User login
Insulin may be toxic to the placenta in early pregnancy
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
, according to findings from an experimental in vitro study published in Fertility and Sterility.
“Collectively these results demonstrate that insulin itself may be directly toxic to the early human placenta but that metformin can prevent these deleterious effects,” wrote Mario Vega, MD, of Columbia University Fertility Center, New York, and his colleagues. “If confirmed in animal and human studies, this would indicate that screening and treatment for insulin resistance should focus on hyperinsulinemia.”
Dr. Vega and his colleagues cultivated trophoblast cells from three healthy women scheduled for manual vacuum aspiration during the first trimester of pregnancy to study the effects of insulin exposure alone, while trophoblast cells were cultured from a different set of women for the insulin and metformin follow-up experiments. The researchers tested each experiment against a control group of cultivated lung fibroblast cells. Insulin was measured in doses of 0.2 nmol, 1 nmol, and 5 nmol, while metformin was measured at 10 micromol. The primary outcome measures examined were gamma-H2AX for DNA damage, cell proliferation assay for cell survival, and cleaved caspase-3 for apoptosis.
Within 48 hours, the cultures showed DNA damage and induction of apoptosis when exposed to 1 nmol of insulin, but researchers said pretreatment with metformin prevented these effects. Exposing cells to metformin after insulin reduced but did not eliminate the effects of insulin.
The researchers noted the study is limited because the effects of insulin and metformin have not been examined in vivo, and it is not known at what level insulin causes damage. In addition, they suggested downregulation of genes in trophoblasts caused by insulin could cause apoptosis and DNA damage to trophoblast cells.
“Although studies performed on kidney and colon cells suggest that one possible mechanism of action for insulin-mediated genotoxicity is through AKT activation of mitochondria and subsequent reactive oxygen species production, the exact mechanism is poorly understood,” Dr. Vega and colleagues said. “Future studies will be necessary to determine variability among subjects, as well as mechanisms of action through which insulin exerts its cytotoxicity and genotoxicity.”
This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported no relevant financial disclosures.
SOURCE: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
FROM FERTILITY & STERILITY
Key clinical point: Trophoblasts cultured during the first trimester of pregnancy exposed to insulin were more likely to have increased apoptosis, DNA damage, and decreased cell survival, while pretreatment with metformin prior to exposure with insulin prevented these effects.
Major finding: DNA damage and rate of apoptosis increased in trophoblast cells exposed to 1 nmol of insulin, and cell survival decreased, compared with primary lung fibroblast cells; treating the cells with metformin prior to exposure with insulin resulted in prevention of these effects.
Study details: An experimental in vitro study of first trimester trophoblast cells exposed to insulin and metformin.
Disclosures: This study was funded by a grant from the National Institutes of Health Human Placenta Project. The authors reported they had no relevant financial disclosures.
Source: Vega M et al. Fertil Steril. 2019. doi: 10.1016/j.fertnstert.2018.11.032.
Stopping TNF inhibitors before 20 weeks’ gestation not linked to worsening RA, JIA
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
In pregnant women with arthritis, discontinuing tumor necrosis factor inhibitors prior to gestational week 20 seems feasible without an increased risk of disease worsening, particularly in those with well-controlled disease, according to authors of a recent analysis of a prospective cohort study.
Stopping tumor necrosis factor inhibitor (TNFi) treatment at that point in the second trimester was not linked to any clinically important worsening of patient-reported outcomes later in pregnancy for women with rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA), the researchers said.
However, continuing a TNFi past gestational week 20 may also be warranted for some patients, according to the researchers, led by Frauke Förger, MD, of the University of Bern (Switzerland).
“In case of active disease, the continuation of TNF inhibitors beyond gestational week 20 seems reasonable from the standpoint of improved disease activity in the third trimester, which may in turn lead to improved pregnancy outcomes,” Dr. Förger and her coinvestigators wrote in Arthritis & Rheumatology.
These findings stand in contrast to those of another recent study, in which stopping a TNF inhibitor after a positive pregnancy test was linked to disease flares in women with RA, according to the authors.
“The timing of drug discontinuation during pregnancy may be of importance,” they said in their report.
Beyond these studies, there are very limited data on the effects of discontinuing TNF inhibitors in pregnant women with rheumatoid arthritis, and “a lack of any data” in pregnant women with JIA, they said.
The current investigation by Dr. Förger and her colleagues included 490 pregnant women in the United States or Canada who were enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study. Of those women, 397 had RA and 93 had JIA.
About one-quarter of the women (122, or 24.9%) discontinued TNF inhibitor therapy prior to gestational week 20, while 41% continued on TNF inhibitors beyond that point, and 34.1% did not use a TNF inhibitor in pregnancy.
For those women who discontinued TNF inhibitors before gestational week 20, scores on the Patient Activity Scale (PAS) were stable over time, Dr. Förger and her colleagues reported.
Women who continued TNF inhibitor treatment past gestational week 20 had improved PAS scores in the third trimester, according to results of a univariate analysis (P = .02). However, the improvement appeared to be attenuated after adjustment for factors including race, smoking, use of prednisone or disease-modifying antirheumatic drugs, and gestational age, the investigators said.
They were unable to analyze the effects of ongoing TNFi treatment or discontinuation on patients with JIA separately because of the limited number of such patients in each group.
Another limitation of the study is that a high proportion of women – nearly three-quarters – had low disease activity at the start of pregnancy, according to the investigators, who said that group of women might expect some degree of improvement in the third trimester with or without TNF inhibitor discontinuation.
“In this context, the ameliorating effect of pregnancy on RA and JIA, which is most pronounced in the third trimester, may play a role,” they explained.
A certain proportion of women choose to discontinue certain arthritis treatments during pregnancy because of concerns that the medication may lead to fetal harm, but that may be changing, the investigators noted in their report.
“In recent years, more patients requiring treatment have been continuing on effective TNF inhibitors beyond conception as the available data on the safety of TNF inhibitors during pregnancy has increased,” they wrote.
Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
SOURCE: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: In contrast to a previous report,
Major finding: Patient Activity Scale (PAS) scores were stable over time in women who discontinued TNF inhibitors before gestational week 20. Those who continued past week 20 had improved PAS scores in the third trimester (univariate analysis; P = .02).
Study details: Analysis including 490 pregnant women in the United States or Canada who enrolled in the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases in Pregnancy Project, a prospective cohort study.
Disclosures: Dr. Förger and her coauthors reported no financial disclosures or conflicts of interest. Financial support for the OTIS Collaborative Research Group comes from industry sources including AbbVie, Bristol-Myers Squibb, Celgene, Hoffman La Roche-Genentech, Janssen, Pfizer, Regeneron, Sandoz, and UCB, among others.
Source: Förger F et al. Arthritis Rheumatol. 2019 Jan 21. doi: 10.1002/art.40821.
Women with RA have reduced chance of live birth after assisted reproduction treatment
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Women with rheumatoid arthritis who undergo assisted reproduction treatment have a decreased likelihood of live births versus women without rheumatoid arthritis, according to authors of a recent Denmark-wide cohort study.
The problem might be caused by an impaired chance of embryo implantation, authors of the study reported in the Annals of the Rheumatic Diseases.
Corticosteroids prescribed before embryo transfer might have improved the likelihood of live birth in these women with rheumatoid arthritis, according to the investigators, led by Professor Bente Mertz Nørgård of the center for clinical epidemiology at the Odense (Denmark) University Hospital.
However, findings with regard to that potential effect of corticosteroids were “not unambiguous,” said Prof. Nørgård and her coauthors said in their report.
The cohort study, based on Danish registry data from 1994-2017, included 1,149 embryo transfers in women and rheumatoid arthritis with 198,941 embryo transfers in women without rheumatoid arthritis.
Live births per embryo transfer were less likely in women with rheumatoid arthritis versus those they were in women with no rheumatoid arthritis, with an adjusted odds ratio of 0.78 (95% confidence interval, 0.65-0.92), according to investigators.
Chances of biochemical and clinical pregnancies were also lower in women with rheumatoid arthritis, with odds ratios of 0.81 (95% CI, 0.68-0.95) and 0.82 (95% CI, 0.59-1.15), respectively, the investigators found in an analysis of secondary outcomes in the study.
Corticosteroids prescribed before embryo transfer increased odds of live birth, with an adjusted odds ratio of 1.32 (95% CI, 0.85-2.05), though the underlying reason why corticosteroids were prescribed could not be established in this data set, investigators cautioned.
“The impact of corticosteroid prior to embryo transfer, found in our study, could be due to a suppression of ‘abnormalities in the immune system’ in women with RA, but we have to underline that this is speculative,” Prof. Nørgård and her colleagues said in a discussion of their results.
Future investigations are needed to clarify the role of corticosteroids in women with rheumatoid arthritis undergoing assisted reproduction treatment, they added.
Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense University Hospital. Dr. Nørgård and her coauthors said they had no competing interests related to the research.
SOURCE: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Women with rheumatoid arthritis (RA) who undergo assisted reproduction treatment have decreased chances of live births, compared with women without RA.
Major finding: The odds ratio for live births per embryo transfer in women with RA, as compared to women without RA, was 0.78 (95% confidence interval, 0.65-0.92).
Study details: A nationwide cohort study including 1,149 embryo transfers in women with rheumatoid arthritis and 198,941 without rheumatoid arthritis
Disclosures: Study authors had no disclosures. Support for the study came from the Research Foundation of the Region of Southern Denmark and the Free Research Foundation at Odense (Denmark) University Hospital.
Source: Nørgård BM et al. Ann Rheum Dis. 2019 Jan 12. doi: 10.1136/annrheumdis-2018-214619.
Management of hypertensive disorders in pregnancy
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 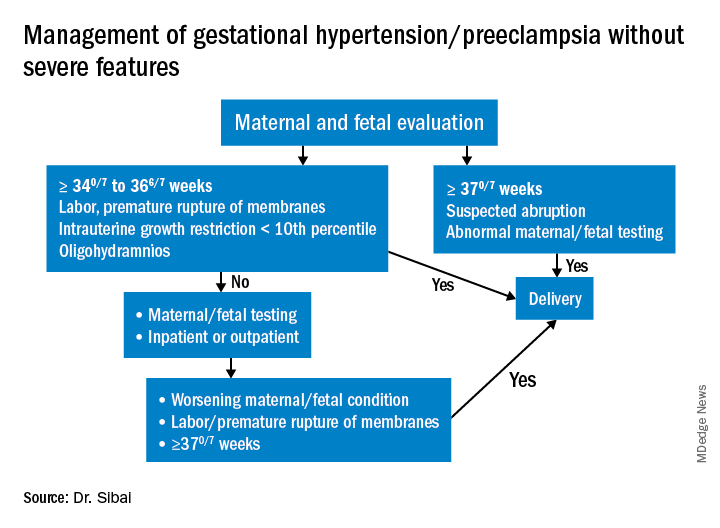
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 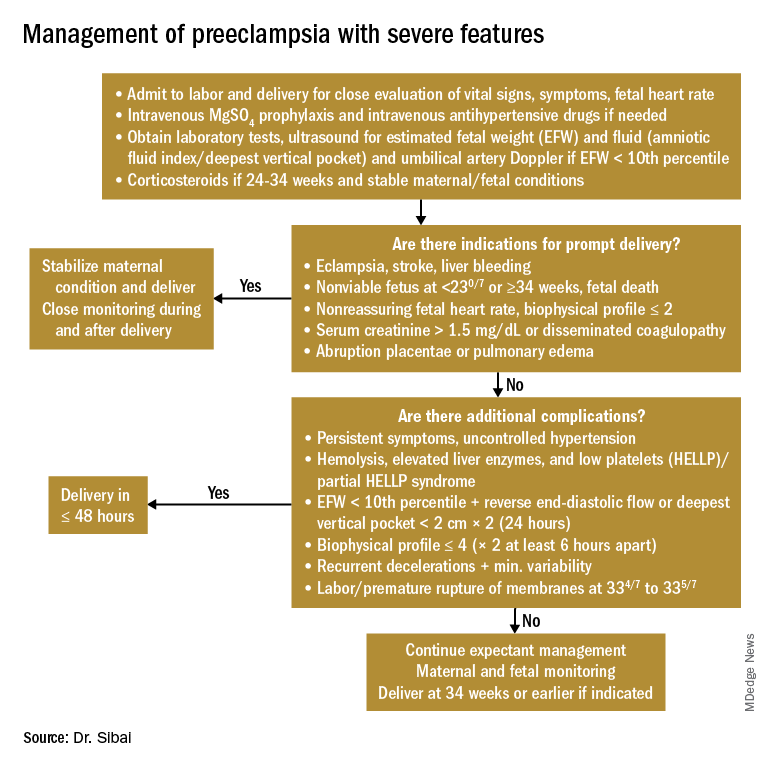
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.
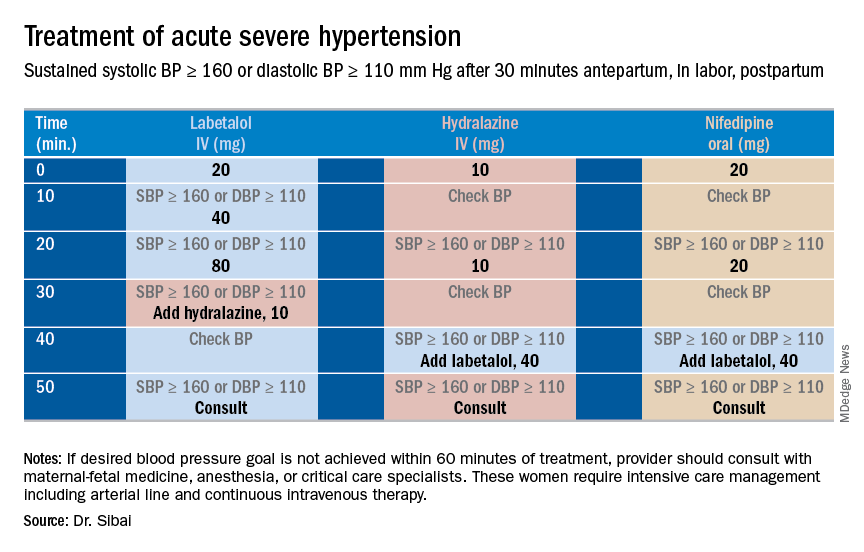
Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.

Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
In the last installment of the Master Class, I addressed the importance of clarity in the classification of hypertensive disorders in pregnancy, and proposed several key diagnostic definitions. Here, I address the management of “mild” gestational hypertension (GHTN) and preeclampsia without severe features, which I believe should be managed similarly. I also address the management of preeclampsia with severe features, and I share an algorithm that I have developed and fine-tuned over the years to control acute severe hypertension with the use of intravenous labetalol, intravenous hydralazine, or oral nifedipine.
Management of “mild” gestational hypertension/Preeclampsia without severe features
Mild gestational hypertension in and of itself has little effect on maternal or perinatal morbidity and mortality when it develops at or beyond 37 weeks’ gestation. However, approximately 40% of patients diagnosed with preterm GHTN will subsequently develop preeclampsia or progress to severe GHTN. In addition, these pregnancies may result in fetal growth restriction and placental abruption.
Antihypertensive drugs should not be used during ambulatory management of women with GHTN. Patients who receive antihypertensive therapy, including those diagnosed with severe GHTN, should be hospitalized and initially treated as having preeclampsia with or without severe features. Subsequent management will depend on initial response to therapy, blood pressure values after treatment, gestational age, and laboratory findings.
Preeclampsia without severe features is usually managed as in those with GHTN. (See related figure.) 
Close surveillance is warranted, as either type may progress to fulminant disease. Maternal surveillance should include blood pressure measurements twice per week, and CBC, liver enzymes, and serum creatinine measurements once every week. Patients also should be instructed to immediately report any of these symptoms: Persistent severe headaches; right upper quadrant or epigastric pain, nausea, and vomiting; scotomata, blurred vision, photophobia, or double vision; shortness of breath or orthopnea; altered mental changes; decreased fetal movement; rupture of membranes; vaginal bleeding; or regular uterine contractions.
Fetal evaluation for patients with GHTN/preeclampsia includes ultrasound at the time of diagnosis for evaluation of fetal growth and amniotic fluid value (deepest vertical pocket, or DVP) as well as fetal movement count and non-stress testing (NST). Subsequently, NST and DVP need to be checked twice per week. A decision for delivery will depend on gestational age, fetal status, and development of severe disease.
Management of preeclampsia with severe features
Any patient who has preeclampsia with severe features should be admitted and initially observed in a labor and delivery unit. (See related figure.) 
Initial workup should include assessment for fetal well-being, monitoring of maternal blood pressure and symptomatology, and laboratory evaluation. Laboratory assessment should include hematocrit, platelet count, serum creatinine, and aspartate aminotransferase (AST). An ultrasound for fetal growth and amniotic fluid index/DVP also should be obtained. Candidates for expectant management should be carefully selected, counseled regarding its risks and benefits, and managed only at tertiary care hospitals.
Fetal well-being should be assessed on a daily basis by NST and on a weekly basis with amniotic fluid/DVP determination. An ultrasound for fetal growth should be performed every 2-3 weeks. Maternal laboratory evaluation should be done daily or every other day. If the patient maintains a stable maternal and fetal course, she may be expectantly managed until 34 weeks. Worsening maternal or fetal status warrants delivery, regardless of gestational age.
Maternal blood pressure (BP) control is essential with expectant management or during delivery. Medications can be given orally or intravenously, as necessary, to maintain a systolic BP of 140-150 mm Hg and a diastolic BP of 90-100 mm Hg. The most commonly used intravenous medications for this purpose are labetalol and hydralazine. Other medications can include oral rapid-acting nifedipine. Subsequent management can include oral medications such as labetalol and long-acting nifedipine. Care should be taken not to drop the blood pressure too rapidly to avoid reduced renal and placental perfusion.
A trial of labor is indicated in patients with severe preeclampsia if gestational age is greater than 30 weeks and/or if cervical Bishop Score is greater than or equal to 6. However, an appropriate time frame should be established regarding achievement of active labor.
Patients should be closely monitored for at least 24 hours post partum. Post partum eclampsia occurs in 30% of patients; thus, women who are receiving magnesium sulfate should continue it for 24 hours after delivery. In addition, women with preeclampsia who are receiving magnesium sulfate are at risk for postpartum hemorrhage due to uterine atony and should be managed accordingly.
Some patients with severe preeclampsia also are at risk for pulmonary edema and exacerbation of severe hypertension 3-5 days post partum. Therefore, all patients should receive frequent monitoring of intake and output.
Control of acute severe hypertension antepartum, in labor, or post partum
Uncontrolled severe hypertension for several hours may be associated with stroke and pulmonary edema. Therefore, several guidelines recommend initiation of antihypertensive medications for acute lowering of maternal blood pressure within 30-60 minutes. Several antihypertensive agents are available for the control of sustained severe hypertension before, during, and after delivery. It is important to be familiar with the maternal and fetal side effects, as well as mode of action of each agent, to select the best one. Antihypertensive agents can exert an effect by decreasing cardiac output, peripheral vascular resistance, and central blood pressure, or by inhibiting angiotensin production. Indications for therapy and commonly used drugs in pregnancy are listed in the accompanying table.

Several trials have compared the efficacy and side effects of intravenous bolus injections of hydralazine to either IV labetalol or oral rapid-acting nifedipine as well as oral nifedipine to IV labetalol. The results of these studies suggest that any of these three medications can be used to treat severe hypertension in pregnancy as long as the physician is familiar with the doses to be used, the expected onset of action, and potential side effects.
Because both hydralazine and nifedipine are associated with tachycardia, it is recommended that these agents not be used in patients with a heart rate above 105-110 beats per minute (bpm). It also is important to be attentive to patients with generalized swelling and/or hemoconcentration (hematocrit great than or equal to 40%), as these patients usually have marked reduction in plasma volume and can develop an excessive hypotensive response, with secondary reduction in tissue perfusion and uteroplacental blood flow, when treated with a combination of rapid-acting vasodilators (hydralazine or nifedipine). Such patients may require a bolus infusion of 250-500 mL of isotonic saline prior to the administration of vasodilators. In these patients, labetalol may be the appropriate drug to use.
Labetalol should be avoided in patients with bradycardia (heart rate less than 60 bpm), in those with moderate to severe asthma, and in those with heart failure. In these patients, either hydralazine or nifedipine is the drug of choice. If an intravenous access is not available or difficult to obtain, oral nifedipine should be the drug of choice. In addition, because nifedipine is associated with improved renal blood flow with resultant increase in urine output, it is the drug of choice for treatment in those with decreased urine output, and for treatment of severe hypertension in the postpartum period.
In a third and final installment, I will elaborate on the postpartum management of women who have experienced hypertension with or without associated symptoms. Recently, postpartum hypertension has become a major cause of hospital readmission, as well as severe maternal morbidity and mortality.
Dr. Sibai is professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston.
Treating preeclampsia
Preeclampsia is such a complicated and insidious disease – and one with such serious implications for the fetus, the infant at birth, and the mother – that we decided to run a three-part series on its diagnosis and management. The complication can have an acute onset in many patients, and this acute onset may rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for obstetricians to be able to affect the health and well-being of both the mother and fetus.
I have invited Dr. Baha M. Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to deliver this series. Our first installment addressed diagnostic criteria and attempted to clarify confusion that may have been introduced with the 2013 publication of the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy. It is important that the diagnostic criteria are well established and understood because the management of patients is very much based on accurate placement within these diagnostic criteria.
This second installment of our series focuses on the application of appropriate therapeutic measures for various diagnostic groups. Dr. Sibai has spent decades studying hypertensive disorders in pregnancy and developing practical clinical strategies for management. It is our hope that the guidance and algorithms presented here will be useful for improving patient care and outcomes of this serious obstetrical syndrome. A third installment on postpartum management will come later.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Preeclampsia is such a complicated and insidious disease – and one with such serious implications for the fetus, the infant at birth, and the mother – that we decided to run a three-part series on its diagnosis and management. The complication can have an acute onset in many patients, and this acute onset may rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for obstetricians to be able to affect the health and well-being of both the mother and fetus.
I have invited Dr. Baha M. Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to deliver this series. Our first installment addressed diagnostic criteria and attempted to clarify confusion that may have been introduced with the 2013 publication of the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy. It is important that the diagnostic criteria are well established and understood because the management of patients is very much based on accurate placement within these diagnostic criteria.
This second installment of our series focuses on the application of appropriate therapeutic measures for various diagnostic groups. Dr. Sibai has spent decades studying hypertensive disorders in pregnancy and developing practical clinical strategies for management. It is our hope that the guidance and algorithms presented here will be useful for improving patient care and outcomes of this serious obstetrical syndrome. A third installment on postpartum management will come later.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Preeclampsia is such a complicated and insidious disease – and one with such serious implications for the fetus, the infant at birth, and the mother – that we decided to run a three-part series on its diagnosis and management. The complication can have an acute onset in many patients, and this acute onset may rapidly progress to eclampsia and to severe consequences, including maternal death. In addition, the disorder can occur as early as the late second trimester and can thus impact the timing of delivery and fetal age at birth. A full knowledge of the disease state – its pathophysiology, clinical manifestations, and various therapeutic options, both medical and surgical – is critical for obstetricians to be able to affect the health and well-being of both the mother and fetus.
I have invited Dr. Baha M. Sibai, professor of obstetrics, gynecology, and reproductive sciences at the University of Texas McGovern Medical School, Houston, to deliver this series. Our first installment addressed diagnostic criteria and attempted to clarify confusion that may have been introduced with the 2013 publication of the American College of Obstetricians and Gynecologists’ Task Force Report on Hypertension in Pregnancy. It is important that the diagnostic criteria are well established and understood because the management of patients is very much based on accurate placement within these diagnostic criteria.
This second installment of our series focuses on the application of appropriate therapeutic measures for various diagnostic groups. Dr. Sibai has spent decades studying hypertensive disorders in pregnancy and developing practical clinical strategies for management. It is our hope that the guidance and algorithms presented here will be useful for improving patient care and outcomes of this serious obstetrical syndrome. A third installment on postpartum management will come later.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
Endometrial scratching doesn’t lead to higher live birth rate for IVF patients
Endometrial scratching prior to a fresh embryo or frozen embryo transfer did not result in a higher rate of live births for women undergoing in vitro fertilization (IVF), according to results from a recent randomized controlled trial published in the New England Journal of Medicine.
Sarah Lensen, PhD, of the University of Auckland in New Zealand, and her colleagues recruited 1,364 women from 13 sites in 5 countries in 2014-2017 who did not have a recent history of disruptive intrauterine instrumentation such as hysteroscopy or endometrial biopsy and were planning an IVF cycle with a fresh or frozen embryo transfer. The women were randomized to receive endometrial scratching through pipelle biopsy between day 3 of the cycle prior to IVF and day 3 of the IVF cycle. Live birth was the primary outcome, while secondary outcomes measured included ongoing pregnancy, clinical pregnancy, multiple pregnancy, ectopic pregnancy, and biochemical pregnancy, as well as miscarriage, termination of pregnancy, stillbirth, and other maternal and neonatal outcomes.
For the endometrial scratch group, the rate of live birth was 26% (180 of 690 women), compared with 26% (176 of 674 women) in the control group (adjusted odds ratio, 1.00; 95% confidence interval, 0.78-1.27, P = .97). The rate of ongoing pregnancy, clinical pregnancy, ectopic pregnancy, and miscarriage also did not significantly differ between groups.
Among women who underwent endometrial scratching, there was a median pain score of 3.5 points from a range of 0-10 points; 37 women reported a pain scale score of 0, while 6 said their pain score was a 10. Adverse reactions to endometrial scratching included fainting, dizziness and/or nausea (7 women); excessive pain (5 women), including 1 woman who went to the emergency department after a concurrent endometrial scratch and sonohysterogram procedure; and excessive bleeding (2 women).
The researchers noted several limitations in their study, including its unblinded design; tracking of adverse outcomes in the endometrial scratch group only; and a definition of implantation failure not based on embryo or transfer quality, but on the number of previous unsuccessful transfers. There were also “imbalances favoring the endometrial scratch group” based on the number of available oocytes per participant and willingness to begin their IVF cycle.
“Women in the endometrial scratch group may have been more likely to start their cycle in order to capitalize on their exposure to the endometrial scratch. However, results were materially unchanged in a per-protocol analysis,” the researchers said.
This study was funded in part by the University of Auckland, the A+ Trust, Auckland District Health Board, the Nurture Foundation, and the Maurice and Phyllis Paykel Trust. Dr. Priya Bhide received personal fees from Ferring Pharmaceuticals, and grants from Bart’s Charity, Pharmasure Pharmaceuticals, and Finox Pharmaceuticals. The other authors reported no relevant financial disclosures.
SOURCE: Lensen S et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1808737.
The results of a large, pragmatic trial by Lensen et al., examining the effects of endometrial scratching, which used current standards of care for in vitro fertilization (IVF) and included women undergoing treatment for the first time in addition to those who have had previously failed cycles, should be trusted despite contrary data from other studies, Ben W. Mol, MD, PhD, and Kurt T. Barnhart, MD, MSCE, wrote in a related editorial.
Although a Cochrane systematic review of 14 randomized controlled trials found evidence of an increased live birth rate for women undergoing IVF after an endometrial scratch procedure (risk ratio, 1.42; 95% confidence interval, 1.08-1.85), many trials in the meta-analysis had “unrealistic” large effect sizes within limited sample sizes, were not optimally randomized, were stopped prematurely, or were not prospectively registered, they noted.
“Rigorous synthesis of bad data cannot overcome bias from uncontrolled or poorly conducted studies; it may result only in tighter confidence around a spurious conclusion, an answer that is precisely wrong,” they said.
However, as the results from Lensen et al. show, there were a low number of reported adverse events, and “on the basis of the current report, the IVF community can take solace in the observation that ‘scratching’ apparently caused no harm, other than some procedure-associated pain and bleeding.”
Any adjuvant to IVF should be “evaluated carefully before being offered to infertility couples” and it is still an unanswered question as to whether IVF doctors should continue to offer unevaluated adjuvants to their patients, Dr. Mol and Dr. Barnhart said.
“The population affected by infertility has been described as vulnerable. The goals of reproductive medicine are the same as those of other fields of medicine: Provide compassionate and effective care, do no harm, and do not offer false hope or sell snake oil,” the authors concluded.
Dr. Mol is at Monash University in Clayton, Australia, and Dr. Barnhart is at the University of Pennsylvania in Philadelphia. This commentary summarizes their editorial on the study by Lensen et al. (N Eng J Med. 2019. doi: 10.1056/NEJMe1815042 ). Dr. Mol disclosed ties with Merck, Guerbet, and ObsEva, outside the submitted work. Dr. Barnhart had no relevant disclosures.
The results of a large, pragmatic trial by Lensen et al., examining the effects of endometrial scratching, which used current standards of care for in vitro fertilization (IVF) and included women undergoing treatment for the first time in addition to those who have had previously failed cycles, should be trusted despite contrary data from other studies, Ben W. Mol, MD, PhD, and Kurt T. Barnhart, MD, MSCE, wrote in a related editorial.
Although a Cochrane systematic review of 14 randomized controlled trials found evidence of an increased live birth rate for women undergoing IVF after an endometrial scratch procedure (risk ratio, 1.42; 95% confidence interval, 1.08-1.85), many trials in the meta-analysis had “unrealistic” large effect sizes within limited sample sizes, were not optimally randomized, were stopped prematurely, or were not prospectively registered, they noted.
“Rigorous synthesis of bad data cannot overcome bias from uncontrolled or poorly conducted studies; it may result only in tighter confidence around a spurious conclusion, an answer that is precisely wrong,” they said.
However, as the results from Lensen et al. show, there were a low number of reported adverse events, and “on the basis of the current report, the IVF community can take solace in the observation that ‘scratching’ apparently caused no harm, other than some procedure-associated pain and bleeding.”
Any adjuvant to IVF should be “evaluated carefully before being offered to infertility couples” and it is still an unanswered question as to whether IVF doctors should continue to offer unevaluated adjuvants to their patients, Dr. Mol and Dr. Barnhart said.
“The population affected by infertility has been described as vulnerable. The goals of reproductive medicine are the same as those of other fields of medicine: Provide compassionate and effective care, do no harm, and do not offer false hope or sell snake oil,” the authors concluded.
Dr. Mol is at Monash University in Clayton, Australia, and Dr. Barnhart is at the University of Pennsylvania in Philadelphia. This commentary summarizes their editorial on the study by Lensen et al. (N Eng J Med. 2019. doi: 10.1056/NEJMe1815042 ). Dr. Mol disclosed ties with Merck, Guerbet, and ObsEva, outside the submitted work. Dr. Barnhart had no relevant disclosures.
The results of a large, pragmatic trial by Lensen et al., examining the effects of endometrial scratching, which used current standards of care for in vitro fertilization (IVF) and included women undergoing treatment for the first time in addition to those who have had previously failed cycles, should be trusted despite contrary data from other studies, Ben W. Mol, MD, PhD, and Kurt T. Barnhart, MD, MSCE, wrote in a related editorial.
Although a Cochrane systematic review of 14 randomized controlled trials found evidence of an increased live birth rate for women undergoing IVF after an endometrial scratch procedure (risk ratio, 1.42; 95% confidence interval, 1.08-1.85), many trials in the meta-analysis had “unrealistic” large effect sizes within limited sample sizes, were not optimally randomized, were stopped prematurely, or were not prospectively registered, they noted.
“Rigorous synthesis of bad data cannot overcome bias from uncontrolled or poorly conducted studies; it may result only in tighter confidence around a spurious conclusion, an answer that is precisely wrong,” they said.
However, as the results from Lensen et al. show, there were a low number of reported adverse events, and “on the basis of the current report, the IVF community can take solace in the observation that ‘scratching’ apparently caused no harm, other than some procedure-associated pain and bleeding.”
Any adjuvant to IVF should be “evaluated carefully before being offered to infertility couples” and it is still an unanswered question as to whether IVF doctors should continue to offer unevaluated adjuvants to their patients, Dr. Mol and Dr. Barnhart said.
“The population affected by infertility has been described as vulnerable. The goals of reproductive medicine are the same as those of other fields of medicine: Provide compassionate and effective care, do no harm, and do not offer false hope or sell snake oil,” the authors concluded.
Dr. Mol is at Monash University in Clayton, Australia, and Dr. Barnhart is at the University of Pennsylvania in Philadelphia. This commentary summarizes their editorial on the study by Lensen et al. (N Eng J Med. 2019. doi: 10.1056/NEJMe1815042 ). Dr. Mol disclosed ties with Merck, Guerbet, and ObsEva, outside the submitted work. Dr. Barnhart had no relevant disclosures.
Endometrial scratching prior to a fresh embryo or frozen embryo transfer did not result in a higher rate of live births for women undergoing in vitro fertilization (IVF), according to results from a recent randomized controlled trial published in the New England Journal of Medicine.
Sarah Lensen, PhD, of the University of Auckland in New Zealand, and her colleagues recruited 1,364 women from 13 sites in 5 countries in 2014-2017 who did not have a recent history of disruptive intrauterine instrumentation such as hysteroscopy or endometrial biopsy and were planning an IVF cycle with a fresh or frozen embryo transfer. The women were randomized to receive endometrial scratching through pipelle biopsy between day 3 of the cycle prior to IVF and day 3 of the IVF cycle. Live birth was the primary outcome, while secondary outcomes measured included ongoing pregnancy, clinical pregnancy, multiple pregnancy, ectopic pregnancy, and biochemical pregnancy, as well as miscarriage, termination of pregnancy, stillbirth, and other maternal and neonatal outcomes.
For the endometrial scratch group, the rate of live birth was 26% (180 of 690 women), compared with 26% (176 of 674 women) in the control group (adjusted odds ratio, 1.00; 95% confidence interval, 0.78-1.27, P = .97). The rate of ongoing pregnancy, clinical pregnancy, ectopic pregnancy, and miscarriage also did not significantly differ between groups.
Among women who underwent endometrial scratching, there was a median pain score of 3.5 points from a range of 0-10 points; 37 women reported a pain scale score of 0, while 6 said their pain score was a 10. Adverse reactions to endometrial scratching included fainting, dizziness and/or nausea (7 women); excessive pain (5 women), including 1 woman who went to the emergency department after a concurrent endometrial scratch and sonohysterogram procedure; and excessive bleeding (2 women).
The researchers noted several limitations in their study, including its unblinded design; tracking of adverse outcomes in the endometrial scratch group only; and a definition of implantation failure not based on embryo or transfer quality, but on the number of previous unsuccessful transfers. There were also “imbalances favoring the endometrial scratch group” based on the number of available oocytes per participant and willingness to begin their IVF cycle.
“Women in the endometrial scratch group may have been more likely to start their cycle in order to capitalize on their exposure to the endometrial scratch. However, results were materially unchanged in a per-protocol analysis,” the researchers said.
This study was funded in part by the University of Auckland, the A+ Trust, Auckland District Health Board, the Nurture Foundation, and the Maurice and Phyllis Paykel Trust. Dr. Priya Bhide received personal fees from Ferring Pharmaceuticals, and grants from Bart’s Charity, Pharmasure Pharmaceuticals, and Finox Pharmaceuticals. The other authors reported no relevant financial disclosures.
SOURCE: Lensen S et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1808737.
Endometrial scratching prior to a fresh embryo or frozen embryo transfer did not result in a higher rate of live births for women undergoing in vitro fertilization (IVF), according to results from a recent randomized controlled trial published in the New England Journal of Medicine.
Sarah Lensen, PhD, of the University of Auckland in New Zealand, and her colleagues recruited 1,364 women from 13 sites in 5 countries in 2014-2017 who did not have a recent history of disruptive intrauterine instrumentation such as hysteroscopy or endometrial biopsy and were planning an IVF cycle with a fresh or frozen embryo transfer. The women were randomized to receive endometrial scratching through pipelle biopsy between day 3 of the cycle prior to IVF and day 3 of the IVF cycle. Live birth was the primary outcome, while secondary outcomes measured included ongoing pregnancy, clinical pregnancy, multiple pregnancy, ectopic pregnancy, and biochemical pregnancy, as well as miscarriage, termination of pregnancy, stillbirth, and other maternal and neonatal outcomes.
For the endometrial scratch group, the rate of live birth was 26% (180 of 690 women), compared with 26% (176 of 674 women) in the control group (adjusted odds ratio, 1.00; 95% confidence interval, 0.78-1.27, P = .97). The rate of ongoing pregnancy, clinical pregnancy, ectopic pregnancy, and miscarriage also did not significantly differ between groups.
Among women who underwent endometrial scratching, there was a median pain score of 3.5 points from a range of 0-10 points; 37 women reported a pain scale score of 0, while 6 said their pain score was a 10. Adverse reactions to endometrial scratching included fainting, dizziness and/or nausea (7 women); excessive pain (5 women), including 1 woman who went to the emergency department after a concurrent endometrial scratch and sonohysterogram procedure; and excessive bleeding (2 women).
The researchers noted several limitations in their study, including its unblinded design; tracking of adverse outcomes in the endometrial scratch group only; and a definition of implantation failure not based on embryo or transfer quality, but on the number of previous unsuccessful transfers. There were also “imbalances favoring the endometrial scratch group” based on the number of available oocytes per participant and willingness to begin their IVF cycle.
“Women in the endometrial scratch group may have been more likely to start their cycle in order to capitalize on their exposure to the endometrial scratch. However, results were materially unchanged in a per-protocol analysis,” the researchers said.
This study was funded in part by the University of Auckland, the A+ Trust, Auckland District Health Board, the Nurture Foundation, and the Maurice and Phyllis Paykel Trust. Dr. Priya Bhide received personal fees from Ferring Pharmaceuticals, and grants from Bart’s Charity, Pharmasure Pharmaceuticals, and Finox Pharmaceuticals. The other authors reported no relevant financial disclosures.
SOURCE: Lensen S et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1808737.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The live birth rate for women in the endometrial scratch group was 26% of 690 participants, compared with 26% of 674 women in the control group, a nonsignificant difference.
Study details: A multicenter, open-label, randomized controlled trial of 1,364 women undergoing IVF at 13 different sites in 5 countries.
Disclosures: This study was funded in part by the University of Auckland, the A+ Trust, Auckland District Health Board, the Nurture Foundation, and the Maurice and Phyllis Paykel Trust. Dr. Priya Bhide received personal fees from Ferring Pharmaceuticals, and grants from Bart’s Charity, Pharmasure Pharmaceuticals, and Finox Pharmaceuticals. The other authors reported no relevant financial disclosures.
Source: Lensen S et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1808737.
Violence against women: Gail Robinson
Dr. Robinson is professor of psychiatry and obstetrics/gynecology and professor of equality, gender, and population at the University of Toronto. She’s also chair of GAP’s Committee on Gender & Mental Health. In this episode, Dr. Robinson delves into strategies for interacting with survivors of violence, the roots of the “Me Too” movement, as well as rising rates of maternal mortality in the United States.
Amazon
Apple Podcasts
Google Podcasts
Spotify
Dr. Robinson is professor of psychiatry and obstetrics/gynecology and professor of equality, gender, and population at the University of Toronto. She’s also chair of GAP’s Committee on Gender & Mental Health. In this episode, Dr. Robinson delves into strategies for interacting with survivors of violence, the roots of the “Me Too” movement, as well as rising rates of maternal mortality in the United States.
Amazon
Apple Podcasts
Google Podcasts
Spotify
Dr. Robinson is professor of psychiatry and obstetrics/gynecology and professor of equality, gender, and population at the University of Toronto. She’s also chair of GAP’s Committee on Gender & Mental Health. In this episode, Dr. Robinson delves into strategies for interacting with survivors of violence, the roots of the “Me Too” movement, as well as rising rates of maternal mortality in the United States.
Amazon
Apple Podcasts
Google Podcasts
Spotify
Intrapartum molecular GBS screening reduced newborn early-onset disease, antibiotic use
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
Point-of-care intrapartum molecular screening of group B Streptococcus reduced the incidence of early-onset disease cases and antibiotic use, according to research published in Obstetrics & Gynecology.
Najoua El Helali, PharmD, from the Service de Microbiologie Clinique at Groupe Hospitalier Paris Saint-Joseph, and her colleagues measured the rate of early-onset disease group B Streptococcus (GBS) in a single-center study analyzing antenatal culture screening for 4 years prior to implementation (2006-2009) of polymerase chain reaction (PCR) screening (2010-2015). There were 11,226 deliveries (11,818 live births) during the antenatal screening period and 18,835 deliveries (18,980 live births) during the PCR screening period. Overall, 4% of deliveries during the antenatal period and 0.1% of deliveries during the intrapartum period were not screened for GBS (P less than .001).
During 2006-2015, the rate of early-onset disease of GBS decreased to 0.21/1,000 cases from 1.01/1,000 cases (risk ratio, 0.25; 95% confidence interval, 0.14-0.43; P = .026), while the rate of probable early-onset disease GBS decreased to 0.73/1,000 cases from 2.8/1,000 cases (RR, 0.25; (95% CI, 0.14-0.43; P less than .001).
For patients with early-onset GBS, length of stay in hospital decreased by 64%, and antibiotic therapy decreased by 60%, but there was no significant difference in average length of stay or duration of antibiotic therapy during the study period. There was a reduction in annual delivery- and treatment-associated costs of early-onset disease GBS from $41,875 to $11,945, while the estimated extra cost of PCR screening to avoid one additional case of early-onset disease GBS was $5,819 and a cost increase of $49 per newborn.
“The additional PCR costs were offset in part by the reduction in early-onset GBS disease treatment costs,” the investigators said.
“A randomized, controlled multicenter study is probably needed to evaluate the cost-effectiveness of this prevention strategy and demonstrate a better efficacy in populations where poorly followed women are of unknown GBS status at presentation for delivery,” the researchers said. “In term newborns, however, using infection rate as an endpoint is problematic given the sample size needed.”
The researchers said their study was potentially limited by lack of a control group and population selection, and described mothers in their center as “mostly well-informed and well-monitored during their pregnancy.”
The authors reported no relevant conflicts of interest.
SOURCE: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: The rate of early-onset disease group B Streptococcus decreased from 1.01/1,000 cases to 0.21/1,000 cases across the antenatal and intrapartum periods.
Study details: A single-center study of antenatal culture screening for 11,226 deliveries during 2006-2009 and intrapartum PCR screening for 18,835 deliveries during 2010-2015.
Disclosures: The authors reported no relevant conflicts of interest.
Source: El Helali N et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003057.
Delayed first contraception use raises unwanted pregnancy risk
Young women who delay starting contraception when they start sexual activity are at increased risk of unwanted pregnancy, according to data from a cross-sectional study of more than 26,000 women in the United States.
Unintended pregnancy in the United States is associated with delayed prenatal care, premature birth, and low birth weight and remains more common among African American and Hispanic women than among white women, and it also is more common among low-income women than among high income women, wrote Mara E. Murray Horwitz, MD, of Harvard Pilgrim Health Care Institute in Boston and her colleagues.
“Reducing unintended pregnancy and the associated socioeconomic disparities is a national public health priority,” they wrote.
In a study published in Pediatrics, the researchers reviewed data from four cycles of the National Survey of Family Growth between 2002 and 2015. They examined self-reported responses from 26,359 women aged 15-44 years with sexual debuts during 1970-2014, including the dates of sexual debut, initiation of contraceptives, and rates of unwanted pregnancy. Timely contraceptive initiation was defined as use within a month of starting sexual activity.
Overall, one in five women reported delayed initiation of contraception. This delay was significantly associated with an increased unwanted pregnancy risk within 3 months of starting sexual activity, compared with timely use of contraception (adjusted risk ratio, 3.7). The average age of sexual debut was 17 years.
When the researchers examined subgroups, they found that one in four respondents who were African American, Hispanic, or low income reported delayed contraceptive initiation.
No association with unwanted pregnancy was found between effective versus less effective contraception methods. Timely contraceptive use increased during the study period from less than 10% in the 1970s to more than 25% in the 2000s, but condoms accounted for most of this increase. Use of other methods including long-acting reversible and short-acting hormonal options was low, especially among African American, Hispanic, and low-income women, Dr. Murray Horwitz and her colleagues noted.
The study was limited by several factors including the use of self-reports, lack of data on the exact start of contraceptive initiation, and the lack of association between contraceptive method and unwanted pregnancy, the researchers noted. However, the findings suggest that clinicians can help by intervening with young patients and educating them about early adoption of pregnancy prevention strategies.
The study was funded by the National Institutes of Health; Dr. Murray Horwitz was supported by an award from the NIH and Harvard Pilgrim Health Care Institute. Another researcher received support from Harvard Pilgrim Health Care Institute to provide mentorship for the study. The remaining researcher had no relevant financial disclosures.
SOURCE: Murray Horwitz M et al. Pediatrics. 2019;143(2):e20182463.
Despite a declining teen birth rate in the last several decades, the United States has the highest teen birth rate among industrialized nations. While many factors play into this rate, we know that, in many European countries with low teen birth rates, adolescents often initiate contraceptive methods before their sexual debut. As we often tell teenagers, they can become pregnant the “first time,” which makes initiating contraception early – and preferably before sexual debut – an important strategy to preventing unplanned pregnancy.
This study identifies the trends over time in the initiation of contraception in relationship to sexual debut and examines its effects on unplanned teen pregnancy. Understanding these trends can help clinicians more effectively target teen pregnancy.
I was pleasantly surprised to see that rates of timely contraceptive initiation have increased since 1970. Sadly, this rise is largely because of condom use. Use of effective forms of contraception – especially long-acting reversible forms of contraception (LARC), such as the IUD or the etonogestrel rod – still remain low at the time of sexual debut. While we continue to encourage LARCs as first line for pregnancy prevention, many patients are not getting the message about these highly effective, safe methods. Unsurprisingly, there are significant differences based on race/ethnicity and socioeconomic status on timely initiation of contraceptive methods, especially highly effective methods. This supports prior research which has shown significant barriers in access to contraception to these groups, which leads to higher rates of unplanned pregnancies.
Dr. Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma, Oklahoma City. She is a member of the Pediatric News editorial advisory board and was asked to comment on the study by Murray Horwitz et al. Dr. Curran had no relevant financial disclosures.
Despite a declining teen birth rate in the last several decades, the United States has the highest teen birth rate among industrialized nations. While many factors play into this rate, we know that, in many European countries with low teen birth rates, adolescents often initiate contraceptive methods before their sexual debut. As we often tell teenagers, they can become pregnant the “first time,” which makes initiating contraception early – and preferably before sexual debut – an important strategy to preventing unplanned pregnancy.
This study identifies the trends over time in the initiation of contraception in relationship to sexual debut and examines its effects on unplanned teen pregnancy. Understanding these trends can help clinicians more effectively target teen pregnancy.
I was pleasantly surprised to see that rates of timely contraceptive initiation have increased since 1970. Sadly, this rise is largely because of condom use. Use of effective forms of contraception – especially long-acting reversible forms of contraception (LARC), such as the IUD or the etonogestrel rod – still remain low at the time of sexual debut. While we continue to encourage LARCs as first line for pregnancy prevention, many patients are not getting the message about these highly effective, safe methods. Unsurprisingly, there are significant differences based on race/ethnicity and socioeconomic status on timely initiation of contraceptive methods, especially highly effective methods. This supports prior research which has shown significant barriers in access to contraception to these groups, which leads to higher rates of unplanned pregnancies.
Dr. Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma, Oklahoma City. She is a member of the Pediatric News editorial advisory board and was asked to comment on the study by Murray Horwitz et al. Dr. Curran had no relevant financial disclosures.
Despite a declining teen birth rate in the last several decades, the United States has the highest teen birth rate among industrialized nations. While many factors play into this rate, we know that, in many European countries with low teen birth rates, adolescents often initiate contraceptive methods before their sexual debut. As we often tell teenagers, they can become pregnant the “first time,” which makes initiating contraception early – and preferably before sexual debut – an important strategy to preventing unplanned pregnancy.
This study identifies the trends over time in the initiation of contraception in relationship to sexual debut and examines its effects on unplanned teen pregnancy. Understanding these trends can help clinicians more effectively target teen pregnancy.
I was pleasantly surprised to see that rates of timely contraceptive initiation have increased since 1970. Sadly, this rise is largely because of condom use. Use of effective forms of contraception – especially long-acting reversible forms of contraception (LARC), such as the IUD or the etonogestrel rod – still remain low at the time of sexual debut. While we continue to encourage LARCs as first line for pregnancy prevention, many patients are not getting the message about these highly effective, safe methods. Unsurprisingly, there are significant differences based on race/ethnicity and socioeconomic status on timely initiation of contraceptive methods, especially highly effective methods. This supports prior research which has shown significant barriers in access to contraception to these groups, which leads to higher rates of unplanned pregnancies.
Dr. Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma, Oklahoma City. She is a member of the Pediatric News editorial advisory board and was asked to comment on the study by Murray Horwitz et al. Dr. Curran had no relevant financial disclosures.
Young women who delay starting contraception when they start sexual activity are at increased risk of unwanted pregnancy, according to data from a cross-sectional study of more than 26,000 women in the United States.
Unintended pregnancy in the United States is associated with delayed prenatal care, premature birth, and low birth weight and remains more common among African American and Hispanic women than among white women, and it also is more common among low-income women than among high income women, wrote Mara E. Murray Horwitz, MD, of Harvard Pilgrim Health Care Institute in Boston and her colleagues.
“Reducing unintended pregnancy and the associated socioeconomic disparities is a national public health priority,” they wrote.
In a study published in Pediatrics, the researchers reviewed data from four cycles of the National Survey of Family Growth between 2002 and 2015. They examined self-reported responses from 26,359 women aged 15-44 years with sexual debuts during 1970-2014, including the dates of sexual debut, initiation of contraceptives, and rates of unwanted pregnancy. Timely contraceptive initiation was defined as use within a month of starting sexual activity.
Overall, one in five women reported delayed initiation of contraception. This delay was significantly associated with an increased unwanted pregnancy risk within 3 months of starting sexual activity, compared with timely use of contraception (adjusted risk ratio, 3.7). The average age of sexual debut was 17 years.
When the researchers examined subgroups, they found that one in four respondents who were African American, Hispanic, or low income reported delayed contraceptive initiation.
No association with unwanted pregnancy was found between effective versus less effective contraception methods. Timely contraceptive use increased during the study period from less than 10% in the 1970s to more than 25% in the 2000s, but condoms accounted for most of this increase. Use of other methods including long-acting reversible and short-acting hormonal options was low, especially among African American, Hispanic, and low-income women, Dr. Murray Horwitz and her colleagues noted.
The study was limited by several factors including the use of self-reports, lack of data on the exact start of contraceptive initiation, and the lack of association between contraceptive method and unwanted pregnancy, the researchers noted. However, the findings suggest that clinicians can help by intervening with young patients and educating them about early adoption of pregnancy prevention strategies.
The study was funded by the National Institutes of Health; Dr. Murray Horwitz was supported by an award from the NIH and Harvard Pilgrim Health Care Institute. Another researcher received support from Harvard Pilgrim Health Care Institute to provide mentorship for the study. The remaining researcher had no relevant financial disclosures.
SOURCE: Murray Horwitz M et al. Pediatrics. 2019;143(2):e20182463.
Young women who delay starting contraception when they start sexual activity are at increased risk of unwanted pregnancy, according to data from a cross-sectional study of more than 26,000 women in the United States.
Unintended pregnancy in the United States is associated with delayed prenatal care, premature birth, and low birth weight and remains more common among African American and Hispanic women than among white women, and it also is more common among low-income women than among high income women, wrote Mara E. Murray Horwitz, MD, of Harvard Pilgrim Health Care Institute in Boston and her colleagues.
“Reducing unintended pregnancy and the associated socioeconomic disparities is a national public health priority,” they wrote.
In a study published in Pediatrics, the researchers reviewed data from four cycles of the National Survey of Family Growth between 2002 and 2015. They examined self-reported responses from 26,359 women aged 15-44 years with sexual debuts during 1970-2014, including the dates of sexual debut, initiation of contraceptives, and rates of unwanted pregnancy. Timely contraceptive initiation was defined as use within a month of starting sexual activity.
Overall, one in five women reported delayed initiation of contraception. This delay was significantly associated with an increased unwanted pregnancy risk within 3 months of starting sexual activity, compared with timely use of contraception (adjusted risk ratio, 3.7). The average age of sexual debut was 17 years.
When the researchers examined subgroups, they found that one in four respondents who were African American, Hispanic, or low income reported delayed contraceptive initiation.
No association with unwanted pregnancy was found between effective versus less effective contraception methods. Timely contraceptive use increased during the study period from less than 10% in the 1970s to more than 25% in the 2000s, but condoms accounted for most of this increase. Use of other methods including long-acting reversible and short-acting hormonal options was low, especially among African American, Hispanic, and low-income women, Dr. Murray Horwitz and her colleagues noted.
The study was limited by several factors including the use of self-reports, lack of data on the exact start of contraceptive initiation, and the lack of association between contraceptive method and unwanted pregnancy, the researchers noted. However, the findings suggest that clinicians can help by intervening with young patients and educating them about early adoption of pregnancy prevention strategies.
The study was funded by the National Institutes of Health; Dr. Murray Horwitz was supported by an award from the NIH and Harvard Pilgrim Health Care Institute. Another researcher received support from Harvard Pilgrim Health Care Institute to provide mentorship for the study. The remaining researcher had no relevant financial disclosures.
SOURCE: Murray Horwitz M et al. Pediatrics. 2019;143(2):e20182463.
FROM PEDIATRICS
Key clinical point: Women who delayed using contraception were significantly more likely to become pregnant within 3 months of starting sexual activity than were those who had initiated contraception use, especially black, Hispanic, and low-income women.
Major finding: Unwanted pregnancy within 3 months of sexual debut was 3.7 times more likely in women who delayed initial contraception use, compared with those who had timely initiation.
Study details: The data come from a cross-sectional study including 26,359 women with sexual debuts between 1970 and 2014.
Disclosures: The study was funded by the National Institutes of Health; Dr. Murray Horwitz was supported by an award from the NIH and Harvard Pilgrim Health Care Institute. Another researcher received support from Harvard Pilgrim Health Care Institute to provide mentorship for the study. The remaining researcher had no relevant financial disclosures.
Source: Horwitz M et al. Pediatrics. 2019;143(2):e20182463.
SMFM and ACOG team up for interpregnancy care guidance
A new consensus statement places renewed focus on maternal interpregnancy care, with a goal of extending care past the postpartum period to provide a wellness-maximizing continuum of care.
The document, developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine (SMFM), recognizes that pregnancy is part of the lifelong continuum of health and wellness. Although not all women will go on to have another pregnancy, the concept of interpregnancy care recognizes that ob.gyns. have a vital role that extends past the postpartum period.
“This is a shift in what we used to think was our job. We used to think that our job ended when the baby came out,” said the first author of the obstetric care consensus statement, Judette Marie Louis, MD, an ob.gyn. faculty member at the University of South Florida, Tampa, and a SMFM board member. “For too long, our focus was just the baby; we need to tell women, ‘You’re important too,’ ” she said in an interview.
“The interpregnancy period is an opportunity to address these complications of medical issues that have developed during pregnancy, to assess a woman’s mental and physical well-being, and to optimize her health along her life course,” Dr. Louis and her coauthors wrote in Obstetrics & Gynecology.
Conceptually, the opportunity for interpregnancy care arises after any pregnancy, no matter the outcome, and is part of the continuum of care for women of reproductive age. For women who do not intend a future pregnancy, well-woman care is the focus, while women who currently intend to become pregnant again receive interpregnancy care. Women may move from one arm of the continuum to the other if their intentions change or if they become pregnant, explained Dr. Louis and her coauthors.
Birth spacing
The new consensus document includes an emphasis on long-term health outcomes as well as maternal and neonatal outcomes in future pregnancies. Although the evidence is of no more than moderate quality, women should be advised to have an interpregnancy interval of at least 6 months and offered counseling about family planning before delivery. Risks and benefits of an interpregnancy interval of less than 18 months should be reviewed as well.
For women with a history of preterm birth and those who have had a prior cesarean delivery and desire a subsequent trial of labor, birth spacing is particularly important, noted Dr. Louis and her colleagues. There is higher-risk evidence for uterine rupture after cesarean delivery if delivery-to-delivery intervals are 18-24 months or less.
Recommendations regarding the length of the interpregnancy interval generally should not be affected by a history of prior infertility.
Other recommendations
High-quality evidence supports breast feeding’s salutary effects on maternal and child health, so women should receive anticipatory support and guidance to enable breastfeeding, according to the document.
In accordance with other guidelines, the document recommends that all women be screened for depression post partum and as part of well-woman care in the interpregnancy interval. Procedures for effective diagnosis, treatment, and follow-up should accompany the screening.
Best practices dictate that women are asked about alcohol, prescription, and nonprescription drug use as a routine matter. High-quality evidence supports offering smoking cessation support to women who smoke and giving specific advice about nutrition and physical activity that’s based on “proven behavioral techniques.”
Evidence is of moderate quality that women should be encouraged to reach their prepregnancy weight by a year after delivery, with the ultimate goal of having a body mass index of 18.5-24.9.
High-quality evidence backs encouragement to engage in safe sex practices, with care providers also advised to facilitate partner screening and treatment for STIs.
Screening for STIs should follow guidance put forward by the Centers for Disease Control and Prevention and should be offered to women at high risk for STIs; those with a history of STI should have a careful history taken to determine risks for current or repeat infections, wrote Dr. Louis and her coauthors. These strong recommendations have high-quality evidence behind them.
The consensus statement recommends screening women for intimate partner violence, with moderate-quality evidence to support the recommendation. Patient navigators, expert medical interpretation, and other health educators can be offered to women with health literacy or language and communication challenges, but the evidence backing the recommendation is of low quality.
A subset of the interpregnancy care recommendations gives additional guidance regarding women with a history of high-risk pregnancy. All women planning pregnancy – or who could become pregnant – should take 500 mcg of folic acid daily beginning 1 month before fertilization and continuing through the 12th week of pregnancy. Folic acid supplementation for women who have had children with neural tube defects should begin at least 3 months before fertilization and continue through 12 weeks of pregnancy, at a dose of 400 mg.
All prescription and nonprescription medications, as well as potential environmental teratogens, should be reviewed before a repeat pregnancy. This, as well as the folic acid recommendations, are strong recommendations backed by high-quality evidence.
When appropriate, genetic counseling should be offered to women who have had prior pregnancies with genetic disorders or congenital anomalies. Asymptomatic genitourinary infections should not be treated in women with a history of preterm birth during the interpregnancy interval. These are strong recommendations, but are backed by low to moderate quality evidence, wrote Dr. Louis and her colleagues.
Another section of the consensus document specifically addresses specific health conditions, including diabetes, gestational diabetes, gestational and chronic hypertension, and preeclampsia, as well as mental health disorders and overweight or obesity.
For each, Dr. Louis and her coauthors recommend counseling that reviews complications and risk for future disease; for example, not only does prior preeclampsia increase risk for that complication in future pregnancies, risk for later cardiovascular disease is also doubled. The document outlines recommended interpregnancy testing, management considerations and medications of concern for health care providers caring for women with these conditions, and condition-specific goals.
Of the association between gestational diabetes, hypertension, and preeclampsia with later disease, Dr. Louis said in the interview, “We don’t know. ... It may be that pregnancy accelerates these diseases. We do know that normal changes in pregnancy stress your body, and that preeclampsia damages your vessels. Pregnancy can give you a warning, but we don’t have enough information to predict the outcome” in later life. “We do know there is some advice we can give: stop smoking and maintain a normal body weight.”
Other conditions such as HIV, renal disease, epilepsy, autoimmune and thyroid disease, and thrombophilias and antiphospholipid antibody syndrome are also addressed in this section of the consensus document.
Specific attention also is given to psychosocial risks, such as socioeconomic disadvantages and being a member of a racial or ethnic minority. Social determinants of health are complex, said Dr. Louis, but socioeconomic and racial stressors can include the added burden of caring for loved ones with constrained resources. Additionally, there can be access issues: Women can get emergent care by presenting to the ED but receiving continued primary and specialty care can be much more of a challenge.
Regarding racism, Dr. Louis said, “We all come into caring for these women with certain ideas.” For example, it’s not enough to say of a patient, “She’s noncompliant. You need to ask why.” When the “why” question is asked, then you may discover, “there’s something you can help the patient with,” she said. “We need to ask why, and then take steps to help our patients.”
Document building
The working group for the consensus document felt it was important to include nonphysicians who care for women, Dr. Louis said, so drafts were reviewed by and input received from the American College of Nurse-Midwives and the National Association of Nurse Practitioners in Women’s Health. Both groups endorsed the document.
The collaborative process of putting drafts together and then reviewing and revising the document was a big part of the reason it took 2 years to produce the interpregnancy care consensus statement. “It was the equivalent of two full gestations with a short interpregnancy interval!” said Dr. Louis, laughing. But seeking input from all stakeholders strengthened the final product.
Dr. Louis reported no conflicts of interest and no outside sources of funding were reported.
SOURCE: Louis JM et al. Obstet Gynecol. 2019;133:e51-72.
A new consensus statement places renewed focus on maternal interpregnancy care, with a goal of extending care past the postpartum period to provide a wellness-maximizing continuum of care.
The document, developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine (SMFM), recognizes that pregnancy is part of the lifelong continuum of health and wellness. Although not all women will go on to have another pregnancy, the concept of interpregnancy care recognizes that ob.gyns. have a vital role that extends past the postpartum period.
“This is a shift in what we used to think was our job. We used to think that our job ended when the baby came out,” said the first author of the obstetric care consensus statement, Judette Marie Louis, MD, an ob.gyn. faculty member at the University of South Florida, Tampa, and a SMFM board member. “For too long, our focus was just the baby; we need to tell women, ‘You’re important too,’ ” she said in an interview.
“The interpregnancy period is an opportunity to address these complications of medical issues that have developed during pregnancy, to assess a woman’s mental and physical well-being, and to optimize her health along her life course,” Dr. Louis and her coauthors wrote in Obstetrics & Gynecology.
Conceptually, the opportunity for interpregnancy care arises after any pregnancy, no matter the outcome, and is part of the continuum of care for women of reproductive age. For women who do not intend a future pregnancy, well-woman care is the focus, while women who currently intend to become pregnant again receive interpregnancy care. Women may move from one arm of the continuum to the other if their intentions change or if they become pregnant, explained Dr. Louis and her coauthors.
Birth spacing
The new consensus document includes an emphasis on long-term health outcomes as well as maternal and neonatal outcomes in future pregnancies. Although the evidence is of no more than moderate quality, women should be advised to have an interpregnancy interval of at least 6 months and offered counseling about family planning before delivery. Risks and benefits of an interpregnancy interval of less than 18 months should be reviewed as well.
For women with a history of preterm birth and those who have had a prior cesarean delivery and desire a subsequent trial of labor, birth spacing is particularly important, noted Dr. Louis and her colleagues. There is higher-risk evidence for uterine rupture after cesarean delivery if delivery-to-delivery intervals are 18-24 months or less.
Recommendations regarding the length of the interpregnancy interval generally should not be affected by a history of prior infertility.
Other recommendations
High-quality evidence supports breast feeding’s salutary effects on maternal and child health, so women should receive anticipatory support and guidance to enable breastfeeding, according to the document.
In accordance with other guidelines, the document recommends that all women be screened for depression post partum and as part of well-woman care in the interpregnancy interval. Procedures for effective diagnosis, treatment, and follow-up should accompany the screening.
Best practices dictate that women are asked about alcohol, prescription, and nonprescription drug use as a routine matter. High-quality evidence supports offering smoking cessation support to women who smoke and giving specific advice about nutrition and physical activity that’s based on “proven behavioral techniques.”
Evidence is of moderate quality that women should be encouraged to reach their prepregnancy weight by a year after delivery, with the ultimate goal of having a body mass index of 18.5-24.9.
High-quality evidence backs encouragement to engage in safe sex practices, with care providers also advised to facilitate partner screening and treatment for STIs.
Screening for STIs should follow guidance put forward by the Centers for Disease Control and Prevention and should be offered to women at high risk for STIs; those with a history of STI should have a careful history taken to determine risks for current or repeat infections, wrote Dr. Louis and her coauthors. These strong recommendations have high-quality evidence behind them.
The consensus statement recommends screening women for intimate partner violence, with moderate-quality evidence to support the recommendation. Patient navigators, expert medical interpretation, and other health educators can be offered to women with health literacy or language and communication challenges, but the evidence backing the recommendation is of low quality.
A subset of the interpregnancy care recommendations gives additional guidance regarding women with a history of high-risk pregnancy. All women planning pregnancy – or who could become pregnant – should take 500 mcg of folic acid daily beginning 1 month before fertilization and continuing through the 12th week of pregnancy. Folic acid supplementation for women who have had children with neural tube defects should begin at least 3 months before fertilization and continue through 12 weeks of pregnancy, at a dose of 400 mg.
All prescription and nonprescription medications, as well as potential environmental teratogens, should be reviewed before a repeat pregnancy. This, as well as the folic acid recommendations, are strong recommendations backed by high-quality evidence.
When appropriate, genetic counseling should be offered to women who have had prior pregnancies with genetic disorders or congenital anomalies. Asymptomatic genitourinary infections should not be treated in women with a history of preterm birth during the interpregnancy interval. These are strong recommendations, but are backed by low to moderate quality evidence, wrote Dr. Louis and her colleagues.
Another section of the consensus document specifically addresses specific health conditions, including diabetes, gestational diabetes, gestational and chronic hypertension, and preeclampsia, as well as mental health disorders and overweight or obesity.
For each, Dr. Louis and her coauthors recommend counseling that reviews complications and risk for future disease; for example, not only does prior preeclampsia increase risk for that complication in future pregnancies, risk for later cardiovascular disease is also doubled. The document outlines recommended interpregnancy testing, management considerations and medications of concern for health care providers caring for women with these conditions, and condition-specific goals.
Of the association between gestational diabetes, hypertension, and preeclampsia with later disease, Dr. Louis said in the interview, “We don’t know. ... It may be that pregnancy accelerates these diseases. We do know that normal changes in pregnancy stress your body, and that preeclampsia damages your vessels. Pregnancy can give you a warning, but we don’t have enough information to predict the outcome” in later life. “We do know there is some advice we can give: stop smoking and maintain a normal body weight.”
Other conditions such as HIV, renal disease, epilepsy, autoimmune and thyroid disease, and thrombophilias and antiphospholipid antibody syndrome are also addressed in this section of the consensus document.
Specific attention also is given to psychosocial risks, such as socioeconomic disadvantages and being a member of a racial or ethnic minority. Social determinants of health are complex, said Dr. Louis, but socioeconomic and racial stressors can include the added burden of caring for loved ones with constrained resources. Additionally, there can be access issues: Women can get emergent care by presenting to the ED but receiving continued primary and specialty care can be much more of a challenge.
Regarding racism, Dr. Louis said, “We all come into caring for these women with certain ideas.” For example, it’s not enough to say of a patient, “She’s noncompliant. You need to ask why.” When the “why” question is asked, then you may discover, “there’s something you can help the patient with,” she said. “We need to ask why, and then take steps to help our patients.”
Document building
The working group for the consensus document felt it was important to include nonphysicians who care for women, Dr. Louis said, so drafts were reviewed by and input received from the American College of Nurse-Midwives and the National Association of Nurse Practitioners in Women’s Health. Both groups endorsed the document.
The collaborative process of putting drafts together and then reviewing and revising the document was a big part of the reason it took 2 years to produce the interpregnancy care consensus statement. “It was the equivalent of two full gestations with a short interpregnancy interval!” said Dr. Louis, laughing. But seeking input from all stakeholders strengthened the final product.
Dr. Louis reported no conflicts of interest and no outside sources of funding were reported.
SOURCE: Louis JM et al. Obstet Gynecol. 2019;133:e51-72.
A new consensus statement places renewed focus on maternal interpregnancy care, with a goal of extending care past the postpartum period to provide a wellness-maximizing continuum of care.
The document, developed by the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine (SMFM), recognizes that pregnancy is part of the lifelong continuum of health and wellness. Although not all women will go on to have another pregnancy, the concept of interpregnancy care recognizes that ob.gyns. have a vital role that extends past the postpartum period.
“This is a shift in what we used to think was our job. We used to think that our job ended when the baby came out,” said the first author of the obstetric care consensus statement, Judette Marie Louis, MD, an ob.gyn. faculty member at the University of South Florida, Tampa, and a SMFM board member. “For too long, our focus was just the baby; we need to tell women, ‘You’re important too,’ ” she said in an interview.
“The interpregnancy period is an opportunity to address these complications of medical issues that have developed during pregnancy, to assess a woman’s mental and physical well-being, and to optimize her health along her life course,” Dr. Louis and her coauthors wrote in Obstetrics & Gynecology.
Conceptually, the opportunity for interpregnancy care arises after any pregnancy, no matter the outcome, and is part of the continuum of care for women of reproductive age. For women who do not intend a future pregnancy, well-woman care is the focus, while women who currently intend to become pregnant again receive interpregnancy care. Women may move from one arm of the continuum to the other if their intentions change or if they become pregnant, explained Dr. Louis and her coauthors.
Birth spacing
The new consensus document includes an emphasis on long-term health outcomes as well as maternal and neonatal outcomes in future pregnancies. Although the evidence is of no more than moderate quality, women should be advised to have an interpregnancy interval of at least 6 months and offered counseling about family planning before delivery. Risks and benefits of an interpregnancy interval of less than 18 months should be reviewed as well.
For women with a history of preterm birth and those who have had a prior cesarean delivery and desire a subsequent trial of labor, birth spacing is particularly important, noted Dr. Louis and her colleagues. There is higher-risk evidence for uterine rupture after cesarean delivery if delivery-to-delivery intervals are 18-24 months or less.
Recommendations regarding the length of the interpregnancy interval generally should not be affected by a history of prior infertility.
Other recommendations
High-quality evidence supports breast feeding’s salutary effects on maternal and child health, so women should receive anticipatory support and guidance to enable breastfeeding, according to the document.
In accordance with other guidelines, the document recommends that all women be screened for depression post partum and as part of well-woman care in the interpregnancy interval. Procedures for effective diagnosis, treatment, and follow-up should accompany the screening.
Best practices dictate that women are asked about alcohol, prescription, and nonprescription drug use as a routine matter. High-quality evidence supports offering smoking cessation support to women who smoke and giving specific advice about nutrition and physical activity that’s based on “proven behavioral techniques.”
Evidence is of moderate quality that women should be encouraged to reach their prepregnancy weight by a year after delivery, with the ultimate goal of having a body mass index of 18.5-24.9.
High-quality evidence backs encouragement to engage in safe sex practices, with care providers also advised to facilitate partner screening and treatment for STIs.
Screening for STIs should follow guidance put forward by the Centers for Disease Control and Prevention and should be offered to women at high risk for STIs; those with a history of STI should have a careful history taken to determine risks for current or repeat infections, wrote Dr. Louis and her coauthors. These strong recommendations have high-quality evidence behind them.
The consensus statement recommends screening women for intimate partner violence, with moderate-quality evidence to support the recommendation. Patient navigators, expert medical interpretation, and other health educators can be offered to women with health literacy or language and communication challenges, but the evidence backing the recommendation is of low quality.
A subset of the interpregnancy care recommendations gives additional guidance regarding women with a history of high-risk pregnancy. All women planning pregnancy – or who could become pregnant – should take 500 mcg of folic acid daily beginning 1 month before fertilization and continuing through the 12th week of pregnancy. Folic acid supplementation for women who have had children with neural tube defects should begin at least 3 months before fertilization and continue through 12 weeks of pregnancy, at a dose of 400 mg.
All prescription and nonprescription medications, as well as potential environmental teratogens, should be reviewed before a repeat pregnancy. This, as well as the folic acid recommendations, are strong recommendations backed by high-quality evidence.
When appropriate, genetic counseling should be offered to women who have had prior pregnancies with genetic disorders or congenital anomalies. Asymptomatic genitourinary infections should not be treated in women with a history of preterm birth during the interpregnancy interval. These are strong recommendations, but are backed by low to moderate quality evidence, wrote Dr. Louis and her colleagues.
Another section of the consensus document specifically addresses specific health conditions, including diabetes, gestational diabetes, gestational and chronic hypertension, and preeclampsia, as well as mental health disorders and overweight or obesity.
For each, Dr. Louis and her coauthors recommend counseling that reviews complications and risk for future disease; for example, not only does prior preeclampsia increase risk for that complication in future pregnancies, risk for later cardiovascular disease is also doubled. The document outlines recommended interpregnancy testing, management considerations and medications of concern for health care providers caring for women with these conditions, and condition-specific goals.
Of the association between gestational diabetes, hypertension, and preeclampsia with later disease, Dr. Louis said in the interview, “We don’t know. ... It may be that pregnancy accelerates these diseases. We do know that normal changes in pregnancy stress your body, and that preeclampsia damages your vessels. Pregnancy can give you a warning, but we don’t have enough information to predict the outcome” in later life. “We do know there is some advice we can give: stop smoking and maintain a normal body weight.”
Other conditions such as HIV, renal disease, epilepsy, autoimmune and thyroid disease, and thrombophilias and antiphospholipid antibody syndrome are also addressed in this section of the consensus document.
Specific attention also is given to psychosocial risks, such as socioeconomic disadvantages and being a member of a racial or ethnic minority. Social determinants of health are complex, said Dr. Louis, but socioeconomic and racial stressors can include the added burden of caring for loved ones with constrained resources. Additionally, there can be access issues: Women can get emergent care by presenting to the ED but receiving continued primary and specialty care can be much more of a challenge.
Regarding racism, Dr. Louis said, “We all come into caring for these women with certain ideas.” For example, it’s not enough to say of a patient, “She’s noncompliant. You need to ask why.” When the “why” question is asked, then you may discover, “there’s something you can help the patient with,” she said. “We need to ask why, and then take steps to help our patients.”
Document building
The working group for the consensus document felt it was important to include nonphysicians who care for women, Dr. Louis said, so drafts were reviewed by and input received from the American College of Nurse-Midwives and the National Association of Nurse Practitioners in Women’s Health. Both groups endorsed the document.
The collaborative process of putting drafts together and then reviewing and revising the document was a big part of the reason it took 2 years to produce the interpregnancy care consensus statement. “It was the equivalent of two full gestations with a short interpregnancy interval!” said Dr. Louis, laughing. But seeking input from all stakeholders strengthened the final product.
Dr. Louis reported no conflicts of interest and no outside sources of funding were reported.
SOURCE: Louis JM et al. Obstet Gynecol. 2019;133:e51-72.
FROM OBSTETRICS & GYNECOLOGY