User login
Periconception smoking found to affect birth defect risk
SAN DIEGO – Smoking during the period of fetal organogenesis, during the first trimester of pregnancy, is associated with increased risk of some birth defects, results from a large retrospective analysis demonstrated.
Ms. Perry, a second-year medical student at the University of Cincinnati and her associates conducted a population-based retrospective cohort analysis of 1,436,036 live births in Ohio during 2006-2015. They compared the rates of major defects between births to nonsmoking mothers and those who smoked only during the 3-month preconception period and not in the first trimester; and in the preconception period plus throughout the first trimester. They used multivariate logistic regression to quantify the relationship between smoking and birth defects after adjustment for maternal race, age, pregestational diabetes, and socioeconomic factors.
The researchers observed that 23.3% of women smoked during pregnancy; 6.0% during preconception only and 17.3% smoked through the first trimester, as well. Smoking during the preconception period only, even without first trimester exposure, was associated with a 40% increased risk of gastroschisis (adjusted risk ratio, 1.4), but no other individual birth defects. However, smoking through the first trimester was associated with a modest but significantly increased risk of several defects, including gastroschisis (adjusted RR, 1.9), limb reduction (adjusted RR, 1.6), congenital diaphragmatic hernia (adjusted RR, 1.4), and cleft palate (adjusted RR, 1.2), even after adjustment for coexisting factors.
“It was surprising to see that, even when women stop smoking when they find out they are pregnant, and therefore are not smoking during the period of fetal organogenesis, there is still an increased risk of some congenital birth defects to the fetus,” Ms. Perry said. “My hope is that this study serves as a launching point for future research and public health efforts. It’s important to encourage smoking cessation in women of reproductive age, whether pregnant or not. Furthermore, it’s valuable to be able to explain to patients that along with adverse effects to their own health, smoking even before conception poses a risk to the fetus.”
She acknowledged certain limitations of the study, including its observational design. “There could exist unmeasurable influences that we were unable to adjust for,” Ms. Perry said. She reported having no financial disclosures.
SAN DIEGO – Smoking during the period of fetal organogenesis, during the first trimester of pregnancy, is associated with increased risk of some birth defects, results from a large retrospective analysis demonstrated.
Ms. Perry, a second-year medical student at the University of Cincinnati and her associates conducted a population-based retrospective cohort analysis of 1,436,036 live births in Ohio during 2006-2015. They compared the rates of major defects between births to nonsmoking mothers and those who smoked only during the 3-month preconception period and not in the first trimester; and in the preconception period plus throughout the first trimester. They used multivariate logistic regression to quantify the relationship between smoking and birth defects after adjustment for maternal race, age, pregestational diabetes, and socioeconomic factors.
The researchers observed that 23.3% of women smoked during pregnancy; 6.0% during preconception only and 17.3% smoked through the first trimester, as well. Smoking during the preconception period only, even without first trimester exposure, was associated with a 40% increased risk of gastroschisis (adjusted risk ratio, 1.4), but no other individual birth defects. However, smoking through the first trimester was associated with a modest but significantly increased risk of several defects, including gastroschisis (adjusted RR, 1.9), limb reduction (adjusted RR, 1.6), congenital diaphragmatic hernia (adjusted RR, 1.4), and cleft palate (adjusted RR, 1.2), even after adjustment for coexisting factors.
“It was surprising to see that, even when women stop smoking when they find out they are pregnant, and therefore are not smoking during the period of fetal organogenesis, there is still an increased risk of some congenital birth defects to the fetus,” Ms. Perry said. “My hope is that this study serves as a launching point for future research and public health efforts. It’s important to encourage smoking cessation in women of reproductive age, whether pregnant or not. Furthermore, it’s valuable to be able to explain to patients that along with adverse effects to their own health, smoking even before conception poses a risk to the fetus.”
She acknowledged certain limitations of the study, including its observational design. “There could exist unmeasurable influences that we were unable to adjust for,” Ms. Perry said. She reported having no financial disclosures.
SAN DIEGO – Smoking during the period of fetal organogenesis, during the first trimester of pregnancy, is associated with increased risk of some birth defects, results from a large retrospective analysis demonstrated.
Ms. Perry, a second-year medical student at the University of Cincinnati and her associates conducted a population-based retrospective cohort analysis of 1,436,036 live births in Ohio during 2006-2015. They compared the rates of major defects between births to nonsmoking mothers and those who smoked only during the 3-month preconception period and not in the first trimester; and in the preconception period plus throughout the first trimester. They used multivariate logistic regression to quantify the relationship between smoking and birth defects after adjustment for maternal race, age, pregestational diabetes, and socioeconomic factors.
The researchers observed that 23.3% of women smoked during pregnancy; 6.0% during preconception only and 17.3% smoked through the first trimester, as well. Smoking during the preconception period only, even without first trimester exposure, was associated with a 40% increased risk of gastroschisis (adjusted risk ratio, 1.4), but no other individual birth defects. However, smoking through the first trimester was associated with a modest but significantly increased risk of several defects, including gastroschisis (adjusted RR, 1.9), limb reduction (adjusted RR, 1.6), congenital diaphragmatic hernia (adjusted RR, 1.4), and cleft palate (adjusted RR, 1.2), even after adjustment for coexisting factors.
“It was surprising to see that, even when women stop smoking when they find out they are pregnant, and therefore are not smoking during the period of fetal organogenesis, there is still an increased risk of some congenital birth defects to the fetus,” Ms. Perry said. “My hope is that this study serves as a launching point for future research and public health efforts. It’s important to encourage smoking cessation in women of reproductive age, whether pregnant or not. Furthermore, it’s valuable to be able to explain to patients that along with adverse effects to their own health, smoking even before conception poses a risk to the fetus.”
She acknowledged certain limitations of the study, including its observational design. “There could exist unmeasurable influences that we were unable to adjust for,” Ms. Perry said. She reported having no financial disclosures.
AT ACOG 2017
Key clinical point:
Major finding: Smoking during only the preconception period was associated with a 40% increased risk of gastroschisis (adjusted RR, 1.4), while smoking during the first trimester of pregnancy was associated with a significantly increased risk of gastroschisis (adjusted RR, 1.9) and several other birth defects.
Data source: A retrospective cohort analysis of 1,436,036 live births in Ohio during 2006-2015.
Disclosures: Ms. Perry reported having no financial disclosures.
Start offering antenatal corticosteroids to women delivering between 34 0/7 and 36 6/7 weeks of gestation to improve newborn outcomes
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3

A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3

A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Antenatal corticosteroid treat-ment prior to preterm birth is the most important pharmacologic intervention available to obstetricians to improve newborn health. Antenatal corticosteroids reduce preterm newborn morbidity and mortality.1 The American College of Obstetricians and Gynecologists (ACOG) recently has summarized updated recommendations for the use of antenatal steroid treatment.2
ACOG guidance includes:
- “A single course of corticosteroids is recommended for pregnant women between 24 0/7 weeks and 33 6/7 weeks of gestation, including for those with ruptured membranes and multiple gestations.” This guidance is supported by many high-quality trials and meta-analyses.1
- A single course of corticosteroids “may be considered for pregnant women starting at 23 0/7 weeks of gestation who are at risk of preterm delivery within 7 days.”
- “A single repeat course of antenatal corticosteroids should be considered in women who are less than 34 0/7 weeks of gestation who have an imminent risk of preterm delivery within the next 7 days and whose prior course of antenatal corticosteroids was administered more than 14 days previously.” A repeat course of corticosteroids could be considered as early as 7 days from the prior dose.
- No more than 2 courses of antenatal steroids should be administered.
An important new ACOG recommendation is:
- “A single course of betamethasone is recommended for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk of preterm birth within 7 days, and who have not received a previous course of antenatal corticosteroids.”
This recommendation is based, in part, on a high-quality, randomized trial including 2,831 women at high risk for preterm birth between 34 0/7 and 36 6/7 weeks of gestation who were randomly assigned to receive a course of betamethasone or placebo. The newborn and maternal outcomes observed in this study are summarized in the TABLE.3

A few points relevant to the Antenatal Late Preterm Steroids study bear emphasizing. The women enrolled in this trial were at high risk for preterm delivery based on preterm labor with a cervical dilation of ≥3 cm or 75% effacement, spontaneous rupture of the membranes, or a planned late preterm delivery by cesarean or induction. No tocolytics were administered to women in this study, and approximately 40% of the women delivered within 24 hours of entry into the trial and only received 1 dose of corticosteroid or placebo.
Women with multiple gestations, pregestational diabetes, or a prior course of corticosteroids were not included in the trial; therefore, this study cannot guide our clinical practice for these subgroups of women. Of note, betamethasone should not be administered to women in the late preterm who have chorioamnionitis.
Related article:
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The investigators calculated that 35 women would need to be treated to prevent one case of the primary outcome: a composite score of the use of respiratory support. Consequently, 34 fetuses who do not benefit from treatment are exposed in utero to betamethasone. Long-term follow-up of infants born to mothers participating in this study is currently underway.
A recent meta-analysis of 3 trials including 3,200 women at high risk for preterm delivery at 34 0/7 to 36 6/7 weeks of gestation reported that the corticosteroid administration reduced newborn risk for transient tachypnea of the newborn (relative risk [RR], 0.72; 95% confidence interval [CI], 0.56−0.92), severe respiratory distress syndrome (RR, 0.60; 95% CI, 0.33−0.94), and use of surfactant (RR, 0.61; 95% CI, 0.38−0.99).4
The recommendation to offer a single course of betamethasone for pregnant women between 34 0/7 and 36 6/7 weeks of gestation at risk for preterm birth has not been embraced enthusiastically by all obstetricians. Many experts have emphasized that the known risks of late preterm betamethasone, including neonatal hypoglycemia and the unknown long-term risks of treatment, including suboptimal neurodevelopmental, cardiovascular, and metabolic outcomes should dampen enthusiasm for embracing the new ACOG recommendation.5 Experts also emphasize that late preterm newborns are less likely to benefit from antenatal corticosteroid treatment than babies born at less than 34 weeks. Hence, many late preterm newborns will be exposed to a potentially harmful intervention and have only a small chance of benefiting from the treatment.6
Many neonatologists believe that for the newborn, the benefits of maternal corticosteroid treatment outweigh the risks.7–9 In a 30-year follow-up of 534 newborns participating in antenatal corticosteroid trials, treatment had no effect on body size, blood lipids, blood pressure, plasma cortisol, prevalence of diabetes, lung function, history of cardiovascular disease, educational attainment, or socioeconomic status. Corticosteroid treatment was associated with increased insulin secretion in response to a glucose load.10 In this study, the mothers received treatment at a median of 33 weeks of gestation and births occurred at a median of 35 weeks. Hence this study is relevant to the issue of late preterm corticosteroid treatment.
Balancing risks and benefits is complex. Balancing immediate benefits against long-term risks is most challenging. Regarding antenatal steroid use there are many unknowns, including optimal dose, drug formulation, and timing from treatment to delivery. In addition we need more high-quality data delineating the long-term effects of antenatal corticosteroids on childhood and adult health.
Read about 3 options to use in your practice
Consider these 3 options for your practice
As noted, the Antenatal Late Preterm Steroids trial investigators are pursuing long-term follow-up of the children born after maternal treatment with antenatal glucocorticoids. Both ACOG and the Society for Maternal-Fetal Medicine (SMFM)11 recommend administration of antenatal glucocorticoids to women at high risk for late preterm delivery. However, since some experts are concerned that a great number of babies born late preterm will have been exposed to glucocorticoids, whose long-term risks are not well known, with only a few babies having a modest short-term benefit, 3 options could be considered for your clinical practice.
Related article:
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
Option 1
Follow the ACOG and SMFM suggestion that all women with a high risk of late preterm birth be offered antenatal corticosteroids. Counsel the mother and family about the potential risks and benefits and involve them in the decision.
Two alternative options are to limit antenatal corticosteroid treatment to subgroups of late preterm babies most likely to benefit from treatment, those born by cesarean delivery and those born at the earliest gestational ages.
Option 2
Limit the use of antenatal corticosteroids in the late preterm to women who are scheduled for a cesarean delivery for an obstetric indication between 34 0/7 weeks and 36 6/7 weeks of gestation. This approach greatly reduces the number of babies born in the late preterm that will be exposed to antenatal corticosteroids and focuses the treatment on a subset of babies who are certain to be born preterm and most likely to benefit.
Option 3
Limit the use of antenatal corticosteroids to women at high risk for preterm birth whose newborns are most likely to benefit from treatment—women at 34 0/7 to 35 6/7 weeks of gestation. Neonates born in the 34th or 35th week of gestation are at higher risk for morbidity than those born in the 36th week of gestation and are likely to derive the greatest benefit from antenatal corticosteroid treatment.3,12
My advice
Yogi Berra advised, “It is tough to make predictions, especially about the future.” Although ACOG and SMFM have recommended administration of glucocorticoids to women at high risk for late preterm birth, many experts caution that until the long-term effects of antenatal corticosteroids are better characterized we should limit the use of corticosteroids in the late preterm.5,6,13 My prediction is that long-term follow-up studies will not document significant adverse effects of one course of late preterm antenatal glucocorticoid treatment on children. My advice is to start offering antenatal corticosteroids to some women at high risk for late preterm delivery.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;CD004454.
- American College of Obstetricians and Gynecologists' Committee on Obstetrics Practice; Society for Maternal−Fetal Medicine. Committee Opinion No. 677: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2016;128(4):e187−e194.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311−1320.
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
- Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423−430.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications? BJOG. 2016;123(7):1067−1069.
- Dalziel SR, Lim VK, Lambert A, McCarthy D, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Dalziel SR, Rea HH, Walker NK, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61(8):678−683.
- McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE; ACTORDS Study Group. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405−e415.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856−1862.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the later preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215(2):B13−B15.
- Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595.
- Nowik CM, Davies GA, Smith GN. We should proceed with caution when it comes to antenatal corticosteroids after 34 weeks. J Obstet Gynaecol Can. 2018;39(1):49−51.
Safety of corticosteroids in pregnancy: Is it the drug or the disease?
Corticosteroids such as prednisone are relatively frequently administered in pregnancy for their immunosuppressive and anti-inflammatory effects. Treatment may be initiated on a short-term basis for acute conditions. Alternatively, treatment may be more or less ongoing for severe chronic diseases such as asthma or a variety of other autoimmune conditions when disease symptoms do not remit in pregnancy. However, the safety of corticosteroid use with respect to risk of specific birth defects, preterm delivery, and low birth weight has been the subject of debate over some time.
Concerns about the teratogenicity of corticosteroids were raised as early as the 1950s, based on animal studies suggesting an increased risk for oral clefts. The association between corticosteroids and oral clefts has also been observed in some human epidemiologic studies. However, results of these studies have been inconsistent.
Similar to the NBDPS findings, in a large Danish cohort study covering 832,636 live births from 1996 to 2008, exposure to any corticosteroids during the first trimester was not associated with an increased risk for cleft lip or cleft palate. Only those exposed to topical corticosteroids had a higher risk of cleft lip with or without cleft palate (odds ratio, 1.45; 95% CI, 1.03-2.05).3 Another, smaller Danish study covered primiparous births from 1999 to 2009 (n = 83,043). The unadjusted odds of oral clefts following exposure to any corticosteroids (inhaled or oral) in the first trimester was null (OR, 0.4; 95% CI, 0.1-2.8).4
Inconsistencies across these studies, as speculated by authors of the NBDPS analysis, may result from a lack of information on the dose of drug used by the mother, the indication for its use, or any measure of the severity of the underlying maternal disease for which the corticosteroids were prescribed. It is possible that maternal disease or disease activity in and of itself is a direct cause of oral clefts or that corticosteroids are linked to increased risk for clefts through co-occurring other exposures such as smoking, alcohol, or obesity. However, these questions have yet to be answered.
With respect to other birth outcomes, a few disease-specific studies have examined birth weight or intrauterine growth restriction following corticosteroid use. In general, study findings have been reassuring. Among Danish women with Crohn’s disease, corticosteroids were not associated with reduced birth weight after adjusting for gestational age and disease activity (adjusted risk ratio, 1.1; 95% CI, 0.2-5.7).5 In another study of pregnant women with rheumatoid arthritis, birth weight was not associated with prednisone use after adjustment for gestational age at delivery and sex of the newborn.6 In a third cohort study of pregnant women with systemic lupus erythematosus, there was no a significant elevation in odds of intrauterine growth restriction following prednisone use.7
Several disease-specific studies have also examined corticosteroid use and risk of preterm birth. From the Danish cohort of pregnant women with Crohn’s disease, the researchers reported no association between prednisolone and preterm birth after adjustment for covariates. In contrast, in a separate Danish cohort of pregnant women with irritable bowel disease, there was an increased risk of preterm delivery following systemic corticosteroid use, compared with women without disease (adjusted hazard ratio, 6.3; 95% CI, 3.1-12.7).8 However, data were not available to address underlying disease severity as a possible contributing factor. Of note, among women with irritable bowel disease who did not use medication in pregnancy, there was a 50% increase in the risk of preterm birth, compared with women without disease (aHR, 1.5; 95% CI, 1.0-2.4). This suggests that the disease itself contributed to the increased risk of preterm birth.
Currently available data regarding corticosteroid use and adverse birth outcomes are generally reassuring. Recent estimates for oral clefts suggest a low elevation in risk, if any at all. This translates to a very low absolute risk for clefts, which occur in the general population in approximately 1 in 1,000 births. The clinical benefit of adequate treatment in the first trimester for inflammatory or immune-mediated diseases may far outweigh any small and tenuous risks for oral clefts.
With respect to reduced birth weight and preterm delivery, available evidence suggests either no association or that maternal disease and disease severity are driving any increased risks noted for these outcomes. Future studies of pregnancy safety for medications used to treat maternal diseases that themselves are potentially linked to adverse outcomes must incorporate appropriate measures of disease type and disease severity in the study designs.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at [email protected].
References
1. Teratology. 2000 Dec;62(6):385-92.
2. Birth Defects Res A Clin Mol Teratol. 2014 Jun;100(6):499-506.
3. CMAJ. 2011 Apr 19;183(7):796-804.
4. Am J Ther. 2014 Mar-Apr;21(2):73-80.
5. Am J Gastroenterol. 2007 Jul;102(7):1406-13.
6. Arthritis Rheum. 2009 Nov;60(11):3196-206.
7. Lupus. 2010 Dec;19(14):1665-73.
8. PLoS One. 2015 Jun 17;10(6):e0129567.
Corticosteroids such as prednisone are relatively frequently administered in pregnancy for their immunosuppressive and anti-inflammatory effects. Treatment may be initiated on a short-term basis for acute conditions. Alternatively, treatment may be more or less ongoing for severe chronic diseases such as asthma or a variety of other autoimmune conditions when disease symptoms do not remit in pregnancy. However, the safety of corticosteroid use with respect to risk of specific birth defects, preterm delivery, and low birth weight has been the subject of debate over some time.
Concerns about the teratogenicity of corticosteroids were raised as early as the 1950s, based on animal studies suggesting an increased risk for oral clefts. The association between corticosteroids and oral clefts has also been observed in some human epidemiologic studies. However, results of these studies have been inconsistent.
Similar to the NBDPS findings, in a large Danish cohort study covering 832,636 live births from 1996 to 2008, exposure to any corticosteroids during the first trimester was not associated with an increased risk for cleft lip or cleft palate. Only those exposed to topical corticosteroids had a higher risk of cleft lip with or without cleft palate (odds ratio, 1.45; 95% CI, 1.03-2.05).3 Another, smaller Danish study covered primiparous births from 1999 to 2009 (n = 83,043). The unadjusted odds of oral clefts following exposure to any corticosteroids (inhaled or oral) in the first trimester was null (OR, 0.4; 95% CI, 0.1-2.8).4
Inconsistencies across these studies, as speculated by authors of the NBDPS analysis, may result from a lack of information on the dose of drug used by the mother, the indication for its use, or any measure of the severity of the underlying maternal disease for which the corticosteroids were prescribed. It is possible that maternal disease or disease activity in and of itself is a direct cause of oral clefts or that corticosteroids are linked to increased risk for clefts through co-occurring other exposures such as smoking, alcohol, or obesity. However, these questions have yet to be answered.
With respect to other birth outcomes, a few disease-specific studies have examined birth weight or intrauterine growth restriction following corticosteroid use. In general, study findings have been reassuring. Among Danish women with Crohn’s disease, corticosteroids were not associated with reduced birth weight after adjusting for gestational age and disease activity (adjusted risk ratio, 1.1; 95% CI, 0.2-5.7).5 In another study of pregnant women with rheumatoid arthritis, birth weight was not associated with prednisone use after adjustment for gestational age at delivery and sex of the newborn.6 In a third cohort study of pregnant women with systemic lupus erythematosus, there was no a significant elevation in odds of intrauterine growth restriction following prednisone use.7
Several disease-specific studies have also examined corticosteroid use and risk of preterm birth. From the Danish cohort of pregnant women with Crohn’s disease, the researchers reported no association between prednisolone and preterm birth after adjustment for covariates. In contrast, in a separate Danish cohort of pregnant women with irritable bowel disease, there was an increased risk of preterm delivery following systemic corticosteroid use, compared with women without disease (adjusted hazard ratio, 6.3; 95% CI, 3.1-12.7).8 However, data were not available to address underlying disease severity as a possible contributing factor. Of note, among women with irritable bowel disease who did not use medication in pregnancy, there was a 50% increase in the risk of preterm birth, compared with women without disease (aHR, 1.5; 95% CI, 1.0-2.4). This suggests that the disease itself contributed to the increased risk of preterm birth.
Currently available data regarding corticosteroid use and adverse birth outcomes are generally reassuring. Recent estimates for oral clefts suggest a low elevation in risk, if any at all. This translates to a very low absolute risk for clefts, which occur in the general population in approximately 1 in 1,000 births. The clinical benefit of adequate treatment in the first trimester for inflammatory or immune-mediated diseases may far outweigh any small and tenuous risks for oral clefts.
With respect to reduced birth weight and preterm delivery, available evidence suggests either no association or that maternal disease and disease severity are driving any increased risks noted for these outcomes. Future studies of pregnancy safety for medications used to treat maternal diseases that themselves are potentially linked to adverse outcomes must incorporate appropriate measures of disease type and disease severity in the study designs.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at [email protected].
References
1. Teratology. 2000 Dec;62(6):385-92.
2. Birth Defects Res A Clin Mol Teratol. 2014 Jun;100(6):499-506.
3. CMAJ. 2011 Apr 19;183(7):796-804.
4. Am J Ther. 2014 Mar-Apr;21(2):73-80.
5. Am J Gastroenterol. 2007 Jul;102(7):1406-13.
6. Arthritis Rheum. 2009 Nov;60(11):3196-206.
7. Lupus. 2010 Dec;19(14):1665-73.
8. PLoS One. 2015 Jun 17;10(6):e0129567.
Corticosteroids such as prednisone are relatively frequently administered in pregnancy for their immunosuppressive and anti-inflammatory effects. Treatment may be initiated on a short-term basis for acute conditions. Alternatively, treatment may be more or less ongoing for severe chronic diseases such as asthma or a variety of other autoimmune conditions when disease symptoms do not remit in pregnancy. However, the safety of corticosteroid use with respect to risk of specific birth defects, preterm delivery, and low birth weight has been the subject of debate over some time.
Concerns about the teratogenicity of corticosteroids were raised as early as the 1950s, based on animal studies suggesting an increased risk for oral clefts. The association between corticosteroids and oral clefts has also been observed in some human epidemiologic studies. However, results of these studies have been inconsistent.
Similar to the NBDPS findings, in a large Danish cohort study covering 832,636 live births from 1996 to 2008, exposure to any corticosteroids during the first trimester was not associated with an increased risk for cleft lip or cleft palate. Only those exposed to topical corticosteroids had a higher risk of cleft lip with or without cleft palate (odds ratio, 1.45; 95% CI, 1.03-2.05).3 Another, smaller Danish study covered primiparous births from 1999 to 2009 (n = 83,043). The unadjusted odds of oral clefts following exposure to any corticosteroids (inhaled or oral) in the first trimester was null (OR, 0.4; 95% CI, 0.1-2.8).4
Inconsistencies across these studies, as speculated by authors of the NBDPS analysis, may result from a lack of information on the dose of drug used by the mother, the indication for its use, or any measure of the severity of the underlying maternal disease for which the corticosteroids were prescribed. It is possible that maternal disease or disease activity in and of itself is a direct cause of oral clefts or that corticosteroids are linked to increased risk for clefts through co-occurring other exposures such as smoking, alcohol, or obesity. However, these questions have yet to be answered.
With respect to other birth outcomes, a few disease-specific studies have examined birth weight or intrauterine growth restriction following corticosteroid use. In general, study findings have been reassuring. Among Danish women with Crohn’s disease, corticosteroids were not associated with reduced birth weight after adjusting for gestational age and disease activity (adjusted risk ratio, 1.1; 95% CI, 0.2-5.7).5 In another study of pregnant women with rheumatoid arthritis, birth weight was not associated with prednisone use after adjustment for gestational age at delivery and sex of the newborn.6 In a third cohort study of pregnant women with systemic lupus erythematosus, there was no a significant elevation in odds of intrauterine growth restriction following prednisone use.7
Several disease-specific studies have also examined corticosteroid use and risk of preterm birth. From the Danish cohort of pregnant women with Crohn’s disease, the researchers reported no association between prednisolone and preterm birth after adjustment for covariates. In contrast, in a separate Danish cohort of pregnant women with irritable bowel disease, there was an increased risk of preterm delivery following systemic corticosteroid use, compared with women without disease (adjusted hazard ratio, 6.3; 95% CI, 3.1-12.7).8 However, data were not available to address underlying disease severity as a possible contributing factor. Of note, among women with irritable bowel disease who did not use medication in pregnancy, there was a 50% increase in the risk of preterm birth, compared with women without disease (aHR, 1.5; 95% CI, 1.0-2.4). This suggests that the disease itself contributed to the increased risk of preterm birth.
Currently available data regarding corticosteroid use and adverse birth outcomes are generally reassuring. Recent estimates for oral clefts suggest a low elevation in risk, if any at all. This translates to a very low absolute risk for clefts, which occur in the general population in approximately 1 in 1,000 births. The clinical benefit of adequate treatment in the first trimester for inflammatory or immune-mediated diseases may far outweigh any small and tenuous risks for oral clefts.
With respect to reduced birth weight and preterm delivery, available evidence suggests either no association or that maternal disease and disease severity are driving any increased risks noted for these outcomes. Future studies of pregnancy safety for medications used to treat maternal diseases that themselves are potentially linked to adverse outcomes must incorporate appropriate measures of disease type and disease severity in the study designs.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at [email protected].
References
1. Teratology. 2000 Dec;62(6):385-92.
2. Birth Defects Res A Clin Mol Teratol. 2014 Jun;100(6):499-506.
3. CMAJ. 2011 Apr 19;183(7):796-804.
4. Am J Ther. 2014 Mar-Apr;21(2):73-80.
5. Am J Gastroenterol. 2007 Jul;102(7):1406-13.
6. Arthritis Rheum. 2009 Nov;60(11):3196-206.
7. Lupus. 2010 Dec;19(14):1665-73.
8. PLoS One. 2015 Jun 17;10(6):e0129567.
When to consider external cephalic version
Editor’s Note: This is the fourth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material often tested on board exams. In February 2016, ACOG issued a revised Practice Bulletin (#161) on external cephalic version outlining clinical considerations and recommendations and providing an algorithm for patient management.1 We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: According to the Practice Bulletin, which of the following is TRUE about external cephalic version (ECV)?
A. Success rate is lower in women with a previous cesarean delivery.
B. Placental location affects the success rate.
C. External cephalic version should be stopped after 15 minutes.
D. Women at 37 weeks’ gestation are preferred candidates.
E. Tocolysis decreases success rate.
The correct answer is D.
Women at 37 weeks’ gestation are the preferred candidates for an ECV because spontaneous version would likely have already occurred by this time and the risk of spontaneous reversion is lower. Answers A-C and E are incorrect statements.
Key points
Women at 37 weeks’ gestation are the preferred candidates for an ECV.
The overall pooled success rate for ECV is 58% with a 6% pooled complication rate.
The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
Literature summary
ECV is a procedure designed to turn a fetus into vertex presentation by applying external pressure to a woman’s abdomen. Women at 37 weeks’ gestation are the preferred candidates. At this gestational age, spontaneous version is most likely to have occurred, and there is decreased risk of spontaneous reversion after the ECV. All patients who are near term and found to have a fetus in a nonvertex presentation should be offered an ECV as long as there are no contraindications to the procedure. ECV is not appropriate for women who have a contraindication to a vaginal delivery.
There are limited studies of ECV in women who undergo the procedure in early labor and in those who have had a previous uterine surgery. ECV success rates are not affected by a previous cesarean delivery, though the risks of uterine rupture are not clear. The procedure should be attempted only in settings where cesarean delivery services are immediately available.
The success rates of ECV have been reported to be anywhere from 16% to 100%, with an overall pooled success of 58% with a 6% pooled complication rate. Some studies have documented higher success rates with higher parity and a transverse or oblique fetal lie. However, placental location, maternal weight, and amniotic fluid volume have not been consistently found to be predictive of ECV success. The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
ECV should be stopped in the face of a prolonged or significant fetal bradycardia or if the patient is experiencing intolerable levels of discomfort. However, there are no guidelines to recommend the total time limit of the procedure. After the ECV, there should be fetal heart rate monitoring for at least 30 minutes and anti-D immune globulin should be administered to those women who are Rh-negative if delivery is not anticipated in the next 72 hours.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fourth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material often tested on board exams. In February 2016, ACOG issued a revised Practice Bulletin (#161) on external cephalic version outlining clinical considerations and recommendations and providing an algorithm for patient management.1 We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: According to the Practice Bulletin, which of the following is TRUE about external cephalic version (ECV)?
A. Success rate is lower in women with a previous cesarean delivery.
B. Placental location affects the success rate.
C. External cephalic version should be stopped after 15 minutes.
D. Women at 37 weeks’ gestation are preferred candidates.
E. Tocolysis decreases success rate.
The correct answer is D.
Women at 37 weeks’ gestation are the preferred candidates for an ECV because spontaneous version would likely have already occurred by this time and the risk of spontaneous reversion is lower. Answers A-C and E are incorrect statements.
Key points
Women at 37 weeks’ gestation are the preferred candidates for an ECV.
The overall pooled success rate for ECV is 58% with a 6% pooled complication rate.
The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
Literature summary
ECV is a procedure designed to turn a fetus into vertex presentation by applying external pressure to a woman’s abdomen. Women at 37 weeks’ gestation are the preferred candidates. At this gestational age, spontaneous version is most likely to have occurred, and there is decreased risk of spontaneous reversion after the ECV. All patients who are near term and found to have a fetus in a nonvertex presentation should be offered an ECV as long as there are no contraindications to the procedure. ECV is not appropriate for women who have a contraindication to a vaginal delivery.
There are limited studies of ECV in women who undergo the procedure in early labor and in those who have had a previous uterine surgery. ECV success rates are not affected by a previous cesarean delivery, though the risks of uterine rupture are not clear. The procedure should be attempted only in settings where cesarean delivery services are immediately available.
The success rates of ECV have been reported to be anywhere from 16% to 100%, with an overall pooled success of 58% with a 6% pooled complication rate. Some studies have documented higher success rates with higher parity and a transverse or oblique fetal lie. However, placental location, maternal weight, and amniotic fluid volume have not been consistently found to be predictive of ECV success. The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
ECV should be stopped in the face of a prolonged or significant fetal bradycardia or if the patient is experiencing intolerable levels of discomfort. However, there are no guidelines to recommend the total time limit of the procedure. After the ECV, there should be fetal heart rate monitoring for at least 30 minutes and anti-D immune globulin should be administered to those women who are Rh-negative if delivery is not anticipated in the next 72 hours.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fourth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte Inc. The series will cover issues in reproductive endocrinology and infertility, maternal-fetal medicine, gynecologic oncology, and female pelvic medicine, as well as general test-taking and study tips.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material often tested on board exams. In February 2016, ACOG issued a revised Practice Bulletin (#161) on external cephalic version outlining clinical considerations and recommendations and providing an algorithm for patient management.1 We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: According to the Practice Bulletin, which of the following is TRUE about external cephalic version (ECV)?
A. Success rate is lower in women with a previous cesarean delivery.
B. Placental location affects the success rate.
C. External cephalic version should be stopped after 15 minutes.
D. Women at 37 weeks’ gestation are preferred candidates.
E. Tocolysis decreases success rate.
The correct answer is D.
Women at 37 weeks’ gestation are the preferred candidates for an ECV because spontaneous version would likely have already occurred by this time and the risk of spontaneous reversion is lower. Answers A-C and E are incorrect statements.
Key points
Women at 37 weeks’ gestation are the preferred candidates for an ECV.
The overall pooled success rate for ECV is 58% with a 6% pooled complication rate.
The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
Literature summary
ECV is a procedure designed to turn a fetus into vertex presentation by applying external pressure to a woman’s abdomen. Women at 37 weeks’ gestation are the preferred candidates. At this gestational age, spontaneous version is most likely to have occurred, and there is decreased risk of spontaneous reversion after the ECV. All patients who are near term and found to have a fetus in a nonvertex presentation should be offered an ECV as long as there are no contraindications to the procedure. ECV is not appropriate for women who have a contraindication to a vaginal delivery.
There are limited studies of ECV in women who undergo the procedure in early labor and in those who have had a previous uterine surgery. ECV success rates are not affected by a previous cesarean delivery, though the risks of uterine rupture are not clear. The procedure should be attempted only in settings where cesarean delivery services are immediately available.
The success rates of ECV have been reported to be anywhere from 16% to 100%, with an overall pooled success of 58% with a 6% pooled complication rate. Some studies have documented higher success rates with higher parity and a transverse or oblique fetal lie. However, placental location, maternal weight, and amniotic fluid volume have not been consistently found to be predictive of ECV success. The use of parenteral tocolysis has been associated with increased success rates of ECV, though there are not enough data to make a recommendation regarding use of regional anesthesia with the procedure.
ECV should be stopped in the face of a prolonged or significant fetal bradycardia or if the patient is experiencing intolerable levels of discomfort. However, there are no guidelines to recommend the total time limit of the procedure. After the ECV, there should be fetal heart rate monitoring for at least 30 minutes and anti-D immune globulin should be administered to those women who are Rh-negative if delivery is not anticipated in the next 72 hours.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Bromocriptine shows efficacy, safety for peripartum cardiomyopathy
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
[email protected]
On Twitter @mitchelzoler
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
This is a very important trial that was extremely difficult to conduct, and the results are exciting. It represents an effective bedside to bench to bedside sequence of research. The problem of peripartum cardiomyopathy was recognized in clinical practice, understood through basic research that led to a potential treatment, and the treatment is now confirmed through clinical testing. The results provide a reason for hope for the women who develop this disease.
These trial results are important for all mothers, for all women, and for anyone born from a woman.
Mariell Jessup, MD, is a heart failure specialist and chief scientific officer of the Leducq organization in Boston. She had no disclosures. She made these comments as designated discussant for the report by Dr. Hilfiker-Kleiner.
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
[email protected]
On Twitter @mitchelzoler
PARIS – Two regimens of bromocriptine treatment administered with anticoagulation and standard heart failure management were safe, and often effective, for normalizing ejection fraction levels in women diagnosed with peripartum cardiomyopathy in a study with 63 women and designed to be the pivotal trial for this management strategy.
“Bromocriptine therapy applied for 1 week seems sufficient to promote healing from PPCM [peripartum cardiomyopathy] in most patients, although critically ill patients may profit from prolonged [8 week] treatment,” Denise Hilfiker-Kleiner, PhD, said at a meeting of the Heart Failure Association of the ESC.
Women who are suspected of having PPCM but with less compromised ventricular output, with an ejection fraction of 40%-45%, should be closely followed with repeated clinical examinations for 6 months and echocardiography examinations at 3 and 6 months to see if their cardiac output worsens enough to justify initiating a bromocriptine regimen, she advised. “We don’t recommend that every woman with an postpartum ejection fraction of 45% needs to immediately stop lactation [with bromocriptine treatment], but she should be frequently seen by a cardiologist to see whether she recovers or deteriorates further.”
The PPCM (Effect of Bromocriptine on Left Ventricular Function in Women With Peripartum Cardiomyopathy) trial enrolled 63 women with PPCM and severely depressed left ventricular ejection fraction at 12 German centers. Randomization placed 32 women into a group assigned to received 1 week of bromocriptine treatment, with 26 completing the study, and 31 in a group treated with bromocriptine for 8 weeks, with all 31 completing the study. The patients averaged 34 years of age. All patients also received standard heart failure treatment.
The study’s primary endpoint, the change in left ventricular ejection fraction from baseline to 6-month follow-up, was similar in the two treatment groups, with the 1-week regimen leading to an average 21% improvement in ejection fraction and the 8-week regimen averaging a 24% gain in pump function. Among a subgroup of 37 women who entered the study with a left ventricular ejection fraction of less than 30%, slightly more than 60% achieved full heart function by 6-month follow-up with an ejection fraction of 50% or greater, and an additional 35% had partial recovery, with a follow-up ejection fraction of 35%-49%, Dr. Hilfiker-Kleiner reported.
No women in the study developed a thrombotic complication, a potential danger of the bromocriptine intervention. All participants received antithrombotic prophylaxis with either warfarin or subcutaneous heparin. Although the bromocriptine strategy has already been adopted for routine treatment of PPCM in Germany and in many other parts of the world, its uptake in the United States has lagged, largely because of concerns about thrombotic complications, noted Dr. Sliwa, professor of medicine and director of the Hatter Institute for Cardiovascular Research in Africa at the University of Cape Town, South Africa.
Among all 57 women available for a follow-up echocardiography assessment 6 months after the start of treatment, roughly 60% had a left ventricular ejection fraction of 50% or greater, and more than 20% achieved an ejection fraction of 35%-49%. The remaining roughly 18% of women either did not have a 6-month follow-up or failed to reach at least a 35% ejection fraction at 6 months.
PPCM can be a diagnostic challenge, said Dr. Hilfiker-Kleiner, but it is relatively common, with an average worldwide incidence of about 1 case for every 1,000 deliveries. The incidence may be even higher with many cases going undetected, often because the clinical signs of PPCM, including fatigue, difficulty sleeping, edema, and dyspnea, can be dismissed as the results of recent pregnancy or caring for a newborn baby. Certain racial or ethnic groups appear to have an increased incidence of the disease, including African and Hispanic women, likely because of genetic factors, said Dr. Sliwa. Clinical factors that boost risk include pre-eclampsia, smoking, obesity, older age, and multiparity, but not diabetes.
Testing for N-terminal-pro B-type natriuretic peptide levels appears to be a good screen for women who have developed PPCM, with a level of at least 500 pg/mL high enough to warrant further assessment, Dr. Sliwa said. She recommended running an NT-proBNP test on any recent postpartum women with a clinical or demographic risk factor or suggestive clinical presentation, but she also stressed that PPCM can occur in younger, totally healthy, and athletic women who appear to have a normal delivery.
A significant concern about bromocriptine treatment is that it precludes breastfeeding, a reason not to use the drug in women with an ejection fraction of 40% or greater, especially in settings where access to safe baby formula is a challenge.
The PPCM trial enrolled 63 women at 12 German centers.
The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT HEART FAILURE 2017
Key clinical point:
Major finding: At 6-month follow-up, more than 80% of patients had full or partial restoration of their left ventricular function.
Data source: The PPCM trial, which enrolled 63 women at 12 German centers.
Disclosures: The trial received no commercial funding. Dr. Hilfiker-Kleiner and Dr. Sliwa had no disclosures.
Preeclampsia/eclampsia rate highest in black women
The rate of preeclampsia and eclampsia for black women is 61% higher than it is for white women and 50% higher than for women overall, according to the Agency for Healthcare Research and Quality.
In 2014, black women experienced preeclampsia/eclampsia in 69.8 of every 1,000 deliveries, compared with 43.3 per 1,000 deliveries in white women and 46.6 per 1,000 for all women. Hispanic women were just above the overall rate at 46.8 per 1,000 deliveries, and Asian/Pacific Islander women were 38% lower than the overall rate at 28.8 per 1,000 deliveries, AHRQ said in its report. The overall rate was up 21% over the 38.4 per 1,000 reported in 2005.
Looking at degree of severity, 1.7% of all preeclampsia/eclampsia births in black women were eclampsia, compared with 1.4% for white and Hispanic women and 0.9% for Asian/Pacific Islanders. Severe preeclampsia was most common in Asian/Pacific Islanders – 40.4% of those diagnosed – with Hispanics at 40.3%, blacks at 38.5%, and whites at 34.3%. Mild or unspecified preeclampsia was diagnosed in 52% of preeclamptic/eclamptic white women, 46% of Hispanic women, 45% of Asians/Pacific Islanders, and 37% of black women, the AHRQ said in its analysis of data from the National Inpatient Sample.
Altogether, there were almost 177,000 delivery hospitalizations with preeclampsia/eclampsia in 2014, representing 4.7% of all deliveries and making it the most common hypertension-related diagnosis, followed by gestational hypertension (3.8%) and preexisting hypertension (1.7%). Compared with hospital stays for all other deliveries, those complicated by preeclampsia/eclampsia were 70% longer (mean, 4.4 vs. 2.6 days) and 70% more expensive (mean, $7,500 vs. $4,400), according to the report. 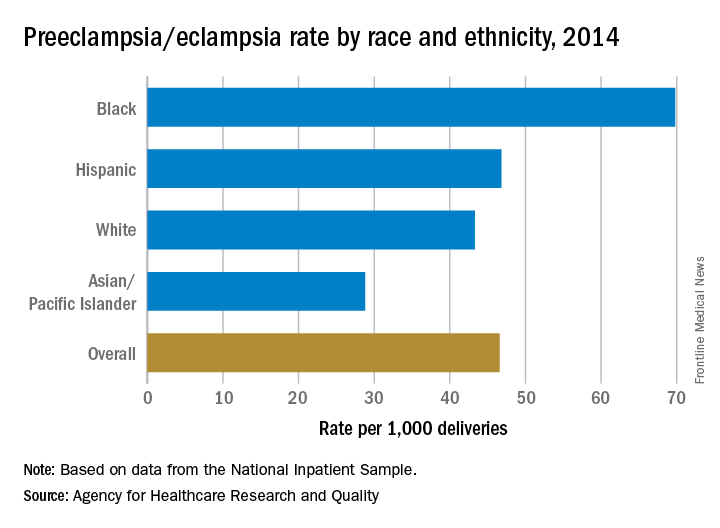
The rate of preeclampsia and eclampsia for black women is 61% higher than it is for white women and 50% higher than for women overall, according to the Agency for Healthcare Research and Quality.
In 2014, black women experienced preeclampsia/eclampsia in 69.8 of every 1,000 deliveries, compared with 43.3 per 1,000 deliveries in white women and 46.6 per 1,000 for all women. Hispanic women were just above the overall rate at 46.8 per 1,000 deliveries, and Asian/Pacific Islander women were 38% lower than the overall rate at 28.8 per 1,000 deliveries, AHRQ said in its report. The overall rate was up 21% over the 38.4 per 1,000 reported in 2005.
Looking at degree of severity, 1.7% of all preeclampsia/eclampsia births in black women were eclampsia, compared with 1.4% for white and Hispanic women and 0.9% for Asian/Pacific Islanders. Severe preeclampsia was most common in Asian/Pacific Islanders – 40.4% of those diagnosed – with Hispanics at 40.3%, blacks at 38.5%, and whites at 34.3%. Mild or unspecified preeclampsia was diagnosed in 52% of preeclamptic/eclamptic white women, 46% of Hispanic women, 45% of Asians/Pacific Islanders, and 37% of black women, the AHRQ said in its analysis of data from the National Inpatient Sample.
Altogether, there were almost 177,000 delivery hospitalizations with preeclampsia/eclampsia in 2014, representing 4.7% of all deliveries and making it the most common hypertension-related diagnosis, followed by gestational hypertension (3.8%) and preexisting hypertension (1.7%). Compared with hospital stays for all other deliveries, those complicated by preeclampsia/eclampsia were 70% longer (mean, 4.4 vs. 2.6 days) and 70% more expensive (mean, $7,500 vs. $4,400), according to the report. 
The rate of preeclampsia and eclampsia for black women is 61% higher than it is for white women and 50% higher than for women overall, according to the Agency for Healthcare Research and Quality.
In 2014, black women experienced preeclampsia/eclampsia in 69.8 of every 1,000 deliveries, compared with 43.3 per 1,000 deliveries in white women and 46.6 per 1,000 for all women. Hispanic women were just above the overall rate at 46.8 per 1,000 deliveries, and Asian/Pacific Islander women were 38% lower than the overall rate at 28.8 per 1,000 deliveries, AHRQ said in its report. The overall rate was up 21% over the 38.4 per 1,000 reported in 2005.
Looking at degree of severity, 1.7% of all preeclampsia/eclampsia births in black women were eclampsia, compared with 1.4% for white and Hispanic women and 0.9% for Asian/Pacific Islanders. Severe preeclampsia was most common in Asian/Pacific Islanders – 40.4% of those diagnosed – with Hispanics at 40.3%, blacks at 38.5%, and whites at 34.3%. Mild or unspecified preeclampsia was diagnosed in 52% of preeclamptic/eclamptic white women, 46% of Hispanic women, 45% of Asians/Pacific Islanders, and 37% of black women, the AHRQ said in its analysis of data from the National Inpatient Sample.
Altogether, there were almost 177,000 delivery hospitalizations with preeclampsia/eclampsia in 2014, representing 4.7% of all deliveries and making it the most common hypertension-related diagnosis, followed by gestational hypertension (3.8%) and preexisting hypertension (1.7%). Compared with hospital stays for all other deliveries, those complicated by preeclampsia/eclampsia were 70% longer (mean, 4.4 vs. 2.6 days) and 70% more expensive (mean, $7,500 vs. $4,400), according to the report. 
USPSTF says check BP at each visit to screen for preeclampsia
Blood pressure should be checked at every prenatal visit to screen for preeclampsia, according to a new recommendation from the U.S. Preventive Services Task Force.
The USPSTF commissioned a review of the current literature to update its initial recommendation regarding preeclampsia screening, which was issued in 1996. The update was considered important “given changes to screening, diagnosis, and management practices, as well as population health, over the past two decades.”
However, the evidence regarding different screening approaches is still quite limited, and the USPSTF did not change its recommendation except to indicate blood pressure checks at every visit rather than “periodically.” The current advice is a “B” recommendation, meaning that the USPSTF recommends the service.
Blood pressure assessment remains the most reliable, least harmful strategy for identifying preeclampsia as early as possible, said Kirsten Bobbins-Domingo, MD, PhD, chair of the USPSTF and lead author of the recommendation statement, and her associates.
“The USPSTF concludes with moderate certainty that screening for preeclampsia in pregnant women with blood pressure measurements has a substantial net benefit,” the researchers wrote (JAMA. 2017;317[16]:1661-7).
The evidence report supporting this recommendation included 21 studies involving 13,982 pregnant women. But no studies directly compared the effectiveness of blood pressure screening between screened and unscreened populations, Jillian T. Henderson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates said in the report.
Fourteen studies assessed tests for protein in the urine to identify preeclampsia, but the accuracy of such tests was highly variable. Sensitivity ranged from 22% to 100% and specificity ranged from 36% to 100%, so the USPSTF did not recommend urine testing for proteinuria as a screen for preeclampsia.
In addition, Dr. Henderson and her associates reviewed four studies (involving 7,123 women) assessing 16 different risk-prediction models. Again, the findings did not support the routine use of such tools in clinical practice: their positive predictive value was just 4% in the largest validation cohort, the investigators reported (JAMA. 2017;317[16]:1668-83).
The American College of Obstetricians and Gynecologists recommends monitoring blood pressure at every prenatal visit to screen for preeclampsia, as well as obtaining a detailed medical history to assess risk factors for the disorder. In contrast, the Society of Obstetricians and Gynaecologists of Canada and the National Institute for Health and Care Excellence in the United Kingdom recommend urinalysis for proteinuria in addition to blood pressure screening.
The full recommendation statement and evidence report are available at www.uspreventiveservicestaskforce.org.
The use of blood pressure measurement at every prenatal visit is a simple and effective way to identify and treat at-risk women without adding any substantial risks. In contrast, the risks related to preeclampsia are very high. Although risk assessment tools for preeclampsia are available, their low positive predictive value currently limits their usefulness for screening. Given the incidence of preeclampsia and the lack of predictability, it is important that all pregnant women have routine blood pressure assessments at every visit during the prenatal period.
The emerging data relating preeclampsia to the risk of future cardiovascular disease make this pregnancy-related issue important not just for obstetricians for the acute risks but also for internists and cardiologists for the long-term risks.
Martha Gulati, MD, is in the division of cardiology at the University of Arizona, Phoenix. She reported having no relevant financial disclosures. These remarks are excerpted from an editorial (JAMA Cardiol. 2017 Apr 25. doi: 10.1001/jamacardio.2017.1276).
The use of blood pressure measurement at every prenatal visit is a simple and effective way to identify and treat at-risk women without adding any substantial risks. In contrast, the risks related to preeclampsia are very high. Although risk assessment tools for preeclampsia are available, their low positive predictive value currently limits their usefulness for screening. Given the incidence of preeclampsia and the lack of predictability, it is important that all pregnant women have routine blood pressure assessments at every visit during the prenatal period.
The emerging data relating preeclampsia to the risk of future cardiovascular disease make this pregnancy-related issue important not just for obstetricians for the acute risks but also for internists and cardiologists for the long-term risks.
Martha Gulati, MD, is in the division of cardiology at the University of Arizona, Phoenix. She reported having no relevant financial disclosures. These remarks are excerpted from an editorial (JAMA Cardiol. 2017 Apr 25. doi: 10.1001/jamacardio.2017.1276).
The use of blood pressure measurement at every prenatal visit is a simple and effective way to identify and treat at-risk women without adding any substantial risks. In contrast, the risks related to preeclampsia are very high. Although risk assessment tools for preeclampsia are available, their low positive predictive value currently limits their usefulness for screening. Given the incidence of preeclampsia and the lack of predictability, it is important that all pregnant women have routine blood pressure assessments at every visit during the prenatal period.
The emerging data relating preeclampsia to the risk of future cardiovascular disease make this pregnancy-related issue important not just for obstetricians for the acute risks but also for internists and cardiologists for the long-term risks.
Martha Gulati, MD, is in the division of cardiology at the University of Arizona, Phoenix. She reported having no relevant financial disclosures. These remarks are excerpted from an editorial (JAMA Cardiol. 2017 Apr 25. doi: 10.1001/jamacardio.2017.1276).
Blood pressure should be checked at every prenatal visit to screen for preeclampsia, according to a new recommendation from the U.S. Preventive Services Task Force.
The USPSTF commissioned a review of the current literature to update its initial recommendation regarding preeclampsia screening, which was issued in 1996. The update was considered important “given changes to screening, diagnosis, and management practices, as well as population health, over the past two decades.”
However, the evidence regarding different screening approaches is still quite limited, and the USPSTF did not change its recommendation except to indicate blood pressure checks at every visit rather than “periodically.” The current advice is a “B” recommendation, meaning that the USPSTF recommends the service.
Blood pressure assessment remains the most reliable, least harmful strategy for identifying preeclampsia as early as possible, said Kirsten Bobbins-Domingo, MD, PhD, chair of the USPSTF and lead author of the recommendation statement, and her associates.
“The USPSTF concludes with moderate certainty that screening for preeclampsia in pregnant women with blood pressure measurements has a substantial net benefit,” the researchers wrote (JAMA. 2017;317[16]:1661-7).
The evidence report supporting this recommendation included 21 studies involving 13,982 pregnant women. But no studies directly compared the effectiveness of blood pressure screening between screened and unscreened populations, Jillian T. Henderson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates said in the report.
Fourteen studies assessed tests for protein in the urine to identify preeclampsia, but the accuracy of such tests was highly variable. Sensitivity ranged from 22% to 100% and specificity ranged from 36% to 100%, so the USPSTF did not recommend urine testing for proteinuria as a screen for preeclampsia.
In addition, Dr. Henderson and her associates reviewed four studies (involving 7,123 women) assessing 16 different risk-prediction models. Again, the findings did not support the routine use of such tools in clinical practice: their positive predictive value was just 4% in the largest validation cohort, the investigators reported (JAMA. 2017;317[16]:1668-83).
The American College of Obstetricians and Gynecologists recommends monitoring blood pressure at every prenatal visit to screen for preeclampsia, as well as obtaining a detailed medical history to assess risk factors for the disorder. In contrast, the Society of Obstetricians and Gynaecologists of Canada and the National Institute for Health and Care Excellence in the United Kingdom recommend urinalysis for proteinuria in addition to blood pressure screening.
The full recommendation statement and evidence report are available at www.uspreventiveservicestaskforce.org.
Blood pressure should be checked at every prenatal visit to screen for preeclampsia, according to a new recommendation from the U.S. Preventive Services Task Force.
The USPSTF commissioned a review of the current literature to update its initial recommendation regarding preeclampsia screening, which was issued in 1996. The update was considered important “given changes to screening, diagnosis, and management practices, as well as population health, over the past two decades.”
However, the evidence regarding different screening approaches is still quite limited, and the USPSTF did not change its recommendation except to indicate blood pressure checks at every visit rather than “periodically.” The current advice is a “B” recommendation, meaning that the USPSTF recommends the service.
Blood pressure assessment remains the most reliable, least harmful strategy for identifying preeclampsia as early as possible, said Kirsten Bobbins-Domingo, MD, PhD, chair of the USPSTF and lead author of the recommendation statement, and her associates.
“The USPSTF concludes with moderate certainty that screening for preeclampsia in pregnant women with blood pressure measurements has a substantial net benefit,” the researchers wrote (JAMA. 2017;317[16]:1661-7).
The evidence report supporting this recommendation included 21 studies involving 13,982 pregnant women. But no studies directly compared the effectiveness of blood pressure screening between screened and unscreened populations, Jillian T. Henderson, PhD, of Kaiser Permanente Center for Health Research, Portland, Ore., and her associates said in the report.
Fourteen studies assessed tests for protein in the urine to identify preeclampsia, but the accuracy of such tests was highly variable. Sensitivity ranged from 22% to 100% and specificity ranged from 36% to 100%, so the USPSTF did not recommend urine testing for proteinuria as a screen for preeclampsia.
In addition, Dr. Henderson and her associates reviewed four studies (involving 7,123 women) assessing 16 different risk-prediction models. Again, the findings did not support the routine use of such tools in clinical practice: their positive predictive value was just 4% in the largest validation cohort, the investigators reported (JAMA. 2017;317[16]:1668-83).
The American College of Obstetricians and Gynecologists recommends monitoring blood pressure at every prenatal visit to screen for preeclampsia, as well as obtaining a detailed medical history to assess risk factors for the disorder. In contrast, the Society of Obstetricians and Gynaecologists of Canada and the National Institute for Health and Care Excellence in the United Kingdom recommend urinalysis for proteinuria in addition to blood pressure screening.
The full recommendation statement and evidence report are available at www.uspreventiveservicestaskforce.org.
Key clinical point:
Major finding: The 14 studies about testing for proteinuria and the 4 studies about 16 different risk prediction tools did not yield evidence to support either approach as a screen for preeclampsia.
Data source: A review of 21 studies involving 13,982 pregnant women, performed since the initial USPSTF recommendation was issued in 1996.
Disclosures: The USPSTF is an independent voluntary group supported by the Agency for Healthcare Research and Quality.
Beyond Residency: No more black and white
The obstetrics and gynecology written board exam made everything seem cut and dry. A patient with fibroids causing heavy bleeding? Management options include hormone treatment, minor surgical procedures, or major surgical procedures like myomectomy or hysterectomy. A pregnant patient in labor with a fetal heart rate deceleration? The next step is to shut off the oxytocin infusion, turn the patient on her left side, administer intravenous fluids, and give her oxygen via a nasal cannula. A patient who has ruptured her membranes at 28 weeks? That’s an easy one: magnesium for neuroprotection, latency antibiotics, prenatal steroids, neonatalogy consult. Straightforward.
At the end of June, I was grateful for my residency experience – even though some of it seemed hectic and haphazard – because it ensured that I understood the reasoning behind these multiple-choice questions. But then I started my maternal-fetal medicine fellowship this past July. I was learning the names of new residents, attendings, and nurses, and having to orient myself to an entirely different hospital system. Even labor and delivery board sign-out was completely different. I reassured myself by thinking: Obstetrics is obstetrics. The rules and guidelines of obstetrics are universal, practiced at every level, and always make sense, right?
One day I had a patient come in with chronic, refractory immune thrombocytopenia. Her plan for delivery was induction at 37 weeks after our hematology colleagues used medications we had never heard of to finally get her platelets into the 100s. But upon admission, her platelets were down to the 70s. I wondered, should we induce anyway because her platelets are likely to drop even further if we wait? Or do we give her the slew of medications that didn’t completely work initially as a last-ditch effort to boost her platelets again before delivery? I looked at practice bulletins, hematology guidelines, and numerous other publications and still I could not find a protocol for this specific kind of patient. After discussion with anesthesiologists, hematologists, maternal-fetal medicine specialists, labor and delivery nurses, and the patient herself, we came up with a plan. We gave her additional doses of thrombopoietic agents and steroids and continued to monitor her platelet count. Within a week, she had an uncomplicated vaginal delivery with an epidural.*
Taking a lead role in the decision-making process and organizing a management plan made me feel like I was the quarterback of a football team, with a healthy mother and baby substituting for a game-winning touchdown. The decisions we make in maternal-fetal medicine are not supposed to be easy. However, I’ve heard over and over that when our colleagues ask for our input, the guidance they want to hear is “deliver” or “don’t deliver.” Over the last several months, I’ve learned that there is so much more to it than that. I now examine the entire patient and fetus in two ways: as one physiologically inseparable unit, and as two patients, weighing the neonatal risks of delivery against the maternal risks of the pregnancy itself. Determining an appropriate time for delivery is just part of it.
There are also the questions about antepartum fetal testing. Should we do additional monitoring for patients with isolated polyhydramnios? What about patients who are at advanced maternal age? What about patients whose fetuses have “decreased growth velocity” but not growth restriction? Should these patients get umbilical artery Dopplers, too? ACOG and the SMFM often do not give us specific monitoring guidelines, which forces us to make a plan based on each individual clinical scenario.
In other words, we must practice in that ever-changing, ever-frustrating, and confusing gray area. I hope in fellowship I learn to not only navigate through this area effectively, but to one day confidently hold out my hand to others to help guide them through it, too.
Dr. Grossman recently completed her residency in obstetrics and gynecology at Albert Einstein College of Medicine–Montefiore Hospital in the Bronx, N.Y., and is currently a first-year maternal-fetal medicine fellow at Weill Cornell Medical College in New York. She reported having no financial disclosures.
*This article was update April 21, 2107.
The obstetrics and gynecology written board exam made everything seem cut and dry. A patient with fibroids causing heavy bleeding? Management options include hormone treatment, minor surgical procedures, or major surgical procedures like myomectomy or hysterectomy. A pregnant patient in labor with a fetal heart rate deceleration? The next step is to shut off the oxytocin infusion, turn the patient on her left side, administer intravenous fluids, and give her oxygen via a nasal cannula. A patient who has ruptured her membranes at 28 weeks? That’s an easy one: magnesium for neuroprotection, latency antibiotics, prenatal steroids, neonatalogy consult. Straightforward.
At the end of June, I was grateful for my residency experience – even though some of it seemed hectic and haphazard – because it ensured that I understood the reasoning behind these multiple-choice questions. But then I started my maternal-fetal medicine fellowship this past July. I was learning the names of new residents, attendings, and nurses, and having to orient myself to an entirely different hospital system. Even labor and delivery board sign-out was completely different. I reassured myself by thinking: Obstetrics is obstetrics. The rules and guidelines of obstetrics are universal, practiced at every level, and always make sense, right?
One day I had a patient come in with chronic, refractory immune thrombocytopenia. Her plan for delivery was induction at 37 weeks after our hematology colleagues used medications we had never heard of to finally get her platelets into the 100s. But upon admission, her platelets were down to the 70s. I wondered, should we induce anyway because her platelets are likely to drop even further if we wait? Or do we give her the slew of medications that didn’t completely work initially as a last-ditch effort to boost her platelets again before delivery? I looked at practice bulletins, hematology guidelines, and numerous other publications and still I could not find a protocol for this specific kind of patient. After discussion with anesthesiologists, hematologists, maternal-fetal medicine specialists, labor and delivery nurses, and the patient herself, we came up with a plan. We gave her additional doses of thrombopoietic agents and steroids and continued to monitor her platelet count. Within a week, she had an uncomplicated vaginal delivery with an epidural.*
Taking a lead role in the decision-making process and organizing a management plan made me feel like I was the quarterback of a football team, with a healthy mother and baby substituting for a game-winning touchdown. The decisions we make in maternal-fetal medicine are not supposed to be easy. However, I’ve heard over and over that when our colleagues ask for our input, the guidance they want to hear is “deliver” or “don’t deliver.” Over the last several months, I’ve learned that there is so much more to it than that. I now examine the entire patient and fetus in two ways: as one physiologically inseparable unit, and as two patients, weighing the neonatal risks of delivery against the maternal risks of the pregnancy itself. Determining an appropriate time for delivery is just part of it.
There are also the questions about antepartum fetal testing. Should we do additional monitoring for patients with isolated polyhydramnios? What about patients who are at advanced maternal age? What about patients whose fetuses have “decreased growth velocity” but not growth restriction? Should these patients get umbilical artery Dopplers, too? ACOG and the SMFM often do not give us specific monitoring guidelines, which forces us to make a plan based on each individual clinical scenario.
In other words, we must practice in that ever-changing, ever-frustrating, and confusing gray area. I hope in fellowship I learn to not only navigate through this area effectively, but to one day confidently hold out my hand to others to help guide them through it, too.
Dr. Grossman recently completed her residency in obstetrics and gynecology at Albert Einstein College of Medicine–Montefiore Hospital in the Bronx, N.Y., and is currently a first-year maternal-fetal medicine fellow at Weill Cornell Medical College in New York. She reported having no financial disclosures.
*This article was update April 21, 2107.
The obstetrics and gynecology written board exam made everything seem cut and dry. A patient with fibroids causing heavy bleeding? Management options include hormone treatment, minor surgical procedures, or major surgical procedures like myomectomy or hysterectomy. A pregnant patient in labor with a fetal heart rate deceleration? The next step is to shut off the oxytocin infusion, turn the patient on her left side, administer intravenous fluids, and give her oxygen via a nasal cannula. A patient who has ruptured her membranes at 28 weeks? That’s an easy one: magnesium for neuroprotection, latency antibiotics, prenatal steroids, neonatalogy consult. Straightforward.
At the end of June, I was grateful for my residency experience – even though some of it seemed hectic and haphazard – because it ensured that I understood the reasoning behind these multiple-choice questions. But then I started my maternal-fetal medicine fellowship this past July. I was learning the names of new residents, attendings, and nurses, and having to orient myself to an entirely different hospital system. Even labor and delivery board sign-out was completely different. I reassured myself by thinking: Obstetrics is obstetrics. The rules and guidelines of obstetrics are universal, practiced at every level, and always make sense, right?
One day I had a patient come in with chronic, refractory immune thrombocytopenia. Her plan for delivery was induction at 37 weeks after our hematology colleagues used medications we had never heard of to finally get her platelets into the 100s. But upon admission, her platelets were down to the 70s. I wondered, should we induce anyway because her platelets are likely to drop even further if we wait? Or do we give her the slew of medications that didn’t completely work initially as a last-ditch effort to boost her platelets again before delivery? I looked at practice bulletins, hematology guidelines, and numerous other publications and still I could not find a protocol for this specific kind of patient. After discussion with anesthesiologists, hematologists, maternal-fetal medicine specialists, labor and delivery nurses, and the patient herself, we came up with a plan. We gave her additional doses of thrombopoietic agents and steroids and continued to monitor her platelet count. Within a week, she had an uncomplicated vaginal delivery with an epidural.*
Taking a lead role in the decision-making process and organizing a management plan made me feel like I was the quarterback of a football team, with a healthy mother and baby substituting for a game-winning touchdown. The decisions we make in maternal-fetal medicine are not supposed to be easy. However, I’ve heard over and over that when our colleagues ask for our input, the guidance they want to hear is “deliver” or “don’t deliver.” Over the last several months, I’ve learned that there is so much more to it than that. I now examine the entire patient and fetus in two ways: as one physiologically inseparable unit, and as two patients, weighing the neonatal risks of delivery against the maternal risks of the pregnancy itself. Determining an appropriate time for delivery is just part of it.
There are also the questions about antepartum fetal testing. Should we do additional monitoring for patients with isolated polyhydramnios? What about patients who are at advanced maternal age? What about patients whose fetuses have “decreased growth velocity” but not growth restriction? Should these patients get umbilical artery Dopplers, too? ACOG and the SMFM often do not give us specific monitoring guidelines, which forces us to make a plan based on each individual clinical scenario.
In other words, we must practice in that ever-changing, ever-frustrating, and confusing gray area. I hope in fellowship I learn to not only navigate through this area effectively, but to one day confidently hold out my hand to others to help guide them through it, too.
Dr. Grossman recently completed her residency in obstetrics and gynecology at Albert Einstein College of Medicine–Montefiore Hospital in the Bronx, N.Y., and is currently a first-year maternal-fetal medicine fellow at Weill Cornell Medical College in New York. She reported having no financial disclosures.
*This article was update April 21, 2107.
FDA warns against use of codeine, tramadol in children
The Food and Drug Administration is restricting the use of two opiates – codeine and tramadol – in children, and also warns they are potentially dangerous to infants of breastfeeding women.
Codeine is approved to treat pain and cough; tramadol is approved to treat pain.
“We understand that there are limited options when it comes to treating pain or cough in children, and that these changes may raise some questions for health care providers and parents. However, please know that our decision today was made based on the latest evidence and with this goal in mind: keeping our kids safe,” Douglas Throckmorton, MD, the deputy center director for regulatory programs at the FDA Center for Drug Evaluation and Research, said in a statement.
In 2013, the FDA updated prescription codeine labeling to contain a boxed warning and contraindication for children up to age 18 years regarding the risk of slowed breathing from codeine prescribed after tonsillectomy and/or adenoidectomy. And in 2015, the agency issued warnings about the risk of serious breathing problems in children who had ultrarapid metabolism of codeine and tramadol.
In the current safety statement, the FDA said it will require additional labeling changes, including contraindications for use of codeine or tramadol in all children younger than 12 years of age and a new contraindication for tramadol in children younger than 18 years being treated for pain after tonsillectomy and/or adenoidectomy, warnings about their use in children 12-18 years of age with certain medical conditions, and a stronger warning recommending against their use in nursing mothers.
Single-ingredient codeine and all tramadol-containing products are FDA-approved only for use in adults, the agency noted.
The agency cited particular concerns over those who are “ultra-rapid metabolizers” of substrates of the cytochrome P450 isoenzyme 2D6 (CYP2D6) genotype. These people more quickly convert codeine into potentially dangerously high levels of morphine, which can lead to fatal respiratory depression.
Supporting the restrictions and warning were data on 64 cases of respiratory depression that occurred worldwide between 1969 and 2015, when a codeine-based medicine was used in children younger than 18 years. Twenty-four of these children died.
The most frequently cited medicines in the cases were acetaminophen with codeine and promethazine with codeine with or without phenylephrine, either given to soothe postoperative pain, general pain, sore or strep throat pain, or cough and cold.
Ten of the 64 cases implicated the CYP2D6 genotype. Seven of the patients were “ultrarapid metabolizers,” five of whom died.
Data cited on tramadol included nine worldwide cases of tramadol-related respiratory depression occurring between 1969 and 2016, resulting in the deaths of three children, all under the age of 6 years, and all of whom were taking the drug for postoperative pain or fever.
All but one case of respiratory depression occurred within the first 24 hours of taking the drug. One of the patients was genotyped for CYP2D6, and found to have three functional alleles consistent with ultrarapid metabolism.
Mothers who are ultrarapid metabolizers of codeine can have high levels of morphine in their breast milk that are dangerous to their breastfed infants, whereas there is less of a threat posed by women with normal codeine metabolism because the amount of codeine secreted into the breast milk is low and dose dependent.
The FDA stated that data reveal numerous reports of respiratory depression and at least one death in infants of breastfeeding mothers taking these medicines, particularly mothers with the CYP2D6 genotype. Although there are FDA-cleared tests for ultrarapid metabolism, they are not commonly used.
The agency stated that breastfeeding women taking any opioid pain medicine, including codeine or tramadol should contact their health care provider if they notice the infant is sleeping more than 4 hours at a time, or if the infant is having difficulty breastfeeding or seems limp.
Clinicians are urged to report side effects that occur while using codeine or tramadol to the FDA’s MedWatch Adverse Event Reporting program.
[email protected]
On Twitter @whitneymcknight
The Food and Drug Administration is restricting the use of two opiates – codeine and tramadol – in children, and also warns they are potentially dangerous to infants of breastfeeding women.
Codeine is approved to treat pain and cough; tramadol is approved to treat pain.
“We understand that there are limited options when it comes to treating pain or cough in children, and that these changes may raise some questions for health care providers and parents. However, please know that our decision today was made based on the latest evidence and with this goal in mind: keeping our kids safe,” Douglas Throckmorton, MD, the deputy center director for regulatory programs at the FDA Center for Drug Evaluation and Research, said in a statement.
In 2013, the FDA updated prescription codeine labeling to contain a boxed warning and contraindication for children up to age 18 years regarding the risk of slowed breathing from codeine prescribed after tonsillectomy and/or adenoidectomy. And in 2015, the agency issued warnings about the risk of serious breathing problems in children who had ultrarapid metabolism of codeine and tramadol.
In the current safety statement, the FDA said it will require additional labeling changes, including contraindications for use of codeine or tramadol in all children younger than 12 years of age and a new contraindication for tramadol in children younger than 18 years being treated for pain after tonsillectomy and/or adenoidectomy, warnings about their use in children 12-18 years of age with certain medical conditions, and a stronger warning recommending against their use in nursing mothers.
Single-ingredient codeine and all tramadol-containing products are FDA-approved only for use in adults, the agency noted.
The agency cited particular concerns over those who are “ultra-rapid metabolizers” of substrates of the cytochrome P450 isoenzyme 2D6 (CYP2D6) genotype. These people more quickly convert codeine into potentially dangerously high levels of morphine, which can lead to fatal respiratory depression.
Supporting the restrictions and warning were data on 64 cases of respiratory depression that occurred worldwide between 1969 and 2015, when a codeine-based medicine was used in children younger than 18 years. Twenty-four of these children died.
The most frequently cited medicines in the cases were acetaminophen with codeine and promethazine with codeine with or without phenylephrine, either given to soothe postoperative pain, general pain, sore or strep throat pain, or cough and cold.
Ten of the 64 cases implicated the CYP2D6 genotype. Seven of the patients were “ultrarapid metabolizers,” five of whom died.
Data cited on tramadol included nine worldwide cases of tramadol-related respiratory depression occurring between 1969 and 2016, resulting in the deaths of three children, all under the age of 6 years, and all of whom were taking the drug for postoperative pain or fever.
All but one case of respiratory depression occurred within the first 24 hours of taking the drug. One of the patients was genotyped for CYP2D6, and found to have three functional alleles consistent with ultrarapid metabolism.
Mothers who are ultrarapid metabolizers of codeine can have high levels of morphine in their breast milk that are dangerous to their breastfed infants, whereas there is less of a threat posed by women with normal codeine metabolism because the amount of codeine secreted into the breast milk is low and dose dependent.
The FDA stated that data reveal numerous reports of respiratory depression and at least one death in infants of breastfeeding mothers taking these medicines, particularly mothers with the CYP2D6 genotype. Although there are FDA-cleared tests for ultrarapid metabolism, they are not commonly used.
The agency stated that breastfeeding women taking any opioid pain medicine, including codeine or tramadol should contact their health care provider if they notice the infant is sleeping more than 4 hours at a time, or if the infant is having difficulty breastfeeding or seems limp.
Clinicians are urged to report side effects that occur while using codeine or tramadol to the FDA’s MedWatch Adverse Event Reporting program.
[email protected]
On Twitter @whitneymcknight
The Food and Drug Administration is restricting the use of two opiates – codeine and tramadol – in children, and also warns they are potentially dangerous to infants of breastfeeding women.
Codeine is approved to treat pain and cough; tramadol is approved to treat pain.
“We understand that there are limited options when it comes to treating pain or cough in children, and that these changes may raise some questions for health care providers and parents. However, please know that our decision today was made based on the latest evidence and with this goal in mind: keeping our kids safe,” Douglas Throckmorton, MD, the deputy center director for regulatory programs at the FDA Center for Drug Evaluation and Research, said in a statement.
In 2013, the FDA updated prescription codeine labeling to contain a boxed warning and contraindication for children up to age 18 years regarding the risk of slowed breathing from codeine prescribed after tonsillectomy and/or adenoidectomy. And in 2015, the agency issued warnings about the risk of serious breathing problems in children who had ultrarapid metabolism of codeine and tramadol.
In the current safety statement, the FDA said it will require additional labeling changes, including contraindications for use of codeine or tramadol in all children younger than 12 years of age and a new contraindication for tramadol in children younger than 18 years being treated for pain after tonsillectomy and/or adenoidectomy, warnings about their use in children 12-18 years of age with certain medical conditions, and a stronger warning recommending against their use in nursing mothers.
Single-ingredient codeine and all tramadol-containing products are FDA-approved only for use in adults, the agency noted.
The agency cited particular concerns over those who are “ultra-rapid metabolizers” of substrates of the cytochrome P450 isoenzyme 2D6 (CYP2D6) genotype. These people more quickly convert codeine into potentially dangerously high levels of morphine, which can lead to fatal respiratory depression.
Supporting the restrictions and warning were data on 64 cases of respiratory depression that occurred worldwide between 1969 and 2015, when a codeine-based medicine was used in children younger than 18 years. Twenty-four of these children died.
The most frequently cited medicines in the cases were acetaminophen with codeine and promethazine with codeine with or without phenylephrine, either given to soothe postoperative pain, general pain, sore or strep throat pain, or cough and cold.
Ten of the 64 cases implicated the CYP2D6 genotype. Seven of the patients were “ultrarapid metabolizers,” five of whom died.
Data cited on tramadol included nine worldwide cases of tramadol-related respiratory depression occurring between 1969 and 2016, resulting in the deaths of three children, all under the age of 6 years, and all of whom were taking the drug for postoperative pain or fever.
All but one case of respiratory depression occurred within the first 24 hours of taking the drug. One of the patients was genotyped for CYP2D6, and found to have three functional alleles consistent with ultrarapid metabolism.
Mothers who are ultrarapid metabolizers of codeine can have high levels of morphine in their breast milk that are dangerous to their breastfed infants, whereas there is less of a threat posed by women with normal codeine metabolism because the amount of codeine secreted into the breast milk is low and dose dependent.
The FDA stated that data reveal numerous reports of respiratory depression and at least one death in infants of breastfeeding mothers taking these medicines, particularly mothers with the CYP2D6 genotype. Although there are FDA-cleared tests for ultrarapid metabolism, they are not commonly used.
The agency stated that breastfeeding women taking any opioid pain medicine, including codeine or tramadol should contact their health care provider if they notice the infant is sleeping more than 4 hours at a time, or if the infant is having difficulty breastfeeding or seems limp.
Clinicians are urged to report side effects that occur while using codeine or tramadol to the FDA’s MedWatch Adverse Event Reporting program.
[email protected]
On Twitter @whitneymcknight
Teens’ marijuana use higher during pregnancy
The prevalence of marijuana use among pregnant teenagers is more than double that among teens who are not pregnant, according to a study involving 410,000 females aged 12-44 years.
For pregnant teens aged 12-17 years, the past-month prevalence of marijuana use was 14% between 2002 and 2015, compared with 6.5% for their nonpregnant peers, Nora D. Volkow, MD, director of the National Institute on Drug Abuse in Bethesda, Md., and her associates reported in a letter on April 17 (Ann Intern Med. 2017 Apr 17. doi: 10.7326/L17-0067).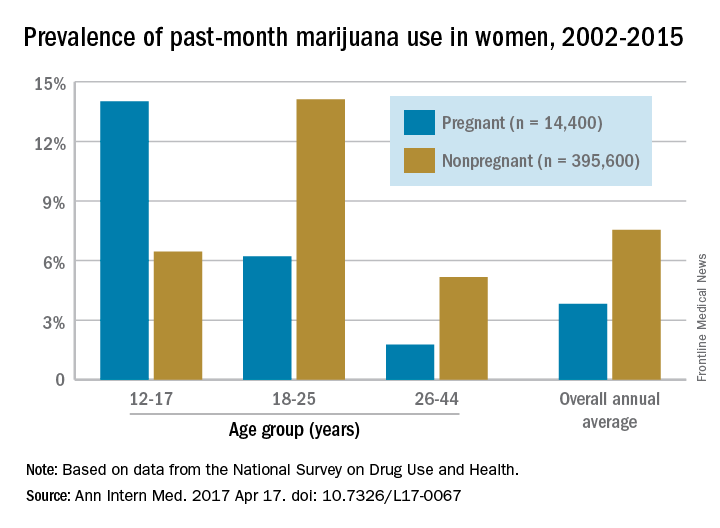
The pattern of use in the youngest age group “may reflect underlying risky behavior common to both teen pregnancy and early substance use and suggests the importance of intervention for teenagers,” the researchers wrote. “Because of consistent overlap between use of marijuana and other substances, identification of marijuana use during pregnancy warrants evaluation for comorbid substance abuse.”
The overall annual average prevalence of marijuana use was 3.8% among the 14,400 pregnant women and 7.5% for the 395,600 nonpregnant women who responded to the survey from 2002 to 2015. The investigators also found that marijuana use was higher in the first trimester (6.4%) than in the second (3.3%) or third (1.8%) trimesters and that use was higher in pregnant black women (6.5%) than in white (3.8%) or Hispanic women(2.9%) or women of other races/ethnicities (1.4%).
Although evidence on the effects of marijuana on prenatal development is limited, “pregnant females and those considering becoming pregnant should be advised not to use marijuana or other cannabinoids recreationally or to treat nausea,” Dr. Volkow and her associates wrote.
The study was sponsored by the National Institute on Drug Abuse and the Substance Abuse and Mental Health Services Administration. Dr. Volkow reported having no financial disclosures. One coauthor reported stock ownership of Pfizer, and another reported stock ownership of Sanofi and Eli Lilly.
The prevalence of marijuana use among pregnant teenagers is more than double that among teens who are not pregnant, according to a study involving 410,000 females aged 12-44 years.
For pregnant teens aged 12-17 years, the past-month prevalence of marijuana use was 14% between 2002 and 2015, compared with 6.5% for their nonpregnant peers, Nora D. Volkow, MD, director of the National Institute on Drug Abuse in Bethesda, Md., and her associates reported in a letter on April 17 (Ann Intern Med. 2017 Apr 17. doi: 10.7326/L17-0067).
The pattern of use in the youngest age group “may reflect underlying risky behavior common to both teen pregnancy and early substance use and suggests the importance of intervention for teenagers,” the researchers wrote. “Because of consistent overlap between use of marijuana and other substances, identification of marijuana use during pregnancy warrants evaluation for comorbid substance abuse.”
The overall annual average prevalence of marijuana use was 3.8% among the 14,400 pregnant women and 7.5% for the 395,600 nonpregnant women who responded to the survey from 2002 to 2015. The investigators also found that marijuana use was higher in the first trimester (6.4%) than in the second (3.3%) or third (1.8%) trimesters and that use was higher in pregnant black women (6.5%) than in white (3.8%) or Hispanic women(2.9%) or women of other races/ethnicities (1.4%).
Although evidence on the effects of marijuana on prenatal development is limited, “pregnant females and those considering becoming pregnant should be advised not to use marijuana or other cannabinoids recreationally or to treat nausea,” Dr. Volkow and her associates wrote.
The study was sponsored by the National Institute on Drug Abuse and the Substance Abuse and Mental Health Services Administration. Dr. Volkow reported having no financial disclosures. One coauthor reported stock ownership of Pfizer, and another reported stock ownership of Sanofi and Eli Lilly.
The prevalence of marijuana use among pregnant teenagers is more than double that among teens who are not pregnant, according to a study involving 410,000 females aged 12-44 years.
For pregnant teens aged 12-17 years, the past-month prevalence of marijuana use was 14% between 2002 and 2015, compared with 6.5% for their nonpregnant peers, Nora D. Volkow, MD, director of the National Institute on Drug Abuse in Bethesda, Md., and her associates reported in a letter on April 17 (Ann Intern Med. 2017 Apr 17. doi: 10.7326/L17-0067).
The pattern of use in the youngest age group “may reflect underlying risky behavior common to both teen pregnancy and early substance use and suggests the importance of intervention for teenagers,” the researchers wrote. “Because of consistent overlap between use of marijuana and other substances, identification of marijuana use during pregnancy warrants evaluation for comorbid substance abuse.”
The overall annual average prevalence of marijuana use was 3.8% among the 14,400 pregnant women and 7.5% for the 395,600 nonpregnant women who responded to the survey from 2002 to 2015. The investigators also found that marijuana use was higher in the first trimester (6.4%) than in the second (3.3%) or third (1.8%) trimesters and that use was higher in pregnant black women (6.5%) than in white (3.8%) or Hispanic women(2.9%) or women of other races/ethnicities (1.4%).
Although evidence on the effects of marijuana on prenatal development is limited, “pregnant females and those considering becoming pregnant should be advised not to use marijuana or other cannabinoids recreationally or to treat nausea,” Dr. Volkow and her associates wrote.
The study was sponsored by the National Institute on Drug Abuse and the Substance Abuse and Mental Health Services Administration. Dr. Volkow reported having no financial disclosures. One coauthor reported stock ownership of Pfizer, and another reported stock ownership of Sanofi and Eli Lilly.
FROM ANNALS OF INTERNAL MEDICINE















