User login
Evidence Suggests Pregnancies Can Survive Maternal Cancer Treatment
Emerging data on pregnancy and cancer can now help women and their doctors chart a safer course between effective treatment and protecting the developing fetus.
Two registries – one in the United States and another in Europe – agree: It’s not only possible to save a pregnancy in many situations, but children born to these women appear to be largely unaffected by in utero chemotherapy exposure. The combined studies followed more than 200 exposed children for up to 18 years; neither one found any elevated risk of congenital anomaly or any kind of cognitive or developmental delay.
"This is practice-changing information," Dr. Frédéric Amant, primary author on the European paper, said in an interview. "Until now, physicians were reluctant to administer chemotherapy and usually opted for termination, or at least for a delay in treatment or premature delivery in order to get treatment going," he said.
Dr. Amant’s study leads off a special section on cancer in pregnancy, published in the March issue of Lancet Oncology. The paper examined evidence that both supports and questions the clinical wisdom of treating a disease that threatens two very different patients – an adult woman and the fetus she carries.
Every case is different, depending on the type of cancer, its grade and potential aggressiveness, the stage of pregnancy, and the woman’s own desires, said Dr. Elyce Cardonick, an ob.gyn. at Cooper University Hospital in Camden, N.J., and the lead investigator of the Pregnancy and Cancer Registry.
"With solid tumors you usually have time to do the surgery, get the pathologic diagnosis, and let the patient recover. But if there is leukemia, for instance, you have to move faster. The first question should be ‘Could we delay treatment for this patient if she was not pregnant?’ I don’t want to limit their treatment, but at the same time, it’s true that they might not be getting the most up-to-date treatment – the newest agent on the block – because you would want to go with something there is at least some experience with. But this doesn’t necessarily mean they are going to do worse."
Cancer occurs in about 1 in 1,000 pregnancies, said Dr. Sarah Temkin, a gynecologic oncologist at the University of Maryland, Baltimore. Many of her patients are referred after a routine screening during early pregnancy finds something abnormal, or when a woman with an existing cancer is incidentally found to be pregnant. But signs that might raise a red flag in other situations don’t necessarily alert physicians to danger in pregnant women, she said in an interview. Pregnancy could obfuscate some symptoms, which might be further downplayed in light of a mother’s relatively young age. Breast cancer is a prime example.
"There are two problems, especially for breast cancers, which are the most common ones we see in pregnancy. First, a woman’s breasts are changing anyway during that time. Breast cancer is so rare in women of earlier childbearing age that both the patient and the doctor tend to disregard any new lumps and bumps."
But despite its rarity, cancer in all forms appears to be increasing among pregnant women, she said. This is probably a direct relation to age. "Cancer rates increase with increasing age, and women are becoming mothers at older and older ages."
When cancer coincides with pregnancy, Dr. Temkin views the mother’s health as paramount. "The mother is the person with cancer, and she deserves whatever the standard of care is for that particular cancer – the best care that would be offered to her if she was not pregnant."
Chemotherapy and Fetal Outcomes
To examine long-term neurodevelopmental risks associated with maternal cancer treatment, Dr. Amant, a gynecologic oncologist at the Leuven (Belgium) Cancer Institute, and his colleagues, are following 70 children from the age of 18 months until 18 years. In the newly published interim analysis, the mean follow-up time is 22 months. All the children had intrauterine exposure to chemotherapy, radiation, oncologic surgery, or combinations of these.
The analysis, which also appeared in Lancet Oncology’s special issue, includes data on all the children, including 18 who are now older than 9 years (Lancet Oncol. 2012;13:256-64).
The children – 68 singletons and one set of twins – were born from pregnancies exposed to a total of 236 chemotherapy cycles. Exposure varied by the mother’s cancer type and its stage at diagnosis. In all, 34 mothers were treated with only chemotherapy, 27 had chemotherapy and surgery, 1 received chemotherapy and radiation, and 6 women were treated with all three modalities. Most of the children also had in utero exposure to multiple imaging studies, including MRI, ultrasound, echocardiography, CT, and mammography. Chemotherapy regimens included doxorubicin, epirubicin, idarubicin and daunorubicin.
Fetuses also were exposed to a variety of other drugs, including antibiotics, antiemetics, pain medications, colony stimulating factors, and anxiolytics.
Breast cancer was the most common disease type (35). There were 18 cases of hematologic cancers, 6 ovarian cancers, 4 cervical cancers, and 1 each of basal cell carcinoma, brain tumor, Ewing’s sarcoma, colorectal cancer, and nasopharyngeal cancer.
The mean gestational age at cancer diagnosis was 18 weeks, although fetuses ranged in age from 2 to 33 weeks when their mothers were diagnosed. About a third of the babies (23) were born at term.
Seven were born at 28-32 weeks, nine at 32-34 weeks, and 31 at 34-37 weeks. Weight for gestational age was below the 10th percentile in 14 children (21%).
Seven congenital anomalies were found in the group of 70 children (10%) – a rate not significantly different from that in the background population. There were only two major malformations, for a 2.9% rate. Malformations included the following:
• Hip subluxation, pectus excavatum and hemangioma, associated with chemotherapy only.
• Bilateral partial syndactyly, associated with chemotherapy plus radiotherapy.
• Bilateral small protuberance on one finger, and rectal atresia, associated with chemotherapy plus surgery.
• Bilateral double cartilage ring in a child exposed to chemotherapy, surgery and radiotherapy.
None of the children showed any congenital cardiac issues.
All of the children showed neurocognitive development that was within normal range, except for the set of twins, who were delivered by cesarean section at 32.5 weeks after a preterm premature rupture of membranes. These children were so delayed that they were not able to complete cognitive testing. Their mother developed an acute myeloid leukemia – one of the true "emergency" cancers diagnosed in pregnancy, Dr. Amant said. The babies had been exposed to idarubicin and cytosine arabinoside at 15.5, 21.5, 26.5, and 31.5 weeks’ gestation.
The boy, who weighed 1,640 g at birth, had a normal karyotype but, at 3 years, brain imaging showed a unilateral polymicrogyria in the left perisylvian area. He showed an early developmental delay; at age 9 years, he had the developmental capacity of a 12-month old.
The girl, who weighed 1,390 g at birth, also had an early developmental delay, but at age 9 years she attended school with support. Her parents refused brain imaging.
"The other 68 children did well," Dr. Amant said. "This doesn’t mean they were all normal in every way, but in any population you will see learning and developmental delay issues. We think the problem for the delayed children was not related to chemotherapy exposure, but more likely to their prematurity."
Dr. Amant saw a direct correlation between gestational age and intelligence quota. "When we controlled for age, gender, and country of birth, we found that the IQ score increased by almost 12 points for each additional month of gestation."
The U.S. Experience
Dr. Cardonick has found similar results. Her registry now contains information on 280 women who were enrolled over a 13-year period. The children born from these pregnancies have been assessed annually since birth. It also includes 70 controls – children whose mothers had cancer but who were not exposed to chemotherapy.
She published interim results of the registry in 2010 (Cancer J. 2010;16:76-82). At that point, it contained information on 231 women and 157 children. The most common malignancy was breast cancer (128); the mean gestational age at diagnosis was 13 weeks. About a third of the women (54) were advised to terminate their pregnancy; 12 did so.
Among those who continued both pregnancy and treatment, neonatal outcomes were generally good. There were nine premature deliveries related to preterm labor or premature rupture of membranes. The congenital anomaly rate born was 4%, which was in line with the normal background rate and slightly lower than that seen in Dr. Amant’s cohort.
The infants’ mean birth weight was 2,647 g, which was significantly less than the mean 2,873 g in the control group, but probably clinically irrelevant, Dr. Cardonick wrote in the paper.
She continues to follow these children annually. At this point, the children are a mean 5 years old; her oldest subject is 14 years. So far, the rate of neurocognitive issues in the group is no different than would be observed in any other group, and none of the children has developed any health problem that could be conclusively tied to intrauterine chemotherapy exposure.
Her experience stresses several key factors that must be considered in this situation. Her patients received chemotherapy at a mean of 20 weeks’ gestation – safely outside the critical early period. Only two women received chemotherapy before 10 weeks; both were treated before they knew they were pregnant. Both children born of these pregnancies were considered well. One with intrauterine exposure to cytarabine was developmentally normal at age 7 years. The other, who was exposed to oxaliplatin and capecitabine, was normal at age 2 years.
Treatment also was stopped a mean of 40 days before delivery, to allow the mother’s bone marrow to fully recover before giving birth.
A second U.S. study was reported at the 2011 meeting of the American Society of Clinical Oncology. A poster by Dr. Jennifer Litton and her colleagues examined physiological outcomes in 81 children exposed to chemotherapy for maternal breast cancer. The mothers had taken a standardized chemotherapy regimen of 5-fluorouracil, doxorubicin and cyclophosphamide (FAC) given during the second and third trimesters (J. Clin. Oncol. 2011;29[May 20 suppl.]:abstract 1099).
One child was born with Down syndrome, one with a club foot, and one with ureteral reflux. Three parents reported language delay in later follow-up surveys. Other reported health issues included 15 children with allergies and/or eczema, 2 with asthma, and 1 with absence seizures.
Dr. Litton, a breast oncologist at the University of Texas M.D. Anderson Cancer Center in Houston, also cowrote a 2010 review of breast cancer treatment in pregnancy, in which she discusses maternal and fetal outcomes from several cohorts, and the possible impact of intrauterine exposure to a variety of chemotherapy agents (Oncologist 2010;15:1238-47).
Risks Vary With Cancer Type
Breast cancer during pregnancy may be the simplest to treat. If the cancer is caught very early, it may be reasonable to delay treatment until the fetus has passed the critical first trimester, waiting until organs are formed and the risk of chemically induced damage is reduced, Dr. Temkin said. "It’s safe to do breast surgery during pregnancy and it’s safe to give chemotherapy after the first trimester."
But physicians can miss a new breast tumor during a prenatal exam, so some present at a more advanced stage, according to Dr. Amant, who is also the lead author of the Lancet’s breast cancer report (Lancet 2012;379:570-9).
Infiltrating ductal adenocarcinomas account for more than 70% of the breast cancers diagnosed during pregnancy. These can be aggressive, said Dr. Amant. Estrogen receptor status is probably no different in pregnant and nonpregnant women.
If the tumor is discovered early and is pathologically favorable, chemotherapy probably can be delayed until 14 weeks’ gestation, allowing nearly complete fetal organogenesis without worsening the mother’s outcome. Women also may elect an early termination if the pathology is unfavorable, or for other personal reasons, Dr. Temkin said. "I think a lot of it depends on when the cancer is diagnosed. Patients of mine who already have a diagnosis and then become pregnant almost always elect to terminate. But if the cancer is discovered when the pregnancy is farther along, most will continue, especially if the woman is highly emotionally invested," she noted.
Tougher Cancers, Tougher Choices
Treating gynecologic cancers during pregnancy often comes down to a choice between the mother’s health and maintenance of the pregnancy, Dr. Temkin said. "The standard of care for ovarian cancers is surgery or radiation to the pelvis, where the fetus is. Cervical cancer is treated with a hysterectomy or radiation, and neither treatment is compatible with keeping a pregnancy. Neoadjuvant therapy is not considered standard of care for these tumors. These are complex decisions for the patient: ‘Do I accept a different treatment [that might not be as effective] or maintain the pregnancy?’ "
In early cervical cancers without nodal spread, the most common tactic is close observation with periodic imaging to rule out spread; therapy is given after delivery, Dr. Phillippe Morice wrote in the Lancet section’s review on gynecologic malignancies (Lancet 2012;379:558-69).
"Delayed treatment until fetal maturation for patients with stage IA disease has an excellent prognosis and is now the standard of care," wrote Dr. Morice of the Institut de Cancérologie Gustave-Roussy in Villejuif, France.
Locally advanced disease is often not compatible with pregnancy. "The main treatment choice is either neoadjuvant chemotherapy or chemotherapy and radiotherapy. In pregnant patients, this approach means that the pregnancy must be ended before the initiation of therapy, but in exceptional cases in which surgery to end the pregnancy is not technically feasible ([that is], a bulky cervical tumor), radiation therapy can be delivered with the fetus in utero, resulting in a spontaneous abortion in about 3 weeks," he wrote.
Ovarian tumors can be surgically staged and – if it is of low malignant potential – can be laparoscopically removed, usually without endangering the pregnancy. Large tumors or those with aggressive pathology, like epithelial tumors, are much more difficult. Advanced or large tumors often have uterine and pelvic involvement, and treatment usually means a hysterectomy.
The literature contains reports of a very few women who have undergone chemotherapy to control peritoneal spread while keeping a pregnancy. However, despite giving birth to normally developed children, a number of these women died from recurrent disease, Dr. Morice noted.
Hematologic Cancers: True Emergency
Cancers of the blood are rare in pregnancy, occurring in only 1 of every 6,000. But when they do occur, they can be devastating, Dr. Benjamin Brenner wrote in the special series (Lancet 2012;379:580-7).
Pregnant patients with Hodgkin’s lymphoma generally do as well as their nonpregnant counterparts and can receive the same chemotherapy regimens, observing the first-trimester delay to favor the fetus.
Those who present with non-Hodgkin’s lymphoma are likely to have a very poor outlook. This disease is very rare in pregnant women, and symptoms can overlap with Hodgkin’s. Those factors, combined with a desire to avoid imaging, can delay diagnosis until the cancer is more advanced, said Dr. Brenner of the Rambam Health Care Campus, Haifa, Israel.
Acute leukemia is also rare, but demands urgent attention regardless of gestational stage, Dr. Brenner warned. "Patients diagnosed with acute leukemia during the first trimester are recommended to terminate the pregnancy, in view of the high risk of toxic effects on the fetus and mother, along with the expected need for further intensive treatment including stem-cell transplantation, which is absolutely contraindicated during gestation."
Talking It Out
Despite the emerging positive evidence, treating cancer during pregnancy can be a tough sell, Dr. Amant said. "Women have been told over and over to avoid taking so much as an aspirin. It’s very difficult to convince them that a fetus can not only survive a mother’s cancer treatment, but have a good chance of developing normally."
The stress of a cancer diagnosis during a desired pregnancy is very hard on patients, Dr. Temkin added. "Pregnancy is a time when many women come to grips with their own mortality as well as that of giving new life. Adding a diagnosis of cancer of top of that – especially in the face of a much-desired pregnancy – can be devastating."
These women are faced with two options: terminate the pregnancy and concentrate on their own treatment, or continue the pregnancy knowing that their unborn child will be exposed to the possible risks of radiation, chemotherapy, and surgery. Either option can "inflict terrible guilt on a pregnant woman. We can try to minimize that to some degree, but it’s important to know from the outset that what is the right solution for one patient is not right for the next."
Connecting with other women who have experienced the same situation can be of immense value, Dr. Cardonick said. She participates in an online support group called "Hope for Two."
The organization’s main goal is to link new patients with survivors who can help educate them as well as lend emotional support. Patients call in or fill out a secure online request for a personal match-up with a survivor, who is often a woman who has had the same type of cancer.
The website also contains links to news and medical articles, books, and financial assistance sources, and allows new patients to securely contact Dr. Cardonick’s pregnancy registry. "We keep in touch with the baby’s pediatrician and the mom every year, to see how things are going and [to] collect information," she said. "The best way to treat these women in the future depends on the information we continue to gather in the present."
None of the researchers interviewed for this article had any relevant financial disclosures. Dr. Litton noted that she had no financial disclosures for her 2011 ASCO poster.
Emerging data on pregnancy and cancer can now help women and their doctors chart a safer course between effective treatment and protecting the developing fetus.
Two registries – one in the United States and another in Europe – agree: It’s not only possible to save a pregnancy in many situations, but children born to these women appear to be largely unaffected by in utero chemotherapy exposure. The combined studies followed more than 200 exposed children for up to 18 years; neither one found any elevated risk of congenital anomaly or any kind of cognitive or developmental delay.
"This is practice-changing information," Dr. Frédéric Amant, primary author on the European paper, said in an interview. "Until now, physicians were reluctant to administer chemotherapy and usually opted for termination, or at least for a delay in treatment or premature delivery in order to get treatment going," he said.
Dr. Amant’s study leads off a special section on cancer in pregnancy, published in the March issue of Lancet Oncology. The paper examined evidence that both supports and questions the clinical wisdom of treating a disease that threatens two very different patients – an adult woman and the fetus she carries.
Every case is different, depending on the type of cancer, its grade and potential aggressiveness, the stage of pregnancy, and the woman’s own desires, said Dr. Elyce Cardonick, an ob.gyn. at Cooper University Hospital in Camden, N.J., and the lead investigator of the Pregnancy and Cancer Registry.
"With solid tumors you usually have time to do the surgery, get the pathologic diagnosis, and let the patient recover. But if there is leukemia, for instance, you have to move faster. The first question should be ‘Could we delay treatment for this patient if she was not pregnant?’ I don’t want to limit their treatment, but at the same time, it’s true that they might not be getting the most up-to-date treatment – the newest agent on the block – because you would want to go with something there is at least some experience with. But this doesn’t necessarily mean they are going to do worse."
Cancer occurs in about 1 in 1,000 pregnancies, said Dr. Sarah Temkin, a gynecologic oncologist at the University of Maryland, Baltimore. Many of her patients are referred after a routine screening during early pregnancy finds something abnormal, or when a woman with an existing cancer is incidentally found to be pregnant. But signs that might raise a red flag in other situations don’t necessarily alert physicians to danger in pregnant women, she said in an interview. Pregnancy could obfuscate some symptoms, which might be further downplayed in light of a mother’s relatively young age. Breast cancer is a prime example.
"There are two problems, especially for breast cancers, which are the most common ones we see in pregnancy. First, a woman’s breasts are changing anyway during that time. Breast cancer is so rare in women of earlier childbearing age that both the patient and the doctor tend to disregard any new lumps and bumps."
But despite its rarity, cancer in all forms appears to be increasing among pregnant women, she said. This is probably a direct relation to age. "Cancer rates increase with increasing age, and women are becoming mothers at older and older ages."
When cancer coincides with pregnancy, Dr. Temkin views the mother’s health as paramount. "The mother is the person with cancer, and she deserves whatever the standard of care is for that particular cancer – the best care that would be offered to her if she was not pregnant."
Chemotherapy and Fetal Outcomes
To examine long-term neurodevelopmental risks associated with maternal cancer treatment, Dr. Amant, a gynecologic oncologist at the Leuven (Belgium) Cancer Institute, and his colleagues, are following 70 children from the age of 18 months until 18 years. In the newly published interim analysis, the mean follow-up time is 22 months. All the children had intrauterine exposure to chemotherapy, radiation, oncologic surgery, or combinations of these.
The analysis, which also appeared in Lancet Oncology’s special issue, includes data on all the children, including 18 who are now older than 9 years (Lancet Oncol. 2012;13:256-64).
The children – 68 singletons and one set of twins – were born from pregnancies exposed to a total of 236 chemotherapy cycles. Exposure varied by the mother’s cancer type and its stage at diagnosis. In all, 34 mothers were treated with only chemotherapy, 27 had chemotherapy and surgery, 1 received chemotherapy and radiation, and 6 women were treated with all three modalities. Most of the children also had in utero exposure to multiple imaging studies, including MRI, ultrasound, echocardiography, CT, and mammography. Chemotherapy regimens included doxorubicin, epirubicin, idarubicin and daunorubicin.
Fetuses also were exposed to a variety of other drugs, including antibiotics, antiemetics, pain medications, colony stimulating factors, and anxiolytics.
Breast cancer was the most common disease type (35). There were 18 cases of hematologic cancers, 6 ovarian cancers, 4 cervical cancers, and 1 each of basal cell carcinoma, brain tumor, Ewing’s sarcoma, colorectal cancer, and nasopharyngeal cancer.
The mean gestational age at cancer diagnosis was 18 weeks, although fetuses ranged in age from 2 to 33 weeks when their mothers were diagnosed. About a third of the babies (23) were born at term.
Seven were born at 28-32 weeks, nine at 32-34 weeks, and 31 at 34-37 weeks. Weight for gestational age was below the 10th percentile in 14 children (21%).
Seven congenital anomalies were found in the group of 70 children (10%) – a rate not significantly different from that in the background population. There were only two major malformations, for a 2.9% rate. Malformations included the following:
• Hip subluxation, pectus excavatum and hemangioma, associated with chemotherapy only.
• Bilateral partial syndactyly, associated with chemotherapy plus radiotherapy.
• Bilateral small protuberance on one finger, and rectal atresia, associated with chemotherapy plus surgery.
• Bilateral double cartilage ring in a child exposed to chemotherapy, surgery and radiotherapy.
None of the children showed any congenital cardiac issues.
All of the children showed neurocognitive development that was within normal range, except for the set of twins, who were delivered by cesarean section at 32.5 weeks after a preterm premature rupture of membranes. These children were so delayed that they were not able to complete cognitive testing. Their mother developed an acute myeloid leukemia – one of the true "emergency" cancers diagnosed in pregnancy, Dr. Amant said. The babies had been exposed to idarubicin and cytosine arabinoside at 15.5, 21.5, 26.5, and 31.5 weeks’ gestation.
The boy, who weighed 1,640 g at birth, had a normal karyotype but, at 3 years, brain imaging showed a unilateral polymicrogyria in the left perisylvian area. He showed an early developmental delay; at age 9 years, he had the developmental capacity of a 12-month old.
The girl, who weighed 1,390 g at birth, also had an early developmental delay, but at age 9 years she attended school with support. Her parents refused brain imaging.
"The other 68 children did well," Dr. Amant said. "This doesn’t mean they were all normal in every way, but in any population you will see learning and developmental delay issues. We think the problem for the delayed children was not related to chemotherapy exposure, but more likely to their prematurity."
Dr. Amant saw a direct correlation between gestational age and intelligence quota. "When we controlled for age, gender, and country of birth, we found that the IQ score increased by almost 12 points for each additional month of gestation."
The U.S. Experience
Dr. Cardonick has found similar results. Her registry now contains information on 280 women who were enrolled over a 13-year period. The children born from these pregnancies have been assessed annually since birth. It also includes 70 controls – children whose mothers had cancer but who were not exposed to chemotherapy.
She published interim results of the registry in 2010 (Cancer J. 2010;16:76-82). At that point, it contained information on 231 women and 157 children. The most common malignancy was breast cancer (128); the mean gestational age at diagnosis was 13 weeks. About a third of the women (54) were advised to terminate their pregnancy; 12 did so.
Among those who continued both pregnancy and treatment, neonatal outcomes were generally good. There were nine premature deliveries related to preterm labor or premature rupture of membranes. The congenital anomaly rate born was 4%, which was in line with the normal background rate and slightly lower than that seen in Dr. Amant’s cohort.
The infants’ mean birth weight was 2,647 g, which was significantly less than the mean 2,873 g in the control group, but probably clinically irrelevant, Dr. Cardonick wrote in the paper.
She continues to follow these children annually. At this point, the children are a mean 5 years old; her oldest subject is 14 years. So far, the rate of neurocognitive issues in the group is no different than would be observed in any other group, and none of the children has developed any health problem that could be conclusively tied to intrauterine chemotherapy exposure.
Her experience stresses several key factors that must be considered in this situation. Her patients received chemotherapy at a mean of 20 weeks’ gestation – safely outside the critical early period. Only two women received chemotherapy before 10 weeks; both were treated before they knew they were pregnant. Both children born of these pregnancies were considered well. One with intrauterine exposure to cytarabine was developmentally normal at age 7 years. The other, who was exposed to oxaliplatin and capecitabine, was normal at age 2 years.
Treatment also was stopped a mean of 40 days before delivery, to allow the mother’s bone marrow to fully recover before giving birth.
A second U.S. study was reported at the 2011 meeting of the American Society of Clinical Oncology. A poster by Dr. Jennifer Litton and her colleagues examined physiological outcomes in 81 children exposed to chemotherapy for maternal breast cancer. The mothers had taken a standardized chemotherapy regimen of 5-fluorouracil, doxorubicin and cyclophosphamide (FAC) given during the second and third trimesters (J. Clin. Oncol. 2011;29[May 20 suppl.]:abstract 1099).
One child was born with Down syndrome, one with a club foot, and one with ureteral reflux. Three parents reported language delay in later follow-up surveys. Other reported health issues included 15 children with allergies and/or eczema, 2 with asthma, and 1 with absence seizures.
Dr. Litton, a breast oncologist at the University of Texas M.D. Anderson Cancer Center in Houston, also cowrote a 2010 review of breast cancer treatment in pregnancy, in which she discusses maternal and fetal outcomes from several cohorts, and the possible impact of intrauterine exposure to a variety of chemotherapy agents (Oncologist 2010;15:1238-47).
Risks Vary With Cancer Type
Breast cancer during pregnancy may be the simplest to treat. If the cancer is caught very early, it may be reasonable to delay treatment until the fetus has passed the critical first trimester, waiting until organs are formed and the risk of chemically induced damage is reduced, Dr. Temkin said. "It’s safe to do breast surgery during pregnancy and it’s safe to give chemotherapy after the first trimester."
But physicians can miss a new breast tumor during a prenatal exam, so some present at a more advanced stage, according to Dr. Amant, who is also the lead author of the Lancet’s breast cancer report (Lancet 2012;379:570-9).
Infiltrating ductal adenocarcinomas account for more than 70% of the breast cancers diagnosed during pregnancy. These can be aggressive, said Dr. Amant. Estrogen receptor status is probably no different in pregnant and nonpregnant women.
If the tumor is discovered early and is pathologically favorable, chemotherapy probably can be delayed until 14 weeks’ gestation, allowing nearly complete fetal organogenesis without worsening the mother’s outcome. Women also may elect an early termination if the pathology is unfavorable, or for other personal reasons, Dr. Temkin said. "I think a lot of it depends on when the cancer is diagnosed. Patients of mine who already have a diagnosis and then become pregnant almost always elect to terminate. But if the cancer is discovered when the pregnancy is farther along, most will continue, especially if the woman is highly emotionally invested," she noted.
Tougher Cancers, Tougher Choices
Treating gynecologic cancers during pregnancy often comes down to a choice between the mother’s health and maintenance of the pregnancy, Dr. Temkin said. "The standard of care for ovarian cancers is surgery or radiation to the pelvis, where the fetus is. Cervical cancer is treated with a hysterectomy or radiation, and neither treatment is compatible with keeping a pregnancy. Neoadjuvant therapy is not considered standard of care for these tumors. These are complex decisions for the patient: ‘Do I accept a different treatment [that might not be as effective] or maintain the pregnancy?’ "
In early cervical cancers without nodal spread, the most common tactic is close observation with periodic imaging to rule out spread; therapy is given after delivery, Dr. Phillippe Morice wrote in the Lancet section’s review on gynecologic malignancies (Lancet 2012;379:558-69).
"Delayed treatment until fetal maturation for patients with stage IA disease has an excellent prognosis and is now the standard of care," wrote Dr. Morice of the Institut de Cancérologie Gustave-Roussy in Villejuif, France.
Locally advanced disease is often not compatible with pregnancy. "The main treatment choice is either neoadjuvant chemotherapy or chemotherapy and radiotherapy. In pregnant patients, this approach means that the pregnancy must be ended before the initiation of therapy, but in exceptional cases in which surgery to end the pregnancy is not technically feasible ([that is], a bulky cervical tumor), radiation therapy can be delivered with the fetus in utero, resulting in a spontaneous abortion in about 3 weeks," he wrote.
Ovarian tumors can be surgically staged and – if it is of low malignant potential – can be laparoscopically removed, usually without endangering the pregnancy. Large tumors or those with aggressive pathology, like epithelial tumors, are much more difficult. Advanced or large tumors often have uterine and pelvic involvement, and treatment usually means a hysterectomy.
The literature contains reports of a very few women who have undergone chemotherapy to control peritoneal spread while keeping a pregnancy. However, despite giving birth to normally developed children, a number of these women died from recurrent disease, Dr. Morice noted.
Hematologic Cancers: True Emergency
Cancers of the blood are rare in pregnancy, occurring in only 1 of every 6,000. But when they do occur, they can be devastating, Dr. Benjamin Brenner wrote in the special series (Lancet 2012;379:580-7).
Pregnant patients with Hodgkin’s lymphoma generally do as well as their nonpregnant counterparts and can receive the same chemotherapy regimens, observing the first-trimester delay to favor the fetus.
Those who present with non-Hodgkin’s lymphoma are likely to have a very poor outlook. This disease is very rare in pregnant women, and symptoms can overlap with Hodgkin’s. Those factors, combined with a desire to avoid imaging, can delay diagnosis until the cancer is more advanced, said Dr. Brenner of the Rambam Health Care Campus, Haifa, Israel.
Acute leukemia is also rare, but demands urgent attention regardless of gestational stage, Dr. Brenner warned. "Patients diagnosed with acute leukemia during the first trimester are recommended to terminate the pregnancy, in view of the high risk of toxic effects on the fetus and mother, along with the expected need for further intensive treatment including stem-cell transplantation, which is absolutely contraindicated during gestation."
Talking It Out
Despite the emerging positive evidence, treating cancer during pregnancy can be a tough sell, Dr. Amant said. "Women have been told over and over to avoid taking so much as an aspirin. It’s very difficult to convince them that a fetus can not only survive a mother’s cancer treatment, but have a good chance of developing normally."
The stress of a cancer diagnosis during a desired pregnancy is very hard on patients, Dr. Temkin added. "Pregnancy is a time when many women come to grips with their own mortality as well as that of giving new life. Adding a diagnosis of cancer of top of that – especially in the face of a much-desired pregnancy – can be devastating."
These women are faced with two options: terminate the pregnancy and concentrate on their own treatment, or continue the pregnancy knowing that their unborn child will be exposed to the possible risks of radiation, chemotherapy, and surgery. Either option can "inflict terrible guilt on a pregnant woman. We can try to minimize that to some degree, but it’s important to know from the outset that what is the right solution for one patient is not right for the next."
Connecting with other women who have experienced the same situation can be of immense value, Dr. Cardonick said. She participates in an online support group called "Hope for Two."
The organization’s main goal is to link new patients with survivors who can help educate them as well as lend emotional support. Patients call in or fill out a secure online request for a personal match-up with a survivor, who is often a woman who has had the same type of cancer.
The website also contains links to news and medical articles, books, and financial assistance sources, and allows new patients to securely contact Dr. Cardonick’s pregnancy registry. "We keep in touch with the baby’s pediatrician and the mom every year, to see how things are going and [to] collect information," she said. "The best way to treat these women in the future depends on the information we continue to gather in the present."
None of the researchers interviewed for this article had any relevant financial disclosures. Dr. Litton noted that she had no financial disclosures for her 2011 ASCO poster.
Emerging data on pregnancy and cancer can now help women and their doctors chart a safer course between effective treatment and protecting the developing fetus.
Two registries – one in the United States and another in Europe – agree: It’s not only possible to save a pregnancy in many situations, but children born to these women appear to be largely unaffected by in utero chemotherapy exposure. The combined studies followed more than 200 exposed children for up to 18 years; neither one found any elevated risk of congenital anomaly or any kind of cognitive or developmental delay.
"This is practice-changing information," Dr. Frédéric Amant, primary author on the European paper, said in an interview. "Until now, physicians were reluctant to administer chemotherapy and usually opted for termination, or at least for a delay in treatment or premature delivery in order to get treatment going," he said.
Dr. Amant’s study leads off a special section on cancer in pregnancy, published in the March issue of Lancet Oncology. The paper examined evidence that both supports and questions the clinical wisdom of treating a disease that threatens two very different patients – an adult woman and the fetus she carries.
Every case is different, depending on the type of cancer, its grade and potential aggressiveness, the stage of pregnancy, and the woman’s own desires, said Dr. Elyce Cardonick, an ob.gyn. at Cooper University Hospital in Camden, N.J., and the lead investigator of the Pregnancy and Cancer Registry.
"With solid tumors you usually have time to do the surgery, get the pathologic diagnosis, and let the patient recover. But if there is leukemia, for instance, you have to move faster. The first question should be ‘Could we delay treatment for this patient if she was not pregnant?’ I don’t want to limit their treatment, but at the same time, it’s true that they might not be getting the most up-to-date treatment – the newest agent on the block – because you would want to go with something there is at least some experience with. But this doesn’t necessarily mean they are going to do worse."
Cancer occurs in about 1 in 1,000 pregnancies, said Dr. Sarah Temkin, a gynecologic oncologist at the University of Maryland, Baltimore. Many of her patients are referred after a routine screening during early pregnancy finds something abnormal, or when a woman with an existing cancer is incidentally found to be pregnant. But signs that might raise a red flag in other situations don’t necessarily alert physicians to danger in pregnant women, she said in an interview. Pregnancy could obfuscate some symptoms, which might be further downplayed in light of a mother’s relatively young age. Breast cancer is a prime example.
"There are two problems, especially for breast cancers, which are the most common ones we see in pregnancy. First, a woman’s breasts are changing anyway during that time. Breast cancer is so rare in women of earlier childbearing age that both the patient and the doctor tend to disregard any new lumps and bumps."
But despite its rarity, cancer in all forms appears to be increasing among pregnant women, she said. This is probably a direct relation to age. "Cancer rates increase with increasing age, and women are becoming mothers at older and older ages."
When cancer coincides with pregnancy, Dr. Temkin views the mother’s health as paramount. "The mother is the person with cancer, and she deserves whatever the standard of care is for that particular cancer – the best care that would be offered to her if she was not pregnant."
Chemotherapy and Fetal Outcomes
To examine long-term neurodevelopmental risks associated with maternal cancer treatment, Dr. Amant, a gynecologic oncologist at the Leuven (Belgium) Cancer Institute, and his colleagues, are following 70 children from the age of 18 months until 18 years. In the newly published interim analysis, the mean follow-up time is 22 months. All the children had intrauterine exposure to chemotherapy, radiation, oncologic surgery, or combinations of these.
The analysis, which also appeared in Lancet Oncology’s special issue, includes data on all the children, including 18 who are now older than 9 years (Lancet Oncol. 2012;13:256-64).
The children – 68 singletons and one set of twins – were born from pregnancies exposed to a total of 236 chemotherapy cycles. Exposure varied by the mother’s cancer type and its stage at diagnosis. In all, 34 mothers were treated with only chemotherapy, 27 had chemotherapy and surgery, 1 received chemotherapy and radiation, and 6 women were treated with all three modalities. Most of the children also had in utero exposure to multiple imaging studies, including MRI, ultrasound, echocardiography, CT, and mammography. Chemotherapy regimens included doxorubicin, epirubicin, idarubicin and daunorubicin.
Fetuses also were exposed to a variety of other drugs, including antibiotics, antiemetics, pain medications, colony stimulating factors, and anxiolytics.
Breast cancer was the most common disease type (35). There were 18 cases of hematologic cancers, 6 ovarian cancers, 4 cervical cancers, and 1 each of basal cell carcinoma, brain tumor, Ewing’s sarcoma, colorectal cancer, and nasopharyngeal cancer.
The mean gestational age at cancer diagnosis was 18 weeks, although fetuses ranged in age from 2 to 33 weeks when their mothers were diagnosed. About a third of the babies (23) were born at term.
Seven were born at 28-32 weeks, nine at 32-34 weeks, and 31 at 34-37 weeks. Weight for gestational age was below the 10th percentile in 14 children (21%).
Seven congenital anomalies were found in the group of 70 children (10%) – a rate not significantly different from that in the background population. There were only two major malformations, for a 2.9% rate. Malformations included the following:
• Hip subluxation, pectus excavatum and hemangioma, associated with chemotherapy only.
• Bilateral partial syndactyly, associated with chemotherapy plus radiotherapy.
• Bilateral small protuberance on one finger, and rectal atresia, associated with chemotherapy plus surgery.
• Bilateral double cartilage ring in a child exposed to chemotherapy, surgery and radiotherapy.
None of the children showed any congenital cardiac issues.
All of the children showed neurocognitive development that was within normal range, except for the set of twins, who were delivered by cesarean section at 32.5 weeks after a preterm premature rupture of membranes. These children were so delayed that they were not able to complete cognitive testing. Their mother developed an acute myeloid leukemia – one of the true "emergency" cancers diagnosed in pregnancy, Dr. Amant said. The babies had been exposed to idarubicin and cytosine arabinoside at 15.5, 21.5, 26.5, and 31.5 weeks’ gestation.
The boy, who weighed 1,640 g at birth, had a normal karyotype but, at 3 years, brain imaging showed a unilateral polymicrogyria in the left perisylvian area. He showed an early developmental delay; at age 9 years, he had the developmental capacity of a 12-month old.
The girl, who weighed 1,390 g at birth, also had an early developmental delay, but at age 9 years she attended school with support. Her parents refused brain imaging.
"The other 68 children did well," Dr. Amant said. "This doesn’t mean they were all normal in every way, but in any population you will see learning and developmental delay issues. We think the problem for the delayed children was not related to chemotherapy exposure, but more likely to their prematurity."
Dr. Amant saw a direct correlation between gestational age and intelligence quota. "When we controlled for age, gender, and country of birth, we found that the IQ score increased by almost 12 points for each additional month of gestation."
The U.S. Experience
Dr. Cardonick has found similar results. Her registry now contains information on 280 women who were enrolled over a 13-year period. The children born from these pregnancies have been assessed annually since birth. It also includes 70 controls – children whose mothers had cancer but who were not exposed to chemotherapy.
She published interim results of the registry in 2010 (Cancer J. 2010;16:76-82). At that point, it contained information on 231 women and 157 children. The most common malignancy was breast cancer (128); the mean gestational age at diagnosis was 13 weeks. About a third of the women (54) were advised to terminate their pregnancy; 12 did so.
Among those who continued both pregnancy and treatment, neonatal outcomes were generally good. There were nine premature deliveries related to preterm labor or premature rupture of membranes. The congenital anomaly rate born was 4%, which was in line with the normal background rate and slightly lower than that seen in Dr. Amant’s cohort.
The infants’ mean birth weight was 2,647 g, which was significantly less than the mean 2,873 g in the control group, but probably clinically irrelevant, Dr. Cardonick wrote in the paper.
She continues to follow these children annually. At this point, the children are a mean 5 years old; her oldest subject is 14 years. So far, the rate of neurocognitive issues in the group is no different than would be observed in any other group, and none of the children has developed any health problem that could be conclusively tied to intrauterine chemotherapy exposure.
Her experience stresses several key factors that must be considered in this situation. Her patients received chemotherapy at a mean of 20 weeks’ gestation – safely outside the critical early period. Only two women received chemotherapy before 10 weeks; both were treated before they knew they were pregnant. Both children born of these pregnancies were considered well. One with intrauterine exposure to cytarabine was developmentally normal at age 7 years. The other, who was exposed to oxaliplatin and capecitabine, was normal at age 2 years.
Treatment also was stopped a mean of 40 days before delivery, to allow the mother’s bone marrow to fully recover before giving birth.
A second U.S. study was reported at the 2011 meeting of the American Society of Clinical Oncology. A poster by Dr. Jennifer Litton and her colleagues examined physiological outcomes in 81 children exposed to chemotherapy for maternal breast cancer. The mothers had taken a standardized chemotherapy regimen of 5-fluorouracil, doxorubicin and cyclophosphamide (FAC) given during the second and third trimesters (J. Clin. Oncol. 2011;29[May 20 suppl.]:abstract 1099).
One child was born with Down syndrome, one with a club foot, and one with ureteral reflux. Three parents reported language delay in later follow-up surveys. Other reported health issues included 15 children with allergies and/or eczema, 2 with asthma, and 1 with absence seizures.
Dr. Litton, a breast oncologist at the University of Texas M.D. Anderson Cancer Center in Houston, also cowrote a 2010 review of breast cancer treatment in pregnancy, in which she discusses maternal and fetal outcomes from several cohorts, and the possible impact of intrauterine exposure to a variety of chemotherapy agents (Oncologist 2010;15:1238-47).
Risks Vary With Cancer Type
Breast cancer during pregnancy may be the simplest to treat. If the cancer is caught very early, it may be reasonable to delay treatment until the fetus has passed the critical first trimester, waiting until organs are formed and the risk of chemically induced damage is reduced, Dr. Temkin said. "It’s safe to do breast surgery during pregnancy and it’s safe to give chemotherapy after the first trimester."
But physicians can miss a new breast tumor during a prenatal exam, so some present at a more advanced stage, according to Dr. Amant, who is also the lead author of the Lancet’s breast cancer report (Lancet 2012;379:570-9).
Infiltrating ductal adenocarcinomas account for more than 70% of the breast cancers diagnosed during pregnancy. These can be aggressive, said Dr. Amant. Estrogen receptor status is probably no different in pregnant and nonpregnant women.
If the tumor is discovered early and is pathologically favorable, chemotherapy probably can be delayed until 14 weeks’ gestation, allowing nearly complete fetal organogenesis without worsening the mother’s outcome. Women also may elect an early termination if the pathology is unfavorable, or for other personal reasons, Dr. Temkin said. "I think a lot of it depends on when the cancer is diagnosed. Patients of mine who already have a diagnosis and then become pregnant almost always elect to terminate. But if the cancer is discovered when the pregnancy is farther along, most will continue, especially if the woman is highly emotionally invested," she noted.
Tougher Cancers, Tougher Choices
Treating gynecologic cancers during pregnancy often comes down to a choice between the mother’s health and maintenance of the pregnancy, Dr. Temkin said. "The standard of care for ovarian cancers is surgery or radiation to the pelvis, where the fetus is. Cervical cancer is treated with a hysterectomy or radiation, and neither treatment is compatible with keeping a pregnancy. Neoadjuvant therapy is not considered standard of care for these tumors. These are complex decisions for the patient: ‘Do I accept a different treatment [that might not be as effective] or maintain the pregnancy?’ "
In early cervical cancers without nodal spread, the most common tactic is close observation with periodic imaging to rule out spread; therapy is given after delivery, Dr. Phillippe Morice wrote in the Lancet section’s review on gynecologic malignancies (Lancet 2012;379:558-69).
"Delayed treatment until fetal maturation for patients with stage IA disease has an excellent prognosis and is now the standard of care," wrote Dr. Morice of the Institut de Cancérologie Gustave-Roussy in Villejuif, France.
Locally advanced disease is often not compatible with pregnancy. "The main treatment choice is either neoadjuvant chemotherapy or chemotherapy and radiotherapy. In pregnant patients, this approach means that the pregnancy must be ended before the initiation of therapy, but in exceptional cases in which surgery to end the pregnancy is not technically feasible ([that is], a bulky cervical tumor), radiation therapy can be delivered with the fetus in utero, resulting in a spontaneous abortion in about 3 weeks," he wrote.
Ovarian tumors can be surgically staged and – if it is of low malignant potential – can be laparoscopically removed, usually without endangering the pregnancy. Large tumors or those with aggressive pathology, like epithelial tumors, are much more difficult. Advanced or large tumors often have uterine and pelvic involvement, and treatment usually means a hysterectomy.
The literature contains reports of a very few women who have undergone chemotherapy to control peritoneal spread while keeping a pregnancy. However, despite giving birth to normally developed children, a number of these women died from recurrent disease, Dr. Morice noted.
Hematologic Cancers: True Emergency
Cancers of the blood are rare in pregnancy, occurring in only 1 of every 6,000. But when they do occur, they can be devastating, Dr. Benjamin Brenner wrote in the special series (Lancet 2012;379:580-7).
Pregnant patients with Hodgkin’s lymphoma generally do as well as their nonpregnant counterparts and can receive the same chemotherapy regimens, observing the first-trimester delay to favor the fetus.
Those who present with non-Hodgkin’s lymphoma are likely to have a very poor outlook. This disease is very rare in pregnant women, and symptoms can overlap with Hodgkin’s. Those factors, combined with a desire to avoid imaging, can delay diagnosis until the cancer is more advanced, said Dr. Brenner of the Rambam Health Care Campus, Haifa, Israel.
Acute leukemia is also rare, but demands urgent attention regardless of gestational stage, Dr. Brenner warned. "Patients diagnosed with acute leukemia during the first trimester are recommended to terminate the pregnancy, in view of the high risk of toxic effects on the fetus and mother, along with the expected need for further intensive treatment including stem-cell transplantation, which is absolutely contraindicated during gestation."
Talking It Out
Despite the emerging positive evidence, treating cancer during pregnancy can be a tough sell, Dr. Amant said. "Women have been told over and over to avoid taking so much as an aspirin. It’s very difficult to convince them that a fetus can not only survive a mother’s cancer treatment, but have a good chance of developing normally."
The stress of a cancer diagnosis during a desired pregnancy is very hard on patients, Dr. Temkin added. "Pregnancy is a time when many women come to grips with their own mortality as well as that of giving new life. Adding a diagnosis of cancer of top of that – especially in the face of a much-desired pregnancy – can be devastating."
These women are faced with two options: terminate the pregnancy and concentrate on their own treatment, or continue the pregnancy knowing that their unborn child will be exposed to the possible risks of radiation, chemotherapy, and surgery. Either option can "inflict terrible guilt on a pregnant woman. We can try to minimize that to some degree, but it’s important to know from the outset that what is the right solution for one patient is not right for the next."
Connecting with other women who have experienced the same situation can be of immense value, Dr. Cardonick said. She participates in an online support group called "Hope for Two."
The organization’s main goal is to link new patients with survivors who can help educate them as well as lend emotional support. Patients call in or fill out a secure online request for a personal match-up with a survivor, who is often a woman who has had the same type of cancer.
The website also contains links to news and medical articles, books, and financial assistance sources, and allows new patients to securely contact Dr. Cardonick’s pregnancy registry. "We keep in touch with the baby’s pediatrician and the mom every year, to see how things are going and [to] collect information," she said. "The best way to treat these women in the future depends on the information we continue to gather in the present."
None of the researchers interviewed for this article had any relevant financial disclosures. Dr. Litton noted that she had no financial disclosures for her 2011 ASCO poster.
Stop performing median episiotomy!
Advocates of the liberal use of episiotomy have hypothesized that the procedure has many benefits, including:
Most studies do not provide strong support for these claims.
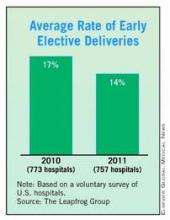
Utilization has declined. Over the past decades, the liberal use of episiotomy has given way to a pattern of practice that emphasizes restricted use.1-4 In the 1980s, in the United States, episiotomy incisions were performed in approximately 40% of vaginal deliveries5; in 2010, the rate at most obstetric facilities was <10%.
When the episiotomy rate was 40%, a median incision made sense: A very limited incision was sufficient to provide extra room for the passage of an average-sized fetus through an average-sized birth outlet. With the rate below 10% today, however, the likelihood is greater that episiotomy is being reserved for cases in which a significant clinical problem exists—most often, mismatch between the birth outlet and fetal size—and the appropriateness of median episiotomy comes into question. By restricting episiotomy incisions to the most complex clinical situations, the risk is greater that the episiotomy will be associated with a severe perineal laceration, such as a laceration of the anal sphincter (third-degree) or the rectal mucosa (fourth-degree)—or both.
A spotlight on severe lacerations. Over the past decade, practitioners of obstetrics have refocused attention on reducing the risk of third- and fourth-degree perineal lacerations—severe injuries that are associated with significant maternal morbidity. Clinical variables that increase the risk of a third- or fourth-degree perineal laceration include:
- nulliparity
- forceps delivery
- median episiotomy
- macrosomia
- persistent occiput posterior position.6
Many studies have reported that a median episiotomy is associated with a higher rate of third- and fourth-degree lacerations than either 1) deliveries without an episiotomy or 2) deliveries with a mediolateral episiotomy.1,7-9 With the modern practice of reserving episiotomy for the most complex vaginal deliveries and renewed attention to reducing the rate of third- and fourth-degree lacerations, the time has come for us to stop using the median episiotomy and switch to using a mediolateral episiotomy incision.
STOP: using the median episiotomy
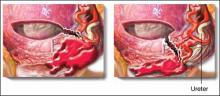
Numerous studies have reported that the median episiotomy is associated with an increased risk of laceration of the anal sphincter (third-degree) and rectal mucosa (fourth-degree), compared with mediolateral episiotomy.
Randomized study of incisions. In a clinical trial, 407 nulliparous women were randomized to median or mediolateral episiotomy incision.10 The incisions were made at a time during the second stage of labor that was judged by the clinician to be most appropriate—typically immediately before delivery of the fetal head.
To perform mediolateral episiotomy, clinicians in this study used a pair of straight scissors to make an incision that began in the midline and was carried to the right side of the anal sphincter for 3 to 4 cm, at an angle >45°. Median episiotomy was performed by incision of the perineal tissues for 2 to 3 cm, directly in the midline.
The clinical protocol resulted in more women assigned to mediolateral episiotomy (n=244) than to the midline episiotomy (n=159), but the two groups were well matched on such major clinical characteristics as age, gestational age at delivery, duration of the second stage, rate of operative delivery, and anesthesia used.
Compared to what was found with mediolateral episiotomy, the median episiotomy incision was associated with a statistically significant increase in the frequency of complete third-degree tears (median, 6.1%; mediolateral, 1.6%) and fourth-degree tears (median, 5.5%; mediolateral, 0.4%).
Further comparisons. Many clinicians avoid mediolateral episiotomy. Why? Because, compared with median episiotomy, a mediolateral incision is believed to be associated with greater postpartum pain, increased severity and incidence of dyspareunia, and more disfiguring scars.11
But subjects in the randomized trial that I just described, in which mediolateral and median episiotomy were compared,10 reported postpartum pain, postpartum use of pain medicine, and impaired bowel function at similar rates regardless of the type of incision.
Investigators determined that, in the first month after delivery, more women who had a median episiotomy resumed vaginal intercourse (18.1%, compared to 6.3% who had a mediolateral incision). In the second month after delivery, however, women in both the median and mediolateral episiotomy groups reported similar resumption of sexual intercourse (median, 82.8%; mediolateral, 80.8%).
Three months after delivery, physical examination determined that a higher percentage of subjects in the median incision group (43%) had what was judged to be a “good” appearance to the scar (compared to 27% in the mediolateral group). Last, subjects in the median group had a higher rate of perineal laxity (7%) than did women in the mediolateral group (1.6%).
START: using a mediolateral incision when episiotomy is necessary
Reducing the risk of severe perineal laceration is an important clinical goal because severe lacerations are associated with significant morbidity. In a study of 390 women who had a fourth-degree perineal laceration, 5% had significant complications that, in most cases, required additional surgery.12 Furthermore, in that study:
- 1.8% of women had a breakdown of the repair
- 2.8% had infection plus breakdown of the repair
- 0.8% had infection only.
In another study, 31% of women who sustained a fourth-degree laceration reported poor bowel control postpartum.7
Given the focus on reducing the rate of severe perineal laceration, I recommend that, in most cases, you reduce the use of median episiotomy and increase the use of mediolateral episiotomy.
Use a mediolateral incision for episiotomy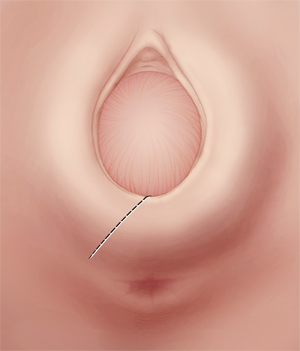
Make the incision at a 45°-angle; incisions made at >35° angle are associated with less of a risk of severe perineal laceration. Avoid using chromic sutures; rapid-absorption polyglactin 910 suture might offer better healing.
Mediolateral episiotomy: Technique
Begin the mediolateral episiotomy in the midline or slightly lateral to the midline (see the FIGURE). Insert two fingers into the vagina to distend the tissue of the birth outlet; using a pair of sharp, straight scissors, cut an incision 4 or 5 cm long at a 45° angle, directing it toward the ipsilateral ischial tuberosity. In most women, this incision cuts a portion of the bulbospongiosus muscle and, occasionally, reaches the ischioanal fossa.
Proper angle is key. The angle of the mediolateral episiotomy, in relation to the midline, is an important variable that influences the possibility that the patient will have a severe perineal laceration. Most experts recommend that the angle of the incision be at least 45° from the midline. If you use a shallow angle (<35°) from the midline to perform mediolateral episiotomy, you increase the risk of a severe perineal laceration, compared with incisions made at an angle >35° degrees from the midline (again, the FIGURE). In one report, the risk of a third-degree tear was about 10% with a 25°-angle mediolateral episiotomy, but less than 1% when the angle was >35°.13
Repairing the incision. After delivery, begin repair of a mediolateral incision by assessing the extent of vaginal, anal sphincter, rectal, and periurethral lacerations. Then, use two fingers, with or without a retractor, to spread the edges of the incision so that you can fully determine the length and depth of the episiotomy.
Place a 2-0 or 3-0 suture just above the apex of the incision. Use a running suture to close the vaginal mucosa and submucosal tissue. As you approach the introitus, suspend the running mucosal–submucosal suture and turn your attention to approximating the deeper submucosal space.
In mediolateral episiotomy, the upper-lateral edge of the incision contains more tissue than the lower-medial edge. To improve healing of the incision, use diagonal, rather than horizontal, sutures to provide better approximation of the submucosa. The fascia of the bulbocavernosus and superficial transveralis muscles might need to be reapproximated with individual sutures. Then, resume closing the skin and submucosa of the introitus and perineum.14 Perioperative antibiotic prophylaxis might be warranted—before you repair a complex perineal laceration.15
Concern about suture material. Using chromic suture to repair an episiotomy incision is associated with increased postdelivery pain, compared with the use of rapid-absorption polyglactin 910 suture (Vicryl Rapide).16 In fact, most OBs have stopped using chromic suture to repair episiotomy incisions. Rapid-absorption polyglactin 910 suture (average time to absorption, 42 days) might be associated with less of a need to remove suture that migrates through the incision than what is seen with standard-absorption polyglactin 910 sutures (average time to absorption, 63 days).16,17
When an episiotomy is indicated
There is renewed emphasis on reducing the rate of third- and fourth-degree perineal lacerations at delivery, because these adverse outcomes are associated with:
- an increased risk of wound breakdown that requires surgical repair
- incontinence of flatus or stool, or both.
At a time when the use of episiotomy has become limited, continuing to use a median incision will get you more third- and fourth-degree lacerations than if you use a mediolateral episiotomy.
A mediolateral episiotomy might cause more perineal pain immediately postpartum but, within a few months after delivery, patients mostly have recovered from either type of episiotomy.
To recap: If an episiotomy is indicated, use a mediolateral incision. I urge you to stop performing median episiotomy incisions.
For many OBs, episiotomy is one of the most common operative procedures that they will perform during their career. Precisely because the procedure is common, and because it is considered minor surgery, coding for the creation of the incision and subsequent repair is, under Current Procedural Terminology (CPT) rules, considered integral to the services provided during delivery.
Repair of an intentional episiotomy closely compares to the repair required for a first- or second-degree laceration. For that reason, payers will not reimburse separately for this level of repair—even when made necessary by laceration of tissues and not by intentional episiotomy.
On the other hand, most payers do reimburse for repair of third- and fourth-degree lacerations and, at times, for a more complex repair of an extension to an intentional episiotomy.
Details explained
Coding options for more extensive intentional episiotomies and for third- and fourth-degree lacerations vary by payer. The simplest coding option is to add modifier -22 (increased procedural services) to the delivery or the global OB care code (for example, 59400-22 or 59409-22). To support use of this modifier, your documentation must include:
- the reason for the additional work (increased intensity, time, technical difficulty of procedure, severity of patient’s condition, physical and mental effort required)
- description of the significant additional work.
Some payers allow you to bill separately for the repair; do this by reporting the integumentary repair codes by type of repair:
- 12001-12007, for simple repair
- 12041-12047, for intermediate repair
- 13131-13132, for complex repair.
Select a code based on the total length of the repair, which must be documented as part of the description of the repair.
When repair is performed at the same time as the delivery, add modifier -51 to the separate repair code because this is considered a multiple procedure.
When repair is made after delivery with a return to the operating room, append a modifier -78 to the repair code.
Note that the CPT code 59300, Episiotomy or vaginal repair, by other than the attending physician, can never be reported by the attending OB or a physician who is covering for this physician; doing so will always result in a denial of the service.
MELANIE WITT, RN, CPC, COBGC, MA
Faced with a difficult vaginal delivery, would you use a median or mediolateral episiotomy incision? Why?
1. American College of Obstetricians and Gynecologists. Clinical Management Guidelines for Obstetrician-Gynecologists: Episiotomy. No. 71. Obstet Gynecol. 2006;107(4):957-962.
2. Myles TD, Santolaya J. Maternal and neonatal outcomes in patients with prolonged second stage of labor. Obstet Gynecol. 2003;102(1):52-58.
3. Bodner-Adler B, Bodner K, Kimberger O, Wagenbichler P, Mayerhofer K. Management of the perineum during forceps delivery. Association of episiotomy with the frequency and severity of perineal trauma in women undergoing forceps delivery. J Reprod Med. 2003;48(4):239-242.
4. Hartmann K, Viswanathan M, Palmieri R, Gartlehner G, Thorpe J, Jr, Lohr KN. Outcomes of routine episiotomy a systematic review. JAMA. 2005;293(17):2141-2148.
5. Barbieri RL. It’s time to restrict the use of episiotomy. OBG Manage. 2006;18(9):8-12.
6. De Leeuw JW, Struijik PC, Vierhout ME, Wallenburgh HC. Risk factors for third degree perineal ruptures during delivery. BJOG. 2001;108(4):383-387.
7. Fenner DE, Genberg B, Brahma P, Marek L, DeLancey JO. Fecal and urinary incontinence after vaginal delivery with anal sphincter disruption in an obstetrics unit in the United States. Am J Obstet Gynecol. 2003;189(6):1543-1550.
8. Shiono P, Klebenoff MA, Carey JC. Midline episiotomies: more harm than good? Obstet Gynecol. 1990;75(5):765-770.
9. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third-degree perineal tears in vaginal delivery, with an analysis of episiotomy types. J Reprod Med. 2001;46(8):752-756.
10. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87(5):408-412.
11. Thacker SB, Banta HD. Benefits and risks of episiotomy: an interpretive review of the English language literature, 1860–1980. Obstet Gynecol Surv. 1983;38(6):322-338.
12. Goldaber KG, Wendel PJ, McIntire DD, Wendel GD. Postpartum perineal morbidity after fourth degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489-493.
13. Eogan M, Daly L, O’Connell PR, O’Herlihy C. Does the angle of episiotomy affect the incidence of anal sphincter injury? BJOG. 2006;113(2):190-194.
14. Hale RW, Ling FW. Episiotomy: Procedure and repair techniques. Washington DC: American College of Obstetricians and Gynecologists; 2007:1-24.
15. Duggal N, Mercado C, Daniels K, Bujor A, Caughey AB, El-Sayed YY. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1268-1273.
16. 1Greenberg JA, Lieberman E, Cohen AP, Ecker JL. Randomized comparison of chromic versus fast-absorbing polyglactin 910 for postpartum perineal repair. Obstet Gynecol. 2004;103(6):1308-1313.
17. Mackrodt C, Gordon B, Fern E, Ayers S, Truesdale A, Grant A. The Ipswich childbirth study 2. A randomised comparison of polyglactin 910 with chromic catgut for postpartum perineal repair. Br J Obstet Gynecol. 1998;105(4):441-445.
Advocates of the liberal use of episiotomy have hypothesized that the procedure has many benefits, including:
Most studies do not provide strong support for these claims.
Utilization has declined. Over the past decades, the liberal use of episiotomy has given way to a pattern of practice that emphasizes restricted use.1-4 In the 1980s, in the United States, episiotomy incisions were performed in approximately 40% of vaginal deliveries5; in 2010, the rate at most obstetric facilities was <10%.
When the episiotomy rate was 40%, a median incision made sense: A very limited incision was sufficient to provide extra room for the passage of an average-sized fetus through an average-sized birth outlet. With the rate below 10% today, however, the likelihood is greater that episiotomy is being reserved for cases in which a significant clinical problem exists—most often, mismatch between the birth outlet and fetal size—and the appropriateness of median episiotomy comes into question. By restricting episiotomy incisions to the most complex clinical situations, the risk is greater that the episiotomy will be associated with a severe perineal laceration, such as a laceration of the anal sphincter (third-degree) or the rectal mucosa (fourth-degree)—or both.
A spotlight on severe lacerations. Over the past decade, practitioners of obstetrics have refocused attention on reducing the risk of third- and fourth-degree perineal lacerations—severe injuries that are associated with significant maternal morbidity. Clinical variables that increase the risk of a third- or fourth-degree perineal laceration include:
- nulliparity
- forceps delivery
- median episiotomy
- macrosomia
- persistent occiput posterior position.6
Many studies have reported that a median episiotomy is associated with a higher rate of third- and fourth-degree lacerations than either 1) deliveries without an episiotomy or 2) deliveries with a mediolateral episiotomy.1,7-9 With the modern practice of reserving episiotomy for the most complex vaginal deliveries and renewed attention to reducing the rate of third- and fourth-degree lacerations, the time has come for us to stop using the median episiotomy and switch to using a mediolateral episiotomy incision.
STOP: using the median episiotomy
Numerous studies have reported that the median episiotomy is associated with an increased risk of laceration of the anal sphincter (third-degree) and rectal mucosa (fourth-degree), compared with mediolateral episiotomy.
Randomized study of incisions. In a clinical trial, 407 nulliparous women were randomized to median or mediolateral episiotomy incision.10 The incisions were made at a time during the second stage of labor that was judged by the clinician to be most appropriate—typically immediately before delivery of the fetal head.
To perform mediolateral episiotomy, clinicians in this study used a pair of straight scissors to make an incision that began in the midline and was carried to the right side of the anal sphincter for 3 to 4 cm, at an angle >45°. Median episiotomy was performed by incision of the perineal tissues for 2 to 3 cm, directly in the midline.
The clinical protocol resulted in more women assigned to mediolateral episiotomy (n=244) than to the midline episiotomy (n=159), but the two groups were well matched on such major clinical characteristics as age, gestational age at delivery, duration of the second stage, rate of operative delivery, and anesthesia used.
Compared to what was found with mediolateral episiotomy, the median episiotomy incision was associated with a statistically significant increase in the frequency of complete third-degree tears (median, 6.1%; mediolateral, 1.6%) and fourth-degree tears (median, 5.5%; mediolateral, 0.4%).
Further comparisons. Many clinicians avoid mediolateral episiotomy. Why? Because, compared with median episiotomy, a mediolateral incision is believed to be associated with greater postpartum pain, increased severity and incidence of dyspareunia, and more disfiguring scars.11
But subjects in the randomized trial that I just described, in which mediolateral and median episiotomy were compared,10 reported postpartum pain, postpartum use of pain medicine, and impaired bowel function at similar rates regardless of the type of incision.
Investigators determined that, in the first month after delivery, more women who had a median episiotomy resumed vaginal intercourse (18.1%, compared to 6.3% who had a mediolateral incision). In the second month after delivery, however, women in both the median and mediolateral episiotomy groups reported similar resumption of sexual intercourse (median, 82.8%; mediolateral, 80.8%).
Three months after delivery, physical examination determined that a higher percentage of subjects in the median incision group (43%) had what was judged to be a “good” appearance to the scar (compared to 27% in the mediolateral group). Last, subjects in the median group had a higher rate of perineal laxity (7%) than did women in the mediolateral group (1.6%).
START: using a mediolateral incision when episiotomy is necessary
Reducing the risk of severe perineal laceration is an important clinical goal because severe lacerations are associated with significant morbidity. In a study of 390 women who had a fourth-degree perineal laceration, 5% had significant complications that, in most cases, required additional surgery.12 Furthermore, in that study:
- 1.8% of women had a breakdown of the repair
- 2.8% had infection plus breakdown of the repair
- 0.8% had infection only.
In another study, 31% of women who sustained a fourth-degree laceration reported poor bowel control postpartum.7
Given the focus on reducing the rate of severe perineal laceration, I recommend that, in most cases, you reduce the use of median episiotomy and increase the use of mediolateral episiotomy.
Use a mediolateral incision for episiotomy
Make the incision at a 45°-angle; incisions made at >35° angle are associated with less of a risk of severe perineal laceration. Avoid using chromic sutures; rapid-absorption polyglactin 910 suture might offer better healing.
Mediolateral episiotomy: Technique
Begin the mediolateral episiotomy in the midline or slightly lateral to the midline (see the FIGURE). Insert two fingers into the vagina to distend the tissue of the birth outlet; using a pair of sharp, straight scissors, cut an incision 4 or 5 cm long at a 45° angle, directing it toward the ipsilateral ischial tuberosity. In most women, this incision cuts a portion of the bulbospongiosus muscle and, occasionally, reaches the ischioanal fossa.
Proper angle is key. The angle of the mediolateral episiotomy, in relation to the midline, is an important variable that influences the possibility that the patient will have a severe perineal laceration. Most experts recommend that the angle of the incision be at least 45° from the midline. If you use a shallow angle (<35°) from the midline to perform mediolateral episiotomy, you increase the risk of a severe perineal laceration, compared with incisions made at an angle >35° degrees from the midline (again, the FIGURE). In one report, the risk of a third-degree tear was about 10% with a 25°-angle mediolateral episiotomy, but less than 1% when the angle was >35°.13
Repairing the incision. After delivery, begin repair of a mediolateral incision by assessing the extent of vaginal, anal sphincter, rectal, and periurethral lacerations. Then, use two fingers, with or without a retractor, to spread the edges of the incision so that you can fully determine the length and depth of the episiotomy.
Place a 2-0 or 3-0 suture just above the apex of the incision. Use a running suture to close the vaginal mucosa and submucosal tissue. As you approach the introitus, suspend the running mucosal–submucosal suture and turn your attention to approximating the deeper submucosal space.
In mediolateral episiotomy, the upper-lateral edge of the incision contains more tissue than the lower-medial edge. To improve healing of the incision, use diagonal, rather than horizontal, sutures to provide better approximation of the submucosa. The fascia of the bulbocavernosus and superficial transveralis muscles might need to be reapproximated with individual sutures. Then, resume closing the skin and submucosa of the introitus and perineum.14 Perioperative antibiotic prophylaxis might be warranted—before you repair a complex perineal laceration.15
Concern about suture material. Using chromic suture to repair an episiotomy incision is associated with increased postdelivery pain, compared with the use of rapid-absorption polyglactin 910 suture (Vicryl Rapide).16 In fact, most OBs have stopped using chromic suture to repair episiotomy incisions. Rapid-absorption polyglactin 910 suture (average time to absorption, 42 days) might be associated with less of a need to remove suture that migrates through the incision than what is seen with standard-absorption polyglactin 910 sutures (average time to absorption, 63 days).16,17
When an episiotomy is indicated
There is renewed emphasis on reducing the rate of third- and fourth-degree perineal lacerations at delivery, because these adverse outcomes are associated with:
- an increased risk of wound breakdown that requires surgical repair
- incontinence of flatus or stool, or both.
At a time when the use of episiotomy has become limited, continuing to use a median incision will get you more third- and fourth-degree lacerations than if you use a mediolateral episiotomy.
A mediolateral episiotomy might cause more perineal pain immediately postpartum but, within a few months after delivery, patients mostly have recovered from either type of episiotomy.
To recap: If an episiotomy is indicated, use a mediolateral incision. I urge you to stop performing median episiotomy incisions.
For many OBs, episiotomy is one of the most common operative procedures that they will perform during their career. Precisely because the procedure is common, and because it is considered minor surgery, coding for the creation of the incision and subsequent repair is, under Current Procedural Terminology (CPT) rules, considered integral to the services provided during delivery.
Repair of an intentional episiotomy closely compares to the repair required for a first- or second-degree laceration. For that reason, payers will not reimburse separately for this level of repair—even when made necessary by laceration of tissues and not by intentional episiotomy.
On the other hand, most payers do reimburse for repair of third- and fourth-degree lacerations and, at times, for a more complex repair of an extension to an intentional episiotomy.
Details explained
Coding options for more extensive intentional episiotomies and for third- and fourth-degree lacerations vary by payer. The simplest coding option is to add modifier -22 (increased procedural services) to the delivery or the global OB care code (for example, 59400-22 or 59409-22). To support use of this modifier, your documentation must include:
- the reason for the additional work (increased intensity, time, technical difficulty of procedure, severity of patient’s condition, physical and mental effort required)
- description of the significant additional work.
Some payers allow you to bill separately for the repair; do this by reporting the integumentary repair codes by type of repair:
- 12001-12007, for simple repair
- 12041-12047, for intermediate repair
- 13131-13132, for complex repair.
Select a code based on the total length of the repair, which must be documented as part of the description of the repair.
When repair is performed at the same time as the delivery, add modifier -51 to the separate repair code because this is considered a multiple procedure.
When repair is made after delivery with a return to the operating room, append a modifier -78 to the repair code.
Note that the CPT code 59300, Episiotomy or vaginal repair, by other than the attending physician, can never be reported by the attending OB or a physician who is covering for this physician; doing so will always result in a denial of the service.
MELANIE WITT, RN, CPC, COBGC, MA
Faced with a difficult vaginal delivery, would you use a median or mediolateral episiotomy incision? Why?
Advocates of the liberal use of episiotomy have hypothesized that the procedure has many benefits, including:
Most studies do not provide strong support for these claims.
Utilization has declined. Over the past decades, the liberal use of episiotomy has given way to a pattern of practice that emphasizes restricted use.1-4 In the 1980s, in the United States, episiotomy incisions were performed in approximately 40% of vaginal deliveries5; in 2010, the rate at most obstetric facilities was <10%.
When the episiotomy rate was 40%, a median incision made sense: A very limited incision was sufficient to provide extra room for the passage of an average-sized fetus through an average-sized birth outlet. With the rate below 10% today, however, the likelihood is greater that episiotomy is being reserved for cases in which a significant clinical problem exists—most often, mismatch between the birth outlet and fetal size—and the appropriateness of median episiotomy comes into question. By restricting episiotomy incisions to the most complex clinical situations, the risk is greater that the episiotomy will be associated with a severe perineal laceration, such as a laceration of the anal sphincter (third-degree) or the rectal mucosa (fourth-degree)—or both.
A spotlight on severe lacerations. Over the past decade, practitioners of obstetrics have refocused attention on reducing the risk of third- and fourth-degree perineal lacerations—severe injuries that are associated with significant maternal morbidity. Clinical variables that increase the risk of a third- or fourth-degree perineal laceration include:
- nulliparity
- forceps delivery
- median episiotomy
- macrosomia
- persistent occiput posterior position.6
Many studies have reported that a median episiotomy is associated with a higher rate of third- and fourth-degree lacerations than either 1) deliveries without an episiotomy or 2) deliveries with a mediolateral episiotomy.1,7-9 With the modern practice of reserving episiotomy for the most complex vaginal deliveries and renewed attention to reducing the rate of third- and fourth-degree lacerations, the time has come for us to stop using the median episiotomy and switch to using a mediolateral episiotomy incision.
STOP: using the median episiotomy
Numerous studies have reported that the median episiotomy is associated with an increased risk of laceration of the anal sphincter (third-degree) and rectal mucosa (fourth-degree), compared with mediolateral episiotomy.
Randomized study of incisions. In a clinical trial, 407 nulliparous women were randomized to median or mediolateral episiotomy incision.10 The incisions were made at a time during the second stage of labor that was judged by the clinician to be most appropriate—typically immediately before delivery of the fetal head.
To perform mediolateral episiotomy, clinicians in this study used a pair of straight scissors to make an incision that began in the midline and was carried to the right side of the anal sphincter for 3 to 4 cm, at an angle >45°. Median episiotomy was performed by incision of the perineal tissues for 2 to 3 cm, directly in the midline.
The clinical protocol resulted in more women assigned to mediolateral episiotomy (n=244) than to the midline episiotomy (n=159), but the two groups were well matched on such major clinical characteristics as age, gestational age at delivery, duration of the second stage, rate of operative delivery, and anesthesia used.
Compared to what was found with mediolateral episiotomy, the median episiotomy incision was associated with a statistically significant increase in the frequency of complete third-degree tears (median, 6.1%; mediolateral, 1.6%) and fourth-degree tears (median, 5.5%; mediolateral, 0.4%).
Further comparisons. Many clinicians avoid mediolateral episiotomy. Why? Because, compared with median episiotomy, a mediolateral incision is believed to be associated with greater postpartum pain, increased severity and incidence of dyspareunia, and more disfiguring scars.11
But subjects in the randomized trial that I just described, in which mediolateral and median episiotomy were compared,10 reported postpartum pain, postpartum use of pain medicine, and impaired bowel function at similar rates regardless of the type of incision.
Investigators determined that, in the first month after delivery, more women who had a median episiotomy resumed vaginal intercourse (18.1%, compared to 6.3% who had a mediolateral incision). In the second month after delivery, however, women in both the median and mediolateral episiotomy groups reported similar resumption of sexual intercourse (median, 82.8%; mediolateral, 80.8%).
Three months after delivery, physical examination determined that a higher percentage of subjects in the median incision group (43%) had what was judged to be a “good” appearance to the scar (compared to 27% in the mediolateral group). Last, subjects in the median group had a higher rate of perineal laxity (7%) than did women in the mediolateral group (1.6%).
START: using a mediolateral incision when episiotomy is necessary
Reducing the risk of severe perineal laceration is an important clinical goal because severe lacerations are associated with significant morbidity. In a study of 390 women who had a fourth-degree perineal laceration, 5% had significant complications that, in most cases, required additional surgery.12 Furthermore, in that study:
- 1.8% of women had a breakdown of the repair
- 2.8% had infection plus breakdown of the repair
- 0.8% had infection only.
In another study, 31% of women who sustained a fourth-degree laceration reported poor bowel control postpartum.7
Given the focus on reducing the rate of severe perineal laceration, I recommend that, in most cases, you reduce the use of median episiotomy and increase the use of mediolateral episiotomy.
Use a mediolateral incision for episiotomy
Make the incision at a 45°-angle; incisions made at >35° angle are associated with less of a risk of severe perineal laceration. Avoid using chromic sutures; rapid-absorption polyglactin 910 suture might offer better healing.
Mediolateral episiotomy: Technique
Begin the mediolateral episiotomy in the midline or slightly lateral to the midline (see the FIGURE). Insert two fingers into the vagina to distend the tissue of the birth outlet; using a pair of sharp, straight scissors, cut an incision 4 or 5 cm long at a 45° angle, directing it toward the ipsilateral ischial tuberosity. In most women, this incision cuts a portion of the bulbospongiosus muscle and, occasionally, reaches the ischioanal fossa.
Proper angle is key. The angle of the mediolateral episiotomy, in relation to the midline, is an important variable that influences the possibility that the patient will have a severe perineal laceration. Most experts recommend that the angle of the incision be at least 45° from the midline. If you use a shallow angle (<35°) from the midline to perform mediolateral episiotomy, you increase the risk of a severe perineal laceration, compared with incisions made at an angle >35° degrees from the midline (again, the FIGURE). In one report, the risk of a third-degree tear was about 10% with a 25°-angle mediolateral episiotomy, but less than 1% when the angle was >35°.13
Repairing the incision. After delivery, begin repair of a mediolateral incision by assessing the extent of vaginal, anal sphincter, rectal, and periurethral lacerations. Then, use two fingers, with or without a retractor, to spread the edges of the incision so that you can fully determine the length and depth of the episiotomy.
Place a 2-0 or 3-0 suture just above the apex of the incision. Use a running suture to close the vaginal mucosa and submucosal tissue. As you approach the introitus, suspend the running mucosal–submucosal suture and turn your attention to approximating the deeper submucosal space.
In mediolateral episiotomy, the upper-lateral edge of the incision contains more tissue than the lower-medial edge. To improve healing of the incision, use diagonal, rather than horizontal, sutures to provide better approximation of the submucosa. The fascia of the bulbocavernosus and superficial transveralis muscles might need to be reapproximated with individual sutures. Then, resume closing the skin and submucosa of the introitus and perineum.14 Perioperative antibiotic prophylaxis might be warranted—before you repair a complex perineal laceration.15
Concern about suture material. Using chromic suture to repair an episiotomy incision is associated with increased postdelivery pain, compared with the use of rapid-absorption polyglactin 910 suture (Vicryl Rapide).16 In fact, most OBs have stopped using chromic suture to repair episiotomy incisions. Rapid-absorption polyglactin 910 suture (average time to absorption, 42 days) might be associated with less of a need to remove suture that migrates through the incision than what is seen with standard-absorption polyglactin 910 sutures (average time to absorption, 63 days).16,17
When an episiotomy is indicated
There is renewed emphasis on reducing the rate of third- and fourth-degree perineal lacerations at delivery, because these adverse outcomes are associated with:
- an increased risk of wound breakdown that requires surgical repair
- incontinence of flatus or stool, or both.
At a time when the use of episiotomy has become limited, continuing to use a median incision will get you more third- and fourth-degree lacerations than if you use a mediolateral episiotomy.
A mediolateral episiotomy might cause more perineal pain immediately postpartum but, within a few months after delivery, patients mostly have recovered from either type of episiotomy.
To recap: If an episiotomy is indicated, use a mediolateral incision. I urge you to stop performing median episiotomy incisions.
For many OBs, episiotomy is one of the most common operative procedures that they will perform during their career. Precisely because the procedure is common, and because it is considered minor surgery, coding for the creation of the incision and subsequent repair is, under Current Procedural Terminology (CPT) rules, considered integral to the services provided during delivery.
Repair of an intentional episiotomy closely compares to the repair required for a first- or second-degree laceration. For that reason, payers will not reimburse separately for this level of repair—even when made necessary by laceration of tissues and not by intentional episiotomy.
On the other hand, most payers do reimburse for repair of third- and fourth-degree lacerations and, at times, for a more complex repair of an extension to an intentional episiotomy.
Details explained
Coding options for more extensive intentional episiotomies and for third- and fourth-degree lacerations vary by payer. The simplest coding option is to add modifier -22 (increased procedural services) to the delivery or the global OB care code (for example, 59400-22 or 59409-22). To support use of this modifier, your documentation must include:
- the reason for the additional work (increased intensity, time, technical difficulty of procedure, severity of patient’s condition, physical and mental effort required)
- description of the significant additional work.
Some payers allow you to bill separately for the repair; do this by reporting the integumentary repair codes by type of repair:
- 12001-12007, for simple repair
- 12041-12047, for intermediate repair
- 13131-13132, for complex repair.
Select a code based on the total length of the repair, which must be documented as part of the description of the repair.
When repair is performed at the same time as the delivery, add modifier -51 to the separate repair code because this is considered a multiple procedure.
When repair is made after delivery with a return to the operating room, append a modifier -78 to the repair code.
Note that the CPT code 59300, Episiotomy or vaginal repair, by other than the attending physician, can never be reported by the attending OB or a physician who is covering for this physician; doing so will always result in a denial of the service.
MELANIE WITT, RN, CPC, COBGC, MA
Faced with a difficult vaginal delivery, would you use a median or mediolateral episiotomy incision? Why?
1. American College of Obstetricians and Gynecologists. Clinical Management Guidelines for Obstetrician-Gynecologists: Episiotomy. No. 71. Obstet Gynecol. 2006;107(4):957-962.
2. Myles TD, Santolaya J. Maternal and neonatal outcomes in patients with prolonged second stage of labor. Obstet Gynecol. 2003;102(1):52-58.
3. Bodner-Adler B, Bodner K, Kimberger O, Wagenbichler P, Mayerhofer K. Management of the perineum during forceps delivery. Association of episiotomy with the frequency and severity of perineal trauma in women undergoing forceps delivery. J Reprod Med. 2003;48(4):239-242.
4. Hartmann K, Viswanathan M, Palmieri R, Gartlehner G, Thorpe J, Jr, Lohr KN. Outcomes of routine episiotomy a systematic review. JAMA. 2005;293(17):2141-2148.
5. Barbieri RL. It’s time to restrict the use of episiotomy. OBG Manage. 2006;18(9):8-12.
6. De Leeuw JW, Struijik PC, Vierhout ME, Wallenburgh HC. Risk factors for third degree perineal ruptures during delivery. BJOG. 2001;108(4):383-387.
7. Fenner DE, Genberg B, Brahma P, Marek L, DeLancey JO. Fecal and urinary incontinence after vaginal delivery with anal sphincter disruption in an obstetrics unit in the United States. Am J Obstet Gynecol. 2003;189(6):1543-1550.
8. Shiono P, Klebenoff MA, Carey JC. Midline episiotomies: more harm than good? Obstet Gynecol. 1990;75(5):765-770.
9. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third-degree perineal tears in vaginal delivery, with an analysis of episiotomy types. J Reprod Med. 2001;46(8):752-756.
10. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87(5):408-412.
11. Thacker SB, Banta HD. Benefits and risks of episiotomy: an interpretive review of the English language literature, 1860–1980. Obstet Gynecol Surv. 1983;38(6):322-338.
12. Goldaber KG, Wendel PJ, McIntire DD, Wendel GD. Postpartum perineal morbidity after fourth degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489-493.
13. Eogan M, Daly L, O’Connell PR, O’Herlihy C. Does the angle of episiotomy affect the incidence of anal sphincter injury? BJOG. 2006;113(2):190-194.
14. Hale RW, Ling FW. Episiotomy: Procedure and repair techniques. Washington DC: American College of Obstetricians and Gynecologists; 2007:1-24.
15. Duggal N, Mercado C, Daniels K, Bujor A, Caughey AB, El-Sayed YY. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1268-1273.
16. 1Greenberg JA, Lieberman E, Cohen AP, Ecker JL. Randomized comparison of chromic versus fast-absorbing polyglactin 910 for postpartum perineal repair. Obstet Gynecol. 2004;103(6):1308-1313.
17. Mackrodt C, Gordon B, Fern E, Ayers S, Truesdale A, Grant A. The Ipswich childbirth study 2. A randomised comparison of polyglactin 910 with chromic catgut for postpartum perineal repair. Br J Obstet Gynecol. 1998;105(4):441-445.
1. American College of Obstetricians and Gynecologists. Clinical Management Guidelines for Obstetrician-Gynecologists: Episiotomy. No. 71. Obstet Gynecol. 2006;107(4):957-962.
2. Myles TD, Santolaya J. Maternal and neonatal outcomes in patients with prolonged second stage of labor. Obstet Gynecol. 2003;102(1):52-58.
3. Bodner-Adler B, Bodner K, Kimberger O, Wagenbichler P, Mayerhofer K. Management of the perineum during forceps delivery. Association of episiotomy with the frequency and severity of perineal trauma in women undergoing forceps delivery. J Reprod Med. 2003;48(4):239-242.
4. Hartmann K, Viswanathan M, Palmieri R, Gartlehner G, Thorpe J, Jr, Lohr KN. Outcomes of routine episiotomy a systematic review. JAMA. 2005;293(17):2141-2148.
5. Barbieri RL. It’s time to restrict the use of episiotomy. OBG Manage. 2006;18(9):8-12.
6. De Leeuw JW, Struijik PC, Vierhout ME, Wallenburgh HC. Risk factors for third degree perineal ruptures during delivery. BJOG. 2001;108(4):383-387.
7. Fenner DE, Genberg B, Brahma P, Marek L, DeLancey JO. Fecal and urinary incontinence after vaginal delivery with anal sphincter disruption in an obstetrics unit in the United States. Am J Obstet Gynecol. 2003;189(6):1543-1550.
8. Shiono P, Klebenoff MA, Carey JC. Midline episiotomies: more harm than good? Obstet Gynecol. 1990;75(5):765-770.
9. Bodner-Adler B, Bodner K, Kaider A, et al. Risk factors for third-degree perineal tears in vaginal delivery, with an analysis of episiotomy types. J Reprod Med. 2001;46(8):752-756.
10. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87(5):408-412.
11. Thacker SB, Banta HD. Benefits and risks of episiotomy: an interpretive review of the English language literature, 1860–1980. Obstet Gynecol Surv. 1983;38(6):322-338.
12. Goldaber KG, Wendel PJ, McIntire DD, Wendel GD. Postpartum perineal morbidity after fourth degree perineal repair. Am J Obstet Gynecol. 1993;168(2):489-493.
13. Eogan M, Daly L, O’Connell PR, O’Herlihy C. Does the angle of episiotomy affect the incidence of anal sphincter injury? BJOG. 2006;113(2):190-194.
14. Hale RW, Ling FW. Episiotomy: Procedure and repair techniques. Washington DC: American College of Obstetricians and Gynecologists; 2007:1-24.
15. Duggal N, Mercado C, Daniels K, Bujor A, Caughey AB, El-Sayed YY. Antibiotic prophylaxis for prevention of postpartum perineal wound complications: a randomized controlled trial. Obstet Gynecol. 2008;111(6):1268-1273.
16. 1Greenberg JA, Lieberman E, Cohen AP, Ecker JL. Randomized comparison of chromic versus fast-absorbing polyglactin 910 for postpartum perineal repair. Obstet Gynecol. 2004;103(6):1308-1313.
17. Mackrodt C, Gordon B, Fern E, Ayers S, Truesdale A, Grant A. The Ipswich childbirth study 2. A randomised comparison of polyglactin 910 with chromic catgut for postpartum perineal repair. Br J Obstet Gynecol. 1998;105(4):441-445.
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
For more insight and advice on the management of a short cervix in pregnancy, see “Update on Obstetrics.”
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
This study by Romero and colleagues represents yet another attempt by obstetricians to successfully address the important issue of preterm delivery.
My main objection to this attempt?
It’s a systematic review of already published data, with no original findings presented.
The investigators argue that the use of individual patient data strengthens their analysis. They observe that this approach “has been considered the gold standard for summarizing evidence across clinical studies since it offers several advantages, both statistically and clinically, over conventional meta-analyses, which are based on published aggregate data.”
Their methodology included a literature search of multiple databases, including MEDLINE, EMBASE, CINAHL, LILACS, and the Cochrane Central Register of Controlled Trials. Two of the authors (Romero and Conde-Agudelo) made every effort to select only methodologically rigorous articles; about half of the articles identified initially were excluded.
The primary outcome of interest was preterm birth at less than 33 weeks of gestation. After exhaustive analysis, the investigators concluded that universal cervical-length assessment, followed by administration of vaginal progesterone in cases involving a cervical length of 10 to 20 mm, is effective, economical, and without risk.
Although this conclusion sounds promising, it must be viewed with caution—for more than a few reasons.
Weaknesses of the analysis
Here are some of my reservations about this study:
- Meta-analyses should always be interpreted with caution, as should papers that rely on composite outcomes and secondary analyses to bolster their case, as this investigation does
- The true cost of the proposal for universal cervical-length screening is unclear. The figures the investigators present—that, “for every 100,000 women screened, 22 cases of neonatal death or long-term neurologic deficits could be prevented, and approximately $19 million could potentially be saved”—are not universally agreed on.
- Our ability to offer reliable cervical-length screening throughout the US health-care system to all obstetric patients is questionable, and I worry about the bottlenecks such screening would create in the provision of health care
- The US Food and Drug Administration (FDA) recently decided against approving vaginal progesterone.1 Their reasons were numerous, but the most important reasons are summarized by the following FDA committee statements:
This latter point refers to the fact that preterm-birth reduction in this Phase-3 study was meaningful predominantly in centers outside the United States.
What are we to do?
For now, we lack sufficient data to support universal cervical-length screening and vaginal progesterone administration in cases involving a cervical length of 10 to 20 mm to prevent preterm delivery. That said, the FDA committee found this approach to lack statistically significant differences in the incidence of adverse events (maternal, fetal, and neonatal) and conceded, therefore, that a properly informed and counseled patient could be offered this treatment until more definitive data are available.
A completely unscientific approach would be to give vaginal progesterone to every pregnant woman in the United States and, at the end of 1 year, assess the change (or lack thereof) in the rate of prematurity, the cost of care, and neonatal morbidity and mortality. That would settle this issue once and for all—unlike clinical trials or repetitive analyses of already completed clinical trials, which seem unlikely to accomplish this end.
Women who have a history of a short cervix are at very high risk of preterm delivery. A patient who also has a history of prior preterm delivery as a complicating factor is at particularly high risk of recurrent preterm delivery. More puzzling is what to do with the asymptomatic short cervix in a nulliparous patient. For now, I would recommend that physicians discuss with these patients the options available, including the risks, benefits, and limitations of each. Depending on cervical length, these options may include vaginal progesterone, cerclage, or expectant management, with or without serial cervical-length measurement.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
Reference
1. Background document for the meeting of the Advisory Committee for Reproductive Health Drugs. January 20, 2012.
For more insight and advice on the management of a short cervix in pregnancy, see “Update on Obstetrics.”
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
This study by Romero and colleagues represents yet another attempt by obstetricians to successfully address the important issue of preterm delivery.
My main objection to this attempt?
It’s a systematic review of already published data, with no original findings presented.
The investigators argue that the use of individual patient data strengthens their analysis. They observe that this approach “has been considered the gold standard for summarizing evidence across clinical studies since it offers several advantages, both statistically and clinically, over conventional meta-analyses, which are based on published aggregate data.”
Their methodology included a literature search of multiple databases, including MEDLINE, EMBASE, CINAHL, LILACS, and the Cochrane Central Register of Controlled Trials. Two of the authors (Romero and Conde-Agudelo) made every effort to select only methodologically rigorous articles; about half of the articles identified initially were excluded.
The primary outcome of interest was preterm birth at less than 33 weeks of gestation. After exhaustive analysis, the investigators concluded that universal cervical-length assessment, followed by administration of vaginal progesterone in cases involving a cervical length of 10 to 20 mm, is effective, economical, and without risk.
Although this conclusion sounds promising, it must be viewed with caution—for more than a few reasons.
Weaknesses of the analysis
Here are some of my reservations about this study:
- Meta-analyses should always be interpreted with caution, as should papers that rely on composite outcomes and secondary analyses to bolster their case, as this investigation does
- The true cost of the proposal for universal cervical-length screening is unclear. The figures the investigators present—that, “for every 100,000 women screened, 22 cases of neonatal death or long-term neurologic deficits could be prevented, and approximately $19 million could potentially be saved”—are not universally agreed on.
- Our ability to offer reliable cervical-length screening throughout the US health-care system to all obstetric patients is questionable, and I worry about the bottlenecks such screening would create in the provision of health care
- The US Food and Drug Administration (FDA) recently decided against approving vaginal progesterone.1 Their reasons were numerous, but the most important reasons are summarized by the following FDA committee statements:
This latter point refers to the fact that preterm-birth reduction in this Phase-3 study was meaningful predominantly in centers outside the United States.
What are we to do?
For now, we lack sufficient data to support universal cervical-length screening and vaginal progesterone administration in cases involving a cervical length of 10 to 20 mm to prevent preterm delivery. That said, the FDA committee found this approach to lack statistically significant differences in the incidence of adverse events (maternal, fetal, and neonatal) and conceded, therefore, that a properly informed and counseled patient could be offered this treatment until more definitive data are available.
A completely unscientific approach would be to give vaginal progesterone to every pregnant woman in the United States and, at the end of 1 year, assess the change (or lack thereof) in the rate of prematurity, the cost of care, and neonatal morbidity and mortality. That would settle this issue once and for all—unlike clinical trials or repetitive analyses of already completed clinical trials, which seem unlikely to accomplish this end.
Women who have a history of a short cervix are at very high risk of preterm delivery. A patient who also has a history of prior preterm delivery as a complicating factor is at particularly high risk of recurrent preterm delivery. More puzzling is what to do with the asymptomatic short cervix in a nulliparous patient. For now, I would recommend that physicians discuss with these patients the options available, including the risks, benefits, and limitations of each. Depending on cervical length, these options may include vaginal progesterone, cerclage, or expectant management, with or without serial cervical-length measurement.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
For more insight and advice on the management of a short cervix in pregnancy, see “Update on Obstetrics.”
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
This study by Romero and colleagues represents yet another attempt by obstetricians to successfully address the important issue of preterm delivery.
My main objection to this attempt?
It’s a systematic review of already published data, with no original findings presented.
The investigators argue that the use of individual patient data strengthens their analysis. They observe that this approach “has been considered the gold standard for summarizing evidence across clinical studies since it offers several advantages, both statistically and clinically, over conventional meta-analyses, which are based on published aggregate data.”
Their methodology included a literature search of multiple databases, including MEDLINE, EMBASE, CINAHL, LILACS, and the Cochrane Central Register of Controlled Trials. Two of the authors (Romero and Conde-Agudelo) made every effort to select only methodologically rigorous articles; about half of the articles identified initially were excluded.
The primary outcome of interest was preterm birth at less than 33 weeks of gestation. After exhaustive analysis, the investigators concluded that universal cervical-length assessment, followed by administration of vaginal progesterone in cases involving a cervical length of 10 to 20 mm, is effective, economical, and without risk.
Although this conclusion sounds promising, it must be viewed with caution—for more than a few reasons.
Weaknesses of the analysis
Here are some of my reservations about this study:
- Meta-analyses should always be interpreted with caution, as should papers that rely on composite outcomes and secondary analyses to bolster their case, as this investigation does
- The true cost of the proposal for universal cervical-length screening is unclear. The figures the investigators present—that, “for every 100,000 women screened, 22 cases of neonatal death or long-term neurologic deficits could be prevented, and approximately $19 million could potentially be saved”—are not universally agreed on.
- Our ability to offer reliable cervical-length screening throughout the US health-care system to all obstetric patients is questionable, and I worry about the bottlenecks such screening would create in the provision of health care
- The US Food and Drug Administration (FDA) recently decided against approving vaginal progesterone.1 Their reasons were numerous, but the most important reasons are summarized by the following FDA committee statements:
This latter point refers to the fact that preterm-birth reduction in this Phase-3 study was meaningful predominantly in centers outside the United States.
What are we to do?
For now, we lack sufficient data to support universal cervical-length screening and vaginal progesterone administration in cases involving a cervical length of 10 to 20 mm to prevent preterm delivery. That said, the FDA committee found this approach to lack statistically significant differences in the incidence of adverse events (maternal, fetal, and neonatal) and conceded, therefore, that a properly informed and counseled patient could be offered this treatment until more definitive data are available.
A completely unscientific approach would be to give vaginal progesterone to every pregnant woman in the United States and, at the end of 1 year, assess the change (or lack thereof) in the rate of prematurity, the cost of care, and neonatal morbidity and mortality. That would settle this issue once and for all—unlike clinical trials or repetitive analyses of already completed clinical trials, which seem unlikely to accomplish this end.
Women who have a history of a short cervix are at very high risk of preterm delivery. A patient who also has a history of prior preterm delivery as a complicating factor is at particularly high risk of recurrent preterm delivery. More puzzling is what to do with the asymptomatic short cervix in a nulliparous patient. For now, I would recommend that physicians discuss with these patients the options available, including the risks, benefits, and limitations of each. Depending on cervical length, these options may include vaginal progesterone, cerclage, or expectant management, with or without serial cervical-length measurement.
JOHN T. REPKE, MD
We want to hear from you! Tell us what you think.
Reference
1. Background document for the meeting of the Advisory Committee for Reproductive Health Drugs. January 20, 2012.
Reference
1. Background document for the meeting of the Advisory Committee for Reproductive Health Drugs. January 20, 2012.
Baby Can Wait: Hospitals Curb Early Elective Deliveries
At North Shore Medical Center in Salem, Mass., interest in reducing elective deliveries before 39 weeks’ gestation started with a newborn with respiratory problems.
A few years ago, when a patient was induced at about 38 weeks’ gestation, the newborn ended up having significant respiratory complications and had to be transferred to a tertiary care center, said Dr. Allyson L. Preston, chair of obstetrics and gynecology there.
Although the baby recovered and did fine, a review of the case led to a deeper examination of the data on elective deliveries at 37-39 weeks. The result was a growing realization that early elective deliveries weren’t as benign as previously thought, Dr. Preston said in an interview.
After hearing about the success that Intermountain Healthcare in Utah had in reducing early elective deliveries, Dr. Preston worked with her physician and nursing colleagues in the department of ob.gyn. to develop a policy not to schedule either elective inductions or cesarean sections if the patient was at less than 39 weeks’ gestation, unless there was a medical or obstetrical indication that early delivery was necessary.
In early 2010, the hospital staff sent out new booking forms to area obstetrical offices. The new forms required physicians to note the indications for early delivery and the gestational age. The charge nurses at North Shore Medical Center were given the authority not to schedule procedures if a patient was at less than 39 weeks’ gestation and the physician did not include an accepted medical or obstetrical indication for delivery. Physicians who took exception to the policy were referred to Dr. Preston. For the most part, physicians have eagerly adopted the policy, she said.
"I think people realized we were serious about it," Dr. Preston said. "They realized it was the right thing to do."
The results since the policy was implemented have been fairly dramatic. Data released earlier this year by the Leapfrog Group show that in 2010, the early elective newborn delivery rate was 31.6% at North Shore, compared with just 1.6% in 2011. That means that among deliveries done at 37-39 weeks, more than 98% are now medically indicated, according to Dr. Preston.
Other hospitals around the country are experiencing similar success, according to data from the Leapfrog Group’s annual hospital survey. The survey of 757 hospitals found that 39% of reporting hospitals had early elective delivery rates of 5% or less in 2011, compared with 30% the previous year. The rate of elective deliveries represents the percentage of nonmedically indicated births at 37-39 weeks’ gestation delivered by caesarean section or induction. The average rate for early elective deliveries among these hospitals dropped from 17% to 14% between 2010 and 2011.
Despite the progress already being made to curb early deliveries in some hospitals, there was still a wide variation among the hospitals in the Leapfrog survey, with early elective delivery rates ranging from a low of less than 5% to more than 40%, according to the 2011 data.
The Leapfrog Group (a coalition of public and private purchasers of employee health benefits that works on health care quality improvement) began collecting data on early elective deliveries in 2009 and first publicly reported hospital data in 2010. The group has identified the trend toward early deliveries as both a safety and an economic issue. For instance, women who are induced in weeks 37 and 38 have a higher risk of needing a cesarean section than do women who go into labor on their own. Studies also have linked early elective deliveries to postpartum complications such as hematoma, wound dehiscence, anemia, endometriosis, urinary tract infections, and sepsis, according to the Leapfrog Group.
The Leapfrog Group is not the only organization that has made the reduction of early elective deliveries a priority. For many years, the American College of Obstetricians and Gynecologists has recommended against elective deliveries before 39 weeks. In February, the federal government added its weight to the push to reduce these types of elective deliveries with the launch of its Strong Start initiative. Under the new effort, officials at the Department of Health and Human Services will work with outside partners to test a nationwide awareness campaign on best practices to reduce the rate of early elective deliveries before 39 weeks among all pregnant women. They will also test "enhanced prenatal care" approaches for Medicaid beneficiaries who are at risk for preterm birth.
The latest Leapfrog data are consistent with what other efforts that are aimed at reducing early elective deliveries have been able to show, said Dr. Donald Dudley, professor and vice chair for research in the department of ob.gyn. at the University of Texas Health Sciences Center in San Antonio.
Dr. Dudley also has been active in the March of Dimes Big 5 Prematurity Collaborative, which has been working to reduce preterm births through evidence-based quality improvement projects.
The Big 5 Prematurity Collaborative worked with 25 hospitals in California, Florida, Illinois, New York, and Texas to implement a policy of not performing elective deliveries before 39 weeks unless they are medically indicated. They found that hospitals – regardless of their size or location – were able to rapidly reduce early elective deliveries. The key, Dr. Dudley said, was making physicians and nurses aware of the data on complications from early deliveries.
Dr. Dudley said some physicians are skeptical that those last few weeks make that big a difference. He had been one of them. But once he read through the studies, it became clear that deliveries before 39 weeks were not the safest path for mothers and babies. "You’ll come away saying it is really not a good idea to do elective deliveries at less than 39 weeks," he said.
Once the buy-in from providers is there, making the change is fairly simple and relatively cheap, Dr. Dudley said. Hospitals can take advantage of free toolkits like the one from the California Maternal Quality Care Collaborative and the March of Dimes.
The most costly and time-intensive part of any effort to reduce early elective deliveries is the data collection, he said. Hospitals need to know how they doing at the start, but many community hospitals don’t routinely collect those data.
Dr. Dudley cautioned hospitals to be careful not to push the policy so hard that they discourage physicians from exercising their clinical judgment. "One of the things we have to be really careful about is not to swing the pendulum so far the other way that we disengage our brains."
If there’s a reason for a patient to be delivered at less than 39 weeks, then the delivery is indicated, not elective, he said. And there is a long list of appropriate indications for an early delivery.
For example, Dr. Dudley said he recently had a patient whom he had been monitoring and hoping to deliver after 39 weeks, but at 38 and a half weeks her blood pressure began creeping up enough that it was necessary to do the delivery early. The patient had a history of hypertension, and the risk of waiting outweighed the risks of early induction, he said. "You don’t want to delay acting when there’s an acceptable indication."
Policies aimed at reducing early elective deliveries can introduce a reluctance to induce (either by the physician or the patient) that may result in delaying an induction that is actually indicated, said Dr. William H. Barth Jr., chief of the division of maternal-fetal medicine at Massachusetts General Hospital, Boston, where the early elective newborn delivery rate has declined from 8% to 4% between 2010 and 2011. "Recommending an induction is a complex decision," he said. "The recent push to decrease elective inductions is sort of one more factor in the mix of that complex decision."
But the other issue is simple math, Dr. Barth said. If more inductions are delayed – indicated or not – there will be more stillbirths. He pointed to a retrospective cohort study published last year in Obstetrics & Gynecology that looked at the effect of a policy limiting elective delivery before 39 weeks’ gestation. The researchers found a significant decrease in admissions to the neonatal ICU, but they also detected an increase in stillbirths at 37 and 38 weeks (Obstet. Gynecol. 2011;118:1047-55).
Dr. Barth advised hospitals considering formal policies to reduce early elective deliveries to track not only the number of elective deliveries, but also stillbirths. "Shared decision making between patients and physicians is best conducted with all of the information – good and bad," he said.
At North Shore Medical Center in Salem, Mass., interest in reducing elective deliveries before 39 weeks’ gestation started with a newborn with respiratory problems.
A few years ago, when a patient was induced at about 38 weeks’ gestation, the newborn ended up having significant respiratory complications and had to be transferred to a tertiary care center, said Dr. Allyson L. Preston, chair of obstetrics and gynecology there.
Although the baby recovered and did fine, a review of the case led to a deeper examination of the data on elective deliveries at 37-39 weeks. The result was a growing realization that early elective deliveries weren’t as benign as previously thought, Dr. Preston said in an interview.
After hearing about the success that Intermountain Healthcare in Utah had in reducing early elective deliveries, Dr. Preston worked with her physician and nursing colleagues in the department of ob.gyn. to develop a policy not to schedule either elective inductions or cesarean sections if the patient was at less than 39 weeks’ gestation, unless there was a medical or obstetrical indication that early delivery was necessary.
In early 2010, the hospital staff sent out new booking forms to area obstetrical offices. The new forms required physicians to note the indications for early delivery and the gestational age. The charge nurses at North Shore Medical Center were given the authority not to schedule procedures if a patient was at less than 39 weeks’ gestation and the physician did not include an accepted medical or obstetrical indication for delivery. Physicians who took exception to the policy were referred to Dr. Preston. For the most part, physicians have eagerly adopted the policy, she said.
"I think people realized we were serious about it," Dr. Preston said. "They realized it was the right thing to do."
The results since the policy was implemented have been fairly dramatic. Data released earlier this year by the Leapfrog Group show that in 2010, the early elective newborn delivery rate was 31.6% at North Shore, compared with just 1.6% in 2011. That means that among deliveries done at 37-39 weeks, more than 98% are now medically indicated, according to Dr. Preston.
Other hospitals around the country are experiencing similar success, according to data from the Leapfrog Group’s annual hospital survey. The survey of 757 hospitals found that 39% of reporting hospitals had early elective delivery rates of 5% or less in 2011, compared with 30% the previous year. The rate of elective deliveries represents the percentage of nonmedically indicated births at 37-39 weeks’ gestation delivered by caesarean section or induction. The average rate for early elective deliveries among these hospitals dropped from 17% to 14% between 2010 and 2011.
Despite the progress already being made to curb early deliveries in some hospitals, there was still a wide variation among the hospitals in the Leapfrog survey, with early elective delivery rates ranging from a low of less than 5% to more than 40%, according to the 2011 data.
The Leapfrog Group (a coalition of public and private purchasers of employee health benefits that works on health care quality improvement) began collecting data on early elective deliveries in 2009 and first publicly reported hospital data in 2010. The group has identified the trend toward early deliveries as both a safety and an economic issue. For instance, women who are induced in weeks 37 and 38 have a higher risk of needing a cesarean section than do women who go into labor on their own. Studies also have linked early elective deliveries to postpartum complications such as hematoma, wound dehiscence, anemia, endometriosis, urinary tract infections, and sepsis, according to the Leapfrog Group.
The Leapfrog Group is not the only organization that has made the reduction of early elective deliveries a priority. For many years, the American College of Obstetricians and Gynecologists has recommended against elective deliveries before 39 weeks. In February, the federal government added its weight to the push to reduce these types of elective deliveries with the launch of its Strong Start initiative. Under the new effort, officials at the Department of Health and Human Services will work with outside partners to test a nationwide awareness campaign on best practices to reduce the rate of early elective deliveries before 39 weeks among all pregnant women. They will also test "enhanced prenatal care" approaches for Medicaid beneficiaries who are at risk for preterm birth.
The latest Leapfrog data are consistent with what other efforts that are aimed at reducing early elective deliveries have been able to show, said Dr. Donald Dudley, professor and vice chair for research in the department of ob.gyn. at the University of Texas Health Sciences Center in San Antonio.
Dr. Dudley also has been active in the March of Dimes Big 5 Prematurity Collaborative, which has been working to reduce preterm births through evidence-based quality improvement projects.
The Big 5 Prematurity Collaborative worked with 25 hospitals in California, Florida, Illinois, New York, and Texas to implement a policy of not performing elective deliveries before 39 weeks unless they are medically indicated. They found that hospitals – regardless of their size or location – were able to rapidly reduce early elective deliveries. The key, Dr. Dudley said, was making physicians and nurses aware of the data on complications from early deliveries.
Dr. Dudley said some physicians are skeptical that those last few weeks make that big a difference. He had been one of them. But once he read through the studies, it became clear that deliveries before 39 weeks were not the safest path for mothers and babies. "You’ll come away saying it is really not a good idea to do elective deliveries at less than 39 weeks," he said.
Once the buy-in from providers is there, making the change is fairly simple and relatively cheap, Dr. Dudley said. Hospitals can take advantage of free toolkits like the one from the California Maternal Quality Care Collaborative and the March of Dimes.
The most costly and time-intensive part of any effort to reduce early elective deliveries is the data collection, he said. Hospitals need to know how they doing at the start, but many community hospitals don’t routinely collect those data.
Dr. Dudley cautioned hospitals to be careful not to push the policy so hard that they discourage physicians from exercising their clinical judgment. "One of the things we have to be really careful about is not to swing the pendulum so far the other way that we disengage our brains."
If there’s a reason for a patient to be delivered at less than 39 weeks, then the delivery is indicated, not elective, he said. And there is a long list of appropriate indications for an early delivery.
For example, Dr. Dudley said he recently had a patient whom he had been monitoring and hoping to deliver after 39 weeks, but at 38 and a half weeks her blood pressure began creeping up enough that it was necessary to do the delivery early. The patient had a history of hypertension, and the risk of waiting outweighed the risks of early induction, he said. "You don’t want to delay acting when there’s an acceptable indication."
Policies aimed at reducing early elective deliveries can introduce a reluctance to induce (either by the physician or the patient) that may result in delaying an induction that is actually indicated, said Dr. William H. Barth Jr., chief of the division of maternal-fetal medicine at Massachusetts General Hospital, Boston, where the early elective newborn delivery rate has declined from 8% to 4% between 2010 and 2011. "Recommending an induction is a complex decision," he said. "The recent push to decrease elective inductions is sort of one more factor in the mix of that complex decision."
But the other issue is simple math, Dr. Barth said. If more inductions are delayed – indicated or not – there will be more stillbirths. He pointed to a retrospective cohort study published last year in Obstetrics & Gynecology that looked at the effect of a policy limiting elective delivery before 39 weeks’ gestation. The researchers found a significant decrease in admissions to the neonatal ICU, but they also detected an increase in stillbirths at 37 and 38 weeks (Obstet. Gynecol. 2011;118:1047-55).
Dr. Barth advised hospitals considering formal policies to reduce early elective deliveries to track not only the number of elective deliveries, but also stillbirths. "Shared decision making between patients and physicians is best conducted with all of the information – good and bad," he said.
At North Shore Medical Center in Salem, Mass., interest in reducing elective deliveries before 39 weeks’ gestation started with a newborn with respiratory problems.
A few years ago, when a patient was induced at about 38 weeks’ gestation, the newborn ended up having significant respiratory complications and had to be transferred to a tertiary care center, said Dr. Allyson L. Preston, chair of obstetrics and gynecology there.
Although the baby recovered and did fine, a review of the case led to a deeper examination of the data on elective deliveries at 37-39 weeks. The result was a growing realization that early elective deliveries weren’t as benign as previously thought, Dr. Preston said in an interview.
After hearing about the success that Intermountain Healthcare in Utah had in reducing early elective deliveries, Dr. Preston worked with her physician and nursing colleagues in the department of ob.gyn. to develop a policy not to schedule either elective inductions or cesarean sections if the patient was at less than 39 weeks’ gestation, unless there was a medical or obstetrical indication that early delivery was necessary.
In early 2010, the hospital staff sent out new booking forms to area obstetrical offices. The new forms required physicians to note the indications for early delivery and the gestational age. The charge nurses at North Shore Medical Center were given the authority not to schedule procedures if a patient was at less than 39 weeks’ gestation and the physician did not include an accepted medical or obstetrical indication for delivery. Physicians who took exception to the policy were referred to Dr. Preston. For the most part, physicians have eagerly adopted the policy, she said.
"I think people realized we were serious about it," Dr. Preston said. "They realized it was the right thing to do."
The results since the policy was implemented have been fairly dramatic. Data released earlier this year by the Leapfrog Group show that in 2010, the early elective newborn delivery rate was 31.6% at North Shore, compared with just 1.6% in 2011. That means that among deliveries done at 37-39 weeks, more than 98% are now medically indicated, according to Dr. Preston.
Other hospitals around the country are experiencing similar success, according to data from the Leapfrog Group’s annual hospital survey. The survey of 757 hospitals found that 39% of reporting hospitals had early elective delivery rates of 5% or less in 2011, compared with 30% the previous year. The rate of elective deliveries represents the percentage of nonmedically indicated births at 37-39 weeks’ gestation delivered by caesarean section or induction. The average rate for early elective deliveries among these hospitals dropped from 17% to 14% between 2010 and 2011.
Despite the progress already being made to curb early deliveries in some hospitals, there was still a wide variation among the hospitals in the Leapfrog survey, with early elective delivery rates ranging from a low of less than 5% to more than 40%, according to the 2011 data.
The Leapfrog Group (a coalition of public and private purchasers of employee health benefits that works on health care quality improvement) began collecting data on early elective deliveries in 2009 and first publicly reported hospital data in 2010. The group has identified the trend toward early deliveries as both a safety and an economic issue. For instance, women who are induced in weeks 37 and 38 have a higher risk of needing a cesarean section than do women who go into labor on their own. Studies also have linked early elective deliveries to postpartum complications such as hematoma, wound dehiscence, anemia, endometriosis, urinary tract infections, and sepsis, according to the Leapfrog Group.
The Leapfrog Group is not the only organization that has made the reduction of early elective deliveries a priority. For many years, the American College of Obstetricians and Gynecologists has recommended against elective deliveries before 39 weeks. In February, the federal government added its weight to the push to reduce these types of elective deliveries with the launch of its Strong Start initiative. Under the new effort, officials at the Department of Health and Human Services will work with outside partners to test a nationwide awareness campaign on best practices to reduce the rate of early elective deliveries before 39 weeks among all pregnant women. They will also test "enhanced prenatal care" approaches for Medicaid beneficiaries who are at risk for preterm birth.
The latest Leapfrog data are consistent with what other efforts that are aimed at reducing early elective deliveries have been able to show, said Dr. Donald Dudley, professor and vice chair for research in the department of ob.gyn. at the University of Texas Health Sciences Center in San Antonio.
Dr. Dudley also has been active in the March of Dimes Big 5 Prematurity Collaborative, which has been working to reduce preterm births through evidence-based quality improvement projects.
The Big 5 Prematurity Collaborative worked with 25 hospitals in California, Florida, Illinois, New York, and Texas to implement a policy of not performing elective deliveries before 39 weeks unless they are medically indicated. They found that hospitals – regardless of their size or location – were able to rapidly reduce early elective deliveries. The key, Dr. Dudley said, was making physicians and nurses aware of the data on complications from early deliveries.
Dr. Dudley said some physicians are skeptical that those last few weeks make that big a difference. He had been one of them. But once he read through the studies, it became clear that deliveries before 39 weeks were not the safest path for mothers and babies. "You’ll come away saying it is really not a good idea to do elective deliveries at less than 39 weeks," he said.
Once the buy-in from providers is there, making the change is fairly simple and relatively cheap, Dr. Dudley said. Hospitals can take advantage of free toolkits like the one from the California Maternal Quality Care Collaborative and the March of Dimes.
The most costly and time-intensive part of any effort to reduce early elective deliveries is the data collection, he said. Hospitals need to know how they doing at the start, but many community hospitals don’t routinely collect those data.
Dr. Dudley cautioned hospitals to be careful not to push the policy so hard that they discourage physicians from exercising their clinical judgment. "One of the things we have to be really careful about is not to swing the pendulum so far the other way that we disengage our brains."
If there’s a reason for a patient to be delivered at less than 39 weeks, then the delivery is indicated, not elective, he said. And there is a long list of appropriate indications for an early delivery.
For example, Dr. Dudley said he recently had a patient whom he had been monitoring and hoping to deliver after 39 weeks, but at 38 and a half weeks her blood pressure began creeping up enough that it was necessary to do the delivery early. The patient had a history of hypertension, and the risk of waiting outweighed the risks of early induction, he said. "You don’t want to delay acting when there’s an acceptable indication."
Policies aimed at reducing early elective deliveries can introduce a reluctance to induce (either by the physician or the patient) that may result in delaying an induction that is actually indicated, said Dr. William H. Barth Jr., chief of the division of maternal-fetal medicine at Massachusetts General Hospital, Boston, where the early elective newborn delivery rate has declined from 8% to 4% between 2010 and 2011. "Recommending an induction is a complex decision," he said. "The recent push to decrease elective inductions is sort of one more factor in the mix of that complex decision."
But the other issue is simple math, Dr. Barth said. If more inductions are delayed – indicated or not – there will be more stillbirths. He pointed to a retrospective cohort study published last year in Obstetrics & Gynecology that looked at the effect of a policy limiting elective delivery before 39 weeks’ gestation. The researchers found a significant decrease in admissions to the neonatal ICU, but they also detected an increase in stillbirths at 37 and 38 weeks (Obstet. Gynecol. 2011;118:1047-55).
Dr. Barth advised hospitals considering formal policies to reduce early elective deliveries to track not only the number of elective deliveries, but also stillbirths. "Shared decision making between patients and physicians is best conducted with all of the information – good and bad," he said.
The fine points of delayed cord clamping
Related article Does the timing of umbilical cord clamping at delivery affect an infant’s long-term iron status? Athol P. Kent, MBChB, MPhil, and David L. Woods, MBChB, MD, DCH (Examining the Evidence, March 2012)
Related article Does the timing of umbilical cord clamping at delivery affect an infant’s long-term iron status? Athol P. Kent, MBChB, MPhil, and David L. Woods, MBChB, MD, DCH (Examining the Evidence, March 2012)
Related article Does the timing of umbilical cord clamping at delivery affect an infant’s long-term iron status? Athol P. Kent, MBChB, MPhil, and David L. Woods, MBChB, MD, DCH (Examining the Evidence, March 2012)
10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)
Cesarean delivery is not risk-free, despite its high prevalence (30% overall, but almost 100% in women who have more than two prior cesareans). It increases the risks of adhesions, severe blood loss, and injury to the bowel, bladder and ureters, particularly among women undergoing the procedure for the second or third time.
Morbidly obese women (i.e., those who have a body mass index [BMI] of 40 or above) are in a particular bind: They have an elevated risk of cesarean delivery, and when they undergo the procedure, they have a significantly heightened risk of cardiopulmonary complications, anesthetic complications, wound complications, thromboembolism, and prolonged skin incision-to-delivery time.
A number of studies have described the technical aspects of cesarean delivery, but debate continues about a number of issues:
- the risks and benefits of types of skin incision
- whether the rectus muscle should be separated bluntly or sharply
- whether or not to close the peritoneum
- the best method of closing the skin (i.e., subcuticular sutures or staples).
In this review, I offer 10 practical, evidence-based recommendations that help clarify these issues, including several that focus on the morbidl obese population.
1. Anticipate anesthetic complications
In morbidly obese pregnant women, plan for potential complications
Vricella LK, Lois JM, Mercer BM, Bolden N. Anesthesia complications during scheduled cesarean delivery for morbidly obese women. Am J Obstet Gynecol. 2010;203(3):276.e1–5.
Knight M, Kurinczuk JJ, Spark P, Brocklehurst P; UK Obstetric Surveillance System. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol. 2010;115(5):989–997.
In a national cohort study of 665 women who had a BMI of 50 or above, 11% experienced problems with epidural anesthesia, including failure; 6% required general anesthesia; and 3% required admission to intensive care. A similar, but retrospective, study of 142 morbidly obese women found an anesthesia complication rate of 8.5%.
These studies suggest that planning and antenatal consultation with anesthesiologists are important to help avert anesthetic complications during cesarean delivery. Requirements include detailed evaluation at admission, early placement of an epidural catheter, preparation for general anesthesia in case of failure of regional anesthesia, and ensuring the availability of an anesthesiologist who has expertise in this population.
2. Reduce the interval from decision to delivery
Plan, implement, and rehearse a protocol to move from decision to incision and delivery in 30 minutes in morbidly obese women
Lucas DN. The 30-minute decision to delivery time is unrealistic in morbidly obese women. Int J Obstet Anesth. 2010;19(4):431–435. Comment by: Dresner M. Int J Obstet Anesth. 2010;19(4):435–437.
Although several national bodies recommend a decision-to-incision or delivery interval of 30 minutes or less, this approach is not backed by definitive data. Moreover, the 30-minute goal poses major challenges to the nursing, anesthesia, and surgical teams that provide care to morbidly obese women who require emergent cesarean delivery. This is especially true in cases that involve catastrophic events, such as abruptio placentae, cord prolapse, uterine rupture, or vasa previa—where minutes matter.
Nevertheless, efforts to reduce this interval are vital. Consider four phases:
- how long it takes to transfer the patient to the operating room
- the time it takes to position and prepare the patient for surgery
- the time required to administer anesthesia
- how long it takes to move from skin incision to delivery of the fetus.
Because all four phases will be prolonged in morbidly obese patients, it is prudent for obstetric units to develop protocols to identify and flag women who are at risk, and to have policies and procedures in place to reduce these times. This will necessitate drills for rehearsal and testing of response times and skills of the various providers. In addition, whenever emergent cesarean is performed, the actual response time and effectiveness of interventions should be evaluated.
3. Consider a transverse skin incision
In morbidly obese women who undergo emergent cesarean delivery, a transverse skin incision may provide benefit
Wylie BJ, Gilbert S, Landon MB, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Comparison of transverse and vertical skin incision for emergency cesarean delivery. Obstet Gynecol. 2010;115(6):1134–1140.
Bell J, Bell S, Vahratian A, Awonuga AO. Abdominal surgical incisions and perioperative morbidity among morbidly obese women undergoing cesarean delivery. Eur J Obstet Gynecol Reprod Biol. 2011;154(1):16–19.
No randomized trials have compared the benefits and risks of vertical and transverse skin incisions during cesarean delivery. In general, a vertical incision is believed to shorten the time to delivery, but is associated with a greater need for transfusion, greater postoperative pain, and higher rates of wound dehiscence and infection, compared with a transverse incision.
A prospective cohort study of all emergent cesarean deliveries performed at 13 medical centers compared maternal and neonatal outcomes between 2,498 women who had a vertical incision and 1,027 who had a transverse incision. The use of a vertical incision shortened the median incision-to-delivery interval by 1 minute (3 vs 4 minutes; P < .001) for primary cesarean and by 2 minutes (3 vs 5 minutes; P < .001) for repeat cesarean. However, a vertical incision was associated with higher rates of endometritis (15% vs 11%; P = .006) and postpartum transfusion (7% vs 5%; P = .01) for primary cesarean, as well as a higher rate of postpartum transfusion (15% vs 8%; P = .02) for repeat cesarean. No differences in the rates of wound hematoma and infection were noted.
A retrospective cohort study in 424 morbidly obese women compared maternal morbidity between 41 women who had a vertical skin incision and 383 women who had a transverse incision for cesarean delivery. A vertical incision was associated with a dramatic increase in the risk of a classical uterine incision (65.9% vs 7.3%; P < .001), but there were no differences in the rates of blood transfusion or wound breakdown or infection between the two groups. However, these findings should be interpreted with caution because women who received a vertical incision were older (31.0 ± 6.2 years vs 27.1 ± 6.7 years; P <.001), and there was no mention of the type of vertical skin incision in relation to the umbilicus or the use of drains. A randomized trial is needed to determine the optimal skin incision in morbidly obese women.
4. Use blunt, not sharp, expansion of the uterine incision
Blunt expansion is associated with less blood loss
Sekhavat L, Firouzabadi RD, Mojiri P. Effect of expansion technique of uterine incision on maternal blood loss in cesarean section. Arch Gynecol Obstet. 2010;282(5):475–479.
A prospective, randomized trial explored the rate of lateral extension of the uterine incision and estimated blood loss in 200 full-term primiparas undergoing cesarean delivery. Women were assigned to blunt expansion (n = 100) or sharp expansion (n = 100). Blunt expansion was associated with lower estimated blood loss (375 ± 95 mL vs 443 ± 86 mL; P <.05) but no differences in the rate of lateral extension (5% vs 6%). These findings reveal that blunt expansion of the uterine incision in primiparas is safer and easier than sharp expansion.
Nonclosure after cesarean delivery is associated with a higher rate of adhesion formation
Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):3–8.
Shi Z, Ma L, Yang Y, Wang H, et al. Adhesion formation after previous caesarean section—a meta-analysis and systematic review. BJOG. 2011;118(4):410–422. doi: 10.1111/j.1471-0528.2010.02808.x.
A systematic review and meta-analysis that included two randomized trials and one prospective study compared the rate of adhesions after cesarean delivery between women who had peritoneal closure (n = 110) and those who did not (n = 139). Nonclosure was associated with a substantial increase in the rate of subsequent adhesion formation (adjusted odds ratio, 4.23; 95% confidence interval [CI], 2.06–8.69). However, this review did not consider risk factors such as creation of a bladder flap or type of uterine incision.
A subsequent systematic review (n = 4,423) compared the rate of adhesions associated with closure and nonclosure of the peritoneum according to cesarean technique (Stark’s, modified Stark’s, or classic lower-segment). The classic lower-segment technique involves dissecting the bladder off the uterus and closure of both peritoneal layers (visceral and peritoneal). Neither Stark’s technique nor the modified Stark’s technique dissects the bladder from the uterus; both techniques use single-layer closure of the uterine incision. Stark’s technique leaves the peritoneal layer open, whereas the modified Stark’s technique closes the peritoneal layer. This review revealed that closing the peritoneum in modified Stark’s cesarean delivery was associated with a lower rate of subsequent adhesions—both in terms of total adhesions and individual grades of adhesions.
6. Use double-layer uterine closure
Despite its lack of effect on maternal morbidity, double-layer closure reduces risk of rupture during VBAC
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
This large multicenter, randomized trial evaluated maternal infectious morbidity in women undergoing single- (n = 1,483) and double-layer (n = 1,496) closure of the uterine incision. The total rates of maternal infectious morbidity (16.1% vs 16.9%), wound infection (12.8% vs 12.7%), severe morbidity (0.5% vs 0.7%), and readmission within 6 weeks (2.6% vs 2.7%) were similar between groups for single- and double-layer closure, respectively. However, retrospective and case-control studies reveal that double-layer closure is associated with lower rates of uterine dehiscence and rupture during vaginal birth after cesarean (VBAC).
7. Keep the risk of adhesions in mind
Closing the peritoneum may reduce long-term adhesion formation
The CAESAR study collaborative group. Caesarean section surgical techniques: a randomised factorial trial (CAESAR). BJOG. 2010;117(11):1366–1376. doi: 10.1111/j.1471-0528.2010.02686.x.
According to the findings of this large, multicenter, randomized trial, the rate of maternal infectious morbidity did not change whether or not a drain was used or the peritoneum was closed. The study evaluated only maternal infectious morbidity related to peritoneal closure—not long-term adhesion formation. Nevertheless, it appears that closure of the peritoneum at the time of cesarean delivery is associated with a lower rate of long-term adhesion formation.
8. Forget adhesion barriers
Their use to prevent intra-abdominal adhesions is ill-advised
Albright CM, Rouse DJ. Adhesion barriers at cesarean delivery: advertising compared with the evidence. Obstet Gynecol. 2011;118(1):157–160.
After cesarean delivery, there is a potential for intra-abdominal adhesions to form, which can lead to pain, small bowel obstruction, and injury during repeat surgery. A review of the literature suggests that the use of adhesion barriers at cesarean delivery is not cost-effective. Randomized trials are needed.
9. Be vigilant for bladder and ureteral injuries
Know the risk factors and preventive strategies for these injuries
Gungorduk K, Asicioglu O, Celikkol O, et al. Iatrogenic bladder injuries during caesarean delivery: a case control study. J Obstet Gynaecol. 2010;30(7):667–670.
Karram M. Avoiding and managing lower urinary tract injury during vaginal and abdominal deliveries. In: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011:179–187.
The reported incidence of bladder injury at the time of cesarean delivery ranges from 0.13% to 0.31% during primary cesarean and reaches 0.6% during repeat cesarean. During primary cesarean, bladder injury usually occurs during entry into the peritoneal cavity and involves the high extraperitoneal aspect of the bladder. In repeat cesarean, it usually occurs during dissection of the bladder flap and involves the intraperitoneal aspect of the bladder (dependent portion).
Bladder and ureteral injuries are more likely to occur in the presence of one or more of the following risk factors:
- emergency or crash cesarean delivery
- cesarean delivery after prolonged pushing
- history of uterine or abdominal surgery
- central placenta previa or accreta
- lateral or downward extension of the uterine incision
- uterine rupture
- need for hysterectomy.
Inadvertent bladder injury during entrance into the peritoneum should be managed with layered closure of the cystotomy. Because such injury occurs high in the bladder, it requires only a short period of postoperative drainage. In contrast, injury to the base of the bladder requires appropriate mobilization of the bladder off any adherent structures to allow tension-free closure of the injury. Since this type of injury lies in the dependent portion of the bladder, it requires postoperative drainage for 10 to 14 days. Close the injury in two layers, using fine, delayed, absorbable suture in interrupted or running fashion, with the first layer approximating the mucosa and the second layer imbricating the muscularis.
Ureteral injury is rare during cesarean delivery. When it does occur, it usually occurs during repair of lacerations from the uterine incision or control of excessive bleeding from the lower segment or broad ligament (FIGURE). The most common site of injury from uterine lacerations is at the level of the uterine vessels, whereas the most common site of injury at cesarean hysterectomy is the lower portion of the ureter near the uterosacral ligaments.
If you suspect injury, confirm ureteral patency by making a cystotomy in the bladder dome to visualize the orifices, and attempt to pass a ureteral catheter or pediatric feeding tube through the orifice into the ureter until you reach a point above the area of concern. If there is an obstruction, kinking, or transection, consult a urogynecologist or urologist.
Risks of lateral and downward extension
Avoid lateral and downward extension of the uterine incision, which may injure the blood vessels or ureter.
Source: Sibai BM, ed. Management of Acute Obstetric Emergencies. Philadelphia, Pa: Elsevier-Saunders; 2011.
10. Close the skin with subcuticular suture
This approach is associated with lower risk of wound separation and infection than is closure with staples
Tuuli MG, Rampersad RM, Carbone JF, Stamilio D, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2011;117(3):682–690.
Wound complications following cesarean can include hematoma, seroma, complete separation, or infection (superficial or deep). Wound complications may be more likely with staple closure of a transverse skin incision than with subcuticular suture. Other issues to consider when choosing a type of skin closure include postoperative pain, cosmesis, and how long it takes to deliver the infant.
This systematic review and meta-analysis of five randomized trials and one prospective study compared outcomes after skin staple closure (n = 803) with those after subcuticular suture closure (n = 684) in women who had a transverse incision. Staple closure was associated with a higher rate of wound infection or separation (13.4% vs 6.6%; pooled odds ratio, 2.06; 95% CI, 1.43–2.98). Staple closure was associated with a shorter operating time (range, 3.3–9.3 minutes). Both techniques were similar in terms of postoperative pain, cosmetic appearance, and patient satisfaction.
We want to hear from you! Tell us what you think.
- 10 practical recommendations to improve maternal and perinatal outcomes in patients with eclampsia
Baha M. Sibai, MD (November 2011) - 10 (+1) practical, evidence-based recommendations for you to improve contraceptive care now
Colleen Krajewski, MD; Mark D. Walters, MD (August 2011) - 10 practical, evidence-based recommendations for the management of severe postpartum hemorrhage
Baha M. Sibai, MD (June 2011) - 10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011) - 10 practical, evidence-based suggestions to improve your gyn practice now
Mark D. Walters, MD (January 2011)
Does the timing of umbilical cord clamping at delivery affect an infant’s long-term iron status?
Andersson and colleagues begin their published report by explaining the importance of adequate iron status to child development, particularly neurodevelopment. As they note, “Young children are at particular risk of iron deficiency because of high iron requirements during rapid growth in combination with low iron intake.” The investigators also point out that the prevalence of iron-deficiency anemia (3%–7%) and iron deficiency (as high as 26%) among young children in Europe, where this study was conducted, has troubling implications. Iron-deficiency anemia, in particular, may produce enduring cognitive and behavioral deficits.
This is the first randomized, controlled trial to explore the timing of umbilical cord clamping in a high-income country. Earlier studies in low- and middle-income populations have found that delayed cord clamping leads to an increased serum level of ferritin at 3 to 6 months of age.1-5
The rationale for late clamping
Delayed clamping allows for placental transfusion of almost one third of the infant’s blood volume. Infants need the iron in all their red blood cells (RBCs)—circulating systemically and in the placenta—to guard against iron deficiency in the first few months of life. This need is unrelated to the mother’s health, iron status, and socioeconomic station.
Without the full quota of RBCs, an infant’s iron stores—reflected most accurately by ferritin levels at 4 to 6 months of age—will be suboptimal. Early clamping deprives newborns of RBCs remaining in the placenta and the iron they contain, which is essential for the development of the hematopoietic and central nervous systems.
The standard routine of administering oxytocin with early cord clamping, followed by controlled cord traction, was introduced as part of the active management of the third stage of labor. Until recently, there were no randomized, controlled trials demonstrating how early clamping disadvantaged the infant.
Details of the trial
The trial was conducted in a Swedish county hospital in an affluent, high-income population. Four hundred full-term, singleton infants, born after low-risk pregnancy, were randomized to early or late cord clamping.
Intravenous oxytocin (10 IU) was given immediately after the cord was clamped. The time from full delivery of the baby to the start of cord clamping was measured by an assistant to the midwife.
Blood samples were taken from the newborns 48 to 72 hours after delivery and again at 4 months of age.
A clear benefit from delayed clamping
The infants’ hemoglobin concentration and packed RBC volume were higher 2 days after delivery in the delayed-clamping group, compared with early clamping, and this translated into improved iron stores at the 4-month follow-up. Specifically, the rate of anemia at a median age of 2.4 days was 1.2% in the delayed-clamping group versus 6.3% for early clamping (P = .02; relative risk reduction 0.80; 95% confidence interval [CI], 0.22–0.95). And the geometric mean serum ferritin concentration at 4 months of age was 45% higher in the delayed-clamping group (117 μg/L vs 81 μg/L; P < .001; 95% CI, 23%–71%). Iron deficiency was significantly more prevalent in the early-clamping group, but the prevalence of anemia was similar between groups. To prevent one case of iron deficiency—with or without anemia—the number needed to treat was 20 (95% CI, 17–67).
Other growth parameters were similar. Additional long-term tracking is planned.
One of the arguments against delayed clamping is that the practice raises the risks of respiratory symptoms, polycythemia, and hyperbilirubinemia, and increases the need for phototherapy. However, this study demonstrated that late clamping does not increase these risks.
The advantages and risks of delayed cord clamping in infants who are either preterm, growth-restricted, or hypoxic remain to be determined.
Delaying umbilical cord clamping for 3 minutes after delivery allows for substantial transfusion of blood from the placenta to the newborn. This extra blood protects the child from iron deficiency, producing a 45% increase in iron stores that is measurable at 4 months of age. The iron is required for optimal hematopoietic and central nervous system function. The benefits of delayed clamping are evident in both low- and high-income countries.
Delayed clamping is not associated with adverse effects in a healthy term newborn or the mother. It should become standard practice for term infants from uncomplicated pregnancies.
ATHOL P. KENT, MBChB, MPhil, AND DAVID L. WOODS, MBChB, MD, DCH
We want to hear from you! Tell us what you think.
1. Gupta R, Ramji S. Effect of cord clamping on iron stores in infants born to anemic mothers: a randomized controlled trial. Indian Pediatr. 2002;39(2):130-135.
2. Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Líz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomized controlled trial. Lancet. 2006;367(9527):1997-2004.
3. Grajeda R, Perez-Escamilla R, Dewey KG. Delayed clamping of the umbilical cord improves hematologic status of Guatemalan infants at 2 mo of age. Am J Clin Nutr. 1997;65(2):425-431.
4. McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;2:CD004074.-
5. Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297(11):1241-1252.
Andersson and colleagues begin their published report by explaining the importance of adequate iron status to child development, particularly neurodevelopment. As they note, “Young children are at particular risk of iron deficiency because of high iron requirements during rapid growth in combination with low iron intake.” The investigators also point out that the prevalence of iron-deficiency anemia (3%–7%) and iron deficiency (as high as 26%) among young children in Europe, where this study was conducted, has troubling implications. Iron-deficiency anemia, in particular, may produce enduring cognitive and behavioral deficits.
This is the first randomized, controlled trial to explore the timing of umbilical cord clamping in a high-income country. Earlier studies in low- and middle-income populations have found that delayed cord clamping leads to an increased serum level of ferritin at 3 to 6 months of age.1-5
The rationale for late clamping
Delayed clamping allows for placental transfusion of almost one third of the infant’s blood volume. Infants need the iron in all their red blood cells (RBCs)—circulating systemically and in the placenta—to guard against iron deficiency in the first few months of life. This need is unrelated to the mother’s health, iron status, and socioeconomic station.
Without the full quota of RBCs, an infant’s iron stores—reflected most accurately by ferritin levels at 4 to 6 months of age—will be suboptimal. Early clamping deprives newborns of RBCs remaining in the placenta and the iron they contain, which is essential for the development of the hematopoietic and central nervous systems.
The standard routine of administering oxytocin with early cord clamping, followed by controlled cord traction, was introduced as part of the active management of the third stage of labor. Until recently, there were no randomized, controlled trials demonstrating how early clamping disadvantaged the infant.
Details of the trial
The trial was conducted in a Swedish county hospital in an affluent, high-income population. Four hundred full-term, singleton infants, born after low-risk pregnancy, were randomized to early or late cord clamping.
Intravenous oxytocin (10 IU) was given immediately after the cord was clamped. The time from full delivery of the baby to the start of cord clamping was measured by an assistant to the midwife.
Blood samples were taken from the newborns 48 to 72 hours after delivery and again at 4 months of age.
A clear benefit from delayed clamping
The infants’ hemoglobin concentration and packed RBC volume were higher 2 days after delivery in the delayed-clamping group, compared with early clamping, and this translated into improved iron stores at the 4-month follow-up. Specifically, the rate of anemia at a median age of 2.4 days was 1.2% in the delayed-clamping group versus 6.3% for early clamping (P = .02; relative risk reduction 0.80; 95% confidence interval [CI], 0.22–0.95). And the geometric mean serum ferritin concentration at 4 months of age was 45% higher in the delayed-clamping group (117 μg/L vs 81 μg/L; P < .001; 95% CI, 23%–71%). Iron deficiency was significantly more prevalent in the early-clamping group, but the prevalence of anemia was similar between groups. To prevent one case of iron deficiency—with or without anemia—the number needed to treat was 20 (95% CI, 17–67).
Other growth parameters were similar. Additional long-term tracking is planned.
One of the arguments against delayed clamping is that the practice raises the risks of respiratory symptoms, polycythemia, and hyperbilirubinemia, and increases the need for phototherapy. However, this study demonstrated that late clamping does not increase these risks.
The advantages and risks of delayed cord clamping in infants who are either preterm, growth-restricted, or hypoxic remain to be determined.
Delaying umbilical cord clamping for 3 minutes after delivery allows for substantial transfusion of blood from the placenta to the newborn. This extra blood protects the child from iron deficiency, producing a 45% increase in iron stores that is measurable at 4 months of age. The iron is required for optimal hematopoietic and central nervous system function. The benefits of delayed clamping are evident in both low- and high-income countries.
Delayed clamping is not associated with adverse effects in a healthy term newborn or the mother. It should become standard practice for term infants from uncomplicated pregnancies.
ATHOL P. KENT, MBChB, MPhil, AND DAVID L. WOODS, MBChB, MD, DCH
We want to hear from you! Tell us what you think.
Andersson and colleagues begin their published report by explaining the importance of adequate iron status to child development, particularly neurodevelopment. As they note, “Young children are at particular risk of iron deficiency because of high iron requirements during rapid growth in combination with low iron intake.” The investigators also point out that the prevalence of iron-deficiency anemia (3%–7%) and iron deficiency (as high as 26%) among young children in Europe, where this study was conducted, has troubling implications. Iron-deficiency anemia, in particular, may produce enduring cognitive and behavioral deficits.
This is the first randomized, controlled trial to explore the timing of umbilical cord clamping in a high-income country. Earlier studies in low- and middle-income populations have found that delayed cord clamping leads to an increased serum level of ferritin at 3 to 6 months of age.1-5
The rationale for late clamping
Delayed clamping allows for placental transfusion of almost one third of the infant’s blood volume. Infants need the iron in all their red blood cells (RBCs)—circulating systemically and in the placenta—to guard against iron deficiency in the first few months of life. This need is unrelated to the mother’s health, iron status, and socioeconomic station.
Without the full quota of RBCs, an infant’s iron stores—reflected most accurately by ferritin levels at 4 to 6 months of age—will be suboptimal. Early clamping deprives newborns of RBCs remaining in the placenta and the iron they contain, which is essential for the development of the hematopoietic and central nervous systems.
The standard routine of administering oxytocin with early cord clamping, followed by controlled cord traction, was introduced as part of the active management of the third stage of labor. Until recently, there were no randomized, controlled trials demonstrating how early clamping disadvantaged the infant.
Details of the trial
The trial was conducted in a Swedish county hospital in an affluent, high-income population. Four hundred full-term, singleton infants, born after low-risk pregnancy, were randomized to early or late cord clamping.
Intravenous oxytocin (10 IU) was given immediately after the cord was clamped. The time from full delivery of the baby to the start of cord clamping was measured by an assistant to the midwife.
Blood samples were taken from the newborns 48 to 72 hours after delivery and again at 4 months of age.
A clear benefit from delayed clamping
The infants’ hemoglobin concentration and packed RBC volume were higher 2 days after delivery in the delayed-clamping group, compared with early clamping, and this translated into improved iron stores at the 4-month follow-up. Specifically, the rate of anemia at a median age of 2.4 days was 1.2% in the delayed-clamping group versus 6.3% for early clamping (P = .02; relative risk reduction 0.80; 95% confidence interval [CI], 0.22–0.95). And the geometric mean serum ferritin concentration at 4 months of age was 45% higher in the delayed-clamping group (117 μg/L vs 81 μg/L; P < .001; 95% CI, 23%–71%). Iron deficiency was significantly more prevalent in the early-clamping group, but the prevalence of anemia was similar between groups. To prevent one case of iron deficiency—with or without anemia—the number needed to treat was 20 (95% CI, 17–67).
Other growth parameters were similar. Additional long-term tracking is planned.
One of the arguments against delayed clamping is that the practice raises the risks of respiratory symptoms, polycythemia, and hyperbilirubinemia, and increases the need for phototherapy. However, this study demonstrated that late clamping does not increase these risks.
The advantages and risks of delayed cord clamping in infants who are either preterm, growth-restricted, or hypoxic remain to be determined.
Delaying umbilical cord clamping for 3 minutes after delivery allows for substantial transfusion of blood from the placenta to the newborn. This extra blood protects the child from iron deficiency, producing a 45% increase in iron stores that is measurable at 4 months of age. The iron is required for optimal hematopoietic and central nervous system function. The benefits of delayed clamping are evident in both low- and high-income countries.
Delayed clamping is not associated with adverse effects in a healthy term newborn or the mother. It should become standard practice for term infants from uncomplicated pregnancies.
ATHOL P. KENT, MBChB, MPhil, AND DAVID L. WOODS, MBChB, MD, DCH
We want to hear from you! Tell us what you think.
1. Gupta R, Ramji S. Effect of cord clamping on iron stores in infants born to anemic mothers: a randomized controlled trial. Indian Pediatr. 2002;39(2):130-135.
2. Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Líz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomized controlled trial. Lancet. 2006;367(9527):1997-2004.
3. Grajeda R, Perez-Escamilla R, Dewey KG. Delayed clamping of the umbilical cord improves hematologic status of Guatemalan infants at 2 mo of age. Am J Clin Nutr. 1997;65(2):425-431.
4. McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;2:CD004074.-
5. Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297(11):1241-1252.
1. Gupta R, Ramji S. Effect of cord clamping on iron stores in infants born to anemic mothers: a randomized controlled trial. Indian Pediatr. 2002;39(2):130-135.
2. Chaparro CM, Neufeld LM, Tena Alavez G, Eguia-Líz Cedillo R, Dewey KG. Effect of timing of umbilical cord clamping on iron status in Mexican infants: a randomized controlled trial. Lancet. 2006;367(9527):1997-2004.
3. Grajeda R, Perez-Escamilla R, Dewey KG. Delayed clamping of the umbilical cord improves hematologic status of Guatemalan infants at 2 mo of age. Am J Clin Nutr. 1997;65(2):425-431.
4. McDonald SJ, Middleton P. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev. 2008;2:CD004074.-
5. Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297(11):1241-1252.
Act fast when confronted by a coagulopathy postpartum
“Have you made best use of the Bakri balloon in PPH?”
Robert L. Barbieri, MD (Editorial, July 2011)
A nurse-midwife delivered a macrosomic fetus and identified multiple cervical, vaginal, and perineal tears. After spending approximately 30 minutes suturing a few of the vaginal lacerations, she realized that she needed an experienced obstetrician to complete the complex repair. She has consulted you.
You introduce yourself to the patient, obtain consent, and begin to assess the situation. You note that she has a tear of the anterior cervix at its intersection with the vagina; a few deep vaginal lacerations; and a fourth-degree tear. The uterus is well-contracted and the abdomen is not distended.
As you sit to begin the repair, you notice diffuse oozing of blood from all areas of vaginal and perineal trauma. You suture the cervical laceration and notice that, after tying the stitch, bleeding is continuing from the closed laceration.
Based on what you’re seeing, you become suspicious that the patient has a coagulopathy. What should you do?
Approximately 1 of every 300 deliveries is complicated by development of a clinically significant coagulopathy. Typically, these cases occur in conjunction with postpartum hemorrhage.
Because postpartum coagulopathy does not occur often, it’s difficult for an OB to gain extensive personal experience with this disorder. Yet recognizing postpartum coagulopathy early helps ensure a good outcome.
Diseases that can cause postpartum coagulopathy include:
- placental abruption
- preeclampsia
- amniotic fluid embolism
- acute fatty liver of pregnancy
- prolonged intrauterine retention of a fetal demise
- sepsis
- a previously undiagnosed coagulation disorder.
In addition postpartum hemorrhage of any cause—placenta previa, placenta accreta, postpartum uterine atony—can cause coagulopathy.1
Do you suspect postpartum coagulopathy? If so, you should review the above list of possible conditions and diseases for the likely cause, because treatment of any one of them must be tailored to the individual patient. If placenta accreta is present, for example, hysterectomy may be necessary to save the life of the mother.
In this Editorial, I review three approaches to identifying postpartum coagulopathy—any one or more of which might be necessary for a given patient:
- clinical diagnosis
- the whole blood clotting test
- clinical laboratory measurement of the coagulation profile.
Clinical observation and diagnosis—key to early, rapid recognition
An experienced clinician often has an inkling that a coagulopathy is present when she observes evidence of abnormal clotting:
- blood oozes excessively from many areas of minor trauma
- suturing lacerations fails to stanch bleeding
- blood is more “watery” and less deeply red than ordinarily encountered (the so-called Kool-Aid sign).
Direct observation of any of these findings might prompt an experienced clinician to immediately activate a postpartum coagulopathy protocol, as I describe below, without waiting for additional test results. Delay in treating the coagulopathy could result in an acceleration of a dire cycle of bleeding and a worsening coagulation defect, causing even more bleeding.
On the other hand, some clinicians prefer to wait for a laboratory test to confirm a coagulopathy before they activate a postpartum coagulopathy protocol.
Whole-blood clotting test
This is the so-called red-top–tube test—a simple test that can be performed at the patient’s bedside to identify a coagulopathy; it’s also known as the Lee and White test.2-4 Obtain a sample of venous blood in a red-top tube (a glass tube without additives); at the same time, send a specimen of venous blood to the lab for a stat coagulation profile. In people who have normal hematologic function, the median time for blood to clot in the red-top tube, at room temperature (65ºF to 90ºF), is approximately 6.5 minutes (range, 5 to 8 minutes).
When blood in the red-top tube takes longer than 10 minutes to clot, the patient has a coagulopathy. If the blood clots but the clot then lyses over the following hour, a disorder of fibrinolysis, a type of coagulopathy, is likely.
Coagulation laboratory panel
If you suspect a coagulopathy, have blood drawn for stat measurement of hemoglobin and hematocrit, platelet count, prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen.
Regrettably, it might take as long as 40 minutes from the time blood is drawn to receive the complete panel of results. Such a delay might necessitate your deciding on a course of action based on your clinical observation and diagnosis, rather than waiting for test results. To delay initiating the transfusion of clotting factors creates the risk of having the coagulopathy cause more bleeding, resulting in a worsening coagulopathy—a cycle that can spiral into a clinical disaster.
Activate the postpartum hemorrhage protocol!
Most obstetric services have developed a formal approach to managing postpartum hemorrhage. That protocol can also guide the treatment of a postpartum coagulopathy.
After vaginal delivery, the standard postpartum hemorrhage algorithm includes:
- administration of uterotonics
- fundal massage
- placement of an additional large-bore IV catheter
- volume replacement
- insertion of an arterial line
- moving the patient from a labor room to a fully equipped operating room.
Procedures that might need to be performed include:
- a sonogram to determine whether retained products are in the uterus or if free fluid is in the peritoneal cavity
- a dilatation and curettage to remove retained products of conception
- placement of an intrauterine tamponade balloon
- repair of vaginal and cervical lacerations
- uterine artery embolization.
After vaginal delivery and postpartum hemorrhage, additional surgical procedures that might be necessary include:
- exploratory laparotomy
- uterine compression stitches
- sequential devascularization of the uterus with O’Leary stitches
- hypogastric artery ligation
- hysterectomy.
Additional steps in the postpartum hemorrhage protocol often include recruiting additional staff to the OR, including an advanced general surgeon or gyn surgeon; an additional anesthesiologist; and the director of the blood bank.
In hospitals that provide OB services but have a blood bank with limited transfusion products, stocking RiaSTAP (lyophilized fibrinogen concentrate [human]; CSL Behring) may provide a reliable source of fibrinogen for transfusion.
In many OB cases marked by coagulopathy, a major contributor to the disorder is hypofibrinogenemia. At hospitals with limited blood bank resources, the in-house supply of fresh frozen plasma and cryoprecipitate might be depleted before an obstetrical patient’s coagulopathy is fully corrected. RiaSTAP can provide a stable, readily available alternative source of fibrinogen for transfusion.1
One major disadvantage of RiaSTAP: It is a more expensive source of fibrinogen than FFP and cryoprecipitate.
Reference
Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct
hypofibrinogenaemia rapidly during obstetric hemorrhage. Int J Obstet Anesth. 2010;19(2):218–223.
Blood product transfusion protocol. An additional key feature of the emergency hemorrhage protocol is activation of a standardized blood product transfusion protocol. Rapid replacement of blood products is essential to support effective surgical management (see “Lyophilized fibrinogen concentrate: Another source of fibrinogen,” above, and the TABLE).
Modern transfusion guidelines in a case of massive hemorrhage after trauma call for transfusing fresh frozen plasma (FFP) at a ratio of FFP to red blood cells (RBCs) of >1:1.5.5-7 At my hospital (Brigham and Women’s Hospital), the blood bank is alerted to activate the protocol when a clinician announces that an “emergency obstetric hemorrhage” is in progress. The blood bank emergently transports 2 units of FFP and 2 units of RBCs to the delivery suite and begins to prepare a cooler with 6 units of RBCs, 2 units of FFP, and 1 dose of cryoprecipitate.
Because a postpartum coagulopathy is not a common occurrence, it is of great value to practice the OB hemorrhage protocol using simulation exercises.
The clinical impact of various blood replacement products1-3
| Product | How provided | Clinical effect |
|---|---|---|
| Red blood cells | 1 unit (bag) contains 300–350 mL | 1 unit raises the hemoglobin concentration by 1 g/dL and the hematocrit by 3% |
| Fresh frozen plasma (all clotting factors) | 1 unit (bag) contains 200–300 mL | 1 unit raises the fibrinogen level by 7 to 10 mg/dL |
| Cryoprecipitate (fibrinogen, factor VIII, factor XIII, and von Willebrand factor) | 1 dose (as provided by the Red Cross) comprises 2 120–158 mL bags of 5 units each; 1 dose contains protein precipitate from 10 units of fresh plasma | 1 dose raises the fibrinogen level by 70 mg/dL in a 70-kg person |
| Platelets | 1 unit (bag) contains 300 mL, from 6 units of whole blood or one apheresis donor | 1 unit raises the platelet count by 30 × 103/μL in an adult whose surface area is 2 m2 |
| References | ||
CASE Resolved
You recognize the diffuse oozing and failure of the oozing to stop after a laceration is properly sutured as a sign of a developing coagulopathy.
You decide to send a blood specimen for a stat set of coagulation studies. You discontinue repair of the lacerations and pack the vagina with three laparotomy sponges tied together.
The patient is moved to the OR, and you activate the emergency transfusion protocol. You immediately receive 2 units of FFP, which you transfuse.
The blood bank sends a cooler with 4 units of FFP; 8 units of RBCs; and 2 bags of cryoprecipitate to the OR. The anesthesiologists begin the transfusion of these products.
The pretransfusion coagulation profile eventually returns: hemoglobin, 9.8 g/dL; platelet count, 79 X 103 μL; PT, 23.1 sec; International Normalized Ratio (INR), 2.0; PTT, 49 sec; and fibrinogen, 60 mg/dL.
After transfusing 4 units of FFP and the bag of cryoprecipitate, you remove the vaginal packs and note that diffuse bleeding has stopped. You resume repair of cervical and vaginal lacerations. During the course of the repair, all products in the cooler and 2 bags of platelets are transfused.
At the conclusion of your repair, the patient is no longer bleeding.
A final coagulation profile shows: hemoglobin, 9.1 g/dL; platelet count, 100 X 103 μL; PT, 16.6 sec; INR, 1.3; PTT, 36 sec; and fibrinogen, 210 mg/dL. The patient is discharged on the third postpartum day.
What were the key clinical interventions that helped you save the life of a woman who had a severe coagulopathy postpartum?
1. Thachil J, Toh CH. Disseminated intravascular coagulation in obstetric disorders and its acute haematological management. Blood Rev. 2009;23(4):167-176.
2. Lee RI, White PD. A clinical study of the coagulation time of blood. Am J Med Sci. 1913;145(4):494-503.
3. Weiner AE, Reid DE, Roby CC. Incoagulable blood in severe premature separation of the placenta: a method of management. Am J Obstet Gynecol. 1953;66(3):475-499.
4. Poe MF. Clot observation test for clinical diagnosis of clotting defects. Anesthesiology. 1959;20:825-829.
5. Sperry JL, Ochoa JB, Gunn SR, et al. An FFP: PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986-993.
6. Gonzalez EA, Moore FA, Holbomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112-119.
7. Moore FA, Nelson T, McKinley BA, et al. Is there a role for aggressive use of fresh frozen plasma in massive transfusion of civilian trauma patients. Am J Surg. 2008;196(6):948-960.
“Have you made best use of the Bakri balloon in PPH?”
Robert L. Barbieri, MD (Editorial, July 2011)
A nurse-midwife delivered a macrosomic fetus and identified multiple cervical, vaginal, and perineal tears. After spending approximately 30 minutes suturing a few of the vaginal lacerations, she realized that she needed an experienced obstetrician to complete the complex repair. She has consulted you.
You introduce yourself to the patient, obtain consent, and begin to assess the situation. You note that she has a tear of the anterior cervix at its intersection with the vagina; a few deep vaginal lacerations; and a fourth-degree tear. The uterus is well-contracted and the abdomen is not distended.
As you sit to begin the repair, you notice diffuse oozing of blood from all areas of vaginal and perineal trauma. You suture the cervical laceration and notice that, after tying the stitch, bleeding is continuing from the closed laceration.
Based on what you’re seeing, you become suspicious that the patient has a coagulopathy. What should you do?
Approximately 1 of every 300 deliveries is complicated by development of a clinically significant coagulopathy. Typically, these cases occur in conjunction with postpartum hemorrhage.
Because postpartum coagulopathy does not occur often, it’s difficult for an OB to gain extensive personal experience with this disorder. Yet recognizing postpartum coagulopathy early helps ensure a good outcome.
Diseases that can cause postpartum coagulopathy include:
- placental abruption
- preeclampsia
- amniotic fluid embolism
- acute fatty liver of pregnancy
- prolonged intrauterine retention of a fetal demise
- sepsis
- a previously undiagnosed coagulation disorder.
In addition postpartum hemorrhage of any cause—placenta previa, placenta accreta, postpartum uterine atony—can cause coagulopathy.1
Do you suspect postpartum coagulopathy? If so, you should review the above list of possible conditions and diseases for the likely cause, because treatment of any one of them must be tailored to the individual patient. If placenta accreta is present, for example, hysterectomy may be necessary to save the life of the mother.
In this Editorial, I review three approaches to identifying postpartum coagulopathy—any one or more of which might be necessary for a given patient:
- clinical diagnosis
- the whole blood clotting test
- clinical laboratory measurement of the coagulation profile.
Clinical observation and diagnosis—key to early, rapid recognition
An experienced clinician often has an inkling that a coagulopathy is present when she observes evidence of abnormal clotting:
- blood oozes excessively from many areas of minor trauma
- suturing lacerations fails to stanch bleeding
- blood is more “watery” and less deeply red than ordinarily encountered (the so-called Kool-Aid sign).
Direct observation of any of these findings might prompt an experienced clinician to immediately activate a postpartum coagulopathy protocol, as I describe below, without waiting for additional test results. Delay in treating the coagulopathy could result in an acceleration of a dire cycle of bleeding and a worsening coagulation defect, causing even more bleeding.
On the other hand, some clinicians prefer to wait for a laboratory test to confirm a coagulopathy before they activate a postpartum coagulopathy protocol.
Whole-blood clotting test
This is the so-called red-top–tube test—a simple test that can be performed at the patient’s bedside to identify a coagulopathy; it’s also known as the Lee and White test.2-4 Obtain a sample of venous blood in a red-top tube (a glass tube without additives); at the same time, send a specimen of venous blood to the lab for a stat coagulation profile. In people who have normal hematologic function, the median time for blood to clot in the red-top tube, at room temperature (65ºF to 90ºF), is approximately 6.5 minutes (range, 5 to 8 minutes).
When blood in the red-top tube takes longer than 10 minutes to clot, the patient has a coagulopathy. If the blood clots but the clot then lyses over the following hour, a disorder of fibrinolysis, a type of coagulopathy, is likely.
Coagulation laboratory panel
If you suspect a coagulopathy, have blood drawn for stat measurement of hemoglobin and hematocrit, platelet count, prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen.
Regrettably, it might take as long as 40 minutes from the time blood is drawn to receive the complete panel of results. Such a delay might necessitate your deciding on a course of action based on your clinical observation and diagnosis, rather than waiting for test results. To delay initiating the transfusion of clotting factors creates the risk of having the coagulopathy cause more bleeding, resulting in a worsening coagulopathy—a cycle that can spiral into a clinical disaster.
Activate the postpartum hemorrhage protocol!
Most obstetric services have developed a formal approach to managing postpartum hemorrhage. That protocol can also guide the treatment of a postpartum coagulopathy.
After vaginal delivery, the standard postpartum hemorrhage algorithm includes:
- administration of uterotonics
- fundal massage
- placement of an additional large-bore IV catheter
- volume replacement
- insertion of an arterial line
- moving the patient from a labor room to a fully equipped operating room.
Procedures that might need to be performed include:
- a sonogram to determine whether retained products are in the uterus or if free fluid is in the peritoneal cavity
- a dilatation and curettage to remove retained products of conception
- placement of an intrauterine tamponade balloon
- repair of vaginal and cervical lacerations
- uterine artery embolization.
After vaginal delivery and postpartum hemorrhage, additional surgical procedures that might be necessary include:
- exploratory laparotomy
- uterine compression stitches
- sequential devascularization of the uterus with O’Leary stitches
- hypogastric artery ligation
- hysterectomy.
Additional steps in the postpartum hemorrhage protocol often include recruiting additional staff to the OR, including an advanced general surgeon or gyn surgeon; an additional anesthesiologist; and the director of the blood bank.
In hospitals that provide OB services but have a blood bank with limited transfusion products, stocking RiaSTAP (lyophilized fibrinogen concentrate [human]; CSL Behring) may provide a reliable source of fibrinogen for transfusion.
In many OB cases marked by coagulopathy, a major contributor to the disorder is hypofibrinogenemia. At hospitals with limited blood bank resources, the in-house supply of fresh frozen plasma and cryoprecipitate might be depleted before an obstetrical patient’s coagulopathy is fully corrected. RiaSTAP can provide a stable, readily available alternative source of fibrinogen for transfusion.1
One major disadvantage of RiaSTAP: It is a more expensive source of fibrinogen than FFP and cryoprecipitate.
Reference
Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct
hypofibrinogenaemia rapidly during obstetric hemorrhage. Int J Obstet Anesth. 2010;19(2):218–223.
Blood product transfusion protocol. An additional key feature of the emergency hemorrhage protocol is activation of a standardized blood product transfusion protocol. Rapid replacement of blood products is essential to support effective surgical management (see “Lyophilized fibrinogen concentrate: Another source of fibrinogen,” above, and the TABLE).
Modern transfusion guidelines in a case of massive hemorrhage after trauma call for transfusing fresh frozen plasma (FFP) at a ratio of FFP to red blood cells (RBCs) of >1:1.5.5-7 At my hospital (Brigham and Women’s Hospital), the blood bank is alerted to activate the protocol when a clinician announces that an “emergency obstetric hemorrhage” is in progress. The blood bank emergently transports 2 units of FFP and 2 units of RBCs to the delivery suite and begins to prepare a cooler with 6 units of RBCs, 2 units of FFP, and 1 dose of cryoprecipitate.
Because a postpartum coagulopathy is not a common occurrence, it is of great value to practice the OB hemorrhage protocol using simulation exercises.
The clinical impact of various blood replacement products1-3
| Product | How provided | Clinical effect |
|---|---|---|
| Red blood cells | 1 unit (bag) contains 300–350 mL | 1 unit raises the hemoglobin concentration by 1 g/dL and the hematocrit by 3% |
| Fresh frozen plasma (all clotting factors) | 1 unit (bag) contains 200–300 mL | 1 unit raises the fibrinogen level by 7 to 10 mg/dL |
| Cryoprecipitate (fibrinogen, factor VIII, factor XIII, and von Willebrand factor) | 1 dose (as provided by the Red Cross) comprises 2 120–158 mL bags of 5 units each; 1 dose contains protein precipitate from 10 units of fresh plasma | 1 dose raises the fibrinogen level by 70 mg/dL in a 70-kg person |
| Platelets | 1 unit (bag) contains 300 mL, from 6 units of whole blood or one apheresis donor | 1 unit raises the platelet count by 30 × 103/μL in an adult whose surface area is 2 m2 |
| References | ||
CASE Resolved
You recognize the diffuse oozing and failure of the oozing to stop after a laceration is properly sutured as a sign of a developing coagulopathy.
You decide to send a blood specimen for a stat set of coagulation studies. You discontinue repair of the lacerations and pack the vagina with three laparotomy sponges tied together.
The patient is moved to the OR, and you activate the emergency transfusion protocol. You immediately receive 2 units of FFP, which you transfuse.
The blood bank sends a cooler with 4 units of FFP; 8 units of RBCs; and 2 bags of cryoprecipitate to the OR. The anesthesiologists begin the transfusion of these products.
The pretransfusion coagulation profile eventually returns: hemoglobin, 9.8 g/dL; platelet count, 79 X 103 μL; PT, 23.1 sec; International Normalized Ratio (INR), 2.0; PTT, 49 sec; and fibrinogen, 60 mg/dL.
After transfusing 4 units of FFP and the bag of cryoprecipitate, you remove the vaginal packs and note that diffuse bleeding has stopped. You resume repair of cervical and vaginal lacerations. During the course of the repair, all products in the cooler and 2 bags of platelets are transfused.
At the conclusion of your repair, the patient is no longer bleeding.
A final coagulation profile shows: hemoglobin, 9.1 g/dL; platelet count, 100 X 103 μL; PT, 16.6 sec; INR, 1.3; PTT, 36 sec; and fibrinogen, 210 mg/dL. The patient is discharged on the third postpartum day.
What were the key clinical interventions that helped you save the life of a woman who had a severe coagulopathy postpartum?
“Have you made best use of the Bakri balloon in PPH?”
Robert L. Barbieri, MD (Editorial, July 2011)
A nurse-midwife delivered a macrosomic fetus and identified multiple cervical, vaginal, and perineal tears. After spending approximately 30 minutes suturing a few of the vaginal lacerations, she realized that she needed an experienced obstetrician to complete the complex repair. She has consulted you.
You introduce yourself to the patient, obtain consent, and begin to assess the situation. You note that she has a tear of the anterior cervix at its intersection with the vagina; a few deep vaginal lacerations; and a fourth-degree tear. The uterus is well-contracted and the abdomen is not distended.
As you sit to begin the repair, you notice diffuse oozing of blood from all areas of vaginal and perineal trauma. You suture the cervical laceration and notice that, after tying the stitch, bleeding is continuing from the closed laceration.
Based on what you’re seeing, you become suspicious that the patient has a coagulopathy. What should you do?
Approximately 1 of every 300 deliveries is complicated by development of a clinically significant coagulopathy. Typically, these cases occur in conjunction with postpartum hemorrhage.
Because postpartum coagulopathy does not occur often, it’s difficult for an OB to gain extensive personal experience with this disorder. Yet recognizing postpartum coagulopathy early helps ensure a good outcome.
Diseases that can cause postpartum coagulopathy include:
- placental abruption
- preeclampsia
- amniotic fluid embolism
- acute fatty liver of pregnancy
- prolonged intrauterine retention of a fetal demise
- sepsis
- a previously undiagnosed coagulation disorder.
In addition postpartum hemorrhage of any cause—placenta previa, placenta accreta, postpartum uterine atony—can cause coagulopathy.1
Do you suspect postpartum coagulopathy? If so, you should review the above list of possible conditions and diseases for the likely cause, because treatment of any one of them must be tailored to the individual patient. If placenta accreta is present, for example, hysterectomy may be necessary to save the life of the mother.
In this Editorial, I review three approaches to identifying postpartum coagulopathy—any one or more of which might be necessary for a given patient:
- clinical diagnosis
- the whole blood clotting test
- clinical laboratory measurement of the coagulation profile.
Clinical observation and diagnosis—key to early, rapid recognition
An experienced clinician often has an inkling that a coagulopathy is present when she observes evidence of abnormal clotting:
- blood oozes excessively from many areas of minor trauma
- suturing lacerations fails to stanch bleeding
- blood is more “watery” and less deeply red than ordinarily encountered (the so-called Kool-Aid sign).
Direct observation of any of these findings might prompt an experienced clinician to immediately activate a postpartum coagulopathy protocol, as I describe below, without waiting for additional test results. Delay in treating the coagulopathy could result in an acceleration of a dire cycle of bleeding and a worsening coagulation defect, causing even more bleeding.
On the other hand, some clinicians prefer to wait for a laboratory test to confirm a coagulopathy before they activate a postpartum coagulopathy protocol.
Whole-blood clotting test
This is the so-called red-top–tube test—a simple test that can be performed at the patient’s bedside to identify a coagulopathy; it’s also known as the Lee and White test.2-4 Obtain a sample of venous blood in a red-top tube (a glass tube without additives); at the same time, send a specimen of venous blood to the lab for a stat coagulation profile. In people who have normal hematologic function, the median time for blood to clot in the red-top tube, at room temperature (65ºF to 90ºF), is approximately 6.5 minutes (range, 5 to 8 minutes).
When blood in the red-top tube takes longer than 10 minutes to clot, the patient has a coagulopathy. If the blood clots but the clot then lyses over the following hour, a disorder of fibrinolysis, a type of coagulopathy, is likely.
Coagulation laboratory panel
If you suspect a coagulopathy, have blood drawn for stat measurement of hemoglobin and hematocrit, platelet count, prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen.
Regrettably, it might take as long as 40 minutes from the time blood is drawn to receive the complete panel of results. Such a delay might necessitate your deciding on a course of action based on your clinical observation and diagnosis, rather than waiting for test results. To delay initiating the transfusion of clotting factors creates the risk of having the coagulopathy cause more bleeding, resulting in a worsening coagulopathy—a cycle that can spiral into a clinical disaster.
Activate the postpartum hemorrhage protocol!
Most obstetric services have developed a formal approach to managing postpartum hemorrhage. That protocol can also guide the treatment of a postpartum coagulopathy.
After vaginal delivery, the standard postpartum hemorrhage algorithm includes:
- administration of uterotonics
- fundal massage
- placement of an additional large-bore IV catheter
- volume replacement
- insertion of an arterial line
- moving the patient from a labor room to a fully equipped operating room.
Procedures that might need to be performed include:
- a sonogram to determine whether retained products are in the uterus or if free fluid is in the peritoneal cavity
- a dilatation and curettage to remove retained products of conception
- placement of an intrauterine tamponade balloon
- repair of vaginal and cervical lacerations
- uterine artery embolization.
After vaginal delivery and postpartum hemorrhage, additional surgical procedures that might be necessary include:
- exploratory laparotomy
- uterine compression stitches
- sequential devascularization of the uterus with O’Leary stitches
- hypogastric artery ligation
- hysterectomy.
Additional steps in the postpartum hemorrhage protocol often include recruiting additional staff to the OR, including an advanced general surgeon or gyn surgeon; an additional anesthesiologist; and the director of the blood bank.
In hospitals that provide OB services but have a blood bank with limited transfusion products, stocking RiaSTAP (lyophilized fibrinogen concentrate [human]; CSL Behring) may provide a reliable source of fibrinogen for transfusion.
In many OB cases marked by coagulopathy, a major contributor to the disorder is hypofibrinogenemia. At hospitals with limited blood bank resources, the in-house supply of fresh frozen plasma and cryoprecipitate might be depleted before an obstetrical patient’s coagulopathy is fully corrected. RiaSTAP can provide a stable, readily available alternative source of fibrinogen for transfusion.1
One major disadvantage of RiaSTAP: It is a more expensive source of fibrinogen than FFP and cryoprecipitate.
Reference
Bell SF, Rayment R, Collins PW, Collis RE. The use of fibrinogen concentrate to correct
hypofibrinogenaemia rapidly during obstetric hemorrhage. Int J Obstet Anesth. 2010;19(2):218–223.
Blood product transfusion protocol. An additional key feature of the emergency hemorrhage protocol is activation of a standardized blood product transfusion protocol. Rapid replacement of blood products is essential to support effective surgical management (see “Lyophilized fibrinogen concentrate: Another source of fibrinogen,” above, and the TABLE).
Modern transfusion guidelines in a case of massive hemorrhage after trauma call for transfusing fresh frozen plasma (FFP) at a ratio of FFP to red blood cells (RBCs) of >1:1.5.5-7 At my hospital (Brigham and Women’s Hospital), the blood bank is alerted to activate the protocol when a clinician announces that an “emergency obstetric hemorrhage” is in progress. The blood bank emergently transports 2 units of FFP and 2 units of RBCs to the delivery suite and begins to prepare a cooler with 6 units of RBCs, 2 units of FFP, and 1 dose of cryoprecipitate.
Because a postpartum coagulopathy is not a common occurrence, it is of great value to practice the OB hemorrhage protocol using simulation exercises.
The clinical impact of various blood replacement products1-3
| Product | How provided | Clinical effect |
|---|---|---|
| Red blood cells | 1 unit (bag) contains 300–350 mL | 1 unit raises the hemoglobin concentration by 1 g/dL and the hematocrit by 3% |
| Fresh frozen plasma (all clotting factors) | 1 unit (bag) contains 200–300 mL | 1 unit raises the fibrinogen level by 7 to 10 mg/dL |
| Cryoprecipitate (fibrinogen, factor VIII, factor XIII, and von Willebrand factor) | 1 dose (as provided by the Red Cross) comprises 2 120–158 mL bags of 5 units each; 1 dose contains protein precipitate from 10 units of fresh plasma | 1 dose raises the fibrinogen level by 70 mg/dL in a 70-kg person |
| Platelets | 1 unit (bag) contains 300 mL, from 6 units of whole blood or one apheresis donor | 1 unit raises the platelet count by 30 × 103/μL in an adult whose surface area is 2 m2 |
| References | ||
CASE Resolved
You recognize the diffuse oozing and failure of the oozing to stop after a laceration is properly sutured as a sign of a developing coagulopathy.
You decide to send a blood specimen for a stat set of coagulation studies. You discontinue repair of the lacerations and pack the vagina with three laparotomy sponges tied together.
The patient is moved to the OR, and you activate the emergency transfusion protocol. You immediately receive 2 units of FFP, which you transfuse.
The blood bank sends a cooler with 4 units of FFP; 8 units of RBCs; and 2 bags of cryoprecipitate to the OR. The anesthesiologists begin the transfusion of these products.
The pretransfusion coagulation profile eventually returns: hemoglobin, 9.8 g/dL; platelet count, 79 X 103 μL; PT, 23.1 sec; International Normalized Ratio (INR), 2.0; PTT, 49 sec; and fibrinogen, 60 mg/dL.
After transfusing 4 units of FFP and the bag of cryoprecipitate, you remove the vaginal packs and note that diffuse bleeding has stopped. You resume repair of cervical and vaginal lacerations. During the course of the repair, all products in the cooler and 2 bags of platelets are transfused.
At the conclusion of your repair, the patient is no longer bleeding.
A final coagulation profile shows: hemoglobin, 9.1 g/dL; platelet count, 100 X 103 μL; PT, 16.6 sec; INR, 1.3; PTT, 36 sec; and fibrinogen, 210 mg/dL. The patient is discharged on the third postpartum day.
What were the key clinical interventions that helped you save the life of a woman who had a severe coagulopathy postpartum?
1. Thachil J, Toh CH. Disseminated intravascular coagulation in obstetric disorders and its acute haematological management. Blood Rev. 2009;23(4):167-176.
2. Lee RI, White PD. A clinical study of the coagulation time of blood. Am J Med Sci. 1913;145(4):494-503.
3. Weiner AE, Reid DE, Roby CC. Incoagulable blood in severe premature separation of the placenta: a method of management. Am J Obstet Gynecol. 1953;66(3):475-499.
4. Poe MF. Clot observation test for clinical diagnosis of clotting defects. Anesthesiology. 1959;20:825-829.
5. Sperry JL, Ochoa JB, Gunn SR, et al. An FFP: PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986-993.
6. Gonzalez EA, Moore FA, Holbomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112-119.
7. Moore FA, Nelson T, McKinley BA, et al. Is there a role for aggressive use of fresh frozen plasma in massive transfusion of civilian trauma patients. Am J Surg. 2008;196(6):948-960.
1. Thachil J, Toh CH. Disseminated intravascular coagulation in obstetric disorders and its acute haematological management. Blood Rev. 2009;23(4):167-176.
2. Lee RI, White PD. A clinical study of the coagulation time of blood. Am J Med Sci. 1913;145(4):494-503.
3. Weiner AE, Reid DE, Roby CC. Incoagulable blood in severe premature separation of the placenta: a method of management. Am J Obstet Gynecol. 1953;66(3):475-499.
4. Poe MF. Clot observation test for clinical diagnosis of clotting defects. Anesthesiology. 1959;20:825-829.
5. Sperry JL, Ochoa JB, Gunn SR, et al. An FFP: PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65(5):986-993.
6. Gonzalez EA, Moore FA, Holbomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62(1):112-119.
7. Moore FA, Nelson T, McKinley BA, et al. Is there a role for aggressive use of fresh frozen plasma in massive transfusion of civilian trauma patients. Am J Surg. 2008;196(6):948-960.
17P Not Effective Once Preterm Labor Begins
DALLAS – Biweekly injections of 17-alpha-hydroxyprogesterone caproate, or 17P, did not significantly prolong pregnancy in a randomized open-label French study involving 188 women with an episode of successfully arrested preterm labor and a short cervix.
The median time from randomization to delivery was 64 days in 94 women who received 17P, and 67 days in 94 control subjects, Dr. Patrick Rozenberg reported at the annual meeting of the Society for Maternal-Fetal Medicine.
The rates of delivery prior to 37, 34, and 32 weeks’ gestation also did not differ significantly between the groups, with 39% and 38%, 16% and 20%, and 9% and 14% in the treatment and control groups, respectively, delivering at those time points, said Dr. Rozenberg of Poissy (France) Saint-Germain Hospital.
The findings contrast with those from several smaller trials that suggested 17P might reduce the risk of preterm delivery in women with preterm labor arrested by tocolysis.
Women in the current study had singleton pregnancies at 24-32 weeks’ gestation and a cervical length less than 25 mm, and were admitted at one of 13 French university hospitals with preterm labor. All were successfully treated with 12 mg of intramuscular betamethasone, which was repeated after 24 hours. The women were then randomized to receive or not receive 17P.
The 17P treatment group received 500 mg of intramuscular 17P beginning after tocolysis ended, and then twice weekly until 36 weeks’ gestation or until delivery. The control group received no progesterone, but all other management in both groups was left to the discretion of the attending physician, Dr. Rozenberg said, noting that clinical characteristics, including gestational age and cervical length, were similar in both the treatment and control groups, as were additional management, need for readmission for preterm labor, and tocolysis.
Neonatal outcomes, including birth weight and complications, also were similar between the groups, and there were no adverse effects associated with treatment.
Although several studies suggest that progesterone can reduce the rate of prematurity, many questions remain about optimal timing, mode of administration, dosing, and indications, Dr. Rozenberg noted. The findings of this study, however, do not support its use in women with a short cervix and an episode of preterm labor successfully treated with tocolysis.
"We could say that treatment with progesterone in patients with preterm labor and a short cervix below 25 mm did not prolong pregnancy, did not reduce the rate of prematurity and related complications, and was not associated with adverse effects. In conclusion, once the pathologic process of preterm labor begins, progesterone is no longer effective," he said.
Dr. Rozenberg said he had no relevant financial disclosures.
DALLAS – Biweekly injections of 17-alpha-hydroxyprogesterone caproate, or 17P, did not significantly prolong pregnancy in a randomized open-label French study involving 188 women with an episode of successfully arrested preterm labor and a short cervix.
The median time from randomization to delivery was 64 days in 94 women who received 17P, and 67 days in 94 control subjects, Dr. Patrick Rozenberg reported at the annual meeting of the Society for Maternal-Fetal Medicine.
The rates of delivery prior to 37, 34, and 32 weeks’ gestation also did not differ significantly between the groups, with 39% and 38%, 16% and 20%, and 9% and 14% in the treatment and control groups, respectively, delivering at those time points, said Dr. Rozenberg of Poissy (France) Saint-Germain Hospital.
The findings contrast with those from several smaller trials that suggested 17P might reduce the risk of preterm delivery in women with preterm labor arrested by tocolysis.
Women in the current study had singleton pregnancies at 24-32 weeks’ gestation and a cervical length less than 25 mm, and were admitted at one of 13 French university hospitals with preterm labor. All were successfully treated with 12 mg of intramuscular betamethasone, which was repeated after 24 hours. The women were then randomized to receive or not receive 17P.
The 17P treatment group received 500 mg of intramuscular 17P beginning after tocolysis ended, and then twice weekly until 36 weeks’ gestation or until delivery. The control group received no progesterone, but all other management in both groups was left to the discretion of the attending physician, Dr. Rozenberg said, noting that clinical characteristics, including gestational age and cervical length, were similar in both the treatment and control groups, as were additional management, need for readmission for preterm labor, and tocolysis.
Neonatal outcomes, including birth weight and complications, also were similar between the groups, and there were no adverse effects associated with treatment.
Although several studies suggest that progesterone can reduce the rate of prematurity, many questions remain about optimal timing, mode of administration, dosing, and indications, Dr. Rozenberg noted. The findings of this study, however, do not support its use in women with a short cervix and an episode of preterm labor successfully treated with tocolysis.
"We could say that treatment with progesterone in patients with preterm labor and a short cervix below 25 mm did not prolong pregnancy, did not reduce the rate of prematurity and related complications, and was not associated with adverse effects. In conclusion, once the pathologic process of preterm labor begins, progesterone is no longer effective," he said.
Dr. Rozenberg said he had no relevant financial disclosures.
DALLAS – Biweekly injections of 17-alpha-hydroxyprogesterone caproate, or 17P, did not significantly prolong pregnancy in a randomized open-label French study involving 188 women with an episode of successfully arrested preterm labor and a short cervix.
The median time from randomization to delivery was 64 days in 94 women who received 17P, and 67 days in 94 control subjects, Dr. Patrick Rozenberg reported at the annual meeting of the Society for Maternal-Fetal Medicine.
The rates of delivery prior to 37, 34, and 32 weeks’ gestation also did not differ significantly between the groups, with 39% and 38%, 16% and 20%, and 9% and 14% in the treatment and control groups, respectively, delivering at those time points, said Dr. Rozenberg of Poissy (France) Saint-Germain Hospital.
The findings contrast with those from several smaller trials that suggested 17P might reduce the risk of preterm delivery in women with preterm labor arrested by tocolysis.
Women in the current study had singleton pregnancies at 24-32 weeks’ gestation and a cervical length less than 25 mm, and were admitted at one of 13 French university hospitals with preterm labor. All were successfully treated with 12 mg of intramuscular betamethasone, which was repeated after 24 hours. The women were then randomized to receive or not receive 17P.
The 17P treatment group received 500 mg of intramuscular 17P beginning after tocolysis ended, and then twice weekly until 36 weeks’ gestation or until delivery. The control group received no progesterone, but all other management in both groups was left to the discretion of the attending physician, Dr. Rozenberg said, noting that clinical characteristics, including gestational age and cervical length, were similar in both the treatment and control groups, as were additional management, need for readmission for preterm labor, and tocolysis.
Neonatal outcomes, including birth weight and complications, also were similar between the groups, and there were no adverse effects associated with treatment.
Although several studies suggest that progesterone can reduce the rate of prematurity, many questions remain about optimal timing, mode of administration, dosing, and indications, Dr. Rozenberg noted. The findings of this study, however, do not support its use in women with a short cervix and an episode of preterm labor successfully treated with tocolysis.
"We could say that treatment with progesterone in patients with preterm labor and a short cervix below 25 mm did not prolong pregnancy, did not reduce the rate of prematurity and related complications, and was not associated with adverse effects. In conclusion, once the pathologic process of preterm labor begins, progesterone is no longer effective," he said.
Dr. Rozenberg said he had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR MATERNAL-FETAL MEDICINE
Major Finding: The median time from randomization to delivery was 64 days in 94 women who received 17P, and 67 days in 94 control subjects.
Data Source: Findings were from an open-label randomized study.
Disclosures: Dr. Rozenberg said he had no relevant financial disclosures.
Labor Pattern During TOLAC May Signal Uterine Rupture Risk
DALLAS – Slow progression of labor beyond 7 cm of dilation in women attempting a trial of labor after cesarean section may signal risk for uterine rupture, findings from a case-control study suggest.
In 99 women who experienced uterine rupture while attempting a trial of labor after cesarean section (TOLAC) and 309 controls who did not experience uterine rupture during TOLAC, the time to progress 1 cm of dilation – after adjustment for prior vaginal delivery – was similar until 7 cm of dilation. Once the women reached 7 cm of dilation, however, a 1 cm progression took significantly longer in those who experienced rupture.
For example, progression from 7 cm to 8 cm took a median of 0.33 vs. 0.15 hours in the uterine rupture patients and the controls, respectively, and the progression from 8 cm to 9 cm took a median of 0.24 vs. 0.1 hours in the groups, respectively, Dr. Lorie Harper of Washington University in St. Louis reported at the annual meeting of the Society for Maternal-Fetal Medicine.
"This works out to approx 10-13 minutes longer for the median time to progress 1 cm in the uterine rupture group," she said, noting that the differences were particularly striking at the 95th percentile of time per 1 cm of dilation; for women with a successful trial of labor, each 1 cm of dilation after 7 cm took less than 1 hour, while at the 95th percentile, progression of each 1 cm of dilation after 7 cm took at least 1 hour.
The findings are from a secondary analysis of data from a nested case-control study of women attempting TOLAC within a 17-center retrospective cohort study of women with a prior low transverse cesarean section. Cases included women who experienced uterine rupture, and controls included women attempting TOLAC who reached 10 cm dilation. An additional reference group included 110 women with a failed TOLAC who ultimately underwent repeat cesarean section, and no significant difference was seen between this group and the cases in terms of time to progress after 7 cm. All subjects had only one prior cesarean section.
Uterine rupture for this study was explicitly defined, a priori, as a full-thickness disruption of the uterine wall accompanied by clinical signs, Dr. Harper said.
The cases and controls from both reference groups were similar with respect to age, gravidity, diabetes, hypertension, and type of hospital, although the rate of prior vaginal delivery and black race both were more common in the reference groups. The three groups also were comparable with respect to intrapartum characteristics such as epidural use and gestational age at delivery, although those who experienced uterine rupture were more likely to have been induced and to have received Pitocin, she noted.
Although the case-control design of this study has inherent limitations such as possible selection bias, the study also has strengths, including the large study population of nearly 14,000 women in the original retrospective cohort, she said.
The findings suggest that a protracted labor prior to 7 cm of dilation does not necessarily signify uterine rupture in women attempting a TOLAC, as it was not uncommon during this period for the women to require 2 hours to progress 1 cm, and some still reached 10 cm, Dr. Harper said.
After 7 cm, however, labor dystocia should raise suspicion for uterine rupture in this population, she said.
Although the findings don’t necessarily suggest that intervention such as cesarean delivery is indicated in those with slow progression after 7 cm, they do underscore the need for more careful and frequent monitoring for signs of uterine rupture during active labor in women attempting a TOLAC.
"Instead of waiting 2 hours to check them, maybe they should be checked in an hour to make sure they’re progressing," she said.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Harper said she had no relevant financial disclosures.
DALLAS – Slow progression of labor beyond 7 cm of dilation in women attempting a trial of labor after cesarean section may signal risk for uterine rupture, findings from a case-control study suggest.
In 99 women who experienced uterine rupture while attempting a trial of labor after cesarean section (TOLAC) and 309 controls who did not experience uterine rupture during TOLAC, the time to progress 1 cm of dilation – after adjustment for prior vaginal delivery – was similar until 7 cm of dilation. Once the women reached 7 cm of dilation, however, a 1 cm progression took significantly longer in those who experienced rupture.
For example, progression from 7 cm to 8 cm took a median of 0.33 vs. 0.15 hours in the uterine rupture patients and the controls, respectively, and the progression from 8 cm to 9 cm took a median of 0.24 vs. 0.1 hours in the groups, respectively, Dr. Lorie Harper of Washington University in St. Louis reported at the annual meeting of the Society for Maternal-Fetal Medicine.
"This works out to approx 10-13 minutes longer for the median time to progress 1 cm in the uterine rupture group," she said, noting that the differences were particularly striking at the 95th percentile of time per 1 cm of dilation; for women with a successful trial of labor, each 1 cm of dilation after 7 cm took less than 1 hour, while at the 95th percentile, progression of each 1 cm of dilation after 7 cm took at least 1 hour.
The findings are from a secondary analysis of data from a nested case-control study of women attempting TOLAC within a 17-center retrospective cohort study of women with a prior low transverse cesarean section. Cases included women who experienced uterine rupture, and controls included women attempting TOLAC who reached 10 cm dilation. An additional reference group included 110 women with a failed TOLAC who ultimately underwent repeat cesarean section, and no significant difference was seen between this group and the cases in terms of time to progress after 7 cm. All subjects had only one prior cesarean section.
Uterine rupture for this study was explicitly defined, a priori, as a full-thickness disruption of the uterine wall accompanied by clinical signs, Dr. Harper said.
The cases and controls from both reference groups were similar with respect to age, gravidity, diabetes, hypertension, and type of hospital, although the rate of prior vaginal delivery and black race both were more common in the reference groups. The three groups also were comparable with respect to intrapartum characteristics such as epidural use and gestational age at delivery, although those who experienced uterine rupture were more likely to have been induced and to have received Pitocin, she noted.
Although the case-control design of this study has inherent limitations such as possible selection bias, the study also has strengths, including the large study population of nearly 14,000 women in the original retrospective cohort, she said.
The findings suggest that a protracted labor prior to 7 cm of dilation does not necessarily signify uterine rupture in women attempting a TOLAC, as it was not uncommon during this period for the women to require 2 hours to progress 1 cm, and some still reached 10 cm, Dr. Harper said.
After 7 cm, however, labor dystocia should raise suspicion for uterine rupture in this population, she said.
Although the findings don’t necessarily suggest that intervention such as cesarean delivery is indicated in those with slow progression after 7 cm, they do underscore the need for more careful and frequent monitoring for signs of uterine rupture during active labor in women attempting a TOLAC.
"Instead of waiting 2 hours to check them, maybe they should be checked in an hour to make sure they’re progressing," she said.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Harper said she had no relevant financial disclosures.
DALLAS – Slow progression of labor beyond 7 cm of dilation in women attempting a trial of labor after cesarean section may signal risk for uterine rupture, findings from a case-control study suggest.
In 99 women who experienced uterine rupture while attempting a trial of labor after cesarean section (TOLAC) and 309 controls who did not experience uterine rupture during TOLAC, the time to progress 1 cm of dilation – after adjustment for prior vaginal delivery – was similar until 7 cm of dilation. Once the women reached 7 cm of dilation, however, a 1 cm progression took significantly longer in those who experienced rupture.
For example, progression from 7 cm to 8 cm took a median of 0.33 vs. 0.15 hours in the uterine rupture patients and the controls, respectively, and the progression from 8 cm to 9 cm took a median of 0.24 vs. 0.1 hours in the groups, respectively, Dr. Lorie Harper of Washington University in St. Louis reported at the annual meeting of the Society for Maternal-Fetal Medicine.
"This works out to approx 10-13 minutes longer for the median time to progress 1 cm in the uterine rupture group," she said, noting that the differences were particularly striking at the 95th percentile of time per 1 cm of dilation; for women with a successful trial of labor, each 1 cm of dilation after 7 cm took less than 1 hour, while at the 95th percentile, progression of each 1 cm of dilation after 7 cm took at least 1 hour.
The findings are from a secondary analysis of data from a nested case-control study of women attempting TOLAC within a 17-center retrospective cohort study of women with a prior low transverse cesarean section. Cases included women who experienced uterine rupture, and controls included women attempting TOLAC who reached 10 cm dilation. An additional reference group included 110 women with a failed TOLAC who ultimately underwent repeat cesarean section, and no significant difference was seen between this group and the cases in terms of time to progress after 7 cm. All subjects had only one prior cesarean section.
Uterine rupture for this study was explicitly defined, a priori, as a full-thickness disruption of the uterine wall accompanied by clinical signs, Dr. Harper said.
The cases and controls from both reference groups were similar with respect to age, gravidity, diabetes, hypertension, and type of hospital, although the rate of prior vaginal delivery and black race both were more common in the reference groups. The three groups also were comparable with respect to intrapartum characteristics such as epidural use and gestational age at delivery, although those who experienced uterine rupture were more likely to have been induced and to have received Pitocin, she noted.
Although the case-control design of this study has inherent limitations such as possible selection bias, the study also has strengths, including the large study population of nearly 14,000 women in the original retrospective cohort, she said.
The findings suggest that a protracted labor prior to 7 cm of dilation does not necessarily signify uterine rupture in women attempting a TOLAC, as it was not uncommon during this period for the women to require 2 hours to progress 1 cm, and some still reached 10 cm, Dr. Harper said.
After 7 cm, however, labor dystocia should raise suspicion for uterine rupture in this population, she said.
Although the findings don’t necessarily suggest that intervention such as cesarean delivery is indicated in those with slow progression after 7 cm, they do underscore the need for more careful and frequent monitoring for signs of uterine rupture during active labor in women attempting a TOLAC.
"Instead of waiting 2 hours to check them, maybe they should be checked in an hour to make sure they’re progressing," she said.
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Harper said she had no relevant financial disclosures.
FROM THE ANNUAL MEETING OF THE SOCIETY FOR MATERNAL-FETAL MEDICINE
Major Finding: In 99 women who experienced uterine rupture while attempting a trial of labor after cesarean section (TOLAC) and 309 controls who did not experience uterine rupture during TOLAC, the time to progress 1 cm of dilation – after adjusting for prior vaginal delivery – was similar until 7 cm of dilation. Progression from 7 cm to 8 cm took a median of 0.33 vs. 0.15 hours in the uterine rupture patients and the controls, respectively, and the progression from 8 cm to 9 cm took a median of 0.24 vs. 0.1 hours in the groups, respectively.
Data Source: A nested case-control study of women attempting TOLAC within a 17-center retrospective cohort study of women with a prior low transverse cesarean section.
Disclosures: The study was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development. Dr. Harper said she had no relevant financial disclosures.