User login
Using telemedicine to improve maternal safety
SAN DIEGO – Utah hospitals reported improved implementation of an obstetrics hemorrhage bundle following a series of teleconferencing sessions.
“There is an increasing body of evidence to support the use of protocols and bundles in obstetrics to improve outcomes for pregnant women and their babies,” Brett D. Einerson, MD, MPH, lead study author, said in an interview. “In Utah and throughout the Mountain West, we face the unique challenge of disseminating information and education on the latest evidence-based treatments to smaller rural hospitals that still need to be prepared for events like severe postpartum hemorrhage but do not have the volume, or sometime the resources, to be adequately prepared.”
“Telehealth allowed us to reach providers who otherwise could not travel the distance to attend frequent training sessions and gave the whole state access to expertise at the region’s large tertiary care hospitals,” Dr. Einerson said. “As far as we know, this is one of the first uses of telehealth as a tool for disseminating patient safety and quality improvement education for health care providers on a statewide scale.”
Dr. Einerson and his associates invited all Utah hospitals to participate in the Obstetric Hemorrhage Collaborative, an evidence-based educational program aimed at facilitating implementation of the obstetric hemorrhage bundle. The program involved two in-person training meetings and twice-monthly teleconferencing with expert mentorship over 6 months. In-person sessions consisted of hands-on training and strategy building, while telehealth sessions were led by regional and national leaders in the field of obstetric hemorrhage.
A statewide self-assessment survey of 38 bundle elements was administered before initiation of the project and after completion. The researchers used modified Likert scales to describe participant responses. Means and proportions were compared before and after the training.
Of Utah’s obstetric hospitals, representing every hospital system in the state, 27 (61%) completed the needs-assessment survey, and 15 (34%) participated in the Obstetric Hemorrhage Collaborative, which included four bundle domains:
- Recognition and Prevention: Conducting a risk assessment and active management of the Third Stage of labor.
- Response: Creating a checklist and a rapid response team.
- Readiness: Establishing a blood bank, hemorrhage cart, and conducting simulation/team drills.
- Reporting and Learning: Fostering a culture of debriefing, conducting a multidisciplinary review, and measuring outcomes and processes.
Hospitals reported implementation, or progress toward implementation, of significantly more elements of the bundle after the educational program, compared with before the collaborative (a mean of 33.3 vs. 19 bundle elements; P less than 0.001). Hospitals reported increased implementation of elements in all four bundle domains. All participants (100%) reported that teleconferencing sessions were “very helpful,” and 14 (93%) said that they were “very satisfied” with the collaborative.
“Hospitals in the state of Utah generally had the right tools to treat and prevent obstetric hemorrhage but did not have the systems in place to be sure that the tools were used correctly,” Dr. Einerson said. “For instance, 80% of hospitals had access to a cart with supplies for treating bleeding, but less than 15% were systematically measuring blood loss after delivery. What surprised me most, however, was that most hospitals did not track their rates of postpartum bleeding. In my mind, you can’t set goals for treatment until you know how good – or bad – you are doing. Knowing your baseline rate of outcomes can help set goals and measure progress toward achieving them. Before training, less than 50% of Utah hospitals knew their own rate of hemorrhage, but all participating hospitals reported tracking their rates after the intervention.”
He acknowledged certain limitations of the study, including the fact that it did not measure obstetric outcomes. “We are in the process of measuring the effectiveness of our telehealth intervention by monitoring hemorrhage rates and complications over time,” Dr. Einerson said. “This survey of participants in the statewide telehealth bundle program is the first step.”
Dr. Einerson reported having no financial disclosures.
SAN DIEGO – Utah hospitals reported improved implementation of an obstetrics hemorrhage bundle following a series of teleconferencing sessions.
“There is an increasing body of evidence to support the use of protocols and bundles in obstetrics to improve outcomes for pregnant women and their babies,” Brett D. Einerson, MD, MPH, lead study author, said in an interview. “In Utah and throughout the Mountain West, we face the unique challenge of disseminating information and education on the latest evidence-based treatments to smaller rural hospitals that still need to be prepared for events like severe postpartum hemorrhage but do not have the volume, or sometime the resources, to be adequately prepared.”
“Telehealth allowed us to reach providers who otherwise could not travel the distance to attend frequent training sessions and gave the whole state access to expertise at the region’s large tertiary care hospitals,” Dr. Einerson said. “As far as we know, this is one of the first uses of telehealth as a tool for disseminating patient safety and quality improvement education for health care providers on a statewide scale.”
Dr. Einerson and his associates invited all Utah hospitals to participate in the Obstetric Hemorrhage Collaborative, an evidence-based educational program aimed at facilitating implementation of the obstetric hemorrhage bundle. The program involved two in-person training meetings and twice-monthly teleconferencing with expert mentorship over 6 months. In-person sessions consisted of hands-on training and strategy building, while telehealth sessions were led by regional and national leaders in the field of obstetric hemorrhage.
A statewide self-assessment survey of 38 bundle elements was administered before initiation of the project and after completion. The researchers used modified Likert scales to describe participant responses. Means and proportions were compared before and after the training.
Of Utah’s obstetric hospitals, representing every hospital system in the state, 27 (61%) completed the needs-assessment survey, and 15 (34%) participated in the Obstetric Hemorrhage Collaborative, which included four bundle domains:
- Recognition and Prevention: Conducting a risk assessment and active management of the Third Stage of labor.
- Response: Creating a checklist and a rapid response team.
- Readiness: Establishing a blood bank, hemorrhage cart, and conducting simulation/team drills.
- Reporting and Learning: Fostering a culture of debriefing, conducting a multidisciplinary review, and measuring outcomes and processes.
Hospitals reported implementation, or progress toward implementation, of significantly more elements of the bundle after the educational program, compared with before the collaborative (a mean of 33.3 vs. 19 bundle elements; P less than 0.001). Hospitals reported increased implementation of elements in all four bundle domains. All participants (100%) reported that teleconferencing sessions were “very helpful,” and 14 (93%) said that they were “very satisfied” with the collaborative.
“Hospitals in the state of Utah generally had the right tools to treat and prevent obstetric hemorrhage but did not have the systems in place to be sure that the tools were used correctly,” Dr. Einerson said. “For instance, 80% of hospitals had access to a cart with supplies for treating bleeding, but less than 15% were systematically measuring blood loss after delivery. What surprised me most, however, was that most hospitals did not track their rates of postpartum bleeding. In my mind, you can’t set goals for treatment until you know how good – or bad – you are doing. Knowing your baseline rate of outcomes can help set goals and measure progress toward achieving them. Before training, less than 50% of Utah hospitals knew their own rate of hemorrhage, but all participating hospitals reported tracking their rates after the intervention.”
He acknowledged certain limitations of the study, including the fact that it did not measure obstetric outcomes. “We are in the process of measuring the effectiveness of our telehealth intervention by monitoring hemorrhage rates and complications over time,” Dr. Einerson said. “This survey of participants in the statewide telehealth bundle program is the first step.”
Dr. Einerson reported having no financial disclosures.
SAN DIEGO – Utah hospitals reported improved implementation of an obstetrics hemorrhage bundle following a series of teleconferencing sessions.
“There is an increasing body of evidence to support the use of protocols and bundles in obstetrics to improve outcomes for pregnant women and their babies,” Brett D. Einerson, MD, MPH, lead study author, said in an interview. “In Utah and throughout the Mountain West, we face the unique challenge of disseminating information and education on the latest evidence-based treatments to smaller rural hospitals that still need to be prepared for events like severe postpartum hemorrhage but do not have the volume, or sometime the resources, to be adequately prepared.”
“Telehealth allowed us to reach providers who otherwise could not travel the distance to attend frequent training sessions and gave the whole state access to expertise at the region’s large tertiary care hospitals,” Dr. Einerson said. “As far as we know, this is one of the first uses of telehealth as a tool for disseminating patient safety and quality improvement education for health care providers on a statewide scale.”
Dr. Einerson and his associates invited all Utah hospitals to participate in the Obstetric Hemorrhage Collaborative, an evidence-based educational program aimed at facilitating implementation of the obstetric hemorrhage bundle. The program involved two in-person training meetings and twice-monthly teleconferencing with expert mentorship over 6 months. In-person sessions consisted of hands-on training and strategy building, while telehealth sessions were led by regional and national leaders in the field of obstetric hemorrhage.
A statewide self-assessment survey of 38 bundle elements was administered before initiation of the project and after completion. The researchers used modified Likert scales to describe participant responses. Means and proportions were compared before and after the training.
Of Utah’s obstetric hospitals, representing every hospital system in the state, 27 (61%) completed the needs-assessment survey, and 15 (34%) participated in the Obstetric Hemorrhage Collaborative, which included four bundle domains:
- Recognition and Prevention: Conducting a risk assessment and active management of the Third Stage of labor.
- Response: Creating a checklist and a rapid response team.
- Readiness: Establishing a blood bank, hemorrhage cart, and conducting simulation/team drills.
- Reporting and Learning: Fostering a culture of debriefing, conducting a multidisciplinary review, and measuring outcomes and processes.
Hospitals reported implementation, or progress toward implementation, of significantly more elements of the bundle after the educational program, compared with before the collaborative (a mean of 33.3 vs. 19 bundle elements; P less than 0.001). Hospitals reported increased implementation of elements in all four bundle domains. All participants (100%) reported that teleconferencing sessions were “very helpful,” and 14 (93%) said that they were “very satisfied” with the collaborative.
“Hospitals in the state of Utah generally had the right tools to treat and prevent obstetric hemorrhage but did not have the systems in place to be sure that the tools were used correctly,” Dr. Einerson said. “For instance, 80% of hospitals had access to a cart with supplies for treating bleeding, but less than 15% were systematically measuring blood loss after delivery. What surprised me most, however, was that most hospitals did not track their rates of postpartum bleeding. In my mind, you can’t set goals for treatment until you know how good – or bad – you are doing. Knowing your baseline rate of outcomes can help set goals and measure progress toward achieving them. Before training, less than 50% of Utah hospitals knew their own rate of hemorrhage, but all participating hospitals reported tracking their rates after the intervention.”
He acknowledged certain limitations of the study, including the fact that it did not measure obstetric outcomes. “We are in the process of measuring the effectiveness of our telehealth intervention by monitoring hemorrhage rates and complications over time,” Dr. Einerson said. “This survey of participants in the statewide telehealth bundle program is the first step.”
Dr. Einerson reported having no financial disclosures.
AT ACOG 2017
Key clinical point:
Major finding: Hospitals reported implementation, or progress toward implementation, of significantly more elements of the bundle after the educational program, compared with before the collaborative (a mean of 33.3 vs. 19 bundle elements; P less than 0.001).
Data source: Results from 15 Utah hospitals that participated in the Obstetric Hemorrhage Collaborative.
Disclosures: The researchers reported having no financial disclosures.
More than one-third of genetic tests misordered, study finds
SAN DIEGO – A review of genetic tests ordered during a 3-month period found that more than one-third were misordered, leading to more than $20,000 in unnecessary health care costs, results from a single-center quality improvement project showed.
“We know there is an ever-expanding number of genetic tests available for clinicians to order, and there is more direct marketing to the patient,” Kathleen Ruzzo, MD, the lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “It can be difficult to stay on top of that as we have so many different clinical responsibilities.”
Of genetic tests ordered for the 114 patients, 44 (39%) were deemed to be misordered based on published clinical practice guidelines. Of the rest, 24 tests were misordered/not indicated, 8 tests were misordered/false reassurance, and 12 tests were misordered/inadequate.
The costs of ordered genetic testing totaled approximately $75,000, while the cost of recommended testing following the chart review was approximately $54,000, a difference of more than $20,000.
When Dr. Ruzzo shared results of the study with her colleagues at Naval Medical Center San Diego, “I think it opened a lot of people’s eyes … to be more meticulous about [genetic] testing and to ask for help when you need help,” she said. “Having trained individuals, reviewing genetic tests could save money in the health care system more broadly. We could also approve the appropriate testing for the patient.”
She acknowledged certain limitations of the study, including the fact that it “reviewed a very narrow scope of [genetic] tests for a short amount of time, so we think we underestimated the appropriate health care expenditures. Additionally, we didn’t focus on the clinical ramifications of the misordering for patients.”
Study coauthor Monica A. Lutgendorf, MD, a maternal-fetal medicine physician at the medical center, characterized the study findings as “a call to action in general for ob.gyns. to get additional training and resources to handle the ever-expanding number of [genetic] tests,” she said. “I don’t think that this is unique to any specific institution. I think this is part of the new environment of practice that we’re in.”
Physicians can learn more about genetic testing from ACOG and from the Perinatal Quality Foundation, Dr. Lutgendorf said. The study won first prize among oral abstracts presented at the ACOG meeting. The researchers reported having no financial disclosures.
SAN DIEGO – A review of genetic tests ordered during a 3-month period found that more than one-third were misordered, leading to more than $20,000 in unnecessary health care costs, results from a single-center quality improvement project showed.
“We know there is an ever-expanding number of genetic tests available for clinicians to order, and there is more direct marketing to the patient,” Kathleen Ruzzo, MD, the lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “It can be difficult to stay on top of that as we have so many different clinical responsibilities.”
Of genetic tests ordered for the 114 patients, 44 (39%) were deemed to be misordered based on published clinical practice guidelines. Of the rest, 24 tests were misordered/not indicated, 8 tests were misordered/false reassurance, and 12 tests were misordered/inadequate.
The costs of ordered genetic testing totaled approximately $75,000, while the cost of recommended testing following the chart review was approximately $54,000, a difference of more than $20,000.
When Dr. Ruzzo shared results of the study with her colleagues at Naval Medical Center San Diego, “I think it opened a lot of people’s eyes … to be more meticulous about [genetic] testing and to ask for help when you need help,” she said. “Having trained individuals, reviewing genetic tests could save money in the health care system more broadly. We could also approve the appropriate testing for the patient.”
She acknowledged certain limitations of the study, including the fact that it “reviewed a very narrow scope of [genetic] tests for a short amount of time, so we think we underestimated the appropriate health care expenditures. Additionally, we didn’t focus on the clinical ramifications of the misordering for patients.”
Study coauthor Monica A. Lutgendorf, MD, a maternal-fetal medicine physician at the medical center, characterized the study findings as “a call to action in general for ob.gyns. to get additional training and resources to handle the ever-expanding number of [genetic] tests,” she said. “I don’t think that this is unique to any specific institution. I think this is part of the new environment of practice that we’re in.”
Physicians can learn more about genetic testing from ACOG and from the Perinatal Quality Foundation, Dr. Lutgendorf said. The study won first prize among oral abstracts presented at the ACOG meeting. The researchers reported having no financial disclosures.
SAN DIEGO – A review of genetic tests ordered during a 3-month period found that more than one-third were misordered, leading to more than $20,000 in unnecessary health care costs, results from a single-center quality improvement project showed.
“We know there is an ever-expanding number of genetic tests available for clinicians to order, and there is more direct marketing to the patient,” Kathleen Ruzzo, MD, the lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “It can be difficult to stay on top of that as we have so many different clinical responsibilities.”
Of genetic tests ordered for the 114 patients, 44 (39%) were deemed to be misordered based on published clinical practice guidelines. Of the rest, 24 tests were misordered/not indicated, 8 tests were misordered/false reassurance, and 12 tests were misordered/inadequate.
The costs of ordered genetic testing totaled approximately $75,000, while the cost of recommended testing following the chart review was approximately $54,000, a difference of more than $20,000.
When Dr. Ruzzo shared results of the study with her colleagues at Naval Medical Center San Diego, “I think it opened a lot of people’s eyes … to be more meticulous about [genetic] testing and to ask for help when you need help,” she said. “Having trained individuals, reviewing genetic tests could save money in the health care system more broadly. We could also approve the appropriate testing for the patient.”
She acknowledged certain limitations of the study, including the fact that it “reviewed a very narrow scope of [genetic] tests for a short amount of time, so we think we underestimated the appropriate health care expenditures. Additionally, we didn’t focus on the clinical ramifications of the misordering for patients.”
Study coauthor Monica A. Lutgendorf, MD, a maternal-fetal medicine physician at the medical center, characterized the study findings as “a call to action in general for ob.gyns. to get additional training and resources to handle the ever-expanding number of [genetic] tests,” she said. “I don’t think that this is unique to any specific institution. I think this is part of the new environment of practice that we’re in.”
Physicians can learn more about genetic testing from ACOG and from the Perinatal Quality Foundation, Dr. Lutgendorf said. The study won first prize among oral abstracts presented at the ACOG meeting. The researchers reported having no financial disclosures.
AT ACOG 2017
Key clinical point:
Major finding: Of genetic tests ordered by clinicians, 39% were deemed to be misordered.
Data source: A review of 114 genetic tests ordered over a 3-month period at a single center.
Disclosures: The researchers reported having no financial disclosures.
Should recent evidence of improved outcomes for neonates born during the periviable period change our approach to these deliveries?
EXPERT COMMENTARY
Pregnancy management when delivery appears to be imminent at 22 to 26 weeks’ gestation—a window defined as the periviable period—is among the most challenging situations that obstetricians face. Expert guidance exists both at a national level in a shared guideline from the American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine and, ideally, at a local level where teams of obstetricians and neonatologists have considered in their facility what represents best care
Among the most important yet often missing data points are outcomes of neonates born in the periviable period. Surveys suggest that obstetric care providers often underestimate the chance of survival following periviable delivery.2 Understanding and weighing anticipated outcomes inform decision making regarding management and planned obstetric and neonatal interventions, including plans for neonatal resuscitation.
Not surprisingly, perhaps, survival of periviable neonates has been linked clearly to willingness to undertake resuscitation.3 Yet decisions are not and should not be all about survival. Patients and providers want to know about short- and long-term morbidity, especially neurologic health, among survivors. Available collections of morbidity and mortality data, however, often are limited by whether all cases are captured or just those from specialized centers with particular management approaches, which outcomes are included and how they are defined, and the inevitable reality that the outcome of death “competes” with the outcome of neurologic development (that is, those neonates who die are not at risk for later abnormal neurologic outcome).
Given the need for more and better information, the data from a recent study by Younge and colleagues is especially welcome. The investigators reported on survival and neurologic outcome among more than 4,000 births between 22 and 24 weeks’ gestation at 11 centers in the United States.
Details of the study
The authors compared outcomes among three 3-year epochs between 2000 and 2011 and reported that the rate of survival without neurodevelopmental impairment increased over this period while the rate of survival with such impairment did not change. This argues that the observed overall increase in survival over these 12 years was not simply a tradeoff for life with significant impairment.
Within that overall message, however, the details of the data are important. Survival without neurodevelopmental impairment did improve from epoch 1 to epoch 3, but just from 16% to 20% (95% confidence interval [CI], 18–23; P = .001). Most neonates in the 2008–2011 epoch died (64%; 95% CI, 61–66; P<.001) or were severely impaired (16%; 95% CI, 14–18; P = .29). This led the authors to conclude that “despite improvements over time, the incidence of death, neurodevelopmental impairment, and other adverse outcomes remains high.” Examined separately, outcomes for infants born at 22 0/7 to 22 6/7 weeks’ gestation were very limited and unchanged over the 3 epochs studied, with death rates of 97% to 98% and survival without neurodevelopmental impairment of just 1%. In my own practice I do not encourage neonatal resuscitation, cesarean delivery, or many other interventions at less than 23 weeks’ gestation.
By contrast, the study showed that at 24 0/7 to 24 6/7 weeks’ gestation in the 2008–2011 epoch, 55% of neonates survived and, overall, 32% of infants survived without evidence of neurodevelopmental impairment at 18 to 22 months of age.
Related Article:
Is expectant management a safe alternative to immediate delivery in patients with PPROM close to term?
Study strengths and weaknesses
It is important to note that the definition of neurodevelopmental impairment used in the Younge study included only what many would classify as severe impairment, and survivors in this cohort “without” neurodevelopmental impairment may still have had important neurologic and other health concerns. In addition, the study did not track outcomes of the children at school age or beyond, when other developmental issues may become evident. As well, the study data may not be generalizable, for it included births from just 11 specialized centers, albeit a consortium accounting for 4% to 5% of periviable births in the United States.
Nevertheless, in supporting findings from other US and European analyses, these new data will help inform counseling conversations in the years to come. Such conversations should consider options for resuscitation, palliative care, and, at less than 24 weeks’ gestation, pregnancy termination. In individual cases these and many other decisions will be informed by both specific clinical circumstances—estimated fetal weight, fetal sex, presence of infection, use of antenatal steroids—and, perhaps most important, individual and family values and preferences. Despite these new data, managing periviable gestations will remain a great and important challenge.
--Jeffrey L. Ecker, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Obstetric Care Consensus No. 4: Periviable birth. Obstet Gynecol. 2016;127(6):e157-e169.
- Haywood JL, Goldenberg RL, Bronstein J, Nelson KG, Carlo WA. Comparison of perceived and actual rates of survival and freedom from handicap in premature infants. Am J Obstet Gynecol. 1994;171(2):432-439.
- Rysavy MA, Li L, Bell EF, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Neonatal Research Unit. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801-1811.
EXPERT COMMENTARY
Pregnancy management when delivery appears to be imminent at 22 to 26 weeks’ gestation—a window defined as the periviable period—is among the most challenging situations that obstetricians face. Expert guidance exists both at a national level in a shared guideline from the American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine and, ideally, at a local level where teams of obstetricians and neonatologists have considered in their facility what represents best care
Among the most important yet often missing data points are outcomes of neonates born in the periviable period. Surveys suggest that obstetric care providers often underestimate the chance of survival following periviable delivery.2 Understanding and weighing anticipated outcomes inform decision making regarding management and planned obstetric and neonatal interventions, including plans for neonatal resuscitation.
Not surprisingly, perhaps, survival of periviable neonates has been linked clearly to willingness to undertake resuscitation.3 Yet decisions are not and should not be all about survival. Patients and providers want to know about short- and long-term morbidity, especially neurologic health, among survivors. Available collections of morbidity and mortality data, however, often are limited by whether all cases are captured or just those from specialized centers with particular management approaches, which outcomes are included and how they are defined, and the inevitable reality that the outcome of death “competes” with the outcome of neurologic development (that is, those neonates who die are not at risk for later abnormal neurologic outcome).
Given the need for more and better information, the data from a recent study by Younge and colleagues is especially welcome. The investigators reported on survival and neurologic outcome among more than 4,000 births between 22 and 24 weeks’ gestation at 11 centers in the United States.
Details of the study
The authors compared outcomes among three 3-year epochs between 2000 and 2011 and reported that the rate of survival without neurodevelopmental impairment increased over this period while the rate of survival with such impairment did not change. This argues that the observed overall increase in survival over these 12 years was not simply a tradeoff for life with significant impairment.
Within that overall message, however, the details of the data are important. Survival without neurodevelopmental impairment did improve from epoch 1 to epoch 3, but just from 16% to 20% (95% confidence interval [CI], 18–23; P = .001). Most neonates in the 2008–2011 epoch died (64%; 95% CI, 61–66; P<.001) or were severely impaired (16%; 95% CI, 14–18; P = .29). This led the authors to conclude that “despite improvements over time, the incidence of death, neurodevelopmental impairment, and other adverse outcomes remains high.” Examined separately, outcomes for infants born at 22 0/7 to 22 6/7 weeks’ gestation were very limited and unchanged over the 3 epochs studied, with death rates of 97% to 98% and survival without neurodevelopmental impairment of just 1%. In my own practice I do not encourage neonatal resuscitation, cesarean delivery, or many other interventions at less than 23 weeks’ gestation.
By contrast, the study showed that at 24 0/7 to 24 6/7 weeks’ gestation in the 2008–2011 epoch, 55% of neonates survived and, overall, 32% of infants survived without evidence of neurodevelopmental impairment at 18 to 22 months of age.
Related Article:
Is expectant management a safe alternative to immediate delivery in patients with PPROM close to term?
Study strengths and weaknesses
It is important to note that the definition of neurodevelopmental impairment used in the Younge study included only what many would classify as severe impairment, and survivors in this cohort “without” neurodevelopmental impairment may still have had important neurologic and other health concerns. In addition, the study did not track outcomes of the children at school age or beyond, when other developmental issues may become evident. As well, the study data may not be generalizable, for it included births from just 11 specialized centers, albeit a consortium accounting for 4% to 5% of periviable births in the United States.
Nevertheless, in supporting findings from other US and European analyses, these new data will help inform counseling conversations in the years to come. Such conversations should consider options for resuscitation, palliative care, and, at less than 24 weeks’ gestation, pregnancy termination. In individual cases these and many other decisions will be informed by both specific clinical circumstances—estimated fetal weight, fetal sex, presence of infection, use of antenatal steroids—and, perhaps most important, individual and family values and preferences. Despite these new data, managing periviable gestations will remain a great and important challenge.
--Jeffrey L. Ecker, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Pregnancy management when delivery appears to be imminent at 22 to 26 weeks’ gestation—a window defined as the periviable period—is among the most challenging situations that obstetricians face. Expert guidance exists both at a national level in a shared guideline from the American College of Obstetricians and Gynecologists and the Society of Maternal Fetal Medicine and, ideally, at a local level where teams of obstetricians and neonatologists have considered in their facility what represents best care
Among the most important yet often missing data points are outcomes of neonates born in the periviable period. Surveys suggest that obstetric care providers often underestimate the chance of survival following periviable delivery.2 Understanding and weighing anticipated outcomes inform decision making regarding management and planned obstetric and neonatal interventions, including plans for neonatal resuscitation.
Not surprisingly, perhaps, survival of periviable neonates has been linked clearly to willingness to undertake resuscitation.3 Yet decisions are not and should not be all about survival. Patients and providers want to know about short- and long-term morbidity, especially neurologic health, among survivors. Available collections of morbidity and mortality data, however, often are limited by whether all cases are captured or just those from specialized centers with particular management approaches, which outcomes are included and how they are defined, and the inevitable reality that the outcome of death “competes” with the outcome of neurologic development (that is, those neonates who die are not at risk for later abnormal neurologic outcome).
Given the need for more and better information, the data from a recent study by Younge and colleagues is especially welcome. The investigators reported on survival and neurologic outcome among more than 4,000 births between 22 and 24 weeks’ gestation at 11 centers in the United States.
Details of the study
The authors compared outcomes among three 3-year epochs between 2000 and 2011 and reported that the rate of survival without neurodevelopmental impairment increased over this period while the rate of survival with such impairment did not change. This argues that the observed overall increase in survival over these 12 years was not simply a tradeoff for life with significant impairment.
Within that overall message, however, the details of the data are important. Survival without neurodevelopmental impairment did improve from epoch 1 to epoch 3, but just from 16% to 20% (95% confidence interval [CI], 18–23; P = .001). Most neonates in the 2008–2011 epoch died (64%; 95% CI, 61–66; P<.001) or were severely impaired (16%; 95% CI, 14–18; P = .29). This led the authors to conclude that “despite improvements over time, the incidence of death, neurodevelopmental impairment, and other adverse outcomes remains high.” Examined separately, outcomes for infants born at 22 0/7 to 22 6/7 weeks’ gestation were very limited and unchanged over the 3 epochs studied, with death rates of 97% to 98% and survival without neurodevelopmental impairment of just 1%. In my own practice I do not encourage neonatal resuscitation, cesarean delivery, or many other interventions at less than 23 weeks’ gestation.
By contrast, the study showed that at 24 0/7 to 24 6/7 weeks’ gestation in the 2008–2011 epoch, 55% of neonates survived and, overall, 32% of infants survived without evidence of neurodevelopmental impairment at 18 to 22 months of age.
Related Article:
Is expectant management a safe alternative to immediate delivery in patients with PPROM close to term?
Study strengths and weaknesses
It is important to note that the definition of neurodevelopmental impairment used in the Younge study included only what many would classify as severe impairment, and survivors in this cohort “without” neurodevelopmental impairment may still have had important neurologic and other health concerns. In addition, the study did not track outcomes of the children at school age or beyond, when other developmental issues may become evident. As well, the study data may not be generalizable, for it included births from just 11 specialized centers, albeit a consortium accounting for 4% to 5% of periviable births in the United States.
Nevertheless, in supporting findings from other US and European analyses, these new data will help inform counseling conversations in the years to come. Such conversations should consider options for resuscitation, palliative care, and, at less than 24 weeks’ gestation, pregnancy termination. In individual cases these and many other decisions will be informed by both specific clinical circumstances—estimated fetal weight, fetal sex, presence of infection, use of antenatal steroids—and, perhaps most important, individual and family values and preferences. Despite these new data, managing periviable gestations will remain a great and important challenge.
--Jeffrey L. Ecker, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Obstetric Care Consensus No. 4: Periviable birth. Obstet Gynecol. 2016;127(6):e157-e169.
- Haywood JL, Goldenberg RL, Bronstein J, Nelson KG, Carlo WA. Comparison of perceived and actual rates of survival and freedom from handicap in premature infants. Am J Obstet Gynecol. 1994;171(2):432-439.
- Rysavy MA, Li L, Bell EF, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Neonatal Research Unit. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801-1811.
- Obstetric Care Consensus No. 4: Periviable birth. Obstet Gynecol. 2016;127(6):e157-e169.
- Haywood JL, Goldenberg RL, Bronstein J, Nelson KG, Carlo WA. Comparison of perceived and actual rates of survival and freedom from handicap in premature infants. Am J Obstet Gynecol. 1994;171(2):432-439.
- Rysavy MA, Li L, Bell EF, et al; Eunice Kennedy Schriver National Institute of Child Health and Human Development Neonatal Research Unit. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. 2015;372(19):1801-1811.
Endometriosis: From Identification to Management
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
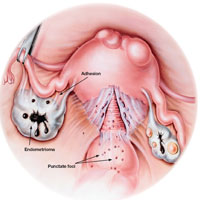
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3
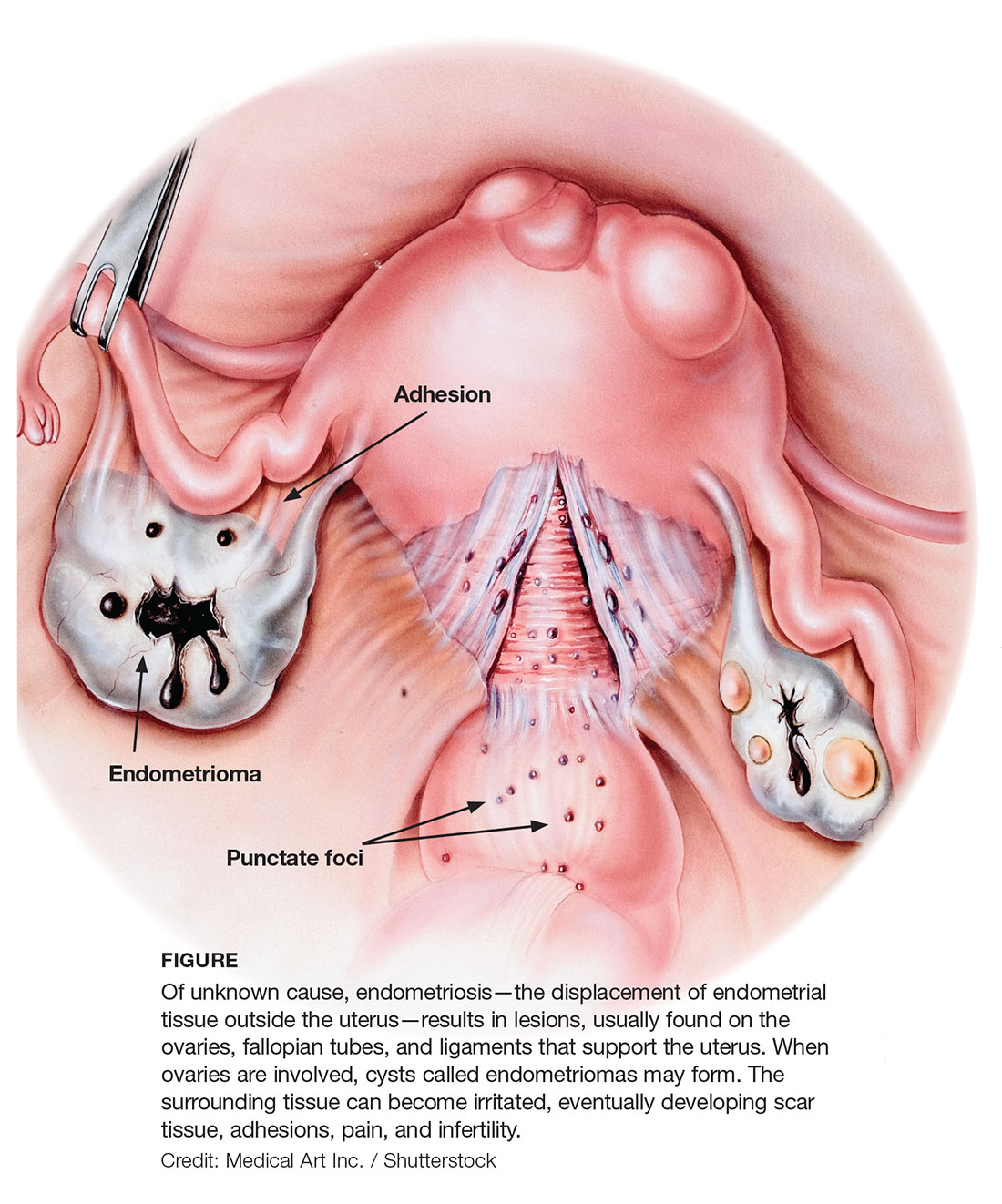
Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
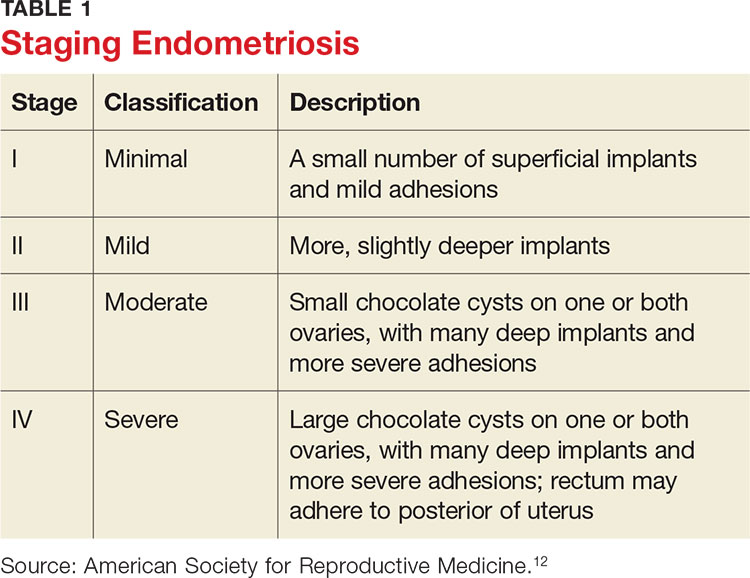
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12
DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
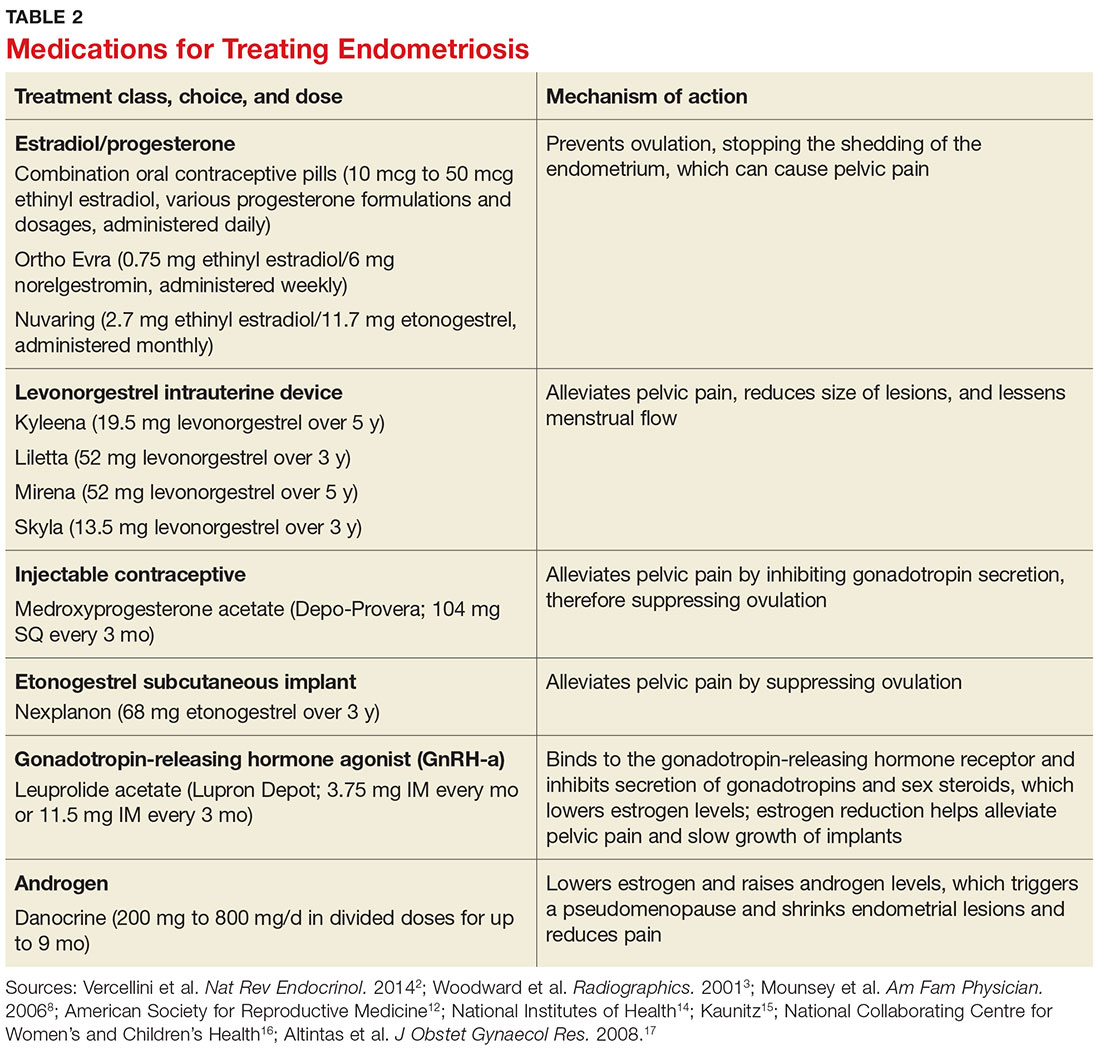
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.
Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3

Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12

DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.

Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
IN THIS ARTICLE
- Staging endometriosis
- Medications for treating endometriosis
- Complications
Endometriosis is a gynecologic disorder characterized by the presence and growth of endometrial tissue outside the uterine cavity (ie, endometrial implants), most commonly found on the ovaries. Although its pathophysiology is not completely understood, the disease is associated with dysmenorrhea, dyspareunia, and infertility.1,2 Endometriosis is an estrogen-dependent disorder, predominantly affecting women of childbearing age. It occurs in 10% to 15% of the general female population, but prevalence is even higher (35% to 50%) among women who experience pelvic pain and/or infertility.1-4 Although endometriosis mainly affects women in their mid-to-late 20s, it can also manifest in adolescence.3,5 Nearly half of all adolescents with intractable dysmenorrhea are diagnosed with endometriosis.5
ETIOLOGY
The etiology of endometriosis, while not completely understood, is likely multifactorial. Factors that may influence its development include gene expression, tissue response to hormones, neuronal tissue involvement, lack of protective factors, inflammation, and cellular oxidative stress.6,7
Several theories regarding the etiology of endometriosis have been proposed; the most widely accepted is the transplantation theory, which suggests that endometriosis results from retrograde flow of menstrual tissue through the fallopian tubes. During menstruation, fragments of the endometrium are driven through the fallopian tubes and into the pelvic cavity, where they can implant onto the pelvic structures, leading to further growth and invasion.2,6,8 Women who have polymenorrhea, prolonged menses, and early menarche therefore have an increased risk for endometriosis.8 This theory does not account for the fact that although nearly 90% of women have some elements of retrograde menstrual flow, only a fraction of them develop endometriosis.6
Two other plausible explanations are the coelomic metaplasia and embryonic rest theories. In the coelomic metaplasia theory, the mesothelium (coelomic epithelium)—which encases the ovaries—invaginates into the ovaries and undergoes a metaplastic change to endometrial tissue. This could explain the development of endometriosis in patients with the congenital malformation Müllerian agenesis. In the embryonic rest theory, Müllerian remnants in the rectovaginal area, left behind by the Müllerian duct system, have the potential to differentiate into endometrial tissue.2,5,6,8
Another theory involving lymphatic or hematologic spread has been proposed, which would explain the presence of endometrial implants at sites distant from the uterus (eg, the pleural cavity and brain). However, this theory is not widely understood
The two most recent hypotheses on endometriosis are associated with an abnormal immune system and a possible genetic predisposition. The peritoneal fluid of women with endometriosis has different levels of prostanoids, cytokines, growth factors, and interleukins than that of women who do not have the condition. It is uncertain whether the relationship between peritoneal fluid changes and endometriosis is causal.6 A genetic correlation has been suggested, based on an increased prevalence of endometriosis in women with an affected first-degree relative; in a case-control study on family incidence of endometriosis, 5.9% to 9.6% of first-degree relatives and 1.3% of second-degree relatives were affected.9 The Oxford Endometriosis Gene (OXEGENE) study is currently investigating susceptible loci for endometriosis genes, which could provide a better understanding of the disease process.6
CLINICAL PRESENTATION
The most common symptoms of endometriosis are dysmenorrhea, deep dyspareunia, chronic pelvic pain, and infertility, but 20% to 25% of affected women are asymptomatic.4,10,11 Pelvic pain in women most often heralds onset of menses and worsens during menstruation.1 Other symptoms include back pain, dyschezia, dysuria, nausea, lethargy, and chronic fatigue.4,8,10
Endometriosis is concomitant with infertility; endometrial adhesions that attach to pelvic organs cause distortion of pelvic structures and impaired ovum release and pick-up, and are believed to reduce fecundity. Additionally, women with endometriosis have low ovarian reserve and low-quality oocytes.6,8 Altered chemical elements (ie, prostanoids, cytokines, growth factors, and interleukins) may also contribute to endometrial-related infertility; intrapelvic growth factors could affect the fallopian tubes or pelvic environment, and thus the oocytes in a similar fashion.6
In adolescents, endometriosis can present as cyclic or acyclic pain; severe dysmenorrhea; dysmenorrhea that responds poorly to medications (eg, oral contraceptive pills [OCPs] or NSAIDs); and prolonged menstruation with premenstrual spotting.1
The physical exam may reveal tender nodules in the posterior vaginal fornix; cervical motion tenderness; a fixed uterus, cervix, or adnexa; uterine motion tenderness; thickening, pain, tenderness, or nodularity of the uterosacral ligament; or tender adnexal masses due to endometriomas.8,10
PATHOLOGIC CHARACTERISTICS AND STAGING
Gross pathology of endometriosis varies based on duration of disease and depth of implants or lesions. Implants range from punctate foci to small stellate patches that vary in color but typically measure less than 2 cm. They manifest most commonly in the ovaries, followed by the anterior and posterior cul-de-sac, posterior broad ligament, and uterosacral ligament. Implants can also be located on the uterus, fallopian tubes, sigmoid colon, ureter, small intestine, lungs, and brain (see Figure).3

Due to recurrent cyclic hemorrhage within a deep implant, endometriomas typically appear in the ovaries, entirely replacing normal ovarian tissue. Endometriomas are composed of dark, thick, degenerated blood products that result in a brown cyst—hence their designation as chocolate cysts. Microscopically, they are comprised of endometrial glands, stroma, and sometimes smooth muscle.3
Staging of endometriosis is determined by the volume, depth, location, and size of the implants (see Table 1). It is important to note that staging does not necessarily reflect symptom severity.12

DIAGNOSIS
There are several approaches to the diagnostic evaluation of endometriosis, all of which should be guided by the clinical presentation and physical examination. Clinical characteristics can be nonspecific and highly variable, warranting more reliable diagnostic methods.
Laparoscopy is the diagnostic gold standard for endometriosis, and biopsy of implants revealing endometrial tissue is confirmatory. Less invasive diagnostic methods include ultrasound and MRI—but without confirmatory histologic sampling, these only yield a presumptive diagnosis.
With ultrasonography, a transvaginal approach should be taken. While endometriomas have a variety of presentations on ultrasound, most appear as a homogenous, hypoechoic, focal lesion within the ovary. MRI has greater specificity than ultrasound for diagnosis of endometriomas. However, “shading,” or loss of signal, within an endometrioma is a feature commonly found on MRI.3
Other tests that aid in the diagnosis, but are not definitive, include sedimentation rate and tumor marker CA-125. These are both commonly elevated in patients with endometriosis. Measurement of CA-125 is helpful for identifying patients with infertility and severe endometriosis, who would therefore benefit from early surgical intervention.8
TREATMENT
There is no permanent cure for endometriosis; treatment entails nonsurgical and surgical approaches to symptom resolution. Treatment is directed by the patient’s desire to maintain fertility.
Conservative treatment of pelvic pain with NSAIDs is a common approach. Progestins are also used to treat pelvic pain; they create an acyclic, hypo-estrogenic environment by blocking ovarian estrogen secretion and subsequent endometrial cell proliferation. In addition to alleviating pain, progestins also prevent disease recurrence after surgery.2,13 Options include combination OCPs, levonorgestrel intrauterine devices, medroxyprogesterone acetate, and etonogestrel implants. Combination OCPs and medroxyprogesterone acetate are considered to be firstline treatment.8
Gonadotropin-releasing hormone agonists (GnRH-a), such as leuprolide acetate, and androgenic agents, such as danocrine, are also indicated for relief of pain resulting from biopsy-confirmed endometriosis. Danocrine has been shown to ameliorate pain in up to 92% of patients.3,8 Other unconventional treatment modalities include aromatase inhibitors, selective estrogen receptor modulators, anti-inflammatory agents, and immunomodulators.2 For an outline of the medication choices and their mechanisms of action, see Table 2.

Surgery, or ablation of the implants, is another viable treatment option; it can be performed via laparoscopy or laparotomy. Although the success rate is high, implants recur in 28% of patients 18 months after surgery and in 40% of patients after nine years; 40% to 50% of patients have adhesion recurrence.3
Patients who have concomitant infertility can be treated with advanced reproductive techniques, including intrauterine insemination and ovarian hyperstimulation. The monthly fecundity rate with such techniques is 9% to 18%.3 Laparoscopic surgery with ablation of endometrial implants may increase fertility in patients with endometriosis.8
Hysterectomy and bilateral salpingo-oophorectomy are definitive treatment options reserved for patients with intractable pain and those who do not wish to maintain fertility.3,8 Recurrent symptoms occur in 10% of patients 10 years after hysterectomy with bilateral salpingectomy, compared with 62% of those who have hysterectomy alone.8 Complete surgical removal of endometriomas, and ovary if affected, can reduce risk for epithelial ovarian cancer in the future.2
COMPLICATIONS
Adhesions are a common complication of endometriosis. Ultrasound can be used for diagnosis and to determine whether pelvic organs are fixed (ie, fixed retroverted uterus). MRI may also be used; adhesions appear as “speculated low-signal-intensity stranding that obscures organ interfaces.”3 Other suggestive findings on MRI include posterior displacement of the pelvic organs, elevation of the posterior vaginal fornix, hydrosalpinx, loculated fluid collections, and angulated bowel loops.3
Malignant transformation is rare, affecting fewer than 1% of patients with endometriosis. Most malignancies arise from ovarian endometriosis and can be related to unopposed estrogen therapy; they are typically large and have a solid component. The most common endometriosis-related malignant neoplasm is endometrioid carcinoma, followed by clear-cell carcinoma.3
CONCLUSION
Patients with endometriosis often present with complaints such as dysmenorrhea, deep dyspareunia, and chronic pelvic pain, but surgical and histologic findings indicate that symptom severity does not necessarily equate to disease severity. Definitive diagnosis requires an invasive surgical procedure.
In the absence of a cure, endometriosis treatment focuses on symptom control and improvement in quality of life. Familiarity with the disease process and knowledge of treatment options will help health care providers achieve this goal for patients who experience the potentially life-altering effects of endometriosis.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
1. Janssen EB, Rijkers AC, Hoppenbrouwers K, et al. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum Reprod Update. 2013;19(5):570-582.
2. Vercellini P, Viganò P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014; 10(5):261-275.
3. Woodward PJ, Sohaey R, Mezzetti TP. Endometriosis: radiologic-pathologic correlation. Radiographics. 2001;21(1):193-216.
4. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441-447.
5. Ahn SH, Monsanto SP, Miller C, et al. Pathophysiology and immune dysfunction in endometriosis. BioMed Res Int. 2014;2015:1-12.
6. Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis, and treatment. Drugs. 2001;61(12):1735-1750.
7. Farrell E, Garad R. Clinical update: endometriosis. Aust Nurs J. 2012;20(5):37-39.
8. Mounsey AL, Wilgus A, Slawson DC. Diagnosis and management of endometriosis. Am Fam Physician. 2006;74(4):594-600.
9. Nouri K, Ott J, Krupitz B, et al. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8(85):1-7.
10. Riazi H, Tehranian N, Ziaei S, et al. Clinical diagnosis of pelvic endometriosis: a scoping review. BMC Women’s Health. 2015;15(39):1-12.
11. Acién P, Velasco I. Endometriosis: a disease that remains enigmatic. ISRN Obstet Gynecol. 2013;2013:1-12.
12. American Society for Reproductive Medicine. Endometriosis: a guide for patients. www.conceive.ca/wp-content/uploads/2013/09/ASRM-endometriosis.pdf. Accessed April 19, 2017.
13. Angioni S, Cofelice V, Pontis A, et al. New trends of progestins treatment of endometriosis. Gynecol Endocrinol. 2014; 30(11):769-773.
14. National Institutes of Health. What are the treatments for endometriosis? www.nichd.nih.gov/health/topics/endometri/conditioninfo/Pages/treatment.aspx. Accessed April 19, 2017.
15. Kaunitz AM. Depot medroxyprogesterone acetate for contraception. UpToDate. www.uptodate.com/contents/depot-medroxyprogesterone-acetate-for-contraception. Accessed April 19, 2017.
16. National Collaborating Centre for Women’s and Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London, England: RCOG Press; 2005. www.ncbi.nlm.nih.gov/books/NBK51051/pdf/Bookshelf_NBK51051.pdf. Accessed April 19, 2017.
17. Altintas D, Kokcu A, Tosun M, Kandemir B. Comparison of the effects of cetrorelix, a GnRH antagonist, and leuprolide, a GnRH agonist, on experimental endometriosis. J Obstet Gynaecol Res. 2008;34(6):1014-1019.
Changing gloves before closure cut wound-related cesarean complications
SAN DIEGO – Changing gloves before closure during cesarean deliveries can reduce overall wound morbidity, a study showed.
Glove changing during the study had a number needed to treat (NNT) of 14 to see benefit, no demonstrated risk, and a total cost per procedure of about $5.
Researchers led by Buvana Reddy, MD, an ob.gyn. in group practice in Woodbury, Minn., used a randomized trial design to determine whether donning a fresh pair of gloves makes a difference in the rate of surgical complications during cesarean delivery. .
Just one previous study had shown a reduced wound infection rate when gloves were changed after placental delivery (J Reprod Med. 2004 Jan;49[1]:13-6). The study showed a statistically significant drop in wound infections, but Dr. Scrafford said that the sample size was small, and the point at which glove changing was performed is not very practical in terms of the flow of surgery.
Building on this study, Dr. Scrafford and his colleagues designed a trial that was meant to be larger, incorporated a composite wound outcome score rather than just focusing strictly on infection, and switched the timing of glove change to just before abdominal closure.
The randomized controlled trial included all women who underwent nonemergent cesarean delivery at the investigators’ home facility during a 15-month period. The patient allocation was not known until the presurgical “time-out.” Patients allocated to the control condition did not have any change in operative procedures; the surgical team of patients allocated to the intervention arm changed their outer gloves immediately before peritoneal or fascial closure.
The composite primary outcome measure assessed wound seroma, hematoma, or dehiscence (defined as a separation of at least 1 cm), as well as wound infection and any other wound abnormality. Secondary outcome measures included whether patients had a fever or peri-incisional cellulitis.
A total of 553 patients were randomized. Follow-up was high, so Dr. Scrafford and his colleagues were able to analyze data from a total of 250 patients in the control arm and 236 patients who received the glove-changing intervention.
Patient demographics and comorbidities were similar between groups, as was the surgical time and estimated blood loss. Dr. Scrafford noted that significantly more patients in the glove-changing group than in the control group received presurgical vaginal preparation with antiseptic solution (20.3% vs. 10.5%; P = .001). However, he reported, statistical analysis showed that this difference had no effect on the results.
Overall, 15 (6.4%) of the patients in the glove-changing group met the criteria for wound complication, compared with 34 (13.6%) in the control group (P = .008). Of the individual components of the primary outcome measure, the difference in incidence of skin separation was also significant; separation was experienced by five patients (2.1%) in the glove-changing group, compared with 14 (5.6%) in the control group (P = .01).
The rate of wound infections was numerically lower in the intervention group, compared with the control group (8 vs. 14), but the difference was not statistically significant.
There were no significant differences between the groups when secondary outcomes were analyzed, Dr. Scrafford said.
The lack of block randomization in the study left open the potential for historical bias, Dr. Scrafford said. Since surgeons couldn’t be blinded as to study arm, the potential for observer bias also existed. Still, he said, the study’s strengths included its large size, and the real-world applicability of the broad inclusion criteria and diverse patient population.
The study authors reported no outside sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
SAN DIEGO – Changing gloves before closure during cesarean deliveries can reduce overall wound morbidity, a study showed.
Glove changing during the study had a number needed to treat (NNT) of 14 to see benefit, no demonstrated risk, and a total cost per procedure of about $5.
Researchers led by Buvana Reddy, MD, an ob.gyn. in group practice in Woodbury, Minn., used a randomized trial design to determine whether donning a fresh pair of gloves makes a difference in the rate of surgical complications during cesarean delivery. .
Just one previous study had shown a reduced wound infection rate when gloves were changed after placental delivery (J Reprod Med. 2004 Jan;49[1]:13-6). The study showed a statistically significant drop in wound infections, but Dr. Scrafford said that the sample size was small, and the point at which glove changing was performed is not very practical in terms of the flow of surgery.
Building on this study, Dr. Scrafford and his colleagues designed a trial that was meant to be larger, incorporated a composite wound outcome score rather than just focusing strictly on infection, and switched the timing of glove change to just before abdominal closure.
The randomized controlled trial included all women who underwent nonemergent cesarean delivery at the investigators’ home facility during a 15-month period. The patient allocation was not known until the presurgical “time-out.” Patients allocated to the control condition did not have any change in operative procedures; the surgical team of patients allocated to the intervention arm changed their outer gloves immediately before peritoneal or fascial closure.
The composite primary outcome measure assessed wound seroma, hematoma, or dehiscence (defined as a separation of at least 1 cm), as well as wound infection and any other wound abnormality. Secondary outcome measures included whether patients had a fever or peri-incisional cellulitis.
A total of 553 patients were randomized. Follow-up was high, so Dr. Scrafford and his colleagues were able to analyze data from a total of 250 patients in the control arm and 236 patients who received the glove-changing intervention.
Patient demographics and comorbidities were similar between groups, as was the surgical time and estimated blood loss. Dr. Scrafford noted that significantly more patients in the glove-changing group than in the control group received presurgical vaginal preparation with antiseptic solution (20.3% vs. 10.5%; P = .001). However, he reported, statistical analysis showed that this difference had no effect on the results.
Overall, 15 (6.4%) of the patients in the glove-changing group met the criteria for wound complication, compared with 34 (13.6%) in the control group (P = .008). Of the individual components of the primary outcome measure, the difference in incidence of skin separation was also significant; separation was experienced by five patients (2.1%) in the glove-changing group, compared with 14 (5.6%) in the control group (P = .01).
The rate of wound infections was numerically lower in the intervention group, compared with the control group (8 vs. 14), but the difference was not statistically significant.
There were no significant differences between the groups when secondary outcomes were analyzed, Dr. Scrafford said.
The lack of block randomization in the study left open the potential for historical bias, Dr. Scrafford said. Since surgeons couldn’t be blinded as to study arm, the potential for observer bias also existed. Still, he said, the study’s strengths included its large size, and the real-world applicability of the broad inclusion criteria and diverse patient population.
The study authors reported no outside sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
SAN DIEGO – Changing gloves before closure during cesarean deliveries can reduce overall wound morbidity, a study showed.
Glove changing during the study had a number needed to treat (NNT) of 14 to see benefit, no demonstrated risk, and a total cost per procedure of about $5.
Researchers led by Buvana Reddy, MD, an ob.gyn. in group practice in Woodbury, Minn., used a randomized trial design to determine whether donning a fresh pair of gloves makes a difference in the rate of surgical complications during cesarean delivery. .
Just one previous study had shown a reduced wound infection rate when gloves were changed after placental delivery (J Reprod Med. 2004 Jan;49[1]:13-6). The study showed a statistically significant drop in wound infections, but Dr. Scrafford said that the sample size was small, and the point at which glove changing was performed is not very practical in terms of the flow of surgery.
Building on this study, Dr. Scrafford and his colleagues designed a trial that was meant to be larger, incorporated a composite wound outcome score rather than just focusing strictly on infection, and switched the timing of glove change to just before abdominal closure.
The randomized controlled trial included all women who underwent nonemergent cesarean delivery at the investigators’ home facility during a 15-month period. The patient allocation was not known until the presurgical “time-out.” Patients allocated to the control condition did not have any change in operative procedures; the surgical team of patients allocated to the intervention arm changed their outer gloves immediately before peritoneal or fascial closure.
The composite primary outcome measure assessed wound seroma, hematoma, or dehiscence (defined as a separation of at least 1 cm), as well as wound infection and any other wound abnormality. Secondary outcome measures included whether patients had a fever or peri-incisional cellulitis.
A total of 553 patients were randomized. Follow-up was high, so Dr. Scrafford and his colleagues were able to analyze data from a total of 250 patients in the control arm and 236 patients who received the glove-changing intervention.
Patient demographics and comorbidities were similar between groups, as was the surgical time and estimated blood loss. Dr. Scrafford noted that significantly more patients in the glove-changing group than in the control group received presurgical vaginal preparation with antiseptic solution (20.3% vs. 10.5%; P = .001). However, he reported, statistical analysis showed that this difference had no effect on the results.
Overall, 15 (6.4%) of the patients in the glove-changing group met the criteria for wound complication, compared with 34 (13.6%) in the control group (P = .008). Of the individual components of the primary outcome measure, the difference in incidence of skin separation was also significant; separation was experienced by five patients (2.1%) in the glove-changing group, compared with 14 (5.6%) in the control group (P = .01).
The rate of wound infections was numerically lower in the intervention group, compared with the control group (8 vs. 14), but the difference was not statistically significant.
There were no significant differences between the groups when secondary outcomes were analyzed, Dr. Scrafford said.
The lack of block randomization in the study left open the potential for historical bias, Dr. Scrafford said. Since surgeons couldn’t be blinded as to study arm, the potential for observer bias also existed. Still, he said, the study’s strengths included its large size, and the real-world applicability of the broad inclusion criteria and diverse patient population.
The study authors reported no outside sources of funding and no conflicts of interest.
[email protected]
On Twitter @karioakes
Key clinical point:
Major finding: The number needed to treat to see benefit from glove changing was 14; the cost per procedure was about $5.
Data source: A randomized controlled trial of 553 patients undergoing nonemergent cesarean delivery.
Disclosures: The study authors reported no outside sources of funding and no conflicts of interest.
Breastfeeding among factors that modify neonatal abstinence syndrome
SAN FRANCISCO – The good news of a prospective, multicenter study presented at the Pediatric Academic Societies meeting was that neonates who were breastfed were less likely to require neonatal abstinence syndrome (NAS) treatment and displayed milder symptoms.
The study also identified other risk factors associated with the need for NAS treatment and the severity of NAS.
“These findings are important because many of these risk factors are modifiable. Prenatal care providers strive to provide the best treatments for both the mother and the fetus. Our findings regarding the association of NAS with some maternal drug exposures can be shared with opiate-dependent mothers during general counseling about tobacco and illicit use in pregnancy and in counseling about NAS,” explained Megan Stover, MD, a fellow in maternal-fetal medicine and genetics at Tufts Medical Center in Boston.
The current standard of care for opioid-dependent pregnant women is medication-assisted treatment with either methadone or buprenorphine. The intervention can be effective in curbing continued opioid abuse and preventing relapse. However, for many of their unborn children, the damage has already been done.
The scope of the problem in the United States is staggering. Between 2004 and 2013, there was a fourfold to fivefold increase in the rate of admissions to neonatal intensive care units (NICUs) for NAS. “It has been estimated that, every minute in the United States, one neonate will require treatment for NAS,” said Dr. Stover. The glum reality for the Tufts researchers is that 50%-80% of opiate-exposed infants will require treatment for NAS. The aim is to reduce this rate.
Dr. Stover was part of a study conducted at hospitals on the U.S. East Coast that aimed to clarify factors before and after birth that were associated with NAS. The enrolled mothers had been treated during their third trimester or following admission for birth for opioid dependence or had received an opioid for relief of chronic pain. They had given birth to their child at term.
Neonates who were born prematurely or who had comorbidities judged to be significant were not part of the analyses. Of the 306 neonates included, 52% required treatment for NAS and 48% did not. The two groups were similar in age of the mother and for neonatal characteristics of gestational age, sex, ethnicity, and body measurements at birth. The severity of NAS was gauged in two ways. One was the number of days of treatment required to free the neonates from the opioid-induced symptoms, with less than 10 days indicating mild NAS, greater than 30 days indicating severe NAS, and the intervening days indicating moderate NAS. Severe NAS also was indicated by the use of two or more medications.
There was good news. Neonates were significantly less likely to require NAS treatment if they were breastfed exclusively, compared with formula fed babies (15% vs 67%; P less than .0001). NAS was usually mild in breastfed babies and often severe in formula-fed babies (P less than .002).
“Our findings regarding the favorable outcomes seen with breastfeeding support recent research regarding the influence of nonpharmacologic approaches to the prevention and management of NAS, namely that more soothing environments, like those outside the NICU, may be more optimal settings for infants undergoing surveillance for NAS,” said Dr. Stover.
Neonatal treatment was more prevalent for women whose opioid substitution therapy involved methadone (54% vs 28% of untreated neonates; P less than .0001). When therapy used buprenorphine, 62% of the neonates did not display NAS. The drug used for substitution therapy had no effect on the length of treatment of the neonates.
“Our data regarding methadone exposure [versus buprenorphine] adds to a growing literature surrounding more favorable neonatal effects seen with this opiate maintenance agent over methadone,” commented Dr. Stover.
NAS treatment was more prevalent for mothers who smoked during pregnancy, compared with those who did not (76% vs 42%; P equal to .02), and for maternal use of illicit drugs (50% vs 34%; P equal to .002), with no effect on length of neonatal treatment.
Maternal psychiatric diagnosis was associated with neonatal NAS (P equal to .03), as was prescription benzodiazepine use in the third trimester of pregnancy (P equal to .02). Benzodiazepine use did not influence the length of treatment. However, maternal alprazolam use did, as it was associated with more severe NAS (P less than .001). Use of selective serotonin reuptake inhibitor during pregnancy was also associated with more severe NAS (P equal to 0.01).
The researchers are currently sifting through the genetic data gathered in the study. The goal is to combine the clinical and genetic data to create a risk score that will be used to tailor care before birth and in the early weeks following birth.
The study was sponsored by Tufts Medical Center and was funded by National Institute on Drug Abuse. Dr. Stover reported having no relevant financial disclosures.
SAN FRANCISCO – The good news of a prospective, multicenter study presented at the Pediatric Academic Societies meeting was that neonates who were breastfed were less likely to require neonatal abstinence syndrome (NAS) treatment and displayed milder symptoms.
The study also identified other risk factors associated with the need for NAS treatment and the severity of NAS.
“These findings are important because many of these risk factors are modifiable. Prenatal care providers strive to provide the best treatments for both the mother and the fetus. Our findings regarding the association of NAS with some maternal drug exposures can be shared with opiate-dependent mothers during general counseling about tobacco and illicit use in pregnancy and in counseling about NAS,” explained Megan Stover, MD, a fellow in maternal-fetal medicine and genetics at Tufts Medical Center in Boston.
The current standard of care for opioid-dependent pregnant women is medication-assisted treatment with either methadone or buprenorphine. The intervention can be effective in curbing continued opioid abuse and preventing relapse. However, for many of their unborn children, the damage has already been done.
The scope of the problem in the United States is staggering. Between 2004 and 2013, there was a fourfold to fivefold increase in the rate of admissions to neonatal intensive care units (NICUs) for NAS. “It has been estimated that, every minute in the United States, one neonate will require treatment for NAS,” said Dr. Stover. The glum reality for the Tufts researchers is that 50%-80% of opiate-exposed infants will require treatment for NAS. The aim is to reduce this rate.
Dr. Stover was part of a study conducted at hospitals on the U.S. East Coast that aimed to clarify factors before and after birth that were associated with NAS. The enrolled mothers had been treated during their third trimester or following admission for birth for opioid dependence or had received an opioid for relief of chronic pain. They had given birth to their child at term.
Neonates who were born prematurely or who had comorbidities judged to be significant were not part of the analyses. Of the 306 neonates included, 52% required treatment for NAS and 48% did not. The two groups were similar in age of the mother and for neonatal characteristics of gestational age, sex, ethnicity, and body measurements at birth. The severity of NAS was gauged in two ways. One was the number of days of treatment required to free the neonates from the opioid-induced symptoms, with less than 10 days indicating mild NAS, greater than 30 days indicating severe NAS, and the intervening days indicating moderate NAS. Severe NAS also was indicated by the use of two or more medications.
There was good news. Neonates were significantly less likely to require NAS treatment if they were breastfed exclusively, compared with formula fed babies (15% vs 67%; P less than .0001). NAS was usually mild in breastfed babies and often severe in formula-fed babies (P less than .002).
“Our findings regarding the favorable outcomes seen with breastfeeding support recent research regarding the influence of nonpharmacologic approaches to the prevention and management of NAS, namely that more soothing environments, like those outside the NICU, may be more optimal settings for infants undergoing surveillance for NAS,” said Dr. Stover.
Neonatal treatment was more prevalent for women whose opioid substitution therapy involved methadone (54% vs 28% of untreated neonates; P less than .0001). When therapy used buprenorphine, 62% of the neonates did not display NAS. The drug used for substitution therapy had no effect on the length of treatment of the neonates.
“Our data regarding methadone exposure [versus buprenorphine] adds to a growing literature surrounding more favorable neonatal effects seen with this opiate maintenance agent over methadone,” commented Dr. Stover.
NAS treatment was more prevalent for mothers who smoked during pregnancy, compared with those who did not (76% vs 42%; P equal to .02), and for maternal use of illicit drugs (50% vs 34%; P equal to .002), with no effect on length of neonatal treatment.
Maternal psychiatric diagnosis was associated with neonatal NAS (P equal to .03), as was prescription benzodiazepine use in the third trimester of pregnancy (P equal to .02). Benzodiazepine use did not influence the length of treatment. However, maternal alprazolam use did, as it was associated with more severe NAS (P less than .001). Use of selective serotonin reuptake inhibitor during pregnancy was also associated with more severe NAS (P equal to 0.01).
The researchers are currently sifting through the genetic data gathered in the study. The goal is to combine the clinical and genetic data to create a risk score that will be used to tailor care before birth and in the early weeks following birth.
The study was sponsored by Tufts Medical Center and was funded by National Institute on Drug Abuse. Dr. Stover reported having no relevant financial disclosures.
SAN FRANCISCO – The good news of a prospective, multicenter study presented at the Pediatric Academic Societies meeting was that neonates who were breastfed were less likely to require neonatal abstinence syndrome (NAS) treatment and displayed milder symptoms.
The study also identified other risk factors associated with the need for NAS treatment and the severity of NAS.
“These findings are important because many of these risk factors are modifiable. Prenatal care providers strive to provide the best treatments for both the mother and the fetus. Our findings regarding the association of NAS with some maternal drug exposures can be shared with opiate-dependent mothers during general counseling about tobacco and illicit use in pregnancy and in counseling about NAS,” explained Megan Stover, MD, a fellow in maternal-fetal medicine and genetics at Tufts Medical Center in Boston.
The current standard of care for opioid-dependent pregnant women is medication-assisted treatment with either methadone or buprenorphine. The intervention can be effective in curbing continued opioid abuse and preventing relapse. However, for many of their unborn children, the damage has already been done.
The scope of the problem in the United States is staggering. Between 2004 and 2013, there was a fourfold to fivefold increase in the rate of admissions to neonatal intensive care units (NICUs) for NAS. “It has been estimated that, every minute in the United States, one neonate will require treatment for NAS,” said Dr. Stover. The glum reality for the Tufts researchers is that 50%-80% of opiate-exposed infants will require treatment for NAS. The aim is to reduce this rate.
Dr. Stover was part of a study conducted at hospitals on the U.S. East Coast that aimed to clarify factors before and after birth that were associated with NAS. The enrolled mothers had been treated during their third trimester or following admission for birth for opioid dependence or had received an opioid for relief of chronic pain. They had given birth to their child at term.
Neonates who were born prematurely or who had comorbidities judged to be significant were not part of the analyses. Of the 306 neonates included, 52% required treatment for NAS and 48% did not. The two groups were similar in age of the mother and for neonatal characteristics of gestational age, sex, ethnicity, and body measurements at birth. The severity of NAS was gauged in two ways. One was the number of days of treatment required to free the neonates from the opioid-induced symptoms, with less than 10 days indicating mild NAS, greater than 30 days indicating severe NAS, and the intervening days indicating moderate NAS. Severe NAS also was indicated by the use of two or more medications.
There was good news. Neonates were significantly less likely to require NAS treatment if they were breastfed exclusively, compared with formula fed babies (15% vs 67%; P less than .0001). NAS was usually mild in breastfed babies and often severe in formula-fed babies (P less than .002).
“Our findings regarding the favorable outcomes seen with breastfeeding support recent research regarding the influence of nonpharmacologic approaches to the prevention and management of NAS, namely that more soothing environments, like those outside the NICU, may be more optimal settings for infants undergoing surveillance for NAS,” said Dr. Stover.
Neonatal treatment was more prevalent for women whose opioid substitution therapy involved methadone (54% vs 28% of untreated neonates; P less than .0001). When therapy used buprenorphine, 62% of the neonates did not display NAS. The drug used for substitution therapy had no effect on the length of treatment of the neonates.
“Our data regarding methadone exposure [versus buprenorphine] adds to a growing literature surrounding more favorable neonatal effects seen with this opiate maintenance agent over methadone,” commented Dr. Stover.
NAS treatment was more prevalent for mothers who smoked during pregnancy, compared with those who did not (76% vs 42%; P equal to .02), and for maternal use of illicit drugs (50% vs 34%; P equal to .002), with no effect on length of neonatal treatment.
Maternal psychiatric diagnosis was associated with neonatal NAS (P equal to .03), as was prescription benzodiazepine use in the third trimester of pregnancy (P equal to .02). Benzodiazepine use did not influence the length of treatment. However, maternal alprazolam use did, as it was associated with more severe NAS (P less than .001). Use of selective serotonin reuptake inhibitor during pregnancy was also associated with more severe NAS (P equal to 0.01).
The researchers are currently sifting through the genetic data gathered in the study. The goal is to combine the clinical and genetic data to create a risk score that will be used to tailor care before birth and in the early weeks following birth.
The study was sponsored by Tufts Medical Center and was funded by National Institute on Drug Abuse. Dr. Stover reported having no relevant financial disclosures.
AT PAS 2017
Key clinical point: Clinical factors associated with the need for treatment of neonatal abstinence syndrome and for its severity were identified.
Major finding: Breastfeeding was also associated with less severe NAS (P equal to .0003).
Data source: Prospective cohort analysis as part of a multisite trial.
Disclosures: The study was sponsored by Tufts Medical Center and was funded by the National Institute on Drug Abuse. Dr. Stover reported having no relevant financial disclosures.
Breast milk bacteria seed infant gut microbiome
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
FROM JAMA PEDIATRICS
Key clinical point: Beneficial bacteria in the mother’s breast milk and on the areola seed and maintain the infant gut microbiome.
Major finding: During their first 30 days of life, breastfed infants obtained a mean of 28% of their gut bacteria from breast milk and 10% from the mothers’ areolar skin.
Data source: A prospective longitudinal study involving 107 healthy mother-infant pairs.
Disclosures: This study was supported by the National Institutes of Health and the University of Pennsylvania Center for AIDS Research. Dr. Pannaraj reported ties to AstraZeneca and Pfizer.
Weight loss by obese women linked to perinatal improvements
SAN DIEGO – Among obese women, weight loss, stable weight, or weight gain below Institute of Medicine guidelines may result in more favorable or similar perinatal outcomes, compared with weight gain within IOM guidelines, but not for small for gestational age, results from a retrospective cohort study showed.
“This study adds to the limited body of evidence regarding associations of low weight gain and perinatal outcomes among obese women,” Emilia G. Wilkins, MD, the lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “This is the first study that uses data collected after the 2009 IOM guidelines were published. Although there have been some studies published after that time, they used older cohorts. We have a large, diverse cohort and had access to robust electronic health records, which allowed for inclusion of multiple relevant covariates.”
Dr. Wilkins, a fourth-year ob.gyn. resident at Kaiser Permanente in Oakland, Calif., reported that 59% of obese women gained weight above the IOM guidelines and that gestational weight gain above the IOM guidelines was consistently associated with worse perinatal outcomes.
Among class III obese women (BMI of 40 or greater), gestational weight gain below –2 kg, compared with gestational weight gain within IOM guidelines, was associated with lower odds of large-for-gestational-age infants (OR, 0.4; 95% CI, 0.3–0.7), preeclampsia/eclampsia (OR, 0.5; 95% CI, 0.3–0.9), cesarean section (OR, 0.5; 95% CI, 0.4–0.7), NICU admission (OR, 0.7; 95% CI, 0.5–1.1), and length of stay greater than 3 days (OR, 0.5; 95% CI, 0.4–0.8), but higher odds of small for gestational age (OR, 2.61; 95% CI, 1.11–6.20). Findings were similar for other obesity classes.
“It is surprising that the protective associations for weight loss were so strong,” Dr. Wilkins said. “At least 50% lower odds of adverse outcomes [were seen], including large for gestational age, cesarean delivery, preeclampsia/eclampsia, and extended neonatal hospital stay – all of which are clinically significant and could have a major implications for maternal and child health. The degree of increased odds (2- to 2.7-fold higher) of small for gestational age was also a surprising finding and indicates the need to further assess whether the health of the small-for-gestational-age babies differed across the gestational weight gain categories.”
One surprise was that, although the weight loss group had higher odds of delivering a small for gestational age infant, there was no association with increased NICU admission or increased neonatal hospital stay,” Dr. Wilkins added.
She acknowledged certain limitations of the study, including the fact that approximately 10% of the prepregnancy weights were self-measured. Additionally, there was low statistical power to evaluate NICU and length of stay data, and maternal conditions associated with prior weight loss such as bariatric surgery were not evaluated.
Dr. Wilkins reported having no financial disclosures.
SAN DIEGO – Among obese women, weight loss, stable weight, or weight gain below Institute of Medicine guidelines may result in more favorable or similar perinatal outcomes, compared with weight gain within IOM guidelines, but not for small for gestational age, results from a retrospective cohort study showed.
“This study adds to the limited body of evidence regarding associations of low weight gain and perinatal outcomes among obese women,” Emilia G. Wilkins, MD, the lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “This is the first study that uses data collected after the 2009 IOM guidelines were published. Although there have been some studies published after that time, they used older cohorts. We have a large, diverse cohort and had access to robust electronic health records, which allowed for inclusion of multiple relevant covariates.”
Dr. Wilkins, a fourth-year ob.gyn. resident at Kaiser Permanente in Oakland, Calif., reported that 59% of obese women gained weight above the IOM guidelines and that gestational weight gain above the IOM guidelines was consistently associated with worse perinatal outcomes.
Among class III obese women (BMI of 40 or greater), gestational weight gain below –2 kg, compared with gestational weight gain within IOM guidelines, was associated with lower odds of large-for-gestational-age infants (OR, 0.4; 95% CI, 0.3–0.7), preeclampsia/eclampsia (OR, 0.5; 95% CI, 0.3–0.9), cesarean section (OR, 0.5; 95% CI, 0.4–0.7), NICU admission (OR, 0.7; 95% CI, 0.5–1.1), and length of stay greater than 3 days (OR, 0.5; 95% CI, 0.4–0.8), but higher odds of small for gestational age (OR, 2.61; 95% CI, 1.11–6.20). Findings were similar for other obesity classes.
“It is surprising that the protective associations for weight loss were so strong,” Dr. Wilkins said. “At least 50% lower odds of adverse outcomes [were seen], including large for gestational age, cesarean delivery, preeclampsia/eclampsia, and extended neonatal hospital stay – all of which are clinically significant and could have a major implications for maternal and child health. The degree of increased odds (2- to 2.7-fold higher) of small for gestational age was also a surprising finding and indicates the need to further assess whether the health of the small-for-gestational-age babies differed across the gestational weight gain categories.”
One surprise was that, although the weight loss group had higher odds of delivering a small for gestational age infant, there was no association with increased NICU admission or increased neonatal hospital stay,” Dr. Wilkins added.
She acknowledged certain limitations of the study, including the fact that approximately 10% of the prepregnancy weights were self-measured. Additionally, there was low statistical power to evaluate NICU and length of stay data, and maternal conditions associated with prior weight loss such as bariatric surgery were not evaluated.
Dr. Wilkins reported having no financial disclosures.
SAN DIEGO – Among obese women, weight loss, stable weight, or weight gain below Institute of Medicine guidelines may result in more favorable or similar perinatal outcomes, compared with weight gain within IOM guidelines, but not for small for gestational age, results from a retrospective cohort study showed.
“This study adds to the limited body of evidence regarding associations of low weight gain and perinatal outcomes among obese women,” Emilia G. Wilkins, MD, the lead study author, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. “This is the first study that uses data collected after the 2009 IOM guidelines were published. Although there have been some studies published after that time, they used older cohorts. We have a large, diverse cohort and had access to robust electronic health records, which allowed for inclusion of multiple relevant covariates.”
Dr. Wilkins, a fourth-year ob.gyn. resident at Kaiser Permanente in Oakland, Calif., reported that 59% of obese women gained weight above the IOM guidelines and that gestational weight gain above the IOM guidelines was consistently associated with worse perinatal outcomes.
Among class III obese women (BMI of 40 or greater), gestational weight gain below –2 kg, compared with gestational weight gain within IOM guidelines, was associated with lower odds of large-for-gestational-age infants (OR, 0.4; 95% CI, 0.3–0.7), preeclampsia/eclampsia (OR, 0.5; 95% CI, 0.3–0.9), cesarean section (OR, 0.5; 95% CI, 0.4–0.7), NICU admission (OR, 0.7; 95% CI, 0.5–1.1), and length of stay greater than 3 days (OR, 0.5; 95% CI, 0.4–0.8), but higher odds of small for gestational age (OR, 2.61; 95% CI, 1.11–6.20). Findings were similar for other obesity classes.
“It is surprising that the protective associations for weight loss were so strong,” Dr. Wilkins said. “At least 50% lower odds of adverse outcomes [were seen], including large for gestational age, cesarean delivery, preeclampsia/eclampsia, and extended neonatal hospital stay – all of which are clinically significant and could have a major implications for maternal and child health. The degree of increased odds (2- to 2.7-fold higher) of small for gestational age was also a surprising finding and indicates the need to further assess whether the health of the small-for-gestational-age babies differed across the gestational weight gain categories.”
One surprise was that, although the weight loss group had higher odds of delivering a small for gestational age infant, there was no association with increased NICU admission or increased neonatal hospital stay,” Dr. Wilkins added.
She acknowledged certain limitations of the study, including the fact that approximately 10% of the prepregnancy weights were self-measured. Additionally, there was low statistical power to evaluate NICU and length of stay data, and maternal conditions associated with prior weight loss such as bariatric surgery were not evaluated.
Dr. Wilkins reported having no financial disclosures.
AT ACOG 2017
Key clinical point:
Major finding: Among class III obese women, gestational weight gain below –2 kg, compared with gestational weight gain within IOM guidelines, was associated with lower odds of large-for-gestational-age infants (OR, 0.4), preeclampsia/eclampsia (OR, 0.5), cesarean section (OR, 0.5), NICU admission (OR, 0.7), and length of stay greater than 3 days (OR, 0.5), but higher odds of small for gestational age (OR, 2.61).
Data source: A retrospective cohort analysis of 19,810 obese women who delivered singleton, live births greater than 35 weeks between 2009 and 2012.
Disclosures: Dr. Wilkins reported having no financial disclosures.
Vulnerable patients face higher risk of 30-day postpartum readmission
SAN DIEGO – Vulnerable patients experienced higher 30-day postpartum readmission rates, regardless of site of care, results from a large national analysis demonstrated.
“Although childbirth is the most common indication for inpatient admission in the United States – accounting for up to 4 million inpatient stays per year – national 30-day postpartum readmission rates, reasons for readmissions, and variation in readmission rates according to patient clinical and demographic characteristics on a national scale remain unknown,” Anju Ranjit, MD, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Ranjit, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston, and her associates used multivariable logistic regression to compare differences in 30-day readmissions among 499,578 patients treated at hospitals serving a higher proportion of vulnerable patients (safety net hospitals) with the 30-day readmissions among 877,325 patients treated at hospitals serving a higher proportion of nonvulnerable patients (non–safety net hospitals). Models were adjusted for differences in patient demographics, comorbidities, pregnancy risk-status, type of deliveries, and hospital characteristics.
The researchers included 1,374,903 deliveries in the analysis, which were weighted to represent 3,113,047 deliveries nationwide. The national 30-day postpartum readmission rate was 1.4/100 deliveries. The top three reasons for readmission were wound infection (20.8%), infection (7.7%), and hypertension-related complications (6.2%).
Dr. Ranjit reported that in 2013, a total of 724,833 (53.3%) deliveries were to vulnerable patients and the remaining 652,070 (47.4%) to nonvulnerable patients. The rate of 30-day postpartum readmissions were higher among vulnerable patients, compared with their nonvulnerable counterparts (1.52% vs. 1.18%, respectively; adjusted odds ratio, 1.31; confidence interval, 1.26-1.37) and among patients who were treated at hospitals managing a higher proportion of vulnerable patients (1.51% vs. 1.27%; adjusted OR 1.19; CI, 1.12-1.27).*
“Higher readmission rates seen among vulnerable patients across institutions speak to the need to address disparities in perinatal care,” Dr. Ranjit said. “Quality improvement interventions targeted at safety net hospitals, where the majority of vulnerable patients seek care, could be helpful in reducing readmission rates among vulnerable populations.”
She acknowledged certain limitations of study, including the use of administrative data that is subject to coding error and that lacks clinical granularity.
“We were unable to control for patient’s race and region of residence due of lack of information,” she added. “Classification of hospitals into ‘safety net’ and ‘non–safety net’ hospitals was done – based on the proportion of vulnerable patients served, as identified in the national readmissions database – for the purpose of this study only.”
Dr. Ranjit reported having no financial disclosures.
* Correction, 05/08/17: An earlier version of this article incorrectly described the patient populations.
[email protected]
SAN DIEGO – Vulnerable patients experienced higher 30-day postpartum readmission rates, regardless of site of care, results from a large national analysis demonstrated.
“Although childbirth is the most common indication for inpatient admission in the United States – accounting for up to 4 million inpatient stays per year – national 30-day postpartum readmission rates, reasons for readmissions, and variation in readmission rates according to patient clinical and demographic characteristics on a national scale remain unknown,” Anju Ranjit, MD, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Ranjit, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston, and her associates used multivariable logistic regression to compare differences in 30-day readmissions among 499,578 patients treated at hospitals serving a higher proportion of vulnerable patients (safety net hospitals) with the 30-day readmissions among 877,325 patients treated at hospitals serving a higher proportion of nonvulnerable patients (non–safety net hospitals). Models were adjusted for differences in patient demographics, comorbidities, pregnancy risk-status, type of deliveries, and hospital characteristics.
The researchers included 1,374,903 deliveries in the analysis, which were weighted to represent 3,113,047 deliveries nationwide. The national 30-day postpartum readmission rate was 1.4/100 deliveries. The top three reasons for readmission were wound infection (20.8%), infection (7.7%), and hypertension-related complications (6.2%).
Dr. Ranjit reported that in 2013, a total of 724,833 (53.3%) deliveries were to vulnerable patients and the remaining 652,070 (47.4%) to nonvulnerable patients. The rate of 30-day postpartum readmissions were higher among vulnerable patients, compared with their nonvulnerable counterparts (1.52% vs. 1.18%, respectively; adjusted odds ratio, 1.31; confidence interval, 1.26-1.37) and among patients who were treated at hospitals managing a higher proportion of vulnerable patients (1.51% vs. 1.27%; adjusted OR 1.19; CI, 1.12-1.27).*
“Higher readmission rates seen among vulnerable patients across institutions speak to the need to address disparities in perinatal care,” Dr. Ranjit said. “Quality improvement interventions targeted at safety net hospitals, where the majority of vulnerable patients seek care, could be helpful in reducing readmission rates among vulnerable populations.”
She acknowledged certain limitations of study, including the use of administrative data that is subject to coding error and that lacks clinical granularity.
“We were unable to control for patient’s race and region of residence due of lack of information,” she added. “Classification of hospitals into ‘safety net’ and ‘non–safety net’ hospitals was done – based on the proportion of vulnerable patients served, as identified in the national readmissions database – for the purpose of this study only.”
Dr. Ranjit reported having no financial disclosures.
* Correction, 05/08/17: An earlier version of this article incorrectly described the patient populations.
[email protected]
SAN DIEGO – Vulnerable patients experienced higher 30-day postpartum readmission rates, regardless of site of care, results from a large national analysis demonstrated.
“Although childbirth is the most common indication for inpatient admission in the United States – accounting for up to 4 million inpatient stays per year – national 30-day postpartum readmission rates, reasons for readmissions, and variation in readmission rates according to patient clinical and demographic characteristics on a national scale remain unknown,” Anju Ranjit, MD, said in an interview prior to the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Ranjit, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston, and her associates used multivariable logistic regression to compare differences in 30-day readmissions among 499,578 patients treated at hospitals serving a higher proportion of vulnerable patients (safety net hospitals) with the 30-day readmissions among 877,325 patients treated at hospitals serving a higher proportion of nonvulnerable patients (non–safety net hospitals). Models were adjusted for differences in patient demographics, comorbidities, pregnancy risk-status, type of deliveries, and hospital characteristics.
The researchers included 1,374,903 deliveries in the analysis, which were weighted to represent 3,113,047 deliveries nationwide. The national 30-day postpartum readmission rate was 1.4/100 deliveries. The top three reasons for readmission were wound infection (20.8%), infection (7.7%), and hypertension-related complications (6.2%).
Dr. Ranjit reported that in 2013, a total of 724,833 (53.3%) deliveries were to vulnerable patients and the remaining 652,070 (47.4%) to nonvulnerable patients. The rate of 30-day postpartum readmissions were higher among vulnerable patients, compared with their nonvulnerable counterparts (1.52% vs. 1.18%, respectively; adjusted odds ratio, 1.31; confidence interval, 1.26-1.37) and among patients who were treated at hospitals managing a higher proportion of vulnerable patients (1.51% vs. 1.27%; adjusted OR 1.19; CI, 1.12-1.27).*
“Higher readmission rates seen among vulnerable patients across institutions speak to the need to address disparities in perinatal care,” Dr. Ranjit said. “Quality improvement interventions targeted at safety net hospitals, where the majority of vulnerable patients seek care, could be helpful in reducing readmission rates among vulnerable populations.”
She acknowledged certain limitations of study, including the use of administrative data that is subject to coding error and that lacks clinical granularity.
“We were unable to control for patient’s race and region of residence due of lack of information,” she added. “Classification of hospitals into ‘safety net’ and ‘non–safety net’ hospitals was done – based on the proportion of vulnerable patients served, as identified in the national readmissions database – for the purpose of this study only.”
Dr. Ranjit reported having no financial disclosures.
* Correction, 05/08/17: An earlier version of this article incorrectly described the patient populations.
[email protected]
AT ACOG 2017
Key clinical point:
Major finding: Vulnerable patients and safety net hospitals had higher rates and adjusted odds of readmission within 30 postdischarge days after delivery.
Data source: An analysis of the Nationwide Readmissions Database to identify deliveries during January 2013-October 2013.
Disclosures: Dr. Ranjit reported having no financial disclosures.
Lower limb compression halved epidural-associated hypotension
SAN DIEGO – Use of sequential compression devices reduced by half cases of epidural-associated hypotension in laboring women in a small real-world, randomized controlled trial.
The results showed that patients who received sequential compression devices (SCD) and kept them on for at least an hour after epidural analgesia placement had half the hypotension of patients who had no lower limb compression (33.3% vs. 66.7%; P .022).
“Lower limb compression using SCDs significantly decreased the incidence of maternal hypotension in laboring patients receiving epidural anesthesia,” said Margaret Steinmetz, MD, a third-year resident at the State University of New York at Buffalo.
The study, which was presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, was a multisite, randomized controlled trial that used a randomized block design to assign women to three groups. The control group received no intervention; the remainder of patients received either thromboembolic deterrent (TED) stockings or sequential compression devices (SCDs) set to intermittent compression and applied before receiving epidural anesthesia.
The facilities’ usual protocols were followed both for epidural placement and for patient management, except for the lower limb compression and the study’s timed blood pressure checks.
Pregnant women who were at term and had requested epidural anesthesia were included if they had a singleton pregnancy; no history of hypertension, cardiovascular disease; and no contraindication to lower limb compression.
Hypotension – defined as at least one decrease in either systolic or diastolic blood pressure of more than 20% from baseline – was tracked by obtaining sequential blood pressure readings. A baseline was established with an average of three readings obtained before epidural placement. Following the epidural bolus, blood pressures were measured at minutes 1, 5, 15, 30, 45, and 60.
The investigators used an intention-to-treat analysis, meaning that they included patients allocated to each group whether or not they actually received lower limb compression. Patients with missing data were excluded.
A total of 82 patients were randomized:
- 28 to the control arm (no lower limb compression); 1 had missing data.
- 26 to receive TEDs (6 of whom did not don the stockings); 5 were excluded from analysis because of missing data or no epidural placement.
- 28 patients to receive SCDs (8 of whom did not have SCDs applied); 5 were excluded from analysis because of missing data or no epidural placement.
While the SCDs cut the incidence of hypotension in half, compared with no compression, women in the TEDs group saw an intermediate result, with a 52.4% incidence of hypotension.
Dr. Steinmetz noted that knee-high TEDs were used. The choice was made in part because the labor and delivery nursing staff were not enthusiastic about the prospect of placing thigh-high TEDs on a woman in labor, she added.
Patient age, mean body mass index, and gestational age did not differ significantly between the study arms. Logistic regression analysis performed to control for clinical site, method of delivery, gestational age, and maternal age and body mass index did not affect the analysis, Dr. Steinmetz added.
Older data showed that about 30% of women getting epidurals in labor experience hypotension, though Dr. Steinmetz said that she believes that the 66.7% seen in this study is probably closer to an accurate estimate.
SUNY Buffalo is looking at changing the labor and delivery protocol to include lower limb compression with epidurals, Dr. Steinmetz said, adding “When I’m on labor and delivery, I definitely encourage the placement of SCDs.”
Some facilities also use lower limb compression to reduce hypotension when patients receive regional anesthesia for cesarean deliveries. Dr. Steinmetz said that there are studies that support that practice, but the literature is not conclusive. Still, it makes sense in this setting too, she said. “C-section patients sit up, they get their spinal, then we lay them down and put in a Foley, and they’re vomiting as we put in the Foley because they’re hypotensive from that spinal. ... For me, myself, when I go into practice in 2 months, yes; I will be wanting to do this.”
Dr. Steinmetz reported no relevant disclosures.
[email protected]
On Twitter @karioakes
SAN DIEGO – Use of sequential compression devices reduced by half cases of epidural-associated hypotension in laboring women in a small real-world, randomized controlled trial.
The results showed that patients who received sequential compression devices (SCD) and kept them on for at least an hour after epidural analgesia placement had half the hypotension of patients who had no lower limb compression (33.3% vs. 66.7%; P .022).
“Lower limb compression using SCDs significantly decreased the incidence of maternal hypotension in laboring patients receiving epidural anesthesia,” said Margaret Steinmetz, MD, a third-year resident at the State University of New York at Buffalo.
The study, which was presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, was a multisite, randomized controlled trial that used a randomized block design to assign women to three groups. The control group received no intervention; the remainder of patients received either thromboembolic deterrent (TED) stockings or sequential compression devices (SCDs) set to intermittent compression and applied before receiving epidural anesthesia.
The facilities’ usual protocols were followed both for epidural placement and for patient management, except for the lower limb compression and the study’s timed blood pressure checks.
Pregnant women who were at term and had requested epidural anesthesia were included if they had a singleton pregnancy; no history of hypertension, cardiovascular disease; and no contraindication to lower limb compression.
Hypotension – defined as at least one decrease in either systolic or diastolic blood pressure of more than 20% from baseline – was tracked by obtaining sequential blood pressure readings. A baseline was established with an average of three readings obtained before epidural placement. Following the epidural bolus, blood pressures were measured at minutes 1, 5, 15, 30, 45, and 60.
The investigators used an intention-to-treat analysis, meaning that they included patients allocated to each group whether or not they actually received lower limb compression. Patients with missing data were excluded.
A total of 82 patients were randomized:
- 28 to the control arm (no lower limb compression); 1 had missing data.
- 26 to receive TEDs (6 of whom did not don the stockings); 5 were excluded from analysis because of missing data or no epidural placement.
- 28 patients to receive SCDs (8 of whom did not have SCDs applied); 5 were excluded from analysis because of missing data or no epidural placement.
While the SCDs cut the incidence of hypotension in half, compared with no compression, women in the TEDs group saw an intermediate result, with a 52.4% incidence of hypotension.
Dr. Steinmetz noted that knee-high TEDs were used. The choice was made in part because the labor and delivery nursing staff were not enthusiastic about the prospect of placing thigh-high TEDs on a woman in labor, she added.
Patient age, mean body mass index, and gestational age did not differ significantly between the study arms. Logistic regression analysis performed to control for clinical site, method of delivery, gestational age, and maternal age and body mass index did not affect the analysis, Dr. Steinmetz added.
Older data showed that about 30% of women getting epidurals in labor experience hypotension, though Dr. Steinmetz said that she believes that the 66.7% seen in this study is probably closer to an accurate estimate.
SUNY Buffalo is looking at changing the labor and delivery protocol to include lower limb compression with epidurals, Dr. Steinmetz said, adding “When I’m on labor and delivery, I definitely encourage the placement of SCDs.”
Some facilities also use lower limb compression to reduce hypotension when patients receive regional anesthesia for cesarean deliveries. Dr. Steinmetz said that there are studies that support that practice, but the literature is not conclusive. Still, it makes sense in this setting too, she said. “C-section patients sit up, they get their spinal, then we lay them down and put in a Foley, and they’re vomiting as we put in the Foley because they’re hypotensive from that spinal. ... For me, myself, when I go into practice in 2 months, yes; I will be wanting to do this.”
Dr. Steinmetz reported no relevant disclosures.
[email protected]
On Twitter @karioakes
SAN DIEGO – Use of sequential compression devices reduced by half cases of epidural-associated hypotension in laboring women in a small real-world, randomized controlled trial.
The results showed that patients who received sequential compression devices (SCD) and kept them on for at least an hour after epidural analgesia placement had half the hypotension of patients who had no lower limb compression (33.3% vs. 66.7%; P .022).
“Lower limb compression using SCDs significantly decreased the incidence of maternal hypotension in laboring patients receiving epidural anesthesia,” said Margaret Steinmetz, MD, a third-year resident at the State University of New York at Buffalo.
The study, which was presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, was a multisite, randomized controlled trial that used a randomized block design to assign women to three groups. The control group received no intervention; the remainder of patients received either thromboembolic deterrent (TED) stockings or sequential compression devices (SCDs) set to intermittent compression and applied before receiving epidural anesthesia.
The facilities’ usual protocols were followed both for epidural placement and for patient management, except for the lower limb compression and the study’s timed blood pressure checks.
Pregnant women who were at term and had requested epidural anesthesia were included if they had a singleton pregnancy; no history of hypertension, cardiovascular disease; and no contraindication to lower limb compression.
Hypotension – defined as at least one decrease in either systolic or diastolic blood pressure of more than 20% from baseline – was tracked by obtaining sequential blood pressure readings. A baseline was established with an average of three readings obtained before epidural placement. Following the epidural bolus, blood pressures were measured at minutes 1, 5, 15, 30, 45, and 60.
The investigators used an intention-to-treat analysis, meaning that they included patients allocated to each group whether or not they actually received lower limb compression. Patients with missing data were excluded.
A total of 82 patients were randomized:
- 28 to the control arm (no lower limb compression); 1 had missing data.
- 26 to receive TEDs (6 of whom did not don the stockings); 5 were excluded from analysis because of missing data or no epidural placement.
- 28 patients to receive SCDs (8 of whom did not have SCDs applied); 5 were excluded from analysis because of missing data or no epidural placement.
While the SCDs cut the incidence of hypotension in half, compared with no compression, women in the TEDs group saw an intermediate result, with a 52.4% incidence of hypotension.
Dr. Steinmetz noted that knee-high TEDs were used. The choice was made in part because the labor and delivery nursing staff were not enthusiastic about the prospect of placing thigh-high TEDs on a woman in labor, she added.
Patient age, mean body mass index, and gestational age did not differ significantly between the study arms. Logistic regression analysis performed to control for clinical site, method of delivery, gestational age, and maternal age and body mass index did not affect the analysis, Dr. Steinmetz added.
Older data showed that about 30% of women getting epidurals in labor experience hypotension, though Dr. Steinmetz said that she believes that the 66.7% seen in this study is probably closer to an accurate estimate.
SUNY Buffalo is looking at changing the labor and delivery protocol to include lower limb compression with epidurals, Dr. Steinmetz said, adding “When I’m on labor and delivery, I definitely encourage the placement of SCDs.”
Some facilities also use lower limb compression to reduce hypotension when patients receive regional anesthesia for cesarean deliveries. Dr. Steinmetz said that there are studies that support that practice, but the literature is not conclusive. Still, it makes sense in this setting too, she said. “C-section patients sit up, they get their spinal, then we lay them down and put in a Foley, and they’re vomiting as we put in the Foley because they’re hypotensive from that spinal. ... For me, myself, when I go into practice in 2 months, yes; I will be wanting to do this.”
Dr. Steinmetz reported no relevant disclosures.
[email protected]
On Twitter @karioakes
AT ACOG 2017
Key clinical point:
Major finding: The rate of hypotension for women in labor who received epidurals was 66.7% with no lower limb compression, compared with 33.3% when sequential compression devices were used (P –.02).
Data source: Multisite randomized controlled trial of 82 patients who received epidurals while in labor.
Disclosures: The study authors reported no outside sources of funding, and reported no conflicts of interest.