currently the generally accepted formulas.4
A rise in SCr is exponential; for each doubling of the SCr, a reduction in kidney function of approximately 50% occurs. This means that a rise in SCr from 4 to 8 mg/dL is equivalent (in the proportion of loss of kidney function) to a rise of SCr from 0.5 to 1 mg/dL. Commonly, patients are not referred to nephrology until the SCr doubles to 4 or 6 mg/dL—whereas the rise in SCr from 0.5 to 1 mg/dL should be of greatest concern to the primary care provider, prompting a referral.9 Early recognition of reduced renal function allows for identification of potentially reversible etiologies and the slowing of CKD progression.
INDICATIONS FOR RENAL REPLACEMENT THERAPY
Not all patients with an eGFR below 15 mL/min/1.73 m2 are started on dialysis immediately. The decision to initiate dialysis is guided by assessment of a constellation of uremic manifestations—not eGFR alone. Newer data suggest that early initiation of dialysis may be associated with increased mortality.10-15
The IDEAL study,1 a well-designed, randomized, controlled trial of all patients who started dialysis in Australia and New Zealand over a multiyear period, was designed to determine the optimal time to initiate dialysis. Patients were randomized to start dialysis (hemodialysis or peritoneal dialysis) at an eGFR between 10 and 14 mL/min/1.73 m2 or between 5 and 7 mL/min/1.73 m2. While there was some overlap and some patients were started on dialysis outside their randomized eGFR (eg, earlier than the allotted time because symptoms developed), what the IDEAL investigators found surprised the entire nephrology community: Early dialysis starts did not enhance survival and, in some cases, it hastened death.1
The indications for initiation of dialysis often develop long after the patient has progressed within CKD 5, commonly with an eGFR of 10 mL/min/1.73 m2 or less. Medicare has acknowledged this by reimbursing dialysis only in eligible patients whose eGFR is below 10 mL/min/1.73 m2.16 There are also acute indications for initiation of dialysis, such as uremic pericarditis, hyperkalemia, bleeding related to uremic platelet dysfunction, and metabolic encephalopathy related to uremia (and reimbursement for dialysis can be justified), but these are uncommon.
RENAL REPLACEMENT THERAPY CHOICES
The choices of treatment for kidney failure (note: treatment, not cure) are medical management, hemodialysis, peritoneal dialysis, and transplant. Each choice has its advantages and disadvantages, and all patients should receive clear explanations of what they can expect from each modality.
While younger, higher-functioning individuals are likely to benefit from dialysis, patients with extensive comorbid illnesses and/or low functional status tend to respond poorly17; medical management may be the best choice for these patients. In one recent study, the functional status of residents in skilled nursing homes was examined, before and after initiation of dialysis. At one year, 58% of the nursing home residents who underwent dialysis had died, and only 13% had maintained their pre-dialysis functional status.18
Another research team recently compared conservative management of CKD 5 (ie, medical therapy without dialysis) with dialysis in elderly patients. For patients 75 and older with extensive comorbid illness, the researchers found no statistically significant survival benefit to dialysis.19
Dialysis, whether administered as hemodialysis or peritoneal dialysis, is a rigorous, intensive medical therapy. Dialysis does not necessarily prolong life in patients with extensive comorbidities, and it does not always enhance quality of life.18,20-23 The decision to undergo dialysis is personal and individual, and both the patient and family should be actively involved in making it. Primary care providers should be the nephrologist’s ally in the discussion of therapy for renal failure; often, they have cared for the patient for years, and they understand the patient and family dynamics.
An important message the nephrology practitioner must communicate is that choosing medical management without dialysis is not withholding care; sometimes it is a more humane choice.24 Dying of kidney failure can be a peaceful and comfortable death: As the uremic toxins build up (with eGFR ≤ 2 mL/min/1.73 m2, although this can take many years), the patient becomes confused and slowly slips into a sleepiness that leads to death.25 Hospice is usually involved to support the patient and family.
Hemodialysis
Hemodialysis is the most common, best-known treatment modality for CKD in the US, with about 94% of patients choosing it.3 In this process, blood is removed from the body (approximately 500 cc at a time) and filtered through a semipermeable membrane that removes uremic toxins and excess fluid, normalizing the metabolic and electrolyte derangements. The filtered blood is then returned to the patient.
The average dialysis session is four hours long and is conducted three times per week, following recommendations from the 2002 Hemodialysis Study (HEMO).26 Most patients come to a free-standing dialysis center on a Monday/Wednesday/Friday or a Tuesday/Thursday/Saturday schedule.
In theory, there is no such thing as too much dialysis (since the kidneys work 24 hours per day, seven days per week); thus, researchers have recently examined lengthening dialysis in an attempt to extend survival.27-29 According to study results, patients who undergo longer dialysis times generally enjoy better nutrition with a more liberal diet, require fewer medications, have reduced incidence of increased left ventricular mass (a marker for coronary artery disease), and report better quality of life, all in addition to a survival benefit; however, the latter was not considered statistically significant in any of the studies.27-29 Additionally, this survival benefit may not extend to daily hemodialysis; in a recent publication, daily hemodialysis was associated with a 60% higher death rate.30
A number of dialysis units have begun to offer nocturnal dialysis. In this option, patients sleep at the unit for eight hours, three nights per week, for a total of 24 hours of dialysis (vs the typical 12 hours per week). Some patients receive dialysis at home, allowing them to dialyze for six weekly sessions of two to three hours. This strategy attempts to mimic a more “natural” state.
One of the primary challenges associated with hemodialysis is establishing and maintaining a vascular access. An arteriovenous (AV) fistula is the access of choice because its use reduces the likelihood of clotting, improves access survival, and increases clearances during dialysis.31 However, the AV fistula is most effective if it is placed a minimum of six months before use.32
For many patients, an AV fistula is created surgically when the eGFR falls to 15 mL/min/1.73 m2. Fistula placement in preparation for the initiation of hemodialysis is important to reduce the need for hemodialysis catheters, which are associated with higher risk for infection and poorer outcomes.33
Peritoneal Dialysis
Peritoneal dialysis (PD) filters out uremic toxins and normalizes the metabolic and electrolyte derangements by using the patient’s peritoneal membrane as a filter. Dialysate is instilled through a catheter into the peritoneal cavity where it is allowed to dwell, often for four to six hours (see Figure 334). Exchanges can be performed three to four times per day (allowing six to eight hours of dwell time per exchange for the dialysate), or by way of a cycler at night.35,36
Performing exchanges by way of a cycler requires the patient to be connected via the PD catheter for eight hours; thus the advantage of performing the exchange during sleep. The cycler has a soft, whooshing sound that many patients describe as “white noise” that does not disturb their sleep.
The principal advantage of peritoneal dialysis is extended survival of the patient.37-39 PD also allows the patient a more liberal diet. Although PD must be performed every day, exchanges can be adjusted to the patient’s timing preference. PD reduces cost and time, since the patient need not travel to a dialysis center. PD also preserves residual renal function,39 which is associated with a survival benefit and contributes to the patient’s overall health and well-being. The use of PD requires a committed, competent patient in a clean home environment.
This intervention may not be suitable for patients with a history of abdominal surgeries. However, as a therapy considered gentler than hemodialysis, PD is an excellent choice for the patient with congestive heart failure.40 The PD catheter is not placed until two to four weeks before it will be needed.41
Kidney Transplantation
Last, but certainly not least, is transplant. As entire books have been written on this modality, only the highlights will be addressed here.42 Survival rates following transplantation are reported to exceed those associated with any other treatment modality, even when controlling for comorbidities and patient selection bias.43 A patient can receive a kidney from either a living or a deceased donor. Apart from the perioperative risks associated with transplantation surgery, long-term surgical or medical problems are not common for the living kidney donor.44-46
Since 2002, as a result of a policy change from the United Network for Organ Sharing (UNOS),47 the donor pool has been expanded by including kidneys from what are commonly referred to as extended criteria donors: those who are older than 60, or who are 50 to 59 and have two of the following factors: cerebrovascular accident as the cause of death; preexisting hypertension; or an SCr level exceeding 1.5 mg/dL. These kidneys may be given to any recipients but are primarily used for those age 50 or older.47
Graft survival is statistically shorter in a cadaver kidney than in a living donor kidney.48 However, the larger problem is the long wait time for a cadaver transplant—seven to eight years (or possibly longer) for some centers and some blood types.49 A patient can be referred to a transplant center when the eGFR falls below 25 mL/min/1.73 m2; he or she will then be actively listed for transplant when eGFR reaches 20 mL/min/1.73 m2 or lower.
Early referral for transplant listing buys the patient time before dialysis becomes essential. Patients can receive preemptive transplants (ie, transplantation before dialysis is initiated); these are becoming increasingly popular because they generally extend survival for the recipient.50,51
UNOS (www.unos.org) has set few limits on transplant recipients: patients are required to undergo an extensive medical work-up, but there are no upper age limits (although some centers do set their own) and usually no limits on patients infected with HIV, hepatitis B, or hepatitis C (although there may be separate listing requirements). Patients who are not US citizens are not denied.
Because more than 100,000 patients are currently on the wait list, domino kidney transplants are now being offered at many centers.52-54 These organ exchanges involve altruistic donors who do not match their targeted kidney recipients. Since publication of the first article describing this procedure at Johns Hopkins Medical Center,53 there have been two-way, three-way, and up to 32-way domino transplants. However, patients wishing to engage in this type of trade require a donor; not every patient has access to an altruistic donor.
CKD PATIENT EDUCATION
When Congress passed the Medicare Improvements for Patients and Providers Act of 2008,55 a component little noted outside the nephrology community was the offer of kidney disease education (KDE) classes to Medicare-eligible patients with CKD 4.56-58 Medicare patients with an eGFR of 15 to 30 mL/min/1.73 m2 are eligible to attend KDE classes presented by a physician, an NP, a PA, or a clinical nurse specialist. Medicare will reimburse for six hours of education.
This groundbreaking educational benefit, the first such program paid for by Medicare, was championed by the National Kidney Foundation and other nephrology groups. Many nephrology practices now offer these classes to all their CKD patients, regardless of health insurance status. Instructors educate patients on the choices of renal replacement therapy and help patients select their best option.
KDE classes can be conducted in the office setting or at the patient’s home, and they can be billed on the same day as an evaluation and management (E/M) visit.56,57 This may help explain why, in 2010, gerontologists billed for more home KDE classes than did nephrologists.58
FUTURE TRENDS AND ONGOING TRIALS
The overarching goal of therapy for CKD patients is to diagnose accurately and treat effectively in order to slow or prevent progression to end-stage renal disease (ESRD). Following is a brief review of a selection of new diagnostic tools and therapeutic interventions that may impact the management of CKD in the coming years.
The QxMD Kidney Failure Risk Equation is a newly developed, well-validated predictive model that offers relatively accurate prediction of a patient’s likelihood of progression to ESRD, based on age, sex, eGFR, and levels of albuminuria, SCr, serum phosphorus, serum bicarbonate, and serum albumin59 (see table59). This tool can be accessed online at www.qxmd.com/calculate-online/nephrology/kidney-failure-risk-equation.
Increased awareness of the role that phosphorus plays in the development of vascular calcifications has led to an emphasis on earlier, more aggressive control of dietary phosphorus. Historically, dietary phosphorus control and phosphorus binders were recommended only when the patient’s serum phosphorus exceeded normal limits. Dietary phosphorus control may now be advised as early as CKD 3, based on the understanding that phosphorus is a key component in driving the development of vascular calcifications—which in turn contribute to the high incidence of cardiovascular disease in patients with CKD.60
Bardoxolone is a new medication developed for treatment of diabetic nephropathy.61,62 It works by inhibiting proinflammatory mediators and moderating the effects of oxidative stress, thus interrupting the cascade of inflammation and cellular injury that result in diabetic nephropathy.62 Early clinical trials examining this agent have shown promise in terms of raising eGFR and reducing serum creatinine, but tolerability and toxicity profiles have been an issue.61 Additionally, some reduction in serum creatinine may have been attributable to weight loss as opposed to true improvement of renal function.62
Data have recently been released regarding the use of the herb silymarin for treatment of patients with macroalbuminuria related to diabetic nephropathy. Results from a small (n = 60), randomized, nonblinded clinical trial by Fallahzadeh et al63 indicate that silymarin decreases proteinuria when used in combination with an ACE inhibitor or an angiotensin receptor blocker (ARB). Silymarin, an extract from milk thistle, has been used medicinally for centuries for its antioxidant, anti-inflammatory, and antiviral properties in those with liver “ailments.” In this clinical trial, silymarin was well tolerated and associated with a reduction in pro-inflammatory markers.63
AST-120 is another agent intended to slow progression of CKD. It promotes intestinal adsorption and fecal excretion of the uremic toxin indoxyl sulfate.64 A recently published study demonstrated an association between use of AST-120 and a delay in required initiation of hemodialysis, but no survival benefit.65 This was a nonrandomized trial, and previous research showed no effect of AST-120 on progression of CKD.66 However, patients close to requiring dialysis are likely to welcome a means to delay it.
A growing body of evidence suggests that reversal of CKD-associated acidemia by administering sodium bicarbonate may actually forestall progression of CKD.67 However, treatment with sodium bicarbonate can lead to complications of hypertension and fluid volume overload. In one study, patients at risk for these complications derived equivalent benefit by increasing dietary fruit and vegetable intake to reduce renal acid load.68 However, providers must be mindful of patients’ serum potassium levels, especially patients who are taking an ACE inhibitor or an ARB.
The Provider’s Current Role
Despite the hope offered by new and novel therapies, the mainstay of treatment for CKD continues to be aggressive management of diabetes, hypertension, and the cardiovascular risk profile. As a result of vigorous preventive strategies to address the cardiovascular risks inherent in this patient population, the patient with type 2 diabetes is now more likely to progress to ESRD than to die of a cardiovascular event.69
The well-informed clinician can be instrumental in providing evidence-based medical therapy and excellent patient education from the time of initial CKD diagnosis through CKD 5.
PATIENT OUTCOME
The case patient was started on injections of epoietin alfa for her anemia. She attended KDE classes led by an advanced practitioner in her nephrology group and decided to undergo peritoneal dialysis, since it would allow her to maintain her travel schedule. When last heard from, the patient was preparing for a trip to see the Great Barrier Reef in Australia.
CONCLUSION
The decision to begin renal replacement therapy—whether a form of dialysis, or another management option—depends not on SCr or eGFR alone. Rather, a number of uremic manifestations, comorbidities, lifestyle factors, and other variables must be carefully weighed before the patient, the family, and the clinicians involved can decide on the management plan most likely to enhance the patient’s quality of life and extend survival.
The Initiating Dialysis Early and Late (IDEAL) study in Australia and New Zealand1 examined the optimal time to initiate dialysis. This well-designed, randomized, controlled trial gave the nephrology community guidelines for treating patients as they progress through chronic kidney disease to stage 5 (CKD 5). The IDEAL investigators demonstrated that planned early initiation of dialysis did not enhance survival—and in some cases, it hastened death.1
Although most patients have a nephrology provider by the time they reach CKD 5 (ie, kidney failure), primary care providers can be instrumental in preparing the patient for what lies ahead as kidney failure progresses. Presented here is an overview of diagnosis and management of the patient with CKD 5 in the 21st century.
CASE PRESENTATION
A 70-year-old woman with an extensive history of diabetes and hypertension presents to her primary care clinician complaining of a lack of energy. She has just returned from a trip to Disney World, where she says she was unable to keep up with her grandchildren. She sat in the shade while they enjoyed Space Mountain and other attractions, and because of uncustomary fatigue, she required a nap every afternoon.
Physical examination shows an elderly female in no acute distress. Cardiac exam shows a regular heart rate and rhythm, the patient’s lungs are clear, and she has 1+ pitting leg edema bilaterally. The patient’s blood pressure is slightly elevated at 142/92 mm Hg, and no protein is detected on spot urine testing.
Blood work is ordered, including a complete blood count, a comprehensive metabolic panel, and an A1C. Results include a hemoglobin level of 8.7 g/dL (reference range for women, 12.0 to 16.0 g/dL), a serum creatinine level (SCr) of 3 mg/dL (range, 0.6 to 1.2 mg/dL; estimated glomerular filtration rate [eGFR], 15 mL/min/1.73 m2), and an A1C value of 7.5% (normal, & < 7%).
The patient is told that she is in kidney failure and is referred immediately to a nephrology practice, where she is seen the following day. After her appointment, she returns to the primary care office. When she is asked when she will be starting dialysis, she seems surprised, saying, “They told me I was doing well. I need some shots for my blood, but they didn’t seem worried at all.”
Is this patient being managed correctly by the nephrology practitioner?
EPIDEMIOLOGY
Presently, more than 1 million persons in the United States have CKD 5, and 500,000 are undergoing dialysis. The number of patients with CDK 5 who are on dialysis has doubled since 1994 and is projected to reach 774,000 by 20202,3 (see Figure 13 and Figure 23).
CKD 5 is defined as an eGFR below 15 mL/min/1.73 m2, according to the Modification of Diet in Renal Disease (MDRD) study group formula; or as a creatinine clearance of less than 15 mL/min, using the Cockcroft-Gault formula.4-6 Both formulas have limitations, since they are not fully accurate at extremes of age, with variations in weight, for some racial mixes, or for the very malnourished patient.7,8 However, they do correct for normal age-related loss of kidney function, gender, and SCr, and they are currently the generally accepted formulas.4
A rise in SCr is exponential; for each doubling of the SCr, a reduction in kidney function of approximately 50% occurs. This means that a rise in SCr from 4 to 8 mg/dL is equivalent (in the proportion of loss of kidney function) to a rise of SCr from 0.5 to 1 mg/dL. Commonly, patients are not referred to nephrology until the SCr doubles to 4 or 6 mg/dL—whereas the rise in SCr from 0.5 to 1 mg/dL should be of greatest concern to the primary care provider, prompting a referral.9 Early recognition of reduced renal function allows for identification of potentially reversible etiologies and the slowing of CKD progression.
INDICATIONS FOR RENAL REPLACEMENT THERAPY
Not all patients with an eGFR below 15 mL/min/1.73 m2 are started on dialysis immediately. The decision to initiate dialysis is guided by assessment of a constellation of uremic manifestations—not eGFR alone. Newer data suggest that early initiation of dialysis may be associated with increased mortality.10-15
The IDEAL study,1 a well-designed, randomized, controlled trial of all patients who started dialysis in Australia and New Zealand over a multiyear period, was designed to determine the optimal time to initiate dialysis. Patients were randomized to start dialysis (hemodialysis or peritoneal dialysis) at an eGFR between 10 and 14 mL/min/1.73 m2 or between 5 and 7 mL/min/1.73 m2. While there was some overlap and some patients were started on dialysis outside their randomized eGFR (eg, earlier than the allotted time because symptoms developed), what the IDEAL investigators found surprised the entire nephrology community: Early dialysis starts did not enhance survival and, in some cases, it hastened death.1
The indications for initiation of dialysis often develop long after the patient has progressed within CKD 5, commonly with an eGFR of 10 mL/min/1.73 m2 or less. Medicare has acknowledged this by reimbursing dialysis only in eligible patients whose eGFR is below 10 mL/min/1.73 m2.16 There are also acute indications for initiation of dialysis, such as uremic pericarditis, hyperkalemia, bleeding related to uremic platelet dysfunction, and metabolic encephalopathy related to uremia (and reimbursement for dialysis can be justified), but these are uncommon.
RENAL REPLACEMENT THERAPY CHOICES
The choices of treatment for kidney failure (note: treatment, not cure) are medical management, hemodialysis, peritoneal dialysis, and transplant. Each choice has its advantages and disadvantages, and all patients should receive clear explanations of what they can expect from each modality.
While younger, higher-functioning individuals are likely to benefit from dialysis, patients with extensive comorbid illnesses and/or low functional status tend to respond poorly17; medical management may be the best choice for these patients. In one recent study, the functional status of residents in skilled nursing homes was examined, before and after initiation of dialysis. At one year, 58% of the nursing home residents who underwent dialysis had died, and only 13% had maintained their pre-dialysis functional status.18
Another research team recently compared conservative management of CKD 5 (ie, medical therapy without dialysis) with dialysis in elderly patients. For patients 75 and older with extensive comorbid illness, the researchers found no statistically significant survival benefit to dialysis.19
Dialysis, whether administered as hemodialysis or peritoneal dialysis, is a rigorous, intensive medical therapy. Dialysis does not necessarily prolong life in patients with extensive comorbidities, and it does not always enhance quality of life.18,20-23 The decision to undergo dialysis is personal and individual, and both the patient and family should be actively involved in making it. Primary care providers should be the nephrologist’s ally in the discussion of therapy for renal failure; often, they have cared for the patient for years, and they understand the patient and family dynamics.
An important message the nephrology practitioner must communicate is that choosing medical management without dialysis is not withholding care; sometimes it is a more humane choice.24 Dying of kidney failure can be a peaceful and comfortable death: As the uremic toxins build up (with eGFR ≤ 2 mL/min/1.73 m2, although this can take many years), the patient becomes confused and slowly slips into a sleepiness that leads to death.25 Hospice is usually involved to support the patient and family.
Hemodialysis
Hemodialysis is the most common, best-known treatment modality for CKD in the US, with about 94% of patients choosing it.3 In this process, blood is removed from the body (approximately 500 cc at a time) and filtered through a semipermeable membrane that removes uremic toxins and excess fluid, normalizing the metabolic and electrolyte derangements. The filtered blood is then returned to the patient.
The average dialysis session is four hours long and is conducted three times per week, following recommendations from the 2002 Hemodialysis Study (HEMO).26 Most patients come to a free-standing dialysis center on a Monday/Wednesday/Friday or a Tuesday/Thursday/Saturday schedule.
In theory, there is no such thing as too much dialysis (since the kidneys work 24 hours per day, seven days per week); thus, researchers have recently examined lengthening dialysis in an attempt to extend survival.27-29 According to study results, patients who undergo longer dialysis times generally enjoy better nutrition with a more liberal diet, require fewer medications, have reduced incidence of increased left ventricular mass (a marker for coronary artery disease), and report better quality of life, all in addition to a survival benefit; however, the latter was not considered statistically significant in any of the studies.27-29 Additionally, this survival benefit may not extend to daily hemodialysis; in a recent publication, daily hemodialysis was associated with a 60% higher death rate.30
A number of dialysis units have begun to offer nocturnal dialysis. In this option, patients sleep at the unit for eight hours, three nights per week, for a total of 24 hours of dialysis (vs the typical 12 hours per week). Some patients receive dialysis at home, allowing them to dialyze for six weekly sessions of two to three hours. This strategy attempts to mimic a more “natural” state.
One of the primary challenges associated with hemodialysis is establishing and maintaining a vascular access. An arteriovenous (AV) fistula is the access of choice because its use reduces the likelihood of clotting, improves access survival, and increases clearances during dialysis.31 However, the AV fistula is most effective if it is placed a minimum of six months before use.32
For many patients, an AV fistula is created surgically when the eGFR falls to 15 mL/min/1.73 m2. Fistula placement in preparation for the initiation of hemodialysis is important to reduce the need for hemodialysis catheters, which are associated with higher risk for infection and poorer outcomes.33
Peritoneal Dialysis
Peritoneal dialysis (PD) filters out uremic toxins and normalizes the metabolic and electrolyte derangements by using the patient’s peritoneal membrane as a filter. Dialysate is instilled through a catheter into the peritoneal cavity where it is allowed to dwell, often for four to six hours (see Figure 334). Exchanges can be performed three to four times per day (allowing six to eight hours of dwell time per exchange for the dialysate), or by way of a cycler at night.35,36
Performing exchanges by way of a cycler requires the patient to be connected via the PD catheter for eight hours; thus the advantage of performing the exchange during sleep. The cycler has a soft, whooshing sound that many patients describe as “white noise” that does not disturb their sleep.
The principal advantage of peritoneal dialysis is extended survival of the patient.37-39 PD also allows the patient a more liberal diet. Although PD must be performed every day, exchanges can be adjusted to the patient’s timing preference. PD reduces cost and time, since the patient need not travel to a dialysis center. PD also preserves residual renal function,39 which is associated with a survival benefit and contributes to the patient’s overall health and well-being. The use of PD requires a committed, competent patient in a clean home environment.
This intervention may not be suitable for patients with a history of abdominal surgeries. However, as a therapy considered gentler than hemodialysis, PD is an excellent choice for the patient with congestive heart failure.40 The PD catheter is not placed until two to four weeks before it will be needed.41
Kidney Transplantation
Last, but certainly not least, is transplant. As entire books have been written on this modality, only the highlights will be addressed here.42 Survival rates following transplantation are reported to exceed those associated with any other treatment modality, even when controlling for comorbidities and patient selection bias.43 A patient can receive a kidney from either a living or a deceased donor. Apart from the perioperative risks associated with transplantation surgery, long-term surgical or medical problems are not common for the living kidney donor.44-46
Since 2002, as a result of a policy change from the United Network for Organ Sharing (UNOS),47 the donor pool has been expanded by including kidneys from what are commonly referred to as extended criteria donors: those who are older than 60, or who are 50 to 59 and have two of the following factors: cerebrovascular accident as the cause of death; preexisting hypertension; or an SCr level exceeding 1.5 mg/dL. These kidneys may be given to any recipients but are primarily used for those age 50 or older.47
Graft survival is statistically shorter in a cadaver kidney than in a living donor kidney.48 However, the larger problem is the long wait time for a cadaver transplant—seven to eight years (or possibly longer) for some centers and some blood types.49 A patient can be referred to a transplant center when the eGFR falls below 25 mL/min/1.73 m2; he or she will then be actively listed for transplant when eGFR reaches 20 mL/min/1.73 m2 or lower.
Early referral for transplant listing buys the patient time before dialysis becomes essential. Patients can receive preemptive transplants (ie, transplantation before dialysis is initiated); these are becoming increasingly popular because they generally extend survival for the recipient.50,51
UNOS (www.unos.org) has set few limits on transplant recipients: patients are required to undergo an extensive medical work-up, but there are no upper age limits (although some centers do set their own) and usually no limits on patients infected with HIV, hepatitis B, or hepatitis C (although there may be separate listing requirements). Patients who are not US citizens are not denied.
Because more than 100,000 patients are currently on the wait list, domino kidney transplants are now being offered at many centers.52-54 These organ exchanges involve altruistic donors who do not match their targeted kidney recipients. Since publication of the first article describing this procedure at Johns Hopkins Medical Center,53 there have been two-way, three-way, and up to 32-way domino transplants. However, patients wishing to engage in this type of trade require a donor; not every patient has access to an altruistic donor.
CKD PATIENT EDUCATION
When Congress passed the Medicare Improvements for Patients and Providers Act of 2008,55 a component little noted outside the nephrology community was the offer of kidney disease education (KDE) classes to Medicare-eligible patients with CKD 4.56-58 Medicare patients with an eGFR of 15 to 30 mL/min/1.73 m2 are eligible to attend KDE classes presented by a physician, an NP, a PA, or a clinical nurse specialist. Medicare will reimburse for six hours of education.
This groundbreaking educational benefit, the first such program paid for by Medicare, was championed by the National Kidney Foundation and other nephrology groups. Many nephrology practices now offer these classes to all their CKD patients, regardless of health insurance status. Instructors educate patients on the choices of renal replacement therapy and help patients select their best option.
KDE classes can be conducted in the office setting or at the patient’s home, and they can be billed on the same day as an evaluation and management (E/M) visit.56,57 This may help explain why, in 2010, gerontologists billed for more home KDE classes than did nephrologists.58
FUTURE TRENDS AND ONGOING TRIALS
The overarching goal of therapy for CKD patients is to diagnose accurately and treat effectively in order to slow or prevent progression to end-stage renal disease (ESRD). Following is a brief review of a selection of new diagnostic tools and therapeutic interventions that may impact the management of CKD in the coming years.
The QxMD Kidney Failure Risk Equation is a newly developed, well-validated predictive model that offers relatively accurate prediction of a patient’s likelihood of progression to ESRD, based on age, sex, eGFR, and levels of albuminuria, SCr, serum phosphorus, serum bicarbonate, and serum albumin59 (see table59). This tool can be accessed online at www.qxmd.com/calculate-online/nephrology/kidney-failure-risk-equation.
Increased awareness of the role that phosphorus plays in the development of vascular calcifications has led to an emphasis on earlier, more aggressive control of dietary phosphorus. Historically, dietary phosphorus control and phosphorus binders were recommended only when the patient’s serum phosphorus exceeded normal limits. Dietary phosphorus control may now be advised as early as CKD 3, based on the understanding that phosphorus is a key component in driving the development of vascular calcifications—which in turn contribute to the high incidence of cardiovascular disease in patients with CKD.60
Bardoxolone is a new medication developed for treatment of diabetic nephropathy.61,62 It works by inhibiting proinflammatory mediators and moderating the effects of oxidative stress, thus interrupting the cascade of inflammation and cellular injury that result in diabetic nephropathy.62 Early clinical trials examining this agent have shown promise in terms of raising eGFR and reducing serum creatinine, but tolerability and toxicity profiles have been an issue.61 Additionally, some reduction in serum creatinine may have been attributable to weight loss as opposed to true improvement of renal function.62
Data have recently been released regarding the use of the herb silymarin for treatment of patients with macroalbuminuria related to diabetic nephropathy. Results from a small (n = 60), randomized, nonblinded clinical trial by Fallahzadeh et al63 indicate that silymarin decreases proteinuria when used in combination with an ACE inhibitor or an angiotensin receptor blocker (ARB). Silymarin, an extract from milk thistle, has been used medicinally for centuries for its antioxidant, anti-inflammatory, and antiviral properties in those with liver “ailments.” In this clinical trial, silymarin was well tolerated and associated with a reduction in pro-inflammatory markers.63
AST-120 is another agent intended to slow progression of CKD. It promotes intestinal adsorption and fecal excretion of the uremic toxin indoxyl sulfate.64 A recently published study demonstrated an association between use of AST-120 and a delay in required initiation of hemodialysis, but no survival benefit.65 This was a nonrandomized trial, and previous research showed no effect of AST-120 on progression of CKD.66 However, patients close to requiring dialysis are likely to welcome a means to delay it.
A growing body of evidence suggests that reversal of CKD-associated acidemia by administering sodium bicarbonate may actually forestall progression of CKD.67 However, treatment with sodium bicarbonate can lead to complications of hypertension and fluid volume overload. In one study, patients at risk for these complications derived equivalent benefit by increasing dietary fruit and vegetable intake to reduce renal acid load.68 However, providers must be mindful of patients’ serum potassium levels, especially patients who are taking an ACE inhibitor or an ARB.
The Provider’s Current Role
Despite the hope offered by new and novel therapies, the mainstay of treatment for CKD continues to be aggressive management of diabetes, hypertension, and the cardiovascular risk profile. As a result of vigorous preventive strategies to address the cardiovascular risks inherent in this patient population, the patient with type 2 diabetes is now more likely to progress to ESRD than to die of a cardiovascular event.69
The well-informed clinician can be instrumental in providing evidence-based medical therapy and excellent patient education from the time of initial CKD diagnosis through CKD 5.
PATIENT OUTCOME
The case patient was started on injections of epoietin alfa for her anemia. She attended KDE classes led by an advanced practitioner in her nephrology group and decided to undergo peritoneal dialysis, since it would allow her to maintain her travel schedule. When last heard from, the patient was preparing for a trip to see the Great Barrier Reef in Australia.
CONCLUSION
The decision to begin renal replacement therapy—whether a form of dialysis, or another management option—depends not on SCr or eGFR alone. Rather, a number of uremic manifestations, comorbidities, lifestyle factors, and other variables must be carefully weighed before the patient, the family, and the clinicians involved can decide on the management plan most likely to enhance the patient’s quality of life and extend survival.
1. Cooper BA, Branley P, Bulfone L, et al; IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010; 363(7):609-619.
2. Olan G. Policy Update: ASN to CDC: data collection of creatinine levels will advance research. ASN Kidney News. 2012;4(6):16. www.asn-online.org/publications/kidneynews/archives/2012/KN_jun2012.pdf. Accessed August 27, 2012.
3. US Renal Data System, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. 2011 Atlas of CKD & ESRD. Vol 2. Chap 1: Incidence, prevalence, patient characteristics, and modalities. www.usrds.org/2011/pdf/v2_ch01_11.pdf. Accessed September 26, 2012.
4. National Kidney Foundation. Kidney Disease Outcomes Quality Initiative (KDOQI). Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. ?Part 4. Definition and classification of the stages of chronic kidney disease. 2002:43-80. www.kidney.org/professionals/kdoqi/pdf/ckd_evalua tion_classification_stratification.pdf. Accessed August 27, 2012.
5. Levey AS, Bosch JP, Lewis JB, et al; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Internal Med. 1999;130 (6):461-470.
6. Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763-773.
7. Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16(5):1413-1419.
8. Zuo L, Ma YC, Zhou YH, et al. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45(3):463-472.
9. Stevens LA, Stoycheff N, Levey AS. Staging and management of chronic kidney disease. In: Greenberg A, ed. Primer of Kidney Diseases: Expert Consult. 5th ed. Philadelphia, PA: WB Saunders; 2009:436-445.
10. Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47-53.
11. Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14(9):2305-2312.
12. Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887-896.
13. Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77(8):700-707.
14. Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24 (10):3175-3182.
15. Traynor JP, Simpson K, Geddes CC, et al. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13(8):2125-2132.
16. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. CMS Form 2728. End-Stage Renal Disease Medical Evidence Report: Medicare Entitlement and/or Patient Registration. www.usrds.org/2008/rg/forms/02_2728_1965.pdf. Accessed September 25, 2012.
17. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline. 2nd ed. Rockville, MD: Renal Physicians Association; Oct 2010:1-12.
18. Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
19. Chandna SM, Da Silva-Gane M, Marshall C, et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614.
20. Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95:c40-c46.
21. Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356:1543-1550.
22. O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012; 15(2):228-235.
23. Jassal SV, Trpeski L, Zhu N, et al. Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ. 2007;177(9):?1033-1038.
24. Schmidt RJ. Informing our elders about dialysis: is an age-attuned approach warranted? Clin J Am Soc Nephrol. 2012;7(1):185-191.
25. Holley JL. Providing optimal care before and after discontinuation. Semin Dial. 2012;25(1):?33-34.
26. Eknoyan G, Beck GJ, Cheung AK, et al; Hemodialysis (HEMO) Study Group. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):?2010-2019.
27. Lacson E, Lazarus M. Dialysis time: does it matter? A reappraisal of existing literature. Curr Opin Nephrol Hypertens. 2011;20(2):189-194.
28. Chertow GM, Levin NW, Beck GJ, et al; FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):22878-2300.
29. Lacson E Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23(4):687-695.
30. Suri RS, Lindsay RM, Bieber BA, et al. A multinational cohort study of in-center daily hemodialysis and patient survival. Kidney Int. 2012 Sep 12. [Epub ahead of print]
31. Sidawy AN, Spergel LM, Besarab A, et al; Society for Vascular Surgery. Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. ?J Vasc Surg. 2008;48(5 Suppl):2S-25S.
32. Heaf JG. Algorithm for optimal dialysis access timing. Clin Nephrol. 2007;67(2):96-104.
33. Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60(1):1-13.
34. National Kidney Foundation. Peritoneal dialysis: what you need to know (2006;11-50-0215). www.kidney.org/atoz/pdf/PeritonealDialysis.pdf. Accessed September 24, 2012.
35. Davison SN, Ghangri GS, Jindal K, Pannu N. Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2009;4(6):1044-1050.
36. Juergensen P, Eras J, McClure B, et al. The impact of various cycling regimens on phosphorus removal in chronic peritoneal dialysis patients. Int J Artif Organs. 2005;28(12):1219-1223.
37. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006 Nov;(103):S3-S11.
38. Heaf J, Løkkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(1):112-117.
39. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726-1732.
40. Cnossen N, Kooman JP, Konings CJ, et al. Peritoneal dialysis in patients with congestive heart failure. Nephrol Dial Transplant. 2006;21 suppl 2: ii63-ii66.
1. Cooper BA, Branley P, Bulfone L, et al; IDEAL Study. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010; 363(7):609-619.
2. Olan G. Policy Update: ASN to CDC: data collection of creatinine levels will advance research. ASN Kidney News. 2012;4(6):16. www.asn-online.org/publications/kidneynews/archives/2012/KN_jun2012.pdf. Accessed August 27, 2012.
3. US Renal Data System, National Institute of Diabetes and Digestive and Kidney Diseases, NIH. 2011 Atlas of CKD & ESRD. Vol 2. Chap 1: Incidence, prevalence, patient characteristics, and modalities. www.usrds.org/2011/pdf/v2_ch01_11.pdf. Accessed September 26, 2012.
4. National Kidney Foundation. Kidney Disease Outcomes Quality Initiative (KDOQI). Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. ?Part 4. Definition and classification of the stages of chronic kidney disease. 2002:43-80. www.kidney.org/professionals/kdoqi/pdf/ckd_evalua tion_classification_stratification.pdf. Accessed August 27, 2012.
5. Levey AS, Bosch JP, Lewis JB, et al; Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Internal Med. 1999;130 (6):461-470.
6. Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the Modification of Diet in Renal Disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763-773.
7. Jafar TH, Schmid CH, Levey AS. Serum creatinine as marker of kidney function in South Asians: a study of reduced GFR in adults in Pakistan. J Am Soc Nephrol. 2005;16(5):1413-1419.
8. Zuo L, Ma YC, Zhou YH, et al. Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis. 2005;45(3):463-472.
9. Stevens LA, Stoycheff N, Levey AS. Staging and management of chronic kidney disease. In: Greenberg A, ed. Primer of Kidney Diseases: Expert Consult. 5th ed. Philadelphia, PA: WB Saunders; 2009:436-445.
10. Clark WF, Na Y, Rosansky SJ, et al. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ. 2011;183(1):47-53.
11. Beddhu S, Samore MH, Roberts MS, et al. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14(9):2305-2312.
12. Kazmi WH, Gilbertson DT, Obrador GT, et al. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46(5):887-896.
13. Lassalle M, Labeeuw M, Frimat L, et al. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77(8):700-707.
14. Stel VS, Dekker FW, Ansell D, et al. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24 (10):3175-3182.
15. Traynor JP, Simpson K, Geddes CC, et al. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13(8):2125-2132.
16. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. CMS Form 2728. End-Stage Renal Disease Medical Evidence Report: Medicare Entitlement and/or Patient Registration. www.usrds.org/2008/rg/forms/02_2728_1965.pdf. Accessed September 25, 2012.
17. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis: Clinical Practice Guideline. 2nd ed. Rockville, MD: Renal Physicians Association; Oct 2010:1-12.
18. Kurella Tamura M, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547.
19. Chandna SM, Da Silva-Gane M, Marshall C, et al. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614.
20. Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract. 2003;95:c40-c46.
21. Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet. 2000;356:1543-1550.
22. O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012; 15(2):228-235.
23. Jassal SV, Trpeski L, Zhu N, et al. Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ. 2007;177(9):?1033-1038.
24. Schmidt RJ. Informing our elders about dialysis: is an age-attuned approach warranted? Clin J Am Soc Nephrol. 2012;7(1):185-191.
25. Holley JL. Providing optimal care before and after discontinuation. Semin Dial. 2012;25(1):?33-34.
26. Eknoyan G, Beck GJ, Cheung AK, et al; Hemodialysis (HEMO) Study Group. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):?2010-2019.
27. Lacson E, Lazarus M. Dialysis time: does it matter? A reappraisal of existing literature. Curr Opin Nephrol Hypertens. 2011;20(2):189-194.
28. Chertow GM, Levin NW, Beck GJ, et al; FHN Trial Group. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):22878-2300.
29. Lacson E Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23(4):687-695.
30. Suri RS, Lindsay RM, Bieber BA, et al. A multinational cohort study of in-center daily hemodialysis and patient survival. Kidney Int. 2012 Sep 12. [Epub ahead of print]
31. Sidawy AN, Spergel LM, Besarab A, et al; Society for Vascular Surgery. Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. ?J Vasc Surg. 2008;48(5 Suppl):2S-25S.
32. Heaf JG. Algorithm for optimal dialysis access timing. Clin Nephrol. 2007;67(2):96-104.
33. Nassar GM, Ayus JC. Infectious complications of the hemodialysis access. Kidney Int. 2001;60(1):1-13.
34. National Kidney Foundation. Peritoneal dialysis: what you need to know (2006;11-50-0215). www.kidney.org/atoz/pdf/PeritonealDialysis.pdf. Accessed September 24, 2012.
35. Davison SN, Ghangri GS, Jindal K, Pannu N. Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2009;4(6):1044-1050.
36. Juergensen P, Eras J, McClure B, et al. The impact of various cycling regimens on phosphorus removal in chronic peritoneal dialysis patients. Int J Artif Organs. 2005;28(12):1219-1223.
37. Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int Suppl. 2006 Nov;(103):S3-S11.
38. Heaf J, Løkkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant. 2002;17(1):112-117.
39. Wang AY, Lai KN. The importance of residual renal function in dialysis patients. Kidney Int. 2006;69(10):1726-1732.
40. Cnossen N, Kooman JP, Konings CJ, et al. Peritoneal dialysis in patients with congestive heart failure. Nephrol Dial Transplant. 2006;21 suppl 2: ii63-ii66.
Should N-acetylcysteine be used routinely to prevent contrast-induced acute kidney injury?
No. Using N-acetylcysteine (NAC) routinely to prevent contrast-induced acute kidney injury is not supported by the evidence at this time.1,2 However, there is evidence to suggest using it for patients at high risk, ie, those with significant baseline renal dysfunction.3,4
INCIDENCE AND IMPACT OF ACUTE KIDNEY INJURY
Intraarterial use of contrast is associated with a higher risk of acute kidney injury than intravenous use. Most studies of NAC for the prevention of contrast-induced acute kidney injury have focused on patients receiving contrast intraarterially. The reported rates of contrast-induced acute kidney injury also vary depending on how acute kidney injury was defined.
Although the incidence is low (1% to 2%) in patients with normal renal function, it can be as high as 25% in patients with renal impairment or a chronic condition such as diabetes or congestive heart failure, or in elderly patients.5
The development of acute kidney injury after percutaneous coronary intervention is associated with a longer hospital stay, a higher cost of care, and higher rates of morbidity and death.6
RATIONALE FOR USING N-ACETYLCYSTEINE
Contrast-induced acute kidney injury is thought to involve vasoconstriction and medullary ischemia mediated by reactive oxygen species.5 As an antioxidant and a scavenger of free radicals, NAC showed early promise in reducing the risk of this complication, but subsequent trials raised doubts about its efficacy. 1,2 In clinical practice, the drug is often used to prevent acute kidney injury because it is easy to give, cheap, and has few side effects. Recently, however, there have been suggestions that giving it intravenously may be associated with adverse effects that include anaphylactoid reactions.7
THE POSITIVE TRIALS
Tepel et al3 performed one of the earliest trials that found that NAC prevented contrast-induced acute kidney injury. The trial included 83 patients with stable chronic kidney disease (mean serum creatinine 2.4 mg/dL) who underwent computed tomography with about 75 mL of a nonionic, low-osmolality contrast agent. Participants were randomized to receive either NAC (600 mg orally twice daily) and 0.45% saline intravenously or placebo and saline. Acute kidney injury was defined as an increase of at least 0.5 mg/dL in the serum creatinine level 48 hours after the contrast dye was given.
The rate of acute kidney injury was significantly lower in the treatment group (2% vs 21%, P = .01). None of the patients who developed acute kidney injury needed hemodialysis.
Shyu et al4 studied 121 patients with chronic kidney disease (mean serum creatinine 2.8 mg/dL) who underwent a coronary procedure. Patients were randomized to receive NAC 400 mg orally twice daily or placebo in addition to 0.45% saline in both groups. Two (3.3%) of the 60 patients in the treated group and 15 (24.6%) of the 61 patients in the control group had an increase in creatinine concentration greater than 0.5 mg/dL at 48 hours (P < .001).
Both of these single-center studies were limited by small sample sizes and very short follow-up. Further, the impact of the drug on important clinical outcomes such as death and progression of chronic kidney disease was not reported.
Marenzi et al8 randomized 354 patients undergoing coronary angioplasty as the primary treatment for acute myocardial infarction to one of three treatment groups:
- NAC in a standard dosage (a 600-mg intravenous bolus before the procedure and then 600 mg orally twice daily for 48 hours afterward)
- NAC in a high dosage (a 1,200-mg intravenous bolus and then 1,200 mg orally twice daily for 48 hours)
- Placebo.
The two treatment groups had significantly lower rates of acute kidney injury than the placebo group. In addition, the hospital mortality rate and the rate of a composite end point of death, need for renal replacement therapy, or need for mechanical ventilation were significantly lower in the treated groups. However, the number of events was small, and a beneficial effect on the death rate has not been confirmed by other studies.5
THE NEGATIVE TRIALS
Several studies found that NAC did not prevent contrast-induced acute kidney injury.1,2,9
The Acetylcysteine for Contrast-induced Nephropathy Trial (ACT), published in 2011,1 was the largest of these trials. It included 2,308 patients undergoing an angiographic procedure who had at least one risk factor for contrast-induced acute kidney injury (age > 70, renal failure, diabetes mellitus, heart failure, or hypotension). Patients were randomly assigned to receive the drug (1,200 mg by mouth) or placebo.
The incidence of contrast-induced acute kidney injury was 12.7% in the treated group and 12.7% in the control group (relative risk 1.00; 95% confidence interval 0.81–1.25; P = .97). The rate of a combined end point of death or need for dialysis at 30 days was also similar in both groups (2.2% with treatment vs 2.3% with placebo).
Importantly, only about 15% of patients had a baseline serum creatinine greater than 1.5 mg/dL. Of these, most had an estimated glomerular filtration rate between 45 and 60 mL/min. Indeed, most patients in the ACT were at low risk of contrast-induced acute kidney injury. As a result, there were low event rates and, not surprisingly, no differences between the control and treatment groups.
Subgroup analysis did not suggest a benefit of treatment in those with a baseline serum creatinine greater than 1.5 mg/dL. However, as the authors pointed out, this subgroup was small, so definitive statistically powered conclusions cannot be drawn. There was no significant difference in the primary end point among several other predefined subgroups (age > 70, female sex, diabetes).1
The ACT differed from the “positive” study by Marenzi et al8 in several ways. The ACT patients were at lower risk, the coronary catheterizations were being done mainly for diagnosis rather than intervention, a lower volume of contrast dye was used (100 mL in the ACT vs 250 mL in the Marenzi study), and patients with ST-elevation myocardial infarction were excluded. Other weaknesses of the ACT include use of a baseline serum creatinine within 3 months of study entry, variations in the hydration protocol, and the use of a high-osmolar contrast agent in some patients.
Webb et al2 found, in a large, randomized trial, that intravenous NAC did not prevent contrast-induced acute kidney injury. Patients with renal dysfunction (mean serum creatinine around 1.6 mg/dL) undergoing cardiac catheterization were randomly assigned to receive either NAC 500 mg or placebo immediately before the procedure. All patients first received isotonic saline 200 mL, then 1.5 mL/kg per hour for 6 hours, unless contraindicated. The study was terminated early because of a determination of futility.
Gurm et al9 found that a database of 90,578 consecutive patients undergoing nonemergency coronary angiography from 2006 to 2009 did not show differences in the rate of contrast-induced acute kidney injury between patients who received NAC and those who did not (5.5% vs 5.5%, P = .99). There was also no difference in the rate of death or the need for dialysis. These negative findings were consistent across many prespecified subgroups.
MIXED RESULTS IN META-ANALYSES
Results from meta-analyses have been mixed,10,11 mainly because of study heterogeneity (eg, baseline risk, end points, dose of the drug) and publication bias. None of the previous meta-analyses included the recent negative results from the ACT.
CURRENT GUIDELINES
After the publication of the ACT, the joint guidelines of the American College of Cardiology and the American Heart Association were updated, designating NAC as class III (no benefit) and level of evidence A.12
However, recently published guidelines from the Kidney Disease: Improving Global Outcomes Acute Kidney Injury Working Group recommend using the drug together with intravenous isotonic crystalloids in patients at high risk of contrast-induced acute kidney injury, although the level of evidence is 2D (2 = suggestion, D = quality of evidence very low).5
WHAT WE RECOMMEND
The routine use of NAC to prevent contrast-induced acute kidney injury is not supported by the current evidence. However, clarification of its efficacy in high-risk patients is needed, especially those with baseline renal dysfunction and diabetes mellitus.
The Prevention of Serious Adverse Events Following Angiography (PRESERVE) study (ClinTrials.gov identifier NCT01467466) may clarify the role of this drug in a high-risk cohort using the important clinical outcomes of death, need for acute dialysis, or persistent decline in kidney function after angiography. This important study was set to begin in July 2012, with an anticipated enrollment of more than 8,000 patients who have glomerular filtration rates of 15 to 59 mL/min/1.73 m2.
In the meantime, we recommend the following in patients at high risk of contrast-induced acute kidney injury:
- Clarify whether contrast is truly needed
- When possible, limit the volume of contrast, avoid repeated doses over a short time frame, and use an iso-osmolar or low-osmolar contrast agent
- Discontinue nephrotoxic agents
- Provide an evidence-based intravenous crystalloid regimen with isotonic sodium bicarbonate or saline
- Although it is not strictly evidence-based, use NAC in patients with significant baseline renal dysfunction (glomerular filtration rate < 45 mL/min/1.73 m2), multiple concurrent risk factors such as hypotension, diabetes, preexisting kidney injury, or congestive heart failure that limits the use of intravenous fluids, or who need a high volume of contrast dye
- Avoid using intravenous NAC, given its lack of benefit and risk of anaphylactoid reactions.7,13
We do not yet have clear evidence on the optimal dosing regimen. But based on the limited data, we recommend 600 to 1,200 mg twice a day for 1 day before and 1 day after the dye is given.
- ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT). Circulation 2011; 124:1250–1259.
- Webb JG, Pate GE, Humphries KH, et al. A randomized controlled trial of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy after cardiac catheterization: lack of effect. Am Heart J 2004; 148:422–429.
- Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000; 343:180–184.
- Shyu KG, Cheng JJ, Kuan P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol 2002; 40:1383–1388.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2(suppl 1):1–138.
- Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002; 105:2259–2264.
- Baker CS, Wragg A, Kumar S, De Palma R, Baker LR, Knight CJ. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol 2003; 41:2114–2118.
- Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006; 354:2773–2782.
- Gurm HS, Smith DE, Berwanger O, et al; BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Contemporary use and effectiveness of N-acetylcysteine in preventing contrast-induced nephropathy among patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv 2012; 5:98–104.
- Duong MH, MacKenzie TA, Malenka DJ. N-acetylcysteine prophylaxis significantly reduces the risk of radiocontrast-induced nephropathy: comprehensive meta-analysis. Catheter Cardiovasc Interv 2005; 64:471–479.
- Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med 2007; 5:32.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:e574–e651.
- Kanter MZ. Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm 2006; 63:1821–1827.
No. Using N-acetylcysteine (NAC) routinely to prevent contrast-induced acute kidney injury is not supported by the evidence at this time.1,2 However, there is evidence to suggest using it for patients at high risk, ie, those with significant baseline renal dysfunction.3,4
INCIDENCE AND IMPACT OF ACUTE KIDNEY INJURY
Intraarterial use of contrast is associated with a higher risk of acute kidney injury than intravenous use. Most studies of NAC for the prevention of contrast-induced acute kidney injury have focused on patients receiving contrast intraarterially. The reported rates of contrast-induced acute kidney injury also vary depending on how acute kidney injury was defined.
Although the incidence is low (1% to 2%) in patients with normal renal function, it can be as high as 25% in patients with renal impairment or a chronic condition such as diabetes or congestive heart failure, or in elderly patients.5
The development of acute kidney injury after percutaneous coronary intervention is associated with a longer hospital stay, a higher cost of care, and higher rates of morbidity and death.6
RATIONALE FOR USING N-ACETYLCYSTEINE
Contrast-induced acute kidney injury is thought to involve vasoconstriction and medullary ischemia mediated by reactive oxygen species.5 As an antioxidant and a scavenger of free radicals, NAC showed early promise in reducing the risk of this complication, but subsequent trials raised doubts about its efficacy. 1,2 In clinical practice, the drug is often used to prevent acute kidney injury because it is easy to give, cheap, and has few side effects. Recently, however, there have been suggestions that giving it intravenously may be associated with adverse effects that include anaphylactoid reactions.7
THE POSITIVE TRIALS
Tepel et al3 performed one of the earliest trials that found that NAC prevented contrast-induced acute kidney injury. The trial included 83 patients with stable chronic kidney disease (mean serum creatinine 2.4 mg/dL) who underwent computed tomography with about 75 mL of a nonionic, low-osmolality contrast agent. Participants were randomized to receive either NAC (600 mg orally twice daily) and 0.45% saline intravenously or placebo and saline. Acute kidney injury was defined as an increase of at least 0.5 mg/dL in the serum creatinine level 48 hours after the contrast dye was given.
The rate of acute kidney injury was significantly lower in the treatment group (2% vs 21%, P = .01). None of the patients who developed acute kidney injury needed hemodialysis.
Shyu et al4 studied 121 patients with chronic kidney disease (mean serum creatinine 2.8 mg/dL) who underwent a coronary procedure. Patients were randomized to receive NAC 400 mg orally twice daily or placebo in addition to 0.45% saline in both groups. Two (3.3%) of the 60 patients in the treated group and 15 (24.6%) of the 61 patients in the control group had an increase in creatinine concentration greater than 0.5 mg/dL at 48 hours (P < .001).
Both of these single-center studies were limited by small sample sizes and very short follow-up. Further, the impact of the drug on important clinical outcomes such as death and progression of chronic kidney disease was not reported.
Marenzi et al8 randomized 354 patients undergoing coronary angioplasty as the primary treatment for acute myocardial infarction to one of three treatment groups:
- NAC in a standard dosage (a 600-mg intravenous bolus before the procedure and then 600 mg orally twice daily for 48 hours afterward)
- NAC in a high dosage (a 1,200-mg intravenous bolus and then 1,200 mg orally twice daily for 48 hours)
- Placebo.
The two treatment groups had significantly lower rates of acute kidney injury than the placebo group. In addition, the hospital mortality rate and the rate of a composite end point of death, need for renal replacement therapy, or need for mechanical ventilation were significantly lower in the treated groups. However, the number of events was small, and a beneficial effect on the death rate has not been confirmed by other studies.5
THE NEGATIVE TRIALS
Several studies found that NAC did not prevent contrast-induced acute kidney injury.1,2,9
The Acetylcysteine for Contrast-induced Nephropathy Trial (ACT), published in 2011,1 was the largest of these trials. It included 2,308 patients undergoing an angiographic procedure who had at least one risk factor for contrast-induced acute kidney injury (age > 70, renal failure, diabetes mellitus, heart failure, or hypotension). Patients were randomly assigned to receive the drug (1,200 mg by mouth) or placebo.
The incidence of contrast-induced acute kidney injury was 12.7% in the treated group and 12.7% in the control group (relative risk 1.00; 95% confidence interval 0.81–1.25; P = .97). The rate of a combined end point of death or need for dialysis at 30 days was also similar in both groups (2.2% with treatment vs 2.3% with placebo).
Importantly, only about 15% of patients had a baseline serum creatinine greater than 1.5 mg/dL. Of these, most had an estimated glomerular filtration rate between 45 and 60 mL/min. Indeed, most patients in the ACT were at low risk of contrast-induced acute kidney injury. As a result, there were low event rates and, not surprisingly, no differences between the control and treatment groups.
Subgroup analysis did not suggest a benefit of treatment in those with a baseline serum creatinine greater than 1.5 mg/dL. However, as the authors pointed out, this subgroup was small, so definitive statistically powered conclusions cannot be drawn. There was no significant difference in the primary end point among several other predefined subgroups (age > 70, female sex, diabetes).1
The ACT differed from the “positive” study by Marenzi et al8 in several ways. The ACT patients were at lower risk, the coronary catheterizations were being done mainly for diagnosis rather than intervention, a lower volume of contrast dye was used (100 mL in the ACT vs 250 mL in the Marenzi study), and patients with ST-elevation myocardial infarction were excluded. Other weaknesses of the ACT include use of a baseline serum creatinine within 3 months of study entry, variations in the hydration protocol, and the use of a high-osmolar contrast agent in some patients.
Webb et al2 found, in a large, randomized trial, that intravenous NAC did not prevent contrast-induced acute kidney injury. Patients with renal dysfunction (mean serum creatinine around 1.6 mg/dL) undergoing cardiac catheterization were randomly assigned to receive either NAC 500 mg or placebo immediately before the procedure. All patients first received isotonic saline 200 mL, then 1.5 mL/kg per hour for 6 hours, unless contraindicated. The study was terminated early because of a determination of futility.
Gurm et al9 found that a database of 90,578 consecutive patients undergoing nonemergency coronary angiography from 2006 to 2009 did not show differences in the rate of contrast-induced acute kidney injury between patients who received NAC and those who did not (5.5% vs 5.5%, P = .99). There was also no difference in the rate of death or the need for dialysis. These negative findings were consistent across many prespecified subgroups.
MIXED RESULTS IN META-ANALYSES
Results from meta-analyses have been mixed,10,11 mainly because of study heterogeneity (eg, baseline risk, end points, dose of the drug) and publication bias. None of the previous meta-analyses included the recent negative results from the ACT.
CURRENT GUIDELINES
After the publication of the ACT, the joint guidelines of the American College of Cardiology and the American Heart Association were updated, designating NAC as class III (no benefit) and level of evidence A.12
However, recently published guidelines from the Kidney Disease: Improving Global Outcomes Acute Kidney Injury Working Group recommend using the drug together with intravenous isotonic crystalloids in patients at high risk of contrast-induced acute kidney injury, although the level of evidence is 2D (2 = suggestion, D = quality of evidence very low).5
WHAT WE RECOMMEND
The routine use of NAC to prevent contrast-induced acute kidney injury is not supported by the current evidence. However, clarification of its efficacy in high-risk patients is needed, especially those with baseline renal dysfunction and diabetes mellitus.
The Prevention of Serious Adverse Events Following Angiography (PRESERVE) study (ClinTrials.gov identifier NCT01467466) may clarify the role of this drug in a high-risk cohort using the important clinical outcomes of death, need for acute dialysis, or persistent decline in kidney function after angiography. This important study was set to begin in July 2012, with an anticipated enrollment of more than 8,000 patients who have glomerular filtration rates of 15 to 59 mL/min/1.73 m2.
In the meantime, we recommend the following in patients at high risk of contrast-induced acute kidney injury:
- Clarify whether contrast is truly needed
- When possible, limit the volume of contrast, avoid repeated doses over a short time frame, and use an iso-osmolar or low-osmolar contrast agent
- Discontinue nephrotoxic agents
- Provide an evidence-based intravenous crystalloid regimen with isotonic sodium bicarbonate or saline
- Although it is not strictly evidence-based, use NAC in patients with significant baseline renal dysfunction (glomerular filtration rate < 45 mL/min/1.73 m2), multiple concurrent risk factors such as hypotension, diabetes, preexisting kidney injury, or congestive heart failure that limits the use of intravenous fluids, or who need a high volume of contrast dye
- Avoid using intravenous NAC, given its lack of benefit and risk of anaphylactoid reactions.7,13
We do not yet have clear evidence on the optimal dosing regimen. But based on the limited data, we recommend 600 to 1,200 mg twice a day for 1 day before and 1 day after the dye is given.
No. Using N-acetylcysteine (NAC) routinely to prevent contrast-induced acute kidney injury is not supported by the evidence at this time.1,2 However, there is evidence to suggest using it for patients at high risk, ie, those with significant baseline renal dysfunction.3,4
INCIDENCE AND IMPACT OF ACUTE KIDNEY INJURY
Intraarterial use of contrast is associated with a higher risk of acute kidney injury than intravenous use. Most studies of NAC for the prevention of contrast-induced acute kidney injury have focused on patients receiving contrast intraarterially. The reported rates of contrast-induced acute kidney injury also vary depending on how acute kidney injury was defined.
Although the incidence is low (1% to 2%) in patients with normal renal function, it can be as high as 25% in patients with renal impairment or a chronic condition such as diabetes or congestive heart failure, or in elderly patients.5
The development of acute kidney injury after percutaneous coronary intervention is associated with a longer hospital stay, a higher cost of care, and higher rates of morbidity and death.6
RATIONALE FOR USING N-ACETYLCYSTEINE
Contrast-induced acute kidney injury is thought to involve vasoconstriction and medullary ischemia mediated by reactive oxygen species.5 As an antioxidant and a scavenger of free radicals, NAC showed early promise in reducing the risk of this complication, but subsequent trials raised doubts about its efficacy. 1,2 In clinical practice, the drug is often used to prevent acute kidney injury because it is easy to give, cheap, and has few side effects. Recently, however, there have been suggestions that giving it intravenously may be associated with adverse effects that include anaphylactoid reactions.7
THE POSITIVE TRIALS
Tepel et al3 performed one of the earliest trials that found that NAC prevented contrast-induced acute kidney injury. The trial included 83 patients with stable chronic kidney disease (mean serum creatinine 2.4 mg/dL) who underwent computed tomography with about 75 mL of a nonionic, low-osmolality contrast agent. Participants were randomized to receive either NAC (600 mg orally twice daily) and 0.45% saline intravenously or placebo and saline. Acute kidney injury was defined as an increase of at least 0.5 mg/dL in the serum creatinine level 48 hours after the contrast dye was given.
The rate of acute kidney injury was significantly lower in the treatment group (2% vs 21%, P = .01). None of the patients who developed acute kidney injury needed hemodialysis.
Shyu et al4 studied 121 patients with chronic kidney disease (mean serum creatinine 2.8 mg/dL) who underwent a coronary procedure. Patients were randomized to receive NAC 400 mg orally twice daily or placebo in addition to 0.45% saline in both groups. Two (3.3%) of the 60 patients in the treated group and 15 (24.6%) of the 61 patients in the control group had an increase in creatinine concentration greater than 0.5 mg/dL at 48 hours (P < .001).
Both of these single-center studies were limited by small sample sizes and very short follow-up. Further, the impact of the drug on important clinical outcomes such as death and progression of chronic kidney disease was not reported.
Marenzi et al8 randomized 354 patients undergoing coronary angioplasty as the primary treatment for acute myocardial infarction to one of three treatment groups:
- NAC in a standard dosage (a 600-mg intravenous bolus before the procedure and then 600 mg orally twice daily for 48 hours afterward)
- NAC in a high dosage (a 1,200-mg intravenous bolus and then 1,200 mg orally twice daily for 48 hours)
- Placebo.
The two treatment groups had significantly lower rates of acute kidney injury than the placebo group. In addition, the hospital mortality rate and the rate of a composite end point of death, need for renal replacement therapy, or need for mechanical ventilation were significantly lower in the treated groups. However, the number of events was small, and a beneficial effect on the death rate has not been confirmed by other studies.5
THE NEGATIVE TRIALS
Several studies found that NAC did not prevent contrast-induced acute kidney injury.1,2,9
The Acetylcysteine for Contrast-induced Nephropathy Trial (ACT), published in 2011,1 was the largest of these trials. It included 2,308 patients undergoing an angiographic procedure who had at least one risk factor for contrast-induced acute kidney injury (age > 70, renal failure, diabetes mellitus, heart failure, or hypotension). Patients were randomly assigned to receive the drug (1,200 mg by mouth) or placebo.
The incidence of contrast-induced acute kidney injury was 12.7% in the treated group and 12.7% in the control group (relative risk 1.00; 95% confidence interval 0.81–1.25; P = .97). The rate of a combined end point of death or need for dialysis at 30 days was also similar in both groups (2.2% with treatment vs 2.3% with placebo).
Importantly, only about 15% of patients had a baseline serum creatinine greater than 1.5 mg/dL. Of these, most had an estimated glomerular filtration rate between 45 and 60 mL/min. Indeed, most patients in the ACT were at low risk of contrast-induced acute kidney injury. As a result, there were low event rates and, not surprisingly, no differences between the control and treatment groups.
Subgroup analysis did not suggest a benefit of treatment in those with a baseline serum creatinine greater than 1.5 mg/dL. However, as the authors pointed out, this subgroup was small, so definitive statistically powered conclusions cannot be drawn. There was no significant difference in the primary end point among several other predefined subgroups (age > 70, female sex, diabetes).1
The ACT differed from the “positive” study by Marenzi et al8 in several ways. The ACT patients were at lower risk, the coronary catheterizations were being done mainly for diagnosis rather than intervention, a lower volume of contrast dye was used (100 mL in the ACT vs 250 mL in the Marenzi study), and patients with ST-elevation myocardial infarction were excluded. Other weaknesses of the ACT include use of a baseline serum creatinine within 3 months of study entry, variations in the hydration protocol, and the use of a high-osmolar contrast agent in some patients.
Webb et al2 found, in a large, randomized trial, that intravenous NAC did not prevent contrast-induced acute kidney injury. Patients with renal dysfunction (mean serum creatinine around 1.6 mg/dL) undergoing cardiac catheterization were randomly assigned to receive either NAC 500 mg or placebo immediately before the procedure. All patients first received isotonic saline 200 mL, then 1.5 mL/kg per hour for 6 hours, unless contraindicated. The study was terminated early because of a determination of futility.
Gurm et al9 found that a database of 90,578 consecutive patients undergoing nonemergency coronary angiography from 2006 to 2009 did not show differences in the rate of contrast-induced acute kidney injury between patients who received NAC and those who did not (5.5% vs 5.5%, P = .99). There was also no difference in the rate of death or the need for dialysis. These negative findings were consistent across many prespecified subgroups.
MIXED RESULTS IN META-ANALYSES
Results from meta-analyses have been mixed,10,11 mainly because of study heterogeneity (eg, baseline risk, end points, dose of the drug) and publication bias. None of the previous meta-analyses included the recent negative results from the ACT.
CURRENT GUIDELINES
After the publication of the ACT, the joint guidelines of the American College of Cardiology and the American Heart Association were updated, designating NAC as class III (no benefit) and level of evidence A.12
However, recently published guidelines from the Kidney Disease: Improving Global Outcomes Acute Kidney Injury Working Group recommend using the drug together with intravenous isotonic crystalloids in patients at high risk of contrast-induced acute kidney injury, although the level of evidence is 2D (2 = suggestion, D = quality of evidence very low).5
WHAT WE RECOMMEND
The routine use of NAC to prevent contrast-induced acute kidney injury is not supported by the current evidence. However, clarification of its efficacy in high-risk patients is needed, especially those with baseline renal dysfunction and diabetes mellitus.
The Prevention of Serious Adverse Events Following Angiography (PRESERVE) study (ClinTrials.gov identifier NCT01467466) may clarify the role of this drug in a high-risk cohort using the important clinical outcomes of death, need for acute dialysis, or persistent decline in kidney function after angiography. This important study was set to begin in July 2012, with an anticipated enrollment of more than 8,000 patients who have glomerular filtration rates of 15 to 59 mL/min/1.73 m2.
In the meantime, we recommend the following in patients at high risk of contrast-induced acute kidney injury:
- Clarify whether contrast is truly needed
- When possible, limit the volume of contrast, avoid repeated doses over a short time frame, and use an iso-osmolar or low-osmolar contrast agent
- Discontinue nephrotoxic agents
- Provide an evidence-based intravenous crystalloid regimen with isotonic sodium bicarbonate or saline
- Although it is not strictly evidence-based, use NAC in patients with significant baseline renal dysfunction (glomerular filtration rate < 45 mL/min/1.73 m2), multiple concurrent risk factors such as hypotension, diabetes, preexisting kidney injury, or congestive heart failure that limits the use of intravenous fluids, or who need a high volume of contrast dye
- Avoid using intravenous NAC, given its lack of benefit and risk of anaphylactoid reactions.7,13
We do not yet have clear evidence on the optimal dosing regimen. But based on the limited data, we recommend 600 to 1,200 mg twice a day for 1 day before and 1 day after the dye is given.
- ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT). Circulation 2011; 124:1250–1259.
- Webb JG, Pate GE, Humphries KH, et al. A randomized controlled trial of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy after cardiac catheterization: lack of effect. Am Heart J 2004; 148:422–429.
- Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000; 343:180–184.
- Shyu KG, Cheng JJ, Kuan P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol 2002; 40:1383–1388.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2(suppl 1):1–138.
- Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002; 105:2259–2264.
- Baker CS, Wragg A, Kumar S, De Palma R, Baker LR, Knight CJ. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol 2003; 41:2114–2118.
- Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006; 354:2773–2782.
- Gurm HS, Smith DE, Berwanger O, et al; BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Contemporary use and effectiveness of N-acetylcysteine in preventing contrast-induced nephropathy among patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv 2012; 5:98–104.
- Duong MH, MacKenzie TA, Malenka DJ. N-acetylcysteine prophylaxis significantly reduces the risk of radiocontrast-induced nephropathy: comprehensive meta-analysis. Catheter Cardiovasc Interv 2005; 64:471–479.
- Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med 2007; 5:32.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:e574–e651.
- Kanter MZ. Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm 2006; 63:1821–1827.
- ACT Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT). Circulation 2011; 124:1250–1259.
- Webb JG, Pate GE, Humphries KH, et al. A randomized controlled trial of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy after cardiac catheterization: lack of effect. Am Heart J 2004; 148:422–429.
- Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med 2000; 343:180–184.
- Shyu KG, Cheng JJ, Kuan P. Acetylcysteine protects against acute renal damage in patients with abnormal renal function undergoing a coronary procedure. J Am Coll Cardiol 2002; 40:1383–1388.
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2(suppl 1):1–138.
- Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002; 105:2259–2264.
- Baker CS, Wragg A, Kumar S, De Palma R, Baker LR, Knight CJ. A rapid protocol for the prevention of contrast-induced renal dysfunction: the RAPPID study. J Am Coll Cardiol 2003; 41:2114–2118.
- Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006; 354:2773–2782.
- Gurm HS, Smith DE, Berwanger O, et al; BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium). Contemporary use and effectiveness of N-acetylcysteine in preventing contrast-induced nephropathy among patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv 2012; 5:98–104.
- Duong MH, MacKenzie TA, Malenka DJ. N-acetylcysteine prophylaxis significantly reduces the risk of radiocontrast-induced nephropathy: comprehensive meta-analysis. Catheter Cardiovasc Interv 2005; 64:471–479.
- Gonzales DA, Norsworthy KJ, Kern SJ, et al. A meta-analysis of N-acetylcysteine in contrast-induced nephrotoxicity: unsupervised clustering to resolve heterogeneity. BMC Med 2007; 5:32.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:e574–e651.
- Kanter MZ. Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm 2006; 63:1821–1827.
Estimated GFR, Albuminuria Predict Mortality Across All Age Groups
Kidney measures such as low estimated glomerular filtration rate and high albuminuria are strongly associated with mortality and end-stage renal disease across all age groups – even in the elderly, according to a collaborative meta-analysis reported online Oct. 30 in JAMA and presented simultaneously at Kidney Week.
The risk for chronic kidney disease, which in turn is closely allied with the risk for cardiovascular disease and all-cause mortality, typically is gauged by assessing estimated GFR (eGFR) and albuminuria levels. But there has been substantial controversy regarding the accuracy of these measures for predicting mortality and CKD risk in the elderly, because kidney function appears to decline markedly even in apparently healthy people as they age, said Dr. Stein I. Hallan and his associates in the Chronic Kidney Disease Prognosis Consortium.
Some experts even hold that reduced GFR might simply be part of the natural aging process and that the kidneys undergo an inevitable senescence, rendering "normal" markers of kidney function unusable in the elderly, said Dr. Hallan, of St. Olav University Hospital and the Norwegian University of Science and Technology, both in Trondheim, and his colleagues.
To examine whether aging modifies the usefulness of estimated GFR and albuminuria in assessing the risks for mortality and CKD, Dr. Hallan and his associates analyzed data from 46 different cohorts worldwide that included the entire adult age range (18-108 years). The Chronic Kidney Disease Prognosis Consortium includes data on 20 North American, 12 European, 10 Asian, 1 Australian, and 3 multinational cohorts comprising more than 2 million study subjects followed for a mean of 6 years.
Among the study cohorts, 26 involved people from the general population, 8 involved patients at high risk for vascular disease, and the remaining 12 involved patients with CKD.
During follow-up there were 112,325 deaths in the general population and the high-risk cohorts, as well as 9,037 deaths in the CKD cohorts. There were 2,766 end-stage renal disease (ESRD) events in the general population and high-risk cohorts, as well as 5,962 ESRD events in the CKD cohorts.
Both mortality risk and the risk of ESRD events strongly increased with decreasing GFR across all age groups, even though the study populations had widely divergent demographic and clinical characteristics, the investigators said. These risks declined with increasing age (JAMA 2012 Oct. 30 [doi: 10.1001/jama.2012.16817]).
This correlation remained robust when the data were adjusted to account for patient sex, race, history of cardiovascular disease, blood pressure, serum cholesterol levels, body mass index, smoking status, and diabetes status. For example, the adjusted hazard ratio for all-cause mortality in subjects with an eGFR of 45 (compared with 80) mL/min per 1.73 m2 was 3.50 in those aged 18-54 years, and declined with age to 1.35 in those aged at least 75 years.
The findings were similar for albuminuria levels, with high levels predicting mortality and ESRD events across all age groups.
"Although some variation in management of CKD should be considered by age, based on cost and benefits, with respect to risk of mortality and ESRD, our data support a common definition and staging of CKD based on eGFR and albuminuria for all age groups," they said.
These results contradict the concern "that CKD guidelines should be used with caution in older individuals and that low eGFR reflects only natural aging." They also support recommendations that CKD measures be added to mortality risk equations.
In addition, "the strong increase in mortality along with kidney measures at older ages suggests that older adults should not be left out from management strategies of CKD. Previous data show that low eGFR in the very old is associated with classical CKD complications like anemia, acidosis, hyperparathyroidism, and hyperphosphatemia," the researchers said.
This study was supported by a variety of government agencies, medical research councils, and industry sponsors. The 16 authors reported numerous ties to industry sources.
The medical community should conclude from this important new data that older adults with impaired kidney function are at high risk of death.
Since their excess mortality usually takes the form of cardiovascular disease, all appropriate preventive efforts should be taken in this patient population, including lifestyle modifications, blood pressure–lowering medications, renin-angiotensin system inhibitors if proteinuria is present, and lipid-lowering medications.
Furthermore, more study should be undertaken to assess the effects of commonly used glucose-lowering therapies in elderly patients, who have generally been excluded from clinical trials.
Dr. Ian H. de Boer is at the Kidney Research Institute at the University of Washington, Seattle. He reported receiving research funding from Abbott Laboratories. These remarks were taken from his editorial accompanying Dr. Hallan’s report (JAMA 2012 Oct. 30 [doi: 20.2002/jama.2012.30761]).
The medical community should conclude from this important new data that older adults with impaired kidney function are at high risk of death.
Since their excess mortality usually takes the form of cardiovascular disease, all appropriate preventive efforts should be taken in this patient population, including lifestyle modifications, blood pressure–lowering medications, renin-angiotensin system inhibitors if proteinuria is present, and lipid-lowering medications.
Furthermore, more study should be undertaken to assess the effects of commonly used glucose-lowering therapies in elderly patients, who have generally been excluded from clinical trials.
Dr. Ian H. de Boer is at the Kidney Research Institute at the University of Washington, Seattle. He reported receiving research funding from Abbott Laboratories. These remarks were taken from his editorial accompanying Dr. Hallan’s report (JAMA 2012 Oct. 30 [doi: 20.2002/jama.2012.30761]).
The medical community should conclude from this important new data that older adults with impaired kidney function are at high risk of death.
Since their excess mortality usually takes the form of cardiovascular disease, all appropriate preventive efforts should be taken in this patient population, including lifestyle modifications, blood pressure–lowering medications, renin-angiotensin system inhibitors if proteinuria is present, and lipid-lowering medications.
Furthermore, more study should be undertaken to assess the effects of commonly used glucose-lowering therapies in elderly patients, who have generally been excluded from clinical trials.
Dr. Ian H. de Boer is at the Kidney Research Institute at the University of Washington, Seattle. He reported receiving research funding from Abbott Laboratories. These remarks were taken from his editorial accompanying Dr. Hallan’s report (JAMA 2012 Oct. 30 [doi: 20.2002/jama.2012.30761]).
Kidney measures such as low estimated glomerular filtration rate and high albuminuria are strongly associated with mortality and end-stage renal disease across all age groups – even in the elderly, according to a collaborative meta-analysis reported online Oct. 30 in JAMA and presented simultaneously at Kidney Week.
The risk for chronic kidney disease, which in turn is closely allied with the risk for cardiovascular disease and all-cause mortality, typically is gauged by assessing estimated GFR (eGFR) and albuminuria levels. But there has been substantial controversy regarding the accuracy of these measures for predicting mortality and CKD risk in the elderly, because kidney function appears to decline markedly even in apparently healthy people as they age, said Dr. Stein I. Hallan and his associates in the Chronic Kidney Disease Prognosis Consortium.
Some experts even hold that reduced GFR might simply be part of the natural aging process and that the kidneys undergo an inevitable senescence, rendering "normal" markers of kidney function unusable in the elderly, said Dr. Hallan, of St. Olav University Hospital and the Norwegian University of Science and Technology, both in Trondheim, and his colleagues.
To examine whether aging modifies the usefulness of estimated GFR and albuminuria in assessing the risks for mortality and CKD, Dr. Hallan and his associates analyzed data from 46 different cohorts worldwide that included the entire adult age range (18-108 years). The Chronic Kidney Disease Prognosis Consortium includes data on 20 North American, 12 European, 10 Asian, 1 Australian, and 3 multinational cohorts comprising more than 2 million study subjects followed for a mean of 6 years.
Among the study cohorts, 26 involved people from the general population, 8 involved patients at high risk for vascular disease, and the remaining 12 involved patients with CKD.
During follow-up there were 112,325 deaths in the general population and the high-risk cohorts, as well as 9,037 deaths in the CKD cohorts. There were 2,766 end-stage renal disease (ESRD) events in the general population and high-risk cohorts, as well as 5,962 ESRD events in the CKD cohorts.
Both mortality risk and the risk of ESRD events strongly increased with decreasing GFR across all age groups, even though the study populations had widely divergent demographic and clinical characteristics, the investigators said. These risks declined with increasing age (JAMA 2012 Oct. 30 [doi: 10.1001/jama.2012.16817]).
This correlation remained robust when the data were adjusted to account for patient sex, race, history of cardiovascular disease, blood pressure, serum cholesterol levels, body mass index, smoking status, and diabetes status. For example, the adjusted hazard ratio for all-cause mortality in subjects with an eGFR of 45 (compared with 80) mL/min per 1.73 m2 was 3.50 in those aged 18-54 years, and declined with age to 1.35 in those aged at least 75 years.
The findings were similar for albuminuria levels, with high levels predicting mortality and ESRD events across all age groups.
"Although some variation in management of CKD should be considered by age, based on cost and benefits, with respect to risk of mortality and ESRD, our data support a common definition and staging of CKD based on eGFR and albuminuria for all age groups," they said.
These results contradict the concern "that CKD guidelines should be used with caution in older individuals and that low eGFR reflects only natural aging." They also support recommendations that CKD measures be added to mortality risk equations.
In addition, "the strong increase in mortality along with kidney measures at older ages suggests that older adults should not be left out from management strategies of CKD. Previous data show that low eGFR in the very old is associated with classical CKD complications like anemia, acidosis, hyperparathyroidism, and hyperphosphatemia," the researchers said.
This study was supported by a variety of government agencies, medical research councils, and industry sponsors. The 16 authors reported numerous ties to industry sources.
Kidney measures such as low estimated glomerular filtration rate and high albuminuria are strongly associated with mortality and end-stage renal disease across all age groups – even in the elderly, according to a collaborative meta-analysis reported online Oct. 30 in JAMA and presented simultaneously at Kidney Week.
The risk for chronic kidney disease, which in turn is closely allied with the risk for cardiovascular disease and all-cause mortality, typically is gauged by assessing estimated GFR (eGFR) and albuminuria levels. But there has been substantial controversy regarding the accuracy of these measures for predicting mortality and CKD risk in the elderly, because kidney function appears to decline markedly even in apparently healthy people as they age, said Dr. Stein I. Hallan and his associates in the Chronic Kidney Disease Prognosis Consortium.
Some experts even hold that reduced GFR might simply be part of the natural aging process and that the kidneys undergo an inevitable senescence, rendering "normal" markers of kidney function unusable in the elderly, said Dr. Hallan, of St. Olav University Hospital and the Norwegian University of Science and Technology, both in Trondheim, and his colleagues.
To examine whether aging modifies the usefulness of estimated GFR and albuminuria in assessing the risks for mortality and CKD, Dr. Hallan and his associates analyzed data from 46 different cohorts worldwide that included the entire adult age range (18-108 years). The Chronic Kidney Disease Prognosis Consortium includes data on 20 North American, 12 European, 10 Asian, 1 Australian, and 3 multinational cohorts comprising more than 2 million study subjects followed for a mean of 6 years.
Among the study cohorts, 26 involved people from the general population, 8 involved patients at high risk for vascular disease, and the remaining 12 involved patients with CKD.
During follow-up there were 112,325 deaths in the general population and the high-risk cohorts, as well as 9,037 deaths in the CKD cohorts. There were 2,766 end-stage renal disease (ESRD) events in the general population and high-risk cohorts, as well as 5,962 ESRD events in the CKD cohorts.
Both mortality risk and the risk of ESRD events strongly increased with decreasing GFR across all age groups, even though the study populations had widely divergent demographic and clinical characteristics, the investigators said. These risks declined with increasing age (JAMA 2012 Oct. 30 [doi: 10.1001/jama.2012.16817]).
This correlation remained robust when the data were adjusted to account for patient sex, race, history of cardiovascular disease, blood pressure, serum cholesterol levels, body mass index, smoking status, and diabetes status. For example, the adjusted hazard ratio for all-cause mortality in subjects with an eGFR of 45 (compared with 80) mL/min per 1.73 m2 was 3.50 in those aged 18-54 years, and declined with age to 1.35 in those aged at least 75 years.
The findings were similar for albuminuria levels, with high levels predicting mortality and ESRD events across all age groups.
"Although some variation in management of CKD should be considered by age, based on cost and benefits, with respect to risk of mortality and ESRD, our data support a common definition and staging of CKD based on eGFR and albuminuria for all age groups," they said.
These results contradict the concern "that CKD guidelines should be used with caution in older individuals and that low eGFR reflects only natural aging." They also support recommendations that CKD measures be added to mortality risk equations.
In addition, "the strong increase in mortality along with kidney measures at older ages suggests that older adults should not be left out from management strategies of CKD. Previous data show that low eGFR in the very old is associated with classical CKD complications like anemia, acidosis, hyperparathyroidism, and hyperphosphatemia," the researchers said.
This study was supported by a variety of government agencies, medical research councils, and industry sponsors. The 16 authors reported numerous ties to industry sources.
FROM JAMA
Major Finding: The risks of both mortality and ESRD strongly correlated with low estimated GFR and high albuminuria across all age groups, including the elderly.
Data Source: This was a meta-analysis of 46 large cohorts involving over 2 million study subjects followed for a mean of 6 years, to assess a possible effect of patient age on risk prediction of mortality and ESRD.
Disclosures: This study was supported by a variety of government agencies, medical research councils, and industry sponsors. The 16 authors reported numerous ties to industry sources.
EULAR's Lupus Nephritis Guidelines Emphasize Early Biopsy
The European League Against Rheumatism has issued its first guidelines on management of lupus nephritis, and they advise renal biopsy at the first sign of kidney involvement, unlike the guidance issued by the American College of Rheumatology, which leaves timing of that testing up to the clinician’s judgment.
Other bright spots in EULAR’s guidelines, which it issued jointly with the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) are their position that mycophenolic acid should be the first choice for immunosuppressive therapy, the precise recommendations for steroid dosage, stratification of treatment plans according to disease severity, and explicit advice on switching therapies after one drug has failed.
The European guidelines were published in the November issue of Annals of the Rheumatic Diseases (2012;71:1771-82) and are intended for rheumatologists, nephrologists, and internists managing adult and pediatric patients with lupus nephritis. The EULAR guidelines differ in several key ways from those issued by the American College of Rheumatology (ACR) in June (Arthritis Care Res. 2012;64:797-808).
The EULAR guidelines are unequivocal in their support for renal biopsy at any sign of renal involvement, including when unexplained renal insufficiency is accompanied by normal urinary findings. The ACR guidelines, by contrast, allow for more physician latitude in determining whether to biopsy.
"This can be an emotional issue because rheumatologists do not do biopsies and may try to avoid them," said the EULAR guidelines’ lead author, rheumatologist Dr. Dimitrios T. Boumpas, who is professor of medicine and director of internal medicine/rheumatology at the University of Crete in Heraklion, Greece, in an interview. "We felt that someone should make a position statement. Biopsy is simply best care. If you’re dealing with something severe like LN [lupus nephritis] and you avoid biopsy, that’s not good medicine."
Dr. Boumpas said the EULAR task force had also moved to put mycophenolic acid in the first position as an initial immunosuppressant treatment for most cases of class III-IV LN. Low-dose intravenous cyclophosphamide in combination with steroids is also recommended for this patient group.
There are no 5-year data for mycophenolic acid as there are for cyclophosphamide, Dr. Boumpas said. But the task force that developed the guidelines weighed data on safety and efficacy and found mycophenolic acid "as the clear first choice, while at the same time recognizing the limitations of the studies," he noted.
The ACR guidelines do not recommend use of azathioprine (AZA) as induction treatment. The EULAR recommendations acknowledge that AZA has been associated with a higher risk of renal flares, and call for its use in certain patients who have no adverse clinical or histological risk factors. Patients treated with AZA need close follow-up. "This is particularly important for countries without access to MPA [mycophenolic acid]," Dr. Boumpas said.
The EULAR guidelines also recommend switching to an alternative agent when patients fail to improve in 3-4 months or do not achieve partial response after 6-12 months, or a complete response after 2 years.
"This is based on evidence from both controlled trials and observational cohort studies, which highlight the fact that immunosuppressive agents, particularly cyclophosphamide, may take up to 2 years to achieve complete renal response," Dr. George K. Bertsias, also of the University of Crete in Heraklion and the first author of the guidelines, said in an interview. "On the other hand, lack of improvement at early time points (3-6 months) is associated with adverse prognosis and should evoke discussions for treatment intensification or switch."
This is a different timetable from that described in the ACR guidelines, which advocate switching after patients fail to respond after 6 months of treatment based on the treating physician’s clinical impression.
For patients not responding to mycophenolic acid or cyclophosphamide, treatment may be switched from mycophenolic acid to cyclophosphamide or from cyclophosphamide to mycophenolic acid, according to the guidelines.
If switching fails, rituximab, a biological agent, may be given either as an add-on treatment or as monotherapy. Although randomized controlled trials have failed to demonstrate the superiority of rituximab over standard treatment in lupus nephritis, "there is culminating evidence from several uncontrolled studies and several groups worldwide that rituximab works in about half of patients with nephritis refractory to conventional immunosuppressive therapy," Dr. Boumpas said. "Since rituximab does not have adverse effects on the gonads – a significant issue in the care of young women with lupus – the committee decided to recommend it as an additional treatment resource."
The EULAR guidelines, in contrast to the ACR guidelines, contain specific dosing advice on steroids, advocating pulse steroids (500-1,000 mg of methylprednisolone daily for three doses) in combination with initial immunosuppressive therapy, followed by daily oral glucocorticoids (0.5-1.0 mg/kg per day), afterward tapering to the minimal amount necessary to control disease.
The EULAR guidelines also contain specific recommendations for patients planning pregnancy. In addition, they cover diagnosis and management of pediatric lupus nephritis, which largely follow adult recommendations. The pediatric recommendations are based on evidence in adults, and on nonrandomized evidence in children.
Work on the recommendations was funded by EULAR and the ERA-EDTA. The authors declared no conflicts of interest.
European Renal Association-European Dialysis and Transplant Association, ERA-EDTA, mycophenolic acid, immunosuppressive therapy, Annals of the Rheumatic Diseases, Dr. Dimitrios T. Boumpas,
The European League Against Rheumatism has issued its first guidelines on management of lupus nephritis, and they advise renal biopsy at the first sign of kidney involvement, unlike the guidance issued by the American College of Rheumatology, which leaves timing of that testing up to the clinician’s judgment.
Other bright spots in EULAR’s guidelines, which it issued jointly with the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) are their position that mycophenolic acid should be the first choice for immunosuppressive therapy, the precise recommendations for steroid dosage, stratification of treatment plans according to disease severity, and explicit advice on switching therapies after one drug has failed.
The European guidelines were published in the November issue of Annals of the Rheumatic Diseases (2012;71:1771-82) and are intended for rheumatologists, nephrologists, and internists managing adult and pediatric patients with lupus nephritis. The EULAR guidelines differ in several key ways from those issued by the American College of Rheumatology (ACR) in June (Arthritis Care Res. 2012;64:797-808).
The EULAR guidelines are unequivocal in their support for renal biopsy at any sign of renal involvement, including when unexplained renal insufficiency is accompanied by normal urinary findings. The ACR guidelines, by contrast, allow for more physician latitude in determining whether to biopsy.
"This can be an emotional issue because rheumatologists do not do biopsies and may try to avoid them," said the EULAR guidelines’ lead author, rheumatologist Dr. Dimitrios T. Boumpas, who is professor of medicine and director of internal medicine/rheumatology at the University of Crete in Heraklion, Greece, in an interview. "We felt that someone should make a position statement. Biopsy is simply best care. If you’re dealing with something severe like LN [lupus nephritis] and you avoid biopsy, that’s not good medicine."
Dr. Boumpas said the EULAR task force had also moved to put mycophenolic acid in the first position as an initial immunosuppressant treatment for most cases of class III-IV LN. Low-dose intravenous cyclophosphamide in combination with steroids is also recommended for this patient group.
There are no 5-year data for mycophenolic acid as there are for cyclophosphamide, Dr. Boumpas said. But the task force that developed the guidelines weighed data on safety and efficacy and found mycophenolic acid "as the clear first choice, while at the same time recognizing the limitations of the studies," he noted.
The ACR guidelines do not recommend use of azathioprine (AZA) as induction treatment. The EULAR recommendations acknowledge that AZA has been associated with a higher risk of renal flares, and call for its use in certain patients who have no adverse clinical or histological risk factors. Patients treated with AZA need close follow-up. "This is particularly important for countries without access to MPA [mycophenolic acid]," Dr. Boumpas said.
The EULAR guidelines also recommend switching to an alternative agent when patients fail to improve in 3-4 months or do not achieve partial response after 6-12 months, or a complete response after 2 years.
"This is based on evidence from both controlled trials and observational cohort studies, which highlight the fact that immunosuppressive agents, particularly cyclophosphamide, may take up to 2 years to achieve complete renal response," Dr. George K. Bertsias, also of the University of Crete in Heraklion and the first author of the guidelines, said in an interview. "On the other hand, lack of improvement at early time points (3-6 months) is associated with adverse prognosis and should evoke discussions for treatment intensification or switch."
This is a different timetable from that described in the ACR guidelines, which advocate switching after patients fail to respond after 6 months of treatment based on the treating physician’s clinical impression.
For patients not responding to mycophenolic acid or cyclophosphamide, treatment may be switched from mycophenolic acid to cyclophosphamide or from cyclophosphamide to mycophenolic acid, according to the guidelines.
If switching fails, rituximab, a biological agent, may be given either as an add-on treatment or as monotherapy. Although randomized controlled trials have failed to demonstrate the superiority of rituximab over standard treatment in lupus nephritis, "there is culminating evidence from several uncontrolled studies and several groups worldwide that rituximab works in about half of patients with nephritis refractory to conventional immunosuppressive therapy," Dr. Boumpas said. "Since rituximab does not have adverse effects on the gonads – a significant issue in the care of young women with lupus – the committee decided to recommend it as an additional treatment resource."
The EULAR guidelines, in contrast to the ACR guidelines, contain specific dosing advice on steroids, advocating pulse steroids (500-1,000 mg of methylprednisolone daily for three doses) in combination with initial immunosuppressive therapy, followed by daily oral glucocorticoids (0.5-1.0 mg/kg per day), afterward tapering to the minimal amount necessary to control disease.
The EULAR guidelines also contain specific recommendations for patients planning pregnancy. In addition, they cover diagnosis and management of pediatric lupus nephritis, which largely follow adult recommendations. The pediatric recommendations are based on evidence in adults, and on nonrandomized evidence in children.
Work on the recommendations was funded by EULAR and the ERA-EDTA. The authors declared no conflicts of interest.
The European League Against Rheumatism has issued its first guidelines on management of lupus nephritis, and they advise renal biopsy at the first sign of kidney involvement, unlike the guidance issued by the American College of Rheumatology, which leaves timing of that testing up to the clinician’s judgment.
Other bright spots in EULAR’s guidelines, which it issued jointly with the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) are their position that mycophenolic acid should be the first choice for immunosuppressive therapy, the precise recommendations for steroid dosage, stratification of treatment plans according to disease severity, and explicit advice on switching therapies after one drug has failed.
The European guidelines were published in the November issue of Annals of the Rheumatic Diseases (2012;71:1771-82) and are intended for rheumatologists, nephrologists, and internists managing adult and pediatric patients with lupus nephritis. The EULAR guidelines differ in several key ways from those issued by the American College of Rheumatology (ACR) in June (Arthritis Care Res. 2012;64:797-808).
The EULAR guidelines are unequivocal in their support for renal biopsy at any sign of renal involvement, including when unexplained renal insufficiency is accompanied by normal urinary findings. The ACR guidelines, by contrast, allow for more physician latitude in determining whether to biopsy.
"This can be an emotional issue because rheumatologists do not do biopsies and may try to avoid them," said the EULAR guidelines’ lead author, rheumatologist Dr. Dimitrios T. Boumpas, who is professor of medicine and director of internal medicine/rheumatology at the University of Crete in Heraklion, Greece, in an interview. "We felt that someone should make a position statement. Biopsy is simply best care. If you’re dealing with something severe like LN [lupus nephritis] and you avoid biopsy, that’s not good medicine."
Dr. Boumpas said the EULAR task force had also moved to put mycophenolic acid in the first position as an initial immunosuppressant treatment for most cases of class III-IV LN. Low-dose intravenous cyclophosphamide in combination with steroids is also recommended for this patient group.
There are no 5-year data for mycophenolic acid as there are for cyclophosphamide, Dr. Boumpas said. But the task force that developed the guidelines weighed data on safety and efficacy and found mycophenolic acid "as the clear first choice, while at the same time recognizing the limitations of the studies," he noted.
The ACR guidelines do not recommend use of azathioprine (AZA) as induction treatment. The EULAR recommendations acknowledge that AZA has been associated with a higher risk of renal flares, and call for its use in certain patients who have no adverse clinical or histological risk factors. Patients treated with AZA need close follow-up. "This is particularly important for countries without access to MPA [mycophenolic acid]," Dr. Boumpas said.
The EULAR guidelines also recommend switching to an alternative agent when patients fail to improve in 3-4 months or do not achieve partial response after 6-12 months, or a complete response after 2 years.
"This is based on evidence from both controlled trials and observational cohort studies, which highlight the fact that immunosuppressive agents, particularly cyclophosphamide, may take up to 2 years to achieve complete renal response," Dr. George K. Bertsias, also of the University of Crete in Heraklion and the first author of the guidelines, said in an interview. "On the other hand, lack of improvement at early time points (3-6 months) is associated with adverse prognosis and should evoke discussions for treatment intensification or switch."
This is a different timetable from that described in the ACR guidelines, which advocate switching after patients fail to respond after 6 months of treatment based on the treating physician’s clinical impression.
For patients not responding to mycophenolic acid or cyclophosphamide, treatment may be switched from mycophenolic acid to cyclophosphamide or from cyclophosphamide to mycophenolic acid, according to the guidelines.
If switching fails, rituximab, a biological agent, may be given either as an add-on treatment or as monotherapy. Although randomized controlled trials have failed to demonstrate the superiority of rituximab over standard treatment in lupus nephritis, "there is culminating evidence from several uncontrolled studies and several groups worldwide that rituximab works in about half of patients with nephritis refractory to conventional immunosuppressive therapy," Dr. Boumpas said. "Since rituximab does not have adverse effects on the gonads – a significant issue in the care of young women with lupus – the committee decided to recommend it as an additional treatment resource."
The EULAR guidelines, in contrast to the ACR guidelines, contain specific dosing advice on steroids, advocating pulse steroids (500-1,000 mg of methylprednisolone daily for three doses) in combination with initial immunosuppressive therapy, followed by daily oral glucocorticoids (0.5-1.0 mg/kg per day), afterward tapering to the minimal amount necessary to control disease.
The EULAR guidelines also contain specific recommendations for patients planning pregnancy. In addition, they cover diagnosis and management of pediatric lupus nephritis, which largely follow adult recommendations. The pediatric recommendations are based on evidence in adults, and on nonrandomized evidence in children.
Work on the recommendations was funded by EULAR and the ERA-EDTA. The authors declared no conflicts of interest.
European Renal Association-European Dialysis and Transplant Association, ERA-EDTA, mycophenolic acid, immunosuppressive therapy, Annals of the Rheumatic Diseases, Dr. Dimitrios T. Boumpas,
European Renal Association-European Dialysis and Transplant Association, ERA-EDTA, mycophenolic acid, immunosuppressive therapy, Annals of the Rheumatic Diseases, Dr. Dimitrios T. Boumpas,
FROM ANNALS OF THE RHEUMATIC DISEASES
Pancreas/Kidney Graft Improves Survival for Type 1 Patients
BERLIN – In patients with type 1 diabetes and end-stage renal disease, a combined pancreas/kidney transplant was associated with significantly better 15-year survival than a single kidney graft alone.
Patients who got the simultaneous transplant were 30% more likely to survive to 15 years than were those who received a living donor kidney. Those patients who got a single kidney from a deceased donor, however, were 30% less likely to survive for 15 years after the operation, Dr. Trond Jenssen said at the annual meeting of the European Association for the Study of Diabetes.
The combined graft is the preferred method of treating end-stage renal disease (ESRD) caused by diabetic nephropathy, said Dr. Jenssen of Oslo University. Not all patients are suited for it, however.
"According to the algorithm at our center, if you are older than 55 or too sick, you have to decide between the living and deceased donor single kidney graft. Patients who are younger and have less comorbidity are the ones considered for a combined transplant," he explained.
Since those who have the dual-organ operation almost always normalize their glycemic values afterward, Dr. Jenssen said, it’s assumed that they will live longer than those who get only a kidney, but studies are divided on the finding.
"The literature over the past 10 years has differed," he said. Comparing studies among institutions is impossible because of the differences in surgical technique and immunosuppressive regimens; the patient populations can also vary widely.
Oslo University is in a unique place to study the issue, Dr. Jenssen suggested. The facility is the national transplant center, with nearly 30 years of full follow-up data on 630 type 1 diabetes patients who were transplanted for ESRD. All of the patients are followed at least annually and their information is entered into the Norwegian Renal Registry.
"Because patients in Norway tend to be very faithful to their doctors, we have not lost a single one of these to follow-up," he said.
Of the entire group, 222 received the simultaneous transplants, 171 received a living donor single kidney, and 237 got a deceased donor kidney. Patients who received the simultaneous transplant were younger than the living or deceased single graft groups (41 years vs. 45 and 55 years, respectively).
The study controlled for the evolution of surgical techniques and immunosuppressant regimens. Before 1989, all pancreases were transplanted with occluded ducts. From 1989 to 1999, the exocrine duct drained into the bladder, and since 2000, into the intestine. The pancreas has always been connected to the systemic circulation by the iliac artery and vein.
Before 2000, the immunosuppressive regiment consisted of cyclosporine and azathioprine; afterwards, tacrolimus and mycophenolate. All patients from both eras take a daily dose of prednisone as well.
There was no induction therapy before 2000, Dr. Jenssen said. After that time, patients receiving a single kidney began to receive basiliximab and the dual-transplant patients got thymoglobulin.
The overall 15-year survival rate was 50% in the simultaneous-graft group, 30% in the living donor kidney group, and 12% in the deceased donor kidney group.
Dr. Jenssen presented two regression models. In the first one, which controlled for recipient age, time on dialysis, and the transplant era, patients who got the dual graft were significantly more likely to survive to 15 years than were those who got the single live donor kidney (hazard ratio, 0.70). Patients who received a single deceased donor kidney were 31% less likely to survive (HR, 1.29).
These differences were no longer significant in a second model, which also controlled for donor age, but Dr. Jenssen said that the difference was not clinically meaningful.
Among the 317 patients who died during the study, the most common cause was cardiovascular disease (59%). Infections claimed 15% and malignancy 8%. The remainder of the patients died from causes that he did not specify.
Dr. Jenssen said that he did not control for glycemic index because the hemoglobin A1c test was unavailable during a large part of the follow-up period. He intends to work that into the model eventually, he added.
Dr. Jenssen had no financial disclosures.
simultaneous transplant, Dr. Trond Jenssen, European Association for the Study of Diabetes, combined graft, end-stage renal disease, (ESRD), diabetic nephropathy,
BERLIN – In patients with type 1 diabetes and end-stage renal disease, a combined pancreas/kidney transplant was associated with significantly better 15-year survival than a single kidney graft alone.
Patients who got the simultaneous transplant were 30% more likely to survive to 15 years than were those who received a living donor kidney. Those patients who got a single kidney from a deceased donor, however, were 30% less likely to survive for 15 years after the operation, Dr. Trond Jenssen said at the annual meeting of the European Association for the Study of Diabetes.
The combined graft is the preferred method of treating end-stage renal disease (ESRD) caused by diabetic nephropathy, said Dr. Jenssen of Oslo University. Not all patients are suited for it, however.
"According to the algorithm at our center, if you are older than 55 or too sick, you have to decide between the living and deceased donor single kidney graft. Patients who are younger and have less comorbidity are the ones considered for a combined transplant," he explained.
Since those who have the dual-organ operation almost always normalize their glycemic values afterward, Dr. Jenssen said, it’s assumed that they will live longer than those who get only a kidney, but studies are divided on the finding.
"The literature over the past 10 years has differed," he said. Comparing studies among institutions is impossible because of the differences in surgical technique and immunosuppressive regimens; the patient populations can also vary widely.
Oslo University is in a unique place to study the issue, Dr. Jenssen suggested. The facility is the national transplant center, with nearly 30 years of full follow-up data on 630 type 1 diabetes patients who were transplanted for ESRD. All of the patients are followed at least annually and their information is entered into the Norwegian Renal Registry.
"Because patients in Norway tend to be very faithful to their doctors, we have not lost a single one of these to follow-up," he said.
Of the entire group, 222 received the simultaneous transplants, 171 received a living donor single kidney, and 237 got a deceased donor kidney. Patients who received the simultaneous transplant were younger than the living or deceased single graft groups (41 years vs. 45 and 55 years, respectively).
The study controlled for the evolution of surgical techniques and immunosuppressant regimens. Before 1989, all pancreases were transplanted with occluded ducts. From 1989 to 1999, the exocrine duct drained into the bladder, and since 2000, into the intestine. The pancreas has always been connected to the systemic circulation by the iliac artery and vein.
Before 2000, the immunosuppressive regiment consisted of cyclosporine and azathioprine; afterwards, tacrolimus and mycophenolate. All patients from both eras take a daily dose of prednisone as well.
There was no induction therapy before 2000, Dr. Jenssen said. After that time, patients receiving a single kidney began to receive basiliximab and the dual-transplant patients got thymoglobulin.
The overall 15-year survival rate was 50% in the simultaneous-graft group, 30% in the living donor kidney group, and 12% in the deceased donor kidney group.
Dr. Jenssen presented two regression models. In the first one, which controlled for recipient age, time on dialysis, and the transplant era, patients who got the dual graft were significantly more likely to survive to 15 years than were those who got the single live donor kidney (hazard ratio, 0.70). Patients who received a single deceased donor kidney were 31% less likely to survive (HR, 1.29).
These differences were no longer significant in a second model, which also controlled for donor age, but Dr. Jenssen said that the difference was not clinically meaningful.
Among the 317 patients who died during the study, the most common cause was cardiovascular disease (59%). Infections claimed 15% and malignancy 8%. The remainder of the patients died from causes that he did not specify.
Dr. Jenssen said that he did not control for glycemic index because the hemoglobin A1c test was unavailable during a large part of the follow-up period. He intends to work that into the model eventually, he added.
Dr. Jenssen had no financial disclosures.
BERLIN – In patients with type 1 diabetes and end-stage renal disease, a combined pancreas/kidney transplant was associated with significantly better 15-year survival than a single kidney graft alone.
Patients who got the simultaneous transplant were 30% more likely to survive to 15 years than were those who received a living donor kidney. Those patients who got a single kidney from a deceased donor, however, were 30% less likely to survive for 15 years after the operation, Dr. Trond Jenssen said at the annual meeting of the European Association for the Study of Diabetes.
The combined graft is the preferred method of treating end-stage renal disease (ESRD) caused by diabetic nephropathy, said Dr. Jenssen of Oslo University. Not all patients are suited for it, however.
"According to the algorithm at our center, if you are older than 55 or too sick, you have to decide between the living and deceased donor single kidney graft. Patients who are younger and have less comorbidity are the ones considered for a combined transplant," he explained.
Since those who have the dual-organ operation almost always normalize their glycemic values afterward, Dr. Jenssen said, it’s assumed that they will live longer than those who get only a kidney, but studies are divided on the finding.
"The literature over the past 10 years has differed," he said. Comparing studies among institutions is impossible because of the differences in surgical technique and immunosuppressive regimens; the patient populations can also vary widely.
Oslo University is in a unique place to study the issue, Dr. Jenssen suggested. The facility is the national transplant center, with nearly 30 years of full follow-up data on 630 type 1 diabetes patients who were transplanted for ESRD. All of the patients are followed at least annually and their information is entered into the Norwegian Renal Registry.
"Because patients in Norway tend to be very faithful to their doctors, we have not lost a single one of these to follow-up," he said.
Of the entire group, 222 received the simultaneous transplants, 171 received a living donor single kidney, and 237 got a deceased donor kidney. Patients who received the simultaneous transplant were younger than the living or deceased single graft groups (41 years vs. 45 and 55 years, respectively).
The study controlled for the evolution of surgical techniques and immunosuppressant regimens. Before 1989, all pancreases were transplanted with occluded ducts. From 1989 to 1999, the exocrine duct drained into the bladder, and since 2000, into the intestine. The pancreas has always been connected to the systemic circulation by the iliac artery and vein.
Before 2000, the immunosuppressive regiment consisted of cyclosporine and azathioprine; afterwards, tacrolimus and mycophenolate. All patients from both eras take a daily dose of prednisone as well.
There was no induction therapy before 2000, Dr. Jenssen said. After that time, patients receiving a single kidney began to receive basiliximab and the dual-transplant patients got thymoglobulin.
The overall 15-year survival rate was 50% in the simultaneous-graft group, 30% in the living donor kidney group, and 12% in the deceased donor kidney group.
Dr. Jenssen presented two regression models. In the first one, which controlled for recipient age, time on dialysis, and the transplant era, patients who got the dual graft were significantly more likely to survive to 15 years than were those who got the single live donor kidney (hazard ratio, 0.70). Patients who received a single deceased donor kidney were 31% less likely to survive (HR, 1.29).
These differences were no longer significant in a second model, which also controlled for donor age, but Dr. Jenssen said that the difference was not clinically meaningful.
Among the 317 patients who died during the study, the most common cause was cardiovascular disease (59%). Infections claimed 15% and malignancy 8%. The remainder of the patients died from causes that he did not specify.
Dr. Jenssen said that he did not control for glycemic index because the hemoglobin A1c test was unavailable during a large part of the follow-up period. He intends to work that into the model eventually, he added.
Dr. Jenssen had no financial disclosures.
simultaneous transplant, Dr. Trond Jenssen, European Association for the Study of Diabetes, combined graft, end-stage renal disease, (ESRD), diabetic nephropathy,
simultaneous transplant, Dr. Trond Jenssen, European Association for the Study of Diabetes, combined graft, end-stage renal disease, (ESRD), diabetic nephropathy,
AT THE ANNUAL MEETING OF THE EUROPEAN ASSOCIATION FOR THE STUDY OF DIABETES
Major Finding: Type 1 diabetes patients who got a combined pancreas/kidney transplant were 30% more likely to survive for 15 years than were patients who received a single living donor kidney.
Data Source: Findings are based on 27 years of follow-up among 630 patients.
Disclosures: Dr. Jenssen had no financial disclosures.
Management of Hypertensive Urgency and Emergency
An estimated 1% to 2% of patients with chronic hypertension will at some time develop hypertensive urgency or emergency.1 According to recent data from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2010,2 the prevalence of hypertension has remained stable at 30.5% among men and 28.5% among women in the United States; however, 74% of the hypertensive population is unaware of having this condition. Furthermore, 71.6% of hypertensive patients are managed for the condition, and in only 46.5% is blood pressure well controlled.2
In 2006, essential hypertension was estimated to account for more than 44 million emergency department visits in the US. The direct and indirect costs of hypertension totaled $73 billion in 2009.3,4
NEW TERMINOLOGY AND CLASSIFICATION
The terms malignant hypertension, hypertensive crisis, and accelerated hypertension have been replaced by hypertensive urgency or hypertensive emergency. Hypertensive urgency and emergency are differentiated by the absence or presence of acute end-organ damage, respectively.
Given the inconsistent terminology used, database searches can be challenging. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7),5,6 published in 2003, is considered the gold standard for categorizing hypertension in the outpatient setting. The JNC7 authorsclassify normal blood pressure as < 120/< 80 mm Hg. The document further classifies blood pressure into the stages shown in Table 1.5,6 Blood pressure higher than 180 mm Hg systolic and/or 120 mm Hg diastolic is generally considered severe hypertension— a designation that includes hypertensive urgency and hypertensive emergency.6
What Defines Hypertensive Urgency/Emergency?
Hypertensive urgency is defined as a diastolic blood pressure of 110 mm Hg or greater without the acute signs of end-organ damage.7 Some sources suggest that a patient must also have certain risk factors (eg, heart disease, renal disease) to be given this diagnosis.8 The presence of acute and rapidly evolving end-organ damage with an elevated diastolic blood pressure, usually greater than 120 mm Hg, establishes a diagnosis of hypertensive emergency.6,8,9
No specific blood pressure measurement indicates a hypertensive emergency, however; rather, the defining feature of this diagnosis is the presence of progressive target end-organ damage.7 This is most commonly manifested in cardiopulmonary, central nervous system, and/or renal findings; for the specific forms of end-organ damage, see Table 2.5,6,10,11 Preeclampsia and eclampsia are also considered manifestations of hypertensive end-organ damage but are beyond the scope of this article.5,11
The most common form of organ damage associated with hypertension is ischemic heart disease, in the form of either heart failure or acute coronary syndrome.12
PATHOPHYSIOLOGY
Blood pressure is calculated by cardiac output (ie, stroke volume multiplied by heart rate) multiplied by total peripheral resistance. Total peripheral resistance is influenced by a variety of humoral and neural factors, also known as vasoactive substances (see Figure 13,4). During an episode of acute hypertension, a failure of autoregulatory function occurs, precipitated by one or more of a host of potential causes. This failure of autoregulation then leads to increased systemic vascular resistance. In the setting of end-organ damage, release of inflammatory markers ensues, which ultimately causes endovascular injury and fibrin necrosis of arterioles.4,10,11
The renin-angiotensin-aldosterone system also plays a significant role in the cascade of hypertension, stimulating decreased renal perfusion and lowering tubular sodium concentration. This in turn stimulates aldosterone to increase blood pressure by maintaining excess volume through sodium retention and potassium excretion, further potentiating the cycle of uncontrolled blood pressure.4,13,14
Patients with chronically elevated blood pressures have a compensatory response, lying in the threshold mechanism, that protects against end-organ damage. Acute changes in blood pressure are better tolerated in these patients because of their decreased propensity for hypoperfusion.4 In contrast, normotensive patients who experience precipitous changes in blood pressure are at increased risk for organ hypoperfusion. The main concern regarding organ hypoperfusion is that it can lead to ischemia4 (see Figure 23,4).
PATIENT HISTORY
Acute hypertensive urgency or emergency can be triggered by many factors. Systemic etiologies (including kidney disease) caused by immunologic mediators or renal artery stenosis can cause or exacerbate hypertension. The patient should be asked about his or her normal blood pressure range, as this may offer clues to medication compliance. Rebound hypertension can be seen in patients who abruptly discontinue medications such as clonidine or β-blockers, as this causes an increase in sympathetic outflow.9,15
All patients should be queried regarding their use of OTC medications and other drug use, including cocaine, methamphetamines, phencyclidine, and alcohol.1,4,11,16 Patients taking monoamine oxidase inhibitors (MAOIs) are at increased risk for serious medication interactions; concomitant administration of MAOIs with other antidepressants can lead to a hypertensive reaction, but also to serotonin syndrome.1,17 Because MAOIs inhibit the breakdown of tyramine, patients taking them should avoid tyramine-containing foods and herbal supplements (including, but not limited to, St. John's wort, ginseng, and yohimbine).1,15,18
Acute hypertensive episodes can also occur as a result of preeclampsia or eclampsia in pregnant women, pheochromocytoma, primary aldosteronism, glucocorticoid excess (Cushing syndrome), or central nervous system disorders (eg, cerebrovascular accident, head trauma, brain tumors).9,11,19
PHYSICAL EXAMINATION
The purpose of the physical examination is to determine whether end-organ damage is present.1,11 The fundoscopic exam may reveal papilledema, a sign of increased intracranial pressure (ICP). Flame hemorrhages, cotton wool spots or arteriovenous nicking suggest a long-standing history of uncontrolled hypertension or diabetes.7,9 The neck should be assessed for jugular venous distention, which may be elevated in decompensated heart failure or pulmonary edema.11
The cardiac exam may reveal an irregular rate and rhythm, displaced apical pulse, gallop, or murmur. On pulmonary exam, rales may be auscultated, suggestive of pulmonary edema.9,15
The abdominal exam should include listening for a renal artery bruit.1 The neurologic exam may demonstrate altered mental status (possibly indicating hypertensive encephalopathy) or focal findings, if the patient has had an underlying ischemic or hemorrhagic event.9
LABORATORY STUDIES AND IMAGING
In most cases, a serum chemistry panel is warranted to identify any renal dysfunction. Urinalysis may reveal proteinuria, possibly indicating renal damage.4,9,15
Any patient complaining of chest pain should have an ECG to look for ischemic changes or presence of a left bundle branch block, and serial cardiac enzymes to rule out acute coronary syndrome.15 Access to previous ECGs is helpful in differentiating between new and old conductive abnormalities.
A chest x-ray should be performed in patients who complain of shortness of breath and/or chest pain. A widened mediastinum can represent aortic dissection.4,15 Evidence of pulmonary edema should prompt the clinician to assess for left ventricular dysfunction or valvular insufficiencies by echocardiogram. Chest CT should be pursued in patients with clinical suspicion for dissection.1,15,20
Patients presenting with a headache or focal neurologic abnormalities warrant a head CT to rule out stroke.15 Urine drug screening is appropriate if the patient history suggests illicit drug use.12
"FIRST, DO NO HARM"
Treatment of hypertensive emergency and urgency varies from traditional treatment for hypertension. Aggressive blood pressure control in patients presenting with acute ischemic stroke has been associated with poorer patient outcomes.21,22 Thus, treating the patient and not the numbers is the first general recommendation for treatment of hypertensive emergency and urgency. It is important for the clinician to remember the Hippocratic oath, "First do no harm," when treating these patients.
Other general recommendations are derived from theory, physiology, and smaller clinical trials; their application must be individualized according to the patient's needs. These recommendations include aiming for a reduction in mean arterial blood pressure of no more than 10% to 25% within the first hour, a goal blood pressure of 160/90 mm Hg within the first 8 hours, and normalization of blood pressure over 8 to 24 hours.12
While the use of pharmacologic agents may be warranted, it is important to consider that elevated blood pressure may be a reaction to pain or stress and may be best treated alternatively. Recommendations for permissive hypertension in acute ischemic stroke will be discussed below.
TREATMENT: HYPERTENSIVE URGENCY
The treatment of hypertensive urgency is usually immediate and warrants close follow-up. Although elevated blood pressures can be alarming to the patient, hypertensive urgency usually develops over days to weeks.8 In this setting, it is not necessary to lower blood pressure acutely.12 A rapid decrease in blood pressure can actually cause symptomatic hypotension, resulting in hypoperfusion to the brain.5,6,8
After ruling out end-organ damage, the next step is to treat according to the guidelines for hypertensive urgency.5,6 These recommendations include the use of rapid-onset oral antihypertensive agents, such as clonidine, labetalol, or captopril.23 Use of these agents is only suggested for gradual, short-term reduction of blood pressure (ie, over 24 to 48 hours) while the patient is being monitored for potential hypertension-related organ damage, either in the emergency department or in an observational hospital setting.5,6,23
Once the short-acting agents have adequately reduced blood pressure, long-term agents can be chosen to prevent rebound hypertension.16 Patients are typically monitored for 24 hours in the hospital during this transition. Upon discharge, the patient should be scheduled for follow-up within one to two days.11 Patient education, including a discussion of medication adherence, weight loss, and reduced dietary salt, is key to prevent recurrences and optimize overall treatment compliance.
TREATMENT: HYPERTENSIVE EMERGENCY
Treatment of hypertensive emergency always warrants hospitalization, usually in the ICU.5,6 IV antihypertensive medications (eg, nicardipine, fenoldopam, labetalol, esmolol, phentolamine) are preferred. Their use often necessitates continuous blood pressure monitoring via arterial line, allowing the clinician to perform ongoing medication titration. In hypertensive emergencies, the purpose of treatment is to preserve brain, kidney, and heart function.4
Goal-directed therapy is initiated even before the patient evaluation has been fully completed. Patient assessment continues after treatment is begun to avoid overly aggressive blood pressure reduction, which can increase the risk for patient demise or morbidity.4
Exceptions in the treatment of hypertensive emergencies (particularly of specific disease states) will be discussed below, along with other treatment considerations. Patient comorbidities, for example, must be considered in the choice of antihypertensive agents.
Focused Treatment for Specific Hypertensive Emergencies
Hypertensive encephalopathy. This condition, associated with severe hypertension, is indicated by an abrupt change in mental status. During this acute end-organ damage event, a failure of cerebral autoregulation occurs, with increased pressure in the vascular endothelium leading to arteriole dilation that in turn can result in hyperperfusion of the brain, cerebral edema, and microhemorrhages.23
Because presentation of hypertensive encephalopathy may be similar to that in patients with acute stroke, hemorrhage, or brain lesions, these and other potential causes must be ruled out. While blood pressure treatment goals correspond with general recommendations,5,6 caution must be taken not to reduce blood pressure too swiftly; thus, continuous monitoring is warranted. If the patient's neurologic function worsens, treatment should be suspended and blood pressure allowed to rise slowly.4
Preferred antihypertensives for patients with hypertensive encephalopathy include labetalol, nicardipine, and fenoldopam23 (see Table 34-6,21,23). Centrally acting antihypertensives, such as clonidine, methyldopa, or reserpine,24 should not be used, as they can cause central nervous system depression and may cloud the patient's sensorium further.
Myocardial ischemia/infarction. During an acute hypertensive event, the workload on the heart and activation of the renin-angiotensin-aldosterone system can lead to acute coronary ischemia or infarction.23 Treatment is aimed at increasing blood flow to the myocardium and reducing the workload on the heart. Antihypertensives are combined with reperfusion (eg, angioplasty) and/or thrombolytics to preserve myocardial structure and function. Standard agents to reduce blood pressure include IV nitroglycerin and β-blockers. Systolic blood pressure is reduced until symptoms subside or diastolic blood pressure is reduced to 100 mm Hg or lower. Adjuncts such as morphine and oxygen are used to reduce patient discomfort and improve oxygen delivery to the myocardium.4
Acute left ventricular failure. In this potential manifestation of hypertensive emergency, the left ventricle initially attempts to compensate for rising blood pressure and becomes hypertrophic. Once the myocardium can no longer meet the demand, left ventricular function decompensates, causing a flow backup that leads to acute pulmonary edema.23
Blood pressure goals mirror those in the general treatment recommendations but focus specifically on reducing preload and afterload, improving myocardial contractility and decreasing peripheral vascular resistance. The preferred agents in this setting are IV nitroglycerin and ACE inhibitors, along with loop diuretics, morphine, and oxygen.4 Medications that increase workload on the heart (eg, hydralazine, clonidine) should be avoided.23
Aortic dissection. This is a true medical emergency that can result in significant morbidity and mortality. Type A dissection occurs proximally, at the ascending aorta, whereas type B dissection occurs at the level of the descending aorta. Typically, type B dissection is managed medically, as surgical treatment carries a significant risk for paralysis.4 Both types of aortic dissection are strongly associated with uncontrolled hypertension and in some patients may be precipitated by an acute hypertensive event. In such cases, the goal for blood pressure reduction is to decrease the shearing forces associated with the dissection. This is accomplished by lowering both blood pressure and pulse rate.12
While cases of type A dissection are usually managed surgically, all affected patients will require some component of medical management and tight blood pressure control. The current recommendation for blood pressure in aortic dissection is swift downward titration to a goal systolic blood pressure of 100 to 110 mm Hg.4 A β-blocker in combination with a vasodilator, administered intravenously, should be used for swift blood pressure reduction.4,25
IV nitroprusside, a potent vasodilator, is the preferred agent, but its use requires intra-arterial blood pressure monitoring.23 Because nitroprusside is metabolized to cyanide, its use can lead to lethal toxicity, especially in patients with hepatic or renal impairment.11 In this patient population, IV labetalol or esmolol may be used instead.4,25
Acute renal failure. In the setting of an acute hypertensive episode, it is often difficult to determine whether acute renal failure is the cause or the effect. Regardless, rapid reduction in blood pressure is warranted to preserve renal function and to stop the cycle of microvascular kidney destruction. Blood pressure goals are aligned with the general treatment recommendations. The preferred antihypertensive agent is IV fenoldopam, a dopamine receptor agonist that directly dilates renal arterioles, improving renal perfusion and promoting diuresis.4,26 Nicardipine, a calcium channel blocker, may be considered as an alternative.23,27
Treatment Considerations in Stroke
Elevated blood pressure is common in the early stages of stroke. Numerous studies have analyzed overall outcomes in patients presenting with ischemic stroke and uncontrolled hypertension. In this setting, evidence suggests a poorer prognosis in patients treated aggressively with antihypertensive agents.4,21,22
The association between dramatic reduction in blood pressure and poor prognoses lies in the theory of the ischemic penumbra. This is an area around the core of ischemic tissue that receives enough blood flow to maintain neuronal activity for a few hours after initial injury, but this tissue is susceptible to further infarction. Precipitous drops in blood pressure can reduce blood flow to collateral vessels, resulting in hypoperfusion of the penumbra and leading to further neurologic damage.
Details and current treatment recommendations for each of the various types of stroke follow.
Acute intracerebral hemorrhage. Uncontrolled hypertension is often associated with intracerebral hemorrhage (ICH), either as a risk factor or a factor that contributes to the event. Once a patient has experienced an acute brain insult, blood pressure can become even more uncontrolled. Extension of the hematoma and a worsening outcome are the main concerns in treating the patient with concomitant blood pressure elevation and ICH. Also of concern is maintaining adequate perfusion to the penumbra. Additionally, transient hypoperfusion can develop when the ICP is elevated and the mean arterial pressure (MAP) is acutely lowered, thus reducing the cerebral perfusion pressure (CPP; CPP = MAP - ICP).
Researchers have acknowledged there is insufficient evidence to offer management guidelines for blood pressure reduction in patients with ICH.28 The 2007 recommendations from the American Heart Association/ American Stroke Association (AHA/ASA) for blood pressure management in patients with acute ICH29 are as follows: In the setting of ICH in patients with uncontrolled blood pressure, treatment should be aggressive if systolic blood pressure exceeds 200 mm Hg or MAP exceeds 150 mm Hg. A treatment goal to consider is reducing systolic blood pres sure to 160 mm Hg or less (or MAP to below 130 mm Hg).29 Patients with elevated ICP should undergo placement of a ventriculostomy to maintain a CPP between 60 and 80 mm Hg, although the risk for infection or intracerebral hemorrhage must be weighed against the potential benefits.29,30
When blood pressure reduction is required, the MAP should not be lowered more than 20% in a 24-hour period. Recommended agents include IV nicardipine, labetalol, enalapril, hydralazine, or esmolol.31
Acute ischemic stroke. Long-term control of blood pressure in patients who have experienced stroke remains undisputed, as it improves outcomes. However, in the setting of acute ischemic stroke (AIS), initiating blood pressure control is more liberal. Optimal control of blood pressure during management of AIS is imperative to reduce morbidity and mortality.32 Areas affected by edematous brain tissue are at increased risk for bleeding (ie, hemorrhagic expansion).
Patients who present with AIS require careful history taking to elicit their average blood pressure range; this will help the clinician determine goal pressures during management of the acute stroke phase.33 The primary rationale for treating blood pressure in this acute setting is to prevent hemorrhagic expansion at sites with potential for bleeding.34
According to the 2007 AHA/ ASA recommendations for management of blood pressure in AIS,35 patients who are eligible for thrombolysis should have a systolic blood pressure goal below 180 mm Hg and diastolic blood pressure below 105 mm Hg. Patients who will not receive thrombolytics should have blood pressure lowered only if systolic blood pressure exceeds 220 mm Hg or diastolic blood pressure exceeds 110 mm Hg.35,36 Appropriately refraining from reducing blood pressure is known as permissive hypertension.
Given the fragility of the cerebral brain tissue after AIS, permissive hypertension is intended to protect the penumbra and preserve cerebral blood flow. In patients who require blood pressure reduction because of other medical conditions (eg, decompensated heart failure), blood pressure should not be lowered more than 10% to 15% in a 24-hour period.9,31,36 No specific antihypertensives are preferred in patients with AIS: IV enalapril, esmolol, labetalol, or nicardipine can be used.31
Subarachnoid hemorrhage. The two complications of a subarachnoid hemorrhage (SAH) that most contribute to morbidity and mortality are rebleeding and vasospasms; elevated blood pressure can contribute to both. Thus, blood pressure control in patients with SAH is imperative.
Patients with acute SAH often require blood pressure monitoring via arterial line, as well as ICP monitoring. Blood pressure goals are similar to those in patients with ICH. The preferred agent for blood pressure control is nimodipine, which offers the secondary benefit of vasospasm prevention.37
CONCLUSION
Patients presenting with urgent or emergent hypertension need expeditious evaluation to avoid the significant morbidity and mortality associated with acute end-organ damage. Hypertensive urgency is defined as a diastolic blood pressure of greater than 120 mm Hg without evidence of end-organ damage.
In cases of hypertensive emergency, in which acute end-organ damage is present, lowering blood pressure should be directed by the type of end-organ damage and/or underlying comorbidities. In general, blood pressure should not be lowered more than 10% to 25% within the first hour, with normalization achieved over the next 8 to 24 hours.
In cases of acute ischemic stroke, permissive hypertension is recommended. Above all, treat patients, not numbers, bearing in mind the Hippocratic oath: Primum non nocere, or "First, do no harm."
REFERENCES
- Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6): 1949-1962.
- Guo F, He D, Zhang W, Walton RG. Trends in Prevalence, Awareness, Management, and Control of Hypertension Among United States Adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599-606.
- Flanigan JS, Vitberg D. Hypertensive emergency and severe hypertension: what to treat, who to treat, and how to treat. Med Clin North Am. 2006;90(3):439-451.
- Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
- National High Blood Pressure Education Program Coordinating Committee, National Heart Lung and Blood Institute, NIH. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No. 04-5230. August 2004. www .nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed September 20, 2012.
- Houston M. Hypertensive emergencies and urgencies: pathophysiology and clinical aspects. Am Heart J. 1986;111(1):205-210.
- Kessler CS, Joudeh Y. Evaluation and treatment of severe asymptomatic hypertension. Am Fam Physician. 2010;81(4):470-476.
- Vaidya CK, Ouellette JR. Hypertensive urgency and emergency. Hosp Physician. Mar 2007:43-50. www.turner-white.com/memberfile.php?Pub Code=hp_mar07_hypertensive.pdf. Accessed September 20, 2012.
- Perez MI, Musini VM. Pharmacological interventions for hypertensive emergencies: a Cochrane systematic review. J Hum Hypertens. 2008;22(9):596-607.
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411-417.
- Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006; 33(3):613-623.
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 suppl B):9-20.
- Flack JM. Epidemiology and unmet needs in hypertension. J Manag Care Pharm. 2007;13(e suppl B):2-8.
- Haas AR, Marik PE. Current diagnosis and management of hypertensive emergency. Semin Dial. 2006;19(6):502-512.
- Hebert CJ, Vidt DG. Hypertensive crises. Prim Care. 2008;35(3):475-487.
- Shulman KI, Fischer HD, Herrmann N, et al. Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiatry. 2009;70(12):1681-1696.
- Musso NR, Vergassola C, Pende A, Lotti G. Yohimbine effects on blood pressure and plasma catecholamines in human hypertension. Am J Hypertens. 1995;8(6):565-571.
- Baid S, Nieman LK. Glucocorticoid excess and hypertension. Curr Hypertens Rep. 2004;6(6): 493-499.
- Society of Critical Care Medicine. Fundamental Critical Care Support Course. www.sccm.org/ fccs_and_training_courses/fccs/pages/default .aspx. Accessed September 20, 2012.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.
- Bernardini GL, Yavagal DR. Management of ischemic stroke: current concepts and treatment options. Hosp Physician. Sep 2006:13-23. www.turner-white.com/memberfile.php?PubCode=hp_sep06_is chemic.pdf. Accessed September 20, 2012.
- Varon J. Treatment of acute severe hypertension: current and newer agents. Drugs. 2008;68(3): 283-297.
- Webster J, Koch HF. Aspects of tolerability of centrally acting antihypertensive drugs. J Cardiovasc Pharmacol. 1996;27 suppl 3:S49-S54.
- Gupta PK, Gupta H, Khoynezhad A. Hypertensive emergency in aortic dissection and thoracic aortic aneurysm: a review of management. Pharmaceuticals. 2009;2(3):66-76.
- Post JB 4th, Frishman WH. Fenoldopam: a new dopamine agonist for the treatment of hypertensive urgencies and emergencies. J Clin Pharmacol. 1998;38(1):2-13.
- Suzuki S, Ohtsuka S, Ishikawa K, Yamaguchi I. Effects of nicardipine on coronary, vertebral and renal arterial flows in patients with essential hypertension. Hypertens Res. 2003;26(3):193-199.
- Anderson CS, Huang Y, Arima H, et al; INTERACT Investigators. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41(2):307-312.
- Broderick J, Connolly S, Feldmann E, et al. Quality of Care and Outcomes in Research Interdisciplinary Working Group Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):e391-413.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Brott T, Lu M, Kothari R, et al. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29(8):1504-1509.
- Aiyagari V, Badruddin A. Management of hypertension in acute stroke. Expert Rev Cardiovasc Ther. 2009;7(6):637-646.
- Castillo J, Leira R, García MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35(2):520-526.
- Bonita R, Beaglehole R. The enigma of the decline in stroke deaths in the United States the search for an explanation. Stroke. 1996;27(3): 370-372.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655-1711.
- Heitsch L, Jauch EC. Management of hypertension in the setting of acute ischemic stroke. Curr Hypertens Rep. 2007;9(6):506-511.
- Barker FG II, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405-414.
An estimated 1% to 2% of patients with chronic hypertension will at some time develop hypertensive urgency or emergency.1 According to recent data from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2010,2 the prevalence of hypertension has remained stable at 30.5% among men and 28.5% among women in the United States; however, 74% of the hypertensive population is unaware of having this condition. Furthermore, 71.6% of hypertensive patients are managed for the condition, and in only 46.5% is blood pressure well controlled.2
In 2006, essential hypertension was estimated to account for more than 44 million emergency department visits in the US. The direct and indirect costs of hypertension totaled $73 billion in 2009.3,4
NEW TERMINOLOGY AND CLASSIFICATION
The terms malignant hypertension, hypertensive crisis, and accelerated hypertension have been replaced by hypertensive urgency or hypertensive emergency. Hypertensive urgency and emergency are differentiated by the absence or presence of acute end-organ damage, respectively.
Given the inconsistent terminology used, database searches can be challenging. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7),5,6 published in 2003, is considered the gold standard for categorizing hypertension in the outpatient setting. The JNC7 authorsclassify normal blood pressure as < 120/< 80 mm Hg. The document further classifies blood pressure into the stages shown in Table 1.5,6 Blood pressure higher than 180 mm Hg systolic and/or 120 mm Hg diastolic is generally considered severe hypertension— a designation that includes hypertensive urgency and hypertensive emergency.6
What Defines Hypertensive Urgency/Emergency?
Hypertensive urgency is defined as a diastolic blood pressure of 110 mm Hg or greater without the acute signs of end-organ damage.7 Some sources suggest that a patient must also have certain risk factors (eg, heart disease, renal disease) to be given this diagnosis.8 The presence of acute and rapidly evolving end-organ damage with an elevated diastolic blood pressure, usually greater than 120 mm Hg, establishes a diagnosis of hypertensive emergency.6,8,9
No specific blood pressure measurement indicates a hypertensive emergency, however; rather, the defining feature of this diagnosis is the presence of progressive target end-organ damage.7 This is most commonly manifested in cardiopulmonary, central nervous system, and/or renal findings; for the specific forms of end-organ damage, see Table 2.5,6,10,11 Preeclampsia and eclampsia are also considered manifestations of hypertensive end-organ damage but are beyond the scope of this article.5,11
The most common form of organ damage associated with hypertension is ischemic heart disease, in the form of either heart failure or acute coronary syndrome.12
PATHOPHYSIOLOGY
Blood pressure is calculated by cardiac output (ie, stroke volume multiplied by heart rate) multiplied by total peripheral resistance. Total peripheral resistance is influenced by a variety of humoral and neural factors, also known as vasoactive substances (see Figure 13,4). During an episode of acute hypertension, a failure of autoregulatory function occurs, precipitated by one or more of a host of potential causes. This failure of autoregulation then leads to increased systemic vascular resistance. In the setting of end-organ damage, release of inflammatory markers ensues, which ultimately causes endovascular injury and fibrin necrosis of arterioles.4,10,11
The renin-angiotensin-aldosterone system also plays a significant role in the cascade of hypertension, stimulating decreased renal perfusion and lowering tubular sodium concentration. This in turn stimulates aldosterone to increase blood pressure by maintaining excess volume through sodium retention and potassium excretion, further potentiating the cycle of uncontrolled blood pressure.4,13,14
Patients with chronically elevated blood pressures have a compensatory response, lying in the threshold mechanism, that protects against end-organ damage. Acute changes in blood pressure are better tolerated in these patients because of their decreased propensity for hypoperfusion.4 In contrast, normotensive patients who experience precipitous changes in blood pressure are at increased risk for organ hypoperfusion. The main concern regarding organ hypoperfusion is that it can lead to ischemia4 (see Figure 23,4).
PATIENT HISTORY
Acute hypertensive urgency or emergency can be triggered by many factors. Systemic etiologies (including kidney disease) caused by immunologic mediators or renal artery stenosis can cause or exacerbate hypertension. The patient should be asked about his or her normal blood pressure range, as this may offer clues to medication compliance. Rebound hypertension can be seen in patients who abruptly discontinue medications such as clonidine or β-blockers, as this causes an increase in sympathetic outflow.9,15
All patients should be queried regarding their use of OTC medications and other drug use, including cocaine, methamphetamines, phencyclidine, and alcohol.1,4,11,16 Patients taking monoamine oxidase inhibitors (MAOIs) are at increased risk for serious medication interactions; concomitant administration of MAOIs with other antidepressants can lead to a hypertensive reaction, but also to serotonin syndrome.1,17 Because MAOIs inhibit the breakdown of tyramine, patients taking them should avoid tyramine-containing foods and herbal supplements (including, but not limited to, St. John's wort, ginseng, and yohimbine).1,15,18
Acute hypertensive episodes can also occur as a result of preeclampsia or eclampsia in pregnant women, pheochromocytoma, primary aldosteronism, glucocorticoid excess (Cushing syndrome), or central nervous system disorders (eg, cerebrovascular accident, head trauma, brain tumors).9,11,19
PHYSICAL EXAMINATION
The purpose of the physical examination is to determine whether end-organ damage is present.1,11 The fundoscopic exam may reveal papilledema, a sign of increased intracranial pressure (ICP). Flame hemorrhages, cotton wool spots or arteriovenous nicking suggest a long-standing history of uncontrolled hypertension or diabetes.7,9 The neck should be assessed for jugular venous distention, which may be elevated in decompensated heart failure or pulmonary edema.11
The cardiac exam may reveal an irregular rate and rhythm, displaced apical pulse, gallop, or murmur. On pulmonary exam, rales may be auscultated, suggestive of pulmonary edema.9,15
The abdominal exam should include listening for a renal artery bruit.1 The neurologic exam may demonstrate altered mental status (possibly indicating hypertensive encephalopathy) or focal findings, if the patient has had an underlying ischemic or hemorrhagic event.9
LABORATORY STUDIES AND IMAGING
In most cases, a serum chemistry panel is warranted to identify any renal dysfunction. Urinalysis may reveal proteinuria, possibly indicating renal damage.4,9,15
Any patient complaining of chest pain should have an ECG to look for ischemic changes or presence of a left bundle branch block, and serial cardiac enzymes to rule out acute coronary syndrome.15 Access to previous ECGs is helpful in differentiating between new and old conductive abnormalities.
A chest x-ray should be performed in patients who complain of shortness of breath and/or chest pain. A widened mediastinum can represent aortic dissection.4,15 Evidence of pulmonary edema should prompt the clinician to assess for left ventricular dysfunction or valvular insufficiencies by echocardiogram. Chest CT should be pursued in patients with clinical suspicion for dissection.1,15,20
Patients presenting with a headache or focal neurologic abnormalities warrant a head CT to rule out stroke.15 Urine drug screening is appropriate if the patient history suggests illicit drug use.12
"FIRST, DO NO HARM"
Treatment of hypertensive emergency and urgency varies from traditional treatment for hypertension. Aggressive blood pressure control in patients presenting with acute ischemic stroke has been associated with poorer patient outcomes.21,22 Thus, treating the patient and not the numbers is the first general recommendation for treatment of hypertensive emergency and urgency. It is important for the clinician to remember the Hippocratic oath, "First do no harm," when treating these patients.
Other general recommendations are derived from theory, physiology, and smaller clinical trials; their application must be individualized according to the patient's needs. These recommendations include aiming for a reduction in mean arterial blood pressure of no more than 10% to 25% within the first hour, a goal blood pressure of 160/90 mm Hg within the first 8 hours, and normalization of blood pressure over 8 to 24 hours.12
While the use of pharmacologic agents may be warranted, it is important to consider that elevated blood pressure may be a reaction to pain or stress and may be best treated alternatively. Recommendations for permissive hypertension in acute ischemic stroke will be discussed below.
TREATMENT: HYPERTENSIVE URGENCY
The treatment of hypertensive urgency is usually immediate and warrants close follow-up. Although elevated blood pressures can be alarming to the patient, hypertensive urgency usually develops over days to weeks.8 In this setting, it is not necessary to lower blood pressure acutely.12 A rapid decrease in blood pressure can actually cause symptomatic hypotension, resulting in hypoperfusion to the brain.5,6,8
After ruling out end-organ damage, the next step is to treat according to the guidelines for hypertensive urgency.5,6 These recommendations include the use of rapid-onset oral antihypertensive agents, such as clonidine, labetalol, or captopril.23 Use of these agents is only suggested for gradual, short-term reduction of blood pressure (ie, over 24 to 48 hours) while the patient is being monitored for potential hypertension-related organ damage, either in the emergency department or in an observational hospital setting.5,6,23
Once the short-acting agents have adequately reduced blood pressure, long-term agents can be chosen to prevent rebound hypertension.16 Patients are typically monitored for 24 hours in the hospital during this transition. Upon discharge, the patient should be scheduled for follow-up within one to two days.11 Patient education, including a discussion of medication adherence, weight loss, and reduced dietary salt, is key to prevent recurrences and optimize overall treatment compliance.
TREATMENT: HYPERTENSIVE EMERGENCY
Treatment of hypertensive emergency always warrants hospitalization, usually in the ICU.5,6 IV antihypertensive medications (eg, nicardipine, fenoldopam, labetalol, esmolol, phentolamine) are preferred. Their use often necessitates continuous blood pressure monitoring via arterial line, allowing the clinician to perform ongoing medication titration. In hypertensive emergencies, the purpose of treatment is to preserve brain, kidney, and heart function.4
Goal-directed therapy is initiated even before the patient evaluation has been fully completed. Patient assessment continues after treatment is begun to avoid overly aggressive blood pressure reduction, which can increase the risk for patient demise or morbidity.4
Exceptions in the treatment of hypertensive emergencies (particularly of specific disease states) will be discussed below, along with other treatment considerations. Patient comorbidities, for example, must be considered in the choice of antihypertensive agents.
Focused Treatment for Specific Hypertensive Emergencies
Hypertensive encephalopathy. This condition, associated with severe hypertension, is indicated by an abrupt change in mental status. During this acute end-organ damage event, a failure of cerebral autoregulation occurs, with increased pressure in the vascular endothelium leading to arteriole dilation that in turn can result in hyperperfusion of the brain, cerebral edema, and microhemorrhages.23
Because presentation of hypertensive encephalopathy may be similar to that in patients with acute stroke, hemorrhage, or brain lesions, these and other potential causes must be ruled out. While blood pressure treatment goals correspond with general recommendations,5,6 caution must be taken not to reduce blood pressure too swiftly; thus, continuous monitoring is warranted. If the patient's neurologic function worsens, treatment should be suspended and blood pressure allowed to rise slowly.4
Preferred antihypertensives for patients with hypertensive encephalopathy include labetalol, nicardipine, and fenoldopam23 (see Table 34-6,21,23). Centrally acting antihypertensives, such as clonidine, methyldopa, or reserpine,24 should not be used, as they can cause central nervous system depression and may cloud the patient's sensorium further.
Myocardial ischemia/infarction. During an acute hypertensive event, the workload on the heart and activation of the renin-angiotensin-aldosterone system can lead to acute coronary ischemia or infarction.23 Treatment is aimed at increasing blood flow to the myocardium and reducing the workload on the heart. Antihypertensives are combined with reperfusion (eg, angioplasty) and/or thrombolytics to preserve myocardial structure and function. Standard agents to reduce blood pressure include IV nitroglycerin and β-blockers. Systolic blood pressure is reduced until symptoms subside or diastolic blood pressure is reduced to 100 mm Hg or lower. Adjuncts such as morphine and oxygen are used to reduce patient discomfort and improve oxygen delivery to the myocardium.4
Acute left ventricular failure. In this potential manifestation of hypertensive emergency, the left ventricle initially attempts to compensate for rising blood pressure and becomes hypertrophic. Once the myocardium can no longer meet the demand, left ventricular function decompensates, causing a flow backup that leads to acute pulmonary edema.23
Blood pressure goals mirror those in the general treatment recommendations but focus specifically on reducing preload and afterload, improving myocardial contractility and decreasing peripheral vascular resistance. The preferred agents in this setting are IV nitroglycerin and ACE inhibitors, along with loop diuretics, morphine, and oxygen.4 Medications that increase workload on the heart (eg, hydralazine, clonidine) should be avoided.23
Aortic dissection. This is a true medical emergency that can result in significant morbidity and mortality. Type A dissection occurs proximally, at the ascending aorta, whereas type B dissection occurs at the level of the descending aorta. Typically, type B dissection is managed medically, as surgical treatment carries a significant risk for paralysis.4 Both types of aortic dissection are strongly associated with uncontrolled hypertension and in some patients may be precipitated by an acute hypertensive event. In such cases, the goal for blood pressure reduction is to decrease the shearing forces associated with the dissection. This is accomplished by lowering both blood pressure and pulse rate.12
While cases of type A dissection are usually managed surgically, all affected patients will require some component of medical management and tight blood pressure control. The current recommendation for blood pressure in aortic dissection is swift downward titration to a goal systolic blood pressure of 100 to 110 mm Hg.4 A β-blocker in combination with a vasodilator, administered intravenously, should be used for swift blood pressure reduction.4,25
IV nitroprusside, a potent vasodilator, is the preferred agent, but its use requires intra-arterial blood pressure monitoring.23 Because nitroprusside is metabolized to cyanide, its use can lead to lethal toxicity, especially in patients with hepatic or renal impairment.11 In this patient population, IV labetalol or esmolol may be used instead.4,25
Acute renal failure. In the setting of an acute hypertensive episode, it is often difficult to determine whether acute renal failure is the cause or the effect. Regardless, rapid reduction in blood pressure is warranted to preserve renal function and to stop the cycle of microvascular kidney destruction. Blood pressure goals are aligned with the general treatment recommendations. The preferred antihypertensive agent is IV fenoldopam, a dopamine receptor agonist that directly dilates renal arterioles, improving renal perfusion and promoting diuresis.4,26 Nicardipine, a calcium channel blocker, may be considered as an alternative.23,27
Treatment Considerations in Stroke
Elevated blood pressure is common in the early stages of stroke. Numerous studies have analyzed overall outcomes in patients presenting with ischemic stroke and uncontrolled hypertension. In this setting, evidence suggests a poorer prognosis in patients treated aggressively with antihypertensive agents.4,21,22
The association between dramatic reduction in blood pressure and poor prognoses lies in the theory of the ischemic penumbra. This is an area around the core of ischemic tissue that receives enough blood flow to maintain neuronal activity for a few hours after initial injury, but this tissue is susceptible to further infarction. Precipitous drops in blood pressure can reduce blood flow to collateral vessels, resulting in hypoperfusion of the penumbra and leading to further neurologic damage.
Details and current treatment recommendations for each of the various types of stroke follow.
Acute intracerebral hemorrhage. Uncontrolled hypertension is often associated with intracerebral hemorrhage (ICH), either as a risk factor or a factor that contributes to the event. Once a patient has experienced an acute brain insult, blood pressure can become even more uncontrolled. Extension of the hematoma and a worsening outcome are the main concerns in treating the patient with concomitant blood pressure elevation and ICH. Also of concern is maintaining adequate perfusion to the penumbra. Additionally, transient hypoperfusion can develop when the ICP is elevated and the mean arterial pressure (MAP) is acutely lowered, thus reducing the cerebral perfusion pressure (CPP; CPP = MAP - ICP).
Researchers have acknowledged there is insufficient evidence to offer management guidelines for blood pressure reduction in patients with ICH.28 The 2007 recommendations from the American Heart Association/ American Stroke Association (AHA/ASA) for blood pressure management in patients with acute ICH29 are as follows: In the setting of ICH in patients with uncontrolled blood pressure, treatment should be aggressive if systolic blood pressure exceeds 200 mm Hg or MAP exceeds 150 mm Hg. A treatment goal to consider is reducing systolic blood pres sure to 160 mm Hg or less (or MAP to below 130 mm Hg).29 Patients with elevated ICP should undergo placement of a ventriculostomy to maintain a CPP between 60 and 80 mm Hg, although the risk for infection or intracerebral hemorrhage must be weighed against the potential benefits.29,30
When blood pressure reduction is required, the MAP should not be lowered more than 20% in a 24-hour period. Recommended agents include IV nicardipine, labetalol, enalapril, hydralazine, or esmolol.31
Acute ischemic stroke. Long-term control of blood pressure in patients who have experienced stroke remains undisputed, as it improves outcomes. However, in the setting of acute ischemic stroke (AIS), initiating blood pressure control is more liberal. Optimal control of blood pressure during management of AIS is imperative to reduce morbidity and mortality.32 Areas affected by edematous brain tissue are at increased risk for bleeding (ie, hemorrhagic expansion).
Patients who present with AIS require careful history taking to elicit their average blood pressure range; this will help the clinician determine goal pressures during management of the acute stroke phase.33 The primary rationale for treating blood pressure in this acute setting is to prevent hemorrhagic expansion at sites with potential for bleeding.34
According to the 2007 AHA/ ASA recommendations for management of blood pressure in AIS,35 patients who are eligible for thrombolysis should have a systolic blood pressure goal below 180 mm Hg and diastolic blood pressure below 105 mm Hg. Patients who will not receive thrombolytics should have blood pressure lowered only if systolic blood pressure exceeds 220 mm Hg or diastolic blood pressure exceeds 110 mm Hg.35,36 Appropriately refraining from reducing blood pressure is known as permissive hypertension.
Given the fragility of the cerebral brain tissue after AIS, permissive hypertension is intended to protect the penumbra and preserve cerebral blood flow. In patients who require blood pressure reduction because of other medical conditions (eg, decompensated heart failure), blood pressure should not be lowered more than 10% to 15% in a 24-hour period.9,31,36 No specific antihypertensives are preferred in patients with AIS: IV enalapril, esmolol, labetalol, or nicardipine can be used.31
Subarachnoid hemorrhage. The two complications of a subarachnoid hemorrhage (SAH) that most contribute to morbidity and mortality are rebleeding and vasospasms; elevated blood pressure can contribute to both. Thus, blood pressure control in patients with SAH is imperative.
Patients with acute SAH often require blood pressure monitoring via arterial line, as well as ICP monitoring. Blood pressure goals are similar to those in patients with ICH. The preferred agent for blood pressure control is nimodipine, which offers the secondary benefit of vasospasm prevention.37
CONCLUSION
Patients presenting with urgent or emergent hypertension need expeditious evaluation to avoid the significant morbidity and mortality associated with acute end-organ damage. Hypertensive urgency is defined as a diastolic blood pressure of greater than 120 mm Hg without evidence of end-organ damage.
In cases of hypertensive emergency, in which acute end-organ damage is present, lowering blood pressure should be directed by the type of end-organ damage and/or underlying comorbidities. In general, blood pressure should not be lowered more than 10% to 25% within the first hour, with normalization achieved over the next 8 to 24 hours.
In cases of acute ischemic stroke, permissive hypertension is recommended. Above all, treat patients, not numbers, bearing in mind the Hippocratic oath: Primum non nocere, or "First, do no harm."
REFERENCES
- Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6): 1949-1962.
- Guo F, He D, Zhang W, Walton RG. Trends in Prevalence, Awareness, Management, and Control of Hypertension Among United States Adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599-606.
- Flanigan JS, Vitberg D. Hypertensive emergency and severe hypertension: what to treat, who to treat, and how to treat. Med Clin North Am. 2006;90(3):439-451.
- Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
- National High Blood Pressure Education Program Coordinating Committee, National Heart Lung and Blood Institute, NIH. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No. 04-5230. August 2004. www .nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed September 20, 2012.
- Houston M. Hypertensive emergencies and urgencies: pathophysiology and clinical aspects. Am Heart J. 1986;111(1):205-210.
- Kessler CS, Joudeh Y. Evaluation and treatment of severe asymptomatic hypertension. Am Fam Physician. 2010;81(4):470-476.
- Vaidya CK, Ouellette JR. Hypertensive urgency and emergency. Hosp Physician. Mar 2007:43-50. www.turner-white.com/memberfile.php?Pub Code=hp_mar07_hypertensive.pdf. Accessed September 20, 2012.
- Perez MI, Musini VM. Pharmacological interventions for hypertensive emergencies: a Cochrane systematic review. J Hum Hypertens. 2008;22(9):596-607.
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411-417.
- Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006; 33(3):613-623.
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 suppl B):9-20.
- Flack JM. Epidemiology and unmet needs in hypertension. J Manag Care Pharm. 2007;13(e suppl B):2-8.
- Haas AR, Marik PE. Current diagnosis and management of hypertensive emergency. Semin Dial. 2006;19(6):502-512.
- Hebert CJ, Vidt DG. Hypertensive crises. Prim Care. 2008;35(3):475-487.
- Shulman KI, Fischer HD, Herrmann N, et al. Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiatry. 2009;70(12):1681-1696.
- Musso NR, Vergassola C, Pende A, Lotti G. Yohimbine effects on blood pressure and plasma catecholamines in human hypertension. Am J Hypertens. 1995;8(6):565-571.
- Baid S, Nieman LK. Glucocorticoid excess and hypertension. Curr Hypertens Rep. 2004;6(6): 493-499.
- Society of Critical Care Medicine. Fundamental Critical Care Support Course. www.sccm.org/ fccs_and_training_courses/fccs/pages/default .aspx. Accessed September 20, 2012.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.
- Bernardini GL, Yavagal DR. Management of ischemic stroke: current concepts and treatment options. Hosp Physician. Sep 2006:13-23. www.turner-white.com/memberfile.php?PubCode=hp_sep06_is chemic.pdf. Accessed September 20, 2012.
- Varon J. Treatment of acute severe hypertension: current and newer agents. Drugs. 2008;68(3): 283-297.
- Webster J, Koch HF. Aspects of tolerability of centrally acting antihypertensive drugs. J Cardiovasc Pharmacol. 1996;27 suppl 3:S49-S54.
- Gupta PK, Gupta H, Khoynezhad A. Hypertensive emergency in aortic dissection and thoracic aortic aneurysm: a review of management. Pharmaceuticals. 2009;2(3):66-76.
- Post JB 4th, Frishman WH. Fenoldopam: a new dopamine agonist for the treatment of hypertensive urgencies and emergencies. J Clin Pharmacol. 1998;38(1):2-13.
- Suzuki S, Ohtsuka S, Ishikawa K, Yamaguchi I. Effects of nicardipine on coronary, vertebral and renal arterial flows in patients with essential hypertension. Hypertens Res. 2003;26(3):193-199.
- Anderson CS, Huang Y, Arima H, et al; INTERACT Investigators. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41(2):307-312.
- Broderick J, Connolly S, Feldmann E, et al. Quality of Care and Outcomes in Research Interdisciplinary Working Group Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):e391-413.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Brott T, Lu M, Kothari R, et al. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29(8):1504-1509.
- Aiyagari V, Badruddin A. Management of hypertension in acute stroke. Expert Rev Cardiovasc Ther. 2009;7(6):637-646.
- Castillo J, Leira R, García MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35(2):520-526.
- Bonita R, Beaglehole R. The enigma of the decline in stroke deaths in the United States the search for an explanation. Stroke. 1996;27(3): 370-372.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655-1711.
- Heitsch L, Jauch EC. Management of hypertension in the setting of acute ischemic stroke. Curr Hypertens Rep. 2007;9(6):506-511.
- Barker FG II, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405-414.
An estimated 1% to 2% of patients with chronic hypertension will at some time develop hypertensive urgency or emergency.1 According to recent data from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2010,2 the prevalence of hypertension has remained stable at 30.5% among men and 28.5% among women in the United States; however, 74% of the hypertensive population is unaware of having this condition. Furthermore, 71.6% of hypertensive patients are managed for the condition, and in only 46.5% is blood pressure well controlled.2
In 2006, essential hypertension was estimated to account for more than 44 million emergency department visits in the US. The direct and indirect costs of hypertension totaled $73 billion in 2009.3,4
NEW TERMINOLOGY AND CLASSIFICATION
The terms malignant hypertension, hypertensive crisis, and accelerated hypertension have been replaced by hypertensive urgency or hypertensive emergency. Hypertensive urgency and emergency are differentiated by the absence or presence of acute end-organ damage, respectively.
Given the inconsistent terminology used, database searches can be challenging. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7),5,6 published in 2003, is considered the gold standard for categorizing hypertension in the outpatient setting. The JNC7 authorsclassify normal blood pressure as < 120/< 80 mm Hg. The document further classifies blood pressure into the stages shown in Table 1.5,6 Blood pressure higher than 180 mm Hg systolic and/or 120 mm Hg diastolic is generally considered severe hypertension— a designation that includes hypertensive urgency and hypertensive emergency.6
What Defines Hypertensive Urgency/Emergency?
Hypertensive urgency is defined as a diastolic blood pressure of 110 mm Hg or greater without the acute signs of end-organ damage.7 Some sources suggest that a patient must also have certain risk factors (eg, heart disease, renal disease) to be given this diagnosis.8 The presence of acute and rapidly evolving end-organ damage with an elevated diastolic blood pressure, usually greater than 120 mm Hg, establishes a diagnosis of hypertensive emergency.6,8,9
No specific blood pressure measurement indicates a hypertensive emergency, however; rather, the defining feature of this diagnosis is the presence of progressive target end-organ damage.7 This is most commonly manifested in cardiopulmonary, central nervous system, and/or renal findings; for the specific forms of end-organ damage, see Table 2.5,6,10,11 Preeclampsia and eclampsia are also considered manifestations of hypertensive end-organ damage but are beyond the scope of this article.5,11
The most common form of organ damage associated with hypertension is ischemic heart disease, in the form of either heart failure or acute coronary syndrome.12
PATHOPHYSIOLOGY
Blood pressure is calculated by cardiac output (ie, stroke volume multiplied by heart rate) multiplied by total peripheral resistance. Total peripheral resistance is influenced by a variety of humoral and neural factors, also known as vasoactive substances (see Figure 13,4). During an episode of acute hypertension, a failure of autoregulatory function occurs, precipitated by one or more of a host of potential causes. This failure of autoregulation then leads to increased systemic vascular resistance. In the setting of end-organ damage, release of inflammatory markers ensues, which ultimately causes endovascular injury and fibrin necrosis of arterioles.4,10,11
The renin-angiotensin-aldosterone system also plays a significant role in the cascade of hypertension, stimulating decreased renal perfusion and lowering tubular sodium concentration. This in turn stimulates aldosterone to increase blood pressure by maintaining excess volume through sodium retention and potassium excretion, further potentiating the cycle of uncontrolled blood pressure.4,13,14
Patients with chronically elevated blood pressures have a compensatory response, lying in the threshold mechanism, that protects against end-organ damage. Acute changes in blood pressure are better tolerated in these patients because of their decreased propensity for hypoperfusion.4 In contrast, normotensive patients who experience precipitous changes in blood pressure are at increased risk for organ hypoperfusion. The main concern regarding organ hypoperfusion is that it can lead to ischemia4 (see Figure 23,4).
PATIENT HISTORY
Acute hypertensive urgency or emergency can be triggered by many factors. Systemic etiologies (including kidney disease) caused by immunologic mediators or renal artery stenosis can cause or exacerbate hypertension. The patient should be asked about his or her normal blood pressure range, as this may offer clues to medication compliance. Rebound hypertension can be seen in patients who abruptly discontinue medications such as clonidine or β-blockers, as this causes an increase in sympathetic outflow.9,15
All patients should be queried regarding their use of OTC medications and other drug use, including cocaine, methamphetamines, phencyclidine, and alcohol.1,4,11,16 Patients taking monoamine oxidase inhibitors (MAOIs) are at increased risk for serious medication interactions; concomitant administration of MAOIs with other antidepressants can lead to a hypertensive reaction, but also to serotonin syndrome.1,17 Because MAOIs inhibit the breakdown of tyramine, patients taking them should avoid tyramine-containing foods and herbal supplements (including, but not limited to, St. John's wort, ginseng, and yohimbine).1,15,18
Acute hypertensive episodes can also occur as a result of preeclampsia or eclampsia in pregnant women, pheochromocytoma, primary aldosteronism, glucocorticoid excess (Cushing syndrome), or central nervous system disorders (eg, cerebrovascular accident, head trauma, brain tumors).9,11,19
PHYSICAL EXAMINATION
The purpose of the physical examination is to determine whether end-organ damage is present.1,11 The fundoscopic exam may reveal papilledema, a sign of increased intracranial pressure (ICP). Flame hemorrhages, cotton wool spots or arteriovenous nicking suggest a long-standing history of uncontrolled hypertension or diabetes.7,9 The neck should be assessed for jugular venous distention, which may be elevated in decompensated heart failure or pulmonary edema.11
The cardiac exam may reveal an irregular rate and rhythm, displaced apical pulse, gallop, or murmur. On pulmonary exam, rales may be auscultated, suggestive of pulmonary edema.9,15
The abdominal exam should include listening for a renal artery bruit.1 The neurologic exam may demonstrate altered mental status (possibly indicating hypertensive encephalopathy) or focal findings, if the patient has had an underlying ischemic or hemorrhagic event.9
LABORATORY STUDIES AND IMAGING
In most cases, a serum chemistry panel is warranted to identify any renal dysfunction. Urinalysis may reveal proteinuria, possibly indicating renal damage.4,9,15
Any patient complaining of chest pain should have an ECG to look for ischemic changes or presence of a left bundle branch block, and serial cardiac enzymes to rule out acute coronary syndrome.15 Access to previous ECGs is helpful in differentiating between new and old conductive abnormalities.
A chest x-ray should be performed in patients who complain of shortness of breath and/or chest pain. A widened mediastinum can represent aortic dissection.4,15 Evidence of pulmonary edema should prompt the clinician to assess for left ventricular dysfunction or valvular insufficiencies by echocardiogram. Chest CT should be pursued in patients with clinical suspicion for dissection.1,15,20
Patients presenting with a headache or focal neurologic abnormalities warrant a head CT to rule out stroke.15 Urine drug screening is appropriate if the patient history suggests illicit drug use.12
"FIRST, DO NO HARM"
Treatment of hypertensive emergency and urgency varies from traditional treatment for hypertension. Aggressive blood pressure control in patients presenting with acute ischemic stroke has been associated with poorer patient outcomes.21,22 Thus, treating the patient and not the numbers is the first general recommendation for treatment of hypertensive emergency and urgency. It is important for the clinician to remember the Hippocratic oath, "First do no harm," when treating these patients.
Other general recommendations are derived from theory, physiology, and smaller clinical trials; their application must be individualized according to the patient's needs. These recommendations include aiming for a reduction in mean arterial blood pressure of no more than 10% to 25% within the first hour, a goal blood pressure of 160/90 mm Hg within the first 8 hours, and normalization of blood pressure over 8 to 24 hours.12
While the use of pharmacologic agents may be warranted, it is important to consider that elevated blood pressure may be a reaction to pain or stress and may be best treated alternatively. Recommendations for permissive hypertension in acute ischemic stroke will be discussed below.
TREATMENT: HYPERTENSIVE URGENCY
The treatment of hypertensive urgency is usually immediate and warrants close follow-up. Although elevated blood pressures can be alarming to the patient, hypertensive urgency usually develops over days to weeks.8 In this setting, it is not necessary to lower blood pressure acutely.12 A rapid decrease in blood pressure can actually cause symptomatic hypotension, resulting in hypoperfusion to the brain.5,6,8
After ruling out end-organ damage, the next step is to treat according to the guidelines for hypertensive urgency.5,6 These recommendations include the use of rapid-onset oral antihypertensive agents, such as clonidine, labetalol, or captopril.23 Use of these agents is only suggested for gradual, short-term reduction of blood pressure (ie, over 24 to 48 hours) while the patient is being monitored for potential hypertension-related organ damage, either in the emergency department or in an observational hospital setting.5,6,23
Once the short-acting agents have adequately reduced blood pressure, long-term agents can be chosen to prevent rebound hypertension.16 Patients are typically monitored for 24 hours in the hospital during this transition. Upon discharge, the patient should be scheduled for follow-up within one to two days.11 Patient education, including a discussion of medication adherence, weight loss, and reduced dietary salt, is key to prevent recurrences and optimize overall treatment compliance.
TREATMENT: HYPERTENSIVE EMERGENCY
Treatment of hypertensive emergency always warrants hospitalization, usually in the ICU.5,6 IV antihypertensive medications (eg, nicardipine, fenoldopam, labetalol, esmolol, phentolamine) are preferred. Their use often necessitates continuous blood pressure monitoring via arterial line, allowing the clinician to perform ongoing medication titration. In hypertensive emergencies, the purpose of treatment is to preserve brain, kidney, and heart function.4
Goal-directed therapy is initiated even before the patient evaluation has been fully completed. Patient assessment continues after treatment is begun to avoid overly aggressive blood pressure reduction, which can increase the risk for patient demise or morbidity.4
Exceptions in the treatment of hypertensive emergencies (particularly of specific disease states) will be discussed below, along with other treatment considerations. Patient comorbidities, for example, must be considered in the choice of antihypertensive agents.
Focused Treatment for Specific Hypertensive Emergencies
Hypertensive encephalopathy. This condition, associated with severe hypertension, is indicated by an abrupt change in mental status. During this acute end-organ damage event, a failure of cerebral autoregulation occurs, with increased pressure in the vascular endothelium leading to arteriole dilation that in turn can result in hyperperfusion of the brain, cerebral edema, and microhemorrhages.23
Because presentation of hypertensive encephalopathy may be similar to that in patients with acute stroke, hemorrhage, or brain lesions, these and other potential causes must be ruled out. While blood pressure treatment goals correspond with general recommendations,5,6 caution must be taken not to reduce blood pressure too swiftly; thus, continuous monitoring is warranted. If the patient's neurologic function worsens, treatment should be suspended and blood pressure allowed to rise slowly.4
Preferred antihypertensives for patients with hypertensive encephalopathy include labetalol, nicardipine, and fenoldopam23 (see Table 34-6,21,23). Centrally acting antihypertensives, such as clonidine, methyldopa, or reserpine,24 should not be used, as they can cause central nervous system depression and may cloud the patient's sensorium further.
Myocardial ischemia/infarction. During an acute hypertensive event, the workload on the heart and activation of the renin-angiotensin-aldosterone system can lead to acute coronary ischemia or infarction.23 Treatment is aimed at increasing blood flow to the myocardium and reducing the workload on the heart. Antihypertensives are combined with reperfusion (eg, angioplasty) and/or thrombolytics to preserve myocardial structure and function. Standard agents to reduce blood pressure include IV nitroglycerin and β-blockers. Systolic blood pressure is reduced until symptoms subside or diastolic blood pressure is reduced to 100 mm Hg or lower. Adjuncts such as morphine and oxygen are used to reduce patient discomfort and improve oxygen delivery to the myocardium.4
Acute left ventricular failure. In this potential manifestation of hypertensive emergency, the left ventricle initially attempts to compensate for rising blood pressure and becomes hypertrophic. Once the myocardium can no longer meet the demand, left ventricular function decompensates, causing a flow backup that leads to acute pulmonary edema.23
Blood pressure goals mirror those in the general treatment recommendations but focus specifically on reducing preload and afterload, improving myocardial contractility and decreasing peripheral vascular resistance. The preferred agents in this setting are IV nitroglycerin and ACE inhibitors, along with loop diuretics, morphine, and oxygen.4 Medications that increase workload on the heart (eg, hydralazine, clonidine) should be avoided.23
Aortic dissection. This is a true medical emergency that can result in significant morbidity and mortality. Type A dissection occurs proximally, at the ascending aorta, whereas type B dissection occurs at the level of the descending aorta. Typically, type B dissection is managed medically, as surgical treatment carries a significant risk for paralysis.4 Both types of aortic dissection are strongly associated with uncontrolled hypertension and in some patients may be precipitated by an acute hypertensive event. In such cases, the goal for blood pressure reduction is to decrease the shearing forces associated with the dissection. This is accomplished by lowering both blood pressure and pulse rate.12
While cases of type A dissection are usually managed surgically, all affected patients will require some component of medical management and tight blood pressure control. The current recommendation for blood pressure in aortic dissection is swift downward titration to a goal systolic blood pressure of 100 to 110 mm Hg.4 A β-blocker in combination with a vasodilator, administered intravenously, should be used for swift blood pressure reduction.4,25
IV nitroprusside, a potent vasodilator, is the preferred agent, but its use requires intra-arterial blood pressure monitoring.23 Because nitroprusside is metabolized to cyanide, its use can lead to lethal toxicity, especially in patients with hepatic or renal impairment.11 In this patient population, IV labetalol or esmolol may be used instead.4,25
Acute renal failure. In the setting of an acute hypertensive episode, it is often difficult to determine whether acute renal failure is the cause or the effect. Regardless, rapid reduction in blood pressure is warranted to preserve renal function and to stop the cycle of microvascular kidney destruction. Blood pressure goals are aligned with the general treatment recommendations. The preferred antihypertensive agent is IV fenoldopam, a dopamine receptor agonist that directly dilates renal arterioles, improving renal perfusion and promoting diuresis.4,26 Nicardipine, a calcium channel blocker, may be considered as an alternative.23,27
Treatment Considerations in Stroke
Elevated blood pressure is common in the early stages of stroke. Numerous studies have analyzed overall outcomes in patients presenting with ischemic stroke and uncontrolled hypertension. In this setting, evidence suggests a poorer prognosis in patients treated aggressively with antihypertensive agents.4,21,22
The association between dramatic reduction in blood pressure and poor prognoses lies in the theory of the ischemic penumbra. This is an area around the core of ischemic tissue that receives enough blood flow to maintain neuronal activity for a few hours after initial injury, but this tissue is susceptible to further infarction. Precipitous drops in blood pressure can reduce blood flow to collateral vessels, resulting in hypoperfusion of the penumbra and leading to further neurologic damage.
Details and current treatment recommendations for each of the various types of stroke follow.
Acute intracerebral hemorrhage. Uncontrolled hypertension is often associated with intracerebral hemorrhage (ICH), either as a risk factor or a factor that contributes to the event. Once a patient has experienced an acute brain insult, blood pressure can become even more uncontrolled. Extension of the hematoma and a worsening outcome are the main concerns in treating the patient with concomitant blood pressure elevation and ICH. Also of concern is maintaining adequate perfusion to the penumbra. Additionally, transient hypoperfusion can develop when the ICP is elevated and the mean arterial pressure (MAP) is acutely lowered, thus reducing the cerebral perfusion pressure (CPP; CPP = MAP - ICP).
Researchers have acknowledged there is insufficient evidence to offer management guidelines for blood pressure reduction in patients with ICH.28 The 2007 recommendations from the American Heart Association/ American Stroke Association (AHA/ASA) for blood pressure management in patients with acute ICH29 are as follows: In the setting of ICH in patients with uncontrolled blood pressure, treatment should be aggressive if systolic blood pressure exceeds 200 mm Hg or MAP exceeds 150 mm Hg. A treatment goal to consider is reducing systolic blood pres sure to 160 mm Hg or less (or MAP to below 130 mm Hg).29 Patients with elevated ICP should undergo placement of a ventriculostomy to maintain a CPP between 60 and 80 mm Hg, although the risk for infection or intracerebral hemorrhage must be weighed against the potential benefits.29,30
When blood pressure reduction is required, the MAP should not be lowered more than 20% in a 24-hour period. Recommended agents include IV nicardipine, labetalol, enalapril, hydralazine, or esmolol.31
Acute ischemic stroke. Long-term control of blood pressure in patients who have experienced stroke remains undisputed, as it improves outcomes. However, in the setting of acute ischemic stroke (AIS), initiating blood pressure control is more liberal. Optimal control of blood pressure during management of AIS is imperative to reduce morbidity and mortality.32 Areas affected by edematous brain tissue are at increased risk for bleeding (ie, hemorrhagic expansion).
Patients who present with AIS require careful history taking to elicit their average blood pressure range; this will help the clinician determine goal pressures during management of the acute stroke phase.33 The primary rationale for treating blood pressure in this acute setting is to prevent hemorrhagic expansion at sites with potential for bleeding.34
According to the 2007 AHA/ ASA recommendations for management of blood pressure in AIS,35 patients who are eligible for thrombolysis should have a systolic blood pressure goal below 180 mm Hg and diastolic blood pressure below 105 mm Hg. Patients who will not receive thrombolytics should have blood pressure lowered only if systolic blood pressure exceeds 220 mm Hg or diastolic blood pressure exceeds 110 mm Hg.35,36 Appropriately refraining from reducing blood pressure is known as permissive hypertension.
Given the fragility of the cerebral brain tissue after AIS, permissive hypertension is intended to protect the penumbra and preserve cerebral blood flow. In patients who require blood pressure reduction because of other medical conditions (eg, decompensated heart failure), blood pressure should not be lowered more than 10% to 15% in a 24-hour period.9,31,36 No specific antihypertensives are preferred in patients with AIS: IV enalapril, esmolol, labetalol, or nicardipine can be used.31
Subarachnoid hemorrhage. The two complications of a subarachnoid hemorrhage (SAH) that most contribute to morbidity and mortality are rebleeding and vasospasms; elevated blood pressure can contribute to both. Thus, blood pressure control in patients with SAH is imperative.
Patients with acute SAH often require blood pressure monitoring via arterial line, as well as ICP monitoring. Blood pressure goals are similar to those in patients with ICH. The preferred agent for blood pressure control is nimodipine, which offers the secondary benefit of vasospasm prevention.37
CONCLUSION
Patients presenting with urgent or emergent hypertension need expeditious evaluation to avoid the significant morbidity and mortality associated with acute end-organ damage. Hypertensive urgency is defined as a diastolic blood pressure of greater than 120 mm Hg without evidence of end-organ damage.
In cases of hypertensive emergency, in which acute end-organ damage is present, lowering blood pressure should be directed by the type of end-organ damage and/or underlying comorbidities. In general, blood pressure should not be lowered more than 10% to 25% within the first hour, with normalization achieved over the next 8 to 24 hours.
In cases of acute ischemic stroke, permissive hypertension is recommended. Above all, treat patients, not numbers, bearing in mind the Hippocratic oath: Primum non nocere, or "First, do no harm."
REFERENCES
- Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6): 1949-1962.
- Guo F, He D, Zhang W, Walton RG. Trends in Prevalence, Awareness, Management, and Control of Hypertension Among United States Adults, 1999 to 2010. J Am Coll Cardiol. 2012;60(7):599-606.
- Flanigan JS, Vitberg D. Hypertensive emergency and severe hypertension: what to treat, who to treat, and how to treat. Med Clin North Am. 2006;90(3):439-451.
- Aggarwal M, Khan IA. Hypertensive crisis: hypertensive emergencies and urgencies. Cardiol Clin. 2006;24(1):135-146.
- Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572.
- National High Blood Pressure Education Program Coordinating Committee, National Heart Lung and Blood Institute, NIH. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. NIH Publication No. 04-5230. August 2004. www .nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf. Accessed September 20, 2012.
- Houston M. Hypertensive emergencies and urgencies: pathophysiology and clinical aspects. Am Heart J. 1986;111(1):205-210.
- Kessler CS, Joudeh Y. Evaluation and treatment of severe asymptomatic hypertension. Am Fam Physician. 2010;81(4):470-476.
- Vaidya CK, Ouellette JR. Hypertensive urgency and emergency. Hosp Physician. Mar 2007:43-50. www.turner-white.com/memberfile.php?Pub Code=hp_mar07_hypertensive.pdf. Accessed September 20, 2012.
- Perez MI, Musini VM. Pharmacological interventions for hypertensive emergencies: a Cochrane systematic review. J Hum Hypertens. 2008;22(9):596-607.
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411-417.
- Stewart DL, Feinstein SE, Colgan R. Hypertensive urgencies and emergencies. Prim Care. 2006; 33(3):613-623.
- Atlas SA. The renin-angiotensin aldosterone system: pathophysiological role and pharmacologic inhibition. J Manag Care Pharm. 2007;13(8 suppl B):9-20.
- Flack JM. Epidemiology and unmet needs in hypertension. J Manag Care Pharm. 2007;13(e suppl B):2-8.
- Haas AR, Marik PE. Current diagnosis and management of hypertensive emergency. Semin Dial. 2006;19(6):502-512.
- Hebert CJ, Vidt DG. Hypertensive crises. Prim Care. 2008;35(3):475-487.
- Shulman KI, Fischer HD, Herrmann N, et al. Current prescription patterns and safety profile of irreversible monoamine oxidase inhibitors: a population-based cohort study of older adults. J Clin Psychiatry. 2009;70(12):1681-1696.
- Musso NR, Vergassola C, Pende A, Lotti G. Yohimbine effects on blood pressure and plasma catecholamines in human hypertension. Am J Hypertens. 1995;8(6):565-571.
- Baid S, Nieman LK. Glucocorticoid excess and hypertension. Curr Hypertens Rep. 2004;6(6): 493-499.
- Society of Critical Care Medicine. Fundamental Critical Care Support Course. www.sccm.org/ fccs_and_training_courses/fccs/pages/default .aspx. Accessed September 20, 2012.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577-617.
- Bernardini GL, Yavagal DR. Management of ischemic stroke: current concepts and treatment options. Hosp Physician. Sep 2006:13-23. www.turner-white.com/memberfile.php?PubCode=hp_sep06_is chemic.pdf. Accessed September 20, 2012.
- Varon J. Treatment of acute severe hypertension: current and newer agents. Drugs. 2008;68(3): 283-297.
- Webster J, Koch HF. Aspects of tolerability of centrally acting antihypertensive drugs. J Cardiovasc Pharmacol. 1996;27 suppl 3:S49-S54.
- Gupta PK, Gupta H, Khoynezhad A. Hypertensive emergency in aortic dissection and thoracic aortic aneurysm: a review of management. Pharmaceuticals. 2009;2(3):66-76.
- Post JB 4th, Frishman WH. Fenoldopam: a new dopamine agonist for the treatment of hypertensive urgencies and emergencies. J Clin Pharmacol. 1998;38(1):2-13.
- Suzuki S, Ohtsuka S, Ishikawa K, Yamaguchi I. Effects of nicardipine on coronary, vertebral and renal arterial flows in patients with essential hypertension. Hypertens Res. 2003;26(3):193-199.
- Anderson CS, Huang Y, Arima H, et al; INTERACT Investigators. Effects of early intensive blood pressure-lowering treatment on the growth of hematoma and perihematomal edema in acute intracerebral hemorrhage: the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT). Stroke. 2010;41(2):307-312.
- Broderick J, Connolly S, Feldmann E, et al. Quality of Care and Outcomes in Research Interdisciplinary Working Group Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):e391-413.
- Morgenstern LB, Hemphill JC 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108-2129.
- Brott T, Lu M, Kothari R, et al. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29(8):1504-1509.
- Aiyagari V, Badruddin A. Management of hypertension in acute stroke. Expert Rev Cardiovasc Ther. 2009;7(6):637-646.
- Castillo J, Leira R, García MM, et al. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35(2):520-526.
- Bonita R, Beaglehole R. The enigma of the decline in stroke deaths in the United States the search for an explanation. Stroke. 1996;27(3): 370-372.
- Adams HP Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(5):1655-1711.
- Heitsch L, Jauch EC. Management of hypertension in the setting of acute ischemic stroke. Curr Hypertens Rep. 2007;9(6):506-511.
- Barker FG II, Ogilvy CS. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405-414.
Developing Renal Education Classes
Q: We are trying to develop renal education classes in our hospital’s general medical clinic. Participating patients (pre-renal) will be those we hope can be managed by their primary care providers in coordination with our nephrology specialists before their initial renal clinic visits. Our team of educators will include an RN, an NP, a primary care physician, and a nephrologist. Any information, models, and/or links to educational resources would be much appreciated.
Everyone loses 1% of kidney function per year after age 40. If we lived long enough, all of us would need renal education!
As you try to develop classes, one of your first concerns will be whether you want to charge for them. If they are meant to be billed for, they will take a much different form than a free kidney disease education class would. Let’s explore both.
PAID CLASSES
Only Medicare pays for education classes, and patients must be at stage 4 kidney disease (ie, glomerular filtration rate [GFR], 15 to 30 mL/dL). The class can be taught in a group or an individualized format, and an RN, a dietician, or a social worker can assist—but the bulk of the class must be taught by a practitioner with a National Provider Identifier billing number (an NP, a PA, or a physician).
Medicare specifies the content of the classes and has set certain requirements regarding a class’s site and length. In addition, there must be preevaluation and postevaluation tools in place, and the number of classes over a patient’s lifetime is limited to six.
The best program available (one that contains all the needed tools, slide sets, and handouts) is Your Treatment, Your Choice8 from the National Kidney Foundation (www.kidney.org/profes sionals/KLS/YTYC.cfm). It is free, but you must be a PA, an NP, or an MD to request it.
NONPAID CLASSES AND PROGRAMS
These can be given by anybody, and the format is up to the teacher. Prevention always trumps a cure, and preventing advanced kidney disease (GFR < 60 mL/dL) fits in very well in general practice. Promoting good health habits is a common goal. To that end, instruction regarding diet, blood pressure control, blood sugar control, and smoking cessation all help slow kidney disease progression.
What’s best about offering classes like these is that you don’t have to reinvent the wheel. There are some fantastic free programs out there. Some of our favorites are available through the National Kidney Disease Education Program (NKDEP) Web site: http://nkdep.nih.gov/resources.shtml. This is a division of one of the National Institutes of Health, paid for by your tax dollars, and it offers free or very inexpensive handouts, videos, and slide sets, all written at an eighth-grade reading level.
Among the materials offered is a phenomenal tear-off sheet, “Explaining Your Kidney Test Results,” which is available in English, Spanish, Chinese, and Vietnamese (with the first five copies free, then $1 each). It illustrates the stages of kidney function using the traffic light scenario: green, yellow, or red (stage 5 CKD is the red zone) and explains what patients can do to “stay out of the red.” We consider this one of the most effective tools we can use.
NKDEP also offers free handouts listing foods high in potassium, phosphorus, protein, and sodium. Nothing is as good as a renal dietician, but these forms are an excellent alternative.
NKDEP allows you to download and reprint almost all of their information free, or you can request 50 copies of just about any item at no cost. Put your best shopper on the Web site. The amount of materials offered is truly wonderful, and you can’t beat the price.
Another program is called Kidney School (http://kidneyschool
.org), a nonprofit organization set up by the kidney community that offers all kinds of videos and slide sets at no charge.
Last, but certainly not least, is Seymour Jones and the Temple of CKD, a five-minute video put out by the Renal Support Network (RSN; www.rsnhope.org). You can request the video from RSN or find it on YouTube (www.youtube.com/watch?v=lDJZHIVTNzo). Though hilarious, it makes excellent points about the symptoms of chronic kidney disease.
As you can see, there are many wonderful and varied (and free!) programs out there.
With the double-whammy of an aging population and increasing obesity, the number of people with kidney disease is growing exponentially; the past 20 years have seen a 67% increase in the number of patients with CKD, which now affects more than 20 million Americans. Yet in that same 20-year period, effective treatments have been developed for CKD that “can delay and, in some cases, prevent ESRD.”9 Patients with CKD need not assume there will be dialysis in their future.
Most importantly of all, we need to get out there and talk up prevention.
Kim Zuber, PA-C; Jane S. Davis, DNP, CRNP
REFERENCES
1. K/DOQI [Kidney Disease Outcome Quality Initiative] clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
2. Reilly RF, Jackson EK. Ch 25. Regulation of renal function and vascular volume. In: Chabner BA, Brunton LL, Knollman BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill Professional; 2010.
3. Sica DA, Gehr TW. Diuretic use in stage 5 chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2003;12(5): 483-490.
4. Cohen DL, Townsend RR. Treatment of hypertension in patients with chronic kidney disease. US Cardiology. 2009;6(2):54-58.
5. Wickersham RM, ed. Drug Facts and Comparisons. St. Louis, MO: Wolters Kluwer Health; 2009.
6. Comparison of commonly used diuretics (Detail Document). Pharmacist’s Letter/Prescriber’s Letter. February 2012.
7. DRUGDEX® System [Internet database]. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc. Updated periodically.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed September 19, 2012.
9. Turner JM, Bauer C, Abramowitz MK, et al. Treatment of chronic kidney disease. Kidney Int. 2012;81(4):351-362.
Q: We are trying to develop renal education classes in our hospital’s general medical clinic. Participating patients (pre-renal) will be those we hope can be managed by their primary care providers in coordination with our nephrology specialists before their initial renal clinic visits. Our team of educators will include an RN, an NP, a primary care physician, and a nephrologist. Any information, models, and/or links to educational resources would be much appreciated.
Everyone loses 1% of kidney function per year after age 40. If we lived long enough, all of us would need renal education!
As you try to develop classes, one of your first concerns will be whether you want to charge for them. If they are meant to be billed for, they will take a much different form than a free kidney disease education class would. Let’s explore both.
PAID CLASSES
Only Medicare pays for education classes, and patients must be at stage 4 kidney disease (ie, glomerular filtration rate [GFR], 15 to 30 mL/dL). The class can be taught in a group or an individualized format, and an RN, a dietician, or a social worker can assist—but the bulk of the class must be taught by a practitioner with a National Provider Identifier billing number (an NP, a PA, or a physician).
Medicare specifies the content of the classes and has set certain requirements regarding a class’s site and length. In addition, there must be preevaluation and postevaluation tools in place, and the number of classes over a patient’s lifetime is limited to six.
The best program available (one that contains all the needed tools, slide sets, and handouts) is Your Treatment, Your Choice8 from the National Kidney Foundation (www.kidney.org/profes sionals/KLS/YTYC.cfm). It is free, but you must be a PA, an NP, or an MD to request it.
NONPAID CLASSES AND PROGRAMS
These can be given by anybody, and the format is up to the teacher. Prevention always trumps a cure, and preventing advanced kidney disease (GFR < 60 mL/dL) fits in very well in general practice. Promoting good health habits is a common goal. To that end, instruction regarding diet, blood pressure control, blood sugar control, and smoking cessation all help slow kidney disease progression.
What’s best about offering classes like these is that you don’t have to reinvent the wheel. There are some fantastic free programs out there. Some of our favorites are available through the National Kidney Disease Education Program (NKDEP) Web site: http://nkdep.nih.gov/resources.shtml. This is a division of one of the National Institutes of Health, paid for by your tax dollars, and it offers free or very inexpensive handouts, videos, and slide sets, all written at an eighth-grade reading level.
Among the materials offered is a phenomenal tear-off sheet, “Explaining Your Kidney Test Results,” which is available in English, Spanish, Chinese, and Vietnamese (with the first five copies free, then $1 each). It illustrates the stages of kidney function using the traffic light scenario: green, yellow, or red (stage 5 CKD is the red zone) and explains what patients can do to “stay out of the red.” We consider this one of the most effective tools we can use.
NKDEP also offers free handouts listing foods high in potassium, phosphorus, protein, and sodium. Nothing is as good as a renal dietician, but these forms are an excellent alternative.
NKDEP allows you to download and reprint almost all of their information free, or you can request 50 copies of just about any item at no cost. Put your best shopper on the Web site. The amount of materials offered is truly wonderful, and you can’t beat the price.
Another program is called Kidney School (http://kidneyschool
.org), a nonprofit organization set up by the kidney community that offers all kinds of videos and slide sets at no charge.
Last, but certainly not least, is Seymour Jones and the Temple of CKD, a five-minute video put out by the Renal Support Network (RSN; www.rsnhope.org). You can request the video from RSN or find it on YouTube (www.youtube.com/watch?v=lDJZHIVTNzo). Though hilarious, it makes excellent points about the symptoms of chronic kidney disease.
As you can see, there are many wonderful and varied (and free!) programs out there.
With the double-whammy of an aging population and increasing obesity, the number of people with kidney disease is growing exponentially; the past 20 years have seen a 67% increase in the number of patients with CKD, which now affects more than 20 million Americans. Yet in that same 20-year period, effective treatments have been developed for CKD that “can delay and, in some cases, prevent ESRD.”9 Patients with CKD need not assume there will be dialysis in their future.
Most importantly of all, we need to get out there and talk up prevention.
Kim Zuber, PA-C; Jane S. Davis, DNP, CRNP
REFERENCES
1. K/DOQI [Kidney Disease Outcome Quality Initiative] clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
2. Reilly RF, Jackson EK. Ch 25. Regulation of renal function and vascular volume. In: Chabner BA, Brunton LL, Knollman BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill Professional; 2010.
3. Sica DA, Gehr TW. Diuretic use in stage 5 chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2003;12(5): 483-490.
4. Cohen DL, Townsend RR. Treatment of hypertension in patients with chronic kidney disease. US Cardiology. 2009;6(2):54-58.
5. Wickersham RM, ed. Drug Facts and Comparisons. St. Louis, MO: Wolters Kluwer Health; 2009.
6. Comparison of commonly used diuretics (Detail Document). Pharmacist’s Letter/Prescriber’s Letter. February 2012.
7. DRUGDEX® System [Internet database]. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc. Updated periodically.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed September 19, 2012.
9. Turner JM, Bauer C, Abramowitz MK, et al. Treatment of chronic kidney disease. Kidney Int. 2012;81(4):351-362.
Q: We are trying to develop renal education classes in our hospital’s general medical clinic. Participating patients (pre-renal) will be those we hope can be managed by their primary care providers in coordination with our nephrology specialists before their initial renal clinic visits. Our team of educators will include an RN, an NP, a primary care physician, and a nephrologist. Any information, models, and/or links to educational resources would be much appreciated.
Everyone loses 1% of kidney function per year after age 40. If we lived long enough, all of us would need renal education!
As you try to develop classes, one of your first concerns will be whether you want to charge for them. If they are meant to be billed for, they will take a much different form than a free kidney disease education class would. Let’s explore both.
PAID CLASSES
Only Medicare pays for education classes, and patients must be at stage 4 kidney disease (ie, glomerular filtration rate [GFR], 15 to 30 mL/dL). The class can be taught in a group or an individualized format, and an RN, a dietician, or a social worker can assist—but the bulk of the class must be taught by a practitioner with a National Provider Identifier billing number (an NP, a PA, or a physician).
Medicare specifies the content of the classes and has set certain requirements regarding a class’s site and length. In addition, there must be preevaluation and postevaluation tools in place, and the number of classes over a patient’s lifetime is limited to six.
The best program available (one that contains all the needed tools, slide sets, and handouts) is Your Treatment, Your Choice8 from the National Kidney Foundation (www.kidney.org/profes sionals/KLS/YTYC.cfm). It is free, but you must be a PA, an NP, or an MD to request it.
NONPAID CLASSES AND PROGRAMS
These can be given by anybody, and the format is up to the teacher. Prevention always trumps a cure, and preventing advanced kidney disease (GFR < 60 mL/dL) fits in very well in general practice. Promoting good health habits is a common goal. To that end, instruction regarding diet, blood pressure control, blood sugar control, and smoking cessation all help slow kidney disease progression.
What’s best about offering classes like these is that you don’t have to reinvent the wheel. There are some fantastic free programs out there. Some of our favorites are available through the National Kidney Disease Education Program (NKDEP) Web site: http://nkdep.nih.gov/resources.shtml. This is a division of one of the National Institutes of Health, paid for by your tax dollars, and it offers free or very inexpensive handouts, videos, and slide sets, all written at an eighth-grade reading level.
Among the materials offered is a phenomenal tear-off sheet, “Explaining Your Kidney Test Results,” which is available in English, Spanish, Chinese, and Vietnamese (with the first five copies free, then $1 each). It illustrates the stages of kidney function using the traffic light scenario: green, yellow, or red (stage 5 CKD is the red zone) and explains what patients can do to “stay out of the red.” We consider this one of the most effective tools we can use.
NKDEP also offers free handouts listing foods high in potassium, phosphorus, protein, and sodium. Nothing is as good as a renal dietician, but these forms are an excellent alternative.
NKDEP allows you to download and reprint almost all of their information free, or you can request 50 copies of just about any item at no cost. Put your best shopper on the Web site. The amount of materials offered is truly wonderful, and you can’t beat the price.
Another program is called Kidney School (http://kidneyschool
.org), a nonprofit organization set up by the kidney community that offers all kinds of videos and slide sets at no charge.
Last, but certainly not least, is Seymour Jones and the Temple of CKD, a five-minute video put out by the Renal Support Network (RSN; www.rsnhope.org). You can request the video from RSN or find it on YouTube (www.youtube.com/watch?v=lDJZHIVTNzo). Though hilarious, it makes excellent points about the symptoms of chronic kidney disease.
As you can see, there are many wonderful and varied (and free!) programs out there.
With the double-whammy of an aging population and increasing obesity, the number of people with kidney disease is growing exponentially; the past 20 years have seen a 67% increase in the number of patients with CKD, which now affects more than 20 million Americans. Yet in that same 20-year period, effective treatments have been developed for CKD that “can delay and, in some cases, prevent ESRD.”9 Patients with CKD need not assume there will be dialysis in their future.
Most importantly of all, we need to get out there and talk up prevention.
Kim Zuber, PA-C; Jane S. Davis, DNP, CRNP
REFERENCES
1. K/DOQI [Kidney Disease Outcome Quality Initiative] clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1-S290.
2. Reilly RF, Jackson EK. Ch 25. Regulation of renal function and vascular volume. In: Chabner BA, Brunton LL, Knollman BC, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw-Hill Professional; 2010.
3. Sica DA, Gehr TW. Diuretic use in stage 5 chronic kidney disease and end-stage renal disease. Curr Opin Nephrol Hypertens. 2003;12(5): 483-490.
4. Cohen DL, Townsend RR. Treatment of hypertension in patients with chronic kidney disease. US Cardiology. 2009;6(2):54-58.
5. Wickersham RM, ed. Drug Facts and Comparisons. St. Louis, MO: Wolters Kluwer Health; 2009.
6. Comparison of commonly used diuretics (Detail Document). Pharmacist’s Letter/Prescriber’s Letter. February 2012.
7. DRUGDEX® System [Internet database]. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc. Updated periodically.
8. National Kidney Foundation. MIPPA Kidney Disease Education Benefit. Your Treatment, Your Choice (2010). www.kidney.org/professionals/KLS/YTYC.cfm. Accessed September 19, 2012.
9. Turner JM, Bauer C, Abramowitz MK, et al. Treatment of chronic kidney disease. Kidney Int. 2012;81(4):351-362.