User login
17P Failed to Help Women With 'Longer Short Cervix'
DALLAS – The drug 17-hydroxyprogesterone caproate, or 17P, is not effective for reducing the risk of preterm delivery in nulliparous women with a cervix length less than the 10th percentile, findings from a randomized placebo-controlled study involving 657 women have shown.
Based on findings from prior studies, 17P is indicated in women with a cervical length of 10-20 mm, which represents only a very small proportion of patients. The objective of the current study was to determine if the benefits of 17P might extend to those with a "longer short cervix," Dr. William Grobman said at the annual meeting of the Society for Maternal-Fetal Medicine.
"Using a cervical length cutoff of the 10th percentile potentially expands the benefits of progesterone to a larger proportion of the population," said Dr. Grobman of the department of obstetrics and gynecology at Northwestern University, Chicago, who was speaking on behalf of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network.
Between 16 and 22 weeks’ gestation, when routine anatomic surveys are performed, the 10th percentile for cervical length is 30 mm, he explained.
Of 15,436 women screened at the 14 centers that are part of the Maternal-Fetal Medicine Units Network, 1,588 had a cervical length of less than 30 mm, and 657 consented to randomization. The frequency of preterm birth, defined as delivery prior to 37 weeks’ gestation, was 25.1% in 327 women randomized to receive 17P, and 24.5% in the 330 who received placebo.
There also were no differences in the rates of preterm birth at less than 32 weeks and less than 28 weeks, and no difference in the survival curve – defined as the number of days the women remained pregnant following 17P injection, Dr. Grobman said.
Based on these findings, enrollment in the study was stopped at the third interim analysis, he noted.
As for neonatal outcomes, no difference was seen between the treatment and placebo groups in regard to a composite outcome including, but not limited to, respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, sepsis, retinopathy of prematurity, and perinatal death.
When neonatal outcomes were analyzed individually, a significant difference was seen between the treatment and placebo groups with regard to sepsis; those in the 17P group had a lower risk of developing sepsis, Dr. Grobman noted.
Women in this study had a singleton pregnancy, and were screened between 16 and 223/7 weeks by a certified sonographer. They had no major fetal anomalies, no increased probability of indicated preterm birth, and no prior loop electrosurgical excision procedure or müllerian anomalies.
Those who consented to participate received an injection of 1 mL of placebo, and those who returned at least 3 days later but before 23 weeks’ gestation were randomized to receive 250 mg of intramuscular 17P weekly, or identically appearing placebo. All received weekly injections until delivery or 37 weeks.
Those in the treatment group were slightly, but significantly older. All other characteristics, including body mass index, race/ethnicity, and estimated gestational age at randomization were similar in the treatment and placebo groups.
The mean cervical length was 24 mm in both groups; less than 10% had a cervical length less than 15 mm, and less than 20% of those had the cervical funnel visualized, Dr. Grobman said.
"Based on these data, we do conclude that weekly intramuscular 17P does not reduce the frequency of preterm birth in nulliparous women with a cervix less than 30 mm," he concluded.
"This is really a study more than anything of women with a longer short cervix," he added, explaining during a question and answer session that the study isn’t powered to look at outcomes for those with other cervical lengths, for example, less than 20 mm or less than 15 mm. About 85% of women in this study had a cervical length between 25 and 30 mm.
Dr. Grobman said he had no relevant financial disclosures.
DALLAS – The drug 17-hydroxyprogesterone caproate, or 17P, is not effective for reducing the risk of preterm delivery in nulliparous women with a cervix length less than the 10th percentile, findings from a randomized placebo-controlled study involving 657 women have shown.
Based on findings from prior studies, 17P is indicated in women with a cervical length of 10-20 mm, which represents only a very small proportion of patients. The objective of the current study was to determine if the benefits of 17P might extend to those with a "longer short cervix," Dr. William Grobman said at the annual meeting of the Society for Maternal-Fetal Medicine.
"Using a cervical length cutoff of the 10th percentile potentially expands the benefits of progesterone to a larger proportion of the population," said Dr. Grobman of the department of obstetrics and gynecology at Northwestern University, Chicago, who was speaking on behalf of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network.
Between 16 and 22 weeks’ gestation, when routine anatomic surveys are performed, the 10th percentile for cervical length is 30 mm, he explained.
Of 15,436 women screened at the 14 centers that are part of the Maternal-Fetal Medicine Units Network, 1,588 had a cervical length of less than 30 mm, and 657 consented to randomization. The frequency of preterm birth, defined as delivery prior to 37 weeks’ gestation, was 25.1% in 327 women randomized to receive 17P, and 24.5% in the 330 who received placebo.
There also were no differences in the rates of preterm birth at less than 32 weeks and less than 28 weeks, and no difference in the survival curve – defined as the number of days the women remained pregnant following 17P injection, Dr. Grobman said.
Based on these findings, enrollment in the study was stopped at the third interim analysis, he noted.
As for neonatal outcomes, no difference was seen between the treatment and placebo groups in regard to a composite outcome including, but not limited to, respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, sepsis, retinopathy of prematurity, and perinatal death.
When neonatal outcomes were analyzed individually, a significant difference was seen between the treatment and placebo groups with regard to sepsis; those in the 17P group had a lower risk of developing sepsis, Dr. Grobman noted.
Women in this study had a singleton pregnancy, and were screened between 16 and 223/7 weeks by a certified sonographer. They had no major fetal anomalies, no increased probability of indicated preterm birth, and no prior loop electrosurgical excision procedure or müllerian anomalies.
Those who consented to participate received an injection of 1 mL of placebo, and those who returned at least 3 days later but before 23 weeks’ gestation were randomized to receive 250 mg of intramuscular 17P weekly, or identically appearing placebo. All received weekly injections until delivery or 37 weeks.
Those in the treatment group were slightly, but significantly older. All other characteristics, including body mass index, race/ethnicity, and estimated gestational age at randomization were similar in the treatment and placebo groups.
The mean cervical length was 24 mm in both groups; less than 10% had a cervical length less than 15 mm, and less than 20% of those had the cervical funnel visualized, Dr. Grobman said.
"Based on these data, we do conclude that weekly intramuscular 17P does not reduce the frequency of preterm birth in nulliparous women with a cervix less than 30 mm," he concluded.
"This is really a study more than anything of women with a longer short cervix," he added, explaining during a question and answer session that the study isn’t powered to look at outcomes for those with other cervical lengths, for example, less than 20 mm or less than 15 mm. About 85% of women in this study had a cervical length between 25 and 30 mm.
Dr. Grobman said he had no relevant financial disclosures.
DALLAS – The drug 17-hydroxyprogesterone caproate, or 17P, is not effective for reducing the risk of preterm delivery in nulliparous women with a cervix length less than the 10th percentile, findings from a randomized placebo-controlled study involving 657 women have shown.
Based on findings from prior studies, 17P is indicated in women with a cervical length of 10-20 mm, which represents only a very small proportion of patients. The objective of the current study was to determine if the benefits of 17P might extend to those with a "longer short cervix," Dr. William Grobman said at the annual meeting of the Society for Maternal-Fetal Medicine.
"Using a cervical length cutoff of the 10th percentile potentially expands the benefits of progesterone to a larger proportion of the population," said Dr. Grobman of the department of obstetrics and gynecology at Northwestern University, Chicago, who was speaking on behalf of the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network.
Between 16 and 22 weeks’ gestation, when routine anatomic surveys are performed, the 10th percentile for cervical length is 30 mm, he explained.
Of 15,436 women screened at the 14 centers that are part of the Maternal-Fetal Medicine Units Network, 1,588 had a cervical length of less than 30 mm, and 657 consented to randomization. The frequency of preterm birth, defined as delivery prior to 37 weeks’ gestation, was 25.1% in 327 women randomized to receive 17P, and 24.5% in the 330 who received placebo.
There also were no differences in the rates of preterm birth at less than 32 weeks and less than 28 weeks, and no difference in the survival curve – defined as the number of days the women remained pregnant following 17P injection, Dr. Grobman said.
Based on these findings, enrollment in the study was stopped at the third interim analysis, he noted.
As for neonatal outcomes, no difference was seen between the treatment and placebo groups in regard to a composite outcome including, but not limited to, respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, sepsis, retinopathy of prematurity, and perinatal death.
When neonatal outcomes were analyzed individually, a significant difference was seen between the treatment and placebo groups with regard to sepsis; those in the 17P group had a lower risk of developing sepsis, Dr. Grobman noted.
Women in this study had a singleton pregnancy, and were screened between 16 and 223/7 weeks by a certified sonographer. They had no major fetal anomalies, no increased probability of indicated preterm birth, and no prior loop electrosurgical excision procedure or müllerian anomalies.
Those who consented to participate received an injection of 1 mL of placebo, and those who returned at least 3 days later but before 23 weeks’ gestation were randomized to receive 250 mg of intramuscular 17P weekly, or identically appearing placebo. All received weekly injections until delivery or 37 weeks.
Those in the treatment group were slightly, but significantly older. All other characteristics, including body mass index, race/ethnicity, and estimated gestational age at randomization were similar in the treatment and placebo groups.
The mean cervical length was 24 mm in both groups; less than 10% had a cervical length less than 15 mm, and less than 20% of those had the cervical funnel visualized, Dr. Grobman said.
"Based on these data, we do conclude that weekly intramuscular 17P does not reduce the frequency of preterm birth in nulliparous women with a cervix less than 30 mm," he concluded.
"This is really a study more than anything of women with a longer short cervix," he added, explaining during a question and answer session that the study isn’t powered to look at outcomes for those with other cervical lengths, for example, less than 20 mm or less than 15 mm. About 85% of women in this study had a cervical length between 25 and 30 mm.
Dr. Grobman said he had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR MATERNAL-FETAL MEDICINE
Major Finding: The frequency of preterm birth, defined as delivery prior to 37 weeks’ gestation, was 25.1% in 327 women randomized to receive 17P, and 24.5% in the 330 who received placebo. The mean cervical length was 24 mm in both groups.
Data Source: This was a randomized placebo-controlled trial of 657 pregnant women who were part of the NICHD Maternal-Fetal Medicine Units Network.
Disclosures: Dr. Grobman said he had no relevant financial disclosures.
Pregnancy Does Not Preclude Safe DVT Treatment
MIAMI BEACH – Women who develop a deep vein thrombosis while pregnant can safely undergo removal of the clot without jeopardizing their pregnancy, based on a recent experience with 11 cases at one U.S. center.
"Not many vascular surgeons are willing to perform thrombectomy in pregnant women, and most physicians fear thrombolytic therapy in pregnant patients," Dr. Anthony J. Comerota said at ISET 2012, an international symposium on endovascular therapy.
But in reality, there is no evidence that thrombolysis poses any special risk during pregnancy, in part because the drugs that are usually used – urokinase, streptokinase, or tissue plasminogen activator (TPA) – do not cross the placenta, and therefore do not exert any fibrinolytic effect on a fetus, he said. In addition, the relatively recent introduction of pharmacomechanical methods for thrombolysis have "accelerated our enthusiasm and strengthened our confidence that this is an effective and safe strategy for treating extensive DVT [deep vein thrombosis] during pregnancy," said Dr. Comerota, a vascular surgeon and director of the Jobst Vascular Institute in Toledo, Ohio.
"Pregnancy need not be a major contraindication for successful management of extensive DVT. We need to minimize radiation, monitor the fetus, and control uterine contractions, but we should treat healthy, pregnant women [who have] a DVT the same way we treat healthy nonpregnant women" who have a DVT, he said in an interview. "The extraordinary concern for risk to the pregnancy [from clot removal] seems substantially overstated."
Because pregnancy produces a prothrombotic state, the risk that a healthy, pregnant woman has for DVT is about sixfold higher than her risk when not pregnant. Until now, most physicians deferred endovascular or surgical management of women who develop a DVT during pregnancy, and have relied exclusively on medical treatment with heparin or low-molecular-weight heparin, a strategy that is often ineffective.
"What pushed me to treat women earlier during their pregnancy was that I saw patients who did not receive therapy until after delivery, and by then the clot became more organized – a mix of collagen and scar inside the vein – -and caused severe postthrombotic disability," Dr. Comerota said.
Recently, he treated 11 pregnant women (10 with an iliofemoral thrombosis and 1 with the clot in her superior vena cava). One woman was in the first trimester, two were in the second, and eight were in the third trimester. Three patients underwent clot removal by open surgery, with the other eight treated by endovascular methods, including six who were treated with catheter-delivered TPA and one who received urokinase via catheter. Other endovascular tools that were used as needed included ultrasound clot treatment and rheolytic thrombectomy. In all cases, these treatments successfully removed the clot. One women developed hematuria and required a transfusion following her clot removal, and another patient developed a popliteal artery pseudoaneurysm. None of the patients had a recurrent DVT; two developed mild postthrombotic symptoms.
In one case, the fetus spontaneously aborted 5 days after the procedure secondary to antiphospholipid antibody syndrome, which had also triggered the thrombosis. The other 10 cases resulted in successful pregnancies and live deliveries, one surgically and the other nine vaginally. Three of the women had successful subsequent pregnancies, Dr. Comerota said.
Dr. Comerota said that he has been a speaker for and a consultant to Covidien, a company that makes some of the devices used in these procedures.
MIAMI BEACH – Women who develop a deep vein thrombosis while pregnant can safely undergo removal of the clot without jeopardizing their pregnancy, based on a recent experience with 11 cases at one U.S. center.
"Not many vascular surgeons are willing to perform thrombectomy in pregnant women, and most physicians fear thrombolytic therapy in pregnant patients," Dr. Anthony J. Comerota said at ISET 2012, an international symposium on endovascular therapy.
But in reality, there is no evidence that thrombolysis poses any special risk during pregnancy, in part because the drugs that are usually used – urokinase, streptokinase, or tissue plasminogen activator (TPA) – do not cross the placenta, and therefore do not exert any fibrinolytic effect on a fetus, he said. In addition, the relatively recent introduction of pharmacomechanical methods for thrombolysis have "accelerated our enthusiasm and strengthened our confidence that this is an effective and safe strategy for treating extensive DVT [deep vein thrombosis] during pregnancy," said Dr. Comerota, a vascular surgeon and director of the Jobst Vascular Institute in Toledo, Ohio.
"Pregnancy need not be a major contraindication for successful management of extensive DVT. We need to minimize radiation, monitor the fetus, and control uterine contractions, but we should treat healthy, pregnant women [who have] a DVT the same way we treat healthy nonpregnant women" who have a DVT, he said in an interview. "The extraordinary concern for risk to the pregnancy [from clot removal] seems substantially overstated."
Because pregnancy produces a prothrombotic state, the risk that a healthy, pregnant woman has for DVT is about sixfold higher than her risk when not pregnant. Until now, most physicians deferred endovascular or surgical management of women who develop a DVT during pregnancy, and have relied exclusively on medical treatment with heparin or low-molecular-weight heparin, a strategy that is often ineffective.
"What pushed me to treat women earlier during their pregnancy was that I saw patients who did not receive therapy until after delivery, and by then the clot became more organized – a mix of collagen and scar inside the vein – -and caused severe postthrombotic disability," Dr. Comerota said.
Recently, he treated 11 pregnant women (10 with an iliofemoral thrombosis and 1 with the clot in her superior vena cava). One woman was in the first trimester, two were in the second, and eight were in the third trimester. Three patients underwent clot removal by open surgery, with the other eight treated by endovascular methods, including six who were treated with catheter-delivered TPA and one who received urokinase via catheter. Other endovascular tools that were used as needed included ultrasound clot treatment and rheolytic thrombectomy. In all cases, these treatments successfully removed the clot. One women developed hematuria and required a transfusion following her clot removal, and another patient developed a popliteal artery pseudoaneurysm. None of the patients had a recurrent DVT; two developed mild postthrombotic symptoms.
In one case, the fetus spontaneously aborted 5 days after the procedure secondary to antiphospholipid antibody syndrome, which had also triggered the thrombosis. The other 10 cases resulted in successful pregnancies and live deliveries, one surgically and the other nine vaginally. Three of the women had successful subsequent pregnancies, Dr. Comerota said.
Dr. Comerota said that he has been a speaker for and a consultant to Covidien, a company that makes some of the devices used in these procedures.
MIAMI BEACH – Women who develop a deep vein thrombosis while pregnant can safely undergo removal of the clot without jeopardizing their pregnancy, based on a recent experience with 11 cases at one U.S. center.
"Not many vascular surgeons are willing to perform thrombectomy in pregnant women, and most physicians fear thrombolytic therapy in pregnant patients," Dr. Anthony J. Comerota said at ISET 2012, an international symposium on endovascular therapy.
But in reality, there is no evidence that thrombolysis poses any special risk during pregnancy, in part because the drugs that are usually used – urokinase, streptokinase, or tissue plasminogen activator (TPA) – do not cross the placenta, and therefore do not exert any fibrinolytic effect on a fetus, he said. In addition, the relatively recent introduction of pharmacomechanical methods for thrombolysis have "accelerated our enthusiasm and strengthened our confidence that this is an effective and safe strategy for treating extensive DVT [deep vein thrombosis] during pregnancy," said Dr. Comerota, a vascular surgeon and director of the Jobst Vascular Institute in Toledo, Ohio.
"Pregnancy need not be a major contraindication for successful management of extensive DVT. We need to minimize radiation, monitor the fetus, and control uterine contractions, but we should treat healthy, pregnant women [who have] a DVT the same way we treat healthy nonpregnant women" who have a DVT, he said in an interview. "The extraordinary concern for risk to the pregnancy [from clot removal] seems substantially overstated."
Because pregnancy produces a prothrombotic state, the risk that a healthy, pregnant woman has for DVT is about sixfold higher than her risk when not pregnant. Until now, most physicians deferred endovascular or surgical management of women who develop a DVT during pregnancy, and have relied exclusively on medical treatment with heparin or low-molecular-weight heparin, a strategy that is often ineffective.
"What pushed me to treat women earlier during their pregnancy was that I saw patients who did not receive therapy until after delivery, and by then the clot became more organized – a mix of collagen and scar inside the vein – -and caused severe postthrombotic disability," Dr. Comerota said.
Recently, he treated 11 pregnant women (10 with an iliofemoral thrombosis and 1 with the clot in her superior vena cava). One woman was in the first trimester, two were in the second, and eight were in the third trimester. Three patients underwent clot removal by open surgery, with the other eight treated by endovascular methods, including six who were treated with catheter-delivered TPA and one who received urokinase via catheter. Other endovascular tools that were used as needed included ultrasound clot treatment and rheolytic thrombectomy. In all cases, these treatments successfully removed the clot. One women developed hematuria and required a transfusion following her clot removal, and another patient developed a popliteal artery pseudoaneurysm. None of the patients had a recurrent DVT; two developed mild postthrombotic symptoms.
In one case, the fetus spontaneously aborted 5 days after the procedure secondary to antiphospholipid antibody syndrome, which had also triggered the thrombosis. The other 10 cases resulted in successful pregnancies and live deliveries, one surgically and the other nine vaginally. Three of the women had successful subsequent pregnancies, Dr. Comerota said.
Dr. Comerota said that he has been a speaker for and a consultant to Covidien, a company that makes some of the devices used in these procedures.
FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY
Major Finding: Treatment of 11 pregnant women with DVT resulted in 10 successful live births.
Data Source: A retrospective review of 11 cases managed at one U.S. center.
Disclosures: Dr. Comerota said that he has been a speaker for and a consultant to Covidien, a company that makes some of the devices used in these procedures.
Is the hCG discriminatory zone a reliable indicator of intrauterine or ectopic pregnancy?
Often used in the evaluation of early pregnancy, the hCG discriminatory zone is based on the assumption that a serum ß-hCG level exceeding 1,000–2,000 mIU/mL in a woman who has a normal intrauterine pregnancy should be accompanied by a gestational sac that is visible via transvaginal US. When such a sac is not visible in the uterus, many practitioners conclude that the pregnancy is ectopic and tailor management accordingly.
In this study of women who were assessed between the years 2000 and 2010, investigators reviewed the records of those who underwent US and hCG measurement on the same (index) day, had detectable hCG, had no US evidence of intrauterine pregnancy on the index day, and were subsequently found to have a viable intrauterine pregnancy. Among 202 women who met these criteria, the hCG level fell into the following ranges:
- below 1,000 mIU/mL (80.2%)
- 1,000–1,499 mIU/mL (9.4%)
- 1,500–1,999 mIU/mL (5.9%)
- 2,000 mIU/mL or higher (4.5%).
The highest hCG value observed was 6,567 mIU/mL; the highest level of hCG observed in a woman who later delivered a term infant was 4,336 mIU/mL.
A case for abandoning the zone?
Many clinicians (and malpractice attorneys) are familiar with unfortunate cases of women who underwent uterine curettage or were given methotrexate, based on an hCG level found to be in the discriminatory zone without accompanying evidence of intrauterine pregnancy, only to lose what was, in fact, a potentially viable pregnancy.
By demonstrating the high variability in hCG levels among women who had early intrauterine pregnancy without definitive findings on US, the authors make a strong case for abandoning the concept of the discriminatory zone altogether.
Women’s health providers often encounter hemodynamically stable patients who have early pregnancy of unknown viability or implantation site and who lack ultrasonographic (US) evidence of hemoperitoneum. It is not appropriate to perform uterine curettage or administer methotrexate in this setting. Instead, counsel these patients that the earliness of the pregnancy precludes definitive assessment of gestational status. Review with the patient the signs and symptoms of ruptured ectopic pregnancy, and arrange for follow-up hCG measurement and US assessment.
ANDREW M. KAUNITZ, MD
We want to hear from you! Tell us what you think.
Often used in the evaluation of early pregnancy, the hCG discriminatory zone is based on the assumption that a serum ß-hCG level exceeding 1,000–2,000 mIU/mL in a woman who has a normal intrauterine pregnancy should be accompanied by a gestational sac that is visible via transvaginal US. When such a sac is not visible in the uterus, many practitioners conclude that the pregnancy is ectopic and tailor management accordingly.
In this study of women who were assessed between the years 2000 and 2010, investigators reviewed the records of those who underwent US and hCG measurement on the same (index) day, had detectable hCG, had no US evidence of intrauterine pregnancy on the index day, and were subsequently found to have a viable intrauterine pregnancy. Among 202 women who met these criteria, the hCG level fell into the following ranges:
- below 1,000 mIU/mL (80.2%)
- 1,000–1,499 mIU/mL (9.4%)
- 1,500–1,999 mIU/mL (5.9%)
- 2,000 mIU/mL or higher (4.5%).
The highest hCG value observed was 6,567 mIU/mL; the highest level of hCG observed in a woman who later delivered a term infant was 4,336 mIU/mL.
A case for abandoning the zone?
Many clinicians (and malpractice attorneys) are familiar with unfortunate cases of women who underwent uterine curettage or were given methotrexate, based on an hCG level found to be in the discriminatory zone without accompanying evidence of intrauterine pregnancy, only to lose what was, in fact, a potentially viable pregnancy.
By demonstrating the high variability in hCG levels among women who had early intrauterine pregnancy without definitive findings on US, the authors make a strong case for abandoning the concept of the discriminatory zone altogether.
Women’s health providers often encounter hemodynamically stable patients who have early pregnancy of unknown viability or implantation site and who lack ultrasonographic (US) evidence of hemoperitoneum. It is not appropriate to perform uterine curettage or administer methotrexate in this setting. Instead, counsel these patients that the earliness of the pregnancy precludes definitive assessment of gestational status. Review with the patient the signs and symptoms of ruptured ectopic pregnancy, and arrange for follow-up hCG measurement and US assessment.
ANDREW M. KAUNITZ, MD
We want to hear from you! Tell us what you think.
Often used in the evaluation of early pregnancy, the hCG discriminatory zone is based on the assumption that a serum ß-hCG level exceeding 1,000–2,000 mIU/mL in a woman who has a normal intrauterine pregnancy should be accompanied by a gestational sac that is visible via transvaginal US. When such a sac is not visible in the uterus, many practitioners conclude that the pregnancy is ectopic and tailor management accordingly.
In this study of women who were assessed between the years 2000 and 2010, investigators reviewed the records of those who underwent US and hCG measurement on the same (index) day, had detectable hCG, had no US evidence of intrauterine pregnancy on the index day, and were subsequently found to have a viable intrauterine pregnancy. Among 202 women who met these criteria, the hCG level fell into the following ranges:
- below 1,000 mIU/mL (80.2%)
- 1,000–1,499 mIU/mL (9.4%)
- 1,500–1,999 mIU/mL (5.9%)
- 2,000 mIU/mL or higher (4.5%).
The highest hCG value observed was 6,567 mIU/mL; the highest level of hCG observed in a woman who later delivered a term infant was 4,336 mIU/mL.
A case for abandoning the zone?
Many clinicians (and malpractice attorneys) are familiar with unfortunate cases of women who underwent uterine curettage or were given methotrexate, based on an hCG level found to be in the discriminatory zone without accompanying evidence of intrauterine pregnancy, only to lose what was, in fact, a potentially viable pregnancy.
By demonstrating the high variability in hCG levels among women who had early intrauterine pregnancy without definitive findings on US, the authors make a strong case for abandoning the concept of the discriminatory zone altogether.
Women’s health providers often encounter hemodynamically stable patients who have early pregnancy of unknown viability or implantation site and who lack ultrasonographic (US) evidence of hemoperitoneum. It is not appropriate to perform uterine curettage or administer methotrexate in this setting. Instead, counsel these patients that the earliness of the pregnancy precludes definitive assessment of gestational status. Review with the patient the signs and symptoms of ruptured ectopic pregnancy, and arrange for follow-up hCG measurement and US assessment.
ANDREW M. KAUNITZ, MD
We want to hear from you! Tell us what you think.
Pregnant Women With Lymphoma Can Have Good Outcomes
SAN DIEGO – Women diagnosed with lymphoma during pregnancy stand a good chance of carrying a healthy child to term even when they opt for treatment during the second or third trimester, according to a retrospective multicenter analysis.
Among 82 women diagnosed with either Hodgkin’s or non-Hodgkin’s lymphoma during pregnancy, 48 opted to start therapy during pregnancy rather than defer it until after delivery, investigators reported at the annual meeting of the American Society of Hematology.
All but one woman had a normal birth, the exception being a severe malformation: microcephaly in the fetus of a woman who had received four cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for diffuse large B-cell lymphoma (DLBCL).
The timing of therapy did not appear to affect overall survival, with the 3-year progression-free survival (PFS) rate being 76% among women who underwent treatment during pregnancy, compared with 79% for those who deferred it, said Dr. Andrew M. Evens of the University of Massachusetts in Worcester.
Respective overall survival rates were 92% and 83%, he reported. For the six women who elected to terminate their pregnancies, the 3-year PFS rate and overall survival rate were each 100%.
Among 39 women with Hodgkin’s lymphoma (HL), the 3-year PFS rate was 90%, and overall survival was 95%. Among 33 patients with B-cell non-Hodgkin’s lymphomas (NHL), 73% were progression free at 3 years; the overall survival rate was 82%. For 10 women with NHL of T-cell histology, the respective figures were 50% and 90%.
"We conclude that standard chemotherapy – non-antimetabolite chemotherapy – and radiation in select cases, in particular localized disease likely above the diaphragm during the second and third trimester, were associated with expected maternal complications and fetal detriment," Dr. Evens said.
Women with low-risk disease, such as indolent NHL, or a diagnosis late in gestation may be able to defer therapy until after delivery, he added.
Cancers in Pregnancy Uncommon. Cancer diagnoses during pregnancy are uncommon, occurring in about 3,500 women annually in the United States. The estimated prevalence is 1 in 1,000 gestations. Hematologic malignancies, primarily lymphomas, account for about 20% of all cancers diagnosed in pregnancy, Dr. Evens said.
He and his colleagues at nine academic medical centers conducted a descriptive retrospective analysis looking at histology, disease characteristics, therapy received, and maternal and fetal complications among pregnant women diagnosed with lymphomas from 1998 through 2011.
Of the 82 women identified for whom follow-up data were available, 43 (52%) were diagnosed with NHL (83% B-cell and 17% T-cell histologies) and 39 (48%) with HL. The median time of diagnosis was at 24 weeks gestation (range 5-40 weeks).
Six patients (4 with NHL and 2 with HL) decided to terminate the pregnancies to have immediate chemotherapy. Five of these patients were diagnosed in the first trimester and required systemic therapy.
The remaining patient was diagnosed early in the second trimester with lymphoma involving the central nervous system and requiring high-dose methotrexate, an antimetabolite in FDA pregnancy category X (positive evidence of fetal harm from animal or human studies and/or clinical experience; contraindicated). Other antimetabolites are classified in category D (positive evidence of fetal risk, but the benefits may warrant use in pregnant women).
A total of 28 patients (34%) chose to defer therapy, including 15 with HL, 5 with follicular lymphoma, 4 with DLBCL, 3 with T-cell lymphoma, and 1 with Burkitt’s lymphoma. The median gestation time at diagnosis in these patients was 34 weeks (range 6-38).
Of the 48 patients who chose to start therapy during pregnancy, 27 patients with NHL received therapy with CHOP, CHOP plus rituximab (Rituxan), modified hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), or similar regimens.
All but 2 patients with HL received the ABVD regimen (doxorubicin, bleomycin, vinblastine, and dacarbazine), and 4 of these patients also received partial-dose radiation therapy with shielding of the fetus. One patient received AVD (no bleomycin), and 1 received ChlVPP (chlorambucil, vinblastine, procarbazine, and prednisone). Treatments ranged from the 13th to the 33rd week of gestation.
Among the 48 treated patients, gestation reached full term in 73% with delivery at a median of 37 weeks (range 31-40); most of the deliveries occurred at or after 35 weeks. Among the 28 patients who deferred therapy, delivery was at a median of 38 weeks (range 26-40), and 86% of these women were able to carry their pregnancies to term.
"The goal in every patient, whether they received therapy or not, was to try and deliver as close to term as possible," Dr. Evens said.
Among all patients, 72% had vaginal delivery, and 28% had cesarean sections.
Labor Induced in Nearly Half of Patients. The most common preterm complication was the need for induction of labor in 45%. Preeclampsia occurred in 8%, 5% had spontaneous rupture of membranes, and 4% had gestational diabetes. There were no reported cases of endometritis or chorioamnionitis. There were no significant differences in preterm events between patients who were treated or deferred therapy.
There was one stillbirth, occurring in a 34-year-old woman with double-hit (two-mutation) NHL at 19 weeks after one cycle of R-CHOP.
One woman died before giving birth. She had very-high-risk DLBCL with significant metastases to the liver. She had been diagnosed at week 29 and died at week 32 from encephalopathy, but delivered a healthy infant before her death.
Outcomes for the fetuses of the 76 women who opted to continue their pregnancies included the aforementioned stillbirth and 1 case of microcephaly in a woman who had received four cycles of CHOP for DLBCL.
The median birth weight of neonates was 2,427 g (range 1,005-5,262 g), and there were no differences between the children of women who underwent antepartum chemotherapy or deferred therapy.
Dr. Evens noted that the investigators looked only at acute fetal outcomes, and have not evaluated long-term developmental measures.
The study was funded by the participating centers. The authors reported no relevant conflicts of interest.
SAN DIEGO – Women diagnosed with lymphoma during pregnancy stand a good chance of carrying a healthy child to term even when they opt for treatment during the second or third trimester, according to a retrospective multicenter analysis.
Among 82 women diagnosed with either Hodgkin’s or non-Hodgkin’s lymphoma during pregnancy, 48 opted to start therapy during pregnancy rather than defer it until after delivery, investigators reported at the annual meeting of the American Society of Hematology.
All but one woman had a normal birth, the exception being a severe malformation: microcephaly in the fetus of a woman who had received four cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for diffuse large B-cell lymphoma (DLBCL).
The timing of therapy did not appear to affect overall survival, with the 3-year progression-free survival (PFS) rate being 76% among women who underwent treatment during pregnancy, compared with 79% for those who deferred it, said Dr. Andrew M. Evens of the University of Massachusetts in Worcester.
Respective overall survival rates were 92% and 83%, he reported. For the six women who elected to terminate their pregnancies, the 3-year PFS rate and overall survival rate were each 100%.
Among 39 women with Hodgkin’s lymphoma (HL), the 3-year PFS rate was 90%, and overall survival was 95%. Among 33 patients with B-cell non-Hodgkin’s lymphomas (NHL), 73% were progression free at 3 years; the overall survival rate was 82%. For 10 women with NHL of T-cell histology, the respective figures were 50% and 90%.
"We conclude that standard chemotherapy – non-antimetabolite chemotherapy – and radiation in select cases, in particular localized disease likely above the diaphragm during the second and third trimester, were associated with expected maternal complications and fetal detriment," Dr. Evens said.
Women with low-risk disease, such as indolent NHL, or a diagnosis late in gestation may be able to defer therapy until after delivery, he added.
Cancers in Pregnancy Uncommon. Cancer diagnoses during pregnancy are uncommon, occurring in about 3,500 women annually in the United States. The estimated prevalence is 1 in 1,000 gestations. Hematologic malignancies, primarily lymphomas, account for about 20% of all cancers diagnosed in pregnancy, Dr. Evens said.
He and his colleagues at nine academic medical centers conducted a descriptive retrospective analysis looking at histology, disease characteristics, therapy received, and maternal and fetal complications among pregnant women diagnosed with lymphomas from 1998 through 2011.
Of the 82 women identified for whom follow-up data were available, 43 (52%) were diagnosed with NHL (83% B-cell and 17% T-cell histologies) and 39 (48%) with HL. The median time of diagnosis was at 24 weeks gestation (range 5-40 weeks).
Six patients (4 with NHL and 2 with HL) decided to terminate the pregnancies to have immediate chemotherapy. Five of these patients were diagnosed in the first trimester and required systemic therapy.
The remaining patient was diagnosed early in the second trimester with lymphoma involving the central nervous system and requiring high-dose methotrexate, an antimetabolite in FDA pregnancy category X (positive evidence of fetal harm from animal or human studies and/or clinical experience; contraindicated). Other antimetabolites are classified in category D (positive evidence of fetal risk, but the benefits may warrant use in pregnant women).
A total of 28 patients (34%) chose to defer therapy, including 15 with HL, 5 with follicular lymphoma, 4 with DLBCL, 3 with T-cell lymphoma, and 1 with Burkitt’s lymphoma. The median gestation time at diagnosis in these patients was 34 weeks (range 6-38).
Of the 48 patients who chose to start therapy during pregnancy, 27 patients with NHL received therapy with CHOP, CHOP plus rituximab (Rituxan), modified hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), or similar regimens.
All but 2 patients with HL received the ABVD regimen (doxorubicin, bleomycin, vinblastine, and dacarbazine), and 4 of these patients also received partial-dose radiation therapy with shielding of the fetus. One patient received AVD (no bleomycin), and 1 received ChlVPP (chlorambucil, vinblastine, procarbazine, and prednisone). Treatments ranged from the 13th to the 33rd week of gestation.
Among the 48 treated patients, gestation reached full term in 73% with delivery at a median of 37 weeks (range 31-40); most of the deliveries occurred at or after 35 weeks. Among the 28 patients who deferred therapy, delivery was at a median of 38 weeks (range 26-40), and 86% of these women were able to carry their pregnancies to term.
"The goal in every patient, whether they received therapy or not, was to try and deliver as close to term as possible," Dr. Evens said.
Among all patients, 72% had vaginal delivery, and 28% had cesarean sections.
Labor Induced in Nearly Half of Patients. The most common preterm complication was the need for induction of labor in 45%. Preeclampsia occurred in 8%, 5% had spontaneous rupture of membranes, and 4% had gestational diabetes. There were no reported cases of endometritis or chorioamnionitis. There were no significant differences in preterm events between patients who were treated or deferred therapy.
There was one stillbirth, occurring in a 34-year-old woman with double-hit (two-mutation) NHL at 19 weeks after one cycle of R-CHOP.
One woman died before giving birth. She had very-high-risk DLBCL with significant metastases to the liver. She had been diagnosed at week 29 and died at week 32 from encephalopathy, but delivered a healthy infant before her death.
Outcomes for the fetuses of the 76 women who opted to continue their pregnancies included the aforementioned stillbirth and 1 case of microcephaly in a woman who had received four cycles of CHOP for DLBCL.
The median birth weight of neonates was 2,427 g (range 1,005-5,262 g), and there were no differences between the children of women who underwent antepartum chemotherapy or deferred therapy.
Dr. Evens noted that the investigators looked only at acute fetal outcomes, and have not evaluated long-term developmental measures.
The study was funded by the participating centers. The authors reported no relevant conflicts of interest.
SAN DIEGO – Women diagnosed with lymphoma during pregnancy stand a good chance of carrying a healthy child to term even when they opt for treatment during the second or third trimester, according to a retrospective multicenter analysis.
Among 82 women diagnosed with either Hodgkin’s or non-Hodgkin’s lymphoma during pregnancy, 48 opted to start therapy during pregnancy rather than defer it until after delivery, investigators reported at the annual meeting of the American Society of Hematology.
All but one woman had a normal birth, the exception being a severe malformation: microcephaly in the fetus of a woman who had received four cycles of CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for diffuse large B-cell lymphoma (DLBCL).
The timing of therapy did not appear to affect overall survival, with the 3-year progression-free survival (PFS) rate being 76% among women who underwent treatment during pregnancy, compared with 79% for those who deferred it, said Dr. Andrew M. Evens of the University of Massachusetts in Worcester.
Respective overall survival rates were 92% and 83%, he reported. For the six women who elected to terminate their pregnancies, the 3-year PFS rate and overall survival rate were each 100%.
Among 39 women with Hodgkin’s lymphoma (HL), the 3-year PFS rate was 90%, and overall survival was 95%. Among 33 patients with B-cell non-Hodgkin’s lymphomas (NHL), 73% were progression free at 3 years; the overall survival rate was 82%. For 10 women with NHL of T-cell histology, the respective figures were 50% and 90%.
"We conclude that standard chemotherapy – non-antimetabolite chemotherapy – and radiation in select cases, in particular localized disease likely above the diaphragm during the second and third trimester, were associated with expected maternal complications and fetal detriment," Dr. Evens said.
Women with low-risk disease, such as indolent NHL, or a diagnosis late in gestation may be able to defer therapy until after delivery, he added.
Cancers in Pregnancy Uncommon. Cancer diagnoses during pregnancy are uncommon, occurring in about 3,500 women annually in the United States. The estimated prevalence is 1 in 1,000 gestations. Hematologic malignancies, primarily lymphomas, account for about 20% of all cancers diagnosed in pregnancy, Dr. Evens said.
He and his colleagues at nine academic medical centers conducted a descriptive retrospective analysis looking at histology, disease characteristics, therapy received, and maternal and fetal complications among pregnant women diagnosed with lymphomas from 1998 through 2011.
Of the 82 women identified for whom follow-up data were available, 43 (52%) were diagnosed with NHL (83% B-cell and 17% T-cell histologies) and 39 (48%) with HL. The median time of diagnosis was at 24 weeks gestation (range 5-40 weeks).
Six patients (4 with NHL and 2 with HL) decided to terminate the pregnancies to have immediate chemotherapy. Five of these patients were diagnosed in the first trimester and required systemic therapy.
The remaining patient was diagnosed early in the second trimester with lymphoma involving the central nervous system and requiring high-dose methotrexate, an antimetabolite in FDA pregnancy category X (positive evidence of fetal harm from animal or human studies and/or clinical experience; contraindicated). Other antimetabolites are classified in category D (positive evidence of fetal risk, but the benefits may warrant use in pregnant women).
A total of 28 patients (34%) chose to defer therapy, including 15 with HL, 5 with follicular lymphoma, 4 with DLBCL, 3 with T-cell lymphoma, and 1 with Burkitt’s lymphoma. The median gestation time at diagnosis in these patients was 34 weeks (range 6-38).
Of the 48 patients who chose to start therapy during pregnancy, 27 patients with NHL received therapy with CHOP, CHOP plus rituximab (Rituxan), modified hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), or similar regimens.
All but 2 patients with HL received the ABVD regimen (doxorubicin, bleomycin, vinblastine, and dacarbazine), and 4 of these patients also received partial-dose radiation therapy with shielding of the fetus. One patient received AVD (no bleomycin), and 1 received ChlVPP (chlorambucil, vinblastine, procarbazine, and prednisone). Treatments ranged from the 13th to the 33rd week of gestation.
Among the 48 treated patients, gestation reached full term in 73% with delivery at a median of 37 weeks (range 31-40); most of the deliveries occurred at or after 35 weeks. Among the 28 patients who deferred therapy, delivery was at a median of 38 weeks (range 26-40), and 86% of these women were able to carry their pregnancies to term.
"The goal in every patient, whether they received therapy or not, was to try and deliver as close to term as possible," Dr. Evens said.
Among all patients, 72% had vaginal delivery, and 28% had cesarean sections.
Labor Induced in Nearly Half of Patients. The most common preterm complication was the need for induction of labor in 45%. Preeclampsia occurred in 8%, 5% had spontaneous rupture of membranes, and 4% had gestational diabetes. There were no reported cases of endometritis or chorioamnionitis. There were no significant differences in preterm events between patients who were treated or deferred therapy.
There was one stillbirth, occurring in a 34-year-old woman with double-hit (two-mutation) NHL at 19 weeks after one cycle of R-CHOP.
One woman died before giving birth. She had very-high-risk DLBCL with significant metastases to the liver. She had been diagnosed at week 29 and died at week 32 from encephalopathy, but delivered a healthy infant before her death.
Outcomes for the fetuses of the 76 women who opted to continue their pregnancies included the aforementioned stillbirth and 1 case of microcephaly in a woman who had received four cycles of CHOP for DLBCL.
The median birth weight of neonates was 2,427 g (range 1,005-5,262 g), and there were no differences between the children of women who underwent antepartum chemotherapy or deferred therapy.
Dr. Evens noted that the investigators looked only at acute fetal outcomes, and have not evaluated long-term developmental measures.
The study was funded by the participating centers. The authors reported no relevant conflicts of interest.
FROM THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: Among women with lymphomas diagnosed during pregnancy, the 3-year progression-free survival rates were 76% in women who underwent immediate treatment and 79% for those who deferred it until after delivery. Respective overall survival rates were 92% and 83%.
Data Source: Retrospective analysis of 82 cases from nine academic health centers.
Disclosures: The study was funded by the participating centers. The authors reported no relevant conflicts of interest.
Preterm Delivery a Risk in Systemic Sclerosis
Many women with systemic sclerosis can have successful pregnancies, but the rates of preterm birth, low birth weight, and intrauterine growth restriction are approximately twice as high in these women compared to the general population of pregnant women, based on data from 109 pregnancies in 99 women with systemic sclerosis.
The findings were published in Arthritis & Rheumatism (Arthritis Rheum. 2011 Dec. 28 [doi:10.1002/art.34350]).
Data from previous studies have suggested negative outcomes for pregnancies in women with systemic sclerosis (SSc), but these have been small case series or large database reviews that did not allow for the identification of individual patients, said Dr. Mara Taraborelli of Spedali Civili and University, Brescia, Italy, and colleagues.
In this prospective study, the researchers followed 99 women with SSc who had 109 pregnancies between 2000 and 2011. The women attended one of 25 participating research centers in Italy.
The average age at conception was 32 years, and most of the women were white. A total of 107 pregnancies were spontaneous, and 2 were achieved with assisted reproductive techniques.
Preterm deliveries were significantly more common in the SSc women, compared to the general obstetric population that served as a control group (25% vs. 12%, respectively). Severe preterm delivery (defined as delivery at less than 34 weeks) also was significantly more common in SSc women, compared to the controls (10% vs. 5%, respectively).
In addition, very low birth weight babies and cases of intrauterine growth restriction were significantly more common in the SSc women, compared to the controls (5% vs. 1%, respectively, and 6% vs. 1%, respectively).
The researchers found no increase in hypertensive disorders of pregnancy or spontaneous pregnancy losses in SSc women, compared to the general pregnant population.
"We observed a low rate of disease progression shortly after the end of pregnancy; this risk might be greater in aSCL-70 positive patients with recent-onset disease," the researchers noted. All four cases of internal organ disease evolution within 12 months after delivery occurred in women who were aSCL-70 positive, and 3 of 23 (13%) of women who were aSCL-70 positive whose disease had lasted less than 3 years had some disease progression after delivery.
A total of six newborns spent a median of 15 days in the intensive care unit. Of these, one was severely premature and died of multi-organ failure.
The study findings were limited by the use of retrospective analysis and the use of controls for only one year, but the results suggest that successful pregnancies are possible for SSc women despite the increased risks for poor maternal and fetal outcomes, with multidisciplinary management, the researchers said. However, pregnancy may not be advisable for patients with severe organ damage or recent onset of SSc, especially those who are antitopoisomerase positive, they added.
The researchers had no financial conflicts to disclose. The study was supported in part by three patients’ associations: the Gruppo Italiano Lotta alla Sclerodermia, Gruppo Lupus Eritematoso Sistemico Lombardia, and the Associazone Lombarda Malati Reumatici.
Findings from this study and other studies on pregnancy in women with systemic sclerosis are important because many affected patients are in their child-bearing years.
This is one of several studies on the topic of SSc and pregnancy, and findings from the other studies do not agree fully with these data. Findings from other studies have shown that the babies of women with SSc are more likely to have low weight for gestational age. Also, data from previous studies have shown that patients with severe SSc disease, just like all patients with any illness in the connective tissue disease realm, need to be very cautious when considering pregnancy. As always, good communication between the patient and physician and good clinical judgment are paramount.
When physicians counsel patients about pregnancy, the discussion should include specifics of cardiac, pulmonary, gastrointestinal, and renal involvement as they relate to the patient’s condition. Other issues include family history, whether there are other children in the family, what kind of support systems the patient has, other medications needed to control disease, and the psychological status of the individual patient.
As for avenues for further research, larger prospective data sets are needed, including data on patients with concomitant illnesses, different medications, serologies, microchimerism, physiology, and genetics when possible. Of course, data are needed on both the short-term and long-term outcomes of the children as well as the mothers. In cases of poor outcomes, studies of tissue are warranted.
Dr. Daniel E. Furst is the Carl M. Pearson professor in rheumatology at the University of California, Los Angeles and a member of the Rheumatology News Editorial Advisory Board.
Dr. Furst has received research grants from multiple companies including Bristol-Myers Squibb, Celgene, Genentech, National Institutes of Health, and UCB. He has served as a consultant for multiple companies including Abbott, Bristol-Myers Squibb, Centocor, Novartis, and Xoma. He has served on the speaker's bureau for Abbott and Genentech, and has received honoraria from Abbott, Actelion, Bristol-Myers Squibb, Genentech, Encysive, and UCB.
Findings from this study and other studies on pregnancy in women with systemic sclerosis are important because many affected patients are in their child-bearing years.
This is one of several studies on the topic of SSc and pregnancy, and findings from the other studies do not agree fully with these data. Findings from other studies have shown that the babies of women with SSc are more likely to have low weight for gestational age. Also, data from previous studies have shown that patients with severe SSc disease, just like all patients with any illness in the connective tissue disease realm, need to be very cautious when considering pregnancy. As always, good communication between the patient and physician and good clinical judgment are paramount.
When physicians counsel patients about pregnancy, the discussion should include specifics of cardiac, pulmonary, gastrointestinal, and renal involvement as they relate to the patient’s condition. Other issues include family history, whether there are other children in the family, what kind of support systems the patient has, other medications needed to control disease, and the psychological status of the individual patient.
As for avenues for further research, larger prospective data sets are needed, including data on patients with concomitant illnesses, different medications, serologies, microchimerism, physiology, and genetics when possible. Of course, data are needed on both the short-term and long-term outcomes of the children as well as the mothers. In cases of poor outcomes, studies of tissue are warranted.
Dr. Daniel E. Furst is the Carl M. Pearson professor in rheumatology at the University of California, Los Angeles and a member of the Rheumatology News Editorial Advisory Board.
Dr. Furst has received research grants from multiple companies including Bristol-Myers Squibb, Celgene, Genentech, National Institutes of Health, and UCB. He has served as a consultant for multiple companies including Abbott, Bristol-Myers Squibb, Centocor, Novartis, and Xoma. He has served on the speaker's bureau for Abbott and Genentech, and has received honoraria from Abbott, Actelion, Bristol-Myers Squibb, Genentech, Encysive, and UCB.
Findings from this study and other studies on pregnancy in women with systemic sclerosis are important because many affected patients are in their child-bearing years.
This is one of several studies on the topic of SSc and pregnancy, and findings from the other studies do not agree fully with these data. Findings from other studies have shown that the babies of women with SSc are more likely to have low weight for gestational age. Also, data from previous studies have shown that patients with severe SSc disease, just like all patients with any illness in the connective tissue disease realm, need to be very cautious when considering pregnancy. As always, good communication between the patient and physician and good clinical judgment are paramount.
When physicians counsel patients about pregnancy, the discussion should include specifics of cardiac, pulmonary, gastrointestinal, and renal involvement as they relate to the patient’s condition. Other issues include family history, whether there are other children in the family, what kind of support systems the patient has, other medications needed to control disease, and the psychological status of the individual patient.
As for avenues for further research, larger prospective data sets are needed, including data on patients with concomitant illnesses, different medications, serologies, microchimerism, physiology, and genetics when possible. Of course, data are needed on both the short-term and long-term outcomes of the children as well as the mothers. In cases of poor outcomes, studies of tissue are warranted.
Dr. Daniel E. Furst is the Carl M. Pearson professor in rheumatology at the University of California, Los Angeles and a member of the Rheumatology News Editorial Advisory Board.
Dr. Furst has received research grants from multiple companies including Bristol-Myers Squibb, Celgene, Genentech, National Institutes of Health, and UCB. He has served as a consultant for multiple companies including Abbott, Bristol-Myers Squibb, Centocor, Novartis, and Xoma. He has served on the speaker's bureau for Abbott and Genentech, and has received honoraria from Abbott, Actelion, Bristol-Myers Squibb, Genentech, Encysive, and UCB.
Many women with systemic sclerosis can have successful pregnancies, but the rates of preterm birth, low birth weight, and intrauterine growth restriction are approximately twice as high in these women compared to the general population of pregnant women, based on data from 109 pregnancies in 99 women with systemic sclerosis.
The findings were published in Arthritis & Rheumatism (Arthritis Rheum. 2011 Dec. 28 [doi:10.1002/art.34350]).
Data from previous studies have suggested negative outcomes for pregnancies in women with systemic sclerosis (SSc), but these have been small case series or large database reviews that did not allow for the identification of individual patients, said Dr. Mara Taraborelli of Spedali Civili and University, Brescia, Italy, and colleagues.
In this prospective study, the researchers followed 99 women with SSc who had 109 pregnancies between 2000 and 2011. The women attended one of 25 participating research centers in Italy.
The average age at conception was 32 years, and most of the women were white. A total of 107 pregnancies were spontaneous, and 2 were achieved with assisted reproductive techniques.
Preterm deliveries were significantly more common in the SSc women, compared to the general obstetric population that served as a control group (25% vs. 12%, respectively). Severe preterm delivery (defined as delivery at less than 34 weeks) also was significantly more common in SSc women, compared to the controls (10% vs. 5%, respectively).
In addition, very low birth weight babies and cases of intrauterine growth restriction were significantly more common in the SSc women, compared to the controls (5% vs. 1%, respectively, and 6% vs. 1%, respectively).
The researchers found no increase in hypertensive disorders of pregnancy or spontaneous pregnancy losses in SSc women, compared to the general pregnant population.
"We observed a low rate of disease progression shortly after the end of pregnancy; this risk might be greater in aSCL-70 positive patients with recent-onset disease," the researchers noted. All four cases of internal organ disease evolution within 12 months after delivery occurred in women who were aSCL-70 positive, and 3 of 23 (13%) of women who were aSCL-70 positive whose disease had lasted less than 3 years had some disease progression after delivery.
A total of six newborns spent a median of 15 days in the intensive care unit. Of these, one was severely premature and died of multi-organ failure.
The study findings were limited by the use of retrospective analysis and the use of controls for only one year, but the results suggest that successful pregnancies are possible for SSc women despite the increased risks for poor maternal and fetal outcomes, with multidisciplinary management, the researchers said. However, pregnancy may not be advisable for patients with severe organ damage or recent onset of SSc, especially those who are antitopoisomerase positive, they added.
The researchers had no financial conflicts to disclose. The study was supported in part by three patients’ associations: the Gruppo Italiano Lotta alla Sclerodermia, Gruppo Lupus Eritematoso Sistemico Lombardia, and the Associazone Lombarda Malati Reumatici.
Many women with systemic sclerosis can have successful pregnancies, but the rates of preterm birth, low birth weight, and intrauterine growth restriction are approximately twice as high in these women compared to the general population of pregnant women, based on data from 109 pregnancies in 99 women with systemic sclerosis.
The findings were published in Arthritis & Rheumatism (Arthritis Rheum. 2011 Dec. 28 [doi:10.1002/art.34350]).
Data from previous studies have suggested negative outcomes for pregnancies in women with systemic sclerosis (SSc), but these have been small case series or large database reviews that did not allow for the identification of individual patients, said Dr. Mara Taraborelli of Spedali Civili and University, Brescia, Italy, and colleagues.
In this prospective study, the researchers followed 99 women with SSc who had 109 pregnancies between 2000 and 2011. The women attended one of 25 participating research centers in Italy.
The average age at conception was 32 years, and most of the women were white. A total of 107 pregnancies were spontaneous, and 2 were achieved with assisted reproductive techniques.
Preterm deliveries were significantly more common in the SSc women, compared to the general obstetric population that served as a control group (25% vs. 12%, respectively). Severe preterm delivery (defined as delivery at less than 34 weeks) also was significantly more common in SSc women, compared to the controls (10% vs. 5%, respectively).
In addition, very low birth weight babies and cases of intrauterine growth restriction were significantly more common in the SSc women, compared to the controls (5% vs. 1%, respectively, and 6% vs. 1%, respectively).
The researchers found no increase in hypertensive disorders of pregnancy or spontaneous pregnancy losses in SSc women, compared to the general pregnant population.
"We observed a low rate of disease progression shortly after the end of pregnancy; this risk might be greater in aSCL-70 positive patients with recent-onset disease," the researchers noted. All four cases of internal organ disease evolution within 12 months after delivery occurred in women who were aSCL-70 positive, and 3 of 23 (13%) of women who were aSCL-70 positive whose disease had lasted less than 3 years had some disease progression after delivery.
A total of six newborns spent a median of 15 days in the intensive care unit. Of these, one was severely premature and died of multi-organ failure.
The study findings were limited by the use of retrospective analysis and the use of controls for only one year, but the results suggest that successful pregnancies are possible for SSc women despite the increased risks for poor maternal and fetal outcomes, with multidisciplinary management, the researchers said. However, pregnancy may not be advisable for patients with severe organ damage or recent onset of SSc, especially those who are antitopoisomerase positive, they added.
The researchers had no financial conflicts to disclose. The study was supported in part by three patients’ associations: the Gruppo Italiano Lotta alla Sclerodermia, Gruppo Lupus Eritematoso Sistemico Lombardia, and the Associazone Lombarda Malati Reumatici.
FROM ARTHRITIS & RHEUMATISM
Major Finding: Preterm deliveries were twice as common in pregnant women with systemic sclerosis, compared with pregnant women in the general population (25% vs. 12%, respectively).
Data Source: A prospective study of 99 women with systemic sclerosis.
Disclosures: The researchers had no financial conflicts to disclose. The study was supported in part by three patients’ associations: the Gruppo Italiano Lotta alla Sclerodermia, Gruppo Lupus Eritematoso Sistemico Lombardia, and the Associazone Lombarda Malati Reumatici.
5 tips for talking to patients about postpartum sexuality
Related article How to prepare your patient for the many nuances of postpartum sexuality (January 2012)
Related article How to prepare your patient for the many nuances of postpartum sexuality (January 2012)
Related article How to prepare your patient for the many nuances of postpartum sexuality (January 2012)
Data on liability claims offer bright spots for ObGyns— and sobering statistics
Good news on the medical liability front, Doctor!
Yes, that’s right, good news.
According to data from the Physician Insurers Association of America (PIAA), the number of claims that were paid in the ObGyn category between 2006 and 2011 was 44% lower than the number of claims paid between 1986 and 1991.
The percentage of claims that were paid also decreased over the same quarter century. During 1986–1990, 37.25% of all claims were paid in the ObGyn category, compared with 31.73% during 2006–2010.1
And when claims for both periods are calculated in 2010 dollars, the amount paid also declined—by more than $138 million!
These are three of the findings that reflect “significant improvement” in obstetrics and gynecology in medical liability, says John B. Stanchfield, MD, an endocrinologist who, for 25 years, was medical director of the Utah Medical Insurance Association (UMIA)—a member company of PIAA. PIAA is the insurance industry trade association.
PIAA member companies in the United States “include large national insurance companies, mid-size regional writers, single-state insurers, and specialty companies that serve specific health care–provider niche markets. Collectively, these companies provide insurance protection to more than 60% of America’s private practice physicians and write approximately 46%, or $5.2 billion, of the total industry premium.”2
The improvement in ObGyn comes as no surprise to Dr. Stanchfield because the specialty was “the first group that got ‘risk-managed’ almost universally across the country,” he says. “In our little company out here in Utah, in 1985, we were told that if we didn’t do something [about lawsuits in obstetrics and gynecology], we weren’t going to be able to insure that class anymore because we wouldn’t be able to collect enough money. That’s what the actuaries told us, but it wasn’t unique to us—it was a nationwide problem.”
Why was the ObGyn specialty, in particular, in need of aggressive risk management? What made ObGyn claims unique?
“It’s infant injury,” says Dr. Stanchfield. “It’s injury to the baby. Those claims, you start talking at a million dollars.”
Another reason may be that some claims in this specialty category involve doctors other than ObGyns who provide obstetric care—for example, family practice physicians.

A risk manager’s perspective
After receiving the warning about ObGyn claims, UMIA got busy. First, it formed a committee comprising perinatologists, ObGyns, family practice physicians, claims specialists, and attorneys. “We analyzed all claims that were paid, looking for common denominators,” says Dr. Stanchfield. Improvement was clearly needed in about 10 areas, so “we basically created a risk-management program and then mandated it.” In the process, the organization published a booklet entitled Insurance Recommendations for Obstetrical Practice, of which Dr. Stanchfield was the editor.3
The booklet offers guidance on 10 potential “problem” areas:
- antepartum testing
- hypertension and pregnancy
- operative vaginal delivery
- breech delivery
- oxytocin administration
- vaginal birth after cesarean
- use of misoprostol
- shoulder dystocia
- preterm labor
- hospital standards.
Fewer claims and more physicians
The declining number and percentage of paid claims in obstetrics and gynecology over 25 years may be even more impressive than the figures suggest, says Dr. Stanchfield.
“Gestalt tells me that through the years there are more practicing physicians rather than fewer,” so the denominator is increasing even as these claims are declining—making the decrease “even more powerful.”
The numbers haven’t improved to the same degree in other specialties, Dr. Stanchfield says. “If you look at global data, the decrease in paid claims might have been 10% to 15%. In ObGyn, if you compare the last 5-year block of data with the first 5-year block, the number of paid claims is almost cut in half.”
What brought about this improvement?
“There weren’t any groundbreaking medical or surgical technological advances during this period. It was just doing it better. And the main push to doing it better in this country, in my opinion, is risk management.”
Slicing the data
Now for the not-so-great news: In 2010 alone, more than $55 million was paid out in the ObGyn category for 10 patient conditions. Topping the list were “pregnancy” and “brain-damaged infant.” The $55 million figure represents the money paid for the top 10 most commonly cited conditions in cases closed during 2010 (TABLE 1).
TABLE 1
Claims categorized under the rather broad category of pregnancy usually were placed there because a more appropriate category was lacking, says Dr. Stanchfield. These claims typically involve “things that happen—usually to the baby—that result in a lawsuit other than brain damage per se.” For example, a claim that involved skull fracture without brain damage might fall into this zone, he says.
Problematic procedures
Slicing the data a different way, problems related to the 10 most commonly cited ObGyn procedures cost PIAA companies more than $120 million dollars in 2010—and that figure is only for the top 10.1 The top three procedures, in terms of number of claims closed in 2010, were operative procedures on the uterus, manually assisted delivery, and cesarean delivery (TABLE 2).
TABLE 2
Manually assisted delivery does not include vacuum extraction or forceps delivery, notes Dr. Stanchfield. “Manually assisted delivery is basically standing there like a quarterback and catching the baby.”
Top 10 “medical misadventures”
And another slice of data reveals the 10 most prevalent medical misadventures in the ObGyn specialty in 2010 (TABLE 3):
- improper performance of a procedure
- no medical misadventure (i.e., no misadventure was identifiable)
- errors in diagnosis
- failure to supervise or monitor a case
- delay in performance of a procedure
- failure to recognize a complication
- surgical foreign body left in a patient after a procedure
- necessary treatment or management was “not performed”
- failure to instruct or communicate with a patient
- medication errors.
The total indemnity paid for these so-called misadventures was more than $136 million.1
TABLE 3
Putting the dollars in perspective
PIAA also collects data on the number of claims reported, and indemnity dollars paid, for other specialties.
“Of the 28 specialty groups included in the database, ObGyn ranks second”—behind internal medicine—“in the number of claims closed between 1985 and 2010,” a PIAA report notes. The ObGyn specialty also ranks second—behind dentists—in the percentage (35%) of those claims that were paid (for dentists, the figure was 46%). Obstetrics and gynecology was also responsible for the single largest indemnity payment—$13,000,000.1
Medical liability: A national disaster?
According to figures from the PIAA Data Sharing Project, an ongoing claim study that includes 22 PIAA member companies, $19.7 billion in losses (total indemnity plus expenses) were reported during the period from 1985 through 2008. Those losses represented approximately 25% of the physicians who were practicing during that time.
“So if you multiply that $19.7 billion figure by four”—to extrapolate it to the full spectrum of physicians practicing between 1985 and 2008—“you’ve got almost $80 billion coming out of the pockets of the doctors in this country,” says Dr. Stanchfield. If you compare that $80 billion figure to the World Trade Center disaster, which involved approximately $42 billion in losses, the need for federal tort reform is highlighted, he says. In 24 years, the physicians “in this country have paid for almost two World Trade Center disasters. That’s an incredible dollar cost.”
From Dr. Stanchfield’s perspective as a risk manager, the best thing physicians can do to protect themselves is to practice medicine wisely.
“One of our speakers used to say, ‘Look, just practice good, middle-of-the-road medicine. Don’t get yourself out on the fringes where you’re doing something questionable. Just practice rock-solid, conservative, safe medicine.’”
We want to hear from you! Tell us what you think.
1. Physician Insurers Association of America. 2011 Risk Management Review. Rockville, Md: PIAA; 2011.
2. Physician Insurers Association of America. PIAA Backgrounder. Rockville, Md: PIAA; 2011.
3. Stanchfied JB, ed. Insurance Recommendations for Obstetrical Practice. Revised ed. Salt Lake City, Utah: Utah Medical Insurance Association; 2009.
Good news on the medical liability front, Doctor!
Yes, that’s right, good news.
According to data from the Physician Insurers Association of America (PIAA), the number of claims that were paid in the ObGyn category between 2006 and 2011 was 44% lower than the number of claims paid between 1986 and 1991.
The percentage of claims that were paid also decreased over the same quarter century. During 1986–1990, 37.25% of all claims were paid in the ObGyn category, compared with 31.73% during 2006–2010.1
And when claims for both periods are calculated in 2010 dollars, the amount paid also declined—by more than $138 million!
These are three of the findings that reflect “significant improvement” in obstetrics and gynecology in medical liability, says John B. Stanchfield, MD, an endocrinologist who, for 25 years, was medical director of the Utah Medical Insurance Association (UMIA)—a member company of PIAA. PIAA is the insurance industry trade association.
PIAA member companies in the United States “include large national insurance companies, mid-size regional writers, single-state insurers, and specialty companies that serve specific health care–provider niche markets. Collectively, these companies provide insurance protection to more than 60% of America’s private practice physicians and write approximately 46%, or $5.2 billion, of the total industry premium.”2
The improvement in ObGyn comes as no surprise to Dr. Stanchfield because the specialty was “the first group that got ‘risk-managed’ almost universally across the country,” he says. “In our little company out here in Utah, in 1985, we were told that if we didn’t do something [about lawsuits in obstetrics and gynecology], we weren’t going to be able to insure that class anymore because we wouldn’t be able to collect enough money. That’s what the actuaries told us, but it wasn’t unique to us—it was a nationwide problem.”
Why was the ObGyn specialty, in particular, in need of aggressive risk management? What made ObGyn claims unique?
“It’s infant injury,” says Dr. Stanchfield. “It’s injury to the baby. Those claims, you start talking at a million dollars.”
Another reason may be that some claims in this specialty category involve doctors other than ObGyns who provide obstetric care—for example, family practice physicians.

A risk manager’s perspective
After receiving the warning about ObGyn claims, UMIA got busy. First, it formed a committee comprising perinatologists, ObGyns, family practice physicians, claims specialists, and attorneys. “We analyzed all claims that were paid, looking for common denominators,” says Dr. Stanchfield. Improvement was clearly needed in about 10 areas, so “we basically created a risk-management program and then mandated it.” In the process, the organization published a booklet entitled Insurance Recommendations for Obstetrical Practice, of which Dr. Stanchfield was the editor.3
The booklet offers guidance on 10 potential “problem” areas:
- antepartum testing
- hypertension and pregnancy
- operative vaginal delivery
- breech delivery
- oxytocin administration
- vaginal birth after cesarean
- use of misoprostol
- shoulder dystocia
- preterm labor
- hospital standards.
Fewer claims and more physicians
The declining number and percentage of paid claims in obstetrics and gynecology over 25 years may be even more impressive than the figures suggest, says Dr. Stanchfield.
“Gestalt tells me that through the years there are more practicing physicians rather than fewer,” so the denominator is increasing even as these claims are declining—making the decrease “even more powerful.”
The numbers haven’t improved to the same degree in other specialties, Dr. Stanchfield says. “If you look at global data, the decrease in paid claims might have been 10% to 15%. In ObGyn, if you compare the last 5-year block of data with the first 5-year block, the number of paid claims is almost cut in half.”
What brought about this improvement?
“There weren’t any groundbreaking medical or surgical technological advances during this period. It was just doing it better. And the main push to doing it better in this country, in my opinion, is risk management.”
Slicing the data
Now for the not-so-great news: In 2010 alone, more than $55 million was paid out in the ObGyn category for 10 patient conditions. Topping the list were “pregnancy” and “brain-damaged infant.” The $55 million figure represents the money paid for the top 10 most commonly cited conditions in cases closed during 2010 (TABLE 1).
TABLE 1
Claims categorized under the rather broad category of pregnancy usually were placed there because a more appropriate category was lacking, says Dr. Stanchfield. These claims typically involve “things that happen—usually to the baby—that result in a lawsuit other than brain damage per se.” For example, a claim that involved skull fracture without brain damage might fall into this zone, he says.
Problematic procedures
Slicing the data a different way, problems related to the 10 most commonly cited ObGyn procedures cost PIAA companies more than $120 million dollars in 2010—and that figure is only for the top 10.1 The top three procedures, in terms of number of claims closed in 2010, were operative procedures on the uterus, manually assisted delivery, and cesarean delivery (TABLE 2).
TABLE 2
Manually assisted delivery does not include vacuum extraction or forceps delivery, notes Dr. Stanchfield. “Manually assisted delivery is basically standing there like a quarterback and catching the baby.”
Top 10 “medical misadventures”
And another slice of data reveals the 10 most prevalent medical misadventures in the ObGyn specialty in 2010 (TABLE 3):
- improper performance of a procedure
- no medical misadventure (i.e., no misadventure was identifiable)
- errors in diagnosis
- failure to supervise or monitor a case
- delay in performance of a procedure
- failure to recognize a complication
- surgical foreign body left in a patient after a procedure
- necessary treatment or management was “not performed”
- failure to instruct or communicate with a patient
- medication errors.
The total indemnity paid for these so-called misadventures was more than $136 million.1
TABLE 3
Putting the dollars in perspective
PIAA also collects data on the number of claims reported, and indemnity dollars paid, for other specialties.
“Of the 28 specialty groups included in the database, ObGyn ranks second”—behind internal medicine—“in the number of claims closed between 1985 and 2010,” a PIAA report notes. The ObGyn specialty also ranks second—behind dentists—in the percentage (35%) of those claims that were paid (for dentists, the figure was 46%). Obstetrics and gynecology was also responsible for the single largest indemnity payment—$13,000,000.1
Medical liability: A national disaster?
According to figures from the PIAA Data Sharing Project, an ongoing claim study that includes 22 PIAA member companies, $19.7 billion in losses (total indemnity plus expenses) were reported during the period from 1985 through 2008. Those losses represented approximately 25% of the physicians who were practicing during that time.
“So if you multiply that $19.7 billion figure by four”—to extrapolate it to the full spectrum of physicians practicing between 1985 and 2008—“you’ve got almost $80 billion coming out of the pockets of the doctors in this country,” says Dr. Stanchfield. If you compare that $80 billion figure to the World Trade Center disaster, which involved approximately $42 billion in losses, the need for federal tort reform is highlighted, he says. In 24 years, the physicians “in this country have paid for almost two World Trade Center disasters. That’s an incredible dollar cost.”
From Dr. Stanchfield’s perspective as a risk manager, the best thing physicians can do to protect themselves is to practice medicine wisely.
“One of our speakers used to say, ‘Look, just practice good, middle-of-the-road medicine. Don’t get yourself out on the fringes where you’re doing something questionable. Just practice rock-solid, conservative, safe medicine.’”
We want to hear from you! Tell us what you think.
Good news on the medical liability front, Doctor!
Yes, that’s right, good news.
According to data from the Physician Insurers Association of America (PIAA), the number of claims that were paid in the ObGyn category between 2006 and 2011 was 44% lower than the number of claims paid between 1986 and 1991.
The percentage of claims that were paid also decreased over the same quarter century. During 1986–1990, 37.25% of all claims were paid in the ObGyn category, compared with 31.73% during 2006–2010.1
And when claims for both periods are calculated in 2010 dollars, the amount paid also declined—by more than $138 million!
These are three of the findings that reflect “significant improvement” in obstetrics and gynecology in medical liability, says John B. Stanchfield, MD, an endocrinologist who, for 25 years, was medical director of the Utah Medical Insurance Association (UMIA)—a member company of PIAA. PIAA is the insurance industry trade association.
PIAA member companies in the United States “include large national insurance companies, mid-size regional writers, single-state insurers, and specialty companies that serve specific health care–provider niche markets. Collectively, these companies provide insurance protection to more than 60% of America’s private practice physicians and write approximately 46%, or $5.2 billion, of the total industry premium.”2
The improvement in ObGyn comes as no surprise to Dr. Stanchfield because the specialty was “the first group that got ‘risk-managed’ almost universally across the country,” he says. “In our little company out here in Utah, in 1985, we were told that if we didn’t do something [about lawsuits in obstetrics and gynecology], we weren’t going to be able to insure that class anymore because we wouldn’t be able to collect enough money. That’s what the actuaries told us, but it wasn’t unique to us—it was a nationwide problem.”
Why was the ObGyn specialty, in particular, in need of aggressive risk management? What made ObGyn claims unique?
“It’s infant injury,” says Dr. Stanchfield. “It’s injury to the baby. Those claims, you start talking at a million dollars.”
Another reason may be that some claims in this specialty category involve doctors other than ObGyns who provide obstetric care—for example, family practice physicians.

A risk manager’s perspective
After receiving the warning about ObGyn claims, UMIA got busy. First, it formed a committee comprising perinatologists, ObGyns, family practice physicians, claims specialists, and attorneys. “We analyzed all claims that were paid, looking for common denominators,” says Dr. Stanchfield. Improvement was clearly needed in about 10 areas, so “we basically created a risk-management program and then mandated it.” In the process, the organization published a booklet entitled Insurance Recommendations for Obstetrical Practice, of which Dr. Stanchfield was the editor.3
The booklet offers guidance on 10 potential “problem” areas:
- antepartum testing
- hypertension and pregnancy
- operative vaginal delivery
- breech delivery
- oxytocin administration
- vaginal birth after cesarean
- use of misoprostol
- shoulder dystocia
- preterm labor
- hospital standards.
Fewer claims and more physicians
The declining number and percentage of paid claims in obstetrics and gynecology over 25 years may be even more impressive than the figures suggest, says Dr. Stanchfield.
“Gestalt tells me that through the years there are more practicing physicians rather than fewer,” so the denominator is increasing even as these claims are declining—making the decrease “even more powerful.”
The numbers haven’t improved to the same degree in other specialties, Dr. Stanchfield says. “If you look at global data, the decrease in paid claims might have been 10% to 15%. In ObGyn, if you compare the last 5-year block of data with the first 5-year block, the number of paid claims is almost cut in half.”
What brought about this improvement?
“There weren’t any groundbreaking medical or surgical technological advances during this period. It was just doing it better. And the main push to doing it better in this country, in my opinion, is risk management.”
Slicing the data
Now for the not-so-great news: In 2010 alone, more than $55 million was paid out in the ObGyn category for 10 patient conditions. Topping the list were “pregnancy” and “brain-damaged infant.” The $55 million figure represents the money paid for the top 10 most commonly cited conditions in cases closed during 2010 (TABLE 1).
TABLE 1
Claims categorized under the rather broad category of pregnancy usually were placed there because a more appropriate category was lacking, says Dr. Stanchfield. These claims typically involve “things that happen—usually to the baby—that result in a lawsuit other than brain damage per se.” For example, a claim that involved skull fracture without brain damage might fall into this zone, he says.
Problematic procedures
Slicing the data a different way, problems related to the 10 most commonly cited ObGyn procedures cost PIAA companies more than $120 million dollars in 2010—and that figure is only for the top 10.1 The top three procedures, in terms of number of claims closed in 2010, were operative procedures on the uterus, manually assisted delivery, and cesarean delivery (TABLE 2).
TABLE 2
Manually assisted delivery does not include vacuum extraction or forceps delivery, notes Dr. Stanchfield. “Manually assisted delivery is basically standing there like a quarterback and catching the baby.”
Top 10 “medical misadventures”
And another slice of data reveals the 10 most prevalent medical misadventures in the ObGyn specialty in 2010 (TABLE 3):
- improper performance of a procedure
- no medical misadventure (i.e., no misadventure was identifiable)
- errors in diagnosis
- failure to supervise or monitor a case
- delay in performance of a procedure
- failure to recognize a complication
- surgical foreign body left in a patient after a procedure
- necessary treatment or management was “not performed”
- failure to instruct or communicate with a patient
- medication errors.
The total indemnity paid for these so-called misadventures was more than $136 million.1
TABLE 3
Putting the dollars in perspective
PIAA also collects data on the number of claims reported, and indemnity dollars paid, for other specialties.
“Of the 28 specialty groups included in the database, ObGyn ranks second”—behind internal medicine—“in the number of claims closed between 1985 and 2010,” a PIAA report notes. The ObGyn specialty also ranks second—behind dentists—in the percentage (35%) of those claims that were paid (for dentists, the figure was 46%). Obstetrics and gynecology was also responsible for the single largest indemnity payment—$13,000,000.1
Medical liability: A national disaster?
According to figures from the PIAA Data Sharing Project, an ongoing claim study that includes 22 PIAA member companies, $19.7 billion in losses (total indemnity plus expenses) were reported during the period from 1985 through 2008. Those losses represented approximately 25% of the physicians who were practicing during that time.
“So if you multiply that $19.7 billion figure by four”—to extrapolate it to the full spectrum of physicians practicing between 1985 and 2008—“you’ve got almost $80 billion coming out of the pockets of the doctors in this country,” says Dr. Stanchfield. If you compare that $80 billion figure to the World Trade Center disaster, which involved approximately $42 billion in losses, the need for federal tort reform is highlighted, he says. In 24 years, the physicians “in this country have paid for almost two World Trade Center disasters. That’s an incredible dollar cost.”
From Dr. Stanchfield’s perspective as a risk manager, the best thing physicians can do to protect themselves is to practice medicine wisely.
“One of our speakers used to say, ‘Look, just practice good, middle-of-the-road medicine. Don’t get yourself out on the fringes where you’re doing something questionable. Just practice rock-solid, conservative, safe medicine.’”
We want to hear from you! Tell us what you think.
1. Physician Insurers Association of America. 2011 Risk Management Review. Rockville, Md: PIAA; 2011.
2. Physician Insurers Association of America. PIAA Backgrounder. Rockville, Md: PIAA; 2011.
3. Stanchfied JB, ed. Insurance Recommendations for Obstetrical Practice. Revised ed. Salt Lake City, Utah: Utah Medical Insurance Association; 2009.
1. Physician Insurers Association of America. 2011 Risk Management Review. Rockville, Md: PIAA; 2011.
2. Physician Insurers Association of America. PIAA Backgrounder. Rockville, Md: PIAA; 2011.
3. Stanchfied JB, ed. Insurance Recommendations for Obstetrical Practice. Revised ed. Salt Lake City, Utah: Utah Medical Insurance Association; 2009.
2012 Update on Obstetrics
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7
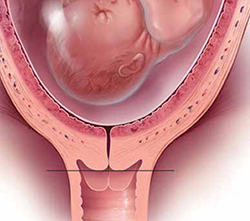
There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7

There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
The authors report no financial relationships relevant to this article.
The year that has followed our inaugural “Update on Obstetrics” [OBG Management, January 2011, available at www.obgmanagement.com] saw a resurgence of interest in a number of aspects of obstetric care. We want to highlight four of them in this Update because we think they are particularly important—given the attention they’ve received in the medical literature and in the consumer media:
- the ever-increasing cesarean delivery rate
- home birth
- postpartum hemorrhage
- measurement of cervical length and the use of progesterone.
Taming the cesarean delivery rate—how can we
accomplish this?
No one should be surprised to learn that the cesarean delivery rate increased nearly sevenfold from 1970 to 2011—from a rate of approximately 5% in 1970 to nearly 35%. Recall that, in the 1990s, the US Public Health Service proposed, as part of Healthy People 2010, a target cesarean rate of 15%, with a vaginal birth after cesarean (VBAC) rate of 60%. Today, the cesarean delivery rate is, as we said, nearly 35% and the VBAC rate is less than 10%.
Many factors have been cited for the rise, including:
- the obesity pandemic
- delaying childbearing
- increasing use of assisted reproduction
- multiple gestation (although the incidence of higher-order multiple gestations is now decreasing, the rate of twin births remains quite high relative to past decades).
So, how did this happen? And what can we do?
For one, VBAC is not likely to gain in popularity. More than 60% of US hospitals that provide OB services handle a volume of fewer than 1,000 deliveries a year. Such low volumes generally will not be able to support (either with dollars or staffing) the resources needed to safely provide VBAC.
Other options have been proposed: Loosen the guidelines for VBAC, change the personnel requirements, gather community groups of doctors, attorneys, and patients to agree on guidelines that, if followed, would protect physicians from being sued1—and so on. The medicolegal reality, however, is that these options have not been shown to be viable. We have concluded that increasing VBAC utilization is not the answer. Rather, addressing ways to prevent primary cesarean delivery holds the most promise for, ultimately, reducing the current rising trend.
On a positive note: The most recent data available from the National Center for Health Statistics suggest that the cesarean delivery rate has dropped slightly: from 32.9% in 2009 to 32.8% in 2010. The drop is truly slight; we’ll watch with interest to see if a trend has begun.
Considering that the cesarean delivery rate in 1970 was 5%, and that the dictum at the time was “once a section, always a section,” it seems clear (to us, at least) that the solution to this problem lies in preventing first cesarean deliveries. How can the specialty and, in some ways, you, in your practice, work toward this goal? Here are possible strategies:
- Eliminate elective inductions of labor when the modified Bishop score is less than 8
- Return to defining “post-term” as 42—not 41—completed weeks’ gestation
- Eliminate all elective inductions before 39 weeks’ gestation
- Provide better and more standardized training of physicians in the interpretation of fetal heart-rate tracings
- Improve communication between obstetricians and anesthesiologists in regard to managing pain during labor
- Institute mandatory review of all cesarean deliveries that are performed in the latent phase of labor and all so-called “stat cesareans”
- Readjust the compensation scale for physicians and hospitals in such a way that successful vaginal delivery is rewarded.
Even if all these measures were implemented, we think it’s unlikely that we will ever see a 5% cesarean delivery rate again—although probably for good reason. But even a return to a more manageable 20% rate seems a reasonable goal.
Home birth: Consider where you stand
We suppose that one way to avoid cesarean delivery would be to deliver at home. The topic, and practice, of home birth has mushroomed in the past few years—for a number of social and economic reasons, probably. It seems to us that there are a few basic issues that must be addressed, however, before it’s possible to come to grips with home birth in the 21st century in an enlightened way:
- In 1935, the maternal mortality rate approached 500 to 600 for every 100,000 births; most of those deaths occurred at home. In 2009, the maternal mortality rate was approximately 8 for every 100,000 births. Both rates are very low, but the difference would be significant to the 492 to 592 women who met a potentially preventable death.
- Methods of identifying who might be an appropriate candidate for a home birth are, at best, imprecise.
- Infrastructure for rapidly transporting mother and baby to a hospital if matters go awry is inadequate.
Although evidence is limited, what data there are suggest that one significant outcome—neonatal death—occurs with higher frequency among home births than among hospital births, even after correcting for anomalies (odds ratio, 2.9 [95% confidence interval, 1.3–6.2]).2 Although women who delivered at home did have fewer episiotomies, fewer third- and fourth-degree perineal lacerations, fewer operative deliveries (vaginal and cesarean) and a lower rate of infection, those reductions seem inconsequential compared to the death of a newborn….
Bottom line? Home birth is legal; home birth may be appropriate for some women who are at low risk and willing to accept a legitimate amount of personal risk; and you, as an OB, are in no way required to participate in or endorse the practice.
Many institutions have addressed this matter by developing a family-centered health care model for obstetrics—so-called hospitals within hospitals—that allow for a less interventionist approach to childbirth within the safety net of a hospital facility, should unforeseeable complications arise. Consider your interest in affiliating with such a facility, based on your acceptance of the practice of home birth and your comfort with being part of this approach.
Formal, systematic planning is key to managing
postpartum hemorrhage
A question for mothers-to-be: What could be worse than having a cesarean delivery in your home?
Answer: Having an associated postpartum bleed.
Perhaps that isn’t the most elegant segue, but postpartum hemorrhage is a significant problem that remains a major contributor to maternal mortality in the United States. And, in fact, a prolonged and unsuccessful labor—the kind that could present to your hospital from an outside birthing facility or home—necessitating a cesarean delivery is a set-up for postpartum hemorrhage.
One of the tenets of emergency management in obstetrics is the three-pronged preparedness of 1) risk identification, 2) foreseeability, and 3) having a plan for taking action. Of late, many institutions have begun to develop a formal plan for managing OB emergencies—in particular, postpartum hemorrhage.
(A note about the potential role of interventional radiology in the management of postpartum hemorrhage: Our experience is limited, but we conjecture that, in most US hospitals that provide OB services, mobilizing an interventional radiology group in an emergency isn’t feasible. That makes it essential to have established medical and surgical management guidelines for such cases.)
To establish a plan on labor and delivery for managing postpartum bleeds, we recommend the following steps and direct you to ACOG’s “Practice Bulletin#76” for more specific information3:
- Establish a list of conditions that predispose a woman to postpartum hemorrhage and post that list throughout labor and delivery to heighten the awareness of team members
- Establish protocols for pharmacotherapeutic intervention—including oxytocin, methylergonovine, misoprostol, and prostaglandin F2a, with dosage and frequency guidelines and algorithms for use—and have those protocols readily available on labor and delivery, either on-line or posted
- Establish an “all-hands-on-deck” protocol for surgical emergencies—actual or potential—that includes what personnel to call and in what order to call them
- Use simulation to practice the all-hands-on-deck protocol and evaluate team and individual performance in managing hemorrhage
- Establish blood product replacement protocols, including order sets for products that are linked to particular diagnoses (e.g., typing and cross-matching for patients coming in to deliver who have a diagnosis of placenta previa; adding products such as fresh frozen plasma and platelets for patients who have complicating diagnoses, such as suspected placenta accreta or severe preeclampsia).
To prevent preterm birth: Cervical length screening
and progesterone
Preterm birth accounts for almost 13% of births in the United States, with spontaneous preterm labor and preterm rupture of membranes accounting for approximately 80% of those cases.4 Once preterm labor has begun, little in the way of successful intervention is possible, beyond short-term prolongation of pregnancy with tocolytic agents to allow for corticosteroid administration. Studies in recent years have, therefore, moved the focus back on prevention, using the same treatments that were used 60 years ago—progesterone supplementation and cerclage—with the addition of transvaginal ultrasonography (US) screening for cervical length.
Several large, randomized trials have examined the use of intramuscular injection or vaginal delivery of progesterone to prevent preterm birth in patients who are at high risk of preterm birth based on their obstetric history.5,6 Both 17a-hydroxyprogesterone caproate and vaginal progesterone suppositories are associated with a significant reduction in the risk of preterm birth in singleton pregnancies. ACOG reconfirmed the value of this finding in a 2011 Committee Opinion, which recommended the use of progesterone supplementation in singleton pregnancies in which there is a history of preterm labor or preterm rupture of membranes.7

There is mounting evidence that cervical length is inversely related to risk of preterm birth.The real question, however, is: What should be done about transvaginal cervical length: Should we be screening, or not? As recently as 2009, a Cochrane Review did not advocate universal screening for cervical length as a predictor for preterm birth4—despite mounting evidence that cervical length is inversely related to risk of preterm birth, with progressively shorter length (starting at <25 mm) associated with significantly higher risk of preterm birth.8,9 Keeping in mind that the decision to screen depends on your ability to treat the condition for which you are screening, what was needed was proof that intervention works.
2011 brought two studies that recommend screening for cervical length based on a successful reduction in preterm birth with a specific intervention. A large, randomized trial of vaginal progesterone gel for the prevention of preterm birth used universal screening for shortened cervical length (10 to 20 mm) as the criterion for randomization to treatment or placebo. The investigators demonstrated a 45% reduction in preterm birth of less than 33 weeks in the treatment arm.10
An interesting aspect of this study: The reduction in preterm birth was not, in fact, seen in patients who had a history of preterm birth, suggesting that this may be a different patient population that benefits from vaginal progesterone.
On the other hand, a recent meta-analysis concluded that patients who meet the criteria of 1) cervical length less than 25 mm and 2) a history of prior spontaneous preterm birth experience a significant reduction in preterm birth and a reduction in perinatal morbidity and mortality if they have cervical cerclage placed.11
Although these publications lead us to hope that there may be some benefit from preventive intervention for preterm birth, the question of how to screen for, and prevent, spontaneous preterm birth remains somewhat nebulous: It hasn’t been determined which patient population will benefit from which combination of screening and intervention. Larger trials for specific populations are still needed.
This is what we know, for now:
- Women who have a history of spontaneous preterm birth should have a thorough evaluation of their OB history to determine possible modifiable risk factors (e.g., smoking, short inter-pregnancy interval) and to determine, as definitively as possible, the likely cause of that preterm birth
- Women who have a singleton pregnancy and a history of either spontaneous preterm labor or preterm rupture of membranes can be offered progesterone supplementation as intramuscular 17a-hydroxyprogesterone or a vaginal preparation to reduce their risk of preterm birth
- Women who have an asymptomatic shortening of the cervix, as measured on transvaginal US at 18 to 24 weeks’ gestation, can be offered vaginal progesterone to reduce their risk of preterm birth
- Women who have a history of preterm birth and cervical shortening may see a reduction in their risk of preterm birth from cerclage placement
- The use of screening for cervical length or progesterone supplementation, or both, in a multiple gestation pregnancy are not recommended because their benefit in this population has not been demonstrated.
Until we fully understand the various etiologic pathways of spontaneous preterm birth, we won’t have a one-size-fits-all solution to this major cause of perinatal morbidity and mortality.
We want to hear from you! Tell us what you think.
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
1. Scott JR. Vaginal birth after cesarean delivery; a common sense approach. Obstet Gynecol. 2011;118(2 Pt 1):342-350.
2. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425-428.
3. American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG Practice Bulletin No. 76: Postpartum hemorrhage. Obstet Gynecol. 2006;108(4):1039-1047.
4. Berghella V, Baxter JK, Hendrix NW. Cervical assessment by ultrasound for preventing preterm delivery (Review). Cochrane Database Syst Rev. 2009;(3):CD007235.-
5. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188(2):419-424.
6. Meis PJ, Klebanoff M, Thom E, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379-2385.
7. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 419: Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112(4):963-935.
8. Owen J, Yost N, Berghella V, et al:. National Institute of Child Health and Human Development, Maternal-Fetal Medicine Units Network. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286(11):1340-1348.
9. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3):1170-1174.
10. Hassan SS, Romero R, Vidyadhari D, et al. PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18-31.
11. Berghella V, Rafael TJ, Szychowski M, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth. Obstet Gynecol. 2011;117(3):663-771
How to prepare your patient for the many nuances of postpartum sexuality
CASE: Waiting for an OK to resume sex
L. L. is a 29-year-old woman, G1P1, who delivered a healthy infant 4 weeks ago by spontaneous vaginal birth. The delivery involved a 2-day induction of labor for preeclampsia and a second-degree tear that was repaired without complication. The patient also experienced postpartum hemorrhage that was managed with bimanual massage and uterotonics and for which she ultimately required transfusion of blood products. Her hospital course was otherwise unremarkable.
Before pregnancy, L. L. had a normal medical history and conceived spontaneously. Her antenatal course was uncomplicated.
Today, she returns for her postpartum visit. She reports being tired and says she still has some pain at the site of the tear, but reports no problems with urinary or fecal continence. She denies being depressed, and her Edinburgh Postnatal Depression Scale (EPDS) score is consistent with that report. She is breastfeeding and appears to be doing well on the progestin-only pill for contraception. She has not yet attempted intercourse because she is complying with instructions to wait until she sees you for her postpartum visit.
How should you counsel her about resuming sexual activity?

Childbirth is a central event in a woman’s life. Pregnancy and delivery are a time of psychological, biological, and physical transformation, and the postpartum period—the “fourth trimester”—is no exception. Sexual function may be affected. In fact, many women who seek assistance for sexual dissatisfaction note that their problem arose in the postpartum period.1
Postpartum sexuality involves considerably more than the physical act of genital stimulation—with or without intromission or penile penetration—and depends on more than the physical state of recovery of the vagina (after vaginal delivery). It also depends on:
- the woman’s sexual drive and motivation
- her general state of health and quality of life
- her emotional readiness to resume sexual intimacy with a partner
- her adaptation to the maternal role and ability to balance her identity as a mother with her identity as a sexual being
- her relationship with her partner.
Given all these contributing factors, many of which fall outside the scope of the clinical practice of obstetrics and gynecology, how do we go about counseling our patients about the resumption of sexual activity?
Other questions:
- How can we help patients manage expectations about the quality of their postpartum sexual function?
- What guidance can we provide regarding the interplay of psychosexual and physical aspects of the puerperium?
- Can we offer a method of screening for sexual dysfunction in the puerperium? If so, will it help prevent sexual problems or hasten their resolution?
This article addresses these issues. Ultimately, the answer to the question of when to resume sexual activity should reflect an awareness of cultural norms and taboos as well as familiarity with empirically based recommendations.
Traditional postpartum sexual education is not evidence-based and has limited effectiveness. More up-to-date strategies can be easily incorporated into even the busiest clinical practice. We offer the following counseling model for you to consider when addressing the sexual health of patients postpartum.
Educate, legitimize, and normalize
The first sexual encounter after childbirth can be an important step for couples to reclaim their intimate relationship.
Adaptation to the parental role, physical healing, hormonal changes, breastfeeding, and sleep deprivation contribute to a profound psychosocial challenge. The resumption of sexual activities and a satisfying postpartum sex life depend on many variables, many of which the patient may not even be aware.
First, do not assume that all patients are heterosexual and that intercourse is their only form of sexual activity.
Second, it is important to be proactive in antepartum and postpartum counseling and to offer anticipatory guidance. Counseling can take place any time during routine prenatal care, as well as at the time of hospital discharge and the postpartum visit.
Reassure the patient that, if sexual activity and frequency are lower during pregnancy and the postpartum period, it is likely a normal transition. Also give the patient time to talk about her expectations and perceptions. Explain to her the normal fluctuations and variability of sexual interest and enjoyment in pregnancy and the puerperium, and suggest that she consider alternative options for intimate expression, non-coital sexual activities, and mutual pleasure within her cultural context.
Be thorough
Take a comprehensive medical, obstetric, psychological, and social history as part of the sexual history. Also perform a physical intake and exam. Questions about urinary and fecal incontinence ought to be part of all postpartum assessment.
Other potential areas to address include the quality of the relationship, prepregnancy sexual function, the support network, planned or unplanned state of the pregnancy, previous pregnancy and delivery outcomes, the health status of current children, and present, previous, and future contraceptive use.29
Consider multiple visits
It is hard to know exactly when to evaluate a patient for postpartum sexual dysfunction, given the impact of pudendal nerve latency, fatigue, and breastfeeding. For this reason, assessment on multiple occasions may be appropriate. Numerous validated scales to assess sexual function can be easily incorporated into clinical practice.
Couples counseling and therapy may be needed in some cases; be aware of referral services in your area for sexual wellness specialists.
The bottom line: A “successful” sexual life does not necessarily mean adequate genital function (e.g., coital orgasm, improved clitoral blood flow, increased sexual frequency) but, rather, a sexual life that is intimate and satisfying to the individual patient.
A paucity of research
To date, research into sexuality during the postpartum period has focused primarily on the physical changes and constraints that affect the mechanics and frequency of intercourse and overall sexual satisfaction and desire.2 This perspective has begun to broaden to include the psychological aspects of sexuality.
TABLE 1
These validated tools can help you measure female sexual dysfunction
| Tool | Area assessed |
|---|---|
| Female Sexual Function Index (FSFI)30 | Desire, arousal, orgasm, and pain |
| Female Sexual Function Index 6-Item (FSFI-6)31 | Desire, arousal, orgasm, and pain |
| McCoy Female Sexual Function Questionnaire*32 | Presence of female sexual disorders |
| Brief Sexual Symptoms Checklist33 | Screener for sexual concerns |
| Female Sexual Distress Scale – Revised34 | Distress |
| Intimate Relationship Scale*35 | Changes in sexual relationship |
| Sexual Quality of Life – Female (SQol-F)36 | Quality of life in women with female sexual dysfunction |
| Golombok Rust Inventory of Sexual Satisfaction (GRISS)37 | Quality of sexual relationship |
| Decreased Sexual Desire Screener38 | Brief diagnostic tool for hypoactive sexual desire disorder |
| * Validated in pregnant and/or postpartum women | |
Women’s sexual health during the postpartum period has generally been under-researched. It wasn’t until the past decade that validated sexual function questionnaires were utilized. Although a number of these instruments are now available (TABLE 1, TABLE 2, FIGURE), it remains unclear whether they can accurately measure postpartum sexual function. Despite these limitations, significant information has been elicited that can be used to counsel patients struggling with postpartum sexual concerns.
TABLE 2
The 6-item Female Sexual Function Index*
| Question | Responses | |||||
|---|---|---|---|---|---|---|
| 0 points | 5 points | 4 points | 3 points | 2 points | 1 point | |
| How would you rate your level of sexual desire or interest? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How would you rate your level of sexual arousal (“turn on”) during sexual activity or intercourse? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How often did you become lubricated (“wet”) during sexual activity or intercourse? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| When you had sexual stimulation or intercourse, how often did you reach orgasm? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| How satisfied have you been overall with your sexual life? | No sexual activity | Very satisfied | Moderately satisfied | About equally satisfied and dissatisfied | Moderately dissatisfied | Very dissatisfied |
| How often did you experience discomfort or pain during vaginal penetration? | Did not attempt intercourse | Almost never or never | A few times | Sometimes | Most times | Almost always or always |
| *The components of this index are to be assessed over the past 4 weeks. The score is the sum of the ordinal responses to the 6 items and ranges from 2 to 30. A score of less than 19 indicates a need for further investigation, including the full-length Female Sexual Function Index. Source: Adapted from Isidori et al.31 | ||||||
Ideal period of abstinence is unknown
Although our knowledge of the female genital tract in the puerperium is based upon histologic evidence, there are no evidence-based policies to outline the ideal period of postpartum coital abstinence. It seems reasonable to assume that our traditional scientific recommendations developed in part to prevent uterine infection and disruption of sutured wounds. These concerns, combined with cultural and societal norms, have led to the routine discouragement of sexual activity until 4 to 6 weeks postpartum.
The possibility of shortening the period of postpartum abstinence was first suggested by the American College of Obstetricians and Gynecologists (ACOG) in 1984.1 In 1985, Pritchard and colleagues wrote about the individualization of postpartum prohibitions of sexual activity in Williams Obstetrics.1 The earliest time at which intercourse may be safely resumed is unknown, but the 23rd edition of Williams Obstetrics states that a woman can resume sexual intercourse as early as 2 weeks, based on her comfort and desire.3 The sixth edition of the American Academy of Pediatrics (AAP) and ACOG guidelines for perinatal care also states that the risks ought to be minimal at 2 weeks postpartum.4
BRIEF SEXUAL SYMPTOMS CHECKLIST FOR WOMEN (BSSC-W)

Reprinted from Hatzichristou et al. 33
Low desire is not unusual
Although a patient may be granted “permission” to engage in coital activity, other variables influence her decision. It is well known that sexual desire may fluctuate during pregnancy and typically decreases significantly during the third trimester.2 Many women enter the postpartum period with lower levels of sexual desire and satisfaction, and these depressed levels may continue for some time.2 Twenty-five percent of women report worsened sexual function, including diminished sexual satisfaction, during pregnancy that persists for 6 to 12 months postpartum.5 By 12 weeks postpartum, 80% to 93% of women have resumed intercourse, but as many as 83% report sexual problems during the first 3 months of the postpartum period. At 6 months, 18% to 30% of these women may still be experiencing sexual problems, including dyspareunia.5,6
In 1998, von Sydow performed a meta-content analysis of all existing studies on parental sexuality during pregnancy and the first 6 months postpartum.7 Using psychological and medical data banks, she brought together information from two branches of science and identified 59 relevant studies in English or German between 1950 and 1996. Although the majority of studies were retrospective and failed to utilize a validated instrument, von Sydow determined that, overall, sexual interest and activity were low or nonexistent during the first months after delivery. There was high variability between individuals, however, and levels of sexual interest and activity of individual women remained relatively constant from the time before pregnancy until 1 year postpartum.7 von Sydow determined that there is great variability in female sexuality during pregnancy and postpartum; this variability may represent fluctuations during this phase of life. She also determined that severe psychosexual and marital problems are much more prevalent in the postpartum period than during pregnancy and persist long after a physical cause can be used as an explanation.7
Fatigue and quality of the relationship have an impact on sexual function
De Judicibus and colleagues identified a broad range of variables that have a detrimental impact on sexuality at 12 weeks postpartum, most particularly:
- marital dissatisfaction
- dyspareunia
- fatigue
- depression
- breastfeeding.2
There is evidence to suggest that the addition of the first child reduces marital quality after the first month postpartum, and this decline in marital satisfaction continues for 6 to 18 months postpartum.2 Witting and coworkers suggested that this decline may represent a transitional phase of parenthood for some couples; data support the positive effects on overall marital satisfaction with the addition of children.8 Women who were more satisfied with their relationships reported higher sexual satisfaction and greater frequency of intercourse.2,8
Fatigue is one of the most common problems women experience during pregnancy and postpartum and is a common reason given for loss of sexual desire and interest, infrequent sexual activity, and lack of enjoyment.5 A high level of exhaustion is found during the first 8 weeks postpartum. Although it declines over the next 6 months, it does not appear to resolve completely in a good number of women.9
Don’t underestimate the impact of obstetric morbidity
Surprisingly, the long-term impact of severe obstetric events on postpartum maternal health is often overlooked. Waterstone and colleagues found that women who have severe obstetric morbidity, such as massive hemorrhage, preeclampsia, sepsis, and uterine rupture, experience significant changes in sexual health and well-being.10 They conducted a prospective cohort study of such women, measuring sexual activity, general health, and postpartum depression. They utilized two validated postnatal questionnaires—the Short Form 36 (SF-36) to measure general health and the EPDS. Women who had uncomplicated pregnancies and childbirth tended to perform well in most SF-36 categories, whereas women who had experienced severe morbidity scored worse in almost every category. These women also reported problems with intercourse. Thirteen percent of women had not resumed sexual relations by 6 to 12 months postpartum; of these women, more than half reported a fear of conceiving as a reason.
The female body undergoes dramatic physiologic, anatomic, and psychological changes immediately following delivery and throughout the restoration of its pre-pregnant state. This fourth trimester usually lasts 6 to 12 weeks.39
Uterus. The uterus undergoes rapid involution after separation of the placenta. By 2 to 4 weeks postpartum, it may no longer be palpable abdominally, and by 6 weeks, it usually has returned to its nonpregnant state and size. Seven to 14 days after delivery, a woman often experiences an episode of heavier vaginal bleeding that corresponds with the sloughing of the placental bed eschar. During this time of involution, myometrial vessels may be 5 mm or larger in diameter.40
Lochia. The postpartum lochia begins to change within days of birth, transitioning through its stages of lochia rubra, serosa, and alba. It decreases by 3 weeks postpartum and is likely completely resolved by 6 weeks.
Prolactin is responsible for lactogenesis. When the prolactin level is maintained through breastfeeding, it depresses ovarian production of estrogen by suppressing pituitary gonadotropin secretion, triggering a period of “steroid starvation” after the loss of estrogen and progesterone production from the placenta.1
Vagina. Early in the postpartum period, the vagina is typically edematous and lax and, as a result of parturition, there may be not only a spontaneous tear or episiotomy that must heal, but superficial small tears that do not require suturing. Ruggae begin to reappear by 3 weeks, and the vaginal epithelium will begin to mature under the influence of estrogen production. Much of this tissue damage is healed by 6 weeks postpartum.
The perception of pregnant and postpartum women’s sexuality varies, based on religious and cultural norms. In some religions and cultures, sexual activity is forbidden for 2 to 3 months postpartum; in others, it is prohibited until the child is weaned from the breast. The postpartum woman and lochia have traditionally been perceived as unclean, and many religions have specific proscriptions regarding the management of this time in a woman’s life.1 Although early cultures did not study these issues specifically, their doctrines suggest that they had some awareness of the natural physiologic transition of a woman’s body after she has given birth.
Exploring the role of body image
Paul and coworkers prospectively assessed female sexual function, body image, and pelvic symptoms from the first trimester until 6 months postpartum.11 They utilized the validated questionnaire instruments of the Female Sexual Function Index (FSFI), the Body Exposure during Sexual Activities Questionnaire (BESAQ), the short forms of the Urogenital Distress Inventory (UDI-6), the Incontinence Impact Questionnaire (IIQ-7), and the Fecal Incontinence Quality of Life Scale (FIQOL). They found that sexual activity and sexual function scores were highest before pregnancy, declined between the first and third trimesters, and did not return to pre-pregnancy baselines even by 6 months postpartum.11
Differences in sexual practices contributed to these patterns. Kissing, fondling, and vaginal intercourse remained stable across pregnancy, whereas oral sex, breast stimulation, and masturbation declined in the third trimester.
The decline of these activities during pregnancy and postpartum has been seen in other studies as well.12
Obstacles to sexual activity also changed across pregnancy and the postpartum period. Vaginal pain was more problematic in the third trimester and postpartum, whereas feelings of unattractiveness and issues of body image were present throughout pregnancy and at their worst in the postpartum period. Sexual function scores based on the FSFI declined during pregnancy and did not return to pre-pregnancy or first-trimester levels by 6 months postpartum. Urinary symptoms, as measured by the UDI-6, were associated with lower sexual function scores during the postpartum period. The association between urinary incontinence and sexual dysfunction has been seen in other studies.13,14
The enduring effects of perineal trauma
Childbirth may physically affect a woman’s sexual function through perineal trauma, pudendal neuropathy, and vaginal dryness associated with breastfeeding. There is an obvious connection between perineal laceration and perineal pain and problems with intercourse.5 Overall, dyspareunia is reported by 41% to 67% of women 2 to 3 months after delivery.15 Women who have an episiotomy complain of increased perineal pain and delayed return of sexual activity, compared with women who deliver with an intact perineum.16
Persistent dyspareunia is strongly associated with the severity of perineal trauma and operative vaginal delivery.3,17 Multiple studies have investigated this association and found a positive correlation 3 to 6 months postpartum,6,9,17 but the long-term effects and association remain unclear.18
Findings from research. Rogers and colleagues prospectively studied the effect of perineal trauma on postpartum sexual function in a midwifery population of women who had a low rate of episiotomy and operative vaginal delivery.6 They utilized the Intimate Relationship Scale (IRS), a validated questionnaire to measure postpartum sexual function in couples. Most women in this study had resumed sexual activity by 3 months postpartum and did not have postpartum inactivity or dysfunction, based on their IRS scores. However, women who were identified as having experienced major trauma (second-, third-, or fourth-degree laceration or a repaired first-degree laceration) had significantly less desire to engage in activities such as touching and stroking with their partner.6
Present-day limits on the routine use of episiotomy and operative vaginal delivery have yielded a lower rate of third- and fourth-degree laceration.19 Second-degree lacerations are common and constitute the majority of perineal trauma in births without episiotomy.20 There is evidence that the use of synthetic absorbable suture, such as polyglactin, rather than chromic suture, results in less postpartum perineal pain, as does leaving the well-approximated perineal skin edges unsutured.20
Signorello and coworkers found that second-, third-, and fourth-degree lacerations increased the risk of postpartum dyspareunia; operative vaginal delivery (forceps or vacuum) was also an independent risk factor for dyspareunia.21
The impact of route of delivery
Some researchers have concluded that the route of delivery has an impact on the long-term pelvic floor health of women.18 In 1986, Snooks and colleagues analyzed possible obstetric risk factors for damage to the innervation of the pelvic floor, which can lead to both stress urinary and anorectal incontinence.22 They found that the process of vaginal delivery causes a compression and stretch type of injury to the pudendal nerve, as well as the possibility of severe perineal lacerations. This injury may be less likely to occur when cesarean delivery is performed before labor, avoiding direct perineal trauma and possible pudendal neuropathy.15 Because the pudendal nerve mediates some of the reflex pathways in the female sexual response, it is plausible that damage to it could result in sexual dysfunction.
Women who deliver vaginally have a higher rate of fecal and urinary incontinence than women who deliver by cesarean.16,23 The presence of incontinence, however, does not always have a significant long-term effect on one’s sexual life.6
In the Term Breech Trial, the route of delivery had no impact on the resumption of intercourse, dyspareunia, or sexual satisfaction.23 Although the trial was randomized and controlled, it had many limitations that call its generalizability into question in regard to postpartum sexual dysfunction.
The National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request indicated that, by 6 months postpartum, there is no difference in sexual function based on the route of delivery.24 However, Lydon-Rochelle and colleagues used the SF-36 to assess reported general health status and found that women who had cesarean delivery or assisted vaginal delivery exhibited significantly poorer postpartum functional status than women who had spontaneous vaginal delivery in five areas at 7 weeks postpartum: physical functioning, mental health, general health perception, bodily pain, social functioning, and ability to perform daily activities.25 Women were more likely to be readmitted to the hospital and more likely to report fatigue during the first 2 months after cesarean delivery.9 It appears that women who undergo cesarean delivery have an elevated risk of nondyspareunia-related causes of sexual dysfunction. Any protective effect of cesarean on sexual function is limited to the early postnatal period and is related to the absence of perineal injury.18
How breastfeeding can affect sexual desire
Evidence is strong that breastfeeding reduces a woman’s sexual desire and the frequency of intercourse.1,5 A high level of prolactin suppresses ovarian production of estrogen, thereby reducing vaginal lubrication. Some women and their partner may identify this loss of lubrication as a lack of arousal. This type of vaginal dryness should be explained, and the use of a lubricant should be encouraged in breastfeeding women.
Nipple sensitivity may develop, making touching and foreplay uncomfortable in some women. One third to one half of mothers find breastfeeding to be an erotic experience, and one fourth feel guilty about this sexual excitement; others stop nursing or wean early due to these feelings.1,7 Women are often not educated about the relationship between the release of oxytocin, uterine contractions, milk ejection, sexual arousal, and orgasm; raising the subject can help to diminish any potential distress over this response.
Sleep disturbances from feeding on demand contribute to fatigue and exhaustion.
Many women may not realize that their loss of interest in sex may be because they are receiving sufficient physical contact or touching through their nurturing interactions with the baby. This may leave the partner feeling isolated and envious of the mother-baby relationship.
Couples should be encouraged to discuss these feelings to avoid misperceptions and to maintain the relationship dyad as a priority to prevent the development of relationship problems.
The majority of women will discuss contraception with a health provider, but only 15% will voluntarily discuss their sexual needs or dysfunction.17 This finding is alarming given that, during the postpartum period, two of every three new mothers will experience at least one problem related to sexual function, including dyspareunia, decreased libido, difficulty achieving orgasm, and vaginal dryness.41 This lack of discussion with a health-care provider may be the result of several variables: incomplete knowledge on the part of the provider about what affects sexual function, poor training in the taking of an effective sexual history, and uneasiness on the part of the patient about discussing the issue.5,42
Postnatal depression takes a toll
Depressed mood and emotional lability in the postpartum period are negatively associated with sexual interest, enjoyment, coital activity, and perceived tenderness of the partner.7 Conversely, reduced sexual interest, desire and satisfaction; a lower frequency of intercourse; and later resumption of intercourse are associated with a higher number of psychiatric symptoms in the postpartum period.2 Between 10% and 15% of women experience postpartum depression (PPD).26 Depression has been associated with a decreased frequency and interest in sexual activity at 8 to 12 weeks postpartum.2,5
Chivers and colleagues assessed sexual functioning and sexual behavior in women with and without symptoms of PPD using the FSFI and EPDS. Although theirs was a small study, they found that women who had depressive symptoms also reported poorer functioning in regard to sexual arousal, orgasm, pain, lubrication, and sexual satisfaction.26 Morof and coworkers found that women who had PPD were less likely to have resumed intercourse by 6 months postpartum; they were also less likely to engage in other sexual activities.27
Role of pharmacotherapy
Many women are started on antidepressant medication near the time of delivery or during the immediate postpartum period. Often, serotonin reuptake inhibitors (SRIs) are used because there is minimal transmission of this class of medication through breast milk. However, the potential sexual side effects of these medications should be discussed because they are the agents most commonly associated with female sexual dysfunction.28
For clinicians
American Association of Sex Educators, Counselors, and Therapists – A not-for-profit, interdisciplinary professional organization comprising sexuality educators, sexuality counselors, sex therapists, physicians, social workers, and other clinicians. Its home page links to a referral page and other resources. http://www.aasect.org" target="_blank">http://www.aasect.org
Association of Reproductive Health Professionals offers a resource for clinicians on postpartum counseling about sexuality. http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception" target="_blank">http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception
For patients
Mayo Clinic provides a fact sheet entitled “Sex after pregnancy: Set your own timeline.” http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146" target="_blank">http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146
Sex and a Healthier You – This site offers information for patients on sexuality and relationships. http://www.sexandahealthieryou.org/sex-health/index.html" target="_blank">http://www.sexandahealthieryou.org/sex-health/index.html
We want to hear from you! Tell us what you think.
1. Reamy KJ, White SE. Sexuality in the puerperium: a review. Arch Sex Behav. 1987;16(2):165-186.
2. De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39(2):94-103.
3. The puerperium. In: Cunningham FG Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill Co.; 2010:646-660.
4. The American Academy of Pediatrics (AAP), American College of Obstetricians Gynecologists (ACOG) Guidelines for perinatal care. 6th ed. Washington DC: AAP, ACOG; 2008.
5. Glazener CM. Sexual function after childbirth: women’s experiences persistent morbidity and lack of professional recognition. Br J Obstet Gynaecol. 1997;104(3):330-335.
6. Rogers RG, Borders, N,, Leeman L, Albers L. Does spontaneous genital tract trauma impact postpartum sexual function? J Midwifery Womens Health. 2009;54(2):98-103.
7. von Sydow K. Sexuality during pregnancy and after childbirth: a metacontent analysis of 59 studies. J Psychosom Res. 1999;47(1):27-49.
8. Witting K, Santtila P, Alanko K, et al. Female sexual function and its associations with number of children, pregnancy, and relationship satisfaction. J Sex Marital Ther. 2008;34(2):89-106.
9. Thompson JF, Roberts CL, Currie M, Elwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29(2):83-94.
10. Waterstone M, Wolfe C, Hooper R, Bewley S. Postnatal morbidity after childbirth and severe obstetric morbidity. BJOG. 2003;110(2):128-133.
11. Pauls RN, Occhino JA, Dryfhout VL. Effects of pregnancy on female sexual function and body image: A prospective study. J Sex Med. 2008;5(8):1915-1922.
12. von Sydow K, Ullmeyer M, Happ N. Sexual activity during pregnancy and after childbirth: Results from the Sexual P Questionnaire. J Psychosom Obstet Gynaecol. 2001;22(1):29-40.
13. Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Rsearch Group. Neurourol Urodyn. 1995;14(2):131-139.
14. Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC; Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281-289.
15. Handa VL. Sexual function and childbirth. Semin Perinatol. 2006;30(5):253-256.
16. Klein MC, Gauthier RJ, Robbins JM, et al. Relationship of episiotomy to perineal trauma and morbidity, sexual dysfunction, and pelvic floor relaxation. Am J Obstet Gynecol. 1994;171(3):591-598.
17. Barrett G, Pendry E, Peacock J, Victor C, Thakar, Manyonda I. Women’s sexual health after childbirth. BJOG. 2000;107(2):186-195.
18. Barrett G, Peacock J, Victor CR, Manyonda I. Cesarean section and postnatal sexual health. Birth. 2005;32(4):306-311.
19. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol. 2000;95(3):464-471.
20. Leeman LM, Rogers RG, Greulich B, Albers LL. Do unsutured second-degree perineal lacerations affect postpartum functional outcomes? J Am Board Fam Med. 2007;20(5):451-457.
21. Signorello L, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: A retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184(5):881-890.
22. Snooks SJ, Swash M, Henry MW, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. In J Colorect Dis. 1986;1(1):20-24.
23. Hannah ME, Whyte H, Hannah WJ, et al. Term Breech Trial Collaborative Group. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):917-927.
24. NIH State-of-the-Science Conference Statement on Cesarean Delivery on Maternal Request NIH Consens Sci Statements. 2006;23:1-29.http://consensus.nih.gov/2006/cesareanstatement.htm. Accessed December 6 2011.
25. Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001;15(3):232-240.
26. Chivers ML, Pittini MD, Grigoriadis S, Villegas L, Ross LE. The relationship between sexual functioning and depressive symptomatology in postpartum women: a pilot study. J Sex Med. 2011;8(3):792-799.
27. Morof D, Barrett G, Peacock J, Victor CR, Manyonda I. Postnatal depression and sexual health after childbirth. Obstet Gynecol. 2003;102(6):1318-1325.
28. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No.119: Female sexual dysfunction. Obstet Gynecol. 2011;117(4):996-1007.
29. Read J. Sexual problems associated with infertility pregnancy and ageing. BMJ. 2004;329(7465):559-561.
30. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
31. Isidori AM, Pozza C, Esposito K, et al. The Female Sexual Function Index (FSFI): Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med. 2010;7(3):1139-11.
32. McCoy NL. The McCoy Female Sexuality Questionnaire. Quality Life Res. 2000;9(suppl 6):739-745.
33. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 1):337-348.
34. DeRogatis LR, Allgood A, Rosen RC, Leiblum S, Zipfel L, Guo CY. Development and evaluation of the Women’s Sexual Interest Diagnostic Interview (WSID): a structured interview to diagnose hypoactive sexual desire disorder (HSDD) in standardized patients. J Sex Med. 2008;5(12):2827-2841.
35. Fischman SH, Rankin EA, Soeken KL, Lenz ER. Changes in sexual relationships in postpartum couples. J Obstet Gynecol Neonatal Nurs. 1986;15(1):58-63.
36. Sillis T, Wunderlich G, Pyke R, et al. The Sexual Interest and Desire Inventory-Female (SIDI-F): item response analyses of data from women diagnosed with hypoactive sexual desire disorder. J Sex Med. 2005;2(6):801-818.
37. Rust J, Golombok S. The Golombok-Rust Inventory of Sexual Satisfaction (GRISS). Br J Clin Psych. 1985;24(Pt 1):63-64.
38. Clayton AH, Balon R. The impact of mental illness and psychotropic medications on sexual functioning: the evidence and management. J Sex Med. 2009;6(5):1200-1213.
39. Jennings B, Edmundson M. The postpartum period: after confinement: the fourth trimester. Clin Obstet Gynecol. 1980;23(4):1093-1103.
40. Oppenheimer LS, Sheriff EA, Goodman JDS, shah D, James CE. The duration of lochia. Br J Obstet Gynaecol. 1986;93(7):754-757.
41. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):263-267.
42. Pancholy AB, Goldenhar L, Fellner AN, Crisp C, Kleeman S, Pauls R. Resident education and training in female sexuality: results of a national survey. J Sex Med. 2011;8(2):361-366.
CASE: Waiting for an OK to resume sex
L. L. is a 29-year-old woman, G1P1, who delivered a healthy infant 4 weeks ago by spontaneous vaginal birth. The delivery involved a 2-day induction of labor for preeclampsia and a second-degree tear that was repaired without complication. The patient also experienced postpartum hemorrhage that was managed with bimanual massage and uterotonics and for which she ultimately required transfusion of blood products. Her hospital course was otherwise unremarkable.
Before pregnancy, L. L. had a normal medical history and conceived spontaneously. Her antenatal course was uncomplicated.
Today, she returns for her postpartum visit. She reports being tired and says she still has some pain at the site of the tear, but reports no problems with urinary or fecal continence. She denies being depressed, and her Edinburgh Postnatal Depression Scale (EPDS) score is consistent with that report. She is breastfeeding and appears to be doing well on the progestin-only pill for contraception. She has not yet attempted intercourse because she is complying with instructions to wait until she sees you for her postpartum visit.
How should you counsel her about resuming sexual activity?

Childbirth is a central event in a woman’s life. Pregnancy and delivery are a time of psychological, biological, and physical transformation, and the postpartum period—the “fourth trimester”—is no exception. Sexual function may be affected. In fact, many women who seek assistance for sexual dissatisfaction note that their problem arose in the postpartum period.1
Postpartum sexuality involves considerably more than the physical act of genital stimulation—with or without intromission or penile penetration—and depends on more than the physical state of recovery of the vagina (after vaginal delivery). It also depends on:
- the woman’s sexual drive and motivation
- her general state of health and quality of life
- her emotional readiness to resume sexual intimacy with a partner
- her adaptation to the maternal role and ability to balance her identity as a mother with her identity as a sexual being
- her relationship with her partner.
Given all these contributing factors, many of which fall outside the scope of the clinical practice of obstetrics and gynecology, how do we go about counseling our patients about the resumption of sexual activity?
Other questions:
- How can we help patients manage expectations about the quality of their postpartum sexual function?
- What guidance can we provide regarding the interplay of psychosexual and physical aspects of the puerperium?
- Can we offer a method of screening for sexual dysfunction in the puerperium? If so, will it help prevent sexual problems or hasten their resolution?
This article addresses these issues. Ultimately, the answer to the question of when to resume sexual activity should reflect an awareness of cultural norms and taboos as well as familiarity with empirically based recommendations.
Traditional postpartum sexual education is not evidence-based and has limited effectiveness. More up-to-date strategies can be easily incorporated into even the busiest clinical practice. We offer the following counseling model for you to consider when addressing the sexual health of patients postpartum.
Educate, legitimize, and normalize
The first sexual encounter after childbirth can be an important step for couples to reclaim their intimate relationship.
Adaptation to the parental role, physical healing, hormonal changes, breastfeeding, and sleep deprivation contribute to a profound psychosocial challenge. The resumption of sexual activities and a satisfying postpartum sex life depend on many variables, many of which the patient may not even be aware.
First, do not assume that all patients are heterosexual and that intercourse is their only form of sexual activity.
Second, it is important to be proactive in antepartum and postpartum counseling and to offer anticipatory guidance. Counseling can take place any time during routine prenatal care, as well as at the time of hospital discharge and the postpartum visit.
Reassure the patient that, if sexual activity and frequency are lower during pregnancy and the postpartum period, it is likely a normal transition. Also give the patient time to talk about her expectations and perceptions. Explain to her the normal fluctuations and variability of sexual interest and enjoyment in pregnancy and the puerperium, and suggest that she consider alternative options for intimate expression, non-coital sexual activities, and mutual pleasure within her cultural context.
Be thorough
Take a comprehensive medical, obstetric, psychological, and social history as part of the sexual history. Also perform a physical intake and exam. Questions about urinary and fecal incontinence ought to be part of all postpartum assessment.
Other potential areas to address include the quality of the relationship, prepregnancy sexual function, the support network, planned or unplanned state of the pregnancy, previous pregnancy and delivery outcomes, the health status of current children, and present, previous, and future contraceptive use.29
Consider multiple visits
It is hard to know exactly when to evaluate a patient for postpartum sexual dysfunction, given the impact of pudendal nerve latency, fatigue, and breastfeeding. For this reason, assessment on multiple occasions may be appropriate. Numerous validated scales to assess sexual function can be easily incorporated into clinical practice.
Couples counseling and therapy may be needed in some cases; be aware of referral services in your area for sexual wellness specialists.
The bottom line: A “successful” sexual life does not necessarily mean adequate genital function (e.g., coital orgasm, improved clitoral blood flow, increased sexual frequency) but, rather, a sexual life that is intimate and satisfying to the individual patient.
A paucity of research
To date, research into sexuality during the postpartum period has focused primarily on the physical changes and constraints that affect the mechanics and frequency of intercourse and overall sexual satisfaction and desire.2 This perspective has begun to broaden to include the psychological aspects of sexuality.
TABLE 1
These validated tools can help you measure female sexual dysfunction
| Tool | Area assessed |
|---|---|
| Female Sexual Function Index (FSFI)30 | Desire, arousal, orgasm, and pain |
| Female Sexual Function Index 6-Item (FSFI-6)31 | Desire, arousal, orgasm, and pain |
| McCoy Female Sexual Function Questionnaire*32 | Presence of female sexual disorders |
| Brief Sexual Symptoms Checklist33 | Screener for sexual concerns |
| Female Sexual Distress Scale – Revised34 | Distress |
| Intimate Relationship Scale*35 | Changes in sexual relationship |
| Sexual Quality of Life – Female (SQol-F)36 | Quality of life in women with female sexual dysfunction |
| Golombok Rust Inventory of Sexual Satisfaction (GRISS)37 | Quality of sexual relationship |
| Decreased Sexual Desire Screener38 | Brief diagnostic tool for hypoactive sexual desire disorder |
| * Validated in pregnant and/or postpartum women | |
Women’s sexual health during the postpartum period has generally been under-researched. It wasn’t until the past decade that validated sexual function questionnaires were utilized. Although a number of these instruments are now available (TABLE 1, TABLE 2, FIGURE), it remains unclear whether they can accurately measure postpartum sexual function. Despite these limitations, significant information has been elicited that can be used to counsel patients struggling with postpartum sexual concerns.
TABLE 2
The 6-item Female Sexual Function Index*
| Question | Responses | |||||
|---|---|---|---|---|---|---|
| 0 points | 5 points | 4 points | 3 points | 2 points | 1 point | |
| How would you rate your level of sexual desire or interest? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How would you rate your level of sexual arousal (“turn on”) during sexual activity or intercourse? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How often did you become lubricated (“wet”) during sexual activity or intercourse? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| When you had sexual stimulation or intercourse, how often did you reach orgasm? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| How satisfied have you been overall with your sexual life? | No sexual activity | Very satisfied | Moderately satisfied | About equally satisfied and dissatisfied | Moderately dissatisfied | Very dissatisfied |
| How often did you experience discomfort or pain during vaginal penetration? | Did not attempt intercourse | Almost never or never | A few times | Sometimes | Most times | Almost always or always |
| *The components of this index are to be assessed over the past 4 weeks. The score is the sum of the ordinal responses to the 6 items and ranges from 2 to 30. A score of less than 19 indicates a need for further investigation, including the full-length Female Sexual Function Index. Source: Adapted from Isidori et al.31 | ||||||
Ideal period of abstinence is unknown
Although our knowledge of the female genital tract in the puerperium is based upon histologic evidence, there are no evidence-based policies to outline the ideal period of postpartum coital abstinence. It seems reasonable to assume that our traditional scientific recommendations developed in part to prevent uterine infection and disruption of sutured wounds. These concerns, combined with cultural and societal norms, have led to the routine discouragement of sexual activity until 4 to 6 weeks postpartum.
The possibility of shortening the period of postpartum abstinence was first suggested by the American College of Obstetricians and Gynecologists (ACOG) in 1984.1 In 1985, Pritchard and colleagues wrote about the individualization of postpartum prohibitions of sexual activity in Williams Obstetrics.1 The earliest time at which intercourse may be safely resumed is unknown, but the 23rd edition of Williams Obstetrics states that a woman can resume sexual intercourse as early as 2 weeks, based on her comfort and desire.3 The sixth edition of the American Academy of Pediatrics (AAP) and ACOG guidelines for perinatal care also states that the risks ought to be minimal at 2 weeks postpartum.4
BRIEF SEXUAL SYMPTOMS CHECKLIST FOR WOMEN (BSSC-W)

Reprinted from Hatzichristou et al. 33
Low desire is not unusual
Although a patient may be granted “permission” to engage in coital activity, other variables influence her decision. It is well known that sexual desire may fluctuate during pregnancy and typically decreases significantly during the third trimester.2 Many women enter the postpartum period with lower levels of sexual desire and satisfaction, and these depressed levels may continue for some time.2 Twenty-five percent of women report worsened sexual function, including diminished sexual satisfaction, during pregnancy that persists for 6 to 12 months postpartum.5 By 12 weeks postpartum, 80% to 93% of women have resumed intercourse, but as many as 83% report sexual problems during the first 3 months of the postpartum period. At 6 months, 18% to 30% of these women may still be experiencing sexual problems, including dyspareunia.5,6
In 1998, von Sydow performed a meta-content analysis of all existing studies on parental sexuality during pregnancy and the first 6 months postpartum.7 Using psychological and medical data banks, she brought together information from two branches of science and identified 59 relevant studies in English or German between 1950 and 1996. Although the majority of studies were retrospective and failed to utilize a validated instrument, von Sydow determined that, overall, sexual interest and activity were low or nonexistent during the first months after delivery. There was high variability between individuals, however, and levels of sexual interest and activity of individual women remained relatively constant from the time before pregnancy until 1 year postpartum.7 von Sydow determined that there is great variability in female sexuality during pregnancy and postpartum; this variability may represent fluctuations during this phase of life. She also determined that severe psychosexual and marital problems are much more prevalent in the postpartum period than during pregnancy and persist long after a physical cause can be used as an explanation.7
Fatigue and quality of the relationship have an impact on sexual function
De Judicibus and colleagues identified a broad range of variables that have a detrimental impact on sexuality at 12 weeks postpartum, most particularly:
- marital dissatisfaction
- dyspareunia
- fatigue
- depression
- breastfeeding.2
There is evidence to suggest that the addition of the first child reduces marital quality after the first month postpartum, and this decline in marital satisfaction continues for 6 to 18 months postpartum.2 Witting and coworkers suggested that this decline may represent a transitional phase of parenthood for some couples; data support the positive effects on overall marital satisfaction with the addition of children.8 Women who were more satisfied with their relationships reported higher sexual satisfaction and greater frequency of intercourse.2,8
Fatigue is one of the most common problems women experience during pregnancy and postpartum and is a common reason given for loss of sexual desire and interest, infrequent sexual activity, and lack of enjoyment.5 A high level of exhaustion is found during the first 8 weeks postpartum. Although it declines over the next 6 months, it does not appear to resolve completely in a good number of women.9
Don’t underestimate the impact of obstetric morbidity
Surprisingly, the long-term impact of severe obstetric events on postpartum maternal health is often overlooked. Waterstone and colleagues found that women who have severe obstetric morbidity, such as massive hemorrhage, preeclampsia, sepsis, and uterine rupture, experience significant changes in sexual health and well-being.10 They conducted a prospective cohort study of such women, measuring sexual activity, general health, and postpartum depression. They utilized two validated postnatal questionnaires—the Short Form 36 (SF-36) to measure general health and the EPDS. Women who had uncomplicated pregnancies and childbirth tended to perform well in most SF-36 categories, whereas women who had experienced severe morbidity scored worse in almost every category. These women also reported problems with intercourse. Thirteen percent of women had not resumed sexual relations by 6 to 12 months postpartum; of these women, more than half reported a fear of conceiving as a reason.
The female body undergoes dramatic physiologic, anatomic, and psychological changes immediately following delivery and throughout the restoration of its pre-pregnant state. This fourth trimester usually lasts 6 to 12 weeks.39
Uterus. The uterus undergoes rapid involution after separation of the placenta. By 2 to 4 weeks postpartum, it may no longer be palpable abdominally, and by 6 weeks, it usually has returned to its nonpregnant state and size. Seven to 14 days after delivery, a woman often experiences an episode of heavier vaginal bleeding that corresponds with the sloughing of the placental bed eschar. During this time of involution, myometrial vessels may be 5 mm or larger in diameter.40
Lochia. The postpartum lochia begins to change within days of birth, transitioning through its stages of lochia rubra, serosa, and alba. It decreases by 3 weeks postpartum and is likely completely resolved by 6 weeks.
Prolactin is responsible for lactogenesis. When the prolactin level is maintained through breastfeeding, it depresses ovarian production of estrogen by suppressing pituitary gonadotropin secretion, triggering a period of “steroid starvation” after the loss of estrogen and progesterone production from the placenta.1
Vagina. Early in the postpartum period, the vagina is typically edematous and lax and, as a result of parturition, there may be not only a spontaneous tear or episiotomy that must heal, but superficial small tears that do not require suturing. Ruggae begin to reappear by 3 weeks, and the vaginal epithelium will begin to mature under the influence of estrogen production. Much of this tissue damage is healed by 6 weeks postpartum.
The perception of pregnant and postpartum women’s sexuality varies, based on religious and cultural norms. In some religions and cultures, sexual activity is forbidden for 2 to 3 months postpartum; in others, it is prohibited until the child is weaned from the breast. The postpartum woman and lochia have traditionally been perceived as unclean, and many religions have specific proscriptions regarding the management of this time in a woman’s life.1 Although early cultures did not study these issues specifically, their doctrines suggest that they had some awareness of the natural physiologic transition of a woman’s body after she has given birth.
Exploring the role of body image
Paul and coworkers prospectively assessed female sexual function, body image, and pelvic symptoms from the first trimester until 6 months postpartum.11 They utilized the validated questionnaire instruments of the Female Sexual Function Index (FSFI), the Body Exposure during Sexual Activities Questionnaire (BESAQ), the short forms of the Urogenital Distress Inventory (UDI-6), the Incontinence Impact Questionnaire (IIQ-7), and the Fecal Incontinence Quality of Life Scale (FIQOL). They found that sexual activity and sexual function scores were highest before pregnancy, declined between the first and third trimesters, and did not return to pre-pregnancy baselines even by 6 months postpartum.11
Differences in sexual practices contributed to these patterns. Kissing, fondling, and vaginal intercourse remained stable across pregnancy, whereas oral sex, breast stimulation, and masturbation declined in the third trimester.
The decline of these activities during pregnancy and postpartum has been seen in other studies as well.12
Obstacles to sexual activity also changed across pregnancy and the postpartum period. Vaginal pain was more problematic in the third trimester and postpartum, whereas feelings of unattractiveness and issues of body image were present throughout pregnancy and at their worst in the postpartum period. Sexual function scores based on the FSFI declined during pregnancy and did not return to pre-pregnancy or first-trimester levels by 6 months postpartum. Urinary symptoms, as measured by the UDI-6, were associated with lower sexual function scores during the postpartum period. The association between urinary incontinence and sexual dysfunction has been seen in other studies.13,14
The enduring effects of perineal trauma
Childbirth may physically affect a woman’s sexual function through perineal trauma, pudendal neuropathy, and vaginal dryness associated with breastfeeding. There is an obvious connection between perineal laceration and perineal pain and problems with intercourse.5 Overall, dyspareunia is reported by 41% to 67% of women 2 to 3 months after delivery.15 Women who have an episiotomy complain of increased perineal pain and delayed return of sexual activity, compared with women who deliver with an intact perineum.16
Persistent dyspareunia is strongly associated with the severity of perineal trauma and operative vaginal delivery.3,17 Multiple studies have investigated this association and found a positive correlation 3 to 6 months postpartum,6,9,17 but the long-term effects and association remain unclear.18
Findings from research. Rogers and colleagues prospectively studied the effect of perineal trauma on postpartum sexual function in a midwifery population of women who had a low rate of episiotomy and operative vaginal delivery.6 They utilized the Intimate Relationship Scale (IRS), a validated questionnaire to measure postpartum sexual function in couples. Most women in this study had resumed sexual activity by 3 months postpartum and did not have postpartum inactivity or dysfunction, based on their IRS scores. However, women who were identified as having experienced major trauma (second-, third-, or fourth-degree laceration or a repaired first-degree laceration) had significantly less desire to engage in activities such as touching and stroking with their partner.6
Present-day limits on the routine use of episiotomy and operative vaginal delivery have yielded a lower rate of third- and fourth-degree laceration.19 Second-degree lacerations are common and constitute the majority of perineal trauma in births without episiotomy.20 There is evidence that the use of synthetic absorbable suture, such as polyglactin, rather than chromic suture, results in less postpartum perineal pain, as does leaving the well-approximated perineal skin edges unsutured.20
Signorello and coworkers found that second-, third-, and fourth-degree lacerations increased the risk of postpartum dyspareunia; operative vaginal delivery (forceps or vacuum) was also an independent risk factor for dyspareunia.21
The impact of route of delivery
Some researchers have concluded that the route of delivery has an impact on the long-term pelvic floor health of women.18 In 1986, Snooks and colleagues analyzed possible obstetric risk factors for damage to the innervation of the pelvic floor, which can lead to both stress urinary and anorectal incontinence.22 They found that the process of vaginal delivery causes a compression and stretch type of injury to the pudendal nerve, as well as the possibility of severe perineal lacerations. This injury may be less likely to occur when cesarean delivery is performed before labor, avoiding direct perineal trauma and possible pudendal neuropathy.15 Because the pudendal nerve mediates some of the reflex pathways in the female sexual response, it is plausible that damage to it could result in sexual dysfunction.
Women who deliver vaginally have a higher rate of fecal and urinary incontinence than women who deliver by cesarean.16,23 The presence of incontinence, however, does not always have a significant long-term effect on one’s sexual life.6
In the Term Breech Trial, the route of delivery had no impact on the resumption of intercourse, dyspareunia, or sexual satisfaction.23 Although the trial was randomized and controlled, it had many limitations that call its generalizability into question in regard to postpartum sexual dysfunction.
The National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request indicated that, by 6 months postpartum, there is no difference in sexual function based on the route of delivery.24 However, Lydon-Rochelle and colleagues used the SF-36 to assess reported general health status and found that women who had cesarean delivery or assisted vaginal delivery exhibited significantly poorer postpartum functional status than women who had spontaneous vaginal delivery in five areas at 7 weeks postpartum: physical functioning, mental health, general health perception, bodily pain, social functioning, and ability to perform daily activities.25 Women were more likely to be readmitted to the hospital and more likely to report fatigue during the first 2 months after cesarean delivery.9 It appears that women who undergo cesarean delivery have an elevated risk of nondyspareunia-related causes of sexual dysfunction. Any protective effect of cesarean on sexual function is limited to the early postnatal period and is related to the absence of perineal injury.18
How breastfeeding can affect sexual desire
Evidence is strong that breastfeeding reduces a woman’s sexual desire and the frequency of intercourse.1,5 A high level of prolactin suppresses ovarian production of estrogen, thereby reducing vaginal lubrication. Some women and their partner may identify this loss of lubrication as a lack of arousal. This type of vaginal dryness should be explained, and the use of a lubricant should be encouraged in breastfeeding women.
Nipple sensitivity may develop, making touching and foreplay uncomfortable in some women. One third to one half of mothers find breastfeeding to be an erotic experience, and one fourth feel guilty about this sexual excitement; others stop nursing or wean early due to these feelings.1,7 Women are often not educated about the relationship between the release of oxytocin, uterine contractions, milk ejection, sexual arousal, and orgasm; raising the subject can help to diminish any potential distress over this response.
Sleep disturbances from feeding on demand contribute to fatigue and exhaustion.
Many women may not realize that their loss of interest in sex may be because they are receiving sufficient physical contact or touching through their nurturing interactions with the baby. This may leave the partner feeling isolated and envious of the mother-baby relationship.
Couples should be encouraged to discuss these feelings to avoid misperceptions and to maintain the relationship dyad as a priority to prevent the development of relationship problems.
The majority of women will discuss contraception with a health provider, but only 15% will voluntarily discuss their sexual needs or dysfunction.17 This finding is alarming given that, during the postpartum period, two of every three new mothers will experience at least one problem related to sexual function, including dyspareunia, decreased libido, difficulty achieving orgasm, and vaginal dryness.41 This lack of discussion with a health-care provider may be the result of several variables: incomplete knowledge on the part of the provider about what affects sexual function, poor training in the taking of an effective sexual history, and uneasiness on the part of the patient about discussing the issue.5,42
Postnatal depression takes a toll
Depressed mood and emotional lability in the postpartum period are negatively associated with sexual interest, enjoyment, coital activity, and perceived tenderness of the partner.7 Conversely, reduced sexual interest, desire and satisfaction; a lower frequency of intercourse; and later resumption of intercourse are associated with a higher number of psychiatric symptoms in the postpartum period.2 Between 10% and 15% of women experience postpartum depression (PPD).26 Depression has been associated with a decreased frequency and interest in sexual activity at 8 to 12 weeks postpartum.2,5
Chivers and colleagues assessed sexual functioning and sexual behavior in women with and without symptoms of PPD using the FSFI and EPDS. Although theirs was a small study, they found that women who had depressive symptoms also reported poorer functioning in regard to sexual arousal, orgasm, pain, lubrication, and sexual satisfaction.26 Morof and coworkers found that women who had PPD were less likely to have resumed intercourse by 6 months postpartum; they were also less likely to engage in other sexual activities.27
Role of pharmacotherapy
Many women are started on antidepressant medication near the time of delivery or during the immediate postpartum period. Often, serotonin reuptake inhibitors (SRIs) are used because there is minimal transmission of this class of medication through breast milk. However, the potential sexual side effects of these medications should be discussed because they are the agents most commonly associated with female sexual dysfunction.28
For clinicians
American Association of Sex Educators, Counselors, and Therapists – A not-for-profit, interdisciplinary professional organization comprising sexuality educators, sexuality counselors, sex therapists, physicians, social workers, and other clinicians. Its home page links to a referral page and other resources. http://www.aasect.org" target="_blank">http://www.aasect.org
Association of Reproductive Health Professionals offers a resource for clinicians on postpartum counseling about sexuality. http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception" target="_blank">http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception
For patients
Mayo Clinic provides a fact sheet entitled “Sex after pregnancy: Set your own timeline.” http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146" target="_blank">http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146
Sex and a Healthier You – This site offers information for patients on sexuality and relationships. http://www.sexandahealthieryou.org/sex-health/index.html" target="_blank">http://www.sexandahealthieryou.org/sex-health/index.html
We want to hear from you! Tell us what you think.
CASE: Waiting for an OK to resume sex
L. L. is a 29-year-old woman, G1P1, who delivered a healthy infant 4 weeks ago by spontaneous vaginal birth. The delivery involved a 2-day induction of labor for preeclampsia and a second-degree tear that was repaired without complication. The patient also experienced postpartum hemorrhage that was managed with bimanual massage and uterotonics and for which she ultimately required transfusion of blood products. Her hospital course was otherwise unremarkable.
Before pregnancy, L. L. had a normal medical history and conceived spontaneously. Her antenatal course was uncomplicated.
Today, she returns for her postpartum visit. She reports being tired and says she still has some pain at the site of the tear, but reports no problems with urinary or fecal continence. She denies being depressed, and her Edinburgh Postnatal Depression Scale (EPDS) score is consistent with that report. She is breastfeeding and appears to be doing well on the progestin-only pill for contraception. She has not yet attempted intercourse because she is complying with instructions to wait until she sees you for her postpartum visit.
How should you counsel her about resuming sexual activity?

Childbirth is a central event in a woman’s life. Pregnancy and delivery are a time of psychological, biological, and physical transformation, and the postpartum period—the “fourth trimester”—is no exception. Sexual function may be affected. In fact, many women who seek assistance for sexual dissatisfaction note that their problem arose in the postpartum period.1
Postpartum sexuality involves considerably more than the physical act of genital stimulation—with or without intromission or penile penetration—and depends on more than the physical state of recovery of the vagina (after vaginal delivery). It also depends on:
- the woman’s sexual drive and motivation
- her general state of health and quality of life
- her emotional readiness to resume sexual intimacy with a partner
- her adaptation to the maternal role and ability to balance her identity as a mother with her identity as a sexual being
- her relationship with her partner.
Given all these contributing factors, many of which fall outside the scope of the clinical practice of obstetrics and gynecology, how do we go about counseling our patients about the resumption of sexual activity?
Other questions:
- How can we help patients manage expectations about the quality of their postpartum sexual function?
- What guidance can we provide regarding the interplay of psychosexual and physical aspects of the puerperium?
- Can we offer a method of screening for sexual dysfunction in the puerperium? If so, will it help prevent sexual problems or hasten their resolution?
This article addresses these issues. Ultimately, the answer to the question of when to resume sexual activity should reflect an awareness of cultural norms and taboos as well as familiarity with empirically based recommendations.
Traditional postpartum sexual education is not evidence-based and has limited effectiveness. More up-to-date strategies can be easily incorporated into even the busiest clinical practice. We offer the following counseling model for you to consider when addressing the sexual health of patients postpartum.
Educate, legitimize, and normalize
The first sexual encounter after childbirth can be an important step for couples to reclaim their intimate relationship.
Adaptation to the parental role, physical healing, hormonal changes, breastfeeding, and sleep deprivation contribute to a profound psychosocial challenge. The resumption of sexual activities and a satisfying postpartum sex life depend on many variables, many of which the patient may not even be aware.
First, do not assume that all patients are heterosexual and that intercourse is their only form of sexual activity.
Second, it is important to be proactive in antepartum and postpartum counseling and to offer anticipatory guidance. Counseling can take place any time during routine prenatal care, as well as at the time of hospital discharge and the postpartum visit.
Reassure the patient that, if sexual activity and frequency are lower during pregnancy and the postpartum period, it is likely a normal transition. Also give the patient time to talk about her expectations and perceptions. Explain to her the normal fluctuations and variability of sexual interest and enjoyment in pregnancy and the puerperium, and suggest that she consider alternative options for intimate expression, non-coital sexual activities, and mutual pleasure within her cultural context.
Be thorough
Take a comprehensive medical, obstetric, psychological, and social history as part of the sexual history. Also perform a physical intake and exam. Questions about urinary and fecal incontinence ought to be part of all postpartum assessment.
Other potential areas to address include the quality of the relationship, prepregnancy sexual function, the support network, planned or unplanned state of the pregnancy, previous pregnancy and delivery outcomes, the health status of current children, and present, previous, and future contraceptive use.29
Consider multiple visits
It is hard to know exactly when to evaluate a patient for postpartum sexual dysfunction, given the impact of pudendal nerve latency, fatigue, and breastfeeding. For this reason, assessment on multiple occasions may be appropriate. Numerous validated scales to assess sexual function can be easily incorporated into clinical practice.
Couples counseling and therapy may be needed in some cases; be aware of referral services in your area for sexual wellness specialists.
The bottom line: A “successful” sexual life does not necessarily mean adequate genital function (e.g., coital orgasm, improved clitoral blood flow, increased sexual frequency) but, rather, a sexual life that is intimate and satisfying to the individual patient.
A paucity of research
To date, research into sexuality during the postpartum period has focused primarily on the physical changes and constraints that affect the mechanics and frequency of intercourse and overall sexual satisfaction and desire.2 This perspective has begun to broaden to include the psychological aspects of sexuality.
TABLE 1
These validated tools can help you measure female sexual dysfunction
| Tool | Area assessed |
|---|---|
| Female Sexual Function Index (FSFI)30 | Desire, arousal, orgasm, and pain |
| Female Sexual Function Index 6-Item (FSFI-6)31 | Desire, arousal, orgasm, and pain |
| McCoy Female Sexual Function Questionnaire*32 | Presence of female sexual disorders |
| Brief Sexual Symptoms Checklist33 | Screener for sexual concerns |
| Female Sexual Distress Scale – Revised34 | Distress |
| Intimate Relationship Scale*35 | Changes in sexual relationship |
| Sexual Quality of Life – Female (SQol-F)36 | Quality of life in women with female sexual dysfunction |
| Golombok Rust Inventory of Sexual Satisfaction (GRISS)37 | Quality of sexual relationship |
| Decreased Sexual Desire Screener38 | Brief diagnostic tool for hypoactive sexual desire disorder |
| * Validated in pregnant and/or postpartum women | |
Women’s sexual health during the postpartum period has generally been under-researched. It wasn’t until the past decade that validated sexual function questionnaires were utilized. Although a number of these instruments are now available (TABLE 1, TABLE 2, FIGURE), it remains unclear whether they can accurately measure postpartum sexual function. Despite these limitations, significant information has been elicited that can be used to counsel patients struggling with postpartum sexual concerns.
TABLE 2
The 6-item Female Sexual Function Index*
| Question | Responses | |||||
|---|---|---|---|---|---|---|
| 0 points | 5 points | 4 points | 3 points | 2 points | 1 point | |
| How would you rate your level of sexual desire or interest? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How would you rate your level of sexual arousal (“turn on”) during sexual activity or intercourse? | No sexual activity | Very high | High | Moderate | Low | Very low or none at all |
| How often did you become lubricated (“wet”) during sexual activity or intercourse? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| When you had sexual stimulation or intercourse, how often did you reach orgasm? | No sexual activity | Almost always or always | Most times | Sometimes | A few times | Almost never or never |
| How satisfied have you been overall with your sexual life? | No sexual activity | Very satisfied | Moderately satisfied | About equally satisfied and dissatisfied | Moderately dissatisfied | Very dissatisfied |
| How often did you experience discomfort or pain during vaginal penetration? | Did not attempt intercourse | Almost never or never | A few times | Sometimes | Most times | Almost always or always |
| *The components of this index are to be assessed over the past 4 weeks. The score is the sum of the ordinal responses to the 6 items and ranges from 2 to 30. A score of less than 19 indicates a need for further investigation, including the full-length Female Sexual Function Index. Source: Adapted from Isidori et al.31 | ||||||
Ideal period of abstinence is unknown
Although our knowledge of the female genital tract in the puerperium is based upon histologic evidence, there are no evidence-based policies to outline the ideal period of postpartum coital abstinence. It seems reasonable to assume that our traditional scientific recommendations developed in part to prevent uterine infection and disruption of sutured wounds. These concerns, combined with cultural and societal norms, have led to the routine discouragement of sexual activity until 4 to 6 weeks postpartum.
The possibility of shortening the period of postpartum abstinence was first suggested by the American College of Obstetricians and Gynecologists (ACOG) in 1984.1 In 1985, Pritchard and colleagues wrote about the individualization of postpartum prohibitions of sexual activity in Williams Obstetrics.1 The earliest time at which intercourse may be safely resumed is unknown, but the 23rd edition of Williams Obstetrics states that a woman can resume sexual intercourse as early as 2 weeks, based on her comfort and desire.3 The sixth edition of the American Academy of Pediatrics (AAP) and ACOG guidelines for perinatal care also states that the risks ought to be minimal at 2 weeks postpartum.4
BRIEF SEXUAL SYMPTOMS CHECKLIST FOR WOMEN (BSSC-W)

Reprinted from Hatzichristou et al. 33
Low desire is not unusual
Although a patient may be granted “permission” to engage in coital activity, other variables influence her decision. It is well known that sexual desire may fluctuate during pregnancy and typically decreases significantly during the third trimester.2 Many women enter the postpartum period with lower levels of sexual desire and satisfaction, and these depressed levels may continue for some time.2 Twenty-five percent of women report worsened sexual function, including diminished sexual satisfaction, during pregnancy that persists for 6 to 12 months postpartum.5 By 12 weeks postpartum, 80% to 93% of women have resumed intercourse, but as many as 83% report sexual problems during the first 3 months of the postpartum period. At 6 months, 18% to 30% of these women may still be experiencing sexual problems, including dyspareunia.5,6
In 1998, von Sydow performed a meta-content analysis of all existing studies on parental sexuality during pregnancy and the first 6 months postpartum.7 Using psychological and medical data banks, she brought together information from two branches of science and identified 59 relevant studies in English or German between 1950 and 1996. Although the majority of studies were retrospective and failed to utilize a validated instrument, von Sydow determined that, overall, sexual interest and activity were low or nonexistent during the first months after delivery. There was high variability between individuals, however, and levels of sexual interest and activity of individual women remained relatively constant from the time before pregnancy until 1 year postpartum.7 von Sydow determined that there is great variability in female sexuality during pregnancy and postpartum; this variability may represent fluctuations during this phase of life. She also determined that severe psychosexual and marital problems are much more prevalent in the postpartum period than during pregnancy and persist long after a physical cause can be used as an explanation.7
Fatigue and quality of the relationship have an impact on sexual function
De Judicibus and colleagues identified a broad range of variables that have a detrimental impact on sexuality at 12 weeks postpartum, most particularly:
- marital dissatisfaction
- dyspareunia
- fatigue
- depression
- breastfeeding.2
There is evidence to suggest that the addition of the first child reduces marital quality after the first month postpartum, and this decline in marital satisfaction continues for 6 to 18 months postpartum.2 Witting and coworkers suggested that this decline may represent a transitional phase of parenthood for some couples; data support the positive effects on overall marital satisfaction with the addition of children.8 Women who were more satisfied with their relationships reported higher sexual satisfaction and greater frequency of intercourse.2,8
Fatigue is one of the most common problems women experience during pregnancy and postpartum and is a common reason given for loss of sexual desire and interest, infrequent sexual activity, and lack of enjoyment.5 A high level of exhaustion is found during the first 8 weeks postpartum. Although it declines over the next 6 months, it does not appear to resolve completely in a good number of women.9
Don’t underestimate the impact of obstetric morbidity
Surprisingly, the long-term impact of severe obstetric events on postpartum maternal health is often overlooked. Waterstone and colleagues found that women who have severe obstetric morbidity, such as massive hemorrhage, preeclampsia, sepsis, and uterine rupture, experience significant changes in sexual health and well-being.10 They conducted a prospective cohort study of such women, measuring sexual activity, general health, and postpartum depression. They utilized two validated postnatal questionnaires—the Short Form 36 (SF-36) to measure general health and the EPDS. Women who had uncomplicated pregnancies and childbirth tended to perform well in most SF-36 categories, whereas women who had experienced severe morbidity scored worse in almost every category. These women also reported problems with intercourse. Thirteen percent of women had not resumed sexual relations by 6 to 12 months postpartum; of these women, more than half reported a fear of conceiving as a reason.
The female body undergoes dramatic physiologic, anatomic, and psychological changes immediately following delivery and throughout the restoration of its pre-pregnant state. This fourth trimester usually lasts 6 to 12 weeks.39
Uterus. The uterus undergoes rapid involution after separation of the placenta. By 2 to 4 weeks postpartum, it may no longer be palpable abdominally, and by 6 weeks, it usually has returned to its nonpregnant state and size. Seven to 14 days after delivery, a woman often experiences an episode of heavier vaginal bleeding that corresponds with the sloughing of the placental bed eschar. During this time of involution, myometrial vessels may be 5 mm or larger in diameter.40
Lochia. The postpartum lochia begins to change within days of birth, transitioning through its stages of lochia rubra, serosa, and alba. It decreases by 3 weeks postpartum and is likely completely resolved by 6 weeks.
Prolactin is responsible for lactogenesis. When the prolactin level is maintained through breastfeeding, it depresses ovarian production of estrogen by suppressing pituitary gonadotropin secretion, triggering a period of “steroid starvation” after the loss of estrogen and progesterone production from the placenta.1
Vagina. Early in the postpartum period, the vagina is typically edematous and lax and, as a result of parturition, there may be not only a spontaneous tear or episiotomy that must heal, but superficial small tears that do not require suturing. Ruggae begin to reappear by 3 weeks, and the vaginal epithelium will begin to mature under the influence of estrogen production. Much of this tissue damage is healed by 6 weeks postpartum.
The perception of pregnant and postpartum women’s sexuality varies, based on religious and cultural norms. In some religions and cultures, sexual activity is forbidden for 2 to 3 months postpartum; in others, it is prohibited until the child is weaned from the breast. The postpartum woman and lochia have traditionally been perceived as unclean, and many religions have specific proscriptions regarding the management of this time in a woman’s life.1 Although early cultures did not study these issues specifically, their doctrines suggest that they had some awareness of the natural physiologic transition of a woman’s body after she has given birth.
Exploring the role of body image
Paul and coworkers prospectively assessed female sexual function, body image, and pelvic symptoms from the first trimester until 6 months postpartum.11 They utilized the validated questionnaire instruments of the Female Sexual Function Index (FSFI), the Body Exposure during Sexual Activities Questionnaire (BESAQ), the short forms of the Urogenital Distress Inventory (UDI-6), the Incontinence Impact Questionnaire (IIQ-7), and the Fecal Incontinence Quality of Life Scale (FIQOL). They found that sexual activity and sexual function scores were highest before pregnancy, declined between the first and third trimesters, and did not return to pre-pregnancy baselines even by 6 months postpartum.11
Differences in sexual practices contributed to these patterns. Kissing, fondling, and vaginal intercourse remained stable across pregnancy, whereas oral sex, breast stimulation, and masturbation declined in the third trimester.
The decline of these activities during pregnancy and postpartum has been seen in other studies as well.12
Obstacles to sexual activity also changed across pregnancy and the postpartum period. Vaginal pain was more problematic in the third trimester and postpartum, whereas feelings of unattractiveness and issues of body image were present throughout pregnancy and at their worst in the postpartum period. Sexual function scores based on the FSFI declined during pregnancy and did not return to pre-pregnancy or first-trimester levels by 6 months postpartum. Urinary symptoms, as measured by the UDI-6, were associated with lower sexual function scores during the postpartum period. The association between urinary incontinence and sexual dysfunction has been seen in other studies.13,14
The enduring effects of perineal trauma
Childbirth may physically affect a woman’s sexual function through perineal trauma, pudendal neuropathy, and vaginal dryness associated with breastfeeding. There is an obvious connection between perineal laceration and perineal pain and problems with intercourse.5 Overall, dyspareunia is reported by 41% to 67% of women 2 to 3 months after delivery.15 Women who have an episiotomy complain of increased perineal pain and delayed return of sexual activity, compared with women who deliver with an intact perineum.16
Persistent dyspareunia is strongly associated with the severity of perineal trauma and operative vaginal delivery.3,17 Multiple studies have investigated this association and found a positive correlation 3 to 6 months postpartum,6,9,17 but the long-term effects and association remain unclear.18
Findings from research. Rogers and colleagues prospectively studied the effect of perineal trauma on postpartum sexual function in a midwifery population of women who had a low rate of episiotomy and operative vaginal delivery.6 They utilized the Intimate Relationship Scale (IRS), a validated questionnaire to measure postpartum sexual function in couples. Most women in this study had resumed sexual activity by 3 months postpartum and did not have postpartum inactivity or dysfunction, based on their IRS scores. However, women who were identified as having experienced major trauma (second-, third-, or fourth-degree laceration or a repaired first-degree laceration) had significantly less desire to engage in activities such as touching and stroking with their partner.6
Present-day limits on the routine use of episiotomy and operative vaginal delivery have yielded a lower rate of third- and fourth-degree laceration.19 Second-degree lacerations are common and constitute the majority of perineal trauma in births without episiotomy.20 There is evidence that the use of synthetic absorbable suture, such as polyglactin, rather than chromic suture, results in less postpartum perineal pain, as does leaving the well-approximated perineal skin edges unsutured.20
Signorello and coworkers found that second-, third-, and fourth-degree lacerations increased the risk of postpartum dyspareunia; operative vaginal delivery (forceps or vacuum) was also an independent risk factor for dyspareunia.21
The impact of route of delivery
Some researchers have concluded that the route of delivery has an impact on the long-term pelvic floor health of women.18 In 1986, Snooks and colleagues analyzed possible obstetric risk factors for damage to the innervation of the pelvic floor, which can lead to both stress urinary and anorectal incontinence.22 They found that the process of vaginal delivery causes a compression and stretch type of injury to the pudendal nerve, as well as the possibility of severe perineal lacerations. This injury may be less likely to occur when cesarean delivery is performed before labor, avoiding direct perineal trauma and possible pudendal neuropathy.15 Because the pudendal nerve mediates some of the reflex pathways in the female sexual response, it is plausible that damage to it could result in sexual dysfunction.
Women who deliver vaginally have a higher rate of fecal and urinary incontinence than women who deliver by cesarean.16,23 The presence of incontinence, however, does not always have a significant long-term effect on one’s sexual life.6
In the Term Breech Trial, the route of delivery had no impact on the resumption of intercourse, dyspareunia, or sexual satisfaction.23 Although the trial was randomized and controlled, it had many limitations that call its generalizability into question in regard to postpartum sexual dysfunction.
The National Institutes of Health (NIH) State-of-the-Science Conference on Cesarean Delivery on Maternal Request indicated that, by 6 months postpartum, there is no difference in sexual function based on the route of delivery.24 However, Lydon-Rochelle and colleagues used the SF-36 to assess reported general health status and found that women who had cesarean delivery or assisted vaginal delivery exhibited significantly poorer postpartum functional status than women who had spontaneous vaginal delivery in five areas at 7 weeks postpartum: physical functioning, mental health, general health perception, bodily pain, social functioning, and ability to perform daily activities.25 Women were more likely to be readmitted to the hospital and more likely to report fatigue during the first 2 months after cesarean delivery.9 It appears that women who undergo cesarean delivery have an elevated risk of nondyspareunia-related causes of sexual dysfunction. Any protective effect of cesarean on sexual function is limited to the early postnatal period and is related to the absence of perineal injury.18
How breastfeeding can affect sexual desire
Evidence is strong that breastfeeding reduces a woman’s sexual desire and the frequency of intercourse.1,5 A high level of prolactin suppresses ovarian production of estrogen, thereby reducing vaginal lubrication. Some women and their partner may identify this loss of lubrication as a lack of arousal. This type of vaginal dryness should be explained, and the use of a lubricant should be encouraged in breastfeeding women.
Nipple sensitivity may develop, making touching and foreplay uncomfortable in some women. One third to one half of mothers find breastfeeding to be an erotic experience, and one fourth feel guilty about this sexual excitement; others stop nursing or wean early due to these feelings.1,7 Women are often not educated about the relationship between the release of oxytocin, uterine contractions, milk ejection, sexual arousal, and orgasm; raising the subject can help to diminish any potential distress over this response.
Sleep disturbances from feeding on demand contribute to fatigue and exhaustion.
Many women may not realize that their loss of interest in sex may be because they are receiving sufficient physical contact or touching through their nurturing interactions with the baby. This may leave the partner feeling isolated and envious of the mother-baby relationship.
Couples should be encouraged to discuss these feelings to avoid misperceptions and to maintain the relationship dyad as a priority to prevent the development of relationship problems.
The majority of women will discuss contraception with a health provider, but only 15% will voluntarily discuss their sexual needs or dysfunction.17 This finding is alarming given that, during the postpartum period, two of every three new mothers will experience at least one problem related to sexual function, including dyspareunia, decreased libido, difficulty achieving orgasm, and vaginal dryness.41 This lack of discussion with a health-care provider may be the result of several variables: incomplete knowledge on the part of the provider about what affects sexual function, poor training in the taking of an effective sexual history, and uneasiness on the part of the patient about discussing the issue.5,42
Postnatal depression takes a toll
Depressed mood and emotional lability in the postpartum period are negatively associated with sexual interest, enjoyment, coital activity, and perceived tenderness of the partner.7 Conversely, reduced sexual interest, desire and satisfaction; a lower frequency of intercourse; and later resumption of intercourse are associated with a higher number of psychiatric symptoms in the postpartum period.2 Between 10% and 15% of women experience postpartum depression (PPD).26 Depression has been associated with a decreased frequency and interest in sexual activity at 8 to 12 weeks postpartum.2,5
Chivers and colleagues assessed sexual functioning and sexual behavior in women with and without symptoms of PPD using the FSFI and EPDS. Although theirs was a small study, they found that women who had depressive symptoms also reported poorer functioning in regard to sexual arousal, orgasm, pain, lubrication, and sexual satisfaction.26 Morof and coworkers found that women who had PPD were less likely to have resumed intercourse by 6 months postpartum; they were also less likely to engage in other sexual activities.27
Role of pharmacotherapy
Many women are started on antidepressant medication near the time of delivery or during the immediate postpartum period. Often, serotonin reuptake inhibitors (SRIs) are used because there is minimal transmission of this class of medication through breast milk. However, the potential sexual side effects of these medications should be discussed because they are the agents most commonly associated with female sexual dysfunction.28
For clinicians
American Association of Sex Educators, Counselors, and Therapists – A not-for-profit, interdisciplinary professional organization comprising sexuality educators, sexuality counselors, sex therapists, physicians, social workers, and other clinicians. Its home page links to a referral page and other resources. http://www.aasect.org" target="_blank">http://www.aasect.org
Association of Reproductive Health Professionals offers a resource for clinicians on postpartum counseling about sexuality. http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception" target="_blank">http://www.arhp.org/publications-and-resources/quick-reference-guide-for-clinicians/postpartum-counseling/contraception
For patients
Mayo Clinic provides a fact sheet entitled “Sex after pregnancy: Set your own timeline.” http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146" target="_blank">http://www.mayoclinic.com/health/sex-after-pregnancy/PR00146
Sex and a Healthier You – This site offers information for patients on sexuality and relationships. http://www.sexandahealthieryou.org/sex-health/index.html" target="_blank">http://www.sexandahealthieryou.org/sex-health/index.html
We want to hear from you! Tell us what you think.
1. Reamy KJ, White SE. Sexuality in the puerperium: a review. Arch Sex Behav. 1987;16(2):165-186.
2. De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39(2):94-103.
3. The puerperium. In: Cunningham FG Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill Co.; 2010:646-660.
4. The American Academy of Pediatrics (AAP), American College of Obstetricians Gynecologists (ACOG) Guidelines for perinatal care. 6th ed. Washington DC: AAP, ACOG; 2008.
5. Glazener CM. Sexual function after childbirth: women’s experiences persistent morbidity and lack of professional recognition. Br J Obstet Gynaecol. 1997;104(3):330-335.
6. Rogers RG, Borders, N,, Leeman L, Albers L. Does spontaneous genital tract trauma impact postpartum sexual function? J Midwifery Womens Health. 2009;54(2):98-103.
7. von Sydow K. Sexuality during pregnancy and after childbirth: a metacontent analysis of 59 studies. J Psychosom Res. 1999;47(1):27-49.
8. Witting K, Santtila P, Alanko K, et al. Female sexual function and its associations with number of children, pregnancy, and relationship satisfaction. J Sex Marital Ther. 2008;34(2):89-106.
9. Thompson JF, Roberts CL, Currie M, Elwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29(2):83-94.
10. Waterstone M, Wolfe C, Hooper R, Bewley S. Postnatal morbidity after childbirth and severe obstetric morbidity. BJOG. 2003;110(2):128-133.
11. Pauls RN, Occhino JA, Dryfhout VL. Effects of pregnancy on female sexual function and body image: A prospective study. J Sex Med. 2008;5(8):1915-1922.
12. von Sydow K, Ullmeyer M, Happ N. Sexual activity during pregnancy and after childbirth: Results from the Sexual P Questionnaire. J Psychosom Obstet Gynaecol. 2001;22(1):29-40.
13. Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Rsearch Group. Neurourol Urodyn. 1995;14(2):131-139.
14. Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC; Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281-289.
15. Handa VL. Sexual function and childbirth. Semin Perinatol. 2006;30(5):253-256.
16. Klein MC, Gauthier RJ, Robbins JM, et al. Relationship of episiotomy to perineal trauma and morbidity, sexual dysfunction, and pelvic floor relaxation. Am J Obstet Gynecol. 1994;171(3):591-598.
17. Barrett G, Pendry E, Peacock J, Victor C, Thakar, Manyonda I. Women’s sexual health after childbirth. BJOG. 2000;107(2):186-195.
18. Barrett G, Peacock J, Victor CR, Manyonda I. Cesarean section and postnatal sexual health. Birth. 2005;32(4):306-311.
19. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol. 2000;95(3):464-471.
20. Leeman LM, Rogers RG, Greulich B, Albers LL. Do unsutured second-degree perineal lacerations affect postpartum functional outcomes? J Am Board Fam Med. 2007;20(5):451-457.
21. Signorello L, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: A retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184(5):881-890.
22. Snooks SJ, Swash M, Henry MW, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. In J Colorect Dis. 1986;1(1):20-24.
23. Hannah ME, Whyte H, Hannah WJ, et al. Term Breech Trial Collaborative Group. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):917-927.
24. NIH State-of-the-Science Conference Statement on Cesarean Delivery on Maternal Request NIH Consens Sci Statements. 2006;23:1-29.http://consensus.nih.gov/2006/cesareanstatement.htm. Accessed December 6 2011.
25. Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001;15(3):232-240.
26. Chivers ML, Pittini MD, Grigoriadis S, Villegas L, Ross LE. The relationship between sexual functioning and depressive symptomatology in postpartum women: a pilot study. J Sex Med. 2011;8(3):792-799.
27. Morof D, Barrett G, Peacock J, Victor CR, Manyonda I. Postnatal depression and sexual health after childbirth. Obstet Gynecol. 2003;102(6):1318-1325.
28. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No.119: Female sexual dysfunction. Obstet Gynecol. 2011;117(4):996-1007.
29. Read J. Sexual problems associated with infertility pregnancy and ageing. BMJ. 2004;329(7465):559-561.
30. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
31. Isidori AM, Pozza C, Esposito K, et al. The Female Sexual Function Index (FSFI): Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med. 2010;7(3):1139-11.
32. McCoy NL. The McCoy Female Sexuality Questionnaire. Quality Life Res. 2000;9(suppl 6):739-745.
33. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 1):337-348.
34. DeRogatis LR, Allgood A, Rosen RC, Leiblum S, Zipfel L, Guo CY. Development and evaluation of the Women’s Sexual Interest Diagnostic Interview (WSID): a structured interview to diagnose hypoactive sexual desire disorder (HSDD) in standardized patients. J Sex Med. 2008;5(12):2827-2841.
35. Fischman SH, Rankin EA, Soeken KL, Lenz ER. Changes in sexual relationships in postpartum couples. J Obstet Gynecol Neonatal Nurs. 1986;15(1):58-63.
36. Sillis T, Wunderlich G, Pyke R, et al. The Sexual Interest and Desire Inventory-Female (SIDI-F): item response analyses of data from women diagnosed with hypoactive sexual desire disorder. J Sex Med. 2005;2(6):801-818.
37. Rust J, Golombok S. The Golombok-Rust Inventory of Sexual Satisfaction (GRISS). Br J Clin Psych. 1985;24(Pt 1):63-64.
38. Clayton AH, Balon R. The impact of mental illness and psychotropic medications on sexual functioning: the evidence and management. J Sex Med. 2009;6(5):1200-1213.
39. Jennings B, Edmundson M. The postpartum period: after confinement: the fourth trimester. Clin Obstet Gynecol. 1980;23(4):1093-1103.
40. Oppenheimer LS, Sheriff EA, Goodman JDS, shah D, James CE. The duration of lochia. Br J Obstet Gynaecol. 1986;93(7):754-757.
41. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):263-267.
42. Pancholy AB, Goldenhar L, Fellner AN, Crisp C, Kleeman S, Pauls R. Resident education and training in female sexuality: results of a national survey. J Sex Med. 2011;8(2):361-366.
1. Reamy KJ, White SE. Sexuality in the puerperium: a review. Arch Sex Behav. 1987;16(2):165-186.
2. De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39(2):94-103.
3. The puerperium. In: Cunningham FG Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY, eds. Williams Obstetrics. 23rd ed. New York, NY: McGraw-Hill Co.; 2010:646-660.
4. The American Academy of Pediatrics (AAP), American College of Obstetricians Gynecologists (ACOG) Guidelines for perinatal care. 6th ed. Washington DC: AAP, ACOG; 2008.
5. Glazener CM. Sexual function after childbirth: women’s experiences persistent morbidity and lack of professional recognition. Br J Obstet Gynaecol. 1997;104(3):330-335.
6. Rogers RG, Borders, N,, Leeman L, Albers L. Does spontaneous genital tract trauma impact postpartum sexual function? J Midwifery Womens Health. 2009;54(2):98-103.
7. von Sydow K. Sexuality during pregnancy and after childbirth: a metacontent analysis of 59 studies. J Psychosom Res. 1999;47(1):27-49.
8. Witting K, Santtila P, Alanko K, et al. Female sexual function and its associations with number of children, pregnancy, and relationship satisfaction. J Sex Marital Ther. 2008;34(2):89-106.
9. Thompson JF, Roberts CL, Currie M, Elwood DA. Prevalence and persistence of health problems after childbirth: associations with parity and method of birth. Birth. 2002;29(2):83-94.
10. Waterstone M, Wolfe C, Hooper R, Bewley S. Postnatal morbidity after childbirth and severe obstetric morbidity. BJOG. 2003;110(2):128-133.
11. Pauls RN, Occhino JA, Dryfhout VL. Effects of pregnancy on female sexual function and body image: A prospective study. J Sex Med. 2008;5(8):1915-1922.
12. von Sydow K, Ullmeyer M, Happ N. Sexual activity during pregnancy and after childbirth: Results from the Sexual P Questionnaire. J Psychosom Obstet Gynaecol. 2001;22(1):29-40.
13. Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Rsearch Group. Neurourol Urodyn. 1995;14(2):131-139.
14. Barber MD, Visco AG, Wyman JF, Fantl JA, Bump RC; Continence Program for Women Research Group. Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281-289.
15. Handa VL. Sexual function and childbirth. Semin Perinatol. 2006;30(5):253-256.
16. Klein MC, Gauthier RJ, Robbins JM, et al. Relationship of episiotomy to perineal trauma and morbidity, sexual dysfunction, and pelvic floor relaxation. Am J Obstet Gynecol. 1994;171(3):591-598.
17. Barrett G, Pendry E, Peacock J, Victor C, Thakar, Manyonda I. Women’s sexual health after childbirth. BJOG. 2000;107(2):186-195.
18. Barrett G, Peacock J, Victor CR, Manyonda I. Cesarean section and postnatal sexual health. Birth. 2005;32(4):306-311.
19. Eason E, Labrecque M, Wells G, Feldman P. Preventing perineal trauma during childbirth: a systematic review. Obstet Gynecol. 2000;95(3):464-471.
20. Leeman LM, Rogers RG, Greulich B, Albers LL. Do unsutured second-degree perineal lacerations affect postpartum functional outcomes? J Am Board Fam Med. 2007;20(5):451-457.
21. Signorello L, Harlow BL, Chekos AK, Repke JT. Postpartum sexual functioning and its relationship to perineal trauma: A retrospective cohort study of primiparous women. Am J Obstet Gynecol. 2001;184(5):881-890.
22. Snooks SJ, Swash M, Henry MW, Setchell M. Risk factors in childbirth causing damage to the pelvic floor innervation. In J Colorect Dis. 1986;1(1):20-24.
23. Hannah ME, Whyte H, Hannah WJ, et al. Term Breech Trial Collaborative Group. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: the international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):917-927.
24. NIH State-of-the-Science Conference Statement on Cesarean Delivery on Maternal Request NIH Consens Sci Statements. 2006;23:1-29.http://consensus.nih.gov/2006/cesareanstatement.htm. Accessed December 6 2011.
25. Lydon-Rochelle MT, Holt VL, Martin DP. Delivery method and self-reported postpartum general health status among primiparous women. Paediatr Perinat Epidemiol. 2001;15(3):232-240.
26. Chivers ML, Pittini MD, Grigoriadis S, Villegas L, Ross LE. The relationship between sexual functioning and depressive symptomatology in postpartum women: a pilot study. J Sex Med. 2011;8(3):792-799.
27. Morof D, Barrett G, Peacock J, Victor CR, Manyonda I. Postnatal depression and sexual health after childbirth. Obstet Gynecol. 2003;102(6):1318-1325.
28. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin No.119: Female sexual dysfunction. Obstet Gynecol. 2011;117(4):996-1007.
29. Read J. Sexual problems associated with infertility pregnancy and ageing. BMJ. 2004;329(7465):559-561.
30. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191-208.
31. Isidori AM, Pozza C, Esposito K, et al. The Female Sexual Function Index (FSFI): Development and validation of a 6-item version of the female sexual function index (FSFI) as a diagnostic tool for female sexual dysfunction. J Sex Med. 2010;7(3):1139-11.
32. McCoy NL. The McCoy Female Sexuality Questionnaire. Quality Life Res. 2000;9(suppl 6):739-745.
33. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 1):337-348.
34. DeRogatis LR, Allgood A, Rosen RC, Leiblum S, Zipfel L, Guo CY. Development and evaluation of the Women’s Sexual Interest Diagnostic Interview (WSID): a structured interview to diagnose hypoactive sexual desire disorder (HSDD) in standardized patients. J Sex Med. 2008;5(12):2827-2841.
35. Fischman SH, Rankin EA, Soeken KL, Lenz ER. Changes in sexual relationships in postpartum couples. J Obstet Gynecol Neonatal Nurs. 1986;15(1):58-63.
36. Sillis T, Wunderlich G, Pyke R, et al. The Sexual Interest and Desire Inventory-Female (SIDI-F): item response analyses of data from women diagnosed with hypoactive sexual desire disorder. J Sex Med. 2005;2(6):801-818.
37. Rust J, Golombok S. The Golombok-Rust Inventory of Sexual Satisfaction (GRISS). Br J Clin Psych. 1985;24(Pt 1):63-64.
38. Clayton AH, Balon R. The impact of mental illness and psychotropic medications on sexual functioning: the evidence and management. J Sex Med. 2009;6(5):1200-1213.
39. Jennings B, Edmundson M. The postpartum period: after confinement: the fourth trimester. Clin Obstet Gynecol. 1980;23(4):1093-1103.
40. Oppenheimer LS, Sheriff EA, Goodman JDS, shah D, James CE. The duration of lochia. Br J Obstet Gynaecol. 1986;93(7):754-757.
41. Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16(4):263-267.
42. Pancholy AB, Goldenhar L, Fellner AN, Crisp C, Kleeman S, Pauls R. Resident education and training in female sexuality: results of a national survey. J Sex Med. 2011;8(2):361-366.
Does folic acid supplementation have long-term benefit to offspring?
Prenatal folic acid supplementation to prevent open neural tube defects is accepted as standard obstetric practice. The possibility that relative folic acid deficiency in pregnancy could cause long-term childhood neurologic dysfunction (including severe language delay) without detectable anatomic abnormality is explored in this prospective pregnancy cohort study.
Details of the study
The Norwegian Mother and Child Cohort Study (MoBa) collected pregnancy and infant outcome data, including self-reported information on questionnaires, from 1999 to 2010. A total of 38,954 children were included in the study. The incidence of severe language delay—defined as use of only one word or unintelligible utterances—at 3 years of age was compared between pregnancies that involved folic acid supplementation (from 4 weeks before to 8 weeks after conception) and those that did not.
The incidence of severe language delay among children whose mothers took folic acid during pregnancy (n = 26,232) was 0.4%. Among children whose mothers did not take folic acid (n = 11,532), the incidence was 0.9%; this difference was found to be significant.
Care was taken to verify the accuracy of collected data and to assess the potential effects of confounding variables. When results were adjusted for maternal variables such as education, the initiation of folic acid after conception no longer had a protective effect, but initiation at 4 weeks prior to conception continued to provide benefit.
US mandate for folic acid enrichment reduced open neural tube defects
In 1996, the US Food and Drug Administration (FDA) mandated that folic acid be added to all cereals, flours, breads, and pastas.1 This action seems to have reduced the incidence of open neural tube defects in the United States.2 The MoBa study was carried out in Norway, which has declined to mandate folic acid enrichment of the food supply. Although the FDA mandate for folic acid fortification of food appears to have reduced the rate of open neural tube defects, the effect of folic acid supplementation on language development has not yet been explored.
Certain findings of the MoBa study suggest areas for future study or intervention. For example, paternal education of less than 12 years was associated with decreased maternal folic acid supplementation. Also, the incidence of severe language delay was more than three times greater in male children than in females. The study seemed to demonstrate improvement in groups at risk (e.g., male fetuses) with preconception and early pregnancy folic acid supplementation.
Severe language delay is a rare but severe childhood condition; folic acid may protect against such delay. In the United States, the FDA mandate that food be fortified with folic acid may provide some protection, but we lack direct evidence of its precise effects. Consequently, ideal preparation for pregnancy includes the use of folic acid (preferably, 1 mg/day), starting by 4 weeks prior to conception, in all countries.
PAUL L. OGBURN, JR, MD
We want to hear from you! Tell us what you think.
1. Food and Drug Administration. Food standards: Amendment of standards of identity for enriched grain products to require addition of folic acid; final rule (21 CFR Parts 136, 137, and 139). Federal Register. 1996;61:8781-8797.
2. Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LC. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981-2986.
Prenatal folic acid supplementation to prevent open neural tube defects is accepted as standard obstetric practice. The possibility that relative folic acid deficiency in pregnancy could cause long-term childhood neurologic dysfunction (including severe language delay) without detectable anatomic abnormality is explored in this prospective pregnancy cohort study.
Details of the study
The Norwegian Mother and Child Cohort Study (MoBa) collected pregnancy and infant outcome data, including self-reported information on questionnaires, from 1999 to 2010. A total of 38,954 children were included in the study. The incidence of severe language delay—defined as use of only one word or unintelligible utterances—at 3 years of age was compared between pregnancies that involved folic acid supplementation (from 4 weeks before to 8 weeks after conception) and those that did not.
The incidence of severe language delay among children whose mothers took folic acid during pregnancy (n = 26,232) was 0.4%. Among children whose mothers did not take folic acid (n = 11,532), the incidence was 0.9%; this difference was found to be significant.
Care was taken to verify the accuracy of collected data and to assess the potential effects of confounding variables. When results were adjusted for maternal variables such as education, the initiation of folic acid after conception no longer had a protective effect, but initiation at 4 weeks prior to conception continued to provide benefit.
US mandate for folic acid enrichment reduced open neural tube defects
In 1996, the US Food and Drug Administration (FDA) mandated that folic acid be added to all cereals, flours, breads, and pastas.1 This action seems to have reduced the incidence of open neural tube defects in the United States.2 The MoBa study was carried out in Norway, which has declined to mandate folic acid enrichment of the food supply. Although the FDA mandate for folic acid fortification of food appears to have reduced the rate of open neural tube defects, the effect of folic acid supplementation on language development has not yet been explored.
Certain findings of the MoBa study suggest areas for future study or intervention. For example, paternal education of less than 12 years was associated with decreased maternal folic acid supplementation. Also, the incidence of severe language delay was more than three times greater in male children than in females. The study seemed to demonstrate improvement in groups at risk (e.g., male fetuses) with preconception and early pregnancy folic acid supplementation.
Severe language delay is a rare but severe childhood condition; folic acid may protect against such delay. In the United States, the FDA mandate that food be fortified with folic acid may provide some protection, but we lack direct evidence of its precise effects. Consequently, ideal preparation for pregnancy includes the use of folic acid (preferably, 1 mg/day), starting by 4 weeks prior to conception, in all countries.
PAUL L. OGBURN, JR, MD
We want to hear from you! Tell us what you think.
Prenatal folic acid supplementation to prevent open neural tube defects is accepted as standard obstetric practice. The possibility that relative folic acid deficiency in pregnancy could cause long-term childhood neurologic dysfunction (including severe language delay) without detectable anatomic abnormality is explored in this prospective pregnancy cohort study.
Details of the study
The Norwegian Mother and Child Cohort Study (MoBa) collected pregnancy and infant outcome data, including self-reported information on questionnaires, from 1999 to 2010. A total of 38,954 children were included in the study. The incidence of severe language delay—defined as use of only one word or unintelligible utterances—at 3 years of age was compared between pregnancies that involved folic acid supplementation (from 4 weeks before to 8 weeks after conception) and those that did not.
The incidence of severe language delay among children whose mothers took folic acid during pregnancy (n = 26,232) was 0.4%. Among children whose mothers did not take folic acid (n = 11,532), the incidence was 0.9%; this difference was found to be significant.
Care was taken to verify the accuracy of collected data and to assess the potential effects of confounding variables. When results were adjusted for maternal variables such as education, the initiation of folic acid after conception no longer had a protective effect, but initiation at 4 weeks prior to conception continued to provide benefit.
US mandate for folic acid enrichment reduced open neural tube defects
In 1996, the US Food and Drug Administration (FDA) mandated that folic acid be added to all cereals, flours, breads, and pastas.1 This action seems to have reduced the incidence of open neural tube defects in the United States.2 The MoBa study was carried out in Norway, which has declined to mandate folic acid enrichment of the food supply. Although the FDA mandate for folic acid fortification of food appears to have reduced the rate of open neural tube defects, the effect of folic acid supplementation on language development has not yet been explored.
Certain findings of the MoBa study suggest areas for future study or intervention. For example, paternal education of less than 12 years was associated with decreased maternal folic acid supplementation. Also, the incidence of severe language delay was more than three times greater in male children than in females. The study seemed to demonstrate improvement in groups at risk (e.g., male fetuses) with preconception and early pregnancy folic acid supplementation.
Severe language delay is a rare but severe childhood condition; folic acid may protect against such delay. In the United States, the FDA mandate that food be fortified with folic acid may provide some protection, but we lack direct evidence of its precise effects. Consequently, ideal preparation for pregnancy includes the use of folic acid (preferably, 1 mg/day), starting by 4 weeks prior to conception, in all countries.
PAUL L. OGBURN, JR, MD
We want to hear from you! Tell us what you think.
1. Food and Drug Administration. Food standards: Amendment of standards of identity for enriched grain products to require addition of folic acid; final rule (21 CFR Parts 136, 137, and 139). Federal Register. 1996;61:8781-8797.
2. Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LC. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981-2986.
1. Food and Drug Administration. Food standards: Amendment of standards of identity for enriched grain products to require addition of folic acid; final rule (21 CFR Parts 136, 137, and 139). Federal Register. 1996;61:8781-8797.
2. Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LC. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001;285(23):2981-2986.




