User login
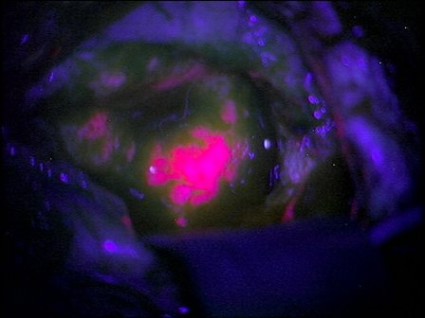
Brain Tumors Glow 'Like Lava' With New Surgical Probe
Neurosurgeons can now follow a glowing road map that points the way to cancerous brain tissue, leading thereby to a more effective surgical excision.
Researchers at the Norris Cotton Cancer Center and the Thayer School of Engineering at Dartmouth College, Hanover, N.H., have developed a probe that uses protoporphyrin IX fluorescence, oxygen saturation, hemoglobin concentration, and cell morphology to differentiate cancerous tissue from normal.
The probe identified 94% of glioma tissue in a small pilot study (J. Biomed. Opt. 2012 May 4 [doi:10.1117/1.JBO.17.5.056008]).
Research in Germany 15 years ago suggested that such a tool would identify only highly metabolic primary tumors. But augmenting the fluorescence technique with a computer algorithm that added the other cellular features gave surgeons a "jaw-dropping" view of low-grade tumors.
"The tumor glowed like lava," said Keith Paulsen, Ph.D., a professor of biomedical engineering at the school of engineering and a member of the cancer imaging and radiobiology research program at Norris Cotton Cancer Center.
The team will next evaluate their technique on lung cancers, with investigations of other tumor types to follow.
Neurosurgeons can now follow a glowing road map that points the way to cancerous brain tissue, leading thereby to a more effective surgical excision.
Researchers at the Norris Cotton Cancer Center and the Thayer School of Engineering at Dartmouth College, Hanover, N.H., have developed a probe that uses protoporphyrin IX fluorescence, oxygen saturation, hemoglobin concentration, and cell morphology to differentiate cancerous tissue from normal.
The probe identified 94% of glioma tissue in a small pilot study (J. Biomed. Opt. 2012 May 4 [doi:10.1117/1.JBO.17.5.056008]).
Research in Germany 15 years ago suggested that such a tool would identify only highly metabolic primary tumors. But augmenting the fluorescence technique with a computer algorithm that added the other cellular features gave surgeons a "jaw-dropping" view of low-grade tumors.
"The tumor glowed like lava," said Keith Paulsen, Ph.D., a professor of biomedical engineering at the school of engineering and a member of the cancer imaging and radiobiology research program at Norris Cotton Cancer Center.
The team will next evaluate their technique on lung cancers, with investigations of other tumor types to follow.
Neurosurgeons can now follow a glowing road map that points the way to cancerous brain tissue, leading thereby to a more effective surgical excision.
Researchers at the Norris Cotton Cancer Center and the Thayer School of Engineering at Dartmouth College, Hanover, N.H., have developed a probe that uses protoporphyrin IX fluorescence, oxygen saturation, hemoglobin concentration, and cell morphology to differentiate cancerous tissue from normal.
The probe identified 94% of glioma tissue in a small pilot study (J. Biomed. Opt. 2012 May 4 [doi:10.1117/1.JBO.17.5.056008]).
Research in Germany 15 years ago suggested that such a tool would identify only highly metabolic primary tumors. But augmenting the fluorescence technique with a computer algorithm that added the other cellular features gave surgeons a "jaw-dropping" view of low-grade tumors.
"The tumor glowed like lava," said Keith Paulsen, Ph.D., a professor of biomedical engineering at the school of engineering and a member of the cancer imaging and radiobiology research program at Norris Cotton Cancer Center.
The team will next evaluate their technique on lung cancers, with investigations of other tumor types to follow.
FROM THE JOURNAL OF BIOMEDICAL OPTICS
Brain Mets in Breast Cancer: Breaking Through the Barrier
BOSTON – Although brain metastasis is a notoriously formidable foe in the battle against breast cancer, recent breakthroughs in some patient subgroups suggest a challenge to its reign of terror.
As the ranks of women living with advanced breast cancer have swelled, thanks to treatment advances that alter the natural history of the disease, so has the incidence of tumor progression in the brain, according to Dr. Nancy U. Lin. The brain is a particularly hospitable sanctuary for cancer cells because few of the current chemotherapy agents penetrate the blood-brain barrier.
Current strategies for managing brain metastases have not substantially altered patient outcomes, Dr. Lin told attendees at a breast cancer program sponsored by Harvard Medical School in Boston.
But improved understanding of the biology of primary tumors and the specific sensitivities of tumor subtypes to various therapies (along with the identification of mediators of CNS progression) promises to usher in a new era in the treatment – and possibly prevention – of brain metastases in breast cancer, predicted Dr. Lin of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston.
Most Common in HER2 and Triple-Negative Disease
A critical consideration in the development of treatment and prevention strategies is tumor subtype. The highest incidences of brain metastases in breast cancer occur in women with HER2-positive disease and those with triple-negative (estrogen receptor–, progesterone receptor–, and HER2-negative) disease, Dr. Lin said, noting that there is also significant variation in prognosis within these subtypes.
Although estimates for median survival after a diagnosis of brain metastasis from breast cancer have been 6 months on average in historical series, many recent series have reported a median survival of 1-2 years for metastatic breast cancer patients who are HER2-positive, she said. The median survival for patients with triple-negative disease is less than 6 months, however.
These survival differences have important management implications, Dr. Lin explained. Effective, targeted, extracranial therapies extend the survival of many HER2-positive patients relative to historical estimates, and ultimately, more than half of these patients die as a result of CNS progression. In contrast, the shorter survival of women with triple-negative disease is generally attributable to progression in both the brain and distant sites.
For this reason, she said, therapies targeting the central nervous system specifically are warranted in the HER2-positive setting, whereas improved systemic treatments that target all metastatic sites are needed for patients with triple-negative disease.
Despite advances in the understanding of brain metastasis in breast cancer, the currently available treatment options are limited; as yet, no guidelines have been published for the management of brain metastasis associated specifically with breast cancer, according to Dr. Lin.
Surgery, stereotactic radiosurgery, and whole-brain radiotherapy are potential local therapeutic options, depending on the number, size, and site of metastatic lesions, she said; conventional breast cancer chemotherapies also have demonstrated efficacy in the first-line setting.
Along with her Dana-Farber colleague, Dr. ElgeneLim, she recently published a review article outlining the range of local and systemic therapeutic considerations for this patient population (Oncology 2012 July 12 [Epub ahead of print]). First-line therapies "typically have better response rates than therapies in heavily pretreated disease, and there is a need to identify therapies that will work in CNS disease that progresses following local and systemic therapies," the authors observed.
Lapatinib Studied for CNS Penetration
Among HER2-directed therapies, the small-molecule TKI (tyrosine kinase inhibitor) lapatinib (Tykerb) continues to pique interest. Because of its micromolecular structure, the agent can achieve greater CNS penetration than can the macromolecular agents, including monoclonal antibodies.
To date, this theoretical advantage has been associated with modest clinical benefit in trials. For example, in a clinical trial of single agent lapatinib, Dr. Lin and colleagues demonstrated a 2.6% objective response and an approximately 20% clinical benefit (J. Clin. Oncol. 2008;26:1993-9).
In a trial comparing the drug in combination with capecitabine (Xeloda) or topotecan (Hycamtin) in patients with brain metastasis following radiotherapy (J. Neurooncol. 2011;105: 613-20), "the objective central nervous system response was 38%," in the lapatinib plus capecitabine arm, Dr. Lin said.
Although the study was closed early because of toxicity and lack of efficacy in the topotecan arm, the observation of CNS activity in the lapatinib-capecitabine arm was promising, as were updated results from the pivotal randomized registration trial of lapatinib, suggesting that lapatinib may delay the onset of brain metastases in breast cancer patients with HER2-positive metastatic disease (Oncologist 2010;15:924-34).
In an editorial accompanying the review article by Dr. Lim and Dr. Lin, Dr. Mark D. Pegram of Stanford (Calif.) University observed that the lapatinib data suggest that "an ‘adjuvant’ HER2-TKI immediately following primary neurosurgery and/or radiotherapy for newly diagnosed [breast cancer brain metastasis] might be a more compelling treatment strategy than waiting for measurable relapse to occur following primary local therapy for HER2-positive CNS metastasis"(Oncology 2012 July 12 [Epub ahead of print]).
The ongoing ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization) trial, comparing adjuvant trastuzumab (Herceptin) with trastuzumab plus lapatinib, will offer important insight into the potential preventive value of lapatinib with respect to CNS relapse in early-stage HER2-positive disease, he wrote.
New Agents Also in Trials
Additional systemic and combination therapies representing a range of potential approaches are also under investigation, said Dr. Lin. These include the novel HER2-targeted therapies neratinib and afatinib, both of which are being evaluated in phase II trials, as well as cytotoxic agents targeting brain metastases specifically. The latter agents include the peptide-taxane conjugate GRN1005, the glutathione-pegylated liposomal doxorubicin 2B3-101, and the third-generation taxane TPI 287 – all three of which are being evaluated in phase I and II trials.
Also under investigation, she added, are PIK3CA inhibitors, mTOR (mammalian target of rapamycin) inhibitors, PARP (poly[ADP-ribose] polymerase) inhibitors, and VEGF (vascular endothelial growth factor)–targeting agents.
In order for research in this arena to bear fruit that translates into clinically effective strategies, the improvement of clinical trial availability and access should be a top priority, Dr. Lin stressed.
"Breast cancer patients with brain metastasis have been routinely excluded from clinical trials," which limits investigators’ ability to evaluate novel therapies, she said. "Our goal should be to increase and continue efforts to study novel agents in patients with brain metastases to establish better historical control data."
Dr. Lin disclosed financial relationships with GlaxoSmithKline, Genentech, Geron, Boehringer Ingelheim, Bayer, and Novartis.
BOSTON – Although brain metastasis is a notoriously formidable foe in the battle against breast cancer, recent breakthroughs in some patient subgroups suggest a challenge to its reign of terror.
As the ranks of women living with advanced breast cancer have swelled, thanks to treatment advances that alter the natural history of the disease, so has the incidence of tumor progression in the brain, according to Dr. Nancy U. Lin. The brain is a particularly hospitable sanctuary for cancer cells because few of the current chemotherapy agents penetrate the blood-brain barrier.
Current strategies for managing brain metastases have not substantially altered patient outcomes, Dr. Lin told attendees at a breast cancer program sponsored by Harvard Medical School in Boston.
But improved understanding of the biology of primary tumors and the specific sensitivities of tumor subtypes to various therapies (along with the identification of mediators of CNS progression) promises to usher in a new era in the treatment – and possibly prevention – of brain metastases in breast cancer, predicted Dr. Lin of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston.
Most Common in HER2 and Triple-Negative Disease
A critical consideration in the development of treatment and prevention strategies is tumor subtype. The highest incidences of brain metastases in breast cancer occur in women with HER2-positive disease and those with triple-negative (estrogen receptor–, progesterone receptor–, and HER2-negative) disease, Dr. Lin said, noting that there is also significant variation in prognosis within these subtypes.
Although estimates for median survival after a diagnosis of brain metastasis from breast cancer have been 6 months on average in historical series, many recent series have reported a median survival of 1-2 years for metastatic breast cancer patients who are HER2-positive, she said. The median survival for patients with triple-negative disease is less than 6 months, however.
These survival differences have important management implications, Dr. Lin explained. Effective, targeted, extracranial therapies extend the survival of many HER2-positive patients relative to historical estimates, and ultimately, more than half of these patients die as a result of CNS progression. In contrast, the shorter survival of women with triple-negative disease is generally attributable to progression in both the brain and distant sites.
For this reason, she said, therapies targeting the central nervous system specifically are warranted in the HER2-positive setting, whereas improved systemic treatments that target all metastatic sites are needed for patients with triple-negative disease.
Despite advances in the understanding of brain metastasis in breast cancer, the currently available treatment options are limited; as yet, no guidelines have been published for the management of brain metastasis associated specifically with breast cancer, according to Dr. Lin.
Surgery, stereotactic radiosurgery, and whole-brain radiotherapy are potential local therapeutic options, depending on the number, size, and site of metastatic lesions, she said; conventional breast cancer chemotherapies also have demonstrated efficacy in the first-line setting.
Along with her Dana-Farber colleague, Dr. ElgeneLim, she recently published a review article outlining the range of local and systemic therapeutic considerations for this patient population (Oncology 2012 July 12 [Epub ahead of print]). First-line therapies "typically have better response rates than therapies in heavily pretreated disease, and there is a need to identify therapies that will work in CNS disease that progresses following local and systemic therapies," the authors observed.
Lapatinib Studied for CNS Penetration
Among HER2-directed therapies, the small-molecule TKI (tyrosine kinase inhibitor) lapatinib (Tykerb) continues to pique interest. Because of its micromolecular structure, the agent can achieve greater CNS penetration than can the macromolecular agents, including monoclonal antibodies.
To date, this theoretical advantage has been associated with modest clinical benefit in trials. For example, in a clinical trial of single agent lapatinib, Dr. Lin and colleagues demonstrated a 2.6% objective response and an approximately 20% clinical benefit (J. Clin. Oncol. 2008;26:1993-9).
In a trial comparing the drug in combination with capecitabine (Xeloda) or topotecan (Hycamtin) in patients with brain metastasis following radiotherapy (J. Neurooncol. 2011;105: 613-20), "the objective central nervous system response was 38%," in the lapatinib plus capecitabine arm, Dr. Lin said.
Although the study was closed early because of toxicity and lack of efficacy in the topotecan arm, the observation of CNS activity in the lapatinib-capecitabine arm was promising, as were updated results from the pivotal randomized registration trial of lapatinib, suggesting that lapatinib may delay the onset of brain metastases in breast cancer patients with HER2-positive metastatic disease (Oncologist 2010;15:924-34).
In an editorial accompanying the review article by Dr. Lim and Dr. Lin, Dr. Mark D. Pegram of Stanford (Calif.) University observed that the lapatinib data suggest that "an ‘adjuvant’ HER2-TKI immediately following primary neurosurgery and/or radiotherapy for newly diagnosed [breast cancer brain metastasis] might be a more compelling treatment strategy than waiting for measurable relapse to occur following primary local therapy for HER2-positive CNS metastasis"(Oncology 2012 July 12 [Epub ahead of print]).
The ongoing ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization) trial, comparing adjuvant trastuzumab (Herceptin) with trastuzumab plus lapatinib, will offer important insight into the potential preventive value of lapatinib with respect to CNS relapse in early-stage HER2-positive disease, he wrote.
New Agents Also in Trials
Additional systemic and combination therapies representing a range of potential approaches are also under investigation, said Dr. Lin. These include the novel HER2-targeted therapies neratinib and afatinib, both of which are being evaluated in phase II trials, as well as cytotoxic agents targeting brain metastases specifically. The latter agents include the peptide-taxane conjugate GRN1005, the glutathione-pegylated liposomal doxorubicin 2B3-101, and the third-generation taxane TPI 287 – all three of which are being evaluated in phase I and II trials.
Also under investigation, she added, are PIK3CA inhibitors, mTOR (mammalian target of rapamycin) inhibitors, PARP (poly[ADP-ribose] polymerase) inhibitors, and VEGF (vascular endothelial growth factor)–targeting agents.
In order for research in this arena to bear fruit that translates into clinically effective strategies, the improvement of clinical trial availability and access should be a top priority, Dr. Lin stressed.
"Breast cancer patients with brain metastasis have been routinely excluded from clinical trials," which limits investigators’ ability to evaluate novel therapies, she said. "Our goal should be to increase and continue efforts to study novel agents in patients with brain metastases to establish better historical control data."
Dr. Lin disclosed financial relationships with GlaxoSmithKline, Genentech, Geron, Boehringer Ingelheim, Bayer, and Novartis.
BOSTON – Although brain metastasis is a notoriously formidable foe in the battle against breast cancer, recent breakthroughs in some patient subgroups suggest a challenge to its reign of terror.
As the ranks of women living with advanced breast cancer have swelled, thanks to treatment advances that alter the natural history of the disease, so has the incidence of tumor progression in the brain, according to Dr. Nancy U. Lin. The brain is a particularly hospitable sanctuary for cancer cells because few of the current chemotherapy agents penetrate the blood-brain barrier.
Current strategies for managing brain metastases have not substantially altered patient outcomes, Dr. Lin told attendees at a breast cancer program sponsored by Harvard Medical School in Boston.
But improved understanding of the biology of primary tumors and the specific sensitivities of tumor subtypes to various therapies (along with the identification of mediators of CNS progression) promises to usher in a new era in the treatment – and possibly prevention – of brain metastases in breast cancer, predicted Dr. Lin of the Dana-Farber Cancer Institute and Harvard Medical School, both in Boston.
Most Common in HER2 and Triple-Negative Disease
A critical consideration in the development of treatment and prevention strategies is tumor subtype. The highest incidences of brain metastases in breast cancer occur in women with HER2-positive disease and those with triple-negative (estrogen receptor–, progesterone receptor–, and HER2-negative) disease, Dr. Lin said, noting that there is also significant variation in prognosis within these subtypes.
Although estimates for median survival after a diagnosis of brain metastasis from breast cancer have been 6 months on average in historical series, many recent series have reported a median survival of 1-2 years for metastatic breast cancer patients who are HER2-positive, she said. The median survival for patients with triple-negative disease is less than 6 months, however.
These survival differences have important management implications, Dr. Lin explained. Effective, targeted, extracranial therapies extend the survival of many HER2-positive patients relative to historical estimates, and ultimately, more than half of these patients die as a result of CNS progression. In contrast, the shorter survival of women with triple-negative disease is generally attributable to progression in both the brain and distant sites.
For this reason, she said, therapies targeting the central nervous system specifically are warranted in the HER2-positive setting, whereas improved systemic treatments that target all metastatic sites are needed for patients with triple-negative disease.
Despite advances in the understanding of brain metastasis in breast cancer, the currently available treatment options are limited; as yet, no guidelines have been published for the management of brain metastasis associated specifically with breast cancer, according to Dr. Lin.
Surgery, stereotactic radiosurgery, and whole-brain radiotherapy are potential local therapeutic options, depending on the number, size, and site of metastatic lesions, she said; conventional breast cancer chemotherapies also have demonstrated efficacy in the first-line setting.
Along with her Dana-Farber colleague, Dr. ElgeneLim, she recently published a review article outlining the range of local and systemic therapeutic considerations for this patient population (Oncology 2012 July 12 [Epub ahead of print]). First-line therapies "typically have better response rates than therapies in heavily pretreated disease, and there is a need to identify therapies that will work in CNS disease that progresses following local and systemic therapies," the authors observed.
Lapatinib Studied for CNS Penetration
Among HER2-directed therapies, the small-molecule TKI (tyrosine kinase inhibitor) lapatinib (Tykerb) continues to pique interest. Because of its micromolecular structure, the agent can achieve greater CNS penetration than can the macromolecular agents, including monoclonal antibodies.
To date, this theoretical advantage has been associated with modest clinical benefit in trials. For example, in a clinical trial of single agent lapatinib, Dr. Lin and colleagues demonstrated a 2.6% objective response and an approximately 20% clinical benefit (J. Clin. Oncol. 2008;26:1993-9).
In a trial comparing the drug in combination with capecitabine (Xeloda) or topotecan (Hycamtin) in patients with brain metastasis following radiotherapy (J. Neurooncol. 2011;105: 613-20), "the objective central nervous system response was 38%," in the lapatinib plus capecitabine arm, Dr. Lin said.
Although the study was closed early because of toxicity and lack of efficacy in the topotecan arm, the observation of CNS activity in the lapatinib-capecitabine arm was promising, as were updated results from the pivotal randomized registration trial of lapatinib, suggesting that lapatinib may delay the onset of brain metastases in breast cancer patients with HER2-positive metastatic disease (Oncologist 2010;15:924-34).
In an editorial accompanying the review article by Dr. Lim and Dr. Lin, Dr. Mark D. Pegram of Stanford (Calif.) University observed that the lapatinib data suggest that "an ‘adjuvant’ HER2-TKI immediately following primary neurosurgery and/or radiotherapy for newly diagnosed [breast cancer brain metastasis] might be a more compelling treatment strategy than waiting for measurable relapse to occur following primary local therapy for HER2-positive CNS metastasis"(Oncology 2012 July 12 [Epub ahead of print]).
The ongoing ALTTO (Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization) trial, comparing adjuvant trastuzumab (Herceptin) with trastuzumab plus lapatinib, will offer important insight into the potential preventive value of lapatinib with respect to CNS relapse in early-stage HER2-positive disease, he wrote.
New Agents Also in Trials
Additional systemic and combination therapies representing a range of potential approaches are also under investigation, said Dr. Lin. These include the novel HER2-targeted therapies neratinib and afatinib, both of which are being evaluated in phase II trials, as well as cytotoxic agents targeting brain metastases specifically. The latter agents include the peptide-taxane conjugate GRN1005, the glutathione-pegylated liposomal doxorubicin 2B3-101, and the third-generation taxane TPI 287 – all three of which are being evaluated in phase I and II trials.
Also under investigation, she added, are PIK3CA inhibitors, mTOR (mammalian target of rapamycin) inhibitors, PARP (poly[ADP-ribose] polymerase) inhibitors, and VEGF (vascular endothelial growth factor)–targeting agents.
In order for research in this arena to bear fruit that translates into clinically effective strategies, the improvement of clinical trial availability and access should be a top priority, Dr. Lin stressed.
"Breast cancer patients with brain metastasis have been routinely excluded from clinical trials," which limits investigators’ ability to evaluate novel therapies, she said. "Our goal should be to increase and continue efforts to study novel agents in patients with brain metastases to establish better historical control data."
Dr. Lin disclosed financial relationships with GlaxoSmithKline, Genentech, Geron, Boehringer Ingelheim, Bayer, and Novartis.
EXPERT ANALYSIS FROM A BREAST CANCER PROGRAM SPONSORED BY HARVARD MEDICAL SCHOOL
CNS Events Not Immediately Fatal in Mantle Cell Lymphomas
AMSTERDAM – Although it is rare and the prognosis for patients is often poor, the presence of central nervous system involvement at the time of diagnosis of mantle cell lymphoma is not always immediately fatal, according to findings from an international study.
According to the European Mantle Cell Lymphoma Network (EMCLN) findings, the crude prevalence of CNS events was 0.9% at the time of diagnosis and 4.1% at any time. The multicenter, retrospective study found that the median time to a CNS event’s occurring was 15.2 months, with an overall survival of 3.9 months after the event was identified, but some patients were still alive 2 years later.
"We now have some better descriptors about what the expectations and outcome of patients with central nervous system involvement are," Dr. John Seymour said in an interview at the annual congress of the European Hematology Association.
Dr. Seymour, professor and chair of the hematology service at Australia’s Peter MacCallum Cancer Centre in East Melbourne, Victoria, added that even though the overall prognosis of patients who develop CNS involvement is very poor, there are some patients who do better than others.
There is "a subgroup [of patients] who are able to receive high-dose ara-c [cytarabine] or high-dose methotrexate treatment, who are young and fit enough, who do somewhat better," Dr. Seymour said. "A proportion will be alive at 2 years, so it’s not an inevitably, rapidly fatal, and ... futile situation."
Mantle cell lymphomas are a rare type of non-Hodgkin’s lymphoma (NHL), accounting for just less than 3% of all NHL cases in the United States and affecting primarily more elderly patients (Cancer 2008;113:791-8).CNS involvement is also a rare and often devastating event, but it has not previously been very well characterized. As a result, it’s not known whether CNS prophylaxis is of benefit to patients.
The aim of the EMCLN study, therefore, was to look at the problem in more detail, to determine the prevalence of CNS involvement, and to look for any clinically defining features, effect of treatment, and patient outcomes.
A retrospective database review by EMCLN members in 12 centers identified 1,396 patients with mantle cell lymphoma, of whom 1,339 had no CNS involvement. Of the 57 patients with CNS involvement, most (44) developed it at some point during the course of their follow-up.
At diagnosis of mantle cell lymphoma, the patients who developed CNS involvement had a median age of 61 years, but this ranged from 38 years to 82 years; patients were predominantly men (70%), with stage IV (91%) disease, and 28% had blastoid histology. Isolated CNS involvement occurred in 15 cases.
Prominent features were a high MIPI (Mantle Cell Lymphoma International Prognostic Index) score (61% of cases), a Ki-67 greater than 30% in 69% of patients, and increased beta2-microglobulin and lactate dehydrogenase in 77% and 75% of cases, respectively. The bone marrow and the peripheral blood were the most common extranodal sites involved, affecting two or more sites in 61% of patients.
At diagnosis of CNS involvement, patients’ neurologic symptoms included weakness, altered mental state, headache, and ocular problems such as double vision. Other symptoms – such as sensory disturbances, pain, sciatica, dizziness, vertigo, ataxia, seizure, and dysphagia – occurred but were less frequent.
CSF cytology and flow cytometry showed a high sensitivity for identifying CNS involvement, with 85% having positive cytology and 91% a positive flow cytometry result.
"Patients had a range of chemotherapies prior to developing central nervous system involvement, but receipt of these regimens was not totally protective," Dr. Seymour said. Data were not collected to enable the relative risk of CNS development with the regimens received.
Chemotherapy was the most frequent treatment strategy to allay CNS disease (67%), and some patients did appear to achieve a complete remission of the CNS disease as a result. In an exploratory analysis, these patients also tended to have improved overall survival, as did those with lower white cell counts (less than 10.9 x 109/L), and who received treatment with high-dose antimetabolites.
"In the longer term, these data will provide a foundation for us to identify predictive factors, to identify – ahead of the event – those people at increased risk," Dr. Seymour said, adding his hope that this will allow preventive steps to be taken.
Dr. Seymour had no conflicts of interest.
AMSTERDAM – Although it is rare and the prognosis for patients is often poor, the presence of central nervous system involvement at the time of diagnosis of mantle cell lymphoma is not always immediately fatal, according to findings from an international study.
According to the European Mantle Cell Lymphoma Network (EMCLN) findings, the crude prevalence of CNS events was 0.9% at the time of diagnosis and 4.1% at any time. The multicenter, retrospective study found that the median time to a CNS event’s occurring was 15.2 months, with an overall survival of 3.9 months after the event was identified, but some patients were still alive 2 years later.
"We now have some better descriptors about what the expectations and outcome of patients with central nervous system involvement are," Dr. John Seymour said in an interview at the annual congress of the European Hematology Association.
Dr. Seymour, professor and chair of the hematology service at Australia’s Peter MacCallum Cancer Centre in East Melbourne, Victoria, added that even though the overall prognosis of patients who develop CNS involvement is very poor, there are some patients who do better than others.
There is "a subgroup [of patients] who are able to receive high-dose ara-c [cytarabine] or high-dose methotrexate treatment, who are young and fit enough, who do somewhat better," Dr. Seymour said. "A proportion will be alive at 2 years, so it’s not an inevitably, rapidly fatal, and ... futile situation."
Mantle cell lymphomas are a rare type of non-Hodgkin’s lymphoma (NHL), accounting for just less than 3% of all NHL cases in the United States and affecting primarily more elderly patients (Cancer 2008;113:791-8).CNS involvement is also a rare and often devastating event, but it has not previously been very well characterized. As a result, it’s not known whether CNS prophylaxis is of benefit to patients.
The aim of the EMCLN study, therefore, was to look at the problem in more detail, to determine the prevalence of CNS involvement, and to look for any clinically defining features, effect of treatment, and patient outcomes.
A retrospective database review by EMCLN members in 12 centers identified 1,396 patients with mantle cell lymphoma, of whom 1,339 had no CNS involvement. Of the 57 patients with CNS involvement, most (44) developed it at some point during the course of their follow-up.
At diagnosis of mantle cell lymphoma, the patients who developed CNS involvement had a median age of 61 years, but this ranged from 38 years to 82 years; patients were predominantly men (70%), with stage IV (91%) disease, and 28% had blastoid histology. Isolated CNS involvement occurred in 15 cases.
Prominent features were a high MIPI (Mantle Cell Lymphoma International Prognostic Index) score (61% of cases), a Ki-67 greater than 30% in 69% of patients, and increased beta2-microglobulin and lactate dehydrogenase in 77% and 75% of cases, respectively. The bone marrow and the peripheral blood were the most common extranodal sites involved, affecting two or more sites in 61% of patients.
At diagnosis of CNS involvement, patients’ neurologic symptoms included weakness, altered mental state, headache, and ocular problems such as double vision. Other symptoms – such as sensory disturbances, pain, sciatica, dizziness, vertigo, ataxia, seizure, and dysphagia – occurred but were less frequent.
CSF cytology and flow cytometry showed a high sensitivity for identifying CNS involvement, with 85% having positive cytology and 91% a positive flow cytometry result.
"Patients had a range of chemotherapies prior to developing central nervous system involvement, but receipt of these regimens was not totally protective," Dr. Seymour said. Data were not collected to enable the relative risk of CNS development with the regimens received.
Chemotherapy was the most frequent treatment strategy to allay CNS disease (67%), and some patients did appear to achieve a complete remission of the CNS disease as a result. In an exploratory analysis, these patients also tended to have improved overall survival, as did those with lower white cell counts (less than 10.9 x 109/L), and who received treatment with high-dose antimetabolites.
"In the longer term, these data will provide a foundation for us to identify predictive factors, to identify – ahead of the event – those people at increased risk," Dr. Seymour said, adding his hope that this will allow preventive steps to be taken.
Dr. Seymour had no conflicts of interest.
AMSTERDAM – Although it is rare and the prognosis for patients is often poor, the presence of central nervous system involvement at the time of diagnosis of mantle cell lymphoma is not always immediately fatal, according to findings from an international study.
According to the European Mantle Cell Lymphoma Network (EMCLN) findings, the crude prevalence of CNS events was 0.9% at the time of diagnosis and 4.1% at any time. The multicenter, retrospective study found that the median time to a CNS event’s occurring was 15.2 months, with an overall survival of 3.9 months after the event was identified, but some patients were still alive 2 years later.
"We now have some better descriptors about what the expectations and outcome of patients with central nervous system involvement are," Dr. John Seymour said in an interview at the annual congress of the European Hematology Association.
Dr. Seymour, professor and chair of the hematology service at Australia’s Peter MacCallum Cancer Centre in East Melbourne, Victoria, added that even though the overall prognosis of patients who develop CNS involvement is very poor, there are some patients who do better than others.
There is "a subgroup [of patients] who are able to receive high-dose ara-c [cytarabine] or high-dose methotrexate treatment, who are young and fit enough, who do somewhat better," Dr. Seymour said. "A proportion will be alive at 2 years, so it’s not an inevitably, rapidly fatal, and ... futile situation."
Mantle cell lymphomas are a rare type of non-Hodgkin’s lymphoma (NHL), accounting for just less than 3% of all NHL cases in the United States and affecting primarily more elderly patients (Cancer 2008;113:791-8).CNS involvement is also a rare and often devastating event, but it has not previously been very well characterized. As a result, it’s not known whether CNS prophylaxis is of benefit to patients.
The aim of the EMCLN study, therefore, was to look at the problem in more detail, to determine the prevalence of CNS involvement, and to look for any clinically defining features, effect of treatment, and patient outcomes.
A retrospective database review by EMCLN members in 12 centers identified 1,396 patients with mantle cell lymphoma, of whom 1,339 had no CNS involvement. Of the 57 patients with CNS involvement, most (44) developed it at some point during the course of their follow-up.
At diagnosis of mantle cell lymphoma, the patients who developed CNS involvement had a median age of 61 years, but this ranged from 38 years to 82 years; patients were predominantly men (70%), with stage IV (91%) disease, and 28% had blastoid histology. Isolated CNS involvement occurred in 15 cases.
Prominent features were a high MIPI (Mantle Cell Lymphoma International Prognostic Index) score (61% of cases), a Ki-67 greater than 30% in 69% of patients, and increased beta2-microglobulin and lactate dehydrogenase in 77% and 75% of cases, respectively. The bone marrow and the peripheral blood were the most common extranodal sites involved, affecting two or more sites in 61% of patients.
At diagnosis of CNS involvement, patients’ neurologic symptoms included weakness, altered mental state, headache, and ocular problems such as double vision. Other symptoms – such as sensory disturbances, pain, sciatica, dizziness, vertigo, ataxia, seizure, and dysphagia – occurred but were less frequent.
CSF cytology and flow cytometry showed a high sensitivity for identifying CNS involvement, with 85% having positive cytology and 91% a positive flow cytometry result.
"Patients had a range of chemotherapies prior to developing central nervous system involvement, but receipt of these regimens was not totally protective," Dr. Seymour said. Data were not collected to enable the relative risk of CNS development with the regimens received.
Chemotherapy was the most frequent treatment strategy to allay CNS disease (67%), and some patients did appear to achieve a complete remission of the CNS disease as a result. In an exploratory analysis, these patients also tended to have improved overall survival, as did those with lower white cell counts (less than 10.9 x 109/L), and who received treatment with high-dose antimetabolites.
"In the longer term, these data will provide a foundation for us to identify predictive factors, to identify – ahead of the event – those people at increased risk," Dr. Seymour said, adding his hope that this will allow preventive steps to be taken.
Dr. Seymour had no conflicts of interest.
AT THE ANNUAL CONGRESS OF THE EUROPEAN HEMATOLOGY ASSOCIATION
Major Finding: Crude prevalences of CNS involvement at diagnosis and overall were 0.9% and 4.1%, respectively, with a median time to an event of 15.2 months and overall survival thereafter of 3.9 months.
Data Source: The EMCLN conducted a retrospective database review of 1,396 patients with mantle cell lymphoma in 12 centers.
Disclosures: Dr. Seymour had no conflicts of interest.
PET Radiotracer Identifies Glioma Treatment Response
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
A PET imaging protocol using an amino acid analog radiotracer in patients with recurrent high-grade gliomas identified responses to treatment with bevacizumab as early as 2 weeks after starting therapy in a prospective study.
In the 28-patient pilot study, the metabolic tumor volume measured in follow-up PET scans with 6-18F-fluoro-L-DOPA (18F-FDOPA) at 2 and 6 weeks after the baseline scan proved to be the most significant predictor of survival with the method.
There is currently no reliable way to predict treatment response noninvasively in patients with malignant glioma, which has only 6% overall survival at 5 years. Chemotherapeutics have toxic side effects and are expensive, "so from the patient’s point of view, if a treatment doesn’t work, it’s important to get that information as early as possible," said the senior investigator of the study, Dr. Wei Chen of the division of molecular and medical pharmacology at the University of California, Los Angeles.
The ability to detect treatment response only 2 weeks after the start of treatment is the shortest interval yet reported, Dr. Chen said. In a study published last year, she and her colleagues reported that another PET radiotracer, 3´-deoxy-3´-[18F]-fluorothymidine (18F-FLT), could be used to monitor the response of recurrent high-grade gliomas to treatment (J. Nucl. Med. 2012;53:29-36). However, change in response to treatment with bevacizumab (Avastin) could not be detected with 18F-FLT until 6 weeks after starting therapy in that study, compared with 2 weeks for 18F-FDOPA in the current study.
The 18F-FDOPA technique of assessing metabolic tumor volume as early as 2 weeks after starting bevacizumab proved to be a significant predictor of overall and progression-free survival. The 17 metabolic responders survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders. In comparison, when MRI was used to determine response, the survival difference shrank (12.9 months vs. 9.0 months). All patients in the study eventually died.
The investigators chose to use bevacizumab because "it is the most effective treatment," with a significant treatment response of 50% instead of 5%-10% with other drugs, Dr. Chen said in an interview. "But in terms of monitoring, it doesn’t matter which agent is used for treatment."
18F-FDOPA is normally used to assess the striatal dopaminergic system in patients with movement disorders. But it works in assessing tumor treatment response because the higher metabolic rate of cancer cells causes greater uptake of the tracer through a phenylalanine and tyrosine transporter. Conventional MRI assessments for tumor recurrence cannot distinguish tumor from scar tissue left by surgery or radiation, and cannot determine the amount of change until 1.5 to 3 months, according to Dr. Chen.
In eight of nine discrepant cases between PET and MRI, 18F-FDOPA PET demonstrated treatment response earlier than MRI.
The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
AT THE ANNUAL MEETING OF THE SOCIETY OF NUCLEAR MEDICINE AND MOLECULAR IMAGING
Major Finding: The 17 patients identified as responders to bevacizumab by 18F-FDOPA PET after 2 weeks of treatment survived a median of 12.1 months, compared with 3.5 months for 11 nonresponders.
Data Source: This pilot study involved 28 patients with recurrent high-grade gliomas who underwent MRI and 18F-FDOPA PET at baseline and after 2 and 6 weeks.
Disclosures: The study was supported by grants from the National Cancer Institute and the Department of Energy. Dr. Chen had no relevant disclosures.
Chemotherapy-Induced Neuropathy Linked to Falls
CHICAGO – Cancer survivors with chemotherapy-induced peripheral neuropathy may be headed for a fall, researchers cautioned at the annual meeting of the American Society of Clinical Oncology.
About 12% of patients with chemotherapy-induced peripheral neuropathy (CIPN) had at least one fall, and nearly 60% experienced some kind of physical problem related to CIPN, reported Dr. Supriya Mohile of the department of hematology/oncology at the University of Rochester (N.Y.).
"We should in our clinics longitudinally evaluate patients not only for toxicities from neuropathy, but also for physical functioning and falls," she said.
Dr. Mohile urged providing patients with balance and mobility training throughout chemotherapy and minimizing fall risk by recommending assistive devices and home-safety evaluations and modifications as necessary.
She and her colleagues evaluated 421 patients who had reported baseline data as part of a randomized phase III trial for a topical cream. The patients had completed chemotherapy and had self-reported CIPN of 4 or greater on an 11-point scale. The patients were not on significant medications for either pain or neuropathy, and those with other possible causes of neuropathy, such as diabetes, were excluded.
They found that about one-third of patients had a CIPN-related problem such as difficulty stooping, walking for one-fourth of a mile, or performing tasks requiring heavy lifting.
Additionally, more than 25% reported a functional loss, limiting their ability to shop, manage money, walk across a room, do light housework, or bathe themselves.
Comparing 260 patients who reported falls or physical problems with 161 who did not, the investigators identified pain, sensory neuropathy, and motor neuropathy as toxicities independently associated with falls and/or physical problems (P less than .001 for all three comparisons).
In a multivariate analysis controlling for age, sex, race, ethnicity, marital status, education, history of taxane exposure, previous surgery and radiation, pain, and sensory neuropathy, the investigators found that breast cancer (odds ratio, 2.776; P = .045) and motor neuropathy (OR, 1.138; P = .006) were independently associated with falls. Factors associated with having a physical performance problem were previous surgery (OR, 2.536; P = .013) and motor neuropathy (OR, 1.325; P less than .001).
Functional losses were more likely to occur among Hispanics (OR, 5.318; P = –.048), patients with any physical performance problem (OR, 4.942; P less than .001), and those with motor neuropathy (OR, 1.191; P = .0001).
The study was limited by the heterogeneity of the cancer sample, its cross-sectional design that precludes determination of causality or of a temporal relationship between chemotherapy and neuropathies, and self-report of CIPN toxicities, Dr. Mohile said.
Commenting on the study, Dr. Charles L. Loprinzi, emeritus chair of the division of medical oncology at the Mayo Clinic in Rochester, Minn., said that it supports earlier findings of a relationship between epidermal nerve fiber loss and deficits in sensory and motor function leading, and that "it makes sense" that such losses would lead to functional losses.*
The study was funded by grants from the National Cancer Institute. Dr. Mohile reported no relevant disclosures. Dr. Loprinzi disclosed receiving research funding from Abbott, Amgen, Bristol-Myers Squibb, Eisai, Novartis, Ortho Biotech, Pfizer, Roche, and Sanofi.
* Dr. Loprinzi's title was corrected on June 18, 2012.
balance and mobility training, fall risk,
CHICAGO – Cancer survivors with chemotherapy-induced peripheral neuropathy may be headed for a fall, researchers cautioned at the annual meeting of the American Society of Clinical Oncology.
About 12% of patients with chemotherapy-induced peripheral neuropathy (CIPN) had at least one fall, and nearly 60% experienced some kind of physical problem related to CIPN, reported Dr. Supriya Mohile of the department of hematology/oncology at the University of Rochester (N.Y.).
"We should in our clinics longitudinally evaluate patients not only for toxicities from neuropathy, but also for physical functioning and falls," she said.
Dr. Mohile urged providing patients with balance and mobility training throughout chemotherapy and minimizing fall risk by recommending assistive devices and home-safety evaluations and modifications as necessary.
She and her colleagues evaluated 421 patients who had reported baseline data as part of a randomized phase III trial for a topical cream. The patients had completed chemotherapy and had self-reported CIPN of 4 or greater on an 11-point scale. The patients were not on significant medications for either pain or neuropathy, and those with other possible causes of neuropathy, such as diabetes, were excluded.
They found that about one-third of patients had a CIPN-related problem such as difficulty stooping, walking for one-fourth of a mile, or performing tasks requiring heavy lifting.
Additionally, more than 25% reported a functional loss, limiting their ability to shop, manage money, walk across a room, do light housework, or bathe themselves.
Comparing 260 patients who reported falls or physical problems with 161 who did not, the investigators identified pain, sensory neuropathy, and motor neuropathy as toxicities independently associated with falls and/or physical problems (P less than .001 for all three comparisons).
In a multivariate analysis controlling for age, sex, race, ethnicity, marital status, education, history of taxane exposure, previous surgery and radiation, pain, and sensory neuropathy, the investigators found that breast cancer (odds ratio, 2.776; P = .045) and motor neuropathy (OR, 1.138; P = .006) were independently associated with falls. Factors associated with having a physical performance problem were previous surgery (OR, 2.536; P = .013) and motor neuropathy (OR, 1.325; P less than .001).
Functional losses were more likely to occur among Hispanics (OR, 5.318; P = –.048), patients with any physical performance problem (OR, 4.942; P less than .001), and those with motor neuropathy (OR, 1.191; P = .0001).
The study was limited by the heterogeneity of the cancer sample, its cross-sectional design that precludes determination of causality or of a temporal relationship between chemotherapy and neuropathies, and self-report of CIPN toxicities, Dr. Mohile said.
Commenting on the study, Dr. Charles L. Loprinzi, emeritus chair of the division of medical oncology at the Mayo Clinic in Rochester, Minn., said that it supports earlier findings of a relationship between epidermal nerve fiber loss and deficits in sensory and motor function leading, and that "it makes sense" that such losses would lead to functional losses.*
The study was funded by grants from the National Cancer Institute. Dr. Mohile reported no relevant disclosures. Dr. Loprinzi disclosed receiving research funding from Abbott, Amgen, Bristol-Myers Squibb, Eisai, Novartis, Ortho Biotech, Pfizer, Roche, and Sanofi.
* Dr. Loprinzi's title was corrected on June 18, 2012.
CHICAGO – Cancer survivors with chemotherapy-induced peripheral neuropathy may be headed for a fall, researchers cautioned at the annual meeting of the American Society of Clinical Oncology.
About 12% of patients with chemotherapy-induced peripheral neuropathy (CIPN) had at least one fall, and nearly 60% experienced some kind of physical problem related to CIPN, reported Dr. Supriya Mohile of the department of hematology/oncology at the University of Rochester (N.Y.).
"We should in our clinics longitudinally evaluate patients not only for toxicities from neuropathy, but also for physical functioning and falls," she said.
Dr. Mohile urged providing patients with balance and mobility training throughout chemotherapy and minimizing fall risk by recommending assistive devices and home-safety evaluations and modifications as necessary.
She and her colleagues evaluated 421 patients who had reported baseline data as part of a randomized phase III trial for a topical cream. The patients had completed chemotherapy and had self-reported CIPN of 4 or greater on an 11-point scale. The patients were not on significant medications for either pain or neuropathy, and those with other possible causes of neuropathy, such as diabetes, were excluded.
They found that about one-third of patients had a CIPN-related problem such as difficulty stooping, walking for one-fourth of a mile, or performing tasks requiring heavy lifting.
Additionally, more than 25% reported a functional loss, limiting their ability to shop, manage money, walk across a room, do light housework, or bathe themselves.
Comparing 260 patients who reported falls or physical problems with 161 who did not, the investigators identified pain, sensory neuropathy, and motor neuropathy as toxicities independently associated with falls and/or physical problems (P less than .001 for all three comparisons).
In a multivariate analysis controlling for age, sex, race, ethnicity, marital status, education, history of taxane exposure, previous surgery and radiation, pain, and sensory neuropathy, the investigators found that breast cancer (odds ratio, 2.776; P = .045) and motor neuropathy (OR, 1.138; P = .006) were independently associated with falls. Factors associated with having a physical performance problem were previous surgery (OR, 2.536; P = .013) and motor neuropathy (OR, 1.325; P less than .001).
Functional losses were more likely to occur among Hispanics (OR, 5.318; P = –.048), patients with any physical performance problem (OR, 4.942; P less than .001), and those with motor neuropathy (OR, 1.191; P = .0001).
The study was limited by the heterogeneity of the cancer sample, its cross-sectional design that precludes determination of causality or of a temporal relationship between chemotherapy and neuropathies, and self-report of CIPN toxicities, Dr. Mohile said.
Commenting on the study, Dr. Charles L. Loprinzi, emeritus chair of the division of medical oncology at the Mayo Clinic in Rochester, Minn., said that it supports earlier findings of a relationship between epidermal nerve fiber loss and deficits in sensory and motor function leading, and that "it makes sense" that such losses would lead to functional losses.*
The study was funded by grants from the National Cancer Institute. Dr. Mohile reported no relevant disclosures. Dr. Loprinzi disclosed receiving research funding from Abbott, Amgen, Bristol-Myers Squibb, Eisai, Novartis, Ortho Biotech, Pfizer, Roche, and Sanofi.
* Dr. Loprinzi's title was corrected on June 18, 2012.
balance and mobility training, fall risk,
balance and mobility training, fall risk,
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: About 12% of patients with chemotherapy-induced peripheral neuropathy had at least one fall, and nearly 60% experienced some kind of physical problem related to CIPN.
Data Source: This was an analysis of baseline assessments from a phase III randomized trial.
Disclosures: The study was funded by grants from the National Cancer Institute. Dr. Mohile reported no relevant disclosures. Dr. Loprinzi disclosed receiving research funding from Abbott, Amgen, Bristol-Myers Squibb, Eisai, Novartis, Ortho Biotech, Pfizer, Roche, and Sanofi.
Duloxetine Eases Pain of Chemo-Induced Neuropathy
CHICAGO – The antidepressant duloxetine reduces chronic pain from chemotherapy-induced peripheral neuropathy with fewer side effects than with current therapies, according to results of a phase III, double-blind trial.
Among evaluable patients, 59% reported less pain with duloxetine (Cymbalta), compared with 38% with placebo. A pain reduction of 30% or more – a measure considered clinically significant – was reported by 33% and 17%, respectively.
"Duloxetine 60 mg a day is the first drug to be shown to be effective in painful chemotherapy-induced peripheral neuropathy based on the results of a randomized trial," Ellen Lavoie Smith, Ph.D., said at a press conference at the annual meeting of the American Society of Clinical Oncology.
A variety of other agents – including gabapentin and tricyclic antidepressants – have been shown to be effective in treating neuropathic pain and are routinely pressed into service in oncology practice, but have failed to demonstrate efficacy in randomized trials of peripheral neuropathy caused by chemotherapy, she noted.
"Some patients endure this painful neuropathy for months and possibly for as long as years following completion of chemotherapy," she said. "It’s chronic, it’s very distressing and it’s disabling. And there is nothing to date effective in treating this problem."
Duloxetine is approved to treat major depressive disorder, with an additional indication for chronic musculoskeletal pain added in 2010. The safety label was revised in September 2011 to include warnings for severe skin reactions including Stevens-Johnson Syndrome and erythema multiforme.
The Cancer and Leukemia Group B (CALGB) 170601 trial randomized 231 patients with painful neuropathy after receiving single-agent paclitaxel (Taxol) or oxaliplatin (Eloxatin) chemotherapy to duloxetine 30 mg for 1 week and 60 mg for 4 weeks, followed by crossover to placebo after a 1-week washout period or the same regimen in the opposite order. Pain levels were assessed weekly using the Brief Pain Inventory-Short Form (BPI-SF) in 220 patients.
The average change in BPI-SF score, the study’s primary outcome, was -1.09 in patients given duloxetine, compared with –0.33 given placebo (P = .003), reported Dr. Smith, with the University of Michigan, Ann Arbor.
During duloxetine treatment, patients also experienced a significant reduction in the BPI-SF pain interference score, a sum of seven items including interference with general activity, mood, walking, normal work, relations with people, sleep and enjoyment of life. There was no difference in duloxetine efficacy based on the specific neurotoxic chemotherapeutic agent received.
In all, 21% of patients said their pain was cut by at least one-half with duloxetine, while only 9% taking placebo did.
Interestingly, 11% of patients saw their pain increase with the serotonin-norepinephrine reuptake inhibitor, compared with 28% with placebo. The reason for the finding is unclear, Dr. Smith said in an interview.
"Pain is a very complicated thing to study," she said. "There are many things that go into it – psychosocial issues, environmental issues, cultural issues, but again, there may be patients who are more likely to respond to these drugs because their central nervous system isn’t really working normally."
Overall, duloxetine was well tolerated, but was associated with significantly more grade 2 or greater fatigue than placebo (11% vs. 3%; P = .029). Dr. Smith pointed out that the overall incidence of side effects was lower than observed in two studies of diabetes-related peripheral neuropathy, likely because they used the 60 mg dose without the lower 30 mg starting dose.
Dr. Hope Rugo, an oncologist at the University of California, San Francisco, who was not involved in the study, said one of the advantages of duloxetine is the lack of somnolence observed in the study – a side effect that is bothersome to many of her breast cancer patients taking gabapentin for chronic neuropathic pain induced by taxanes or platinum-based therapy.
Press briefing moderator Dr. Nicholas Vogelzang, head of genitourinary cancer at the Nevada Cancer Institute in Las Vegas, echoed those remarks and said that neuropathy is fairly common among his patients treated with platinum-based chemotherapy. Duloxetine is an addition to the oncologist’s armamentarium, he said, adding "I’m certainly going to use this when I get back to the office."
Dr. Smith acknowledged that not everyone responded to duloxetine, but said that the dual serotonin norepinephrine reuptake inhibitor improves compliance with chemotherapy treatment and that most patients saw improved function and quality of life.
Patients with depression were excluded from the trial, so the effect of duloxetine was not simply because of improved mood, she said. Instead, it is thought that the drug eases pain by increasing serotonin and norepinephrine, and that responders may have an abnormality in the way their brain processes pain because of lower levels of these two pain-inhibiting neurotransmitters. Future work will try to determine which patients are most likely to respond to the antidepressant.
CALBG 170601 was supported by the National Cancer Institute division of cancer prevention and Lilly Pharmaceuticals. Dr. Smith reported no conflicts of interest. A coauthor reported research funding from Merck. Dr. Rugo has received research funding from Genentech/Roche, Abraxis BioScience, and Bristol-Myers Squibb.
CHICAGO – The antidepressant duloxetine reduces chronic pain from chemotherapy-induced peripheral neuropathy with fewer side effects than with current therapies, according to results of a phase III, double-blind trial.
Among evaluable patients, 59% reported less pain with duloxetine (Cymbalta), compared with 38% with placebo. A pain reduction of 30% or more – a measure considered clinically significant – was reported by 33% and 17%, respectively.
"Duloxetine 60 mg a day is the first drug to be shown to be effective in painful chemotherapy-induced peripheral neuropathy based on the results of a randomized trial," Ellen Lavoie Smith, Ph.D., said at a press conference at the annual meeting of the American Society of Clinical Oncology.
A variety of other agents – including gabapentin and tricyclic antidepressants – have been shown to be effective in treating neuropathic pain and are routinely pressed into service in oncology practice, but have failed to demonstrate efficacy in randomized trials of peripheral neuropathy caused by chemotherapy, she noted.
"Some patients endure this painful neuropathy for months and possibly for as long as years following completion of chemotherapy," she said. "It’s chronic, it’s very distressing and it’s disabling. And there is nothing to date effective in treating this problem."
Duloxetine is approved to treat major depressive disorder, with an additional indication for chronic musculoskeletal pain added in 2010. The safety label was revised in September 2011 to include warnings for severe skin reactions including Stevens-Johnson Syndrome and erythema multiforme.
The Cancer and Leukemia Group B (CALGB) 170601 trial randomized 231 patients with painful neuropathy after receiving single-agent paclitaxel (Taxol) or oxaliplatin (Eloxatin) chemotherapy to duloxetine 30 mg for 1 week and 60 mg for 4 weeks, followed by crossover to placebo after a 1-week washout period or the same regimen in the opposite order. Pain levels were assessed weekly using the Brief Pain Inventory-Short Form (BPI-SF) in 220 patients.
The average change in BPI-SF score, the study’s primary outcome, was -1.09 in patients given duloxetine, compared with –0.33 given placebo (P = .003), reported Dr. Smith, with the University of Michigan, Ann Arbor.
During duloxetine treatment, patients also experienced a significant reduction in the BPI-SF pain interference score, a sum of seven items including interference with general activity, mood, walking, normal work, relations with people, sleep and enjoyment of life. There was no difference in duloxetine efficacy based on the specific neurotoxic chemotherapeutic agent received.
In all, 21% of patients said their pain was cut by at least one-half with duloxetine, while only 9% taking placebo did.
Interestingly, 11% of patients saw their pain increase with the serotonin-norepinephrine reuptake inhibitor, compared with 28% with placebo. The reason for the finding is unclear, Dr. Smith said in an interview.
"Pain is a very complicated thing to study," she said. "There are many things that go into it – psychosocial issues, environmental issues, cultural issues, but again, there may be patients who are more likely to respond to these drugs because their central nervous system isn’t really working normally."
Overall, duloxetine was well tolerated, but was associated with significantly more grade 2 or greater fatigue than placebo (11% vs. 3%; P = .029). Dr. Smith pointed out that the overall incidence of side effects was lower than observed in two studies of diabetes-related peripheral neuropathy, likely because they used the 60 mg dose without the lower 30 mg starting dose.
Dr. Hope Rugo, an oncologist at the University of California, San Francisco, who was not involved in the study, said one of the advantages of duloxetine is the lack of somnolence observed in the study – a side effect that is bothersome to many of her breast cancer patients taking gabapentin for chronic neuropathic pain induced by taxanes or platinum-based therapy.
Press briefing moderator Dr. Nicholas Vogelzang, head of genitourinary cancer at the Nevada Cancer Institute in Las Vegas, echoed those remarks and said that neuropathy is fairly common among his patients treated with platinum-based chemotherapy. Duloxetine is an addition to the oncologist’s armamentarium, he said, adding "I’m certainly going to use this when I get back to the office."
Dr. Smith acknowledged that not everyone responded to duloxetine, but said that the dual serotonin norepinephrine reuptake inhibitor improves compliance with chemotherapy treatment and that most patients saw improved function and quality of life.
Patients with depression were excluded from the trial, so the effect of duloxetine was not simply because of improved mood, she said. Instead, it is thought that the drug eases pain by increasing serotonin and norepinephrine, and that responders may have an abnormality in the way their brain processes pain because of lower levels of these two pain-inhibiting neurotransmitters. Future work will try to determine which patients are most likely to respond to the antidepressant.
CALBG 170601 was supported by the National Cancer Institute division of cancer prevention and Lilly Pharmaceuticals. Dr. Smith reported no conflicts of interest. A coauthor reported research funding from Merck. Dr. Rugo has received research funding from Genentech/Roche, Abraxis BioScience, and Bristol-Myers Squibb.
CHICAGO – The antidepressant duloxetine reduces chronic pain from chemotherapy-induced peripheral neuropathy with fewer side effects than with current therapies, according to results of a phase III, double-blind trial.
Among evaluable patients, 59% reported less pain with duloxetine (Cymbalta), compared with 38% with placebo. A pain reduction of 30% or more – a measure considered clinically significant – was reported by 33% and 17%, respectively.
"Duloxetine 60 mg a day is the first drug to be shown to be effective in painful chemotherapy-induced peripheral neuropathy based on the results of a randomized trial," Ellen Lavoie Smith, Ph.D., said at a press conference at the annual meeting of the American Society of Clinical Oncology.
A variety of other agents – including gabapentin and tricyclic antidepressants – have been shown to be effective in treating neuropathic pain and are routinely pressed into service in oncology practice, but have failed to demonstrate efficacy in randomized trials of peripheral neuropathy caused by chemotherapy, she noted.
"Some patients endure this painful neuropathy for months and possibly for as long as years following completion of chemotherapy," she said. "It’s chronic, it’s very distressing and it’s disabling. And there is nothing to date effective in treating this problem."
Duloxetine is approved to treat major depressive disorder, with an additional indication for chronic musculoskeletal pain added in 2010. The safety label was revised in September 2011 to include warnings for severe skin reactions including Stevens-Johnson Syndrome and erythema multiforme.
The Cancer and Leukemia Group B (CALGB) 170601 trial randomized 231 patients with painful neuropathy after receiving single-agent paclitaxel (Taxol) or oxaliplatin (Eloxatin) chemotherapy to duloxetine 30 mg for 1 week and 60 mg for 4 weeks, followed by crossover to placebo after a 1-week washout period or the same regimen in the opposite order. Pain levels were assessed weekly using the Brief Pain Inventory-Short Form (BPI-SF) in 220 patients.
The average change in BPI-SF score, the study’s primary outcome, was -1.09 in patients given duloxetine, compared with –0.33 given placebo (P = .003), reported Dr. Smith, with the University of Michigan, Ann Arbor.
During duloxetine treatment, patients also experienced a significant reduction in the BPI-SF pain interference score, a sum of seven items including interference with general activity, mood, walking, normal work, relations with people, sleep and enjoyment of life. There was no difference in duloxetine efficacy based on the specific neurotoxic chemotherapeutic agent received.
In all, 21% of patients said their pain was cut by at least one-half with duloxetine, while only 9% taking placebo did.
Interestingly, 11% of patients saw their pain increase with the serotonin-norepinephrine reuptake inhibitor, compared with 28% with placebo. The reason for the finding is unclear, Dr. Smith said in an interview.
"Pain is a very complicated thing to study," she said. "There are many things that go into it – psychosocial issues, environmental issues, cultural issues, but again, there may be patients who are more likely to respond to these drugs because their central nervous system isn’t really working normally."
Overall, duloxetine was well tolerated, but was associated with significantly more grade 2 or greater fatigue than placebo (11% vs. 3%; P = .029). Dr. Smith pointed out that the overall incidence of side effects was lower than observed in two studies of diabetes-related peripheral neuropathy, likely because they used the 60 mg dose without the lower 30 mg starting dose.
Dr. Hope Rugo, an oncologist at the University of California, San Francisco, who was not involved in the study, said one of the advantages of duloxetine is the lack of somnolence observed in the study – a side effect that is bothersome to many of her breast cancer patients taking gabapentin for chronic neuropathic pain induced by taxanes or platinum-based therapy.
Press briefing moderator Dr. Nicholas Vogelzang, head of genitourinary cancer at the Nevada Cancer Institute in Las Vegas, echoed those remarks and said that neuropathy is fairly common among his patients treated with platinum-based chemotherapy. Duloxetine is an addition to the oncologist’s armamentarium, he said, adding "I’m certainly going to use this when I get back to the office."
Dr. Smith acknowledged that not everyone responded to duloxetine, but said that the dual serotonin norepinephrine reuptake inhibitor improves compliance with chemotherapy treatment and that most patients saw improved function and quality of life.
Patients with depression were excluded from the trial, so the effect of duloxetine was not simply because of improved mood, she said. Instead, it is thought that the drug eases pain by increasing serotonin and norepinephrine, and that responders may have an abnormality in the way their brain processes pain because of lower levels of these two pain-inhibiting neurotransmitters. Future work will try to determine which patients are most likely to respond to the antidepressant.
CALBG 170601 was supported by the National Cancer Institute division of cancer prevention and Lilly Pharmaceuticals. Dr. Smith reported no conflicts of interest. A coauthor reported research funding from Merck. Dr. Rugo has received research funding from Genentech/Roche, Abraxis BioScience, and Bristol-Myers Squibb.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding: A pain reduction of 30% or more was seen in 33% of patients on duloxetine vs. 17% on placebo.
Data Source: Investigators conducted a double-blind, phase III randomized controlled trial in 231 patients with peripheral neuropathy.
Disclosures: CALBG 170601 was supported by the National Cancer Institute division of cancer prevention and Lilly Pharmaceuticals. Dr. Smith reported no conflicts of interest. A co-author reported research funding from Merck.
Adjuvant PCV Chemo Hikes Oligodendroglioma Survival
CHICAGO – Patients with newly diagnosed anaplastic oligodendroglial tumors with chromosome 1p and 19q deletions live dramatically longer lives if PCV chemotherapy is added before or after standard radiation therapy, long-term follow-up from two large, prospective trials shows.*
The survival benefit was not statistically significant in patients without the signature co-deletions, which occur in about 50%-60% of patients with this rare, slow-growing brain tumor.
Median overall survival in the 80 patients harboring the 1p/19q co-deletions was 9.3 years with radiation, but had not been reached in those also treated with PCV chemotherapy (hazard ratio 0.56; P value = .059).
Overall survival was 21 months and 25 months, respectively, among the 236 patients without the co-deletions (HR 0.83; P = .19) in the European Organization for Research and Treatment of Cancer (EORTC) 26951 trial.
"This opens the venue for personalized medicine not based on the histology of the tumor, but the molecular signatures of these tumors," Dr. Martin van den Bent said at a press conference at the annual meeting of the American Society of Clinical Oncology.
A survival advantage with combination therapy was also seen in patients with MGMT (O6-methylguanine DNA methyltransferase) methylated tumors and IDH (isocitrate dehydrogenase) mutations, but further study will be needed to confirm this, he added.
The most pressing question for clinicians is whether to use the older, more toxic PCV chemotherapy regimen of procarbazine (Matulane), lomustine (CeeNU) and vincristine (Oncovin) for its known survival advantage or to substitute the less toxic, oral alkylating agent temozolomide (Temodar), which has replaced PCV since the phase III trial was launched some 17 years ago.
Dr. van den Bent said in an interview that PCV plus radiation should be the standard of care for anaplastic oligodendroglial (AOD) patients but admits it’s a difficult question to answer because of the toxicity associated with PCV, including weight loss and bone marrow suppression, which he described as mostly asymptomatic. Nearly half or 46% of patients treated with PCV in the trial experienced grade 3 or 4 hematologic toxicity.
He noted, however, that the results were the same regardless of the number of PCV cycles given in the trial, raising the question of how much PCV chemotherapy is actually needed.
"The thing that’s important is that we now know that we get this huge increase in survival with PCV," he said. "The question for the physicians at home is whether they are willing to trade a certain survival benefit for an unknown survival benefit, and I expect that there will be a lot of discussions in the coming months where this will be one of the primary questions."
Dr. Mark R. Gilbert, a professor of neuro-oncology at the University of Texas M.D. Anderson Cancer Center in Houston, who was invited to discuss the plenary abstract, said the previous standard of radiation alone is no longer adequate for patients with AOD tumors with 1p/19q co-deletions, and that the existing data support the first-line treatment of these patients with radiation and chemotherapy.
He added that the optimal chemo-radiation treatment has not been established, with data available on chemotherapy given before or after radiation and the place for temozolomide in this disease yet to be determined.
"If we decide temozolomide has a role, should it be used as we do for grade IV glioma, which is concurrently with radiation followed by adjuvant treatment?" he asked.
EORTC 26951 randomized patients to radiotherapy 59.4 Gy alone or followed by six cycles of lomustine 110 mg/m2 on day 1, procarbazine 60 mg/m2 on day 8-21 and IV vincristine 1.4 mg/m2 on days 8 and 28. The median follow-up was 140 months, with 24% of patients still alive in 2012. In all, 75% of patients who progressed in the radiation arm crossed over to PCV.
In the intent-to-treat population, median overall survival increased from 31 months with radiation alone to 42 months with the addition of PCV chemotherapy (HR 0.75; P = .018). The overall survival benefit was observed despite the crossover treatment (risk reduction 0.75), observed Dr. van den Bent, professor of neuro-oncology at Erasmus University Medical Center, Rotterdam, the Netherlands.
Progression-free survival nearly doubled from 13 months to 24 months with adjuvant PCV (HR 0.66; P = .003).
In patients with the 1p/19q co-deletions, median overall survival was 112 months with radiation alone, but has not been reached in those treated also given chemotherapy (P = .059; relative risk reduction 0.56).
Among those without 1p/19q co-deletions, the addition of PCV delayed progression from a median of 50 months with radiation alone to 157 months, (HR 0.42). In the non-deleted patients, progression was prolonged from 9 months to only 15 months (HR 0.73).
In univariate analysis 1p/19q co-deletions, IDH, and MGMT were all independent prognostic factors for survival (P less than .0001), with only MGMT falling out in multivariate analysis.
Virtually all 1p/19q co-deleted tumors show IDH mutation and virtually all IDH-mutated tumors show MGMT promoter methylation, Dr. van den Bent pointed out. Post-hoc testing revealed IDH mutations in 46% of 178 patients and MGMT methylation in 74% of 183 patients tested.
In a separate presentation at the meeting, North American investigators reported that patients with 1p/19q co-deletions lived twice as long or 14.7 years with PCV chemotherapy followed by radiation, compared with 7.3 years with radiation alone in the phase III, 291-patient Radiation Therapy Oncology Group (RTOG) 9402 trial (HR 0.59; P = .03).
Survival times were not significantly different at 2.6 years and 2.7 years, respectively, in patients without such deletions (HR 0.86; P = .39).
In all, 64% of patients given PCV experienced grade III-IV toxicity, although salvage treatment was more common with radiation only (81% vs. 57%; P = .04).
The overall survival benefit was observed after a median follow-up of 11.3 years, reversing an early analysis in 2006 that showed no overall survival benefit for the combined therapy, according to lead author Dr. J. Gregory Cairncross, professor and head of clinical neurosciences at University of Calgary (Alta.).
The same phenomenon was reported in the EORTC cohort.
"It could be because, after 6 or 7 years, the effects of radiation therapy begin to wear off and then the beneficial effects of chemotherapy kick in," Dr. van den Bent speculated. "We don’t know. It’s a unique phenomenon. I can’t recall having seen this before, but we have two trials showing exactly the same separation of the survival curves after only 6 years."
Press conference moderator Dr. Bruce J. Roth, an oncology professor at Washington University in St. Louis, said most AOD patients in the United States are treated with radiation after surgery, and that the minority of those who do receive chemotherapy will likely continue to receive temozolomide because of the ease of administration and lower toxicity.
Dr. van den Bent said the ongoing intergroup phase III CATNON trial of concurrent and adjuvant temozolomide in patients with non-1p/19q co-deleted tumors should further define which patients benefit from chemotherapy, but that results will not be available for many years.
Dr. Van den Bent reports consulting with and honoraria from MSD. A co-author reports consulting with MSD and honoraria from GlaxoSmithKline and Roche Diagnostics.
RTOG 9402 was supported by grants from the National Cancer Institute, North Central Cancer Treatment Group, Southwest Oncology Group, Eastern Cooperative Oncology Group and the NCIC Clinical Trials Group.
* This story has been updated and revised on 6/8/2012.
CHICAGO – Patients with newly diagnosed anaplastic oligodendroglial tumors with chromosome 1p and 19q deletions live dramatically longer lives if PCV chemotherapy is added before or after standard radiation therapy, long-term follow-up from two large, prospective trials shows.*
The survival benefit was not statistically significant in patients without the signature co-deletions, which occur in about 50%-60% of patients with this rare, slow-growing brain tumor.
Median overall survival in the 80 patients harboring the 1p/19q co-deletions was 9.3 years with radiation, but had not been reached in those also treated with PCV chemotherapy (hazard ratio 0.56; P value = .059).
Overall survival was 21 months and 25 months, respectively, among the 236 patients without the co-deletions (HR 0.83; P = .19) in the European Organization for Research and Treatment of Cancer (EORTC) 26951 trial.
"This opens the venue for personalized medicine not based on the histology of the tumor, but the molecular signatures of these tumors," Dr. Martin van den Bent said at a press conference at the annual meeting of the American Society of Clinical Oncology.
A survival advantage with combination therapy was also seen in patients with MGMT (O6-methylguanine DNA methyltransferase) methylated tumors and IDH (isocitrate dehydrogenase) mutations, but further study will be needed to confirm this, he added.
The most pressing question for clinicians is whether to use the older, more toxic PCV chemotherapy regimen of procarbazine (Matulane), lomustine (CeeNU) and vincristine (Oncovin) for its known survival advantage or to substitute the less toxic, oral alkylating agent temozolomide (Temodar), which has replaced PCV since the phase III trial was launched some 17 years ago.
Dr. van den Bent said in an interview that PCV plus radiation should be the standard of care for anaplastic oligodendroglial (AOD) patients but admits it’s a difficult question to answer because of the toxicity associated with PCV, including weight loss and bone marrow suppression, which he described as mostly asymptomatic. Nearly half or 46% of patients treated with PCV in the trial experienced grade 3 or 4 hematologic toxicity.
He noted, however, that the results were the same regardless of the number of PCV cycles given in the trial, raising the question of how much PCV chemotherapy is actually needed.
"The thing that’s important is that we now know that we get this huge increase in survival with PCV," he said. "The question for the physicians at home is whether they are willing to trade a certain survival benefit for an unknown survival benefit, and I expect that there will be a lot of discussions in the coming months where this will be one of the primary questions."
Dr. Mark R. Gilbert, a professor of neuro-oncology at the University of Texas M.D. Anderson Cancer Center in Houston, who was invited to discuss the plenary abstract, said the previous standard of radiation alone is no longer adequate for patients with AOD tumors with 1p/19q co-deletions, and that the existing data support the first-line treatment of these patients with radiation and chemotherapy.
He added that the optimal chemo-radiation treatment has not been established, with data available on chemotherapy given before or after radiation and the place for temozolomide in this disease yet to be determined.
"If we decide temozolomide has a role, should it be used as we do for grade IV glioma, which is concurrently with radiation followed by adjuvant treatment?" he asked.
EORTC 26951 randomized patients to radiotherapy 59.4 Gy alone or followed by six cycles of lomustine 110 mg/m2 on day 1, procarbazine 60 mg/m2 on day 8-21 and IV vincristine 1.4 mg/m2 on days 8 and 28. The median follow-up was 140 months, with 24% of patients still alive in 2012. In all, 75% of patients who progressed in the radiation arm crossed over to PCV.
In the intent-to-treat population, median overall survival increased from 31 months with radiation alone to 42 months with the addition of PCV chemotherapy (HR 0.75; P = .018). The overall survival benefit was observed despite the crossover treatment (risk reduction 0.75), observed Dr. van den Bent, professor of neuro-oncology at Erasmus University Medical Center, Rotterdam, the Netherlands.
Progression-free survival nearly doubled from 13 months to 24 months with adjuvant PCV (HR 0.66; P = .003).
In patients with the 1p/19q co-deletions, median overall survival was 112 months with radiation alone, but has not been reached in those treated also given chemotherapy (P = .059; relative risk reduction 0.56).
Among those without 1p/19q co-deletions, the addition of PCV delayed progression from a median of 50 months with radiation alone to 157 months, (HR 0.42). In the non-deleted patients, progression was prolonged from 9 months to only 15 months (HR 0.73).
In univariate analysis 1p/19q co-deletions, IDH, and MGMT were all independent prognostic factors for survival (P less than .0001), with only MGMT falling out in multivariate analysis.
Virtually all 1p/19q co-deleted tumors show IDH mutation and virtually all IDH-mutated tumors show MGMT promoter methylation, Dr. van den Bent pointed out. Post-hoc testing revealed IDH mutations in 46% of 178 patients and MGMT methylation in 74% of 183 patients tested.
In a separate presentation at the meeting, North American investigators reported that patients with 1p/19q co-deletions lived twice as long or 14.7 years with PCV chemotherapy followed by radiation, compared with 7.3 years with radiation alone in the phase III, 291-patient Radiation Therapy Oncology Group (RTOG) 9402 trial (HR 0.59; P = .03).
Survival times were not significantly different at 2.6 years and 2.7 years, respectively, in patients without such deletions (HR 0.86; P = .39).
In all, 64% of patients given PCV experienced grade III-IV toxicity, although salvage treatment was more common with radiation only (81% vs. 57%; P = .04).
The overall survival benefit was observed after a median follow-up of 11.3 years, reversing an early analysis in 2006 that showed no overall survival benefit for the combined therapy, according to lead author Dr. J. Gregory Cairncross, professor and head of clinical neurosciences at University of Calgary (Alta.).
The same phenomenon was reported in the EORTC cohort.
"It could be because, after 6 or 7 years, the effects of radiation therapy begin to wear off and then the beneficial effects of chemotherapy kick in," Dr. van den Bent speculated. "We don’t know. It’s a unique phenomenon. I can’t recall having seen this before, but we have two trials showing exactly the same separation of the survival curves after only 6 years."
Press conference moderator Dr. Bruce J. Roth, an oncology professor at Washington University in St. Louis, said most AOD patients in the United States are treated with radiation after surgery, and that the minority of those who do receive chemotherapy will likely continue to receive temozolomide because of the ease of administration and lower toxicity.
Dr. van den Bent said the ongoing intergroup phase III CATNON trial of concurrent and adjuvant temozolomide in patients with non-1p/19q co-deleted tumors should further define which patients benefit from chemotherapy, but that results will not be available for many years.
Dr. Van den Bent reports consulting with and honoraria from MSD. A co-author reports consulting with MSD and honoraria from GlaxoSmithKline and Roche Diagnostics.
RTOG 9402 was supported by grants from the National Cancer Institute, North Central Cancer Treatment Group, Southwest Oncology Group, Eastern Cooperative Oncology Group and the NCIC Clinical Trials Group.
* This story has been updated and revised on 6/8/2012.
CHICAGO – Patients with newly diagnosed anaplastic oligodendroglial tumors with chromosome 1p and 19q deletions live dramatically longer lives if PCV chemotherapy is added before or after standard radiation therapy, long-term follow-up from two large, prospective trials shows.*
The survival benefit was not statistically significant in patients without the signature co-deletions, which occur in about 50%-60% of patients with this rare, slow-growing brain tumor.
Median overall survival in the 80 patients harboring the 1p/19q co-deletions was 9.3 years with radiation, but had not been reached in those also treated with PCV chemotherapy (hazard ratio 0.56; P value = .059).
Overall survival was 21 months and 25 months, respectively, among the 236 patients without the co-deletions (HR 0.83; P = .19) in the European Organization for Research and Treatment of Cancer (EORTC) 26951 trial.
"This opens the venue for personalized medicine not based on the histology of the tumor, but the molecular signatures of these tumors," Dr. Martin van den Bent said at a press conference at the annual meeting of the American Society of Clinical Oncology.
A survival advantage with combination therapy was also seen in patients with MGMT (O6-methylguanine DNA methyltransferase) methylated tumors and IDH (isocitrate dehydrogenase) mutations, but further study will be needed to confirm this, he added.
The most pressing question for clinicians is whether to use the older, more toxic PCV chemotherapy regimen of procarbazine (Matulane), lomustine (CeeNU) and vincristine (Oncovin) for its known survival advantage or to substitute the less toxic, oral alkylating agent temozolomide (Temodar), which has replaced PCV since the phase III trial was launched some 17 years ago.
Dr. van den Bent said in an interview that PCV plus radiation should be the standard of care for anaplastic oligodendroglial (AOD) patients but admits it’s a difficult question to answer because of the toxicity associated with PCV, including weight loss and bone marrow suppression, which he described as mostly asymptomatic. Nearly half or 46% of patients treated with PCV in the trial experienced grade 3 or 4 hematologic toxicity.
He noted, however, that the results were the same regardless of the number of PCV cycles given in the trial, raising the question of how much PCV chemotherapy is actually needed.
"The thing that’s important is that we now know that we get this huge increase in survival with PCV," he said. "The question for the physicians at home is whether they are willing to trade a certain survival benefit for an unknown survival benefit, and I expect that there will be a lot of discussions in the coming months where this will be one of the primary questions."
Dr. Mark R. Gilbert, a professor of neuro-oncology at the University of Texas M.D. Anderson Cancer Center in Houston, who was invited to discuss the plenary abstract, said the previous standard of radiation alone is no longer adequate for patients with AOD tumors with 1p/19q co-deletions, and that the existing data support the first-line treatment of these patients with radiation and chemotherapy.
He added that the optimal chemo-radiation treatment has not been established, with data available on chemotherapy given before or after radiation and the place for temozolomide in this disease yet to be determined.
"If we decide temozolomide has a role, should it be used as we do for grade IV glioma, which is concurrently with radiation followed by adjuvant treatment?" he asked.
EORTC 26951 randomized patients to radiotherapy 59.4 Gy alone or followed by six cycles of lomustine 110 mg/m2 on day 1, procarbazine 60 mg/m2 on day 8-21 and IV vincristine 1.4 mg/m2 on days 8 and 28. The median follow-up was 140 months, with 24% of patients still alive in 2012. In all, 75% of patients who progressed in the radiation arm crossed over to PCV.
In the intent-to-treat population, median overall survival increased from 31 months with radiation alone to 42 months with the addition of PCV chemotherapy (HR 0.75; P = .018). The overall survival benefit was observed despite the crossover treatment (risk reduction 0.75), observed Dr. van den Bent, professor of neuro-oncology at Erasmus University Medical Center, Rotterdam, the Netherlands.
Progression-free survival nearly doubled from 13 months to 24 months with adjuvant PCV (HR 0.66; P = .003).
In patients with the 1p/19q co-deletions, median overall survival was 112 months with radiation alone, but has not been reached in those treated also given chemotherapy (P = .059; relative risk reduction 0.56).
Among those without 1p/19q co-deletions, the addition of PCV delayed progression from a median of 50 months with radiation alone to 157 months, (HR 0.42). In the non-deleted patients, progression was prolonged from 9 months to only 15 months (HR 0.73).
In univariate analysis 1p/19q co-deletions, IDH, and MGMT were all independent prognostic factors for survival (P less than .0001), with only MGMT falling out in multivariate analysis.
Virtually all 1p/19q co-deleted tumors show IDH mutation and virtually all IDH-mutated tumors show MGMT promoter methylation, Dr. van den Bent pointed out. Post-hoc testing revealed IDH mutations in 46% of 178 patients and MGMT methylation in 74% of 183 patients tested.
In a separate presentation at the meeting, North American investigators reported that patients with 1p/19q co-deletions lived twice as long or 14.7 years with PCV chemotherapy followed by radiation, compared with 7.3 years with radiation alone in the phase III, 291-patient Radiation Therapy Oncology Group (RTOG) 9402 trial (HR 0.59; P = .03).
Survival times were not significantly different at 2.6 years and 2.7 years, respectively, in patients without such deletions (HR 0.86; P = .39).
In all, 64% of patients given PCV experienced grade III-IV toxicity, although salvage treatment was more common with radiation only (81% vs. 57%; P = .04).
The overall survival benefit was observed after a median follow-up of 11.3 years, reversing an early analysis in 2006 that showed no overall survival benefit for the combined therapy, according to lead author Dr. J. Gregory Cairncross, professor and head of clinical neurosciences at University of Calgary (Alta.).
The same phenomenon was reported in the EORTC cohort.
"It could be because, after 6 or 7 years, the effects of radiation therapy begin to wear off and then the beneficial effects of chemotherapy kick in," Dr. van den Bent speculated. "We don’t know. It’s a unique phenomenon. I can’t recall having seen this before, but we have two trials showing exactly the same separation of the survival curves after only 6 years."
Press conference moderator Dr. Bruce J. Roth, an oncology professor at Washington University in St. Louis, said most AOD patients in the United States are treated with radiation after surgery, and that the minority of those who do receive chemotherapy will likely continue to receive temozolomide because of the ease of administration and lower toxicity.
Dr. van den Bent said the ongoing intergroup phase III CATNON trial of concurrent and adjuvant temozolomide in patients with non-1p/19q co-deleted tumors should further define which patients benefit from chemotherapy, but that results will not be available for many years.
Dr. Van den Bent reports consulting with and honoraria from MSD. A co-author reports consulting with MSD and honoraria from GlaxoSmithKline and Roche Diagnostics.
RTOG 9402 was supported by grants from the National Cancer Institute, North Central Cancer Treatment Group, Southwest Oncology Group, Eastern Cooperative Oncology Group and the NCIC Clinical Trials Group.
* This story has been updated and revised on 6/8/2012.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF CLINICAL ONCOLOGY
Major Finding:. In the intent-to-treat population, median overall survival increased from 31 months with radiation alone to 42 months with the addition of PCV chemotherapy (P = .018).
Data Source: Phase III EORTC 26951 and RTOG 9402 trials of PCV chemotherapy plus radiation for anaplastic oligodendroglial tumors.
Disclosures: Dr. Van den Bent reports consulting with and honoraria from MSD. A co-author reports consulting with MSD, and honoraria from GlaxoSmithKline and Roche Diagnostics. RTOG 9402 was supported by grants from the National Cancer Institute, North Central Cancer Treatment Group, Southwest Oncology Group, Eastern Cooperative Oncology Group and the NCIC Clinical Trials Group.
In the Pipeline: Immunotherapy Slows Childhood Gliomas
CHICAGO – Immunotherapy with peptide vaccines may offer a much-needed therapeutic option for gliomas in children, which carry a poor prognosis despite current treatments.
In a pilot trial of subcutaneous vaccinations with peptides for glioma-associated antigen (GAA) epitopes in children who have been newly diagnosed with brain stem gliomas, cerebral high-grade gliomas, or recurrent gliomas, the immunotherapy was well tolerated and demonstrated immunologic and clinical activity, Dr. Ian F. Pollack reported April 2 at the annual meeting of the American Association for Cancer Research.
Based on significant experience with immunotherapy for adult gliomas, "we extended these insights to childhood gliomas – malignant astrocytomas of the brain stem and cerebral hemispheres and recurrent low-grade gliomas – based on our observations of their glioma-associated antigen expression profiles," Dr. Pollack explained during a press conference.
For the pilot study, Dr. Pollack, chief of pediatric neurosurgery at Children’s Hospital of Pittsburgh Brain Care Institute, and his colleagues have enrolled 27 human leukocyte antigen (HLA) A2–positive children to date, including 16 who are newly diagnosed with brain stem gliomas, 5 with newly diagnosed high-grade gliomas, and 6 with recurrent gliomas. The GAAs were EphA2, interleukin-13 receptor alpha 2 (IL-13RA2), and survivin, he said.
All of the children received eight courses of subcutaneous vaccinations with peptides for GAA epitopes emulsified in Montanide-ISA-51 every 3 weeks, along with intramuscular injections of the immunoadjuvant poly-ICLC, which promotes the infiltration of effector T cells into intracranial gliomas.
"Our primary end points were safety and T-cell response against vaccine-targeted [GAAs]," he said, noting that treatment response was assessed clinically and by MRI.
The preliminary results reported at the meeting are based on an interim analysis of 22 evaluable patients. To date, no non–central nervous system toxicities have limited the vaccine dosages, Dr. Pollack said. Of the 22 children, 4 showed signs of rapidly progressive disease, 14 had stable disease for more than 3 months, 3 had sustained partial responses, and 1 had prolonged disease-free status after surgery, he reported.
Symptomatic "pseudoprogression" – consisting of transient neurologic deterioration and tumor enlargement, followed by tumor regression and stabilization on decreasing steroid doses with sustained partial response – was observed in four of the children with brain stem glioma, said Dr. Pollack, who is also codirector of the University of Pittsburgh Cancer Institute’s brain tumor program.
Results of the ELISPOT (enzyme-linked immunosorbent spot) assay, which was completed in seven of the children, showed responses in six of them. Specifically, the investigators observed responses to IL-13RA2 in five cases, EphA2 in three cases, and survivin in three cases, Dr. Pollack said.
"Based on what we’ve seen so far, it seems that these kids are able to mount immune responses to the vaccine at high rates, possibly higher than we’ve seen in adult studies," likely because of their robust immune systems, he said in an interview.
The observation of immunologic and clinical response – particularly the evidence of tumor shrinkage in children with very high-risk tumors – "has been extremely encouraging and somewhat surprising," Dr. Pollack reported. "This is the first study of its type that examined peptide vaccine therapy for children with brain tumors like this."
The findings are especially notable because children with these tumors generally do not respond well to standard chemotherapy, he said, stressing that if further study validates the early findings, "immunotherapy may be a promising strategy to control tumor growth."
The study was funded by the National Institutes of Health. Dr. Pollack disclosed having no potential conflicts of interest.
CHICAGO – Immunotherapy with peptide vaccines may offer a much-needed therapeutic option for gliomas in children, which carry a poor prognosis despite current treatments.
In a pilot trial of subcutaneous vaccinations with peptides for glioma-associated antigen (GAA) epitopes in children who have been newly diagnosed with brain stem gliomas, cerebral high-grade gliomas, or recurrent gliomas, the immunotherapy was well tolerated and demonstrated immunologic and clinical activity, Dr. Ian F. Pollack reported April 2 at the annual meeting of the American Association for Cancer Research.
Based on significant experience with immunotherapy for adult gliomas, "we extended these insights to childhood gliomas – malignant astrocytomas of the brain stem and cerebral hemispheres and recurrent low-grade gliomas – based on our observations of their glioma-associated antigen expression profiles," Dr. Pollack explained during a press conference.
For the pilot study, Dr. Pollack, chief of pediatric neurosurgery at Children’s Hospital of Pittsburgh Brain Care Institute, and his colleagues have enrolled 27 human leukocyte antigen (HLA) A2–positive children to date, including 16 who are newly diagnosed with brain stem gliomas, 5 with newly diagnosed high-grade gliomas, and 6 with recurrent gliomas. The GAAs were EphA2, interleukin-13 receptor alpha 2 (IL-13RA2), and survivin, he said.
All of the children received eight courses of subcutaneous vaccinations with peptides for GAA epitopes emulsified in Montanide-ISA-51 every 3 weeks, along with intramuscular injections of the immunoadjuvant poly-ICLC, which promotes the infiltration of effector T cells into intracranial gliomas.
"Our primary end points were safety and T-cell response against vaccine-targeted [GAAs]," he said, noting that treatment response was assessed clinically and by MRI.
The preliminary results reported at the meeting are based on an interim analysis of 22 evaluable patients. To date, no non–central nervous system toxicities have limited the vaccine dosages, Dr. Pollack said. Of the 22 children, 4 showed signs of rapidly progressive disease, 14 had stable disease for more than 3 months, 3 had sustained partial responses, and 1 had prolonged disease-free status after surgery, he reported.
Symptomatic "pseudoprogression" – consisting of transient neurologic deterioration and tumor enlargement, followed by tumor regression and stabilization on decreasing steroid doses with sustained partial response – was observed in four of the children with brain stem glioma, said Dr. Pollack, who is also codirector of the University of Pittsburgh Cancer Institute’s brain tumor program.
Results of the ELISPOT (enzyme-linked immunosorbent spot) assay, which was completed in seven of the children, showed responses in six of them. Specifically, the investigators observed responses to IL-13RA2 in five cases, EphA2 in three cases, and survivin in three cases, Dr. Pollack said.
"Based on what we’ve seen so far, it seems that these kids are able to mount immune responses to the vaccine at high rates, possibly higher than we’ve seen in adult studies," likely because of their robust immune systems, he said in an interview.
The observation of immunologic and clinical response – particularly the evidence of tumor shrinkage in children with very high-risk tumors – "has been extremely encouraging and somewhat surprising," Dr. Pollack reported. "This is the first study of its type that examined peptide vaccine therapy for children with brain tumors like this."
The findings are especially notable because children with these tumors generally do not respond well to standard chemotherapy, he said, stressing that if further study validates the early findings, "immunotherapy may be a promising strategy to control tumor growth."
The study was funded by the National Institutes of Health. Dr. Pollack disclosed having no potential conflicts of interest.
CHICAGO – Immunotherapy with peptide vaccines may offer a much-needed therapeutic option for gliomas in children, which carry a poor prognosis despite current treatments.
In a pilot trial of subcutaneous vaccinations with peptides for glioma-associated antigen (GAA) epitopes in children who have been newly diagnosed with brain stem gliomas, cerebral high-grade gliomas, or recurrent gliomas, the immunotherapy was well tolerated and demonstrated immunologic and clinical activity, Dr. Ian F. Pollack reported April 2 at the annual meeting of the American Association for Cancer Research.
Based on significant experience with immunotherapy for adult gliomas, "we extended these insights to childhood gliomas – malignant astrocytomas of the brain stem and cerebral hemispheres and recurrent low-grade gliomas – based on our observations of their glioma-associated antigen expression profiles," Dr. Pollack explained during a press conference.
For the pilot study, Dr. Pollack, chief of pediatric neurosurgery at Children’s Hospital of Pittsburgh Brain Care Institute, and his colleagues have enrolled 27 human leukocyte antigen (HLA) A2–positive children to date, including 16 who are newly diagnosed with brain stem gliomas, 5 with newly diagnosed high-grade gliomas, and 6 with recurrent gliomas. The GAAs were EphA2, interleukin-13 receptor alpha 2 (IL-13RA2), and survivin, he said.
All of the children received eight courses of subcutaneous vaccinations with peptides for GAA epitopes emulsified in Montanide-ISA-51 every 3 weeks, along with intramuscular injections of the immunoadjuvant poly-ICLC, which promotes the infiltration of effector T cells into intracranial gliomas.
"Our primary end points were safety and T-cell response against vaccine-targeted [GAAs]," he said, noting that treatment response was assessed clinically and by MRI.
The preliminary results reported at the meeting are based on an interim analysis of 22 evaluable patients. To date, no non–central nervous system toxicities have limited the vaccine dosages, Dr. Pollack said. Of the 22 children, 4 showed signs of rapidly progressive disease, 14 had stable disease for more than 3 months, 3 had sustained partial responses, and 1 had prolonged disease-free status after surgery, he reported.
Symptomatic "pseudoprogression" – consisting of transient neurologic deterioration and tumor enlargement, followed by tumor regression and stabilization on decreasing steroid doses with sustained partial response – was observed in four of the children with brain stem glioma, said Dr. Pollack, who is also codirector of the University of Pittsburgh Cancer Institute’s brain tumor program.
Results of the ELISPOT (enzyme-linked immunosorbent spot) assay, which was completed in seven of the children, showed responses in six of them. Specifically, the investigators observed responses to IL-13RA2 in five cases, EphA2 in three cases, and survivin in three cases, Dr. Pollack said.
"Based on what we’ve seen so far, it seems that these kids are able to mount immune responses to the vaccine at high rates, possibly higher than we’ve seen in adult studies," likely because of their robust immune systems, he said in an interview.
The observation of immunologic and clinical response – particularly the evidence of tumor shrinkage in children with very high-risk tumors – "has been extremely encouraging and somewhat surprising," Dr. Pollack reported. "This is the first study of its type that examined peptide vaccine therapy for children with brain tumors like this."
The findings are especially notable because children with these tumors generally do not respond well to standard chemotherapy, he said, stressing that if further study validates the early findings, "immunotherapy may be a promising strategy to control tumor growth."
The study was funded by the National Institutes of Health. Dr. Pollack disclosed having no potential conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN ASSOCIATION FOR CANCER RESEARCH
Major Finding: Of 22 children with HLA A2–positive gliomas receiving eight courses of subcutaneous peptide vaccination, 4 showed signs of rapidly progressive disease, 14 had stable disease for more than 3 months, 3 had sustained partial responses, and 1 had prolonged disease-free status.
Data Source: The interim results are from a pilot trial of subcutaneous peptide vaccine in children with newly diagnosed gliomas.
Disclosures: Dr. Pollack disclosed no conflicts of interest.
'Scrambler' Device Reduces Refractory Neuropathic Pain
DENVER – Electrocutaneous direct nerve stimulation via a device that scrambles "pain" and "no pain" signals reduced pain scores in preliminary studies involving patients with refractory chemotherapy-induced peripheral neuropathy, post-herpetic neuralgia, and other forms of chronic, disabling neuropathic pain.
"What I saw in our pilot study in patients with chemotherapy-induced peripheral neuropathy was about a 60% reduction in pain, and you knew right away – within the first 3 days – whether it was going to work," said Dr. Thomas J. Smith, professor of oncology and director of palliative care at Johns Hopkins University, Baltimore.
The device, known as the MC5-A Calmare Scrambler, received Food and Drug Administration clearance in February 2009 and has a Medicare payment code. The Scrambler has been used worldwide to treat more than 4,000 patients with no reported serious side effects. Yet few American physicians have heard of the therapy because Medicare fixed the payment so low (at $44) that there is little financial incentive to adopt it, according to Dr. Smith.
"There’s not much you can do in the hospital outpatient setting for $44. That barely covers the cost of the electrodes and maybe a technician’s time," he said at the annual meeting of the American Academy of Hospice and Palliative Care Medicine. "They set the reimbursement so low that it’s almost guaranteed not to be accepted by physicians until we get more evidence. But there are a significant number of studies in the works."
Chemotherapy-induced peripheral neuropathy affects up to 30%-40% of treated cancer patients. Dr. Smith’s pilot study involved 16 patients with refractory chemotherapy-induced peripheral neuropathy that lasted from 3 months to 8 years. The most common drugs involved were taxanes, platinum-based agents, and bortezomib (Velcade). Patients received hour-long Scrambler sessions daily on 10 consecutive working days.
Pain scores fell from a mean baseline score of 5.8 on a 10-point scale to 2.4 at the end of 10 days. Four patients had a pain score of 0, and 15 of the 16 patients had at least a 20% reduction in their pain score on day 10. There was no toxicity (J. Pain Symptom Manage. 2010;40:883-91).
"When you see that sort of effect size, you can’t believe it’s real," Dr. Smith observed. "That’s in fact what the first reviewer said of the article. He said, ‘I’ve never heard of this therapy, and I don’t believe it.’ I volunteered to give him the case reports."
Neuropathic pain reductions in the 60% range are what are seen with permanent implanted spinal cord stimulation devices, a therapy that runs about $40,000, he added.
Dr. Smith’s Scrambler pilot study results were confirmed in an Italian study involving 40 patients with refractory cancer and 33 with non–cancer-related pain. Their mean pretreatment pain scale score was 6.2, plunging to 1.6 after the 10th day of treatment, and rebounding to 2.9 at follow-up 2 weeks after the final treatment session. Again, there were no side effects (Support Care Cancer. 2012;20:405-12).
Separately, a group led by the Scrambler’s inventor, Giuseppe Marineo, Ph.D., of the University of Rome Tor Vergata, reported on a randomized but unblinded trial involving 52 patients with chronic failed back syndrome, post-herpetic neuralgia, or spinal cord stenosis. Subjects assigned to the control arm received pharmacotherapy according to European Federation of Neurological Societies guidelines (Eur. J. Neurol. 2010;17:1113-e88), while the intervention arm received 10 daily Scrambler sessions.
The pretreatment mean visual analog pain score fell in 1 month from 8.1 in a control group and 8.0 in a group treated with the Scrambler to 5.8 in controls and to 0.7 in the Scrambler group. At 2 and 3 months of follow-up, the mean pain scores were 1.4 and 2.0 points, respectively, in the Scrambler group but were still 5.7 and 5.9 points in controls. A marked and persistent reduction in allodynia was also documented in response to Scrambler therapy (J. Pain Symptom Manage. 2012;43:87-95).
With regard to post-herpetic neuralgia, Dr. Smith presented a series of 10 treated patients. Their mean pain scores dropped from 8 at baseline to less than 1 at 1 month, holding steady with a score of 2 at 2 and 3 months’ follow-up.
The Scrambler entails application of 16 ECG-like electrode pads placed along the dermatome above and below the site of pain. The Scrambler machine is designed to feed 16 different nerve potentials in rapid sequence, essentially in order to confuse a firing nerve. The electrical charge is individually adjusted to patient tolerance. It feels like a bee sting, according to Dr. Smith. The mechanism of benefit isn’t well defined as yet.
"It’s likely acting like direct spinal cord stimulation, raising the gate threshold," he continued. "My hypothesis – and the inventor’s hypothesis as well – is that the therapy resets the damaged nerves at several sites. You see some effect within the first 30 minutes, and that rapid onset suggests biochemical change. And the long-lasting nature of the pain relief suggests remodeling as well as adaptation to the pain."
Clearly, further studies need to be done – free of industry sponsorship, on larger numbers of patients, and with sham-treated controls – in order to fully assess the Scrambler therapy’s efficacy, mechanism of action, and optimal schedule.
"The Scrambler is one of several neurocutaneous direct nerve stimulation techniques that are interesting but absolutely require further testing," Dr. Smith said.
Dr. Charles L. Loprinzi, professor of oncology at the Mayo Clinic, Rochester, Minn., has started such studies, he noted. "He was at least as skeptical of this as I was, and he’s been impressed with results from this machine," Dr. Smith said, adding that Dr. Loprinzi’s research team is expected to present data later this year at the annual meeting of the American Society of Clinical Oncology.
Competitive Technologies Inc., based in Fairfield, Conn., has worldwide rights to the device. Dr. Smith reported having no financial conflicts.
DENVER – Electrocutaneous direct nerve stimulation via a device that scrambles "pain" and "no pain" signals reduced pain scores in preliminary studies involving patients with refractory chemotherapy-induced peripheral neuropathy, post-herpetic neuralgia, and other forms of chronic, disabling neuropathic pain.
"What I saw in our pilot study in patients with chemotherapy-induced peripheral neuropathy was about a 60% reduction in pain, and you knew right away – within the first 3 days – whether it was going to work," said Dr. Thomas J. Smith, professor of oncology and director of palliative care at Johns Hopkins University, Baltimore.
The device, known as the MC5-A Calmare Scrambler, received Food and Drug Administration clearance in February 2009 and has a Medicare payment code. The Scrambler has been used worldwide to treat more than 4,000 patients with no reported serious side effects. Yet few American physicians have heard of the therapy because Medicare fixed the payment so low (at $44) that there is little financial incentive to adopt it, according to Dr. Smith.
"There’s not much you can do in the hospital outpatient setting for $44. That barely covers the cost of the electrodes and maybe a technician’s time," he said at the annual meeting of the American Academy of Hospice and Palliative Care Medicine. "They set the reimbursement so low that it’s almost guaranteed not to be accepted by physicians until we get more evidence. But there are a significant number of studies in the works."
Chemotherapy-induced peripheral neuropathy affects up to 30%-40% of treated cancer patients. Dr. Smith’s pilot study involved 16 patients with refractory chemotherapy-induced peripheral neuropathy that lasted from 3 months to 8 years. The most common drugs involved were taxanes, platinum-based agents, and bortezomib (Velcade). Patients received hour-long Scrambler sessions daily on 10 consecutive working days.
Pain scores fell from a mean baseline score of 5.8 on a 10-point scale to 2.4 at the end of 10 days. Four patients had a pain score of 0, and 15 of the 16 patients had at least a 20% reduction in their pain score on day 10. There was no toxicity (J. Pain Symptom Manage. 2010;40:883-91).
"When you see that sort of effect size, you can’t believe it’s real," Dr. Smith observed. "That’s in fact what the first reviewer said of the article. He said, ‘I’ve never heard of this therapy, and I don’t believe it.’ I volunteered to give him the case reports."
Neuropathic pain reductions in the 60% range are what are seen with permanent implanted spinal cord stimulation devices, a therapy that runs about $40,000, he added.
Dr. Smith’s Scrambler pilot study results were confirmed in an Italian study involving 40 patients with refractory cancer and 33 with non–cancer-related pain. Their mean pretreatment pain scale score was 6.2, plunging to 1.6 after the 10th day of treatment, and rebounding to 2.9 at follow-up 2 weeks after the final treatment session. Again, there were no side effects (Support Care Cancer. 2012;20:405-12).
Separately, a group led by the Scrambler’s inventor, Giuseppe Marineo, Ph.D., of the University of Rome Tor Vergata, reported on a randomized but unblinded trial involving 52 patients with chronic failed back syndrome, post-herpetic neuralgia, or spinal cord stenosis. Subjects assigned to the control arm received pharmacotherapy according to European Federation of Neurological Societies guidelines (Eur. J. Neurol. 2010;17:1113-e88), while the intervention arm received 10 daily Scrambler sessions.
The pretreatment mean visual analog pain score fell in 1 month from 8.1 in a control group and 8.0 in a group treated with the Scrambler to 5.8 in controls and to 0.7 in the Scrambler group. At 2 and 3 months of follow-up, the mean pain scores were 1.4 and 2.0 points, respectively, in the Scrambler group but were still 5.7 and 5.9 points in controls. A marked and persistent reduction in allodynia was also documented in response to Scrambler therapy (J. Pain Symptom Manage. 2012;43:87-95).
With regard to post-herpetic neuralgia, Dr. Smith presented a series of 10 treated patients. Their mean pain scores dropped from 8 at baseline to less than 1 at 1 month, holding steady with a score of 2 at 2 and 3 months’ follow-up.
The Scrambler entails application of 16 ECG-like electrode pads placed along the dermatome above and below the site of pain. The Scrambler machine is designed to feed 16 different nerve potentials in rapid sequence, essentially in order to confuse a firing nerve. The electrical charge is individually adjusted to patient tolerance. It feels like a bee sting, according to Dr. Smith. The mechanism of benefit isn’t well defined as yet.
"It’s likely acting like direct spinal cord stimulation, raising the gate threshold," he continued. "My hypothesis – and the inventor’s hypothesis as well – is that the therapy resets the damaged nerves at several sites. You see some effect within the first 30 minutes, and that rapid onset suggests biochemical change. And the long-lasting nature of the pain relief suggests remodeling as well as adaptation to the pain."
Clearly, further studies need to be done – free of industry sponsorship, on larger numbers of patients, and with sham-treated controls – in order to fully assess the Scrambler therapy’s efficacy, mechanism of action, and optimal schedule.
"The Scrambler is one of several neurocutaneous direct nerve stimulation techniques that are interesting but absolutely require further testing," Dr. Smith said.
Dr. Charles L. Loprinzi, professor of oncology at the Mayo Clinic, Rochester, Minn., has started such studies, he noted. "He was at least as skeptical of this as I was, and he’s been impressed with results from this machine," Dr. Smith said, adding that Dr. Loprinzi’s research team is expected to present data later this year at the annual meeting of the American Society of Clinical Oncology.
Competitive Technologies Inc., based in Fairfield, Conn., has worldwide rights to the device. Dr. Smith reported having no financial conflicts.
DENVER – Electrocutaneous direct nerve stimulation via a device that scrambles "pain" and "no pain" signals reduced pain scores in preliminary studies involving patients with refractory chemotherapy-induced peripheral neuropathy, post-herpetic neuralgia, and other forms of chronic, disabling neuropathic pain.
"What I saw in our pilot study in patients with chemotherapy-induced peripheral neuropathy was about a 60% reduction in pain, and you knew right away – within the first 3 days – whether it was going to work," said Dr. Thomas J. Smith, professor of oncology and director of palliative care at Johns Hopkins University, Baltimore.
The device, known as the MC5-A Calmare Scrambler, received Food and Drug Administration clearance in February 2009 and has a Medicare payment code. The Scrambler has been used worldwide to treat more than 4,000 patients with no reported serious side effects. Yet few American physicians have heard of the therapy because Medicare fixed the payment so low (at $44) that there is little financial incentive to adopt it, according to Dr. Smith.
"There’s not much you can do in the hospital outpatient setting for $44. That barely covers the cost of the electrodes and maybe a technician’s time," he said at the annual meeting of the American Academy of Hospice and Palliative Care Medicine. "They set the reimbursement so low that it’s almost guaranteed not to be accepted by physicians until we get more evidence. But there are a significant number of studies in the works."
Chemotherapy-induced peripheral neuropathy affects up to 30%-40% of treated cancer patients. Dr. Smith’s pilot study involved 16 patients with refractory chemotherapy-induced peripheral neuropathy that lasted from 3 months to 8 years. The most common drugs involved were taxanes, platinum-based agents, and bortezomib (Velcade). Patients received hour-long Scrambler sessions daily on 10 consecutive working days.
Pain scores fell from a mean baseline score of 5.8 on a 10-point scale to 2.4 at the end of 10 days. Four patients had a pain score of 0, and 15 of the 16 patients had at least a 20% reduction in their pain score on day 10. There was no toxicity (J. Pain Symptom Manage. 2010;40:883-91).
"When you see that sort of effect size, you can’t believe it’s real," Dr. Smith observed. "That’s in fact what the first reviewer said of the article. He said, ‘I’ve never heard of this therapy, and I don’t believe it.’ I volunteered to give him the case reports."
Neuropathic pain reductions in the 60% range are what are seen with permanent implanted spinal cord stimulation devices, a therapy that runs about $40,000, he added.
Dr. Smith’s Scrambler pilot study results were confirmed in an Italian study involving 40 patients with refractory cancer and 33 with non–cancer-related pain. Their mean pretreatment pain scale score was 6.2, plunging to 1.6 after the 10th day of treatment, and rebounding to 2.9 at follow-up 2 weeks after the final treatment session. Again, there were no side effects (Support Care Cancer. 2012;20:405-12).
Separately, a group led by the Scrambler’s inventor, Giuseppe Marineo, Ph.D., of the University of Rome Tor Vergata, reported on a randomized but unblinded trial involving 52 patients with chronic failed back syndrome, post-herpetic neuralgia, or spinal cord stenosis. Subjects assigned to the control arm received pharmacotherapy according to European Federation of Neurological Societies guidelines (Eur. J. Neurol. 2010;17:1113-e88), while the intervention arm received 10 daily Scrambler sessions.
The pretreatment mean visual analog pain score fell in 1 month from 8.1 in a control group and 8.0 in a group treated with the Scrambler to 5.8 in controls and to 0.7 in the Scrambler group. At 2 and 3 months of follow-up, the mean pain scores were 1.4 and 2.0 points, respectively, in the Scrambler group but were still 5.7 and 5.9 points in controls. A marked and persistent reduction in allodynia was also documented in response to Scrambler therapy (J. Pain Symptom Manage. 2012;43:87-95).
With regard to post-herpetic neuralgia, Dr. Smith presented a series of 10 treated patients. Their mean pain scores dropped from 8 at baseline to less than 1 at 1 month, holding steady with a score of 2 at 2 and 3 months’ follow-up.
The Scrambler entails application of 16 ECG-like electrode pads placed along the dermatome above and below the site of pain. The Scrambler machine is designed to feed 16 different nerve potentials in rapid sequence, essentially in order to confuse a firing nerve. The electrical charge is individually adjusted to patient tolerance. It feels like a bee sting, according to Dr. Smith. The mechanism of benefit isn’t well defined as yet.
"It’s likely acting like direct spinal cord stimulation, raising the gate threshold," he continued. "My hypothesis – and the inventor’s hypothesis as well – is that the therapy resets the damaged nerves at several sites. You see some effect within the first 30 minutes, and that rapid onset suggests biochemical change. And the long-lasting nature of the pain relief suggests remodeling as well as adaptation to the pain."
Clearly, further studies need to be done – free of industry sponsorship, on larger numbers of patients, and with sham-treated controls – in order to fully assess the Scrambler therapy’s efficacy, mechanism of action, and optimal schedule.
"The Scrambler is one of several neurocutaneous direct nerve stimulation techniques that are interesting but absolutely require further testing," Dr. Smith said.
Dr. Charles L. Loprinzi, professor of oncology at the Mayo Clinic, Rochester, Minn., has started such studies, he noted. "He was at least as skeptical of this as I was, and he’s been impressed with results from this machine," Dr. Smith said, adding that Dr. Loprinzi’s research team is expected to present data later this year at the annual meeting of the American Society of Clinical Oncology.
Competitive Technologies Inc., based in Fairfield, Conn., has worldwide rights to the device. Dr. Smith reported having no financial conflicts.
EXPERT ANALYSIS FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF HOSPICE AND PALLIATIVE CARE MEDICINE
Gamma Knife Surgery Cuts Seizures in Tumor Patients
BALTIMORE – Gamma Knife surgery significantly reduced the number of seizures in a subset of patients with rare congenital tumors, based on data from a prospective trial of 64 patients presented at the annual meeting of the American Epilepsy Society.
Hypothalamic hamartomas (congenital tumors that are attached to functional brain tissue) can cause a range of complications, including intractable seizures, said Dr. Jean Régis of Timone University Hospital in Marseilles, France.
Dr. Régis’s center is one of the few in the world where Gamma Knife surgery is performed on patients with hypothalamic hamartomas (HH). His ongoing study includes patients as young as age 3 years who have undergone surgery for HH. After a median of 62 months’ follow-up, the number of seizures in this group dropped from a median of 92 per month to a median of 6 per month.
But the benefits of the surgery extended beyond seizure reduction, Dr. Régis emphasized. Global psychiatric and cognitive comorbidity was considered cured in 28% of patients, improved in 56% of patients, and stable in 8% of patients at postsurgical follow-up.
Hyperkinetic behavior was identified in 34 patients at baseline. After surgery, 35% of patients were cured of hyperkinetic behavior and 30% were very much improved, Dr. Régis said. In addition, heteroaggressive behavior was noted in 56 patients at baseline, but after surgery, the behavior completely disappeared in 53% of these patients and was dramatically reduced in 32%, he added.
The specific approach for Gamma Knife surgery depends on the anatomy of the lesion, Dr. Régis noted. Most of the patients in this study had hamartomas of types I-IV, which are the smaller lesions, he said. The median marginal dose of radiation was 17 Gy and the median volume was 419 mm3.
"Longer follow-up remains mandatory due to the young age of this population," Dr. Régis said.
"Beyond seizure reduction, the improvement of the psychiatric, cognitive condition, and school and social insertion is turning out to be the major benefit of GKS in this frequently catastrophic epilepsy group," he added.
Dr. Régis is the president of the International Stereotactic Radiosurgery Society (ISRS) and chairman of its 2011 congress. He said he has raised major congress funding from the following manufacturers of radiosurgery devices: Accuray, BrainLab, Elekta, and Radionic.
BALTIMORE – Gamma Knife surgery significantly reduced the number of seizures in a subset of patients with rare congenital tumors, based on data from a prospective trial of 64 patients presented at the annual meeting of the American Epilepsy Society.
Hypothalamic hamartomas (congenital tumors that are attached to functional brain tissue) can cause a range of complications, including intractable seizures, said Dr. Jean Régis of Timone University Hospital in Marseilles, France.
Dr. Régis’s center is one of the few in the world where Gamma Knife surgery is performed on patients with hypothalamic hamartomas (HH). His ongoing study includes patients as young as age 3 years who have undergone surgery for HH. After a median of 62 months’ follow-up, the number of seizures in this group dropped from a median of 92 per month to a median of 6 per month.
But the benefits of the surgery extended beyond seizure reduction, Dr. Régis emphasized. Global psychiatric and cognitive comorbidity was considered cured in 28% of patients, improved in 56% of patients, and stable in 8% of patients at postsurgical follow-up.
Hyperkinetic behavior was identified in 34 patients at baseline. After surgery, 35% of patients were cured of hyperkinetic behavior and 30% were very much improved, Dr. Régis said. In addition, heteroaggressive behavior was noted in 56 patients at baseline, but after surgery, the behavior completely disappeared in 53% of these patients and was dramatically reduced in 32%, he added.
The specific approach for Gamma Knife surgery depends on the anatomy of the lesion, Dr. Régis noted. Most of the patients in this study had hamartomas of types I-IV, which are the smaller lesions, he said. The median marginal dose of radiation was 17 Gy and the median volume was 419 mm3.
"Longer follow-up remains mandatory due to the young age of this population," Dr. Régis said.
"Beyond seizure reduction, the improvement of the psychiatric, cognitive condition, and school and social insertion is turning out to be the major benefit of GKS in this frequently catastrophic epilepsy group," he added.
Dr. Régis is the president of the International Stereotactic Radiosurgery Society (ISRS) and chairman of its 2011 congress. He said he has raised major congress funding from the following manufacturers of radiosurgery devices: Accuray, BrainLab, Elekta, and Radionic.
BALTIMORE – Gamma Knife surgery significantly reduced the number of seizures in a subset of patients with rare congenital tumors, based on data from a prospective trial of 64 patients presented at the annual meeting of the American Epilepsy Society.
Hypothalamic hamartomas (congenital tumors that are attached to functional brain tissue) can cause a range of complications, including intractable seizures, said Dr. Jean Régis of Timone University Hospital in Marseilles, France.
Dr. Régis’s center is one of the few in the world where Gamma Knife surgery is performed on patients with hypothalamic hamartomas (HH). His ongoing study includes patients as young as age 3 years who have undergone surgery for HH. After a median of 62 months’ follow-up, the number of seizures in this group dropped from a median of 92 per month to a median of 6 per month.
But the benefits of the surgery extended beyond seizure reduction, Dr. Régis emphasized. Global psychiatric and cognitive comorbidity was considered cured in 28% of patients, improved in 56% of patients, and stable in 8% of patients at postsurgical follow-up.
Hyperkinetic behavior was identified in 34 patients at baseline. After surgery, 35% of patients were cured of hyperkinetic behavior and 30% were very much improved, Dr. Régis said. In addition, heteroaggressive behavior was noted in 56 patients at baseline, but after surgery, the behavior completely disappeared in 53% of these patients and was dramatically reduced in 32%, he added.
The specific approach for Gamma Knife surgery depends on the anatomy of the lesion, Dr. Régis noted. Most of the patients in this study had hamartomas of types I-IV, which are the smaller lesions, he said. The median marginal dose of radiation was 17 Gy and the median volume was 419 mm3.
"Longer follow-up remains mandatory due to the young age of this population," Dr. Régis said.
"Beyond seizure reduction, the improvement of the psychiatric, cognitive condition, and school and social insertion is turning out to be the major benefit of GKS in this frequently catastrophic epilepsy group," he added.
Dr. Régis is the president of the International Stereotactic Radiosurgery Society (ISRS) and chairman of its 2011 congress. He said he has raised major congress funding from the following manufacturers of radiosurgery devices: Accuray, BrainLab, Elekta, and Radionic.
FROM THE ANNUAL MEETING OF THE AMERICAN EPILEPSY SOCIETY
Major Finding: After a median of 62 months’ follow-up, the number of seizures in this group dropped from a median of 92 per month to a median of 6 per month.
Data Source: A prospective trial of 64 patients with hypothalamic hamartomas who underwent Gamma Knife surgery.
Disclosures: Dr. Régis is the president of the International Stereotactic Radiosurgery Society (ISRS) and chairman of its 2011 congress. He said he has raised major congress funding from the following manufacturers of radiosurgery devices: Accuray, BrainLab, Elekta, and Radionic.