User login
Obesity in Pregnancy Linked to Offspring's Language Scores
HOUSTON – Second-trimester maternal obesity was associated with lower scores in offspring on neurocognitive tests in early childhood.
This observation initially came as an unexpected finding, a byproduct of a case-control study set up for another purpose. But the disturbing finding, particularly in light of the ongoing obesity epidemic, led researchers to take a second look at the issue using an entirely separate data set. Those findings were confirmatory, Wendy Y. Craig, Ph.D., of the Foundation for Blood Research in Scarborough, Maine, reported at the annual meeting of the Endocrine Society.
The initial study involved a cohort of 101 children who underwent neurocognitive testing at age 2 years using the Bayley Scales of Infant Development, Third Edition (BSID-III). Their mothers, who were pregnant during 2004-2006, had a 30% prevalence of second trimester obesity.
The mean BSID-III cognitive and motor scores didn’t differ significantly between offspring of mothers who were normal-weight, overweight, or obese in pregnancy. However, mean BSID-III language scores averaged 110.6 in the children of normal-weight mothers, 107.2 in those whose mothers were overweight, and 98.0 in children born to women with second trimester obesity; that difference was highly statistically significant (P = .009).
Moreover, after adjustment for potential confounders in a multivariate regression analysis, it was apparent that the proportion of children with a BSID-III composite score below 85 rose with increasing maternal weight in pregnancy. The rate was 3.1% in the offspring of normal weight mothers, 7.7% in kids whose mothers were overweight, and 33.3% in those whose mothers were obese.
The second study included 118 children tested at age 8 years using the Wechsler Intelligence Scale for Children (WISC-III). Their mothers had been pregnant during 1987-1990, or 15 years earlier than in the first study population, and their prevalence of second-trimester obesity in that earlier, leaner era was a mere 10%.
The mean unadjusted WISC-III performance IQ score for children whose mothers were obese in pregnancy was 10.7 points lower than children of normal-weight mothers. Similarly, the full scale IQ and verbal subscale scores were lower by an average of 9.2 and 6.4 points, respectively.
"Although we cannot rule out the possibility that other covariates not measured in this study were responsible for the observed relationships, an independent effect of maternal obesity on the child’s early neurocognitive development deserves further investigation," Dr. Craig concluded.
All participants in both studies were recruited from the Maine statewide pregnancy project. The studies were supported by the National Institute of Child Health and Human Development, the Thrasher Fund, and Knoll Pharmaceuticals. Dr. Craig reported having no financial conflicts.
HOUSTON – Second-trimester maternal obesity was associated with lower scores in offspring on neurocognitive tests in early childhood.
This observation initially came as an unexpected finding, a byproduct of a case-control study set up for another purpose. But the disturbing finding, particularly in light of the ongoing obesity epidemic, led researchers to take a second look at the issue using an entirely separate data set. Those findings were confirmatory, Wendy Y. Craig, Ph.D., of the Foundation for Blood Research in Scarborough, Maine, reported at the annual meeting of the Endocrine Society.
The initial study involved a cohort of 101 children who underwent neurocognitive testing at age 2 years using the Bayley Scales of Infant Development, Third Edition (BSID-III). Their mothers, who were pregnant during 2004-2006, had a 30% prevalence of second trimester obesity.
The mean BSID-III cognitive and motor scores didn’t differ significantly between offspring of mothers who were normal-weight, overweight, or obese in pregnancy. However, mean BSID-III language scores averaged 110.6 in the children of normal-weight mothers, 107.2 in those whose mothers were overweight, and 98.0 in children born to women with second trimester obesity; that difference was highly statistically significant (P = .009).
Moreover, after adjustment for potential confounders in a multivariate regression analysis, it was apparent that the proportion of children with a BSID-III composite score below 85 rose with increasing maternal weight in pregnancy. The rate was 3.1% in the offspring of normal weight mothers, 7.7% in kids whose mothers were overweight, and 33.3% in those whose mothers were obese.
The second study included 118 children tested at age 8 years using the Wechsler Intelligence Scale for Children (WISC-III). Their mothers had been pregnant during 1987-1990, or 15 years earlier than in the first study population, and their prevalence of second-trimester obesity in that earlier, leaner era was a mere 10%.
The mean unadjusted WISC-III performance IQ score for children whose mothers were obese in pregnancy was 10.7 points lower than children of normal-weight mothers. Similarly, the full scale IQ and verbal subscale scores were lower by an average of 9.2 and 6.4 points, respectively.
"Although we cannot rule out the possibility that other covariates not measured in this study were responsible for the observed relationships, an independent effect of maternal obesity on the child’s early neurocognitive development deserves further investigation," Dr. Craig concluded.
All participants in both studies were recruited from the Maine statewide pregnancy project. The studies were supported by the National Institute of Child Health and Human Development, the Thrasher Fund, and Knoll Pharmaceuticals. Dr. Craig reported having no financial conflicts.
HOUSTON – Second-trimester maternal obesity was associated with lower scores in offspring on neurocognitive tests in early childhood.
This observation initially came as an unexpected finding, a byproduct of a case-control study set up for another purpose. But the disturbing finding, particularly in light of the ongoing obesity epidemic, led researchers to take a second look at the issue using an entirely separate data set. Those findings were confirmatory, Wendy Y. Craig, Ph.D., of the Foundation for Blood Research in Scarborough, Maine, reported at the annual meeting of the Endocrine Society.
The initial study involved a cohort of 101 children who underwent neurocognitive testing at age 2 years using the Bayley Scales of Infant Development, Third Edition (BSID-III). Their mothers, who were pregnant during 2004-2006, had a 30% prevalence of second trimester obesity.
The mean BSID-III cognitive and motor scores didn’t differ significantly between offspring of mothers who were normal-weight, overweight, or obese in pregnancy. However, mean BSID-III language scores averaged 110.6 in the children of normal-weight mothers, 107.2 in those whose mothers were overweight, and 98.0 in children born to women with second trimester obesity; that difference was highly statistically significant (P = .009).
Moreover, after adjustment for potential confounders in a multivariate regression analysis, it was apparent that the proportion of children with a BSID-III composite score below 85 rose with increasing maternal weight in pregnancy. The rate was 3.1% in the offspring of normal weight mothers, 7.7% in kids whose mothers were overweight, and 33.3% in those whose mothers were obese.
The second study included 118 children tested at age 8 years using the Wechsler Intelligence Scale for Children (WISC-III). Their mothers had been pregnant during 1987-1990, or 15 years earlier than in the first study population, and their prevalence of second-trimester obesity in that earlier, leaner era was a mere 10%.
The mean unadjusted WISC-III performance IQ score for children whose mothers were obese in pregnancy was 10.7 points lower than children of normal-weight mothers. Similarly, the full scale IQ and verbal subscale scores were lower by an average of 9.2 and 6.4 points, respectively.
"Although we cannot rule out the possibility that other covariates not measured in this study were responsible for the observed relationships, an independent effect of maternal obesity on the child’s early neurocognitive development deserves further investigation," Dr. Craig concluded.
All participants in both studies were recruited from the Maine statewide pregnancy project. The studies were supported by the National Institute of Child Health and Human Development, the Thrasher Fund, and Knoll Pharmaceuticals. Dr. Craig reported having no financial conflicts.
AT THE ANNUAL MEETING OF THE ENDOCRINE SOCIETY
Major Finding: At age 2 years, children whose mothers were obese during the second trimester averaged a 12.6-point lower score on the language component of the Bayley Scales of Infant Development (BSID-III) than did the offspring of normal-weight mothers.
Data Source: The results came from retrospective studies involving prospectively collected data from the Maine pregnancy project.
Disclosures: The studies were supported by the National Institute of Child Health and Human Development, the Thrasher Fund, and Knoll Pharmaceuticals. Dr. Craig reported having no financial conflicts.
Are You What You Eat?: Pica in Pregnancy
PRODUCT UPDATE
DISPOSABLE EXTRA-SMALL SPECULUM
Welch Allyn introduced the industry’s first extra-small KleenSpec® Disposable Vaginal Speculum for virginal and pediatric patients as well as postmenopausal and posthysterectomy women. Available in August, this speculum joins the company’s suite of vaginal specula designed to work with the Welch Allyn Cordless Illumination System. The KleenSpec line is constructed of smooth, molded acrylic with wide handles for improved ergonomics and manipulation, says Welch Allyn.
FOR MORE INFORMATION, VISIT www.welchallyn.com
SCARAWAY® SILICONE SCAR SHEETS
ScarAway® Silicone Scar Sheets are an effective, affordable, over-the-counter treatment to reduce and prevent hypertrophic and keloid scars that may result from surgery, injuries, or burns, reports Mitchell-Vance Laboratories. Unlike scar-minimizing creams and ointments, ScarAway delivers a slight pressure directly to the scar, and mimics the natural barrier function of healthy skin, diminishing the scar’s appearance and restoring skin to its natural texture and color. The sheeting is nongreasy, ultra-thin, flexible, breathable, and durable with a soft fabric backing.
FOR MORE INFORMATION, VISIT www.MyScarAway.com
PREMAMA LIQUID PRENATAL VITAMINS
Premama, a flavorless prenatal vitamin drink mix, can be added to juices, milk, iced tea, and more. Premama includes the necessary vitamins and minerals, including folic acid, iron, DHA, CoQ10, and calcium, to support prepregnant, pregnant, and breast-feeding mothers. The unique mix of ingredients has been shown to help relieve common prenatal vitamin side-effects, such as nausea and constipation. Premama is 100% natural, lactate-free, gluten-free, and certified kosher.
FOR MORE INFORMATION, VISIT www.drinkpremama.com
HARMONY™ PRENATAL TEST DETECTS COMMON FETAL TRISOMIES
The Harmony Prenatal Test from Ariosa Diagnostics and available through LabCorp, is designed as a safe and highly accurate option to test pregnant woman for fetal anomalies using a directed approach to analyze cell-free DNA (cfDNA) in maternal blood. The Harmony Prenatal Test detects common fetal trisomies such as Trisomy 21 (associated with Down Syndrome) in pregnant women of at least 10 weeks’ gestation with a single fetus conceived without the use of an egg donor.
FOR MORE INFORMATION, VISIT www.ariosadx.com
GE HEALTHCARE’S NEW VSCAN: MORE SCAN TIME, BETTER RESOLUTION, EXPORT OPTION
GE Healthcare has upgraded Vscan, its pocket-sized, hand-held ultrasound (US) tool. New features include 50% more scan time (extended battery life), a more user-friendly interface, enhanced resolution, and an “export to PDF” option that allows physicians to more efficiently document findings. Vscan provides black and white anatomic and color-coded blood flow images. While Vscan is not intended to replace a full fetal US survey, it can provide physicians a quick look to assess fetal viability, says the manufacturer.
FOR MORE INFORMATION, VISIT https://vscan.gehealthcare.com
SODEXO HEALTH CARE SERVICES
Sodexo Health Care Services provides food and facilities services to hospitals across the country. Using the latest technologies, Sodexo employees implement environmental services and cleaning programs that have reduced hospital-acquired infections, improved patient and staff satisfaction, and contributed to cost savings for more than 1,700 client hospitals.
FOR MORE INFORMATION, VISIT www.sodexousa.com
DISPOSABLE EXTRA-SMALL SPECULUM
Welch Allyn introduced the industry’s first extra-small KleenSpec® Disposable Vaginal Speculum for virginal and pediatric patients as well as postmenopausal and posthysterectomy women. Available in August, this speculum joins the company’s suite of vaginal specula designed to work with the Welch Allyn Cordless Illumination System. The KleenSpec line is constructed of smooth, molded acrylic with wide handles for improved ergonomics and manipulation, says Welch Allyn.
FOR MORE INFORMATION, VISIT www.welchallyn.com
SCARAWAY® SILICONE SCAR SHEETS
ScarAway® Silicone Scar Sheets are an effective, affordable, over-the-counter treatment to reduce and prevent hypertrophic and keloid scars that may result from surgery, injuries, or burns, reports Mitchell-Vance Laboratories. Unlike scar-minimizing creams and ointments, ScarAway delivers a slight pressure directly to the scar, and mimics the natural barrier function of healthy skin, diminishing the scar’s appearance and restoring skin to its natural texture and color. The sheeting is nongreasy, ultra-thin, flexible, breathable, and durable with a soft fabric backing.
FOR MORE INFORMATION, VISIT www.MyScarAway.com
PREMAMA LIQUID PRENATAL VITAMINS
Premama, a flavorless prenatal vitamin drink mix, can be added to juices, milk, iced tea, and more. Premama includes the necessary vitamins and minerals, including folic acid, iron, DHA, CoQ10, and calcium, to support prepregnant, pregnant, and breast-feeding mothers. The unique mix of ingredients has been shown to help relieve common prenatal vitamin side-effects, such as nausea and constipation. Premama is 100% natural, lactate-free, gluten-free, and certified kosher.
FOR MORE INFORMATION, VISIT www.drinkpremama.com
HARMONY™ PRENATAL TEST DETECTS COMMON FETAL TRISOMIES
The Harmony Prenatal Test from Ariosa Diagnostics and available through LabCorp, is designed as a safe and highly accurate option to test pregnant woman for fetal anomalies using a directed approach to analyze cell-free DNA (cfDNA) in maternal blood. The Harmony Prenatal Test detects common fetal trisomies such as Trisomy 21 (associated with Down Syndrome) in pregnant women of at least 10 weeks’ gestation with a single fetus conceived without the use of an egg donor.
FOR MORE INFORMATION, VISIT www.ariosadx.com
GE HEALTHCARE’S NEW VSCAN: MORE SCAN TIME, BETTER RESOLUTION, EXPORT OPTION
GE Healthcare has upgraded Vscan, its pocket-sized, hand-held ultrasound (US) tool. New features include 50% more scan time (extended battery life), a more user-friendly interface, enhanced resolution, and an “export to PDF” option that allows physicians to more efficiently document findings. Vscan provides black and white anatomic and color-coded blood flow images. While Vscan is not intended to replace a full fetal US survey, it can provide physicians a quick look to assess fetal viability, says the manufacturer.
FOR MORE INFORMATION, VISIT https://vscan.gehealthcare.com
SODEXO HEALTH CARE SERVICES
Sodexo Health Care Services provides food and facilities services to hospitals across the country. Using the latest technologies, Sodexo employees implement environmental services and cleaning programs that have reduced hospital-acquired infections, improved patient and staff satisfaction, and contributed to cost savings for more than 1,700 client hospitals.
FOR MORE INFORMATION, VISIT www.sodexousa.com
DISPOSABLE EXTRA-SMALL SPECULUM
Welch Allyn introduced the industry’s first extra-small KleenSpec® Disposable Vaginal Speculum for virginal and pediatric patients as well as postmenopausal and posthysterectomy women. Available in August, this speculum joins the company’s suite of vaginal specula designed to work with the Welch Allyn Cordless Illumination System. The KleenSpec line is constructed of smooth, molded acrylic with wide handles for improved ergonomics and manipulation, says Welch Allyn.
FOR MORE INFORMATION, VISIT www.welchallyn.com
SCARAWAY® SILICONE SCAR SHEETS
ScarAway® Silicone Scar Sheets are an effective, affordable, over-the-counter treatment to reduce and prevent hypertrophic and keloid scars that may result from surgery, injuries, or burns, reports Mitchell-Vance Laboratories. Unlike scar-minimizing creams and ointments, ScarAway delivers a slight pressure directly to the scar, and mimics the natural barrier function of healthy skin, diminishing the scar’s appearance and restoring skin to its natural texture and color. The sheeting is nongreasy, ultra-thin, flexible, breathable, and durable with a soft fabric backing.
FOR MORE INFORMATION, VISIT www.MyScarAway.com
PREMAMA LIQUID PRENATAL VITAMINS
Premama, a flavorless prenatal vitamin drink mix, can be added to juices, milk, iced tea, and more. Premama includes the necessary vitamins and minerals, including folic acid, iron, DHA, CoQ10, and calcium, to support prepregnant, pregnant, and breast-feeding mothers. The unique mix of ingredients has been shown to help relieve common prenatal vitamin side-effects, such as nausea and constipation. Premama is 100% natural, lactate-free, gluten-free, and certified kosher.
FOR MORE INFORMATION, VISIT www.drinkpremama.com
HARMONY™ PRENATAL TEST DETECTS COMMON FETAL TRISOMIES
The Harmony Prenatal Test from Ariosa Diagnostics and available through LabCorp, is designed as a safe and highly accurate option to test pregnant woman for fetal anomalies using a directed approach to analyze cell-free DNA (cfDNA) in maternal blood. The Harmony Prenatal Test detects common fetal trisomies such as Trisomy 21 (associated with Down Syndrome) in pregnant women of at least 10 weeks’ gestation with a single fetus conceived without the use of an egg donor.
FOR MORE INFORMATION, VISIT www.ariosadx.com
GE HEALTHCARE’S NEW VSCAN: MORE SCAN TIME, BETTER RESOLUTION, EXPORT OPTION
GE Healthcare has upgraded Vscan, its pocket-sized, hand-held ultrasound (US) tool. New features include 50% more scan time (extended battery life), a more user-friendly interface, enhanced resolution, and an “export to PDF” option that allows physicians to more efficiently document findings. Vscan provides black and white anatomic and color-coded blood flow images. While Vscan is not intended to replace a full fetal US survey, it can provide physicians a quick look to assess fetal viability, says the manufacturer.
FOR MORE INFORMATION, VISIT https://vscan.gehealthcare.com
SODEXO HEALTH CARE SERVICES
Sodexo Health Care Services provides food and facilities services to hospitals across the country. Using the latest technologies, Sodexo employees implement environmental services and cleaning programs that have reduced hospital-acquired infections, improved patient and staff satisfaction, and contributed to cost savings for more than 1,700 client hospitals.
FOR MORE INFORMATION, VISIT www.sodexousa.com
Routine use of oxytocin at birth: just the right amount to prevent postpartum hemorrhage
Postpartum hemorrhage is a common complication of birth—annually, it occurs in about 11% of natural, unmedicated childbirths and accounts for more than 50,000 maternal deaths worldwide. Administration of a uterotonic agent, such as oxytocin, at the time the anterior shoulder is delivered or after the birth of the baby reduces the risk of postpartum hemorrhage by approximately 66%.1 Administration of a uterotonic also reduces the risk of severe maternal hemorrhage (blood loss >1000 mL) and the risk of maternal transfusion by approximately 66% and 65%, respectively.1 It had been thought that early cord clamping and controlled cord traction also might help reduce the risk of postpartum hemorrhage, but recent data indicate that administering a uterotonic is the key intervention and cord traction has little or no effect on reducing the risk of hemorrhage.2
When considering the optimal approach to uterotonic administration for the purpose of postpartum hemorrhage prevention, we must ask:
- Why do so few clinicians administer a uterotonic?
- What is the optimal time for uterotonic initiation?
- If using oxytocin, what is the optimal dose and route of administration?
- Is there a dose of oxytocin that is too much?
- If postpartum hemorrhage follows oxytocin administration, what is the next step?
These questions are the focus of this editorial.
Too little oxytocin
Why do so few clinicians administer a uterotonic agent?
Across the globe, many deliveries occur without the administration of a postpartum uterotonic. In the United States, beliefs about avoiding any nonphysiologic interventions during the birth process contribute to many birthing women not receiving a uterotonic. Deliveries that occur outside of a hospital and at birthing centers are more likely to be associated with the failure to administer a uterotonic, which contributes to unnecessary postpartum hemorrhage. In these settings, administration of uterotonics used in resource-poor settings (see “Alternative Uterotonics”) may be warranted.
Bottom line: All obstetric care providers should ensure that every woman receive a uterotonic agent at birth.
Timing of oxytocin initiation
The overarching clinical goal is to ensure that every birthing mother receives a uterotonic agent; the timing of administration is of secondary importance. In the United States, oxytocin is the uterotonic most often administered at birth. It is commonly administered: 1) after delivery of the baby’s anterior shoulder, 2) after delivery of the baby but before delivery of the placenta, or 3) after delivery of the placenta.
Of the three options, the last one is the least studied. There are insufficient data to identify the optimal timing for drug initiation conclusively, but most experts conclude it should be administered somewhere between delivery of the anterior shoulder and delivery of the placenta. Clinician preference is appropriate for selecting the timing of initiation.
Optimal route, dose, and duration
The most studied routes and doses of oxytocin are a single 10-unit intramuscular (IM) dose or an intravenous (IV) infusion of 20 to 40 units oxytocin in 1000 mL of saline or lactated Ringers solution, often infused at a rate of about 125 mL/hr. The onset of action of the IM dose is typically 3 to 5 minutes, while the onset of action of the IV dose is about 1 minute.
The optimal interval for administering the oxytocin IV infusion has not been well studied. I recommend the infusion be continued for at least 4 hours following delivery. My recommendation is based on the observation that when oxytocin is discontinued shortly after birth, the risk of a delayed postpartum hemorrhage increases significantly.
Too much oxytocin
Avoid using a 5- or 10-unit IV bolus
IV boluses of oxytocin, at doses of 5 to 10 units, have been reported to be followed by hypotension,3 ischemic changes detected by electrocardiogram,4,5 and maternal death.6 Since an IV infusion of oxytocin appears to be as effective as a bolus or bolus plus IV infusion, it may be preferable to avoid the bolus and use only an IV infusion.7
Bottom line: Avoid IV bolus administration of oxytocin at doses of 5 units or 10 units due to adverse effects.
Alternative uterotonics
In addition to oxytocin, the following agents reduce the risk of postpartum hemorrhage:
- misoprostol (Cytotec) 600 μg orally (or rectally)
- methylergonovine (Methergine, a methyl derivative of ergonovine) 0.2 mg by IM injection
- ergonovine 0.2 to 0.5 mg.
Misoprostol is likely somewhat less effective than oxytocin in reducing the risk of postpartum hemorrhage,8 but it is more effective than placebo.9
Resource-limited settings. IV infusion or IM injection of a uterotonic may be difficult to perform outside of a hospital, in a resource-limited setting. In these environments, misoprostol may be useful because it can be administered orally (or rectally) and is stable at a wide range of temperatures.
In addition, a self-contained, single-use Uniject (BD, Franklin Lakes, New Jersey) injection device containing oxytocin has been developed specifically for use in resource-limited settings (FIGURE). The device can be used by minimally trained personnel and stored at room temperature for up to 2 months.
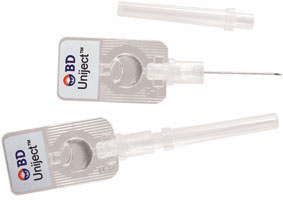
Single-use injection device
Uniject can be filled with oxytocin for use outside of a hospital setting. Photo courtesy of and © Becton, Dickinson and Company.
What’s your next step if oxytocin isn’t enough?
There is a high probability that administering oxytocin alone may not prevent postpartum hemorrhage in settings of:
- prolonged use of oxytocin for the purposes of labor induction
- a prolonged labor in which oxytocin has been used to augment labor
- chorioamnionitis.
Rather than continue to administer more and more oxytocin in these situations, consider administering a second agent, such as methlyergonovine (Methergine), misoprostol (Cytotec), or carboprost tromethamine (Hemabate, 0.25 mg IM).
Additionally, mechanical massage of the uterus can help to increase uterine tone and reduce bleeding. When postpartum hemorrhage fails to respond to the administration of multiple uterotonics, rapid institution of a postpartum bleeding protocol is warranted.10,11
Review your medication order sets for oxytocin administration
It is likely that your hospital has a well-established order set for the administration of oxytocin at birth. Reviewing your hospital’s protocols for administering oxytocin to prevent postpartum hemorrhage can help to ensure that all patients receive a uterotonic at a dose and duration that maximizes the benefits and reduces side effects and theoretical risks. Anesthesiologists play an important role in the administration of a uterotonic during cesarean delivery, and they should be involved in the review of the medication order entry sets for prevention of postpartum hemorrhage.
Prevention of postpartum hemorrhage is a top priority for all obstetric providers. Routine administration of an optimal dose of oxytocin, or another uterotonic, should be a standard clinical process in all birthing units throughout the world.
- Act fast when confronted by a coagulopathy postpartum
Robert L. Barbieri, MD (Editorial, March 2012) - Update on Obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012) - Have you made best use of the Bakri balloon in PPH?
Robert L. Barbieri, MD (Editorial, July 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai (June 2011) - Postpartum hemorrhage: 11 critical questions, answered by an expert
Q&A with Haywood L. Brown, MD (January 2011)
1. Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011;(11):CD007412.-
2. Gulmezoglu AM, Lumbiganon P, Landoulsi S, et al. Active management of the third stage of labour with and without controlled cord traction: a randomised, controlled, non-inferiority trial [published correction appears in Lancet. 2012;379(9827):1704]. Lancet. 2012;379(9827):1721-1727.
3. Archer TL, Knape K, Liles D, Wheeler AS, Carter B. The hemodynamics of oxytocin and other vasoactive agents during neuraxial anesthesia for cesarean delivery: findings in six cases. Int J Obstet Anesth. 2008;17(3):247-254.
4. Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomized controlled trial. BJOG. 2010;117(1):76-83.
5. Svanström MC, Biber B, Hanes M, Johansson G, Naslund U, Balfours EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008;100(5):683-689.
6. Lewis G, Drife J. Why Mothers Die 1977-1999. The confidential inquiries into maternal deaths in the United Kingdom. London England: RCOG Press; 2001.
7. King KJ, Douglas MJ, Unger W, Wong A, King RA. Five unit bolus oxytocin at cesarean delivery in women a risk of atony: a randomized double-blind, controlled trial. Anesth Analg. 2010;111(6):1460-1466.
8. Gülmezoglu AM, Villar J, Ngoc NT, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet. 2001;358(9283):689-695.
9. Mobeen N, Durocher J, Zuberi N, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG. 2011;118(3):353-361.
10. Barbieri RL. Act fast when confronted by a coagulopathy postpartum. OBG Manage. 2012;24(3):8, 10-11.
11. Barbieri RL. Have you made the best use of the Bakri balloon in postpartum hemorrhage? OBG Manage. 2011;23(7):6, 8-9, 19.
Postpartum hemorrhage is a common complication of birth—annually, it occurs in about 11% of natural, unmedicated childbirths and accounts for more than 50,000 maternal deaths worldwide. Administration of a uterotonic agent, such as oxytocin, at the time the anterior shoulder is delivered or after the birth of the baby reduces the risk of postpartum hemorrhage by approximately 66%.1 Administration of a uterotonic also reduces the risk of severe maternal hemorrhage (blood loss >1000 mL) and the risk of maternal transfusion by approximately 66% and 65%, respectively.1 It had been thought that early cord clamping and controlled cord traction also might help reduce the risk of postpartum hemorrhage, but recent data indicate that administering a uterotonic is the key intervention and cord traction has little or no effect on reducing the risk of hemorrhage.2
When considering the optimal approach to uterotonic administration for the purpose of postpartum hemorrhage prevention, we must ask:
- Why do so few clinicians administer a uterotonic?
- What is the optimal time for uterotonic initiation?
- If using oxytocin, what is the optimal dose and route of administration?
- Is there a dose of oxytocin that is too much?
- If postpartum hemorrhage follows oxytocin administration, what is the next step?
These questions are the focus of this editorial.
Too little oxytocin
Why do so few clinicians administer a uterotonic agent?
Across the globe, many deliveries occur without the administration of a postpartum uterotonic. In the United States, beliefs about avoiding any nonphysiologic interventions during the birth process contribute to many birthing women not receiving a uterotonic. Deliveries that occur outside of a hospital and at birthing centers are more likely to be associated with the failure to administer a uterotonic, which contributes to unnecessary postpartum hemorrhage. In these settings, administration of uterotonics used in resource-poor settings (see “Alternative Uterotonics”) may be warranted.
Bottom line: All obstetric care providers should ensure that every woman receive a uterotonic agent at birth.
Timing of oxytocin initiation
The overarching clinical goal is to ensure that every birthing mother receives a uterotonic agent; the timing of administration is of secondary importance. In the United States, oxytocin is the uterotonic most often administered at birth. It is commonly administered: 1) after delivery of the baby’s anterior shoulder, 2) after delivery of the baby but before delivery of the placenta, or 3) after delivery of the placenta.
Of the three options, the last one is the least studied. There are insufficient data to identify the optimal timing for drug initiation conclusively, but most experts conclude it should be administered somewhere between delivery of the anterior shoulder and delivery of the placenta. Clinician preference is appropriate for selecting the timing of initiation.
Optimal route, dose, and duration
The most studied routes and doses of oxytocin are a single 10-unit intramuscular (IM) dose or an intravenous (IV) infusion of 20 to 40 units oxytocin in 1000 mL of saline or lactated Ringers solution, often infused at a rate of about 125 mL/hr. The onset of action of the IM dose is typically 3 to 5 minutes, while the onset of action of the IV dose is about 1 minute.
The optimal interval for administering the oxytocin IV infusion has not been well studied. I recommend the infusion be continued for at least 4 hours following delivery. My recommendation is based on the observation that when oxytocin is discontinued shortly after birth, the risk of a delayed postpartum hemorrhage increases significantly.
Too much oxytocin
Avoid using a 5- or 10-unit IV bolus
IV boluses of oxytocin, at doses of 5 to 10 units, have been reported to be followed by hypotension,3 ischemic changes detected by electrocardiogram,4,5 and maternal death.6 Since an IV infusion of oxytocin appears to be as effective as a bolus or bolus plus IV infusion, it may be preferable to avoid the bolus and use only an IV infusion.7
Bottom line: Avoid IV bolus administration of oxytocin at doses of 5 units or 10 units due to adverse effects.
Alternative uterotonics
In addition to oxytocin, the following agents reduce the risk of postpartum hemorrhage:
- misoprostol (Cytotec) 600 μg orally (or rectally)
- methylergonovine (Methergine, a methyl derivative of ergonovine) 0.2 mg by IM injection
- ergonovine 0.2 to 0.5 mg.
Misoprostol is likely somewhat less effective than oxytocin in reducing the risk of postpartum hemorrhage,8 but it is more effective than placebo.9
Resource-limited settings. IV infusion or IM injection of a uterotonic may be difficult to perform outside of a hospital, in a resource-limited setting. In these environments, misoprostol may be useful because it can be administered orally (or rectally) and is stable at a wide range of temperatures.
In addition, a self-contained, single-use Uniject (BD, Franklin Lakes, New Jersey) injection device containing oxytocin has been developed specifically for use in resource-limited settings (FIGURE). The device can be used by minimally trained personnel and stored at room temperature for up to 2 months.

Single-use injection device
Uniject can be filled with oxytocin for use outside of a hospital setting. Photo courtesy of and © Becton, Dickinson and Company.
What’s your next step if oxytocin isn’t enough?
There is a high probability that administering oxytocin alone may not prevent postpartum hemorrhage in settings of:
- prolonged use of oxytocin for the purposes of labor induction
- a prolonged labor in which oxytocin has been used to augment labor
- chorioamnionitis.
Rather than continue to administer more and more oxytocin in these situations, consider administering a second agent, such as methlyergonovine (Methergine), misoprostol (Cytotec), or carboprost tromethamine (Hemabate, 0.25 mg IM).
Additionally, mechanical massage of the uterus can help to increase uterine tone and reduce bleeding. When postpartum hemorrhage fails to respond to the administration of multiple uterotonics, rapid institution of a postpartum bleeding protocol is warranted.10,11
Review your medication order sets for oxytocin administration
It is likely that your hospital has a well-established order set for the administration of oxytocin at birth. Reviewing your hospital’s protocols for administering oxytocin to prevent postpartum hemorrhage can help to ensure that all patients receive a uterotonic at a dose and duration that maximizes the benefits and reduces side effects and theoretical risks. Anesthesiologists play an important role in the administration of a uterotonic during cesarean delivery, and they should be involved in the review of the medication order entry sets for prevention of postpartum hemorrhage.
Prevention of postpartum hemorrhage is a top priority for all obstetric providers. Routine administration of an optimal dose of oxytocin, or another uterotonic, should be a standard clinical process in all birthing units throughout the world.
- Act fast when confronted by a coagulopathy postpartum
Robert L. Barbieri, MD (Editorial, March 2012) - Update on Obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012) - Have you made best use of the Bakri balloon in PPH?
Robert L. Barbieri, MD (Editorial, July 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai (June 2011) - Postpartum hemorrhage: 11 critical questions, answered by an expert
Q&A with Haywood L. Brown, MD (January 2011)
Postpartum hemorrhage is a common complication of birth—annually, it occurs in about 11% of natural, unmedicated childbirths and accounts for more than 50,000 maternal deaths worldwide. Administration of a uterotonic agent, such as oxytocin, at the time the anterior shoulder is delivered or after the birth of the baby reduces the risk of postpartum hemorrhage by approximately 66%.1 Administration of a uterotonic also reduces the risk of severe maternal hemorrhage (blood loss >1000 mL) and the risk of maternal transfusion by approximately 66% and 65%, respectively.1 It had been thought that early cord clamping and controlled cord traction also might help reduce the risk of postpartum hemorrhage, but recent data indicate that administering a uterotonic is the key intervention and cord traction has little or no effect on reducing the risk of hemorrhage.2
When considering the optimal approach to uterotonic administration for the purpose of postpartum hemorrhage prevention, we must ask:
- Why do so few clinicians administer a uterotonic?
- What is the optimal time for uterotonic initiation?
- If using oxytocin, what is the optimal dose and route of administration?
- Is there a dose of oxytocin that is too much?
- If postpartum hemorrhage follows oxytocin administration, what is the next step?
These questions are the focus of this editorial.
Too little oxytocin
Why do so few clinicians administer a uterotonic agent?
Across the globe, many deliveries occur without the administration of a postpartum uterotonic. In the United States, beliefs about avoiding any nonphysiologic interventions during the birth process contribute to many birthing women not receiving a uterotonic. Deliveries that occur outside of a hospital and at birthing centers are more likely to be associated with the failure to administer a uterotonic, which contributes to unnecessary postpartum hemorrhage. In these settings, administration of uterotonics used in resource-poor settings (see “Alternative Uterotonics”) may be warranted.
Bottom line: All obstetric care providers should ensure that every woman receive a uterotonic agent at birth.
Timing of oxytocin initiation
The overarching clinical goal is to ensure that every birthing mother receives a uterotonic agent; the timing of administration is of secondary importance. In the United States, oxytocin is the uterotonic most often administered at birth. It is commonly administered: 1) after delivery of the baby’s anterior shoulder, 2) after delivery of the baby but before delivery of the placenta, or 3) after delivery of the placenta.
Of the three options, the last one is the least studied. There are insufficient data to identify the optimal timing for drug initiation conclusively, but most experts conclude it should be administered somewhere between delivery of the anterior shoulder and delivery of the placenta. Clinician preference is appropriate for selecting the timing of initiation.
Optimal route, dose, and duration
The most studied routes and doses of oxytocin are a single 10-unit intramuscular (IM) dose or an intravenous (IV) infusion of 20 to 40 units oxytocin in 1000 mL of saline or lactated Ringers solution, often infused at a rate of about 125 mL/hr. The onset of action of the IM dose is typically 3 to 5 minutes, while the onset of action of the IV dose is about 1 minute.
The optimal interval for administering the oxytocin IV infusion has not been well studied. I recommend the infusion be continued for at least 4 hours following delivery. My recommendation is based on the observation that when oxytocin is discontinued shortly after birth, the risk of a delayed postpartum hemorrhage increases significantly.
Too much oxytocin
Avoid using a 5- or 10-unit IV bolus
IV boluses of oxytocin, at doses of 5 to 10 units, have been reported to be followed by hypotension,3 ischemic changes detected by electrocardiogram,4,5 and maternal death.6 Since an IV infusion of oxytocin appears to be as effective as a bolus or bolus plus IV infusion, it may be preferable to avoid the bolus and use only an IV infusion.7
Bottom line: Avoid IV bolus administration of oxytocin at doses of 5 units or 10 units due to adverse effects.
Alternative uterotonics
In addition to oxytocin, the following agents reduce the risk of postpartum hemorrhage:
- misoprostol (Cytotec) 600 μg orally (or rectally)
- methylergonovine (Methergine, a methyl derivative of ergonovine) 0.2 mg by IM injection
- ergonovine 0.2 to 0.5 mg.
Misoprostol is likely somewhat less effective than oxytocin in reducing the risk of postpartum hemorrhage,8 but it is more effective than placebo.9
Resource-limited settings. IV infusion or IM injection of a uterotonic may be difficult to perform outside of a hospital, in a resource-limited setting. In these environments, misoprostol may be useful because it can be administered orally (or rectally) and is stable at a wide range of temperatures.
In addition, a self-contained, single-use Uniject (BD, Franklin Lakes, New Jersey) injection device containing oxytocin has been developed specifically for use in resource-limited settings (FIGURE). The device can be used by minimally trained personnel and stored at room temperature for up to 2 months.

Single-use injection device
Uniject can be filled with oxytocin for use outside of a hospital setting. Photo courtesy of and © Becton, Dickinson and Company.
What’s your next step if oxytocin isn’t enough?
There is a high probability that administering oxytocin alone may not prevent postpartum hemorrhage in settings of:
- prolonged use of oxytocin for the purposes of labor induction
- a prolonged labor in which oxytocin has been used to augment labor
- chorioamnionitis.
Rather than continue to administer more and more oxytocin in these situations, consider administering a second agent, such as methlyergonovine (Methergine), misoprostol (Cytotec), or carboprost tromethamine (Hemabate, 0.25 mg IM).
Additionally, mechanical massage of the uterus can help to increase uterine tone and reduce bleeding. When postpartum hemorrhage fails to respond to the administration of multiple uterotonics, rapid institution of a postpartum bleeding protocol is warranted.10,11
Review your medication order sets for oxytocin administration
It is likely that your hospital has a well-established order set for the administration of oxytocin at birth. Reviewing your hospital’s protocols for administering oxytocin to prevent postpartum hemorrhage can help to ensure that all patients receive a uterotonic at a dose and duration that maximizes the benefits and reduces side effects and theoretical risks. Anesthesiologists play an important role in the administration of a uterotonic during cesarean delivery, and they should be involved in the review of the medication order entry sets for prevention of postpartum hemorrhage.
Prevention of postpartum hemorrhage is a top priority for all obstetric providers. Routine administration of an optimal dose of oxytocin, or another uterotonic, should be a standard clinical process in all birthing units throughout the world.
- Act fast when confronted by a coagulopathy postpartum
Robert L. Barbieri, MD (Editorial, March 2012) - Update on Obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012) - Have you made best use of the Bakri balloon in PPH?
Robert L. Barbieri, MD (Editorial, July 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai (June 2011) - Postpartum hemorrhage: 11 critical questions, answered by an expert
Q&A with Haywood L. Brown, MD (January 2011)
1. Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011;(11):CD007412.-
2. Gulmezoglu AM, Lumbiganon P, Landoulsi S, et al. Active management of the third stage of labour with and without controlled cord traction: a randomised, controlled, non-inferiority trial [published correction appears in Lancet. 2012;379(9827):1704]. Lancet. 2012;379(9827):1721-1727.
3. Archer TL, Knape K, Liles D, Wheeler AS, Carter B. The hemodynamics of oxytocin and other vasoactive agents during neuraxial anesthesia for cesarean delivery: findings in six cases. Int J Obstet Anesth. 2008;17(3):247-254.
4. Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomized controlled trial. BJOG. 2010;117(1):76-83.
5. Svanström MC, Biber B, Hanes M, Johansson G, Naslund U, Balfours EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008;100(5):683-689.
6. Lewis G, Drife J. Why Mothers Die 1977-1999. The confidential inquiries into maternal deaths in the United Kingdom. London England: RCOG Press; 2001.
7. King KJ, Douglas MJ, Unger W, Wong A, King RA. Five unit bolus oxytocin at cesarean delivery in women a risk of atony: a randomized double-blind, controlled trial. Anesth Analg. 2010;111(6):1460-1466.
8. Gülmezoglu AM, Villar J, Ngoc NT, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet. 2001;358(9283):689-695.
9. Mobeen N, Durocher J, Zuberi N, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG. 2011;118(3):353-361.
10. Barbieri RL. Act fast when confronted by a coagulopathy postpartum. OBG Manage. 2012;24(3):8, 10-11.
11. Barbieri RL. Have you made the best use of the Bakri balloon in postpartum hemorrhage? OBG Manage. 2011;23(7):6, 8-9, 19.
1. Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011;(11):CD007412.-
2. Gulmezoglu AM, Lumbiganon P, Landoulsi S, et al. Active management of the third stage of labour with and without controlled cord traction: a randomised, controlled, non-inferiority trial [published correction appears in Lancet. 2012;379(9827):1704]. Lancet. 2012;379(9827):1721-1727.
3. Archer TL, Knape K, Liles D, Wheeler AS, Carter B. The hemodynamics of oxytocin and other vasoactive agents during neuraxial anesthesia for cesarean delivery: findings in six cases. Int J Obstet Anesth. 2008;17(3):247-254.
4. Jonsson M, Hanson U, Lidell C, Norden-Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomized controlled trial. BJOG. 2010;117(1):76-83.
5. Svanström MC, Biber B, Hanes M, Johansson G, Naslund U, Balfours EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double-blind comparison of oxytocin and methylergometrine during Caesarean section. Br J Anaesth. 2008;100(5):683-689.
6. Lewis G, Drife J. Why Mothers Die 1977-1999. The confidential inquiries into maternal deaths in the United Kingdom. London England: RCOG Press; 2001.
7. King KJ, Douglas MJ, Unger W, Wong A, King RA. Five unit bolus oxytocin at cesarean delivery in women a risk of atony: a randomized double-blind, controlled trial. Anesth Analg. 2010;111(6):1460-1466.
8. Gülmezoglu AM, Villar J, Ngoc NT, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet. 2001;358(9283):689-695.
9. Mobeen N, Durocher J, Zuberi N, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo-controlled trial. BJOG. 2011;118(3):353-361.
10. Barbieri RL. Act fast when confronted by a coagulopathy postpartum. OBG Manage. 2012;24(3):8, 10-11.
11. Barbieri RL. Have you made the best use of the Bakri balloon in postpartum hemorrhage? OBG Manage. 2011;23(7):6, 8-9, 19.
Noninvasive Prenatal DNA Test Detects Trisomy 18 and 21
A noninvasive DNA screening test effectively detected chromosome abnormalities in fetuses of women with singleton pregnancies, based on data from approximately 3,000 women published online in June in the American Journal of Obstetrics and Gynecology.
Data from previous case-control studies have shown that the Digital Analysis of Selected Regions (DANSR) assay can identify chromosome abnormalities by evaluating specific fragments of maternal cell-free DNA (cfDNA), said Dr. Mary E. Norton of Stanford (Calif.) University and her colleagues.
In this multicenter, prospective cohort study, the researchers included pregnant women aged 18-50 years with singleton pregnancies who were planning to undergo invasive prenatal diagnostic testing for any reason. Women with multiples, those with known chromosome abnormalities, or those who had already undergone an invasive prenatal test in the current pregnancy were excluded. The final analysis included samples from 3,228 women with a mean maternal age of 34 years and a mean gestational age of 17 weeks (Am. J. Obstet. Gynecol. 2012 [doi:10.1016/j.ajog.2012.05.021]).
The sensitivity of the test was 100% for trisomy 21 malformations. A total of 81 cases of trisomy 21 were identified and all were classified as high risk, with one false positive out of 2,888 normal cases, for a false positive rate of 0.3% (specificity 99.97%). Similarly, the sensitivity was 97% for trisomy 18. A total of 38 cases of trisomy 18 were identified, and 37 were classified as high-risk, with 2 false positives for a false positive rate of 0.07% (specificity 99.93%).
The positive predictive values for trisomy 21 and trisomy 18 were 98.8% and 94.9%, respectively. The negative predictive values for trisomy 21 and trisomy 18 were 100% and 99.96%, respectively.
Another 73 cases of other chromosomal abnormalities were identified, but they did not affect the analysis of risk for trisomy 18 or 21, Dr. Norton and her associates noted.
The test involved collecting 20 mL of blood from each patient before she underwent any invasive diagnostic testing procedure. DNA samples were classified as high risk or low risk based on a predefined cutoff value of 1% based on previous analyses. Results from the DANSR assay were compared with results from invasive tests as a reference.
"With the methods reported here, the higher throughput and lower cost make this technique potentially scalable for population screening," Dr. Norton and her associates wrote. However, even the high detection rates achieved in the study do not compare with those obtained with invasive diagnoses and may not provide much in the way of additional information for women who then have invasive tests, they noted.
"Further experience in larger populations of average-risk women is needed to clarify the role and utility of cfDNA in clinical practice," they said.
Dr. Norton said she had no relevant financial disclosures. The study was funded by Ariosa Diagnostics, which makes the test used in the study. Several of her coauthors are employees, consultants, or board members of Ariosa Diagnostics.
Current methods of screening for common chromosomal abnormalities require multiple steps with ultrasound and blood work. This complex algorithm gives a risk for trisomy 21 that provides a sensitivity of only 88%-95%, with a 5% false-positive rate for Down syndrome. As families face the decision to choose invasive testing and the risk of fetal loss associated with these procedures, a screening test that provides more reliable results might allow parents to feel more comfortable with their decision to choose or not choose invasive testing.
Dr. Norton and her colleagues present their multicenter trial for the detection of fetal chromosomal abnormalities using analysis of cell-free DNA within maternal serum. They report a sensitivity for detection of Down syndrome of 100% for high-risk cases and 99.97% in the low risk group (with a false positive rate of 0.03%). The prediction of trisomy 18 was less sensitive, with a sensitivity of 97.4%. These tests, however, are done on women already undergoing amniocentesis for a specific reason.
Effective, low-cost screening methods are ideal in screening for chromosomal abnormalities. And this type of testing may be promising for the detection of chromosomal abnormalities other than trisomy 21 and trisomy 18. We must be reminded that this testing remains a screening test. The only way to definitively determine the karyotype is through invasive prenatal diagnosis. And is another screening test the right answer?
As we consider this testing modality, it must be studied in routine populations for low-risk patients who are not undergoing invasive testing – the clinical setting in which most of our patients would be. Although cell-free DNA testing modalities offer promise for screening, they are not yet ready to replace invasive testing for definitive diagnosis of karyotypic abnormalities.
Kristin Atkins, M.D., is in the department of obstetrics, gynecology, and reproductive sciences, and the division of maternal/fetal medicine, at the University of Maryland, Baltimore. She said she had no relevant financial disclosures.
Current methods of screening for common chromosomal abnormalities require multiple steps with ultrasound and blood work. This complex algorithm gives a risk for trisomy 21 that provides a sensitivity of only 88%-95%, with a 5% false-positive rate for Down syndrome. As families face the decision to choose invasive testing and the risk of fetal loss associated with these procedures, a screening test that provides more reliable results might allow parents to feel more comfortable with their decision to choose or not choose invasive testing.
Dr. Norton and her colleagues present their multicenter trial for the detection of fetal chromosomal abnormalities using analysis of cell-free DNA within maternal serum. They report a sensitivity for detection of Down syndrome of 100% for high-risk cases and 99.97% in the low risk group (with a false positive rate of 0.03%). The prediction of trisomy 18 was less sensitive, with a sensitivity of 97.4%. These tests, however, are done on women already undergoing amniocentesis for a specific reason.
Effective, low-cost screening methods are ideal in screening for chromosomal abnormalities. And this type of testing may be promising for the detection of chromosomal abnormalities other than trisomy 21 and trisomy 18. We must be reminded that this testing remains a screening test. The only way to definitively determine the karyotype is through invasive prenatal diagnosis. And is another screening test the right answer?
As we consider this testing modality, it must be studied in routine populations for low-risk patients who are not undergoing invasive testing – the clinical setting in which most of our patients would be. Although cell-free DNA testing modalities offer promise for screening, they are not yet ready to replace invasive testing for definitive diagnosis of karyotypic abnormalities.
Kristin Atkins, M.D., is in the department of obstetrics, gynecology, and reproductive sciences, and the division of maternal/fetal medicine, at the University of Maryland, Baltimore. She said she had no relevant financial disclosures.
Current methods of screening for common chromosomal abnormalities require multiple steps with ultrasound and blood work. This complex algorithm gives a risk for trisomy 21 that provides a sensitivity of only 88%-95%, with a 5% false-positive rate for Down syndrome. As families face the decision to choose invasive testing and the risk of fetal loss associated with these procedures, a screening test that provides more reliable results might allow parents to feel more comfortable with their decision to choose or not choose invasive testing.
Dr. Norton and her colleagues present their multicenter trial for the detection of fetal chromosomal abnormalities using analysis of cell-free DNA within maternal serum. They report a sensitivity for detection of Down syndrome of 100% for high-risk cases and 99.97% in the low risk group (with a false positive rate of 0.03%). The prediction of trisomy 18 was less sensitive, with a sensitivity of 97.4%. These tests, however, are done on women already undergoing amniocentesis for a specific reason.
Effective, low-cost screening methods are ideal in screening for chromosomal abnormalities. And this type of testing may be promising for the detection of chromosomal abnormalities other than trisomy 21 and trisomy 18. We must be reminded that this testing remains a screening test. The only way to definitively determine the karyotype is through invasive prenatal diagnosis. And is another screening test the right answer?
As we consider this testing modality, it must be studied in routine populations for low-risk patients who are not undergoing invasive testing – the clinical setting in which most of our patients would be. Although cell-free DNA testing modalities offer promise for screening, they are not yet ready to replace invasive testing for definitive diagnosis of karyotypic abnormalities.
Kristin Atkins, M.D., is in the department of obstetrics, gynecology, and reproductive sciences, and the division of maternal/fetal medicine, at the University of Maryland, Baltimore. She said she had no relevant financial disclosures.
A noninvasive DNA screening test effectively detected chromosome abnormalities in fetuses of women with singleton pregnancies, based on data from approximately 3,000 women published online in June in the American Journal of Obstetrics and Gynecology.
Data from previous case-control studies have shown that the Digital Analysis of Selected Regions (DANSR) assay can identify chromosome abnormalities by evaluating specific fragments of maternal cell-free DNA (cfDNA), said Dr. Mary E. Norton of Stanford (Calif.) University and her colleagues.
In this multicenter, prospective cohort study, the researchers included pregnant women aged 18-50 years with singleton pregnancies who were planning to undergo invasive prenatal diagnostic testing for any reason. Women with multiples, those with known chromosome abnormalities, or those who had already undergone an invasive prenatal test in the current pregnancy were excluded. The final analysis included samples from 3,228 women with a mean maternal age of 34 years and a mean gestational age of 17 weeks (Am. J. Obstet. Gynecol. 2012 [doi:10.1016/j.ajog.2012.05.021]).
The sensitivity of the test was 100% for trisomy 21 malformations. A total of 81 cases of trisomy 21 were identified and all were classified as high risk, with one false positive out of 2,888 normal cases, for a false positive rate of 0.3% (specificity 99.97%). Similarly, the sensitivity was 97% for trisomy 18. A total of 38 cases of trisomy 18 were identified, and 37 were classified as high-risk, with 2 false positives for a false positive rate of 0.07% (specificity 99.93%).
The positive predictive values for trisomy 21 and trisomy 18 were 98.8% and 94.9%, respectively. The negative predictive values for trisomy 21 and trisomy 18 were 100% and 99.96%, respectively.
Another 73 cases of other chromosomal abnormalities were identified, but they did not affect the analysis of risk for trisomy 18 or 21, Dr. Norton and her associates noted.
The test involved collecting 20 mL of blood from each patient before she underwent any invasive diagnostic testing procedure. DNA samples were classified as high risk or low risk based on a predefined cutoff value of 1% based on previous analyses. Results from the DANSR assay were compared with results from invasive tests as a reference.
"With the methods reported here, the higher throughput and lower cost make this technique potentially scalable for population screening," Dr. Norton and her associates wrote. However, even the high detection rates achieved in the study do not compare with those obtained with invasive diagnoses and may not provide much in the way of additional information for women who then have invasive tests, they noted.
"Further experience in larger populations of average-risk women is needed to clarify the role and utility of cfDNA in clinical practice," they said.
Dr. Norton said she had no relevant financial disclosures. The study was funded by Ariosa Diagnostics, which makes the test used in the study. Several of her coauthors are employees, consultants, or board members of Ariosa Diagnostics.
A noninvasive DNA screening test effectively detected chromosome abnormalities in fetuses of women with singleton pregnancies, based on data from approximately 3,000 women published online in June in the American Journal of Obstetrics and Gynecology.
Data from previous case-control studies have shown that the Digital Analysis of Selected Regions (DANSR) assay can identify chromosome abnormalities by evaluating specific fragments of maternal cell-free DNA (cfDNA), said Dr. Mary E. Norton of Stanford (Calif.) University and her colleagues.
In this multicenter, prospective cohort study, the researchers included pregnant women aged 18-50 years with singleton pregnancies who were planning to undergo invasive prenatal diagnostic testing for any reason. Women with multiples, those with known chromosome abnormalities, or those who had already undergone an invasive prenatal test in the current pregnancy were excluded. The final analysis included samples from 3,228 women with a mean maternal age of 34 years and a mean gestational age of 17 weeks (Am. J. Obstet. Gynecol. 2012 [doi:10.1016/j.ajog.2012.05.021]).
The sensitivity of the test was 100% for trisomy 21 malformations. A total of 81 cases of trisomy 21 were identified and all were classified as high risk, with one false positive out of 2,888 normal cases, for a false positive rate of 0.3% (specificity 99.97%). Similarly, the sensitivity was 97% for trisomy 18. A total of 38 cases of trisomy 18 were identified, and 37 were classified as high-risk, with 2 false positives for a false positive rate of 0.07% (specificity 99.93%).
The positive predictive values for trisomy 21 and trisomy 18 were 98.8% and 94.9%, respectively. The negative predictive values for trisomy 21 and trisomy 18 were 100% and 99.96%, respectively.
Another 73 cases of other chromosomal abnormalities were identified, but they did not affect the analysis of risk for trisomy 18 or 21, Dr. Norton and her associates noted.
The test involved collecting 20 mL of blood from each patient before she underwent any invasive diagnostic testing procedure. DNA samples were classified as high risk or low risk based on a predefined cutoff value of 1% based on previous analyses. Results from the DANSR assay were compared with results from invasive tests as a reference.
"With the methods reported here, the higher throughput and lower cost make this technique potentially scalable for population screening," Dr. Norton and her associates wrote. However, even the high detection rates achieved in the study do not compare with those obtained with invasive diagnoses and may not provide much in the way of additional information for women who then have invasive tests, they noted.
"Further experience in larger populations of average-risk women is needed to clarify the role and utility of cfDNA in clinical practice," they said.
Dr. Norton said she had no relevant financial disclosures. The study was funded by Ariosa Diagnostics, which makes the test used in the study. Several of her coauthors are employees, consultants, or board members of Ariosa Diagnostics.
FROM THE AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
FDA Affirms Safety of Compounded Preterm Labor Drug
No major safety problems were identified in the Food and Drug Administration’s review of compounded formulations of hydroxyprogesterone caproate, but the agency continues to emphasize that the approved version of the product, known as Makena, used to reduce the risk of preterm birth in certain patients, is more reliable.
A statement issued by the FDA on June 15 says that the agency’s analyses of a "limited sample" of compounded formulations of hydroxyprogesterone caproate and samples of the active ingredient used to make these formulations – obtained from compounding pharmacies, physician’s offices, distributors of the active pharmaceutical ingredient (API) and imported APIs – found that most had met potency and purity standards. However, approved products such as Makena, the FDA-approved version of hydroxyprogesterone caproate, "provide a greater assurance of safety and effectiveness than do compounded products," the statement said.
Hydroxyprogesterone caproate, a progestin, is the active ingredient in Makena, which was approved by the FDA in February 2011, for reducing the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth; it is administered intramuscularly once a week. Before the approval of Makena, formulations of hydroxyprogesterone caproate compounded by pharmacists had been used for years, and the availability of a reliable approved version was welcomed, but enthusiasm was quickly tempered by the high price tag of the approved version.
In November 2011, the FDA announced that it had started to conduct analyses of compounded hydroxyprogesterone caproate products and bulk APIs, and that it would review data submitted by K-V Pharmaceuticals, the sponsor of Makena, which had found "variability" in their purity and potency.
In the June 15 statement, the FDA provided the final results of its testing and analyses: All 16 samples of the hydroxyprogesterone caproate API passed the tests for potency as specified by the United States Pharmacopeia (USP), and passed the potency tests used in the approval application for Makena. All 16 of these samples also passed the standard for total purity that was used in the Makena application, but "failed" to meet the limit for unidentified impurities used in the Makena application. The FDA also identified four impurities that exceeded the levels allowed in the Makena application, but they "do not raise safety concerns," the statement said,
Of the 13 compounded hydroxyprogesterone caproate products from eight pharmacies, one was subpotent, at about 80% of declared potency. All samples met the standard for total purity in the Makena application, and 2 of the 13 samples did not meet the standard for unidentified impurities used in the Makena application.
In the FDA’s analysis of the 26 samples of the compounded product from the laboratories that had conducted the testing for K-V, 3 failed to meet the potency standard with the method used in the Makena new drug application (NDA), and 7 of these samples failed the standard for unidentified purities that were used in the Makena NDA.
"Although the analysis of this limited sample of compounded hydroxyprogesterone caproate products and APIs did not identify any major safety problems, approved drug products, such as Makena, provide a greater assurance of safety and effectiveness than do compounded products," the FDA statement said. "Before approving the Makena NDA, FDA reviewed manufacturing information, such as the source of the API used by its manufacturer, proposed manufacturing processes, and the firm’s adherence to current good manufacturing practice."
Compounded products are not FDA approved, and compounding of any drug "should not exceed the scope of traditional pharmacy compounding," according to the FDA.
The full FDA statement is available.
No major safety problems were identified in the Food and Drug Administration’s review of compounded formulations of hydroxyprogesterone caproate, but the agency continues to emphasize that the approved version of the product, known as Makena, used to reduce the risk of preterm birth in certain patients, is more reliable.
A statement issued by the FDA on June 15 says that the agency’s analyses of a "limited sample" of compounded formulations of hydroxyprogesterone caproate and samples of the active ingredient used to make these formulations – obtained from compounding pharmacies, physician’s offices, distributors of the active pharmaceutical ingredient (API) and imported APIs – found that most had met potency and purity standards. However, approved products such as Makena, the FDA-approved version of hydroxyprogesterone caproate, "provide a greater assurance of safety and effectiveness than do compounded products," the statement said.
Hydroxyprogesterone caproate, a progestin, is the active ingredient in Makena, which was approved by the FDA in February 2011, for reducing the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth; it is administered intramuscularly once a week. Before the approval of Makena, formulations of hydroxyprogesterone caproate compounded by pharmacists had been used for years, and the availability of a reliable approved version was welcomed, but enthusiasm was quickly tempered by the high price tag of the approved version.
In November 2011, the FDA announced that it had started to conduct analyses of compounded hydroxyprogesterone caproate products and bulk APIs, and that it would review data submitted by K-V Pharmaceuticals, the sponsor of Makena, which had found "variability" in their purity and potency.
In the June 15 statement, the FDA provided the final results of its testing and analyses: All 16 samples of the hydroxyprogesterone caproate API passed the tests for potency as specified by the United States Pharmacopeia (USP), and passed the potency tests used in the approval application for Makena. All 16 of these samples also passed the standard for total purity that was used in the Makena application, but "failed" to meet the limit for unidentified impurities used in the Makena application. The FDA also identified four impurities that exceeded the levels allowed in the Makena application, but they "do not raise safety concerns," the statement said,
Of the 13 compounded hydroxyprogesterone caproate products from eight pharmacies, one was subpotent, at about 80% of declared potency. All samples met the standard for total purity in the Makena application, and 2 of the 13 samples did not meet the standard for unidentified impurities used in the Makena application.
In the FDA’s analysis of the 26 samples of the compounded product from the laboratories that had conducted the testing for K-V, 3 failed to meet the potency standard with the method used in the Makena new drug application (NDA), and 7 of these samples failed the standard for unidentified purities that were used in the Makena NDA.
"Although the analysis of this limited sample of compounded hydroxyprogesterone caproate products and APIs did not identify any major safety problems, approved drug products, such as Makena, provide a greater assurance of safety and effectiveness than do compounded products," the FDA statement said. "Before approving the Makena NDA, FDA reviewed manufacturing information, such as the source of the API used by its manufacturer, proposed manufacturing processes, and the firm’s adherence to current good manufacturing practice."
Compounded products are not FDA approved, and compounding of any drug "should not exceed the scope of traditional pharmacy compounding," according to the FDA.
The full FDA statement is available.
No major safety problems were identified in the Food and Drug Administration’s review of compounded formulations of hydroxyprogesterone caproate, but the agency continues to emphasize that the approved version of the product, known as Makena, used to reduce the risk of preterm birth in certain patients, is more reliable.
A statement issued by the FDA on June 15 says that the agency’s analyses of a "limited sample" of compounded formulations of hydroxyprogesterone caproate and samples of the active ingredient used to make these formulations – obtained from compounding pharmacies, physician’s offices, distributors of the active pharmaceutical ingredient (API) and imported APIs – found that most had met potency and purity standards. However, approved products such as Makena, the FDA-approved version of hydroxyprogesterone caproate, "provide a greater assurance of safety and effectiveness than do compounded products," the statement said.
Hydroxyprogesterone caproate, a progestin, is the active ingredient in Makena, which was approved by the FDA in February 2011, for reducing the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth; it is administered intramuscularly once a week. Before the approval of Makena, formulations of hydroxyprogesterone caproate compounded by pharmacists had been used for years, and the availability of a reliable approved version was welcomed, but enthusiasm was quickly tempered by the high price tag of the approved version.
In November 2011, the FDA announced that it had started to conduct analyses of compounded hydroxyprogesterone caproate products and bulk APIs, and that it would review data submitted by K-V Pharmaceuticals, the sponsor of Makena, which had found "variability" in their purity and potency.
In the June 15 statement, the FDA provided the final results of its testing and analyses: All 16 samples of the hydroxyprogesterone caproate API passed the tests for potency as specified by the United States Pharmacopeia (USP), and passed the potency tests used in the approval application for Makena. All 16 of these samples also passed the standard for total purity that was used in the Makena application, but "failed" to meet the limit for unidentified impurities used in the Makena application. The FDA also identified four impurities that exceeded the levels allowed in the Makena application, but they "do not raise safety concerns," the statement said,
Of the 13 compounded hydroxyprogesterone caproate products from eight pharmacies, one was subpotent, at about 80% of declared potency. All samples met the standard for total purity in the Makena application, and 2 of the 13 samples did not meet the standard for unidentified impurities used in the Makena application.
In the FDA’s analysis of the 26 samples of the compounded product from the laboratories that had conducted the testing for K-V, 3 failed to meet the potency standard with the method used in the Makena new drug application (NDA), and 7 of these samples failed the standard for unidentified purities that were used in the Makena NDA.
"Although the analysis of this limited sample of compounded hydroxyprogesterone caproate products and APIs did not identify any major safety problems, approved drug products, such as Makena, provide a greater assurance of safety and effectiveness than do compounded products," the FDA statement said. "Before approving the Makena NDA, FDA reviewed manufacturing information, such as the source of the API used by its manufacturer, proposed manufacturing processes, and the firm’s adherence to current good manufacturing practice."
Compounded products are not FDA approved, and compounding of any drug "should not exceed the scope of traditional pharmacy compounding," according to the FDA.
The full FDA statement is available.
Third-Trimester Ultrasound Predicts Shoulder Dystocia
SAN DIEGO – Biometric findings on third-trimester ultrasound predicted increased risk for shoulder dystocia at term in a chart review of 132 pregnancies in women without diabetes.
The risk of shoulder dystocia doubled with every unit deviation from the mean for the ratio of femur length to head circumference and the ratio of head circumference to abdominal circumference, Dr. Stephanie M. Melka and her associates reported in a poster presentation at the annual meeting of the American College of Obstetricians and Gynecologists.
Other factors that independently predicted a statistically significant 4%-17% increased risk for shoulder dystocia at term included estimated fetal weight, femur length, abdominal circumference, and the ratio of femur length to biparietal diameter, reported Dr. Melka of Mount Sinai School of Medicine, New York.
The only standard measurements and ratios on prenatal ultrasound that were not significantly associated with increased risk for shoulder dystocia were head circumference and the ratio of femur length to abdominal circumference.
Several previous studies have shown that prenatal sonographic findings can predict shoulder dystocia at term in diabetic mothers, but this appears to be the first study of elective third-trimester sonography in low-risk nondiabetic women, the investigators noted.
The study identified 44 cases of shoulder dystocia during vaginal delivery at Mount Sinai in 2008-2009 in which the mothers underwent ultrasound examination early in the third trimester prior to 37 weeks. Each case was matched with two women who had a third-trimester ultrasound on the same day, for a total of 88 women in the control group.
The ratio of femur length to head circumference averaged 21 in the control group and 21.6 in the shoulder dystocia group. The ratio of head circumference to abdominal circumference averaged 1.05 in the control group and 1 in the dystocia group.
Biometric measurements and estimated fetal weight ascertained from third-trimester prenatal ultrasound can be used to counsel mothers on the risk for shoulder dystocia, the investigators concluded.
Other, classic risk factors for shoulder dystocia include maternal diabetes, obesity, a history of shoulder dystocia, and excessive weight gain during pregnancy.
The study did not control for the effects of obesity or weight gain on the risk for shoulder dystocia. It also did not assess the influence of differences in gestational age at the time of the sonograms.
Shoulder dystocia occurs in approximately 1% of all deliveries. Half of cases have been thought to be unpredictable.
Dr. Melka reported having no relevant financial disclosures.
femur length, head circumference, head circumference, abdominal circumference, Dr. Stephanie M. Melka, the American College of Obstetricians and Gynecologists, prenatal sonographic findings, diabetic mothers, elective third-trimester sonography, vaginal delivery,
SAN DIEGO – Biometric findings on third-trimester ultrasound predicted increased risk for shoulder dystocia at term in a chart review of 132 pregnancies in women without diabetes.
The risk of shoulder dystocia doubled with every unit deviation from the mean for the ratio of femur length to head circumference and the ratio of head circumference to abdominal circumference, Dr. Stephanie M. Melka and her associates reported in a poster presentation at the annual meeting of the American College of Obstetricians and Gynecologists.
Other factors that independently predicted a statistically significant 4%-17% increased risk for shoulder dystocia at term included estimated fetal weight, femur length, abdominal circumference, and the ratio of femur length to biparietal diameter, reported Dr. Melka of Mount Sinai School of Medicine, New York.
The only standard measurements and ratios on prenatal ultrasound that were not significantly associated with increased risk for shoulder dystocia were head circumference and the ratio of femur length to abdominal circumference.
Several previous studies have shown that prenatal sonographic findings can predict shoulder dystocia at term in diabetic mothers, but this appears to be the first study of elective third-trimester sonography in low-risk nondiabetic women, the investigators noted.
The study identified 44 cases of shoulder dystocia during vaginal delivery at Mount Sinai in 2008-2009 in which the mothers underwent ultrasound examination early in the third trimester prior to 37 weeks. Each case was matched with two women who had a third-trimester ultrasound on the same day, for a total of 88 women in the control group.
The ratio of femur length to head circumference averaged 21 in the control group and 21.6 in the shoulder dystocia group. The ratio of head circumference to abdominal circumference averaged 1.05 in the control group and 1 in the dystocia group.
Biometric measurements and estimated fetal weight ascertained from third-trimester prenatal ultrasound can be used to counsel mothers on the risk for shoulder dystocia, the investigators concluded.
Other, classic risk factors for shoulder dystocia include maternal diabetes, obesity, a history of shoulder dystocia, and excessive weight gain during pregnancy.
The study did not control for the effects of obesity or weight gain on the risk for shoulder dystocia. It also did not assess the influence of differences in gestational age at the time of the sonograms.
Shoulder dystocia occurs in approximately 1% of all deliveries. Half of cases have been thought to be unpredictable.
Dr. Melka reported having no relevant financial disclosures.
SAN DIEGO – Biometric findings on third-trimester ultrasound predicted increased risk for shoulder dystocia at term in a chart review of 132 pregnancies in women without diabetes.
The risk of shoulder dystocia doubled with every unit deviation from the mean for the ratio of femur length to head circumference and the ratio of head circumference to abdominal circumference, Dr. Stephanie M. Melka and her associates reported in a poster presentation at the annual meeting of the American College of Obstetricians and Gynecologists.
Other factors that independently predicted a statistically significant 4%-17% increased risk for shoulder dystocia at term included estimated fetal weight, femur length, abdominal circumference, and the ratio of femur length to biparietal diameter, reported Dr. Melka of Mount Sinai School of Medicine, New York.
The only standard measurements and ratios on prenatal ultrasound that were not significantly associated with increased risk for shoulder dystocia were head circumference and the ratio of femur length to abdominal circumference.
Several previous studies have shown that prenatal sonographic findings can predict shoulder dystocia at term in diabetic mothers, but this appears to be the first study of elective third-trimester sonography in low-risk nondiabetic women, the investigators noted.
The study identified 44 cases of shoulder dystocia during vaginal delivery at Mount Sinai in 2008-2009 in which the mothers underwent ultrasound examination early in the third trimester prior to 37 weeks. Each case was matched with two women who had a third-trimester ultrasound on the same day, for a total of 88 women in the control group.
The ratio of femur length to head circumference averaged 21 in the control group and 21.6 in the shoulder dystocia group. The ratio of head circumference to abdominal circumference averaged 1.05 in the control group and 1 in the dystocia group.
Biometric measurements and estimated fetal weight ascertained from third-trimester prenatal ultrasound can be used to counsel mothers on the risk for shoulder dystocia, the investigators concluded.
Other, classic risk factors for shoulder dystocia include maternal diabetes, obesity, a history of shoulder dystocia, and excessive weight gain during pregnancy.
The study did not control for the effects of obesity or weight gain on the risk for shoulder dystocia. It also did not assess the influence of differences in gestational age at the time of the sonograms.
Shoulder dystocia occurs in approximately 1% of all deliveries. Half of cases have been thought to be unpredictable.
Dr. Melka reported having no relevant financial disclosures.
femur length, head circumference, head circumference, abdominal circumference, Dr. Stephanie M. Melka, the American College of Obstetricians and Gynecologists, prenatal sonographic findings, diabetic mothers, elective third-trimester sonography, vaginal delivery,
femur length, head circumference, head circumference, abdominal circumference, Dr. Stephanie M. Melka, the American College of Obstetricians and Gynecologists, prenatal sonographic findings, diabetic mothers, elective third-trimester sonography, vaginal delivery,
AT THE ANNUAL MEETING OF THE AMERICAN COLLEGE OF OBSTETRICIANS AND GYNECOLOGISTS
At 6 Months Postcesarean, 3% Have Chronic Pain
MONTEREY, CALIF. – Chronic pain after cesarean delivery is uncommon and generally mild, new data show. Additionally, women undergoing repeat cesarean do not have worse pain than their counterparts undergoing primary cesarean.
These were among the key findings of a pair of prospective longitudinal studies that investigators reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
The studies took place among healthy women from Brazil and the United States who were having scheduled elective cesarean deliveries with no prior labor, using standardized spinal anesthesia, surgical techniques, and multimodal postoperative analgesia.
Prevalence and description of chronic pain
In the first study, investigators led by Dr. Clemens M. Ortner of the department of anesthesiology and pain medicine at the University of Washington Medical Center in Seattle, followed 360 women from São Paulo, Brazil.
They used two tools recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), which is endorsed by the Food and Drug Administration: the Brief Pain Inventory (BPI), which captures the effect of pain on physical functioning, and the Short-Form McGill Pain Questionnaire 2 (SF-MPQ-2), which evaluates the nature of pain using 22 descriptors that are grouped into four overarching types of pain.
The women were a mean 31 years. For the large majority (70%), the cesarean was their first. Mean surgical time was 40 minutes.
Results with the BPI showed that 3% of women had pain at 6 months, Dr. Ortner reported. However, in the majority of cases, the pain was rated as mild.
Data from the SF-MPQ-2 suggested that the pain was most often of the neuropathic type and seldom of the continuous, intermittent, or affective types.
The leading descriptor the women selected for the pain, cited by 38% of those affected, was numbness. "Some would actually even argue [that numbness] does not qualify as a pain symptom," commented Dr. Ortner. Descriptors such as throbbing, tender, cramping, stabbing, and piercing were rarely used.
"This is the first prospective longitudinal study to characterize chronic pain after cesarean delivery using the SF-MPQ-2, and to our knowledge, is actually the first study that uses the SF-MPQ-2 that tries to characterize chronic postoperative pain," Dr. Ortner noted.
"Our incidence of chronic pain was very low at 6 months: It was 3%. However, this population was a very healthy population that had no other risk factors, like prior sensitization or other chronic pain syndromes," he cautioned.
"We can take this information from our study as a control for future analysis where we can use the SF-MPQ-2 to investigate chronic pain in high-risk populations undergoing cesarean delivery," he added.
Dr. Kenneth E. Nelson, session moderator and an obstetrical anesthesiologist at Wake Forest Baptist Medical Center in Winston Salem, N.C., wondered if the pain descriptors of the SF-MPQ-2 had the same meaning in the Brazilian context. "Some of those words are pretty esoteric. How culturally sensitive are they? Do you see a difference in different cultures using those words to describe pain, and is that potentially an issue with comparing one of these studies to another?" he asked.
"It is true that the SF-MPQ-2 was validated in an English-speaking population. However, these descriptors have been retrieved from a very, very large population of patients, how they describe their pain," Dr. Ortner replied. Additionally, the questionnaire underwent a rigorous validation process, whereby it was translated for a Portuguese-speaking population and then translated back to English. "However, of course, cultural differences can have an impact on these results, yes."
Pain with primary vs. repeat cesarean
In the second study – the only prospective evaluation of pain among healthy women after primary vs. repeat cesareans – a team led by Dr. Ruth Landau studied 451 women from São Paulo, Brazil, and Seattle.
Subsequent cesarean deliveries are noteworthy among surgical procedures in that surgeons typically go through the same scar again, a process that some speculate may contribute to greater pain, she noted.
"The hypothesis is that central sensitization as can be elicited by scar hyperalgesia after a previous cesarean section could result in increased pain if the surgery is repeated," she explained. "And the clinical implications for that would be that analgesic regimens may need to be adapted in women undergoing a repeat cesarean delivery, and that we should tailor our postoperative analgesia based on whether it’s a primary or repeat cesarean section."
Overall, 67% of the women studied were undergoing a primary cesarean delivery, whereas 33% were undergoing a repeat cesarean delivery. They had a mean age of about 30 years.
Results on the BPI showed relatively low scores (2-5 points on a 10-point scale) for pain – at rest, while sitting, and specifically for uterine cramping – at 12 hours and at 24 hours postoperatively, with no significant differences between the primary and repeat cesarean groups, reported Dr. Landau, who is professor and director of obstetric anesthesiology and clinical genetics, anesthesiology, and pain medicine at the University of Washington Medical Center.
At 48 hours, there was a trend whereby women undergoing repeat cesarean had a higher level of pain while at rest relative to women undergoing primary cesarean, but pain while sitting, uterine cramping, wound hyperalgesia, and opioid use were essentially the same.
There also were no significant differences between the primary and repeat cesarean groups with respect to average pain in the past week, worst pain in the past week, and pain now, at either 8 weeks or at 6 months postoperatively.
"In this cohort of healthy women undergoing an elective cesarean delivery under what is considered the best modality to have good intraoperative anesthesia and postoperative analgesia, we did not find a relevant difference in pain scores or analgesic use in the first 48 hours. Therefore, I cannot recommend we should change our practice in giving everybody the same thing," said Dr. Landau.
"I would however caution that we shouldn’t yet extrapolate these results to other clinical contexts, in particular, women who have high risk for pain, prior pain, a nonelective primary cesarean delivery, or women [operated on] with different surgical techniques," she added. "And I would propose ... that we do look into scar hyperalgesia in those who have had a previous cesarean delivery, in trying to tease out women who are potentially at risk for more severe pain postoperatively."
Session moderator Dr. Dennis C. Shay in a group anesthesia practice in San Diego asked, "Did you look at activity levels? I would imagine with a repeat C-section, they have a young child, so if the child is visiting, maybe they are getting up and walking around a lot more than if it was just their first child. And maybe that would make a difference."
It would be challenging to interpret how activity data relate to pain data, Dr. Landau replied. For example, women could be less active because they have more pain or because they are more comfortable.
Dr. Ortner and Dr. Landau said they had no relevant financial conflicts of interest.
MONTEREY, CALIF. – Chronic pain after cesarean delivery is uncommon and generally mild, new data show. Additionally, women undergoing repeat cesarean do not have worse pain than their counterparts undergoing primary cesarean.
These were among the key findings of a pair of prospective longitudinal studies that investigators reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
The studies took place among healthy women from Brazil and the United States who were having scheduled elective cesarean deliveries with no prior labor, using standardized spinal anesthesia, surgical techniques, and multimodal postoperative analgesia.
Prevalence and description of chronic pain
In the first study, investigators led by Dr. Clemens M. Ortner of the department of anesthesiology and pain medicine at the University of Washington Medical Center in Seattle, followed 360 women from São Paulo, Brazil.
They used two tools recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), which is endorsed by the Food and Drug Administration: the Brief Pain Inventory (BPI), which captures the effect of pain on physical functioning, and the Short-Form McGill Pain Questionnaire 2 (SF-MPQ-2), which evaluates the nature of pain using 22 descriptors that are grouped into four overarching types of pain.
The women were a mean 31 years. For the large majority (70%), the cesarean was their first. Mean surgical time was 40 minutes.
Results with the BPI showed that 3% of women had pain at 6 months, Dr. Ortner reported. However, in the majority of cases, the pain was rated as mild.
Data from the SF-MPQ-2 suggested that the pain was most often of the neuropathic type and seldom of the continuous, intermittent, or affective types.
The leading descriptor the women selected for the pain, cited by 38% of those affected, was numbness. "Some would actually even argue [that numbness] does not qualify as a pain symptom," commented Dr. Ortner. Descriptors such as throbbing, tender, cramping, stabbing, and piercing were rarely used.
"This is the first prospective longitudinal study to characterize chronic pain after cesarean delivery using the SF-MPQ-2, and to our knowledge, is actually the first study that uses the SF-MPQ-2 that tries to characterize chronic postoperative pain," Dr. Ortner noted.
"Our incidence of chronic pain was very low at 6 months: It was 3%. However, this population was a very healthy population that had no other risk factors, like prior sensitization or other chronic pain syndromes," he cautioned.
"We can take this information from our study as a control for future analysis where we can use the SF-MPQ-2 to investigate chronic pain in high-risk populations undergoing cesarean delivery," he added.
Dr. Kenneth E. Nelson, session moderator and an obstetrical anesthesiologist at Wake Forest Baptist Medical Center in Winston Salem, N.C., wondered if the pain descriptors of the SF-MPQ-2 had the same meaning in the Brazilian context. "Some of those words are pretty esoteric. How culturally sensitive are they? Do you see a difference in different cultures using those words to describe pain, and is that potentially an issue with comparing one of these studies to another?" he asked.
"It is true that the SF-MPQ-2 was validated in an English-speaking population. However, these descriptors have been retrieved from a very, very large population of patients, how they describe their pain," Dr. Ortner replied. Additionally, the questionnaire underwent a rigorous validation process, whereby it was translated for a Portuguese-speaking population and then translated back to English. "However, of course, cultural differences can have an impact on these results, yes."
Pain with primary vs. repeat cesarean
In the second study – the only prospective evaluation of pain among healthy women after primary vs. repeat cesareans – a team led by Dr. Ruth Landau studied 451 women from São Paulo, Brazil, and Seattle.
Subsequent cesarean deliveries are noteworthy among surgical procedures in that surgeons typically go through the same scar again, a process that some speculate may contribute to greater pain, she noted.
"The hypothesis is that central sensitization as can be elicited by scar hyperalgesia after a previous cesarean section could result in increased pain if the surgery is repeated," she explained. "And the clinical implications for that would be that analgesic regimens may need to be adapted in women undergoing a repeat cesarean delivery, and that we should tailor our postoperative analgesia based on whether it’s a primary or repeat cesarean section."
Overall, 67% of the women studied were undergoing a primary cesarean delivery, whereas 33% were undergoing a repeat cesarean delivery. They had a mean age of about 30 years.
Results on the BPI showed relatively low scores (2-5 points on a 10-point scale) for pain – at rest, while sitting, and specifically for uterine cramping – at 12 hours and at 24 hours postoperatively, with no significant differences between the primary and repeat cesarean groups, reported Dr. Landau, who is professor and director of obstetric anesthesiology and clinical genetics, anesthesiology, and pain medicine at the University of Washington Medical Center.
At 48 hours, there was a trend whereby women undergoing repeat cesarean had a higher level of pain while at rest relative to women undergoing primary cesarean, but pain while sitting, uterine cramping, wound hyperalgesia, and opioid use were essentially the same.
There also were no significant differences between the primary and repeat cesarean groups with respect to average pain in the past week, worst pain in the past week, and pain now, at either 8 weeks or at 6 months postoperatively.
"In this cohort of healthy women undergoing an elective cesarean delivery under what is considered the best modality to have good intraoperative anesthesia and postoperative analgesia, we did not find a relevant difference in pain scores or analgesic use in the first 48 hours. Therefore, I cannot recommend we should change our practice in giving everybody the same thing," said Dr. Landau.
"I would however caution that we shouldn’t yet extrapolate these results to other clinical contexts, in particular, women who have high risk for pain, prior pain, a nonelective primary cesarean delivery, or women [operated on] with different surgical techniques," she added. "And I would propose ... that we do look into scar hyperalgesia in those who have had a previous cesarean delivery, in trying to tease out women who are potentially at risk for more severe pain postoperatively."
Session moderator Dr. Dennis C. Shay in a group anesthesia practice in San Diego asked, "Did you look at activity levels? I would imagine with a repeat C-section, they have a young child, so if the child is visiting, maybe they are getting up and walking around a lot more than if it was just their first child. And maybe that would make a difference."
It would be challenging to interpret how activity data relate to pain data, Dr. Landau replied. For example, women could be less active because they have more pain or because they are more comfortable.
Dr. Ortner and Dr. Landau said they had no relevant financial conflicts of interest.
MONTEREY, CALIF. – Chronic pain after cesarean delivery is uncommon and generally mild, new data show. Additionally, women undergoing repeat cesarean do not have worse pain than their counterparts undergoing primary cesarean.
These were among the key findings of a pair of prospective longitudinal studies that investigators reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
The studies took place among healthy women from Brazil and the United States who were having scheduled elective cesarean deliveries with no prior labor, using standardized spinal anesthesia, surgical techniques, and multimodal postoperative analgesia.
Prevalence and description of chronic pain
In the first study, investigators led by Dr. Clemens M. Ortner of the department of anesthesiology and pain medicine at the University of Washington Medical Center in Seattle, followed 360 women from São Paulo, Brazil.
They used two tools recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT), which is endorsed by the Food and Drug Administration: the Brief Pain Inventory (BPI), which captures the effect of pain on physical functioning, and the Short-Form McGill Pain Questionnaire 2 (SF-MPQ-2), which evaluates the nature of pain using 22 descriptors that are grouped into four overarching types of pain.
The women were a mean 31 years. For the large majority (70%), the cesarean was their first. Mean surgical time was 40 minutes.
Results with the BPI showed that 3% of women had pain at 6 months, Dr. Ortner reported. However, in the majority of cases, the pain was rated as mild.
Data from the SF-MPQ-2 suggested that the pain was most often of the neuropathic type and seldom of the continuous, intermittent, or affective types.
The leading descriptor the women selected for the pain, cited by 38% of those affected, was numbness. "Some would actually even argue [that numbness] does not qualify as a pain symptom," commented Dr. Ortner. Descriptors such as throbbing, tender, cramping, stabbing, and piercing were rarely used.
"This is the first prospective longitudinal study to characterize chronic pain after cesarean delivery using the SF-MPQ-2, and to our knowledge, is actually the first study that uses the SF-MPQ-2 that tries to characterize chronic postoperative pain," Dr. Ortner noted.
"Our incidence of chronic pain was very low at 6 months: It was 3%. However, this population was a very healthy population that had no other risk factors, like prior sensitization or other chronic pain syndromes," he cautioned.
"We can take this information from our study as a control for future analysis where we can use the SF-MPQ-2 to investigate chronic pain in high-risk populations undergoing cesarean delivery," he added.
Dr. Kenneth E. Nelson, session moderator and an obstetrical anesthesiologist at Wake Forest Baptist Medical Center in Winston Salem, N.C., wondered if the pain descriptors of the SF-MPQ-2 had the same meaning in the Brazilian context. "Some of those words are pretty esoteric. How culturally sensitive are they? Do you see a difference in different cultures using those words to describe pain, and is that potentially an issue with comparing one of these studies to another?" he asked.
"It is true that the SF-MPQ-2 was validated in an English-speaking population. However, these descriptors have been retrieved from a very, very large population of patients, how they describe their pain," Dr. Ortner replied. Additionally, the questionnaire underwent a rigorous validation process, whereby it was translated for a Portuguese-speaking population and then translated back to English. "However, of course, cultural differences can have an impact on these results, yes."
Pain with primary vs. repeat cesarean
In the second study – the only prospective evaluation of pain among healthy women after primary vs. repeat cesareans – a team led by Dr. Ruth Landau studied 451 women from São Paulo, Brazil, and Seattle.
Subsequent cesarean deliveries are noteworthy among surgical procedures in that surgeons typically go through the same scar again, a process that some speculate may contribute to greater pain, she noted.
"The hypothesis is that central sensitization as can be elicited by scar hyperalgesia after a previous cesarean section could result in increased pain if the surgery is repeated," she explained. "And the clinical implications for that would be that analgesic regimens may need to be adapted in women undergoing a repeat cesarean delivery, and that we should tailor our postoperative analgesia based on whether it’s a primary or repeat cesarean section."
Overall, 67% of the women studied were undergoing a primary cesarean delivery, whereas 33% were undergoing a repeat cesarean delivery. They had a mean age of about 30 years.
Results on the BPI showed relatively low scores (2-5 points on a 10-point scale) for pain – at rest, while sitting, and specifically for uterine cramping – at 12 hours and at 24 hours postoperatively, with no significant differences between the primary and repeat cesarean groups, reported Dr. Landau, who is professor and director of obstetric anesthesiology and clinical genetics, anesthesiology, and pain medicine at the University of Washington Medical Center.
At 48 hours, there was a trend whereby women undergoing repeat cesarean had a higher level of pain while at rest relative to women undergoing primary cesarean, but pain while sitting, uterine cramping, wound hyperalgesia, and opioid use were essentially the same.
There also were no significant differences between the primary and repeat cesarean groups with respect to average pain in the past week, worst pain in the past week, and pain now, at either 8 weeks or at 6 months postoperatively.
"In this cohort of healthy women undergoing an elective cesarean delivery under what is considered the best modality to have good intraoperative anesthesia and postoperative analgesia, we did not find a relevant difference in pain scores or analgesic use in the first 48 hours. Therefore, I cannot recommend we should change our practice in giving everybody the same thing," said Dr. Landau.
"I would however caution that we shouldn’t yet extrapolate these results to other clinical contexts, in particular, women who have high risk for pain, prior pain, a nonelective primary cesarean delivery, or women [operated on] with different surgical techniques," she added. "And I would propose ... that we do look into scar hyperalgesia in those who have had a previous cesarean delivery, in trying to tease out women who are potentially at risk for more severe pain postoperatively."
Session moderator Dr. Dennis C. Shay in a group anesthesia practice in San Diego asked, "Did you look at activity levels? I would imagine with a repeat C-section, they have a young child, so if the child is visiting, maybe they are getting up and walking around a lot more than if it was just their first child. And maybe that would make a difference."
It would be challenging to interpret how activity data relate to pain data, Dr. Landau replied. For example, women could be less active because they have more pain or because they are more comfortable.
Dr. Ortner and Dr. Landau said they had no relevant financial conflicts of interest.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATOLOGY
Major Finding: Only 3% of women in the first study had pain at 6 months, and it was usually mild.
Data Source: Results were taken from one of a pair of prospective longitudinal studies. The first study was based on a cohort of 360 healthy women undergoing elective cesarean delivery with standardized spinal anesthesia, surgical techniques, and multimodal postoperative analgesia.
Disclosures: Dr. Ortner and Dr. Landau disclosed no relevant conflicts of interest.
Early Epidural Doubles C-Section Risk After Induction
SAN DIEGO – The risk of C-section doubled in women undergoing induction of labor who received an epidural before 4 -cm dilation, compared with later epidural administration, a retrospective study found.
In 281 singleton pregnancies at gestational ages greater than 36 weeks that underwent induction of labor from 2008 to 2009 at one institution, 83% of mothers received an epidural. The C-section rate was significantly higher among the 233 women who received an epidural, compared with the 48 women who did not get an epidural – 30% vs. 8%.
In unadjusted results for women who got an epidural, "early" administration before 4-cm dilation was associated with a 38% chance of C-section, compared with a 24% C-section rate with "late" epidural administration when dilation reached at least 4 cm, Monica Rincon and her associates reported at the annual meeting of the American College of Obstetricians and Gynecologists.
After adjusting for the effects of age, race, body mass index (BMI), parity, and Bishop score, the risk for C-section was doubled with early vs. late epidural, said Ms. Rincon, a senior research assistant in the women’s health research unit at Oregon Health and Science University, Portland.
A non-reassuring fetal heart tracing was significantly more likely to be an indication for C-section in the early epidural group (18%), compared with the late epidural group (9%). Arrest of labor was the indication for C-section in 25% of the early epidural group and 21% of the late epidural group, which was not a statistically significant difference, she said.
Women in the early epidural group were significantly more likely to be white (80%), compared with the late epidural group (66%).
Several other factors besides timing of the epidural were associated with significantly increased risk for C-section after adjusting for confounders. The risk doubled with a Bishop score less than 5 at the time of admission, compared with higher Bishop scores. Maternal age of at least 35 years tripled the risk for C-section, compared with younger age. The risk for C-section quadrupled with a body mass index of at least 30 kg/m2, compared with lower BMI. Nonwhite women had a fivefold increased risk for C-section, compared with white women. Nulliparous women had a 13-fold increased risk for C-section, compared with multiparous women.
The early and late epidural groups did not differ significantly by age, BMI, gestational age, birth weight, reason for induction of labor, or the proportions of women who were nulliparous or who had a Bishop score greater than 5 at admission.
Among all 281 deliveries (including those without an epidural), 68% were spontaneous vaginal deliveries, 6% were assisted vaginal deliveries, 8% were C-sections for non-reassuring fetal heart tracings, and 18% were C-sections because of arrest of labor.
Approximately 22% of U.S. labors in 2006 were induced, Ms. Rincon said. Previous studies of nulliparous women have shown a higher rate of C-section after labor induction, compared with spontaneous labor. Studies of multiparous women with induced labor provide conflicting results for the risk of C-section, with some reporting no increase in risk of C-section and others reporting increased C-section rates.
Among 2,231 deliveries in 2011 at Oregon Health and Science University, 41% involved labor induction, 56% used an epidural, and 27% were C-section deliveries, she said.
The current study excluded twin deliveries, vaginal births after C-section deliveries, and patients whose epidural status was unknown.
Ms. Rincon reported having no financial disclosures.
early administration before 4-cm dilation, C-section, Monica Rincon, American College of Obstetricians and Gynecologists,
SAN DIEGO – The risk of C-section doubled in women undergoing induction of labor who received an epidural before 4 -cm dilation, compared with later epidural administration, a retrospective study found.
In 281 singleton pregnancies at gestational ages greater than 36 weeks that underwent induction of labor from 2008 to 2009 at one institution, 83% of mothers received an epidural. The C-section rate was significantly higher among the 233 women who received an epidural, compared with the 48 women who did not get an epidural – 30% vs. 8%.
In unadjusted results for women who got an epidural, "early" administration before 4-cm dilation was associated with a 38% chance of C-section, compared with a 24% C-section rate with "late" epidural administration when dilation reached at least 4 cm, Monica Rincon and her associates reported at the annual meeting of the American College of Obstetricians and Gynecologists.
After adjusting for the effects of age, race, body mass index (BMI), parity, and Bishop score, the risk for C-section was doubled with early vs. late epidural, said Ms. Rincon, a senior research assistant in the women’s health research unit at Oregon Health and Science University, Portland.
A non-reassuring fetal heart tracing was significantly more likely to be an indication for C-section in the early epidural group (18%), compared with the late epidural group (9%). Arrest of labor was the indication for C-section in 25% of the early epidural group and 21% of the late epidural group, which was not a statistically significant difference, she said.
Women in the early epidural group were significantly more likely to be white (80%), compared with the late epidural group (66%).
Several other factors besides timing of the epidural were associated with significantly increased risk for C-section after adjusting for confounders. The risk doubled with a Bishop score less than 5 at the time of admission, compared with higher Bishop scores. Maternal age of at least 35 years tripled the risk for C-section, compared with younger age. The risk for C-section quadrupled with a body mass index of at least 30 kg/m2, compared with lower BMI. Nonwhite women had a fivefold increased risk for C-section, compared with white women. Nulliparous women had a 13-fold increased risk for C-section, compared with multiparous women.
The early and late epidural groups did not differ significantly by age, BMI, gestational age, birth weight, reason for induction of labor, or the proportions of women who were nulliparous or who had a Bishop score greater than 5 at admission.
Among all 281 deliveries (including those without an epidural), 68% were spontaneous vaginal deliveries, 6% were assisted vaginal deliveries, 8% were C-sections for non-reassuring fetal heart tracings, and 18% were C-sections because of arrest of labor.
Approximately 22% of U.S. labors in 2006 were induced, Ms. Rincon said. Previous studies of nulliparous women have shown a higher rate of C-section after labor induction, compared with spontaneous labor. Studies of multiparous women with induced labor provide conflicting results for the risk of C-section, with some reporting no increase in risk of C-section and others reporting increased C-section rates.
Among 2,231 deliveries in 2011 at Oregon Health and Science University, 41% involved labor induction, 56% used an epidural, and 27% were C-section deliveries, she said.
The current study excluded twin deliveries, vaginal births after C-section deliveries, and patients whose epidural status was unknown.
Ms. Rincon reported having no financial disclosures.
SAN DIEGO – The risk of C-section doubled in women undergoing induction of labor who received an epidural before 4 -cm dilation, compared with later epidural administration, a retrospective study found.
In 281 singleton pregnancies at gestational ages greater than 36 weeks that underwent induction of labor from 2008 to 2009 at one institution, 83% of mothers received an epidural. The C-section rate was significantly higher among the 233 women who received an epidural, compared with the 48 women who did not get an epidural – 30% vs. 8%.
In unadjusted results for women who got an epidural, "early" administration before 4-cm dilation was associated with a 38% chance of C-section, compared with a 24% C-section rate with "late" epidural administration when dilation reached at least 4 cm, Monica Rincon and her associates reported at the annual meeting of the American College of Obstetricians and Gynecologists.
After adjusting for the effects of age, race, body mass index (BMI), parity, and Bishop score, the risk for C-section was doubled with early vs. late epidural, said Ms. Rincon, a senior research assistant in the women’s health research unit at Oregon Health and Science University, Portland.
A non-reassuring fetal heart tracing was significantly more likely to be an indication for C-section in the early epidural group (18%), compared with the late epidural group (9%). Arrest of labor was the indication for C-section in 25% of the early epidural group and 21% of the late epidural group, which was not a statistically significant difference, she said.
Women in the early epidural group were significantly more likely to be white (80%), compared with the late epidural group (66%).
Several other factors besides timing of the epidural were associated with significantly increased risk for C-section after adjusting for confounders. The risk doubled with a Bishop score less than 5 at the time of admission, compared with higher Bishop scores. Maternal age of at least 35 years tripled the risk for C-section, compared with younger age. The risk for C-section quadrupled with a body mass index of at least 30 kg/m2, compared with lower BMI. Nonwhite women had a fivefold increased risk for C-section, compared with white women. Nulliparous women had a 13-fold increased risk for C-section, compared with multiparous women.
The early and late epidural groups did not differ significantly by age, BMI, gestational age, birth weight, reason for induction of labor, or the proportions of women who were nulliparous or who had a Bishop score greater than 5 at admission.
Among all 281 deliveries (including those without an epidural), 68% were spontaneous vaginal deliveries, 6% were assisted vaginal deliveries, 8% were C-sections for non-reassuring fetal heart tracings, and 18% were C-sections because of arrest of labor.
Approximately 22% of U.S. labors in 2006 were induced, Ms. Rincon said. Previous studies of nulliparous women have shown a higher rate of C-section after labor induction, compared with spontaneous labor. Studies of multiparous women with induced labor provide conflicting results for the risk of C-section, with some reporting no increase in risk of C-section and others reporting increased C-section rates.
Among 2,231 deliveries in 2011 at Oregon Health and Science University, 41% involved labor induction, 56% used an epidural, and 27% were C-section deliveries, she said.
The current study excluded twin deliveries, vaginal births after C-section deliveries, and patients whose epidural status was unknown.
Ms. Rincon reported having no financial disclosures.
early administration before 4-cm dilation, C-section, Monica Rincon, American College of Obstetricians and Gynecologists,
early administration before 4-cm dilation, C-section, Monica Rincon, American College of Obstetricians and Gynecologists,
FROM THE ANNUAL MEETING OF AMERICAN COLLEGE OF OBSTETRICIANS AND GYNECOLOGISTS
Drug Combination Improved Local Analgesia After Cesarean
MONTEREY, CALIF. – Two drugs work better than one when it comes to reducing pain and inflammation with local therapy after cesarean delivery, researchers reported.
In a randomized trial of 60 women who used subcutaneous drug delivery, the area under the curve for postoperative pain scores while sitting was roughly 30% less and supplemental opioid requirements were roughly 40% less when the nonsteroidal anti-inflammatory drug ketorolac was added to bupivacaine. Wound exudate levels of the inflammatory markers interleukin-6 and -10 also were lower.
"Giving a low dose peripherally of nonsteroidal ketorolac has both an anti-inflammatory and an analgesic effect," commented lead author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital in Stanford, Calif.
"This suggests that there is a local mediation effect – this is not a systemic effect – and it may give birth to the whole concept of being able to give very small doses directly in the wound," he said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
"You could even create a multimodal analgesic protocol with small doses of drugs that will not have the high side effects based on big systemic doses, and they can work exactly in the area that we know is most important for inflammation and pain propagation."
In contrast, there were no significant improvements in study outcomes when subcutaneous hydromorphone was added to bupivacaine. "This probably could have been anticipated if you look at the balance of the literature, looking at the efficacy of opioids and nonsteroidals peripherally administered," Dr. Carvalho said.
Session attendee Dr. David R. Gambling of the Sharp Mary Birch Hospital for Women in San Diego questioned the impact of the catheter used to deliver the drugs. "Do you think that having that foreign body in place affects the release of the mediators you were measuring, and if that’s the case, do you think patient movement and irritation of the foreign body could have had an effect on the result?" he asked.
The point is a good one, Dr. Carvalho acknowledged. However, "to be able to follow someone continuously [without a catheter in place] would require invasive [needle procedures] as well as punch biopsies. ... There’s sort of a long way to go, and maybe the whole idea behind this is to encourage people to think of better ways of doing this."
Dr. Andrea Fuller of the University of Colorado Hospital in Aurora, who also attended the session, said, "I’m wondering clinically how to use this. If you don’t have an ON-Q pump [to deliver the drug], are you doing things like asking your obstetricians to put some ketorolac in infiltrate right at the site, or even putting it in a TAP [transversus abdominus plane] block – it might be close enough."
"It’s way too early to say," Dr. Carvalho replied. "I am not recommending the use [of a nonsteroidal agent peripherally] yet, until we truly understand what these drugs are doing. ... It’s a growing area, and I think a few years from now, a lot of the drug administration will be where it should be as opposed to systemically."
The trial participants were 60 healthy women with term singleton pregnancies who underwent elective cesarean delivery with spinal anesthesia. The investigators placed elastomeric ON-Q pumps subcutaneously in the incisional wound to permit collection of wound exudate and instillation of agents.
The women were randomized equally into three groups given continuous subcutaneous instillation for 48 hours after surgery of bupivacaine at 10 mg/hr only (as an active control); bupivacaine plus ketorolac at 0.6 mg/hr; or bupivacaine plus hydromorphone at 0.04 mg/hr.
The drugs were intentionally "given in very small doses, which we believe would not be systemically effective, so it’s not a systemic absorption effect," Dr. Carvalho noted.
Pain intensity was measured at 4, 24, and 48 hours post surgery, both at rest and during activity, using the verbal pain scale, on which values range from 0 to 10. Wound exudate was collected at the same time points and assayed for a wide variety of cytokines.
On average, the women were about 32 years old and had a parity of 1. Eighty percent had had a previous cesarean delivery.
Trial results showed that the area under the curve for postoperative pain scores while sitting was approximately 250 for women given bupivacaine only, versus 175 for women given bupivacaine plus ketorolac (P = .018), Dr. Carvalho reported. There were no significant differences in pain scores while at rest.
Compared with bupivacaine alone, bupivacaine plus ketorolac was associated with lower levels of interleukin-6 (P = .012) and interleukin-10 (P = .004) in wound exudate.
Postoperative supplemental opioid analgesic use was approximately 25 mg of morphine equivalents for women in the bupivacaine-ketorolac group, compared with 40 mg for their counterparts in the bupivacaine-only group (P = .020).
None of the women had significant adverse events or study-related complications, and there were no hematomas, infections, or delays in wound healing. One woman developed a subincisional fluid collection.
"The majority of patients we enrolled had had previous cesarean deliveries, which may [have introduced] bias, as patients having second C-sections may respond differently from someone who is undergoing a cesarean for the first time," Dr. Carvalho acknowledged. Additionally, there was some "empiric guessing" involved in the selection of drug doses, and the study focused only on the acute inflammatory period.
"The next plan is to compare systemic versus peripheral administration of various other drugs, and to start understanding the mechanisms behind what is happening in the periphery," he concluded. "We have a very delicate balance where if we inhibit inflammation too much, then we have delayed wound healing. If we accelerate it too much, we might get hypertrophic scarring. So it’s a delicate balance that we can intervene on and maybe improve analgesia, but we mustn’t affect the balance of wound healing."
Dr. Carvalho disclosed that he has a relationship with I-Flow, manufacturer of the pump used in the study.
MONTEREY, CALIF. – Two drugs work better than one when it comes to reducing pain and inflammation with local therapy after cesarean delivery, researchers reported.
In a randomized trial of 60 women who used subcutaneous drug delivery, the area under the curve for postoperative pain scores while sitting was roughly 30% less and supplemental opioid requirements were roughly 40% less when the nonsteroidal anti-inflammatory drug ketorolac was added to bupivacaine. Wound exudate levels of the inflammatory markers interleukin-6 and -10 also were lower.
"Giving a low dose peripherally of nonsteroidal ketorolac has both an anti-inflammatory and an analgesic effect," commented lead author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital in Stanford, Calif.
"This suggests that there is a local mediation effect – this is not a systemic effect – and it may give birth to the whole concept of being able to give very small doses directly in the wound," he said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
"You could even create a multimodal analgesic protocol with small doses of drugs that will not have the high side effects based on big systemic doses, and they can work exactly in the area that we know is most important for inflammation and pain propagation."
In contrast, there were no significant improvements in study outcomes when subcutaneous hydromorphone was added to bupivacaine. "This probably could have been anticipated if you look at the balance of the literature, looking at the efficacy of opioids and nonsteroidals peripherally administered," Dr. Carvalho said.
Session attendee Dr. David R. Gambling of the Sharp Mary Birch Hospital for Women in San Diego questioned the impact of the catheter used to deliver the drugs. "Do you think that having that foreign body in place affects the release of the mediators you were measuring, and if that’s the case, do you think patient movement and irritation of the foreign body could have had an effect on the result?" he asked.
The point is a good one, Dr. Carvalho acknowledged. However, "to be able to follow someone continuously [without a catheter in place] would require invasive [needle procedures] as well as punch biopsies. ... There’s sort of a long way to go, and maybe the whole idea behind this is to encourage people to think of better ways of doing this."
Dr. Andrea Fuller of the University of Colorado Hospital in Aurora, who also attended the session, said, "I’m wondering clinically how to use this. If you don’t have an ON-Q pump [to deliver the drug], are you doing things like asking your obstetricians to put some ketorolac in infiltrate right at the site, or even putting it in a TAP [transversus abdominus plane] block – it might be close enough."
"It’s way too early to say," Dr. Carvalho replied. "I am not recommending the use [of a nonsteroidal agent peripherally] yet, until we truly understand what these drugs are doing. ... It’s a growing area, and I think a few years from now, a lot of the drug administration will be where it should be as opposed to systemically."
The trial participants were 60 healthy women with term singleton pregnancies who underwent elective cesarean delivery with spinal anesthesia. The investigators placed elastomeric ON-Q pumps subcutaneously in the incisional wound to permit collection of wound exudate and instillation of agents.
The women were randomized equally into three groups given continuous subcutaneous instillation for 48 hours after surgery of bupivacaine at 10 mg/hr only (as an active control); bupivacaine plus ketorolac at 0.6 mg/hr; or bupivacaine plus hydromorphone at 0.04 mg/hr.
The drugs were intentionally "given in very small doses, which we believe would not be systemically effective, so it’s not a systemic absorption effect," Dr. Carvalho noted.
Pain intensity was measured at 4, 24, and 48 hours post surgery, both at rest and during activity, using the verbal pain scale, on which values range from 0 to 10. Wound exudate was collected at the same time points and assayed for a wide variety of cytokines.
On average, the women were about 32 years old and had a parity of 1. Eighty percent had had a previous cesarean delivery.
Trial results showed that the area under the curve for postoperative pain scores while sitting was approximately 250 for women given bupivacaine only, versus 175 for women given bupivacaine plus ketorolac (P = .018), Dr. Carvalho reported. There were no significant differences in pain scores while at rest.
Compared with bupivacaine alone, bupivacaine plus ketorolac was associated with lower levels of interleukin-6 (P = .012) and interleukin-10 (P = .004) in wound exudate.
Postoperative supplemental opioid analgesic use was approximately 25 mg of morphine equivalents for women in the bupivacaine-ketorolac group, compared with 40 mg for their counterparts in the bupivacaine-only group (P = .020).
None of the women had significant adverse events or study-related complications, and there were no hematomas, infections, or delays in wound healing. One woman developed a subincisional fluid collection.
"The majority of patients we enrolled had had previous cesarean deliveries, which may [have introduced] bias, as patients having second C-sections may respond differently from someone who is undergoing a cesarean for the first time," Dr. Carvalho acknowledged. Additionally, there was some "empiric guessing" involved in the selection of drug doses, and the study focused only on the acute inflammatory period.
"The next plan is to compare systemic versus peripheral administration of various other drugs, and to start understanding the mechanisms behind what is happening in the periphery," he concluded. "We have a very delicate balance where if we inhibit inflammation too much, then we have delayed wound healing. If we accelerate it too much, we might get hypertrophic scarring. So it’s a delicate balance that we can intervene on and maybe improve analgesia, but we mustn’t affect the balance of wound healing."
Dr. Carvalho disclosed that he has a relationship with I-Flow, manufacturer of the pump used in the study.
MONTEREY, CALIF. – Two drugs work better than one when it comes to reducing pain and inflammation with local therapy after cesarean delivery, researchers reported.
In a randomized trial of 60 women who used subcutaneous drug delivery, the area under the curve for postoperative pain scores while sitting was roughly 30% less and supplemental opioid requirements were roughly 40% less when the nonsteroidal anti-inflammatory drug ketorolac was added to bupivacaine. Wound exudate levels of the inflammatory markers interleukin-6 and -10 also were lower.
"Giving a low dose peripherally of nonsteroidal ketorolac has both an anti-inflammatory and an analgesic effect," commented lead author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital in Stanford, Calif.
"This suggests that there is a local mediation effect – this is not a systemic effect – and it may give birth to the whole concept of being able to give very small doses directly in the wound," he said at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
"You could even create a multimodal analgesic protocol with small doses of drugs that will not have the high side effects based on big systemic doses, and they can work exactly in the area that we know is most important for inflammation and pain propagation."
In contrast, there were no significant improvements in study outcomes when subcutaneous hydromorphone was added to bupivacaine. "This probably could have been anticipated if you look at the balance of the literature, looking at the efficacy of opioids and nonsteroidals peripherally administered," Dr. Carvalho said.
Session attendee Dr. David R. Gambling of the Sharp Mary Birch Hospital for Women in San Diego questioned the impact of the catheter used to deliver the drugs. "Do you think that having that foreign body in place affects the release of the mediators you were measuring, and if that’s the case, do you think patient movement and irritation of the foreign body could have had an effect on the result?" he asked.
The point is a good one, Dr. Carvalho acknowledged. However, "to be able to follow someone continuously [without a catheter in place] would require invasive [needle procedures] as well as punch biopsies. ... There’s sort of a long way to go, and maybe the whole idea behind this is to encourage people to think of better ways of doing this."
Dr. Andrea Fuller of the University of Colorado Hospital in Aurora, who also attended the session, said, "I’m wondering clinically how to use this. If you don’t have an ON-Q pump [to deliver the drug], are you doing things like asking your obstetricians to put some ketorolac in infiltrate right at the site, or even putting it in a TAP [transversus abdominus plane] block – it might be close enough."
"It’s way too early to say," Dr. Carvalho replied. "I am not recommending the use [of a nonsteroidal agent peripherally] yet, until we truly understand what these drugs are doing. ... It’s a growing area, and I think a few years from now, a lot of the drug administration will be where it should be as opposed to systemically."
The trial participants were 60 healthy women with term singleton pregnancies who underwent elective cesarean delivery with spinal anesthesia. The investigators placed elastomeric ON-Q pumps subcutaneously in the incisional wound to permit collection of wound exudate and instillation of agents.
The women were randomized equally into three groups given continuous subcutaneous instillation for 48 hours after surgery of bupivacaine at 10 mg/hr only (as an active control); bupivacaine plus ketorolac at 0.6 mg/hr; or bupivacaine plus hydromorphone at 0.04 mg/hr.
The drugs were intentionally "given in very small doses, which we believe would not be systemically effective, so it’s not a systemic absorption effect," Dr. Carvalho noted.
Pain intensity was measured at 4, 24, and 48 hours post surgery, both at rest and during activity, using the verbal pain scale, on which values range from 0 to 10. Wound exudate was collected at the same time points and assayed for a wide variety of cytokines.
On average, the women were about 32 years old and had a parity of 1. Eighty percent had had a previous cesarean delivery.
Trial results showed that the area under the curve for postoperative pain scores while sitting was approximately 250 for women given bupivacaine only, versus 175 for women given bupivacaine plus ketorolac (P = .018), Dr. Carvalho reported. There were no significant differences in pain scores while at rest.
Compared with bupivacaine alone, bupivacaine plus ketorolac was associated with lower levels of interleukin-6 (P = .012) and interleukin-10 (P = .004) in wound exudate.
Postoperative supplemental opioid analgesic use was approximately 25 mg of morphine equivalents for women in the bupivacaine-ketorolac group, compared with 40 mg for their counterparts in the bupivacaine-only group (P = .020).
None of the women had significant adverse events or study-related complications, and there were no hematomas, infections, or delays in wound healing. One woman developed a subincisional fluid collection.
"The majority of patients we enrolled had had previous cesarean deliveries, which may [have introduced] bias, as patients having second C-sections may respond differently from someone who is undergoing a cesarean for the first time," Dr. Carvalho acknowledged. Additionally, there was some "empiric guessing" involved in the selection of drug doses, and the study focused only on the acute inflammatory period.
"The next plan is to compare systemic versus peripheral administration of various other drugs, and to start understanding the mechanisms behind what is happening in the periphery," he concluded. "We have a very delicate balance where if we inhibit inflammation too much, then we have delayed wound healing. If we accelerate it too much, we might get hypertrophic scarring. So it’s a delicate balance that we can intervene on and maybe improve analgesia, but we mustn’t affect the balance of wound healing."
Dr. Carvalho disclosed that he has a relationship with I-Flow, manufacturer of the pump used in the study.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATOLOGY

