User login
Therese Borden is the editor of CHEST Physician. After 20 years of research, writing, and editing in the field of international development and economics, she began working in the field of medical editing and has held a variety of editorial positions with the company. She holds a PhD in International Economics from American University, Washington, and a BA in history from the University of Washington, Seattle.
Sleepless in the pandemic
Sleep difficulties during the COVID-19 crisis may be exacerbated by media overexposure and other factors causing fear and stress, according to findings from a large survey of French individuals.
“Physicians usually recommend coping with sleep disorders by exercising, going outside, avoiding screen time, and having a regular schedule – all recommendations difficult to apply during lockdown. Being forced to stay home and the ensuing boredom and loneliness may have led to increased [media exposure], especially among disadvantaged people and overexposure to media COVID-19 content may have contributed to fright and emotional distress,” Damien Leger of the Centre du Sommeil et de la Vigilance, Hôtel Dieu APHP, Université de Paris, and his colleagues wrote in the journal Sleep.
The investigators analyzed data from survey respondents about their sleep problems since the COVID-19 lockdown and other topics such as employment, daily activities, and sleep medications. The survey was part of a large research project, COCONEL, that has been developed to study the French population on a variety of behaviors and comprises 750,000 permanent panelists who respond to surveys. The survey was sent to random sample of panelists with no topic label to avoid selection bias. Of the 25,800 surveys sent, 1,005 responses were recorded.
Respondents were classified as having severe sleep problems if they reported that their daytime activities were affected or if their sleeping medications had increased since the lockdown. While 73% of respondents reported poor sleep in the 8 previous days, 25% reported severe sleep problems, and 54% reported that their sleep problems had worsened during the COVID-19 lockdown.
A media exposure score was created with a Likert scale (strongly agree, agree, disagree, strongly disagree) about media exposures of different types. The investigators also queried respondents about the degree to which they found media coverage of the pandemic provoked a fear response. Overall, 68% of respondents agreed that media images and stories about COVD-19 were frightening.
The researchers found a strong association between severe sleeping problems and a high media exposure score (risk ratio, 1.49; 95% confidence interval, 1.10-2.01; P < .05).
In addition, trepidation and fear from media exposure to COVID-19 news were also associated with severe sleep problems (RR, 1.27; 95% CI, 0.92-1.75; P < .05). “Suffering from sleep problems may have increased media use at night, and thus increased stress and/or psychological distress and reinforced sleeping problems,” the investigators wrote.
Not surprisingly, respondents with financial difficulties due to the pandemic also reported severe sleeping difficulties (RR, 1.99; 95% CI, 1.49-2.65; P < .05).
For individuals who have been treated for sleep problems, the COVID-19 pandemic may ratchet up their sleep challenges. The strongest association with severe sleep problems was found in those respondents who were already taking sleeping medications before the pandemic (RR, 2.72; 95% CI, 2.04-3.61; P < .05).
The COCONEL survey has been funded by the French and National Agency for Research, the Fondation de France, and the National Research Institute for Sustainable Development.
SOURCE: Leger D et al. Sleep. 2020, Jul 25. doi: 10.1093/sleep/zsaa125.
Sleep difficulties during the COVID-19 crisis may be exacerbated by media overexposure and other factors causing fear and stress, according to findings from a large survey of French individuals.
“Physicians usually recommend coping with sleep disorders by exercising, going outside, avoiding screen time, and having a regular schedule – all recommendations difficult to apply during lockdown. Being forced to stay home and the ensuing boredom and loneliness may have led to increased [media exposure], especially among disadvantaged people and overexposure to media COVID-19 content may have contributed to fright and emotional distress,” Damien Leger of the Centre du Sommeil et de la Vigilance, Hôtel Dieu APHP, Université de Paris, and his colleagues wrote in the journal Sleep.
The investigators analyzed data from survey respondents about their sleep problems since the COVID-19 lockdown and other topics such as employment, daily activities, and sleep medications. The survey was part of a large research project, COCONEL, that has been developed to study the French population on a variety of behaviors and comprises 750,000 permanent panelists who respond to surveys. The survey was sent to random sample of panelists with no topic label to avoid selection bias. Of the 25,800 surveys sent, 1,005 responses were recorded.
Respondents were classified as having severe sleep problems if they reported that their daytime activities were affected or if their sleeping medications had increased since the lockdown. While 73% of respondents reported poor sleep in the 8 previous days, 25% reported severe sleep problems, and 54% reported that their sleep problems had worsened during the COVID-19 lockdown.
A media exposure score was created with a Likert scale (strongly agree, agree, disagree, strongly disagree) about media exposures of different types. The investigators also queried respondents about the degree to which they found media coverage of the pandemic provoked a fear response. Overall, 68% of respondents agreed that media images and stories about COVD-19 were frightening.
The researchers found a strong association between severe sleeping problems and a high media exposure score (risk ratio, 1.49; 95% confidence interval, 1.10-2.01; P < .05).
In addition, trepidation and fear from media exposure to COVID-19 news were also associated with severe sleep problems (RR, 1.27; 95% CI, 0.92-1.75; P < .05). “Suffering from sleep problems may have increased media use at night, and thus increased stress and/or psychological distress and reinforced sleeping problems,” the investigators wrote.
Not surprisingly, respondents with financial difficulties due to the pandemic also reported severe sleeping difficulties (RR, 1.99; 95% CI, 1.49-2.65; P < .05).
For individuals who have been treated for sleep problems, the COVID-19 pandemic may ratchet up their sleep challenges. The strongest association with severe sleep problems was found in those respondents who were already taking sleeping medications before the pandemic (RR, 2.72; 95% CI, 2.04-3.61; P < .05).
The COCONEL survey has been funded by the French and National Agency for Research, the Fondation de France, and the National Research Institute for Sustainable Development.
SOURCE: Leger D et al. Sleep. 2020, Jul 25. doi: 10.1093/sleep/zsaa125.
Sleep difficulties during the COVID-19 crisis may be exacerbated by media overexposure and other factors causing fear and stress, according to findings from a large survey of French individuals.
“Physicians usually recommend coping with sleep disorders by exercising, going outside, avoiding screen time, and having a regular schedule – all recommendations difficult to apply during lockdown. Being forced to stay home and the ensuing boredom and loneliness may have led to increased [media exposure], especially among disadvantaged people and overexposure to media COVID-19 content may have contributed to fright and emotional distress,” Damien Leger of the Centre du Sommeil et de la Vigilance, Hôtel Dieu APHP, Université de Paris, and his colleagues wrote in the journal Sleep.
The investigators analyzed data from survey respondents about their sleep problems since the COVID-19 lockdown and other topics such as employment, daily activities, and sleep medications. The survey was part of a large research project, COCONEL, that has been developed to study the French population on a variety of behaviors and comprises 750,000 permanent panelists who respond to surveys. The survey was sent to random sample of panelists with no topic label to avoid selection bias. Of the 25,800 surveys sent, 1,005 responses were recorded.
Respondents were classified as having severe sleep problems if they reported that their daytime activities were affected or if their sleeping medications had increased since the lockdown. While 73% of respondents reported poor sleep in the 8 previous days, 25% reported severe sleep problems, and 54% reported that their sleep problems had worsened during the COVID-19 lockdown.
A media exposure score was created with a Likert scale (strongly agree, agree, disagree, strongly disagree) about media exposures of different types. The investigators also queried respondents about the degree to which they found media coverage of the pandemic provoked a fear response. Overall, 68% of respondents agreed that media images and stories about COVD-19 were frightening.
The researchers found a strong association between severe sleeping problems and a high media exposure score (risk ratio, 1.49; 95% confidence interval, 1.10-2.01; P < .05).
In addition, trepidation and fear from media exposure to COVID-19 news were also associated with severe sleep problems (RR, 1.27; 95% CI, 0.92-1.75; P < .05). “Suffering from sleep problems may have increased media use at night, and thus increased stress and/or psychological distress and reinforced sleeping problems,” the investigators wrote.
Not surprisingly, respondents with financial difficulties due to the pandemic also reported severe sleeping difficulties (RR, 1.99; 95% CI, 1.49-2.65; P < .05).
For individuals who have been treated for sleep problems, the COVID-19 pandemic may ratchet up their sleep challenges. The strongest association with severe sleep problems was found in those respondents who were already taking sleeping medications before the pandemic (RR, 2.72; 95% CI, 2.04-3.61; P < .05).
The COCONEL survey has been funded by the French and National Agency for Research, the Fondation de France, and the National Research Institute for Sustainable Development.
SOURCE: Leger D et al. Sleep. 2020, Jul 25. doi: 10.1093/sleep/zsaa125.
FROM SLEEP
‘Collateral damage’: COVID-19 threatens patients with COPD
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
according to a commentary published in CHEST (2020 May 28. doi: 10.1016/j.chest.2020.05.549) by a group of physicians who study COPD.
Not only is COPD among the most prevalent underlying diseases among hospitalized COVID-19 patients (Clin Microbiol Infect. 2020 Jun 8. doi: 10.1016/j.cmi.2020.05.041), but other unanticipated factors of treatment put these patients at extra risk. Valerie Press, MD, assistant professor of medicine and pediatrics at the University of Chicago, and colleagues aimed to alert physicians to be aware of potential negative effects, or collateral damage, that the pandemic can have on their patients with COPD, even those without a COVID-19 diagnosis.
These concerns include that patients may delay presenting to the ED with acute exacerbations of COPD and once they present they may be at later stages of the exacerbation. Further, evaluation for COVID-19 as a possible trigger of acute exacerbations of COPD (AECOPD) is essential; however, implementing proven AECOPD therapies remains challenging. For instance, routine therapy with corticosteroids for AECOPD may be delayed due to diagnostic uncertainty and hesitation to treat COVID-19 with steroids while COVID-19 testing is pending,” Dr. Press and her colleagues stated.
Shortages and scarcity of medications such as albuterol inhalers to treat COPD have been reported. In addition, patients with COPD are currently less likely to access their health care providers because of fear of COVID-19 infection. This barrier to care and the current higher threshold for presenting to the hospital may to lead to more cases of AECOPD and worsening health in these patients, according to the authors.
Dr. Press said in an interview: “Access to medications delivered through inhalers is challenging even without the pandemic due to high cost of medications. Generic medications are key to improving access for patients with chronic lung disease, so once the generic albuterol becomes available, this should help with access. In the meantime, some companies help provide medications at reduced cost, but usually only on a short time basis. In addition, some pharmacies have lower-cost albuterol inhalers, but these are often not supplied with a full month of dosing.”
In addition to all these concerns is the economic toll this pandemic is taking on patients. The association between COPD and socioeconomic status has been studied in depth (Am J Respir Crit Care Med. 2019; 199[8]:961-69) and would indicate that low-income patients with COPD would face an increased burden during an economic downturn. The authors noted, “Historic rapid job loss and unemployment in the U.S., coupled with a health system of employment-integrated health insurance coverage, makes it more likely that people with COPD will not be able to afford their medication.”
Dr. Press stressed that the COVID pandemic has highlighted critically important disparities in access to health care and disparities in health. “Many of the recommendations regarding stay-at-home and other safety mechanisms to prevent contracting and spreading COVID-19 have not been feasible for all sub-populations in the United States. Those that were essential workers did not have the ability to stay home. Further, those that rely on public transportation had less opportunities to social distance. Finally, while telemedicine opportunities have advanced for clinical care, not all patients have equal access to these capabilities and health disparities could widen in this regard as well. Clinicians have a responsibility to identify social determinants of health that increase risks to our patients’ health and limit their safety.”*
The authors offer some concrete suggestions of how physicians can address some of these concerns, including the following:
- Be alert to potential barriers to accessing medication and be aware of generic albuterol inhaler recently approved by the FDA in response to COVID-19–related shortages.
- Use telemedicine to monitor patients and improvement of home self-management. Clinicians should help patients “seek care with worsening symptoms and have clear management guidelines regarding seeking phone/video visits; implementing therapy with corticosteroids, antibiotics, or inhalers and nebulizers; COVID-19 testing recommendations; and thresholds for seeking emergent, urgent, or outpatient care in person,” Dr. Press added, “Building on the work of nurse advice lines and case management and other support services for high-risk patients with COPD may continue via telehealth and telephone visits.”
- Ensure that untried therapy for COVID-19 “does not displace proven and necessary treatments for patients with COPD, hence placing them at increased risk for poor outcomes.”
Dr. Press is also concerned about the post–COVID-19 period for patients with COPD. “It is too early to know if there are specific after effects of the COVID infection on patients with COPD, but given the damage the virus does to even healthy lungs, there is reason to have concern that COVID could cause worsening damage to the lungs of individuals with COPD.”
She noted, “Post-ICU [PICU] syndrome has been recognized in patients with ARDS generally, and patients who recover from critical illness may have long-lasting (and permanent) effects on strength, cognition, disability, and pulmonary function. Whether the PICU syndrome in patients with ARDS due to COVID-19 specifically is different from the PICU syndrome due to other causes remains unknown. But clinicians whose patients with COPD survive COVID-19 may expect long-lasting effects and slow recovery in cases where COVID-19 led to severe ARDS and a prolonged ICU stay. Assessment of overall patient recovery and functional capacity (beyond lung function and dyspnea symptoms) including deconditioning, anxiety, PTSD, weakness, and malnutrition will need to be addressed. Additionally, clinicians may help patients and their families understand the expected recovery and help facilitate family conversations about residual effects of COVID-19.”
The authors had no disclosures.
SOURCE: Press V et al. Chest. 2020 May 28. doi:10.1016/j.chest.2020.05.549.
CORRECTION: *This story was updated with further comments and clarifications from Dr. Press. 6/23/2020
FROM CHEST
Trial undertaken to better predict pulmonary hypertension prognosis
A research team at Vanderbilt University Medical Center has begun a trial to compare the value of tracking daily activity and the Six Minute Walk Distance to predict pulmonary hypertension prognosis. The Longitudinal Pulmonary Vascular Disease Phenomics Program (L-PVDOMICS), a prospective, longitudinal, observational study will track daily activity and patient-reported outcomes in participants enrolled. Patients with pulmonary hypertension and healthy participants will undergo activity monitoring for 12 weeks once a year for 4 years. Metrics will include patient-reported outcomes including quality of life (emphasis-10, Minnesota Living with Heart Failure, and SF-36 surveys), medication changes, hospitalization, and death.
The study is designed to establish the clinical utility of daily activity tracking in patients with pulmonary hypertension and to identify clinical factors associated with reduced daily activity. Five hundred patients are expected to enroll and the estimated closing date is June 2023. The hypothesis for the study is that daily activity will have stronger prognostic value after 12 weeks than the Six Minute Walk Distance in patients with pulmonary hypertension. Participants will wear an accelerometer to record activity level to determine daily activities and will also engage in the Six Minute Walk Distance Test.
Individuals that are pregnant or have been hospitalized within the past 3 months will be excluded. Participants are currently being recruited.
The trial sponsor is Vanderbilt University Medical Center.
A research team at Vanderbilt University Medical Center has begun a trial to compare the value of tracking daily activity and the Six Minute Walk Distance to predict pulmonary hypertension prognosis. The Longitudinal Pulmonary Vascular Disease Phenomics Program (L-PVDOMICS), a prospective, longitudinal, observational study will track daily activity and patient-reported outcomes in participants enrolled. Patients with pulmonary hypertension and healthy participants will undergo activity monitoring for 12 weeks once a year for 4 years. Metrics will include patient-reported outcomes including quality of life (emphasis-10, Minnesota Living with Heart Failure, and SF-36 surveys), medication changes, hospitalization, and death.
The study is designed to establish the clinical utility of daily activity tracking in patients with pulmonary hypertension and to identify clinical factors associated with reduced daily activity. Five hundred patients are expected to enroll and the estimated closing date is June 2023. The hypothesis for the study is that daily activity will have stronger prognostic value after 12 weeks than the Six Minute Walk Distance in patients with pulmonary hypertension. Participants will wear an accelerometer to record activity level to determine daily activities and will also engage in the Six Minute Walk Distance Test.
Individuals that are pregnant or have been hospitalized within the past 3 months will be excluded. Participants are currently being recruited.
The trial sponsor is Vanderbilt University Medical Center.
A research team at Vanderbilt University Medical Center has begun a trial to compare the value of tracking daily activity and the Six Minute Walk Distance to predict pulmonary hypertension prognosis. The Longitudinal Pulmonary Vascular Disease Phenomics Program (L-PVDOMICS), a prospective, longitudinal, observational study will track daily activity and patient-reported outcomes in participants enrolled. Patients with pulmonary hypertension and healthy participants will undergo activity monitoring for 12 weeks once a year for 4 years. Metrics will include patient-reported outcomes including quality of life (emphasis-10, Minnesota Living with Heart Failure, and SF-36 surveys), medication changes, hospitalization, and death.
The study is designed to establish the clinical utility of daily activity tracking in patients with pulmonary hypertension and to identify clinical factors associated with reduced daily activity. Five hundred patients are expected to enroll and the estimated closing date is June 2023. The hypothesis for the study is that daily activity will have stronger prognostic value after 12 weeks than the Six Minute Walk Distance in patients with pulmonary hypertension. Participants will wear an accelerometer to record activity level to determine daily activities and will also engage in the Six Minute Walk Distance Test.
Individuals that are pregnant or have been hospitalized within the past 3 months will be excluded. Participants are currently being recruited.
The trial sponsor is Vanderbilt University Medical Center.
Lombardy ICU capacity stressed to breaking point by COVID-19 outbreak
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
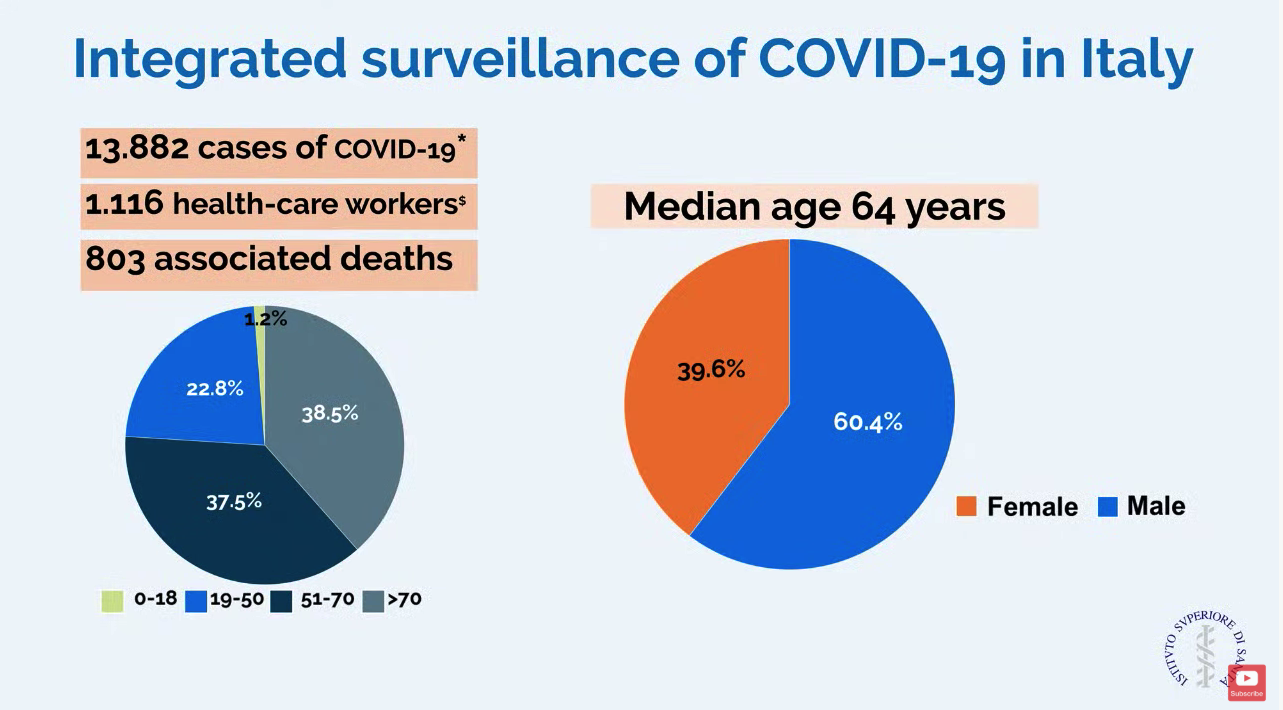
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.
Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
The outbreak of COVID-19 in the Lombardy region of Italy has severely stressed the medical system and the current level of activity may not be sustainable for long, according to Maurizio Cecconi, MD, of the department of anesthesia and intensive care, Humanitas Research Hospital, Milan. Dr. Cecconi spoke via JAMA Live Stream interview with Howard Bauchner, MD, the Editor in Chief of JAMA.

A summary of comments by Dr. Cecconi and two colleagues was simultaneously published in JAMA (2020 Mar 13. doi: 10.1001/jama.2020.4031).
Dr. Cecconi discussed the progress and medical response to the swiftly expanding outbreak that began on Feb. 20. A man in his 30s was admitted to the Codogno Hospital, Lodi, Lombardy, Italy, in respiratory distress. He tested positive for a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19). In less than 24 hours, the hospital had 36 cases of COVID-19.
In a slide provided by the Italian National Health Service, the number of cases in Italy stands at 13,882 with 803 associated deaths.
ICU resources have been severely stressed. Before the outbreak, Lombardy had 720 ICU beds (about 5% of total beds). Within 48 hours of the first case, ICU cohorts were formed in 15 hub hospitals totaling 130 COVID-19 ICU beds. By March 7, the total number of dedicated cohorted COVID-19 ICU beds was 482.
“The proportion of ICU admissions represents 12% of the total positive cases, and 16% of all hospitalized patients,” compared with about 5% of ICU admissions reported from China. The difference may be attributable to different criteria for ICU admissions in Italy, compared with China, according to Dr. Cecconi and colleagues.
Dr. Cecconi mentioned that there were relatively few cases in children, and they had relatively mild disease. The death rate among patients remained under 1% up to age 59. For patients aged 60-69 years, the rate was 2.7%; for patients aged 70-79 years, the rate was 9.6%; for those aged 80-89, the rate was much higher at 16.6%.

Modeled forecasts of the potential number of cases in Lombardy are daunting. “The linear model forecasts that approximately 869 ICU admissions could occur by March 20, 2020, whereas the exponential model growth projects that approximately 14,542 ICU admissions could occur by then. Even though these projections are hypothetical and involve various assumptions, any substantial increase in the number of critically ill patients would rapidly exceed total ICU capacity, without even considering other critical admissions, such as for trauma, stroke, and other emergencies,” wrote Dr. Cecconi and his colleagues in JAMA. He said, “We could be on our knees very soon,” referring to the potential dramatic increase in cases.
Dr. Cecconi had some recommendations for other countries in which a major outbreak has not yet occurred. He recommended going beyond expanding ICU and isolation capacity and focus on training staff with simulation for treating these highly contagious patients. His medical center has worked hard to protect staff but 1,116 health care workers have tested positive for the virus. Conditions for staff are very difficult in full protective gear, and Dr. Cecconi commended the heroic work by these doctors and nurses.
In addition, Dr. Cecconi is focused on supportive care for patients and does not recommend using untried approaches on these patients that could cause harm. “Everyone wants to find a specific drug for these patients, but I say there is not particular drug at the moment.” He stressed that, despite the crisis, doctors should focus on evidence-based treatment and tried-and-true supportive care.
Disclosures by Dr. Cecconi are available on the JAMA website.
CORRECTION 3/13/2020 2.18 P.M. The death rate for patients aged 70-79 was corrected.
REPORTING FROM JAMA LIVE STREAM
Second U.S. coronavirus patient confirmed
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
Washington state patient is first U.S. case of novel coronavirus
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
The first case of the novel coronavirus, named 2019-nCoV, in the United States has been diagnosed in a traveler from China who came through Seattle-Tacoma International Airport on Jan 15, the Centers for Disease Control and Prevention announced today at a press briefing.
The outbreak began at a animal and meat market in China and now has spread to at least three other countries, including Thailand, Japan and South Korea. While originally thought to be spreading from animal to person, it appears that limited person-to-person transmission is occurring, although it is currently unknown how easily this virus spreads between people.
More than 300 cases have been reported and six deaths have occurred. Fourteen health care workers have been infected.
Scott Lindquist, MD, MPH, Washington state epidemiologist, said at the briefing that the patient, a man who had been in Wuhan, arrived at Sea-Tac on Jan. 15, 2 days before airport screening had been initiated. He was symptom free at the time of his arrival and probably would not have been identified as infected with 2019-nCoV. The patient had been aware of the public health and news media coverage of 2019-nCoV and, after developing symptoms, contacted his health care provider on Jan. 19. The patient did not fly directly from Wuhan, but Dr. Lindquist said that he has been fully cooperative and has been helpful to authorities in tracing his route and contacts. The man is being treated at Providence Regional Medical Center, Everett, Wash.
The CDC obtained a specimen from the patient immediately and identified the 2019-nCoV within 24 hours.
Screening at airports is part of a multipart strategy to address this type of infection that includes public health information dissemination, patient education, as well as hospital preparation and training exercises. Currently, a strategy referred to as “funneling” is being implemented wherein travelers from China are rerouted and reticketed to one of the five airports conducting screening. At present, JFK in New York, San Francisco International, Los Angeles International, Hartsfield-Jackson Atlanta International Airport, and Chicago O’Hare International Airport are conducting inbound traveler screening.
The CDC is working in close cooperation with the Department of Homeland Security and the Federal Aviation Administration to coordinate travel screenings and reroutings. In addition, the CDC is working with the World Health Organization and the international global health community to share information about this outbreak. The CDC also has staff on site in Wuhan and is communicating with local health authorities. The CDC has activated its Emergency Operations Center to better provide ongoing support to the 2019-nCoV response. Currently, the focus is on tracing contacts and the means of transmission of this virus.
Updates on the outbreak will be posted on the CDC coronavirus website.
CORRECTION: 1/21/2020: The name of the medical center where the 2019-nCoV patient is being treated was corrected.
REPORTING FROM CDC
Two new cases of coronavirus pneumonia in Thailand, Japan
Health authorities in Wuhan, Hubei Province, China, identified the novel coronavirus, 2019-nCoV, responsible for the outbreak of a mysterious pneumonia that resulted in hospitalization of more than 40 patients and one death, according to the Centers for Disease Control and Prevention in a statement on the CDC website.
On Jan. 13, the Thailand’s Ministry of Public Health reported the first imported case of lab-confirmed 2019-nCoV from Wuhan. The Centers for Disease Control and Prevention stated: “The traveler with febrile illness was detected on the same day by thermal surveillance at Suvarnabhumi Airport, Thailand, and was hospitalized the same day. After temperature check and initial assessment, she was transferred to the hospital for further investigations and treatment.”
Samples from this patient tested positive for coronaviruses by reverse transcriptase-polymerase chain reaction. The genomic sequencing analysis was performed by Emerging Infectious Diseases Health Science Center, the Thai Red Cross Society, and the Thai National Institute of Health. The patient is reported to be in stable condition.
The New York Times has reported a case of 2019-nCoV in Japan in a traveler returning from Wuhan. That patient is reported to have recovered and been discharged.
Chinese health authorities transmitted the full genome of “2019 novel coronavirus,” or “2019-nCoV,” to GenBank, the genetic sequence database managed by the National Institutes of Health, and in the Global Initiative on Sharing All Influenza Data portal.
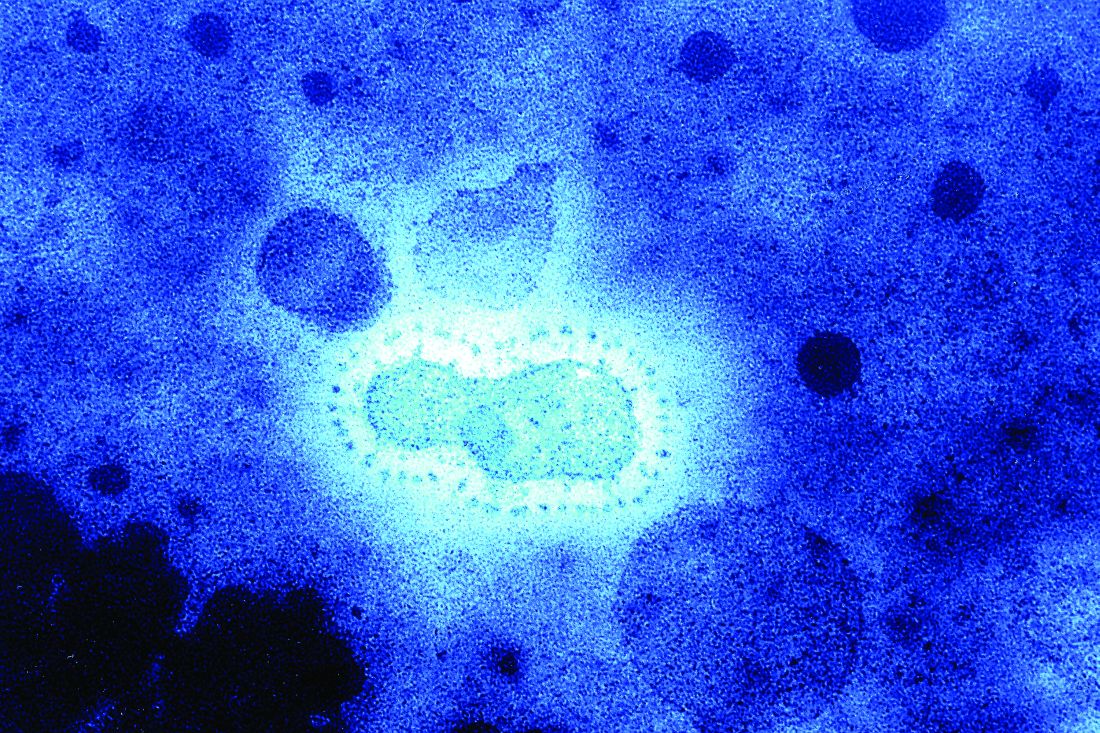
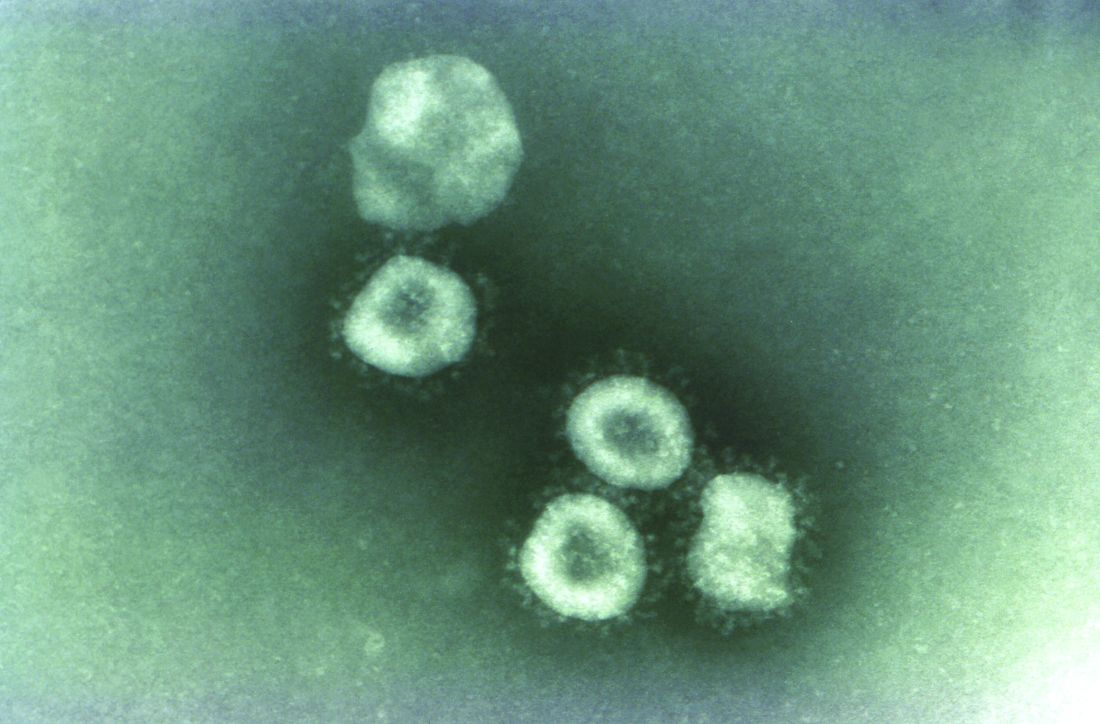
Coronaviruses are a large family of viruses. Most known human coronaviruses only cause mild respiratory disease, such as the common cold. But several coronaviruses have emerged to infect people and cause severe disease, such as has been seen with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). The cases in the Wuhan pneumonia outbreak have tested negative for both SARS and MERS.
The outbreak in Wuhan appears to be contained. The World Health Organization reported that the Wuhan health authorities identified and followed 763 close contacts, including health care workers. No additional cases of infection with the novel coronavirus have been identified. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has issued a Level 1 travel alert and recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
Health authorities in Wuhan, Hubei Province, China, identified the novel coronavirus, 2019-nCoV, responsible for the outbreak of a mysterious pneumonia that resulted in hospitalization of more than 40 patients and one death, according to the Centers for Disease Control and Prevention in a statement on the CDC website.
On Jan. 13, the Thailand’s Ministry of Public Health reported the first imported case of lab-confirmed 2019-nCoV from Wuhan. The Centers for Disease Control and Prevention stated: “The traveler with febrile illness was detected on the same day by thermal surveillance at Suvarnabhumi Airport, Thailand, and was hospitalized the same day. After temperature check and initial assessment, she was transferred to the hospital for further investigations and treatment.”
Samples from this patient tested positive for coronaviruses by reverse transcriptase-polymerase chain reaction. The genomic sequencing analysis was performed by Emerging Infectious Diseases Health Science Center, the Thai Red Cross Society, and the Thai National Institute of Health. The patient is reported to be in stable condition.
The New York Times has reported a case of 2019-nCoV in Japan in a traveler returning from Wuhan. That patient is reported to have recovered and been discharged.
Chinese health authorities transmitted the full genome of “2019 novel coronavirus,” or “2019-nCoV,” to GenBank, the genetic sequence database managed by the National Institutes of Health, and in the Global Initiative on Sharing All Influenza Data portal.
Coronaviruses are a large family of viruses. Most known human coronaviruses only cause mild respiratory disease, such as the common cold. But several coronaviruses have emerged to infect people and cause severe disease, such as has been seen with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). The cases in the Wuhan pneumonia outbreak have tested negative for both SARS and MERS.
The outbreak in Wuhan appears to be contained. The World Health Organization reported that the Wuhan health authorities identified and followed 763 close contacts, including health care workers. No additional cases of infection with the novel coronavirus have been identified. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has issued a Level 1 travel alert and recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
Health authorities in Wuhan, Hubei Province, China, identified the novel coronavirus, 2019-nCoV, responsible for the outbreak of a mysterious pneumonia that resulted in hospitalization of more than 40 patients and one death, according to the Centers for Disease Control and Prevention in a statement on the CDC website.
On Jan. 13, the Thailand’s Ministry of Public Health reported the first imported case of lab-confirmed 2019-nCoV from Wuhan. The Centers for Disease Control and Prevention stated: “The traveler with febrile illness was detected on the same day by thermal surveillance at Suvarnabhumi Airport, Thailand, and was hospitalized the same day. After temperature check and initial assessment, she was transferred to the hospital for further investigations and treatment.”
Samples from this patient tested positive for coronaviruses by reverse transcriptase-polymerase chain reaction. The genomic sequencing analysis was performed by Emerging Infectious Diseases Health Science Center, the Thai Red Cross Society, and the Thai National Institute of Health. The patient is reported to be in stable condition.
The New York Times has reported a case of 2019-nCoV in Japan in a traveler returning from Wuhan. That patient is reported to have recovered and been discharged.
Chinese health authorities transmitted the full genome of “2019 novel coronavirus,” or “2019-nCoV,” to GenBank, the genetic sequence database managed by the National Institutes of Health, and in the Global Initiative on Sharing All Influenza Data portal.
Coronaviruses are a large family of viruses. Most known human coronaviruses only cause mild respiratory disease, such as the common cold. But several coronaviruses have emerged to infect people and cause severe disease, such as has been seen with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). The cases in the Wuhan pneumonia outbreak have tested negative for both SARS and MERS.
The outbreak in Wuhan appears to be contained. The World Health Organization reported that the Wuhan health authorities identified and followed 763 close contacts, including health care workers. No additional cases of infection with the novel coronavirus have been identified. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has issued a Level 1 travel alert and recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
Mystery pneumonia in China has health officials on alert
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
An according to a statement from the Centers for Disease Control and Prevention.
As of Jan. 5, 2020, 59 cases of the disease have been reported by the Wuhan Municipal Health Commission. The cluster of cases is linked to the Wuhan South China Seafood City market where – in addition to seafood – chickens, bats, marmots, and other animals were sold. That market has been closed since Jan. 1, 2020, for cleaning and disinfection.
Wuhan health authorities are closely monitoring over 150 contacts for symptoms. Laboratory results have been negative for influenza, avian influenza, adenovirus, and the viruses that caused SARS (severe acute respiratory syndrome) and MERS (Middle East respiratory syndrome). So far, there are no reports of person-to-person transmission or health care worker infection of this pneumonia.
The World Health Organization reported that, as of Dec. 31, 2019, about one-quarter of patients were severely ill with the pneumonia and the rest were stable. Symptoms reported include fever, difficulty breathing, and chest radiographs showing invasive lesions in both lungs. All patients are being treated in isolation and efforts to identify the pathogen are ongoing.
The WHO is monitoring the situation closely and is in close contact with Chinese health authorities.
The CDC has recommended that travelers to Wuhan, a city of over 19 million people, avoid animal and meat markets, avoid contact with sick people, and wash hands often with soap and water. Travelers who have been in Wuhan recently and who experience respiratory symptoms should notify the local health department immediately. In addition, the CDC has issued a Level 1 travel alert, which recommends travelers observe usual precautions against infectious disease.
In addition, the CDC recommends that, for symptomatic patients with a history of travel to Wuhan, caution should be exercised in the health care setting. “Ask such patients to don a surgical mask as soon as they are identified. Conduct their evaluation in a private room with the door closed. Personnel entering the room to evaluate the patient should use contact precautions and wear an N95 disposable facepiece respirator. For patients admitted for inpatient care, implement contact and airborne isolation precautions, in addition to standard precautions, until further information becomes available. For additional infection control guidance see: www.cdc.gov/infectioncontrol/guidelines/isolation/index.html.”
The measles comeback of 2019
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
Measles made a comeback in 2019.
The Centers for Disease Control and Prevention reported that, as of Dec. 5, 2019, 1,276 individual cases of measles of measles were confirmed in 31 states, the largest number since 1992. This number is a major uptick in cases, compared with previous years since 2000 when the CDC declared measles eliminated from the United States. No deaths have been reported for 2019.
Three-quarters of these cases in 2019 were linked to recent outbreaks in New York and occurred in primarily in underimmunized, close-knit communities and in patients with links to international travel. A total of 124 of the people who got measles this year were hospitalized, and 61 reported having complications, including pneumonia and encephalitis. The overall median patient age was 6 years (31% aged 1-4 years, 27% aged 5-17 years, and 29% aged at least 18 years).
The good news is that most of these cases occurred in unvaccinated patients. The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.7% for two doses of the MMR vaccine, falling just short of the CDC recommended 95% vaccination rate threshold. The CDC reported an approximate 2.5% rate of vaccination exemptions among school-age children.
The bad news is that, despite the high rate of MMR vaccination rates among U.S. children, there are gaps in measles protection in the U.S. population because of factors leaving patients immunocompromised and antivaccination sentiment that has led some parents to defer or refuse the MMR.
In addition, adults who were vaccinated prior to 1968 with either inactivated measles vaccine or measles vaccine of unknown type may have limited immunity. The inactivated measles vaccine, which was available in 1963-1967, did not achieve effective measles protection.
A global measles surge
While antivaccination sentiment contributed to the 2019 measles cases, a more significant factor may be the global surge of measles. More than 140,000 people worldwide died from measles in 2018, according to the World Health Organization and the CDC.
“[Recent data on measles] indicates that during the first 6 months of the year there have been more measles cases reported worldwide than in any year since 2006. From Jan. 1 to July 31, 2019, 182 countries reported 364,808 measles cases to the WHO. This surpasses the 129,239 reported during the same time period in 2018. WHO regions with the biggest increases in cases include the African region (900%), the Western Pacific region (230%), and the European region (150%),” according to a CDC report.
Studies on hospitalization and complications linked to measles in the United States are scarce, but two outbreaks in Minnesota (2011 and 2017) provided some data on what to expect if the measles surge continues into 2020. The investigators found that poor feeding was a primary reason for admission (97%); additional complications included otitis media (42%), pneumonia (30%), and tracheitis (6%). Three-quarters received antibiotics, 30% required oxygen, and 21% received vitamin A. Median length of stay was 3.7 days (range, 1.1-26.2 days) (Pediatr Infect Dis J. 2019 Jun;38[6]:547-52. doi: 10.1097/INF.0000000000002221).
‘Immunological amnesia’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science (2019 Nov 1;366[6465]599-606).
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
Maternal-acquired immunity fades
In another study of measles immunity, maternal antibodies were found to be insufficient to provide immunity to infants after 6 months.
The study of 196 infants showed that maternal measles antibodies had dropped below the protective threshold by 3 months of age – well before the recommended age of 12-15 months for the first dose of MMR vaccine.
The odds of inadequate protection doubled for each additional month of age, Michelle Science, MD, of the University of Toronto and associates reported in Pediatrics (2019 Dec 1. doi 10.1542/peds.2019-0630).
“The widening gap between loss of maternal antibodies and measles vaccination described in our study leaves infants vulnerable to measles for much of their infancy and highlights the need for further research to support public health policy,” Dr. Science and colleagues wrote.
The researchers randomly selected 25 samples for each of eight different age groups: up to 30 days old; 1 month (31-60 days), 2 months (61-89 days), 3 months (90-119 days), 4 months, 5 months, 6-9 months, and 9-11 months.
Just over half the babies (56%) were male, and 35% had an underlying condition, but none had conditions that might affect antibody levels. The conditions were primarily a developmental delay or otherwise affecting the central nervous system, liver, or gastrointestinal function. Mean maternal age was 32 years.
To ensure high test sensitivity, the researchers used the plaque-reduction neutralization test to test for measles-neutralizing antibodies instead of using enzyme-linked immunosorbent assay, because “ELISA sensitivity decreases as antibody titers decrease,” Dr. Science and colleagues wrote. They used a neutralization titer of less than 192 mIU/mL as the threshold for protection against measles.
When the researchers calculated the predicted standardized mean antibody titer for infants with a mother aged 32 years, they determined their mean to be 541 mIU/mL at 1 month, 142 mIU/mL at 3 months (below the measles threshold of susceptibility of 192 mIU/mL), and 64 mIU/mL at 6 months. None of the infants had measles antibodies above the protective threshold at 6 months old, the authors noted.
Children’s odds of susceptibility to measles doubled for each additional month of age, after adjustment for infant sex and maternal age (odds ratio, 2.13). Children’s likelihood of susceptibility to measles modestly increased as maternal age increased in 5-year increments from 25 to 40 years.
Children with an underlying conditions had greater susceptibility to measles (83%), compared with those without a comorbidity (68%, P = .03). No difference in susceptibility existed between males and females or based on gestational age at birth (ranging from 37 to 41 weeks).
The Advisory Committee on Immunization Practices permits measles vaccination “as early as 6 months for infants who plan to travel internationally, infants with ongoing risk for exposure during measles outbreaks and as postexposure prophylaxis,” Huong Q. McLean, PhD, of Marshfield (Wisc.) Clinic Research Institute, and Walter A. Orenstein, MD, of Emory University, Atlanta, noted in an editorial.
The research was funded by the Public Health Ontario Project Initiation Fund. The authors had no relevant financial disclosures.
Bianca Nogrady and Tara Haelle contributed to this story.
EVALI outbreak ongoing, but new cases decline
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).
CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).

CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
The vaping lung disease outbreak continues, but according to the Centers for Disease Control and Prevention, it may have peaked and the number of new hospitalized cases reported to the CDC may be decreasing.
In the Dec. 6, 2019, Morbidity and Mortality Weekly Report, the CDC has updated information about cases of e-cigarette, or vaping, product use–associated lung injury (EVALI): As of Dec. 3, there have been 2,291 cases reported from all 50 states, Washington, D.C., and two U.S. territories (Puerto Rico and U.S. Virgin Islands). A total of 48 deaths have been confirmed in 25 states and Washington, D.C., the CDC reported.
The largest number of weekly hospitalized cases occurred during the week of Sept. 15, 2019; since then, hospitalized cases have steadily declined. “Among all hospitalized EVALI patients reported to CDC weekly, the percentage of recent cases (patients hospitalized within the preceding 3 weeks) declined from 58% reported November 12 to 30% reported December 3,” the report stated.
About 80%of hospitalized EVALI patients reported using tetrahydrocannabinol (THC)–containing e-cigarette, or vaping, products. “Dank Vapes,” counterfeit THC-containing products of unknown origin, were the most commonly reported THC-containing branded products used. Dank Vapes were used by 56% of hospitalized EVALI patients nationwide, followed by TKO brand (15%), Smart Cart (13%), and Rove (12%).
Of EVALI patients for whom data were available, 67% were male, and the median age was 24 years (range, 13-77 years); 78% were aged under 35 years and 16% were under 18 years. About 75% of EVALI patients were non-Hispanic white and 16% were Hispanic. Among the 48 deaths, 54% of patients were male, and the median age was 52 years (range, 17-75 years).

CDC research on EVALI continues to be limited by the self-reported data, lack of data on substances used, missing data, loss to follow-up, and reporting lags, but the intensive investigation and data collection is ongoing.
The report concludes: “While the investigation continues, persons should consider refraining from the use of all e-cigarette, or vaping, products. Adults using e-cigarette, or vaping, products to quit smoking should not return to smoking cigarettes; they should weigh all risks and benefits and consider using [Food and Drug Administration]–approved cessation medications. Adults who continue to use e-cigarette, or vaping, products should carefully monitor themselves for symptoms and see a health care provider immediately if they develop symptoms similar to those reported in this outbreak. Irrespective of the ongoing investigation, e-cigarette, or vaping, products should never be used by youths, young adults or pregnant women.”
Information on the current investigation, reporting of cases, and other resources can be found on the CDC website.
SOURCE: Lozier MJ et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 6. doi: 10.15585/mmwr.mm6849e1.
FROM THE MMWR