User login
Chagas disease: An unusual and dangerous infection for both mother and baby
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
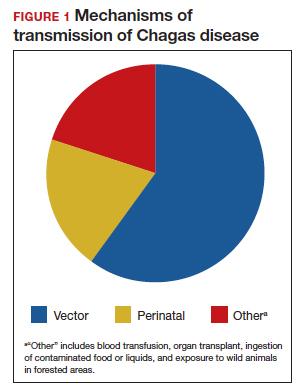
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
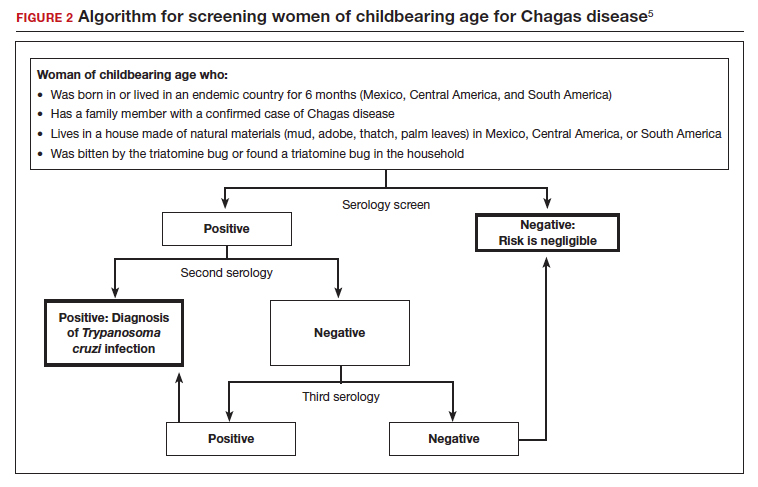
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.
Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7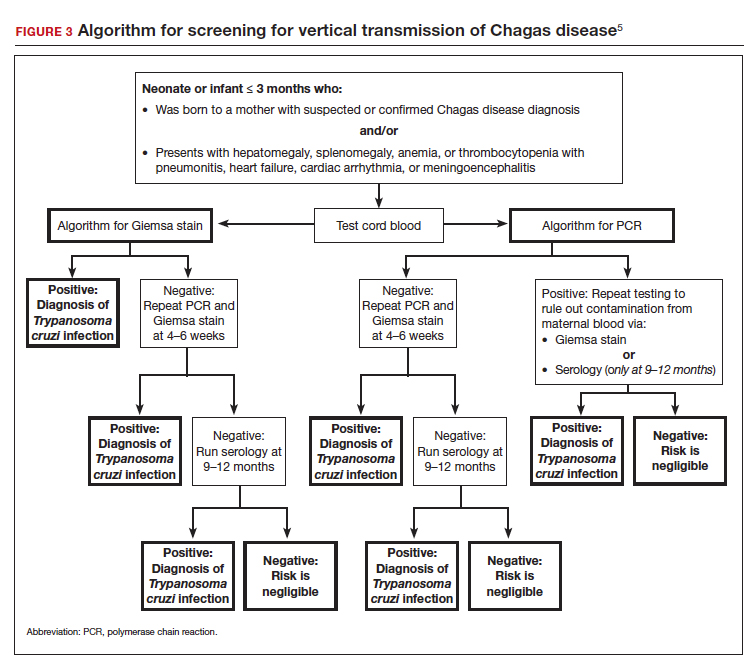
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.

Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.

Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.
RSV vaccine given during pregnancy protects newborns: Pfizer
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
New trial data from drugmaker Pfizer shows promising results of a vaccine given to mothers during pregnancy that later protects infants in their first months from the worst effects of respiratory syncytial virus, or RSV.
Pfizer will apply for FDA approval by the end of the year, the company said in a statement Nov. 1.
Trial results are so promising that – after talking with government regulators – the company will stop enrolling new people in the study.
Specifically, the company reported that the vaccine prevented severe illness particularly well during the first 90 days of life, with measurable protection against severe illness continuing through 6 months of age. (That period is when infants are the most fragile if they get sick with RSV.)
RSV is a respiratory illness than can affect anyone, usually resulting in no symptoms or those similar to the common cold. But it can be particularly dangerous – and even deadly – for babies and for people over the age of 65. Pfizer and another drug company, GSK, are developing promising vaccines for older adults, the Washington Post reported.
RSV is the leading cause of hospitalization for infants, the Post noted.
The Pfizer study, called MATISSE, enrolled 7,400 pregnant women in 18 countries worldwide. Those who received the vaccine were given it during the late second to third trimester of pregnancy. Women in the study were monitored for safety through the rest of their pregnancy and 6 months after their children were born. Infants were monitored for at least 1 year for safety and effectiveness; more than half of them were monitored for 2 years.
The Pfizer vaccine works by passing maternal antibodies to the infant during pregnancy, the Post reported, noting that other vaccines transmitted via maternal immunization include those for influenza, diphtheria, tetanus, and pertussis.
Annually, RSV has a devastating impact on young children, hospitalizing tens of thousands and causing up to 300 deaths, data show.
For every 100 children who get RSV under 6 months of age, one or two of them may need to be hospitalized, according to the CDC. Those hospitalized infants may need oxygen, intubation, or even mechanical ventilation to help with breathing.
“Most improve with this type of supportive care and are discharged in a few days,” the CDC said.
“I think this is a big step for protecting babies against RSV and improving overall lung health,” vaccine researcher Barney Graham, PhD, told the Post. “Overall, it’s an exciting time for RSV. It’s also a troubling time, because you see how the patterns of infection have been changed by COVID, and we’re having an earlier, bigger season this year than we have for a couple of years – and it’s causing a lot of hospitalization and misery for people.”
As many as four RSV vaccines may have applications submitted to the FDA in 2022, according to CNN. Also in development is an antibody shot given to infants just after they are born, the news outlet reported.
Pfizer’s data, announced Tuesday, has not yet been published or peer-reviewed, but the company said it is seeking peer-reviewed publication.
“We are thrilled by these data, as this is the first-ever investigational vaccine shown to help protect newborns against severe RSV-related respiratory illness immediately at birth,” Annaliesa Anderson, PhD, Pfizer chief scientific officer for vaccine research & development, said in a statement. “We look forward to working with the FDA and other regulatory agencies to bring this vaccine candidate to expectant mothers to help protect their infants against severe RSV during their most vulnerable first six months of life, which has the highest burden of RSV illness in infants.”
A version of this article first appeared on WebMD.com.
Access to abortion clinics declines sharply
Estimated travel time to abortion facilities in the United States has increased significantly since the Supreme Court overturned Roe v. Wade, according to results from an original investigation published online in JAMA.
In the wake of the ruling, many clinics have closed and now 33.3% of females of reproductive age live more than an hour from an abortion facility, more than double the 14.6% who lived that far before the Dobbs v. Jackson Women’s Health Organization court ruling, the paper states.
A 2022 study found that when people live 50 miles or more from an abortion facility they “were more likely to still be seeking an abortion on a 4-week follow-up than those who lived closer to an abortion facility,” wrote the authors, led by Benjamin Rader, MPH, from the Computational Epidemiology Lab at Boston Children’s Hospital.
Of 1,134 abortion facilities in the United States, 749 were considered active before the ruling and 671 were considered active in a simulated post-Dobbs period.
More than 15 states have total or partial bans
The researchers accounted for the closure of abortion facilities in states with total bans or 6-week abortion bans, compared with the period before the ruling, “during which all facilities providing abortions in 2021 were considered active.” The authors noted that more than 15 states have such bans.
Researchers found median and mean travel times to abortion facilities were estimated to be 10.9 minutes (interquartile ratio, 4.3-32.4) and 27.8 (standard deviation, 42.0) minutes before the ruling and used a paired sample t test (P < .001) to estimate the increase to a median of 17.0 (IQR, 4.9-124.5) minutes and a mean 100.4 (SD, 161.5) minutes after the ruling.
The numbers “highlight the catastrophe in terms of where we are,” Catherine Cansino, MD, MPH, professor, obstetrics and gynecology at the University of California, Davis, said in an interview.
Behind those numbers, she said, are brick walls for people who can’t take off work to drive that far or can’t leave their responsibilities of care for dependents or don’t have a car or even a driver’s license. It also calculates only land travel (car or public transportation) and doesn’t capture the financial and logistical burdens for some to fly to other states.
Dr. Cansino serves on the board of the Society of Family Planning, which publishes #WeCount, a national reporting effort that attempts to capture the effect of the Dobbs decision on abortion access. In a report published Oct. 28, #WeCount stated the numbers show that since the decision, there were 5,270 fewer abortions in July and 5,400 fewer in August, for a total of 10,670 fewer people in the United States who had abortions in the 2 months.
For Dr. Cansino, the numbers are only one measure of the wider problem.
“If it affects one person, it’s really the spirit of the consequence,” she said. “It’s difficult to wrap your mind around these numbers but the bottom line is that someone other than the person experiencing this health issue is making a decision for them.
“You will see physicians leaving states,” she said, “because their hands are tied in giving care.”
Glimpse of future from Texas example
The experience of abortion restrictions in Texas, described in another original investigation published in JAMA, provides a window into what could happen as access to abortions continues to decrease.
Texas has banned abortions after detectable embryonic cardiac activity since Sept. 1, 2021. Researchers obtained data on 80,107 abortions performed between September 2020 and February 2022.
In the first month following implementation of the Texas law, SB-8, the number of abortions in Texas dropped by 50%, compared with September 2020, and many pregnant Texas residents traveled out of state for abortion care.
But out-of-state abortions didn’t fully offset the overall drop in facility-based abortions.
“This decrease in facility-based abortion care suggests that many Texas residents continued their pregnancies, traveled beyond a neighboring state, or self-managed their abortion,” the authors wrote.
Increased time comes with costs
Sarah W. Prager, MD, professor in obstetrics and gynecology at University of Washington, Seattle, and director of the family planning division, explained that the travel time has to be seen in addition to the time it takes to complete the procedure.
Depending on state law, an abortion may take more than one visit to a clinic, which may mean adding lodging costs and overnight hours, or taking time off work, or finding childcare.
“A typical time to be at a clinic is upwards of 6 hours,” Dr. Prager explained, including paperwork, counseling, consent, the procedure, and recovery. That time is growing as active clinics overbook with others closing, she noted.
“We already know that 75% of people getting abortions are economically burdened at baseline. Gas is super expensive so the farther they have to drive – if they have their own car – that’s going to be expensive,” she noted.
In Washington, she said, abortion access is centralized in the western part of the state and located primarily between Seattle and Olympia. Though Oregon to the south has some of the nation’s most supportive laws for abortion, the other surrounding states have restrictive laws.
People in Alaska, Wyoming, Idaho, and Montana all have restrictive access, she noted, so people seeking abortions from those states have long distances to drive to western Washington and Oregon.
“Even for people living in eastern Washington, they are sometimes driving hours to get abortion care,” she said. “We’re really looking at health care that is dictated by geography, not by evidence, medicine, or science.”
The study by Dr. White and colleagues was supported by grants from the Susan Thompson Buffett Foundation and Collaborative for Gender + Reproductive Equity, as well as a center grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the Population Research Center at the University of Texas at Austin. One coauthor reported receiving compensation from the University of Texas at Austin for providing data during the conduct of the study, as well as grants from Merck and Gynuity Health Projects and personal fees from Merck and Organon outside the submitted work; another reported being named plaintiff in the case Planned Parenthood of Montana v State of Montana, a lawsuit challenging abortion restrictions in that state. No other disclosures were reported. Dr. Cansino and Dr. Prager reported no relevant financial relationships.
Estimated travel time to abortion facilities in the United States has increased significantly since the Supreme Court overturned Roe v. Wade, according to results from an original investigation published online in JAMA.
In the wake of the ruling, many clinics have closed and now 33.3% of females of reproductive age live more than an hour from an abortion facility, more than double the 14.6% who lived that far before the Dobbs v. Jackson Women’s Health Organization court ruling, the paper states.
A 2022 study found that when people live 50 miles or more from an abortion facility they “were more likely to still be seeking an abortion on a 4-week follow-up than those who lived closer to an abortion facility,” wrote the authors, led by Benjamin Rader, MPH, from the Computational Epidemiology Lab at Boston Children’s Hospital.
Of 1,134 abortion facilities in the United States, 749 were considered active before the ruling and 671 were considered active in a simulated post-Dobbs period.
More than 15 states have total or partial bans
The researchers accounted for the closure of abortion facilities in states with total bans or 6-week abortion bans, compared with the period before the ruling, “during which all facilities providing abortions in 2021 were considered active.” The authors noted that more than 15 states have such bans.
Researchers found median and mean travel times to abortion facilities were estimated to be 10.9 minutes (interquartile ratio, 4.3-32.4) and 27.8 (standard deviation, 42.0) minutes before the ruling and used a paired sample t test (P < .001) to estimate the increase to a median of 17.0 (IQR, 4.9-124.5) minutes and a mean 100.4 (SD, 161.5) minutes after the ruling.
The numbers “highlight the catastrophe in terms of where we are,” Catherine Cansino, MD, MPH, professor, obstetrics and gynecology at the University of California, Davis, said in an interview.
Behind those numbers, she said, are brick walls for people who can’t take off work to drive that far or can’t leave their responsibilities of care for dependents or don’t have a car or even a driver’s license. It also calculates only land travel (car or public transportation) and doesn’t capture the financial and logistical burdens for some to fly to other states.
Dr. Cansino serves on the board of the Society of Family Planning, which publishes #WeCount, a national reporting effort that attempts to capture the effect of the Dobbs decision on abortion access. In a report published Oct. 28, #WeCount stated the numbers show that since the decision, there were 5,270 fewer abortions in July and 5,400 fewer in August, for a total of 10,670 fewer people in the United States who had abortions in the 2 months.
For Dr. Cansino, the numbers are only one measure of the wider problem.
“If it affects one person, it’s really the spirit of the consequence,” she said. “It’s difficult to wrap your mind around these numbers but the bottom line is that someone other than the person experiencing this health issue is making a decision for them.
“You will see physicians leaving states,” she said, “because their hands are tied in giving care.”
Glimpse of future from Texas example
The experience of abortion restrictions in Texas, described in another original investigation published in JAMA, provides a window into what could happen as access to abortions continues to decrease.
Texas has banned abortions after detectable embryonic cardiac activity since Sept. 1, 2021. Researchers obtained data on 80,107 abortions performed between September 2020 and February 2022.
In the first month following implementation of the Texas law, SB-8, the number of abortions in Texas dropped by 50%, compared with September 2020, and many pregnant Texas residents traveled out of state for abortion care.
But out-of-state abortions didn’t fully offset the overall drop in facility-based abortions.
“This decrease in facility-based abortion care suggests that many Texas residents continued their pregnancies, traveled beyond a neighboring state, or self-managed their abortion,” the authors wrote.
Increased time comes with costs
Sarah W. Prager, MD, professor in obstetrics and gynecology at University of Washington, Seattle, and director of the family planning division, explained that the travel time has to be seen in addition to the time it takes to complete the procedure.
Depending on state law, an abortion may take more than one visit to a clinic, which may mean adding lodging costs and overnight hours, or taking time off work, or finding childcare.
“A typical time to be at a clinic is upwards of 6 hours,” Dr. Prager explained, including paperwork, counseling, consent, the procedure, and recovery. That time is growing as active clinics overbook with others closing, she noted.
“We already know that 75% of people getting abortions are economically burdened at baseline. Gas is super expensive so the farther they have to drive – if they have their own car – that’s going to be expensive,” she noted.
In Washington, she said, abortion access is centralized in the western part of the state and located primarily between Seattle and Olympia. Though Oregon to the south has some of the nation’s most supportive laws for abortion, the other surrounding states have restrictive laws.
People in Alaska, Wyoming, Idaho, and Montana all have restrictive access, she noted, so people seeking abortions from those states have long distances to drive to western Washington and Oregon.
“Even for people living in eastern Washington, they are sometimes driving hours to get abortion care,” she said. “We’re really looking at health care that is dictated by geography, not by evidence, medicine, or science.”
The study by Dr. White and colleagues was supported by grants from the Susan Thompson Buffett Foundation and Collaborative for Gender + Reproductive Equity, as well as a center grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the Population Research Center at the University of Texas at Austin. One coauthor reported receiving compensation from the University of Texas at Austin for providing data during the conduct of the study, as well as grants from Merck and Gynuity Health Projects and personal fees from Merck and Organon outside the submitted work; another reported being named plaintiff in the case Planned Parenthood of Montana v State of Montana, a lawsuit challenging abortion restrictions in that state. No other disclosures were reported. Dr. Cansino and Dr. Prager reported no relevant financial relationships.
Estimated travel time to abortion facilities in the United States has increased significantly since the Supreme Court overturned Roe v. Wade, according to results from an original investigation published online in JAMA.
In the wake of the ruling, many clinics have closed and now 33.3% of females of reproductive age live more than an hour from an abortion facility, more than double the 14.6% who lived that far before the Dobbs v. Jackson Women’s Health Organization court ruling, the paper states.
A 2022 study found that when people live 50 miles or more from an abortion facility they “were more likely to still be seeking an abortion on a 4-week follow-up than those who lived closer to an abortion facility,” wrote the authors, led by Benjamin Rader, MPH, from the Computational Epidemiology Lab at Boston Children’s Hospital.
Of 1,134 abortion facilities in the United States, 749 were considered active before the ruling and 671 were considered active in a simulated post-Dobbs period.
More than 15 states have total or partial bans
The researchers accounted for the closure of abortion facilities in states with total bans or 6-week abortion bans, compared with the period before the ruling, “during which all facilities providing abortions in 2021 were considered active.” The authors noted that more than 15 states have such bans.
Researchers found median and mean travel times to abortion facilities were estimated to be 10.9 minutes (interquartile ratio, 4.3-32.4) and 27.8 (standard deviation, 42.0) minutes before the ruling and used a paired sample t test (P < .001) to estimate the increase to a median of 17.0 (IQR, 4.9-124.5) minutes and a mean 100.4 (SD, 161.5) minutes after the ruling.
The numbers “highlight the catastrophe in terms of where we are,” Catherine Cansino, MD, MPH, professor, obstetrics and gynecology at the University of California, Davis, said in an interview.
Behind those numbers, she said, are brick walls for people who can’t take off work to drive that far or can’t leave their responsibilities of care for dependents or don’t have a car or even a driver’s license. It also calculates only land travel (car or public transportation) and doesn’t capture the financial and logistical burdens for some to fly to other states.
Dr. Cansino serves on the board of the Society of Family Planning, which publishes #WeCount, a national reporting effort that attempts to capture the effect of the Dobbs decision on abortion access. In a report published Oct. 28, #WeCount stated the numbers show that since the decision, there were 5,270 fewer abortions in July and 5,400 fewer in August, for a total of 10,670 fewer people in the United States who had abortions in the 2 months.
For Dr. Cansino, the numbers are only one measure of the wider problem.
“If it affects one person, it’s really the spirit of the consequence,” she said. “It’s difficult to wrap your mind around these numbers but the bottom line is that someone other than the person experiencing this health issue is making a decision for them.
“You will see physicians leaving states,” she said, “because their hands are tied in giving care.”
Glimpse of future from Texas example
The experience of abortion restrictions in Texas, described in another original investigation published in JAMA, provides a window into what could happen as access to abortions continues to decrease.
Texas has banned abortions after detectable embryonic cardiac activity since Sept. 1, 2021. Researchers obtained data on 80,107 abortions performed between September 2020 and February 2022.
In the first month following implementation of the Texas law, SB-8, the number of abortions in Texas dropped by 50%, compared with September 2020, and many pregnant Texas residents traveled out of state for abortion care.
But out-of-state abortions didn’t fully offset the overall drop in facility-based abortions.
“This decrease in facility-based abortion care suggests that many Texas residents continued their pregnancies, traveled beyond a neighboring state, or self-managed their abortion,” the authors wrote.
Increased time comes with costs
Sarah W. Prager, MD, professor in obstetrics and gynecology at University of Washington, Seattle, and director of the family planning division, explained that the travel time has to be seen in addition to the time it takes to complete the procedure.
Depending on state law, an abortion may take more than one visit to a clinic, which may mean adding lodging costs and overnight hours, or taking time off work, or finding childcare.
“A typical time to be at a clinic is upwards of 6 hours,” Dr. Prager explained, including paperwork, counseling, consent, the procedure, and recovery. That time is growing as active clinics overbook with others closing, she noted.
“We already know that 75% of people getting abortions are economically burdened at baseline. Gas is super expensive so the farther they have to drive – if they have their own car – that’s going to be expensive,” she noted.
In Washington, she said, abortion access is centralized in the western part of the state and located primarily between Seattle and Olympia. Though Oregon to the south has some of the nation’s most supportive laws for abortion, the other surrounding states have restrictive laws.
People in Alaska, Wyoming, Idaho, and Montana all have restrictive access, she noted, so people seeking abortions from those states have long distances to drive to western Washington and Oregon.
“Even for people living in eastern Washington, they are sometimes driving hours to get abortion care,” she said. “We’re really looking at health care that is dictated by geography, not by evidence, medicine, or science.”
The study by Dr. White and colleagues was supported by grants from the Susan Thompson Buffett Foundation and Collaborative for Gender + Reproductive Equity, as well as a center grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the Population Research Center at the University of Texas at Austin. One coauthor reported receiving compensation from the University of Texas at Austin for providing data during the conduct of the study, as well as grants from Merck and Gynuity Health Projects and personal fees from Merck and Organon outside the submitted work; another reported being named plaintiff in the case Planned Parenthood of Montana v State of Montana, a lawsuit challenging abortion restrictions in that state. No other disclosures were reported. Dr. Cansino and Dr. Prager reported no relevant financial relationships.
FROM JAMA
Commentary: Hypertension, morbidity in MTOP, and hypothyroidism risk in obstetric emergencies, November 2022
Richards and colleagues explored the effects of aspirin prophylaxis in women with chronic hypertension. They did not detect a lowered risk for preeclampsia but did note a significantly decreased risk for preterm birth in the aspirin group. This was a systematic review and meta-analysis of nine studies (including retrospective cohort and randomized controlled trials). The mixed quality of the source data did limit the meta-analysis. However, this finding suggests that further research is warranted, and we may have a new role for aspirin in helping to decrease preterm birth in women with chronic hypertension.
Cleary and colleagues investigated the use of 30 mg oral nifedipine ER given every 24 hours until delivery in patients with preE with SF. In this randomized, triple-blinded, placebo-controlled trial, 110 patients were randomly assigned to nifedipine treatment or placebo. The results suggest a role for this medication early in the treatment of preE with SF, as the treated patients were much less likely to require acute therapy for severe-range blood pressure. The researchers also noted a trend toward fewer cesarean deliveries (20.8% vs 34.7%) and lower neonatal intensive care unit admissions (29.1% vs 47.1%) in the nifedipine ER group. This favors the use of nifedipine ER in patients with preE with SF.
Stewart and colleagues examined the more common morbidities associated with MTOP after 20 weeks estimated gestational age using a 10-year retrospective cohort study involving 407 patients. They found that 99% of the women had a successful vaginal delivery; however, 25% had some morbidity. Additionally, 16% of the women needed manual removal of placental tissue, 11% had postpartum hemorrhage, and 1.3% experienced severe maternal morbidity (including amniotic fluid embolism), but no maternal deaths occurred. Increased surveillance for postpartum hemorrhage in this patient population should be considered.
Righini and colleagues provide reassurance regarding a commonly used test to rule out pulmonary embolism in pregnant women. They present ancillary data from a prospective management outcome study of 149 women who underwent CT pulmonary angiography testing in pregnancy. There have been concerns raised regarding potential harmful effects related to intravenous iodinated contrast agents on thyroid function. None of the infants born to these patients had evidence of neonatal hypothyroidism (assessed via thyroid-stimulating hormone measurements). This gives reassurance that the use of CT pulmonary angiography testing for pulmonary embolism in pregnancy is safe.
Richards and colleagues explored the effects of aspirin prophylaxis in women with chronic hypertension. They did not detect a lowered risk for preeclampsia but did note a significantly decreased risk for preterm birth in the aspirin group. This was a systematic review and meta-analysis of nine studies (including retrospective cohort and randomized controlled trials). The mixed quality of the source data did limit the meta-analysis. However, this finding suggests that further research is warranted, and we may have a new role for aspirin in helping to decrease preterm birth in women with chronic hypertension.
Cleary and colleagues investigated the use of 30 mg oral nifedipine ER given every 24 hours until delivery in patients with preE with SF. In this randomized, triple-blinded, placebo-controlled trial, 110 patients were randomly assigned to nifedipine treatment or placebo. The results suggest a role for this medication early in the treatment of preE with SF, as the treated patients were much less likely to require acute therapy for severe-range blood pressure. The researchers also noted a trend toward fewer cesarean deliveries (20.8% vs 34.7%) and lower neonatal intensive care unit admissions (29.1% vs 47.1%) in the nifedipine ER group. This favors the use of nifedipine ER in patients with preE with SF.
Stewart and colleagues examined the more common morbidities associated with MTOP after 20 weeks estimated gestational age using a 10-year retrospective cohort study involving 407 patients. They found that 99% of the women had a successful vaginal delivery; however, 25% had some morbidity. Additionally, 16% of the women needed manual removal of placental tissue, 11% had postpartum hemorrhage, and 1.3% experienced severe maternal morbidity (including amniotic fluid embolism), but no maternal deaths occurred. Increased surveillance for postpartum hemorrhage in this patient population should be considered.
Righini and colleagues provide reassurance regarding a commonly used test to rule out pulmonary embolism in pregnant women. They present ancillary data from a prospective management outcome study of 149 women who underwent CT pulmonary angiography testing in pregnancy. There have been concerns raised regarding potential harmful effects related to intravenous iodinated contrast agents on thyroid function. None of the infants born to these patients had evidence of neonatal hypothyroidism (assessed via thyroid-stimulating hormone measurements). This gives reassurance that the use of CT pulmonary angiography testing for pulmonary embolism in pregnancy is safe.
Richards and colleagues explored the effects of aspirin prophylaxis in women with chronic hypertension. They did not detect a lowered risk for preeclampsia but did note a significantly decreased risk for preterm birth in the aspirin group. This was a systematic review and meta-analysis of nine studies (including retrospective cohort and randomized controlled trials). The mixed quality of the source data did limit the meta-analysis. However, this finding suggests that further research is warranted, and we may have a new role for aspirin in helping to decrease preterm birth in women with chronic hypertension.
Cleary and colleagues investigated the use of 30 mg oral nifedipine ER given every 24 hours until delivery in patients with preE with SF. In this randomized, triple-blinded, placebo-controlled trial, 110 patients were randomly assigned to nifedipine treatment or placebo. The results suggest a role for this medication early in the treatment of preE with SF, as the treated patients were much less likely to require acute therapy for severe-range blood pressure. The researchers also noted a trend toward fewer cesarean deliveries (20.8% vs 34.7%) and lower neonatal intensive care unit admissions (29.1% vs 47.1%) in the nifedipine ER group. This favors the use of nifedipine ER in patients with preE with SF.
Stewart and colleagues examined the more common morbidities associated with MTOP after 20 weeks estimated gestational age using a 10-year retrospective cohort study involving 407 patients. They found that 99% of the women had a successful vaginal delivery; however, 25% had some morbidity. Additionally, 16% of the women needed manual removal of placental tissue, 11% had postpartum hemorrhage, and 1.3% experienced severe maternal morbidity (including amniotic fluid embolism), but no maternal deaths occurred. Increased surveillance for postpartum hemorrhage in this patient population should be considered.
Righini and colleagues provide reassurance regarding a commonly used test to rule out pulmonary embolism in pregnant women. They present ancillary data from a prospective management outcome study of 149 women who underwent CT pulmonary angiography testing in pregnancy. There have been concerns raised regarding potential harmful effects related to intravenous iodinated contrast agents on thyroid function. None of the infants born to these patients had evidence of neonatal hypothyroidism (assessed via thyroid-stimulating hormone measurements). This gives reassurance that the use of CT pulmonary angiography testing for pulmonary embolism in pregnancy is safe.
New consensus on managing nausea and vomiting in pregnancy
Although the nausea and vomiting associated with pregnancy are usually mild, they are more severe (hyperemesis gravidarum) in around one-third of women and require hospitalization in the first trimester for 0.3%-3.6% of these women in France. Given the diversity of practical care, a working group from the National College of French Gynecologists and Obstetricians (CNGOF) has established a consensus on the definition and management of these symptoms.
Definition and severity
Nausea and vomiting during pregnancy are defined as those emerging in the first trimester of pregnancy and for which there is no other etiology.
The severity of these symptoms should be assessed through weight loss from the beginning of the pregnancy, clinical signs of dehydration (thirst, skin turgor, hypotension, oliguria, etc.), and modified PUQE (Pregnancy-Unique Quantification of Emesis and Nausea) score. This is a three-question score rated from 0 to 15, available in the full text of the expert consensus.
Severe nausea and vomiting are not considered complicated when weight loss is < 5%, with no clinical signs of dehydration, and combined with a PUQE score of ≤ 6. In contrast, hyperemesis gravidarum is distinguished from nausea and vomiting during pregnancy by weight loss of ≥ 5 % or signs of dehydration or a PUQE score of ≥ 7.
Treating hyperemesis gravidarum
A laboratory workup should be ordered, along with an assay of blood potassium, blood sodium ions, and creatinine levels, as well as a complete dipstick urinalysis.
If symptoms persist or worsen despite well-managed treatment, an additional assessment is recommended, including an abdominal ultrasound and laboratory workup (white blood cell count, transaminases, lipase, CRP, TSH, T4).
Hospitalization is proposed when at least one of the following criteria is met: weight loss ≥ 10%, one or more clinical signs of dehydration, PUQE score of ≥ 13, hypokalemia < 3.0 mmol/L, hyponatremia < 120 mmol/L, elevated serum creatinine > 100 micromol/L, or resistance to treatment.
Which treatment?
Prenatal vitamins and iron supplementation should be stopped, as the latter seems to make symptoms worse. This step should be taken without stopping folic acid supplementation.
Women are free to adapt their diets and lifestyles according to their symptoms, since no such changes have been reported to improve symptoms.
If the PUQE score is < 6, even in the absence of proof of their benefit, ginger or B6 vitamin can be used. The same applies to acupressure, acupuncture, and electrical stimulation, which should only be considered in women without complications. Aromatherapy is not to be used, because of the potential risks associated with essential oils, and as no efficacy has been demonstrated.
It is proposed that drugs or combinations of drugs associated with the least severe and least frequent side effects should always be chosen in the absence of superiority of one class over another.
To prevent Gayet Wernicke encephalopathy, vitamin B1 must be administered systematically for hyperemesis gravidarum needing parenteral rehydration. Psychological support should be offered to all patients with hyperemesis gravidarum because of the negative impact of this pathology on mental well-being. Patients should be informed that there are patient associations involved in supporting these women and their families.
A version of this article first appeared on Medscape.com and was translated from Univadis France.
Although the nausea and vomiting associated with pregnancy are usually mild, they are more severe (hyperemesis gravidarum) in around one-third of women and require hospitalization in the first trimester for 0.3%-3.6% of these women in France. Given the diversity of practical care, a working group from the National College of French Gynecologists and Obstetricians (CNGOF) has established a consensus on the definition and management of these symptoms.
Definition and severity
Nausea and vomiting during pregnancy are defined as those emerging in the first trimester of pregnancy and for which there is no other etiology.
The severity of these symptoms should be assessed through weight loss from the beginning of the pregnancy, clinical signs of dehydration (thirst, skin turgor, hypotension, oliguria, etc.), and modified PUQE (Pregnancy-Unique Quantification of Emesis and Nausea) score. This is a three-question score rated from 0 to 15, available in the full text of the expert consensus.
Severe nausea and vomiting are not considered complicated when weight loss is < 5%, with no clinical signs of dehydration, and combined with a PUQE score of ≤ 6. In contrast, hyperemesis gravidarum is distinguished from nausea and vomiting during pregnancy by weight loss of ≥ 5 % or signs of dehydration or a PUQE score of ≥ 7.
Treating hyperemesis gravidarum
A laboratory workup should be ordered, along with an assay of blood potassium, blood sodium ions, and creatinine levels, as well as a complete dipstick urinalysis.
If symptoms persist or worsen despite well-managed treatment, an additional assessment is recommended, including an abdominal ultrasound and laboratory workup (white blood cell count, transaminases, lipase, CRP, TSH, T4).
Hospitalization is proposed when at least one of the following criteria is met: weight loss ≥ 10%, one or more clinical signs of dehydration, PUQE score of ≥ 13, hypokalemia < 3.0 mmol/L, hyponatremia < 120 mmol/L, elevated serum creatinine > 100 micromol/L, or resistance to treatment.
Which treatment?
Prenatal vitamins and iron supplementation should be stopped, as the latter seems to make symptoms worse. This step should be taken without stopping folic acid supplementation.
Women are free to adapt their diets and lifestyles according to their symptoms, since no such changes have been reported to improve symptoms.
If the PUQE score is < 6, even in the absence of proof of their benefit, ginger or B6 vitamin can be used. The same applies to acupressure, acupuncture, and electrical stimulation, which should only be considered in women without complications. Aromatherapy is not to be used, because of the potential risks associated with essential oils, and as no efficacy has been demonstrated.
It is proposed that drugs or combinations of drugs associated with the least severe and least frequent side effects should always be chosen in the absence of superiority of one class over another.
To prevent Gayet Wernicke encephalopathy, vitamin B1 must be administered systematically for hyperemesis gravidarum needing parenteral rehydration. Psychological support should be offered to all patients with hyperemesis gravidarum because of the negative impact of this pathology on mental well-being. Patients should be informed that there are patient associations involved in supporting these women and their families.
A version of this article first appeared on Medscape.com and was translated from Univadis France.
Although the nausea and vomiting associated with pregnancy are usually mild, they are more severe (hyperemesis gravidarum) in around one-third of women and require hospitalization in the first trimester for 0.3%-3.6% of these women in France. Given the diversity of practical care, a working group from the National College of French Gynecologists and Obstetricians (CNGOF) has established a consensus on the definition and management of these symptoms.
Definition and severity
Nausea and vomiting during pregnancy are defined as those emerging in the first trimester of pregnancy and for which there is no other etiology.
The severity of these symptoms should be assessed through weight loss from the beginning of the pregnancy, clinical signs of dehydration (thirst, skin turgor, hypotension, oliguria, etc.), and modified PUQE (Pregnancy-Unique Quantification of Emesis and Nausea) score. This is a three-question score rated from 0 to 15, available in the full text of the expert consensus.
Severe nausea and vomiting are not considered complicated when weight loss is < 5%, with no clinical signs of dehydration, and combined with a PUQE score of ≤ 6. In contrast, hyperemesis gravidarum is distinguished from nausea and vomiting during pregnancy by weight loss of ≥ 5 % or signs of dehydration or a PUQE score of ≥ 7.
Treating hyperemesis gravidarum
A laboratory workup should be ordered, along with an assay of blood potassium, blood sodium ions, and creatinine levels, as well as a complete dipstick urinalysis.
If symptoms persist or worsen despite well-managed treatment, an additional assessment is recommended, including an abdominal ultrasound and laboratory workup (white blood cell count, transaminases, lipase, CRP, TSH, T4).
Hospitalization is proposed when at least one of the following criteria is met: weight loss ≥ 10%, one or more clinical signs of dehydration, PUQE score of ≥ 13, hypokalemia < 3.0 mmol/L, hyponatremia < 120 mmol/L, elevated serum creatinine > 100 micromol/L, or resistance to treatment.
Which treatment?
Prenatal vitamins and iron supplementation should be stopped, as the latter seems to make symptoms worse. This step should be taken without stopping folic acid supplementation.
Women are free to adapt their diets and lifestyles according to their symptoms, since no such changes have been reported to improve symptoms.
If the PUQE score is < 6, even in the absence of proof of their benefit, ginger or B6 vitamin can be used. The same applies to acupressure, acupuncture, and electrical stimulation, which should only be considered in women without complications. Aromatherapy is not to be used, because of the potential risks associated with essential oils, and as no efficacy has been demonstrated.
It is proposed that drugs or combinations of drugs associated with the least severe and least frequent side effects should always be chosen in the absence of superiority of one class over another.
To prevent Gayet Wernicke encephalopathy, vitamin B1 must be administered systematically for hyperemesis gravidarum needing parenteral rehydration. Psychological support should be offered to all patients with hyperemesis gravidarum because of the negative impact of this pathology on mental well-being. Patients should be informed that there are patient associations involved in supporting these women and their families.
A version of this article first appeared on Medscape.com and was translated from Univadis France.
BMI and reproduction – weighing the evidence
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Arguably, no topic during an infertility consultation generates more of an emotional reaction than discussing body mass index (BMI), particularly when it is high. Patients have become increasingly sensitive to weight discussions with their physicians because of concerns about body shaming. Among patients with an elevated BMI, criticism on social media of health care professionals’ counseling and a preemptive presentation of “Don’t Weigh Me” cards have become popular responses. Despite the medical evidence on impaired reproduction with an abnormal BMI, patients are choosing to forgo the topic. Research has demonstrated “extensive evidence [of] strong weight bias” in a wide range of health staff.1 A “viral” TikTok study revealed that medical “gaslighting” founded in weight stigma and bias is harmful, as reported on KevinMD.com.2 This month, we review the effect of abnormal BMI, both high and low, on reproduction and pregnancy.
A method to assess relative weight was first described in 1832 as its ratio in kilograms divided by the square of the height in meters, or the Quetelet Index. The search for a functional assessment of relative body weight began after World War II when reports by actuaries noted the increased mortality of overweight policyholders. The relationship between weight and cardiovascular disease was further revealed in epidemiologic studies. The Quetelet Index became the BMI in 1972.3
Weight measurement is a mainstay in the assessment of a patient’s vital signs along with blood pressure, pulse rate, respiration rate, and temperature. Weight is vital to the calculation of medication dosage – for instance, administration of conscious sedative drugs, methotrexate, and gonadotropins. Some state boards of medicine, such as Florida, have a limitation on patient BMI at office-based surgery centers (40 kg/m2).
Obesity is a disease
As reported by the World Health Organization in 2022, the disease of obesity is an epidemic afflicting more than 1 billion people worldwide, or 1 in 8 individuals globally.4 The health implications of an elevated BMI include increased mortality, diabetes, heart disease, and stroke, physical limitations to activities of daily living, and complications affecting reproduction.
Female obesity is related to poorer outcomes in natural and assisted conception, including an increased risk of miscarriage. Compared with normal-weight women, those with obesity are three times more likely to have ovulatory dysfunction,5 infertility,6 a lower chance for conception,7 higher rate of miscarriage, and low birth weight.8,9During pregnancy, women with obesity have three to four times higher rates of gestational diabetes and preeclampsia,10 as well as likelihood of delivering preterm,11 having a fetus with macrosomia and birth defects, and a 1.3- to 2.1-times higher risk of stillbirth.12
Obesity is present in 40%-80% of women with polycystic ovary syndrome,13 the most common cause of ovulatory dysfunction from dysregulation of the hypothalamic-pituitary-ovarian axis. While PCOS is associated with reproductive and metabolic consequences, even in regularly ovulating women, increasing obesity appears to be associated with decreasing spontaneous pregnancy rates and increased time to pregnancy.14
Obesity and IVF
Women with obesity have reduced success with assisted reproductive technology, an increased number of canceled cycles, and poorer quality oocytes retrieved. A prospective cohort study of nearly 2,000 women reported that every 5 kg of body weight increase (from the patient’s baseline weight at age 18) was associated with a 5% increase in the mean duration of time required for conception (95% confidence interval, 3%-7%).15 Given that approximately 90% of these women had regular menstrual cycles, ovulatory dysfunction was not the suspected pathophysiology.
A meta-analysis of 21 cohort studies reported a lower likelihood of live birth following in vitro fertilization for women with obesity, compared with normal-weight women (risk ratio, 0.85; 95% CI, 0.82-0.87).16 A further subgroup analysis that evaluated only women with PCOS showed a reduction in the live birth rate following IVF for individuals with obesity, compared with normal-weight individuals (RR, 0.78; 95% CI, 0.74-0.82).
In a retrospective study of almost 500,000 fresh autologous IVF cycles, women with obesity had a 6% reduction in pregnancy rates and a 13% reduction in live birth rates, compared with normal-weight women. Both high and low BMI were associated with an increased risk of low birth weight and preterm delivery.17 The live birth rates per transfer for normal-weight and higher-weight women were 38% and 33%, respectively.
Contrarily, a randomized controlled trial showed that an intensive weight-reduction program resulted in a large weight loss but did not substantially affect live birth rates in women with obesity scheduled for IVF.18
Low BMI
A noteworthy cause of low BMI is functional hypothalamic amenorrhea (FHA), a disorder with low energy availability either from decreased caloric intake and/or excessive energy expenditure associated with eating disorders, excessive exercise, and stress. Consequently, a reduced GnRH drive results in a decreased pulse frequency and amplitude leading to low levels of follicle-stimulating hormone and luteinizing hormone, resulting in anovulation. Correction of lifestyle behaviors related to FHA can restore menstrual cycles. After normal weight is achieved, it appears unlikely that fertility is affected.19 In 47% of adolescent patients with anorexia, menses spontaneously returned within the first 12 months after admission, with an improved prognosis in secondary over primary amenorrhea.20,21 Interestingly, mildly and significantly underweight infertile women have pregnancy and live birth rates similar to normal-weight patients after IVF treatment.22
Pregnancy is complicated in underweight women, resulting in an increased risk of anemia, fetal growth retardation, and low birth weight, as well as preterm birth.21
Take-home message
The extremes of BMI both impair natural reproduction. Elevated BMI reduces success with IVF but rapid weight loss prior to IVF does not improve outcomes. A normal BMI is the goal for optimal reproductive and pregnancy health.
Dr. Trolice is director of the IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Talumaa B et al. Obesity Rev. 2022;23:e13494.
2. https://bit.ly/3rHCivE.
3. Eknoyan G. Nephrol Dial Transplant. 2008;23:47-51.
4. Wells JCK. Dis Models Mech. 2012;5:595-607.
5. Brewer CJ and Balen AH. Reproduction. 2010;140:347-64.
6. Silvestris E et al. Reprod Biol Endocrinol. 2018;16:22.
7. Wise LA et al. Hum Reprod. 2010;25:253-64.
8. Bellver J. Curr Opin Obstet Gynecol. 2022;34:114-21.
9. Dickey RP et al. Am J Obstet Gynecol. 2013;209:349.e1.
10. Alwash SM et al. Obes Res Clin Pract. 2021;15:425-30.
11. Cnattingius S et al. JAMA. 2013;309:2362-70.
12. Aune D et al. JAMA. 2014;311:1536-46.
13. Sam S. Obes Manag. 2007;3:69-73.
14. van der Steeg JW et al. Hum Reprod. 2008;23:324-8.
15. Gaskins AJ et al. Obstet Gynecol. 2015;126:850-8.
16. Sermondade N et al. Hum Reprod Update. 2019;25:439-519.
17. Kawwass JF et al. Fertil Steril. 2016;106[7]:1742-50.
18. Einarsson S et al. Hum Reprod. 2017;32:1621-30.
19. Chaer R et al. Diseases. 2020;8:46.
20. Dempfle A et al. Psychiatry. 2013;13:308.
21. Verma A and Shrimali L. J Clin Diagn Res. 2012;6:1531-3.
22. Romanski PA et al. Reprod Biomed Online. 2020;42:366-74.
Fertility physicians say they lack access to miscarriage drugs
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
In a survey taken before the Supreme Court’s Dobbs ruling regarding abortion rights, two-thirds of assisted reproduction technology (ART) physicians who don’t offer mifepristone/misoprostol to patients with early pregnancy loss (EPL) reported that they lack access to the drugs.
The numbers are likely higher now. In the wake of the court ruling, some physicians in states with new abortion restrictions fear they won’t be able to properly treat women with miscarriages. Access to mifepristone, a component of medication abortions along with misoprostol, is at the center of their concerns.
“These restrictions that were put in place to restrict abortion care have far-reaching implications regarding miscarriages and early pregnancy loss and the assisted reproduction community is not immune,” obstetrics and gynecology specialist Zachary Anderson, MD, a resident physician at the University of Southern California, Los Angeles, said in an interview. He presented the findings at the American Society for Reproductive Medicine’s 2022 meeting.
Early pregnancy loss – defined as a miscarriage within 12 weeks and 6 days of conception – is common in all pregnancies and affects an estimated 15% of those who rely on in vitro fertilization (IVF). In women who conceive through intrauterine insemination or IVF, “an abnormal karyotype embryo/fetus is the cause of miscarriage in more than two-thirds of cases,” Mark P. Trolice, MD, director of the IVF Center and professor of obstetrics and gynecology at the University of Central Florida, Orlando, said in an interview. “The options of management are observation – with no ability to determine when passage of the products of conception will occur – vs. mifepristone/misoprostol or suction D&C.”
Dr. Trolice added that “most woman select the medical treatment protocol, which is 200 mg mifepristone orally followed by 800 mcg misoprostol vaginally 24 hours later. If no signs of heavy bleeding occur after 3 hours following misoprostol, the patient should repeat the dose of 800 micrograms vaginally.”
According to the Reuters news service, some abortion bans target mifepristone. In October 2022, the American College of Obstetricians and Gynecologists asked the Food and Drug Administration to approve mifepristone for use in miscarriage management; such use is now off label, although it is approved to end early pregnancies in conjunction with misoprostol.
For the new study, researchers sent anonymous surveys to 826 members of the Society of Reproductive Endocrinology and Infertility and received 101 responses (12% response rate, 51% women, 86% non-Hispanic White, average age 52, 52% urban, and 51% in private practice).
More than two-thirds (70%) said they diagnosed early pregnancy loss at least once a week; 47% prefer treatment with misoprostol alone, 18% surgery in an operating room, 15% expectant management (monitoring a miscarriage as it occurs without medical intervention), 10% surgery in the office, and 3% mifepristone-misoprostol.
Of those who don’t offer mifepristone-misoprostol, 68% said they lack access, and 26% said they lack familiarity with the treatment.
Study coauthor Brian T. Nguyen, MD, MSc, assistant professor of obstetrics and gynecology at USC, said in an interview that mifepristone, a highly effective drug, is treated differently from other medications “for no good reason.”
Dr. Anderson, who led the study, urged colleagues to get the appropriate certification to prescribe mifepristone. “Providers overestimate how difficult it is to become certified to prescribe it,” he said.
Dr. Trolice, who is familiar with the study findings, said the response rate is low, and the results might be biased because those with preconceived opinions may be more likely to respond.
However, he said, “The results are not surprising in that medication is more commonly preferred (nearly 50%) given the devastation of a miscarriage and the desire to expedite resolution. Approximately one-third prefer surgical management, which would allow for genetic testing of the embryo/fetus to potentially determine a cause of the pregnancy loss.”
As for the medications used to treat early pregnancy loss, many ART physicians “treat pregnancy loss with misoprostol both pre- and post Dobbs,” he said. “The difficulty in obtaining mifepristone remains.”
The study authors and Dr. Trolice report no disclosures.
FROM ASRM 2022
HPV-positive women who undergo IVF don’t have worse outcomes
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
A new study provides more evidence that HPV infection doesn’t raise the risk of poor outcomes in women who undergo fertility treatment via in vitro fertilization with fresh embryos. In fact, HPV-positive women were somewhat more likely than HPV-negative women to become pregnant (relative risk, 1.20; 95% confidence interval, 1.03-1.39) and have live births (RR, 1.39; 95% CI, 1.13-1.70), researchers reported Oct. 24 at the American Society for Reproductive Medicine’s 2022 meeting .
“This evidence should reassure women that being HPV positive will not affect live birth rates after a fresh embryo transfer cycle,” said study coauthor and ob.gyn. Nina Vyas, MD, a clinical fellow at Weill Cornell Medicine, New York, in an interview.
According to Dr. Vyas, previous studies have offered conflicting results about whether HPV affects pregnancy outcomes. In 2006, for example, her group performed a pilot study (Fertil Steril. Jun 16. doi: 10.1016/j.fertnstert.2006.01.051) that linked lower pregnancy rates to HPV-positive tests on the day of egg retrieval.
“We sought to reevaluate this finding in a retrospective manner,” Dr. Vyas said. “You’re taking eggs out of their home, injecting with sperm, and putting them back. There’s so much that we don’t know, and we want to make sure there’s no extra risk.”
Also, she added, “prior studies had a relatively low sample size. We sought to use our patient volume to address this question on a larger scale. Our current study benefits from a large sample size and using the clinically meaningful endpoint of live birth as our primary outcome.”
For the new study, researchers retrospectively analyzed 1,333 patients (of 2,209 screened) who received first fresh embryo transfers from 2017 to 2019. All had cytology or HPV status documented per cervical cancer screening guidelines within 6 months before embryos were transferred.
The researchers looked at only fresh embryo transfers “so we could account for pregnancy outcomes closest to the documented HPV status at the time of egg retrieval,” Dr. Vyas said.
Ten percent (133) of patients were HPV positive. Of those, 60.1% became pregnant, and 43.6% of them had live births. Of the HPV-negative women (90% of subjects, n = 1,200), 52.2% became pregnant and 33.5% had live births. The researchers didn’t calculate P values, but Dr. Vyas said an analysis determined that the differences between HPV-positive and HPV-negative women were statistically significant.
The study size doesn’t allow researchers to determine whether HPV actually has a protective effect on pregnancy/live birth rates in IVF, Dr. Vyas said. Even if it did, the virus is dangerous.
What else could explain the discrepancy? “Some elements driving this could the smaller sample size of the HPV-positive group, differences in HPV prevalence between the general population and our population,” she said, “or other confounding factors we were not able to appreciate due to the limitations of the retrospective study.”
Researchers also reported that they found “no significant difference in biochemical or spontaneous abortion rates” between HPV-positive and HPV-negative women.
What is the message of the study? “Women with HPV can rest assured that they won’t have worse outcomes than their non-HPV [infected] counterparts after a fresh embryo transfer cycle,” Dr. Vyas said.
In an interview, McGill University, Montreal, epidemiologist Helen Trottier, PhD, MSc, noted that she recently coauthored a study that linked persistent HPV infection in pregnancy to premature births. The findings appear convincing, she said: “I think we can say that HPV is associated with preterm birth.”
She praised the new study but noted “the relative risks that are reported need to be adjusted for race and possibly other factors.”
Dr. Vyas said that kind of adjustment will occur in a future study that’s in progress. “We are now prospectively enrolling patients and collecting cytology data to understand whether there might be a difference for women with higher malignancy potential/different types of HPV genotypes.”
The study authors have no disclosures. Disclosure information for Dr. Trottier was unavailable.
FROM ASRM 2022
Decoding mechanisms of diabetic embryopathy suggests therapeutic targets
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Before the introduction of insulin, there were few reported cases of pregnancy complicated by diabetes because women with the disease too often did not live to childbearing age, and when they did, they were often counseled to terminate their pregnancies. Perinatal and maternal mortality in the limited number of reported pregnancies were 70% and 40%, respectively,1 making the risks of continuing the pregnancy quite high.
After insulin became available, maternal mortality dropped dramatically, down to a few percent. Perinatal mortality also declined, but it took several decades to achieve a similar magnitude of reduction.2 Today, with insulin therapy and tight glucose control as well as improved perinatal care, almost all women with diabetes can contemplate pregnancy with greater hope for normal outcomes.
Problems persist, however. Maternal diabetes continues to cause a variety of adverse outcomes, including infants large for gestational age, prematurity, and structural birth defects. Birth defects and prematurity, in fact, are the top causes of the unacceptably high infant mortality rate in the United States – a rate that is about 70% higher than the average in comparable developed countries.3
Infant mortality is considered an indicator of population health and of the development of a country; to reduce its rate, we must address these two areas.
Women with type 1 and type 2 diabetes are five times more likely to have a child with birth defects than are nondiabetic women.4 Up to 10% of women with preexisting diabetes will have fetuses with a major congenital malformation.5
Over the years we have been striving in our Center for Birth Defects Research to understand the pathomechanisms and the molecular and epigenetic alterations behind the high rates of birth defects in the offspring of women with preexisting diabetes. We have focused on heart defects and neural tube defects (particularly the latter), which together cause significant mortality, morbidity, disability, and human suffering.
Using animal models that mimic human diabetic pregnancy, we have made significant strides in our understanding of the mechanisms, uncovering molecular pathways involving oxidative stress, senescence/premature cellular aging, and epigenetic modifications (Figure 1). Understanding these pathways is providing us, in turn, with potential therapeutic targets and approaches that may be used in the future to prevent birth defects in women who enter pregnancy with type 1 or type 2 diabetes.
Unraveling the role of oxidative stress
Our mouse models accurately reflect the human conditions of diabetes in pregnancy and diabetic embryopathy. Offspring of mice with type 1 and type 2 diabetes have a similarly higher rate of neural tube defects and congenital heart disease, compared to mice without diabetes. We observe a similar incidence of anencephaly and spina bifida, and of cardiac septation defects in the mouse embryo hearts, for instance.
A primary mechanism and causal event of diabetic embryopathy is hyperglycemia-induced apoptosis in embryonic cells. Excessive cell death in the neural epithelium or in the developing heart leads to abnormal organogenesis and dysfunctional developmental events that cause birth defects. We have identified pathways leading to apoptosis, and have found that many of these pathways crosstalk with each other.
Hyperglycemia induces oxidative stress – one of these pathways – by causing sustained generation of reactive oxygen species. The cells’ mitochondrial function is significantly impaired by the hyperglycemia response, and this diabetes-induced mitochondrial dysfunction further increases the production of reactive oxygen species and a weakening of the endogenous cellular antioxidant systems, both of which then exacerbate oxidative stress.
Our research has detailed what happens downstream. We’ve learned that oxidative stress in embryos exposed to maternal diabetes activates a cascade of proapoptotic kinase signaling molecules – for example, protein kinase C isoforms such as PKCalpha; apoptosis signal-regulating kinase 1; and c-Jun-N-terminal kinases – that ultimately lead to abnormal cell death in the neuroepithelium before neural tube closure (Figure 2).5
Hyperglycemia also alters membrane biochemistry in the developing embryo, suppressing lipids including arachidonic acid and myoinositol, and induces the elevation of other molecules that cause newly synthesized proteins to be misfolded. A build-up of misfolded/unfolded proteins triggers or exacerbates endoplasmic reticulum stress, which, like oxidative stress, plays a role in the activation of proapoptotic kinase signaling and apoptosis.6
When we’ve deleted genes for some of the proapoptotic kinase–signaling intermediates, or otherwise inhibited oxidative and endoplasmic reticulum stresses, we’ve been able to ameliorate neural cell apoptosis and the formation of neural tube defects. Studying the processes both forward and backward gives us confidence that the pathways are real and important, and that altering the pathways can alter the outcomes.
Reduced autophagy and induction of cellular senescence
Just as mitochondria are negatively affected by hyperglycemic conditions, so are autophagosomes – organelles that play a key role in removing abnormal or damaged stem cells and cellular components (including unfolded protein aggregates) and in maintaining cellular homeostasis. A high level of autophagy is essential for neural tube closure as well as cardiac morphogenesis.
In our models, maternal diabetes significantly suppressed the process of autophagy in neuroepithelial cells. We have identified responsible molecular intermediates and a key regulating gene for autophagy impairment and have found that deletion of the gene restores autophagy and reduces the development of neural tube defects.4 Administration of a naturally occurring compound, trehalose, which reactivates autophagy, had a similar effect.7Exposure to hyperglycemia not only causes cell death and suppresses autophagy, it also impairs other aspects of cellular function. More recently, we have shown that cells in the neuroepithelium become quiescent and cease proliferating. The quiescent cells, those cells with premature aging markers, also produce cytokines that influence the functioning and development of neighboring cells, causing additional cell death.
All told, premature senescence in the neuroepithelium adversely affects the neurulation process, leading to neural tube defects. In our mouse model, the senomorphic agent rapamycin suppressed cellular senescence, reduced the number of apoptotic neuroepithelial cells, and reduced the formation of neural tube defects.8
The role of epigenetics, future interventions
Epigenetics – the process by which gene expression and function can be modified by environmental conditions without modification of the DNA sequence – has become an additional area of focus in diabetic embryopathy. Our lab has studied the overexpression of both DNA methyltransferases (DNMTs) that cause DNA hypermethylation, and of microRNAs (miRNAs) that can suppress gene expression at the posttranscriptional level. Both are considered to be primary epigenetic mechanisms involved in human diseases and it appears that they are influential in the incidence of birth defects in diabetic mothers.
In our mouse models, maternal diabetes induces DNA hypermethylation via the increase of DNMTs, leading to the silencing of genes essential for neural tube closure and formation of the developing heart. MiRNAs also play a role; in addition to finding altered DNMT activity in the neural epithelium and other tissues of diabetes-exposed embryos, we also found altered miRNA expression. By deleting miRNA genes or by inhibiting DNMT activity through treatment with antioxidants, we saw significant reductions in birth defects.
In one study of the green tea polyphenol epigallocatechin gallate (EGCG), we demonstrated inhibition of diabetes-elevated DNMT expression and activity and suppression of DNA hypermethylation. The expression of genes essential for neural tube closure was restored, with a subsequent reduction in neural tube defects from 29.5% to 2% in embryos treated with EGCG.9
Our interventions to reverse or alter the mechanisms and pathways leading to birth defects have not only helped prove causation, but have given us hope for the future. Antioxidants are among the compounds that could be used as dietary supplements during pregnancy to prevent structural birth defects (Figure 3). Other compounds could activate the process of autophagy (for example, trehalose) and antisenescence compounds similar to rapamycin could be used to reduce numbers of senescent cells in the neuroepithelium or the developing heart.
Dr. Reece and Dr. Yang reported no relevant disclosures.
Dr. Reece, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president, endowed professor and director of CARTI, and codirector of the Center for Birth Defects.
*This story was updated on Nov. 3, 2022
References
1. Z Zhiyong and Reece EA. Clin Lab Med. 2013;33(2)207-33.
2. Reece EA and Coustan DR. Diabetes and obesity in women. Wolters Kluwer: 2019. 4th ed. (https://www.amazon.com/Diabetes-Obesity-Women-Albert-Reece/dp/1496390547).
3. The Peterson-KFF Health System Tracker. www.healthsystemtracker.org.
4. Wang F et al. Nat. Commun. 2017;8:15182.
5. Yang P et al. Am J Obstet Gynecol. 2015;212(5):569-79.
6. Li X et al. Diabetes. 2013 Feb;62(2):599-608.
7. Xu C et al. Am J Physiol Endocrinol Metab. 2013 Sep 1;305(5):E667-78.
8. Xu C et al. Sci Adv. 2021;7(27):eabf5089.
9. Zhong J et al. Am J Obstet Gynecol. 2016 Sep;215(3):368.e1-10.
Discoveries in diabetic embryogenesis
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.
Many issues surrounding pregnancy care of women with preexisting diabetes remain challenging, especially in light of the relentless increase in maternal morbidity and mortality in the United States and globally. Rising rates of death and severe morbidity in diabetic women have continued despite significant advances in insulin pharmacology and administration technology.
However, despite these advances in glucose monitoring and insulin administration, fetal mortality and childhood morbidity rates continue to climb. This is because critical fetal structural anomalies arise from developmental errors occurring in the embryonic period – between 2 and 13 weeks of gestation – a time when most women with preexisting diabetes are just entering into prenatal care, often with suboptimal glycemic control.
Thus, significant future progress in reducing fetal mortality and childhood disability in infants of diabetic mothers will depend upon effective interventions in the first trimester while embryogenesis and critical organ formation are underway.
In this issue of Ob.Gyn. News, the editor of Master Class in Obstetrics, E. Albert Reece MD, PhD, MBA, steps into the role of coauthor. He and his research colleague Peixin Yang, PhD, present exciting insights into the cellular mechanisms underlying structural birth defects in infants of diabetic mothers – especially cardiac and neural tube defects – and also provide a glimpse into some potentially effective maternal pharmacologic interventions. After appropriate human trials, these interventions could be effectively applied from the time of a positive pregnancy test with potentially dramatic results.
Dr. Reece and Dr. Yang, who lead the Center for the Study of Birth Defects at the University of Maryland School of Medicine, share their impressive accumulation of data from embryos of pregnant diabetic rodents. They demonstrate convincingly that, in first-trimester rodent embryos, maternal hyperglycemia induces excessive apoptosis, which in turn leads to structural defects in critical fetal organs. They further found that maternal hyperglycemia reduces embryonic autophagosomes – the developmentally essential organelles that remove abnormal or damaged cells during embryo formation.
These investigators also identified reactivators of these organelles which, when administered maternally in the first trimester, significantly reduced the incidence of neural tube defects. Thus, for optimal development of diabetes-affected embryos, first-trimester administration of reactivators of autophagy could offer a significant, life-changing intervention in the foreseeable future.
Dr. Moore is professor emeritus of maternal-fetal medicine and chair emeritus in the department of obstetrics, gynecology, and reproductive sciences at UC San Diego Health. He reported no disclosures.
*This story was updated on Nov. 3, 2022.