User login
Pregnancy after ventral hernia repair increased the risk for recurrence
concluded the authors of a systematic review of the literature on hernia and pregnancy.
Erling Omoa, MD, of Bispebjerg Hospital and the University of Copenhagen, and his colleagues surveyed 5,189 articles and chose four cohort studies, four case-control studies, and one case-series study that met their criteria of quality, comparability, and outcomes data. Only randomized, controlled trials, analytical observational studies, and large case series were included. The focus was primary ventral (umbilical and epigastric) and incisional hernia surgery before, during, and after pregnancy.
“The prevalence of clinically relevant primary ventral hernias is very low during pregnancy,” the investigators wrote, but there is a lack on consensus concerning the management of hernia repair in women of childbearing age. “The objective of this systematic review was to examine the risk of recurrence following prepregnancy ventral hernia repair, and secondly, to evaluate the prevalence of ventral hernia during pregnancy and the risk of surgical repair before and after childbirth,” they wrote.
The reviewers evaluated pregnancy following ventral hernia repair as a potential risk factor for hernia recurrence. One study found that subsequent pregnancy was associated with a 1.6-fold increased risk of recurrence. Another found that pregnancy was independently associated with a 73% raised risk of recurrence. The risk of recurrence was no different between mesh and suture repair.
The review found the prevalence of primary ventral and inguinal repair during pregnancy to be low. A single-center cohort study of 20,714 pregnant women of which 17 (0.08%) had umbilical hernias and none of these required repair before delivery. A case series of 126 women who underwent this surgery during pregnancy indicated that this procedure was associated with minimal 30-day morbidity and no deaths. No data was available on fetal morbidity or recurrence in this case series.
Case-control studies reporting on umbilical repair concomitant with elective C-section found that, although adding hernia repair to the procedure increased operative time in some studies, there was no additional complication risk.
Overall, the investigators found several areas in which evidence remains weak, such as the long-term risks for recurrence following pregnancy and long-term outcomes of mesh versus suture repairs. They recommended that patients be counseled on the risk of recurrence linked to subsequent pregnancies and that, if possible, ventral hernia repair should be postponed until after a last planned pregnancy. Watchful waiting until after a delivery was deemed safe in many cases.
The investigators reported no conflicts.
SOURCE: Oma E et al. Am J Surg. 2019 Jan;217:163-8.
concluded the authors of a systematic review of the literature on hernia and pregnancy.
Erling Omoa, MD, of Bispebjerg Hospital and the University of Copenhagen, and his colleagues surveyed 5,189 articles and chose four cohort studies, four case-control studies, and one case-series study that met their criteria of quality, comparability, and outcomes data. Only randomized, controlled trials, analytical observational studies, and large case series were included. The focus was primary ventral (umbilical and epigastric) and incisional hernia surgery before, during, and after pregnancy.
“The prevalence of clinically relevant primary ventral hernias is very low during pregnancy,” the investigators wrote, but there is a lack on consensus concerning the management of hernia repair in women of childbearing age. “The objective of this systematic review was to examine the risk of recurrence following prepregnancy ventral hernia repair, and secondly, to evaluate the prevalence of ventral hernia during pregnancy and the risk of surgical repair before and after childbirth,” they wrote.
The reviewers evaluated pregnancy following ventral hernia repair as a potential risk factor for hernia recurrence. One study found that subsequent pregnancy was associated with a 1.6-fold increased risk of recurrence. Another found that pregnancy was independently associated with a 73% raised risk of recurrence. The risk of recurrence was no different between mesh and suture repair.
The review found the prevalence of primary ventral and inguinal repair during pregnancy to be low. A single-center cohort study of 20,714 pregnant women of which 17 (0.08%) had umbilical hernias and none of these required repair before delivery. A case series of 126 women who underwent this surgery during pregnancy indicated that this procedure was associated with minimal 30-day morbidity and no deaths. No data was available on fetal morbidity or recurrence in this case series.
Case-control studies reporting on umbilical repair concomitant with elective C-section found that, although adding hernia repair to the procedure increased operative time in some studies, there was no additional complication risk.
Overall, the investigators found several areas in which evidence remains weak, such as the long-term risks for recurrence following pregnancy and long-term outcomes of mesh versus suture repairs. They recommended that patients be counseled on the risk of recurrence linked to subsequent pregnancies and that, if possible, ventral hernia repair should be postponed until after a last planned pregnancy. Watchful waiting until after a delivery was deemed safe in many cases.
The investigators reported no conflicts.
SOURCE: Oma E et al. Am J Surg. 2019 Jan;217:163-8.
concluded the authors of a systematic review of the literature on hernia and pregnancy.
Erling Omoa, MD, of Bispebjerg Hospital and the University of Copenhagen, and his colleagues surveyed 5,189 articles and chose four cohort studies, four case-control studies, and one case-series study that met their criteria of quality, comparability, and outcomes data. Only randomized, controlled trials, analytical observational studies, and large case series were included. The focus was primary ventral (umbilical and epigastric) and incisional hernia surgery before, during, and after pregnancy.
“The prevalence of clinically relevant primary ventral hernias is very low during pregnancy,” the investigators wrote, but there is a lack on consensus concerning the management of hernia repair in women of childbearing age. “The objective of this systematic review was to examine the risk of recurrence following prepregnancy ventral hernia repair, and secondly, to evaluate the prevalence of ventral hernia during pregnancy and the risk of surgical repair before and after childbirth,” they wrote.
The reviewers evaluated pregnancy following ventral hernia repair as a potential risk factor for hernia recurrence. One study found that subsequent pregnancy was associated with a 1.6-fold increased risk of recurrence. Another found that pregnancy was independently associated with a 73% raised risk of recurrence. The risk of recurrence was no different between mesh and suture repair.
The review found the prevalence of primary ventral and inguinal repair during pregnancy to be low. A single-center cohort study of 20,714 pregnant women of which 17 (0.08%) had umbilical hernias and none of these required repair before delivery. A case series of 126 women who underwent this surgery during pregnancy indicated that this procedure was associated with minimal 30-day morbidity and no deaths. No data was available on fetal morbidity or recurrence in this case series.
Case-control studies reporting on umbilical repair concomitant with elective C-section found that, although adding hernia repair to the procedure increased operative time in some studies, there was no additional complication risk.
Overall, the investigators found several areas in which evidence remains weak, such as the long-term risks for recurrence following pregnancy and long-term outcomes of mesh versus suture repairs. They recommended that patients be counseled on the risk of recurrence linked to subsequent pregnancies and that, if possible, ventral hernia repair should be postponed until after a last planned pregnancy. Watchful waiting until after a delivery was deemed safe in many cases.
The investigators reported no conflicts.
SOURCE: Oma E et al. Am J Surg. 2019 Jan;217:163-8.
FROM THE AMERICAN JOURNAL OF SURGERY
Is oral or IV iron therapy more beneficial for postpartum anemia?
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
EXPERT COMMENTARY
Sultan P, Bampoe S, Shah R, et al. Oral versus intravenous iron therapy for postpartum anemia: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 19, 2018. DOI:10.1016/j.ajog.2018.12.016.
Iron deficiency anemia in pregnancy is associated with increased risk for adverse birth outcomes, including preterm delivery, cesarean delivery, and need for blood transfusion.1,2 Although the outcomes with postpartum iron deficiency anemia are more difficult to study, this condition is associated with increased risk of maternal fatigue and depression, and it is often overlooked as a significant issue during the postpartum period.
In a recent systematic review, Sultan and colleagues sought to provide an updated assessment of IV versus oral iron treatment for postpartum anemia. The 6-week postpartum hemoglobin concentration was the primary outcome.
Details of the study
The authors screened 2,744 articles for randomized controlled trials (RCTs) comparing oral and IV iron in the treatment of postpartum anemia. Fifteen RCTs were included in the review, with 1,001 women receiving oral iron therapy and 1,181 women receiving IV iron. The baseline postpartum hemoglobin concentration in the 15 studies ranged from less than 8 g/dL to 10.5 g/dL.
In all but 1 study, the women in the IV treatment arm experienced a significant increase in postpartum hemoglobin concentration, with the mean difference being 1.0 g/dL at postpartum week 1 (95% confidence interval [CI], 0.5–1.5; P<.0001) and 0.9 g/dL at postpartum week 6 (95% CI, 0.4–1.3; P = .0003).
Only 4 studies were included in the meta-analysis; specifically, 6-week postpartum hemoglobin levels were measured in 251 women who received IV iron and in 134 who received oral iron. Significant differences were seen in the IV iron group compared with the oral iron group for 3 of the secondary outcomes evaluated: flushing (odds ratio [OR], 6.95), decreased constipation (OR, 0.08), and decreased dyspepsia (OR, 0.07).
None of the other secondary outcomes associated with IV iron (muscle cramps, headache, urticaria, rash, or anaphylaxis) occurred at statistically significant rates. Notably, adherence was not assessed in the majority of the studies. Although constipation was increased in the oral iron therapy group, it was reported at only 12%.
Study strengths and weaknesses
Results of this study support previous findings that IV iron is better tolerated, with fewer gastrointestinal adverse effects, than oral iron, and they re-emphasize that IV iron therapy is both safe (the authors identified only 2 cases of anaphylaxis) and effective in improving hematologic indices.
Continue to: The systematic review included...
The systematic review included studies, however, that excluded women treated for antepartum anemia, a group that may benefit from aggressive correction of iron deficiency. Another study weakness is that all the oral iron regimens used were dosed either daily or multiple times per day, which may lead to difficulty with adherence and can decrease overall iron absorption compared with an every-other-day regimen.3
Future studies are needed to determine 1) which women with what level of anemia will benefit the most from postpartum IV iron and 2) the hemoglobin level at which IV iron is a cost-effective therapy.
Given the efficacy and reduced adverse effects associated with IV iron therapy demonstrated in the systematic review by Sultan and colleagues, I recommend treatment with IV iron for women with moderate to severe postpartum anemia (defined in pregnancy as a hemoglobin level less than 10 g/dL and ferritin less than 40 µg/L) who have not received blood products or for women who are unable to tolerate or absorb oral iron (such as those with a history of bariatric surgery, gastritis, or inflammatory bowel disease). In our institution, we frequently give IV iron sucrose 300 mg prior to discharge due to ease of administration. For women with mild iron deficiency anemia (hemoglobin greater than 10 g/dL), I prescribe every-other-day oral iron in the form of ferrous sulfate 325 mg, which effectively raises the hemoglobin level and limits the gastrointestinal side effects associated with more frequent dosing.
Julianna Schantz-Dunn, MD, MPH
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
- Drukker L, Hants Y, Farkash R, et al. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55:2799-2806.
- Rahman MM, Abe SK, Rahman MS, et al. Maternal anemia and risk of adverse birth and health outcomes in low- and middle-income countries: systematic review and meta-analysis. Am J Clin Nutr. 2016;103:495-504.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533.
What is your approach to the persistent occiput posterior malposition?
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
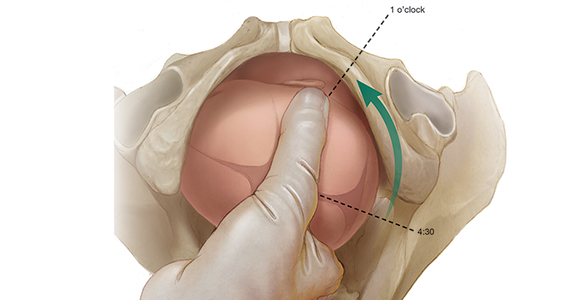
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.
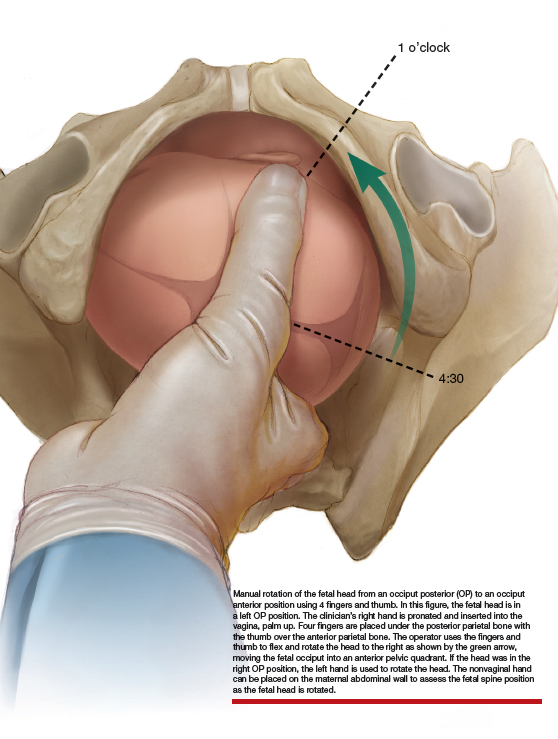
Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.

Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
CASE 7- to 8-lb baby suspected to be in occiput posterior (OP) position
A certified nurse midwife (CNM) asks you to consult on a 37-year-old woman (G1P0) at 41 weeks’ gestation who was admitted to labor and delivery for a late-term induction. The patient had a normal first stage of labor with placement of a combined spinal-epidural anesthetic at a cervical dilation of 4 cm. She has been fully dilated for 3.5 hours and pushing for 2.5 hours with a Category 1 fetal heart rate tracing. The CNM reports that the estimated fetal weight is 7 to 8 lb and the station is +3/5. She suspects that the fetus is in the left OP position. She asks for your advice on how to best deliver the fetus. The patient strongly prefers not to have a cesarean delivery (CD).
What is your recommended approach?
The cardinal movements of labor include cephalic engagement, descent, flexion, internal rotation, extension and rotation of the head at delivery, internal rotation of the shoulders, and expulsion of the body. In the first stage of labor many fetuses are in the OP position. Flexion and internal rotation of the fetal head in a mother with a gynecoid pelvis results in most fetuses assuming an occiput anterior (OA) position with the presenting diameter of the head (occipitobregmatic) being optimal for spontaneous vaginal delivery. Late in the second stage of labor only about 5% of fetuses are in the OP position with the presenting diameter of the head being large (occipitofrontal) with an extended head attitude, thereby reducing the probability of a rapid spontaneous vaginal delivery.
Risk factors for OP position late in the second stage of labor include1,2:
- nulliparity
- body mass index > 29 kg/m2
- gestation age ≥ 41 weeks
- birth weight > 4 kg
- regional anesthesia.
Maternal outcomes associated with persistent OP position include protracted first and second stage of labor, arrest of second stage of labor, and increased rates of operative vaginal delivery, anal sphincter injury, CD, postpartum hemorrhage, chorioamnionitis, and endomyometritis.1,3,4 The neonatal complications of persistent OP position include increased rates of shoulder dystocia, low Apgar score, umbilical artery acidemia, meconium, and admission to a neonatal intensive care unit.1,5
Diagnosis
Many obstetricians report that they can reliably detect a fetus in the OP position based upon abdominal palpation of the fetal spine and digital vaginal examination of the fetal sutures, fontanels, and ears. Such self-confidence may not be wholly warranted, however. Most contemporary data indicate that digital vaginal examination has an error rate of approximately 20% for identifying the position of the cephalic fetus, especially in the presence of fetal caput succedaneum and asynclitism.6-10
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) recommends that cephalic position be determined by transabdominal imaging.11 By placing the ultrasound probe on the maternal abdomen, a view of the fetal body at the level of the chest helps determine the position of the fetal spine. When the probe is placed in a suprapubic position, the observation of the fetal orbits facing the probe indicates an OP position.
When the presenting part is at a very low station, a transperineal ultrasound may be helpful to determine the position of the occiput. The ISUOG recommends that position be defined using a clock face, with positions from 330 h to 830 h being indicative of OP and positions from 930 h to 230 h being indicative of OA.11 The small remaining slivers on the clock face indicate an occiput transverse position (FIGURE).11

Continue to: Approaches to managing the OP position
Approaches to managing the OP position
First stage of labor
Identification of a cephalic-presenting fetus in the OP position in the first stage of labor might warrant increased attention to fetal position in the second stage of labor, but does not usually alter management of the first stage.
Second stage of labor
If an OP position is identified in the second stage of labor, many obstetricians will consider manual rotation of the fetal occiput to an anterior pelvic quadrant to facilitate labor progress. Because a fetus in the OP position may spontaneously rotate to the OA position at any point during the second stage, a judicious interval of waiting is reasonable before attempting a manual rotation in the second stage. For example, allowing the second stage to progress for 60 to 90 min in a nulliparous woman or 30 to 60 min in a multiparous woman will permit some fetuses to rotate to the OA position without intervention.
If the OP position persists beyond these time points, a manual rotation could be considered. There are no high-quality clinical trials to support this maneuver,12 but observational reports suggest that this low-risk maneuver may help reduce the rate of CD and anal sphincter trauma.13-15
Manual rotation from OP to OA. Prior to performing the rotation, the maternal bladder should be emptied and an adequate anesthetic provided. One technique is to use the 4 fingers of the hand as a “spatula” to turn the head. If the fetus is in a left OP position, the operator’s right hand is pronated and inserted into the vagina, palm up. Four fingers are placed under the posterior parietal bone with the thumb over the anterior parietal bone (ILLUSTRATION).4 The operator uses the fingers and thumb to flex and rotate the head to the right, moving the fetal occiput into an anterior pelvic quadrant.4 If the head is in the right OP position, the left hand is used to rotate the head. The nonvaginal hand can be placed on the maternal abdominal wall to assess the fetal spine position as the fetal head is rotated. The fetal head may need to be held in the anterior pelvic quadrant during a few maternal pushes to prevent the head from rotating back into the OP position.

Approaching delivery late in the second stage
If the second stage has progressed for 3 or 4 hours, as in the case described above, and the fetus remains in the OP position, delivery may be indicated to avoid the maternal and fetal complications of an even more prolonged second stage. At some point in a prolonged second stage, expectant management carries more maternal and fetal risks than intervention.
Late in the second stage, options for delivery of the fetus in the OP include: CD, rotational forceps delivery, direct forceps delivery from the OP position, and vacuum delivery.
Cesarean delivery. CD of the fetus in the OP position may be indicated when the fetus is estimated to be macrosomic, the station is high (biparietal diameter palpable on abdominal examination), or when the parturient has an android pelvis (narrow fore-pelvis and anterior convergence of the pelvic bone structures in a wedge shape). During CD, if difficulty is encountered in delivering the fetal head, a hand from below, extension of the uterine incision, or reverse breech extraction may be necessary to complete the delivery. If the clinical situation is conducive to operative vaginal delivery, forceps or vacuum can be used.
Continue to: Rotational forceps delivery...
Rotational forceps delivery. During residency I was told to always use rotational forceps to deliver a fetus in the persistently OP position if the parturient had a gynecoid pelvis (wide oval shape of pelvic bones, wide subpubic arch). Dr. Frederick Irving wrote16:
“Although textbooks almost universally advocate the extraction of the occiput directly posterior without rotation we do not advise it.... Such an extraction maneuver is inartistic and show[s] a lack of regard for the mechanical factors involved in the mechanism of labor. The method used at the Boston Lying-In Hospital presupposes an accurate diagnosis of the primary position. If the fetal back is on the right the head should be rotated to the right; if on the left, toward the left. The head is always rotated in the direction in which the back lies. The forceps are applied as if the occiput was directly anterior. Carrying the forceps handles in a wide sweep the occiput is now rotated to the anterior quadrant of the pelvis or 135 degrees. It will be found that the head turns easily in the way it should go but that it is difficult or impossible to rotate it in the improper direction. The instrument is then reapplied as in the second part of the Scanzoni maneuver.”
Rotation of the fetus from the OP to the OA position may reduce the risk of sphincter injury with vaginal birth. With the waning of rotational forceps skills, many obstetricians prefer a nonrotational approach with direct forceps or vacuum delivery from the OP position.
Direct forceps delivery from the OP position. A fetus in the OP position for 3 to 4 hours of the second stage of labor will often have a significant degree of head molding. The Simpson forceps, with its shallow and longer cephalic curve, accommodates significant fetal head molding and is a good forceps choice in this situation.
Vacuum delivery. In the United States, approximately 5% of vaginal deliveries are performed with a vacuum device, and 1% with forceps.17 Consequently, many obstetricians frequently perform operative vaginal delivery with a vacuum device and infrequently or never perform operative vaginal delivery with forceps. Vacuum vaginal delivery may be the instrument of choice for many obstetricians performing an operative delivery of a fetus in the OP position. However, the vacuum has a higher rate of failure, especially if the OP fetus is at a higher station.18
In some centers, direct forceps delivery from the OP position is preferred over an attempt at vacuum delivery, because in contemporary obstetric practice most centers do not permit the sequential use of vacuum followed by forceps (due to the higher rate of fetal trauma of combination operative delivery). Since vacuum delivery of the fetus in the OP position has a greater rate of failure than forceps, it may be best to initiate operative vaginal delivery of the fetus in the OP position with forceps. If vacuum is used to attempt a vaginal delivery and fails due to too many pop-offs, a CD would be the next step.
Take action when needed to optimize outcomes
The persistent OP position is associated with a longer second stage of labor. It is common during a change of shift for an obstetrician to sign out to the on-coming clinician a case of a prolonged second stage with the fetus in the OP position. In this situation, the on-coming clinician cannot wait hour after hour after hour hoping for a spontaneous delivery. If the on-coming clinician has a clear plan of how to deal with the persistent OP position—including ultrasound confirmation of position and physical examination to determine station, fetal size and adequacy of the pelvis, and timely selection of a delivery technique—the adverse maternal and neonatal outcomes sometimes caused by the persistent OP position will be minimized.
Continue to: CASE Resolved...
CASE Resolved
The consulting obstetrician performed a transabdominal ultrasound and observed the fetal orbits were facing the transducer, confirming an OP position. On physical examination, the station was +3/5, and the fetal weight was confirmed to be approximately 8 lb. The obstetrician recommended a direct forceps delivery from the OP position. The patient and CNM agreed with the plan.
The obstetrician applied Simpson forceps and performed a mediolateral episiotomy just prior to delivery of the head. Following delivery, the rectal sphincter and anal mucosa were intact and the episiotomy was repaired. The newborn, safely delivered, and the mother, having avoided a CD, were transferred to the postpartum floor later in the day.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
- Cheng YW, Hubbard A, Caughey AB, et al. The association between persistent fetal occiput posterior position and perinatal outcomes: An example of propensity score and covariate distance matching. Am J Epidemiol. 2010;171:656-663.
- Cheng YW, Shaffer BL, Caughey AB. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563-568.
- Ponkey SE, Cohen AP, Heffner LJ, et al. Persistent fetal occiput posterior position: obstetric outcomes. Obstet Gynecol. 2003;101:915-920.
- Barth WH Jr. Persistent occiput posterior. Obstet Gynecol. 2015;125:695-709.
- Cheng YW, Shaffer BL, Caughey AB. The association between persistent occiput posterior position and neonatal outcomes. Obstet Gynecol. 2006;107:837-844.
- Ghi T, Dall’Asta A, Masturzo B, et al. Randomised Italian sonography for occiput position trial ante vacuum. Ultrasound Obstet Gynecol. 2018;52:699-705.
- Bellussi F, Ghi T, Youssef A, et al. The use of intrapartum ultrasound to diagnose malpositions and cephalic malpresentations. Am J Obstet Gynecol. 2017;217:633-641.
- Ramphul M, Ooi PV, Burke G, et al. Instrumental delivery and ultrasound: a multicenter randomised controlled trial of ultrasound assessment of the fetal head position versus standard of care as an approach to prevent morbidity at instrumental delivery. BJOG. 2014;121:1029-1038.
- Malvasi A, Tinelli A, Barbera A, et al. Occiput posterior position diagnosis: vaginal examination or intrapartum sonography? A clinical review. J Matern Fetal Neonatal Med. 2014;27:520-526.
- Akmal S, Tsoi E, Kaemtas N, et al. Intrapartum sonography to determine fetal head position. J Matern Fetal Neonatal Med. 2002;12:172-177.
- Ghi T, Eggebo T, Lees C, et al. ISUOG practice guidelines: intrapartum ultrasound. Ultrasound Obstet Gynecol. 2018;52:128-139.
- Phipps H, de Vries B, Hyett J, et al. Prophylactic manual rotation for fetal malposition to reduce operative delivery. Cochrane Database Syst Rev. 2014;CD009298.
- Le Ray C, Serres P, Schmitz T, et al. Manual rotation in occiput posterior or transverse positions. Obstet Gynecol. 2007;110:873-879.
- Shaffer BL, Cheng YW, Vargas JE, et al. Manual rotation to reduce caesarean delivery in persistent occiput posterior or transverse position. J Matern Fetal Neonatal Med. 2011;24:65-72.
- Bertholdt C, Gauchotte E, Dap M, et al. Predictors of successful manual rotation for occiput posterior positions. Int J Gynaecol Obstet. 2019;144:210–215.
- Irving FC. A Textbook of Obstetrics. New York, NY: Macmillan, NY; 1936:426-428.
- Merriam AA, Ananth CV, Wright JD, et al. Trends in operative vaginal delivery, 2005–2013: a population-based study. BJOG. 2017;124:1365-1372.
- Verhoeven CJ, Nuij C, Janssen-Rolf CR, et al. Predictors of failure of vacuum-assisted vaginal delivery: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2016;200:29-34.
No increased pregnancy loss risk for women conceiving soon after stillbirth
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
The interval between pregnancy loss and the next conception may be less important than previously assumed, based on the results of this and other recent studies, according to Mark A Klebanoff, MD, MPH.
“Rather than adhering to hard and fast rules, clinical recommendations should consider a woman’s current health status, her current age in conjunction with her desires regarding child spacing and ultimate family size, and particularly following a loss, her emotional readiness to become pregnant again,” he said in a commentary accompanying the article by Regan et al.
However, these results are specific to high-income countries, and might not extrapolate to women in “less favorable situations” where poor access to quality medical and obstetric care, malnutrition, and untreated chronic conditions are more common, Dr. Klebanoff added.
Another limitation of the study, acknowledged by Regan and coauthors, is the relatively small number of stillbirths in subsequent pregnancies (228) despite this being the largest study of its kind to date.
“The fairly small number of women included in this report dictates that replication, probably using other large, linked, population-level databases, is required,” Dr. Klebanoff said in his commentary.
Dr. Klebanoff is with the Center for Perinatal Research, The Research Institute at Nationwide Children’s Hospital; and the departments of pediatrics and obstetrics and gynecology at the College of Medicine, and division of epidemiology at the College of Public Health, at The Ohio State University, all in Columbus. This is a summarization of his commentary (Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32430-9 ). Dr. Klebanoff said he had a pending grant application to the National Institutes of Health to study the association between interpregnancy interval and birth outcomes, and had no other competing interests.
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
according to authors of a large, international observational study.
There was no significantly increased risk of stillbirth, preterm birth, or small-for-gestational-age birth in the next pregnancy for women who conceived in that 12-month time period, according to results of the study, which was based on birth records for nearly 14,500 women in Finland, Norway, and Australia.
“We hope that our findings can provide reassurance to women who wish to become pregnant or unexpectedly become pregnant shortly after a stillbirth,” study author Annette K. Regan, PhD, of Curtin University, Perth, Australia, said in a statement on the study, which appears in The Lancet.
Judette Marie Louis, MD, MPH, said that while data are conflicting on optimal interpregnancy interval following stillbirth, large population-based studies such as this one may provide an indication of the relative safety of an interval of 12 months or less. (She was not involved in this study.)
“This paper is good news for a lot of women,” Dr. Louis, associate professor of obstetrics and gynecology at the University of South Florida, Tampa, said in an interview. “After a stillbirth, it’s such a traumatic experience that some do want to move on, and these findings suggest that, yes, you don’t have to wait that long to have a successful pregnancy.”
These results are for women living in relatively high-income countries, so the findings might not apply to every population, she added. Dr. Louis was the first author of a recent interpregnancy care consensus statement by the American College of Obstetricians and Gynecologists and the Society of Maternal-Fetal Medicine, and was asked to comment on this study.
The World Health Organization recommends interpregnancy intervals of 2 years or more after live births and 6 months or more after miscarriage, but currently has no specific recommendations on the optimal interpregnancy interval after a stillbirth, according to Dr. Regan and her colleagues.
“Because length of gestation might affect nutrient concentrations and health status in women, it is plausible that the optimal interval after stillbirth is somewhere between the optimal interval after miscarriage and live birth,” they said in their report.
Researchers for two previous studies also have reported on the link between the interpregnancy interval after stillbirth and birth outcomes in the next pregnancy, but neither was specifically designed to evaluate that outcome, they added.
Dr. Regan and her coauthors used birth record data spanning several decades from three high-income countries to identify 14,452 women who had stillbirths. Of those, 63% conceived within the next 12 months, and for 37%, it was as early as 6 months.
Overall, 2% of the subsequent pregnancies ended in stillbirth, while 9% were small-for-gestational-age and 18% were preterm, according to the report.
In analyses adjusted for variables such as age, smoking, and education level, there was no association between short interpregnancy intervals and subsequent stillbirths, compared with longer intervals (24-59 months), with odds ratios of 1.09 for an interval shorter than 6 months and 0.90 for 6-11 months.
Likewise, there was no link between shorter intervals and subsequent small-for-gestational-age birth, with odds ratios of 0.66 for less than 6 months and 0.64 for 6-11 months, and no link between interval and subsequent preterm births, with odds ratios of 0.91 for both short-interval groups.
That data could be useful to health care providers who do postpartum counseling after stillbirths, and could potentially inform future recommendations on pregnancy spacing, Dr. Regan and her coauthors said.
“These results apply to a large proportion of women conceiving after a stillbirth,” they noted.
This study included countries with access to universal health care, with populations that are mostly white, so the results may not apply to low- or middle-income countries without universal health care or with significant ethnic minority populations, they added.
Dr. Regan and her colleagues declared no competing interests related to the study, which was funded the National Health and Medical Research Council of Australia, among other sources.
SOURCE: Regan AK et al. Lancet. 2019 Feb 28. doi: 10.1016/S0140-6736(18)32266-9.
FROM THE LANCET
Many common dermatologic drugs can be safely used during pregnancy
WASHINGTON – With proper counseling and oversight, many drugs used for psoriasis, pemphigus, and atopic dermatitis are safe to use during pregnancy, Jenny Murase, MD, said at the annual meeting of the American Academy of Dermatology.
But it’s important to have an early talk about potential pregnancies, because most dermatologists don’t think about it unless a patient is taking a known teratogen, like isotretinoin, said Dr. Murase of the University of California, San Francisco. “It’s only about 10% of the time that the dermatologist brings it up. In patients with these chronic skin diseases, we need to address family planning proactively. Most women don’t discover they’re pregnant until they’re 2-5 weeks along, and by that time the development of major organs has already started.”
As part of her expertise in this topic, Dr. Murase published two comprehensive reports on the safety of dermatologic drugs in pregnancy and lactation. They were grouped according to the newest federal guidance, the Food and Drug Administration Pregnancy and Lactation Label Ruling. Issued in 2014, it requires the inclusion of any contact information for drug registries and covers reproductive risks or both males and females. Slowly being phased in as new drugs are approved, the ruling is replacing the old category A, B, and C.
The articles were published in the Journal of the American Academy of Dermatology and in the international Journal of Women’s Dermatology, an open-access journal Dr. Murase founded.
Corticosteroids
These dermatology workhorses are largely safe in pregnancy, Dr. Murase said. Some very early reports suggested that systemic cortisone might be associated with oral clefts, but that has never been borne out in prospective data. Prednisone may be the safest as it has the most limited placental transport; betamethasone and dexamethasone cross the placenta easily.
In a Cochrane review, only one study showed an increased risk of orofacial clefts. A 2013 study of about 10,000 women suggested an increased risk of low birth weight associated with a total dose of more than 300 grams during the pregnancy.
Antihistamines
First-generation antihistamines, including hydroxyzine, have been used as antiemetics in pregnant women. Hydroxyzine is generally considered safe, but should be discussed carefully, as it carries a slightly increased risk of congenital malformations (5.8% vs. 2.3% background risk).
Newborns exposed to large doses of hydroxyzine (150 mg or more daily) have also exhibited withdrawal symptoms at birth, including irritability, poor feeding, and tonic-clinic seizures.
“Antihistamines can exert oxytocin-like effects, especially in overdose or when given intravenously, so please avoid using them in the last month of pregnancy. There have also been a few reports of retrolental fibroplasia in preterm infants who were exposed to antihistamines within 2 weeks of delivery,” Dr. Murase said.
Immunosuppressants
Mycophenolate is not compatible with pregnancy. In 2007, the FDA changed the labeling of mycophenolate from category C to D, because of reports of congenital malformations arising from the U.S. National Transplantation Pregnancy Registry and other sources.
“You need to treat these patients like you do someone who is going on isotretinoin,” Dr. Murase said. “Any woman prescribed it should be on mandatory contraception at least 4 weeks before beginning the medication and for 6 weeks after completing treatment.”
Thus far there are no reported pregnancy-related safety issues with dupilumab, although data are scarce.
Pregnancy itself exerts a positive effect on psoriasis in many women, but not all. “About half the time a women will improve,” Dr. Murase said. “A quarter of the time, there’s no change and a quarter of the time, she’ll get worse. But the ones who do improve, often improve dramatically with about 80% body surface area clearance.”
She considers light therapy to be the safest treatment during pregnancy, with one caveat: Ultraviolet light can degrade some vitamins, including folic acid. “Every one of my patients of childbearing age I have on folic acid or a prenatal vitamin just in case. You have to be proactive here.”
Cyclosporine appears to be “quite safe,” she said. The possibility of intrauterine growth restriction seen in some studies is tough to tease out, because it was reported mainly in transplant populations among women with other medical comorbidities. Children from these pregnancies have been followed through toddlerhood and showed no neurodevelopmental or kidney issues.
Apremilast is a category C drug. Some animal data suggested increased spontaneous abortions and fetal demise with doses given at two to four times the human dose.
Biologics
Antibodies are an interesting lot, Dr. Murase noted. Maternal antibodies are transported to the fetus across the villi by Fc receptor; most of this transfer happens during the third trimester. The large, hydrophilic monoclonal antibodies infliximab, adalimumab, and ustekinumab travel this way as well. Cord blood can contain 50% higher serum levels than in maternal blood. Etanercept, however, is a fusion protein that diffuses across the placenta. Cord blood levels generally exceed maternal levels by less than 7%.
There is one published report of a fetal death associated with maternal infliximab for Crohn’s disease. The infant was healthy until it received a Bacillus Calmette–Guerin vaccine. It then developed widespread eczematous dermatitis, head lag, and poor weight gain and died at 4.5 months.
“This is another important counseling point,” Dr. Murase said. “Babies who have been exposed to infliximab in utero can’t have that vaccination in the first 9 months of life.”
Perhaps the safest bet for a pregnant women who needs a biologic is PEGylated certolizumab. “Certolizumab is the only PEGylated anti-TNF [tumor necrosis factor] without an Fc region; study of patients greater than 30 weeks pregnant certolizumab levels were below 0.032 mcg/mL in 13 of 14 infant samples at birth.”
Pemphigus
Pemphigus (impetigo herpetiformous) is a serious dermatologic disorder that can manifest in the third trimester, and affect the fetus as well as the mother. “You have to take even mild cases very seriously, because there’s no distinct correlation between the extent of neonatal involvement and the extent of maternal disease,” Dr. Murase said
Oral pemphigus in the mother is especially worrisome, she added. “Fetal skin shares the same desmoglein-3 profile as adult oral mucosa, and neonatal pemphigus is more likely if mother has oral disease. There’s an increased risk of fetal demise as well.”
Treatment would generally start with topical steroids, progressing to systemic low-dose corticosteroids. If more than 20 mg of prednisone a day is required, consider intravenous immunoglobulin (IVIG), azathioprine, dapsone, or rituximab.
“IVIG is very safe for pregnant women, and in fact reproductive endocrinologists use this to increase the chance of pregnancy for infertility cases,” Dr. Murase said.
She reported relationships with Regeneron, UCB, Dermira, and Genzyme/Sanofi.
WASHINGTON – With proper counseling and oversight, many drugs used for psoriasis, pemphigus, and atopic dermatitis are safe to use during pregnancy, Jenny Murase, MD, said at the annual meeting of the American Academy of Dermatology.
But it’s important to have an early talk about potential pregnancies, because most dermatologists don’t think about it unless a patient is taking a known teratogen, like isotretinoin, said Dr. Murase of the University of California, San Francisco. “It’s only about 10% of the time that the dermatologist brings it up. In patients with these chronic skin diseases, we need to address family planning proactively. Most women don’t discover they’re pregnant until they’re 2-5 weeks along, and by that time the development of major organs has already started.”
As part of her expertise in this topic, Dr. Murase published two comprehensive reports on the safety of dermatologic drugs in pregnancy and lactation. They were grouped according to the newest federal guidance, the Food and Drug Administration Pregnancy and Lactation Label Ruling. Issued in 2014, it requires the inclusion of any contact information for drug registries and covers reproductive risks or both males and females. Slowly being phased in as new drugs are approved, the ruling is replacing the old category A, B, and C.
The articles were published in the Journal of the American Academy of Dermatology and in the international Journal of Women’s Dermatology, an open-access journal Dr. Murase founded.
Corticosteroids
These dermatology workhorses are largely safe in pregnancy, Dr. Murase said. Some very early reports suggested that systemic cortisone might be associated with oral clefts, but that has never been borne out in prospective data. Prednisone may be the safest as it has the most limited placental transport; betamethasone and dexamethasone cross the placenta easily.
In a Cochrane review, only one study showed an increased risk of orofacial clefts. A 2013 study of about 10,000 women suggested an increased risk of low birth weight associated with a total dose of more than 300 grams during the pregnancy.
Antihistamines
First-generation antihistamines, including hydroxyzine, have been used as antiemetics in pregnant women. Hydroxyzine is generally considered safe, but should be discussed carefully, as it carries a slightly increased risk of congenital malformations (5.8% vs. 2.3% background risk).
Newborns exposed to large doses of hydroxyzine (150 mg or more daily) have also exhibited withdrawal symptoms at birth, including irritability, poor feeding, and tonic-clinic seizures.
“Antihistamines can exert oxytocin-like effects, especially in overdose or when given intravenously, so please avoid using them in the last month of pregnancy. There have also been a few reports of retrolental fibroplasia in preterm infants who were exposed to antihistamines within 2 weeks of delivery,” Dr. Murase said.
Immunosuppressants
Mycophenolate is not compatible with pregnancy. In 2007, the FDA changed the labeling of mycophenolate from category C to D, because of reports of congenital malformations arising from the U.S. National Transplantation Pregnancy Registry and other sources.
“You need to treat these patients like you do someone who is going on isotretinoin,” Dr. Murase said. “Any woman prescribed it should be on mandatory contraception at least 4 weeks before beginning the medication and for 6 weeks after completing treatment.”
Thus far there are no reported pregnancy-related safety issues with dupilumab, although data are scarce.
Pregnancy itself exerts a positive effect on psoriasis in many women, but not all. “About half the time a women will improve,” Dr. Murase said. “A quarter of the time, there’s no change and a quarter of the time, she’ll get worse. But the ones who do improve, often improve dramatically with about 80% body surface area clearance.”
She considers light therapy to be the safest treatment during pregnancy, with one caveat: Ultraviolet light can degrade some vitamins, including folic acid. “Every one of my patients of childbearing age I have on folic acid or a prenatal vitamin just in case. You have to be proactive here.”
Cyclosporine appears to be “quite safe,” she said. The possibility of intrauterine growth restriction seen in some studies is tough to tease out, because it was reported mainly in transplant populations among women with other medical comorbidities. Children from these pregnancies have been followed through toddlerhood and showed no neurodevelopmental or kidney issues.
Apremilast is a category C drug. Some animal data suggested increased spontaneous abortions and fetal demise with doses given at two to four times the human dose.
Biologics
Antibodies are an interesting lot, Dr. Murase noted. Maternal antibodies are transported to the fetus across the villi by Fc receptor; most of this transfer happens during the third trimester. The large, hydrophilic monoclonal antibodies infliximab, adalimumab, and ustekinumab travel this way as well. Cord blood can contain 50% higher serum levels than in maternal blood. Etanercept, however, is a fusion protein that diffuses across the placenta. Cord blood levels generally exceed maternal levels by less than 7%.
There is one published report of a fetal death associated with maternal infliximab for Crohn’s disease. The infant was healthy until it received a Bacillus Calmette–Guerin vaccine. It then developed widespread eczematous dermatitis, head lag, and poor weight gain and died at 4.5 months.
“This is another important counseling point,” Dr. Murase said. “Babies who have been exposed to infliximab in utero can’t have that vaccination in the first 9 months of life.”
Perhaps the safest bet for a pregnant women who needs a biologic is PEGylated certolizumab. “Certolizumab is the only PEGylated anti-TNF [tumor necrosis factor] without an Fc region; study of patients greater than 30 weeks pregnant certolizumab levels were below 0.032 mcg/mL in 13 of 14 infant samples at birth.”
Pemphigus
Pemphigus (impetigo herpetiformous) is a serious dermatologic disorder that can manifest in the third trimester, and affect the fetus as well as the mother. “You have to take even mild cases very seriously, because there’s no distinct correlation between the extent of neonatal involvement and the extent of maternal disease,” Dr. Murase said
Oral pemphigus in the mother is especially worrisome, she added. “Fetal skin shares the same desmoglein-3 profile as adult oral mucosa, and neonatal pemphigus is more likely if mother has oral disease. There’s an increased risk of fetal demise as well.”
Treatment would generally start with topical steroids, progressing to systemic low-dose corticosteroids. If more than 20 mg of prednisone a day is required, consider intravenous immunoglobulin (IVIG), azathioprine, dapsone, or rituximab.
“IVIG is very safe for pregnant women, and in fact reproductive endocrinologists use this to increase the chance of pregnancy for infertility cases,” Dr. Murase said.
She reported relationships with Regeneron, UCB, Dermira, and Genzyme/Sanofi.
WASHINGTON – With proper counseling and oversight, many drugs used for psoriasis, pemphigus, and atopic dermatitis are safe to use during pregnancy, Jenny Murase, MD, said at the annual meeting of the American Academy of Dermatology.
But it’s important to have an early talk about potential pregnancies, because most dermatologists don’t think about it unless a patient is taking a known teratogen, like isotretinoin, said Dr. Murase of the University of California, San Francisco. “It’s only about 10% of the time that the dermatologist brings it up. In patients with these chronic skin diseases, we need to address family planning proactively. Most women don’t discover they’re pregnant until they’re 2-5 weeks along, and by that time the development of major organs has already started.”
As part of her expertise in this topic, Dr. Murase published two comprehensive reports on the safety of dermatologic drugs in pregnancy and lactation. They were grouped according to the newest federal guidance, the Food and Drug Administration Pregnancy and Lactation Label Ruling. Issued in 2014, it requires the inclusion of any contact information for drug registries and covers reproductive risks or both males and females. Slowly being phased in as new drugs are approved, the ruling is replacing the old category A, B, and C.
The articles were published in the Journal of the American Academy of Dermatology and in the international Journal of Women’s Dermatology, an open-access journal Dr. Murase founded.
Corticosteroids
These dermatology workhorses are largely safe in pregnancy, Dr. Murase said. Some very early reports suggested that systemic cortisone might be associated with oral clefts, but that has never been borne out in prospective data. Prednisone may be the safest as it has the most limited placental transport; betamethasone and dexamethasone cross the placenta easily.
In a Cochrane review, only one study showed an increased risk of orofacial clefts. A 2013 study of about 10,000 women suggested an increased risk of low birth weight associated with a total dose of more than 300 grams during the pregnancy.
Antihistamines
First-generation antihistamines, including hydroxyzine, have been used as antiemetics in pregnant women. Hydroxyzine is generally considered safe, but should be discussed carefully, as it carries a slightly increased risk of congenital malformations (5.8% vs. 2.3% background risk).
Newborns exposed to large doses of hydroxyzine (150 mg or more daily) have also exhibited withdrawal symptoms at birth, including irritability, poor feeding, and tonic-clinic seizures.
“Antihistamines can exert oxytocin-like effects, especially in overdose or when given intravenously, so please avoid using them in the last month of pregnancy. There have also been a few reports of retrolental fibroplasia in preterm infants who were exposed to antihistamines within 2 weeks of delivery,” Dr. Murase said.
Immunosuppressants
Mycophenolate is not compatible with pregnancy. In 2007, the FDA changed the labeling of mycophenolate from category C to D, because of reports of congenital malformations arising from the U.S. National Transplantation Pregnancy Registry and other sources.
“You need to treat these patients like you do someone who is going on isotretinoin,” Dr. Murase said. “Any woman prescribed it should be on mandatory contraception at least 4 weeks before beginning the medication and for 6 weeks after completing treatment.”
Thus far there are no reported pregnancy-related safety issues with dupilumab, although data are scarce.
Pregnancy itself exerts a positive effect on psoriasis in many women, but not all. “About half the time a women will improve,” Dr. Murase said. “A quarter of the time, there’s no change and a quarter of the time, she’ll get worse. But the ones who do improve, often improve dramatically with about 80% body surface area clearance.”
She considers light therapy to be the safest treatment during pregnancy, with one caveat: Ultraviolet light can degrade some vitamins, including folic acid. “Every one of my patients of childbearing age I have on folic acid or a prenatal vitamin just in case. You have to be proactive here.”
Cyclosporine appears to be “quite safe,” she said. The possibility of intrauterine growth restriction seen in some studies is tough to tease out, because it was reported mainly in transplant populations among women with other medical comorbidities. Children from these pregnancies have been followed through toddlerhood and showed no neurodevelopmental or kidney issues.
Apremilast is a category C drug. Some animal data suggested increased spontaneous abortions and fetal demise with doses given at two to four times the human dose.
Biologics
Antibodies are an interesting lot, Dr. Murase noted. Maternal antibodies are transported to the fetus across the villi by Fc receptor; most of this transfer happens during the third trimester. The large, hydrophilic monoclonal antibodies infliximab, adalimumab, and ustekinumab travel this way as well. Cord blood can contain 50% higher serum levels than in maternal blood. Etanercept, however, is a fusion protein that diffuses across the placenta. Cord blood levels generally exceed maternal levels by less than 7%.
There is one published report of a fetal death associated with maternal infliximab for Crohn’s disease. The infant was healthy until it received a Bacillus Calmette–Guerin vaccine. It then developed widespread eczematous dermatitis, head lag, and poor weight gain and died at 4.5 months.
“This is another important counseling point,” Dr. Murase said. “Babies who have been exposed to infliximab in utero can’t have that vaccination in the first 9 months of life.”
Perhaps the safest bet for a pregnant women who needs a biologic is PEGylated certolizumab. “Certolizumab is the only PEGylated anti-TNF [tumor necrosis factor] without an Fc region; study of patients greater than 30 weeks pregnant certolizumab levels were below 0.032 mcg/mL in 13 of 14 infant samples at birth.”
Pemphigus
Pemphigus (impetigo herpetiformous) is a serious dermatologic disorder that can manifest in the third trimester, and affect the fetus as well as the mother. “You have to take even mild cases very seriously, because there’s no distinct correlation between the extent of neonatal involvement and the extent of maternal disease,” Dr. Murase said
Oral pemphigus in the mother is especially worrisome, she added. “Fetal skin shares the same desmoglein-3 profile as adult oral mucosa, and neonatal pemphigus is more likely if mother has oral disease. There’s an increased risk of fetal demise as well.”
Treatment would generally start with topical steroids, progressing to systemic low-dose corticosteroids. If more than 20 mg of prednisone a day is required, consider intravenous immunoglobulin (IVIG), azathioprine, dapsone, or rituximab.
“IVIG is very safe for pregnant women, and in fact reproductive endocrinologists use this to increase the chance of pregnancy for infertility cases,” Dr. Murase said.
She reported relationships with Regeneron, UCB, Dermira, and Genzyme/Sanofi.
EXPERT ANALYSIS FROM AAD 2019
Texting improves postpregnancy hypertension monitoring in black women
LAS VEGAS – Adi Hirshberg, MD, reported at the Pregnancy Meeting.
The text messaging system increased the compliance rate to 93%, compared with just 30% of those asked to return to the office after hospital discharge. Just as importantly, it completely erased racial disparity in compliance rates, compared with white women, with more than 90% of both groups complying, said Dr. Hirshberg of the University of Pennsylvania, Philadelphia.
“Our study shows that text-based monitoring eliminated the observed racial disparity in postpregnancy hypertension care,” she said at the meeting sponsored by the Society for Maternal-Fetal Medicine. “Using texts as a standard of care would have likely led to medication initiation or adjustment for an additional 20% or more women who missed an office visit. This is an innovative way to equally engage all women in the postpregnancy period.”
Dr. Hirshberg presented a preplanned subanalysis of the Remote Surveillance of Hypertension (TextBP) trial, published last year (BMJ Qual Saf. 2018;27:871-7). The publication gave overall data; this analysis broke results down by race.
TextBP equally randomized 206 postpartum women with pregnancy-induced hypertension to the usual practice of office-based BP monitoring, or to 2 weeks of text-based surveillance using a home BP cuff. It was open to all women with pregnancy-related hypertension who delivered in the Hospital of the University of Pennsylvania. Hypertension classes included gestational hypertension, preeclampsia, chronic hypertension with superimposed preeclampsia, and HELLP syndrome before or during the delivery admission. Women were randomized to usual care or to an office-based BP check within the first postpartum week.
The texting platform was developed through Way to Health, a web-based platform within the institution, with secure technological infrastructure. A starting introductory text message was sent by the Way to Health platform to the phone number provided on day of discharge.
Patients received reminders to text message their blood pressure twice daily for 2 weeks post partum, starting on the day after discharge. If the patient reported a systolic BP of more than 160 mm Hg or diastolic more than 110 mm Hg, the clinician received an alert.
The overall results showed that significantly more women in the texting group reported their blood pressure (92% vs. 44%), compared with the controls. Almost everyone in the texting group (84%) met the established criteria for BP measurement.
Dr. Hirshberg and her colleagues wanted to zero in on black women, to discover if the text reminders could help boost their compliance. This is important for a couple of reasons, she said. For one thing, black women face a higher baseline risk of hypertensive disorders and cardiovascular disease, both in general and during and after pregnancy. Second, “for every 100 black women who require postpartum hypertension surveillance, only about a third are likely to attended an office visit after discharge,” leaving them vulnerable to potentially preventable complications of hypertension.
Even in the original analysis, return rates for postpregnancy BP checks were low in both groups and, in fact, seemed to be declining over several years. In 2012, 56% of nonblack and 33% of black women returned for their checks. By 2014, that number had declined to 34% and 20%. The numbers parallel the increasing disparity in U.S. maternal death rate between black women and nonblack women. From 1989 to 2013, the death rate for black mothers doubled, jumping from 20 to 40 deaths per 100,000 live births. During the same period, the death rate for nonblack mothers increased from about 8 to about 11 per 10,000. Dr. Hirshberg said.
In the subanalysis, the primary outcomes were the percentage of patients in whom a blood pressure was obtained in the first 10 days following discharge. In the control office visit group, about 33% of black women had a measurement, compared with 70% of nonblack women – a significant difference. Text messaging, however, completely eliminated the disparity. In the texting group, 91% of nonblack women and 93% of black women had a BP measurement.
There were no hypertension readmissions in the texting arm; there were four in the office-visit arm, three of which occurred in black women.
“This suggests that the traditional office-based follow-up may have resulted in missed opportunities to start an antihypertensive in about 10 of the 55 women who missed their office visit,” Dr. Hirshberg said, adding that “these kinds of early interventions can reduce maternal morbidity and mortality and increase overall health in all.”
She reported no relevant financial disclosures.
SOURCE: Hirshberg A et al. Am J Obstet Gynecol. 2019 Jan;220(1):S6, Abstract 7.
LAS VEGAS – Adi Hirshberg, MD, reported at the Pregnancy Meeting.
The text messaging system increased the compliance rate to 93%, compared with just 30% of those asked to return to the office after hospital discharge. Just as importantly, it completely erased racial disparity in compliance rates, compared with white women, with more than 90% of both groups complying, said Dr. Hirshberg of the University of Pennsylvania, Philadelphia.
“Our study shows that text-based monitoring eliminated the observed racial disparity in postpregnancy hypertension care,” she said at the meeting sponsored by the Society for Maternal-Fetal Medicine. “Using texts as a standard of care would have likely led to medication initiation or adjustment for an additional 20% or more women who missed an office visit. This is an innovative way to equally engage all women in the postpregnancy period.”
Dr. Hirshberg presented a preplanned subanalysis of the Remote Surveillance of Hypertension (TextBP) trial, published last year (BMJ Qual Saf. 2018;27:871-7). The publication gave overall data; this analysis broke results down by race.
TextBP equally randomized 206 postpartum women with pregnancy-induced hypertension to the usual practice of office-based BP monitoring, or to 2 weeks of text-based surveillance using a home BP cuff. It was open to all women with pregnancy-related hypertension who delivered in the Hospital of the University of Pennsylvania. Hypertension classes included gestational hypertension, preeclampsia, chronic hypertension with superimposed preeclampsia, and HELLP syndrome before or during the delivery admission. Women were randomized to usual care or to an office-based BP check within the first postpartum week.
The texting platform was developed through Way to Health, a web-based platform within the institution, with secure technological infrastructure. A starting introductory text message was sent by the Way to Health platform to the phone number provided on day of discharge.
Patients received reminders to text message their blood pressure twice daily for 2 weeks post partum, starting on the day after discharge. If the patient reported a systolic BP of more than 160 mm Hg or diastolic more than 110 mm Hg, the clinician received an alert.
The overall results showed that significantly more women in the texting group reported their blood pressure (92% vs. 44%), compared with the controls. Almost everyone in the texting group (84%) met the established criteria for BP measurement.
Dr. Hirshberg and her colleagues wanted to zero in on black women, to discover if the text reminders could help boost their compliance. This is important for a couple of reasons, she said. For one thing, black women face a higher baseline risk of hypertensive disorders and cardiovascular disease, both in general and during and after pregnancy. Second, “for every 100 black women who require postpartum hypertension surveillance, only about a third are likely to attended an office visit after discharge,” leaving them vulnerable to potentially preventable complications of hypertension.
Even in the original analysis, return rates for postpregnancy BP checks were low in both groups and, in fact, seemed to be declining over several years. In 2012, 56% of nonblack and 33% of black women returned for their checks. By 2014, that number had declined to 34% and 20%. The numbers parallel the increasing disparity in U.S. maternal death rate between black women and nonblack women. From 1989 to 2013, the death rate for black mothers doubled, jumping from 20 to 40 deaths per 100,000 live births. During the same period, the death rate for nonblack mothers increased from about 8 to about 11 per 10,000. Dr. Hirshberg said.
In the subanalysis, the primary outcomes were the percentage of patients in whom a blood pressure was obtained in the first 10 days following discharge. In the control office visit group, about 33% of black women had a measurement, compared with 70% of nonblack women – a significant difference. Text messaging, however, completely eliminated the disparity. In the texting group, 91% of nonblack women and 93% of black women had a BP measurement.
There were no hypertension readmissions in the texting arm; there were four in the office-visit arm, three of which occurred in black women.
“This suggests that the traditional office-based follow-up may have resulted in missed opportunities to start an antihypertensive in about 10 of the 55 women who missed their office visit,” Dr. Hirshberg said, adding that “these kinds of early interventions can reduce maternal morbidity and mortality and increase overall health in all.”
She reported no relevant financial disclosures.
SOURCE: Hirshberg A et al. Am J Obstet Gynecol. 2019 Jan;220(1):S6, Abstract 7.
LAS VEGAS – Adi Hirshberg, MD, reported at the Pregnancy Meeting.
The text messaging system increased the compliance rate to 93%, compared with just 30% of those asked to return to the office after hospital discharge. Just as importantly, it completely erased racial disparity in compliance rates, compared with white women, with more than 90% of both groups complying, said Dr. Hirshberg of the University of Pennsylvania, Philadelphia.
“Our study shows that text-based monitoring eliminated the observed racial disparity in postpregnancy hypertension care,” she said at the meeting sponsored by the Society for Maternal-Fetal Medicine. “Using texts as a standard of care would have likely led to medication initiation or adjustment for an additional 20% or more women who missed an office visit. This is an innovative way to equally engage all women in the postpregnancy period.”
Dr. Hirshberg presented a preplanned subanalysis of the Remote Surveillance of Hypertension (TextBP) trial, published last year (BMJ Qual Saf. 2018;27:871-7). The publication gave overall data; this analysis broke results down by race.
TextBP equally randomized 206 postpartum women with pregnancy-induced hypertension to the usual practice of office-based BP monitoring, or to 2 weeks of text-based surveillance using a home BP cuff. It was open to all women with pregnancy-related hypertension who delivered in the Hospital of the University of Pennsylvania. Hypertension classes included gestational hypertension, preeclampsia, chronic hypertension with superimposed preeclampsia, and HELLP syndrome before or during the delivery admission. Women were randomized to usual care or to an office-based BP check within the first postpartum week.
The texting platform was developed through Way to Health, a web-based platform within the institution, with secure technological infrastructure. A starting introductory text message was sent by the Way to Health platform to the phone number provided on day of discharge.
Patients received reminders to text message their blood pressure twice daily for 2 weeks post partum, starting on the day after discharge. If the patient reported a systolic BP of more than 160 mm Hg or diastolic more than 110 mm Hg, the clinician received an alert.
The overall results showed that significantly more women in the texting group reported their blood pressure (92% vs. 44%), compared with the controls. Almost everyone in the texting group (84%) met the established criteria for BP measurement.
Dr. Hirshberg and her colleagues wanted to zero in on black women, to discover if the text reminders could help boost their compliance. This is important for a couple of reasons, she said. For one thing, black women face a higher baseline risk of hypertensive disorders and cardiovascular disease, both in general and during and after pregnancy. Second, “for every 100 black women who require postpartum hypertension surveillance, only about a third are likely to attended an office visit after discharge,” leaving them vulnerable to potentially preventable complications of hypertension.
Even in the original analysis, return rates for postpregnancy BP checks were low in both groups and, in fact, seemed to be declining over several years. In 2012, 56% of nonblack and 33% of black women returned for their checks. By 2014, that number had declined to 34% and 20%. The numbers parallel the increasing disparity in U.S. maternal death rate between black women and nonblack women. From 1989 to 2013, the death rate for black mothers doubled, jumping from 20 to 40 deaths per 100,000 live births. During the same period, the death rate for nonblack mothers increased from about 8 to about 11 per 10,000. Dr. Hirshberg said.
In the subanalysis, the primary outcomes were the percentage of patients in whom a blood pressure was obtained in the first 10 days following discharge. In the control office visit group, about 33% of black women had a measurement, compared with 70% of nonblack women – a significant difference. Text messaging, however, completely eliminated the disparity. In the texting group, 91% of nonblack women and 93% of black women had a BP measurement.
There were no hypertension readmissions in the texting arm; there were four in the office-visit arm, three of which occurred in black women.
“This suggests that the traditional office-based follow-up may have resulted in missed opportunities to start an antihypertensive in about 10 of the 55 women who missed their office visit,” Dr. Hirshberg said, adding that “these kinds of early interventions can reduce maternal morbidity and mortality and increase overall health in all.”
She reported no relevant financial disclosures.
SOURCE: Hirshberg A et al. Am J Obstet Gynecol. 2019 Jan;220(1):S6, Abstract 7.
REPORTING FROM THE PREGNANCY MEETING
ACOG: Avoid inductions before 39 weeks unless medically necessary
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
Babies should not be delivered before 39 0/7 weeks’ gestation by means besides spontaneous vaginal delivery, in the absence of medical indications for an earlier delivery.
wrote the authors of the new opinion, developed by the American College of Obstetricians and Gynecologists committee on obstetric practice and the Society for Maternal-Fetal Medicine.
The opinion, which replaces a 2013 statement, clarifies that their recommendations include avoiding cesarean delivery, labor induction, and cervical ripening before 39 0/7 weeks of gestation, unless a medical indication exists for earlier delivery.
The new opinion statement relied, in part, on a recent systematic review finding that late-preterm and early-term children do not fare as well as do term-delivered children in a variety of cognitive and educational domains. The opinion statement acknowledges that it’s not clear why children delivered earlier are showing performance difficulties and that it is possible that medical indications for an earlier delivery contributed to the differences.
Immediate outcomes for neonates delivered in the late preterm and early term period also are worse, compared with those delivered at term, according to several studies cited in the opinion. For example, composite morbidity was higher for infants delivered at both 37 and 38 weeks gestation, compared with those delivered at 39 weeks, with adjusted odds ratios for the composite outcome of 2.1 and 1.5, respectively (N Engl J Med. 2009 Jan 8;360[2]:111-20).
And lung maturity alone should not guide delivery, wrote the authors of the opinion. “Because nonrespiratory morbidities also are increased in early-term deliveries, documentation of fetal pulmonary maturity does not justify an early nonmedically indicated delivery,” they said, adding that physicians should not perform amniocentesis to determine lung maturity in an effort to guide delivery timing.
Because intrapartum demise remains a risk as long as a woman is pregnant, the potential for adverse neonatal outcomes with early delivery has to be balanced against the risk of stillbirth with continued gestation, the opinion authors acknowledged. But, they said, this question has been addressed by “multiple studies using national population level data,” which show that “even as the gestational age at term has increased in response to efforts to reduce early elective delivery, these efforts have not adversely affected stillbirth rates nationally or even in states with the greatest reductions in early elective delivery.”
Formal programs to reduce nonmedically indicated early-term deliveries have been successful. For example, the state of South Carolina achieved a reduction of almost 50% in nonmedically indicated early-term deliveries over the course of just 1 year. The South Carolina Birth Outcomes Initiative led a collaborative effort to institute a “hard-stop” policy against nonmedically indicated early-term deliveries that resulted in an absolute 4.7% decrease in late-preterm birth during 2011-2012. Similar efforts in Oregon and Ohio have reported significant reductions as well, with no increases in adverse neonatal outcomes.
Various policy approaches have been tried to achieve a reduction in nonmedically indicated late-preterm and early-term birth. These range from awareness raising and education, to “soft-stop” policies in which health care providers agree not to deliver before 39 weeks without medical indication, to “hard-stop” policies in which hospitals prohibit the nonindicated deliveries. In one comparative outcomes study, the hard-stop policy was the most effective, with a drop from 8.2% to 1.7% in nonindicated early deliveries, but the soft-stop policy also produced a decrease from 8.4% to 3.3% (P = .007 and .025, respectively). The educational approach didn’t produce a significant drop in nonmedically indicated early deliveries (Am J Obstet Gynecol. 2010 Nov;203[5]:449.e1-6).
In a separate, preexisting statement (Obstet Gynecol. 2019;133:e151-5), ACOG has outlined the management of medically indicated late-preterm and early-term deliveries and has developed an app (www.acog.org/acogapp) as a decision tool for indicated deliveries.
Examples cited by the current opinion statement authors of medical indications for early delivery include maternal factors such as preeclampsia, gestational hypertension, and poorly controlled diabetes. Placentation problems, fetal growth restriction, and prior cesarean deliveries also may warrant earlier delivery, as may a host of other complications. If an earlier delivery is planned, the committee authors recommend full discussion with the patient and clear documentation of the indications and discussion.
FROM OBSTETRICS & GYNECOLOGY
Masterclass: Marlene Freeman on treating bipolar disorder in women
during pregnancy. Dr. Freeman, associate professor of psychiatry at Harvard Medical School, also shares preliminary data on the impact of exposure to atypical antipsychotics from the National Pregnancy Registry for Psychiatric Medications at Massachusetts General Hospital, where she also serves as associate director of Women’s Mental Health.
Amazon
Apple Podcasts
Google Podcasts
Spotify
during pregnancy. Dr. Freeman, associate professor of psychiatry at Harvard Medical School, also shares preliminary data on the impact of exposure to atypical antipsychotics from the National Pregnancy Registry for Psychiatric Medications at Massachusetts General Hospital, where she also serves as associate director of Women’s Mental Health.
Amazon
Apple Podcasts
Google Podcasts
Spotify
during pregnancy. Dr. Freeman, associate professor of psychiatry at Harvard Medical School, also shares preliminary data on the impact of exposure to atypical antipsychotics from the National Pregnancy Registry for Psychiatric Medications at Massachusetts General Hospital, where she also serves as associate director of Women’s Mental Health.
Amazon
Apple Podcasts
Google Podcasts
Spotify
Insulin-treated diabetes in pregnancy carries preterm risk
Women with insulin-treated diabetes are at significantly greater risk of preterm birth and of delivering babies who are large for gestational age (LGA), regardless of prepregnancy body weight, new findings suggest.
Researchers examined the role of maternal diabetes and weight on pregnancy outcomes in the population-based cohort study. The study comprised 649,043 live births in Finland between Jan. 1, 2004, and Dec. 31, 2014, including 4,000 in women with insulin-treated diabetes, 3,740 in women with type 2 diabetes, and 98,568 women with gestational diabetes.
Prepregnancy body mass index was normal for nearly 60% of mothers, while 4% were underweight, 21% were overweight, 8% were moderately obese, and 4% were severely obese.
Overall, the researchers found that women with insulin-treated diabetes had a 43-fold higher odds of having an LGA infant, compared with the reference group of women of normal BMI without diabetes (adjusted odds ratio [aOR], 43.80; 95% confidence interval, 40.88-46.93). And there was an 11-fold greater odds of having a preterm birth in this group (aOR, 11.17; 95% CI, 10.46-11.93).
The findings were published in JAMA Pediatrics.
“Smaller, but clearly statistically significant, increased LGA risks were found also for mothers with type 2 diabetes and gestational diabetes not treated with insulin, especially in combination with prepregnancy overweight or obesity that were stronger for type 2 diabetes than gestational diabetes,” wrote Linghua Kong, MSc, of the department of molecular medicine and surgery at Karolinska Institutet, and coauthors.
The aOR for LGA among women with type 2 diabetes was 9.57 (95% CI, 8.65-10.58), compared with the reference group. And for women with maternal gestational diabetes, the aOR for LGA was 3.80 (95% CI, 3.66-3.96).
Looking at the risk for preterm birth, the researchers found that the aOR among women with type 2 diabetes was 2.12 (95% CI, 1.90-2.36), while there was no association between gestational diabetes and preterm birth.
The researchers also reported that for women with gestational diabetes or no diabetes, the odds of preterm birth increased slightly as maternal prepregnancy BMI increased.
“Maternal glucose metabolism during pregnancy differs from that in the non-pregnant state; insulin resistance is increased, directing fat as the mother’s energy source to ensure adequate carbohydrate supply for the growing fetus,” the researchers wrote. “This increase in insulin resistance is mediated by a number of factors, such as increased levels of progesterone, estrogen, and human placental lactogen.”
The authors noted that their data did not include information on congenital anomalies, maternal complications such as preeclampsia, and grade of diabetes control during pregnancy. In addition, the data on maternal BMI was derived from a single time point.
“These findings may have implications for counseling and managing pregnancies to prevent adverse birth outcomes,” they wrote.
The study and some authors were supported by the THL National Institute for Health and Welfare, the Swedish Research Council, Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. JAMA Pediatr. 2019 Feb 25. doi: 10.1001/jamapediatrics.2018.5541.
Women with insulin-treated diabetes are at significantly greater risk of preterm birth and of delivering babies who are large for gestational age (LGA), regardless of prepregnancy body weight, new findings suggest.
Researchers examined the role of maternal diabetes and weight on pregnancy outcomes in the population-based cohort study. The study comprised 649,043 live births in Finland between Jan. 1, 2004, and Dec. 31, 2014, including 4,000 in women with insulin-treated diabetes, 3,740 in women with type 2 diabetes, and 98,568 women with gestational diabetes.
Prepregnancy body mass index was normal for nearly 60% of mothers, while 4% were underweight, 21% were overweight, 8% were moderately obese, and 4% were severely obese.
Overall, the researchers found that women with insulin-treated diabetes had a 43-fold higher odds of having an LGA infant, compared with the reference group of women of normal BMI without diabetes (adjusted odds ratio [aOR], 43.80; 95% confidence interval, 40.88-46.93). And there was an 11-fold greater odds of having a preterm birth in this group (aOR, 11.17; 95% CI, 10.46-11.93).
The findings were published in JAMA Pediatrics.
“Smaller, but clearly statistically significant, increased LGA risks were found also for mothers with type 2 diabetes and gestational diabetes not treated with insulin, especially in combination with prepregnancy overweight or obesity that were stronger for type 2 diabetes than gestational diabetes,” wrote Linghua Kong, MSc, of the department of molecular medicine and surgery at Karolinska Institutet, and coauthors.
The aOR for LGA among women with type 2 diabetes was 9.57 (95% CI, 8.65-10.58), compared with the reference group. And for women with maternal gestational diabetes, the aOR for LGA was 3.80 (95% CI, 3.66-3.96).
Looking at the risk for preterm birth, the researchers found that the aOR among women with type 2 diabetes was 2.12 (95% CI, 1.90-2.36), while there was no association between gestational diabetes and preterm birth.
The researchers also reported that for women with gestational diabetes or no diabetes, the odds of preterm birth increased slightly as maternal prepregnancy BMI increased.
“Maternal glucose metabolism during pregnancy differs from that in the non-pregnant state; insulin resistance is increased, directing fat as the mother’s energy source to ensure adequate carbohydrate supply for the growing fetus,” the researchers wrote. “This increase in insulin resistance is mediated by a number of factors, such as increased levels of progesterone, estrogen, and human placental lactogen.”
The authors noted that their data did not include information on congenital anomalies, maternal complications such as preeclampsia, and grade of diabetes control during pregnancy. In addition, the data on maternal BMI was derived from a single time point.
“These findings may have implications for counseling and managing pregnancies to prevent adverse birth outcomes,” they wrote.
The study and some authors were supported by the THL National Institute for Health and Welfare, the Swedish Research Council, Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. JAMA Pediatr. 2019 Feb 25. doi: 10.1001/jamapediatrics.2018.5541.
Women with insulin-treated diabetes are at significantly greater risk of preterm birth and of delivering babies who are large for gestational age (LGA), regardless of prepregnancy body weight, new findings suggest.
Researchers examined the role of maternal diabetes and weight on pregnancy outcomes in the population-based cohort study. The study comprised 649,043 live births in Finland between Jan. 1, 2004, and Dec. 31, 2014, including 4,000 in women with insulin-treated diabetes, 3,740 in women with type 2 diabetes, and 98,568 women with gestational diabetes.
Prepregnancy body mass index was normal for nearly 60% of mothers, while 4% were underweight, 21% were overweight, 8% were moderately obese, and 4% were severely obese.
Overall, the researchers found that women with insulin-treated diabetes had a 43-fold higher odds of having an LGA infant, compared with the reference group of women of normal BMI without diabetes (adjusted odds ratio [aOR], 43.80; 95% confidence interval, 40.88-46.93). And there was an 11-fold greater odds of having a preterm birth in this group (aOR, 11.17; 95% CI, 10.46-11.93).
The findings were published in JAMA Pediatrics.
“Smaller, but clearly statistically significant, increased LGA risks were found also for mothers with type 2 diabetes and gestational diabetes not treated with insulin, especially in combination with prepregnancy overweight or obesity that were stronger for type 2 diabetes than gestational diabetes,” wrote Linghua Kong, MSc, of the department of molecular medicine and surgery at Karolinska Institutet, and coauthors.
The aOR for LGA among women with type 2 diabetes was 9.57 (95% CI, 8.65-10.58), compared with the reference group. And for women with maternal gestational diabetes, the aOR for LGA was 3.80 (95% CI, 3.66-3.96).
Looking at the risk for preterm birth, the researchers found that the aOR among women with type 2 diabetes was 2.12 (95% CI, 1.90-2.36), while there was no association between gestational diabetes and preterm birth.
The researchers also reported that for women with gestational diabetes or no diabetes, the odds of preterm birth increased slightly as maternal prepregnancy BMI increased.
“Maternal glucose metabolism during pregnancy differs from that in the non-pregnant state; insulin resistance is increased, directing fat as the mother’s energy source to ensure adequate carbohydrate supply for the growing fetus,” the researchers wrote. “This increase in insulin resistance is mediated by a number of factors, such as increased levels of progesterone, estrogen, and human placental lactogen.”
The authors noted that their data did not include information on congenital anomalies, maternal complications such as preeclampsia, and grade of diabetes control during pregnancy. In addition, the data on maternal BMI was derived from a single time point.
“These findings may have implications for counseling and managing pregnancies to prevent adverse birth outcomes,” they wrote.
The study and some authors were supported by the THL National Institute for Health and Welfare, the Swedish Research Council, Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
SOURCE: Kong L et al. JAMA Pediatr. 2019 Feb 25. doi: 10.1001/jamapediatrics.2018.5541.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: Pregnant women with insulin-treated diabetes have a 43-fold higher odds of having a child who is large for gestational age and 11-fold high risk for preterm birth.
Study details: A population-based cohort study of 649,043 live births in Finland between 2004 and 2014.
Disclosures: The study and some authors were supported by the THL National Institute for Health and Welfare, the Swedish Research Council, Stockholm County Council, the China Scholarship Council, and the Swedish Brain Foundation.
Source: Kong L et al. JAMA Pediatr. 2019 Feb 25. doi: 10.1001/jamapediatrics.2018.5541.
Induction at 41 weeks may cut perinatal complications for low-risk pregnancies
Inducing labor at 41 weeks’ gestation for women with low-risk pregnancies was associated with a 1.4% lower risk of adverse perinatal outcomes, compared with expectant management until 42 weeks, according to results from a randomized, controlled noninferiority trial.
“As with every intervention in the natural birth process, the decision to induce labour must be made with caution, as the expected benefits should outweigh possible adverse effects for both mother and child,” wrote Judit K.J. Keulen, of the department of obstetrics and gynecology at Amsterdam University Medical Center, and her colleagues. “The results of our study should be used to inform women approaching a gestational age of 41 weeks, so they can weigh the respective outcomes and decide whether to be induced at 41 weeks or to continue pregnancy until 42 weeks.”
Ms. Keulen and her colleagues randomized 1,801 women from 123 primary care midwifery practices and 45 hospitals across the Netherlands to receive induction (n = 900) at 41 weeks or expectant management (n = 901) at 42 weeks between 2012 and 2016. The investigators used a composite of perinatal mortality measures, which included Apgar score less than 7 at 5 minutes, arterial pH less than 7.05, meconium aspiration syndrome, neonatal ICU admission, intracranial hemorrhage, and/or brachial plexus injury.
Overall, there were 15 adverse perinatal outcomes in the induction group (1.7%) and 28 adverse outcomes in the expectant management group (3.1%; absolute risk difference, −1.4%). A lower number of infants (n = 11; 1.2%) in the induction group had an Apgar score less than 7 at 5 minutes, compared with infants (n = 23; 2.6%) in the expectant management group (relative risk, 0.48), and there were zero infants and 3 infants (RR, 0.3%) in the induction and expectant management groups, respectively, who had an Apgar score less than 4 at 5 minutes.
Three (0.3%) infants in the induction group and 8 (0.9%) infants in the expectant management group were admitted to the NICU (RR, 0.38). There was one (0.1%) case of fetal death in the induction group and two (0.2%) cases in the expectant management group, but there were no neonatal deaths in either group. With regard to composite adverse maternal outcomes, there were no significant differences between the induction group (n = 122; 14%) and the expectant management group (n = 102; 11%) and both groups had the same number of cesarean sections (n = 97; 11%).
The investigators noted several limitations, such as the noninferiority study design, use of composite adverse perinatal outcome, and lack of stratification by parity that led to an imbalance between the induction and expectant management groups.
“If the composite outcome is interpreted straightforwardly, there is a small benefit of induction at 41 weeks that could justify standard induction at 41 weeks,” Ms. Keulen and colleagues wrote.
“It could be argued, however, that a change of policy to earlier induction, concerning roughly one-fifth of all women with a singleton pregnancy, is too rigorous in light of the relatively low incidence of perinatal mortality, gestational age associated NICU admission, and Apgar score less than 4 at 5 minutes as indicator for encephalopathy,” they added. “This could justify expectant management if women want to avoid induction.”
This study was supported by a grant from the Netherlands Organisation for Health Research and Development ZonMw. Dr. Ben Willem Mol reported a practitioner fellowship with the National Health and Medical Research Council and is a consultant for ObsEva, Merck, and Guerbet. The other authors reported no relevant conflicts of interest.
SOURCE: Keulen JKJ et al. BMJ. 2019 Feb 20. doi: 10.1136/bmj.l344.
In the United States, the current guidelines state that you should consider induction of labor between 41 0/7 and 41 6/7 weeks of gestation and recommend induction between 42 0/7 and 42 6/7 weeks. This study demonstrates that there is a high rate of spontaneous labor among women who are managed with expectant management. Of the women randomized to the expectant management group, only 19% had not gone into labor by 42 weeks and thus, ultimately required induction.
In addition, there is only a 2-day difference in the gestational age of delivery between the induction and expectant management groups. The difference of 2 days does not change the rate of cesarean section or meconium aspiration system. There was a decrease in the rate of the composite neonatal outcome with induction which was mainly related to Apgar less than 7 at 5 minutes. Other significant neonatal outcomes were very rare in the study population (3 vs. 8 neonatal ICU admissions and 0 vs. 2 meconium aspiration).
However, arterial pH, a common marker of adverse neonatal outcomes, was not collected in 70% of the individuals enrolled this study. The rare rate of neonatal complications may reflect the relatively homogenous (about 86% white) and healthy population (about 11% of body mass index greater than or equal to 30).
Further, the lack of difference must be looked at with some caution as the rate of cesarean sections in the study population (11%) is much lower than the cesarean section rate in the United States of 32%. The absolute number of neonates with meconium aspiration system is very low in the study (0.2% for expectant management and none with induction). Previous studies on this subject have demonstrated rates 10-fold higher than in this current study.
In a related editorial, Kenyon et al. are correct in noting that by excluding Apgar scores, the composite adverse neonatal outcome loses its statistical significance (BMJ 2019 Feb 20. doi: 10.1136/bmj.l681). But, the study did not routinely collect arterial pH, which could be an objective measure of neonatal acidemia; thus, Apgar less than 7 at 5 minutes has to remain, as it is associated with neonatal acidemia. Induction should be part of the decision making for patients who are approaching post term. While an induction may alter a birth experience, some individuals opt for this method as evident by 616 individuals who refused enrollment because they desired induction at 41 weeks or the 87 individuals in the expectant management group who desired induction prior to 42 weeks. Thus, this study allows the clinician to provide counseling about the patient’s desires for management of their pregnancy with more information about neonatal outcomes with both expectant management and induction.
This trial, as well as the ARRIVE trial, have studied the effects of induction on a composite neonatal outcome. Both studies note statistical significance with the composite outcome but secondary to rare outcomes, statistical significance is not demonstrated when you look at each individual outcome. The study by Keulen et al. may help guide clinicians in counseling patients about the timing of their induction at late term. In the low-risk patient, these findings may help guide in the timing of induction.
Sarah D. Crimmins, DO, is an assistant professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland, Baltimore, and specializes in maternal-fetal medicine. She was asked to comment on the article by Keulen et al. Dr. Crimmins reported having no conflicts of interest.
In the United States, the current guidelines state that you should consider induction of labor between 41 0/7 and 41 6/7 weeks of gestation and recommend induction between 42 0/7 and 42 6/7 weeks. This study demonstrates that there is a high rate of spontaneous labor among women who are managed with expectant management. Of the women randomized to the expectant management group, only 19% had not gone into labor by 42 weeks and thus, ultimately required induction.
In addition, there is only a 2-day difference in the gestational age of delivery between the induction and expectant management groups. The difference of 2 days does not change the rate of cesarean section or meconium aspiration system. There was a decrease in the rate of the composite neonatal outcome with induction which was mainly related to Apgar less than 7 at 5 minutes. Other significant neonatal outcomes were very rare in the study population (3 vs. 8 neonatal ICU admissions and 0 vs. 2 meconium aspiration).
However, arterial pH, a common marker of adverse neonatal outcomes, was not collected in 70% of the individuals enrolled this study. The rare rate of neonatal complications may reflect the relatively homogenous (about 86% white) and healthy population (about 11% of body mass index greater than or equal to 30).
Further, the lack of difference must be looked at with some caution as the rate of cesarean sections in the study population (11%) is much lower than the cesarean section rate in the United States of 32%. The absolute number of neonates with meconium aspiration system is very low in the study (0.2% for expectant management and none with induction). Previous studies on this subject have demonstrated rates 10-fold higher than in this current study.
In a related editorial, Kenyon et al. are correct in noting that by excluding Apgar scores, the composite adverse neonatal outcome loses its statistical significance (BMJ 2019 Feb 20. doi: 10.1136/bmj.l681). But, the study did not routinely collect arterial pH, which could be an objective measure of neonatal acidemia; thus, Apgar less than 7 at 5 minutes has to remain, as it is associated with neonatal acidemia. Induction should be part of the decision making for patients who are approaching post term. While an induction may alter a birth experience, some individuals opt for this method as evident by 616 individuals who refused enrollment because they desired induction at 41 weeks or the 87 individuals in the expectant management group who desired induction prior to 42 weeks. Thus, this study allows the clinician to provide counseling about the patient’s desires for management of their pregnancy with more information about neonatal outcomes with both expectant management and induction.
This trial, as well as the ARRIVE trial, have studied the effects of induction on a composite neonatal outcome. Both studies note statistical significance with the composite outcome but secondary to rare outcomes, statistical significance is not demonstrated when you look at each individual outcome. The study by Keulen et al. may help guide clinicians in counseling patients about the timing of their induction at late term. In the low-risk patient, these findings may help guide in the timing of induction.
Sarah D. Crimmins, DO, is an assistant professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland, Baltimore, and specializes in maternal-fetal medicine. She was asked to comment on the article by Keulen et al. Dr. Crimmins reported having no conflicts of interest.
In the United States, the current guidelines state that you should consider induction of labor between 41 0/7 and 41 6/7 weeks of gestation and recommend induction between 42 0/7 and 42 6/7 weeks. This study demonstrates that there is a high rate of spontaneous labor among women who are managed with expectant management. Of the women randomized to the expectant management group, only 19% had not gone into labor by 42 weeks and thus, ultimately required induction.
In addition, there is only a 2-day difference in the gestational age of delivery between the induction and expectant management groups. The difference of 2 days does not change the rate of cesarean section or meconium aspiration system. There was a decrease in the rate of the composite neonatal outcome with induction which was mainly related to Apgar less than 7 at 5 minutes. Other significant neonatal outcomes were very rare in the study population (3 vs. 8 neonatal ICU admissions and 0 vs. 2 meconium aspiration).
However, arterial pH, a common marker of adverse neonatal outcomes, was not collected in 70% of the individuals enrolled this study. The rare rate of neonatal complications may reflect the relatively homogenous (about 86% white) and healthy population (about 11% of body mass index greater than or equal to 30).
Further, the lack of difference must be looked at with some caution as the rate of cesarean sections in the study population (11%) is much lower than the cesarean section rate in the United States of 32%. The absolute number of neonates with meconium aspiration system is very low in the study (0.2% for expectant management and none with induction). Previous studies on this subject have demonstrated rates 10-fold higher than in this current study.
In a related editorial, Kenyon et al. are correct in noting that by excluding Apgar scores, the composite adverse neonatal outcome loses its statistical significance (BMJ 2019 Feb 20. doi: 10.1136/bmj.l681). But, the study did not routinely collect arterial pH, which could be an objective measure of neonatal acidemia; thus, Apgar less than 7 at 5 minutes has to remain, as it is associated with neonatal acidemia. Induction should be part of the decision making for patients who are approaching post term. While an induction may alter a birth experience, some individuals opt for this method as evident by 616 individuals who refused enrollment because they desired induction at 41 weeks or the 87 individuals in the expectant management group who desired induction prior to 42 weeks. Thus, this study allows the clinician to provide counseling about the patient’s desires for management of their pregnancy with more information about neonatal outcomes with both expectant management and induction.
This trial, as well as the ARRIVE trial, have studied the effects of induction on a composite neonatal outcome. Both studies note statistical significance with the composite outcome but secondary to rare outcomes, statistical significance is not demonstrated when you look at each individual outcome. The study by Keulen et al. may help guide clinicians in counseling patients about the timing of their induction at late term. In the low-risk patient, these findings may help guide in the timing of induction.
Sarah D. Crimmins, DO, is an assistant professor of obstetrics, gynecology, and reproductive sciences at the University of Maryland, Baltimore, and specializes in maternal-fetal medicine. She was asked to comment on the article by Keulen et al. Dr. Crimmins reported having no conflicts of interest.
Inducing labor at 41 weeks’ gestation for women with low-risk pregnancies was associated with a 1.4% lower risk of adverse perinatal outcomes, compared with expectant management until 42 weeks, according to results from a randomized, controlled noninferiority trial.
“As with every intervention in the natural birth process, the decision to induce labour must be made with caution, as the expected benefits should outweigh possible adverse effects for both mother and child,” wrote Judit K.J. Keulen, of the department of obstetrics and gynecology at Amsterdam University Medical Center, and her colleagues. “The results of our study should be used to inform women approaching a gestational age of 41 weeks, so they can weigh the respective outcomes and decide whether to be induced at 41 weeks or to continue pregnancy until 42 weeks.”
Ms. Keulen and her colleagues randomized 1,801 women from 123 primary care midwifery practices and 45 hospitals across the Netherlands to receive induction (n = 900) at 41 weeks or expectant management (n = 901) at 42 weeks between 2012 and 2016. The investigators used a composite of perinatal mortality measures, which included Apgar score less than 7 at 5 minutes, arterial pH less than 7.05, meconium aspiration syndrome, neonatal ICU admission, intracranial hemorrhage, and/or brachial plexus injury.
Overall, there were 15 adverse perinatal outcomes in the induction group (1.7%) and 28 adverse outcomes in the expectant management group (3.1%; absolute risk difference, −1.4%). A lower number of infants (n = 11; 1.2%) in the induction group had an Apgar score less than 7 at 5 minutes, compared with infants (n = 23; 2.6%) in the expectant management group (relative risk, 0.48), and there were zero infants and 3 infants (RR, 0.3%) in the induction and expectant management groups, respectively, who had an Apgar score less than 4 at 5 minutes.
Three (0.3%) infants in the induction group and 8 (0.9%) infants in the expectant management group were admitted to the NICU (RR, 0.38). There was one (0.1%) case of fetal death in the induction group and two (0.2%) cases in the expectant management group, but there were no neonatal deaths in either group. With regard to composite adverse maternal outcomes, there were no significant differences between the induction group (n = 122; 14%) and the expectant management group (n = 102; 11%) and both groups had the same number of cesarean sections (n = 97; 11%).
The investigators noted several limitations, such as the noninferiority study design, use of composite adverse perinatal outcome, and lack of stratification by parity that led to an imbalance between the induction and expectant management groups.
“If the composite outcome is interpreted straightforwardly, there is a small benefit of induction at 41 weeks that could justify standard induction at 41 weeks,” Ms. Keulen and colleagues wrote.
“It could be argued, however, that a change of policy to earlier induction, concerning roughly one-fifth of all women with a singleton pregnancy, is too rigorous in light of the relatively low incidence of perinatal mortality, gestational age associated NICU admission, and Apgar score less than 4 at 5 minutes as indicator for encephalopathy,” they added. “This could justify expectant management if women want to avoid induction.”
This study was supported by a grant from the Netherlands Organisation for Health Research and Development ZonMw. Dr. Ben Willem Mol reported a practitioner fellowship with the National Health and Medical Research Council and is a consultant for ObsEva, Merck, and Guerbet. The other authors reported no relevant conflicts of interest.
SOURCE: Keulen JKJ et al. BMJ. 2019 Feb 20. doi: 10.1136/bmj.l344.
Inducing labor at 41 weeks’ gestation for women with low-risk pregnancies was associated with a 1.4% lower risk of adverse perinatal outcomes, compared with expectant management until 42 weeks, according to results from a randomized, controlled noninferiority trial.
“As with every intervention in the natural birth process, the decision to induce labour must be made with caution, as the expected benefits should outweigh possible adverse effects for both mother and child,” wrote Judit K.J. Keulen, of the department of obstetrics and gynecology at Amsterdam University Medical Center, and her colleagues. “The results of our study should be used to inform women approaching a gestational age of 41 weeks, so they can weigh the respective outcomes and decide whether to be induced at 41 weeks or to continue pregnancy until 42 weeks.”
Ms. Keulen and her colleagues randomized 1,801 women from 123 primary care midwifery practices and 45 hospitals across the Netherlands to receive induction (n = 900) at 41 weeks or expectant management (n = 901) at 42 weeks between 2012 and 2016. The investigators used a composite of perinatal mortality measures, which included Apgar score less than 7 at 5 minutes, arterial pH less than 7.05, meconium aspiration syndrome, neonatal ICU admission, intracranial hemorrhage, and/or brachial plexus injury.
Overall, there were 15 adverse perinatal outcomes in the induction group (1.7%) and 28 adverse outcomes in the expectant management group (3.1%; absolute risk difference, −1.4%). A lower number of infants (n = 11; 1.2%) in the induction group had an Apgar score less than 7 at 5 minutes, compared with infants (n = 23; 2.6%) in the expectant management group (relative risk, 0.48), and there were zero infants and 3 infants (RR, 0.3%) in the induction and expectant management groups, respectively, who had an Apgar score less than 4 at 5 minutes.
Three (0.3%) infants in the induction group and 8 (0.9%) infants in the expectant management group were admitted to the NICU (RR, 0.38). There was one (0.1%) case of fetal death in the induction group and two (0.2%) cases in the expectant management group, but there were no neonatal deaths in either group. With regard to composite adverse maternal outcomes, there were no significant differences between the induction group (n = 122; 14%) and the expectant management group (n = 102; 11%) and both groups had the same number of cesarean sections (n = 97; 11%).
The investigators noted several limitations, such as the noninferiority study design, use of composite adverse perinatal outcome, and lack of stratification by parity that led to an imbalance between the induction and expectant management groups.
“If the composite outcome is interpreted straightforwardly, there is a small benefit of induction at 41 weeks that could justify standard induction at 41 weeks,” Ms. Keulen and colleagues wrote.
“It could be argued, however, that a change of policy to earlier induction, concerning roughly one-fifth of all women with a singleton pregnancy, is too rigorous in light of the relatively low incidence of perinatal mortality, gestational age associated NICU admission, and Apgar score less than 4 at 5 minutes as indicator for encephalopathy,” they added. “This could justify expectant management if women want to avoid induction.”
This study was supported by a grant from the Netherlands Organisation for Health Research and Development ZonMw. Dr. Ben Willem Mol reported a practitioner fellowship with the National Health and Medical Research Council and is a consultant for ObsEva, Merck, and Guerbet. The other authors reported no relevant conflicts of interest.
SOURCE: Keulen JKJ et al. BMJ. 2019 Feb 20. doi: 10.1136/bmj.l344.
FROM BMJ