User login
Higher rates of group B strep disease found in Black and Asian newborns
Health charities called for action to address racial health disparities after population-wide analysis by the UK Health Security Agency found that Black and Asian neonates had a significantly higher risk of early-onset group B streptococcal disease (GBS), compared with White infants.
One support group said more research was now needed to identify the cause of the disparity, and called for pregnant women to be better informed about the disease and what it could mean for them and their baby.
The study, published in Pediatrics, used UKHSA data on laboratory-confirmed infant group B streptococcal (iGBS) disease cases between Jan. 1, 2016, and Dec. 31, 2020, and were linked to hospital ethnicity records.
Cases of iGBS were defined as isolation of Streptococcus agalactiae from a normally sterile site at 0-6 days of life for early-onset iGBS and 7-90 days for late-onset disease.
Hospital data and parent-reported ethnicity
Researchers found 2,512 iGBS cases in England during the study period, 65.3% were early onset and 34.8% late onset, equivalent to 0.52 and 0.28 cases per 1000 live births respectively.
Researchers were able to link 85.6% of those to ethnicity. Among those 2,149 cases, Black infants had a 48% higher risk, and Asian infants a 40% higher risk of early onset iGBS, compared with White infants. Among those from an Asian background, the risk was 87% higher for Bangladeshi and 38% higher for Pakistani neonates.
Rates of early onset iGBS per 1,000 live births were 0.43 for White infants, 0.63 for Black infants, and 0.60 for those of Asian ethnicity.
In contrast, Indian infants had an early-onset rate of 0.47 per 1,000 live births, which was similar to White infants.
Black infants had 57% higher rates of late-onset iGBS (0.37) than White infants (0.24), the researchers reported.
The study authors highlighted previous research which found higher prevalence of group B streptococcal colonization in mothers from Black and some Asian ethnic groups, but lower prevalence in mothers from the Indian subcontinent. More research was needed to establish causes, the researchers said, including whether higher preterm birth rates in minority ethnic groups led to increased iGBS risk in neonates, or whether maternal group B streptococcal disease led to higher preterm birth rates and subsequent neonatal iGBS.
The researchers concluded: “Understanding the factors underpinning differences in rates of early-onset iGBS within south Asian groups in England may lead to new opportunities for prevention such as prioritized antenatal screening. Strategies to prevent neonatal iGBS must be tailored from high-quality quantitative and qualitative data to reach all women and protect all infants, irrespective of racial or ethnic background.”
‘Shocking but not surprising’
Commenting on the study, Edward Morris, president of the Royal College of Obstetricians and Gynaecologists, said: “This research is striking reading, and is yet another example of how far we have to go to tackle health inequalities within women’s health care.”
Philip Steer, professor emeritus at Imperial College London, said that the results were “consistent with previous reports of higher GBS carriage and higher maternal and neonatal mortality rates in minority groups” and “emphasize the importance of studying not just whether, but why, these differences exist.” He added: “We need to understand the reasons for the differences before we can design much-needed intervention to eliminate them.”
Jane Plumb, chief executive of Group B Strep Support, called the findings “shocking, but unfortunately not surprising” and said that they offered “another example of racial disparities in maternal and neonatal health.” She said: “We’re calling for all pregnant women and birthing people to be informed about GBS and its risks, so they can make empowered choices for themselves and their baby. It is also critical that trusts sign up to take part in the internationally significant [National Institute for Health and Care Research]–funded GBS3 clinical trial, designed to improve the prevention of GBS infection.”
Baroness Shaista Gohir, chief executive of the Muslim Women’s Network, said: “With significantly higher rates of group B Strep infection in Black and Asian babies, greater efforts must be made to improve awareness among pregnant women within these communities.”
A version of this article first appeared on Medscape UK.
Health charities called for action to address racial health disparities after population-wide analysis by the UK Health Security Agency found that Black and Asian neonates had a significantly higher risk of early-onset group B streptococcal disease (GBS), compared with White infants.
One support group said more research was now needed to identify the cause of the disparity, and called for pregnant women to be better informed about the disease and what it could mean for them and their baby.
The study, published in Pediatrics, used UKHSA data on laboratory-confirmed infant group B streptococcal (iGBS) disease cases between Jan. 1, 2016, and Dec. 31, 2020, and were linked to hospital ethnicity records.
Cases of iGBS were defined as isolation of Streptococcus agalactiae from a normally sterile site at 0-6 days of life for early-onset iGBS and 7-90 days for late-onset disease.
Hospital data and parent-reported ethnicity
Researchers found 2,512 iGBS cases in England during the study period, 65.3% were early onset and 34.8% late onset, equivalent to 0.52 and 0.28 cases per 1000 live births respectively.
Researchers were able to link 85.6% of those to ethnicity. Among those 2,149 cases, Black infants had a 48% higher risk, and Asian infants a 40% higher risk of early onset iGBS, compared with White infants. Among those from an Asian background, the risk was 87% higher for Bangladeshi and 38% higher for Pakistani neonates.
Rates of early onset iGBS per 1,000 live births were 0.43 for White infants, 0.63 for Black infants, and 0.60 for those of Asian ethnicity.
In contrast, Indian infants had an early-onset rate of 0.47 per 1,000 live births, which was similar to White infants.
Black infants had 57% higher rates of late-onset iGBS (0.37) than White infants (0.24), the researchers reported.
The study authors highlighted previous research which found higher prevalence of group B streptococcal colonization in mothers from Black and some Asian ethnic groups, but lower prevalence in mothers from the Indian subcontinent. More research was needed to establish causes, the researchers said, including whether higher preterm birth rates in minority ethnic groups led to increased iGBS risk in neonates, or whether maternal group B streptococcal disease led to higher preterm birth rates and subsequent neonatal iGBS.
The researchers concluded: “Understanding the factors underpinning differences in rates of early-onset iGBS within south Asian groups in England may lead to new opportunities for prevention such as prioritized antenatal screening. Strategies to prevent neonatal iGBS must be tailored from high-quality quantitative and qualitative data to reach all women and protect all infants, irrespective of racial or ethnic background.”
‘Shocking but not surprising’
Commenting on the study, Edward Morris, president of the Royal College of Obstetricians and Gynaecologists, said: “This research is striking reading, and is yet another example of how far we have to go to tackle health inequalities within women’s health care.”
Philip Steer, professor emeritus at Imperial College London, said that the results were “consistent with previous reports of higher GBS carriage and higher maternal and neonatal mortality rates in minority groups” and “emphasize the importance of studying not just whether, but why, these differences exist.” He added: “We need to understand the reasons for the differences before we can design much-needed intervention to eliminate them.”
Jane Plumb, chief executive of Group B Strep Support, called the findings “shocking, but unfortunately not surprising” and said that they offered “another example of racial disparities in maternal and neonatal health.” She said: “We’re calling for all pregnant women and birthing people to be informed about GBS and its risks, so they can make empowered choices for themselves and their baby. It is also critical that trusts sign up to take part in the internationally significant [National Institute for Health and Care Research]–funded GBS3 clinical trial, designed to improve the prevention of GBS infection.”
Baroness Shaista Gohir, chief executive of the Muslim Women’s Network, said: “With significantly higher rates of group B Strep infection in Black and Asian babies, greater efforts must be made to improve awareness among pregnant women within these communities.”
A version of this article first appeared on Medscape UK.
Health charities called for action to address racial health disparities after population-wide analysis by the UK Health Security Agency found that Black and Asian neonates had a significantly higher risk of early-onset group B streptococcal disease (GBS), compared with White infants.
One support group said more research was now needed to identify the cause of the disparity, and called for pregnant women to be better informed about the disease and what it could mean for them and their baby.
The study, published in Pediatrics, used UKHSA data on laboratory-confirmed infant group B streptococcal (iGBS) disease cases between Jan. 1, 2016, and Dec. 31, 2020, and were linked to hospital ethnicity records.
Cases of iGBS were defined as isolation of Streptococcus agalactiae from a normally sterile site at 0-6 days of life for early-onset iGBS and 7-90 days for late-onset disease.
Hospital data and parent-reported ethnicity
Researchers found 2,512 iGBS cases in England during the study period, 65.3% were early onset and 34.8% late onset, equivalent to 0.52 and 0.28 cases per 1000 live births respectively.
Researchers were able to link 85.6% of those to ethnicity. Among those 2,149 cases, Black infants had a 48% higher risk, and Asian infants a 40% higher risk of early onset iGBS, compared with White infants. Among those from an Asian background, the risk was 87% higher for Bangladeshi and 38% higher for Pakistani neonates.
Rates of early onset iGBS per 1,000 live births were 0.43 for White infants, 0.63 for Black infants, and 0.60 for those of Asian ethnicity.
In contrast, Indian infants had an early-onset rate of 0.47 per 1,000 live births, which was similar to White infants.
Black infants had 57% higher rates of late-onset iGBS (0.37) than White infants (0.24), the researchers reported.
The study authors highlighted previous research which found higher prevalence of group B streptococcal colonization in mothers from Black and some Asian ethnic groups, but lower prevalence in mothers from the Indian subcontinent. More research was needed to establish causes, the researchers said, including whether higher preterm birth rates in minority ethnic groups led to increased iGBS risk in neonates, or whether maternal group B streptococcal disease led to higher preterm birth rates and subsequent neonatal iGBS.
The researchers concluded: “Understanding the factors underpinning differences in rates of early-onset iGBS within south Asian groups in England may lead to new opportunities for prevention such as prioritized antenatal screening. Strategies to prevent neonatal iGBS must be tailored from high-quality quantitative and qualitative data to reach all women and protect all infants, irrespective of racial or ethnic background.”
‘Shocking but not surprising’
Commenting on the study, Edward Morris, president of the Royal College of Obstetricians and Gynaecologists, said: “This research is striking reading, and is yet another example of how far we have to go to tackle health inequalities within women’s health care.”
Philip Steer, professor emeritus at Imperial College London, said that the results were “consistent with previous reports of higher GBS carriage and higher maternal and neonatal mortality rates in minority groups” and “emphasize the importance of studying not just whether, but why, these differences exist.” He added: “We need to understand the reasons for the differences before we can design much-needed intervention to eliminate them.”
Jane Plumb, chief executive of Group B Strep Support, called the findings “shocking, but unfortunately not surprising” and said that they offered “another example of racial disparities in maternal and neonatal health.” She said: “We’re calling for all pregnant women and birthing people to be informed about GBS and its risks, so they can make empowered choices for themselves and their baby. It is also critical that trusts sign up to take part in the internationally significant [National Institute for Health and Care Research]–funded GBS3 clinical trial, designed to improve the prevention of GBS infection.”
Baroness Shaista Gohir, chief executive of the Muslim Women’s Network, said: “With significantly higher rates of group B Strep infection in Black and Asian babies, greater efforts must be made to improve awareness among pregnant women within these communities.”
A version of this article first appeared on Medscape UK.
FROM PEDIATRICS
Indiana’s new abortion ban may drive some young ob.gyns. to leave a state where they’re needed
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
On a Monday morning, a group of obstetrics and gynecology residents, dressed in blue scrubs and white coats, gathered in an auditorium at Indiana University, Indianapolis. After the usual updates and announcements, Nicole Scott, MD, the residency program director, addressed the elephant in the room. “Any more abortion care questions?” she asked the trainees.
After a few moments of silence, one resident asked: “How’s Dr. Bernard doing?”
“Bernard is actually in really good spirits – I mean, relatively,” Dr. Scott answered. “She has 24/7 security, has her own lawyer.”
They were talking about Caitlin Bernard, MD, an Indiana ob.gyn. who provides abortions and trains residents at the university hospital. Dr. Bernard was recently caught in a political whirlwind after she spoke about an abortion she provided to a 10-year-old rape victim from Ohio. Dr. Bernard was the target of false accusations made on national television by pundits and political leaders, including Indiana’s attorney general.
The doctors interviewed for this article said that they are not speaking on behalf of their school of medicine but rather about their personal experiences during a tumultuous moment that they worry will affect the way they care for their patients.
The vitriol directed at Dr. Bernard hit home for this group of residents. She has mentored most of them for years. Many of the young doctors were certain they wanted to practice in Indiana after their training. But lately, some have been ambivalent about that prospect.
Beatrice Soderholm, DO, a fourth-year ob.gyn. resident, said watching what Dr. Bernard went through was “scary.” “I think that was part of the point for those who were putting her through that,” Dr. Soderholm said. They were trying “to scare other people out of doing the work that she does.”
In early August, Gov. Eric Holcomb, a Republican, signed a near-total abortion ban into law, making Indiana the first state to adopt new restrictions on abortion access since the Supreme Court struck down Roe v. Wade in June. When the ban takes effect Sept. 15, medical providers who violate the law risk losing their licenses or serving up to six years in prison.
These days, Dr. Scott, the residency program director, uses some meeting time with residents to fill them in on political updates and available mental health services. She also reminds them that legal counsel is on call round the clock to help if they’re ever unsure about the care they should provide a patient.
“Our residents are devastated,” Dr. Scott said, holding back tears. “They signed up to provide comprehensive health care to women, and they are being told that they can’t do that.”
She expects this will “deeply impact” how Indiana hospitals recruit and retain medical professionals.
A 2018 report from the March of Dimes found that 27% of Indiana counties are considered maternity care deserts, with no or limited access to maternal care. The state has one of the nation’s highest maternal mortality rates.
Dr. Scott said new laws restricting abortion will only worsen those statistics.
Dr. Scott shared results from a recent survey of nearly 1,400 residents and fellows across all specialties at IU, nearly 80% of the trainees said they were less likely to stay and practice in Indiana after the abortion ban.
Wendy Tian, MD, a third-year resident, said she is worried about her safety. Dr. Tian grew up and went to medical school in Chicago and chose to do her residency in Indiana because the program has a strong family-planning focus. She was open to practicing in Indiana when she completed her training.
But that’s changed.
“I, for sure, don’t know if I would be able to stay in Indiana post graduation with what’s going on,” Dr. Tian said.
Still, she feels guilty for “giving up” on Indiana’s most vulnerable patients.
Even before Roe fell, Dr. Tian said, the climate in Indiana could be hostile and frustrating for ob.gyns. Indiana, like other states with abortion restrictions, allows nearly all health care providers to opt out of providing care to patients having an abortion.
“We encounter other people who we work with on a daily basis who are opposed to what we do,” Dr. Tian said, adding that she and her colleagues have had to cancel scheduled procedures because the nurses on call were not comfortable assisting during an abortion.
Dr. Scott said the ob.gyn. program at the IU has provided residents with comprehensive training, including on abortion care and family planning. Since miscarriages are managed the same way as first-trimester abortions, she said, the training gives residents lots of hands-on experience. “What termination procedures allow you to do is that kind of repetition and that understanding of the female anatomy and how to manage complications that may happen with miscarriages.”
The ban on abortions dramatically reduces the hands-on opportunities for ob.gyn. residents, and that’s a huge concern, she said.
The program is exploring ways to offer training. One option is to send residents to learn in states without abortion restrictions, but Dr. Scott said that would be a logistical nightmare. “This is not as simple as just showing up to an office and saying: ‘Can I observe?’ This includes getting a medical license for out-of-state trainees. This includes funding for travel and lodging,” Dr. Scott said. “It adds a lot to what we already do to educate future ob.gyns.”
Four in 10 of all ob.gyn. residents in the United States are in states where abortion is banned or likely to be banned, so there could be a surge of residents looking to go out of state to make up for lost training opportunities. The Accreditation Council for Graduate Medical Education, the body that accredits residency programs, proposed modifications to the graduation requirements for ob.gyn. residents to account for the changing landscape.
For some of the Indiana ob.gyn. residents – including Veronica Santana, MD, a first-year resident – these political hurdles are a challenge they’re more than willing to take on. Dr. Santana is Latina, grew up in Seattle, and has been involved in community organizing since she was a teenager. One reason she chose obstetrics and gynecology was because of how the field intersects with social justice. “It’s political. It always has been, and it continues to be,” she said, “And, obviously, especially now.”
After Roe was overturned, Dr. Santana, alongside other residents and mentors, took to the streets of Indianapolis to participate in rallies in support of abortion rights.
Indiana could be the perfect battleground for Dr. Santana’s advocacy and social activism. But lately, she said, she is “very unsure” whether staying in Indiana to practice after residency makes sense, since she wants to provide the entire range of ob.gyn. services.
Dr. Soderholm, who grew up in Minnesota, has felt a strong connection to patients at the county hospital in Indianapolis. She had been certain she wanted to practice in Indiana. But her family in Minnesota – where abortion remains largely protected – has recently questioned why she would stay in a state with such a hostile climate for ob.gyns. “There’s been a lot of hesitation,” she said. But the patients make leaving difficult. “Sorry,” she said, starting to cry.
It’s for those patients that Dr. Soderholm decided she’ll likely stay. Other young doctors may make a different decision.
This story is part of a partnership that includes Side Effects Public Media, NPR, and KHN. KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Large study amplifies evidence of COVID vaccine safety in pregnancy
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
The research team wrote in the BMJ that their reassuring findings – drawn from a registry of all births in Ontario over an 8-month period – “can inform evidence-based decision-making” about COVID vaccination during pregnancy.
Previous research has found that pregnant patients are at higher risk of severe complications and death if they become infected with COVID and that vaccination before or during pregnancy prevents such outcomes and reduces the risk of newborn infection, noted Jeffrey Ecker, chief of obstetrics and gynecology at Massachusetts General Hospital, Boston.
This new study “adds to a growing body of information arguing clearly and reassuringly that vaccination during pregnancy is not associated with complications during pregnancy,” said Dr. Ecker, who was not involved in the new study.
He added that it “should help obstetric providers further reassure those who are hesitant that vaccination is safe and best both for the pregnant patient and their pregnancy.”
Methods and results
For the new study, researchers tapped a provincial registry of all live and stillborn infants with a gestational age of at least 20 weeks or birth weight of at least 500 g. Unique health card numbers were used to link birth records to a database of COVID vaccinations.
Of 85,162 infants born from May through December of 2021, 43,099 (50.6%) were born to individuals who received at least one vaccine dose during pregnancy. Among those, 99.7% received an mRNA vaccine such as Pfizer-BioNTech or Moderna.
Vaccination during pregnancy was not associated with greater risk of overall preterm birth (6.5% among vaccinated individuals versus 6.9% among unvaccinated; hazard ratio, 1.02; 95% confidence interval, 0.96-1.08), spontaneous preterm birth (3.7% versus 4.4%; hazard ratio, 0.96; 95% CI, 0.90-1.03) or very preterm birth (0.59% versus 0.89%; hazard ratio, 0.80; 95% CI, 0.67-0.95).
Likewise, no increase was observed in the risk of an infant being small for gestational age at birth (9.1% versus 9.2%; hazard ratio, 0.98; 95% CI, 0.93-1.03).
The researchers observed a reduction in the risk of stillbirth, even after adjusting for potential confounders. Stillbirths occurred in 0.25% of vaccinated individuals, compared with 0.44% of unvaccinated individuals (hazard ratio, 0.65; 95% CI, 0.51-0.84).
A reduced risk of stillbirth – albeit to a smaller degree – was also found in a Scandinavian registry study that included 28,506 babies born to individuals who were vaccinated during pregnancy.
“Collectively, the findings from these two studies are reassuring and are consistent with no increased risk of stillbirth after COVID-19 vaccination during pregnancy. In contrast, COVID-19 disease during pregnancy has been associated with an increased risk of stillbirth,” the researchers wrote.
Findings did not vary by which mRNA vaccine a mother received, the number of doses she received, or the trimester in which a vaccine was given, the researchers reported.
Stillbirth findings will be ‘very reassuring’ for patients
The lead investigator, Deshayne Fell, PhD, said in an interview, the fact that the study comprised the entire population of pregnant people in Ontario during the study period “increases our confidence” about the validity and relevance of the findings for other geographic settings.
Dr. Fell, an associate professor in epidemiology and public health at the University of Ottawa and a scientist at the Children’s Hospital of Eastern Ontario Research Institute, Ottawa, said the evaluation of stillbirth in particular, “a rare but devastating outcome,” will be “very reassuring and useful for clinical counseling.”
A limitation cited by the research team included a lack of data on vaccination prior to pregnancy.
In the new study, Dr, Ecker said, “Though the investigators were able to adjust for many variables they cannot be certain that some unmeasured variable that, accordingly, was not adjusted for does not hide a small risk. This seems very unlikely, however.”
The Canadian research team said similar studies of non-mRNA COVID vaccines “should be a research priority.” However, such studies are not underway in Canada, where only mRNA vaccines are used in pregnancy, Dr. Fell said.
This study was supported by the Public Health Agency of Canada.
Dr. Fell and Dr. Ecker reported no competing financial interests.
FROM BMJ
Postpartum psychosis: Does longitudinal course inform treatment?
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The last 15 years have brought increased effort to screen for postpartum psychiatric illness. That’s exceedingly welcome given the morbidity and potential mortality associated with postpartum psychiatric disorders across the country.
From small community hospitals to major academic centers, screening for postpartum depression is part of the clinical fabric of routine obstetrical care. There is a growing appreciation for the complexity of perinatal psychiatric illness, particularly with respect to the commingling of both mood and anxiety disorders during the postpartum period. However, willingness to treat and appreciation of the urgency to treat with both pharmacologic and nonpharmacologic interventions can vary. For women who suffer from postpartum depression and their families, there are real-world implications of both treating and failing to treat this illness, and there is an urgent need to really help these women “climb out of the darkness” that is and defines postpartum depression.
Less common but of great clinical importance is postpartum psychosis, which occurs in approximately 1 in 1,000-2,000 women based on estimates from several studies. As noted in previous columns, the presentation is a dramatic one, with the typical onset of psychotic symptoms in the first days to weeks post partum. The disorder typically has a mood component and is not an exacerbation of underlying chronic psychotic illness. While there have been few systematic treatment studies, the clinical consensus is treatment usually includes hospitalization to ensure the safety of both the patient and infant. Use of medications, including mood stabilizers, antipsychotics, and benzodiazepines may be appropriate when expeditious treatment is needed.
Appropriate treatment by informed clinical staff is essential, as untreated or incompletely treated postpartum psychosis with its attendant morbidity and potential mortality is a very real concern. As I speak with women across the country with histories of postpartum psychosis, I’m often told of the difficult exchanges that women and their partners have at EDs in various clinical settings where diagnosis was delayed, or treatment was incomplete because of staff without expertise in postpartum psychosis management.
Another dilemma that patients and clinicians face after acute treatment is treatment duration, which is derived from how we conceptualize the illness. Even for experts in the area, there is not a consensus on whether postpartum psychosis should be considered as bipolar disorder or whether it is a circumscribed diagnostic entity. This issue has been hotly debated for many years and is one of the reasons why the illness is not included in the DSM classification system.
At Massachusetts General Hospital, we are systematically studying a large cohort of women with histories of postpartum psychosis as part of the MGH Postpartum Psychosis Project to better understand the phenomenology of postpartum psychosis, and also to understand the possible genomic underpinning of the illness. Most recently, we are conducting a neuroimaging study of women with histories of postpartum psychosis, compared with women in a healthy control group. We hope the results of this novel investigation will help to answer whether there is a neural signature identifiable with neuroimaging techniques such as functional MRI, if those findings are similar to other findings of neural circuitry we see in other forms of psychotic illness, or if the illness has a more distinct neural signature.
A question patients and colleagues often ask is what is the long-term nature of postpartum psychosis. If one considers it clearly to be bipolar disorder, the most intuitive approach would be long-term treatment with mood stabilizers. We now have a growing amount of data on the longitudinal course of postpartum psychosis. In one meta-analysis, 64% of women who had an episode of postpartum psychosis developed episodes of recurrent psychiatric disorder mostly consistent with bipolar illness. However, 36% of women appear to have more circumscribed illness without recurrence. In those women with recurrent disease, the presumption was those patients who had bipolar disorder and their presentation postpartum was simply their index episode of bipolar illness. However, there were other women who looked as if they had developed subsequent illness over the 11-26 years of follow-up, and those women did not receive long-term treatment.
A more recent prospective study of 106 women with postpartum psychosis who had their medication tapered and discontinued showed that 32% of women went on to have recurrent disease with a median time to illness of 20.3 months, and those patients presented primarily with illness that looked like bipolar disorder.
These accumulating data support the impression we’ve had for years that there’s a very strong relationship between bipolar disorder and postpartum psychiatric illness. Regardless of what side of the debate you fall on, the acute treatment is really the same. The real question for the clinician is what to do over the long term. Frequently, patients feel very strongly about a taper and discontinuation of medicine, and even the data show between 30% and 45% of women seem to have relatively circumscribed disease. There may be an issue in terms of prophylaxis if a patient gets pregnant and delivers another child, but that’s a separate issue. The issue is really whether there is a way to “thread the clinical needle” and meet patients where they are who do not want to continue long-term treatment.
I think we are at a point where we could argue the clinical treatment algorithm for patients who present with a new-onset manic-like psychosis postpartum is clear: initial treatment to stabilize, and then treatment with mood stabilizers for at least 12 months to follow is indicated. However, it may also be reasonable to taper treatment at 12-18 months, particularly for patients who have discussed this option with their clinician and who have been totally well for a year. (Women with previously documented bipolar disorder who have episodes of postpartum psychosis should obviously be treated with longer-term treatment aimed at maintenance of euthymia, as discontinuation of mood stabilizer is well known to be associated with risk for relapse.)
It should be noted that the longitudinal course and the treatment implications for women with postpartum psychosis are not etched in stone absent a clear evidence base driving care guidelines. Treatment must still be individualized. Women with underlying mood diatheses will typically declare themselves over time, and others may do well if they discontinue treatment, particularly if they are followed closely and instructed to present to a clinician at the earliest symptoms of mood dysregulation. The good news is we’ve seen an evolution of both interest and expertise in acute management of postpartum psychosis and a richer appreciation of the potential heterogeneity of this sample of women. There may be some variability in terms of long-term course requiring personalized treatment and obviously close follow-up of these women.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
On the Wisconsin-Illinois border: Clinics in neighboring states team up on abortion care
WAUKEGAN, Ill. – Around 2 days a week, Natalee Hartwig, APNP, leaves her home in Madison, Wisconsin, before her son wakes up to travel across the border into Illinois.
“Luckily it’s summer,” said Ms. Hartwig, a nurse midwife at Planned Parenthood of Wisconsin. “For now, he can sleep in. But any getting ready that has to happen will be on my spouse.”
She drives at least 2 hours each way, immersed in audiobooks and podcasts as she heads back and forth from a clinic in this northern Illinois suburb. She spends her days in the recovery room, caring for patients who have had abortions and checking their vitals before they go home. She is also licensed in Illinois and trained to provide medication abortion, something she’ll be able to do virtually through telehealth with patients across Illinois.
Ms. Hartwig is essentially working part time in Illinois because when Roe v. Wade was overturned in June, a state law immediately took effect that bans nearly all abortions in Wisconsin, except to save the life of the pregnant person. Wisconsin providers want to preserve access for patients, while those in Illinois – long an oasis for abortion rights – need more staff to help treat a surge of people arriving from across the U.S.
The Waukegan clinic is Planned Parenthood of Illinois’ busiest for out-of-state abortion patients. After Roe fell, 60% of patients came to this clinic from outside the state – mostly from Wisconsin. In fact, the organization opened in Waukegan 2 years ago with Wisconsin in mind, knowing that if Roe v. Wade did fall, access to abortion in that state would greatly diminish.
After Roe was struck down, Planned Parenthood organizations in both states announced their partnership. More than a dozen employees from Wisconsin – including doctors, nurses, and medical assistants – now commute to Waukegan to help provide care.
“It really required this perfect pairing of supply and demand,” said Kristen Schultz, Planned Parenthood of Illinois’ chief strategy and operations officer. “They had capacity without local demand, and we had the opposite.”
In the month after the U.S. Supreme Court overturned the federal landmark decision, Illinois became even more of an oasis for people seeking abortions. Dozens of clinics closed across the nation as 11 states in the South and Midwest implemented bans, according to the Guttmacher Institute, a nonprofit that supports abortion rights and tracks the issue.
The influx of patients into Illinois has had another effect. For years, abortion providers have been traveling once or twice a month to other states like Kansas, Mississippi, and Oklahoma, where their help was badly needed.
Laura Laursen, MD, an ob.gyn. in Chicago, was one of them.
“Now the script is totally flipped,” said Dr. Laursen, a fellow with Physicians for Reproductive Health. “This is where you are needed more than anywhere else.”
Anti-abortion groups oppose the Planned Parenthood partnership and are preparing for a marathon effort to restrict abortion rights in Illinois. In a statement after the organization’s announcement, Amy Gehrke, executive director of Illinois Right to Life, called it “particularly tragic.”
Some of the Wisconsin providers commute to Waukegan a few times a week; others a few days a month.
For Ms. Hartwig, associate director of clinical services at Planned Parenthood of Wisconsin, she’s able to do more in Illinois for patients than she could back home. Even as a nurse with an advanced degree, she wasn’t allowed to provide medication abortions in Wisconsin. But she can in Illinois, according to the state Department of Financial and Professional Regulation.
“This was really just what I was always supposed to do,” Ms. Hartwig said. “There’s nothing that’s going to keep me from helping our patients.”
Kathy King, MD, Planned Parenthood of Wisconsin’s medical director, said that while her staff is dedicated to providing these services, it comes at a cost.
“It is a burden on our clinicians and nurses and medical assistants who have young children at home,” Dr. King said. “It sounds great. Sure, we’ll all just travel down to Waukegan 5 days a week. But the logistics of that and the sacrifice of doing that on just people’s day-to-day lives takes a toll.”
Still, this sacrifice has helped. With staff from Wisconsin, the Waukegan clinic has doubled the number of abortion appointments available, and it is still ramping up. The support frees up other staffers to treat patients with different needs, like birth control and cancer screenings.
There has been a surge of patients from Wisconsin for abortion appointments at all Planned Parenthood of Illinois clinics – a tenfold increase in the month after Roe was overturned, from about 35 patients a month to 350, Dr. King said. That doesn’t include Wisconsin residents who might have sought abortions with other providers.
The partnership at the Waukegan clinic has ignited interest from abortion providers in other nearby states. Planned Parenthood of Illinois is fielding calls from Indiana, Kentucky, and Ohio, for example, Ms. Schultz said.
What Illinois needs is more staff to treat more patients. The commute from Wisconsin to Waukegan is relatively short compared with abortion providers in Ohio, for example, who’d have to cross Indiana to help relieve the staffing need.
Across the nation, other conversations are happening among providers. The National Abortion Federation, which has about 500 facility members including independent abortion clinics and hospitals, is pairing up people looking for jobs at clinics with those that need workers, said Melissa Fowler, chief program officer at the federation.
Still, she acknowledged moving isn’t a realistic option for everyone.
“People have lives,” Ms. Fowler said. “They have families. They’re deeply rooted in their communities. ... And so a situation like you’re seeing in Illinois and Wisconsin is great because people are able to stay connected to their community, not have to move their family, and still be able to provide care.”
This story is part of a partnership that includes WBEZ Chicago, NPR, and KHN.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
WAUKEGAN, Ill. – Around 2 days a week, Natalee Hartwig, APNP, leaves her home in Madison, Wisconsin, before her son wakes up to travel across the border into Illinois.
“Luckily it’s summer,” said Ms. Hartwig, a nurse midwife at Planned Parenthood of Wisconsin. “For now, he can sleep in. But any getting ready that has to happen will be on my spouse.”
She drives at least 2 hours each way, immersed in audiobooks and podcasts as she heads back and forth from a clinic in this northern Illinois suburb. She spends her days in the recovery room, caring for patients who have had abortions and checking their vitals before they go home. She is also licensed in Illinois and trained to provide medication abortion, something she’ll be able to do virtually through telehealth with patients across Illinois.
Ms. Hartwig is essentially working part time in Illinois because when Roe v. Wade was overturned in June, a state law immediately took effect that bans nearly all abortions in Wisconsin, except to save the life of the pregnant person. Wisconsin providers want to preserve access for patients, while those in Illinois – long an oasis for abortion rights – need more staff to help treat a surge of people arriving from across the U.S.
The Waukegan clinic is Planned Parenthood of Illinois’ busiest for out-of-state abortion patients. After Roe fell, 60% of patients came to this clinic from outside the state – mostly from Wisconsin. In fact, the organization opened in Waukegan 2 years ago with Wisconsin in mind, knowing that if Roe v. Wade did fall, access to abortion in that state would greatly diminish.
After Roe was struck down, Planned Parenthood organizations in both states announced their partnership. More than a dozen employees from Wisconsin – including doctors, nurses, and medical assistants – now commute to Waukegan to help provide care.
“It really required this perfect pairing of supply and demand,” said Kristen Schultz, Planned Parenthood of Illinois’ chief strategy and operations officer. “They had capacity without local demand, and we had the opposite.”
In the month after the U.S. Supreme Court overturned the federal landmark decision, Illinois became even more of an oasis for people seeking abortions. Dozens of clinics closed across the nation as 11 states in the South and Midwest implemented bans, according to the Guttmacher Institute, a nonprofit that supports abortion rights and tracks the issue.
The influx of patients into Illinois has had another effect. For years, abortion providers have been traveling once or twice a month to other states like Kansas, Mississippi, and Oklahoma, where their help was badly needed.
Laura Laursen, MD, an ob.gyn. in Chicago, was one of them.
“Now the script is totally flipped,” said Dr. Laursen, a fellow with Physicians for Reproductive Health. “This is where you are needed more than anywhere else.”
Anti-abortion groups oppose the Planned Parenthood partnership and are preparing for a marathon effort to restrict abortion rights in Illinois. In a statement after the organization’s announcement, Amy Gehrke, executive director of Illinois Right to Life, called it “particularly tragic.”
Some of the Wisconsin providers commute to Waukegan a few times a week; others a few days a month.
For Ms. Hartwig, associate director of clinical services at Planned Parenthood of Wisconsin, she’s able to do more in Illinois for patients than she could back home. Even as a nurse with an advanced degree, she wasn’t allowed to provide medication abortions in Wisconsin. But she can in Illinois, according to the state Department of Financial and Professional Regulation.
“This was really just what I was always supposed to do,” Ms. Hartwig said. “There’s nothing that’s going to keep me from helping our patients.”
Kathy King, MD, Planned Parenthood of Wisconsin’s medical director, said that while her staff is dedicated to providing these services, it comes at a cost.
“It is a burden on our clinicians and nurses and medical assistants who have young children at home,” Dr. King said. “It sounds great. Sure, we’ll all just travel down to Waukegan 5 days a week. But the logistics of that and the sacrifice of doing that on just people’s day-to-day lives takes a toll.”
Still, this sacrifice has helped. With staff from Wisconsin, the Waukegan clinic has doubled the number of abortion appointments available, and it is still ramping up. The support frees up other staffers to treat patients with different needs, like birth control and cancer screenings.
There has been a surge of patients from Wisconsin for abortion appointments at all Planned Parenthood of Illinois clinics – a tenfold increase in the month after Roe was overturned, from about 35 patients a month to 350, Dr. King said. That doesn’t include Wisconsin residents who might have sought abortions with other providers.
The partnership at the Waukegan clinic has ignited interest from abortion providers in other nearby states. Planned Parenthood of Illinois is fielding calls from Indiana, Kentucky, and Ohio, for example, Ms. Schultz said.
What Illinois needs is more staff to treat more patients. The commute from Wisconsin to Waukegan is relatively short compared with abortion providers in Ohio, for example, who’d have to cross Indiana to help relieve the staffing need.
Across the nation, other conversations are happening among providers. The National Abortion Federation, which has about 500 facility members including independent abortion clinics and hospitals, is pairing up people looking for jobs at clinics with those that need workers, said Melissa Fowler, chief program officer at the federation.
Still, she acknowledged moving isn’t a realistic option for everyone.
“People have lives,” Ms. Fowler said. “They have families. They’re deeply rooted in their communities. ... And so a situation like you’re seeing in Illinois and Wisconsin is great because people are able to stay connected to their community, not have to move their family, and still be able to provide care.”
This story is part of a partnership that includes WBEZ Chicago, NPR, and KHN.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
WAUKEGAN, Ill. – Around 2 days a week, Natalee Hartwig, APNP, leaves her home in Madison, Wisconsin, before her son wakes up to travel across the border into Illinois.
“Luckily it’s summer,” said Ms. Hartwig, a nurse midwife at Planned Parenthood of Wisconsin. “For now, he can sleep in. But any getting ready that has to happen will be on my spouse.”
She drives at least 2 hours each way, immersed in audiobooks and podcasts as she heads back and forth from a clinic in this northern Illinois suburb. She spends her days in the recovery room, caring for patients who have had abortions and checking their vitals before they go home. She is also licensed in Illinois and trained to provide medication abortion, something she’ll be able to do virtually through telehealth with patients across Illinois.
Ms. Hartwig is essentially working part time in Illinois because when Roe v. Wade was overturned in June, a state law immediately took effect that bans nearly all abortions in Wisconsin, except to save the life of the pregnant person. Wisconsin providers want to preserve access for patients, while those in Illinois – long an oasis for abortion rights – need more staff to help treat a surge of people arriving from across the U.S.
The Waukegan clinic is Planned Parenthood of Illinois’ busiest for out-of-state abortion patients. After Roe fell, 60% of patients came to this clinic from outside the state – mostly from Wisconsin. In fact, the organization opened in Waukegan 2 years ago with Wisconsin in mind, knowing that if Roe v. Wade did fall, access to abortion in that state would greatly diminish.
After Roe was struck down, Planned Parenthood organizations in both states announced their partnership. More than a dozen employees from Wisconsin – including doctors, nurses, and medical assistants – now commute to Waukegan to help provide care.
“It really required this perfect pairing of supply and demand,” said Kristen Schultz, Planned Parenthood of Illinois’ chief strategy and operations officer. “They had capacity without local demand, and we had the opposite.”
In the month after the U.S. Supreme Court overturned the federal landmark decision, Illinois became even more of an oasis for people seeking abortions. Dozens of clinics closed across the nation as 11 states in the South and Midwest implemented bans, according to the Guttmacher Institute, a nonprofit that supports abortion rights and tracks the issue.
The influx of patients into Illinois has had another effect. For years, abortion providers have been traveling once or twice a month to other states like Kansas, Mississippi, and Oklahoma, where their help was badly needed.
Laura Laursen, MD, an ob.gyn. in Chicago, was one of them.
“Now the script is totally flipped,” said Dr. Laursen, a fellow with Physicians for Reproductive Health. “This is where you are needed more than anywhere else.”
Anti-abortion groups oppose the Planned Parenthood partnership and are preparing for a marathon effort to restrict abortion rights in Illinois. In a statement after the organization’s announcement, Amy Gehrke, executive director of Illinois Right to Life, called it “particularly tragic.”
Some of the Wisconsin providers commute to Waukegan a few times a week; others a few days a month.
For Ms. Hartwig, associate director of clinical services at Planned Parenthood of Wisconsin, she’s able to do more in Illinois for patients than she could back home. Even as a nurse with an advanced degree, she wasn’t allowed to provide medication abortions in Wisconsin. But she can in Illinois, according to the state Department of Financial and Professional Regulation.
“This was really just what I was always supposed to do,” Ms. Hartwig said. “There’s nothing that’s going to keep me from helping our patients.”
Kathy King, MD, Planned Parenthood of Wisconsin’s medical director, said that while her staff is dedicated to providing these services, it comes at a cost.
“It is a burden on our clinicians and nurses and medical assistants who have young children at home,” Dr. King said. “It sounds great. Sure, we’ll all just travel down to Waukegan 5 days a week. But the logistics of that and the sacrifice of doing that on just people’s day-to-day lives takes a toll.”
Still, this sacrifice has helped. With staff from Wisconsin, the Waukegan clinic has doubled the number of abortion appointments available, and it is still ramping up. The support frees up other staffers to treat patients with different needs, like birth control and cancer screenings.
There has been a surge of patients from Wisconsin for abortion appointments at all Planned Parenthood of Illinois clinics – a tenfold increase in the month after Roe was overturned, from about 35 patients a month to 350, Dr. King said. That doesn’t include Wisconsin residents who might have sought abortions with other providers.
The partnership at the Waukegan clinic has ignited interest from abortion providers in other nearby states. Planned Parenthood of Illinois is fielding calls from Indiana, Kentucky, and Ohio, for example, Ms. Schultz said.
What Illinois needs is more staff to treat more patients. The commute from Wisconsin to Waukegan is relatively short compared with abortion providers in Ohio, for example, who’d have to cross Indiana to help relieve the staffing need.
Across the nation, other conversations are happening among providers. The National Abortion Federation, which has about 500 facility members including independent abortion clinics and hospitals, is pairing up people looking for jobs at clinics with those that need workers, said Melissa Fowler, chief program officer at the federation.
Still, she acknowledged moving isn’t a realistic option for everyone.
“People have lives,” Ms. Fowler said. “They have families. They’re deeply rooted in their communities. ... And so a situation like you’re seeing in Illinois and Wisconsin is great because people are able to stay connected to their community, not have to move their family, and still be able to provide care.”
This story is part of a partnership that includes WBEZ Chicago, NPR, and KHN.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Pro-life ob.gyns. say Dobbs not end of abortion struggle
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
Does hidradenitis suppurativa worsen during pregnancy?
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
AT PDA 2022
Hyperthyroidism rebound in pregnancy boosts adverse outcomes
Discontinuing antithyroid drugs during early pregnancy is linked to a possible rebound of hyperthyroidism and a high risk of adverse pregnancy outcomes, new research shows.
“Our study provides preliminary evidence that the risk of rebound increases in women with subnormal thyroid-stimulating hormone (TSH) and/or positive thyrotropin receptor antibody (TRAb) who stop antithyroid drugs in early pregnancy,” first author Xin Hou told this news organization.
“When discussing the pros and cons of antithyroid drug withdrawal early in pregnancy [clinicians] should consider the level of TSH and TRAb in early pregnancy,” said Hou, of the department of endocrinology and metabolism, Institute of Endocrinology, The First Affiliated Hospital of China Medical University, Shenyang.
Suvi Turunen, MD, of the University of Oulu (Finland), who has also conducted research on the issue, said the study adds important insights.
“I find this study very interesting,” Dr. Turunen said in an interview. “It is well known that medical treatment of hyperthyroidism outweighs the potential harms of antithyroid treatment.”
The new findings add to the evidence, she added. “I think that withdrawal of antithyroid drugs should be carefully considered, especially with autoantibody-positive patients,” Dr. Turunen said.
Hyperthyroidism a risk in pregnancy – with or without treatment
The potential risks of hyperthyroidism in pregnancy are well established and can range from preeclampsia to premature birth or miscarriage.
However, antithyroid drugs, including methimazole and propylthiouracil, carry their own risks. In crossing the placental barrier, the drugs can increase the risk of birth defects, particularly during 6-10 weeks of gestation, yet their discontinuation is linked to as much as a 50%-60% risk of relapse, the authors explain.
Because of the risks, the American Thyroid Association recommends that “women with a stable euthyroid state on 5-10 mg methimazole per day achieved within a few months, and a falling TRAb level, are likely candidates to withdraw from antithyroid drug therapy in early pregnancy,” the authors noted.
However, as the recommendations for women who are already pregnant are largely based on evidence from nonpregnant patients, Hou and colleagues sought to evaluate withdrawal among women who were pregnant.
For the study, published in Thyroid, they enrolled 63 women who were pregnant and part of an outpatient service of the department of endocrinology and metabolism at The First Affiliated Hospital of China Medical University, between September 2014 and March 2017, who had well-controlled hyperthyroidism in early pregnancy and discontinued the drugs.
The women were an average age of 27 years, and 28 were multigravida. Twenty-two had a history of miscarriage.
A follow-up of the patients until the end of their pregnancy showed that, overall, 20 (31.7%) had a rebound of hyperthyroidism during their pregnancy after withdrawing from the drugs.
Key factors associated with the highest risk of a rebound after discontinuation included having subnormal TSH levels (TSH < 0.35 mIU/L; odds ratio, 5.12; P = .03) or having positive TRAb (TRAb > 1.75 IU/L; OR, 3.79; P = .02) at the time of medication withdrawal, compared with those with either normal TSH levels or negative TRAb.
The combination of both subnormal TSH and positive TRAb at the time of antithyroid medication withdrawal further boosted the risk of hyperthyroidism rebound (83.3%, 5 of 6), compared with those who had both normal TSH and negative TRAb (13%, 3 of 23; OR, 33.33; P = .003).
Adverse pregnancy outcomes increased
Importantly, among the 20 patients who had a rebound, 11 (55%) had adverse pregnancy outcomes, including miscarriage, premature birth, induced labor, gestational hypertension, and gestational diabetes, compared with only 4 (9.3%) of the 43 who had no rebound (OR, 11.92; P = .0002).
Neonatal abnormalities were also higher among those experiencing a rebound (20% vs. 4.7%), however, the authors noted that “larger prospective studies are required to conclude whether antithyroid drug withdrawal affects fetal outcome.”
In the rebound group, the mean duration of antithyroid medication use was 24.7 months versus 35.1 months in the nonrebound group, however, the difference was not statistically significant (P = .07). And 40% of the rebound group had a history of miscarriage versus 32.6% in the non-rebound group, but was also not significantly different (P = .56).
The authors noted that half of those in the rebound group developed hyperthyroidism more than 4 weeks after their withdrawal from antithyroid medications, “which seemed to have circumvented the most sensitive period of teratogenesis between 6 and 10 weeks of pregnancy.”
Hou added that restarting antithyroid medication did not increase the risk of adverse outcomes for offspring.
“A low dose of antithyroid medications may be a good choice for women with subnormal TSH and/or positive TRAb in early pregnancy,” Hou concluded. “Because of the small size of our study, a larger prospective study is needed to overcome the potential selection bias and to verify the conclusions.”
Findings consistent with Finnish study
In her own recent study, which included 2,144 women in Finland who experienced hyperthyroidism during pregnancy, Dr. Turunen and colleagues found that having hyperthyroidism, with or without antithyroid drug treatment, was associated with an increased odds of pregnancy and/or prenatal complications, compared with those without thyroid disease.
“In our study, we observed an increased risk of adverse pregnancy outcomes also in mothers with previous diagnosis and/or treatment of hyperthyroidism, not only with overt hyperthyroidism treated with antithyroid drugs,” she told this news organization.
“I think that especially those patients with positive antibodies [TRAbs] are at risk even if they are euthyroid,” she noted. “Withdrawal of antithyroid drugs in these patients is a risk.”
“Probably continuing antithyroid treatment with low dose is a better option,” she said.
The authors and Dr. Turunen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Discontinuing antithyroid drugs during early pregnancy is linked to a possible rebound of hyperthyroidism and a high risk of adverse pregnancy outcomes, new research shows.
“Our study provides preliminary evidence that the risk of rebound increases in women with subnormal thyroid-stimulating hormone (TSH) and/or positive thyrotropin receptor antibody (TRAb) who stop antithyroid drugs in early pregnancy,” first author Xin Hou told this news organization.
“When discussing the pros and cons of antithyroid drug withdrawal early in pregnancy [clinicians] should consider the level of TSH and TRAb in early pregnancy,” said Hou, of the department of endocrinology and metabolism, Institute of Endocrinology, The First Affiliated Hospital of China Medical University, Shenyang.
Suvi Turunen, MD, of the University of Oulu (Finland), who has also conducted research on the issue, said the study adds important insights.
“I find this study very interesting,” Dr. Turunen said in an interview. “It is well known that medical treatment of hyperthyroidism outweighs the potential harms of antithyroid treatment.”
The new findings add to the evidence, she added. “I think that withdrawal of antithyroid drugs should be carefully considered, especially with autoantibody-positive patients,” Dr. Turunen said.
Hyperthyroidism a risk in pregnancy – with or without treatment
The potential risks of hyperthyroidism in pregnancy are well established and can range from preeclampsia to premature birth or miscarriage.
However, antithyroid drugs, including methimazole and propylthiouracil, carry their own risks. In crossing the placental barrier, the drugs can increase the risk of birth defects, particularly during 6-10 weeks of gestation, yet their discontinuation is linked to as much as a 50%-60% risk of relapse, the authors explain.
Because of the risks, the American Thyroid Association recommends that “women with a stable euthyroid state on 5-10 mg methimazole per day achieved within a few months, and a falling TRAb level, are likely candidates to withdraw from antithyroid drug therapy in early pregnancy,” the authors noted.
However, as the recommendations for women who are already pregnant are largely based on evidence from nonpregnant patients, Hou and colleagues sought to evaluate withdrawal among women who were pregnant.
For the study, published in Thyroid, they enrolled 63 women who were pregnant and part of an outpatient service of the department of endocrinology and metabolism at The First Affiliated Hospital of China Medical University, between September 2014 and March 2017, who had well-controlled hyperthyroidism in early pregnancy and discontinued the drugs.
The women were an average age of 27 years, and 28 were multigravida. Twenty-two had a history of miscarriage.
A follow-up of the patients until the end of their pregnancy showed that, overall, 20 (31.7%) had a rebound of hyperthyroidism during their pregnancy after withdrawing from the drugs.
Key factors associated with the highest risk of a rebound after discontinuation included having subnormal TSH levels (TSH < 0.35 mIU/L; odds ratio, 5.12; P = .03) or having positive TRAb (TRAb > 1.75 IU/L; OR, 3.79; P = .02) at the time of medication withdrawal, compared with those with either normal TSH levels or negative TRAb.
The combination of both subnormal TSH and positive TRAb at the time of antithyroid medication withdrawal further boosted the risk of hyperthyroidism rebound (83.3%, 5 of 6), compared with those who had both normal TSH and negative TRAb (13%, 3 of 23; OR, 33.33; P = .003).
Adverse pregnancy outcomes increased
Importantly, among the 20 patients who had a rebound, 11 (55%) had adverse pregnancy outcomes, including miscarriage, premature birth, induced labor, gestational hypertension, and gestational diabetes, compared with only 4 (9.3%) of the 43 who had no rebound (OR, 11.92; P = .0002).
Neonatal abnormalities were also higher among those experiencing a rebound (20% vs. 4.7%), however, the authors noted that “larger prospective studies are required to conclude whether antithyroid drug withdrawal affects fetal outcome.”
In the rebound group, the mean duration of antithyroid medication use was 24.7 months versus 35.1 months in the nonrebound group, however, the difference was not statistically significant (P = .07). And 40% of the rebound group had a history of miscarriage versus 32.6% in the non-rebound group, but was also not significantly different (P = .56).
The authors noted that half of those in the rebound group developed hyperthyroidism more than 4 weeks after their withdrawal from antithyroid medications, “which seemed to have circumvented the most sensitive period of teratogenesis between 6 and 10 weeks of pregnancy.”
Hou added that restarting antithyroid medication did not increase the risk of adverse outcomes for offspring.
“A low dose of antithyroid medications may be a good choice for women with subnormal TSH and/or positive TRAb in early pregnancy,” Hou concluded. “Because of the small size of our study, a larger prospective study is needed to overcome the potential selection bias and to verify the conclusions.”
Findings consistent with Finnish study
In her own recent study, which included 2,144 women in Finland who experienced hyperthyroidism during pregnancy, Dr. Turunen and colleagues found that having hyperthyroidism, with or without antithyroid drug treatment, was associated with an increased odds of pregnancy and/or prenatal complications, compared with those without thyroid disease.
“In our study, we observed an increased risk of adverse pregnancy outcomes also in mothers with previous diagnosis and/or treatment of hyperthyroidism, not only with overt hyperthyroidism treated with antithyroid drugs,” she told this news organization.
“I think that especially those patients with positive antibodies [TRAbs] are at risk even if they are euthyroid,” she noted. “Withdrawal of antithyroid drugs in these patients is a risk.”
“Probably continuing antithyroid treatment with low dose is a better option,” she said.
The authors and Dr. Turunen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Discontinuing antithyroid drugs during early pregnancy is linked to a possible rebound of hyperthyroidism and a high risk of adverse pregnancy outcomes, new research shows.
“Our study provides preliminary evidence that the risk of rebound increases in women with subnormal thyroid-stimulating hormone (TSH) and/or positive thyrotropin receptor antibody (TRAb) who stop antithyroid drugs in early pregnancy,” first author Xin Hou told this news organization.
“When discussing the pros and cons of antithyroid drug withdrawal early in pregnancy [clinicians] should consider the level of TSH and TRAb in early pregnancy,” said Hou, of the department of endocrinology and metabolism, Institute of Endocrinology, The First Affiliated Hospital of China Medical University, Shenyang.
Suvi Turunen, MD, of the University of Oulu (Finland), who has also conducted research on the issue, said the study adds important insights.
“I find this study very interesting,” Dr. Turunen said in an interview. “It is well known that medical treatment of hyperthyroidism outweighs the potential harms of antithyroid treatment.”
The new findings add to the evidence, she added. “I think that withdrawal of antithyroid drugs should be carefully considered, especially with autoantibody-positive patients,” Dr. Turunen said.
Hyperthyroidism a risk in pregnancy – with or without treatment
The potential risks of hyperthyroidism in pregnancy are well established and can range from preeclampsia to premature birth or miscarriage.
However, antithyroid drugs, including methimazole and propylthiouracil, carry their own risks. In crossing the placental barrier, the drugs can increase the risk of birth defects, particularly during 6-10 weeks of gestation, yet their discontinuation is linked to as much as a 50%-60% risk of relapse, the authors explain.
Because of the risks, the American Thyroid Association recommends that “women with a stable euthyroid state on 5-10 mg methimazole per day achieved within a few months, and a falling TRAb level, are likely candidates to withdraw from antithyroid drug therapy in early pregnancy,” the authors noted.
However, as the recommendations for women who are already pregnant are largely based on evidence from nonpregnant patients, Hou and colleagues sought to evaluate withdrawal among women who were pregnant.
For the study, published in Thyroid, they enrolled 63 women who were pregnant and part of an outpatient service of the department of endocrinology and metabolism at The First Affiliated Hospital of China Medical University, between September 2014 and March 2017, who had well-controlled hyperthyroidism in early pregnancy and discontinued the drugs.
The women were an average age of 27 years, and 28 were multigravida. Twenty-two had a history of miscarriage.
A follow-up of the patients until the end of their pregnancy showed that, overall, 20 (31.7%) had a rebound of hyperthyroidism during their pregnancy after withdrawing from the drugs.
Key factors associated with the highest risk of a rebound after discontinuation included having subnormal TSH levels (TSH < 0.35 mIU/L; odds ratio, 5.12; P = .03) or having positive TRAb (TRAb > 1.75 IU/L; OR, 3.79; P = .02) at the time of medication withdrawal, compared with those with either normal TSH levels or negative TRAb.
The combination of both subnormal TSH and positive TRAb at the time of antithyroid medication withdrawal further boosted the risk of hyperthyroidism rebound (83.3%, 5 of 6), compared with those who had both normal TSH and negative TRAb (13%, 3 of 23; OR, 33.33; P = .003).
Adverse pregnancy outcomes increased
Importantly, among the 20 patients who had a rebound, 11 (55%) had adverse pregnancy outcomes, including miscarriage, premature birth, induced labor, gestational hypertension, and gestational diabetes, compared with only 4 (9.3%) of the 43 who had no rebound (OR, 11.92; P = .0002).
Neonatal abnormalities were also higher among those experiencing a rebound (20% vs. 4.7%), however, the authors noted that “larger prospective studies are required to conclude whether antithyroid drug withdrawal affects fetal outcome.”
In the rebound group, the mean duration of antithyroid medication use was 24.7 months versus 35.1 months in the nonrebound group, however, the difference was not statistically significant (P = .07). And 40% of the rebound group had a history of miscarriage versus 32.6% in the non-rebound group, but was also not significantly different (P = .56).
The authors noted that half of those in the rebound group developed hyperthyroidism more than 4 weeks after their withdrawal from antithyroid medications, “which seemed to have circumvented the most sensitive period of teratogenesis between 6 and 10 weeks of pregnancy.”
Hou added that restarting antithyroid medication did not increase the risk of adverse outcomes for offspring.
“A low dose of antithyroid medications may be a good choice for women with subnormal TSH and/or positive TRAb in early pregnancy,” Hou concluded. “Because of the small size of our study, a larger prospective study is needed to overcome the potential selection bias and to verify the conclusions.”
Findings consistent with Finnish study
In her own recent study, which included 2,144 women in Finland who experienced hyperthyroidism during pregnancy, Dr. Turunen and colleagues found that having hyperthyroidism, with or without antithyroid drug treatment, was associated with an increased odds of pregnancy and/or prenatal complications, compared with those without thyroid disease.
“In our study, we observed an increased risk of adverse pregnancy outcomes also in mothers with previous diagnosis and/or treatment of hyperthyroidism, not only with overt hyperthyroidism treated with antithyroid drugs,” she told this news organization.
“I think that especially those patients with positive antibodies [TRAbs] are at risk even if they are euthyroid,” she noted. “Withdrawal of antithyroid drugs in these patients is a risk.”
“Probably continuing antithyroid treatment with low dose is a better option,” she said.
The authors and Dr. Turunen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THYROID
Primary cesarean delivery rates in the United States
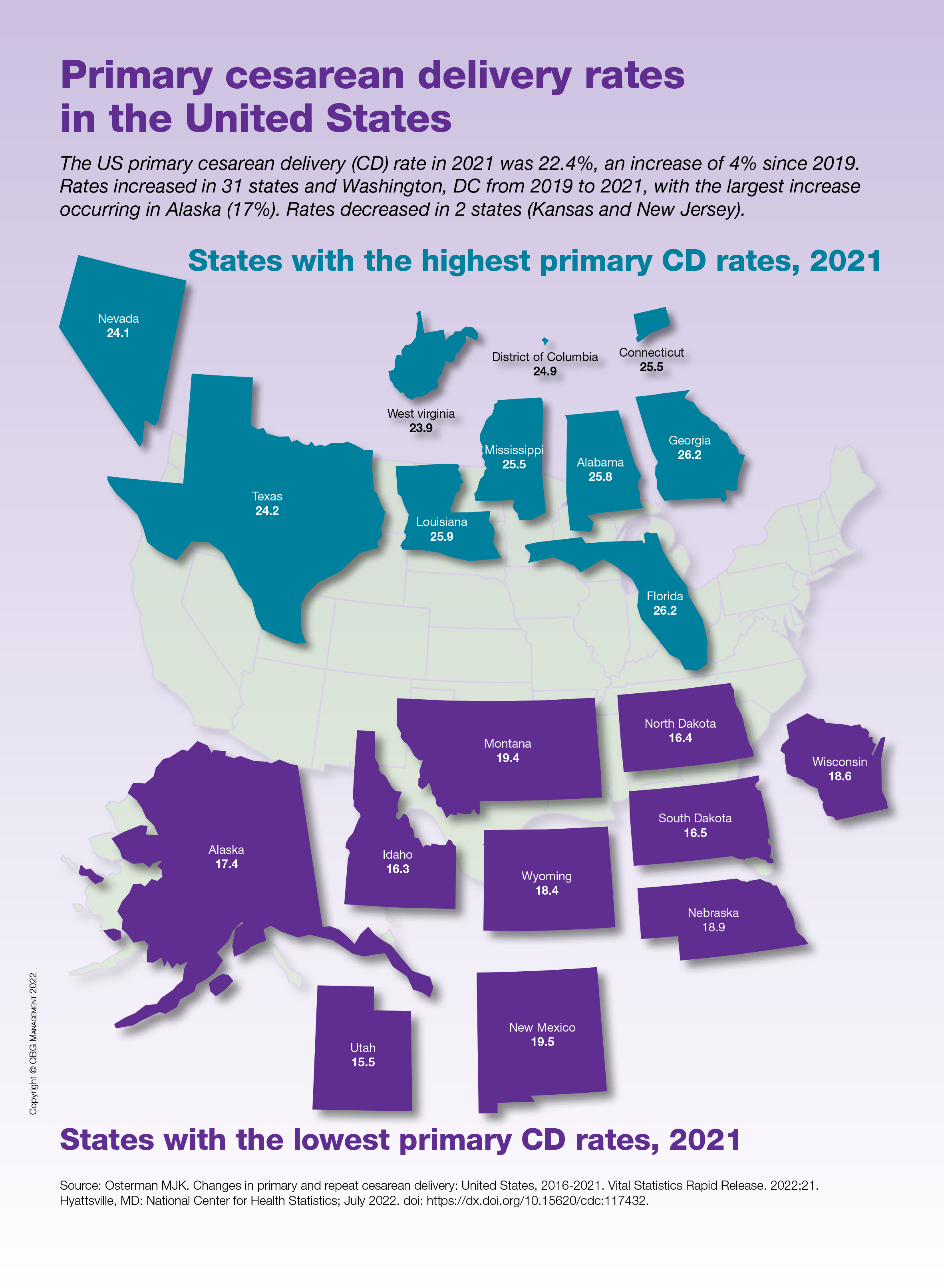


Vaginal birth possible in 50% of women with low-lying placenta
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY


