User login
Effect of Vitamin D on Nonskeletal Tissue Yet Unknown
Available data are not strong enough to show a definitive association between vitamin D levels and risk of obesity, diabetes, cancer, heart disease, or maternal-fetal health, according to a comprehensive review of available research.
"The efficacy issue [of vitamin D] remains a major question mark," said Dr. Clifford J. Rosen in an interview. He chaired the group that wrote the scientific statement published by Endocrine Society (Endocr. Rev. 2012 May 17 [doi:10.1210/er.2012-1000]).
The scientific statement takes a comprehensive look at basic and clinical evidence related to the effect of vitamin D and various organ systems.
Although some observational studies have shown that benefits of vitamin D may extend beyond bone health, the findings are inconsistent, the authors noted.
"We need large randomized controlled trials and dose-response data to test the effects of vitamin D on chronic disease outcomes including autoimmunity, obesity, diabetes, hypertension, and heart disease," said Dr. Rosen, professor of medicine at Tufts University, Boston, in a statement.
Dr. Robert H. Eckel, past president of the American Heart Association, said he agreed with the findings of the report. He said that physicians should be aware of low vitamin D levels and should correct the deficiencies, but they should not make any strong statements about vitamin D levels and risk for conditions such as heart disease or cancer, due to lack of sufficient evidence.
He added that ultimately, data from large, well-designed trials "may be informative in uncovering a relationship that’s more meaningful." Dr. Eckel, who is a professor of medicine at University of Colorado at Denver, Aurora, was not involved in the study.
Interest in vitamin D as a therapeutic option for the prevention of chronic disease has been growing in recent years, the authors noted. "In a 2-month span during the summer of 2011, there were more than 500 publications centered on vitamin D, most of which were related to its relationship to nonskeletal tissues," they wrote. But the results, they added, are confounded and difficult to interpret.
By organ or disorder, the authors came to the following conclusions:
• Skin. "There are no large-scale, randomized, placebo-controlled clinical trials demonstrating that vitamin D metabolites are superior to other types of treatment for various proliferative skin disorders or for the prevention of skin cancer."
• Obesity and diabetes mellitus. "The ever-expanding obesity epidemic has been associated with a rising prevalence of vitamin D deficiency, but a cause-and-effect relationship has not been established. ... There remains a paucity of randomized controlled trials of vitamin D for the prevention of diabetes; hence, few conclusions can be firmly established."
• Fall prevention and improvement in quality of life. Citing the public health implication of fall prevention, and the report by the Institute of Medicine, the authors wrote, "The absolute threshold level of [vitamin D] needed to prevent falls in an elderly population is not known in part because of lack of true dose-ranging studies. ... Selecting patients at risk for falls and defining the appropriate dose remains as areas in need of further research."
• Cancer. "Despite biological plausibility for a role of vitamin D in cancer prevention, most recent systematic reviews and meta-analyses, as well as a comprehensive review by the IOM Committee, have found that the evidence that vitamin D reduces cancer incidence and/or mortality is inconsistent and inconclusive as to causality."
• Cardiovascular disease. Although there is a possibility that vitamin D supplementation may lower cardiovascular risk, "additional research, particularly from randomized trials, is needed."
• The placenta and maternal-fetal health. "There is insufficient evidence to recommend a particular maternal intake of vitamin D or [serum 25-hydroxyvitamin D] blood level during pregnancy to achieve any purported nonskeletal benefit of vitamin D," the authors wrote, but they added that "the biological plausibility may be sufficient to justify clinical trials to test whether vitamin D supplementation during pregnancy will prevent type 1 diabetes in the offspring."
"We’re hopeful that some of the intervention trial [on vitamin D] will get underway, and although they’re expensive, their findings can help change practice," said Dr. Rosen.
Dr. Rosen reported no relevant conflicts of interest.
Available data are not strong enough to show a definitive association between vitamin D levels and risk of obesity, diabetes, cancer, heart disease, or maternal-fetal health, according to a comprehensive review of available research.
"The efficacy issue [of vitamin D] remains a major question mark," said Dr. Clifford J. Rosen in an interview. He chaired the group that wrote the scientific statement published by Endocrine Society (Endocr. Rev. 2012 May 17 [doi:10.1210/er.2012-1000]).
The scientific statement takes a comprehensive look at basic and clinical evidence related to the effect of vitamin D and various organ systems.
Although some observational studies have shown that benefits of vitamin D may extend beyond bone health, the findings are inconsistent, the authors noted.
"We need large randomized controlled trials and dose-response data to test the effects of vitamin D on chronic disease outcomes including autoimmunity, obesity, diabetes, hypertension, and heart disease," said Dr. Rosen, professor of medicine at Tufts University, Boston, in a statement.
Dr. Robert H. Eckel, past president of the American Heart Association, said he agreed with the findings of the report. He said that physicians should be aware of low vitamin D levels and should correct the deficiencies, but they should not make any strong statements about vitamin D levels and risk for conditions such as heart disease or cancer, due to lack of sufficient evidence.
He added that ultimately, data from large, well-designed trials "may be informative in uncovering a relationship that’s more meaningful." Dr. Eckel, who is a professor of medicine at University of Colorado at Denver, Aurora, was not involved in the study.
Interest in vitamin D as a therapeutic option for the prevention of chronic disease has been growing in recent years, the authors noted. "In a 2-month span during the summer of 2011, there were more than 500 publications centered on vitamin D, most of which were related to its relationship to nonskeletal tissues," they wrote. But the results, they added, are confounded and difficult to interpret.
By organ or disorder, the authors came to the following conclusions:
• Skin. "There are no large-scale, randomized, placebo-controlled clinical trials demonstrating that vitamin D metabolites are superior to other types of treatment for various proliferative skin disorders or for the prevention of skin cancer."
• Obesity and diabetes mellitus. "The ever-expanding obesity epidemic has been associated with a rising prevalence of vitamin D deficiency, but a cause-and-effect relationship has not been established. ... There remains a paucity of randomized controlled trials of vitamin D for the prevention of diabetes; hence, few conclusions can be firmly established."
• Fall prevention and improvement in quality of life. Citing the public health implication of fall prevention, and the report by the Institute of Medicine, the authors wrote, "The absolute threshold level of [vitamin D] needed to prevent falls in an elderly population is not known in part because of lack of true dose-ranging studies. ... Selecting patients at risk for falls and defining the appropriate dose remains as areas in need of further research."
• Cancer. "Despite biological plausibility for a role of vitamin D in cancer prevention, most recent systematic reviews and meta-analyses, as well as a comprehensive review by the IOM Committee, have found that the evidence that vitamin D reduces cancer incidence and/or mortality is inconsistent and inconclusive as to causality."
• Cardiovascular disease. Although there is a possibility that vitamin D supplementation may lower cardiovascular risk, "additional research, particularly from randomized trials, is needed."
• The placenta and maternal-fetal health. "There is insufficient evidence to recommend a particular maternal intake of vitamin D or [serum 25-hydroxyvitamin D] blood level during pregnancy to achieve any purported nonskeletal benefit of vitamin D," the authors wrote, but they added that "the biological plausibility may be sufficient to justify clinical trials to test whether vitamin D supplementation during pregnancy will prevent type 1 diabetes in the offspring."
"We’re hopeful that some of the intervention trial [on vitamin D] will get underway, and although they’re expensive, their findings can help change practice," said Dr. Rosen.
Dr. Rosen reported no relevant conflicts of interest.
Available data are not strong enough to show a definitive association between vitamin D levels and risk of obesity, diabetes, cancer, heart disease, or maternal-fetal health, according to a comprehensive review of available research.
"The efficacy issue [of vitamin D] remains a major question mark," said Dr. Clifford J. Rosen in an interview. He chaired the group that wrote the scientific statement published by Endocrine Society (Endocr. Rev. 2012 May 17 [doi:10.1210/er.2012-1000]).
The scientific statement takes a comprehensive look at basic and clinical evidence related to the effect of vitamin D and various organ systems.
Although some observational studies have shown that benefits of vitamin D may extend beyond bone health, the findings are inconsistent, the authors noted.
"We need large randomized controlled trials and dose-response data to test the effects of vitamin D on chronic disease outcomes including autoimmunity, obesity, diabetes, hypertension, and heart disease," said Dr. Rosen, professor of medicine at Tufts University, Boston, in a statement.
Dr. Robert H. Eckel, past president of the American Heart Association, said he agreed with the findings of the report. He said that physicians should be aware of low vitamin D levels and should correct the deficiencies, but they should not make any strong statements about vitamin D levels and risk for conditions such as heart disease or cancer, due to lack of sufficient evidence.
He added that ultimately, data from large, well-designed trials "may be informative in uncovering a relationship that’s more meaningful." Dr. Eckel, who is a professor of medicine at University of Colorado at Denver, Aurora, was not involved in the study.
Interest in vitamin D as a therapeutic option for the prevention of chronic disease has been growing in recent years, the authors noted. "In a 2-month span during the summer of 2011, there were more than 500 publications centered on vitamin D, most of which were related to its relationship to nonskeletal tissues," they wrote. But the results, they added, are confounded and difficult to interpret.
By organ or disorder, the authors came to the following conclusions:
• Skin. "There are no large-scale, randomized, placebo-controlled clinical trials demonstrating that vitamin D metabolites are superior to other types of treatment for various proliferative skin disorders or for the prevention of skin cancer."
• Obesity and diabetes mellitus. "The ever-expanding obesity epidemic has been associated with a rising prevalence of vitamin D deficiency, but a cause-and-effect relationship has not been established. ... There remains a paucity of randomized controlled trials of vitamin D for the prevention of diabetes; hence, few conclusions can be firmly established."
• Fall prevention and improvement in quality of life. Citing the public health implication of fall prevention, and the report by the Institute of Medicine, the authors wrote, "The absolute threshold level of [vitamin D] needed to prevent falls in an elderly population is not known in part because of lack of true dose-ranging studies. ... Selecting patients at risk for falls and defining the appropriate dose remains as areas in need of further research."
• Cancer. "Despite biological plausibility for a role of vitamin D in cancer prevention, most recent systematic reviews and meta-analyses, as well as a comprehensive review by the IOM Committee, have found that the evidence that vitamin D reduces cancer incidence and/or mortality is inconsistent and inconclusive as to causality."
• Cardiovascular disease. Although there is a possibility that vitamin D supplementation may lower cardiovascular risk, "additional research, particularly from randomized trials, is needed."
• The placenta and maternal-fetal health. "There is insufficient evidence to recommend a particular maternal intake of vitamin D or [serum 25-hydroxyvitamin D] blood level during pregnancy to achieve any purported nonskeletal benefit of vitamin D," the authors wrote, but they added that "the biological plausibility may be sufficient to justify clinical trials to test whether vitamin D supplementation during pregnancy will prevent type 1 diabetes in the offspring."
"We’re hopeful that some of the intervention trial [on vitamin D] will get underway, and although they’re expensive, their findings can help change practice," said Dr. Rosen.
Dr. Rosen reported no relevant conflicts of interest.
Some Risk Factors for Failed Epidural Conversion Are Modifiable
MONTEREY, CALIF. – Several factors, some of them modifiable, increase the odds of a failed conversion from epidural analgesia for labor to epidural anesthesia for cesarean delivery, concluded the first systematic review and meta-analysis of this issue.
The analysis found that 1 in every 20 women having an epidural in place and needing a cesarean had to undergo general anesthesia because the epidural could not be used for anesthesia, lead investigator Melissa E.B. Bauer, D.O., reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
Women had a more than tripling of the odds of failed conversion if they needed two or more epidural analgesia boluses (so-called top-ups) during labor, had a general anesthesiologist instead of an obstetric anesthesiologist, or required a cesarean on a more urgent basis.
The findings underscored the need to investigate if a patient needs top-ups, according to Dr. Bauer. If a patient makes two or more requests for more analgesia, has breakthrough pain, and is really uncomfortable, she needs to be assessed to see if she has a nonworking epidural. If so, it needs to be replaced, she said in an interview, while noting that other factors, such as dystocia, may also be a cause.
The higher odds of failed conversion that are seen with a general anesthesiologist suggest that "people who manage labor and delivery more often are going to be a little bit more comfortable troubleshooting epidurals and trying to avoid a general anesthetic," commented Dr. Bauer, who is an obstetric anesthesiologist at the University of Michigan Health System in Ann Arbor.
The risk with an urgent cesarean may be the hardest to modify. "We can’t really do anything about that, except to have better communication of obstetricians and [obstetric] anesthesia; to say, if you tell us [a cesarean is coming], maybe we can run to the room and start bolusing that epidural so that by the time the [patient] gets to the OR, we have enough of a level so that [you] can start and avoid a general," she explained. "So the main points are, having more cooperation and also evaluating the [fetal-monitoring] strip, and saying okay, do we have 5 or 10 minutes to convert [the epidural] or not – those things might be helpful."
Dr. Brenda A. Bucklin, professor of anesthesiology at the University of Colorado at Denver, Aurora, and comoderator of a related poster discussion session, asked, "Are [obstetric] anesthesiologists less comfortable in providing general anesthesia for cesarean delivery?"
"I think that it’s the opposite," Dr. Bauer replied. "Since we provide anesthesia for pregnant patients on a daily or weekly basis, we have more familiarity with the obstetric airway, and we are also called to the main hospital to provide anesthesia on any pregnant patient there as well."
Dr. Bucklin also wanted to know, "Are [obstetric] anesthesiologists more likely to limp along with a bad epidural for cesarean delivery?"
"I would say no, but part of the difference is that we tend to troubleshoot our epidurals sooner because we can look at the fetal strips and say, oh, I think [the baby’s] coming, and we go and evaluate the patient on a regular basis," Dr. Bauer said. "Also, once a C-section is called, we’re in the room and we have some familiarity with the surgeons to see [if this is] really an emergency, is there time for me to bolus this. I think that [because of] those relationships and also our understanding of [obstetrics] in general, we have a decreased rate of general anesthesia."
There are several reasons to want to avoid a failed epidural conversion and have to resort to general anesthesia, she noted in the interview. Managing the airway in obstetric patients can be challenging, and there are risks associated with using another type of anesthesia on top of an epidural. "You always want the mother to be able to participate in the birth as well," she added.
The investigators identified 13 observational studies with a total of 8,628 women that assessed the rate of failed conversion and risk factors for this outcome.
Results showed that the percentage of patients having an epidural catheter in place who still had to undergo general anesthesia for their cesarean averaged 5%, with a range of 0%-21% across studies, reported Dr. Bauer.
Women’s odds of failed conversion increased significantly if they needed at least two clinician-administered top-ups of analgesia during labor vs. no top-ups (odds ratio, 3.2), had a general vs. obstetric anesthesiologist (OR, 4.6), or required a more urgent cesarean delivery (OR, 40.4).
A variety of other factors were not significantly associated with the odds of failed conversion: combined spinal-epidural instead of standard epidural techniques, the duration of epidural analgesia, the extent of cervical dilation at the time of epidural placement, and obesity.
However, Dr. Bauer noted, the lack of association for obesity is uncertain, given that studies varied widely in terms of when they assessed body mass index or weight relative to pregnancy. "Also, most anesthesiologists are not going to let a patient who is really obese have a nonworking epidural because we don’t want to put her to sleep" and use general anesthesia, she added.
Dr. Bauer disclosed no relevant conflicts of interest.
MONTEREY, CALIF. – Several factors, some of them modifiable, increase the odds of a failed conversion from epidural analgesia for labor to epidural anesthesia for cesarean delivery, concluded the first systematic review and meta-analysis of this issue.
The analysis found that 1 in every 20 women having an epidural in place and needing a cesarean had to undergo general anesthesia because the epidural could not be used for anesthesia, lead investigator Melissa E.B. Bauer, D.O., reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
Women had a more than tripling of the odds of failed conversion if they needed two or more epidural analgesia boluses (so-called top-ups) during labor, had a general anesthesiologist instead of an obstetric anesthesiologist, or required a cesarean on a more urgent basis.
The findings underscored the need to investigate if a patient needs top-ups, according to Dr. Bauer. If a patient makes two or more requests for more analgesia, has breakthrough pain, and is really uncomfortable, she needs to be assessed to see if she has a nonworking epidural. If so, it needs to be replaced, she said in an interview, while noting that other factors, such as dystocia, may also be a cause.
The higher odds of failed conversion that are seen with a general anesthesiologist suggest that "people who manage labor and delivery more often are going to be a little bit more comfortable troubleshooting epidurals and trying to avoid a general anesthetic," commented Dr. Bauer, who is an obstetric anesthesiologist at the University of Michigan Health System in Ann Arbor.
The risk with an urgent cesarean may be the hardest to modify. "We can’t really do anything about that, except to have better communication of obstetricians and [obstetric] anesthesia; to say, if you tell us [a cesarean is coming], maybe we can run to the room and start bolusing that epidural so that by the time the [patient] gets to the OR, we have enough of a level so that [you] can start and avoid a general," she explained. "So the main points are, having more cooperation and also evaluating the [fetal-monitoring] strip, and saying okay, do we have 5 or 10 minutes to convert [the epidural] or not – those things might be helpful."
Dr. Brenda A. Bucklin, professor of anesthesiology at the University of Colorado at Denver, Aurora, and comoderator of a related poster discussion session, asked, "Are [obstetric] anesthesiologists less comfortable in providing general anesthesia for cesarean delivery?"
"I think that it’s the opposite," Dr. Bauer replied. "Since we provide anesthesia for pregnant patients on a daily or weekly basis, we have more familiarity with the obstetric airway, and we are also called to the main hospital to provide anesthesia on any pregnant patient there as well."
Dr. Bucklin also wanted to know, "Are [obstetric] anesthesiologists more likely to limp along with a bad epidural for cesarean delivery?"
"I would say no, but part of the difference is that we tend to troubleshoot our epidurals sooner because we can look at the fetal strips and say, oh, I think [the baby’s] coming, and we go and evaluate the patient on a regular basis," Dr. Bauer said. "Also, once a C-section is called, we’re in the room and we have some familiarity with the surgeons to see [if this is] really an emergency, is there time for me to bolus this. I think that [because of] those relationships and also our understanding of [obstetrics] in general, we have a decreased rate of general anesthesia."
There are several reasons to want to avoid a failed epidural conversion and have to resort to general anesthesia, she noted in the interview. Managing the airway in obstetric patients can be challenging, and there are risks associated with using another type of anesthesia on top of an epidural. "You always want the mother to be able to participate in the birth as well," she added.
The investigators identified 13 observational studies with a total of 8,628 women that assessed the rate of failed conversion and risk factors for this outcome.
Results showed that the percentage of patients having an epidural catheter in place who still had to undergo general anesthesia for their cesarean averaged 5%, with a range of 0%-21% across studies, reported Dr. Bauer.
Women’s odds of failed conversion increased significantly if they needed at least two clinician-administered top-ups of analgesia during labor vs. no top-ups (odds ratio, 3.2), had a general vs. obstetric anesthesiologist (OR, 4.6), or required a more urgent cesarean delivery (OR, 40.4).
A variety of other factors were not significantly associated with the odds of failed conversion: combined spinal-epidural instead of standard epidural techniques, the duration of epidural analgesia, the extent of cervical dilation at the time of epidural placement, and obesity.
However, Dr. Bauer noted, the lack of association for obesity is uncertain, given that studies varied widely in terms of when they assessed body mass index or weight relative to pregnancy. "Also, most anesthesiologists are not going to let a patient who is really obese have a nonworking epidural because we don’t want to put her to sleep" and use general anesthesia, she added.
Dr. Bauer disclosed no relevant conflicts of interest.
MONTEREY, CALIF. – Several factors, some of them modifiable, increase the odds of a failed conversion from epidural analgesia for labor to epidural anesthesia for cesarean delivery, concluded the first systematic review and meta-analysis of this issue.
The analysis found that 1 in every 20 women having an epidural in place and needing a cesarean had to undergo general anesthesia because the epidural could not be used for anesthesia, lead investigator Melissa E.B. Bauer, D.O., reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
Women had a more than tripling of the odds of failed conversion if they needed two or more epidural analgesia boluses (so-called top-ups) during labor, had a general anesthesiologist instead of an obstetric anesthesiologist, or required a cesarean on a more urgent basis.
The findings underscored the need to investigate if a patient needs top-ups, according to Dr. Bauer. If a patient makes two or more requests for more analgesia, has breakthrough pain, and is really uncomfortable, she needs to be assessed to see if she has a nonworking epidural. If so, it needs to be replaced, she said in an interview, while noting that other factors, such as dystocia, may also be a cause.
The higher odds of failed conversion that are seen with a general anesthesiologist suggest that "people who manage labor and delivery more often are going to be a little bit more comfortable troubleshooting epidurals and trying to avoid a general anesthetic," commented Dr. Bauer, who is an obstetric anesthesiologist at the University of Michigan Health System in Ann Arbor.
The risk with an urgent cesarean may be the hardest to modify. "We can’t really do anything about that, except to have better communication of obstetricians and [obstetric] anesthesia; to say, if you tell us [a cesarean is coming], maybe we can run to the room and start bolusing that epidural so that by the time the [patient] gets to the OR, we have enough of a level so that [you] can start and avoid a general," she explained. "So the main points are, having more cooperation and also evaluating the [fetal-monitoring] strip, and saying okay, do we have 5 or 10 minutes to convert [the epidural] or not – those things might be helpful."
Dr. Brenda A. Bucklin, professor of anesthesiology at the University of Colorado at Denver, Aurora, and comoderator of a related poster discussion session, asked, "Are [obstetric] anesthesiologists less comfortable in providing general anesthesia for cesarean delivery?"
"I think that it’s the opposite," Dr. Bauer replied. "Since we provide anesthesia for pregnant patients on a daily or weekly basis, we have more familiarity with the obstetric airway, and we are also called to the main hospital to provide anesthesia on any pregnant patient there as well."
Dr. Bucklin also wanted to know, "Are [obstetric] anesthesiologists more likely to limp along with a bad epidural for cesarean delivery?"
"I would say no, but part of the difference is that we tend to troubleshoot our epidurals sooner because we can look at the fetal strips and say, oh, I think [the baby’s] coming, and we go and evaluate the patient on a regular basis," Dr. Bauer said. "Also, once a C-section is called, we’re in the room and we have some familiarity with the surgeons to see [if this is] really an emergency, is there time for me to bolus this. I think that [because of] those relationships and also our understanding of [obstetrics] in general, we have a decreased rate of general anesthesia."
There are several reasons to want to avoid a failed epidural conversion and have to resort to general anesthesia, she noted in the interview. Managing the airway in obstetric patients can be challenging, and there are risks associated with using another type of anesthesia on top of an epidural. "You always want the mother to be able to participate in the birth as well," she added.
The investigators identified 13 observational studies with a total of 8,628 women that assessed the rate of failed conversion and risk factors for this outcome.
Results showed that the percentage of patients having an epidural catheter in place who still had to undergo general anesthesia for their cesarean averaged 5%, with a range of 0%-21% across studies, reported Dr. Bauer.
Women’s odds of failed conversion increased significantly if they needed at least two clinician-administered top-ups of analgesia during labor vs. no top-ups (odds ratio, 3.2), had a general vs. obstetric anesthesiologist (OR, 4.6), or required a more urgent cesarean delivery (OR, 40.4).
A variety of other factors were not significantly associated with the odds of failed conversion: combined spinal-epidural instead of standard epidural techniques, the duration of epidural analgesia, the extent of cervical dilation at the time of epidural placement, and obesity.
However, Dr. Bauer noted, the lack of association for obesity is uncertain, given that studies varied widely in terms of when they assessed body mass index or weight relative to pregnancy. "Also, most anesthesiologists are not going to let a patient who is really obese have a nonworking epidural because we don’t want to put her to sleep" and use general anesthesia, she added.
Dr. Bauer disclosed no relevant conflicts of interest.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATOLOGY
Major Finding: Women’s odds of failed conversion were increased if they received two or more analgesia boluses during labor (OR, 3.2), had a general vs. obstetric anesthesiologist (OR, 4.6), or required a more urgent cesarean delivery (OR, 40.4).
Data Source: The data are from systematic review and meta-analysis of 13 studies with a total of 8,628 women that assessed conversion of epidural labor analgesia to epidural cesarean anesthesia.
Disclosures: Dr. Bauer disclosed no relevant conflicts of interest.
Obstetric Outcomes Fairly Good Despite Maternal CHD
MONTEREY, CALIF. – Parturients with congenital heart disease do have a rockier obstetric course, but generally fare quite well in the peripartum period, a retrospective cohort study found.
Researchers at the University of Colorado Hospital in Denver led by Christine M. Warrick found that roughly 1% of women delivering there over a 4-year period had congenital heart disease.
This group had higher rates of cesarean delivery and neonatal ICU admission than did parturients overall, according to results reported in a poster session at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. Maternal ICU admissions were rare, however, and there were no cases of maternal mortality.
"Congenital heart disease is definitely a risk factor for maternal and fetal complications, but overall the women seemed to do pretty well. The main things to look out for would be neonatal ICU admissions and respiratory distress in the babies," Ms. Warrick commented in an interview.
In additional study findings, although the large majority of women received regional anesthesia for labor and delivery, only a minority had an epidural placed early, before 4 cm of cervical dilation.
"There is some literature that suggests that early epidural placement is beneficial for these women because it decreases the stress on the heart secondary to pain," she noted. "Some of these women come [to the hospital] in labor and they are already beyond 4 cm of dilation, so that may be a big reason why there weren’t so many early epidurals placed."
In a related poster discussion session, comoderator Dr. Katherine W. Arendt of the Mayo Clinic in Rochester, Minn., asked, "Do you believe that the geographical elevation of your hospital improved or worsened your outcomes compared to a sea-level hospital?"
"It’s difficult to say exactly how the elevation affected all of these women, since we had so many different varieties of congenital heart disease," replied Ms. Warrick, who is a fourth-year medical student at the University of Colorado in Aurora. "But it is possible that women with more severe congenital heart disease would not tolerate a lower partial pressure of oxygen in our environment, and there were some women who were advised to deliver at lower altitudes because of this. So that probably improved our outcomes in women who received adequate prenatal care. I also think that points to the importance of prenatal care in these women."
"We know that more women are surviving to childbearing age with congenital heart disease due to the recent advances in surgical repair of these defects, and it is actually one of the major causes of cardiac diseases in pregnant women in the United States," she said. Many of the normal changes of pregnancy, labor, and delivery put stress on the heart and would be expected to exacerbate matters.
The investigators studied 13,109 parturients in the hospital’s perinatal database between October 2005 and December 2009. Medical history and record review identified 75 women, or 0.6%, as having congenital heart disease.
These women had a mean age of 26 years. The majority were non-Hispanic white (60%) and nulliparous (55%). Fully 34% were overweight or obese, and 8% smoked.
According to cardiologists’ notes of symptoms during the third trimester of pregnancy, 60%, 33%, and 7% of the women had New York Heart Association functional class I, II, and III, respectively. The leading congenital anomalies were atrial septal defects (28%), valvular disorders (19%), and tetralogy of Fallot (11%).
In terms of cardiac outcomes, 11% of the women had an arrhythmia in the peripartum period, and 5% required diuresis, Ms. Warrick reported. Although 87% received regional anesthesia for labor and delivery, only 31% received an early epidural.
Some 19% of the women had a preterm birth (one occurring before 37 weeks’ gestation), and 45% had a prolonged hospital stay (lasting more than 2 days after a vaginal delivery or more than 3 days after a cesarean delivery).
Although 3% of the women were admitted to the ICU for prophylactic monitoring, there were no maternal deaths in the peripartum period.
Compared with parturients overall, those with congenital heart disease were more likely to have a cesarean delivery (31% vs. 27%) and a neonatal ICU admission (23% vs. 15%). The majority of the neonatal ICU admissions were for prematurity or respiratory distress.
"In anyone with congenital heart disease, prenatal care is very important, and a lot of these women were followed closely by a team of physicians [specializing in high-risk pregnancies]," noted Ms. Warrick.
"All in all, pregnant women with congenital heart disease can undergo labor and delivery without many complications, but tend to have longer hospital stays and more neonatal ICU admissions," she concluded.
Ms. Warrick disclosed no relevant conflicts of interest.
MONTEREY, CALIF. – Parturients with congenital heart disease do have a rockier obstetric course, but generally fare quite well in the peripartum period, a retrospective cohort study found.
Researchers at the University of Colorado Hospital in Denver led by Christine M. Warrick found that roughly 1% of women delivering there over a 4-year period had congenital heart disease.
This group had higher rates of cesarean delivery and neonatal ICU admission than did parturients overall, according to results reported in a poster session at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. Maternal ICU admissions were rare, however, and there were no cases of maternal mortality.
"Congenital heart disease is definitely a risk factor for maternal and fetal complications, but overall the women seemed to do pretty well. The main things to look out for would be neonatal ICU admissions and respiratory distress in the babies," Ms. Warrick commented in an interview.
In additional study findings, although the large majority of women received regional anesthesia for labor and delivery, only a minority had an epidural placed early, before 4 cm of cervical dilation.
"There is some literature that suggests that early epidural placement is beneficial for these women because it decreases the stress on the heart secondary to pain," she noted. "Some of these women come [to the hospital] in labor and they are already beyond 4 cm of dilation, so that may be a big reason why there weren’t so many early epidurals placed."
In a related poster discussion session, comoderator Dr. Katherine W. Arendt of the Mayo Clinic in Rochester, Minn., asked, "Do you believe that the geographical elevation of your hospital improved or worsened your outcomes compared to a sea-level hospital?"
"It’s difficult to say exactly how the elevation affected all of these women, since we had so many different varieties of congenital heart disease," replied Ms. Warrick, who is a fourth-year medical student at the University of Colorado in Aurora. "But it is possible that women with more severe congenital heart disease would not tolerate a lower partial pressure of oxygen in our environment, and there were some women who were advised to deliver at lower altitudes because of this. So that probably improved our outcomes in women who received adequate prenatal care. I also think that points to the importance of prenatal care in these women."
"We know that more women are surviving to childbearing age with congenital heart disease due to the recent advances in surgical repair of these defects, and it is actually one of the major causes of cardiac diseases in pregnant women in the United States," she said. Many of the normal changes of pregnancy, labor, and delivery put stress on the heart and would be expected to exacerbate matters.
The investigators studied 13,109 parturients in the hospital’s perinatal database between October 2005 and December 2009. Medical history and record review identified 75 women, or 0.6%, as having congenital heart disease.
These women had a mean age of 26 years. The majority were non-Hispanic white (60%) and nulliparous (55%). Fully 34% were overweight or obese, and 8% smoked.
According to cardiologists’ notes of symptoms during the third trimester of pregnancy, 60%, 33%, and 7% of the women had New York Heart Association functional class I, II, and III, respectively. The leading congenital anomalies were atrial septal defects (28%), valvular disorders (19%), and tetralogy of Fallot (11%).
In terms of cardiac outcomes, 11% of the women had an arrhythmia in the peripartum period, and 5% required diuresis, Ms. Warrick reported. Although 87% received regional anesthesia for labor and delivery, only 31% received an early epidural.
Some 19% of the women had a preterm birth (one occurring before 37 weeks’ gestation), and 45% had a prolonged hospital stay (lasting more than 2 days after a vaginal delivery or more than 3 days after a cesarean delivery).
Although 3% of the women were admitted to the ICU for prophylactic monitoring, there were no maternal deaths in the peripartum period.
Compared with parturients overall, those with congenital heart disease were more likely to have a cesarean delivery (31% vs. 27%) and a neonatal ICU admission (23% vs. 15%). The majority of the neonatal ICU admissions were for prematurity or respiratory distress.
"In anyone with congenital heart disease, prenatal care is very important, and a lot of these women were followed closely by a team of physicians [specializing in high-risk pregnancies]," noted Ms. Warrick.
"All in all, pregnant women with congenital heart disease can undergo labor and delivery without many complications, but tend to have longer hospital stays and more neonatal ICU admissions," she concluded.
Ms. Warrick disclosed no relevant conflicts of interest.
MONTEREY, CALIF. – Parturients with congenital heart disease do have a rockier obstetric course, but generally fare quite well in the peripartum period, a retrospective cohort study found.
Researchers at the University of Colorado Hospital in Denver led by Christine M. Warrick found that roughly 1% of women delivering there over a 4-year period had congenital heart disease.
This group had higher rates of cesarean delivery and neonatal ICU admission than did parturients overall, according to results reported in a poster session at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. Maternal ICU admissions were rare, however, and there were no cases of maternal mortality.
"Congenital heart disease is definitely a risk factor for maternal and fetal complications, but overall the women seemed to do pretty well. The main things to look out for would be neonatal ICU admissions and respiratory distress in the babies," Ms. Warrick commented in an interview.
In additional study findings, although the large majority of women received regional anesthesia for labor and delivery, only a minority had an epidural placed early, before 4 cm of cervical dilation.
"There is some literature that suggests that early epidural placement is beneficial for these women because it decreases the stress on the heart secondary to pain," she noted. "Some of these women come [to the hospital] in labor and they are already beyond 4 cm of dilation, so that may be a big reason why there weren’t so many early epidurals placed."
In a related poster discussion session, comoderator Dr. Katherine W. Arendt of the Mayo Clinic in Rochester, Minn., asked, "Do you believe that the geographical elevation of your hospital improved or worsened your outcomes compared to a sea-level hospital?"
"It’s difficult to say exactly how the elevation affected all of these women, since we had so many different varieties of congenital heart disease," replied Ms. Warrick, who is a fourth-year medical student at the University of Colorado in Aurora. "But it is possible that women with more severe congenital heart disease would not tolerate a lower partial pressure of oxygen in our environment, and there were some women who were advised to deliver at lower altitudes because of this. So that probably improved our outcomes in women who received adequate prenatal care. I also think that points to the importance of prenatal care in these women."
"We know that more women are surviving to childbearing age with congenital heart disease due to the recent advances in surgical repair of these defects, and it is actually one of the major causes of cardiac diseases in pregnant women in the United States," she said. Many of the normal changes of pregnancy, labor, and delivery put stress on the heart and would be expected to exacerbate matters.
The investigators studied 13,109 parturients in the hospital’s perinatal database between October 2005 and December 2009. Medical history and record review identified 75 women, or 0.6%, as having congenital heart disease.
These women had a mean age of 26 years. The majority were non-Hispanic white (60%) and nulliparous (55%). Fully 34% were overweight or obese, and 8% smoked.
According to cardiologists’ notes of symptoms during the third trimester of pregnancy, 60%, 33%, and 7% of the women had New York Heart Association functional class I, II, and III, respectively. The leading congenital anomalies were atrial septal defects (28%), valvular disorders (19%), and tetralogy of Fallot (11%).
In terms of cardiac outcomes, 11% of the women had an arrhythmia in the peripartum period, and 5% required diuresis, Ms. Warrick reported. Although 87% received regional anesthesia for labor and delivery, only 31% received an early epidural.
Some 19% of the women had a preterm birth (one occurring before 37 weeks’ gestation), and 45% had a prolonged hospital stay (lasting more than 2 days after a vaginal delivery or more than 3 days after a cesarean delivery).
Although 3% of the women were admitted to the ICU for prophylactic monitoring, there were no maternal deaths in the peripartum period.
Compared with parturients overall, those with congenital heart disease were more likely to have a cesarean delivery (31% vs. 27%) and a neonatal ICU admission (23% vs. 15%). The majority of the neonatal ICU admissions were for prematurity or respiratory distress.
"In anyone with congenital heart disease, prenatal care is very important, and a lot of these women were followed closely by a team of physicians [specializing in high-risk pregnancies]," noted Ms. Warrick.
"All in all, pregnant women with congenital heart disease can undergo labor and delivery without many complications, but tend to have longer hospital stays and more neonatal ICU admissions," she concluded.
Ms. Warrick disclosed no relevant conflicts of interest.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATOLOGY
Why high placement of the cerclage is a key to success
UPDATE: INFECTIOUS DISEASE
- 10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Meghan O. Schimpf (June 2012) - Gaps in Chlamydia testing threaten reproductive health, CDC warns
Janelle Yates, Senior Editor (Web exclusive, May 2012)
Dr. Duff reports no financial relationships relevant to this article.
In this Update, I’ve highlighted four interesting articles about infectious disease management in obstetric and gyn practice that appeared in the medical literature over the past 12 months:
- One describes a study that reminds physicians of the importance of an unusual manifestation of gonococcal infection
- A second article demonstrates the importance of making a change in the prophylactic antibiotic regimen provided to morbidly obese patients who are having a cesarean delivery
- A third describes an exciting development in the treatment of chronic hepatitis C virus infection
- The final article makes interesting observations about the proper duration of treatment for patients who have chorioamnionitis.
N gonorrhoeae causes illness beyond the urogenital tract
Bleich AT, Sheffield JS, Wendel GD, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119(3):597–602.
This article describes a retrospective review of 112 women who were admitted to Parkland Memorial Hospital in Dallas, Texas, from January 1975 through December 2008 and given a diagnosis of disseminated infection with Neisseria gonorrhoeae. Eighty (71%) of these women were not pregnant and were cared for on the internal medicine service; 32 (29%) were pregnant and were treated by faculty members and residents on the ObGyn service.
Over the course of the study, the frequency of disseminated gonococcal infection decreased significantly. Among pregnant women, the rate of infection was 11 for every 100,000 deliveries before 1980 and, after 1985, five for every 100,000 deliveries.
The most common clinical manifestation of disseminated gonococcal infection was arthritis. The most commonly affected joints were the knee, wrist, elbow, and ankle.
Other common clinical manifestations included dermatitis, fever, chills, and a purulent cervical discharge. Notably, the frequency of a purulent joint effusion was 50% in pregnant women and 70% in nonpregnant women—reflecting the fact that the duration of symptoms was approximately 3 days shorter in pregnant women than in nonpregnant women. Otherwise, the clinical presentation in pregnant women did not differ significantly from that of nonpregnant women.
In addition, the clinical course and the response to intravenous (IV) antibiotic therapy did not differ significantly between pregnant and nonpregnant women.
The authors were unable to document that disseminated gonococcal infection had any deleterious effect on the outcome of pregnancy among the patients studied. Although four of the 32 women delivered preterm, in only one instance was delivery related temporally to the disseminated gonococcal infection.
Commentary
Because of their experience treating women who have gonorrhea, I would say that most ObGyns think of N gonorrhoeae as causing localized infection in the lower genital tract (urethritis, endocervicitis, inflammatory proctitis) or upper genital tract (pelvic inflammatory disease). We should recognize, however, that gonorrhea also can cause prominent extra-pelvic findings, such as severe pharyngitis (in patients who practice orogenital intercourse) and perihepatitis (Fitz-Hugh-Curtis syndrome).
In addition, always bear in mind that, in rare instances, gonorrhea can become disseminated, causing quite serious illness. The most common extra-pelvic manifestation of disseminated gonococcal infection is arthritis. As noted in this study of a series of patients, the arthritis is usually polyarticular and affects medium or small joints.
The second most common manifestation of disseminated gonococcal infection is dermatitis. Characteristic lesions are raised, red or purple papules. These lesions are not a simple vasculitis; rather, they contain a high concentration of microorganisms.
Other possible manifestations of disseminated infection include pericarditis, endocarditis, and meningitis.
The diagnosis of disseminated gonococcal infection is usually made by clinical examination and culture of specimens from the genital tract, blood, or joint effusion.
Disseminated gonococcal infection usually responds promptly to intravenous antibiotic therapy.
Recommended therapy is ceftriaxone:
• 25 to 50 mg/kg/d IV for 7 days
or
• a single, daily, 25 to 50 mg/kg intramuscular dose, also for 7 days.
Continue therapy for 10 to 14 days if the patient has meningitis.
An alternative regimen is cefotaxime:
• 25 mg/kg/d IV for 7 days
or
• 25 mg/kg IM every 12 hours, also for 7 days.
Extend treatment for 10 to 14 days if meningitis is present.1
Obesity curtails effectiveness of antibiotic prophylaxis in cesarean delivery
Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
In this prospective study of the influence of an obese habitus on antibiotic prophylaxis during cesarean delivery, researchers divided 29 patients who were scheduled for cesarean into three groups, by body mass index (BMI):
- lean (BMI, <30; n = 10)
- obese (30–39.9; n = 10)
- extremely obese (>40; n = 9).
All patients were given a 2-g dose of IV cefazolin 30 to 60 minutes before surgery.
During delivery, the team took two specimens of adipose tissue: one immediately after the skin incision and one later, after fascia was closed. They also obtained a specimen of myometrial tissue after delivery and a blood specimen after surgery was completed.
The concentration of cefazolin was then measured in adipose and myometrial tissue and in serum.
Findings. The researchers demonstrated that the mean concentration of cefazolin in the initial specimen of adipose tissue was significantly higher in lean patients than in obese and extremely obese patients. All 10 women who had a BMI less than 30 had a serum cefazolin concentration greater than 4 μg/g—the theoretical break-point for defining resistance to cefazolin. The initial adipose tissue specimen from two of the 10 obese patients and three of the nine extremely obese patients showed cefazolin concentrations less than 4 μg/g.
Of particular interest, two women—both of whom had a BMI greater than 40—developed a wound infection that required antibiotic therapy. Their initial and subsequent adipose tissue concentrations of cefazolin were less than the 4 μg/g break-point for resistance.
The concentration of cefazolin in the patients’ myometrial and serum specimens demonstrated a pattern similar to what the researchers observed in adipose tissue, but these results were not statistically significant across BMI groups. In fact, the cefazolin concentration in all groups’ myometrial and serum specimens exceeded the minimum inhibitory concentration for most potential pathogens in the setting of cesarean delivery.
Commentary
Clearly, prophylactic antibiotics are indicated for all women who are having a cesarean delivery. Antibiotics have their greatest impact when administered before the surgical incision is made; to exert their full protective effect against endometritis and wound infection, however, antibiotics should reach a recognized therapeutic concentration—not only in serum and myometrium but in the subcutaneous tissue.
The customary dosage of cefazolin for cesarean delivery prophylaxis has been 1 g. This study demonstrated that, although a 2-g dose of cefazolin reached a therapeutic concentration in myometrial tissue and serum, it did not consistently do so in the adipose tissue of obese and extremely obese patients.
Pending further investigation, I strongly recommend that all women who have a BMI greater than 30 receive a 2-g dose of cefazolin 30 to 60 minutes before cesarean delivery. Future research is needed to determine whether an even higher dosage is necessary to achieve a therapeutic concentration in the subcutaneous tissue of morbidly obese patients.
New therapies promise a better outcome in hepatitis C
Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416.
The authors conducted an international Phase-3, randomized, double-blind, placebo-controlled trial of two different treatment modalities for chronic hepatitis C virus (HCV) infection. The authors assigned 1,088 patients who had HCV genotype-1 infection and who had not received prior therapy to one of three treatment groups:
- telaprevir (Incivek, Vertex Pharmaceuticals), an HCV genotype-1 protease inhibitor, combined with peginterferon alfa-2a (Pegasys, Genetech) plus ribavirin (Copegus, Genetech; Rebetol, Merck; etc.) for 12 weeks; patients then were given peginterferon alfa-2a plus ribavirin only for 12 additional weeks if HCV RNA was undetectable at weeks 4 and 12 or peginterferon alfa-2a plus ribavirin only for 36 weeks if HCV RNA was detectable at either time point (Group 1)
- telaprevir with peginterferon alfa-2a plus ribavirin for 8 weeks, then placebo with peginterferon alfa-2a plus ribavirin for 4 weeks, followed by 12 to 36 weeks of peginterferon alfa-2a plus ribavirin using the HCV RNA criteria applied to Group 1 (Group 2)
- placebo with peginterferon alfa-2a plus ribavirin for 12 weeks, followed by 36 weeks of peginterferon alfa-2a plus ribavirin (Group 3).
The primary endpoint of the trial was the percentage of patients who had undetectable plasma HCV RNA at 24 weeks after the last planned dose of the study drugs. The investigators considered that this endpoint represented a sustained virologic response.
Findings. Seventy-five percent of patients in Group 1 and 69% of those in Group 2 had a sustained virologic response. By comparison, only 44% of patients in Group 3 had a sustained response. The differences in outcome between Group 1 and Group 3, and between Group 2 and Group 3, were highly significant (P<.001). Virologic failure was more common among patients who had HCV genotype-1a infection than among those who had HCV genotype-1b infection.
The most common side effects noted by patients who received telaprevir were gastrointestinal irritation, rash, and anemia. Ten percent of patients in the telaprevir group discontinued therapy, compared with 7% in the peginterferon-ribavirin-alone group.
Commentary
Worldwide, approximately 170 million people have chronic hepatitis C, which is the most common indication for liver transplantation. Until recently, the principal treatments for hepatitis C were pegylated interferon alfa with ribavirin and without ribavirin; the response rate with these regimens was in the range of 55%. This study shows that adding telaprevir to regimens for HCV infection significantly improves prospects for long-term resolution of infection.
In some obstetric and gynecologic populations, HCV is more common than hepatitis B virus. Risk factors for hepatitis C include hepatitis B, intravenous drug abuse, and human immunodeficiency virus infection. HCV-infected women pose a risk to their sex partners; infected pregnant women can transmit the virus to their baby.
Unlike hepatitis A and hepatitis B, immunoprophylaxis is not available for hepatitis C. That reality is what makes the study by Jacobsen and colleagues so compelling: They have clearly demonstrated that multi-agent antiviral therapy might be able to truly cure this infection.
The lesson here for ObGyns? Screen at-risk patients and then refer the hepatitis C-seropositive ones to a specialist in gastroenterology, who can determine candidacy for one of the new treatment regimens.
Clearly, the prognosis for people who have hepatitis C is much better today than it was 20 years ago.
For how long should chorioamnionitis be treated?
Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119(6):1102-1105.
The authors conducted a retrospective review of 423 women who had been treated for chorioamnionitis at the University of Florida from 2005 to 2009.
Patients had been given IV ampicillin (2 g every 6 h) plus IV gentamicin (1.5 mg/kg every 8 h) as soon as the diagnosis of chorioamnionitis was established; postpartum, they were given only the one next scheduled dose of each antibiotic. Patients who had a cesarean received either metronidazole (500 mg) or clindamycin (900 mg) immediately after cord clamping to enhance coverage of anaerobic organisms.
The primary outcome was treatment failure, defined as persistent fever requiring continued antibiotics, surgical intervention, or administration of heparin for septic pelvic-vein thrombophlebitis.
Findings. Here is a breakdown of what the investigators found regarding the 282 women who delivered vaginally and the 141 who underwent cesarean delivery:
- Overall, 399 of the patients (94%; 95% confidence interval [CI], 92% and 96%) were treated successfully; 24 (6%; 95% CI, 3.7% and 8.3%) failed short-course treatment
- Of the 282 patients who delivered vaginally, 279 (99%; 95% CI, 98% and 100%) were cured with short-term therapy
- Of the 141 who delivered by cesarean, 120 (85%; 95% CI, 79% and 91%) were cured (P<.001).
- Seventeen of the total treatment failures had endometritis and responded quickly to continuation of antibiotics. Of the 17 patients with endometritis, 14 had a cesarean delivery.
- Seven patients had more serious complications: four, wound infection; three, septic pelvic-vein thrombophlebitis. All serious complications occurred after cesarean delivery.
- Of the four patients who had a wound infection, three had labor induced by misoprostol; their BMI was 44.8, 31.1, and 48.5, respectively. The fourth had a cesarean delivery at 29 weeks for preterm premature rupture of membranes (PPROM), chorioamnionitis, and malpresentation.
- Of the three patients who had septic pelvic-vein thrombophlebitis, two had labor induced by misoprostol. One had a BMI of 29.2; the other, 31.1. The third patient was delivered secondary to PPROM; her BMI was 40.3.
In addition, of the 21 treatment failures in the cesarean delivery group, 6 had prolonged rupture of membranes (ROM) and 10 had a BMI greater than 30. Six patients had both prolonged ROM and were obese or morbidly obese.
Of the 120 women who had a cesarean delivery and were treated successfully, 3 had prolonged ROM and 39 had a BMI greater than 30. None had both prolonged ROM and a BMI greater than 30.
Last, the difference between treatment failures and treatment successes in regard to the frequency of prolonged ROM or a BMI greater than 30 was highly significant (P<.01).
Commentary
In most published reports of patients who have chorioamnionitis, antibiotic treatment continues until the patient is afebrile and asymptomatic for 24 to 48 hours. This treatment approach has been based largely on expert opinion, however, not on Level-1 or Level-2 evidence.
In 2003, Edwards and Duff published a study of chorioamnionitis antibiotic regimens that compared single-dose postpartum treatment to extended treatment.2 This randomized controlled trial demonstrated that there was no statistically significant difference between patients who had only a single dose of postpartum antibiotics and those who received an extended course of medication (i.e., who were treated until they had been afebrile and asymptomatic for a minimum of 24 hours) in regard to adverse outcomes (2.9% and 4.3%, respectively). The study discussed here extends and refines the observations made in the 2003 Edwards and Duff randomized controlled trial.
The new study shows that a limited course of antibiotics was, overall, effective in treating 94% of patients with chorioamnionitis (95% CI, 92% and 96%). Only 1% of patients who delivered vaginally failed therapy, compared with 15% of patients who delivered by cesarean (P<.001). In the cesarean group, women who failed therapy were likely to 1) be obese or 2) have a relatively long duration of labor or ruptured membranes, or both. These patients may have benefitted from a more extended course of antibiotic therapy.
Based on this investigation, I strongly recommend a limited course of antibiotic therapy (ampicillin plus gentamicin) for women with chorioamnionitis who deliver vaginally. Patients who have had a cesarean delivery—particularly those who are obese or have had an extended duration of labor, or both—should be treated with antibiotics until they have been afebrile and asymptomatic for 24 hours.
We want to hear from you! Tell us what you think.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Edwards RK, Duff P. Single dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 Pt 1):957-961.
- 10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Meghan O. Schimpf (June 2012) - Gaps in Chlamydia testing threaten reproductive health, CDC warns
Janelle Yates, Senior Editor (Web exclusive, May 2012)
Dr. Duff reports no financial relationships relevant to this article.
In this Update, I’ve highlighted four interesting articles about infectious disease management in obstetric and gyn practice that appeared in the medical literature over the past 12 months:
- One describes a study that reminds physicians of the importance of an unusual manifestation of gonococcal infection
- A second article demonstrates the importance of making a change in the prophylactic antibiotic regimen provided to morbidly obese patients who are having a cesarean delivery
- A third describes an exciting development in the treatment of chronic hepatitis C virus infection
- The final article makes interesting observations about the proper duration of treatment for patients who have chorioamnionitis.
N gonorrhoeae causes illness beyond the urogenital tract
Bleich AT, Sheffield JS, Wendel GD, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119(3):597–602.
This article describes a retrospective review of 112 women who were admitted to Parkland Memorial Hospital in Dallas, Texas, from January 1975 through December 2008 and given a diagnosis of disseminated infection with Neisseria gonorrhoeae. Eighty (71%) of these women were not pregnant and were cared for on the internal medicine service; 32 (29%) were pregnant and were treated by faculty members and residents on the ObGyn service.
Over the course of the study, the frequency of disseminated gonococcal infection decreased significantly. Among pregnant women, the rate of infection was 11 for every 100,000 deliveries before 1980 and, after 1985, five for every 100,000 deliveries.
The most common clinical manifestation of disseminated gonococcal infection was arthritis. The most commonly affected joints were the knee, wrist, elbow, and ankle.
Other common clinical manifestations included dermatitis, fever, chills, and a purulent cervical discharge. Notably, the frequency of a purulent joint effusion was 50% in pregnant women and 70% in nonpregnant women—reflecting the fact that the duration of symptoms was approximately 3 days shorter in pregnant women than in nonpregnant women. Otherwise, the clinical presentation in pregnant women did not differ significantly from that of nonpregnant women.
In addition, the clinical course and the response to intravenous (IV) antibiotic therapy did not differ significantly between pregnant and nonpregnant women.
The authors were unable to document that disseminated gonococcal infection had any deleterious effect on the outcome of pregnancy among the patients studied. Although four of the 32 women delivered preterm, in only one instance was delivery related temporally to the disseminated gonococcal infection.
Commentary
Because of their experience treating women who have gonorrhea, I would say that most ObGyns think of N gonorrhoeae as causing localized infection in the lower genital tract (urethritis, endocervicitis, inflammatory proctitis) or upper genital tract (pelvic inflammatory disease). We should recognize, however, that gonorrhea also can cause prominent extra-pelvic findings, such as severe pharyngitis (in patients who practice orogenital intercourse) and perihepatitis (Fitz-Hugh-Curtis syndrome).
In addition, always bear in mind that, in rare instances, gonorrhea can become disseminated, causing quite serious illness. The most common extra-pelvic manifestation of disseminated gonococcal infection is arthritis. As noted in this study of a series of patients, the arthritis is usually polyarticular and affects medium or small joints.
The second most common manifestation of disseminated gonococcal infection is dermatitis. Characteristic lesions are raised, red or purple papules. These lesions are not a simple vasculitis; rather, they contain a high concentration of microorganisms.
Other possible manifestations of disseminated infection include pericarditis, endocarditis, and meningitis.
The diagnosis of disseminated gonococcal infection is usually made by clinical examination and culture of specimens from the genital tract, blood, or joint effusion.
Disseminated gonococcal infection usually responds promptly to intravenous antibiotic therapy.
Recommended therapy is ceftriaxone:
• 25 to 50 mg/kg/d IV for 7 days
or
• a single, daily, 25 to 50 mg/kg intramuscular dose, also for 7 days.
Continue therapy for 10 to 14 days if the patient has meningitis.
An alternative regimen is cefotaxime:
• 25 mg/kg/d IV for 7 days
or
• 25 mg/kg IM every 12 hours, also for 7 days.
Extend treatment for 10 to 14 days if meningitis is present.1
Obesity curtails effectiveness of antibiotic prophylaxis in cesarean delivery
Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
In this prospective study of the influence of an obese habitus on antibiotic prophylaxis during cesarean delivery, researchers divided 29 patients who were scheduled for cesarean into three groups, by body mass index (BMI):
- lean (BMI, <30; n = 10)
- obese (30–39.9; n = 10)
- extremely obese (>40; n = 9).
All patients were given a 2-g dose of IV cefazolin 30 to 60 minutes before surgery.
During delivery, the team took two specimens of adipose tissue: one immediately after the skin incision and one later, after fascia was closed. They also obtained a specimen of myometrial tissue after delivery and a blood specimen after surgery was completed.
The concentration of cefazolin was then measured in adipose and myometrial tissue and in serum.
Findings. The researchers demonstrated that the mean concentration of cefazolin in the initial specimen of adipose tissue was significantly higher in lean patients than in obese and extremely obese patients. All 10 women who had a BMI less than 30 had a serum cefazolin concentration greater than 4 μg/g—the theoretical break-point for defining resistance to cefazolin. The initial adipose tissue specimen from two of the 10 obese patients and three of the nine extremely obese patients showed cefazolin concentrations less than 4 μg/g.
Of particular interest, two women—both of whom had a BMI greater than 40—developed a wound infection that required antibiotic therapy. Their initial and subsequent adipose tissue concentrations of cefazolin were less than the 4 μg/g break-point for resistance.
The concentration of cefazolin in the patients’ myometrial and serum specimens demonstrated a pattern similar to what the researchers observed in adipose tissue, but these results were not statistically significant across BMI groups. In fact, the cefazolin concentration in all groups’ myometrial and serum specimens exceeded the minimum inhibitory concentration for most potential pathogens in the setting of cesarean delivery.
Commentary
Clearly, prophylactic antibiotics are indicated for all women who are having a cesarean delivery. Antibiotics have their greatest impact when administered before the surgical incision is made; to exert their full protective effect against endometritis and wound infection, however, antibiotics should reach a recognized therapeutic concentration—not only in serum and myometrium but in the subcutaneous tissue.
The customary dosage of cefazolin for cesarean delivery prophylaxis has been 1 g. This study demonstrated that, although a 2-g dose of cefazolin reached a therapeutic concentration in myometrial tissue and serum, it did not consistently do so in the adipose tissue of obese and extremely obese patients.
Pending further investigation, I strongly recommend that all women who have a BMI greater than 30 receive a 2-g dose of cefazolin 30 to 60 minutes before cesarean delivery. Future research is needed to determine whether an even higher dosage is necessary to achieve a therapeutic concentration in the subcutaneous tissue of morbidly obese patients.
New therapies promise a better outcome in hepatitis C
Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416.
The authors conducted an international Phase-3, randomized, double-blind, placebo-controlled trial of two different treatment modalities for chronic hepatitis C virus (HCV) infection. The authors assigned 1,088 patients who had HCV genotype-1 infection and who had not received prior therapy to one of three treatment groups:
- telaprevir (Incivek, Vertex Pharmaceuticals), an HCV genotype-1 protease inhibitor, combined with peginterferon alfa-2a (Pegasys, Genetech) plus ribavirin (Copegus, Genetech; Rebetol, Merck; etc.) for 12 weeks; patients then were given peginterferon alfa-2a plus ribavirin only for 12 additional weeks if HCV RNA was undetectable at weeks 4 and 12 or peginterferon alfa-2a plus ribavirin only for 36 weeks if HCV RNA was detectable at either time point (Group 1)
- telaprevir with peginterferon alfa-2a plus ribavirin for 8 weeks, then placebo with peginterferon alfa-2a plus ribavirin for 4 weeks, followed by 12 to 36 weeks of peginterferon alfa-2a plus ribavirin using the HCV RNA criteria applied to Group 1 (Group 2)
- placebo with peginterferon alfa-2a plus ribavirin for 12 weeks, followed by 36 weeks of peginterferon alfa-2a plus ribavirin (Group 3).
The primary endpoint of the trial was the percentage of patients who had undetectable plasma HCV RNA at 24 weeks after the last planned dose of the study drugs. The investigators considered that this endpoint represented a sustained virologic response.
Findings. Seventy-five percent of patients in Group 1 and 69% of those in Group 2 had a sustained virologic response. By comparison, only 44% of patients in Group 3 had a sustained response. The differences in outcome between Group 1 and Group 3, and between Group 2 and Group 3, were highly significant (P<.001). Virologic failure was more common among patients who had HCV genotype-1a infection than among those who had HCV genotype-1b infection.
The most common side effects noted by patients who received telaprevir were gastrointestinal irritation, rash, and anemia. Ten percent of patients in the telaprevir group discontinued therapy, compared with 7% in the peginterferon-ribavirin-alone group.
Commentary
Worldwide, approximately 170 million people have chronic hepatitis C, which is the most common indication for liver transplantation. Until recently, the principal treatments for hepatitis C were pegylated interferon alfa with ribavirin and without ribavirin; the response rate with these regimens was in the range of 55%. This study shows that adding telaprevir to regimens for HCV infection significantly improves prospects for long-term resolution of infection.
In some obstetric and gynecologic populations, HCV is more common than hepatitis B virus. Risk factors for hepatitis C include hepatitis B, intravenous drug abuse, and human immunodeficiency virus infection. HCV-infected women pose a risk to their sex partners; infected pregnant women can transmit the virus to their baby.
Unlike hepatitis A and hepatitis B, immunoprophylaxis is not available for hepatitis C. That reality is what makes the study by Jacobsen and colleagues so compelling: They have clearly demonstrated that multi-agent antiviral therapy might be able to truly cure this infection.
The lesson here for ObGyns? Screen at-risk patients and then refer the hepatitis C-seropositive ones to a specialist in gastroenterology, who can determine candidacy for one of the new treatment regimens.
Clearly, the prognosis for people who have hepatitis C is much better today than it was 20 years ago.
For how long should chorioamnionitis be treated?
Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119(6):1102-1105.
The authors conducted a retrospective review of 423 women who had been treated for chorioamnionitis at the University of Florida from 2005 to 2009.
Patients had been given IV ampicillin (2 g every 6 h) plus IV gentamicin (1.5 mg/kg every 8 h) as soon as the diagnosis of chorioamnionitis was established; postpartum, they were given only the one next scheduled dose of each antibiotic. Patients who had a cesarean received either metronidazole (500 mg) or clindamycin (900 mg) immediately after cord clamping to enhance coverage of anaerobic organisms.
The primary outcome was treatment failure, defined as persistent fever requiring continued antibiotics, surgical intervention, or administration of heparin for septic pelvic-vein thrombophlebitis.
Findings. Here is a breakdown of what the investigators found regarding the 282 women who delivered vaginally and the 141 who underwent cesarean delivery:
- Overall, 399 of the patients (94%; 95% confidence interval [CI], 92% and 96%) were treated successfully; 24 (6%; 95% CI, 3.7% and 8.3%) failed short-course treatment
- Of the 282 patients who delivered vaginally, 279 (99%; 95% CI, 98% and 100%) were cured with short-term therapy
- Of the 141 who delivered by cesarean, 120 (85%; 95% CI, 79% and 91%) were cured (P<.001).
- Seventeen of the total treatment failures had endometritis and responded quickly to continuation of antibiotics. Of the 17 patients with endometritis, 14 had a cesarean delivery.
- Seven patients had more serious complications: four, wound infection; three, septic pelvic-vein thrombophlebitis. All serious complications occurred after cesarean delivery.
- Of the four patients who had a wound infection, three had labor induced by misoprostol; their BMI was 44.8, 31.1, and 48.5, respectively. The fourth had a cesarean delivery at 29 weeks for preterm premature rupture of membranes (PPROM), chorioamnionitis, and malpresentation.
- Of the three patients who had septic pelvic-vein thrombophlebitis, two had labor induced by misoprostol. One had a BMI of 29.2; the other, 31.1. The third patient was delivered secondary to PPROM; her BMI was 40.3.
In addition, of the 21 treatment failures in the cesarean delivery group, 6 had prolonged rupture of membranes (ROM) and 10 had a BMI greater than 30. Six patients had both prolonged ROM and were obese or morbidly obese.
Of the 120 women who had a cesarean delivery and were treated successfully, 3 had prolonged ROM and 39 had a BMI greater than 30. None had both prolonged ROM and a BMI greater than 30.
Last, the difference between treatment failures and treatment successes in regard to the frequency of prolonged ROM or a BMI greater than 30 was highly significant (P<.01).
Commentary
In most published reports of patients who have chorioamnionitis, antibiotic treatment continues until the patient is afebrile and asymptomatic for 24 to 48 hours. This treatment approach has been based largely on expert opinion, however, not on Level-1 or Level-2 evidence.
In 2003, Edwards and Duff published a study of chorioamnionitis antibiotic regimens that compared single-dose postpartum treatment to extended treatment.2 This randomized controlled trial demonstrated that there was no statistically significant difference between patients who had only a single dose of postpartum antibiotics and those who received an extended course of medication (i.e., who were treated until they had been afebrile and asymptomatic for a minimum of 24 hours) in regard to adverse outcomes (2.9% and 4.3%, respectively). The study discussed here extends and refines the observations made in the 2003 Edwards and Duff randomized controlled trial.
The new study shows that a limited course of antibiotics was, overall, effective in treating 94% of patients with chorioamnionitis (95% CI, 92% and 96%). Only 1% of patients who delivered vaginally failed therapy, compared with 15% of patients who delivered by cesarean (P<.001). In the cesarean group, women who failed therapy were likely to 1) be obese or 2) have a relatively long duration of labor or ruptured membranes, or both. These patients may have benefitted from a more extended course of antibiotic therapy.
Based on this investigation, I strongly recommend a limited course of antibiotic therapy (ampicillin plus gentamicin) for women with chorioamnionitis who deliver vaginally. Patients who have had a cesarean delivery—particularly those who are obese or have had an extended duration of labor, or both—should be treated with antibiotics until they have been afebrile and asymptomatic for 24 hours.
We want to hear from you! Tell us what you think.
- 10 practical, evidence-based recommendations for perioperative antibiotic prophylaxis
Meghan O. Schimpf (June 2012) - Gaps in Chlamydia testing threaten reproductive health, CDC warns
Janelle Yates, Senior Editor (Web exclusive, May 2012)
Dr. Duff reports no financial relationships relevant to this article.
In this Update, I’ve highlighted four interesting articles about infectious disease management in obstetric and gyn practice that appeared in the medical literature over the past 12 months:
- One describes a study that reminds physicians of the importance of an unusual manifestation of gonococcal infection
- A second article demonstrates the importance of making a change in the prophylactic antibiotic regimen provided to morbidly obese patients who are having a cesarean delivery
- A third describes an exciting development in the treatment of chronic hepatitis C virus infection
- The final article makes interesting observations about the proper duration of treatment for patients who have chorioamnionitis.
N gonorrhoeae causes illness beyond the urogenital tract
Bleich AT, Sheffield JS, Wendel GD, Sigman A, Cunningham FG. Disseminated gonococcal infection in women. Obstet Gynecol. 2012;119(3):597–602.
This article describes a retrospective review of 112 women who were admitted to Parkland Memorial Hospital in Dallas, Texas, from January 1975 through December 2008 and given a diagnosis of disseminated infection with Neisseria gonorrhoeae. Eighty (71%) of these women were not pregnant and were cared for on the internal medicine service; 32 (29%) were pregnant and were treated by faculty members and residents on the ObGyn service.
Over the course of the study, the frequency of disseminated gonococcal infection decreased significantly. Among pregnant women, the rate of infection was 11 for every 100,000 deliveries before 1980 and, after 1985, five for every 100,000 deliveries.
The most common clinical manifestation of disseminated gonococcal infection was arthritis. The most commonly affected joints were the knee, wrist, elbow, and ankle.
Other common clinical manifestations included dermatitis, fever, chills, and a purulent cervical discharge. Notably, the frequency of a purulent joint effusion was 50% in pregnant women and 70% in nonpregnant women—reflecting the fact that the duration of symptoms was approximately 3 days shorter in pregnant women than in nonpregnant women. Otherwise, the clinical presentation in pregnant women did not differ significantly from that of nonpregnant women.
In addition, the clinical course and the response to intravenous (IV) antibiotic therapy did not differ significantly between pregnant and nonpregnant women.
The authors were unable to document that disseminated gonococcal infection had any deleterious effect on the outcome of pregnancy among the patients studied. Although four of the 32 women delivered preterm, in only one instance was delivery related temporally to the disseminated gonococcal infection.
Commentary
Because of their experience treating women who have gonorrhea, I would say that most ObGyns think of N gonorrhoeae as causing localized infection in the lower genital tract (urethritis, endocervicitis, inflammatory proctitis) or upper genital tract (pelvic inflammatory disease). We should recognize, however, that gonorrhea also can cause prominent extra-pelvic findings, such as severe pharyngitis (in patients who practice orogenital intercourse) and perihepatitis (Fitz-Hugh-Curtis syndrome).
In addition, always bear in mind that, in rare instances, gonorrhea can become disseminated, causing quite serious illness. The most common extra-pelvic manifestation of disseminated gonococcal infection is arthritis. As noted in this study of a series of patients, the arthritis is usually polyarticular and affects medium or small joints.
The second most common manifestation of disseminated gonococcal infection is dermatitis. Characteristic lesions are raised, red or purple papules. These lesions are not a simple vasculitis; rather, they contain a high concentration of microorganisms.
Other possible manifestations of disseminated infection include pericarditis, endocarditis, and meningitis.
The diagnosis of disseminated gonococcal infection is usually made by clinical examination and culture of specimens from the genital tract, blood, or joint effusion.
Disseminated gonococcal infection usually responds promptly to intravenous antibiotic therapy.
Recommended therapy is ceftriaxone:
• 25 to 50 mg/kg/d IV for 7 days
or
• a single, daily, 25 to 50 mg/kg intramuscular dose, also for 7 days.
Continue therapy for 10 to 14 days if the patient has meningitis.
An alternative regimen is cefotaxime:
• 25 mg/kg/d IV for 7 days
or
• 25 mg/kg IM every 12 hours, also for 7 days.
Extend treatment for 10 to 14 days if meningitis is present.1
Obesity curtails effectiveness of antibiotic prophylaxis in cesarean delivery
Pevzner L, Swank M, Krepel C, Wing DA, Chan K, Edmiston CE Jr. Effects of maternal obesity on tissue concentrations of prophylactic cefazolin during cesarean delivery. Obstet Gynecol. 2011;117(4):877–882.
In this prospective study of the influence of an obese habitus on antibiotic prophylaxis during cesarean delivery, researchers divided 29 patients who were scheduled for cesarean into three groups, by body mass index (BMI):
- lean (BMI, <30; n = 10)
- obese (30–39.9; n = 10)
- extremely obese (>40; n = 9).
All patients were given a 2-g dose of IV cefazolin 30 to 60 minutes before surgery.
During delivery, the team took two specimens of adipose tissue: one immediately after the skin incision and one later, after fascia was closed. They also obtained a specimen of myometrial tissue after delivery and a blood specimen after surgery was completed.
The concentration of cefazolin was then measured in adipose and myometrial tissue and in serum.
Findings. The researchers demonstrated that the mean concentration of cefazolin in the initial specimen of adipose tissue was significantly higher in lean patients than in obese and extremely obese patients. All 10 women who had a BMI less than 30 had a serum cefazolin concentration greater than 4 μg/g—the theoretical break-point for defining resistance to cefazolin. The initial adipose tissue specimen from two of the 10 obese patients and three of the nine extremely obese patients showed cefazolin concentrations less than 4 μg/g.
Of particular interest, two women—both of whom had a BMI greater than 40—developed a wound infection that required antibiotic therapy. Their initial and subsequent adipose tissue concentrations of cefazolin were less than the 4 μg/g break-point for resistance.
The concentration of cefazolin in the patients’ myometrial and serum specimens demonstrated a pattern similar to what the researchers observed in adipose tissue, but these results were not statistically significant across BMI groups. In fact, the cefazolin concentration in all groups’ myometrial and serum specimens exceeded the minimum inhibitory concentration for most potential pathogens in the setting of cesarean delivery.
Commentary
Clearly, prophylactic antibiotics are indicated for all women who are having a cesarean delivery. Antibiotics have their greatest impact when administered before the surgical incision is made; to exert their full protective effect against endometritis and wound infection, however, antibiotics should reach a recognized therapeutic concentration—not only in serum and myometrium but in the subcutaneous tissue.
The customary dosage of cefazolin for cesarean delivery prophylaxis has been 1 g. This study demonstrated that, although a 2-g dose of cefazolin reached a therapeutic concentration in myometrial tissue and serum, it did not consistently do so in the adipose tissue of obese and extremely obese patients.
Pending further investigation, I strongly recommend that all women who have a BMI greater than 30 receive a 2-g dose of cefazolin 30 to 60 minutes before cesarean delivery. Future research is needed to determine whether an even higher dosage is necessary to achieve a therapeutic concentration in the subcutaneous tissue of morbidly obese patients.
New therapies promise a better outcome in hepatitis C
Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–2416.
The authors conducted an international Phase-3, randomized, double-blind, placebo-controlled trial of two different treatment modalities for chronic hepatitis C virus (HCV) infection. The authors assigned 1,088 patients who had HCV genotype-1 infection and who had not received prior therapy to one of three treatment groups:
- telaprevir (Incivek, Vertex Pharmaceuticals), an HCV genotype-1 protease inhibitor, combined with peginterferon alfa-2a (Pegasys, Genetech) plus ribavirin (Copegus, Genetech; Rebetol, Merck; etc.) for 12 weeks; patients then were given peginterferon alfa-2a plus ribavirin only for 12 additional weeks if HCV RNA was undetectable at weeks 4 and 12 or peginterferon alfa-2a plus ribavirin only for 36 weeks if HCV RNA was detectable at either time point (Group 1)
- telaprevir with peginterferon alfa-2a plus ribavirin for 8 weeks, then placebo with peginterferon alfa-2a plus ribavirin for 4 weeks, followed by 12 to 36 weeks of peginterferon alfa-2a plus ribavirin using the HCV RNA criteria applied to Group 1 (Group 2)
- placebo with peginterferon alfa-2a plus ribavirin for 12 weeks, followed by 36 weeks of peginterferon alfa-2a plus ribavirin (Group 3).
The primary endpoint of the trial was the percentage of patients who had undetectable plasma HCV RNA at 24 weeks after the last planned dose of the study drugs. The investigators considered that this endpoint represented a sustained virologic response.
Findings. Seventy-five percent of patients in Group 1 and 69% of those in Group 2 had a sustained virologic response. By comparison, only 44% of patients in Group 3 had a sustained response. The differences in outcome between Group 1 and Group 3, and between Group 2 and Group 3, were highly significant (P<.001). Virologic failure was more common among patients who had HCV genotype-1a infection than among those who had HCV genotype-1b infection.
The most common side effects noted by patients who received telaprevir were gastrointestinal irritation, rash, and anemia. Ten percent of patients in the telaprevir group discontinued therapy, compared with 7% in the peginterferon-ribavirin-alone group.
Commentary
Worldwide, approximately 170 million people have chronic hepatitis C, which is the most common indication for liver transplantation. Until recently, the principal treatments for hepatitis C were pegylated interferon alfa with ribavirin and without ribavirin; the response rate with these regimens was in the range of 55%. This study shows that adding telaprevir to regimens for HCV infection significantly improves prospects for long-term resolution of infection.
In some obstetric and gynecologic populations, HCV is more common than hepatitis B virus. Risk factors for hepatitis C include hepatitis B, intravenous drug abuse, and human immunodeficiency virus infection. HCV-infected women pose a risk to their sex partners; infected pregnant women can transmit the virus to their baby.
Unlike hepatitis A and hepatitis B, immunoprophylaxis is not available for hepatitis C. That reality is what makes the study by Jacobsen and colleagues so compelling: They have clearly demonstrated that multi-agent antiviral therapy might be able to truly cure this infection.
The lesson here for ObGyns? Screen at-risk patients and then refer the hepatitis C-seropositive ones to a specialist in gastroenterology, who can determine candidacy for one of the new treatment regimens.
Clearly, the prognosis for people who have hepatitis C is much better today than it was 20 years ago.
For how long should chorioamnionitis be treated?
Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119(6):1102-1105.
The authors conducted a retrospective review of 423 women who had been treated for chorioamnionitis at the University of Florida from 2005 to 2009.
Patients had been given IV ampicillin (2 g every 6 h) plus IV gentamicin (1.5 mg/kg every 8 h) as soon as the diagnosis of chorioamnionitis was established; postpartum, they were given only the one next scheduled dose of each antibiotic. Patients who had a cesarean received either metronidazole (500 mg) or clindamycin (900 mg) immediately after cord clamping to enhance coverage of anaerobic organisms.
The primary outcome was treatment failure, defined as persistent fever requiring continued antibiotics, surgical intervention, or administration of heparin for septic pelvic-vein thrombophlebitis.
Findings. Here is a breakdown of what the investigators found regarding the 282 women who delivered vaginally and the 141 who underwent cesarean delivery:
- Overall, 399 of the patients (94%; 95% confidence interval [CI], 92% and 96%) were treated successfully; 24 (6%; 95% CI, 3.7% and 8.3%) failed short-course treatment
- Of the 282 patients who delivered vaginally, 279 (99%; 95% CI, 98% and 100%) were cured with short-term therapy
- Of the 141 who delivered by cesarean, 120 (85%; 95% CI, 79% and 91%) were cured (P<.001).
- Seventeen of the total treatment failures had endometritis and responded quickly to continuation of antibiotics. Of the 17 patients with endometritis, 14 had a cesarean delivery.
- Seven patients had more serious complications: four, wound infection; three, septic pelvic-vein thrombophlebitis. All serious complications occurred after cesarean delivery.
- Of the four patients who had a wound infection, three had labor induced by misoprostol; their BMI was 44.8, 31.1, and 48.5, respectively. The fourth had a cesarean delivery at 29 weeks for preterm premature rupture of membranes (PPROM), chorioamnionitis, and malpresentation.
- Of the three patients who had septic pelvic-vein thrombophlebitis, two had labor induced by misoprostol. One had a BMI of 29.2; the other, 31.1. The third patient was delivered secondary to PPROM; her BMI was 40.3.
In addition, of the 21 treatment failures in the cesarean delivery group, 6 had prolonged rupture of membranes (ROM) and 10 had a BMI greater than 30. Six patients had both prolonged ROM and were obese or morbidly obese.
Of the 120 women who had a cesarean delivery and were treated successfully, 3 had prolonged ROM and 39 had a BMI greater than 30. None had both prolonged ROM and a BMI greater than 30.
Last, the difference between treatment failures and treatment successes in regard to the frequency of prolonged ROM or a BMI greater than 30 was highly significant (P<.01).
Commentary
In most published reports of patients who have chorioamnionitis, antibiotic treatment continues until the patient is afebrile and asymptomatic for 24 to 48 hours. This treatment approach has been based largely on expert opinion, however, not on Level-1 or Level-2 evidence.
In 2003, Edwards and Duff published a study of chorioamnionitis antibiotic regimens that compared single-dose postpartum treatment to extended treatment.2 This randomized controlled trial demonstrated that there was no statistically significant difference between patients who had only a single dose of postpartum antibiotics and those who received an extended course of medication (i.e., who were treated until they had been afebrile and asymptomatic for a minimum of 24 hours) in regard to adverse outcomes (2.9% and 4.3%, respectively). The study discussed here extends and refines the observations made in the 2003 Edwards and Duff randomized controlled trial.
The new study shows that a limited course of antibiotics was, overall, effective in treating 94% of patients with chorioamnionitis (95% CI, 92% and 96%). Only 1% of patients who delivered vaginally failed therapy, compared with 15% of patients who delivered by cesarean (P<.001). In the cesarean group, women who failed therapy were likely to 1) be obese or 2) have a relatively long duration of labor or ruptured membranes, or both. These patients may have benefitted from a more extended course of antibiotic therapy.
Based on this investigation, I strongly recommend a limited course of antibiotic therapy (ampicillin plus gentamicin) for women with chorioamnionitis who deliver vaginally. Patients who have had a cesarean delivery—particularly those who are obese or have had an extended duration of labor, or both—should be treated with antibiotics until they have been afebrile and asymptomatic for 24 hours.
We want to hear from you! Tell us what you think.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Edwards RK, Duff P. Single dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 Pt 1):957-961.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Edwards RK, Duff P. Single dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 Pt 1):957-961.
A stepwise approach to cervical cerclage
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).
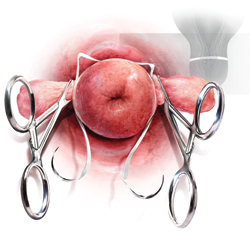
FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).
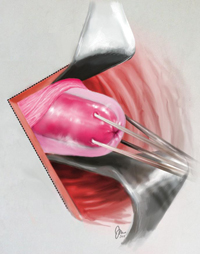
FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)
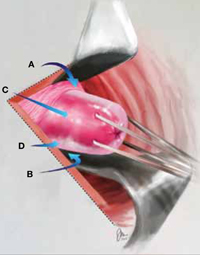
FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.
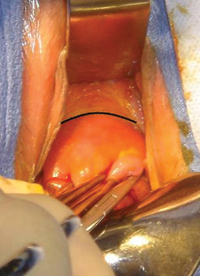
FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).
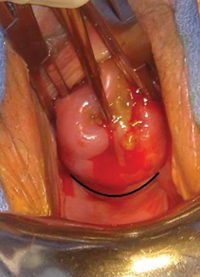
FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)
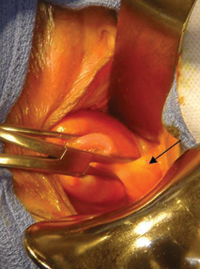
FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)
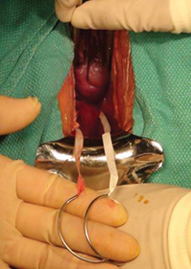
FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)
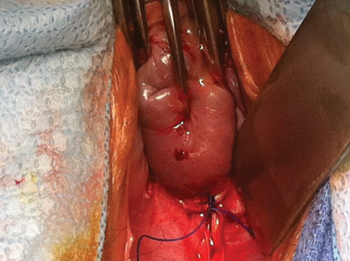
FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.
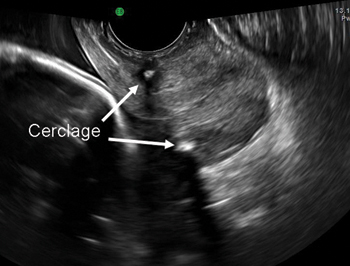
FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).

FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).

FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)

FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.

FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).

FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)

FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)

FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)

FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.

FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).

FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).

FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)

FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.

FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).

FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)

FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)

FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)

FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.

FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
Does elimination of the bladder flap from cesarean delivery increase the risk of complications?
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Cesarean delivery is the most common major surgical procedure performed during pregnancy. In the United States, the rate of cesarean delivery approaches 30%. As this rate rises, it is likely to be accompanied by an increase in the rate of surgical complications, such as pelvic hematoma, infection, and bladder injury, and in the rate of long-term complications, such as adhesion formation.
Several studies have assessed technical aspects of cesarean delivery, but debate continues over whether a bladder flap is a necessary part of the standard procedure.
The bladder flap is developed by incising the peritoneal lining and dissecting the urinary bladder away from the lower uterine segment. Suggested benefits of the bladder flap are easy access to the lower uterine segment and avoidance of bladder injury—but these claims have not been confirmed in retrospective or randomized trials.1,2 On the contrary, some studies suggest that creation of a bladder flap prolongs the duration of surgery and may increase the risk of postoperative infection and adhesion formation, as well as bladder injury at the time of repeat cesarean.3
Details of the trial
This study by Tuuli and colleagues is a single-center, unblinded, randomized, controlled trial designed to explore the risks and benefits of creating a bladder flap versus those of omitting the flap at the time of cesarean delivery. Of the 258 women enrolled in the trial, 131 were allocated to creation of a bladder flap and 127 to omission of the flap.
The primary outcome was total operative time. Secondary outcomes were:
- bladder injury
- incision-to-delivery time
- incision-to-fascial closure time
- estimated blood loss
- postoperative pain
- hospital stay
- endometritis
- urinary tract infection.
Unlike an earlier trial that included only women undergoing primary cesarean, this study included both primary and repeat cesarean deliveries. Sample size for each group was calculated assuming a 5-minute difference in total operating time.
Of the 131 women allocated to the bladder-flap creation group, only 108 (82%) actually had a bladder flap; 23 (18%) did not. Conversely, among the 127 women allocated to the no-flap group, 14 (11%) had a bladder flap created, most commonly because of the presence of scar tissue (n = 9).
Neither group had any bladder injuries nor were there statistical differences in any of the other secondary outcomes studied.
The authors concluded that omission of the bladder flap from primary and repeat cesarean delivery does not increase intraoperative or postoperative complications.
Strengths and limitations
As I mentioned, the rationale for creating a bladder flap is to reduce the rate of bladder injury. Therefore, bladder injury should have been the primary outcome of this trial. However, because the expected rate of bladder injury during cesarean delivery is so low (0.14%–0.35%), a sample size of 40,000 women would have been needed to address this outcome.
Among women who do not have a bladder flap created during cesarean delivery, bladder injury may be more likely when the second stage of labor is prolonged (i.e., when the vertex is wedged low in the pelvis) and when the woman has a history of multiple cesarean deliveries. This study did not include information about the number of women meeting these criteria.
Another limitation of this trial: Adherence to the protocol was inadequate, as 18% of the women assigned to receive a bladder flap did not have one, and 11% of those assigned to receive no flap had a flap created. This failure to adhere to the protocol may explain the lack of significant differences in total delivery time between the two groups, as well as the clinically insignificant difference in the incision-to-delivery interval between groups.
The rationale for omitting a bladder flap is to shorten total operating and incision-to-delivery time and/or to reduce the rate of future adhesions. Regrettably, this trial provided no conclusive evidence regarding any of these benefits. We still need a randomized trial of adequate sample size to address some of the questions raised by this trial.
I agree with the authors of this trial that their findings—along with those of other studies—argue against routine creation of a bladder flap at cesarean delivery.
Consider clinical findings at the time of surgery when deciding whether or not to create a bladder flap. For example, a flap may ease delivery of the fetal head when pushing has been prolonged during the second stage of labor or when operative vaginal delivery has failed. A flap also may help the surgeon avoid injury to the bladder in cases involving accidental extension of the lower-segment incision.
Among women who have a history of cesarean delivery and in whom the bladder flap is attached high above the lower segment, the bladder should be dissected carefully away from the uterus to avoid injury during delivery.
Baha M. Sibai, MD
We want to hear from you! Tell us what you think.
1. Malvasi A, Tinelli A, Gustapane S, et al. Surgical technique to avoid bladder flap formation during cesarean section. G Chir. 2011;32(11–12):498-403.
2. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2011;98(6):1089-1092.
3. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in cesarean section on repeat cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):300-304.
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Cesarean delivery is the most common major surgical procedure performed during pregnancy. In the United States, the rate of cesarean delivery approaches 30%. As this rate rises, it is likely to be accompanied by an increase in the rate of surgical complications, such as pelvic hematoma, infection, and bladder injury, and in the rate of long-term complications, such as adhesion formation.
Several studies have assessed technical aspects of cesarean delivery, but debate continues over whether a bladder flap is a necessary part of the standard procedure.
The bladder flap is developed by incising the peritoneal lining and dissecting the urinary bladder away from the lower uterine segment. Suggested benefits of the bladder flap are easy access to the lower uterine segment and avoidance of bladder injury—but these claims have not been confirmed in retrospective or randomized trials.1,2 On the contrary, some studies suggest that creation of a bladder flap prolongs the duration of surgery and may increase the risk of postoperative infection and adhesion formation, as well as bladder injury at the time of repeat cesarean.3
Details of the trial
This study by Tuuli and colleagues is a single-center, unblinded, randomized, controlled trial designed to explore the risks and benefits of creating a bladder flap versus those of omitting the flap at the time of cesarean delivery. Of the 258 women enrolled in the trial, 131 were allocated to creation of a bladder flap and 127 to omission of the flap.
The primary outcome was total operative time. Secondary outcomes were:
- bladder injury
- incision-to-delivery time
- incision-to-fascial closure time
- estimated blood loss
- postoperative pain
- hospital stay
- endometritis
- urinary tract infection.
Unlike an earlier trial that included only women undergoing primary cesarean, this study included both primary and repeat cesarean deliveries. Sample size for each group was calculated assuming a 5-minute difference in total operating time.
Of the 131 women allocated to the bladder-flap creation group, only 108 (82%) actually had a bladder flap; 23 (18%) did not. Conversely, among the 127 women allocated to the no-flap group, 14 (11%) had a bladder flap created, most commonly because of the presence of scar tissue (n = 9).
Neither group had any bladder injuries nor were there statistical differences in any of the other secondary outcomes studied.
The authors concluded that omission of the bladder flap from primary and repeat cesarean delivery does not increase intraoperative or postoperative complications.
Strengths and limitations
As I mentioned, the rationale for creating a bladder flap is to reduce the rate of bladder injury. Therefore, bladder injury should have been the primary outcome of this trial. However, because the expected rate of bladder injury during cesarean delivery is so low (0.14%–0.35%), a sample size of 40,000 women would have been needed to address this outcome.
Among women who do not have a bladder flap created during cesarean delivery, bladder injury may be more likely when the second stage of labor is prolonged (i.e., when the vertex is wedged low in the pelvis) and when the woman has a history of multiple cesarean deliveries. This study did not include information about the number of women meeting these criteria.
Another limitation of this trial: Adherence to the protocol was inadequate, as 18% of the women assigned to receive a bladder flap did not have one, and 11% of those assigned to receive no flap had a flap created. This failure to adhere to the protocol may explain the lack of significant differences in total delivery time between the two groups, as well as the clinically insignificant difference in the incision-to-delivery interval between groups.
The rationale for omitting a bladder flap is to shorten total operating and incision-to-delivery time and/or to reduce the rate of future adhesions. Regrettably, this trial provided no conclusive evidence regarding any of these benefits. We still need a randomized trial of adequate sample size to address some of the questions raised by this trial.
I agree with the authors of this trial that their findings—along with those of other studies—argue against routine creation of a bladder flap at cesarean delivery.
Consider clinical findings at the time of surgery when deciding whether or not to create a bladder flap. For example, a flap may ease delivery of the fetal head when pushing has been prolonged during the second stage of labor or when operative vaginal delivery has failed. A flap also may help the surgeon avoid injury to the bladder in cases involving accidental extension of the lower-segment incision.
Among women who have a history of cesarean delivery and in whom the bladder flap is attached high above the lower segment, the bladder should be dissected carefully away from the uterus to avoid injury during delivery.
Baha M. Sibai, MD
We want to hear from you! Tell us what you think.
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Baha M. Sibai, MD (March 2012)
Cesarean delivery is the most common major surgical procedure performed during pregnancy. In the United States, the rate of cesarean delivery approaches 30%. As this rate rises, it is likely to be accompanied by an increase in the rate of surgical complications, such as pelvic hematoma, infection, and bladder injury, and in the rate of long-term complications, such as adhesion formation.
Several studies have assessed technical aspects of cesarean delivery, but debate continues over whether a bladder flap is a necessary part of the standard procedure.
The bladder flap is developed by incising the peritoneal lining and dissecting the urinary bladder away from the lower uterine segment. Suggested benefits of the bladder flap are easy access to the lower uterine segment and avoidance of bladder injury—but these claims have not been confirmed in retrospective or randomized trials.1,2 On the contrary, some studies suggest that creation of a bladder flap prolongs the duration of surgery and may increase the risk of postoperative infection and adhesion formation, as well as bladder injury at the time of repeat cesarean.3
Details of the trial
This study by Tuuli and colleagues is a single-center, unblinded, randomized, controlled trial designed to explore the risks and benefits of creating a bladder flap versus those of omitting the flap at the time of cesarean delivery. Of the 258 women enrolled in the trial, 131 were allocated to creation of a bladder flap and 127 to omission of the flap.
The primary outcome was total operative time. Secondary outcomes were:
- bladder injury
- incision-to-delivery time
- incision-to-fascial closure time
- estimated blood loss
- postoperative pain
- hospital stay
- endometritis
- urinary tract infection.
Unlike an earlier trial that included only women undergoing primary cesarean, this study included both primary and repeat cesarean deliveries. Sample size for each group was calculated assuming a 5-minute difference in total operating time.
Of the 131 women allocated to the bladder-flap creation group, only 108 (82%) actually had a bladder flap; 23 (18%) did not. Conversely, among the 127 women allocated to the no-flap group, 14 (11%) had a bladder flap created, most commonly because of the presence of scar tissue (n = 9).
Neither group had any bladder injuries nor were there statistical differences in any of the other secondary outcomes studied.
The authors concluded that omission of the bladder flap from primary and repeat cesarean delivery does not increase intraoperative or postoperative complications.
Strengths and limitations
As I mentioned, the rationale for creating a bladder flap is to reduce the rate of bladder injury. Therefore, bladder injury should have been the primary outcome of this trial. However, because the expected rate of bladder injury during cesarean delivery is so low (0.14%–0.35%), a sample size of 40,000 women would have been needed to address this outcome.
Among women who do not have a bladder flap created during cesarean delivery, bladder injury may be more likely when the second stage of labor is prolonged (i.e., when the vertex is wedged low in the pelvis) and when the woman has a history of multiple cesarean deliveries. This study did not include information about the number of women meeting these criteria.
Another limitation of this trial: Adherence to the protocol was inadequate, as 18% of the women assigned to receive a bladder flap did not have one, and 11% of those assigned to receive no flap had a flap created. This failure to adhere to the protocol may explain the lack of significant differences in total delivery time between the two groups, as well as the clinically insignificant difference in the incision-to-delivery interval between groups.
The rationale for omitting a bladder flap is to shorten total operating and incision-to-delivery time and/or to reduce the rate of future adhesions. Regrettably, this trial provided no conclusive evidence regarding any of these benefits. We still need a randomized trial of adequate sample size to address some of the questions raised by this trial.
I agree with the authors of this trial that their findings—along with those of other studies—argue against routine creation of a bladder flap at cesarean delivery.
Consider clinical findings at the time of surgery when deciding whether or not to create a bladder flap. For example, a flap may ease delivery of the fetal head when pushing has been prolonged during the second stage of labor or when operative vaginal delivery has failed. A flap also may help the surgeon avoid injury to the bladder in cases involving accidental extension of the lower-segment incision.
Among women who have a history of cesarean delivery and in whom the bladder flap is attached high above the lower segment, the bladder should be dissected carefully away from the uterus to avoid injury during delivery.
Baha M. Sibai, MD
We want to hear from you! Tell us what you think.
1. Malvasi A, Tinelli A, Gustapane S, et al. Surgical technique to avoid bladder flap formation during cesarean section. G Chir. 2011;32(11–12):498-403.
2. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2011;98(6):1089-1092.
3. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in cesarean section on repeat cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):300-304.
1. Malvasi A, Tinelli A, Gustapane S, et al. Surgical technique to avoid bladder flap formation during cesarean section. G Chir. 2011;32(11–12):498-403.
2. Hohlagschwandtner M, Ruecklinger E, Husslein P, Joura EA. Is the formation of a bladder flap at cesarean necessary? A randomized trial. Obstet Gynecol. 2011;98(6):1089-1092.
3. Malvasi A, Tinelli A, Guido M, et al. Effect of avoiding bladder flap formation in cesarean section on repeat cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;159(2):300-304.
Latex-Free Works Best to Prevent Anaphylaxis During Cesareans
MONTEREY, CALIF. – A screening questionnaire with targeted replacement of latex-containing medical products dramatically reduces the risk of latex anaphylaxis among women undergoing cesarean delivery, but a latex-free policy appears to be the only surefire way to eliminate this risk entirely, according to one institution’s experience.
Investigators at the tertiary-care Hadassah Hebrew University Medical Center, Ein Kerem campus, Jerusalem, found that 2% of women undergoing cesarean delivery experienced latex anaphylaxis with the center’s usual procedures, involving use of latex products and no special screening.
The incidence was more than two-thirds lower, 0.6%, when the center implemented a screening questionnaire in elective cases to assess latex sensitivity and used latex-free gloves and urinary catheters in women who screened as potentially sensitive, Dr. Carolyn F. Weiniger reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. However, it was even lower, 0%, when the center implemented a latex-free policy for all women undergoing cesareans.
An economic analysis favored use of the questionnaire when the probability of latex anaphylaxis during cesarean was less than 2% and use of the latex-free policy when the probability was greater than that. "The economic analysis was a sensitivity analysis, so it could be used outside of our setting for a wide range of ICU costs. You just need to decide what your ICU costs are," Dr. Weiniger commented in a poster discussion session.
"I think this [study] is very applicable to our practices. ... You can look at your practice and decide if your rate of latex anaphylaxis is greater than or equal to 2%," said session comoderator Dr. Katherine W. Arendt of the Mayo Clinic, Rochester, Minn. She asked the authors what the perceived barriers were to making delivery rooms latex free.
"The main problem is that latex-free gloves, when we started the study, were five times more expensive than regular latex gloves," Dr. Weiniger replied.
Additionally, rewinding a bit, centers must be aware that a problem even exists. "Have a high index of suspicion for latex anaphylaxis because the signs are very subtle. If you have a case of hypotension unexpectedly during surgery that doesn’t have an obvious cause, like bleeding," look into it further, she recommended in an interview.
Centers that do identify a problem should crunch their numbers. "You can use our sensitivity cost analysis, where you just plot your own cost of gloves and cost of an ICU bed, and work out whether you are above or below a cost-benefit position, whereby it would be cheaper for your institution to switch to latex-free gloves as opposed to using latex gloves," she elaborated. Those numbers can then be taken to management to make the case for a change in policy.
But the effort does not necessarily end there, according to Dr. Weiniger. "We knew that all cesarean sections should be done with latex-free gloves, but one patient was rushed to the main OR, and the message hadn’t gotten through, and she had latex anaphylaxis during emergency cesarean section when regular gloves were used. So we had to institute an educational program as well to make sure everybody knew about the policy," she said.
In the study of 1,569 parturients, the investigators analyzed the incidence of latex anaphylaxis among those undergoing elective cesarean delivery during four consecutive periods spanning 40 months.
In the baseline period (n = 460 women), the center followed usual procedures, with use of latex products and no special screening. In the second period (n = 302), it implemented a latex-free policy for cesareans. In the third period (n = 281), it discontinued the latex-free policy because of costs. And in the fourth period (n = 526), it implemented the screening questionnaire with targeted intervention as an alternative approach; the questionnaire contained items validated in previous studies with skin prick test results and/or serum latex IgE antibodies to have high sensitivity and specificity for detection of latex allergy.
Study results showed that the incidence of latex anaphylaxis (possible, probable, or definite) was 2% in the first period, 0% in the second period (P = .003 vs. first), and 0.6% in the fourth period (P = .015 vs. first), Dr. Weiniger reported.
In all, 15% of women completing a questionnaire during the period when it was used had suspected latex sensitivity on the basis of the results.
Women experiencing anaphylaxis received many verbal and written reminders to be evaluated at the center’s allergy clinic, but just 44% did so. All were found to be positive for latex allergy on skin prick testing.
The financial analysis took into account a variety of costs. "When we were looking at ICU costs, we were looking at the costs per treating a case of anaphylaxis, so that could range from very minor to major costs, including lawsuits if there was a fatality," Dr. Weiniger explained. Other costs included those of using latex-free gloves, at $1 per pair and five pair needed per cesarean case.
"The management, when they understood that a cesarean section anaphylaxis could cost them a minimum of $500 per case, and the latex gloves [would cost] $5 per case, they just did the math, with a 2% incidence of latex anaphylaxis in our hospital. They agreed [on a latex-free policy] only for cesarean sections, not for other surgeries, and not for urinary catheters," as the literature suggests that gloves are the main source of the problem.
Many institutions have not assessed anaphylaxis in their cesarean populations, according to Dr. Weiniger. "So we are planning a national study to get other institutions to identify any case of potential anaphylaxis, to do mast cell tryptase to confirm if it was anaphylaxis, and to do latex testing to see if the cause was latex, so the other institutions can identify if they also have a problem," she said.
Dr. Weiniger disclosed no financial conflicts of interests.
MONTEREY, CALIF. – A screening questionnaire with targeted replacement of latex-containing medical products dramatically reduces the risk of latex anaphylaxis among women undergoing cesarean delivery, but a latex-free policy appears to be the only surefire way to eliminate this risk entirely, according to one institution’s experience.
Investigators at the tertiary-care Hadassah Hebrew University Medical Center, Ein Kerem campus, Jerusalem, found that 2% of women undergoing cesarean delivery experienced latex anaphylaxis with the center’s usual procedures, involving use of latex products and no special screening.
The incidence was more than two-thirds lower, 0.6%, when the center implemented a screening questionnaire in elective cases to assess latex sensitivity and used latex-free gloves and urinary catheters in women who screened as potentially sensitive, Dr. Carolyn F. Weiniger reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. However, it was even lower, 0%, when the center implemented a latex-free policy for all women undergoing cesareans.
An economic analysis favored use of the questionnaire when the probability of latex anaphylaxis during cesarean was less than 2% and use of the latex-free policy when the probability was greater than that. "The economic analysis was a sensitivity analysis, so it could be used outside of our setting for a wide range of ICU costs. You just need to decide what your ICU costs are," Dr. Weiniger commented in a poster discussion session.
"I think this [study] is very applicable to our practices. ... You can look at your practice and decide if your rate of latex anaphylaxis is greater than or equal to 2%," said session comoderator Dr. Katherine W. Arendt of the Mayo Clinic, Rochester, Minn. She asked the authors what the perceived barriers were to making delivery rooms latex free.
"The main problem is that latex-free gloves, when we started the study, were five times more expensive than regular latex gloves," Dr. Weiniger replied.
Additionally, rewinding a bit, centers must be aware that a problem even exists. "Have a high index of suspicion for latex anaphylaxis because the signs are very subtle. If you have a case of hypotension unexpectedly during surgery that doesn’t have an obvious cause, like bleeding," look into it further, she recommended in an interview.
Centers that do identify a problem should crunch their numbers. "You can use our sensitivity cost analysis, where you just plot your own cost of gloves and cost of an ICU bed, and work out whether you are above or below a cost-benefit position, whereby it would be cheaper for your institution to switch to latex-free gloves as opposed to using latex gloves," she elaborated. Those numbers can then be taken to management to make the case for a change in policy.
But the effort does not necessarily end there, according to Dr. Weiniger. "We knew that all cesarean sections should be done with latex-free gloves, but one patient was rushed to the main OR, and the message hadn’t gotten through, and she had latex anaphylaxis during emergency cesarean section when regular gloves were used. So we had to institute an educational program as well to make sure everybody knew about the policy," she said.
In the study of 1,569 parturients, the investigators analyzed the incidence of latex anaphylaxis among those undergoing elective cesarean delivery during four consecutive periods spanning 40 months.
In the baseline period (n = 460 women), the center followed usual procedures, with use of latex products and no special screening. In the second period (n = 302), it implemented a latex-free policy for cesareans. In the third period (n = 281), it discontinued the latex-free policy because of costs. And in the fourth period (n = 526), it implemented the screening questionnaire with targeted intervention as an alternative approach; the questionnaire contained items validated in previous studies with skin prick test results and/or serum latex IgE antibodies to have high sensitivity and specificity for detection of latex allergy.
Study results showed that the incidence of latex anaphylaxis (possible, probable, or definite) was 2% in the first period, 0% in the second period (P = .003 vs. first), and 0.6% in the fourth period (P = .015 vs. first), Dr. Weiniger reported.
In all, 15% of women completing a questionnaire during the period when it was used had suspected latex sensitivity on the basis of the results.
Women experiencing anaphylaxis received many verbal and written reminders to be evaluated at the center’s allergy clinic, but just 44% did so. All were found to be positive for latex allergy on skin prick testing.
The financial analysis took into account a variety of costs. "When we were looking at ICU costs, we were looking at the costs per treating a case of anaphylaxis, so that could range from very minor to major costs, including lawsuits if there was a fatality," Dr. Weiniger explained. Other costs included those of using latex-free gloves, at $1 per pair and five pair needed per cesarean case.
"The management, when they understood that a cesarean section anaphylaxis could cost them a minimum of $500 per case, and the latex gloves [would cost] $5 per case, they just did the math, with a 2% incidence of latex anaphylaxis in our hospital. They agreed [on a latex-free policy] only for cesarean sections, not for other surgeries, and not for urinary catheters," as the literature suggests that gloves are the main source of the problem.
Many institutions have not assessed anaphylaxis in their cesarean populations, according to Dr. Weiniger. "So we are planning a national study to get other institutions to identify any case of potential anaphylaxis, to do mast cell tryptase to confirm if it was anaphylaxis, and to do latex testing to see if the cause was latex, so the other institutions can identify if they also have a problem," she said.
Dr. Weiniger disclosed no financial conflicts of interests.
MONTEREY, CALIF. – A screening questionnaire with targeted replacement of latex-containing medical products dramatically reduces the risk of latex anaphylaxis among women undergoing cesarean delivery, but a latex-free policy appears to be the only surefire way to eliminate this risk entirely, according to one institution’s experience.
Investigators at the tertiary-care Hadassah Hebrew University Medical Center, Ein Kerem campus, Jerusalem, found that 2% of women undergoing cesarean delivery experienced latex anaphylaxis with the center’s usual procedures, involving use of latex products and no special screening.
The incidence was more than two-thirds lower, 0.6%, when the center implemented a screening questionnaire in elective cases to assess latex sensitivity and used latex-free gloves and urinary catheters in women who screened as potentially sensitive, Dr. Carolyn F. Weiniger reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology. However, it was even lower, 0%, when the center implemented a latex-free policy for all women undergoing cesareans.
An economic analysis favored use of the questionnaire when the probability of latex anaphylaxis during cesarean was less than 2% and use of the latex-free policy when the probability was greater than that. "The economic analysis was a sensitivity analysis, so it could be used outside of our setting for a wide range of ICU costs. You just need to decide what your ICU costs are," Dr. Weiniger commented in a poster discussion session.
"I think this [study] is very applicable to our practices. ... You can look at your practice and decide if your rate of latex anaphylaxis is greater than or equal to 2%," said session comoderator Dr. Katherine W. Arendt of the Mayo Clinic, Rochester, Minn. She asked the authors what the perceived barriers were to making delivery rooms latex free.
"The main problem is that latex-free gloves, when we started the study, were five times more expensive than regular latex gloves," Dr. Weiniger replied.
Additionally, rewinding a bit, centers must be aware that a problem even exists. "Have a high index of suspicion for latex anaphylaxis because the signs are very subtle. If you have a case of hypotension unexpectedly during surgery that doesn’t have an obvious cause, like bleeding," look into it further, she recommended in an interview.
Centers that do identify a problem should crunch their numbers. "You can use our sensitivity cost analysis, where you just plot your own cost of gloves and cost of an ICU bed, and work out whether you are above or below a cost-benefit position, whereby it would be cheaper for your institution to switch to latex-free gloves as opposed to using latex gloves," she elaborated. Those numbers can then be taken to management to make the case for a change in policy.
But the effort does not necessarily end there, according to Dr. Weiniger. "We knew that all cesarean sections should be done with latex-free gloves, but one patient was rushed to the main OR, and the message hadn’t gotten through, and she had latex anaphylaxis during emergency cesarean section when regular gloves were used. So we had to institute an educational program as well to make sure everybody knew about the policy," she said.
In the study of 1,569 parturients, the investigators analyzed the incidence of latex anaphylaxis among those undergoing elective cesarean delivery during four consecutive periods spanning 40 months.
In the baseline period (n = 460 women), the center followed usual procedures, with use of latex products and no special screening. In the second period (n = 302), it implemented a latex-free policy for cesareans. In the third period (n = 281), it discontinued the latex-free policy because of costs. And in the fourth period (n = 526), it implemented the screening questionnaire with targeted intervention as an alternative approach; the questionnaire contained items validated in previous studies with skin prick test results and/or serum latex IgE antibodies to have high sensitivity and specificity for detection of latex allergy.
Study results showed that the incidence of latex anaphylaxis (possible, probable, or definite) was 2% in the first period, 0% in the second period (P = .003 vs. first), and 0.6% in the fourth period (P = .015 vs. first), Dr. Weiniger reported.
In all, 15% of women completing a questionnaire during the period when it was used had suspected latex sensitivity on the basis of the results.
Women experiencing anaphylaxis received many verbal and written reminders to be evaluated at the center’s allergy clinic, but just 44% did so. All were found to be positive for latex allergy on skin prick testing.
The financial analysis took into account a variety of costs. "When we were looking at ICU costs, we were looking at the costs per treating a case of anaphylaxis, so that could range from very minor to major costs, including lawsuits if there was a fatality," Dr. Weiniger explained. Other costs included those of using latex-free gloves, at $1 per pair and five pair needed per cesarean case.
"The management, when they understood that a cesarean section anaphylaxis could cost them a minimum of $500 per case, and the latex gloves [would cost] $5 per case, they just did the math, with a 2% incidence of latex anaphylaxis in our hospital. They agreed [on a latex-free policy] only for cesarean sections, not for other surgeries, and not for urinary catheters," as the literature suggests that gloves are the main source of the problem.
Many institutions have not assessed anaphylaxis in their cesarean populations, according to Dr. Weiniger. "So we are planning a national study to get other institutions to identify any case of potential anaphylaxis, to do mast cell tryptase to confirm if it was anaphylaxis, and to do latex testing to see if the cause was latex, so the other institutions can identify if they also have a problem," she said.
Dr. Weiniger disclosed no financial conflicts of interests.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATALOGY
Transport Compromises Quality of CPR for Obstetric Patients in Arrest
MONTEREY, CALIF. – The quality of cardiopulmonary resuscitation deteriorates when women experiencing cardiac arrest during labor and delivery are transported to the operating room for emergent cesarean section, suggests a randomized trial using simulation.
Results of the trial, conducted by investigators at Stanford (Calif.) University involving 26 multidisciplinary obstetric teams using mannequins, were reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
Main findings showed that teams that stayed put, starting and continuing CPR in the labor room, administered most chest compressions correctly. In contrast, teams that transported their mannequins on a gurney to the operating room had a marked drop-off in CPR performance, administering only a third of compressions correctly while in motion.
The transport group almost universally experienced interruptions in CPR, whereas the stationary group seldom did. In addition, ventilatory tidal volume fell sharply in the transport group during transport, whereas it remained stable in the stationary group.
"The quality of CPR definitely decreases when you move patients," commented presenting author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital at Stanford. He noted that a similar earlier study, also using simulation, found that the average time to incision for perimortem cesarean was almost doubled by transport to the operating room, from 4:25 minutes to 7:53 minutes (Obstet. Gynecol. 2011;118:1090-4).
Taken together, the studies’ results "would suggest strongly that we would recommend you to perform perimortem cesarean delivery at the site where the arrest occurs, either in labor & delivery or in the operating room, but not to move the patient if they are not in the operating room," he maintained.
Dr. Vilma E. Ortiz, session moderator and an anesthesiologist at the Massachusetts General Hospital in Boston, said, "In general, the overall quality of resuscitation – line placement, intubation, fluids – seems to be better in the operating room. Do you think that in practice, that might offset perhaps the poorer chest compressions [during transport]?"
The earlier study also found that other important tasks were forgotten or performed more poorly under conditions of transport, Dr. Carvalho replied. "Now clearly, it would be easier to do the cesarean in an operating room setting, but these patients are often dead and they are not going to bleed. Once you get the baby out, you can then move them later on if you want to. So I think the importance is getting the delivery, and moving the patient to the operating room will pretty much guarantee you will not be able to do this within 5 minutes [of the arrest], which is the recommendation from the guidelines."
A session attendee noted, "If we are going to do these emergent C-sections in the labor room, one of the advantages to being in the operating room is all the equipment for surgery exists. Do you have some special equipment on your code carts or somewhere else that would help facilitate that cesarean delivery? Can you make recommendations about what we all should have if we are going to do these sections in the delivery room?"
"That’s a very good point," Dr. Carvalho remarked. "If you propose this at your institution, it’s important that you get the surgical pack there so in an arrest situation, when a code comes up, someone’s job is to get the pack there." He noted that his own institution had to deal with logistics to ensure that scalpels for performing cesareans were always available in the labor room. "So you have to work through this and make those logistic changes, whatever works at your institution – each institution is different. But you must think out the scenario before just proposing it as the right scenario."
Institutions should also be aware that changing the place of delivery may change behaviors, he added. For example, in the earlier study, "when the cesareans were done in the labor & delivery room, the vast majority were vertical incisions, as the [obstetricians] have been taught to do in a perimortem section. In the group that moved to the operating room, the majority did Pfannenstiel incisions, because they sort of went back to old habits from the operating room days. So there are behavior changes based on whether you move or don’t move."
In the new study, the investigators created 26 obstetric teams, each having two staff members (obstetricians, nurses, and/or anesthesiologists). The teams were randomly assigned to stationary or transport groups.
The study period had three phases. During phase I, lasting 4 minutes, teams in both groups performed stationary CPR in the labor room. During phase II, lasting 2 minutes, the stationary teams continued with CPR there, whereas the transport teams performed CPR while transporting their mannequin to the operating room. During phase III, lasting 4 minutes, teams in both groups performed stationary CPR at their respective location.
The investigators simulated cardiac arrest using mannequins that provide real-time feedback on the effectiveness of chest compressions and ventilations, according to Dr. Carvalho.
Chest compressions were defined as correctly performed if they were administered at a rate of at least 100/minute, with correct sternal hand placement, a depth of at least 1.5 inches, and a release step.
"The teams were very similar in terms of age, gender, self-reported fitness, and years of experience," Dr. Carvalho reported. Overall, 46% of team members were registered nurses, 15% were obstetricians, and 39% were anesthesiologists. Most (88%) had current certification in advanced cardiac life support.
Study results showed that within the transport group, there was a significant decrease in the percentage of chest compressions performed correctly between phase I and phase II, with a return to baseline in phase III. In contrast, the percentage was generally similar across phases in the stationary group, except for a small fall-off in phase III that he attributed to possible fatigue.
During phase II, the percentage of chest compressions performed correctly was 93% in the stationary group, compared with only 32% in the transport group.
"The reason for [more incorrect compressions] was primarily insufficient depth, but also the sternal hand being too low and no release," Dr. Carvalho commented. "Interestingly, we thought that the rate would be different [between groups], but we did not find significant differences. No one actually did a rate below 100."
Additional analyses showed that there was an interruption in CPR for 92% of teams in the transport group during phase II, compared with just 7% in the stationary group.
In the transport group, team members used a variety of positions when attempting to administer CPR while moving: most knelt next to the mannequin on the gurney, but some straddled the mannequin or ran alongside the gurney. Those running alongside "tended to have a lot of interruptions as they moved past the usual obstructions," he noted.
Mean tidal volume also dropped significantly in the transport group in phase II, whereas it remained essentially constant in the stationary group. However, Dr. Carvalho cautioned, the mannequins were only mask-ventilated and not intubated, which may have affected these results.
"There’s a lot of limitations with any simulated study," he acknowledged. "Obviously, this was a simulated arrest. The mannequin is really just a torso; we can’t administer left uterine displacement, it doesn’t have a pregnant belly, and we didn’t intubate." Also, teams were trained on the mannequin just before the study.
Dr. Carvalho disclosed no relevant conflicts of interest.
MONTEREY, CALIF. – The quality of cardiopulmonary resuscitation deteriorates when women experiencing cardiac arrest during labor and delivery are transported to the operating room for emergent cesarean section, suggests a randomized trial using simulation.
Results of the trial, conducted by investigators at Stanford (Calif.) University involving 26 multidisciplinary obstetric teams using mannequins, were reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
Main findings showed that teams that stayed put, starting and continuing CPR in the labor room, administered most chest compressions correctly. In contrast, teams that transported their mannequins on a gurney to the operating room had a marked drop-off in CPR performance, administering only a third of compressions correctly while in motion.
The transport group almost universally experienced interruptions in CPR, whereas the stationary group seldom did. In addition, ventilatory tidal volume fell sharply in the transport group during transport, whereas it remained stable in the stationary group.
"The quality of CPR definitely decreases when you move patients," commented presenting author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital at Stanford. He noted that a similar earlier study, also using simulation, found that the average time to incision for perimortem cesarean was almost doubled by transport to the operating room, from 4:25 minutes to 7:53 minutes (Obstet. Gynecol. 2011;118:1090-4).
Taken together, the studies’ results "would suggest strongly that we would recommend you to perform perimortem cesarean delivery at the site where the arrest occurs, either in labor & delivery or in the operating room, but not to move the patient if they are not in the operating room," he maintained.
Dr. Vilma E. Ortiz, session moderator and an anesthesiologist at the Massachusetts General Hospital in Boston, said, "In general, the overall quality of resuscitation – line placement, intubation, fluids – seems to be better in the operating room. Do you think that in practice, that might offset perhaps the poorer chest compressions [during transport]?"
The earlier study also found that other important tasks were forgotten or performed more poorly under conditions of transport, Dr. Carvalho replied. "Now clearly, it would be easier to do the cesarean in an operating room setting, but these patients are often dead and they are not going to bleed. Once you get the baby out, you can then move them later on if you want to. So I think the importance is getting the delivery, and moving the patient to the operating room will pretty much guarantee you will not be able to do this within 5 minutes [of the arrest], which is the recommendation from the guidelines."
A session attendee noted, "If we are going to do these emergent C-sections in the labor room, one of the advantages to being in the operating room is all the equipment for surgery exists. Do you have some special equipment on your code carts or somewhere else that would help facilitate that cesarean delivery? Can you make recommendations about what we all should have if we are going to do these sections in the delivery room?"
"That’s a very good point," Dr. Carvalho remarked. "If you propose this at your institution, it’s important that you get the surgical pack there so in an arrest situation, when a code comes up, someone’s job is to get the pack there." He noted that his own institution had to deal with logistics to ensure that scalpels for performing cesareans were always available in the labor room. "So you have to work through this and make those logistic changes, whatever works at your institution – each institution is different. But you must think out the scenario before just proposing it as the right scenario."
Institutions should also be aware that changing the place of delivery may change behaviors, he added. For example, in the earlier study, "when the cesareans were done in the labor & delivery room, the vast majority were vertical incisions, as the [obstetricians] have been taught to do in a perimortem section. In the group that moved to the operating room, the majority did Pfannenstiel incisions, because they sort of went back to old habits from the operating room days. So there are behavior changes based on whether you move or don’t move."
In the new study, the investigators created 26 obstetric teams, each having two staff members (obstetricians, nurses, and/or anesthesiologists). The teams were randomly assigned to stationary or transport groups.
The study period had three phases. During phase I, lasting 4 minutes, teams in both groups performed stationary CPR in the labor room. During phase II, lasting 2 minutes, the stationary teams continued with CPR there, whereas the transport teams performed CPR while transporting their mannequin to the operating room. During phase III, lasting 4 minutes, teams in both groups performed stationary CPR at their respective location.
The investigators simulated cardiac arrest using mannequins that provide real-time feedback on the effectiveness of chest compressions and ventilations, according to Dr. Carvalho.
Chest compressions were defined as correctly performed if they were administered at a rate of at least 100/minute, with correct sternal hand placement, a depth of at least 1.5 inches, and a release step.
"The teams were very similar in terms of age, gender, self-reported fitness, and years of experience," Dr. Carvalho reported. Overall, 46% of team members were registered nurses, 15% were obstetricians, and 39% were anesthesiologists. Most (88%) had current certification in advanced cardiac life support.
Study results showed that within the transport group, there was a significant decrease in the percentage of chest compressions performed correctly between phase I and phase II, with a return to baseline in phase III. In contrast, the percentage was generally similar across phases in the stationary group, except for a small fall-off in phase III that he attributed to possible fatigue.
During phase II, the percentage of chest compressions performed correctly was 93% in the stationary group, compared with only 32% in the transport group.
"The reason for [more incorrect compressions] was primarily insufficient depth, but also the sternal hand being too low and no release," Dr. Carvalho commented. "Interestingly, we thought that the rate would be different [between groups], but we did not find significant differences. No one actually did a rate below 100."
Additional analyses showed that there was an interruption in CPR for 92% of teams in the transport group during phase II, compared with just 7% in the stationary group.
In the transport group, team members used a variety of positions when attempting to administer CPR while moving: most knelt next to the mannequin on the gurney, but some straddled the mannequin or ran alongside the gurney. Those running alongside "tended to have a lot of interruptions as they moved past the usual obstructions," he noted.
Mean tidal volume also dropped significantly in the transport group in phase II, whereas it remained essentially constant in the stationary group. However, Dr. Carvalho cautioned, the mannequins were only mask-ventilated and not intubated, which may have affected these results.
"There’s a lot of limitations with any simulated study," he acknowledged. "Obviously, this was a simulated arrest. The mannequin is really just a torso; we can’t administer left uterine displacement, it doesn’t have a pregnant belly, and we didn’t intubate." Also, teams were trained on the mannequin just before the study.
Dr. Carvalho disclosed no relevant conflicts of interest.
MONTEREY, CALIF. – The quality of cardiopulmonary resuscitation deteriorates when women experiencing cardiac arrest during labor and delivery are transported to the operating room for emergent cesarean section, suggests a randomized trial using simulation.
Results of the trial, conducted by investigators at Stanford (Calif.) University involving 26 multidisciplinary obstetric teams using mannequins, were reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
Main findings showed that teams that stayed put, starting and continuing CPR in the labor room, administered most chest compressions correctly. In contrast, teams that transported their mannequins on a gurney to the operating room had a marked drop-off in CPR performance, administering only a third of compressions correctly while in motion.
The transport group almost universally experienced interruptions in CPR, whereas the stationary group seldom did. In addition, ventilatory tidal volume fell sharply in the transport group during transport, whereas it remained stable in the stationary group.
"The quality of CPR definitely decreases when you move patients," commented presenting author Dr. Brendan Carvalho, an anesthesiologist at Lucile Packard Children’s Hospital at Stanford. He noted that a similar earlier study, also using simulation, found that the average time to incision for perimortem cesarean was almost doubled by transport to the operating room, from 4:25 minutes to 7:53 minutes (Obstet. Gynecol. 2011;118:1090-4).
Taken together, the studies’ results "would suggest strongly that we would recommend you to perform perimortem cesarean delivery at the site where the arrest occurs, either in labor & delivery or in the operating room, but not to move the patient if they are not in the operating room," he maintained.
Dr. Vilma E. Ortiz, session moderator and an anesthesiologist at the Massachusetts General Hospital in Boston, said, "In general, the overall quality of resuscitation – line placement, intubation, fluids – seems to be better in the operating room. Do you think that in practice, that might offset perhaps the poorer chest compressions [during transport]?"
The earlier study also found that other important tasks were forgotten or performed more poorly under conditions of transport, Dr. Carvalho replied. "Now clearly, it would be easier to do the cesarean in an operating room setting, but these patients are often dead and they are not going to bleed. Once you get the baby out, you can then move them later on if you want to. So I think the importance is getting the delivery, and moving the patient to the operating room will pretty much guarantee you will not be able to do this within 5 minutes [of the arrest], which is the recommendation from the guidelines."
A session attendee noted, "If we are going to do these emergent C-sections in the labor room, one of the advantages to being in the operating room is all the equipment for surgery exists. Do you have some special equipment on your code carts or somewhere else that would help facilitate that cesarean delivery? Can you make recommendations about what we all should have if we are going to do these sections in the delivery room?"
"That’s a very good point," Dr. Carvalho remarked. "If you propose this at your institution, it’s important that you get the surgical pack there so in an arrest situation, when a code comes up, someone’s job is to get the pack there." He noted that his own institution had to deal with logistics to ensure that scalpels for performing cesareans were always available in the labor room. "So you have to work through this and make those logistic changes, whatever works at your institution – each institution is different. But you must think out the scenario before just proposing it as the right scenario."
Institutions should also be aware that changing the place of delivery may change behaviors, he added. For example, in the earlier study, "when the cesareans were done in the labor & delivery room, the vast majority were vertical incisions, as the [obstetricians] have been taught to do in a perimortem section. In the group that moved to the operating room, the majority did Pfannenstiel incisions, because they sort of went back to old habits from the operating room days. So there are behavior changes based on whether you move or don’t move."
In the new study, the investigators created 26 obstetric teams, each having two staff members (obstetricians, nurses, and/or anesthesiologists). The teams were randomly assigned to stationary or transport groups.
The study period had three phases. During phase I, lasting 4 minutes, teams in both groups performed stationary CPR in the labor room. During phase II, lasting 2 minutes, the stationary teams continued with CPR there, whereas the transport teams performed CPR while transporting their mannequin to the operating room. During phase III, lasting 4 minutes, teams in both groups performed stationary CPR at their respective location.
The investigators simulated cardiac arrest using mannequins that provide real-time feedback on the effectiveness of chest compressions and ventilations, according to Dr. Carvalho.
Chest compressions were defined as correctly performed if they were administered at a rate of at least 100/minute, with correct sternal hand placement, a depth of at least 1.5 inches, and a release step.
"The teams were very similar in terms of age, gender, self-reported fitness, and years of experience," Dr. Carvalho reported. Overall, 46% of team members were registered nurses, 15% were obstetricians, and 39% were anesthesiologists. Most (88%) had current certification in advanced cardiac life support.
Study results showed that within the transport group, there was a significant decrease in the percentage of chest compressions performed correctly between phase I and phase II, with a return to baseline in phase III. In contrast, the percentage was generally similar across phases in the stationary group, except for a small fall-off in phase III that he attributed to possible fatigue.
During phase II, the percentage of chest compressions performed correctly was 93% in the stationary group, compared with only 32% in the transport group.
"The reason for [more incorrect compressions] was primarily insufficient depth, but also the sternal hand being too low and no release," Dr. Carvalho commented. "Interestingly, we thought that the rate would be different [between groups], but we did not find significant differences. No one actually did a rate below 100."
Additional analyses showed that there was an interruption in CPR for 92% of teams in the transport group during phase II, compared with just 7% in the stationary group.
In the transport group, team members used a variety of positions when attempting to administer CPR while moving: most knelt next to the mannequin on the gurney, but some straddled the mannequin or ran alongside the gurney. Those running alongside "tended to have a lot of interruptions as they moved past the usual obstructions," he noted.
Mean tidal volume also dropped significantly in the transport group in phase II, whereas it remained essentially constant in the stationary group. However, Dr. Carvalho cautioned, the mannequins were only mask-ventilated and not intubated, which may have affected these results.
"There’s a lot of limitations with any simulated study," he acknowledged. "Obviously, this was a simulated arrest. The mannequin is really just a torso; we can’t administer left uterine displacement, it doesn’t have a pregnant belly, and we didn’t intubate." Also, teams were trained on the mannequin just before the study.
Dr. Carvalho disclosed no relevant conflicts of interest.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATOLOGY
Sharp Rise Seen in Rate of Delivery-Related Severe Sepsis
MONTEREY, CALIF. – The rate of severe sepsis among women hospitalized for delivery more than doubled during a recent 10-year period, according to a cohort study reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
In the analysis of more than 9.24 million admissions for delivery from the Nationwide Inpatient Sample, the rate rose from 0.64 to 1.37 per 10,000 deliveries between 1998-2000 and 2007-2008, reported lead investigator Dr. Melissa E.B. Bauer, an obstetric anesthesiologist with the University of Michigan Health System in Ann Arbor.
Several comorbidities and obstetric factors independently increased the risk of severe sepsis. For example, women with congestive heart failure had a more than fourfold higher risk, and women undergoing cesarean delivery had a more than fivefold higher risk.
"The etiology of the increase [in severe sepsis] in this population is unknown," she commented. "Possible hypotheses for the increase ... could be the increased cesarean delivery rate. At the start of the study period, the cesarean delivery rate was 21.2%; by 2008, the national rate was 32.3%."
"There’s also a higher-risk patient population. More women today have comorbidities that increase their risk of severe sepsis," she added. "And there’s an increased microbial resistance that could also be contributing."
Dr. Bauer noted that an important related issue is the difficulty of diagnosing sepsis during pregnancy. Some of the physiologic values typically used to establish the presence of the systemic inflammatory response syndrome (SIRS), often seen in sepsis, are actually considered normal for a pregnant woman.
"As the population at risk for severe sepsis continues to increase, unless we also increase our identification of patients with sepsis in this population, the rate of severe sepsis and death will continue to increase," she maintained. "A priority of future research will be to redefine SIRS in pregnant patients."
Session moderator Dr. Alan C. Santos of St. Luke’s Roosevelt Hospital Center in New York said, "I realize there were limitations to this type of study and what data you were able to extract. Could this all just be related to an increase in the background infections, HIV, and substance abuse among our population?"
Dr. Bauer acknowledged that those factors may have contributed and were not captured by administrative data. Factors often went uncoded "unless there was a reason to code a certain factor, basically, if it complicated the delivery and there would be more reimbursement for that type of situation," she said. For example, the investigators found a code for obesity in only about 1% of the sample, when roughly 20% of the population is obese.
"The only risk factor that appears to be at all modifiable is cesarean delivery, and it may add another nail in the coffin of this conversation," commented session attendee Dr. Stephen Pratt of Beth Israel Deaconess Hospital in Boston. "I was just wondering, are you able to look at either indications for cesarean delivery or even at, more broadly, elective versus intrapartum cesarean delivery? My guess is it’s going to be the intrapartum cesarean deliveries that are high risk for sepsis."
"That's an excellent point," Dr. Bauer replied. "The problem is, it’s difficult to determine exactly." However, it may be possible to get a general sense from measures such as the duration of hospitalization before cesarean. "We are certainly going to tease out more of the data," she said.
Previous research has suggested that sepsis of any severity complicates about 1 in every 8,000 deliveries in the United States. But "this has been determined by single-institution, retrospective cohort studies. To date, there has not been a United States population–based study looking at incidence, temporal trends, and risk factors for sepsis," Dr. Bauer noted.
The investigators identified hospital admissions having pregnancy- and delivery-related diagnostic codes, excluding those pertaining to ectopic or molar pregnancy, or abortion. Within this set, they then assessed outcomes of sepsis, severe sepsis (sepsis with organ failure and/or hypotension), and sepsis-related death.
Results showed that the rate of sepsis remained essentially the same during the study period, affecting about 1 in every 3,300 deliveries, according to Dr. Bauer. However, the rate of severe sepsis doubled.
Multivariate analyses showed a variety of comorbidities and demographic and obstetric factors that were independently associated with an elevated risk of severe sepsis: diabetes (odds ratio, 1.4), black race (1.8), delivery before 37 weeks (1.8), cerclage (2.7), hypertensive diseases of pregnancy (4.5), congestive heart failure (4.5), cesarean delivery (5.1), stillbirth (6.5), retained products of conception (6.9), chronic renal disease (16.2), and chronic liver disease (22.3).
Several other factors – age, insurance, obesity, and preterm premature rupture of membranes (PPROM) – did not significantly affect the risk of this outcome. However, data on PPROM may be misleading because of the heterogeneity of that group, Dr. Bauer cautioned. "It includes any patient who had rupture 24 hours prior to labor; that includes the patient who had rupture 1 day prior to labor and also the patient who has been sitting on the [hospital] floor for several months prior to labor," she explained.
"Of note, teaching-hospital status as well as delivery volume were found to [confer] a mildly increased risk for severe sepsis, but had a minimal effect size when compared to the rest of these variables," Dr. Bauer further reported.
In additional findings, the rate of sepsis-related death among women with sepsis also more than doubled during the study period, from 2.2% to 4.9%. As of 2006-2008, there were 1.31 deaths due to sepsis for every 100,000 deliveries in the United States. "This mirrors the findings in the United Kingdom for increased deaths related to sepsis," she noted (BJOG 2011;118:1-203). There were too few sepsis-related deaths to assess risk factors for this outcome.
Dr. Bauer disclosed no relevant conflicts of interest.
MONTEREY, CALIF. – The rate of severe sepsis among women hospitalized for delivery more than doubled during a recent 10-year period, according to a cohort study reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
In the analysis of more than 9.24 million admissions for delivery from the Nationwide Inpatient Sample, the rate rose from 0.64 to 1.37 per 10,000 deliveries between 1998-2000 and 2007-2008, reported lead investigator Dr. Melissa E.B. Bauer, an obstetric anesthesiologist with the University of Michigan Health System in Ann Arbor.
Several comorbidities and obstetric factors independently increased the risk of severe sepsis. For example, women with congestive heart failure had a more than fourfold higher risk, and women undergoing cesarean delivery had a more than fivefold higher risk.
"The etiology of the increase [in severe sepsis] in this population is unknown," she commented. "Possible hypotheses for the increase ... could be the increased cesarean delivery rate. At the start of the study period, the cesarean delivery rate was 21.2%; by 2008, the national rate was 32.3%."
"There’s also a higher-risk patient population. More women today have comorbidities that increase their risk of severe sepsis," she added. "And there’s an increased microbial resistance that could also be contributing."
Dr. Bauer noted that an important related issue is the difficulty of diagnosing sepsis during pregnancy. Some of the physiologic values typically used to establish the presence of the systemic inflammatory response syndrome (SIRS), often seen in sepsis, are actually considered normal for a pregnant woman.
"As the population at risk for severe sepsis continues to increase, unless we also increase our identification of patients with sepsis in this population, the rate of severe sepsis and death will continue to increase," she maintained. "A priority of future research will be to redefine SIRS in pregnant patients."
Session moderator Dr. Alan C. Santos of St. Luke’s Roosevelt Hospital Center in New York said, "I realize there were limitations to this type of study and what data you were able to extract. Could this all just be related to an increase in the background infections, HIV, and substance abuse among our population?"
Dr. Bauer acknowledged that those factors may have contributed and were not captured by administrative data. Factors often went uncoded "unless there was a reason to code a certain factor, basically, if it complicated the delivery and there would be more reimbursement for that type of situation," she said. For example, the investigators found a code for obesity in only about 1% of the sample, when roughly 20% of the population is obese.
"The only risk factor that appears to be at all modifiable is cesarean delivery, and it may add another nail in the coffin of this conversation," commented session attendee Dr. Stephen Pratt of Beth Israel Deaconess Hospital in Boston. "I was just wondering, are you able to look at either indications for cesarean delivery or even at, more broadly, elective versus intrapartum cesarean delivery? My guess is it’s going to be the intrapartum cesarean deliveries that are high risk for sepsis."
"That's an excellent point," Dr. Bauer replied. "The problem is, it’s difficult to determine exactly." However, it may be possible to get a general sense from measures such as the duration of hospitalization before cesarean. "We are certainly going to tease out more of the data," she said.
Previous research has suggested that sepsis of any severity complicates about 1 in every 8,000 deliveries in the United States. But "this has been determined by single-institution, retrospective cohort studies. To date, there has not been a United States population–based study looking at incidence, temporal trends, and risk factors for sepsis," Dr. Bauer noted.
The investigators identified hospital admissions having pregnancy- and delivery-related diagnostic codes, excluding those pertaining to ectopic or molar pregnancy, or abortion. Within this set, they then assessed outcomes of sepsis, severe sepsis (sepsis with organ failure and/or hypotension), and sepsis-related death.
Results showed that the rate of sepsis remained essentially the same during the study period, affecting about 1 in every 3,300 deliveries, according to Dr. Bauer. However, the rate of severe sepsis doubled.
Multivariate analyses showed a variety of comorbidities and demographic and obstetric factors that were independently associated with an elevated risk of severe sepsis: diabetes (odds ratio, 1.4), black race (1.8), delivery before 37 weeks (1.8), cerclage (2.7), hypertensive diseases of pregnancy (4.5), congestive heart failure (4.5), cesarean delivery (5.1), stillbirth (6.5), retained products of conception (6.9), chronic renal disease (16.2), and chronic liver disease (22.3).
Several other factors – age, insurance, obesity, and preterm premature rupture of membranes (PPROM) – did not significantly affect the risk of this outcome. However, data on PPROM may be misleading because of the heterogeneity of that group, Dr. Bauer cautioned. "It includes any patient who had rupture 24 hours prior to labor; that includes the patient who had rupture 1 day prior to labor and also the patient who has been sitting on the [hospital] floor for several months prior to labor," she explained.
"Of note, teaching-hospital status as well as delivery volume were found to [confer] a mildly increased risk for severe sepsis, but had a minimal effect size when compared to the rest of these variables," Dr. Bauer further reported.
In additional findings, the rate of sepsis-related death among women with sepsis also more than doubled during the study period, from 2.2% to 4.9%. As of 2006-2008, there were 1.31 deaths due to sepsis for every 100,000 deliveries in the United States. "This mirrors the findings in the United Kingdom for increased deaths related to sepsis," she noted (BJOG 2011;118:1-203). There were too few sepsis-related deaths to assess risk factors for this outcome.
Dr. Bauer disclosed no relevant conflicts of interest.
MONTEREY, CALIF. – The rate of severe sepsis among women hospitalized for delivery more than doubled during a recent 10-year period, according to a cohort study reported at the annual meeting of the Society for Obstetric Anesthesia and Perinatology.
In the analysis of more than 9.24 million admissions for delivery from the Nationwide Inpatient Sample, the rate rose from 0.64 to 1.37 per 10,000 deliveries between 1998-2000 and 2007-2008, reported lead investigator Dr. Melissa E.B. Bauer, an obstetric anesthesiologist with the University of Michigan Health System in Ann Arbor.
Several comorbidities and obstetric factors independently increased the risk of severe sepsis. For example, women with congestive heart failure had a more than fourfold higher risk, and women undergoing cesarean delivery had a more than fivefold higher risk.
"The etiology of the increase [in severe sepsis] in this population is unknown," she commented. "Possible hypotheses for the increase ... could be the increased cesarean delivery rate. At the start of the study period, the cesarean delivery rate was 21.2%; by 2008, the national rate was 32.3%."
"There’s also a higher-risk patient population. More women today have comorbidities that increase their risk of severe sepsis," she added. "And there’s an increased microbial resistance that could also be contributing."
Dr. Bauer noted that an important related issue is the difficulty of diagnosing sepsis during pregnancy. Some of the physiologic values typically used to establish the presence of the systemic inflammatory response syndrome (SIRS), often seen in sepsis, are actually considered normal for a pregnant woman.
"As the population at risk for severe sepsis continues to increase, unless we also increase our identification of patients with sepsis in this population, the rate of severe sepsis and death will continue to increase," she maintained. "A priority of future research will be to redefine SIRS in pregnant patients."
Session moderator Dr. Alan C. Santos of St. Luke’s Roosevelt Hospital Center in New York said, "I realize there were limitations to this type of study and what data you were able to extract. Could this all just be related to an increase in the background infections, HIV, and substance abuse among our population?"
Dr. Bauer acknowledged that those factors may have contributed and were not captured by administrative data. Factors often went uncoded "unless there was a reason to code a certain factor, basically, if it complicated the delivery and there would be more reimbursement for that type of situation," she said. For example, the investigators found a code for obesity in only about 1% of the sample, when roughly 20% of the population is obese.
"The only risk factor that appears to be at all modifiable is cesarean delivery, and it may add another nail in the coffin of this conversation," commented session attendee Dr. Stephen Pratt of Beth Israel Deaconess Hospital in Boston. "I was just wondering, are you able to look at either indications for cesarean delivery or even at, more broadly, elective versus intrapartum cesarean delivery? My guess is it’s going to be the intrapartum cesarean deliveries that are high risk for sepsis."
"That's an excellent point," Dr. Bauer replied. "The problem is, it’s difficult to determine exactly." However, it may be possible to get a general sense from measures such as the duration of hospitalization before cesarean. "We are certainly going to tease out more of the data," she said.
Previous research has suggested that sepsis of any severity complicates about 1 in every 8,000 deliveries in the United States. But "this has been determined by single-institution, retrospective cohort studies. To date, there has not been a United States population–based study looking at incidence, temporal trends, and risk factors for sepsis," Dr. Bauer noted.
The investigators identified hospital admissions having pregnancy- and delivery-related diagnostic codes, excluding those pertaining to ectopic or molar pregnancy, or abortion. Within this set, they then assessed outcomes of sepsis, severe sepsis (sepsis with organ failure and/or hypotension), and sepsis-related death.
Results showed that the rate of sepsis remained essentially the same during the study period, affecting about 1 in every 3,300 deliveries, according to Dr. Bauer. However, the rate of severe sepsis doubled.
Multivariate analyses showed a variety of comorbidities and demographic and obstetric factors that were independently associated with an elevated risk of severe sepsis: diabetes (odds ratio, 1.4), black race (1.8), delivery before 37 weeks (1.8), cerclage (2.7), hypertensive diseases of pregnancy (4.5), congestive heart failure (4.5), cesarean delivery (5.1), stillbirth (6.5), retained products of conception (6.9), chronic renal disease (16.2), and chronic liver disease (22.3).
Several other factors – age, insurance, obesity, and preterm premature rupture of membranes (PPROM) – did not significantly affect the risk of this outcome. However, data on PPROM may be misleading because of the heterogeneity of that group, Dr. Bauer cautioned. "It includes any patient who had rupture 24 hours prior to labor; that includes the patient who had rupture 1 day prior to labor and also the patient who has been sitting on the [hospital] floor for several months prior to labor," she explained.
"Of note, teaching-hospital status as well as delivery volume were found to [confer] a mildly increased risk for severe sepsis, but had a minimal effect size when compared to the rest of these variables," Dr. Bauer further reported.
In additional findings, the rate of sepsis-related death among women with sepsis also more than doubled during the study period, from 2.2% to 4.9%. As of 2006-2008, there were 1.31 deaths due to sepsis for every 100,000 deliveries in the United States. "This mirrors the findings in the United Kingdom for increased deaths related to sepsis," she noted (BJOG 2011;118:1-203). There were too few sepsis-related deaths to assess risk factors for this outcome.
Dr. Bauer disclosed no relevant conflicts of interest.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR OBSTETRIC ANESTHESIA AND PERINATOLOGY